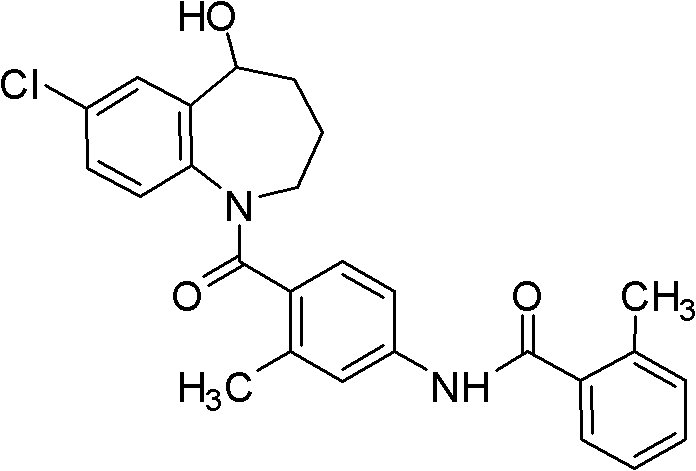
Tolvaptan oral solid medicinal composition and preparation method thereof
A technology of tolvaptan and its composition, which is applied in the field of tolvaptan oral solid pharmaceutical composition and its preparation, can solve the problem that the process of amorphous tolvaptan composition is not easy to control, the preparation method is complicated, and it is not suitable for industrialization Production and other issues, to achieve the effect of being suitable for industrialized large-scale production, simple and easy process, and full absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
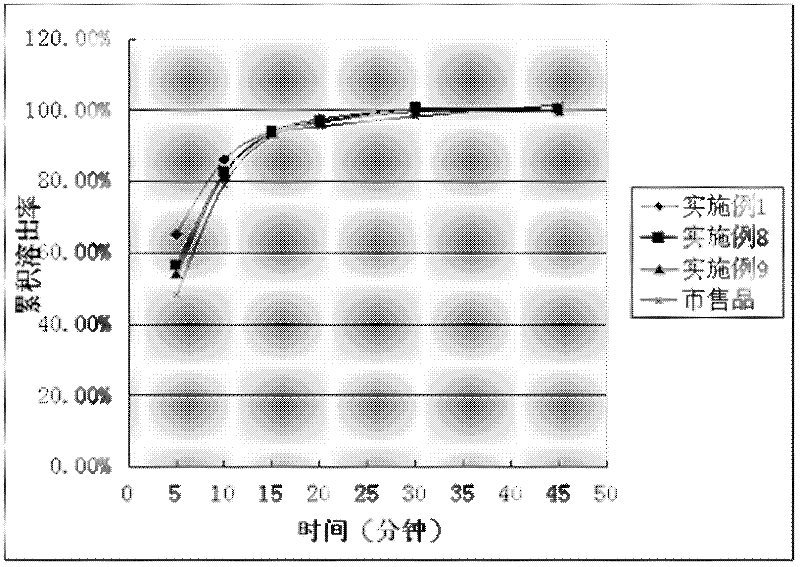
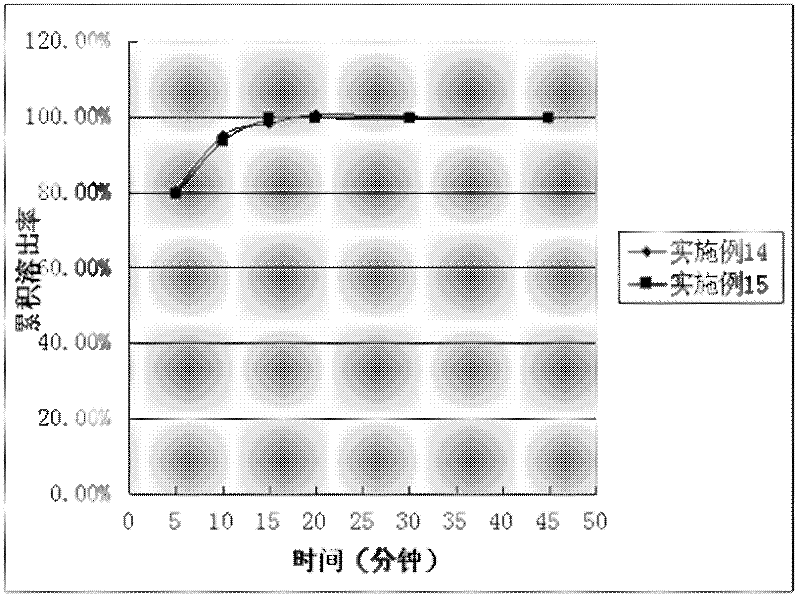
Image
Examples
Embodiment 1
[0071] Embodiment 1: tolvaptan tablet
[0072] Preparation of tolvaptan solution with binder:
[0073] Dissolve 210g of tolvaptan in 6L of pure ethanol solution, heat and stir to make the solution clear, then add 42g of polyvinylpyrrolidone, stir to make it dissolve completely, and obtain a tolvaptan solution containing a binder.
[0074] Preparation of drug-loaded particles:
[0075] 560 g of lactose was placed in a fluidized bed granulator, and the tolvaptan solution containing the binder prepared in the previous step was used for fluidized bed granulation, followed by drying to obtain drug-loaded granules.
[0076] Mixed processing:
[0077] Mix 58g of drug-loaded granules obtained in the previous step, 26g of microcrystalline cellulose, 3.0g of cross-linked polyvinylpyrrolidone and 0.5g of magnesium stearate, punch tablets with 8.5mm dimples to obtain a tablet weight of 175mg containing 30mg of tolvap Tan tablet, about 5Kg hardness.
Embodiment 2
[0078] Embodiment 2: tolvaptan tablet
[0079] Mix the drug-loaded granule obtained in the embodiment 1 of 58g, the microcrystalline cellulose of 26g, the croscarmellose sodium of 3.0g and the magnesium stearate of 0.65g, with 8.5mm dimple punching tablet tablet weight is 175mg tablet containing 30mg tolvaptan, hardness about 5Kg.
Embodiment 3
[0080] Embodiment 3: tolvaptan tablet
[0081] Mix the drug-loaded granules obtained in the embodiment 1 of 58g, the microcrystalline cellulose of 26g, the polacrilin potassium of 3.0g and the magnesium stearate of 0.65g, with 8.5mm dimple punching tablet weight is 175mg and contains 30mg trolley The tablet of vaptan has a hardness of about 5Kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com