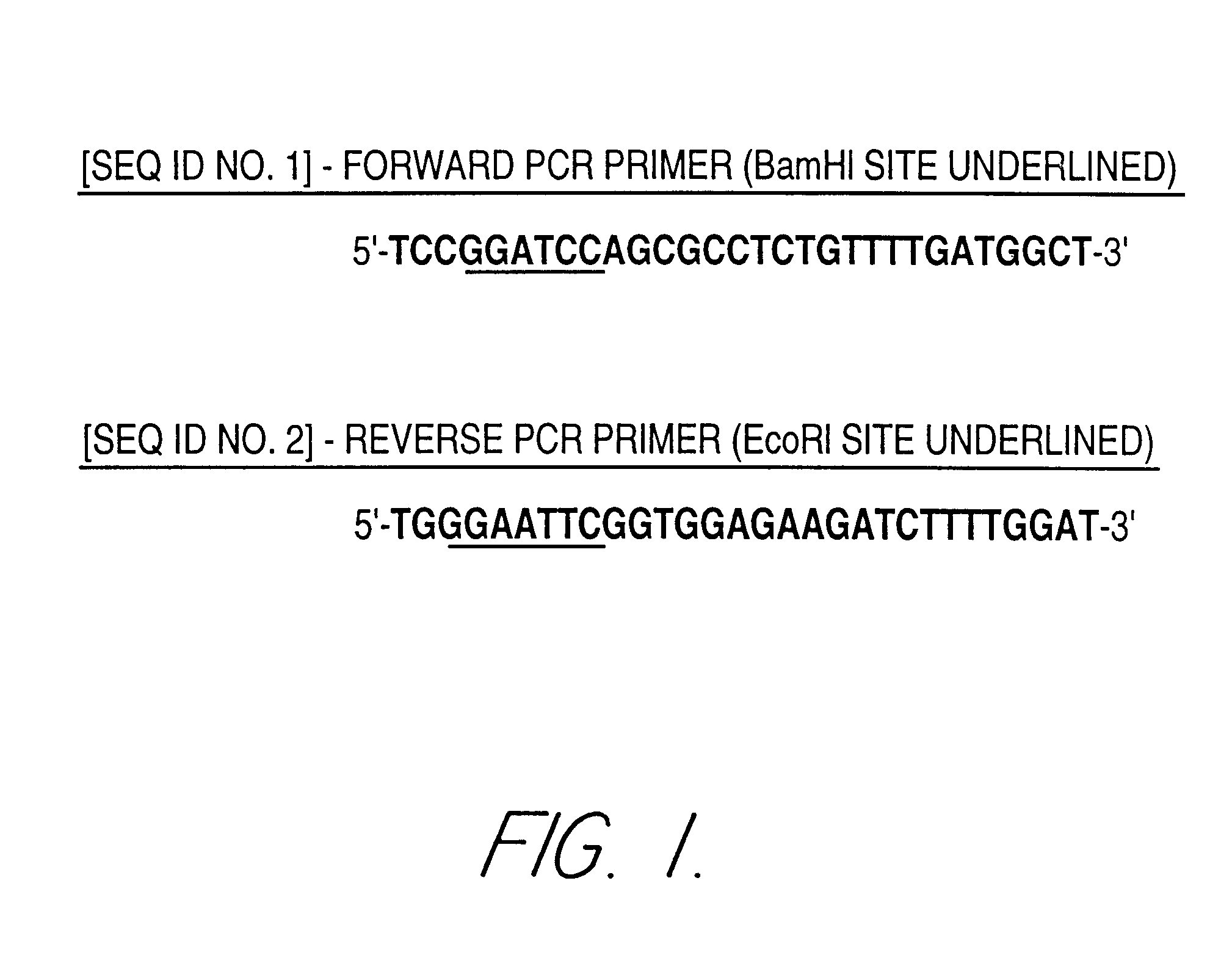
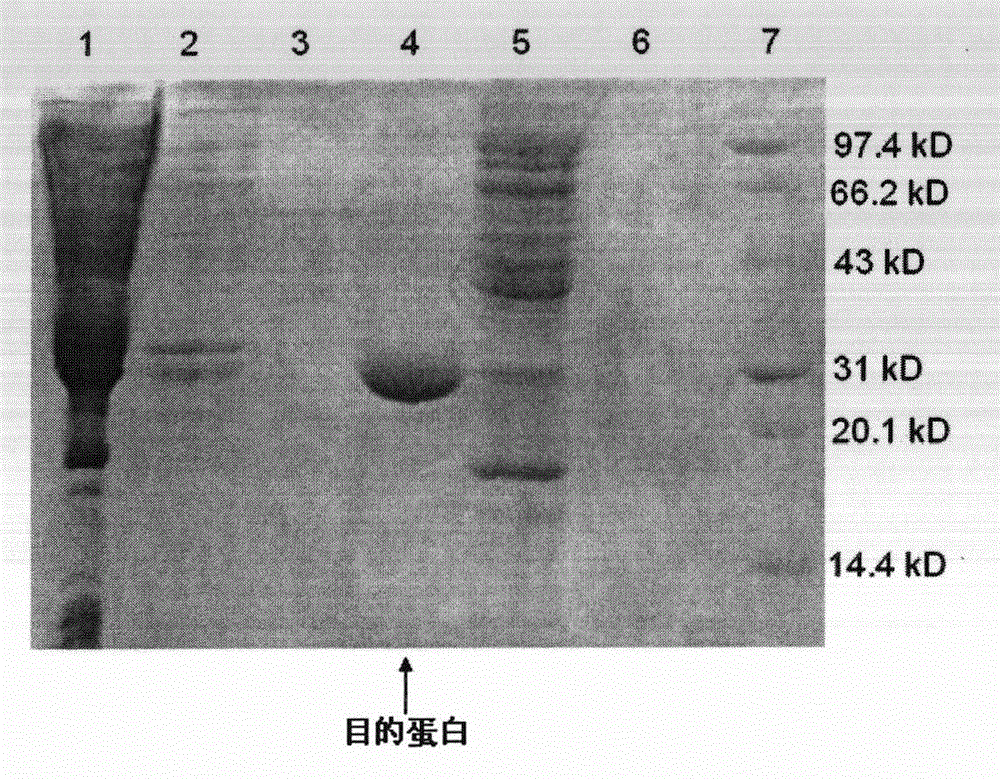
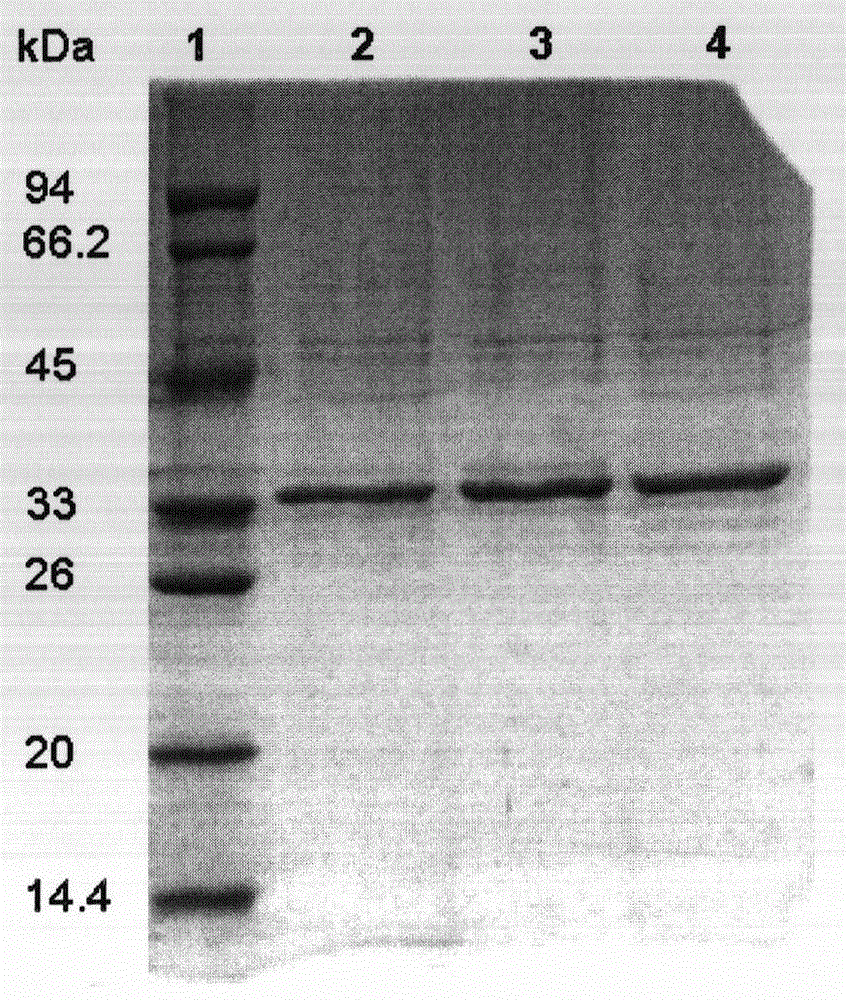
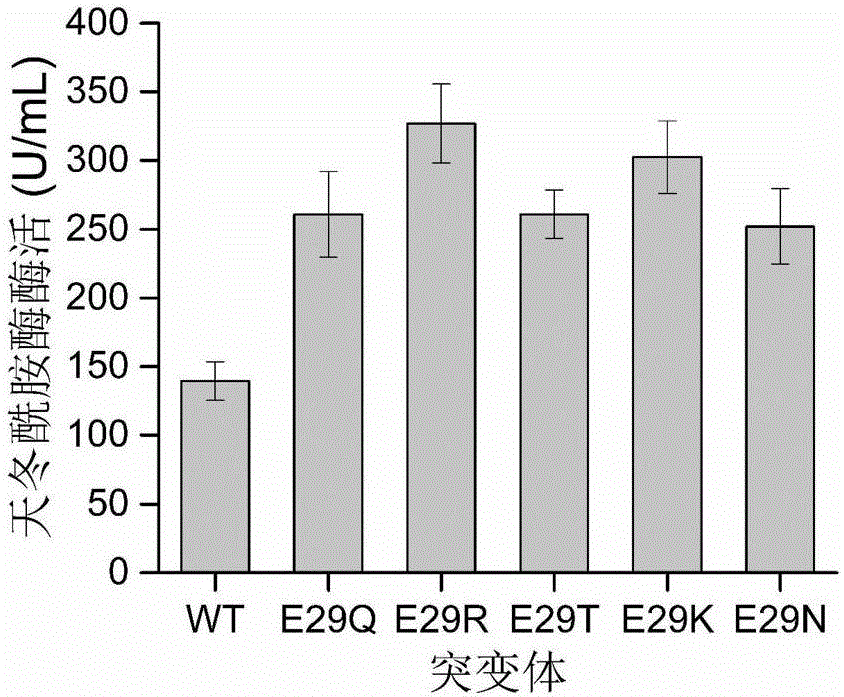
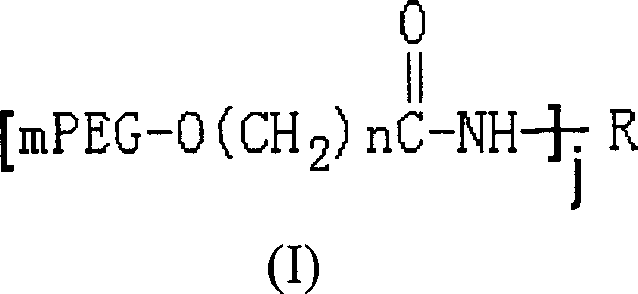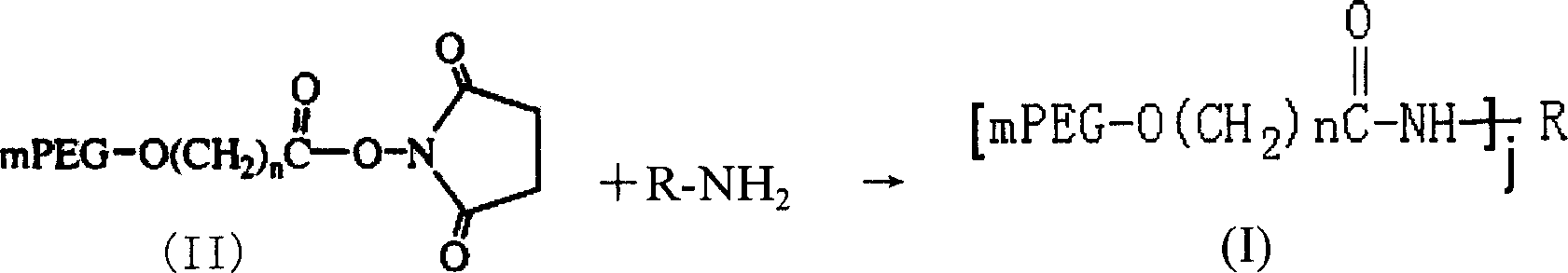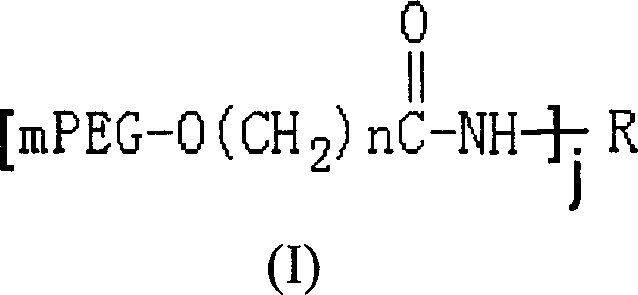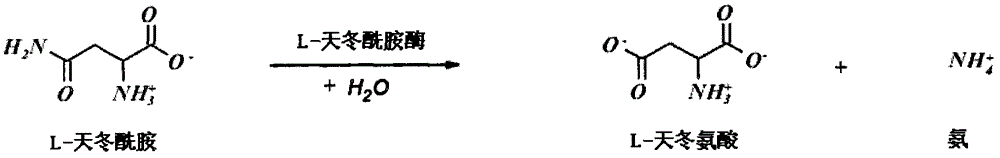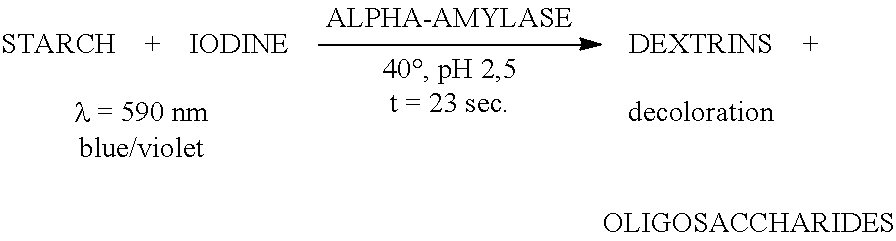Patents
Literature
148 results about "Asparaginase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Asparaginase is used with or without other anticancer (chemotherapy) drugs to treat acute lymphocytic leukemia (ALL).
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Thermal treatment process for tobacco materials
ActiveUS8434496B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Thermal treatment process for tobacco materials
ActiveUS8991403B2Alter natureAlter characterTobacco preparationTobacco treatmentArginineTobacco product
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Thermal treatment process for tobacco materials
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
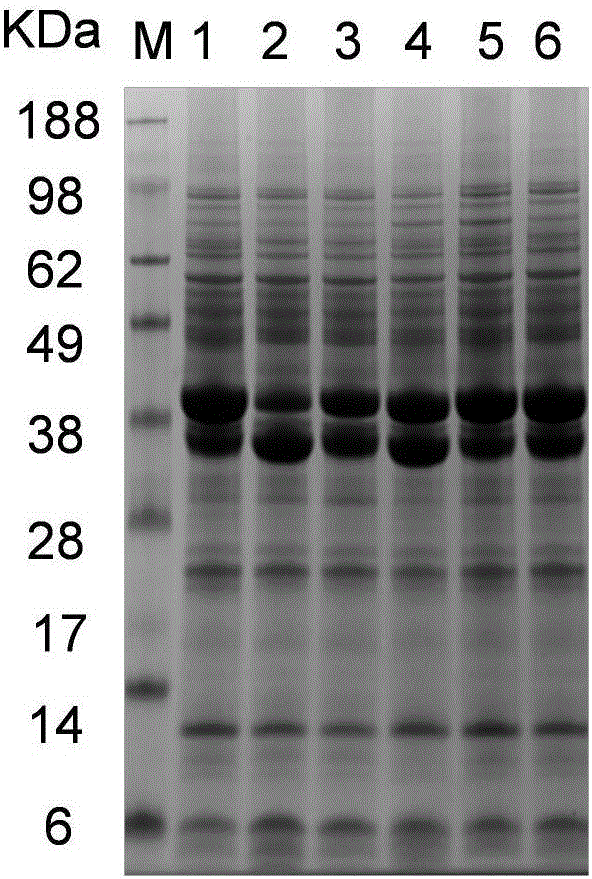
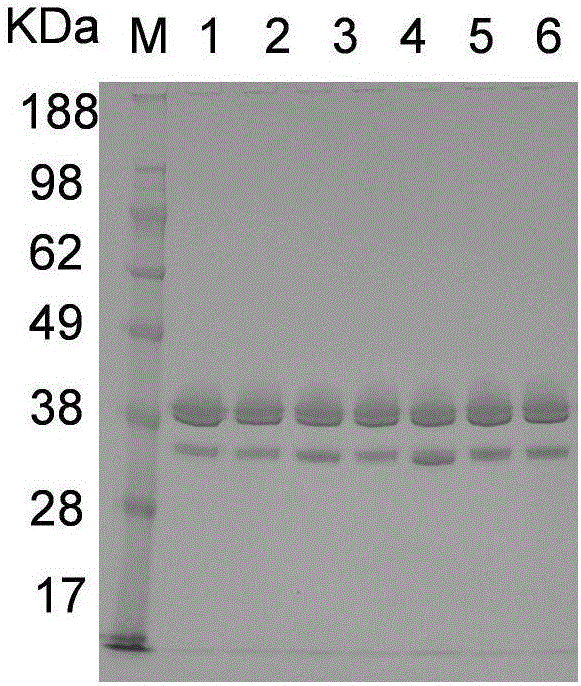
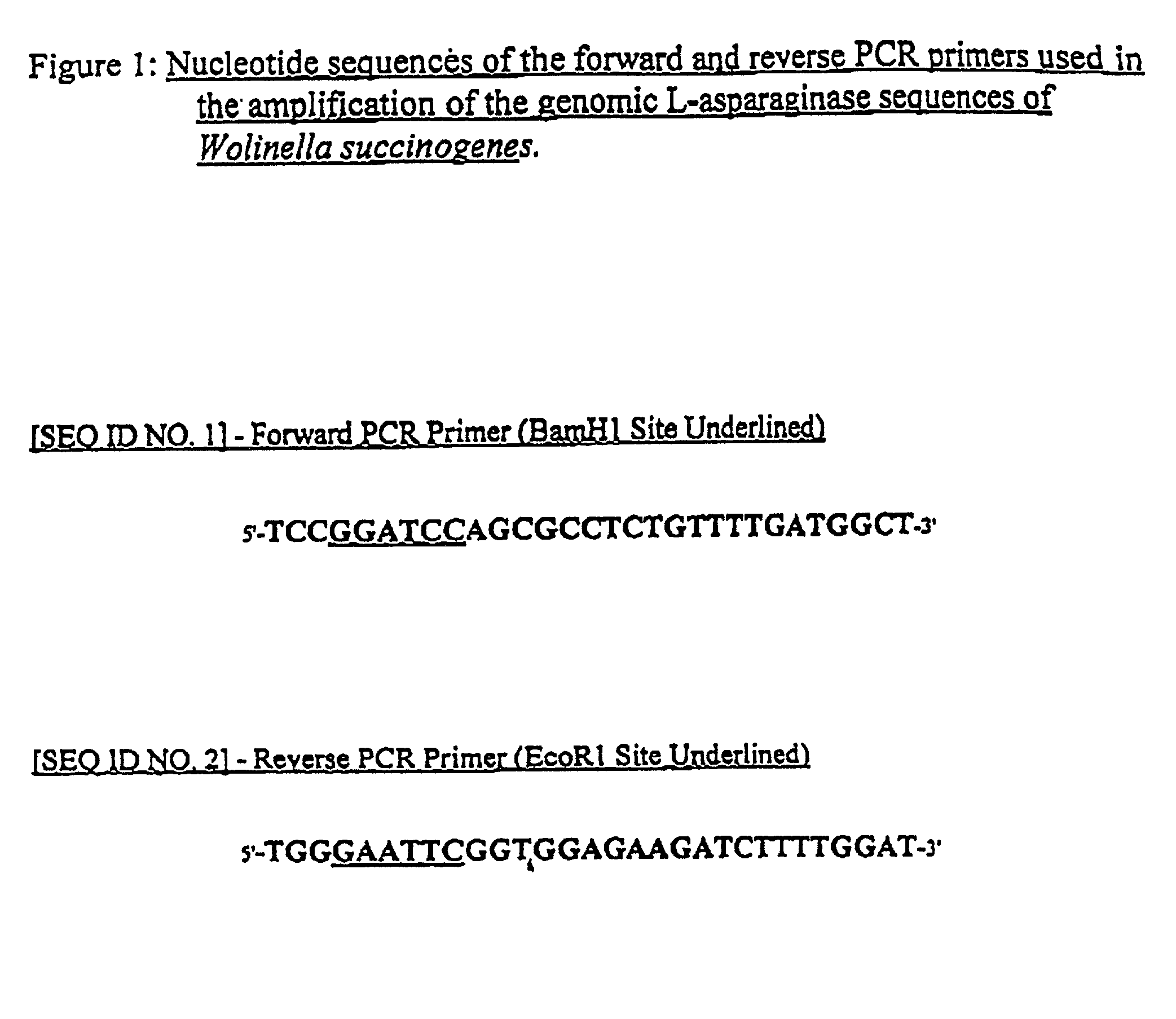
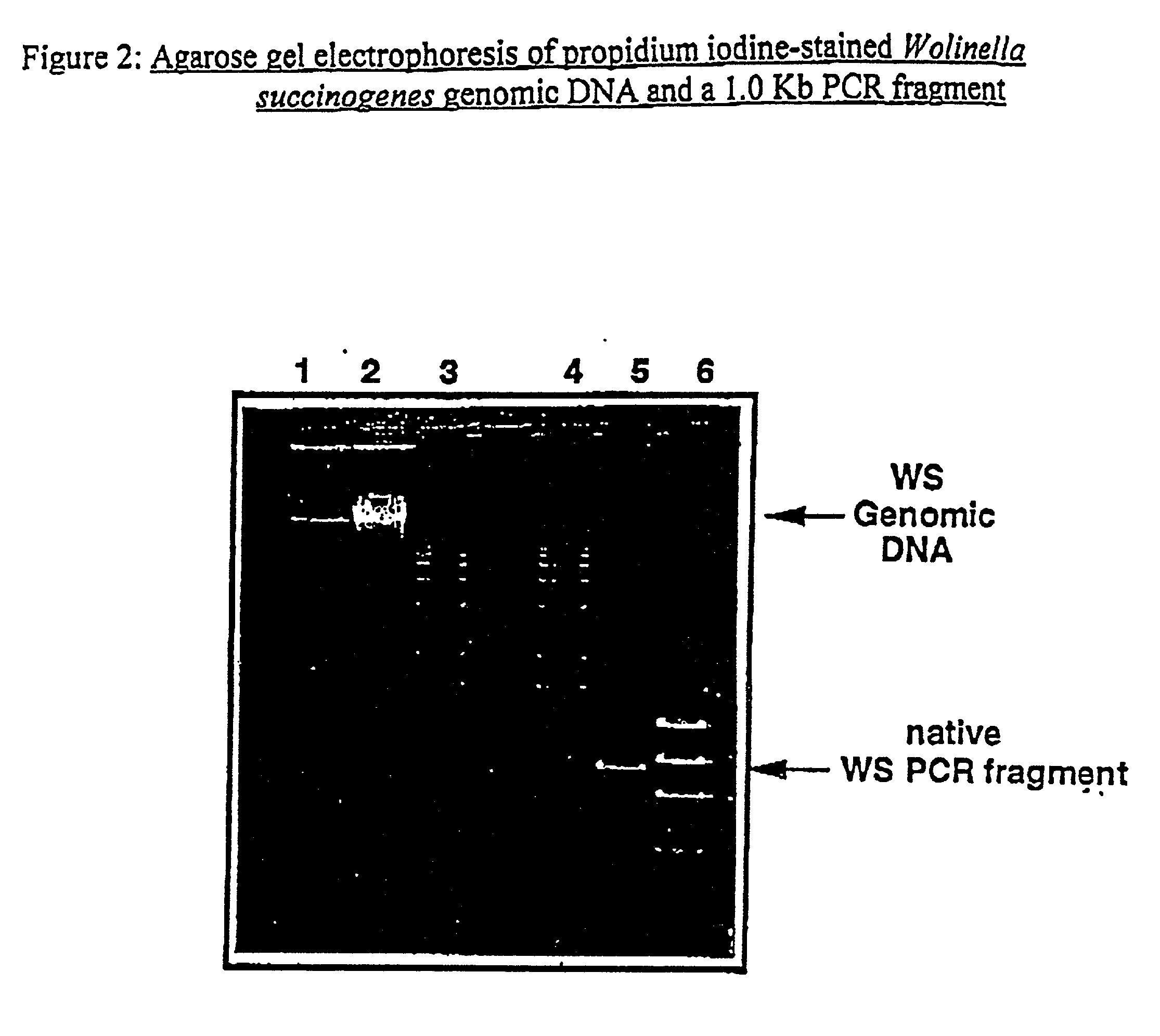
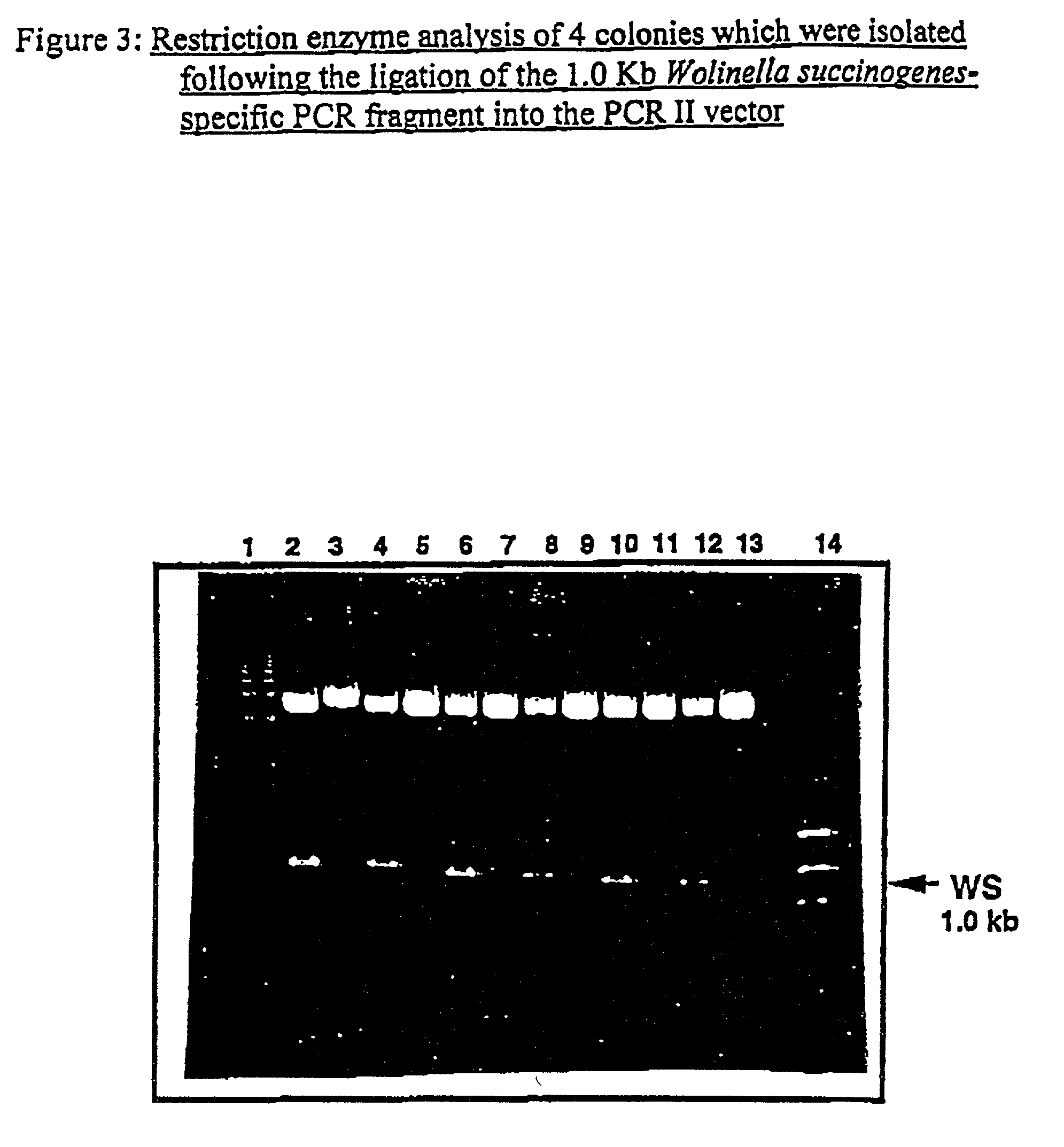
Utilization of Wolinella succinogenes asparaginase to treat diseases associated with asparagine dependence
InactiveUS6251388B1Highly efficaciousLess immunosuppressive activitySugar derivativesBacteriaHomotetramerAutoimmune condition
Described herein are methods for producing recombinant forms of asparaginase derived from Wolinella succinogenes. In addition, methods for covalent modification of proteins, including asparaginases, by acylation are also provided. Certain embodiments provide for epitopic-labeling of the amino terminus of W. succinogenes asparaginase. Additional embodiments concern methods for the therapeutic utilization of the native, homotetrameric form of W. succinogenes asparaginase, as well as the use of epitopically-labeled or non-epitopically-labeled recombinant W. succinogenes asparaginase (or a covalently modified analog thereof) in the therapeutic treatment of malignant and non-malignant hematological disease and other diseases where asparagine depletion or deprivation would be efficacious or which respond to asparagine depletion or deprivation, as well as their potential utilization in the therapeutic treatment of autoimmune diseases such as rheumatoid arthritis, AIDS, and SLE.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES
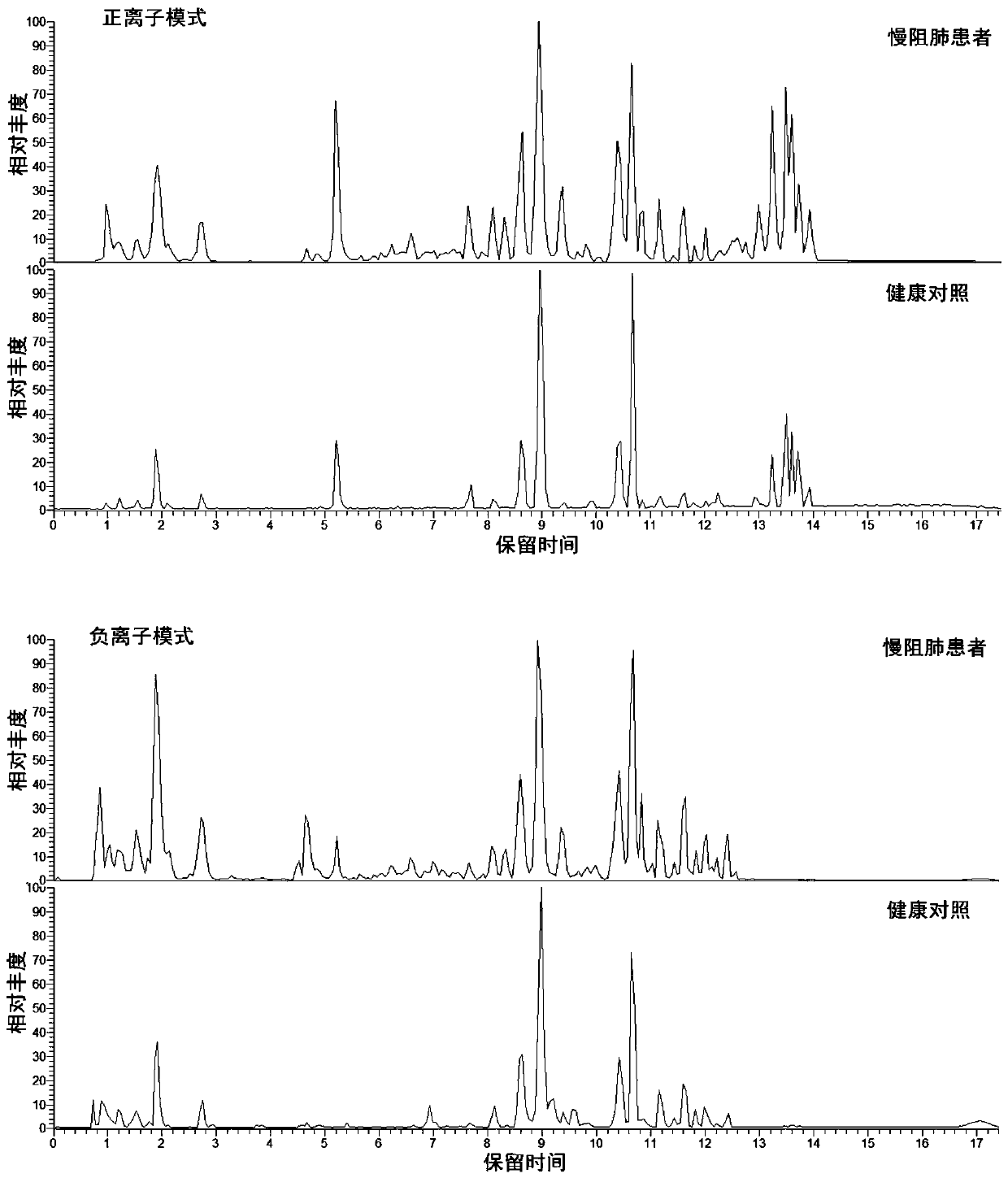
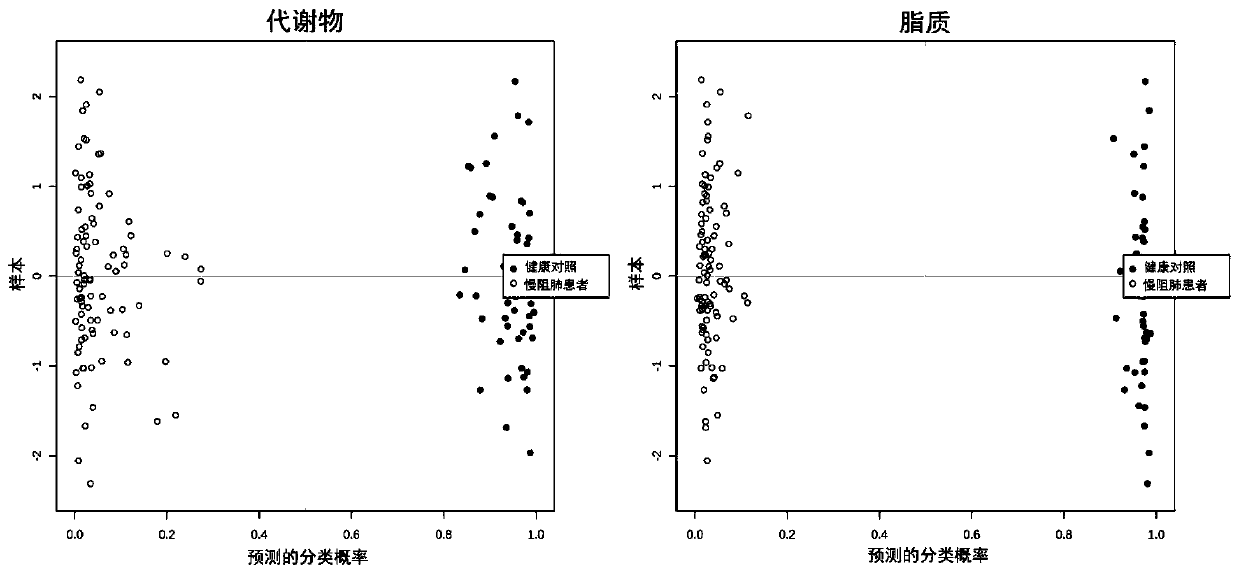
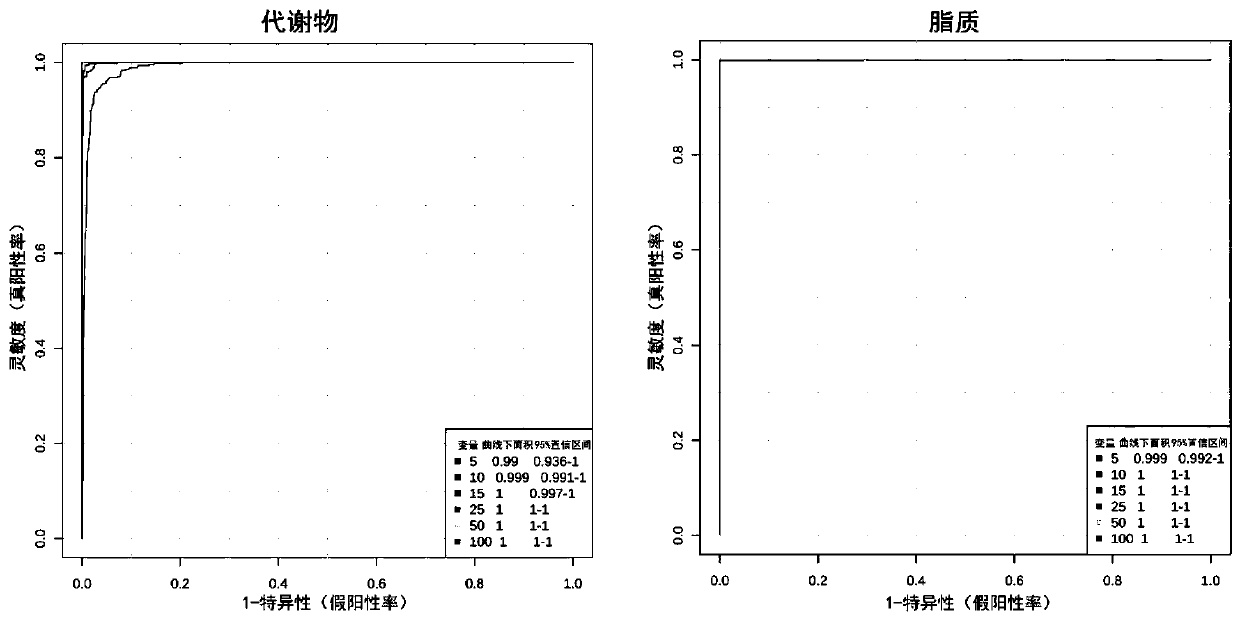
Metabonomics-based early diagnosis marker for chronic obstructive pulmonary disease and application thereof
InactiveCN111289736AExpand clinical applicationHigh promotional valueComponent separationDisease diagnosisDihydrouracilDimethylaniline N-oxide
The invention discloses a metabonomics-based diagnosis marker for chronic obstructive pulmonary disease and application thereof. The diagnosis marker comprises the following 28 blood plasma metabolismmarkers: phosphatidylcholine PC 16:1-36:3, phosphatidylcholine PC 26:0-22:4, phosphatidylcholine PC 26:0-22:3, phosphatidylcholine PC 47:5e, phosphatidylcholine PC 44:11e, phosphatidylcholine PC 16:0-24:5, phosphatidylcholine PC 20:2-20:3, phosphatidylcholine PC 40:10, phosphatidylcholine PC 38:9e, phosphatidylcholine PC 18:1-20:4, phosphatidylcholine PC 16:0-20:3, phenylacetaldehyde, dihydrouracil, nicotinamide, 4-methoxycinnamic acid, ketovaline, threonine, DL-3-aminoisobutyric acid, pyruvic acid, stachyose, caffeic acid, N, N-dimethylaniline, maltotriose, D(-)-gulonate-gamma-lactone, and L-asparaginase. The marker provided by the invention has good classification on metabolome data of patients suffering chronic obstructive pulmonary disease and healthy people, and can accurately distinguish the patients suffering chronic obstructive pulmonary disease from the healthy people.
Owner:PEKING UNIV +1
L-asparaginase mutant with improved enzyme activity and construction method thereof
InactiveCN105062997AIncreased potential for industrial applicationsReduce generationBacteriaHydrolasesGlycineSpecific enzyme
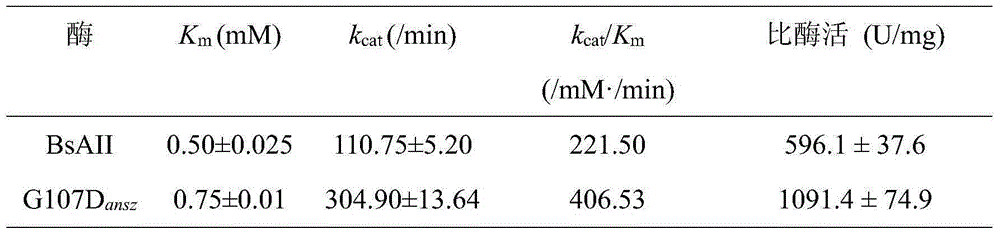
The invention discloses an L-asparaginase mutant with the improved enzyme activity and a construction method thereof, and belongs to the field of gene engineering. According to the L-asparaginase mutant, on the basis of amino acid shown in the SEQ ID NO.2, the 107th glycine is mutated into aspartic acid. The obtained mutant is expressed in bacillus subtilis, fermentation is performed in a shake flask for 24 h and then the enzyme activity is 961 U / mL; the enzyme activity of the mutant is improved by 80%, the appetency of a substrate is decreased by 50% compared with protoenzyme, the catalytic efficiency is improved by 84%, and meanwhile the specific enzyme activity is improved by 83%. According to the L-asparaginase mutant, it is shown that the 107th amino acid residue has a great influence on the enzyme catalytic action, a certain foundation is provided for research on the enzyme catalytic mechanism, and the enzyme industrial application potential is improved.
Owner:JIANGNAN UNIV
Asparaginase enzyme variants and uses thereof
The present invention relates to newly identified asparaginase polypeptide variants of SEQ ID NO: 3 and to polynucleotide sequences that encode such novel asparaginase variants. Furthermore the invention relates to the use of these novel asparaginase variants in industrial processes.
Owner:DSM IP ASSETS BV
Polyethylene glycol modified L-Asparaginasum and modification method thereof
InactiveCN101586099AStrong ligation reactionHigh reaction productHydrolasesFiltrationPolyethylene glycol
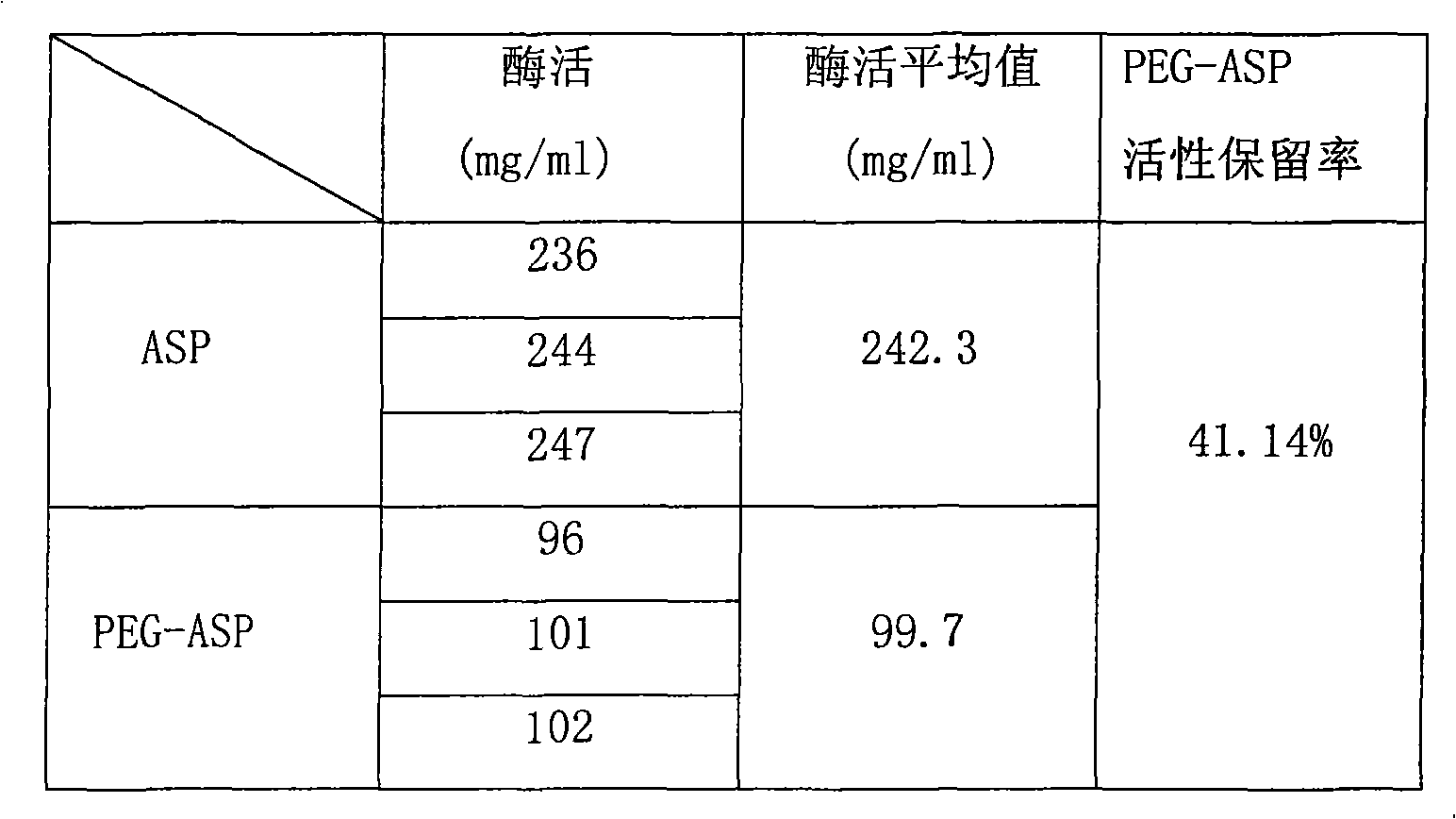
The invention provides polyethylene glycol modified L-Asparaginasum shown as formula (1) and a modification method thereof. The method uses the modification reaction property of polyethylene glycol molecules to modify the L-Asparaginasum under the condition of proper pH value of a reaction system. The polyethylene glycol modified L-Asparaginasum is characterized in that two polyethylene glycol molecules are covalently bound in a reaction product; and the substance has lower glomerular filtration rate and lower immunogenicity compared with a single polyethylene glycol molecule modifier so as to provide better antitumor effect.
Owner:BEIJING SL PHARMA
Compositions and methods related to therapeutic cell systems for tumor growth inhibition
InactiveUS20190160102A1Impairing synthesisReduce concentrationHydrolasesAntibody mimetics/scaffoldsCD33Degradative enzyme
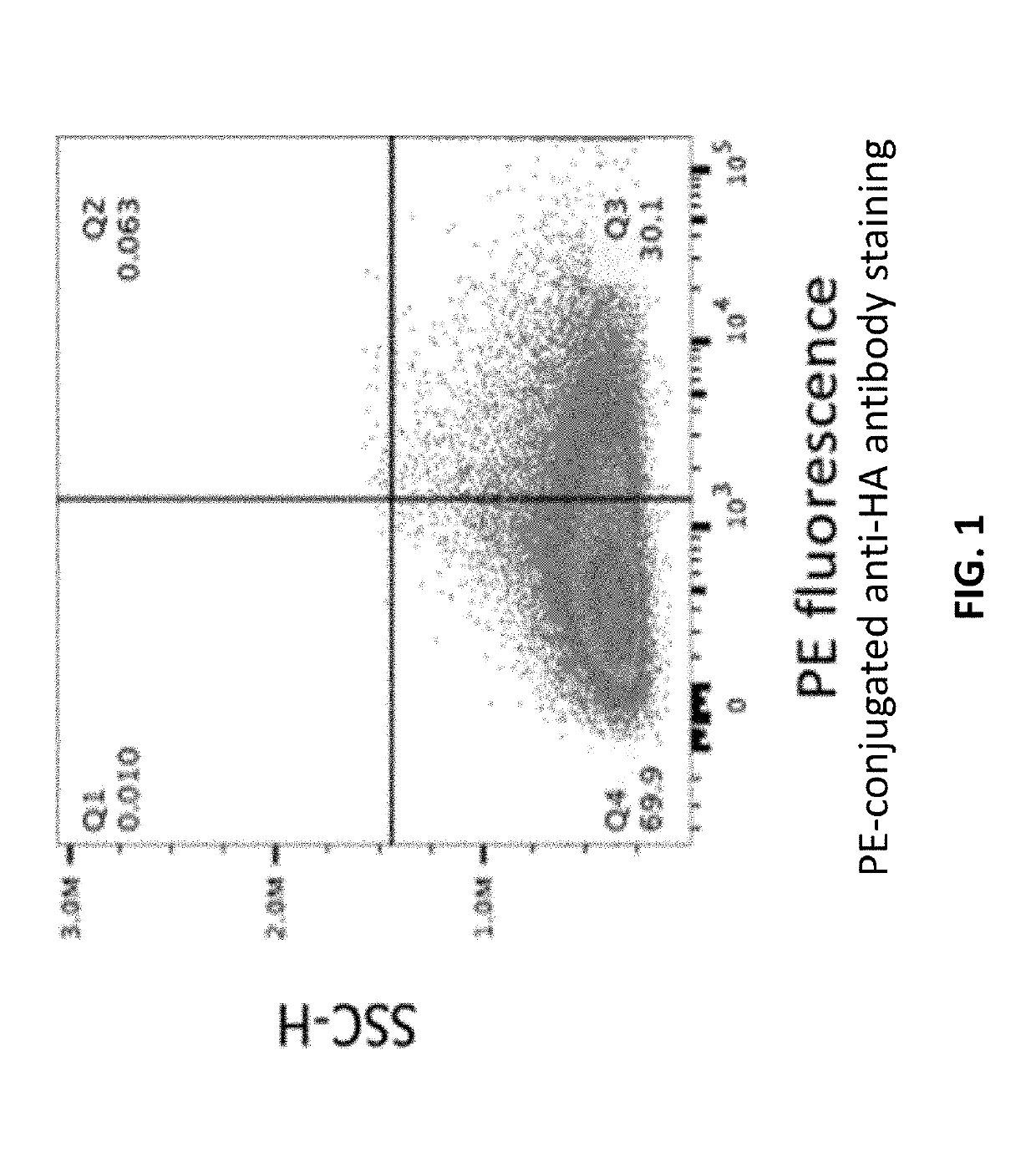
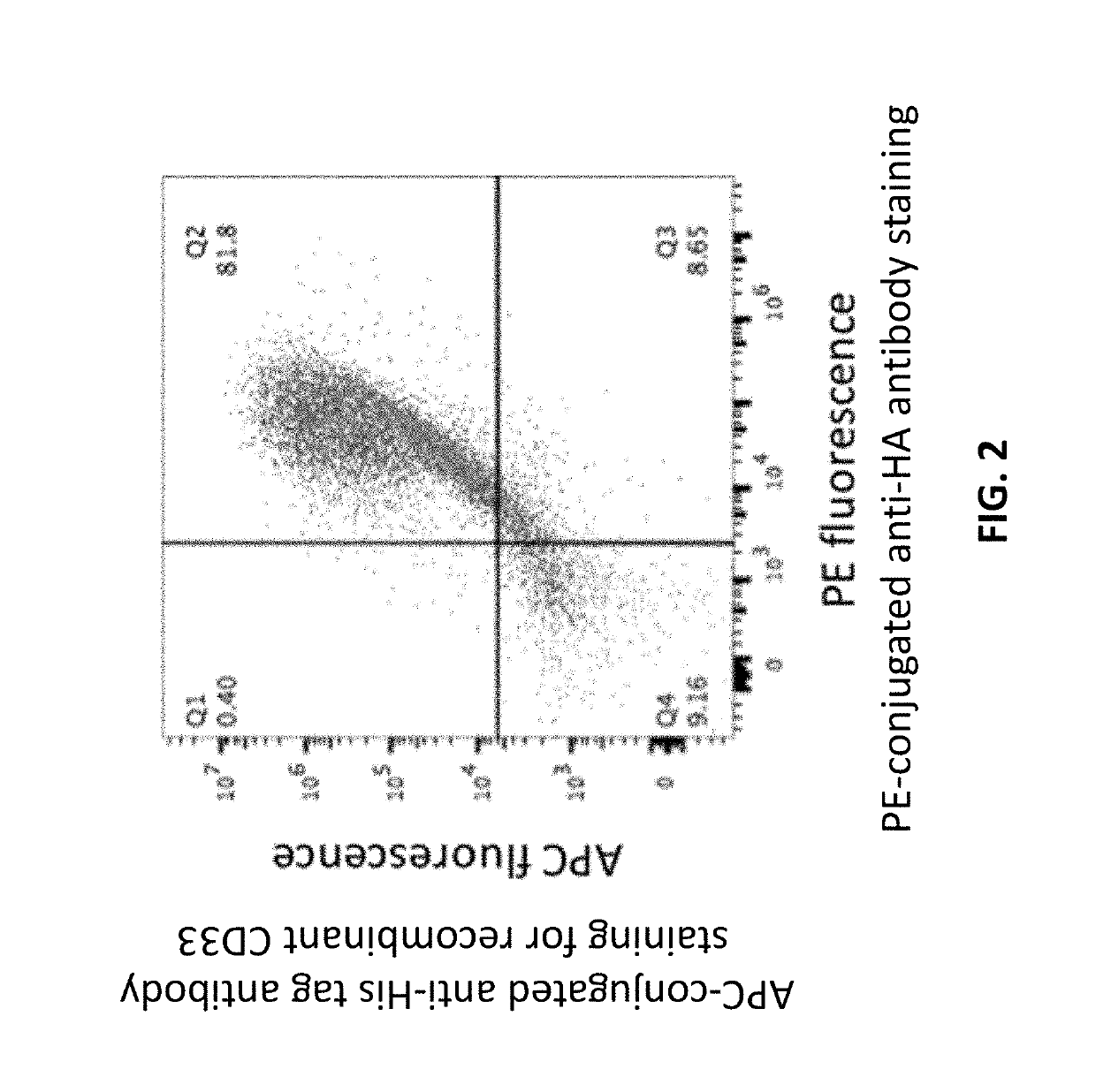
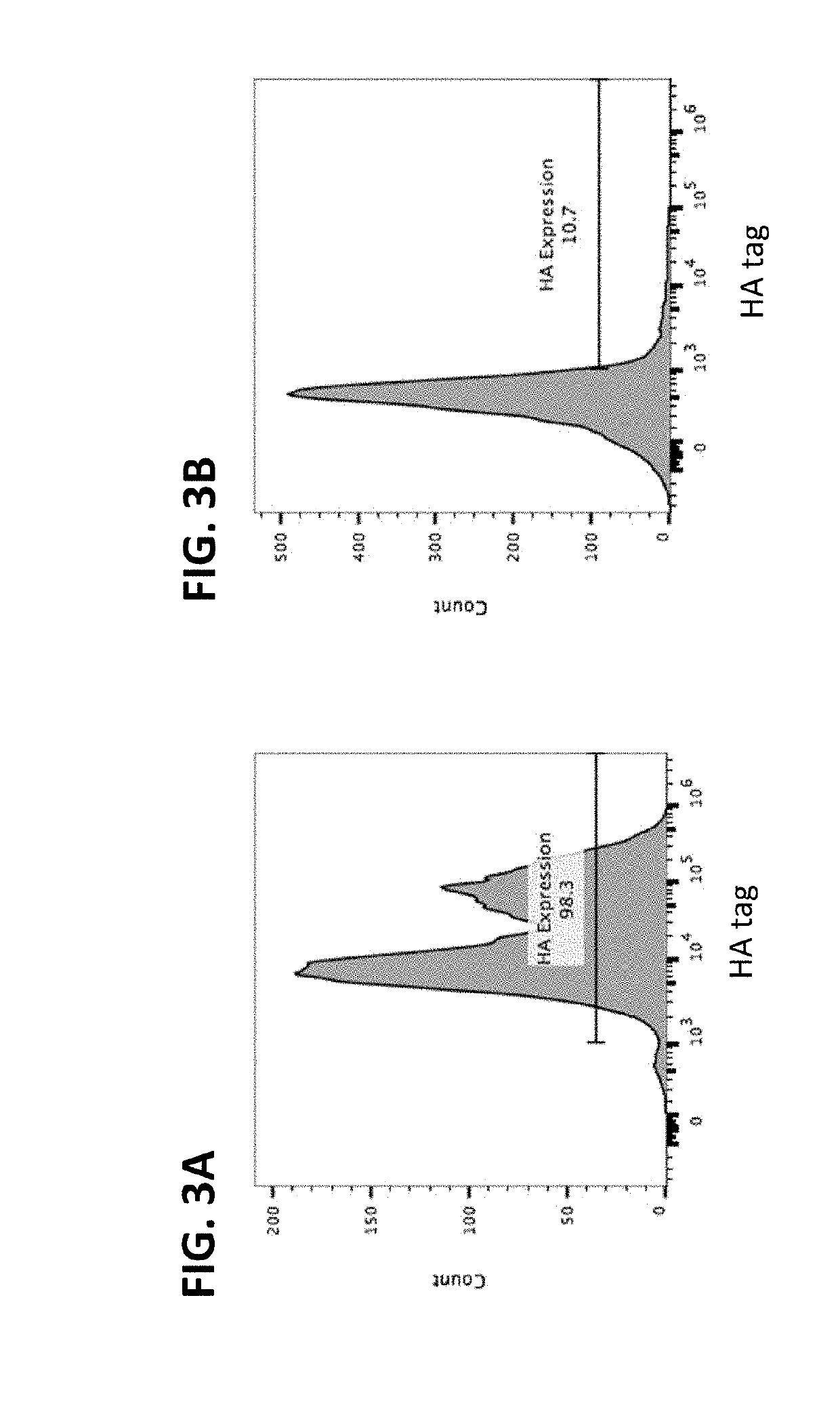
The disclosure provides, e.g., enucleated erythroid cells comprising an amino acid degradative enzyme such as asparaginase and a targeting moiety such as an anti-CD33 antibody molecule. The cells may be used, e.g., to treat cancers such as AML.
Owner:RUBIUS THERAPEUTICS
Signal peptide capable of improving secretion efficiency, and applications thereof
ActiveCN103709236AImprove secretion efficiencyImprove enzyme production capacityBacteriaHydrolasesNucleotideAsparaginase
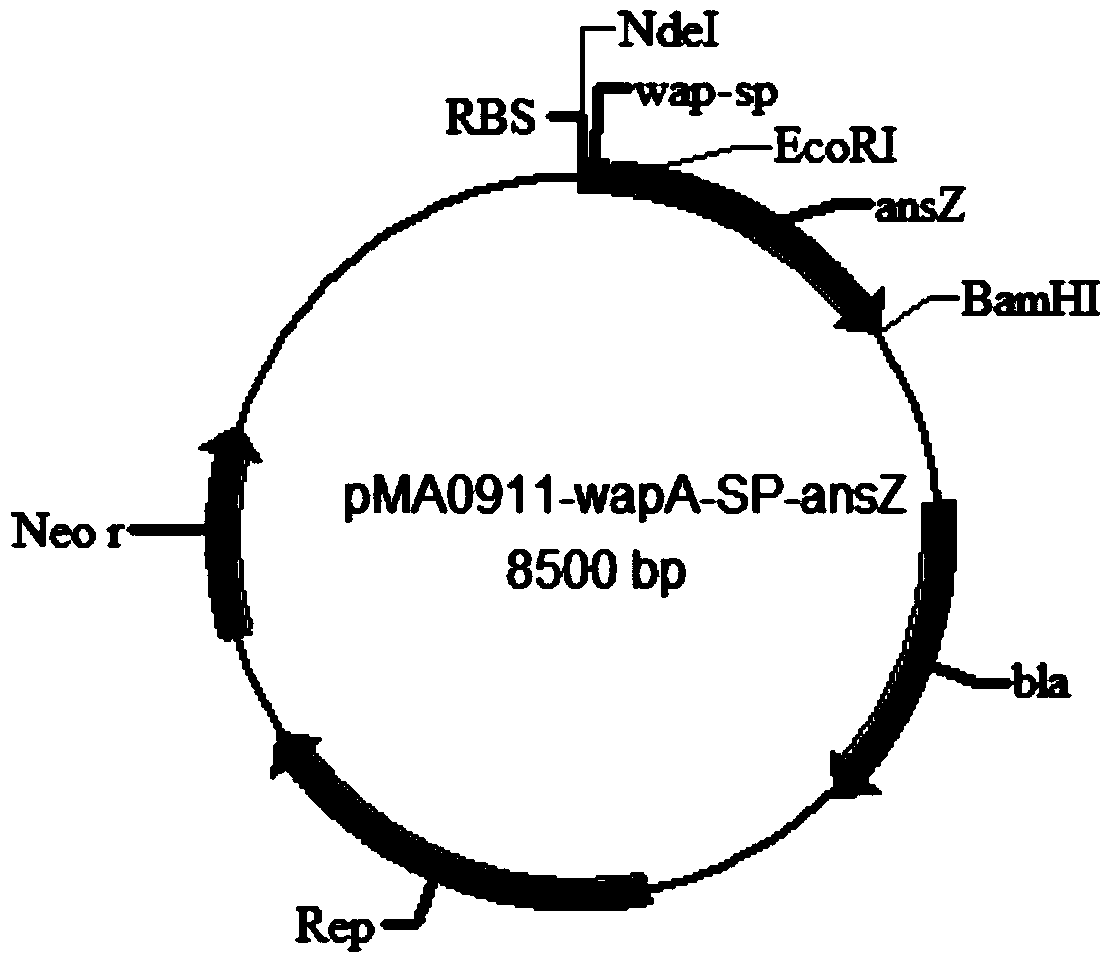
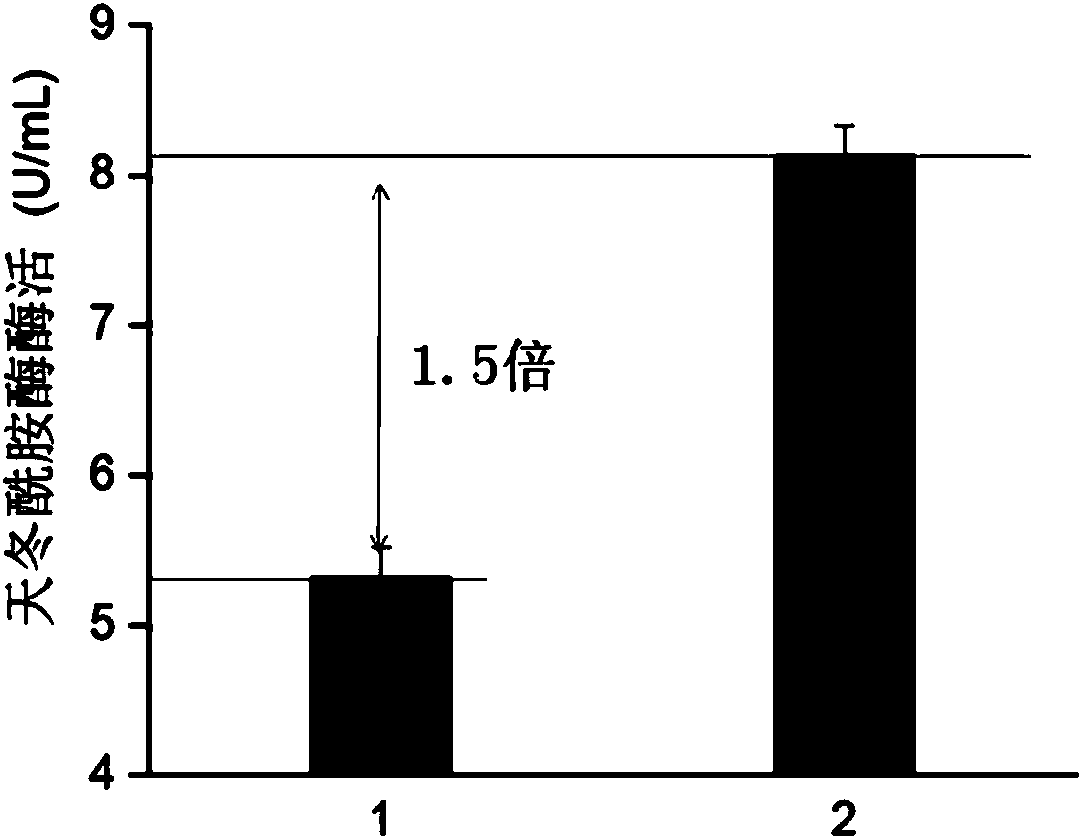
The invention discloses a signal peptide capable of improving secretion efficiency. A nucleotide sequence is shown in 1) or 2): 1) the nucleotide sequence is shown as SEQ ID NO.1; 2) after transforming the SEQ ID NO.1 sequence, the nucleotide sequence still performs the function of signal peptide and the secretion efficiency of the nucleotide sequence does not have fundamental change. The invention provides a signal peptide capable of improving the secretion efficiency, the secretion capability of strains can be remarkably improved after the signal peptide is used, and the enzyme activity of asparaginase can be improved by 1.5 times. The enzyme production capability of the transformed strains is remarkably improved, and the transformed strains are more suitable for industrial application, the production cost can be lowered, and the production efficiency can be improved.
Owner:JIANGNAN UNIV
Thermal treatment process for tobacco materials
InactiveUS20150122271A1Alter natureAlter characterTobacco preparationTobacco treatmentArginineValine
A method of preparing a tobacco material for use in a smoking article is provided, including (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof; (ii) heating the mixture; and (iii) incorporating the heat-treated mixture into a smoking article as a smokable material. A smoking article in the form of a cigarette is also provided that includes a tobacco material pre-treated to inhibit reaction of asparagine to form acrylamide in mainstream smoke. Upon smoking, the smoking article is characterized by an acrylamide content of mainstream smoke that is reduced relative to an untreated control smoking article.
Owner:R J REYNOLDS TOBACCO COMPANY
Polyethylene glycol modified L-asparaginyl amine enzyme
InactiveCN101082043AUniform compositionLow immunogenicityPeptide/protein ingredientsHydrolasesPolyethylene glycolAsparaginase
The present invention discloses one kind of new compound, polyethylene glycol derivative modified L-asparaginase in the structure as shown. The present invention also provides the preparation of the polyethylene glycol derivative modified L-asparaginase and its medicine composition. The compound of the present invention has homogeneous component, high antitumor effect, high chemical stability, and low immunogenicity.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Method for improving secretory expression of L-asparaginase
ActiveCN104611317AImprove enzyme production capacityIncrease productivityBacteriaHydrolasesAsparaginaseMicrobiology
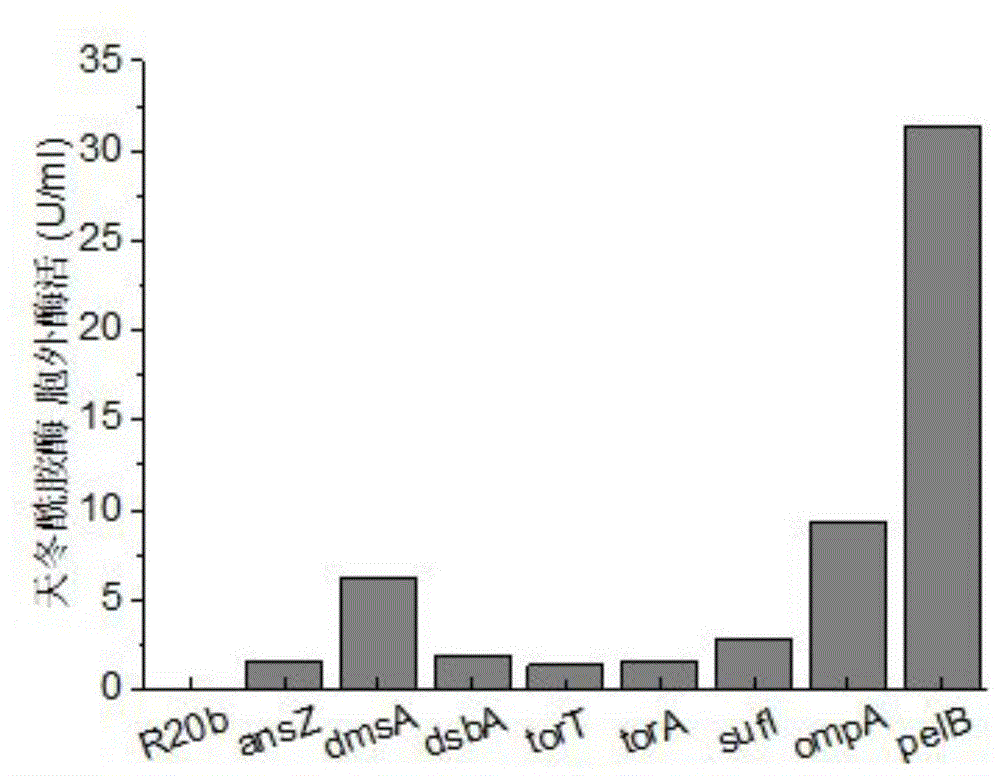
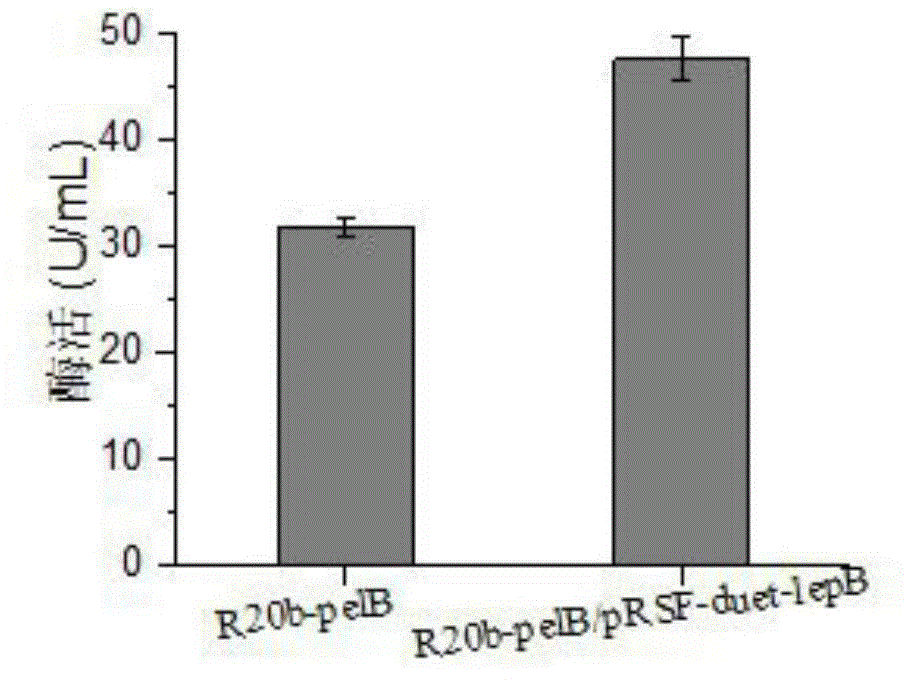
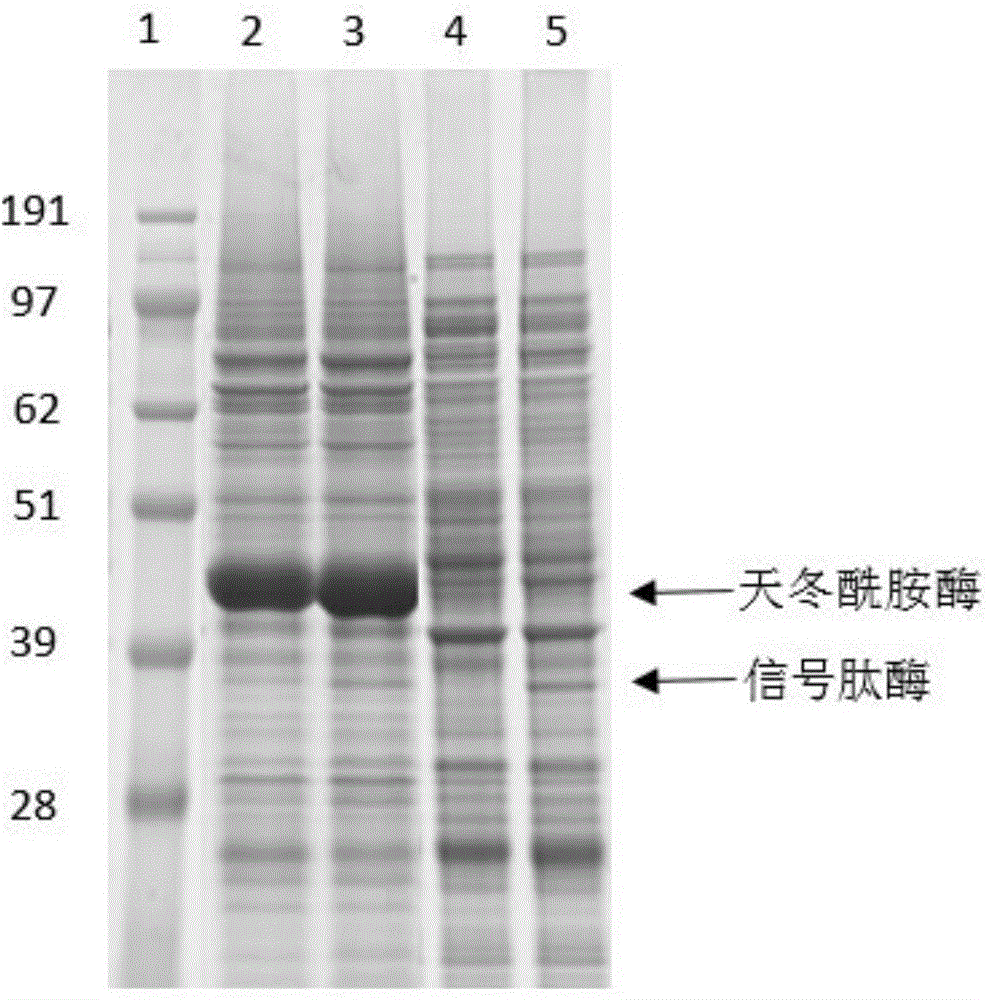
The invention discloses a method for improving secretory expression of L-asparaginase, and belongs to the field of genetic engineering. According to the method, by fusing a pelB signal peptide at an N terminal of asparaginase, the enzyme activity of the extracellular asparaginase of a recombinant bacterium is increased by 20 times, and further through coexpression of lepB signal peptidase, the enzyme activity of the asparaginase can be increased by 1.45 times on the above basis. The enzyme-producing capacity of a modified bacterial strain is significantly improved; the method is more suitable for industrial application; by the method, the production cost can be reduced and the production efficiency can be improved.
Owner:JIANGNAN UNIV
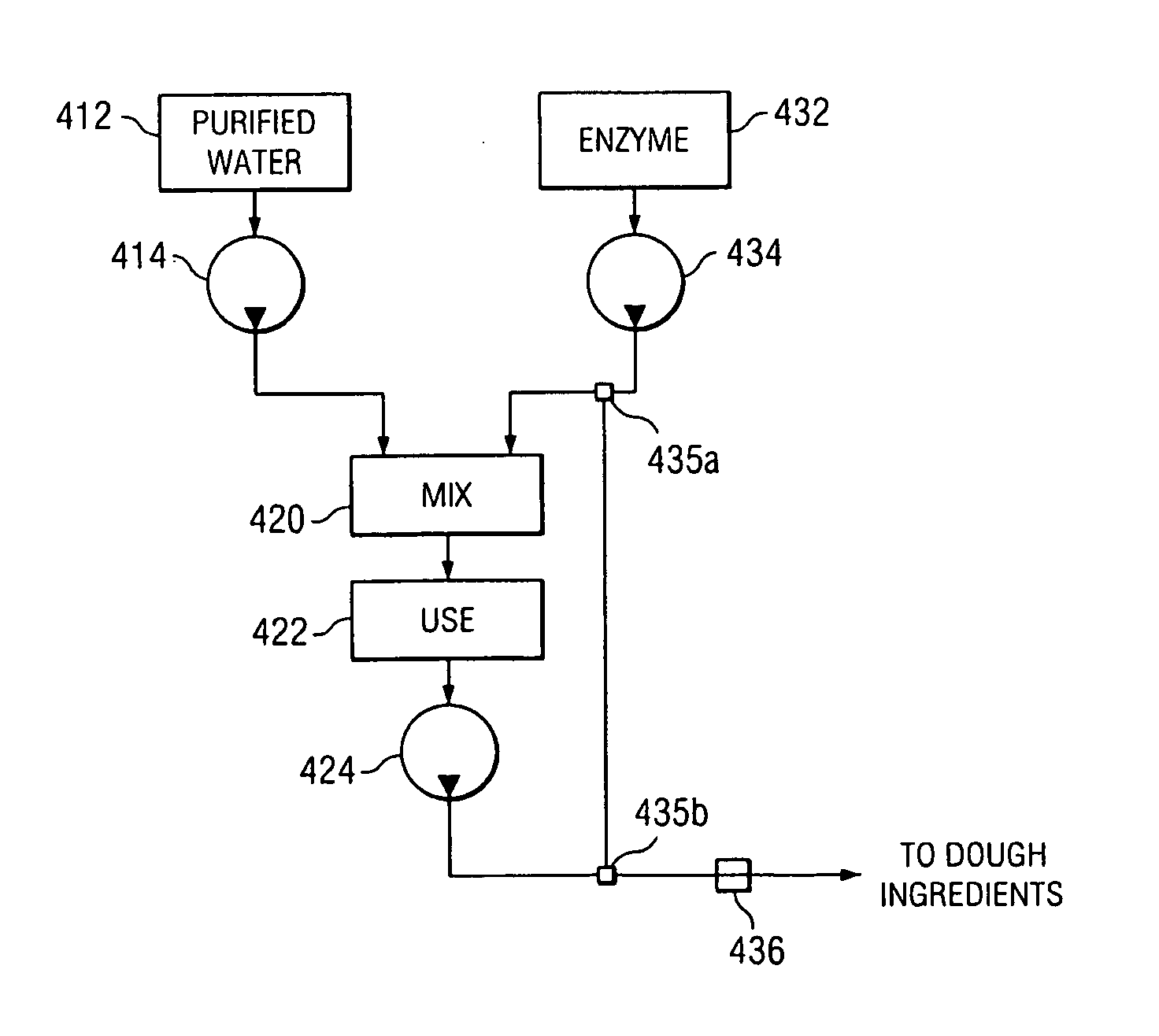
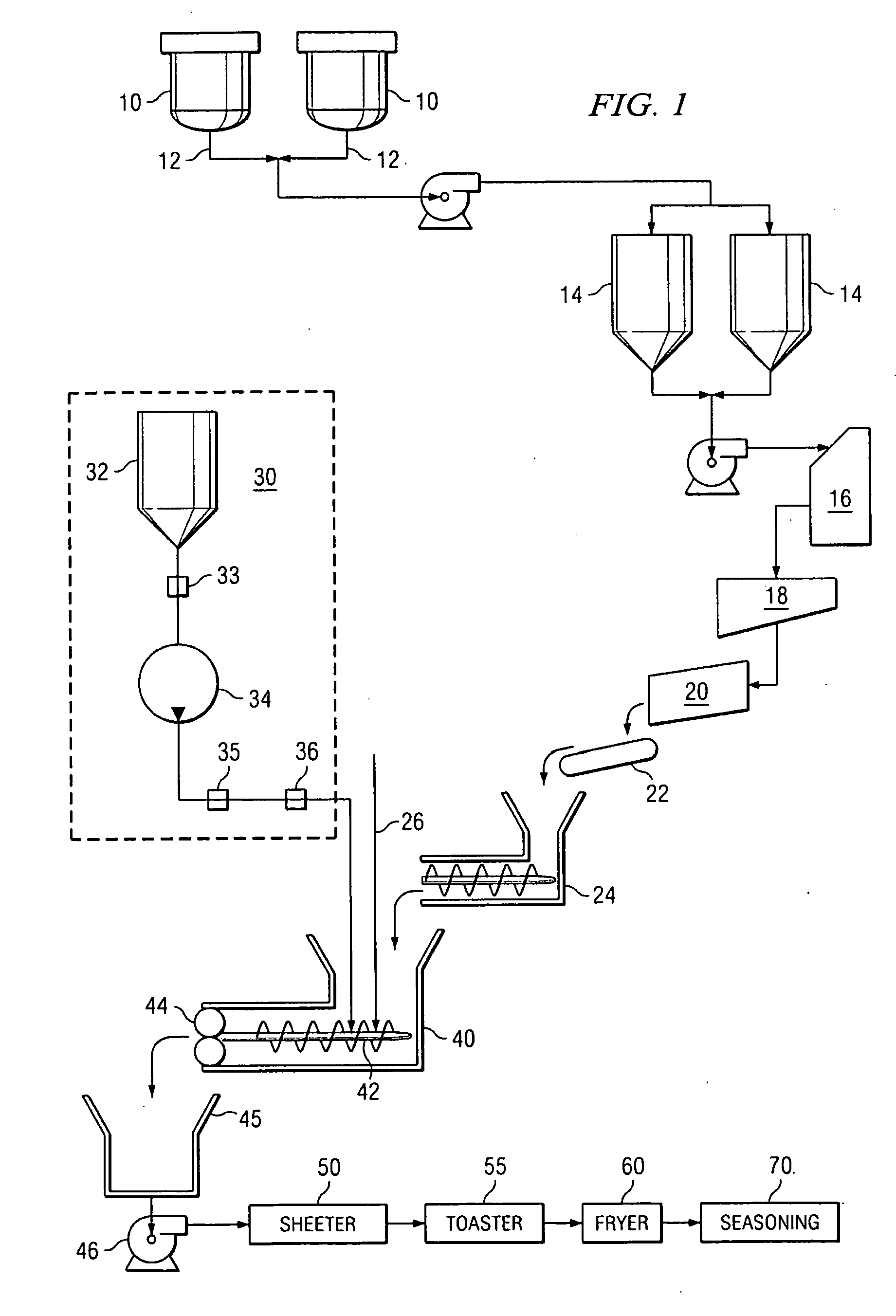
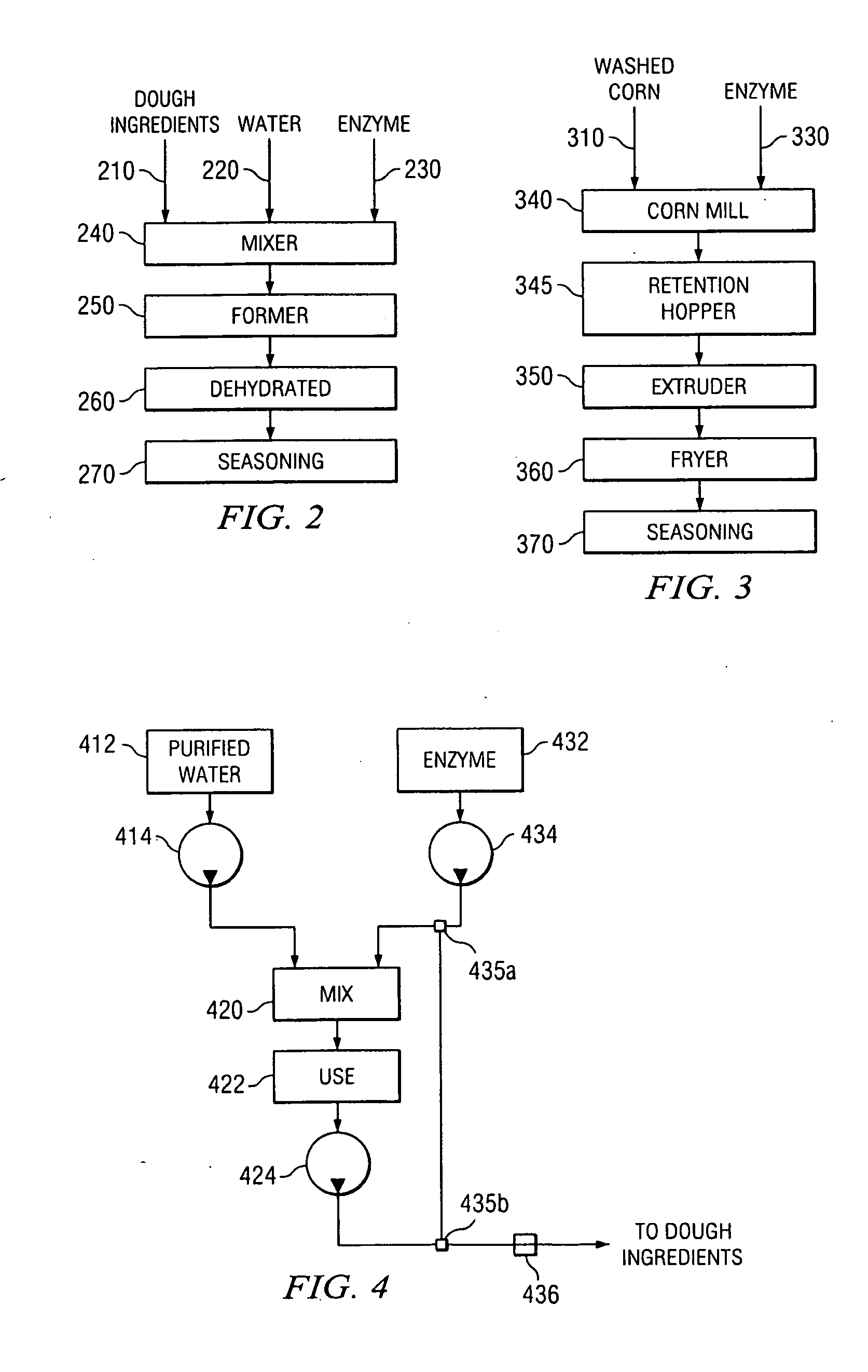
Method and system for the direct injection of asparaginase into a food process
A method and system for the direct injection of a concentrated additive is disclosed. In one aspect, the concentrated additive comprises asparaginase. Because dilution of asparaginase in chlorinated drinking water can reduce the activity of the enzyme thereby making the enzyme less effective the direct injection of the enzyme into a dough can increase the acrylamide reduction in food products.
Owner:FRITO LAY NORTH AMERICA INC
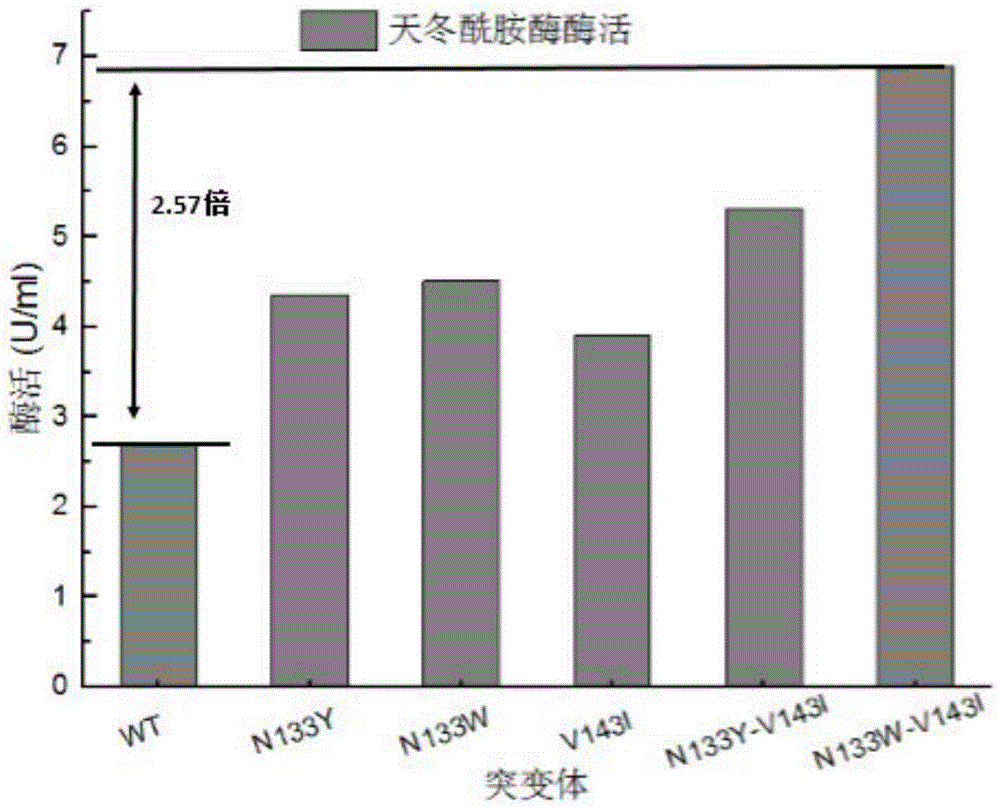
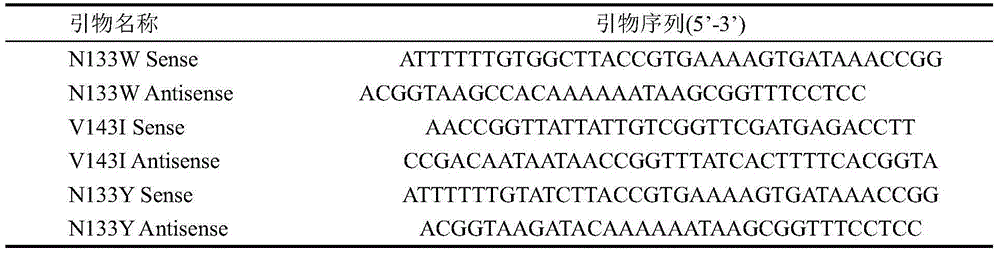
Asparaginase mutant with enhanced enzyme activity
The invention discloses an asparaginase mutant with enhanced enzyme activity, and belongs to the field of enzyme engineering. According to the asparaginase mutant, hydrophilic amino acid asparaginate and valine which are contained in an asparaginase molecule are mutated into tyrosine, tryptophan and isoleucine which have high hydrophobicity in a site-specific mutagenesis mode, so that the hydrophobicity inside the asparaginase molecule is changed, the enzyme activity, which is expressed by a strain, of asparaginase is remarkably improved, and the enzyme activity of the asparaginase is enhanced by 2.57 times. The improved strain is remarkably enhanced in enzyme producing capacity, more suitable for industrial application, reduced in production cost and improved in production efficiency.
Owner:JIANGNAN UNIV
Novel asparaginases and uses thereof
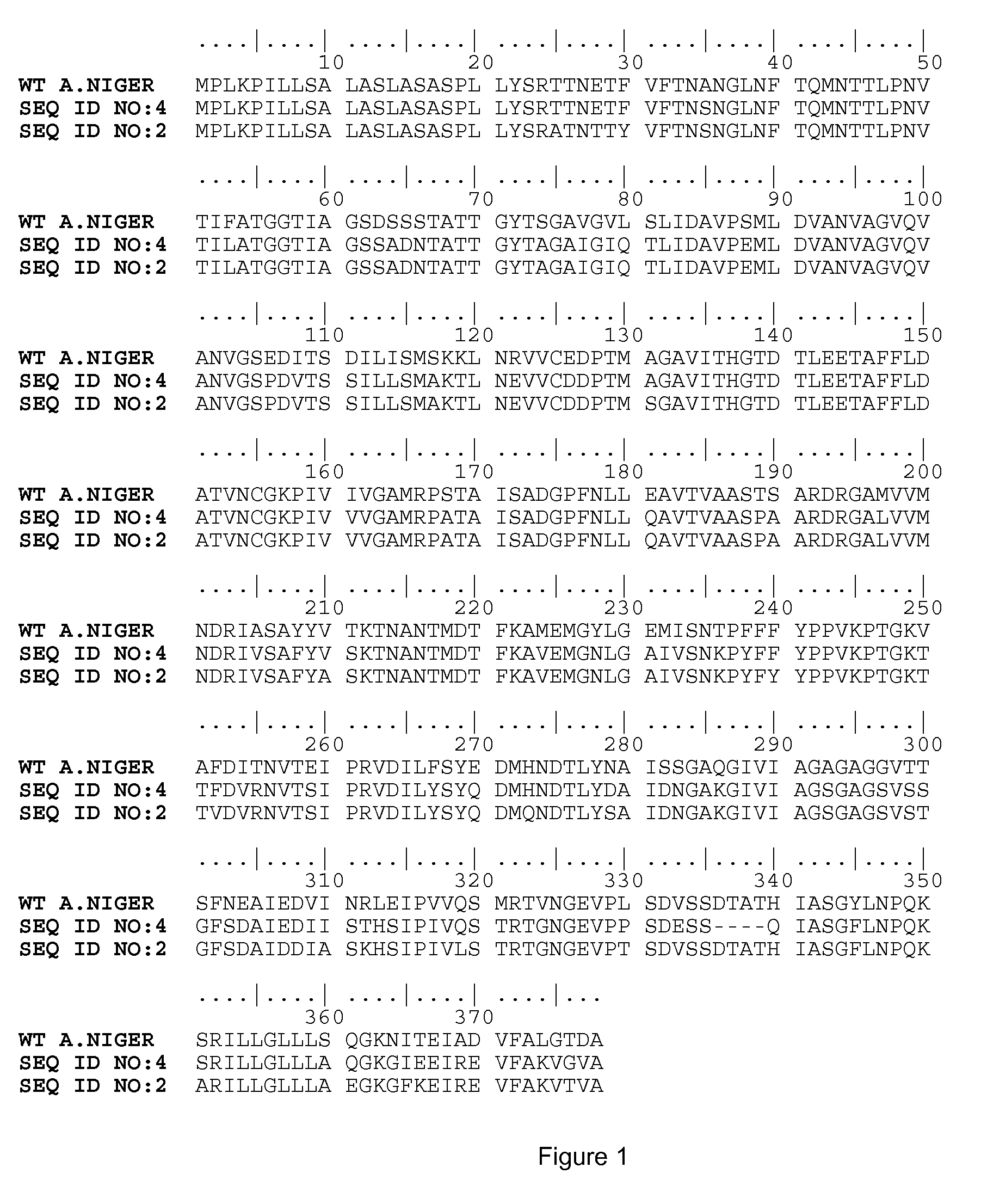
The present invention relates to an asparaginase having the width of the pH activity profile which is at least 3.5. Furthermore the invention relates to newly identified asparaginase polypeptide according to any one of SEQ ID NO: 2 or SEQ ID NO: 4 and to variants thereof and to polynucleotide sequences that encode such novel asparaginase variants. Furthermore the invention relates to the use of these novel asparaginase variants in industrial processes.
Owner:DSM IP ASSETS BV
Method for preparing elspar modilfied by carbowax
InactiveCN1498965AReduce immune antigenicityProlonged biological activity half-lifeHydrolasesPeptide/protein ingredientsChemical reactionChain length
A process for preparing the polyethanediol modified asparaginase includes reaction between asparaaginas and linear polyethanodiol whose linear chain length is 1000-5000 in ratio of 1:5-30, and reaction between linear polyethanediol whose linear chain length is 5000-3000 and biologic macro-molecular compound in ratio of 1:10.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Asparaginases and uses thereof
The present invention relates to an asparaginase having the width of the pH activity profile which is at least 3.5. Furthermore the invention relates to newly identified asparaginase polypeptide according to any one of SEQ ID NO: 2 or SEQ ID NO: 4 and to variants thereof and to polynucleotide sequences that encode such novel asparaginase variants. Furthermore the invention relates to the use of these novel asparaginase variants in industrial processes.
Owner:DSM IP ASSETS BV
Recombinant bacterium for producing L-lysine, constructing method of recombinant bacterium and method for producing L-lysine
The invention relates to a recombinant bacterium for producing L-lysine, a constructing method of the recombinant bacterium and a method for producing the L-lysine. Compared with a starting bacterium,the recombinant bacterium has the effect of improving the expression and / or activity of asparaginase, and the starting bacterium is a strain capable of accumulating lysine. It is observed through fermentation culture that the recombinant bacterium can have the effect of multiply improving the yield and the yield of the L-lysine is obviously improved.
Owner:BEIJING ZHONGKE EPPEN BIOTECH CO LTD +1
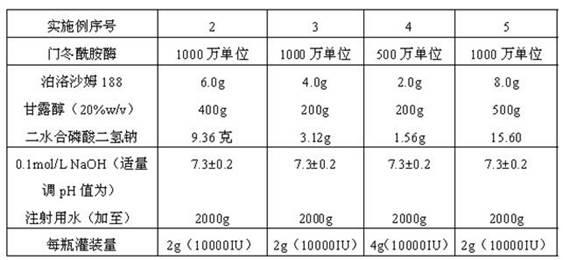
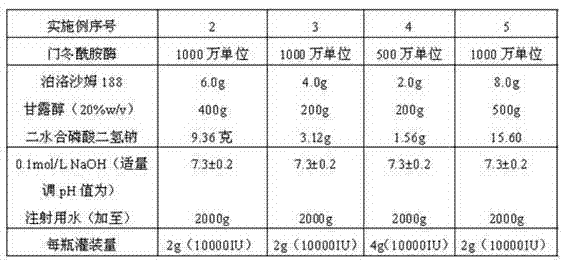
Asparaginase freeze-dried powder injection and preparation method thereof, as well as asparaginase solution
ActiveCN102138909AEasy to useRapid reconstitutionPowder deliveryPeptide/protein ingredientsFreeze-dryingSolvent
The invention discloses an asparaginase freeze-dried powder injection. The injection comprises asparaginase, a buffering agent, a surfactant, medical auxiliary materials and 5% of injection water which is used as a solvent in a preparing process and less than the addition finally, wherein the surfactant is a nonionic surfactant; and the buffering agent and the nonionic surfactant are added into the formula, so that the situation that tiny visible particles exceed standard is prevented when the asparaginase is dissolved and transferred into an injection. The invention also discloses a dedicated solution for dissolving the asparaginase, wherein the solution solvent is the injection water, and the solute comprises the buffering agent and the surfactant. On the premise of not changing the conventional formula of the freeze-dried powder injection, when the freeze-dried powder is dissolved in the solution, and is transferred into sodium chloride or a glucose injection, the situation that tiny visible particles exceed standard is prevented, so that the stimulation to human bodies is reduced in use, and the clinical administration safety is improved.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Stabilization of asparaginase
InactiveUS20110256267A1Improve enzyme stabilityDeclining asparaginase activityHydrolasesTea extractionAsparaginaseBiochemistry
Owner:NOVOZYMES AS
Therapeutic asparaginases
InactiveUS20160213759A1Prolong half-life in vivoIncrease serum stabilityPeptide/protein ingredientsHydrolasesCancer cellAsparaginase
Provided herein are mutant asparaginase enzymes that lack glutaminase activity. Also provided are methods of treating ASNS-negative cancer cells with a glutaminase-free asparaginase.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +2
L-asparaginase variant with increased activity
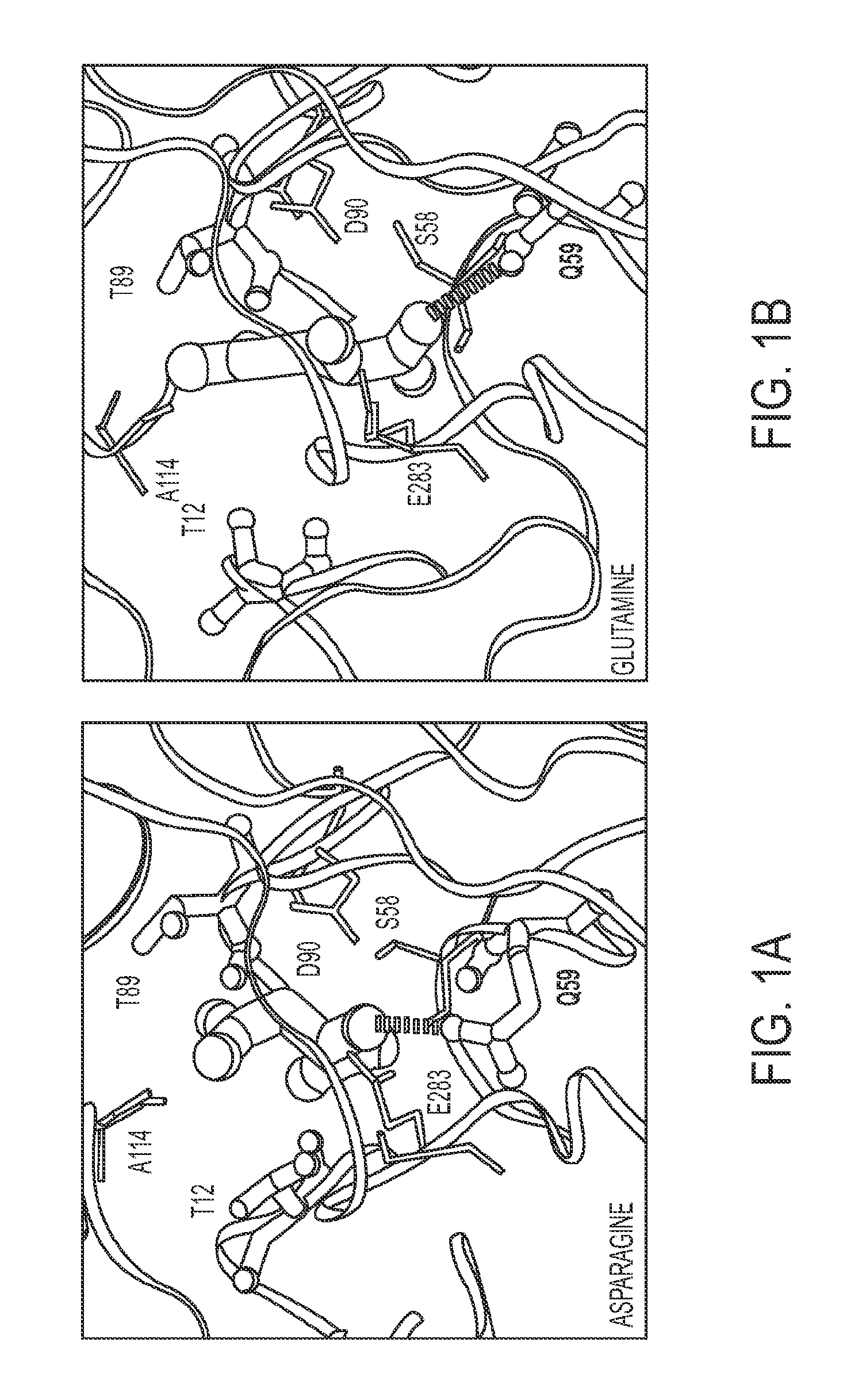
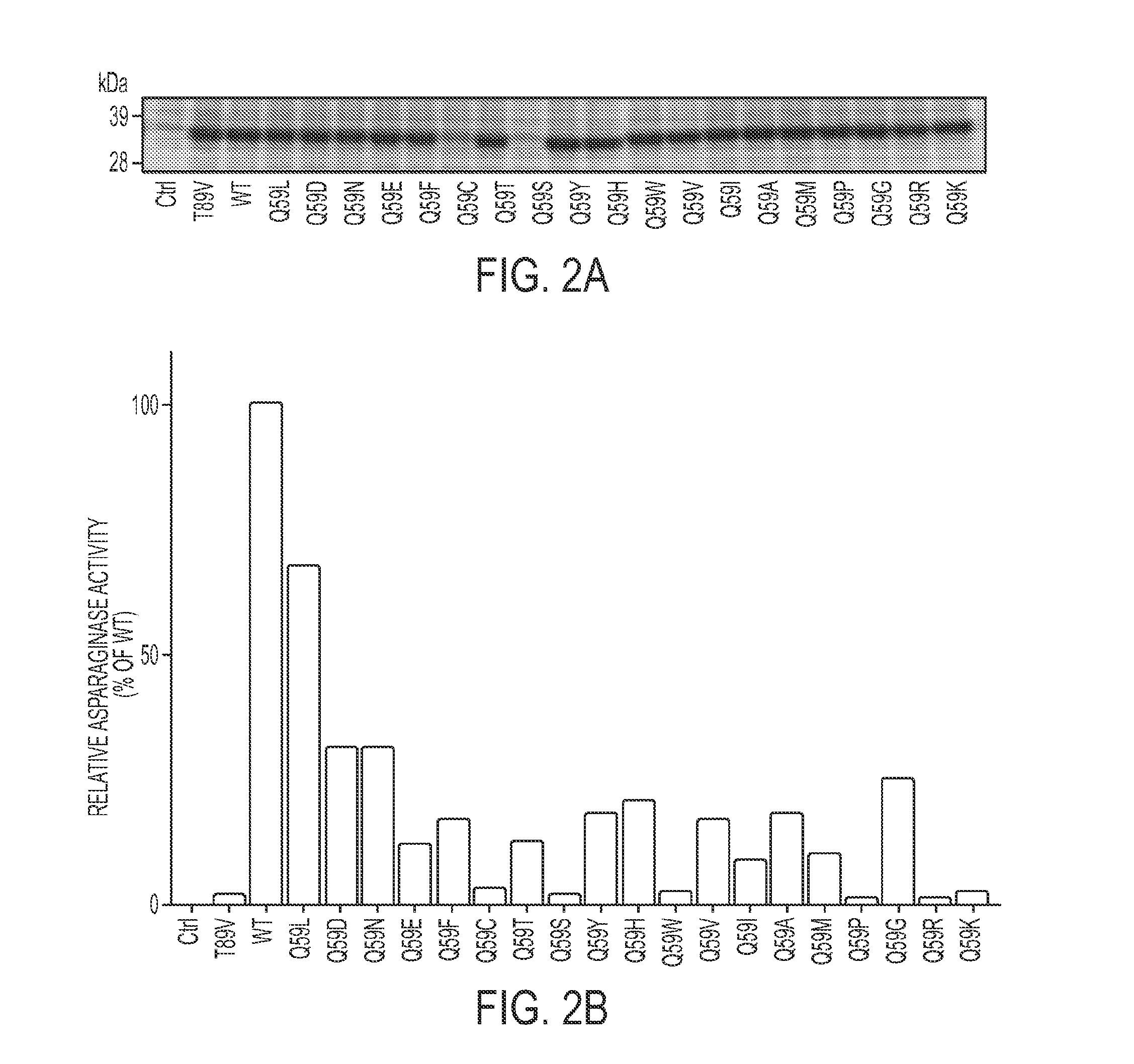
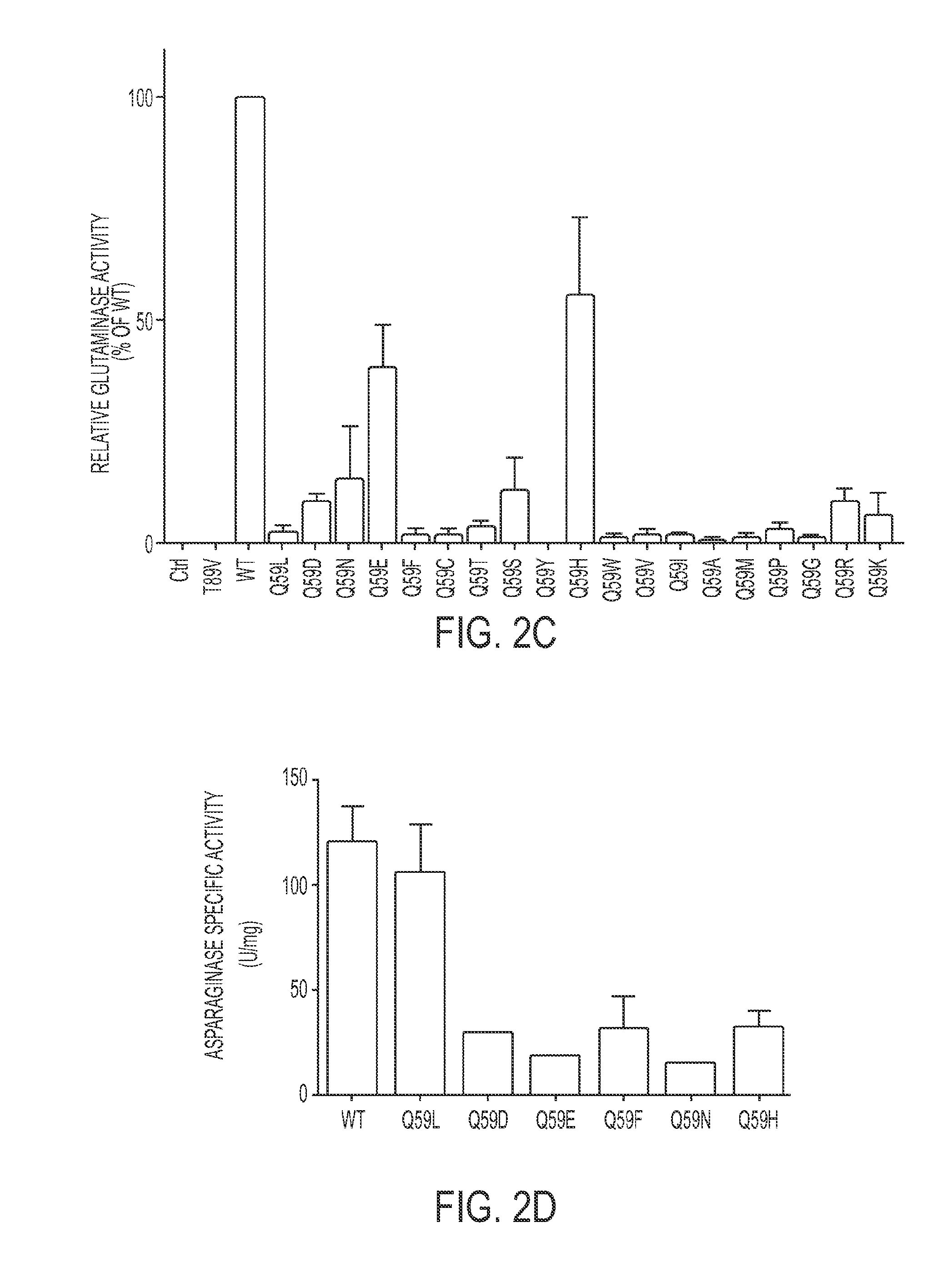
The invention relates to a L-asparaginase variant with increased activity, and is a variant of escherichia coli wild type L-asparaginase shown in a SEQ ID NO:3, and the variant comprises an one or a plurality of amino acid substituted amino acid sequence on 48th site, 49th site, 152nd and 283rd by corresponding to a SEQ ID NO:3. The invention also provides the separated nucleic acid containing the nucleotide sequence which codes the L-asparaginase variant, a recombinant expression construct containing the nucleic acid and a recombinant host cell containing the expression construct. In addition, the invention also provides a method for generating the L-asparaginase variant. The invention also provides a pharmaceutical composition used for treating tumor containing the L-asparaginase variant.
Owner:BEIJING ABZYMO BIOSCIENCES CO LTD
Thermostable Asparaginase Variants and Polynucleotides Encoding Same
ActiveUS20150210995A1Improve propertiesConvenient ligationSugar derivativesBacteriaNucleotideAlpha-amylase
The present invention relates to asparaginase variants. The present invention also relates to polynucleotides encoding the variants; nucleic acid constructs, vectors, and host cells comprising the polynucleotides; and methods of using the variants. The further relates to a process of producing a fermentation product, comprising: liquefying a starch-containing material to dextrins with an alpha-amylase in the presence of an asparaginase of the invention; saccharifying the dextrins to a sugar with a glucoamylase; and fermenting the sugar using a fermenting organism.
Owner:NOVOZYMES AS
Preparation method of L-asparaginase
InactiveCN101831417ASimple manufacturing methodSimple stepsHydrolasesMicroorganism based processesIon exchangeAsparaginase
The invention discloses a preparation method of L-asparaginase. The preparation method comprises the following steps of: carrying out wall breaking on Erwinia thalli by using an extracting solution containing lysozyme; and obtaining high-purity L-asparaginase through ion-exchange chromatography and affinity chromatography. The preparation method of the L-asparaginase is simple and easy to apply and has high efficiency, the yield can reach higher than 60 percent, and the purity of the prepared L-asparaginase can is higher than 99 percent and meets the requirement of the Chinese Pharmacopoeia (2005 Version). The preparation method has no pollution to the environment, is economical and environmenta friendly and can be applied to industrial production.
Owner:广州市微生物研究所
Technology method for improving quality of burley tobacco and application of technology method
ActiveCN109527635AMild process conditionsEasy to implementTobacco preparationTobacco treatmentMaillard reactionIrritation
The invention provides a technology method for improving the quality of burley tobacco and application of the technology method. In the production process of the burley tobacco, during heating and humidifying caused by loosening moisture regain, a compound biological agent solution including saccharomycopsis fibuligera and asparaginase is uniformly sprayed, during pre-mixing and storage, hydrolyzing fermentation is conducted, and during heating and humidifying caused by feeding, an alkalescent asparaginase and amino acid mixed solution is sprayed again; in the strip bulking process, hydrolyzing fermentation and a Maillard reaction are conducted; after shredding, a high-temperature shredding is carried out in the cut tobacco drying process, and burley cut tobacco is obtained. The aroma of the burley cut tobacco is richer, and the characteristic aroma and the characteristic taste of the burley tobacco are greatly mitigated and improved, the burley cut tobacco is moderate, irritation is reduced, and offensive odor is reduced. The burley cut tobacco is mixed with flue-cured type cigarettes in use or mixed with novel heat-not-burn cut tobacco in use, the application of the burley tobacco is expanded, the available quantity is increased, the tobacco scent and strength are improved, irrigation is low, and the offensive odor is reduced.
Owner:HUBEI CHINA TOBACCO IND
Asparaginase mutant with improved enzyme activity
InactiveCN106282148AHigh catalytic efficiencyIncrease productionBacteriaHydrolasesProtein moleculesAsparaginase
The invention discloses an asparaginase mutant with improved enzyme activity, and belongs to the technical field of enzyme engineering. Asparaginase shown as SEQ ID NO.1 is subjected to site-saturation mutagenesis, amino acid residues near protein molecule activity sites are changed, asparaginase catalysis efficiency is improved, and asparaginase yield is further improved. Enzyme activity of the asparaginase can be improved by 2.37 times as compared with that of original strains by recombinant bacillus subtilis with enhanced asparaginase secretion capacity. The enzyme producing ability of modified genetically engineered bacteria is remarkably improved, the enzyme activity of the asparaginase fermented by a shake flask reaches 320U / mL, the yield is the currently reported highest yield of the shake flask, the asparaginase mutant is more suitable for industrial application, production cost can be reduced, and production efficiency is improved.
Owner:JIANGNAN UNIV
Utilization of Wolinella succinogenes asparaginase to treat diseases associated with asparagine dependence
Described herein are methods for producing recombinant forms of asparaginase derived from Wollinella succinogenes. In addition, methods for covalent modification of proteins, including asparaginases, by acylation are also provided. Certain embodiments provide for epitopic-labeling of the amino terminus of W. succinogenes asparaginase. Additional embodiments concern methods for the therapeutic utilization of the native, homotetrameric form of W. succinogenes asparaginase, as well as the use of epitopically-labeled or non-epitopically-labeled recombinant W. succinogenes asparaginase (or a covalently modified analog thereof) in the therapeutic treatment of malignant and non-malignant hematological disease and other diseases where asparagine depletion or deprivation would be efficacious or which respond to asparagine depletion or deprivation, as well as their potential utilization in the therapeutic treatment of autoimmune diseases such as rheumatoid arthritis, AIDS, and SLE.
Owner:DURDEN DONALD L
Asparaginase freeze-dried powder injection and preparation method thereof, as well as asparaginase solution
ActiveCN102138909BEasy to useRapid reconstitutionPowder deliveryPeptide/protein ingredientsForeign matterFreeze-drying
The invention discloses an asparaginase freeze-dried powder injection. The injection comprises asparaginase, a buffering agent, a surfactant, medical auxiliary materials and 5% of injection water which is used as a solvent in a preparing process and less than the addition finally, wherein the surfactant is a nonionic surfactant; and the buffering agent and the nonionic surfactant are added into the formula, so that the situation that tiny visible particles exceed standard is prevented when the asparaginase is dissolved and transferred into an injection. The invention also discloses a dedicated solution for dissolving the asparaginase, wherein the solution solvent is the injection water, and the solute comprises the buffering agent and the surfactant. On the premise of not changing the conventional formula of the freeze-dried powder injection, when the freeze-dried powder is dissolved in the solution, and is transferred into sodium chloride or a glucose injection, the situation that tiny visible particles exceed standard is prevented, so that the stimulation to human bodies is reduced in use, and the clinical administration safety is improved.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com