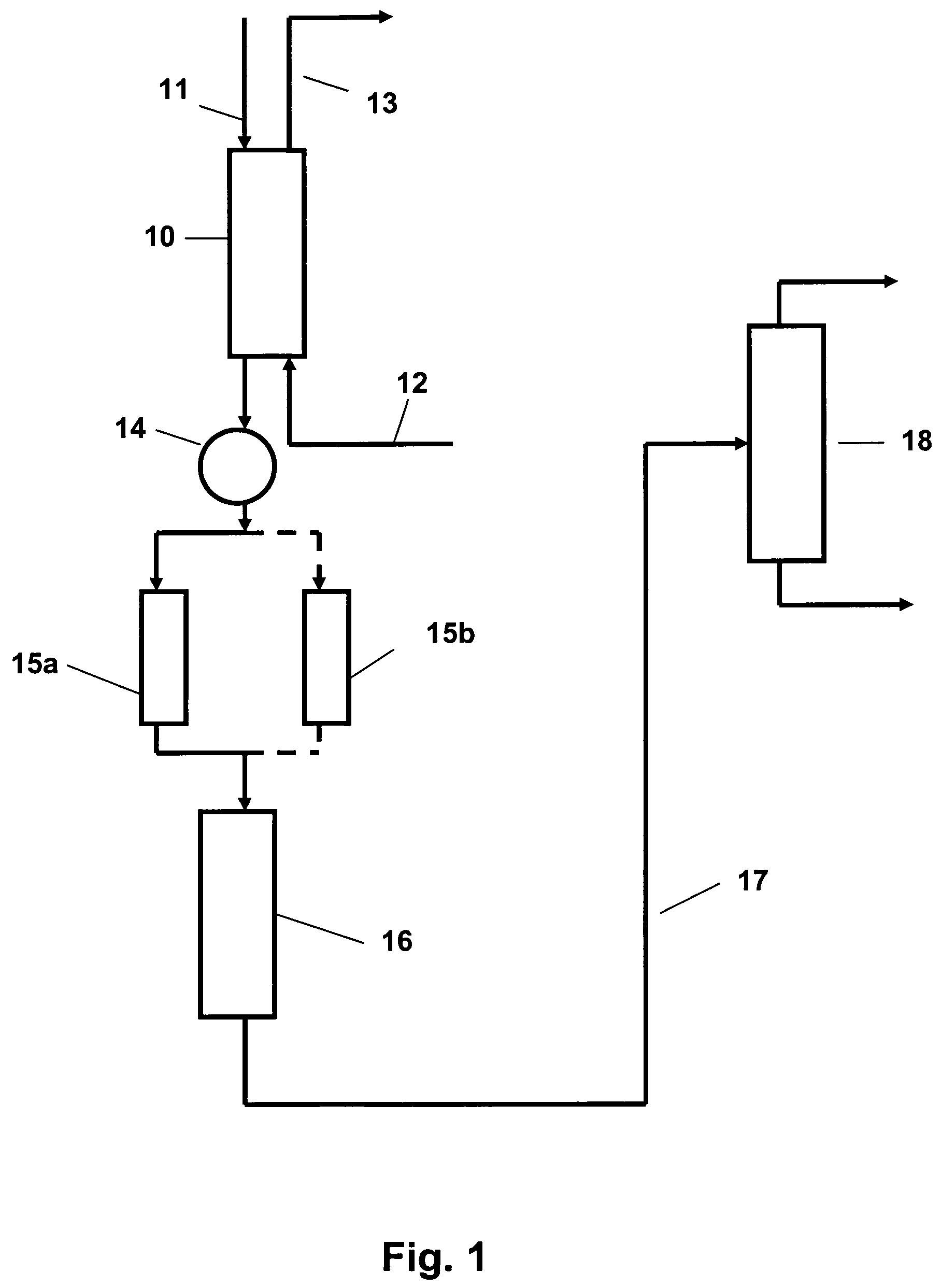
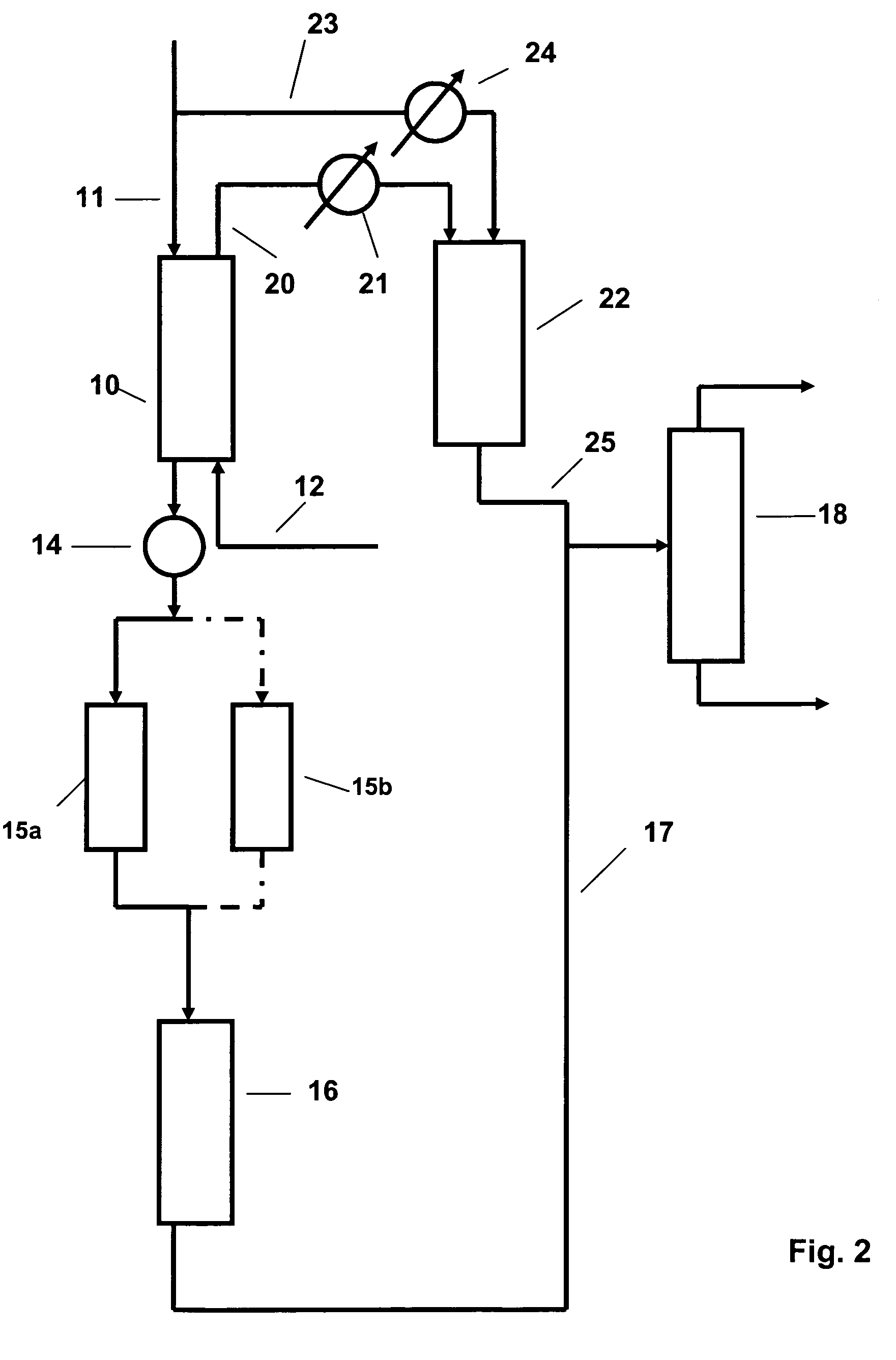
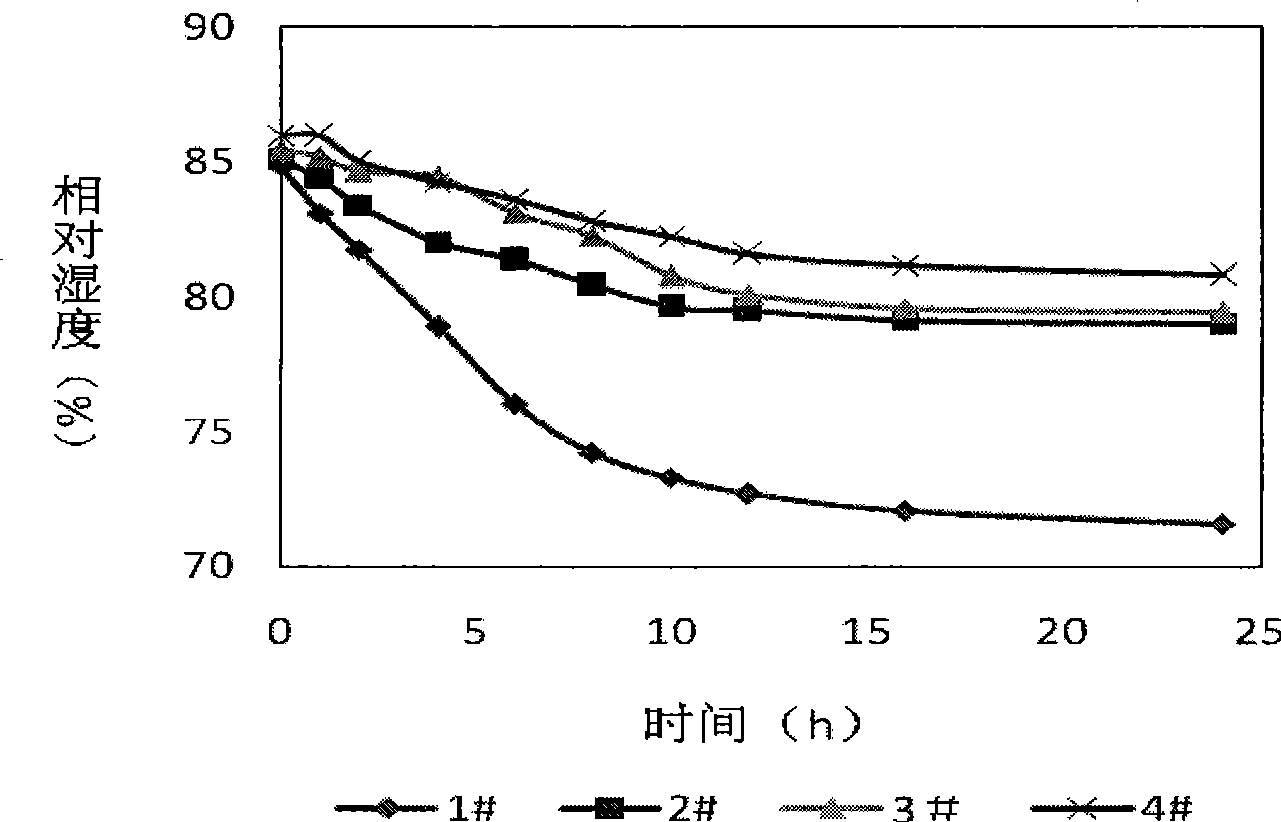
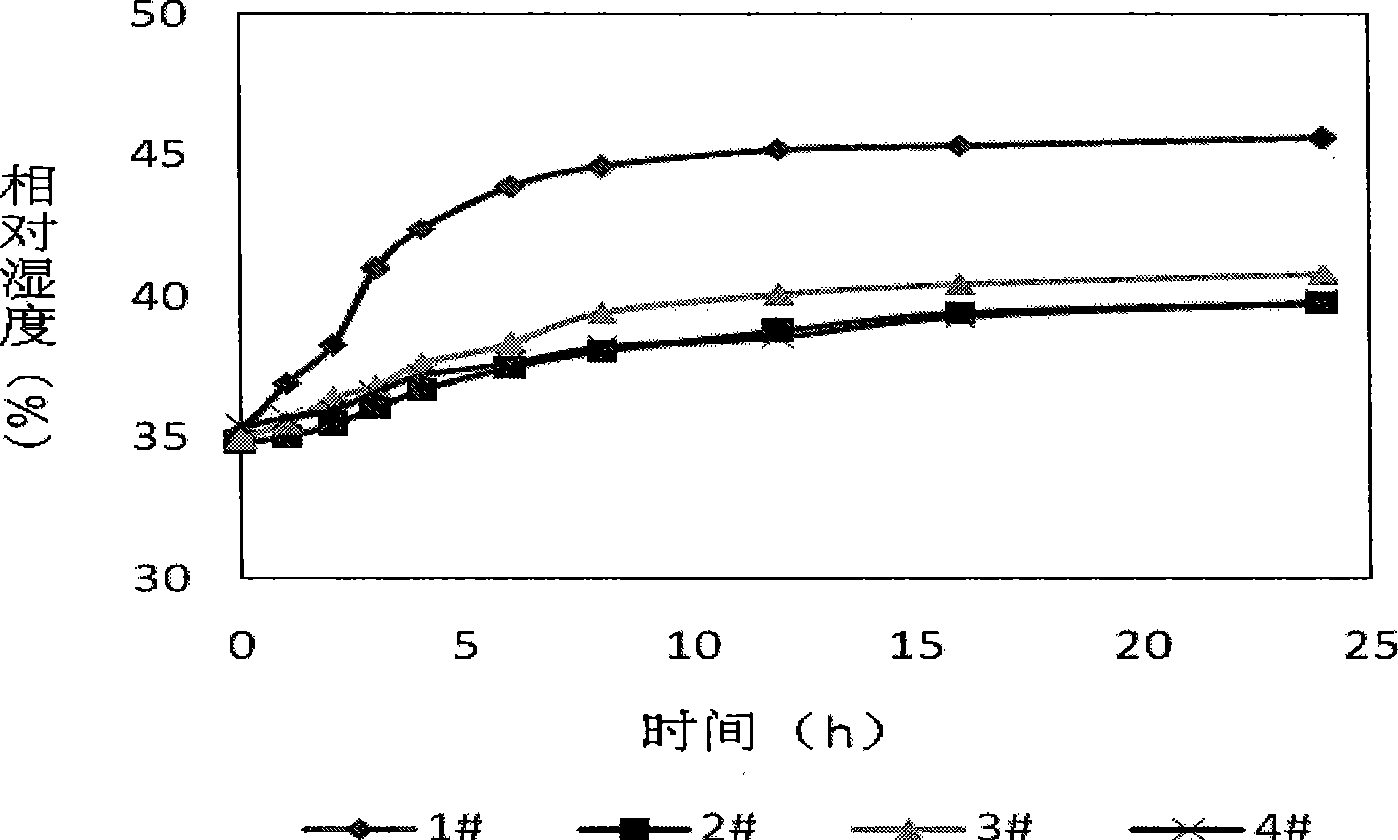
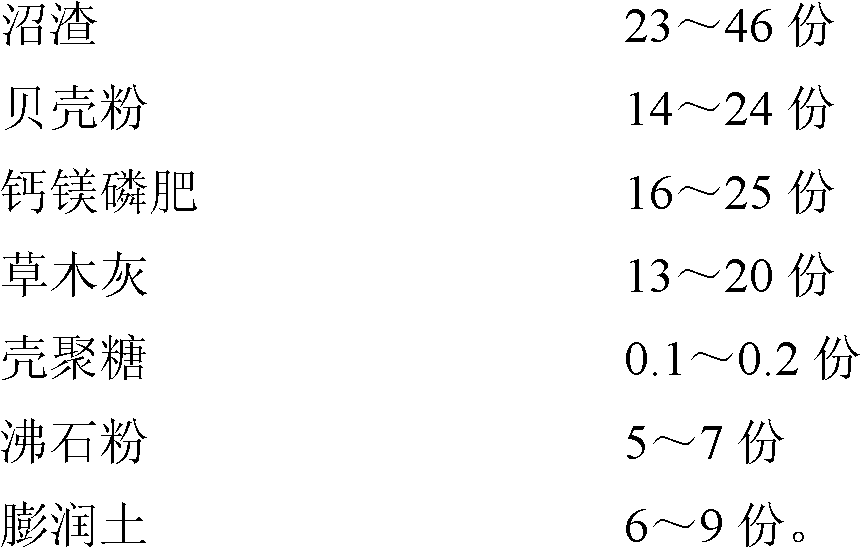Patents
Literature
15489 results about "Zeolite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents and catalysts. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating the material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone". The classic reference for the field has been Breck's book Zeolite Molecular Sieves: Structure, Chemistry, And Use.
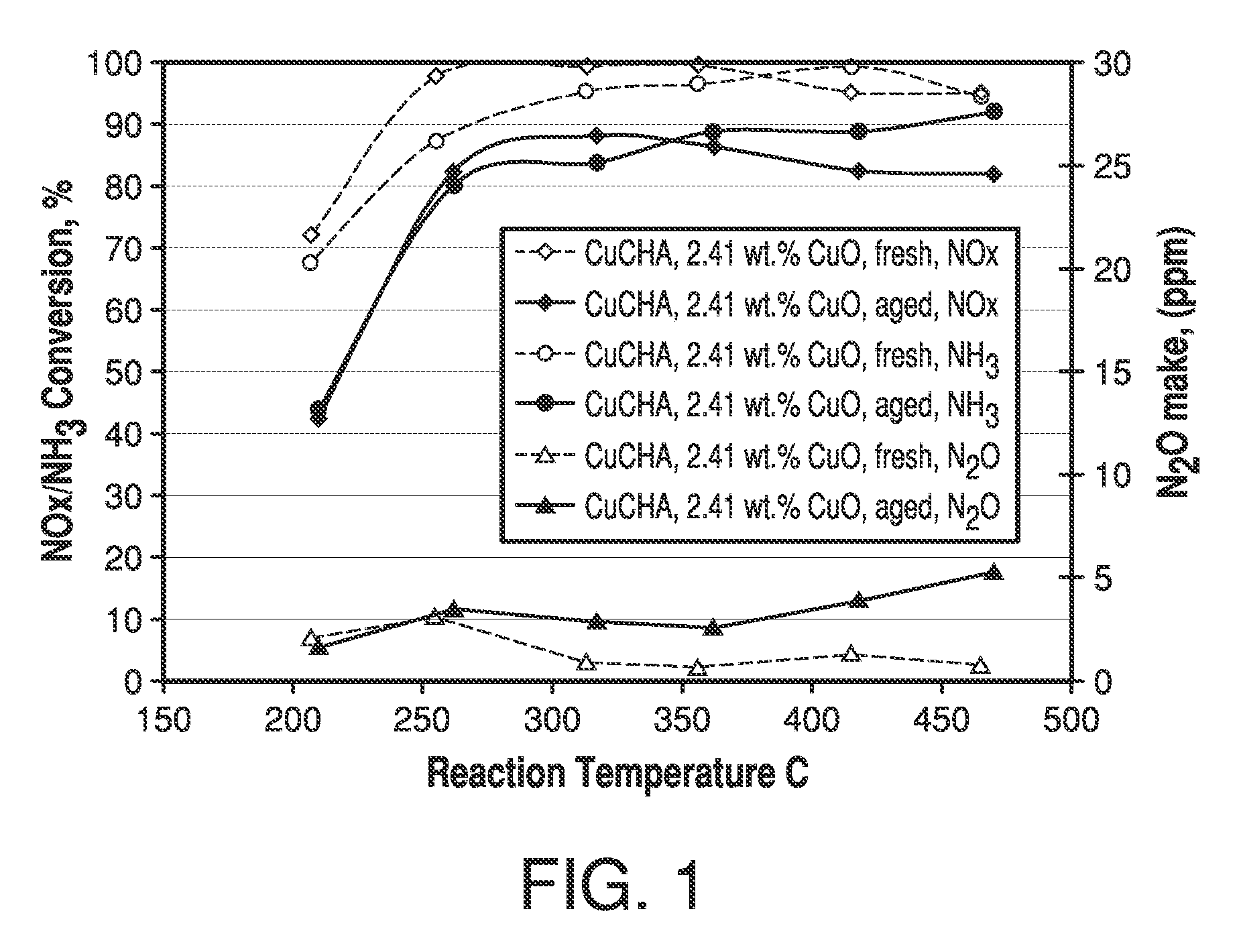
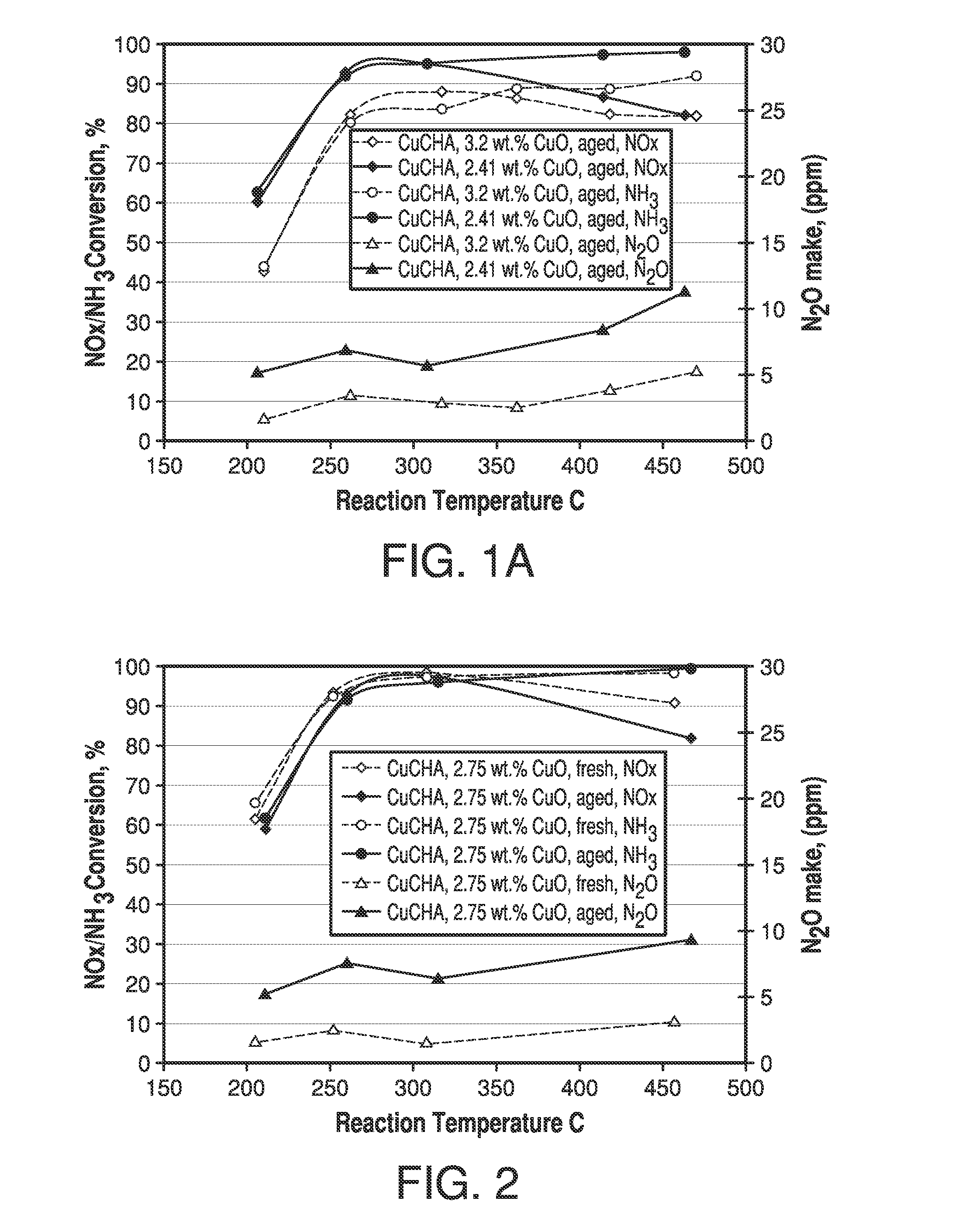
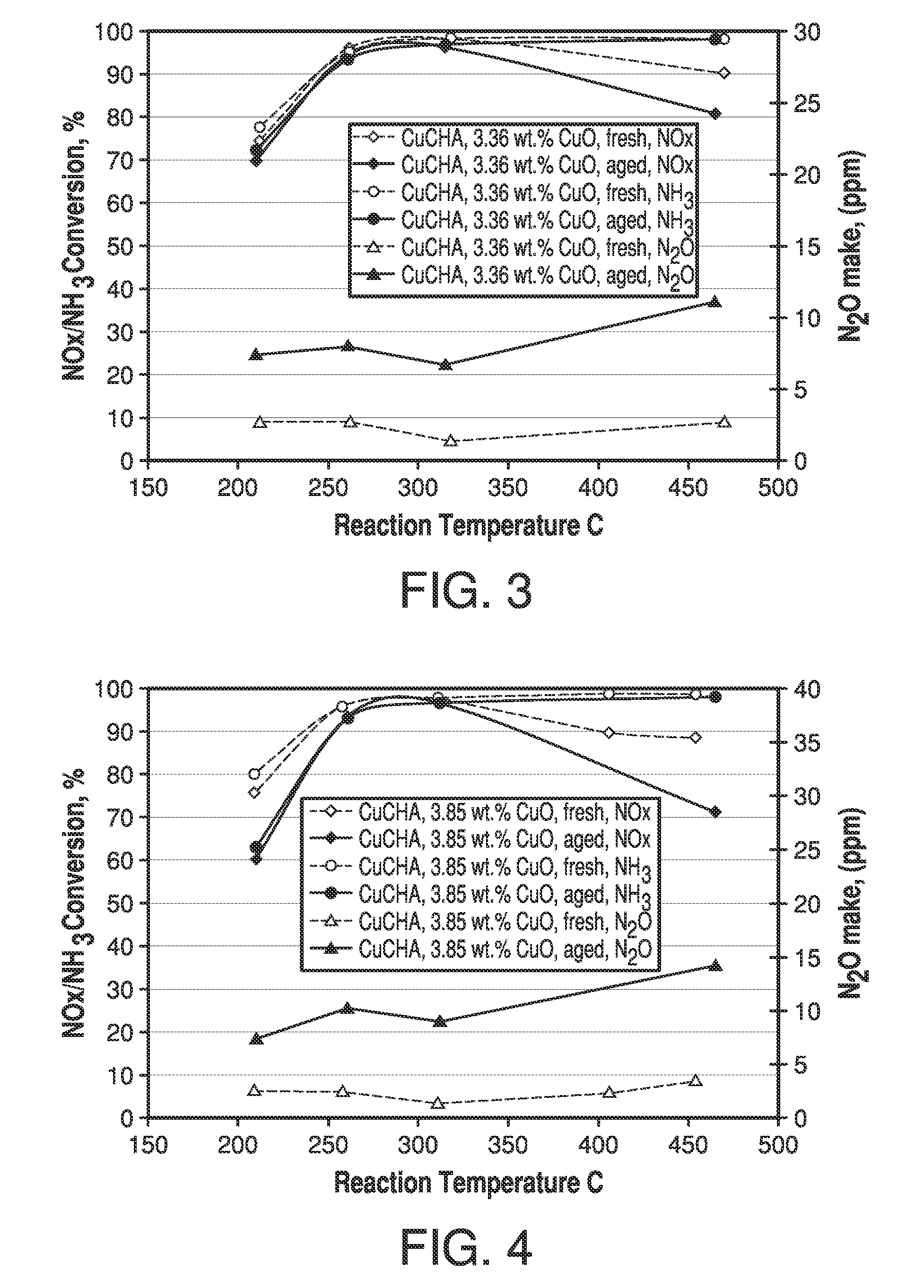
Copper CHA zeolite catalysts
ActiveUS7601662B2Good hydrothermal stabilityHigh catalytic activityCombination devicesAluminium compoundsReaction temperatureCrystal structure
Zeolite catalysts and systems and methods for preparing and using zeolite catalysts having the CHA crystal structure are disclosed. The catalysts can be used to remove nitrogen oxides from a gaseous medium across a broad temperature range and exhibit hydrothermal stable at high reaction temperatures. The zeolite catalysts include a zeolite carrier having a silica to alumina ratio from about 15:1 to about 256:1 and a copper to alumina ratio from about 0.25:1 to about 1:1.
Owner:BASF CORP
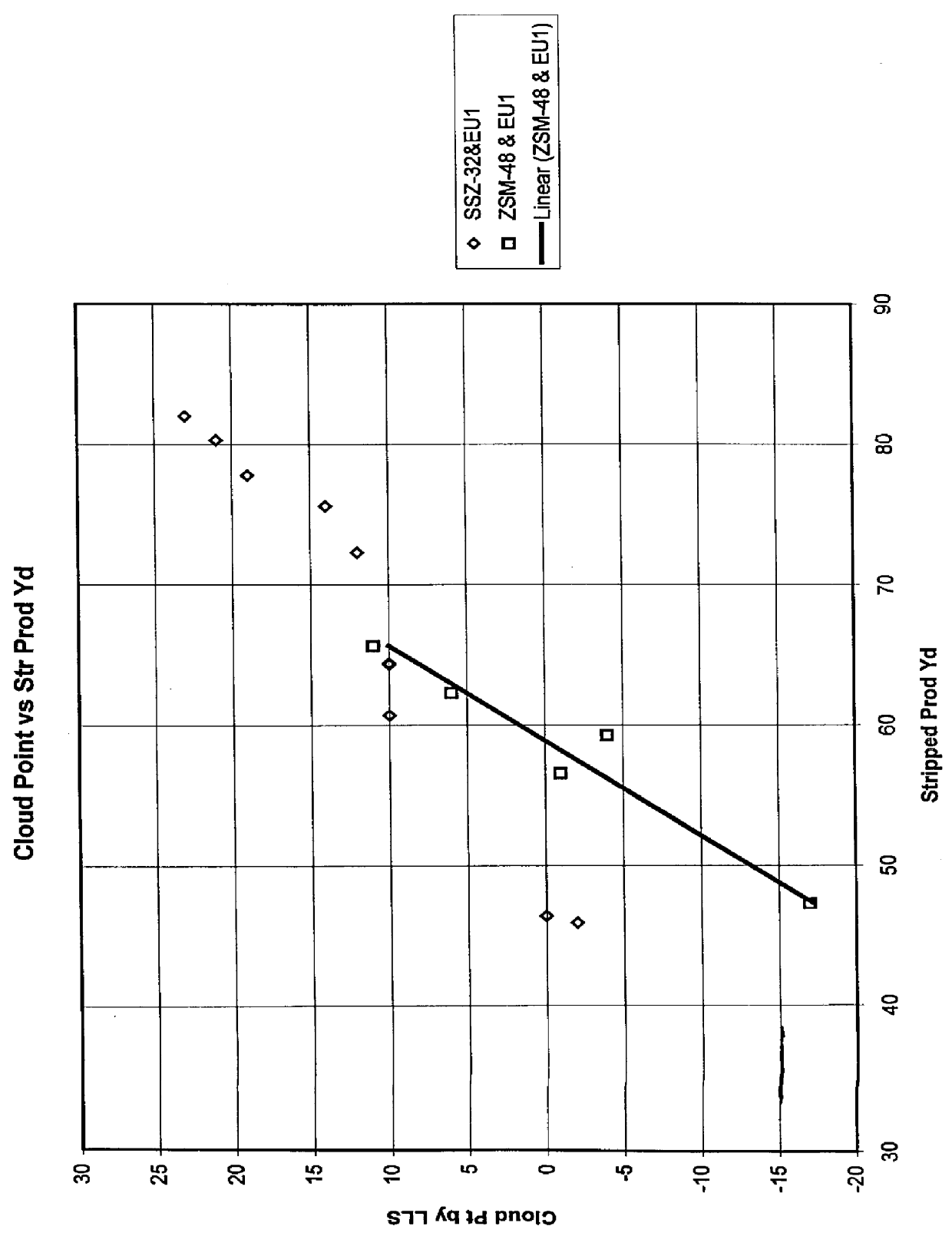
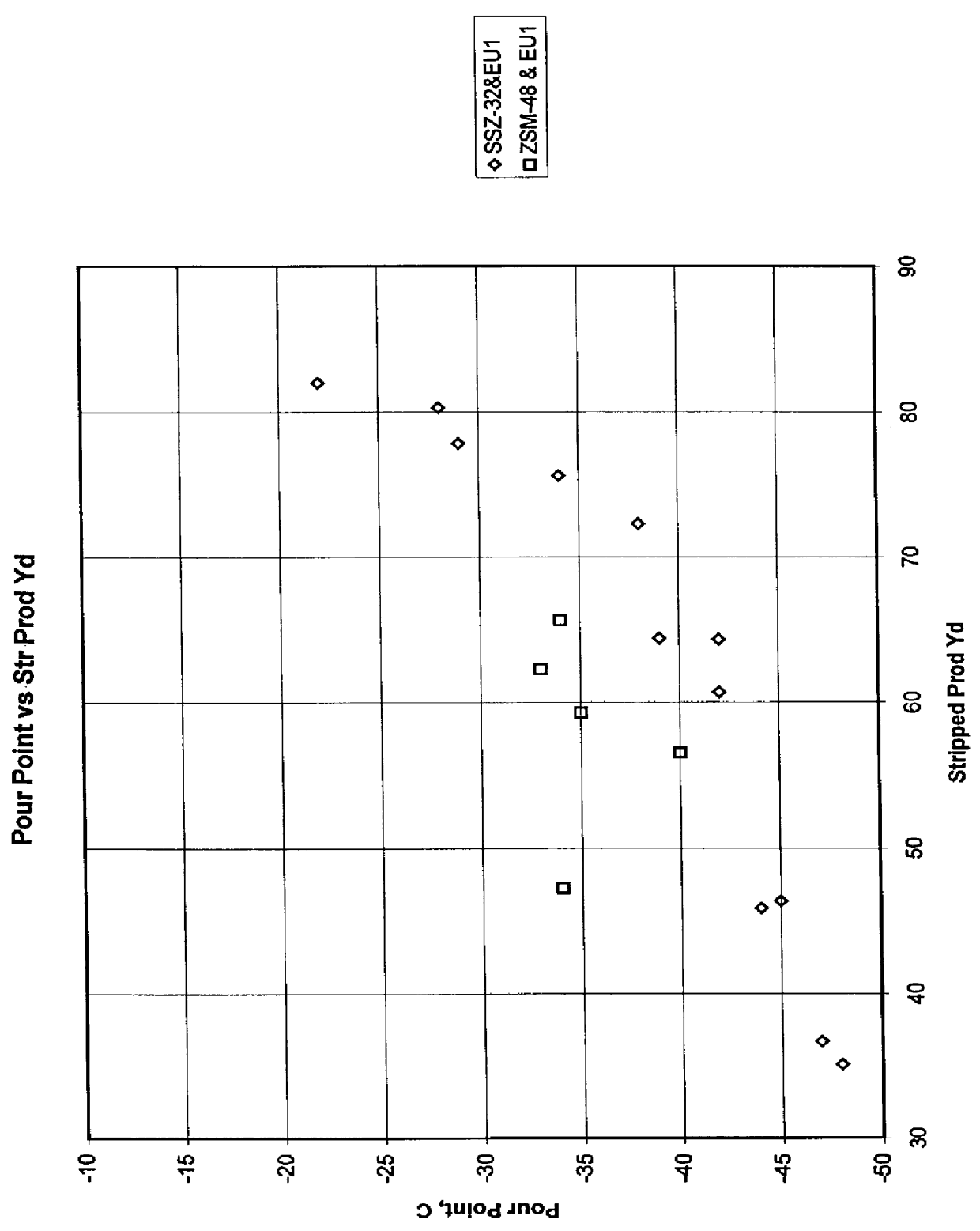
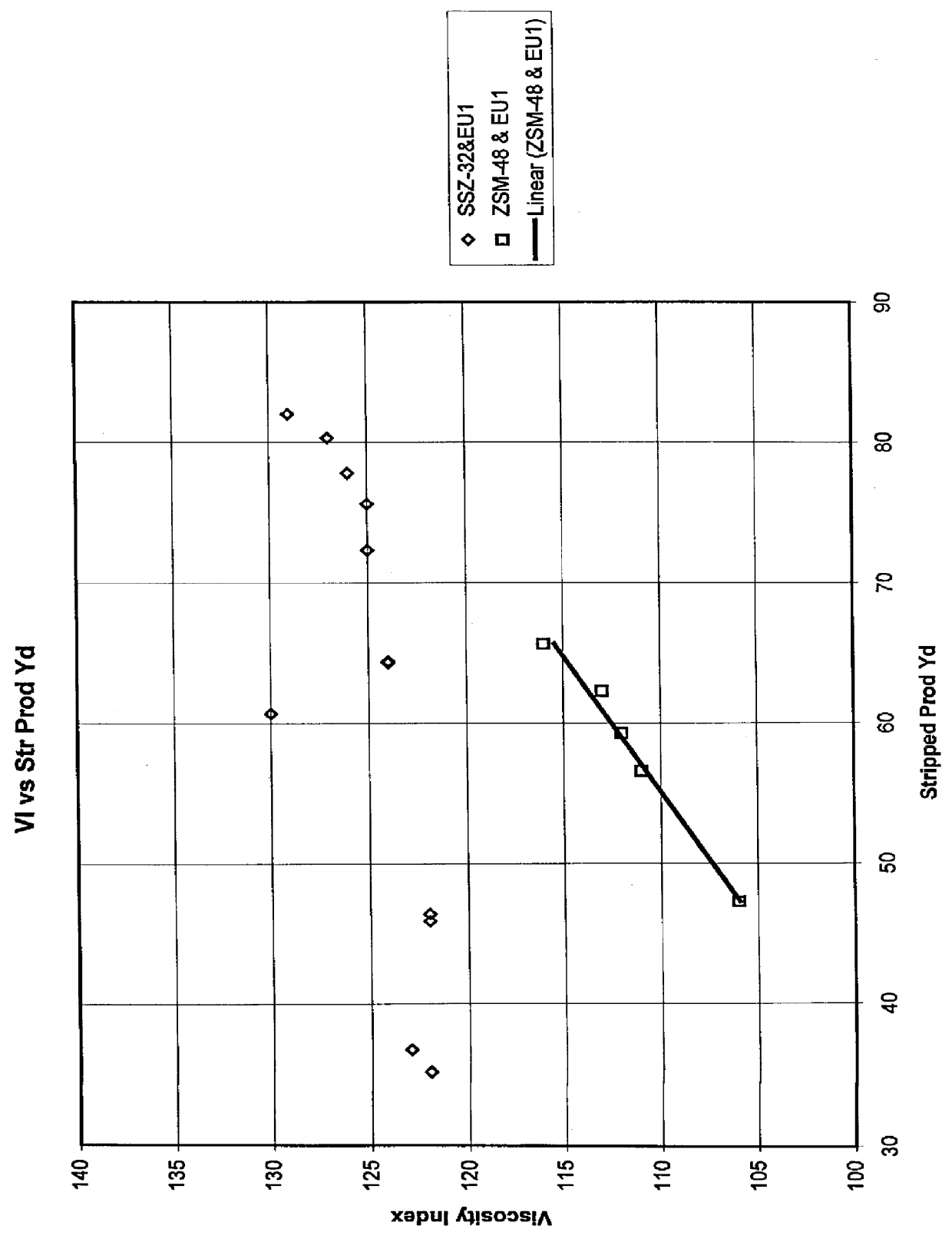
Process for reducing haze point in bright stock
InactiveUS6051129ASuperior lube oil yieldHigh yieldMolecular sieve catalystsRefining to change hydrocarbon structural skeletonHydrogenHaze
Owner:CHEVROU USA INC
Ultrastable Y-type RE molecular sieve active component and its prepn process
InactiveCN1506161AReasonable matching activityIncreased hydrogen transfer activityMolecular sieve catalystsMolecular sieveActive component
The active component of ultrastable Y-type RE molecular sieve is composite modified Y-zeolite containing RE oxide 8-25 wt%, P 0.1-3.0 wt% and sodium oxide 0.3-2.5 wt%, and with degree of crystallization 30-55 % and unit cell parameter 2.455-2.477 nm. It is prepared with Y-zeolite as material and through the steps of exchange with RE and the first roasting to obtain RE-Na Y-zeolite; reaction with RE, reaction with P-containing compound, and the second roasting. The ultrastable Y-type RE molecular sieve is used as the active component of cracking catalyst and has the obvious effect of lowering olefin content in gasoline and the features of resulting in moderate coke yield and high diesel oil yield. The preparation process is simple and high in the utilization of the modifying elements.
Owner:PETROCHINA CO LTD
Zeolite-containing drilling fluids
Methods and compositions for wellbore treating fluids, especially drilling fluids, that comprise zeolite and a carrier fluid.
Owner:HALLIBURTON ENERGY SERVICES INC
Controlled-release nano-diffusion delivery systems for cosmetic and pharmaceutical compositions
InactiveUS20040208902A1High affinityLimited surface areaBiocideCosmetic preparationsAntioxidantAdditive ingredient
The present invention discloses the utilization of zeolites for controlled-release of cosmetic and pharmaceutical compositions by nano-diffusion technology. The treatment and protection of skin surface requires that certain compositions be delivered to the skin surface and allowed to remain on the skin surface for as long as possible before such ingredients are absorbed into deeper layers of skin and carried into the bloodstream. Zeolites do not absorb into the skin, which is useful for topical delivery of cosmetic and pharmaceutical compositions, for example antiaging, anti-wrinkle, antioxidants, skin whitening, acne treatment, rosacea treatment, sun screens, UV blocks, anesthetics, skin soothers, anti-irritants, anti-inflammatory agents, vitamins, hormones, and such that are electronically attached to the outer surfaces of such zeolites and are released to the outer surface of skin by a diffusion-controlled thermodynamic process.
Owner:GUPTA SHYAM K
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
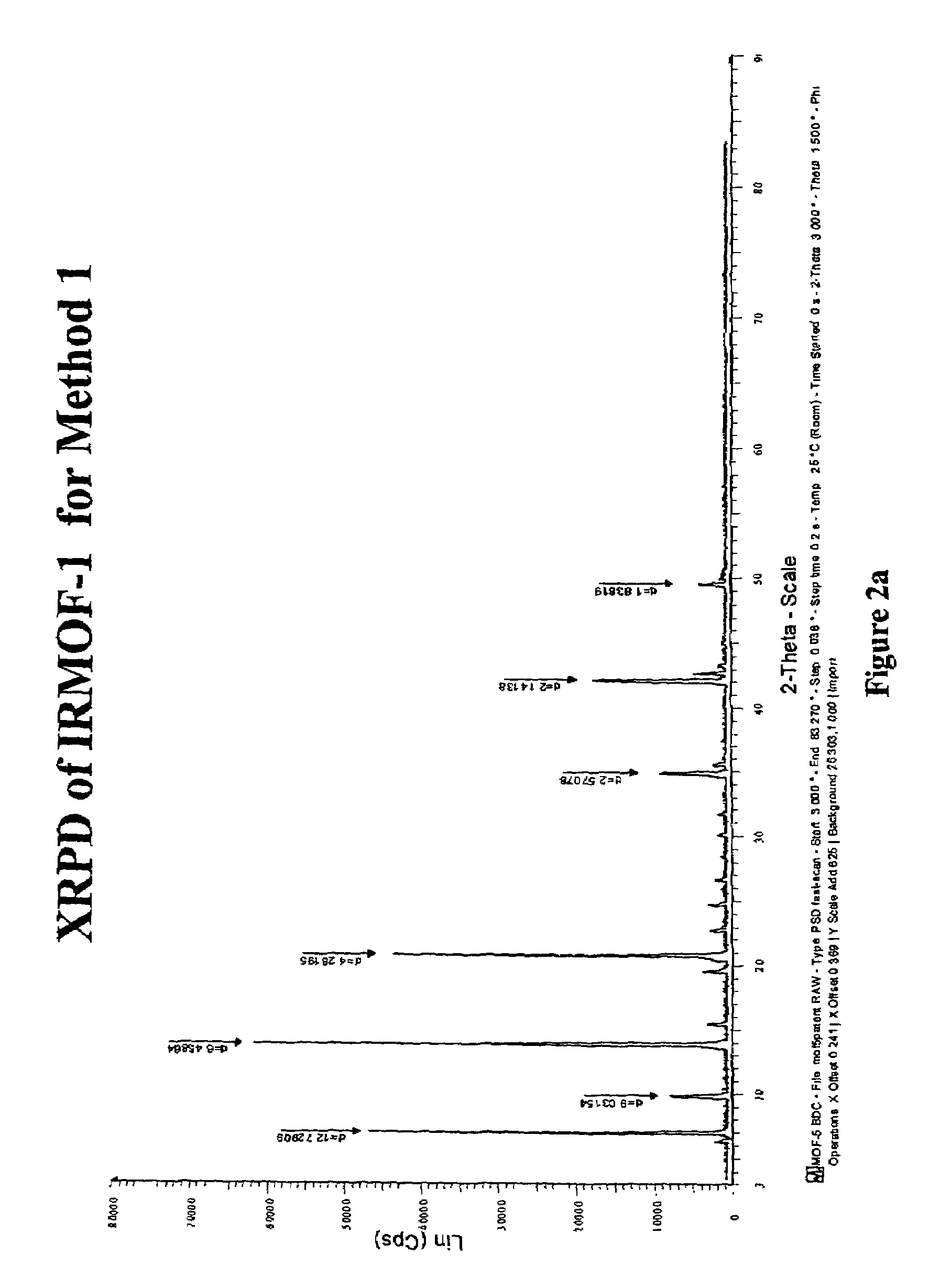
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
an air purifier
InactiveCN103861421BLarger than surface and uniformGood adsorption and decomposition effectOther chemical processesDispersed particle separationActivated carbonHazardous substance
Owner:QINGDAO CHUANSHAN NEW MATERIALS
FCC process with improved yield of light olefins
InactiveUS7312370B2Increase productionImprove olefin selectivityCatalytic crackingHydrocarbonsHydrocarbonZeolite
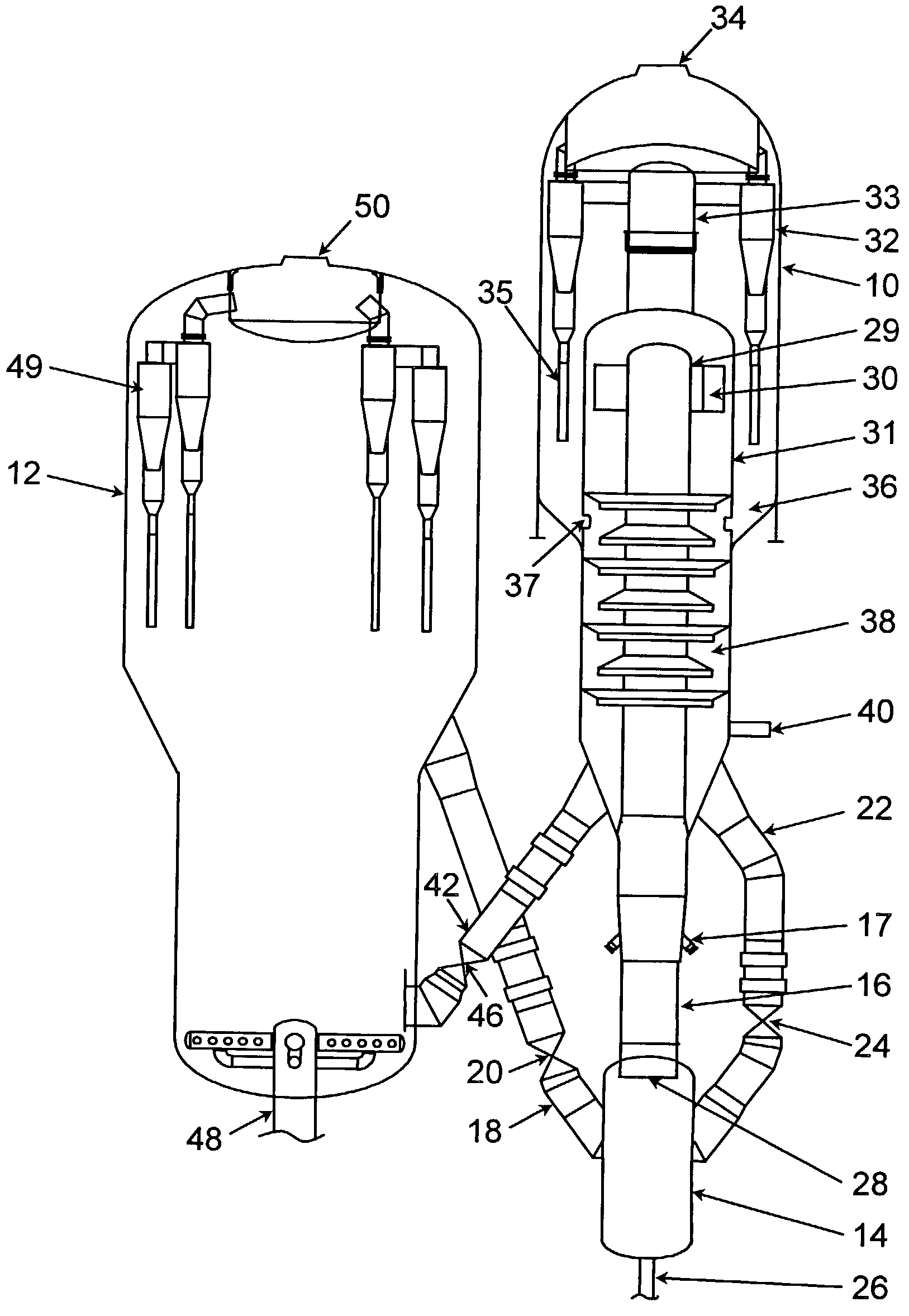
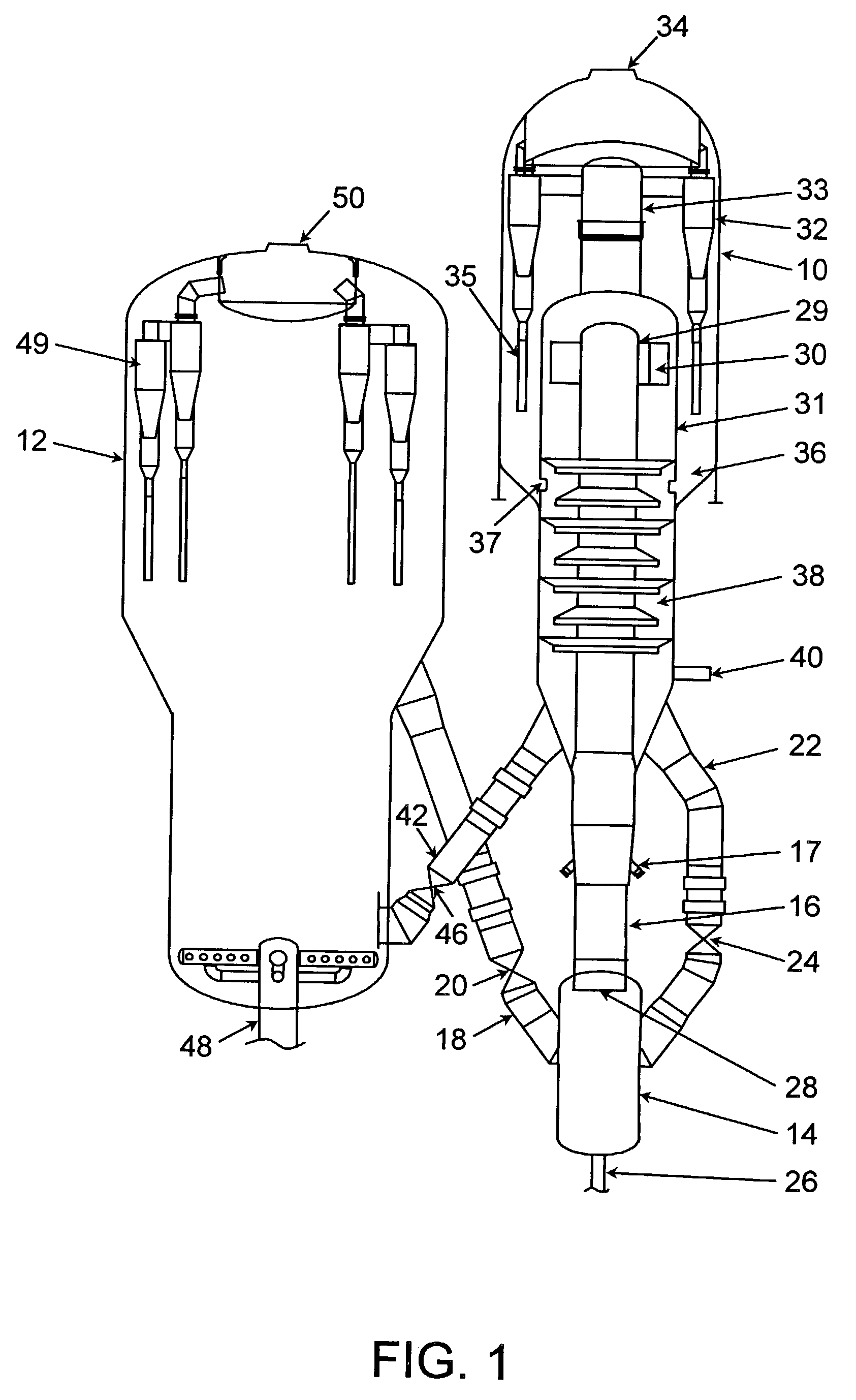
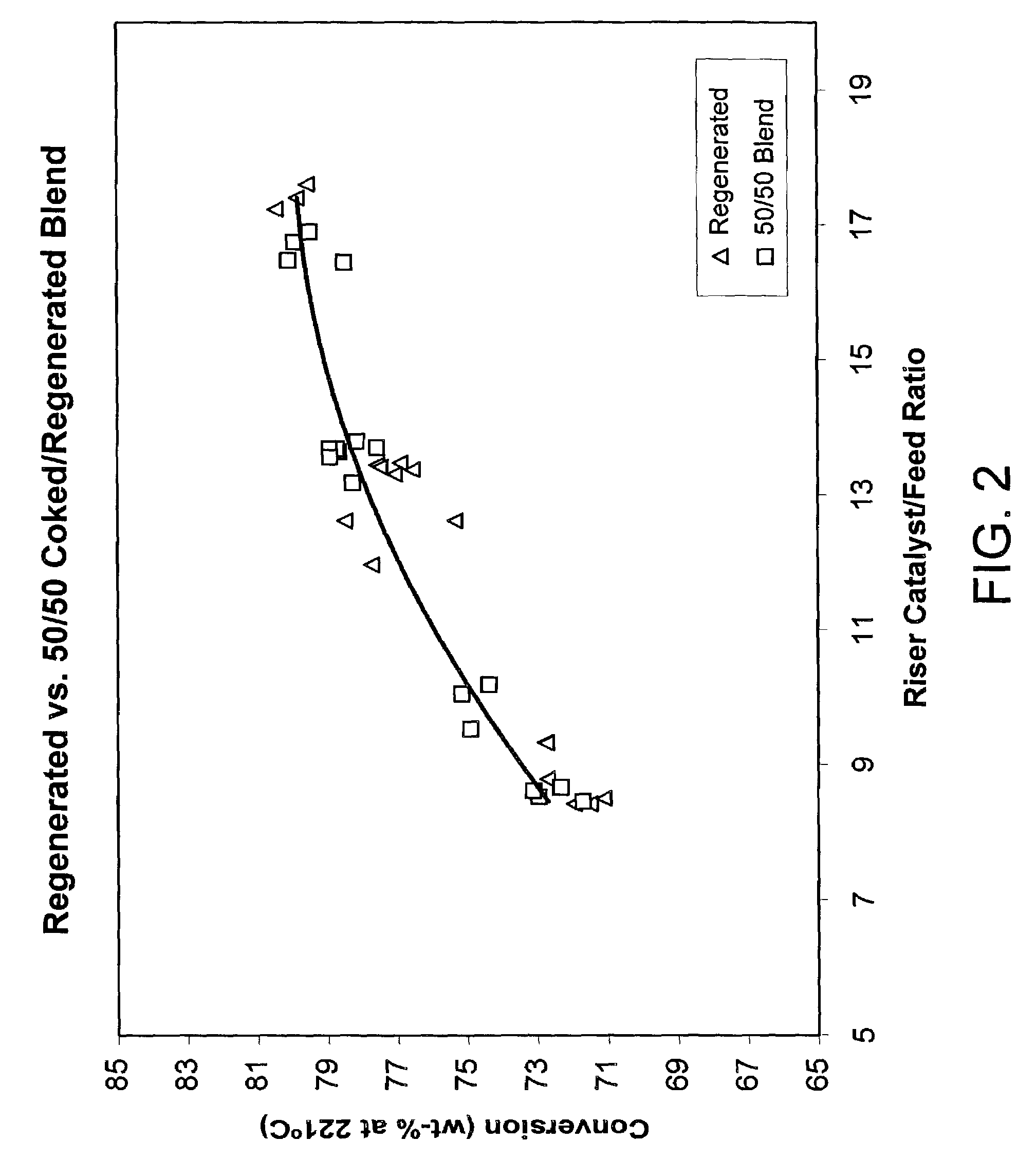
An FCC process for obtaining light olefins comprises contacting a hydrocarbon feed stream with blended catalyst comprising regenerated catalyst and coked catalyst. The catalyst has a composition including a first component and a second component. The second component comprises a zeolite with no greater than medium pore size wherein the zeolite comprises at least 1 wt-% of the catalyst composition. The contacting occurs in a riser to crack hydrocarbons in the feed stream and obtain a cracked stream containing hydrocarbon products including light olefins and coked catalyst. The cracked stream is passed out of an end of the riser such that the hydrocarbon feed stream is in contact with the blended catalyst in the riser for less than or equal to 2 seconds on average. The hydrocarbon products including light olefins are separated from the coked catalyst. The first portion of the coked catalyst is passed to a regeneration zone in which coke is combusted from the catalyst to produce a regenerated catalyst. A second portion of the coked catalyst is blended with the regenerated catalyst and introduced to the riser. The regenerated catalyst has substantially the same relative proportions of the first component and the second component as the blended catalyst that contact the hydrocarbon feed stream.
Owner:UOP LLC
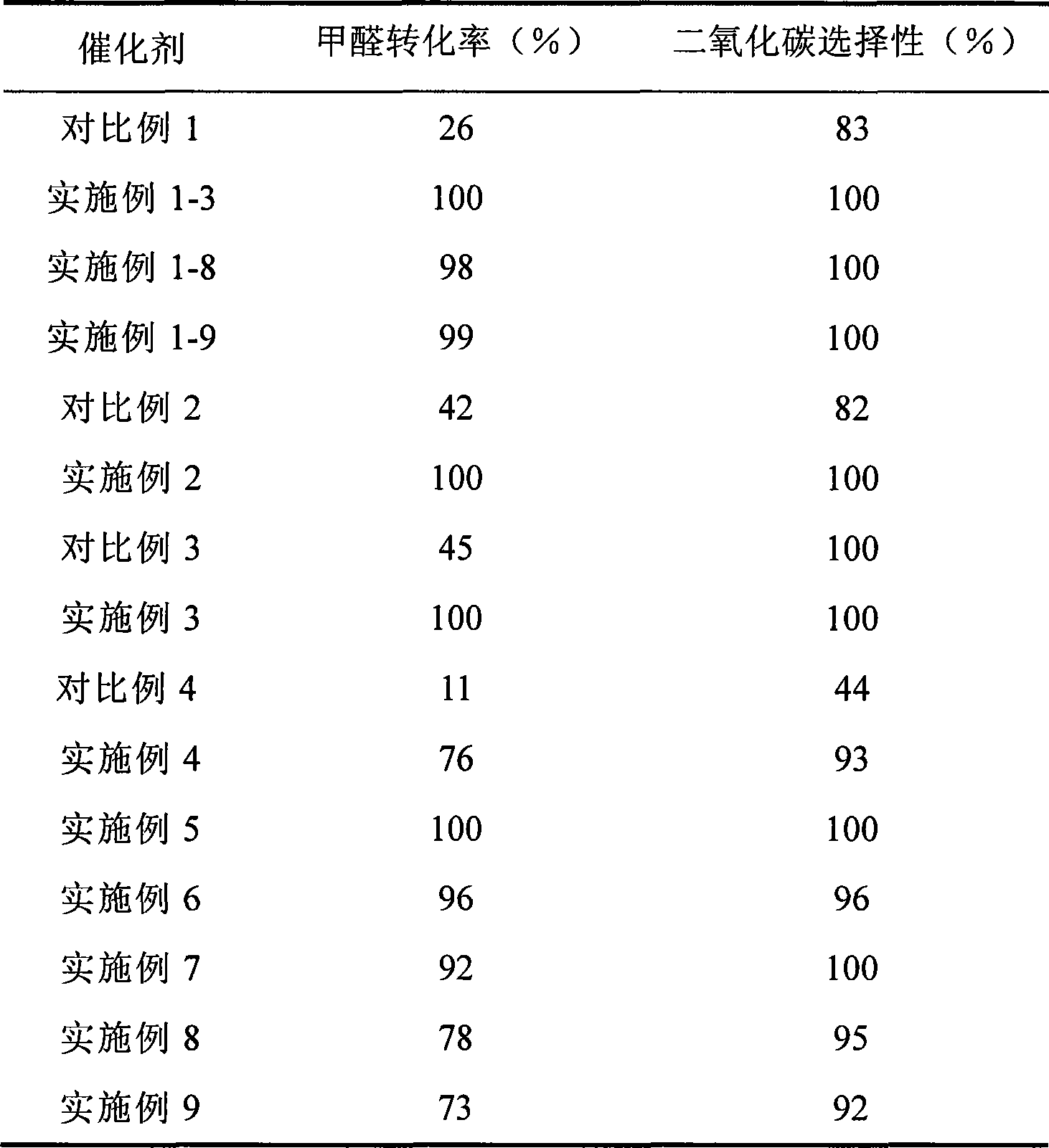
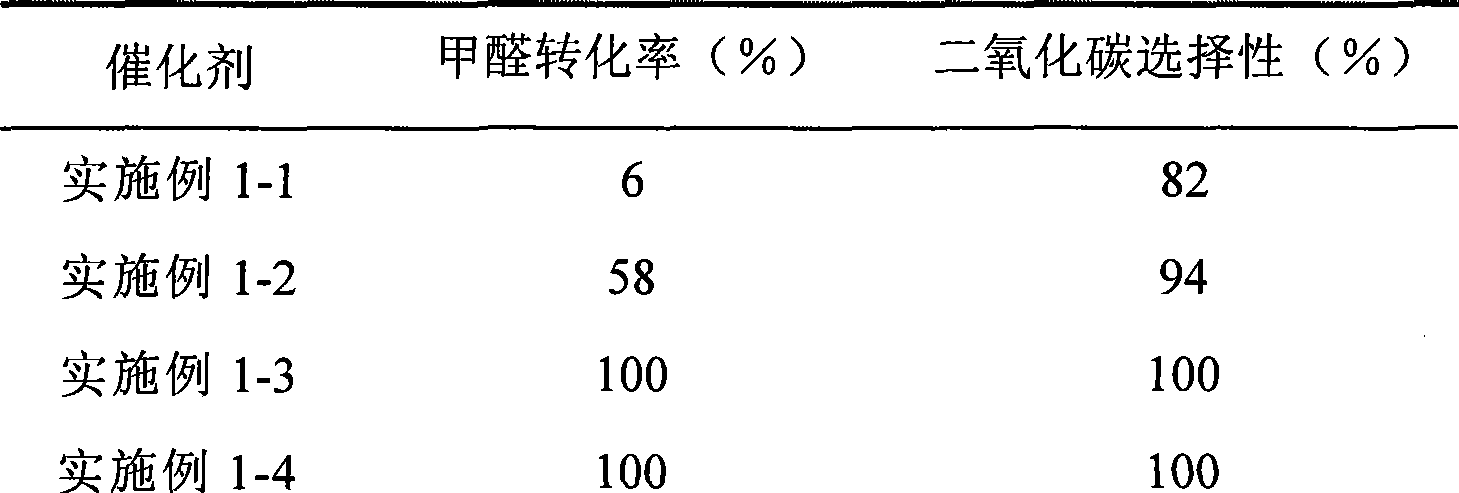
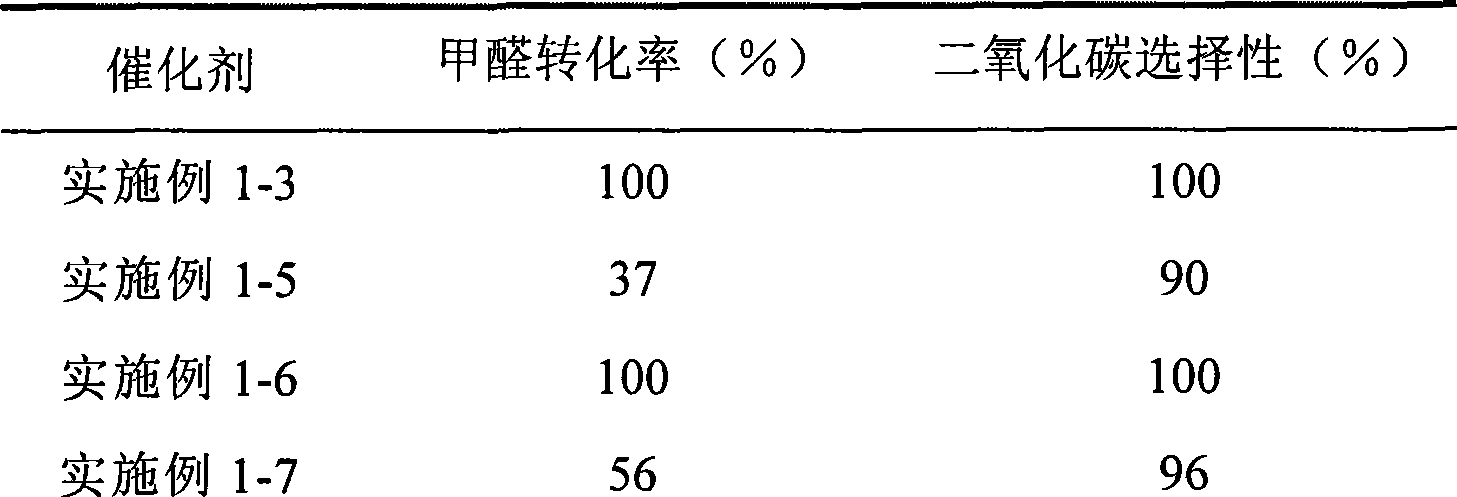
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
Zero Platinum Group Metal Catalysts
Owner:ECS HLDG +1
Catalyst for purifying exhaust gas
InactiveUS6066587AImprove responseImprove purification effectMolecular sieve catalystsInternal combustion piston enginesPlatinumPartial oxidation
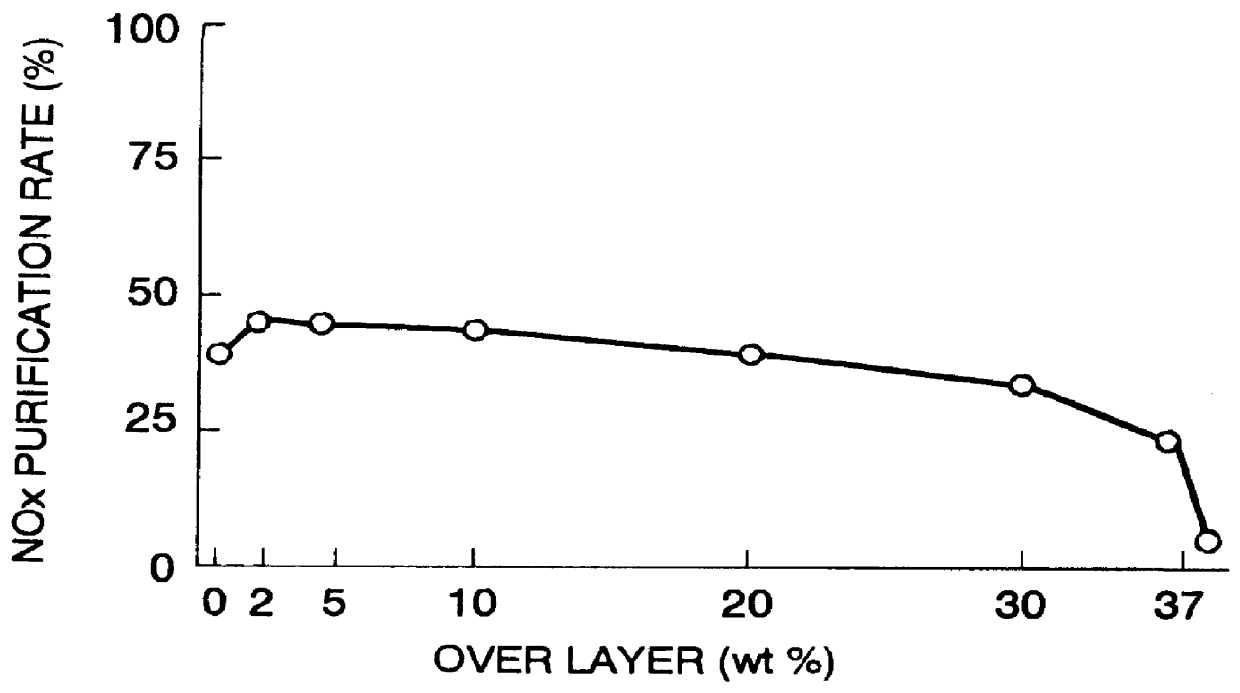
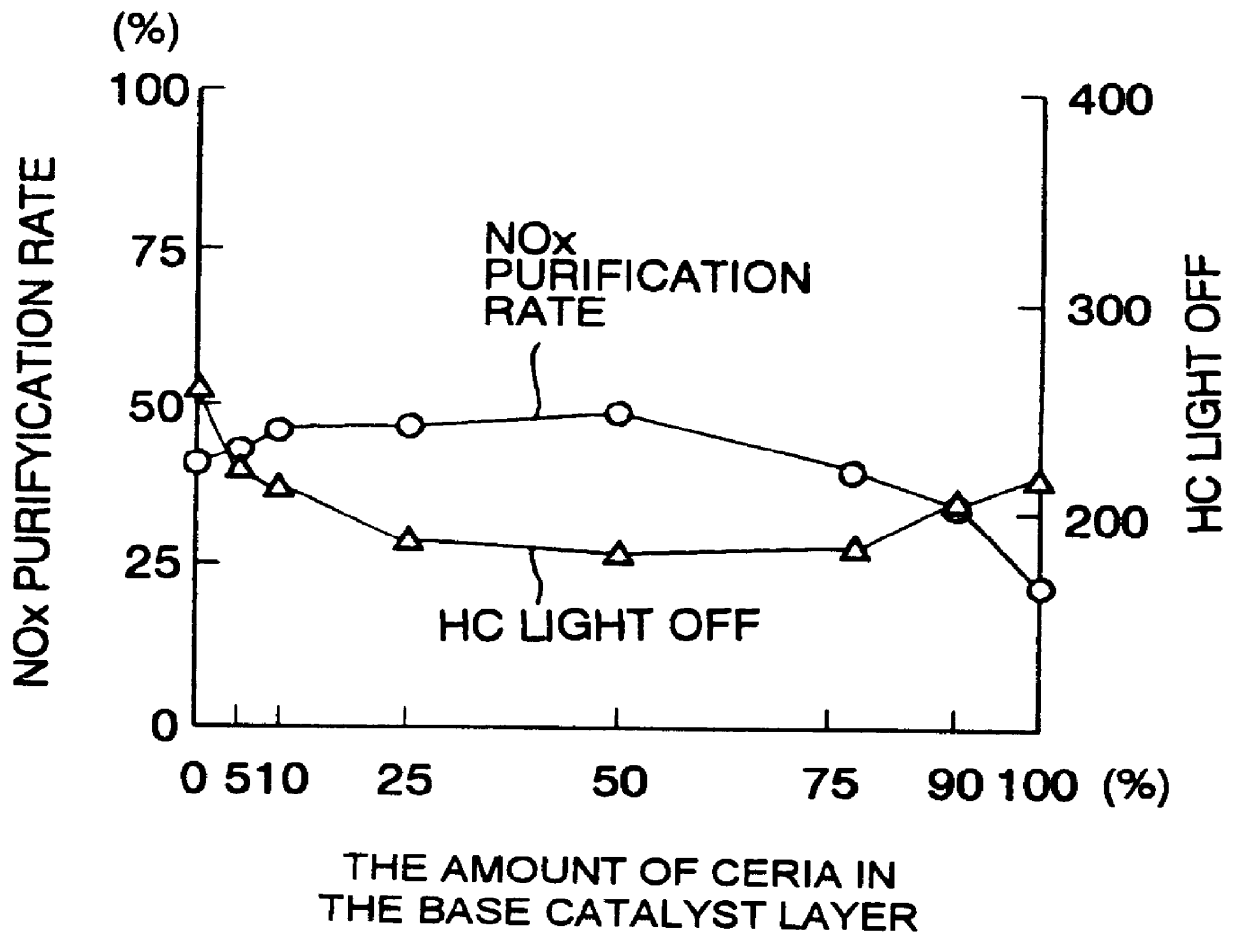
A catalyst has a base catalyst layer containing platinum and barium as precious metal supported by alumina and an over catalyst layer containing platinum and rhodium as precious metal supported by zeolitr. The platinum and rhodium in the over catalyst layer activate NOx and HC so as to make them more reactive in terms of energy, and the barium in the base catalyst layer makes the platinum be more dispersive in the base catalyst layer. Under the existence of dispersive platinum, NOx in exhaust gas is decomposed and purified by reaction with reactive NO2 and partially oxidized HC generated in the over catalyst layer.
Owner:MAZDA MOTOR CORP
Catalytic pyrolysis of solid biomass and related biofuels, aromatic, and olefin compounds
ActiveUS8277643B2Minimize coke productionSolid fuelsHydrocarbon from oxygen organic compoundsCatalytic pyrolysisHigh rate
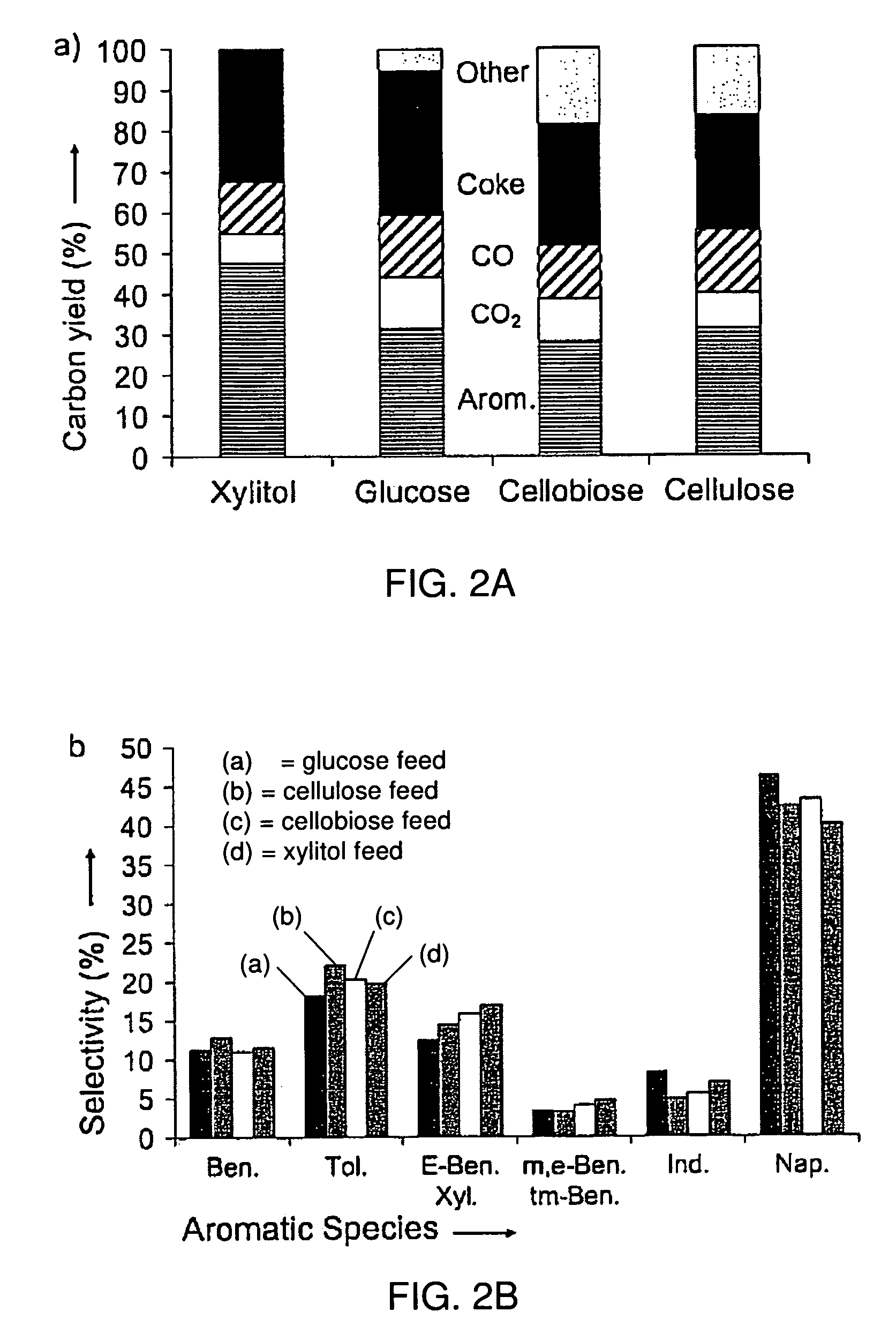
This invention relates to compositions and methods for fluid hydrocarbon product, and more specifically, to compositions and methods for fluid hydrocarbon product via catalytic pyrolysis. Some embodiments relate to methods for the production of specific aromatic products (e.g., benzene, toluene, naphthalene, xylene, etc.) via catalytic pyrolysis. Some such methods may involve the use of a composition comprising a mixture of a solid hydrocarbonaceous material and a heterogeneous pyrolytic catalyst component. In some embodiments, the mixture may be pyrolyzed at high temperatures (e.g., between 500° C. and 1000° C.). The pyrolysis may be conducted for an amount of time at least partially sufficient for production of discrete, identifiable biofuel compounds. Some embodiments involve heating the mixture of catalyst and hydrocarbonaceous material at high rates (e.g., from about 50° C. per second to about 1000° C. per second). The methods described herein may also involve the use of specialized catalysts. For example, in some cases, zeolite catalysts may be used; optionally, the catalysts used herein may have high silica to alumina molar ratios. In some instances, the composition fed to the pyrolysis reactor may have a relatively high catalyst to hydrocarbonaceous material mass ratio (e.g., from about 5:1 to about 20:1).
Owner:UNIV OF MASSACHUSETTS
Fluid cat cracking with high olefins prouduction
InactiveUS20020003103A1Increase productionMaximize lightThermal non-catalytic crackingTreatment with plural serial cracking stages onlyNaphthaOrganic chemistry
The propylene production of a fluid catalytic cracking unit employing a large pore zeolite cracking catalyst, produces more propylene by adding a naphtha cracking riser and a medium pore zeolite catalytic component to the unit, and recycling at least a portion of the naphtha crackate to the naphtha riser. The large pore size zeolite preferably comprises a USY zeolite and the medium pore size is preferably ZSM-5. Propylene production per unit of naphtha feed to the naphtha riser is maximized, by using the 60-300.degree. F. naphtha crackate as the feed.
Owner:EXXON RES & ENG CO
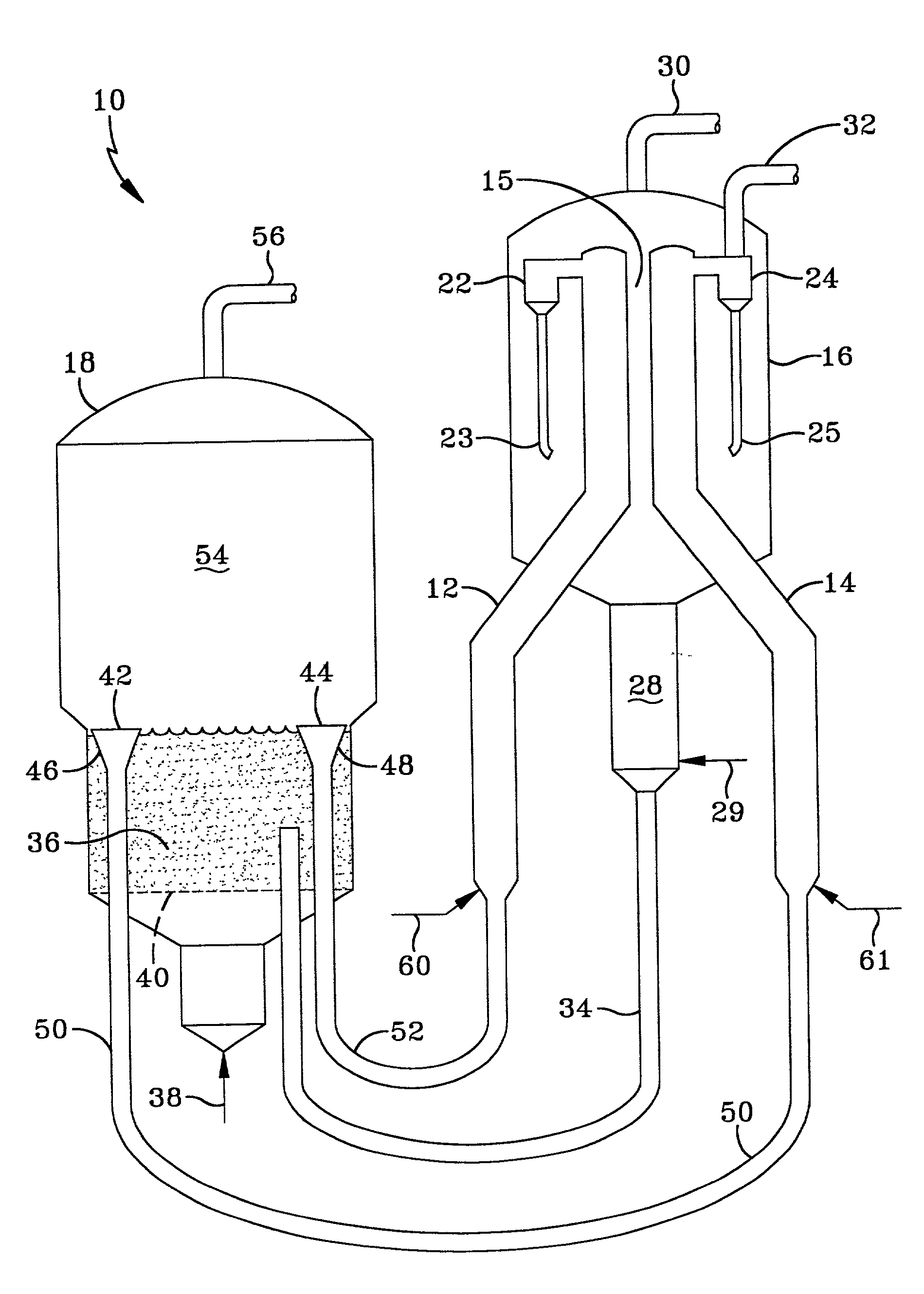
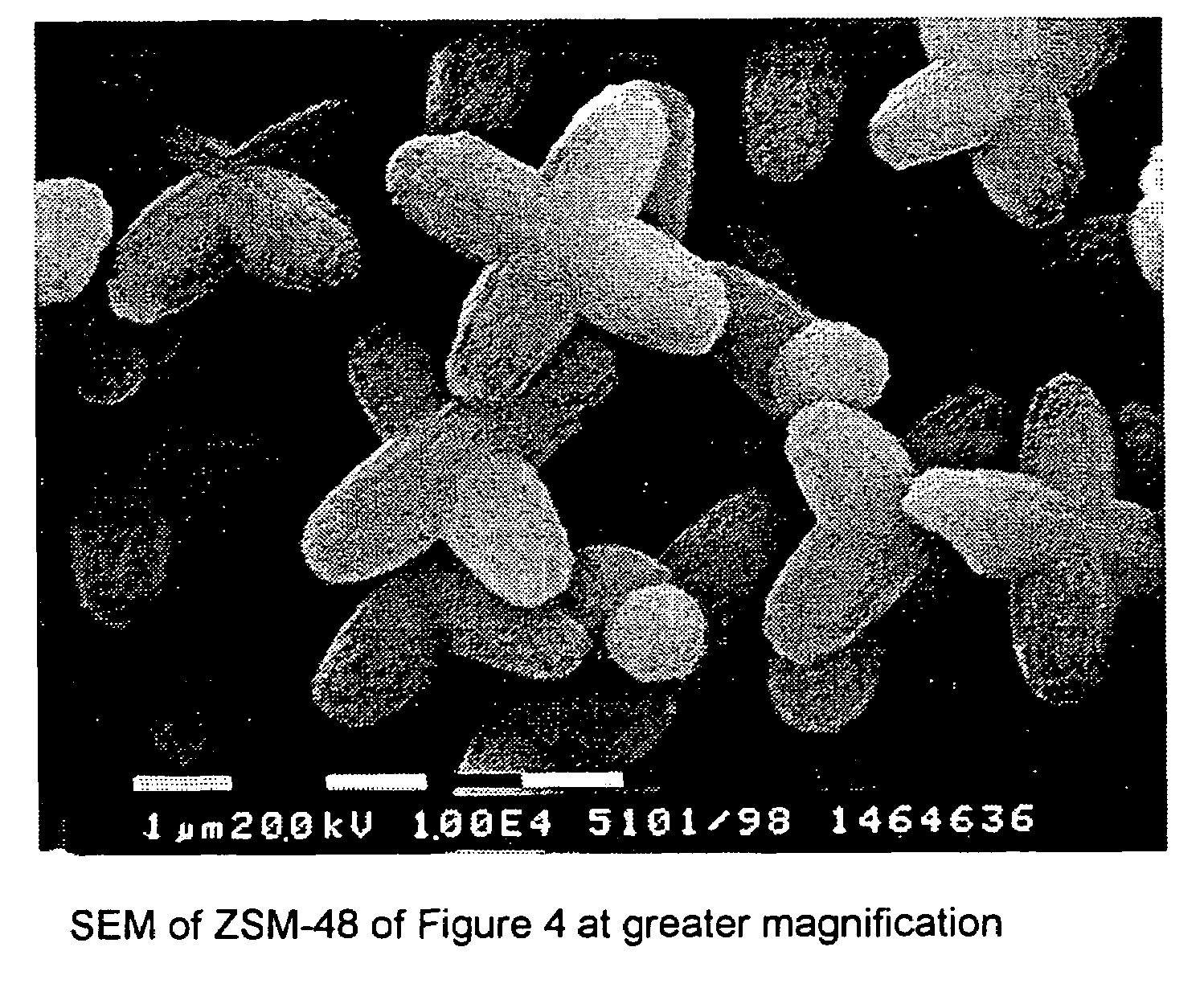
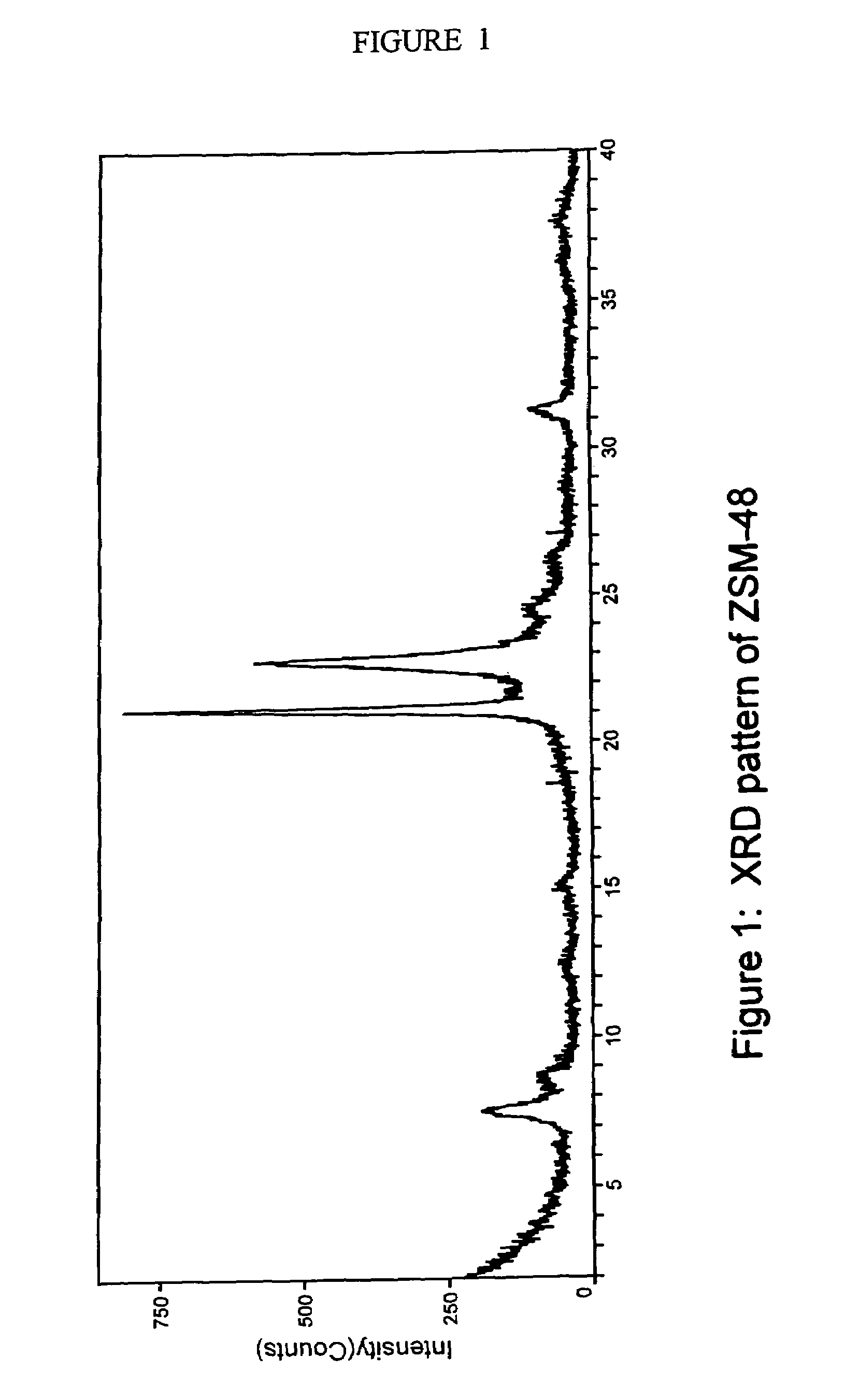
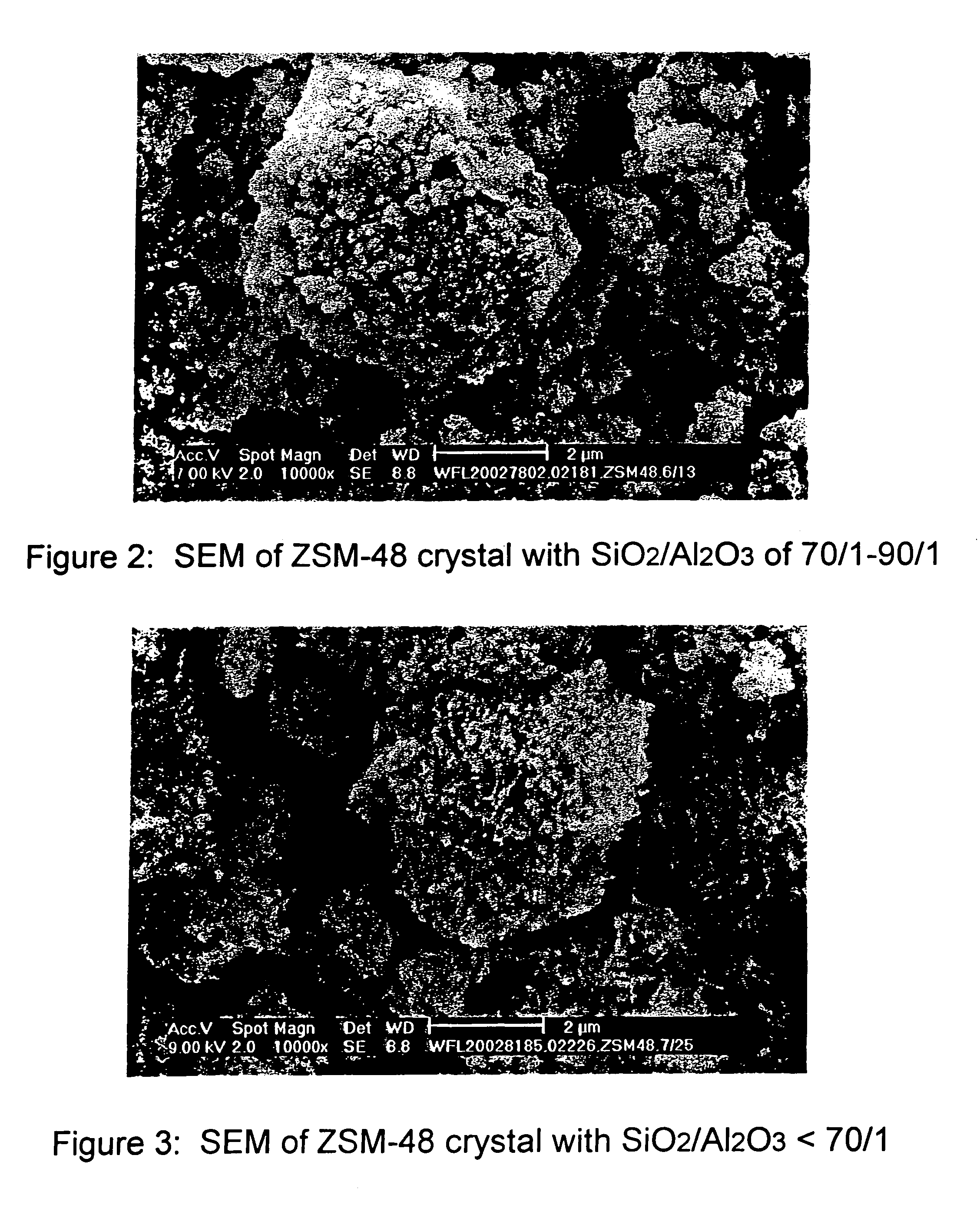
Synthesis of ZSM-48 crystals with heterostructural, non ZSM-48, seeding
ActiveUS6923949B1Suppresses formation of impurityPromotes more aluminum incorporationAluminium compoundsMolecular-sieve and base-exchange compoundsHigh activityZeolite
Pure phase, high activity ZSM-48 crystals having a SiO2 / Al2O3 ratio of less than about 150 / 1, substantially free from ZSM-50 and Kenyaite, and fibrous morphology crystals. The crystals may further have a specific cross morphology. A method for making such crystals using heterostructural, zeolite seeds, other than ZSM-48 and ZSM-50 seeds.
Owner:EXXON RES & ENG CO
Organic environmentally-friendly soil conditioner for improving acidified or acid soil
ActiveCN102321484ARaw material safetyEfficient use ofOrganic fertilisersSoil conditioning compositionsSodium BentoniteHeavy metal poisons
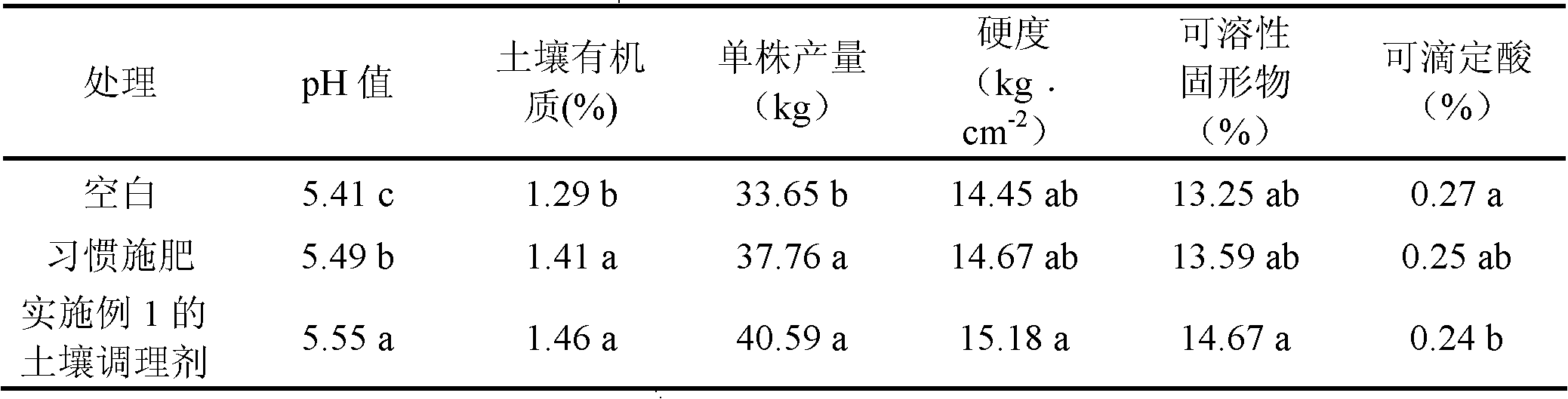
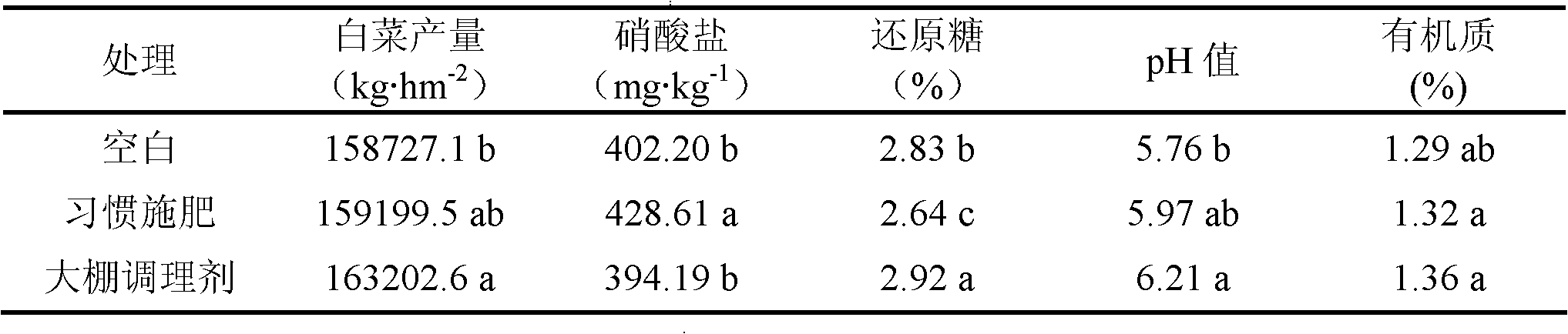
The invention relates to an organic environmentally-friendly soil conditioner for improving acidified or acid soil and a preparation method thereof. The soil conditioner is prepared from the following raw materials in parts by weight: 23-46 parts of biogas residue, 14-24 parts of shell powder, 16-25 parts of calcium magnesium phosphate fertilizer, 13-20 parts of plant ash, 0.1-0.2 part of chitosan, 5-7 parts of zeolite powder and 6-9 parts of bentonite. The soil conditioner is prepared through pretreatment of the biogas residue, pretreatment of the shell powder and the like; through the produced soil conditioner, the pH value of soil can be increased; nutritive elements such as calcium, magnesium, phosphorus, potassium and the like in the soil are increased; the organic matter content in the soil is increased; the water retention and fertilizer retention capacity of the soil is improved; the physicochemical property of the soil is improved; the antiretroviral (diseases, pests, drought and the like) capacity of crops is improved; heavy metal poison is reduced; and the soil conditioner is convenient to apply, is not influenced by wind power and is suitable for mechanical working.
Owner:INST OF AGRI RESOURCES & ENVIRONMENT SHANDONG ACADEMY OF AGRI SCI
Hemostatic textile
ActiveUS20070160653A1Quick activationNon-adhesive dressingsPeptide/protein ingredientsLactideSisal fiber
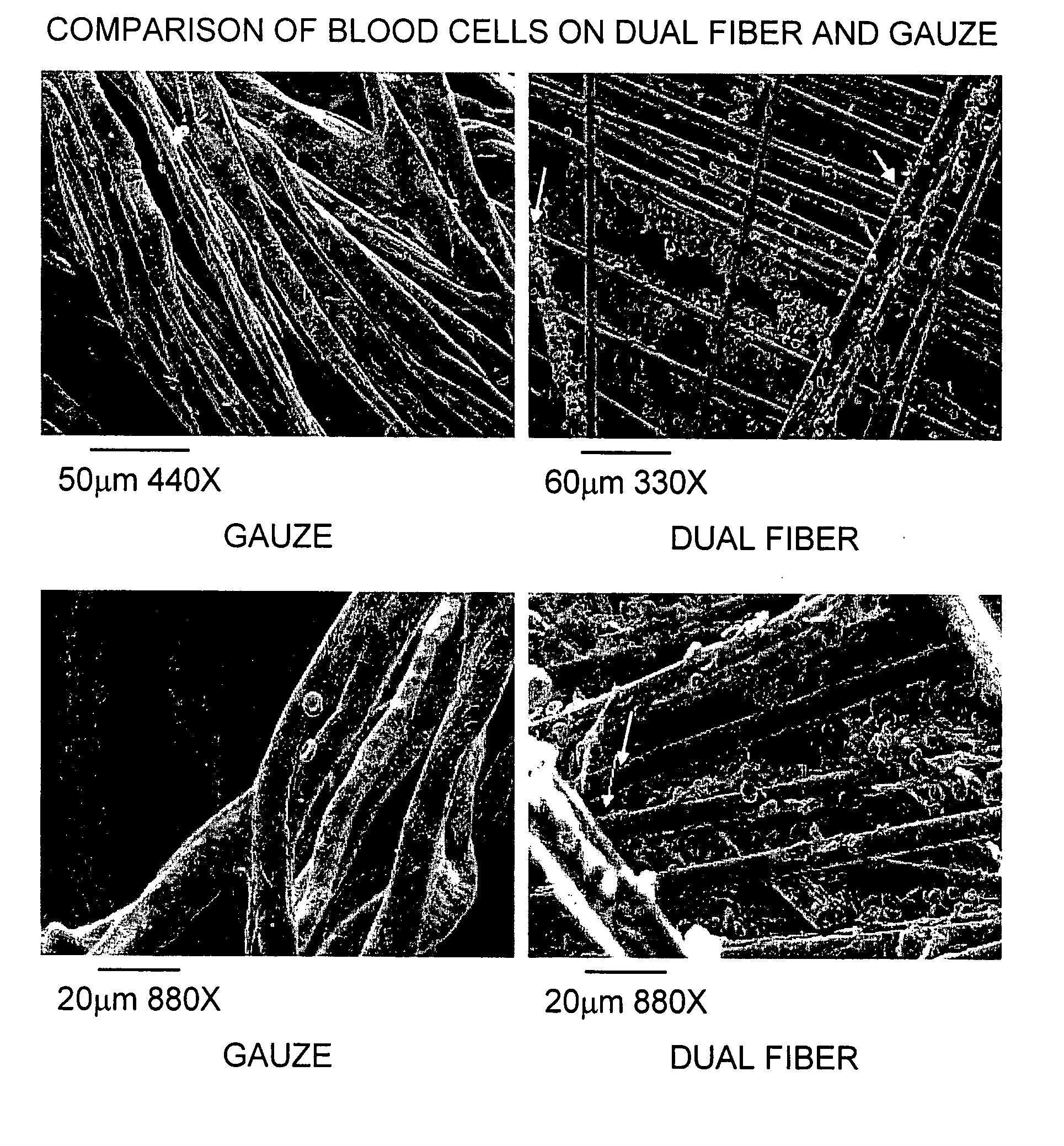
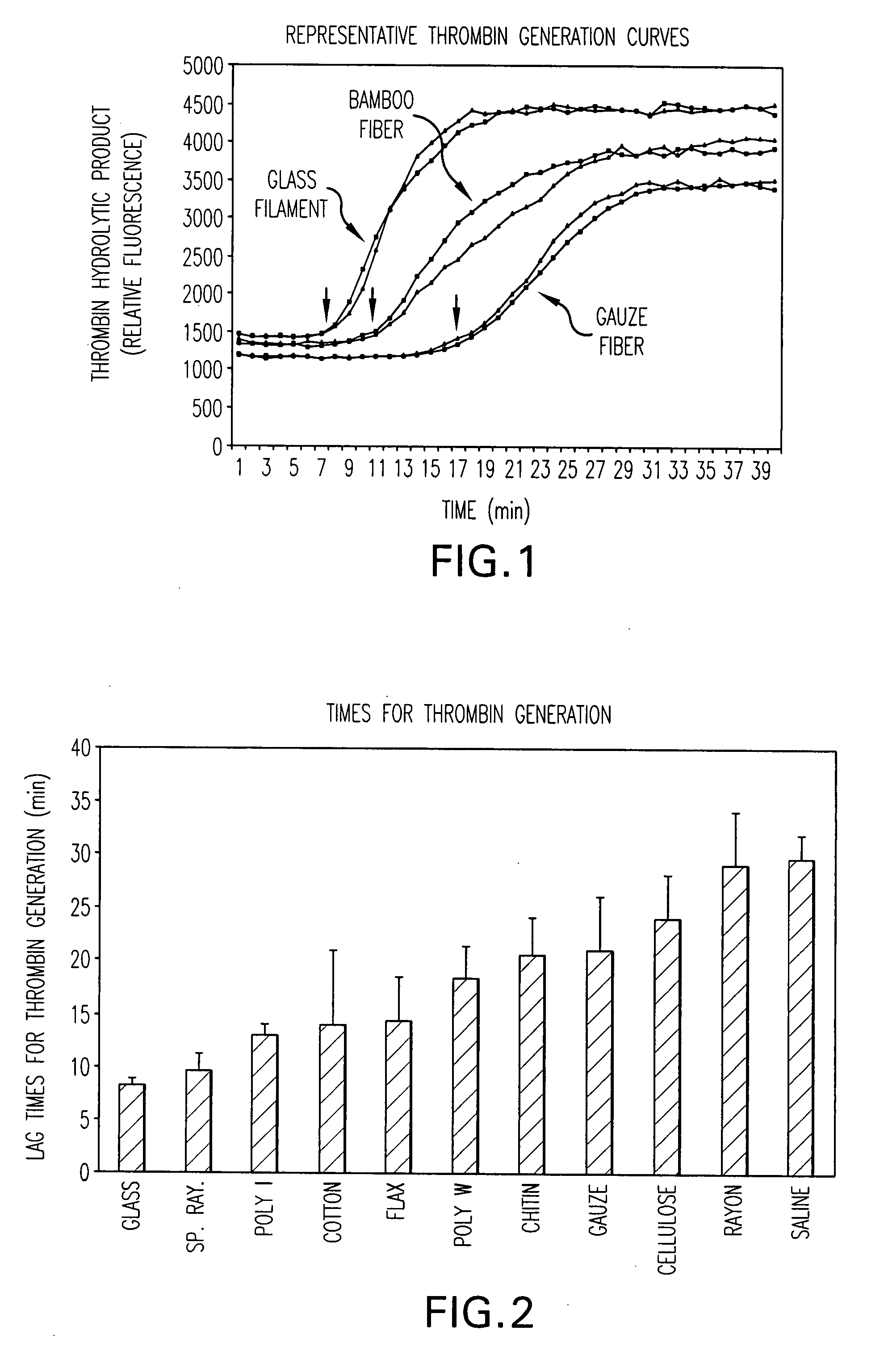
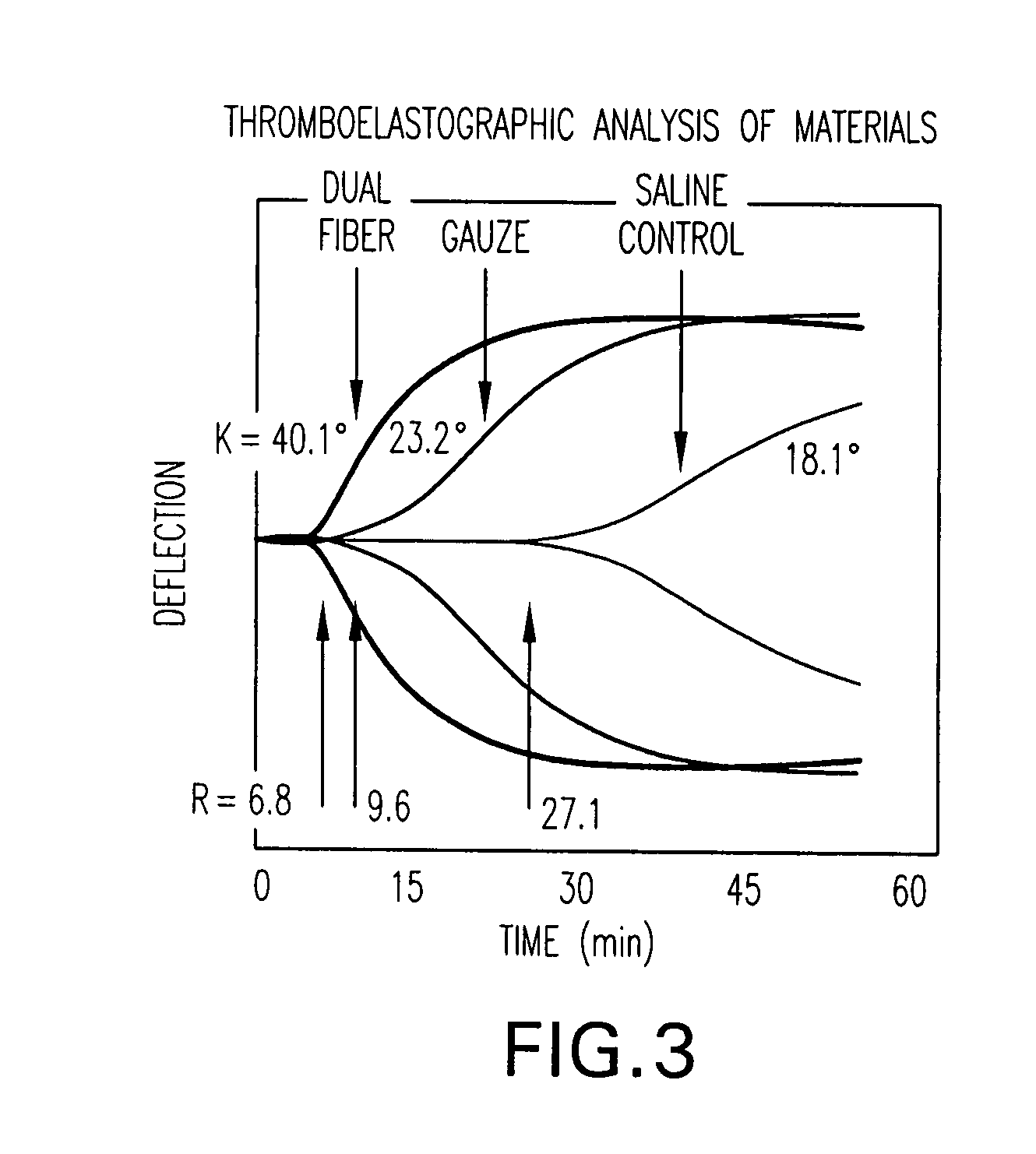
The present invention is directed to a hemostatic textile, comprising: a material comprising a combination of glass fibers and one or more secondary fibers selected from the group consisting of silk fibers; ceramic fibers; raw or regenerated bamboo fibers; cotton fibers; rayon fibers; linen fibers; ramie fibers; jute fibers; sisal fibers; flax fibers; soybean fibers; corn fibers; hemp fibers; lyocel fibers; wool; lactide and / or glycolide polymers; lactide / glycolide copolymers; silicate fibers; polyamide fibers; feldspar fibers; zeolite fibers, zeolite-containing fibers, acetate fibers; and combinations thereof; the hemostatic textile capable of activating hemostatic systems in the body when applied to a wound. Additional cofactors such as thrombin and hemostatic agents such as RL platelets, RL blood cells; fibrin, fibrinogen, and combinations thereof may also be incorporated into the textile. The invention is also directed to methods of producing the textile, and methods of using the textile to stop bleeding.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Baby Care Skin Protectant Compositions for Diaper Rash
The present invention provides a comprehensive solution to skin problems of infants and incontinent adults related to diaper rash, also known as diaper dermatitis. This is based on certain novel divalent metal and quaternary ammonium complexes (ion-pairs) of zeolites (that are made by an in-situ process), which in synergistic combination with certain other compositions, provide a comprehensive treatment for diaper rash that encompasses the following aspects: (1) deactivation of lipase and protease enzymes on skin surface, (2) the controlled-release delivery of skin protectant compositions, such as divalent metal zinc cation, (3) trapping of acidic and alkaline chemicals deposited on skin from body exudates and enzyme activity, (4) controlled-release delivery of anti-inflammatory agents, and COX and LOX enzyme inhibitors, (5) controlled-release delivery of antibacterial and antifungal compositions, and (6) absorption of excess moisture in the diaper zone.
Owner:BIODERM RES
Zeolite-containing remedial compositions
Methods and compositions for wellbore treating fluids, especially remedial compositions such as pills, that include zeolite and at least one carrier fluid.
Owner:HALLIBURTON ENERGY SERVICES INC
Catalyst and process for converting methanol to hydrocarbons
InactiveUS6048816AMolecular sieve catalystsMolecular sieve catalystCrystalline materialsDimethyl ether
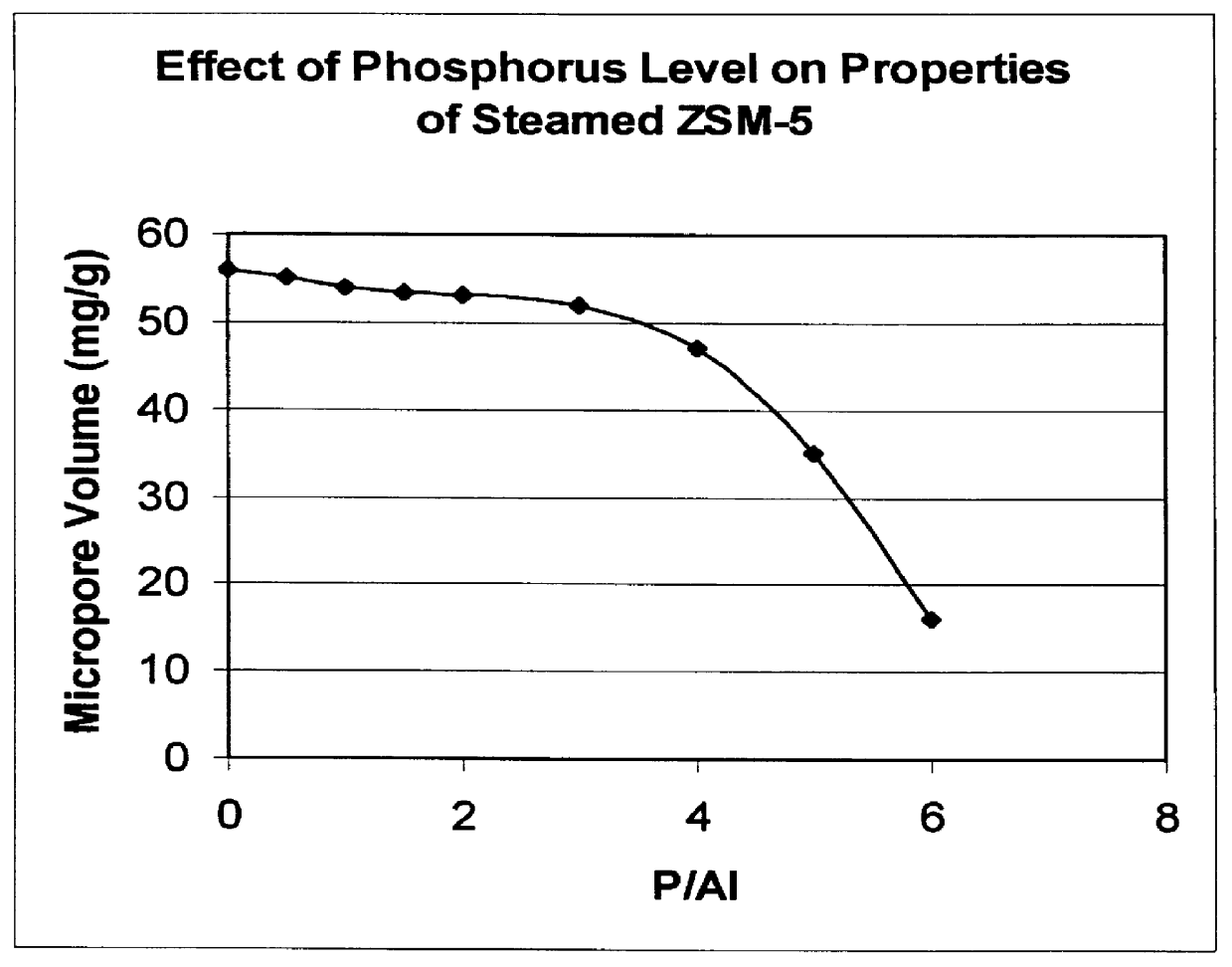
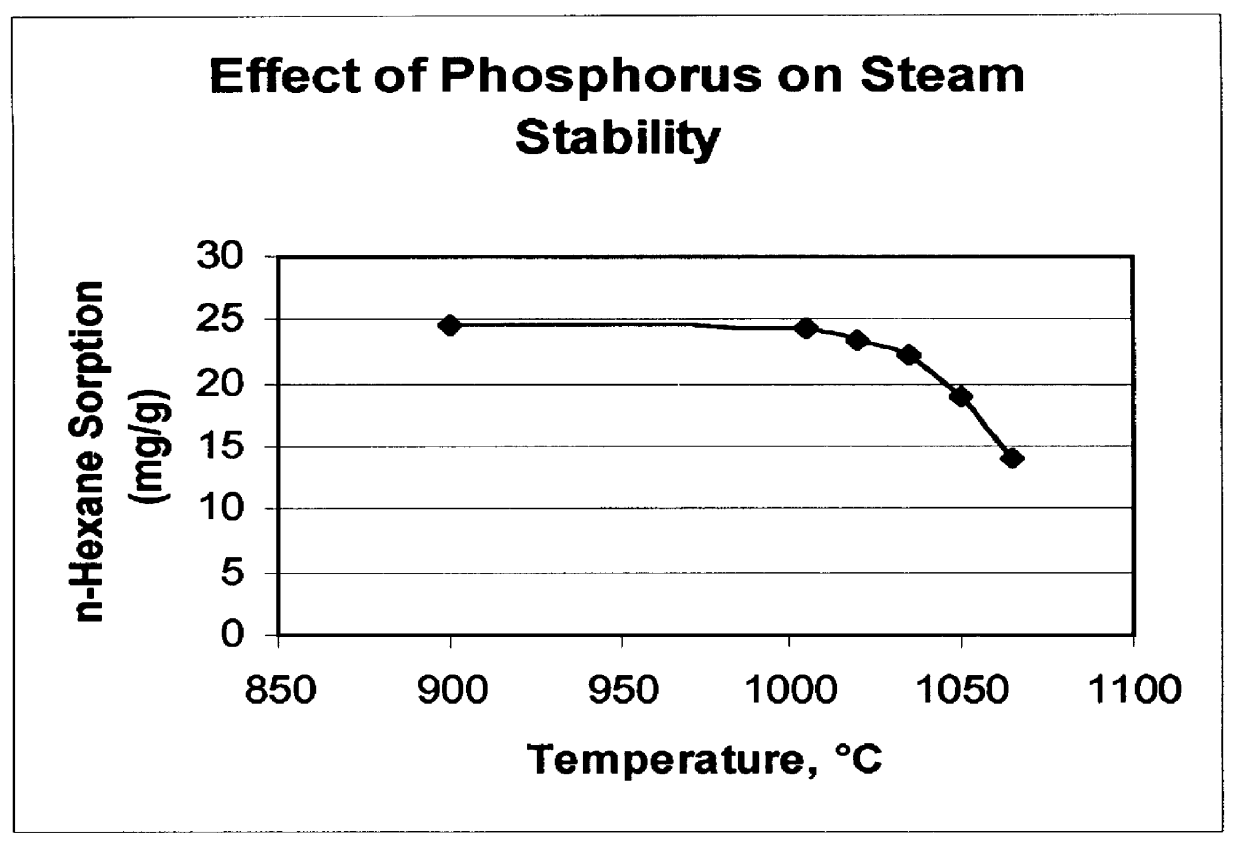
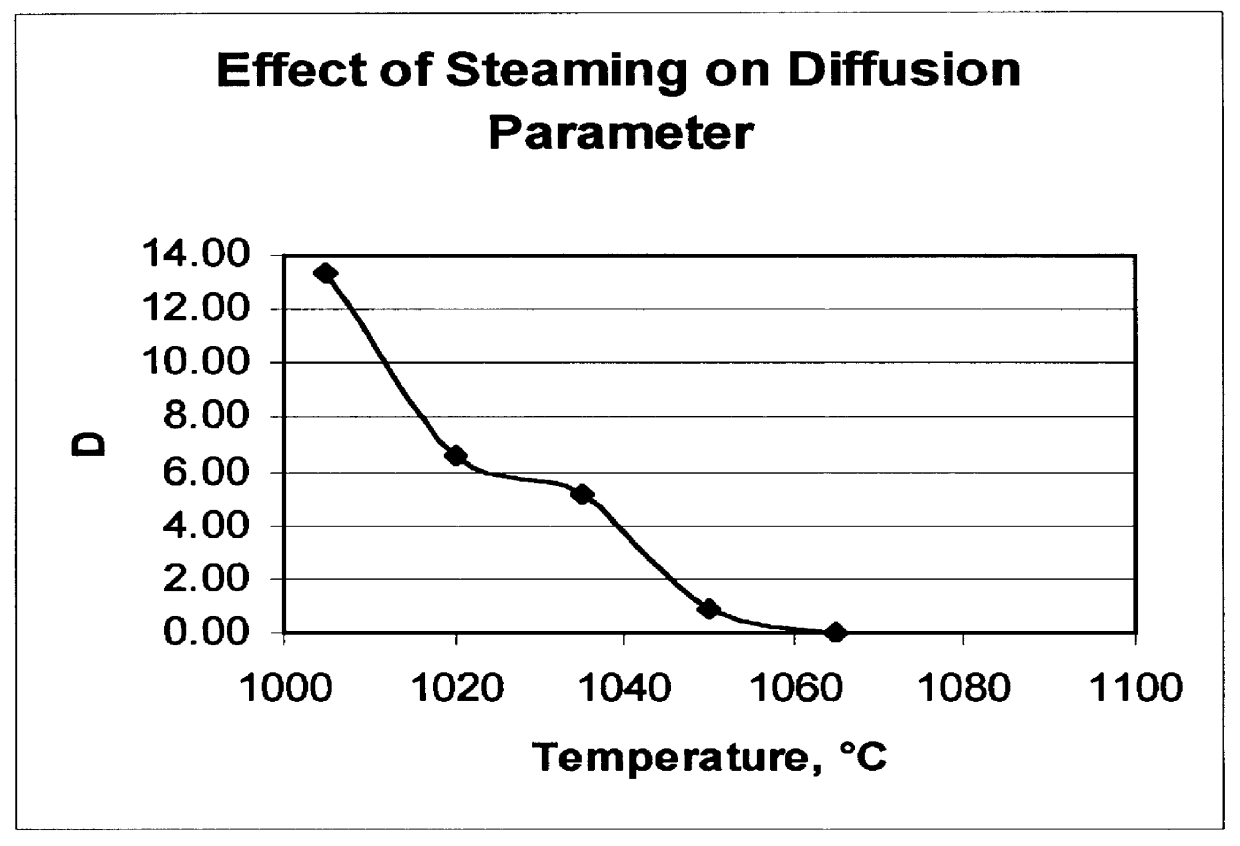
There is provided a catalyst and a process for converting methanol or dimethyl ether to a product containing C2 to C4 olefins. The catalyst comprises a porous crystalline material having a Diffusion Parameter for 2,2-dimethylbutane of about 0.1-20 sec-1 when measured at a temperature of 120 DEG C. and a 2,2-dimethylbutane pressure of 60 torr (8 kPa). In addition, the catalyst is characterized by a hydrothermal stability such that, after steaming the catalyst at 1025 DEG C. for 45 minutes in 1 atmosphere steam, the catalyst exhibits a methanol conversion activity of at least 50% when contacted with methanol at a methanol partial pressure of 1 atmosphere, a temperature of 430 DEG C. and 0.5 WHSV. The porous crystalline material is preferably a medium-pore zeolite, particularly ZSM-5, which contains phosphorus and has been severely steamed at a temperature of at least 950 DEG C.
Owner:MOBIL OIL CORP
Hydrogenating and pour point depressing catalyst and its preparing method
ActiveCN101143333AControl contentUnobstructed channelMolecular sieve catalystsRefining to eliminate hetero atomsPartial oxidationAdhesive
Owner:CHINA PETROLEUM & CHEM CORP +1
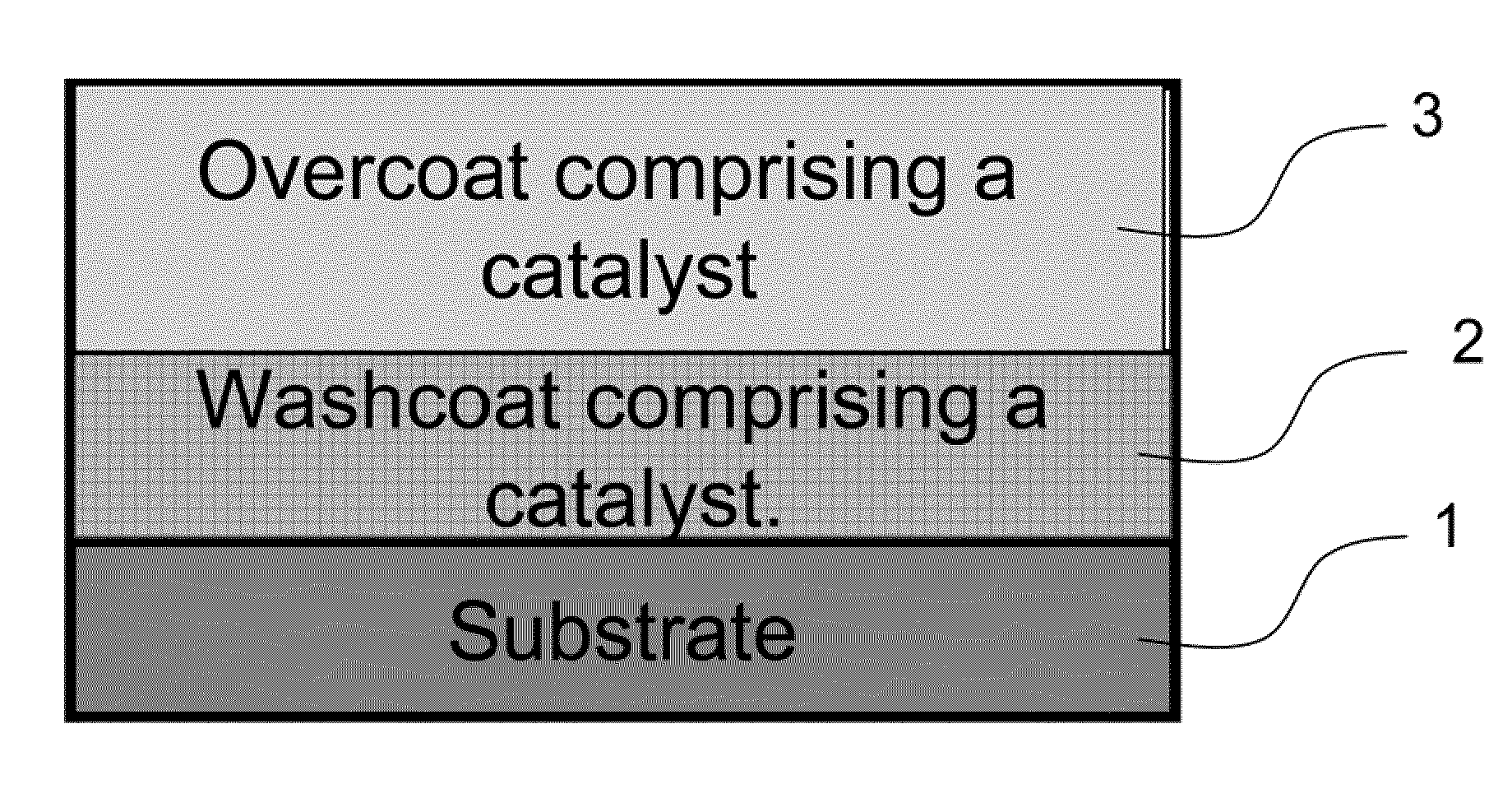
Cold start catalyst and its use in exhaust systems
InactiveUS20120308439A1Emission reductionSpeed up the conversion processCombination devicesMolecular sieve catalystsMetal catalystPlatinum group
A cold start catalyst is disclosed. The cold start catalyst comprises a zeolite catalyst and a supported platinum group metal catalyst. The zeolite catalyst comprises a base metal, a noble metal, and a zeolite. The supported platinum group metal catalyst comprises one or more platinum group metals and one or more inorganic oxide carriers. The invention also includes an exhaust system comprising the cold start catalyst. The cold start catalyst and the process result in improved NOx storage and NOx conversion, improved hydrocarbon storage and conversion, and improved CO oxidation through the cold start period.
Owner:JOHNSON MATTHEY PLC
Gas purifying process and gas purifying apparatus
A method is provided for removing water, carbon monoxide and carbon dioxide out of a gas, such as air, by passing the gas through a packed column so that the gas sequentially contacts a catalyst consisting of platinum or palladium and at least one member selected from the group consisting of iron, cobalt, nickel, manganese, copper, chromium, tin, lead and cerium wherein the catalyst is supported on alumina containing substantially no pores having pore diameters of 110 Angstroms or less under conditions which oxidize the carbon monoxide in the gas into carbon dioxide; an adsorbent selected from the group consisting of silica gel, activated alumina, zeolite and combinations thereof under conditions in which water is adsorbed and removed from the gas and an adsorbent selected from the group consisting of calcium ion exchanged A zeolite; calcium ion exchanged X zeolite; sodium ion exchanged X zeolite and mixtures thereof under conditions which carbon dioxide is adsorbed and removed from the gas. The gas may also be subjected to a catalyst / adsorbent in the packed column to effect oxidation and removal of hydrogen in the gas.
Owner:NIPPON SANSO CORP
Process for BTX purification
InactiveUS6500996B1Lowering indexEfficient executionOrganic chemistry methodsTreatment with plural serial refining stagesCobaltChromium
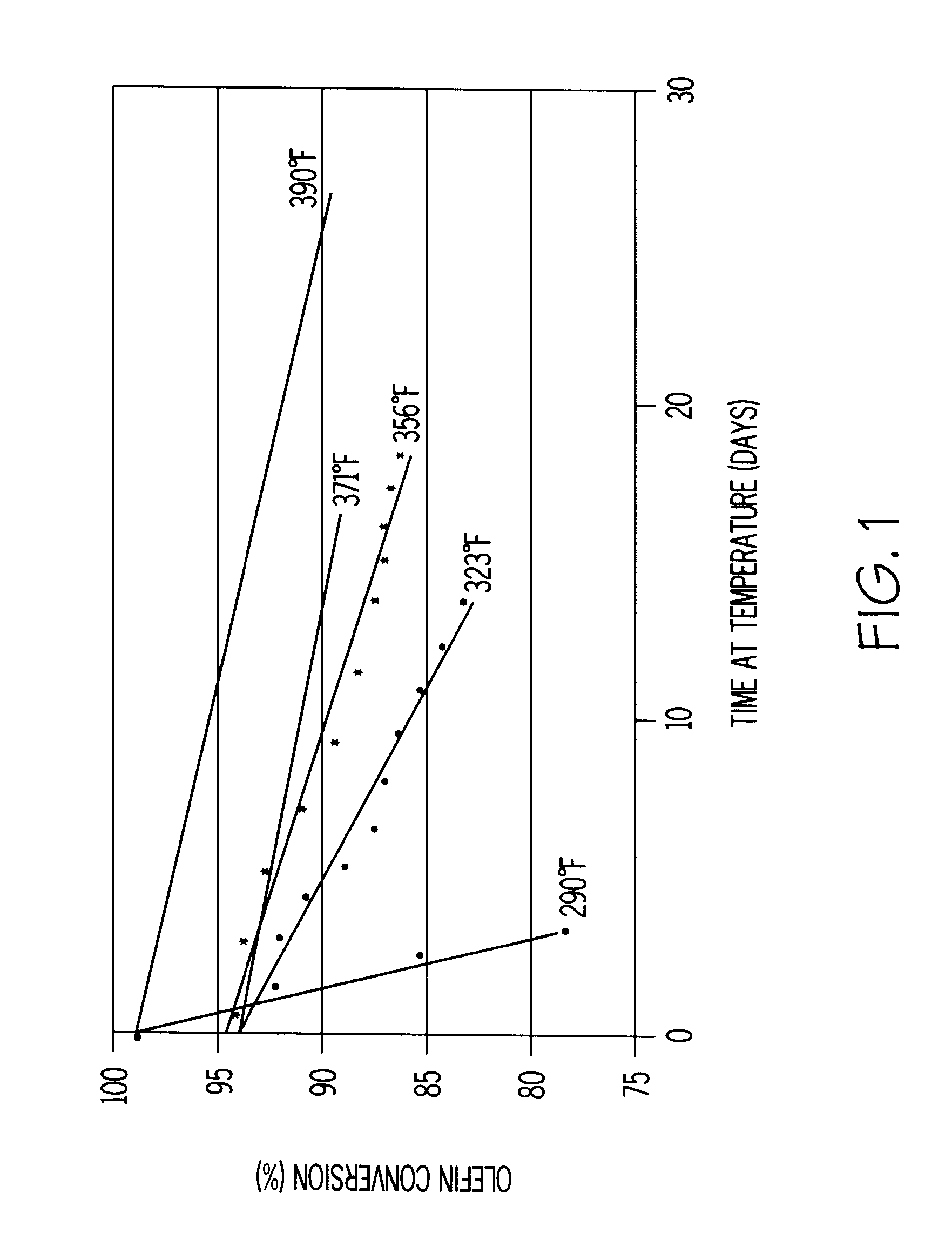
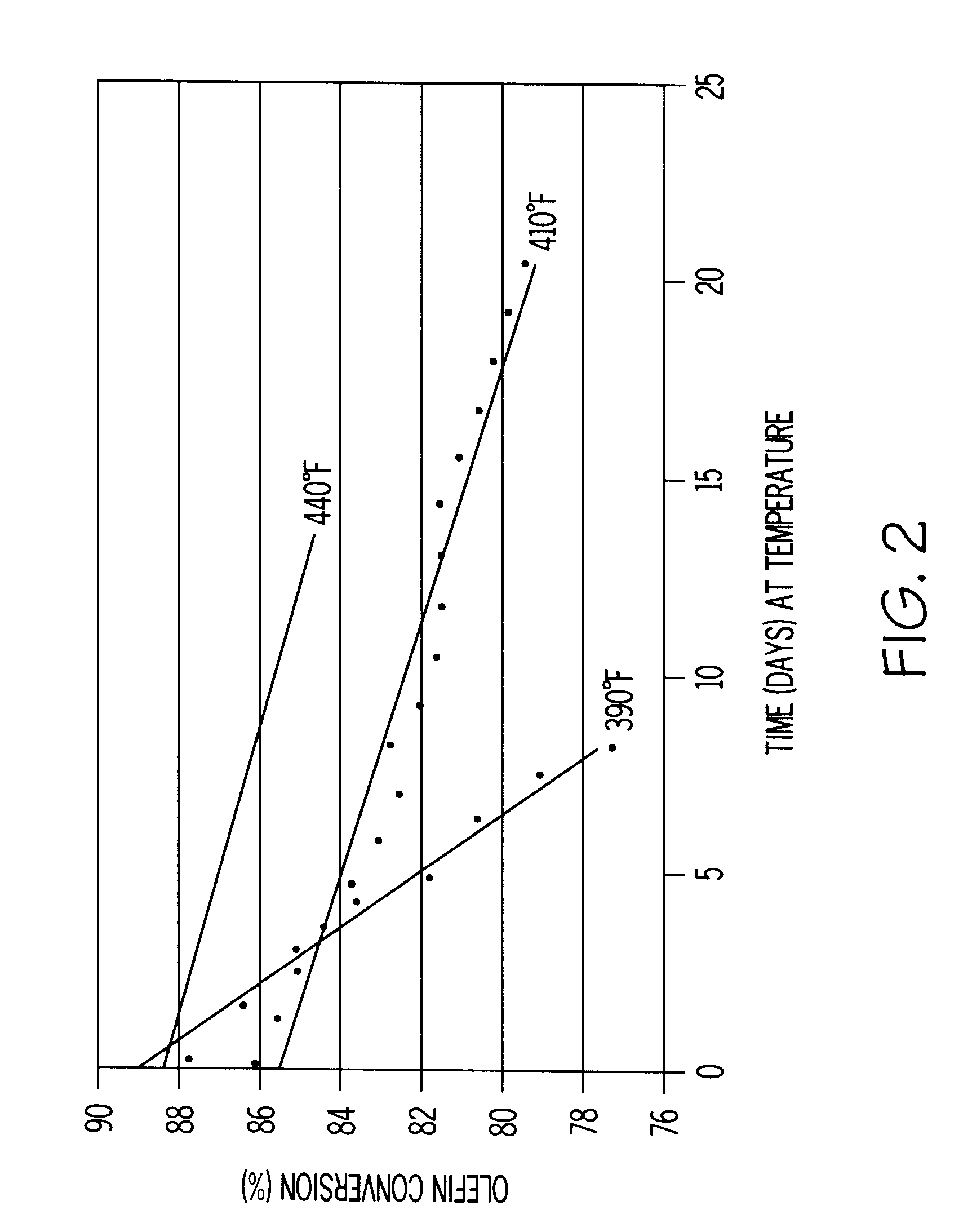
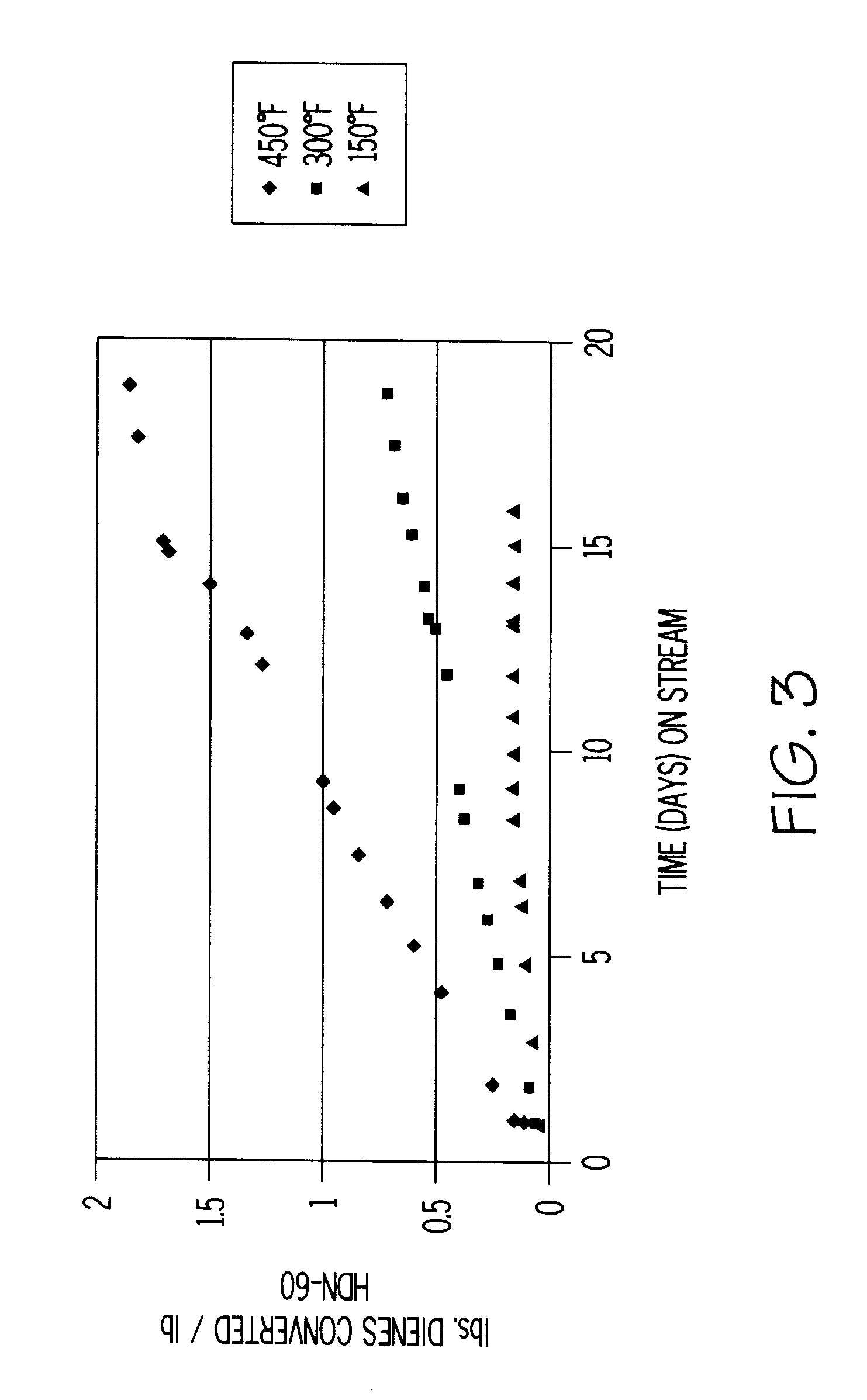
A process for the removal of hydrocarbon contaminants, such as dienes and olefins, from an aromatics reformate by contacting an aromatics reformate stream with a hydrotreating catalyst and / or a molecular sieve. The hydrotreating catalyst substantially converts all dienes to oligomers and partially converts olefins to alkylaromatics. The molecular sieve converts the olefins to alkylaromatics. The process provides an olefin depleted product which can be passed through a clay treater to substantially convert the remaining olefins to alkylaromatics. The hydrotreating catalyst has a metal component of nickel, cobalt, chromium, vanadium, molybdenum, tungsten, nickel-molybdenum, cobalt-nickel-molybdenum, nickel-tungsten, cobalt-molybdenum or nickel-tungsten-titanium, with a nickel molybdenum / alumina catalyst being preferred. The molecular sieve is an intermediate pore size zeolite, preferably MCM-22. The clay treatment can be carried out with any clay suitable for treating hydrocarbons.
Owner:EXXONMOBIL CORP (US)
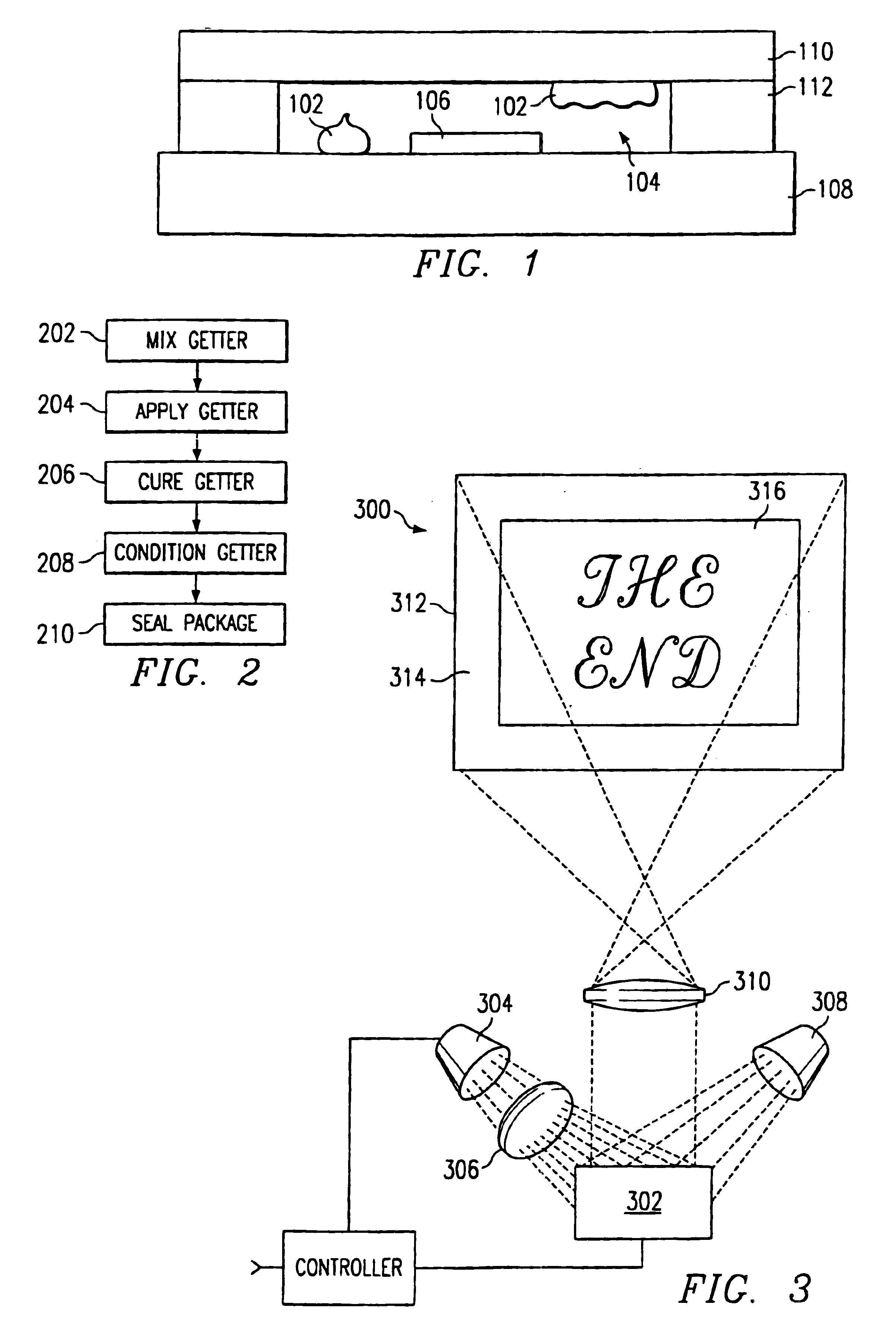
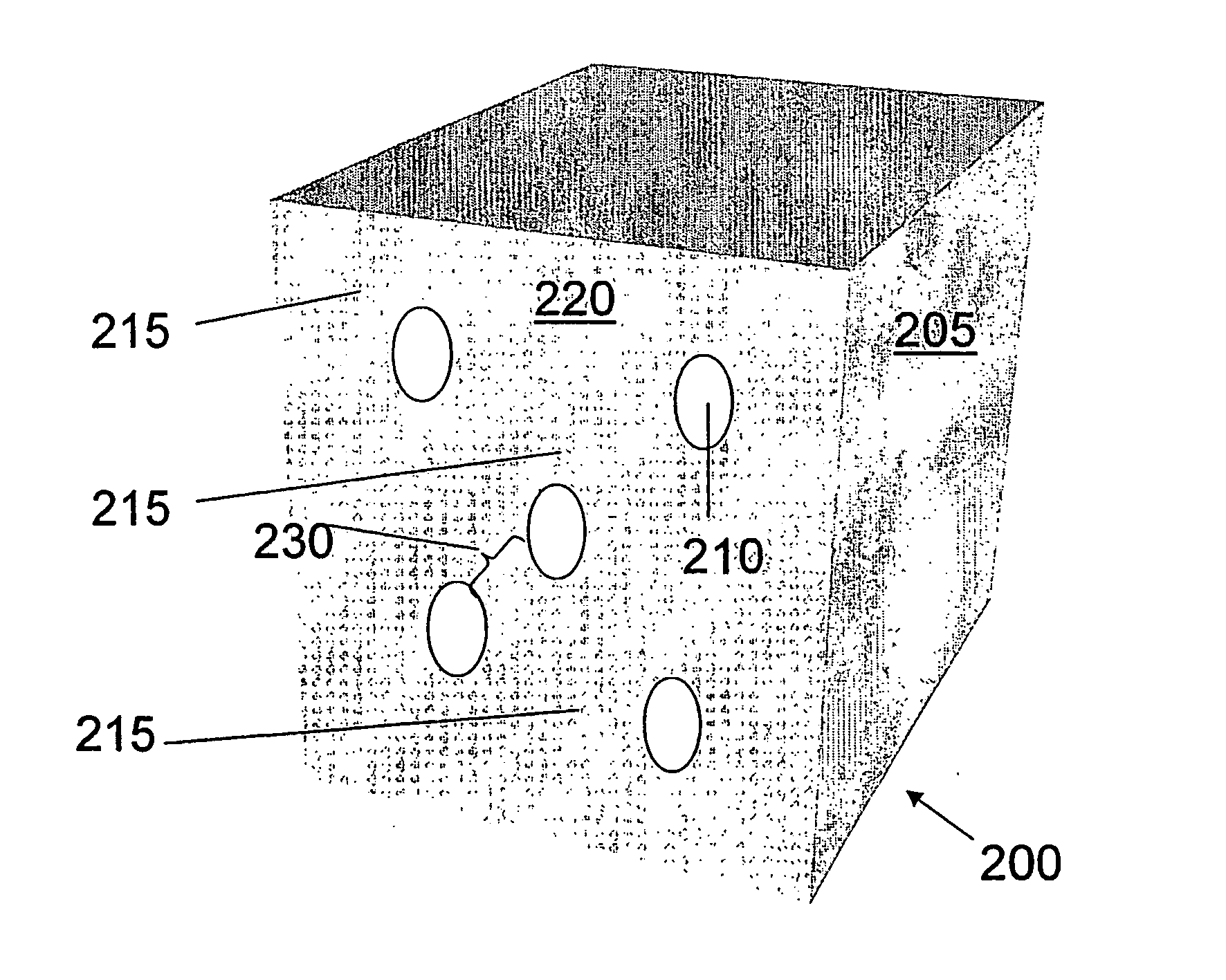
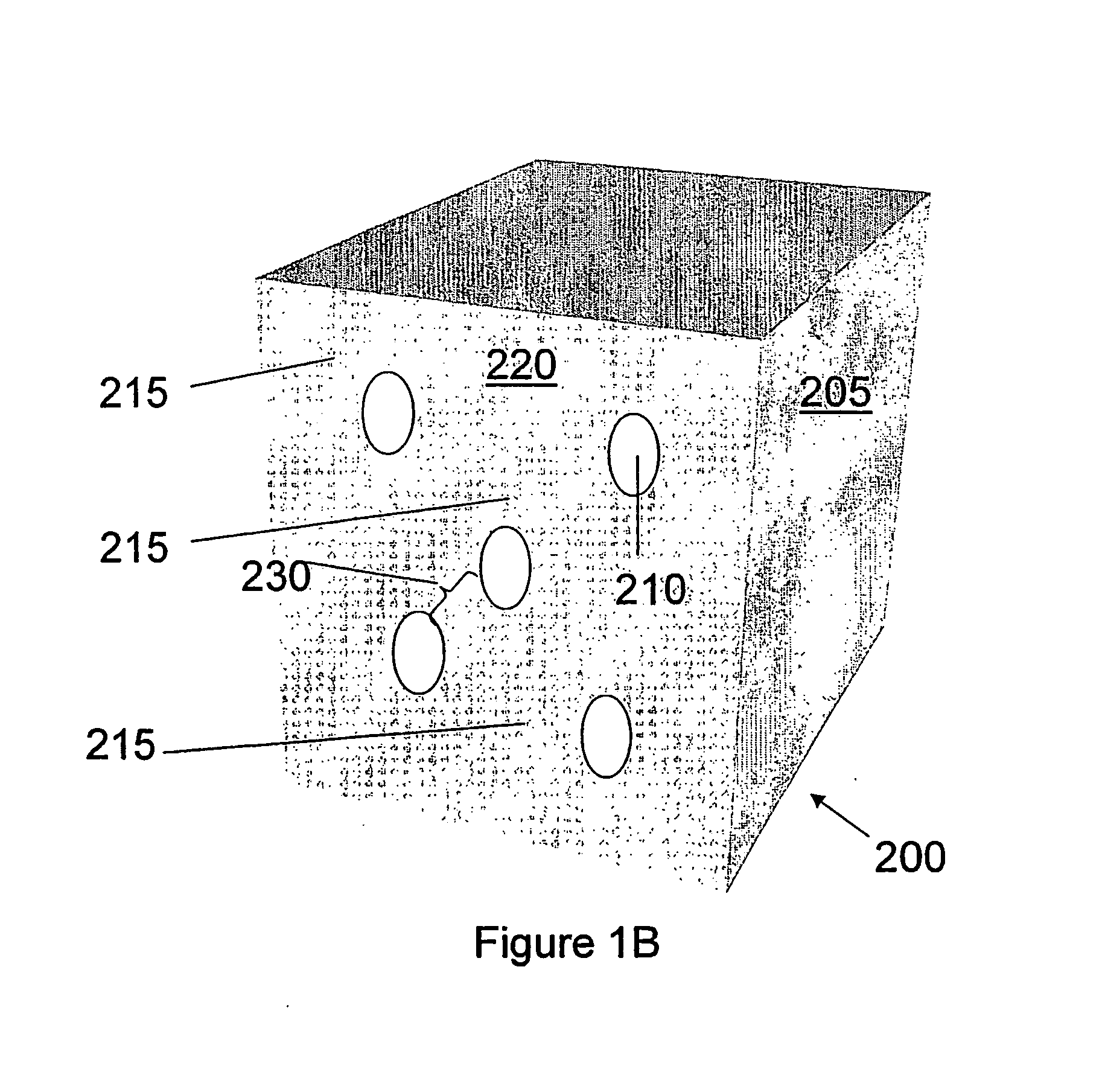
Getter for enhanced micromechanical device performance
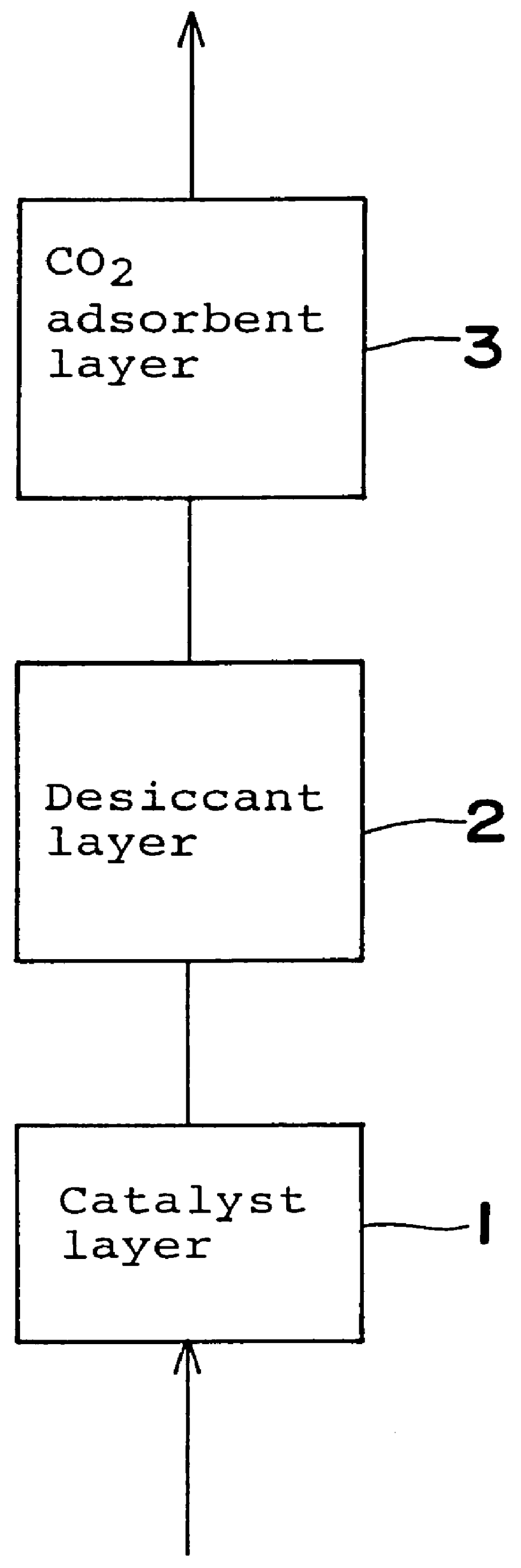
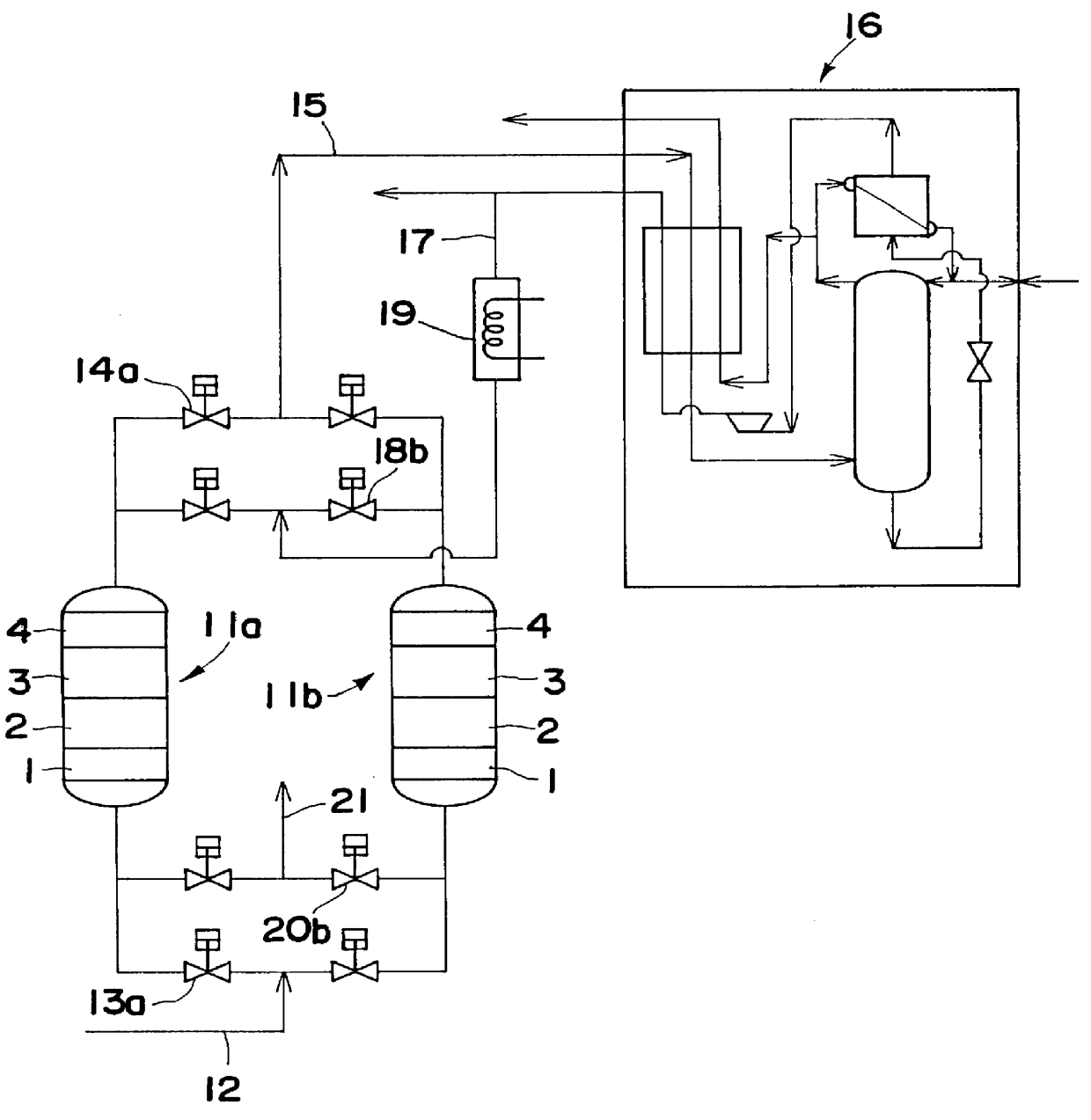
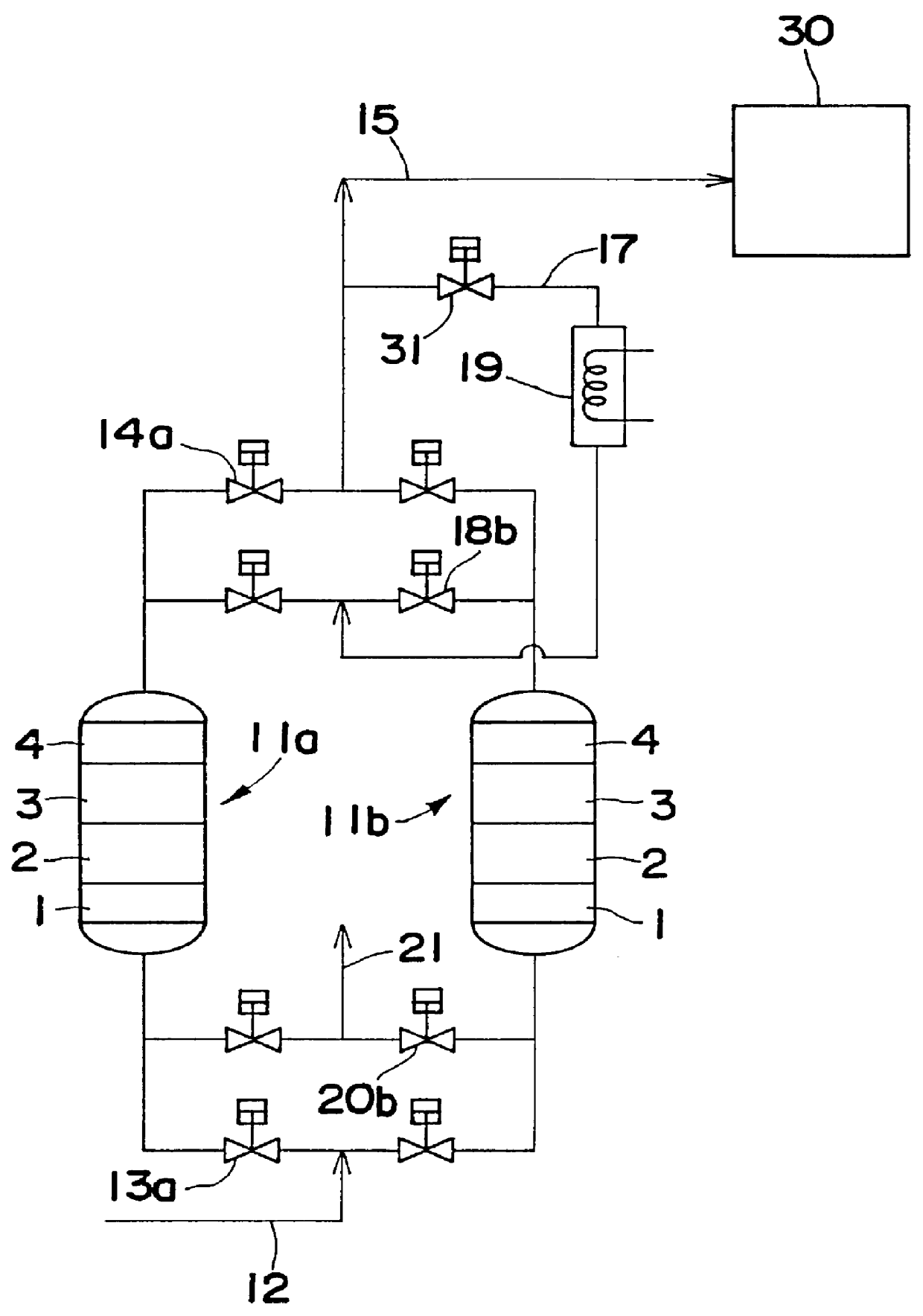
A desiccant compound, image projection system using the desiccant compound, and a method for utilizing the desiccant compound. The desiccant compound formed by mixing (202) a polymer binder selected from the group consisting of polysaccharides (including without limitation structural polysaccharides such as cellulose, chitin, and their functionalized derivatives), polyamines, polysulfones, and polyamides with a drying agent, typically a zeolite, at a polymer to drying agent weight ratio of 1:2.1 to 1:100, or 1:4 to 1:10. After the desiccant compound is mixed (202) it is applied (204) to a surface and cured (206), often through the application of heat and vacuum. The cured desiccant compound is conditioned (208) and the it package is sealed (210).
Owner:TEXAS INSTR INC
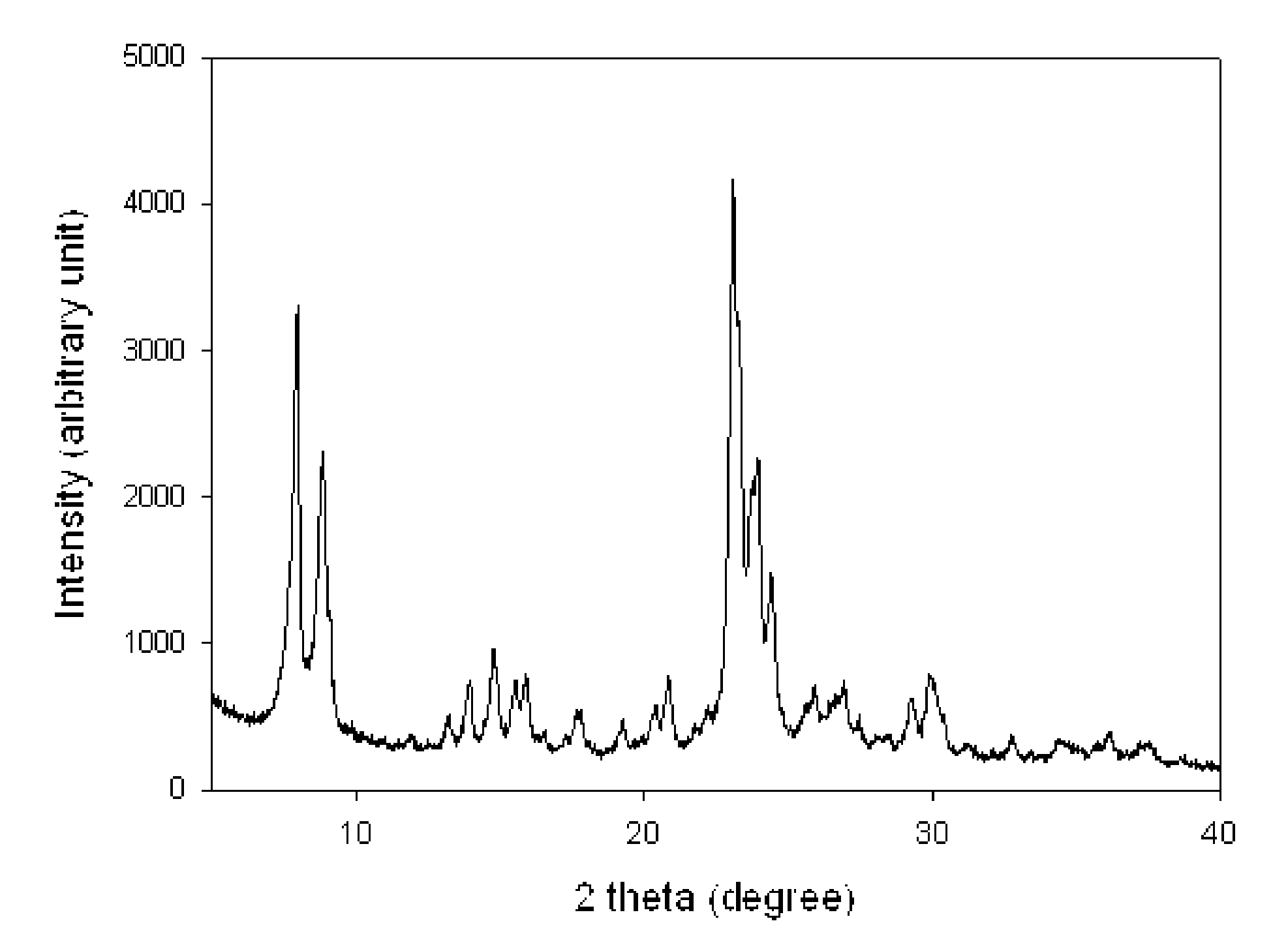
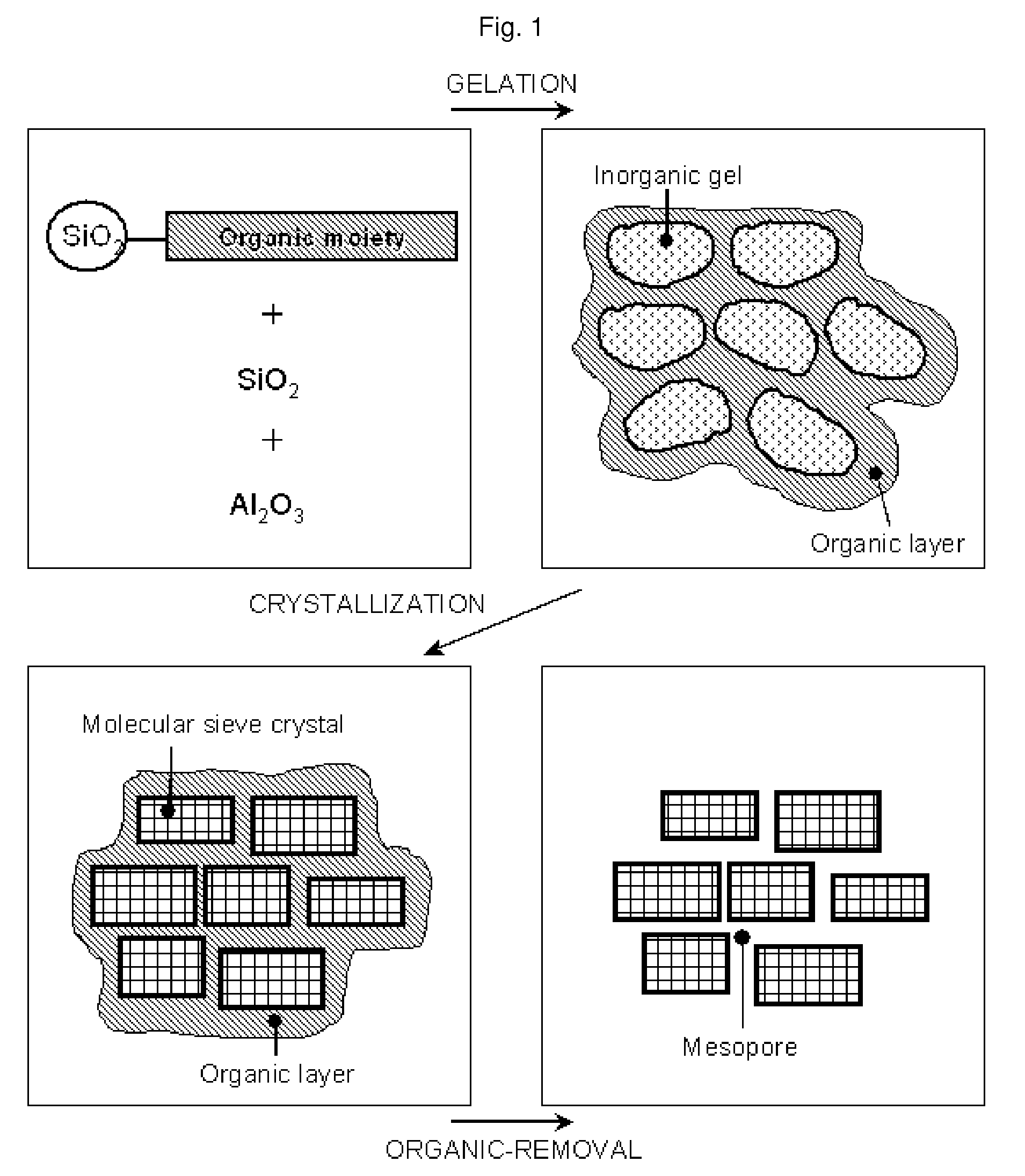
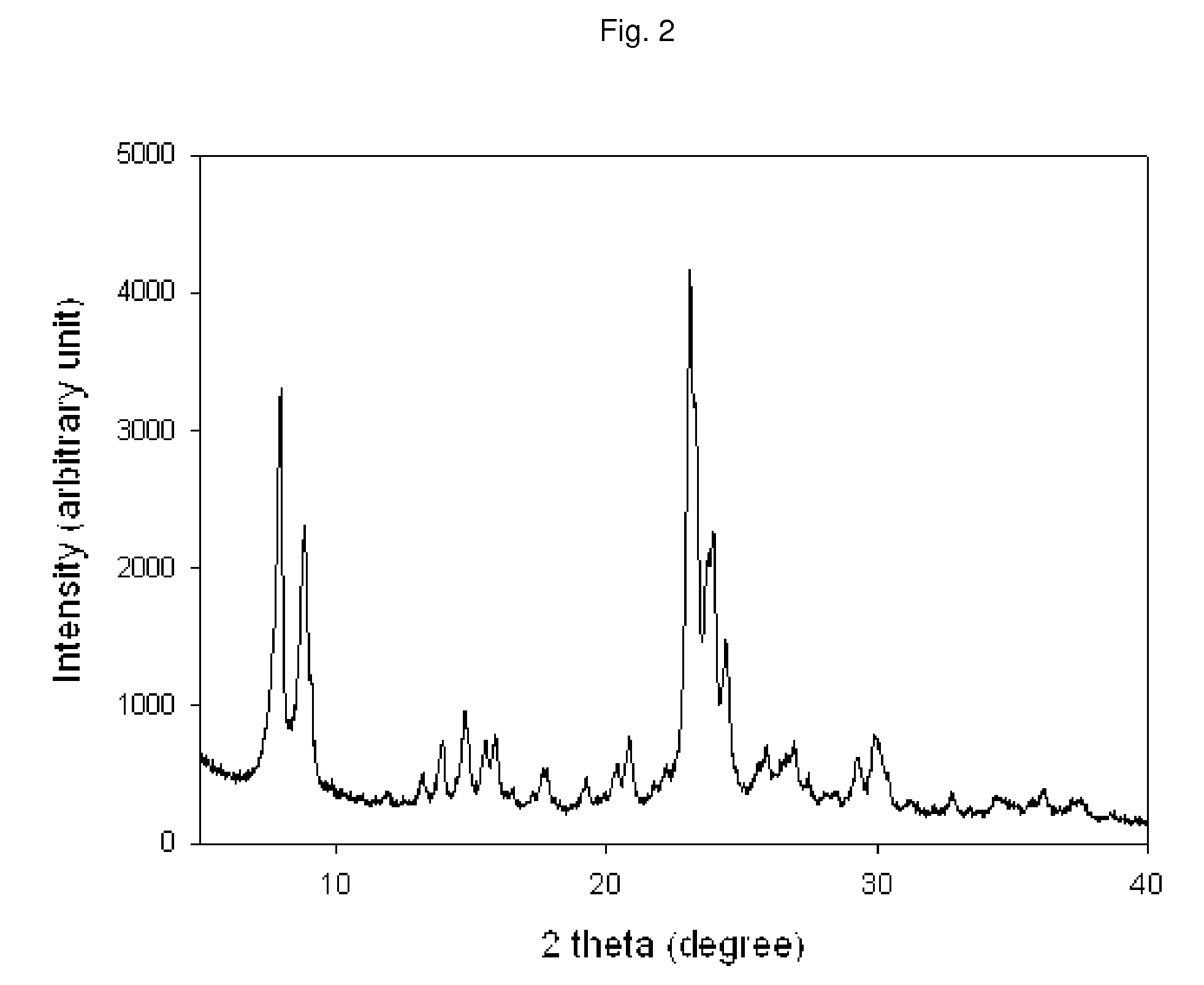
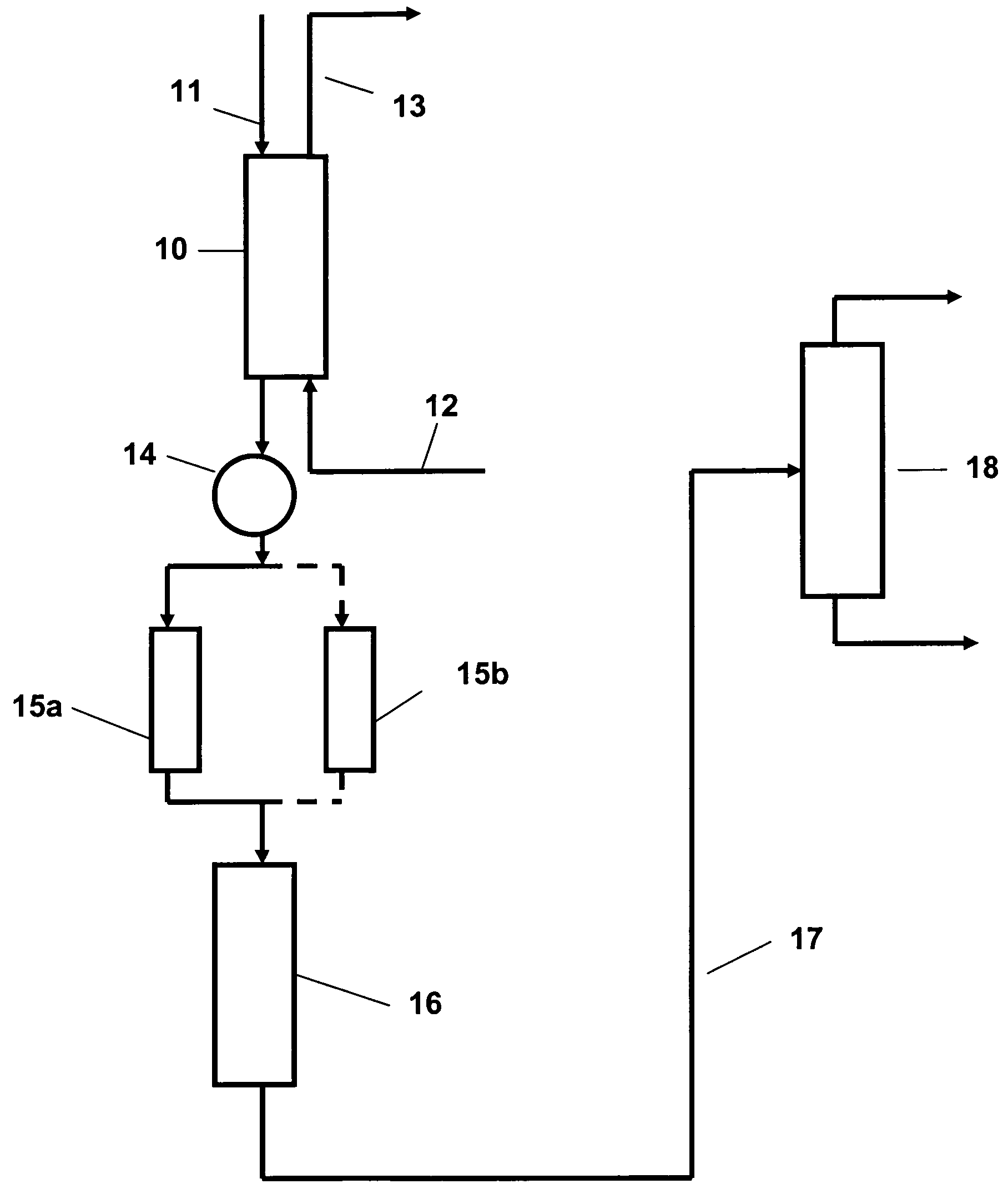
Mesostructured Zeolitic Materials, and Methods of Making and Using the Same
ActiveUS20070227351A1Increase productionConvenient lightingMaterial nanotechnologyCatalytic crackingSimple Organic CompoundsOrganic compound
One aspect of the present invention relates to mesostructured zeolites. The invention also relates to a method of preparing mesostructured zeolites, as well as using them as cracking catalysts for organic compounds and degradation catalysts for polymers.
Owner:MASSACHUSETTS INST OF TECH
Method of the preparation of microporous crystalline molecular sieve possessing mesoporous frameworks
InactiveUS7785563B2Facilitate diffusion and adsorptionImprove overall utilizationAluminium compoundsSilicaMolecular sieveChemical treatment
The present invention relates to a method of preparing a microporous crystalline molecular sieve having mesoporous skeleton, comprising following steps: (a) adding a meso-SDA (meso-Structure Directing Agent) into a gel composition of synthesizing molecular sieve, (b) subjecting the mixture obtained in the above step (a) to crystallization by a hydrothermal reaction, a microwave reaction, a dry-gel synthesis, etc., and (c) removing selectively organic materials from the resulted material obtained in the above step (b) by a calcination or a chemical treatment. Molecular sieve having mesoporous skeleton synthesized by the present invention exhibits, as compared with conventional zeolite, a good molecule diffusion ability and a greatly improved catalytic activity.
Owner:KOREA ADVANCED INST OF SCI & TECH
Liquid phase aromatics alkylation process
InactiveUS7476774B2Improve overall utilizationEasy to useLiquid carbonaceous fuelsHydrocarbonsAlkylationChemistry
A process for the production of high octane number gasoline from light refinery olefins and benzene-containing aromatic streams such as reformate. Light olefins including ethylene and propylene are extracted from refinery off-gases, typically from the catalytic cracking unit, into a light aromatic stream such as reformate containing benzene and other single ring aromatic compounds which is then reacted with the light olefins to form a gasoline boiling range product containing akylaromatics. The alkylation reaction is carried out in the liquid phase with a catalyst which preferably comprises a member of the MWW family of zeolites such as MCM-22 using a fixed catalyst bed.
Owner:EXXON RES & ENG CO
Inorganic putty powder with function of governing humidness
ActiveCN101368014AEffective regulation of relative humidityGood environmental protectionFilling pastesCement coatingsSodium BentoniteMetallurgy
The invention relates to interior wall putty powder with air humidity regulating function, which belongs to the field of building materials. The putty powder is composed of 10 to 50 weight portions of humidity regulating material, 15 to 50 weight portions of inorganic binding material, 15 to 50 weight portions of inorganic filler, and 1 to 5 weight portions of sub divisible rubber powder; the humidity regulating material comprises one type or more than one type of diatomite, sepiolite, attapulgite, vermiculite, zeolite powder, kaolin and bentonite. Because of the environment protection and humidity regulating functions, the multi-pore inorganic mineral material is taken as humidity regulating material and applied in the building interior wall putty powder. With water added in, the putty powder is mixed into lacquer putty and applied on the wall surface, which can effectively regulate the indoor air humidity, so that the indoor air humidity is controlled within the scope which is beneficial to the health of human body; meanwhile, the putty powder has no pollution, no VOC released and can be directly used for decoration.
Owner:CHINA BUILDING MATERIALS ACAD
Catalyst composition, its preparation and use
A catalyst composition which comprises: a) a carrier which comprises at least 30 wt % of a binder selected from silica, zirconia and titania; at least 20 wt % of a pentasil zeolite, having a bulk silica to alumina ratio in the range of from 20 to 150 and being in its H+ form; and less than 10 wt % of other components, all percentages being on the basis of total carrier; b) platinum in an amount in the range of from 0.001 to 0.1 wt %, on the basis of total catalyst; and c) tin in an amount in the range of from 0.01 to 0.5 wt %, on the basis of total catalyst; its preparation and use; are provided.
Owner:SHELL OIL CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com