L-asparaginase mutant with improved enzyme activity and construction method thereof
An asparaginase and mutant technology, which is applied in the field of genetic engineering, can solve the problems of low protein expression and low L-asparaginase enzyme activity, and achieves the effect of reducing the generation of acrylamide and improving the potential of industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1 Contains the construction of the recombinant vector of L-asparaginase mutant
[0015] (1) Obtaining the G107D mutant: using the nucleotide sequence shown in SEQIDNO.4 as a template, Fprimer (sequence shown in SEQIDNO.5) and Rprimer (sequence shown in SEQIDNO.6) as primers, PCR is performed to obtain The recombinant gene shown in SEQ ID NO.3.
[0016] (2) Digest the recombinant gene and pMA5 with BamHI and MluI, respectively, and ligate with T4 DNA ligase overnight at 16°C after purification. The ligation product was chemically transformed into JM109 competent cells. The transformation solution was applied to an LB plate containing kanamycin (50 mg / L), the plasmid was extracted, and the recombinant plasmid constructed was verified by double enzyme digestion, which was named pMA5-G107D. The sequencing work was completed by Shanghai Sangong.
Embodiment 2
[0017] Example 2 Production of L-asparaginase Bacillus subtilis Engineering Bacteria Construction
[0018] The recombinant plasmid pMA5-G107D obtained in Example 1 was chemically transformed into B. subtilis168 competent cells, and the specific method was as follows:
[0019] (1) The solution required for the transformation experiment is as follows (g / L):
[0020] Sp-A: (NH 4 ) 2 SO 4 4,K 2 HPO 4 28. Sodium citrate 12Sp-B: MgSO 4 ·7H 2 O0.4
[0021] 100×CAYE: Casaaminoacid20, yeast powder 100SpI medium: Sp-A49%, Sp-B49%, 50% glucose 2%, 100×CAYE2% SpII medium: SpI medium 98%, 50mmol / LCaCl 2 1%, 250mmol / LMgCl 2 1%. 115°C damp heat sterilization.
[0022] (2) Inoculate a single colony of B.Subtilis168 into 2mL SpI medium (50mL centrifuge tube), and culture overnight at 37°C and 200r / min;
[0023] (3) Take 100 μL of the culture solution into 5 mL of SpI medium, and culture at 37°C and 200 r / min until the logarithmic phase (OD600 value is about 1), about 4 to 5 hours; ...
Embodiment 3
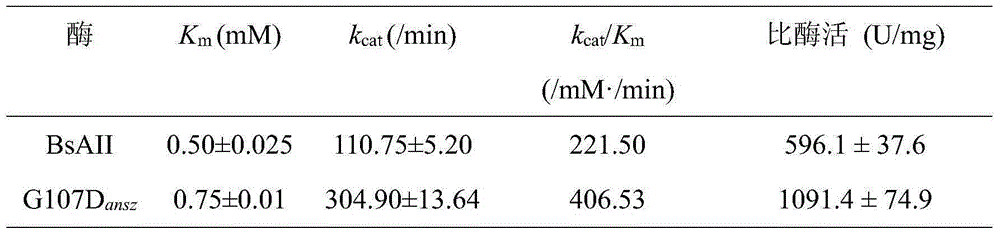
[0025] Example 3 High expression and enzyme activity determination of recombinant bacteria pMA5-G107D / B.subtilis168L-asparaginase.
[0026] (1) The recombinant bacteria pMA5-G107D / B.subtilis168 constructed in Example 2 and the control strain pMA5-ansz / B.subtilis168 expressing unmutated enzymes were inoculated in 10 mL of LB medium containing kanamycin respectively, 37 Shake culture overnight at ℃, transfer to Bacillus subtilis fermentation medium at 4% inoculum the next day, culture at 37℃ for 24h, take the fermentation broth and centrifuge at 10000r / min for 10min at 4℃, the supernatant is crude extracellular enzyme liquid, and the supernatant of broken cells was the intracellular crude enzyme solution, which was used for the determination of enzyme activity.
[0027] (2) Bacillus subtilis fermentation medium: soybean peptone 10g / L, K 2 HPO 4 2.3g / L, KH 2 PO 4 1.7g / L, corn steep liquor 15g / L, urea 3g / L, glucose 40g / L, MgSO 4 0.75g / L, NaCl5g / L. Adjust pH6.8-7.0.
[0028]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com