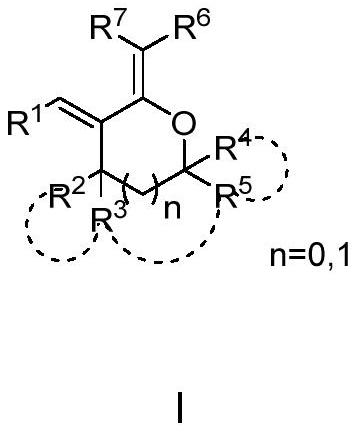
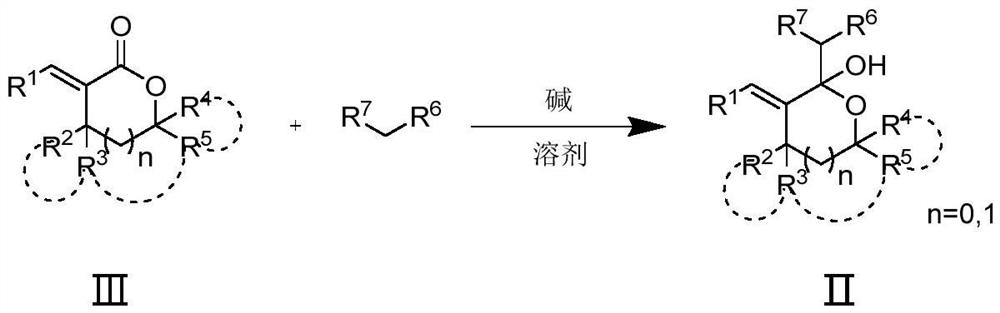
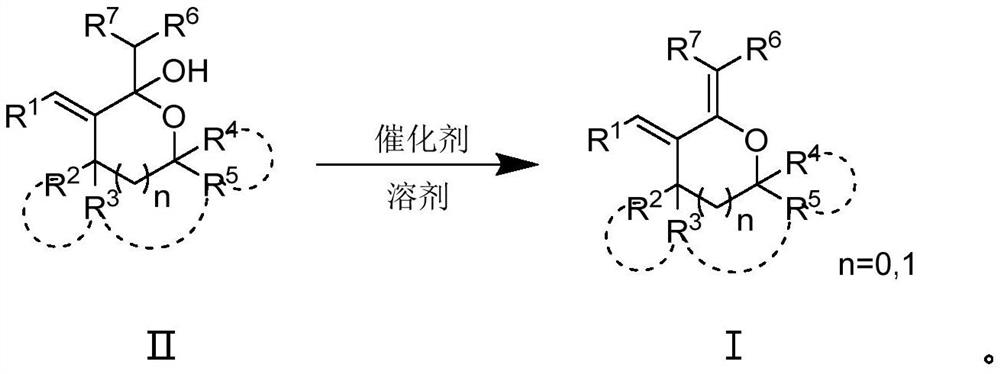
Poly-substituted tetrahydrofuran and tetrahydropyrane diene compound and preparation method thereof
A technology of tetrahydropyrandiene and tetrahydrofuran, which is applied in the directions of organic chemistry, drug combination, antipyretic and the like, and achieves the effects of simple synthesis method, good yield, and cheap and easy-to-obtain catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Target compound:
[0051]
[0052] Preparation:
[0053] Step 1: Dissolve 2.0g (E)-2-benzylidene-3,3-dimethylbutyrolactone in 10ml 1,4-dioxane to form an allyl lactone solution; weigh it into the reaction bottle 1.3g malononitrile, add 10ml 1,4-dioxane, cool down to 0-5°C; weigh 2.2g potassium tert-butoxide into the reaction flask; drop the alkenyl lactone solution into the reaction system, 0- React at 5°C for 10 h; after the reaction, add water to quench, extract with ethyl acetate, evaporate the solvent under reduced pressure to obtain 2.3 g of oily hemiacetal, yield 85%.
[0054] Step 2: Dissolve the hemiacetal obtained in the previous step in 10ml chloroform, add 2g Cu(OTf) 2 , and then heated up to 45° C. for 4 h; after the reaction, quenched with water, extracted with chloroform, and distilled off the solvent under reduced pressure to obtain 2 g of oil, with a yield of 95%.
[0055] Product characterization:
[0056] Colorless liquid. 1 H NMR (400MHz, CDCl...
Embodiment 2
[0058] Target compound:
[0059]
[0060] Preparation:
[0061] Step 1: Dissolve 2.0g (E)-2-benzylidene-3,3-dimethylbutyrolactone in 10ml tetrahydrofuran to form an allyl lactone solution; weigh 0.5g acetonitrile into the reaction bottle, add 10ml tetrahydrofuran ; Weigh n-butyllithium into the reaction flask (the molar ratio of n-butyllithium to (E)-2-benzylidene-3,3-dimethylbutyrolactone is 0.4:1); The ester solution was dripped into the reaction system, and reacted at 10°C for 20 hours; after the reaction was completed, it was quenched with water, extracted with ethyl acetate, and separated by column chromatography to obtain hemiacetal as an oily product with a yield of 83%.
[0062] Step 2: Dissolve the hemiacetal obtained in the previous step in 10ml of dichloromethane, add Zn(OAc) 2 2H 2 O(Zn(OAc) 2 2H 2 The molar ratio of O to hemiacetal is 0.1:1), and then the temperature was raised to 20°C for 48 hours; after the reaction, quenched with water, extracted with e...
Embodiment 3
[0066] Target compound:
[0067]
[0068] Preparation:
[0069] Step 1: Dissolve 2.2g (E)-2-benzylidene-3,3-dimethylvalerolactone in 12ml of ether to form an allyl lactone solution; weigh 2.6g of dimethyl malonate into the reaction bottle ester, add 10ml ether; add sodium hydride into the reaction flask (the molar ratio of sodium hydride to (E)-2-benzylidene-3,3-dimethylvalerolactone is 0.8:1); The lactone solution was dripped into the reaction system, and reacted at 20°C for 12 hours; after the reaction was completed, it was quenched with water, extracted with ethyl acetate, and separated by column chromatography to obtain hemiacetal as an oil with a yield of 84%.
[0070] Step 2: Dissolve the hemiacetal obtained in the previous step in 12ml of n-hexane, add CuSO 4 ·5H 2 O(CuSO 4 ·5H 2 The molar ratio of O to hemiacetal is 0.2:1), and then the temperature was raised to 30°C for 24 hours; after the reaction, quenched with water, extracted with ethyl acetate, and the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com