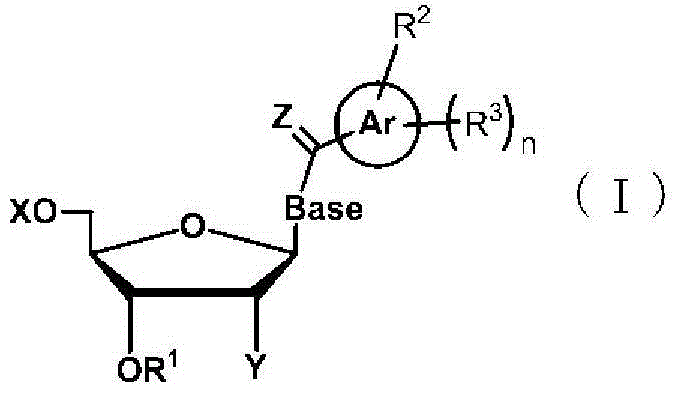
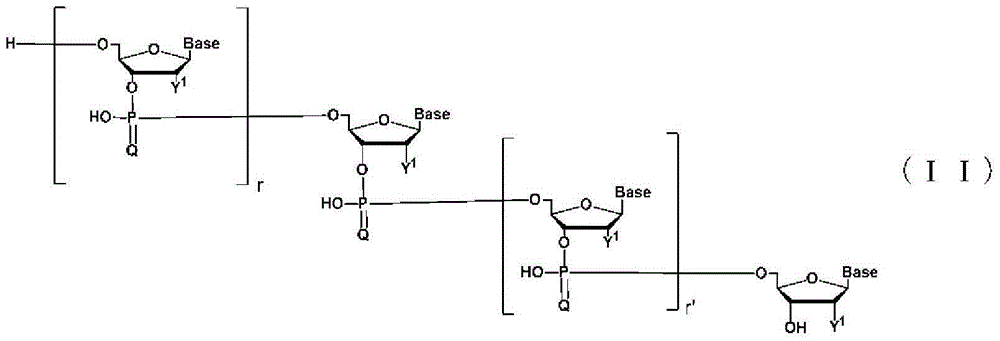
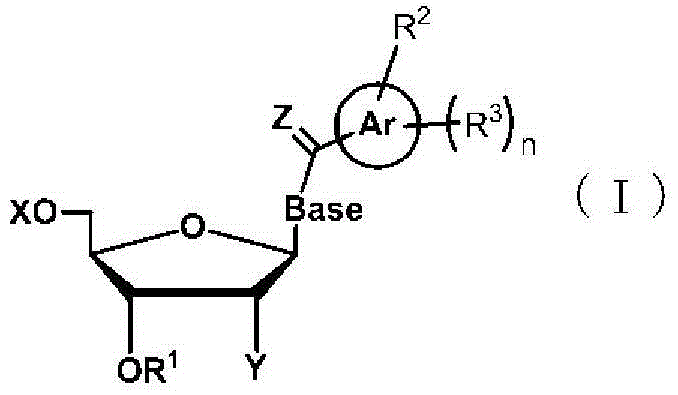
Method for liquid-phase synthesis of nucleic acid
A compound, representative technology, applied in the field of liquid phase synthesis of nucleic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0607] Synthesis of a deoxythymine (dT) type nucleoside monomer compound (compound (E5)) with three octadecyloxy groups
[0608] [Formula 55]
[0609]
[0610] (1) Methyl benzoate substituted by three octadecyloxy groups (compound (E1))
[0611] [Formula 56]
[0612]
[0613] To methyl gallate (9.2 g) and potassium carbonate (103.7 g) was added 1,3-dimethyl-2-imidazolidinone (170 mL), and the mixture was stirred at 80° C. for 30 minutes. 1-Bromooctadecane (69.1 mL) was added thereto, and the mixture was stirred at 80°C for 12 hr. 40° C. hot water was added to the reaction liquid, suspended, and then the precipitate was collected by suction filtration, and the obtained solid was washed with acetonitrile, acetone and methanol to quantitatively obtain the compound represented by E1 (48.0 g).
[0614] 1 H NMR (500MHz, CDCl 3 )δ7.25(s,2H),3.99-4.03(m,6H),3.89(s,3H),1.78-1.84(m,4H),1.71-1.77(m,2H),1.44-1.50(m, 6H), 1.20-1.38(m, 84H), 0.88(t, J=7.0Hz, 9H)
[0615] (2) Sy...
Embodiment 2
[0636] Synthesis of uracil (U) nucleoside monomer compound (compound (E7)) with three octadecyloxy groups
[0637] [Formula 61]
[0638]
[0639] (1) Synthesis of U-shaped nucleosides (compound (E6))
[0640] [Formula 62]
[0641]
[0642] 1-((2R,3R,4R,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-3-((tert-butyldimethyl Silyl)oxy)-4-hydroxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione (1 g) was dissolved in tetrahydrofuran (5 mL). Add levulinic acid (0.272g), N,N'-dimethylaminopyridine (0.0185g) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride in sequence Salt (0.444g), and the mixture was stirred at room temperature for 18 hours. At room temperature, 10% acetic acid / triethylamine (10 mL) and ethyl acetate aqueous solution (10 mL) were added to the reaction liquid, followed by separation and extraction. The obtained organic layer was concentrated under reduced pressure to obtain compound (E6) (1.21 g, yield percentage: 105.7%).
[0643] 1 ...
Embodiment 3
[0650] Synthesis of uracil (U) type nucleoside monomer compound (compound (E8)) with three tetradecyloxy groups
[0651] [Formula 64]
[0652]
[0653] (1) Methyl benzoate substituted by three tetradecyloxy groups (compound (E9))
[0654] [Formula 65]
[0655]
[0656] To methyl gallate (5.5 g) and potassium carbonate (62.2 g) was added 1,3-dimethyl-2-imidazolidinone (100 mL), and the mixture was stirred at 80° C. for 45 minutes. 1-Bromotetradecane (26.9 mL) was added thereto, and the mixture was stirred at 80°C for 12 hr. Water was added to the reaction liquid, suspended, and then the precipitate was collected by suction filtration, and the obtained solid was washed with a 50% aqueous solution of acetonitrile and acetonitrile to obtain compound (E9) (22.6 g).
[0657] 1 H NMR (500MHz, CDCl 3 )δ7.25(2H,s),3.99-4.03(6H,m),3.89(3H,s,COOMe),1.71-1.84(6H,m),1.44-1.50(6H,m),1.23-1.38( 60H, br), 0.88 (9H, t J = 7.0 Hz).
[0658] (2) Synthesis of benzoic acid (compound (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com