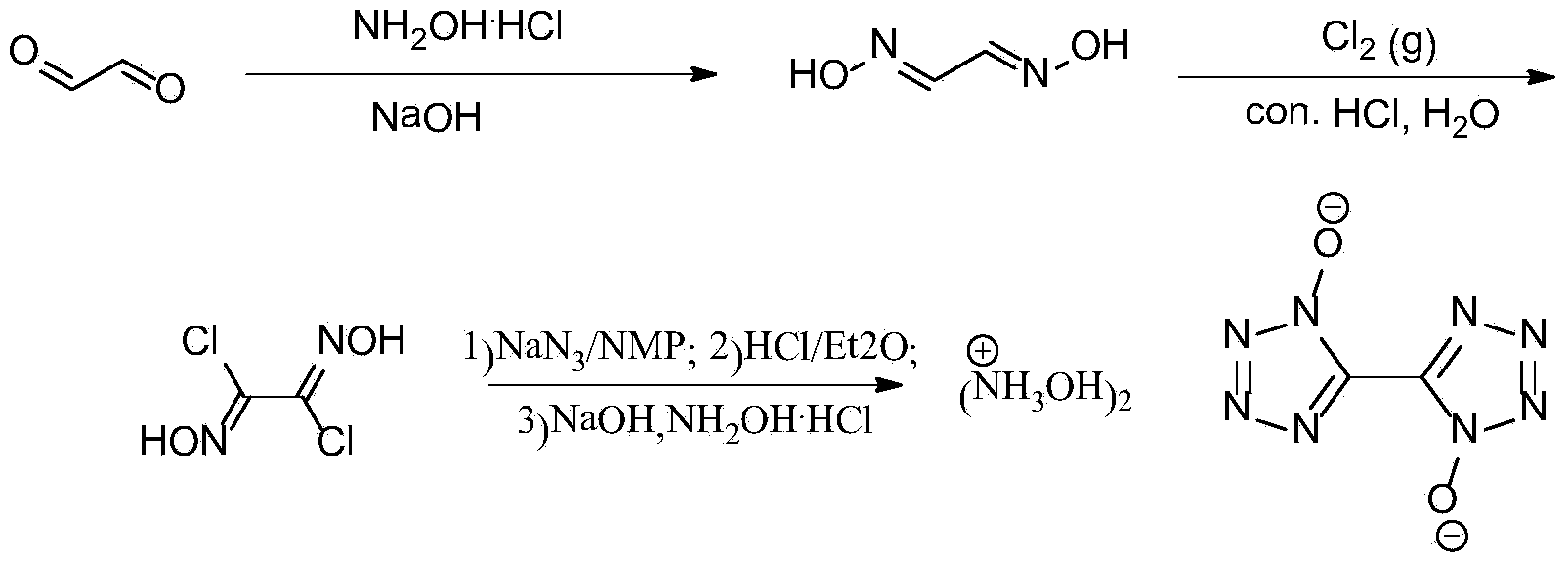
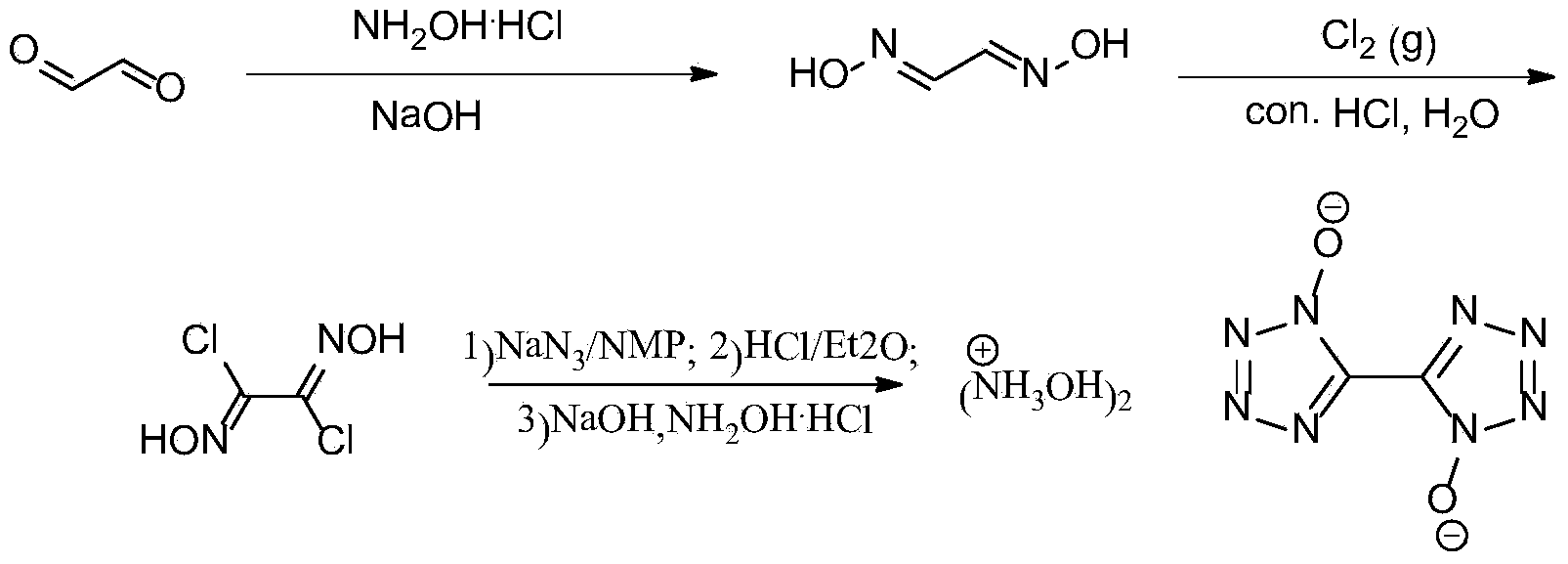
Synthetic method of 5, 5'-bistetrazole-1, 1'-dioxo hydroxyl ammonium salt (TKX-50)
A technology of TKX-50 and dioxyhydroxylamine salt, applied in the direction of organic chemistry, can solve the problems of high mechanical stimulation sensitivity, expensive synthesis, unknown performance, etc., and achieve the effect of easy operation, simple and convenient operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) At room temperature, 165g (412.5mmol) NaOH was dissolved in 450mL water, and the solution was cooled to 0°C in an ice-salt bath, and then 417g (600.1mmol) of hydroxylamine hydrochloride was added to it, and after addition, 435g (299.8mmol) of 40 % glyoxal aqueous solution, control the temperature below 10°C, react for 15 minutes, and react overnight at room temperature. Filter, wash with a small amount of ice water, and dry naturally to obtain 174 g of glyoxime, with a yield of 62%. Melting point: 175-177°C.
[0021] 2) At room temperature, add 100g (1.14mol) of glyoxime obtained in step 1) into 2000mL of water, stir evenly, then add 500mL of concentrated hydrochloric acid, and when the raw materials are almost completely dissolved, cool the mixture to 0°C in an ice-salt bath (a large amount of The raw material is precipitated again, and it is a white turbid liquid), and a large amount of chlorine gas (made by dripping concentrated hydrochloric acid into manganese d...
Embodiment 2
[0024] Same as Example 1, except that the speed of introducing chlorine gas in step 2) is slow, and 81 g of dichloroglyoxime is obtained with a yield of 45%.
Embodiment 3
[0026] Same as Example 1, except that the reaction time of feeding chlorine gas in step 2) was 2 h, and 95 g of dichloroglyoxime was obtained with a yield of 53%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com