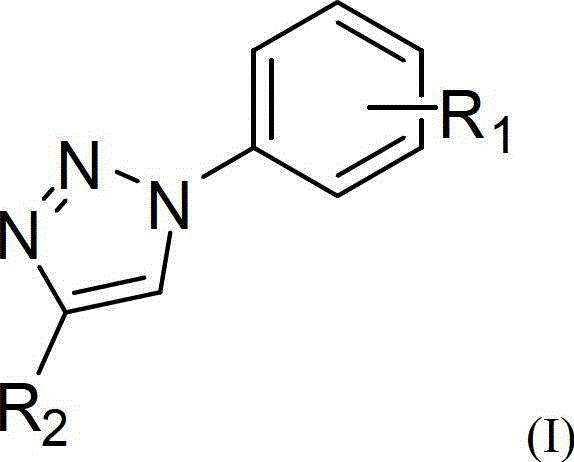
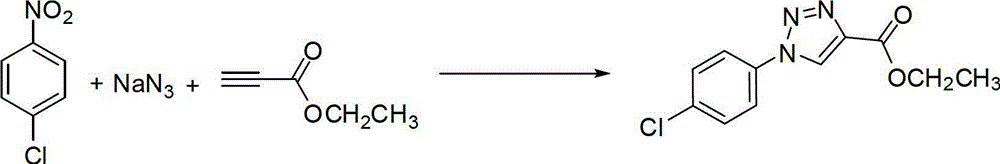
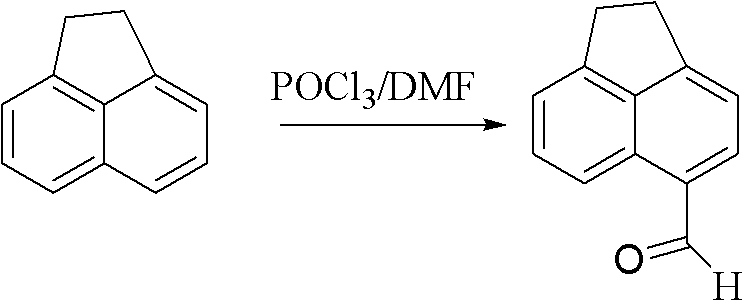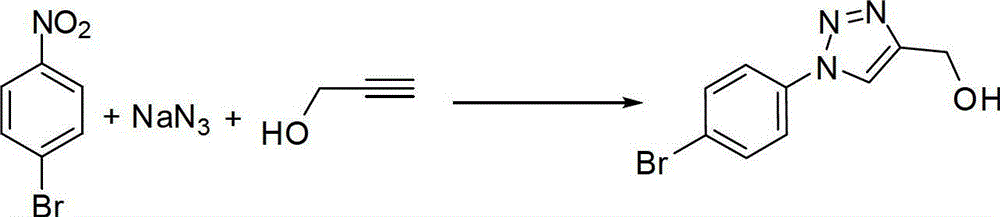Patents
Literature
7712 results about "Organic synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.
Pegylated nanoparticles
ActiveUS8628801B2Good bioadhesionStrong specificityPowder deliveryCosmetic preparationsZeta potentialOrganic synthesis
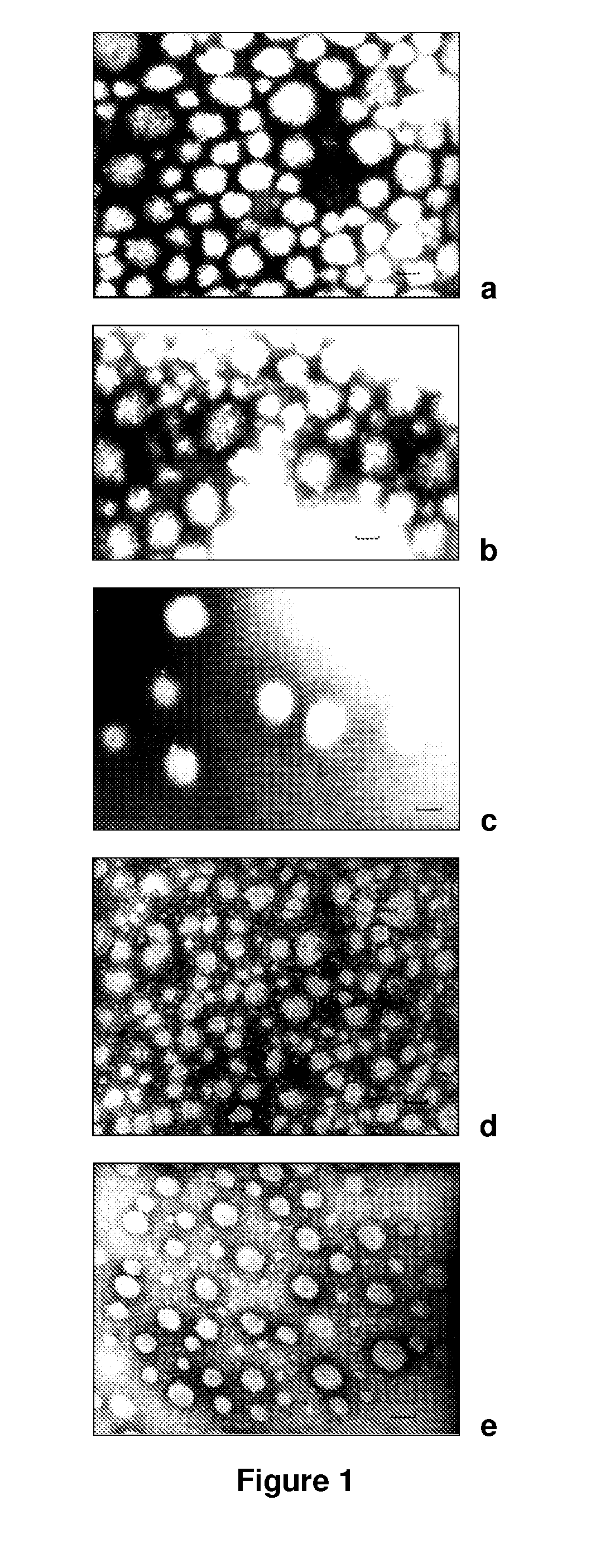
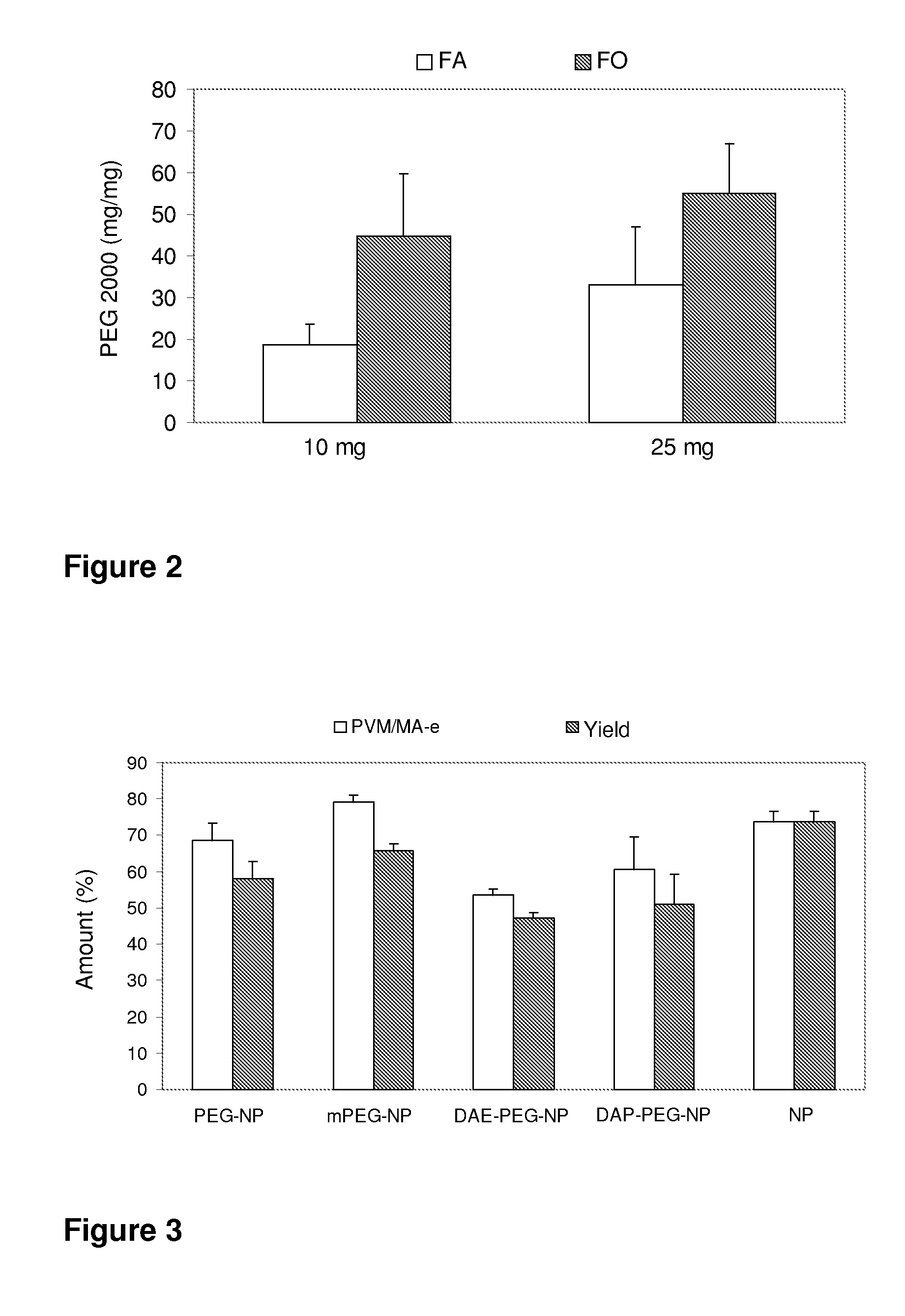
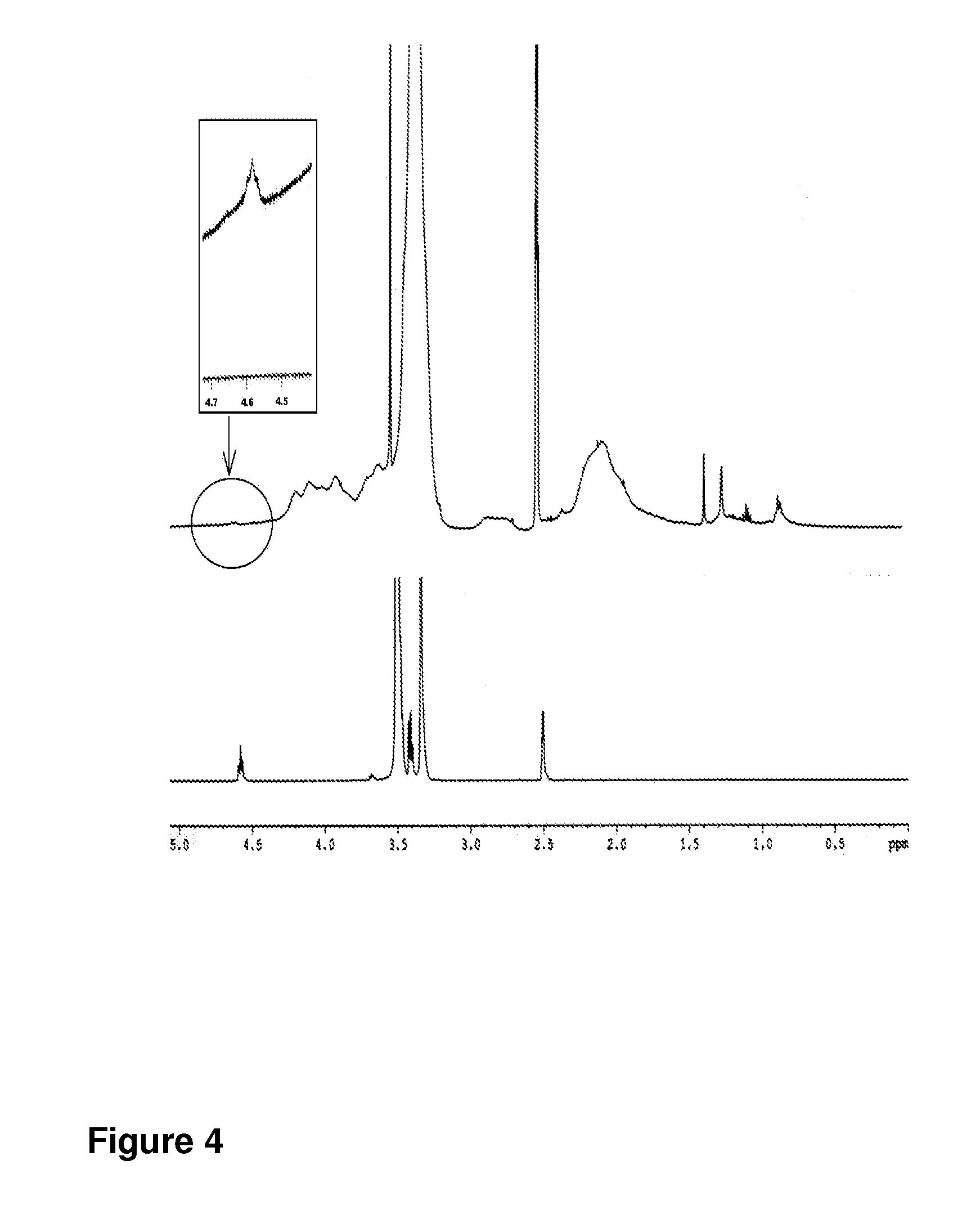
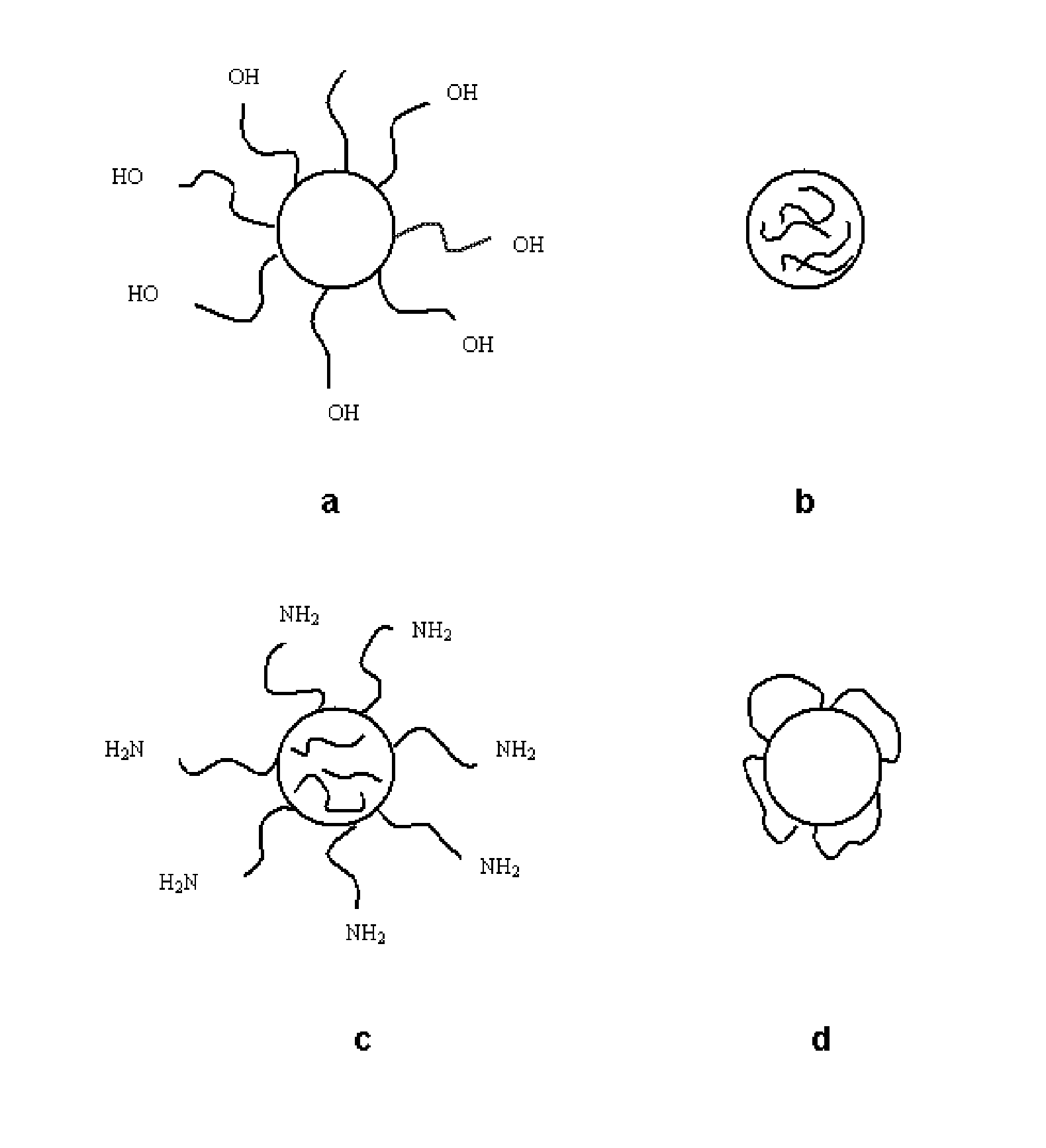
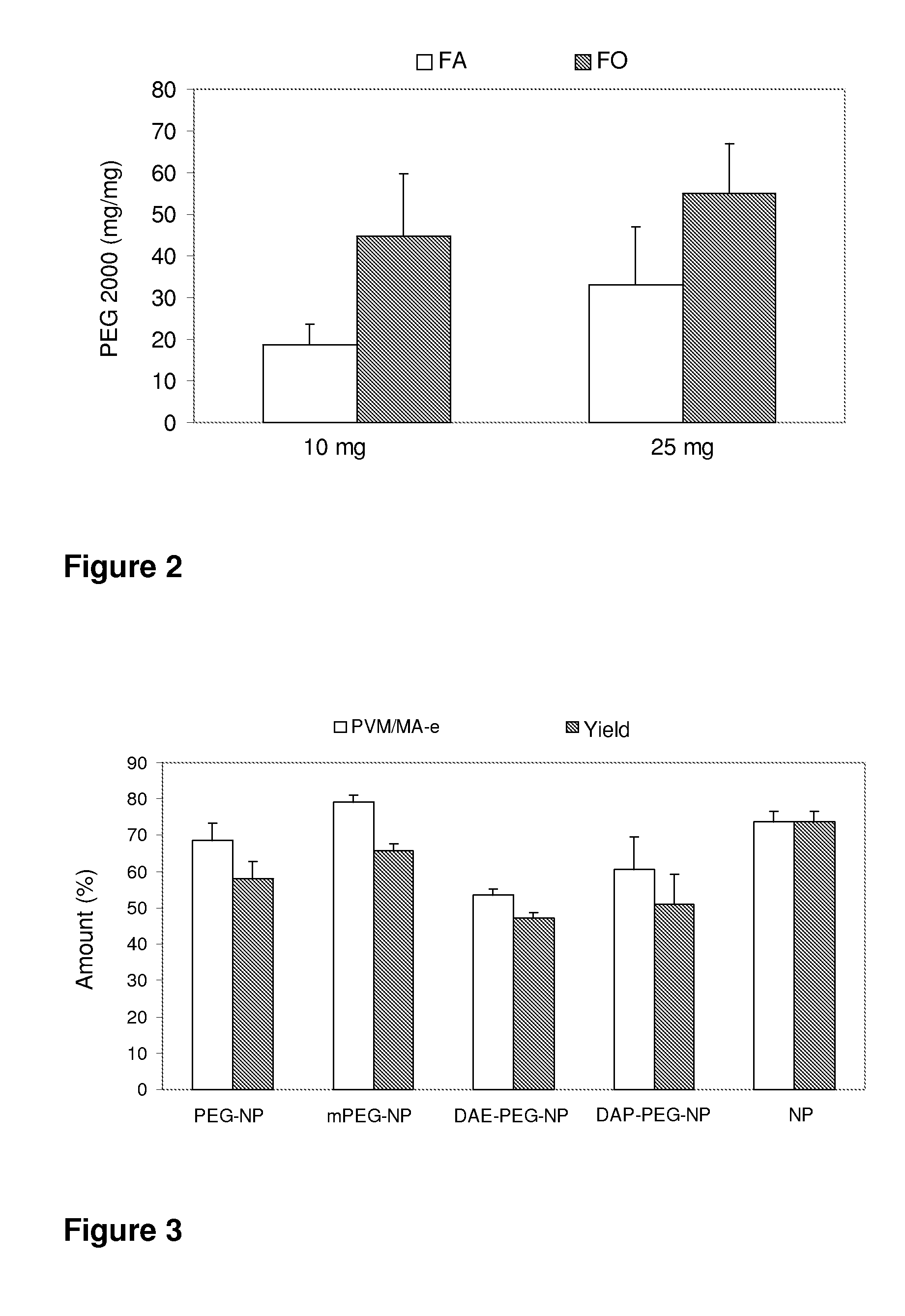
The present invention relates to nanoparticles comprising a biodegradable polymer, preferably the vinyl methyl ether and maleic anhydride (PVM / MA) copolymer, and a polyethylene glycol or derivatives thereof. These nanoparticles are easy to produce and provide excellent bioadhesion, size and zeta potential characteristics making them suitable for the administration of active molecules. The selection of the type of polyethylene glycol used in their production allows suitably modulating the characteristics of these nanoparticles, which can be advantageously used according to the type of drug to be carried and / or the method of administration of the pharmaceutical formulation. pegylation is carried out by simple incubation for a short time period of the two macromolecules in question, without needing to have to resort to the use of organic solvents with high toxicity or long and laborious organic synthesis processes. Furthermore, the pegylation process can be associated to the process of encapsulating the biologically active molecule.
Owner:INNOUP FARMA
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
Method for separation of liquid and solid phases for solid phase organic syntheses
InactiveUS6121054ASequential/parallel process reactionsPeptide/protein ingredientsOrganic synthesisMicrotiter plate
A simple, efficient apparatus and method for separation of solid and liquid phases useful in methods of high-throughput combinatorial organic synthesis of large libraries or megaarrays of organic compounds is disclosed. The apparatus and method are useful, whether as part of an automated, robotic or manual system for combinatorial organic synthesis. In a preferred embodiment, an apparatus and method of removal of liquid phase from solid phase compatible with microtiter plate type array(s) of reaction vessels is disclosed.
Owner:BUCYRUS INTERNATIONAL +1
Mixed-metal oxide particles by liquid feed flame spray pyrolysis of oxide precursors in oxygenated solvents
Liquid feed flame spray pyrolysis of solutions of a metal oxide precursor which is an alkoxide or C1-6 carboxylate and at least one second metal oxide precursor and / or second metal compound dissolved in oxygenated solvent by combustion with oxygen lead to the formation of sub-micron mixed-metal oxide powders not accessible by other processes or by the pyrolysis of metal chlorides or nitrates. The powders have numerous uses in advanced materials applications including particulate solid state lasers, advanced ceramic materials, and as catalysts in organic synthesis and automobile exhaust systems.
Owner:TAL MATERIALS +1
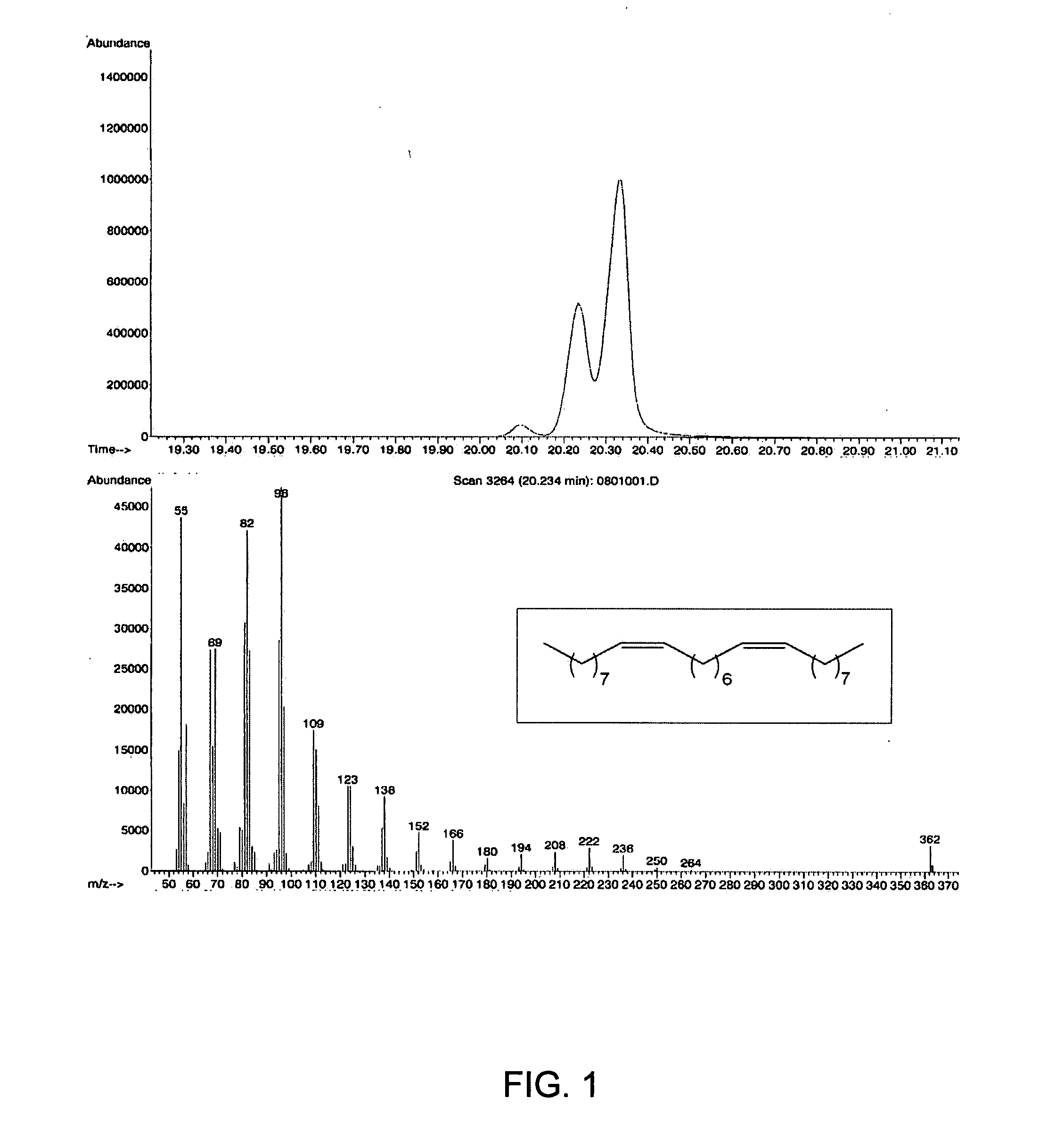
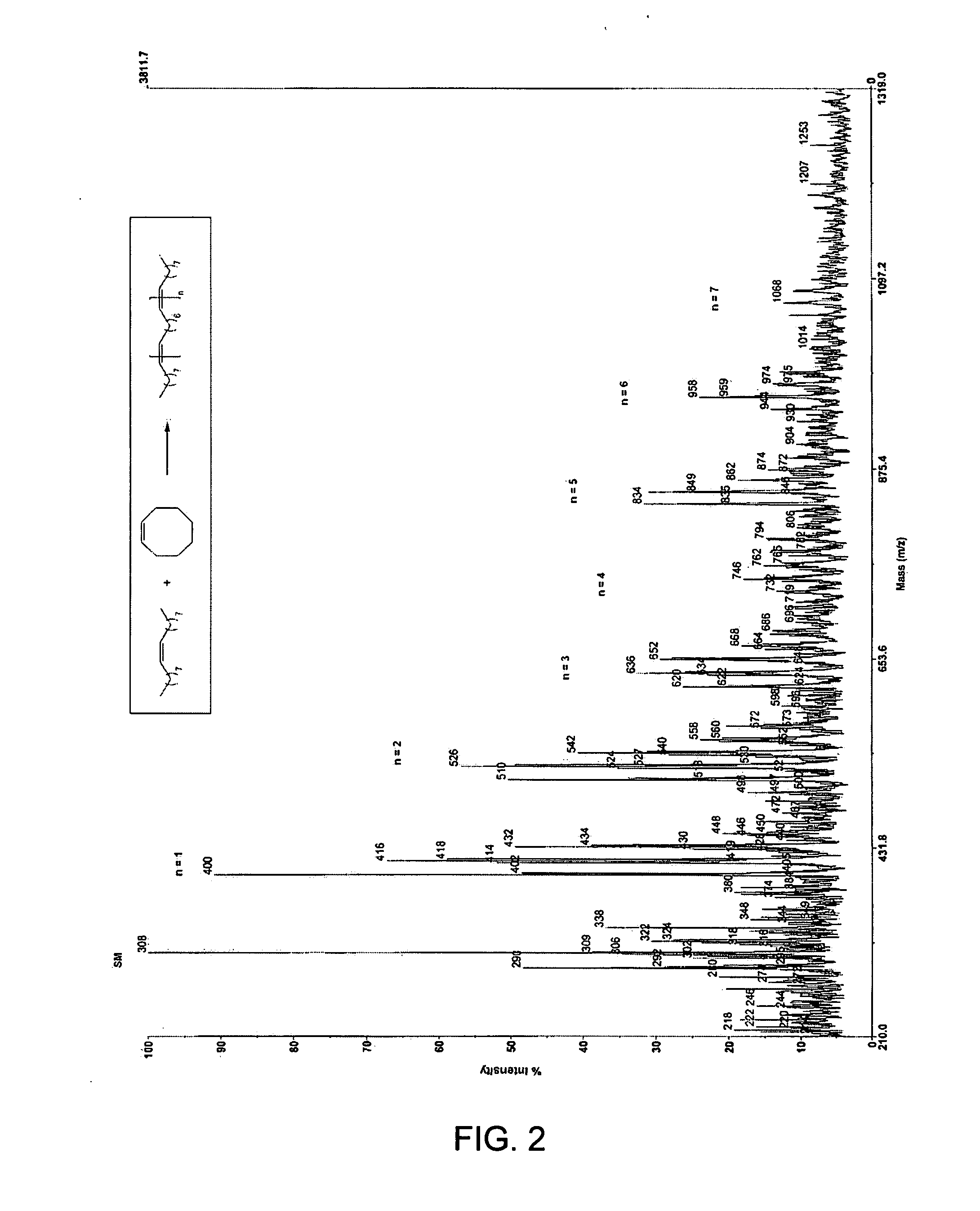
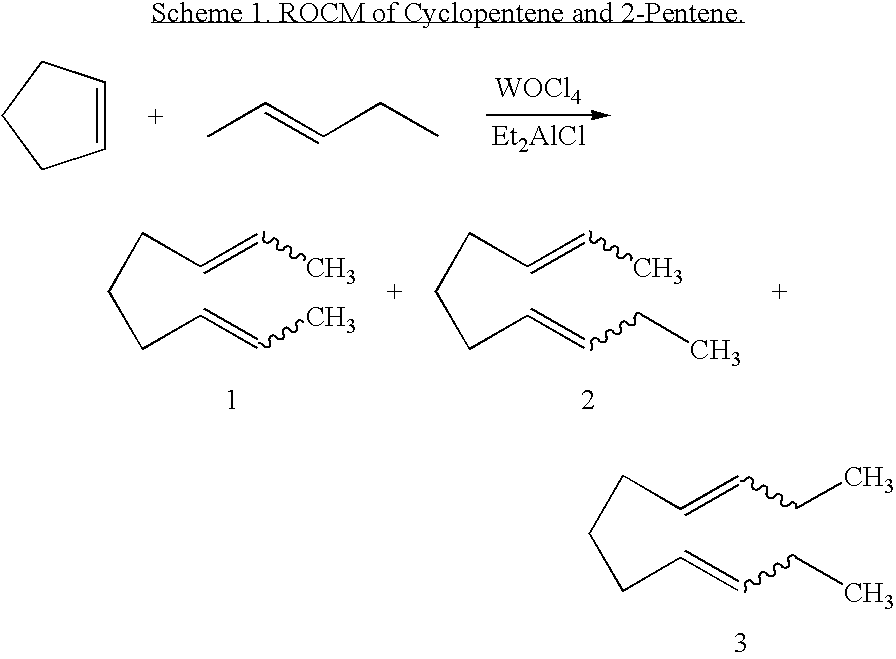
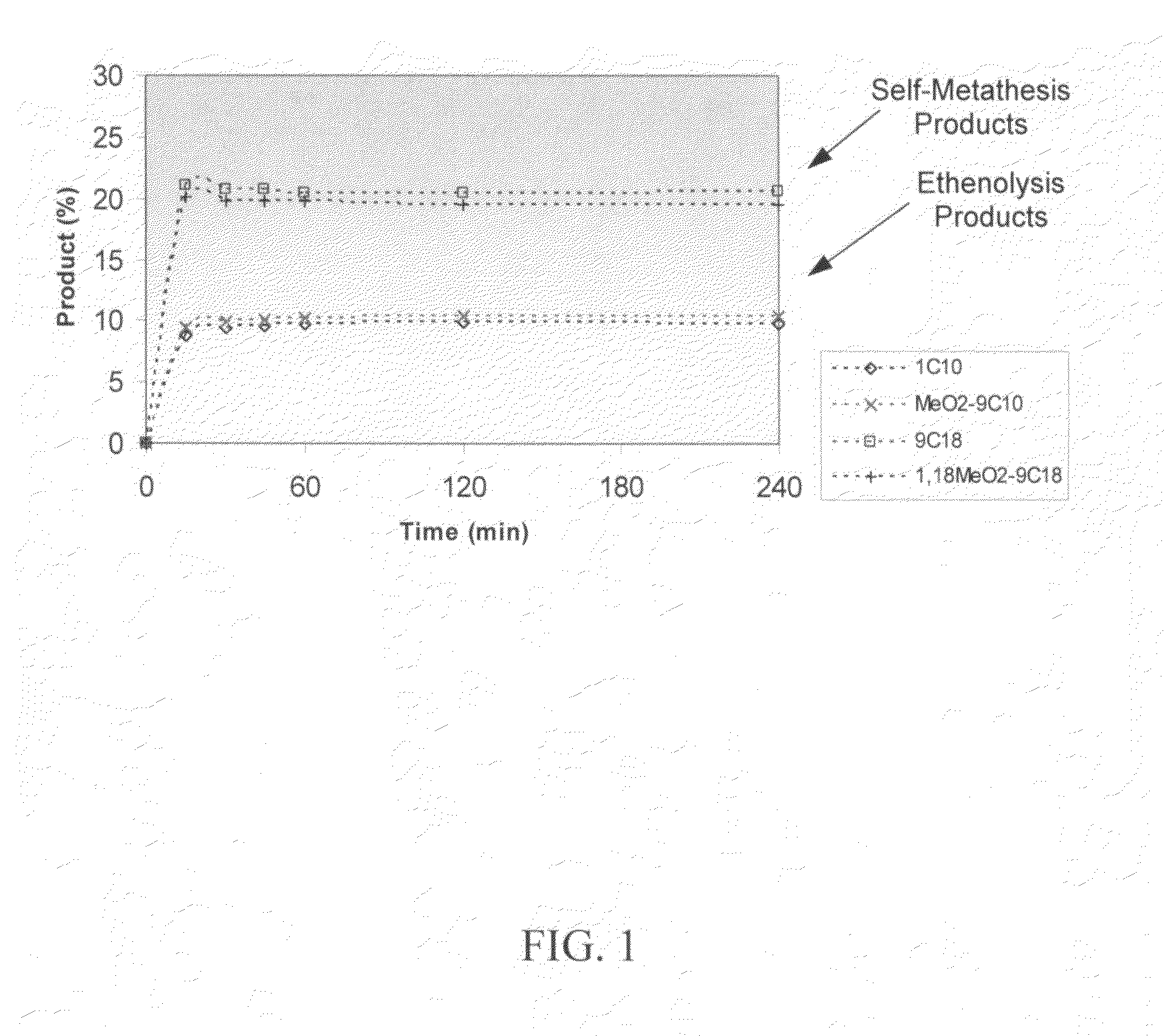
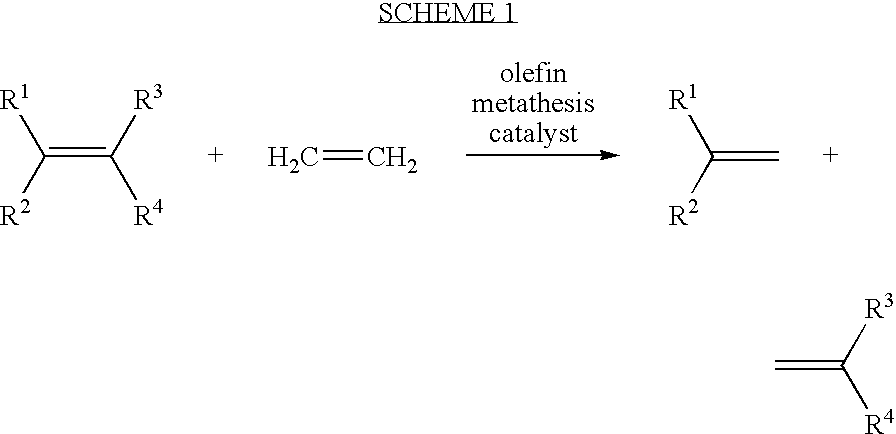
Ring opening cross-metathesis reaction of cyclic olefins with seed oils and the like
ActiveUS20080064891A1Fatty acid esterificationOrganic compound preparationOrganic synthesisRuthenium
This invention relates generally to olefin metathesis, and more particularly relates to the ring-opening, ring insertion cross-metathesis of cyclic olefins with internal olefins such as seed oils and the like. In one embodiment, a method is provided for carrying out a catalytic ring-opening cross-metathesis reaction, comprising contacting at least one olefinic substrate with at least one cyclic olefin as a cross metathesis partner, in the presence of a ruthenium alkylidene olefin metathesis catalyst under conditions effective to allow ring insertion cross metathesis whereby the cyclic olefin is simultaneously opened and inserted into the olefinic substrate. The invention has utility in the fields of catalysis, organic synthesis, and industrial chemistry.
Owner:WILMAR TRADING PTE LTD
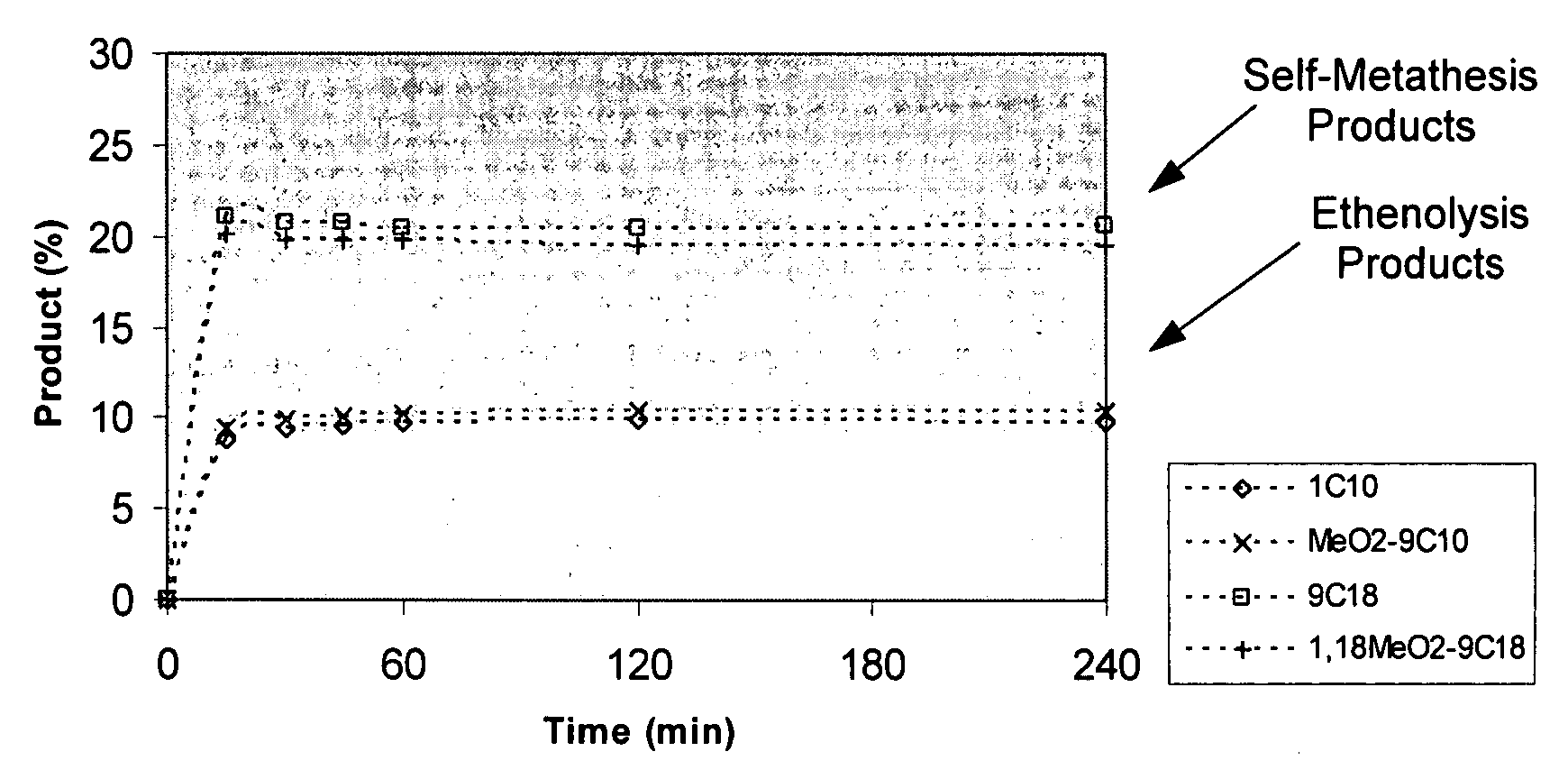
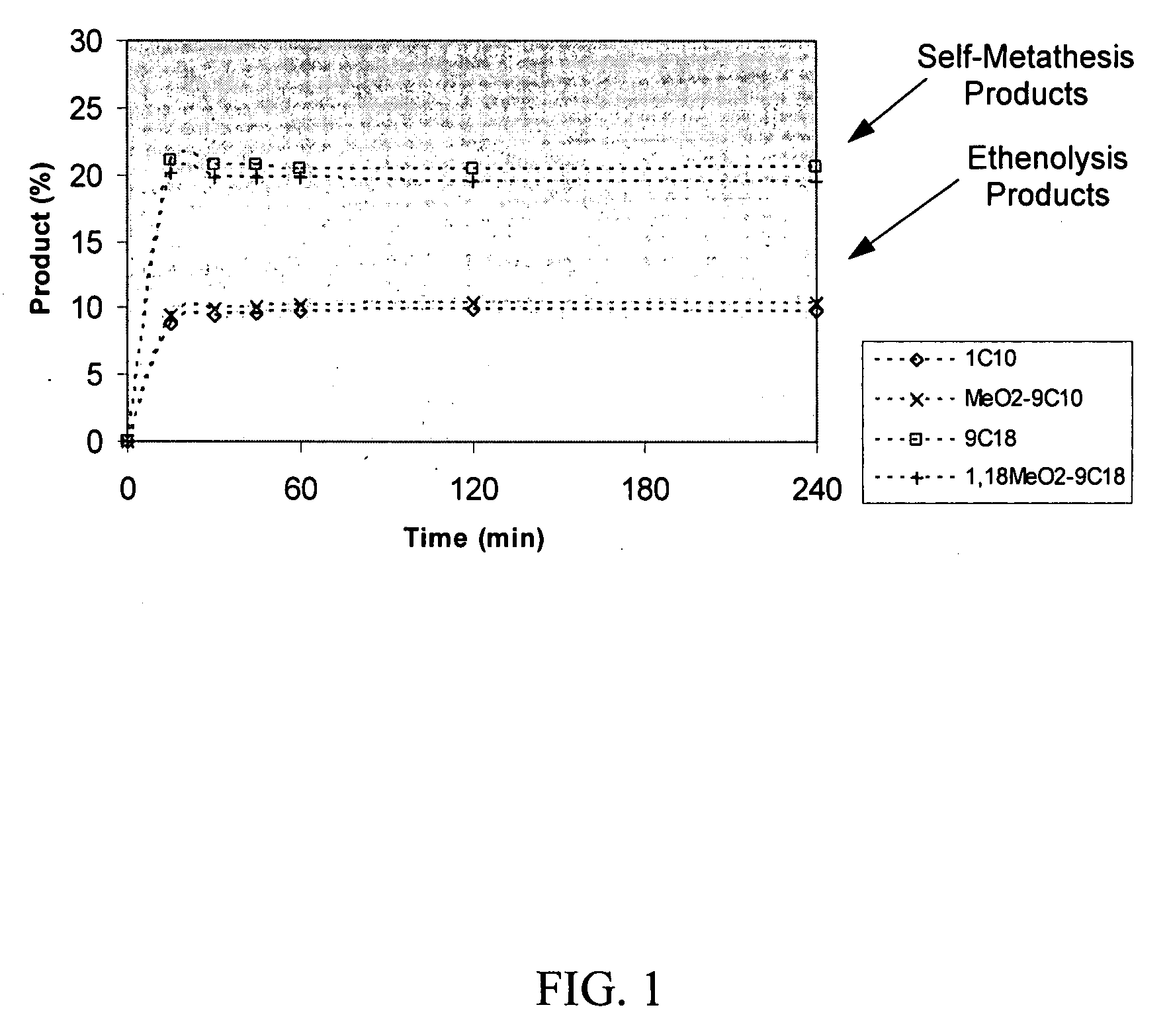
Synthesis of terminal alkenes from internal alkenes and ethylene via olefin metathesis
This invention relates generally to olefin metathesis, and more particularly relates to the synthesis of terminal alkenes from internal alkenes using a cross-metathesis reaction catalyzed by a selected olefin metathesis catalyst. In one embodiment of the invention, for example, a method is provided for synthesizing a terminal olefin, the method comprising contacting an olefinic substrate comprised of at least one internal olefin with ethylene, in the presence of a metathesis catalyst, wherein the catalyst is present in an amount that is less than about 1000 ppm relative to the olefinic substrate, and wherein the metathesis catalyst has the structure of formula (II) wherein the various substituents are as defined herein. The invention has utility, for example, in the fields of catalysis, organic synthesis, and industrial chemistry.
Owner:MATERIA
Slow release scale inhibitor composites and methods of using the same
ActiveUS20060124301A1Prevent and control formationCleaning apparatusFluid removalActivated carbonParticulates
A well treating composition of a composite containing a scale inhibitor adsorbed onto a water-insoluble adsorbent is useful in the control, formation and treatment of inorganic scales in a subterranean formation or wellbore. In addition, the well treating composition is useful in controlling the rate of release of scale inhibitor in the wellbore. The water-insoluble adsorbent may be activated carbon, silica particulate, precipitated silica, zeolite, diatomaceous earth, ground walnut shells, fuller's earth and organic synthetic high molecular weight water-insoluble adsorbents. The composite may be introduced into an oil or gas well with a carrier fluid.
Owner:BAKER HUGHES INC
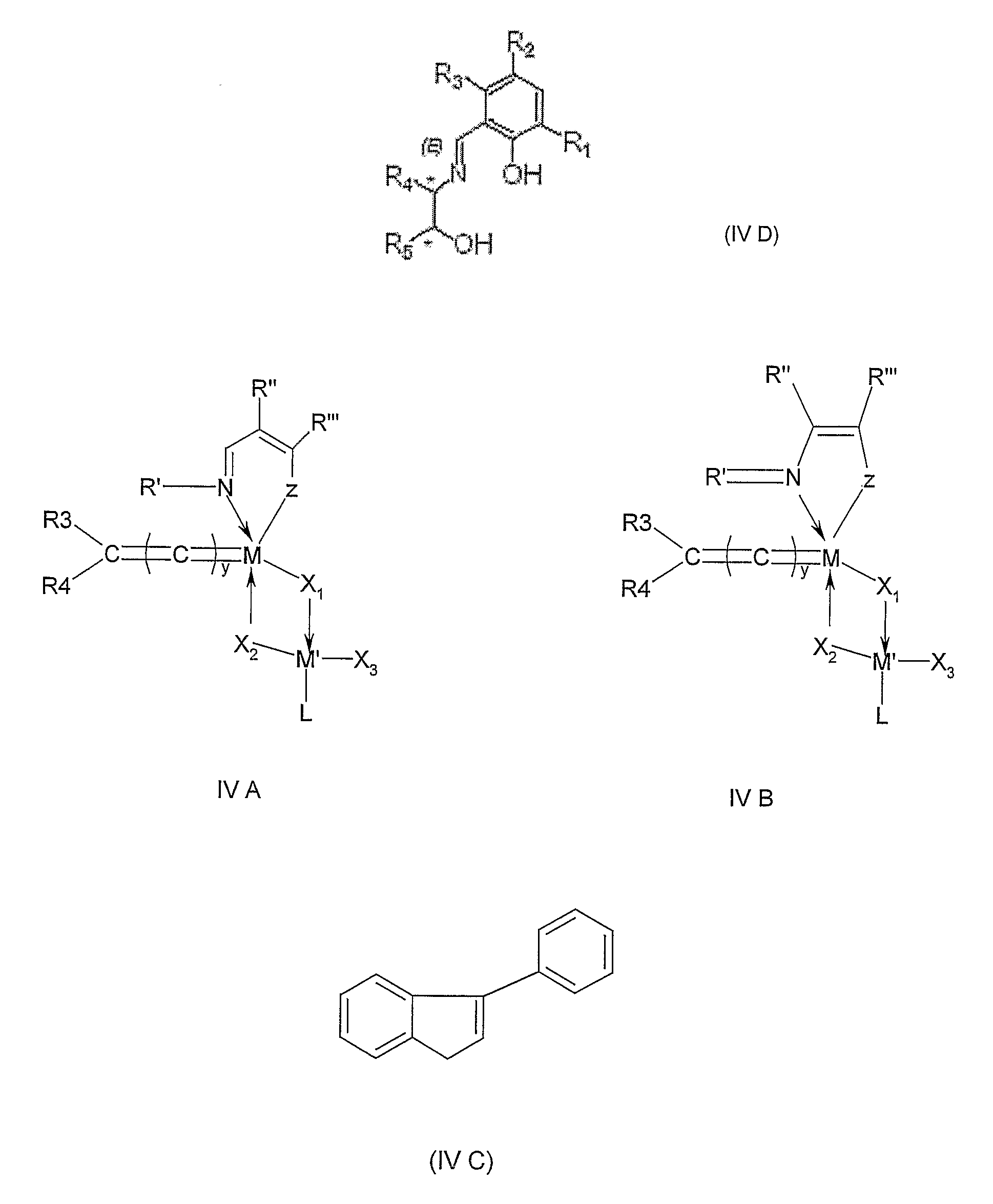
Metal complexes for use in olefin metathesis and atom group transfer reactions
InactiveUS20070185343A1Promote catalysisRuthenium organic compoundsCopper organic compoundsOrganic synthesisAlkene
Improved catalysts useful in a number of organic synthesis reactions such as olefin metathesis and atom or group transfer reactions are made by bringing into contact a multi-coordinated metal complex comprising a multidentate Schiff base ligand, and one or more other ligands, with an acid under conditions such that said acid is able to at least partly cleave a bond between the metal and the multidentate Schiff base ligand of said metal complex, optionally through intermediate protonation of said Schiff base ligand.
Owner:TELENE SAS
Method of inhibiting or controlling formation of inorganic scales
A well treating composition of a composite containing a scale inhibitor adsorbed onto a water-insoluble adsorbent is useful in the control, formation and treatment of inorganic scales in a subterranean formation or wellbore. In addition, the well treating composition is useful in controlling the rate of release of scale inhibitor in the wellbore. The water-insoluble adsorbent may be activated carbon, silica particulate, precipitated silica, zeolite, diatomaceous earth, ground walnut shells, fuller's earth and organic synthetic high molecular weight water-insoluble adsorbents. The composite may be introduced into an oil or gas well with a carrier fluid.
Owner:BAKER HUGHES INC
Evolving new molecular function
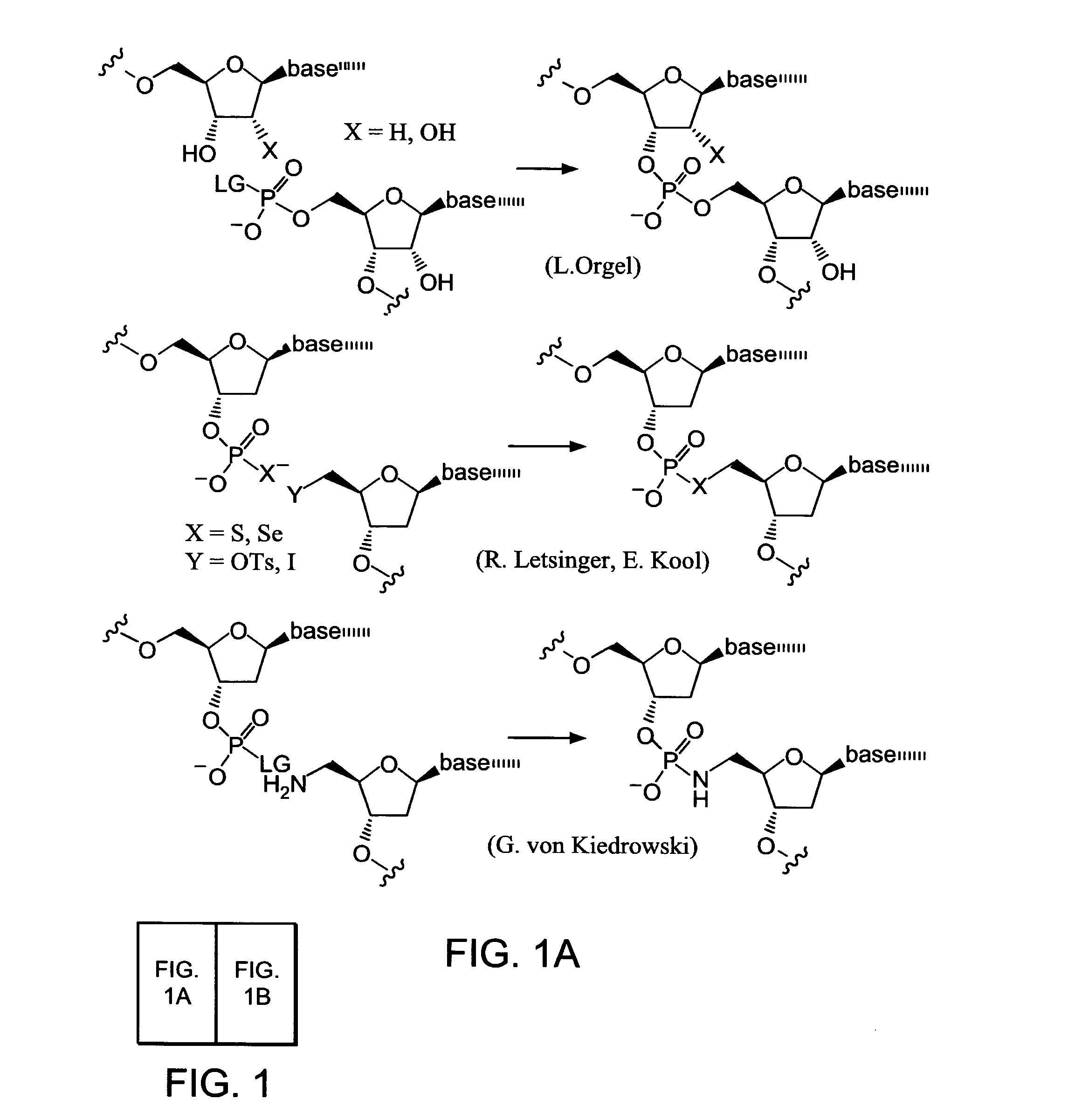
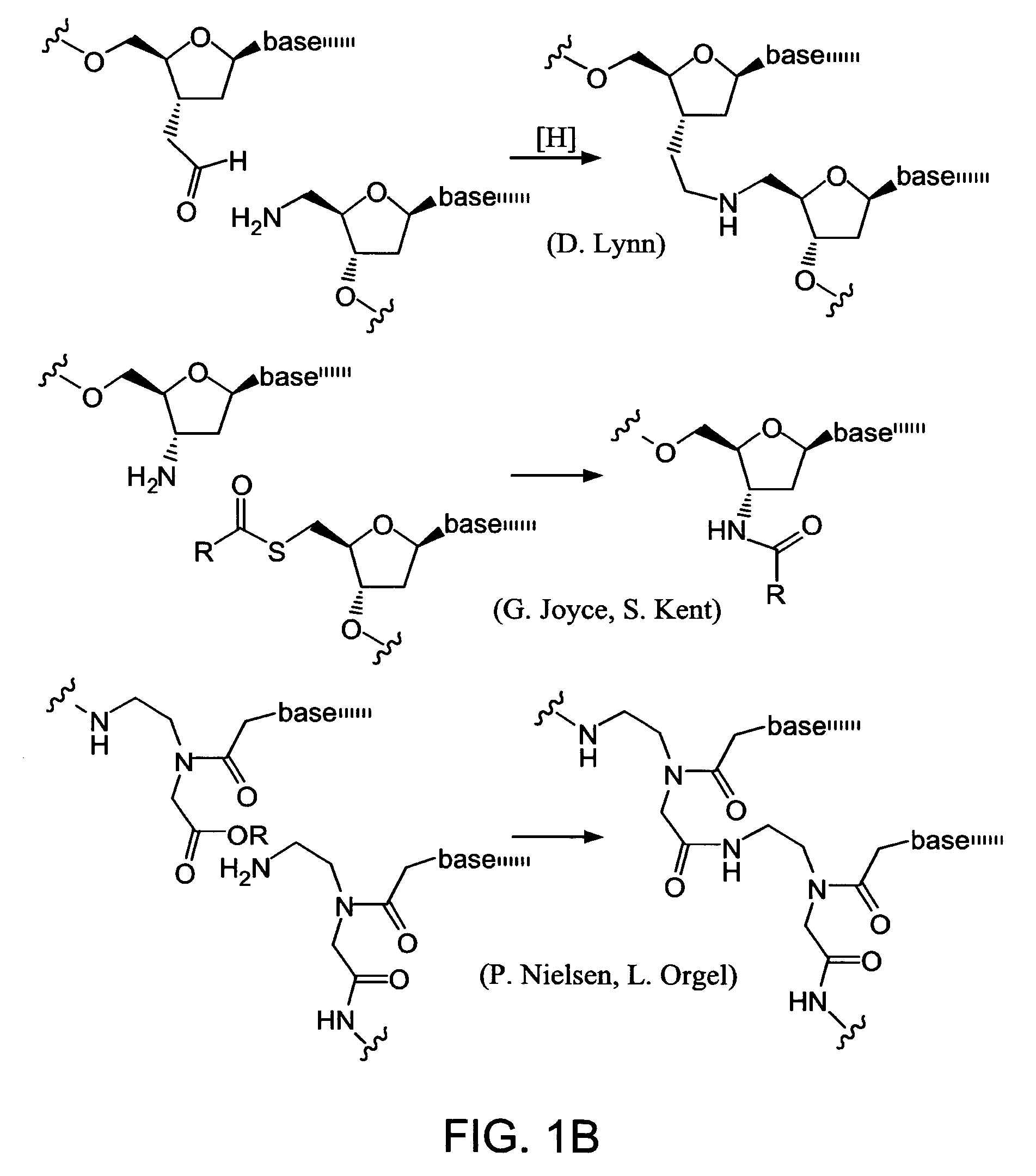
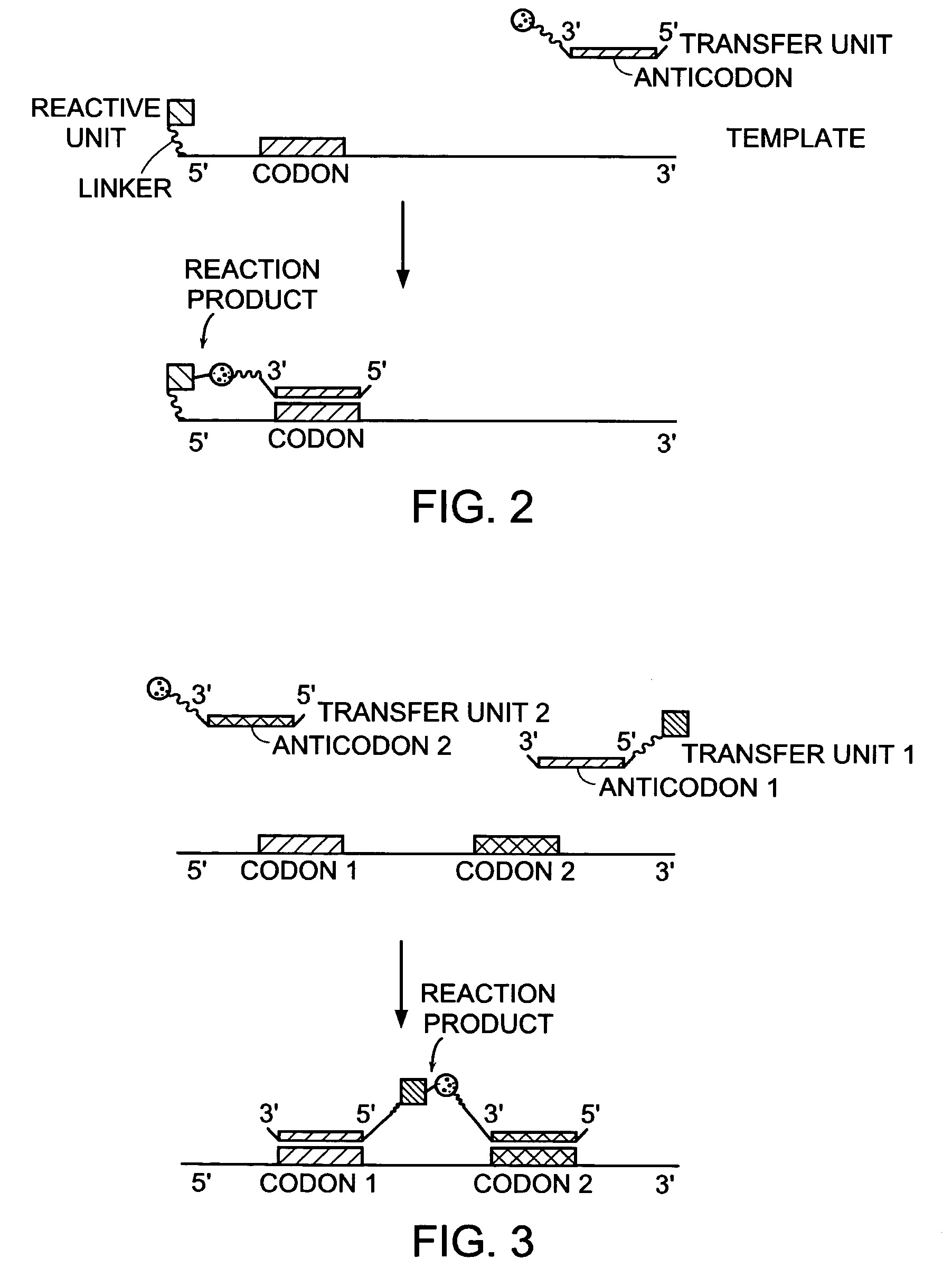
InactiveUS7491494B2High selectivityEnhance covalent bond formationNucleotide librariesMicrobiological testing/measurementBio moleculesChemical reaction
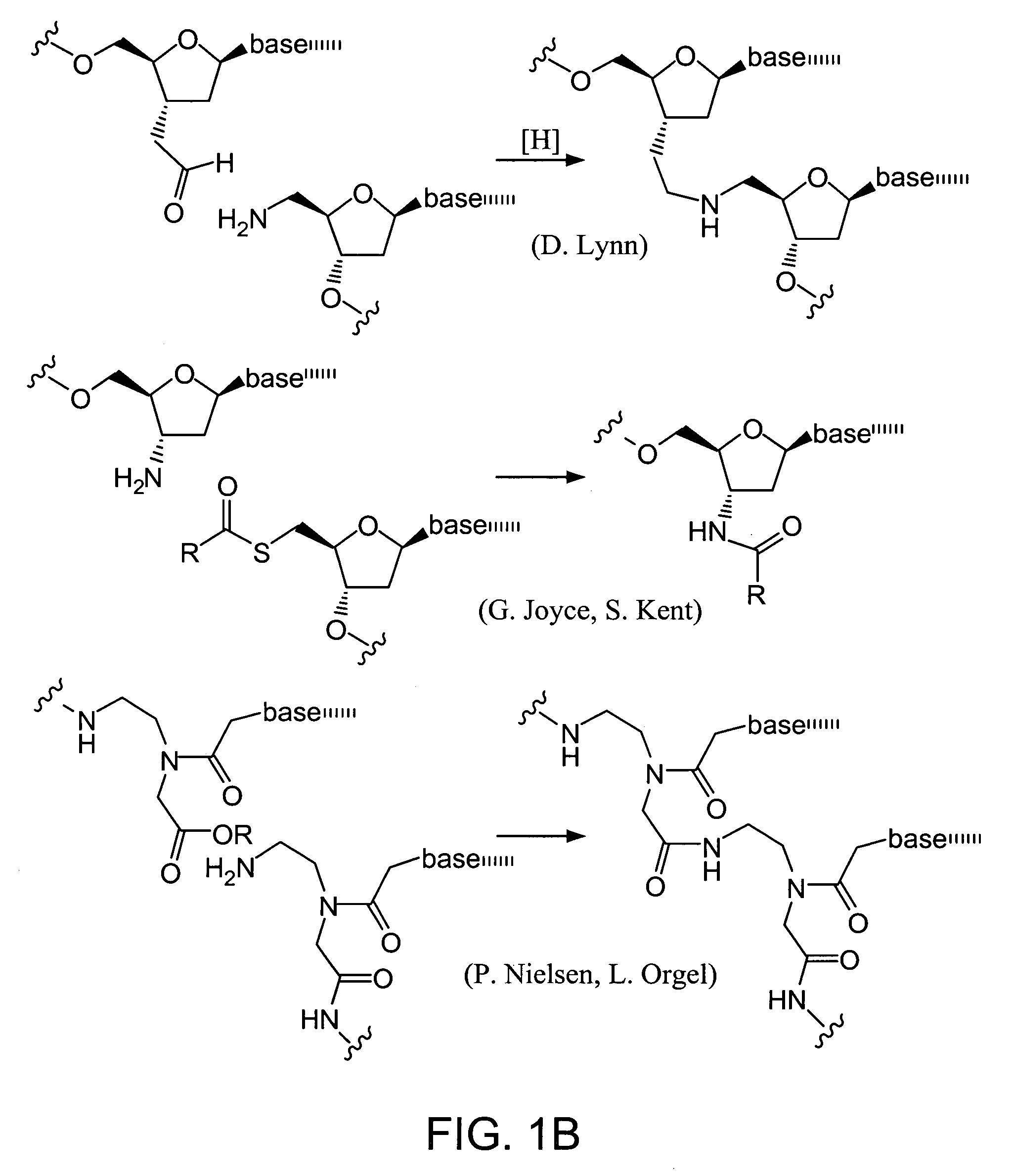
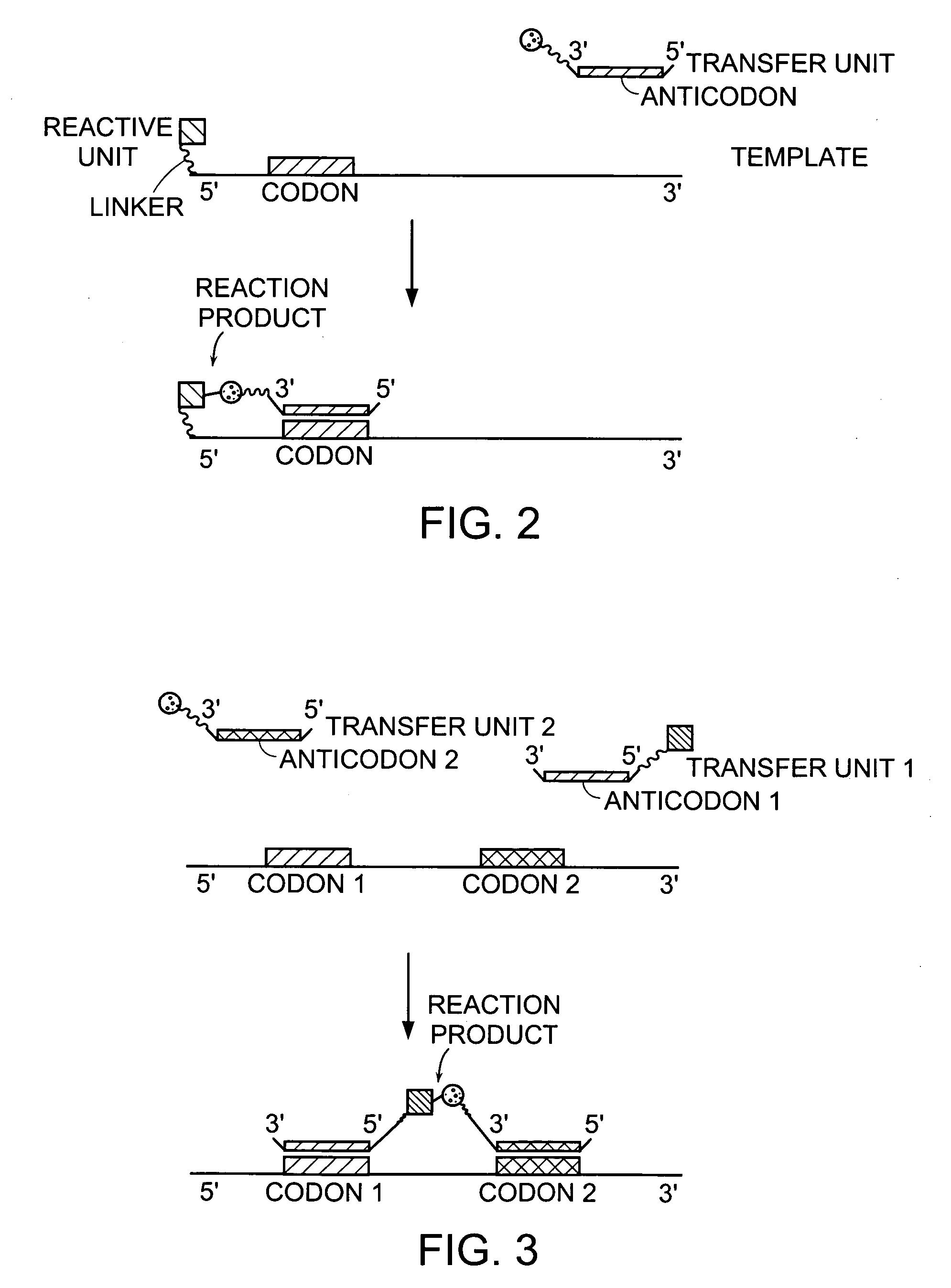
Nature evolves biological molecules such as proteins through iterated rounds of diversification, selection, and amplification. The power of Nature and the flexibility of organic synthesis are combined in nucleic acid-templated synthesis. The present invention provides a variety of template architectures for performing nucleic acid-templated synthesis, methods for increasing the selectivity of nucleic acid-templated reactions, methods for performing stereoselective nucleic acid-templated reactions, methods of selecting for reaction products resulting from nucleic acid-templated synthesis, and methods of identifying new chemical reactions based on nucleic acid-templated synthesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Heat-resistant synthetic fiber sheet
InactiveUS7026033B2Improve heat resistanceNon-fibrous pulp additionNatural cellulose pulp/paperPolymer scienceOrganic synthesis
A heat-resistant fiber paper-like sheet comprises 40 to 97% by mass of heat-resistant organic synthetic polymers staple fibers, 3 to 60% by mass of heat resistant organic synthetic polymer fibrid and or an organic resin binder, in a portion of the staple fibers, each staple fiber having two flat end faces having an inclining angle of 10 degrees or more from a plane crossing the fiber axis at a right angles, and is useful as a base material for laminate materials for electrical circuit boards.
Owner:TEIJIN LTD
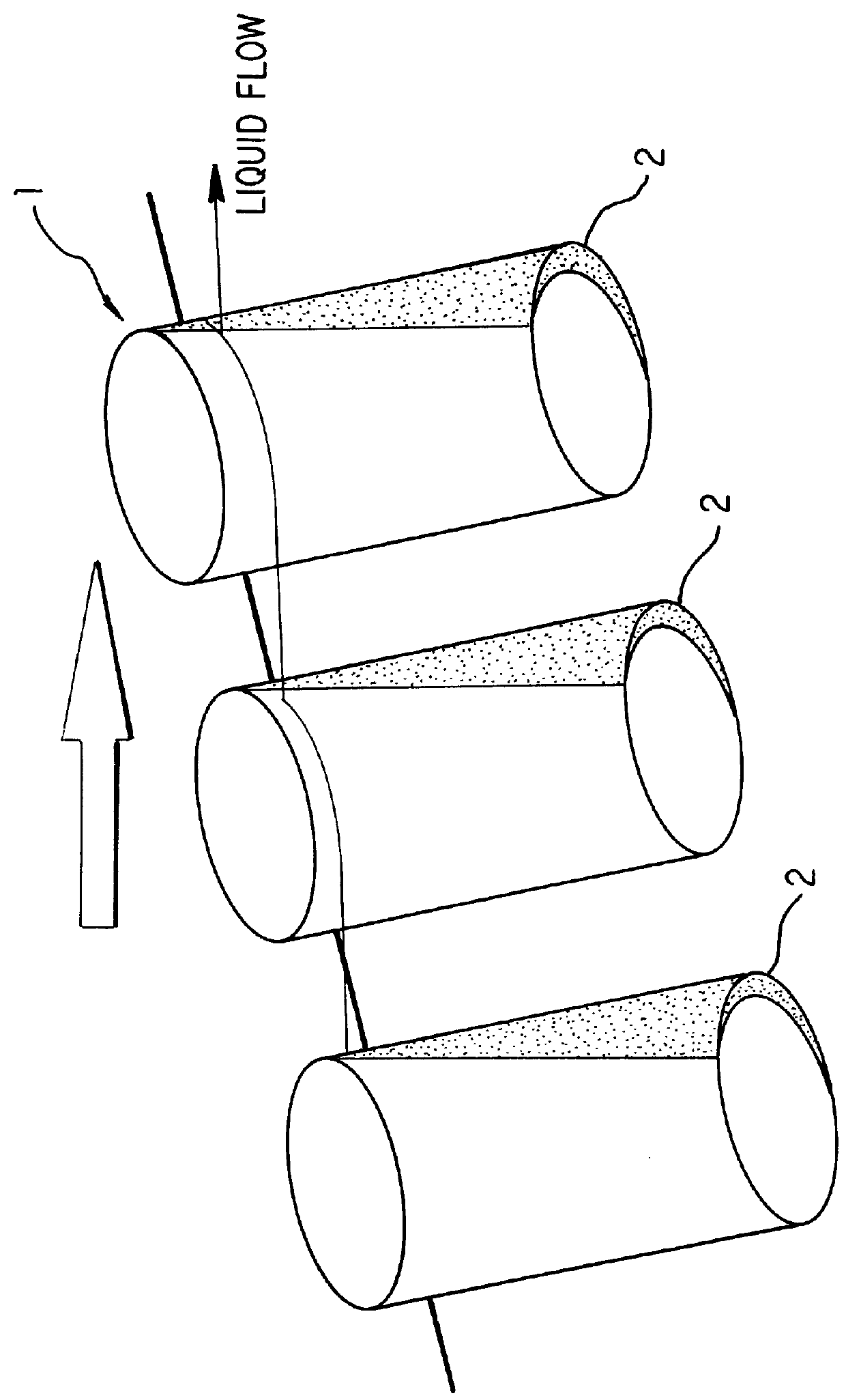
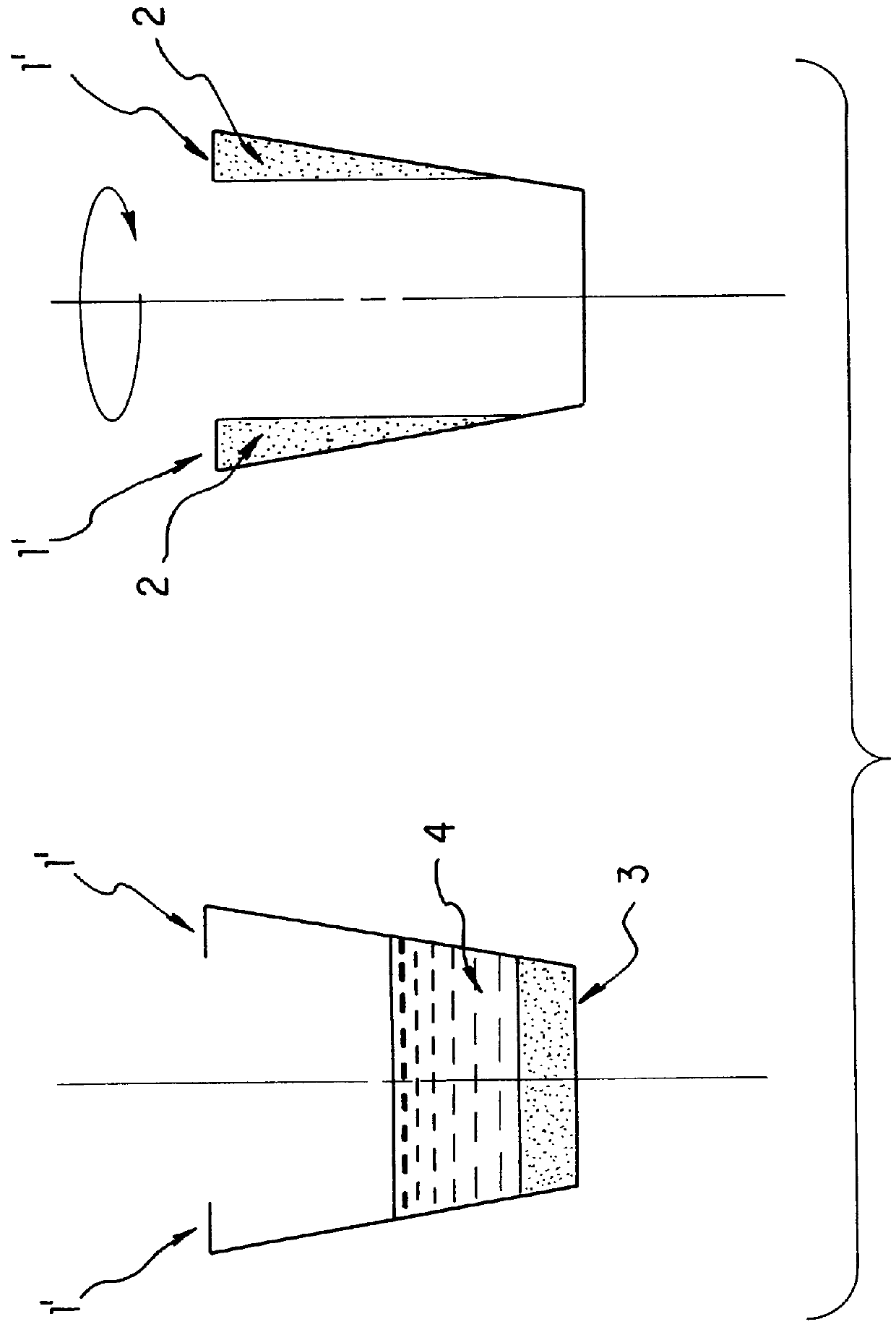
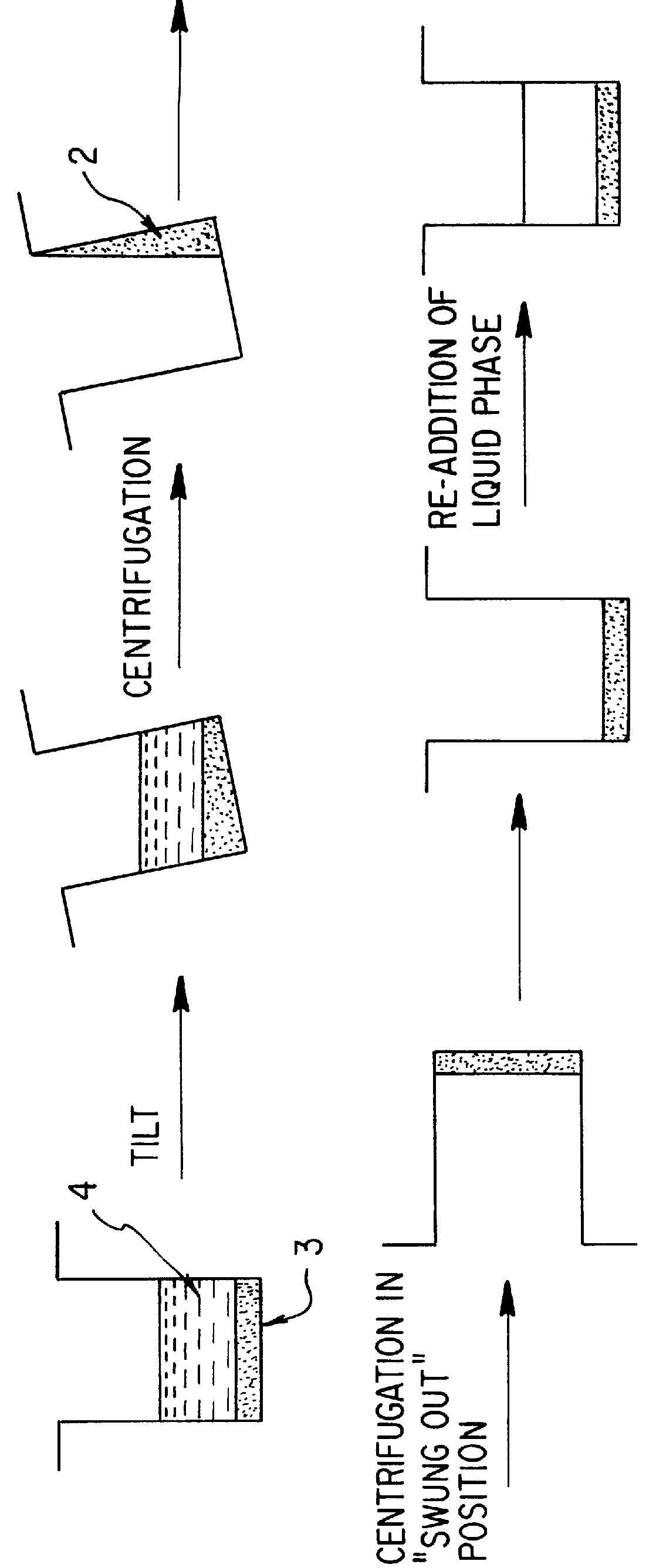
Apparatus and method for separation of liquid phases of different density and for fluorous phase organic syntheses
InactiveUS6846460B1Bioreactor/fermenter combinationsPeptide librariesOrganic synthesisCentrifugation
A simple, efficient apparatus and method for separating layers of immiscible or partially miscible liquids useful in methods of high-throughput combinatorial organic synthesis or parallel extraction of large libraries or megaarrays of organic compounds is disclosed. The apparatus and method are useful, whether as part of an automated, robotic or manual system for combinatorial organic synthesis or purification (extraction). In a preferred embodiment, an apparatus and method for separating layers of immiscible or partially miscible liquids compatible with microtiter plate type array(s) of reaction vessels is disclosed. Another application of centrifugation based liquid removal was found for washing the plates in biological assays or synthesis on modified substrates.
Owner:ILLUMINA INC
Evolving new molecular function
ActiveUS7771935B2High selectivityEnhance covalent bond formationMicrobiological testing/measurementFermentationChemical reactionOrganic synthesis
Nature evolves biological molecules such as proteins through iterated rounds of diversification, selection, and amplification. The power of Nature and the flexibility of organic synthesis are combined in nucleic acid-templated synthesis. The present invention provides a variety of template architectures for performing nucleic acid-templated synthesis, methods for increasing the selectivity of nucleic acid-templated reactions, methods for performing stereoselective nucleic acid-templated reactions, methods of selecting for reaction products resulting from nucleic acid-templated synthesis, and methods of identifying new chemical reactions based on nucleic acid-templated synthesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for undergoing chlorobenzene nitration reaction by using micro-channel reactor
ActiveCN102432471APrevent leakageAvoid dangerNitro compound preparationTemperature controlChlorobenzene
The invention relates to a method for undergoing a chlorobenzene nitration reaction by using a micro-channel reactor, belonging to the technical field of application of organic synthesis. In the method, nitric acid, sulfuric acid, water and chlorobenzene are taken as initial reaction raw materials, and processes such as mixed acid preparation, mixed acid and chlorobenzene preheating, mixed acid and chlorobenzene reacting and the like are completed in a micro-channel reactor system. In the reaction, nitro-sulfuric mixed acid is taken as a nitrating agent, the effective concentration of sulfuric acid in the mixed acid is 50-90 percent, the molar ratio of the nitric acid to the sulfuric acid in the mixed acid is 1:1-1:10, the molar ratio of the chlorobenzene to the nitric acid is 1:1.0-1:2.0, the reaction temperature is 50-100 DEG C, and the reaction time is 30-120 seconds. The chlorobenzene transformation ratio is up to 97 percent, the selectivity of nitrochlorobenzene serving as a product is over 96.5 percent, and the ratio of ortho-para nitrochlorobenzene is over 0.6. A strengthened mixed micro-channel reactor adopted in the invention is particularly suitable for undergoing a continuous nitration reaction, and has the characteristics of stable temperature control and safe process.
Owner:CHANGZHOU UNIV
Amino acid silicon polymer, method for preparing the same, cosmetic particles surface-treated with the same, and cosmetic composition containing the particles
The invention relates to a novel amino acid silicon polymer with good adhesion to the keratin layer of the skin, a method of preparing the amino acid silicon polymer, a cosmetic particles surface-treated with the amino acid silicon polymer, and a color cosmetic composition including the cosmetic particles. According to the present invention, the particles surface-treated up to 3% to the maximum with the amino acid silicon polymer prepared by reacting an amino acid and a functional silicon polymer in the manner of organic synthesis have water resistance and usability peculiar to silicon-treated pigments and take positive surface charges, thereby offering good adhesion to the skin and durability. Due to these characteristics, the cosmetic containing the surface-treated particles of the present invention is superior in the cosmetic effect to that containing the conventional surface-treated particles.
Owner:PACIFIC
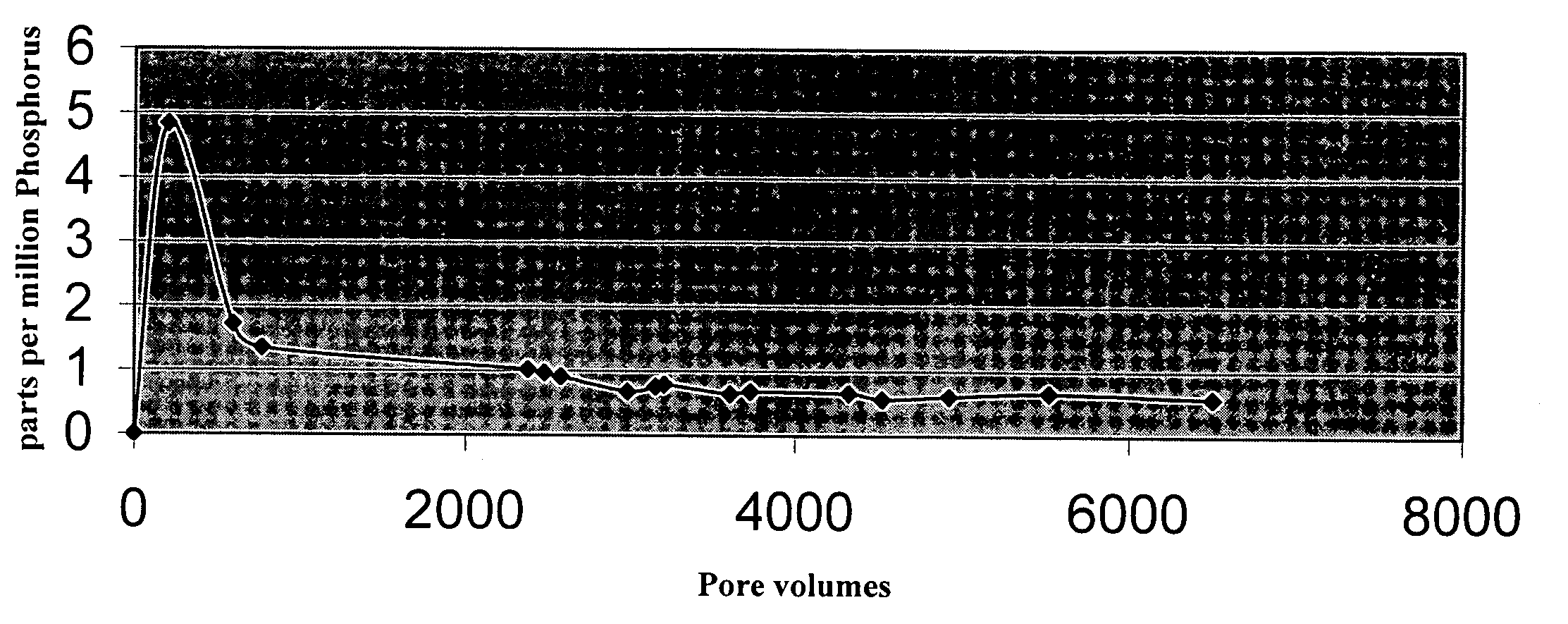
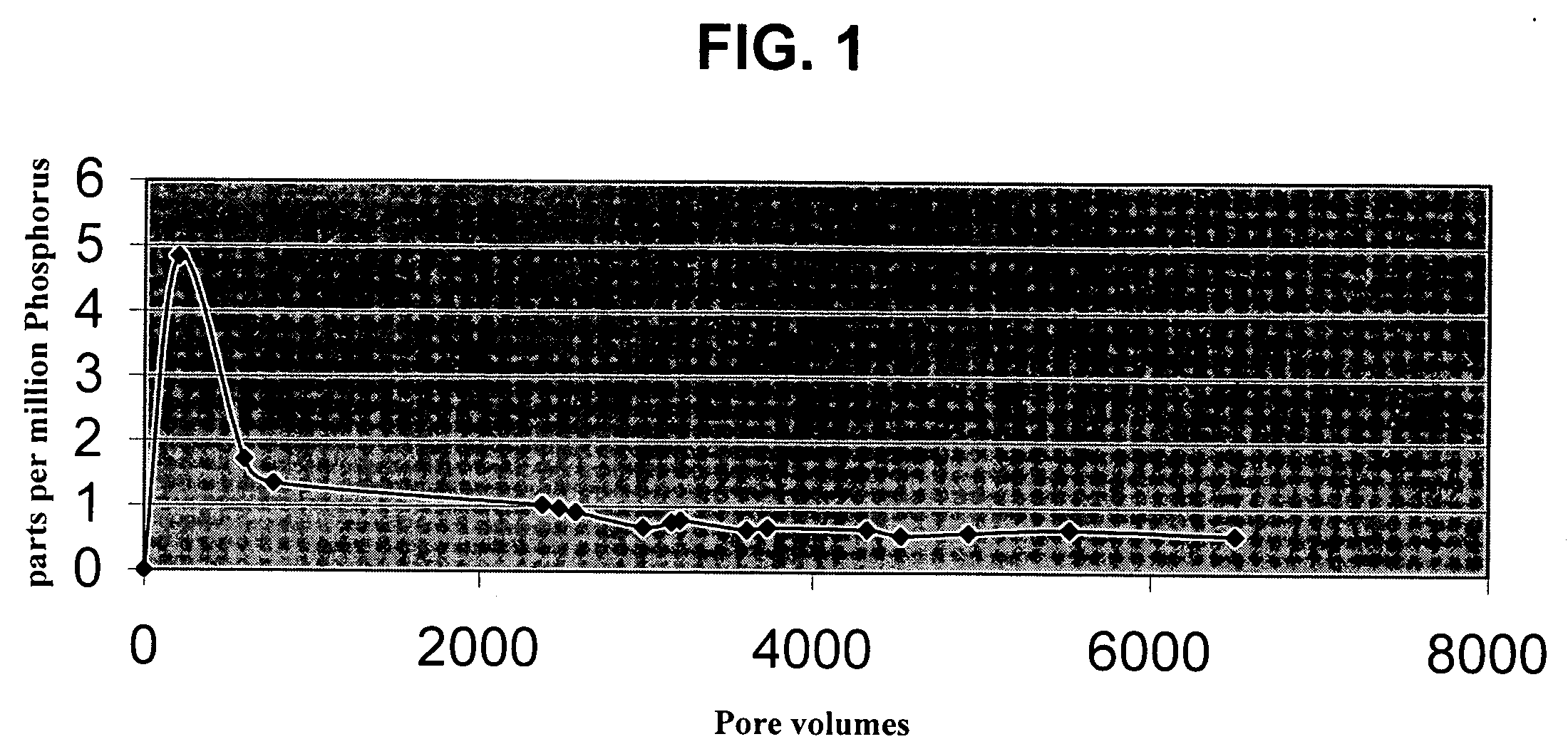
Method of purifying lithium hexafluorosphate
InactiveUS6514474B1Phosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsHydrogen fluoridePhosphor
A method of purifying lithium hexafluorosphate that allows to purify lithium hexafluorophosphate, useful as lithium secondary cell electrolyte, organic synthesis medium or the like, to an extremely high purity is provided. Lithium hexafluorophosphate containing harmful impurities such as oxyfluoride, lithium fluoride is purified by adding phosphoric chloride. The purification is performed in the presence of phosphoric chloride and hydrogen fluoride of the quantity equal or superior to the equivalent amount for reacting them, and then by converting lithium fluoride to lithium hexafluorophosphate with generated phosphor pentafluoride.
Owner:STELLA CHEMIFA CORP
Pegylated Nanoparticles
ActiveUS20080248125A1Easy to operateGood bioadhesionPowder deliveryCosmetic preparationsZeta potentialOrganic synthesis
The present invention relates to nanoparticles comprising a biodegradable polymer, preferably the vinyl methyl ether and maleic anhydride (PVM / MA) copolymer, and a polyethylene glycol or derivatives thereof. These nanoparticles are easy to produce and provide excellent bioadhesion, size and zeta potential characteristics making them suitable for the administration of active molecules. The selection of the type of polyethylene glycol used in their production allows suitably modulating the characteristics of these nanoparticles, which can be advantageously used according to the type of drug to be carried and / or the method of administration of the pharmaceutical formulation. pegylation is carried out by simple incubation for a short time period of the two macromolecules in question, without needing to have to resort to the use of organic solvents with high toxicity or long and laborious organic synthesis processes. Furthermore, the pegylation process can be associated to the process of encapsulating the biologically active molecule.
Owner:INNOUP FARMA
Filter material, method for the production thereof, a filter and filtering method
ActiveUS20080164214A1High propertyLow hydrodynamic resistanceOther chemical processesLayered productsFiberRetention efficiency
The invention concerns the production of filter materials for the purification and disinfection of water, water solutions and other liquids, as well as for sterilizing filtration of injections and other solutions, for concentration of biomolecules in physiological liquids, concentration and extraction of viruses, preparation of apyrogenic water, in biocatalytic diaphragm reactors. The invention solves the problems of a new filter material production, characterized by high sorption properties, high retention efficiency of submicron electronegative particles, microorganisms, submicron non-polar particles and chemical contaminations, and, at the same time, characterized by low hydrodynamic resistance. A base of filter material is the nonwoven organic synthetic polymeric fabric, modified by the aluminum hydroxide particles, fixed to the surface of base fibers for improvement of its sorption properties and for making it positively charged. A method of filter material production comprises: applying the modifying composition onto the fibrous base in the form of organic nonwoven synthetic polymeric fabric, wherein said modifying composition comprises the particles of the aluminum-based material, hydrolysis thereof results to formation and fixation of the aluminum hydroxide particles to the base fibers. A method of fluid filtration is carried out using the filter material as a non-woven organic synthetic polymeric fabric, to the fibers of which the aluminum hydroxide particles are fixed.
Owner:INST OF STRENGTH PHYSICS & MATERIALS SCIENC
Method for producing fine spherical particles of carbonate or hydroxide of nickel, cobalt or copper
InactiveUS6197273B1Amino compound purification/separationOxide/hydroxide preparationSal ammoniacCobalt metal
The invention provides a process for production of fine spherical particles of a carbonate or a hydroxide of nickel, cobalt or copper which comprises: dissolving a carbonate or a hydroxide of nickel, cobalt or copper having the general formula (I)wherein M represents Ni, Co or Cu, and x and y are numerals satisfying the followings: 0<=x<=2, 0<=y<=2 and x+y=2, in aqueous ammonia, converting the resulting solution to a W / O emulsion containing droplets of the solution in a non-aqueous medium, and then removing volatile components including ammonia from within the droplets, thereby precipitating a basic carbonate or a hydroxide of a metal selected from nickel, cobalt and copperin the droplets.The fine spherical particles of a carbonate or a hydroxide of nickel, cobalt or copper obtained according to the process of the invention are especially useful as a precursor for the manufacture of uniform, fine spherical particles of nickel, copper or cobalt metal, as well as useful as themselves as a catalyst for use in organic synthesis, a carrier, a pigment, a filler or a glaze.
Owner:SAKAI CHEM IND CO LTD
Production of high-density topping filtering material from three gradient
InactiveCN1712100AImprove filtration efficiencyExcellent peelabilityFiltration separationHigh densityProcess equipment
Owner:孙熙
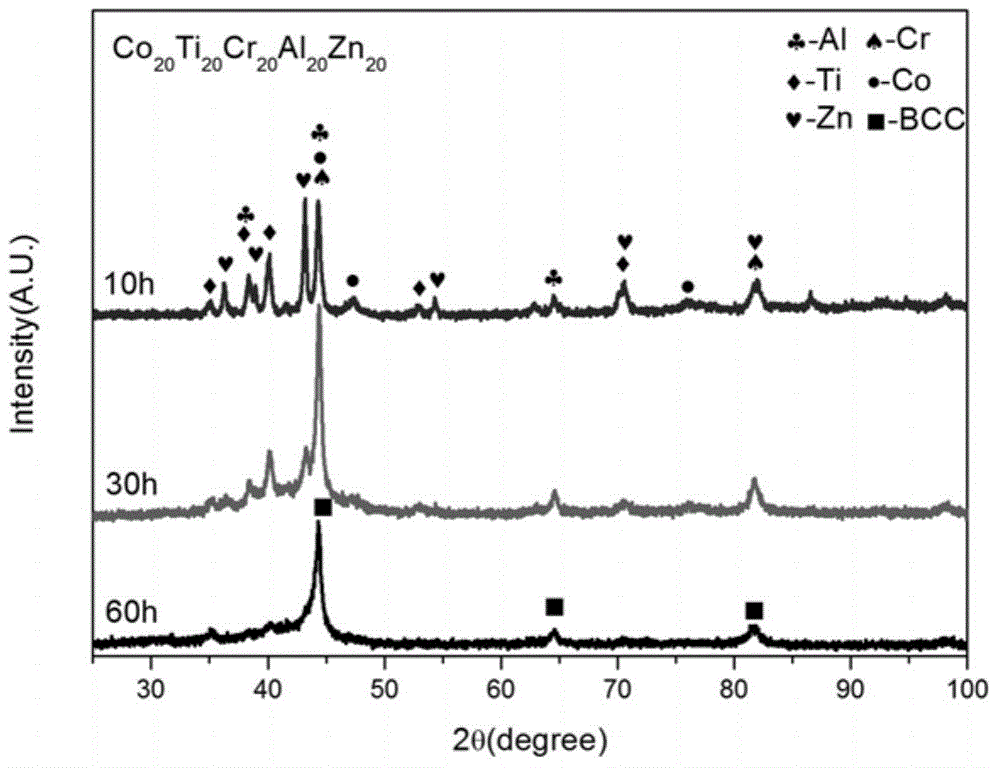
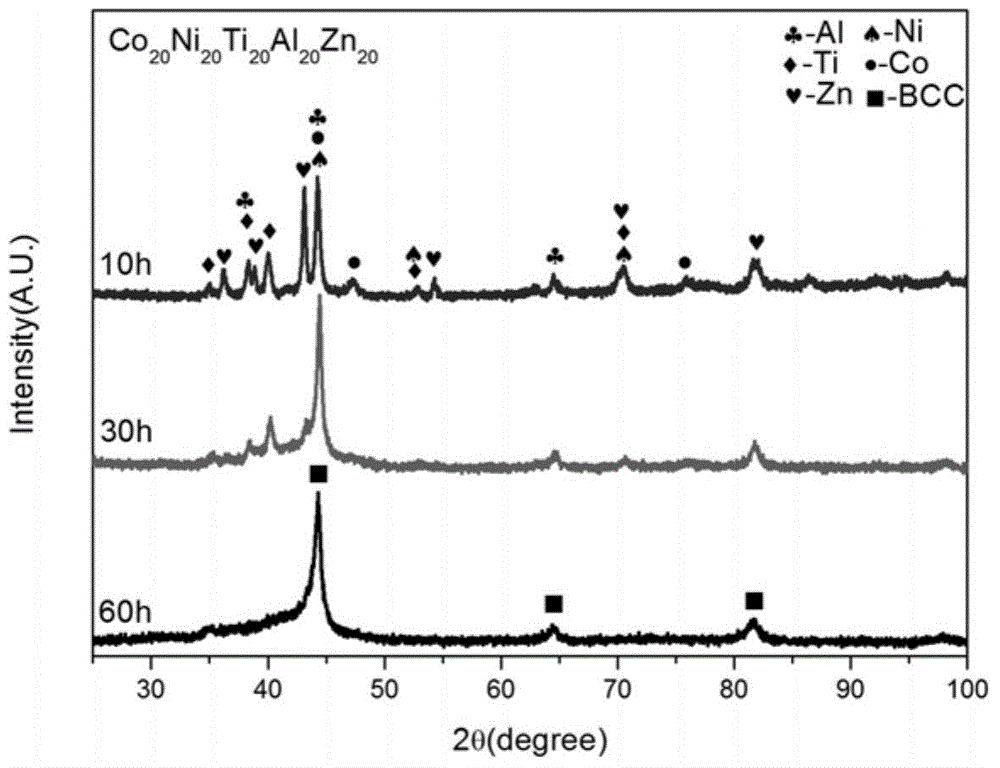
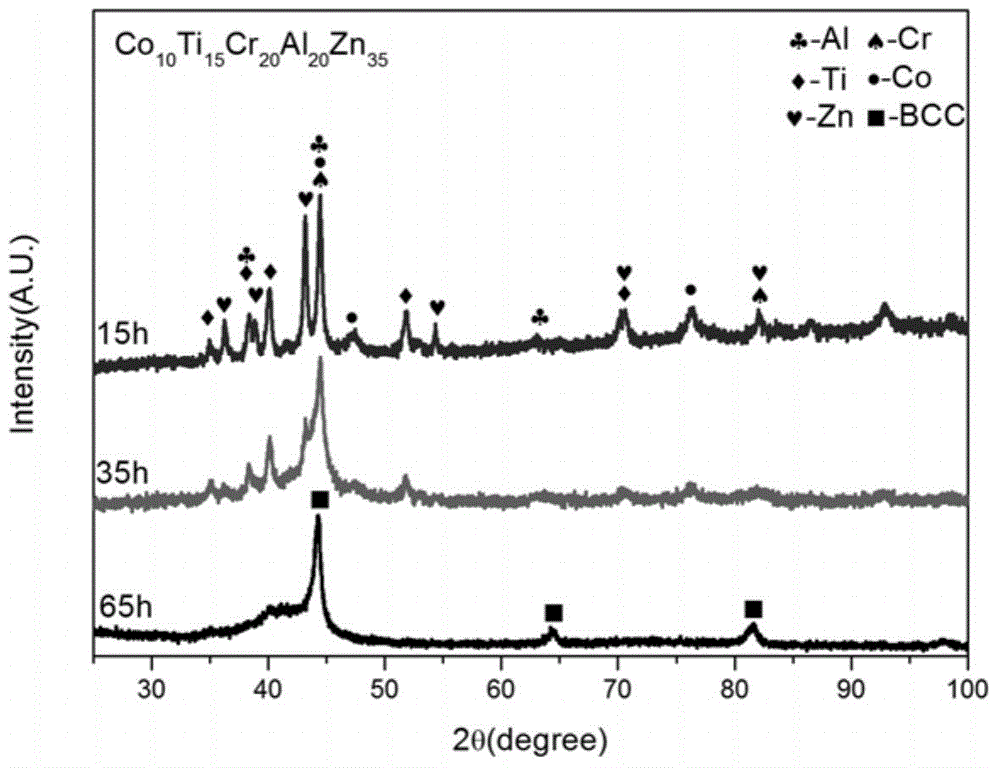
High-entropy alloy for sewage degradation and preparing method thereof
ActiveCN105088048ARaise the ratioStrong degradation efficiencyWater/sewage treatmentIron powderHigh entropy alloys
The invention discloses high-entropy alloy for sewage degradation and a preparing method thereof. The high-entropy alloy at least comprises four components and at least comprises one active metallic element which is Mg, Ca or Zn, and the components are matched based on an equi-atomic ratio or approximate-equi-atomic ratio. The high-entropy alloy is prepared through mechanical alloying. The technology is simple, smelting is not needed, and preparing cost is low. The high-entropy alloy is different from ordinary high-entropy alloy systems in that a dinitrogen bond is broken through redox reaction between the active zero-valent metallic element and the -N=N- perssad in organic synthetic dyestuff, so that a high rate is realized for azo dyestuff and a remarkable effect is realized for degradation of materials similar to organic synthetic dyestuff, and degradation efficiency is improved by over 250 times compared with commercial iron powder which is in common use currently. The composition range of the high-entropy alloy is wide, and appropriate composition adjustment can be conducted according to actual needs. Meanwhile, the high-entropy alloy is high in structural stability and suitable for various working environments and has broad industrial application prospects in sewage treatment.
Owner:UNIV OF SCI & TECH BEIJING
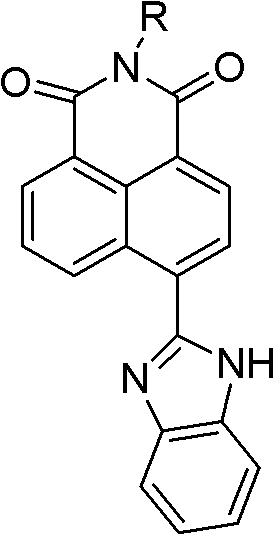
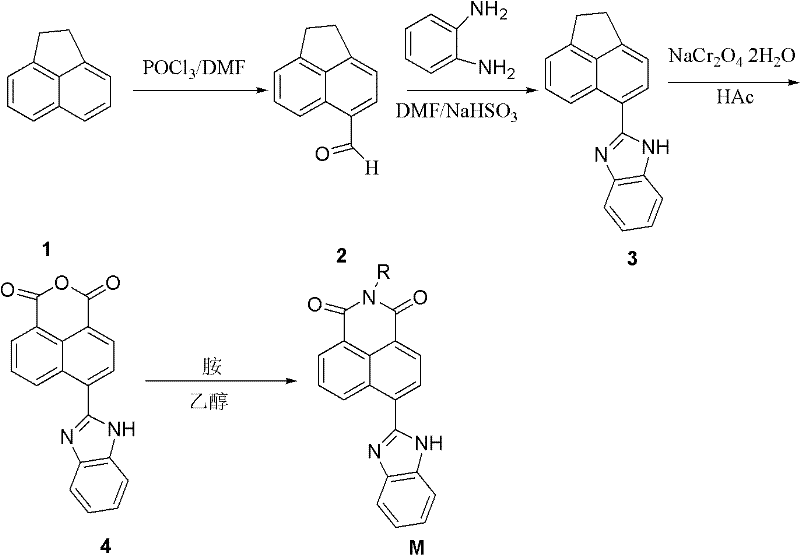
Synthesis of benzimidazole-containing naphthalimide derivatives and applications of benzimidazole-containing naphthalimide derivatives on cancer resistance
InactiveCN102206203AGood choiceGood tumor suppressor activityOrganic active ingredientsOrganic chemistryPheochromocytomaOrganic synthesis
The invention relates to synthesis of benzimidazole-containing naphthalimide derivatives and applications of the benzimidazole-containing naphthalimide derivatives on cancer resistance, and belongs to the field of organic synthesis and pharmaceutical chemistry technology. The benzimidazole-containing naphthalimide derivatives are obtained through a method that a benzimidazole group is introduced into a fourth position of a naphthalene ring of naphthalimide. Experiments of proliferation inhibiting effects of the benzimidazole-containing naphthalimide derivatives on cancer cells adopt a microculture tetrozolium (MTT) reduction method and aim at MCF-7 human breast cancer cells, Hela human cervical cancer cells and PC12 rat adrenal medullary pheochromocytoma differentiated cells. Results of the experiments show that the benzimidazole-containing naphthalimide derivatives have the advantages of good inhibitory activity against cancer cells and good selectivity on cancer cells.
Owner:DALIAN UNIV OF TECH
Plant fiber environment-friendly material as well as preparation method and application thereof
InactiveCN103113753AWide variety of sourcesEvenly distributedWood working apparatusFlat articlesFiberWide field
The invention discloses a plant fiber environment-friendly material and a preparation method thereof as well as application of the material in tableware, toys, furniture, decoration or wall. The preparation method comprises the following steps of: pretreating, grinding and screening the plant fiber; mixing the screened plant fiber powder with an adhesive and a modification aid, and stirring at high temperature, wherein the adhesive mainly adopts hydroxypropyl methyl cellulose; filtering with a riddler, and dehydrating and preheating the screened organic synthesis plant fiber raw material; and performing high-temperature die pressing. According to the plant fiber environment-friendly material as well as the preparation method and application thereof, the product has good performance and additional functionality, can be applied to wider fields, conforms to relatively high requirements on using safety, and facilitates environmental protection so as to overcome the shortcomings of the existing plant fiber environment-friendly material and a preparation method and application thereof.
Owner:王丹 +2
Extraction process of resveratrol from giant knotweed
InactiveCN1384088ARealize the needs of industrialized productionReduce consumptionOrganic chemistryOrganic compound preparationOrganic synthesisGradient elution
During the extraction of resveratrol from giant knotwood, giant knotwood glycoside is hydrolyzed and enriched through organic synthesis. The extraction process includes mixing giant knotwood raw material and composite stuffing, high pressure chromatography in a high-pressure chromatographic column apparatus, gradient elution with chloroform and ethyl acetate and thin chromatographic tracking detection. The said process can reach a kilogram level yield and high product purity. The present invention may be used in extracting resveratrol and other similar product from giant knotwood material.
Owner:杨建文
Diphenylmethane compound
ActiveUS20100249374A1Superior in broad utilityImprove stabilityOrganic compound preparationDiaryl/thriaryl methane dyesDiphenylmethaneOrganic synthesis
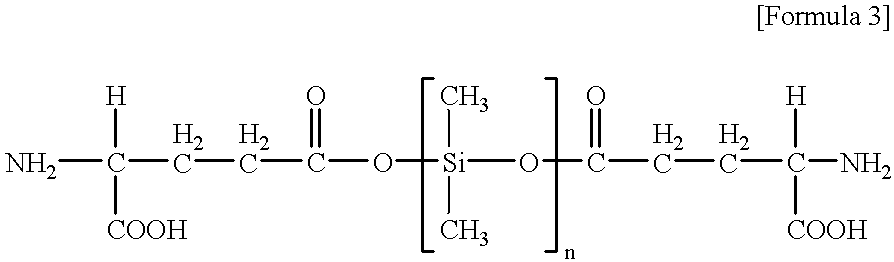
Compounds having a diphenylmethane skeleton are superior in broad utility and stability, and are useful as a protecting reagent (anchor) of amino acid and / or peptide in the liquid phase synthesis and the like of a peptide having a C-terminal etc., which are of a carboxamide(-CONHR)-type, and in organic synthetic reaction methods (particularly peptide liquid phase synthetic methods), and may be contained in a kit for peptide liquid phase synthesis.
Owner:AJINOMOTO CO INC
One-pot synthetic method for 1,2,3-triazole compounds
The invention provides a one-pot method for synthesizing 1,2,3-triazole compounds, and belongs to the technical field of organic synthesis. According to the one-pot synthetic method for the 1,2,3-triazole compounds, nitrobenzene compounds and alkyne compounds serve as starting materials, and a plurality of the 1,2,3-triazole compounds are synthesized conveniently in a one-pot mode under the catalysis of copper. The 1,2,3-triazole compounds synthesized with the one-pot synthetic method are important intermediates for drug synthesis, and have very important application prospects in the fields including biomedicine, medicines and the like. Certain components are already widely used for clinics. The method adopts one-pot preparation, so that diazotization reaction is avoided, organic azides are not directly used, and the one-pot synthetic method for the 1,2,3-triazole compounds is high in safety, simple in operation, mild in conditions, low in cost and easy to use for industrial production and has good application prospects.
Owner:THE CITY VOCATIONAL COLLEGE OF JIANGSU
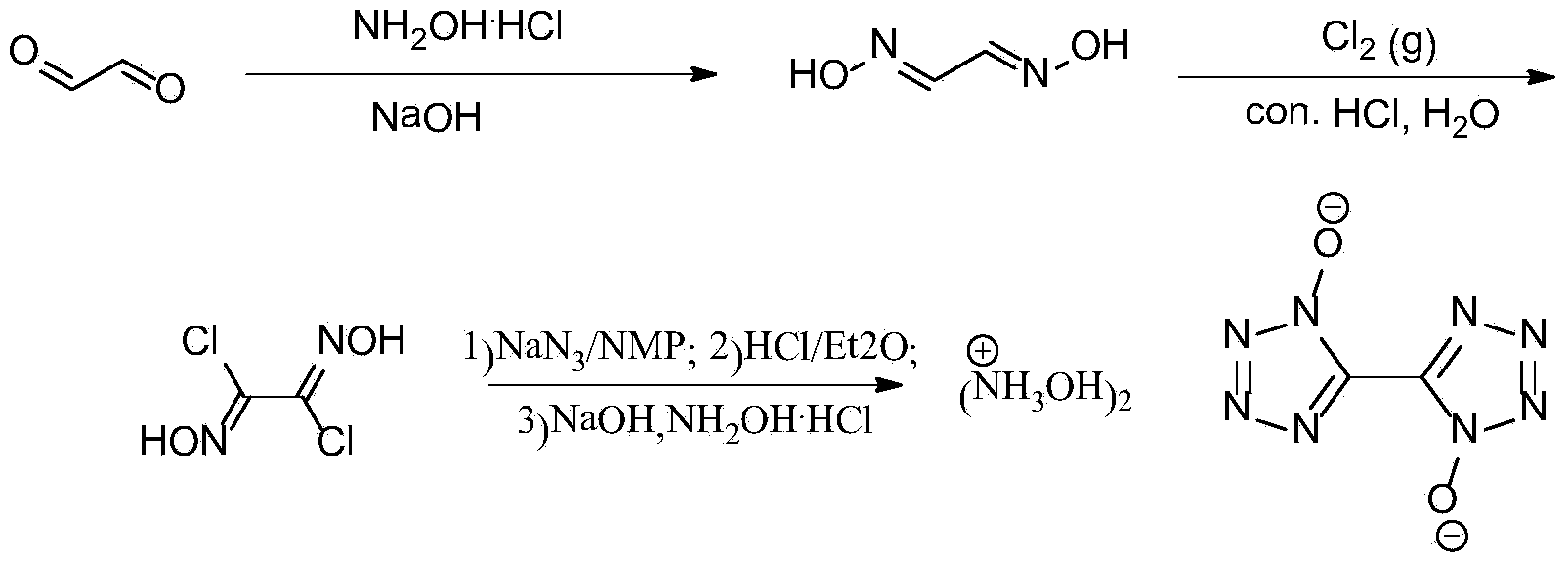
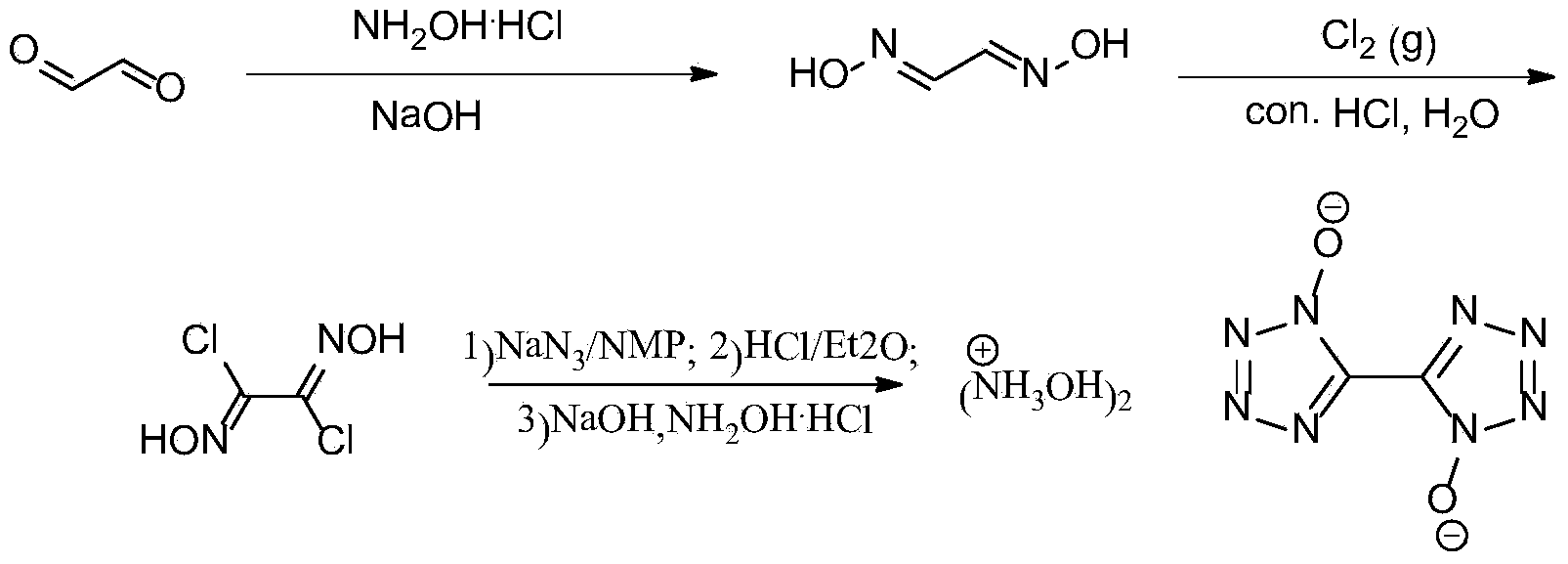
Synthetic method of 5, 5'-bistetrazole-1, 1'-dioxo hydroxyl ammonium salt (TKX-50)
InactiveCN103524444AAvoid purification processEasy to operateOrganic chemistryFiltrationOrganic synthesis
The invention provides a method for preparing 5, 5'-bistetrazole-1, 1'-dioxo hydroxyl ammonium salt (TKX-50), and belongs to the technical field of organic synthesis. The method comprises the steps as follows: step one, glyoxime is prepared, and the yield is 62%; step two, a product obtained in the step one is dissolved in water and concentrated hydrochloric acid, chlorine is introduced at the temperature of 0 DEG C for a reaction for a period of time, and dichloroglyoxime is obtained; and finally, a product obtained in the step two is dissolved in a solvent, and the product and sodium azide have a reaction at the temperature of 0 DEG C for a period of time; after that, the mixture is transferred into diethy ether, and sealed for a reaction at the room temperature overnight after HCl is introduced for a period of time; and after diethyl ether and most HCl gas are volatilized, pH of an aqueous NaOH solution is regulated to be about 8, reflux cooling is performed, filtered and separated solids are dissolved in hydroxylamine hydrochloride to have a reaction for a period of time, and TKX-50 is obtained. According to the method, glyoxal is adopted as a raw material, water is adopted as a solvent for preparation of dichloroglyoxime in the step two, and only direct filtration is required in postprocessing, so that the tedious purification process is prevented, and the cost is reduced; and besides, a target product TKX-50 is synthetized through three steps of reactions, the total yield is up to 34%, the reaction condition is mild, the operation is simple and convenient, and the industrialization is easy to realize.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Synthesis of terminal alkenes from internal alkenes and ethylene via olefin metathesis
This invention relates generally to olefin metathesis, and more particularly relates to the synthesis of terminal alkenes from internal alkenes using a cross-metathesis reaction catalyzed by a selected olefin metathesis catalyst. In one embodiment of the invention, for example, a method is provided for synthesizing a terminal olefin, the method comprising contacting an olefinic substrate comprised of at least one internal olefin with ethylene, in the presence of a metathesis catalyst, wherein the catalyst is present in an amount that is less than about 1000 ppm relative to the olefinic substrate, and wherein the metathesis catalyst has the structure of formula (II)wherein the various substituents are as defined herein. The invention has utility, for example, in the fields of catalysis, organic synthesis, and industrial chemistry.
Owner:MATERIA
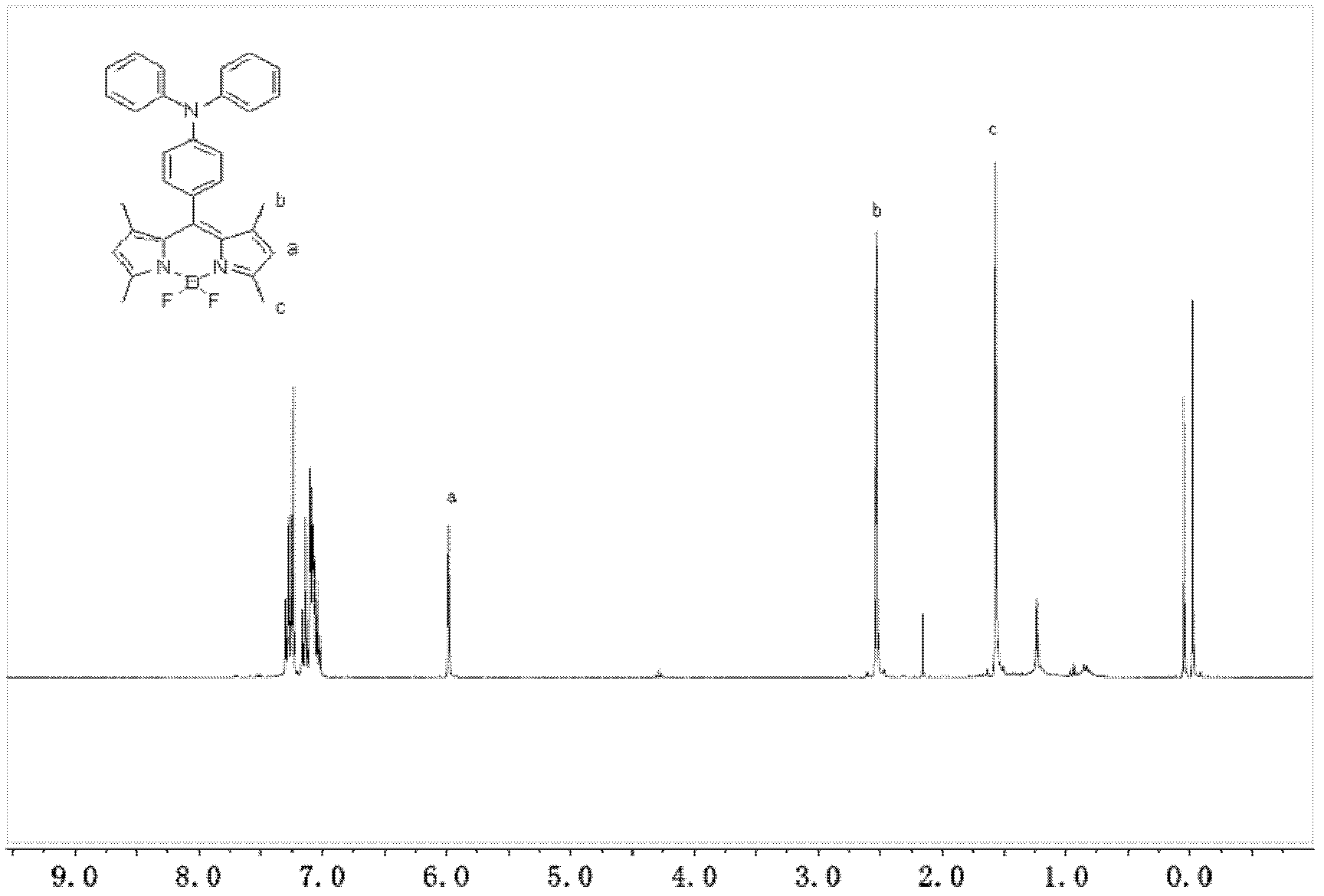
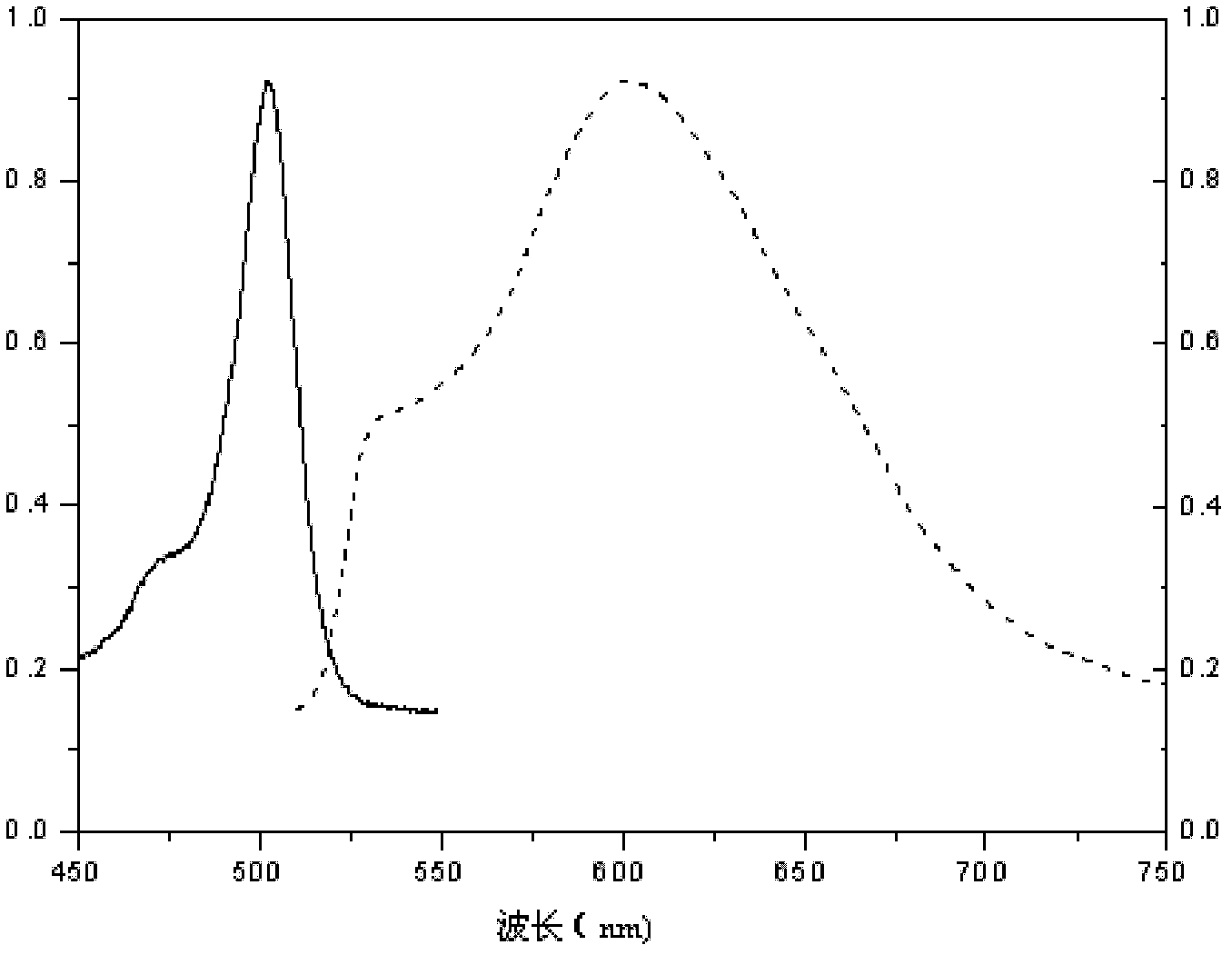
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
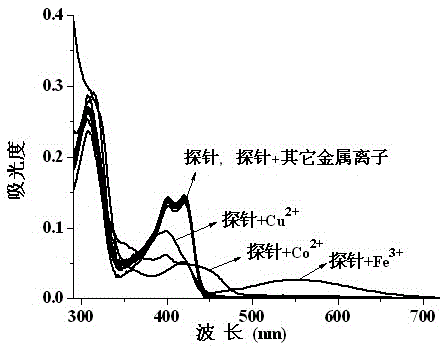
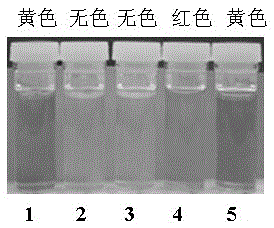
Fluorescent probe reagent for concurrent selection and determination of multiple metal ions, and preparation and appliance
InactiveCN105482812APreparation conditions are easy to controlReduce distractionsOrganic chemistryColor/spectral properties measurementsOrganic synthesisPhotochemistry
The invention discloses a probe reagent for concurrent selection and determination of multiple metal ions, and preparation and appliance, and belongs to the field of organic synthesis and analytical chemistry. Tri (2-aminoethyl) amine serves as a parent, wherein rhodamine B is connected to an amino chain, 2-hydroxy-1-naphthaldehyde groups are connected to other two amino chains respectively, and thus a tripod structured rhodamine-hydroxyl naphthalene derivative probe is prepared. In 1,4-dioxane / water (19 / 1, v / v, pH=7) solution, the probe respectively detects Cu2+, Co2+ and Fe3+ by utilizing rate absorption of different wavelengths, and the detection does not interfere with each other; in acetonitrile / water (19 / 1, v / v) solution, fluorescence emission of different wavelengths under different pHs is utilized, the probe respectively detects Zn2+, Al3+, Hg2+ and Cu2+, and the detection does not interfere with each other; under an ultraviolet lamp of 365 nm, Zn2+, Al3+ and Hg2+ are detected to show blue, pink and orange red fluorescence respectively, and through -Zn2+ mixture detection by the probe, Cu2+ shows blue fluorescence vanishing. The probe structure is as follows.
Owner:GUIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com