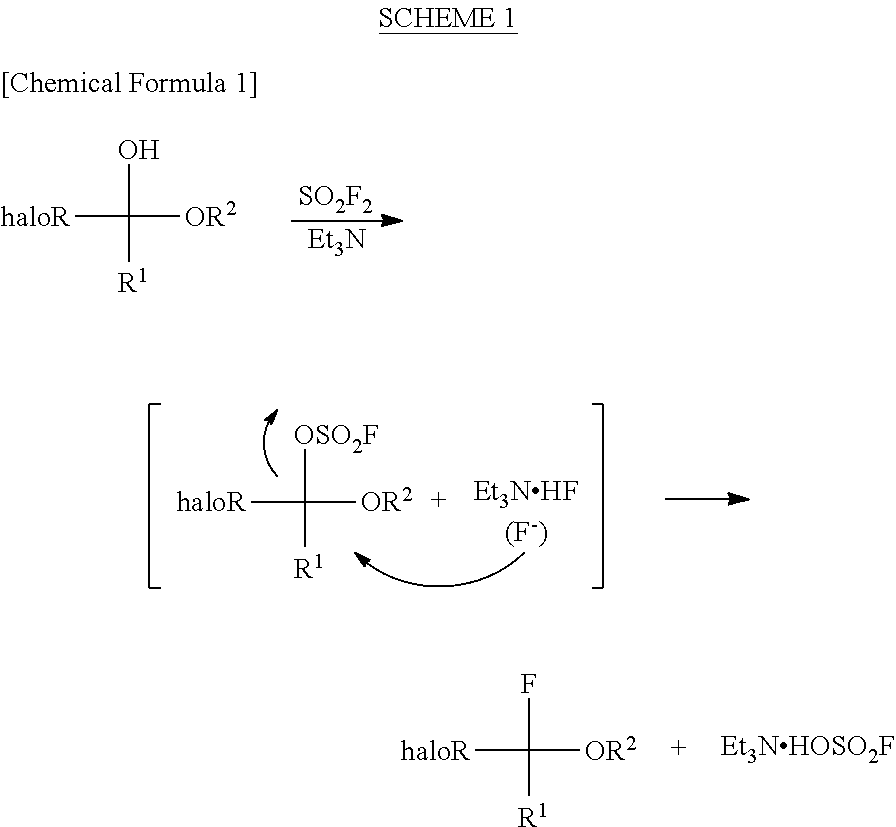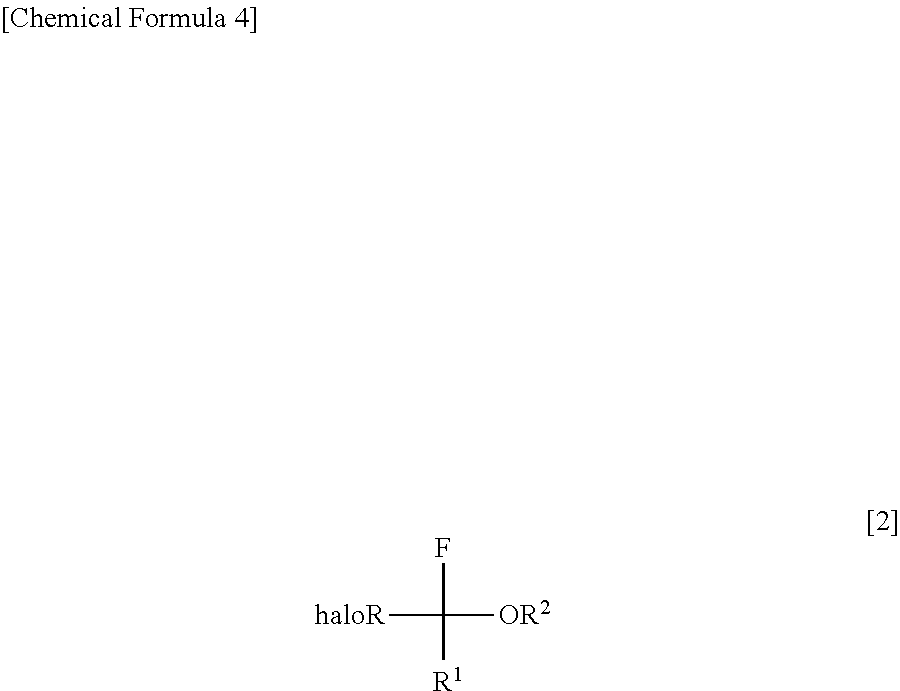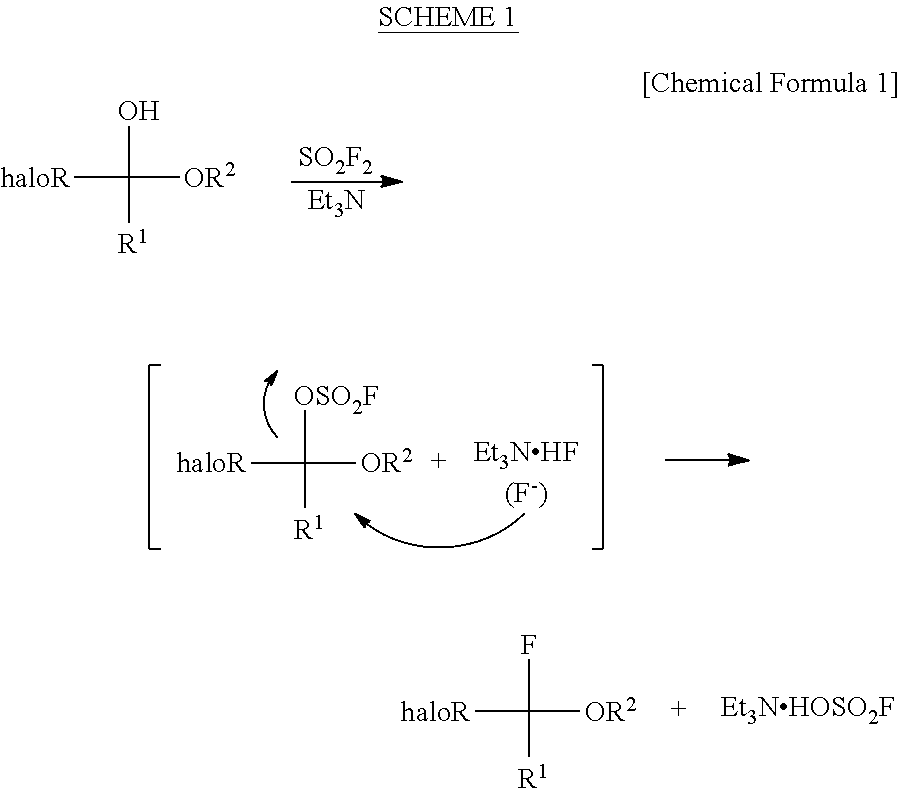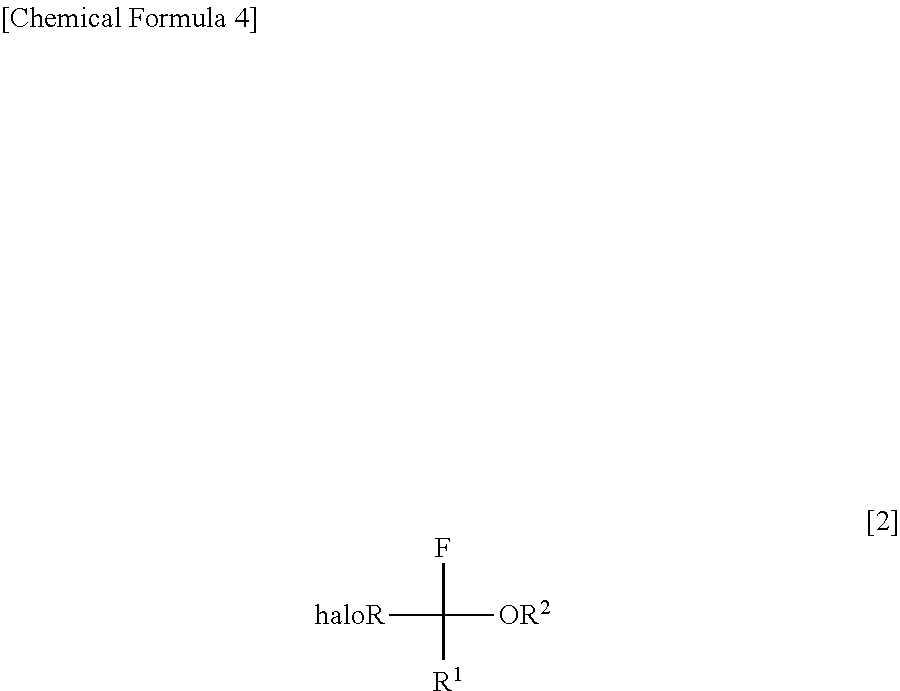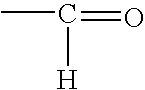Patents
Literature
151 results about "Hemiacetal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
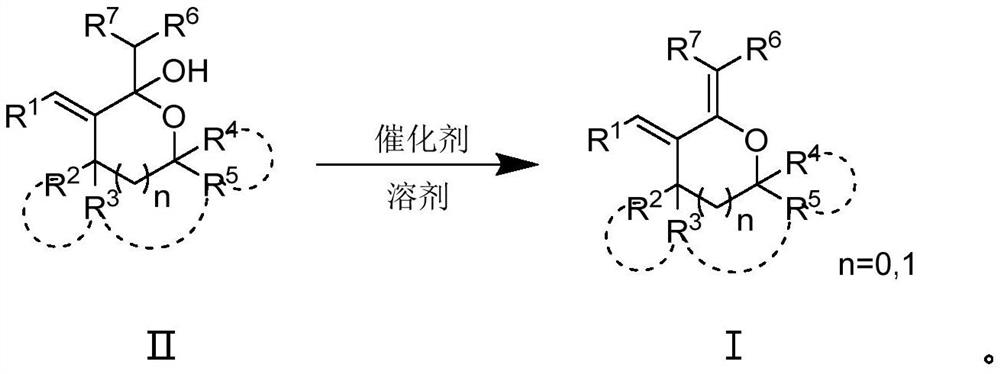
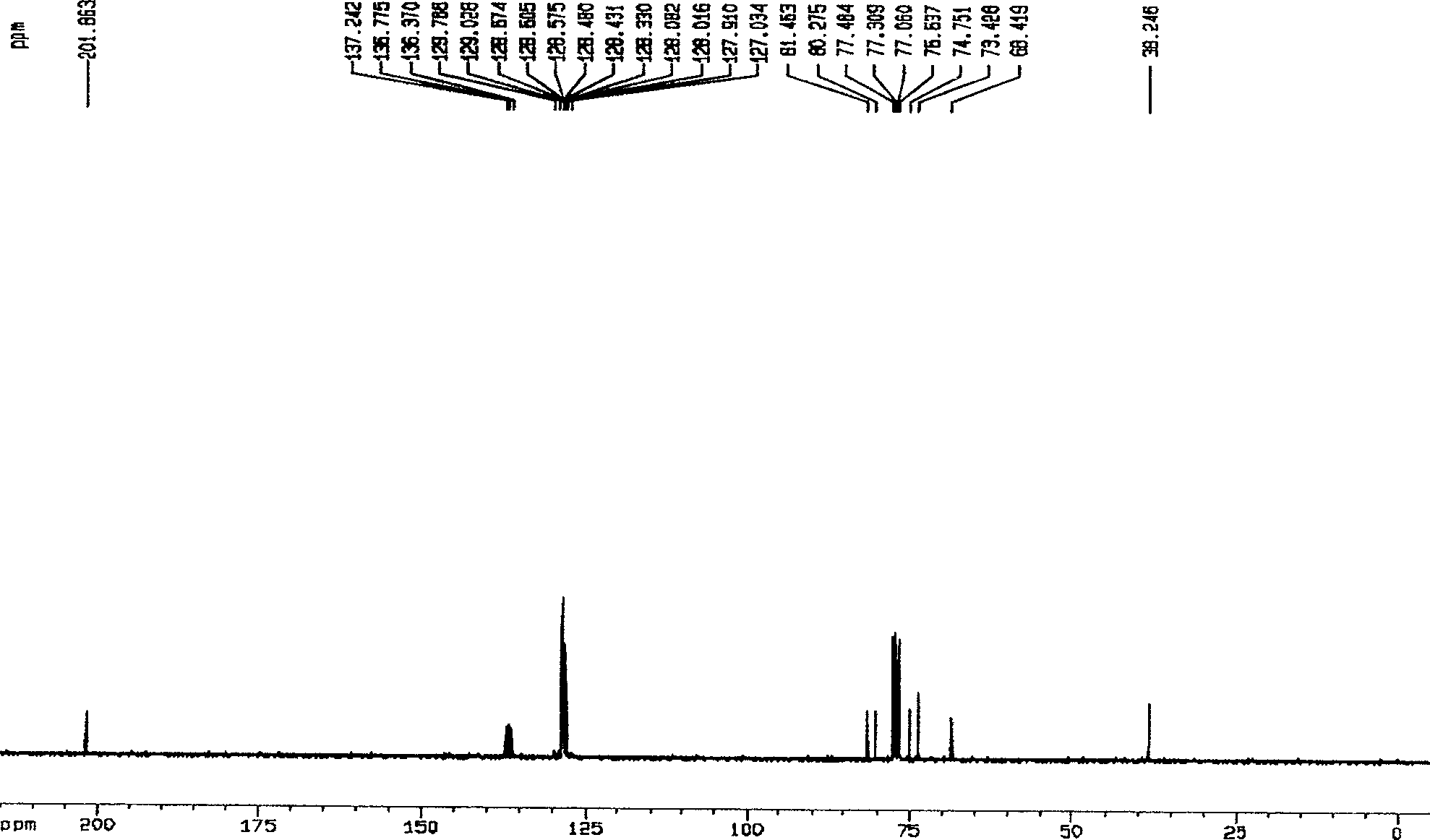
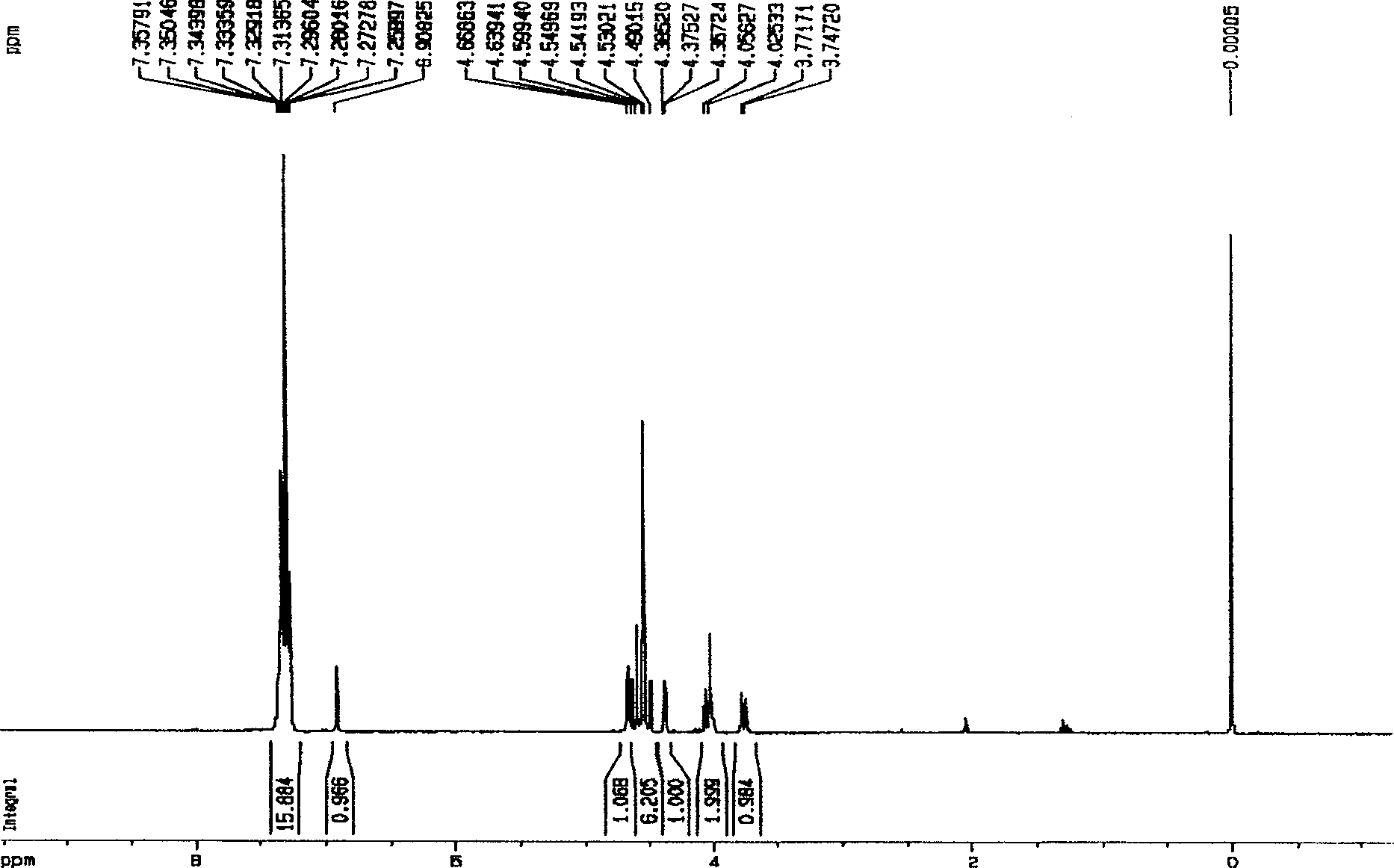
A hemiacetal or a hemiketal is a compound that results from the addition of an alcohol to an aldehyde or a ketone, respectively. The Greek word hèmi, meaning half(semi), refers to the fact that a single alcohol has been added to the carbonyl group, in contrast to acetals or ketals, which are formed when a second alkoxy group has been added to the structure.
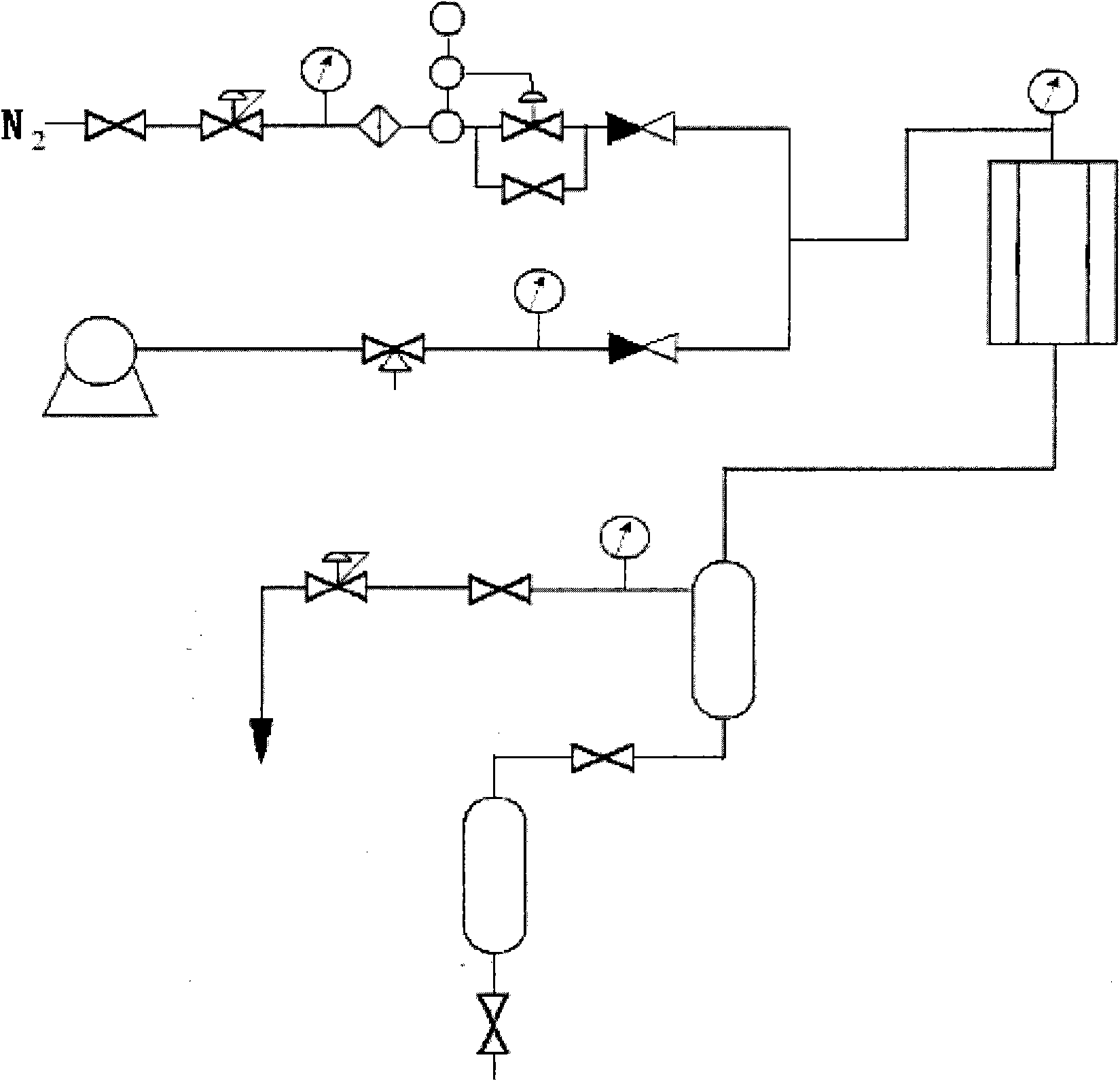
Method for continuous production of polyformaldehyde dimethyl ether
InactiveCN102786397ARealize industrial productionImprove stabilityOrganic chemistryOrganic compound preparationPtru catalystDistillation
The invention provides a method for continuous production of polyformaldehyde dimethyl ether. The method is characterized by comprising the following steps: a) feeding dimethoxymethane and hot-melted paraformaldehyde into a fixed bed reactor and adopting an acidic resin catalyst, so as to prepare polyformaldehyde dimethyl ether (DMM3-8), wherein the reaction temperature is 120-180 DEG C and the pressure is 0.1-10 MPa; b) cooling the reaction product, and then performing adsorptive separation through a dehydrating tower, so as to obtain polyformaldehyde dimethyl ether of which most water, cytidine glycol and hemiacetal are desorbed; c) feeding the polyformaldehyde dimethyl ether subjected to desorption into a distillation tower for separation, wherein most of a low-boiling component (dimethoxymethane (DMM)), poly-di-formaldehyde dimethyl ether (DMM2), a by-product (methanol) and triformol are extracted first, and then the materials in a tower kettle are fed into a rectifying tower in the next step, so as to extract the rest of the DMM2 and the triformol; and d) returning the low-boiling component (dimethoxymethane (DMM)), the methanol, the DMM2 and the triformol, which are evaporated out by the distillation tower and the rectifying tower in the last step, into the fixed bed reactor to continue to react to prepare polyformaldehyde dimethyl ether.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Method for synthesizing polyoxymethylene dimethyl ethers
ActiveCN101898943AImprove conversion rateHigh selectivityOrganic chemistryOrganic compound preparationSynthesis methodsStrong acids
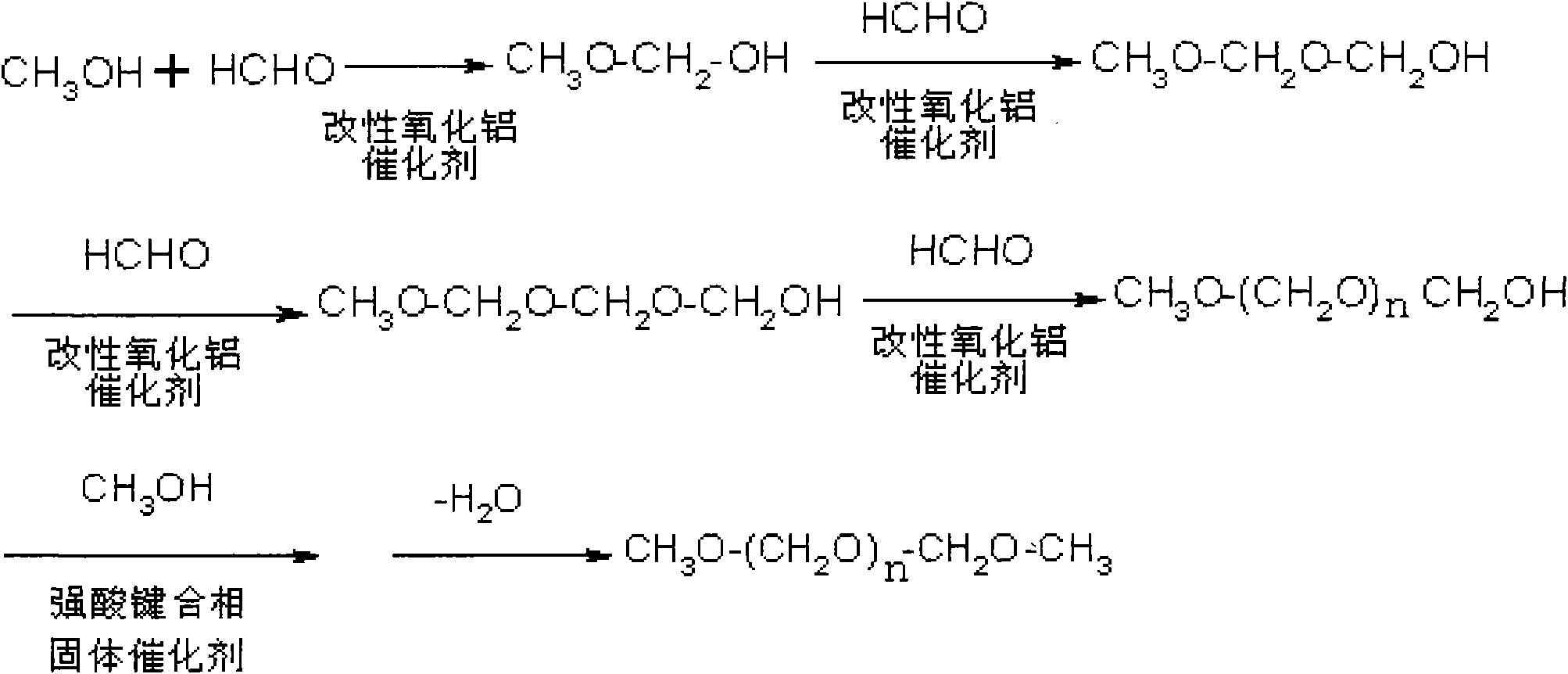
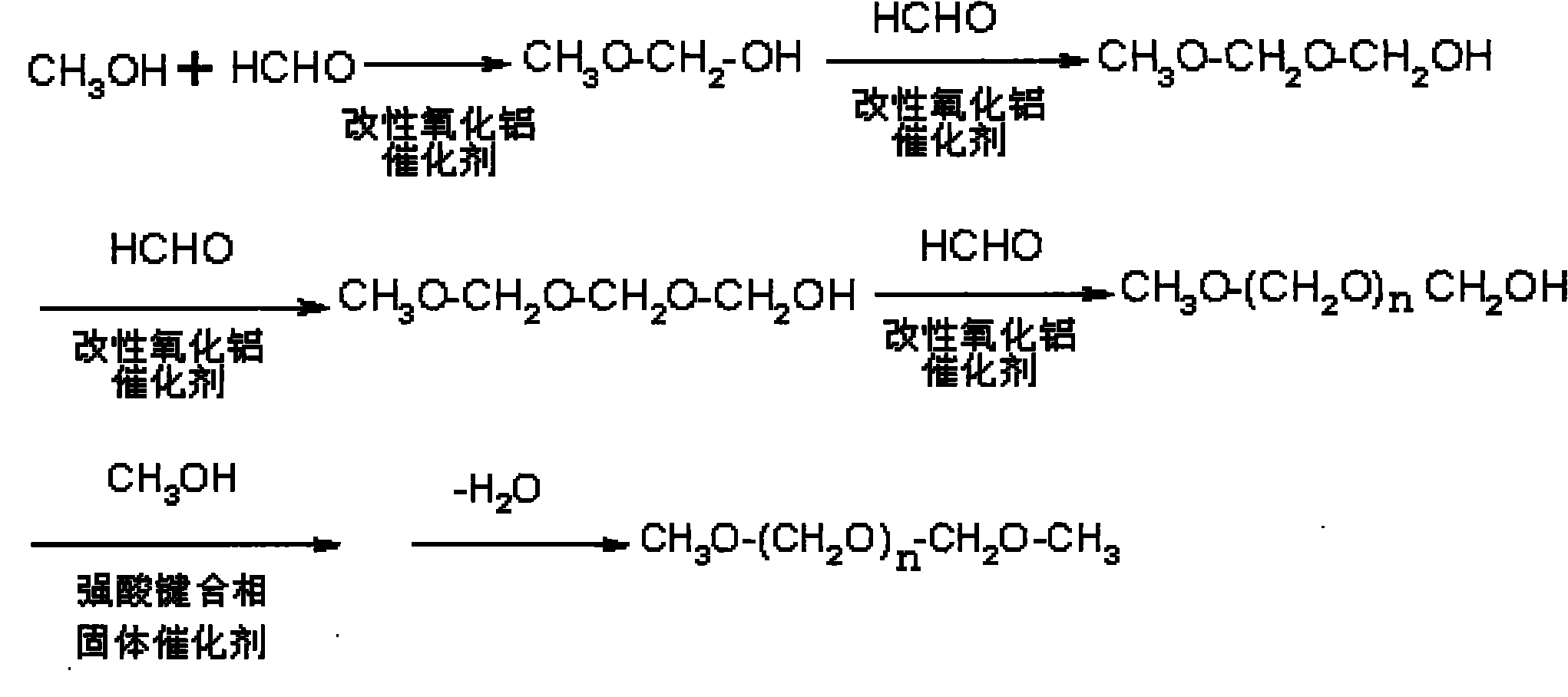
The invention relates to a method for synthesizing polyoxymethylene dimethyl ethers, comprising the following steps: step one, generating hemiacetal by methanol and excessive methanal or paraformaldehyde with low polymerization degree under the existence of a modified alumina catalyst, reacting the hemiacetal with methanal to generate hemiacetal with one more carbon, and successively continuing the above steps to generate poly-hemiacetal with more carbon atoms; and step two, generating the polyoxymethylene dimethyl ethers by the poly-hemiacetal mixture under the existence of methanol and an organic strong acid bonded phase solid catalyst. The invention can achieve the technical targets of improving raw material conversion rate, product selectivity, target product yield and economical efficiency by the two steps of reaction process, adjusts the space velocity by a fixed bed continuous reactor and combines with a catalyst to control the polymerization degree. The synthesis method in the invention has mild reaction conditions and easy control.
Owner:DONGFANG HONGSHENG NEW ENERGY APPL TECH RES INST +1
Method of producing erythromycin derivative
A method of producing an N-demethyl-N-isopropyl-8,9-anhydroerythromycin A-6,9-hemiacetal or a salt thereof, characterized in that an N-demethylerythromycin A or a salt thereof is reacted with an isopropylating agent and subsequently treated under acidic conditions, and a method of producing a substantially pure crystal of an 8,9-anhydroerythromycin A-6,9-hemiacetal derivative represented formula (VI): wherein R1 and R2, whether identical or not, represent an alkyl having 1 to 6 carbon atoms, an alkenyl having 2 to 6 carbon atoms or an alkynyl having 2 to 6 carbon atoms; R3 represents hydrogen or a hydroxyl group; one of R4 and R5 represents hydrogen and the other represents a hydroxyl group, or R4 and R5 bind together to represent O=; R6 represents hydrogen or a hydroxyl group that may be substituted for; R7 represents hydrogen or a hydroxyl group; or a salt thereof, characterized in that a crude crystal of said 8,9-anhydroerythromycin A-6,9-hemiacetal derivative or a salt thereof is recrystallized as a solvation product from hydrated isopropanol.
Owner:TAKEDA PHARMA CO LTD +1
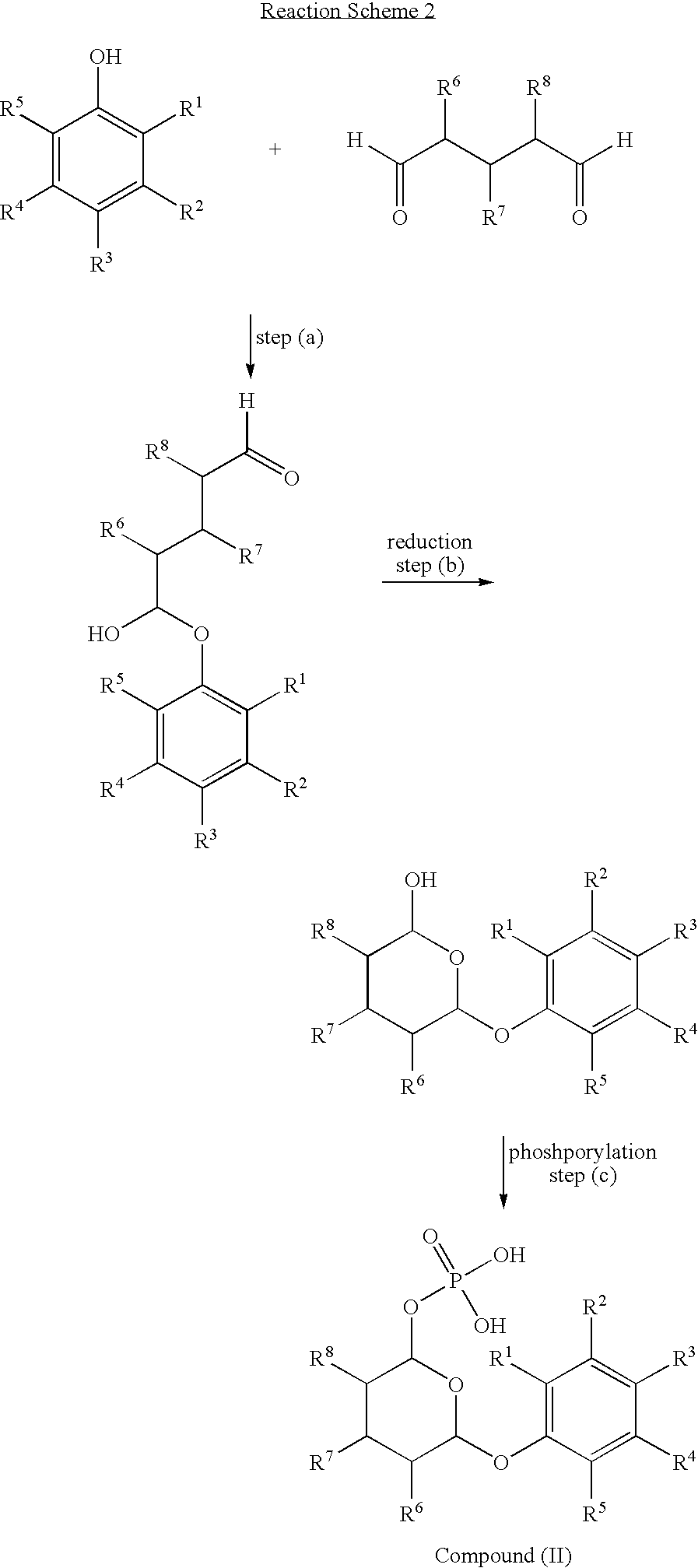
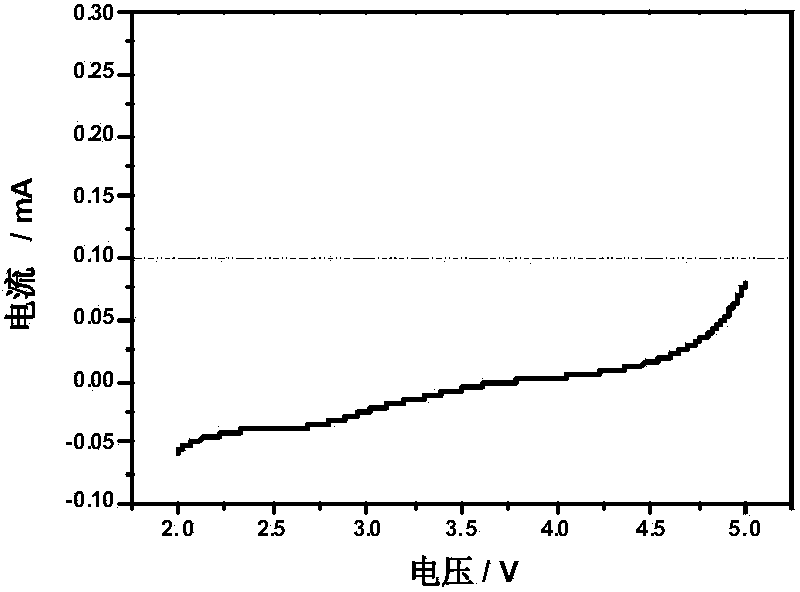
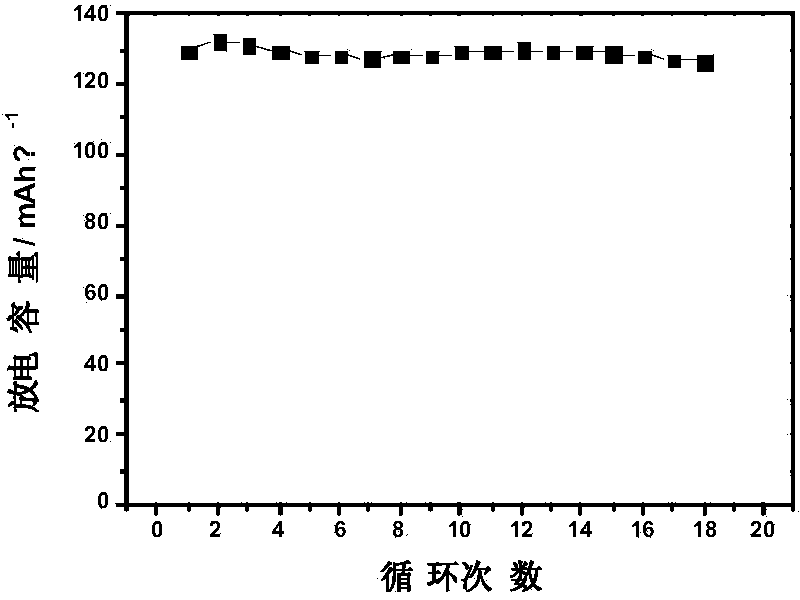
Phosphate derivatives
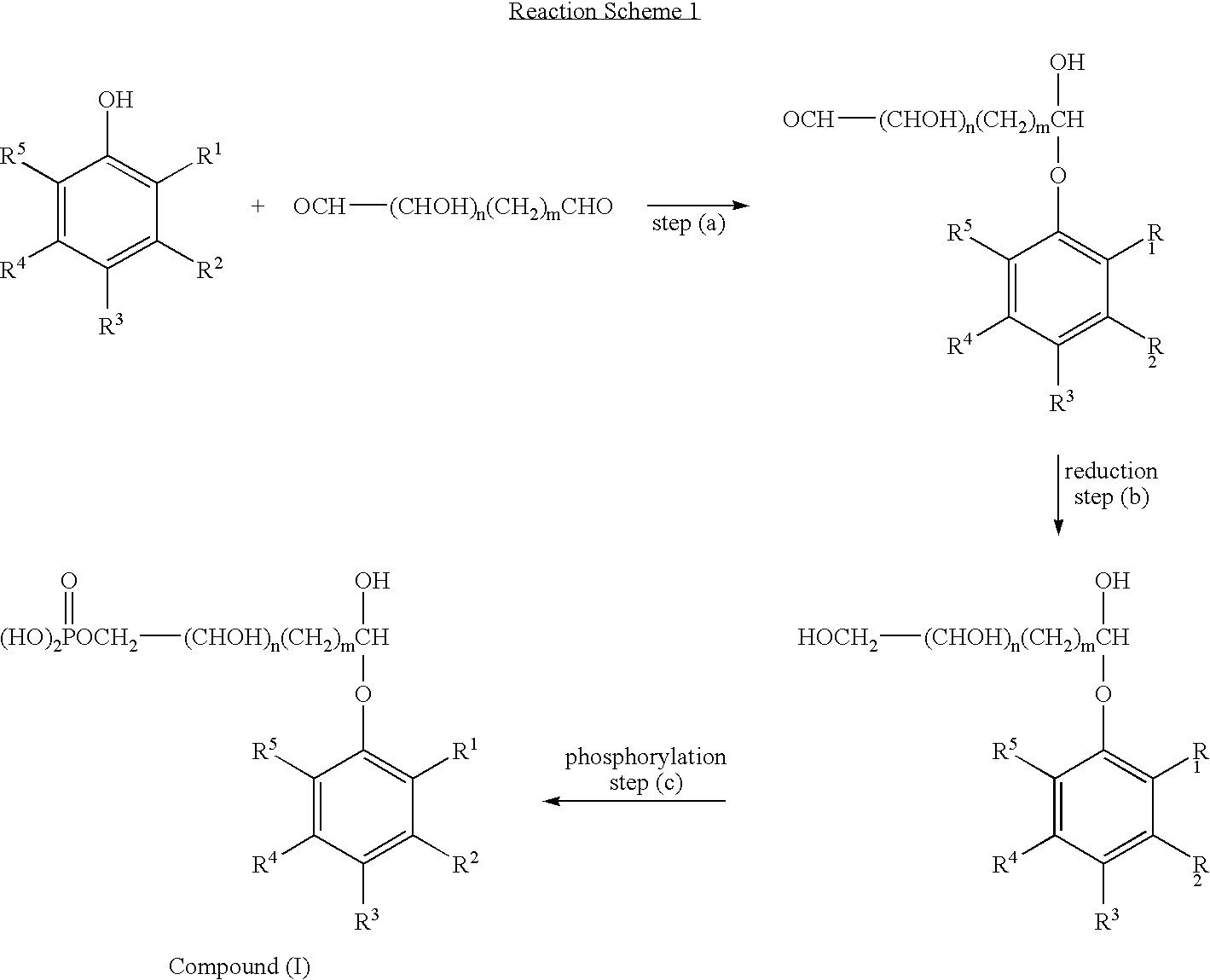
According to the invention, there is provided a phosphate derivative of a phenolic hydroxy compound comprising the reaction product of the following steps: (d) reacting the phenolic hydroxy compound with an alkyl α:ω dialdehyde or a sugar-like polyhydroxy dialdehyde to form a hemiacetal; (e) reducing the terminal aldehyde group on the product from step (a) to a hydroxyl group; and (f) phosphorylating the hydroxyl group formed in step (b) to produce a phosphate derivative of the phenolic hydroxy compound.
Owner:VITAL HEALTH SCIENCES PTY LTD
Preparation method and application of polyvinyl acetal-based gel polymer electrolyte
ActiveCN104319420AIn situ synthesisRealize comprehensive applicationFinal product manufactureElectrolyte accumulators manufacturePolymer dissolutionPolymer science
The invention discloses a preparation method and an application of a polyvinyl acetal-based gel polymer electrolyte. According to the preparation method, the polyvinyl acetal polymers with the structures shown as the general formulae (1), (2), (3) and (4) are dissolved in an organic solvent system; and a reactive diluent and a photoinitiator are added into the liquid electrolyte in a certain ratio and are uniformly mixed to obtain a precursor solution; a lithium ion battery diaphragm is fully swelled and infiltrated by the precursor solution, and then is irradiated under the ultraviolet rays with the wavelength in the range of 200 to 365 nm; chemical cross linking happens to the obtained a diaphragm-supported gel polymer electrolyte, wherein R1 and R2 express a full acetal compound or a hemiacetal compound of aliphatic hydrocarbons or aromatic hydrocarbons with 1 to 13 hydrogen atoms or carbon atoms. The preparation method is easy to operate, and is suitable for continuous scale production of gel polymer electrolyte and in-situ film-forming production of polymer lithium ion batteries; moreover, the prepared gel polymer electrolyte has high ionic conductivity and a wide electrochemical stability window; the matching cycling performance with an electrode material is high; the liquid leakage pollution of the liquid electrolyte battery is avoided; the safety performance is high.
Owner:UNIV OF SCI & TECH BEIJING
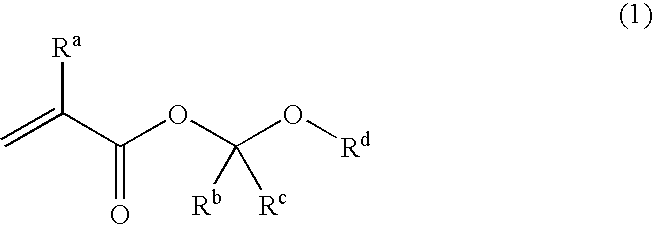
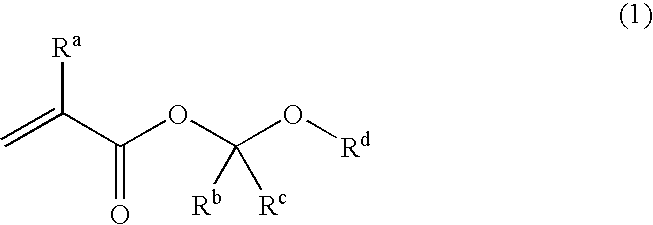
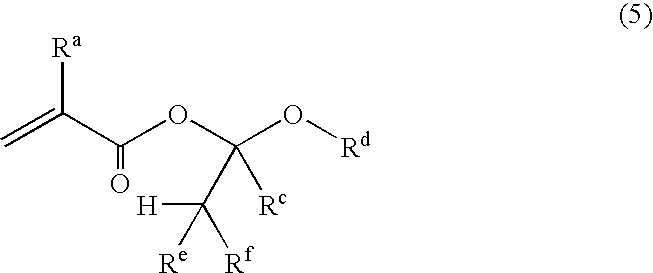
Unsaturated carboxylic acid hemicacetal ester, polymeric compound and photoresist resin composition
InactiveUS20060160247A1Superior acid-eliminating functionFunction increaseOrganic compound preparationPhotosensitive materialsCarbon numberPhotoresist
A polymeric compound having a repeated unit corresponding to an unsaturated carboxylic acid hemiacetal ester represented by the following formula (1); wherein Ra is a hydrogen atom, a halogen atom, an alkyl group of carbon number 1 to 6 or a haloalkyl group of carbon number 1 to 6, Rb is a hydrocarbon group having a hydrogen atom at a first poison, Rc is a hydrogen atom or a hydrocarbon group and Rd is an organic group having a cyclic skeleton. This polymeric compound, further, may have a repeated unit corresponding to at least one monomer selected from a monomer having a lactone skeleton, a monomer having a cyclic ketone skeleton, a monomer having an acid anhydride group and a monomer having an imide group [except for a repeated unit corresponding to the said unsaturated carboxylic acid hemiacetal ester] and / or a repeated unit corresponding to at least one monomer selected from a monomer having a hydroxyl group and others. This polymeric compound shows superior acid-eliminating function in case of using as photoresist.
Owner:DAICEL CHEM IND LTD
Process for preparing cationic polyvinyl acetals
InactiveUS20060264572A1Improve adhesionAvoid thixotropic effect in solutionInksHemiacetalCarboxylic acid
Cationic polyvinyl acetals are prepared by copolymerizing one or more cationic N-alkyldiallylammonium salt monomers with one or more vinyl esters of branched or unbranched carboxylic acids having 1 to 15 carbon atoms, hydrolyzing the resulting copolymers to copolymers containing >50 mol % vinyl alcohol units, and acetalizating the vinyl alcohol units with one or more aliphatic aldehydes having 1 to 15 carbon atoms, their acetals, and / or hemiacetals, wherein the copolymerization takes place in a mixture of water and monovalent aliphatic alcohol having a water content of 2% to 35% by weight.
Owner:KURARAY EURO GMBH
Continuous 3-methyl-3-buten-1-ol production method
ActiveCN105693470APromote depolymerizationGenerate efficientlyOrganic compound preparationHydroxy compound preparationDepolymerizationMethyl group
The invention discloses a continuous 3-methyl-3-buten-1-ol production method.The method includes that paraformaldehyde is put in corresponding alcoholic solution with sodium methylate or sodium ethoxide mass concentration being 1-5% to form methanal hemiacetal solution by depolymerization and condensation; isobutene and methanal hemiacetal are put in a tubular reactor to generate the target product 3-methyl-3-buten-1-ol by one-step reaction under the action of catalysts; the product generated in the reaction stage is cooled and then fed into an isobutene recovery tower, a light component removal tower and a product refining tower sequentially.By the continuous 3-methyl-3-buten-1-ol production method, the defects of low heat and mass transfer efficiency, high reaction pressure, more side reactions, low production efficiency and the like in an intermittent tank process are overcome, and continuous 3-methyl-3-buten-1-ol production with the isobutene and the methanal hemiacetal serving as raw materials is realized.
Owner:JIANGSU SOBUTE NEW MATERIALS +1
Reversibly crosslinked cellulose ethers and process for the production thereof by selective oxidation of vicinal OH groups
Reversibly crosslinked, water-soluble cellulose ethers having at least two different ether components is disclosed. At least one ether component is an alkyl, hydroxyalkyl or carboxymethyl group and at least one is an alkyl group having an aldehyde function which forms hydrolyzable hemiacetals with free OH groups of the cellulose ether. The cellulose ethers are obtainable by selective oxidation of cellulose ethers containing alkyl groups having vicinal OH groups (glycol cleavage). Preferably, water-soluble cellulose ethers are co-etherified simultaneously or subsequently with 2,3-epoxypropanol (glycidol) or 3-chloro-1,2-propanediol and the 2,3-dihydroxypropyl ether groups converted entirely or partly into 2-oxoethyl ether groups by oxidation. Suitable oxidants include periodate, periodic acid or lead tetraacetate. After washing and drying, cellulose ethers reversibly crosslinked via hemiacetals can be dispersed in water or aqueous solutions, going into solution homogeneously with a time delay. No low molecular weight dialdehydes or other problematical crosslinking reagents are liberated on dissolution.
Owner:SE TYLOSE
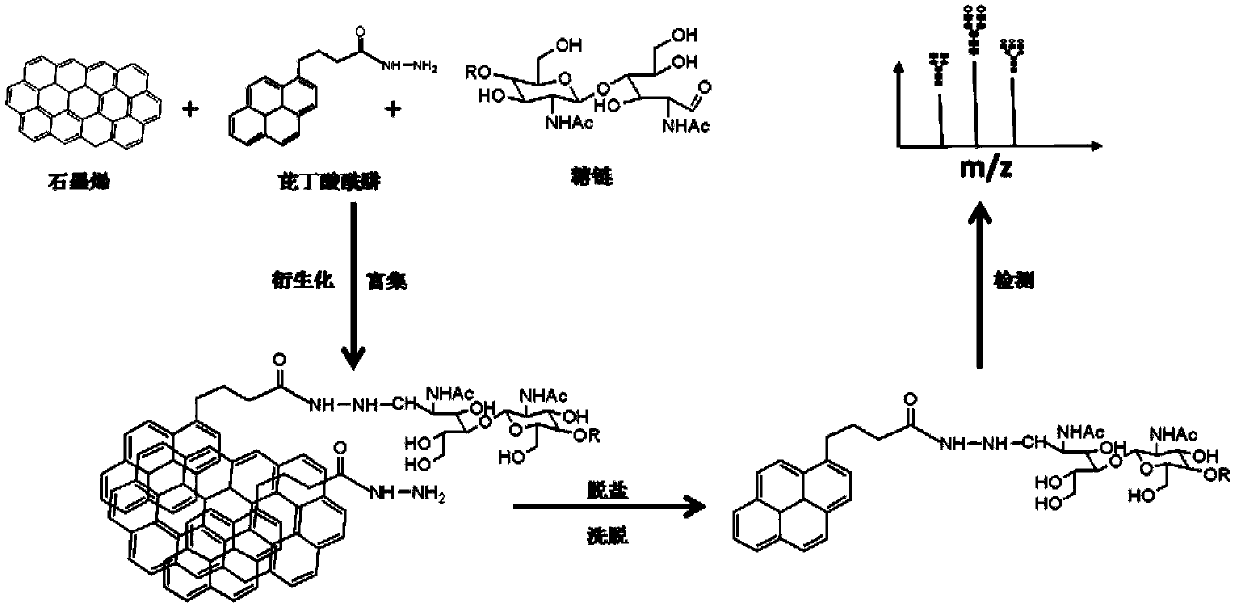
Glycoprotein N-carbohydrate chain one-step enrichment-derivation processing method based on graphene and MALDI-TOF-MS analysis method
ActiveCN104181258AIncrease the areaLarge structureComponent separationChemistryMass spectrum analysis
The invention discloses a glycoprotein N-carbohydrate chain one-step enrichment-derivation processing method based on graphene and an MALDI-TOF-MS analysis method. The chain one-step enrichment-derivation processing method comprises performing enzymatic-hydrolysis release on a glycoprotein N-carbohydrate chain, using graphene and pyrenebutyric hydrazide to performing one-step enrichment and derivation on N-carbohydrate chain, eluting N-carbohydrate chain, performing mass spectrometry, and the like. By utilizing the special pi-pi conjugate interaction of an aromatic compound and graphene and utilizing the specific efficient covalent coupling reaction of an aromatic compound containing a hydrazide or amino functional group and a hemiacetal of the N-carbohydrate chain, one-step enrichment and derivation of N-carbohydrate chain is realized, and efficient derivation of N-carbohydrate chain is finished when specific enrichment is performed. Therefore, the method avoids complicated operation steps, improves sample processing flux and substantially reduces sample loss.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Process for the preparation of a crosslinker composition
ActiveUS20100297356A1High glossReduce yellowingOther chemical processesPretreated surfacesHemiacetalNitrogen
This invention is directed to a process for the preparation of a crosslinker composition, comprising the steps of providing a mixture of an aliphatic alcohol A having at least one hydroxyl group and from 1 to 10 carbon atoms, with at least one multifunctional aldehyde C having at least two aldehyde groups —CHO to form a mixture AC, heating the mixture AC to convert at least a part of the multifunctional aldehyde C to its hemiacetal or to its acetal to form a mixture (AC)′, adding to the mixture (AC)′ least one cyclic urea U or the educts to produce the said cyclic urea U in situ, which cyclic urea U has at least one unsubstituted >NH group, and reacting the mixture thus obtained to form a chemical bond between the nitrogen atom of the at least one unsubstituted >NH group of the at least one cyclic urea U5 and the carbon atom of the least one aldehyde group —CHO of the multifunctional aldehyde C, and coating compositions comprising the said crosslinker composition.
Owner:ALLNEX NETHERLANDS BV
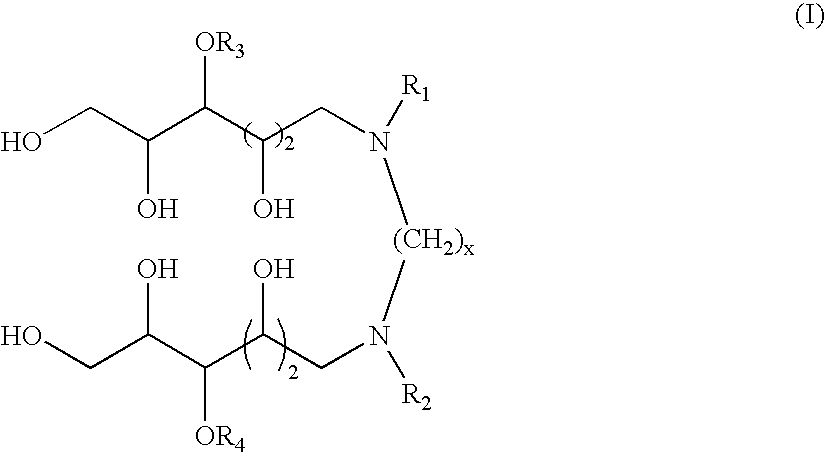
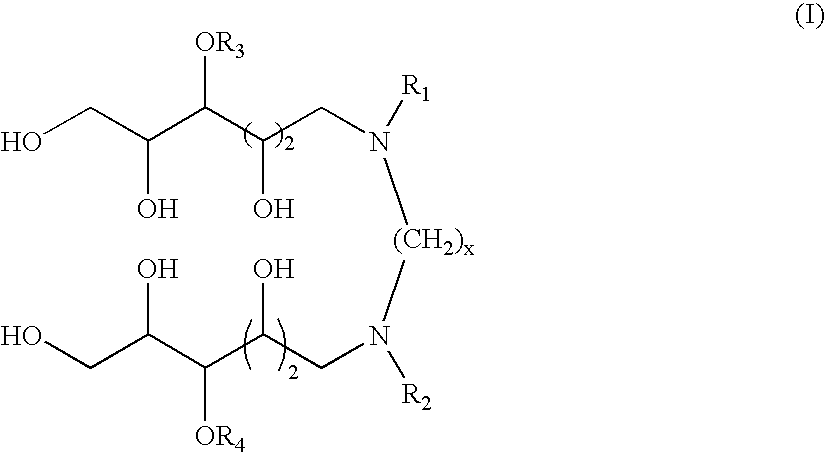
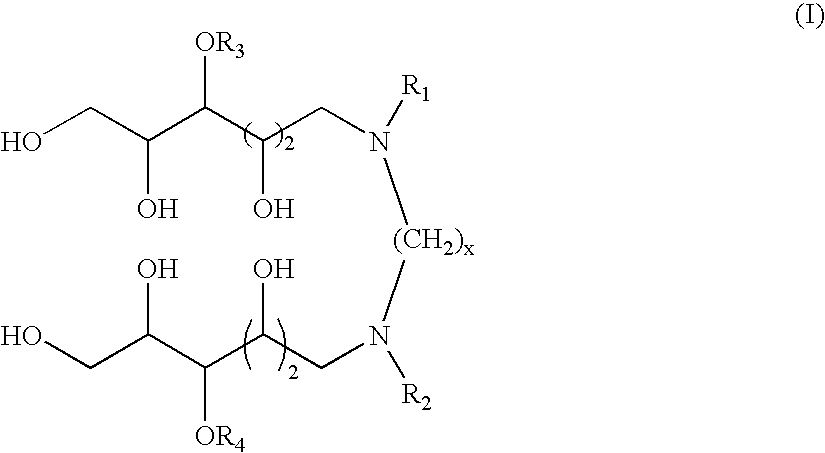
N,N'-dialkyl derivatives of polyhydroxyalkyl alkylenediamines
Surfactant compositions containing compounds according to structure (I), and methods of making them, are disclosed. The compounds provide reduced dynamic and equilibrium surface tension, good solubility, moderate foaming, and good cleaning performance. The methods for making them involve reaction of N-(polyhydroxyalkyl)-alkylamines with dinitriles, dialdehydes, or acetals or hemiacetals of dialdehydes in the presence of hydrogen and a transition metal catalyst. In structure (I), x is an integer from about 1 to 12, R1 and R2 are independently C3-C30 linear alkyl, cyclic alkyl, branched alkyl, alkenyl, aryl, alkylaryl, alkoxyalkyl, and dialkylaminoalkyl; and R3 and R4 are independently hydrogen or a pyranosyl group such as α-D-glucopyranosyl, β-D-glucopyranosyl, or β-D-galactopyranosyl.
Owner:AIR PROD & CHEM INC
Catalytic oxidation of C-H bonds
InactiveUS20040087820A1Increase productionLow yieldOxygen-containing compound preparationPreparation by oxidation reactionsHemiacetalAlcohol
The invention provides a catalytic, chemospecific and stereospecific method of oxidizing a wide variety of substrates without unwanted side reactions. Essentially, the method of the instant invention, under relatively mild reaction conditions, catalytically, stereospecifically and chemospecifically inserts oxygen into a hydrocarbon C-H bond. Oxidation (oxygen insertion) at a tertiary C-H bond to form an alcohol (and in some cases a hemiacetal) at the tertiary carbon is favored. The stereochemistry of an oxidized tertiary carbon is preserved. Ketones are formed by oxidizing a secondary C-H bond and ring-cleaved diones are formed by oxidizing cis tertiary CH bonds.
Owner:PURDUE RES FOUND INC
Method of preparing polyhydroxy annular nitrone
ActiveCN100513395CEasy to manufactureEfficient and high-volume preparationOrganic chemistrySulfonyl chlorideHydroxylamine Hydrochloride
The invention discloses a method for preparing polyhydroxy cyclic nitrones. The method for preparing the polyhydroxy cyclic nitrones of the structure of formula I provided by the present invention comprises the following steps: 1) reacting the hemiacetal of the structure of formula II with O-methylhydroxylamine hydrochloride under alkaline conditions to obtain The methyl oxime ether of the structure of formula III; 2) the methyl oxime ether of the structure of formula III is reacted with methanesulfonyl chloride to obtain the mesylate of the structure of formula IV; 3) The mesylate of the structure of formula IV is subjected to acidic conditions 4) The aldehyde of formula V structure reacts with hydroxylamine hydrochloride under alkaline conditions to obtain the polyhydroxy cyclic nitrone; wherein, R1, R2 and R3 is an alkyl, alkenyl, aryl or substituted aryl group. The present invention can prepare polyhydroxy cyclic nitrones with high reactivity through several steps of reactions by using sugars widely existing in nature as raw materials, thereby realizing the concise, efficient and large-scale preparation of such compounds.
Owner:INST OF CHEM CHINESE ACAD OF SCI
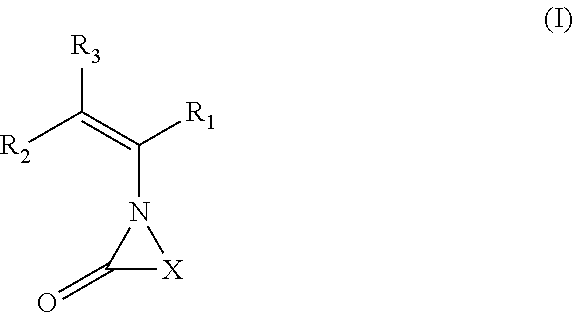
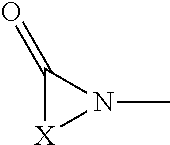
Hemi-aminal ethers and thioethers of n-alkenyl cyclic compounds
Described herein are hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds that may be produced through a reaction comprising: (A) at least one first reactant represented by a structure (I), wherein X is a functionalized or unfunctionalized C1-C5 alkylene group optionally having one or more heteroatoms, and each R1, R2, and R3 is independently selected from the group consisting of hydrogen and functionalized and unfunctionalized alkyl groups optionally having one or more heteroatoms, and (B) at least one second reactant having at least one hydroxyl moiety or thiol moiety. The hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds may comprise a polymerizable moiety, in which case they may be left as-is or used to create homopolymers or non-homopolymers, or they may not comprise a polymerizable moiety. A wide variety of formulations may be created using the hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds, including personal care, oilfield, and construction formulations.
Owner:ISP INVESTMENTS LLC
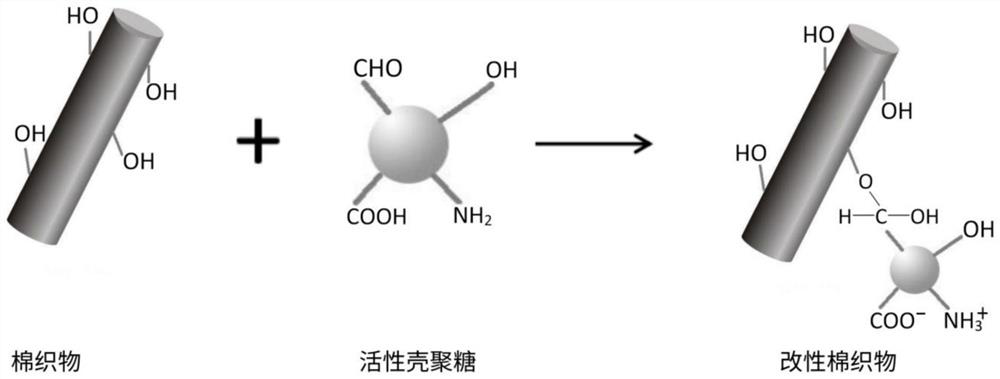
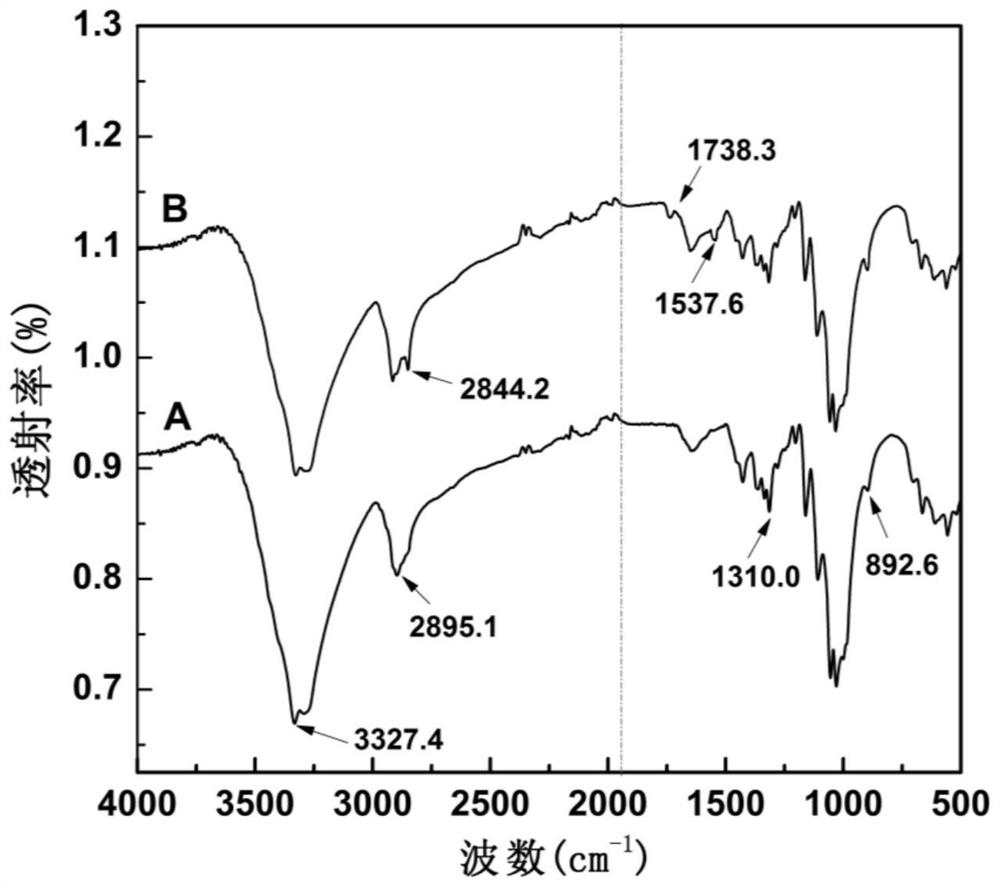
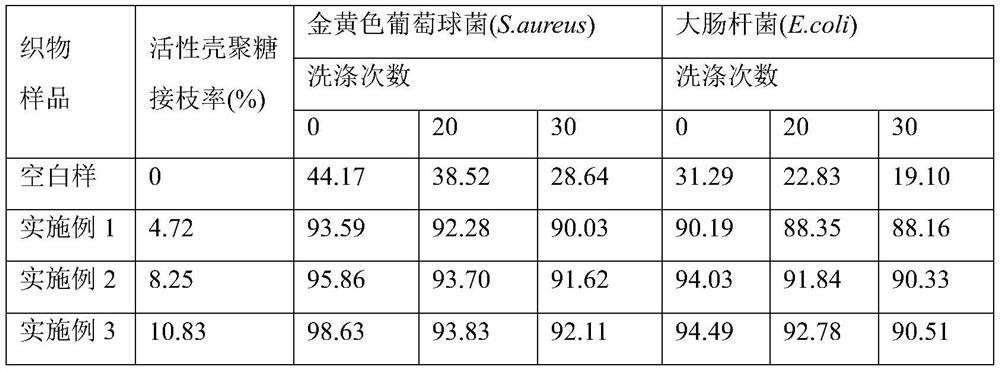
Preparation method of active chitosan modified cotton fabric
PendingCN112160161AAntibacterial lasting and efficientHuman-friendlyBiochemical fibre treatmentGrip property fibresHemiacetalPolymer science
The invention discloses a preparation method of an active chitosan modified cotton fabric. The preparation method comprises the following steps of wetting and swelling a cotton fabric in a mixed medium of 1-ethyl-3-methylimidazolium bisulfate ionic liquid and deionized water, taking out the fabric, and adding active chitosan to dissolve; and forming hemiacetal chemical bonds through reacting between hydroxyl in the cotton fabric with aldehyde groups of the active chitosan. The preparation method is simple and easy to implement, high in grafting rate, free of chemical cross-linking agents and environment-friendly, the prepared active chitosan modified cotton fabric is efficient and durable in antibiosis, good in washing resistance, high in wearing comfort and friendly to the human body, andtherefore the actual application prospect is wide.
Owner:ANHUI AGRICULTURAL UNIVERSITY
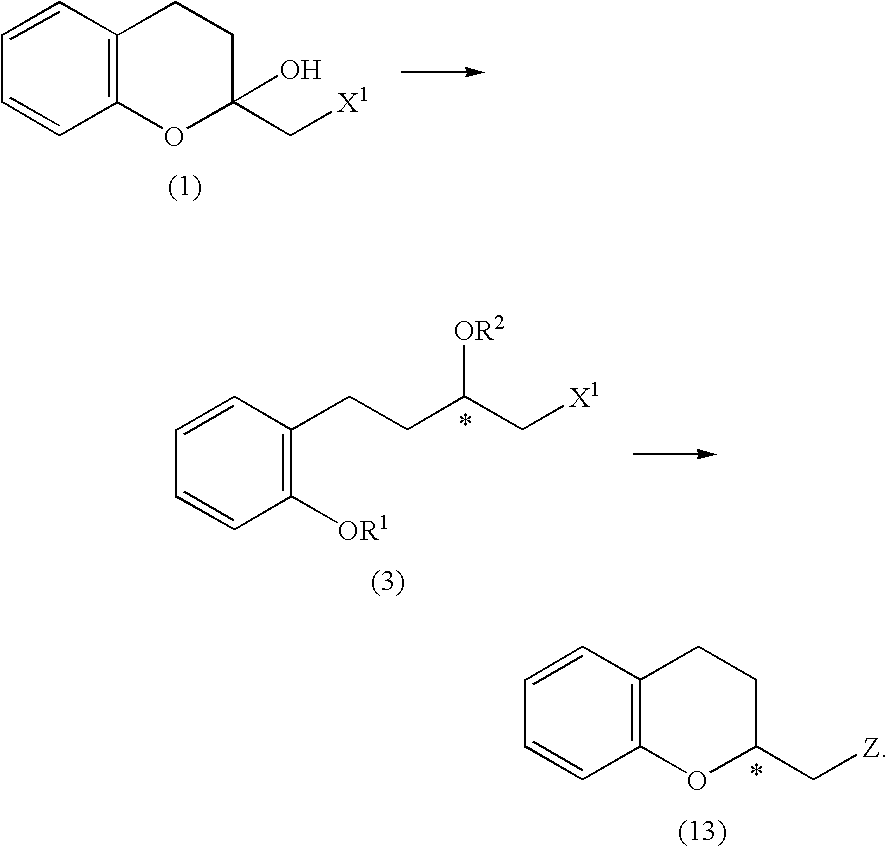
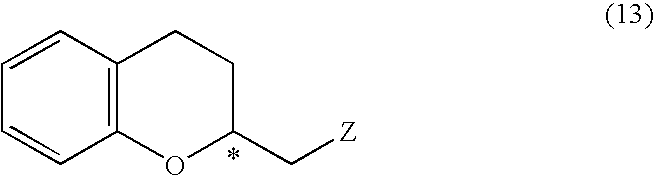
Process for producing optically active chroman derivative and intermediate
InactiveUS20050014818A1Efficient productionHighly practicalBiocideSulfonic acid esters preparationHemiacetalHalohydrin
A process for easily producing various optically active chroman derivatives that are useful as pharmaceutical intermediates from inexpensive starting materials is provided. Cyclic hemiacetal (1) obtained from dihydrocoumarin through one step is asymmetrically reduced to produce an optically active halohydrin derivative (3), and the optically active halohydrin derivative (3) is cyclized to produce an optically active chroman derivative (13):
Owner:KANEKA CORP
Crosslinkable composition and method of producing the same
The instant invention provides crosslinkable compositions, and method of producing the same. The non-aqueous single phase crosslinkable composition comprises: (a) a polyol having an average of 2 or more hydroxyl functional groups; (b) polyaldehyde, or acetal or hemiacetal thereof; and (c) an acid catalyst having pK of less than 6; and (d) optionally one or more organic solvents.
Owner:DOW GLOBAL TECH LLC +1
Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof
InactiveCN102964352AGroup 4/14 element organic compoundsMetabolism disorderMethyl groupDrug biological activity
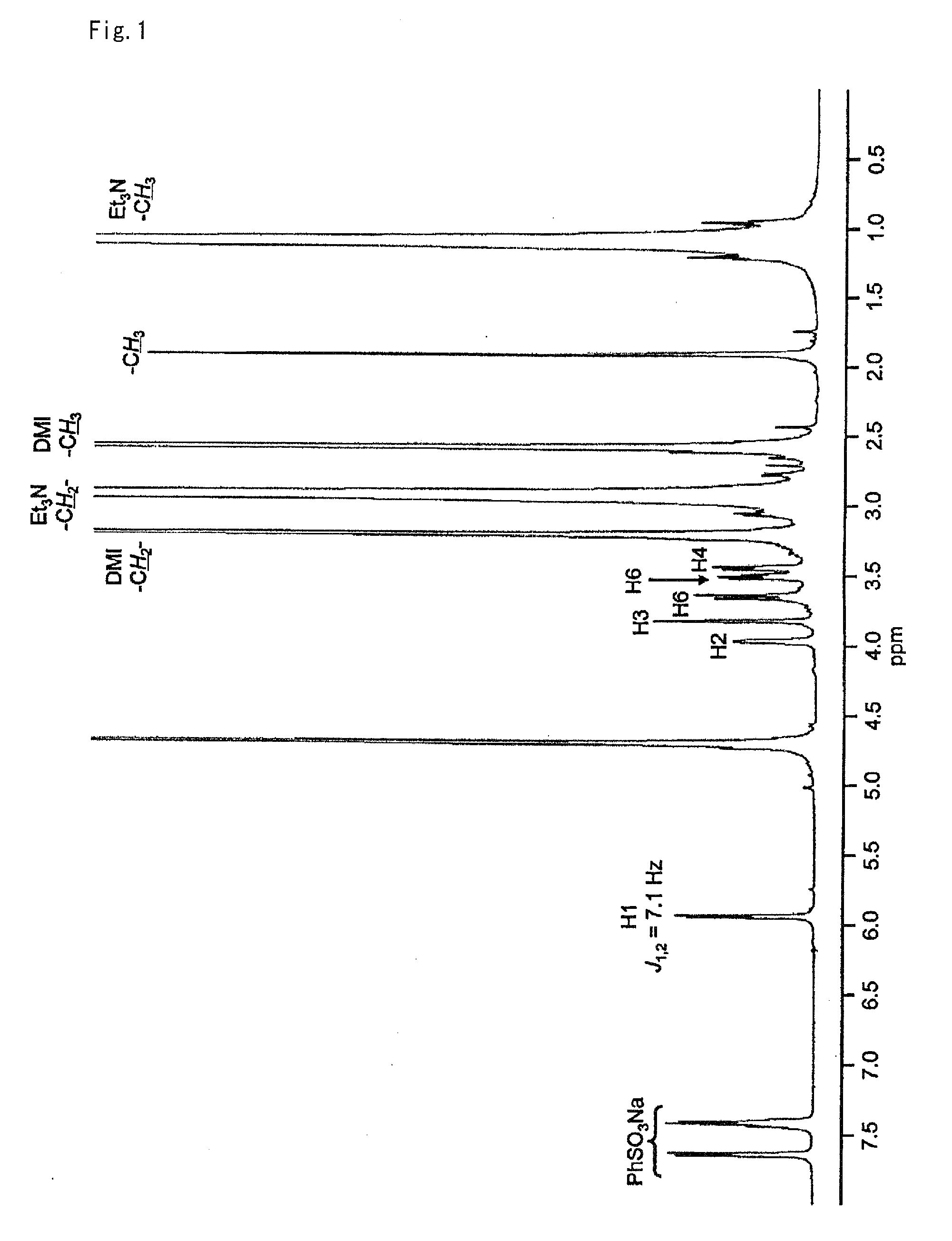
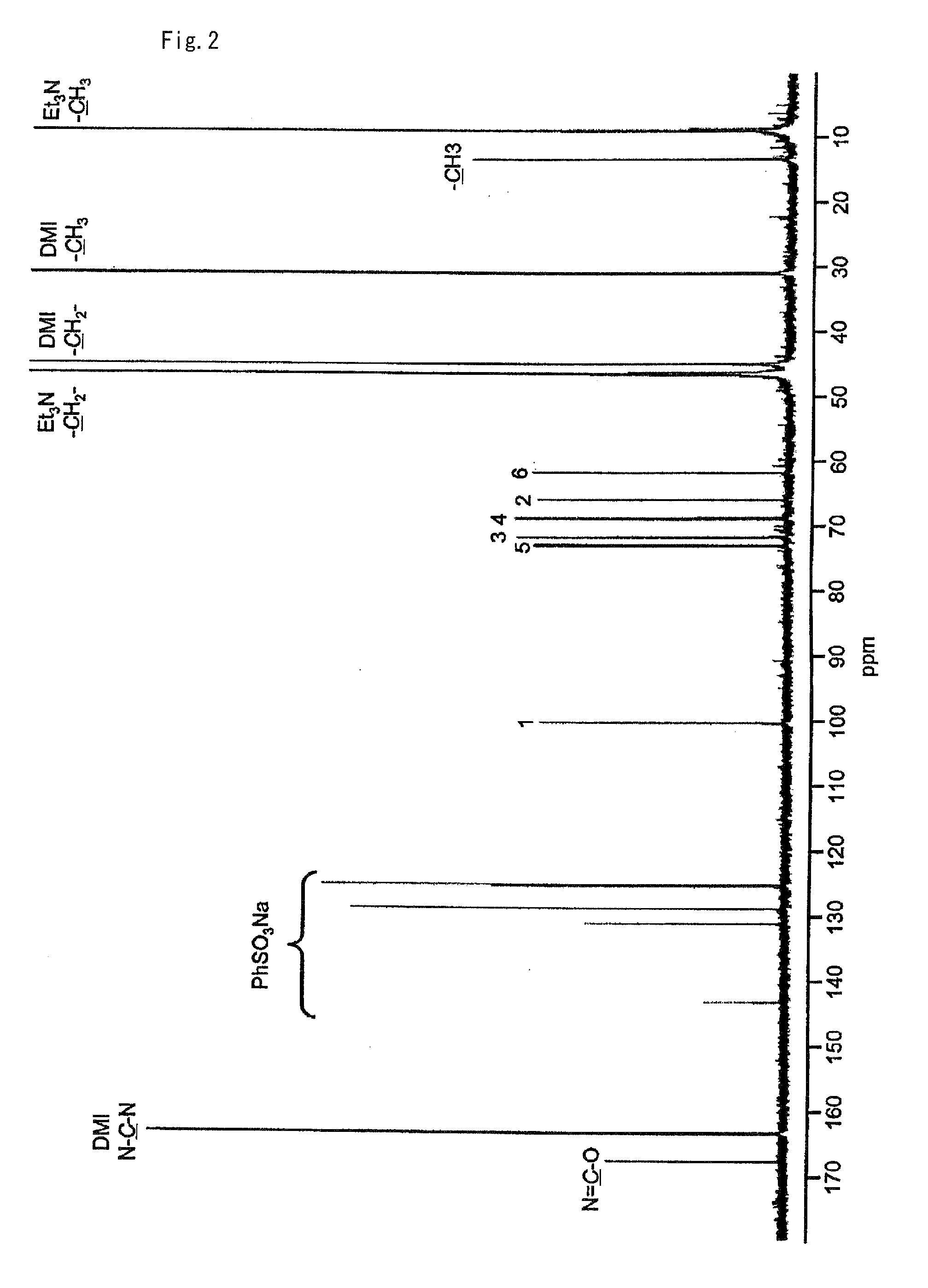
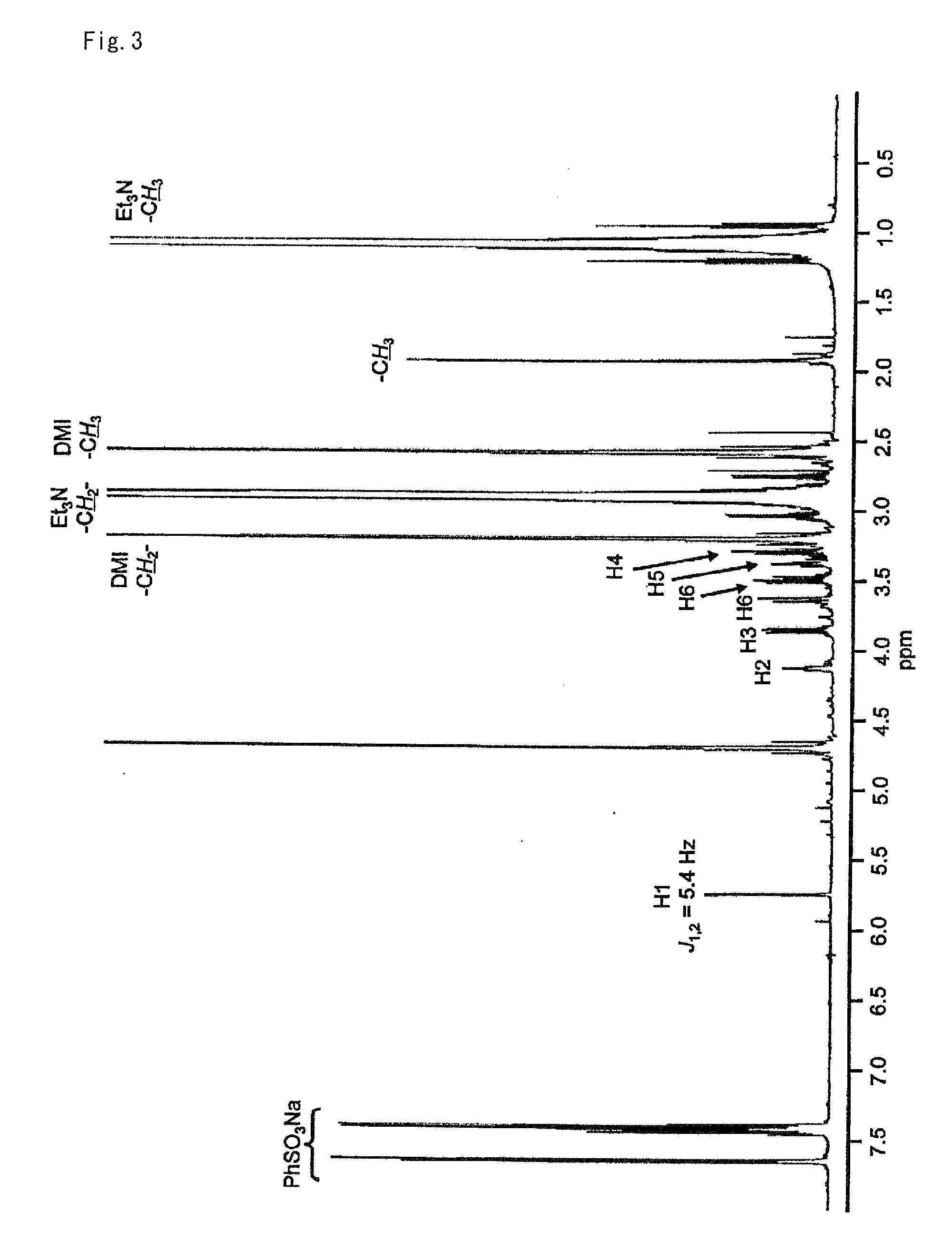
The invention relates to chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with a biological activity and an asymmetric synthesizing method thereof. The derivatives have a structure of a general formula I, in which R1 is hydrogen, 6-methyl, 7-methyl, 8-methyl, 7-methoxyl, 6-fluorine, 6-chlorine, 7-fluorine, 7-chlorine, 7-bromine or 7-benzyloxyl; R2 is methyl, ethyl, isopropyl, cyclohexyl, phenyl, benzyl, dimethyl tertiary butyl siloxy ethyl, benzosuccinimide ethyl or CH2CO2Me; R3 is phenyl, 4-methyl phenyl, 4-methoxyl phenyl, 4-fluorophenyl, 4-chlorphenyl, 4-bromophenyl, 3-bromophenyl, 2-fluorophenyl, 2-thienyl, styryl, ester group or cyclohexyl; and R4 is methyl, ethyl, isopropyl or benzyl. The derivatives are synthesized by Michael-semiacetalation cascade reaction of 3-substituted indole and beta, gamma-unsaturated alpah-keto ester catalyzed by copper trifluoromethanesulfonate and oxazoline ligand.
Owner:HUAZHONG NORMAL UNIV
Process for production of halogenated alpha-fluoroethers
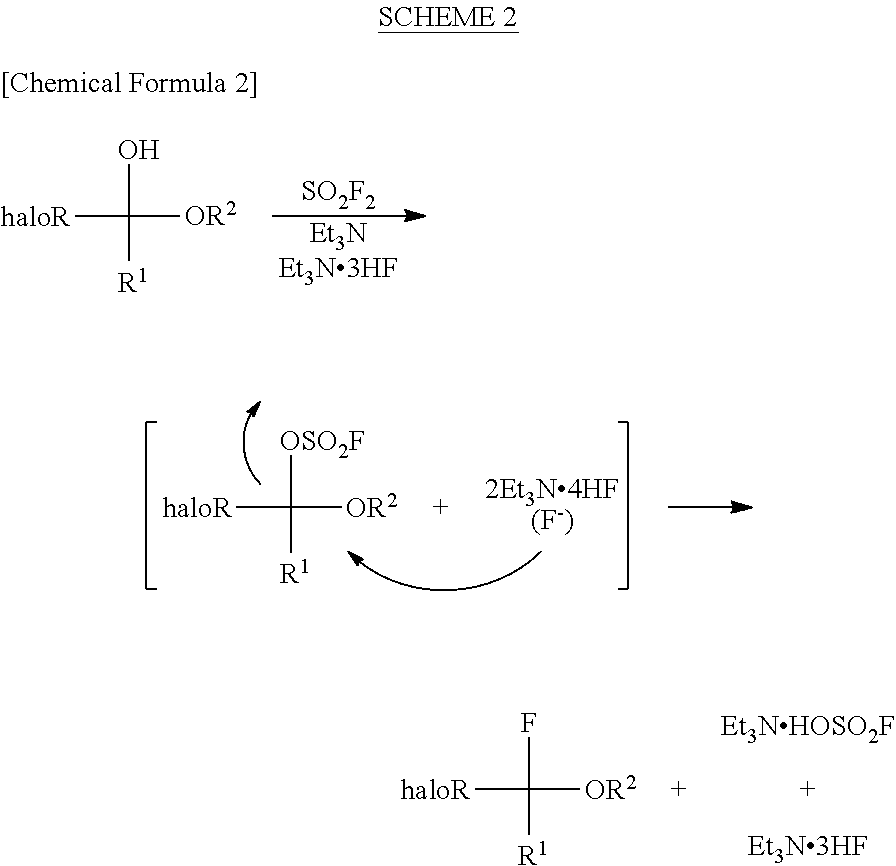
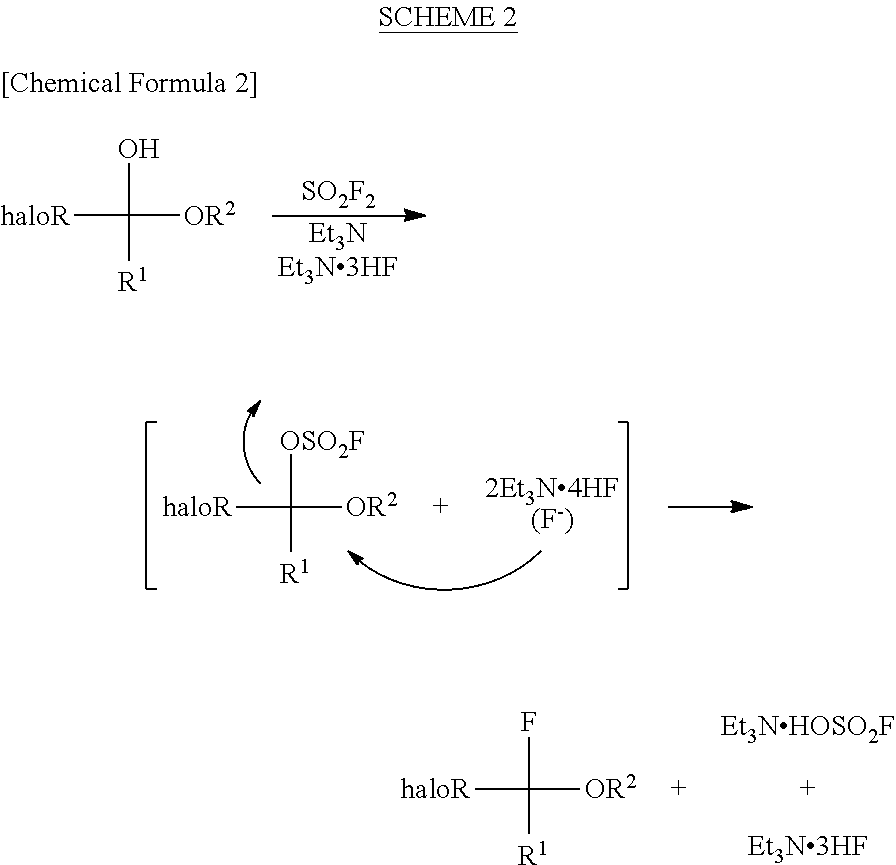
InactiveUS8304576B2Increase productivityHigh yieldOrganic compound preparationCarboxylic acid esters preparationHemiacetalHydrogen fluoride
Halogenated α-fluoroethers (or bis-derivatives thereof) can be produced by reacting a halogenated hemiacetal (or bis-derivative thereof) with sulfuryl fluoride (SO2F2) in the presence of an organic base. The reaction is conducted preferably in the presence of “a salt or complex of an organic base with hydrogen fluoride”, whereby the objective dehydroxyfluorination can proceed extremely favorably. It is still preferable to use as the starting substrate a halogenated hemiacetal prepared from fluoral or 3,3,3-trifluoropyruvic acid ester. Thus, industrially important halogenated α-fluoroethers can be industrially produced with high selectivity and in high yield.
Owner:CENT GLASS CO LTD
Process for Production of Halogenated alpha-Fluoroethers
InactiveUS20110082313A1High selectivityHigh yieldOrganic compound preparationCarboxylic acid esters preparationHemiacetalHydrogen fluoride
Halogenated α-fluoroethers (or bis-derivatives thereof) can be produced by reacting a halogenated hemiacetal (or bis-derivative thereof) with sulfuryl fluoride (S2F2) in the presence of an organic base. The reaction is conducted preferably in the presence of “a salt or complex of an organic base with hydrogen fluoride”, whereby the objective dehydroxyfluorination can proceed extremely favorably. It is still preferable to use as the starting substrate a halogenated hemiacetal prepared from fluoral or 3,3,3-trifluoropyruvic acid ester. Thus, industrially important halogenated α-fluoroethers can be industrially produced with high selectivity and in high yield.
Owner:CENT GLASS CO LTD
Preparation method for high-strength water-resistant starch adhesive for corrugated paperboards
InactiveCN104119816APrevent penetrationImprove initial tack performanceStarch derivtive adhesivesAldehyde/ketone condensation polymer adhesivesPolymer scienceAdhesive
A provided preparation method for a high-strength water-resistant starch adhesive for corrugated paperboards comprises the following steps: 1) preparation of modified starch; 2) preparation of urea-formaldehyde resin; and 3) compounding of modified starch and urea-formaldehyde resin. By adding urea-formaldehyde resin into modified starch glue, dimethylolurea and oligomers in resin and hydroxyl in starch molecules are subjected to crosslinking reaction, aldehyde group in starch and hydroxyl in resin form hemiacetal and acetal structures, and a crosslinking structure participated by starch chains is formed through reaction. Because urea-formaldehyde resin and modified starch mutual react and form a net structure, penetration of starch glue into paper is avoided, initial viscosity and water resistance of starch glue are improved, and drying time is shortened.
Owner:WUXI ALONG PACKAGE PRINTING
Poly-substituted tetrahydrofuran and tetrahydropyrane diene compound and preparation method thereof
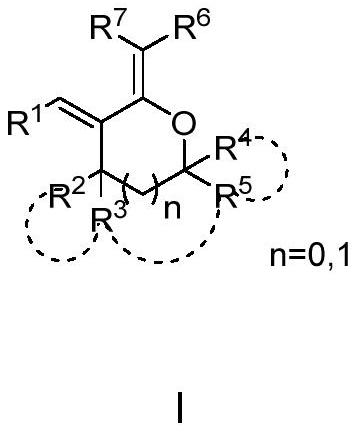
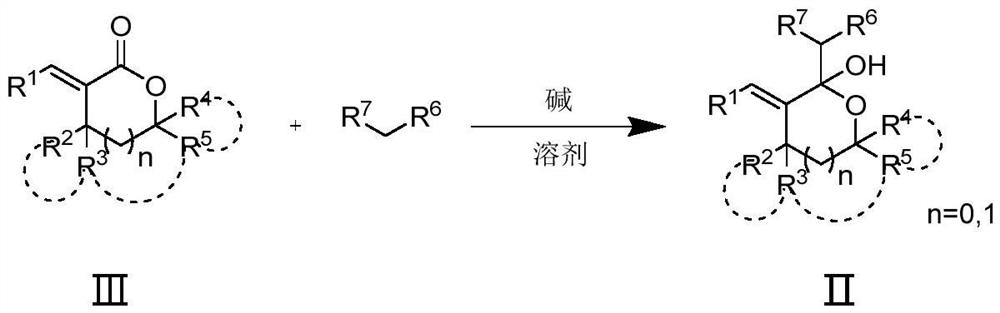
PendingCN112142694AHigh reactivityThe synthesis method is simpleAntimycoticsOrganic chemistryAlkaneProcess equipment
The invention belongs to the field of organic synthesis, and provides a poly-substituted tetrahydrofuran and tetrahydropyrane diene compound as shown in a formula I. In the formula I, R1 is selected from aryl or hetero-aryl; R2 and R3 are selected from hydrogen or C1-C6 alkyl; R4 and R5 are each independently selected from aryl, hetero-aryl, C1-C6 alkyl or hydrogen; and R6 and R7 are selected fromcyano, nitro, C1-C6 alkyl, an ester group or hydrogen. A target product is synthesized through two steps of reaction, and the method comprises the following steps: (1) reacting alpha-alkenyl lactonewith a nucleophilic reagent (nitrile, nitroalkane or ester and the like) for 1-48 hours at -78 to 60 DEG C under the action of alkali to obtain a hemiacetal intermediate; and (2) dehydrating the hemiacetal intermediate under the catalytic action of Lewis acid or Bronsted acid to form the compound shown in the formula I. The method is simple in process equipment, easy to operate, environment-friendly, low in cost and good in yield.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Method for rapidly preparing monodisperse polyvinyl alcohol microspheres at normal temperature
ActiveCN109988323ASimple structureSize adjustable and controllableChemical/physical/physico-chemical microreactorsGel preparationCross-linkMicrosphere
The invention provides a method for rapidly preparing monodisperse polyvinyl alcohol microspheres at normal temperature. The method is carried out by adopting a micro-channel reactor, wherein the micro-channel reactor comprises a liquid drop generation region, a liquid drop internal fluid convection mixing region and a liquid drop pre-crosslinking curing region; and channels of the liquid drop internal fluid convection mixing region comprise a linear channel and a non-linear channel. The method comprises the following steps: pumping a polyvinyl alcohol aqueous solution, a mixed aqueous solution of a cross-linking agent and a catalyst, and an oil phase into the micro-channel reactor to form water-in-oil droplets; rapidly and fully mixing the three components in liquid drops in the liquid drop internal fluid convection mixing region, and then performing a pre-crosslinking reaction in the liquid drop pre-crosslinking curing region to form gel microspheres with linear hemiacetal; carryingout deep crosslinking on the gel microspheres in a collection bottle to obtain an acetal product and further to form polyvinyl alcohol microspheres with a three-dimensional network structure; and conducting washing and separating on the polyvinyl alcohol microspheres to obtain the monodisperse polyvinyl alcohol microspheres. The microsphere has the advantages of controllable particle size, uniformparticle size, good sphericity and the like.
Owner:ENERGY RES INST OF SHANDONG ACAD OF SCI
Process for the preparation of a crosslinker composition
ActiveUS8460758B2Increase resistance and stabilityHigh viscosityOther chemical processesPretreated surfacesHemiacetalNitrogen
This invention is directed to a process for the preparation of a crosslinker composition, comprising the steps of providing a mixture of an aliphatic alcohol A having at least one hydroxyl group and from 1 to 10 carbon atoms, with at least one multifunctional aldehyde C having at least two aldehyde groups —CHO to form a mixture AC, heating the mixture AC to convert at least a part of the multifunctional aldehyde C to its hemiacetal or to its acetal to form a mixture (AC)′, adding to the mixture (AC)′ least one cyclic urea U or the educts to produce the said cyclic urea U in situ, which cyclic urea U has at least one unsubstituted >NH group, and reacting the mixture thus obtained to form a chemical bond between the nitrogen atom of the at least one unsubstituted >NH group of the at least one cyclic urea U5 and the carbon atom of the least one aldehyde group —CHO of the multifunctional aldehyde C, and coating compositions comprising the said crosslinker composition.
Owner:ALLNEX NETHERLANDS BV
Process of refining c6-16 aliphatic diols
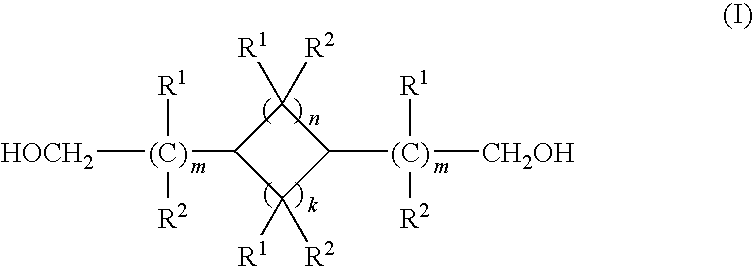
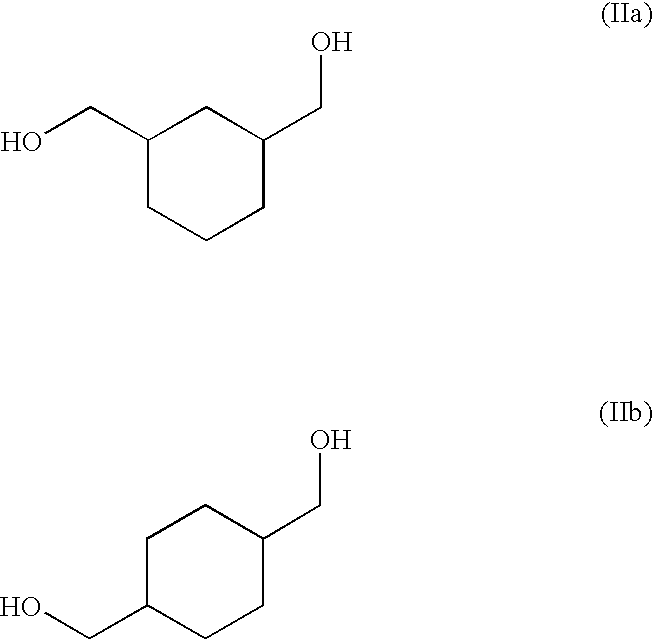
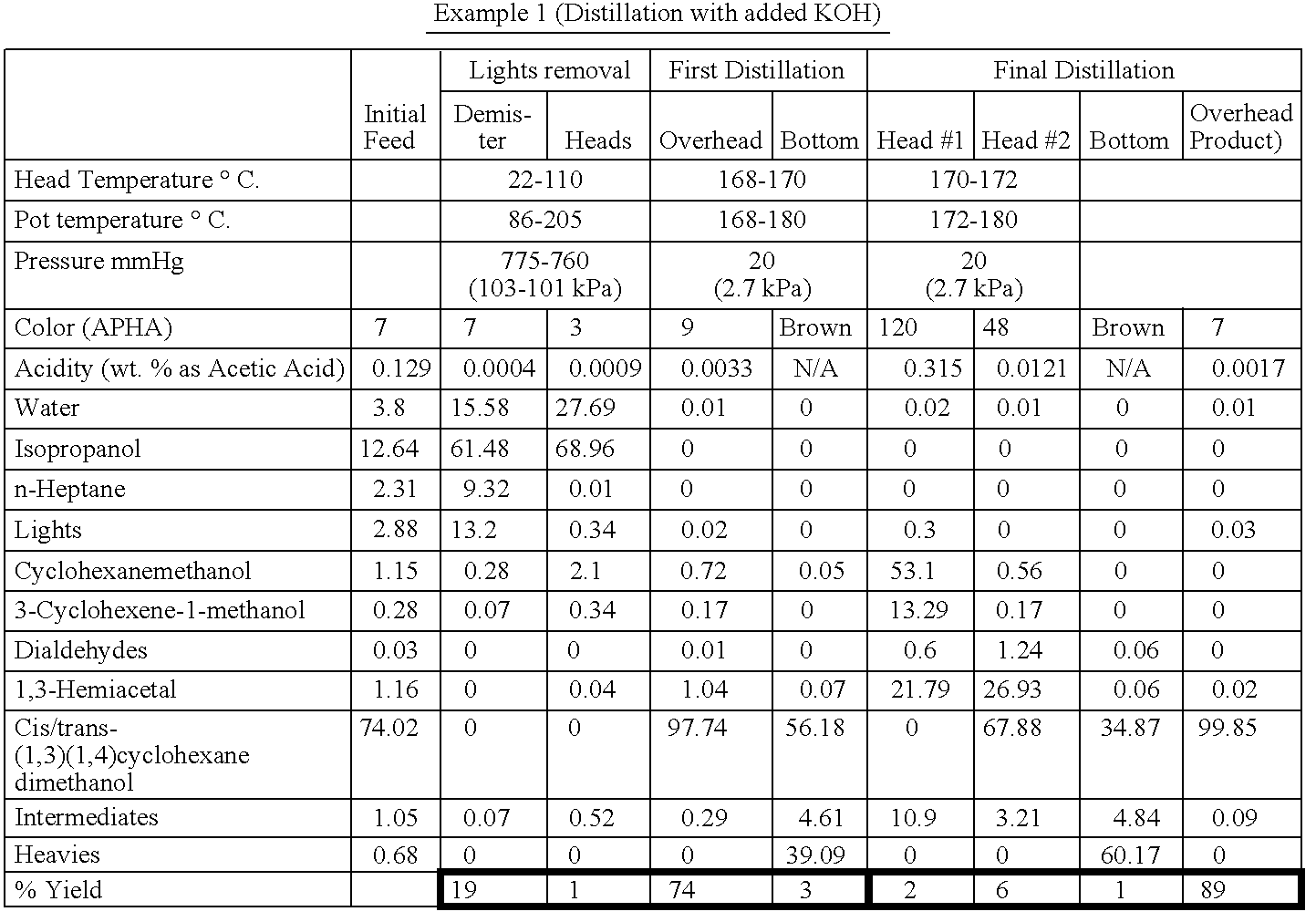
ActiveUS20100267997A1Reduce corrosionAccelerated destructionOrganic compound preparationOrganic chemistry methodsCyclohexanedimethanolHemiacetal
A process of refining a crude C6-C16 aliphatic diol, preferably, a C6-C16 monocyclic aliphatic diol, more preferably, an isomeric mixture of cis / trans-(1,3)(1,4)-cyclohexanedimethanol, containing in addition to the diol one or more impurities selected from phenols, and aliphatic mono-ols, esters, carboxylic acids, and hemiacetals, and mixtures thereof. The refining process involves distilling the crude C6-C16 aliphatic diol in the presence of an alkali or alkaline earth metal compound, preferably, an excess thereof relative to acid equivalents present in the diol.
Owner:DOW GLOBAL TECH LLC
Process for preparing cationic polyvinyl acetals
Cationic polyvinyl acetals are prepared by copolymerizing one or more cationic N-alkyldiallylammonium salt monomers with one or more vinyl esters of branched or unbranched carboxylic acids having 1 to 15 carbon atoms, hydrolyzing the resulting copolymers to copolymers containing ≧50 mol % vinyl alcohol units, and acetalizating the vinyl alcohol units with one or more aliphatic aldehydes having 1 to 15 carbon atoms, their acetals, and / or hemiacetals, wherein the copolymerization takes place in a mixture of water and monovalent aliphatic alcohol having a water content of 2% to 35% by weight.
Owner:KURARAY EURO GMBH
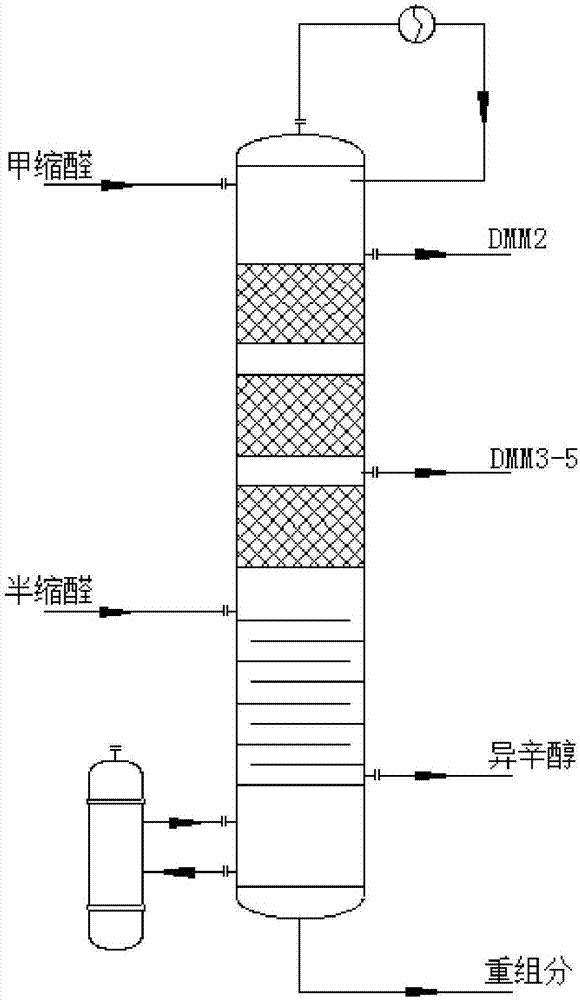
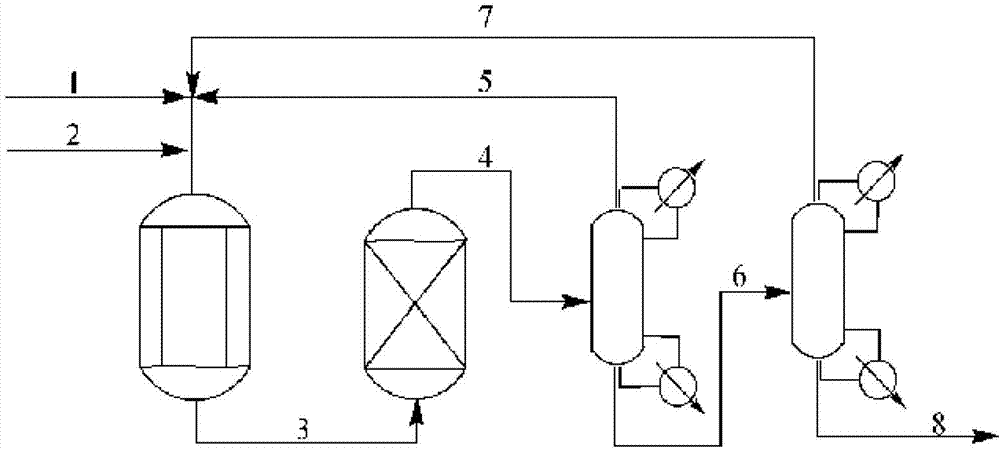
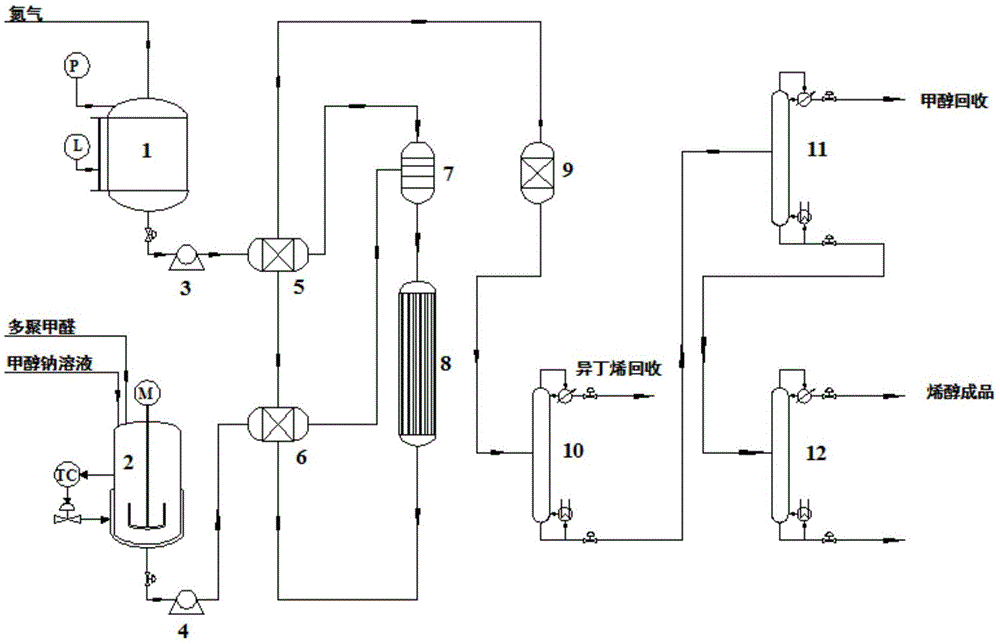
Reaction tower for production of DMMn (polyoxymethylene dimethyl ethers) and production technology
PendingCN107285999AReduce concentration stepsReduce water contentOrganic chemistryOrganic compound preparationHemiacetalPolymer science
The invention discloses a reaction tower for production of DMMn (polyoxymethylene dimethyl ethers) and a production technology. The reaction tower comprises a tower body, wherein the tower body sequentially comprises a re-boiling section, a desorption section, a reaction rectifying section and a tower top section inside from bottom to top; a hemiacetal inlet is arranged at the desorption section of the tower body; a methylal inlet is arranged at the top of the tower body; multiple stages of rectifying sections are arranged at the reaction rectifying section, and solid-acid catalyzed resin is loaded at the reaction rectifying section; a DMM3-5 extraction port and a DMM2 extraction port are sequentially arranged at the reaction rectifying section from bottom to top; an isooctanol outlet is arranged at the bottom of the desorption section; the outside of the re-boiling section at the bottom of the tower body is further connected with a re-boiler, and a heavy component outlet is arranged at the bottom of the re-boiling section. Steps of the synthetic process are reduced, and the energy consumption of the process is reduced.
Owner:青岛卓汇创能新能源科技有限公司
Method for production of sugar oxazoline derivative
Disclosed is a process for producing an oxazoline derivative from a non-protected sugar in a simple manner. Also disclosed is a process for producing a glycoside by utilizing the product of the aforementioned process. A sugar oxazoline derivative is synthesized in one step in an aqueous solution from a sugar having a free hemiacetalic hydroxy group and an amido group by using a haloformamidinium derivative as a dehydrating agent. A glycoside compound is produced by using the oxazoline derivative as a sugar donor and also using a sugar dehydrogenase. The method can be applied to the production of a compound having a long sugar chain, and is therefore useful for the production of a physiologically active oligosaccharide, a carrier for a drug delivery system, a surfactant, a carbohydrate-based drug, a glycopeptide, a glycoprotein, a carbohydrate polymer or the like.
Owner:SEIKAGAKU KOGYO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

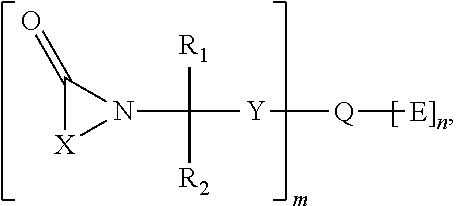
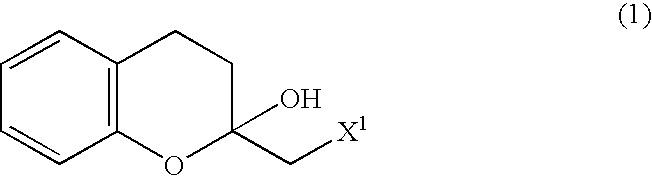
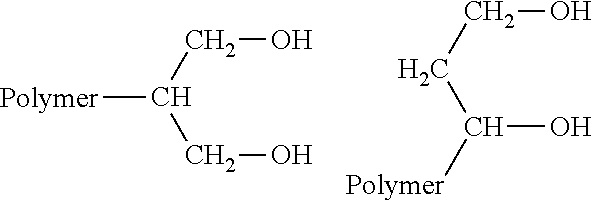
![Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof](https://images-eureka.patsnap.com/patent_img/54540b9c-b29c-4f1b-985d-5c054757f0d1/BDA00002452959900011.PNG)
![Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof](https://images-eureka.patsnap.com/patent_img/54540b9c-b29c-4f1b-985d-5c054757f0d1/BDA00002452959900021.PNG)
![Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof Chiral 2,3-dihydropyrrole[1,2-a] indole derivatives with biological activity and asymmetric synthesizing method thereof](https://images-eureka.patsnap.com/patent_img/54540b9c-b29c-4f1b-985d-5c054757f0d1/BDA00002452959900031.PNG)