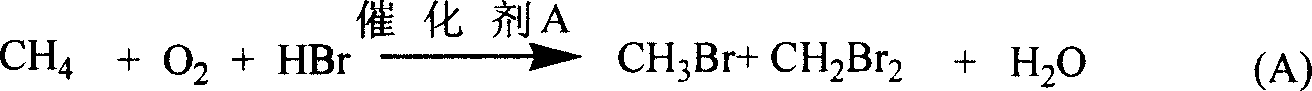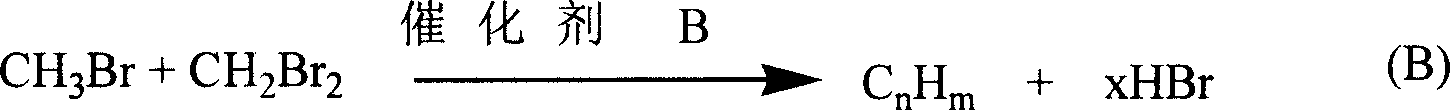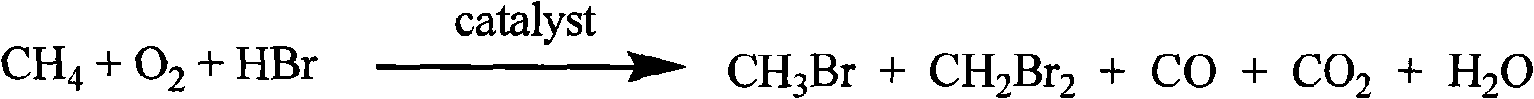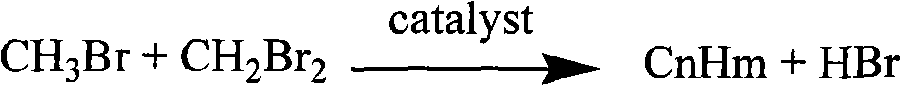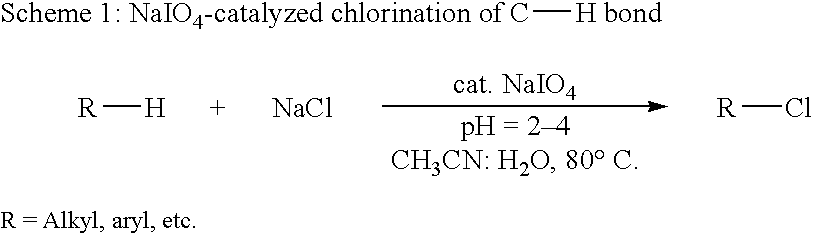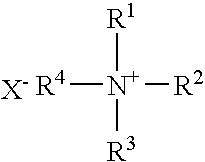Patents
Literature
5071results about "Halogenated hydrocarbon preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
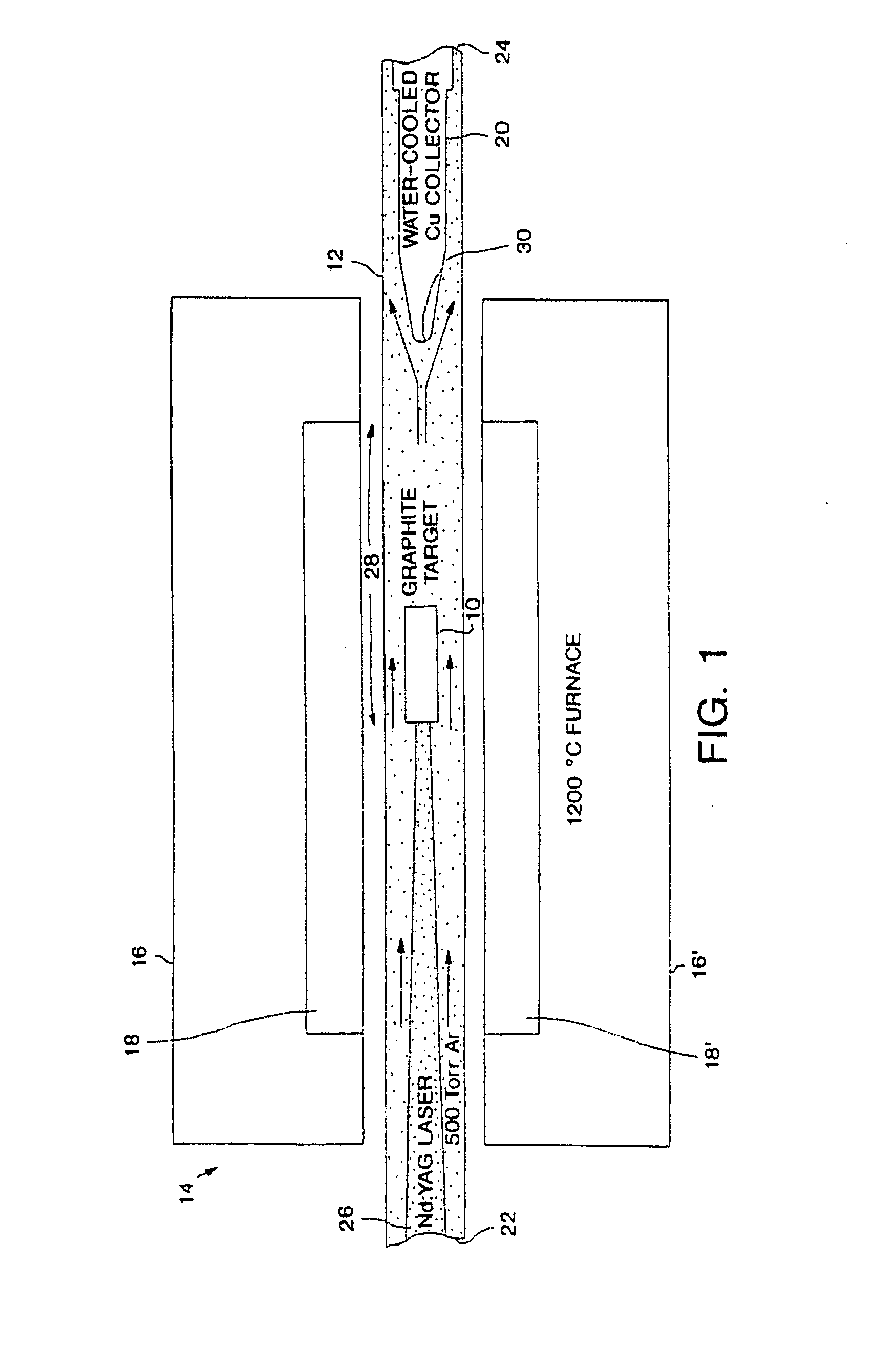
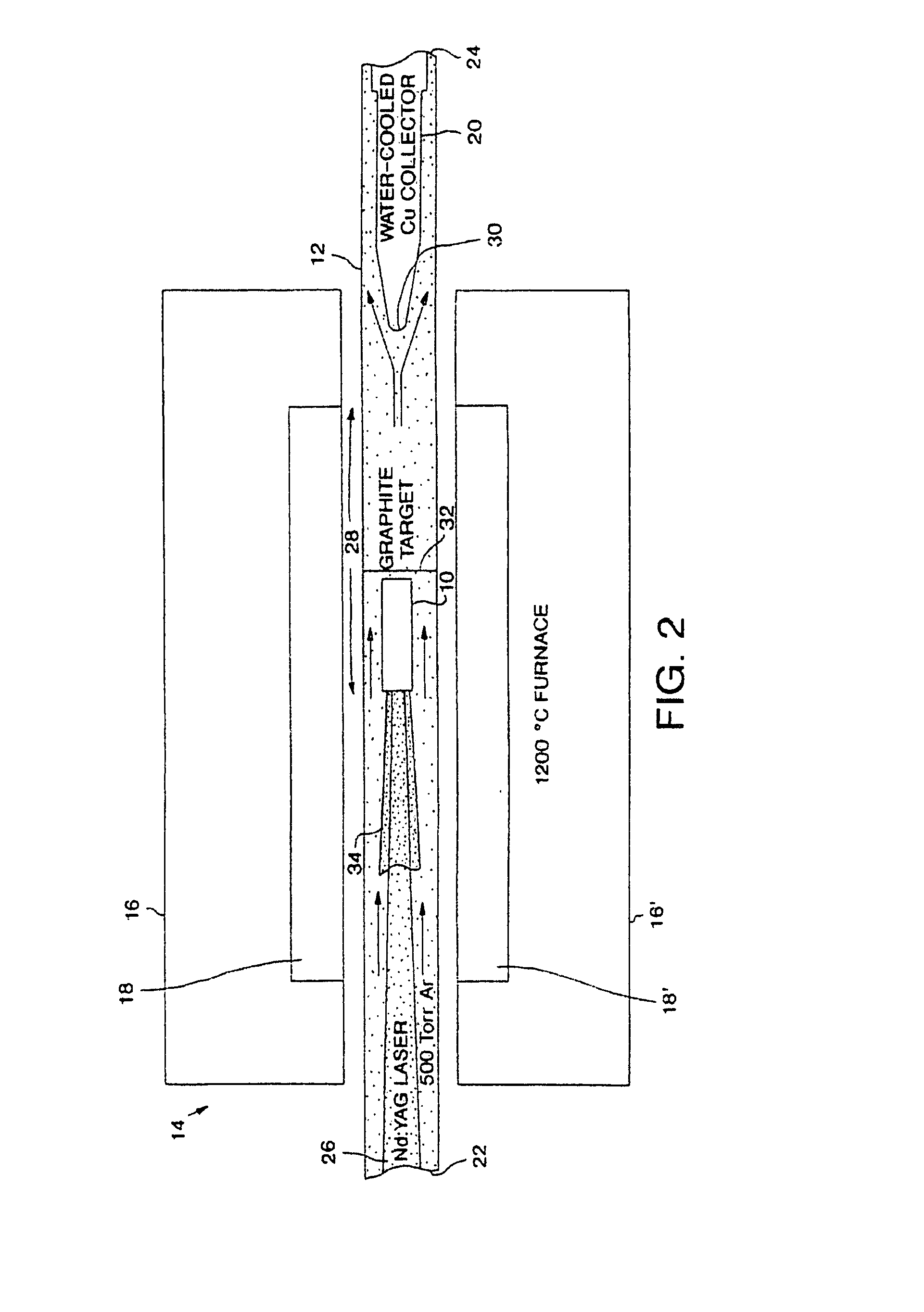
Methods of chemically derivatizing single-wall carbon nanotubes
InactiveUS6841139B2Increase resistanceHigh yieldMaterial nanotechnologyPigmenting treatmentFiberLithium
This invention is directed to making chemical derivatives of carbon nanotubes and to uses for the derivatized nanotubes, including making arrays as a basis for synthesis of carbon fibers. In one embodiment, this invention also provides a method for preparing single wall carbon nanotubes having substituents attached to the side wall of the nanotube by reacting single wall carbon nanotubes with fluorine gas and recovering fluorine derivatized carbon nanotubes, then reacting fluorine derivatized carbon nanotubes with a nucleophile. Some of the fluorine substituents are replaced by nucleophilic substitution. If desired, the remaining fluorine can be completely or partially eliminated to produce single wall carbon nanotubes having substituents attached to the side wall of the nanotube. The substituents will, of course, be dependent on the nucleophile, and preferred nucleophiles include alkyl lithium species such as methyl lithium. Alternatively, fluorine may be fully or partially removed from fluorine derivatized carbon nanotubes by reacting the fluorine derivatized carbon nanotubes with various amounts of hydrazine, substituted hydrazine or alkyl amine. The present invention also provides seed materials for growth of single wall carbon nanotubes comprising a plurality of single wall carbon nanotubes or short tubular molecules having a catalyst precursor moiety covalently bound or physisorbed on the outer surface of the sidewall to provide the optimum metal cluster size under conditions that result in migration of the metal moiety to the tube end.
Owner:RICE UNIV
Fullerene nanotube compositions
InactiveUS20080063585A1High yieldModerate temperatureMaterial nanotechnologyFullerenesFullereneNanotube
This invention relates generally to a fullerene nanotube composition. The fullerene nanotubes may be in the form of a felt, such as a bucky paper. Optionally, the fullerene nanotubes may be derivatized with one or more functional groups. Devices employing the fullerene nanotubes of this invention are also disclosed.
Owner:RICE UNIV
Chemically modifying single wall carbon nanotubes to facilitate dispersal in solvents
InactiveUS6875412B2High yieldIncrease resistanceMaterial nanotechnologyIndividual molecule manipulationFiberCarbon fibers
Owner:RICE UNIV
Flow process for synthesizing C3 to C13 high hydrocarbons by methane through non-synthetic gas method
The invention discloses a new flow path to make C3-C13 hydrocarbonate at the beginning with methane, which is characterized by the following: transmitting methane into CH3Br and CH2Br2 acted by oxygen and HBr / H2O; reacting CH3Br and CH2Br2 in the second reactor to make C3-C13 hydrocarbonate and HBr; adopting HBr as circulated reacting dielectric in the making course of CH3Br and CH2Br2; making the transmitting rate of methane to 32.0% and the selectivity of CH3Br to 80-0% and the selectivity of CH2Br2 to 0.67%; entering the product in the first reactor and residual reacting material into the second reactor directly; making the transmitting rate of methane in the first reactor to 30.0% and total selectivity of CH3Br and CH2Br2 to 88% in the first reactor and the transmitting rate of CH3Br and CH2Br2 to 100% in the second reactor.
Owner:MICROVAST POWER SYST CO LTD
Refrigerant additive compositionis containing perfluoropolyethers
The present invention relates to compositions and processes of using perfluoropolyether to maintain or improve the oil return, lubrication, cooling capacity, or energy efficiency of a refrigeration, air conditioning or heat transfer system.
Owner:THE CHEMOURS CO FC LLC
Bromomethane prepared by bromine oxidation of methane and catalyst for conversing the bromomethane into hydrocarbon
The invention discloses a catalyst which is used when methane is transformed into hydrobromic ether through the bromine oxidation reaction, and the catalyst which is used when preparing heavy hydrocarbons by using the hydrobromic ether further, and belongs to the technology field of the catalyst and the preparation method thereof. The first type of catalyst which is used when the methane is transformed into the hydrobromic ether through the bromine oxidation reaction comprises the preparation step that water-soluble metallic compound precursors such as Rh, Ru, Cu, Zn, Ag, Ce, V, W, Cd, Mo, Mn, Cr, La, etc. are mixed with a silica precursor, the mixture is processed through hydrolyzation, drying and roasting, and then the catalyst is obtained; the first type of catalyst can lead definite masses of reactant composed of the methane, HBr, H2O and oxygen sources (that is O2, air or oxygen-enriched air) to perform the catalytic bromine oxidation reaction, to generate target products such as CH3Br, CH2Br2, etc. The second type of catalyst, which is used when preparing the heavy hydrocarbons by using the hydrobromic ether further, includes the preparation step that molecular sieves such as HZSM-5, HY, H Beta, 3A, 4A, 5A, 13X, etc. load metallic compounds composed of Zn and Mg, so that the catalyst is obtained; the second type of catalyst can lead the methane bromination products composed of the CH3Br and the CH2Br2 to further react so as to generate the heavy hydrocarbons containing C3 to C13 and HBr, wherein, the HBr is used as a circular reaction medium.
Owner:MICROVAST POWER SYST CO LTD
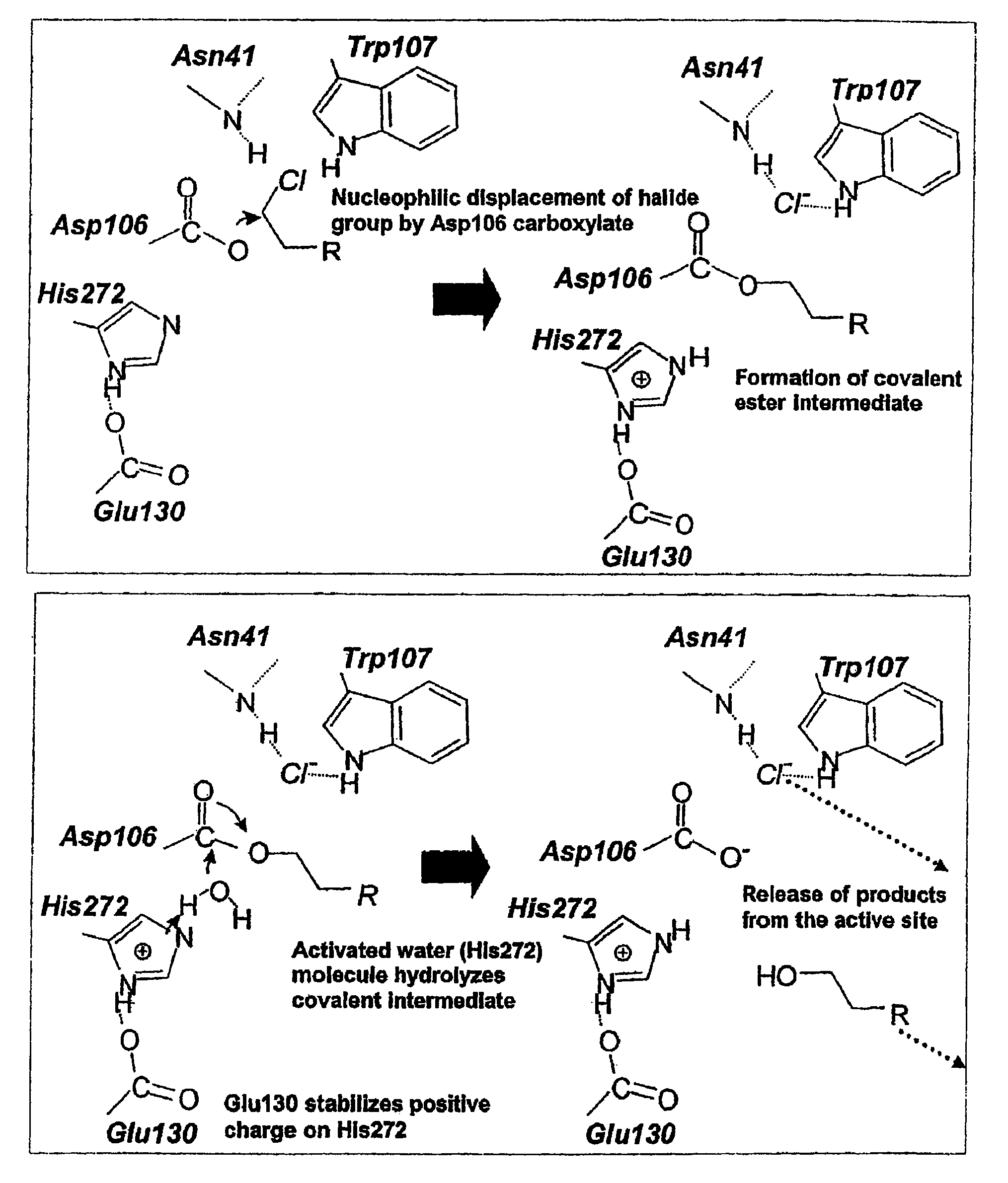
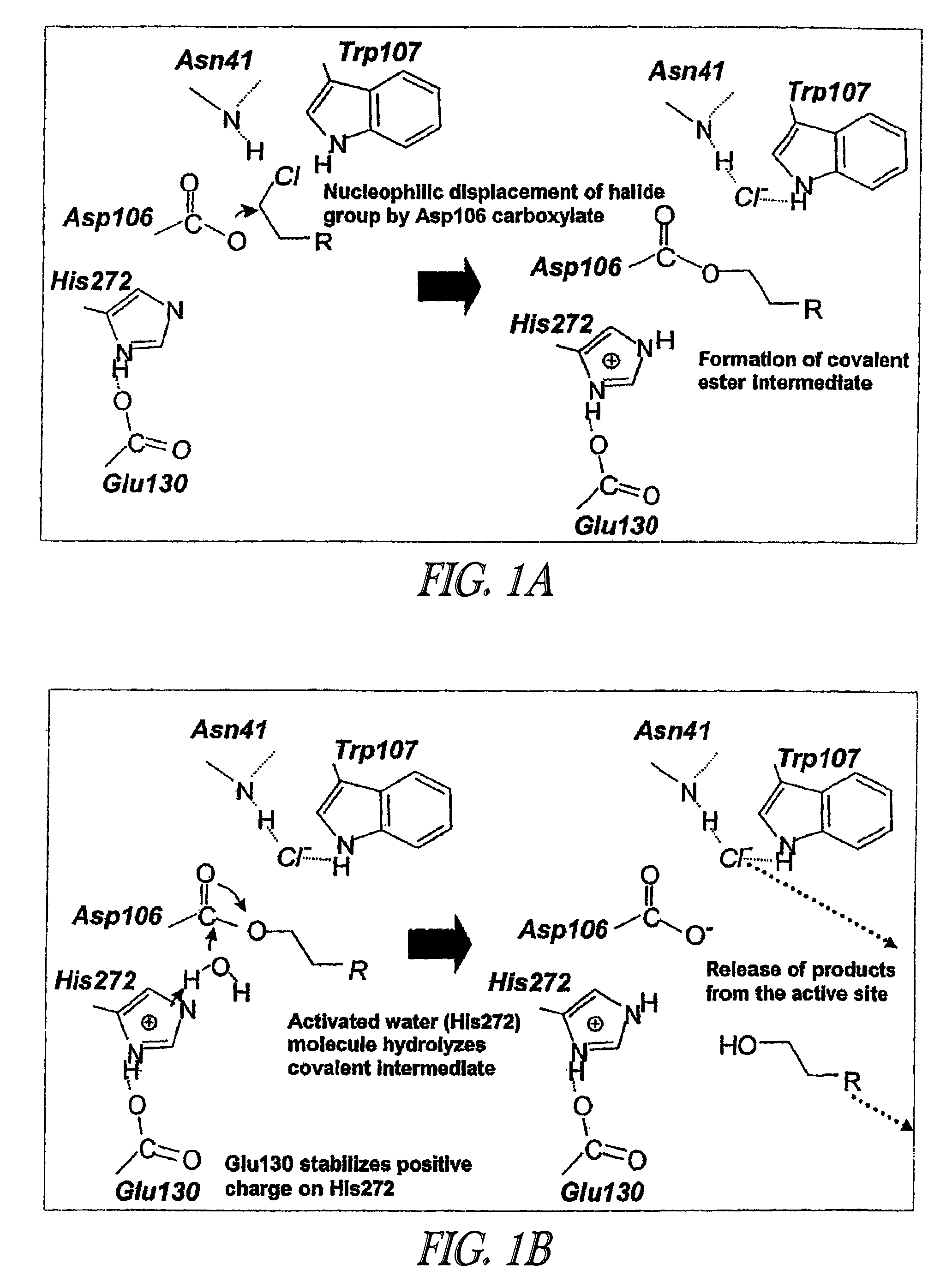
Covalent tethering of functional groups to proteins and substrates therefor
ActiveUS20060024808A1Rapidly and efficiently loaded into and washedStable rateMethine/polymethine dyesBacteriaAmino acid substitutionTethering
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate and has at least two amino acid substitutions relative to the wild-type hydrolase. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA CORP
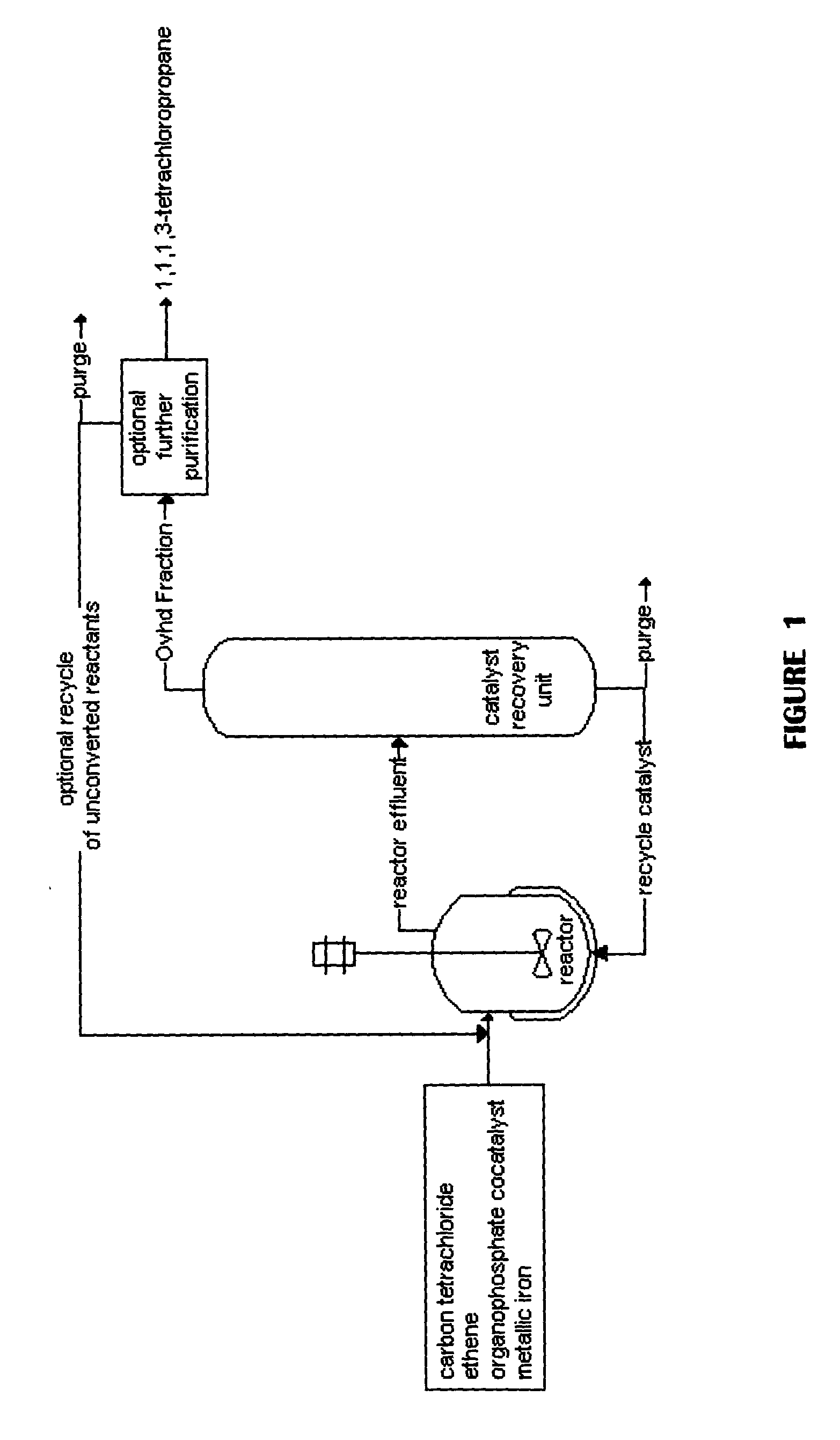
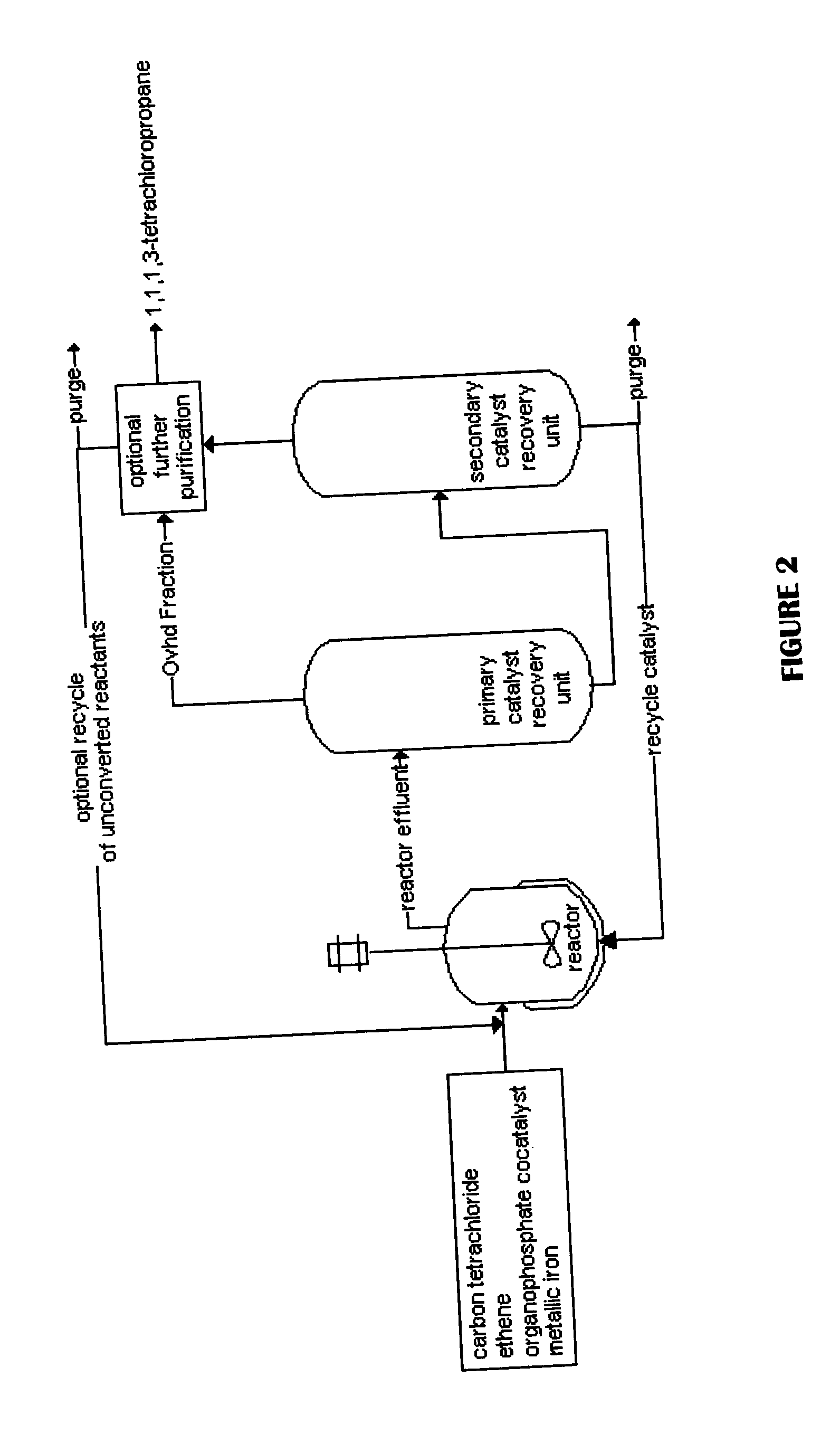
Method for producing 1,1,1,3-tetrachloropropane and other haloalkanes with iron catalyst
InactiveUS20040225166A1Efficient and economical to produceHalogenated hydrocarbon preparationTetrachlorideDistillation
A continuous process is provided for the manufacture of haloalkanes by the reaction of carbon tetrachloride with olefins in the presence of iron metal, trialkylphosphate, and ferric chloride. A fraction of the catalyst and co-catalyst are separated after the reaction and recycled. In a preferred application, the olefin is ethene, and the haloalkane product is 1,1,1,3-tetrachloropropane. Two distillation steps take place in order to enhance the production of 1,1,1,3-tetrachloropropane.
Owner:OCCIDENTAL CHEM CORP
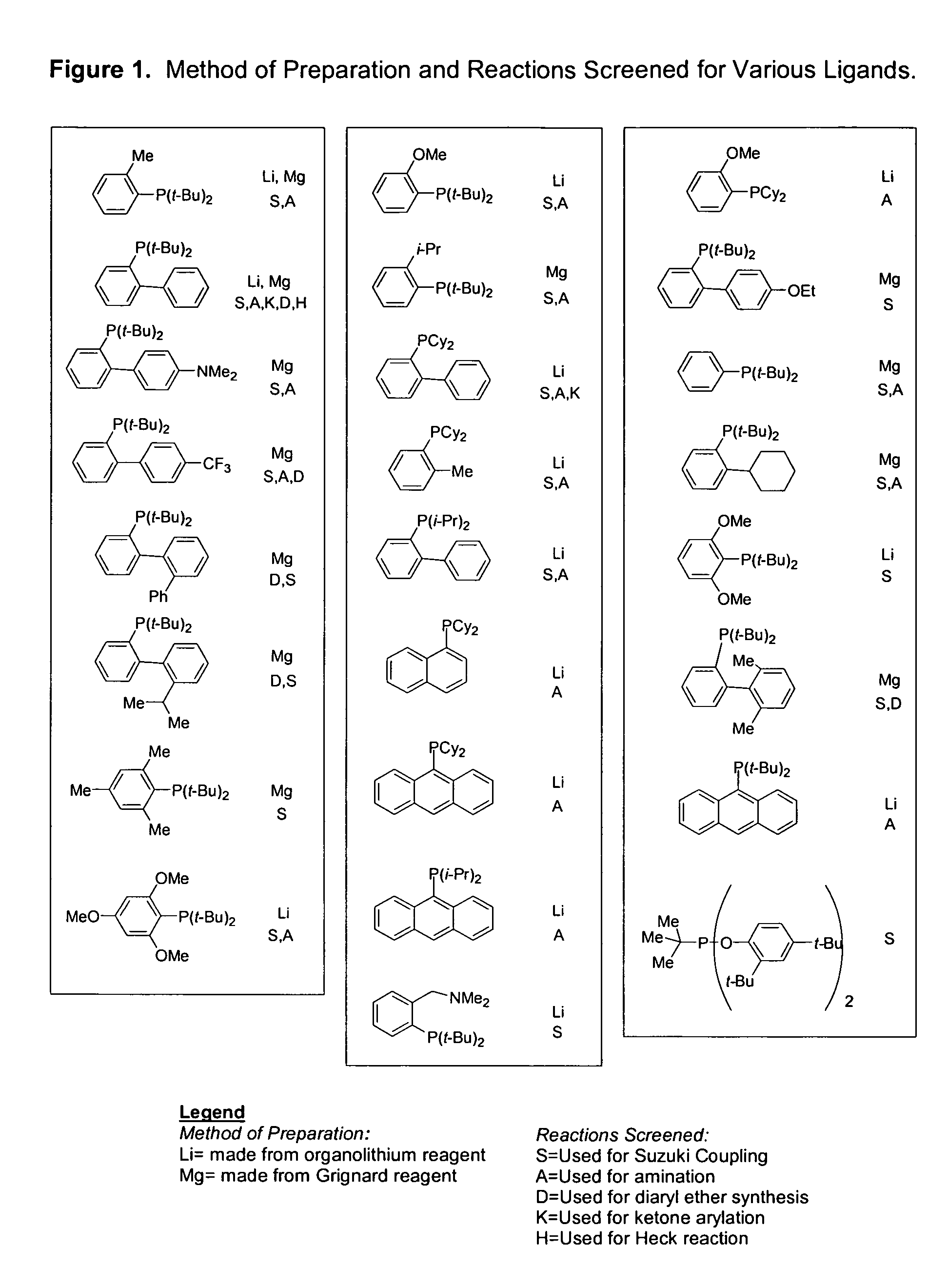
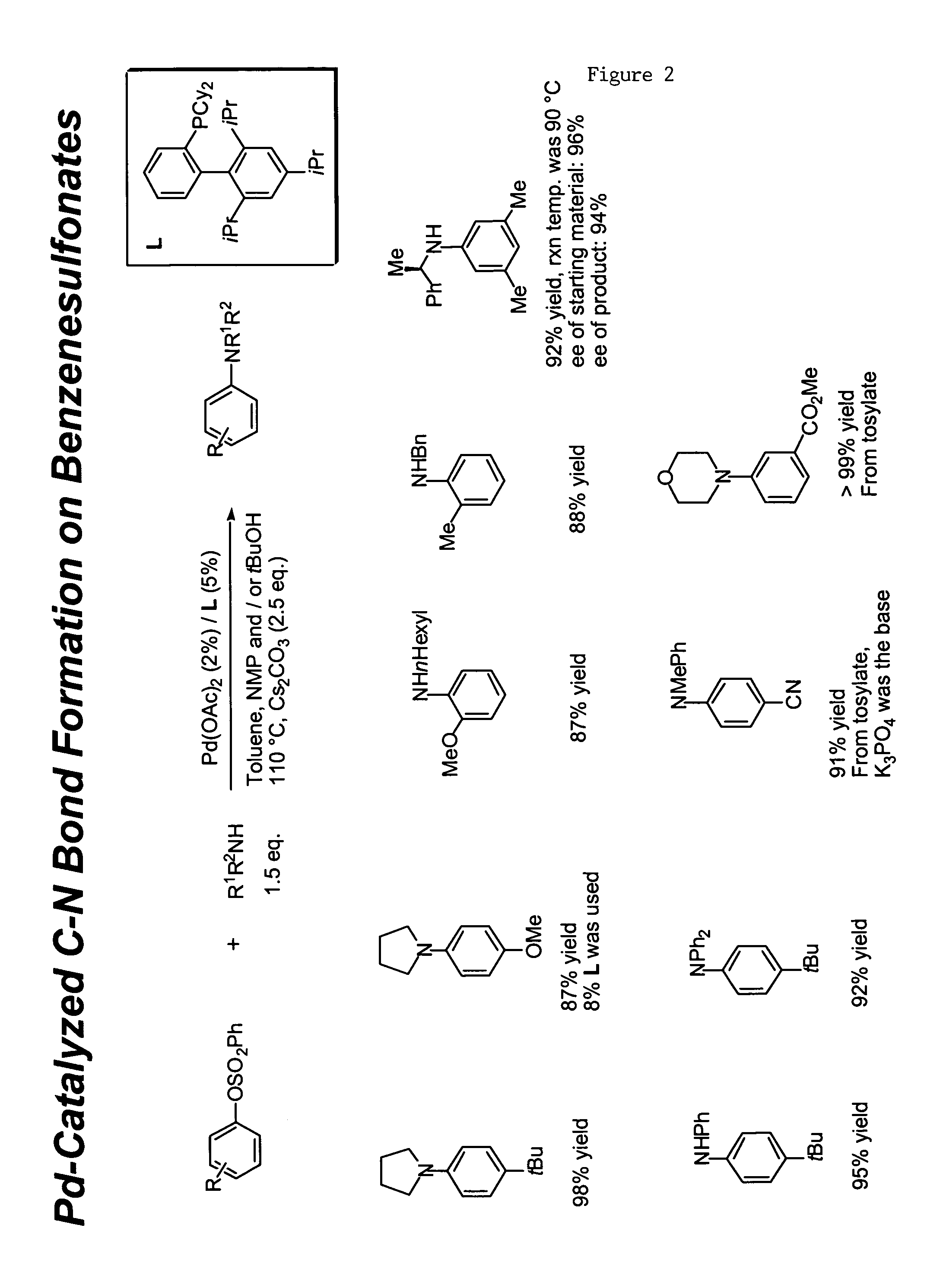
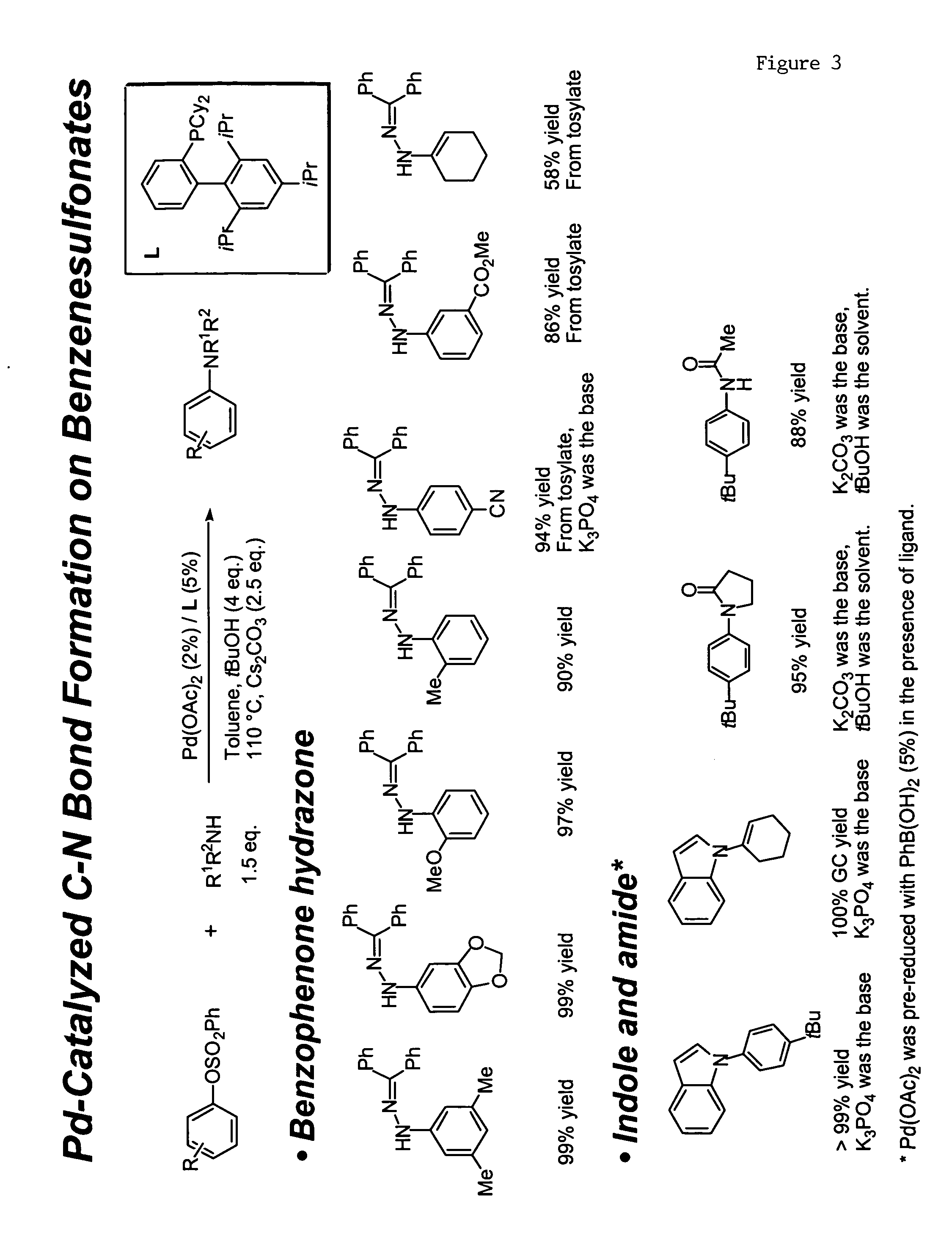
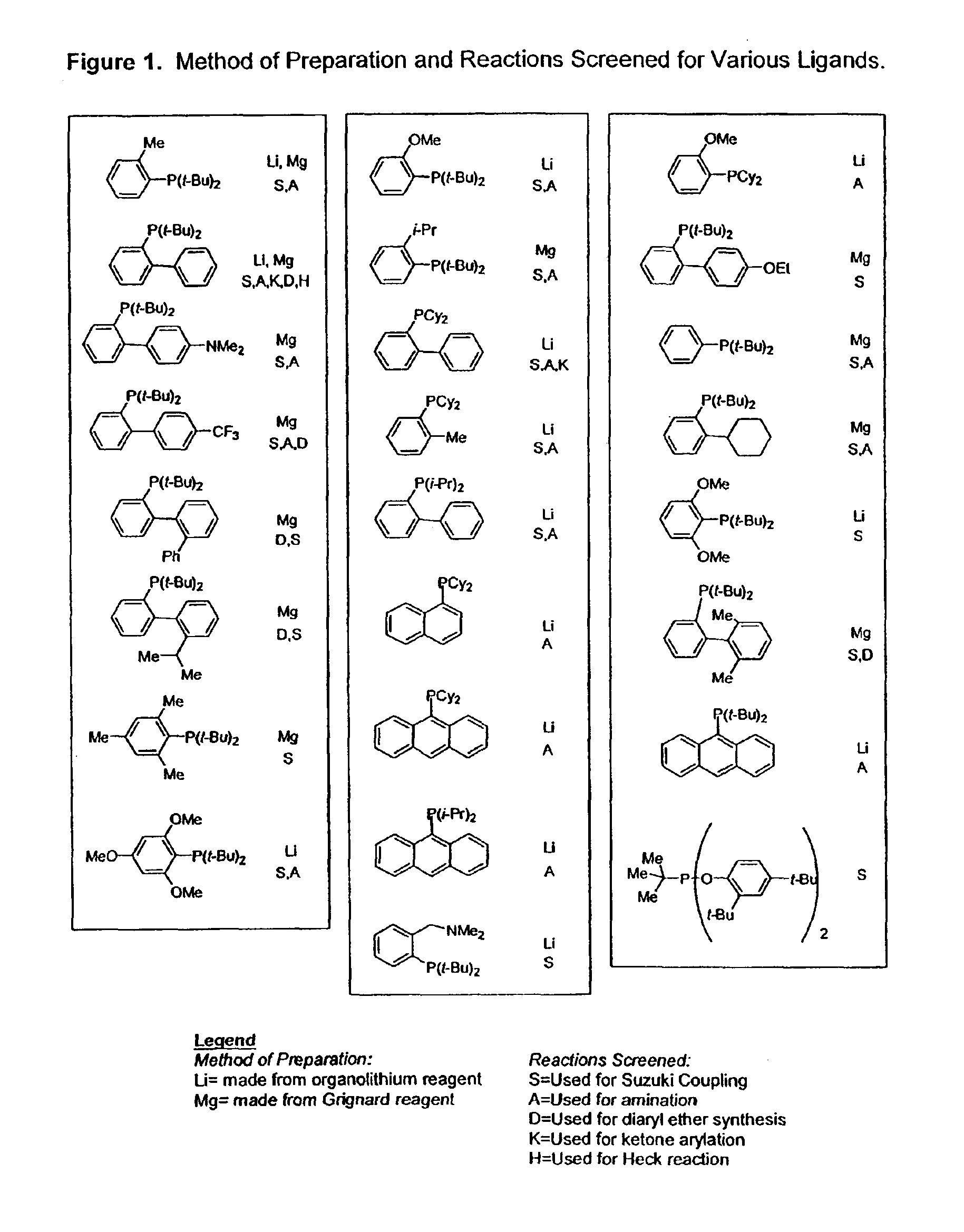
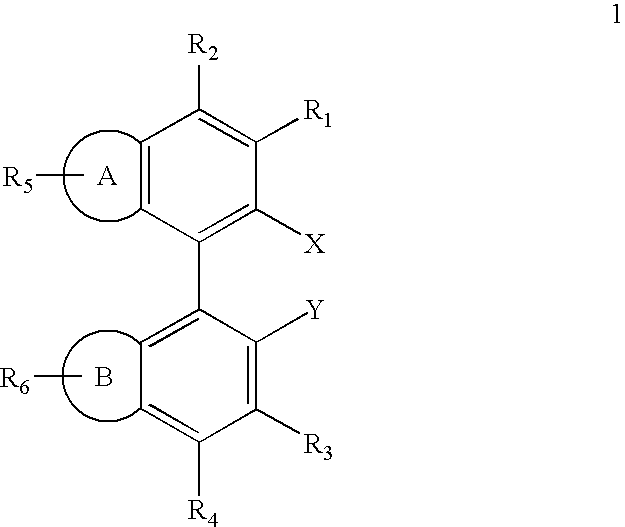
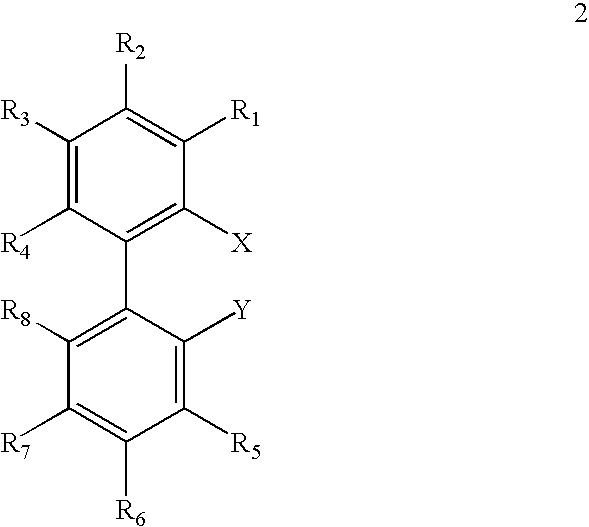
Ligands for metals and improved metal-catalyzed processes based thereon
InactiveUS7223879B2More featureEasy to useCarboxylic acid nitrile preparationCarboxylic acid esters preparationCarbon–carbon bondHeteroatom
One aspect of the present invention relates to ligands for transition metals. A second aspect of the present invention relates to the use of catalysts comprising these ligands in transition metal-catalyzed carbon-heteroatom and carbon-carbon bond-forming reactions. The subject methods provide improvements in many features of the transition metal-catalyzed reactions, including the range of suitable substrates, reaction conditions, and efficiency.
Owner:MASSACHUSETTS INST OF TECH
Ligands for metals and improved metal-catalyzed processes based thereon
InactiveUS6946560B2More featureEasy to useCarboxylic acid nitrile preparationGroup 5/15 element organic compoundsCarbon–carbon bondHeteroatom
One aspect of the present invention relates to novel ligands for transition metals. A second aspect of the present invention relates to the use of catalysts comprising these ligands in transition metal-catalyzed carbon-heteroatom and carbon-carbon bond-forming reactions. The subject methods provide improvements in many features of the transition metal-catalyzed reactions, including the range of suitable substrates, reaction conditions, and efficiency.
Owner:MASSACHUSETTS INST OF TECH
Catalytic process for regiospecific chlorination of alkanes, alkenes and arenes
InactiveUS6825383B1Save energyOrganic compound preparationPreparation by OH and halogen introductionMetal chlorideAlkene
The present invention provides a process for regiospecific chlorination of an aromatic or aliphatic compound with a chlorine source comprising a metal chloride and other than Cl2 and SO2Cl2 in presence of hypervalent iodine catalyst and in acidic medium.
Owner:COUNCIL OF SCI & IND RES
Covalent tethering of functional groups to proteins and substrates therefor
ActiveUS7425436B2Rapidly and efficiently loaded into and washedStable rateMethine/polymethine dyesBacteriaTetheringAmino acid substitution
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate and has at least two amino acid substitutions relative to the wild-type hydrolase. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA CORP
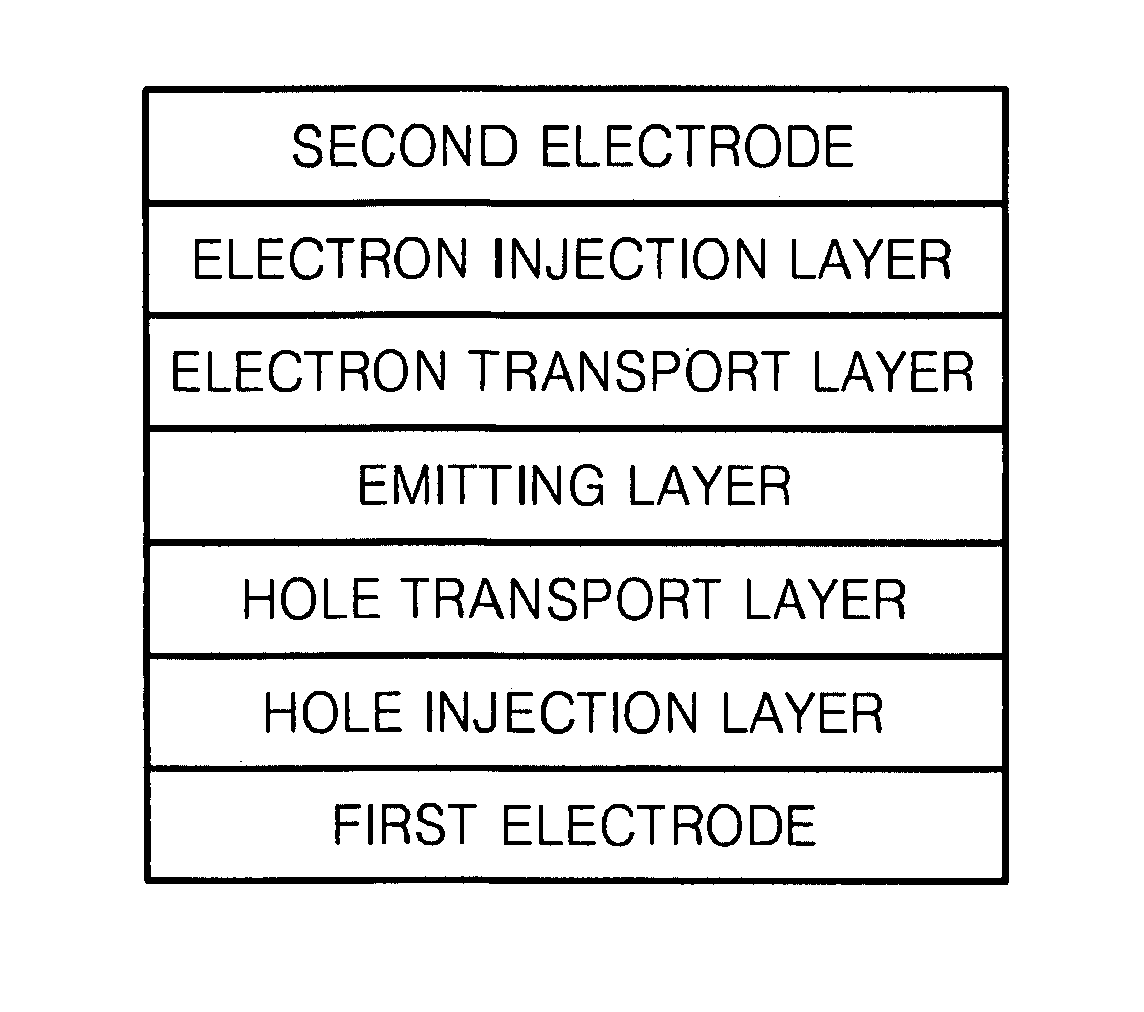
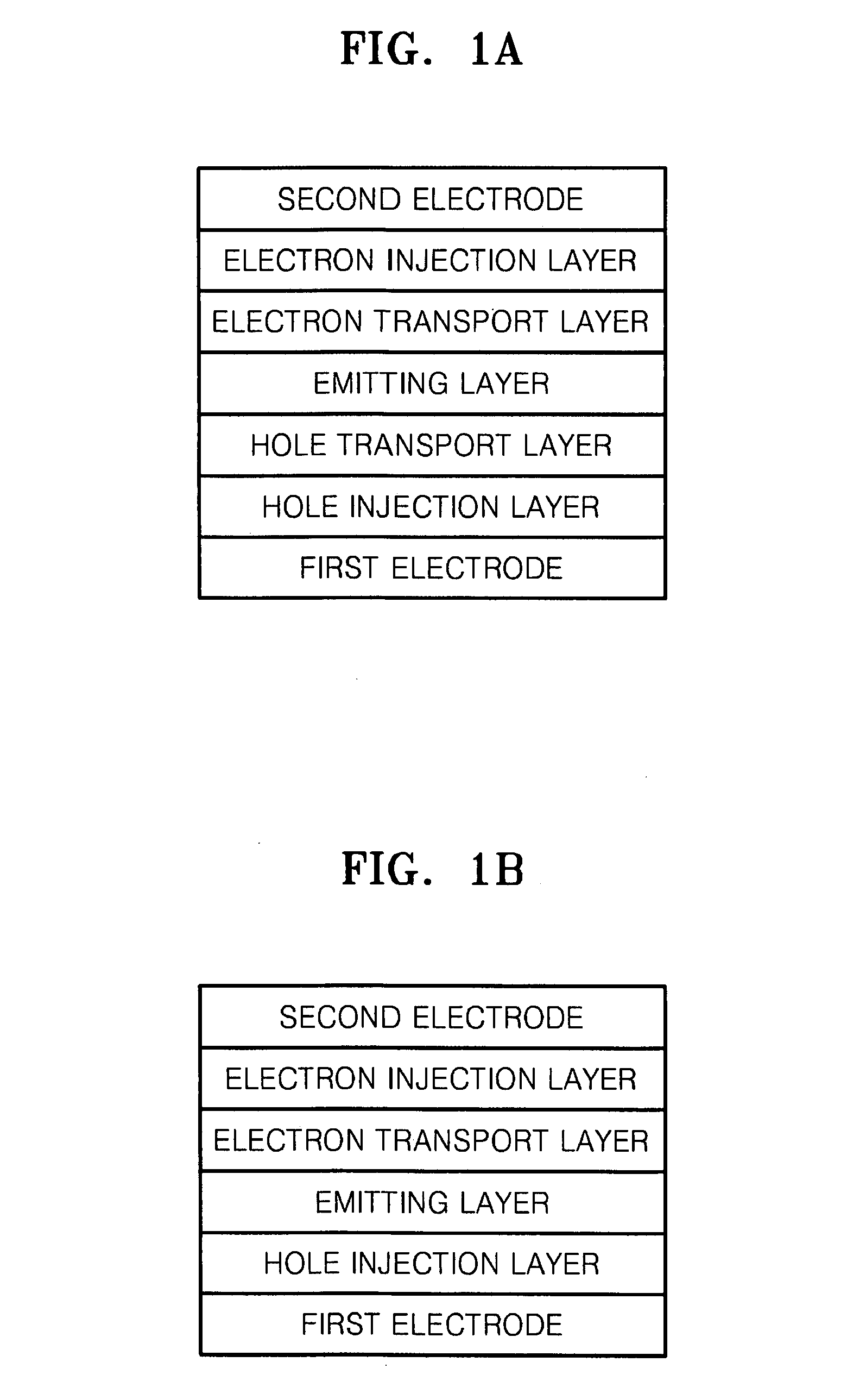
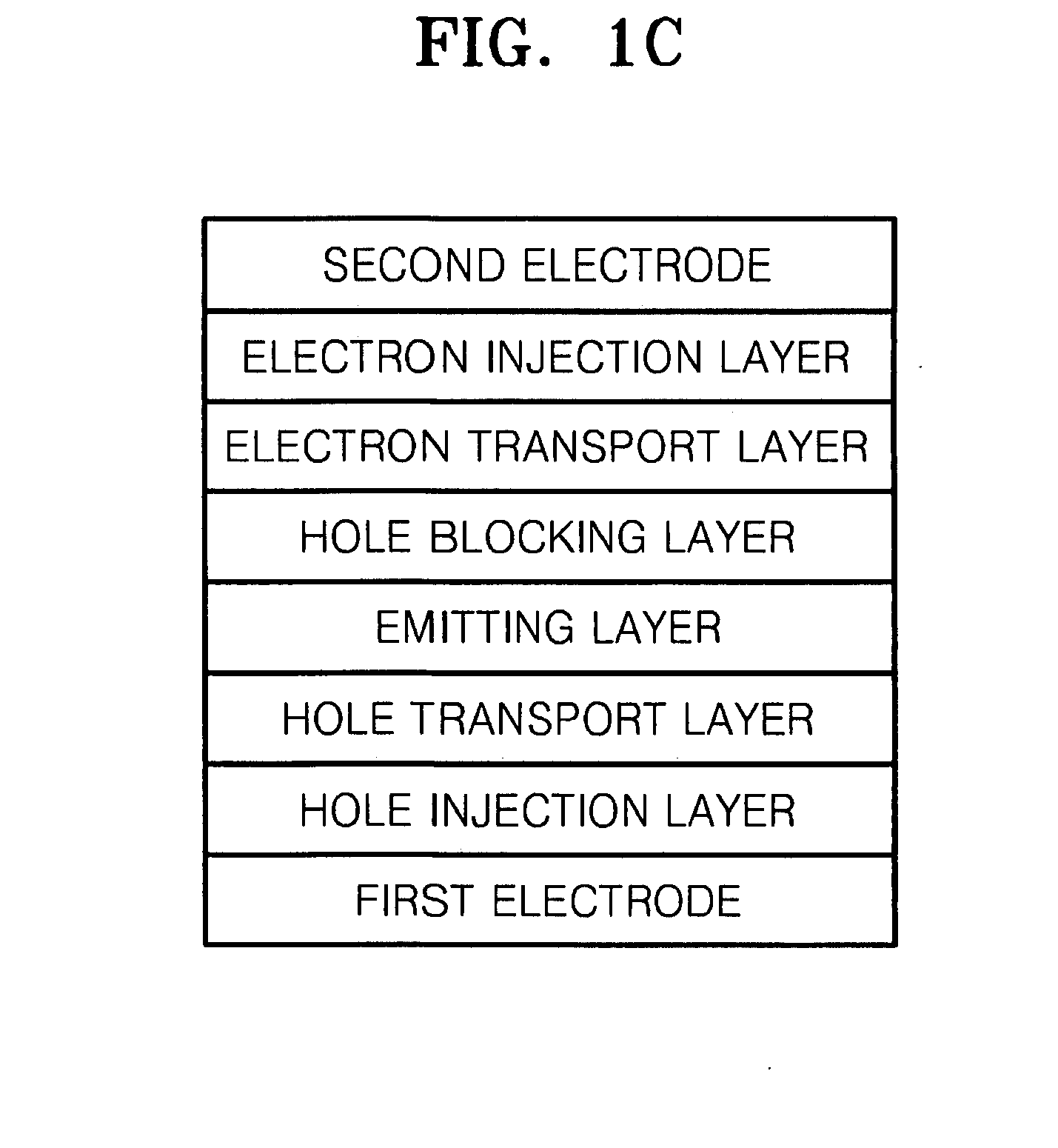
Organic metal complex, and organic light emitting device and display apparatus using the same
ActiveUS20100219407A1Improve efficiencyIncrease brightnessIndium organic compoundsOrganic compound preparationOrganic light emitting devicePhotochemistry
Provided is an organic metal complex having a structure represented by the following general formula (1):MLmL′n (1)where: M represents a metal atom selected from Ir, Pt, Rh, Os, and Zn; L and L′, which are different from each other, each represent a bidentate ligand; m represents an integer of 1 to 3 and n represents an integer of 0 to 2, provided that m+n is 3; a partial structure MLm represents a structure represented by the following general formula (2):and a partial structure ML′n represents a structure including a monovalent bidentate ligand.
Owner:SAMSUNG ELECTRONICS CO LTD
Covalent tethering of functional groups to proteins
ActiveUS7238842B2Rapidly and efficiently loaded intoRapidly and efficiently loaded into and washed outCarbamic acid derivatives preparationHydrolasesTetheringWild type
A mutant hydrolase optionally fused to a protein of interest is provided. The mutant hydrolase is capable of forming a bond with a substrate for the corresponding nonmutant (wild-type) hydrolase which is more stable than the bond formed between the wild-type hydrolase and the substrate. Substrates for hydrolases comprising one or more functional groups are also provided, as well as methods of using the mutant hydrolase and the substrates of the invention. Also provided is a fusion protein capable of forming a stable bond with a substrate and cells which express the fusion protein.
Owner:PROMEGA
Isomerization of 1-Chloro-3,3,3-Trifluoropropene
ActiveUS20100152504A1Organic compounds purification/separation/stabilisationHalogenated hydrocarbon preparationIsomerizationChemistry
Disclosed are processes for an isomerization reaction between (E)1-chloro-3,3,3-trifluoropropene and (Z)1-chloro-3,3,3-trifluoropropene. Some of the disclosed processes include the step of contacting a feed stream with a heated surface, where the feed stream includes (E)1-chloro-3,3,3-trifluoropropene, (Z)1-chloro-3,3,3-trifluoropropene or mixtures thereof. The resulting product stream includes (E)1-chloro-3,3,3-trifluoropropene and (Z)1-chloro-3,3,3-trifluoropropene, where the ratio of (E) isomer to (Z) isomer in the product stream is different than the ratio feed stream. The (E) and (Z) isomers in the product stream may be separated from one another.
Owner:HONEYWELL INT INC
Method for clay exfoliation, compositions therefore, and modified rubber containing same
InactiveUS20060235128A1Reduce air permeabilityLow gas permeabilityPigmenting treatmentLayered productsPolymer scienceButyl rubber
A polymer composition of low gas permeability. The composition includes an exfoliated organically-modified clay, butyl rubber, and a polymeric exfoliant. A method for producing the butyl composition by dry mixing the butyl rubber with the exfoliated clay is also provided.
Owner:BRIDGESTONE CORP
Processes for geometric isomerization of halogenated olefins
ActiveUS20080103342A1Increase conversion rateHigh selectivityHalogenated hydrocarbon preparationGeometric formChemistry
Disclosed are processes for the conversion of isomerizable halogenated C2-C6 olefins from one geometric form to a more preferred geometric form. Preferred process aspects involve converting C2-C6 olefin in a cis-form to a trans-form under conditions effective to convert at least about 50 percent, and even more preferably at least about 70 percent, of the cis-form compound to the trans-form compound. In preferred embodiments the C2-C6 olefin comprises tetrafluoropropene, with cis-1,3,3,3 tetrafluoropropene (cis-HFO-1234ze) being converted, preferably at high conversion rates and high selectivity, to trans-1,3,3,3 tetrafluoropropene (trans-HFO-1234ze). In preferred embodiments the conditions effective to achieve the desired high levels of conversion and selectivity include exposing the feed to a metal based catalyst selected from the group consisting of halogentated metal oxides, Lewis acid metal halides, zero-valent metals, and combinations of these, preferably under reaction conditions, including reaction temperature and residence time, effective to convert at least about 5% of the cis-form of the compound to other compounds and to further achieve a selectivity to the trans-form of the compound of at least about 70%.
Owner:HONEYWELL INT INC
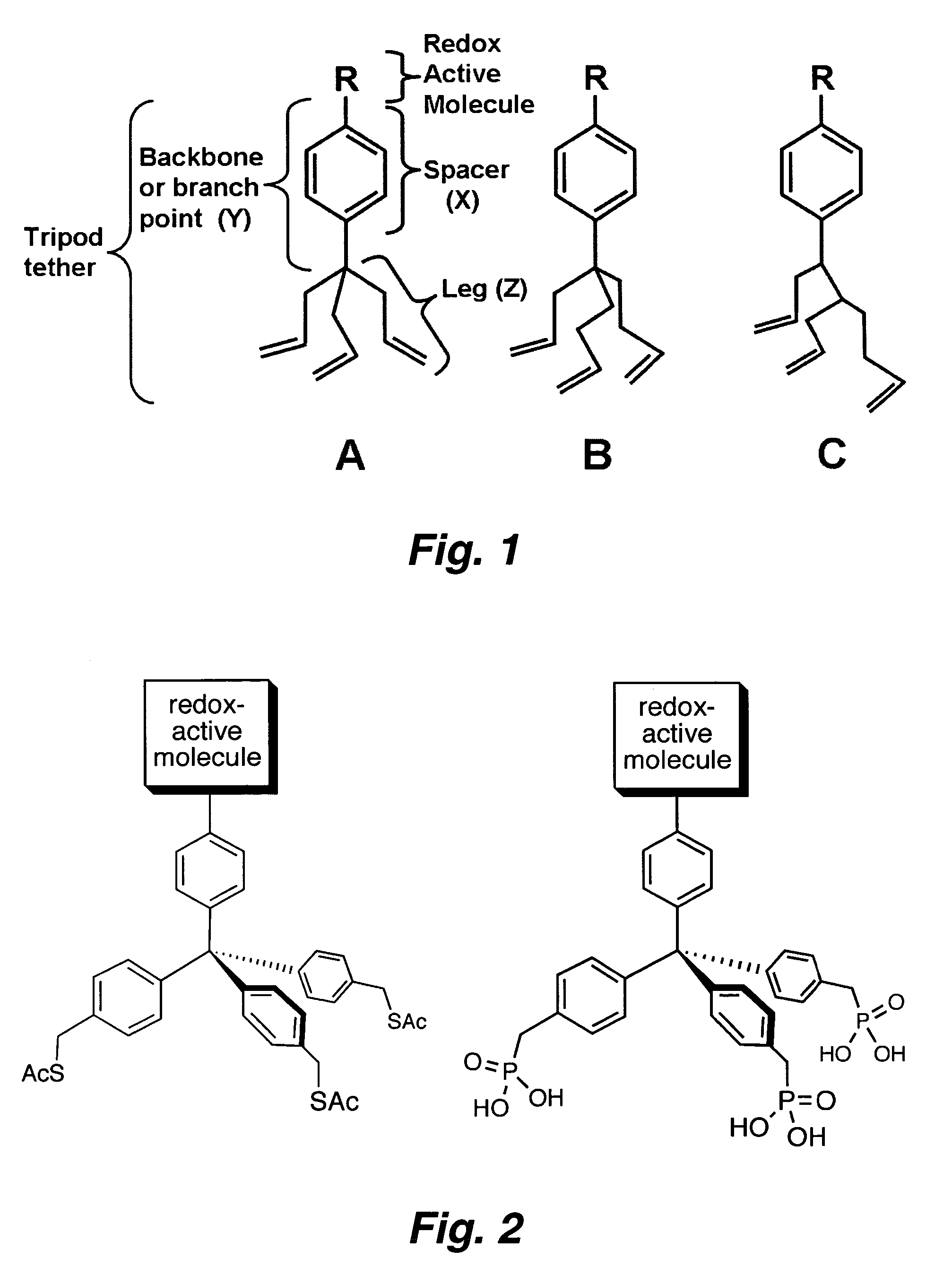
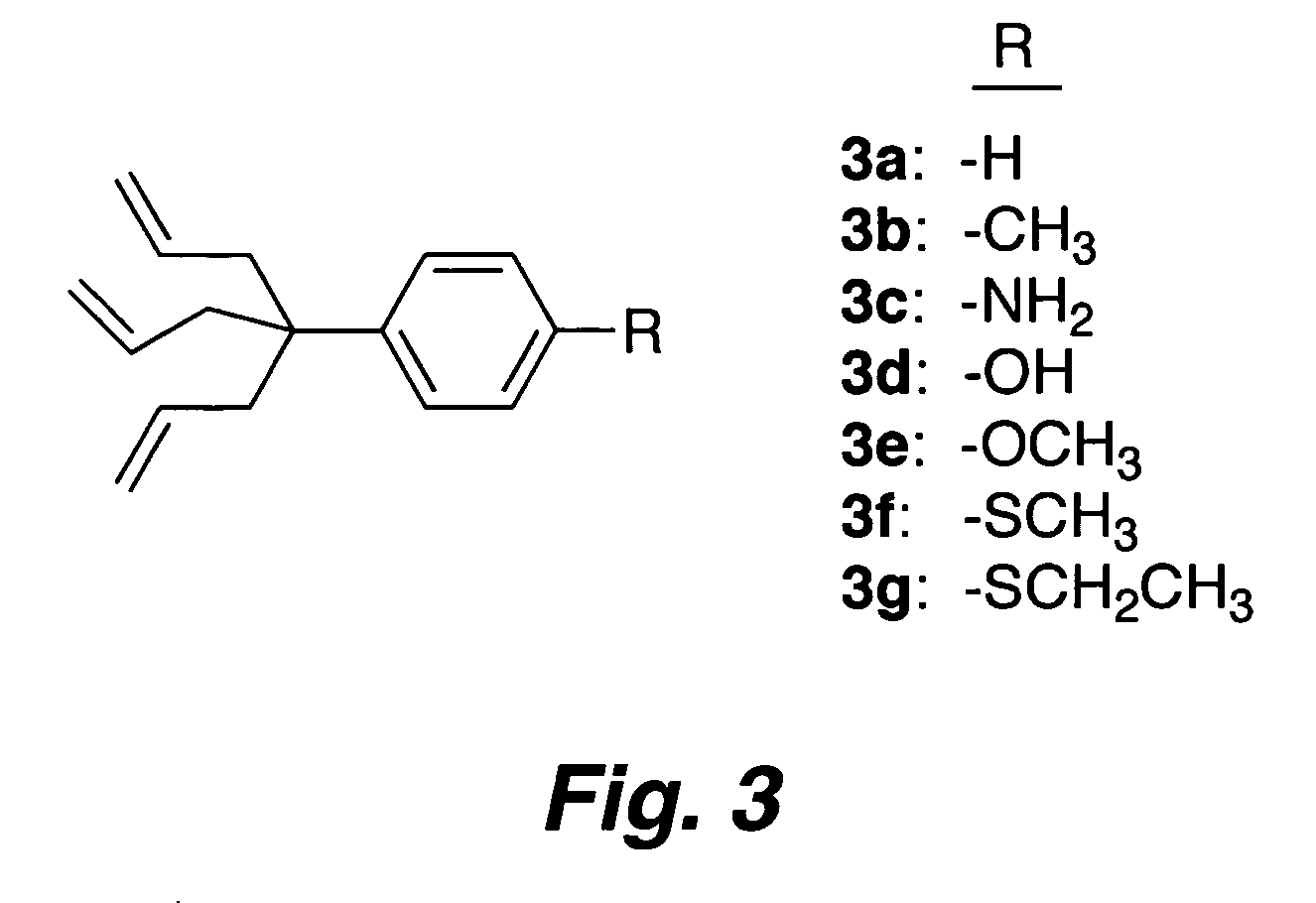
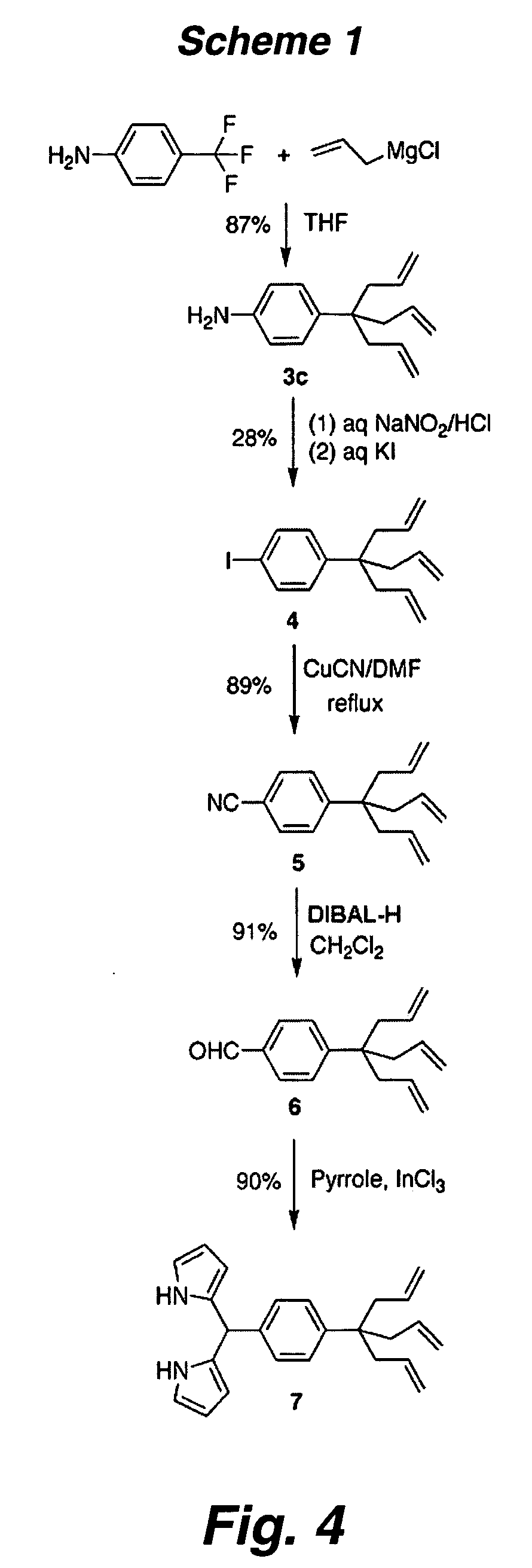
Multypodal tethers for high-density attachment of redox-active moieties to substrates
InactiveUS20070108438A1Increase charge storage densityHigh charge densityMaterial nanotechnologyNanoinformaticsHigh densityCombinatorial chemistry
This invention provides redox-active molecules attached to polypodal (e.g., bipodal, tripodal, quadrapodal, pentapodal, etc.) tethers that can be used for attachment of the redox-active molecules to a substrate (e.g., an electrode). The tethered redox-active molecules are useful for the fabrication of memory devices.
Owner:RGT UNIV OF CALIFORNIA +2
Aromatic Amine Derivative, Organic Electroluminescent Element Employing the Same, and Process for Producing Aromatic Amine Derivative
ActiveUS20070252511A1Extended service lifeImprove efficiencyDischarge tube luminescnet screensCarboxylic acid nitrile preparationOrganic electroluminescenceLight emission
An aromatic amine derivative with a special structure obtained by bonding a diphenylamino group having a substituent to a substituted pyrene structure; and a process for producing the aromatic amine derivative. An organic electroluminescence device which comprises at least one organic thin film layer comprising a light emitting layer sandwiched between a pair of electrodes consisting of an anode and a cathode, wherein at least one of the organic thin film layer comprises the aromatic amine derivative singly or as its mixture component. An organic electroluminescence device having a prolonged lifetime and emits blue light with an enhanced efficiency of light emission and an aromatic amine derivative realizing the device are provided.
Owner:IDEMITSU KOSAN CO LTD
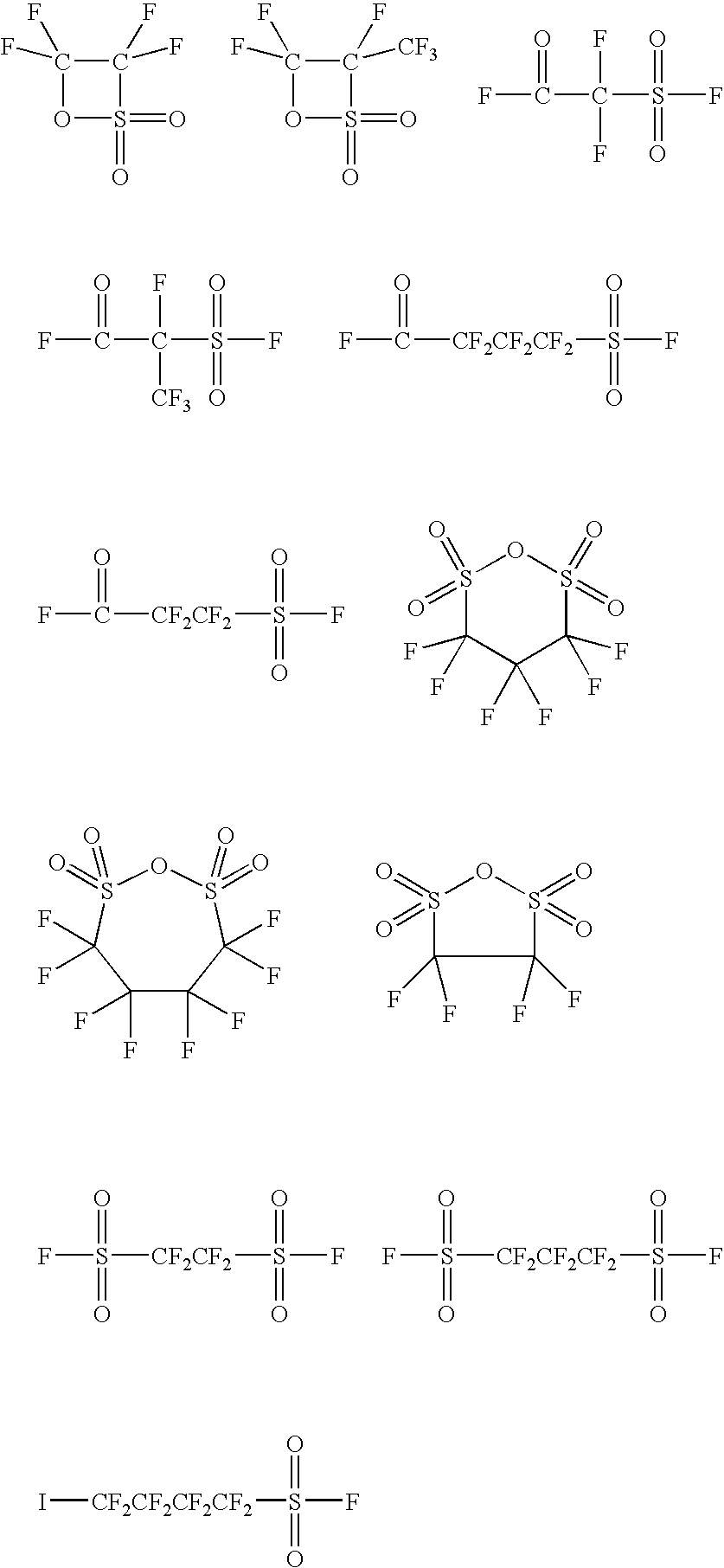
Ionic photoacid generators with segmented hydrocarbon-fluorocarbon sulfonate anions
InactiveUS6841333B2Improve solubilitySolubility in organic compoundsOrganic compound preparationPhotosensitive materialsSolubilityAcid catalyzed
Photoacid generator salts comprising photoactive cationic moieties and segmented, highly fluorinated-hydrocarbon anionic moieties are disclosed which provide high photoacid strength and can be tailored for solubility and polarity. The present invention further relates to photoacid generators as they are used in photoinitiated acid-catalyzed processes for uses such as photoresists for microlithography and photopolymerization.
Owner:3M INNOVATIVE PROPERTIES CO
Organoelectroluminescent compound and organoelectroluminescent device employing the same
ActiveUS20080100207A1Stable film formationImprove solubilityDischarge tube luminescnet screensElectroluminescent light sourcesSolubilityOrganic dye
Provided are a cyclopentaphenanthrene-based compound and an organoelectroluminescent device employing the same. The cyclopentaphenanthrene-based compound is easy to prepare and excellent in solubility, color purity, color stability, and thermal stability. The cyclopentaphenanthrene-based compound is useful as a material for forming an organic layer, in particular, an emitting layer, in an organoelectroluminescent device, and as an organic dye or an electronic material such as a nonlinear optical material.
Owner:SAMSUNG DISPLAY CO LTD
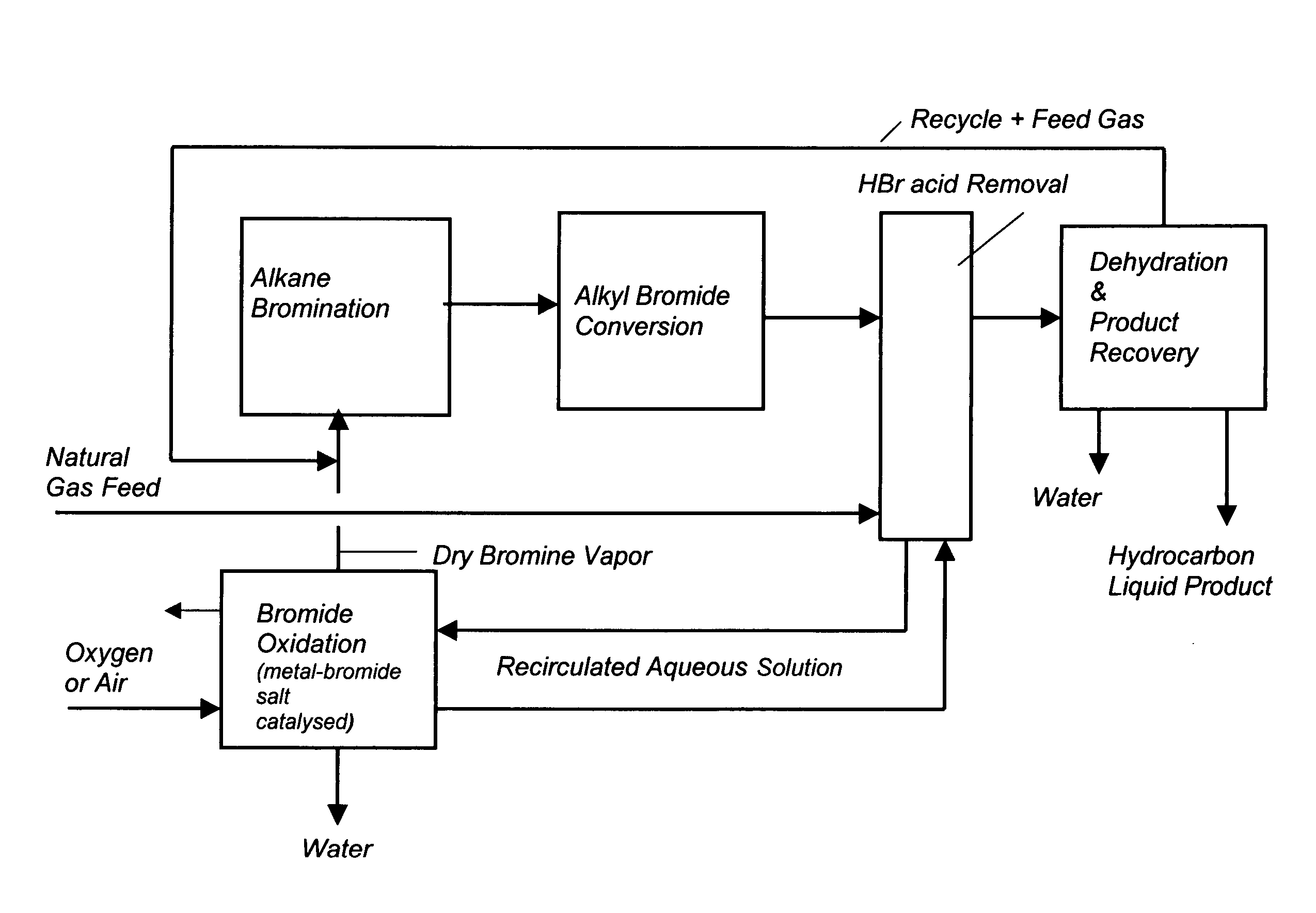
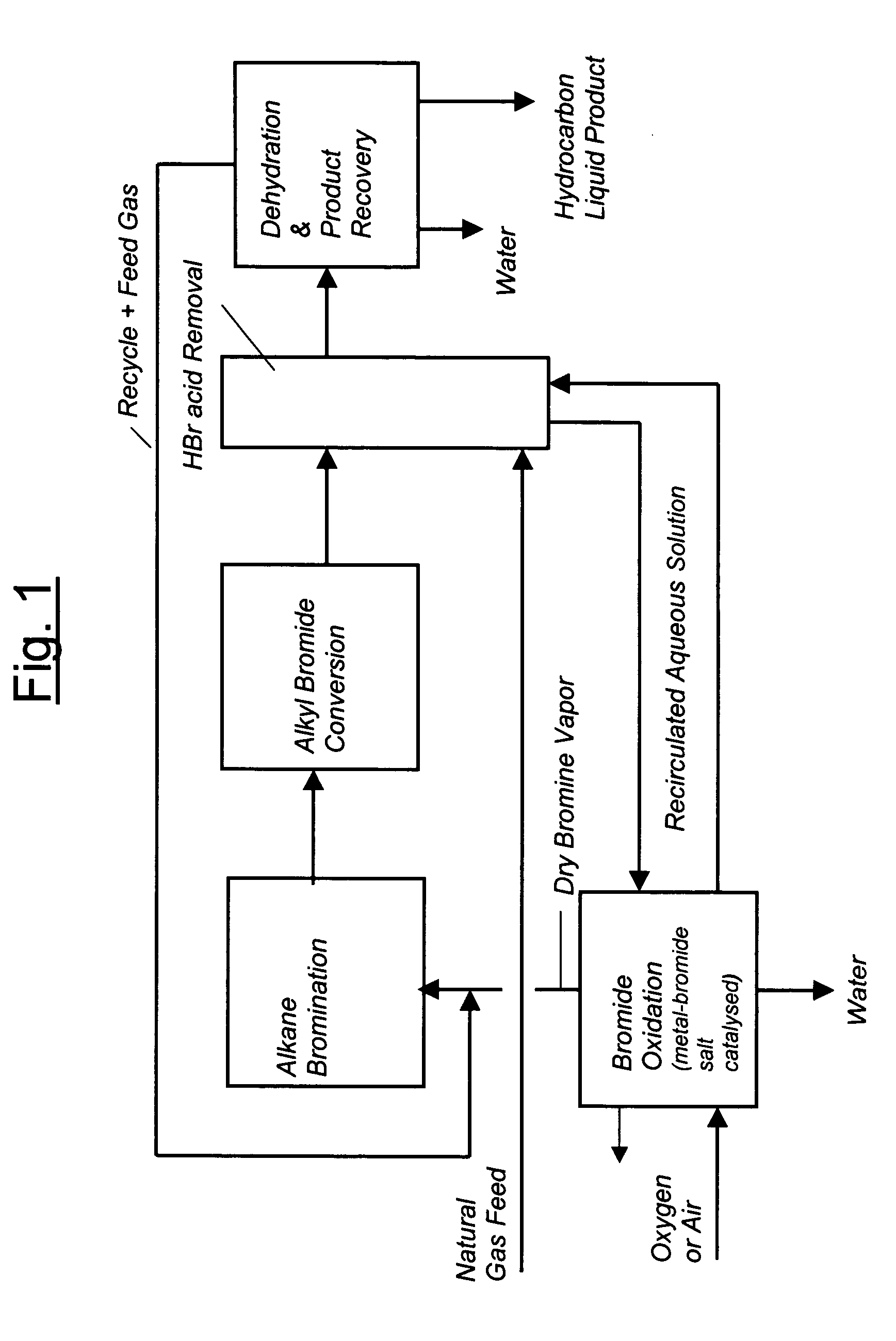
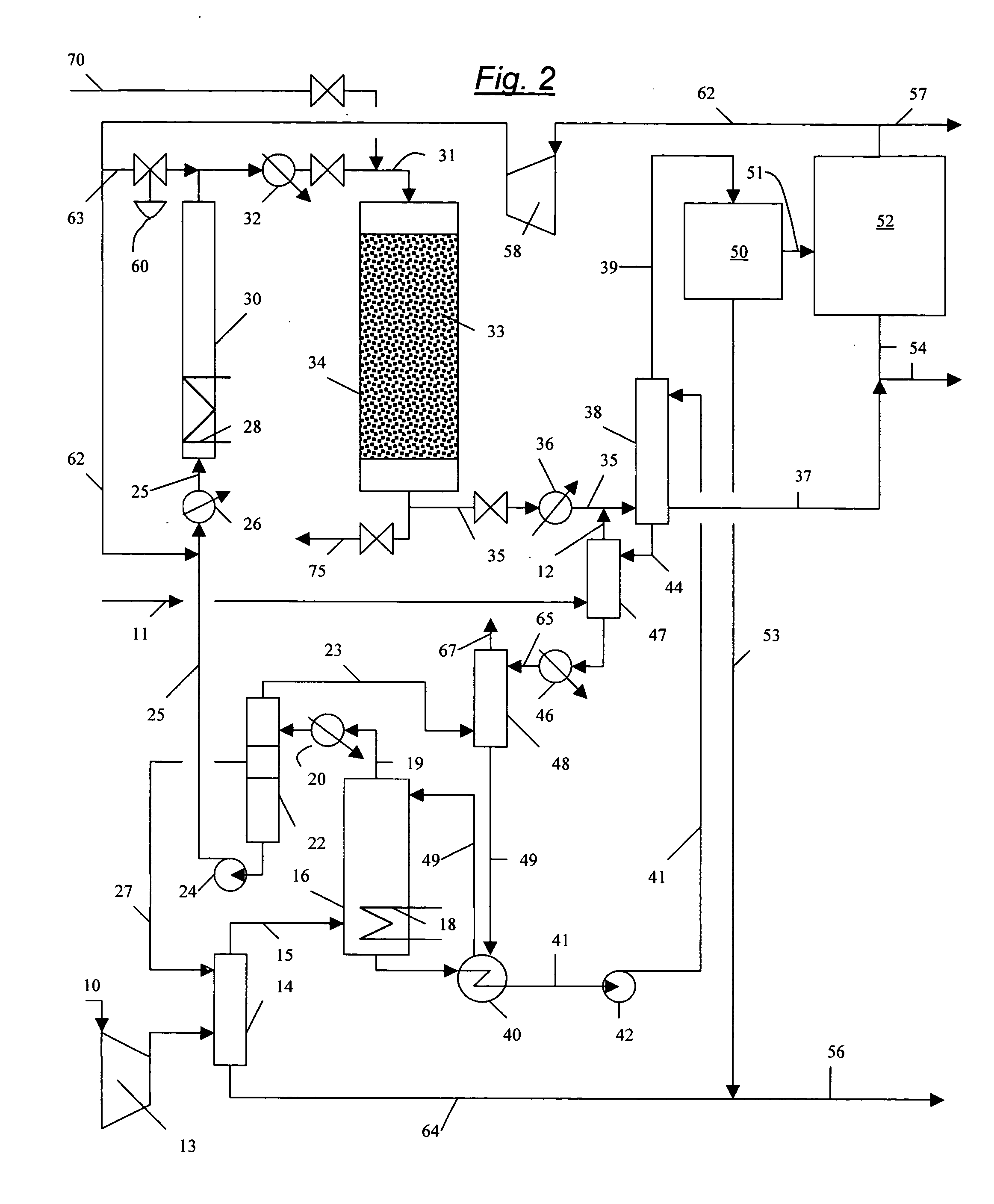
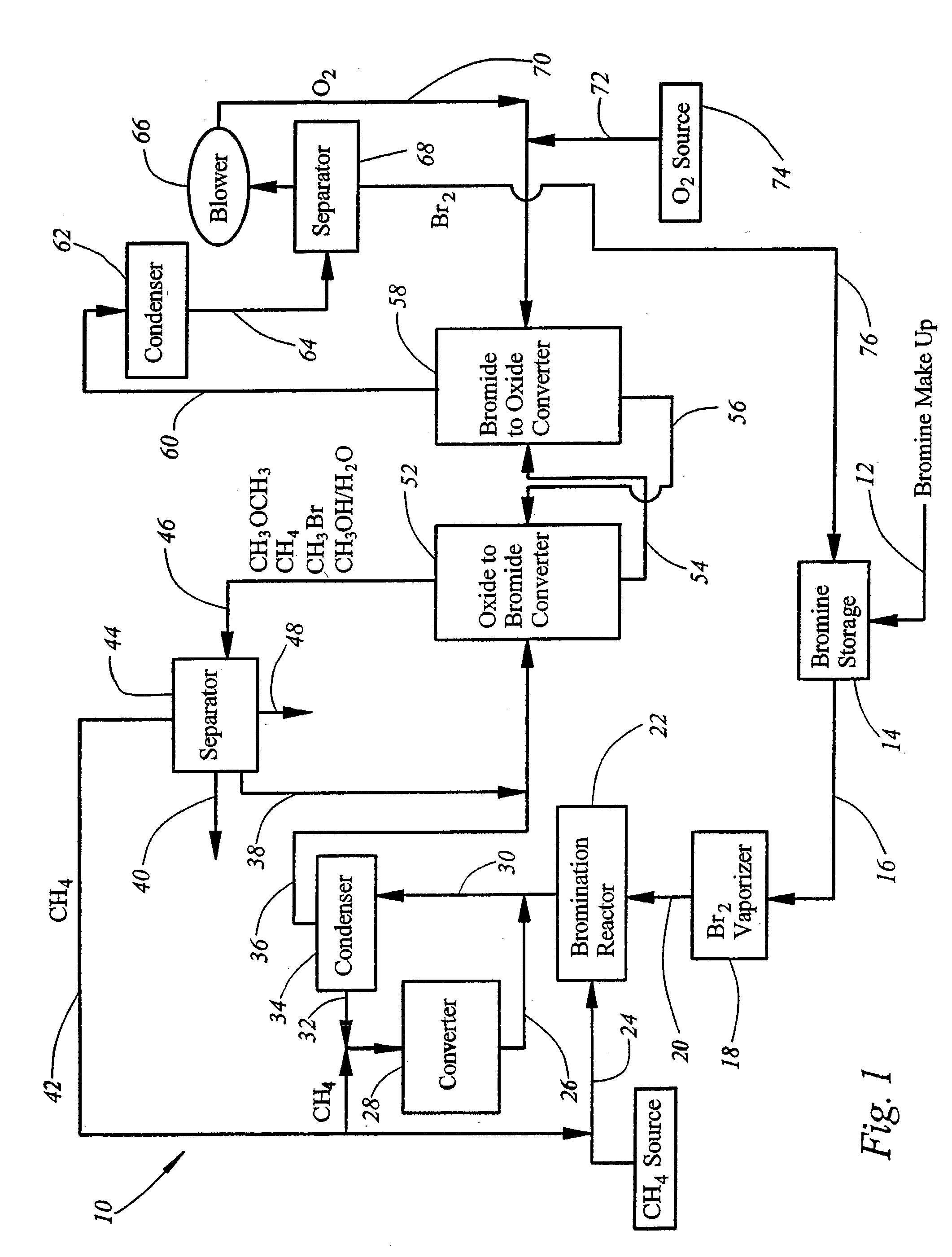
Process for converting gaseous alkanes to liquid hydrocarbons
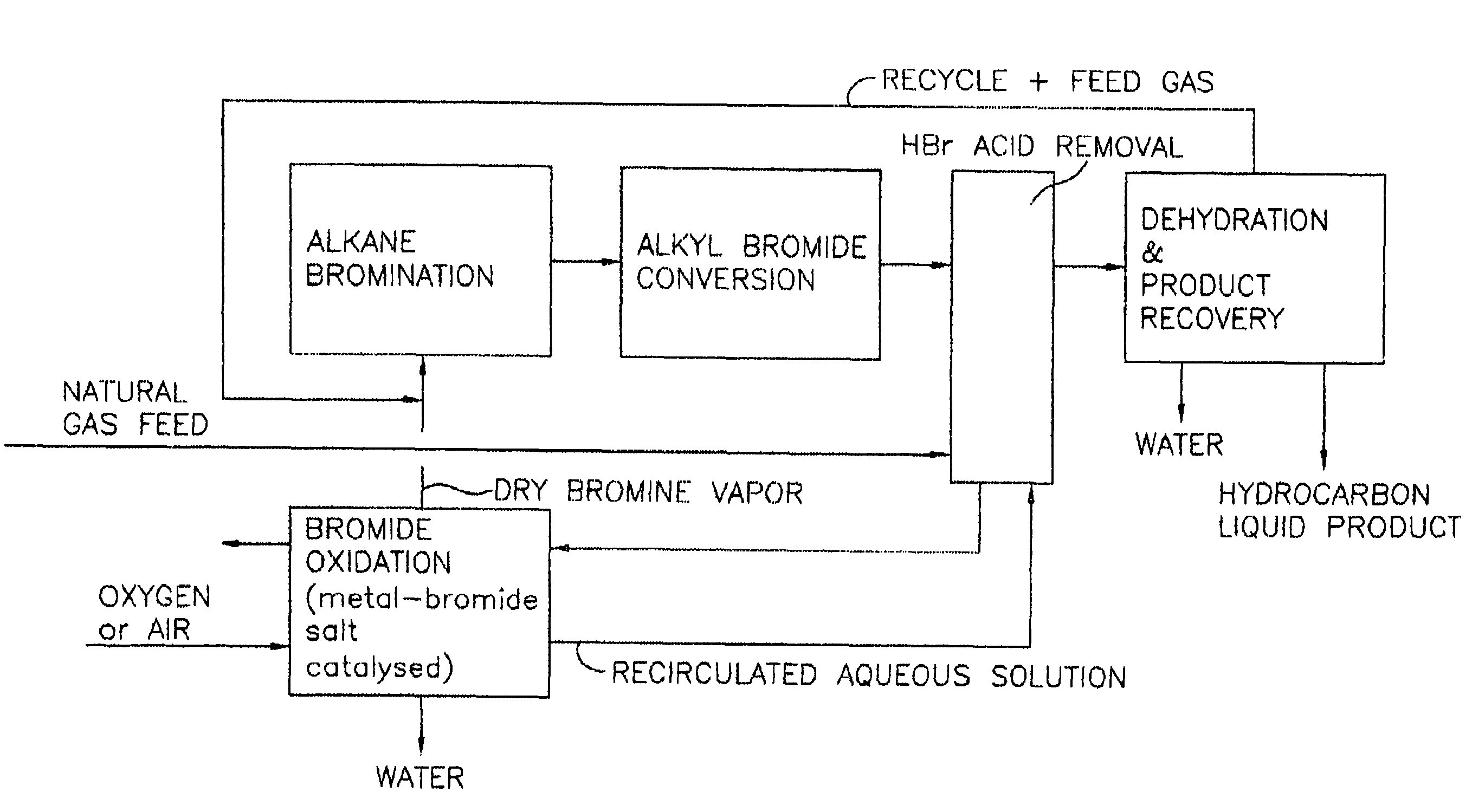
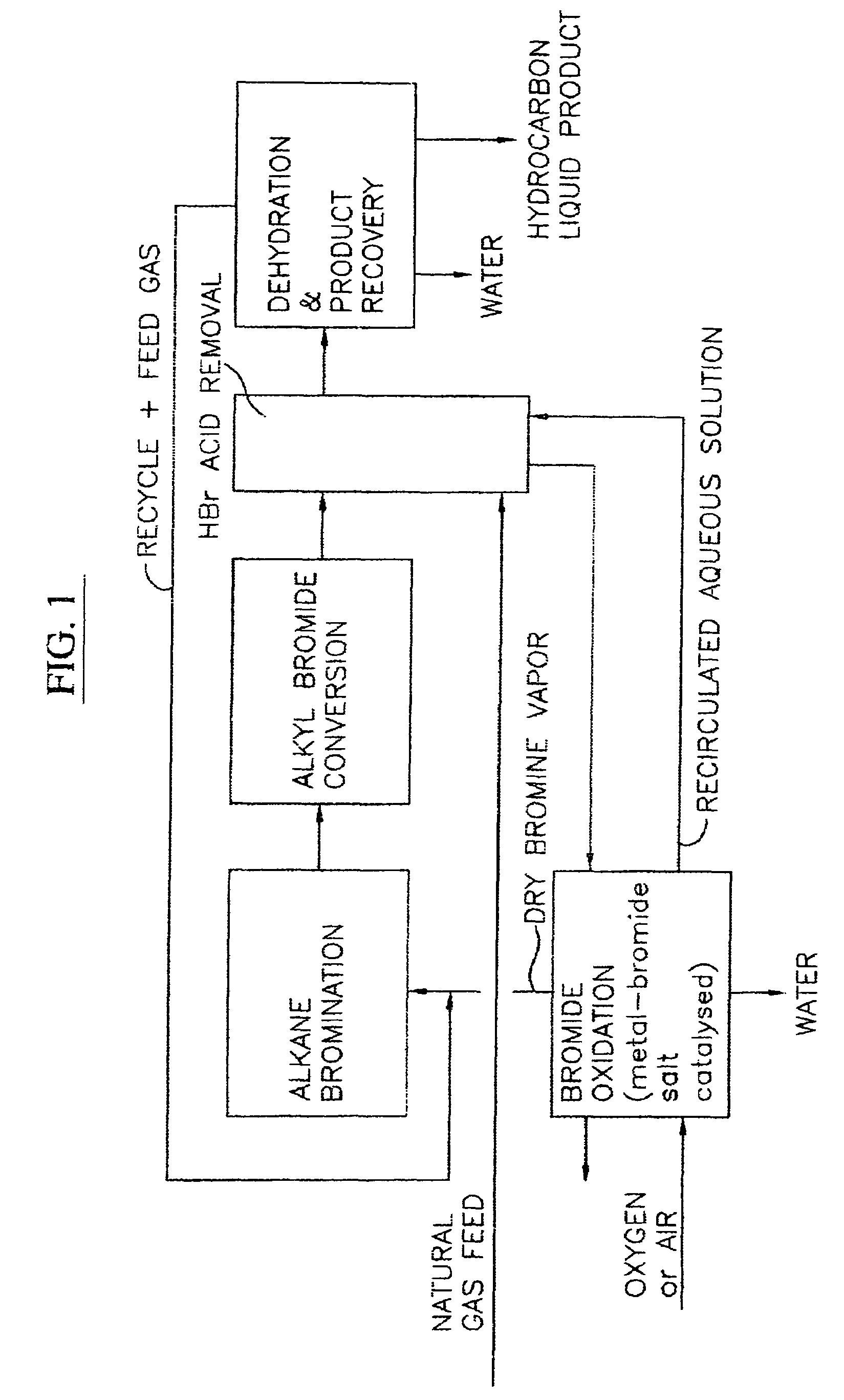
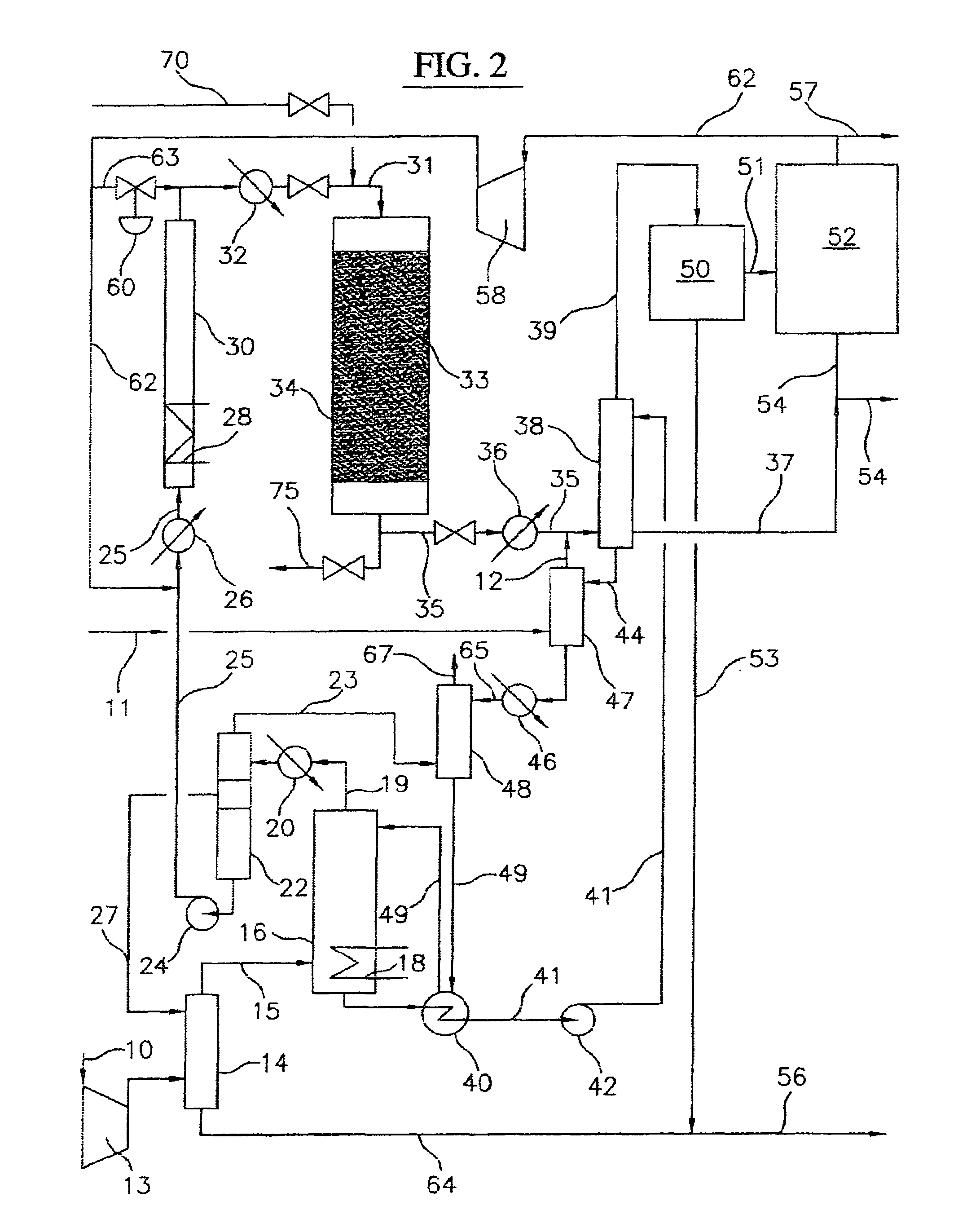
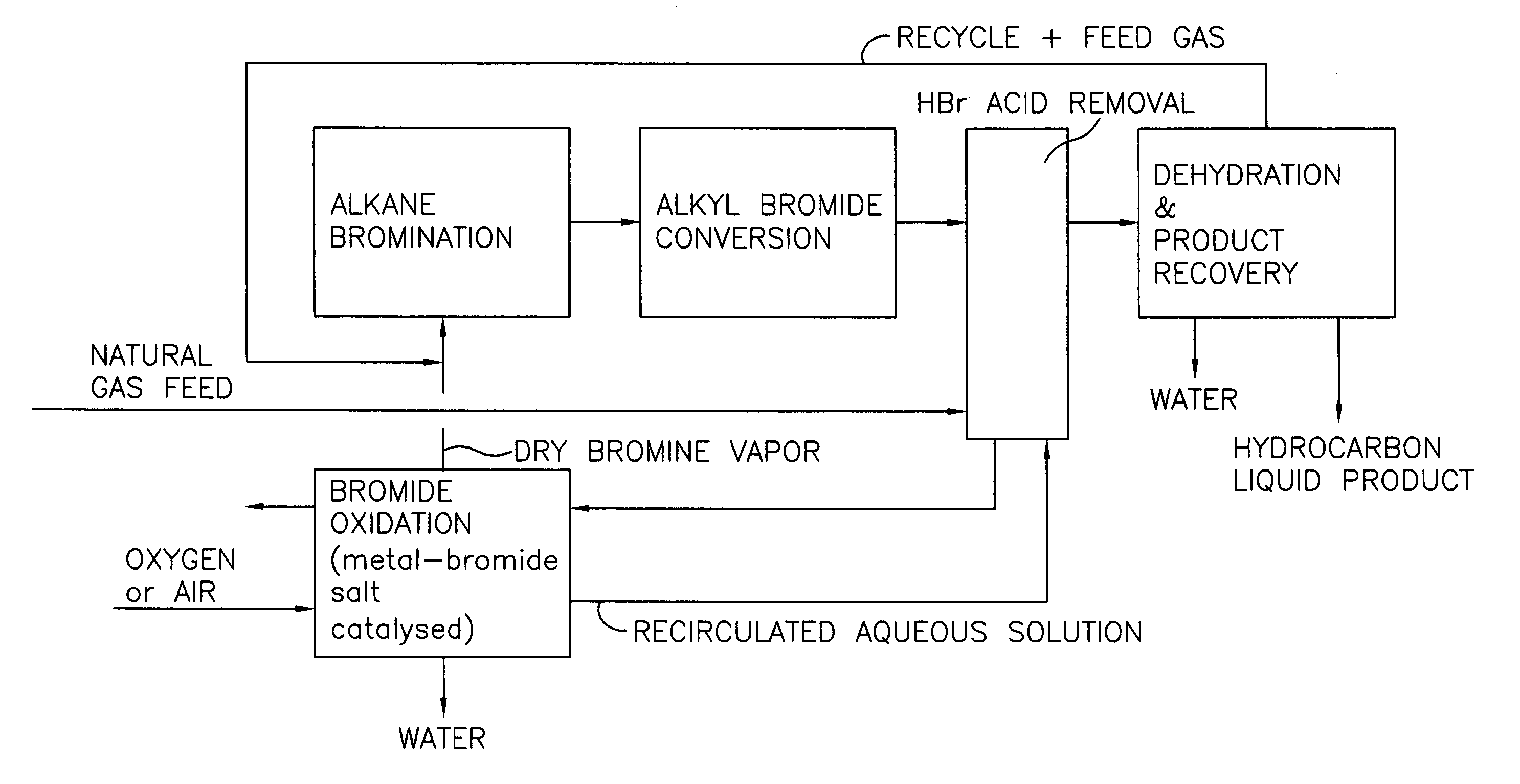
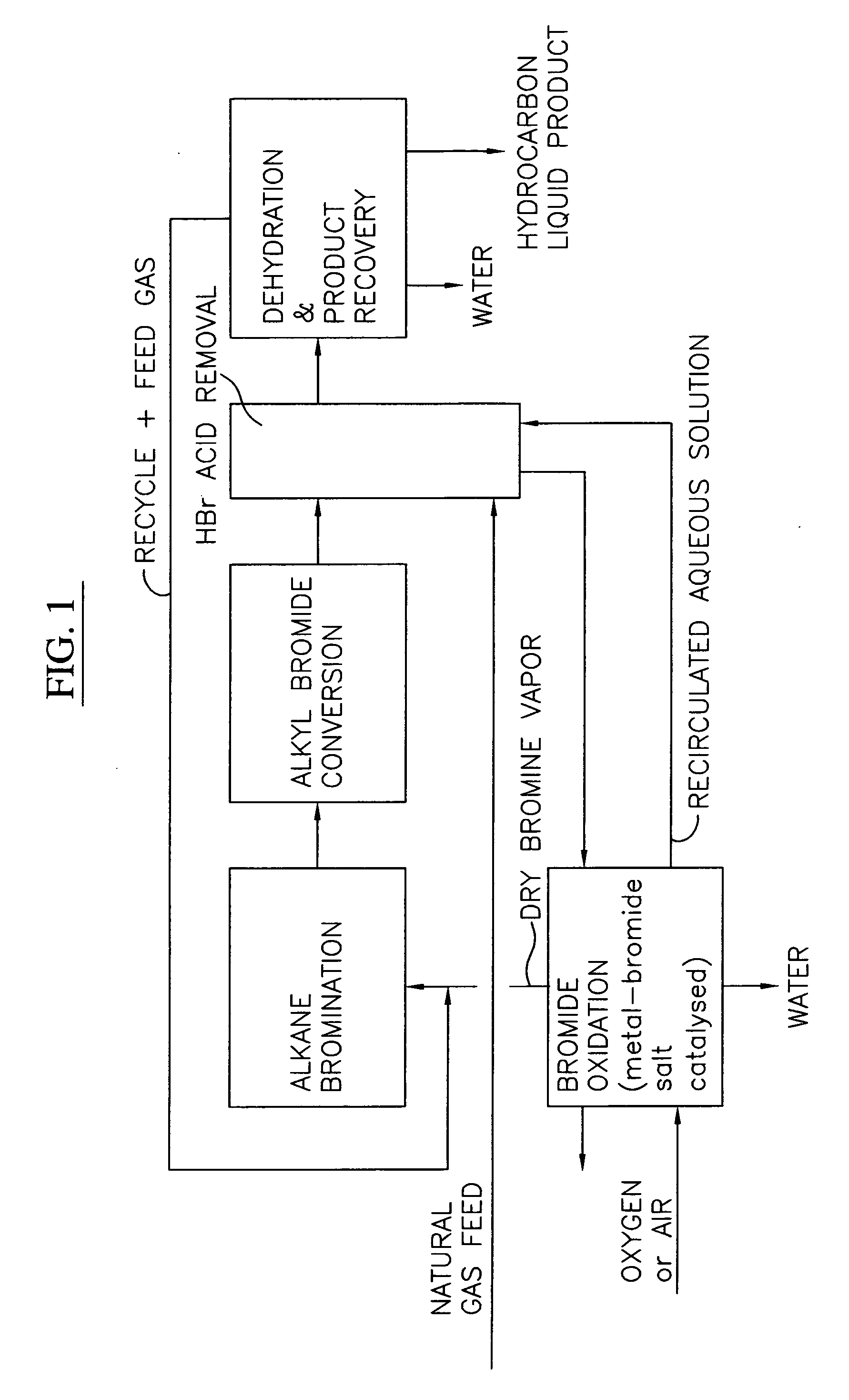
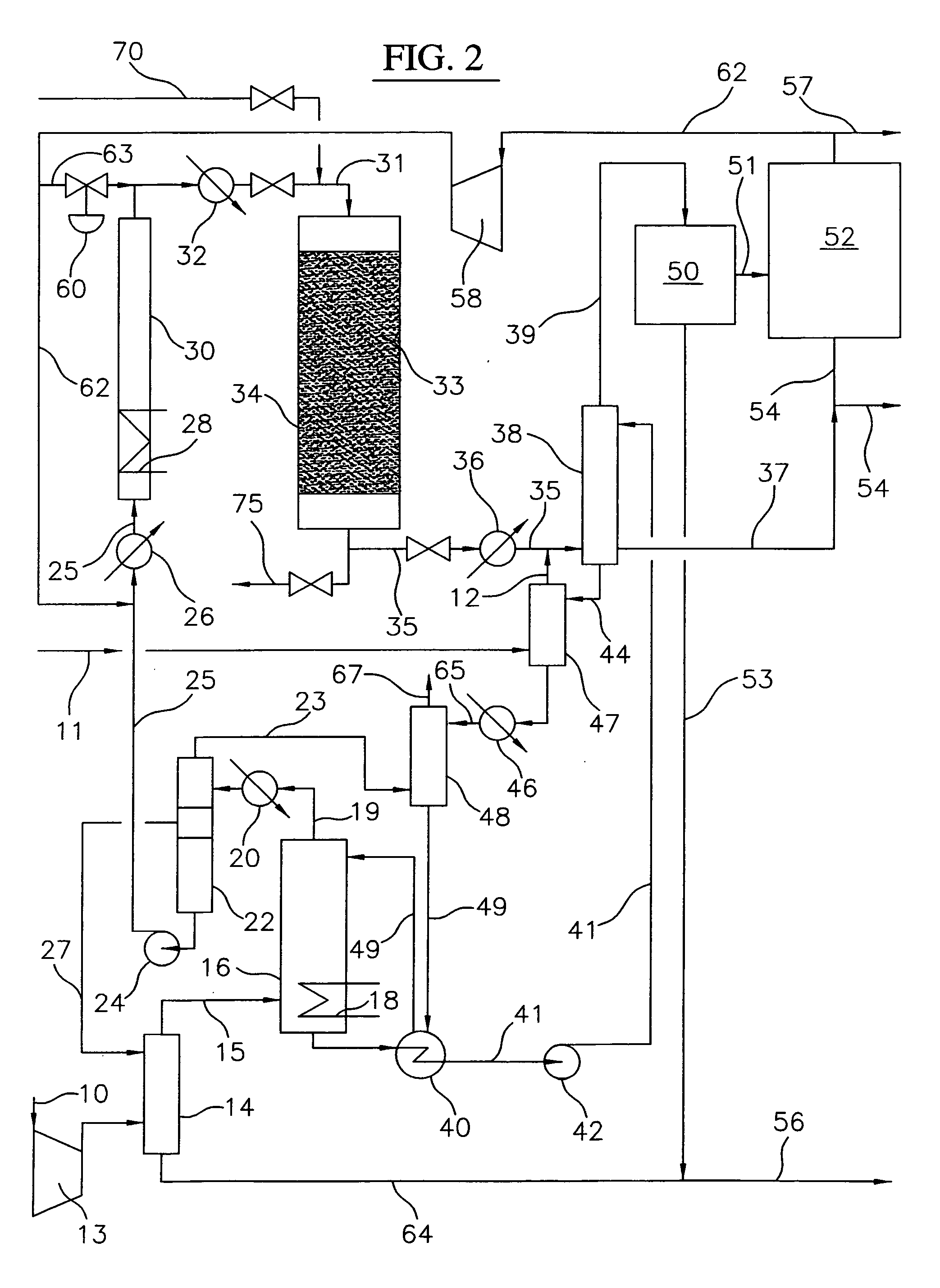
A process for converting gaseous alkanes to liquid hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as a ZSM-5 zeolite, at a temperature of from about 150° C. to about 400° C. so as to form higher molecular weight hydrocarbons and hydrobromic acid vapor. Hydrobromic acid vapor is removed from the higher molecular weight hydrocarbons. A portion of the propane and butane is removed from the higher molecular weight hydrocarbons and reacted with the mixture of alkyl bromides and hydrobromic acid over the synthetic crystalline alumino-silicate catalyst to form C5+ hydrocarbons.
Owner:SULZER MANAGEMENT AG
Process for converting gaseous alkanes to liquid hydrocarbons
Owner:SULZER MANAGEMENT AG
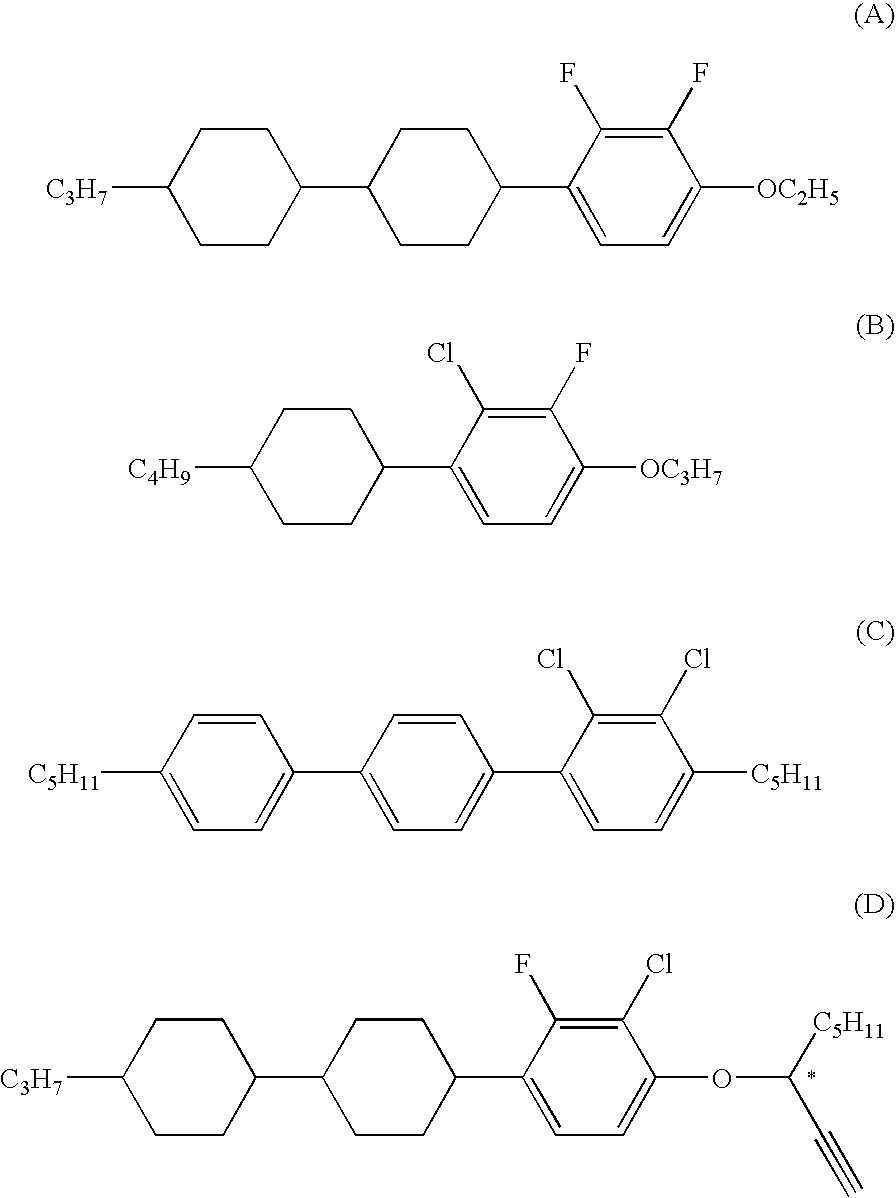
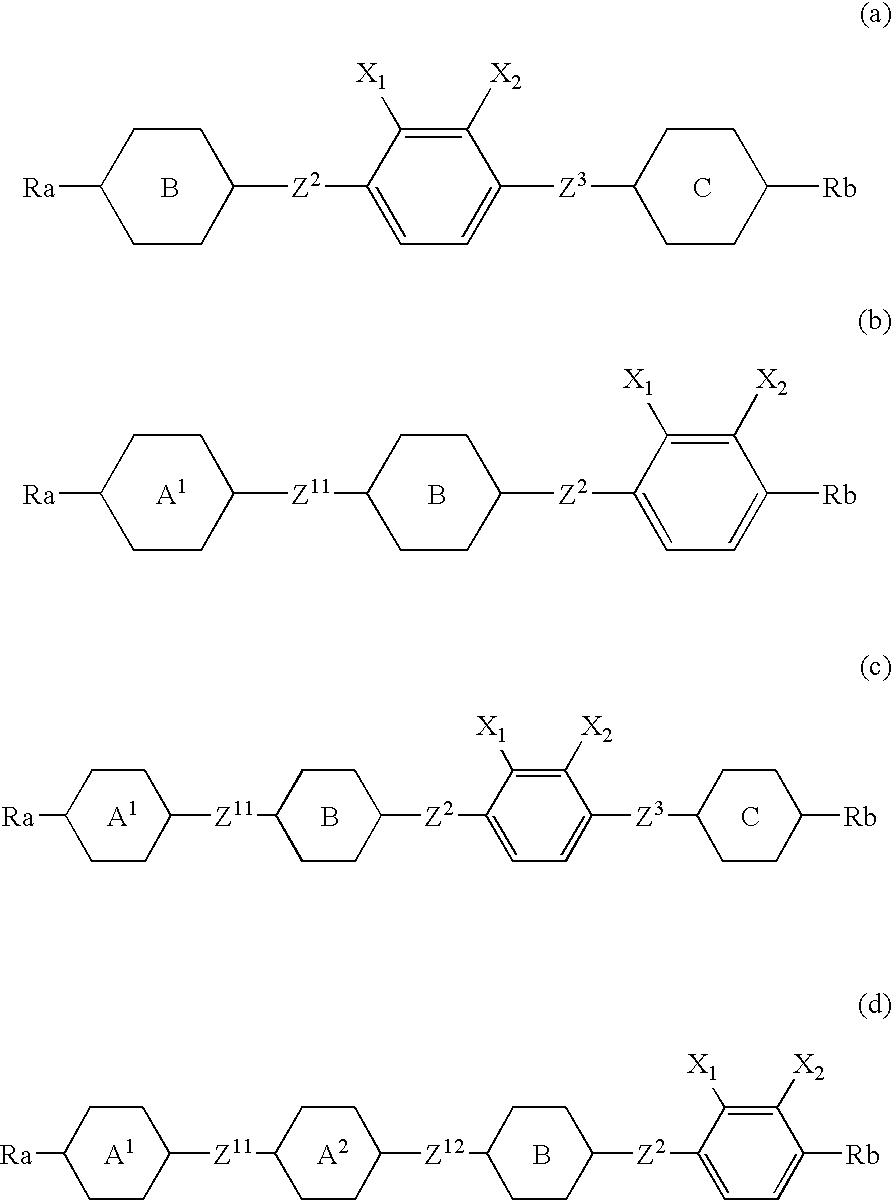
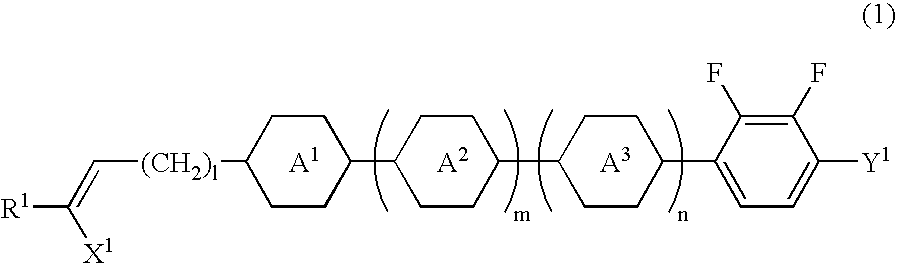
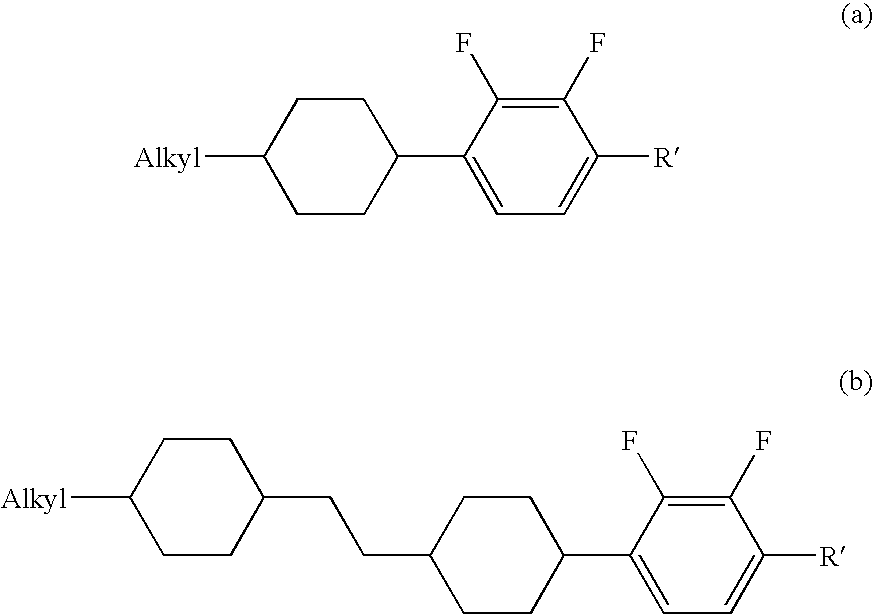
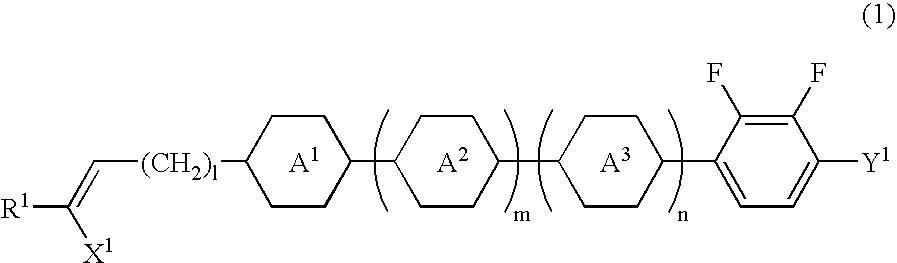
Chlorofluorobenzene liquid crystal compound, liquid crystal composition, and liquid crystal display device
ActiveUS20060198968A1Improve compatibilitySmall viscosityLiquid crystal compositionsHalogenated hydrocarbon preparationCrystallographyDielectric anisotropy
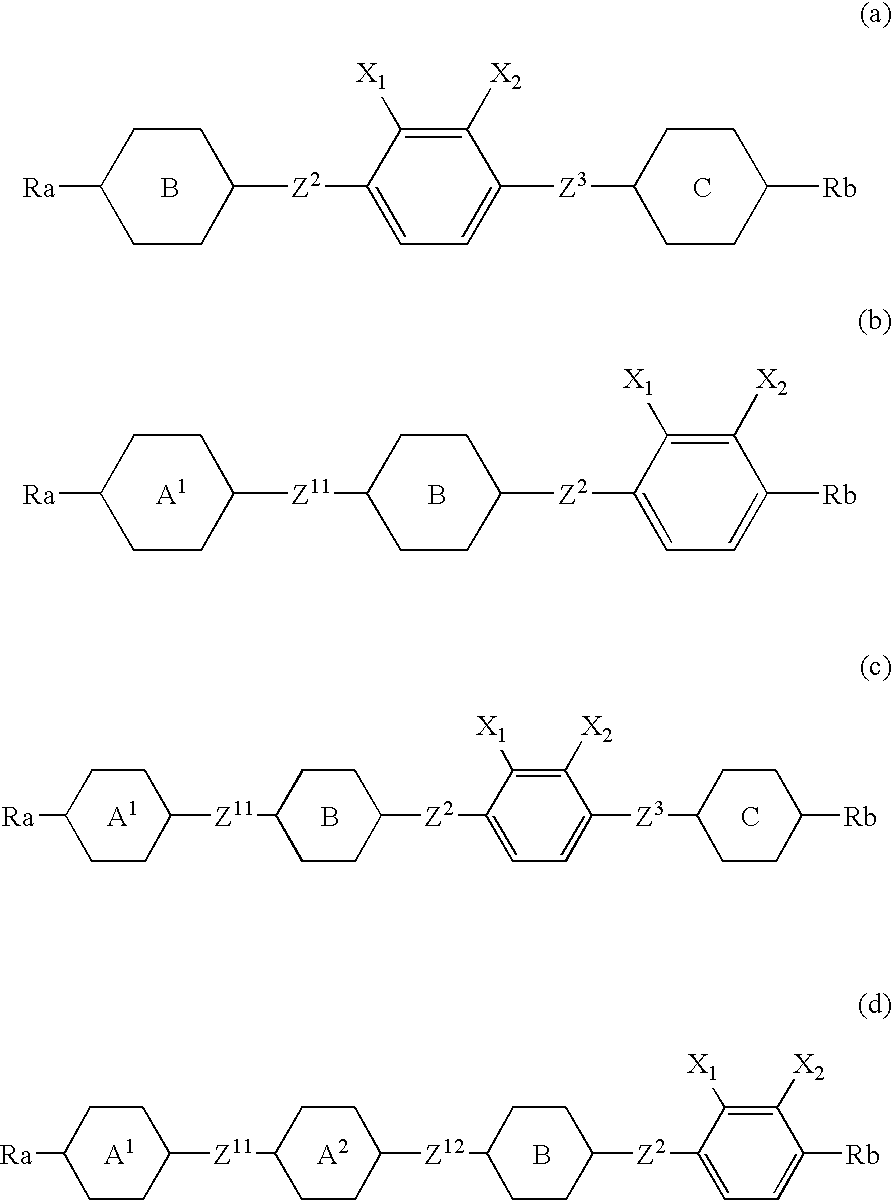
Such a liquid crystal compound is provided that has stability to heat, light and so forth, has a nematic phase in a wide temperature range, has a small viscosity, a suitable optical anisotropy, and suitable elastic constants K33 and K11 (K33: bend elastic constant, K11: splay elastic constant), and has suitable negative dielectric anisotropy and excellent compatibility with other liquid crystal compounds. A liquid crystal composition containing the liquid crystal compound, and a liquid crystal display device containing the liquid crystal composition are also provided. The liquid crystal compound is represented by one of formulas (a) to (d), the liquid crystal composition contains the liquid crystal compound, and the liquid crystal display device contains the liquid crystal composition: wherein Ra and Rb are independently linear alkyl or linear alkoxy, rings A1, A2, B and C are independently trans-1,4-cyclohexylene or 1,4-phenylene, Z11, Z12, Z2 and Z3 are independently a single bond or alkylene, and one of X1 and X2 is fluorine, and the other thereof is chlorine.
Owner:JNC PETROCHEM +1
Alkenyl compound having a negative delta epsilon value, liquid crystal composition, and liquid crystal display device
InactiveUS20040065866A1Low viscosityImprove solubilityLiquid crystal compositionsSilicon organic compoundsLiquid crystallineDithiane
Owner:JNC CORP
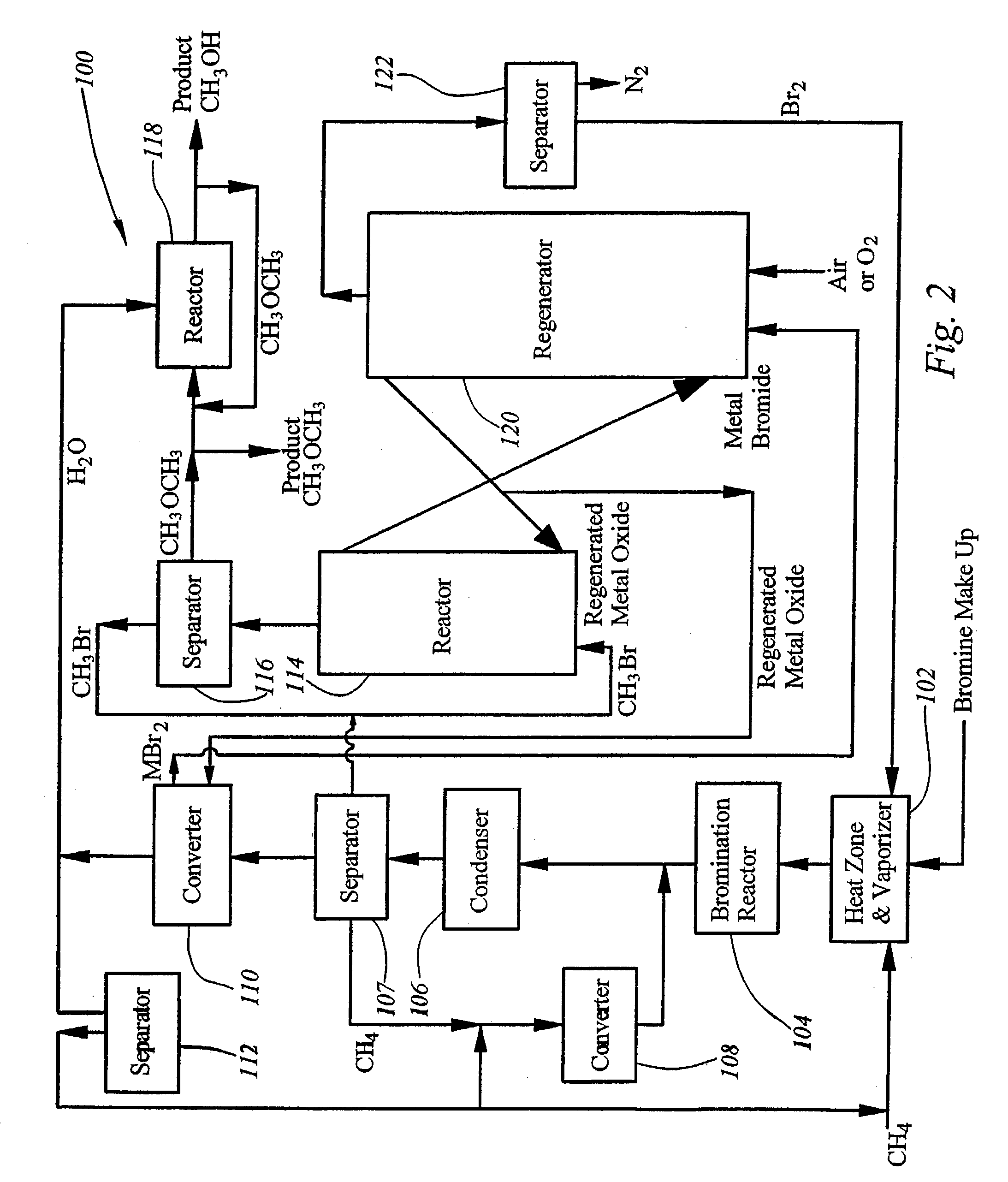
Integrated process for synthesizing alcohols, ethers, aldehydes, and olefins from alkanes
InactiveUS7148390B2Less corrosiveImprove securityOrganic compound preparationChemical industryHydrogen halideAlkane
Alcohols, ethers, aldehydes, and olefins are manufactured from alkanes by mixing an alkane and a halogen selected from the group including chlorine, bromine, and iodine in a reactor to form alkyl halide and hydrogen halide. The alkyl halide only or the alkyl halide and the hydrogen halide are directed into contact with metal oxide to form an alcohol and / or an ether, or an olefin and metal halide. The metal halide is oxidized to form original metal oxide and halogen, both of which are recycled.
Owner:REACTION 35 LLC
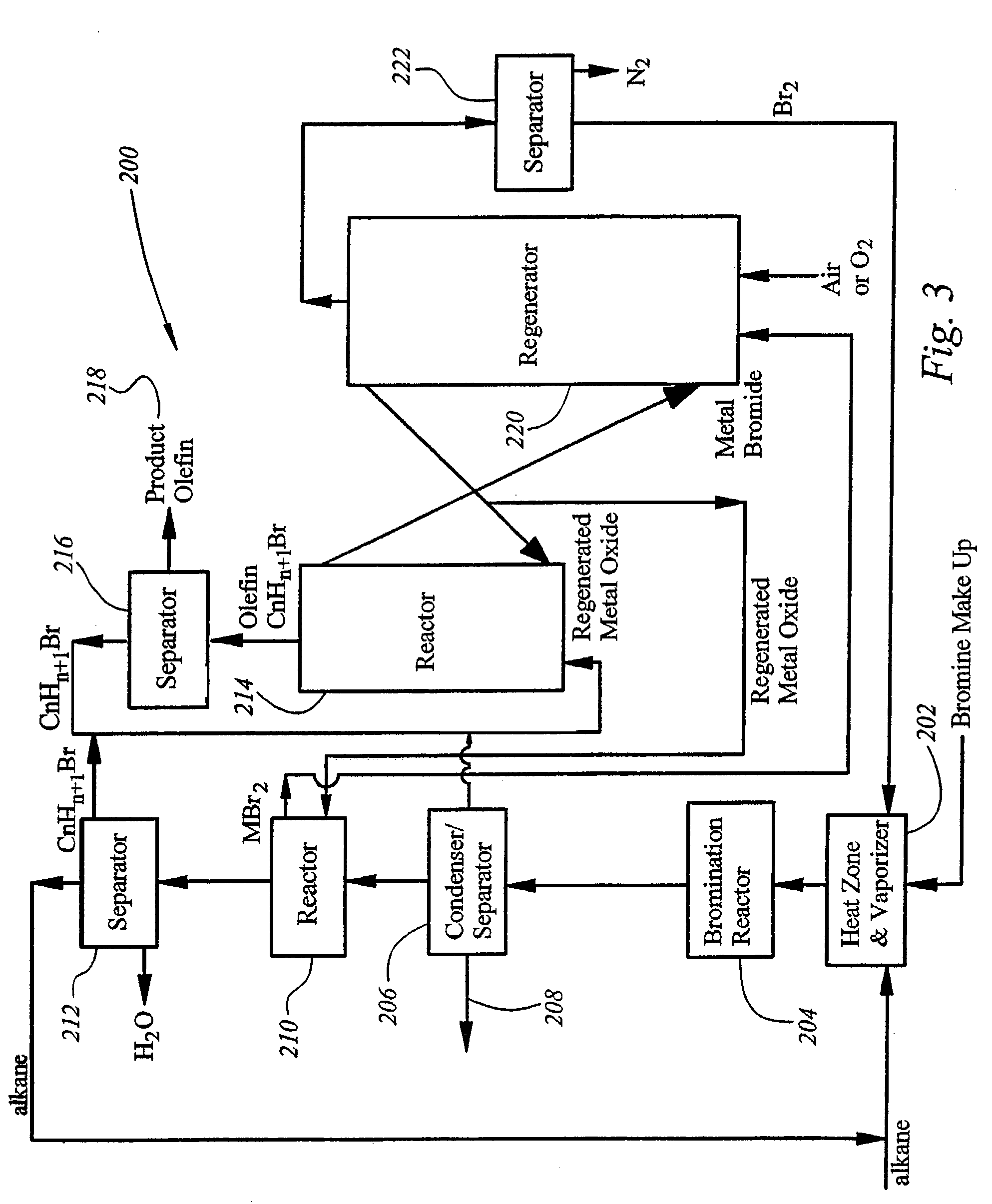
Process for converting gaseous alkanes to olefins and liquid hydrocarbons
InactiveUS20060100469A1High selectivityAvoid disadvantagesBromide preparationRefining with non-metalsAlkaneBromine
A process for converting gaseous alkanes to olefins and liquid hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as an X or Y type zeolite, at a temperature of from about 250° C. to about 500° C. so as to form olefins, higher molecular weight hydrocarbons and hydrobromic acid vapor. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons and to generate bromine from the hydrobromic acid for use in the process.
Owner:MARATHON GTF TECH
Epoxide and fluorinated epoxide stabilizers for fluoroolefins
The present invention relates to compositions comprising at least one fluoroolefin and an effective amount of stabilizer that may be an epoxide, fluorinated epoxide or oxetane, or a mixture thereof with other stabilizers. The stabilized compositions may be useful in cooling apparatus, such as refrigeration, air-conditioning, chillers, and heat pumps, as well as in applications as foam blowing agents, solvents, aerosol propellants, fire extinguishants, and sterilants.
Owner:THE CHEMOURS CO FC LLC
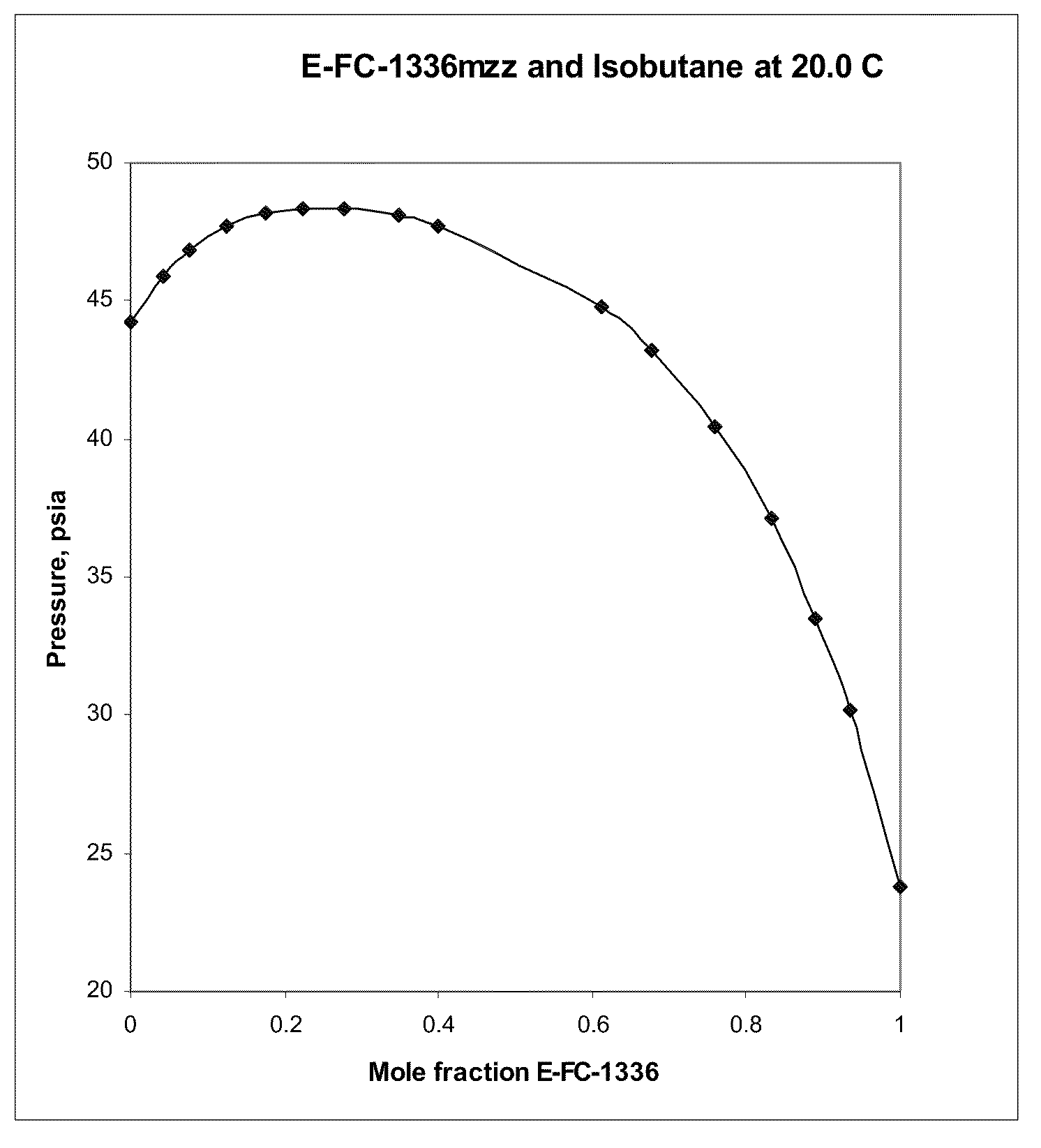
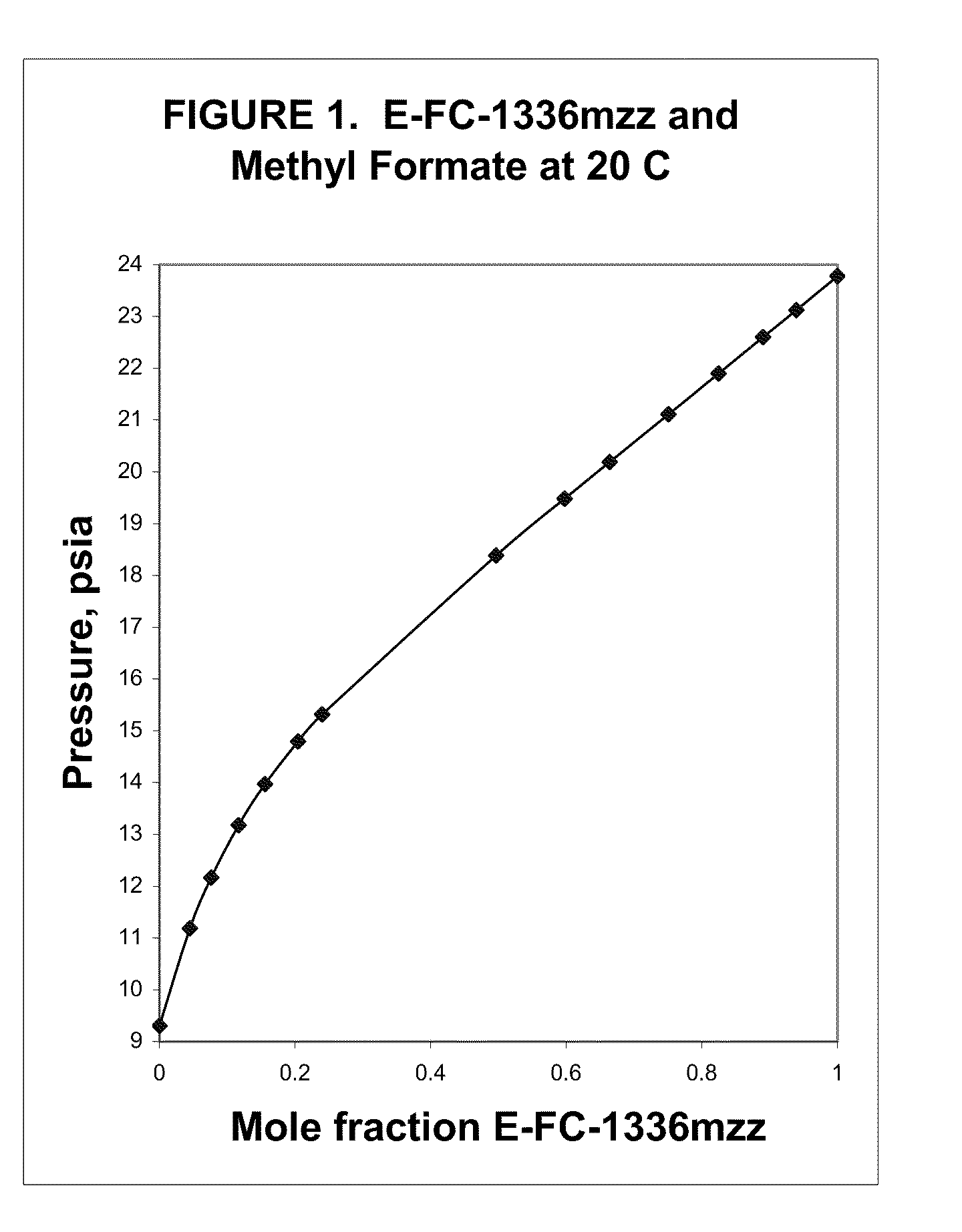
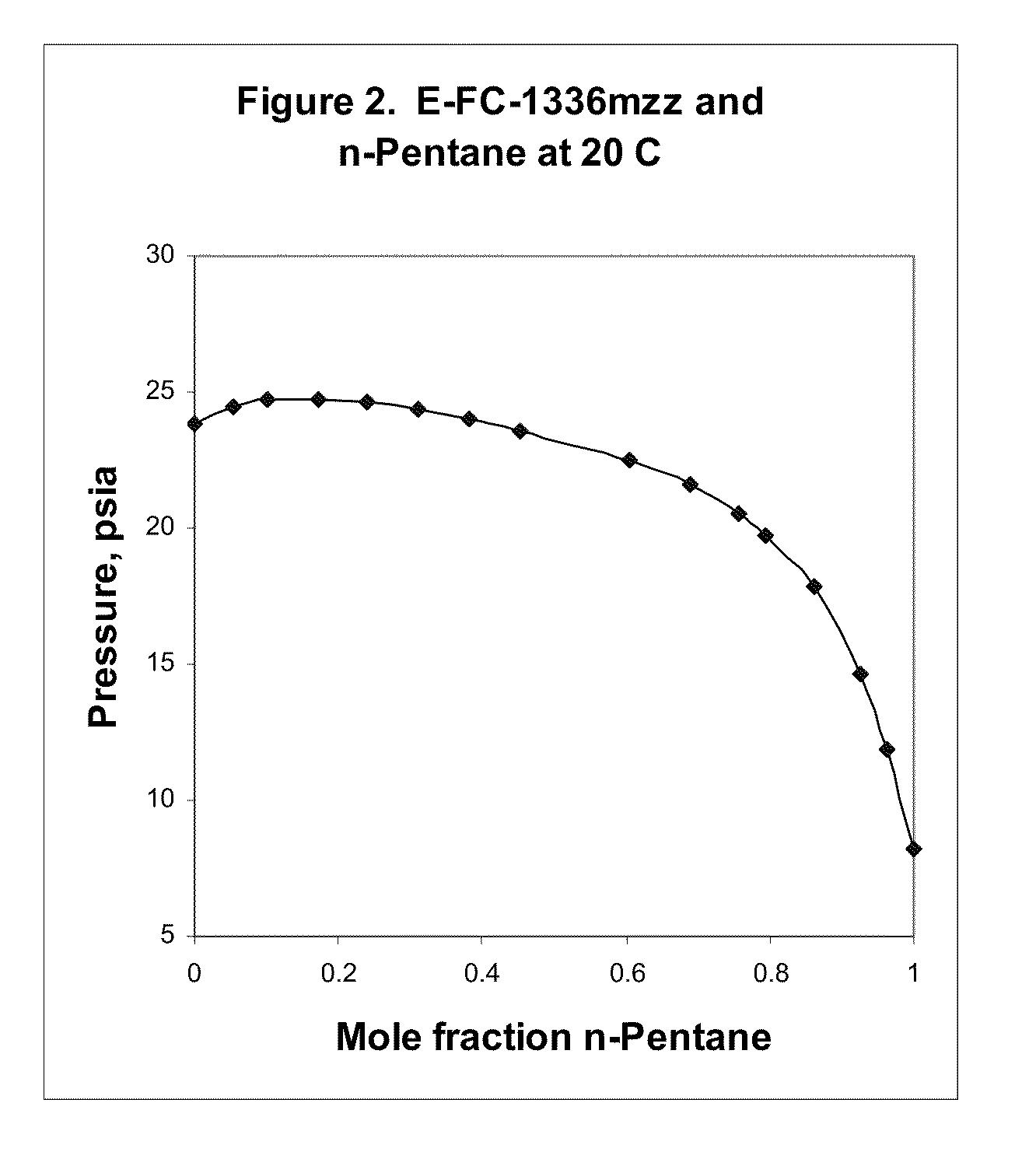
Azeotropic and azeotrope-like compositions of e-1,1,1,4,4,4-hexafluoro-2-butene
Azeotropic or azeotrope-like compositions are disclosed. The azeotropic or azeotrope-like compositions are mixtures of E-1,1,1,4,4,4-hexafluoro-2-butene with methyl formate, n-pentane, 2-methylbutane trans-1,2-dichloroethylene, 1,1,1,3,3-pentafluoropropane, n-butane or isobutane. Also disclosed is a process of preparing a thermoplastic or thermoset foam by using such azeotropic or azeotrope-like compositions as blowing agents. Also disclosed is a process of producing refrigeration by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as solvents. Also disclosed is a process of producing an aerosol product by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as heat transfer media. Also disclosed is a process of extinguishing or suppressing a fire by using such azeotropic or azeotrope-like compositions. Also disclosed is a process of using such azeotropic or azeotrope-like compositions as dielectrics.
Owner:THE CHEMOURS CO FC LLC
Method for preparation of organofluoro compounds in alcohol solvents
InactiveUS20100113763A1Inhibition formationReduced responseIsotope introduction to heterocyclic compoundsIn-vivo radioactive preparationsAlcoholIsotope
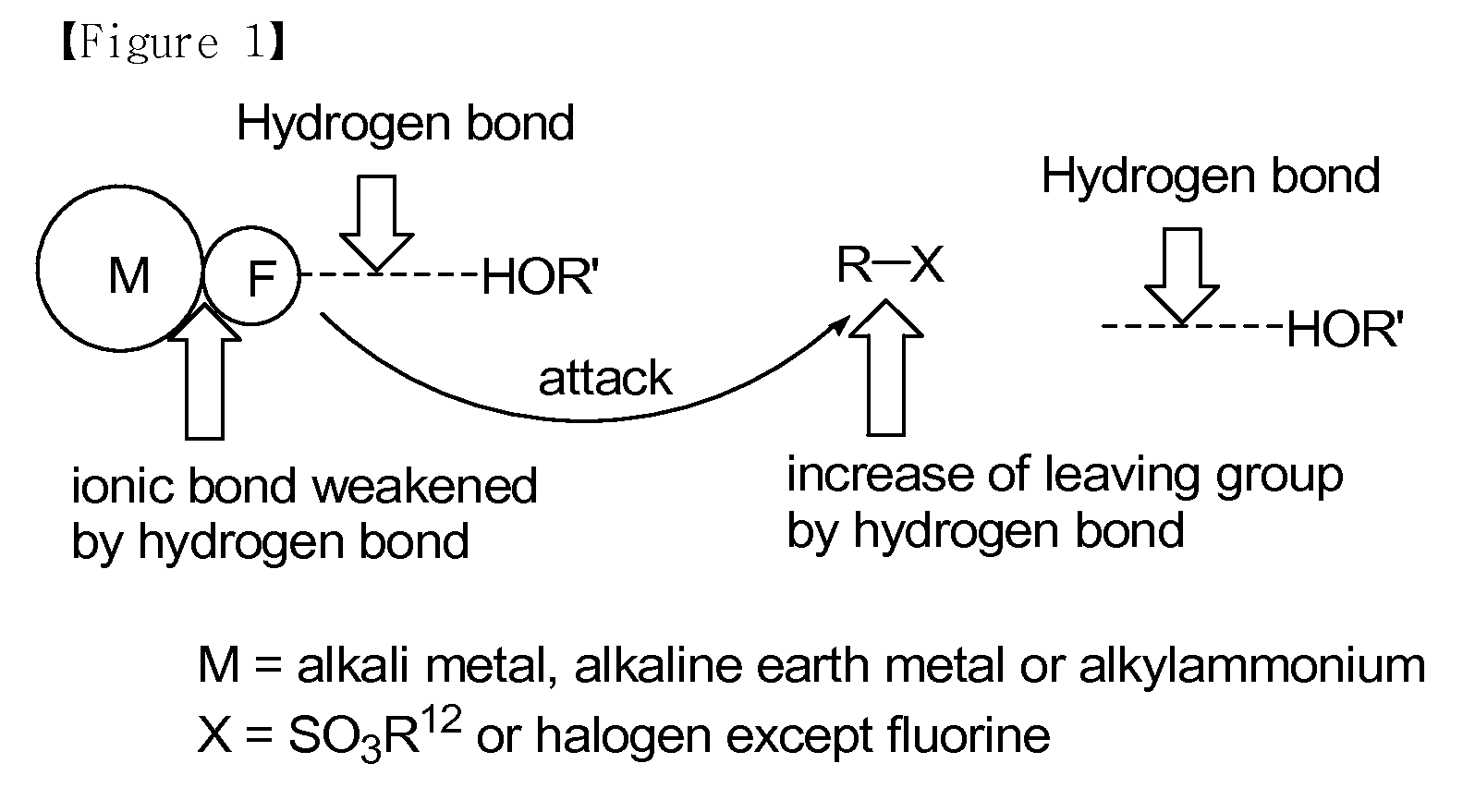
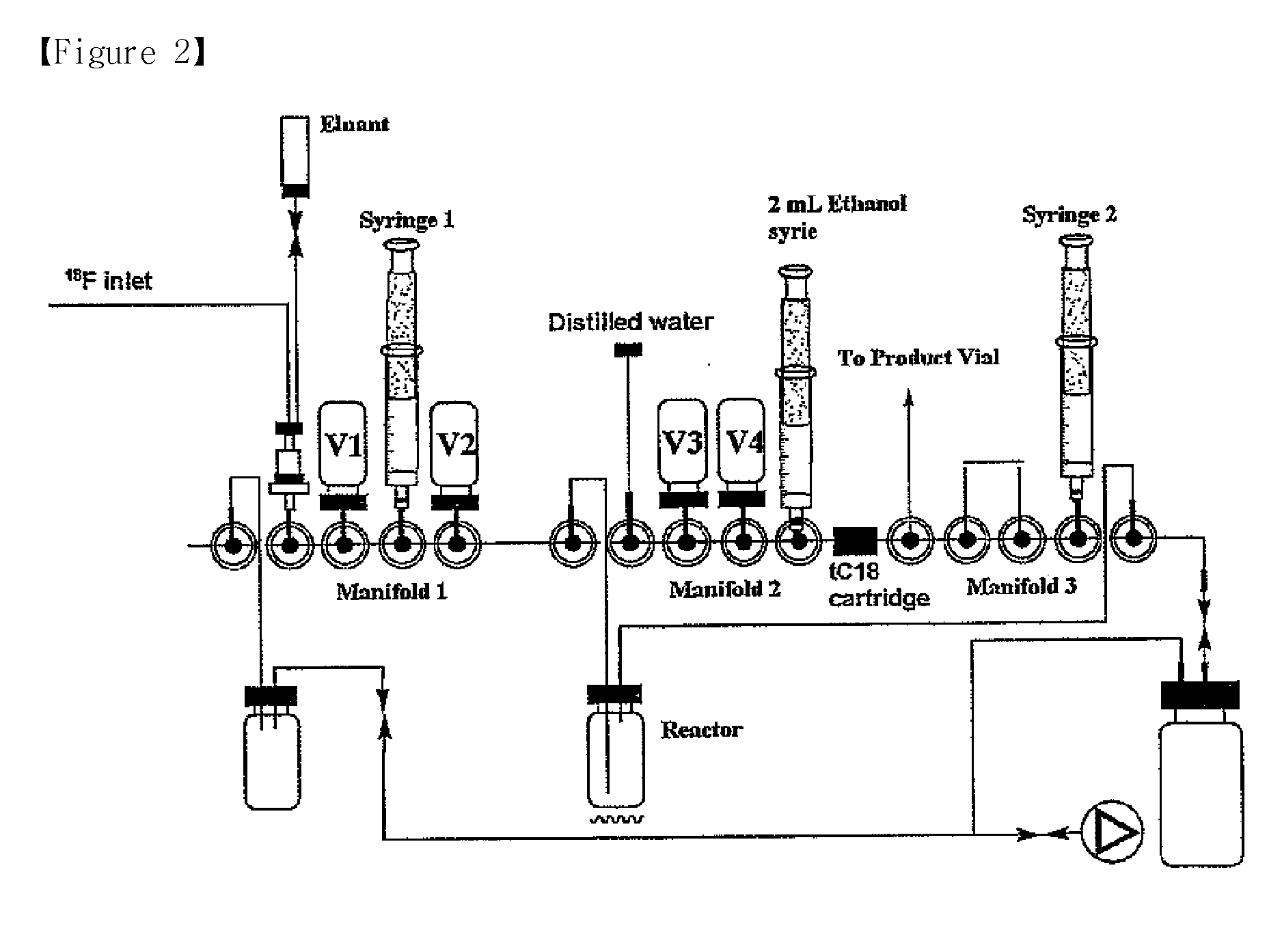
The present invention relates to a method for preparation of organofluoro compounds containing radioactive isotope fluorine-18. More particularly, the present invention relates to a method for preparation of organofluoro compound [18F]florbetaben or [18F]AV-45 having <Chemistry Formula 11> and <Chemistry Formula 12>, respectively, by reacting fluorine salt containing radioactive isotope fluorine-18 with alkyl halide or alkyl sulfonate in the presence of alcohol of Chemistry Formula 1 as a solvent to obtain high yield of organofluoro compound. Synthesis reaction according to the present invention may be carried out under mild condition to give high yield of the organofluoro compounds and the reaction time is decreased, and thereby is suitable for the mass production of the organofluoro compounds.
Owner:FUTURECHEM +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com