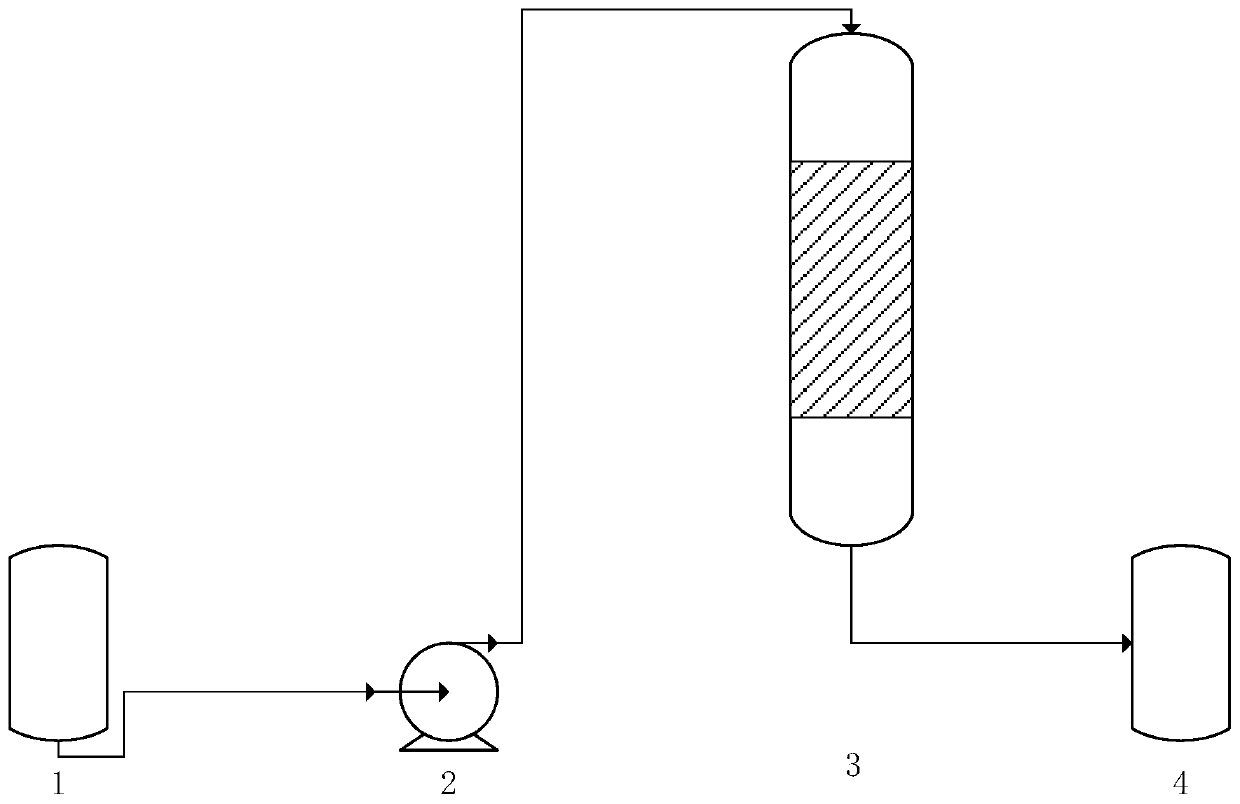
Patents
Literature
315 results about "Intramolecular cyclization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
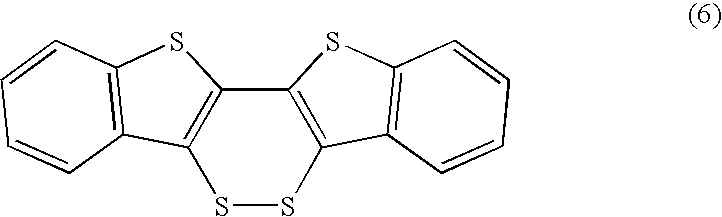
Intramolecular Cyclization. Intramolecular cyclizations are particularly useful for precursors having two components tethered ortho to each other on an aromatic ring, which on ring closure lead to 1,3,6-benzoheterocycles, as exemplified by Schemes 14, 16, 17 and Equation (6), and 1,4,5-benzoheterocycles, as demonstrated in Schemes 13, 19, and 25.
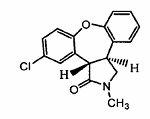
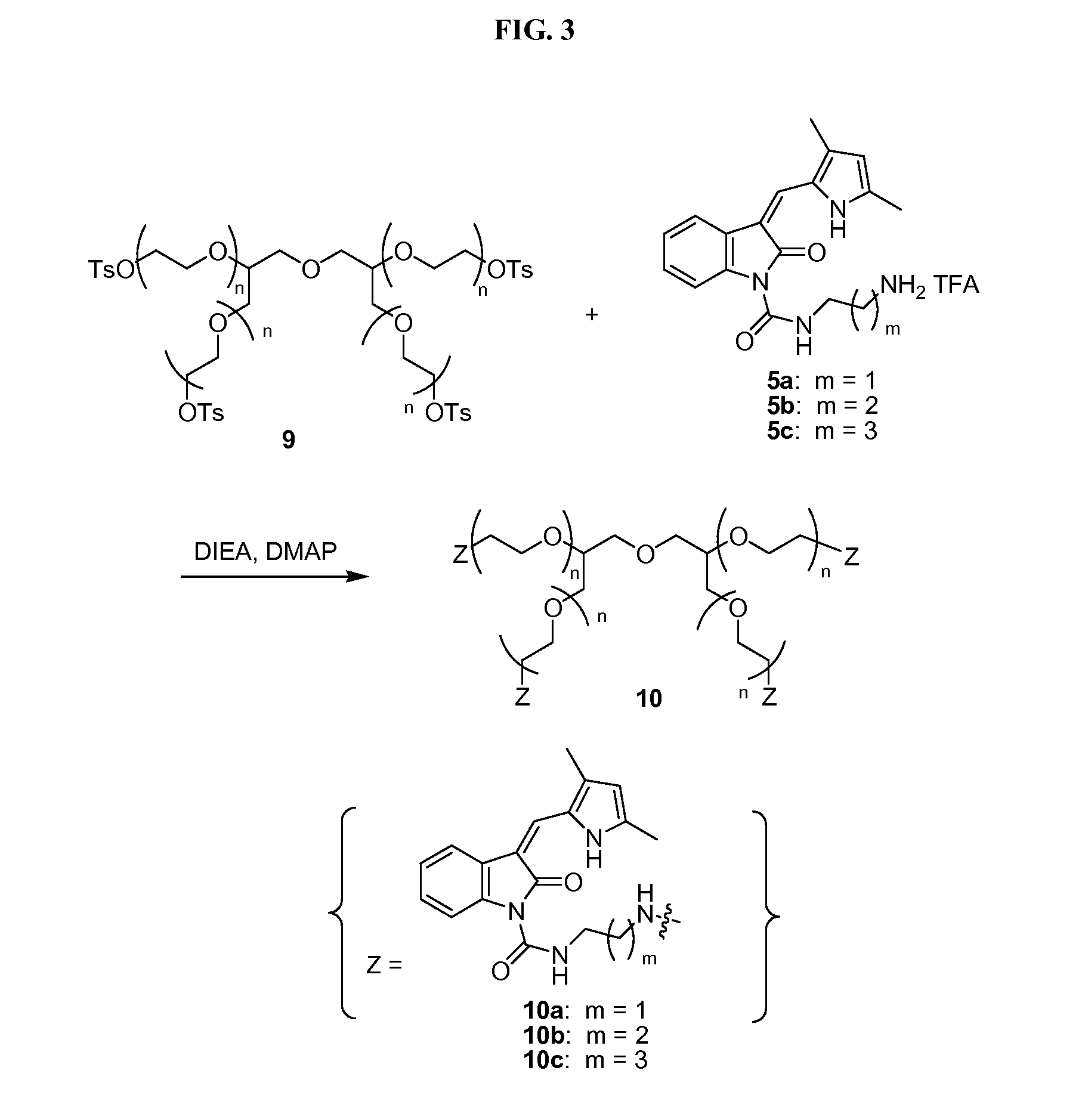
Coated tablet formulation and method
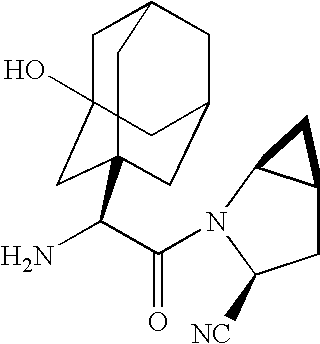
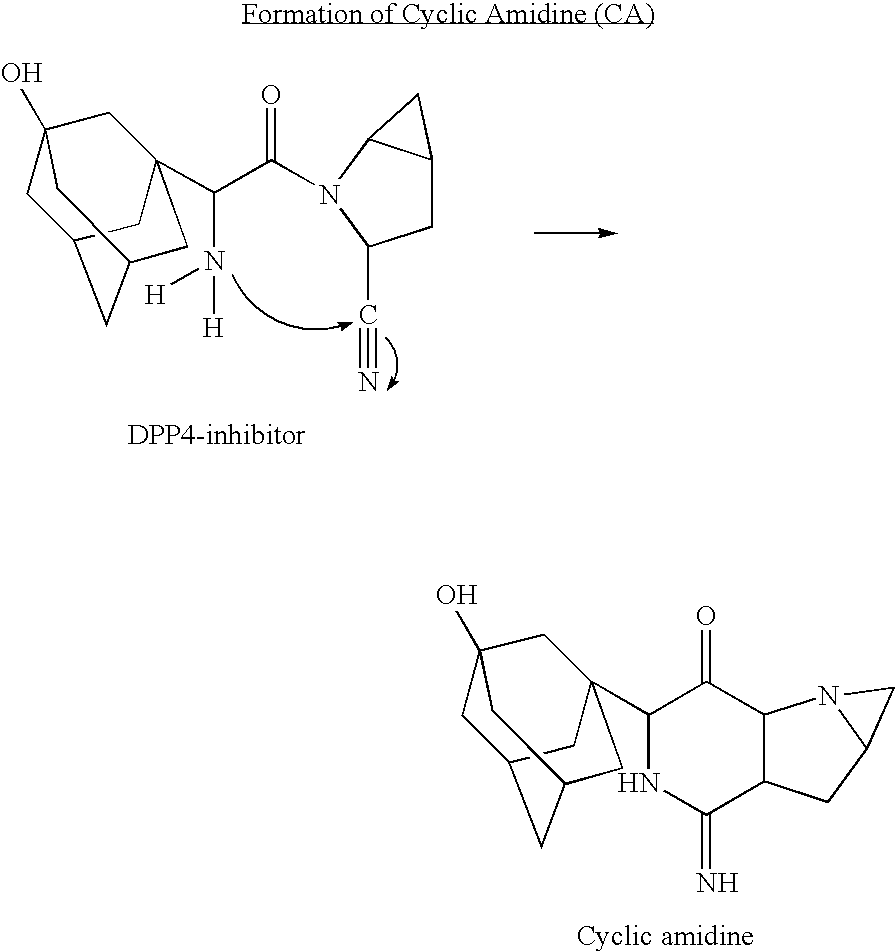
ActiveUS20050266080A1Good chemical stabilityEasy to prepareBiocideMetabolism disorderCoated tabletsExcipient
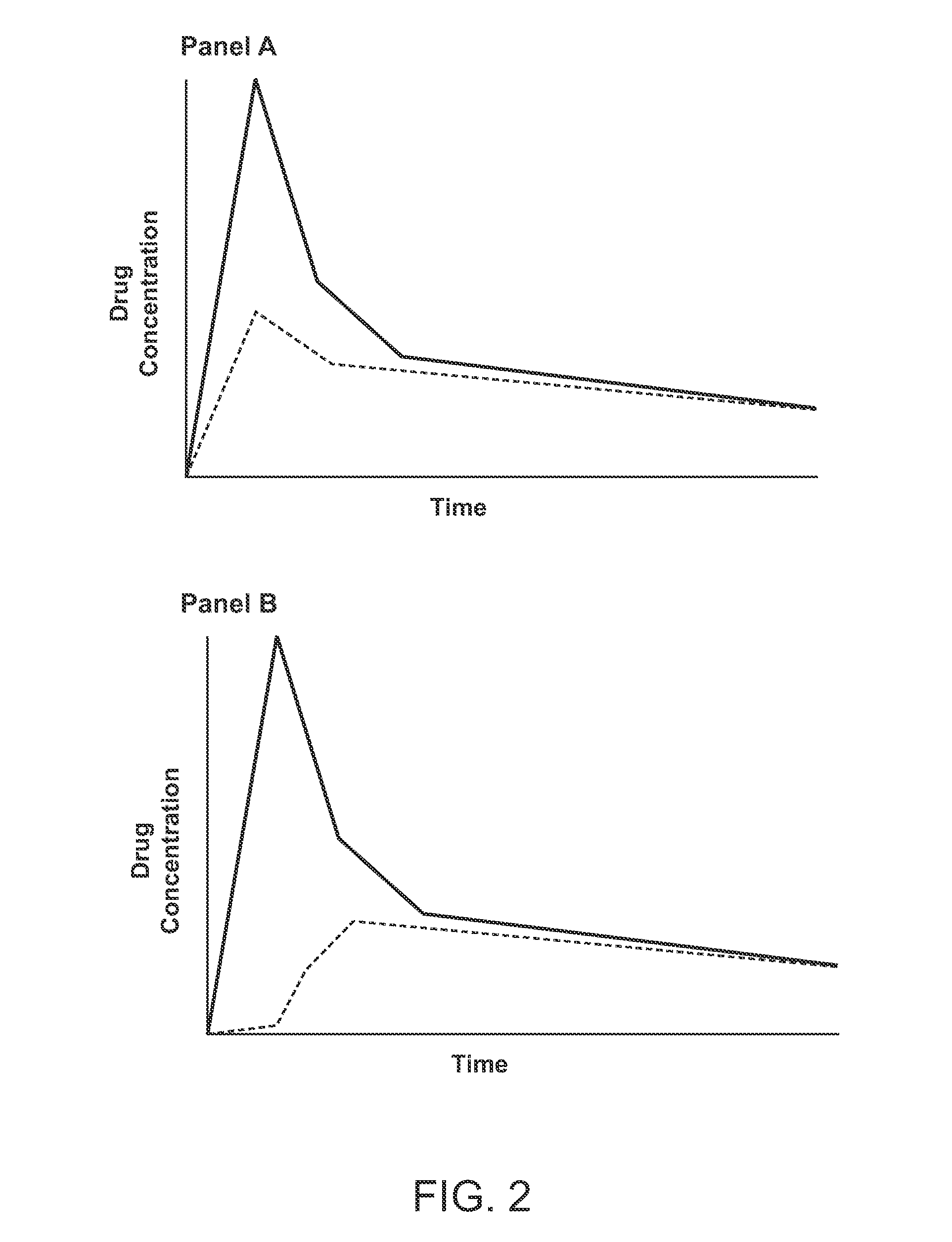
A coated tablet formulation is provided which includes a medicament such as the DPP4-inhibitor, saxaglipitin or its HCl salt, which is subject to intra-molecular cyclization, which formulation includes a tablet core containing one or more fillers, and other conventional excipients, which tablet core includes a coating thereon which may include two or more layers, at least one layer of which is an inner seal coat layer which is formed of one or more coating polymers, a second layer of which is formed of medicament which is the DPP4-inhibitor and one or more coating polymers, and an optional, but preferable third outer protective layer which is formed of one or more coating polymers. A method for forming the coated tablet is also provided.
Owner:ASTRAZENECA AB
Curing treatment method of carbon fiber precursor polyacrylonitrile fiber
InactiveCN102181963AGood for cyclization reactionRaise the degree of orientationFibre chemical featuresAir atmosphereCarbon fibers
The invention relates to a curing treatment method of a carbon fiber precursor polyacrylonitrile fiber. The method comprises the following steps: pre-cyclizing the polyacrylonitrile fiber precursor in the atmosphere of inert gas; carrying out cyclizing and plastic drawing in the atmosphere of inert gas and water vapor; and carrying out oxidation crosslinking in the atmosphere of air, thus obtaining a pre-oxidized polyacrylonitrile fiber. The method has the following beneficial effects: carrying out heat treatment on the fiber in the inert atmosphere is beneficial to implementation of cyclization reaction in the molecules to form rigid ladderlike molecules with regular structure and strong heat resistance, and at the same time, a defined amount of water vapor is introduced to obtain a higher drawing ratio by enhancing the plasticity of the molecular chains in the fiber, thus improving the orientation degree of the rigid molecules along the fiber axis.
Owner:DONGHUA UNIV
Method for stereoselectively producing substituted polycyclic pyridone derivative
ActiveCN111386276AOrganic active ingredientsOrganic chemistry methodsCombinatorial chemistryPerylene derivatives
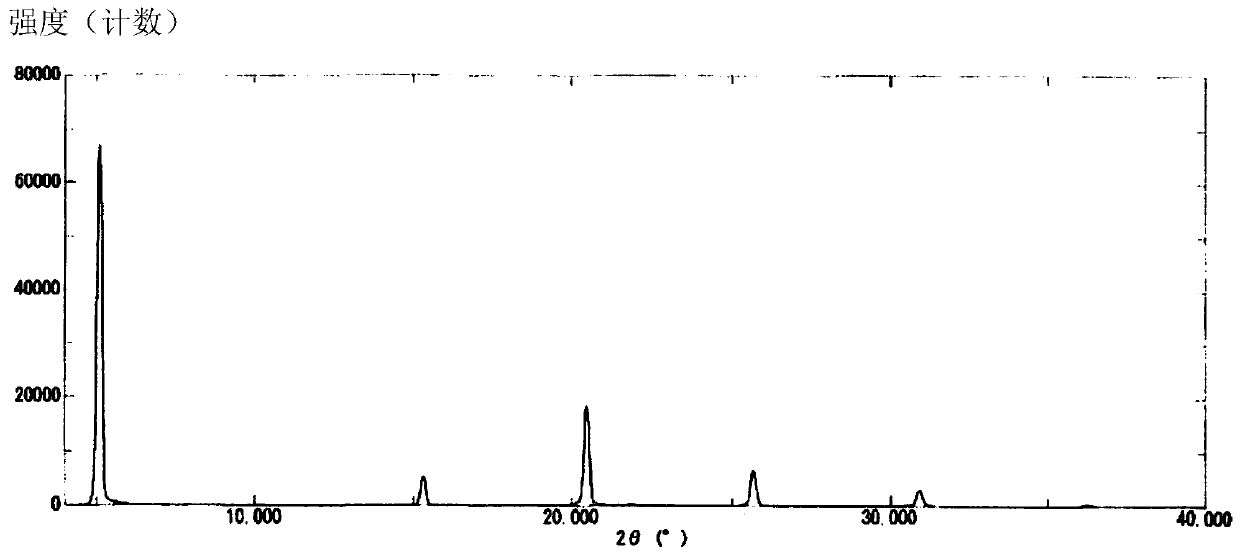
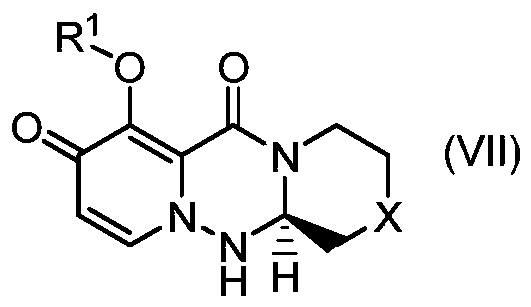
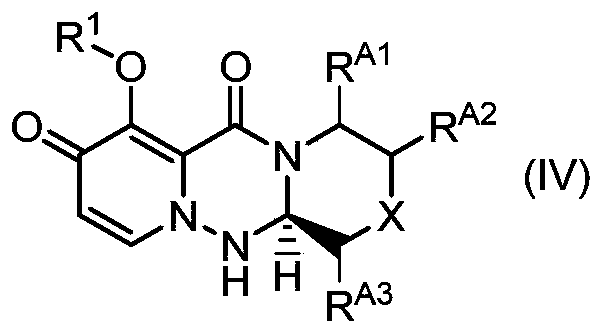
The present invention provides a commercially applicable method for producing an intermediate of a substituted polycyclic pyridone derivative exhibiting cap-dependent endonuclease inhibitory activity.In the production method shown in the reaction formula (in the formula, the symbols are as defined in the description), by means of an intramolecular cyclisation reaction in which the stereochemistryof a compound shown in formula (III) or formula (VI) is controlled, a compound shown in formula (IV) and including an eliminable functional group on an asymmetric carbon is obtained, and said eliminable functional group is eliminated, whereby an optically active substituted 3-ring pyridone derivative shown in formula (VII) is highly enantioselectively obtained in a high yield.
Owner:SHIONOGI & CO LTD
Process for production of thermoplastic copolymer
InactiveUS20100087605A1Improve heat resistanceImprove thermal stabilityGlutaric anhydrideForeign matter
A process for continuously producing a thermoplastic copolymer, in which a copolymer (A) containing unsaturated carboxylic acid alkyl ester units and unsaturated carboxylic acid units is produced and in succession heat-treated to perform intramolecular cyclization reaction by dehydration and / or dealcoholization reaction, for producing a thermoplastic copolymer (B) containing glutaric anhydride units and the unsaturated carboxylic acid alkyl ester units. The obtained copolymer is excellent in heat resistance and colorless transparency and very small in foreign matter content.
Owner:TORAY IND INC
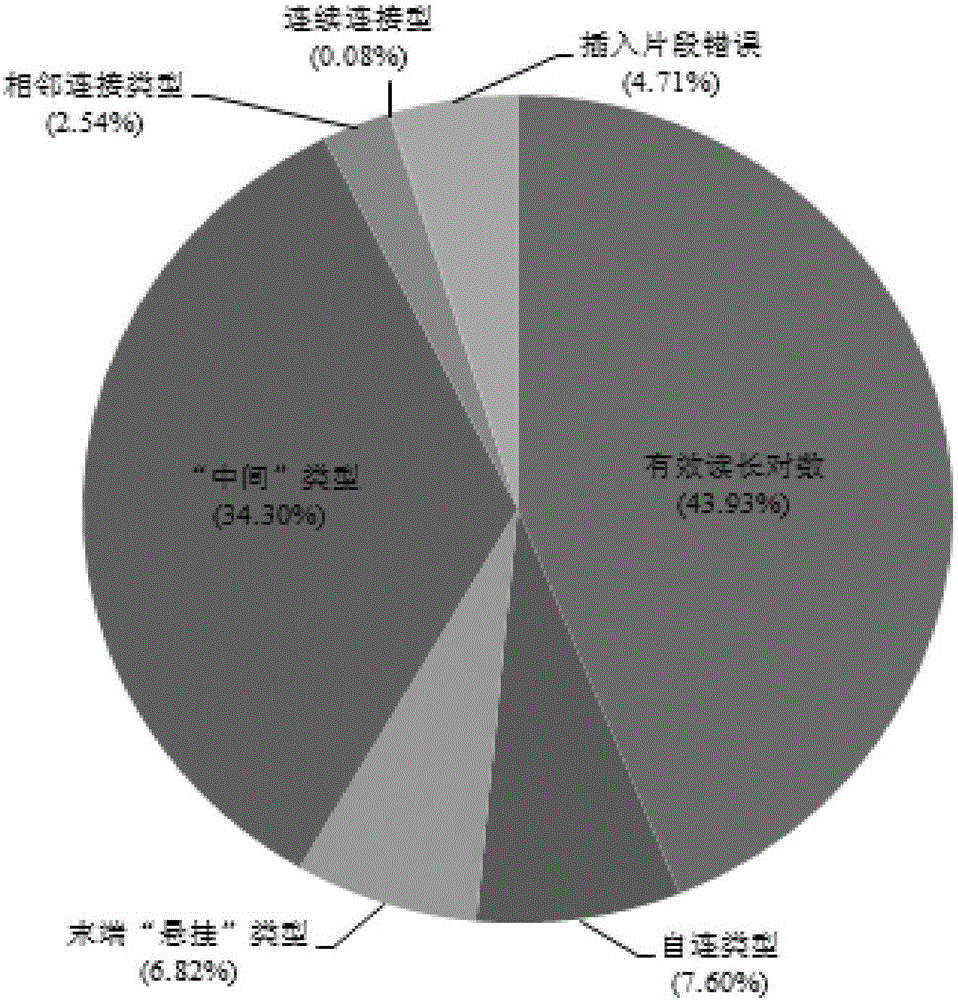
Hi-C high-throughput sequencing and database building method for eukaryote DNA
ActiveCN105839196ACyclization efficiency is lowLow effective data rateMicrobiological testing/measurementLibrary creationAdditive ingredientPolyethylene glycol
The invention relates to a Hi-C high-throughput sequencing and database building method for eukaryote DNA. According to the DNA-cell-nucleuse intramolecular cyclization step, end-repaired DNA and a cyclizing system are mixed, incubated for 2 h-4 h at the temperature of 4 DEG C to 6 DEG C and then incubated for 4 h-6 h at the temperature of 16 DEG C to 22 DEG C, and materials obtained after intramolecular cyclization are obtained, wherein the ingredients of the cyclizing system comprise T4 ligase damping liquid, BSA, T4 DNA ligase and at least one polyethylene glycol selected from PEG 4000 and PEG 6000. By means of the Hi-C high-throughput sequencing and database building method, the character cyclizing efficiency of DNA can be effectively improved, and the effective data rate obviously higher than that of a traditional method is obtained.
Owner:BIOMARKER TECH
New process for synthesis of asenapine
ActiveCN102229613AReaction transposition effect is goodHigh yieldOrganic chemistryLoop closingMethyl group
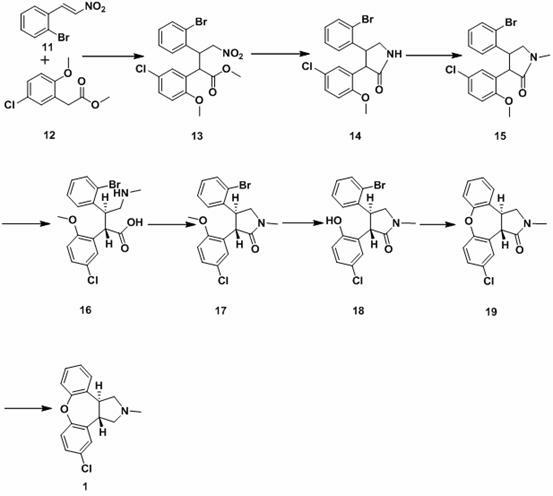
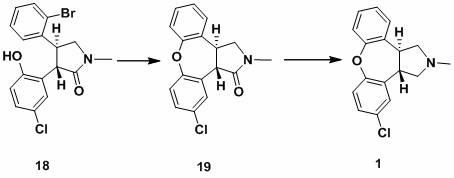
The invention discloses a process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to acondensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:安庆润科生物医药科技有限公司
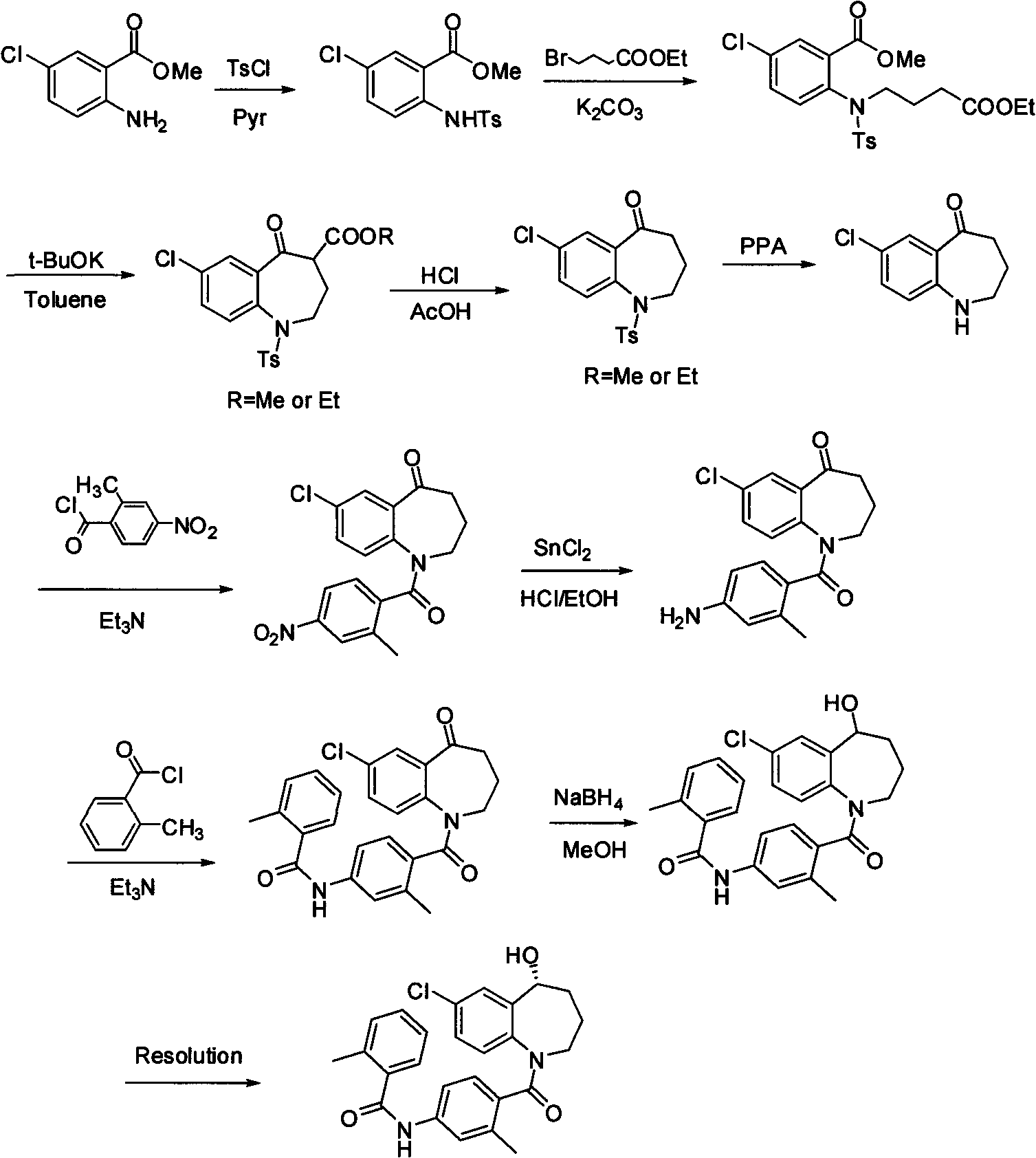
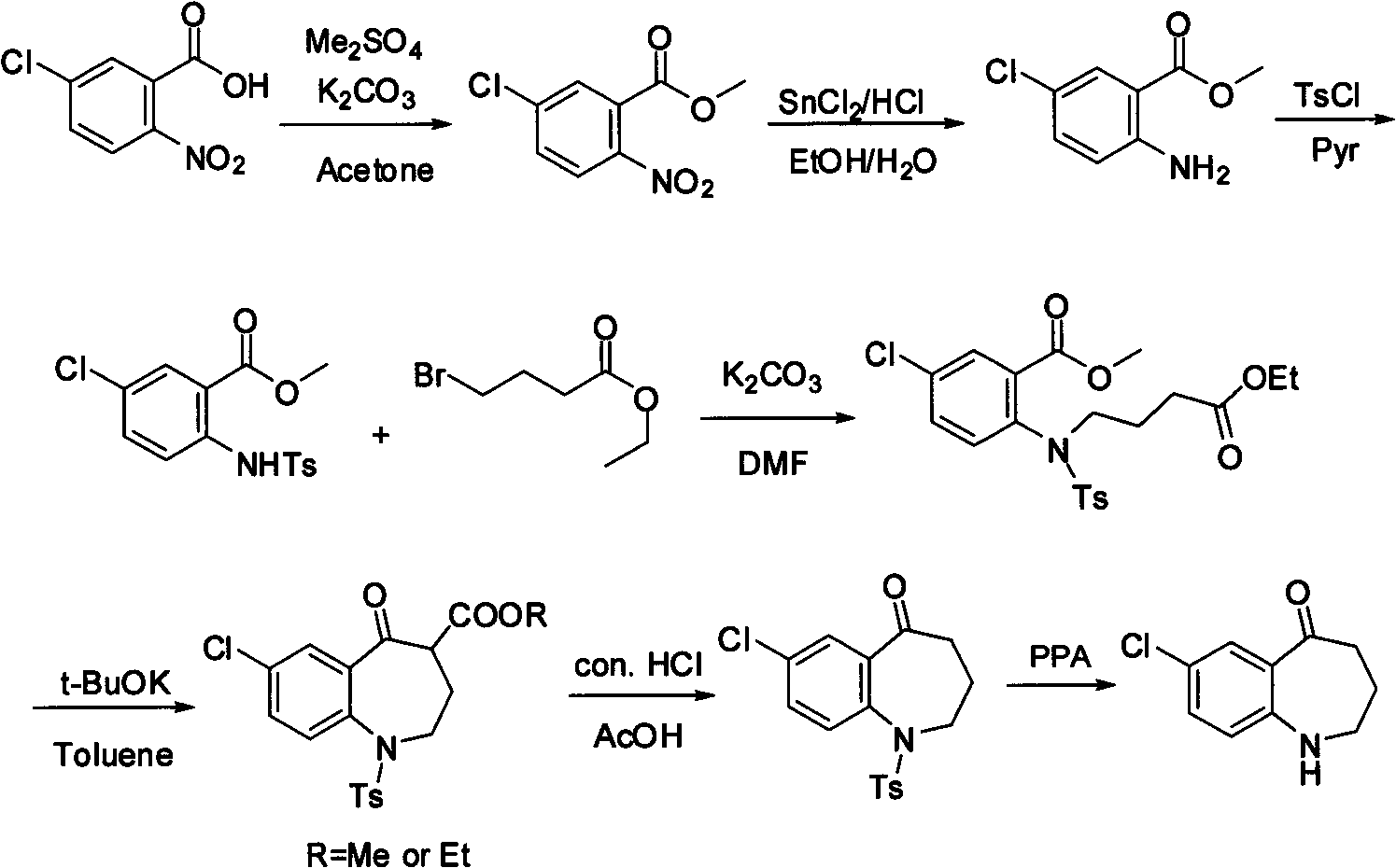
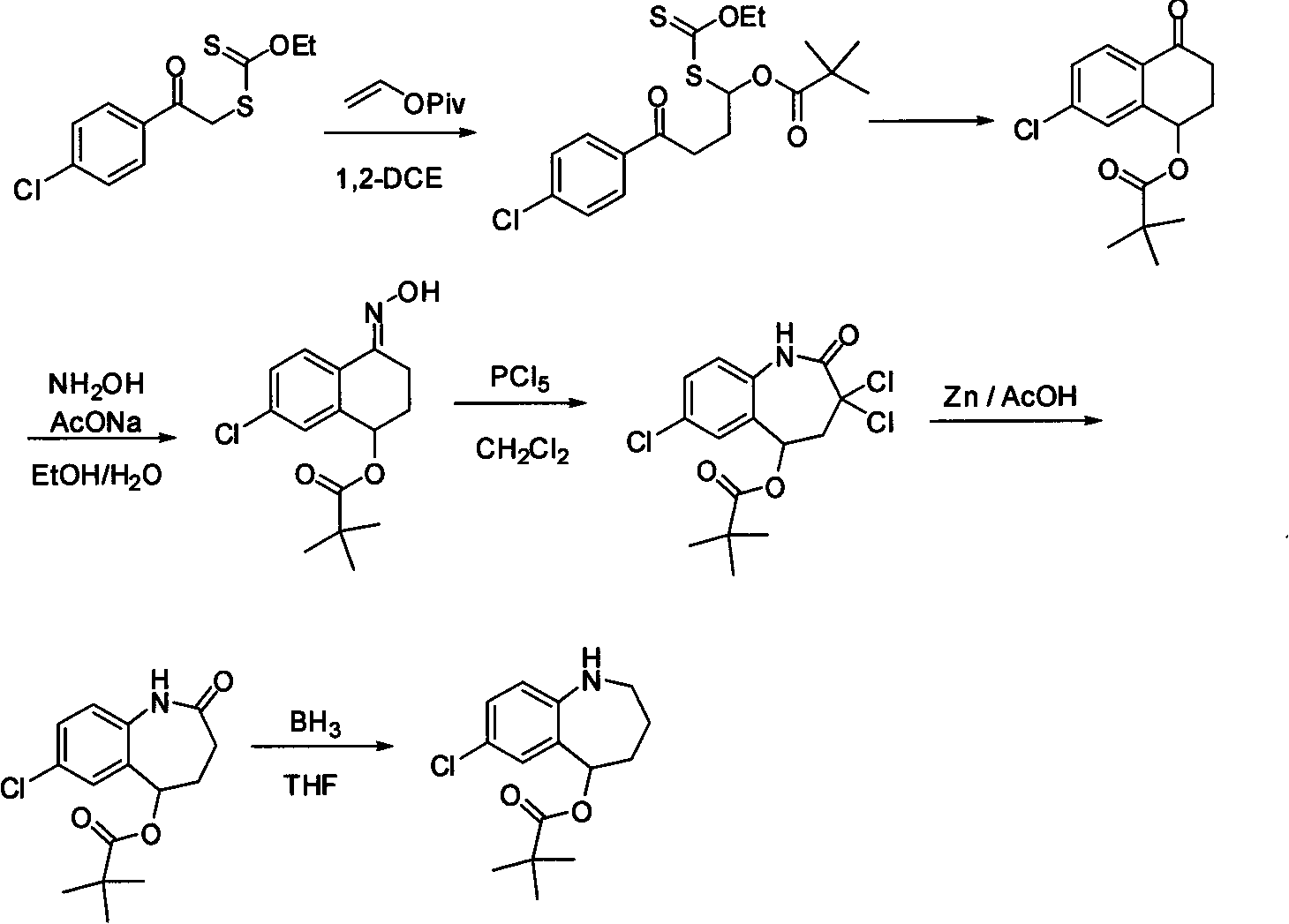
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone
The invention relates to a preparation method of benzazepine compounds, particularly a preparation method of 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone. The 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone can be used as an intermediate for preparing a pitressin antagonist medicament Tolvaptan. The method comprises the following steps: by using parachloroaniline as a raw material, reacting with paratoluensulfonyl chloride in a condensation mode under the alkaline condition to firstly protect amino groups; under the alkaline condition, coupling with ethyl 4-bromobutyrate to obtain a compound; in the presence of alkali, hydrolyzing the obtained compound, and acidifying; in dichloromethane, preparing acyl chloride from the acidified compound under the action of thionyl chloride; under the action of Lewis acid, carrying out Friedel-Crafts acylation reaction to perform intramolecular cyclization; and finally, removing tosyl groups to obtain the target product. The invention has the advantages of simple technique and low cost, and is convenient for industrial large-scale production.
Owner:宁波人健药业集团股份有限公司
Preparation method of praziquantel
The invention belongs to the field of medicine, and relates to a preparation method of a veterinary drug praziquantel, in particular to a novel method for preparing praziquantel. Raw materials such as chloracetyl chloride and glycin used in the method are low in cost and easy to obtain; the reaction is conventional; the operation is simple; the method has no special requirements on equipment; one step that a key intermediate 14 is subjected to intramolecular cyclization to form a compound 15 is designed in a reaction route; the design is brand-new and is not reported previously; the reaction yield is higher in the whole route; the total yield can reach 50%; most solvents used in a reaction process can be recovered, so that the cost is saved, and the environmental pressure is reduced; the cost is lowered extremely; and the route has a very good industrial application prospect.
Owner:迪嘉药业集团股份有限公司
3-aminopyrrolidine compounds, and synthetic method and uses thereof
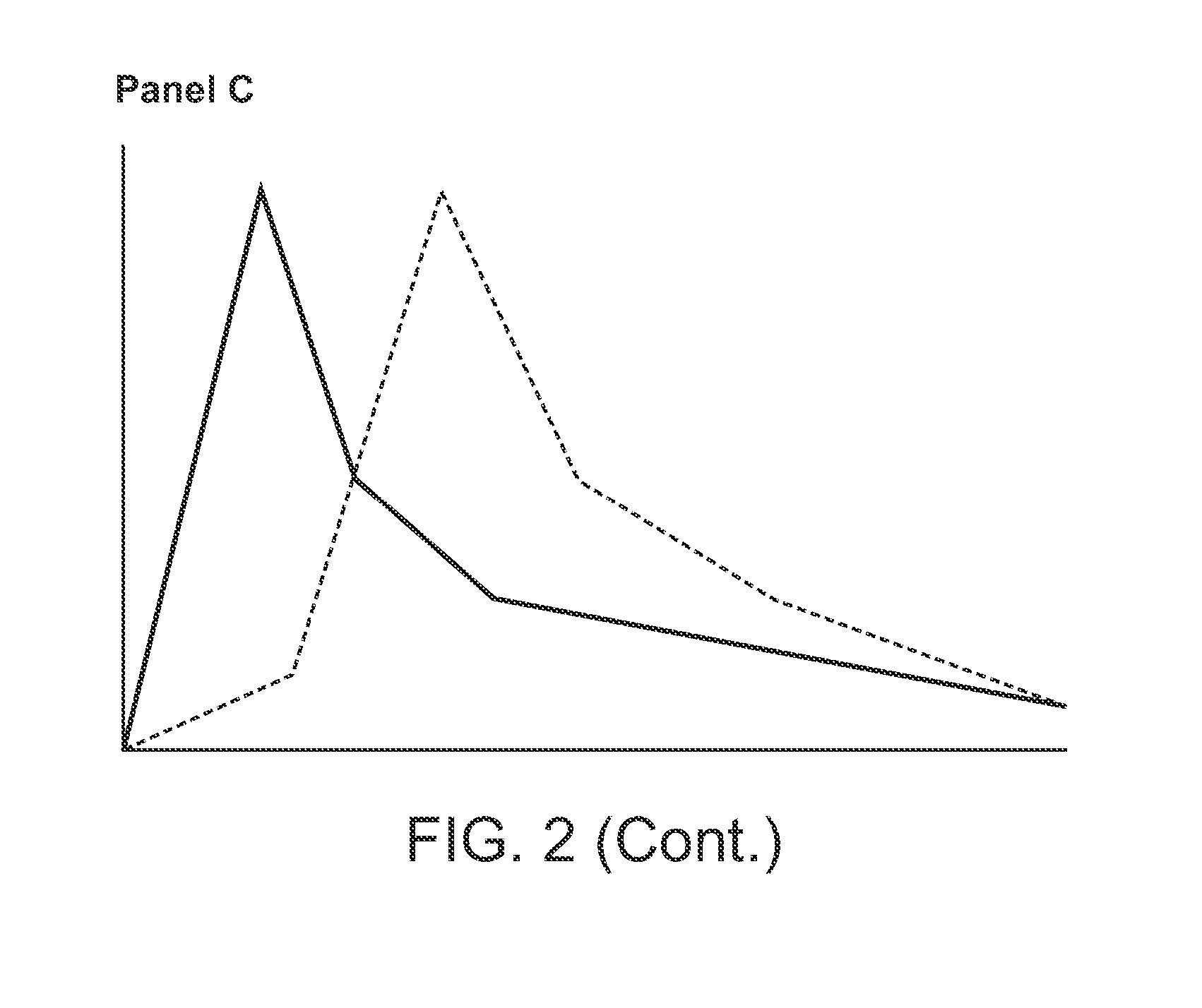
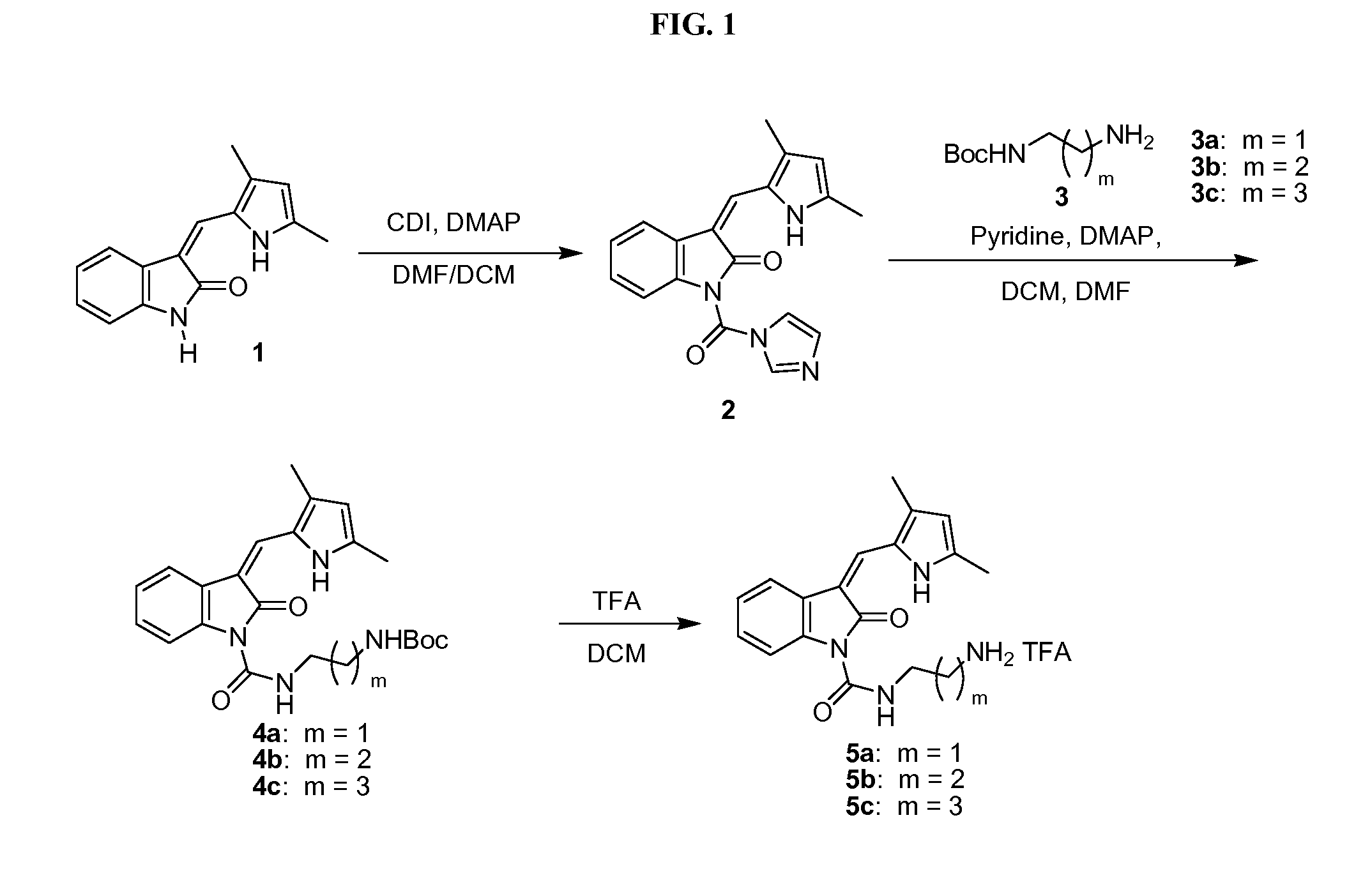
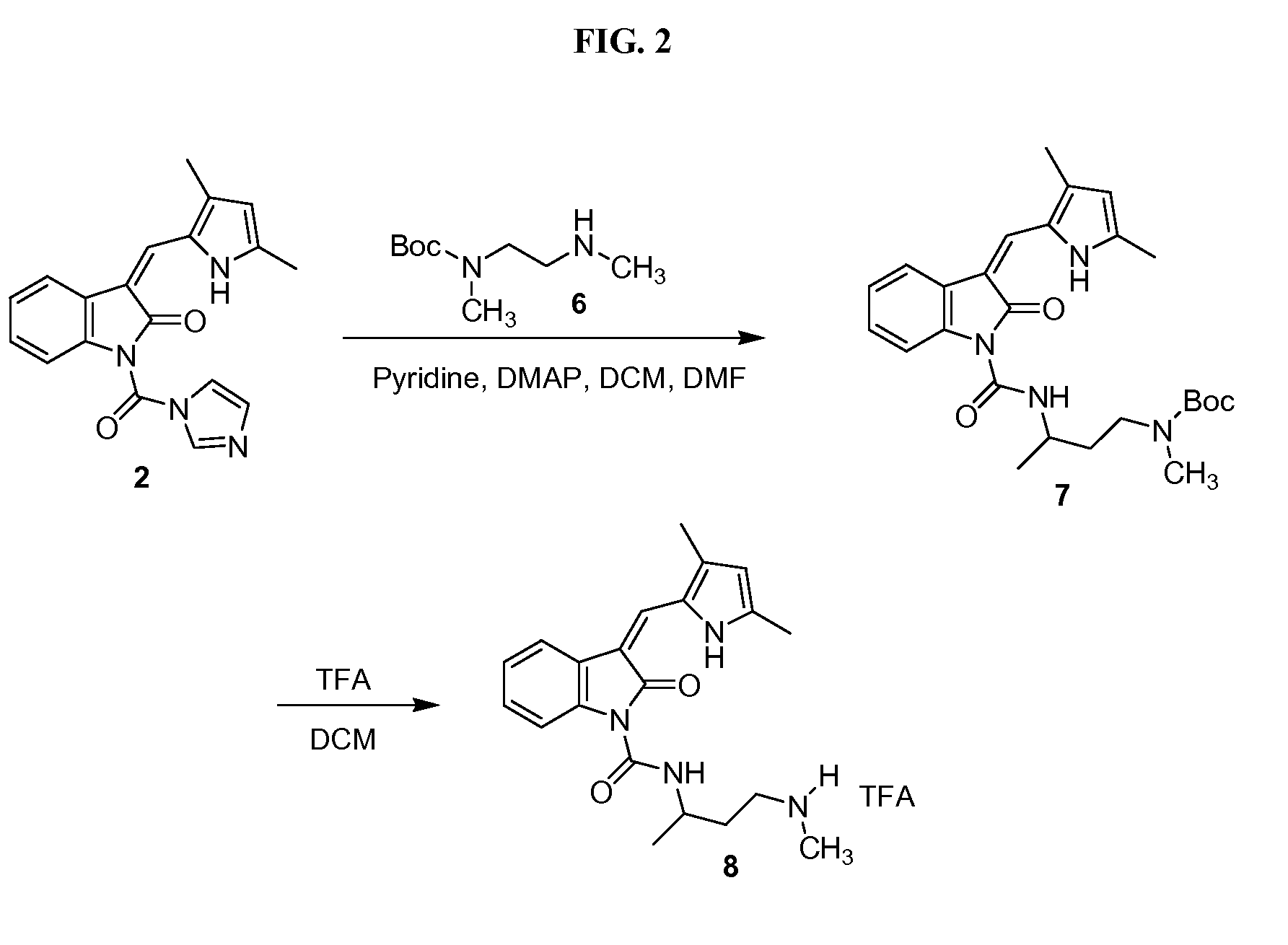
The invention relates to 3-aminopyrrolidine compounds, and a synthetic method and uses thereof. 3-pyrrolidone compounds (compounds IIIa or IIIb) are adopted as substrates and subjected to transaminase reactions under the actions of transaminase and an amino donator to obtain the (S)-3-aminopyrrolidine compounds (compounds II). Then intramolecular cyclization and removal of amino protective groups are performed to obtain (S,S)-2,8-diazabicyclo[4,3,0] nonane (a compound I). According to the 3-aminopyrrolidine compounds, the synthetic method and the uses, a synthetic technology that is low in cost, short in process steps and environmental friendly is provided for a moxifloxacin intermediate, and the synthetic technology will have great market application value and is suitable for large-scale industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Polymer containing azobenzene circlet on side chain and preparation method thereof
ActiveCN103242470AMolecular weight controllableNarrow molecular weight distributionPolymer scienceSide chain
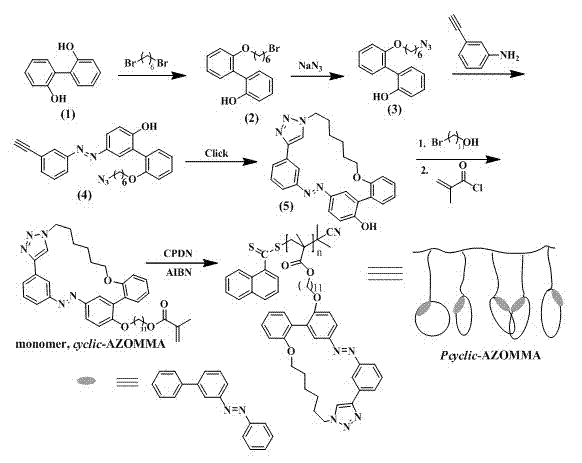
The invention discloses a polymer containing an azobenzene circlet on a side chain and a preparation method thereof. The preparation method comprises the following steps: synthesizing an intermediate compound (4) containing alpha-azido-omega-alkynyl through diazo coupling reaction and azotizing reaction, and then carrying out CuAAC intramolecular cyclization and the like to obtain a monomer containing the azobenzene circlet; and polymerizing the monomer by utilizing an RAFT (Reversible Addition-Fragmentation Chain Transfer Polymerization) method to obtain a functional polymer which contains the azobenzene circlet on the side chain and is definite in structure. Due to adoption of the technical scheme, the polymer is controllable in molecular weight, narrow in molecular weight distribution and definite in structure; cycle tension generated by a cycle structure in the structure of the synthesized polymer which contains the azobenzene circlet on the side chain has special influences on photo-isomerization property, grating property and the like of the polymer and can improve the photo-isomerization property, grating property and the like of the polymer.
Owner:ZHEJIANG KELI NEW MATERIAL TECH CO LTD
Solid-phase synthesis method of coumarin and analogue thereof
InactiveCN102532015AHigh yieldOvercome the disadvantage of low yield of cyclization reactionOrganic chemistryPolystyreneKetone
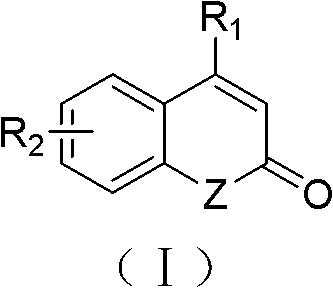
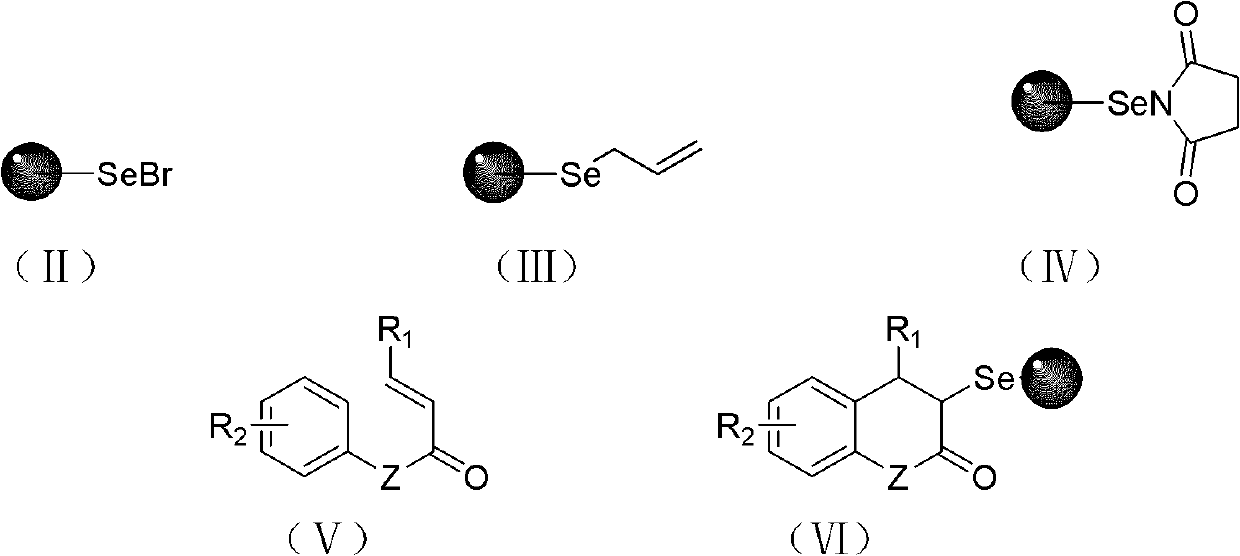
The invention relates to a solid-phase synthesis method of coumarin and an analogue (I) thereof and belongs to the field of organic chemistry. The method comprises the following steps: 1) taking 1% of cross-linked polystyrene resin as a carrier to prepare a polystyrene-supported seleno-succimide reagent (III); 2) using the III to induce phenyl acrylate (V) to perform intramolecular cyclization under the catalysis of trimethylsilyl trifluoromethanesulfonate so as to form 3-polystyrene-supported seleno-3,4-dihydro-benzopyran-2-ketone (VI); and 3) performing oxidation elimination on the VI via an oxidant so as to directly get the coumarin (I) without further separation. When the phenyl acrylate (V) is replaced by N-phenyl acrylamide, the analogue of the coumarin, namely a 2-quinolone compound, can be prepared through the same steps. The solid-phase synthesis method disclosed by the invention has the advantages of easily available raw materials, good product yield, high purity, simplicityand convenience in operation, simple post-treatment and great industrial application prospects.
Owner:YUNNAN UNIV
Heterocylcic derivatives as inhibitors of glutaminyl cyclase
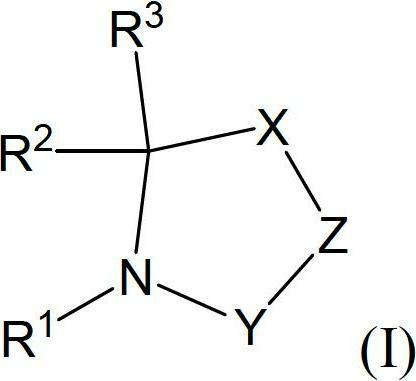
ActiveCN102695546ALasting effectOrganic active ingredientsNervous disorderCyclaseL-Pyroglutamic Acid
The invention relates to novel pyrrolidine derivatives of formula (I), wherein R1, R2 and R3 are as defined herein, as inhibitors of glutaminyl cyclase (QC, EC 2.3.2.5). QC catalyzes the intramolecular cyclization of N-terminal glutamine residues into pyroglutamic acid (5-oxo-prolyl, pGlu*) under liberation of ammonia and the intramolecular cyclization of N-terminal glutamate residues into pyroglutamic acid under liberation of water.
Owner:维沃永治疗公众有限公司
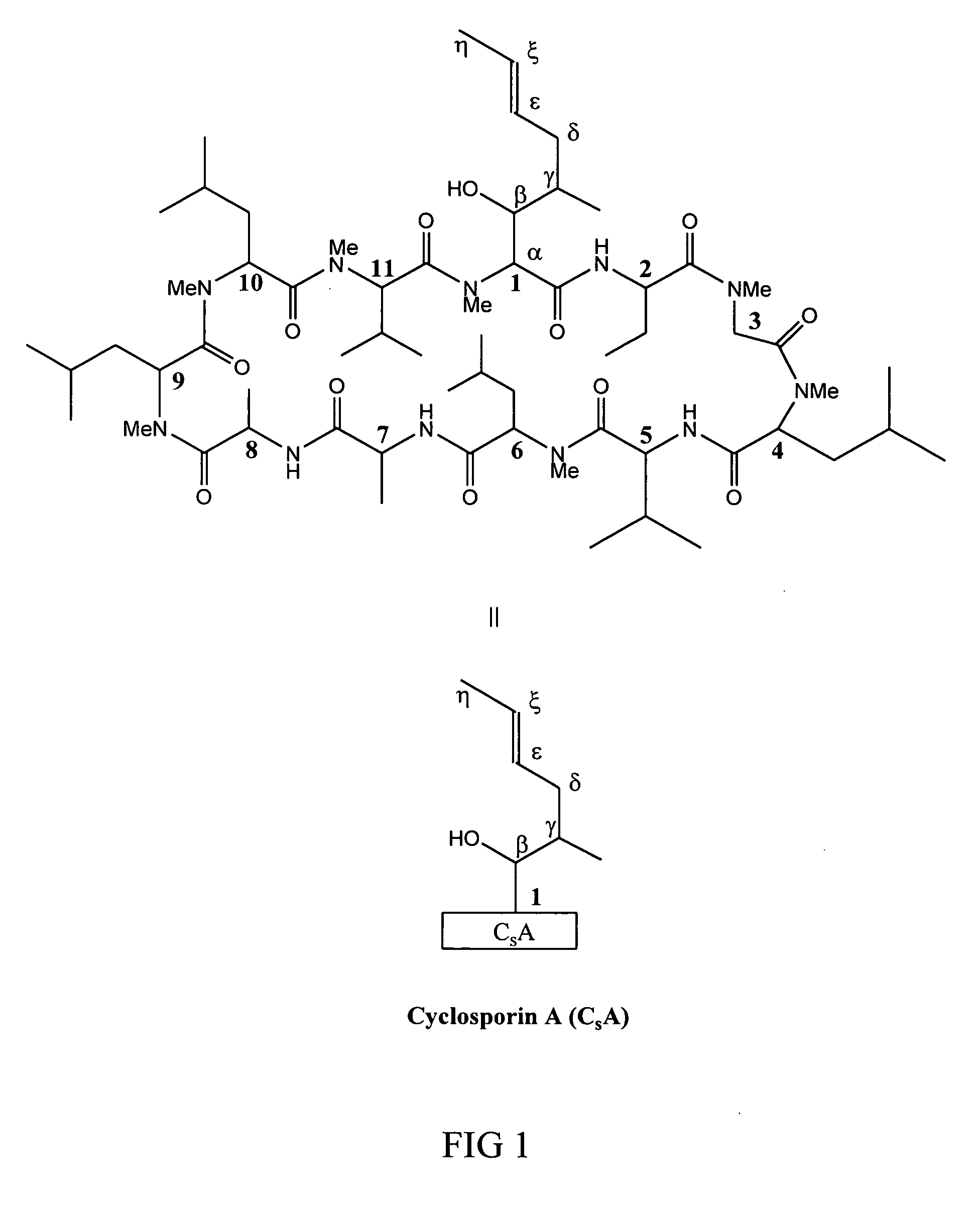
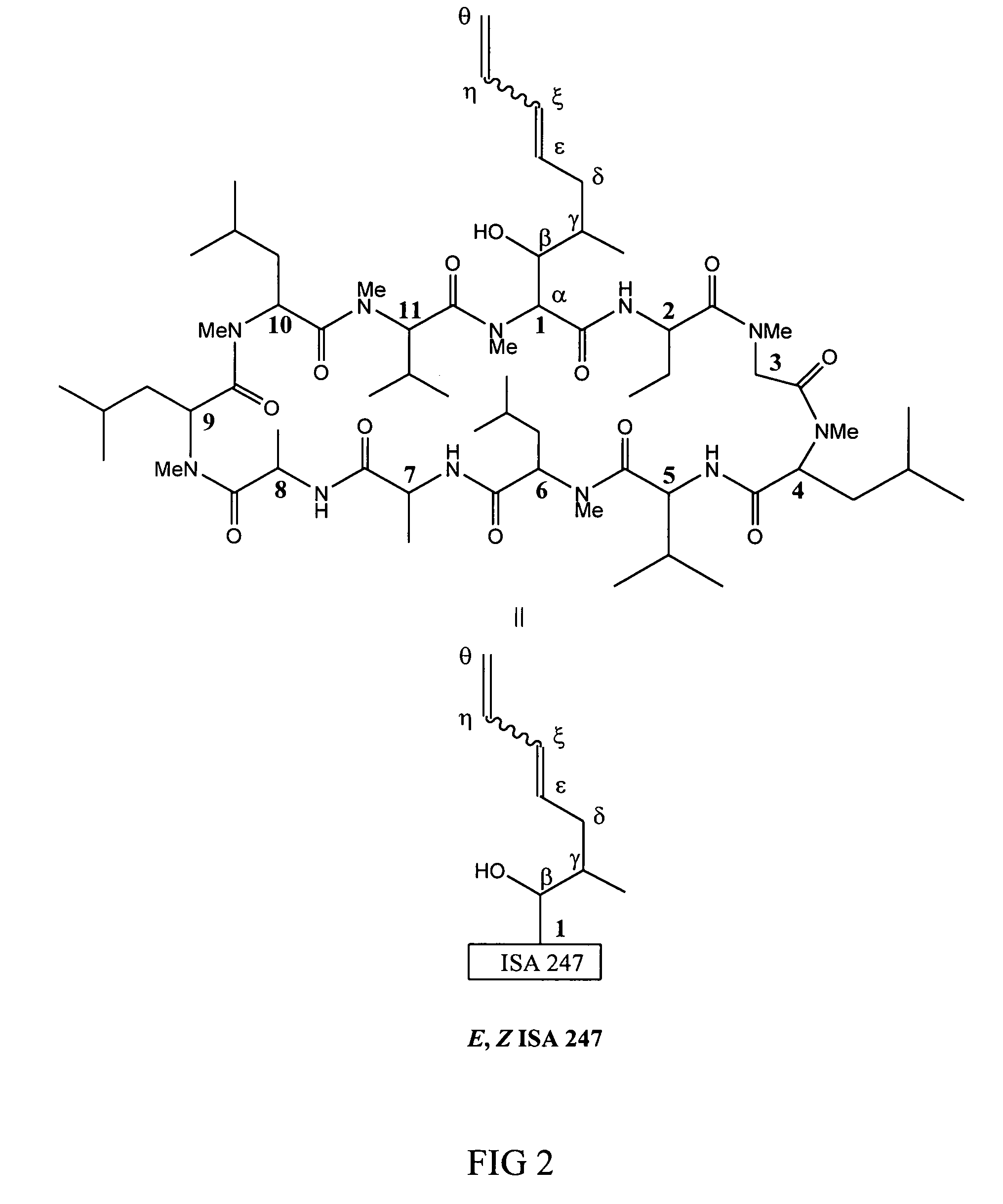
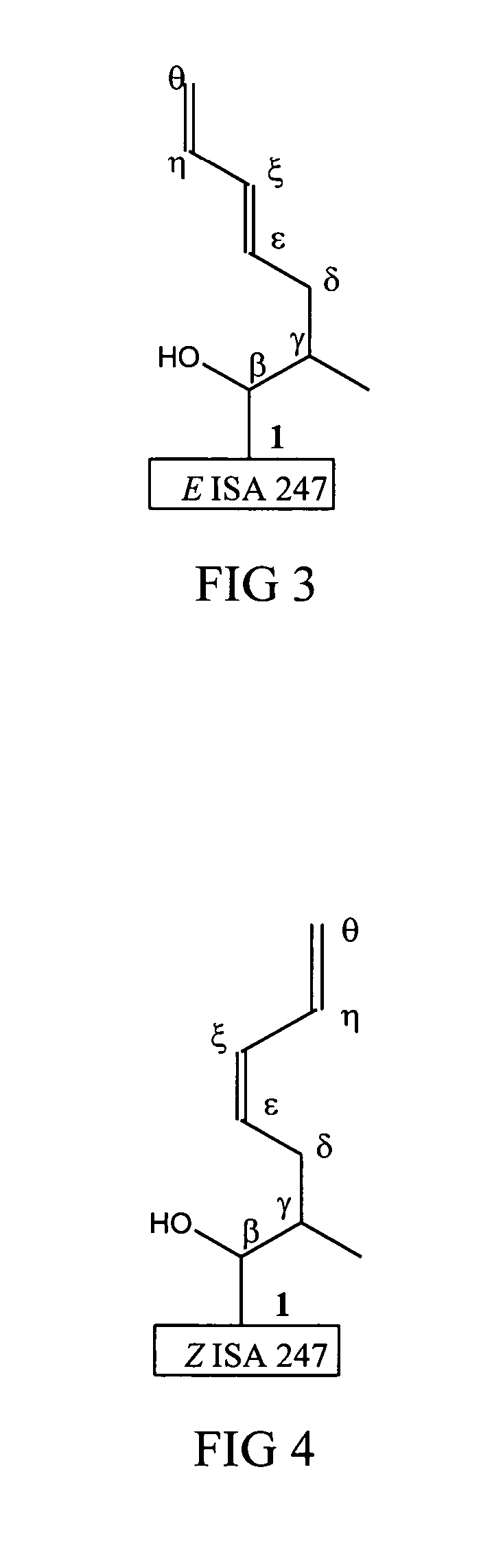
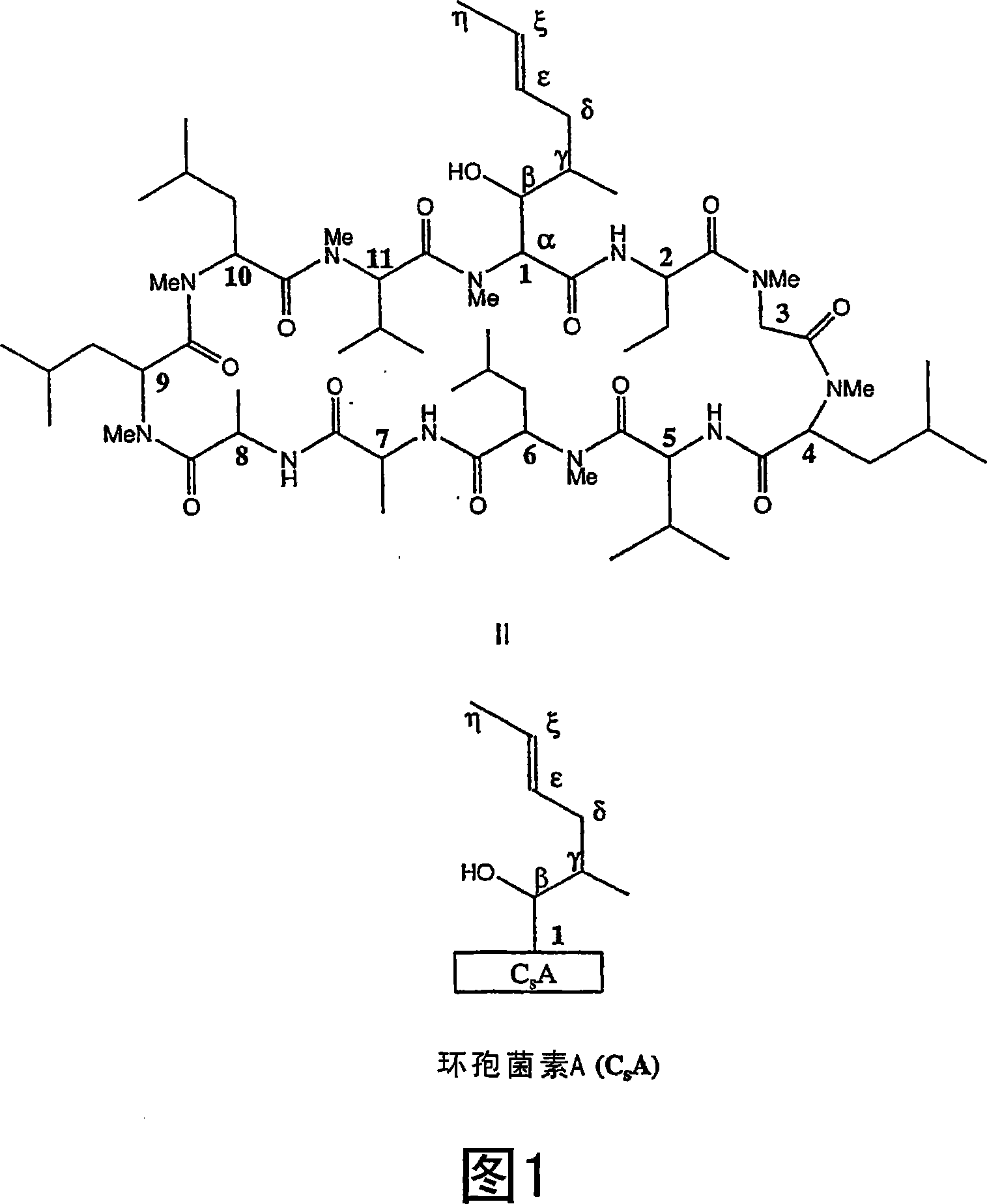
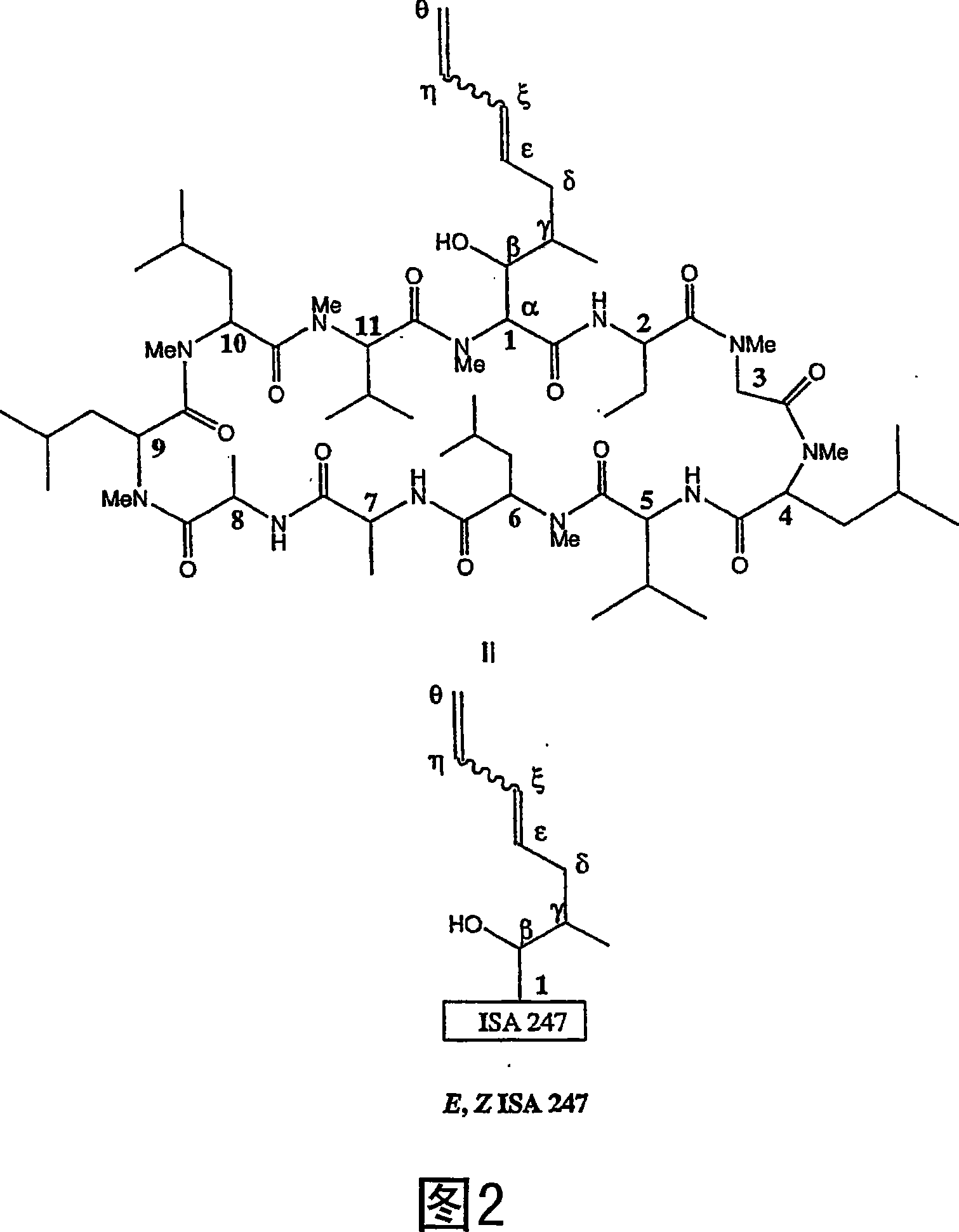
Metabolites of cyclosporin analogs
InactiveUS20060223743A1Useful immunosuppressive activityUseful for developmentCyclic peptide ingredientsImmunoglobulinsPhosphorylationCyclosporins
Isolated metabolites of the cyclosporine analog ISA247 are disclosed, including in vitro methods for their preparation. The metabolites comprise a chemical modification of ISA247, wherein the modification is at least one reaction selected from the group consisting of hydroxylation, N-demethylation, diol formation, epoxide formation, and intramolecular cyclization phosphorylation, sulfation, glucuronide formation and glycosylation. Methods of preparation include semi-synthetic methods, wherein metabolites of ISA247 are produced from the microsomal extracts of animal liver cells, or from cultures using microorganisms, and completely synthetic methods, such as chemically modifying the parent compound or isolated metabolites using organic synthetic methods.
Owner:ISOTECHNIKA INC
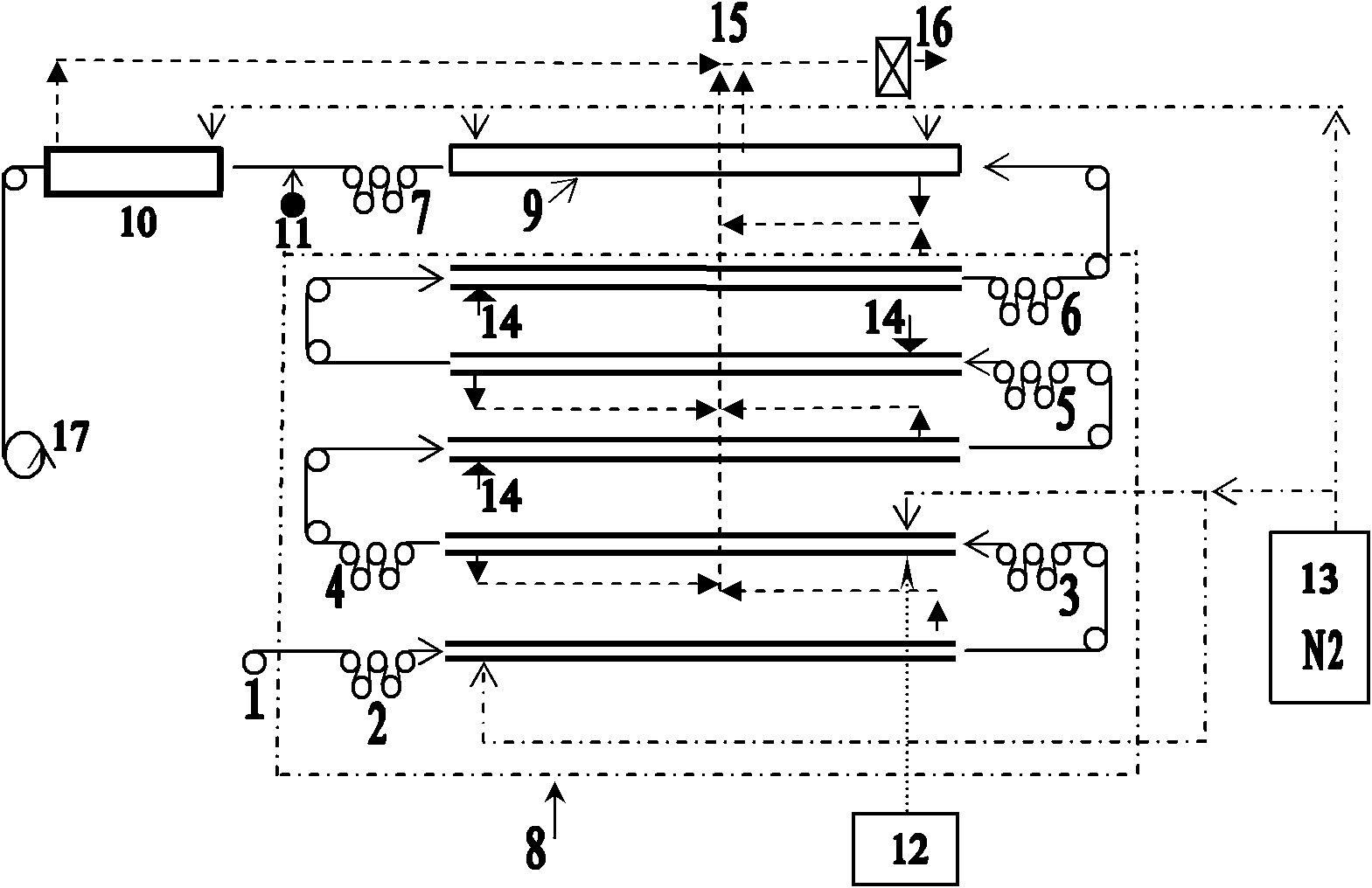
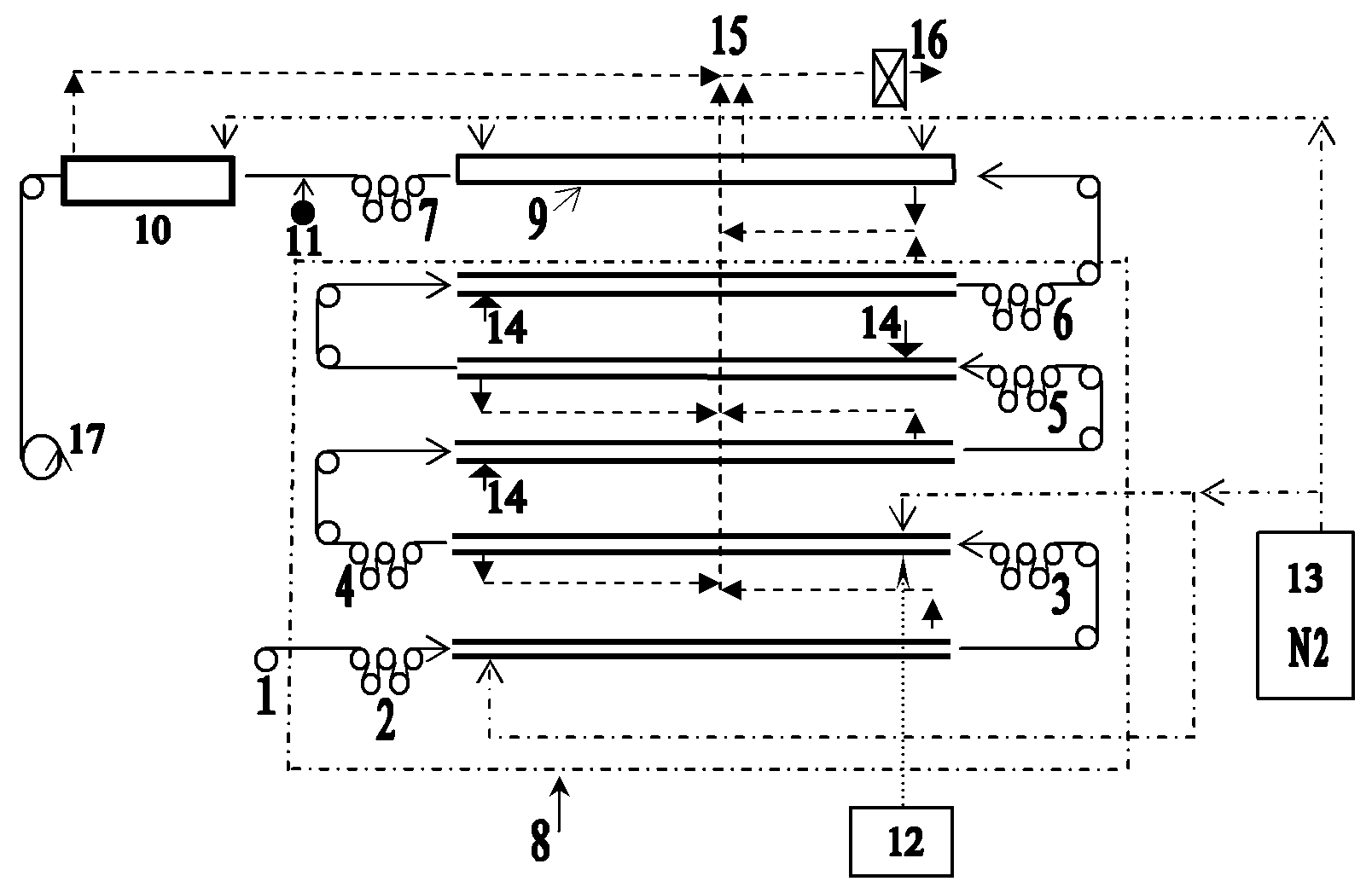
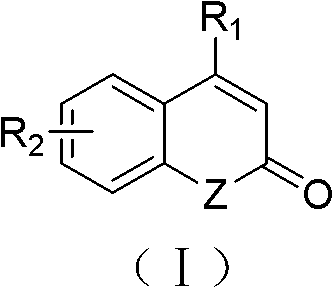
Hydrogenated banded[12]arene compound and preparation method thereof
ActiveCN110372469AMild reaction conditionsEasy to separate and purifyPreparing sample for investigationCatalystsGrignard reagentIntramolecular cyclization
The invention discloses a hydrogenated banded[12]arene compound and a preparation method thereof. The hydrogenated banded[12]arene compound is a compound shown in a formula (I) or a stereoisomer of the compound shown in the formula (I) (please see the specification for the formula). The preparation method includes the steps that an alkenylated calix[6]arene derivative compound shown in a formula (II) or a calix[6]arene alcohol derivative compound shown in a formula (III) (please see the specification for the formulas) reacts with acid in an organic solvent, and through cyclization reaction inmolecules, the hydrogenated banded[12]arene compound shown in the formula (I) can be obtained. Cheap and easily-available raw materials are adopted, from calix[6]reso-arene, a large quantity of calix[6]arene derivative compounds shown in the formula (II) and the formula (III) can be prepared through selective methylation, trifluorosulfonylation, transition metal catalysis coupling, Grignard reagent addition and other steps, and further through Friedel-Crafts alkylation reaction in molecules, the hydrogenated banded[12]arene compound can be conveniently prepared. Reaction conditions are relatively mild, obtained products are stable in air and easy to separate and purify, and good practicability and application prospects are achieved.
Owner:TSINGHUA UNIV
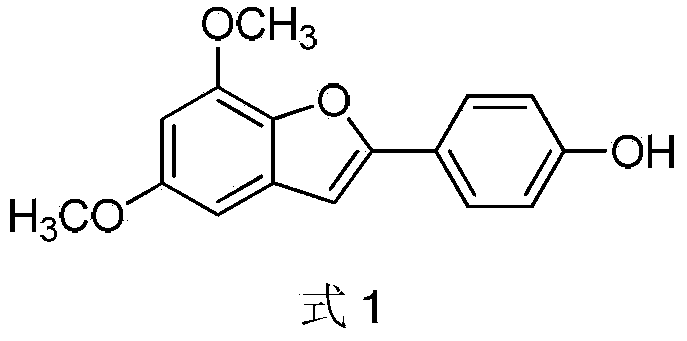
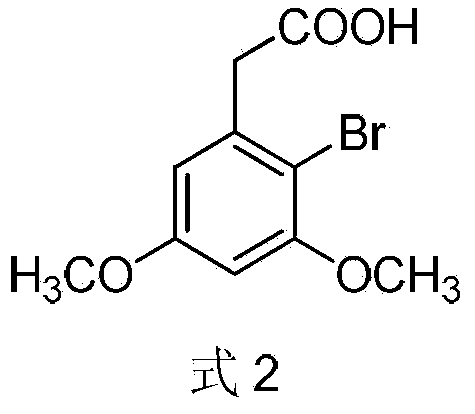
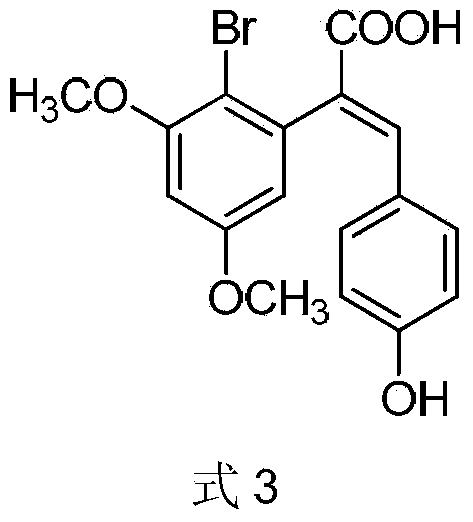
Preparation method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran
The invention discloses a synthetic method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. The synthetic method comprises the following steps of: firstly, performing bromination reaction and Perkin reaction on 3,5-dimethoxy phenylacetic acid and parahydroxyben-zaldehyde which are used as raw materials to obtain 2-(2-bromo-3,5-dimethoxy phenyl)-3-(4-hydroxyphenyl) acroleic acid; then performing series-wound hydroxylation / intramolecular cyclization / dehydrogenation reaction to obtain 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran-3-carboxylic acid; and finally, performing decarboxylic reaction to prepare the 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. According to the method, raw materials are low in price and easy to obtain, the synthetic route is simple, quick and efficient, a noble metal and a ligand are not needed for catalysis, and the method is simple and convenient to operate and good in atom economy.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
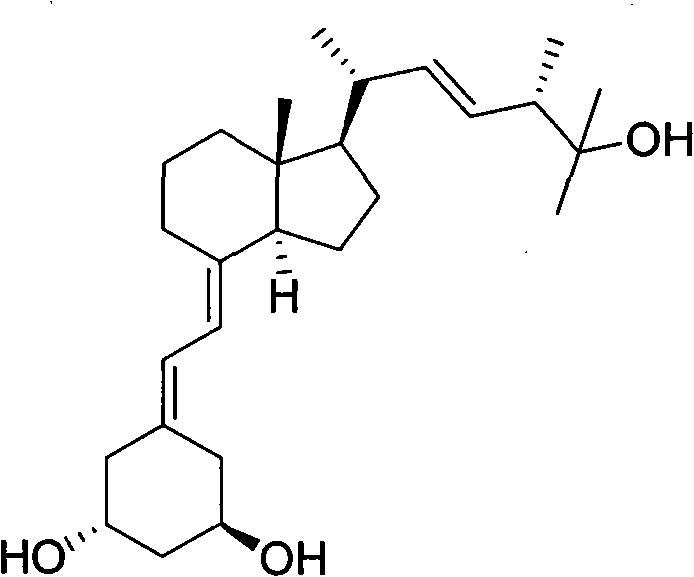
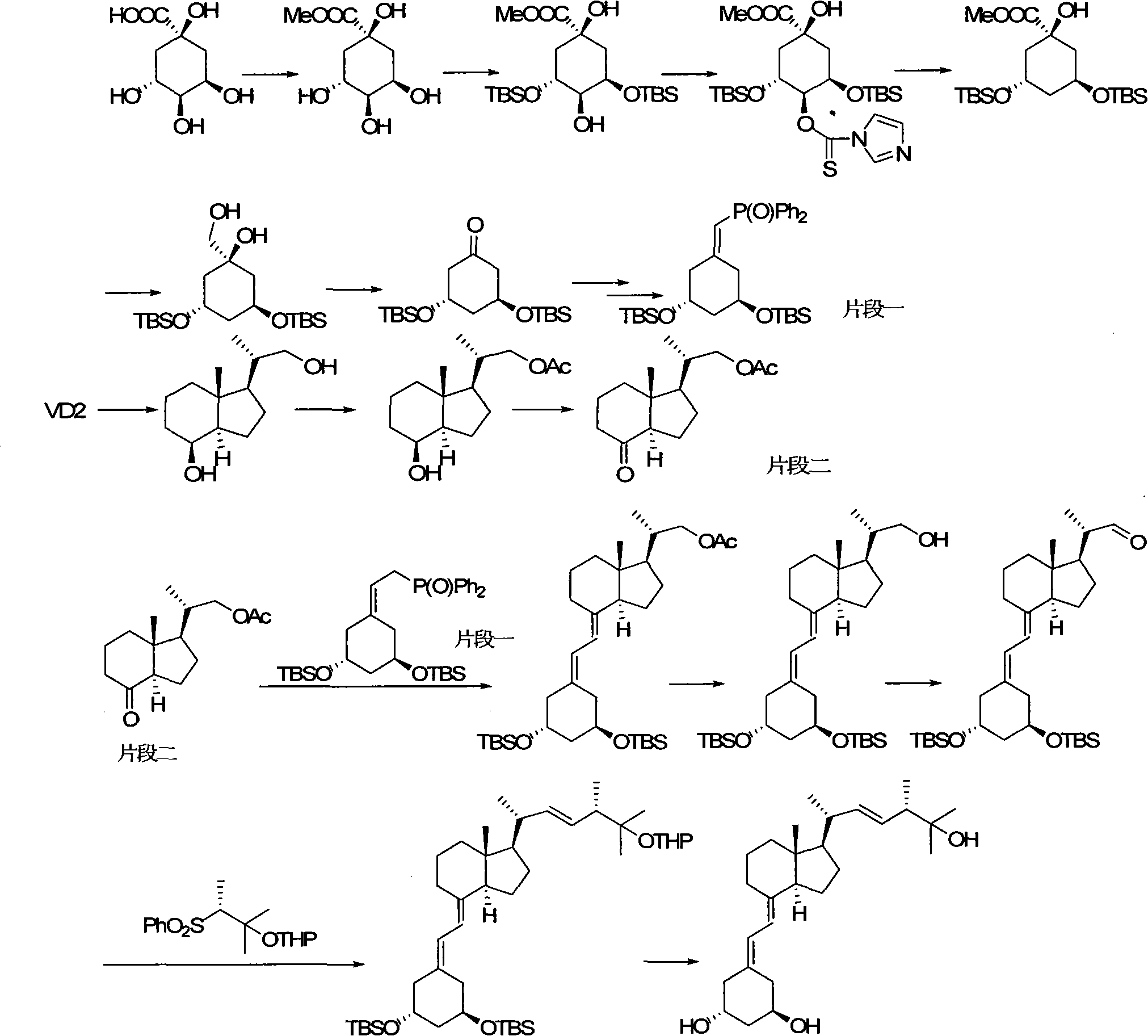
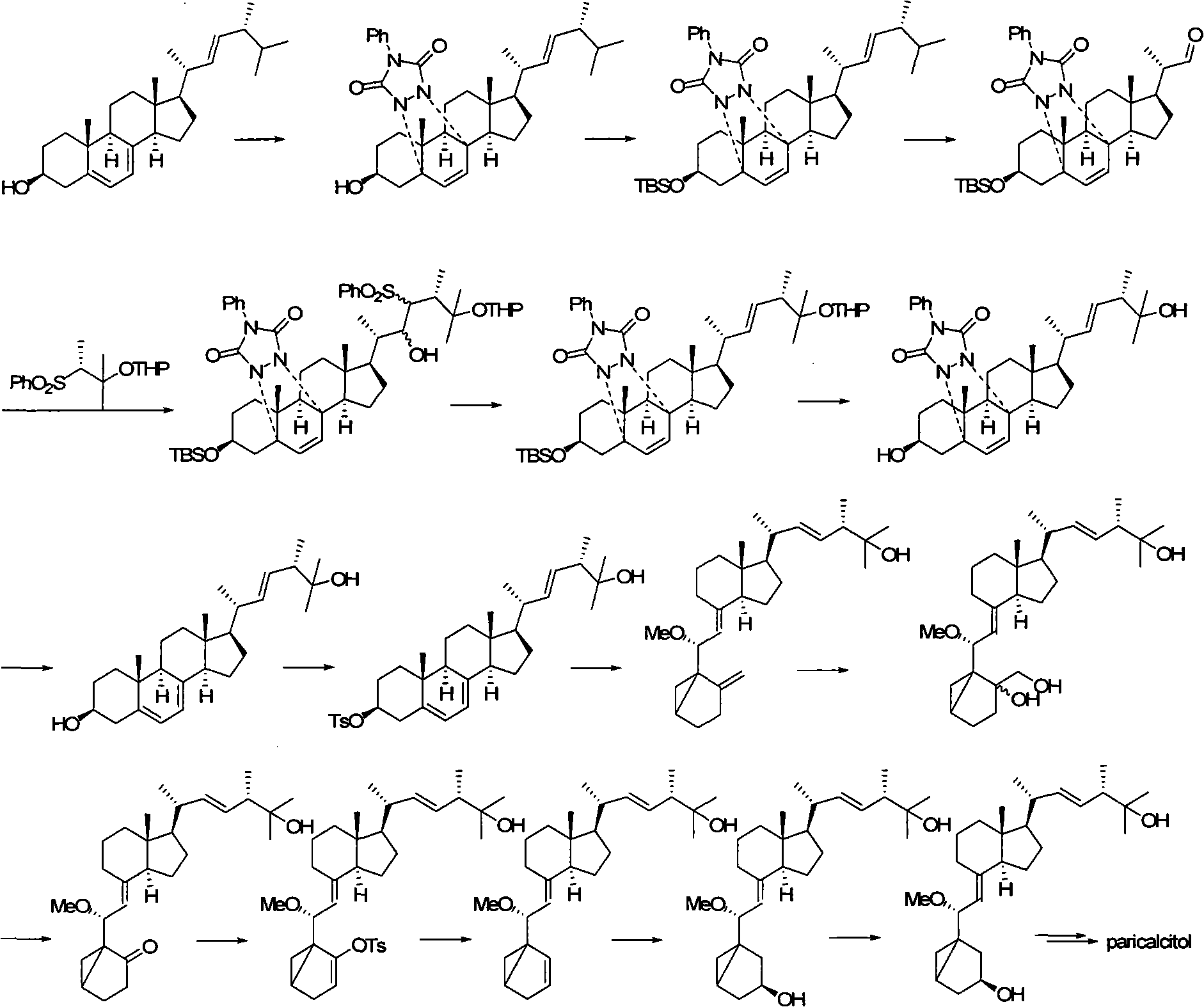
Preparation method of paricalcitol
InactiveCN101880253AEasy to operateLess regional selectivityOrganic chemistryBulk chemical productionSide chainDouble bond
The invention relates to a preparation method of paricalcitol, which is characterized in that after hydroxyl in the vitamin D2 is protected by p-toluenesulfonates, in the presence of alkali, intramolecular cyclization reaction happens in methanol to generate a compound 5; the compound 5 undergoes allylic oxidation and hydroxyl is protected to obtain a key intermediate 7; in the presence of ozone,the side chains and exocyclic terminal double bonds of the key intermediate 7 are cut off to obtain a compound 8; the primary hydroxyl in the compound 8 is selectively protected, a three-membered ring is opened in the presence of acid and then hydroxyl is protected to obtain a key intermediate 11; after the secondary hydroxyl in the key intermediate 11 is protected by sulphonate, a compound 12 isobtained through reduction by LiAlH4; a compound 13 is obtained after the compound 12 is subjected to Swern oxidation and carries out Wittig reaction with the compound 12 to obtain a compound 14; andthe target compound can be obtained by removing the protective group in the compound 14. The reagents used in the method are simple and are convenient to operate, the reactions concerning regioselectivity and stereoselectivity are few, the route is shorter and 12 steps of reactions are carried out.
Owner:CHONGQING TAIHAO PHARM CO LTD
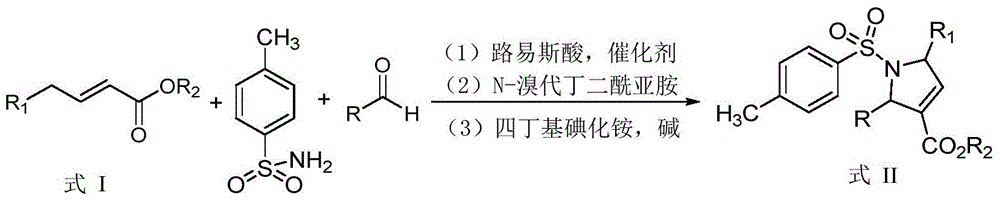
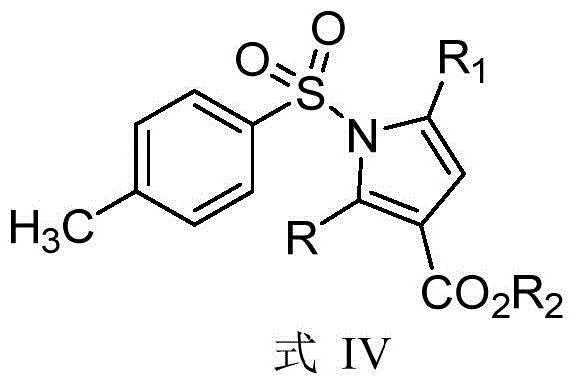
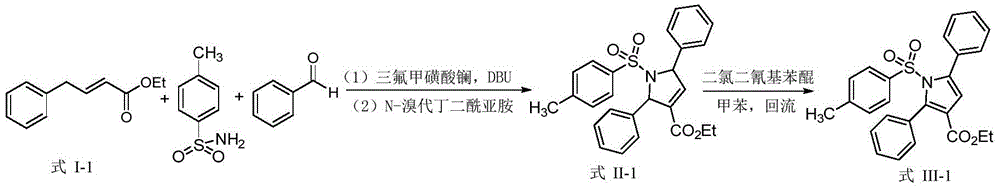
Synthetic method of dihydropyrrole and pyrrole compounds
InactiveCN105712922AHigh yieldHigh stereoselectivityOrganic chemistryIntramolecular cyclizationBenzoquinone
The invention discloses a synthetic method of dihydropyrrole and pyrrole compounds. The method comprises: reacting Alpha,Beta-unsaturated ester as raw material with para toluene sulfonamide and aldehydes under the action of an organic small-molecular catalyst 1,8-diazabicycloundercane-7-ene or 1,5-diazabycyclo[4,3,0]one-5-ene without separating an intermediate product, directly adding N-bromosuccinimide and alkali, and enabling intramolecular cyclization under mild reaction conditions so as to produce multi-group substituted dihydropyrrole compounds difficult to prepare by a conventional method, with high yield and good stereoselectivity. The synthetic method is simple, environment-friendly and high in atomic economy, enables dihydropyrrole compounds to be quickly and efficiently synthesized with one kettle, and the synthesized dihydropyrrole compounds are dehydrogenized under the action of dichloro dicyane benzoquinone to directly obtain pyrrole compounds.
Owner:SHAANXI NORMAL UNIV
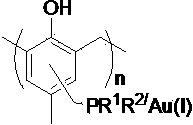
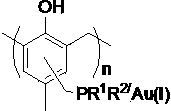
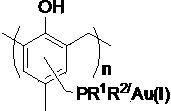
Supported gold catalyst, its preparation and its application
InactiveCN103638975AReduce consumptionReduce pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPropanolamine
The invention discloses a supported gold catalyst, its preparation and its application. The invention is characterized in that the catalyst employs a phosphine ligand for complexation of a monovalent gold compound, then monovalent gold compound is supported on an ordered meso pore phenolic resins material, and then the supported gold catalyst with supporting amount of 2.4-4.0wt% is prepared. The preparation of the supported gold catalyst comprises the steps of ordered meso pore phenolic resins chloromethylation and phosphine ligand; the supported gold catalyst has strong catalytic activity for being used in an intramolecular cyclisation reaction and an intermolecular allyl alcohol amination reaction of 2-alkynyl-1,3-dicarbonyls compounds, which is equivalent to the result of a homogeneous reaction. Compared with the prior art, the preparation method has the advantages of simple process, moderate reaction condition, good activity maintenance and little precious metals consumption, the cycle utilization of the catalyst is realized, the environmental pollution is reduced, and the invention provides the effective solution approach for existed problems of the intramolecular cyclisation reaction and the allyl alcohol amination reaction by traditional homogeneous gold catalysis.
Owner:EAST CHINA NORMAL UNIV
Compositions Comprising Enzyme-Cleavable Ketone-Modified Opioid Prodrugs and Optional Inhibitors Thereof
ActiveUS20120230916A1Eliminate side effectsPatient compliance is goodBiocideNervous disorderControlled releaseDepressant
A method of providing a patient with controlled release of ketone-containing opioid using a prodrug capable, upon enzymatic activation and intramolecular cyclization, of releasing the ketone-containing opioid is disclosed. The disclosure also provides such prodrug compounds and pharmaceutical compositions comprising such compounds. Such pharmaceutical compositions can optionally include an enzyme inhibitor that interacts with the enzyme(s) to mediate the enzymatically-controlled release of the ketone-containing opioid from the prodrug so as to modify enzymatic cleavage of the prodrug. Also included are methods to use such compounds and pharmaceutical compositions.
Owner:SIGNATURE THERAPEUTICS
Polymeric conjugates of aromatic amine containing compounds including releasable urea linker
InactiveUS20120289571A1Extended half-lifeModification for usingBiocideOrganic chemistryActive agentTyrosine-kinase inhibitor
The present invention relates to releasable urea linker systems involving amine-containing chemical compounds and biologically active agents. In particular, the present invention relates to reversibly releasable linkers based on intramolecular cyclization-assisted releasable urea linkages to aromatic amine-containing compounds. The present invention also relates to polymeric conjugates of indolinone-based tyrosine kinase inhibitors.
Owner:BELROSE PHARMA
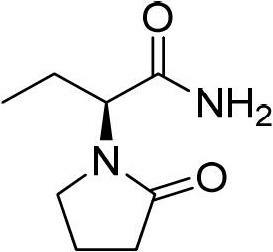
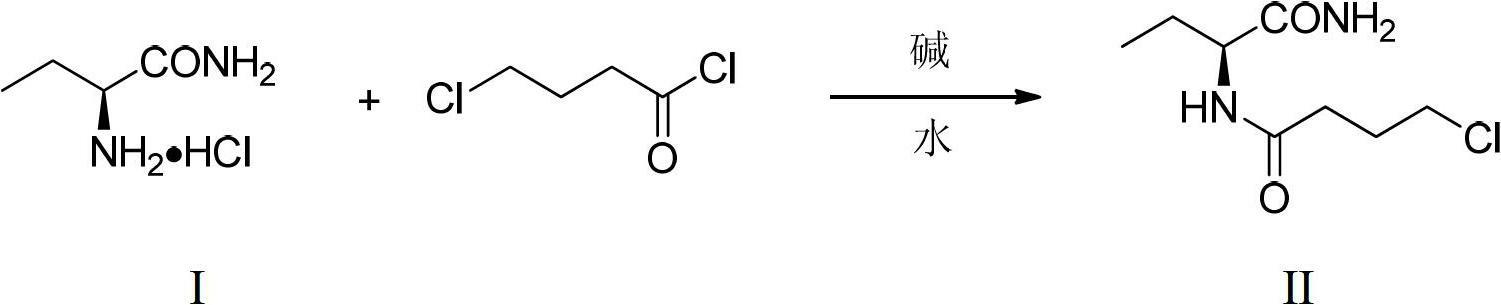
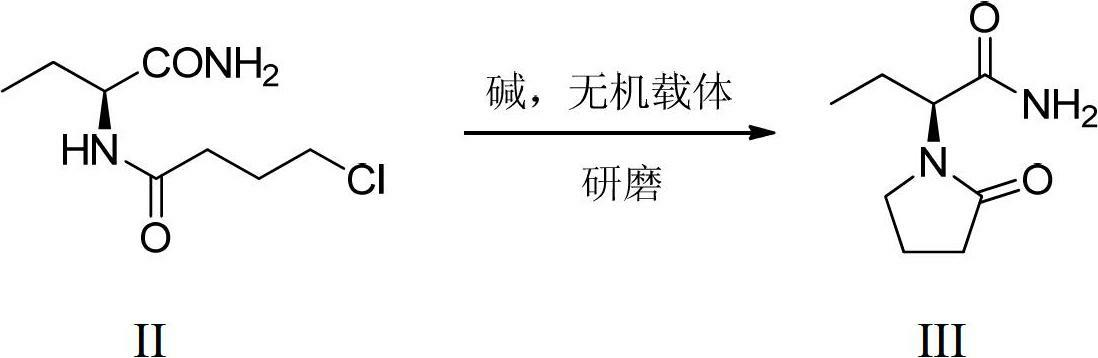
Preparation method of levetiracetam
The invention provides a preparation method of levetiracetam. The method includes utilizing alpha-amino-butyrylamide-hydrochloride and 4-chlorobutyryl chloride as raw materials, first conducting acylation reaction in water solution to obtain a middle body and enabling the middle body, an organic carrier and alkali to be ground in mixing mode to be subjected to intramolecular cyclization reaction to obtain the levetiracetam. The preparation method utilizes water as the catalyst in the acylation reaction, adopts a non-solvent grinding method and does not need adding a phase transfer catalyst in the intramolecular cyclization reaction. Organic solvents are not used in the two steps of reaction, so that the preparation method is environment-friendly, remarkably reduces cost, is simple in operation and high in yield and provides a new choice for preparation of the levetiracetam.
Owner:BEIJING SHIHONG PHARMACEUTICAL CO LTD +1
Method for preparing menthone from citronella aldehyde and catalyst system for the method
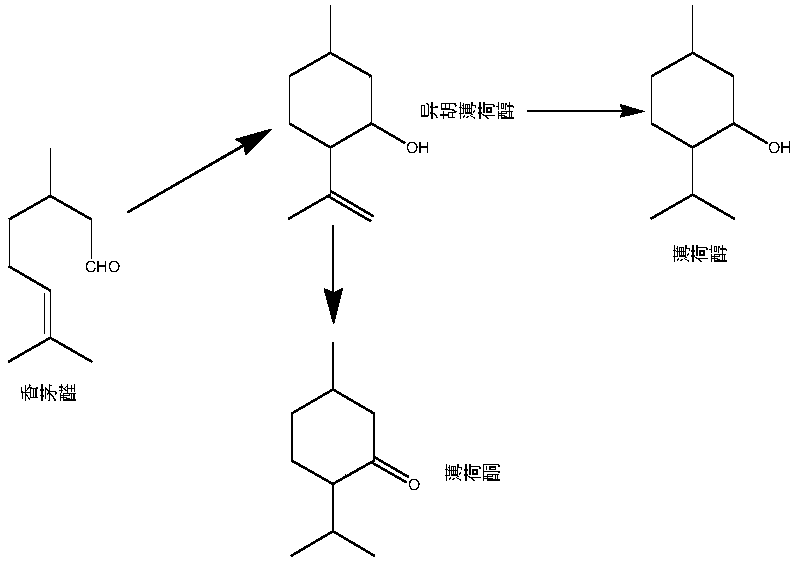
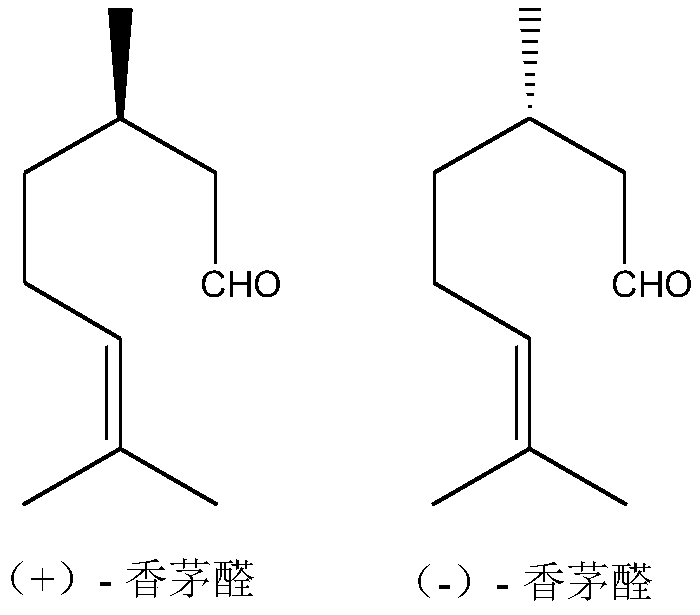
ActiveCN109704944AOrganic compound preparationCarbonyl compound preparationMenthoneSynthesis methods
The invention discloses a synthesis method of menthone and a catalyst system for the method. The method uses a heterogeneous catalyst and adds a small amount of auxiliaries, so that the citronella aldehyde undergoes intramolecular cyclization reaction to directly obtain menthone, the conversion rate can reach more than 90%, and the chemical selectivity of the reaction is more than 96%.
Owner:WANHUA CHEM GRP CO LTD +1
Metabolites of cyclosporin analogs
Isolated metabolites of the cyclosporine analog ISA247 are disclosed, including in vitro methods for their preparation. The metabolites comprise a chemical modification of ISA247, wherein the modification is at least one reaction selected from the group consisting of hydroxylation, N-demethylation, diol formation, epoxide formation, and intramolecular cyclization phosphorylation, sulfation, glucuronide formation and glycosylation. Methods of preparation include semi-synthetic methods, wherein metabolites of ISA247 are produced from the microsomal extracts of animal liver cells, or from cultures using microorganisms, and completely synthetic methods, such as chemically modifying the parent compound or isolated metabolites using organic synthetic methods.
Owner:伊素制药公司
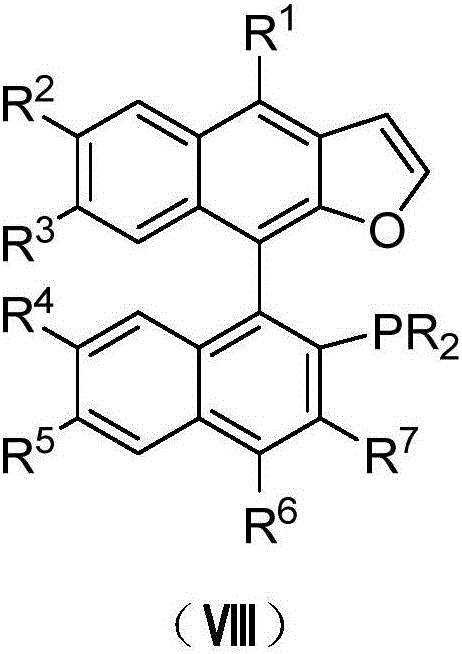
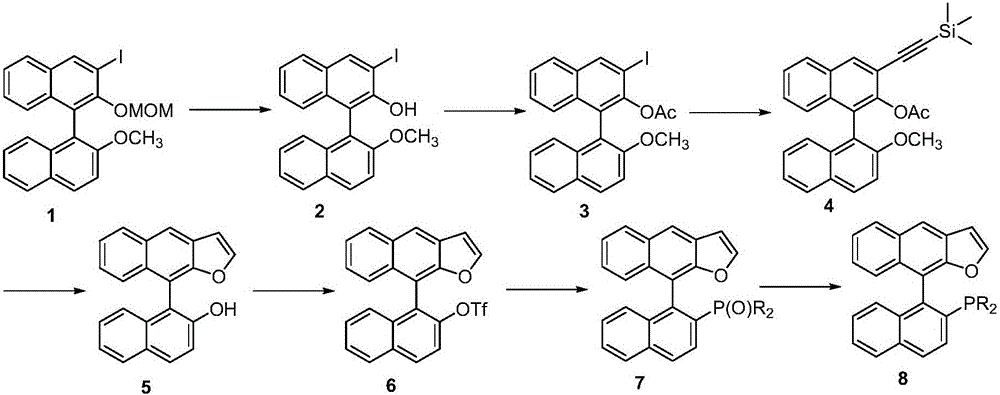
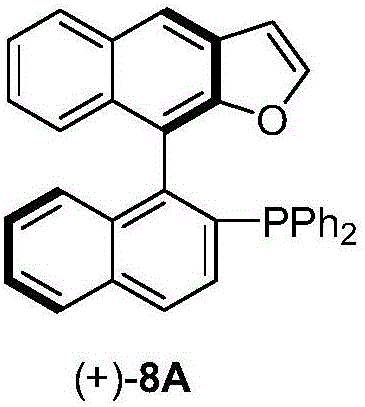
Naphthofuran-structure-containing biaryl monophosphine ligands, and preparation method and application thereof
ActiveCN105968137ANovel structureIncrease steric hindranceOrganic compound preparationGroup 5/15 element organic compoundsFuranSynthesis methods
The invention discloses naphthofuran-structure-containing biaryl monophosphine ligands, and a preparation method and application thereof. The biaryl monophosphine ligands are compounds disclosed the chemical structural formula (VIII) or antipodes or racemates thereof. According to the biaryl monophosphine ligands, the furan ring is introduced and forms a conjugated structure with the dinaphthalene, thereby increasing the steric hindrance of the phosphine ligands (including antipodes or racemates thereof) and the cloud density of the aromatic ring, and enhancing the stability of the complexes. The steric hindrance of the ligands is regulated, so that the ligands have novel structure. The preparation process comprises the steps of Sonogashira reaction, intramolecular cyclization, C-P coupling reaction and the like. The synthesis method is simple; and the obtained ligands have the advantages of high activity, favorable selectivity and the like when being used for Suzuki-Miyuara reaction.
Owner:SUN YAT SEN UNIV
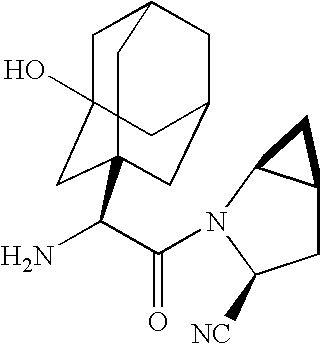
Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane
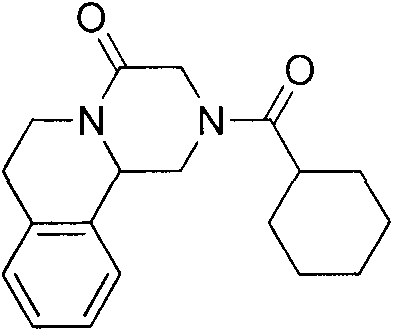
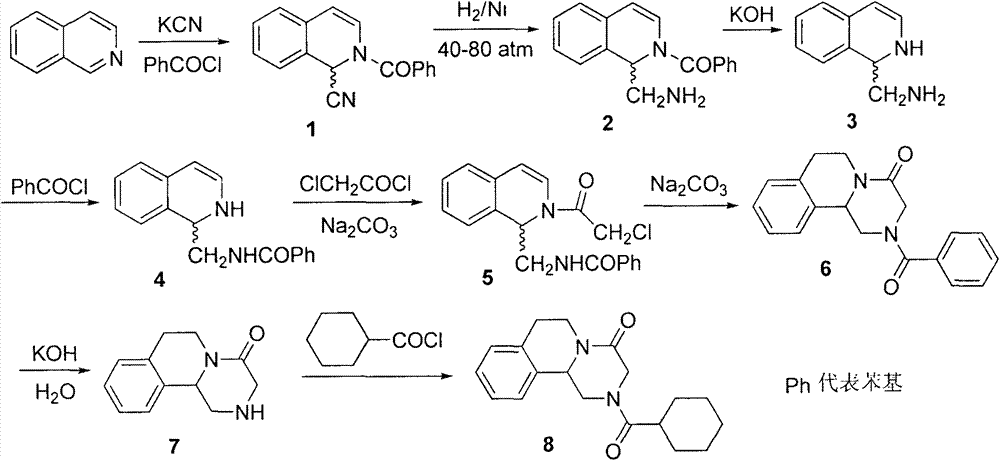
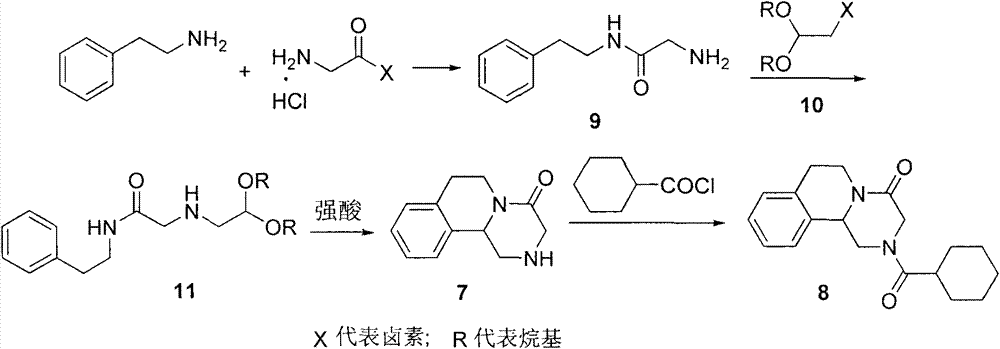
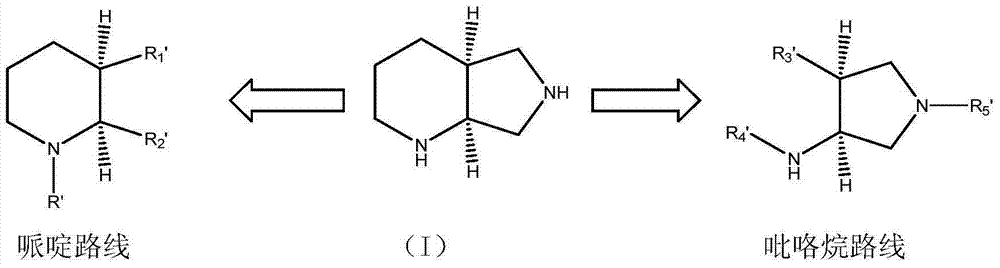
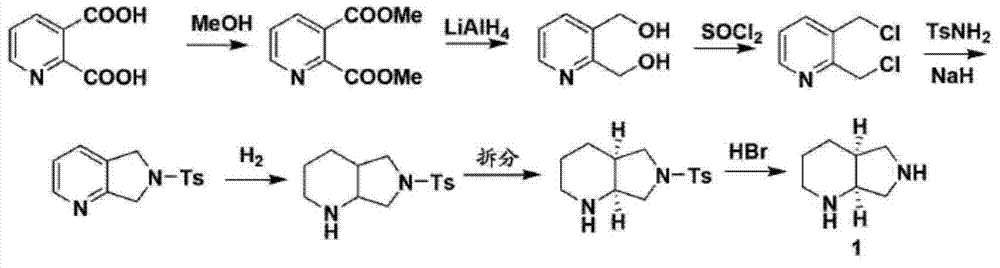
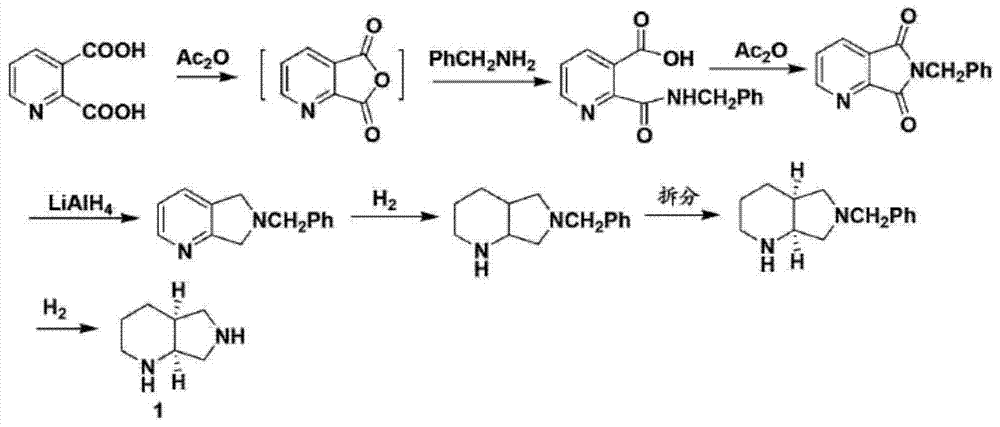
ActiveCN107793414ASimple process routeHigh yieldOrganic chemistryBulk chemical productionHydrogen atomNitrogen
The invention discloses a method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane. The method comprises the following steps of: (a) adopting a compound which is shown in a formula V and comprisesa chiral auxiliary group and an amino protecting group as a raw material, and performing an intramolecular cyclization reaction to obtain a compound shown in a formula VI; (b) removing the chiral auxiliary group and amino protecting group from the compound shown in the formula V so as to obtain a compound shown in a formula VII, wherein when X is a hydrogen atom, the compound shown in the formulaVII is (S,S)-2,8-diazabicyclo[4.3.0]nonane; and (c) when X is an oxygen atom, performing a reduction reaction on the compound shown in the formula VII by using amide so as to obtain (S,S)-2 ,8-diazabicyclo[4.3.0]nonane.
Owner:SHANGYU JINGXIN PHARMA +1
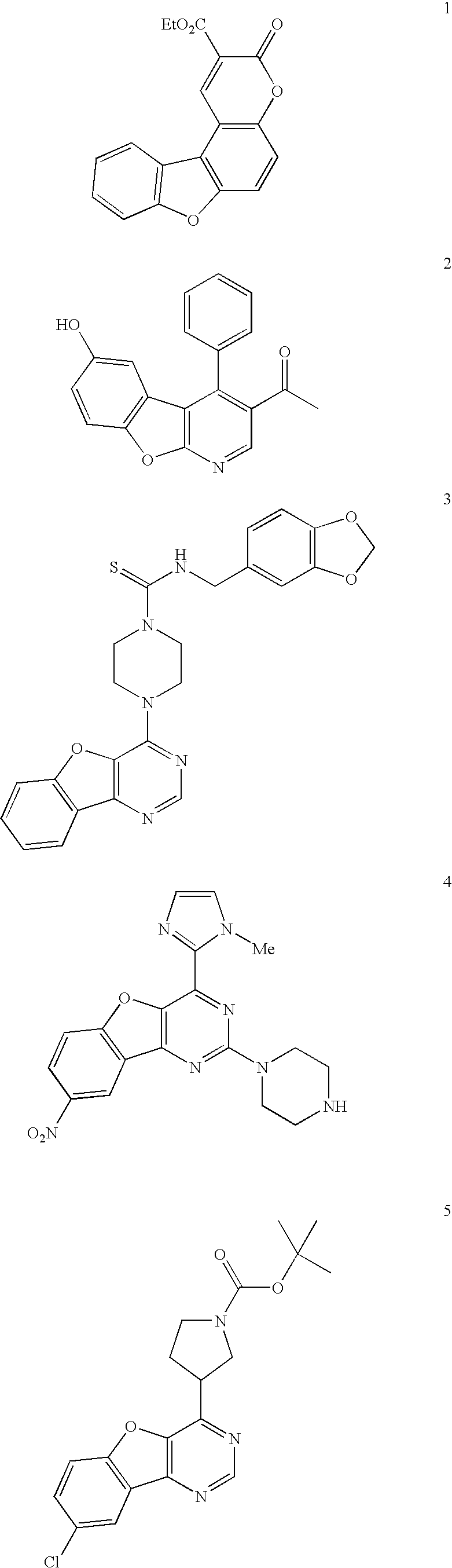
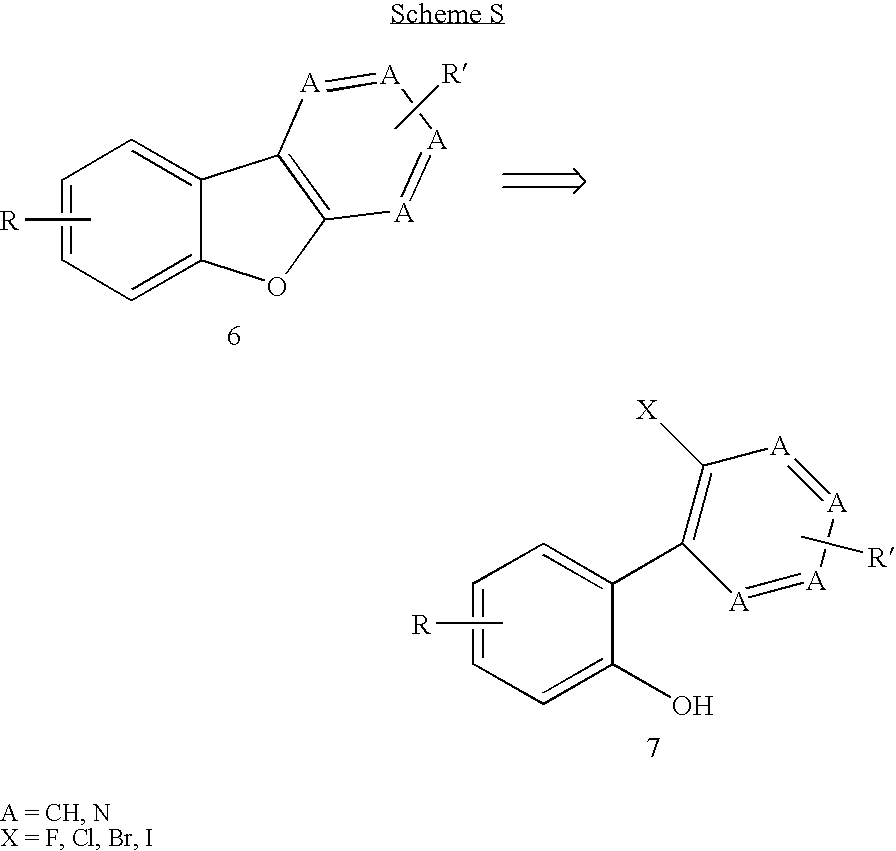
Facile assembly of fused benzofuro-heterocycles
This invention concerns the synthesis of polycyclic structural components of pharmacological compounds, including the synthesis of fused benzofuro-heterocycles, through selective palladium-catalyzed cross-coupling and intramolecular cyclization.
Owner:JANSSEN PHARMA NV
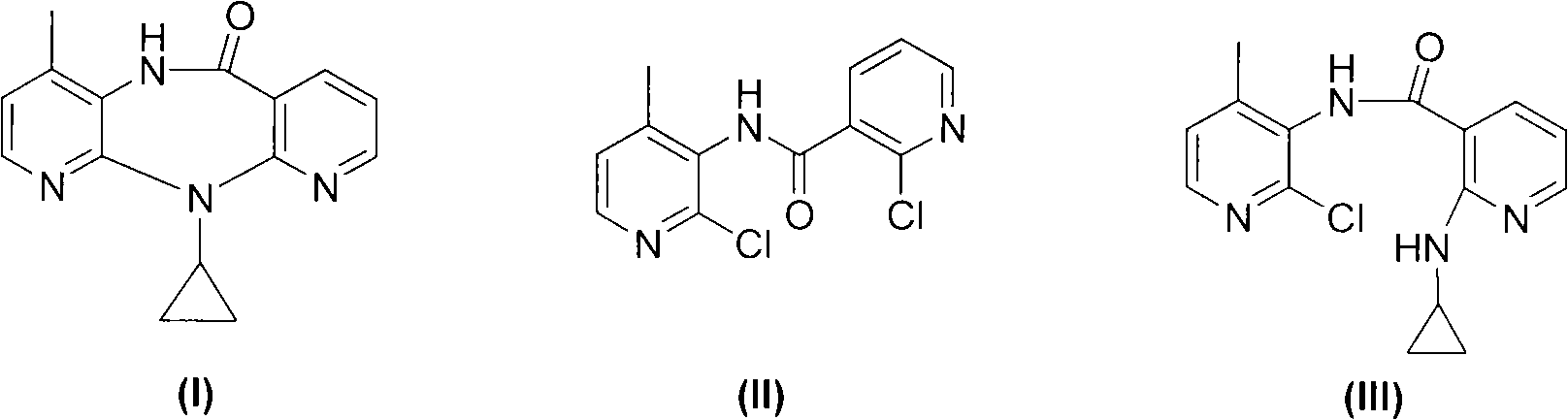
Novel method for preparing Nevirapine
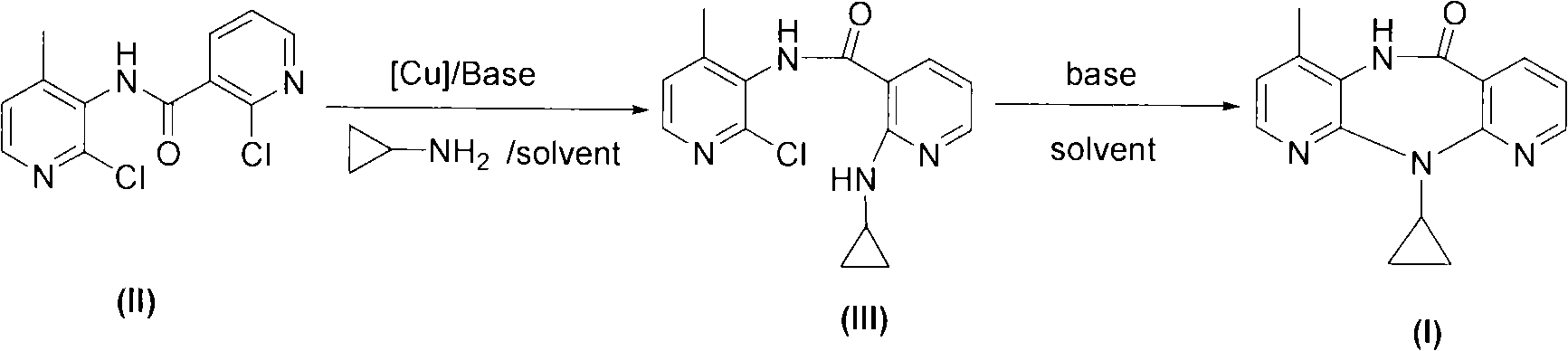
The invention relates to a novel method for preparing Nevirapine and discloses a novel method for preparing 11-cyclopropyl-5,11-dihydrogen-4-methyl-6H-II naphthyridine (3,2-b:2',3'-e)(1,4) azatropylidene (I) starting from 2-chlorin-N-(2-chlorin-4-methyl-3-pyridyl)-3-pyridine carboxamide (II). The method comprises the steps: a compound (II) reacts with cyclopropylamine under the existence of organic solvent and alkali and the catalysis of cupric salt under normal temperature and pressure to produce a compound (III), and the obtained compound (III) produces 11-cyclopropyl-5,11-dihydrogen-4-methyl-6H-II naphthyridine (3,2-b:2',3'-e)(1,4) azatropylidene (I) after intramolecular cyclization under the action of a certain organic solvent and the alkali. The method has low cost, environmental protection and wild reaction condition, thereby being suitable for commercial process.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Environmental-friendly and efficient preparation method of quinolone compound
InactiveCN104262249ASimple processLow costOrganic compound preparationAmino-hyroxy compound preparationIce waterPtru catalyst
The invention discloses an environmental-friendly and efficient preparation method of a quinolone compound. According to the adopted technical scheme, the environmental-friendly and efficient preparation method comprises the following steps: putting an intermediate 1 (I) in a reactor, dropwise adding an intermediate 2 (II) at a room temperature, and mixing and stirring uniformly under the condition without solvent to carry out solvent-free hydroamination; after a detection reaction is completed, carrying out chromatography on reaction liquid through a silicagel column, concentrating through reduced pressure distillation to obtain a yellow oily intermediate 3 (III); then putting the intermediate 3 (III) in the reactor, directly adding a catalyst, heating for dissolution while stirring to carry out an intramolecular cyclization reaction; after the detection reaction is completed, quenching the reaction by using ice water, extracting, concentrating and recrystallizing to obtain a target compound 4 (IV). The preparation method of the quinolone compound disclosed by the invention is environmental-friendly, efficient, simple, convenient and safe to operate, and the produced quinolone compound is low in cost.
Owner:YUNNAN MINZU UNIV
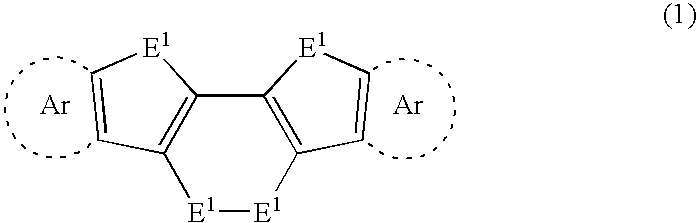
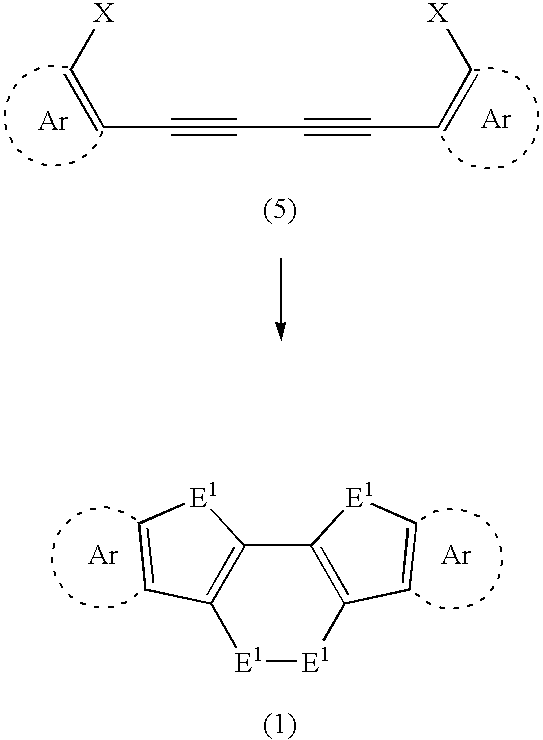
Chalcogen-containing fused polycyclic organic material and method for producing same
ActiveUS20070117973A1Easy to chargeEasy to manufactureOrganic chemistryElectrical apparatusOrganolithium compoundsCompound (substance)
A diacetylene derivative was used as a starting material, and was subjected to dilithiation using an organolithium reagent. The resulting product was allowed to react with an excessive amount of chalcogen. Accordingly, an intramolecular cyclization reaction proceeded simultaneously with formation of skeletons of three rings. As a result, a chalcogen-containing fused polycyclic organic material was found to be obtained which has the three rings and a dichalcogenid bond. Further, by subjecting the resulting compound to a dechalcogenation reaction, a heteroacene was found to be obtained in a satisfactory yield. These synthetic techniques have made it possible to synthesize a series of highly planar chalcogen-containing π-electron system materials. Therefore, it is possible to provide (i) a chalcogen-containing fused polycyclic organic material capable of exhibiting excellent charge-transporting properties and (ii) a method for producing the material.
Owner:JAPAN SCI & TECH CORP
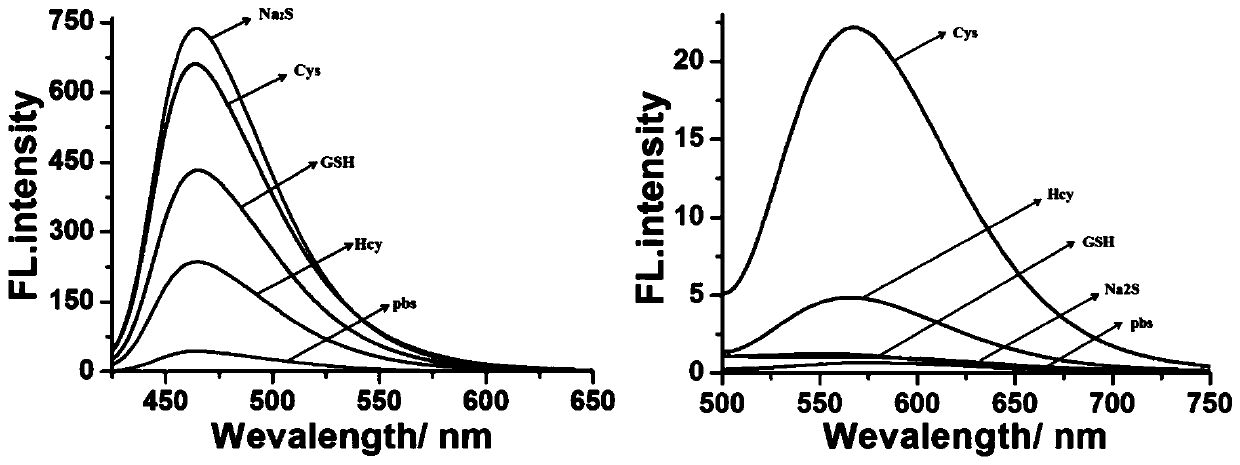
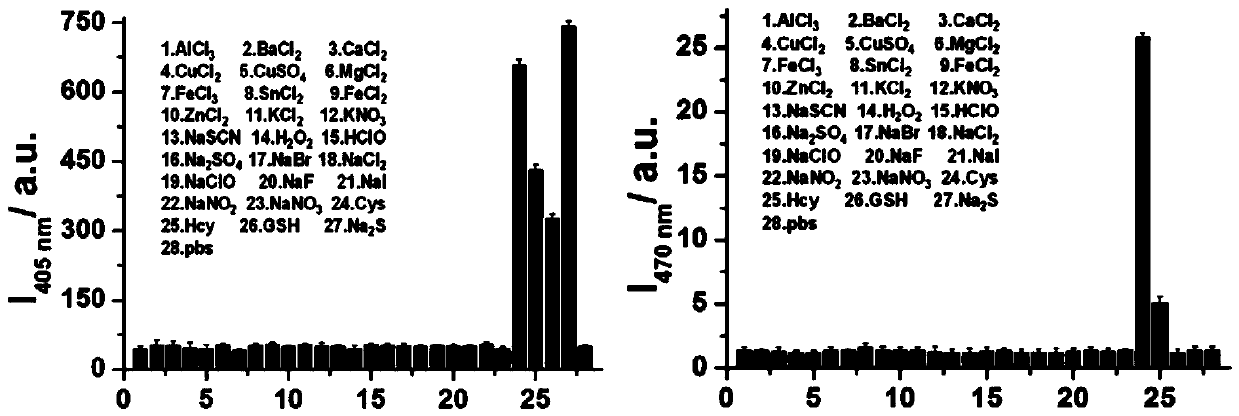
Fluorescent probe for detecting biological mercaptan in lysosome and preparation method and application of fluorescent probe
InactiveCN111072648AImprove targetingQuick responseOrganic chemistryFluorescence/phosphorescenceChemical synthesisFluoProbes
The invention provides a fluorescent probe for detecting biological mercaptan in lysosome. The chemical structural formula of the fluorescent probe is shown in the specification. According to the probe, in the presence of Cys and Hcy, the probe generates two different fluorescence emissions of blue and green at 480nm and 550nm respectively under the excitation of two independent wavelengths. However, when GSH and Na2S are added into the probe, blue fluorescence can only be generated at 480 nm. The difference can be reasonably attributed to the fact that the NBD-GSH / SH intermediate is differentfrom the NBD Cys / Hcy, and an intramolecular cyclization rearrangement reaction cannot occur. Meanwhile, due to the fact that morpholine has lysosome targeting, lysosome can be distinguished from other organelles. The fluorescent probe has the advantages of good lysosome targeting, strong specificity, fast response and the like and can be used for real-time visual determination of biological mercaptan in living cell lysosome, and can be obtained through chemical synthesis and has the advantages of simple and feasible synthesis process, cheap and easily available raw materials, low preparationcost and easiness in popularization.
Owner:UNIV OF JINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Hydrogenated banded[12]arene compound and preparation method thereof Hydrogenated banded[12]arene compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/96820948-cd67-4aaa-bb44-ad5b8da634f7/HDA0002140642040000011.png)
![Hydrogenated banded[12]arene compound and preparation method thereof Hydrogenated banded[12]arene compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/96820948-cd67-4aaa-bb44-ad5b8da634f7/HDA0002140642040000012.png)
![Hydrogenated banded[12]arene compound and preparation method thereof Hydrogenated banded[12]arene compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/96820948-cd67-4aaa-bb44-ad5b8da634f7/HDA0002140642040000021.png)

![Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane](https://images-eureka.patsnap.com/patent_img/274c98d1-31ba-4a2a-b4fc-e857a6b8911c/FDA0001110043610000011.png)
![Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane](https://images-eureka.patsnap.com/patent_img/274c98d1-31ba-4a2a-b4fc-e857a6b8911c/FDA0001110043610000012.png)
![Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane Method for synthesizing (S,S)-2,8-diazabicyclo[4.3.0]nonane](https://images-eureka.patsnap.com/patent_img/274c98d1-31ba-4a2a-b4fc-e857a6b8911c/FDA0001110043610000021.png)