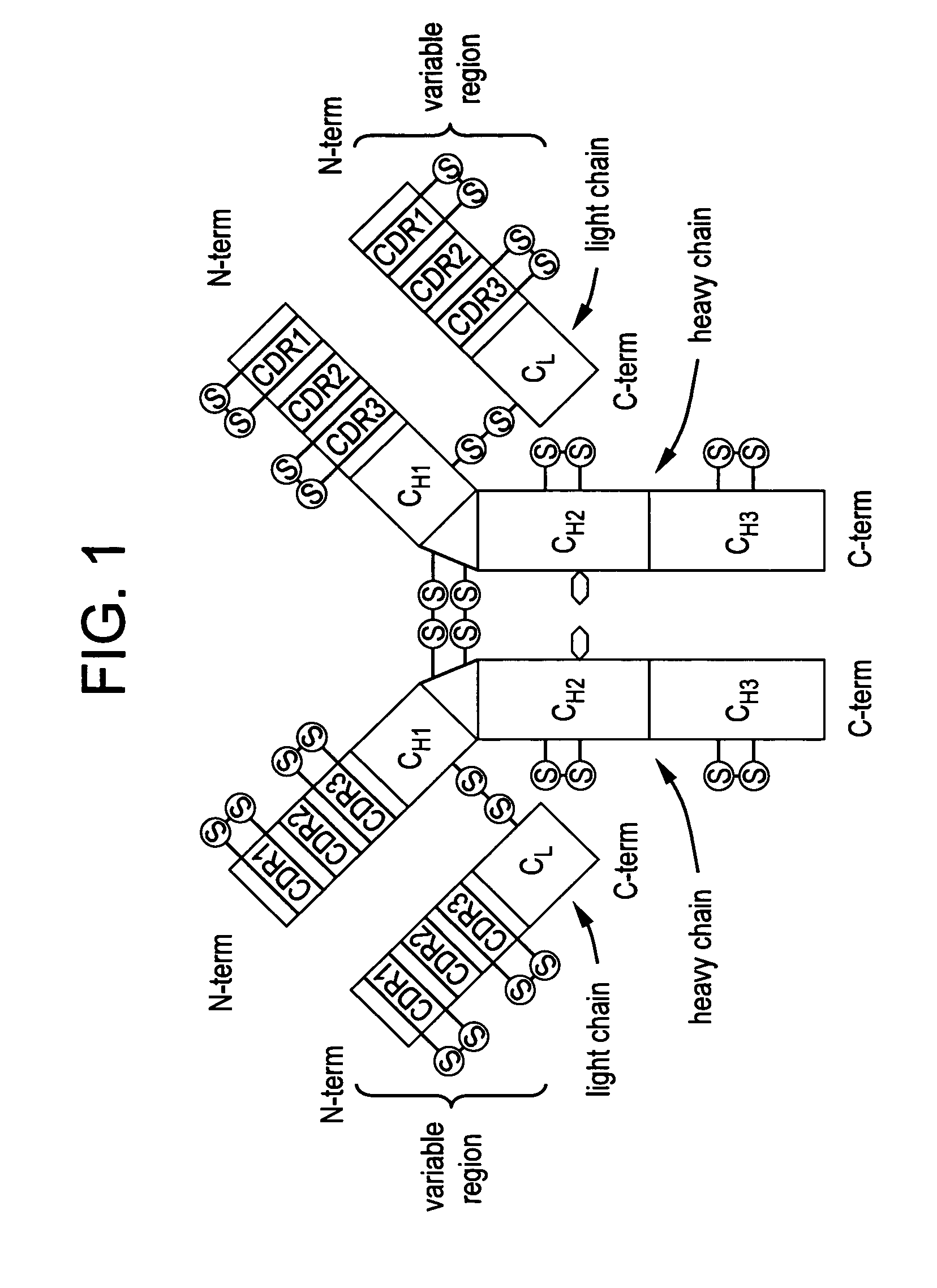
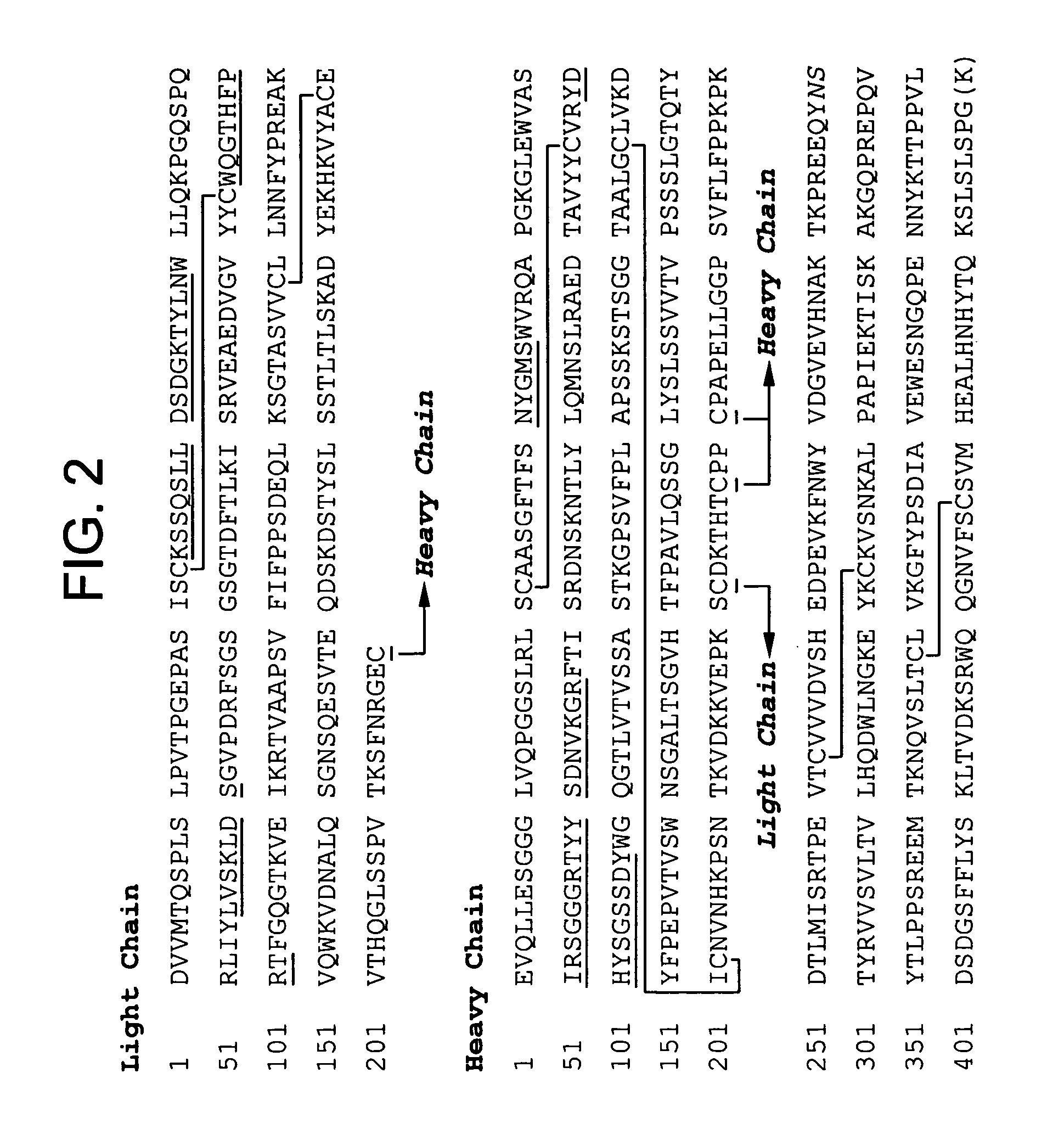
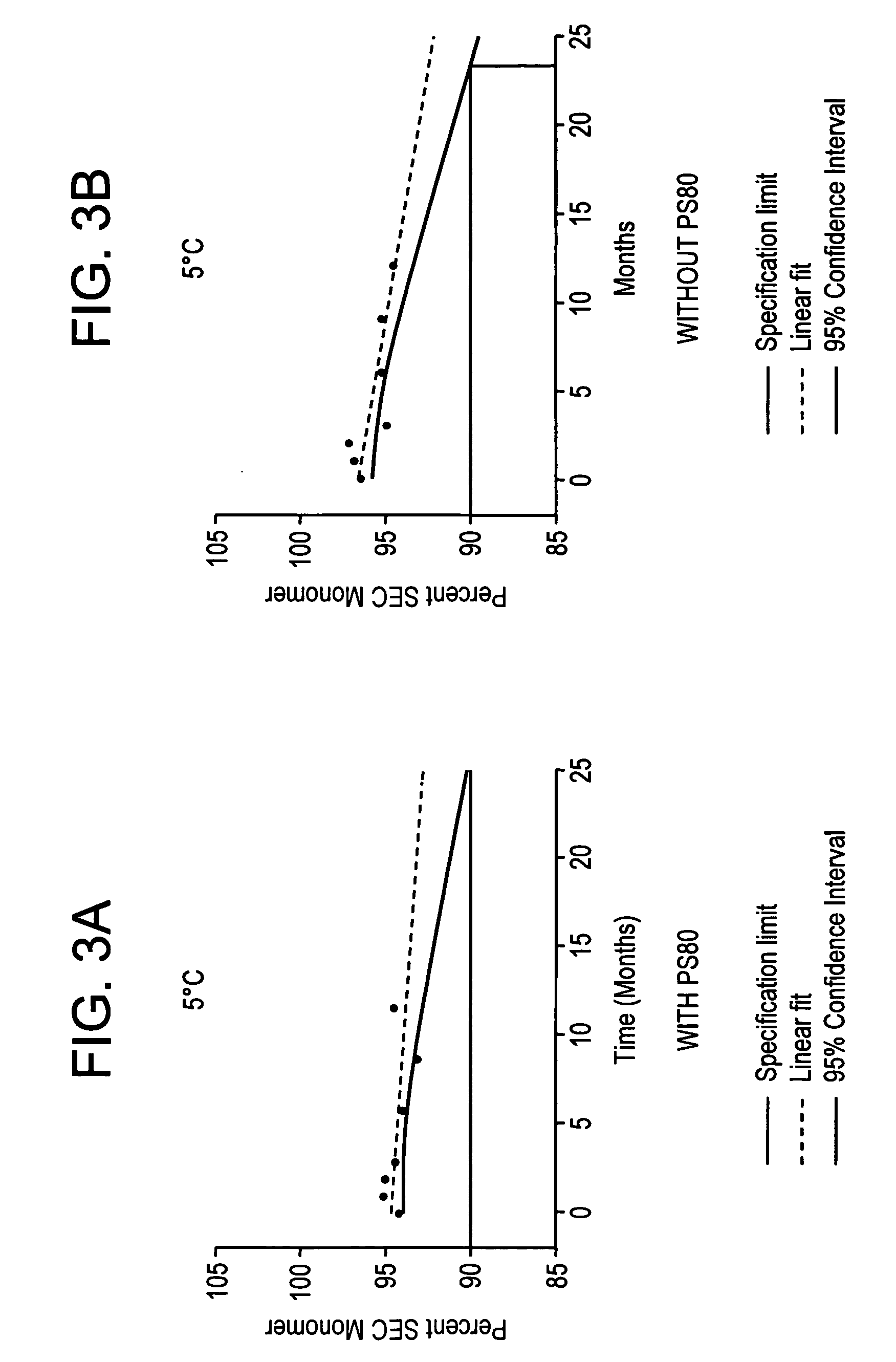
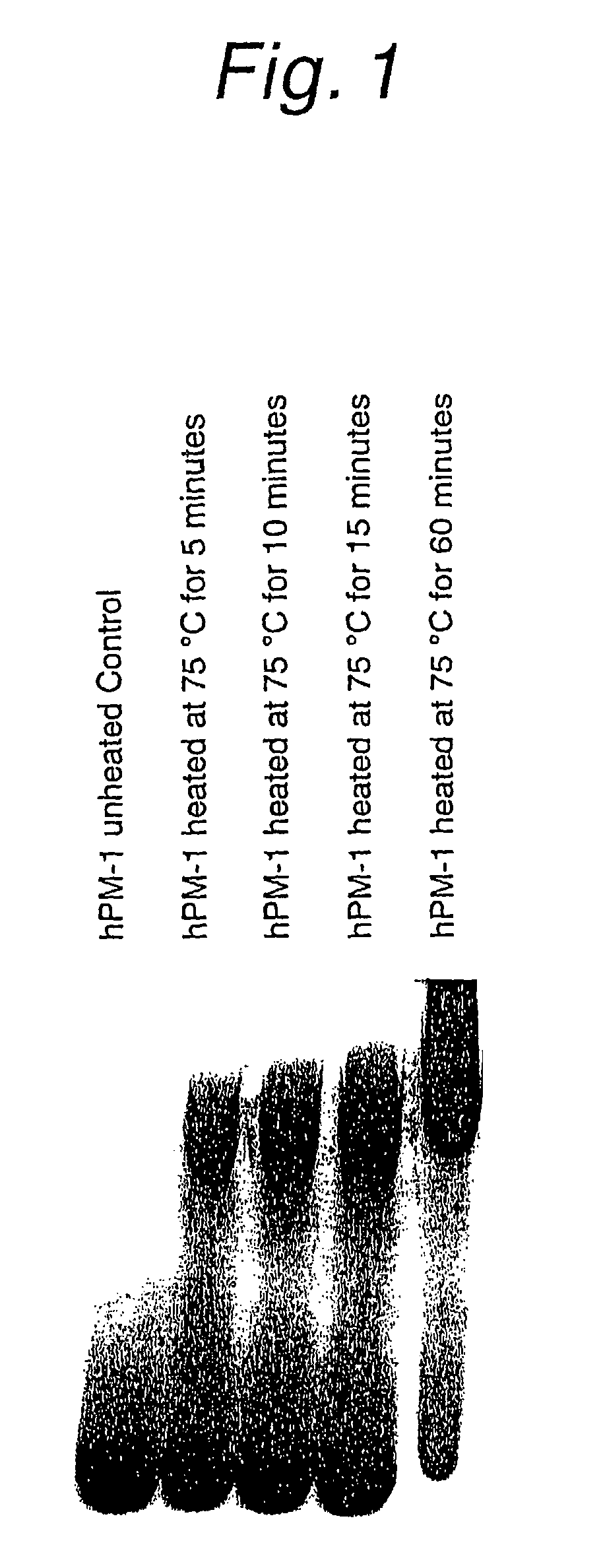
Patents
Literature
2281 results about "Histidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Histidine (symbol His or H) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH₃⁺ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO⁻ form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, longer-term studies have shown it is essential for adults also. It is encoded by the codons CAU and CAC.
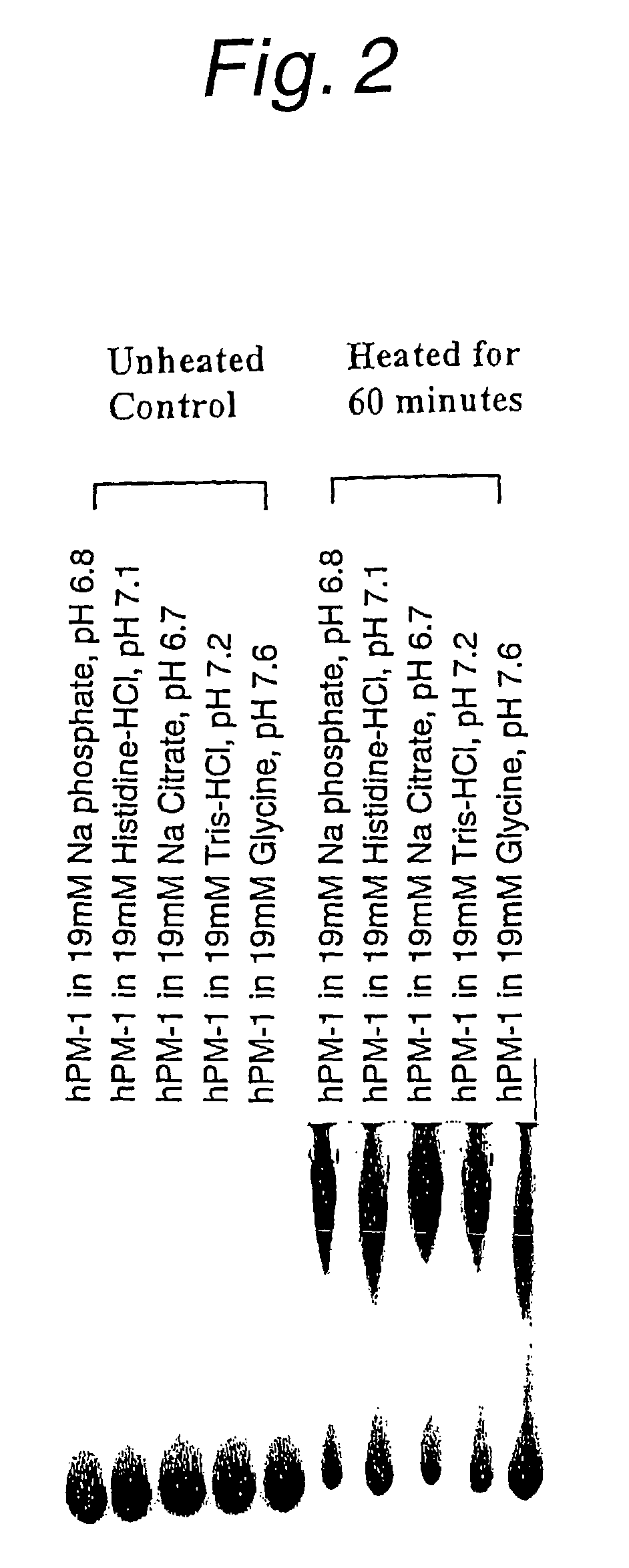
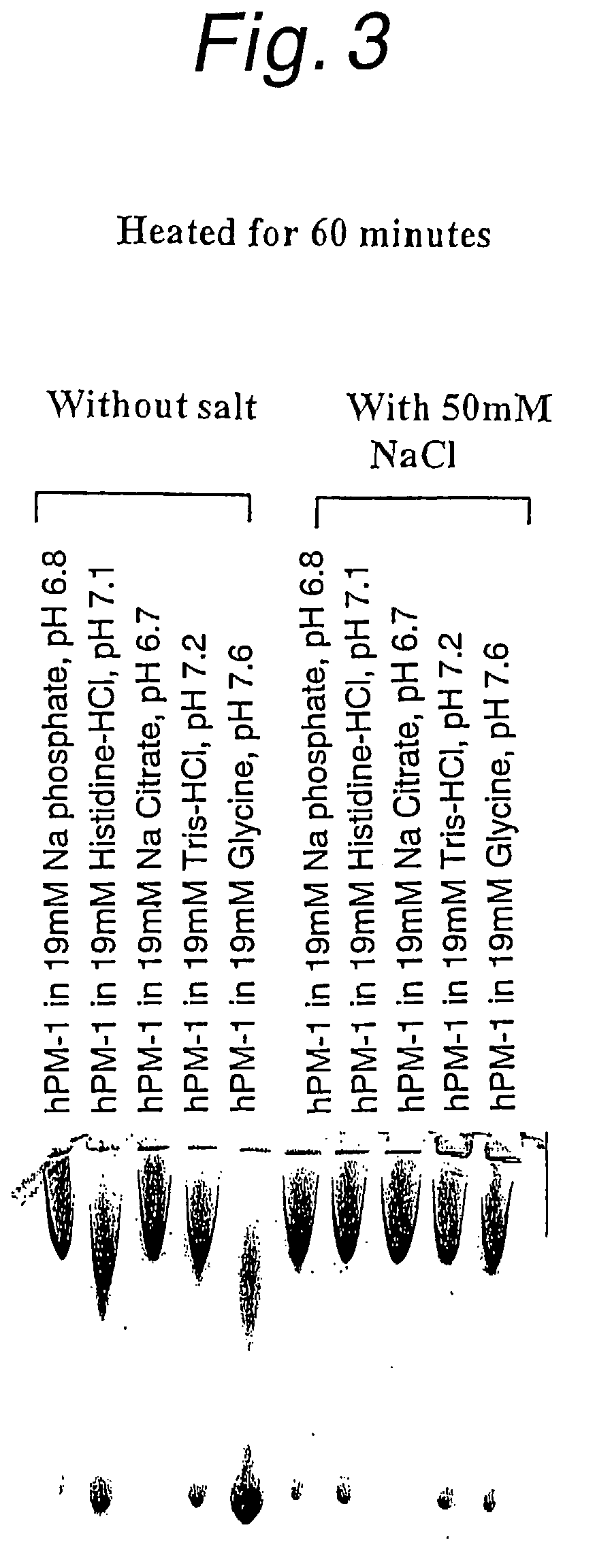
Antibody formulations
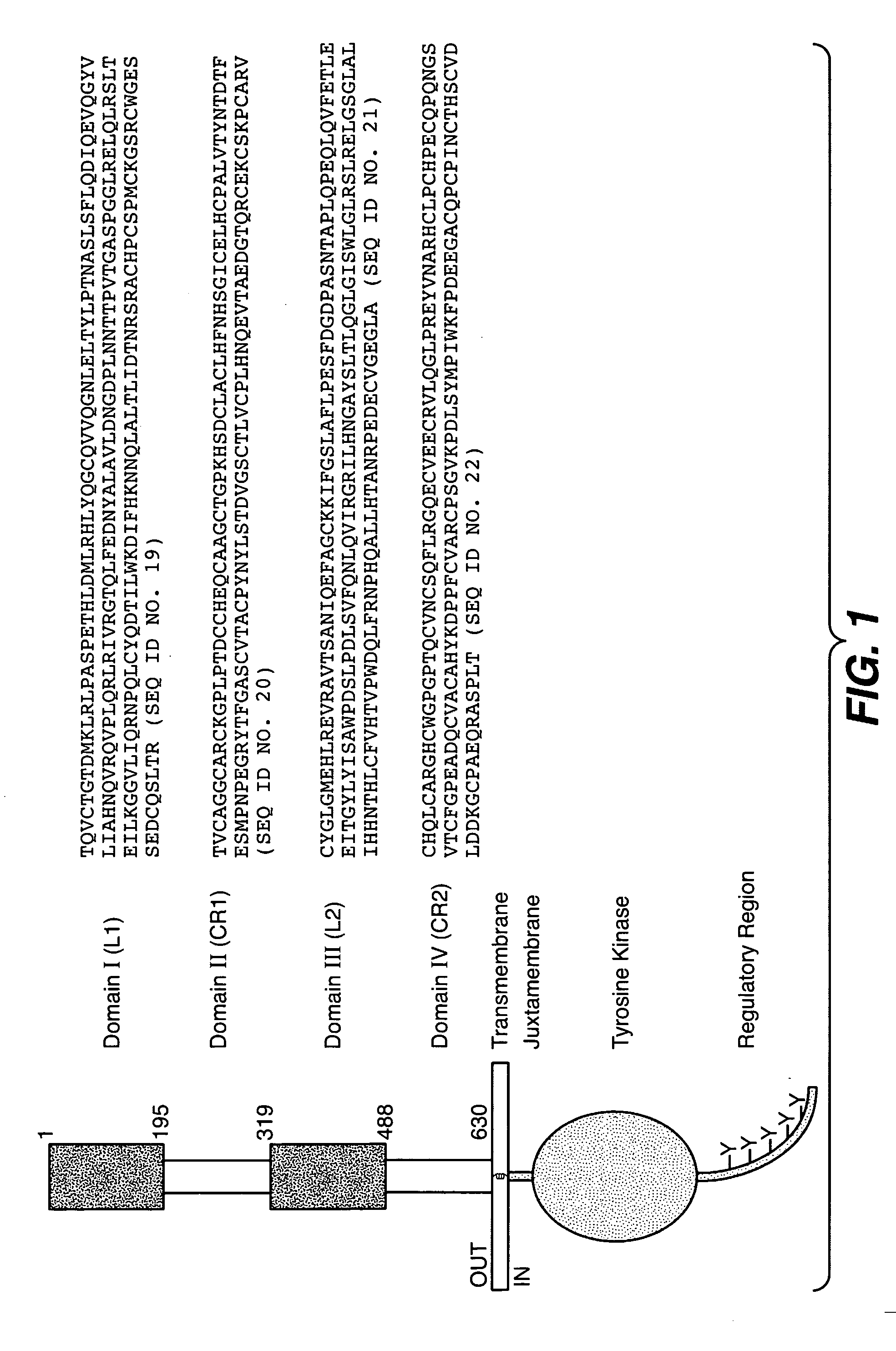
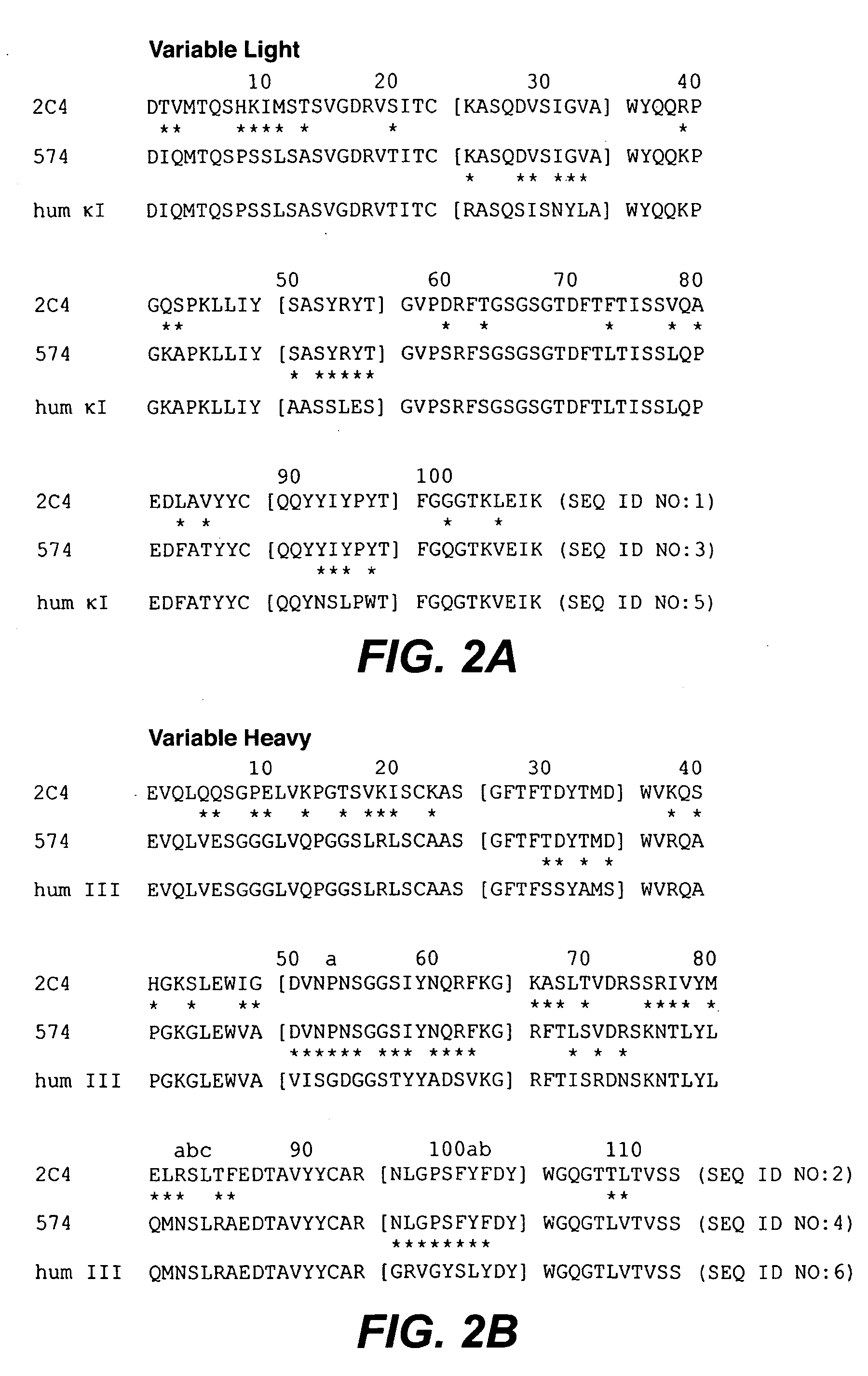
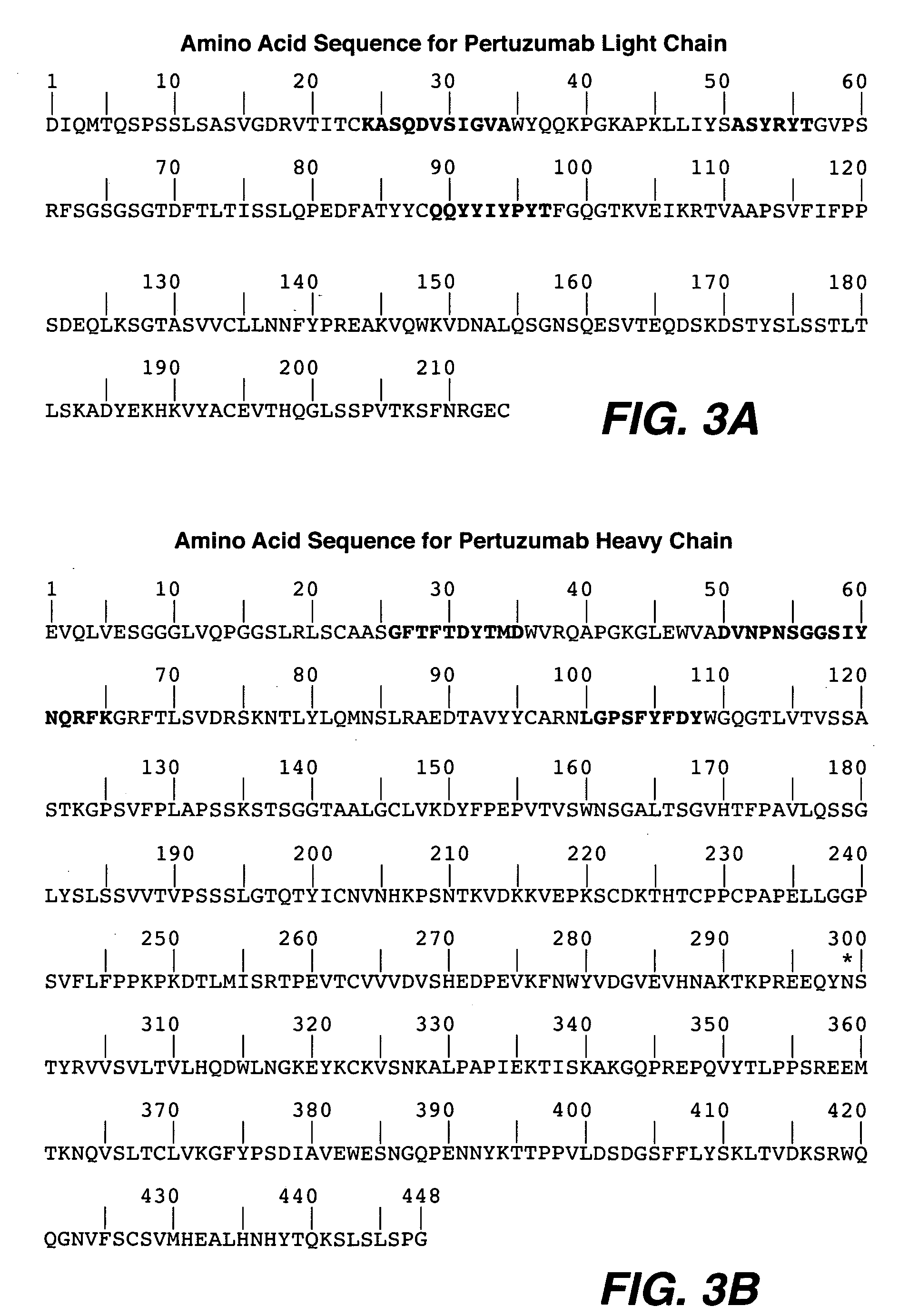
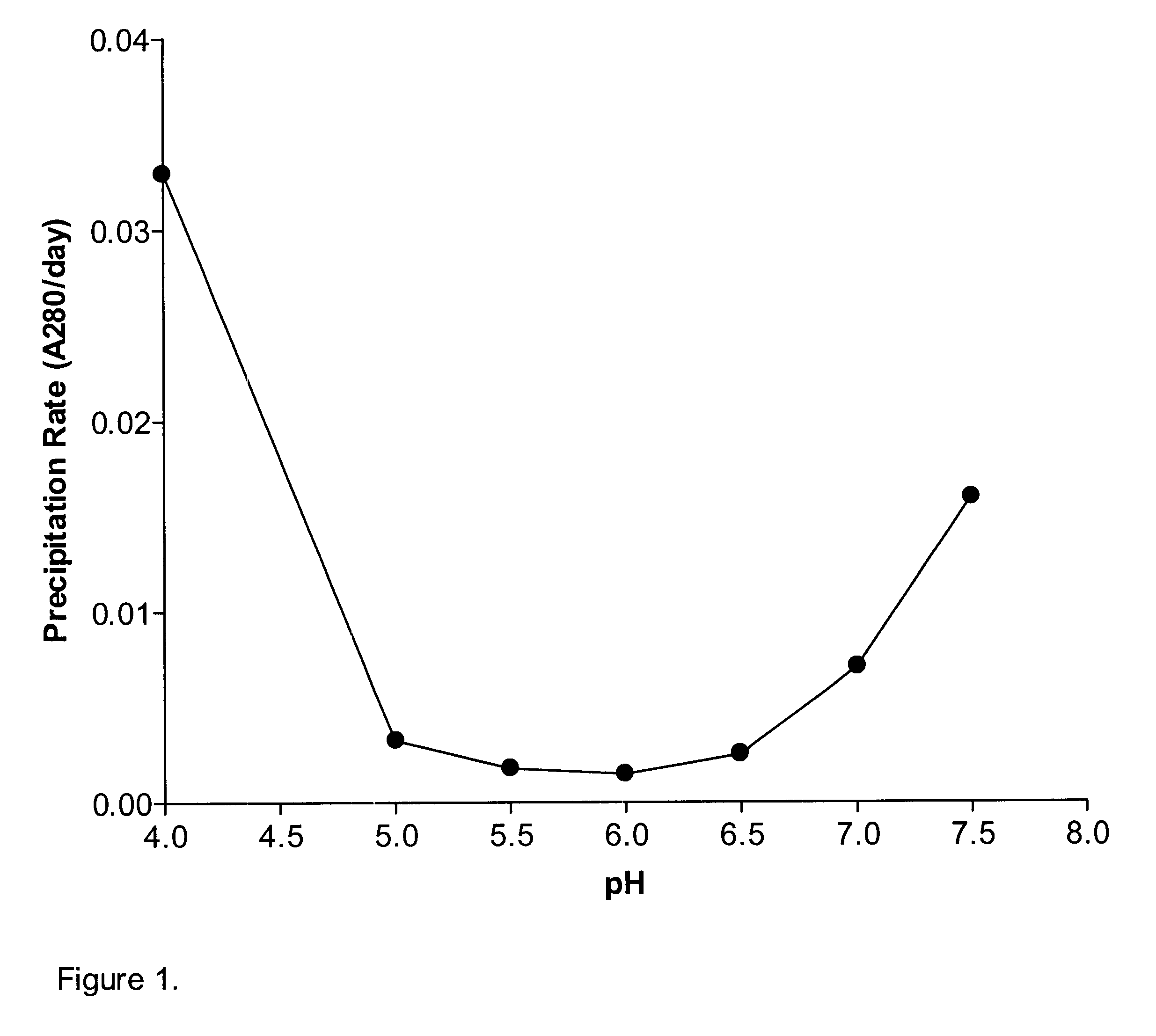
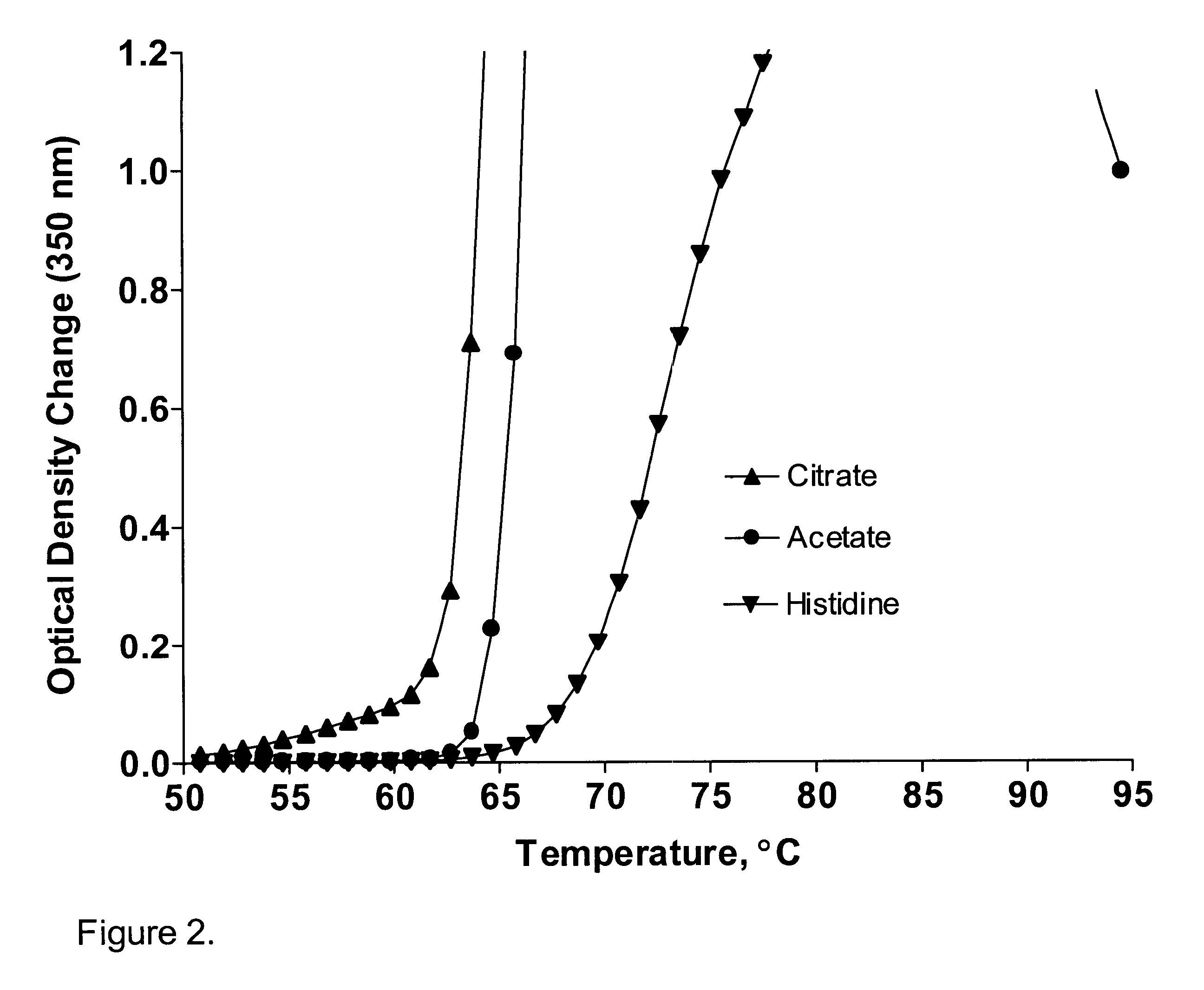
The present application describes antibody formulations, including monoclonal antibodies formulated in histidine-acetate buffer, as well as a formulation comprising an antibody that binds to domain II of HER2 (for example, Pertuzumab), and a formulation comprising an antibody that binds to DR5 (for example, Apomab).
Owner:GENENTECH INC
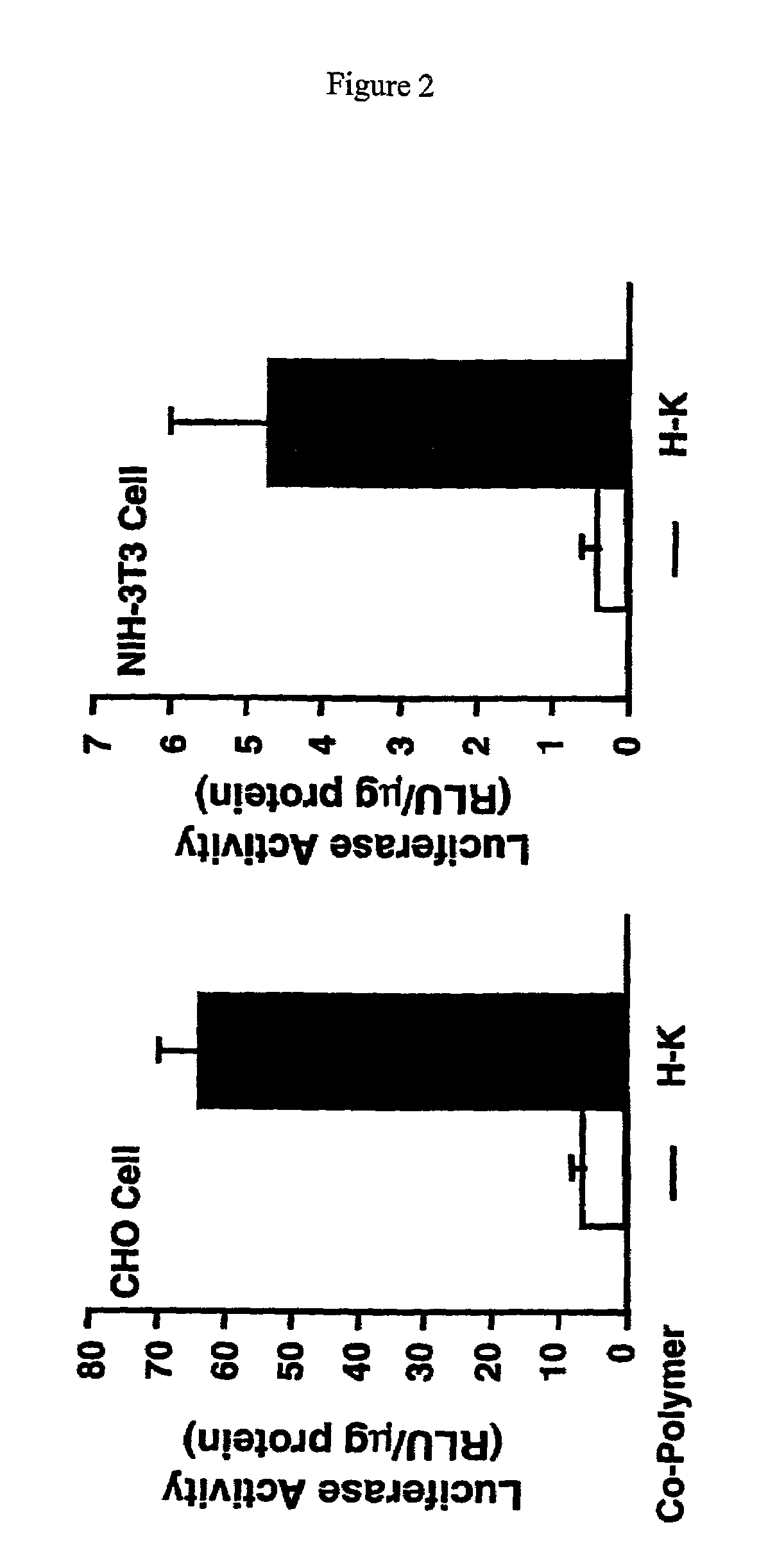
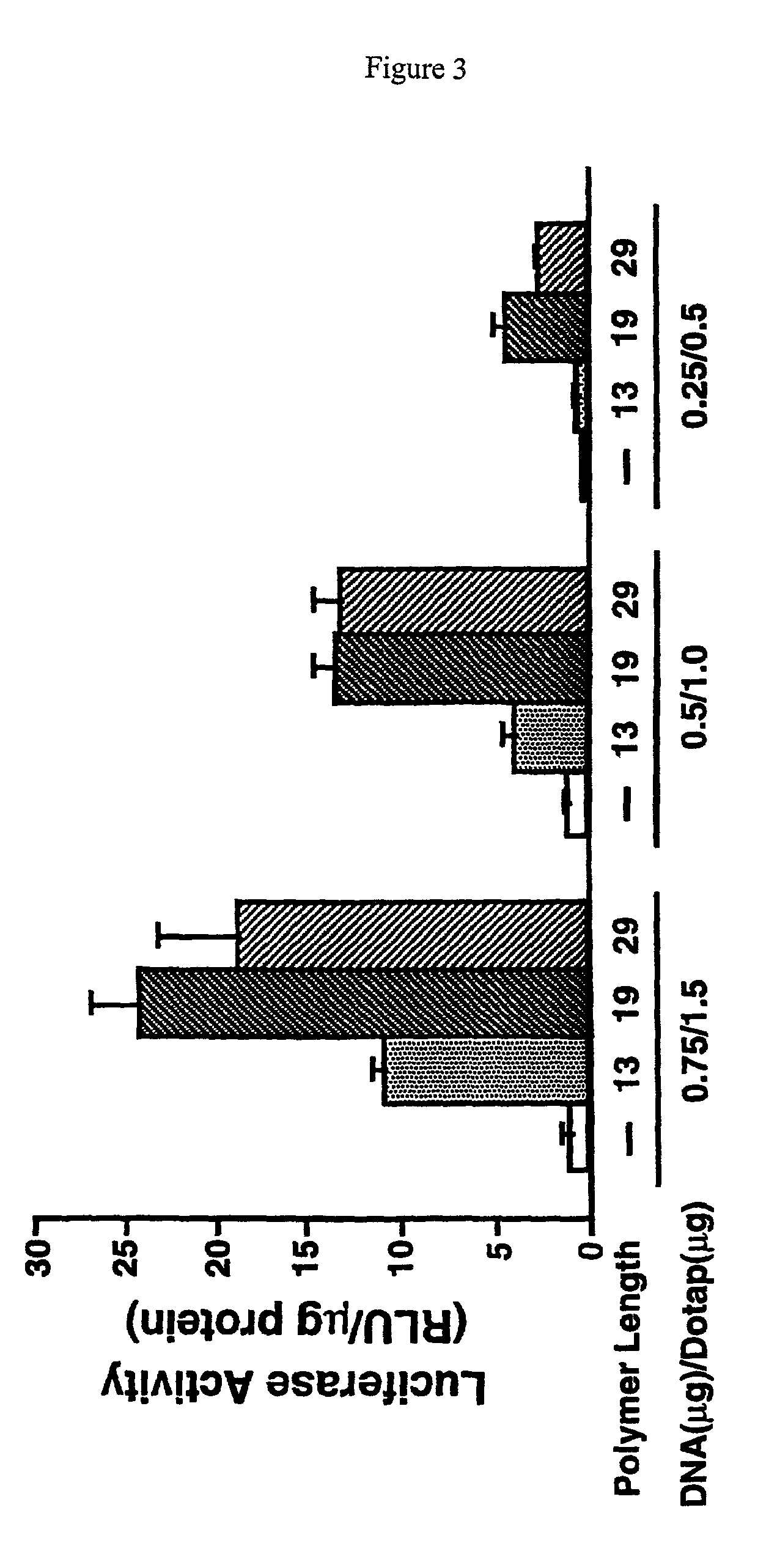
Branched histidine copolymers and methods for using same
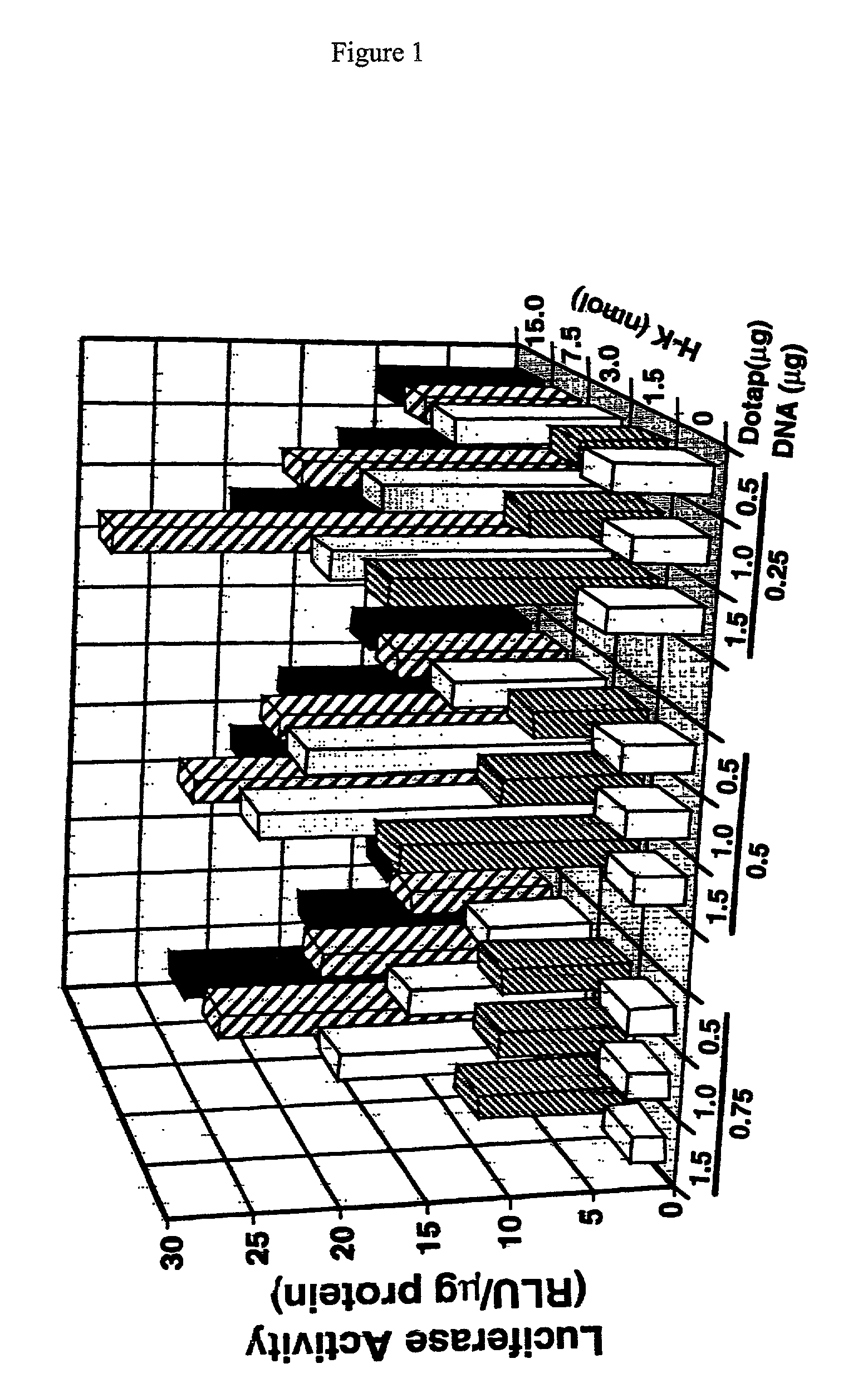
InactiveUS7070807B2Prolong half-life in vivoEnhanced transfectionPowder deliveryPeptide/protein ingredientsIn vivoLocal injection
The invention provides a branched transport polymer characterized as having at least 10 amino acids and a ratio of histidine to non-histidine amino acids greater than 1.5, said branched transport polymer comprising one or more backbones, one or more terminal branches, and optionally, one or more non-terminal branches. The branched transport polymer may be associated with a pharmaceutical agent to form a pharmaceutical agent delivery composition useful for in vivo therapies based on local injection.
Owner:MIXSON A JAMES
Antibody formulations
The present application describes antibody formulations, including monoclonal antibodies formulated in histidine-acetate buffer, as well as a formulation comprising an antibody that binds to domain II of HER2 (for example, Pertuzumab), and a formulation comprising an antibody that binds to DR5 (for example, Apomab).
Owner:GENENTECH INC
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
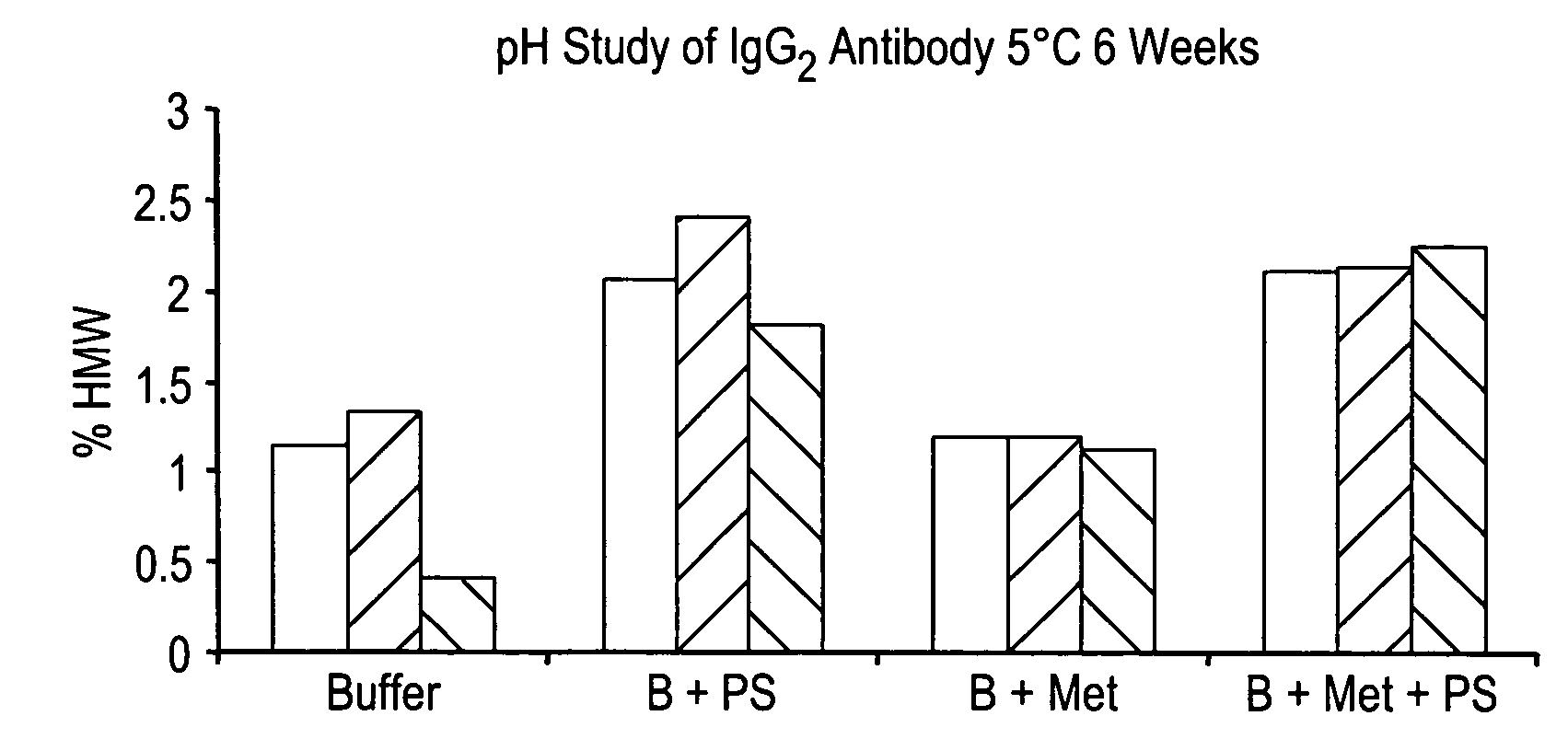
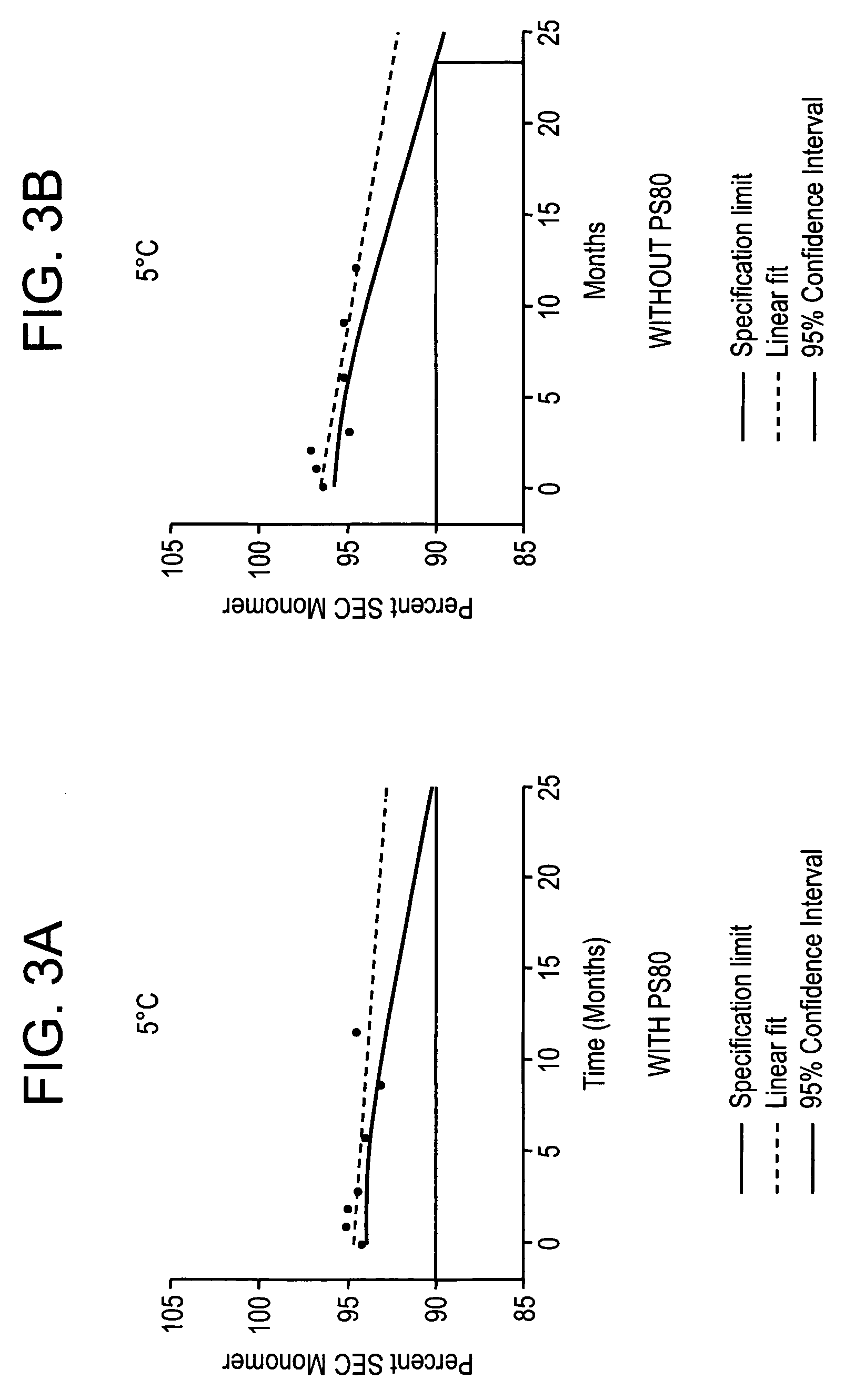
Buffered formulations for concentrating antibodies and methods of use thereof
InactiveUS20060182740A1Increase concentrationConvenient treatmentImmunoglobulins against animals/humansPharmaceutical delivery mechanismHigh concentrationDisease
The present invention provides a method for producing a concentrated antibody preparation that includes the steps of: (a) obtaining an initial antibody preparation that is an aqueous solution of antibodies and histidine or acetate buffer at a concentration in the range of from about 2 mM to about 48 mM; and (b) subjecting the antibody preparation to membrane filtration so as to remove water and buffer but not antibodies from the antibody preparation, thereby producing an antibody preparation having a higher concentration of antibodies than the initial antibody preparation. The concentrated antibody preparations produced by the method have lower viscosity and are more stable than those of other formulations. The invention further includes concentrated antibody preparations produced by the method, pharmaceutical compositions made using such preparations, and therapeutic methods in which such pharmaceutical compositions are administered to treat diseases.
Owner:BIOGEN INC
Subcutaneous anti-HER2 antibody formulations and uses thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Mutant cholera holotoxin as an adjuvant
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
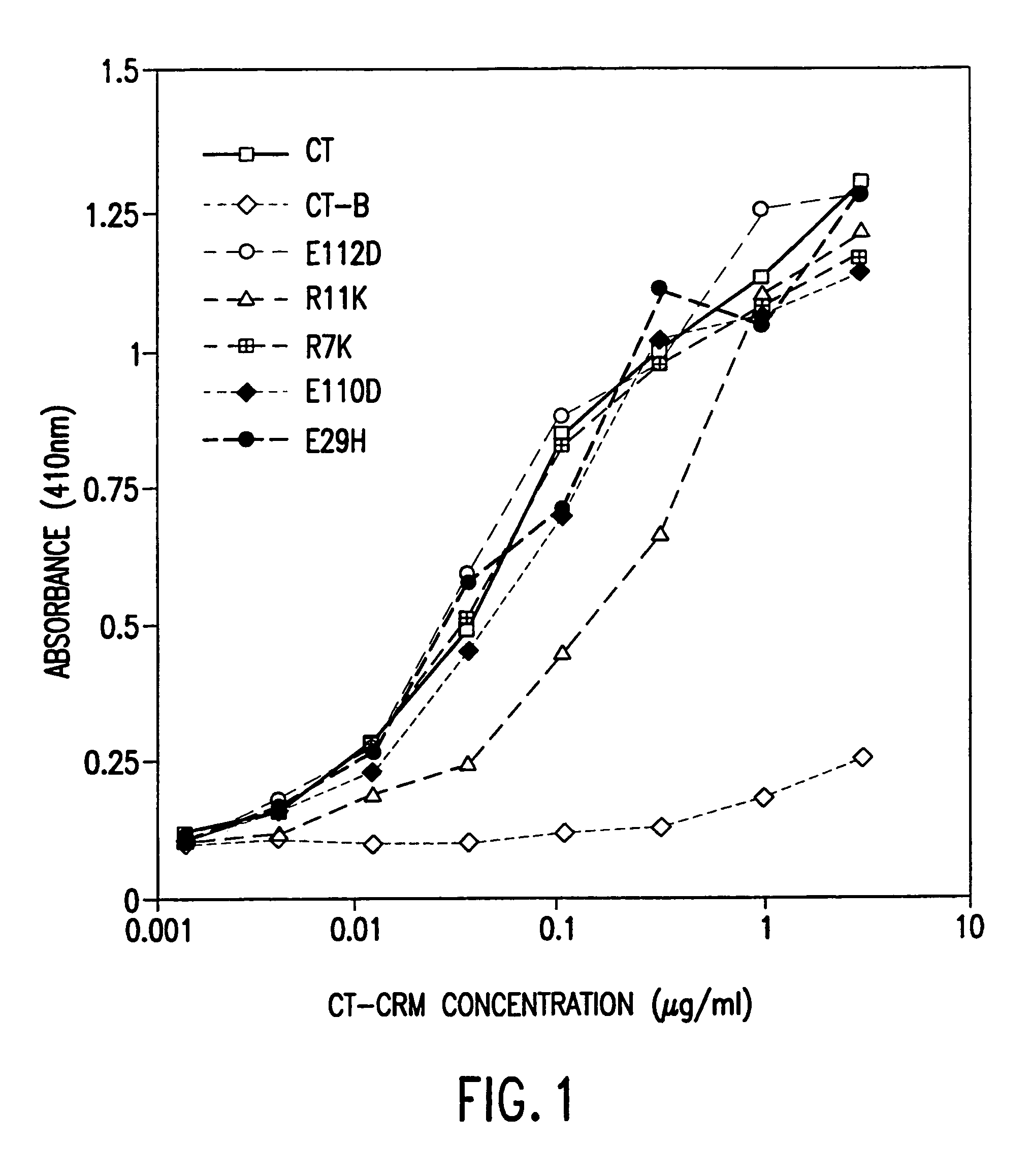
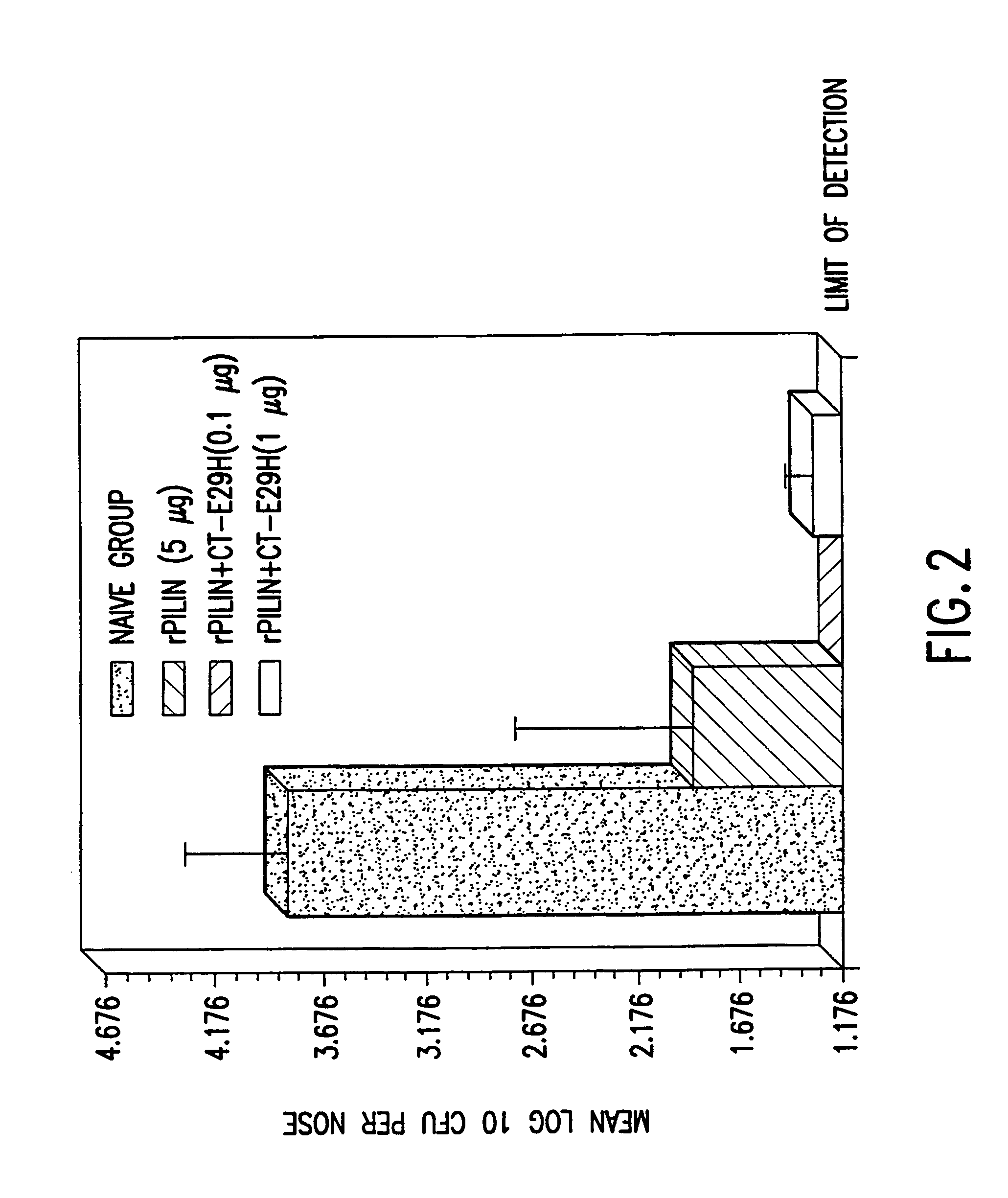
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
Stabilized antibody-containing formulations
ActiveUS20090291076A1Good cakingIncrease of the viscosity of the high-concentration antibody-containing solutionAntibody ingredientsImmunoglobulinsArginineThreonine
The present invention relates to antibody-containing lyophilized formulations free from reducing sugars, non-reducing sugars, sugar alcohols or polysaccharides as excipients and including one or more amino acid selected from the group consisting of arginine, histidine, lysine, serine, proline, glycine, alanine and threonine or a salt thereof.
Owner:CHUGAI PHARMA CO LTD
Novel oral forms of a phosphonic acid derivative
InactiveUS20120190647A1Improved aqueous solubilityIncrease ratingsBiocideOrganic active ingredientsEnprofyllineArginine
Novel solution complexes of zoledronic acid are described which give rise to improved properties of zoledronic acid. The invention includes aqueous solution and molecular complexes of zoledronic acid with and optical isomers of asparagine, histidine, arginine and proline as well as pharmaceutical complexes containing them and methods of treatment using them.
Owner:THAR PHARMA
Method for producing L-amino acids using bacteria of the Enterobacteriaceae family
ActiveUS20060088919A1Improve productivityD-xylose permease is enhancedBacteriaDepsipeptidesL-threonineArginine
There is disclosed a method for producing L-amino acid, for example L-threonine, L-lysine, L-histidine, L-phenylalanine, L-arginine or L-glutamic acid, using a bacterium of the Enterobacteriaceae family, wherein the bacterium has been modified to enhance an activity of D-xylose permease.
Owner:AJINOMOTO CO INC
Vaccines comprising aluminium adjuvants and histidine
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
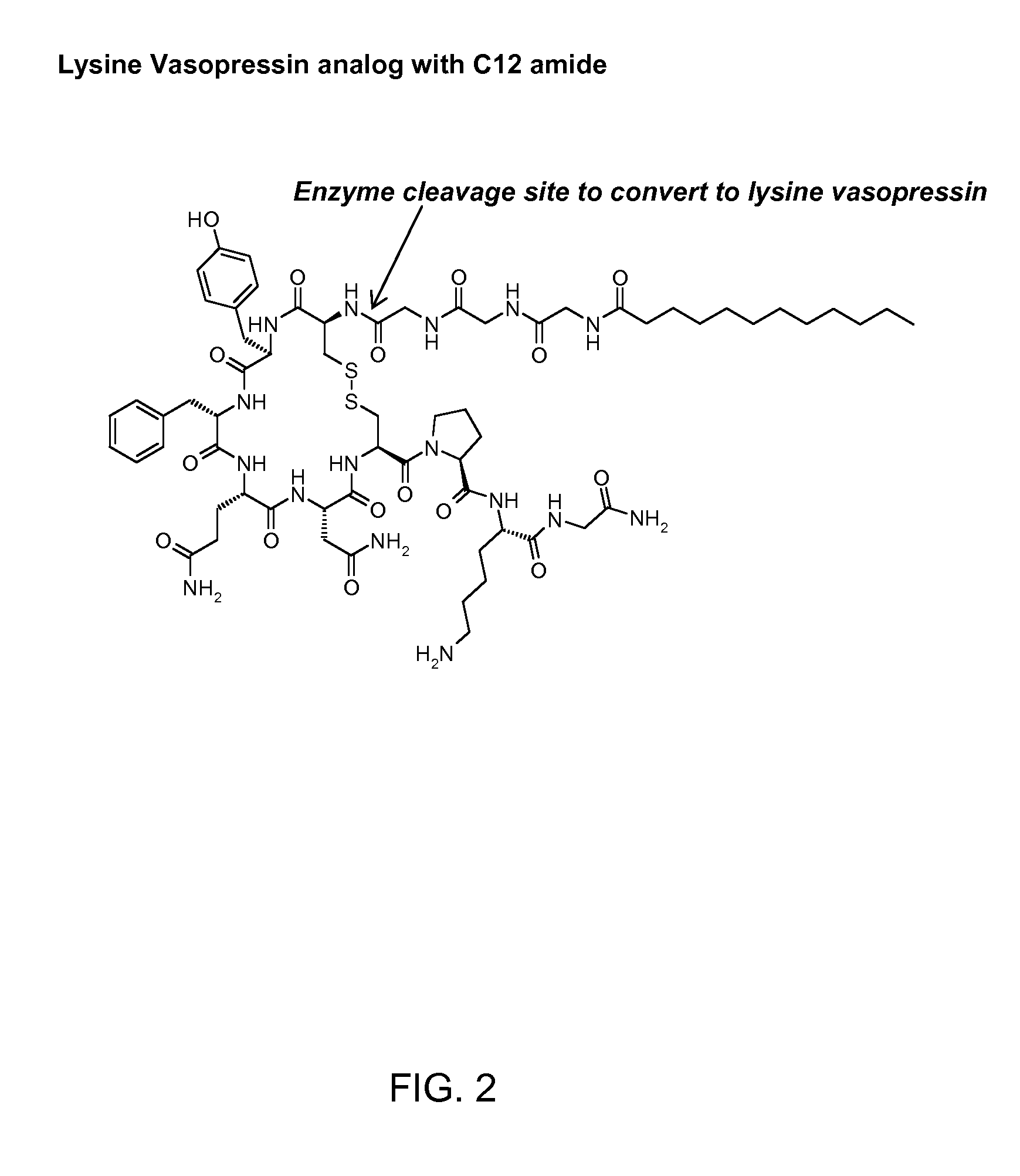
Composition for long-acting peptide analogs
ActiveUS20090088387A1Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
The invention describes compositions of peptide analogs that are active in blood or cleavable in blood to release an active peptide. The peptide analogs have a general formula: A-(Cm)x-Peptide, wherein A is hydrophobic moiety or a metal binding moiety, e.g., a chemical group or moiety containing 1) an alkyl group having 6 to 36 carbon units, 2) a nitrilotriacetic acid group, 3) an imidodiacetic acid group, or 4) a moiety of formula (ZyHisw)p, wherein Z is any amino acid residue other than histidine, His is histidine, y is an integer from 0-6; w is an integer from 1-6; and p is an integer from 1-6; wherein if A has alkyl group with 6 to 36 carbon units x is greater than 0; and Cm is a cleavable moiety consisting of glycine or alanine or lysine or arginine or N-Arginine or N-lysine, wherein x is an integer between 0-6 and N may be any amino acid or none. The peptide analogs are complexed with polymeric carrier to provide enhanced half-life.
Owner:PHARMAIN CORP
Anti a beta antibody formulation
ActiveUS20060193850A1Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
Thermal treatment process for tobacco materials
ActiveUS8434496B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Thermal treatment process for tobacco materials
ActiveUS8991403B2Alter natureAlter characterTobacco preparationTobacco treatmentArginineTobacco product
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Thermal treatment process for tobacco materials
ActiveUS8944072B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of preparing a tobacco material for use in a smoking article is provided, including (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof; (ii) heating the mixture; and (iii) incorporating the heat-treated mixture into a smoking article as a smokable material. A smoking article in the form of a cigarette is also provided that includes a tobacco material pre-treated to inhibit reaction of asparagine to form acrylamide in mainstream smoke. Upon smoking, the smoking article is characterized by an acrylamide content of mainstream smoke that is reduced relative to an untreated control smoking article.
Owner:R J REYNOLDS TOBACCO COMPANY
Stabilized liquid polypeptide formulations
InactiveUS20060210557A1Provide stabilityMaintain biological activityBiocideOrganic active ingredientsMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of polypeptides, in particular, therapeutic antigen-binding polypeptides such as antibodies and the like, for example, anti-Aβ antibodies. The formulations generally include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine, and thus, the formulations are suitable for several different routes of administration.
Owner:WYETH LLC
Stabilized anti-interleukin-6 antibody-containing preparations
InactiveUS8632778B2Reduce aggregationImprove stabilityOrganic active ingredientsBiocideCrystallographyInterleukin 6
The present invention provides stabilized preparations containing an antibody in a glycine buffer and / or a histidine buffer and also provides processes for preparing a protein-containing stabilized preparation, comprising adjusting the pH with a basic amino acid or a basic amino acid derivative or a salt thereof.
Owner:CHUGAI PHARMA CO LTD
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Stable lyophilized pharmaceutical formulation of IgG antibodies
InactiveUS7592004B2Process stabilityAvoid formingPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-IL2 ReceptorHigh concentration
Owner:ABBVIE BIOTHERAPEUTICS
Transgenic plants that exhibit enhanced nitrogen assimilation
InactiveUS20020069430A1Improve growth characteristicsImproved vegetativeClimate change adaptationOther foreign material introduction processesPlant cellThreonine
Transgenic plants containing free amino acids, particularly at least one amino acid selected from among glutamic acid, asparagine, aspartic acid, serine, threonine, alanine and histidine accumulated in a large amount, in edible parts thereof, and a method of producing them are provided. In this method, glutamate dehydrogenase (GDH) gene is introduced into a plant together with a regulator sequence suitable for over expressing the sequence encoding GDH gene in plant cells.
Owner:AJINOMOTO CO INC
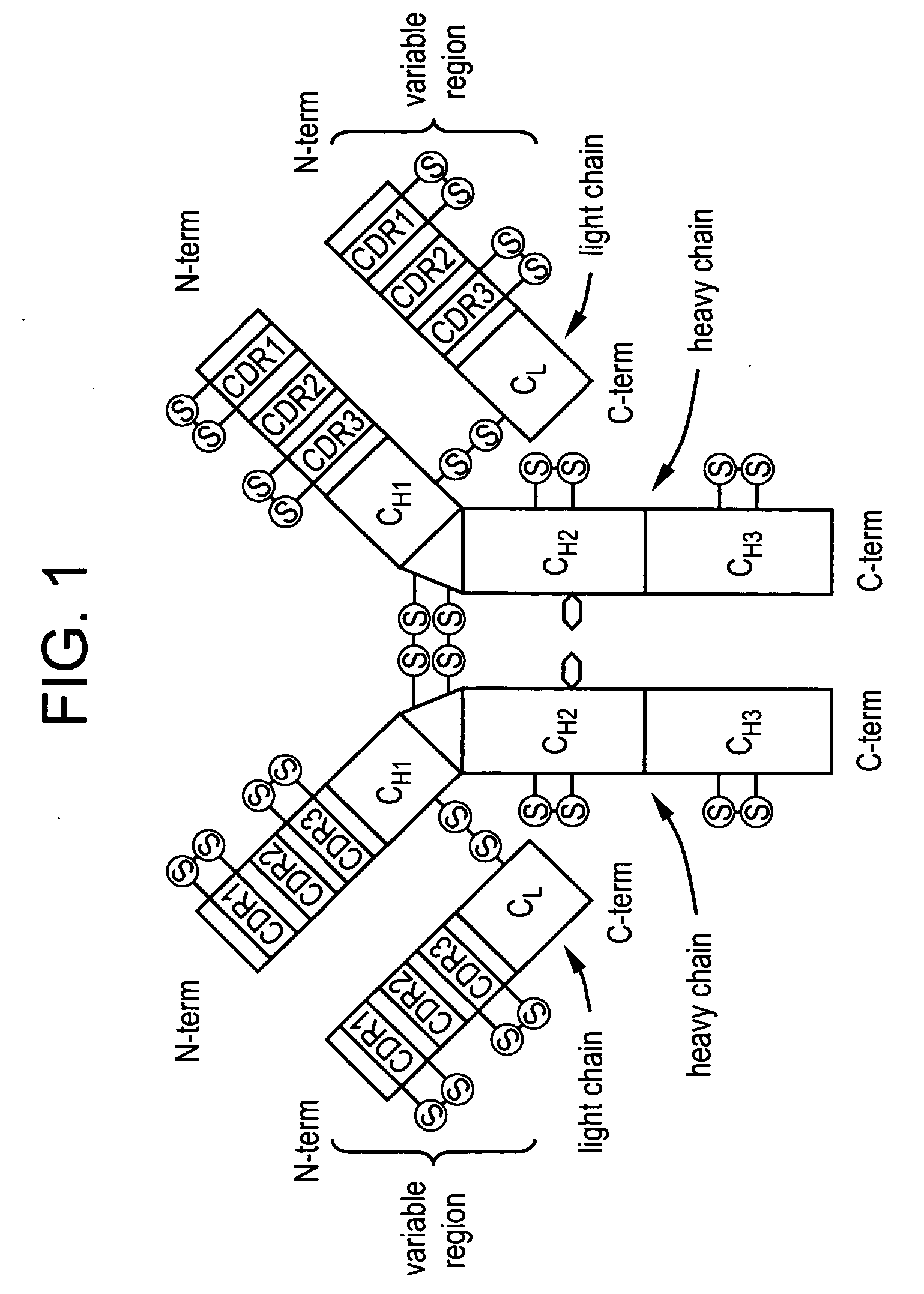
Stable antibody compositions and methods of stabilizing same
InactiveUS20100172862A1Improve propertiesMaintain stabilityOrganic active ingredientsNervous disorderFractionationAntigen binding
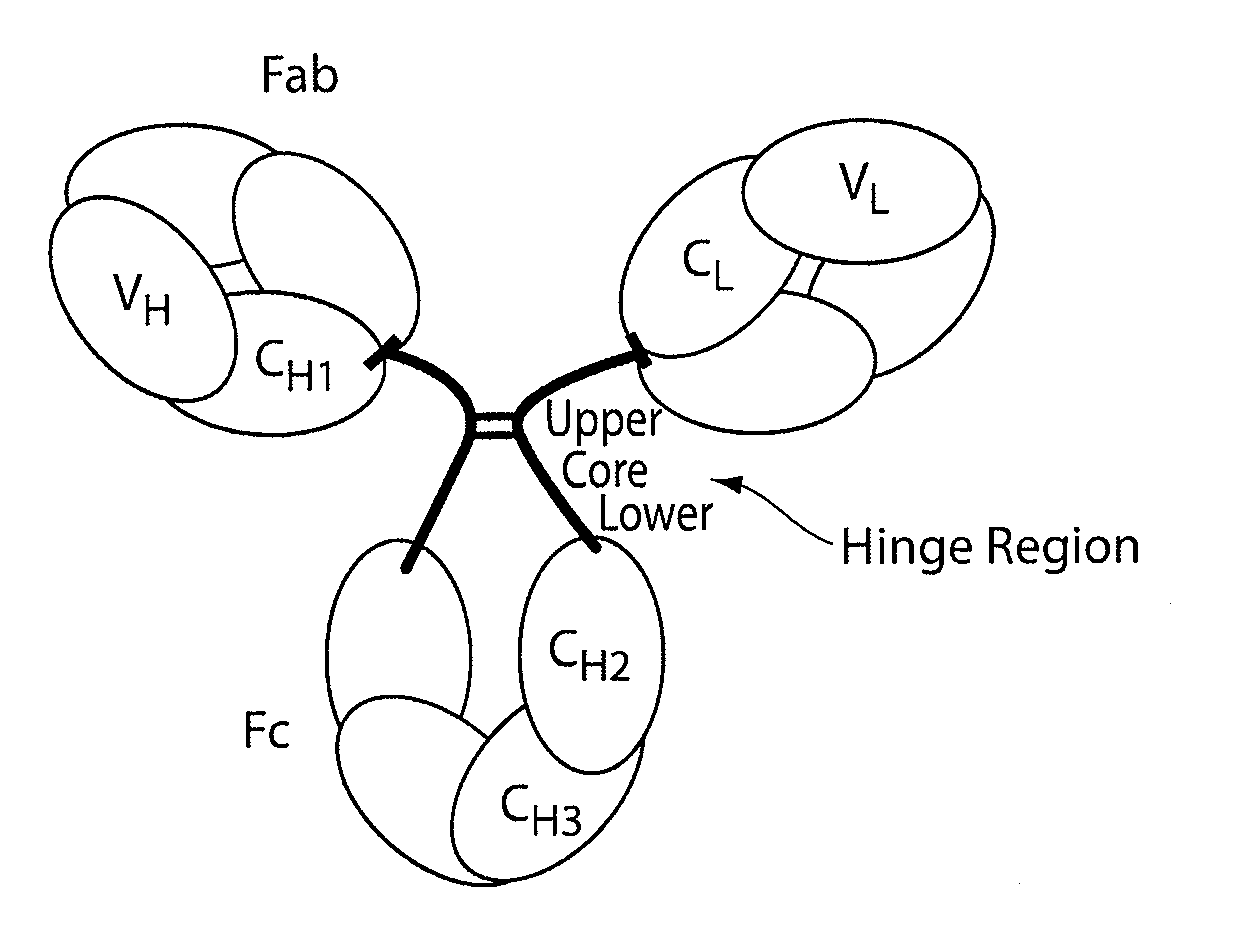
The invention provides compositions and methods for inhibiting fractionation of immunoglobulins comprising a lambda light chain based on the observation that iron, in the presence of histidine, results in increased fragmentation of a recombinant fully human IgG molecule containing a lambda light chain due to cleavage in the hinge region. The invention further provides an aqueous pharmaceutical formulation comprising an antibody, or antigen-binding portion thereof, that binds the p40 subunit of IL-12 / IL-23 and a buffer system comprising histidine, wherein the formulation has enhanced stability, including enhanced resistance to fragmentation.
Owner:ABBVIE INC
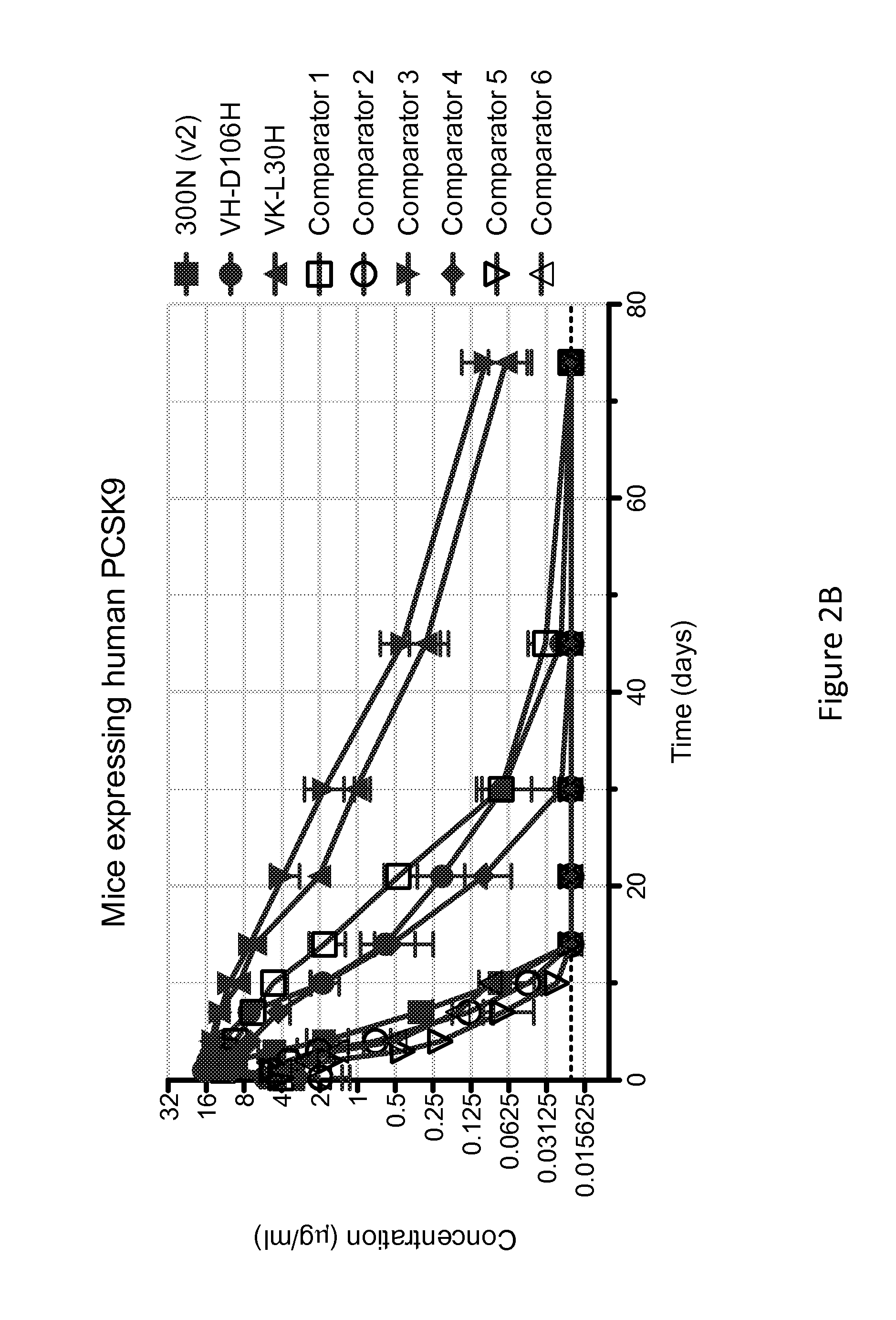
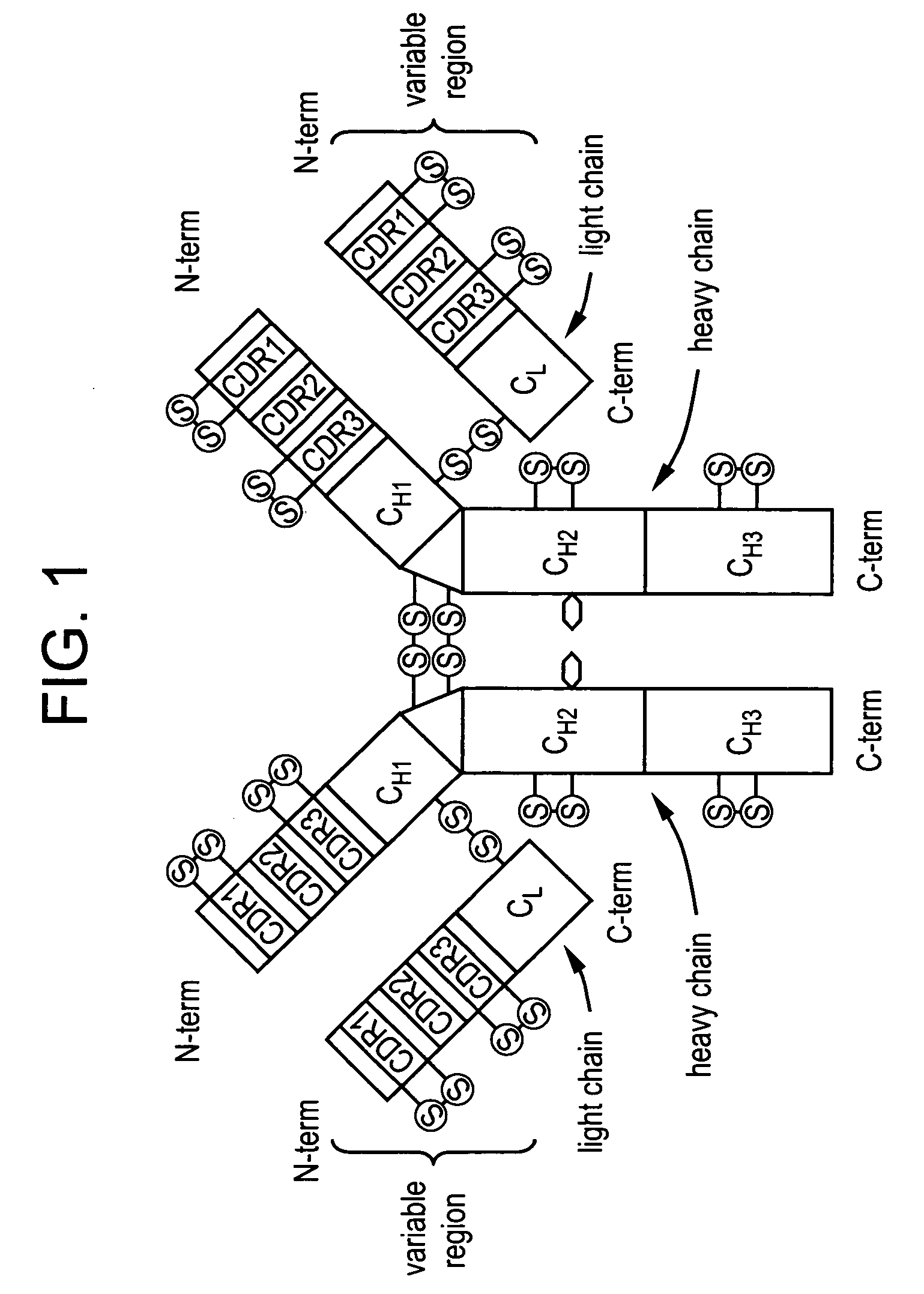
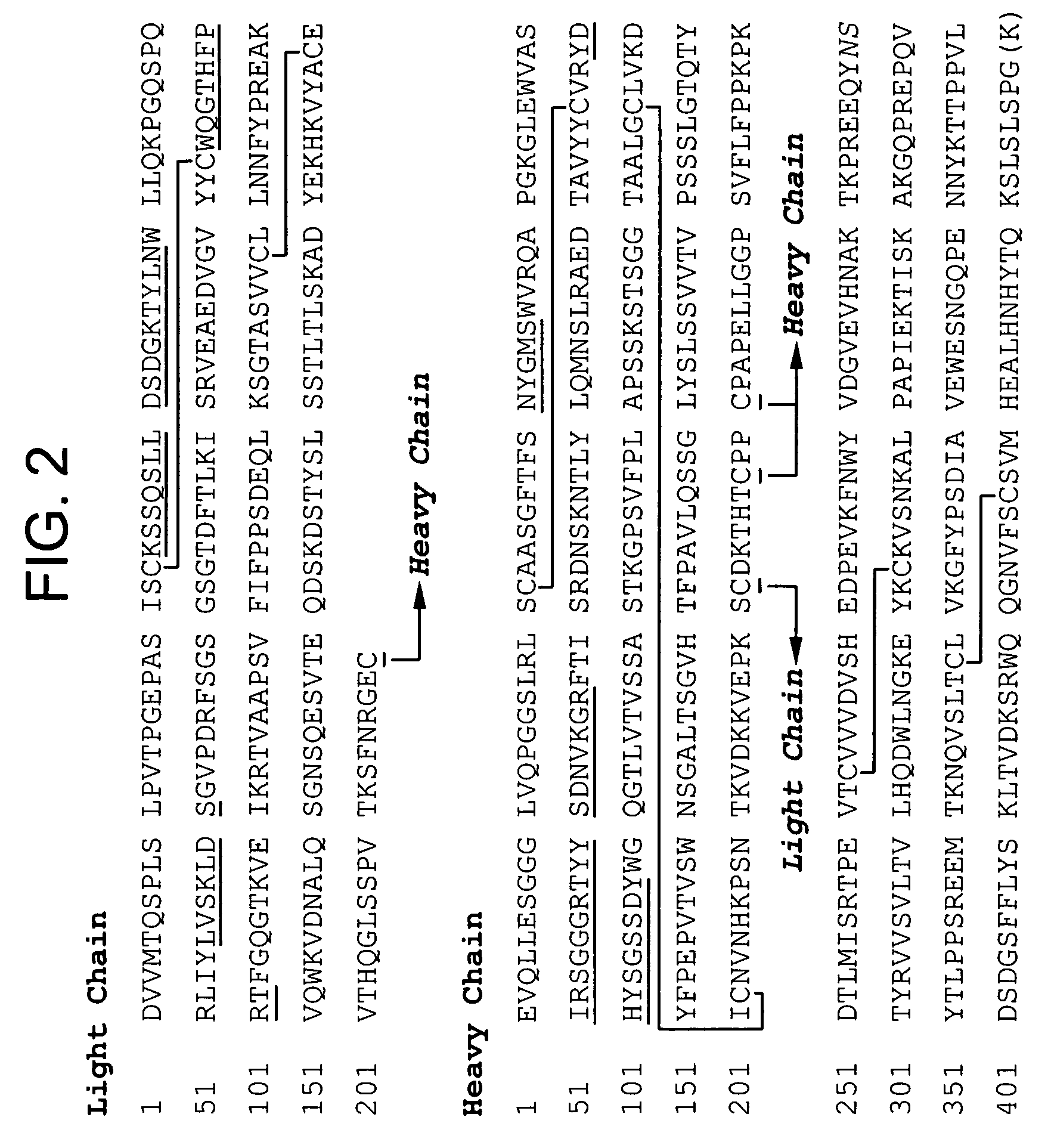
ANTI-PCSK9 ANTIBODIES WITH pH-DEPENDENT BINDING CHARACTERISTICS
ActiveUS20140044730A1High affinityReduced binding affinityMetabolism disorderAntibody ingredientsDiseaseKexin
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
Compositions that include hemagglutinin, methods of making and methods of use thereof
InactiveUS20070224205A1Avoid serious illnessAvoid deathSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininArginine
Compositions comprise a flagellin component or a Toll-like Receptor agonist component that is at least a portion of a flagellin or a Toll-like Receptor agonist, wherein the flagellin component or Toll-like Receptor agonist component includes at least one cysteine residue and whereby the flagellin component or Toll-like Receptor agonist component activates a Toll-like Receptor 5 or Toll-like Receptor. Compositions comprise a flagellin component that is at least a portion of a flagellin, wherein at least one lysine of the flagellin component has been substituted with at least one arginine, serine and histidine, whereby the flagellin component activates Toll-like Receptor 5. Compositions can further include an antigen, such as an influenza antigen, a flavivirus antigen, a pathogen-related antigen, a bacterial capsular antigen and a carrier protein. The compositions are used to stimulate an immune response and a protective immune response in a subject.
Owner:VAXINNATE
L-arginine producing strain and its mutation method and usage in producing L-arginine
InactiveCN1441055AHigh genetic stabilityGood genetic stabilityBacteriaFermentationBiotechnologyArginine
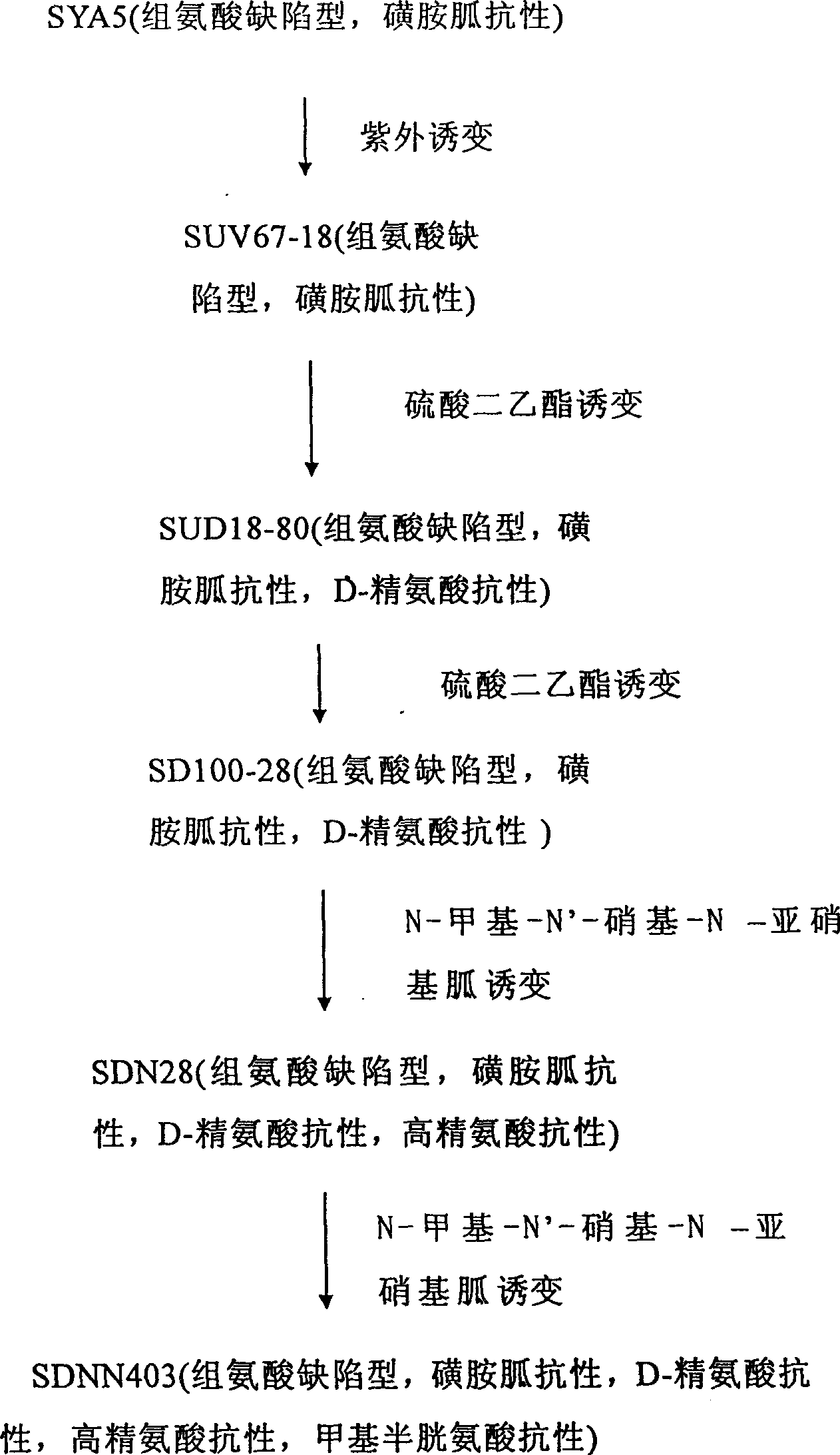
The present invention relates to the fermentation process of producing L-arginine. By using Corynebacterium crenatum SYA5 screened and preserved by the present lab as parent and through conventional physical and chemical mutation process and multiple structural analog resistance screening, mutant strain SDNN403 is obtained. Through culturing of the mutant strain under optimized condition, L-arginine is produced in the yield level of 30-35 g / L. The strain has high genetic stability, stable yield characteristic, high L-arginine yield level, less produced hetero acids and easy technological amplification, and is suitable four industrial production.
Owner:JIANGNAN UNIV
Stabilized interleukin 2
InactiveUS6689353B1Reduce ionic strengthFast rebuildPowder deliveryPeptide/protein ingredientsGlycineSucrose
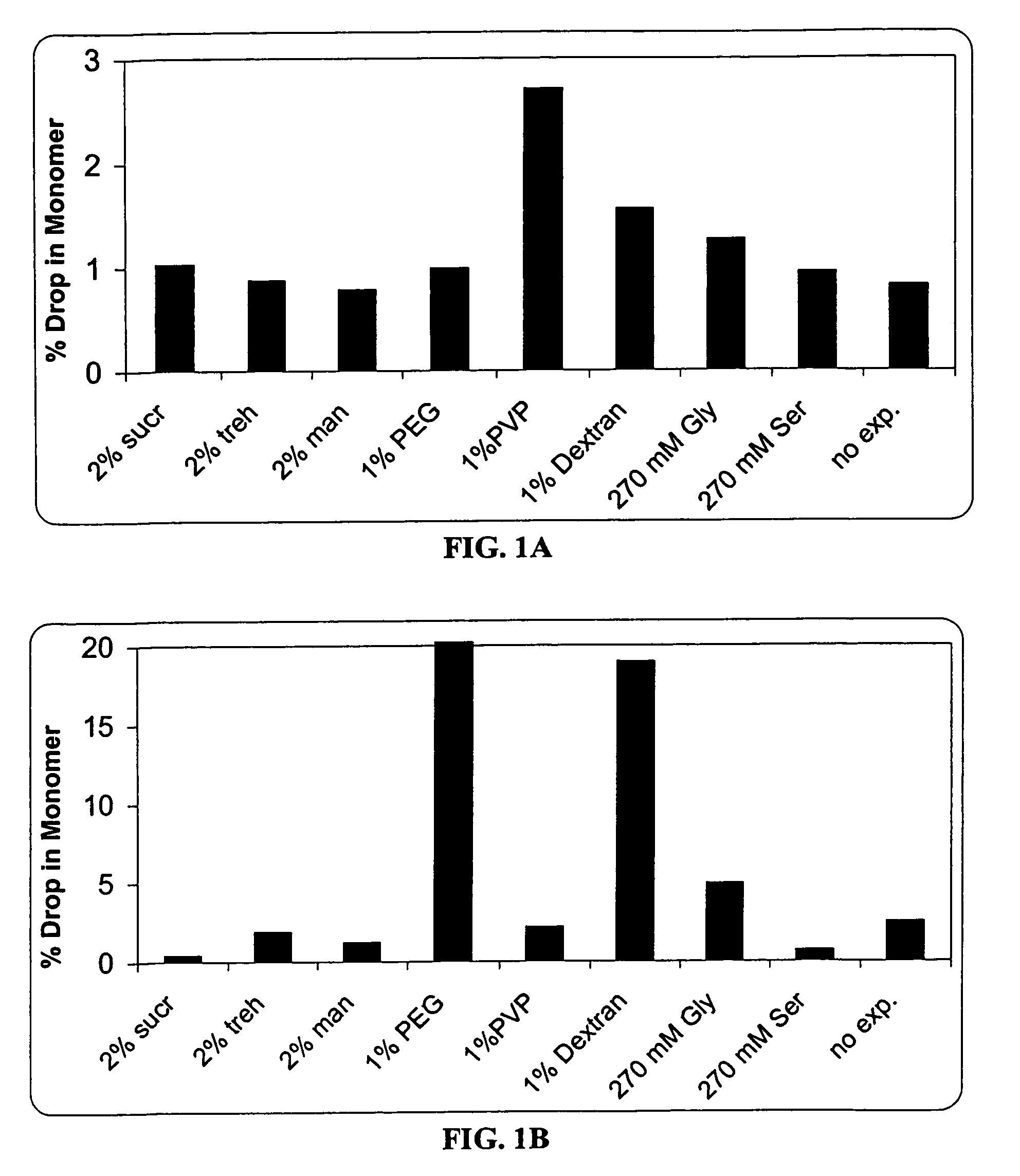
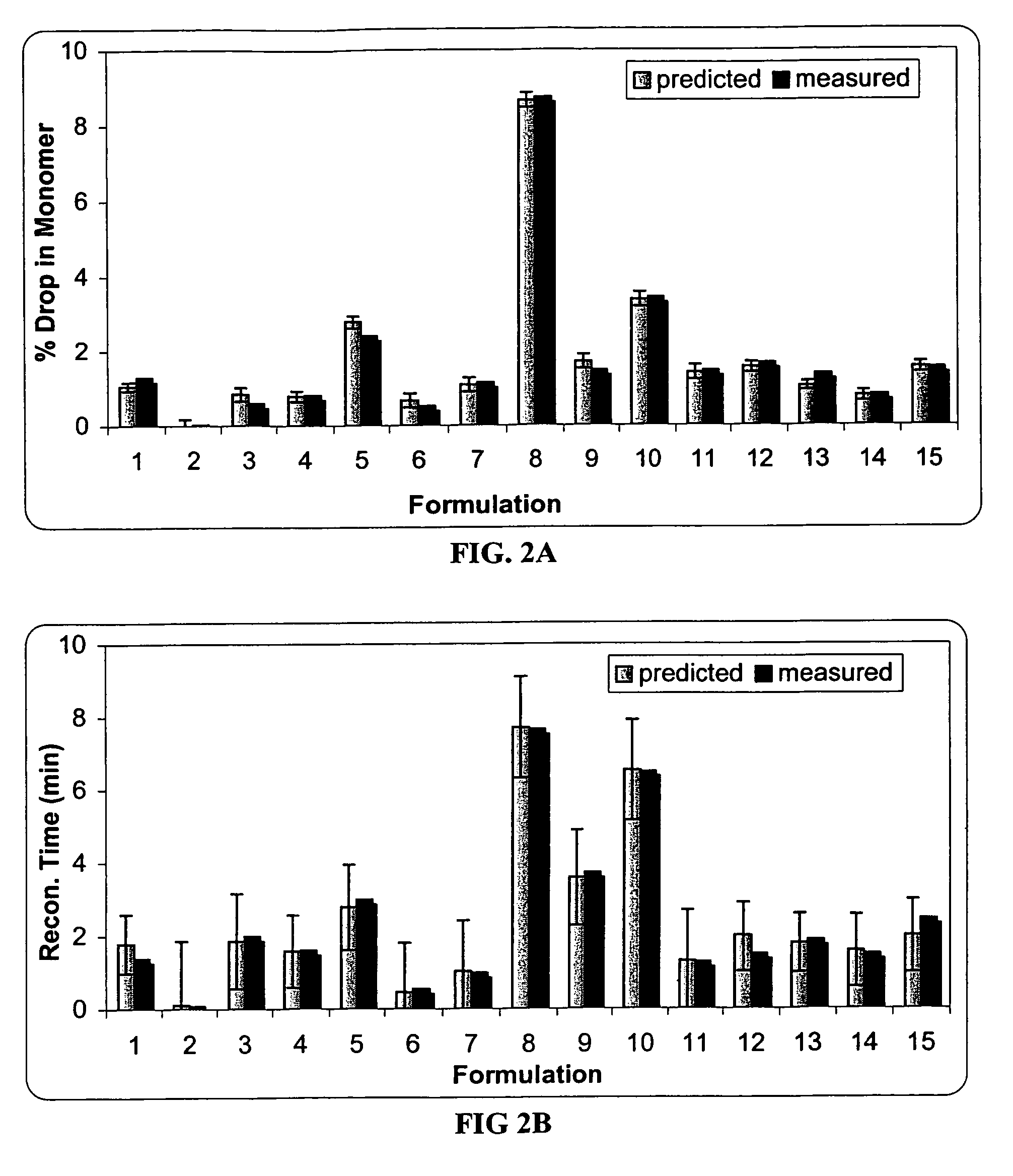
A stable pharmaceutical preparation comprising Human interleukin-2 or a variant thereof and a stabilizing amount of histidine. A preferred formulation includes glycine and sucrose and a variant of IL-2 having a single mutation, N88R. The preferred formulation is in lyophilized form which, on reconstitution with an aqueous diluent, results in a solution having a pH ranging from about 5.0 to 6.5.
Owner:AICURIS GMBH & CO KG
Compositions and methods for glycogen synthesis
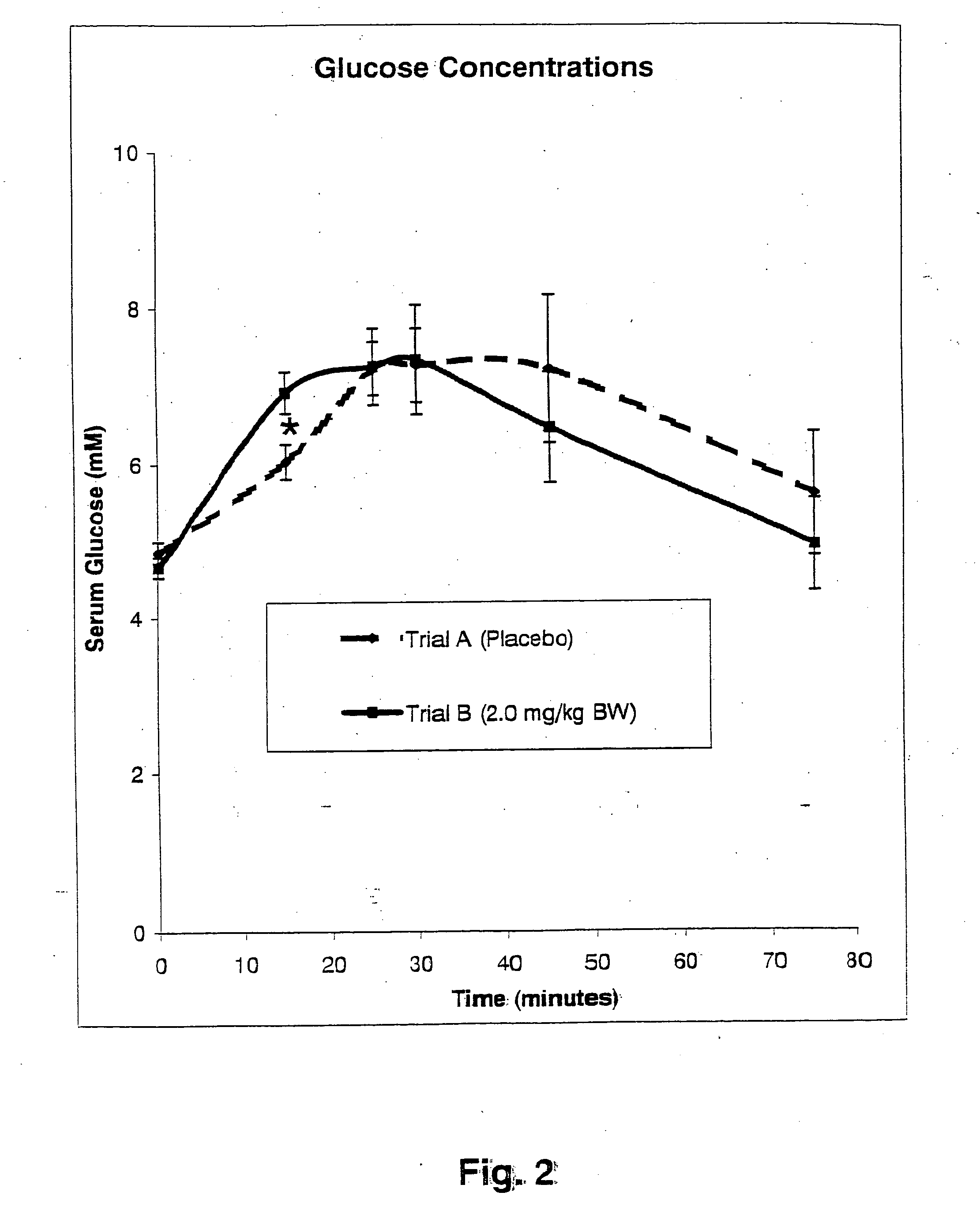
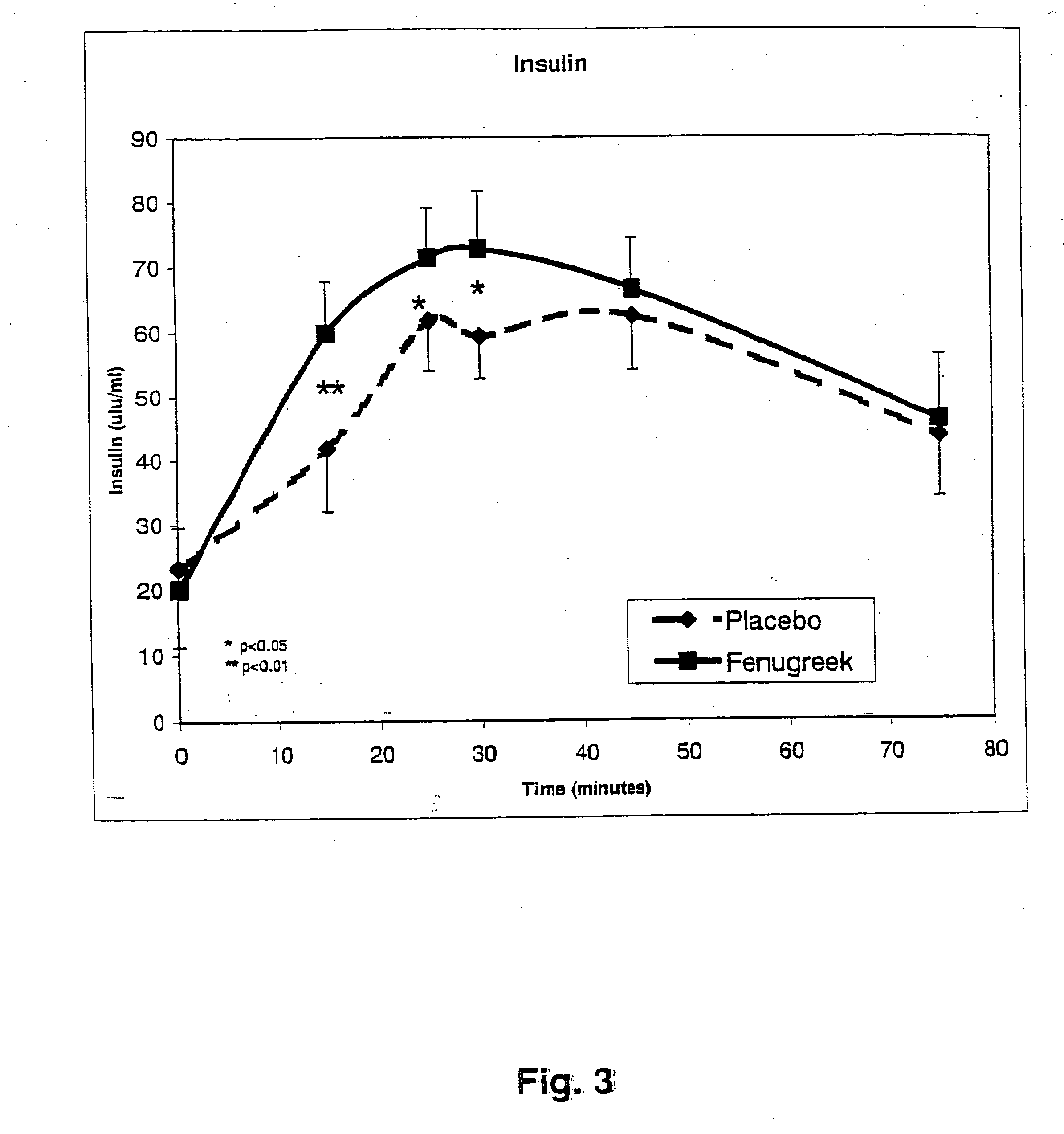
InactiveUS20050176827A1Function increaseIncrease insulin sensitivityBiocideOrganic active ingredientsCysteine thiolateTryptophan
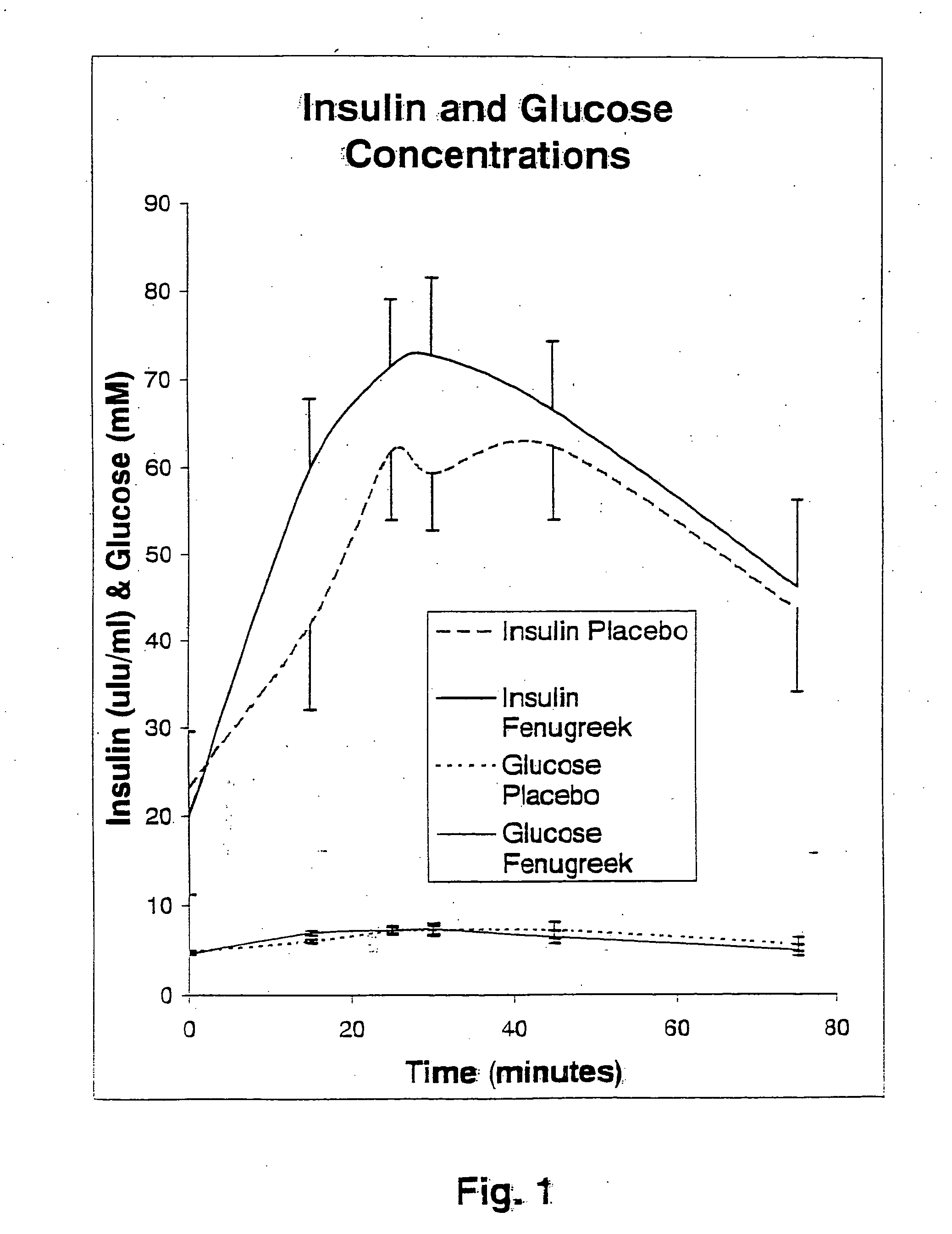
A composition of bio-active compounds and methods for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning. Preferably, the composition of bio-active compounds includes a combination of 4-hydroxyisoleucine with at least one amino acid selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, lysine, histidine, gamma-amino butyrate and tyrosine. In one presently preferred embodiment of the present invention, the combination is derived, isolated, and / or extracted from fenugreek seeds. Methods for using a novel composition of bio-active compounds from fenugreek seed for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning are also disclosed, wherein methods comprise the steps of: (1) providing an effective amount of a composition of bio-active compounds derived, isolated, and / or extracted from fenugreek seeds; and (2) administering the composition to a human or animal.
Owner:TSI INC
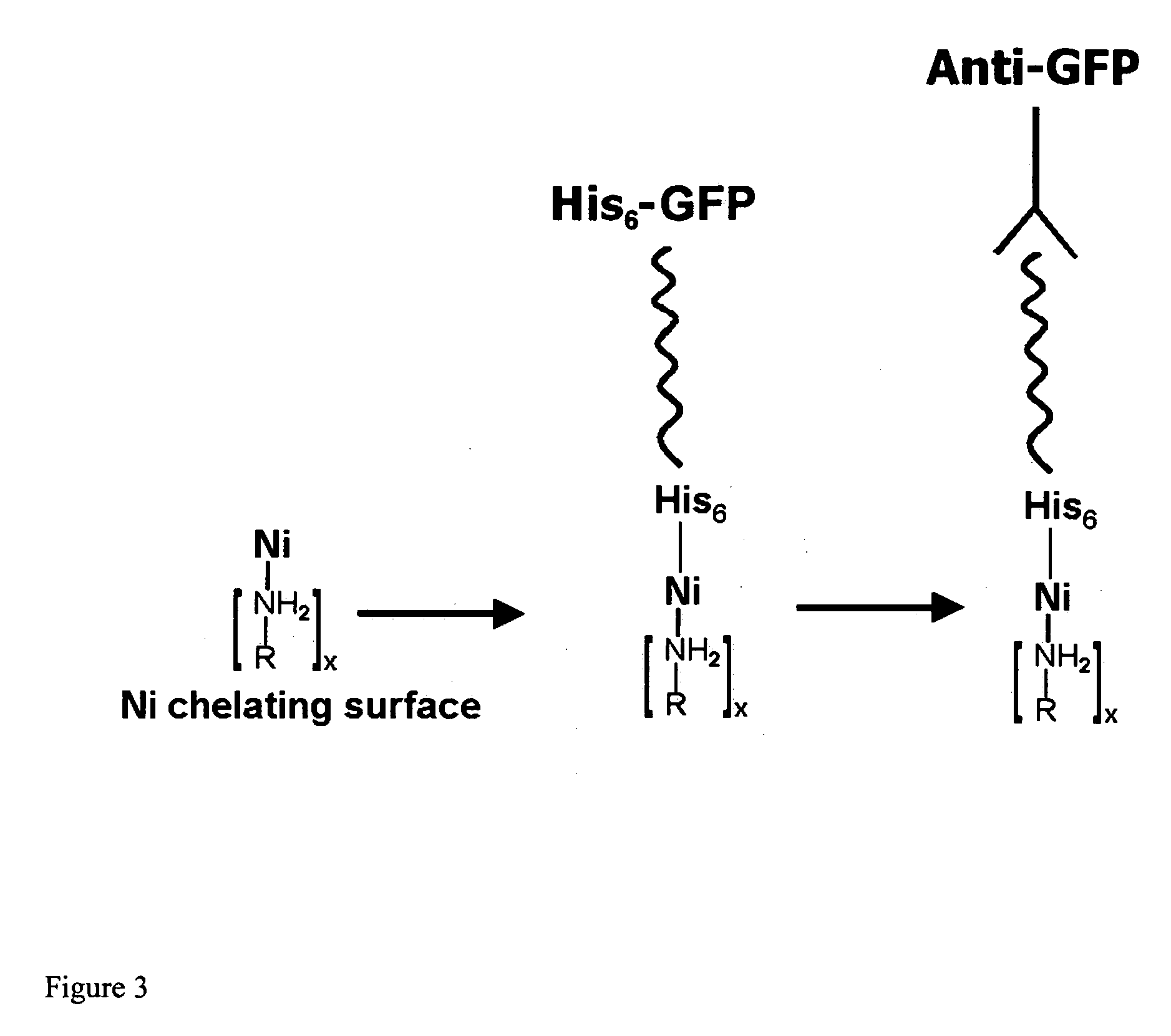
Affinity membrane for capture of a target biomolecule and formation thereof by site-directed immobilization of a capture biomolecule
InactiveUS20060292680A1Increase the number ofEasy to captureGroup 8/9/10/18 element organic compoundsCarrier-bound/immobilised peptidesTyrosineTyrosinase activity
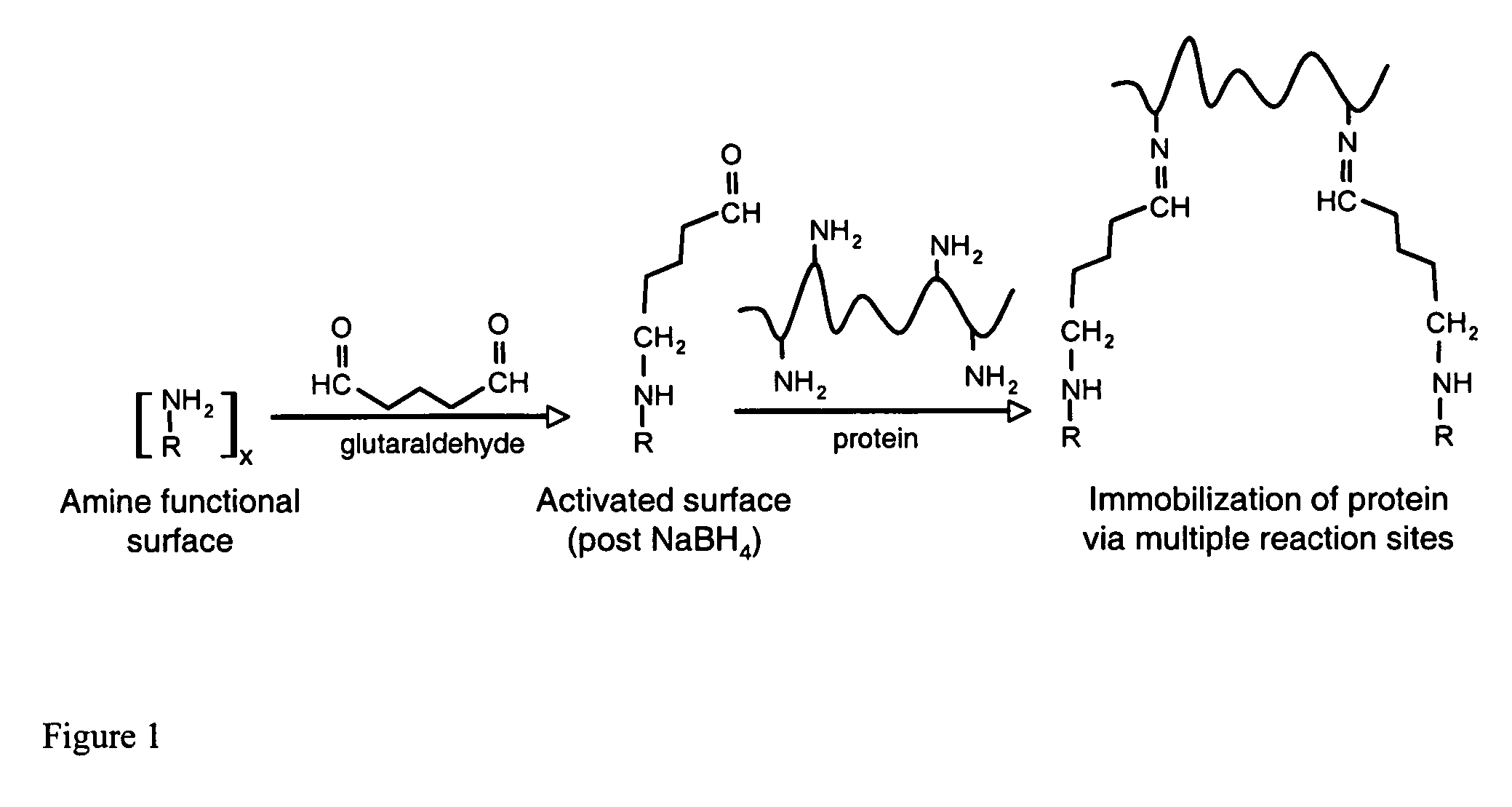
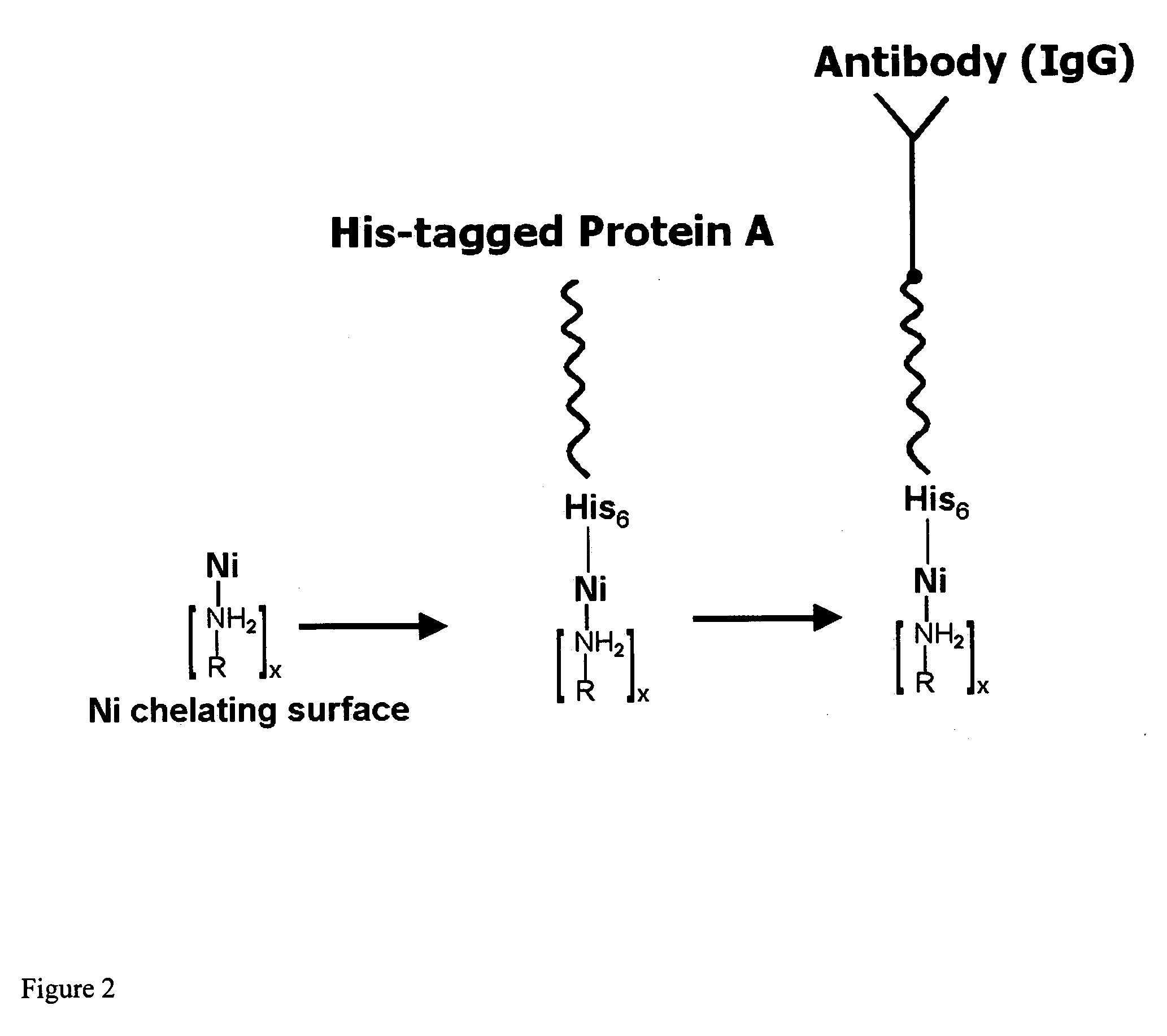
Compositions and methods are taught for directing the orientation of an immobilized capture biomolecule on a hydrophobic membrane. The method comprises layering at least one tie layer on a hydrophobic membrane, adding an amine functional layer on top of at least one tie layer; and attaching an alignment biomolecule to the amine functional layer. The alignment biomolecule has the ability to either capture a target biomolecule itself and thus be considered a capture biomolecule, or bind and orient the immobilized capture biomolecule so as to maximize the binding activity of the immobilized capture biomolecule. In one embodiment, a nickel-coordinated amine functional layer binds with a histidine-tagged alignment biomolecule. In another embodiment, an amine functional layer reacts, via tyrosinase catalysis, with a tyrosine residue in an alignment biomolecule.
Owner:MARYLAND UNIV OF +1
Anti Abeta antibody formulation
ActiveUS7635473B2Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com