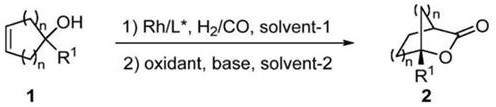
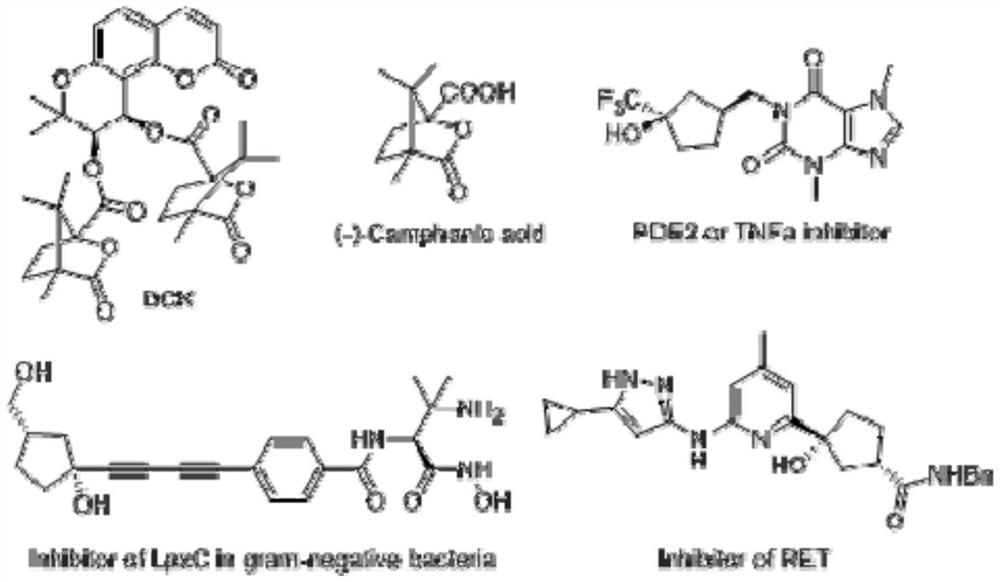
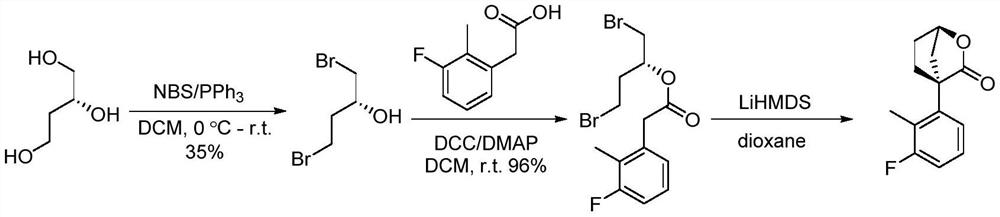
A kind of method of synthesizing chiral bridged cyclic lactone
A chiral and bridged ring technology, which is applied in the field of synthesis of chiral bridged ring lactones, can solve the problems of non-compliance with green chemistry, poor atom economy, long synthesis steps, etc., and achieve high industrial application potential, low cost and simple method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] General preparation method: under high-purity argon atmosphere, Rh(acac)(CO) 2 (0.5 mg, 0.002 mmol) and the chiral ligand (S,R)-DM-YanPhos (3.6 mg, 0.004 mmol) L1 were dissolved in toluene (1 mL), stirred at room temperature for 10 min, and then 1-substituted Cyclopent-3-en-1-ol (0.2 mmol) 1 . The reaction system was placed in an autoclave, and hydrogen and carbon monoxide (H 2 / CO=5 / 5 atm), stirred at 70°C for 24 hours. After the reaction was finished, the gas in the reactor was slowly released, and the reaction solution was poured into the dichloromethane solution (4mL) of pyridine chlorochromate (PCC, 0.5mmol) and triethylamine (0.1mmol), and the reaction was carried out at room temperature. 12 hours. After the reaction, the solvent was removed under reduced pressure, and the pure bridged cyclic lactone 2 was isolated by column chromatography (petroleum ether / ethyl acetate=10:1, V / V).
[0056] Using this general method, the efficient synthesis of 23 bridged cycli...
Embodiment 2
[0106] Under a high-purity argon atmosphere, Rh(acac)(CO) 2 (0.5 mg, 0.002 mmol) and the chiral ligand (S,S)-Ph-BPE (2.0 mg, 0.004 mmol) L2 were dissolved in toluene (1 mL), stirred at room temperature for 10 min, and then 1-benzene was added cyclopent-3-en-1-ol (0.2 mmol) 1a. The reaction system was placed in an autoclave, and hydrogen and carbon monoxide (H 2 / CO=5 / 5 atm), stirred at 70°C for 24 hours. After the reaction was finished, the gas in the reactor was slowly released, and the reaction solution was poured into the dichloromethane solution (4mL) of pyridine chlorochromate (PCC, 0.5mmol) and triethylamine (0.1mmol), and the reaction was carried out at room temperature. 12 hours. After the reaction, the solvent was removed under reduced pressure, and the pure bridged cyclic lactone 2a (88% yield, 94% ee) was isolated by column chromatography (petroleum ether / ethyl acetate=10:1).
[0107] The yield and enantioselectivity for the synthesis of chiral bridged cyclic lac...
Embodiment 3
[0109] Under a high-purity argon atmosphere, Rh(acac)(CO) 2 (0.5 mg, 0.002 mmol) and the chiral ligand (S,R)-DM-YanPhos (3.6 mg, 0.004 mmol) L1 were dissolved in toluene (1 mL), stirred at room temperature for 10 min, and then 1-benzene was added cyclopent-3-en-1-ol (0.2 mmol) 1a. The reaction system was placed in an autoclave, and hydrogen and carbon monoxide (H 2 / CO=5 / 5 atm), stirred at 70°C for 24 hours. After the reaction, slowly release the gas in the reactor, and pour the reaction solution into pyridine chlorochromate (PCC, 0.5mmol) and Cs 2 CO 3 (0.1 mmol) in dichloromethane solution (4 mL), and reacted at room temperature for 12 hours. After the reaction, the solvent was removed under reduced pressure, and the pure bridged cyclic lactone 2a (85% yield, 94% ee) was isolated by column chromatography (petroleum ether / ethyl acetate=10:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com