Patents
Literature
2237 results about "Solid-phase synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
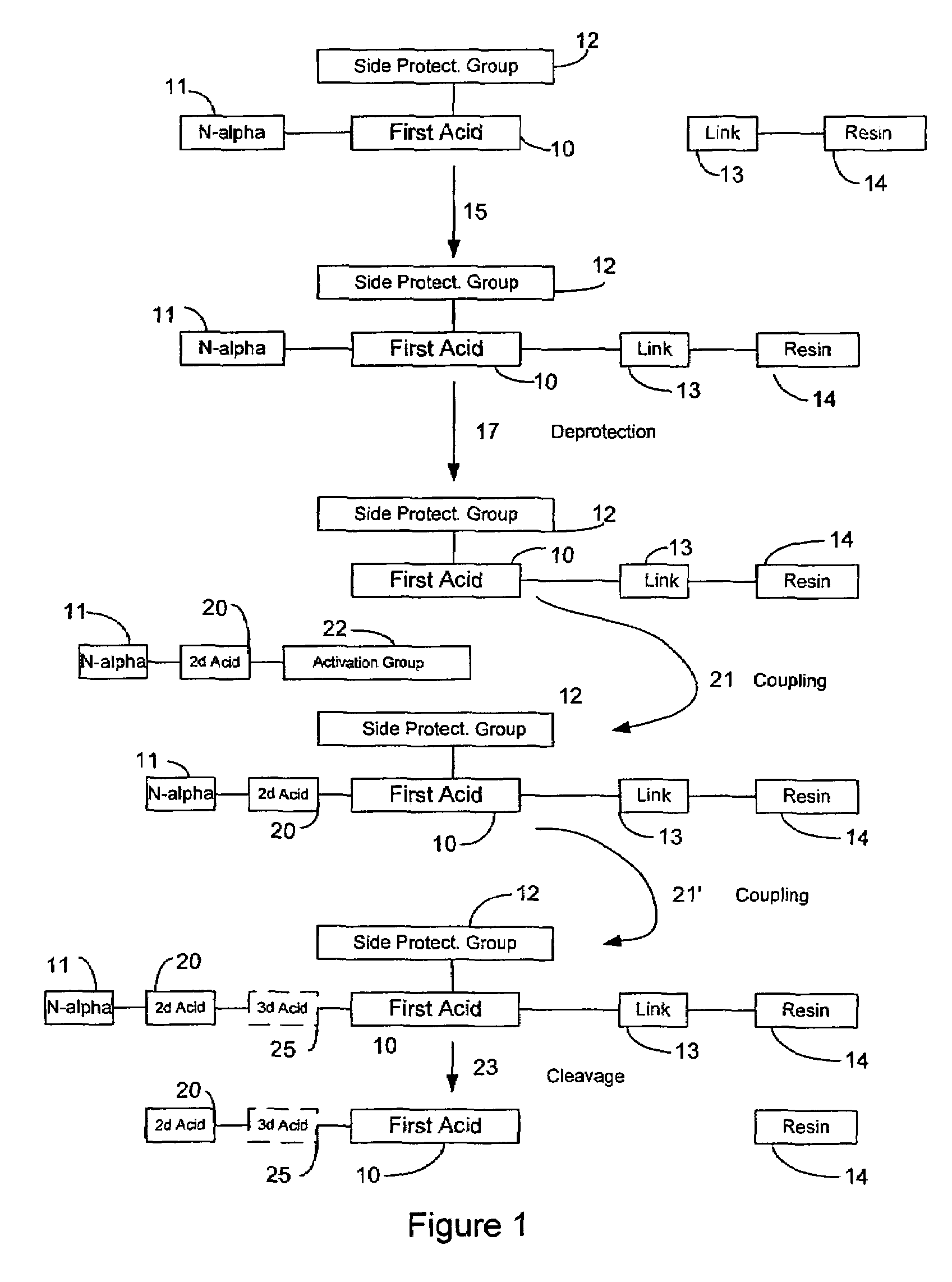
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry.
Support for high performance affinity chromatography and other uses
Multilayered particulate materials are formed by coating a particulate substrate with a metal and adsorbing an organic layer comprising a recognition moiety onto the metal film. The recognition moiety interacts with an analyte of interest allowing for its detection, purification, etc. Suitable recognition moieties can be selected from a range of species including, small molecules, polymers and biomolecules and the like. The novel particulate materials of the invention can be utilized in an array of methods including, ion-exchange, ion-selective ion-exchange, assays, affinity dialysis, size exclusion dialysis, as supports in solid phase synthesis, combinatorial synthesis and screening of compound libraries and the like.
Owner:RGT UNIV OF CALIFORNIA
Topologically segregated, encoded solid phase libraries comprising linkers having an enzymatically susceptible bond
The invention relates to libraries of synthetic test compound attached to separate phase synthesis supports. In particular, the invention relates to libraries of synthetic test compound attached to separate phase synthesis supports that also contain coding molecules that encode the structure of the synthetic test compound. The molecules may be polymers or multiple nonpolymeric molecules. Each of the solid phase synthesis support beads contains a single type of synthetic test compound. The synthetic test compound can have backbone structures with linkages such as amide, urea, carbamate (i.e., urethane), ester, amino, sulfide, disulfide, or carbon-carbon, such as alkane and alkene, or any combination thereof. Examples of subunits suited for the different linkage chemistries are provided. The synthetic test compound can also be molecular scaffolds, such as derivatives of monocyclic of bicyclic carbohydrates, steroids, sugars, heterocyclic structures, polyaromatic structures, or other structures capable of acting as a scaffolding. Examples of suitable molecular scaffolds are provided. The invention also relates to methods of synthesizing such libraries and the use of such libraries to identify and characterize molecules of interest from among the library of synthetic test compound.
Owner:AVENTIS PHARMA INC
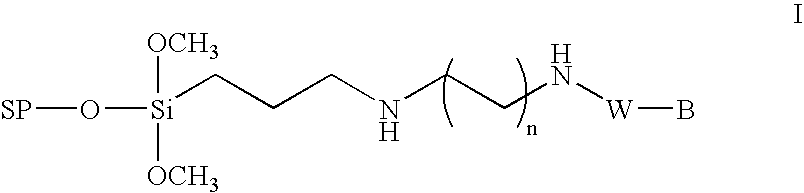
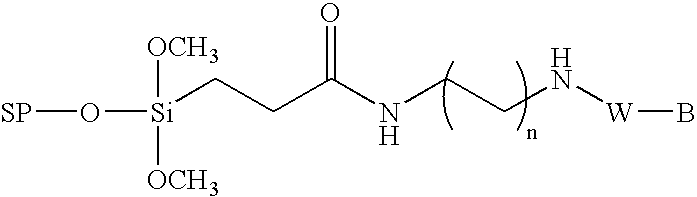
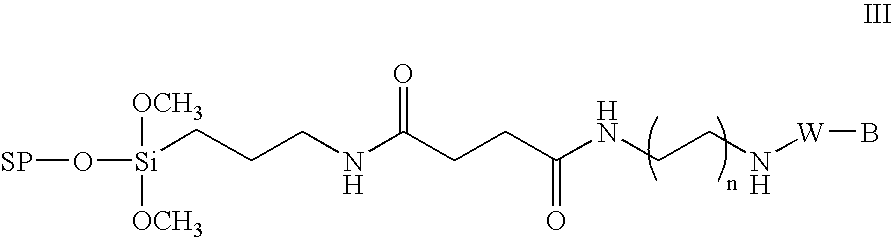
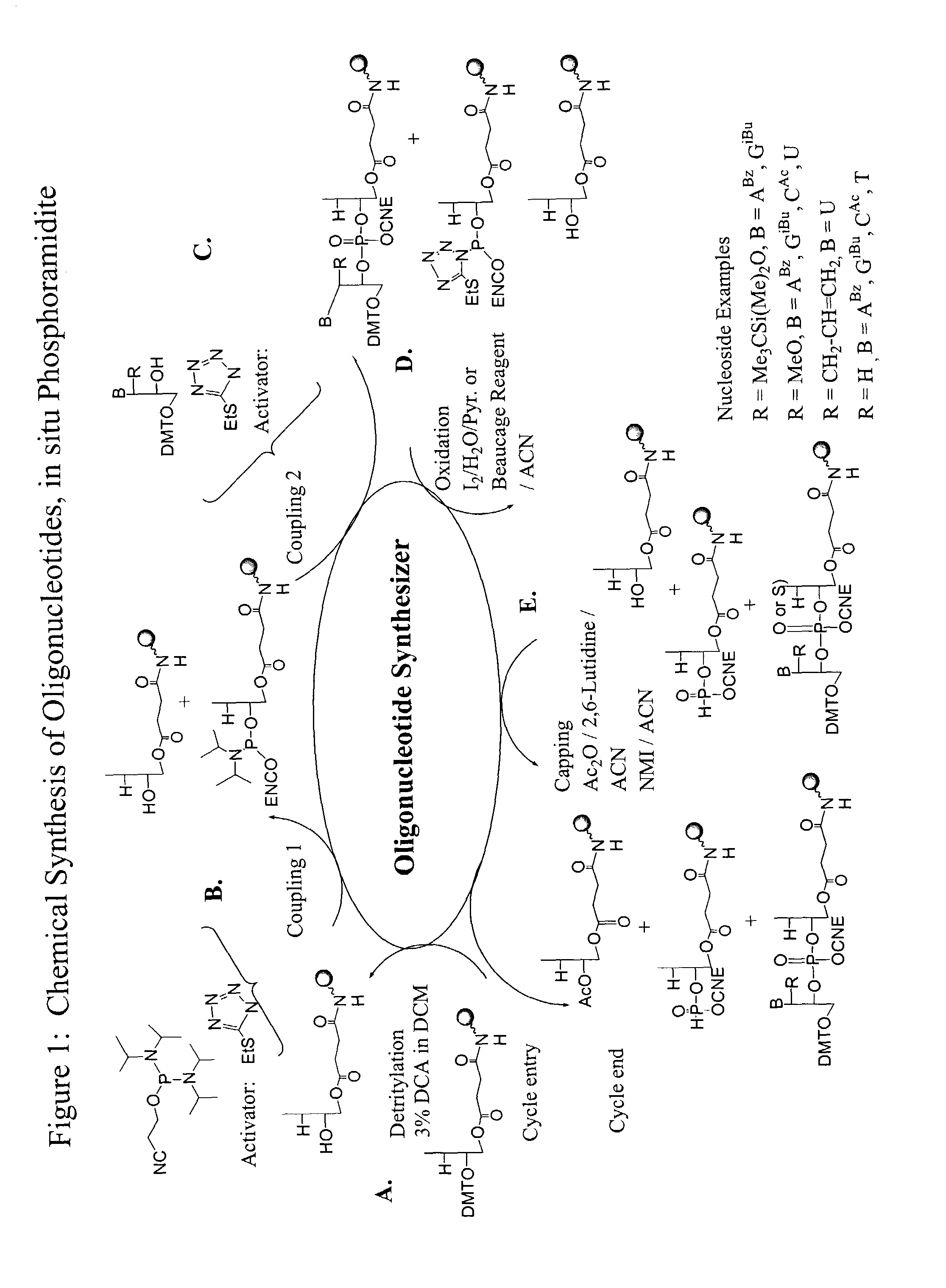
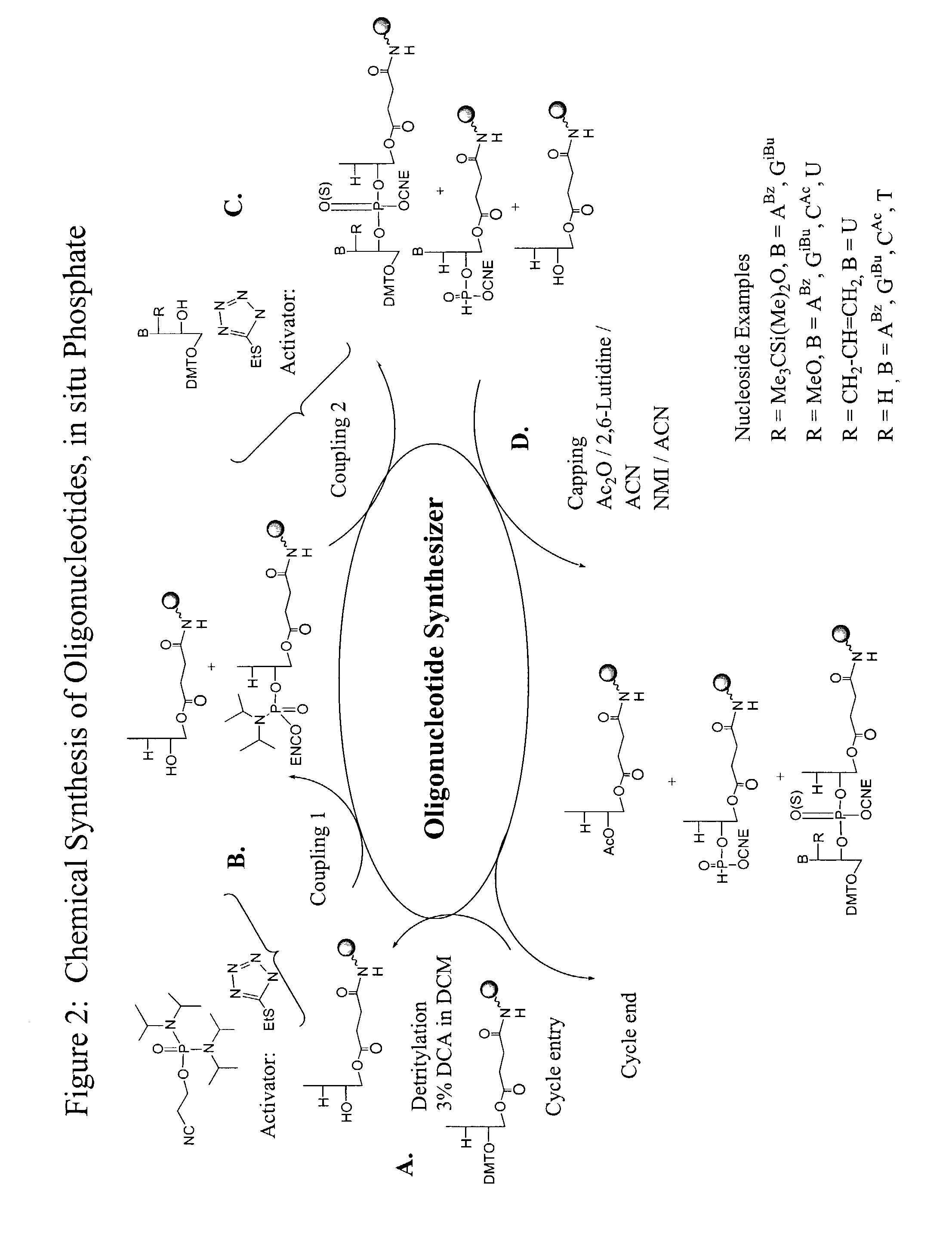
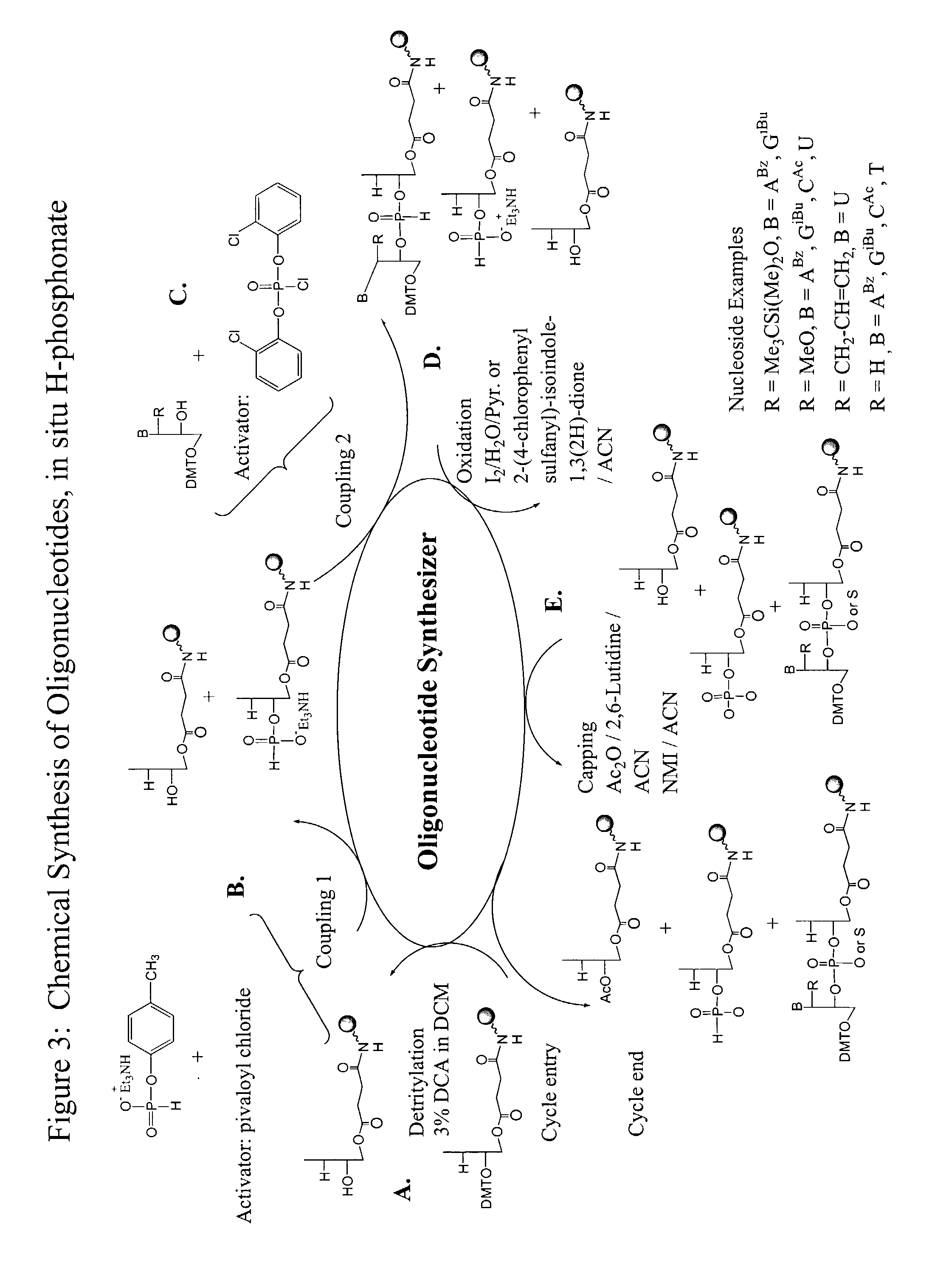
Method for the chemical synthesis of oligonucleotides
InactiveUS6995259B1High yieldHigh purityGroup 4/14 element organic compoundsSugar derivativesChemical synthesisCombinatorial chemistry
Owner:SIRNA THERAPEUTICS INC
Methods and reagents for oligonucleotide synthesis
InactiveUS7205399B1Avoid insufficient lengthSufficient hybridizationSugar derivativesCombinatorial chemistryOligonucleotide synthesis
Owner:SIRNA THERAPEUTICS INC
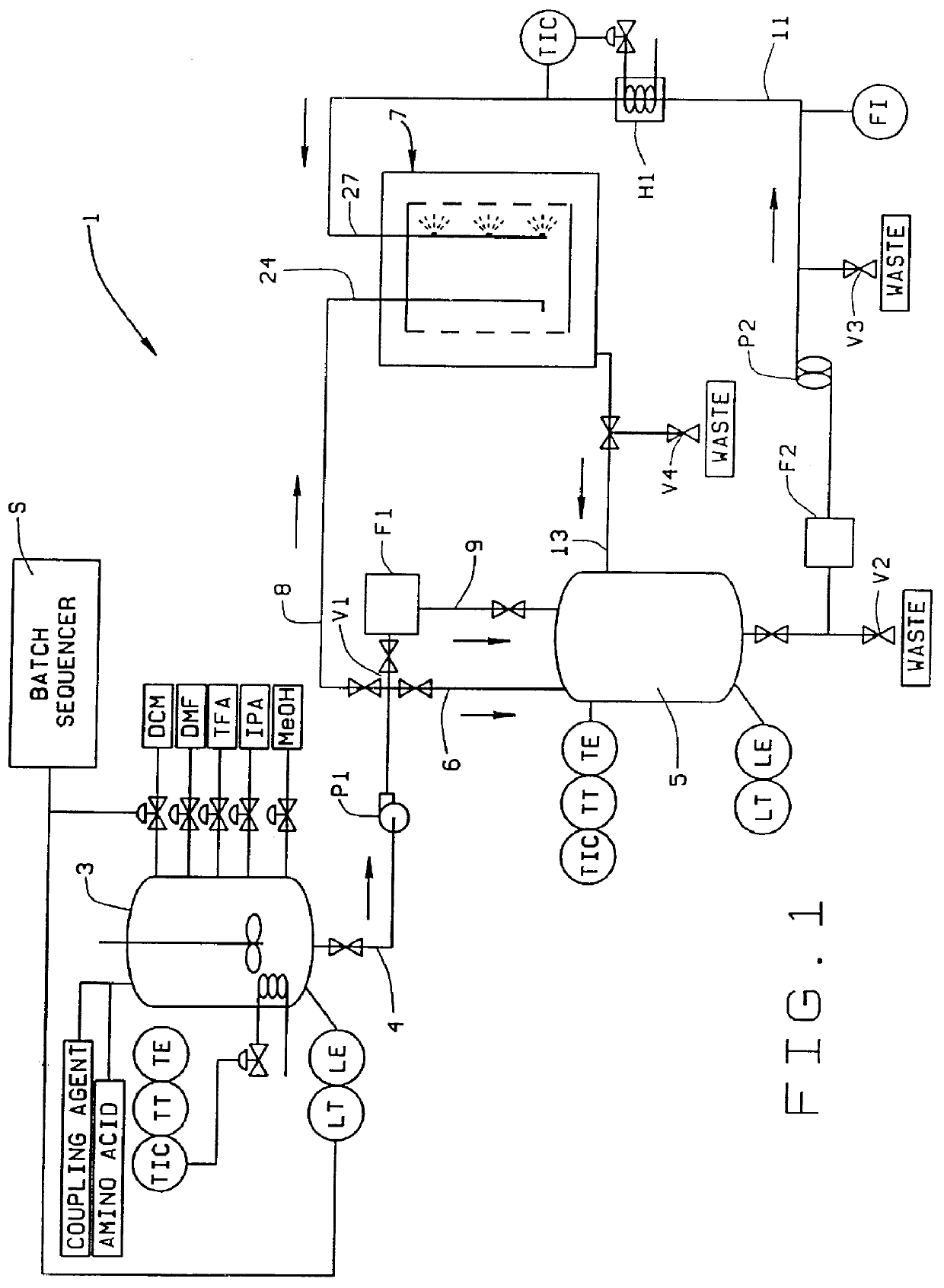
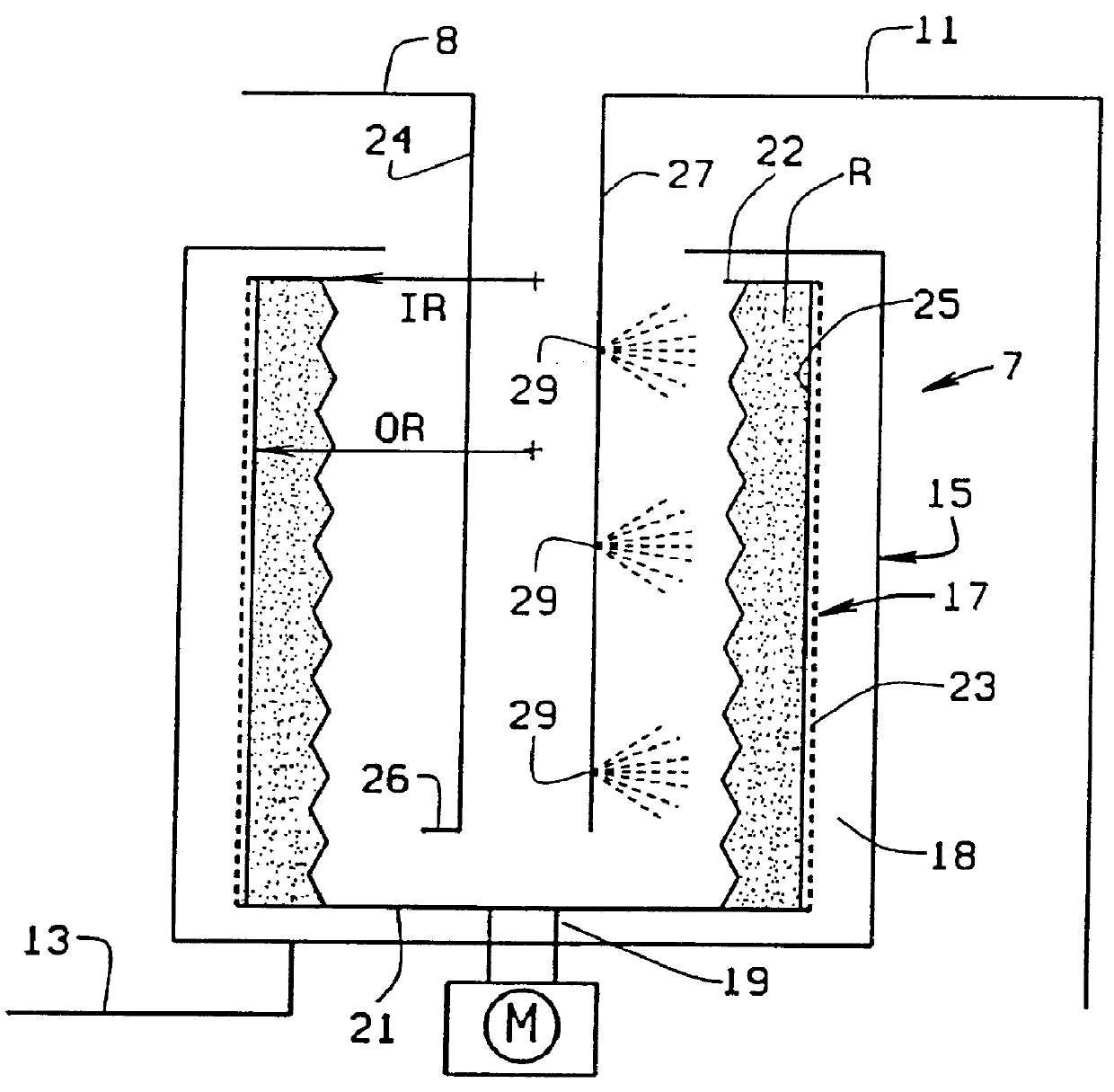
Reactor and method for solid phase peptide synthesis
InactiveUS6028172AIncrease productionHigh yieldPeptide librariesSequential/parallel process reactionsReactor systemEngineering
PCT No. PCT / US98 / 02634 Sec. 371 Date Mar. 9, 1999 Sec. 102(e) Date Mar. 9, 1999 PCT Filed Feb. 10, 1998 PCT Pub. No. WO98 / 34633 PCT Pub. Date Aug. 13, 1998A solid phase peptide synthesis reactor system and method of operating the reactor are provided. The reactor system includes a basket rotatable about an axis within a housing and a receiver which delivers fluid to, and collects fluid from, the housing. The basket has a perforate side wall against which a resin cake for the peptide synthesis is formed. The reactor and receiver form a loop or circuit through which solutions are circulated. The circulation of the solutions prevents the reactor from flooding so that the basket will not be submerged in solution and allows for the use of less liquid. Thus greater amino acid concentrations may be used. The method includes forming a resin cake of uniform depth on the wall of the spinning basket and spraying the solutions against the resin cake while spinning the basket. The solutions will pass through the resin cake and drain to the receiver to be circulated or recycled through the system or discharged from the system. Before a subsequent solution is introduced into the reactor system, the prior solution is purged from the system to help control exposure time of the peptide to the solutions.
Owner:MALLINCKRODT INC
Solid phase synthesis of biomolecule conjugates
InactiveUS20040038331A1Low costImprove efficiencySugar derivativesPeptide preparation methodsWAS PROTEINNucleotide
Processes for the solid state phase formation synthesis of biomolecule conjugates, particularly protein-oligonucleotide conjugates are shown. One of the protein or oligonucleotide is reversibly bound to a solid substrate phase. At least one portion of each of the protein and the oligonucleotide molecules is activated with complementary activation groups. The activated protein and the activated oligonucleotide are then reacted, in a buffered solution resulting in the formation of the desired conjugate which remains reversibly bound to the substrate. The nature of the buffered solution is then modified causing the conjugate to be released from the substrate solid phase.
Owner:BECKMAN COULTER INC
Method for synthesizing dioxalate group lithium borate
InactiveCN1687081AImprove high temperature cycle performanceInhibition of electrochemical co-intercalationGroup 3/13 element organic compoundsChemical reactionSynthesis methods
Owner:SHANDONG HIYI CHEM TECH
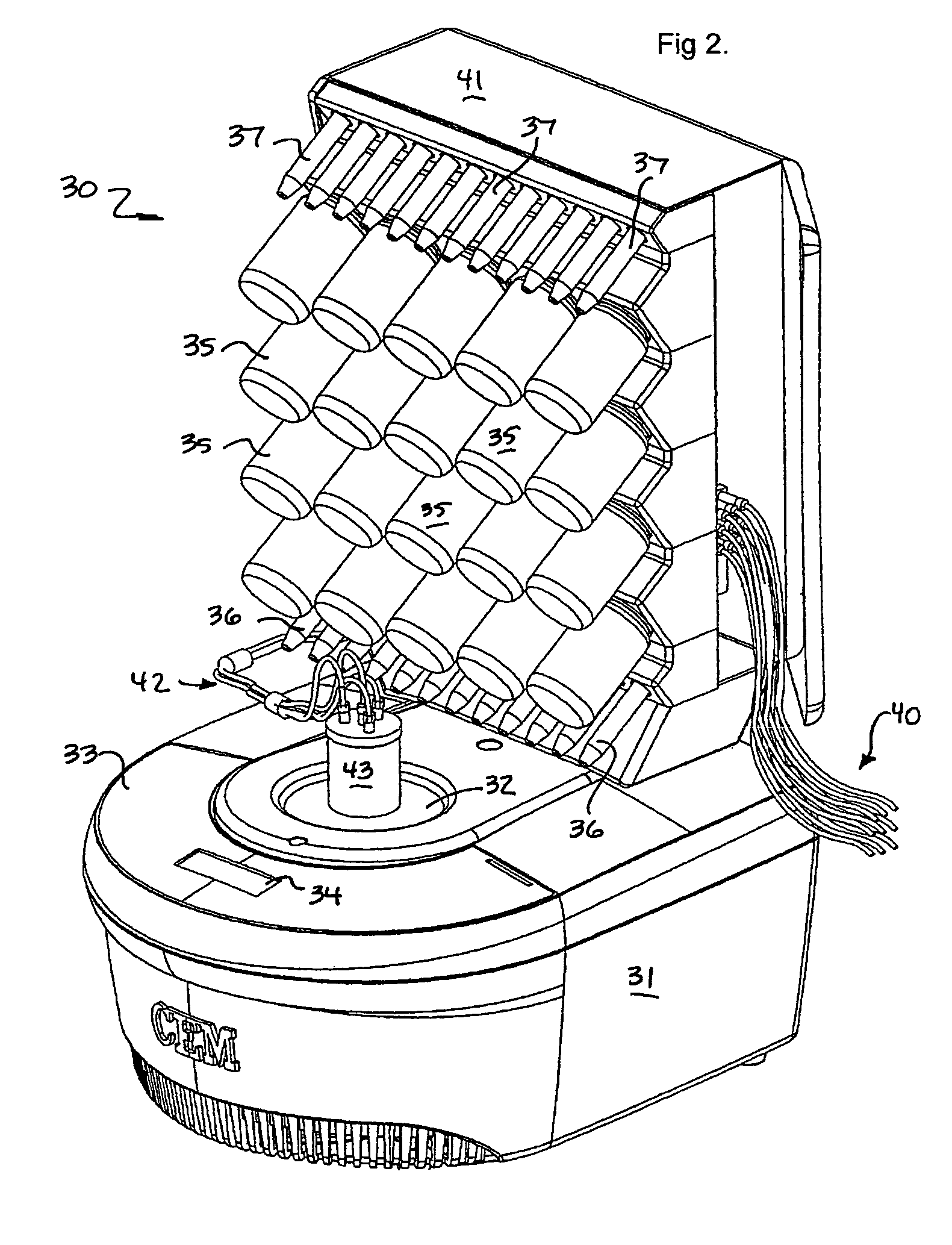
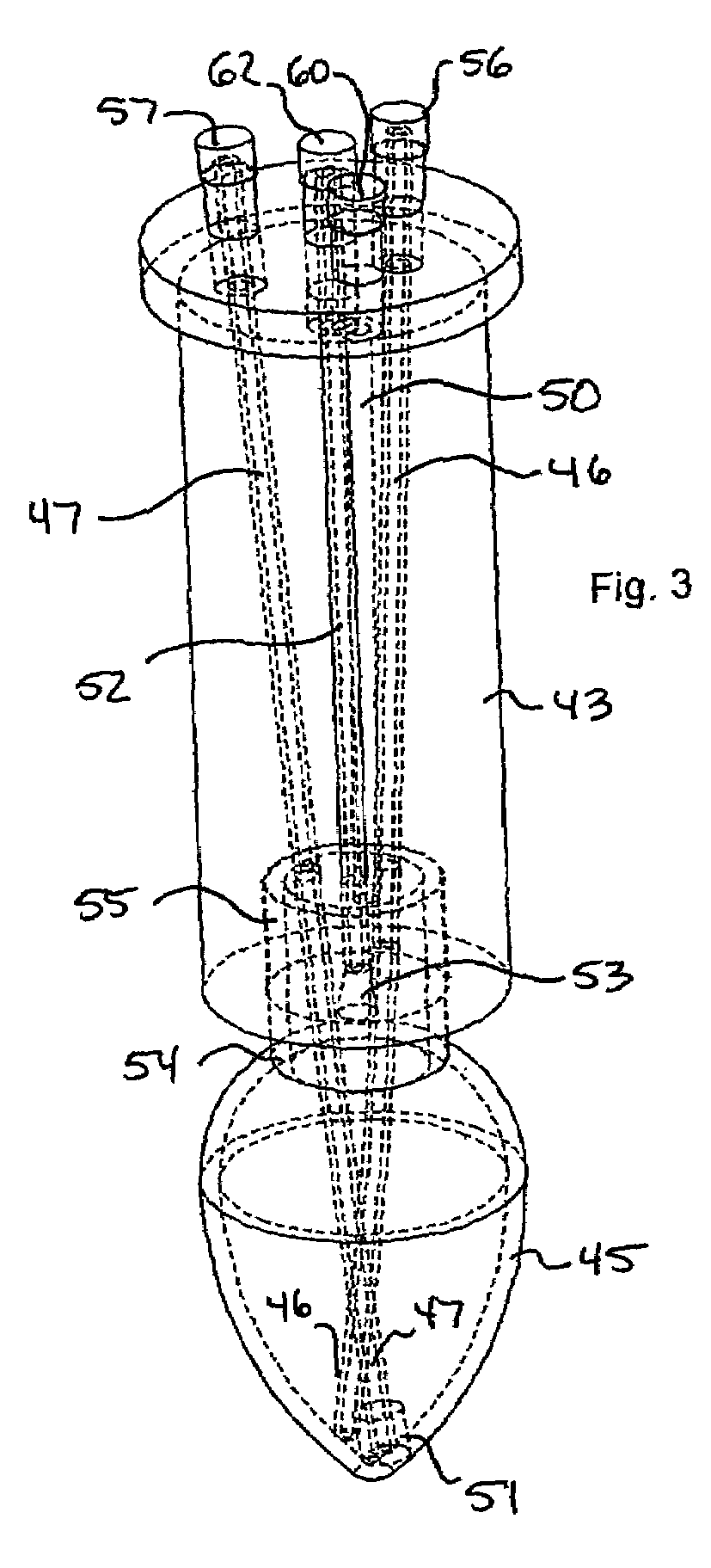
Microwave-assisted peptide synthesis
ActiveUS7393920B2Peptide/protein ingredientsPeptide preparation methodsCombinatorial chemistrySolid-phase synthesis
An instrument and process for accelerating the solid phase synthesis of peptides is disclosed. The method includes the steps of deprotecting a protected first amino acid linked to a solid phase resin by admixing the protected linked acid with a deprotecting solution in a microwave transparent vessel while irradiating the admixed acid and solution with microwaves, then activating a second amino acid by adding the second acid and an activating solution to the same vessel while irradiating the vessel with microwaves, then coupling the second amino acid to the first acid while irradiating the composition in the same vessel with microwaves, and cleaving the linked peptide from the solid phase resin by admixing the linked peptide with a cleaving composition in the same vessel while irradiating the composition with microwaves.
Owner:CEM CORP
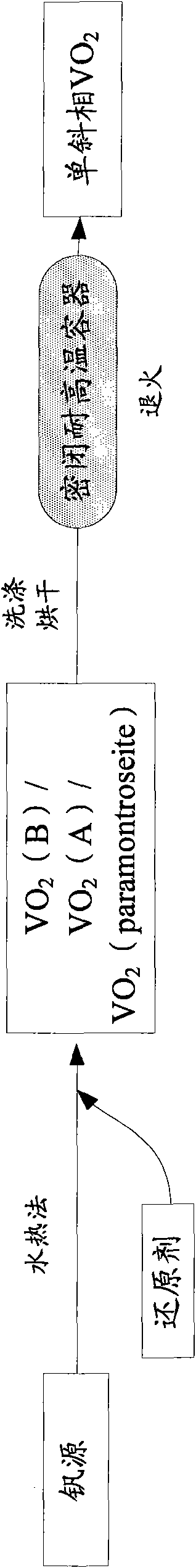
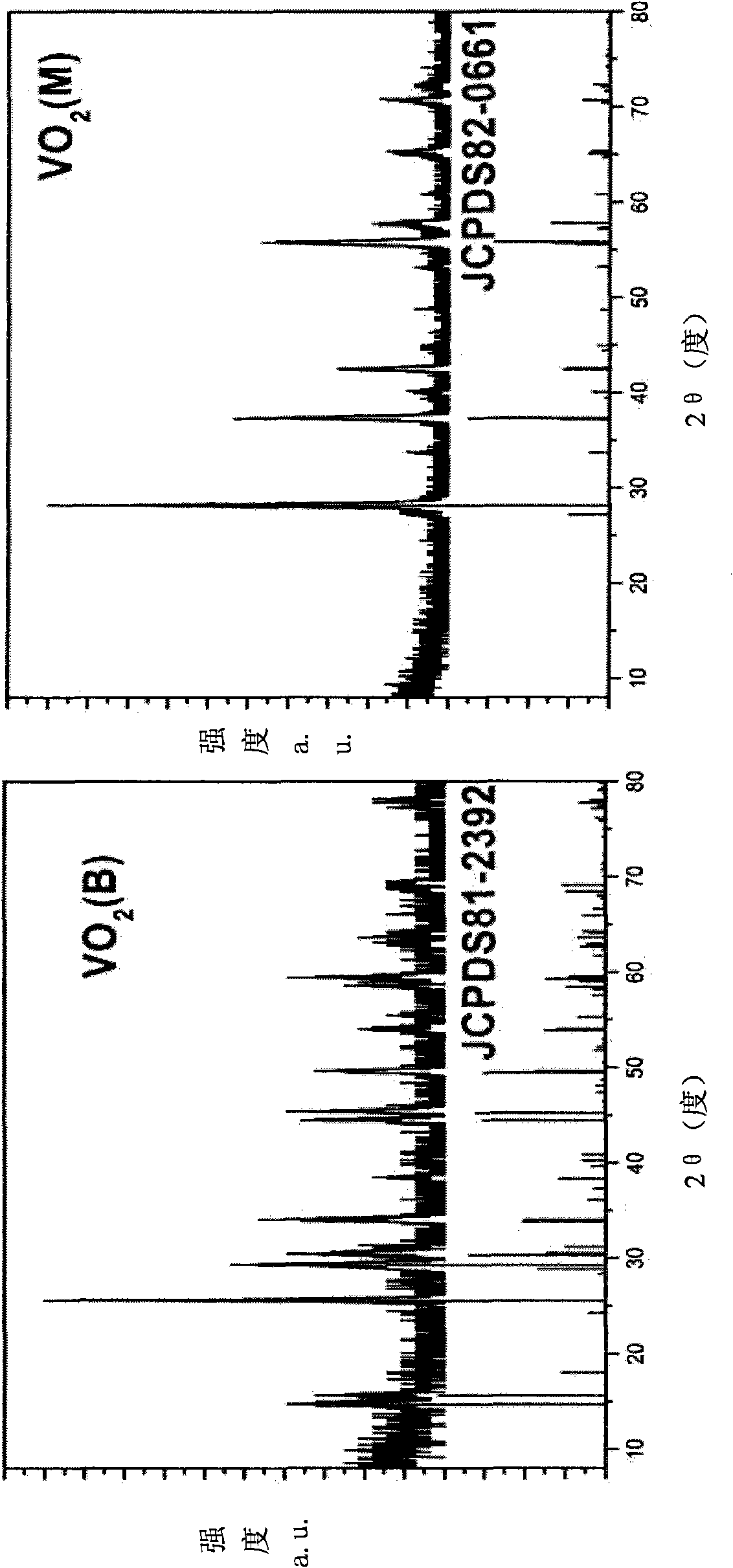
Method for preparing monoclinic phase vanadium dioxide and doped nano powder thereof
InactiveCN101863511ASuperior intelligent infrared controllabilitySave raw materialsVanadium oxidesVanadium dioxideSolid-phase synthesis
The invention provides a method for preparing nano powder of monoclinic phase vanadium dioxide by using a hydrothermal-high temperature solid phase synthesis method. The method comprises the following steps of: a) reacting a vanadium source with a reducing agent to obtain vanadium dioxide (VO2)(B), VO2(A) or VO2 (paramontroseite) by using a hydrothermal method; b) washing the VO2(B), the VO2(A) or the VO2 (paramontroseite) and then thoroughly drying the VO2(B), the VO2(A) or the VO2 (paramontroseite) to form powder; c) vacuum-packaging the powder of the VO2(B), the VO2(A) or the VO2 (paramontroseite) in an airtight and high-temperature resistant container; and d) annealing the container at the temperature of between 350 and 1,200 DEG C for over 3 hours to obtain the nano powder of the monoclinic phase vanadium dioxide. The method has the characteristics of simple production technology, low production cost, environmental friendliness, higher product yield, higher product purity and easy industrial mass production.
Owner:UNIV OF SCI & TECH OF CHINA
Synthesis method of semaglutide
ActiveCN106749613ALow costReduce the number of generatedPeptide preparation methodsBulk chemical productionSynthesis methodsPeptide sequence
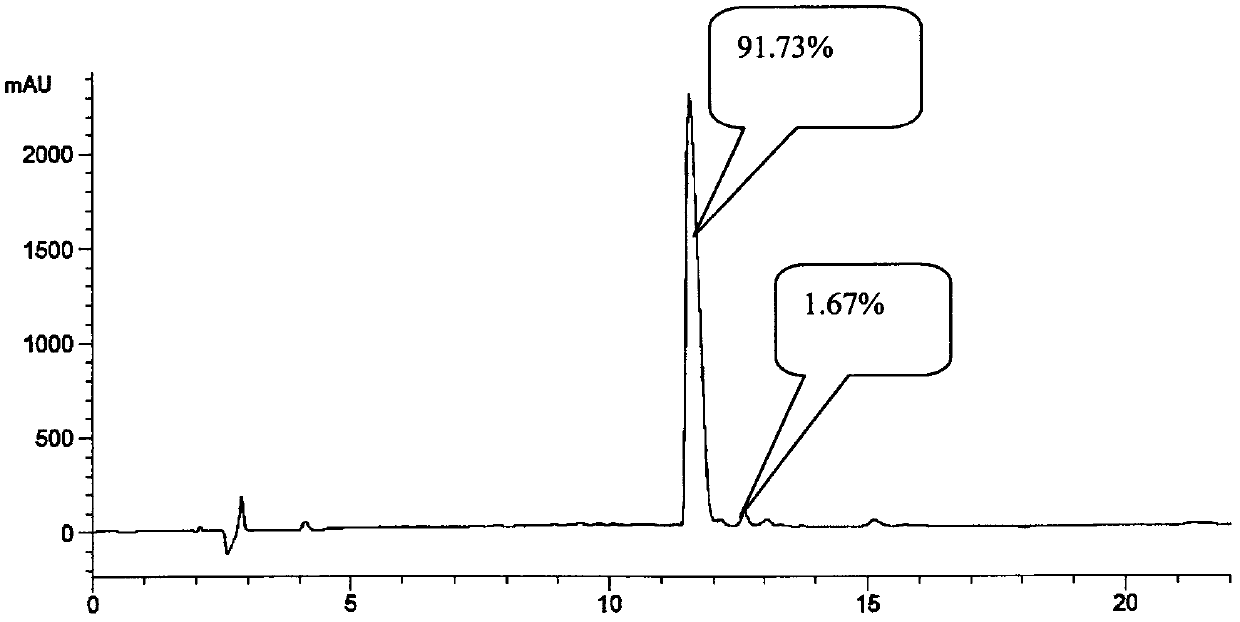
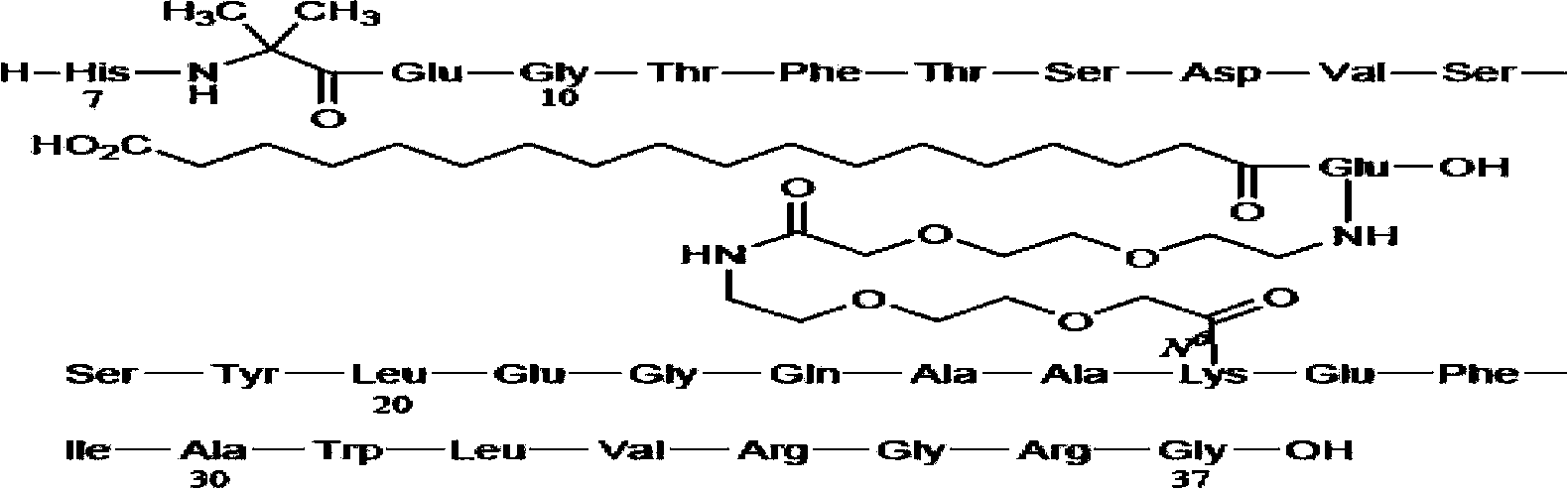
The invention relates to a synthesis method of semaglutide. According to the method, a semaglutide product is synthesized by adopting a solid-liquid phase combination method, and three fragments are simultaneously synthesized in a synthesis manner of 16+6+9 fragments, and therefore, the synthesis time of the product is greatly shortened; moreover, by step-by-step analysis on synthesis factors of His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly, Gln-Ala-Ala-N6-[N-(17-carboxy-1-oxoheptadecyl-L-gama-glutamyl [2-(2-aminoethoxy) ethoxy] acetyl [2-(2-aminoethoxy) ethoxy] acetyl]-Lys-Glu-Phe, Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH and the like, the difficulty in synthesis of a peptide sequence in solid-phase synthesis is reduced, the problem of batch amplification in the solid-phase synthesis is solved, and the synthesis efficiency is improved; and as liquid-phase fragment synthesis is adopted, the purification difficulty is effectively reduced, and the production cost is greatly lowered. The synthesis method disclosed by the invention has the advantages that the synthesis time can be shortened by 40%, the cost of materials is lowered, the generation quantities of deletion peptide and hybrid peptide are decreased, and the synthesis method is suitable for industrial large-scale production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
High-temperature solid-phase synthesis method of high-purity silicon carbide powder
InactiveCN102701208AReduce intrinsic conductivityReduce nitrogen contentPolycrystalline material growthCarbon compoundsCrucibleNitrogen
The invention relates to a high-temperature solid-phase synthesis method of high-purity silicon carbide powder. The high-temperature solid-phase synthesis method comprises the following procedures of: material compounding: uniformly mixing high-purity Si powder and high-purity C powder according to the molar ratio of (1:1)-(1.5:1); high-vacuum heat treatment: placing high-purity Si and C powder into a crucible, placing the crucible in a heating furnace, vacuumizing a growth chamber of the heating furnace to be below 9*10<-4> Pa, increasing the temperature to 600-1300 DEG C and maintaining the temperature for more than 2 h; inert gas cleaning: charging a high-purity inert gas under first specified pressure into the growth chamber, maintaining for more than 1 h, vacuumizing the growth chamber to be below 9*10<-3> Pa once again, and repeating the procedure more than twice; and high-temperature synthesis: in the present of high-purity inert gas under a second specified pressure, reacting for more than 2 h at the reaction temperature of 1500-2500 DEG C, and cooling to room temperature to obtain the high-purity silicon carbide powder with nitrogen content below 15 ppm.
Owner:上海上硅中试基地科技有限公司 +1
Linaclotide synthesis method
ActiveCN102875655AAvoid impuritiesHigh purityPeptide preparation methodsBulk chemical productionOxidized GlutathioneSide chain
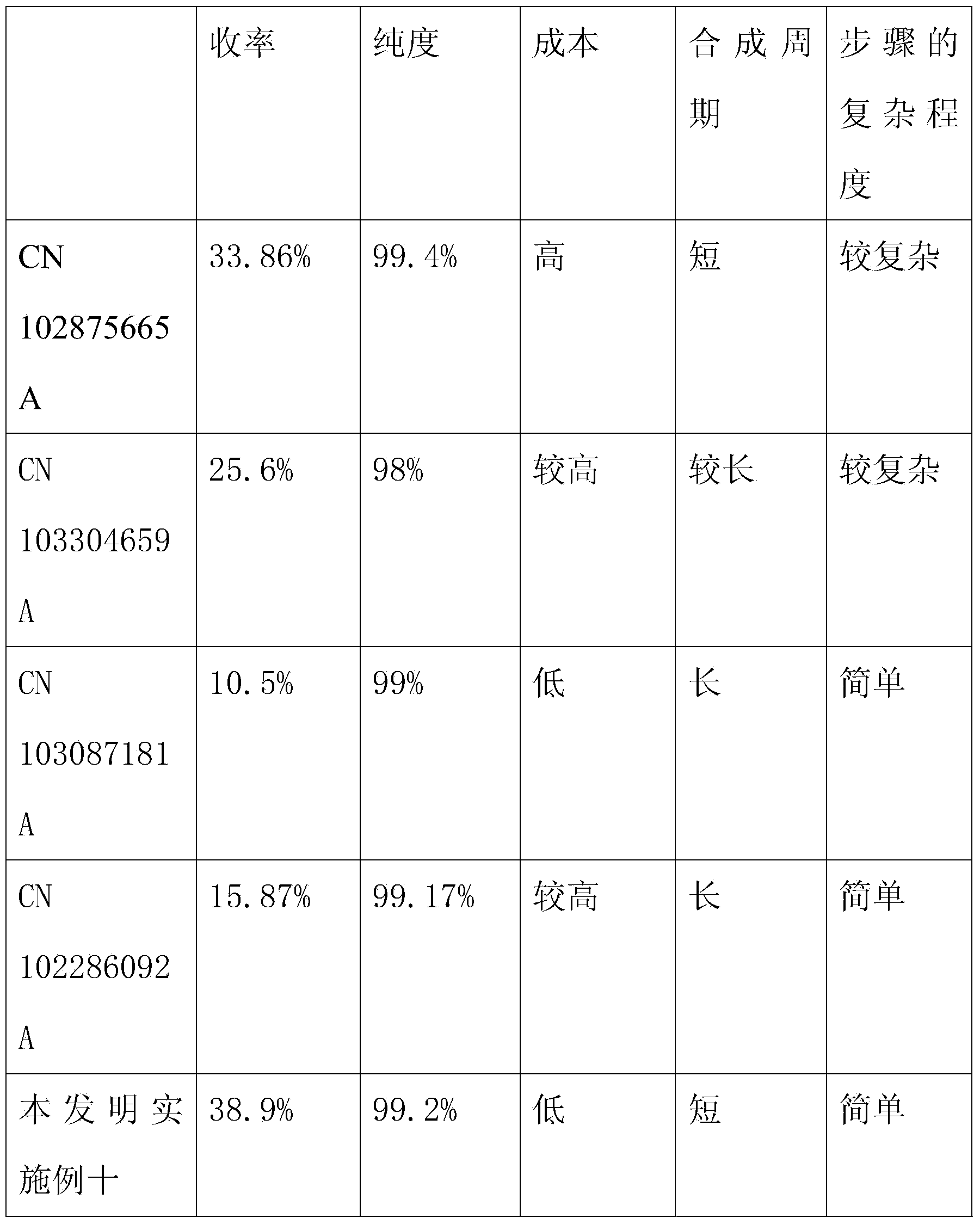
The invention relates to the field of pharmaceutical synthesis and discloses a linaclotide synthesis method. The linaclotide synthesis method includes: performing solid-phase synthesis to obtain linaclotide resin with an N terminal, a Thr side chain, a Cys side chain, an Asn side chain, a Tyr side chain and a Glu side chain of an amino acid sequence shown in SEQ ID NO:1 coupled with protecting groups and with a C terminal coupled with a resin solid-phase carrier, cracking to remove the protecting groups and the resin solid-phase carrier prior to carrying out oxidizing reaction by the aid of a GSH (glutathione) / GSSH (oxidized glutathione) oxidization system to obtain a crude linaclotide product, and purifying the crude linaclotide product so that linaclotide is obtained. By the method, an Mmt protecting group is used for protecting a cysteine side chain, crude linear linaclotide peptide is synthesized by a one-by-one coupling mode, and the linaclotide is obtained by oxidization by the aid of the GSH / GSSH oxidization system. Compared with existing methods, the linaclotide synthesis method has the advantages that purity of the crude linear peptide is improved, the oxidization step can be performed without purification, and purity and yield of the crude linaclotide product are remarkably improved.
Owner:HYBIO PHARMA
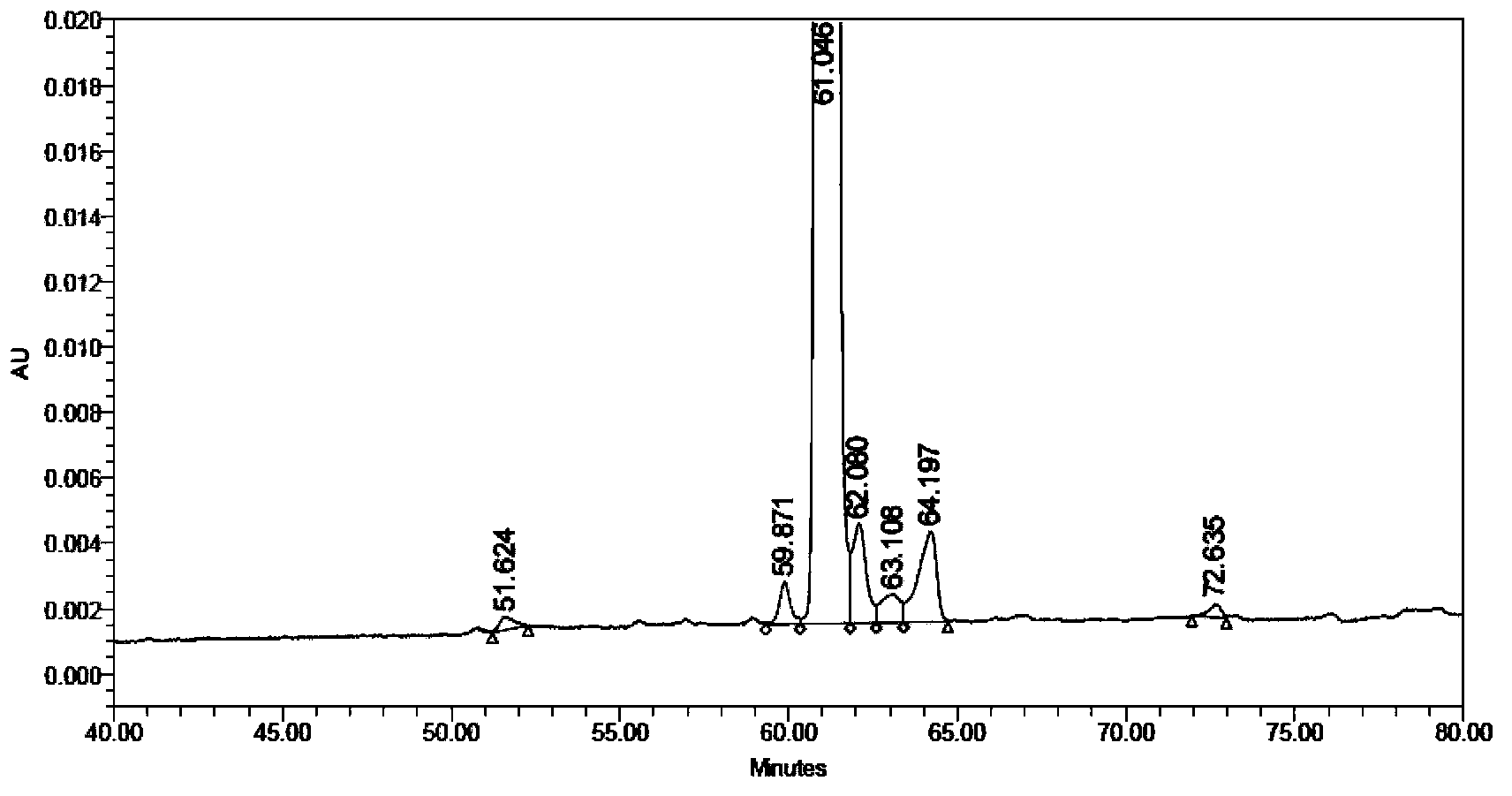
Method for purifying solid-phase synthetic coarse liraglutide
ActiveCN102584982ASolid sorbent liquid separationPeptide preparation methodsAqueous solutionSolid-phase synthesis
The invention relates to the field of biomedicine, in particular to a method for purifying solid-phase synthetic coarse liraglutide. The method comprises the following steps of: dissolving solid-phase synthetic coarse liraglutide into acetonitrile aqueous solution to obtain coarse peptide solution; and purifying through four-step HPLC (High Performance Liquid Chromatography) to obtain the liraglutide. The method has the advantages of high purity and high yield.
Owner:HYBIO PHARMA
Solid-phase synthesis method of Sermaglutide
ActiveCN106478806ASimple processImprove condensation efficiencyPeptide preparation methodsGlucagonsSide chainCombinatorial chemistry
The invention discloses a solid-phase synthesis method of Sermaglutide. The method comprises steps of taking 2-Cl-Resin as an initial resin carrier; through a solid-phase synthesis method, orderly connecting the corresponding amino acids in a Sermaglutide sequence, wherein the lysine takes Dde-Lys (Fmoc)-OH as raw material; after linking the side chain raw material, removing Dde protection base of lysine, and performing continuous condensation reaction of a peptide chain; applying specific microwave technology treatment in a reaction process, and obtaining Sermaglutide-2-Cl-Resin; after cutting and settling Sermaglutide-2-Cl-Resin, freezing and drying, and obtaining crude peptide of Sermaglutide peptide. The method applies Fmoc solid-phase synthesis method, 2-Cl-Resin as solid phase carrier, and DIC / HOBt as the condensating agent, thus the lysine raw material is improved, the technical flow is largely simplified; the specific microwave synthetic technique is applied to the condensation reaction, thus the condensation efficiency is improved; the invention largely shortens the reaction time, improves the product yield, and has considerable economic application value and wide application prospect.
Owner:合肥国肽生物科技有限公司
Solid phase preparation method of carbetocin
ActiveCN101555272AReduce investmentEasy to operatePeptide preparation methodsSexual disorderLithium chlorideFreeze-drying
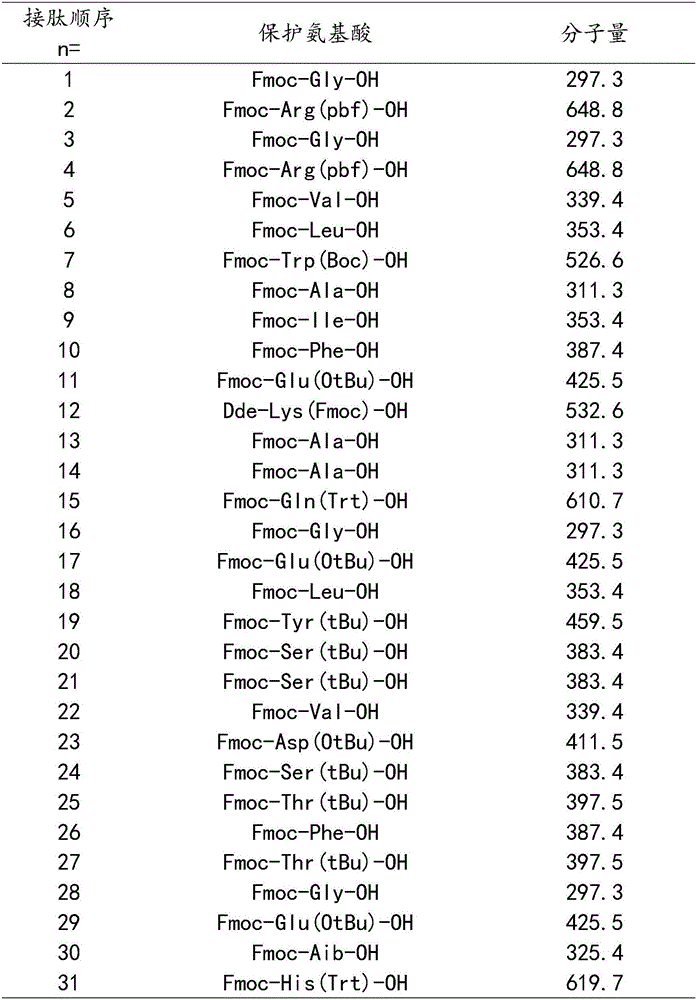
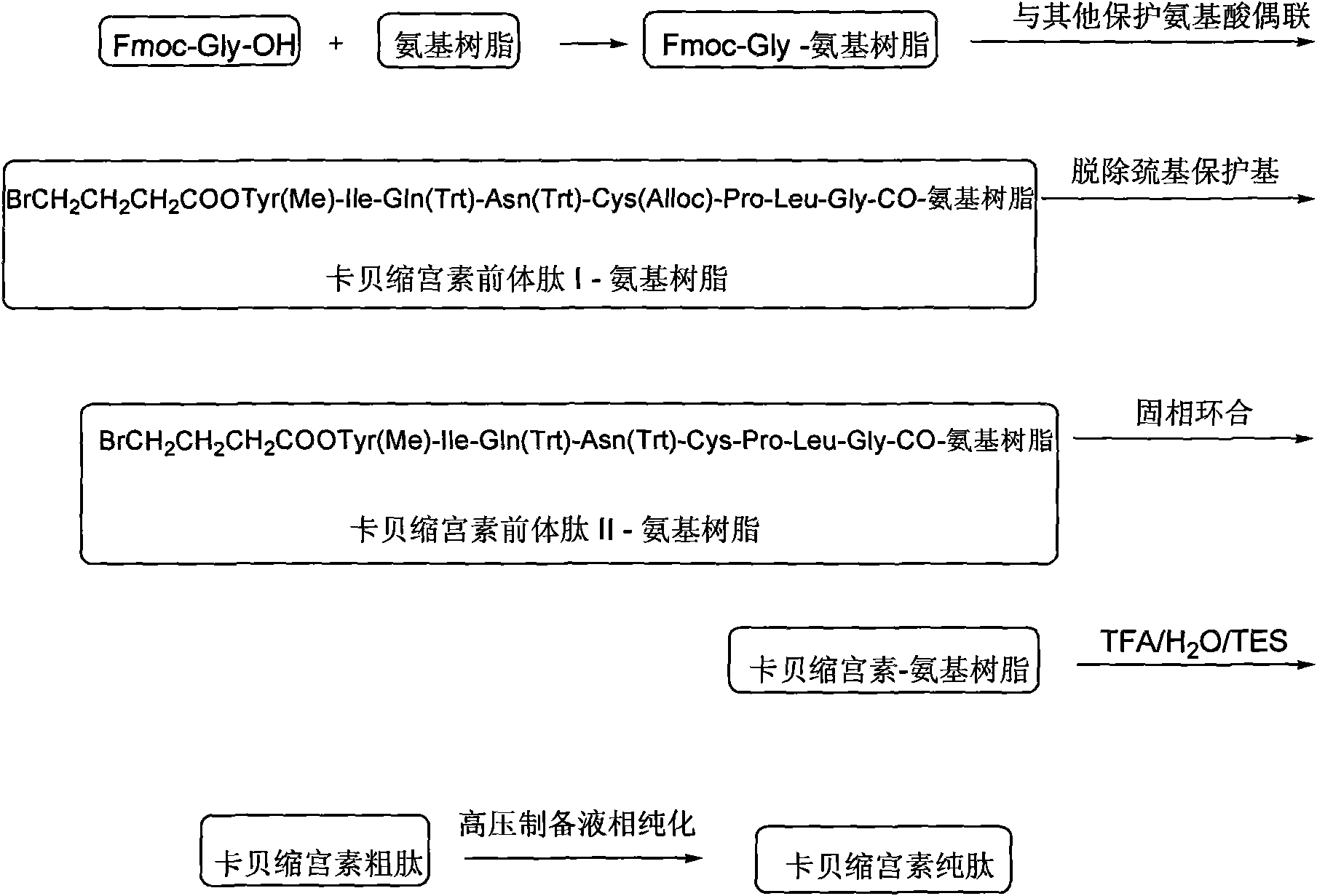
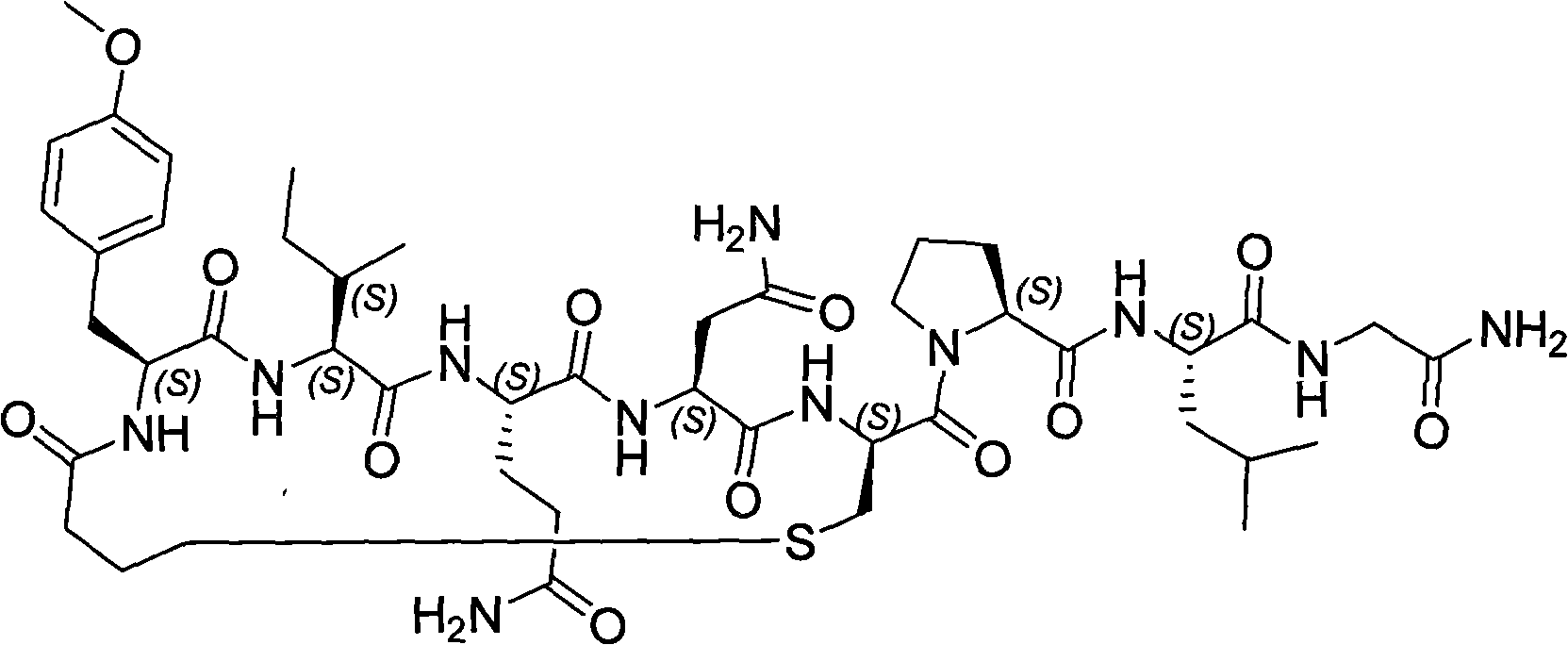
The invention discloses a solid phase synthesis method of carbetocin. The technical proposal comprises the following steps of: obtaining Fmoc-Gly-amino resin by reaction of Fmoc-Gly-OH and amino resin with the substitutability being 0.2 mmol / g-0.9 mmol / g; sequentially connecting amino acids with Fmoc protecting groups by the solid phase synthesis method to obtain carbetocin precursor peptide I-amino resin; stripping off cysteine side chain protecting groups to obtain carbetocin precursor peptide II-amino resin; adding organic alkali and lithium chloride in solvent for cyclization to obtain carbetocin-amino resin; cracking to obtain carbetocin crude peptide; and purifying and freeze-drying to obtain the carbetocin. The method adopts the amino resin to synthesize carbetocin by the solid phase cyclization technology. The process is characterized by simple operation, easy post-treatment, high yield, low cost, and the like, and has considerable economical and practical value and broad application prospect.
Owner:HYBIO PHARMA
Method for the manufacture of amino group containing support matrices, support matrices prepared by the method, and use of the support matrices
InactiveUS6335438B1Simple methodImprove availabilityCatalyst carriersSugar derivativesBackbone chainPolynucleotide
A method for the manufacture of a support matrix exhibiting amino groups I, possibly in acylated form, comprising the step of polymerizing one or more monovinyl monomers (monomer I) with one or more di- tri- or polyvinyl monomers (monomer II). The method is characterized in that that the polymerization is run in the presence of one or more amino-(C0-10)hydrocarbon vinyl aromatic monomers possibly in acylated form (monomer III). The preferred combination of monomers are ethyl vinyl benzene, divinyl benzene and amino styrene. The support matrix comprising a polyvinyl backbone [(-CH2CH2)n] and amino groups or groups derived from said amino groups while retaining the nitrogen of the amino group, each of which groups being attached to said backbone via a link structure containing an arylene group. The matrix is characterized in that each of said groups is directly attached via said nitrogen to said arylene group. Additionally, the use of the support matrix for solid phase synthesis, for instance of an oligo / poly-nucleotide or an oligo / polypeptide.
Owner:LIFE TECH AS
Solid-phase synthesis method for liraglutide
ActiveCN103864918AShort synthesis cycleImprove efficiencyPeptide preparation methodsBulk chemical productionSynthesis methodsSide chain
The invention discloses a solid-phase synthesis method for liraglutide. The solid-phase synthesis method for liraglutide comprises the following steps: 1) firstly synthesizing first to tenth amino acid segments 3, eleventh to nineteenth amino acid segments 2, and twentieth to thirty-first amino acid segments 1, wherein the twentieth lysine adopts Fmoc-Lys(Mtt)-OH, and the first histidine adopts Boc-His(Trt)-OH; 2) synthesizing pal-Glu(OH)-Otbu by adopting a liquid phase synthesis method; 3) selectively removing the Mtt protecting group on the twentieth lysine by use of 5% TFA (Trifluoroacetic Acid), connecting pal-Glu(OH)-Otbu with the side chain of the twentieth lysine to obtain a segment 4; 4) sequentially connecting the segments 2, the segments 3 and the segments 4 to obtain peptide resin completely protected by liraglutide; 5) cracking, purifying, and lyophilizing to obtain the liraglutide product.
Owner:哈尔滨吉象隆生物技术有限公司
Synthesis of cyclic peptides
InactiveUS7589170B1Facilitate cyclisation reactionImprove responseNervous disorderAntipyreticCyclic peptideSolid-phase synthesis
This invention relates to methods for preparing cyclic peptides and peptidomimetic compounds in solution and bound to solid supports, and to cyclic peptide or peptidomimetic libraries for use in drug screening programs. In particular, the invention relates to a generic strategy for synthesis of cyclic peptides or peptidomimetics that enables the efficient synthesis under mild conditions of a wide variety of desired compounds. Two approaches were evaluated for their improvements in solution and solid phase synthesis of small cyclic peptides: positioning reversible N-amide substituents in the sequence; and applying native ligation chemistry in an intramolecular sense. Systematic investigation of the effects of preorganising peptides prior to cyclisation by using peptide cyclisation auxiliaries, and developing new linkers and peptide cyclisation auxiliaries to aid cyclic peptide synthesis gives surprising improvements in both yields and purity of products compared to the prior art methods. The combination of these technologies provides a powerful generic approach for the solution and solid phase synthesis of small cyclic peptides. The ring contraction and N-amide substitution technology of the invention provide improved methods for the synthesis of cyclic peptides and peptidomimetics. When used in conjunction with linker strategies, this combination provides solid-phase avenues to cyclic peptides and peptidomimetics.
Owner:QUEENSLAND THE UNIV OF
Preparation method of lithium transition metal oxide
InactiveCN1493522ASimple preparation processFacilitated DiffusionOxide/hydroxide preparationCell electrodesReaction speedComposite oxide
A process for preparing the composite oxide of Li and transition metals in order to use is as positive electrode of rechargeable Li or Li-ion battery features that the oxides, hydroxides or salts of Li and transition metal (Co, Ni and Mn) are used as raw materials, and the oxide, hydroxide or salt of Co, Ni, Mn, Cr, Al, or Mg is used as doping element, and includes mixing them with fusable salt, heating, constant-temp calcining, cooling, watshing to remove residual salt and baking. Its advantages are high reaction speed, and high specific capacity of product.
Owner:TSINGHUA UNIV
Solid-phase synthetic method of highly ordered mesoporous carbon material
InactiveCN103964414AIncrease the areaHigh pore volumeCarbon preparation/purificationPore diameterSelf-assembly
The invention discloses a solid-phase synthetic method of a highly ordered mesoporous carbon material. The solid-phase synthetic method comprises the following steps: 1) mixing a structure-directing agent with a high molecular monomer used as a carbon source, and grinding for 5-180 min at 10-100 DEG C; 2), heating for 0.5-120 h at 40-380 DEG C; 3) under the protection of inert gases, rising the temperature to 500-2,100 DEG C at the temperature rising speed of 1-40 DEG C / min, and roasting for 2-10 h at high temperature. The functionalization mesoporous carbon material is synthetized through the organic-organic self-assembly between the high molecular monomer and the structure-directing agent and between the low polymer of the high molecular monomer and the structure-directing agent, and the mechanical grinding. The solid-phase synthetic method is simple to operate, efficient, and low in cost, and the prepared mesoporous carbon material has a highly ordered mesoporous channel, high specific surface area (500-2,500 m<2> / g and large pore volume (pore diameter of 2.5-20 nm and pore volume of 0.1-2.5 cm <3> / g).
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Multiplex polynucleotide synthesis
InactiveUS20060234264A1Solve low usageMicrobiological testing/measurementFermentationChemical treatmentDna polymerasen
The invention provides a method of synthesizing complex mixtures of long polynucleotides by separately synthesizing and assembling shorter component oligonucleotides. In one aspect, pairs of oligonucleotides that form components of such polynucleotides are synthesized on one or more microarrays, or other large-scale parallel solid phase synthesis platforms, after which they are released. Members of each pair contain unique complementary barcode sequences that are used match-up pairs in a hybridization reaction to form duplexes. Such duplexes are then extended with a DNA polymerase and the resulting extension product is amplified to form an amplicon. The amplicon may be either used directly as the desired polynucleotide, or it may undergo further processing, such as capture on solid phase supports and / or additional enzymatic or chemical processing, to produce a desired polynucleotide product, such as a circularizing probe for multiplex analysis of genomic DNA, or the like.
Owner:AFFYMETRIX INC
Process for solid phase synthesis of lithium iron phosphate anode materials under high pressure
InactiveCN1884053ALow reaction temperatureReduce manufacturing costCell electrodesPhosphorus compoundsAir atmosphereLithium iron phosphate
The invention discloses a synthesizing method of anode material of high-pressure solid-phase ferric-lithium phosphate, which comprises the following steps: blending lithium salt, ferric salt and phosphate according to 1:1:1 proportion evenly; grinding the material into ball for 6-24 h to obtain priority; disposing priority for 2-10 h at 200-350 deg.c in the air environment; cooling naturally; grinding to obtain powder material; adding carbon material in the powder material with weight percentage of carbon material at 1-20 percent; grinding again for 6-24 h; disposing for 4-24 h at 450-1000 deg.c in the 1-15 Mpa inert environment; cooling naturally to obtain the product.
Owner:SOUTH CHINA UNIV OF TECH
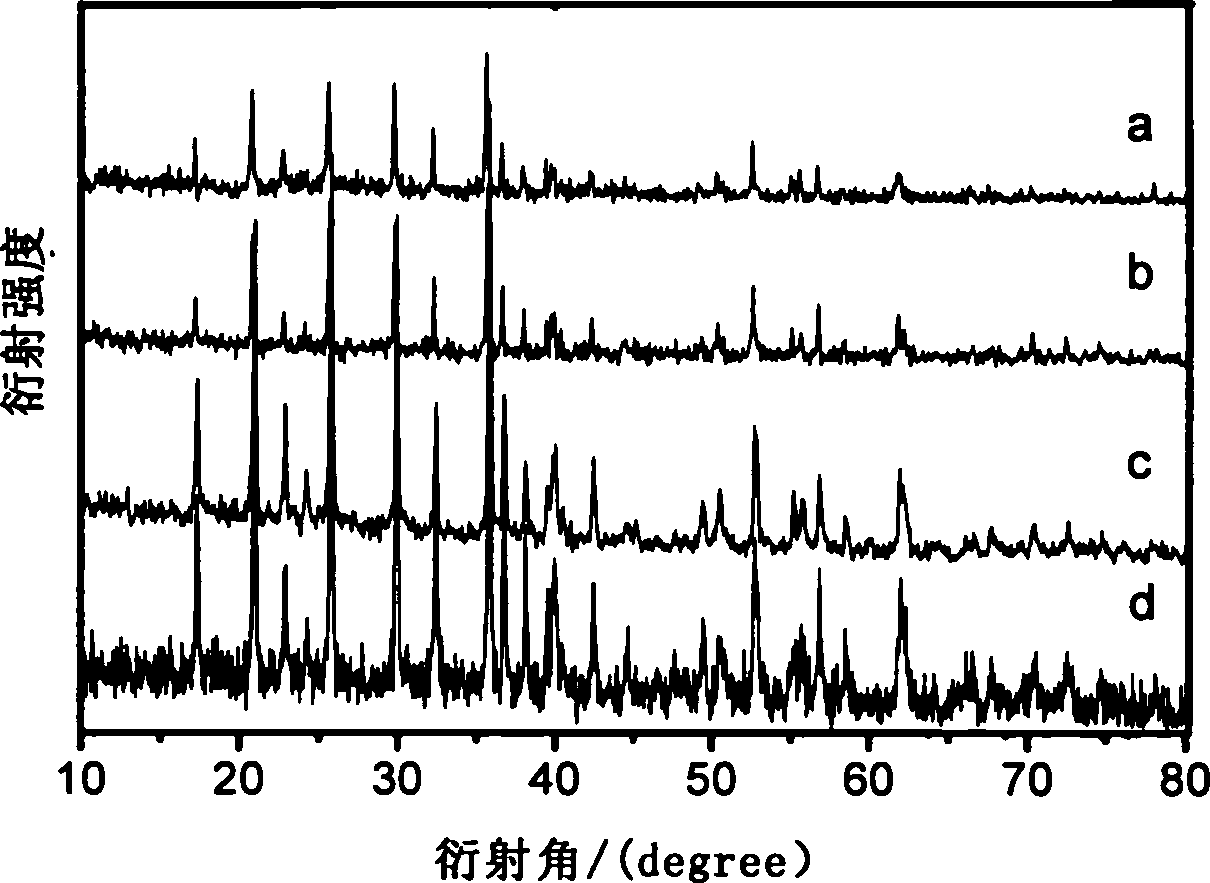
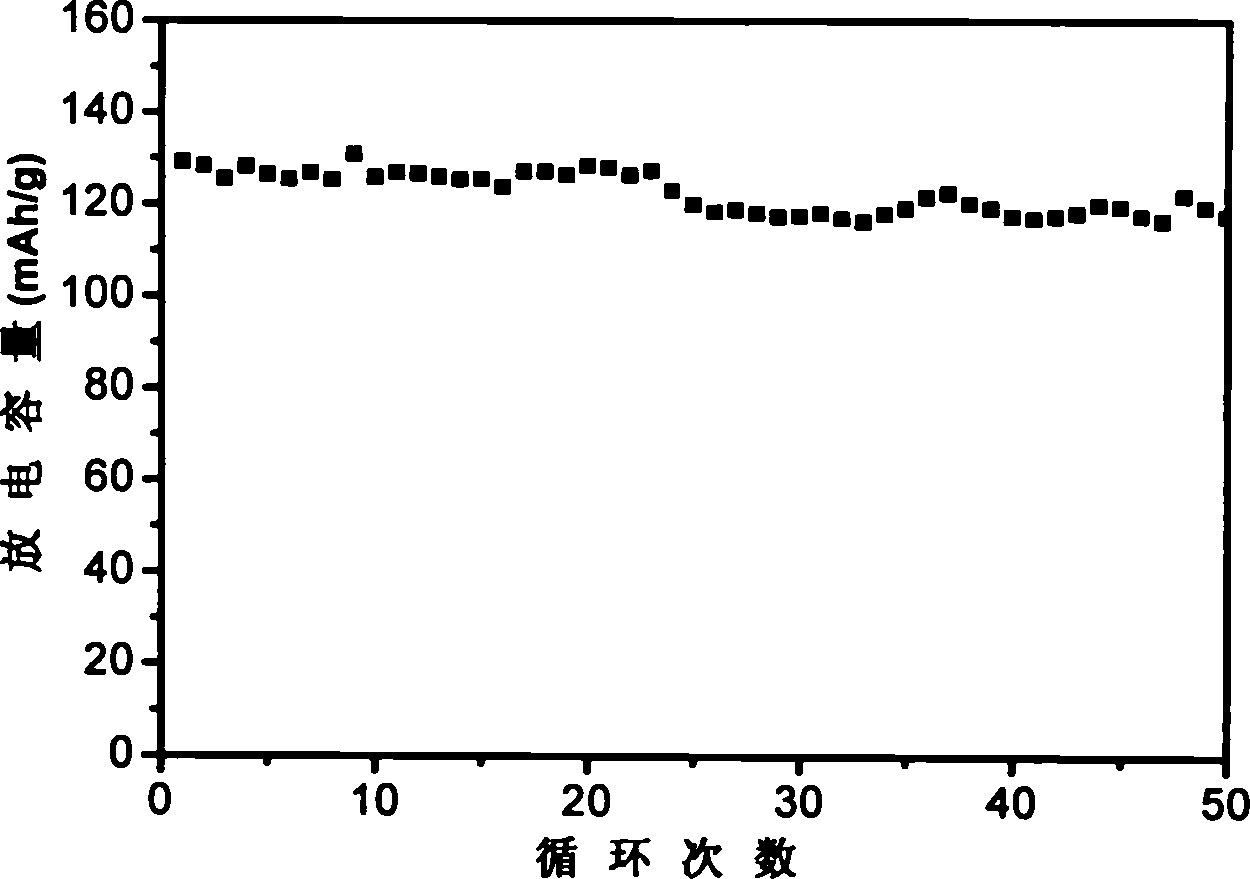
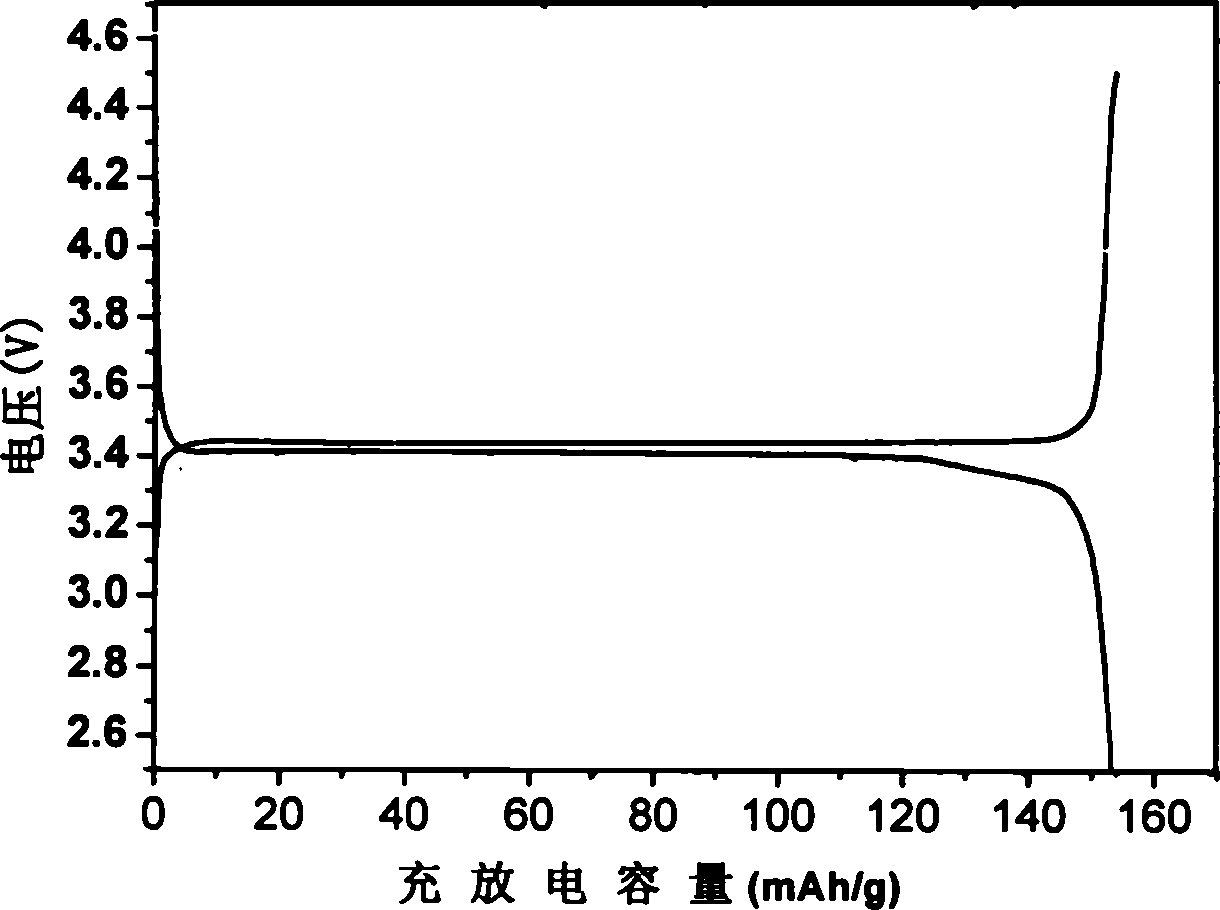
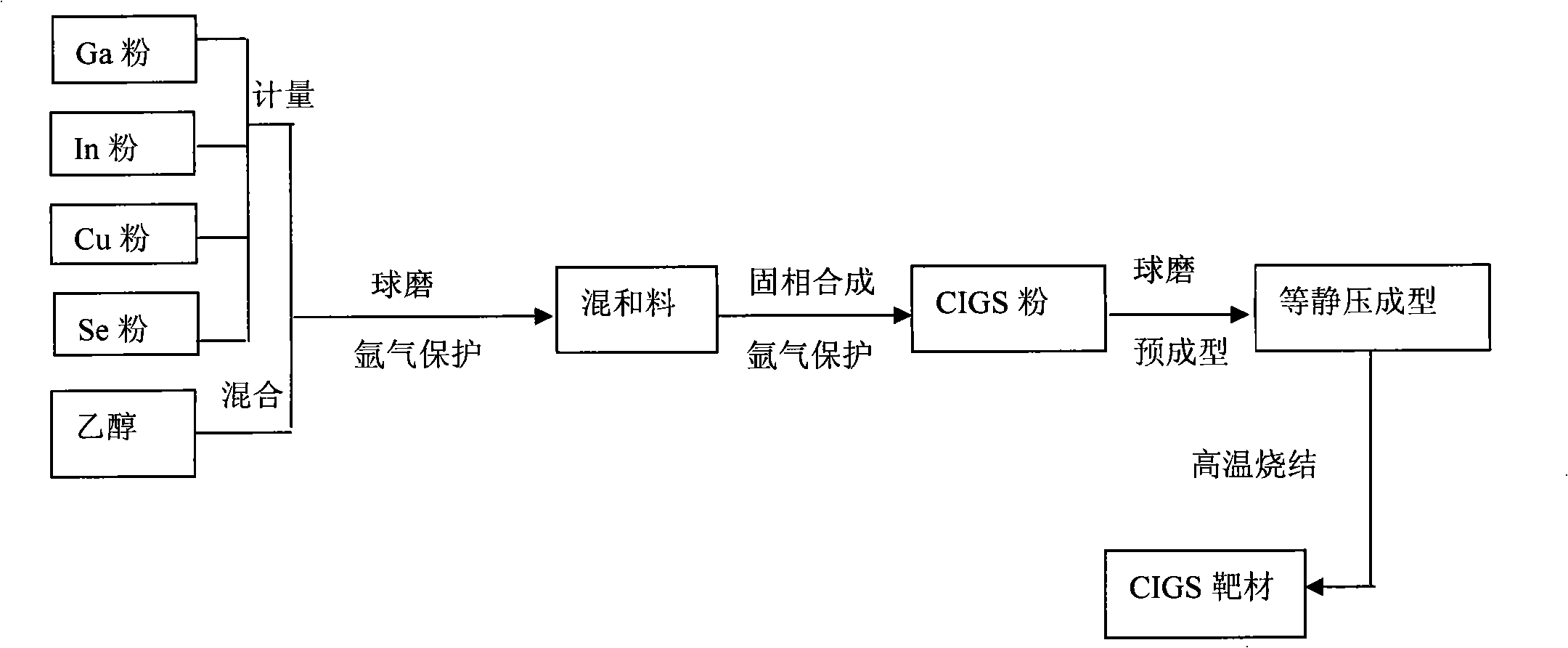
Solar energy battery copper-indium-gallium-selenium film key target material and preparation method thereof
InactiveCN101260513AAvoid quality problemsQuality assuranceVacuum evaporation coatingSputtering coatingIndiumSolar battery
The invention relates to a solar battery cuprum-indium-gallium-selenium film key target material and a preparation method thereof. The target material uses cuprum-indium-gallium-selenium element powders as raw material; CIGS powder is prepared by adopting solid phase synthesis and further isostatic compaction; finally a CIGS target material is prepared by high temperature sintering. The target material is used as raw material and a CIGS film can be achieved by further sputtering. In the invention, the four-element component key target material preparation technology is simplified from 'fractional deposition---selenizing optimization'to'one-step deposition', thereby solving the complex technological process of multiple target replacements and repeated depositions and the quality problem caused by late-stage selenylation; the adjustment of the components is carried out by the target material; the late-stage sputtering technology only needs to ensure the quality of film forming, thereby increasing the controllability of the technology.
Owner:王东生
Synthetic method of liraglutide
ActiveCN103304660AHigh selectivityHigh purityPeptide preparation methodsBulk chemical productionChemical synthesisDrugs levels
The invention discloses a full chemical synthetic method for hybridization of a solid phase and a liquid phase of liraglutide. The method comprises the following steps: chemically synthesizing a liraglutide precursor protected by N terminal and a cetyl derivative; de-protecting to remove tail end protection to obtain a target polypeptide. The liraglutide precursor semi-protected is obtained by polypeptide solid-phase synthesis, and the precursor purified to the drug level enters into the next chemical synthesis.
Owner:SHANGHAI AMBIOPHARM
One-step method based solid-phase polypeptide synthesis method
ActiveCN103374054AHigh purityHigh yieldPeptide preparation methodsChemical reactionSynthesis methods
The invention discloses a one-step method based solid-phase polypeptide synthesis method. The technology of blowing in nitrogen or insert gases to volatilize a volatile solvent and reduce the temperature is adopted in each condensation reaction of connected protected amino acids, the nitrogen or the insert gases are filled into a reaction mixture so that the volatile solvent is volatilized and the temperature of a reaction system can be maintained at 15-20 DEG C, after an auxiliary condensing agent is added to start the condensation reaction, the temperature of the condensation reaction system is always stabilized at 22-28 DEG C by a heat insulation device, and during condensation of protected amino acids, the amino acids are unnecessary to be activated at low temperature in an activator and then be transferred to a condensation reactor, thus all the chemical reactions for synthesizing polypeptide are continuously completed in the same reactor in sequence. The method for synthesizing angiotensinamide is environment-friendly and efficient, has low requirements for equipment and can be applied to large-scale industrial production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Solid synthetic method of semaglutide
ActiveCN103848910AEase of mass productionReduce generationPeptide preparation methodsAnimals/human peptidesAcetic acidSide chain
The invention relates to the technical field of polypeptide synthesis, and particularly relates to a solid synthetic method of semaglutide. The solid synthetic method includes coupling Gly and a resin to obtain a Gly-resin; coupling step by step for the first time an ammonia acid or an amino acid derivative to obtain a first peptide resin the sequence of which is shown as SEQ ID No.1; removing a side chain protective group of Lys; coupling step by step for a second time 2-(2-(2-aminoethoxy)ethoxy) acetic acid, 2-(2-(2-aminoethoxy)ethoxy) acetic acid, Glu and octadecanedioic acid to obtain a second peptide resin; performing pyrolysis; and purifying. The solid synthetic method is simplified in operation step, short in synthetic period, low in cost, reduced in production of waste liquid, low in side products and high in product yield, and is suitable for large-scale production of the semaglutide.
Owner:HYBIO PHARMA
Catalyst for gas-phase synthesis of oxalate and its preparing process
InactiveCN1381310AHigh reactivityHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNitriteGas phase
A catalyst for gas-phase synthesis of dimethyl (or diethyl) oxalate from CO and nitrite is prepared from alpha-Al2O3 as carrier and Ce and Pd as active components through the dipping method. Its advantages are high reaction activity and selectivity, long service life and easy control of reaction.
Owner:EAST CHINA UNIV OF SCI & TECH
Synthetic method of polypeptide thymosin alpha1
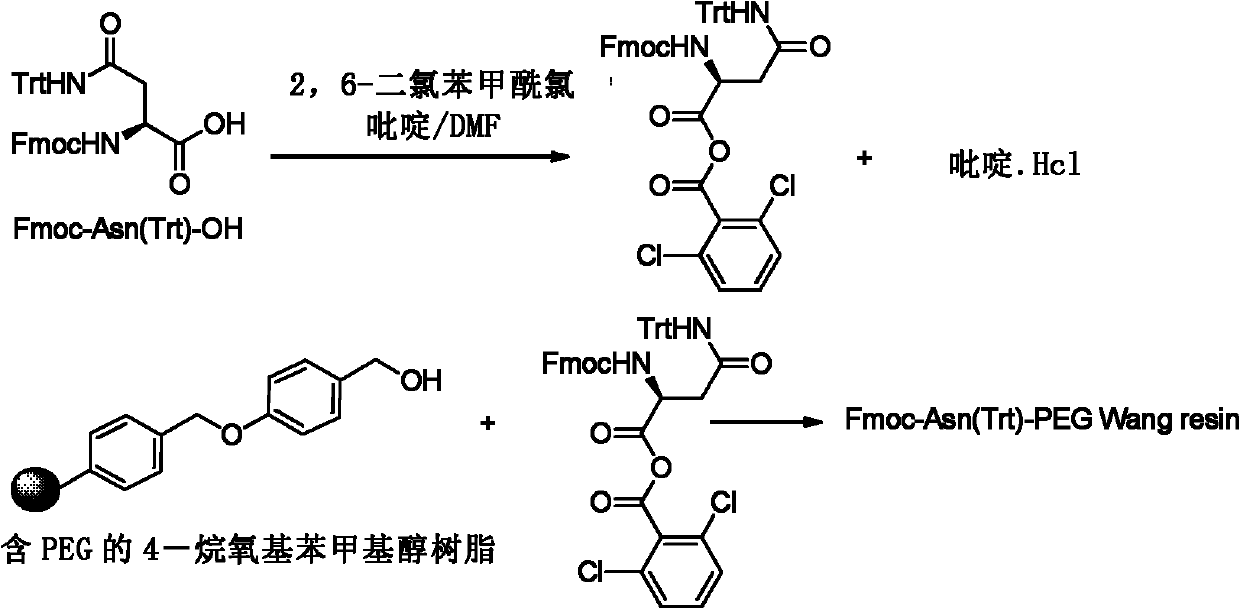
ActiveCN102199205AEasy to prepareHormone peptidesAntiviralsCombinatorial chemistrySolid-phase synthesis
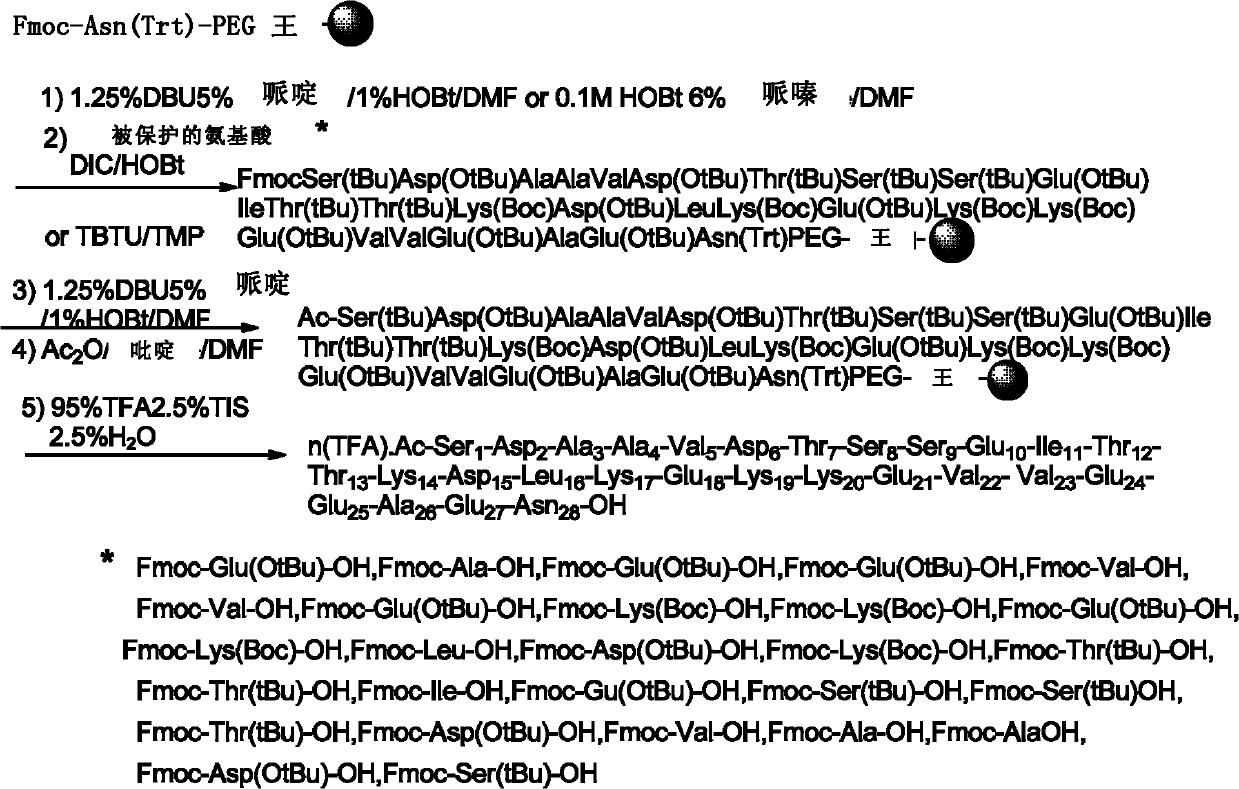
The invention discloses a preparation method of thymosin alpha1 through solid phase polypeptide synthesis, comprising the steps of: (1) mixing Fmoc-Asn(Trt)-OH with hydroxyl functional resin, and performing esterification to the mixture so as to obtain Fmoc-Asn(Trt)- resin; (2) mixing Fmoc-Asn(Trt)- resin with a deprotection agent, thus obtaining Asn(Trt)- resin; (3) condensing Fmoc-Glu(OtBu)-OH and Asn(Trt)- resin, thus obtaining Fmoc-Glu(OtBu)-Asn(Trt)-resin; (4) according to solid phase synthesis method, repeating Fmoc removal in step (2) and condensation of amino acid and polypeptide on resin in step (3), and condensing amino acid from end C to end N in an order of Glu to Ser with polypeptide on resin, thus forming polypeptide resin as shown in formula II; and (5) separating the polypeptide on the polypeptide resin as shown in formula II and resin, thus obtaining thymosin alpha1 as shown in formula I.
Owner:HAINAN SHUANGCHENG PHARMA
Macromolecular arrays on polymeric brushes and methods for preparing the same
InactiveUS6994964B1Bioreactor/fermenter combinationsBiological substance pretreatmentsPorosityCrystallography
Polymeric brush substrates and methods for their preparation are provided. Methods are also provided for preparing macromolecular arrays on such polymeric brush substrates. Using polymeric brush substrates allows control over functional site density as well as wettability and porosity of the substrate. These polymeric brushes are useful in solid-phase synthesis of arrays of peptides, polynucleotides or small organic molecules.
Owner:AFFYMETRIX INC
Method for preparing semaglutide
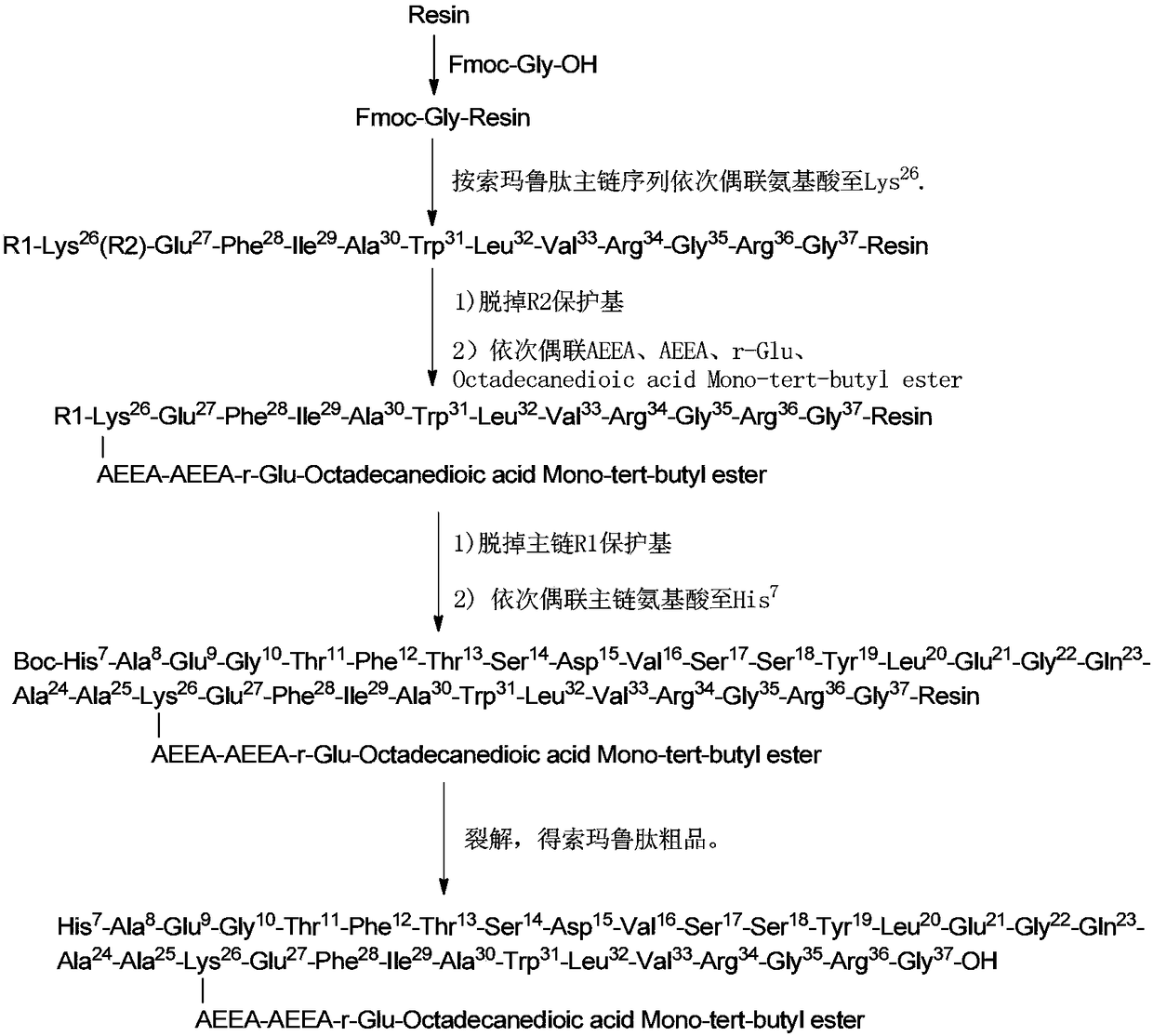
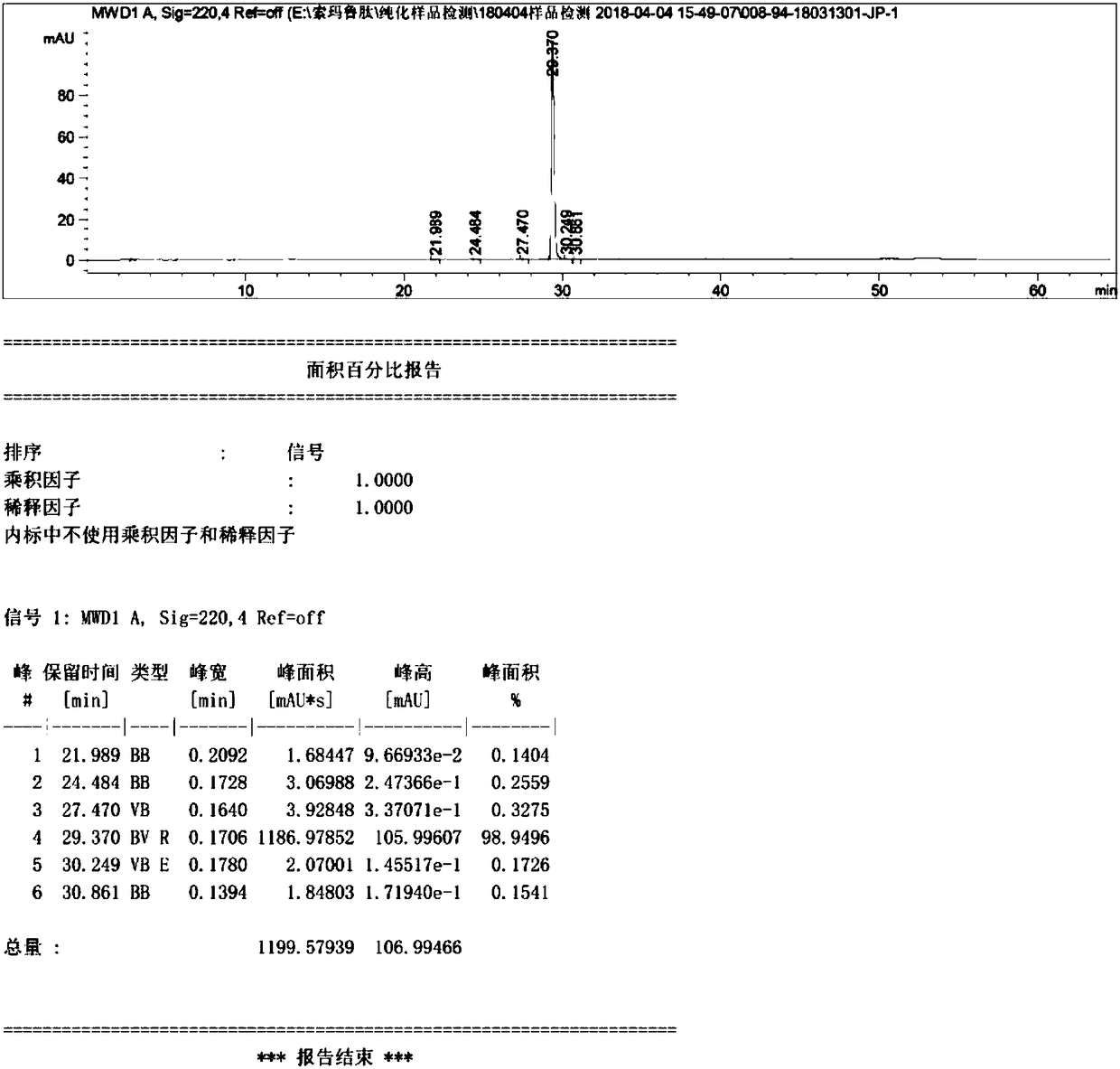
ActiveCN108359006AEnhanced couplingHigh purityPeptide preparation methodsBulk chemical productionSynthesis methodsCombinatorial chemistry
The invention discloses a method for preparing semaglutide. By adopting the method, a specific synthesis method is selected, the purity and the yield of a crude product of semaglutide can be greatly increased, the synthesis cost can be lowered, and the semaglutide is applicable to large-scale production and has wide market application prospects.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com