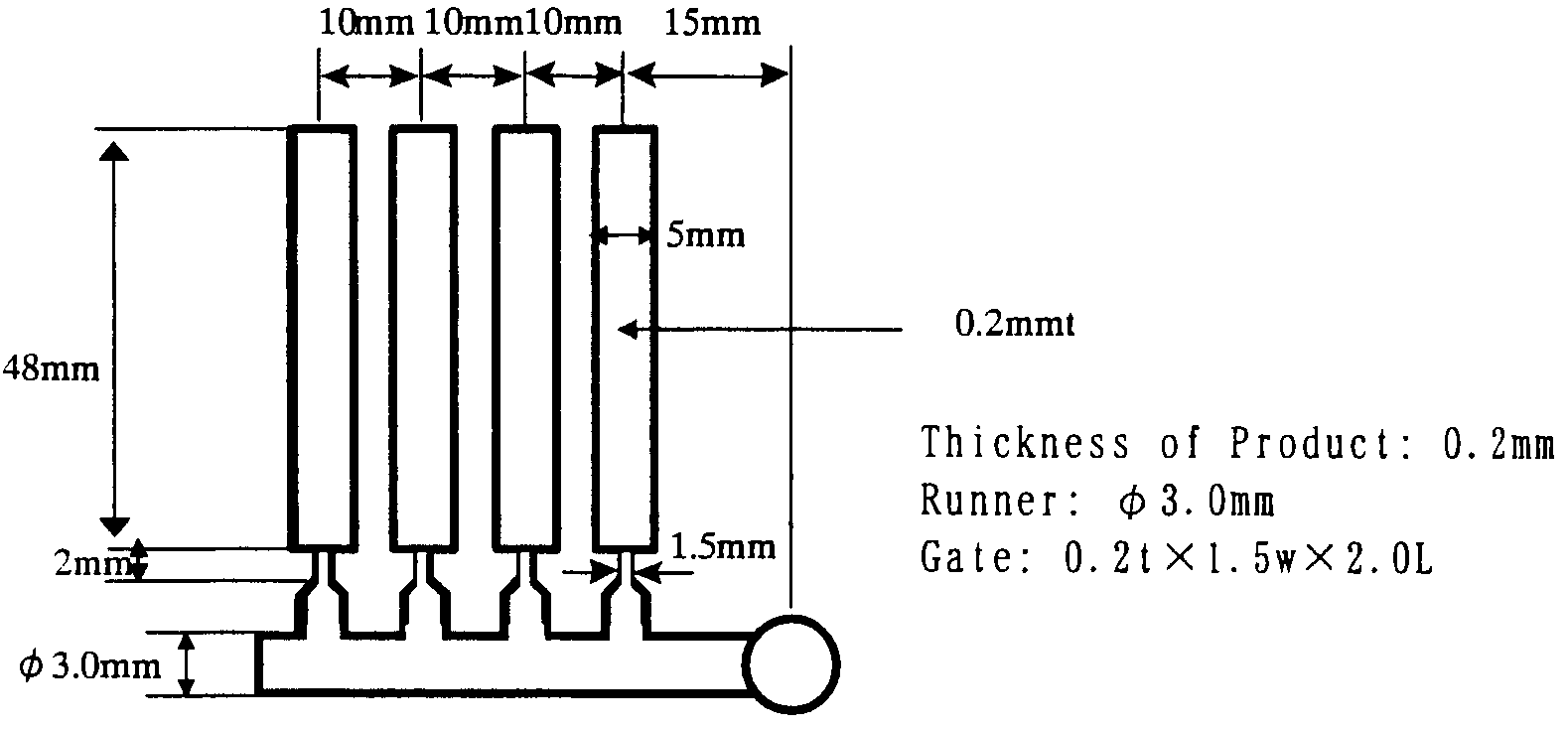
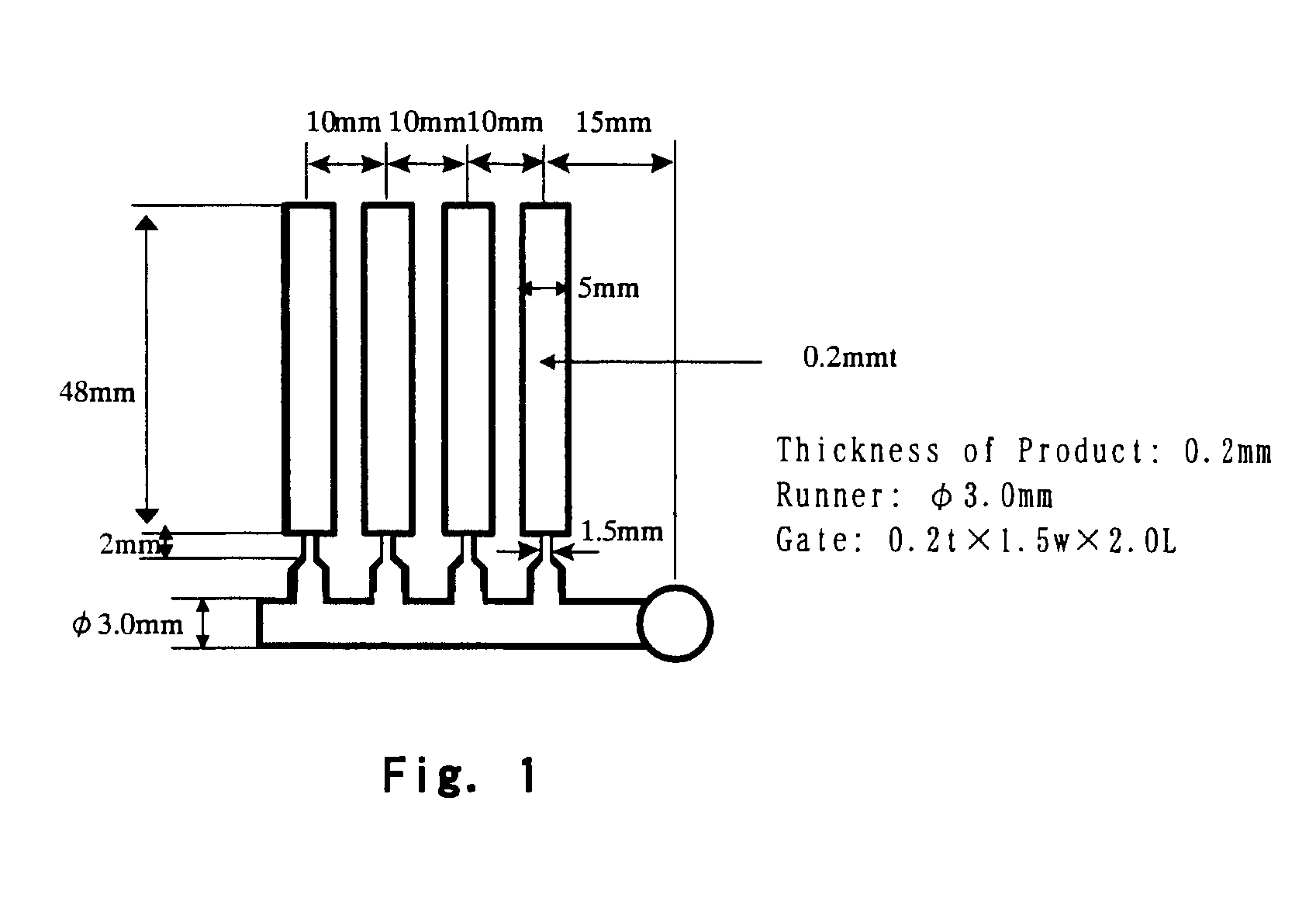
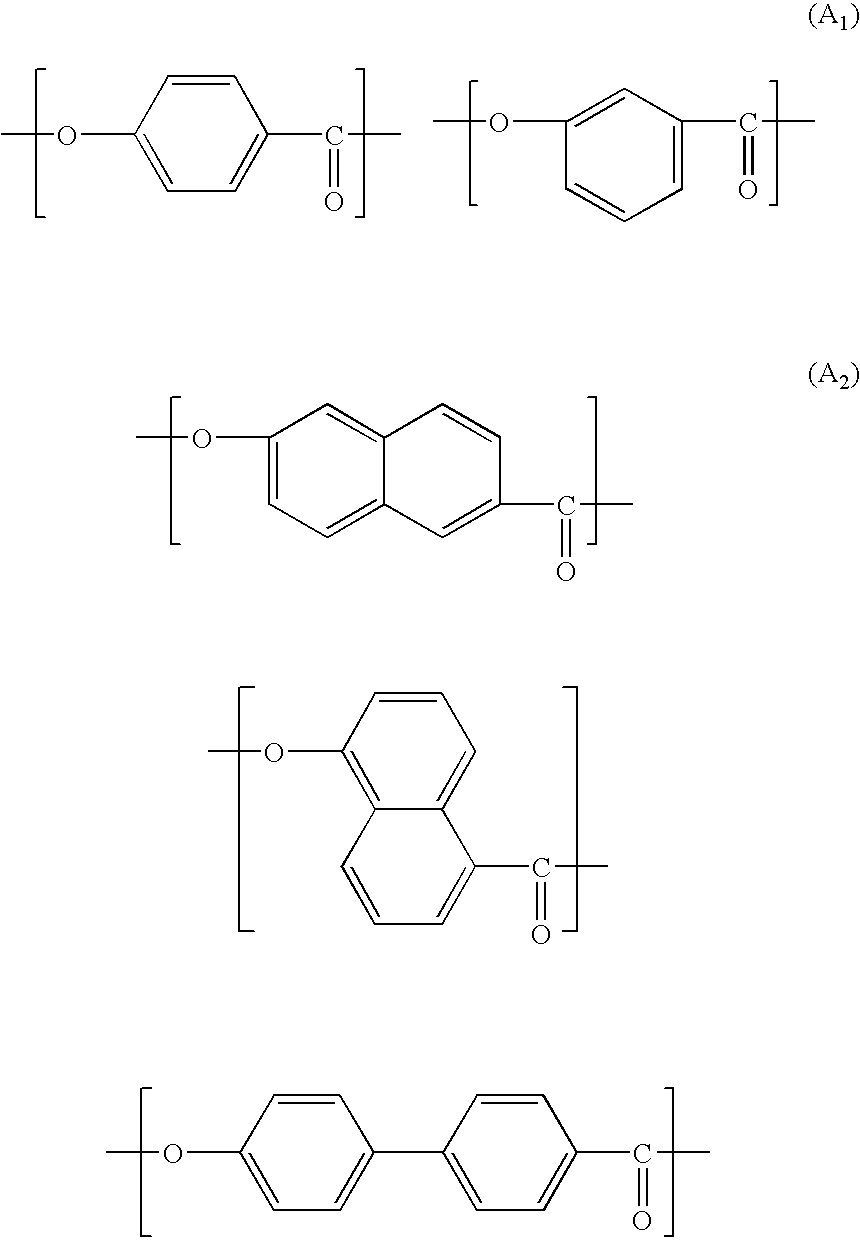
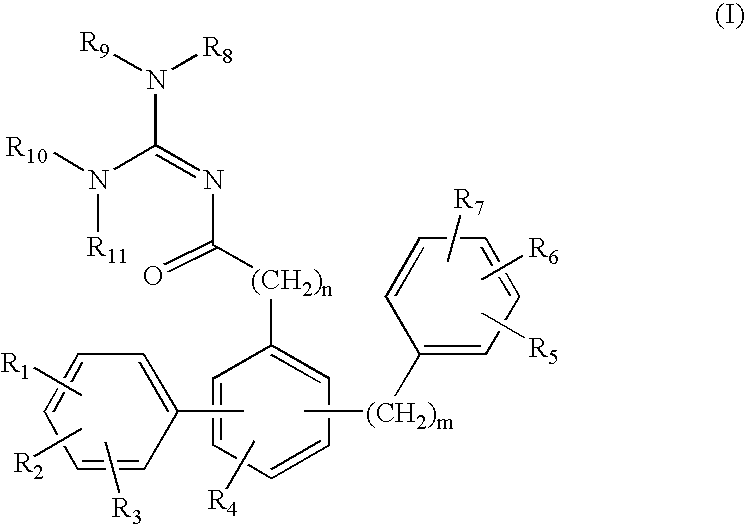
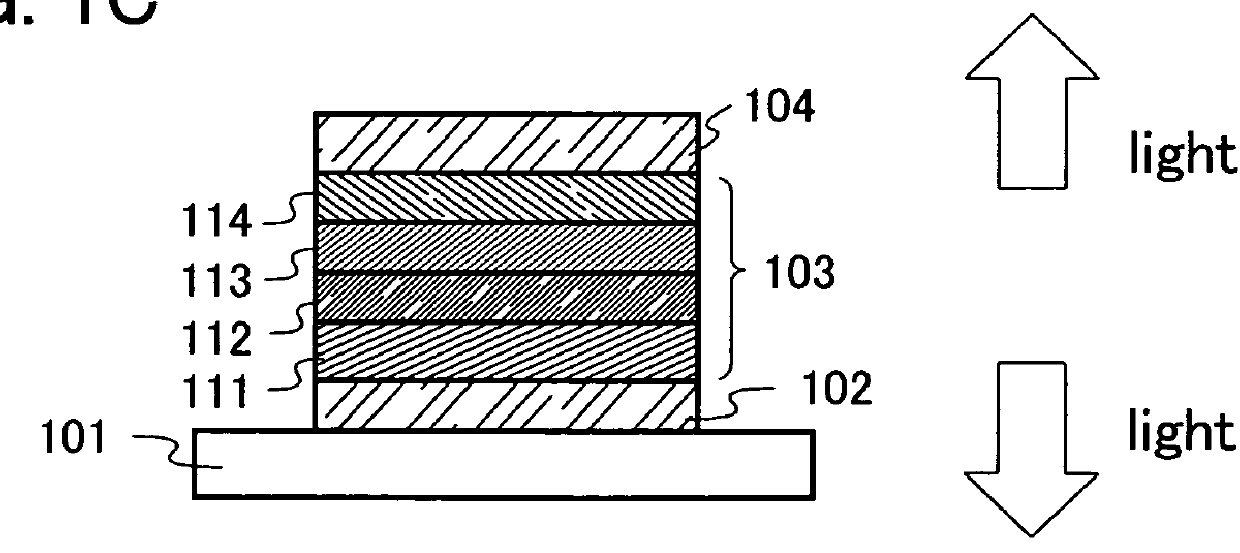
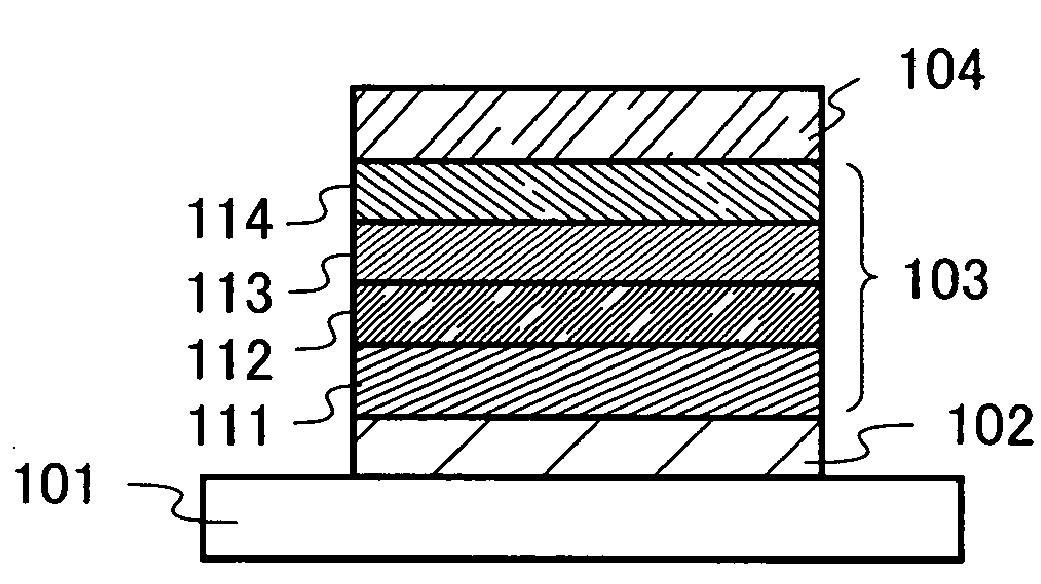
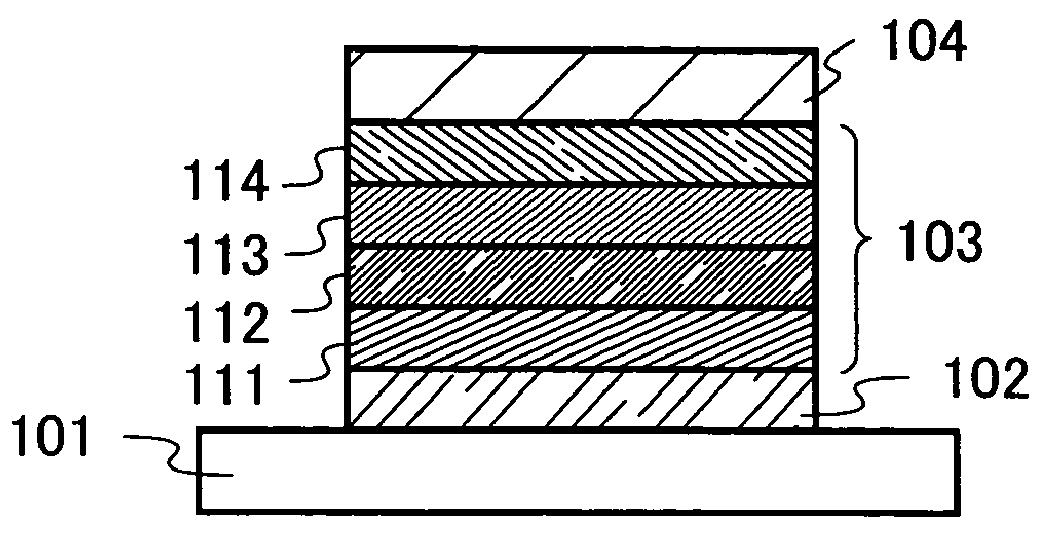
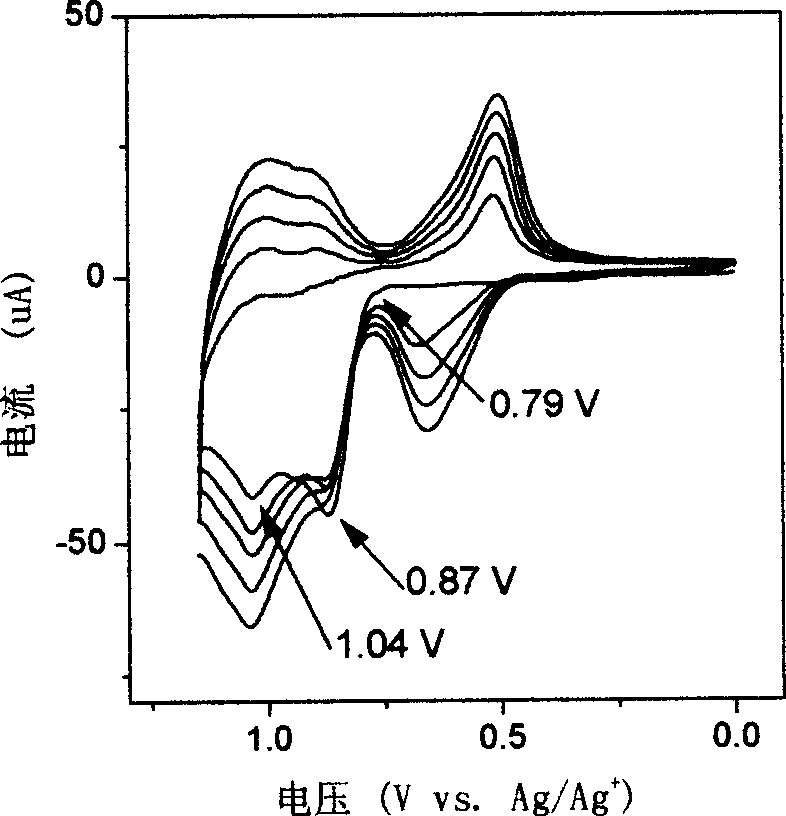
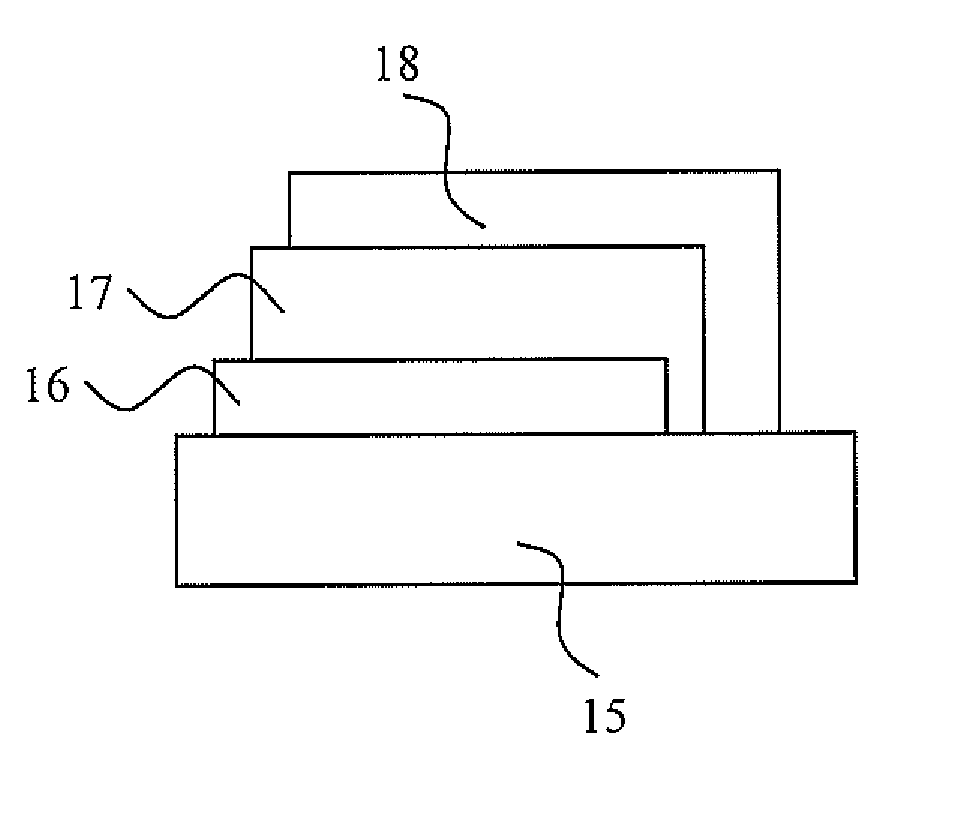
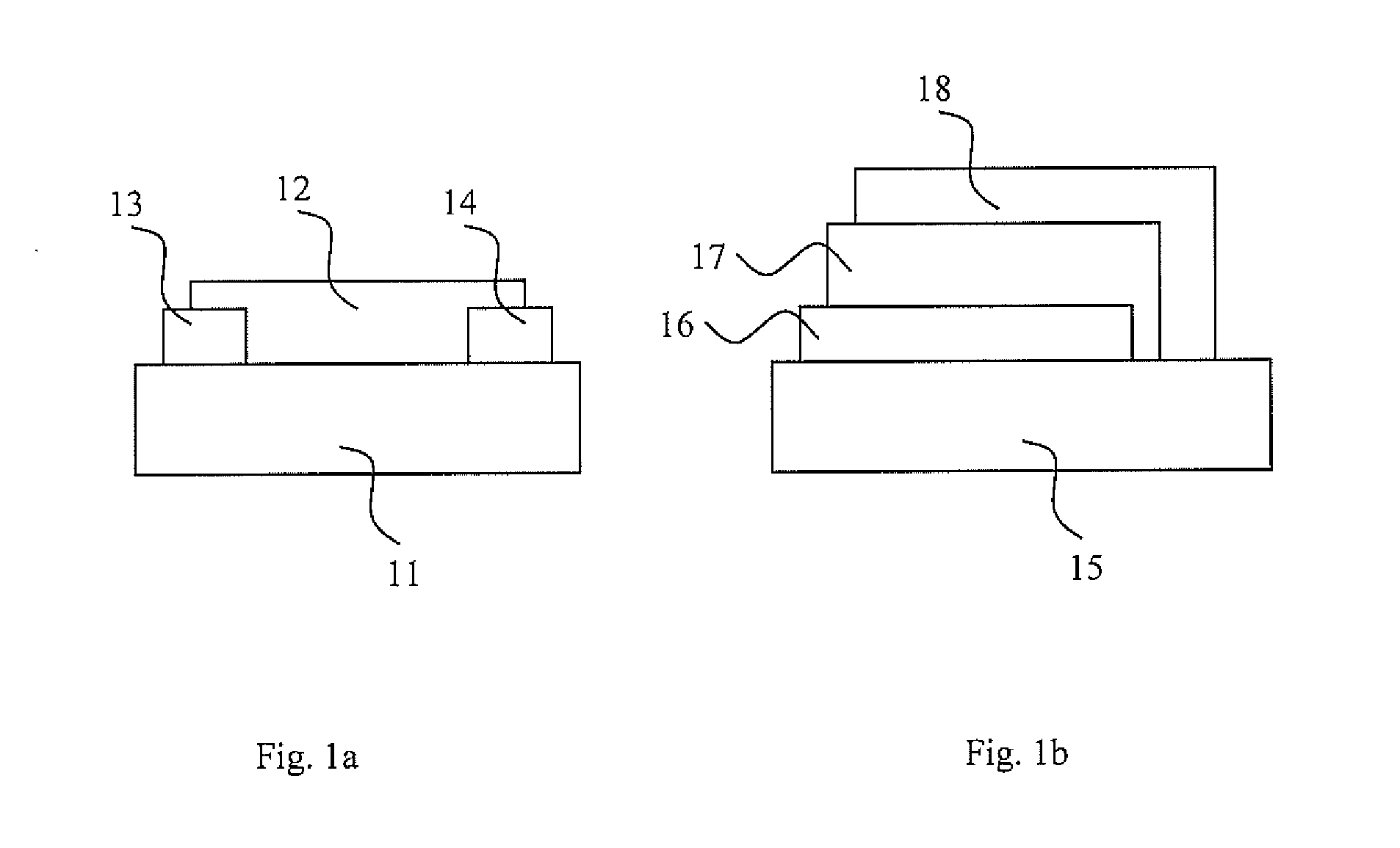
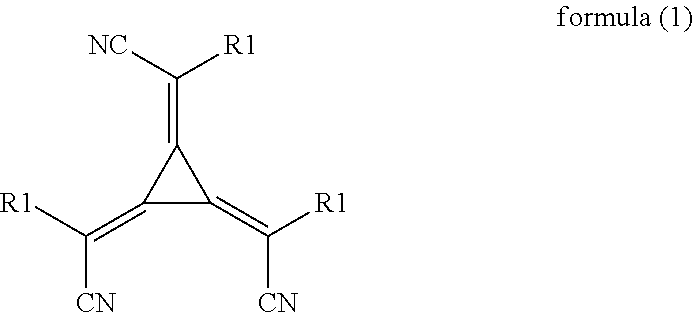
Patents
Literature
381 results about "Terphenyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Terphenyls are a group of closely related aromatic hydrocarbons. Also known as diphenylbenzenes or triphenyls, they consist of a central benzene ring substituted with two phenyl groups. The three isomers are ortho-terphenyl, meta-terphenyl, and para-terphenyl. Commercial grade terphenyl is generally a mixture of the three isomers. This mixture is used in the production of polychlorinated terphenyls, which were formerly used as heat storage and transfer agents.
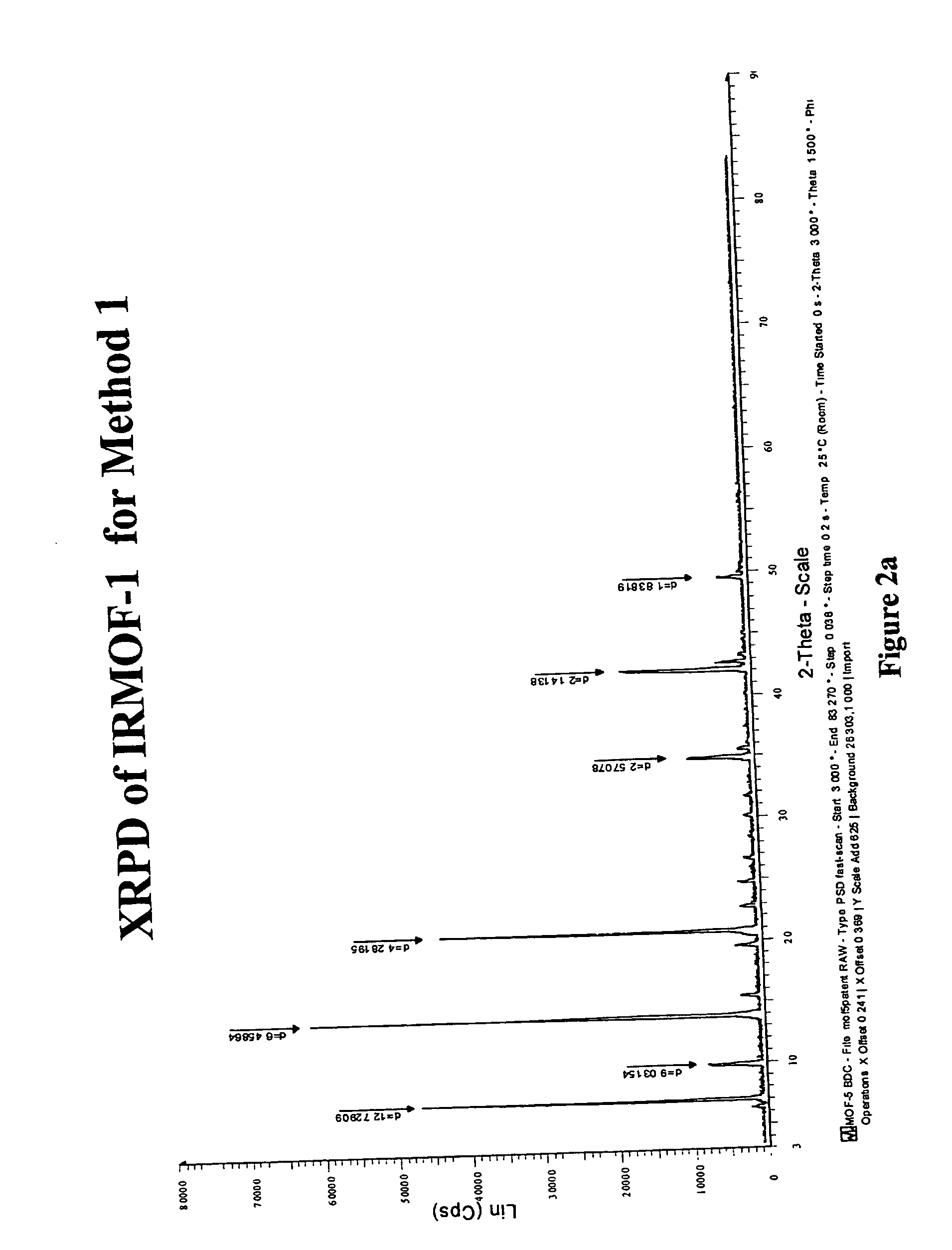
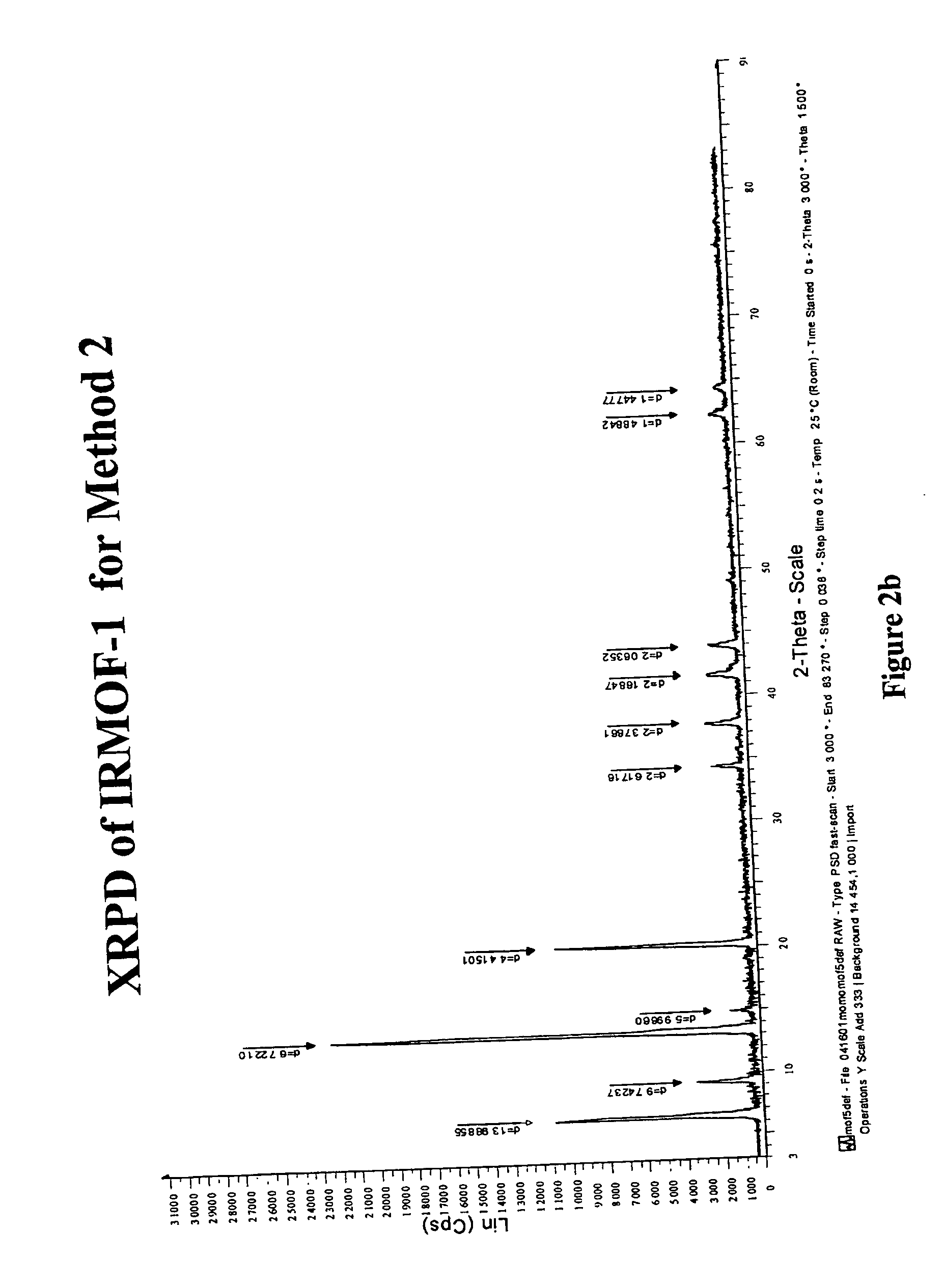
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
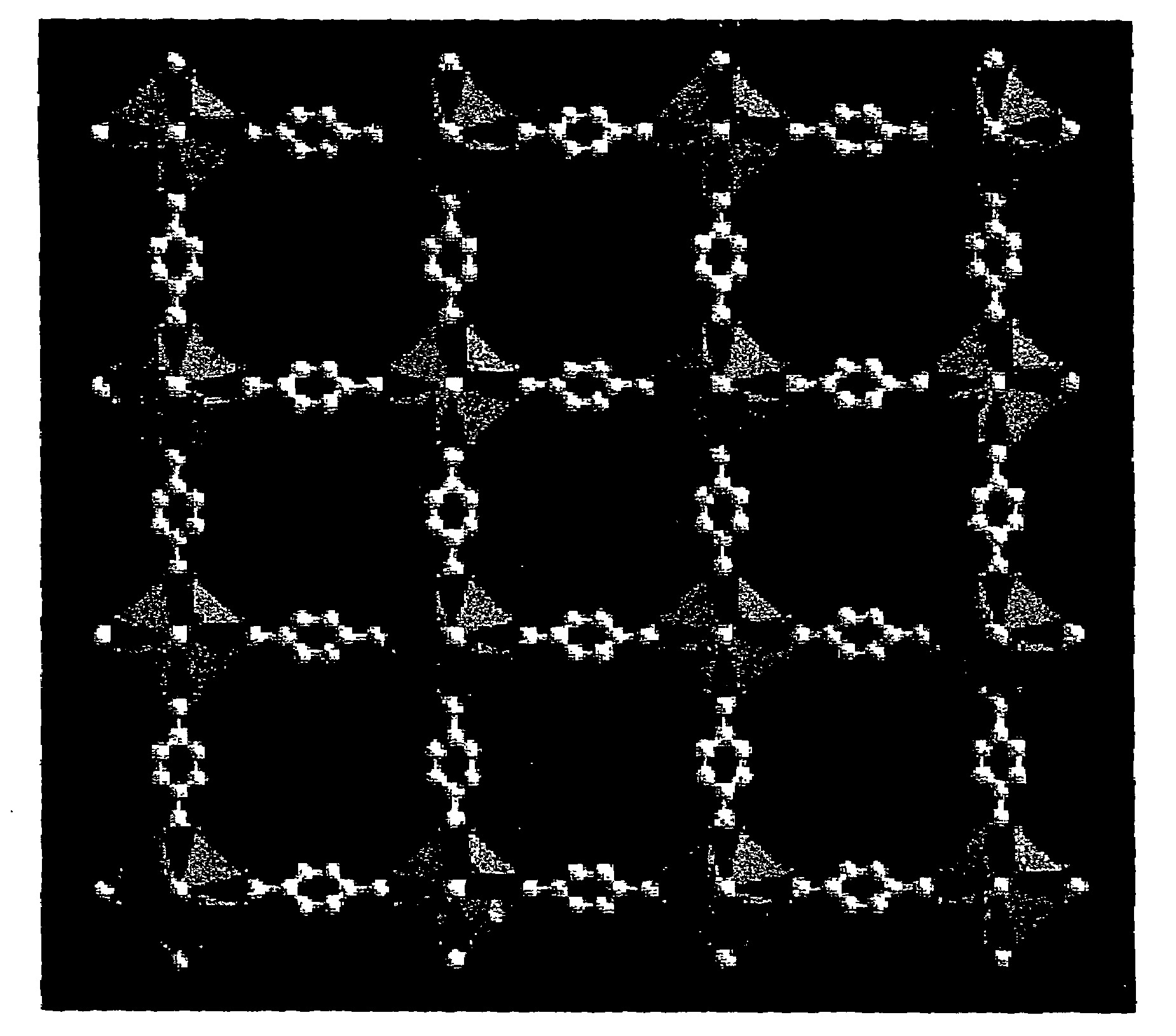
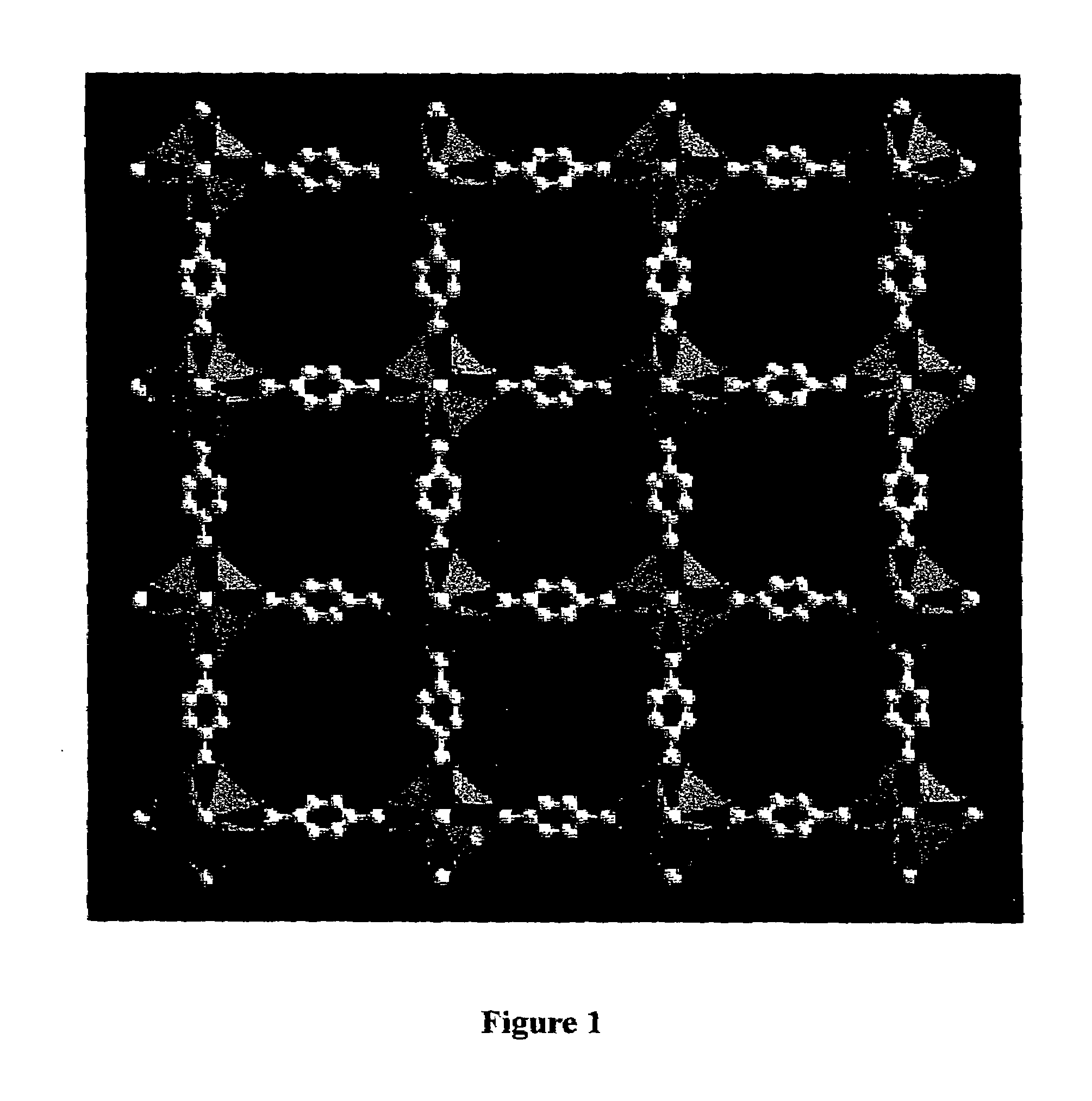
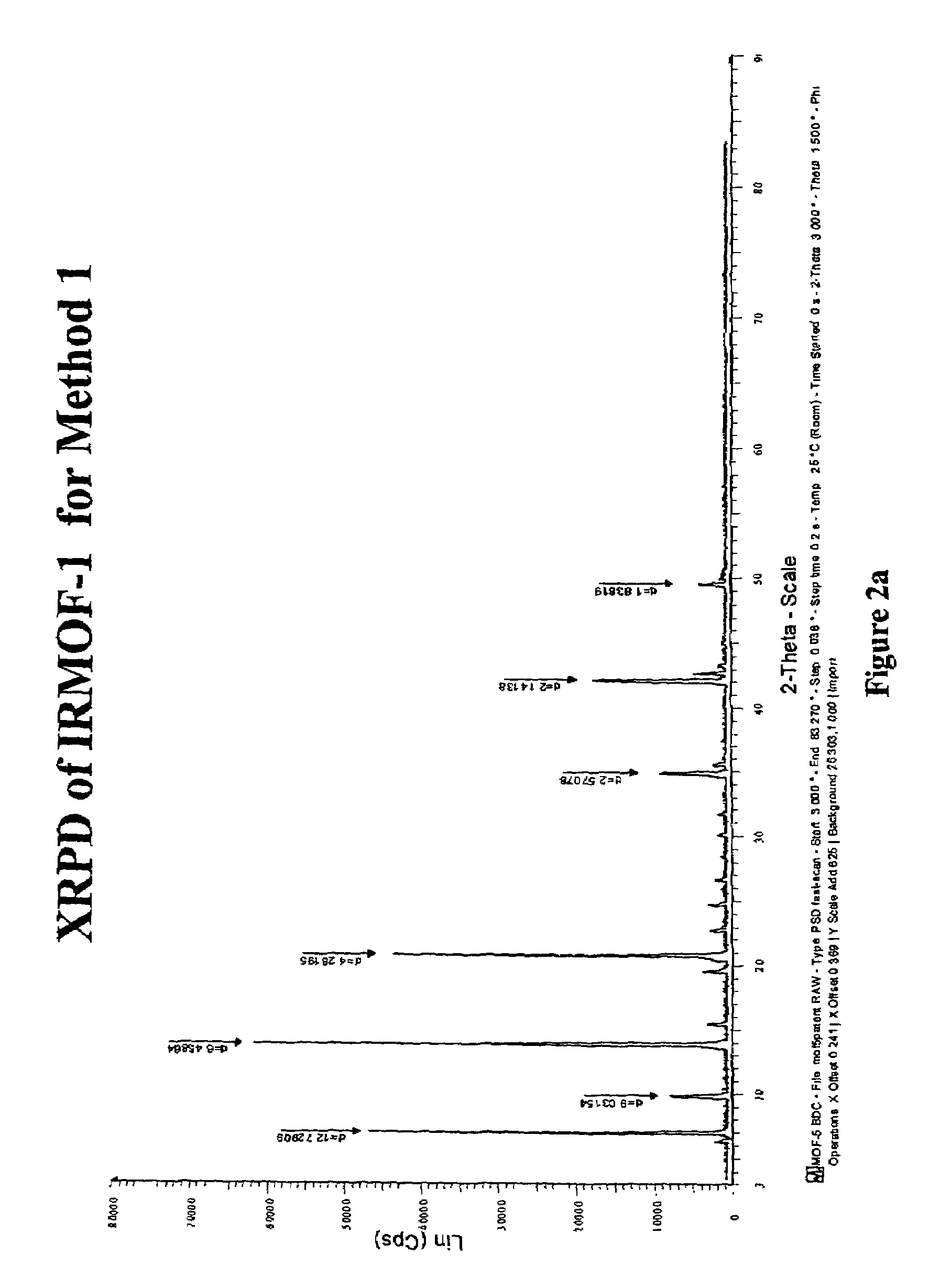
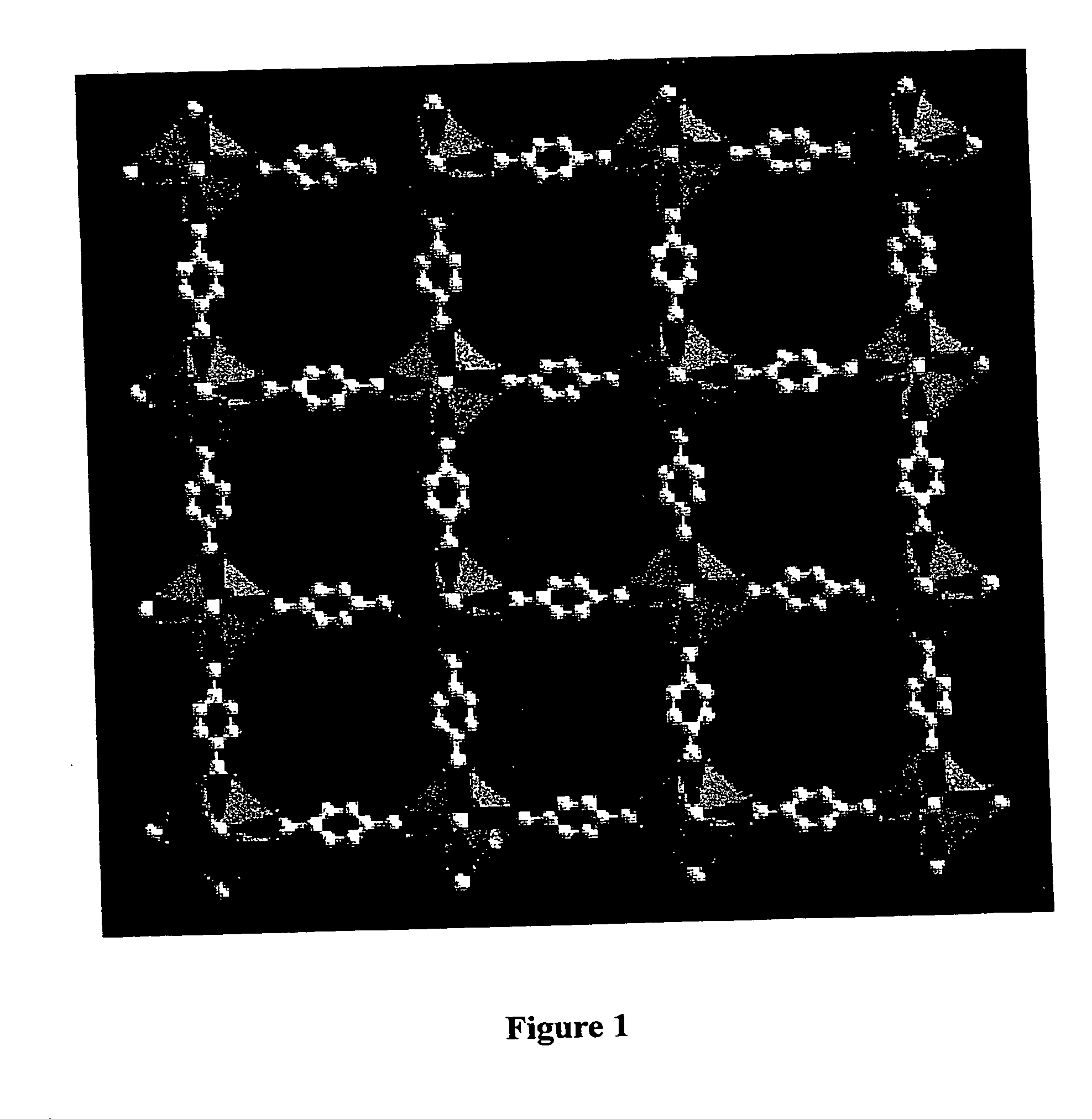
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS20050192175A1Catalyst protectionMolecular sieve catalystsSystems designMetal-organic framework
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
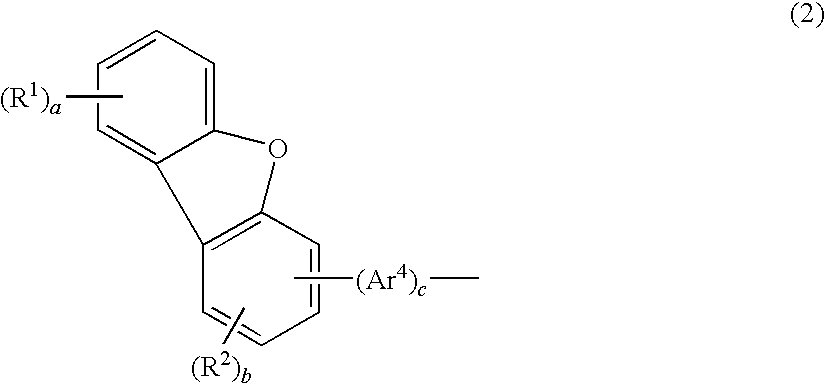
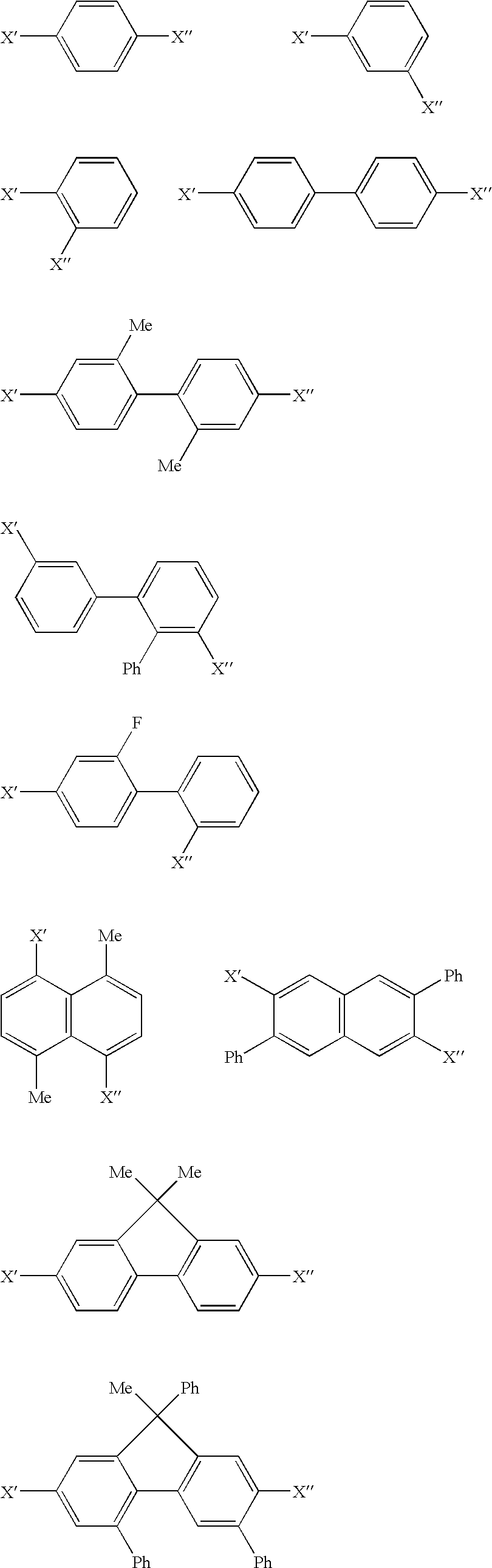
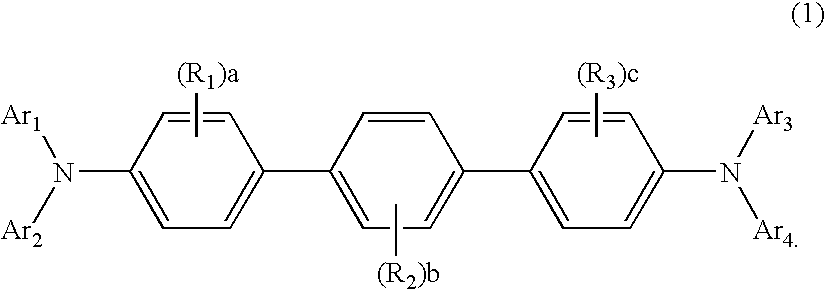
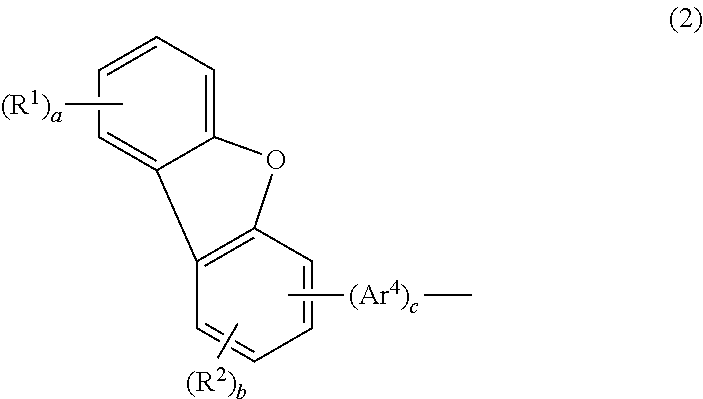
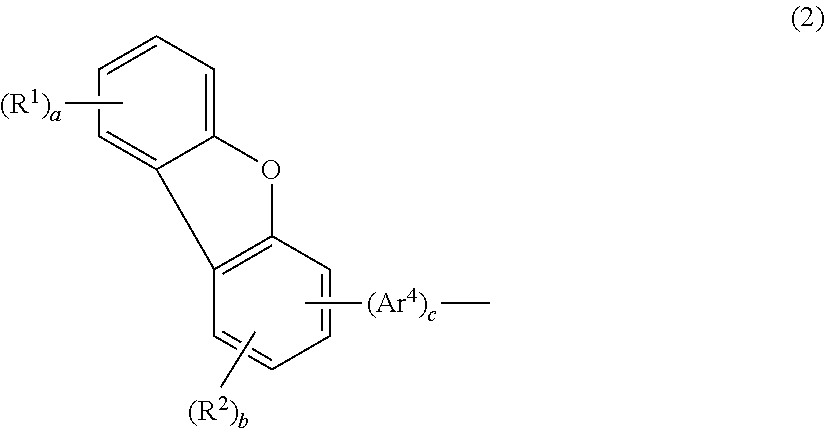
Aromatic amine derivative and organic electroluminescence device
InactiveUS20100001636A1Improves yield upon productionExtended service lifeOrganic chemistryDischarge tube luminescnet screensOrganic filmDibenzofuran
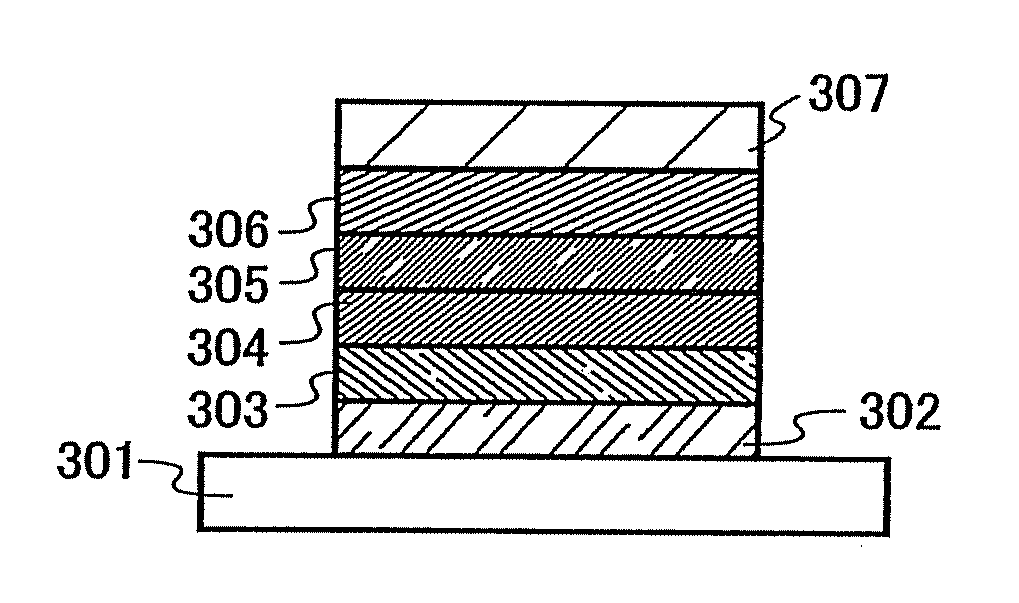
An aromatic amine derivative having a specific structure. An organic electroluminescence device which is composed of one or more organic thin film layers sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers, especially a hole transporting layer, contains the aromatic amine derivative. The aromatic amine derivative has at least one substituted or unsubstituted dibenzofuran skeleton and at least one substituted or unsubstituted terphenylene skeleton. Because the molecules in the aromatic amine derivative hardly crystallize, organic electroluminescence devices improving their production yield and having prolonged lifetime are provided.
Owner:IDEMITSU KOSAN CO LTD
Electrolytic solution for non-aqueous type battery and non-aqueous type secondary battery
InactiveUS20030118912A1Excellent in high-temperature storage characteristicReduced responseCell electrodesOrganic electrolyte cellsNon aqueous electrolytesTerphenyl
In a rechargeable non-aqueous electrolyte secondary battery using positive electrodes, negative electrodes and a non-aqueous electrolytic solution, additives to the electrolytic solution are used in combination, preferably in combination of at least two compounds selected from o-terphenyl, triphenylene, cyclohexylbenzene and biphenyl, and thus there are provided batteries excellent in safety and storage characteristics.
Owner:PANASONIC CORP +1
Organic compositions
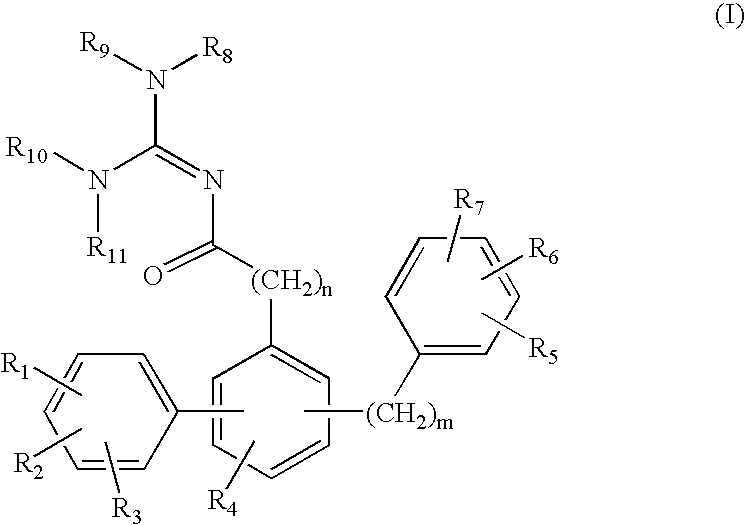
Compositions and methods of forming and using those compositions are provided herein where the composition comprises at least one oligomer or polymer of Formula I wherein E is a cage compound; each Q is the same or different and selected from aryl, branched aryl, and substituted aryl wherein the substituents include hydrogen, halogen, alkyl, aryl, substituted aryl, heteroaryl, aryl ether, alkenyl, alkynyl, alkoxyl, hydroxyalkyl, hydroxyaryl, hydroxyalkenyl, hydroxyalkynyl, hydroxyl, or carboxyl; A is substituted or unsubstituted aryl with substituted or unsubstituted arylalkynyl group (substituents include hydrogen, halogen, alkyl, phenyl or substituted aryl; and aryl includes phenyl, biphenyl, naphthyl, terphenyl, anthracenyl, polyphenylene, polyphenylene ether, or substituted aryl); h is from 0 to 10; i is from 0 to 10; j is from 0 to 10; and w is 0 or 1.
Owner:HONEYWELL INT INC
Liquid crystalline polyester composition
InactiveUS7824572B2High heat resistanceReduce anisotropyLiquid crystal compositionsSynthetic resin layered productsPolyesterLiquid crystalline
The present invention provides a liquid crystalline polyester composition comprising a terphenyl and a liquid crystalline polyester, wherein the terphenyl substantially consists of p-terphenyl, and the p-terphenyl is contained in the composition in the amount of 0.3 to 15 parts by weight on the basis of 100 parts by weight of the liquid crystalline polyester. The liquid crystalline polyester composition has high flowability and low anisotropy, which can also suppress generation of gases when molded.
Owner:SUMITOMO CHEM CO LTD
Aromatic triamine compound and organic electroluminescence device using the same
ActiveUS20060232198A1Excellent hole injectionHigh luminous efficiencyOrganic chemistryDischarge tube luminescnet screensCompound (substance)Organic electroluminescence
Provided are an aromatic triamine compound of a specific structure having at least one terphenyl structure and an organic electroluminescence device in which an organic thin film layer comprising a single layer or plural layers having at least a luminescent layer is interposed between a cathode and an anode, wherein at least one layer of the above organic thin film layers contains the aromatic triamine compound described above in the form of a single component or a mixed component, and provided are the organic electroluminescence device having a high luminous efficiency and a long life and the novel aromatic triamine compound for materializing the same.
Owner:IDEMITSU KOSAN CO LTD
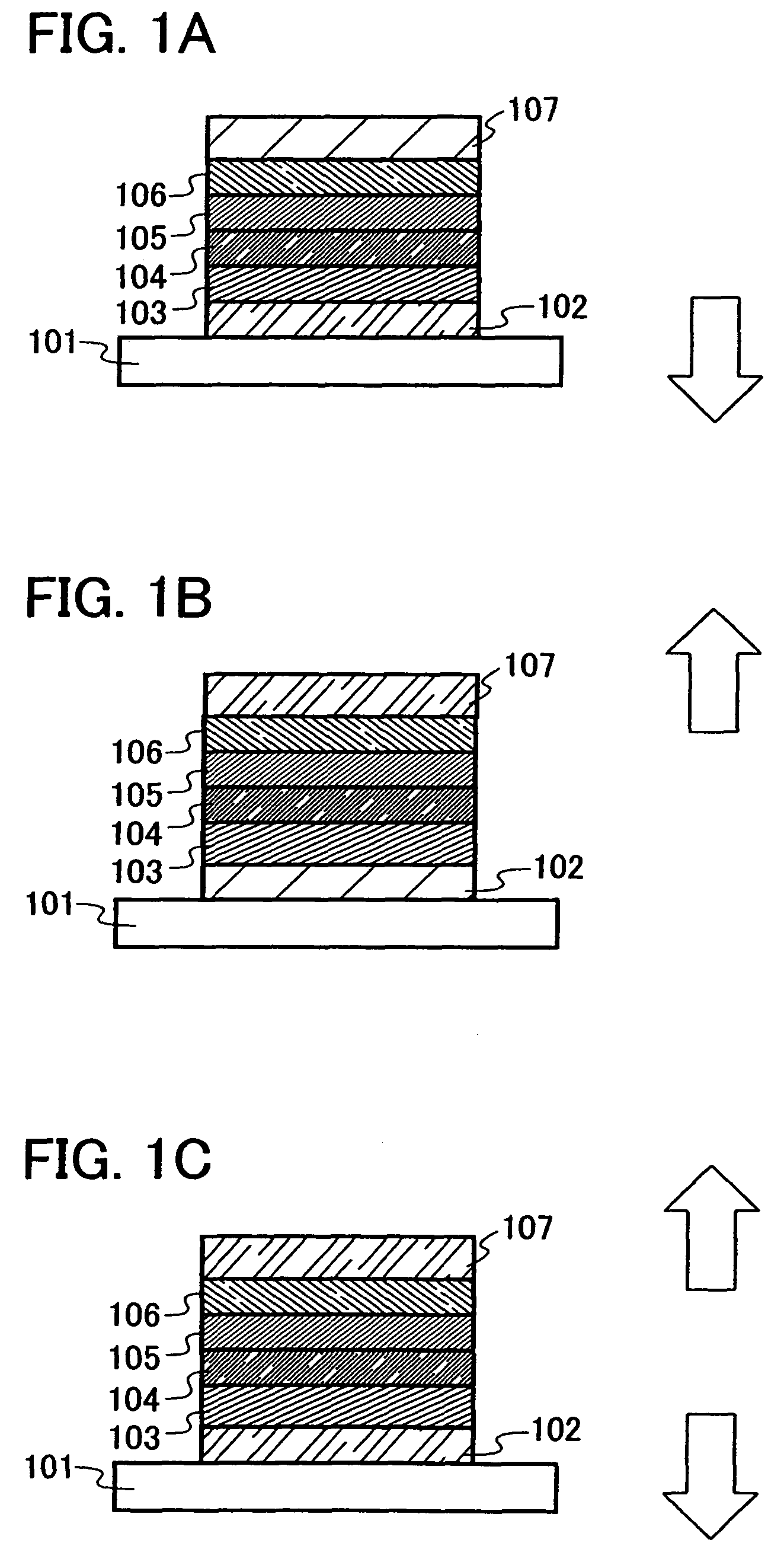
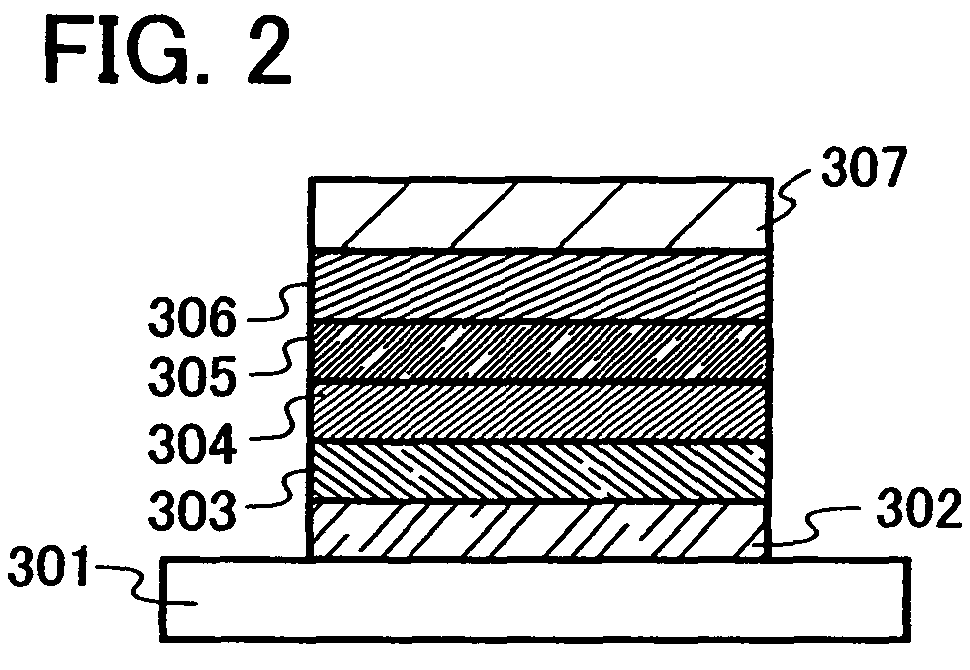
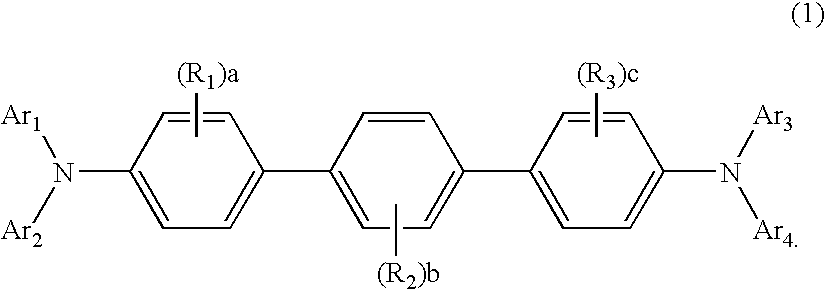
Quinoxaline Derivative, and Light-Emitting Element, Light-Emitting Device, and Electronic Appliance Using the Same
InactiveUS20110186825A1Improve heat resistanceReduce power consumptionOrganic chemistryElectroluminescent light sourcesQuinoxalineAryl
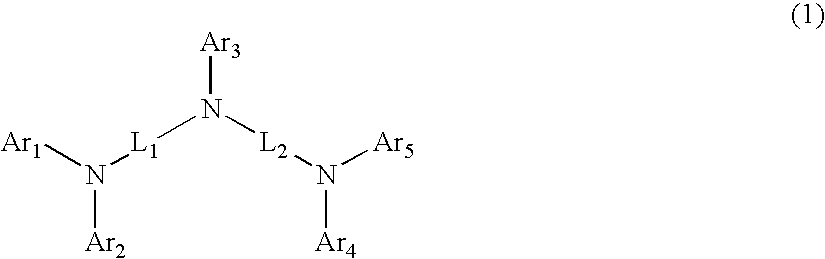
A quinoxaline derivative expressed by the general formula (1) is provided. (Each of R1 to R12 represents one of a hydrogen atom, a halogen atom, an alkyl group, an alkoxyl group, an acyl group, a dialkyl amino group, a diarylamino group, a substituted or unsubstituted vinyl group, a substituted or unsubstituted aryl group, and a substituted or unsubstituted heterocycle group. Ar1 represents one of a substituted or unsubstituted biphenyl group and a substituted or unsubstituted terphenyl group, and Ar2 represents one of a substituted or unsubstituted phenyl group, a substituted or unsubstituted biphenyl group, a substituted or unsubstituted terphenyl group, and a substituted or unsubstituted monocyclic heterocycle group.)
Owner:SEMICON ENERGY LAB CO LTD
Metal organic framework material as well as preparation method and application thereof
ActiveCN107774234AImprove skeletal stabilitySkeleton structure is stableGas treatmentMethane captureBenzeneMetal-organic framework
The invention discloses a metal organic framework material as well as a preparation method and application thereof. The chemical molecular formula of the metal organic framework material is {[M3(P)3(Q)2].5DMF.2H2O}n, wherein M is one or more metal cations selected from the group consisting of Fe<2+>, Co<2+>, Ni<2+>, Cu<2+>, and Zn<2+>; P is an organic ligand 2',3'-dimethyl-p-terphenyl-4,4''-dicarboxylic acid; Q is one or more auxiliary organic ligands selected from the group consisting of 4,4'-dipyridyl, 2-phenylpyridyl, 4-phenylpyridinium, and 1,4-di(p-pyridyl)benzene; DMF is (i)N, N'( / i)-dimethylformamide. The metal organic framework material provided by the invention has good water resistance and thermal stability resistance; due to the presence of 2', 3'-dimethyl group, the process ofan interpenetrating network is restricted to a certain degree, and the excessive orifices in the metal organic framework material are narrowed.
Owner:CHINA PETROLEUM & CHEM CORP +1
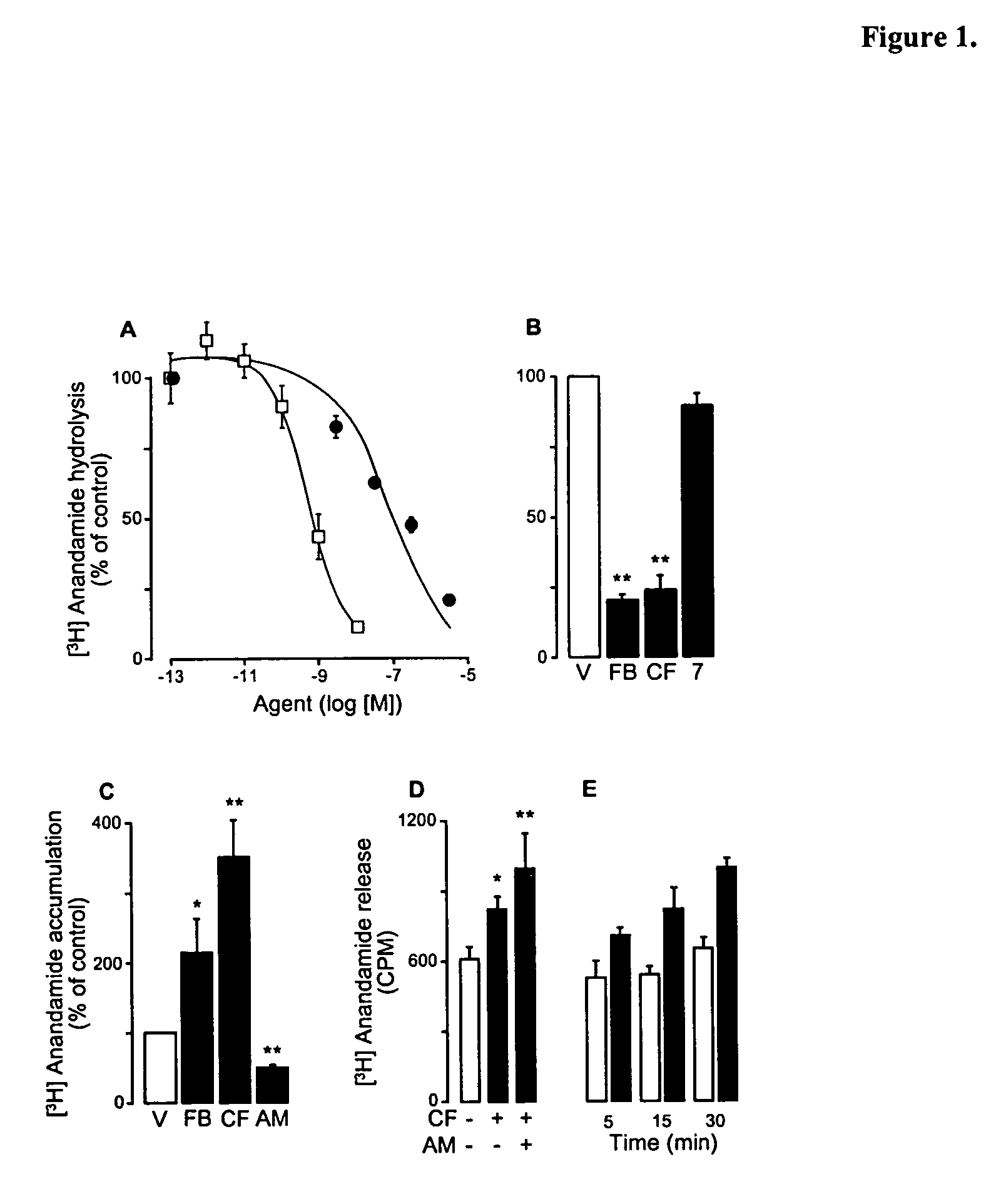
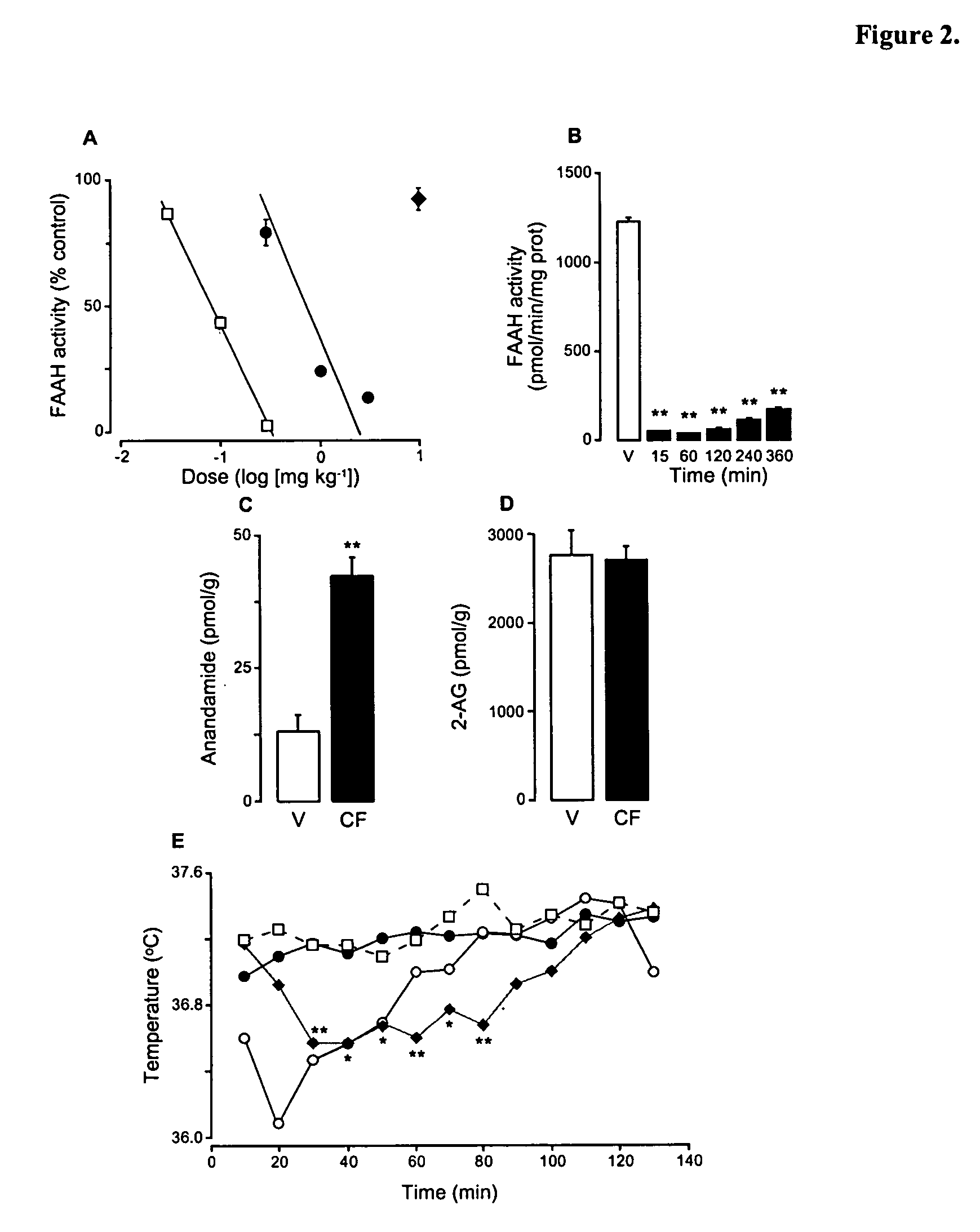
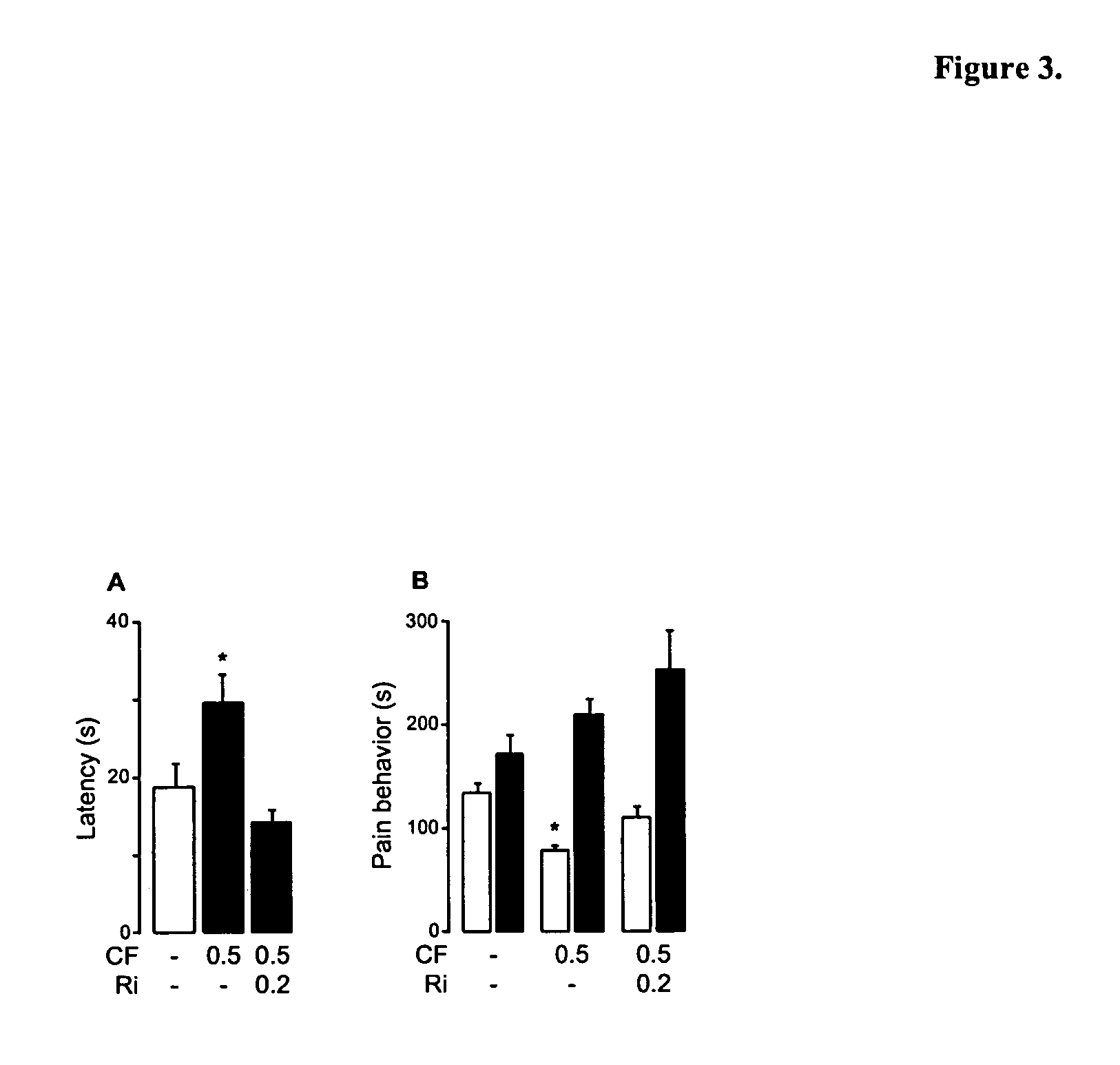
Modulation of anxiety through blockade of anandamide hydrolysis
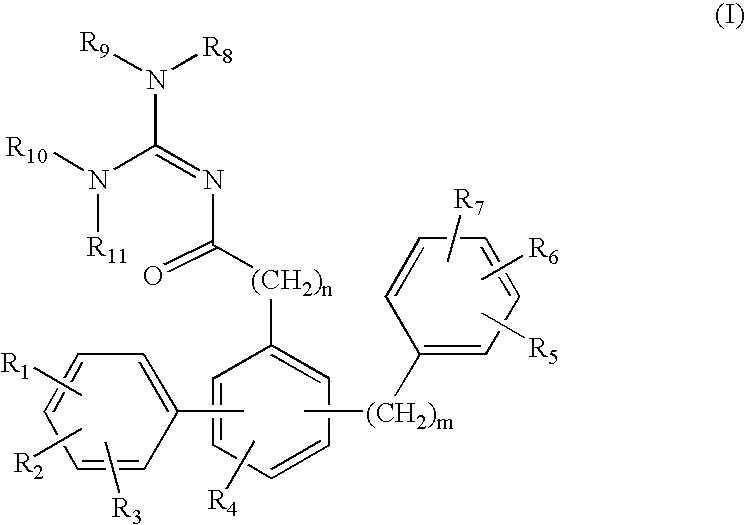
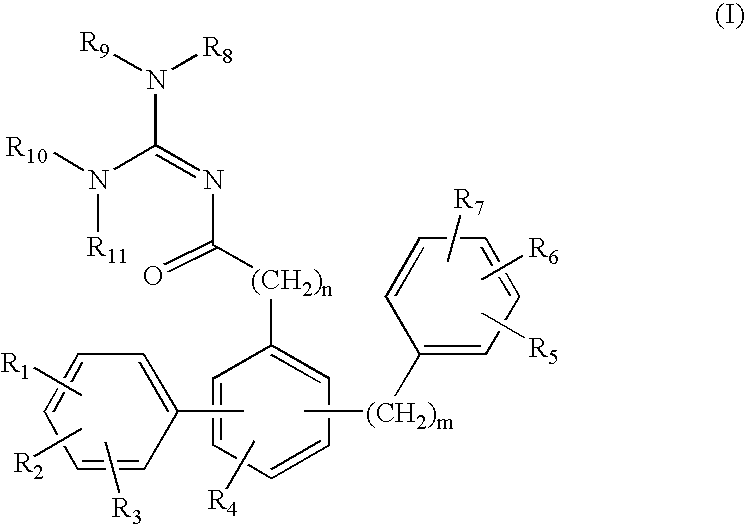
Fatty acid amide hydrolase inhibitors of the Formula:are provided wherein X is NH, CH2, O, or S; Q is O or S; Z is O or N; R is an aromatic moiety selected from the group consisting of substituted or unsubstituted aryl; substituted or unsubstituted biphenylyl, substituted or unsubstituted naphthyl, and substituted or unsubstituted phenyl; substituted or unsubstituted terphenylyl; substituted or unsubstituted cycloalkyl, heteroaryl, or alkyl; and R1 and R2 are independently selected from the group consisting of H, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, and substituted or unsubstituted phenyl, substituted or unsubstituted biphenylyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl; with the proviso that if Z is O, one of R1 and R2 is absent, and that if Z is N, optionally R1 and R2 may optionally be taken together to form a substituted or unsubstituted N-heterocycle or substituted or unsubstituted heteroaryl with the N atom to which they are each attached. Pharmaceutical compositions comprising the compounds of Formula I and methods of using them to inhibit FAAH and / or treat appetite disorders, glaucoma, pain, insomnia, and neurological and psychological disorders including anxiety disorders, epilepsy, and depression are provided.
Owner:UNIV DEGLI STUDI DI PARMA +1
Organic element for electroluminescent devices
ActiveUS7090930B2Solid-state devicesSemiconductor/solid-state device manufacturingHost materialPhenyl group
Disclosed is a useful electroluminescent device comprising a cathode, an anode, and therebetween a light emitting layer containing a host material and a phosphorescent light-emitting material wherein the host material is represented by formula (1):X′-A-X″ (1)wherein:A is selected from the group consisting of an unsubstituted phenylene ring, a biphenylene group, a terphenylene group, a naphthylene group, and a fluorene group; andeach of X′ and X″ is an independently selected aromatic group bearing an ortho aromatic substituent.
Owner:GLOBAL OLED TECH
Quinoxaline derivative, and light emitting element, light emitting device, and electronic appliance using the same
InactiveUS20070059553A1Improve heat resistanceReduce power consumptionOrganic chemistryDischarge tube luminescnet screensQuinoxalineAryl
A quinoxaline derivative expressed by the general formula (1) is provided. (Each of R1 to R12 represents one of a hydrogen atom, a halogen atom, an alkyl group, an alkoxyl group, an acyl group, a dialkyl amino group, a diarylamino group, a substituted or unsubstituted vinyl group, a substituted or unsubstituted aryl group, and a substituted or unsubstituted heterocycle group. Ar1 represents one of a substituted or unsubstituted biphenyl group and a substituted or unsubstituted terphenyl group, and Ar2 represents one of a substituted or unsubstituted phenyl group, a substituted or unsubstituted biphenyl group, a substituted or unsubstituted terphenyl group, and a substituted or unsubstituted monocyclic heterocycle group.)
Owner:SEMICON ENERGY LAB CO LTD
Anhydrous stemming for blast furnace tapping hole
The invention discloses anhydrous stemming for a blast furnace tapping hole, belonging to the technical field of refractory material for blast furnaces. The anhydrous stemming for a blast furnace tapping hole disclosed by the invention is composed of refractory aggregate and bonding agent, wherein the refractory aggregate comprises the following components in parts by mass: 12-14 parts of titanium dioxide, 3-5 parts of titanium carbide, 20-25 parts of corundum, 15-18 parts of silicon carbide, 30-38 parts of mullite, 5-7 parts of calcium-free chromium slag, 6-8 parts of blast furnace gas ash, and 9-11 parts of coke; and the bonding agent comprises the following components in parts by mass: 40-50 parts of asphalt, 4-6 parts of hydrogenated terphenyl, 10-12 parts of organic silicon resin, 8-11 parts of furan resin, and 11-15 parts of melamine-formaldehyde resin, wherein the mass ratio of the refractory aggregate to the bonding agent is 100:13-15. The anhydrous stemming disclosed by the invention has good opening performance, moderate plasticity, can resist scouring and erosion of high-temperature iron slag, does not pollute the environment, and is low in production cost.
Owner:ANHUI SAFE ELECTRONICS
Quinoxaline derivative, and light-emitting element, light-emitting device, and electronic device including quinoxaline derivative
InactiveUS8178216B2Improve balanceReduce the driving voltageOrganic chemistryDischarge tube luminescnet screensQuinoxalineHydrogen atom
An object of the present invention is to provide a quinoxaline derivative represented by a general formula (1). Ar1 represents one of a substituted or unsubstituted biphenyl group, and a substituted or unsubstituted terphenyl group; Ar2 represents one of a substituted or unsubstituted phenyl group, a substituted or unsubstituted biphenyl group, and a substituted or unsubstituted terphenyl group; α1 represents an arylene group having 6 to 25 carbon atoms; and R11 to R15 are the same or different from one another, and each of them represents any one of a hydrogen atom, an alkyl group having 1 to 4 carbon atoms, and a substituted or unsubstituted aryl group having 6 to 25 carbon atoms.
Owner:SEMICON ENERGY LAB CO LTD
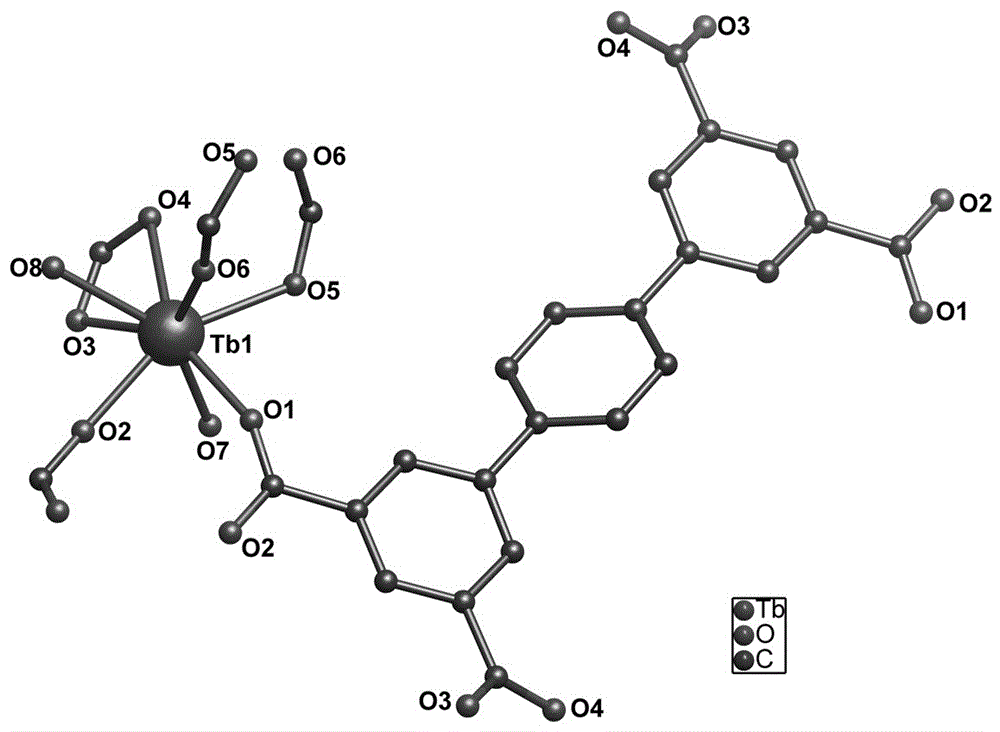
Terbium-based metal organic framework material, preparation method and application of terbium-based metal organic framework material
ActiveCN106279223AEasy temperature controlThe synthesis method is simpleOrganic compound preparationGroup 3/13 organic compounds without C-metal linkagesRare-earth elementDichromate ion
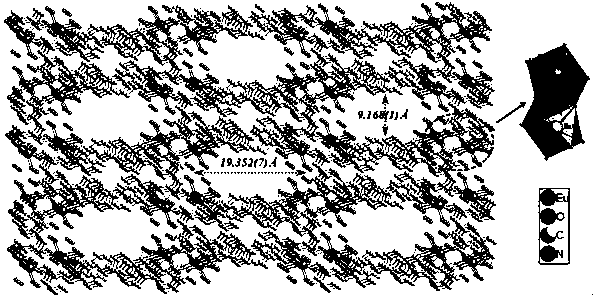
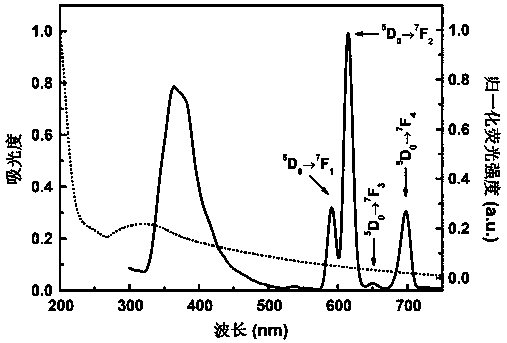
The invention belongs to the field of fluorescence detection materials, and particularly relates to a preparation method of a rare earth terbium-based metal organic framework material and study on the performance of selectively recognizing heavy chromate ions in water. By selecting rare earth terbium emitting green light as metal central ions and selecting p-terphenyl-3,3'',5,5''-tetramethyl acid as organic sensitized ligands, a rare earth porous metal organic framework compound is synthesized under the hydrothermal condition. The compound causes the most obvious fluorescence quenching phenomenon to Cr2O72- anions in different anion solutions, and thus the compound can serve as a novel fluorescence probe for detecting Cr2O72- anions.
Owner:CHINA THREE GORGES UNIV
Preparing organic light emitting film by electrochemical deposition and use in electroluminescence device
InactiveCN1822410AEasy to control area sizeEasy to patternSolid-state devicesSemiconductor/solid-state device manufacturingIridiumRhenium
Present invention relates to an organic light-emitting film electrochemical deposition preparation method and application in preparing electroluminescence device, belonging to organic luminescence field. Said Light-emitting film is formed by electrochemical deposition in electrolytic bath, the monomer for electrochemical polymerization formed by chemical bond connecting electricity activity unit and luminescence unit, wherein electricity activity unit consisting of carbazole, thiophene, pyrrole, ethene, ethyne or phenylamine, diphenylamine, and triphenylamine, luminescence unit being cinnamic dimer, tripolymer, tetramer or terphenyl, quaterphenyl, quinquephenyl, tetracene, pentacene, fluorene dimer, tripolymer or tetramer and terpyridyl (dipyridyl ) rhenium etc, prepared electroluminescence device having simple technology, high device luminous efficiency, easy control area adjustable luminous color etc advantages.
Owner:JILIN UNIV
Organic Semiconducting Material and Electronic Component
ActiveUS20130193414A1Reduce absorptionImprove conductivityElectroluminescent light sourcesSynthetic resin layered productsOrganic semiconductorElectronic component
Organic semiconducting material comprising at least one matrix material and at least one doping material, wherein the doping material is selected from a [3]radialene compound, and wherein the matrix material is selected from a terphenyldiamine compound, as well as an organic component and a mixture for producing a doped semiconductor layer.
Owner:NOVALED GMBH +1
Quinoxaline derivative, and light emitting element, light emitting device, and electronic appliance using the same
InactiveUS7901792B2Improve heat resistanceReduce power consumptionOrganic chemistryElectroluminescent light sourcesQuinoxalineAryl
A quinoxaline derivative expressed by the general formula (1) is provided. (Each of R1 to R12 represents one of a hydrogen atom, a halogen atom, an alkyl group, an alkoxyl group, an acyl group, a dialkyl amino group, a diarylamino group, a substituted or unsubstituted vinyl group, a substituted or unsubstituted aryl group, and a substituted or unsubstituted heterocycle group. Ar1 represents one of a substituted or unsubstituted biphenyl group and a substituted or unsubstituted terphenyl group, and Ar2 represents one of a substituted or unsubstituted phenyl group, a substituted or unsubstituted biphenyl group, a substituted or unsubstituted terphenyl group, and a substituted or unsubstituted monocyclic heterocycle group.)
Owner:SEMICON ENERGY LAB CO LTD
Aromatic amine derivative and organic electroluminescence device using the same
InactiveUS20070296331A1Trend downAdded fabricationOrganic chemistryDischarge tube luminescnet screensOrganic electroluminescenceAromatic amine
A novel aromatic amine derivative which is a diamine compound having a p-terphenyl-4,4″-diyl structure. An organic electroluminescence device which is composed of one or more organic thin film layers including at least one light emitting layer sandwiched between a cathode and an anode. Since at least one of the organic thin film layers contains the aromatic amine derivative singly or combination of two or more, the tendency of molecular crystallization is reduced and the yield in the fabrication of electroluminescence devices is enhanced. The aromatic amine derivative enables the production of electroluminescence devices having a prolonged lifetime and a high efficiency even after storing at high temperatures.
Owner:IDEMITSU KOSAN CO LTD
Reagent for lithiation reaction, preparation method and use thereof
InactiveCN1690694AHigh reactivityPromote reductionAnalysis by subjecting material to chemical reactionInorganic compoundSolvent
The present invention relates to reagent for lithiation reaction. On the condition of room temperature and inert atmosphere, mix lithium, organic compound contained benzene ring and ethers compound, solve and obtain solution; wherein, lithium 0.01~10M / L, organic compound contained benzene ring 0.01~5M / L, other is solvent; the general form is R1OR2 or ethers compound with R1OR2OR3; wherein, R1, R2, R3 are alkyl of C1í½C5; organic compound contained benzene ring aforementioned comprises biphenyl or condensed-nuclei aromatics, optimization of biphenyl and its derivant, terphenyl, quaohenyl, naphthalin, anthracene, and phenanthrene. The lithiation reagent is easy for preparation, storage, cheap of cost and high activity; not only for common reaction, but also can be used to preprocess of electrode material to store lithium cell or prepare new inorganic compound contained lithium; has wide scope of usage.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Aromatic amine derivative and organic electroluminescence device
ActiveUS20140061630A1Improves yield upon productionExtended service lifeOrganic chemistrySolid-state devicesOrganic filmDibenzofuran
An aromatic amine derivative having a specific structure. An organic electroluminescence device which is composed of one or more organic thin film layers sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers, especially a hole transporting layer, contains the aromatic amine derivative. The aromatic amine derivative has at least one substituted or unsubstituted dibenzofuran skeleton and at least one substituted or unsubstituted terphenylene skeleton. Because the molecules in the aromatic amine derivate hardly crystallize, organic electroluminescence devices improving their production yield and having prolonged lifetime are provided.
Owner:IDEMITSU KOSAN CO LTD
Aromatic amine derivative and organic electroluminescence device
ActiveUS20120161119A1Improves yield upon productionExtended service lifeOrganic chemistrySolid-state devicesOrganic filmDibenzofuran
An aromatic amine derivative having a specific structure. An organic electroluminescence device which is composed of one or more organic thin film layers sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers, especially a hole transporting layer, contains the aromatic amine derivative. The aromatic amine derivative has at least one substituted or unsubstituted dibenzofuran skeleton and at least one substituted or unsubstituted terphenylene skeleton. Because the molecules in the aromatic amine derivate hardly crystallize, organic electroluminescence devices improving their production yield and having prolonged lifetime are provided.
Owner:IDEMITSU KOSAN CO LTD
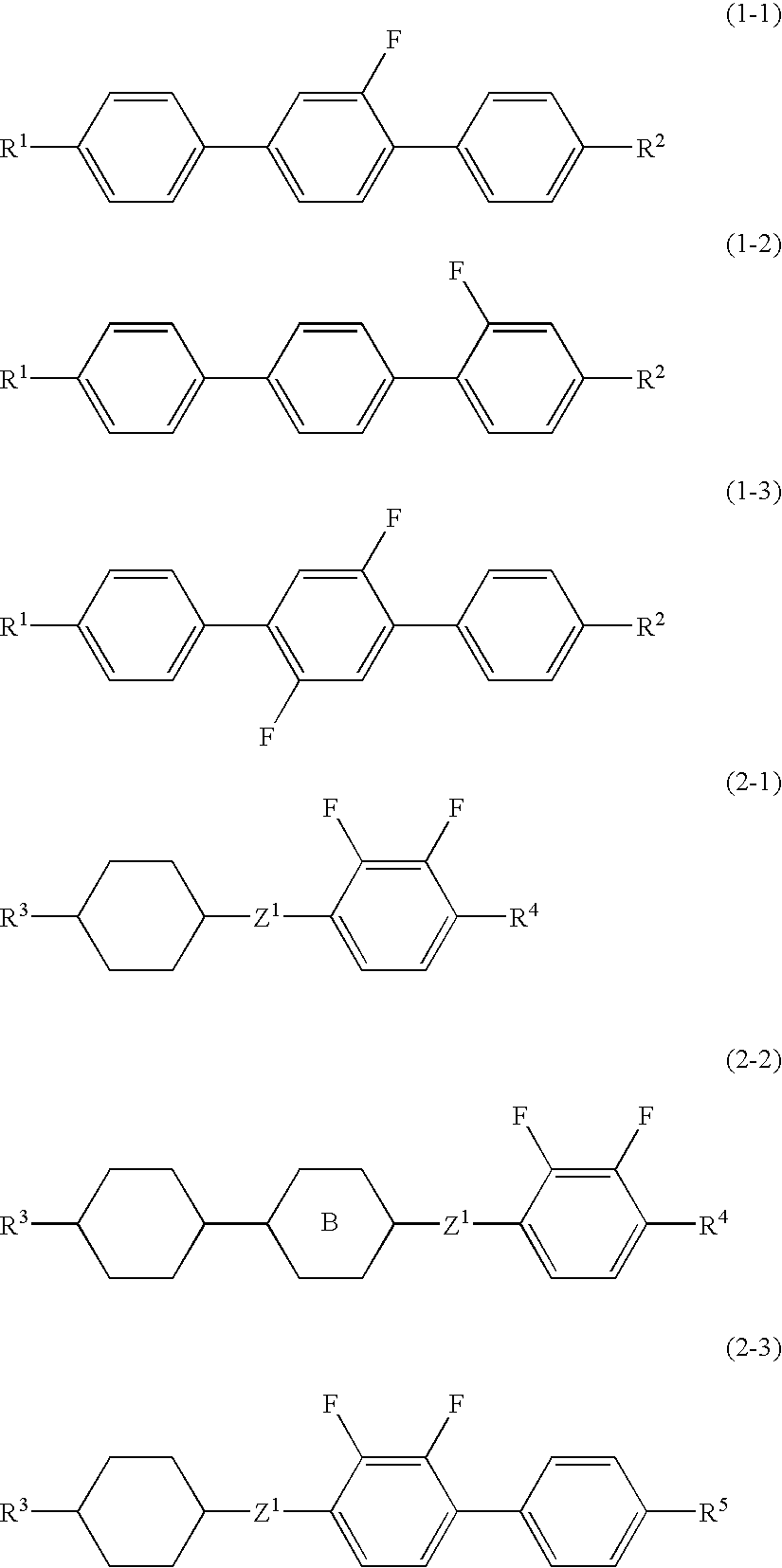
Liquid crystal composition and liquid crystal display device
InactiveUS20090090892A1High resistanceIncrease an optical anisotropyLiquid crystal compositionsThin material handlingCrystallographyDielectric anisotropy
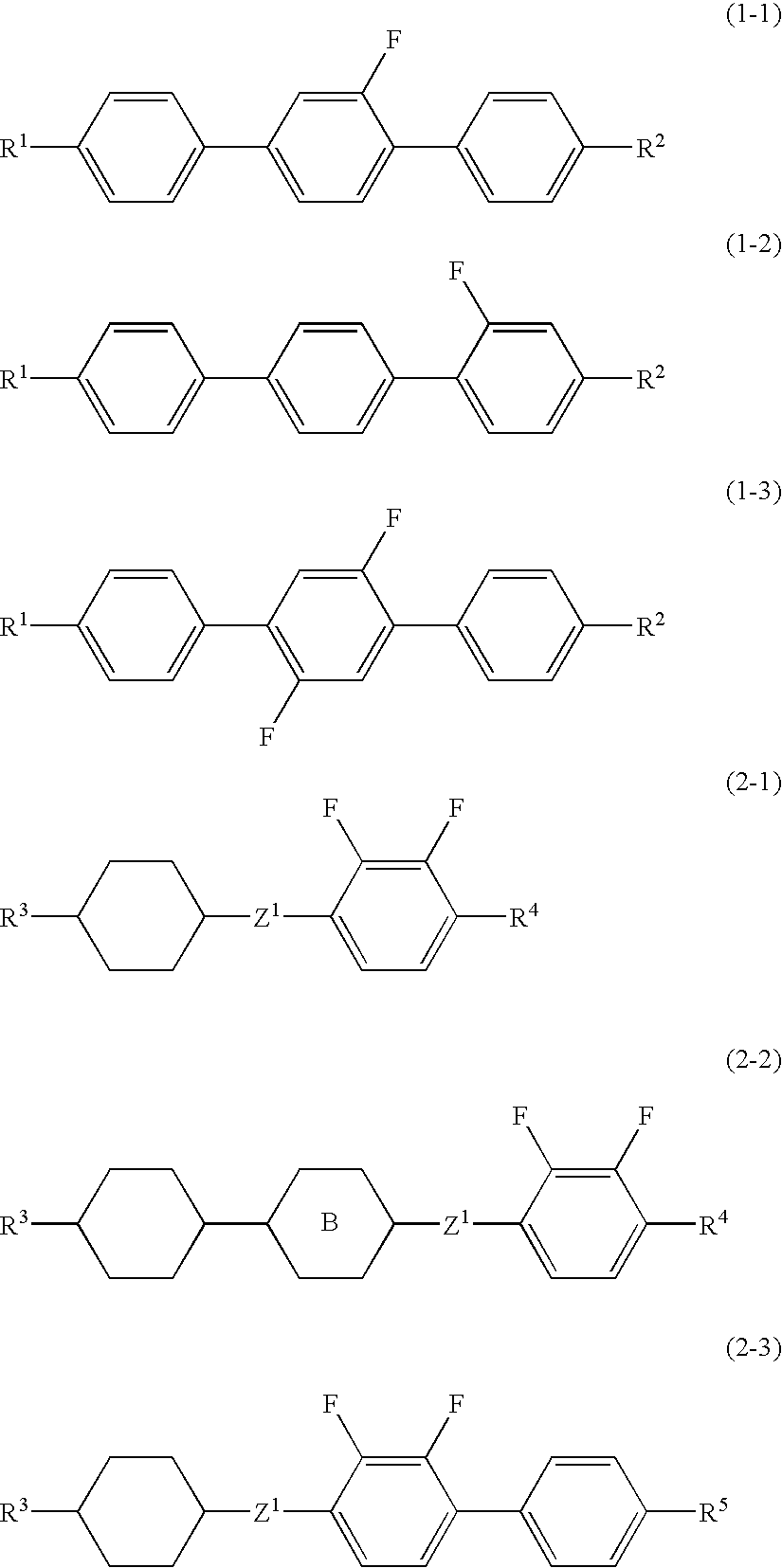
A liquid crystal composition is provided that has a wide temperature range of a nematic phase, a suitable optical anisotropy, a large negative dielectric anisotropy and a large specific resistance, and preferably a liquid crystal composition is provided that has a larger optical anisotropy and a lower minimum temperature of a nematic phase while satisfying the aforementioned characteristics. A liquid crystal display device containing the liquid crystal composition is also provided. The liquid crystal composition contains a liquid crystal compound having terphenyl, in which one or two hydrogens are replaced by fluorines, as the first component, and a liquid crystal compound having phenylene, in which two hydrogens are replaced by fluorine, as the second component, and the liquid crystal display device contains the liquid crystal composition.
Owner:JNC CORP +1
Liquid crystalline polyester composition
InactiveUS20090001317A1Suppressing blister defectImprove heat resistanceLiquid crystal compositionsThin material handlingBenzeneCrystallography
The present invention provides a liquid crystalline polyester composition comprising a terphenyl and a liquid crystalline polyester, wherein the terphenyl substantially consists of p-terphenyl, and the p-terphenyl is contained in the composition in the amount of 0.3 to 15 parts by weight on the basis of 100 parts by weight of the liquid crystalline polyester. The liquid crystalline polyester composition has high flowability and low anisotropy, which can also suppress generation of gases when molded.
Owner:SUMITOMO CHEM CO LTD
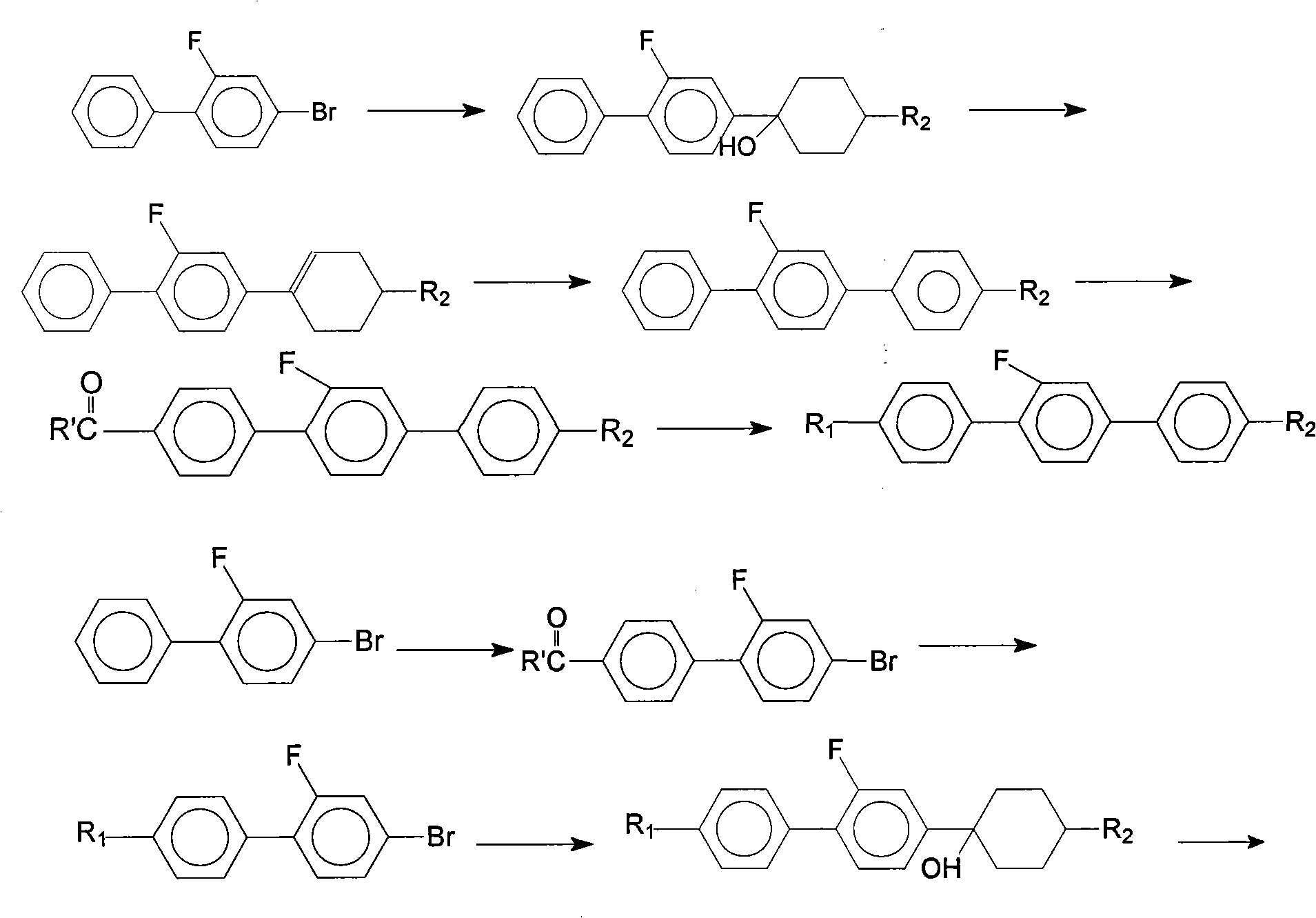
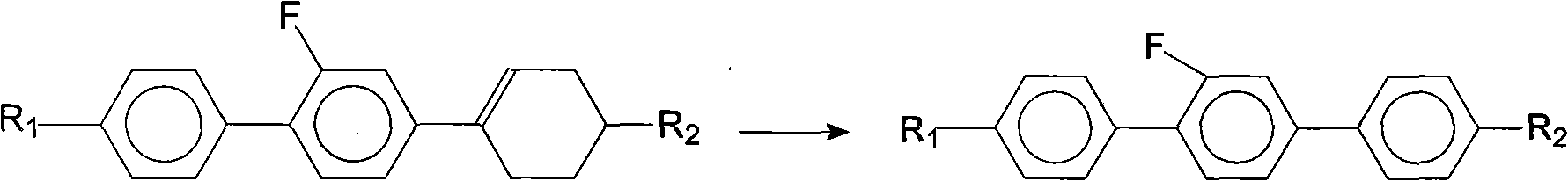
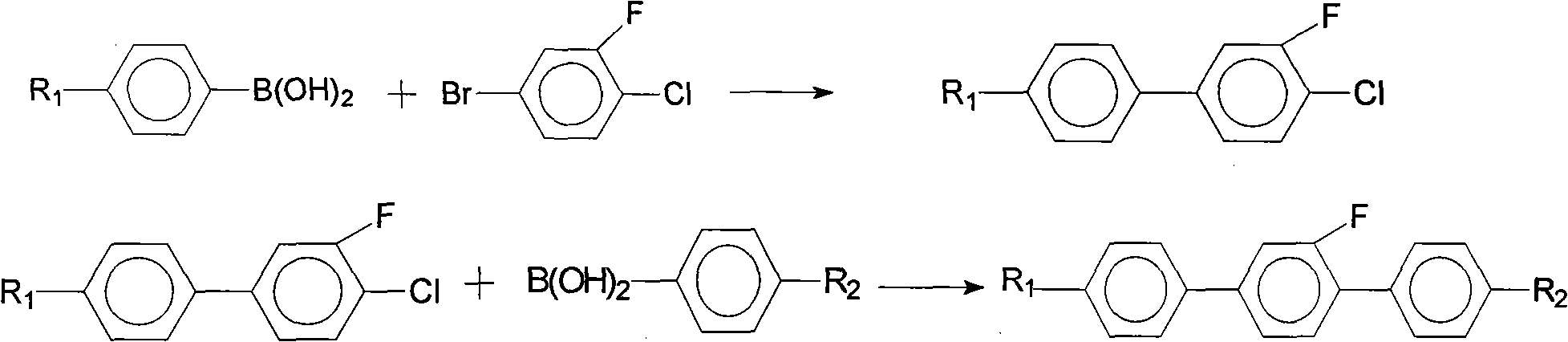
Method for synthesis of 2'-fluoro terphenyl liquid crystal
InactiveCN102399117ALow priceShort reaction pathLiquid crystal compositionsOrganic chemistry methodsBoronic acidReaction temperature
The present invention discloses a method for synthesis of 2'-fluoro terphenyl liquid crystal. With the present invention, problems of more reaction steps, low yield, heavy environmental hazard, expensive catalysts, or hazard on the human body in the prior art are solved. The technical scheme of the present invention is that: a catalyst is added to an organic solvent of the raw material to carry out a suzuki coupling reaction to realize the synthesis of the 2'-fluoro terphenyl liquid crystal. According to the present invention, 4-bromo-3-fluoro iodobenzene, a phenyl boronic acid derivative and a base are adopted as raw materials, and are subjected to two suzuki coupling reactions in the solvent under the effect of the catalyst at the reaction temperature of 70-100 DEG C to obtain the 2'-fluoro terphenyl liquid crystal, wherein the times of the two suzuki coupling reactions are respectively 4-10 hours and 10-20 hours. The method of the present invention has advantages of simple process, mild conditions, low preparation cost, high yield, environmental protection, and strong practicability.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Method for detecting phosphate ions based on dual-emission europium metal organic framework material
InactiveCN108535231AUnique Dual Fluorescent Emission PropertiesHigh selectivityFluorescence/phosphorescenceLuminescent compositionsPhosphate ionTricarboxylic acid
The invention relates to a method for detecting phosphate ions based on a dual-emission europium metal organic framework material. The dual-emission lanthanide europium metal organic framework material is used as a ratio-type fluorescence probe for being applied to high-selectivity high-sensibility detection of the phosphate ions. The chemical general formula of the adopted dual-emission lanthanide europium metal organic framework material is [Eu2(L)2(H20)2(DMF)2], wherein a Eu2O2 cluster is used as an inorganic node, a rigid asymmetric tricarboxylic acid ligand terphenyl-3,4'',5-tricarboxylicacid (H3L) is used as an organic bridging ligand, and preparation is successfully conducted by means of a simple and convenient one-pot solvothermal technology. The invention further discloses application of the metal organic framework material in the aspect of selectively detecting phosphate anions. In a detecting process, along with increase of the content of the added phosphate anions, obviouscolor change from pink to purple which is easily observed with naked eyes is presented under radiation of an ultraviolet lamp, and thus the detecting process becomes more convenient and practical.
Owner:TIANJIN NORMAL UNIVERSITY
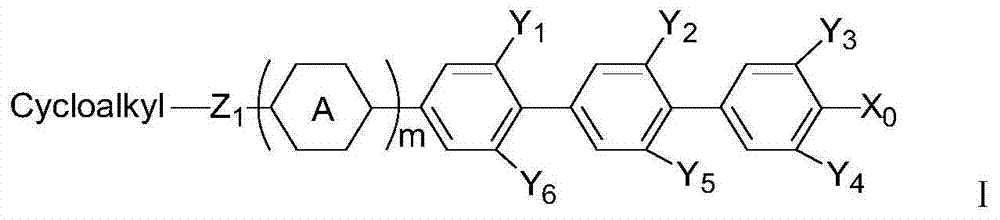
Liquid crystal medium containing triphenyl series compounds and application thereof
InactiveCN104513665AImprove performanceShort response timeLiquid crystal compositionsNon-linear opticsElectricityDielectric anisotropy
The invention relates to a liquid crystal medium containing a terphenyl compound and application thereof, and discloses a liquid crystal medium and application of the liquid crystal medium in liquid crystal display. The liquid crystal medium has positive dielectric anisotropy, and is characterized by containing one or more compounds represented by a structural formula (I) which is shown in the description. The liquid crystal medium provided by the invention is excellent in performance, has wide nematic phase temperature range, high reflective index anisotropy, extremely high electric charge retention rate and low rotary viscosity, can maintain high electric charge retention rate, low power consumption and the like, and has extremely high response speed. The composition disclosed by the invention has wide application prospect and application value in liquid crystal display, and is particularly suitable for manufacturing quick-response active matrix TN-TFT, IPS-TFT, FFS-TFT displays with great refractive index anisotropy.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Anion exchange polymer and preparation method and application thereof
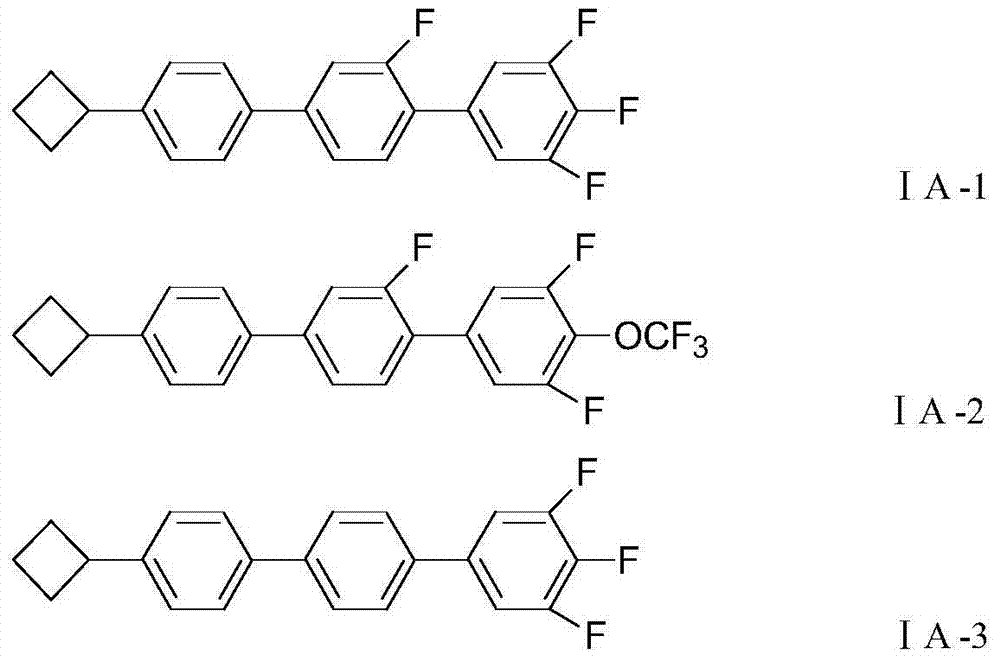
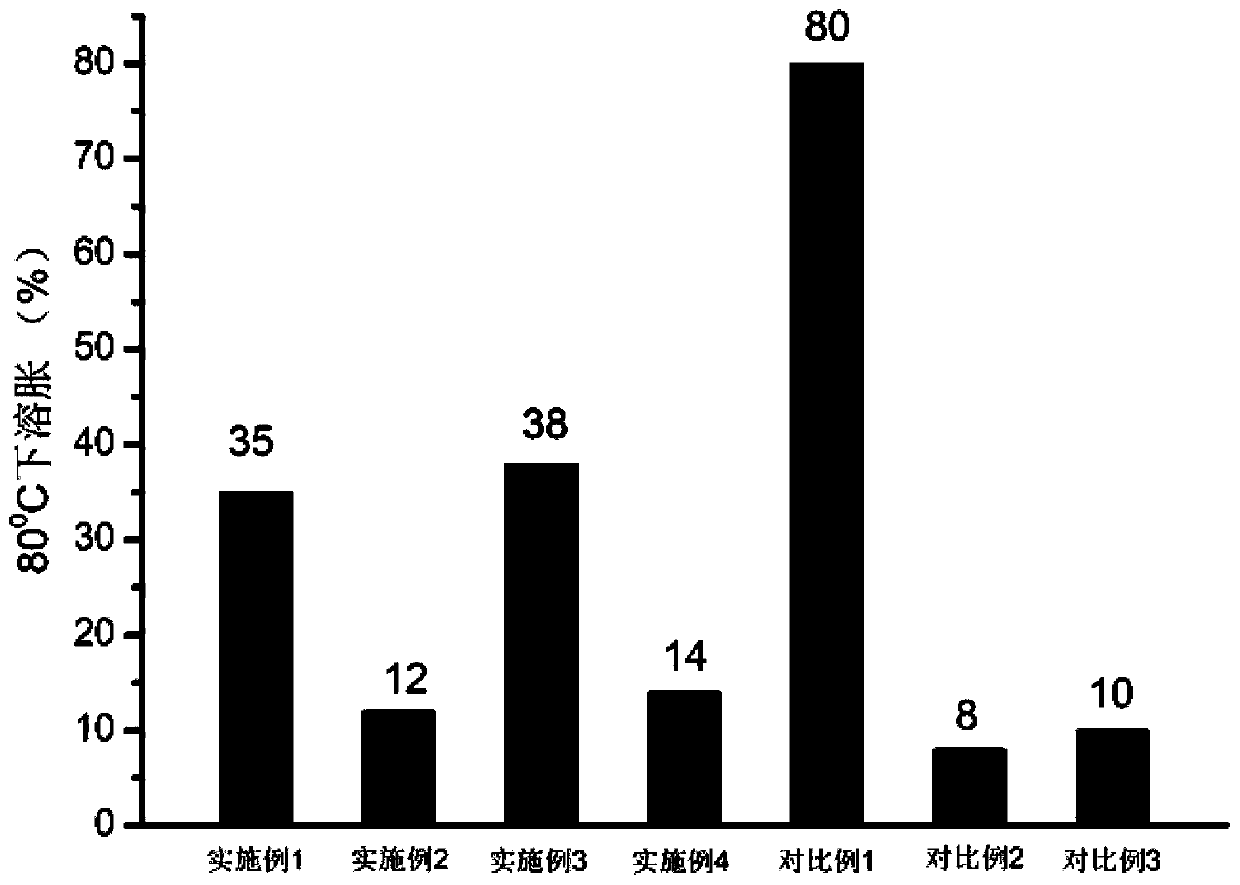
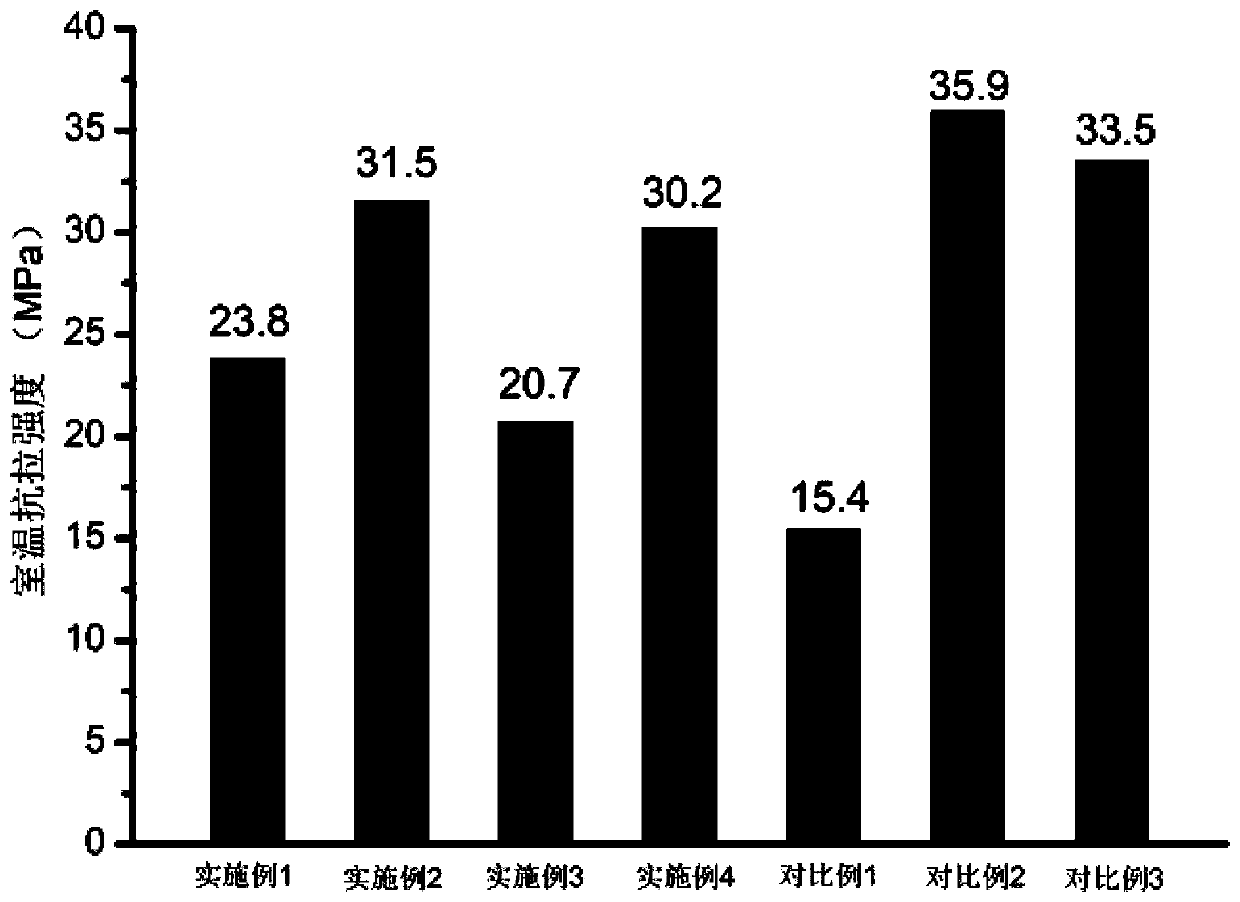
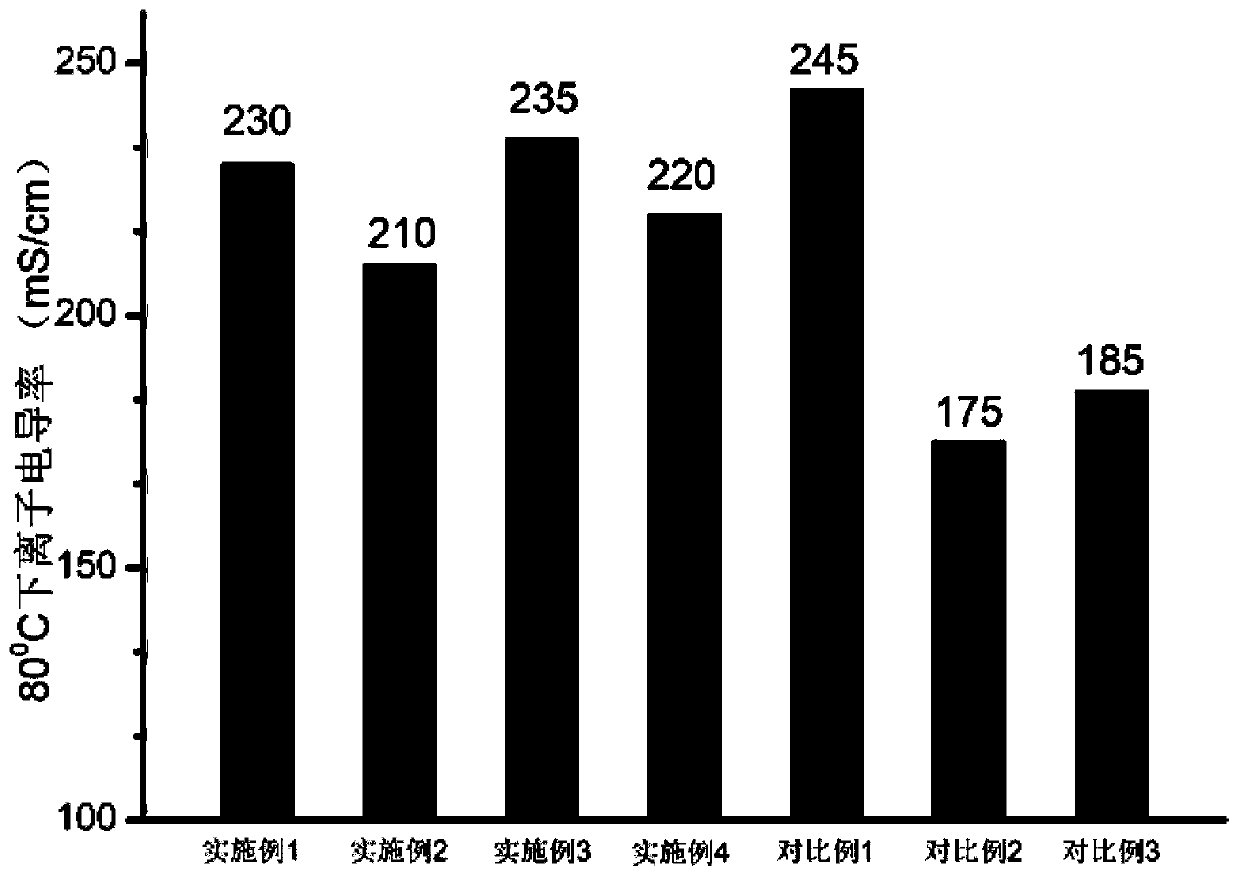
ActiveCN111040137AControllable adjustment of ionic conductivityControllable adjustable mechanical strengthQuaternary ammonium cationIon exchange
The invention provides an anion exchange polymer and a preparation method and application thereof. The anion exchange polymer comprises at least one structural unit as shown in a formula (I) and a structural unit as shown in a formula (II); the anion exchange polymer further comprises at least one structural unit as shown in a formula (III) and / or formula (IV); the main chain of the anion exchangepolymer contains biphenyl, terphenyl and piperidine quaternary ammonium cation structural units at the same time, so the anion exchange polymer has high chemical stability, excellent ionic conductivity, mechanical strength and swelling resistance, and can meet the application requirements of different electrochemical devices such as fuel cells, water electrolyzers and electrodialyzers.
Owner:惠州市亿纬新能源研究院
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com