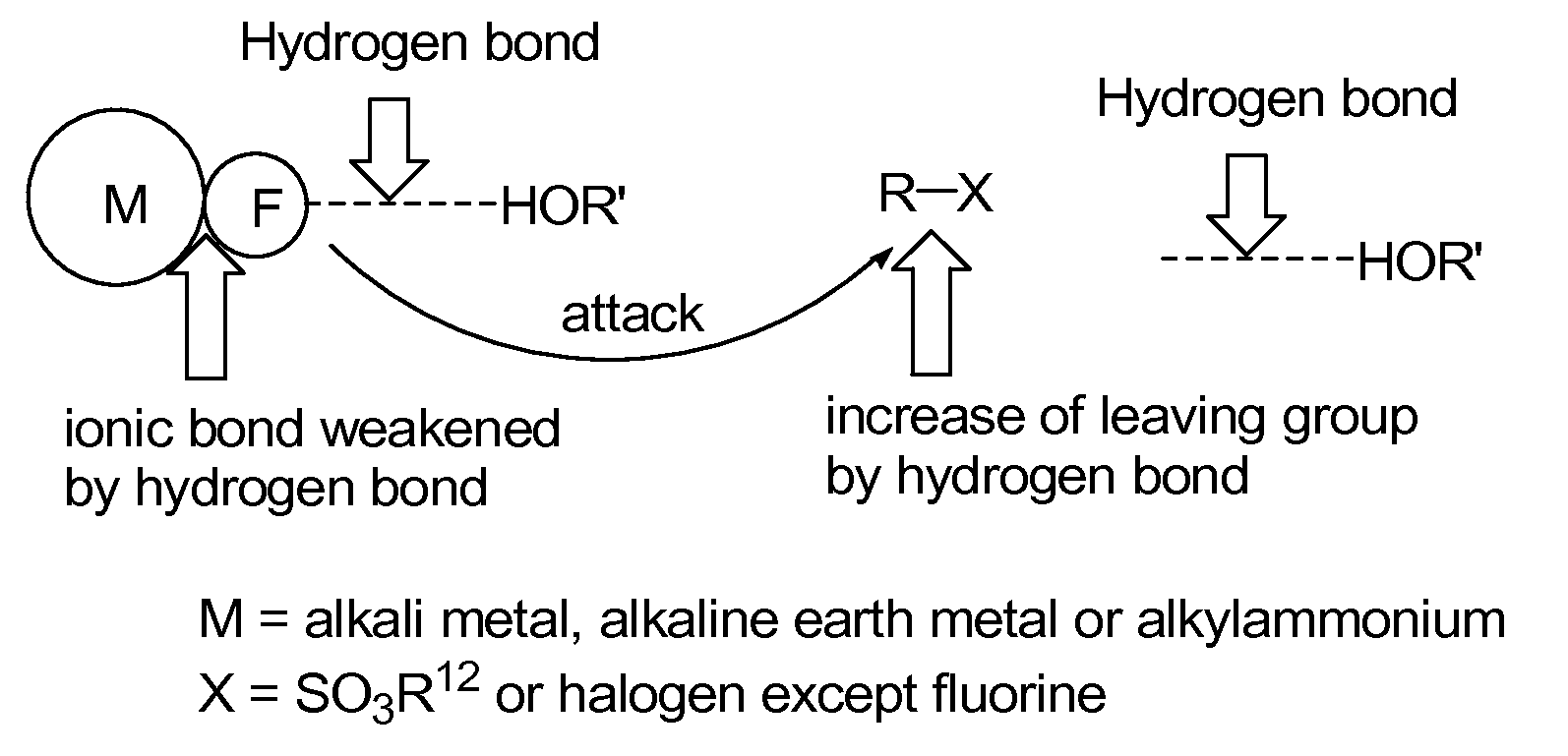
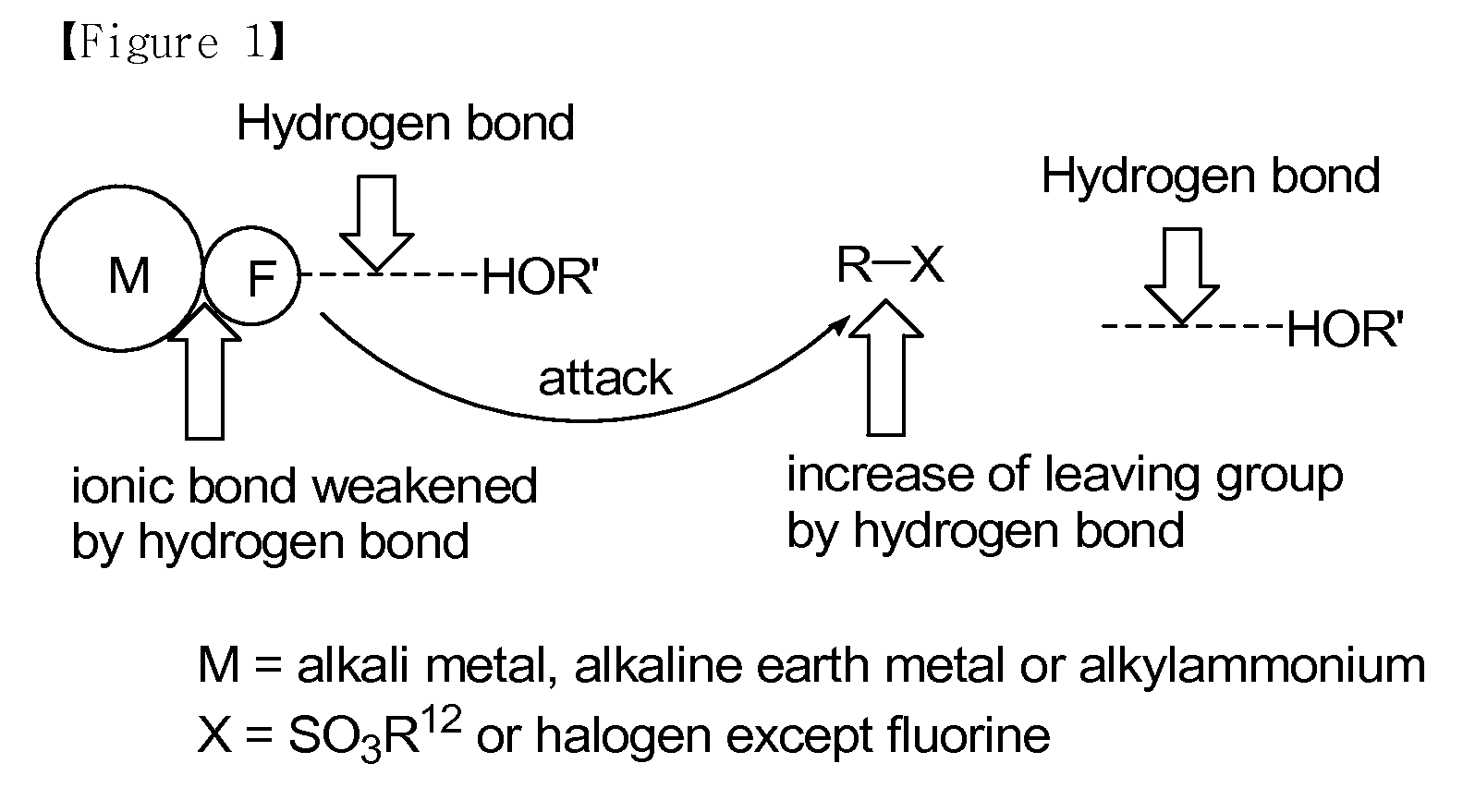
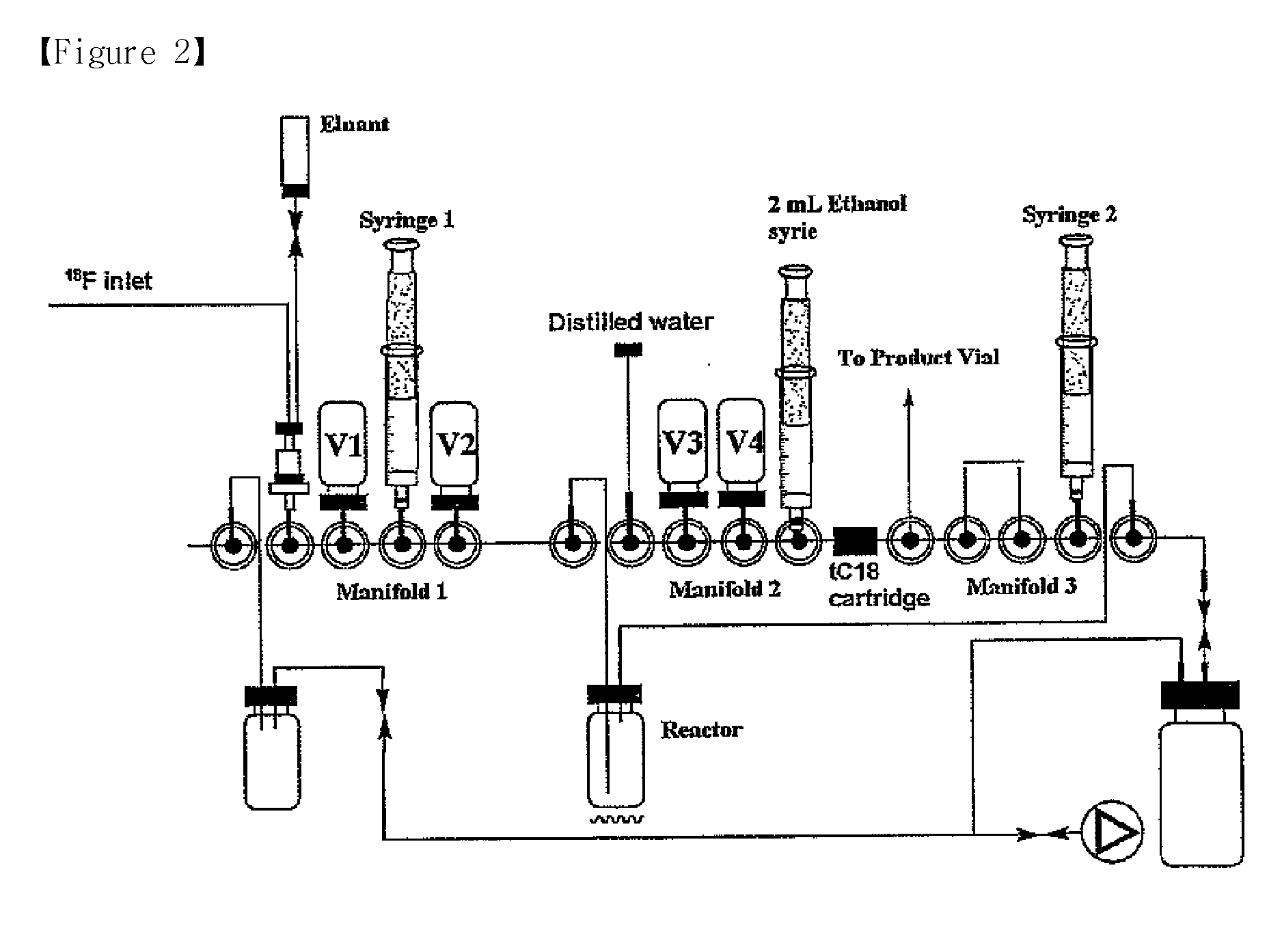
Method for preparation of organofluoro compounds in alcohol solvents
a technology of organofluoro compounds and alcohol solvents, which is applied in the field of preparation of organofluoro compounds in alcohol solvents, can solve the problems of low yield, preparation method, and inability to prepare nucleophilic fluorination reactions, and achieves low reactivity, suppressing side reactions, and increasing the nucleophilic substitution reactivity of fluorine salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Organofluoro Compounds 1
[0060]280 mg (1.0 mmol) of 2-(3-methanesulfonyloxypropoxy)naphthalene and 456 mg (3.0 mmol) of cesium fluoride are added to a solvent of 4.0 mL of t-butanol. The reaction mixture is stirred for 6 hrs at 80° C. 7 mL of ethyl ether is added to the reaction mixture to remove metal salts. After filtration, the filtrate is concentrated by reduced pressure distiller. 188 mg (92% yield) of 2-(3-fluoropropoxy)naphthalene is obtained by column chromatography (ethyl acetate:n-hexane=1:20).
example 2
Preparation of Organofluoro Compounds 2-7
[0061]The reactions are carried out by the same method as described in Example 1 except that kinds of alcohol solvent, reaction temperature and time are same as described in the Table 1. Organofluoro compounds are prepared as shown in the Table 1. Chemical Equation 3 shows 2-(3-fluoropropoxy)naphthalene (A), 2-(3-hydroxypropoxy)naphthalene (B), 2-(3-allyloxy)naphthalene (C) and 2-(3-alkoxypropoxy)naphthalene (D), which are the products obtained in the preparation of the organofluoro compounds.
example 8
Preparation of Organofluoro Compounds 8
[0073]356 mg (1.0 mmol) of 2-(3-toluenesulfonyloxypropoxy)naphthalene and 456 mg (3.0 mmol) of cesium fluoride are added to 4.0 mL of t-amyl alcohol in a reaction vessel. The reaction mixture is stirred for 2 hrs at 90 C. 7 mL of ethyl ether is added to remove metal salt. After filtration, the filtrate is concentrated by a reduced pressure distiller. 190 mg (93% yield) of 2-(3-fluoropropoxy)naphthalene is obtained by column chromatography (ethyl acetate:n-hexane=1:20).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com