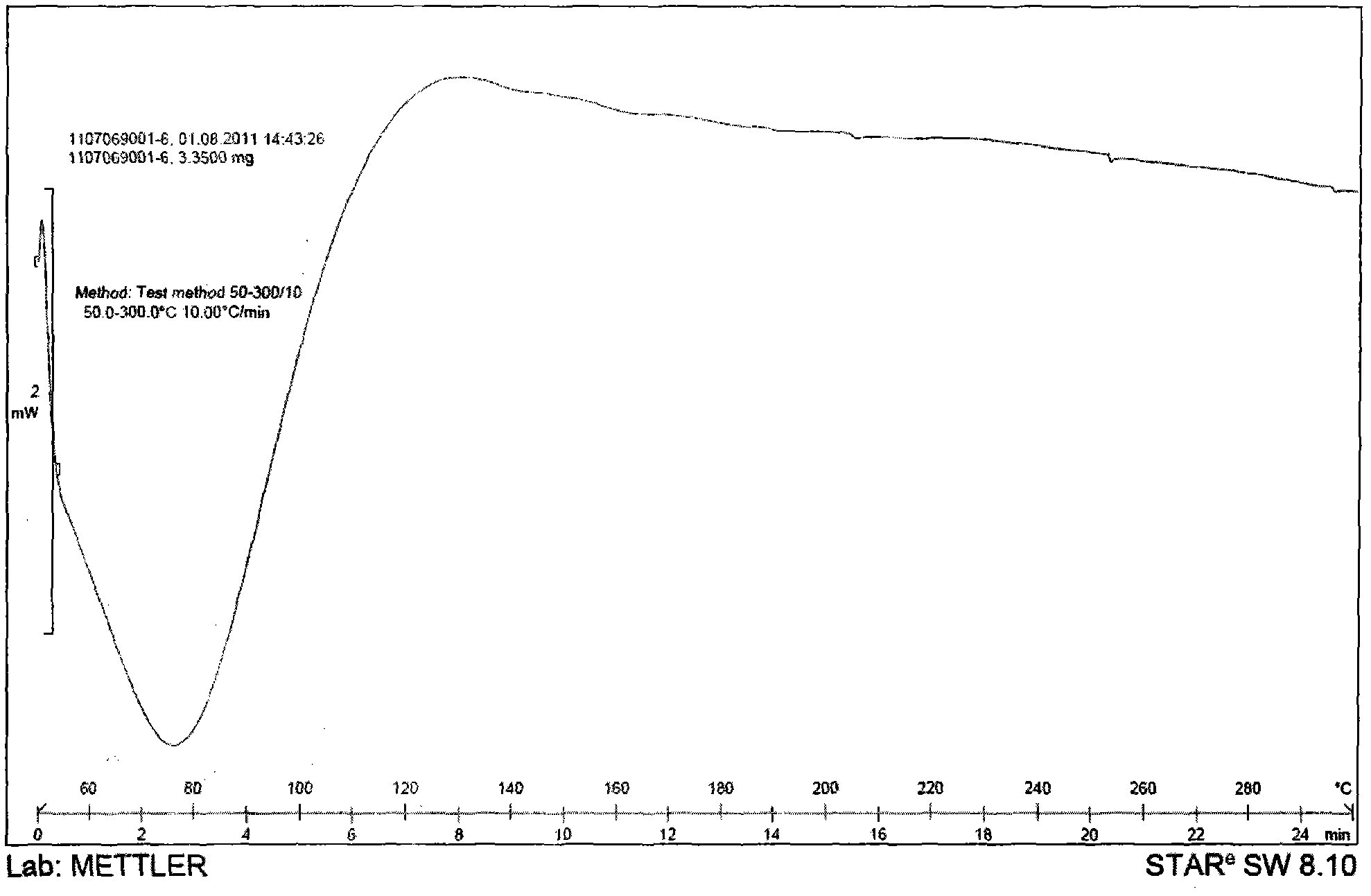
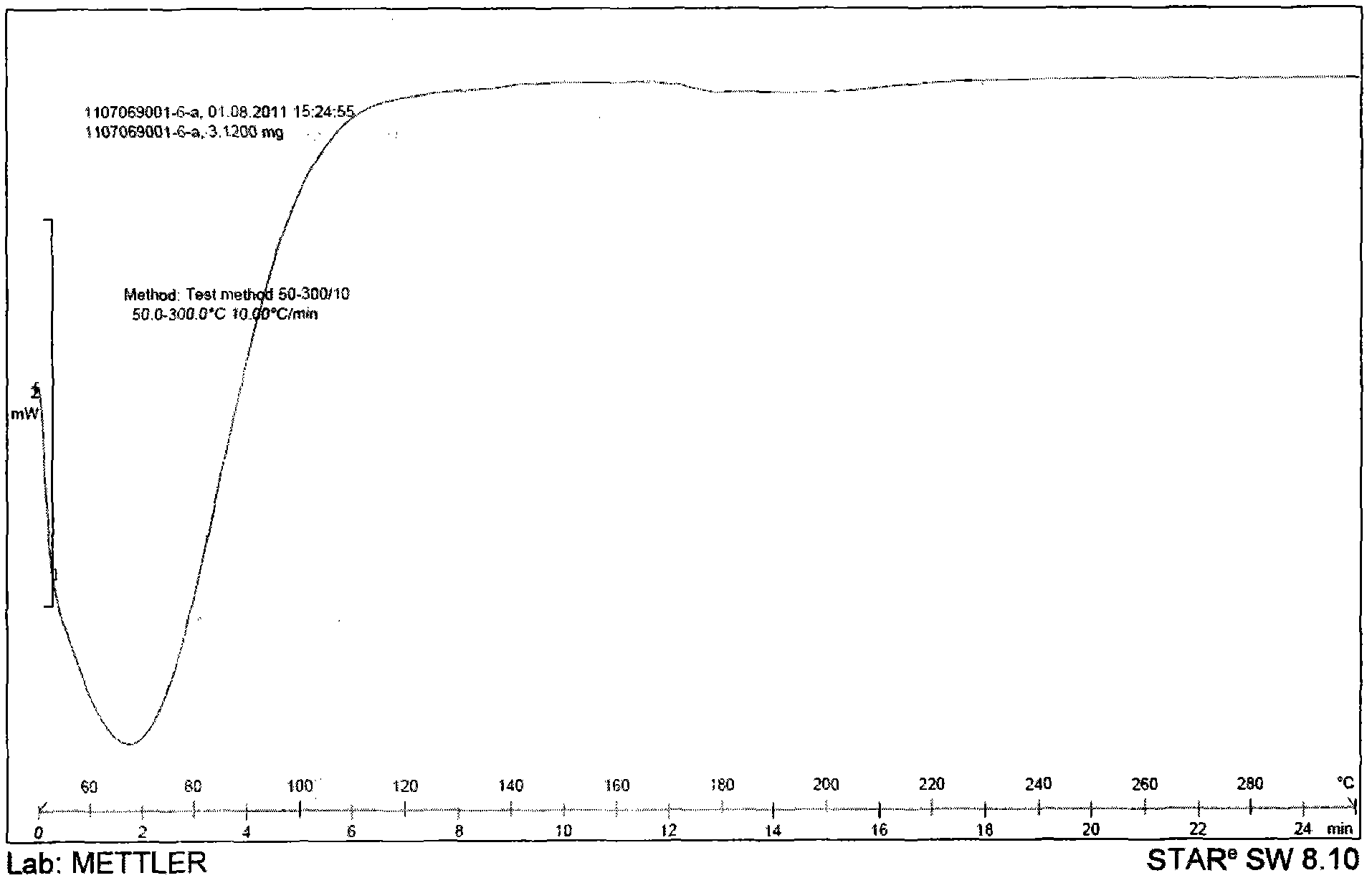
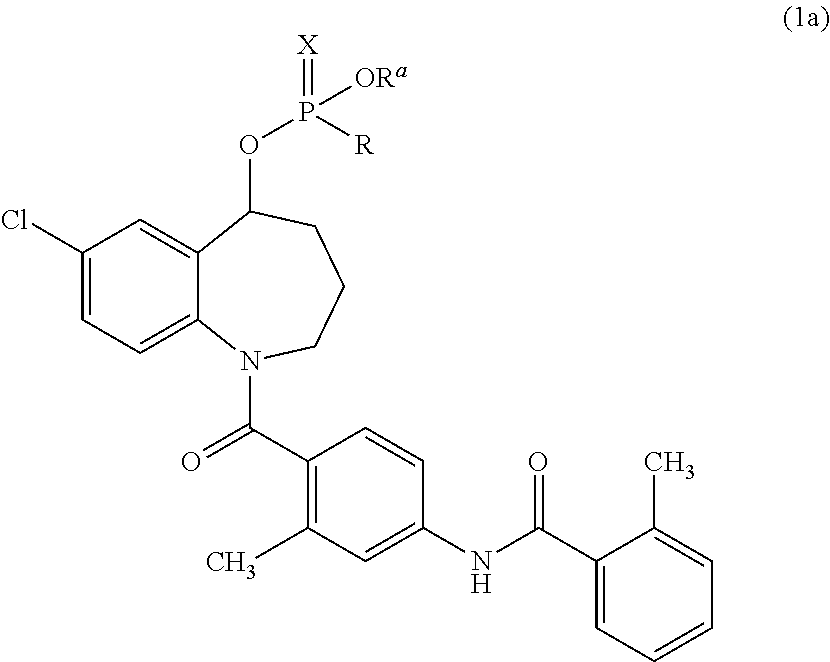
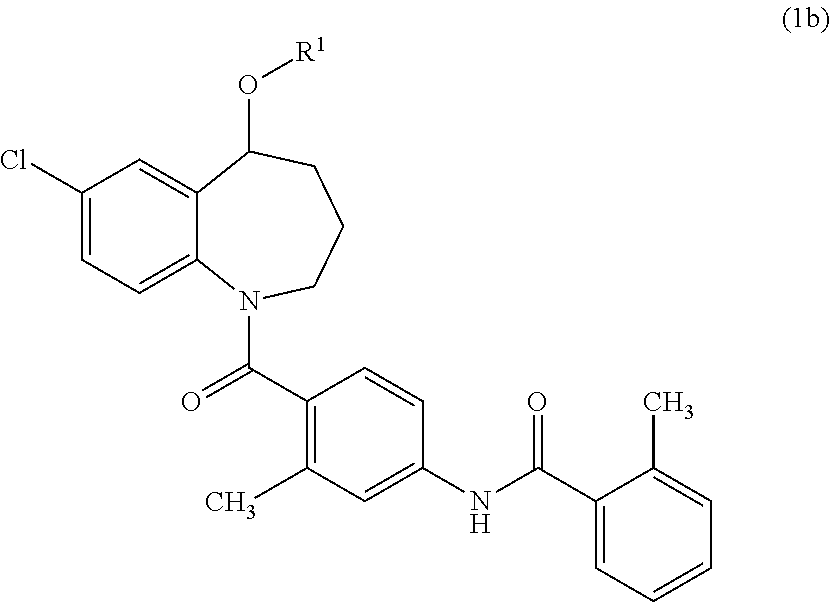
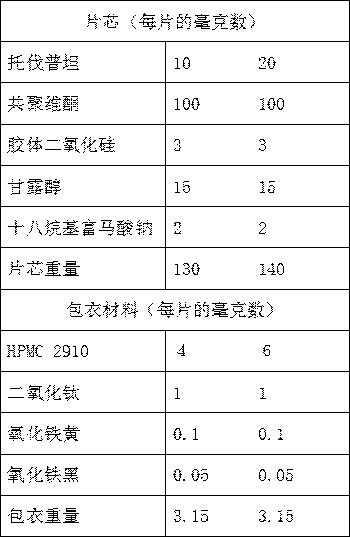
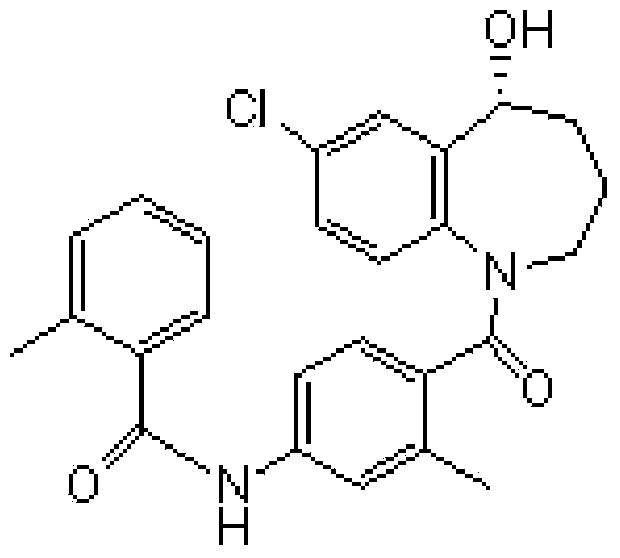
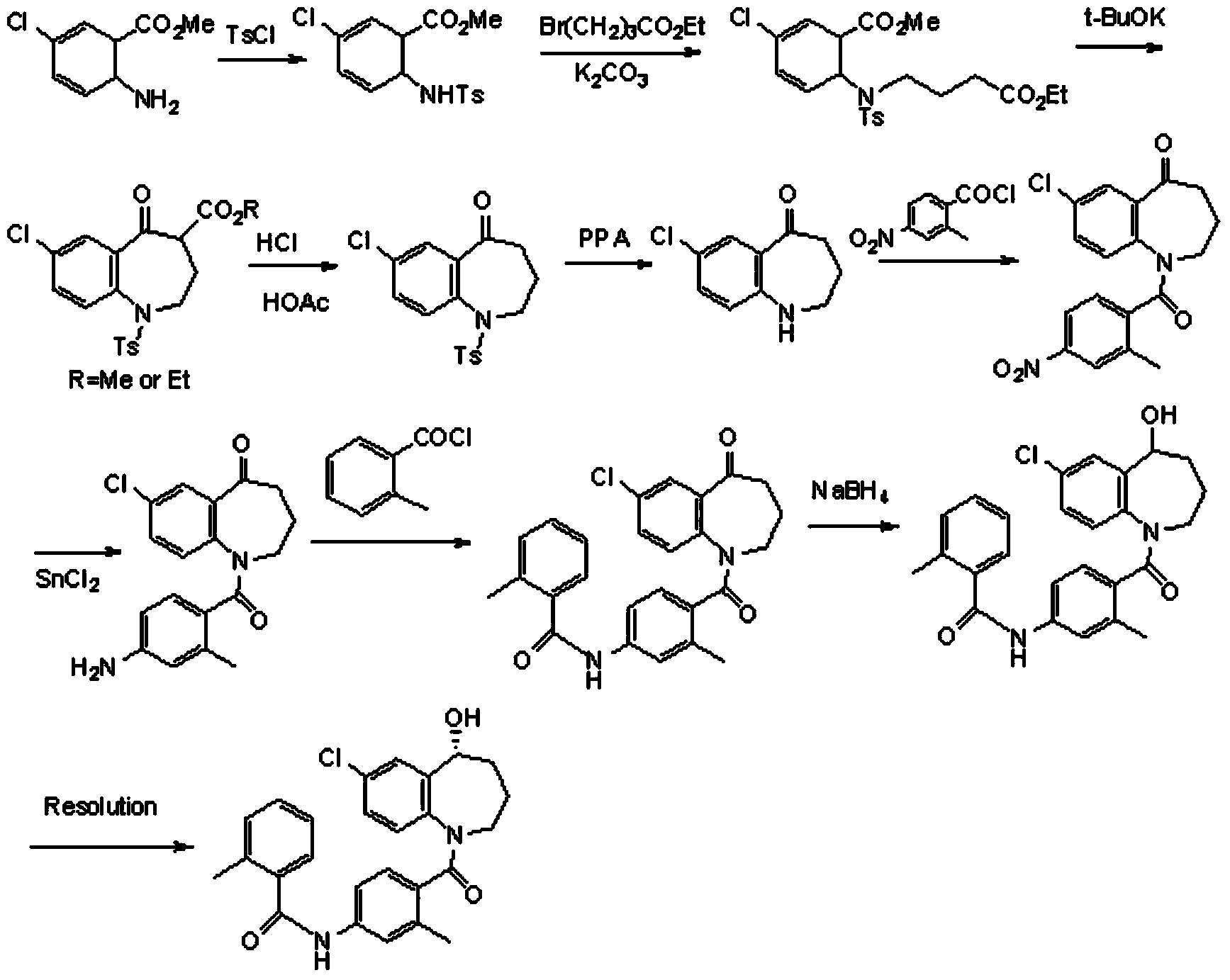
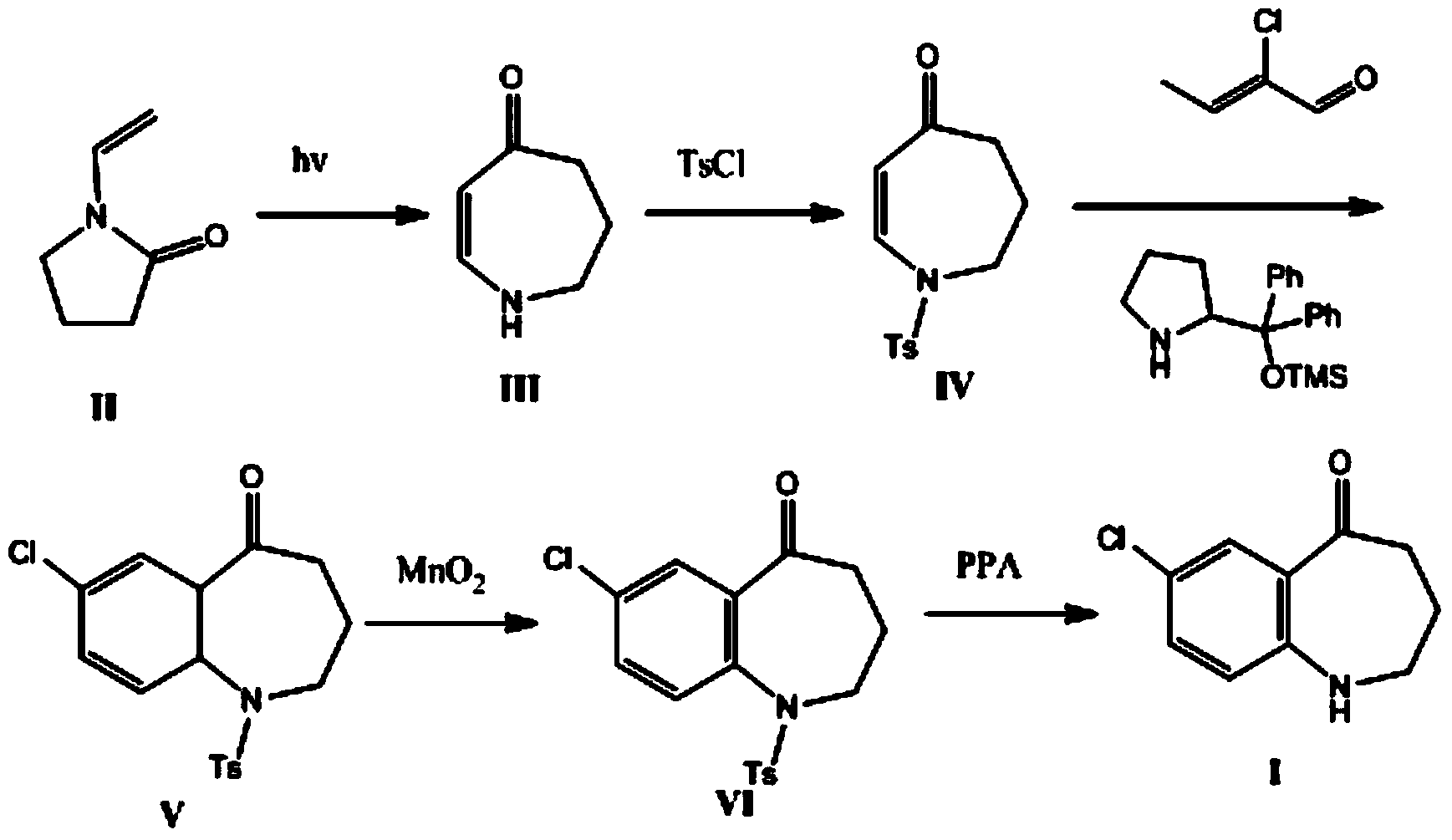
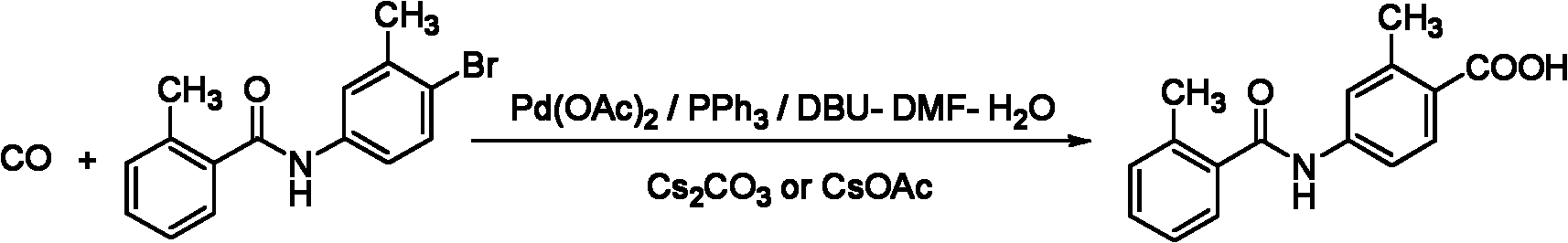Patents
Literature
89 results about "Tolvaptan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
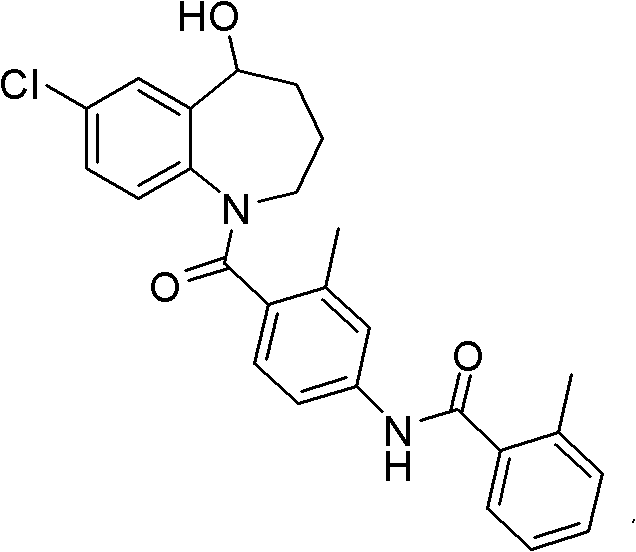
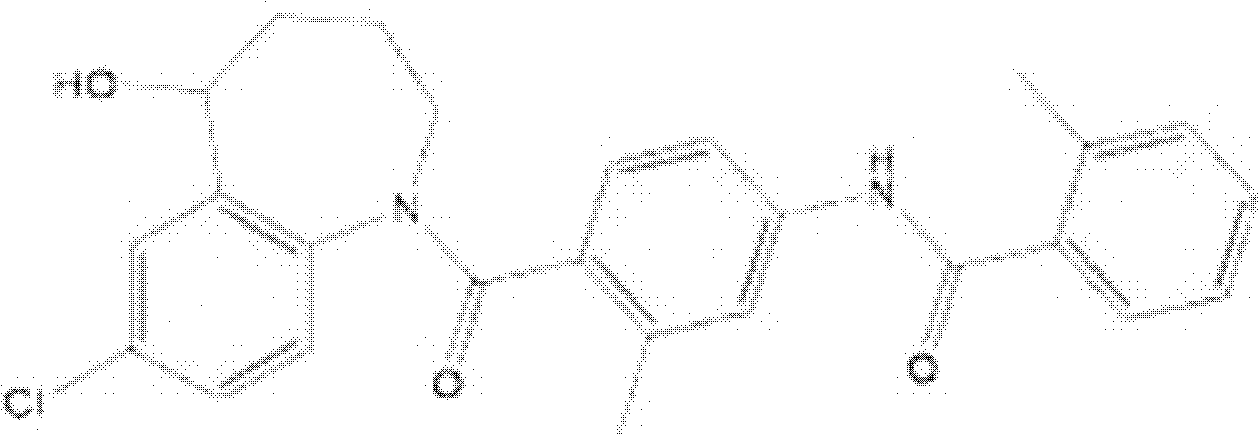
This medication is used by people who have a certain inherited kidney problem (autosomal dominant polycystic kidney disease-ADPKD).
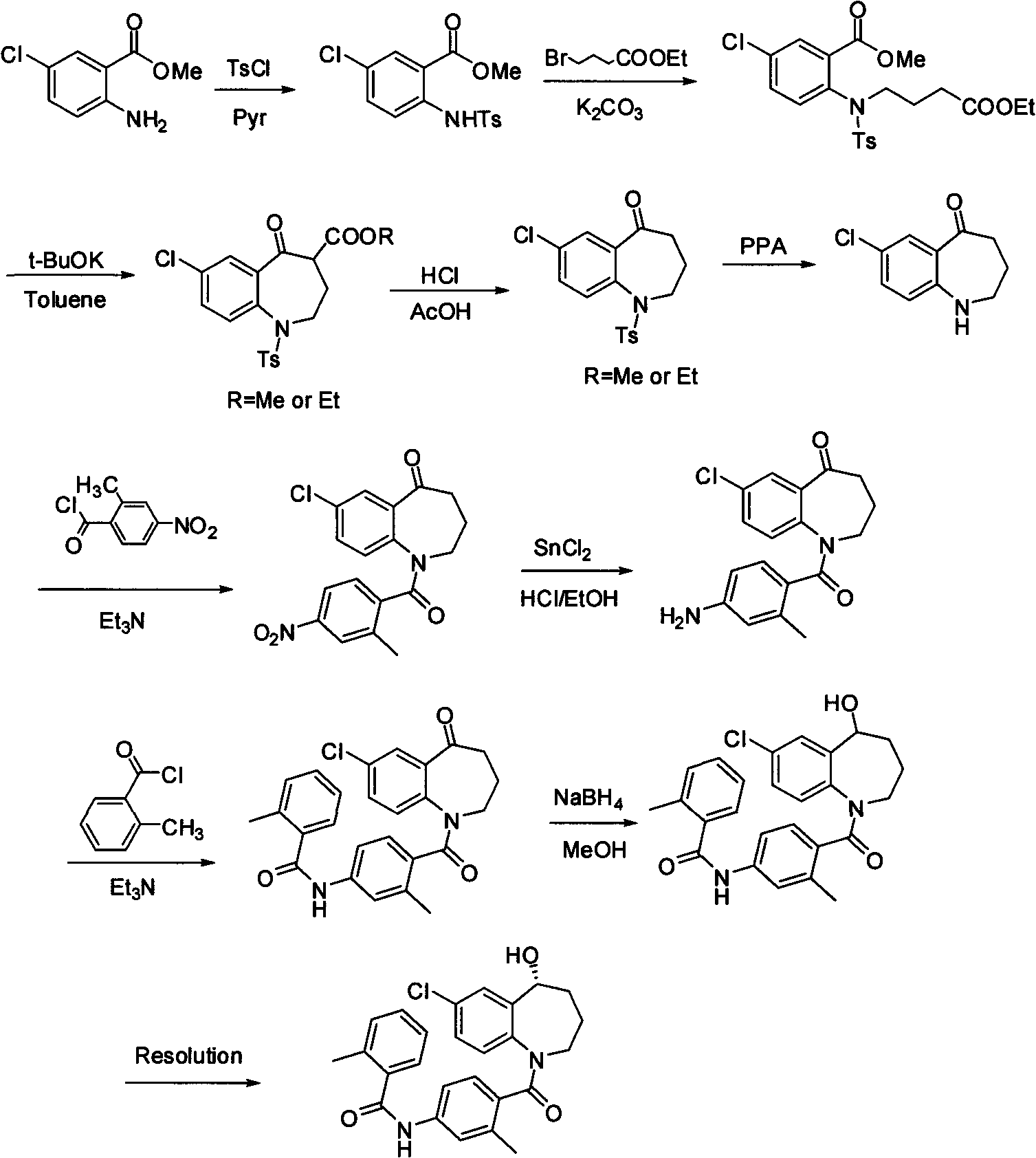
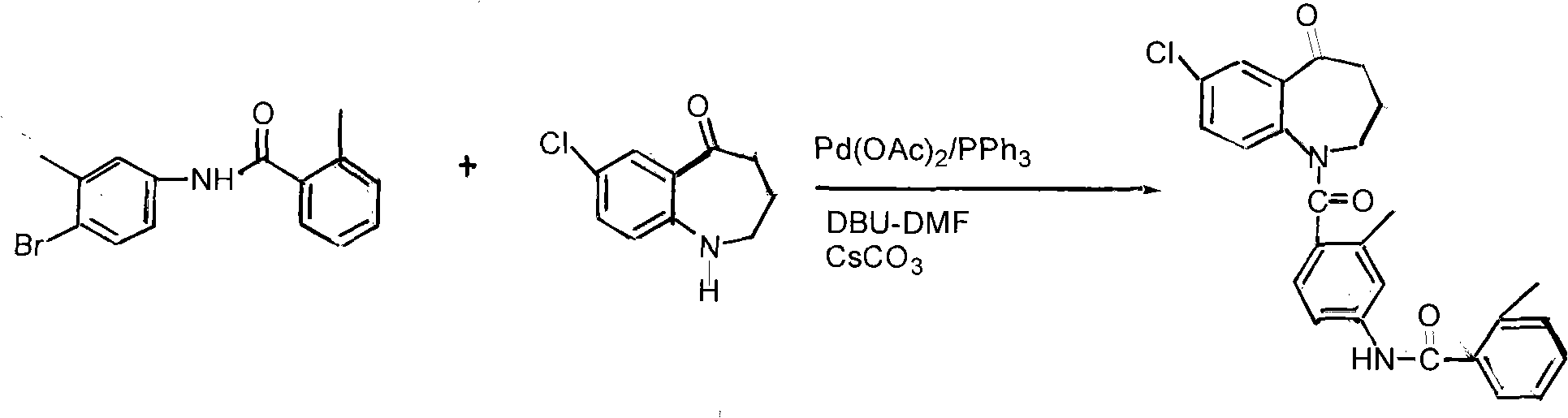
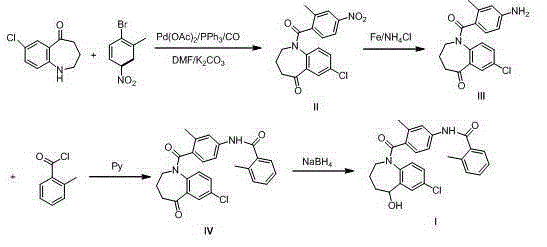
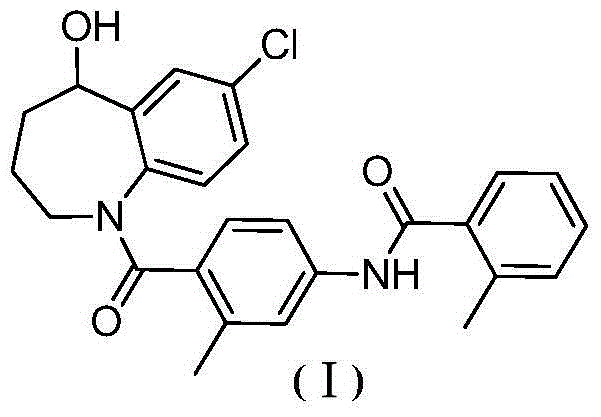
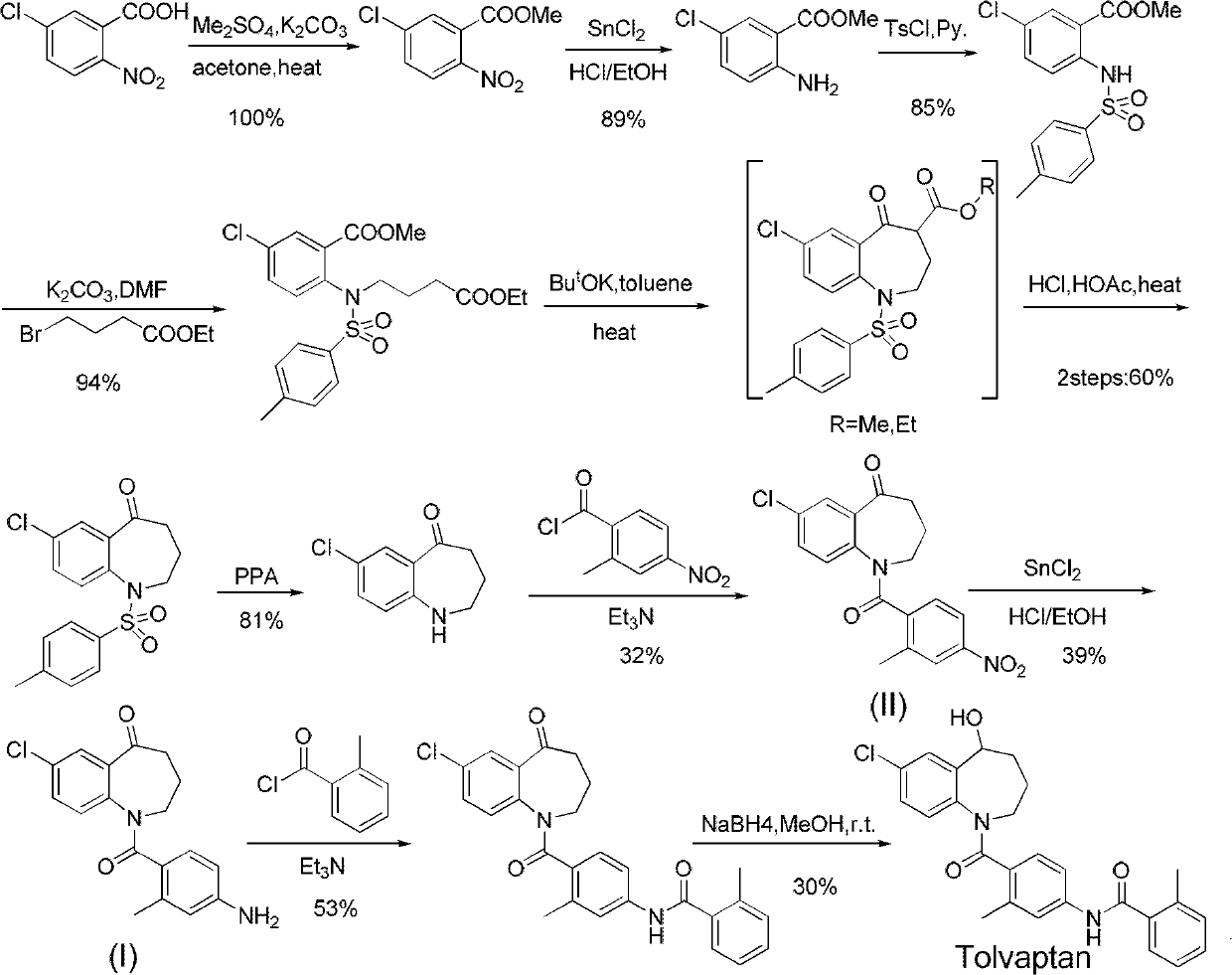
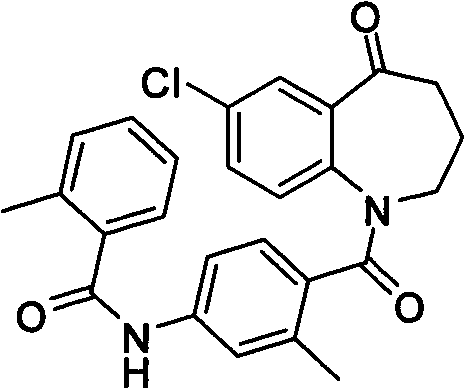
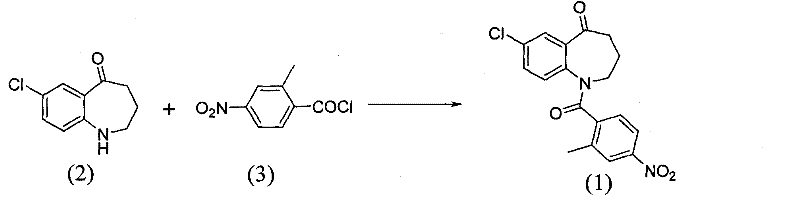
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone
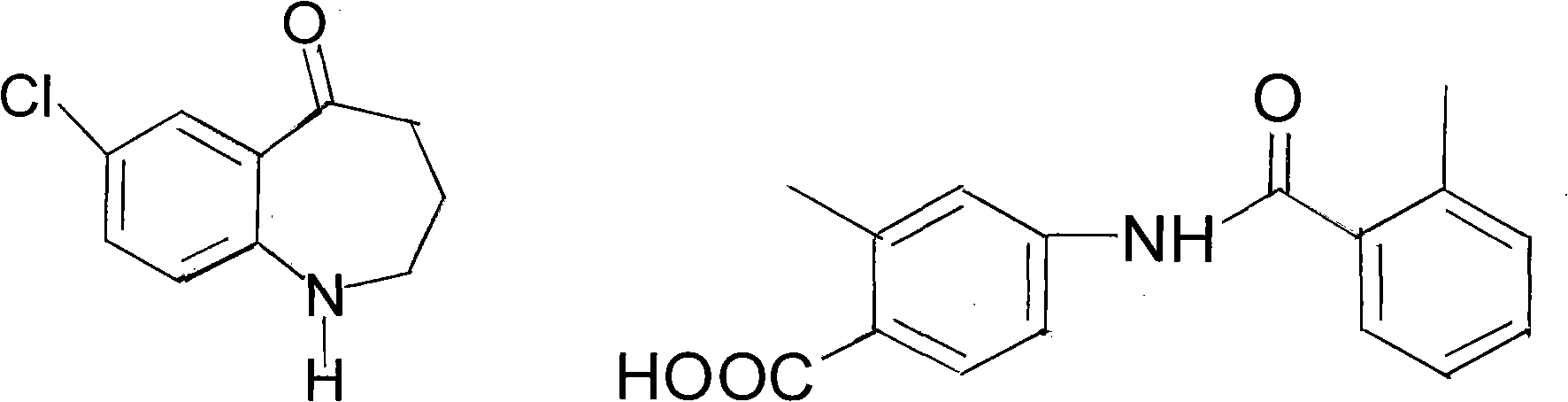
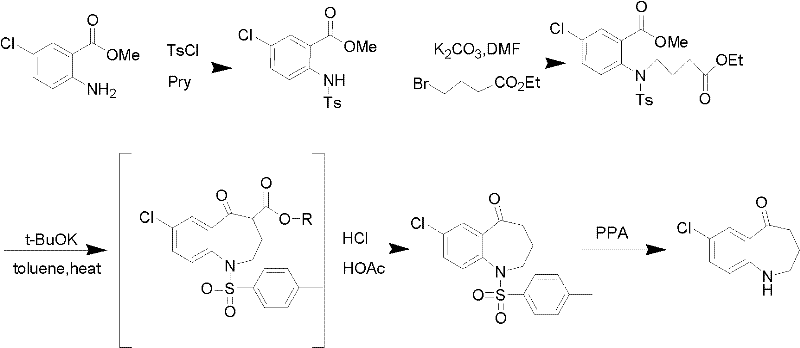
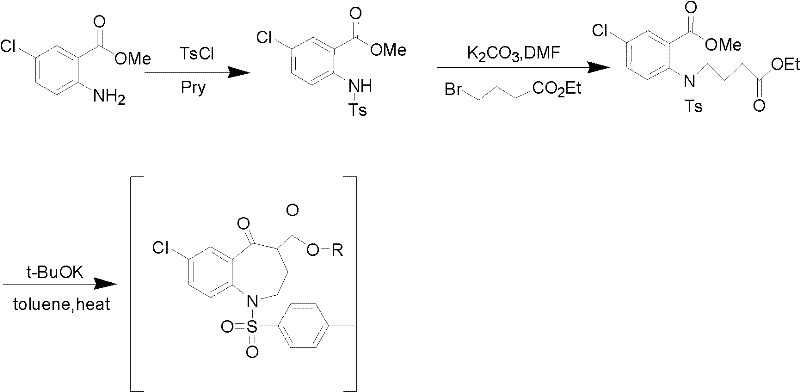
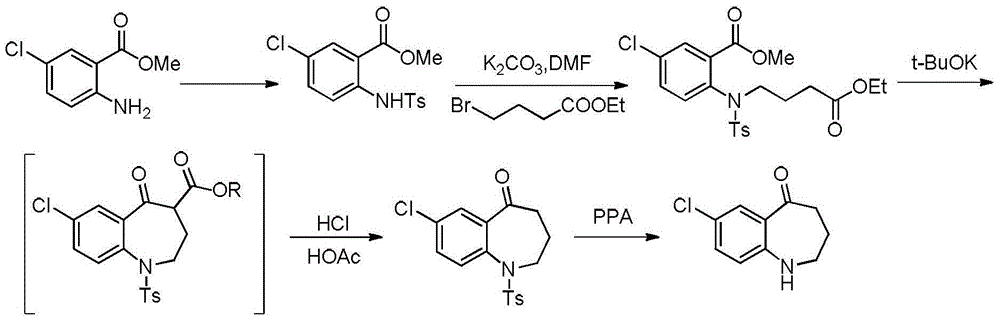
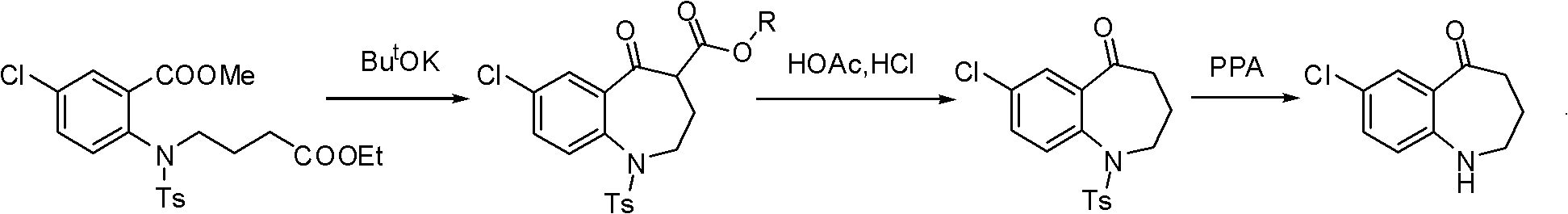
The invention relates to a preparation method of benzazepine compounds, particularly a preparation method of 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone. The 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone can be used as an intermediate for preparing a pitressin antagonist medicament Tolvaptan. The method comprises the following steps: by using parachloroaniline as a raw material, reacting with paratoluensulfonyl chloride in a condensation mode under the alkaline condition to firstly protect amino groups; under the alkaline condition, coupling with ethyl 4-bromobutyrate to obtain a compound; in the presence of alkali, hydrolyzing the obtained compound, and acidifying; in dichloromethane, preparing acyl chloride from the acidified compound under the action of thionyl chloride; under the action of Lewis acid, carrying out Friedel-Crafts acylation reaction to perform intramolecular cyclization; and finally, removing tosyl groups to obtain the target product. The invention has the advantages of simple technique and low cost, and is convenient for industrial large-scale production.
Owner:宁波人健药业集团股份有限公司
Tolvaptan solid preparation
The invention provides a tolvaptan solid preparation. The solid preparation is prepared from tolvaptan amorphous composition which is prepared by dissolving and drying tolvaptan and a carrier, as well as other pharmaceutically acceptable auxiliary materials, wherein the weight ratio of tolvaptan to the carrier is between 1:1.5 and 1:5. The preparation has the advantages of good solubility, good stability and high bioavailability.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for preparing tolvaptan intermediate
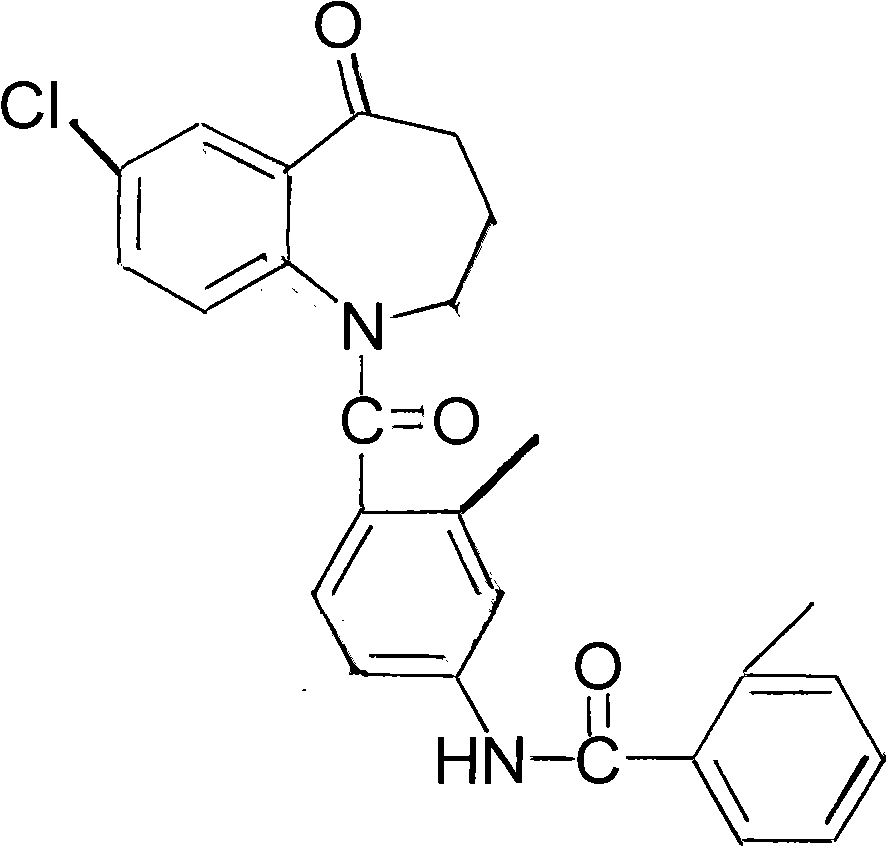
The invention provides a method for preparing tolvaptan intermediate 7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)-benzoyl]2,3,4,5-tetrahydro-1H-1-benzazepine-5-ketone, which comprises the following steps of: reacting a general formula (3) with thionyl chloride to form acetyl chloride solution; and then performing amidation reaction on a general formula (2) and the acetyl chloride solution in an organic solvent in the presence of de-acidifying agent, and treating the reaction product to obtain the tolvaptan intermediate shown in a general formula (1). The tolvaptan intermediate prepared by adopting the method of the invention has the characteristics of low cost, little impurity and high yield, and is more suitable for large-scale industrialized production.
Owner:天津泰普制药有限公司
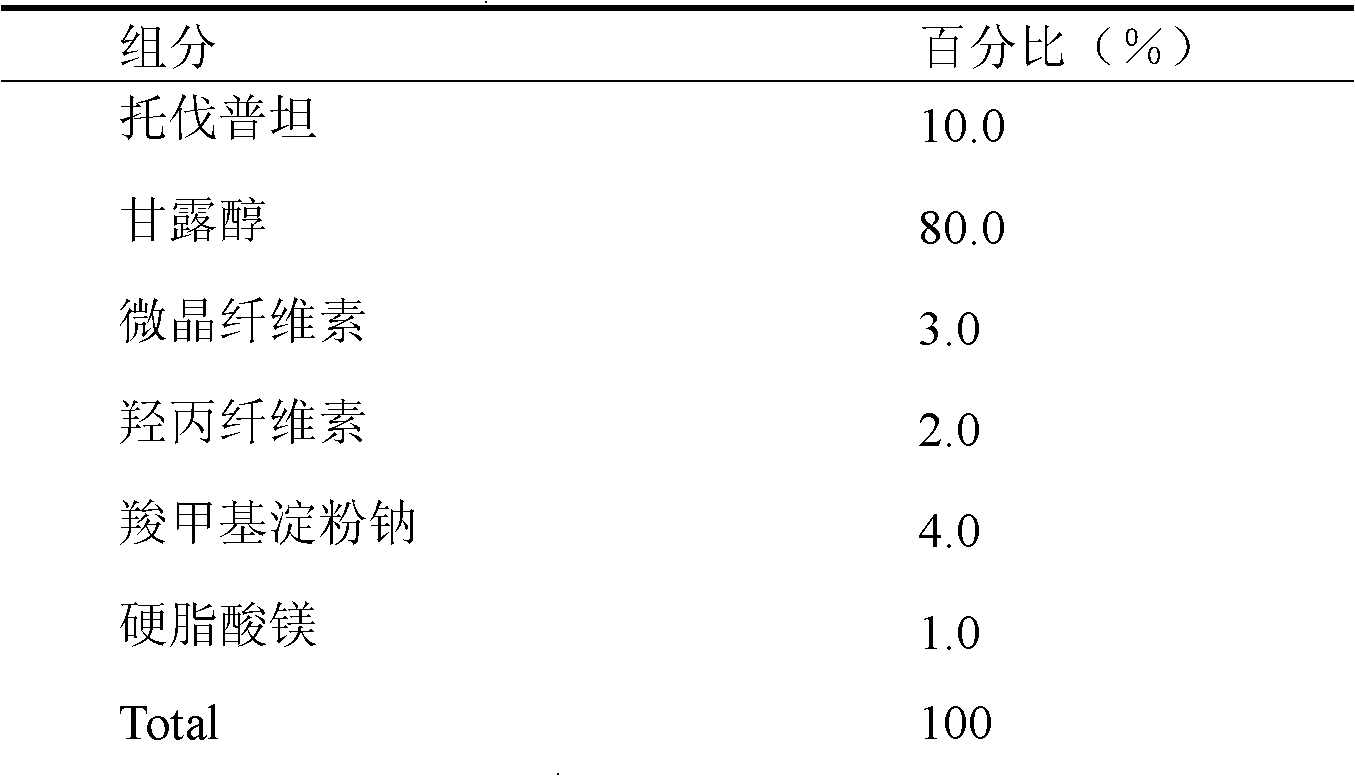
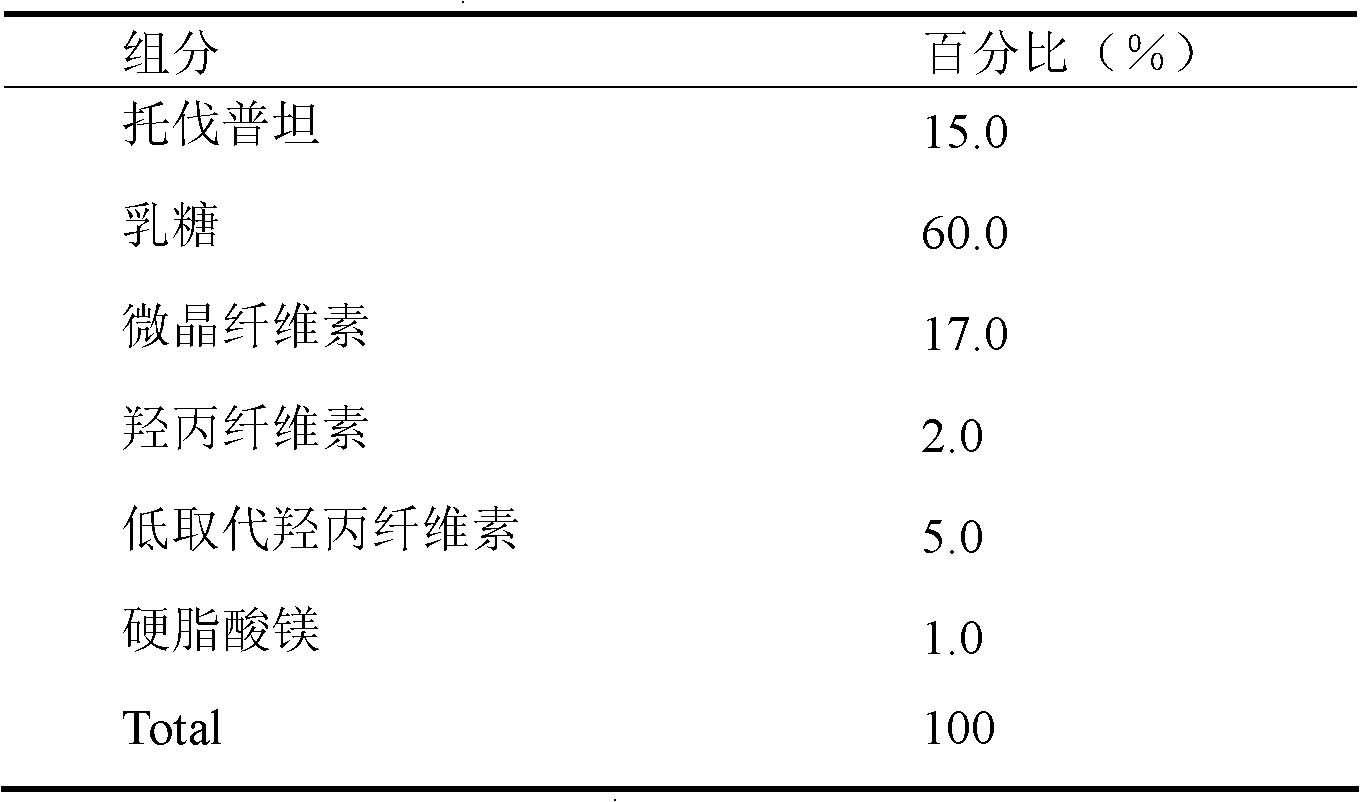
Tolvaptan medicinal composition and preparation method thereof
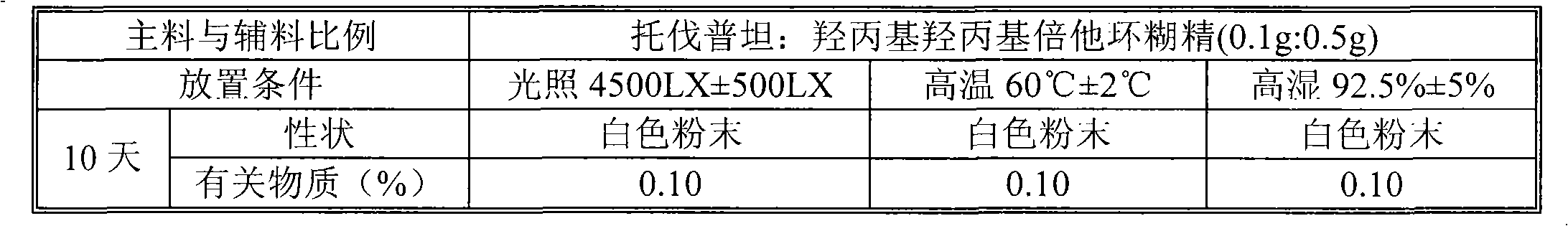
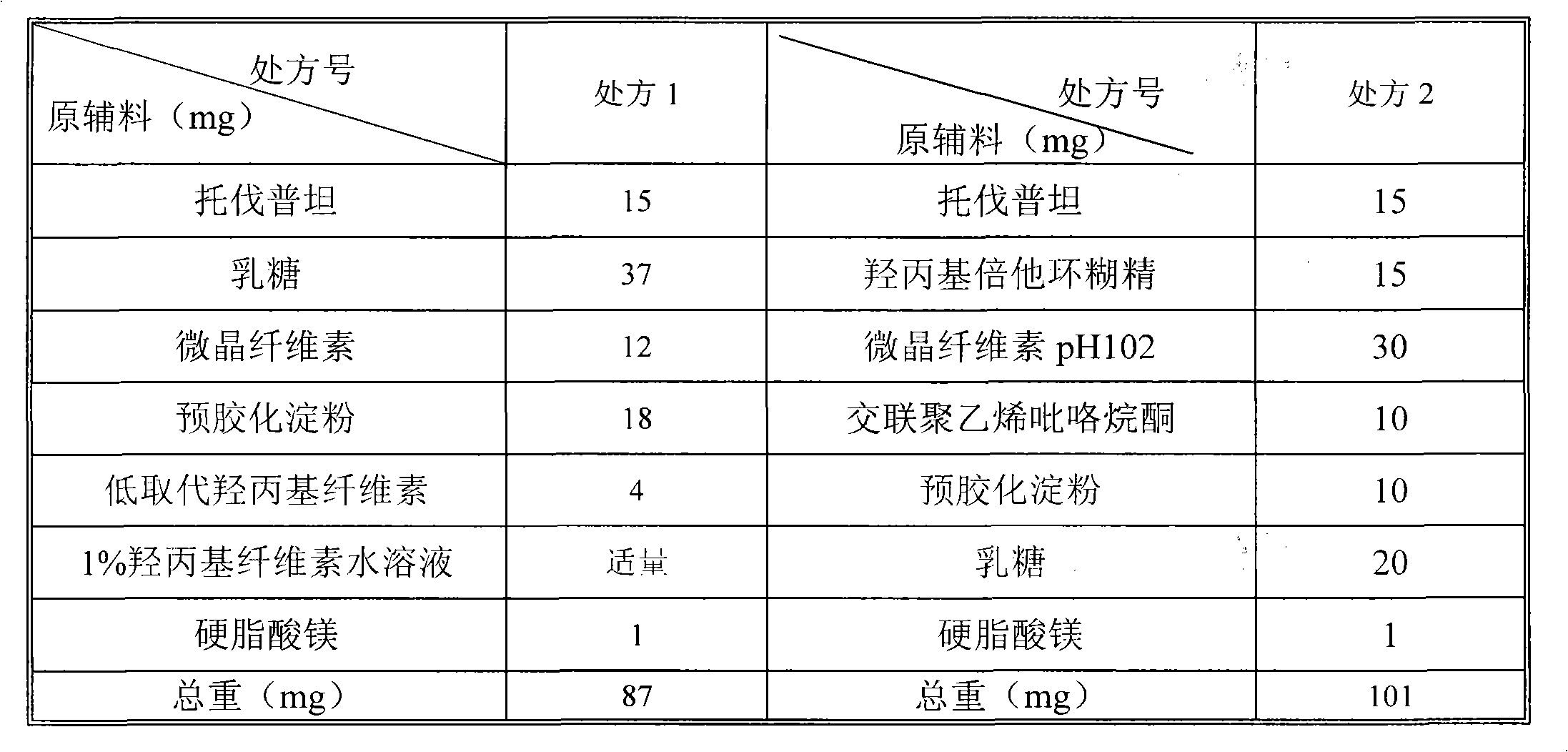
The invention relates to a tolvaptan medicinal composition and a preparation method thereof. 1,000 tablets of the composition consist of 15 to 60 grams of tolvaptan, 15 to 60 grams of hydroxypropyl-beta-cyclodextrin, 30 to 120 grams of microcrystalline cellulose with pH of 102, 10 to 40 grams of cross-linked polyvinylpyrrolidone, 10 to 40 grams of pregelatinized starch, 20 to 80 grams of lactose and 1 to 10 grams of magnesium stearate. In the preparation method, the conventional preparation technology is adopted after the tolvaptan and the hydroxypropyl-beta-cyclodextrin are subjected to special processing. The prepared tolvaptan medicinal composition has the advantages of high fluidity, high dissolution, small tablet weight variation, high stability, high biological utilization degree and good therapeutic effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
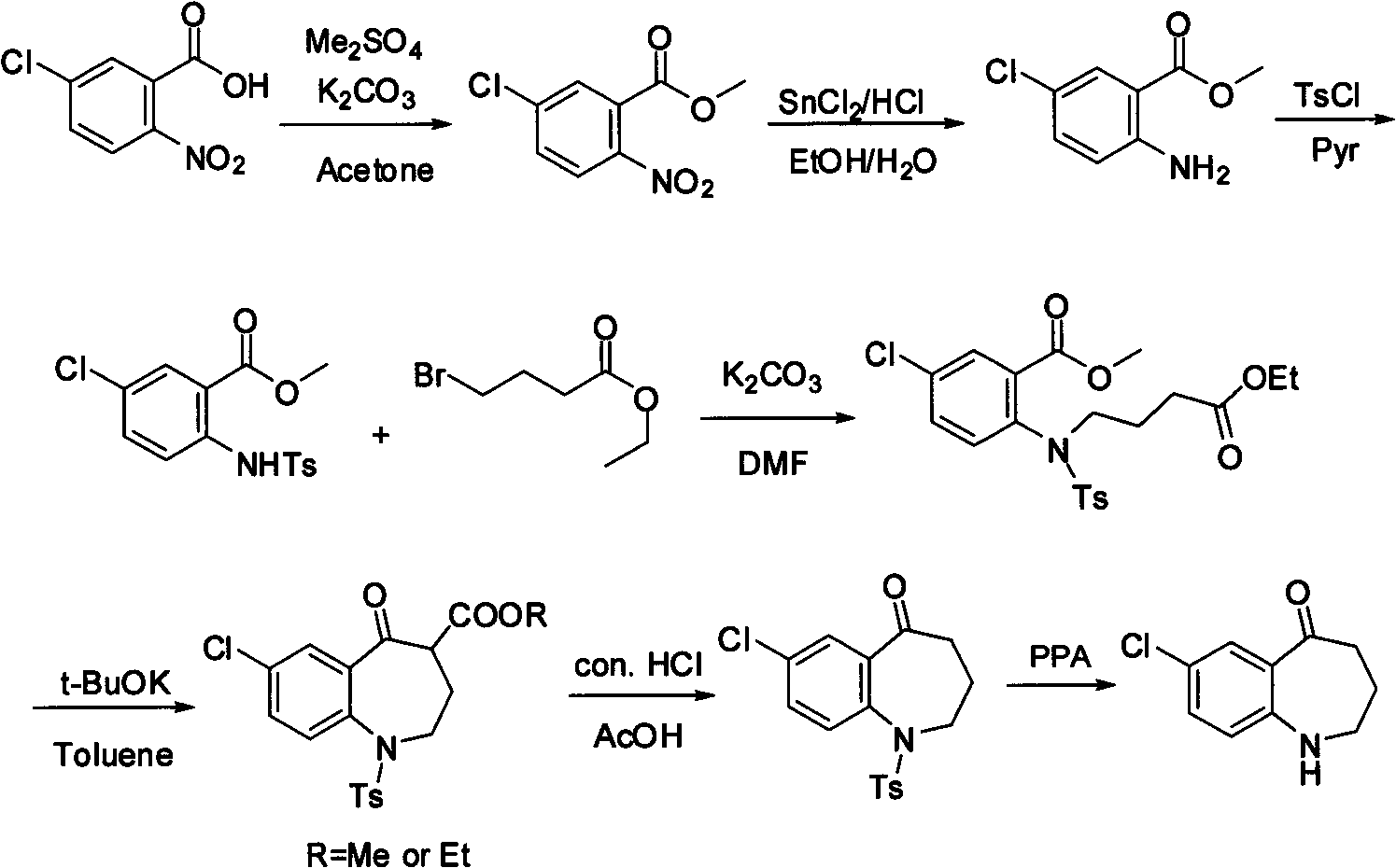
Preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine
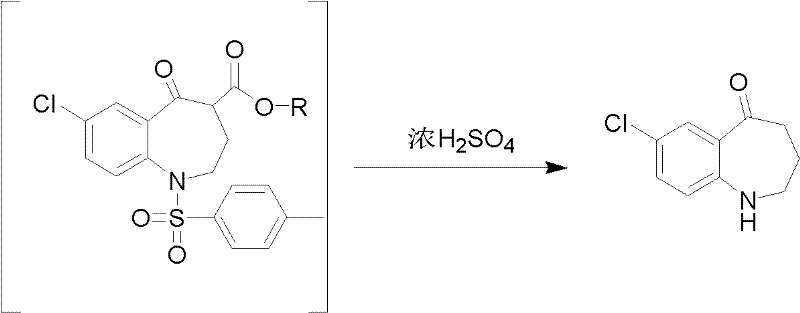
The invention relates to a preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine. The 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine is an important intermediate for preparing arginine pitressin V2 receptor antagonist Tolvaptan. In the preparation method, the target product 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine is prepared by removing tosyl and carbalkoxy from 7-chlorine-5-oxo-4-carbalkoxy-1-tosyl-2,3,4,5-tetrahydro-1-benzoazepine serving as a raw material under the action of sulfuric acid and by a 'one-pot method'. The sulfuric acid is used for performing 'one-pot method' reaction, so reaction steps are reduced and reaction time is greatly shortened. The preparation method has the advantages of simpleness and convenience for operation, equipment and cost saving, simpleness and practicability in separation and purification, short reaction time, high yield and high product purity and is applicable to industrialized production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Tolvaptan oral solid medicinal composition and preparation method thereof
ActiveCN102228423AGood disintegrationShort disintegration timeMetabolism disorderPill deliveryAdhesiveMedicine
The invention provides a tolvaptan oral solid medicinal composition. The tolvaptan oral solid medicinal composition is prepared from the following components in percentage by weight: 3 to 50 percent of tolvaptan, 1 to 10 percent of adhesive, 20 to 80 percent of hydrophilic carrier, 0 to 20 percent of disintegrant, 5 to 60 percent of filler, 0 to 5 percent of flow aid, and 0.1 to 3.0 percent of lubricant. The tolvaptan medicinal composition is an oral solid preparation and is preferably tablets and capsules. The invention also provides a method for preparing the medicinal composition. The tolvaptan oral solid medicinal composition has good disintegration performance and dissolution performance; the composition has lower impurity content and is steadier; the preparation process is simple and easy; and the method is suitable for industrialized mass production.
Owner:福安药业集团庆余堂制药有限公司
Preparation method of tolvaptan
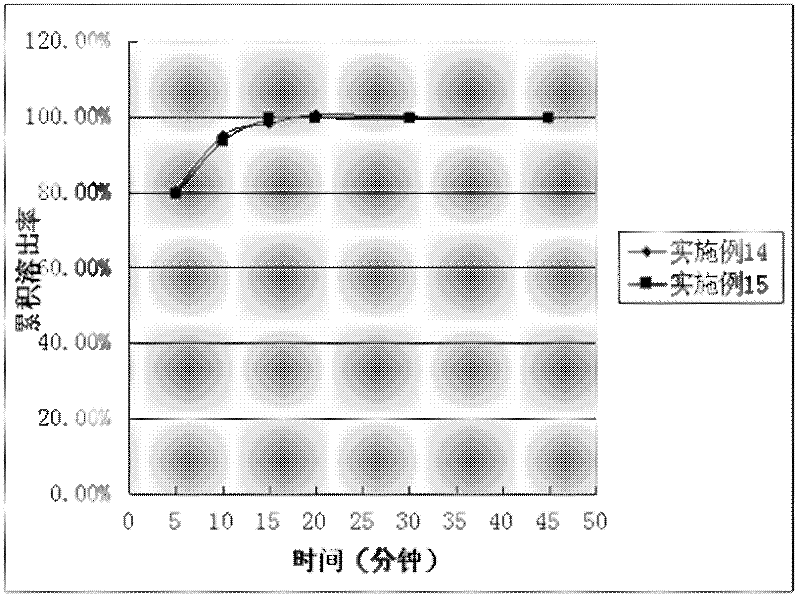
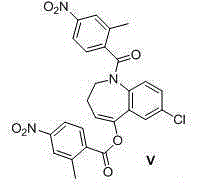
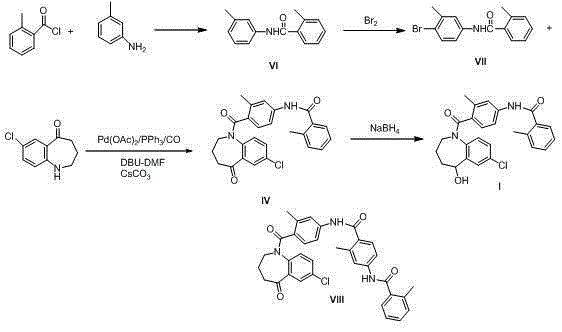
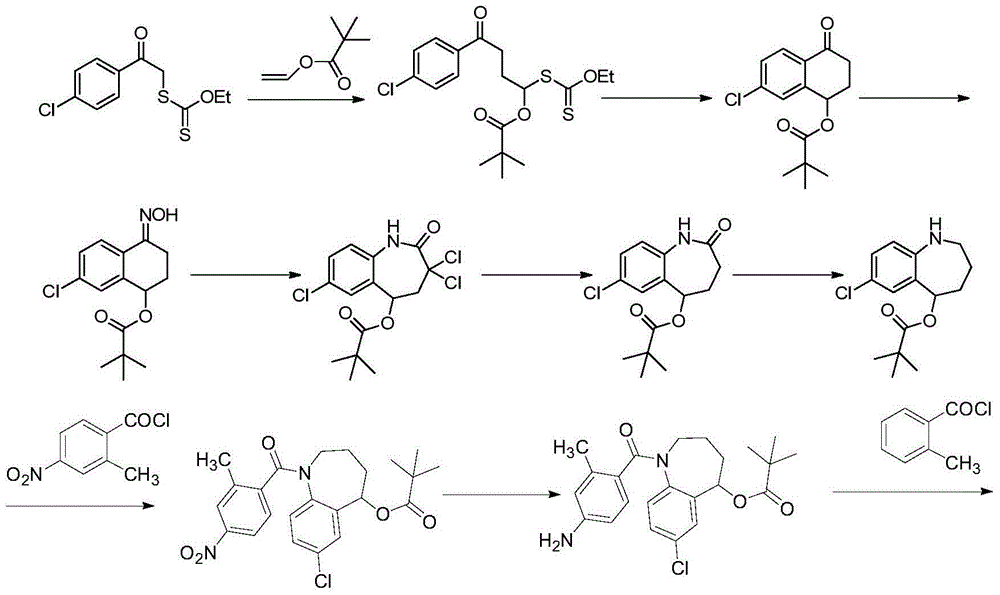
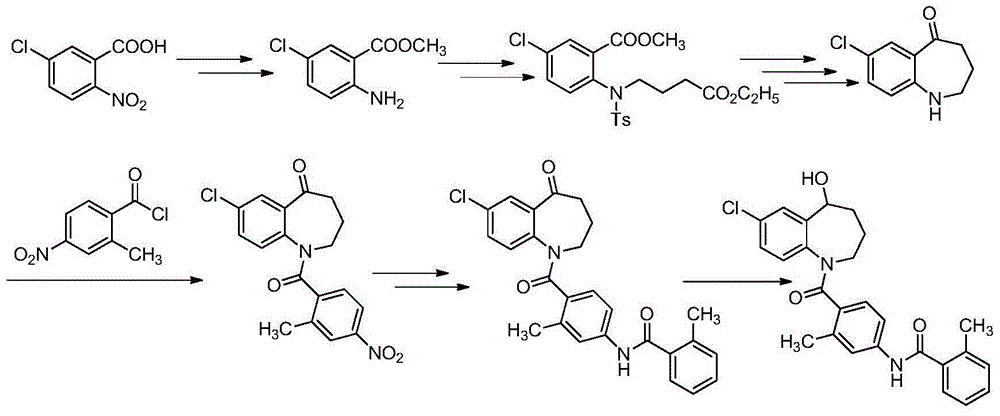
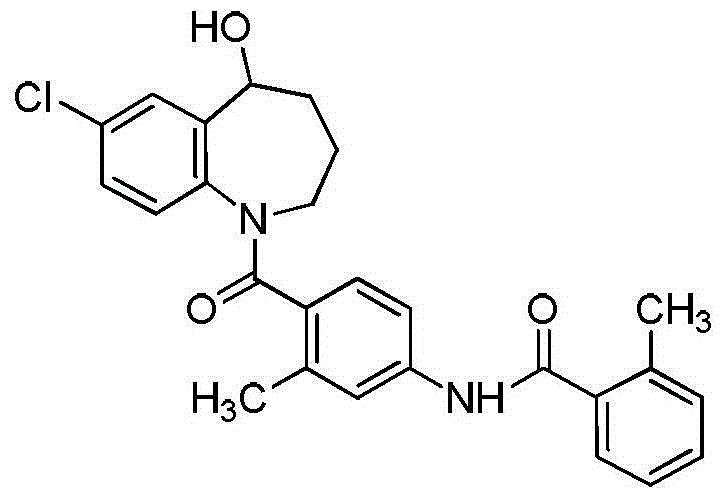
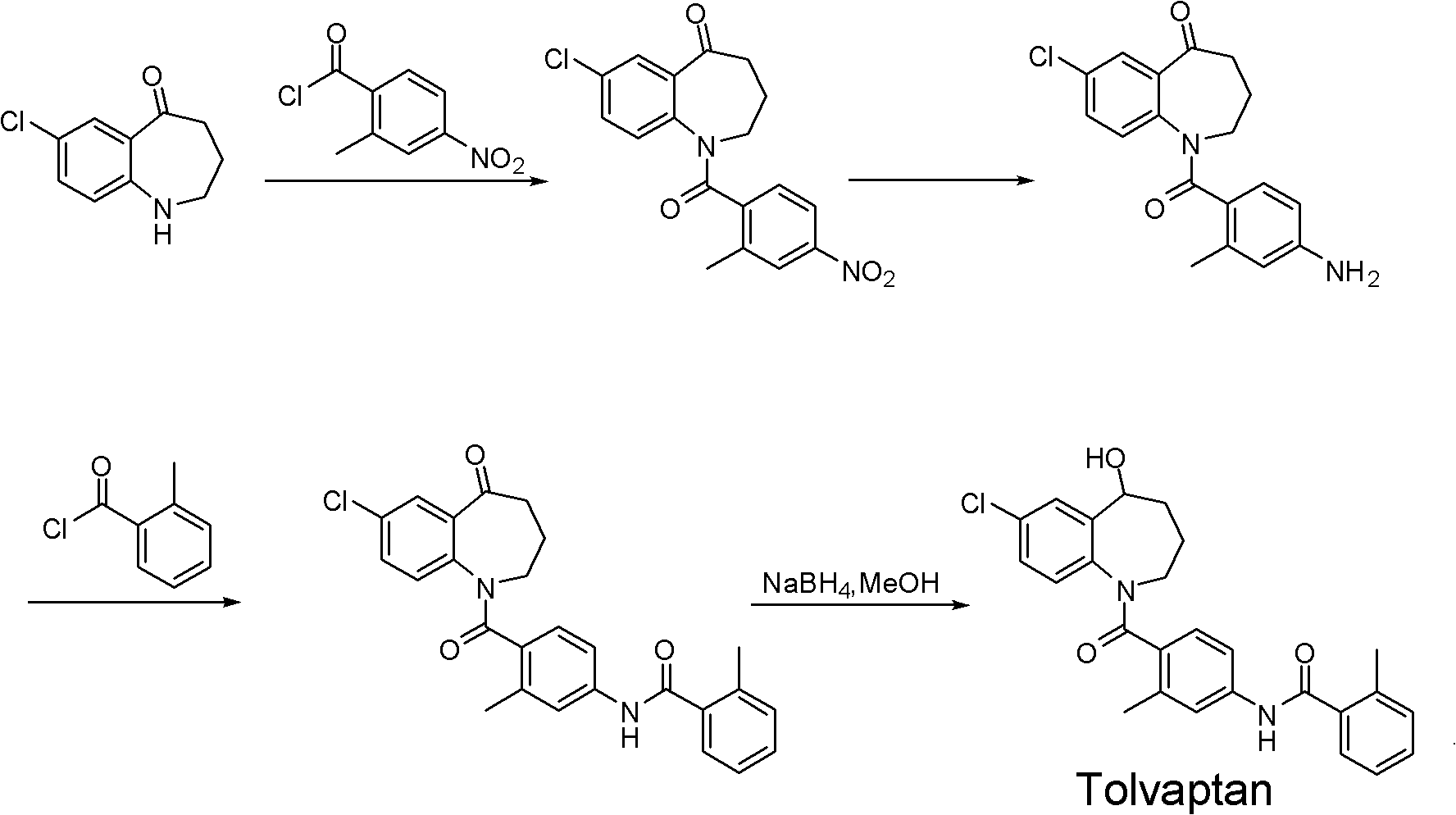
The invention discloses a preparation method of tolvaptan. According to the preparation method, 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one and 4-nitro-2-methyl bromobenzene are taken as the primary raw materials; high purity tolvaptan is obtained after steps of carbonyl inserting reactions, reduction reactions, and acylation reactions, and the yield is high. The preparation method has the advantages that no bromine or tin dichloride is used; the preparation method does not generate a large amount of industrial waste water, and the environment is protected. At the same time, the generation of impurities namely a compound V and a compound VIII is avoided, and the purification becomes easier. No explosive, flammable, and toxic solvent such as chloroform, ether, and the like, is used, the requirements on the protection of workers are lowered, and the safe production is guaranteed. Moreover, the route design is novel, the raw materials are easily available, the operation of the technology is simple and feasible, and a simple and feasible method is provided for the massive industrial production of tolvaptan.
Owner:天津泰普制药有限公司
Preparation method of tolvaptan
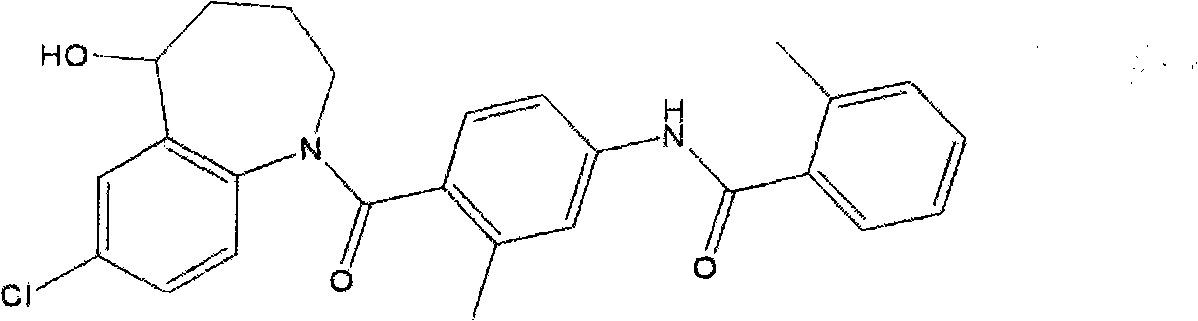
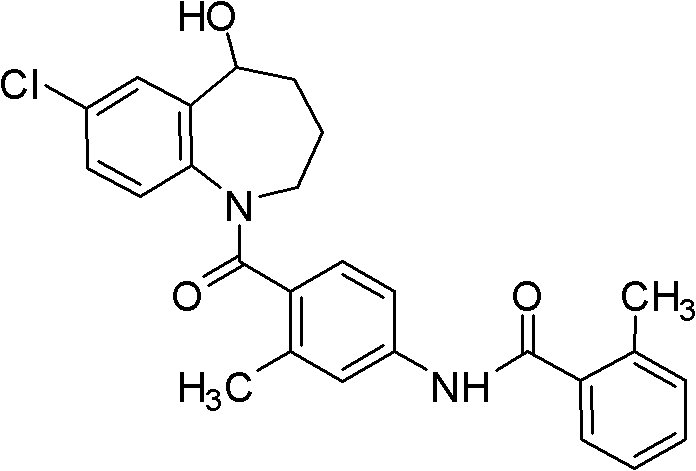
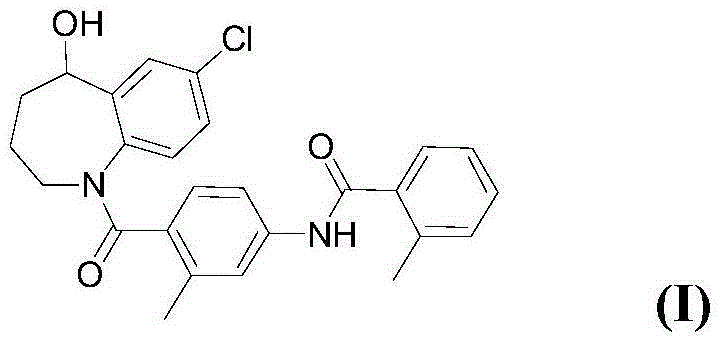
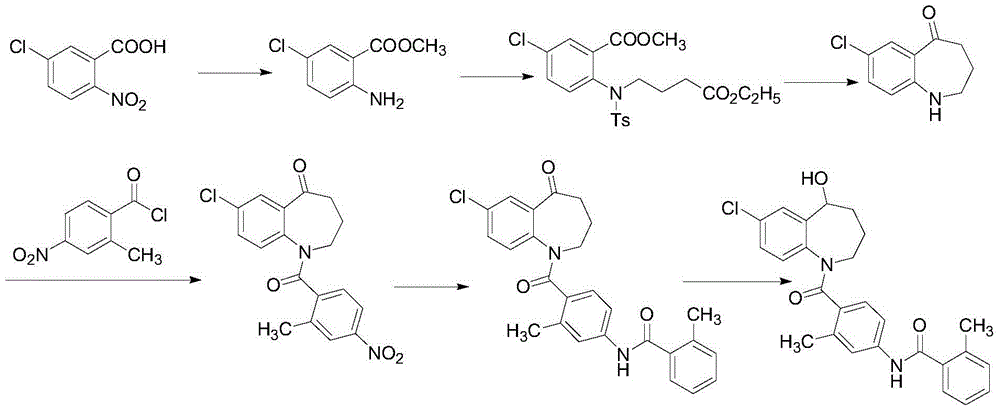
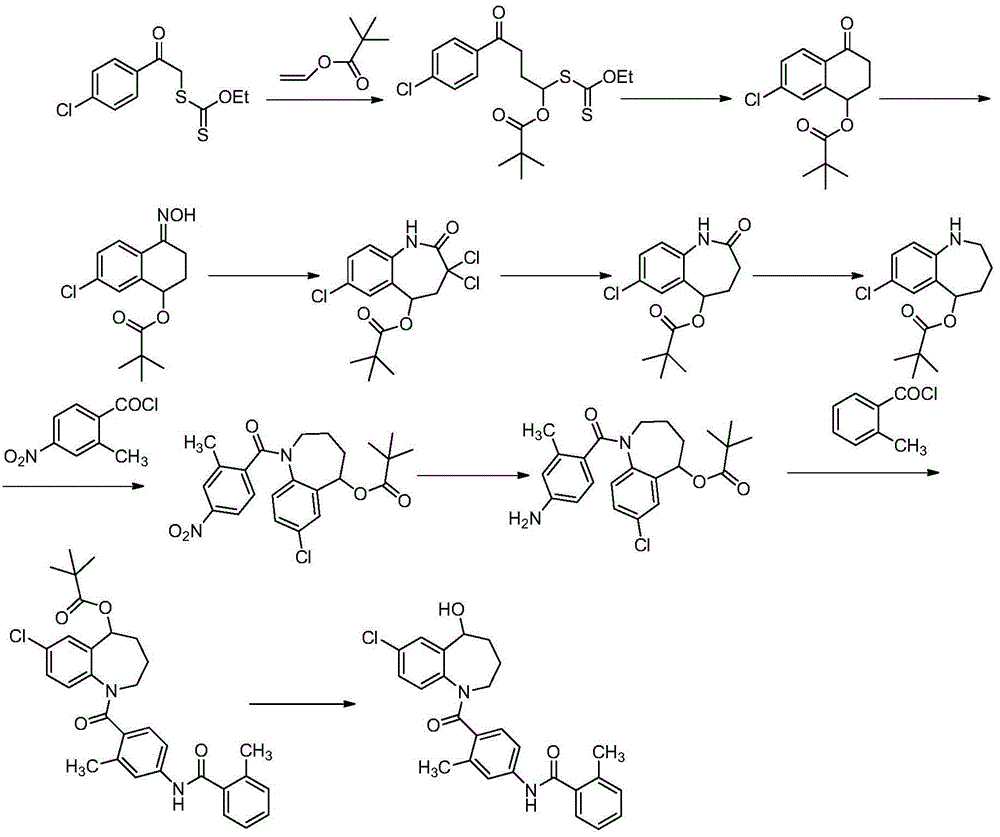
The invention discloses a preparation method of tolvaptan. The method is characterized in that tolvaptan is obtained through an amidation reaction of 7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzoaza and 2-methyl-4-(2-methylphenylamido)benzoyl chloride. The method has the advantages of novel design, work time efficiency increase, reduction of waste liquid discharge, simple operation, easily available raw materials, simple post-treatment and high yield, and is suitable for industrial production.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Preparation method for cardiovascular disease treatment drug
ActiveCN105315169AOrganic compound preparationCarboxylic acid amides preparationTolvaptanRaw material
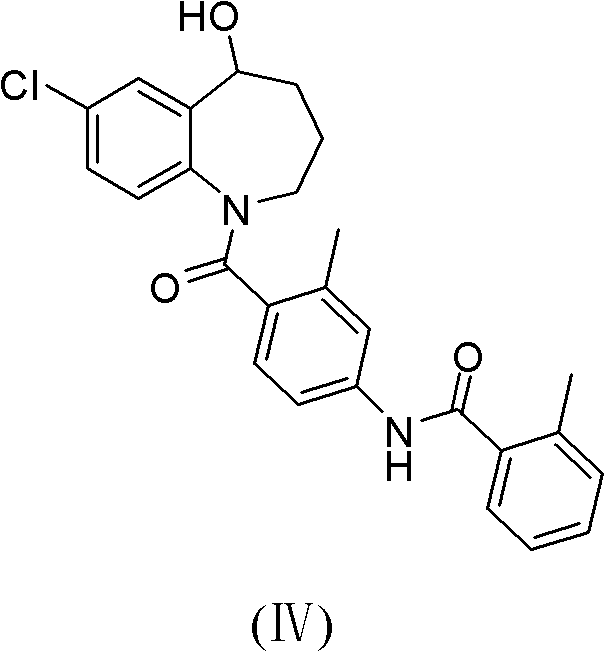
The invention provides a preparation method for a cardiovascular disease treatment drug. Specifically, the invention provides a compound represented by a formula (IV) shown in the description and a method for preparing Tolvaptan from the compound represented by the formula (IV). The method provided by the invention has the advantages of being environment-friendly, being easy in raw material obtaining and high in total yield, and the like, thereby being applicable to the industrialized preparation of Tolvaptan.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Drug for preventing and/or treating polycystic kidney disease
ActiveUS20150141338A1Significant effectHigh therapeutic effectPeptide/protein ingredientsPharmaceutical delivery mechanismTherapeutic effectPolycystic liver disease
An object of the present invention is to provide a combination drug that has remarkably excellent preventive and / or therapeutic effects on polycystic kidney disease. The present invention provides a drug for preventing and / or treating polycystic kidney disease comprising a combination of tolvaptan or a prodrug thereof with a somatostatin derivative, and a method for treating polycystic kidney disease using this drug.
Owner:OTSUKA PHARM CO LTD
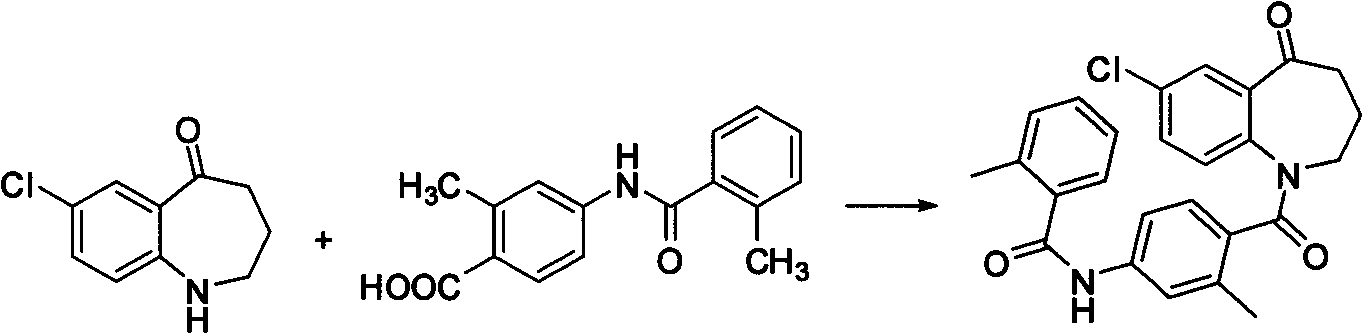
Tolvaptan intermediate and preparation method thereof
ActiveCN103373960AMild responseSimple and fast operationOrganic chemistryCombinatorial chemistryTolvaptan
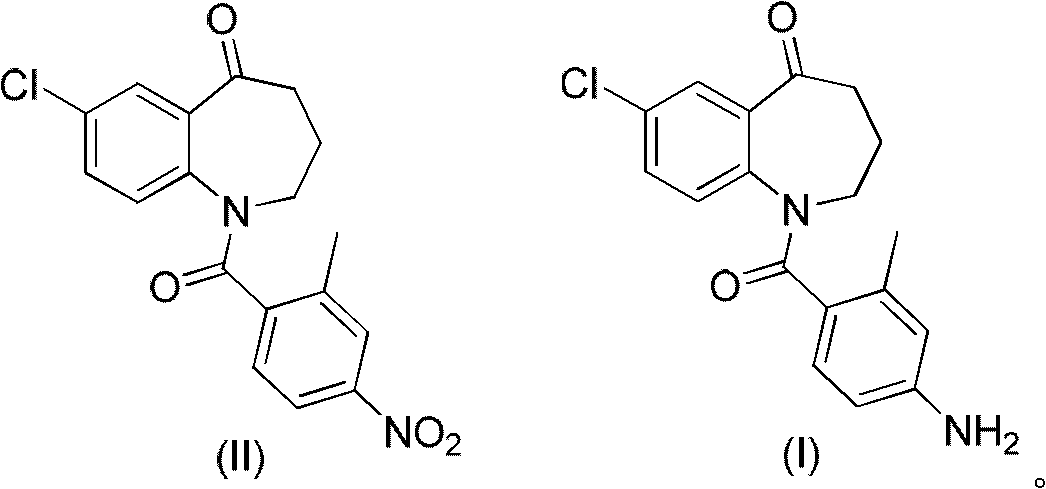
The invention relates to the field of pharmaceutical chemistry and particularly discloses preparation methods for a tolvaptan intermediate 1-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine (I) (shown in the description) and salts thereof. The tolvaptan intermediate prepared by utilizing the method disclosed by the invention has the characteristics of low cost, high yield, environmental friendliness, strong operability and suitability for industrial production.
Owner:JIANGSU KANION PHARMA CO LTD +1
Preparation method of high-purity tolvaptan
The invention discloses a preparation method of high-purity tolvaptan. The preparation method comprises the step of reducing N-[4-[(5R)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1-benazepine-1-formyl]-3-methyl phenyl]-2-methyl benzamide with sodium bis(2-methoxyethoxy) aluminumhydride, thereby obtaining the high-purity tolvaptan with the purity higher than or equal to 99.95%. The preparation method disclosed by the invention has the advantages that sodium bis(2-methoxyethoxy) aluminumhydride is adopted as a reducing agent for preparing tolvaptan by reducing N-[4-[(5R)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1-benazepine-1-formyl]-3-methyl phenyl]-2-methyl benzamide, the production of a dechlorination impurity IV can be extremely effectively inhibited, and finally the high-purity tolvaptan with the purity higher than or equal to 99.95% can be obtained, and high yield being 90% or above can be obtained while tetrahydrofuran or methyltetrahydrofuran is adopted as a reaction solvent.
Owner:CHANGZHOU SUNLIGHT PHARMA
Preparation method of tolvaptan tablet
ActiveCN102366412AImprove bioavailabilityImprove in vitro dissolutionDigestive systemPharmaceutical non-active ingredientsMagnesium stearateStearic acid
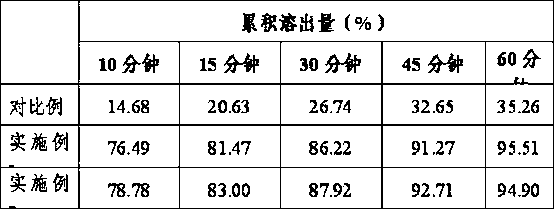
The invention discloses a preparation method of a tolvaptan tablet. The preparation method comprises the following steps: 1, mixing tolvaptan with high-substituted hydroxylpropyl cellulose according to a mass ratio of 1:0.2-0.6, crushing them, and sieving by a 60-100 mesh sieve; 2, dissolving throughs in a mixed solution of waterless ethanol and dichloromethane with a volume ratio of 1:1-4, carrying out spray drying to form powder, carrying out reduced pressure drying at 70-90DEG C until that the content of solvents residual in the sprayed powder is qualified; 3, crushing the sprayed powder, sieving by a 180-220 mesh sieve, accurately weighing the sprayed powder, lactose, microcrystalline cellulose and hydroxypropyl methylcellulose according to prescription amounts, uniformly mixing them, and adding a proper amount of water to prepare a soft material; and 4, sieving the soft material by a 15-25 mesh sieve, preparing a wet particle, drying the wet particle at 50-70DEG C until that the content of water in the wet particle is 2-4%, sieving the dried wet particle by a 15-25 mesh sieve, granulating, adding a prescription amount of magnesium stearate, uniformly mixing, and tabletting. The preparation method of the invention has the advantages of ingenious conception, low production cost, high in vitro dissolubility of the prepared tolvaptan tablet, and good biological availability and clinic curative effect of the tolvaptan tablet.
Owner:SICHUAN BAILI PHARM CO LTD
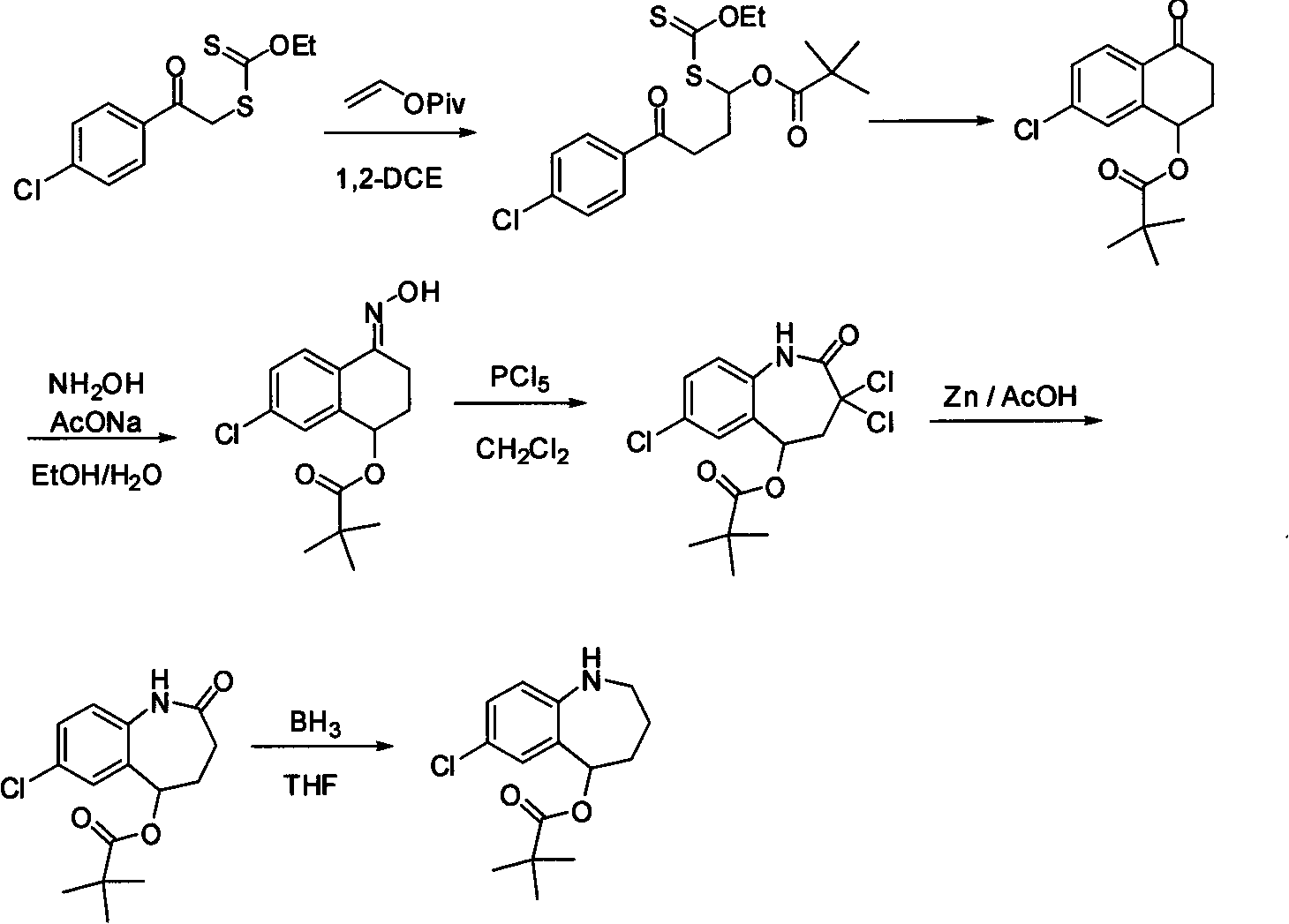
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzoazepine-2,5-diketone
The invention relates to a method for preparing a benzoazepine compound, in particular to a novel method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzoazepine-2,5-diketone. The compound can be used as an intermediate for preparing pitressin antagonist medicament Tolvaptan. By using 4-chloroaniline as a raw material, the method comprises the following steps of: (1) reacting 4-chloroaniline with succinic anhydride in a solvent environment to obtain 4-(4- chlorphenyl amino)-4-oxo-butyric acid; (2) protecting the amino of the compound 4-(4- chlorphenyl amino)-4-oxo-butyric acid by paratoluensulfonyl chloride under the action of alkali in the solvent environment to obtain 4-(N-(4-chlorphenyl)-4-(4-methyl benzolsulfonamido)-4-oxo-butyric acid; and (3) carrying out intramolecular cyclization on the 4-(N-(4-chlorphenyl)-4-(4-methyl benzolsulfonamido)-4-oxo-butyric acid under the action of a condensing agent and removing paratoluensulfonyl to obtain a target product. The process route disclosed by the invention has the advantages of low cost and easiness in acquisition of raw materials, few steps, simple process and convenience for obtaining the target product at high yield and high purity at an industrial production scale.
Owner:宁波人健药业集团股份有限公司
Method for preparing intermediate 2-carboxylic acid-5-(2-methyl-benzoylamino)toluene for tolvaptan
InactiveCN103159641AReduce manufacturing costSuitable for large-scale industrial productionProductsReagentsAfter treatmentOrganic solvent
The present invention provides a method for preparing an intermediate 2-carboxylic acid-5-(2-methyl-benzoylamino)toluene for tolvaptan. The method comprises: in an anhydrous organic solvent, 2-bromo-5-(2-methyl-benzoylamino)toluene is reacted with a n-butyllithium solution at a low temperature, then carbon dioxide is fed in or dry ice is added in, and the intermediate 2-carboxylic acid-5-(2-methyl-benzoylamino)toluene is obtained after treatment. The molar ratio of the 2-bromo-5-(2-methyl-benzoylamino)toluene to the n-butyllithium solution is 1:0.5-5. By using the method of the present invention, the prepared tolvaptan intermediate has characteristics of a low cost, few impurities, and high yields, thereby the method is more suitable for large-scale industrial production.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
Tolvaptan tablet
InactiveCN107432867AReduce the impactStable blood concentrationMetabolism disorderDigestive systemTolvaptanTreatment effect
The present invention relates to a kind of tolvaptan tablet, and the invention belongs to the new technical field of medicine manufacturing, specifically relates to tolvaptan tablet and a kind of preparation method thereof, and this tablet is tolvaptan as main component, is produced by tolvaptan Protan solid dispersion granules and auxiliary materials are directly compressed into tablets. The tolvaptan tablet of the invention solves the problem of in vitro dissolution of the tolvaptan, improves the curative effect of the drug, and has a more ideal therapeutic effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Medicinal solid preparation of tolvaptan and preparation method
PendingCN111888335AImprove bioavailabilityMetabolism disorderPill deliveryMagnesium stearateStearic acid
The invention discloses a medicinal solid preparation containing tolvaptan and a preparation method. The preparation method comprises steps of converting active pharmaceutical ingredients of tolvaptaninto amorphous forms by spray-drying. The preparation method is characterized by controlling the amorphous solid dispersible powder to be 35-80 [mu]m in particle size D90. The preparation method alsocomprises steps of adding lactose, corn starch, microcrystalline cellulose, hydroxypropyl cellulose, low-substituted hydroxypropyl cellulose, magnesium stearate and other auxiliary materials, and conducting granulation through a fluidized bed to obtain the medicinal solid preparation of tolvaptan with good in-vivo solubility and high bioavailability.
Owner:FUAN PHARMA LYBON PHARMA TECH
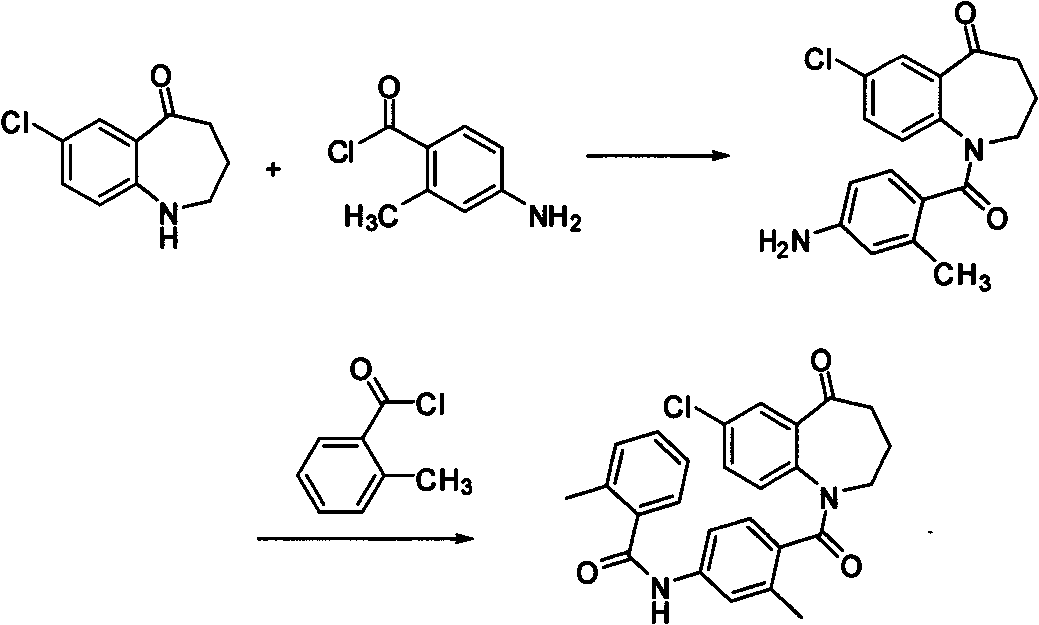
Method for preparing tolvaptan intermediate
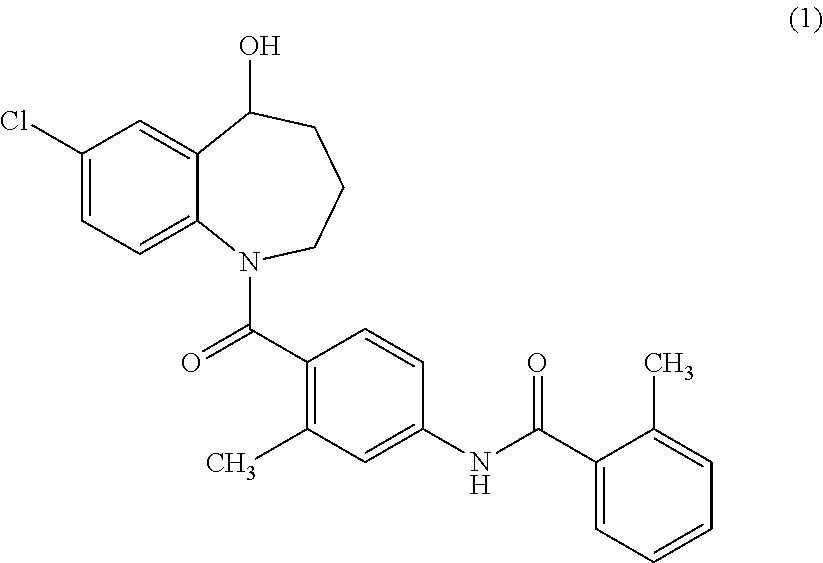
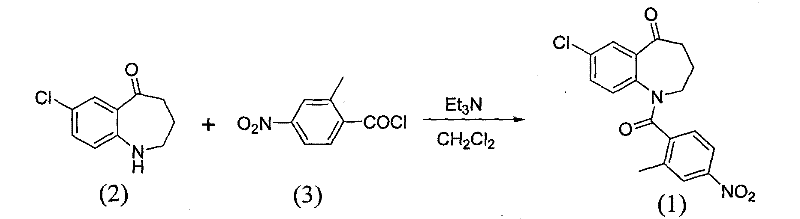
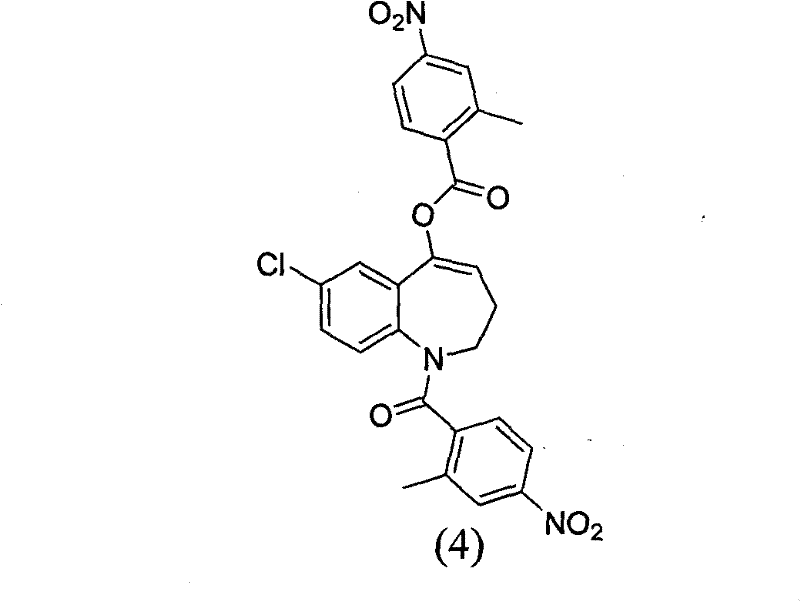
The invention relates to a method for preparing a tolvaptan intermediate, namely 7-chlorine-5-oxo-1-(2-methyl-4-nitrobenzene formyl)-1,2,3,4-tetrahydro-benzo-azepine. The tolvaptan intermediate which is a compound as shown in the formula (1) is obtained by 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzo-azepine (2) reacting with 4-nitryl-2-methylbenzene formyl chloride (3). The tolvaptan intermediate prepared by the method has the advantages of high purity, high yield and short reaction time, thereby being suitable for large-scale industrialized production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Tolvaptan preparation containing micronized tolvaptan and water-soluble auxiliary materials
InactiveCN106880611AImprove in vitro dissolution rateFast dissolutionMetabolism disorderDigestive systemMedicineWater soluble
The invention discloses a tolvaptan preparation containing micronized tolvaptan and water-soluble excipients. Each tablet contains tolvaptan 7.5 mg-60 mg and 90% of the cumulative particle size of tolvaptan is not greater than 75 microns; Each tablet contains no more than 60% of the tablet weight as water-soluble excipients, and the weight ratio of tolvaptan to water-soluble excipients is 1:2.5~1:10. The invention solves the problem of poor compliance during the use of tolvaptan tablets and provides a new and excellent dosage form for clinical use.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
Method for preparing amorphous tolvaptan
ActiveCN103880747AHigh clinical application valueEasy to prepareOrganic chemistryFluidized bedSolvent
The invention discloses a method for preparing amorphous tolvaptan. The method comprises the steps of dissolving a tolvaptan crystalline compound into a solvent I, cooling to the temperature of 25-40 DEG C, so as to obtain a tolvaptan solution, then, adding the obtained tolvaptan solution into a rapidly-stirred solvent II, continuing to stir for 5-15 minutes after the adding is completed, then, filtering and drying, thereby obtaining amorphous tolvaptan, wherein the weight / volume ratio of the tolvaptan crystalline compound to the solvent I is 1g: (5-50)mL, solid precipitation exists during adding, the dosage of the solvent II is 0.5-5 times that of the solvent I, and the temperature of the rapidly-stirred solvent II is kept at 0-5 DEG C. According to the method, through using different solvents respectively at a dissolution stage and a precipitation stage and controlling the temperature change and dosage of the solvents, the yield of amorphous tolvaptan can be higher; moreover, the method disclosed by the invention is simple, is easy in operation and has no need of using large-sized equipment, such as a fluidized bed and the like, thereby being capable of playing a more active role in popularizing the drug.
Owner:CHENGDU BAIYU PHARMA CO LTD
Method for preparing tolvaptan intermediate
ActiveCN102690211ASimple processing methodQuality improvementOrganic compound preparationCarboxylic acid amides preparationHydrolysisMethyl group
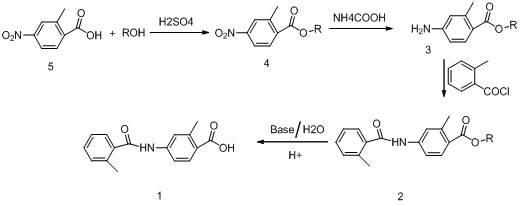
The invention relates to a method for preparing a tolvaptan intermediate. The method comprises the following steps of: reacting 2-methyl-4-nitrobenzoic acid used as an initial raw material and alcohols under the action of a catalyst to generate 2-methyl-4-nitrobenzoate, and reacting under the action of palladium-carbon catalyst by using ammonium formate as hydrogen donor to obtain 2-methyl-4-aminobenzoate compounds; and reacting the 2-methyl-4-aminobenzoate compounds and o-toluoyl chloride under the alkaline condition to generate 2-methyl-4-N-(2-methylbenzoyl)benzoate, adding alkali and a catalyst at presence of water to perform hydrolysis, extracting a water layer by using an organic reagent for one to two times, after layering, collecting a water-layer solution, adjusting the pH of the water-layer solution to be 5 to 6 to separate solid out, filtering an aqueous solution out to obtain solid remnant, and drying to obtain a target product. By the method, the quality and yield of a product are improved; a method for treating the intermediate is simplified; and a simple and feasible process method is provided for industrial production.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Preparation method of tolvaptan key intermediate
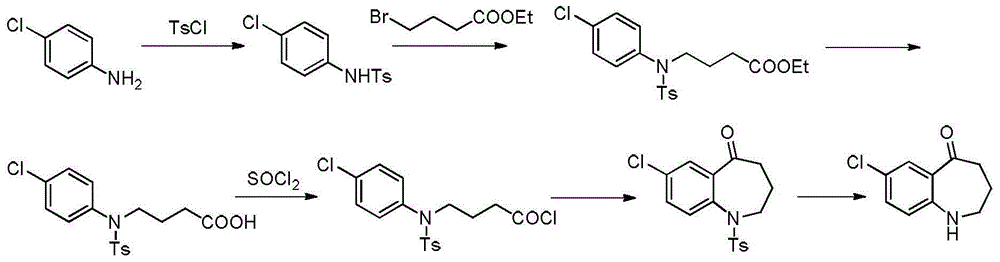
InactiveCN104829533ASimple and fast operationShorten the production cycleOrganic chemistryEthyl ChlorideOxygen
The invention discloses a preparation method of tolvaptan key intermediate 7-chloro-5-oxo-1,2,3,4-tetrahydro-benzazepine. The preparation method comprises the steps of condensing parachloroaniline, which is taken as a starting raw material, with 4-chlorobutyric acid methyl ester, carrying out hydrolysis de-esterification and acylating chlorination, and carrying out ring closure under an acidic condition, so as to obtain a target product, wherein the total yield of the target product can reach above 80%, and the HPLC (High Performance Liquid Chromatography) purity reaches above 99.5%.
Owner:广安凯特制药有限公司
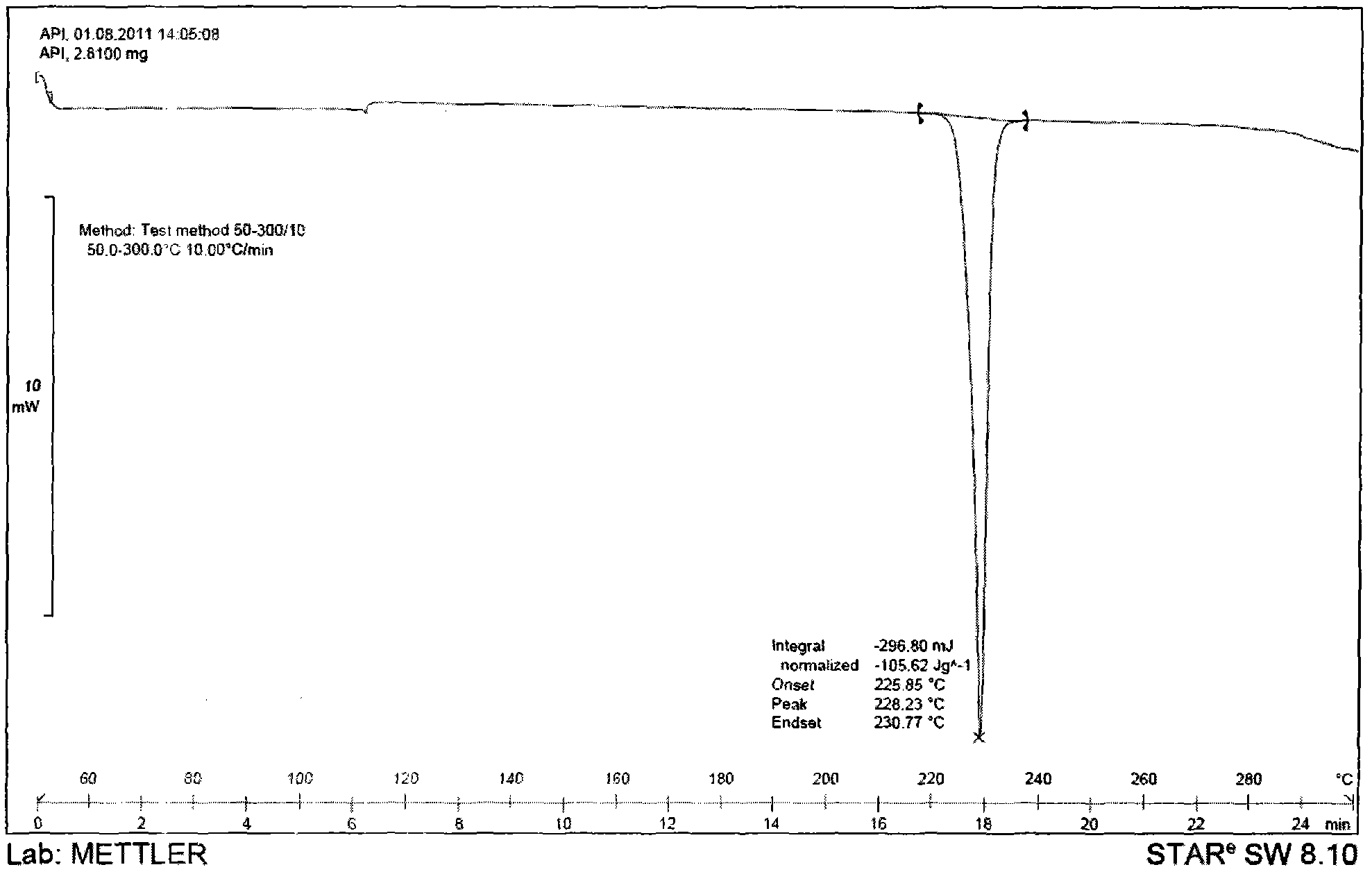
Tolvaptan crystal and medicine composition thereof
ActiveCN102558051AImprove solubilitySimple preparation processOrganic chemistryMetabolism disorderX-rayDifferential scanning calorimetry
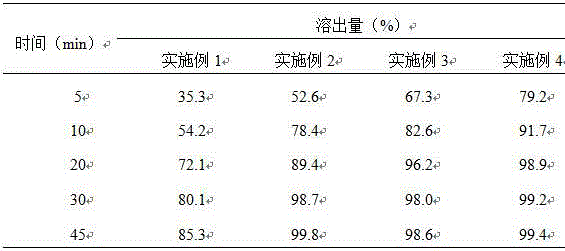
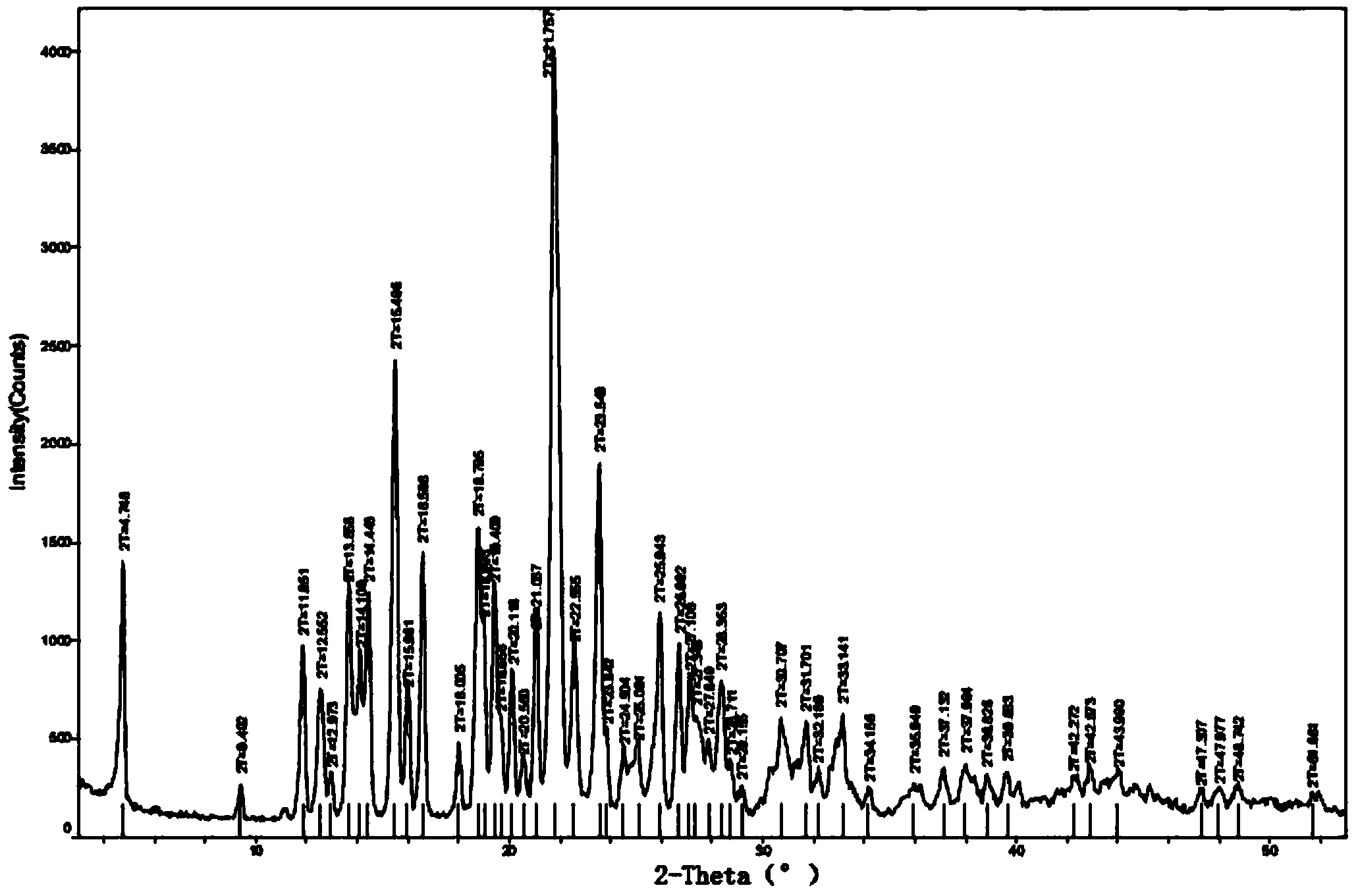
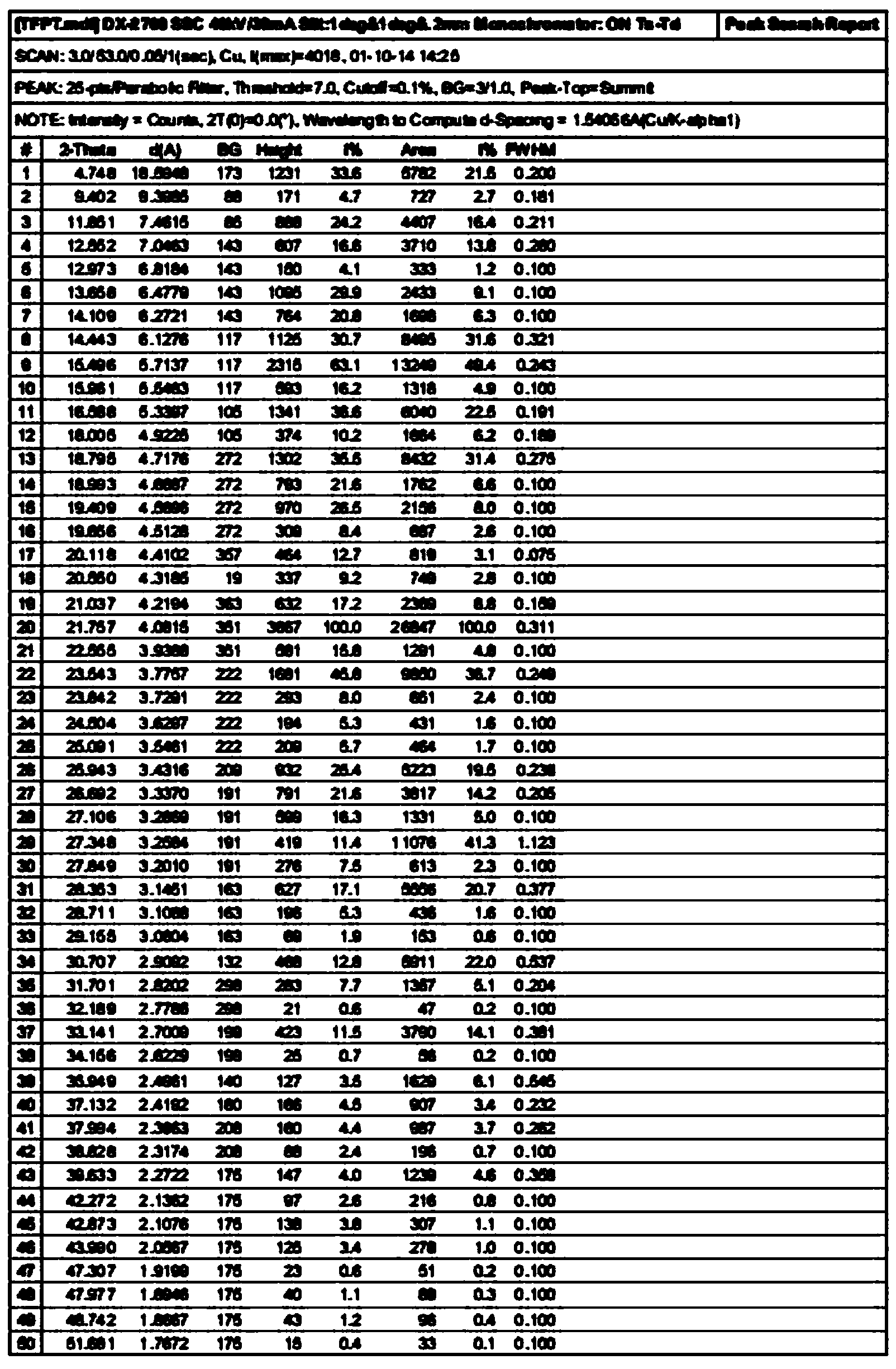
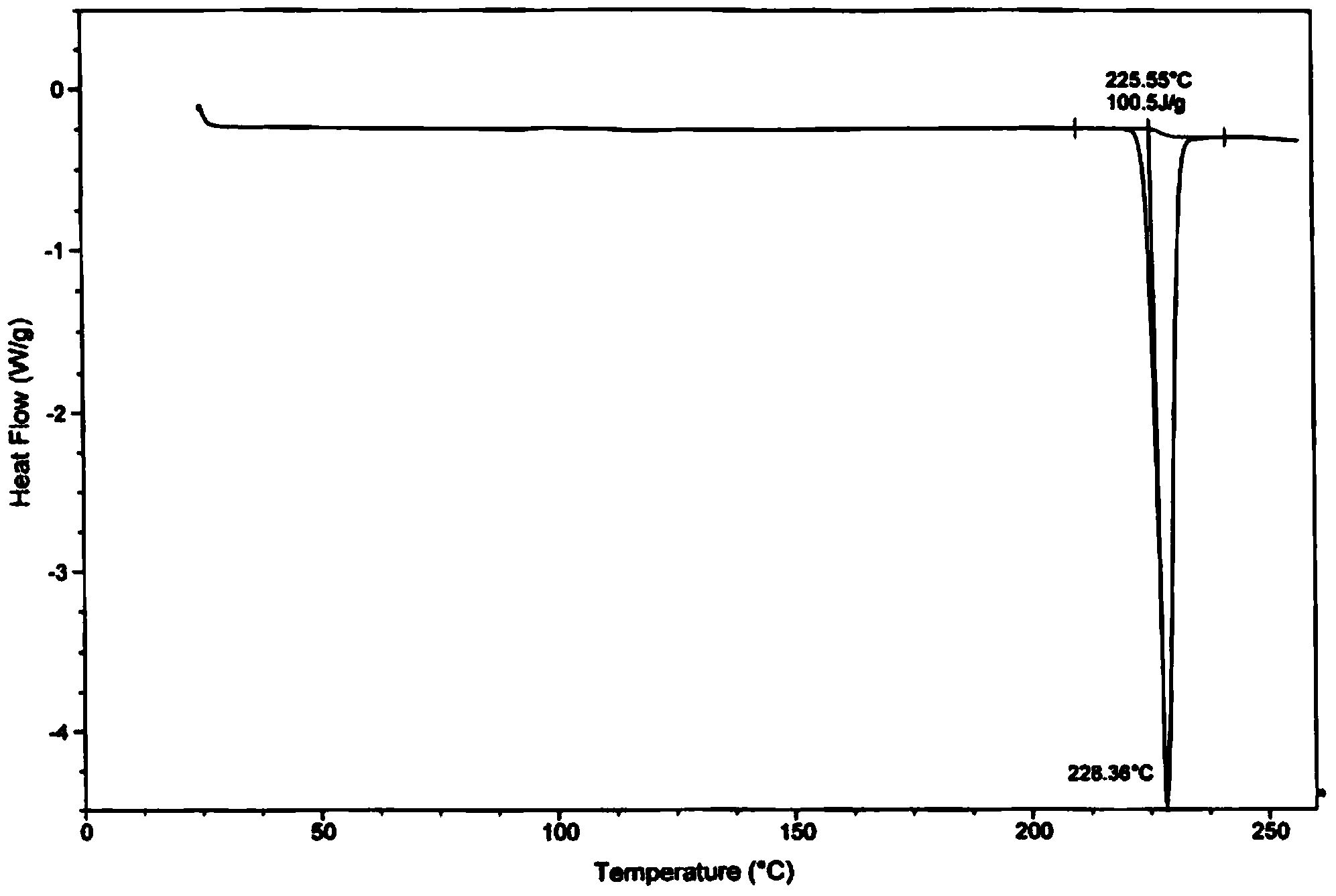
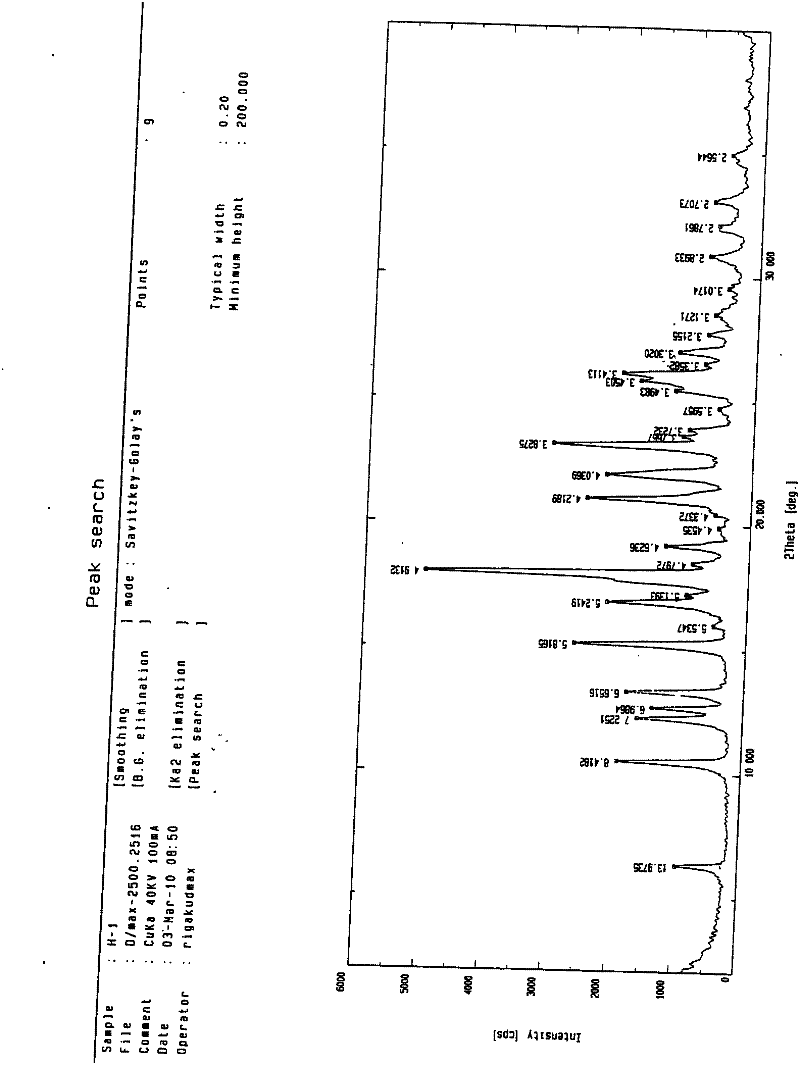
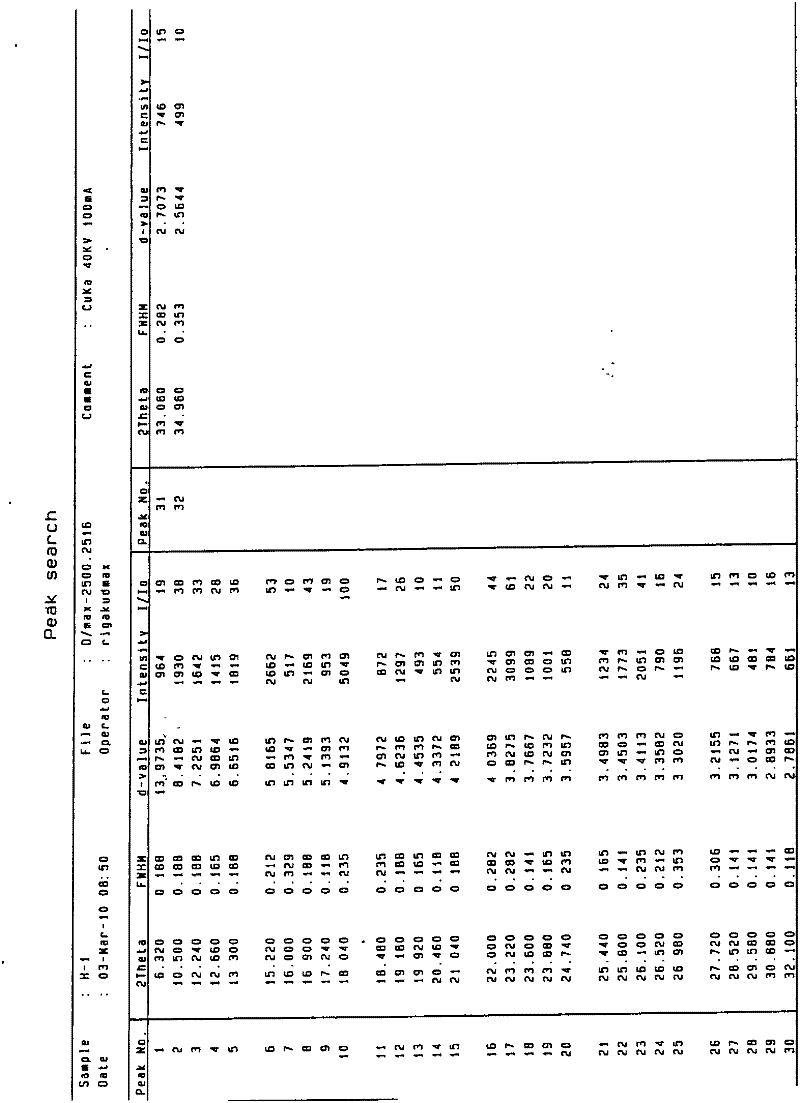
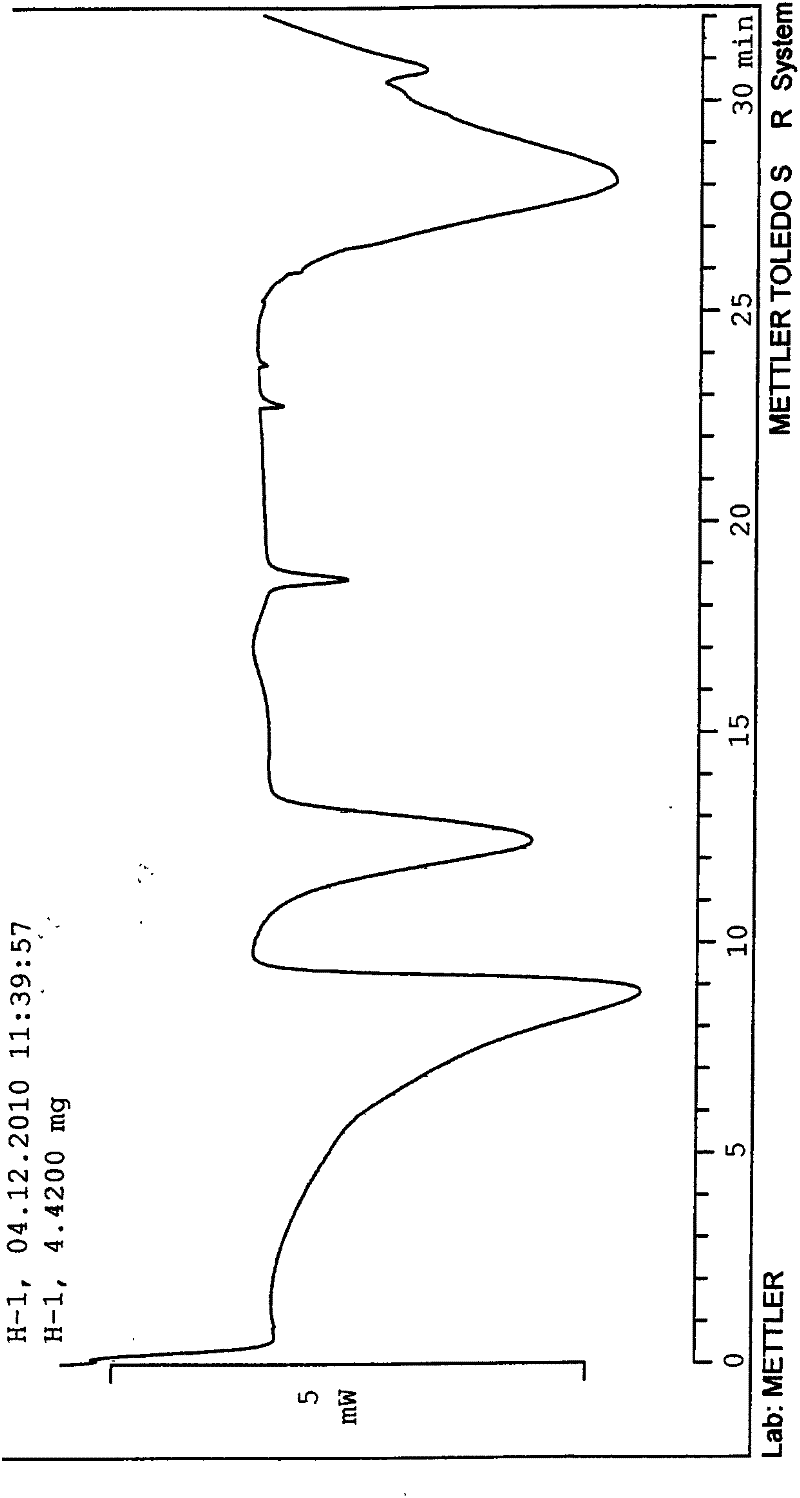
The invention discloses a tolvaptan crystal containing 0.5 crystal water. The crystal has a tolvaptan crystal powder X-ray diffraction pattern represented by a figure 1, a differential scanning calorimetry (DSC) analytical figure represented by the figure 2, and characteristics shown in an infrared spectrum represented by a figure 4. Meanwhile, the invention further discloses 1-100mg of a medicine composition composed of a tolvaptan crystal contains 0.5 crystal water, and one or more pharmaceutically acceptable medicinal carriers. The tolvaptan crystal containing 0.5 crystal water is advantaged in simple preparation technology, good reproducibility, and easy drying. The crystal is stable to light, heat, and humidity. The crystal is suitable for long-time storage, and is suitable for large-scale productions.
Owner:天津泰普制药有限公司
Preparation method of tolvaptan tablet
ActiveCN102366412BImprove bioavailabilityImprove in vitro dissolutionDigestive systemPill deliveryMagnesium stearateStearic acid
The invention discloses a preparation method of a tolvaptan tablet. The preparation method comprises the following steps: 1, mixing tolvaptan with high-substituted hydroxylpropyl cellulose according to a mass ratio of 1:0.2-0.6, crushing them, and sieving by a 60-100 mesh sieve; 2, dissolving throughs in a mixed solution of waterless ethanol and dichloromethane with a volume ratio of 1:1-4, carrying out spray drying to form powder, carrying out reduced pressure drying at 70-90DEG C until that the content of solvents residual in the sprayed powder is qualified; 3, crushing the sprayed powder, sieving by a 180-220 mesh sieve, accurately weighing the sprayed powder, lactose, microcrystalline cellulose and hydroxypropyl methylcellulose according to prescription amounts, uniformly mixing them, and adding a proper amount of water to prepare a soft material; and 4, sieving the soft material by a 15-25 mesh sieve, preparing a wet particle, drying the wet particle at 50-70DEG C until that the content of water in the wet particle is 2-4%, sieving the dried wet particle by a 15-25 mesh sieve, granulating, adding a prescription amount of magnesium stearate, uniformly mixing, and tabletting. The preparation method of the invention has the advantages of ingenious conception, low production cost, high in vitro dissolubility of the prepared tolvaptan tablet, and good biological availability and clinic curative effect of the tolvaptan tablet.
Owner:SICHUAN BAILI PHARM CO LTD
Tolvaptan intermediate and preparation method thereof
Owner:JIANGSU KANION PHARMA CO LTD +1
Preparation method of 2-methyl-4-N-(2-methylbenzoyl)benzoic acid
ActiveCN102093247AReduce usageEase of industrial productionOrganic compound preparationCarboxylic acid amides preparationBenzoic acidAcyl group
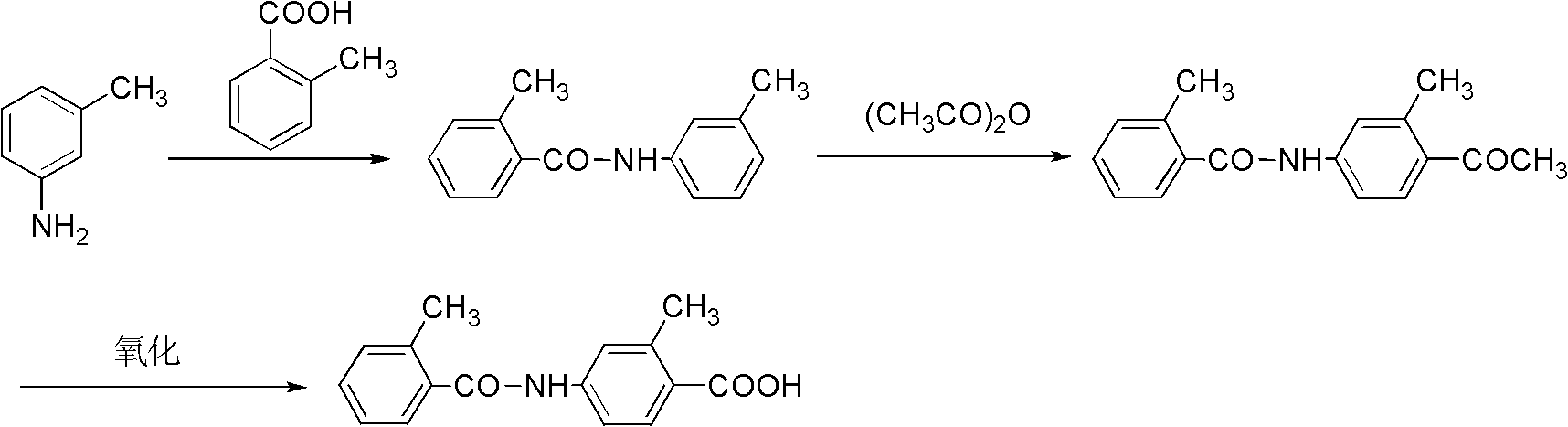
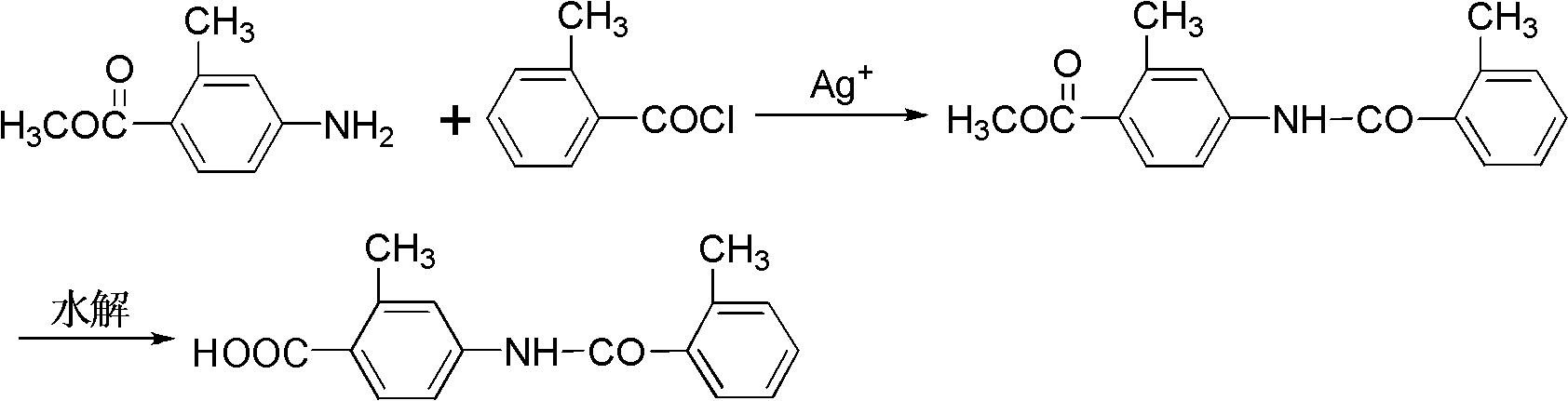
The invention relates to a novel preparation method of a Tolvaptan intermediate 2-methyl-4-N-(2-methylbenzoyl)benzoic acid, which comprises the following step: carrying out N-acylation and hydrolysis on 2-methyl-4-amino methyl benzoate and 2-methyl benzoyl chloride which are used as initial raw materials, thereby obtaining the target compound 2-methyl-4-N-(2-methylbenzoyl)benzoic acid, wherein the N-acylation reaction is carried out under the action of silver salts. Compared with the preparation method provided in the existing literature, the method provided by the invention has the advantages of simple and accessible raw materials, mild reaction conditions, high yield and high purity, is easy and safe for operation, and lowers the requirements for equipment, thereby lowering the cost.
Owner:天津天诚新药评价有限公司
Solid medicine composition of tolvaptan
The invention discloses a solid medicine composition of tolvaptan. The solid medicine composition of tolvaptan comprises granular or unformed powder of tolvaptan, sugar and a sugar alcohol diluent. The preparation method comprises the following steps of: highly dispersing the active component tolvaptan into the diluent to obtain solid dispersoid; and preparing into oral solid preparation with pharmaceutically acceptable auxiliary materials. The solid medicine composition is clinically used for treating hyponatremia.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Suspension for oral administration comprising amorphous tolvaptan
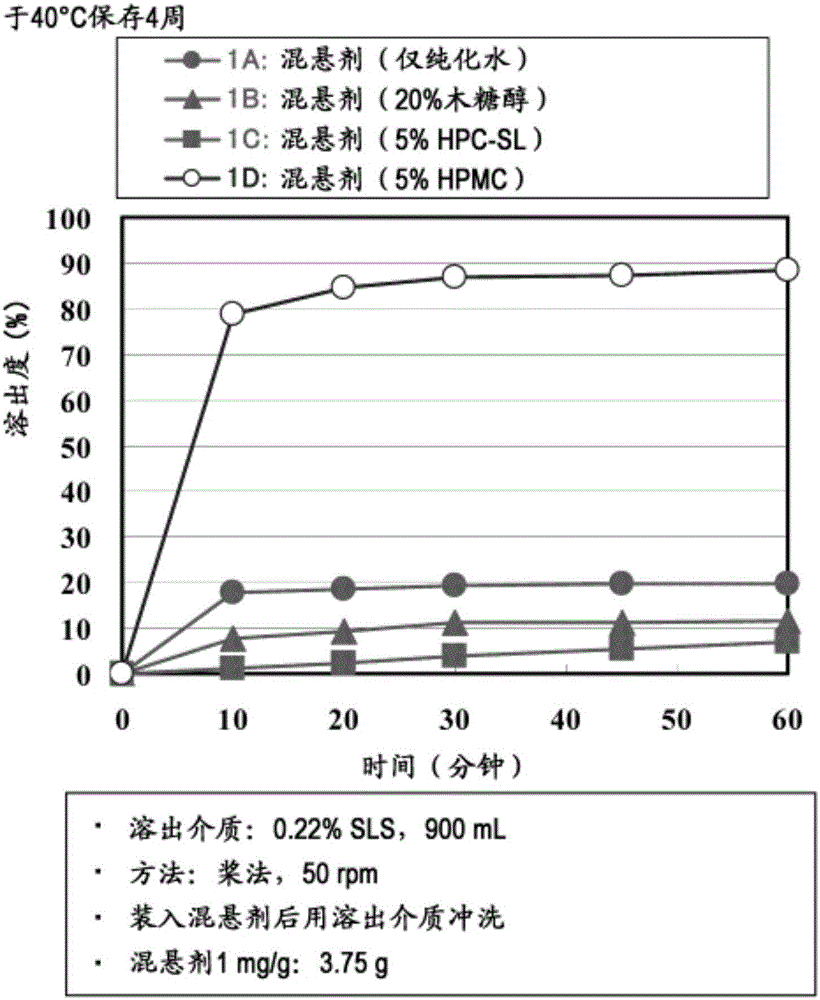
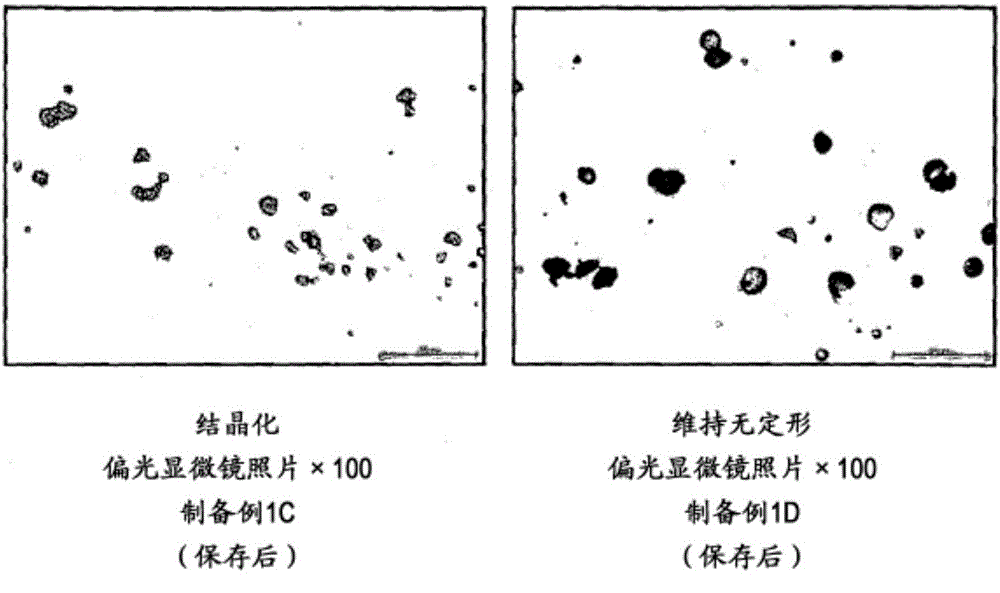
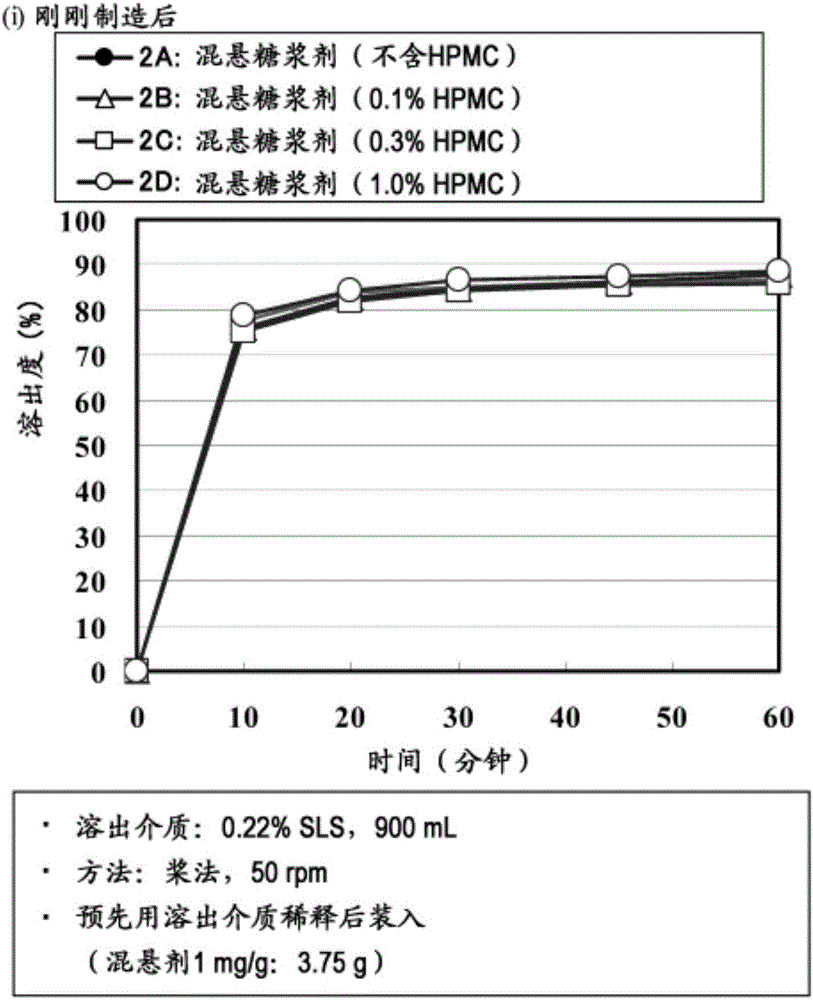
ActiveCN105007897AInhibit or delay crystallizationImprove solubilityDispersion deliveryMetabolism disorderSolubilitySolvent
This invention provides a suspension for oral administration comprising particles containing amorphous tolvaptan that can inhibit or delay crystallization of amorphous tolvaptan over time in the suspension, and stably maintain high solubility of tolvaptan and excellent absorbability of tolvaptan from the gastrointestinal tract; and a solid formulation for oral administration that can be suspended to prepare the suspension for oral administration at the time of use. This invention relates to a suspension for oral administration, in particular, a syrup, comprising (a) particles containing amorphous tolvaptan, (b) hydroxypropyl methylcellulose (HPMC), and (c) a solvent, wherein the amount of the HPMC (b) is 0.1 to 25% by weight based on the total weight of the suspension for oral administration.
Owner:OTSUKA PHARM CO LTD
Tolvaptan solid dispersion preparation and preparation method thereof
The invention relates to the field of pharmaceutical preparations, in particular to a tolvaptan solid dispersion preparation and a preparation method thereof. The tolvaptan solid dispersion preparation comprises tolvaptan, povidone, a specific lubricant, a specific disintegrant and a specific diluent. Owing to the dissolution effect, the bioavailability and the stability, the industrial productionof the tolvaptan solid dispersion preparation is facilitated.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
Preparation method of tolvaptan intermediate
ActiveCN103896842AMild conditionsRaw materials are easy to getOrganic chemistryBenzodiazepineSynthesis methods
The invention belongs to the field of drug synthesis, and particularly relates to a preparation method of a tolvaptan intermediate 7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzodiazepines-5-one. The method comprises the following steps: by taking N-vinylpyrrolidone as an active ingredient, rearranging under light to obtain 4-azecycloheptatrienone, then protecting nitrogen methyl p-toluenesulfinate, reacting with 2-chloro-2-butenal in the presence of diaryl D-prolinol derivatives to generate 7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzodiazepines-5-one, dehydrogenizing and aromatizing in the presence of manganese dioxide, and finally removing protection groups to obtain the 7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzodiazepines-5-one. Due to no Claisen condensation or Friedle-Crafts reaction in the conventional synthesis method, the potassium tert-butanol and aluminum trichloride are omitted, the preparation method is mild in reaction conditions, easily available in raw materials, capable of lowering the cost, and more suitable for industrial production.
Owner:HUBEI GRAND LIFE SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com