Method for synthesis of 2'-fluoro terphenyl liquid crystal
A technology of terphenyls and liquid crystals, which is applied in the direction of liquid crystal materials, organic chemical methods, chemical instruments and methods, etc., can solve the problems of expensive catalysts, cumbersome synthesis, environmental hazards, etc., and achieve low cost, high yield and purity, and reduce pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
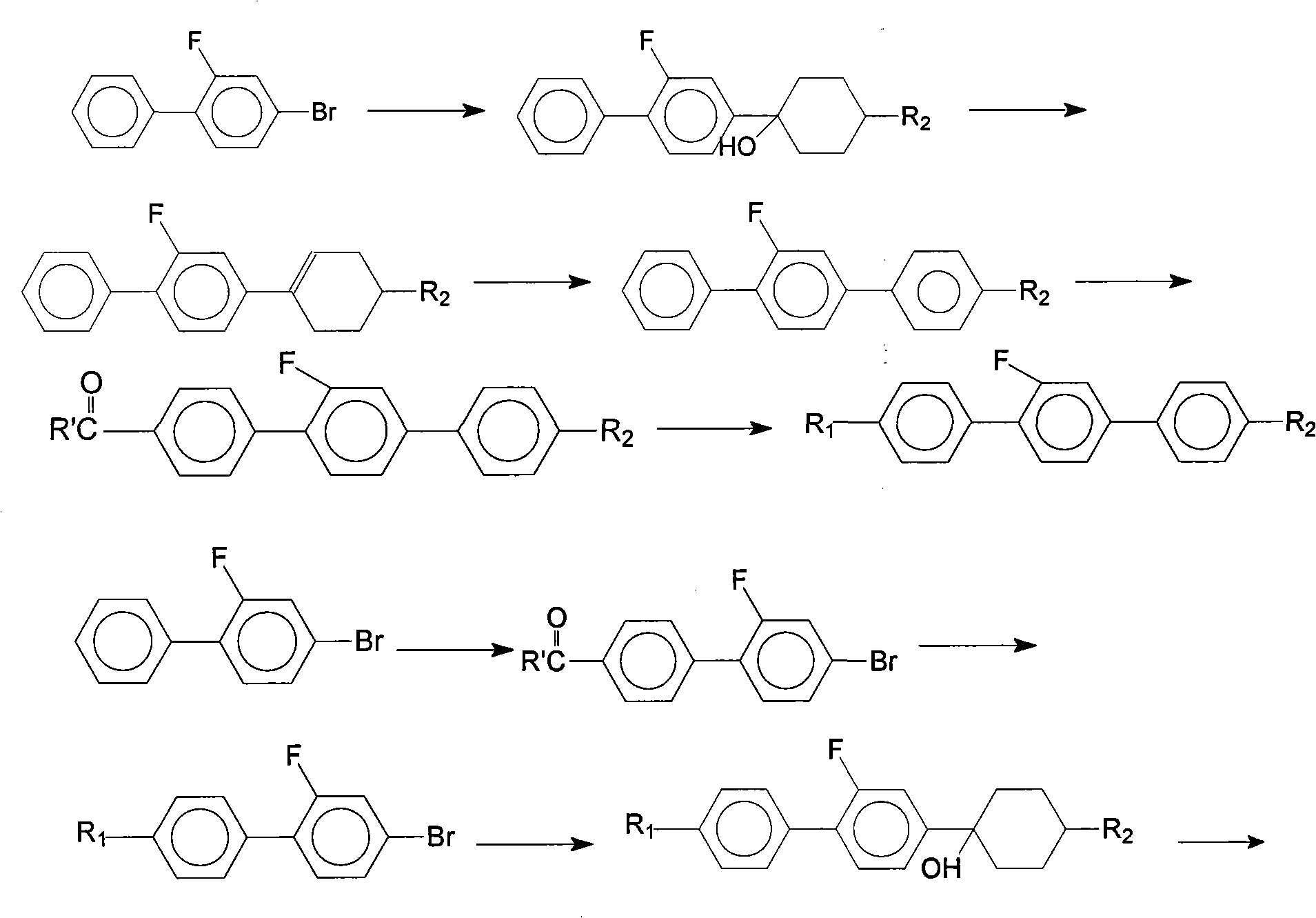
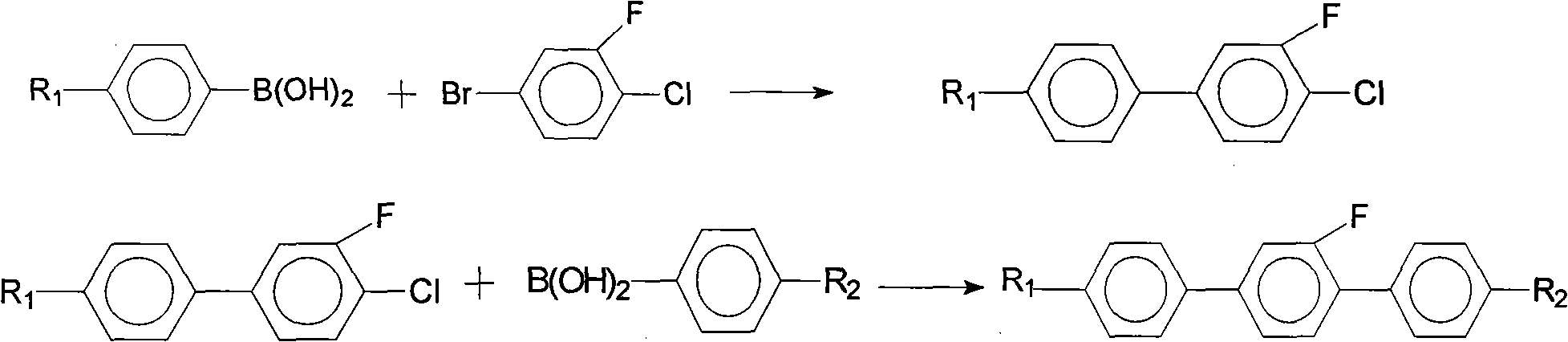
[0035] In this example, the intermediate 4'-propyl-3-fluoro-4-bromobiphenyl is reacted with 4-bromo-3-fluoroiodobenzene and propylphenylboronic acid to obtain 4-ethyl- Taking 2'-fluoro-4"-propyl (1,1',4',1") terphenyl as an example, the synthesis method of the present invention will be further described.
[0036] Step 1, the synthesis of 4'-propyl-3-fluoro-4-bromobiphenyl
[0037] Add 150.5g (0.5mol) of 4-bromo-3-fluoroiodobenzene, 138g of potassium carbonate, 86.1g (0.525mol) of propylphenylboronic acid, and 3g (0.0026mol) of tetrakis(triphenylphosphine) palladium into the three-necked flask , and added 300 g of toluene, 210 g of ethanol, and 240 g of water; then stirred evenly and heated to 70°C, keeping the temperature at 70-75°C for 6 hours. After the solution has dropped to room temperature, carry out liquid separation treatment, wash the organic phase with water until it is neutral, and distill off the toluene, collect the fraction at 150°C / 20Mpa under reduced pressure ...
Embodiment 2
[0054] Step 1, the synthesis of 4'-ethyl-3-fluoro-4-bromobiphenyl
[0055] Add 150.5g (0.5mol) of 4-bromo-3-fluoroiodobenzene, 138g of potassium carbonate, 78.8g (0.0525mol) of ethyl phenylboronic acid, 3.5g (0.003mol) of tetrakis(triphenylphosphine) palladium into the three-necked flask respectively. ), and added 300g of toluene, 210g of ethanol, and 240g of water; then stirred evenly and heated to 70°C, keeping the temperature at 70-75°C for 6 hours. After the solution was cooled to room temperature, liquid separation was performed, the organic phase was washed with water until neutral, and the toluene was evaporated, and the fraction at 145°C / 20Mp was collected under reduced pressure to obtain 4'-ethyl-3-fluoro-4-bromobi Benzene 103.2g, the yield is 74%. The gas chromatographic purity GC was analyzed to be 99%.
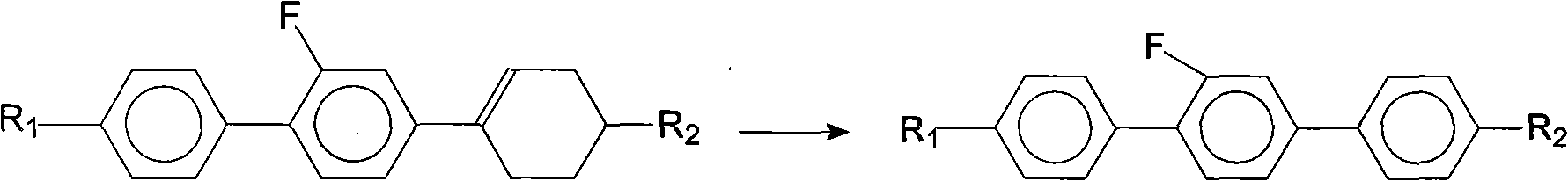
[0056] Step 2, Synthesis of 4-propyl-2'-fluoro-4"-ethyl (1,1',4',1") terphenyl
[0057]
[0058] 83.7g (0.3mol) 4'-ethyl-3-fluoro-4-bromobiphenyl, 54.1g (0.3...
Embodiment 3
[0073] Step 1, Synthesis of 4'-pentyl-3-fluoro-4-bromobiphenyl
[0074] The difference between this step of this example and step one of Example 1 is that the first phenylboronic acid is pentylphenylboronic acid, and the addition amount is 100.8g (0.525mol); in this way, 4′-pentyl-3-fluoro-4 - 115.6 g of brominated biphenyls, the yield is 72%.
[0075] The gas chromatographic purity GC was analyzed to be 99%.
[0076] Step 2, 4-ethyl-2'-fluoro-4"-pentyl (1,1',4',1") terphenyl
[0077]
[0078] 96.3g (0.3mol) 4'-pentyl-3-fluoro-4-bromobiphenyl, 49.5g (0.33mol) p-ethylphenylboronic acid, 5% Pd / C 1.5g (0.0007mol), potassium carbonate 83g , 750g of tetrahydrofuran, and 200g of water were added to a three-necked flask, heated to 70°C, refluxed for 15 hours, cooled and added with 500ml of toluene, separated, washed with water until neutral, and evaporated to clean the toluene. The product was purified by column chromatography, and recrystallized twice with 2 times of ethanol a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com