Synthesis method of acylated GLP-1 compound and modified gene thereof
A technology of GLP-1 and compound, applied in the field of synthesis of acylated GLP-1 compound and its modification group, can solve the problems of many racemic impurities, waste of raw materials, poor activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
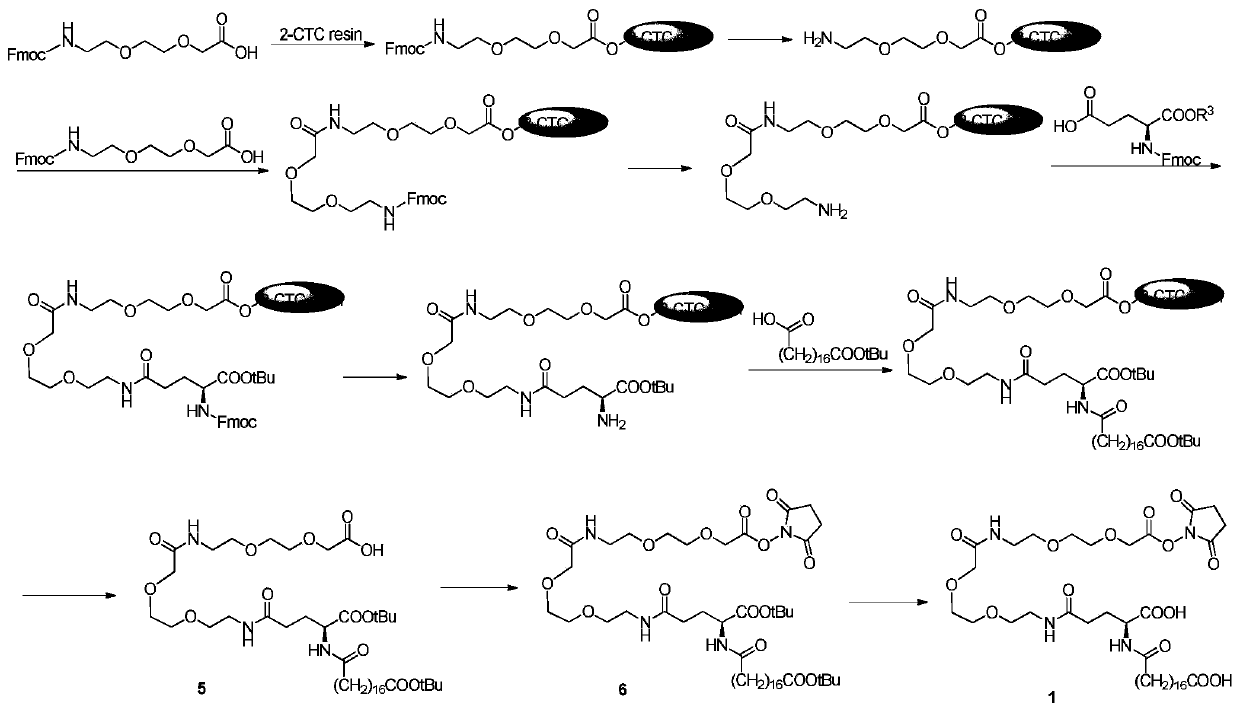
[0057] Synthesis of Compound 5:
[0058] Weigh 15.97g of 2-chlorotriphenyl chloride resin (sub=0.939mmol / g), add it to a solid-phase synthesis column, use Fmoc-AEEA-OH (1.5eq), DIPEA (4.5eq), solvent DCM, Reaction for 1.5h to obtain Fmoc-AEEA-resin, after synthesis, use 20% piperidine / DMF to deprotect, time: 5+15min;
[0059] When condensing the second AEEA, use a reaction system in which the molar ratio of 2-chlorotriphenyl chloride resin / Fmoc-AEEA-OH / HATU / DIPEA is 1:2:2:2.4, and react for 1.5h to obtain Fmoc-AEEA-AEEA- Resin, use 20% piperidine / DMF to deprotect after synthesis, the deprotection time is: 5+15min;
[0060] When condensing γ-Glu, use a reaction system in which the molar ratio of 2-chlorotriphenyl chloride resin / Fmoc-Glu(OH)-OtBu / HATU / DIPEA is 1:2:2:2.4, and react for 1.5h to obtain Fmoc-γ -Glu-AEEA-AEEA-resin, use 20% piperidine / DMF to deprotect after synthesis, the deprotection time is: 5+15min;
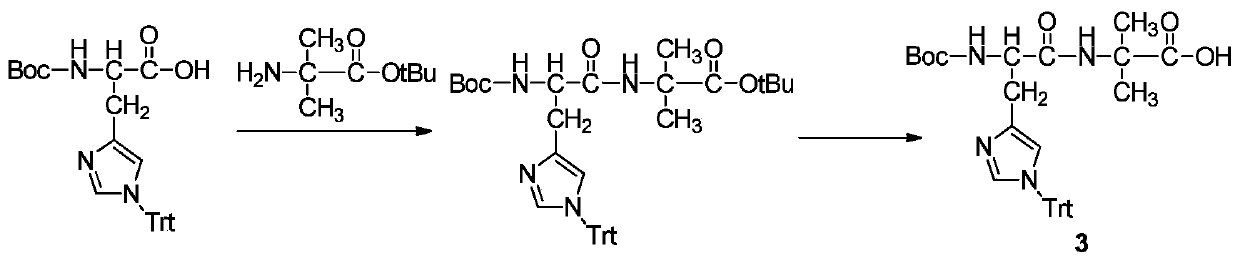
[0061] When condensing mono-tert-butyl octadecanedioate, use...
Embodiment 2
[0064] Synthesis of compound 6:
[0065] Weigh 13.96g of compound 5 (1.0eq) and 2.28g of N-hydroxysuccinimide (1.2eq), add 250mL of dichloromethane and stir to dissolve, add dropwise 4.09g of DCC (1.2eq) in 60ml of DCM solution under ice cooling After dropping, move to room temperature and stir the reaction. After reacting for 15 hours, filter and spin dry the filtrate to obtain a colorless transparent oil, add 430ml of acetonitrile and stir, a small amount of white solid precipitates, filter and spin dry the filtrate to obtain 16.50 g of compound 6 as a colorless oil, yield: 100%.
Embodiment 3
[0067] Synthesis of Compound 1:
[0068] Add 83mL TFA to 53mL DCM and mix well, and cool to 3-5 degrees Celsius, add it to 16.50g crude product of compound 6 under ice bath, then transfer to room temperature and stir for 1.5h, spin dry, add toluene to spin Steam (100mL*3) to obtain a brown oil. Add 300mL of diethyl ether under ice bath and stir to make a slurry, a large amount of white solid precipitates out, centrifuge, add diethyl ether to wash (400mL*3), dry to obtain 13.96g of white solid compound 1, yield: 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com