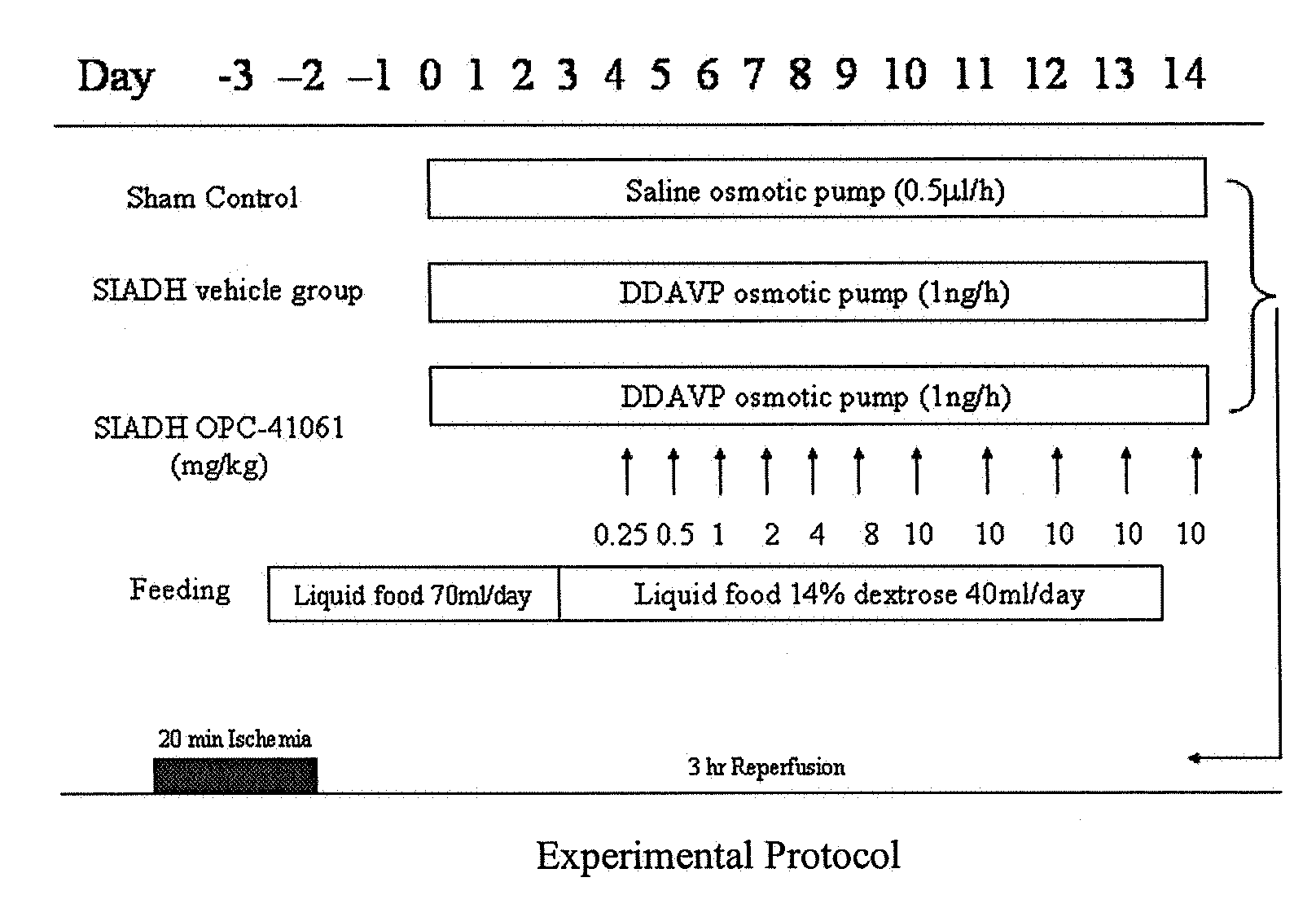
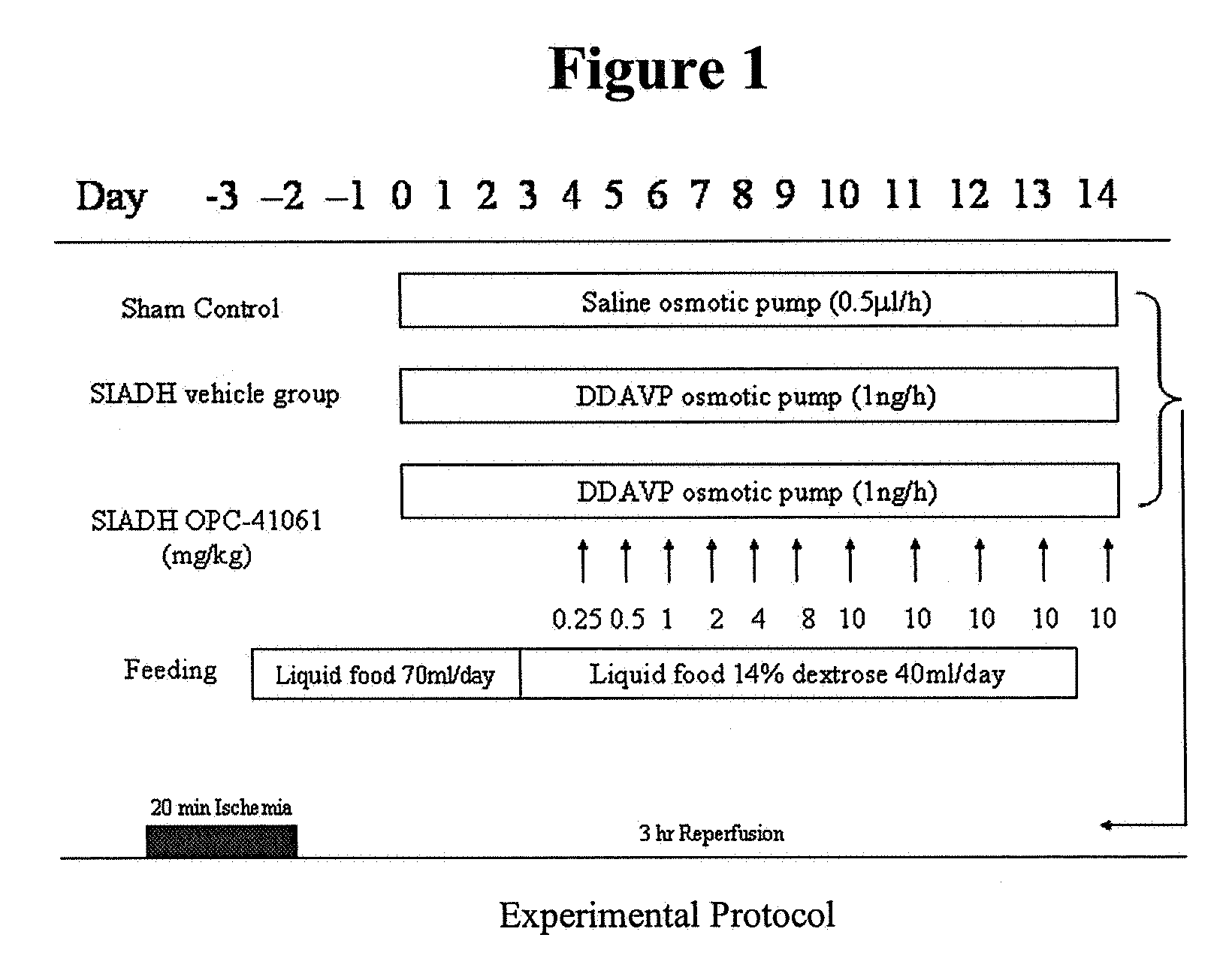
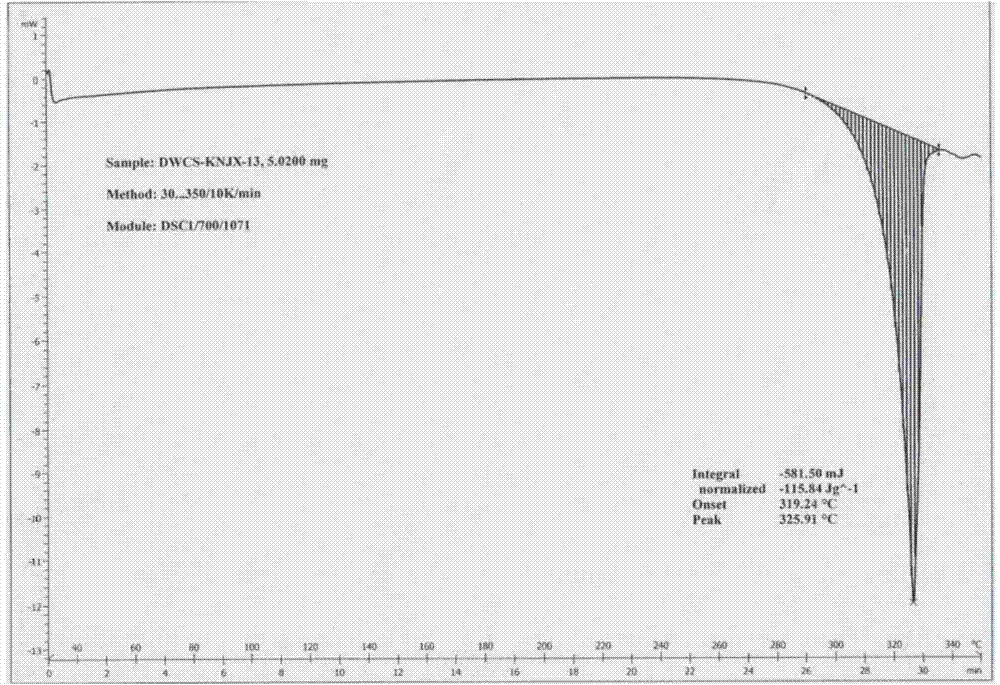
Patents
Literature
34 results about "Hyponatremia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition where sodium levels in the blood are abnormally low.
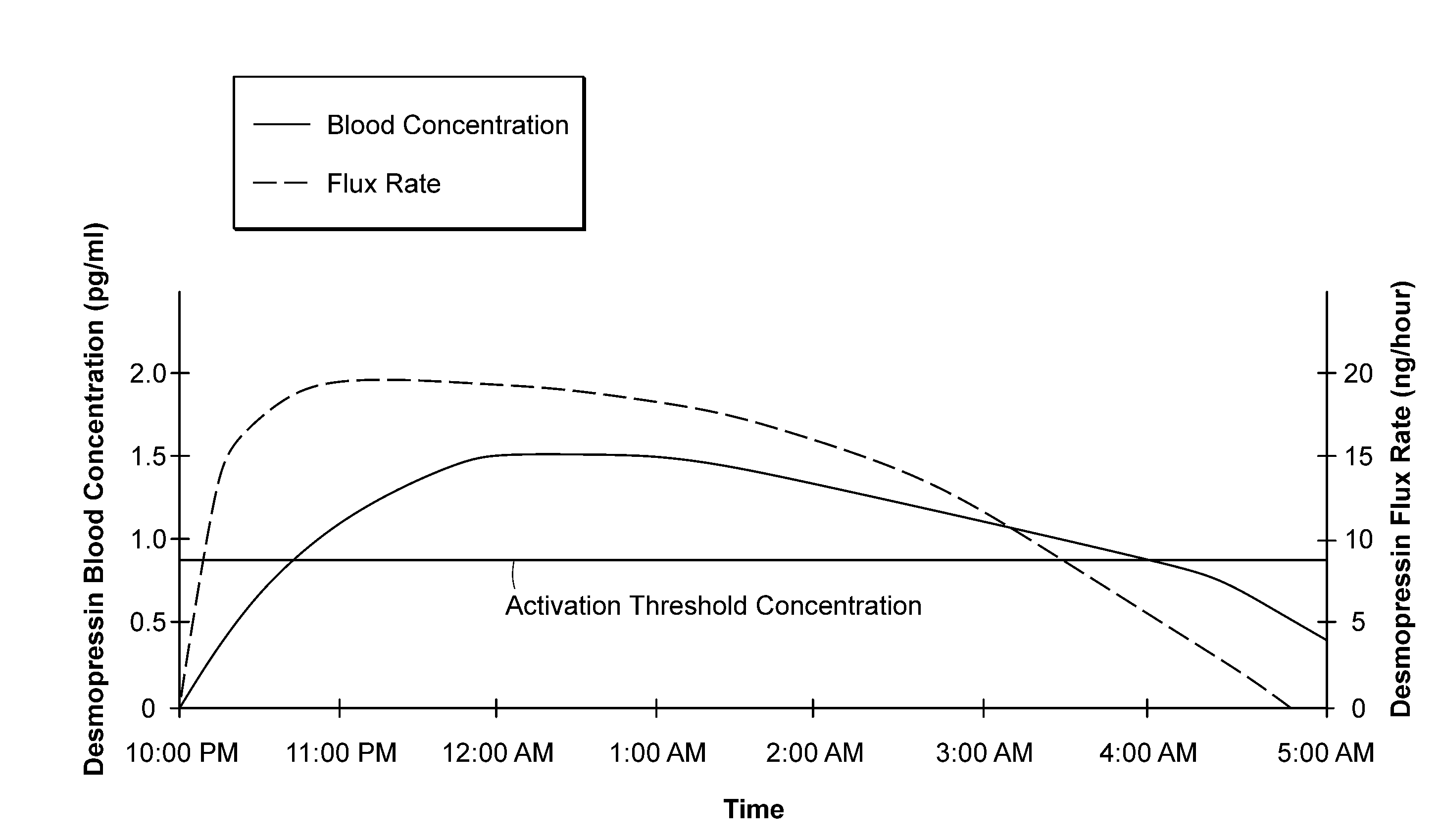
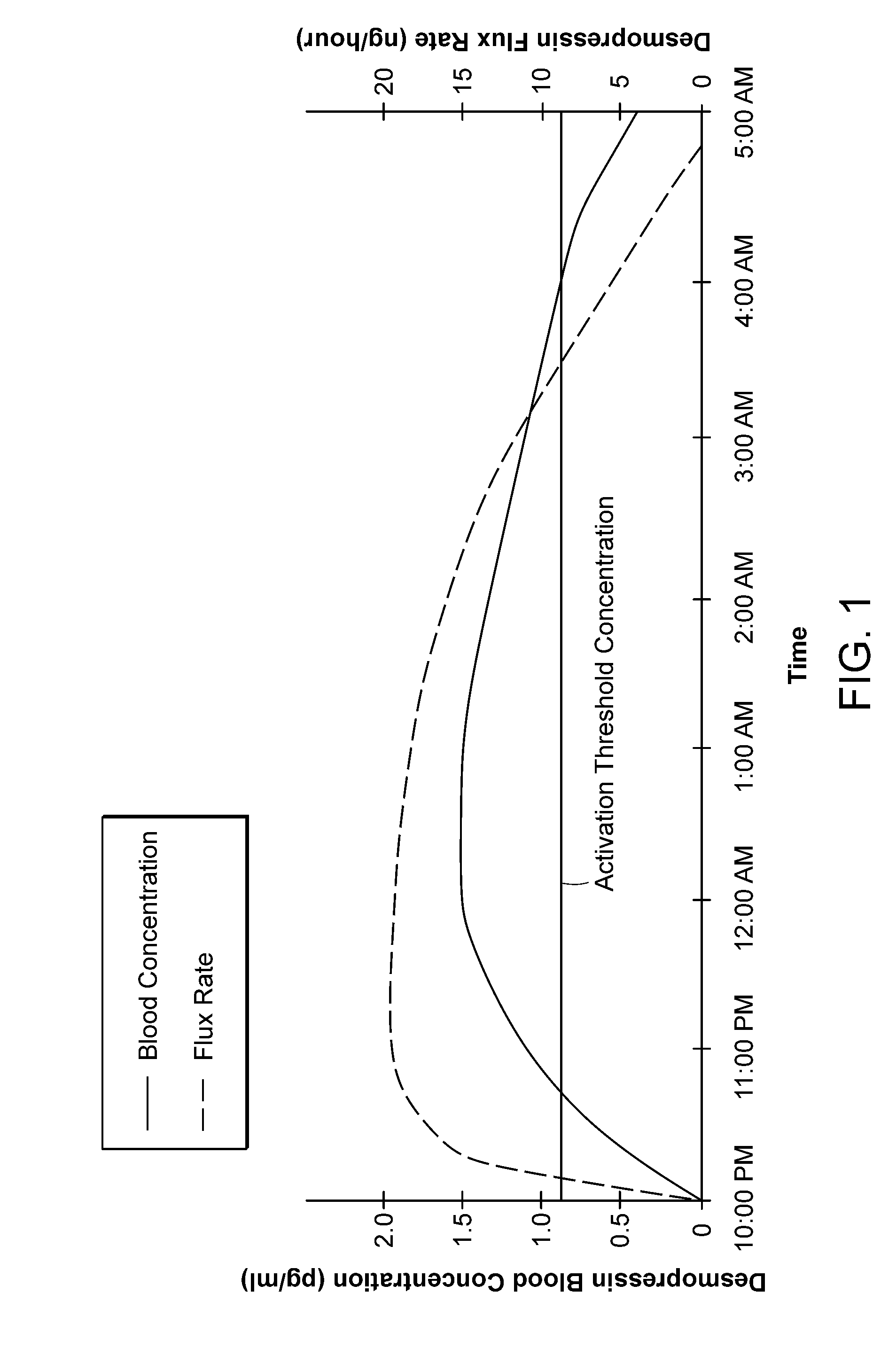
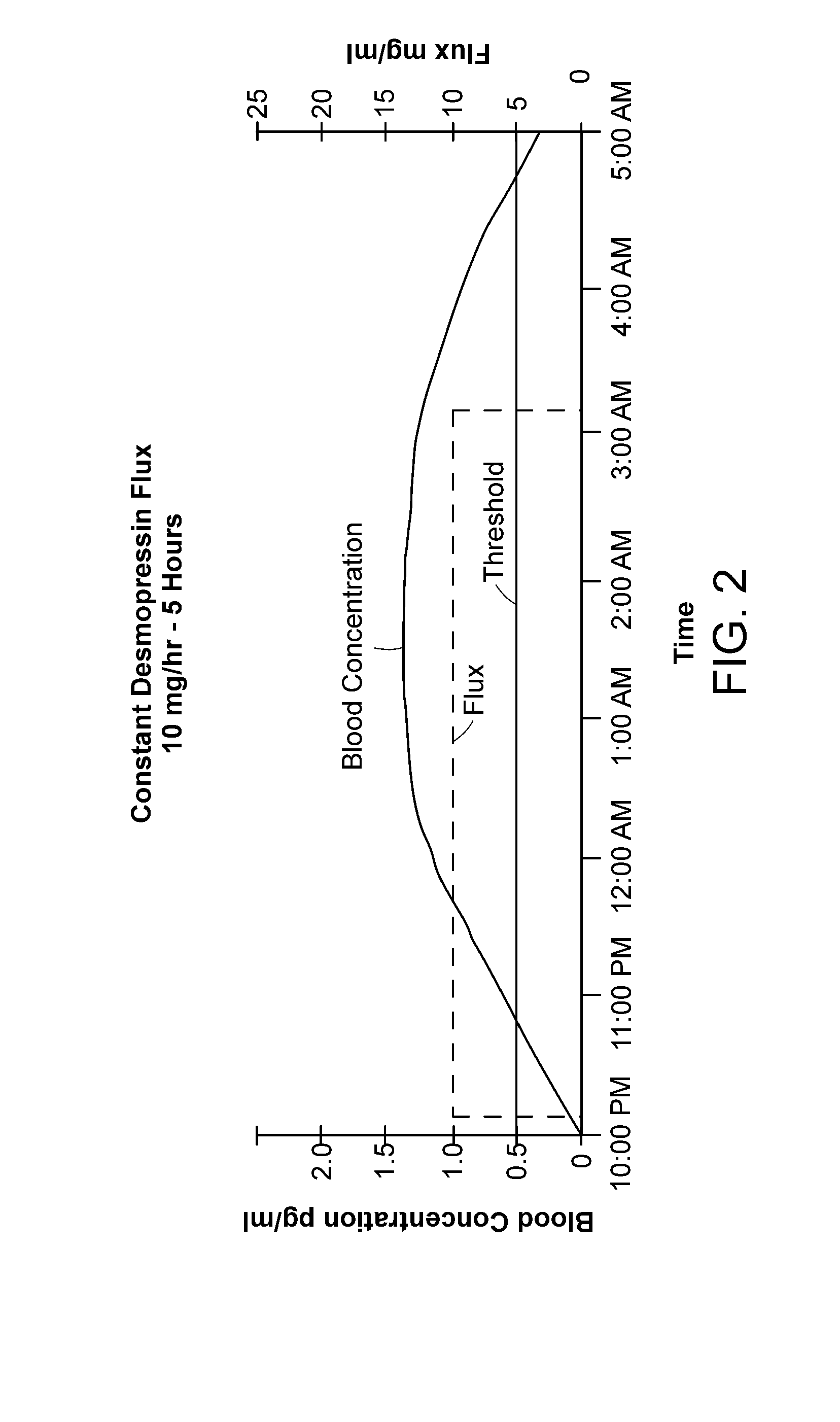
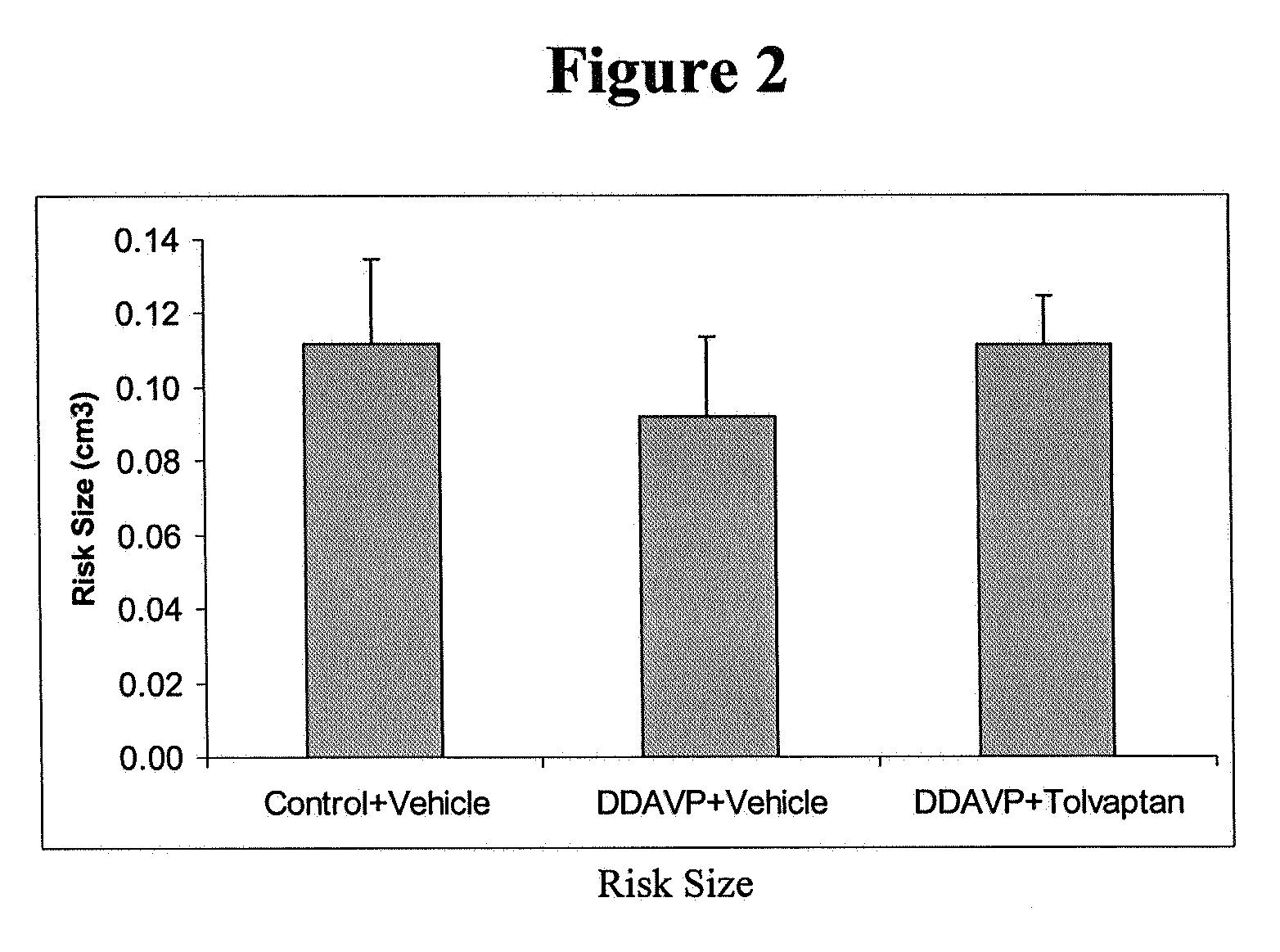
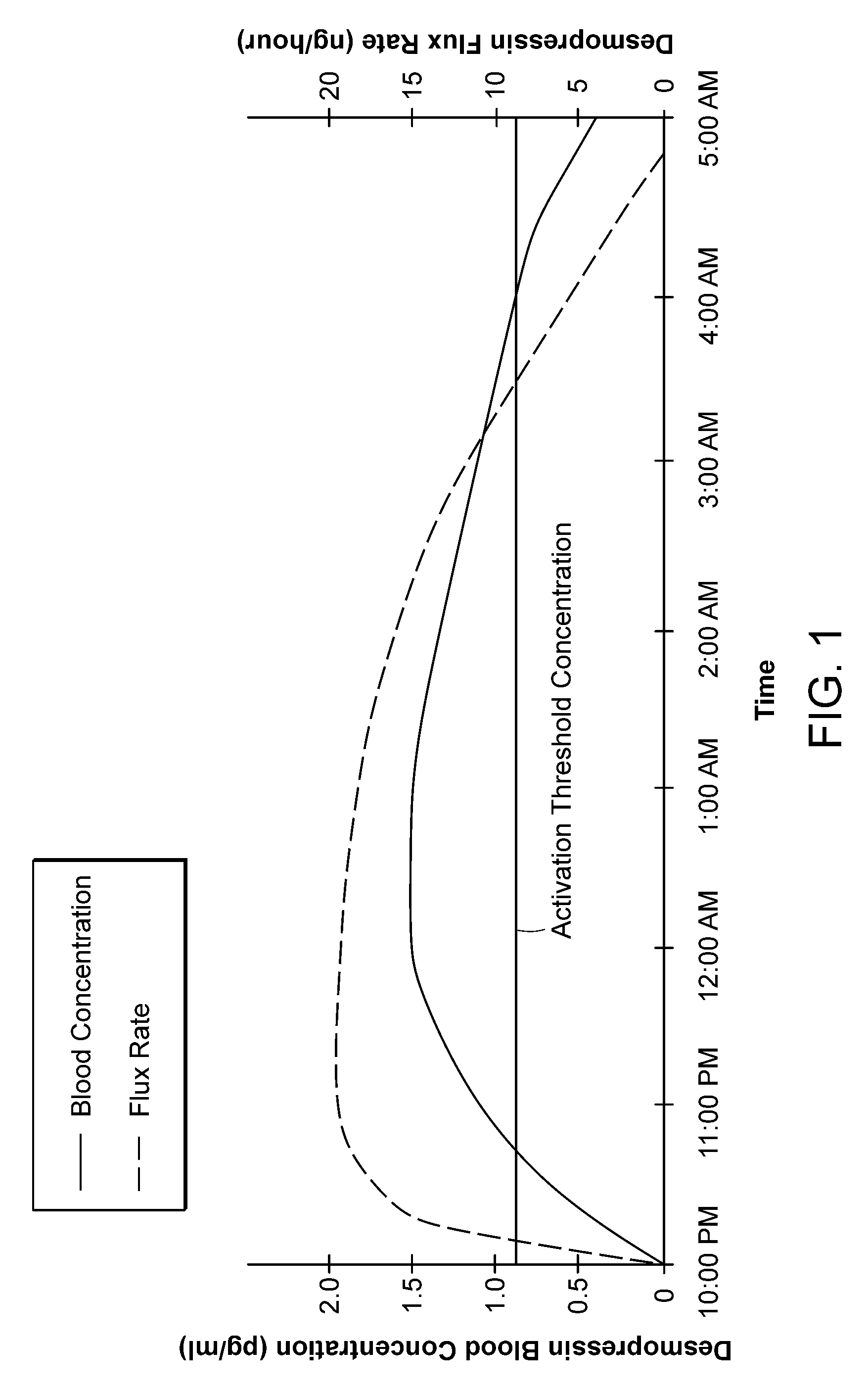
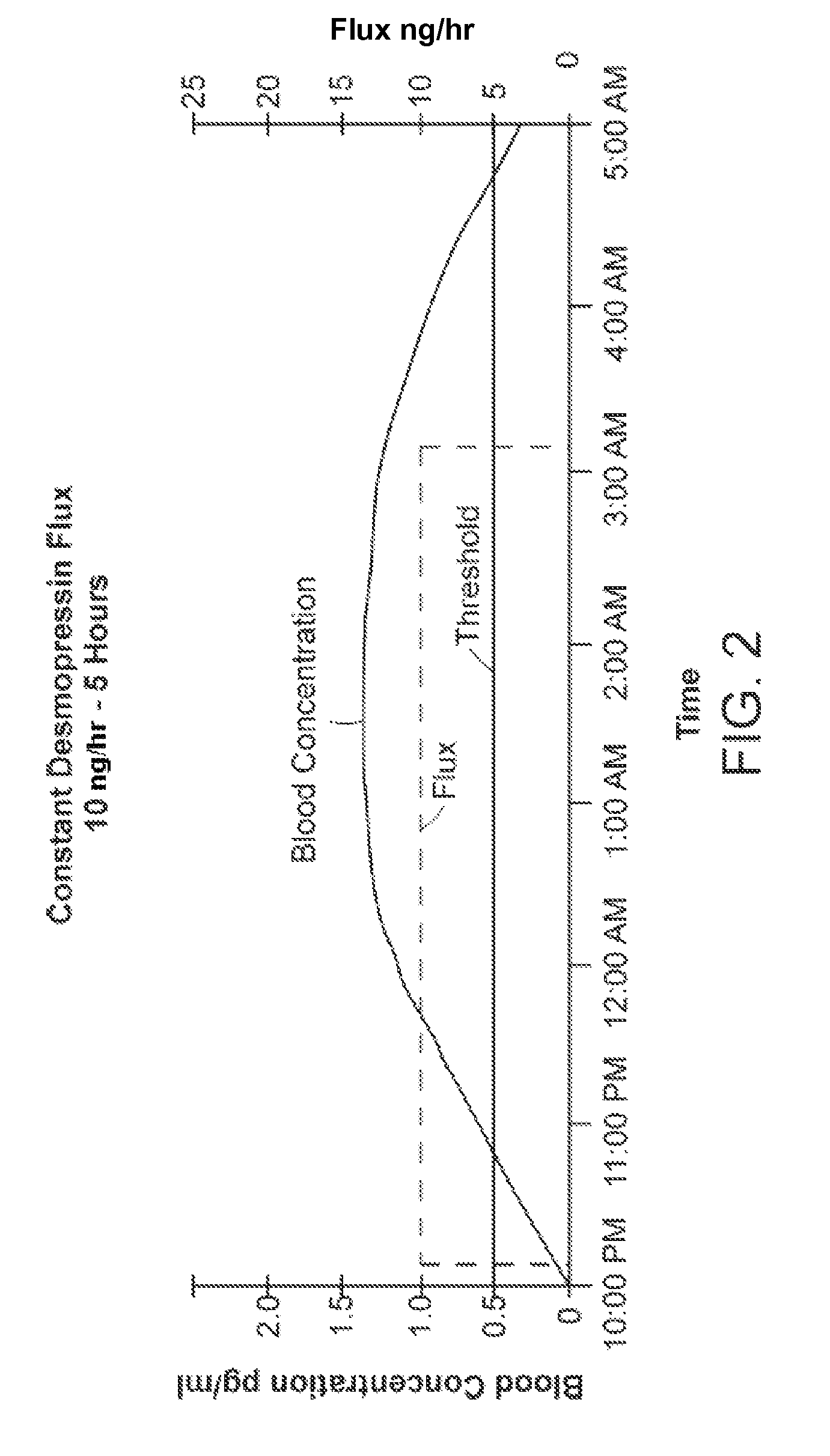
Methods and devices for desmopressin drug delivery
InactiveUS20090042970A1Reduce urine productionRestore normal urine productionBiocidePowder deliveryDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Electrolyte Energy Gel
InactiveUS20050095271A1Food ingredient as viscosity modification agentVitamin food ingredientsDecreased sodiumIntestinal Reabsorption
A nutritional composition in gel form containing carbohydrates, electrolytes, anti-oxidants, vitamins and amino acids for increasing available energy during exercise, for replenishing electrolytes that are depleted during exercise, for promoting fluid retention during exercise, for preventing hyponatremia during exercise and for facilitating intestinal reabsorption of fluids during exercise. The primary electrolyte ion is sodium (Na.sup.+) compounds is in an amount such that the sodium content of the gel is in a ratio in the range of 0.4 to 1.2 parts of the sodium to 100.0 parts of the carbohydrates.
Owner:CRANK SPORTS
SGLT-2 inhibitors, methods of making them, and uses thereof
Owner:ALBANY MOLECULAR RESEARCH INC
Method for reducing infarction using vasopressin antagonist compounds, and compositions and combinations therefor
InactiveUS20080221084A1Reduce infarctionMyocardial infarctionBiocideAnimal repellantsDiseaseDecreased sodium
The present invention relates to a method for reducing infarction comprising administering to a patient ion need thereof a therapeutically effective amount of a composition comprising as an active ingredient a vasopressin antagonist compound and to a composition useful therefor. The present invention also relates to a method for reducing infarction comprising administering to a patient in need thereof a therapeutically effective amount of a combination of a vasopressin antagonist compound and a beta-blocker and to combinations useful therefor. The methods, compositions and combinations of the present invention can be used for reducing infarction in the heart (myocardial infarction) and the brain (stroke). The methods, compositions and combinations of the present invention can also be used for the treatment and / or prevention of hypertension, edema, ascites, heart failure, renal function disorder, vasopressin inappropriate secretion syndrome (SIADH), hepatocirrhosis, hyponatremia, hypokalemia, polycystic kidney disease, diabetes, or circulation disorder.
Owner:OTSUKA PHARM CO LTD
Methods and devices for desmopressin drug delivery
InactiveUS8399410B2Prevent adverse side effectsReduce productionElectrotherapyMicroneedlesDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Cell volume-regulated human kinase h-sgk
InactiveUS6326181B1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDecreased sodiumHypernatremia
The present invention relates to the cloning and characterization of a human serine / threonine kinase (h-sgk: serum and glucocorticoid dependent kinase). The invention furthermore relates to reagents for diagnosing conditions associated with a change in cell volume and / or in "macromolecular crowding" in the body, such as, for example, hypernatremia, hyponatremia, diabetes mellitus, renal failure, hypercatabolism, hepatic encephalopathy, inflammation and microbial or viral infections. The present invention additionally relates to pharmaceuticals comprising the h-sgk, nucleic acids which code for the h-sgk, or receptors, in particular antibodies, which specifically bind to the h-sgk.
Owner:WALDEGGER SIEGFRIED +1
Sglt-2 inhibitors, methods of making them, and uses thereof
InactiveUS20110237527A1Improve the level ofHigh levelSaccharide with heterocyclic radicalsBiocideDiseaseDecreased sodium
The present invention relates to compounds which are inhibitors of sodium dependent glucose co-transporter-2 (SGLT-2). These compounds are used in the treatment of various disorders, including diabetes, impaired glucose tolerance, insulin resistance, retinopathy, nephropathy, neuropathy, cataracts, hyperglycemia, hyperinsulinemia, hyperchlolesterolemia, elevated blood level of free fatty acids or glycerol, hyperlipidemia, hypertriglyceridemia, obesity, wound healing, tissue ischemia, atherosclerosis, and hypertension. These compounds and compositions are also useful for treating and preventing kidney stones, hyperuricemia, gout, and hyponatremia. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
Cell volume-regulated human kinase h-sgk
InactiveUS6855520B2Prevent overcorrectionIncreased osmolarityPeptide/protein ingredientsTransferasesDecreased sodiumHypernatremia
The present invention relates to the cloning and characterization of a human serine / threonine kinase (h-sgk: serum and glucocorticoid dependent kinase). The invention furthermore relates to reagents for diagnosing conditions associated with a change in cell volume and / or in “macromolecular crowding” in the body, such as, for example, hypernatremia, hyponatremia, diabetes mellitus, renal failure, hypercatabolism, hepatic encephalopathy, inflammation and microbial or viral infections. The present invention additionally relates to pharmaceuticals comprising the h-sgk, nucleic acids which code for the h-sgk, or receptors, in particular antibodies, which specifically bind to the h-sgk.
Owner:LANG FLORIAN +1
Sodium Pyruvate Oral Rehydration Salt Composition for Treating Hypovolemia or Hyponatration Associated with Hypohydration
InactiveUS20150105463A1Promote absorptionPromote circulationAntibacterial agentsBiocideBlood pressureD-Glucose
Disclosed in the present invention is a sodium pyruvate oral rehydration salt composition for treating hypovolemia or hyponatration associated with hypohydration, said composition containing the following components: (i) 2.0-6.0 parts by weight of sodium pyruvate; (ii) 1.5-17.0 parts by weight of sodium chloride; (iii) 0-2.0 parts by weight of potassium chloride; and (iv) 10.0-50.0 parts by weight of glucose anhydrous or other carbohydrates. The weight of components (i)+(ii)+(iii)+(iv) constitutes 50-100% of the total weight of the composition.
Owner:ZHOU FANGQIANG
Conivaptan-hydrochloride novel crystal form and preparation method thereof
ActiveCN103497195AImprove stabilityImprove bioavailabilityOrganic chemistryMetabolism disorderSolubilityDecreased sodium
The invention relates to the field of medicinal chemistry, and discloses a conivaptan-hydrochloride novel crystal form and a preparation method of the conivaptan-hydrochloride novel crystal form. The conivaptan-hydrochloride novel crystal form is single in crystal form, high in purity, good in stability, high in solubility and bioavailability, capable of restraining arginine vasopressin V1a and V2 receptors, and capable of being used for preparing medicine for treating hyponatremia. The preparation method of the conivaptan-hydrochloride novel crystal form is simple to operate, avoids complex and fussy crystallization processing conditions, enables solvents to be recycled, is low in production cost, and is suitable for industrial production. Products meet medicinal standards and good in stability.
Owner:BEIJING COLLAB PHARMA
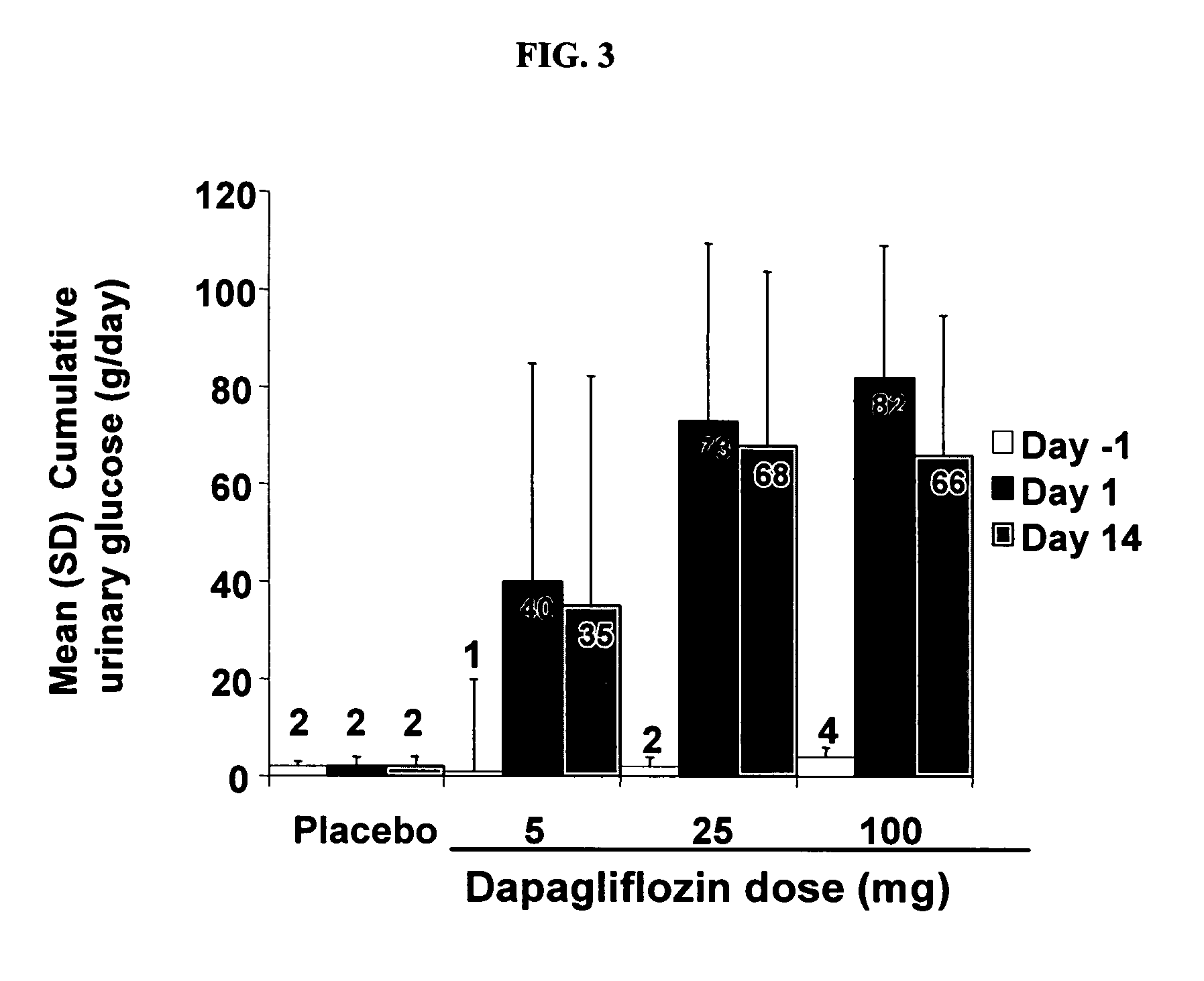
Method for treating hyponatremia employing an SGLT2 inhibitor and composition containing same
Methods are provided for treating hyponatremia, employing an SGLT2 inhibitor alone, or in combination with a supply of carbohydrate, and / or in combination with a diuretic agent. Additionally, compositions comprising an SGLT2 inhibitor, optionally with a supply of carbohydrate, and / or a combination of an SGLT2 inhibitor and a diuretic agent are provided in the instant invention and are provided for use in the inventive methods.
Owner:ASTRAZENECA AB
Dairy product drink and preparation method thereof
The invention provides a dairy product drink and a preparation method thereof and relates to the field of food processing. The dairy product drink contains 10-20 parts by weight of pure milk and 101 parts by weight of yoghourt. The preparation method comprises the following preparation steps: sterilizing, cooling, removing milk skin, inoculating, fermenting, demulsifying, re-fermenting, mixing, sterilizing, cooling and encapsulating. The dairy product drink and the preparation method thereof provided by the invention have the benefits that the original-flavored dairy product drink is made by using common pure milk, does not contain harmful additive and can eliminate multifaceted health problems caused by improper use of a food additive; the dairy product drink is suitable for a wide range of people, has the efficacies of enhancing immunity, nourishing stomach, realizing high protein blood fat removal and blood pressure reduction, strengthening brain, calming nerves, relieving restlessness, improving eyesight, moistening intestine, beautifying and caring skin, and can relieve tension fatigue, dry eye soreness, fatigue, eye fatigue, chronic fatigue syndrome, osteoporosis in the aged, hyponatremia in the aged, postmenopausal osteoporosis and other symptoms.
Owner:BURQIN COUNTY BAHAR BEVERAGE CO LTD
Application of scutellaria baicalensis in preparing medicament for treating ascites due to cirrhosis
InactiveCN105193930APromote excretionImprove liver and kidney functionDigestive systemBlood disorderDecreased sodiumHypoproteinemia
An application of scutellaria baicalensis in preparing a medicament for treating ascites due to cirrhosis relates to a novel application of the scutellaria baicalensis. The invention aims at providing a novel application of the scutellaria baicalensis, and particularly relates to the application of the scutellaria baicalensis in a medicament for treating ascites due to cirrhosis, a medicament for treating liver function damage, a medicament for treating hepatic fibrosis, a medicament for treating liver tissue damage, a medicament for treating hypoproteinemia and a medicament or treating cholestasis. Compared with a modern therapy method, the application of the scutellaria baicalensis has the advantage that the complications, such as hepatic encephalopathy, renal function damage, hyponatremia, hypokalemia or hyperkalemia and the like are avoided, also has the advantages of low treatment cost, simple use method, ideal treatment effect and the like and is conductive to clinical practical application. The scutellaria baicalensis is used for treating ascites due to cirrhosis.
Owner:刘树民
Lixivaptan crystal form I and preparation method and use thereof
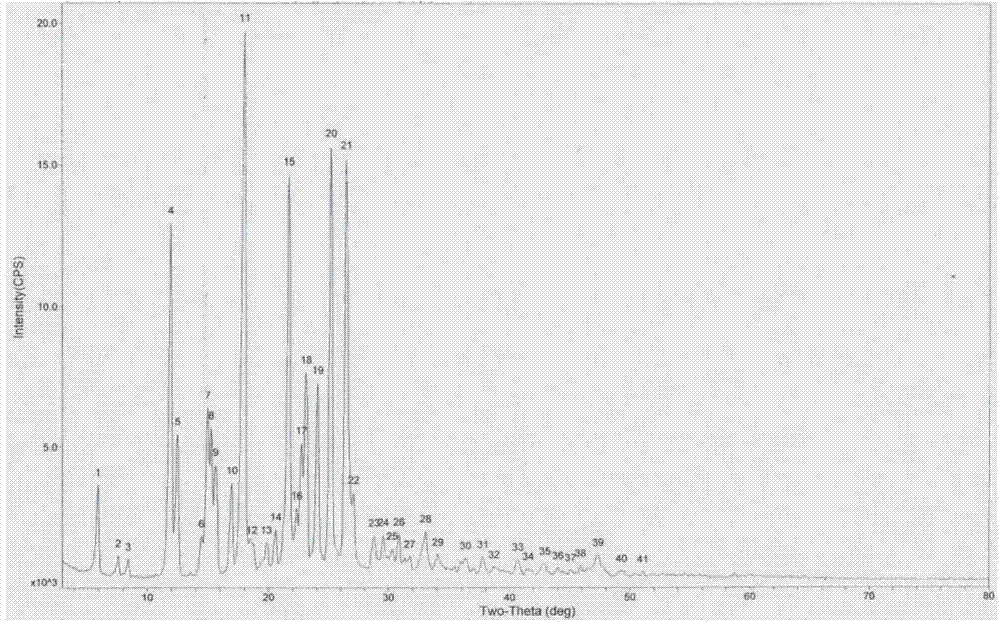
The invention belongs to the field of anti heart failure drugs, and more specifically, relates to lixivaptan crystal form I and a preparation method and use of a pharmaceutical composition containing the lixivaptan crystal form I in preparation of drugs for treating hyponatremia. The lixivaptan crystal form I is prepared from 2-chloro-4-nitro benzoic acid as a starting material by esterification, hydrogenation reduction, acylation, hydrolysis, acyl chlorination and other reaction steps, and the prepared lixivaptan purity is 97.5%. The lixivaptan crystal form I and its powder are characterized by X ray diffraction diagrams, and the lixivaptan crystal form I is important for obtaining of compounds with high purity, very determined crystal form and good reproducibility due to good prospects for development and pharmaceutical value of the lixivaptan crystal form I.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Lixivaptan crystal form II, preparation method and use thereof
The invention belongs to the field of anti-heart failure drugs, and more particularly relates to a lixivaptan crystal form II and a preparation method thereof, a drug composition containing the lixivaptan crystal form II, and a use of the lixivaptan crystal form II in preparation of hyponatremia treatment drugs. According to the present invention, 2-chloro-4-nitrobenzoic acid is adopted as a starting raw material, and esterification, hydrogenation reduction, acylation, hydrolysis, chlorination and other reaction steps are performed to prepare the lixivaptan crystal form II, wherein the purity of the obtained lixivaptan is 97.5%, and the lixivaptan crystal form II is characterized by the powder X-ray diffraction pattern; and due to the good development prospects and the pharmaceutical values of the compound, it is important to obtain the compound with characteristics of high purity, determined crystal form and good reproducibility.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
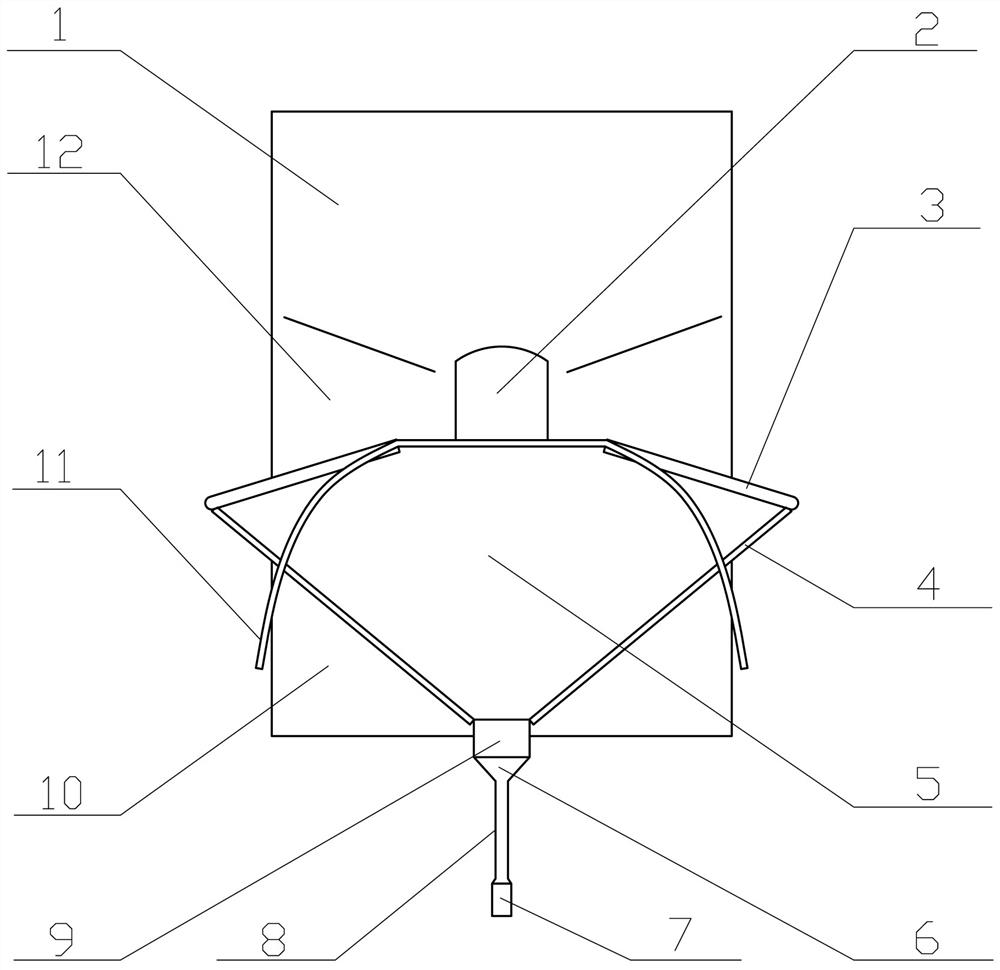
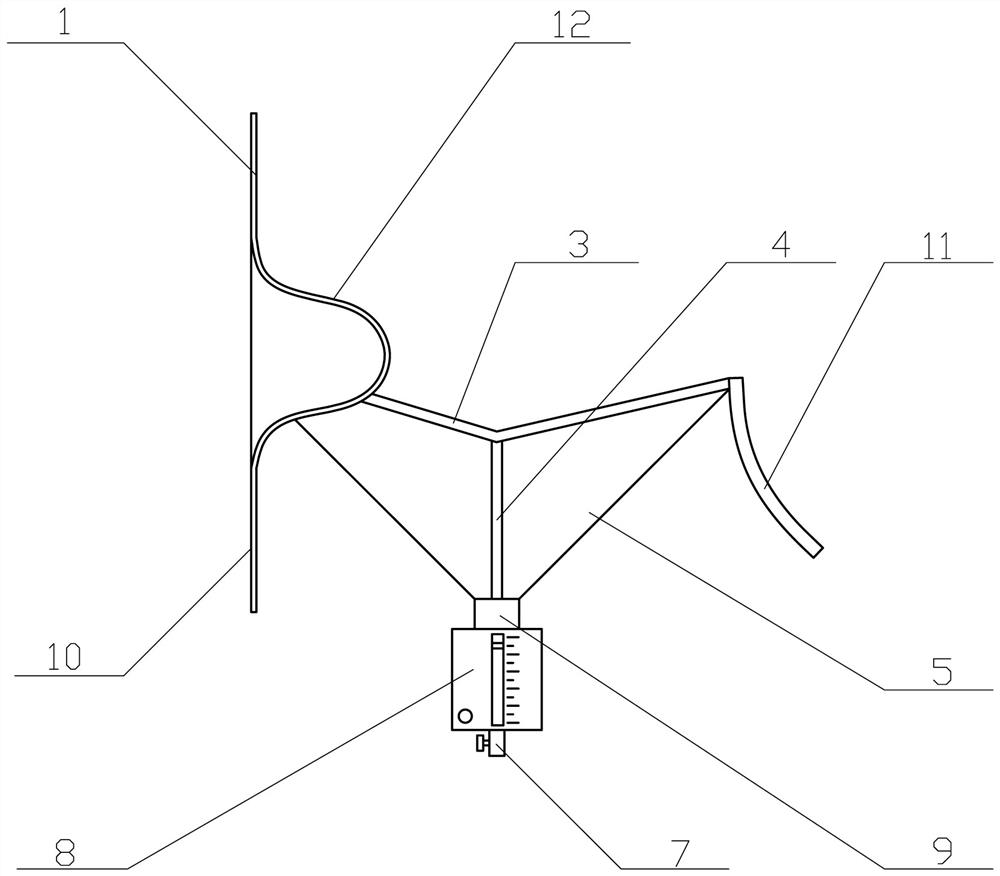
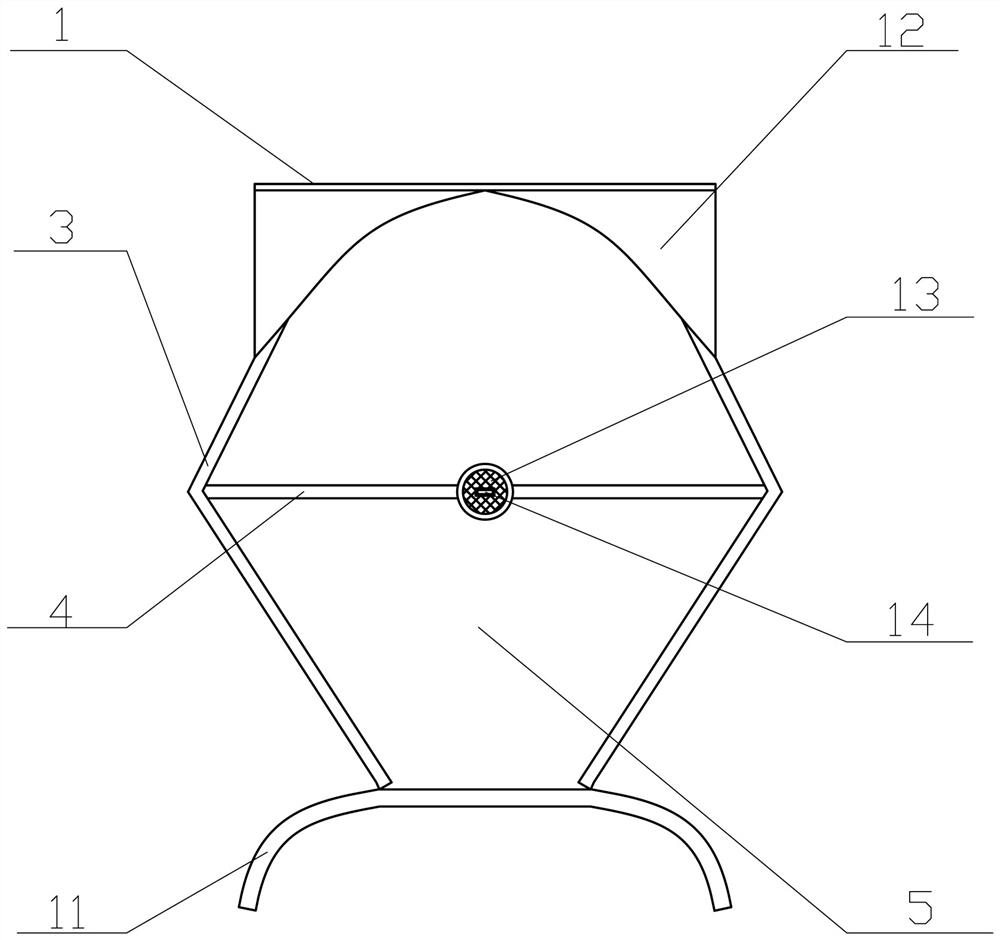
Uterine cavity perfusate recovery and metering, tissue specimen collection and pollution protection device
The invention provides a uterine cavity perfusate recovery and metering, tissue specimen collection and pollution protection device. The uterine cavity perfusate recovery and metering, tissue specimen collection and pollution protection device comprises a funnel body, an upper patch and a lower patch connected with the upper patch. An operation opening is formed in the connecting position of the upper patch and the lower patch, the front edge of the funnel body is connected to the lower part of the operation opening, and a neck tying belt is arranged on the rear edge of the funnel body. Gum is arranged on the sides, away from the funnel body, of the upper patch and the lower patch, and a liquid storage bag with scales is arranged at a ventage of the funnel body. According to the uterine cavity perfusate recovery and metering, tissue specimen collection and pollution protection device, the absorption amount of the perfusate can be accurately obtained, and potential serious risks can be found and treated in time, so that the occurrence of body fluid overload and / or dilution hyponatremia caused by the fact that a large amount of perfusate is absorbed is prevented; operative complications are reduced, and important clinical significance for reducing medical accidents and protecting the life safety of a patient is achieved; and moreover, specimens can be effectively collected, specimen loss is prevented, perfusate splashing pollution is prevented, and high popularization value is achieved.
Owner:THE SECOND HOSPITAL OF HEBEI MEDICAL UNIV
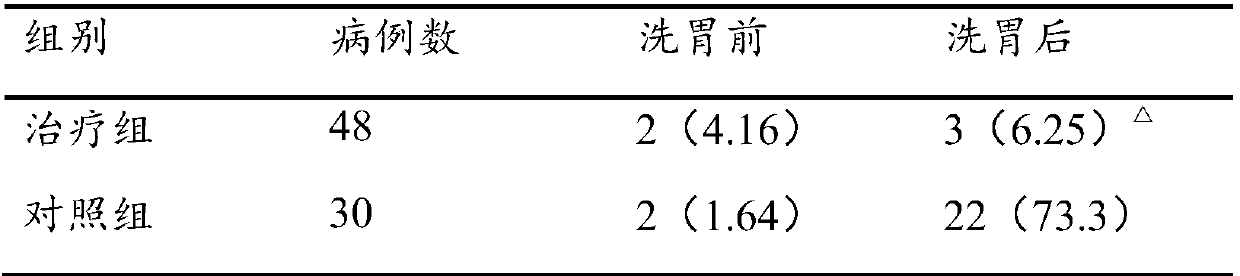
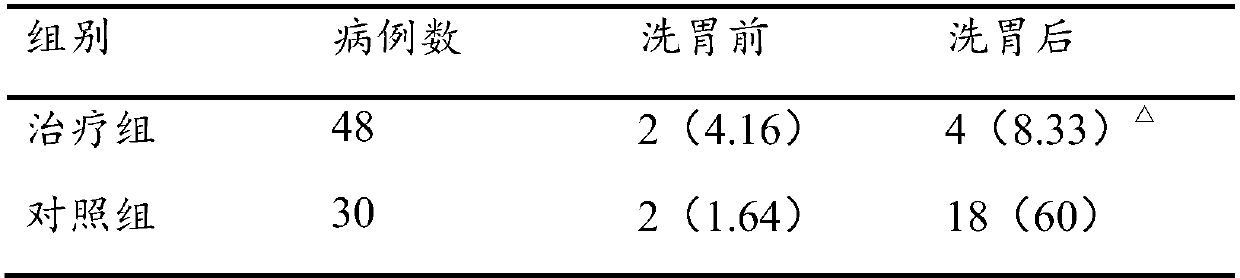
Stomach cleaning combined reagent used for gastroenterology department and preparation method thereof
InactiveCN107929702AImprove antibacterial propertiesPromote secretionDigestive systemBird material medical ingredientsDecreased sodiumAdditive ingredient
The invention discloses a stomach cleaning combined reagent used for the gastroenterology department. The stomach cleaning combined reagent comprises the following ingredients in parts by weight: 10-20 parts of panax notoginseng, 3-9 parts of radix astragali, 5-15 parts of radix codonopsis, 10-15 parts of Mangnolia officinalis, 8-12 parts of radix glycyrrhizae, 5-15 parts of rhizoma corydalis, 12-20 parts of cistanche salsa, 10-15 parts of bitter gourds, 3-8 parts of honeysuckles, 5-10 parts of endothelium corneum gigeriae galli, 8-10 parts of chrysanthemums, 5-10 parts of fructus forsythia, 3-7 parts of royal jelly, 2-8 parts of honey, 5-10 parts of rhizoma cyperi, 5-15 parts of fresh ginger, 10-15 parts of barleys, 50-100 parts of normal saline and 50-100 parts of 10% potassium chloride.Compared with the prior art, the stomach cleaning combined reagent has the beneficial effects that the incidence rate of complications, that is the incidence rate of hyponatremia and hypokalemia in astomach cleaning process can be reduced; meanwhile, the stomach cleaning combined reagent has an effect of nursing a stomach and is beneficial to later recovery of the stomach.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Rehydration salt composition for preventing labor type heatstroke
ActiveCN112933151AGreat tasteConducive to high-intensity training, hydration and salt complianceOrganic active ingredientsAntipyreticBiotechnologySucrose
The invention discloses a rehydration salt composition for preventing labor type heatstroke, and belongs to the technical field of combination of Chinese and western medicines. The rehydration salt composition is prepared from the following raw materials in parts by weight: 0.585 part of sodium chloride, 0.13 part of potassium chloride, 0.0215 part of calcium citrate malate, 0.9785 part of anhydrous dextrose, 0.06 part of DL-malic acid, 0.025 part of sucralose, 0.9785 part of citric acid juice powder, 0.2 part of agastache rugosus extract and 0.1 part of pericarpium citri reticulatae extract. The rehydration salt composition has good taste, and is beneficial to high-intensity training of water and salt supplement compliance; after water and salt are supplemented, the blood volume in the body is increased, hyponatremia is reduced, the sweating amount can be increased, the heat dissipation capacity of the body is improved, and heatstroke and heat stroke caused by excessive temperature rise are avoided; and the traditional Chinese medicine, namely the agastache rugosus has the effect of preventing heatstroke, and the pericarpium citri reticulatae has the effects of promoting water absorption and reducing gastrointestinal discomfort.
Owner:AIR FORCE MEDICAL CENT PLA
Traditional Chinese medicine for treating hyperuricemia complicated with joint swelling
InactiveCN106924608AEasy dischargeCompatibility scienceSkeletal disorderPlant ingredientsDecreased sodiumMedicinal herbs
The invention relates to the technical field of medicines, and in particular relates to a traditional Chinese medicine for treating hyperuricemia complicated with joint swelling. The traditional Chinese medicine is prepared from the following medicinal materials in parts by weight: 20-30 parts of folium isatidis, 20-30 parts of dandelion, 20-30 parts of notopterygium root, 15-25 parts of herba potentillae discoloris cum radice, 15-25 parts of cortex moutan, 13-17 parts of rhizoma anemarrhenae, 13-17 parts of herba centellae, 10-20 parts of indigo naturalis, 10-20 parts of herba houttuyniae, 10-20 parts of fructus trichosanthis, 15-19 parts of radix paeoniae rubra, 12-18 parts of rhizoma atractylodis, 11-17 parts of rhizoma coptidis, 10-14 parts of rhizoma smilacis glabrae, 10-14 parts of rhizoma polygoni cuspidati, 10-14 parts of radix notoginseng, 10-14 parts of black soya beans, 11-17 parts of rhizoma cyperi, 10-18 parts of radix saposhnikoviae, 10-22 parts of herba artemisiae annuae, 5-9 parts of herba plantaginis, and 3-7 parts of licorice root. For the traditional Chinese medicine for treating hyperuricemia complicated with joint swelling, through scientific mixing, the ingredients achieve a synergic effect, so that the traditional Chinese medicine can promote the discharge of uric acid, supplement the elements including sodium, potassium, calcium, zinc and the like, and prevent the side reactions including hyponatremia, hypokalemia, hypocalcemia and the like, and also has the efficacies of relieving swelling and pain, regulating Qi and blood, clearing damp and dissolving turbidity, and treating arthritis.
Owner:QINGDAO ZHITONG SIHAI FURNITURE DESIGN RES & DEV CO LTD
Lixiptan crystal form Ⅱ and its preparation method and use
The invention belongs to the field of anti-heart failure drugs, and more particularly relates to a lixivaptan crystal form II and a preparation method thereof, a drug composition containing the lixivaptan crystal form II, and a use of the lixivaptan crystal form II in preparation of hyponatremia treatment drugs. According to the present invention, 2-chloro-4-nitrobenzoic acid is adopted as a starting raw material, and esterification, hydrogenation reduction, acylation, hydrolysis, chlorination and other reaction steps are performed to prepare the lixivaptan crystal form II, wherein the purity of the obtained lixivaptan is 97.5%, and the lixivaptan crystal form II is characterized by the powder X-ray diffraction pattern; and due to the good development prospects and the pharmaceutical values of the compound, it is important to obtain the compound with characteristics of high purity, determined crystal form and good reproducibility.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
New crystal form of conivaptan hydrochloride and preparation method thereof
ActiveCN103497195BHigh purityImprove stabilityOrganic chemistryMetabolism disorderDecreased sodiumSolubility
The invention relates to the field of medicinal chemistry, and discloses a conivaptan-hydrochloride novel crystal form and a preparation method of the conivaptan-hydrochloride novel crystal form. The conivaptan-hydrochloride novel crystal form is single in crystal form, high in purity, good in stability, high in solubility and bioavailability, capable of restraining arginine vasopressin V1a and V2 receptors, and capable of being used for preparing medicine for treating hyponatremia. The preparation method of the conivaptan-hydrochloride novel crystal form is simple to operate, avoids complex and fussy crystallization processing conditions, enables solvents to be recycled, is low in production cost, and is suitable for industrial production. Products meet medicinal standards and good in stability.
Owner:BEIJING COLLAB PHARMA
A kind of rehydration salt composition for preventing exertional heatstroke
ActiveCN112933151BGreat tasteConducive to high-intensity training, hydration and salt complianceOrganic active ingredientsMetabolism disorderBiotechnologyAgaricus
Owner:AIR FORCE MEDICAL CENT PLA
Oral administration of sodium chloride to prevent complications associated with bowel cleansing with stimulant laxatives
PendingUS20220143079A1Prevents unwanted side effectAvoid side effectsPowder deliveryOrganic active ingredientsBowel cleansingSide effect
A method for preventing unwanted side effects associated with the administration stimulant laxative such as bisacodyl or sodium picosulphate by orally administering sodium chloride to the patient is disclosed. Said side effects include hyponatraemia, hypokalemia, unwanted fluctuations in blood pressure, hypotension and renal failure. In certain embodiments, the sodium chloride is administered in the form of tablets during the two hours period following the administration of the stimulant laxative. This method is particularly useful in preparing patients for a colonoscopy.
Owner:SEAFORD PHARMA INC
A kind of colored functional seasoning low-sodium liquid salt and its preparation method
InactiveCN104000166BReduce intakePrevent morbidityNatural extract food ingredientsFood ingredient functionsDecreased sodiumPlant Sources
The invention relates to a color function seasoning low-sodium liquid salt formula and a preparing method of color function seasoning low-sodium liquid salt. Low-sodium liquid salt mother liquor is used as main raw materials of the low-sodium liquid salt. The low-sodium liquid salt mother liquor comprises (according to the mass percent in the mother liquor salt content total quantity) 60%-80% of sodium chloride, 15%-30% of potassium chloride and 5%-15% of magnesium chloride. The low-sodium liquid salt further comprises a plant source function component with the adding quantity (according to the mass added into 100 mL of low-sodium liquid salt mother liquor) is 0.5%-10%. Optimization technologies such as mixing, homogenization, sterilization and filling are carried out, and color function seasoning low-sodium liquid salt products with function component activity being maintained to the maximum degree are prepared. A controllable-quantity liquid packaging container is used, the salt can be easily and evenly blended into food, the problem that solid salt is dissolved slowly, so that excessive adding or uneven dispersing may happen is solved, and accordingly eating is convenient and flexible. Compared with traditional solid salt production, during a production process of the liquid salt, a large amount of equipment needed for salt drying can be reduced, energy loss is lowered, and waste water discharging is lowered.
Owner:CHINA AGRI UNIV
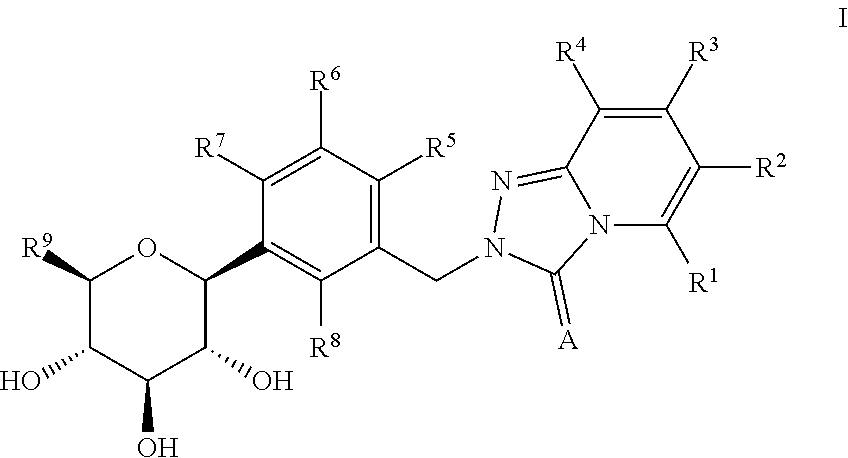
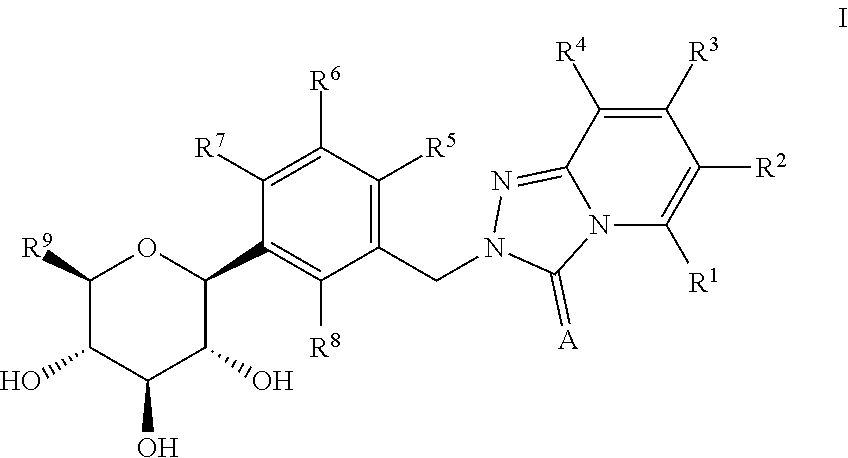
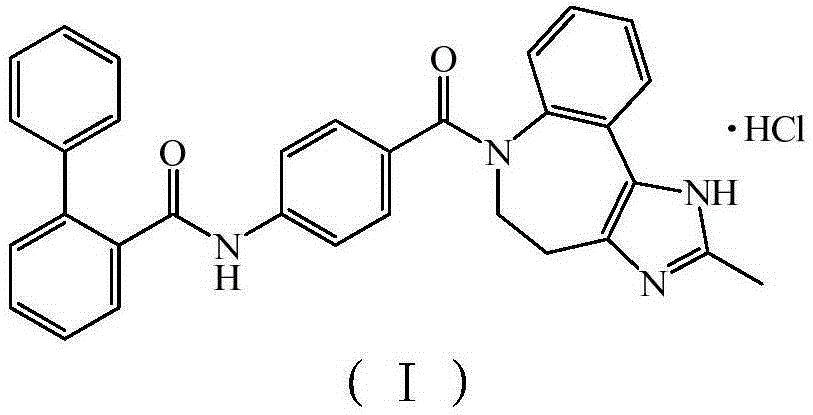
A kind of acyl guanidine compound and its preparation method and application
The invention discloses an acyl guanidine compound with a structure as shown in a formula I (See the specification) and pharmaceutically acceptable salt thereof. The invention also discloses a preparation method of the compound. The invention also discloses a pharmaceutical composition taking the compound or pharmaceutically acceptable salt thereof as an active ingredient, and their application tothe prevention or treatment of diseases (such as hypertension, chronic congestive heart failure, antidiuretic hormone secretion disorder syndrome or hyponatremia caused by chronic heart failure / livercirrhosis / antidiuretic hormone secretion disorder) related to an arginine vasopressin V1a receptor, an arginine vasopressin V1b receptor and an arginine vasopressin V2 receptor.
Owner:天津泰普制药有限公司 +1
Compound sodium acetate electrolyte injection and preparation method thereof
InactiveCN101167740BFast metabolismNo accumulationMetabolism disorderPharmaceutical delivery mechanismSodium acetateDecreased sodium
The utility model relates to the technical field of chemical, in particular to a compound electrolyte injection of sodium acetate and process for preparation, wherein the compound electrolyte injection of sodium acetate takes natrium chloride, potassium chloride, calcium gluconate, magnesium chloride, sodium acetate, sodium citrate and glucose as raw materials. The compound electrolyte injection of sodium acetate of the invention is called sodium acetate equilibrium liquid because pH, osmotic pressure and molar concentration containing various particles are approximate to extra cellular fluidof human body. The invention is used to treat shock caused by oligemia of circulating blood and reduction of tissue fluid caused by operation, trauma, scald and profuse bleeding, to replenish and adjust the extra cellular fluid, to correct metabolic acidosis and to substitute partial blood transfusion as a crystalloid blood volume extended dose. The invention has the advantages of doing effectively organ perfusion, maintaining circularity stable, replenishing electrolyte, improving hyponatremia during shock, preventing and correcting acidosis.
Owner:CHINA OTSUKA PHARM CO LTD
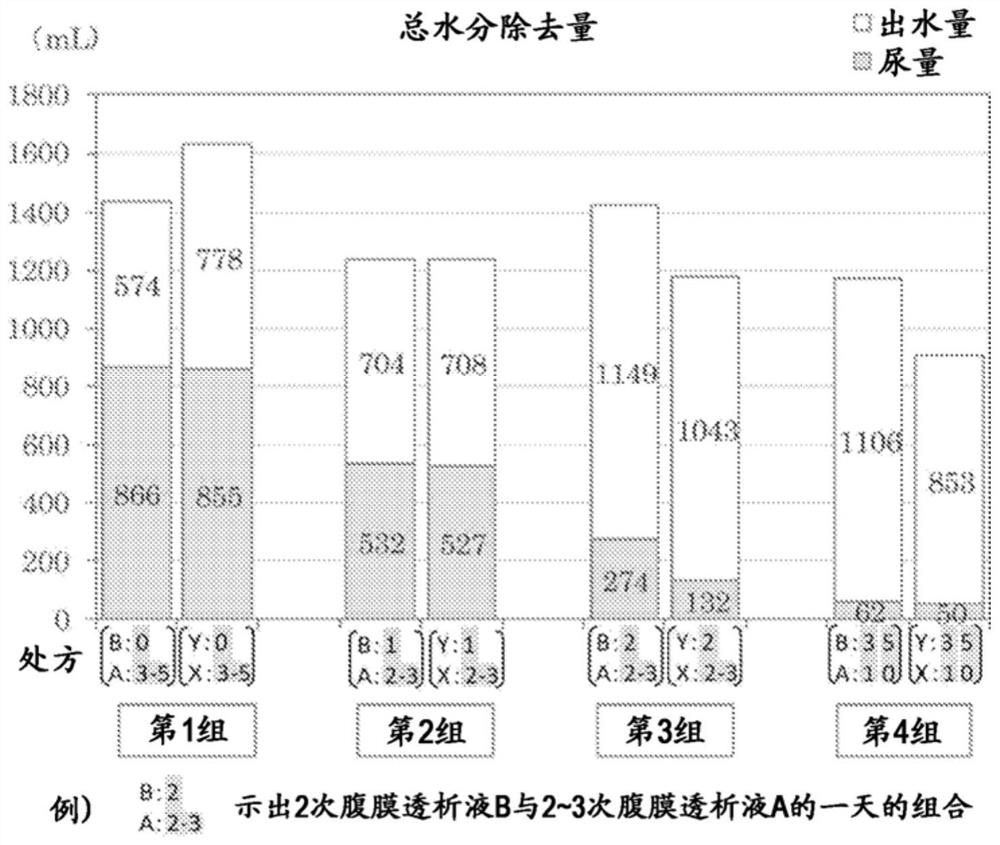
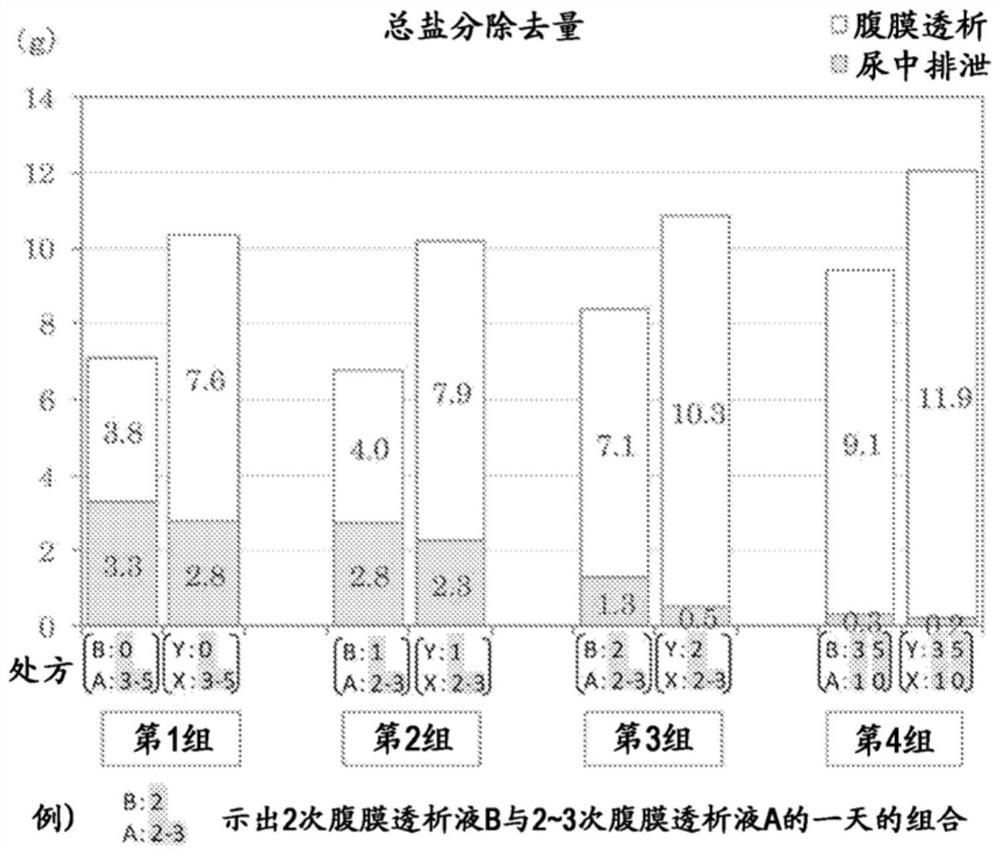
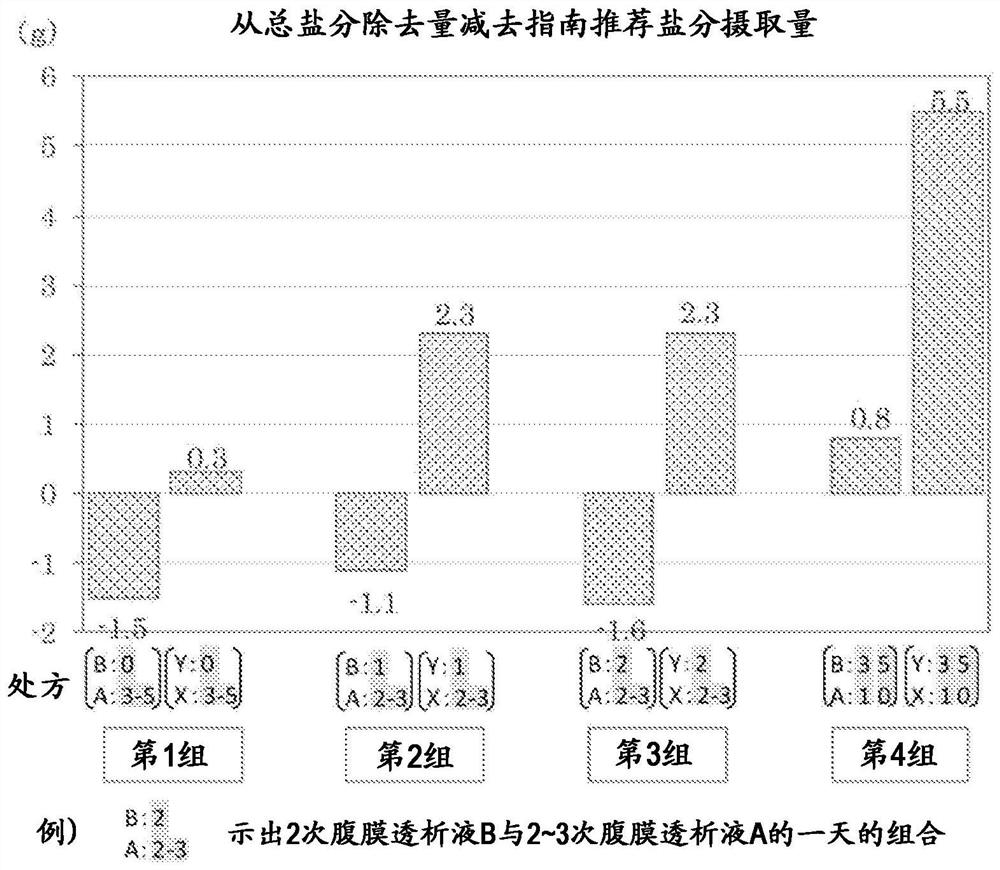
Peritoneal dialysate fluid, peritoneal dialysate fluid set, composition for use in peritoneal dialysis, and peritoneal dialysis method
PendingCN111867650AOrganic active ingredientsInorganic non-active ingredientsPeritoneal dialysis fluidDialysis fluids
The present invention provides a means which can appropriately control the balance between water and sodium in peritoneal dialysis therapies while avoiding adverse effects on the residual renal function and without causing hyponatremia. This peritoneal dialysate fluid contains 1.5 to 1.8 w / v% of glucose and 120 to 130 mEq / L of sodium, and is used alone one or two times a day. The peritoneal dialysate fluid is used in combination with another peritoneal dialysate fluid containing 1.35 to 2.5 w / v% of glucose and 132 to 135 mEq / L of sodium in peritoneal dialysis where the following steps are performed three to five times a day: injecting 1.5 to 2.0 L of a peritoneal dialysate fluid into the abdominal cavity of a subject; retaining the peritoneal dialysate fluid; and discharging the peritonealdialysate fluid.
Owner:TERUMO KK
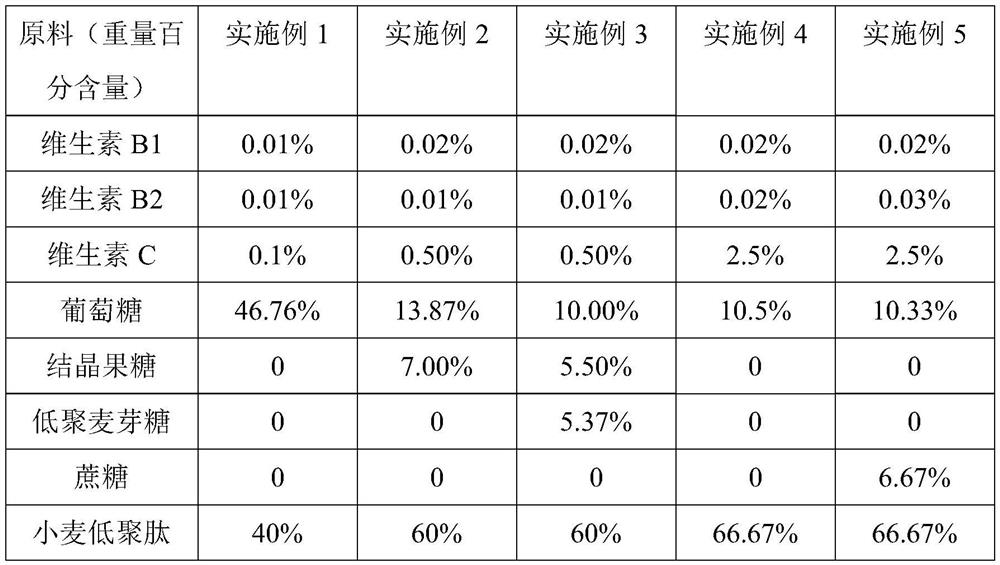
Sports nutrition tablet for improving endurance and preparation method thereof
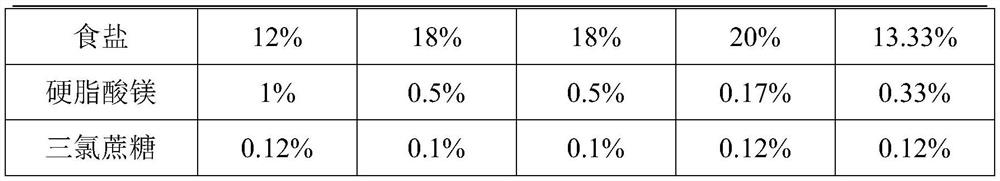
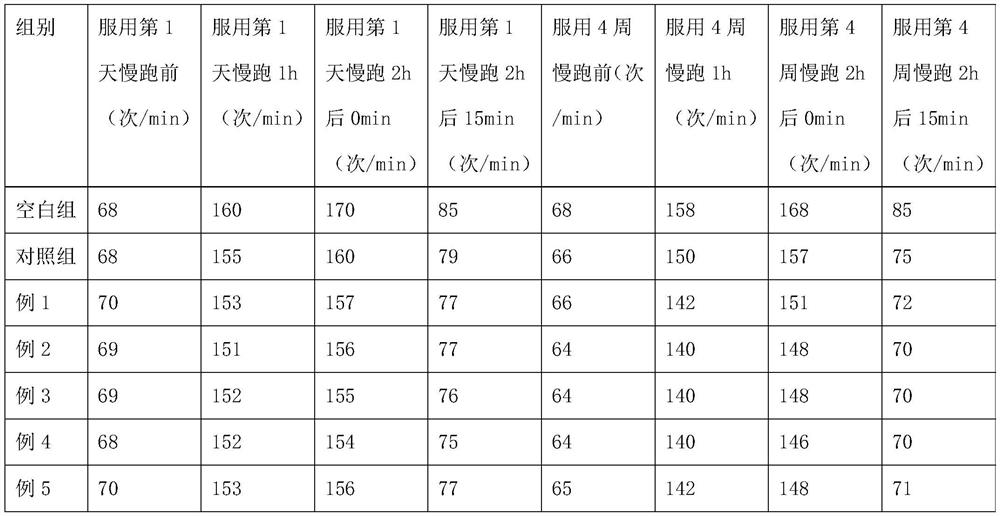
The invention discloses a sports nutrition tablet for improving endurance and a preparation method thereof. The nutrition tablet comprises the following components in percentage by weight: 10-47% of saccharides, 40-70% of wheat oligopeptide, 0.1-0.61% of compound vitamins and 12-20% of salt. The sports nutrition tablet disclosed by the invention is convenient to carry and eat, and can improve metabolism, promote energy substances to participate in aerobic oxidation, accelerate the speed of generating energy by an organism, reduce fatigue feeling, prevent hyponatremia and improve endurance quality.
Owner:广州美春堂医药科技有限公司
Nutritional compositions for the management of hyponatremia
The present invention involves novel urea compositions for oral administration, that are useful for treatment or management of hyponatremia. Also disclosed are novel methods of making the urea compositions.
Owner:NEPHCENTRIC LLC
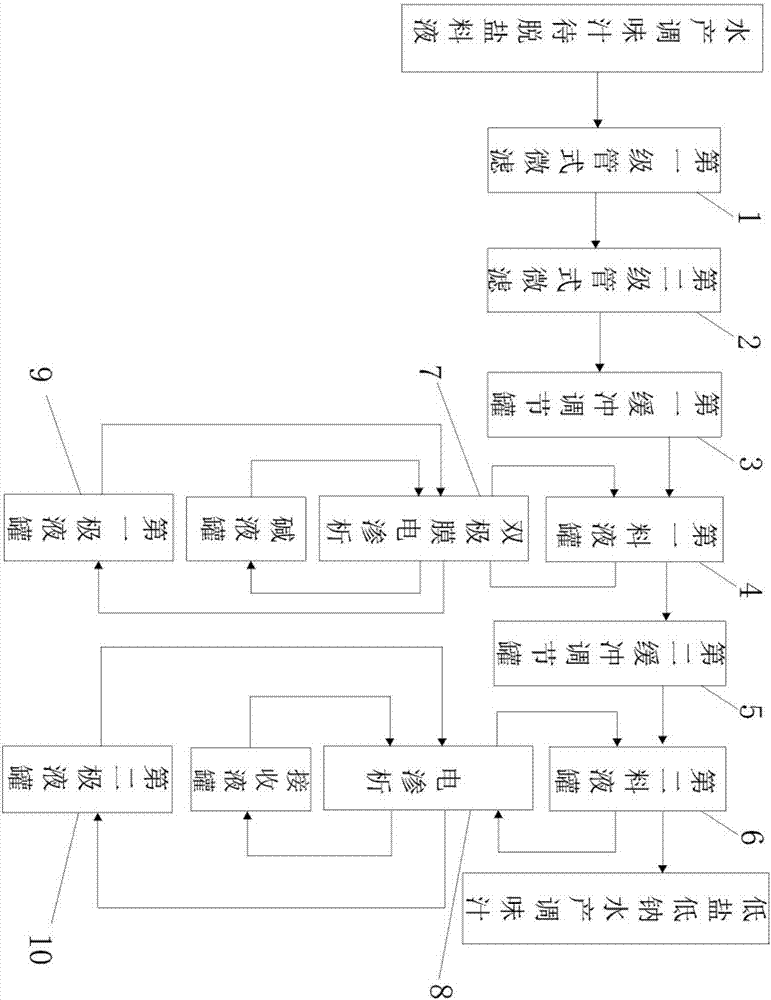
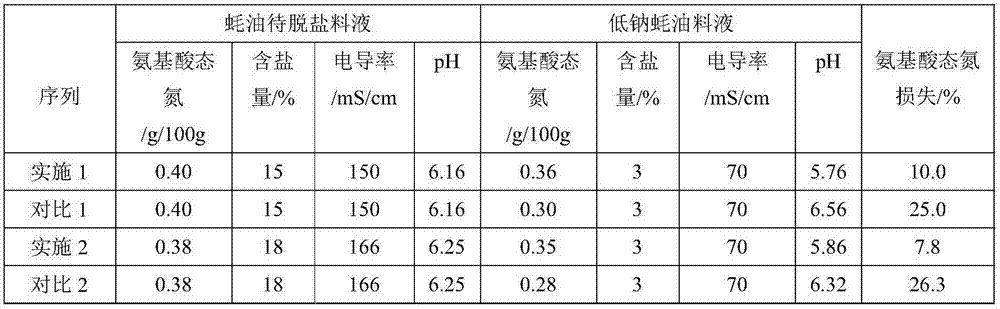
A low-sodium treatment process of aquatic seasoning sauce by electrodialysis
ActiveCN106334451BReduce churnReduce lossesSemi-permeable membranesGeneral water supply conservationDecreased sodiumImpurity
The invention belongs to the field of low sodium treatment of aquatic product sauce and particularly relates to a process for electrodialysis low sodium treatment of aquatic product sauce. The process comprises a step (1) of filtering to-be-desalted feed liquid of the aquatic product sauce sequentially through a primary pipe type microfiltration device and a secondary pipe type microfiltration device to remove impurities such as suspended solids, and obtaining clear aquatic product sauce feed liquid, and causing the clear aquatic product sauce feed liquid to enter a first buffer adjusting tank; a step (2) of causing the clear aquatic product sauce in the first buffer adjusting tank to enter a first feed liquid tank, adjusting pH through a bipolar membrane electroosmosis device, adjusting the pH to an isoelectric point of the aquatic product sauce, obtaining the aquatic product sauce with the pH being at the isoelectric point, and causing the aquatic product sauce to enter a second buffer adjusting tank; a step (3) of causing the aquatic product sauce with the pH being at the isoelectric point in the second buffer adjusting tank, performing desalting treatment through the electroosmosis device, and obtaining a final product which is the low-salt low-sodium aquatic product sauce.
Owner:XIAMEN KENING WOTE WATER TREATMENT TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com