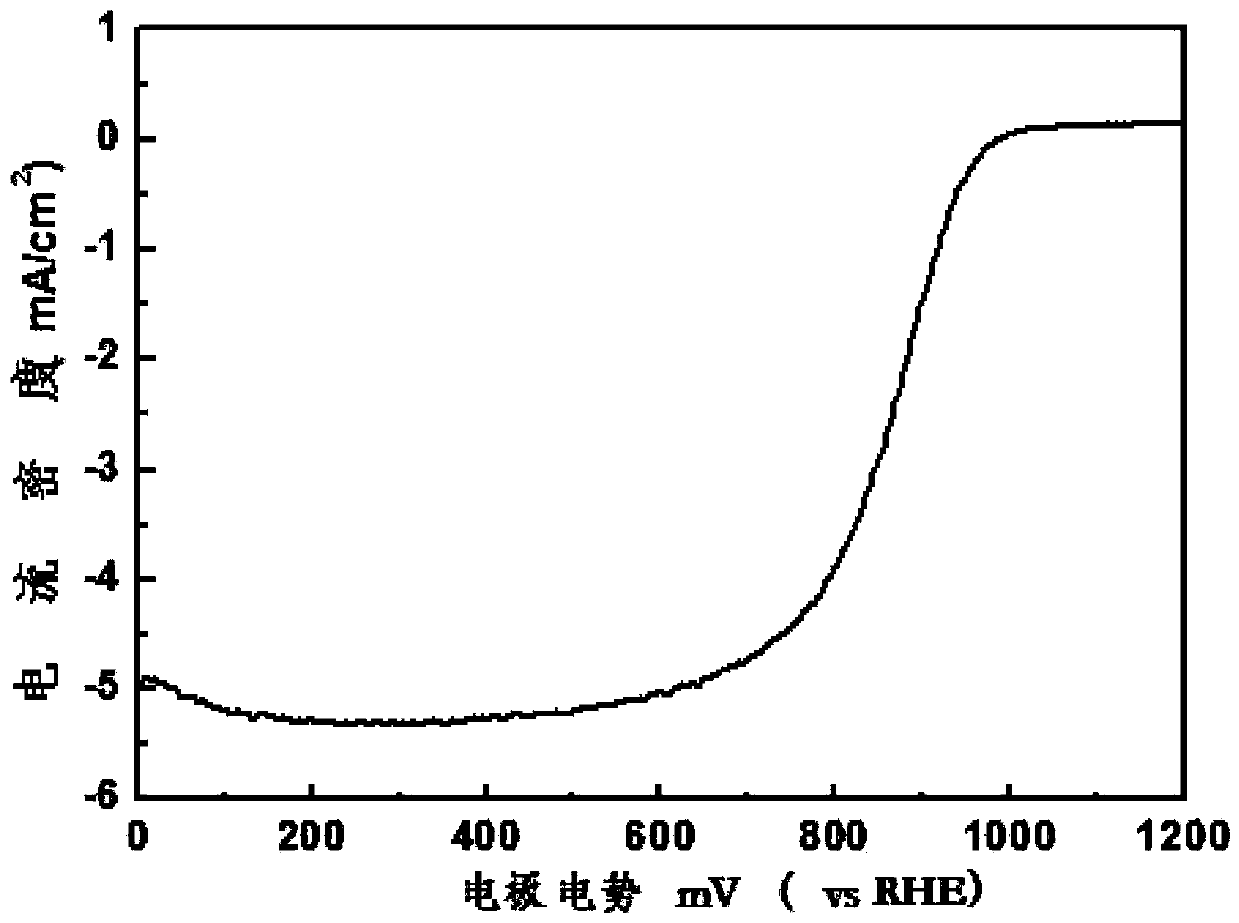
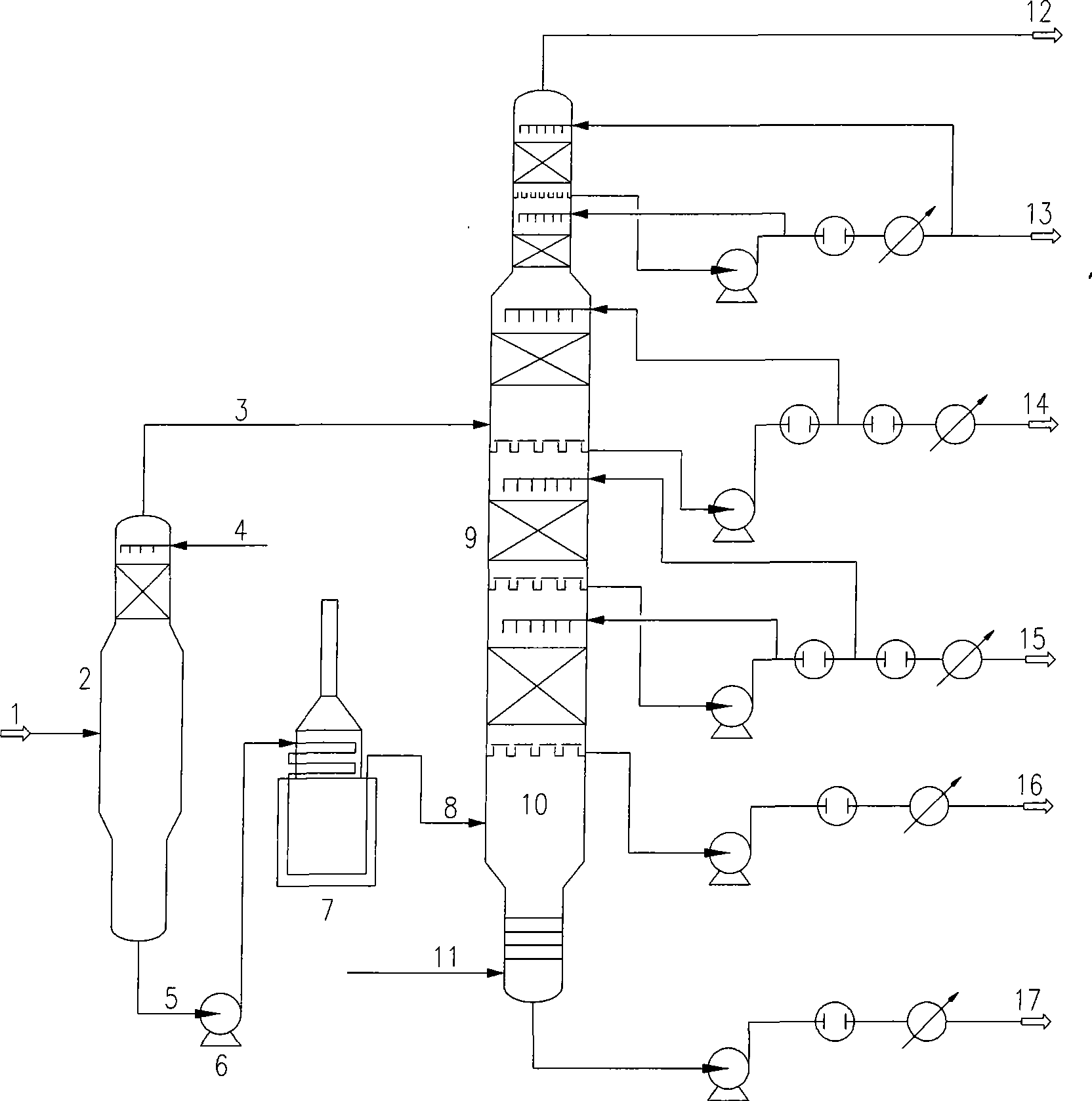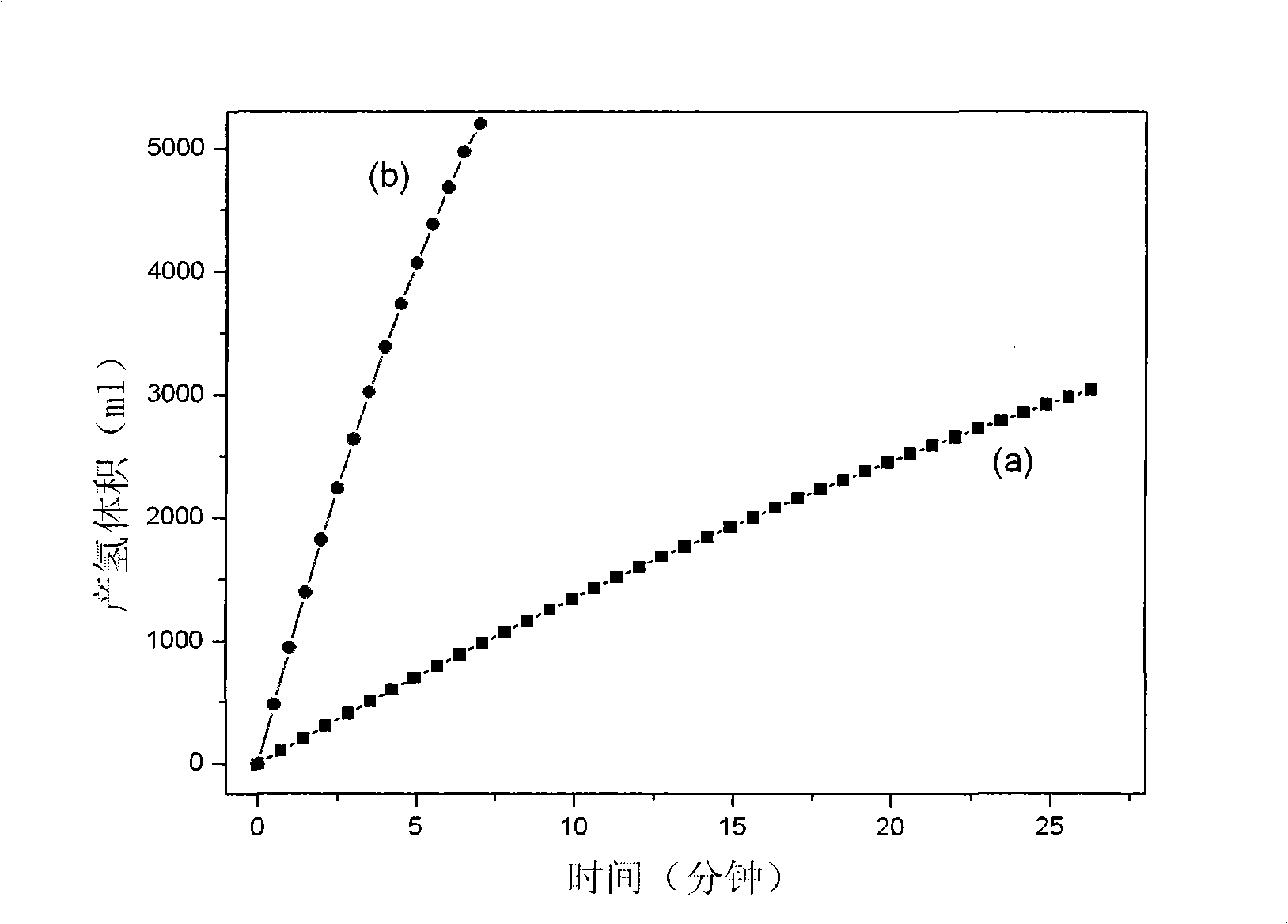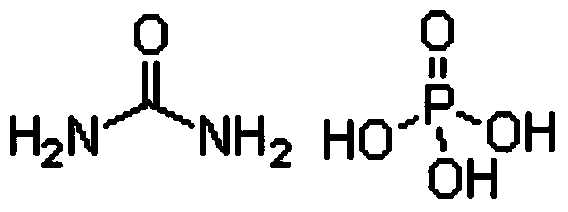Patents
Literature
7047 results about "Side product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Side products are impurities which appear during the reaction as a result of (1) side reactions that can be alternative reaction pathways or (2) further reaction/degradation of the desired product after it has formed. Once we isolate our product, it is likely to contain some impurities, and these can be either byproducts or side products.
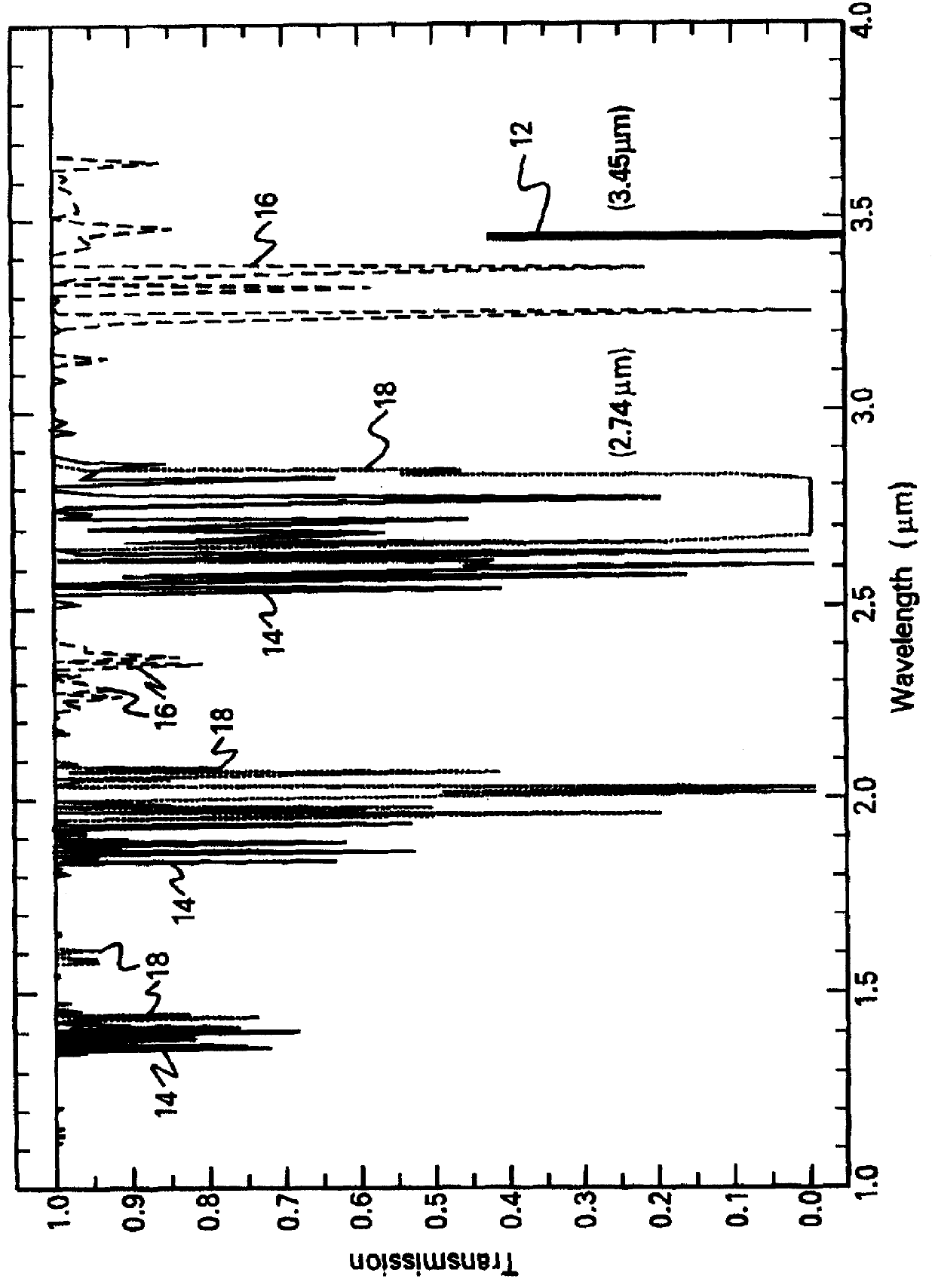
Single-primer nucleic acid amplification methods
ActiveUS7374885B2Reduce appearanceHigh levelMicrobiological testing/measurementFermentationNucleotideNucleic acid sequencing
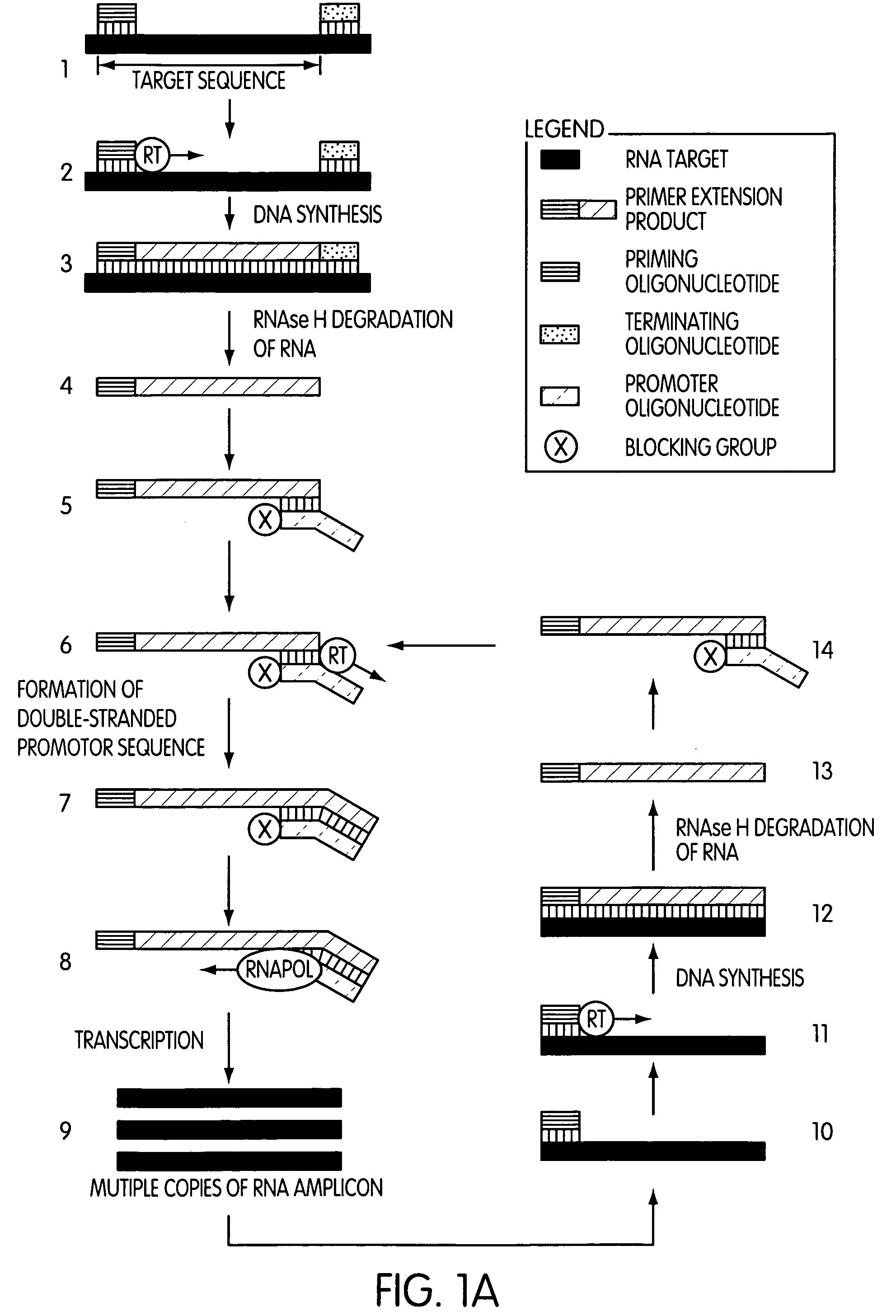
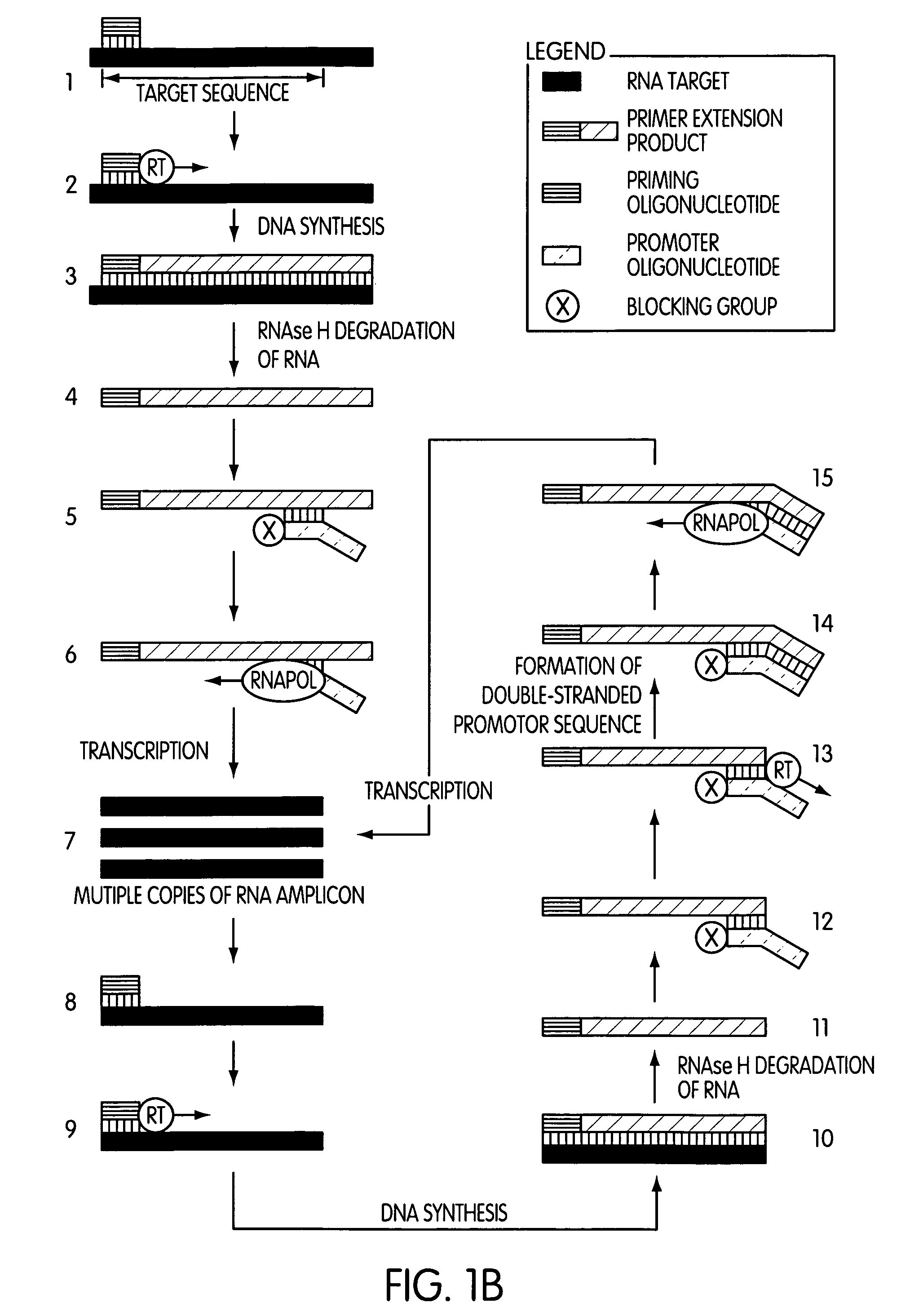
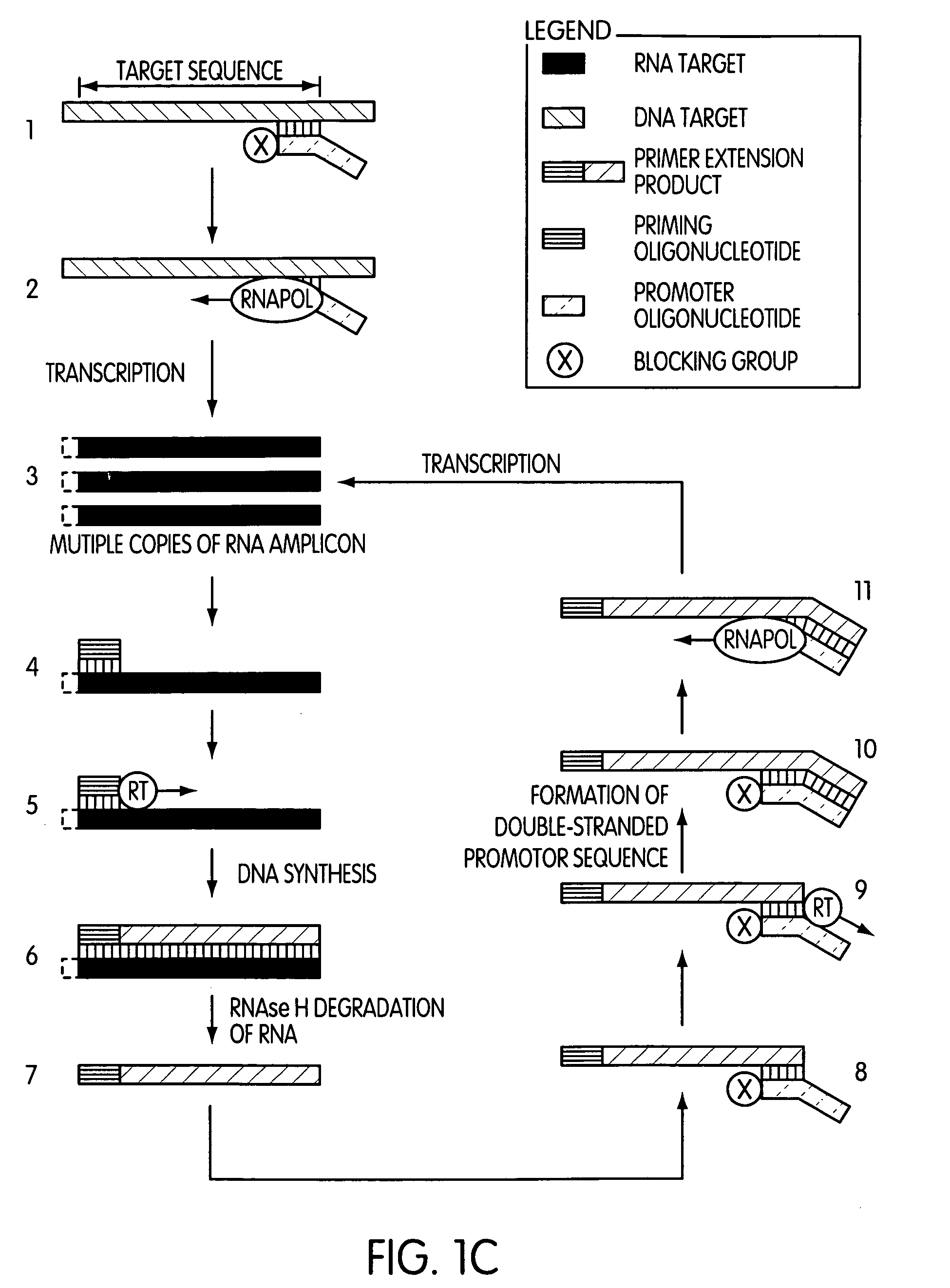
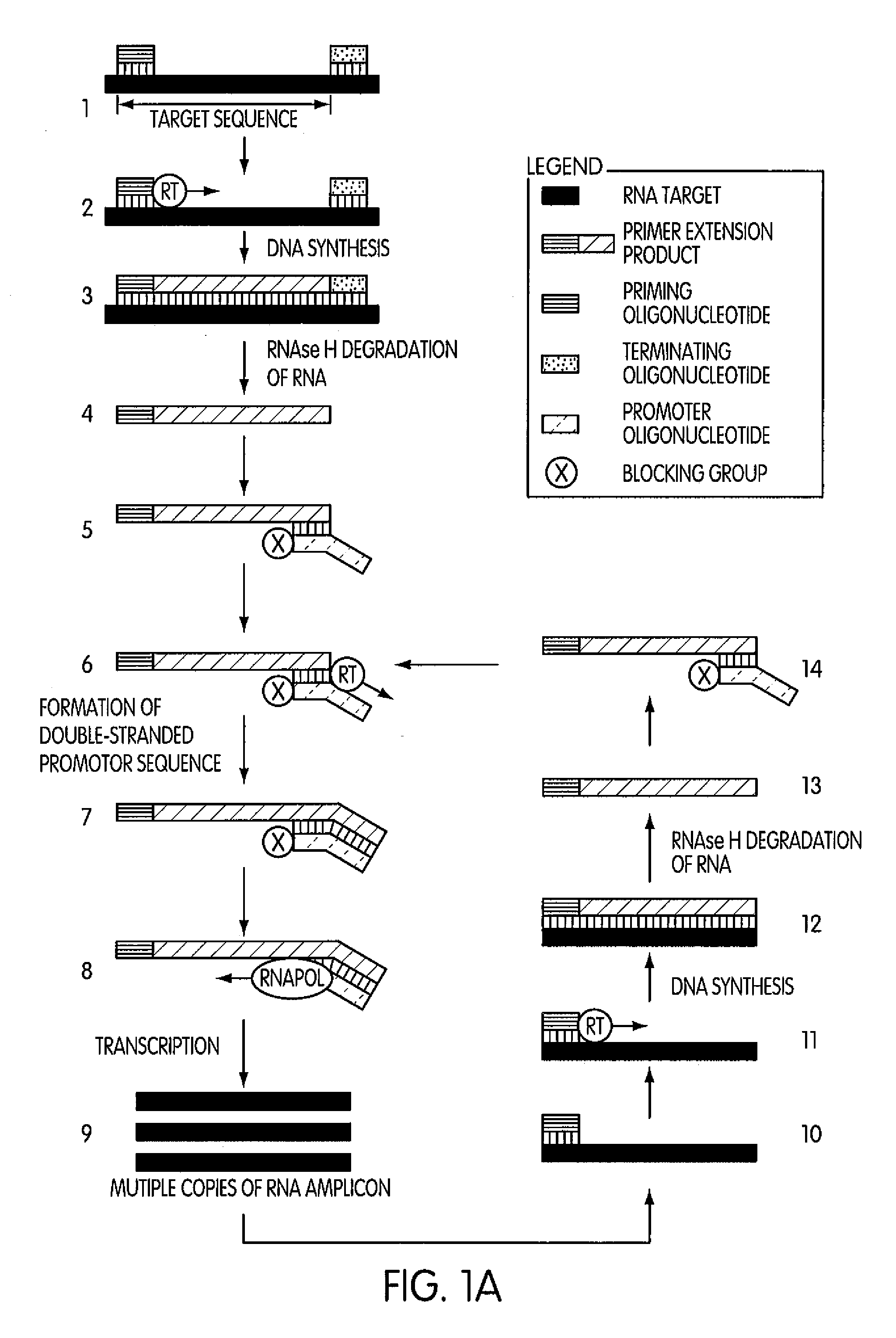
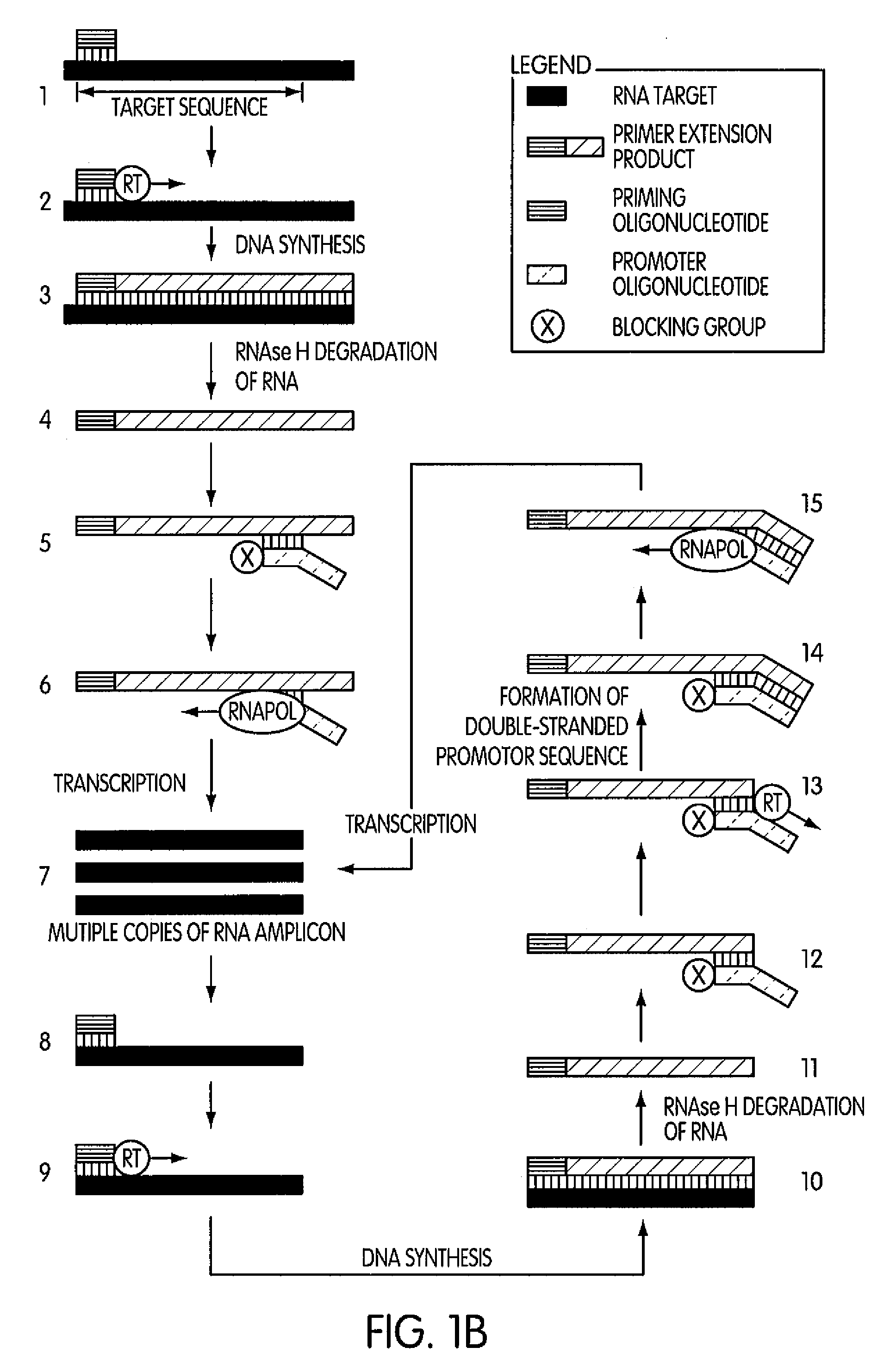
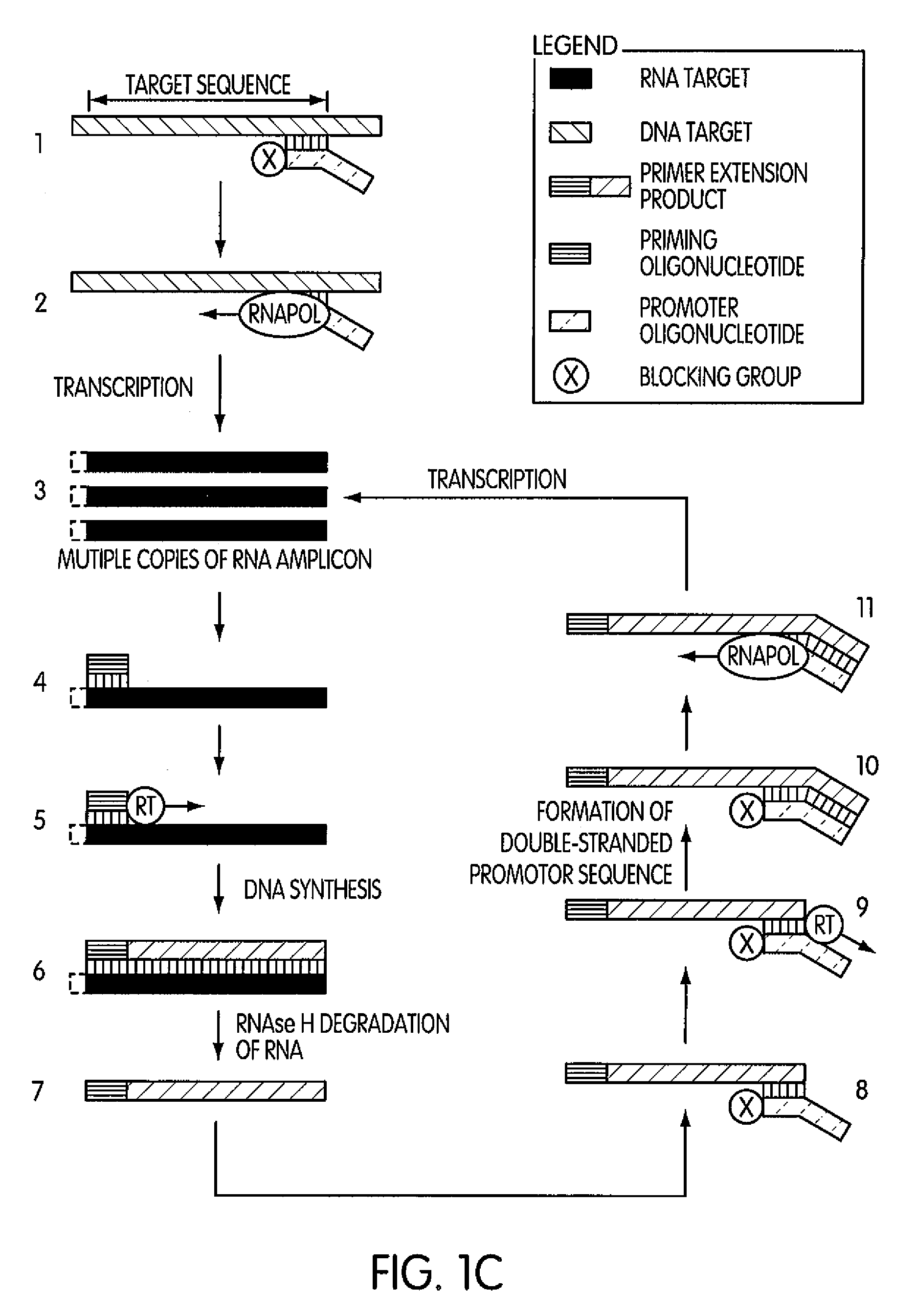
The present invention is directed to novel methods of synthesizing multiple copies of a target nucleic acid sequence which are autocatalytic (i.e., able to cycle automatically without the need to modify reaction conditions such as temperature, pH, or ionic strength and using the product of one cycle in the next one). In particular, the present invention discloses a method of nucleic acid amplification which is robust and efficient, while reducing the appearance of side-products. The method uses only one primer, the “priming oligonucleotide,” a promoter oligonucleotide modified to prevent polymerase extension from its 3′-terminus and, optionally, a means for terminating a primer extension reaction, to amplify RNA or DNA molecules in vitro, while reducing or substantially eliminating the formation of side-products. The method of the present invention minimizes or substantially eliminates the emergence of side-products, thus providing a high level of specificity. Furthermore, the appearance of side-products can complicate the analysis of the amplification reaction by various molecular detection techniques. The present invention minimizes or substantially eliminates this problem, thus providing an enhanced level of sensitivity.
Owner:GEN PROBE INC
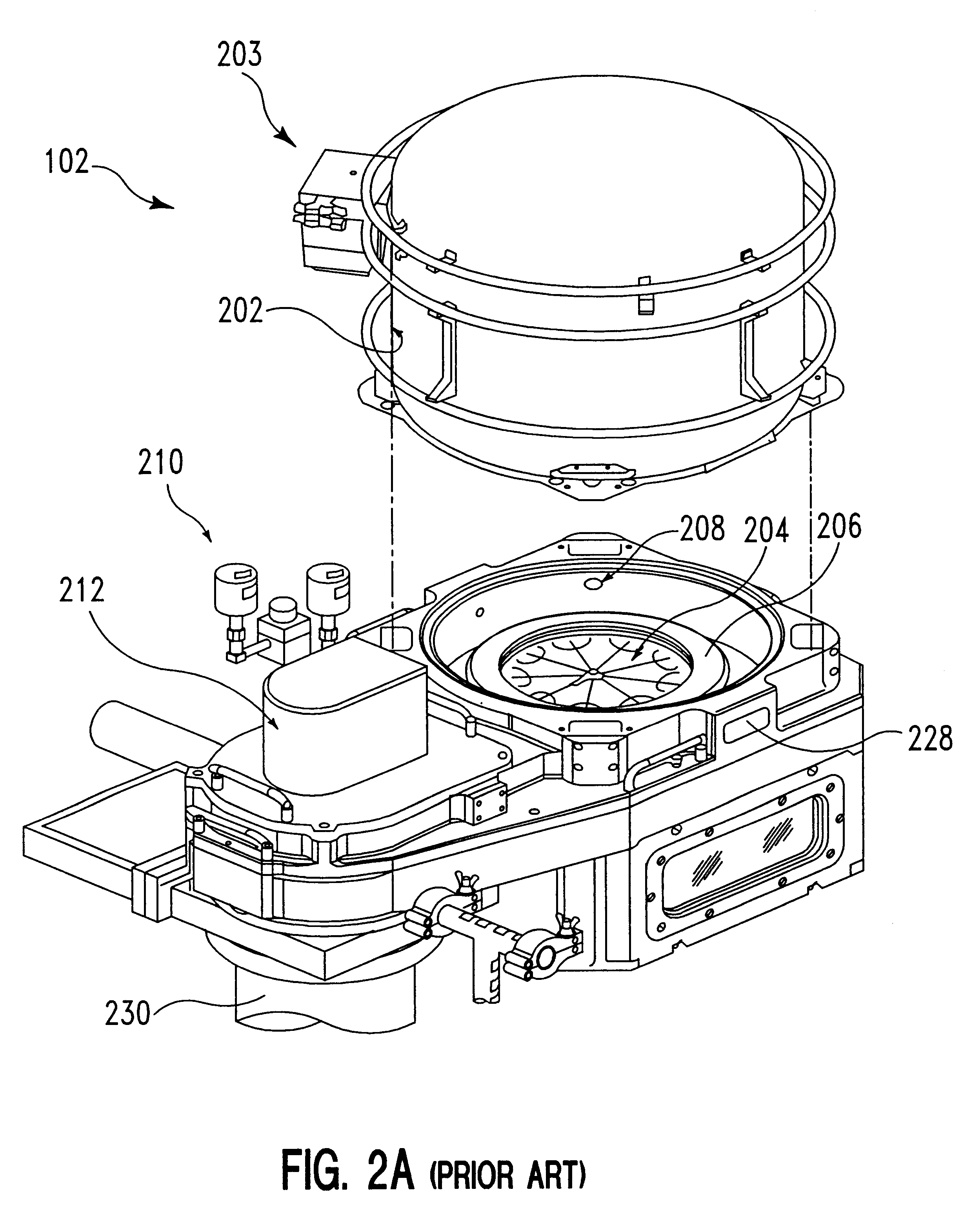
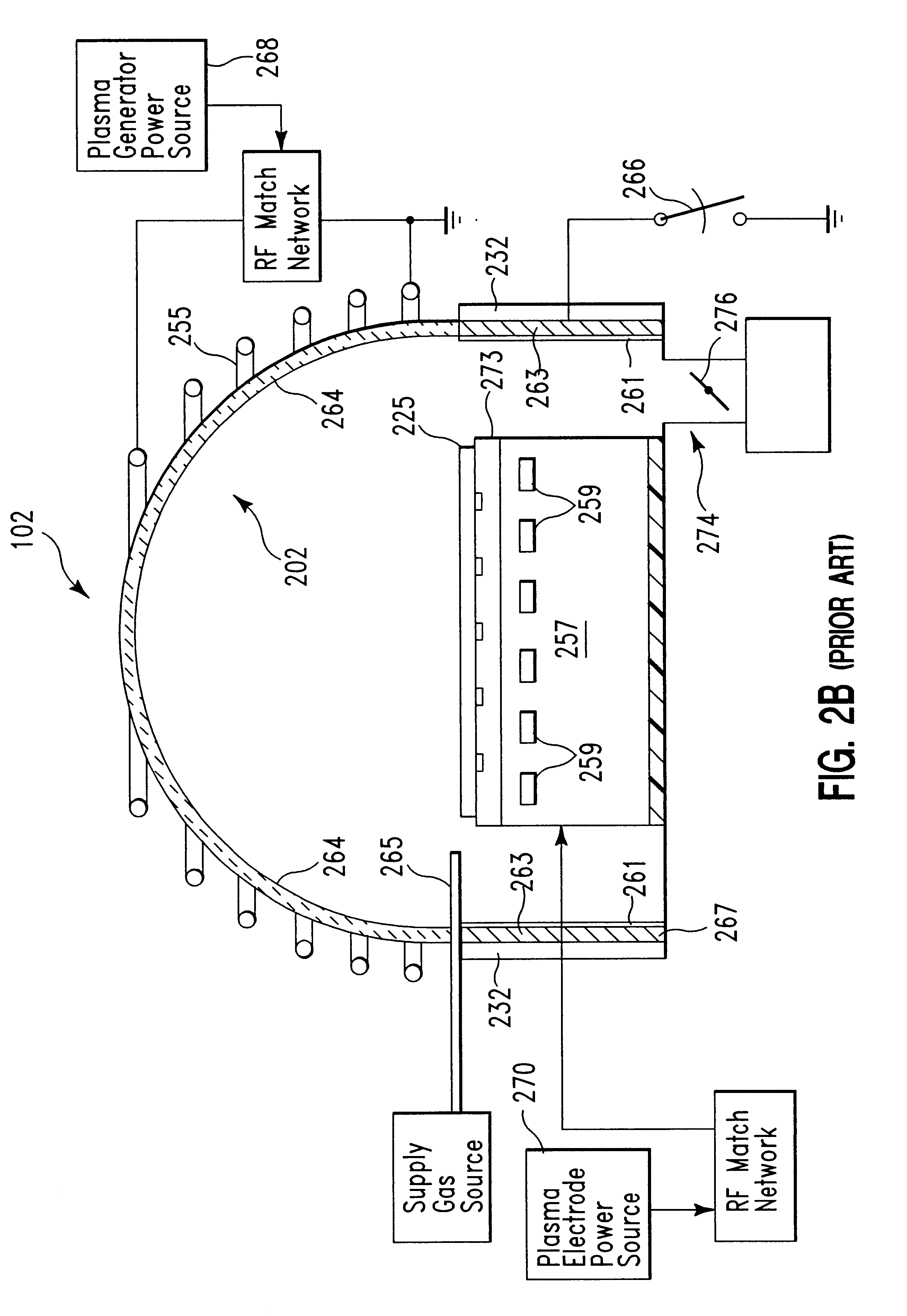
Method of cleaning a semiconductor device processing chamber after a copper etch process
The present invention is a method for removing deposited etch byproducts from surfaces of a semiconductor processing chamber after a copper etch process. The method of the invention comprises the following general steps: (a) an oxidation step, in which interior surfaces of the processing chamber are contacted with an oxidizing plasma; (b) a first non-plasma cleaning step, in which interior surfaces of the processing chamber are contacted with an H+hfac-comprising gas; and (c) a second cleaning step, in which interior surfaces of the processing chamber are contacted with a plasma containing reactive fluorine species, whereby at least a portion of the copper etch byproducts remaining after step (b) are volatilized into gaseous species, which are removed from the processing chamber. The method of the invention is preferably performed at a chamber wall temperature of at least 150° C. in order to achieve optimum cleaning of the chamber at the chamber operating pressures typically used during the cleaning process. The dry cleaning method of the invention can be performed between wafer processing runs without opening the processing chamber, thereby minimizing potential contamination to the chamber as well as chamber downtime.
Owner:APPLIED MATERIALS INC
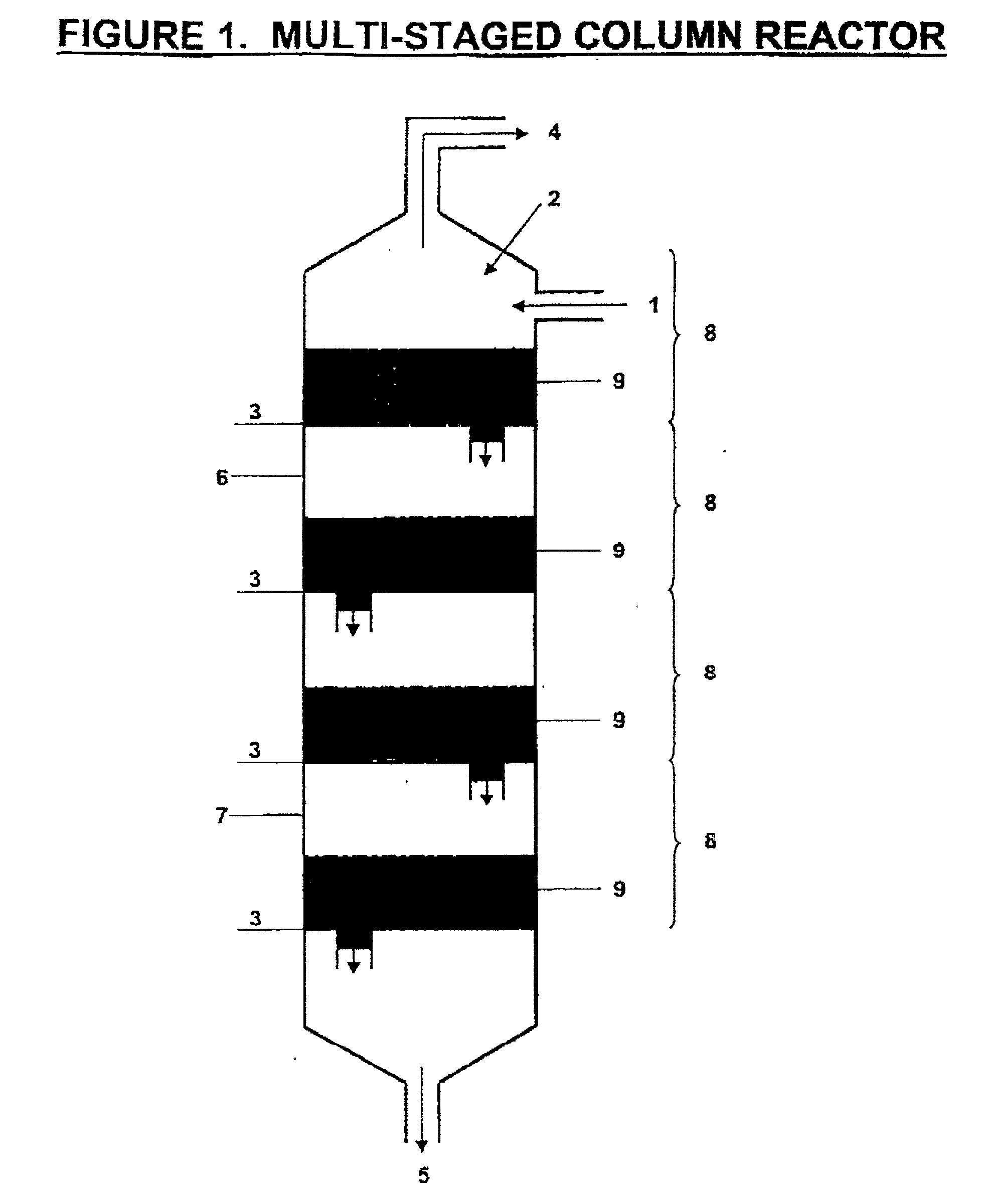
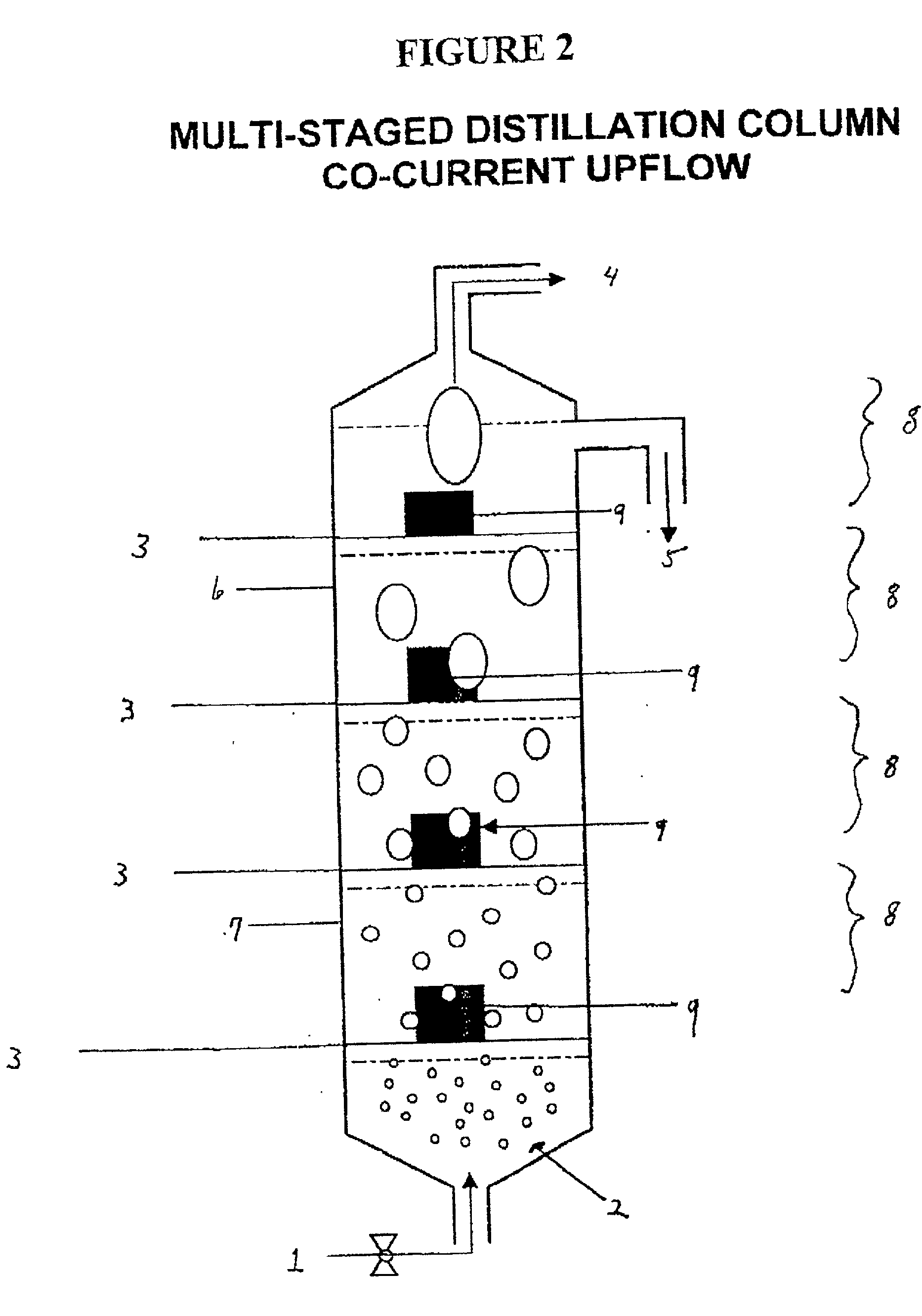
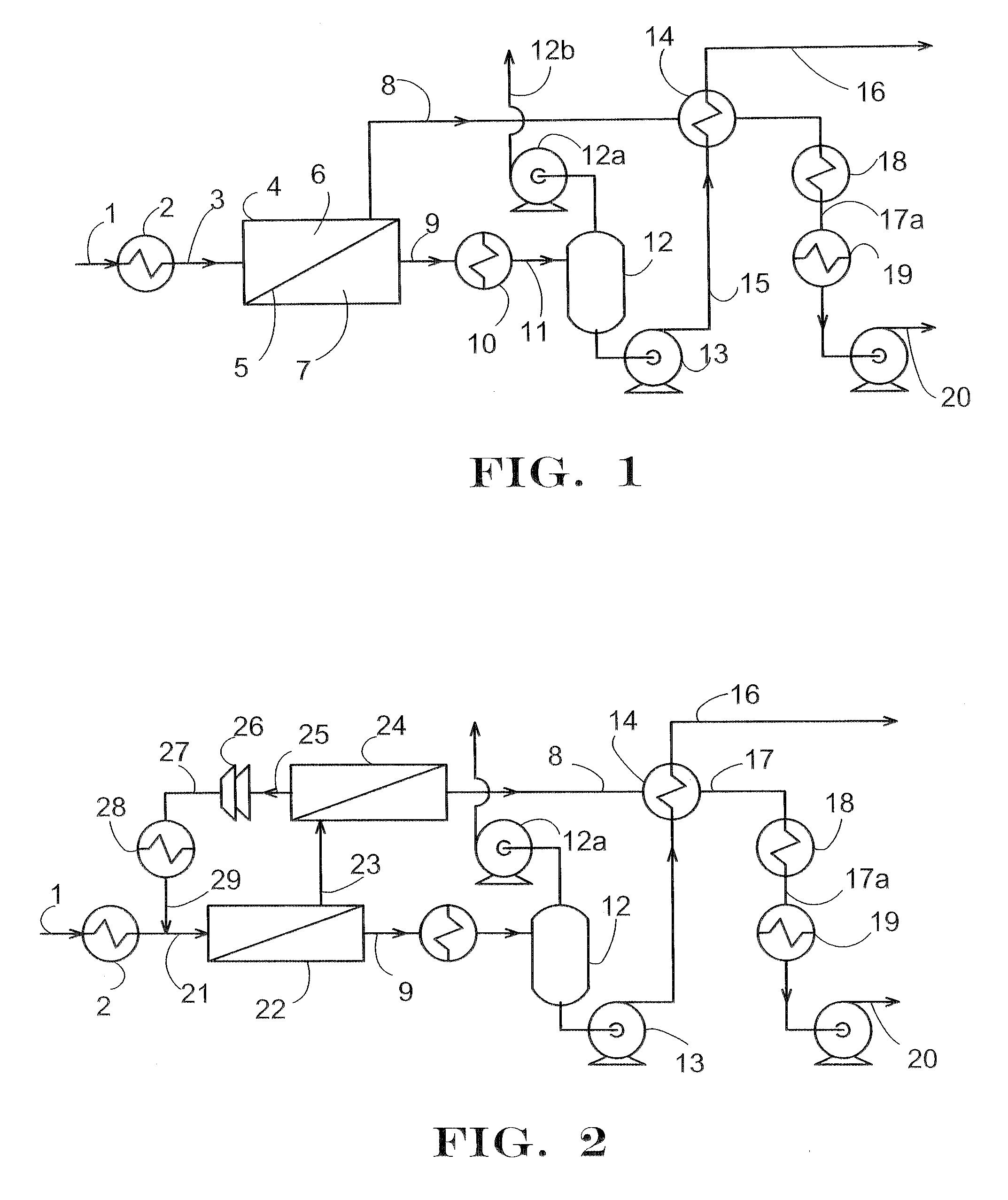
Continuous process for the preparation of polytrimethylene ether glycol
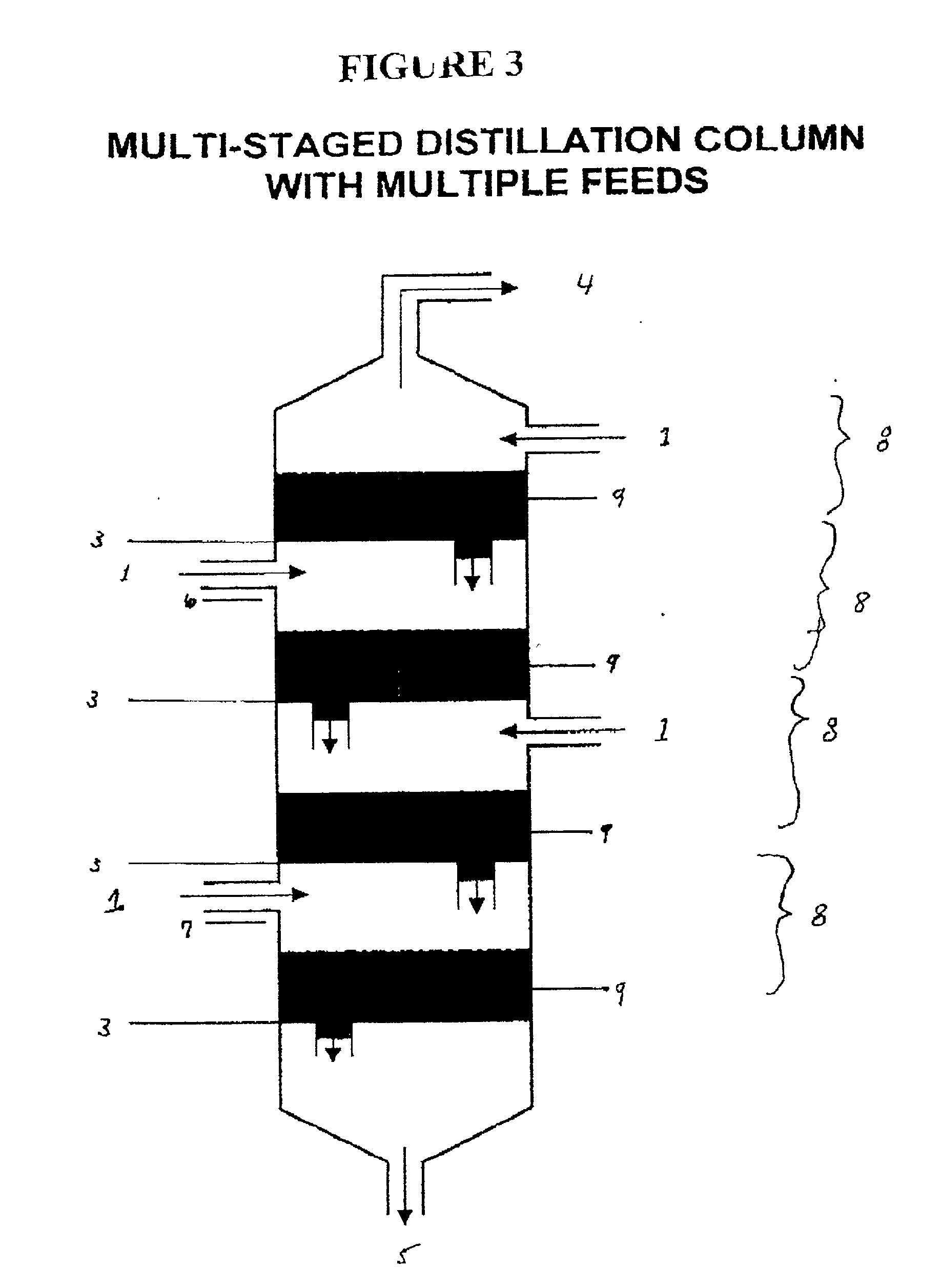
The invention is a continuous process for the preparation of polytrimethylene ether glycol from 1,3-propanediol reactant. In addition, the invention is directed to a continuous multi-stage process comprising reacting at least one reactant in a liquid phase in an up-flow column reactor, and forming a gas or vapor phase by-product wherein the gas or vapor phase by-product is continuously removed at the top and at least one intermediate stage.
Owner:DUPONT CA +1
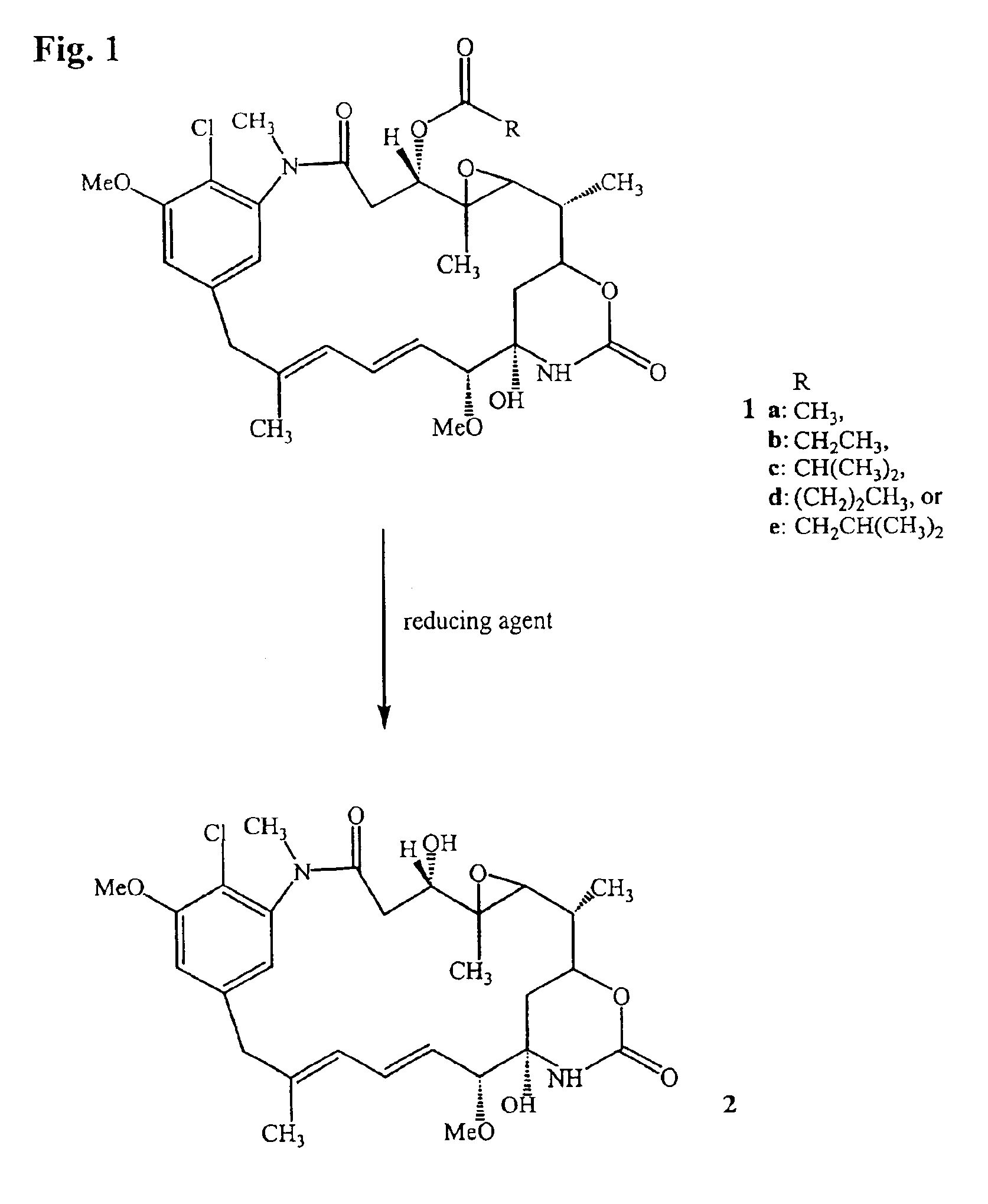
Process for the preparation and purification of thiol-containing maytansinoids
InactiveUSRE39151E1Reduce process complexityAllows scalabilityOrganic active ingredientsOrganic chemistryLithiumThiol
The present invention provides a process for the preparation and purification of thiol-containing maytansinoids comprising the steps of: (1) reductive hydrolysis of a maytansinoid C-3 ester with a reducing agent selected from the group consisting lithium trimethoxyaluminum hydride (LiAl (OMe)3H), lithium triethoxyaluminum hydride (LiAl(OEt)3H), lithium tripropoxyaluminum hydride (LiAl (OPr)3H), sodium trimethoxyaluminum hydride (NaAl (OMe)3H), sodium triethoxyaluminum hydride (NaAl(OEt)3H) and sodium tripropoxyaluminum hydride (NaAl(OPr)3H) to yield a maytansinol; (2) purifying the maytansinol to remove side products when present; (3) esterifying the purified maytansinol with a carboxylic acid to yield a mixture of an L- and a D-aminoacyl ester of maytansinol; (4) separating the L-aminoacyl ester of maytansinol from the reaction mixture in (3); (5) reducing the L-aminoacyl ester of maytansinol to yield a thiol-containing maytansinoid; and (5) purifying the thiol-containing maytansinoid.
Owner:IMMUNOGEN INC
Process for producing sodium sulfate and sodium chloride in Na2SO4-NaCl-H2O system
InactiveCN1944256AIncrease profitReduce energy consumptionAlkali metal sulfite/sulfate purificationAlkali metal halide purificationInorganic saltsSeparation technology
The process of producing sodium sulfate and sodium chloride in a Na2SO4-NaCl-H2O system belongs to the field of mixed inorganic solution evaporating separation technology. Mixed solution of sodium sulfate and sodium chloride as material is first evaporated and then separated to obtain sodium sulfate, sodium chloride and evaporated mother liquor; the evaporated mother liquor is low temperature evaporated and separated to obtain sodium chloride and salt-making mother liquor; and the salt-making mother liquor is evaporated and separated to obtain sodium sulfate and saltpeter-making mother liquor. The present invention has the features of high main and side product quality, high material adaptability, low cost, low cost, no waste draining, etc.
Owner:CHINA LIGHT IND INT ENG CO LTD +1
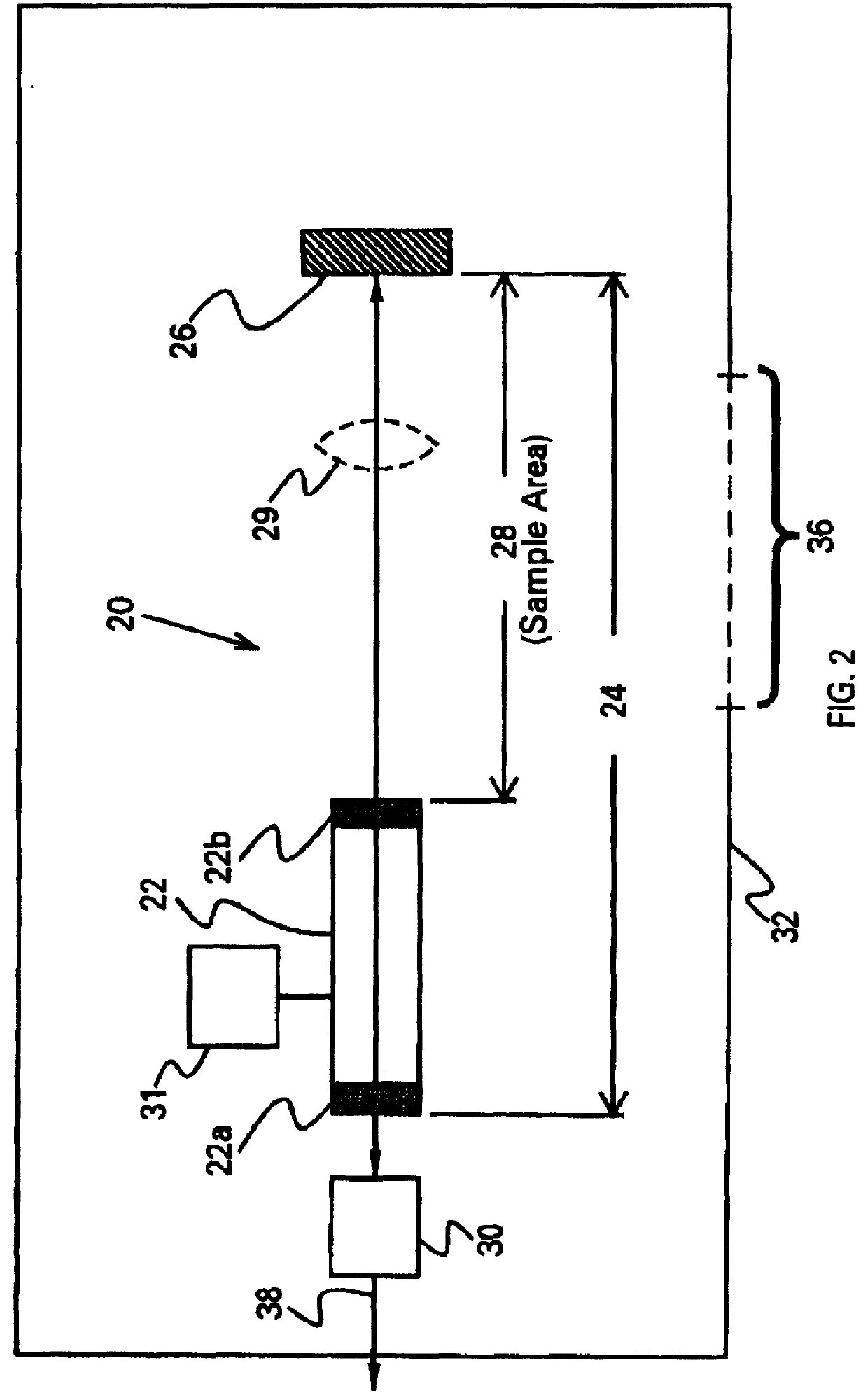
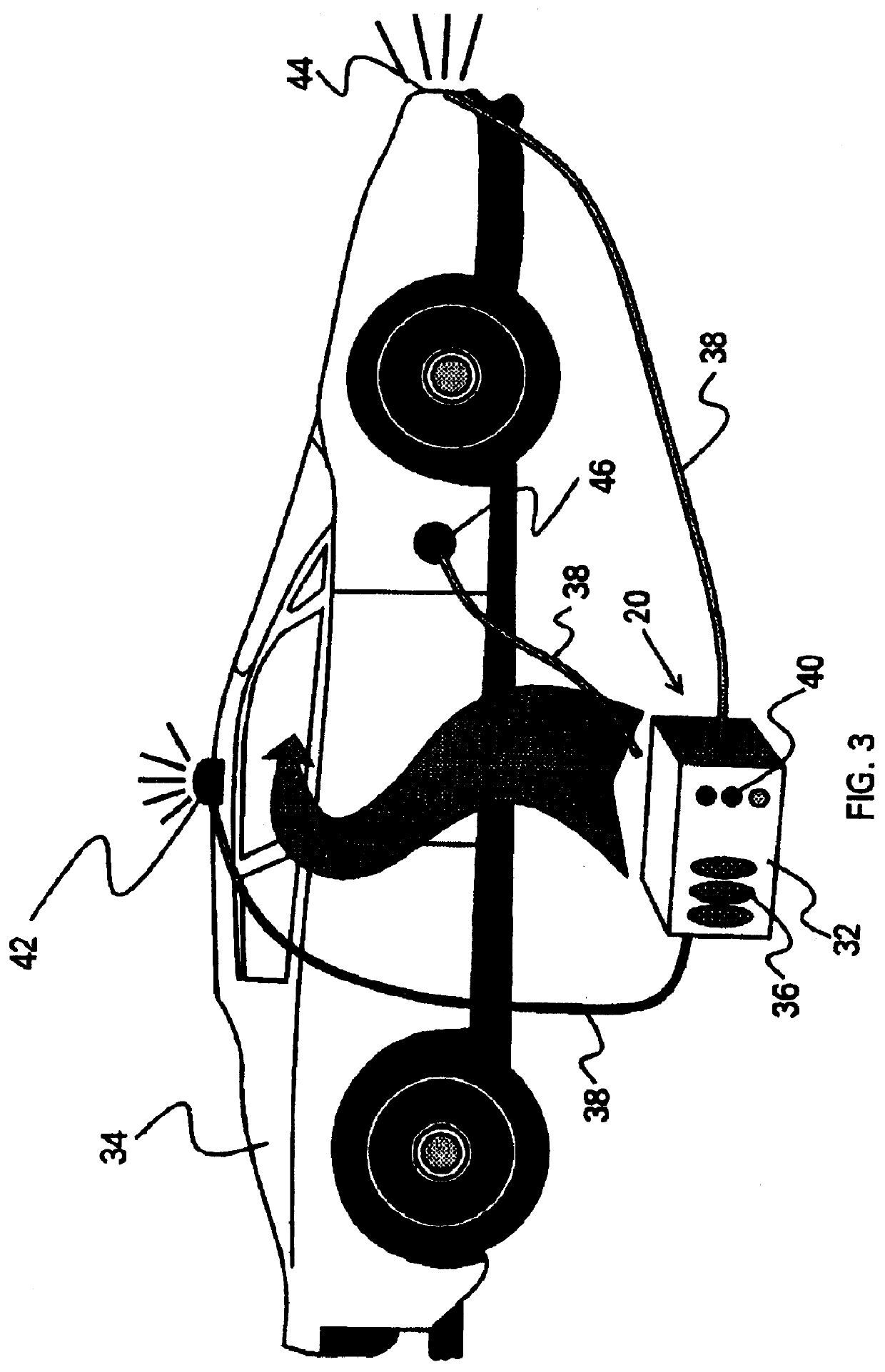
ILS sensors for drug detection within vehicles
On-board ILS sensors for detecting illegal drugs and based on intracavity laser spectroscopy (ILS) are provided for detecting the presence of drugs and their metabolized by-product vapors in an enclosed space, such as a vehicle. The sensor comprises: (a) a laser comprising a gain medium having two opposed facets within a laser resonator and functioning as an intracavity spectroscopic device having a first end and a second end, the first end operatively associated with a partially reflecting (i.e., partially transmitting) surface; (b) a reflective or dispersive optical element (e.g., a mirror or a diffraction grating) operatively associated with the second end to define a broadband wavelength laser resonator between the optical element and the first end and to thereby define an external cavity region between at least one facet of the gain medium and either the first end or the second end or both ends; (c) the external cavity region being exposed to air in the enclosed space to enable any drugs or their metabolized by-product molecules to enter thereinto; (d) a detector spaced from the first end; (e) appropriate electronics for measuring and analyzing the detector signal; (f) a housing for containing at least the laser, the partially reflecting surface, and the optical element, the housing being configured to prevent escape of stray radiation into the enclosed space and to permit air from the enclosed space to continuously circulate through the external cavity region for analysis; and (g) means for driving the laser (e.g., electrical or optical). A method is provided for measuring concentration of drug vapors and their metabolized by-product vapors in the vehicle or other enclosed space employing the on-board sensor. The method comprises: (1) sensing any drugs and their metabolized by-product vapors in the enclosed space by the on-board sensor; and (2) providing a signal indicative of presence of any drugs or metabolized vapors.
Owner:INNOVATIVE LASERS
Method for processing ethylene tar
The invention discloses a method for processing ethylene tar, which selects an appropriate cutting point to fractionate the ethylene tar into a light fraction and a heavy fraction aiming at the characteristics of high content of arene, colloid and carbon residue in the ethylene tar. The light fraction passes through a hydrogenation protection reaction zone, a hydrofining reaction zone and a hydrocracking reaction zone sequentially to obtain a gasoline fraction and a diesel oil fraction; and the heavy fraction is used as a universal type carbon fiber asphalt stock. The method for processing the ethylene tar makes full use of the total fraction of the ethylene tar and increases the additional value of the ethylene tar. In addition, the method enables the arene after hydrostturation in the ethylene tar to open ring, crack moderately and / or isomerize by selecting two types of hydrocracking catalysts to perform grading loading so as to obtain the diesel oil fraction with condensation point less than -40 DEG C in high yield and obtain a side product high-octane gasoline fraction at the same time.
Owner:CHINA PETROLEUM & CHEM CORP +1
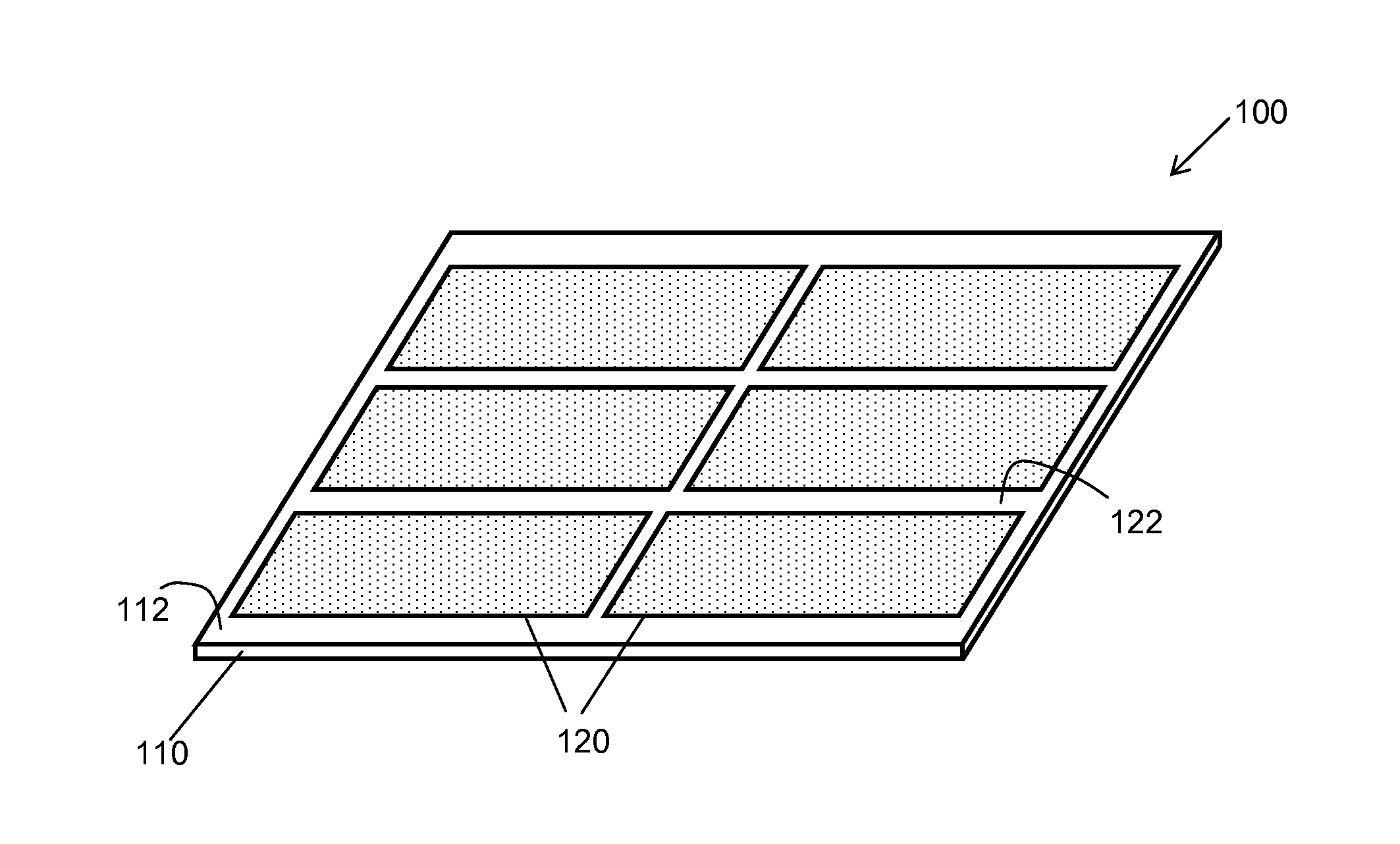
Roofing and Siding Products Having Receptor Zones and Photovoltaic Roofing and Siding Elements and Systems Using Them
ActiveUS20090178350A1Improve adhesionPhotovoltaic supportsSolar heating energyPhotovoltaicsSide product
The present invention relates generally to roofing or siding products. The present invention relates more particularly to roofing or siding products for use with photovoltaic elements, and to photovoltaic systems that include one or more photovoltaic elements joined to a roofing or siding substrate. In one embodiment, a roofing product includes a rigid roofing or siding substrate having a top surface, the top surface having one or more receptor zones thereon, each receptor zone being adapted to receive one or more photovoltaic elements, each receptor zone having a different surfacing than the area of the top surface adjacent to it.
Owner:CERTAIN TEED LLC
Atmospheric vacuum distillation method and apparatus with vacuum flash vaporizer
InactiveCN101376068AReduce the amount of feedEasy to handleVacuum distillation separationVacuum distillationVaporizationPulp and paper industry
The invention relates to an atmospheric and vacuum distillation device with a vacuum flash tower and a method thereof. The atmospheric and vacuum distillation device with a vacuum flash tower is characterized in that the vacuum flash tower arranged in front of a vacuum furnace is connected with the vacuum furnace and a vacuum tower through a pump and a pipeline. Constant bottom oil (1) is introduced into the vacuum flash tower (2)at first, and the operation pressure at the top part of the vacuum flash tower is higher than the operation pressure at the top part of the vacuum tower (9) by10 to 200mmHg; Flash cap gas (3) is introduced into the upper part or the lower part of an outlet for a side product which is similar to Flash cap gas fraction; flash bottom oil (5) is introduced into the vacuum furnace (7) through a flash bottom oil pump (6); when the flash bottom oil is heated to 350 to 430 degrees, air-liquid mixing vacuum tower feed material is obtained through partial vaporization and is introduced into a flash evaporation segment (10) of the vacuum tower through a transfer line (8); and products with different fractions are drawn from the side of the vacuum tower and vacuum residue is drawn from the bottom of the vacuum tower. Through adding the vacuum flash tower to improve the working process of the atmospheric and vacuum distillation device, the invention achieves the advantages of increasing treatment capacity, increasing vacuum distillation yield, and reducing energy consumption.
Owner:TIANJIN UNIV +2
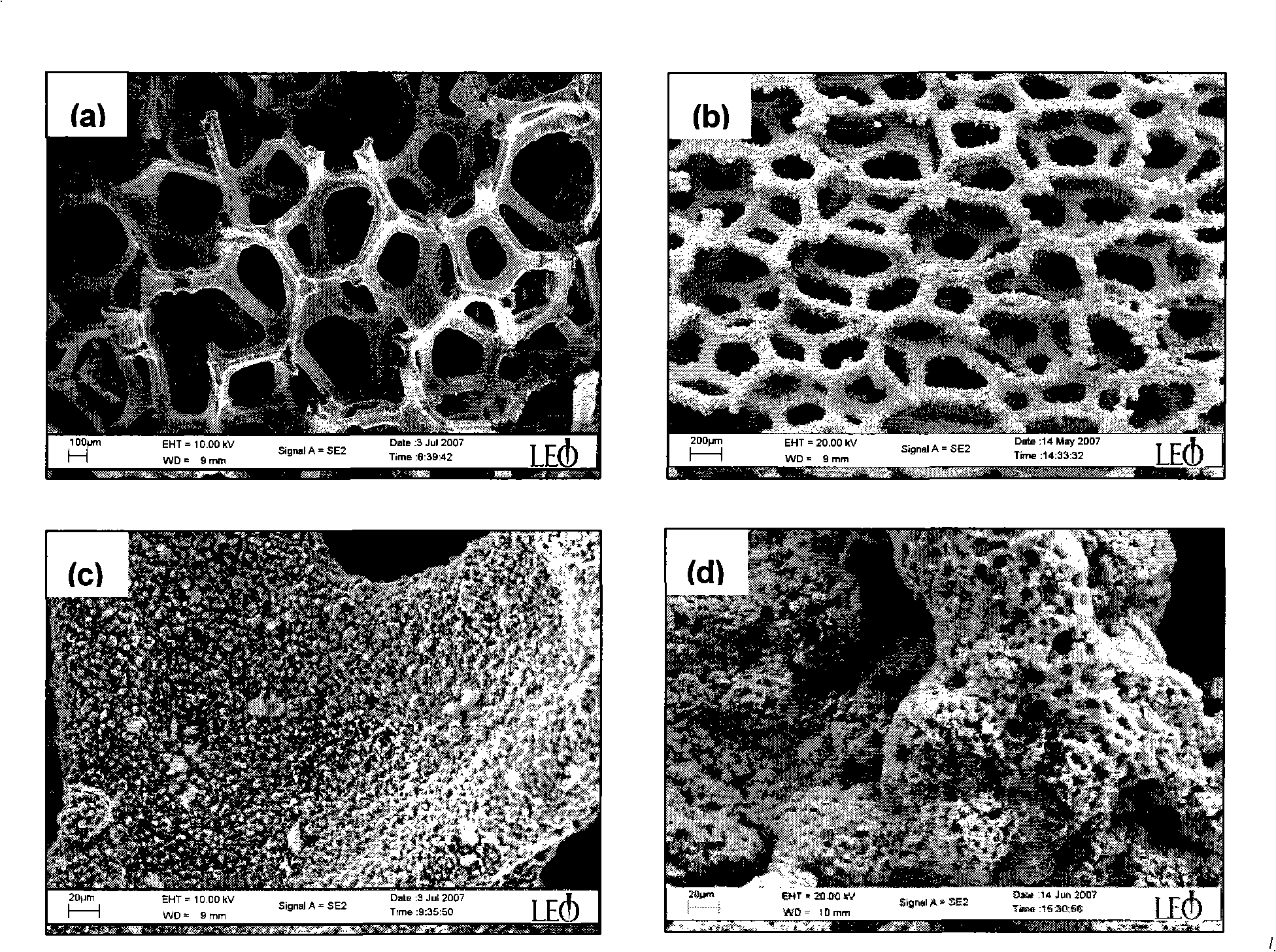
Catalyst for hydrogen production by catalyzing and hydrolyzing borohydride and preparation method thereof
InactiveCN101347736AFast deposition rateIncrease concentrationMetal/metal-oxides/metal-hydroxide catalystsMetal hydridesChemical platingRare earth
The invention relates to hydrogen production and hydrogen storage technologies and materials, in particular to a catalyst for catalytic hydrolysis of borane for the hydrogen production and a preparation method thereof, thereby solving the problems that the direct application of powder catalyst in a catalytic hydrolysis solid-liquid reaction system can cause the loss of the catalyst, the catalytic hydrolysis reaction is difficult to control and the hydrolysis by-products are difficult to be recovered, etc. The catalyst is composed of an active component and a carrier; the active component is a binary, ternary or multinary alloy or a single precious metal or the combination thereof which is composed of one or more transition metals, rare earth metals or precious metals and metalloids; the active component is deposited on the carrier through the improved chemical plating technology, the surface thereof is rough and porous, and the structure of the prepared catalyst is the amorphous or the nanocrystalline structure. The preparation method has simple preparation process, high preparation efficiency and convenient large-scale preparation; the sources of the used raw materials are rich; the catalytic activity of the prepared supported catalyst is high, the real-time control of the catalytic hydrolysis reaction of the borane can be realized, the catalytic performance is stable, and the catalyst can be repeatedly used for a plurality of times.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
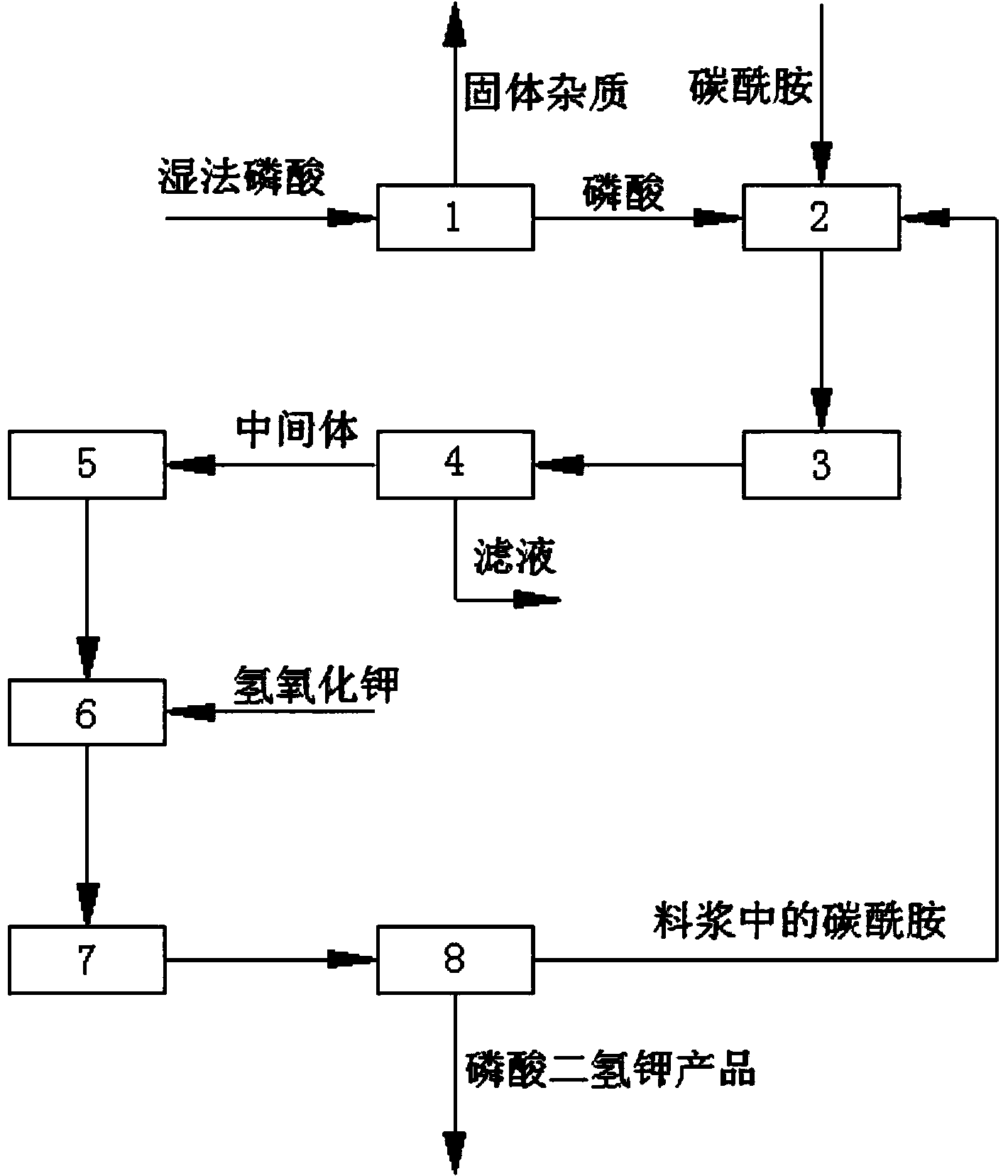
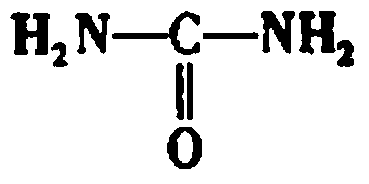
Method for preparing monopotassium phosphate by using wet-process phosphoric acid
InactiveCN103803518AReduce manufacturing costNo pollution in the processPhosphorus compoundsFertilizer mixturesEnvironmental resistanceHigh energy
The invention discloses a method for preparing monopotassium phosphate by using wet-process phosphoric acid. The method comprises the following steps: reacting carbonyl diamide and wet-process phosphoric acid to obtain an intermediate; reacting the intermediate and potassium hydroxide to prepare a monopotassium phosphate product. The method has the advantages of short process route, low energy consumption, stable product quality, low production cost, convenience in operation and safety in production. A side product, namely, slurry can be recycled completely, an entire production process is environment-friendly, clean and free from pollution, and no waste gas, waste water or waste residues are discharged. A response is made to the policy calling of energy saving, emission reduction and clean production, the problems of complex process, instable product quality, high energy consumption and environmental pollution existing in the prior art are solved, and the purity of the obtained monopotassium phosphate product is more than or equal to 98 percent.
Owner:GUIYANG KAILIN FERTILIZER CO LTD +1
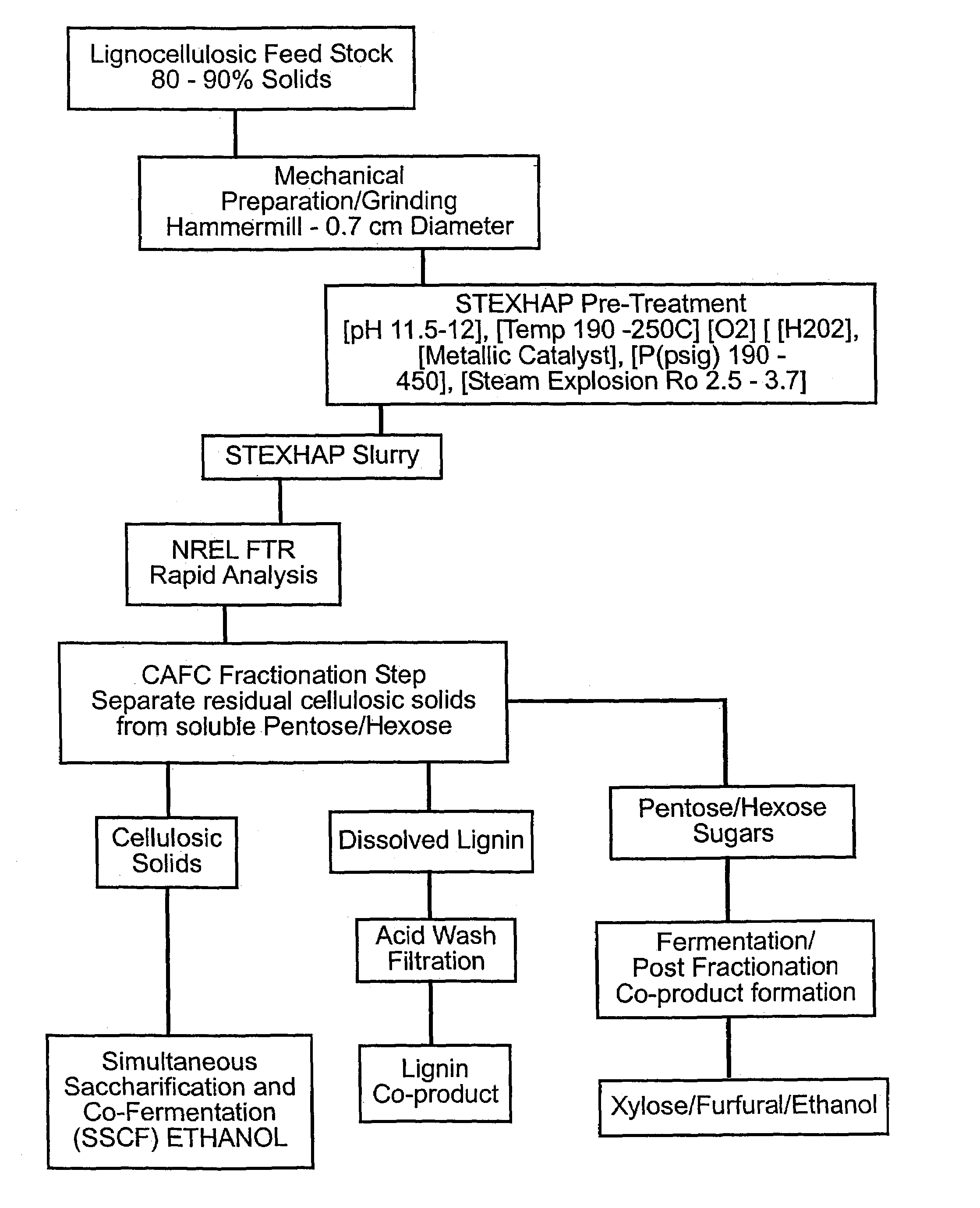
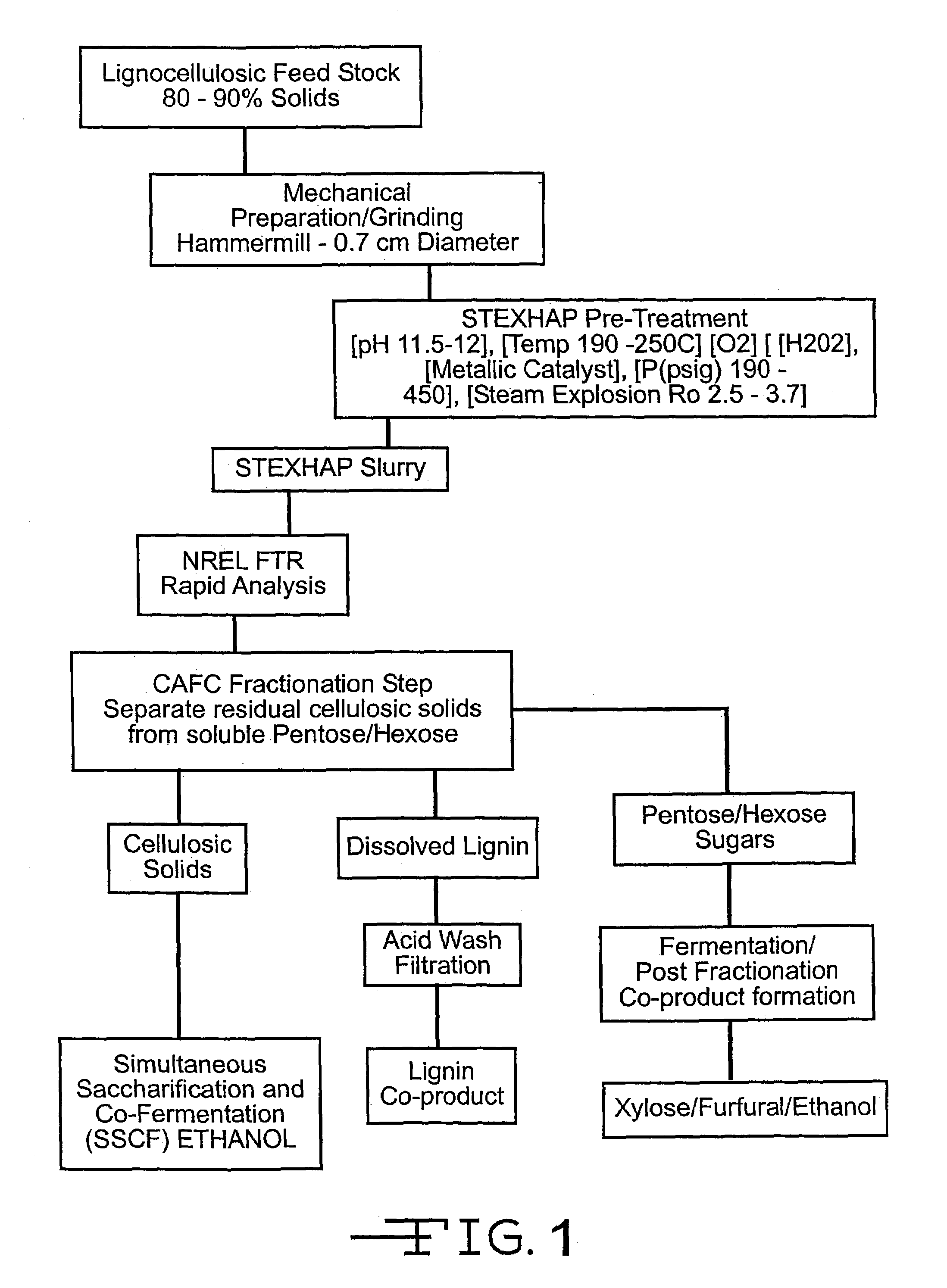
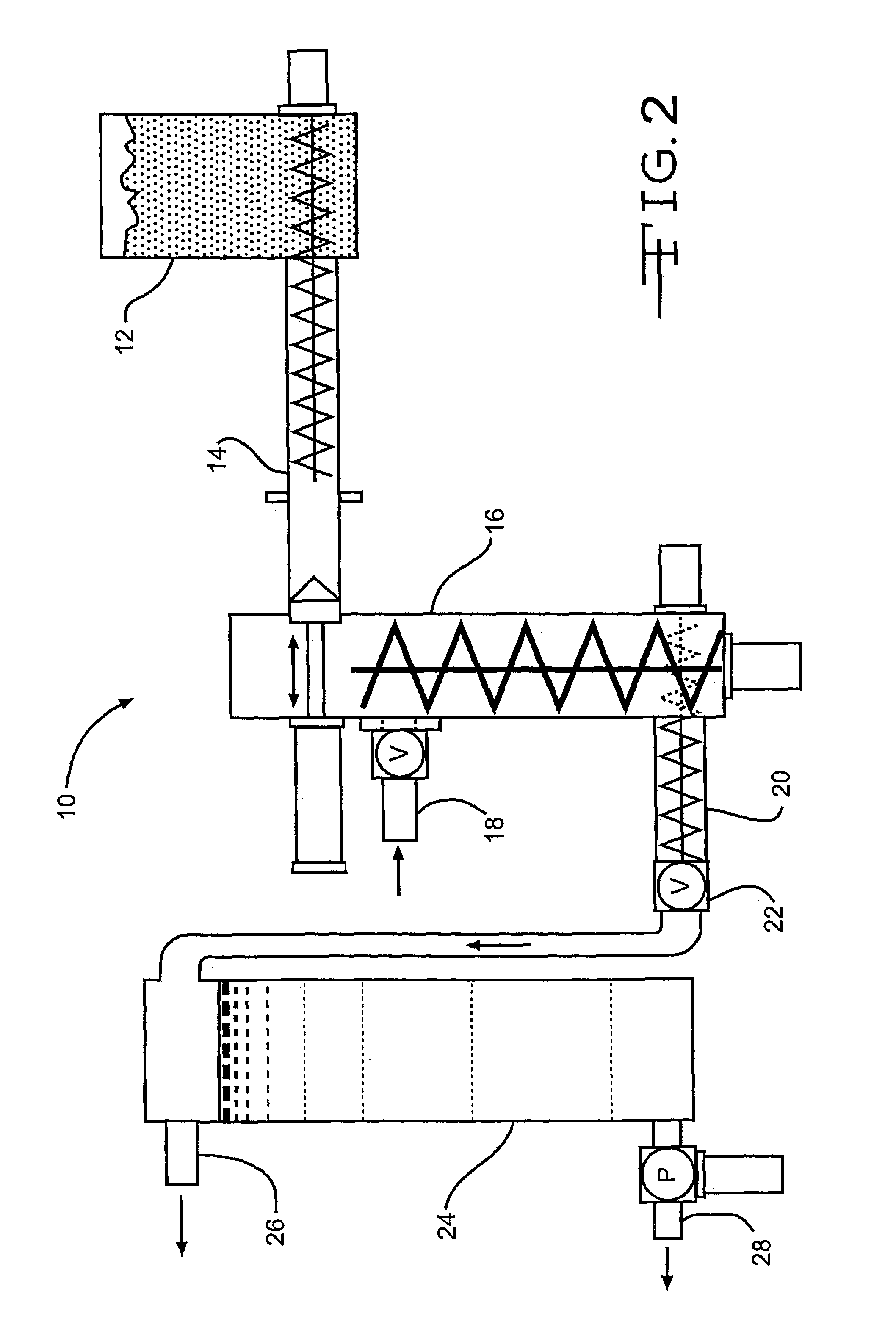
Process of treating lignocellulosic material to produce bio-ethanol
InactiveUS7189306B2Separation efficiency can be improvedEasy to separatePretreatment with water/steamPulp liquor regenerationSide productCo product
This invention relates to a process of treating a lignocellulosic material to produce bio-ethanol. The process includes the steps of: (a) exposing the lignocellulosic material to conditions including a pH not less than about 8, and steam at a first pressure, to produce a step (a) product; (b) explosively discharging the step (a) product to a second pressure less than the first pressure to produce a step (b) product; and (c) further processing the step (b) product to produce bio-ethanol and other co-products. In another embodiment, the invention relates to a conical auger fractionation column. The fractionation column includes a column body having an input and an output. A conical filter is positioned inside the column body, the filter having a larger diameter end directed toward the input and a smaller diameter end directed toward the output. A conical auger is positioned inside the conical filter, the conical auger having an outer diameter which is approximately the same as an inner diameter of the conical filter. The auger and filter are adapted to cooperate to separate cellulosic solids from a liquid stream in a process of producing bio-ethanol from a lignocellulosic material.
Owner:GERVAIS GIBSON W
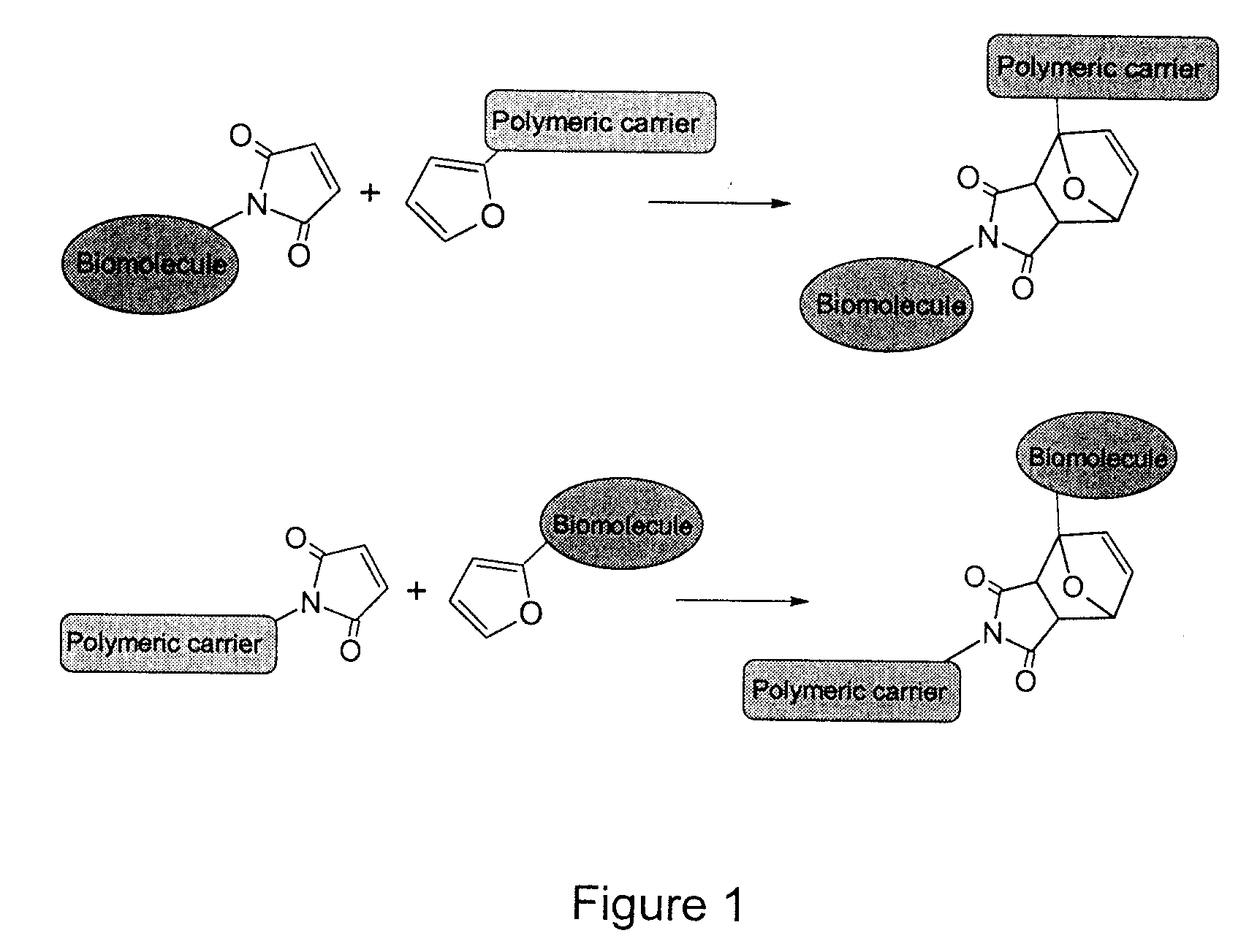
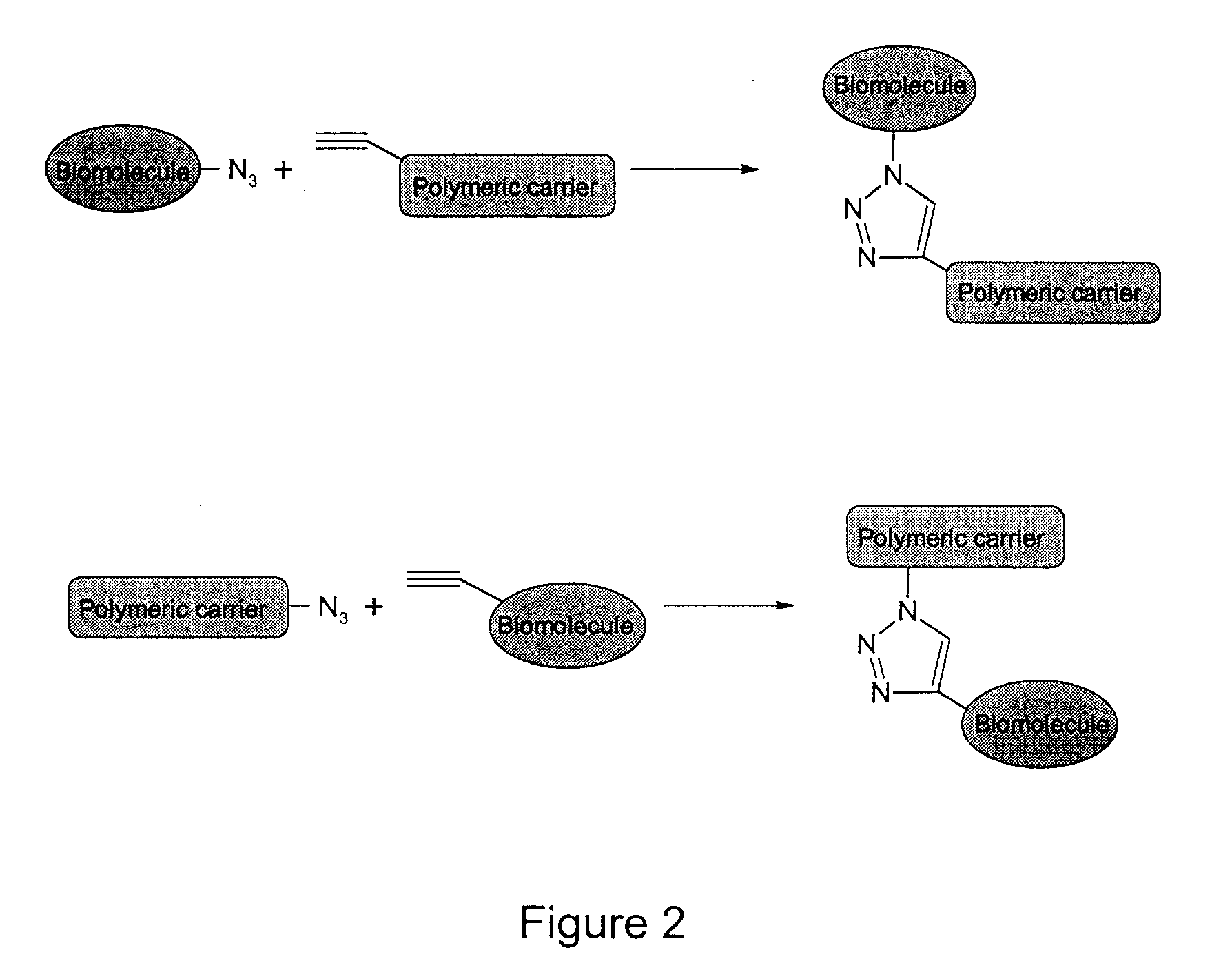
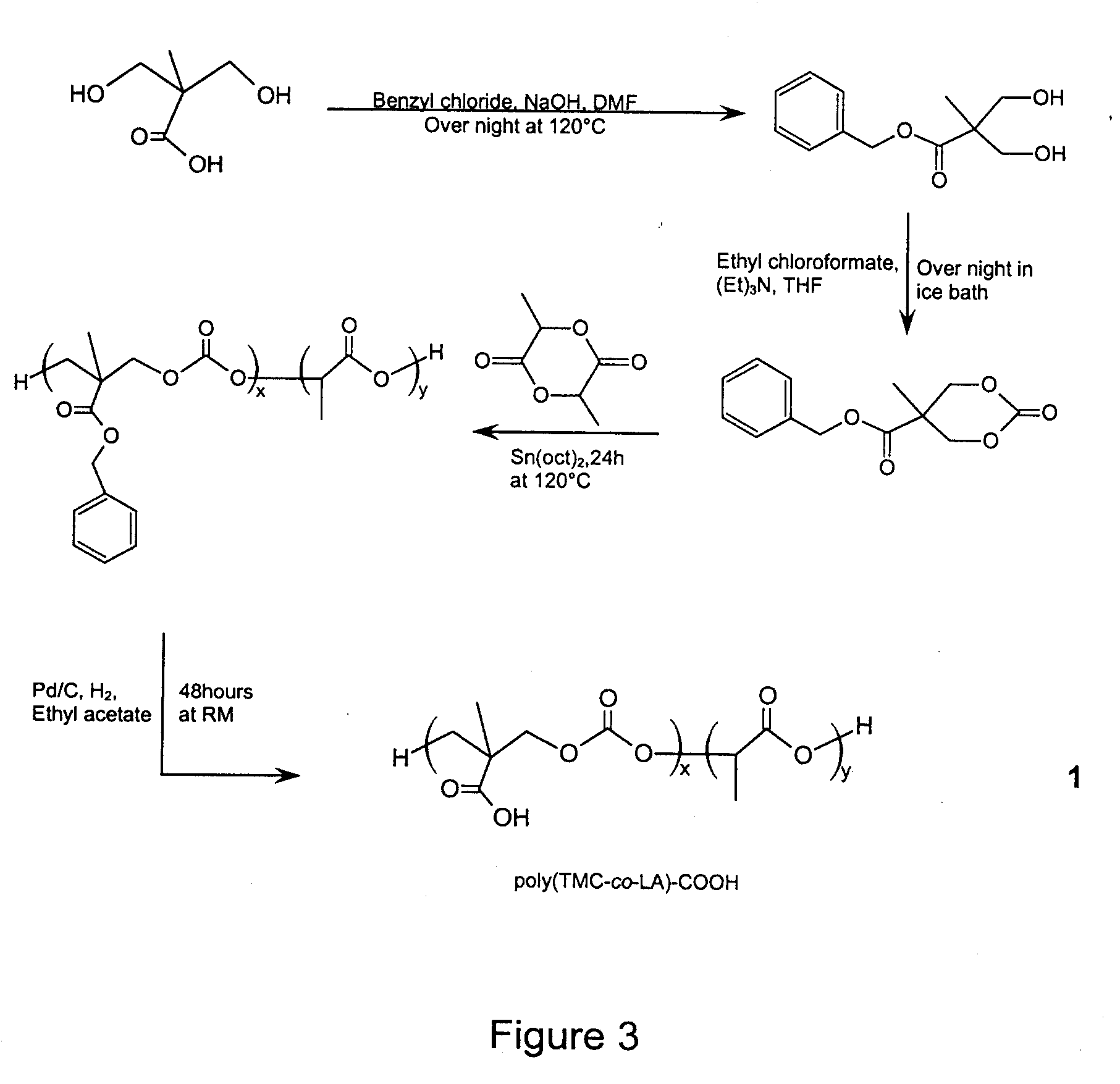
Method of Biomolecule Immobilization On Polymers Using Click-Type Chemistry
ActiveUS20090297609A1Improve efficiencyProcess environmental protectionBiocideOrganic active ingredientsFuranAlkyne
The present invention provides a method for the covalent immobilization of biomolecules on polymers for delivery of the biomolecules, which has the advantage of being simple, highly efficient, environmentally friendly and free of side products relative to traditional immobilization techniques. The invention provides a modified micro / nanoparticle system, which uses a functionalized polymer formed into micro or nanoparticles to bind a molecule to the particles using uses facile chemistry, the Diels-Alder cycloaddition between a diene and a dienophile with the polymer being functionalized with one of them and the molecule with the other, or the Huisgen 1,3-dipolar cycloaddition between a terminal alkyne and an azide to bind the molecule to the particle. The molecules and / or other therapeutic agents may be encapsulated within the polymer particles for intravenous therapeutic delivery. The invention also provides a novel synthetic biodegradable polymer, a furan / alkyne-functionalized poly(trimethylene carbonate) (PTMC)-based polymer, whose composition can be designed to meet the defined physical and chemical property requirements. In one example, the particle system self-aggregates from functionalized PTMC-based copolymers containing poly(ethylene glycol) (PEG) segments. The composition of the copolymers can be designed to meet various particle system requirements, including size, thermodynamic stability, surface PEG density, drug encapsulation capacity and biomolecule immobilization capacity.
Owner:SHOICHET MOLLY S +2
Method for continuous production of polyformaldehyde dimethyl ether
InactiveCN102786397ARealize industrial productionImprove stabilityOrganic chemistryOrganic compound preparationPtru catalystDistillation
The invention provides a method for continuous production of polyformaldehyde dimethyl ether. The method is characterized by comprising the following steps: a) feeding dimethoxymethane and hot-melted paraformaldehyde into a fixed bed reactor and adopting an acidic resin catalyst, so as to prepare polyformaldehyde dimethyl ether (DMM3-8), wherein the reaction temperature is 120-180 DEG C and the pressure is 0.1-10 MPa; b) cooling the reaction product, and then performing adsorptive separation through a dehydrating tower, so as to obtain polyformaldehyde dimethyl ether of which most water, cytidine glycol and hemiacetal are desorbed; c) feeding the polyformaldehyde dimethyl ether subjected to desorption into a distillation tower for separation, wherein most of a low-boiling component (dimethoxymethane (DMM)), poly-di-formaldehyde dimethyl ether (DMM2), a by-product (methanol) and triformol are extracted first, and then the materials in a tower kettle are fed into a rectifying tower in the next step, so as to extract the rest of the DMM2 and the triformol; and d) returning the low-boiling component (dimethoxymethane (DMM)), the methanol, the DMM2 and the triformol, which are evaporated out by the distillation tower and the rectifying tower in the last step, into the fixed bed reactor to continue to react to prepare polyformaldehyde dimethyl ether.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Methods and kits for amplifying DNA
ActiveUS20070202523A1Reduce appearance problemsMinimize and substantially eliminate emergenceMicrobiological testing/measurementFermentationPolymerase LNucleic acid sequencing
Novel methods of synthesizing multiple copies of a target nucleic acid sequence which are autocatalytic are disclosed (i.e., able to cycle automatically without the need to modify reaction conditions such as temperature, pH, or ionic strength and using the product of one cycle in the next one). In particular, methods of nucleic acid amplification are disclosed which are robust and efficient, while reducing the appearance of side-products. In general, the methods use priming oligonucleotides that target only one sense of a target nucleic acid, a promoter oligonucleotide modified to prevent polymerase extension from its 3′-terminus and, optionally, a means for terminating a primer extension reaction, to amplify RNA or DNA molecules in vitro, while reducing or substantially eliminating the formation of side-pro ducts. The disclosed methods minimizes or substantially eliminate the emergence of side-products, thus providing a high level of specificity. Furthermore, the appearance of side-products can complicate the analysis of the amplification reaction by various molecular detection techniques. The disclosed methods minimize or substantially eliminate this problem, thus providing enhanced levels of sensitivity.
Owner:GEN PROBE INC
Reduction of the toxic effect of impurities from raw materials by extractive fermentation
There are provided bioproducts and methods of improving production of the bioproducts from engineered microbial cells, the methods comprising: providing a fermentation broth comprising a crude carbon source; inoculating said fermentation broth with said microbial cells; and incubating the inoculated fermentation broth; wherein said bioproduct is a hydrophobic solvent immiscible with said fermentation broth, and wherein a toxic side product present in said crude carbon source is soluble in said hydrophobic solvent. Also, provided are kits for practicing the methods of improving production of bioproducts.
Owner:LS9 INC +1
Method for recovering carbon-fiber enhanced epoxy resin composite material
The invention relates to a method for recovering a carbon-fiber enhanced epoxy resin composite material. In the existing method, the requirement for equipment is high and the recovery cost is large. The method comprises the following steps of: cutting materials needing to be decomposed into blocks with the volume being 5cm<3>, putting the blocks in a backflow device containing acid liquor, heating for 5-20 minutes at the temperature of boiling point, cleaning and carrying out vacuum drying; then putting the obtained mixture into a reaction kettle, adding an organic solvent and an oxidizing agent, firstly heating, then cooling to normal temperature, and obtaining a primary product; and after cleaning, putting a solid product in the primary product into industrial acetone solution for dipping, obtaining recovered carbon fiber and carrying out pressure-reduced distillation on a liquid product to obtain phenol and derivatives thereof. In the method, reaction under low temperature and low pressure is realized and has the advantages of moderate reaction condition, easy control of reaction, fewer side products, no pollution basically and no corrosion to equipment and the like, so that the method is a green recovering method.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Reduction of the toxic effect of impurities from raw materials by extractive fermentation
There are provided bioproducts and methods of improving production of the bioproducts from engineered microbial cells, the methods comprising: providing a fermentation broth comprising a crude carbon source; inoculating said fermentation broth with said microbial cells; and incubating the inoculated fermentation broth; wherein said bioproduct is a hydrophobic solvent immiscible with said fermentation broth, and wherein a toxic side product present in said crude carbon source is soluble in said hydrophobic solvent. Also, provided are kits for practicing the methods of improving production of bioproducts.
Owner:LS9 INC +1
Removal of Water and Methanol from Fluids
ActiveUS20080099400A1Efficient separationPowerfulMembranesUltrafiltrationChemical reactionInternal combustion engine
A method of removing water and / or methanol from fluid mixtures of the water or methanol with other compounds uses vapor permeation or pervaporation of the water or methanol, as the case may be, from the mixture through a membrane having an amorphous perfluoropolymer selectively permeable layer. The novel process can be applied in such exemplary embodiments as (a) removing water or methanol from mixtures of compounds that have relative volatility of about 1-1.1 or that form azeotropic mixtures with water or methanol, (b) the dehydration of hydrocarbon oil such as hydraulic fluid to concentrations of water less than about 50 ppm, (c) removing water and methanol byproducts of reversible chemical reactions thereby shifting equilibrium to favor high conversion of reactants to desirable products, (d) drying ethanol to less than 0.5 wt. % water as can be used in fuel for internal combustion engines, and (e) controlling the water content to optimum concentration in enzyme-catalyzed chemical reactions carried out in organic media.
Owner:COMPACT MEMBRANE SYST INC
One-step preparation method and application of supported platinum-based multi-metal catalysts
ActiveCN104174392AEasy to operateEnvironmentally friendlyMetal/metal-oxides/metal-hydroxide catalystsPetroleumCompound (substance)
A one-step preparation method and an application of supported platinum-based multi-metal catalysts are provided. The one-step preparation method comprises the specific steps: evenly mixing a carrier with an aqueous solution of a reducing agent, a surfactant, a platinum metal precursor and a non-platinum metal precursor, carrying out a reaction for 0.5-5 hours, and washing for multiple times at a low temperature (less than or equal to 100 DEG C) to enable the surfactant and other by-products in the product to be effectively removed. The platinum-based multi-metal catalysts having different metal loads (10-95 wt%) and different compositions and supported by different carriers are obtained, and the prepared supported platinum-based multi-metal catalysts are nanoparticles evenly dispersed on the surface of the carrier. The supported platinum-based multi-metal catalysts can be prepared through the one-step reaction, the reaction conditions are mild, operations are simple, the reaction is rapid, and synthesis is easy to enlarge. The prepared supported platinum-based multi-metal catalysts can be applied in the fields of petrochemical industry, chemical pharmacy, automobile tail gas purification and fuel cells.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI +1
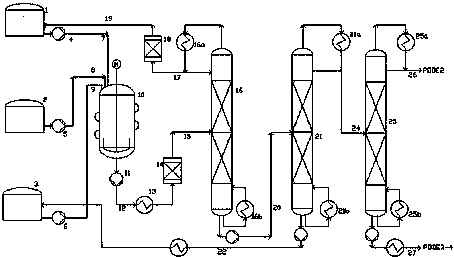
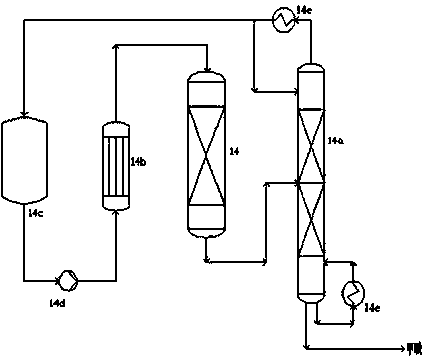
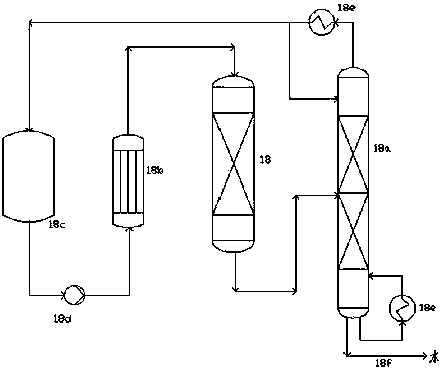
Production device system and production process for polymethoxy dimethyl ether (PODE)
The invention provides a production device system for preparing polymethoxy dimethyl ether (PODE) and a process for preparing the PODE by using the production device system. The production device system comprises a reaction system, an adsorption deacidification system, a first rectification tower system, an adsorption dewatering system, a second rectification tower system and a third rectification tower system. The production device system and the process disclosed by the invention have the advantages that (1) a reaction product is subjected to deacidification before rectification separation, so that decomposition of the reaction product in the rectification separation process is avoided; (2) the reaction product is subjected to deacidification by adopting a fixed bed, so that waste alkali residue produced by an alkali washing method is avoided; (3) un-reacted materials are dewatered before circular reaction, so that excessive hemiacetal byproduct produced due to excessive water of the reaction system is avoided; (4) unreacted methylal, formaldehyde, PODE2 and PODE5-8 are returned to a reactor after rectification separation and reacted, so that the yield of the target product PODE3-4 is improved.
Owner:DONGYING RUNCHENG CARBON MATERIAL TECH +2
Catalyst for preparing olefin with arene as side product by hydrocarbon catalytic cracking, preparing method and uses thereof
InactiveCN1504541AApplicable crackingFlexible and variable choiceCatalytic crackingCarbon numberAlkaline earth metal
A catalyst for preparing lower carbon number hydrocarbons and parallel aromatic hydrocarbons through catalytic hydrocarbon pyrolysis, comprises molecular sieve with bore diameter of 0.45-0.7 nm, nonshaped-set oxide compound and at least two modified elements from phosphor, alkaline-earth metal, lithium and tombarthite. Its preparing process comprises, (1) preparing catalyst base material from crystallization shaped molecular sieve and silicon or aluminum containing amorphous substance or aperture structure modifier through mixing modeling, (2) preparing type-H catalyst base material through sintering, (3) soaking or exchanging catalyst base material by one or more modifying solution through one-step or multistep mode, and drying and sintering for preparation of catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Ultrafine lead oxide prepared by using waste lead plaster and preparation method thereof
InactiveCN103374657AReduce energy consumptionSimple ingredientsReclaiming serviceable partsLead oxidesFiltrationTwo step
The invention discloses an ultrafine lead oxide prepared by using a waste lead plaster and a preparation method thereof. The preparation method comprises the following steps of: carrying out desulphurization process by mixing the waste lead plaster with an aqueous solution containing a composite desulfurizer for reaction; carrying out filtration to remove the desulphurization filtering solution to obtain the desulfurated lead plaster (filter residue); carrying out a leaching and crystal transformation process by adding a citric acid solution and a reducing agent into the desulfurated lead plaster obtained in the process, and carrying out filtration, washing, and drying to obtain the lead citrate after the desulfurated lead plaster reacts with the citric acid solution; carrying out a roasting process by roasting the lead citrate to obtain the ultrafine lead oxide. According to the preparation method disclosed by the invention, the ultrafine lead oxide is prepared from the waste lead storage lead plaster; a two-step leaching process is adopted; the filtering solution is simple in ingredient and can be recycled; a side product is recycled from the desulphurization solution. The preparation method disclosed by the invention is low in energy consumption, simple in equipment, high in lead recycling rate, and high in ultrafine lead product quality, and has the characteristics of good resource recycling effect, environmentally-friendly and pollution-free production process, and capability of clean production.
Owner:湖北金洋冶金股份有限公司 +1
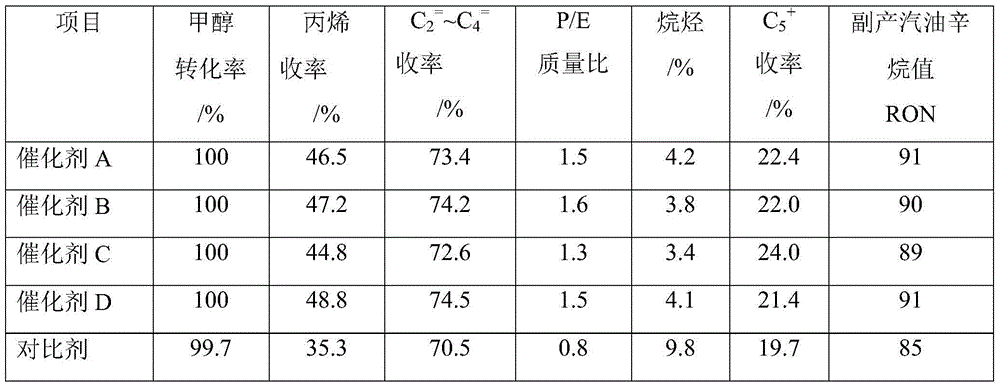
Catalyst for preparing propylene byproduct high-octane gasoline by taking methanol as raw material and preparation method of catalyst
ActiveCN104307560AGood technical effectHigh selectivityMolecular sieve catalystsHydrocarbon from oxygen organic compoundsPtru catalystEngineering
The invention provides a catalyst for preparing propylene byproduct high-octane gasoline by taking methanol as a raw material and a preparation method of the catalyst. The catalyst belongs to a ZSM-5 crystallized aluminosilicate zeolite catalyst. The catalyst is prepared from the following raw materials in parts by mass: 30-89.9 parts of a modified porous-grade MFI structure molecular sieve, 10-60 parts of an inorganic oxide binding agent and 0.1-10 parts of metal oxide. The invention provides the catalyst for preparing the propylene byproduct high-octane gasoline by taking the methanol as the raw material, which has high propylene yield and high P / E weight ratio, few byproducts, good activity stability of the catalyst, high selectivity on propylene and high octane of the byproduct gasoline. The invention provides a preparation method of the catalyst for preparing the propylene byproduct high-octane gasoline by taking the methanol as the raw material, which has the advantages that the equipment investment is saved, the operation is simple and convenient and the production cost is low.
Owner:丁泳
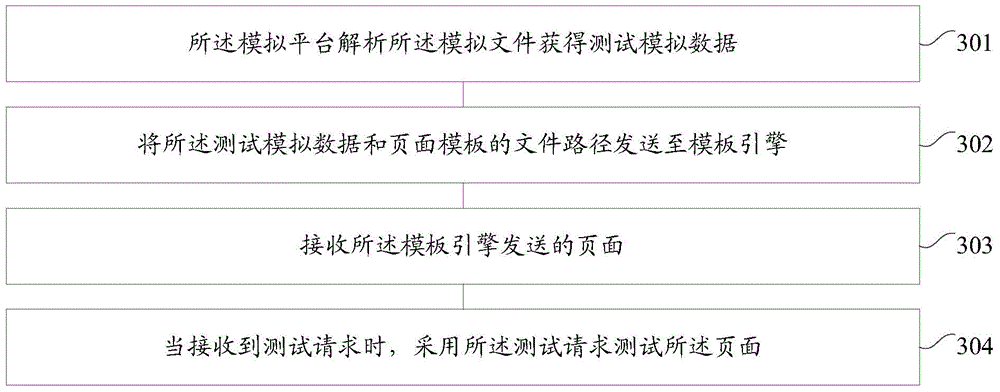
Method and device for page test at front end
ActiveCN105138448ASpeed up developmentImprove development efficiencySoftware testing/debuggingTest requirementsOperating system
Owner:BEIJING AMAZGAME AGE INTERNET TECH CO LTD
Method for preparing alcohols by selectively hydrogenating aldehydes
ActiveCN102408304AHigh selectivityHydrophobicOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSilanesReaction temperature
The invention relates to a method for preparing alcohols by selectively hydrogenating aldehydes, belonging to hydrogenation technologies. In order to meet the requirements of people on two aspects, i.e. the improving of the selectivity on preparing the alcohols by hydrogenating the aldehydes and the prolonging of the service life of a catalyst currently, the method proposes that: the aldehydes are taken as raw materials; the reaction temperature is 20-300 DEG C; the reaction pressure is 0.1-7.0 MPa; the weight space velocity of the aldehydes is 0.02-20 h<-1>; the aldehydes and hydrogen gas are in contact with a hydrogenation catalyst; and the aldehydes are produced into corresponding alcohols through selectively hydrogenating. In the method, the hydrogenation catalyst comprises a carrier, a metal active component and silane groups; the silane groups are grafted through a silylanizing treatment; and the content of the silane groups in the total weight of the catalyst is 0.05 wt% to 25 wt%. Compared with the existing method, with the adoption of the catalyst in the method provided by the invention, the selectivity is high, the amount of byproducts, such as ethers, esters and acetals is greatly lowered; and meanwhile, the generation amount of carbon deposit is little, so that the catalyst has longer service life.
Owner:CHINA PETROLEUM & CHEM CORP +1
Manufacturing method of copolyester for low acetaldehyde content of PET bottles
InactiveUS6489434B2Reduce productionLow in acetaldehydeLayered productsBottlesPolyethylene terephthalate glycolBottle
The present invention provides a manufacturing method of copolyester for low acetaldehyde content of PET bottles. The polyethylene terephthalate (PET) polymer is added with an appropriate modifier in order to decrease the production of acetaldehyde caused by pyrolysis side reaction during the blow molding process of PET bottles. The modifier comprises stabilizer and primary antioxidant, wherein the stabilizer is an inorganic phosphorous compound with an addition quantity of 0.003~0.5 weight % based on the weight of the total copolyester copolymer and the primary antioxidant is a hindered phenolic antioxidant containing Ca+2 with an addition quantity of 0.005~5.0 weight % based on the weight of the total copolyester copolymer. The present invention owns an improving effect of decreasing the production of side product-acetaldehyde at least 30% than those without the addition of said modifier.
Owner:NANYA PLASTICS CORP
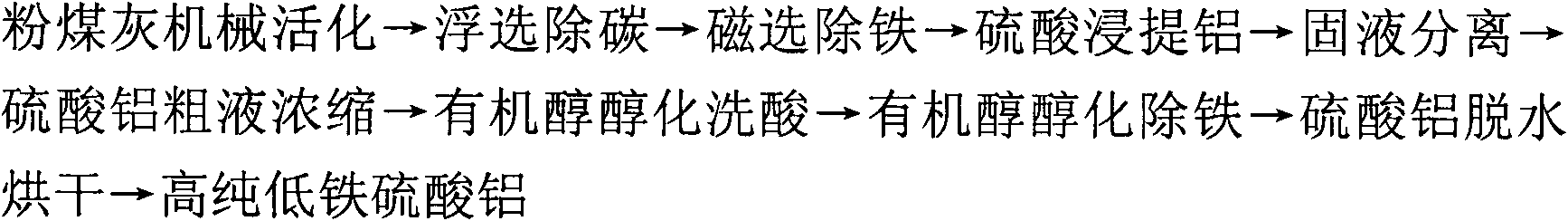
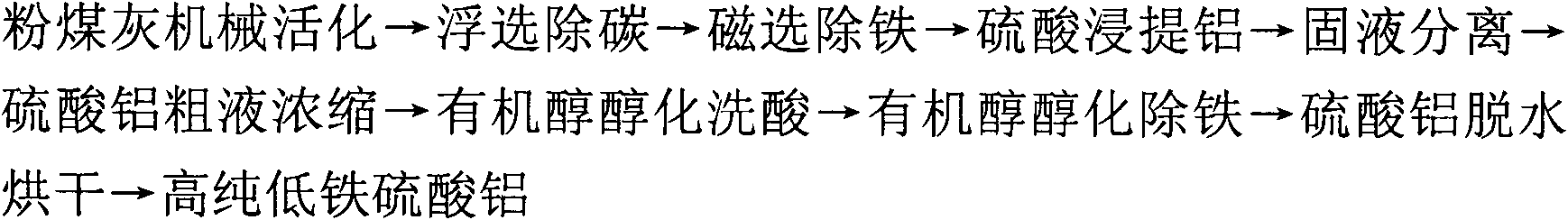
Technological method for producing high-purity low-iron aluminum sulfate by using coal ash and comprehensively utilizing coal ash
InactiveCN102101689AEasy industrial iron removalLow costPigmenting treatmentSolid waste disposalBiological activationCoal
The invention discloses a technological method for producing high-purity low-iron aluminum sulfate by using coal ash and comprehensively utilizing the coal ash, comprising the following steps of: carrying out mechanical activation, flotation decarburization, magnetic separation for deferrization, aluminum extraction with sulfuric acid, solid-liquid separation, concentration of aluminum sulfate crude liquor, organic alcohol alcoholization for acid rinse, organic alcohol alcoholization for deferrization and aluminum sulfate dewatering and drying on the coal ash to obtain the high-purity low-iron aluminum sulfate with low Fe content. The invention solves the problems on impurity removal and purification of the aluminum sulfate in the recycling process of the coal ash, simplifies the process flow, reduces the energy consumption, solves the technical problem of overlarge accumulation of secondary residue quantity, achieves high extraction ratio of aluminum contained in the coal ash, and realizes the recycling of organic alcohol and sulfuric acid and the comprehensive utilization of side products including unburnt black, magnetic iron powder, iron-containing aluminum sulfate crystals, high-silicon-dust active mineral blending materials or novel silicon-magnesium cement, and the like. The technological method has the advantages of simple process, short flow, easiness for control of a production process, high aluminum extraction ratio, low impurity content of products and stable quality.
Owner:内蒙古昶泰资源循环再生利用科技开发有限责任公司 +2
Production of esters of fatty acids and lower alcohols
InactiveUS20080051599A1Impurity can be harmfulBroad processingFatty acid esterificationFatty acids production/refiningTrans esterificationPtru catalyst
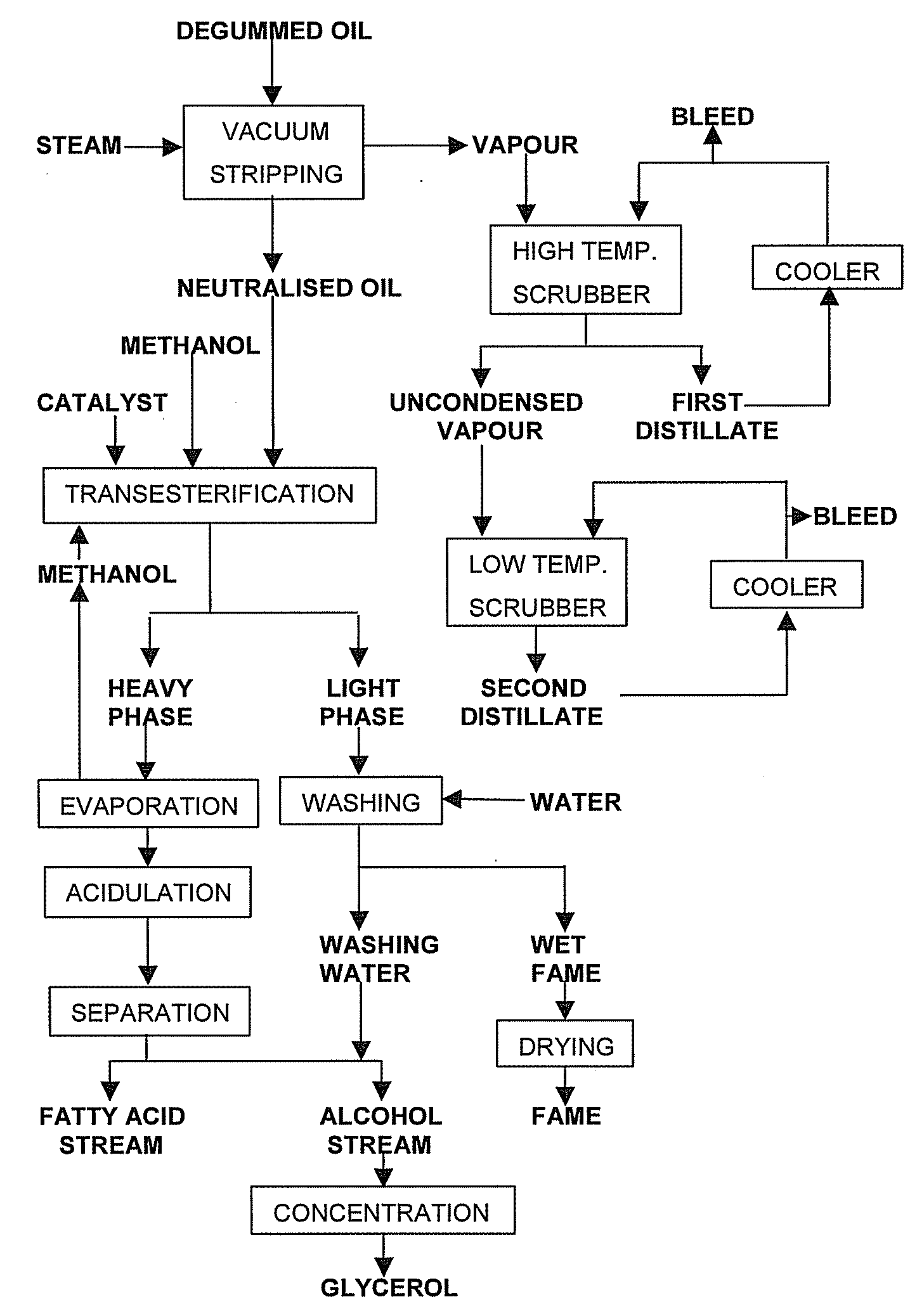
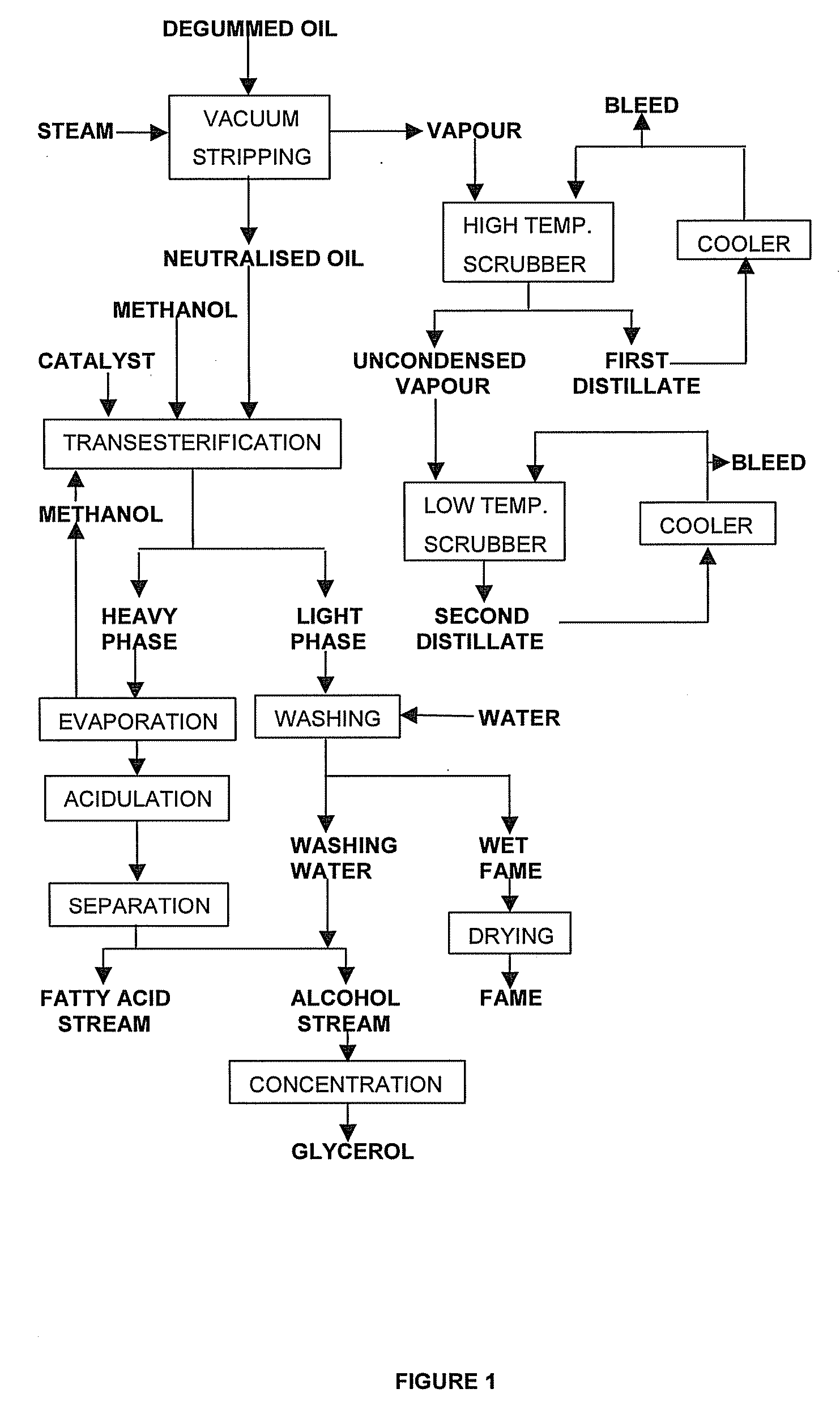
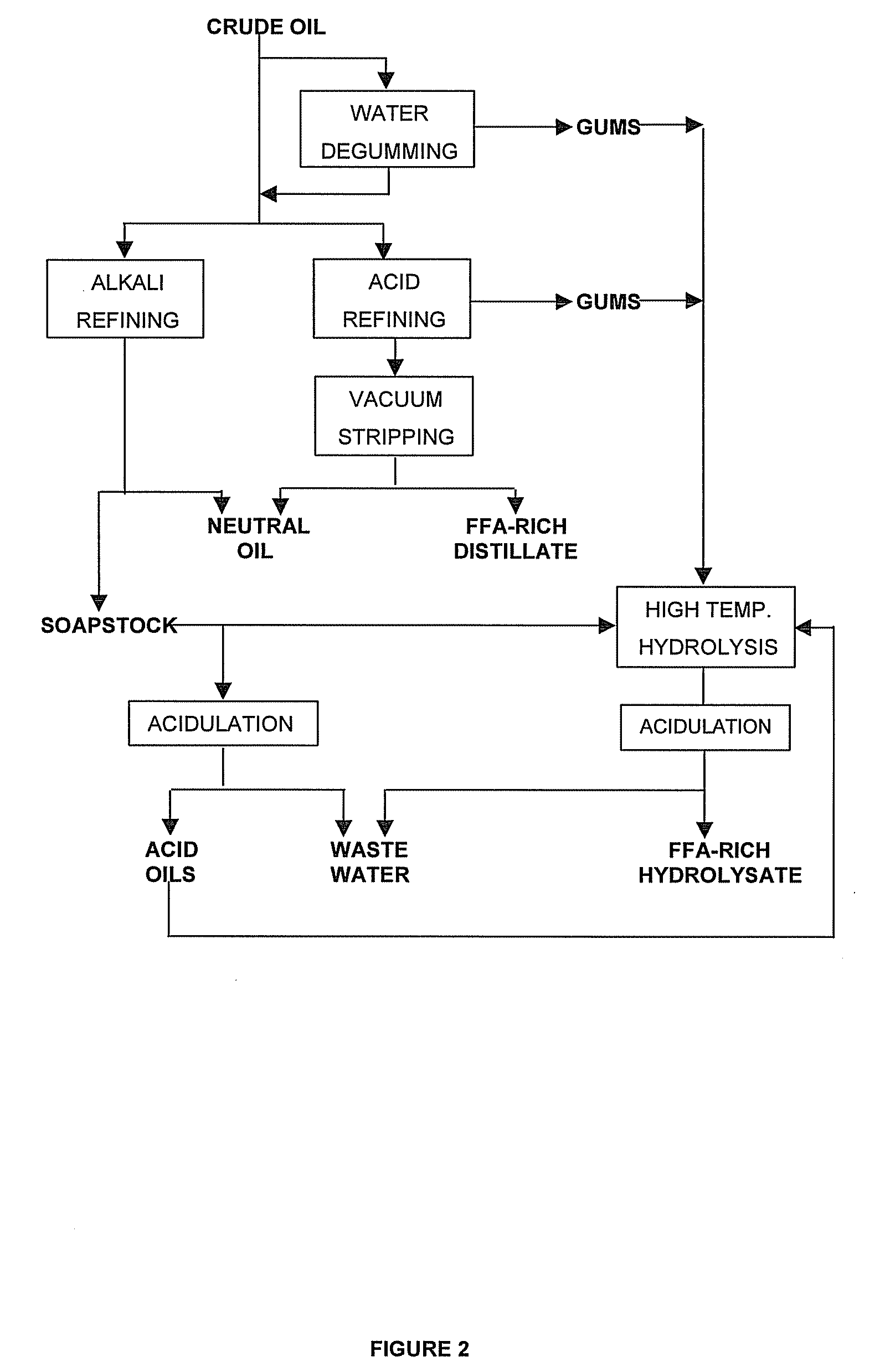
Process for the production of esters of fatty acids and C1-C5 alkyl alcohols comprising the steps of: (a) providing a fatty feed comprising a triglyceride oil or fat, partial glycerides and / or free fatty acids, (b) neutralising said fatty feed by vacuum stripping at a temperature from 200° C. to 280° C., thus providing a vapour stream and a residue, (c) collecting a distillate by scrubbing said vapour stream, (d) transesterifying said residue with a C1-C5 alkyl alcohol while using an alkaline catalyst, (e) separating the transesterification reaction mixture from step (d) into a fraction comprising C1-C5 alkyl esters of fatty acids and an alcoholic fraction (a) wherein at least part of free acids obtained as side products in step (a) and / or (c) and / or (e) are esterifyed with an alcohol using an acid catalyst, the product of this esterification being added to said fatty feed or said residue.
Owner:DESMET BALLESTRA OLEO
Process for reactive esterification distillation
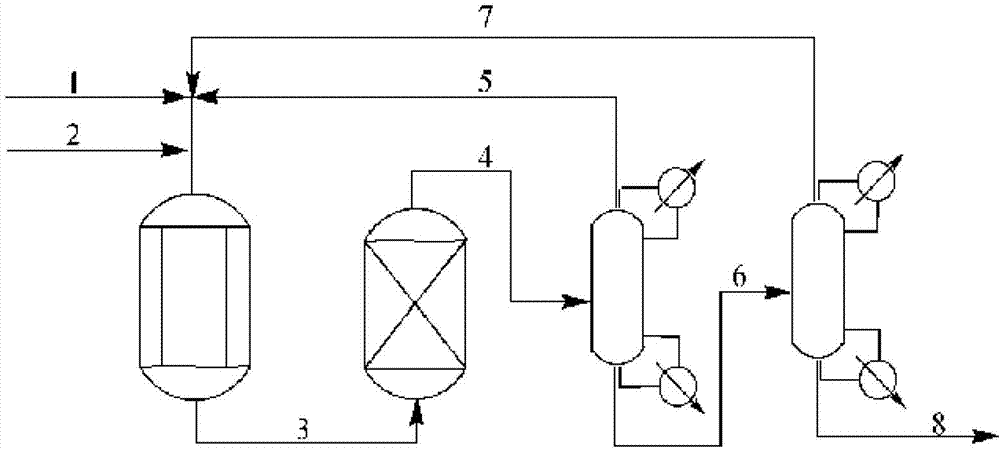
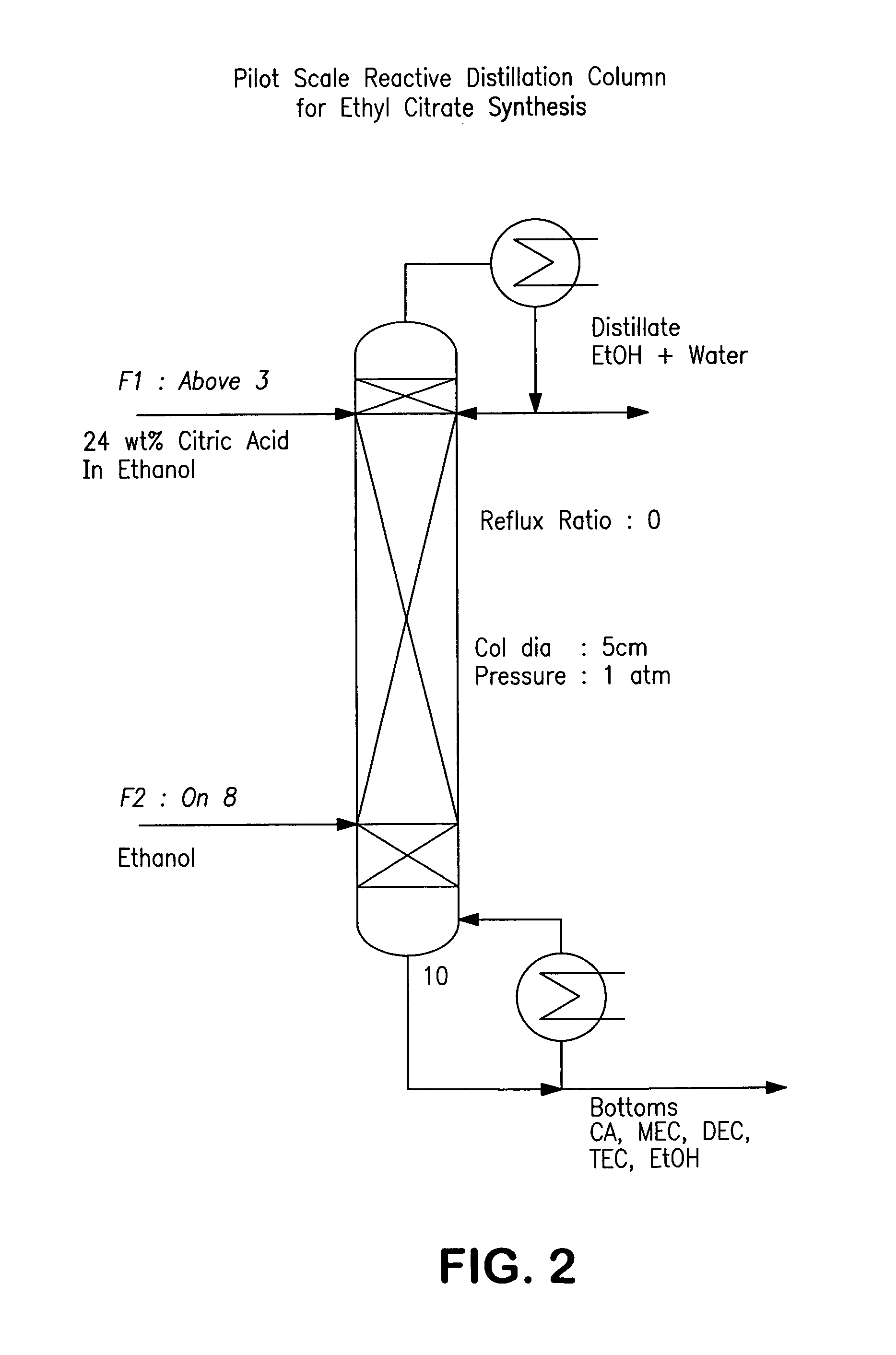
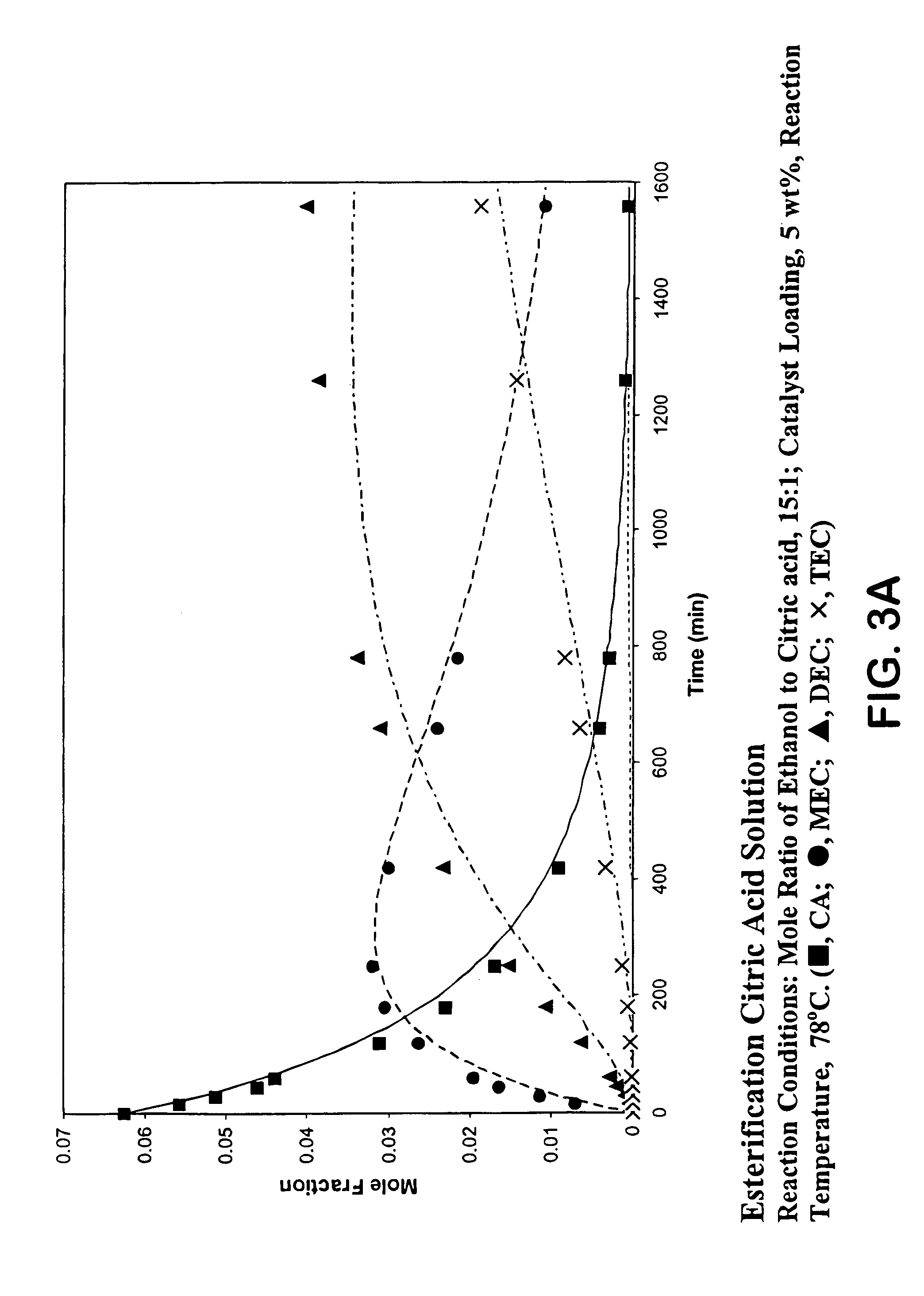
ActiveUS7667068B2Organic compound preparationPreparation by ester-hydroxy reactionOrganic acidChemical reaction
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com