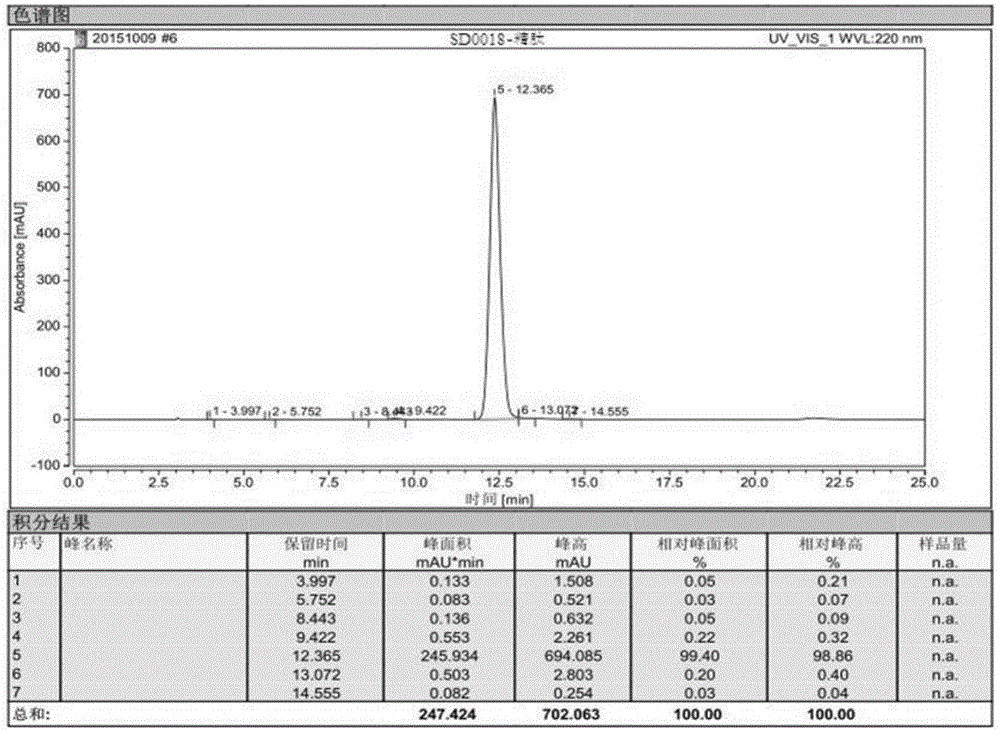
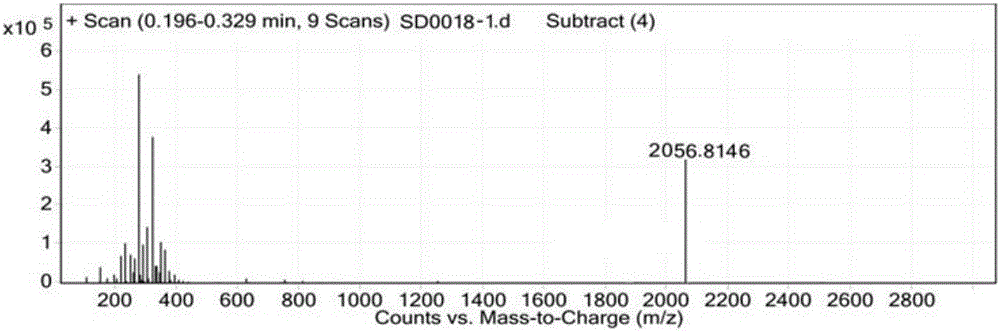
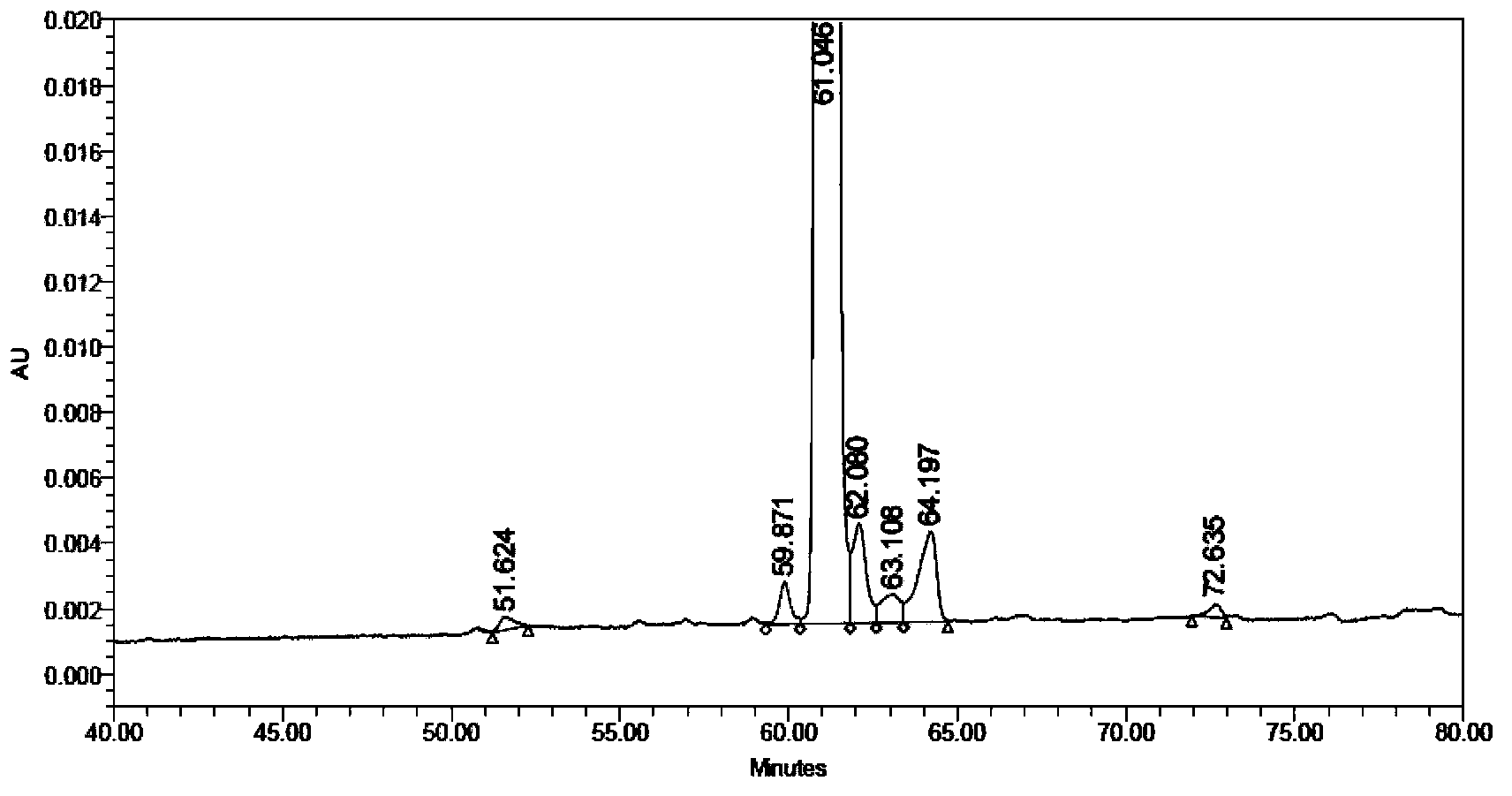
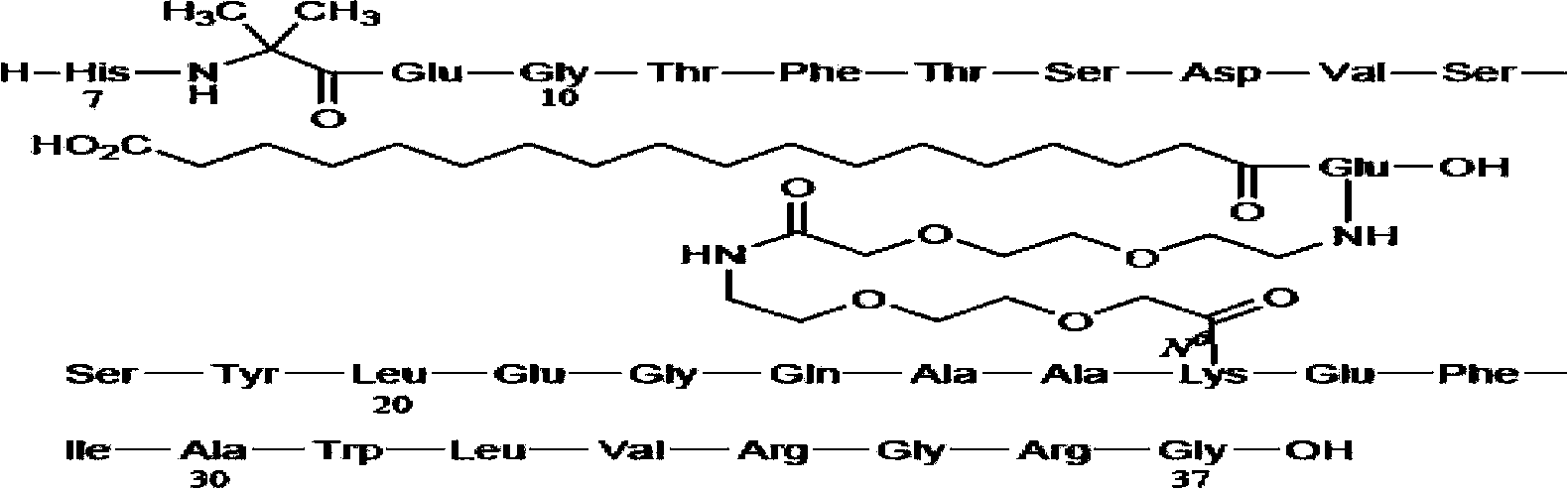
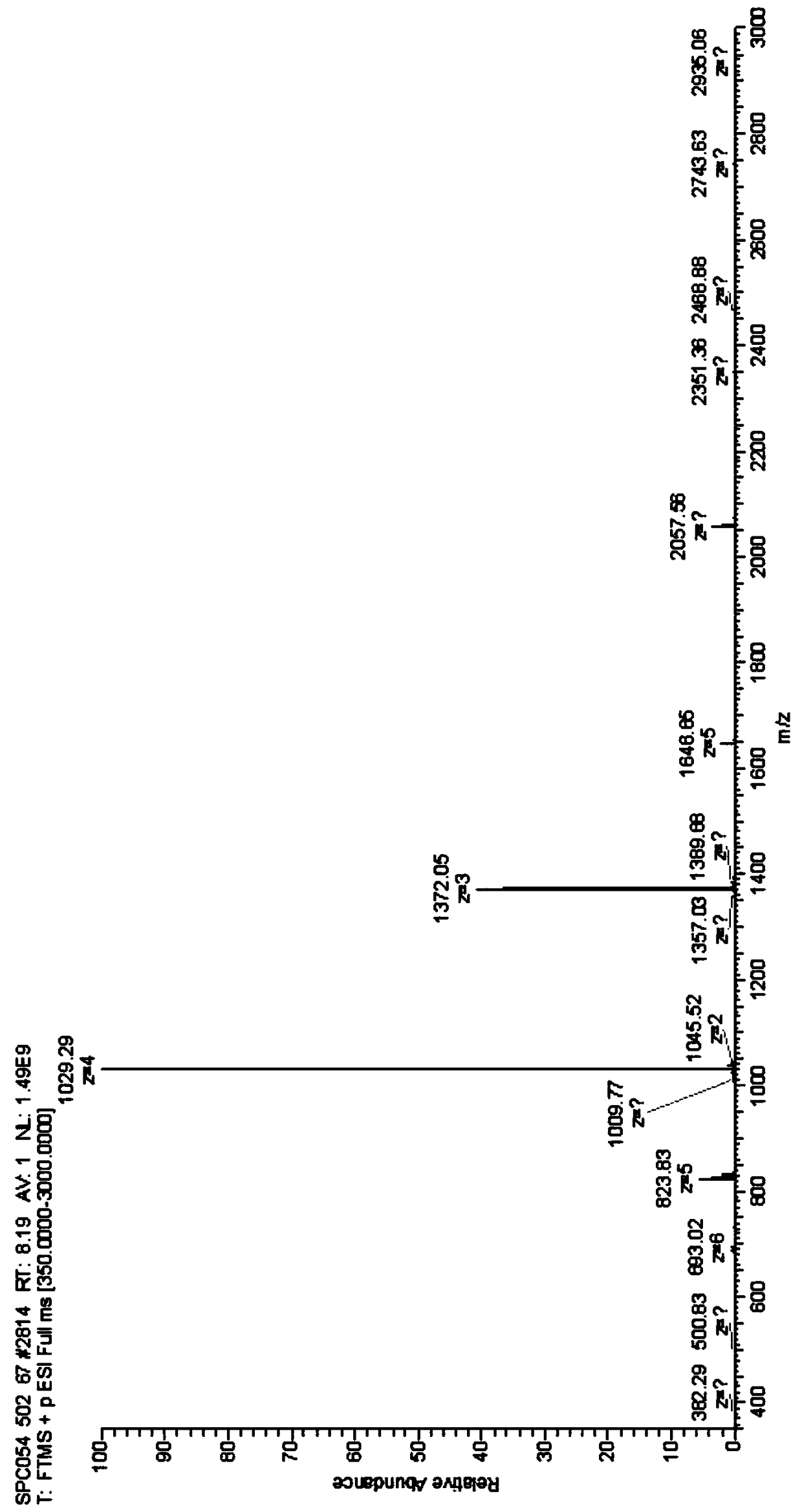
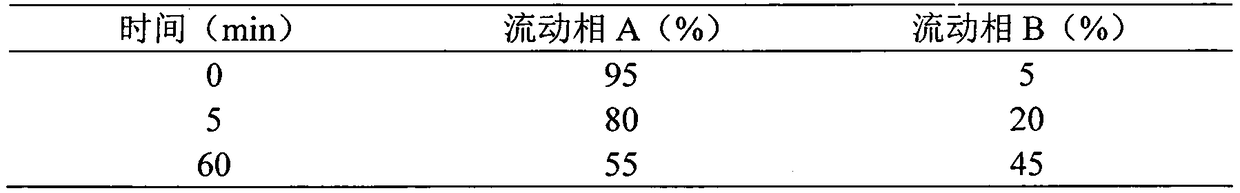
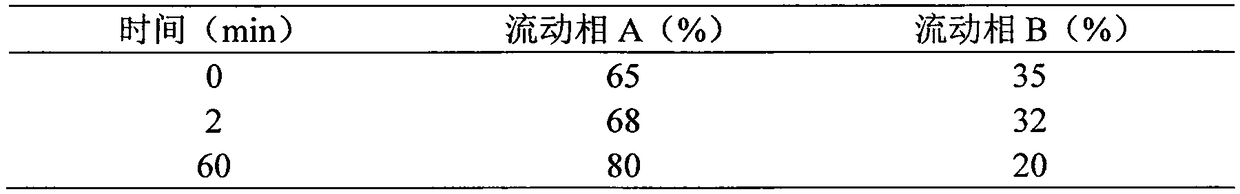
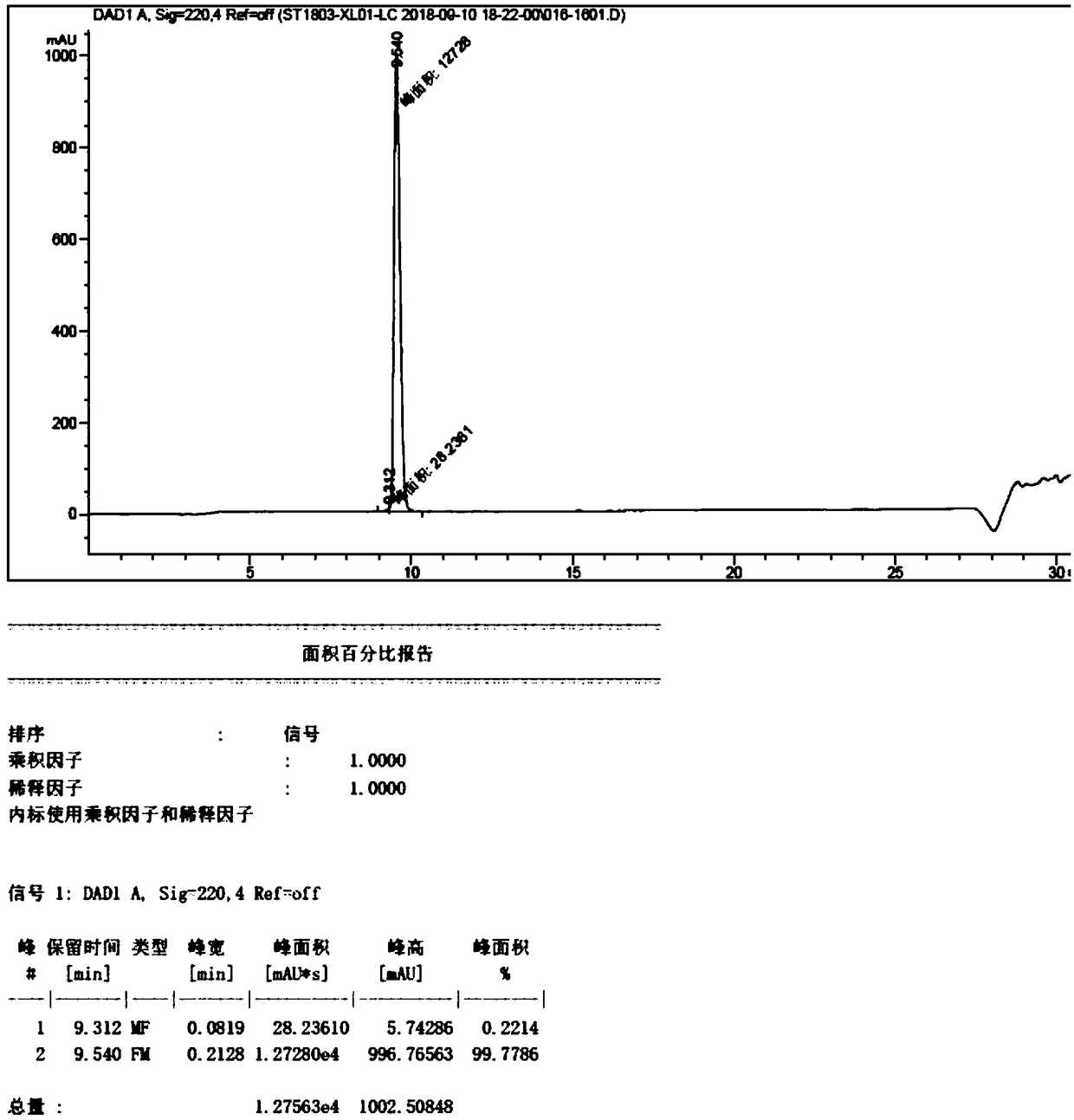
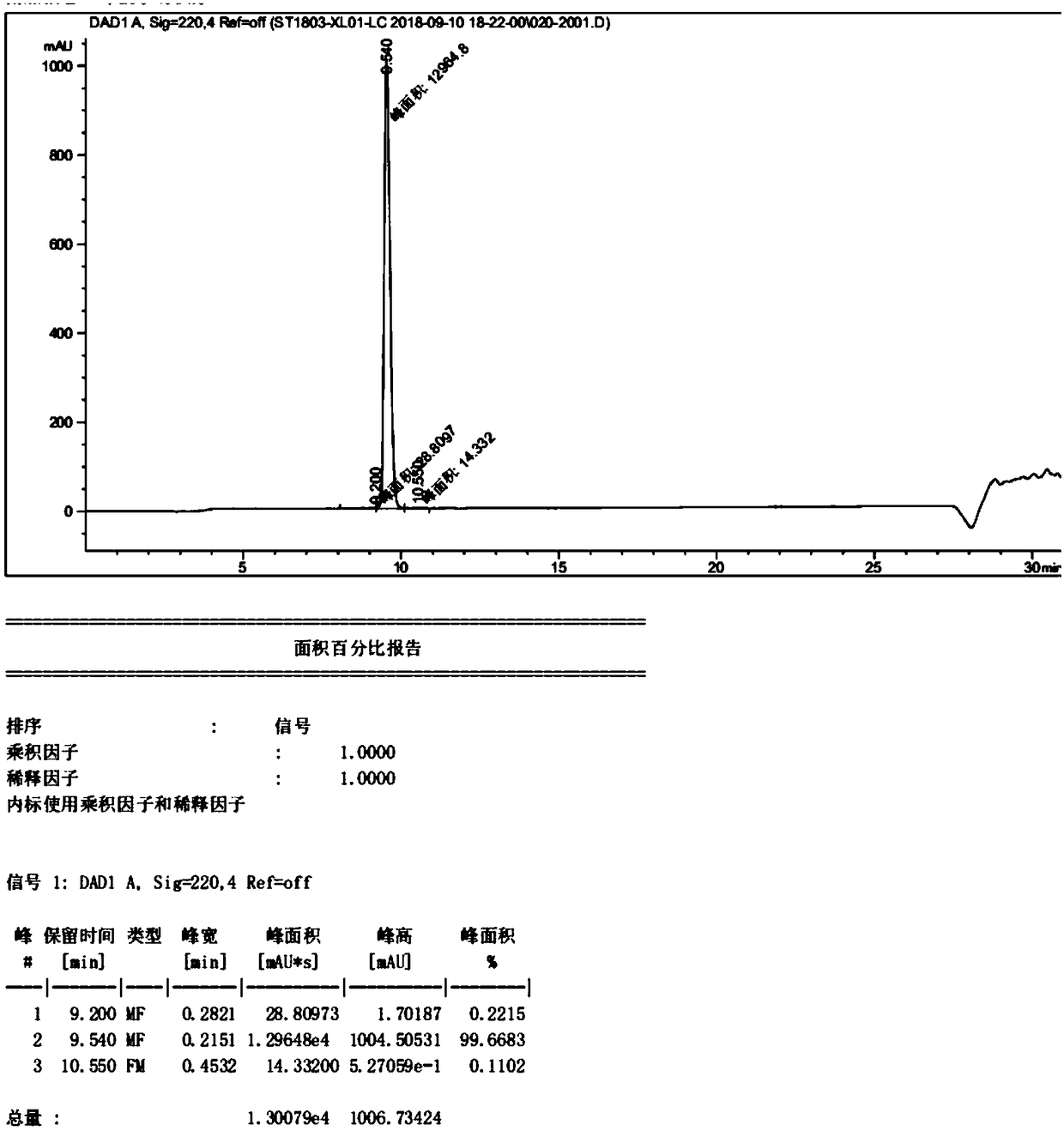
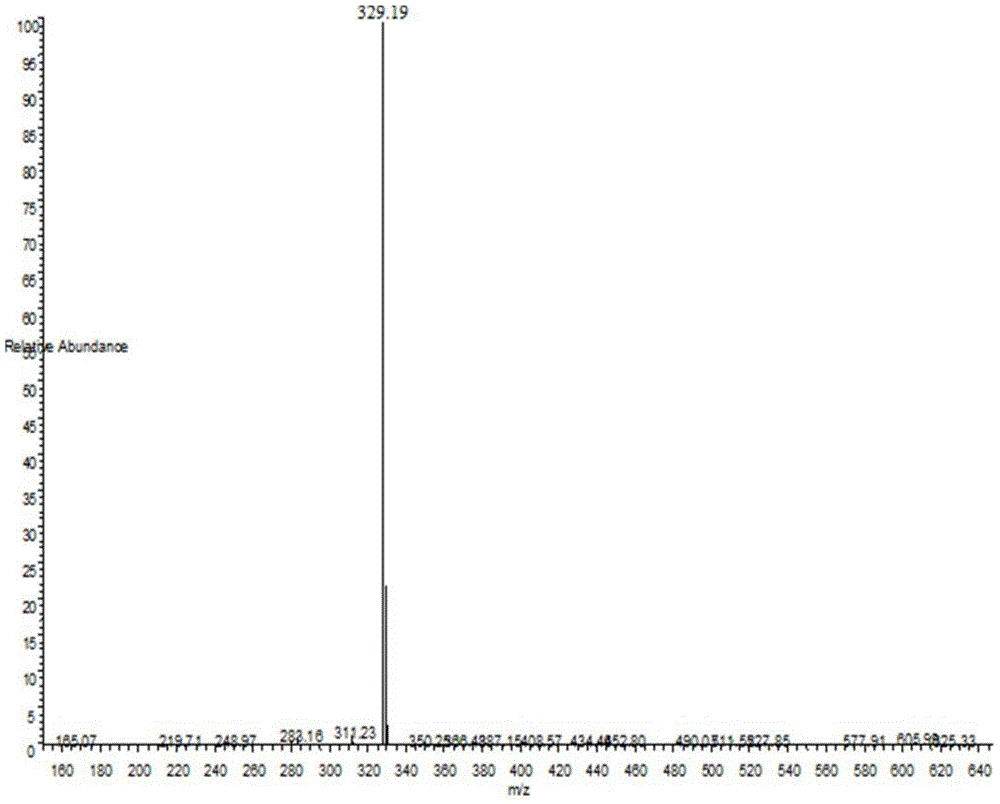
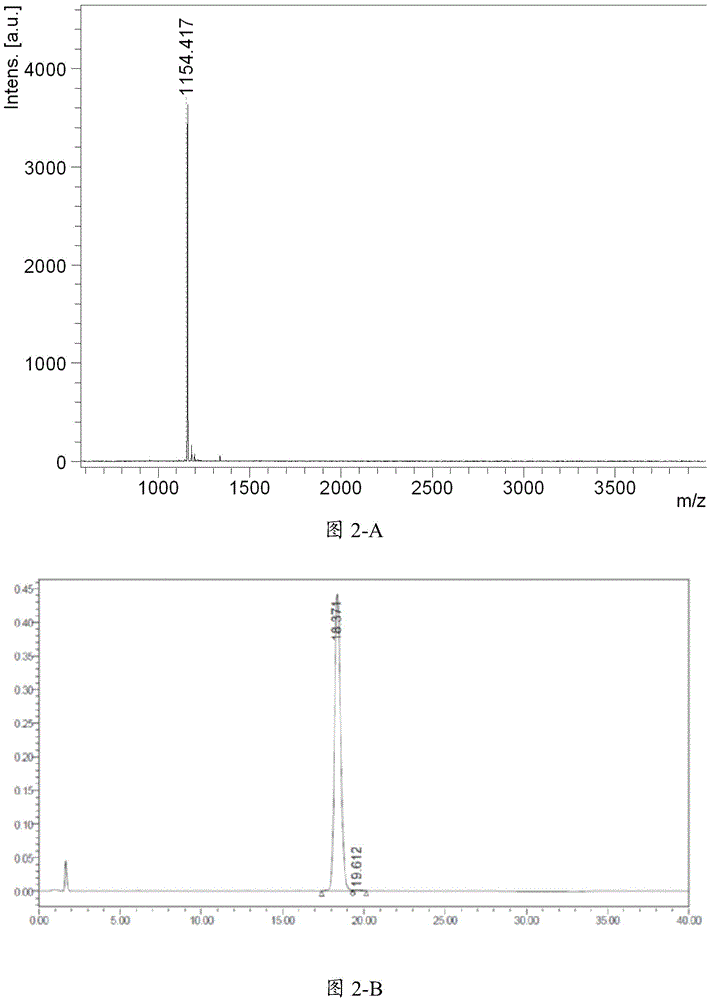
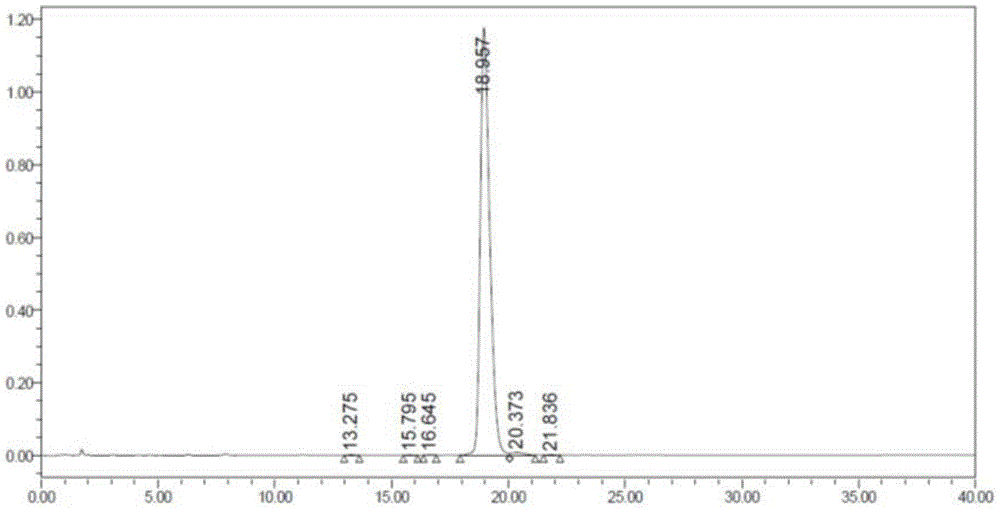
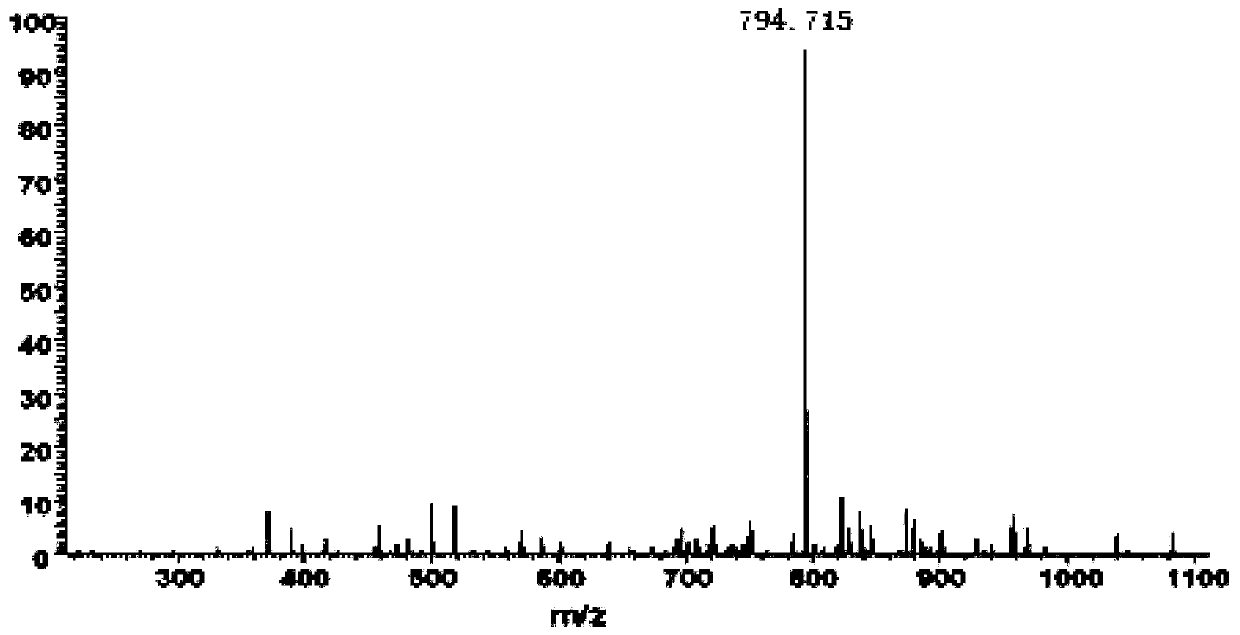
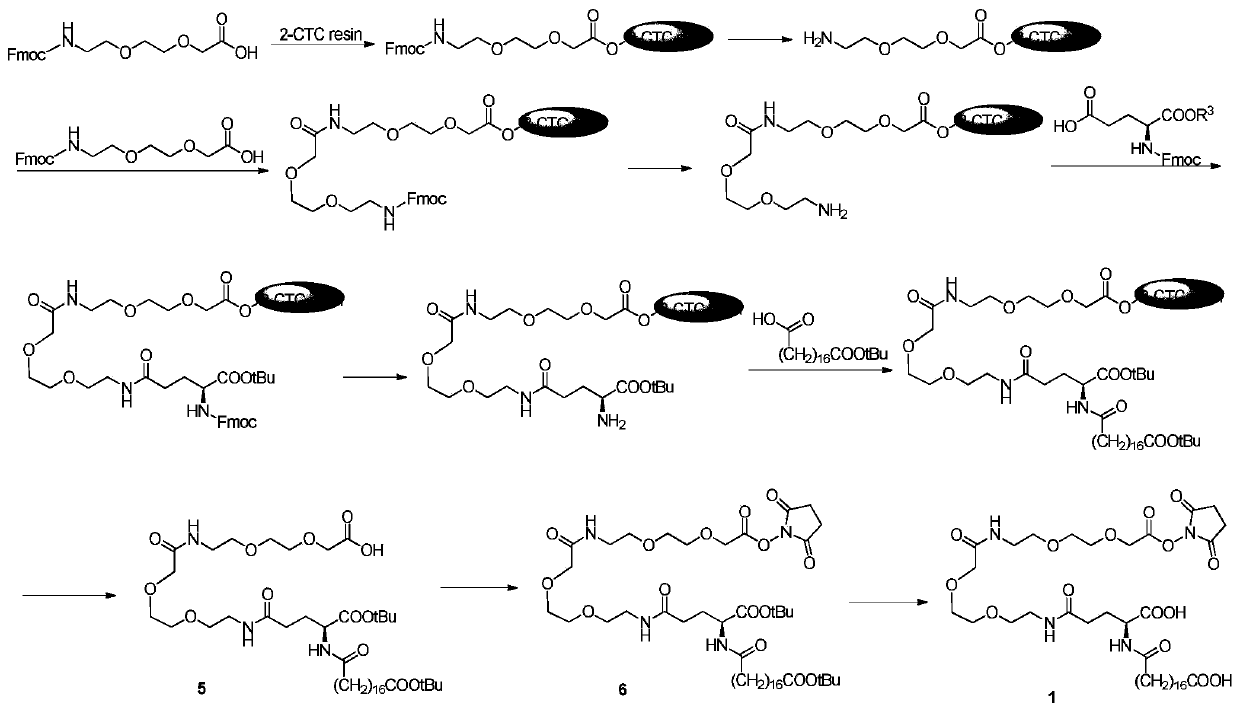
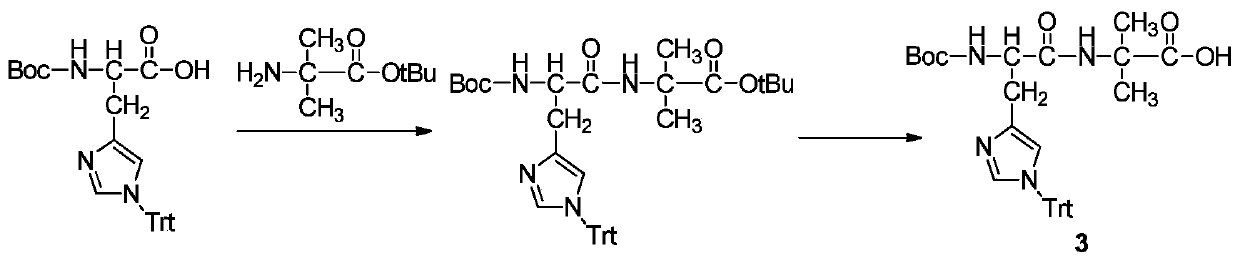
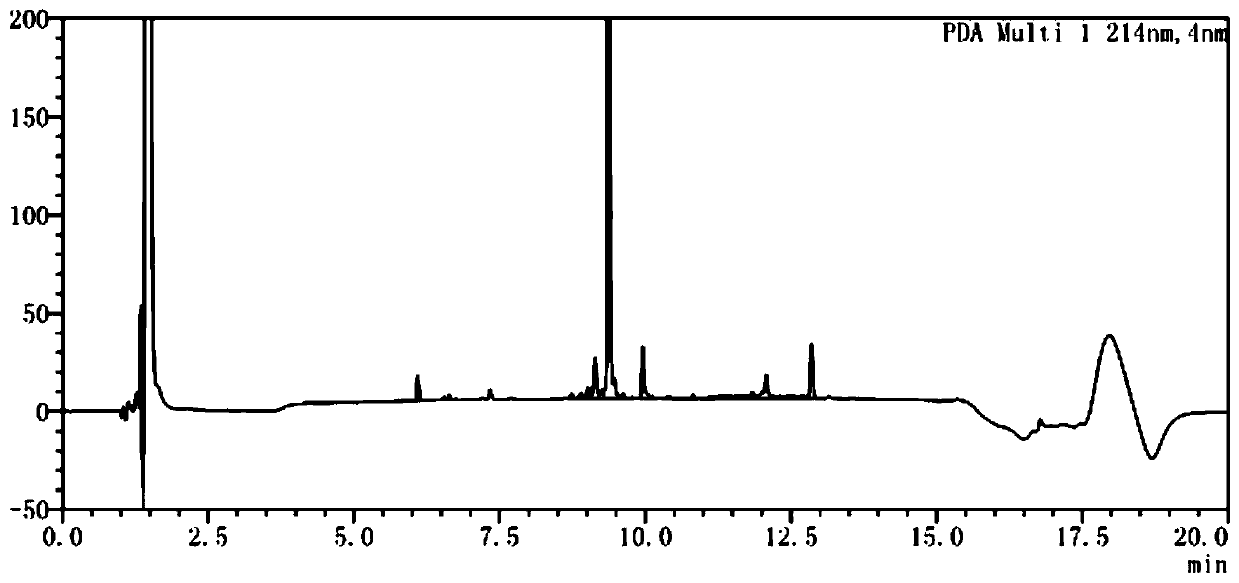
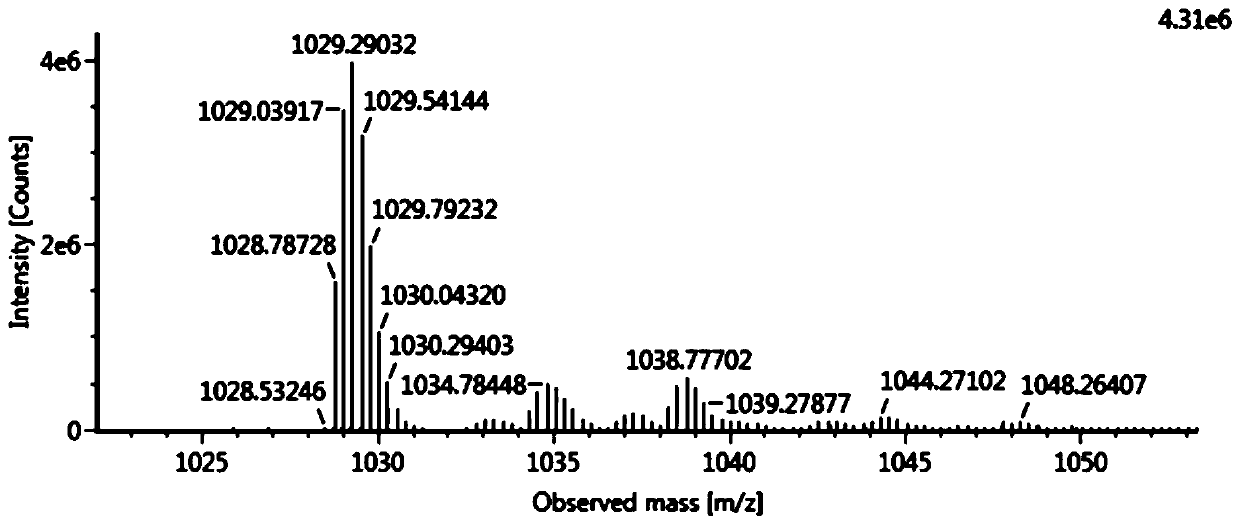
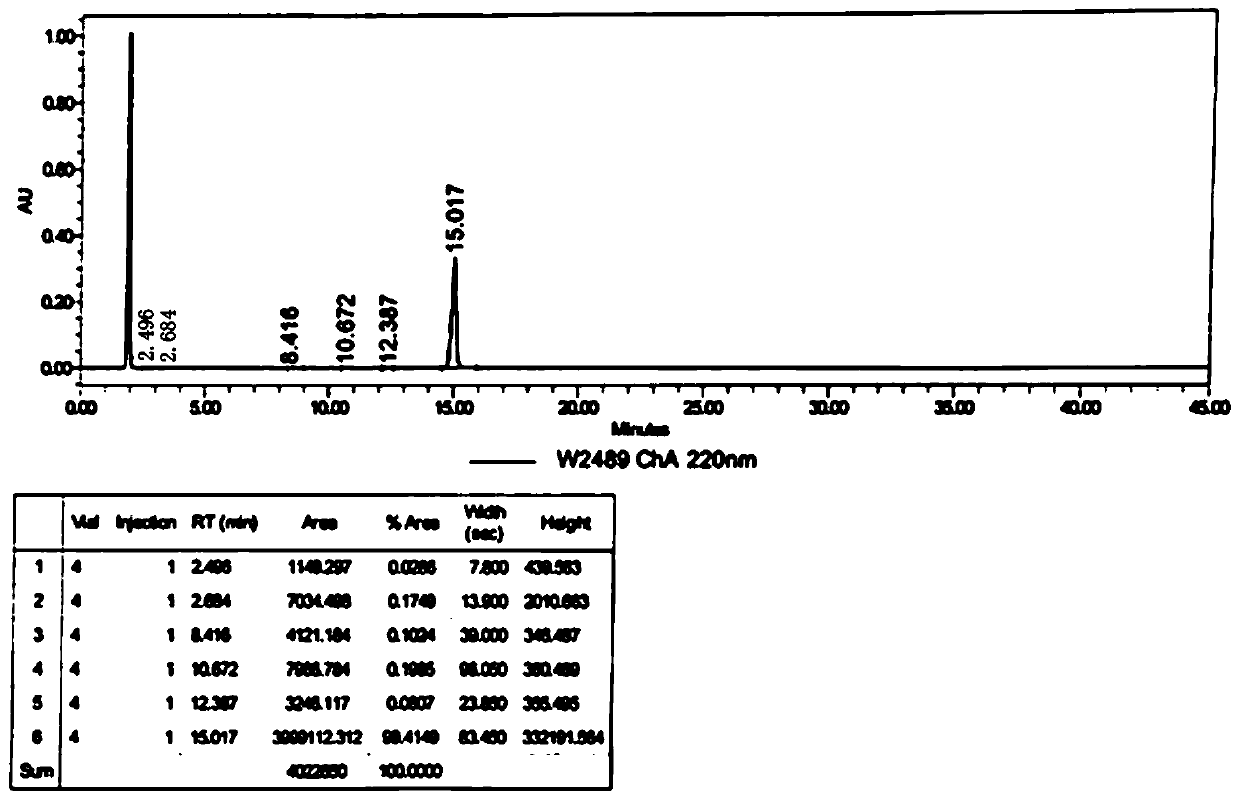
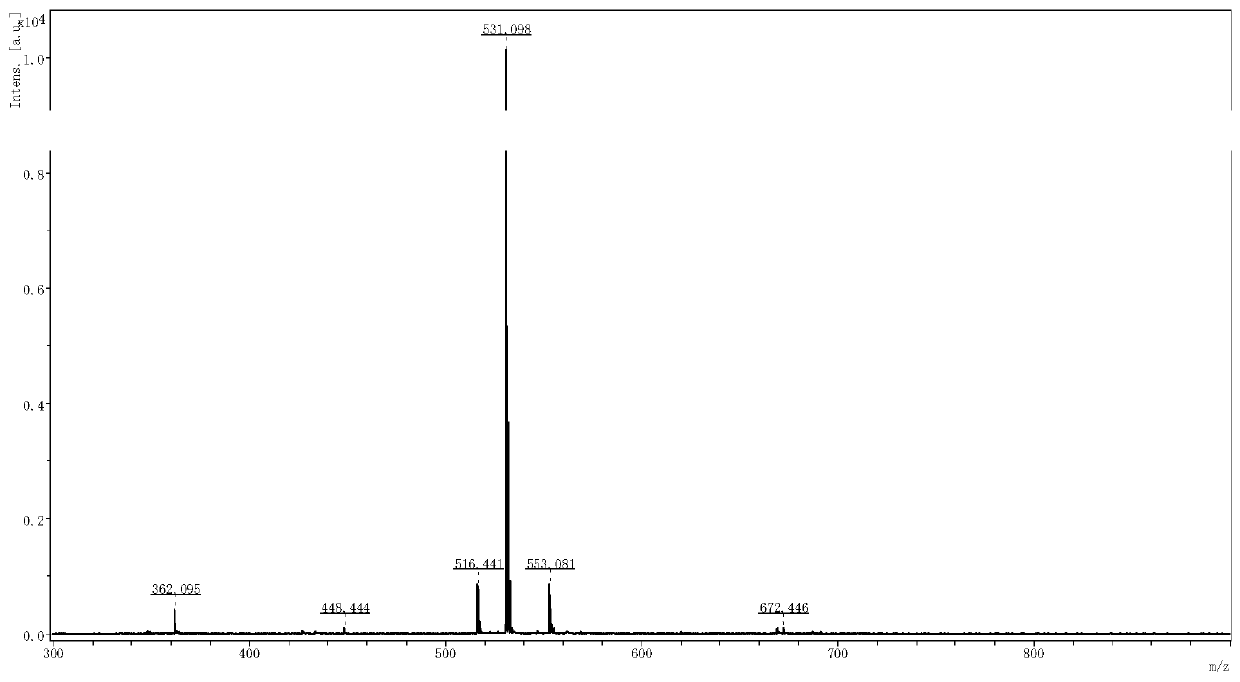
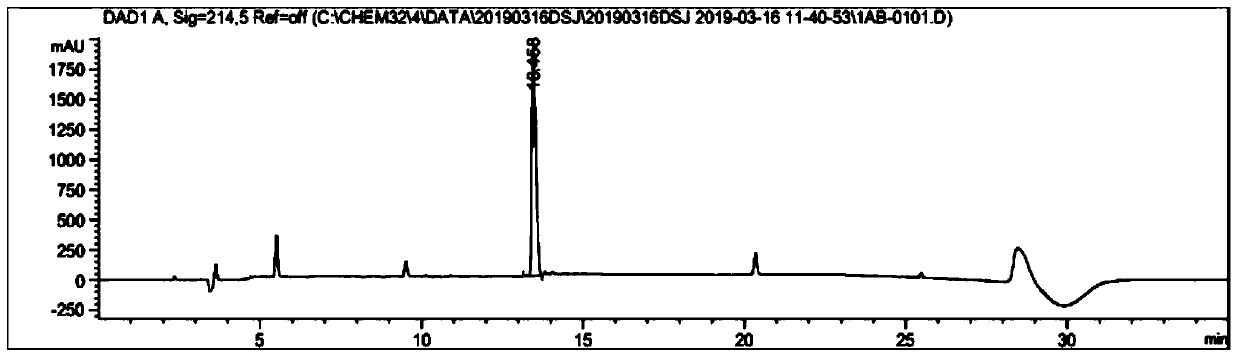
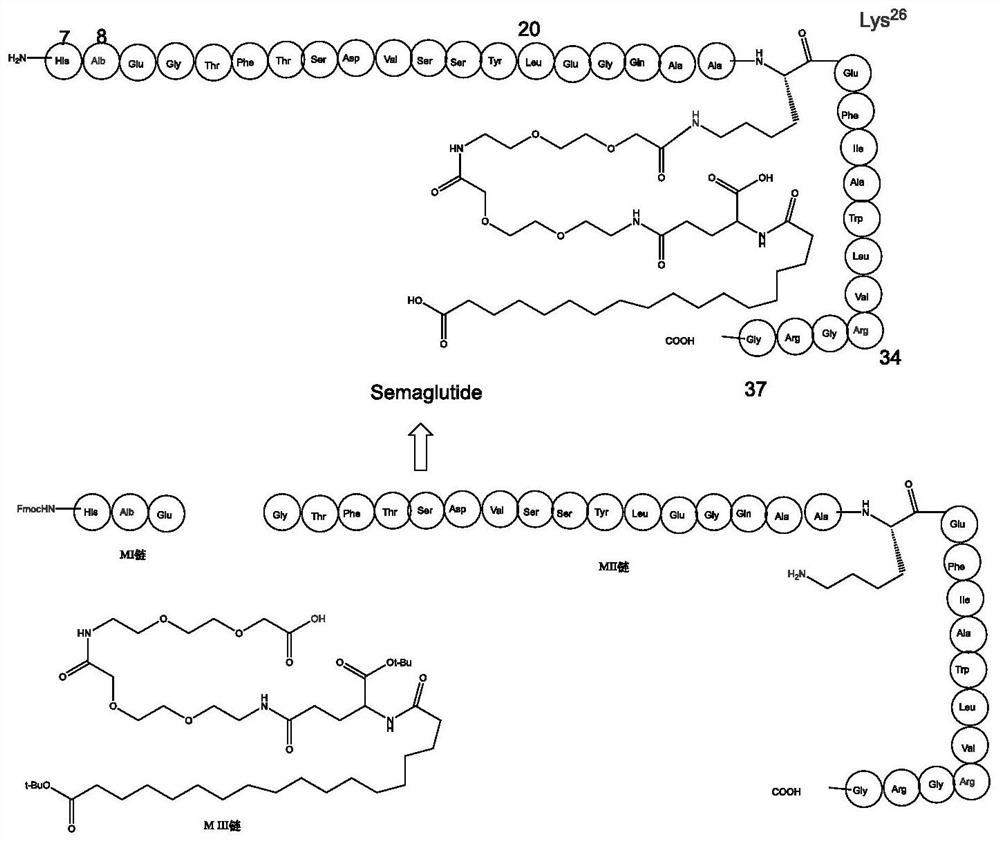
Patents
Literature
79 results about "Semaglutide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
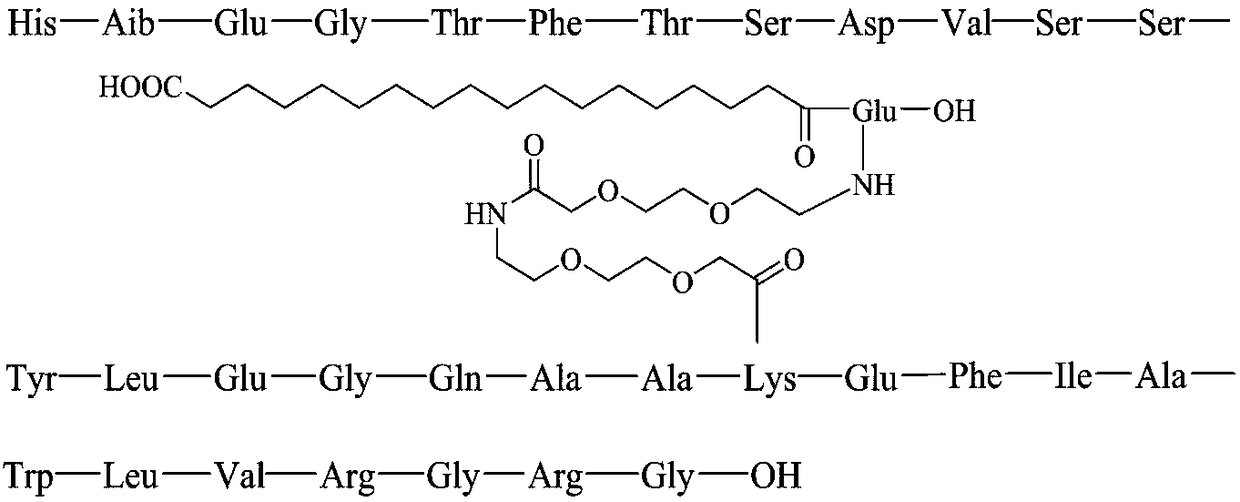
Semaglutide (trade names Rybelsus, Ozempic) is a medication for the treatment of type 2 diabetes. Side effects include medullary thyroid cancer, kidney problems, diabetic retinopathy, allergic reactions, low blood sugar, and pancreatitis.
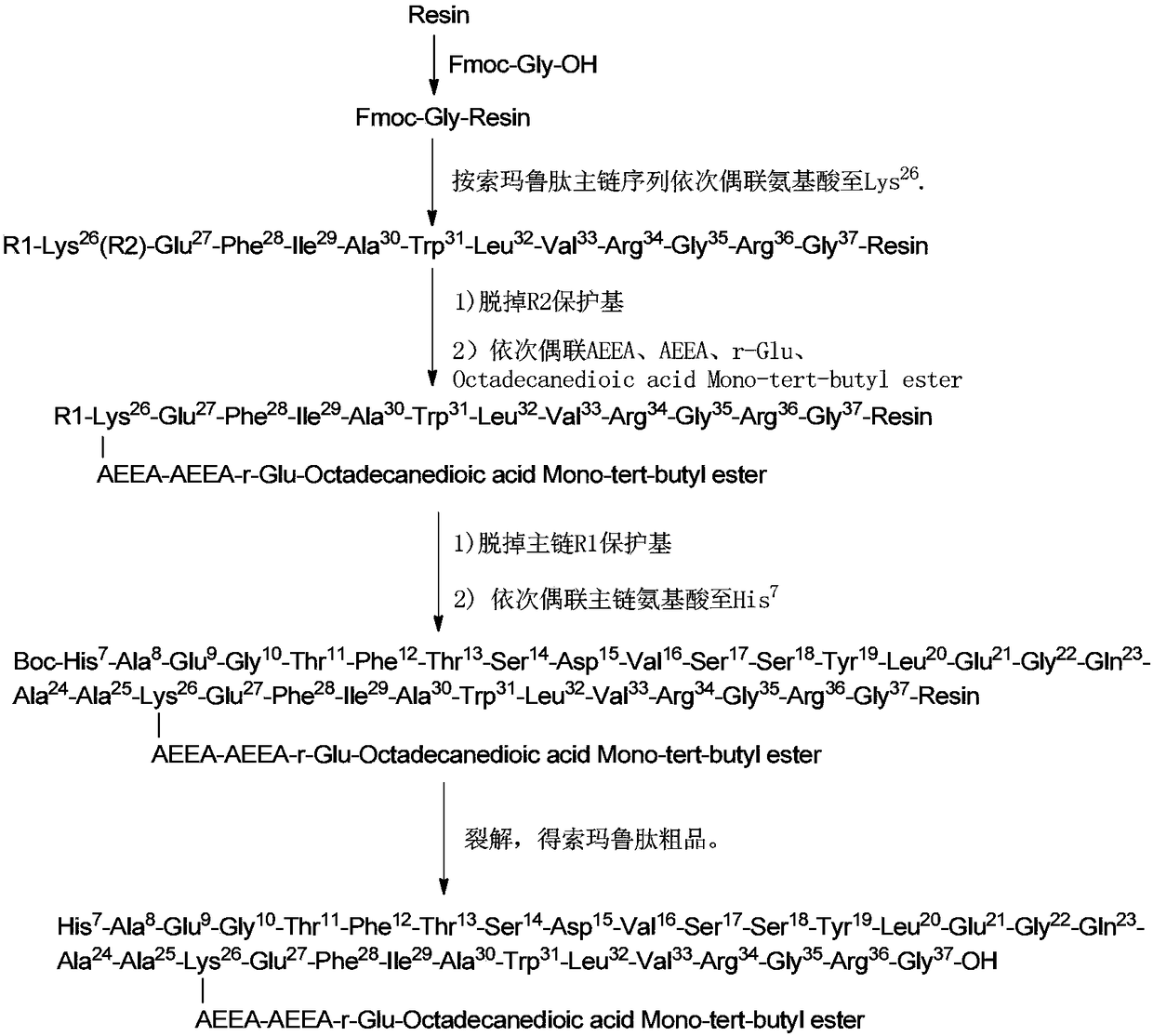
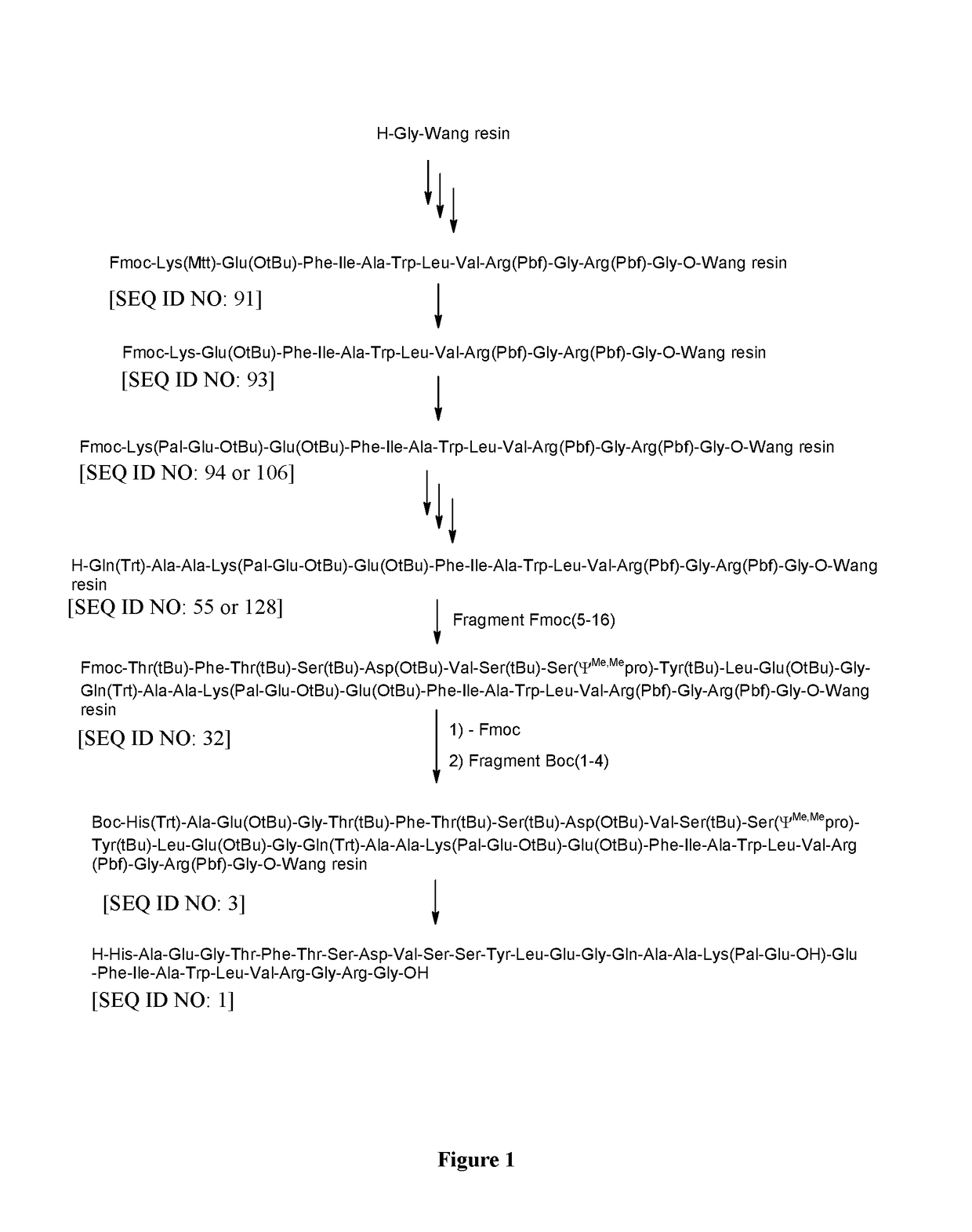
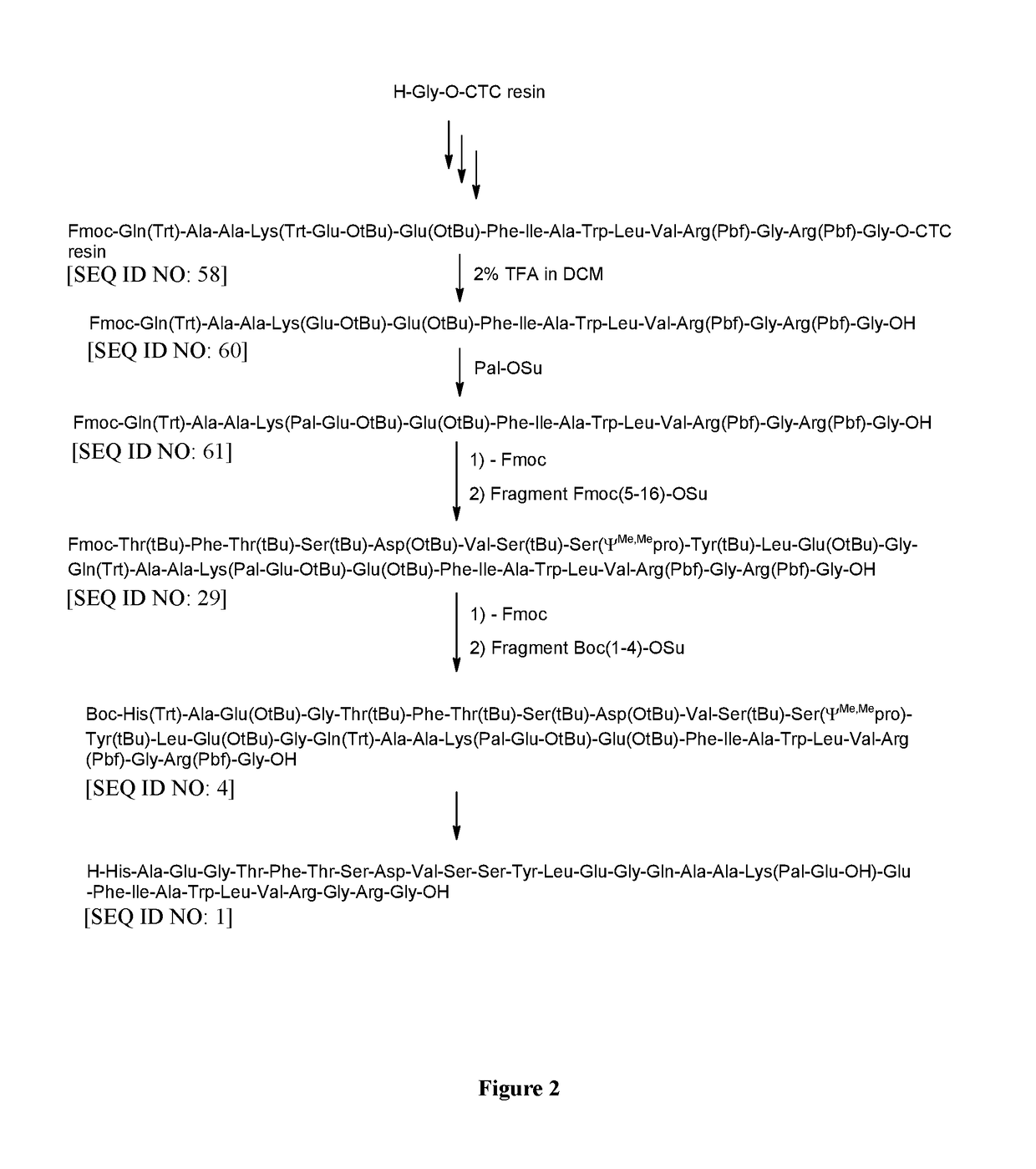
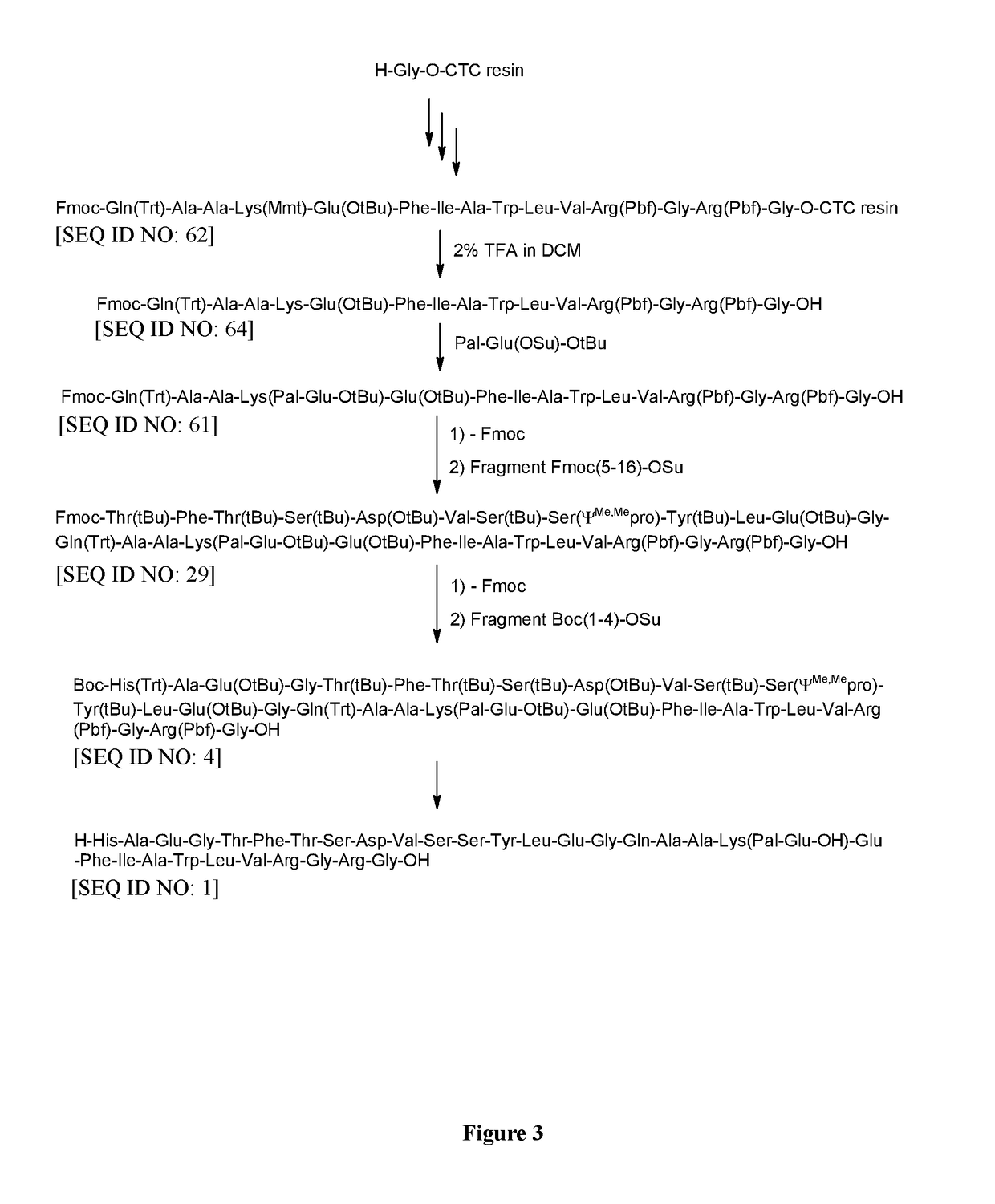
Method for preparing semaglutide
InactiveCN106928343AEasy to operateNo side effectsPeptide-nucleic acidsPeptide preparation methodsSide reactionSemaglutide
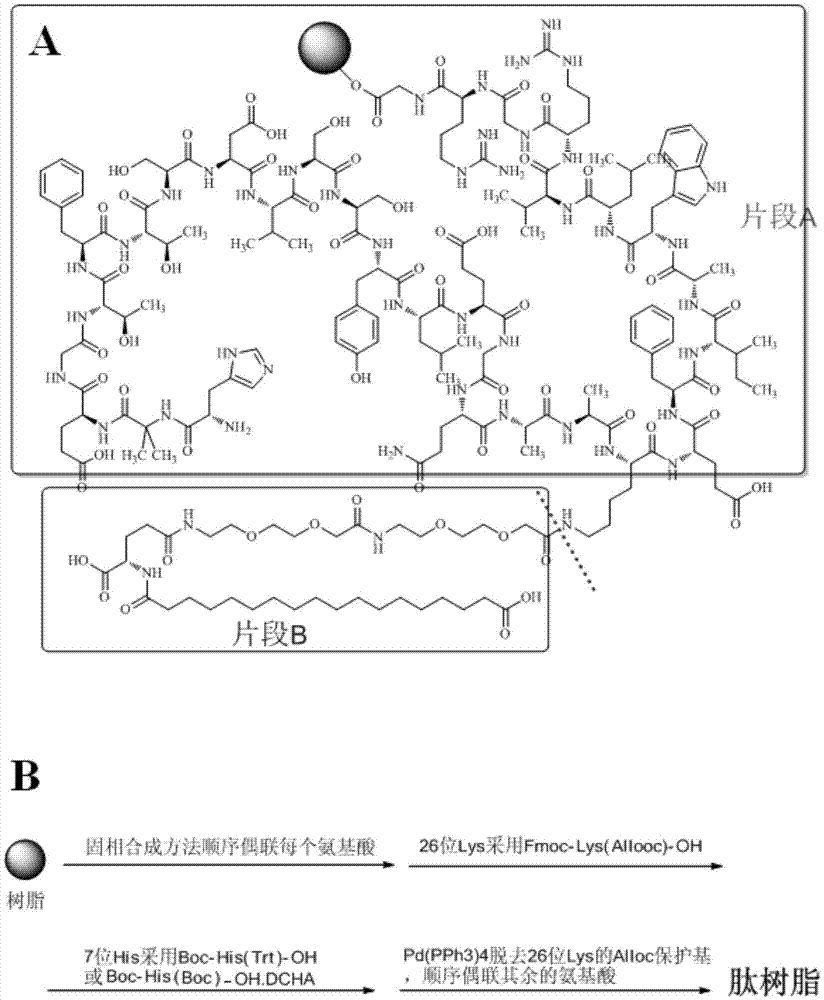
The invention relates to the field of polypeptides, in particular to a method for preparing semaglutide. The method has the advantages that Fmoc-Lys (Alloc)-OH protection amino acid is used as a raw material, de-protection is carried out by the aid of selected Pd (PPh3) 4, accordingly, operation procedures are simple, only 1-2 times of simple elimination reaction operation are required, each elimination reaction operation is carried out for 10-30 min, side reaction is prevented, the operation procedures are safe, and enlarged production can be facilitated; Boc-His (Boc)-OH. DCHA and Boc-His (Trt)-OH are used as raw materials in the procedures, and accordingly His racemization risks can be reduced to the greatest extent; special fragments are coupled, and accordingly the synthesis efficiency can be improved.
Owner:HYBIO PHARMA
Synthesis method of semaglutide
ActiveCN106749613ALow costReduce the number of generatedPeptide preparation methodsBulk chemical productionSynthesis methodsPeptide sequence
The invention relates to a synthesis method of semaglutide. According to the method, a semaglutide product is synthesized by adopting a solid-liquid phase combination method, and three fragments are simultaneously synthesized in a synthesis manner of 16+6+9 fragments, and therefore, the synthesis time of the product is greatly shortened; moreover, by step-by-step analysis on synthesis factors of His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly, Gln-Ala-Ala-N6-[N-(17-carboxy-1-oxoheptadecyl-L-gama-glutamyl [2-(2-aminoethoxy) ethoxy] acetyl [2-(2-aminoethoxy) ethoxy] acetyl]-Lys-Glu-Phe, Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH and the like, the difficulty in synthesis of a peptide sequence in solid-phase synthesis is reduced, the problem of batch amplification in the solid-phase synthesis is solved, and the synthesis efficiency is improved; and as liquid-phase fragment synthesis is adopted, the purification difficulty is effectively reduced, and the production cost is greatly lowered. The synthesis method disclosed by the invention has the advantages that the synthesis time can be shortened by 40%, the cost of materials is lowered, the generation quantities of deletion peptide and hybrid peptide are decreased, and the synthesis method is suitable for industrial large-scale production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Solid synthetic method of semaglutide
ActiveCN103848910AEase of mass productionReduce generationPeptide preparation methodsAnimals/human peptidesAcetic acidSide chain
The invention relates to the technical field of polypeptide synthesis, and particularly relates to a solid synthetic method of semaglutide. The solid synthetic method includes coupling Gly and a resin to obtain a Gly-resin; coupling step by step for the first time an ammonia acid or an amino acid derivative to obtain a first peptide resin the sequence of which is shown as SEQ ID No.1; removing a side chain protective group of Lys; coupling step by step for a second time 2-(2-(2-aminoethoxy)ethoxy) acetic acid, 2-(2-(2-aminoethoxy)ethoxy) acetic acid, Glu and octadecanedioic acid to obtain a second peptide resin; performing pyrolysis; and purifying. The solid synthetic method is simplified in operation step, short in synthetic period, low in cost, reduced in production of waste liquid, low in side products and high in product yield, and is suitable for large-scale production of the semaglutide.
Owner:HYBIO PHARMA
Method for preparing semaglutide
ActiveCN108359006AEnhanced couplingHigh purityPeptide preparation methodsBulk chemical productionSynthesis methodsCombinatorial chemistry
The invention discloses a method for preparing semaglutide. By adopting the method, a specific synthesis method is selected, the purity and the yield of a crude product of semaglutide can be greatly increased, the synthesis cost can be lowered, and the semaglutide is applicable to large-scale production and has wide market application prospects.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
Method for preparing Semaglutide through solid and liquid combination
ActiveCN108059666AReduce generationAvoid it happening againPeptide preparation methodsBulk chemical productionDipeptideSide chain
The invention relates to a method for preparing Semaglutide through solid and liquid combination, and solves the technical problems that in the process for synthesizing long-sequence polypeptide by the existing technology, the synthesis period is long; the purification difficulty is high; the yield is low. The method for preparing Semaglutide through solid and liquid combination provided by the invention is characterized in that firstly, Lys and resin are condensed in an Alloc-Lys(Fmoc)-OH form by adopting a solid phase synthesis method; Fmoc protecting groups on epsilon-NH2 are removed; sidechain connection is performed; cracking is performed to obtain Alloc-Lys(PEG-PEG-gamma-Glu(OtBu)-Monobutyl octadecanate)-OH; meanwhile, 10 dipeptide or tripeptide or tetrapeptide fragments are simultaneously synthesized by a liquid phase synthesis method; then, the condensation reaction of the synthesized peptide fragments and single amino acid is performed by using the resin as a carrier; the 15-step solid phase condensation reaction is reduced in the process; the generation of lacked peptide impurities is reduced; the product purity and the yield are improved; meanwhile, the generation of the impurities of [+Gly]-Semaglutide and [+Ala]-Semaglutide is effectively avoided; the purification difficulty is greatly reduced. The method is widely applied to the technical field of polypeptide medicine preparation.
Owner:润辉生物技术(威海)有限公司
Method for purifying sermaglutide
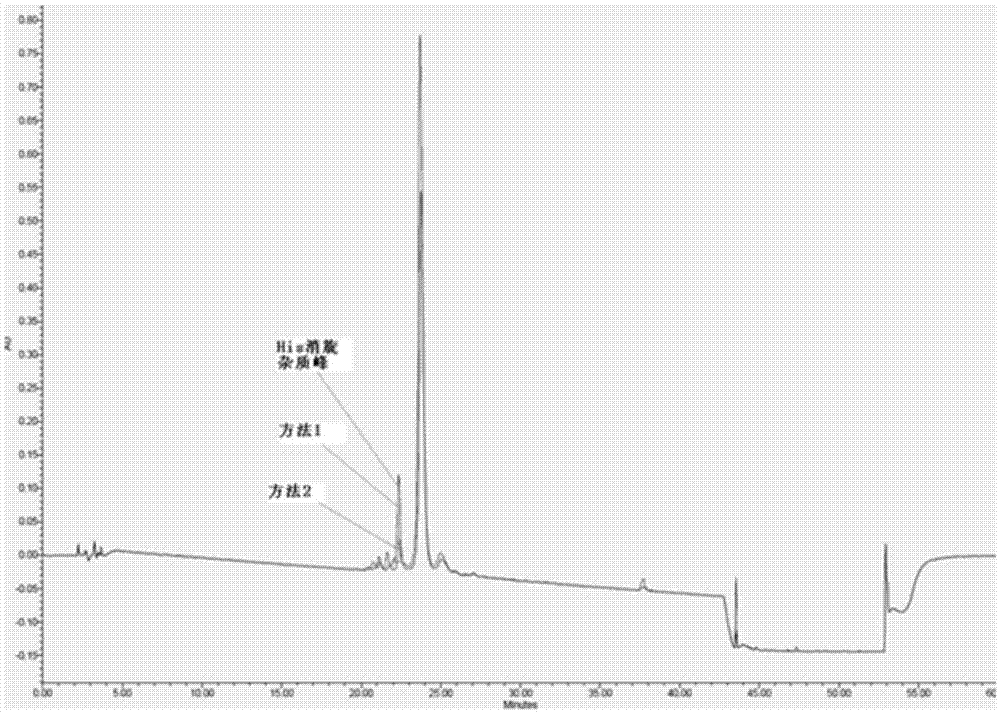
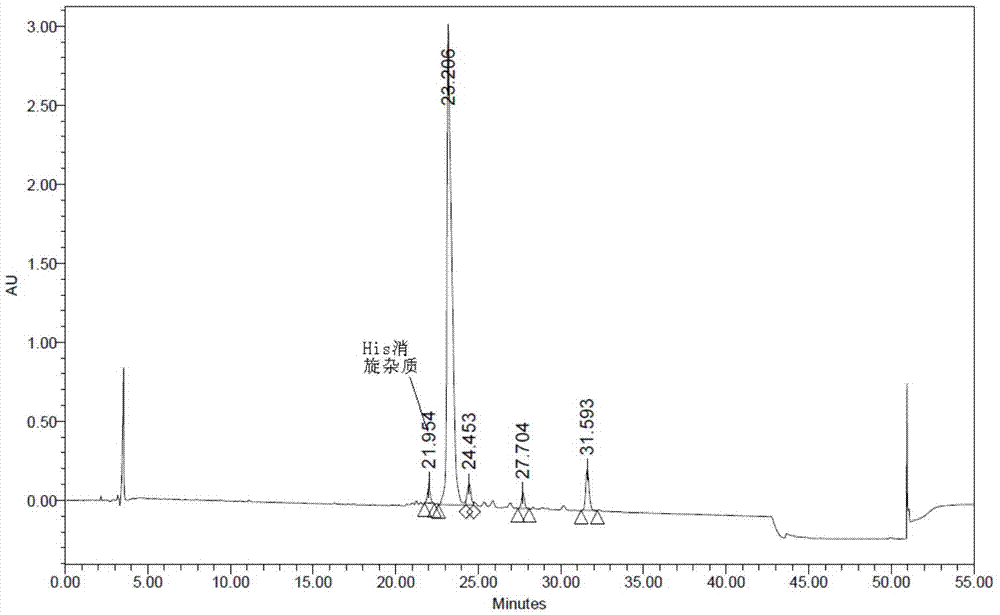
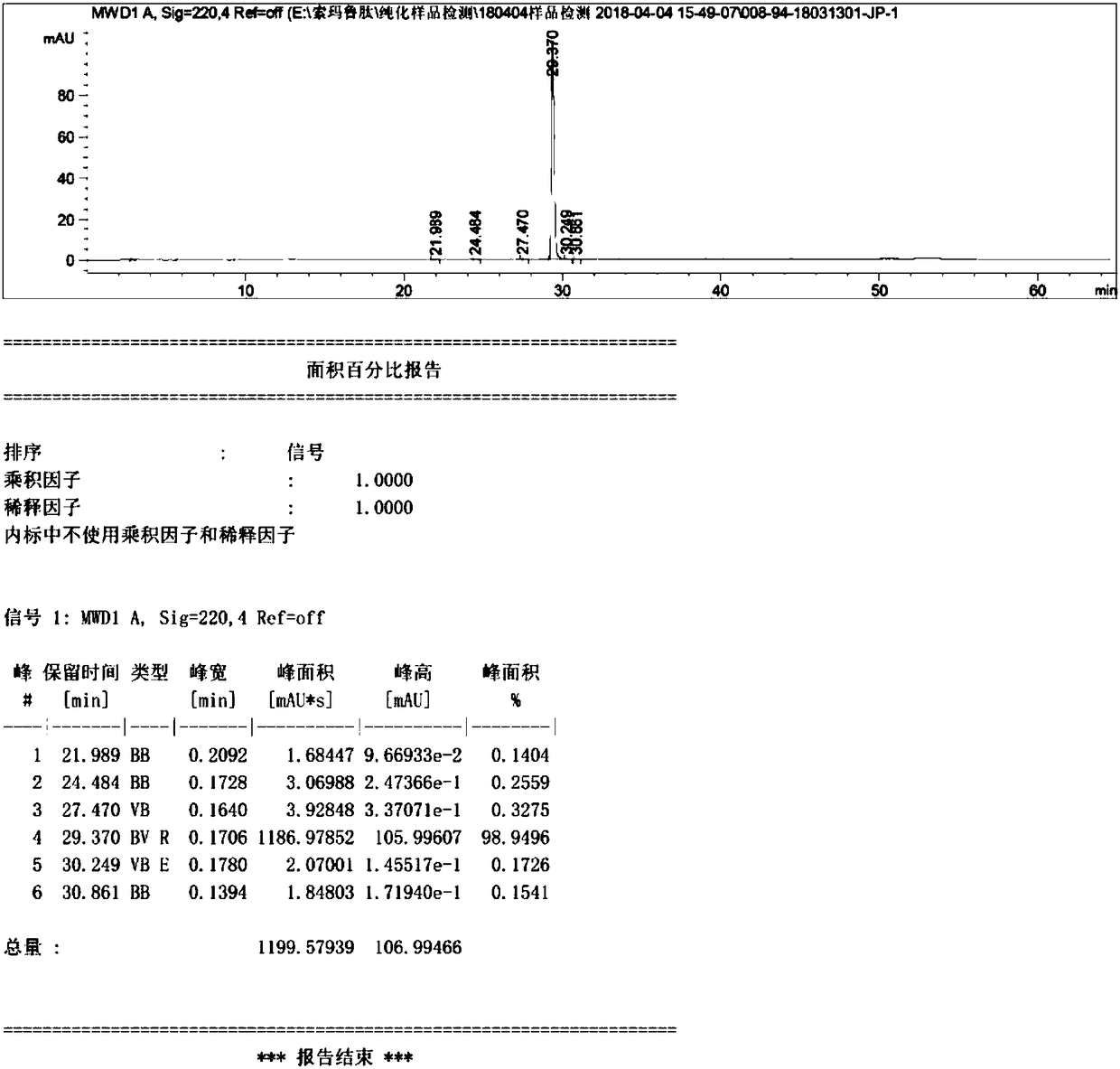
The invention discloses a method for purifying sermaglutide. The method comprises the following steps that in the first step, a sermaglutide crude product is pre-treated, and a sermaglutide crude peptide aqueous solution is obtained; in the second step, a tetralkylsilane bonded silica gel filler serves as a fixed phase, phosphoric acid serves as a mobile phase A, acetonitrile serves as a mobile phase B, and first HPLC (high performance liquid chromatography) purification is conducted so as to remove impurities of sermaglutide segment; in the third step, a solvent is removed, and a first step sample solution of sermaglutide is obtained; in the fourth step, an octane silane bonded silica gel filler serves as a fixed phase, an ammonium acetate solution serves as a mobile phase A, acetonitrileserves as a mobile phase B, and secondary HPLC purification is conducted so as to remove impurities having the similar physical and chemical properties as sermaglutide; and in the fifth step, the solvent is removed to obtain a second step sample solution of sermaglutide, and a sermaglutide sample solution is obtained. HPLC purification is conducted twice, the purity of the sample obtained in thefirst HPLC purification process and the secondary HPLC purification process is 92% and 99% respectively, and thus the purity and the yield of semaglutide are improved.
Owner:HANGZHOU SINOPEP ALLSINO PHARMA TECH DEV CO LTD
Synthetic method for semaglutide
PendingCN109456402ADestroy secondary structureTroubleshoot compositingPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
The invention discloses a synthetic method for semaglutide and belongs to the field of polypeptide synthesis. In the method, amino acids at 1-30 positions of a main sequence of the semaglutide is obtained by adopting solid phase synthesis, Val at 10 position and Ser at 11 position adopt Fmoc-Val10-Ser11 (Psi(Me, Me) pro)-OH; and an Lys side chain at 20 position adopts Fmoc-Lys(X)-OH. The method comprises the following steps of performing cracking precipitation to obtain a semaglutide crude peptide; and performing purification to obtain the semaglutide. In the method, by introducing the Fmoc-Val10-Ser11 (Psi(Me, Me) pro)-OH to solve the problem of difficult sequence synthesis of the semaglutide, difficult sequence synthesis becomes simple and easy; and meanwhile, the yield is greatly increased, the purity of the crude peptide is greatly improved, the production cost is greatly lowered, and industrial amplification production is facilitated.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Synthetic method for semaglutide
ActiveCN109456401AReduce generationNarrow the scope of the studyPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
The invention discloses a synthetic method for semaglutide. The synthetic method for the semaglutide comprises the following steps of (1) synthesizing peptide segment sequences protected by 6 side chains; and (2) gradually coupling various peptide segment sequences in a solid phase to obtain a fully protected semaglutide straight-chain polypeptide. The semaglutide synthesized by utilizing the method is high in purity and yield and low in synthesis cost.
Owner:CHENGDU SINTANOVO BIOTECHNOLOGV CO LTD
Preparation method of semaglutide and intermediate of semaglutide
The invention belongs to the technical field of pharmaceutical chemistry, and discloses a preparation method of semaglutide and an intermediate of semaglutide. The preparation method comprises: obtaining a compound having a structure represented as the formula I by taking a resin, activating the resin, and coupling L-glycine, L-arginine, L-glycine, L-arginine, L-valine, L- leucine, L-tryptophan, L-alanine, L-isoleucine, L-phenylalanine, and L-glutamic acid to the activated resin step by step so as to obtain a first peptide resin; taking the first peptide resin, and coupling amino acids or amino acid derivatives to the second peptide resin step by step; and taking the second peptide resin, and performing cracking and purifying to obtain semaglutide. According to the method, the compound having a structure represented as the formula I is taken as an intermediate and coupling of each amino acid or amino acid derivative step by step through solid-phase synthesis is carried out, thereby improving the yield of semaglutide. In the formula I, R1 is selected from Fmoc, Boc, and ivDde, and R2 is selected from tBu, Dmab, and Bzl.
Owner:HYBIO PHARMA
Synthesis of GLP-1 Peptides
InactiveUS20180057558A1Improve purification effectEfficient productionGlucagonsCombinatorial chemistrySemaglutide
Disclosed are processes for the synthesis of GLP-1 peptides, such as liraglutide and semaglutide, and a process for purifying liraglutide.
Owner:NOVETIDE LTD
Semaglutide purifying method
The invention belongs to the technical field of pharmaceutical chemistry, and discloses a semaglutide purifying method. The purifying method comprises the following steps: taking a semaglutide sample to be purified, allowing the sample to go through a chemically bonded silica gel chromatographic column in order to carry out primary separation, carrying out gradient elution with an A1 solution and B solution mixed solution, and collecting an elution component to obtain a primary purification product; allowing the primary purification product to go through a chemically bonded silica gel chromatographic column in order to carry out secondary separation, carrying out gradient elution with an A2 solution and B solution mixed solution, and collecting an elution component to obtain a semaglutide solution, wherein the above A1 solution is a carbonate solution; the above A2 solution is a phosphate solution; and the B solution is an acetonitrile and isopropanol mixed solution with a volume ratio of acetonitrile to isopropanol of (8:2)-(9:1). The purifying method has the advantages of realization of high purity of the prepared semaglutide, substantial improvement of the semaglutide yield, simple operation, and facilitation of realization of large-scale preparation of semaglutide.
Owner:HYBIO PHARMA
Main peptide chain of semaglutide and preparation method thereof
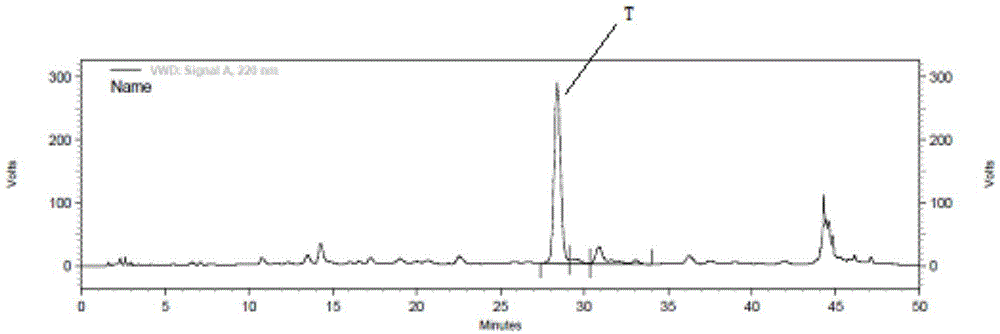
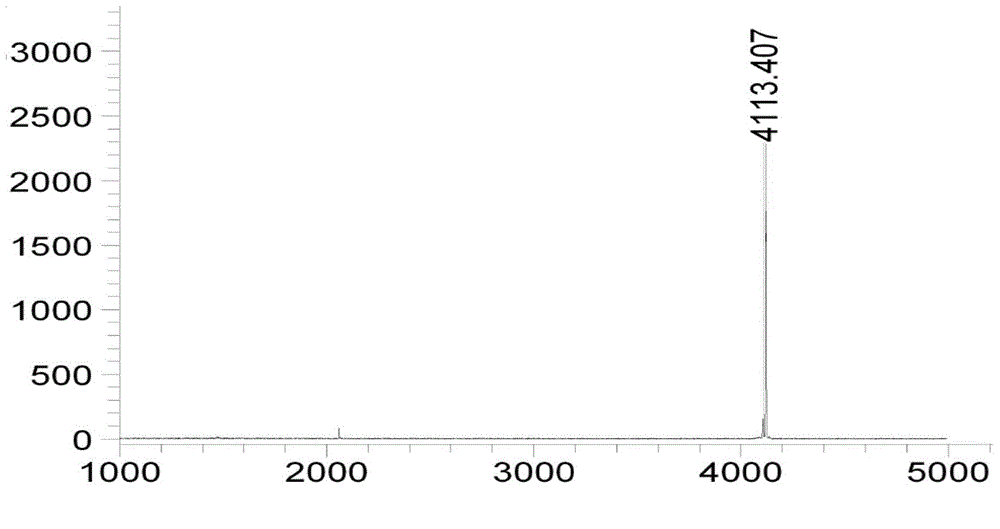
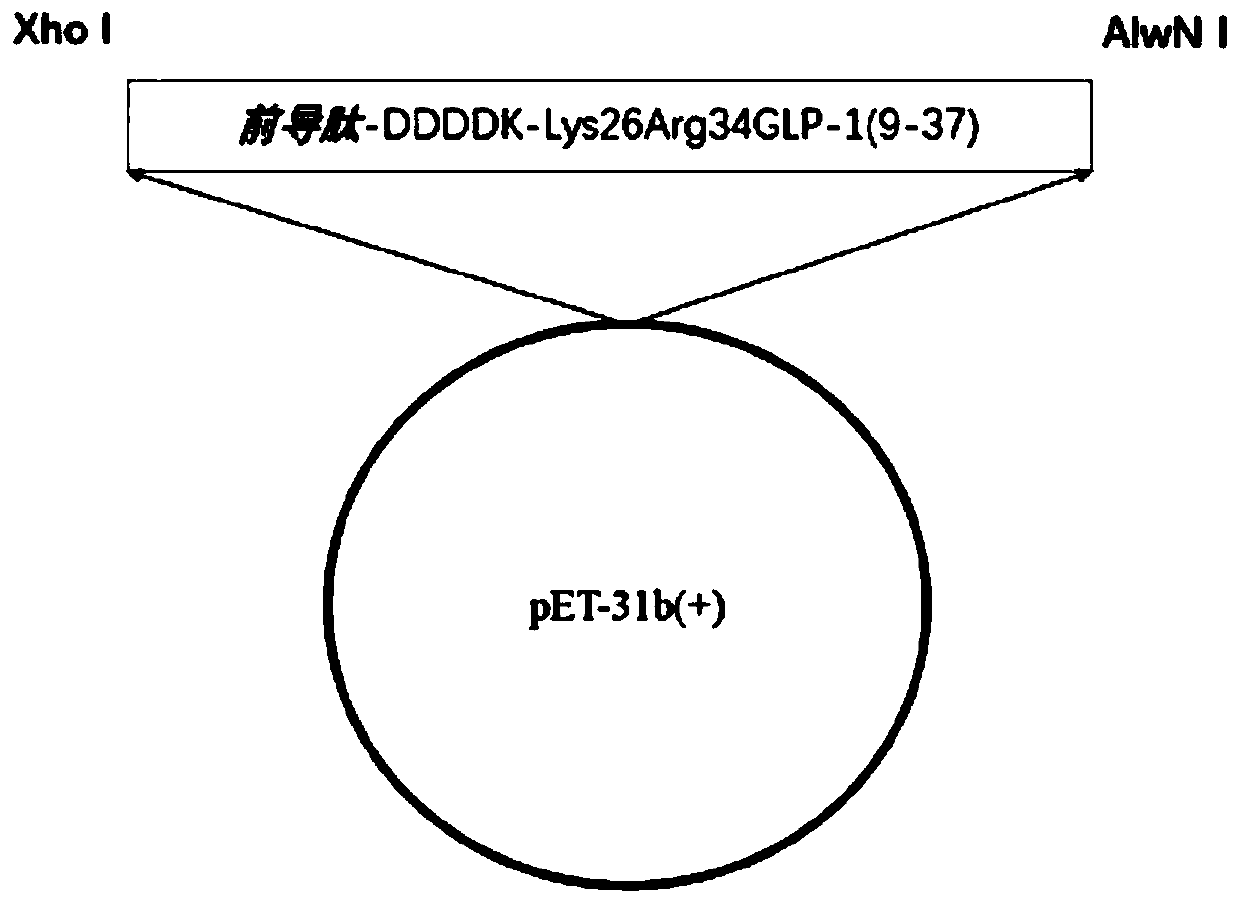
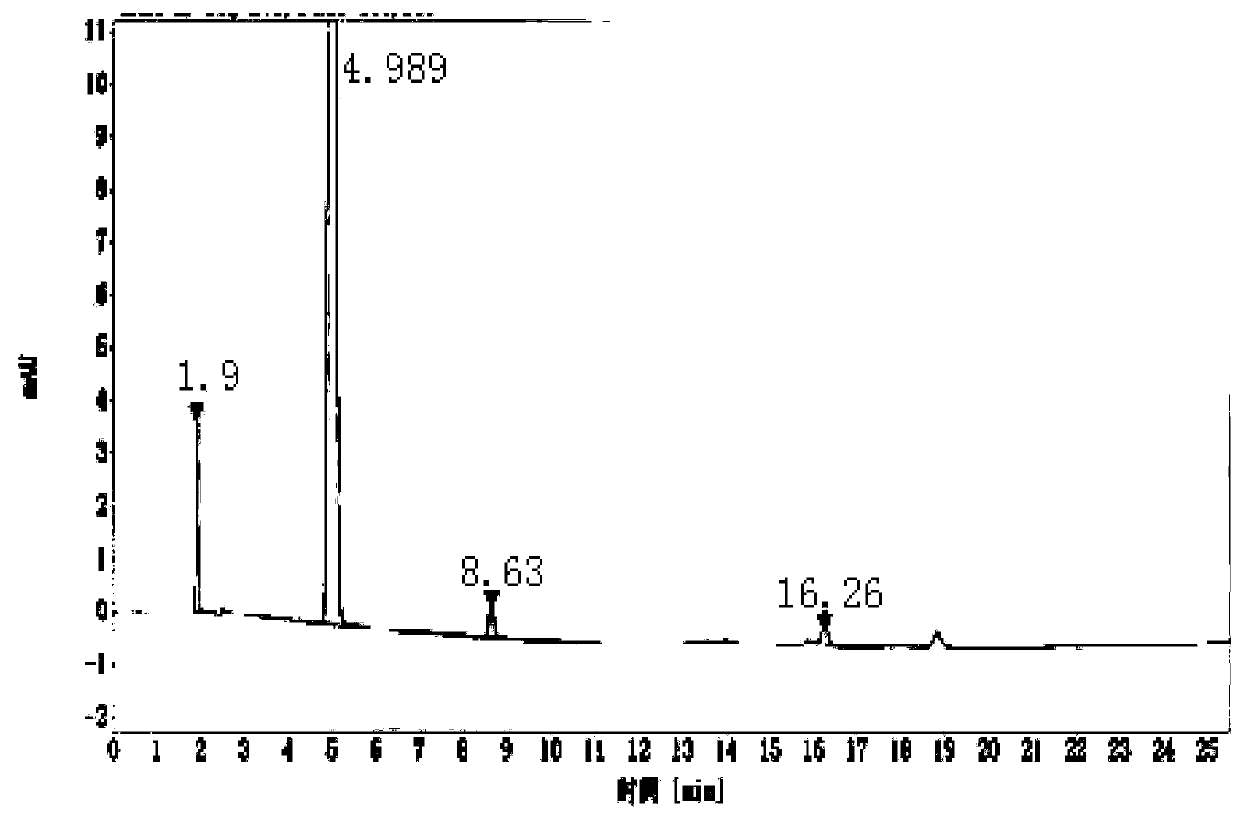
InactiveCN110498849AHigh purityImprove the purification effectBacteriaMicroorganism based processesIon exchangeDsbA
The invention discloses a main peptide chain of semaglutide and a preparation method thereof; and more specifically, the invention discloses recombinant protein of intermediate polypeptide of somaluptide. The recombinant protein of the intermediate polypeptide of the somaluptide is leader peptide-DDDDK-GLP-1 (9-37), and has an amino acid sequence as shown in SEQ ID NO. 1. When the intermediate polypeptide of the somalutide is being prepared, the following leader peptides can be adopted: (1) MFLKGDGYVQGIINFEGLHHLVALGLV; (2) KSI; (3) TrxA; (4) DsbA, (5) DsbC; (6) Sumo; (7) GST; (8) Intein; and (9) KPSTYI. The intermediate polypeptide of the somalutide prepared by the preparation method is high in polypeptide purity that the polypeptide purity can be up to 89% or above; and moreover, yield ofthe intermediate polypeptide can be higher than 82%. By adopting ion-exchange separation and purification, the preparation method has the characteristics of being high in degree of separation, good in purification effects, few in impurities and easy to operate.
Owner:南京迪维奥医药科技有限公司
Synthesis method of acylated GLP-1 compound and modified gene thereof
The invention relates to a synthesis method of an acylated GLP-1 compound and modified gene thereof. The synthesis method of the acylated GLP-1 compound and modified gene thereof is characterized by comprising the following steps of preparing a compound 2, namely, taking GLP-1 (9-37) as an initial raw material, carrying out PEG medication by a compound 1 on Lys of a position 26 of a relative sequence GLP-1 (9-37), controlling the reaction pH and carrying out an acylation coupling reaction to obtain a compound 2; preparing a compound 4, namely, taking the compound 2 as the initial raw material,carrying out modification of GLP-1 (9-37) end amino by a compound 3, and then carrying out a liquid phase acylation coupling reaction with a reaction condensing agent and alkali to obtain a compound4; an preparing Semaglutide, namely, taking the compound 4 as the initial raw material, carrying out deprotection to obtain final Semaglutide. The method is simple and convenient to operate, the sidereaction is reduced, the risk of racemization of the amino acid compound is greatly reduced, and the method has a wide application prospect and is convenient for large-scale production.
Owner:VONSUN PHARMATECH CO LTD +2
Preparation method of Semaglutide
ActiveCN110078816AEnhanced couplingHigh purityPeptide preparation methodsBulk chemical productionSide chainImpurity
The invention provides a preparation method of Semaglutide. By using a short peptide chain Fmoc-Thr<13>-Ser<14>-Asp<15>-OH, impurity deletion caused by the a 14 locus Ser difficult reaction locus is directly avoided; by using a two-step method to couple side chains Fmoc-AEEA-AEEA-OH and Octa(OtBu)-Glu(alpha-OtBu)-gamma-COOH, the problems that the yield and purity are relatively low due to side chain fragment insolubility and low reaction activity are solved, the purification difficulty of the Semaglutide is greatly reduced, the yield and purity of a product are improved, the purity of the obtained high-quality product is greater than 99.0%, the cost of preparing the Semaglutide is also reduced, and the preparation method of the Semaglutide has broad practical value and application prospect.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
Semaglutide liposome and preparation method thereof
InactiveCN104055735AReduce gastrointestinal reactionsImprove stabilityPeptide/protein ingredientsMetabolism disorderMedicineCholesterol
The invention relates to the technical field of medicine, and especially relates to semaglutide liposome and a preparation method thereof. The semaglutide liposome provided in the invention comprises phospholipid, semaglutide, cholesterol and a pharmaceutically-acceptable cryoprotectant, and has a particle size of 50 nm-200 nm. The semaglutide liposome provided in the invention adopts phospholipid and cholesterol as membrane materials, and semaglutide is wrapped in the membrane materials to form liposome with negative charges. Therefore, the damage of semaglutide to pancreas and kidney is reduced; the gastrointestinal reaction after semaglutide administration is alleviated; and the stability and bioavailability of semaglutide are improved.
Owner:HYBIO PHARMA
A kind of solid-phase synthesis method of Samolute
ActiveCN103848910BEase of mass productionReduce generationPeptide preparation methodsAnimals/human peptidesAcetic acidSide chain
The invention relates to the technical field of polypeptide synthesis, and particularly relates to a solid synthetic method of semaglutide. The solid synthetic method includes coupling Gly and a resin to obtain a Gly-resin; coupling step by step for the first time an ammonia acid or an amino acid derivative to obtain a first peptide resin the sequence of which is shown as SEQ ID No.1; removing a side chain protective group of Lys; coupling step by step for a second time 2-(2-(2-aminoethoxy)ethoxy) acetic acid, 2-(2-(2-aminoethoxy)ethoxy) acetic acid, Glu and octadecanedioic acid to obtain a second peptide resin; performing pyrolysis; and purifying. The solid synthetic method is simplified in operation step, short in synthetic period, low in cost, reduced in production of waste liquid, low in side products and high in product yield, and is suitable for large-scale production of the semaglutide.
Owner:HYBIO PHARMA
Synthesis method of semaglutide
ActiveCN110372785AScale upAvoid incomplete couplingPeptide preparation methodsGlucagonsSide chainSynthesis methods
The invention discloses a synthesis method of semaglutide. The method includes the steps that an Oct-gamma-Glu(tBu)-AEEA-AEEA-OH side chain fragment and a Fmoc-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(tBu)-Val-OH hexapeptide fragment are prepared in advance, and then the two fragments are coupled to a semaglutide main chain sequence to obtain the semaglutide. The method can effectively avoid the phenomenon that the amino acids between His and Lys in the sequence cannot be coupled completely due to a secondary structure in thesemaglutide synthesis process, reduces the synthesis difficulty of the product, a large number of missing peptide impurities with very small structural difference and the preparation and purification difficulty of the crude semaglutide product, improves the yield of the product, and ensures the product quality ofasemaglutidebulk drug.
Owner:CHENGDU SINTANOVO BIOTECHNOLOGV CO LTD
GLP-1 analogue and GC-C receptor agonist composite sustained release preparation and preparation method thereof
PendingCN107929718AGood curative effectGood treatment effectPeptide/protein ingredientsMetabolism disorderPlecanatideDiabetes mellitus
The invention a GLP-1 analogue and GC-C receptor agonist composite sustained release preparation and a preparation method thereof. The sustained release microsphere preparation consists of a GLP-1 analogue, a GC-C receptor agonist and a pharmaceutically acceptable polymer material. The GLP-1 analogue is selected from liraglutide or pharmaceutically acceptable salt thereof, semaglutide or pharmaceutically acceptable salt thereof, exenatide or pharmaceutically acceptable salt thereof, trulicity or pharmaceutically acceptable salt thereof and albiglutide or pharmaceutically acceptable salt thereof. The GC-C receptor agonist is selected from Llinaclotide or pharmaceutically acceptable salt thereof, and plecanatide or pharmaceutically acceptable salt thereof. The composition can be used for effectively treating diabetics, and meanwhile, the composition can be used for preventing and relieving diabetic constipation; and the composition has practical significance for relieving patient's painand improving patient's life quality.
Owner:南京星银药业集团有限公司
Preparation method of semaglutide
InactiveCN110922470ASolve the problem of polycondensationReduce the ratioPeptide preparation methodsBulk chemical productionResin acidBiochemical engineering
The invention discloses a preparation method of semaglutide. The preparation method comprises: first preparing a semaglutide resin by a solid-phase peptide synthesis method, and then acidifying the semaglutide resin, and simultaneously removing the resin and a side chain protecting group to obtain crude semaglutide, and then purifying the crude semaglutide to obtain a refined semaglutide. The preparation method greatly reduces difficulties in the synthesis of semaglutide, effectively controls a ratio of racemic impurities, improves purity of the crude semaglutide, reduces amount of the used raw materials, and greatly reduces difficulties in purification, and has wide practical value and application prospect.
Owner:杭州肽佳生物科技有限公司
Method for preparing semaglutide side chain by liquid phase method
InactiveCN111269137AEfficient synthesisWide choiceCarbamic acid derivatives preparationOrganic compound preparationSide chainAklanonic acid
The invention discloses a method for preparing a semaglutide side chain. The preparation method comprises the following steps: protecting amino of an initial raw material 2-(2-aminoethoxy) ethanol byusing R1; then carrying out nucleophilic substitution reaction with alpha halogenated ester to prolong a carbon chain; preparing an aliphatic chain with two protected ends by a one-pot method; removing a protecting group at one end of each aliphatic chain and condensing to obtain a compound 7; removing the R1 protecting group to obtain a compound 8, performing condensation reaction on the compound8 and fluorenylmethoxycarbonyl-L-glutamic acid 1-tert-butyl ester to obtain a compound 10, removing the fluorenylmethoxycarbonyl, performing amidation condensation reaction on the compound 10 and 18-(tert-butoxy)-18-oxooctadecanoic acid to obtain a compound 13, and removing the R2 protecting group to obtain a target product chain 1. Compared with solid-phase synthesis, the method disclosed by theinvention is lower in cost and wider in selection of protecting groups, and has industrial production and application prospects.
Owner:ZHEJIANG UNIV OF TECH
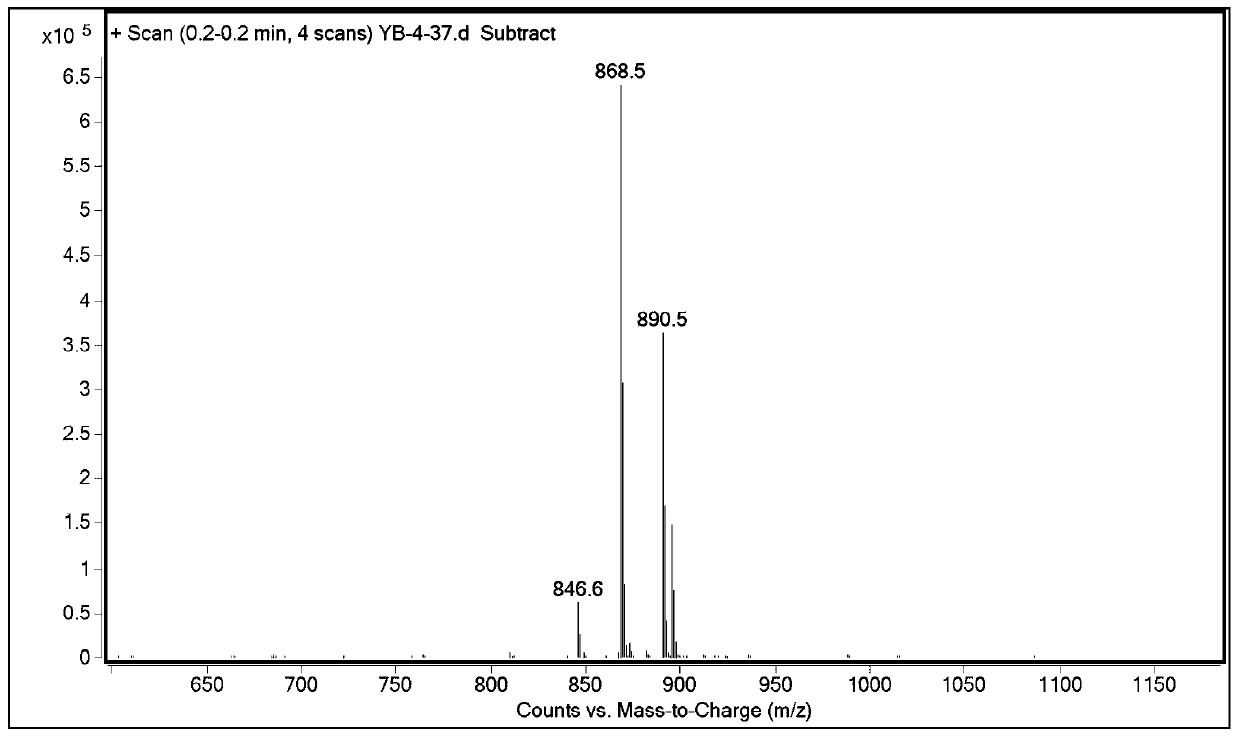
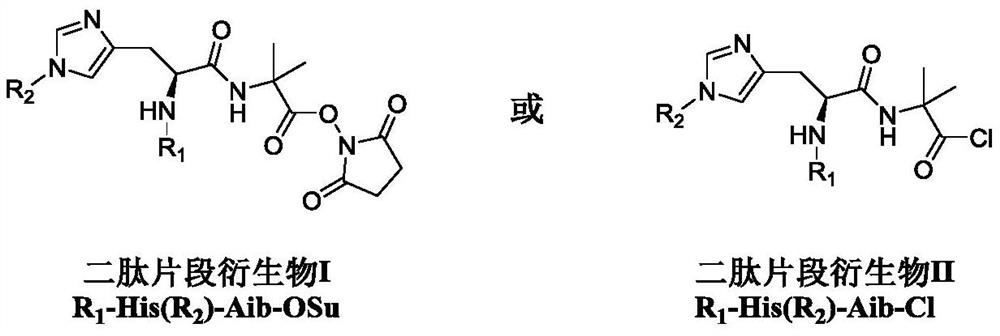
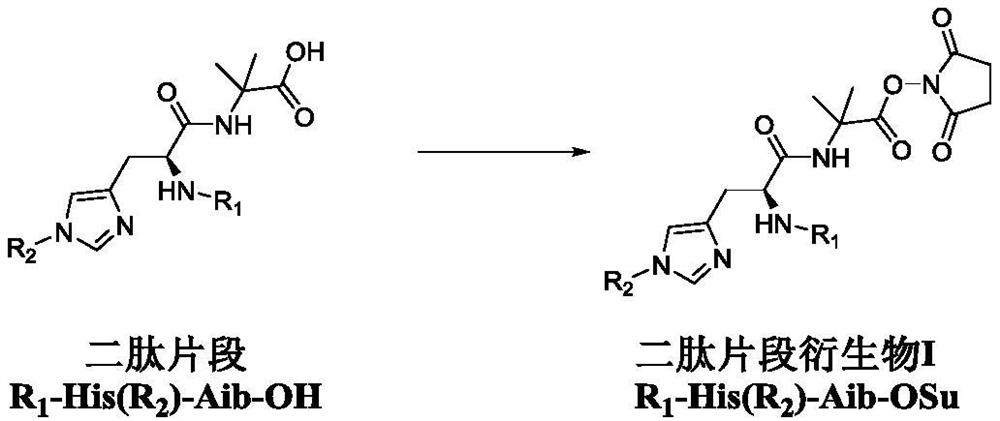
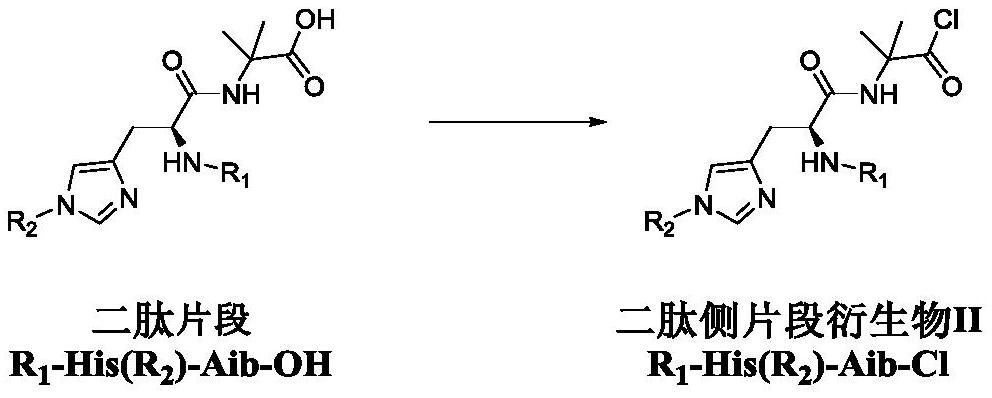
Dipeptide fragment derivative for synthesizing semaglutide and preparation method of dipeptide fragment derivative
PendingCN112625087AImprove reaction efficiencyReduce the probability of racemizationPeptide preparation methodsBulk chemical productionDipeptideCombinatorial chemistry
The invention relates to a dipeptide fragment derivative for synthesizing semaglutide. The dipeptide fragment derivative can directly react with a main chain of semaglutide; and compared with a polypeptide method, the reaction efficiency is greatly improved, carbonyl of histidine does not participate in the reaction any more in the reaction, and racemization is not generated at a chiral site of histidine. The invention also provides a preparation method of the dipeptide fragment derivative. The dipeptide fragment derivative is prepared from a dipeptide fragment R1-His(R2)-Aib-OH with high optical purity. On the other hand, the invention also provides a synthetic method of the dipeptide fragment, and the method is used in the synthetic process of the semaglutide, so that the content of impurities can be reduced, and the product quality of the semaglutide is improved.
Owner:JINAN KANGHE MEDICAL TECH
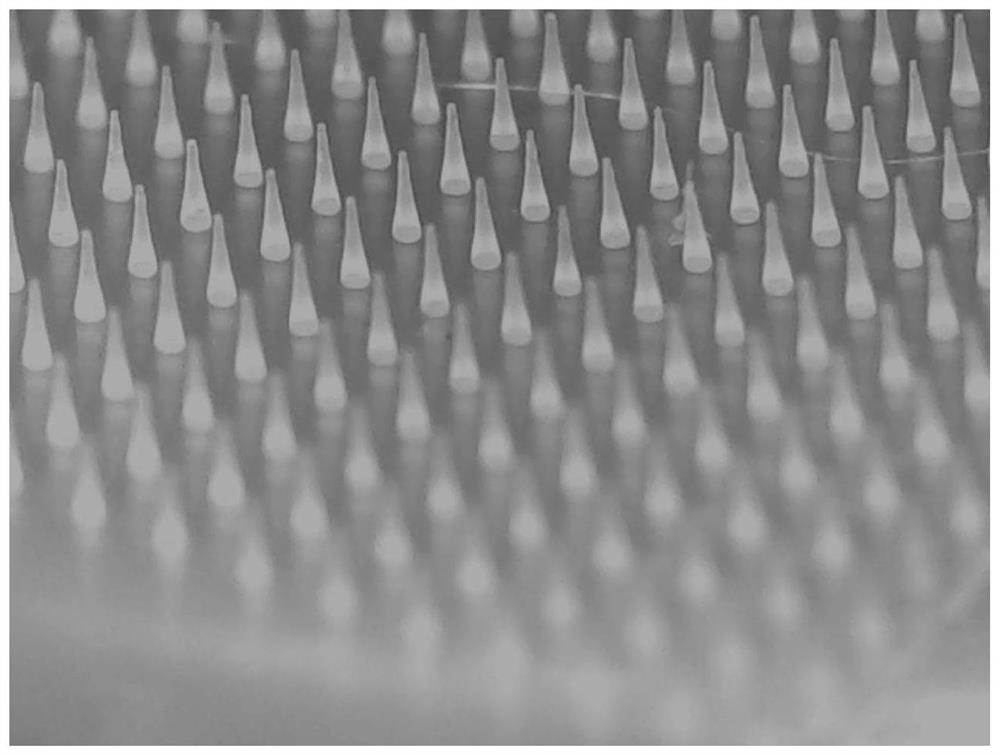
Somaglutide microneedle patch capable of reducing blood glucose and reducing weight as well as preparation method and application thereof
PendingCN112274633AGood hypoglycemic effectHigh mechanical strengthPeptide/protein ingredientsMetabolism disorderMeth-Pyrrolidinones
The invention relates to a somaglutide microneedle patch capable of reducing blood glucose and reducing weight as well as a preparation method and application thereof. The microneedle patch comprisesa microneedle body and a substrate, wherein the microneedle body is prepared from the following components in parts by weight: 0.01-5 part of somaglutide, 0.1-5 part of a penetration promoting component, 10-50 parts of a small molecular protective agent, 45-85 parts of a macromolecular framework material and 65-200 parts of an aqueous solution containing a pH buffer agent; the permeation promotingcomponent is selected from one or more of N-(8-(2-hydroxy benzoyl) amino) caprylate, azone and propylene glycol; the small molecule protective agent is selected from one or more of mannose, glucose,fructose and trehalose; and the macromolecular framework material is selected from one or more of hyaluronic acid or sodium salt thereof, polyvinylpyrrolidone, an N-(2-hydroxypropyl) methacrylamide copolymer and glucan. The microneedle patch can improve the absorption permeability of the somaglutide in the skin so as to reduce the dosage of the somaglutide.
Owner:广州新济薇娜生物科技有限公司 +1
Continuous flow solid-phase reaction preparation of semaglutide
ActiveCN111732650AReduce consumptionEasy to recyclePeptide preparation methodsBulk chemical productionChain structureCombinatorial chemistry
The invention discloses a method for preparing semaglutide by combining a continuous flow solid-phase synthesis system. The method comprises the following steps of firstly, introducing Octadecanedioic(OtBu) acid-gamma-Glu-(OtBu)-AEEA-AEEA into a side chain of Lys26, and then completing a strategy of a main chain, wherein Fmoc-Gly-resin is adopted as a main chain structure, and continuous flow solid-phase synthesis is adopted for polypeptide fragment and semaglutide peptide chain growth.
Owner:苏州金顶生物有限公司 +1
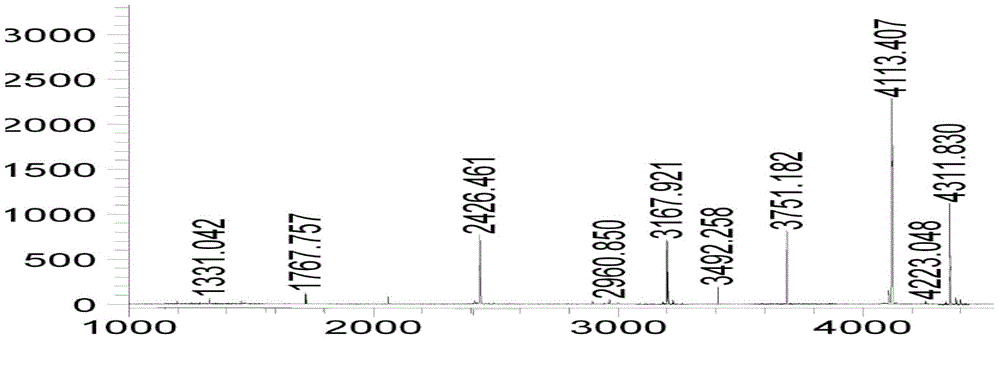
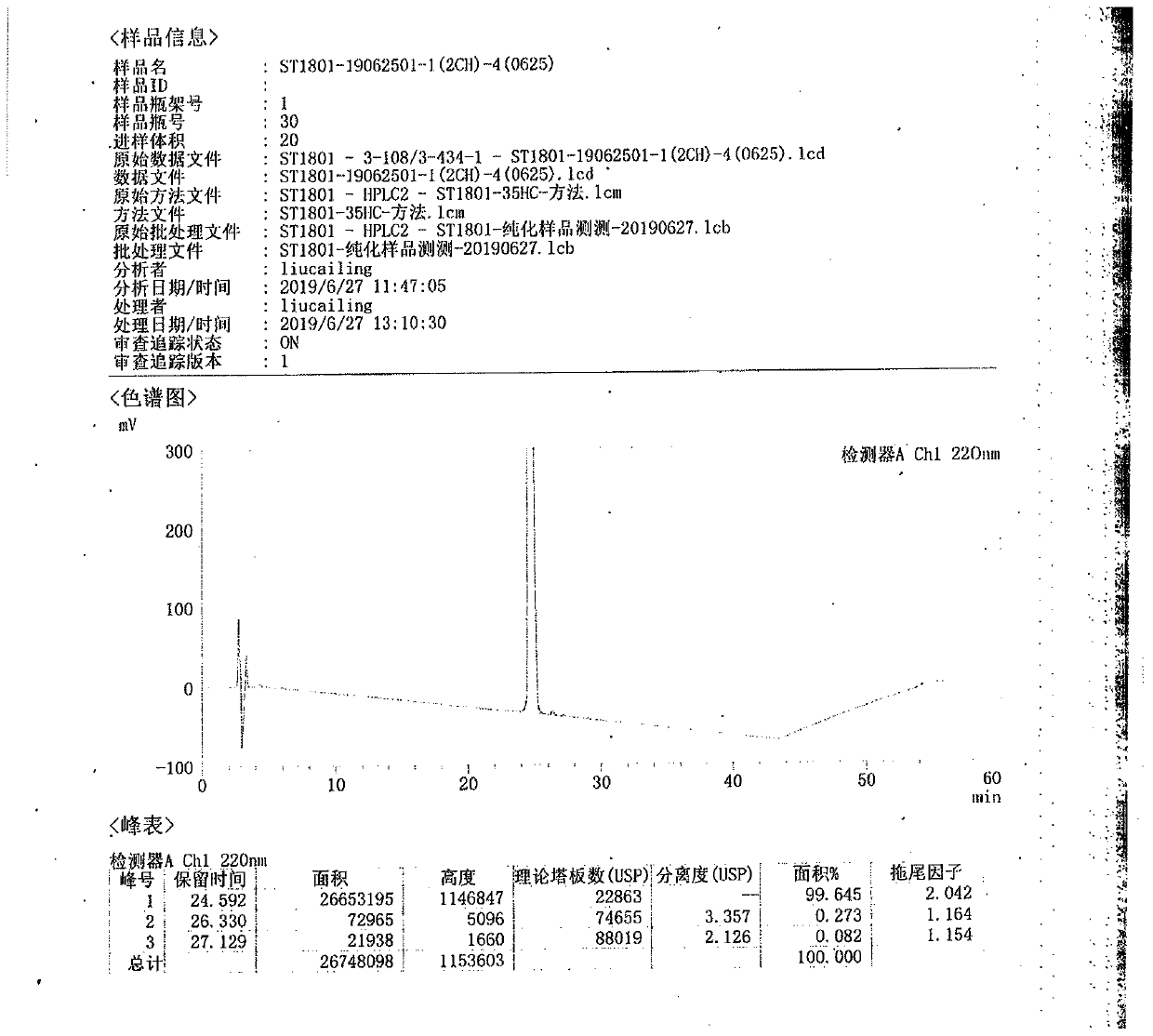
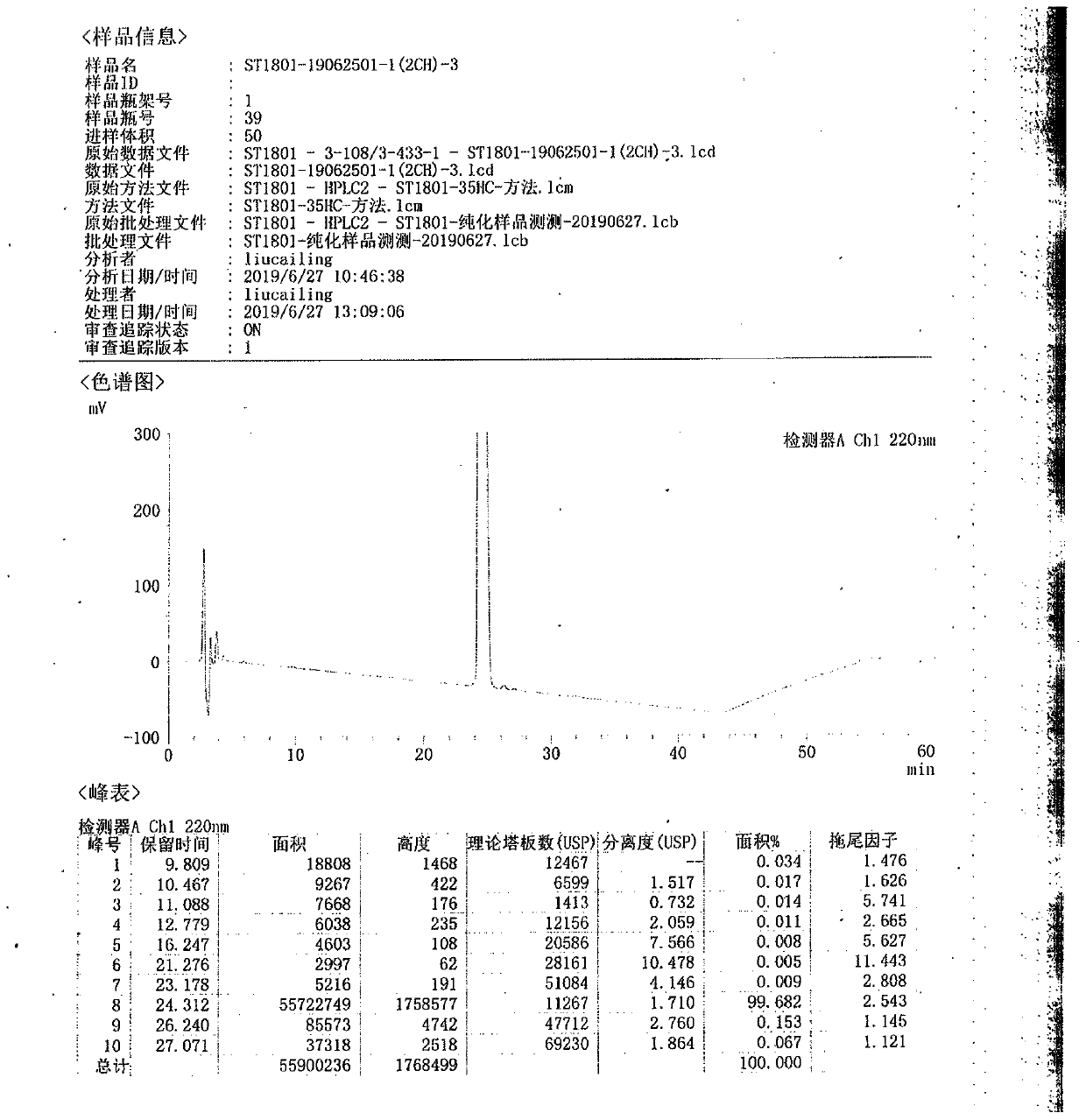
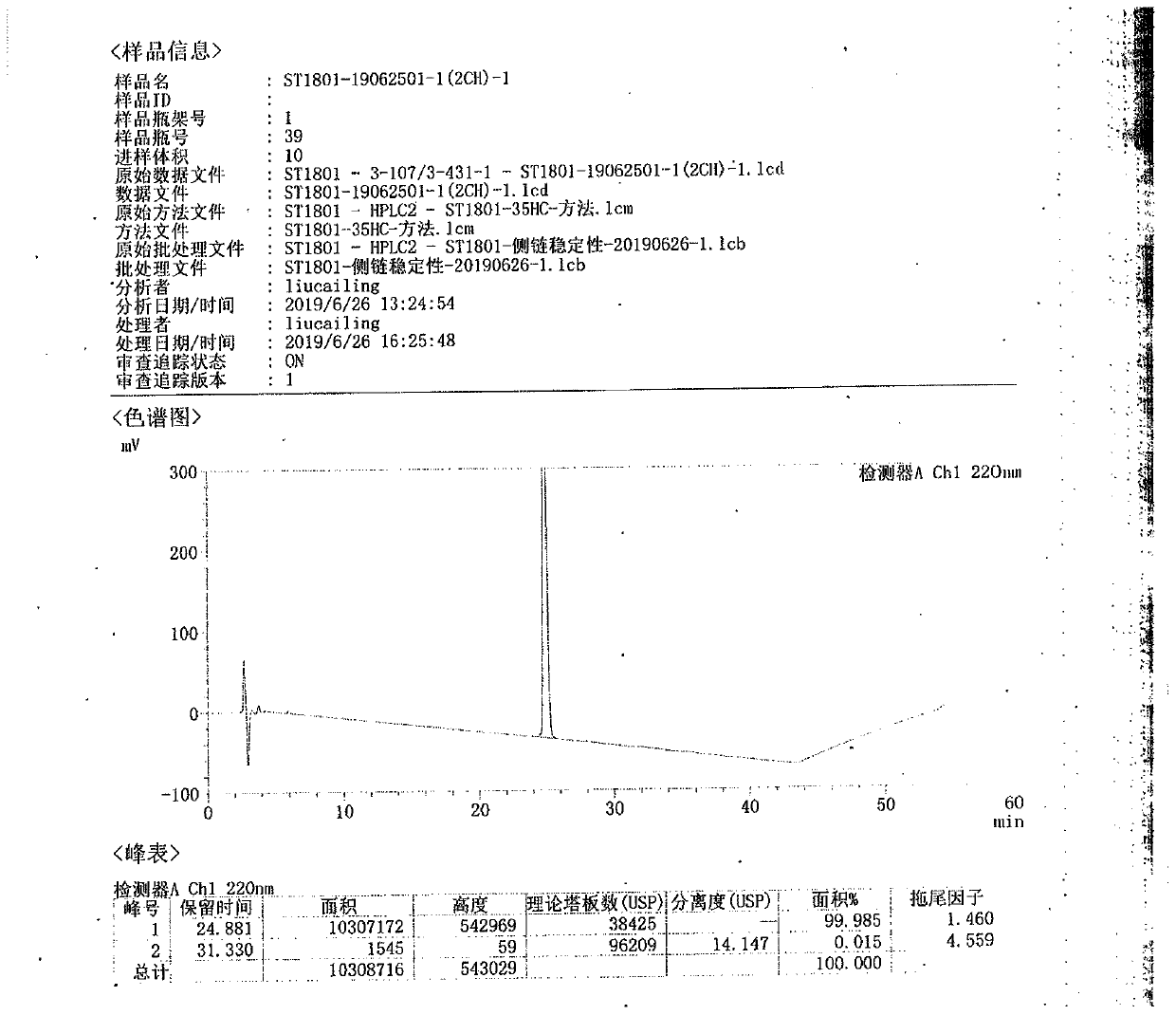
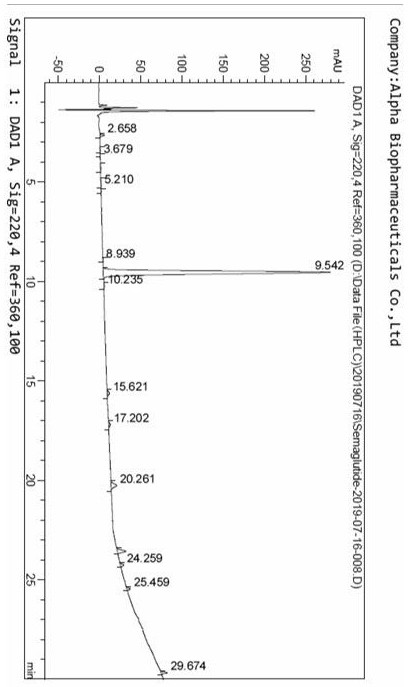
Ultra-high performance liquid chromatography analysis method of semaglutide
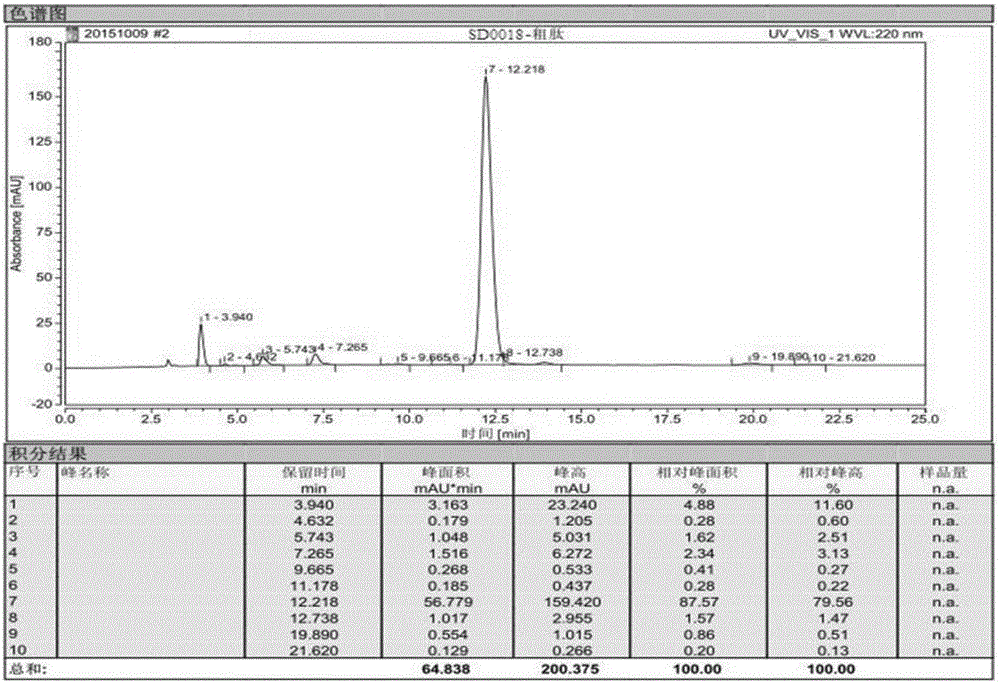
The invention discloses an ultra-high performance liquid chromatography analysis method of semaglutide. The method is characterized by comprising the following steps: in an ultra-high performance liquid chromatograph, respectively mixing a mobile phase A and a mobile phase B according to different proportions to obtain a mobile phase, and carrying out gradient elution on a semaglutide sample, wherein the mobile phase A is selected from a mixed aqueous solution of one or more of trifluoroacetate, perchlorate and hexafluorophosphate, and the pH value is adjusted to be 2-4, and the mobile phase Bis selected from methanol, isopropanol and acetonitrile. According to the method, chromatographic column series connection and multiple buffer salt mixed elution technologies are adopted, and the product quality of the semaglutide can be accurately and effectively detected under specific chromatographic conditions, so that the medication stability of a patient is improved.
Owner:HUBEI JIANXIANG BIOLOGICAL PHARM CO LTD
Method for preparing semaglutide by liquid phase method on basis of soluble carrier
InactiveCN111116731AAvoid missingAvoid elevationPeptide preparation methodsBulk chemical productionFluid phasePharmaceutical drug
The invention provides a method for preparing semaglutide by a liquid phase method on the basis of a soluble carrier. The invention solves the technical problems of tedious method, difficulty in purification and lower yield in a conventional solid phase method for preparing long-chain polypeptides like the semaglutide. According to the invention, the semaglutide is divided into six fragments whichare prepared by taking lipophilic and hydrophobic 2,4-di(docosaalkoxy)benzyl alcohol as a carrier through a one-by-one coupling method. The method provided by the invention is different from a traditional solid phase method, can simply monitor whether a reaction is completely carried out or not through TLC or a liquid phase method in the reaction, effectively avoids the loss or addition of singleamino acid residue impurities, effectively lowers the purification difficulty, enhances the utilization ratio of raw materials, and conforms to the green chemistry concept pushed nowadays. The methodprovided by the invention can also be widely applied to the technical field of preparation of polypeptide drugs.
Owner:SHANDONG UNIV
Purification method of semaglutide
PendingCN112279907AAvoid problems such as poor water solubility of acetateAvoid problems such as poor water solubilityPeptide preparation methodsGlucagonsO-Phosphoric AcidSolvent
The invention discloses a purification method of semaglutide. The method comprises the following steps: (1) dissolving a crude semaglutide product in dilute ammonia water, carrying out water bath andfiltering successively, and collecting a filtrate; (2) performing gradient elution by taking a reverse-phase silica gel filler as a stationary phase, a salt solution with a pH value regulated to 7-9 by ammonia water as a mobile phase A and acetonitrile as a mobile phase B, collecting fractions, and performing rotary evaporation to remove a part of the solvent to obtain a primarily-purified solution; (3) carrying out gradient elution by taking the reversed-phase silica gel filler as the stationary phase, a saline solution with a pH value adjusted to 2-3 by phosphoric acid as the mobile phase Aand a mixed solution of acetonitrile and isopropanol as the mobile phase B, collecting fractions, and carrying out rotary evaporation to remove a part of the solvents so as to obtain a secondarily-purified solution; and (4) carrying out gradient elution by taking the reverse-phase silica gel filler as the stationary phase, an aqueous solution of ammonium bicarbonate as the mobile phase A and acetonitrile as the mobile phase B, collecting fractions, carrying out rotary evaporation, and carrying out freeze-drying so as to obtain saltless refined peptide of semaglutide, wherein the saltless refined peptide has purity of greater than 99.5% and an individual impurity content of less than 0.1%. The method is simple and convenient to operate and facilitates large-scale preparation of semaglutide.
Owner:SHENZHEN JYMED TECH
Semi-synthetic preparation method of semaglutide
ActiveCN111153983AHigh purityHigh yieldPeptide preparation methodsGlucagonsBiochemical engineeringThreonine
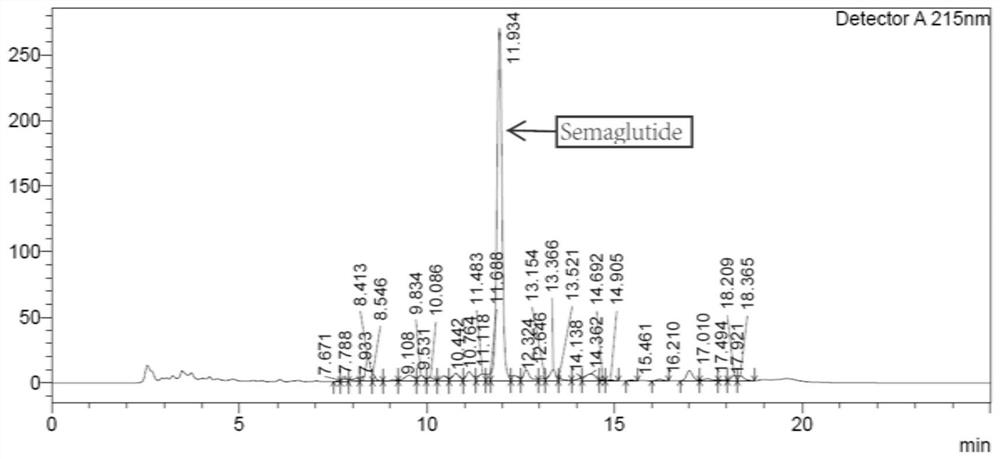
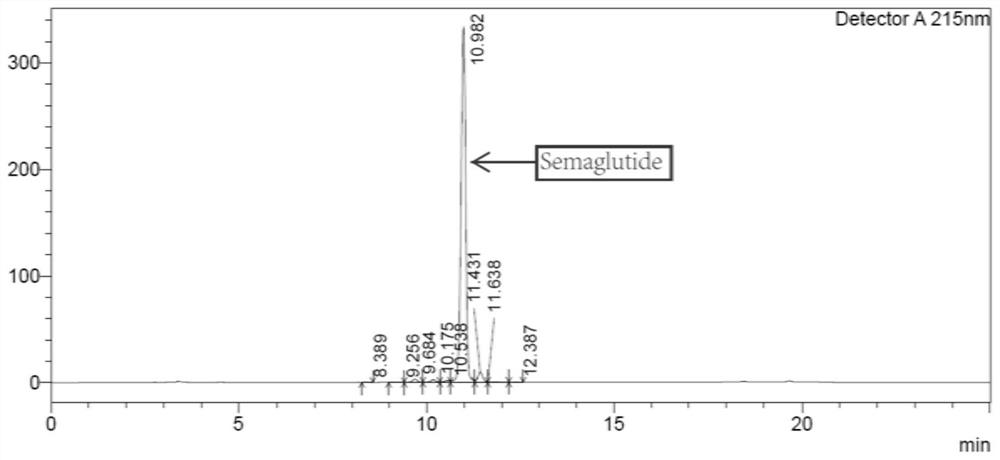
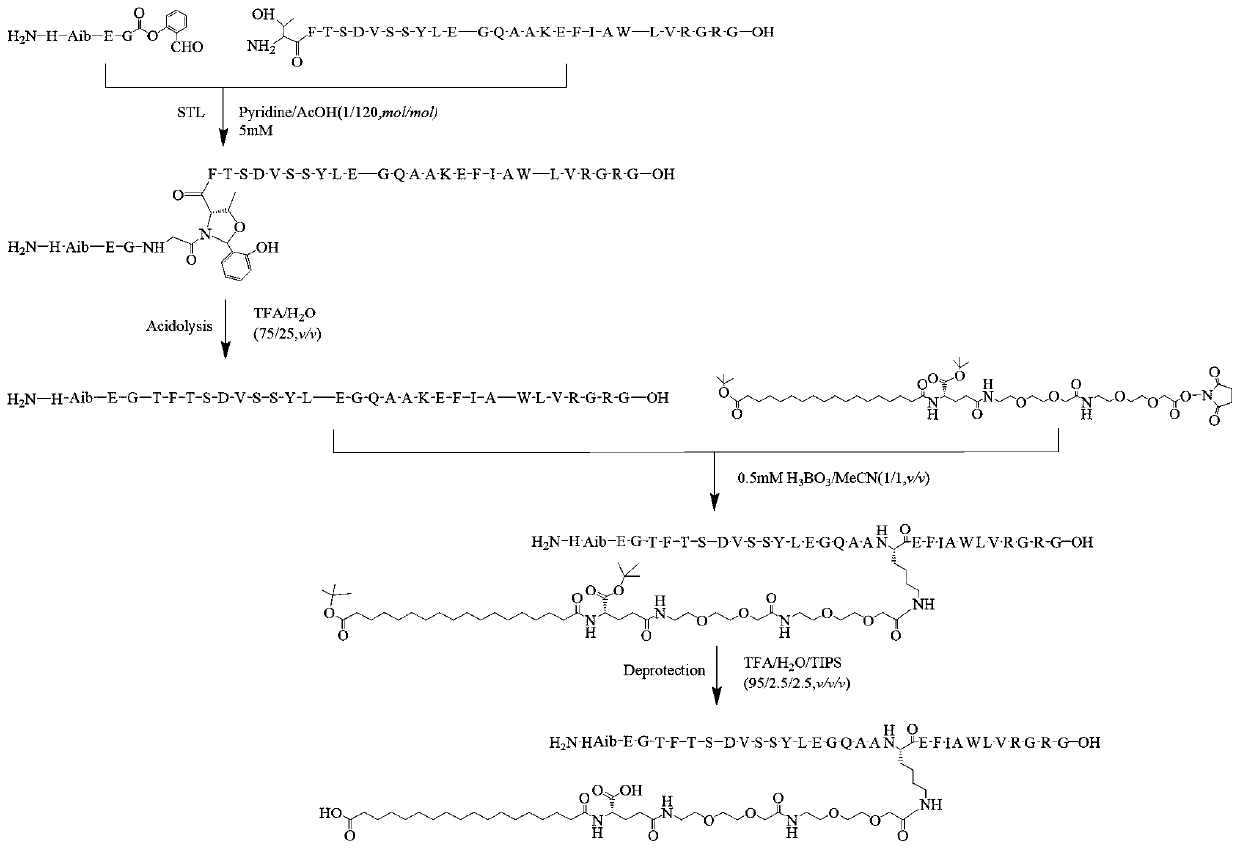
The invention relates to a technology of polypeptide synthesis, and belongs to the technical field of pharmaceutical chemistry. A preparation method of semaglutide is provided. Based on a method (STL)of natural chemical connection of serine and threonine, the semaglutide can be high in purity, high in yield, less in reaction step and simple in operation, so that large-scale preparation of the semaglutide can be realized.
Owner:JIANGNAN UNIV
Method for preparing semaglutide by biochemical method
PendingCN113278061AEasy accessStrong specificityBacteriaMicroorganism based processesEscherichia coliOrganic chemistry
The invention belongs to the field of preparation of recombinant polypeptides, particularly relates to the technical field of preparation of the recombinant polypeptides by a chemistry and biology combined method, and more particularly relates to a method for preparing semaglutide by a biochemical method. By creatively splitting 31 amino acids of a main peptide chain of the semaglutide are into a 3-site amino acid polypeptide chain (MI chain) and a 28-site amino acid polypeptide chain (MII chain), the possibility of efficiently and quickly preparing the semaglutide under an escherichia coli preparation system is realized. After the technical scheme disclosed by the invention is adopted, a semaglutide product can be rapidly, efficiently and highly specifically obtained, and meanwhile, a large number of chemical reagents are not needed, so that the method is a green and environment-friendly preparation method.
Owner:NANJING FORESTRY UNIV +1
Solid-phase synthesis method of semaglutide
ActiveCN112679602AReduce generationLow costPeptide preparation methodsBulk chemical productionDipeptideCombinatorial chemistry
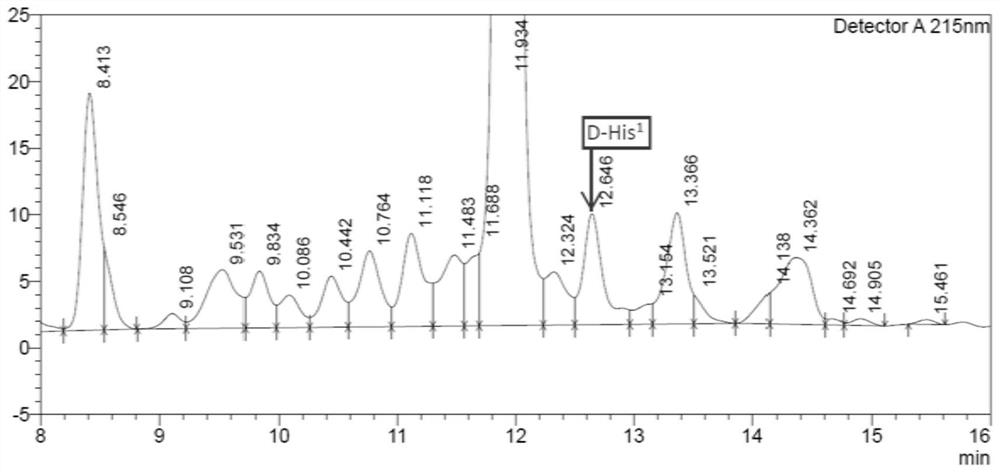
The invention discloses a solid-phase synthesis method of semaglutide. According to the solid-phase synthesis method of the semaglutide, the semaglutide is divided into three fragments to be synthesized simultaneously through a solid-phase fragment synthesis technology, and dipeptide or tripeptide is used for replacing original single amino acid at part of positions during fragment synthesis. According to the solid-phase synthesis method of the semaglutide, the synthesis period is effectively shortened by 50%; and the generation of racemization impurities is greatly reduced, especially formation of [D-His1]-semaglutide is greatly reduced, formation of deletion peptides or polyaminopeptides in conventional one-by-one coupling, especially generation of [+Ser11]-semaglutide, [+Ala19]-semaglutide and [+Gly29]-semaglutide impurities, is effectively avoided, the purity of crude peptides is improved, downstream purification is facilitated, the material cost is greatly reduced, and the solid-phase synthesis method of the semaglutide is suitable for industrial production, and the total yield reaches 35%.
Owner:苏州特瑞药业股份有限公司
Preparation method of semaglutide
ActiveCN114790474AHigh yieldReduce manufacturing costPeptide preparation methodsFermentationBiotechnologyEnzyme digestion
The invention relates to a preparation method of semaglutide. The preparation method comprises the following steps: collecting an inclusion body from a fermentation thallus, treating the inclusion body to prepare a fusion protein, carrying out enzyme digestion and purification treatment on the fusion protein to obtain a semaglutide precursor, and connecting the semaglutide precursor with a side chain and dipeptide to obtain semaglutide. The semaglutide precursor fusion protein prepared by the invention is subjected to enzyme digestion treatment by using a mutant EK enzyme. According to the preparation method provided by the invention, high-expression recombinant engineering bacteria and high-activity and high-yield mutant EK enzyme are combined at the same time, so that the production efficiency of semaglutide can be remarkably improved, and the production cost of semaglutide can be remarkably reduced.
Owner:BEIJING HUIZHIHENG BIOTECHNOLOGY CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com