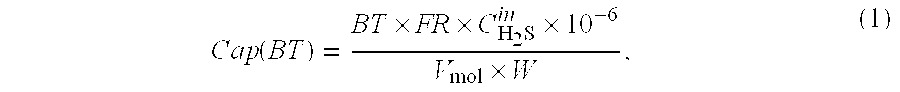Patents
Literature
955 results about "Sorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sorption is a physical and chemical process by which one substance becomes attached to another. The reverse of sorption is desorption.
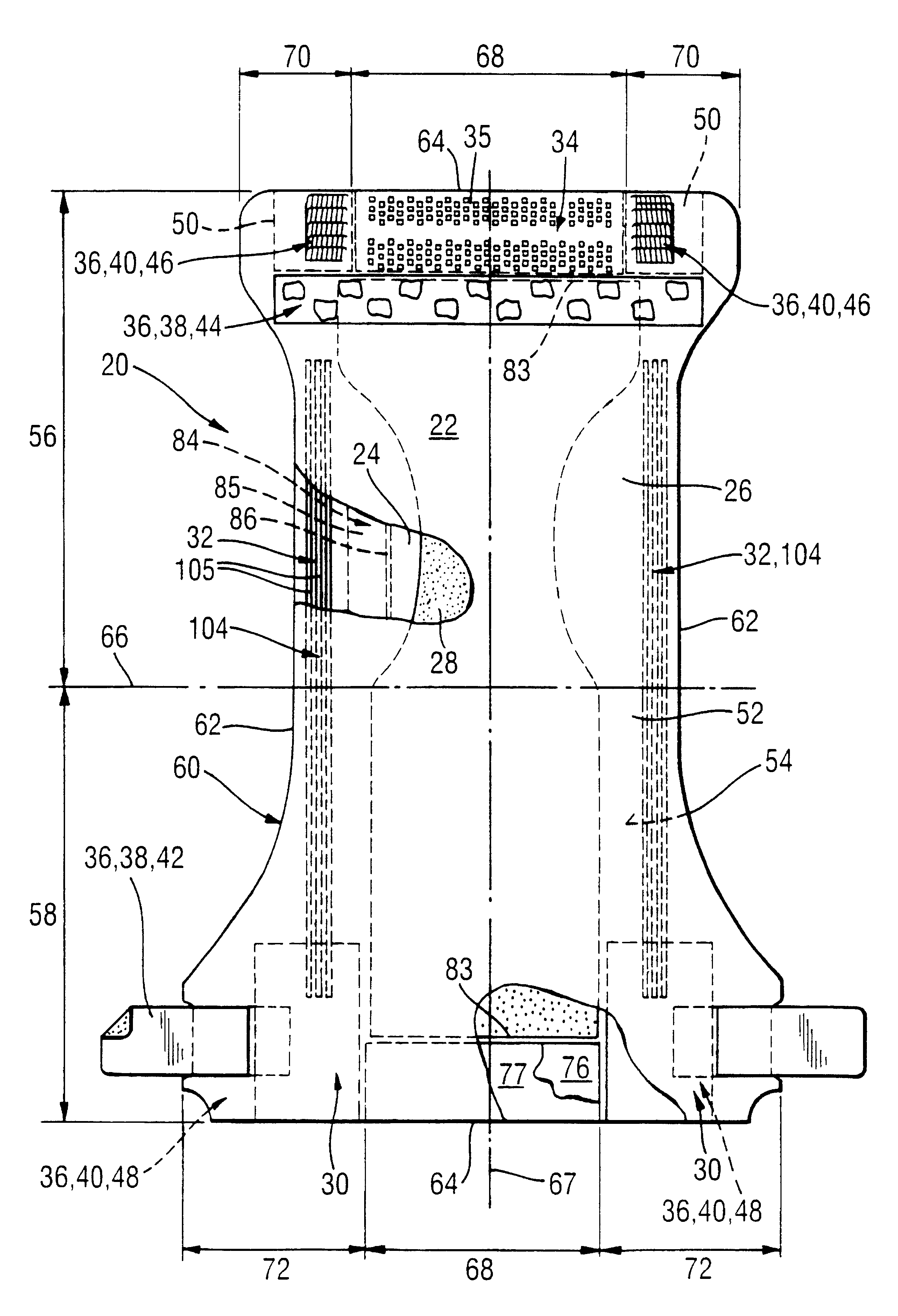
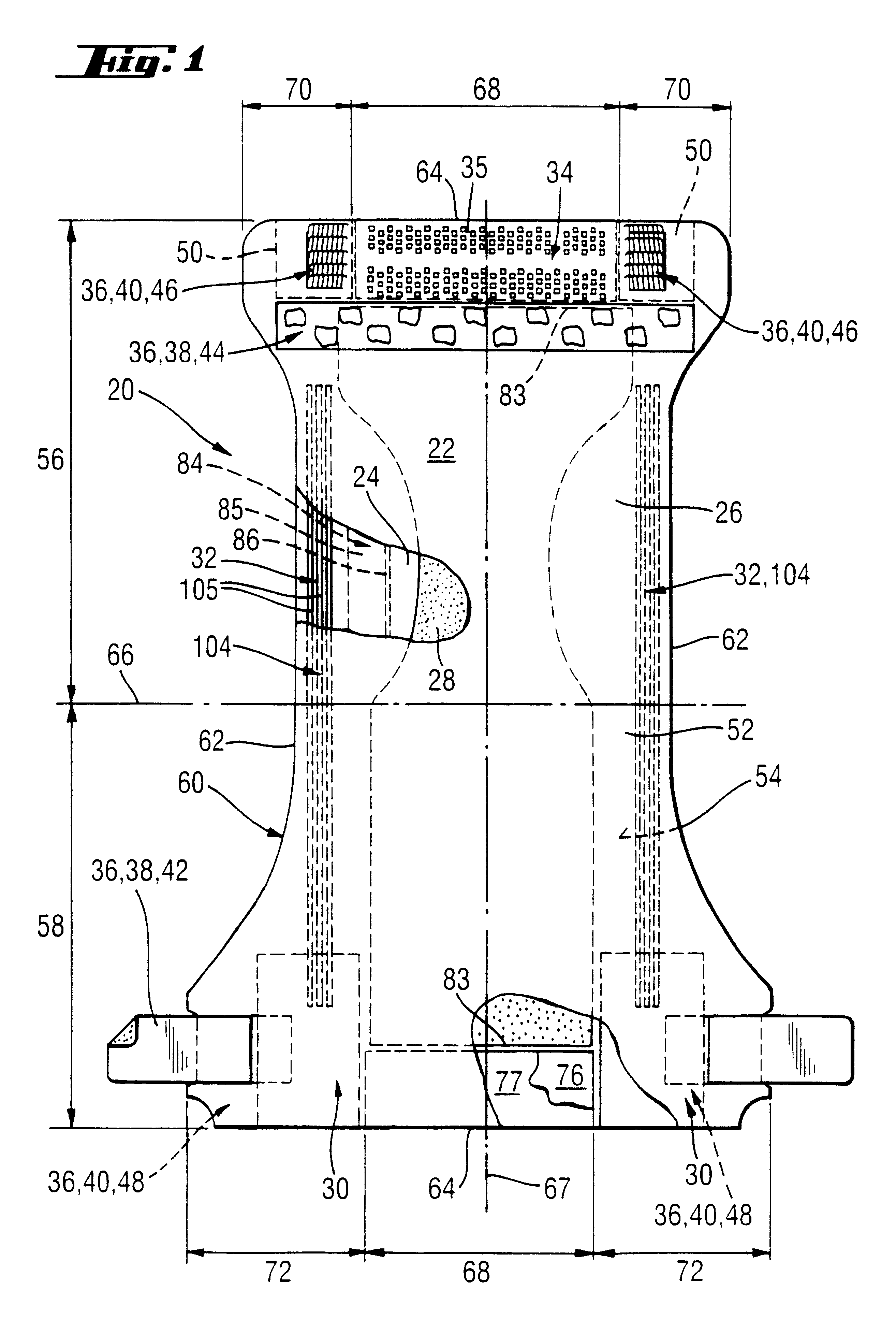
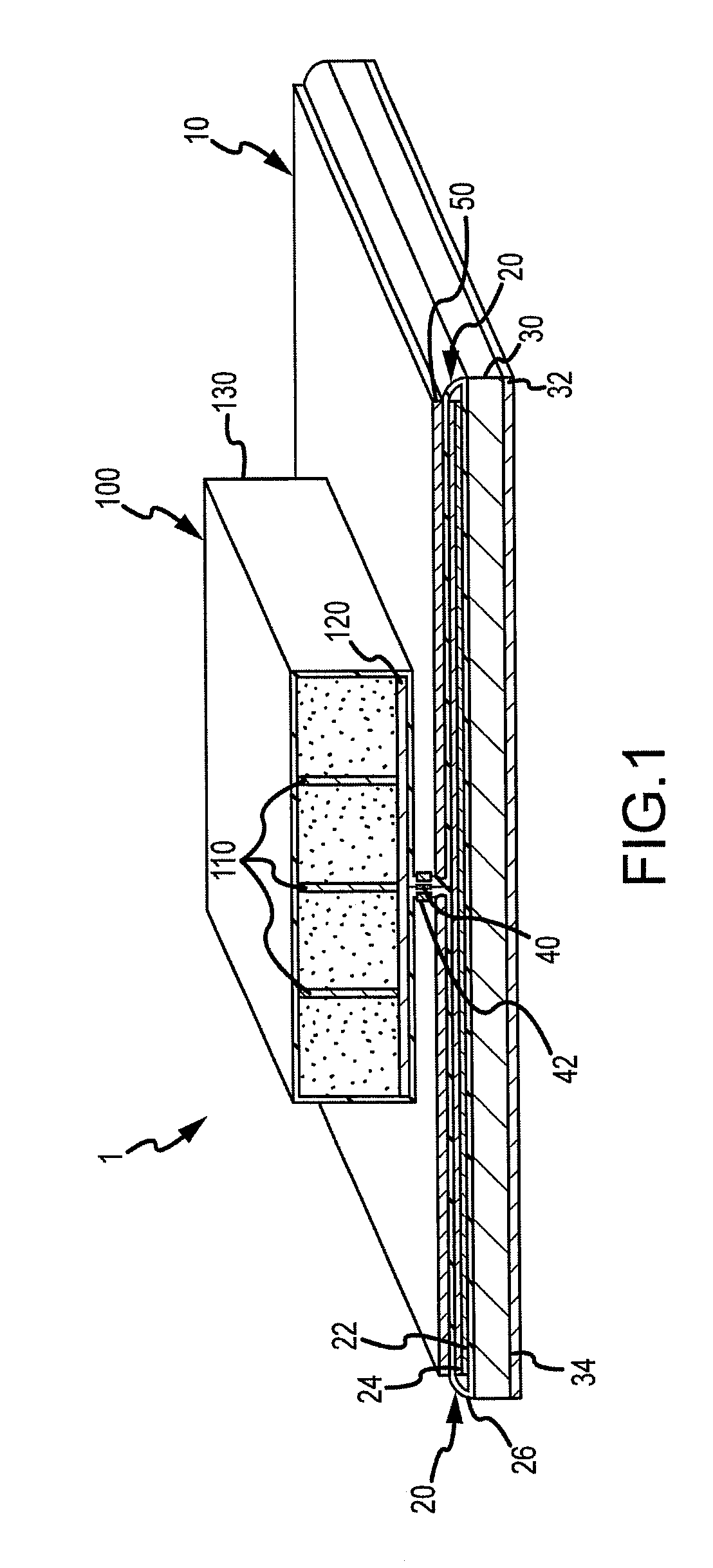
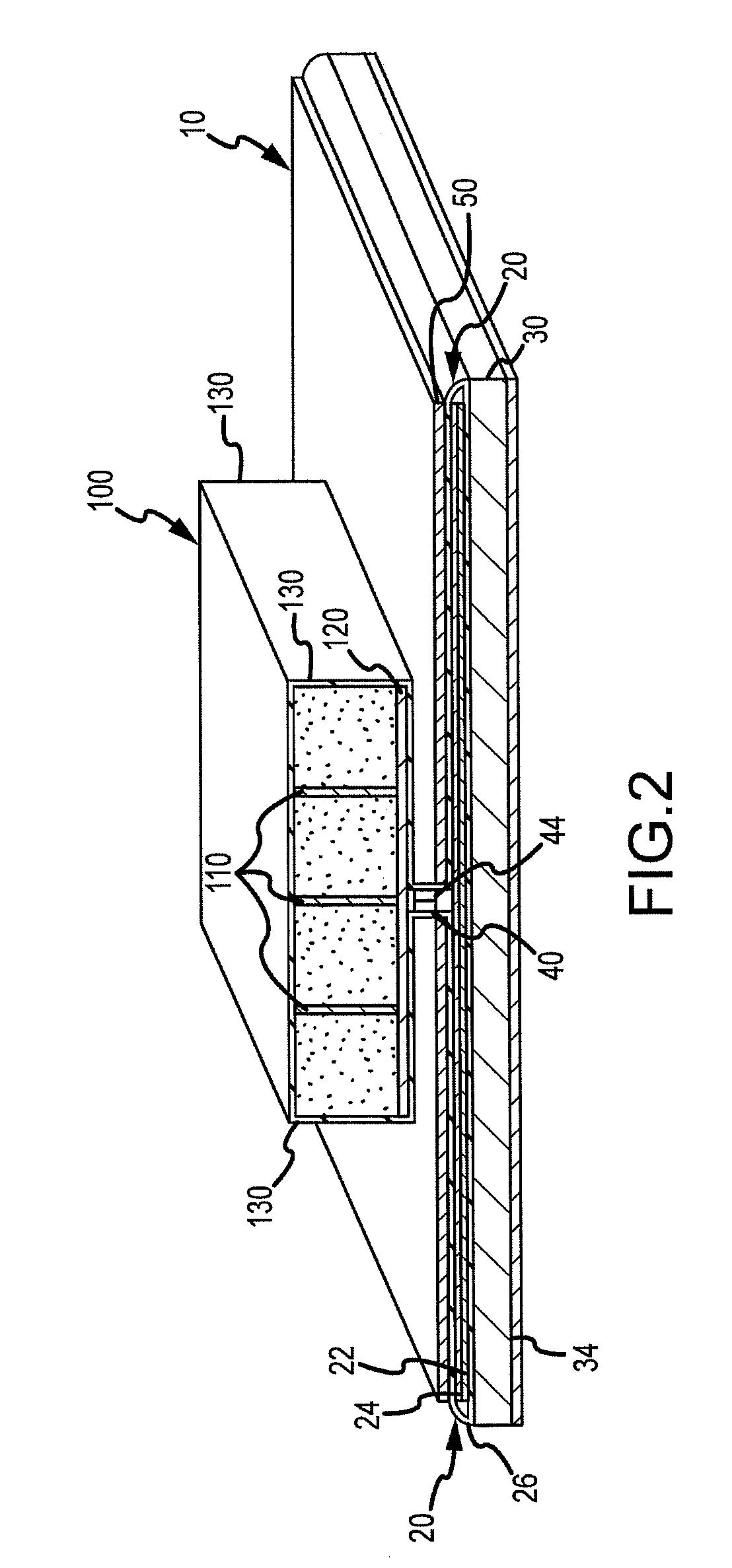
Absorbent articles with distribution materials positioned underneath storage material
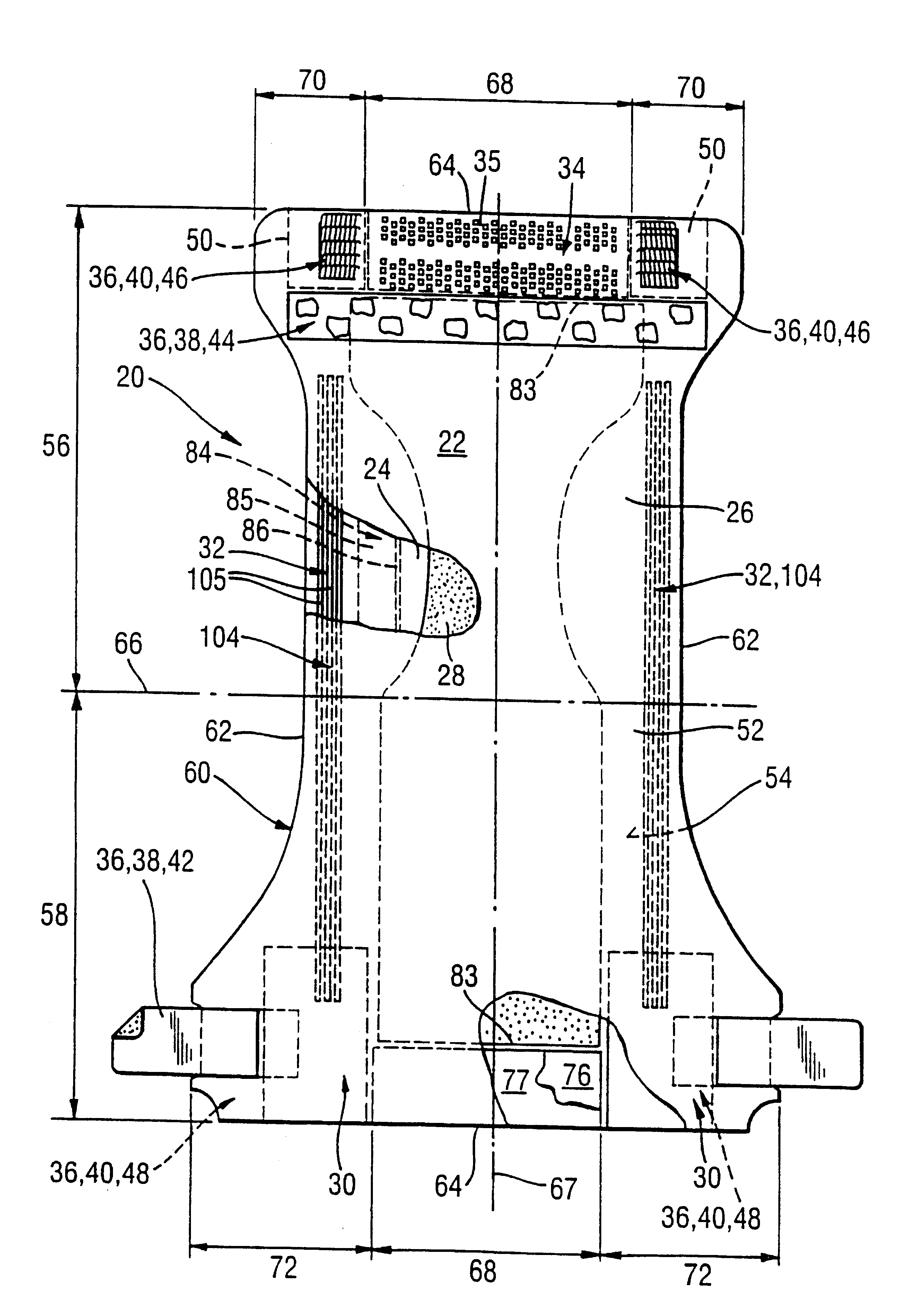
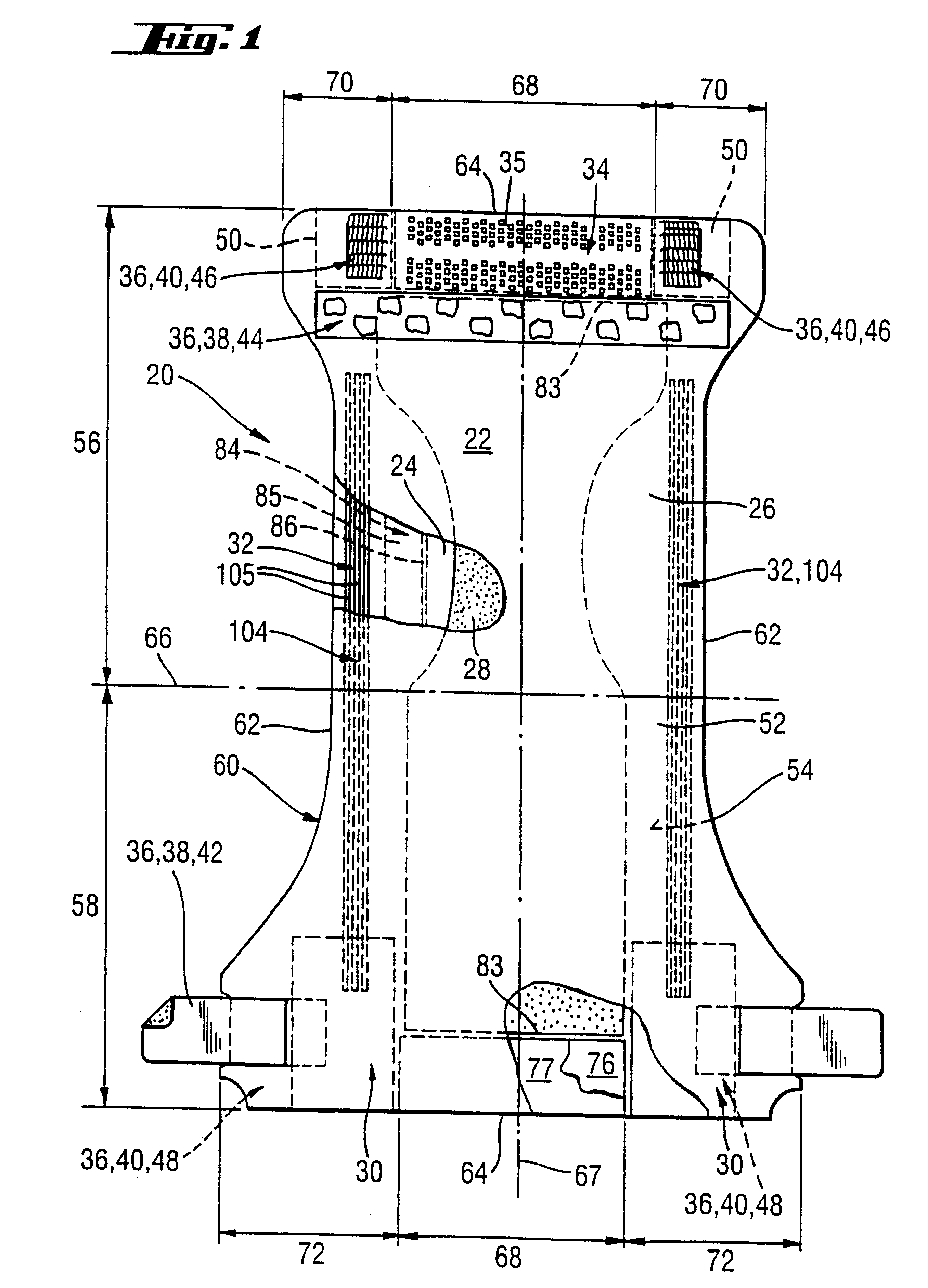
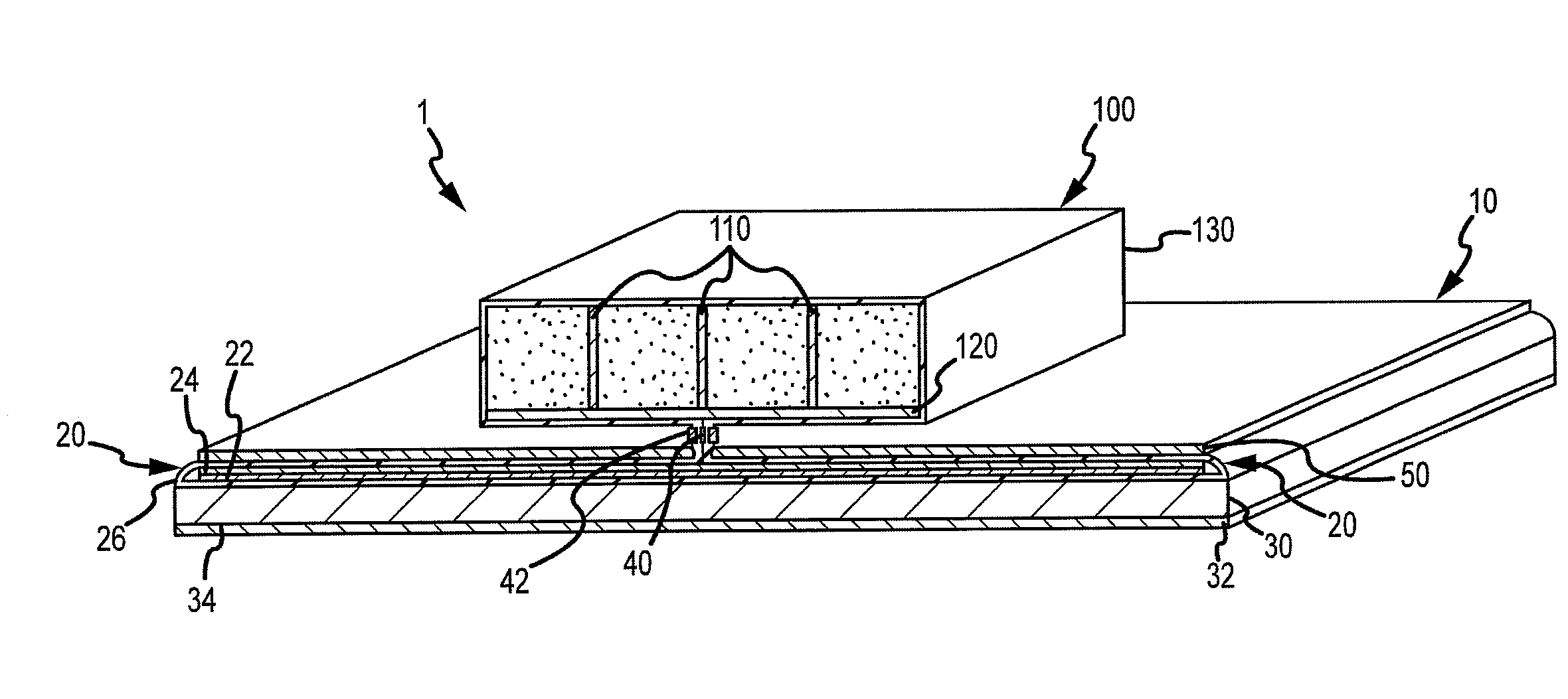
An absorbent article having an ultimate fluid storage region and a fluid distribution region, positioned between the ultimate storage region and the garment oriented surface of the article, in fluid communication with the ultimate fluid storage region, the ultimate fluid storage region includes a material which has: (1) a Capillary Sorption Desorption Capacity at 100 cm (CSDC 100) of at least 10 g / g; (2) a Capillary Sorption Desorption Capacity at 0 cm (CSDC 0) higher than the CSDC 100; (3) a Loosely Bound Liquid Capacity (LBLC); and (4) a Capillary Sorption Desorption Release Height when 50% of the LBLC are released (CSDRH 50) less than 60 cm. Further, the liquid distribution layer material has a Capillary Sorption Absorption Height at 30% of its maximum capacity (CSAH 30) of at least 35 cm. Distribution material can be foam materials, particularly those derived from high internal phase water-in-oil emulsions.
Owner:THE PROCTER & GAMBLE COMPANY
Detection of nucleic acids and nucleic acid units
InactiveUS6127120AReduce riskReducing operator timeSugar derivativesMicrobiological testing/measurementNucleotideBiology
PCT No. PCT / GB96 / 01830 Sec. 371 Date Apr. 21, 1998 Sec. 102(e) Date Apr. 21, 1998 PCT Filed Jul. 25, 1996 PCT Pub. No. WO97 / 05280 PCT Pub. Date Feb. 13, 1997The invention relates to the detection of target nucleic acids or nucleic acid units in a sample, by obtaining a SER(R)S spectrum for a SER(R)S-active complex containing, or derived directly from, the target. The complex includes at least a SER(R)S-active label, and optionally a target binding species containing a nucleic acid or nucleic acid unit. In this detection method, the concentration of the target present in the SER(R)S-active complex, or of the nucleic acid or unit contained in the target binding species in the SER(R)S-active complex, is no higher than 10-10 moles per liter. Additionally or alternatively, one or more of the following features may be used with the method: i) the introduction of a polyamine; ii) modification of the target, and / or of the nucleic acid or nucleic acid unit contained in the target binding species, in a manner that promotes or facilitates its chemi-sorption onto a SER(R)S-active surface; iii) inclusion of a chemi-sorptive functional group in the SER(R)S-active label. The invention also provides SER(R)S-active complexes for use in such a method, a kit for use in carrying out the method or preparing the complexes and a method for sequencing a nucleic acid which comprises the use of the detection method to detect at least one target nucleotide or sequence of nucleotides within the acid.
Owner:RENISHAW DIAGNOSTICS
Method and system for purifying hydraulic-oil
InactiveCN1546198AEasy to installEasy to operateFiltration circuitsLubricant compositionControl systemFuel tank
The invention refers to a method and system for purifying the hydraulic pressure oil in hydraulic pressure machine. The method includes magnetism absorbing step which absorbs the metal particles in the hydraulic oil through magnetism structure onto the magnetism structure and a filter step blocking the impurities in the sift filter core, and finishes the first grade filter, and the second filter step with a sorption filter; the system includes: forward oil boxes connected in series, oil heater, magnetism filter, oil pump, sorption filter, backwards oil boxes and electric control system. Each device is connected through oil pipes and valves, all of which are set in a mobile machine box, moves the box, the purification to hydraulic pressure oil of different locations can be carried on conveniently.
Owner:邝念曾
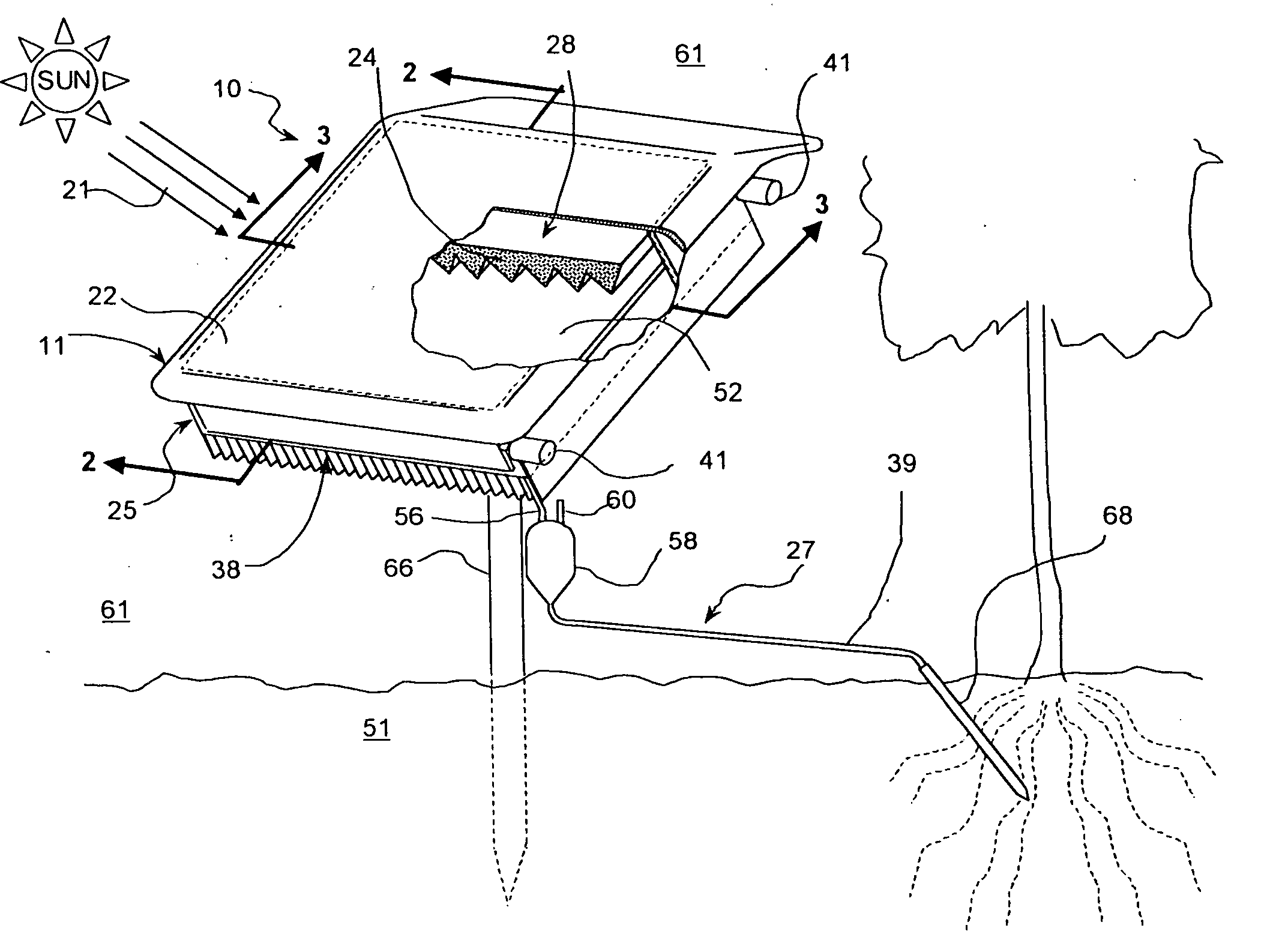
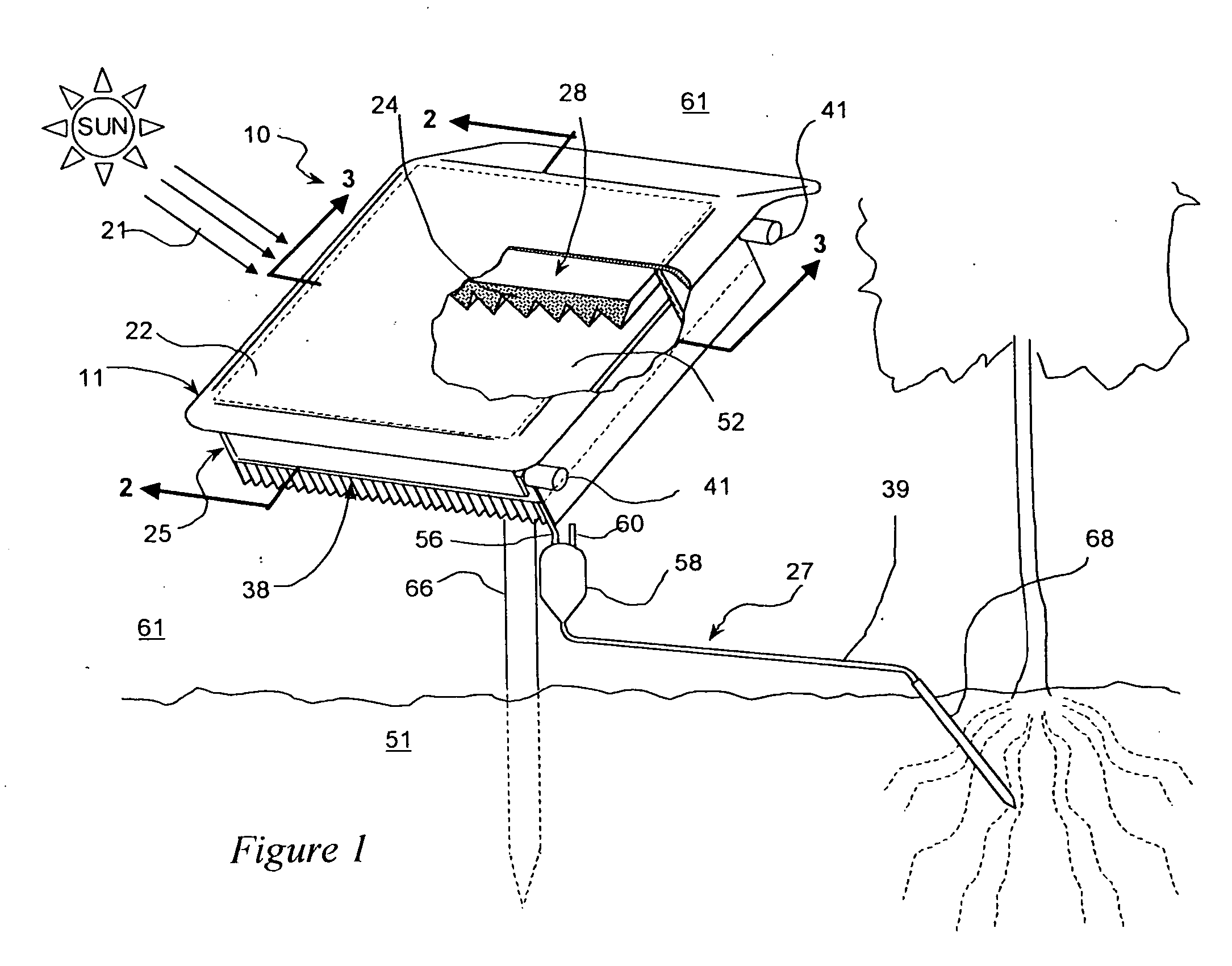
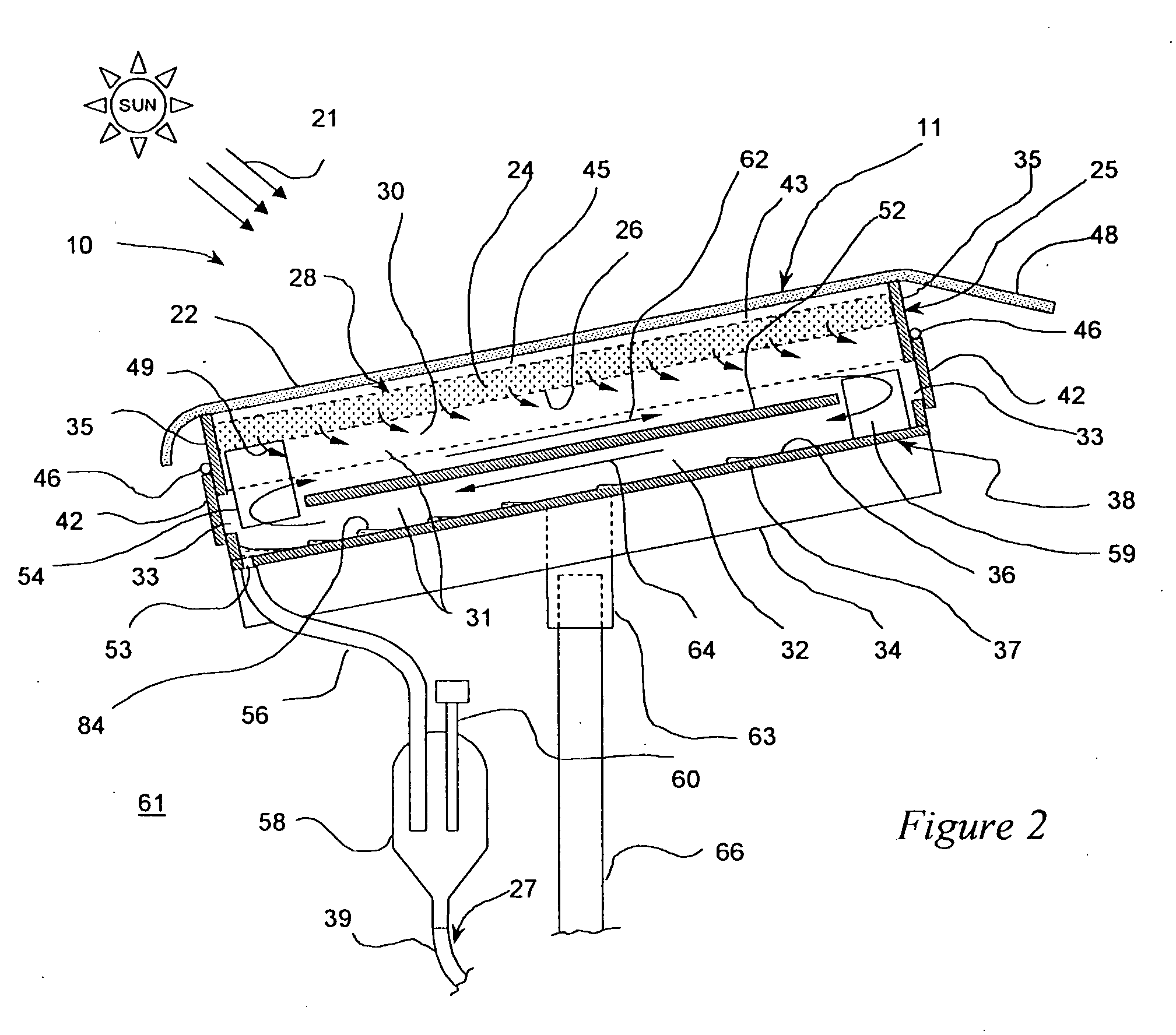
Method and apparatus for producing potable water from air including severely arid and hot climates
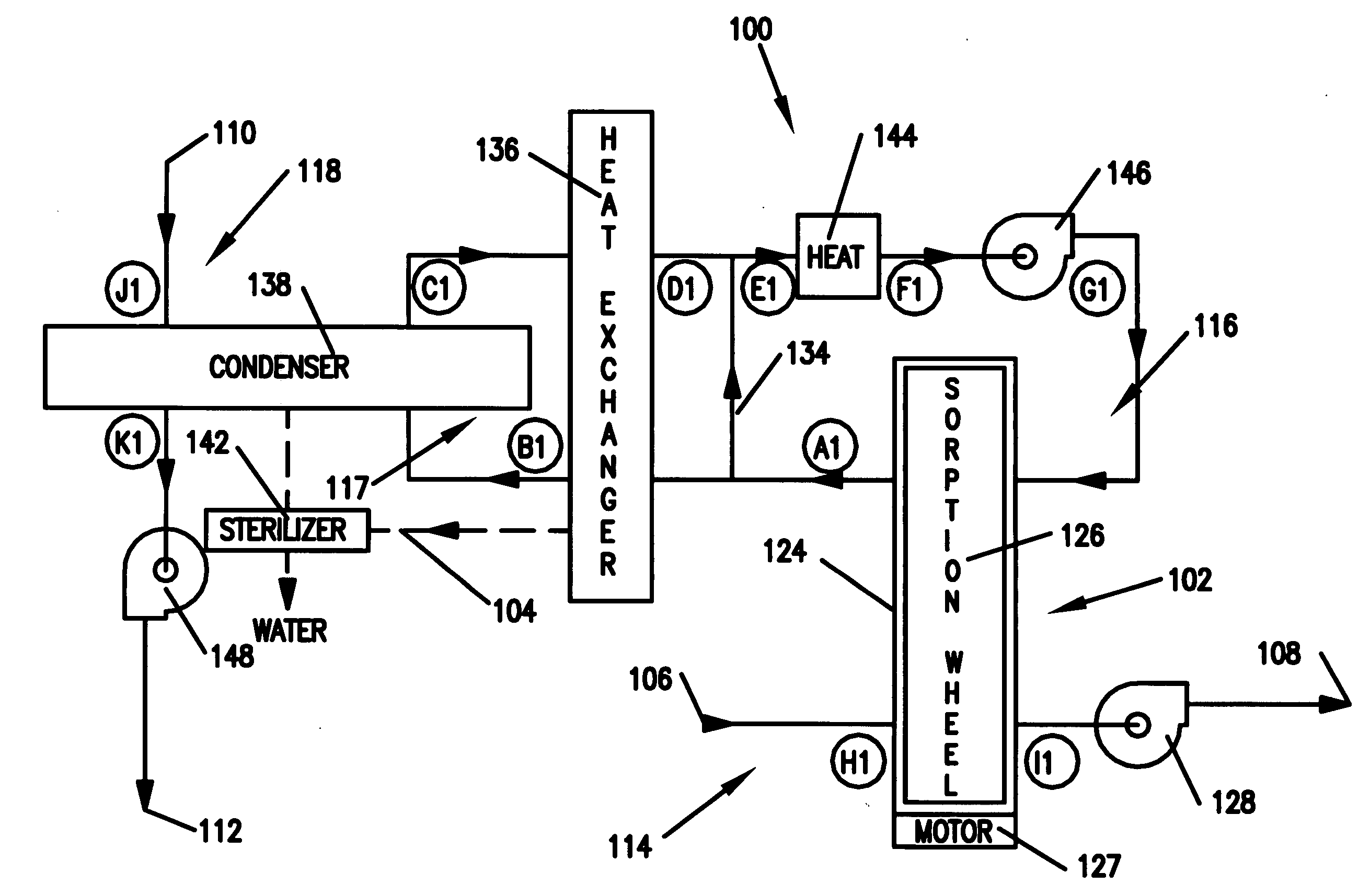
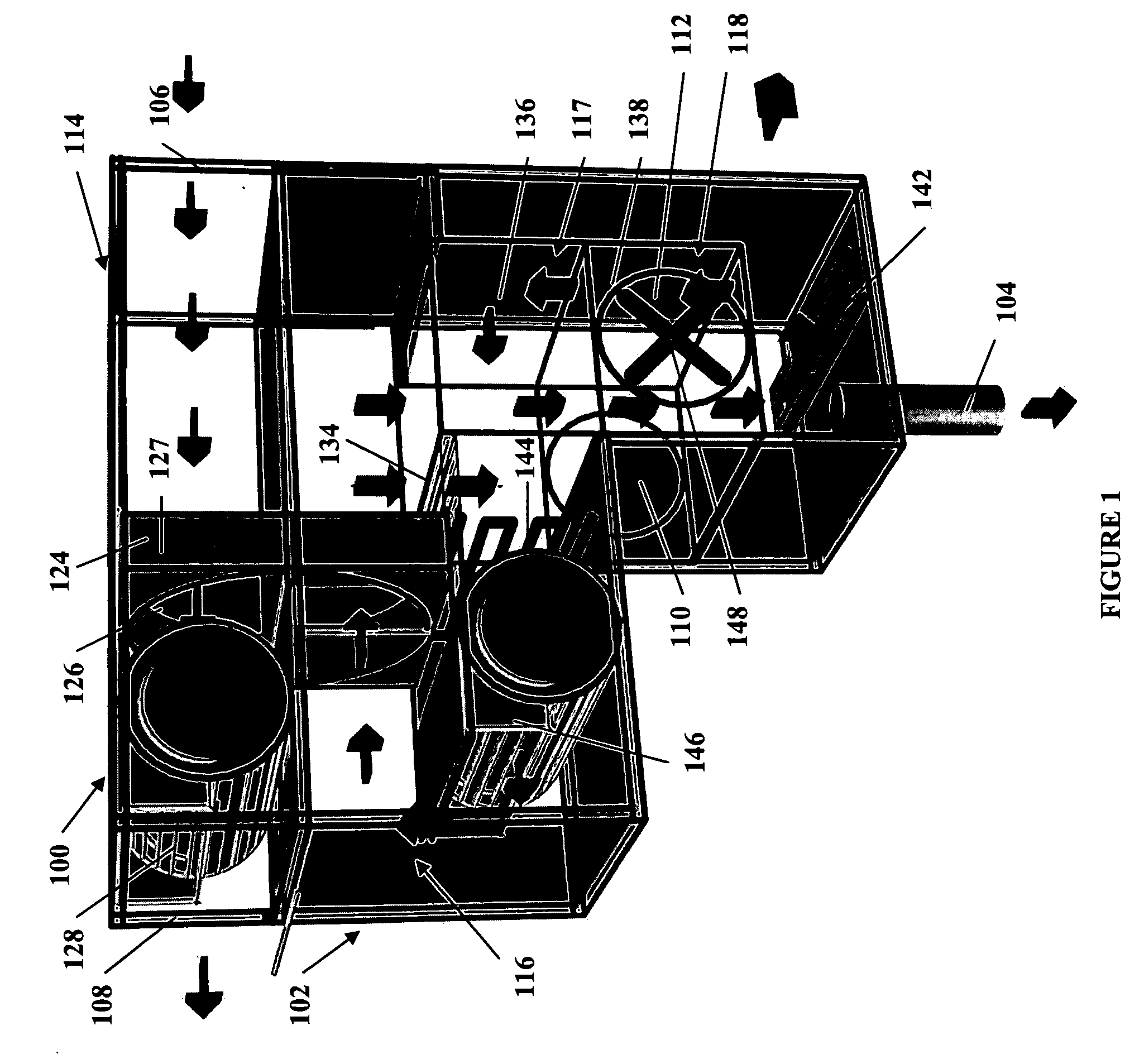
Methods and apparatus for extracting liquid water from ambient air, including ambient air in severely arid and hot climates, are described. An example apparatus uses a sorption-desorption-condensation cycle using a sorption wheel to extract moisture from ambient air and concentrate the water vapor driven off from the sorption material in a circulating gas, with condensation of liquid water from the circulating gas.
Owner:EPLEE DUSTIN M +1
Absorbent structures comprising fluid storage members with improved ability to dewater acquisition/distribution members
InactiveUS6551295B1Effectively and efficiently dewateredImprove distributionOther chemical processesBaby linensAbsorption capacityDesorption
The present invention is an absorbent structure to be used in absorbent articles, having at least a first region for acquisition / distribution of fluid and a second region for storage of fluid. The first region can contain materials which have a relatively high capillary desorption pressure, as the materials in the second region exhibit a sufficiently high capillary absorption pressure so as to still efficiently drain the first region.The first region material has a Capillary Sorption Desorption Height (CSDH 90) of more than 40 cm and the second region material satisfies at least one of following requirements:(a) an absorption capacity of at least 15 g / g at 35 cm in the capsorption test;(b) an absorption capacity of at least 15 g / g at 0 cm in the capsorption test and an absorption efficiency of at least 55% at 40 cm;(c) a Capillary Sorption Absorption height at 50% of its capacity at 0 cm absorption height (CSAH 50) of at least 35 cm in the capsorption test.
Owner:THE PROCTER & GAMBLE COMPANY
Temperature-controlled shipping container and method for using same
InactiveUS6584797B1Container filling methodsDomestic refrigeratorsTemperature controlProcess engineering
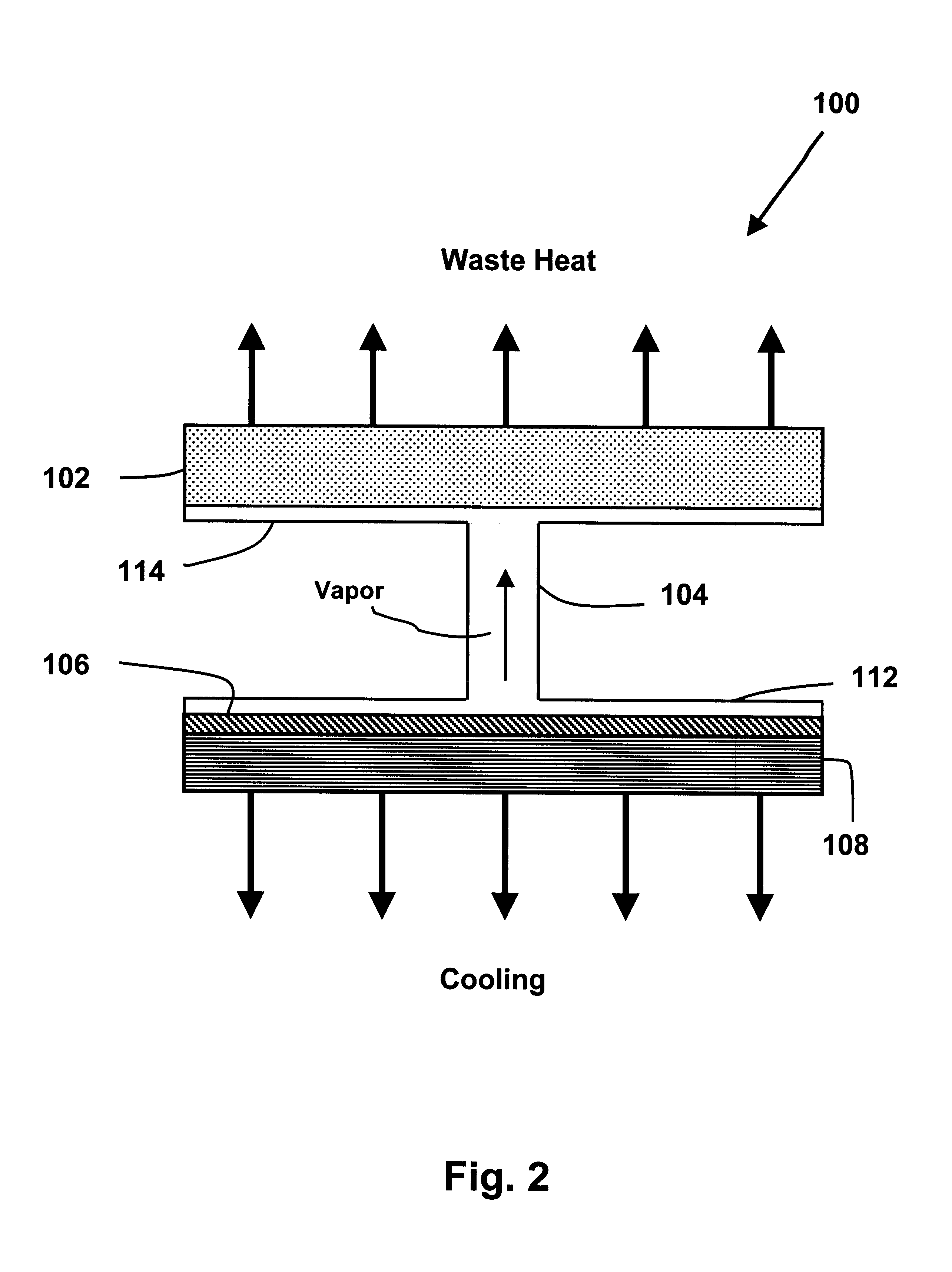
A temperature-controlled container utilizing a sorption cooling unit to maintain the temperature within the container. The sorption cooling unit cools the interior of the container and rejects waste heat to the exterior. The sorption cooling unit provides a lightweight and low volume alternative to the traditional gel pack cooling systems that are commonly used in the modern shipping industry for shipping containers.
Owner:PELICAN NANOCOOL HLDG LLC
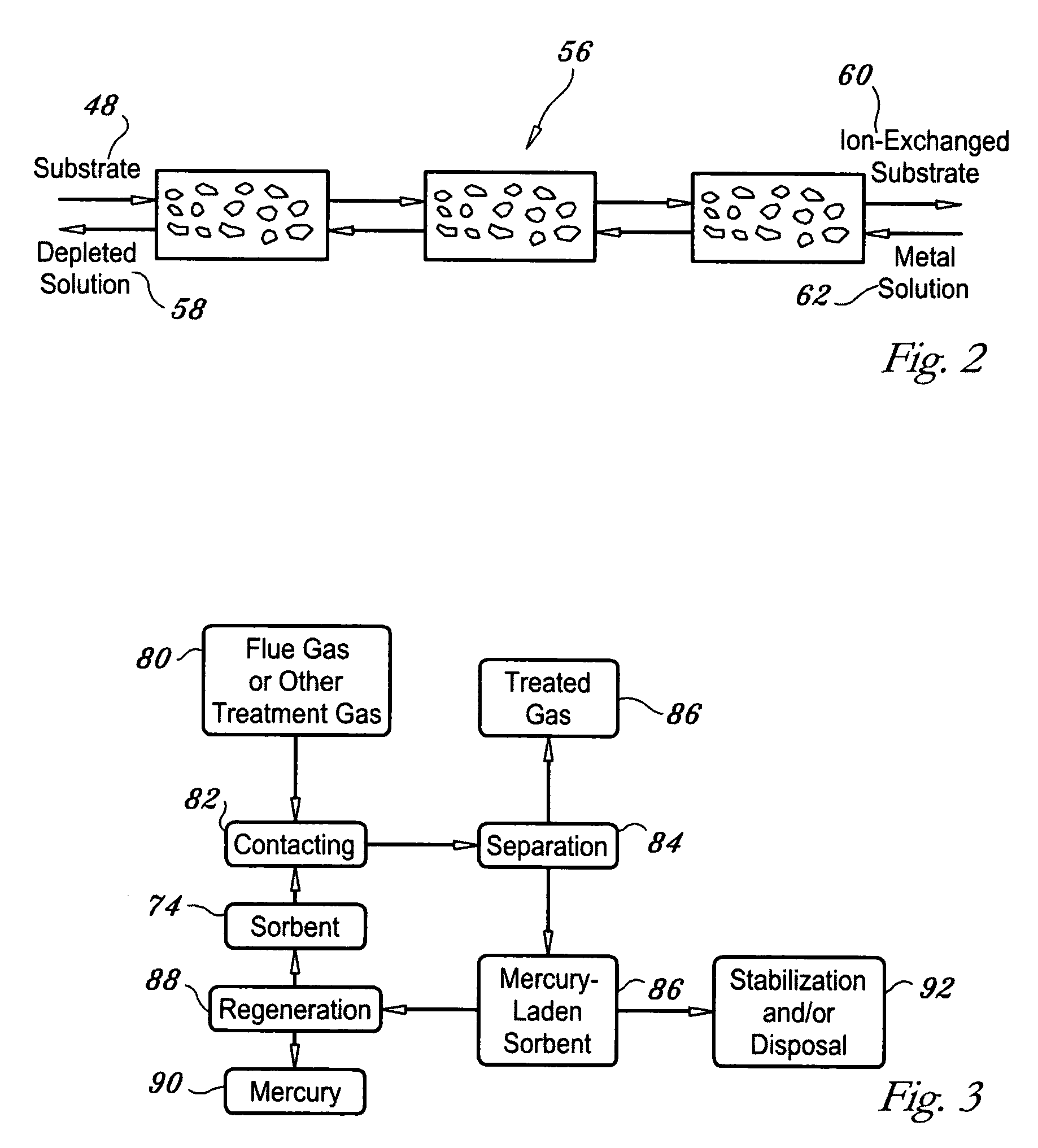
Sorbent for removal of trace hazardous air pollutants from combustion flue gas and preparation method thereof
InactiveUS20070179056A1Low costLow raw material costGas treatmentOther chemical processesSorbentToxic industrial waste
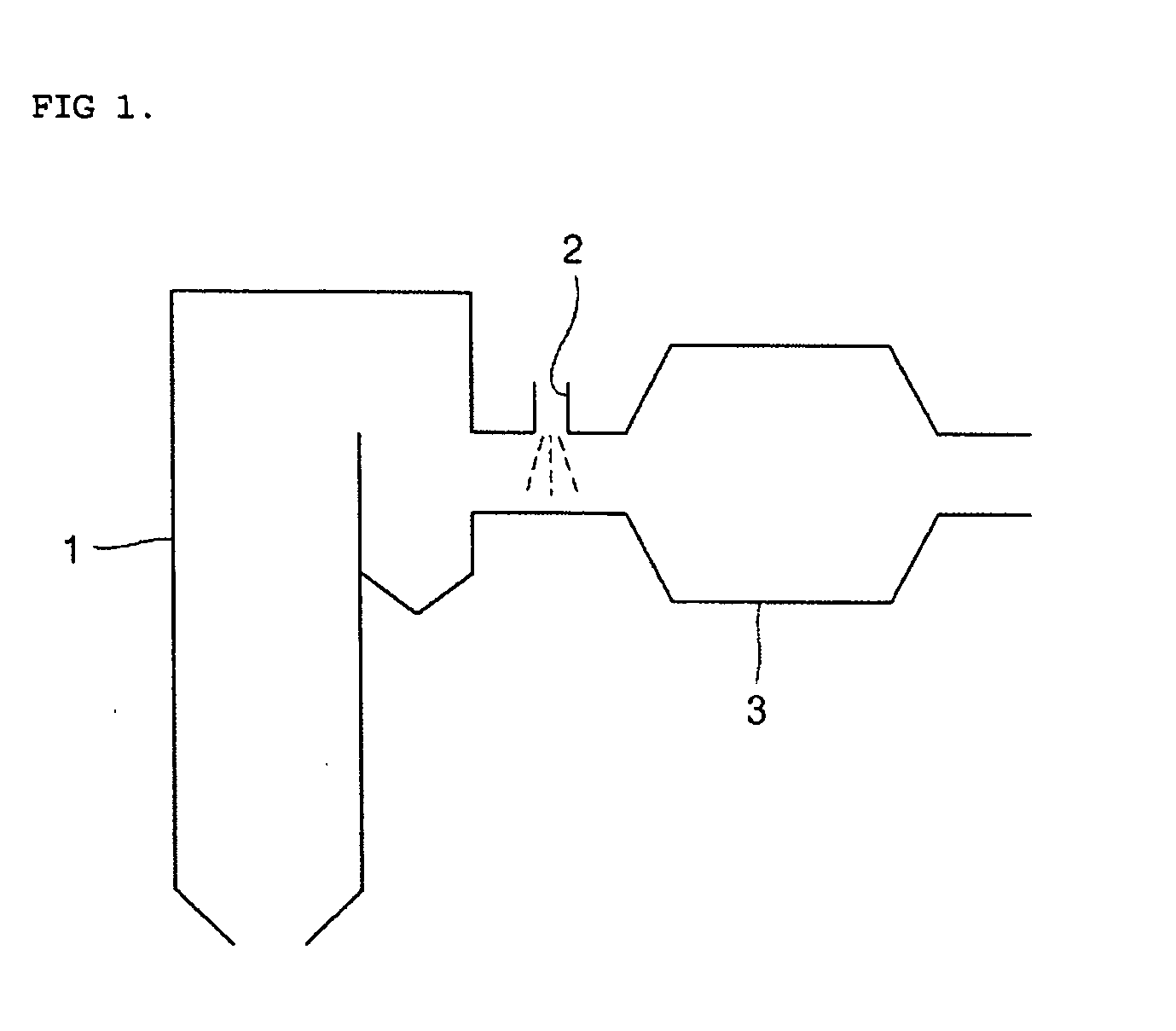
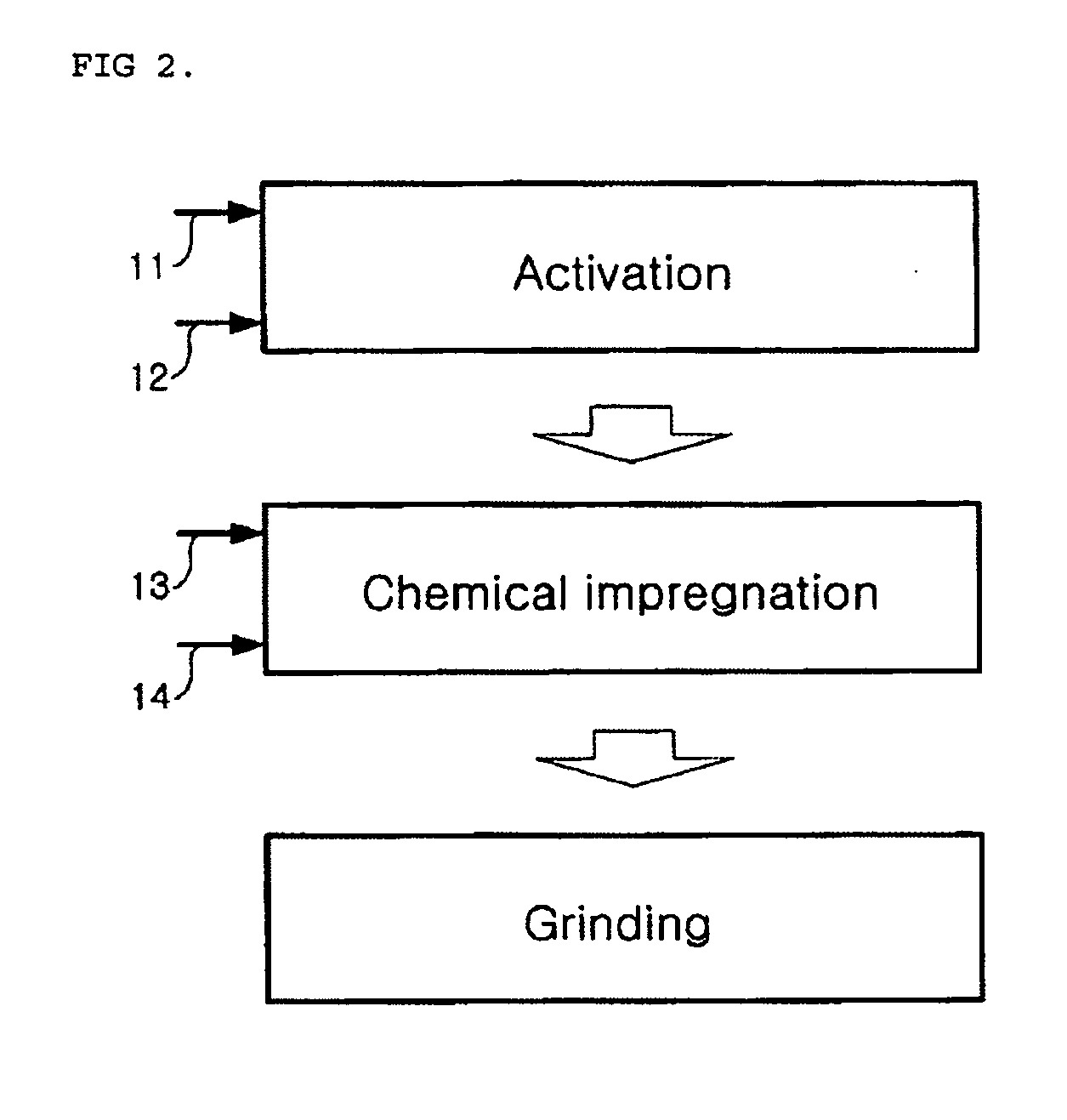
Disclosed is a sorbent for the removal of mercury from combustion flue gas and a preparation method thereof. The sorbent includes an activated heavy oil heavy ash impregnated with 0.1-30% by weight of any chemical substance selected from sulfur, iodine, bromine and chlorine. The sorbent is prepared in an economical manner using heavy oil fly ash, industrial waste generated from heavy oil-fired boilers, and has excellent sorption performance for mercury, so that a low concentration of mercury contained in combustion flue gas discharged from large-scale boilers can be removed by injection of a small amount of the sorbent. Thus, the invention can prevent a reduction in the recycling rate of coal fly ash in coal-fired power plants and minimize operation cost.
Owner:KOREA ELECTRIC POWER CORP
Autonomous water source
InactiveUS20050044862A1Reduce salt contentQuality improvementRoot feedersGeneral water supply conservationForest industryWater vapor
An autonomous water source (AWS) for extracting water from ambient air and delivering it to a plant to support growth. The system is based on an adsorption-desorption-condensation (ADC) cycle using a sorption material to extract moisture from ambient air and condensing the water vapor driven off from the sorption material by subsequent heating and followed by condensation. Liquid condensate produced in this process on the condenser is collected and delivered by gravity to a plant to reduce thermal stress and to support growth. The invention provides a sustainable source of irrigation water for agriculture and forestry, including areas where no water resources exist or are not economically viable. It can be tailored in size, and therefore, output capacity, reflecting the desired water requirements of a particular application, and can be used to replace most agricultural situations now reliant on surface water drip feed systems. The device is simple, rugged, invulnerable to rain, snow, and freezing conditions, and can be designed to last for many years without service as there are few moving parts and power required for operation is provided by sunlight.
Owner:AGWEST
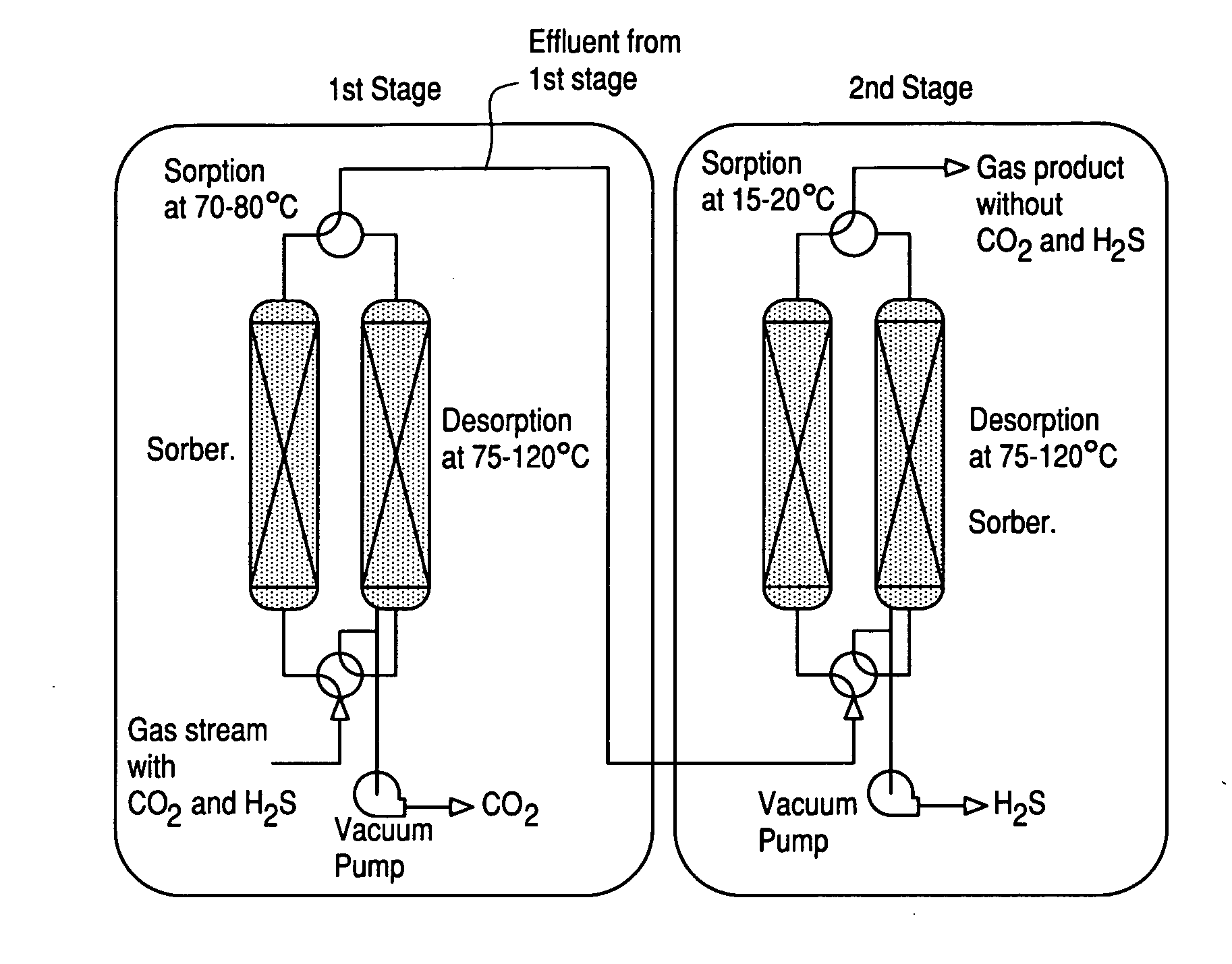
Novel sorbents and purification and bulk separation of gas streams
InactiveUS20080264254A1Large capacityLittle and no corrosive effectNitrous oxide captureGas treatmentSorbentDesorption
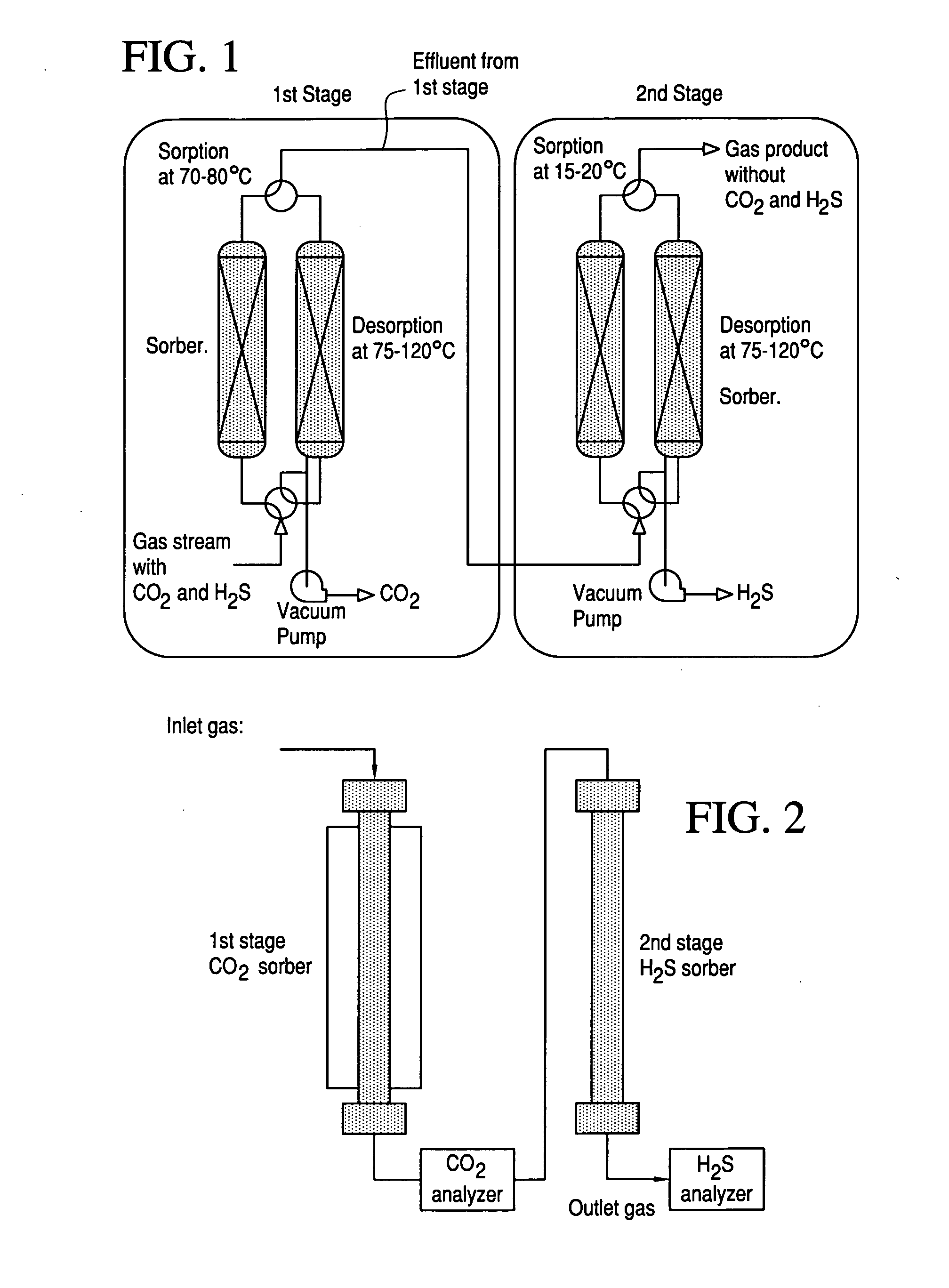
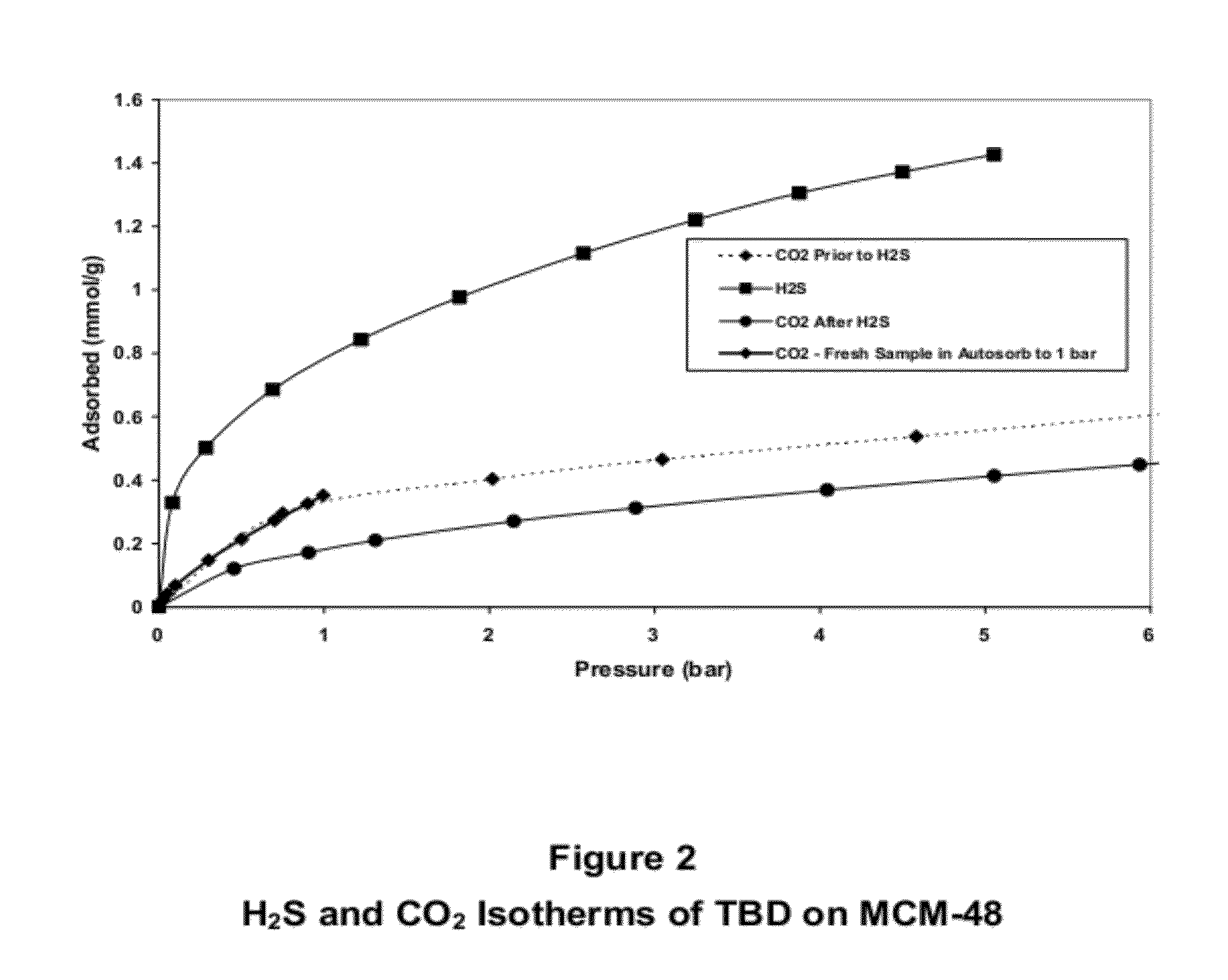
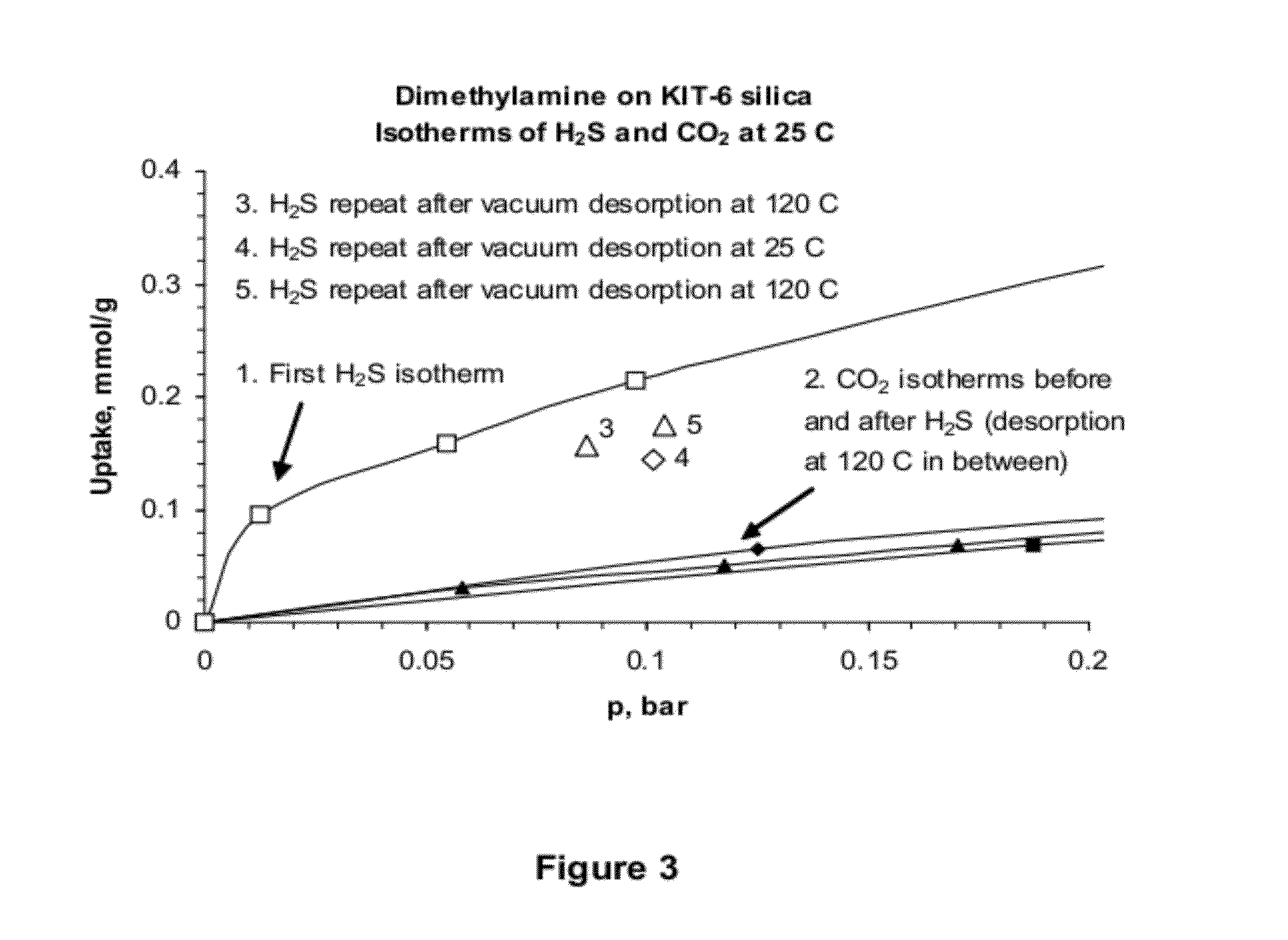
Porous-material-supported polymer sorbents and process for removal of undesirable gases such as H2S, COS, CO2, N2O, NO, NO2, SO2, SO3, HCl, HF, HCN, NH3, H2O, C2H5OH, CH3OH, HCHO, CHCl3, CH2Cl2, CH3Cl, CS2, C4H4S, CH3SH, and CH3—S—CH3 from various gas streams such as natural gas, coal / biomass gasification gas, biogas, landfill gas, coal mine gas, ammonia syngas, H2 and oxo-syngas, Fe ore reduction gas, reformate gas, refinery process gases, indoor air, fuel cell anode fuel gas and cathode air are disclosed. The sorbents have numerous advantages such as high breakthrough capacity, high sorption / desorption rates, little or no corrosive effect and are easily regenerated. The sorbents may be prepared by loading H2S—, COS—, CO2—, N2O, NO—, NO2—, SO2—, SO3—, HCl—, HF—, HCN—, NH3—, H2O—, C2H5OH—, CH3OH—, HCHO—, CHCl3—, CH2Cl2—, CH3Cl—, CS2—, C4H4S—, CH3SH—, CH3—S—CH3-philic polymer(s) or mixtures thereof, as well as any one or more of H2S—, COS—, CO2—, N2O, NO—, NO2—, SO2—, SO3—, HCl—, HF—, HCN—, NH3—, H2O—, C2H5OH—, CH3OH—, HCHO—, CHCl3—, CH2Cl2—, CH3Cl—, CS2—, C4H4S—, CH3SH—, CH3—S—CH3-philic compound(s) or mixtures thereof on to porous materials such as mesoporous, microporous or macroporous materials. The sorbents may be employed in processes such as one-stage and multi-stage processes to remove and recover H2S, COS, CO2, N2O, NO, NO2, SO2, SO3, HCl, HF, HCN, NH3, H2O, C2H5OH, CH3OH, HCHO, CHCl3, CH2Cl2, CH3Cl, CS2, C4H4S, CH3SH and CH3—S—CH3 from gas streams by use of, such as, fixed-bed sorbers, fluidized-bed sorbers, moving-bed sorbers, and rotating-bed sorbers.
Owner:PENN STATE RES FOUND +1
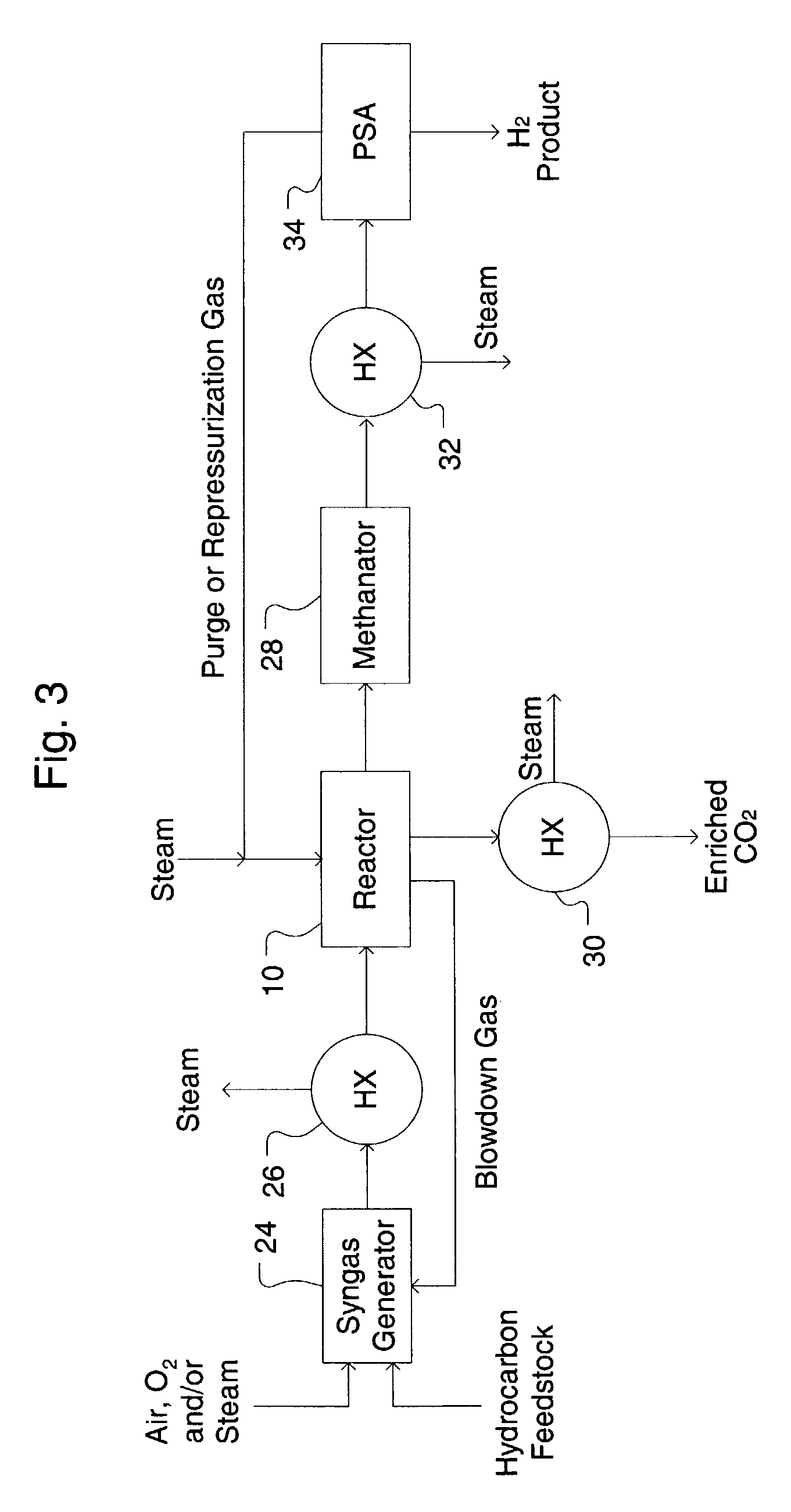
Simultaneous shift-reactive and adsorptive process to produce hydrogen
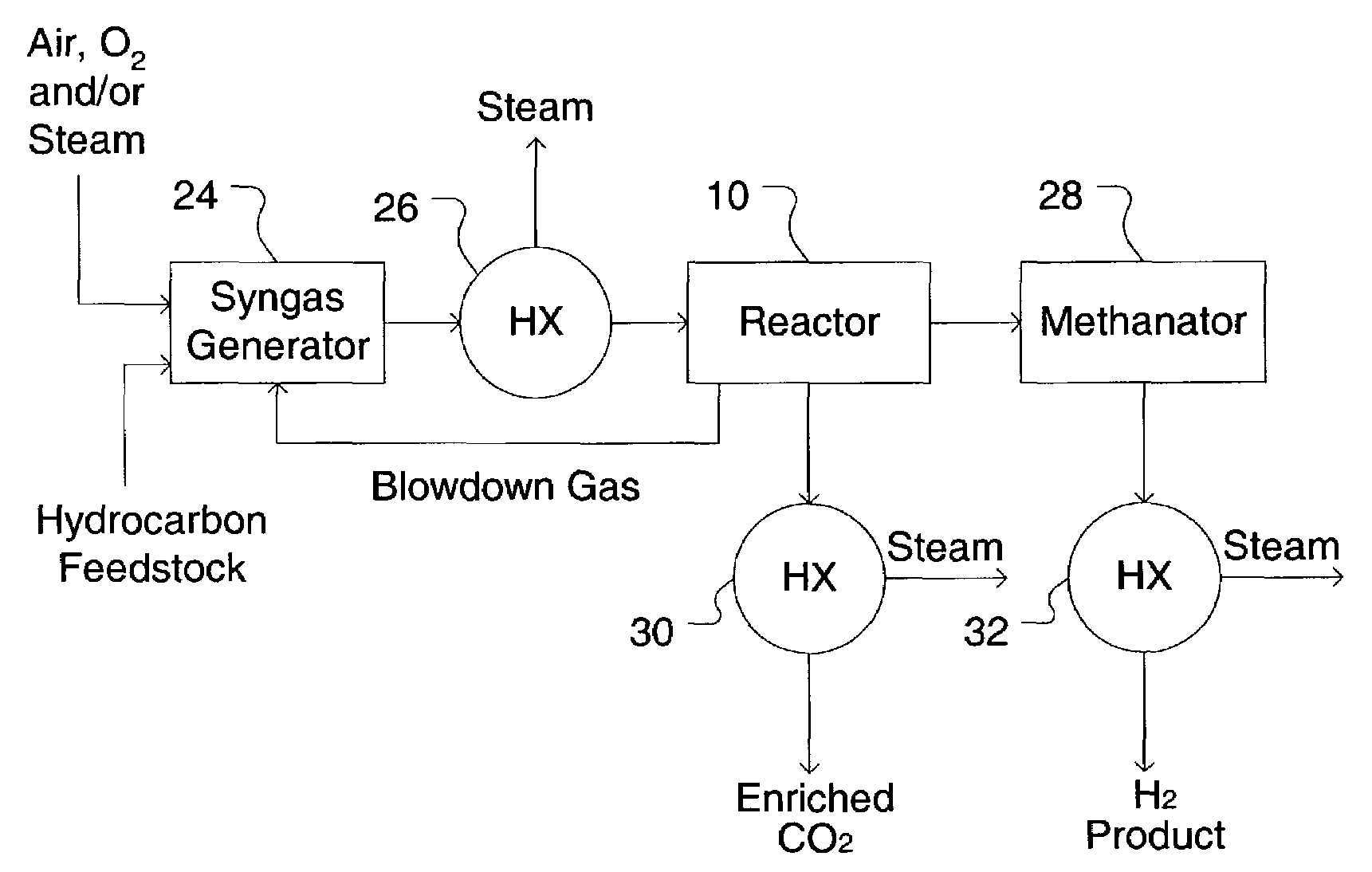
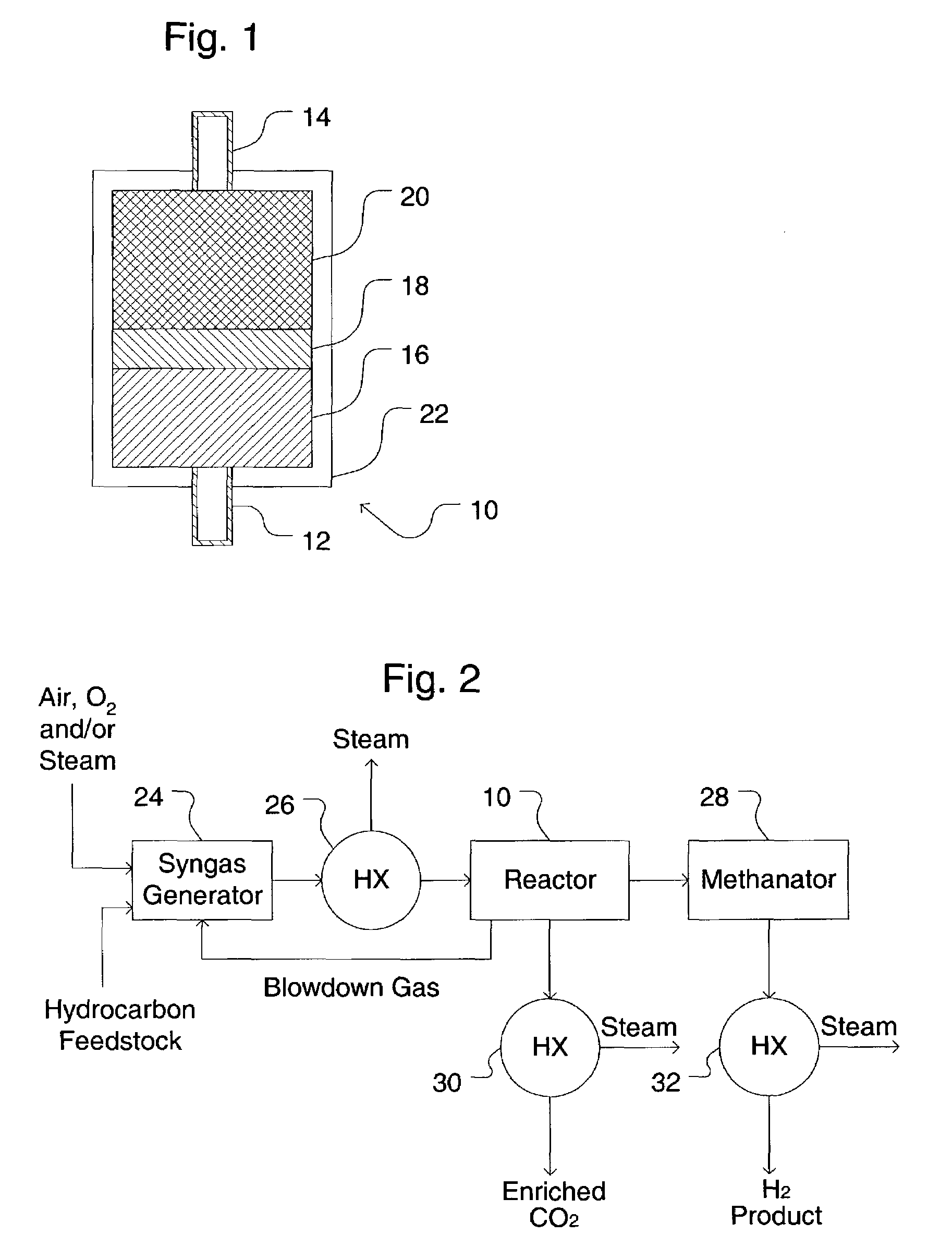
A process for producing a high temperature COx-lean product gas from a high temperature COx-containing feed gas, includes: providing a sorption enhanced reactor containing a first adsorbent, a shift catalyst and a second adsorbent; feeding into the reactor a feed gas containing H2, H2O, CO and CO2; contacting the feed gas with the first adsorbent to provide a CO2 depleted feed gas; contacting the CO2 depleted feed gas with the shift catalyst to form a product mixture comprising CO2 and H2; and contacting the product mixture with a mixture of second adsorbent and shift catalyst to produce the product gas, which contains at least 50 vol. % H2, and less than 5 combined vol. % of CO2 and CO. The adsorbent is a high temperature adsorbent for a Sorption Enhanced Reaction process, such as K2CO3 promoted hydrotalcites, modified double-layered hydroxides, spinels, modified spinels, and magnesium oxides.
Owner:AIR PROD & CHEM INC
Device and method for detecting a substance of a liquid
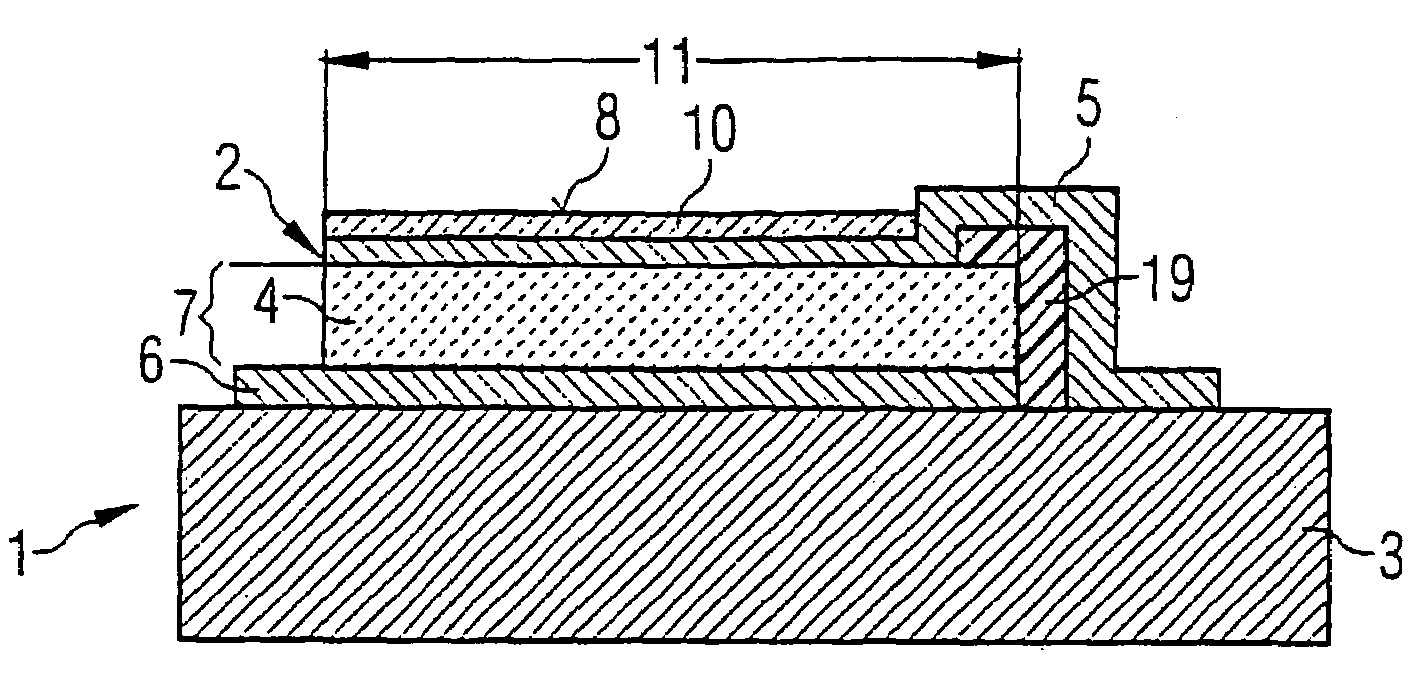
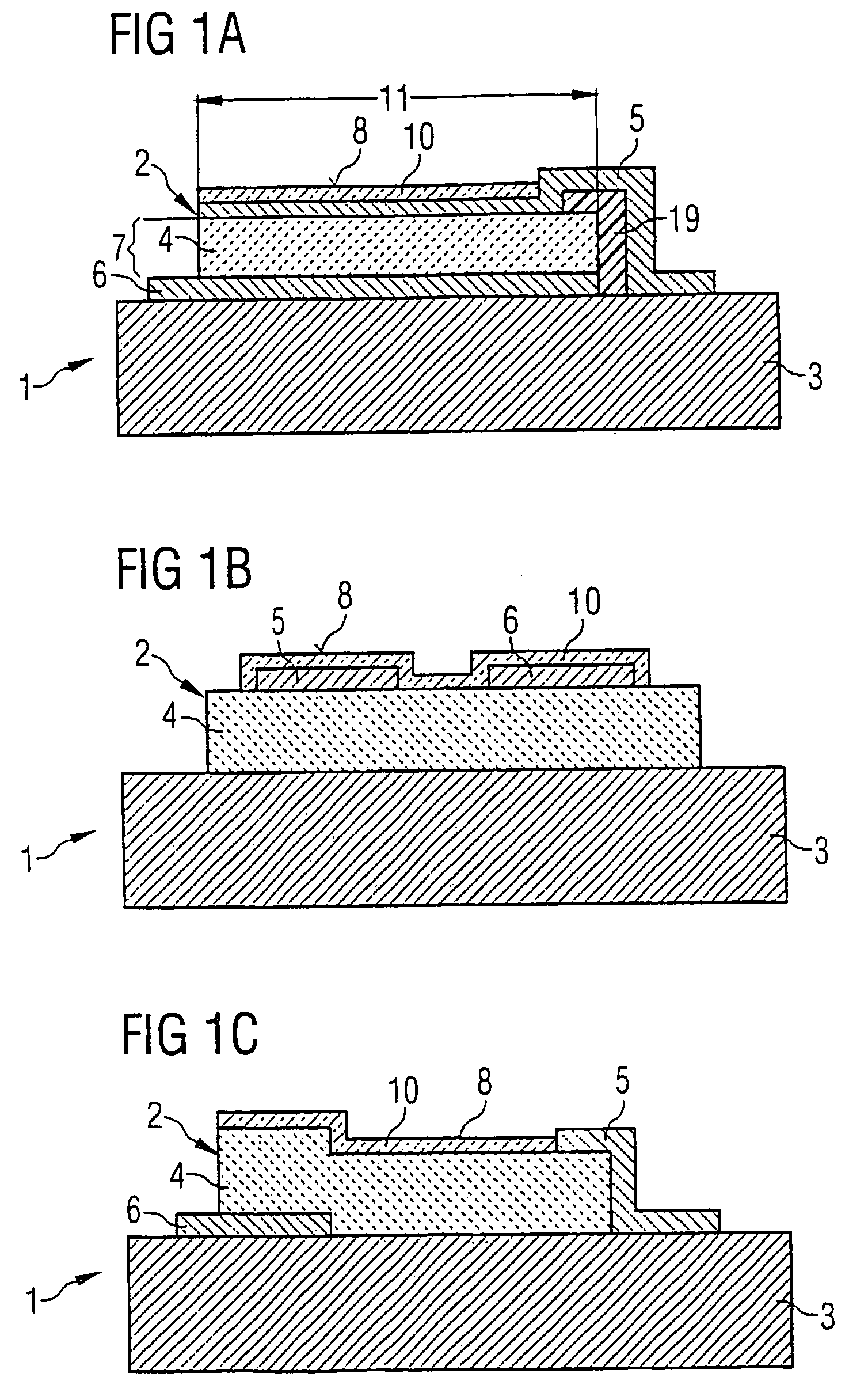
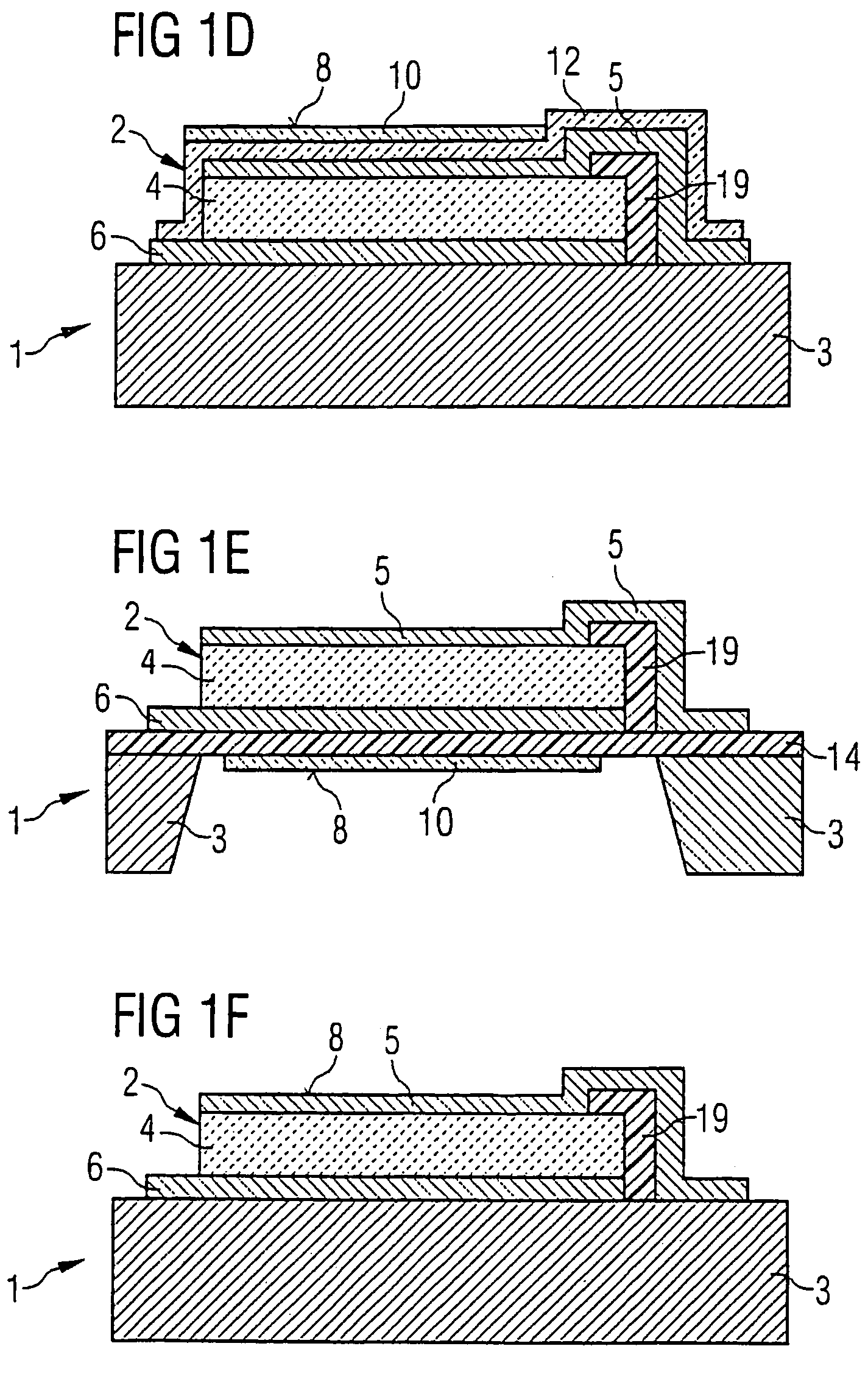
ActiveUS7468608B2Eliminates high-frequency lossEliminates interfering inductanceAnalysing fluids using sonic/ultrasonic/infrasonic wavesShaking/oscillating/vibrating mixersResonancePiezo electric
A device for detecting at least one substance of a fluid includes at least one piezo-acoustic resonator with at least one piezo layer, an electrode arranged on the piezo-electric layer, at least one other electrode arranged on the piezo-electric layer and a surface section used for sorption of the substance of the fluid. The piezo-electric layer, the electrodes and the surface section are disposed in such a way that electric control of the electrodes leads to an oscillation of the resonator at a resonance frequency which depends upon the amount of the substance which is sorbed on the surface section. The thickness of the pioelectric layer is in the region of 0.5 to 20 μm and the resonance frequency of the oscillation ranges from 500 MHz to 2 GHz. The device is a mass sensor with a piezo-acoustic high-frequency thin film resonator.
Owner:BIOMENSIO LTD
Absorbent articles with improved distribution properties under sub-saturation
InactiveUS6570057B1Function increaseNot to impair capillary sorption propertyBaby linensTamponsEngineeringSorption
An absorbent article contains at least one fluid storage member and at least one fluid distribution member. The fluid distribution member has an improved fluid handling property especially under sub-saturation conditions. Such members exhibit, at 50% of their saturation capacity, an increased permeability of at least about 14% of one at saturation. The fluid storage member has a higher Capillary Sorption Absorbency Height than the fluid distribution member.
Owner:THE PROCTER & GAMBLE COMPANY
Temperature controlled shipping containers
InactiveUS6968711B2Improve cooling effectImprove thermal conductivityContainer filling methodsDomestic refrigeratorsTemperature controlSufficient time
Novel sorption cooling devices capable of providing cooling over an extended period of time are disclosed. The sorption cooling devices are particularly useful for temperature-controlled shipping containers that are required to maintain a temperature below ambient for a time sufficient to complete delivery of the container and its contents. The shipping containers can be utilized to cost-effectively transport temperature-sensitive products.
Owner:PELICAN NANOCOOL HLDG LLC
Microchannel with internal fin support for catalyst or sorption medium
InactiveUS7220390B2Fast thermal swingFaster cycle timeCombination devicesMethane captureEngineeringSorption
This invention relates to an apparatus, comprising: at least one process microchannel having a height, width and length, the height being up to about 10 mm, the process microchannel having a base wall extending in one direction along the width of the process microchannel and in another direction along the length of the process microchannel; at least one fin projecting into the process microchannel from the base wall and extending along at least part of the length of the process microchannel; and a catalyst or sorption medium supported by the fin.
Owner:VELOCYS CORPORATION
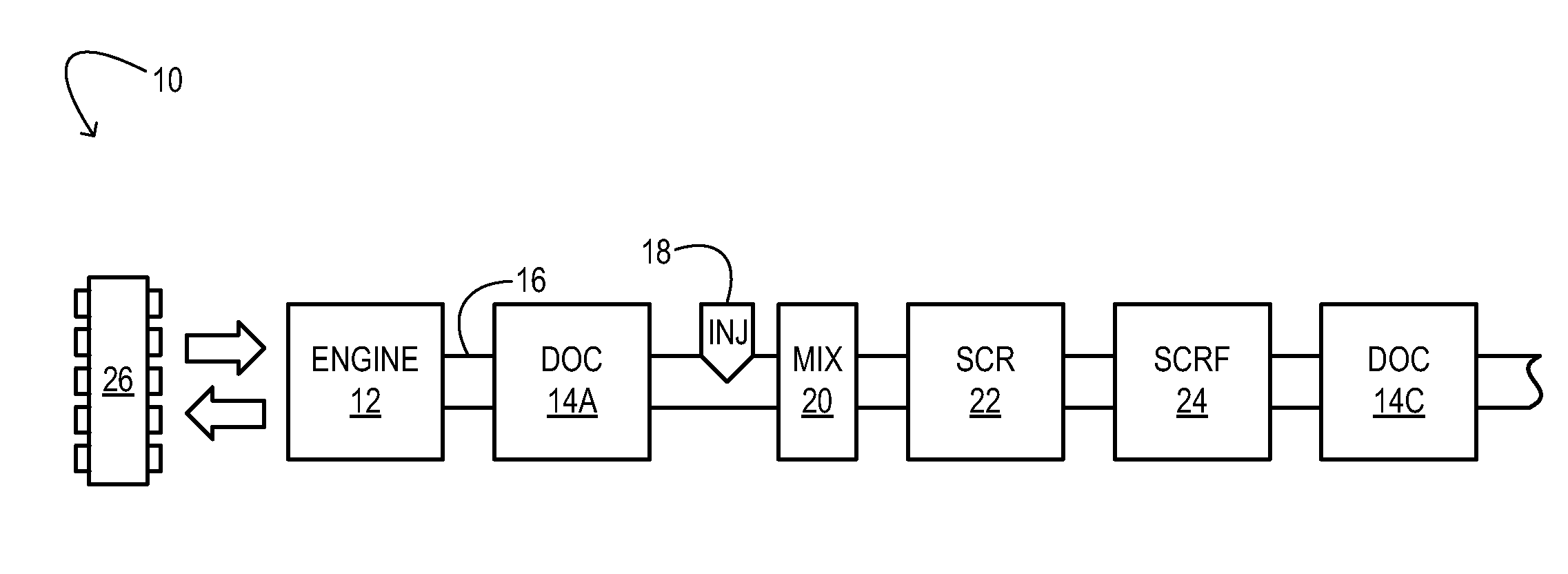
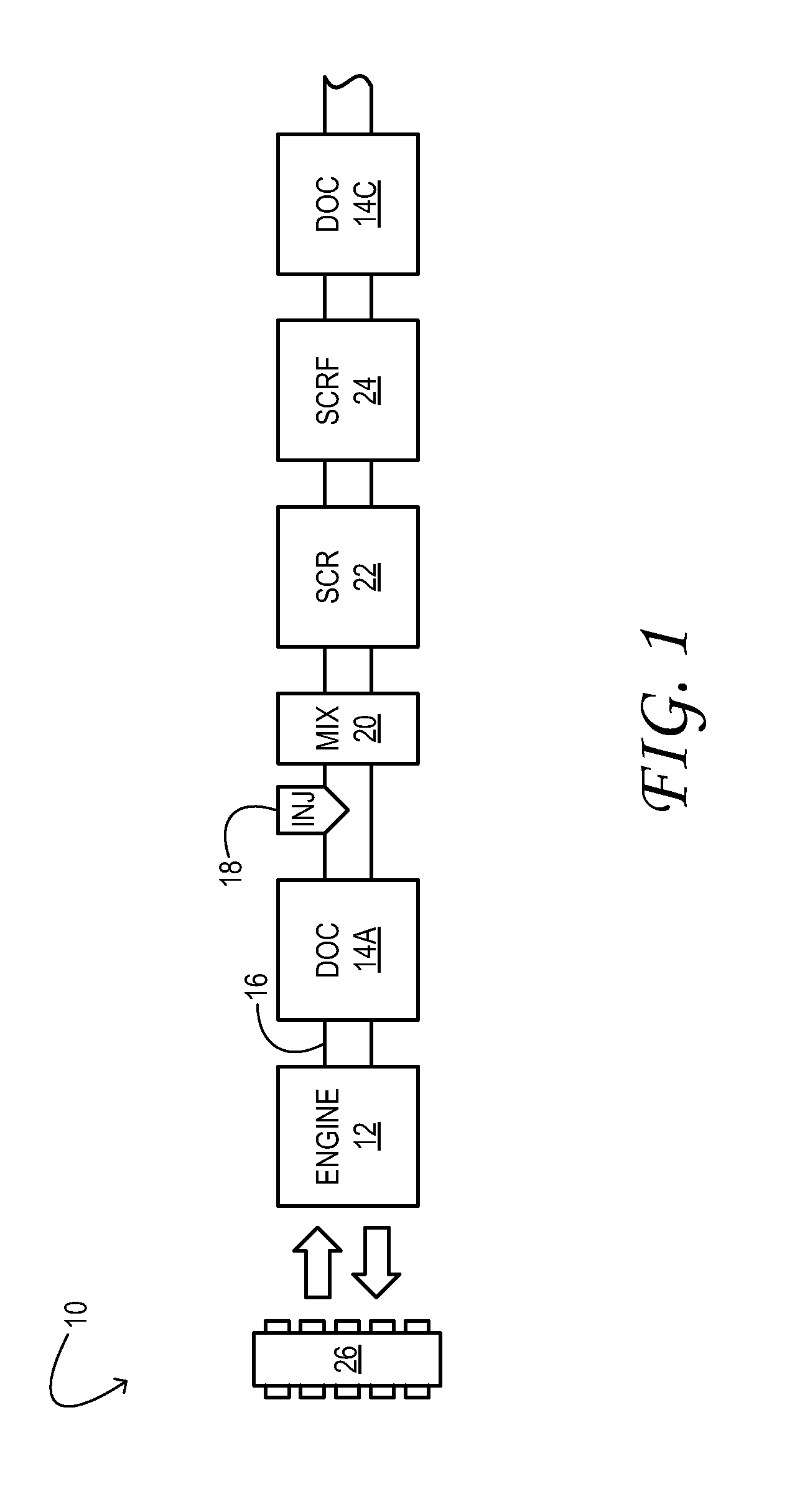
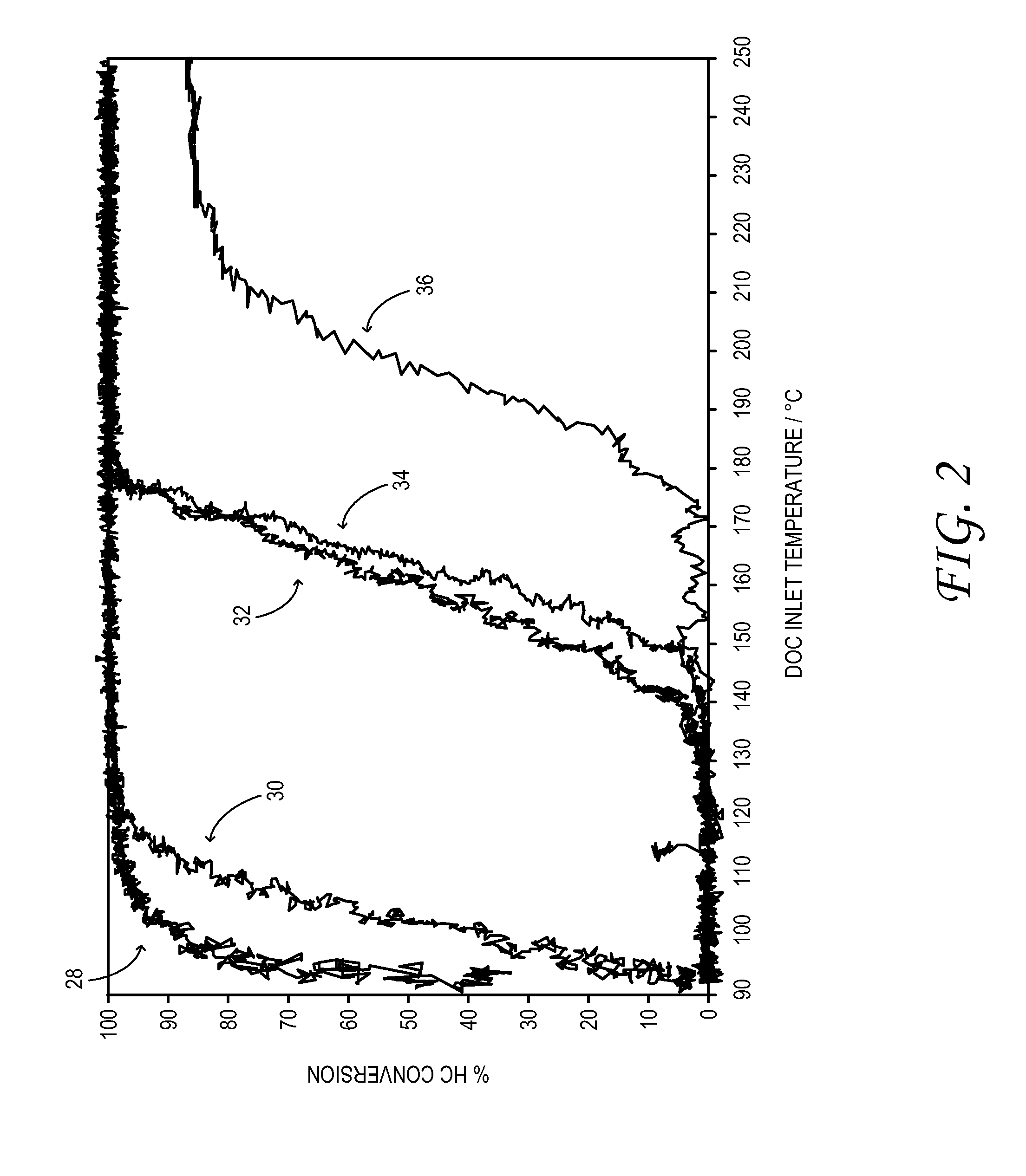
Synergistic SCR / DOC Configurations for Lowering Diesel Emissions
ActiveUS20110162347A1Suppress feed gas emissionReduce NOxInternal combustion piston enginesExhaust apparatusHydrocotyle bowlesioidesSorption
A motor-vehicle engine system comprises a first DOC configured to receive exhaust from an engine and an SCR device coupled downstream of the first DOC in a flow direction of the exhaust. The system further comprises a second DOC coupled downstream of the SCR device. The system takes advantage of hydrocarbon sorption in the SCR catalyst that is a function of temperature to enable reduced hydrocarbon emissions via oxidation at the second DOC.
Owner:FORD GLOBAL TECH LLC
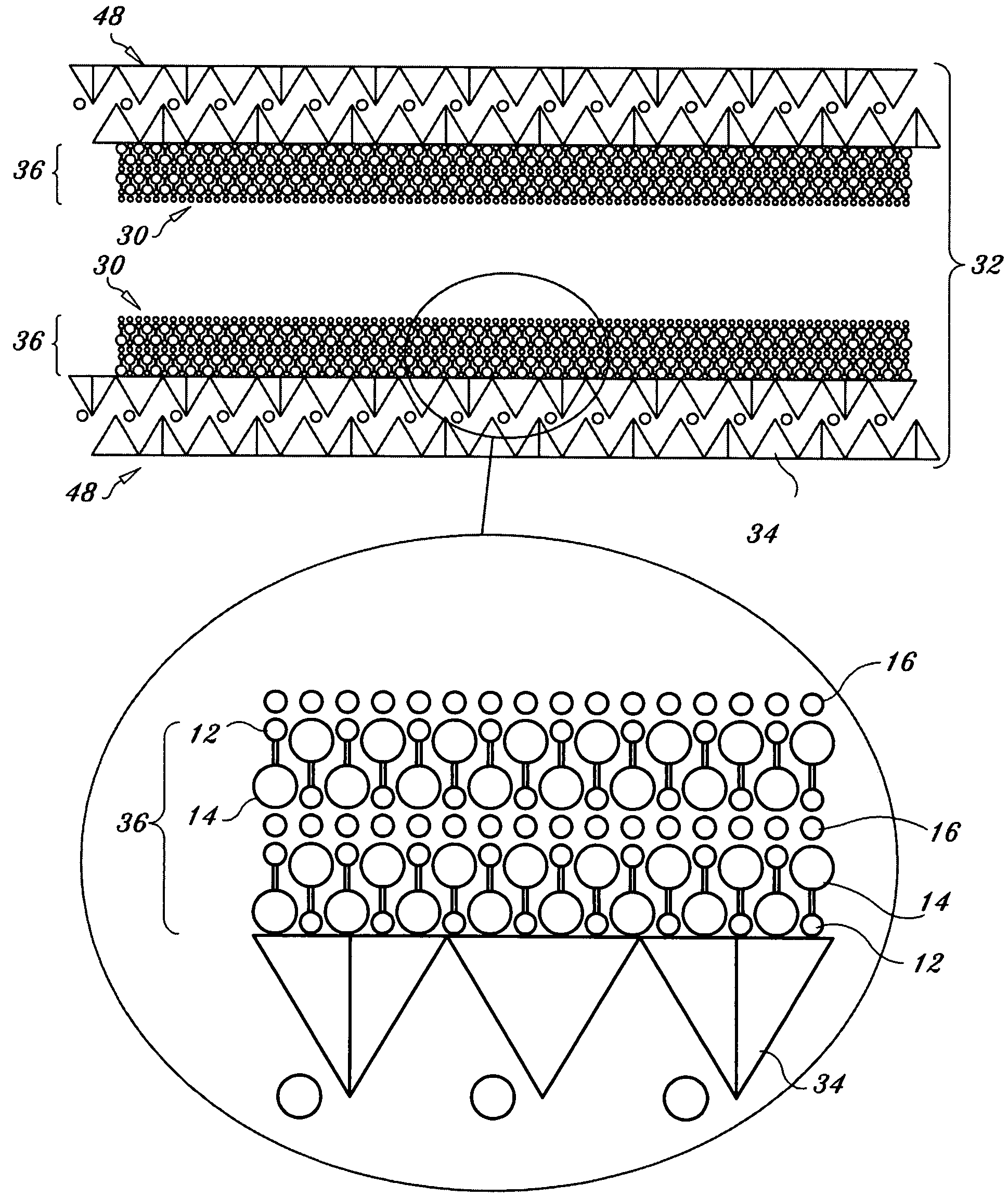
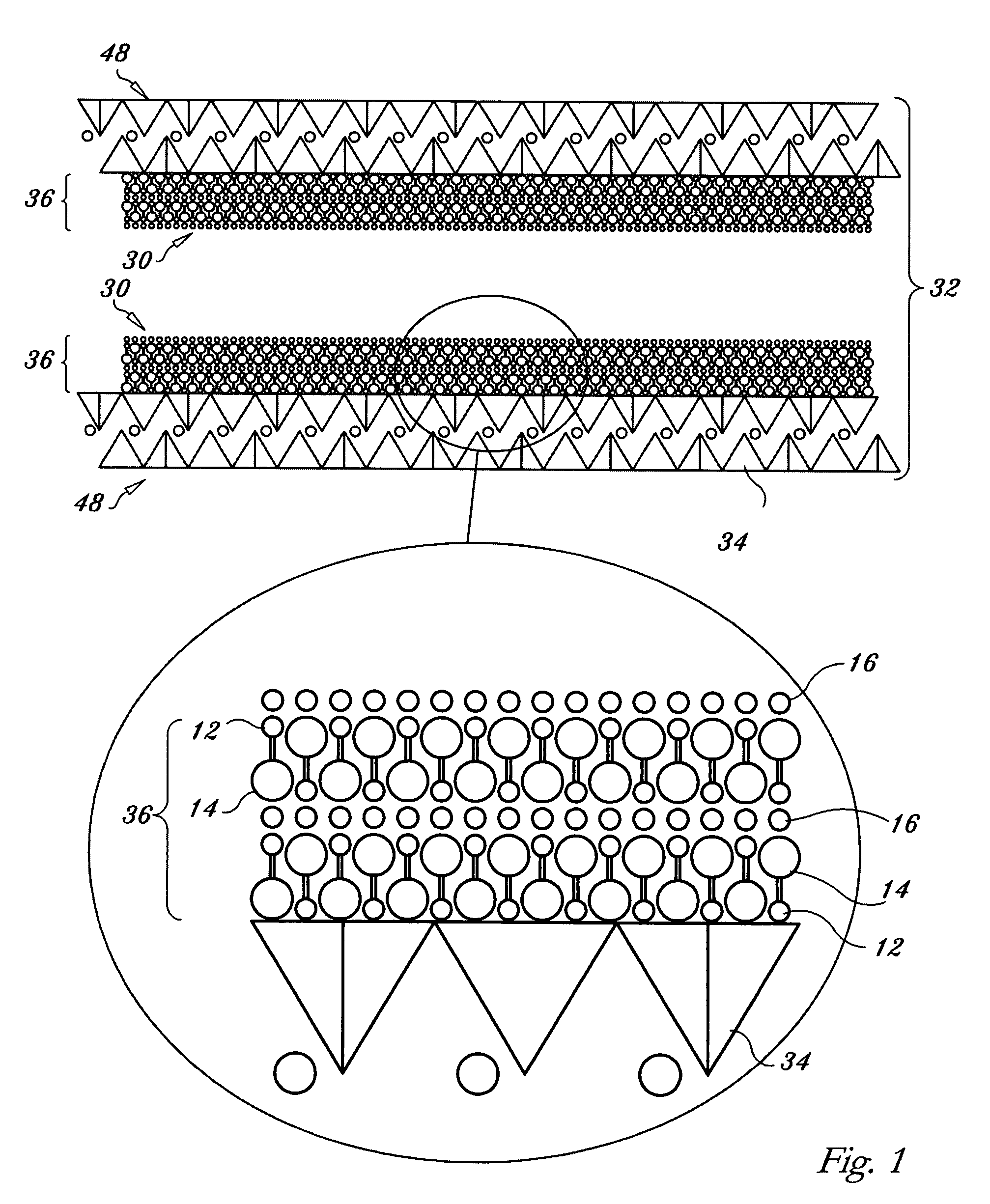
Regenerable high capacity sorbent for removal of mercury from flue gas
InactiveUS7288499B1Low costLower performance requirementsInorganic chemistryGas treatmentSorbentManganese
A regenerable, high-capacity sorbent for removal of mercury from flue gas and processes and systems for making and using the sorbent. A phyllosilicate substrate, for example vermiculite or montmorillinite, acts as an inexpensive support to a thin layer for a polyvalent metal sulfide, ensuring that more of the metal sulfide is engaged in the sorption process. The sorbent is prepared by ion exchange between the silicate substrate material and a solution containing one or more of a group of polyvalent metals including tin (both Sn(II) and Sn(IV)), iron (both Fe(II) and Fe(III)), titanium, manganese, zirconium and molybdenum, dissolved as salts, to produce an exchanged substrate. Controlled reaction of a sulfide ion source with the one or more polyvalent metals that are exchanged on the silicate substrate produces the sorbent. The sorbent is used to absorb elemental mercury or oxidized mercury species such as mercuric chloride from flue gas containing acid gases (e.g., SO2, NO and NO2, and HCl) and other gases over a wide range of temperatures.
Owner:ENVIRONMENTAL ENERGY SERVICES
Selective Sulfur Removal Process
Owner:EXXON RES & ENG CO
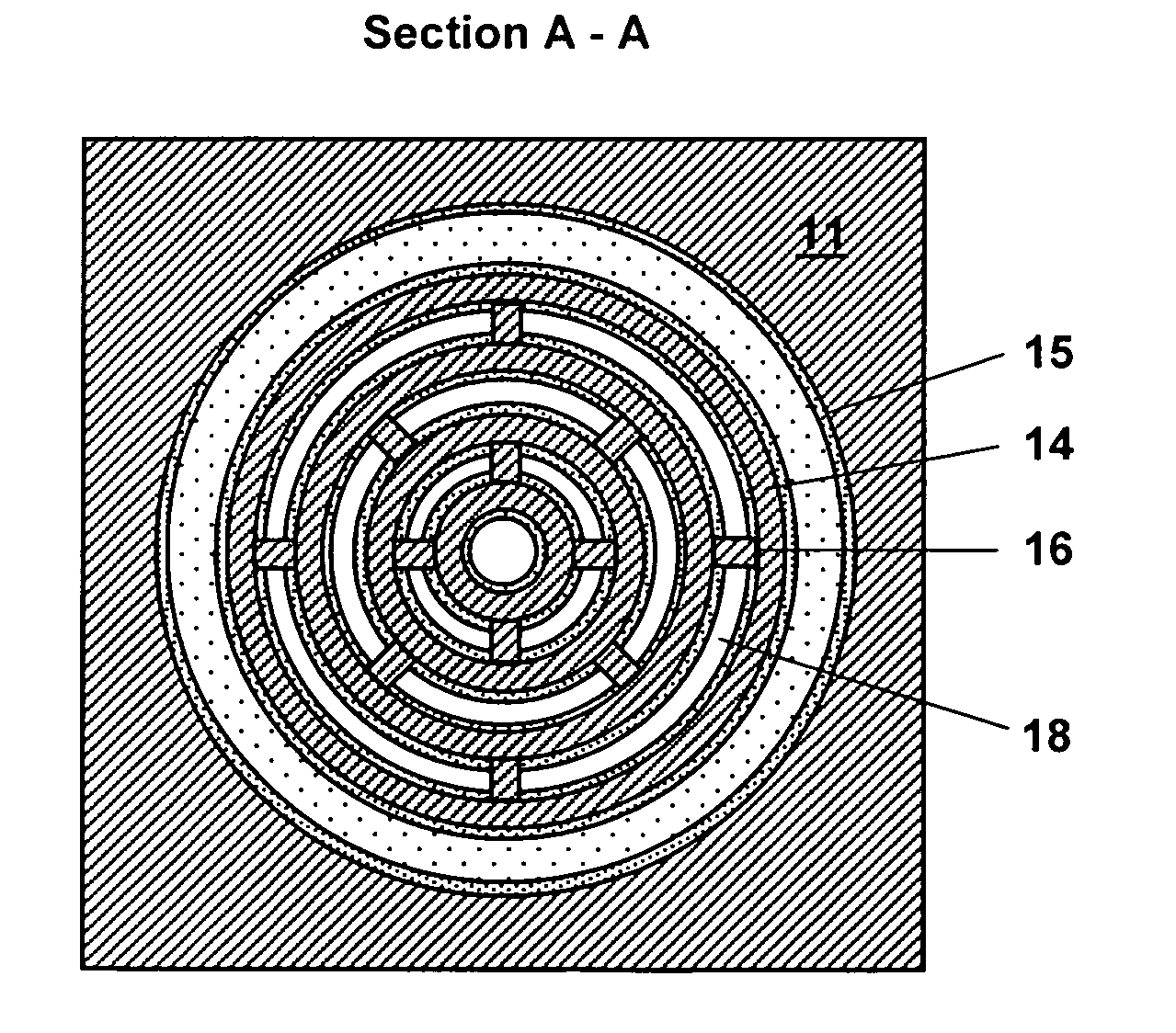
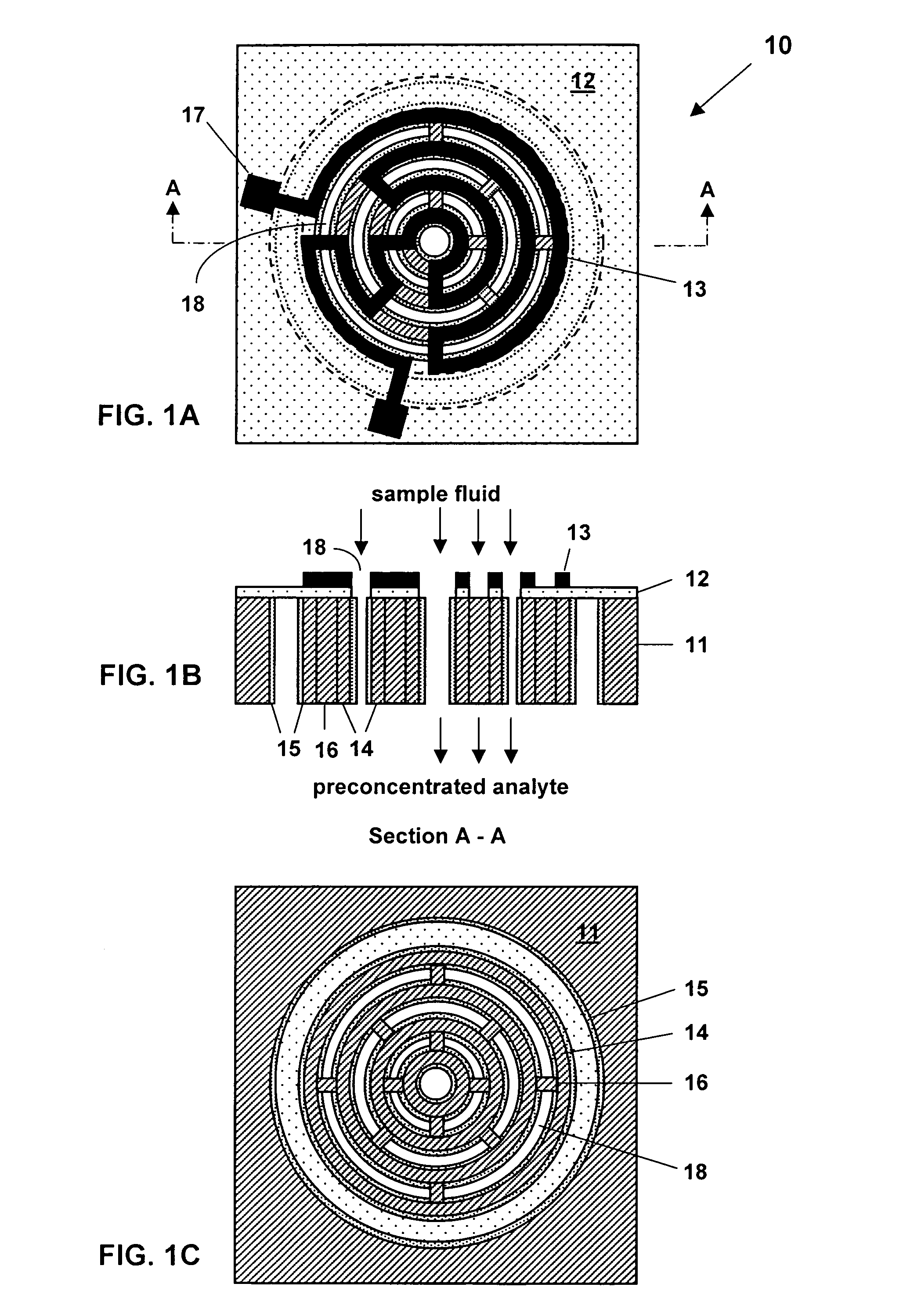
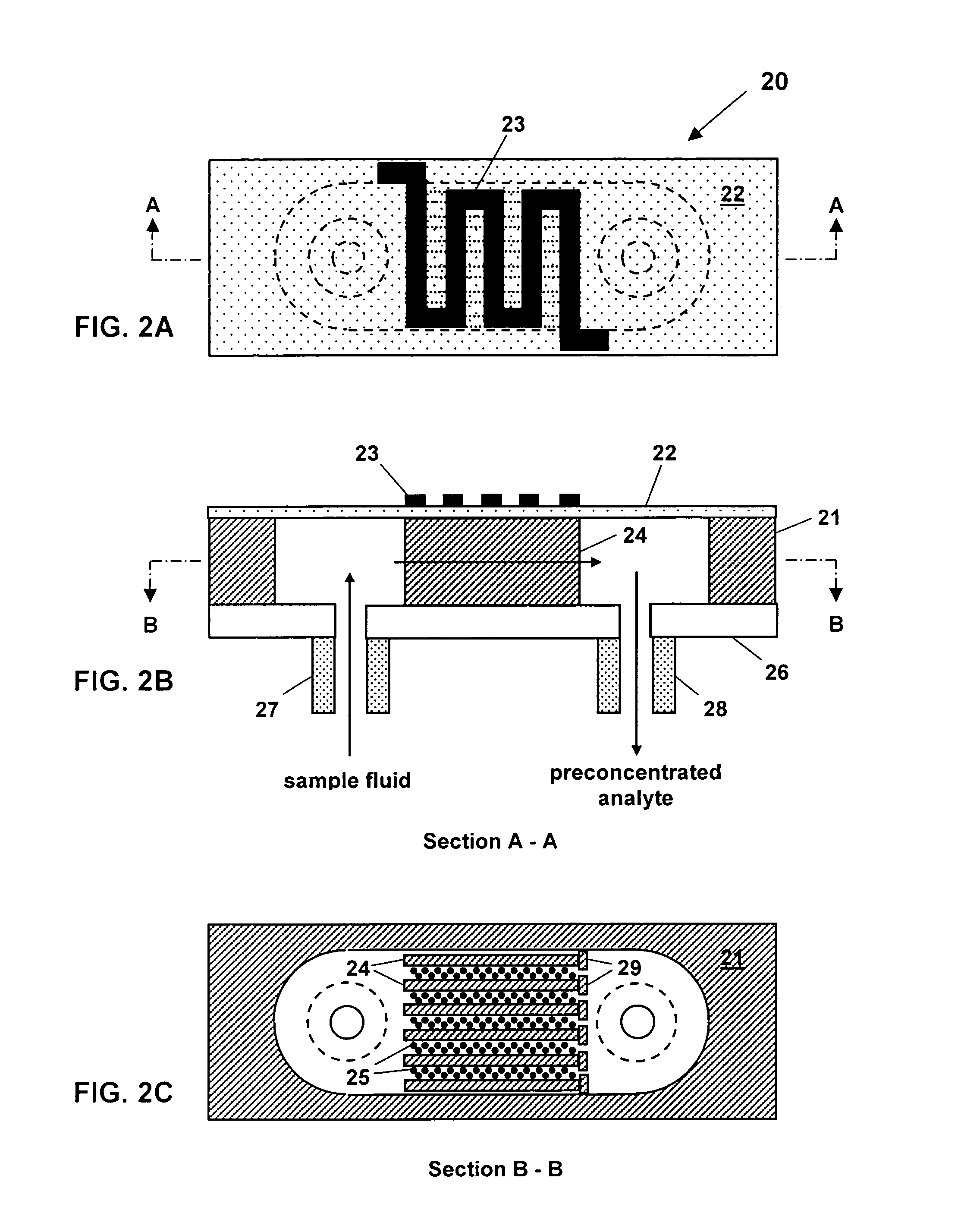
Non-planar chemical preconcentrator
ActiveUS7118712B1Quick releaseComponent separationWithdrawing sample devicesAnalyteCompound (substance)
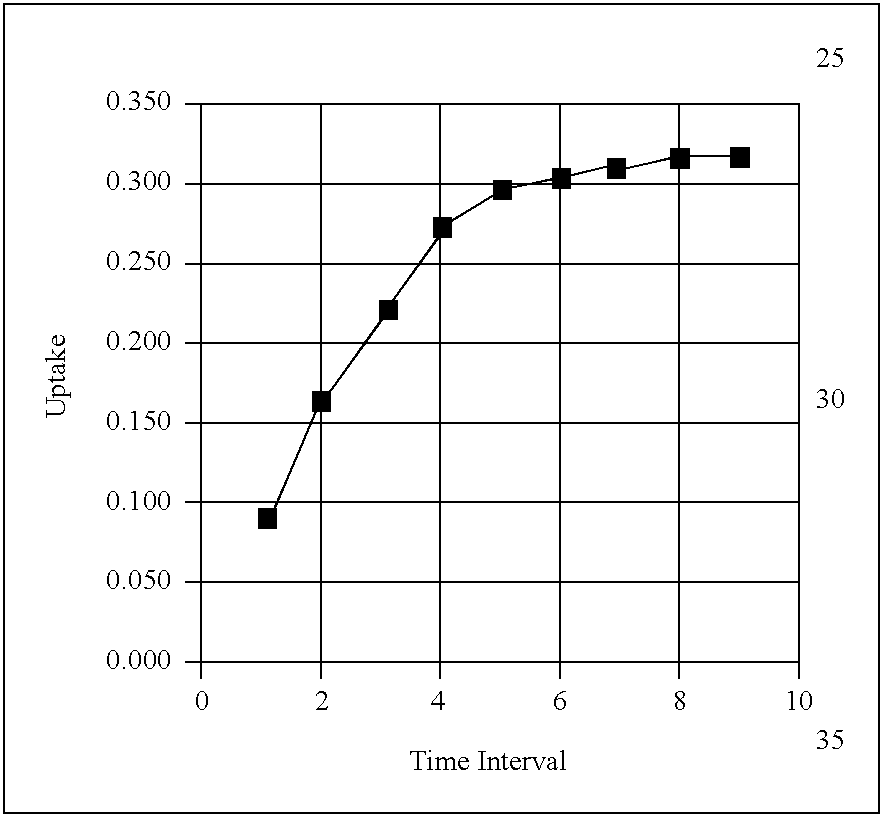
A non-planar chemical preconcentrator comprises a high-surface area, low mass, three-dimensional, flow-through sorption support structure that can be coated or packed with a sorptive material. The sorptive material can collect and concentrate a chemical analyte from a fluid stream and rapidly release it as a very narrow temporal plug for improved separations in a microanalytical system. The non-planar chemical preconcentrator retains most of the thermal and fabrication benefits of a planar preconcentrator, but has improved ruggedness and uptake, while reducing sorptive coating concerns and extending the range of collectible analytes.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
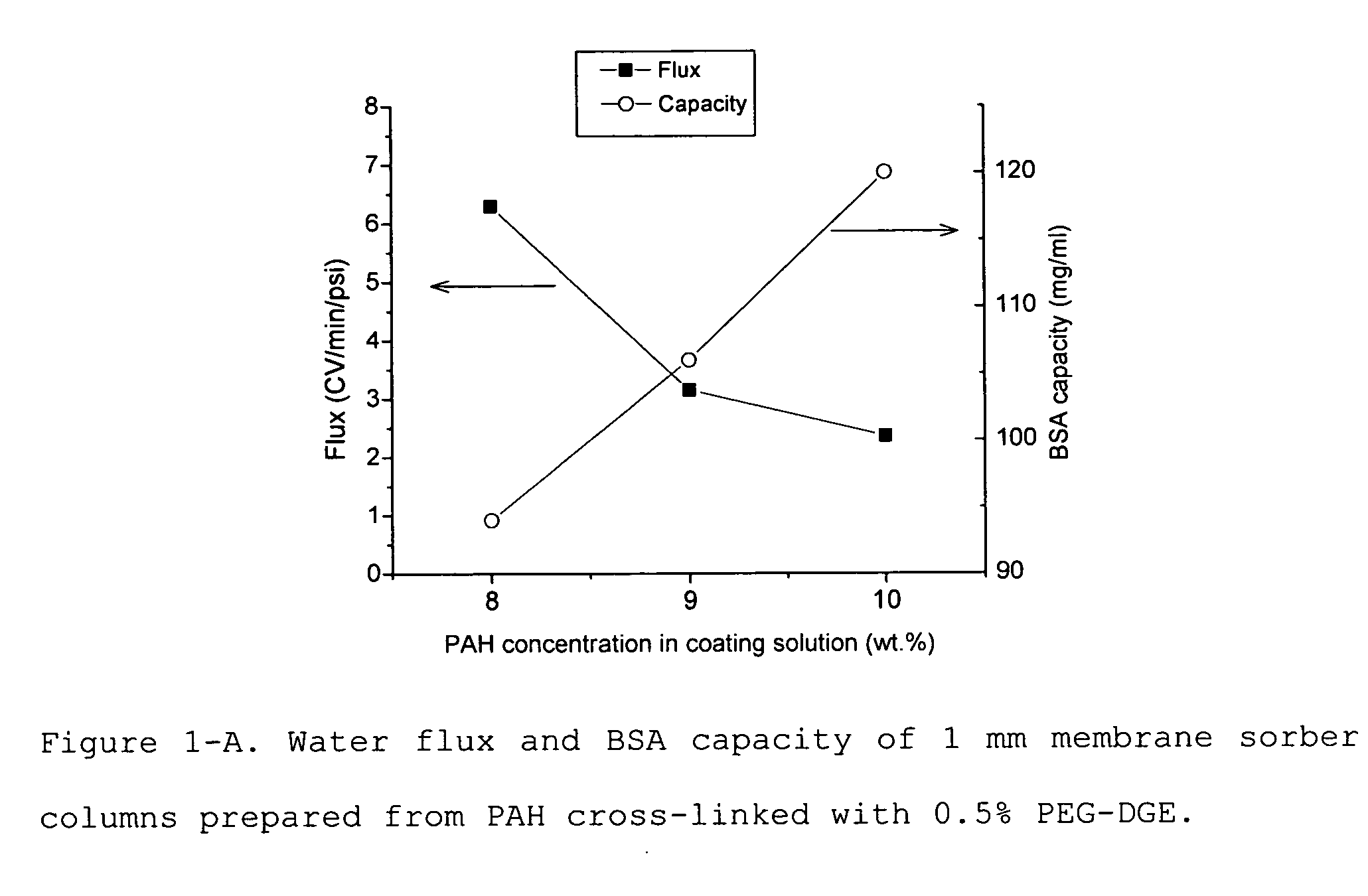
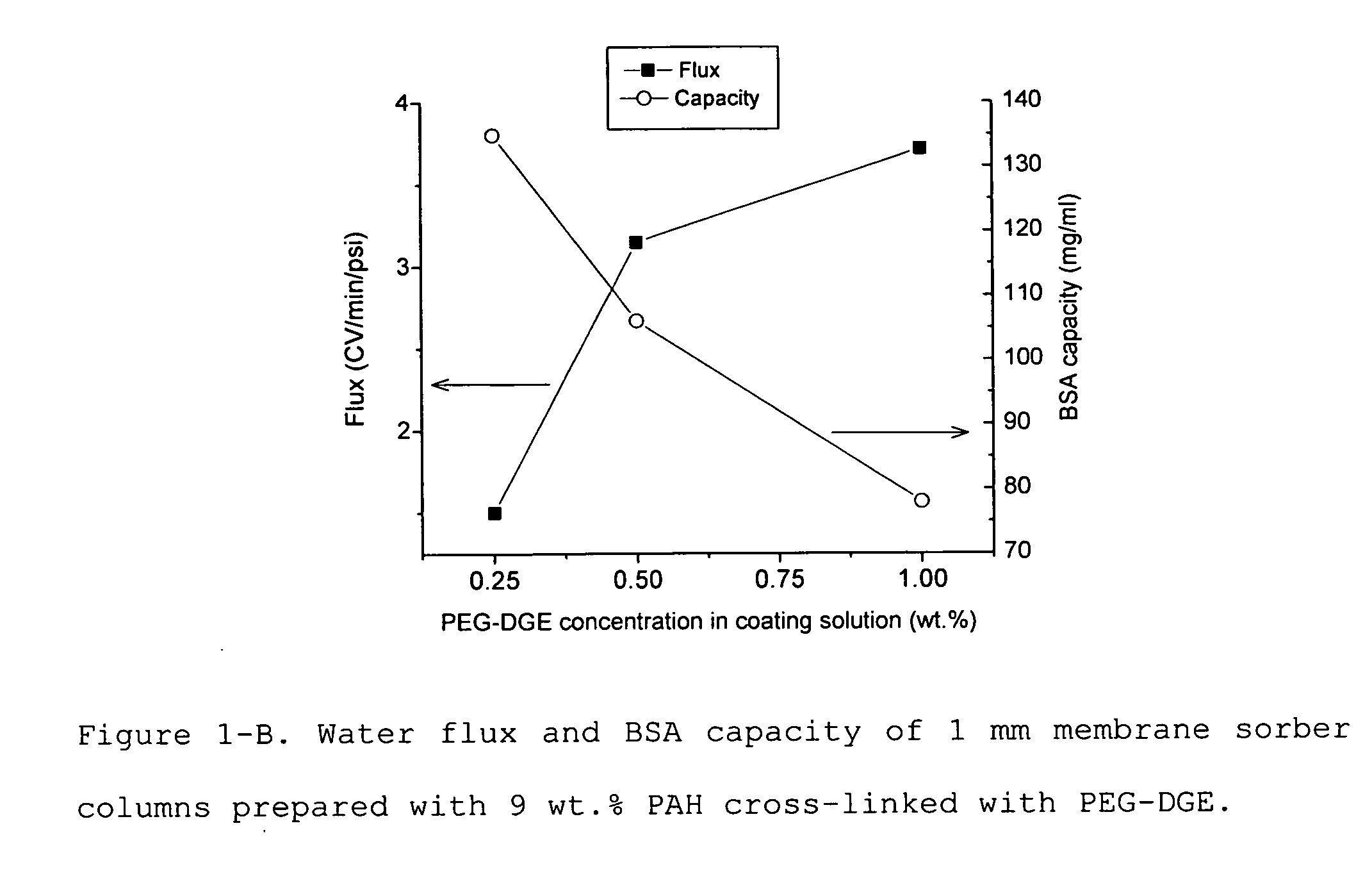
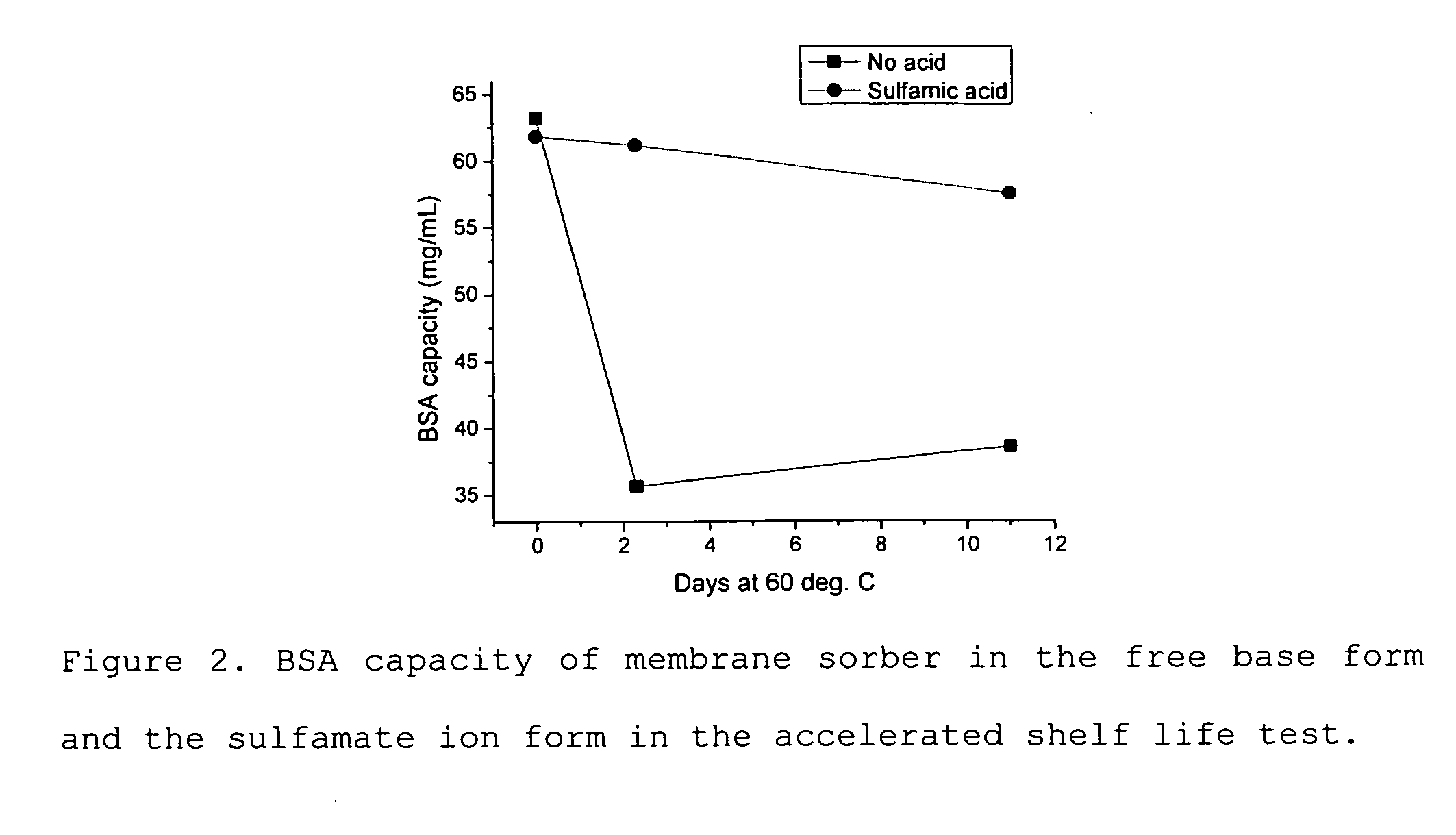
Media for membrane ion exchange chromatography based on polymeric primary amines, sorption device containing that media, and chromatography scheme and purification method using the same
ActiveUS20090050566A1Improve bindingGood removal effectCation exchanger materialsIon-exchanger regenerationPurification methodsSorbent
Media and devices, such as anion exchangers including such media, wherein the media is a membrane having a surface coated with a polymer such as a polyallylamine. The resulting membrane offers stronger binding of protein impurities and superior removal of host cell proteins from biological samples than conventional ligands based on quaternary ammonium salts, including trimethylammonium ligands. Also described is a chromatography scheme and method for purifying monoclonal antibodies, wherein the anion exchange sorber is placed downstream of an affinity column (such as Protein A or Protein G affinity column) and optionally one or more polishing devices such as cationic exchange columns. Little or no dilution of the cation exchanger pool (or affinity column exchange pool where no cation exchanger is used) is necessary to lower the conductivity of the sample. The sorber functions well to strongly bind host cell proteins and other impurities in biological samples even at high conductivities and pH.
Owner:MILLIPORE CORP
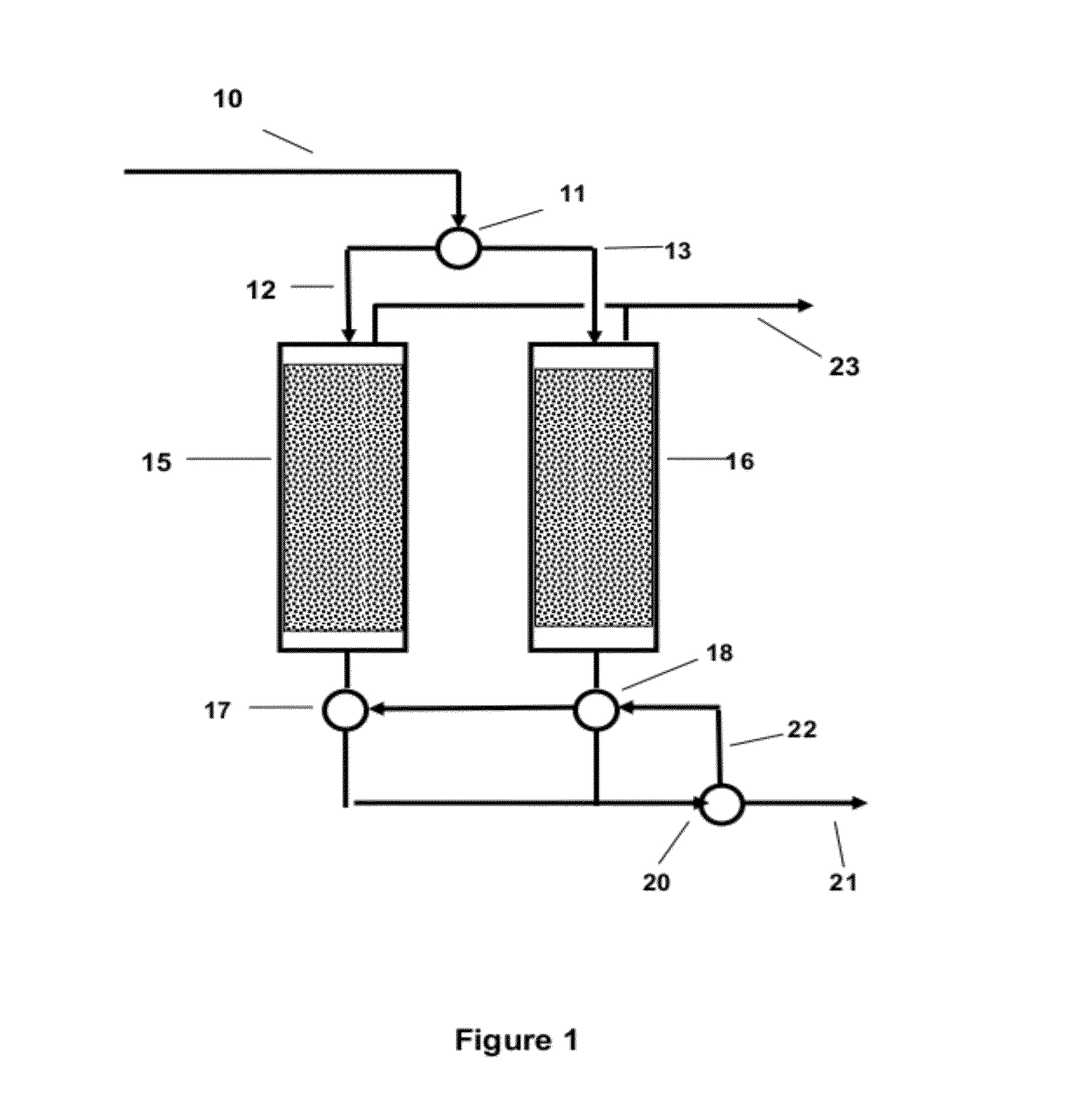
Treatment process for absorbed condensate waste gas
ActiveCN102179129ASo as not to damageShorten heating timeDispersed particle separationVapor condensationNitrogenEnvironmental engineering
The invention provides a treatment process for absorbed condensate waste gas, wherein two active carbon absorbing tanks alternately absorb waste gas; when one active carbon absorbing tank carries out waste gas absorption work, the other active carbon absorbing tank carries out carbon bed reproduction work, wherein the absorbed waste gas in waste gas absorption work is cooled; the carbon bed reproduction work comprises a step of vacuum de-sorption and a step of nitrogen de-sorption in sequence; the step of nitrogen de-sorption comprises flowing heated nitrogen at the temperature of 60-80 DEG Cthrough the carbon bed from top to bottom so that the carbon bed is deeply desorbed, and finally cooling the carbon bed by using unheated cold nitrogen so as to recover adsorptive capacity. The process is simple without providing absorbent on the spot; and the treatment process has high treatment efficiency, and can obtain pure recovery product without causing secondary pollution.
Owner:BAY ENVIRONMENTAL TECH BEIJING
Hygiene product with a probiotic composition
InactiveUS20040243076A1Simple preparation processExtended shelf lifeSanitary towelsBaby linensBacterial strainTampon
A hygiene product, such as a sanitary napkin, diaper, panty liner, tampon, incontinence guard, hygiene tissue and the like, includes a probiotic composition having a bacterial preparation of at least one lactic acid producing bacterial strain and a contact sorption drying carrier dispersed in a lipid phase. A method for producing a hygiene product with lactic acid producing bacteria, dried with the aid of contact sorption drying carriers, in a lipid phase is provided. The manufacturing process for the hygiene product has the advantages of economy, simplicity and bacterial survival during manufacturing and subsequent storage.
Owner:SCA HYGIENE PROD AB
Biorefinery Process for Extraction, Separation, and Recovery of Fermentable Saccharides, other Useful Compounds, and Yield of Improved Lignocellulosic Material from Plant Biomass
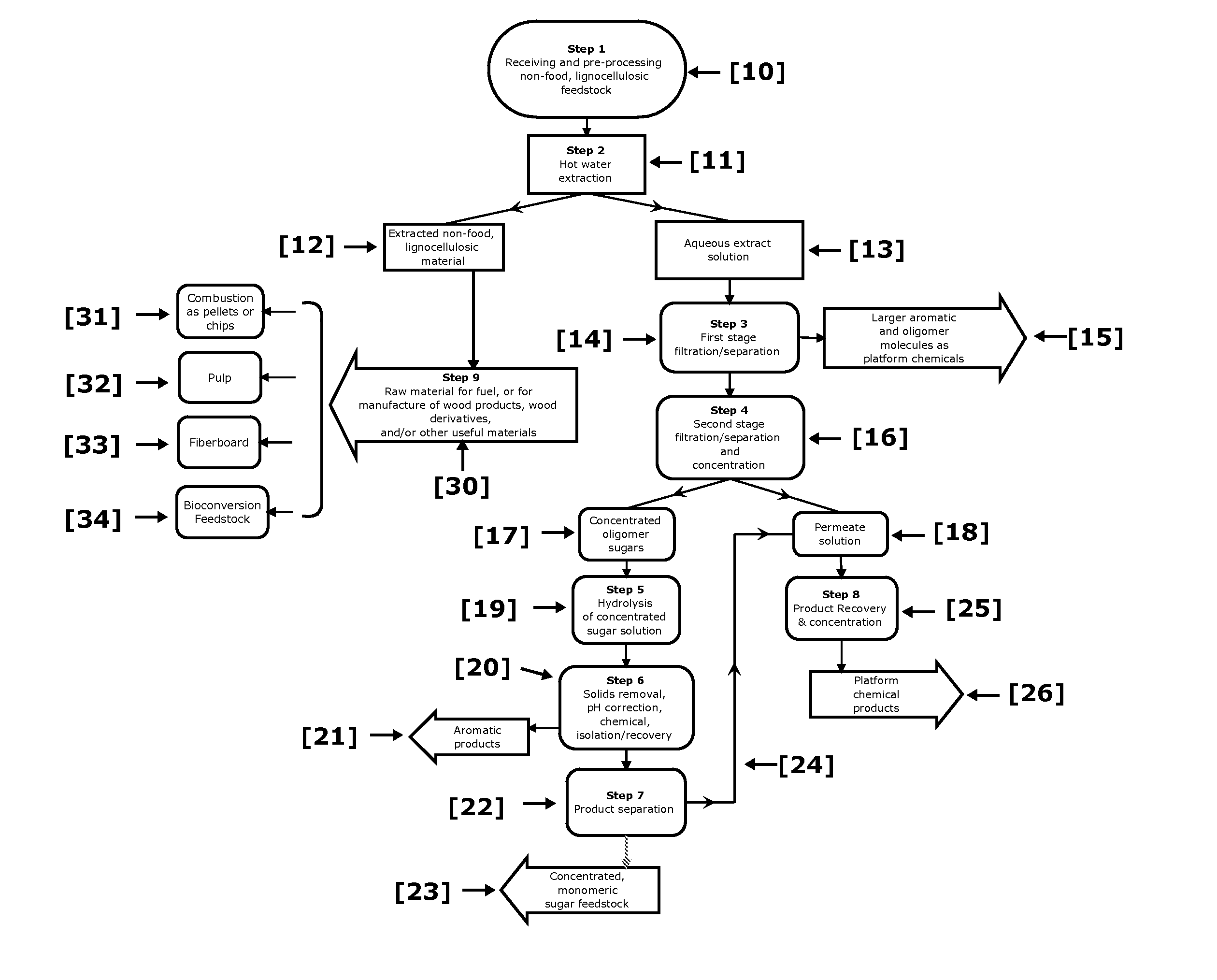
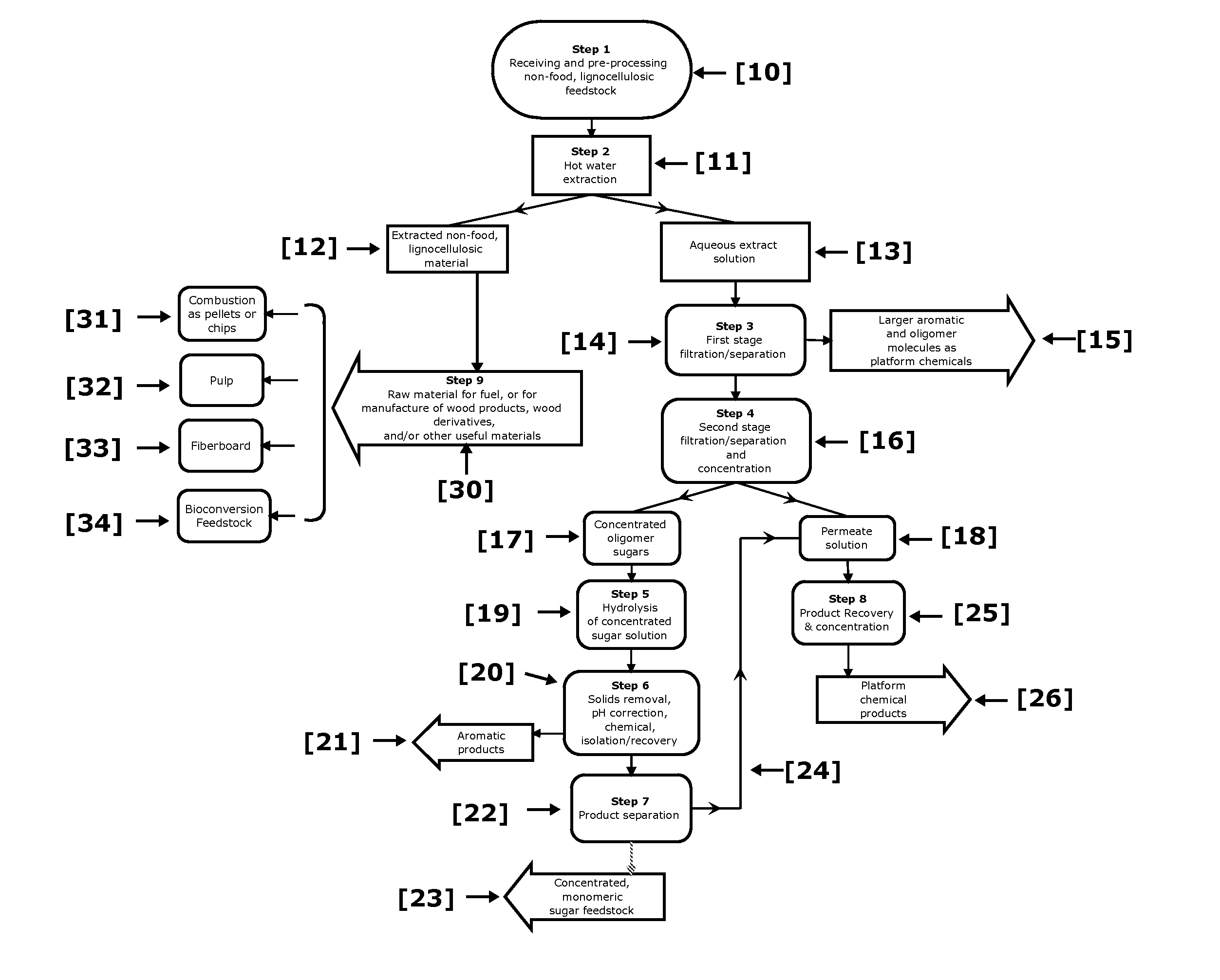
ActiveUS20110129886A1Increase diversityIncrease probabilityCellulosic pulp after-treatmentBiofuelsFurfuralAqueous extract
Non-food plant biomass is subjected to hot-water extraction in a pressurized vessel at an elevated temperature up to about 250° C. and at a pH below about 7.0, to yield an aqueous extract containing hemicellulosic components, other wood-derived compounds, and a lignocellulosic residue. The separated aqueous extract or liquor is purified and concentrated through a multi-step process producing fermentable sugars. At each stage, inhibitory chemicals such as acetic acid, lignin, and furfural are separated and eventually recovered as commercial chemicals. The lignocellulosic residue may be further processed, as a material with enhanced resistance to sorption of water, for manufacture of improved pulp and paper, construction materials, pellet fuel, and / or other useful products.
Owner:APPLIED BIOREFINERY SCI
Method and apparatus for supercharging downhole sample tanks
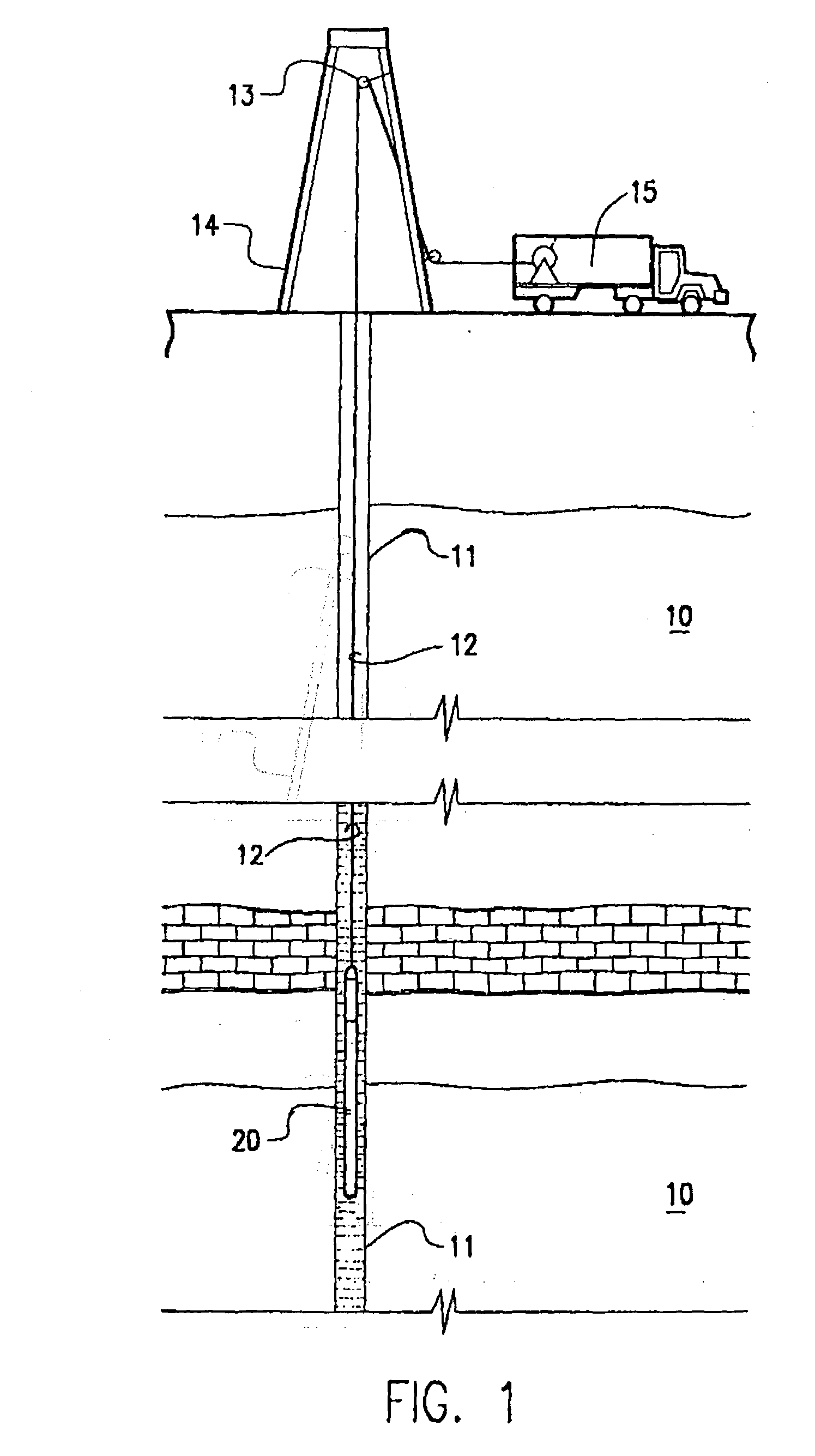
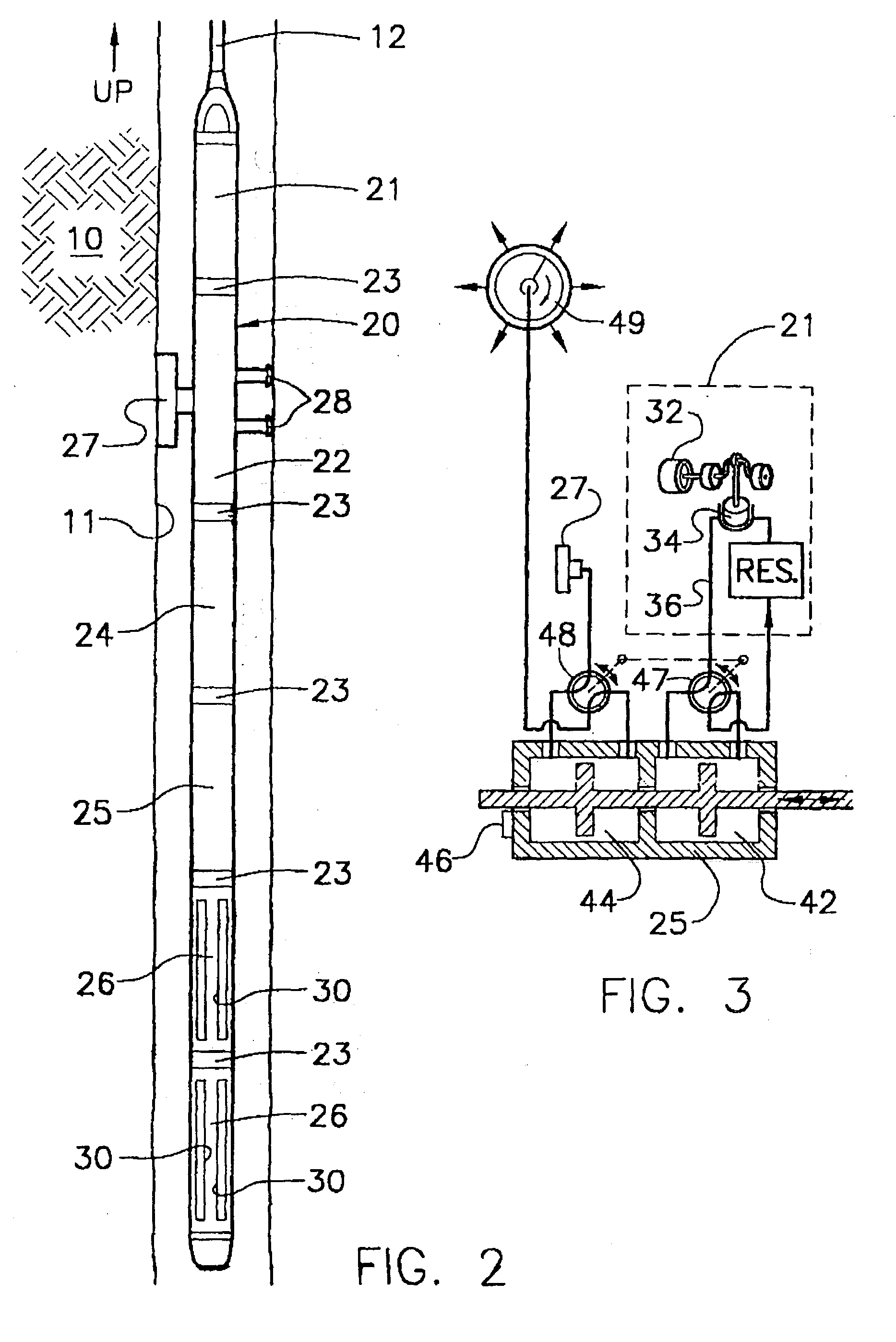
A tank contains both Zeolite and a hydrate in a gas chamber formed beneath a piston in the sample tank. Out of safety considerations, we avoid using source cylinders of nitrogen whose pressures exceed 4000 psi. Thus, the gas chamber of the sample tank is initially pressurized by the source cylinder to no more than 4000 psi of nitrogen at room temperature at the surface. Nitrogen gas is sorbed onto the zeolite at room temperature. As the tank is heated by being lowered downhole, nitrogen desorbs from the zeolite and the gas pressure increases. However, once this tank reaches a temperature high enough to release the hydrate's water of hydration, the released water is preferentially sorbed by zeolite, displacing sorbed nitrogen, and causing the pressure in the gas volume to increase even further. Because well temperatures are not high enough to desorb water from zeolite, any water sorbed onto a Zeolite sorption site will permanently block released nitrogen from resorbing at that site. The process of lowering the tank downhole provides the necessary heating to make the entire process occur. Thus, if returned to the surface at room temperature with the original gas-chamber volume, the tank's pressure would not fall back to the original pressure (e.g., 4000 psi) but would be at a substantially higher pressure (e.g., 6000 psi or more depending on the amount of Zeolite used and gaseous nitrogen gas released).
Owner:BAKER HUGHES INC
Porous poly(aryl ether ketone) membranes, processes for their preparation and use thereof
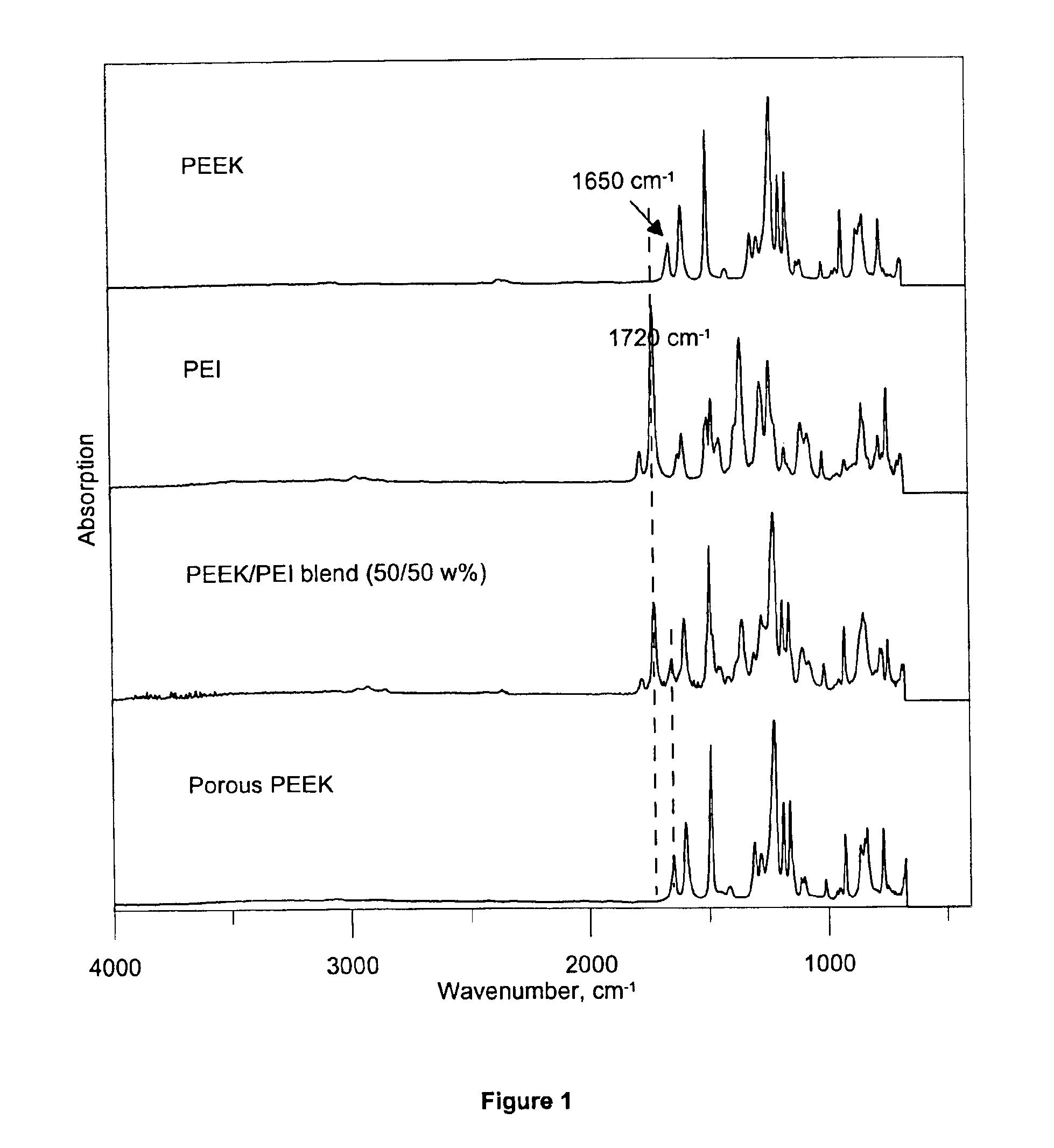
ActiveUS6887408B2Simple and cost-effective and industrially feasibleIncreased porous structureMembranesSemi-permeable membranesArylPorous medium
Porous poly(aryl ether ketone) (PAEK) articles are prepared from PAEK / polyimide blends by selective chemical decomposition and subsequent removal of the polyimide phase. Porous PAEK articles exhibit highly interconnected pore structure and a narrow pore size distribution. The porous PAEK articles of the present invention can be utilized as a porous media for a broad range of applications, including membranes for fluid separations, such as microfiltration, ultrafiltration, nanofiltration, and as a sorption media.
Owner:MASSACHUSETTS DEV FINANCE AGENCY
Oxygen sorbent compositions and methods of using same
InactiveUS7347887B2Extending oxygen sorption/desorption capacityImprove stabilityHydrogenIsotope separationSorbentSorption
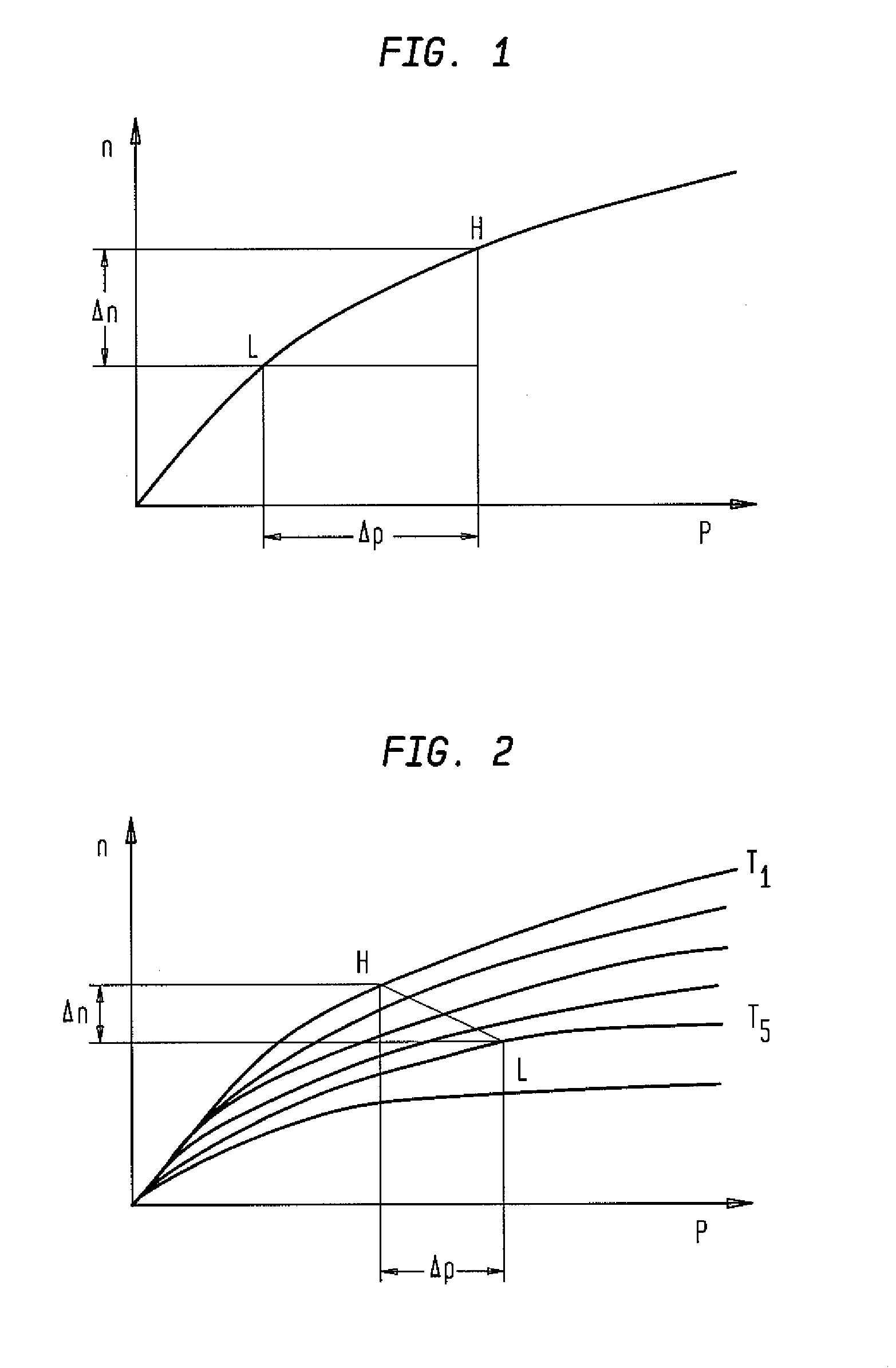
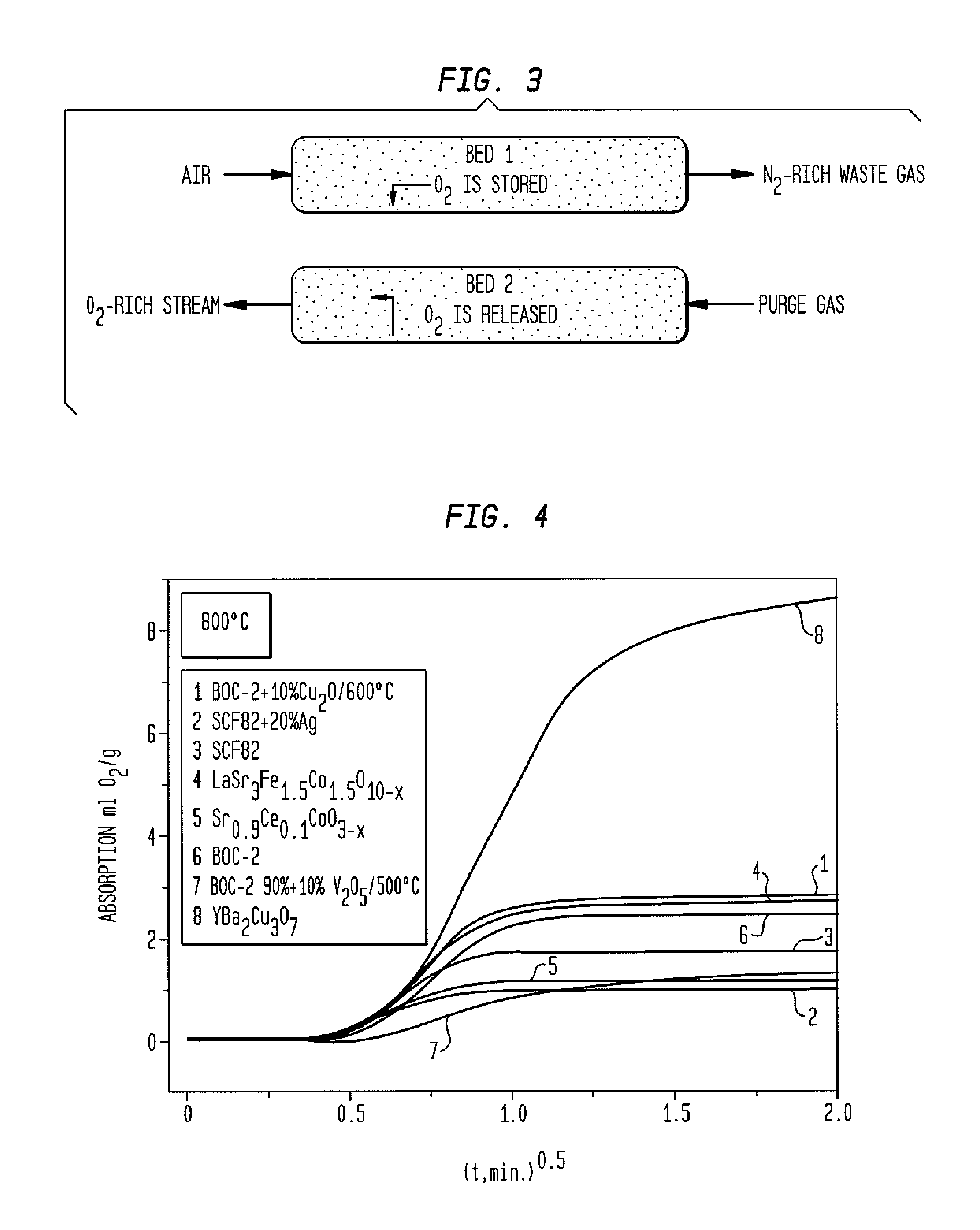
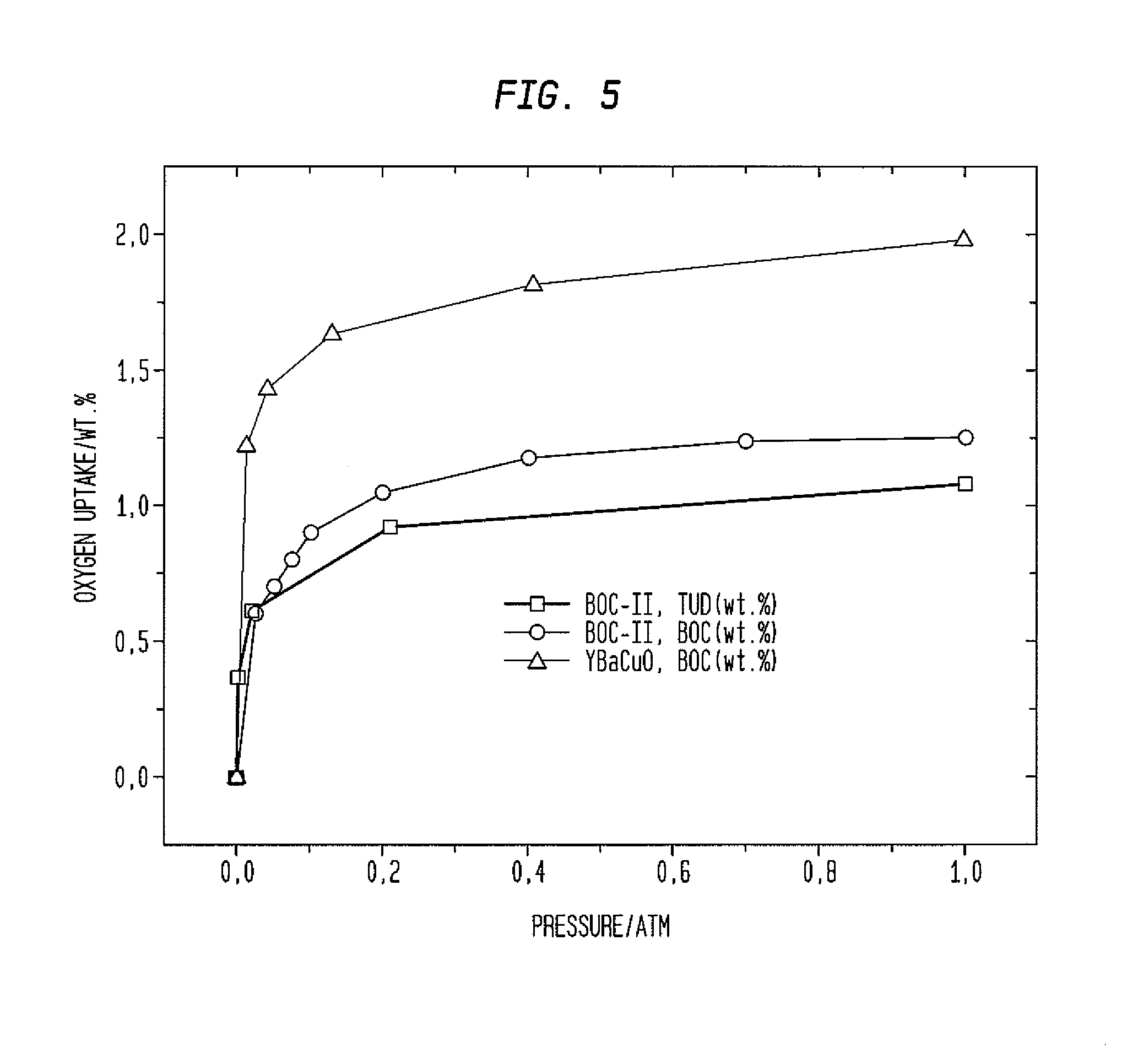
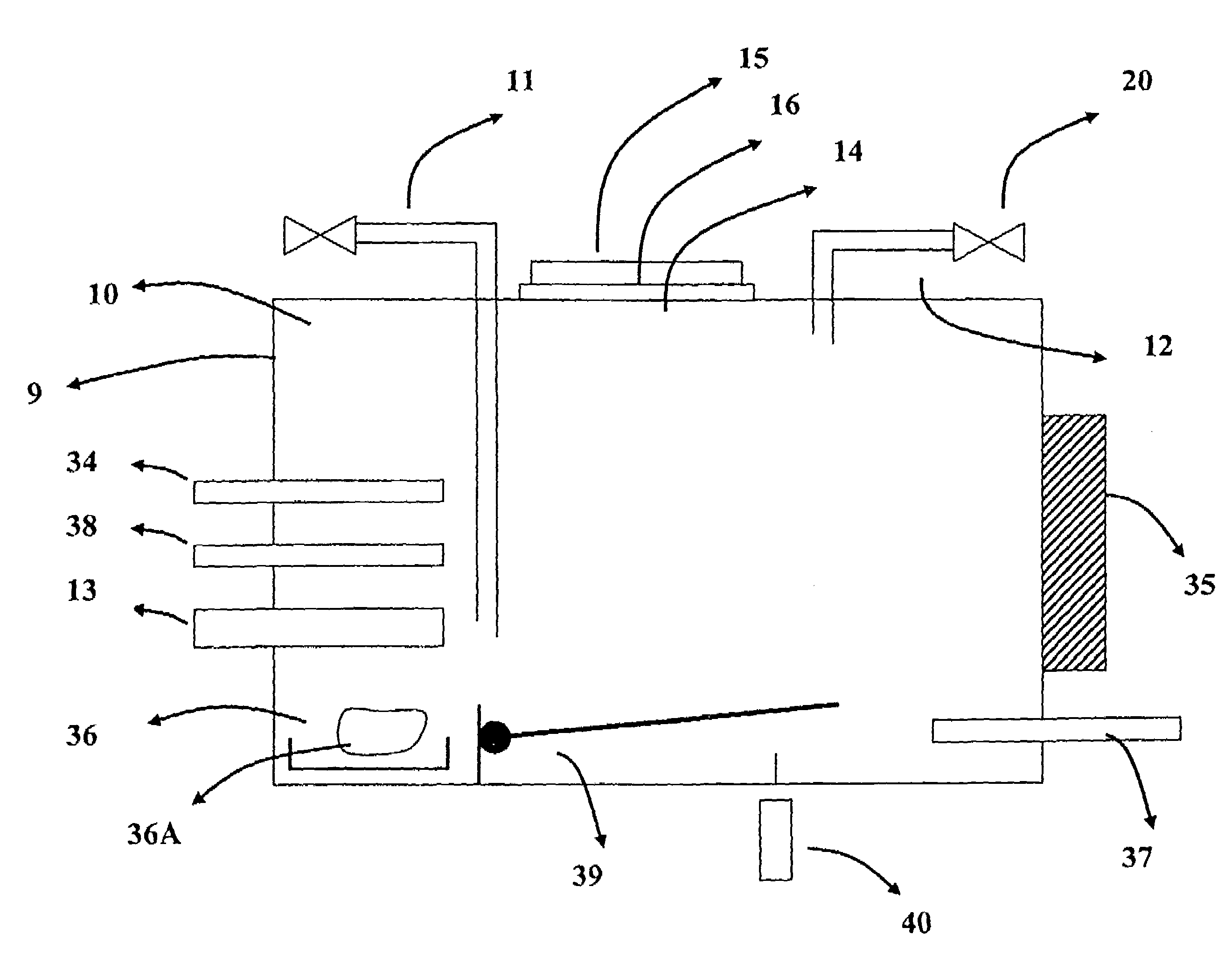
Composites and methods useful for oxygen sorption and other uses are presented, the composites comprising:(A) one or more crystalline ceramic oxides selected from compounds within general formula (1):AxA′x′ByB′y′O3-δ and (1)(B) one or more crystalline ceramic oxides selected from compounds within general formulas (2), (3), (4), (5), (6), (7), and (8):A2BO4-δ (2)A2B2O5-δ (3)AO(ABO3-δ)n (4)AM2Cu3O7-δ (5)Bi4V2(1-x)Me2xO11-3x, (6)A″B″O3 (7)A2B2O7-δ. (8)
Owner:BOC GRP INC
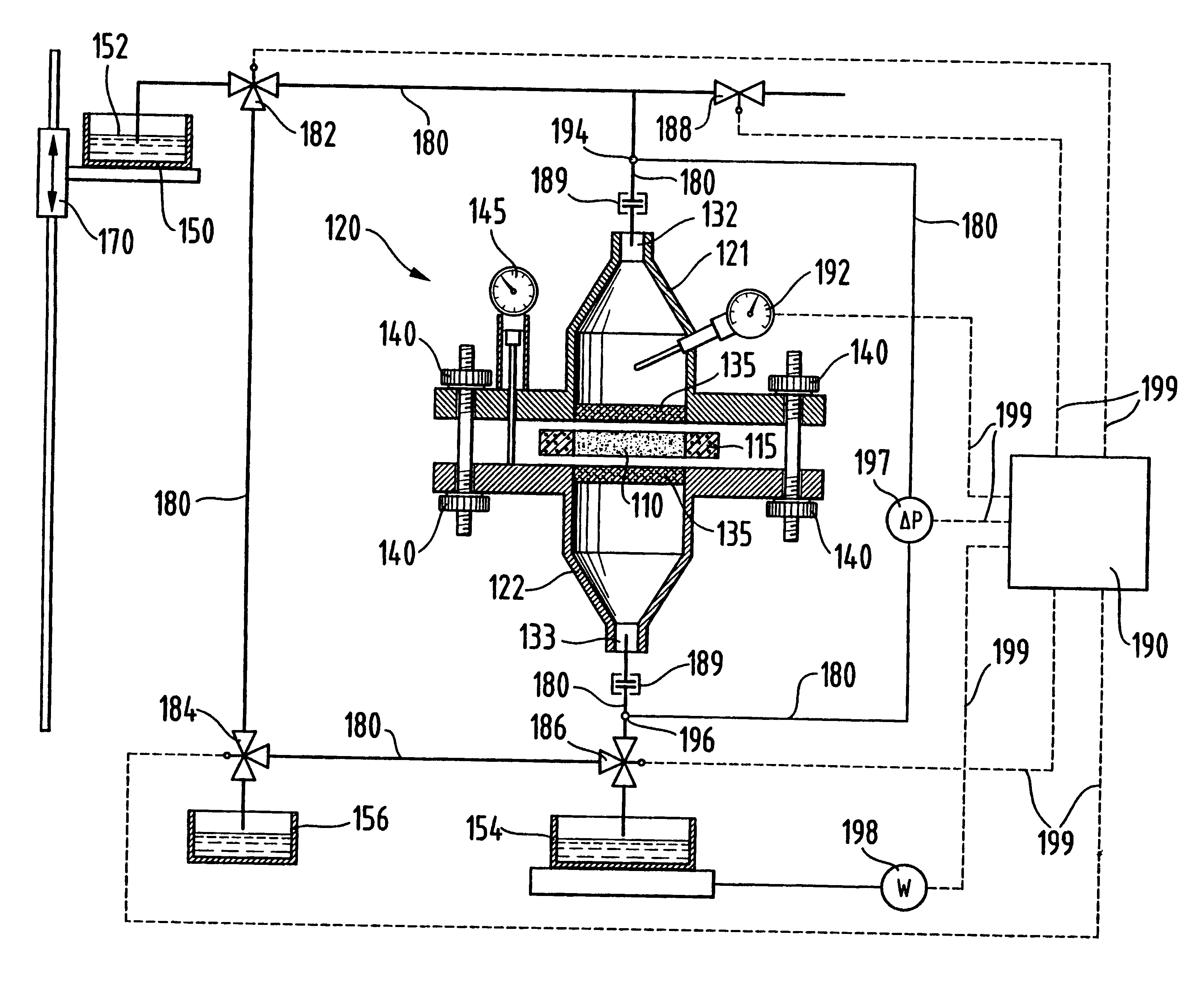
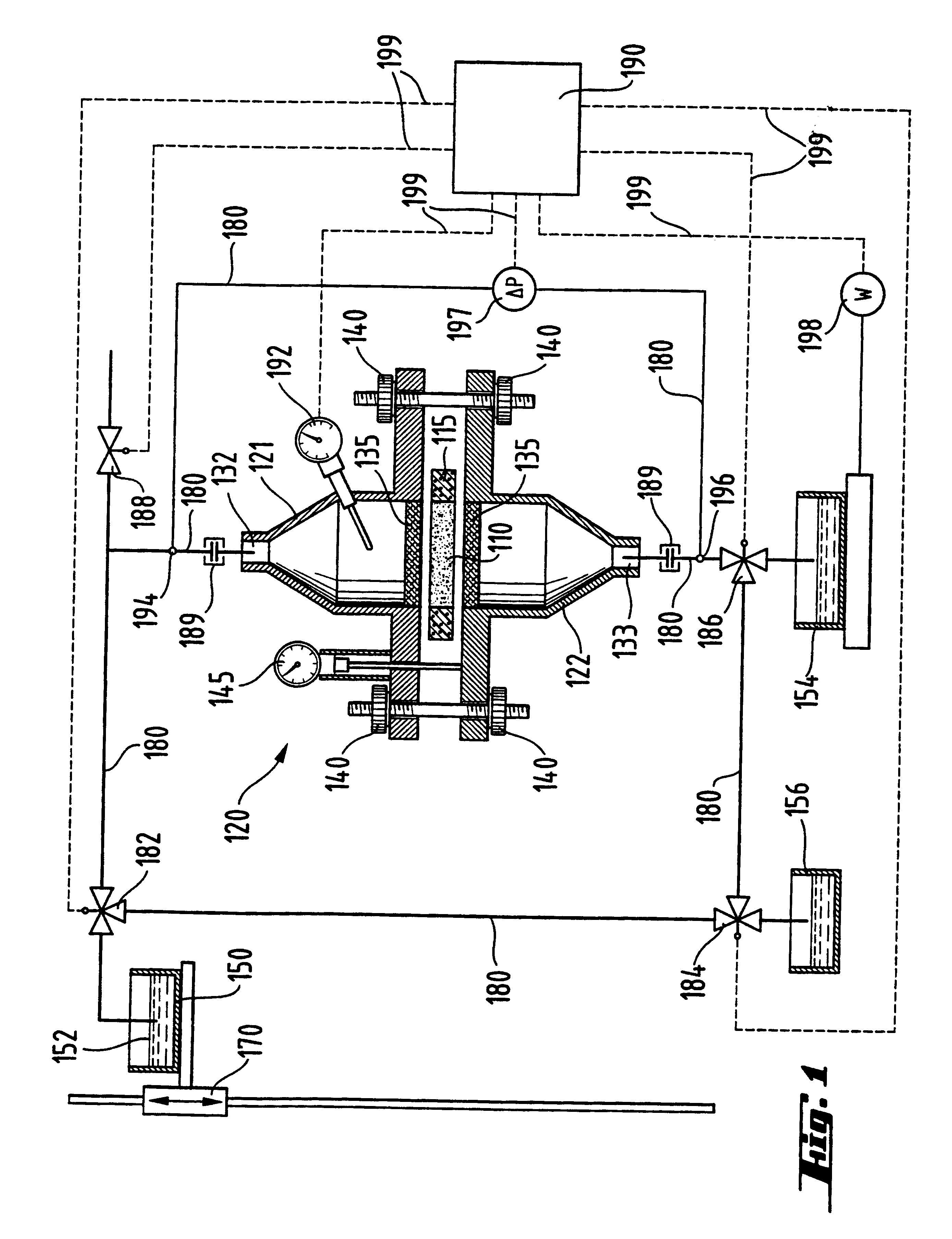
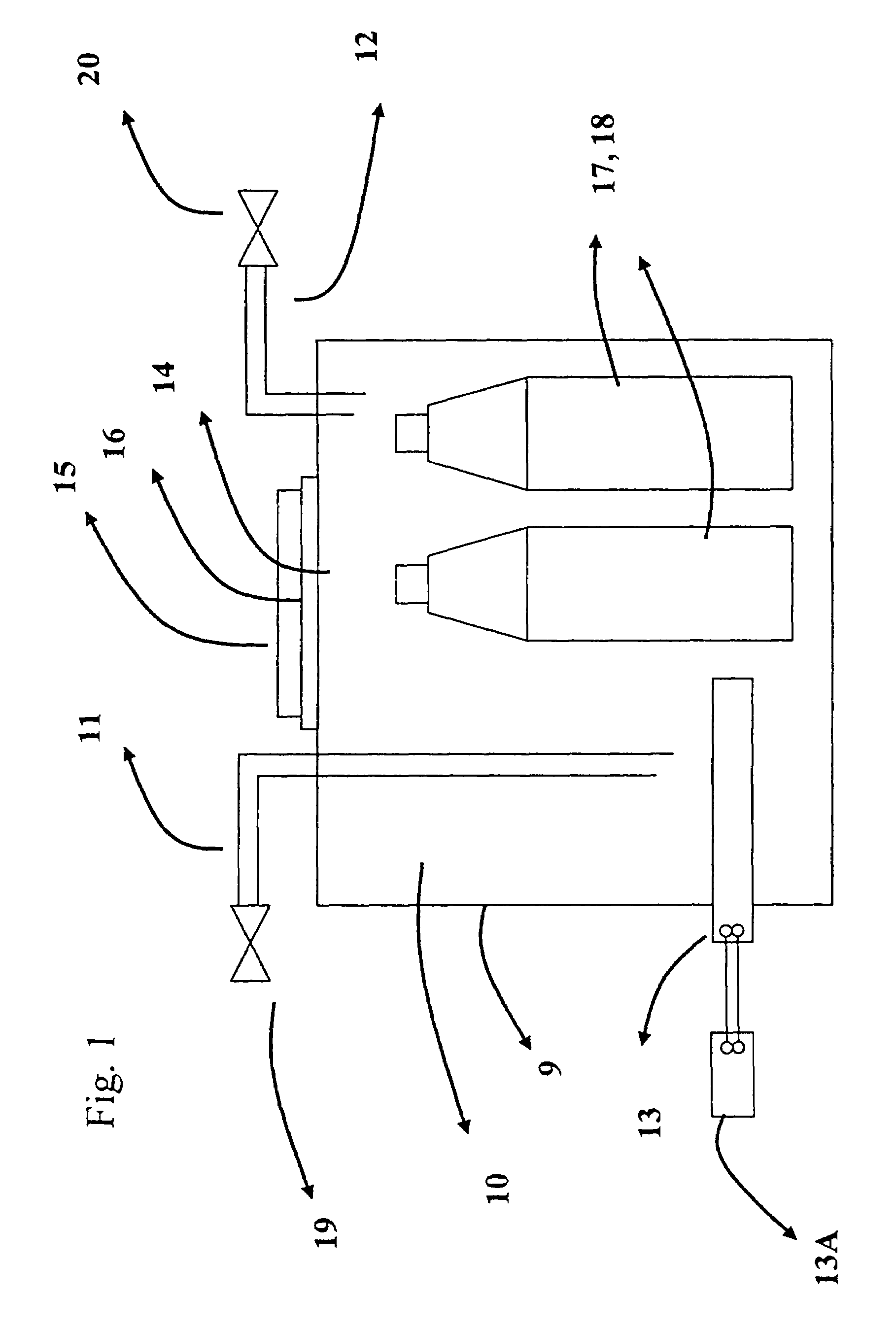
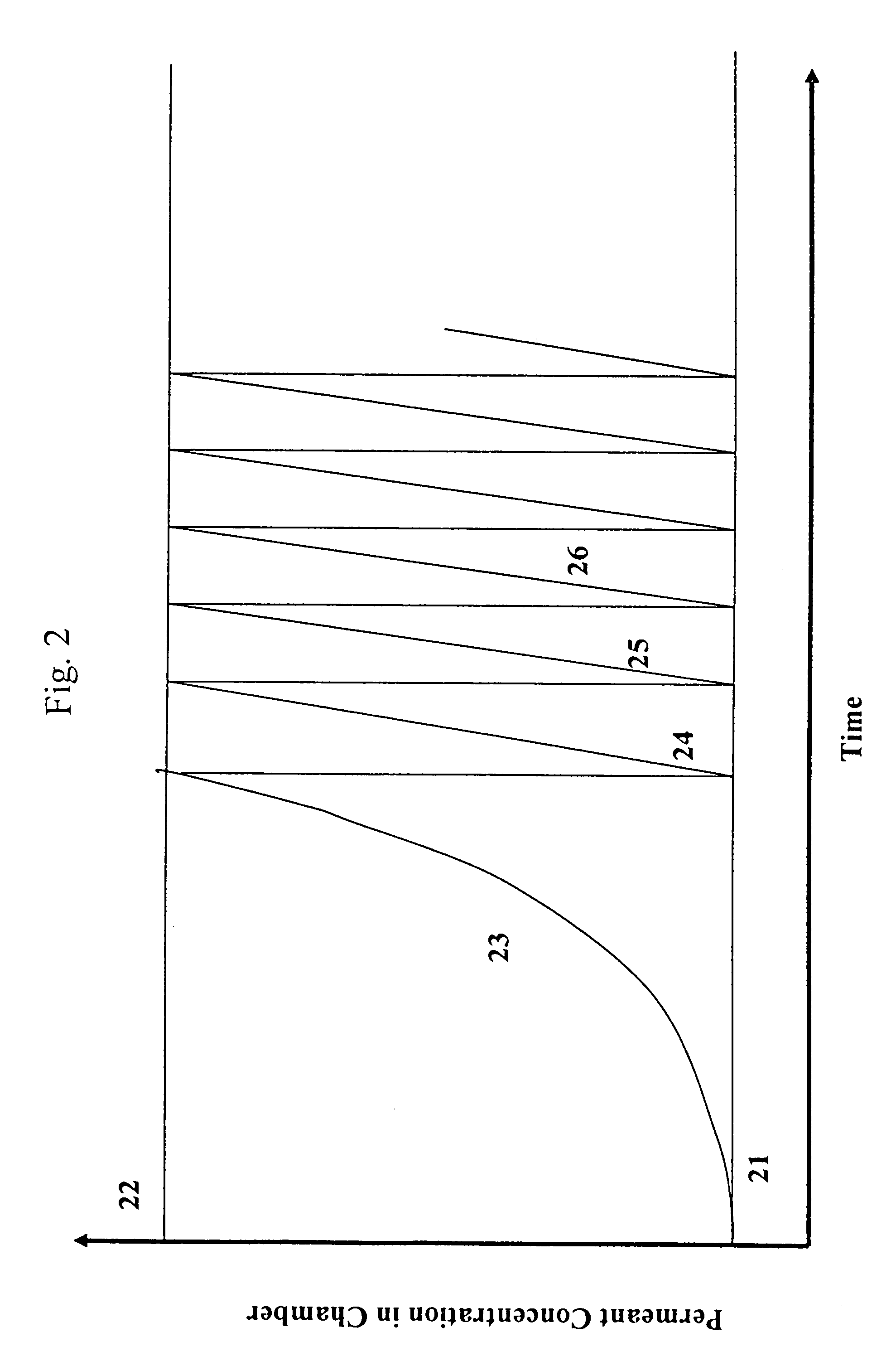
Apparatus and technique for measuring permeability and permeant sorption
InactiveUS6964191B1Extremely accurateDetection of fluid at leakage pointMeasurement of fluid loss/gain rateHigh concentrationEngineering
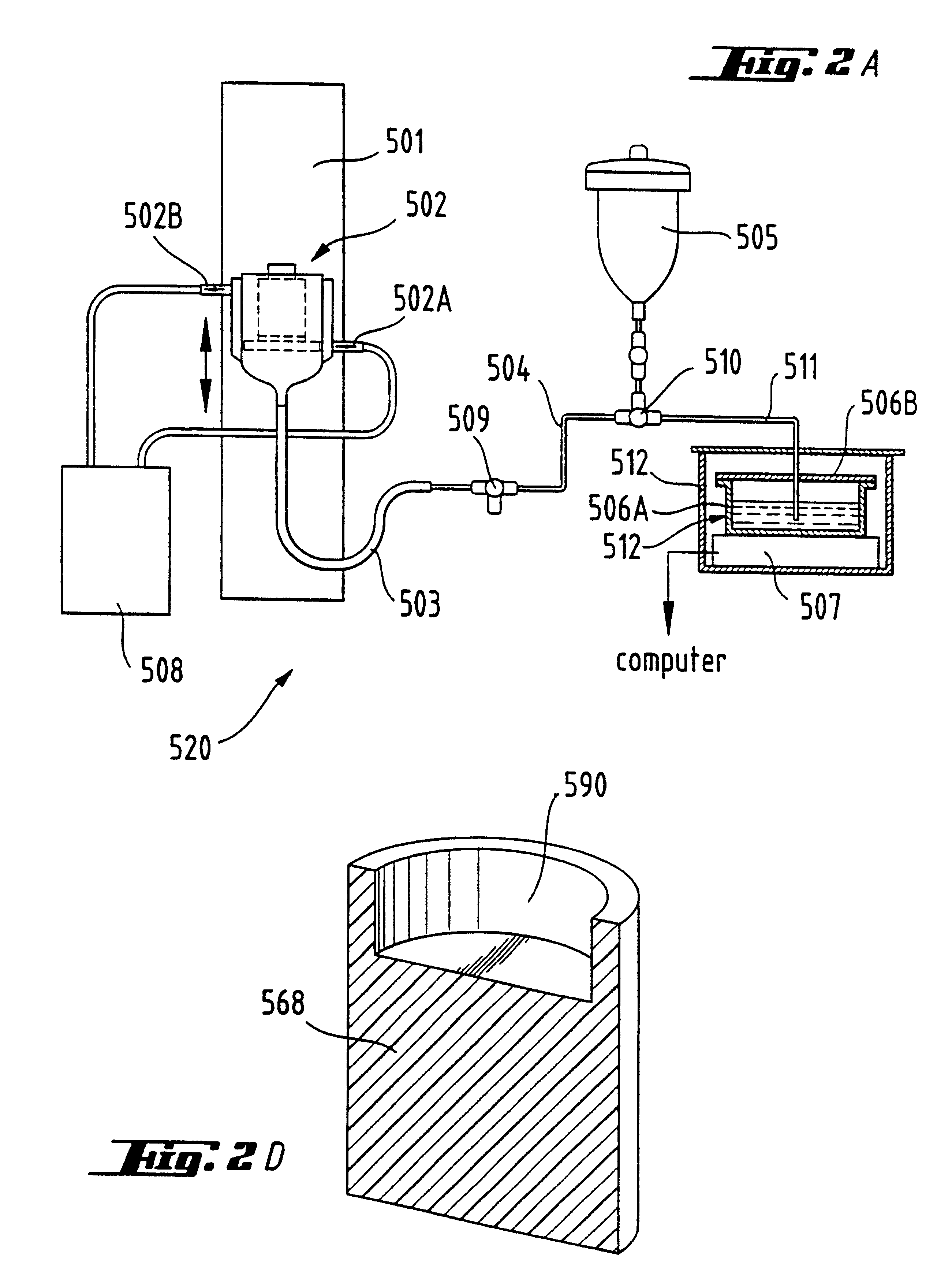
The invention is concerned with apparatus and methods for measuring package permeability and / or permeant sorption. The apparatus includes a housing defining an inner sealed chamber that is substantially impervious to a permeant of interest. The chamber has an inlet for introducing an inert medium substantially free of the permeant and an outlet for removing the inert medium. A sensor is positioned within the chamber and to provide signals indicative of permeant concentrations. A sealable opening is provided in the housing for introducing, within the chamber, packages to be measured for permeability and / or permeant sorption. In the method, testing of permeant concentrations is cycled to determine the slope of a time line between a low and a high concentration of the permeant. Humidity and temperature are preferably maintained constant within the chamber during testing.
Owner:TATA MURTHY
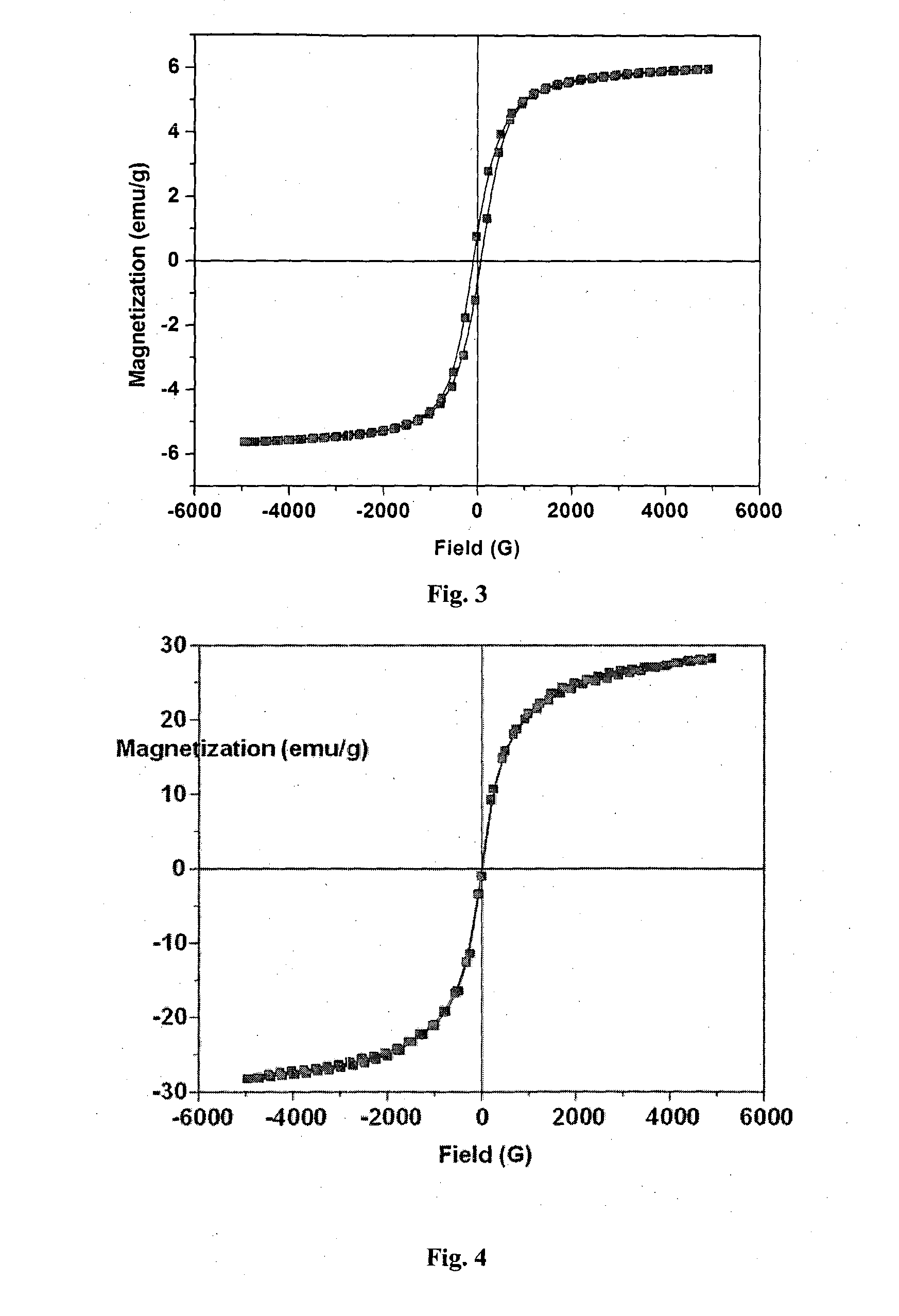
Magnetic nanoparticles decorated activated carbon nanocomposites for purification of water
InactiveUS20160243523A1Material nanotechnologyOther chemical processesSorbentMagnetite Nanoparticles
The present invention relates to the development of water purifying compositions based on magnetic nanoparticles decorated activated carbon nanocomposites which display both magnetic character as well as adsorbent characteristics. The addition of adsorbent to impure water containing dye as pollutant enables the fast adsorption of dye leading to discoloration of water whereas magnetic properties facilitates the rapid isolation of pollutant adsorbed nanocomposites powder from the purified water with the aid of a magnet. The present invention also provides a process for the development of such multifunctional adsorbent using a process which enables decoration of adsorbent with 5-50 weight % of magnetic nanoparticles, the enables the realization of magnetic adsorbent having saturation magnetization in the range 0.09 to 28.3 emu / g, dye removal efficiency of >99%, rapid decolourization of methylene blue (MB) / methyl orange (MO) dye polluted water in less than 1 min, magnetic separation time in the range <0.2 to 60 min and dye sorption capacity in the range of 3.3×10−4 to 116.3×10−4 mol of MB and 3.6×10−4 to 148.6×10−4 mol of MO dye per 100 gram of nanocomposite powder in a rapid adsorption (<1 min) and magnetic separation process. Besides, these nanocomposites could also be useful for other of applications e.g. as separation of catalytic residues from the products, for removal of oil from water, filler for development of thermally / electrically conducting magneto-rheological fluids or for handling of electromagnetic pollution.
Owner:COUNCIL OF SCI & IND RES
Optical MEMS Chemical Sensor Array
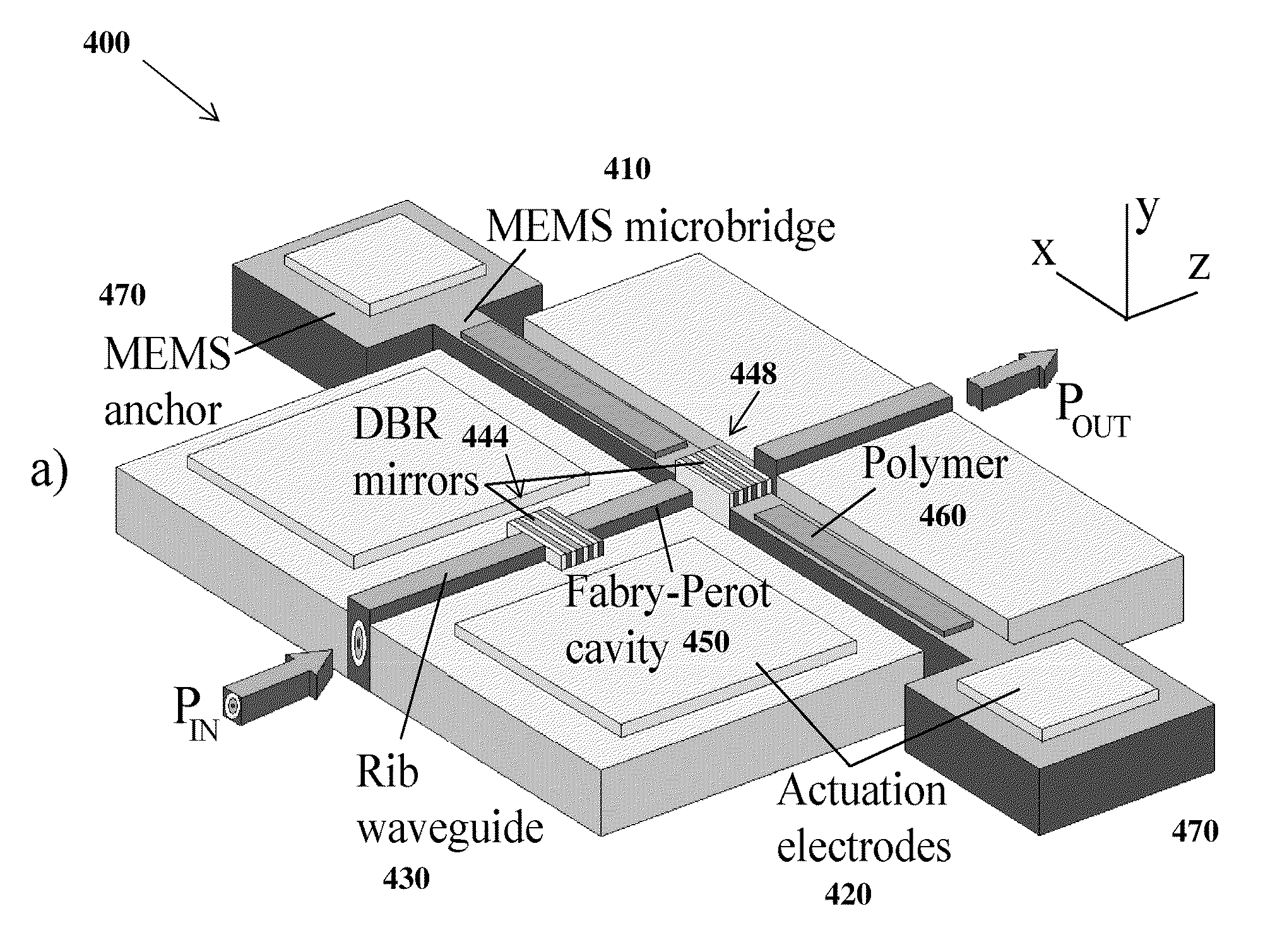
ActiveUS20100238454A1High selectivityIncrease flexibilityMaterial analysis using immobilised reagentsMaterial analysis using sonic/ultrasonic/infrasonic wavesSensor arrayWaveguide
A change in mass of a microbridge in a mass sensor can be sensed by applying a time-varying amplitude modulated electrostatic force to excite the microbridge into resonance at the frequency of amplitude modulation. An optical energy is then transmitted at a wavelength close to a resonant wavelength of a Fabry-Perot microcavity, which is formed by etching a movable reflective mirror into a region of the microbridge and by etching a fixed reflective minor in a region spaced apart from the microbridge. The two mirrors are interconnected by an optical waveguide. The movable mirror and fixed mirror reflect the optical energy to a receiver, and a change in the Fabry-Perot microcavity's reflectivity is interferometrically determined. The change in reflectivity indicates a change in the microbridge's resonant frequency due to increased mass of the microbridge resulting from sorption of a target chemical by a layer of chemoselective material deposited on the microbridge.
Owner:UNITED STATES OF AMERICA
Energy efficient sorption processes and systems
InactiveUS20050262720A1Reduce the temperatureReduce power consumptionSolar heating energyInternal combustion piston enginesSorbentEngineering
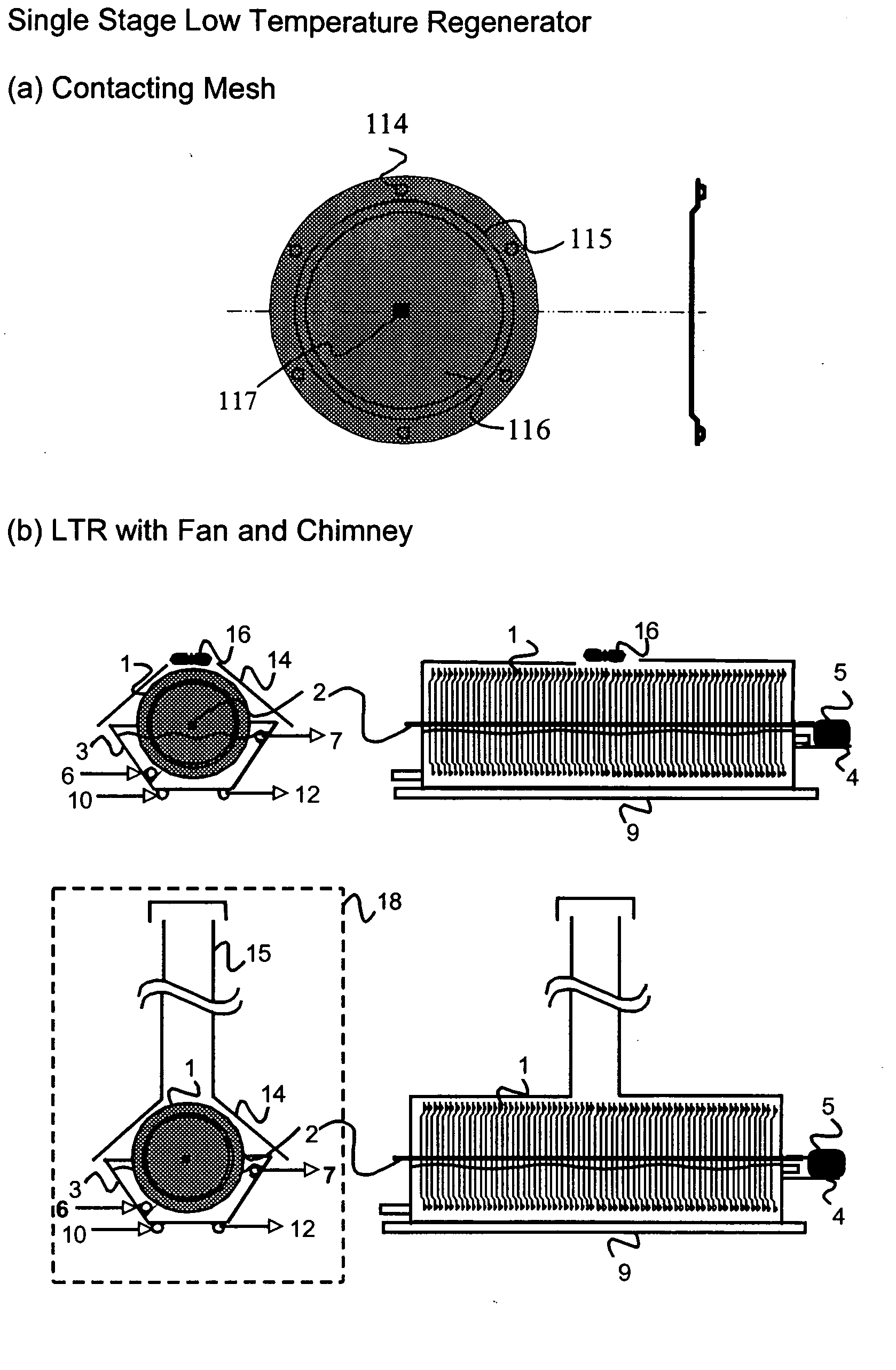
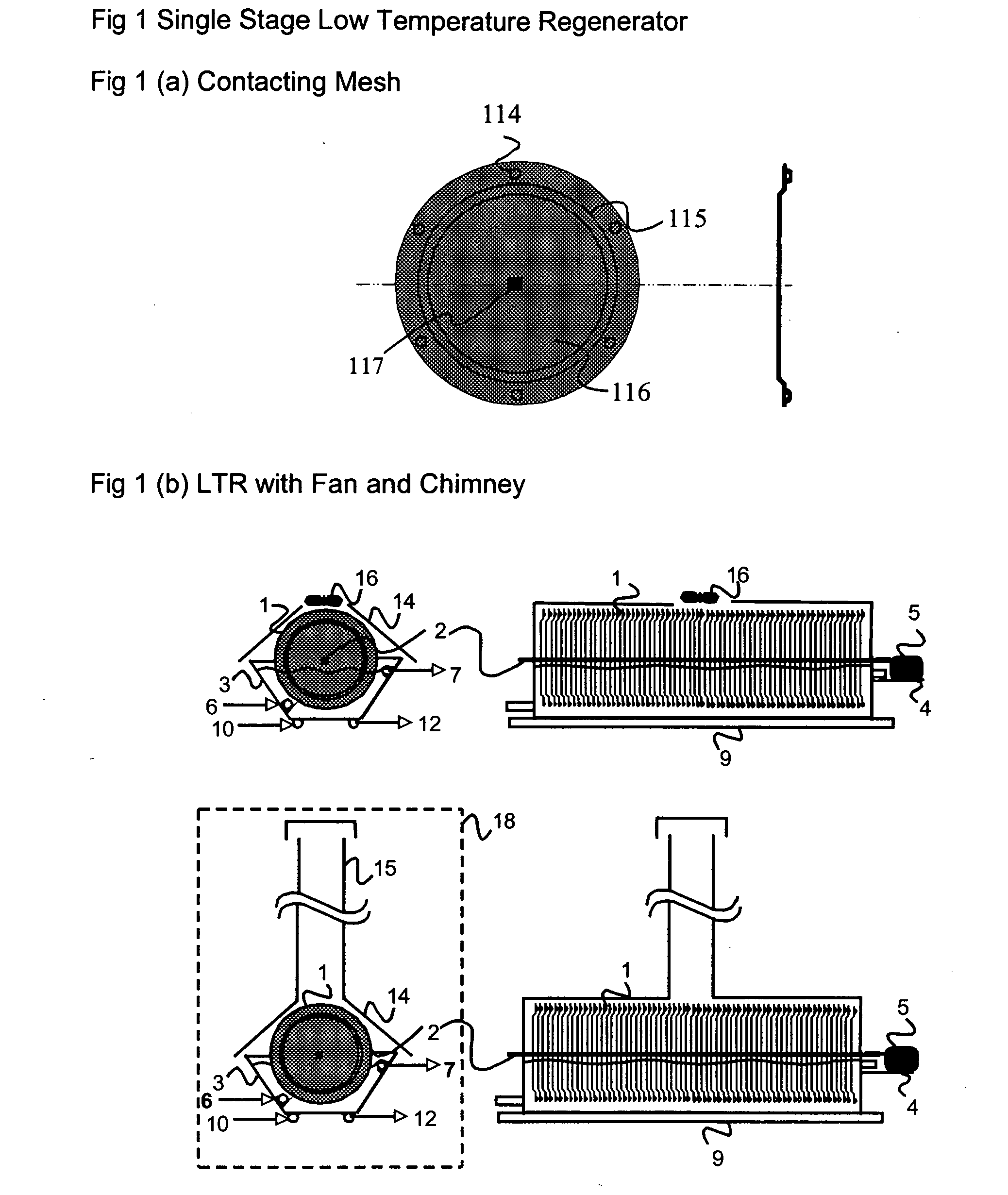
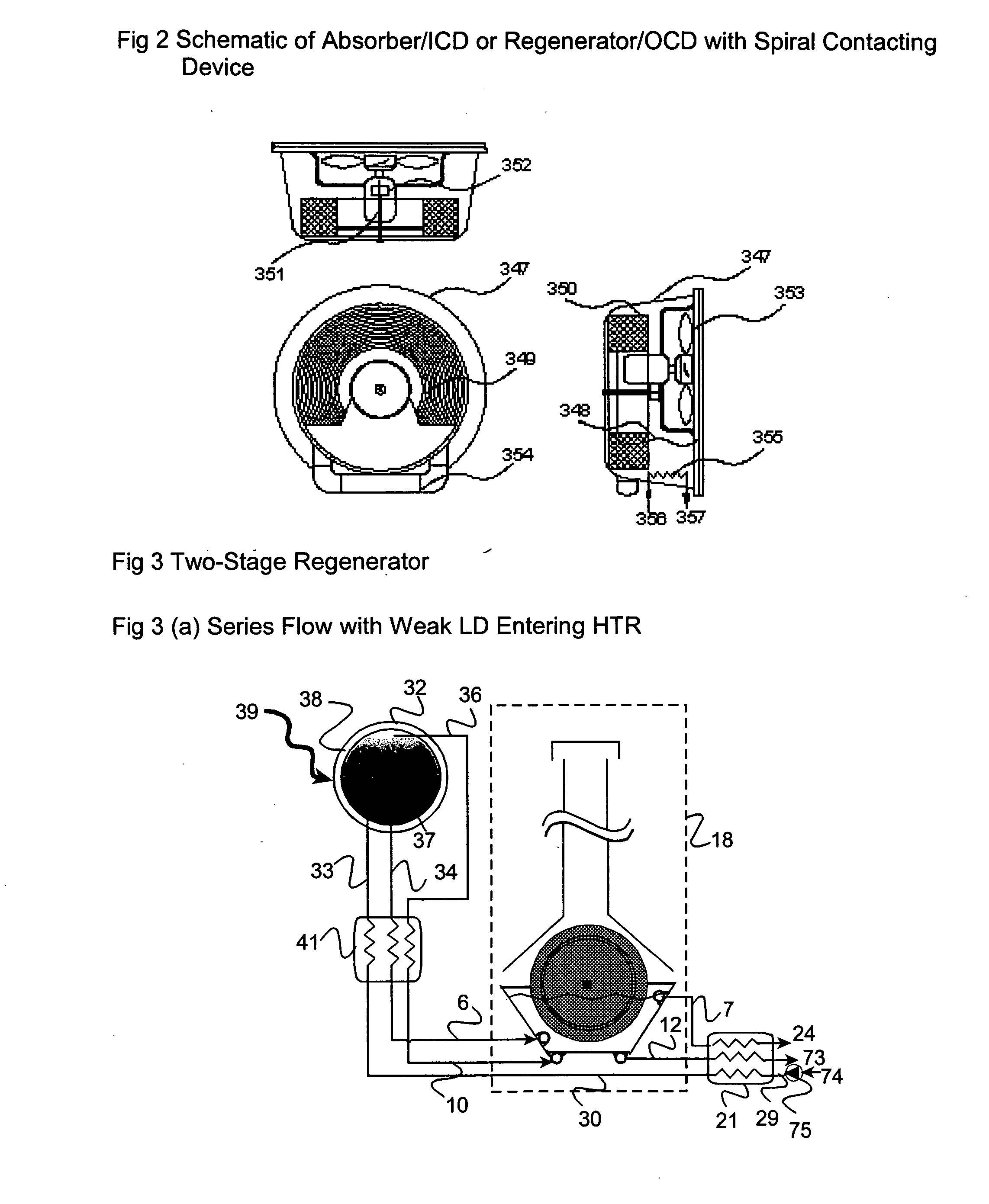
The present invention relates to novel energy efficient sorption processes and systems for cooling, dehumidifying and heating using multistage liquid desiccant regenerators, or hybrid cooling systems or adsorption cooling systems involving appropriate combinations of rotating contacting devises, adsorption modules with heat transfer passages in thermal contact with the adsorption module wall and switchable heat pipes. The sorption processes of this invention help in flexible designing of compact cooling, dehumidifing, heating systems easy operability. The adsorption module of this invention leads to lower cycle times as low as 5 minutes; makes it possible to achieve high system Coefficient of Performance (COP) up to 0.9 due to reduced thermal mass; offers high specific cooling power in the range of 50 to 750 W / kg of AC; is easy to manufacture and operates at low costs. The refrigeration cum heating system of this invention with heat pipe in thermal contact with the adsorption modules increase the heat transfer rates without increasing the thermal mass leading to increase of COP and the single or multistage pressure equalisation increases the internal regeneration of heat thereby increasing the COP, reducing the cycle time resulting in increased specific cooling power (SCP), reducing the required quantity of adsorbent / refrigerant making the module compact and cost effective.
Owner:INDIAN INST OF TECH
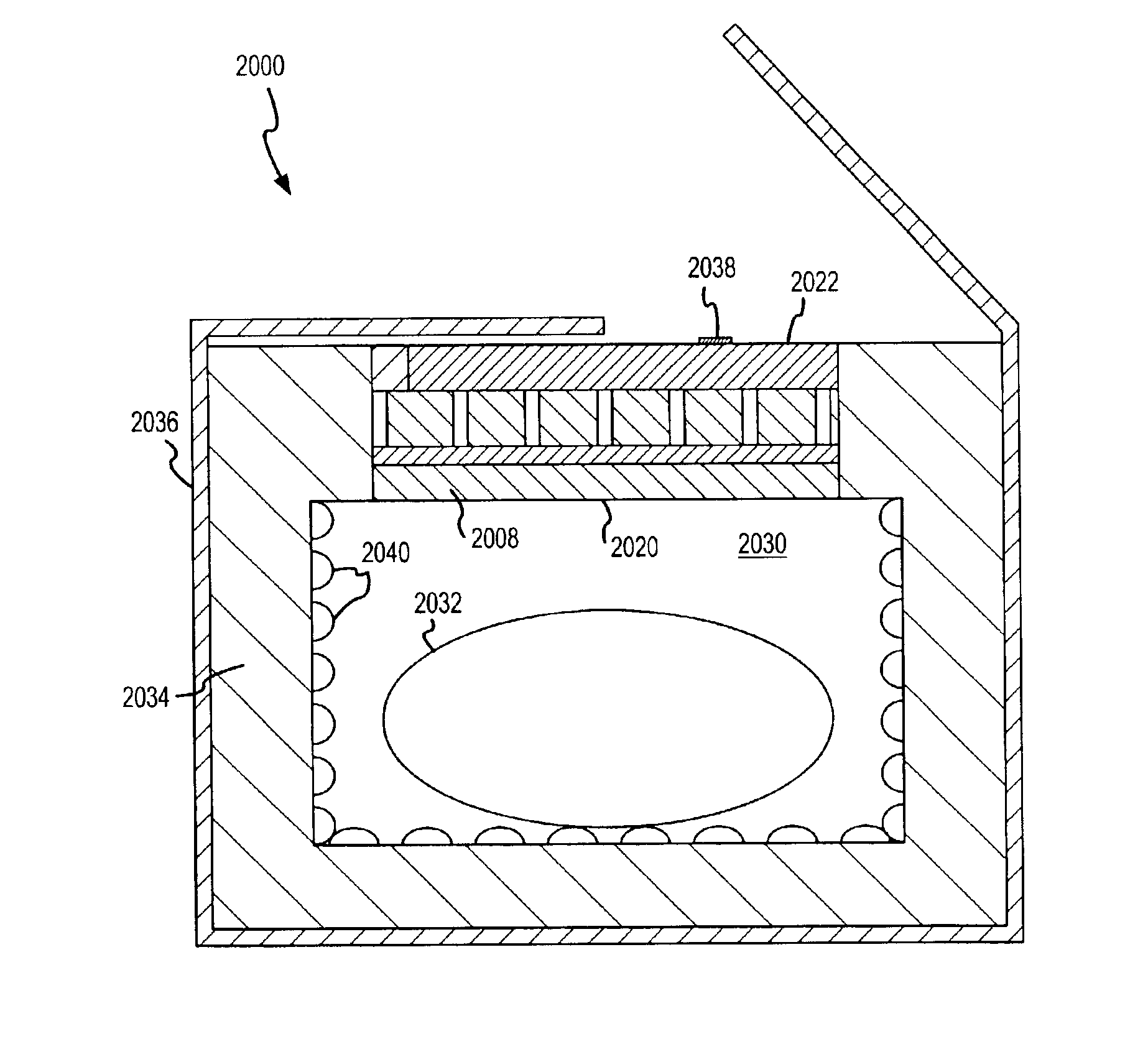
Sorption-based adhesive contact cooling apparatus and method
ActiveUS20080255644A1Light weightHighly effectiveSurgical instruments for coolingTherapeutic coolingInternal pressureInterconnection
A sorption-based, adhesive contact apparatus and method for contact cooling a patient via conductive thermal exchange are described. The apparatus includes a pad, having a fluid containing layer and adhesive contact surface, and a sorption-based device, fluidly interconnectable to the fluid containing layer, for vaporizing fluid contained in the fluid containing layer and for sorping the vaporized fluid. The fluid vaporization and the vapor sorption cools the adhesive contact surface of the pad. A port control member may selectively establish fluid interconnection between the fluid containing layer and sorption-based device. An internal pressure of the sorption-based device may be established lower than an internal pressure of the fluid-containing layer prior to fluid interconnection therebetween. A fluid retention member and path defining members may be included to facilitate vaporization, vapor diffusion and vapor sorption, thereby enhancing uniform cooling.
Owner:MEDIVANCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com