Patents
Literature
883 results about "Renal function" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Renal function is an indication of the kidney's condition and its role in renal physiology. Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. Creatinine clearance rate (CCr or CrCl) is the volume of blood plasma that is cleared of creatinine per unit time and is a useful measure for approximating the GFR. Creatinine clearance exceeds GFR due to creatinine secretion, which can be blocked by cimetidine. Both GFR and CCr may be accurately calculated by comparative measurements of substances in the blood and urine, or estimated by formulas using just a blood test result (eGFR and eCCr) The results of these tests are used to assess the excretory function of the kidneys. Staging of chronic kidney disease is based on categories of GFR as well as albuminuria and cause of kidney disease.
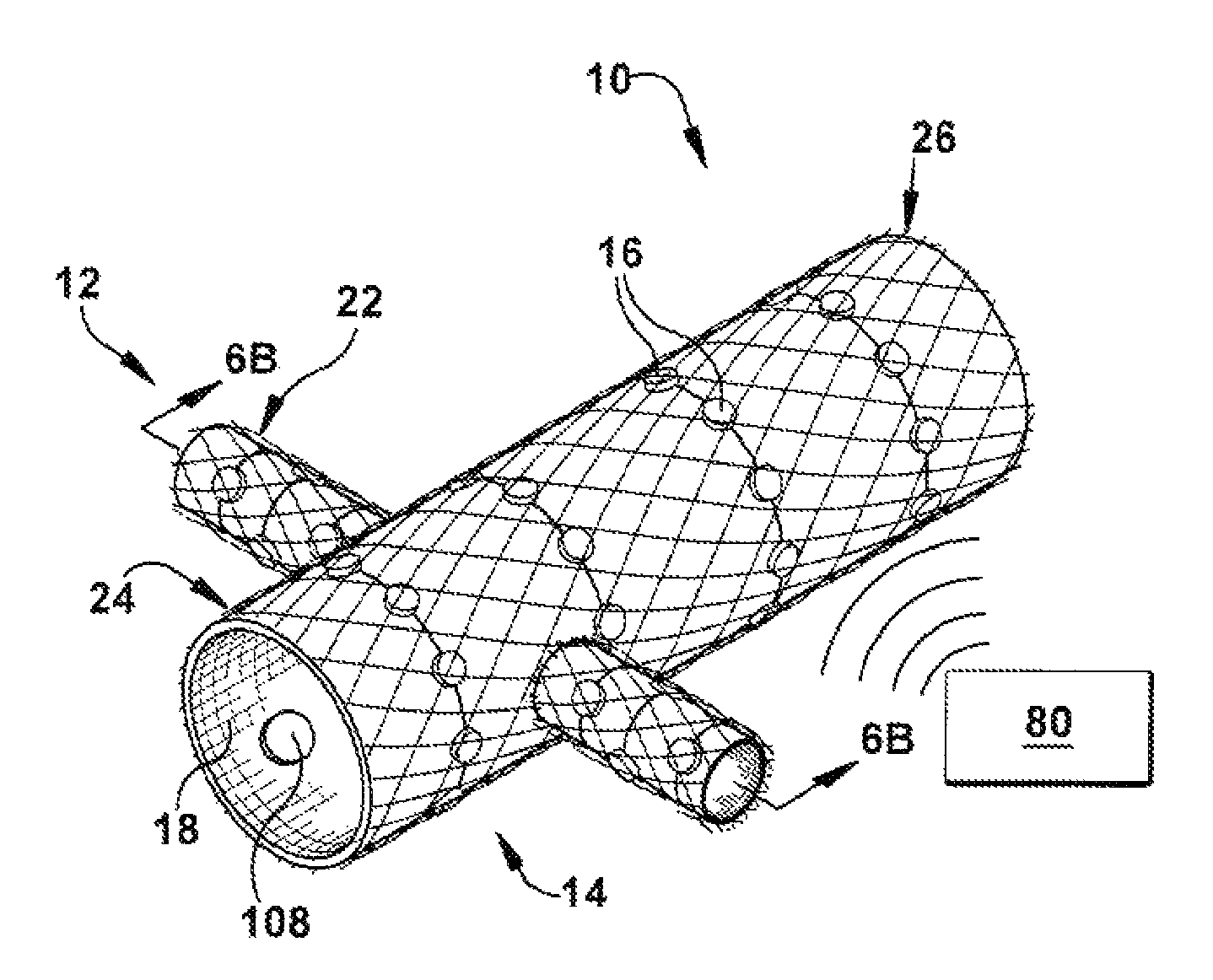
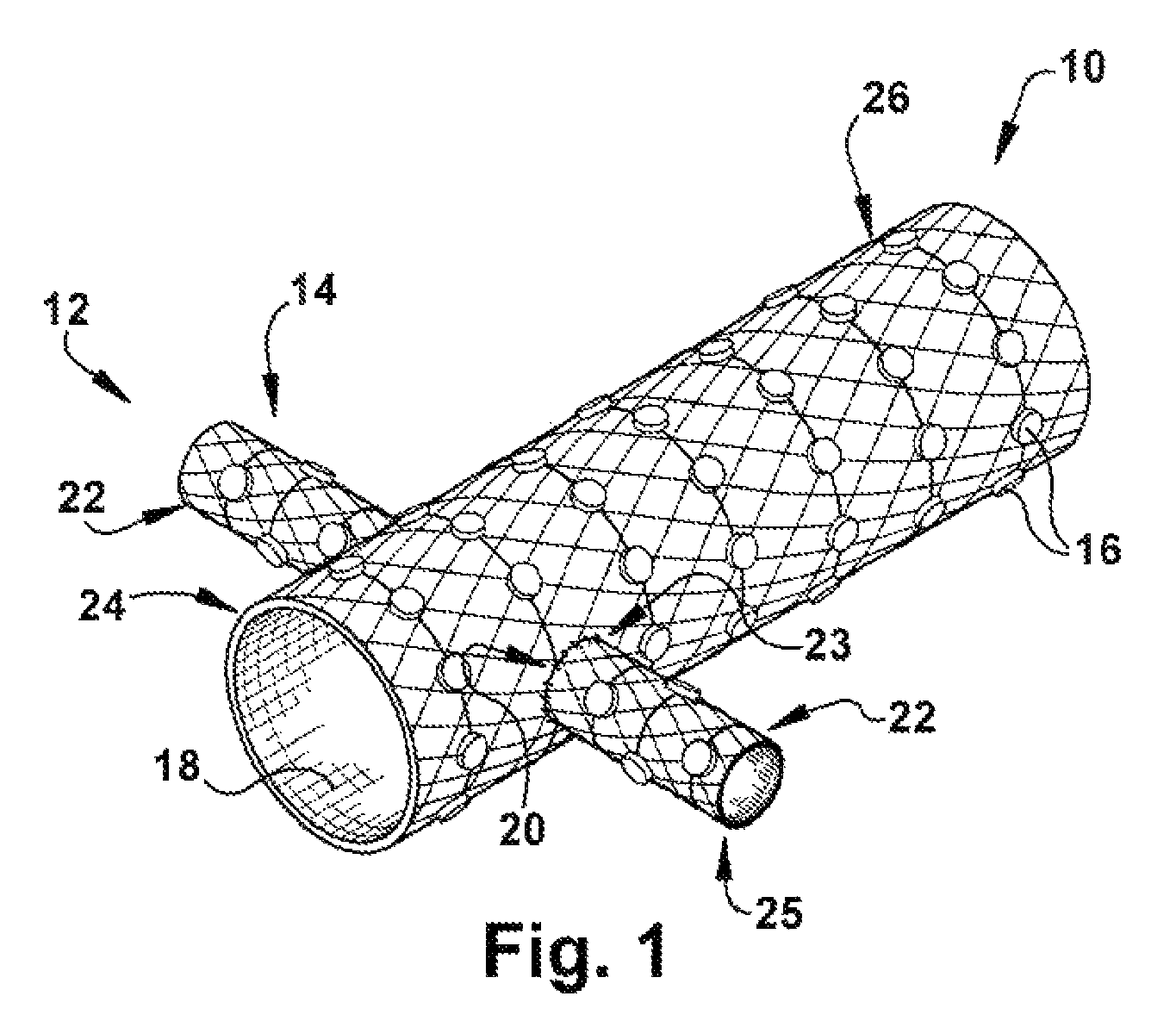
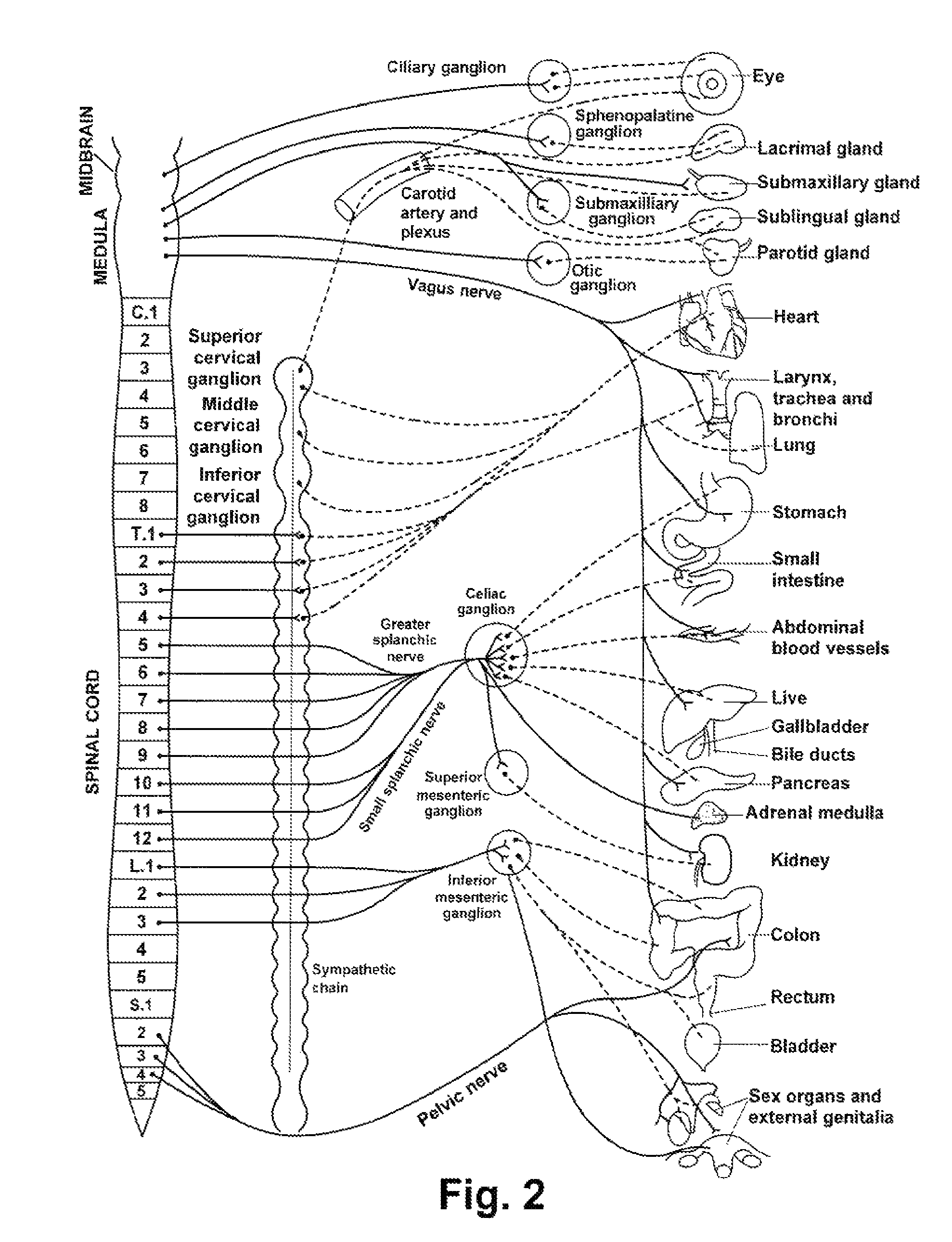
Method and apparatus for renal neuromodulation
An apparatus for renal neuromodulation includes an expandable support member having a main body portion for engaging a wall of a blood vessel proximate a renal vasculature and at least one electrode connected with the main body portion. The at least one electrode is arranged to selectively deliver electric current to a desired location where modulation of the sympathetic nervous system is effective to alter renal function. The apparatus further includes an insulative material attached to at least a portion of the main body portion for isolating blood flow through the vessel from the electric current delivered by the at least one electrode.
Owner:THE CLEVELAND CLINIC FOUND
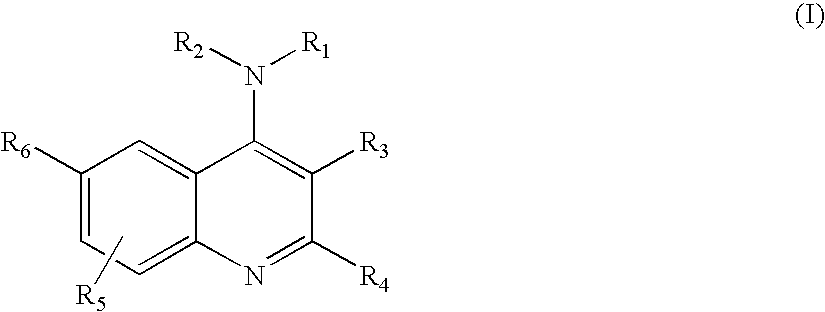
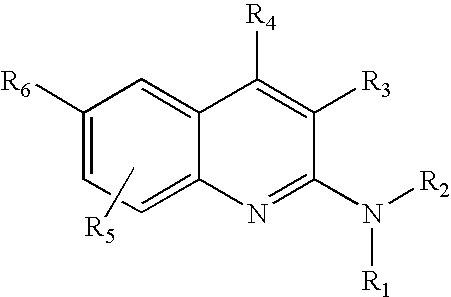
4-Aminoquinoline compounds
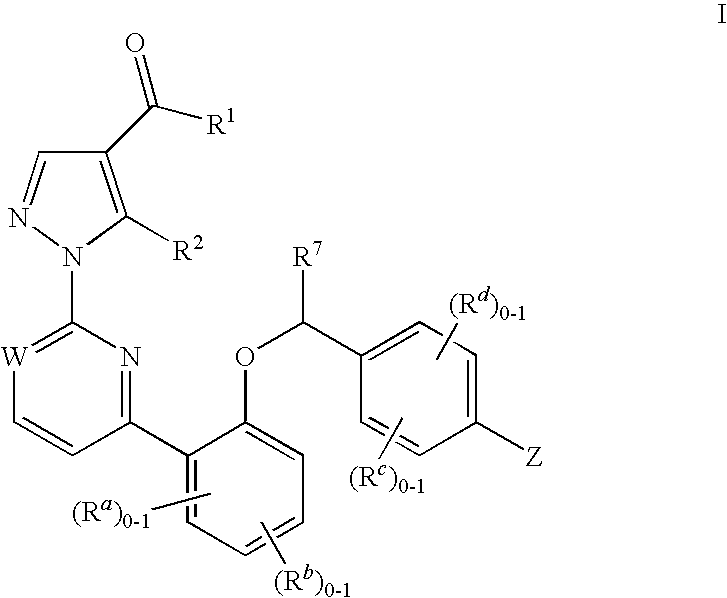
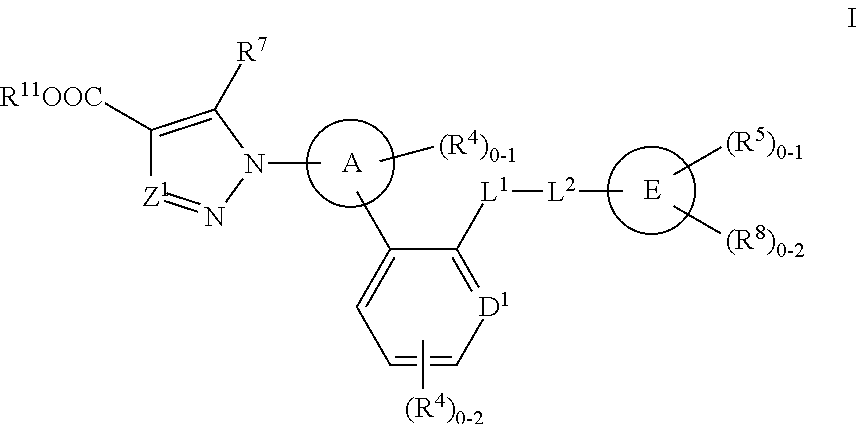
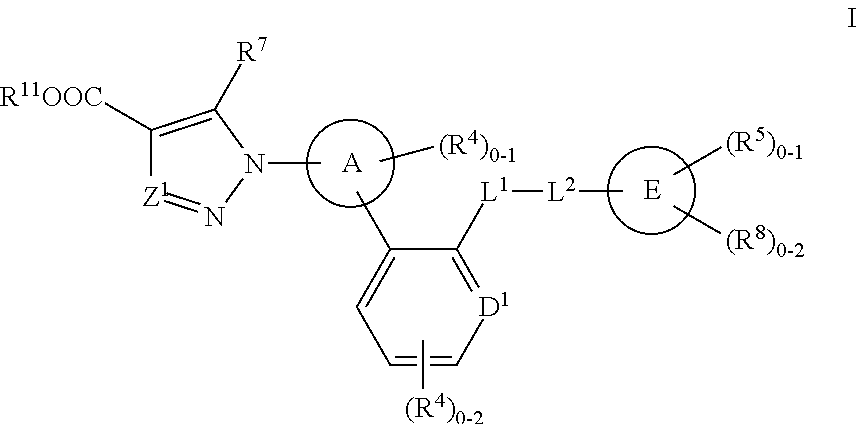
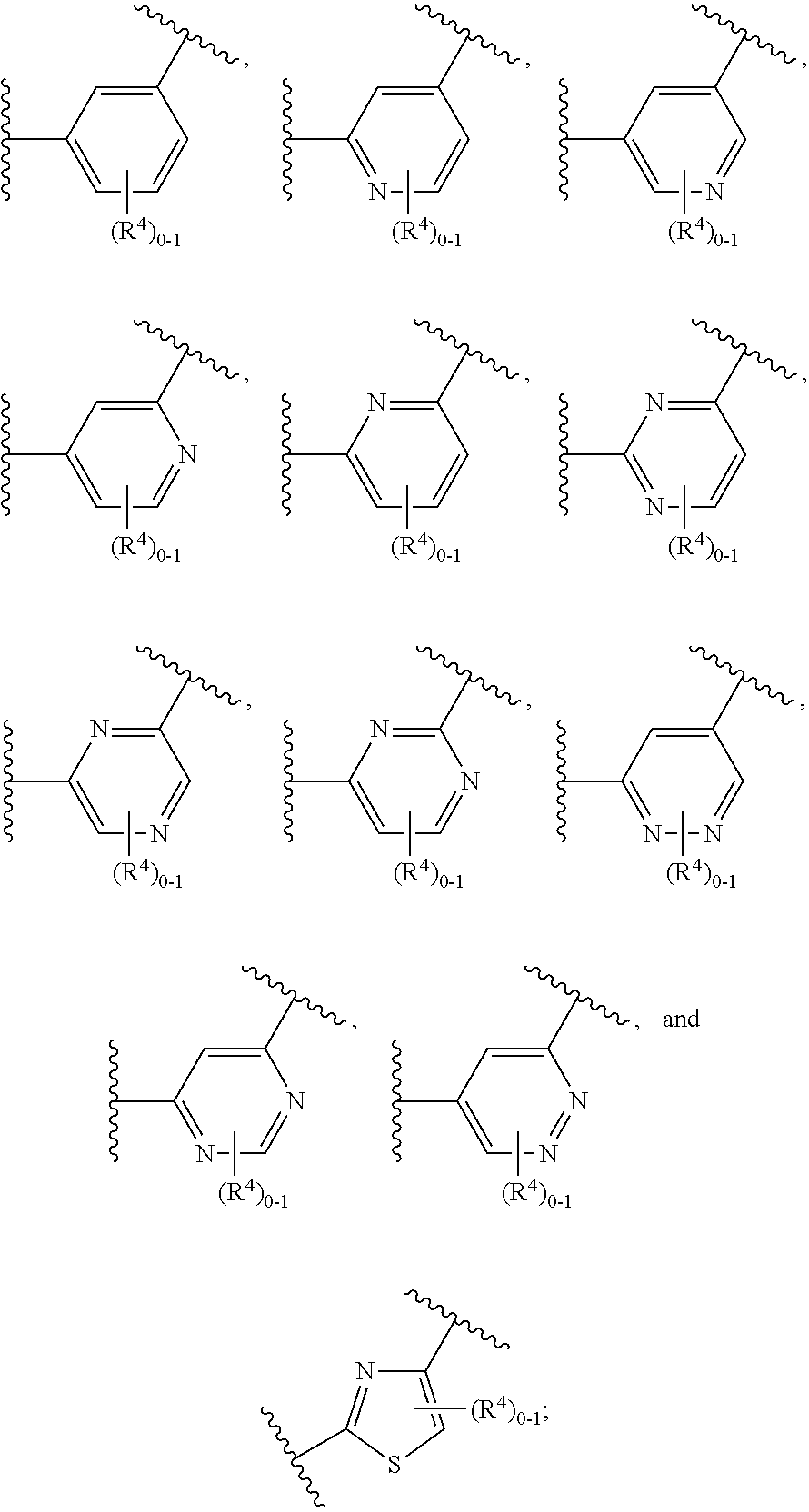
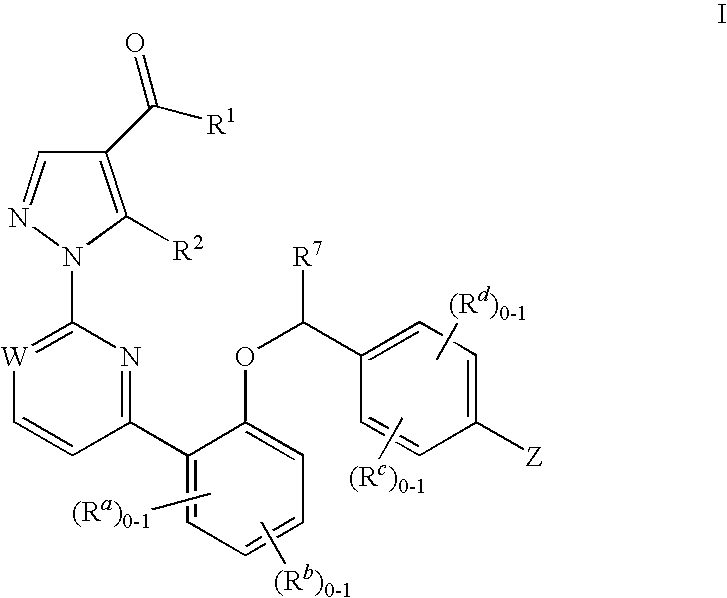
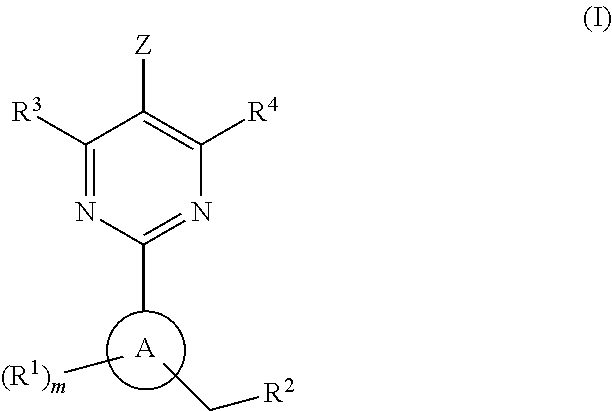
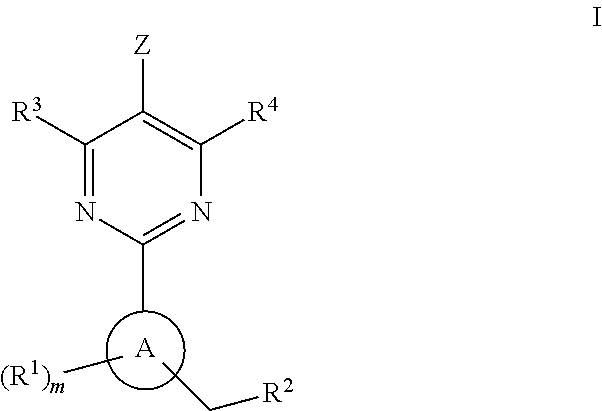
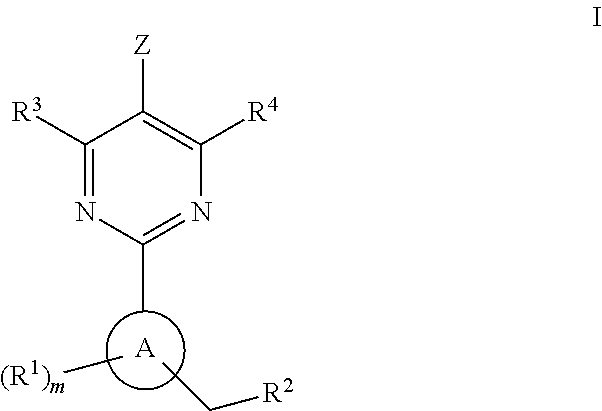
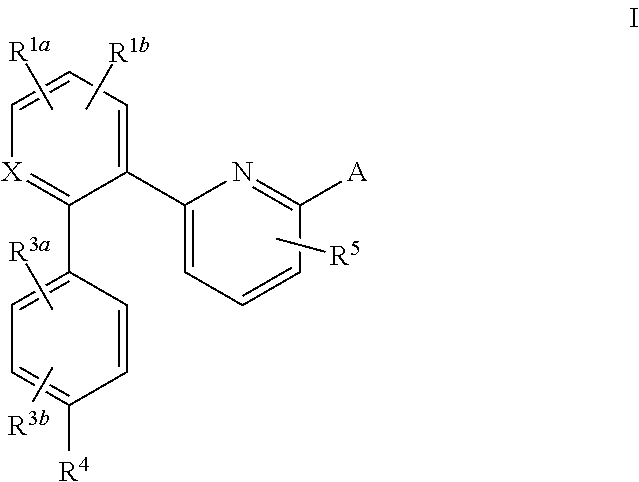
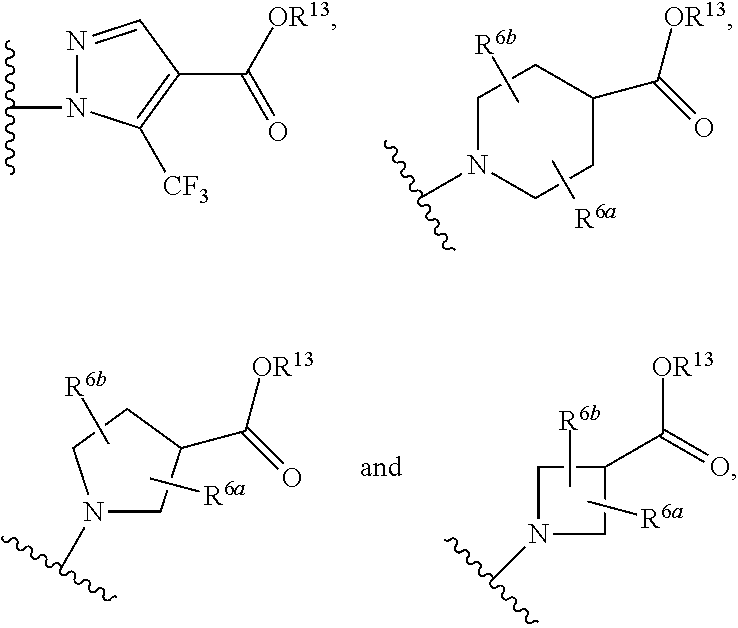
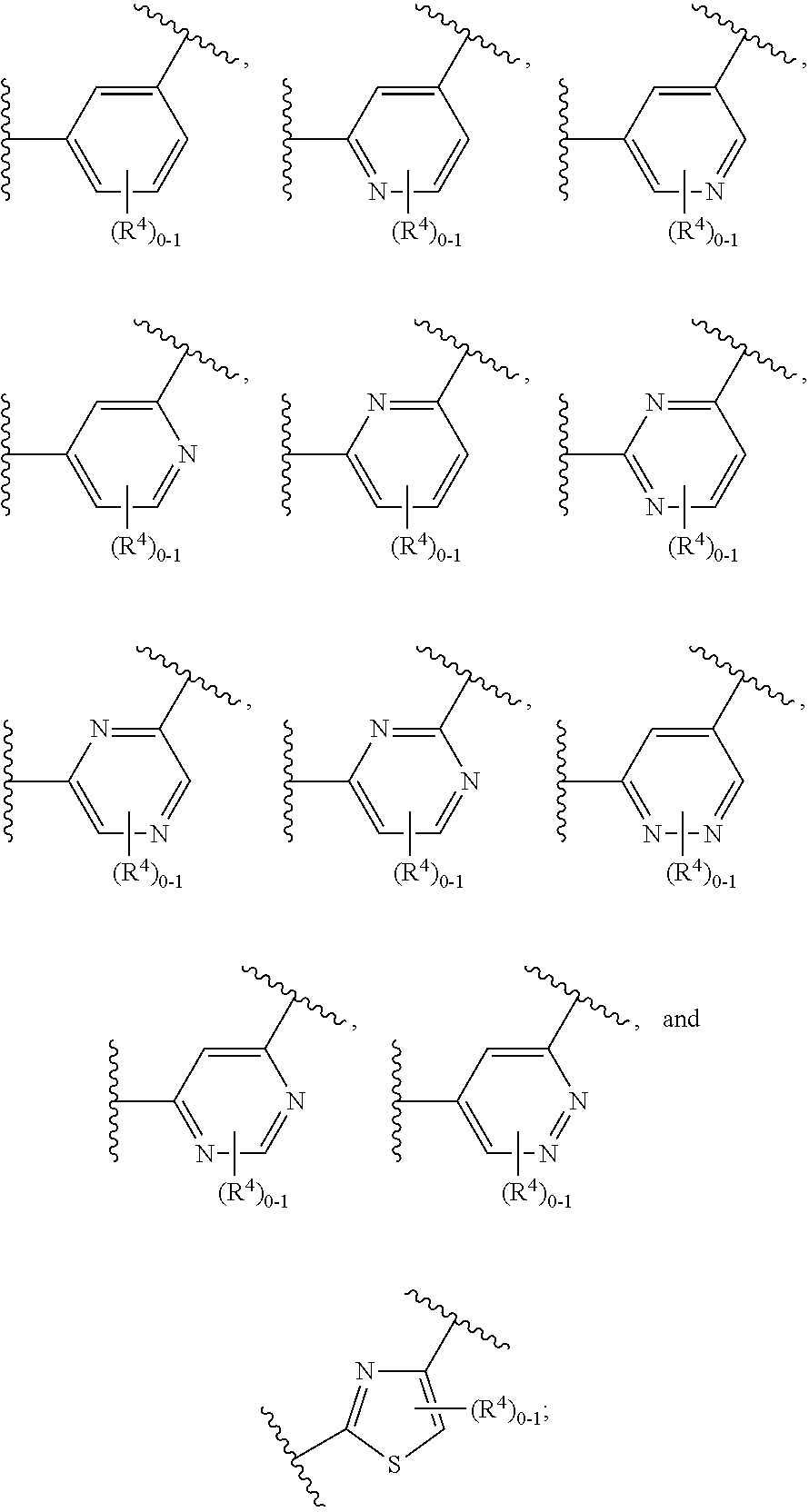
The present invention is concerned with compounds of the general Formula I: and pharmaceutically acceptable salts thereof, which are useful as melanin concentrating hormone receptor antagonists, particularly MCH-1R antagonists. As such, compounds of the present invention are useful for the treatment or prevention of obesity or eating disorders associated with excessive food intake and complications thereof, osteoarthritis, certain cancers, AIDS wasting, cachexia, frailty (particularly in elderly), mental disorders stress, cognitive disorders, sexual function, reproductive function, kidney function, locomotor disorders, attention deficit disorder (ADD), substance abuse disorders and dyskinesias, Huntington's disease, epilepsy, memory function, and spinal muscular atrophy. Compounds of formula I may therefore be used in the treatment of these conditions, and in the manufacture of a medicament useful in treating these conditions. Pharmaceutical formulations comprising one of the compounds of formula (I) as an active ingredient are disclosed, as are processes for preparing these compounds.
Owner:DEVITA ROBERT J +5
Soluble Guanylate Cyclase Activators
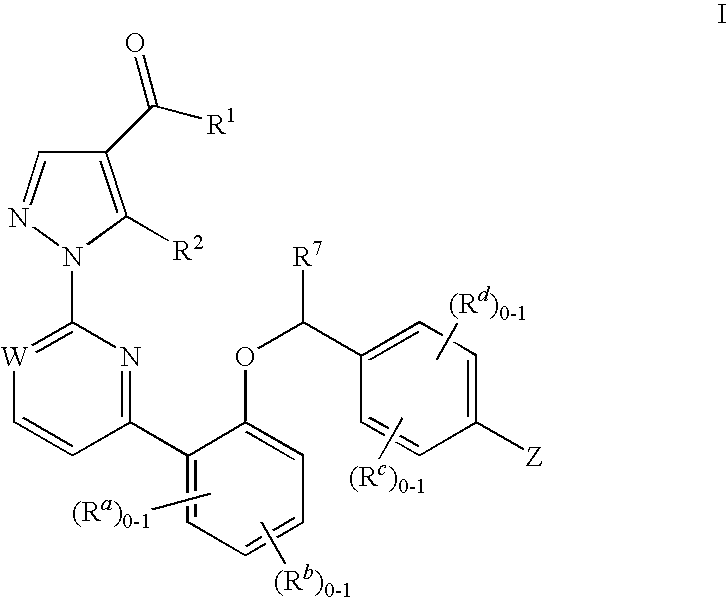
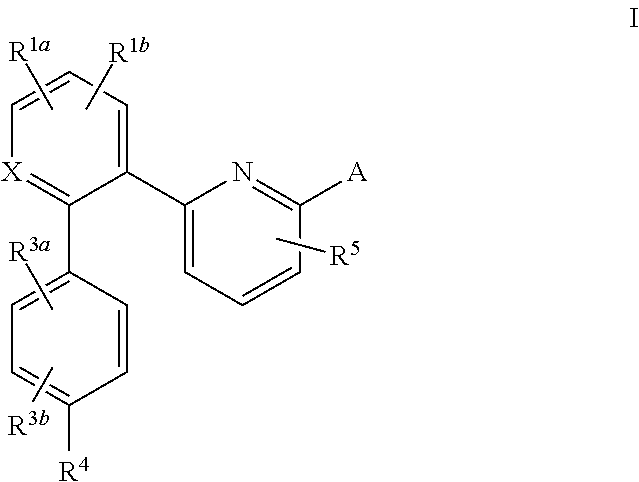
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Dialysis solution for peritoneal dialysis
InactiveUS6284140B1Easy to degradeDwell timeBiocideSolvent extractionHydroxyethyl starchUltrafiltration
The present invention relates to dialysis solutions for peritoneal dialysis, containing hydroxyethyl starch as the osmotically-active substance, electrolytes and, optionally, conventional additives, where the hydroxyethyl starch has a molecular weight Mw in the range from 10,000 to 150,000, a substitution MS in the range from 0.10 to 0.40, a substitution DS in the range from 0.09 to 0.35 and a substitution ratio C2 / C6>=8. With this peritoneal dialysis solution it is possible, with an outstanding ultrafiltration, to maintain a longer dwell time, for example the dialysis solution can be utilized for a period of 12 hours in the CAPD without replacement. In addition, the inventive dialysis solution is also particularly advantageous for patients with residual kidney function. The resorption of the osmotically active substance is clearly diminished and even after a dwell time of 12 hours it amounts to a maximum of 60-70%.
Owner:FRESENIUS AG
Soluble guanylate cyclase activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME CORP
Soluble guanylate cyclase activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME LLC
Ion binding compositions
ActiveUS20060024336A1Powder deliveryPharmaceutical non-active ingredientsEnd stage renal diseaseChronic renal insufficiency
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
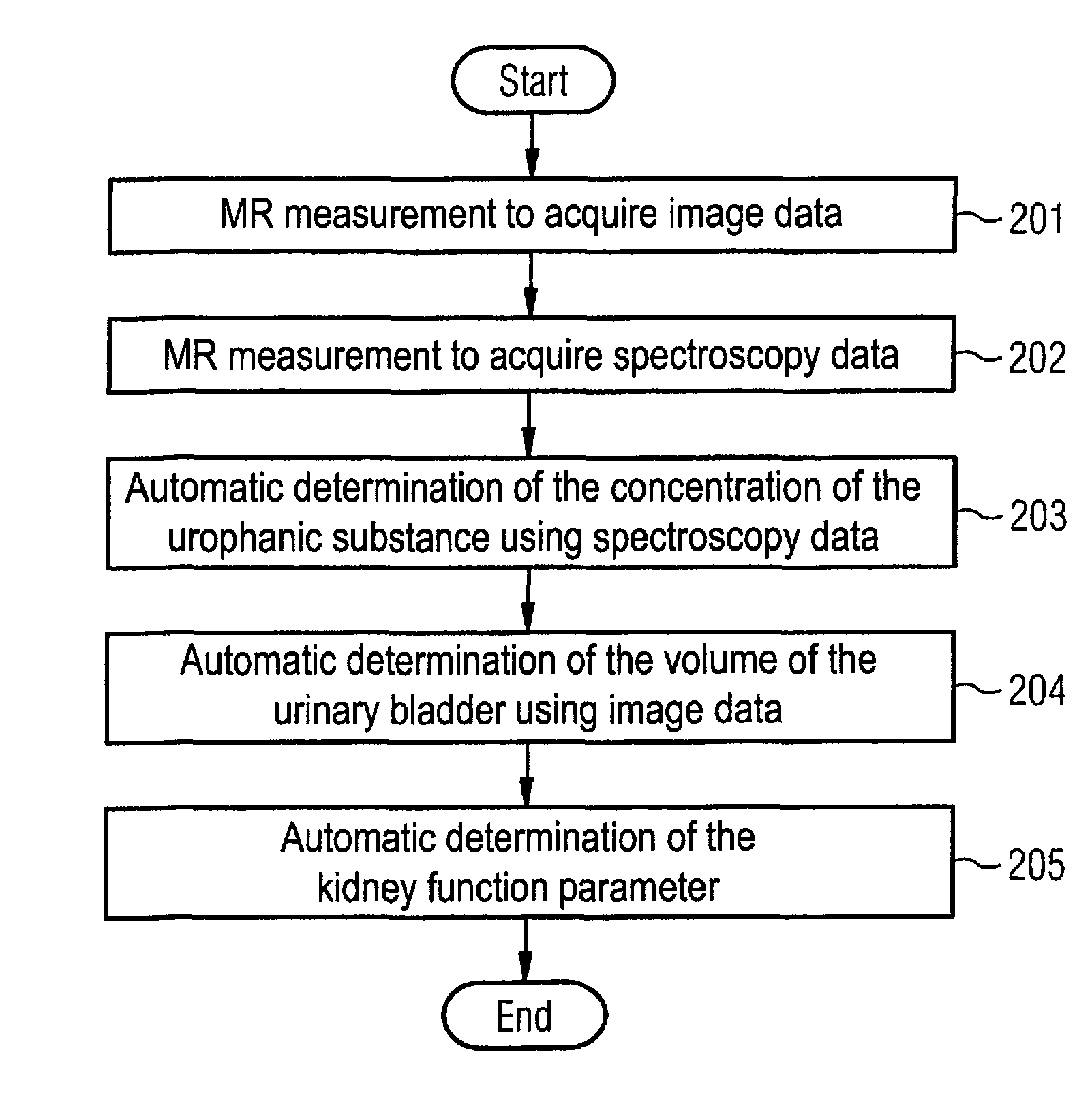
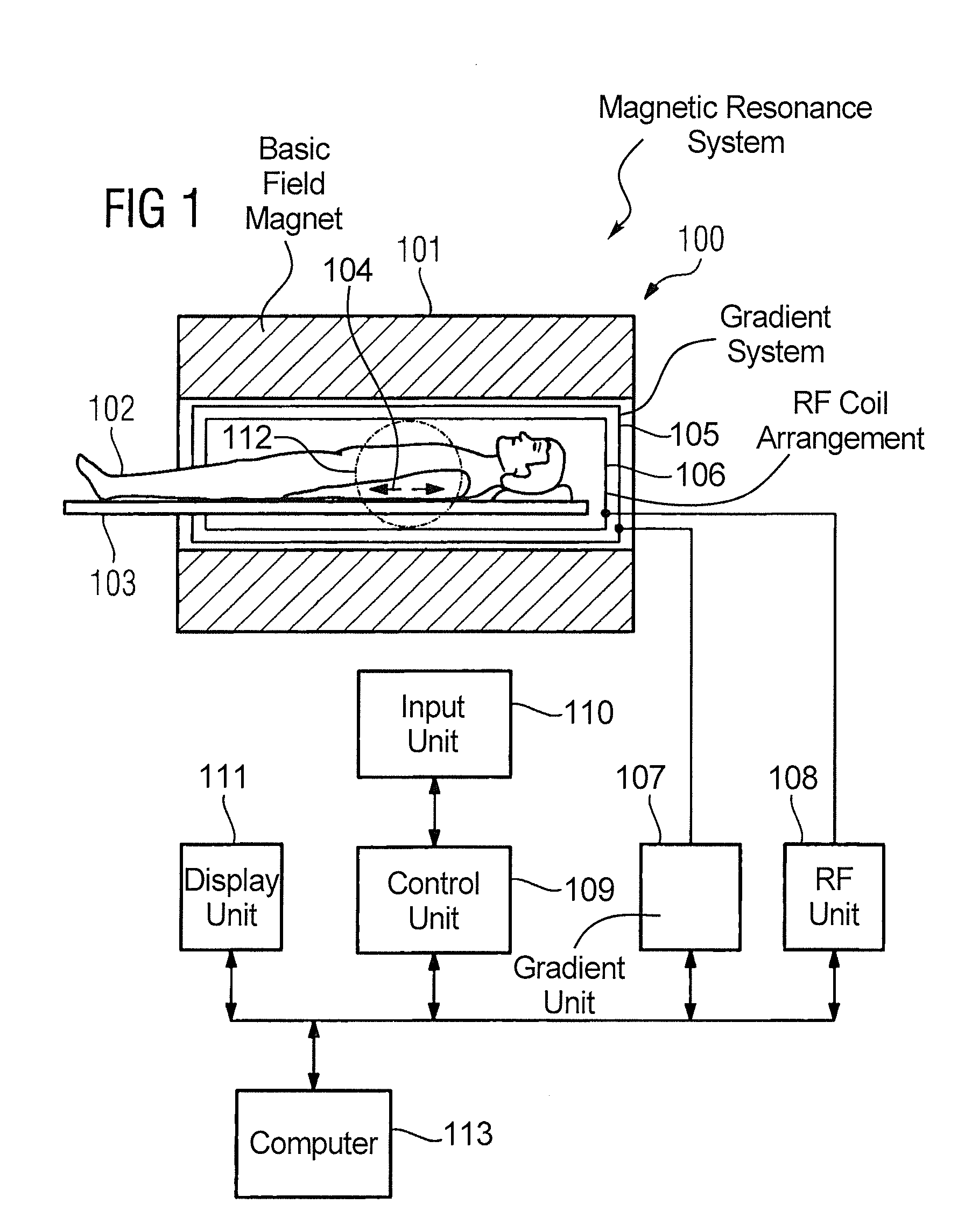
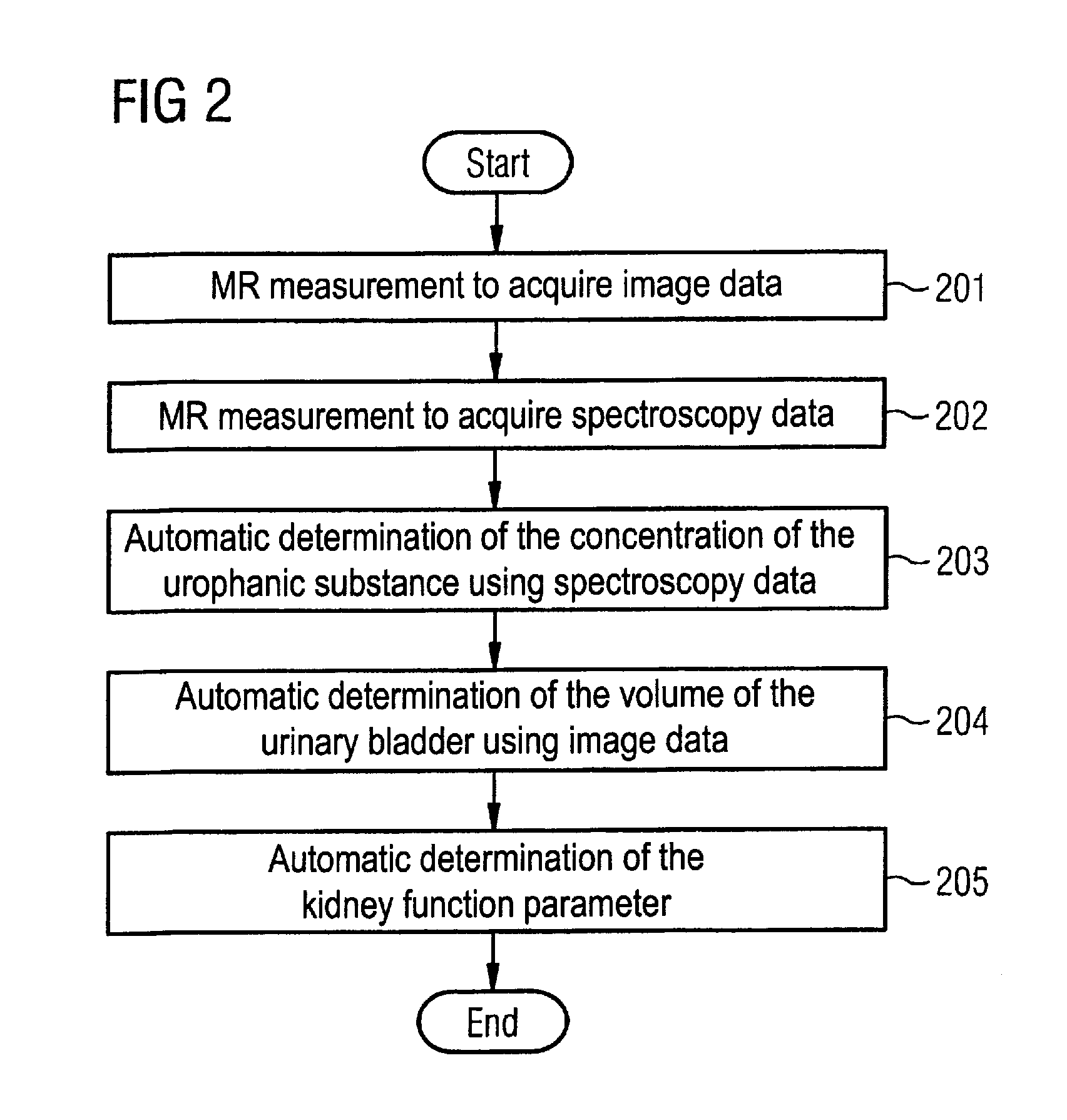
Magnetic resonance method and apparatus for determining a kidney function parameter
ActiveUS8260397B2Accurate measurementShort timeDiagnostic recording/measuringSensorsResonance measurementRenal function
In a method to determine a kidney function parameter of kidneys of an examination person with the aid of magnetic resonance tomography, at least one magnetic resonance measurement is implemented for an examination region of the examination person that comprises a urinary bladder of the examination person, to acquire magnetic resonance data from the examination region that include at least image data. The concentration of a urophanic substance in the urinary bladder of the examination person is automatically determined based on the acquired magnetic resonance data. A volume of the urinary bladder is automatically determined based on the acquired image data. A kidney function parameter of the kidneys of the examination person is automatically determined on the basis of the determined concentration of the urophanic substance in the urinary bladder and of the specific volume of the urinary bladder.
Owner:SIEMENS HEALTHCARE GMBH
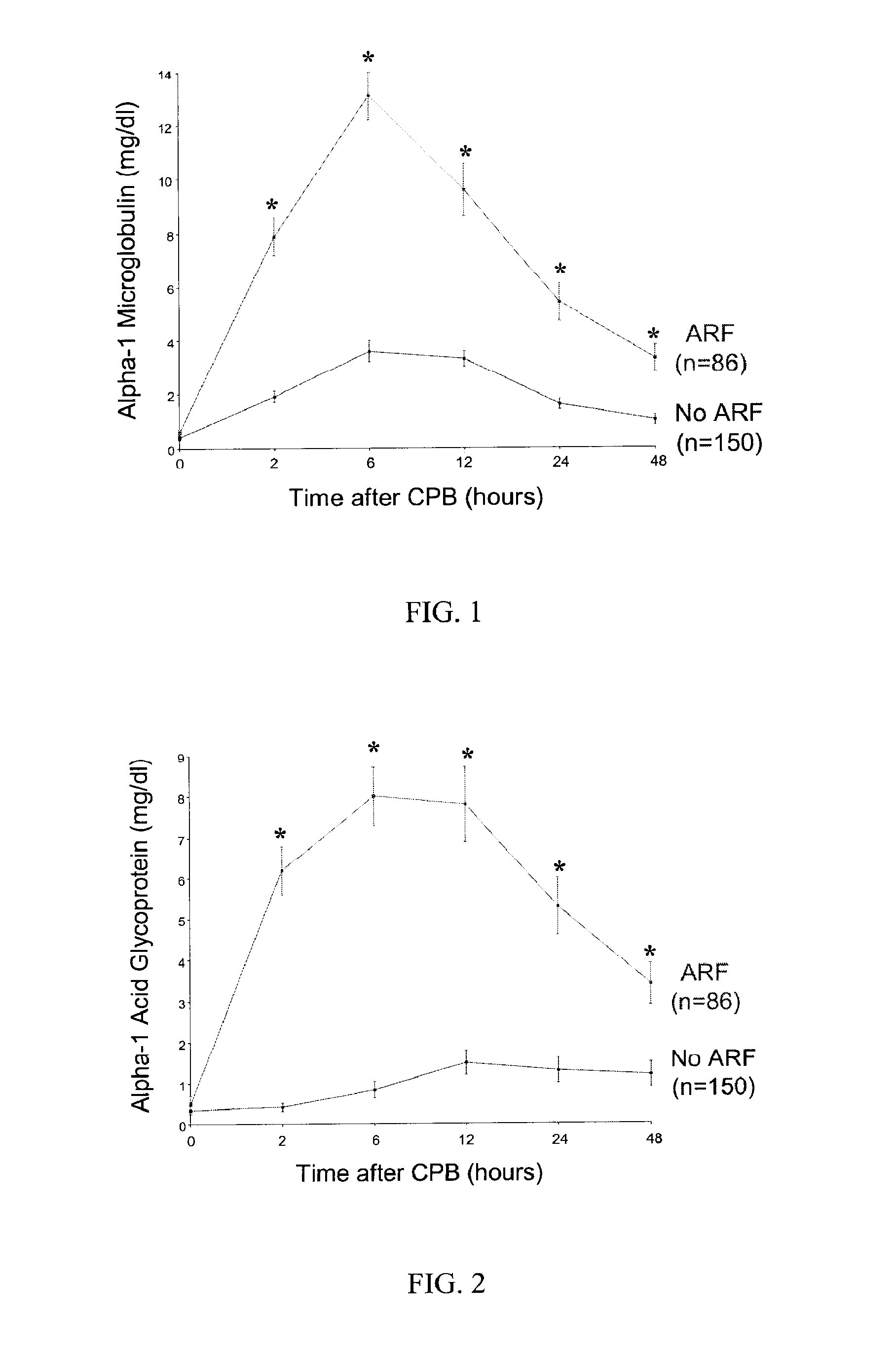
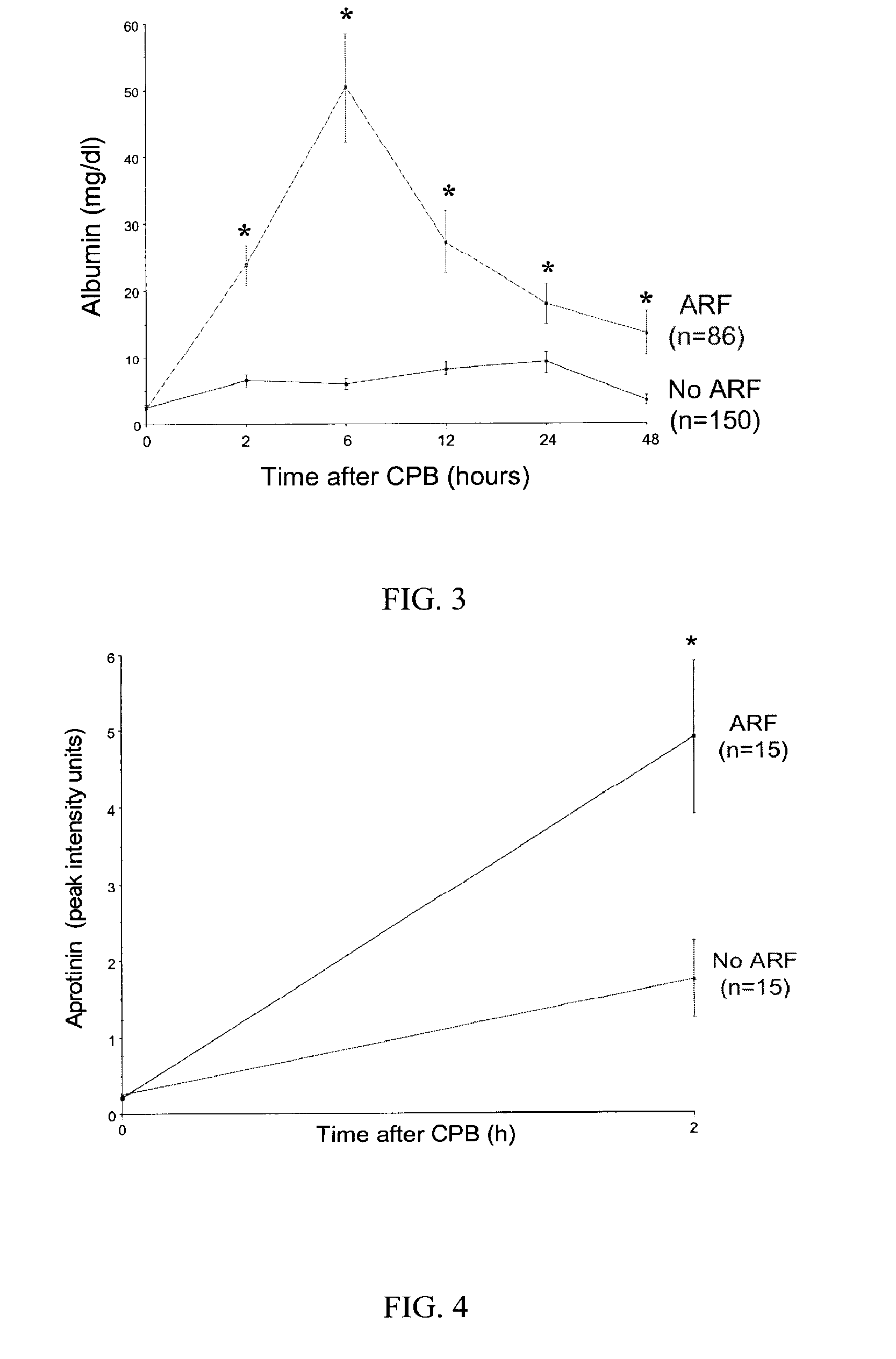
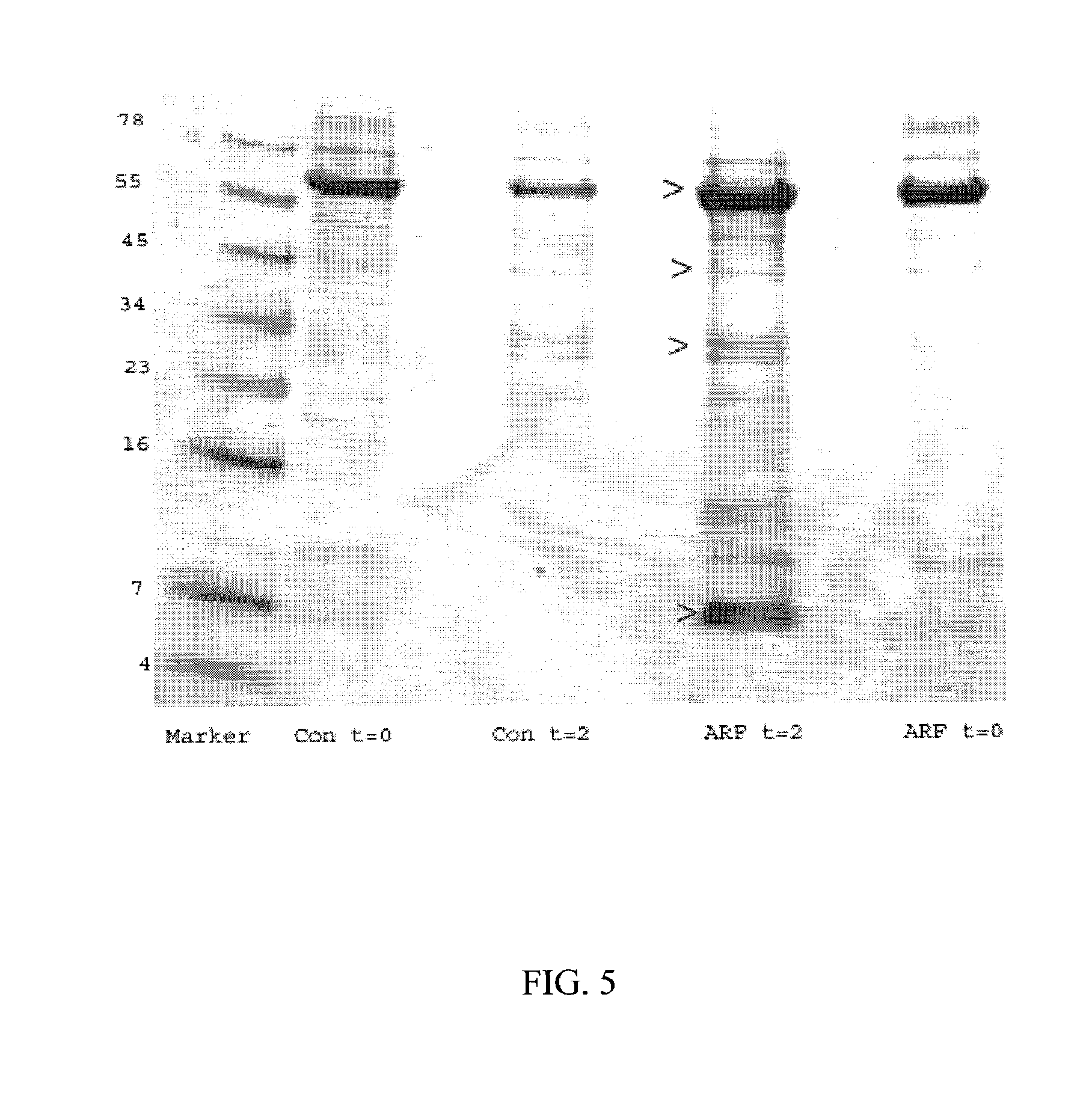
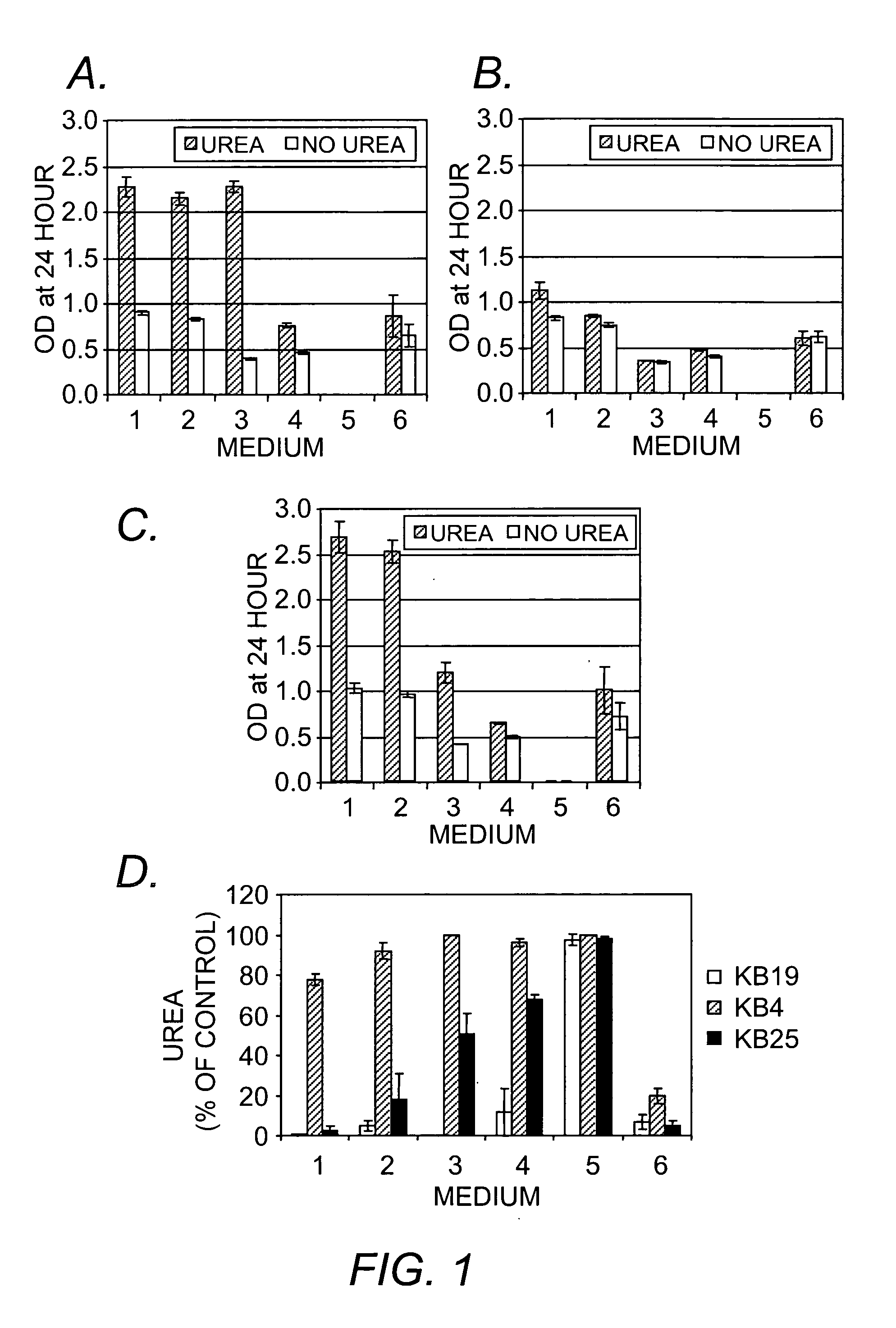
Method and Kit for the Early Detection of Impaired Renal Status
InactiveUS20070248989A1Pharmaceutical containersMedical packagingPoint of careExtracorporeal circulation
A method and kit for identifying the presence of an early biomarker of impaired renal status following a renal event in a mammalian subject. The method typically comprises (a) providing a body fluid sample obtained from a mammalian subject following a renal event; and (b) detecting in the provided sample the presence of a protein selected from the group consisting of aprotinin, alpha-1-microglobulin (A1M), alpha-1-acid-glycoprotein (A1AG), microalbumin, and combinations thereof, the presence thereof serving as an early biomarker of a change in renal status. The method can include a kit for point-of-care detection of the early biomarker of impaired renal status. Identification of the presence or absence of the early biomarker typically directs a caregiver's therapeutic decision regarding managing treatment of the subject for impaired renal status The invention also includes a method of assessing the administration of aprotinin during cardiopulmonary bypass surgery and provides for methods where the level of aprotinin in the subject's urine directs a caregiver's therapeutic decision regarding the intra-operative administration of aprotinin.
Owner:NIH
Soluble guanylate cyclase activators
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Ion binding compositions
The present invention provides methods and compositions for the treatment of ion imbalances. In particular, the invention provides core-shell compositions and pharmaceutical compositions thereof. Methods of use of the core-shell compositions for therapeutic and / or prophylactic benefits are disclosed herein. Examples of these methods include the treatment of phosphate imbalance disorders, hypertension, chronic heart failure, end stage renal disease, liver cirrhosis, chronic renal insufficiency, fluid overload, or sodium overload.
Owner:VIFOR INT AG
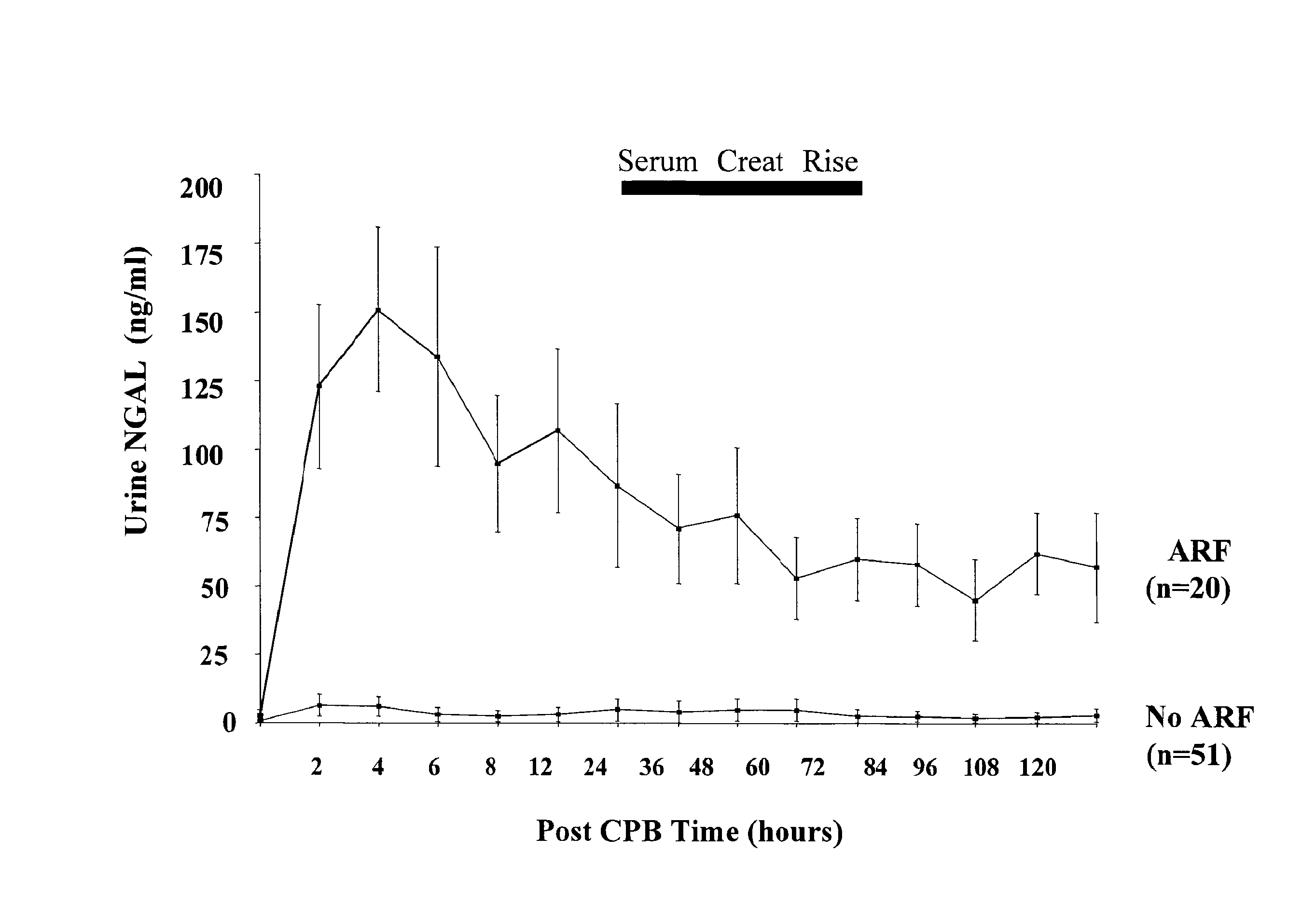
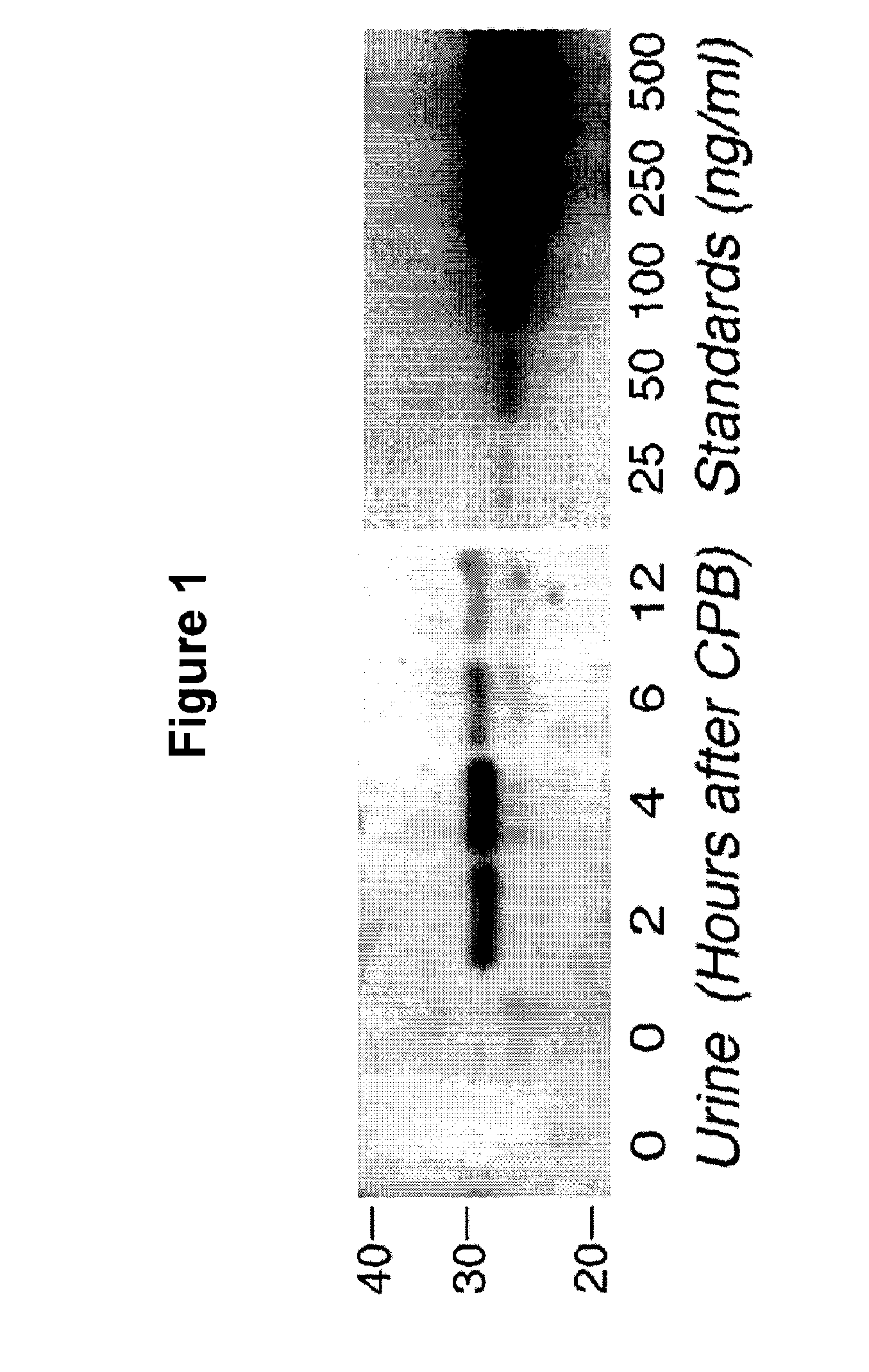
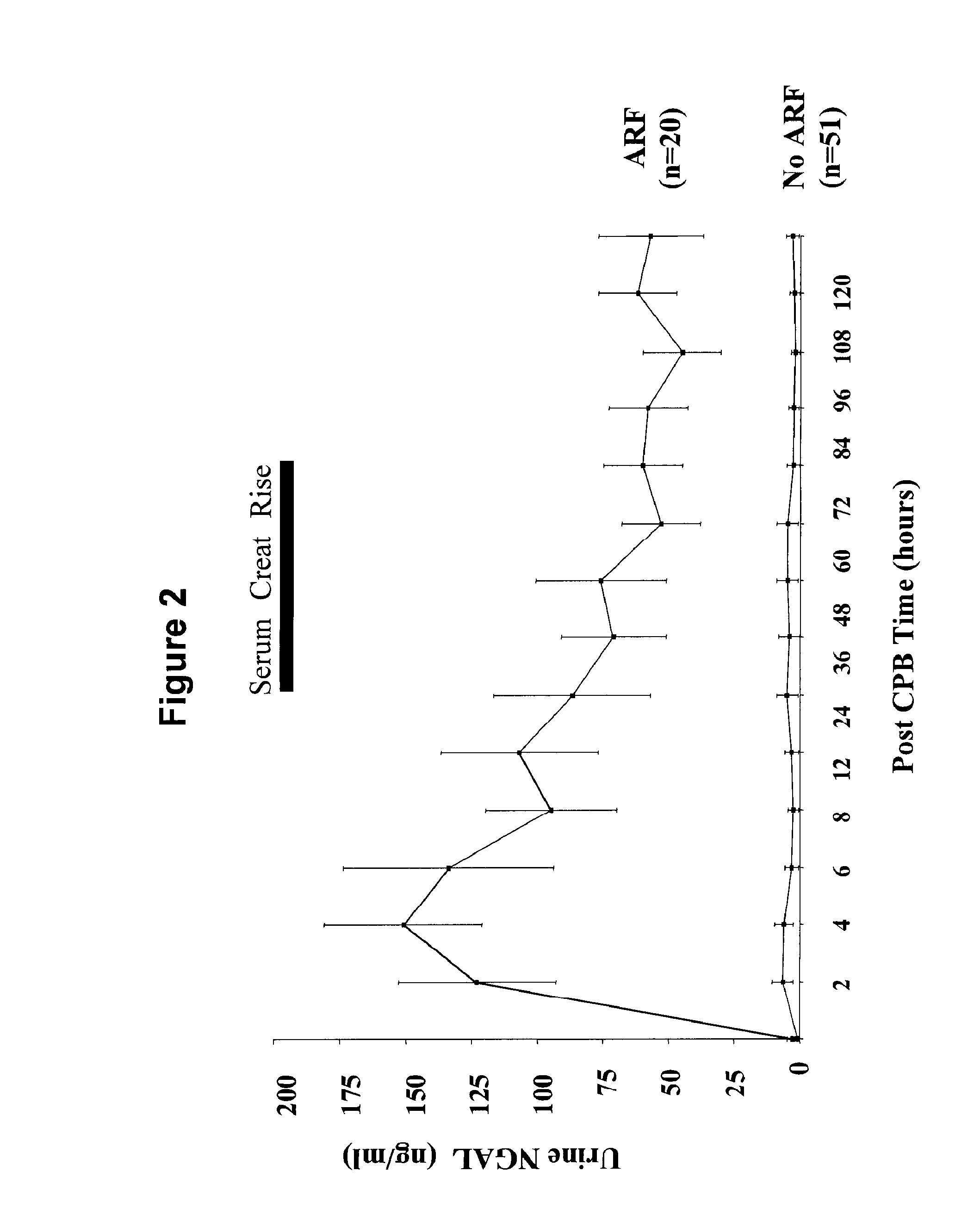
Method for the early detection of renal injury
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:DEVARAJAN PRASAD +1
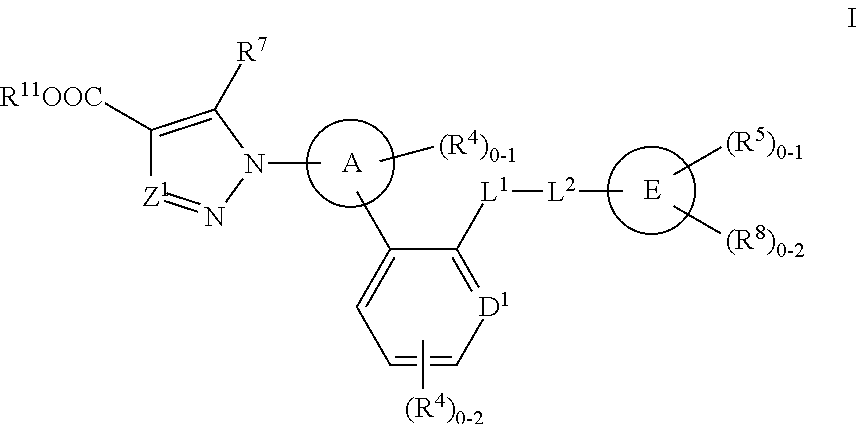
2-Aminoquinoline compounds
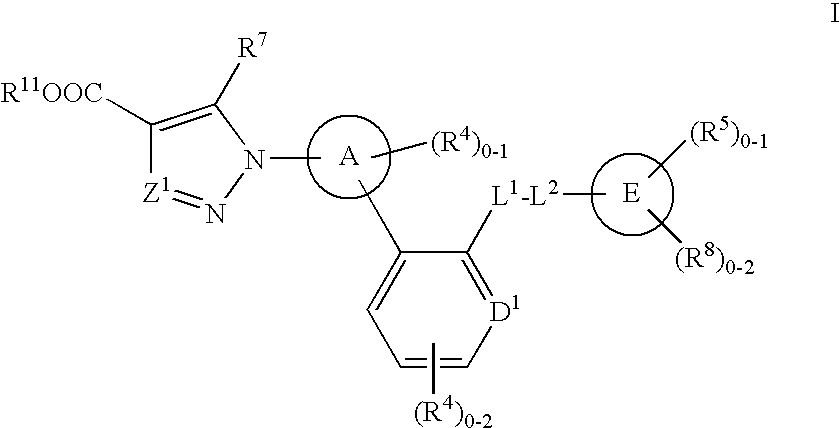
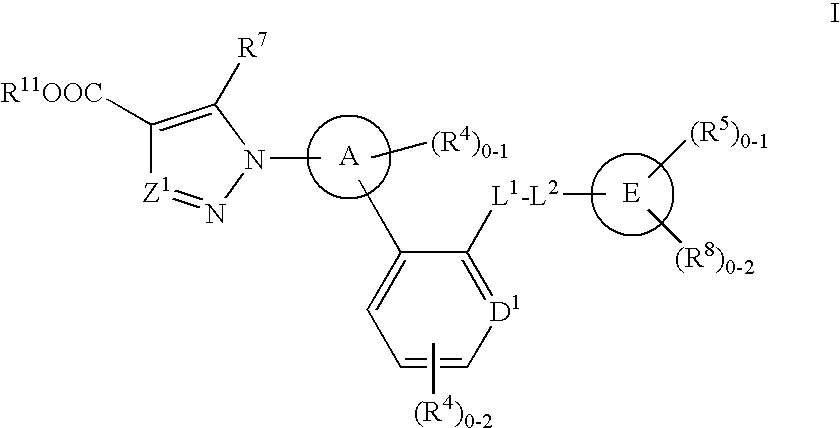
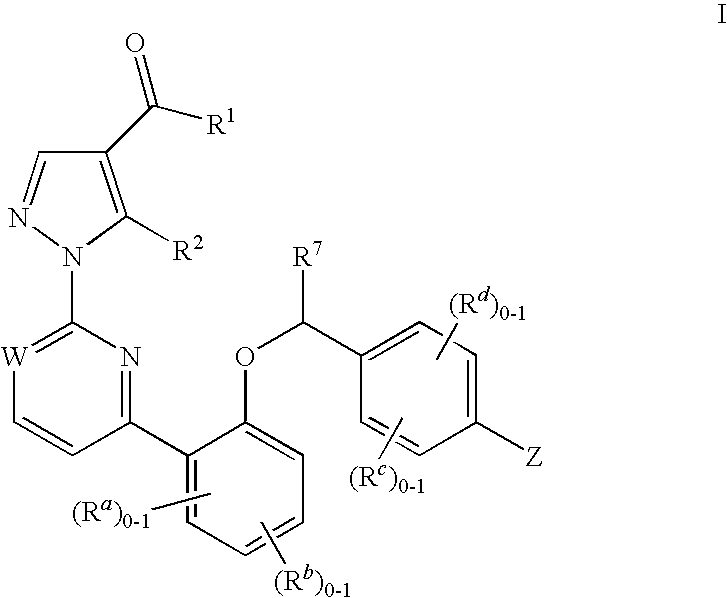
The present invention is concerned with compounds of the general Formula I: and pharmaceutically acceptable salts thereof, which are useful as melanin concentrating hormone receptor antagonists, particularly MCH-1R antagonists. As such, compounds of the present invention are useful for the treatment or prevention of obesity or eating disorders associated with excessive food intake and complications thereof, osteoarthritis, certain cancers, AIDS wasting, cachexia, frailty (particularly in elderly), mental disorders stress, cognitive disorders, sexual function, reproductive function, kidney function, locomotor disorders, attention deficit disorder (ADD), substance abuse disorders and dyskinesias, Huntington's disease, epilepsy, memory function, and spinal muscular atrophy. Compounds of formula I may therefore be used in the treatment of these conditions, and in the manufacture of a medicament useful in treating these conditions. Pharmaceutical formulations comprising one of the compounds of formula (I) as an active ingredient are disclosed, as are processes for preparing these compounds.
Owner:MERCK SHARP & DOHME CORP
Soluble guanylate cyclase activators
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
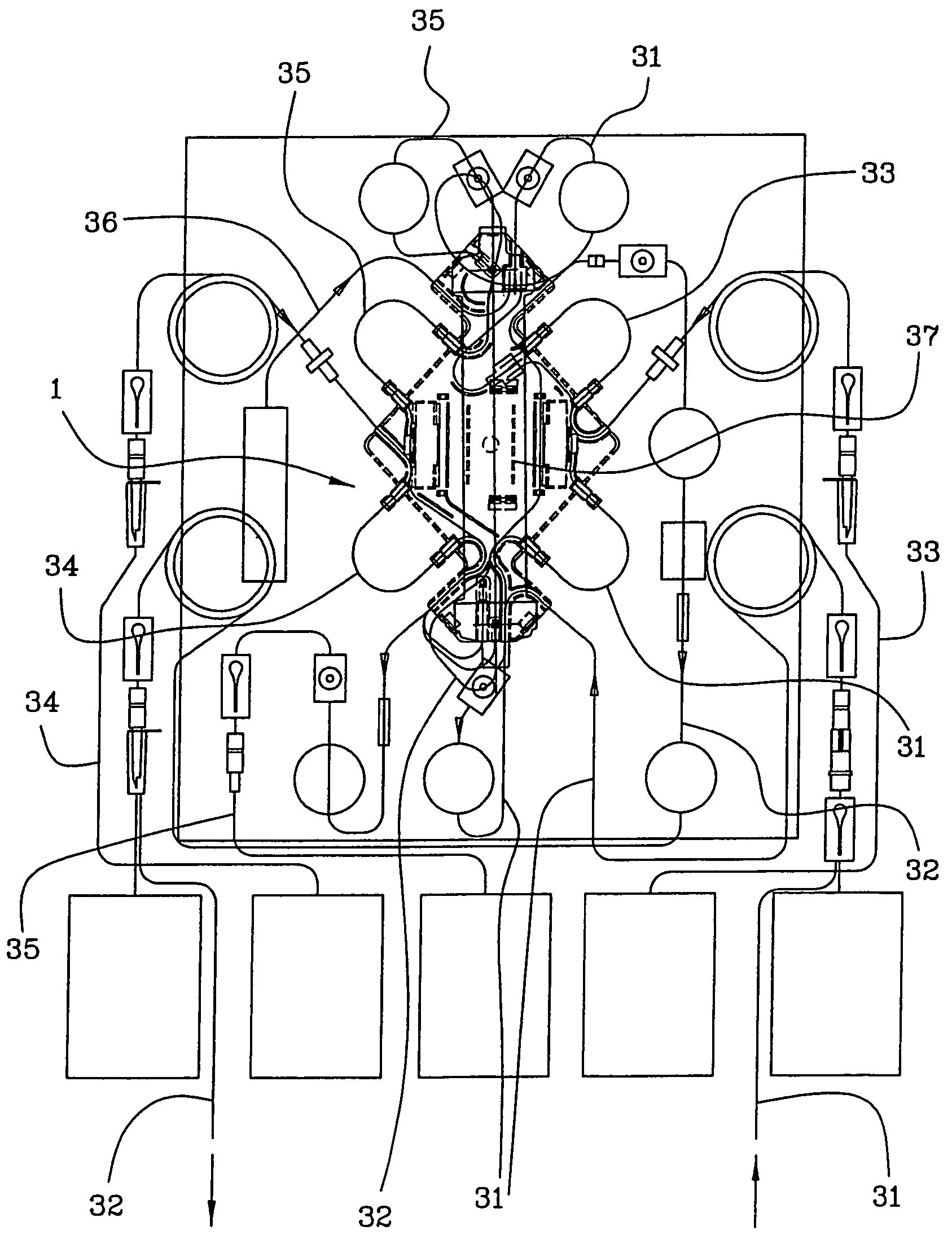
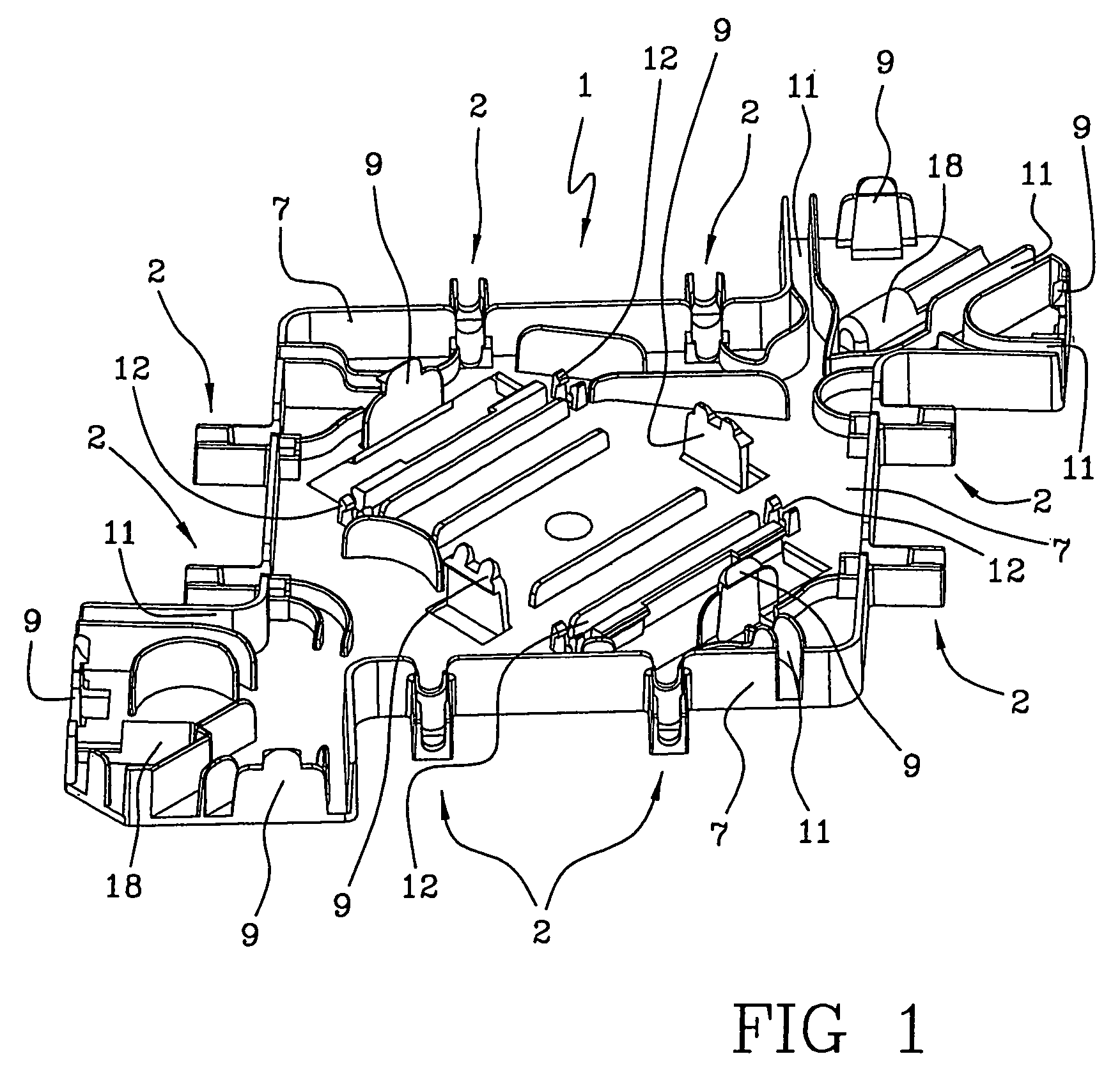
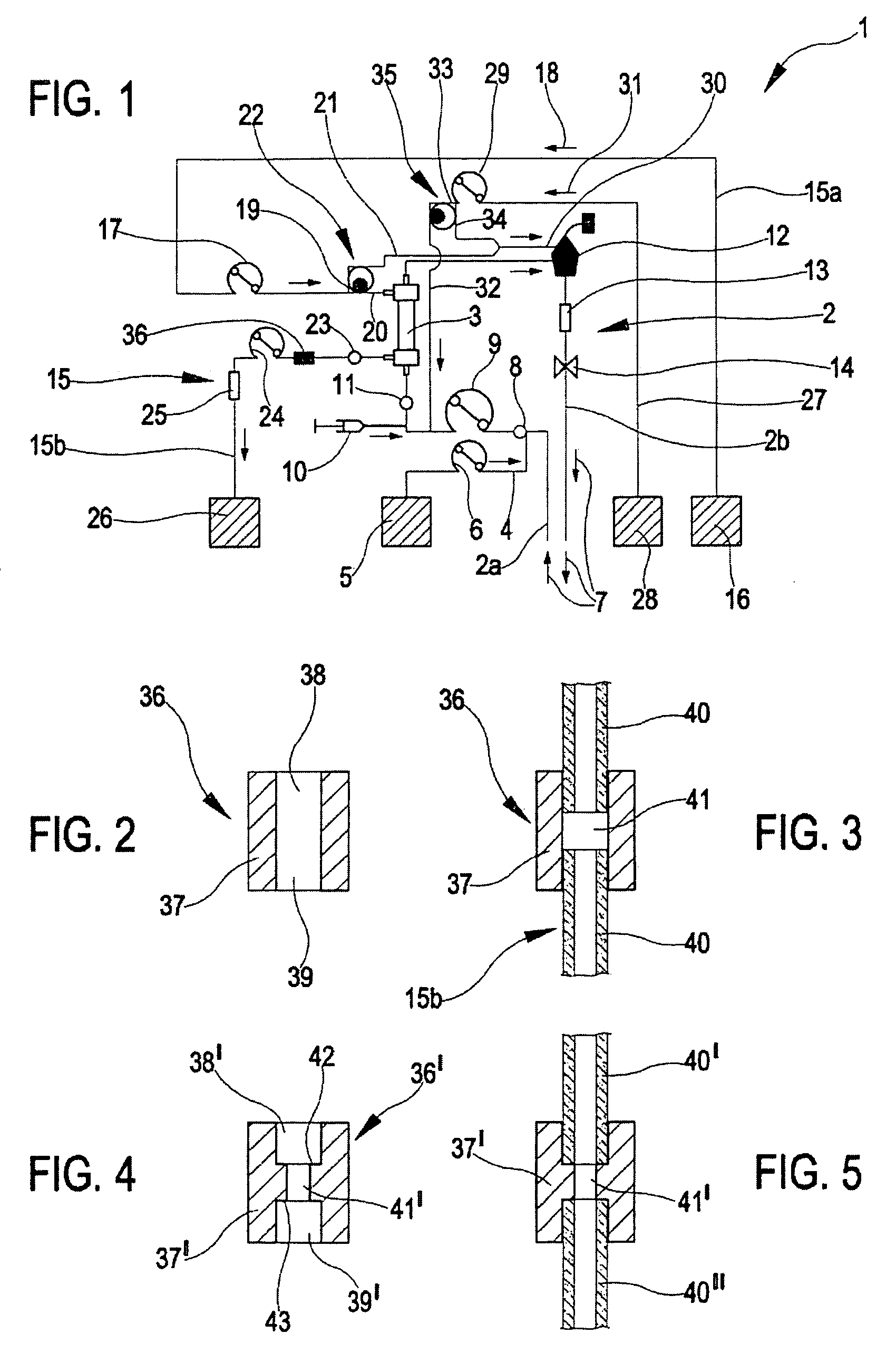
Support element, an integrated module for extracorporeal blood treatment comprising the support element, an apparatus for extracorporeal blood treatment equipped with the integrated module, and an assembly process for an integrated module for extracorporeal blood treatment
ActiveUS7232418B2Quick assemblyEasy to operateEngine diaphragmsSolvent extractionIntensive treatmentBlood treatments
An integrated module for extracorporeal blood treatment has a flat-shaped support element which exhibits on an internal face thereof a complex of fluid distribution lines and on an external face thereof a high-flow dialyzer. The support element has a base body which exhibits fixture seatings, each of which houses an axially extended tract of a fluid distribution line. The tract of the fluid distribution line, with respect to adjacent tracts, has an increased diameter due to the presence of a junction collar made of a rigid material. Each fixture seating exhibits two axial locators for positioning the axially extended tract of a fluid distribution line in a fixed position. The locators interact with the junction collar, and the distribution lines can be fixed to the base body by a resilient fixture of the junction collars in the seatings without gluing. The module is configured to be mounted on an apparatus for intensive treatment of renal insufficiency.
Owner:GAMBRO LUNDIA AB
Bioartificial filtration device for filtering blood to mimic kidney function
InactiveUS6942879B2Long life-timeImmobilised enzymesBioreactor/fermenter combinationsBioartificial liver deviceUltrafiltration
A novel cell seeded hollow fiber bioreactor is described as a potential bioartificial kidney. Endothelial cells along with pericyte, vascular smooth muscle, and / or mesangial cells or any mesenchymally derived support cells are seeded along a hollow fiber in a perfused bioreactor to reproduce the ultrafiltration function and transport function of the kidney. Maintenance of tissue specific function and ultrastructure suggest that this bioreactor provides an economical device for treating renal failure.
Owner:RGT UNIV OF MICHIGAN
Method for treating cachexia with retinoid ligands
InactiveUS20070185055A1BiocideSilicon compound active ingredientsRetinoidObstructive Pulmonary Diseases
The present invention relates to a method of treatment of cachexia in a subject in need of treatment. More specifically, the present invention relates to the use of retinoid compounds that act on retinoid X receptors (RXRs) for the treatment of cachexia in a subject in need of treatment. The cachexia is associated with, in other words a complication of, a primary disease, condition or disorder. Primary diseases, conditions and disorders include, but are not limited to, cancer, AIDS, liver cirrhosis, diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease, chronic cardiac failure, immune system diseases (e.g., rheumatoid arthritis and systemic lupus erythematosus), tuberculosis, cystic fibrosis, gastrointestinal disorders (e.g., irritable bowel syndrome and inflammatory bowel disease), Parkinson's disease, anorexia nervosa, dementia, major depression, an aged condition and sarcopenia.
Owner:JIANG GUANG LIANG +2
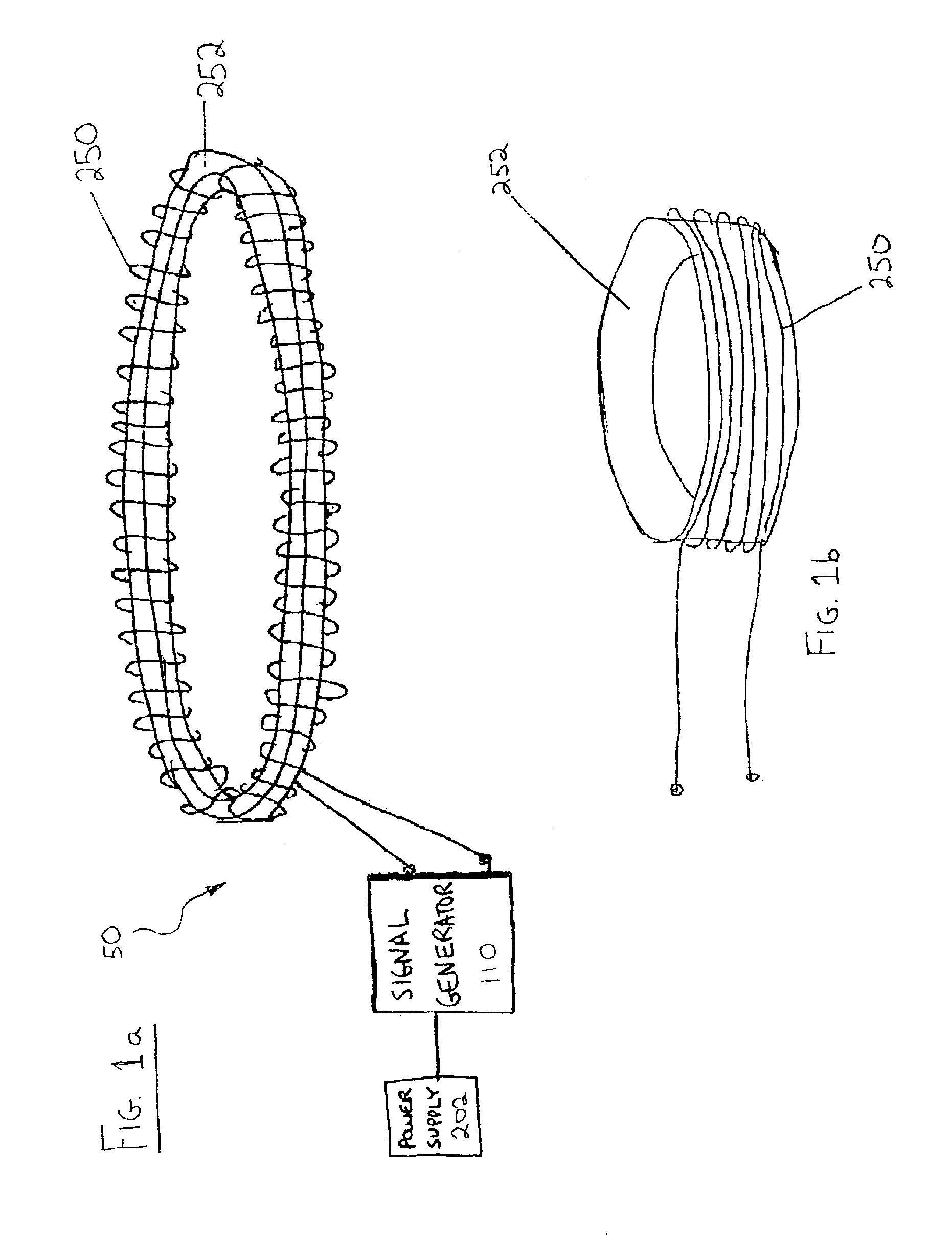
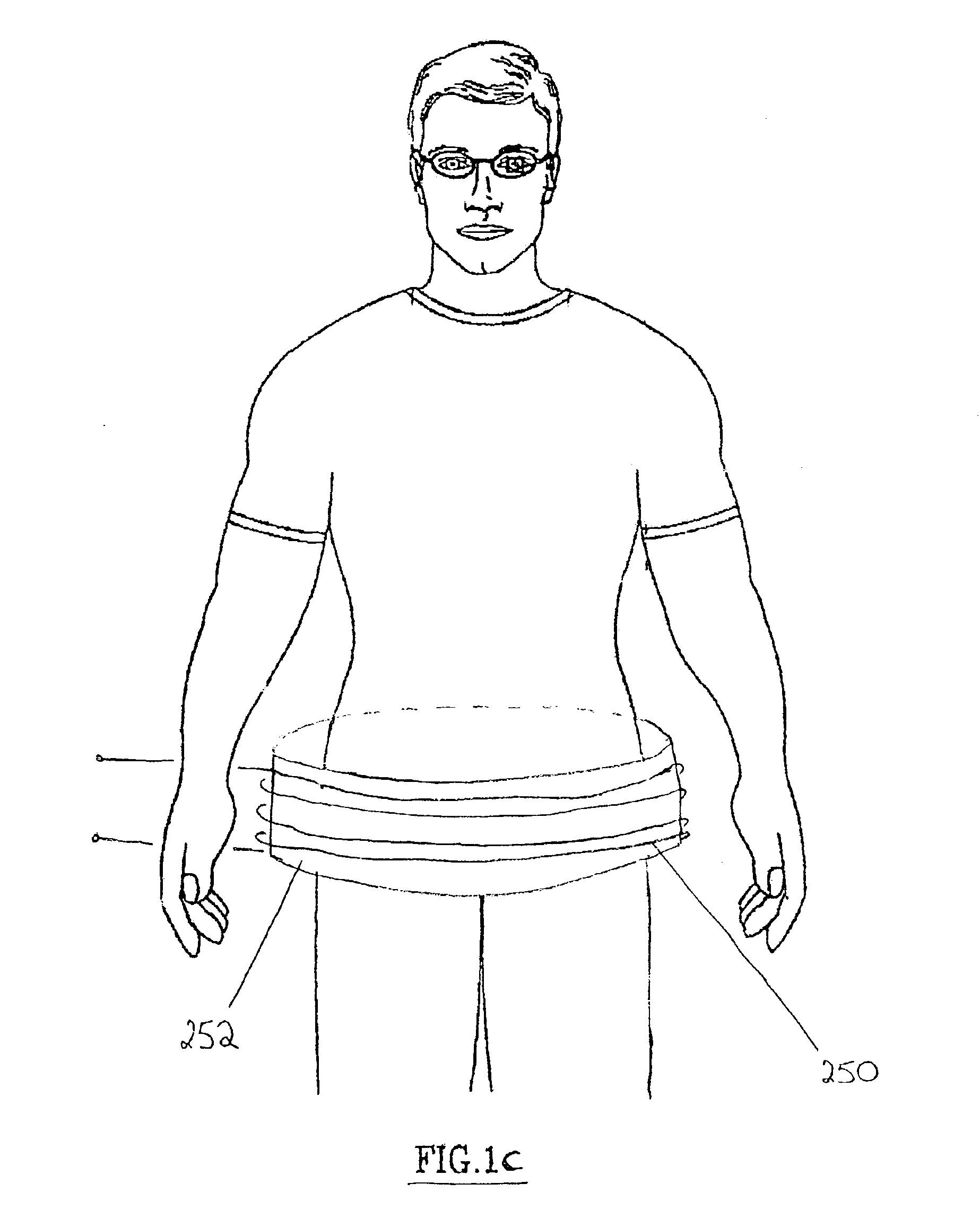
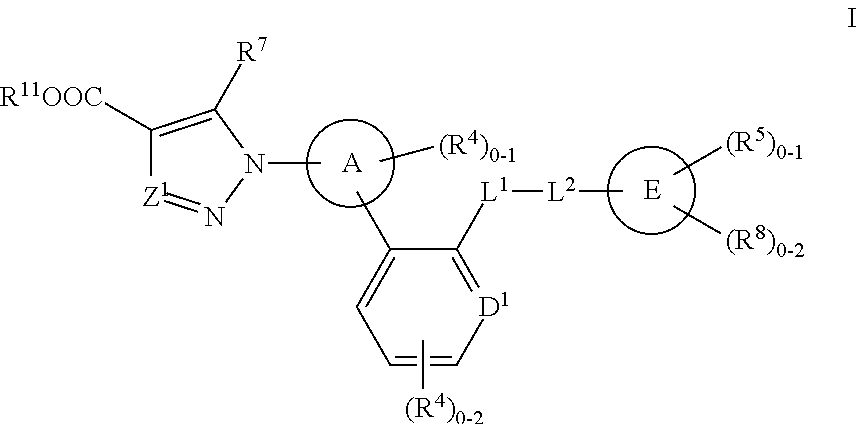
Method and device for restoring kidney function using electromagnetic stimulation
InactiveUS6941172B2Restore kidney functionElectrotherapyMagnetotherapy using coils/electromagnetsProximateRenal function
Owner:NACHUM ZVI
Soluble Guanylate Cyclase Activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME LLC
Compositions and methods of treating chronic kidney disease
InactiveUS20080317725A1Treat and prevent skeletal muscle wastingHigh in proteinBiocideHeavy metal active ingredientsNutritional deficiencyChronic renal disease
The invention relates to nutritional compositions and methods of using these compositions for the treatment of renal disease. More particularly, the invention discloses compositions of vitamins, minerals, amino acids, and / or proteins in amounts that can be used to supplement the nutritional deficiencies observed in patients afflicted with renal disease, renal insufficiency, or end-stage renal disease. The compositions of the invention can also be used as nutritional supplements for patients undergoing dialysis therapy or for patients on a restricted diet. In addition, the compositions can be used in combination or alone as a method of treating or managing a subject in the various stages of chronic renal disease, or throughout disease progression.
Owner:BAUM SETH J
Ion sensor for long term use in complex medium
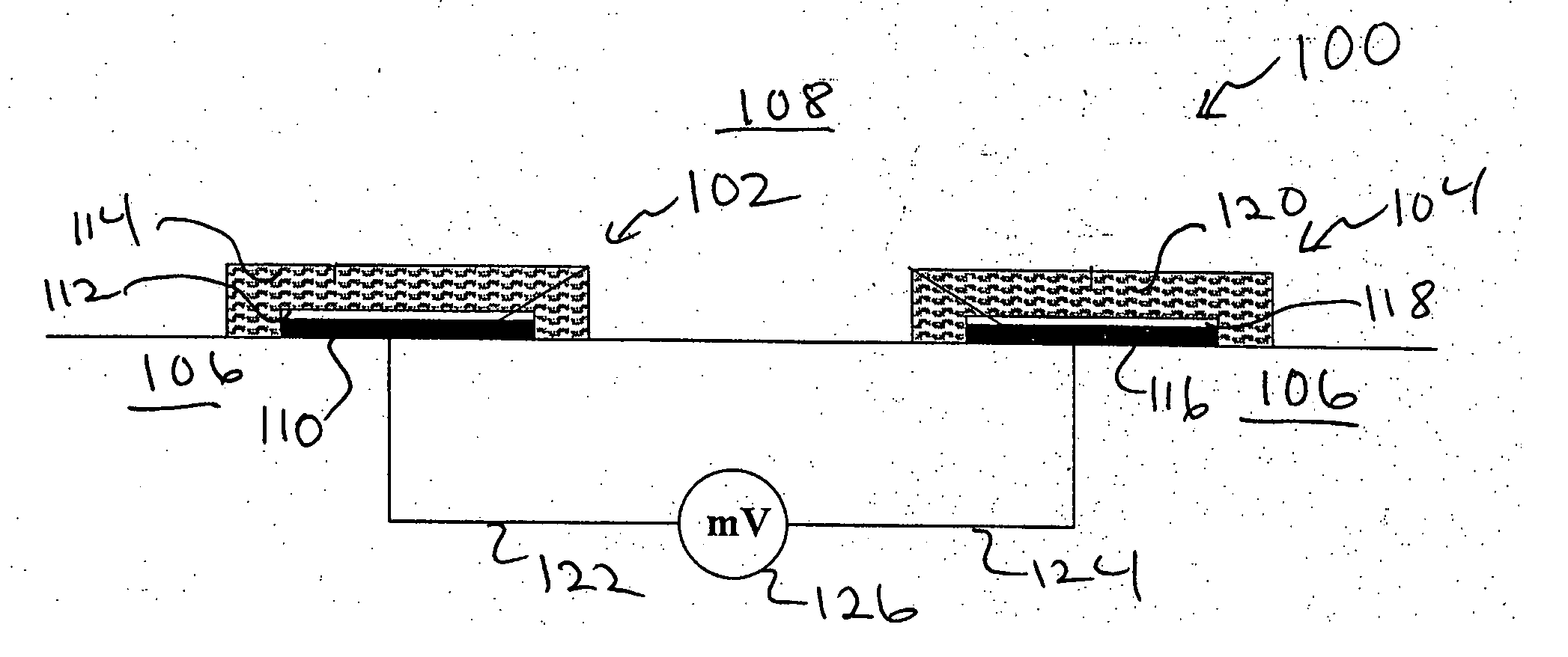
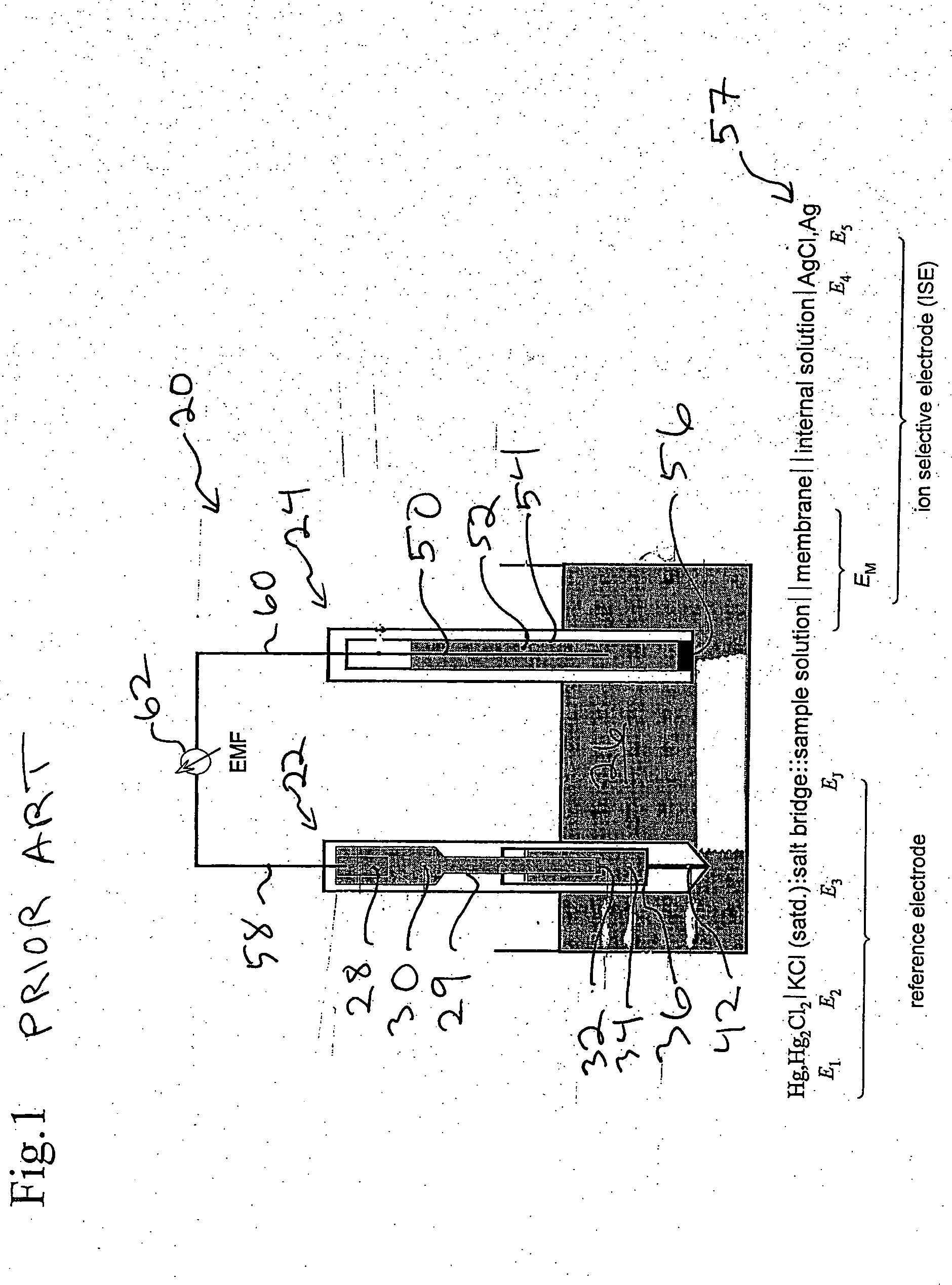
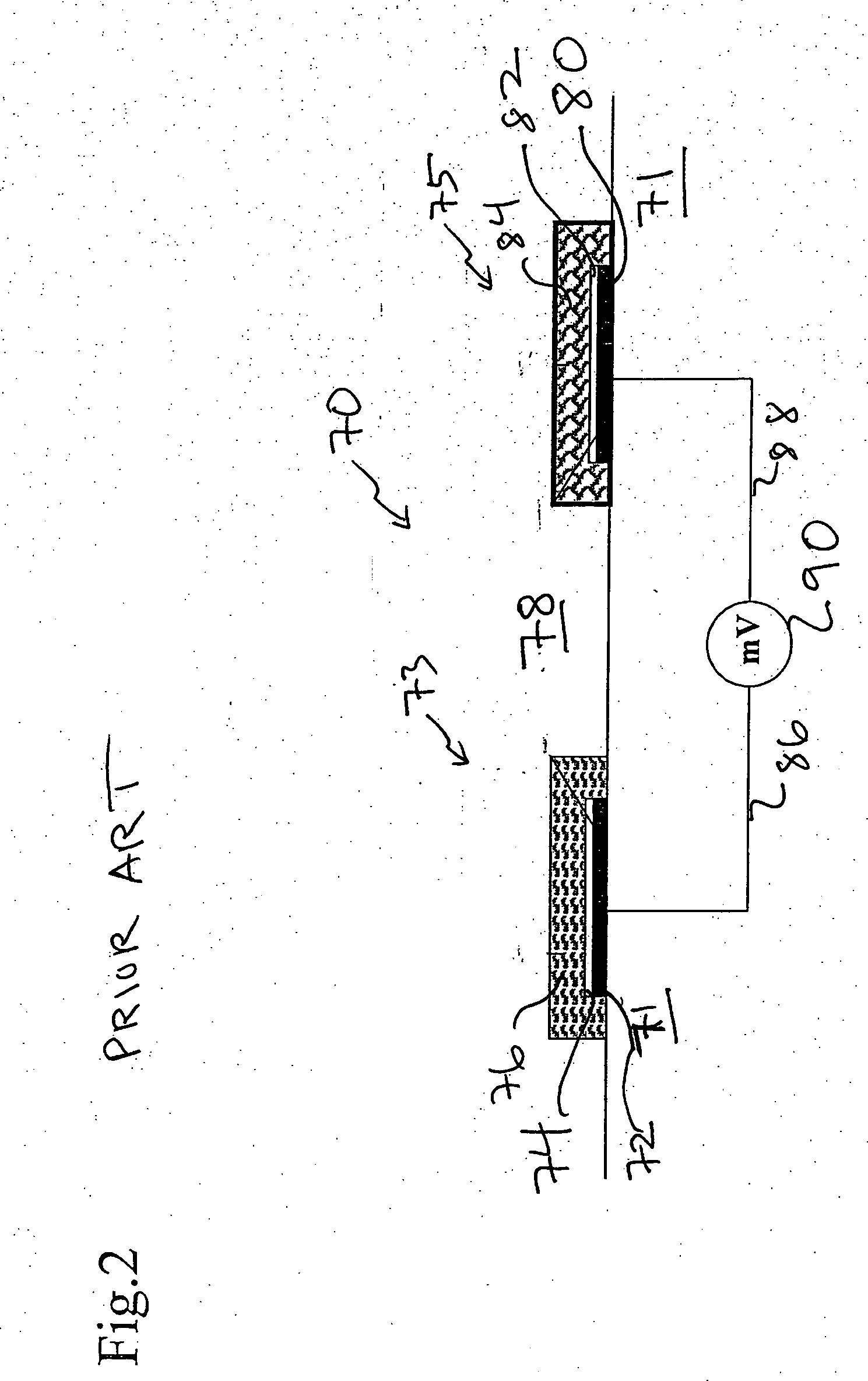
Devices and methods for measuring a target ion concentration uses an electrode pair. The pair includes a working electrode and a reference electrode. The working and reference electrodes are ion-selective electrodes (ISEs). The reference ISE can include a sodium ISE. The ISE pair interacts with body fluids where a target ion concentration changes more than sodium ion concentration over time. Some ISE membranes of a pair vary essentially only in the ionophore. An ISE pair can determine the ratio of a target ion concentration to sodium ion concentration in vivo. Periodic measurement of sodium concentration in drawn blood can be used to calibrate an ISE pair and provide target ion concentration as an output. Or, a potassium / sodium ISE pair beneficially monitors potassium concentration changes over time in heart- or kidney-failure patients. Then, manual or automatic titration of a diuretic material can be implemented to maintain a desired potassium concentration.
Owner:MEDTRONIC INC
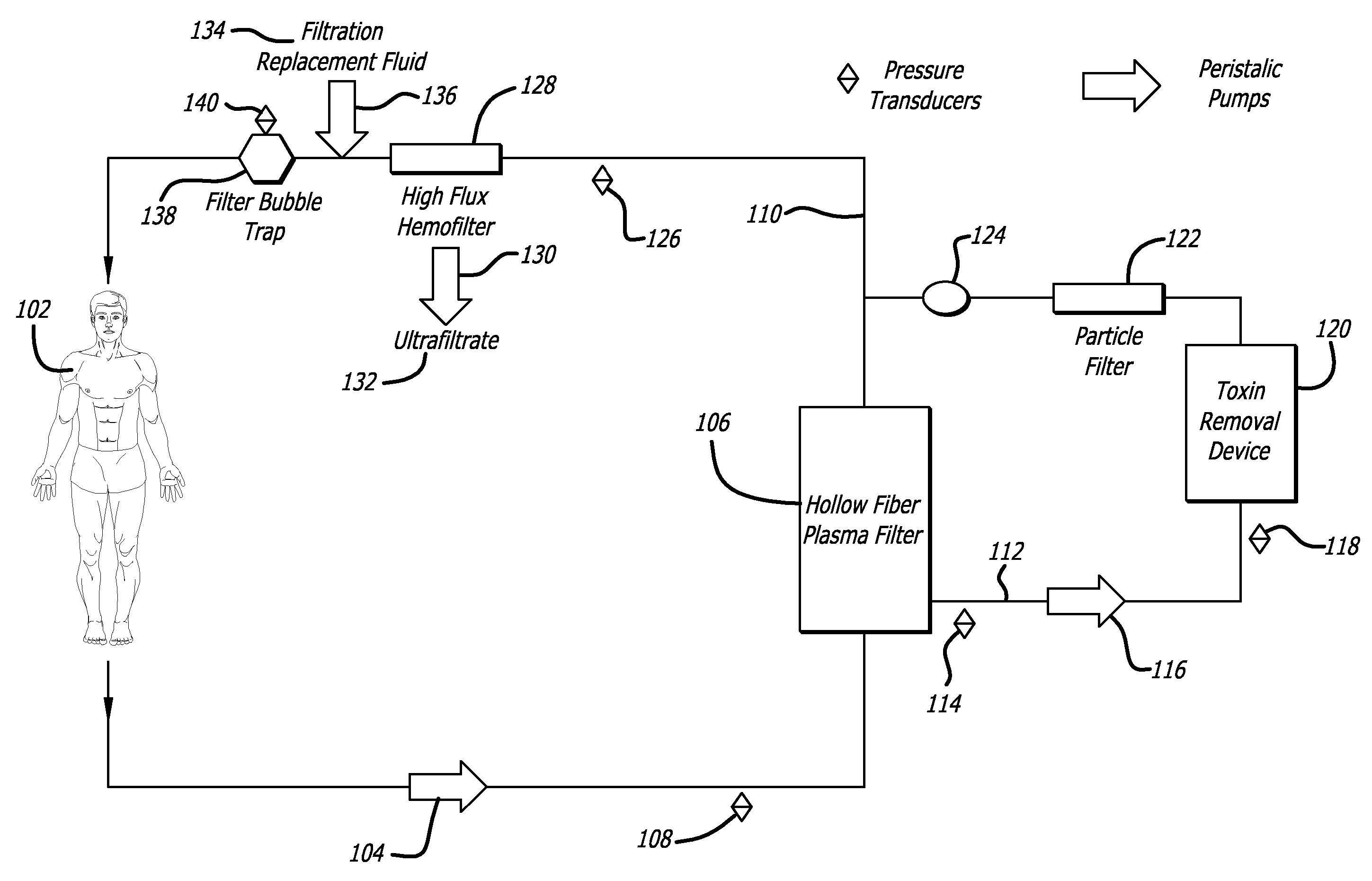
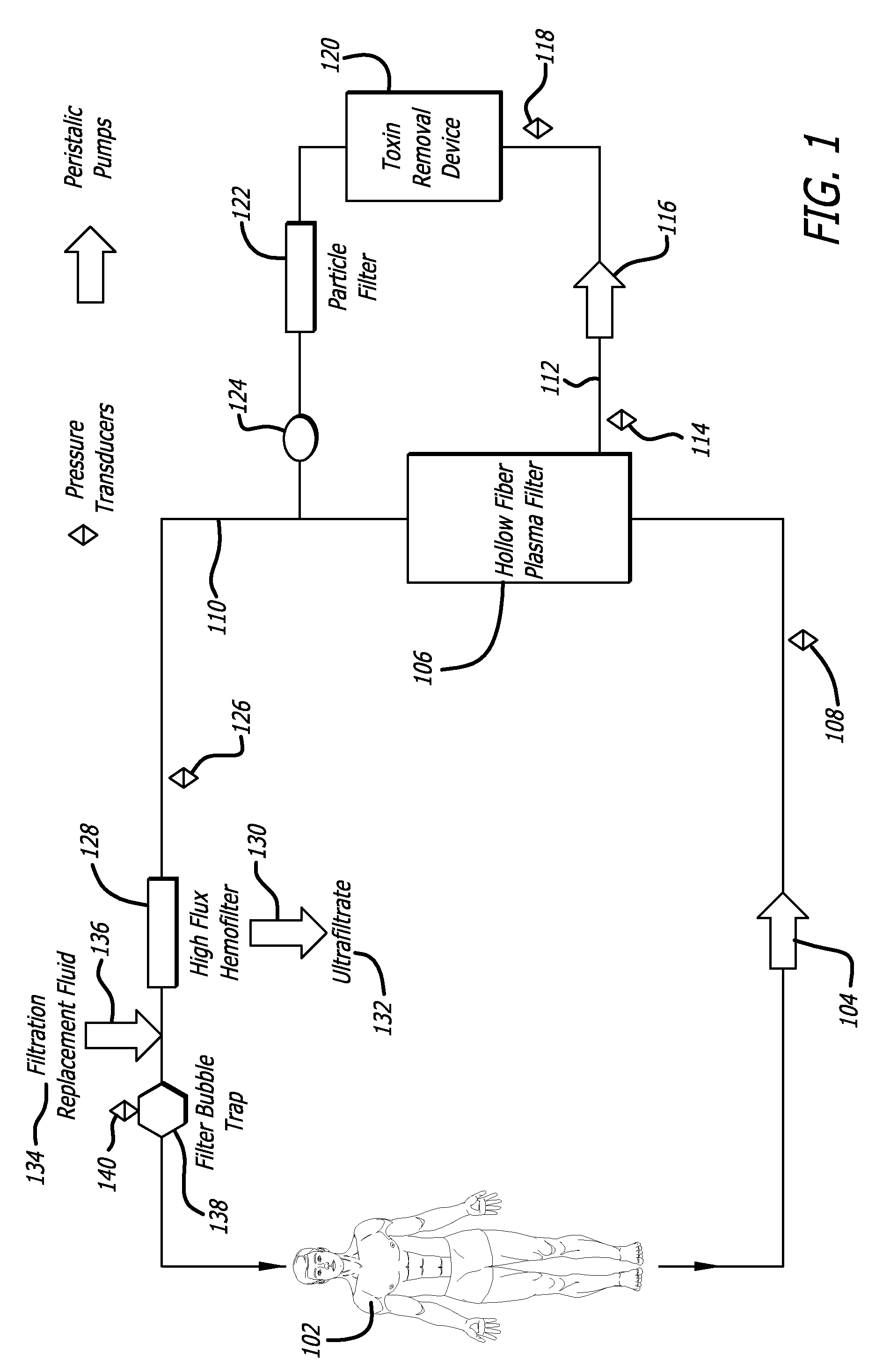
Plasma detoxification and volume control system and methods of use
ActiveUS20070181499A1Effectively detoxify human plasma and balance blood volumeSolvent extractionSolid sorbent liquid separationIon exchangeBlood plasma
An extracorporeal circuit for removing toxins from the blood and plasma volume control in patients suffering from sepsis and renal failure. The extracorporeal circuit disclosed herein comprises a plasma filter, a toxin removal device and optionally a hemofilter that minimizes electrolyte and protein depletion from the treated plasma while effectively removing both free and protein-bound toxins. The toxin removal device comprises adsorbent materials selected from the group consisting of activated carbon, ion exchange resins and non-ionic exchange resins and the adsorbent materials are coated with albumin. Also provided are associated methods for treating patients suffering from sepsis and renal failure using the disclosed extracorporeal circuit and toxin removal device.
Owner:MARKER HLDG AG
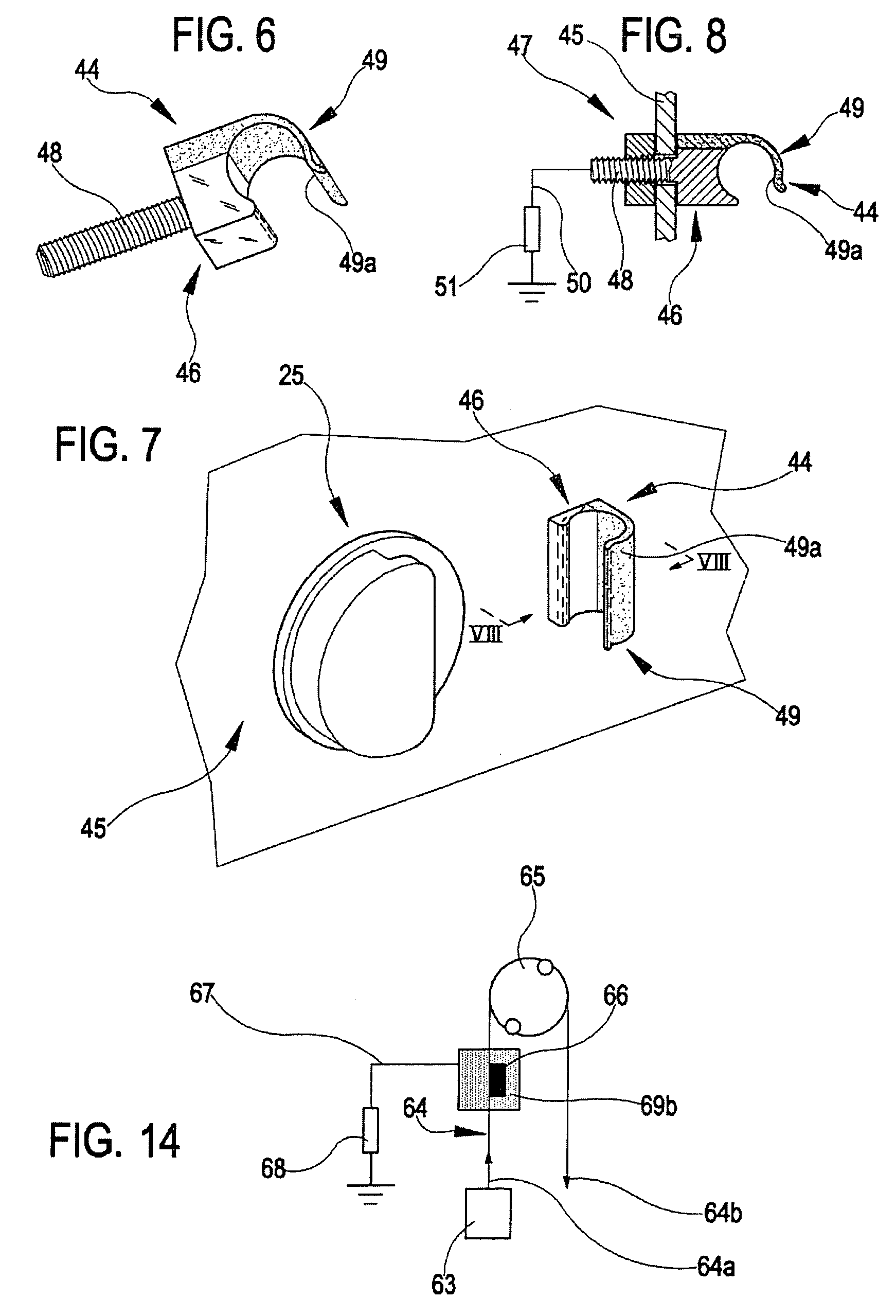
Joint for fluid transport lines for medical use
ActiveUS7291123B2Simple and economical solutionEliminate riskDrilling rodsDialysis systemsPeristaltic pumpIntensive treatment
The joint (36) comprises a tubular body (37) having two connecting zones (38, 39) each connected by an end to a tubular element (40) of a fluid transport line, giving continuity to passage of fluid. The tubular body is made of a mixture of an electrically-conductive material such as PVC, with carbon black to give it electrical conductivity. The joint has an internal surface (41) which is destined to come into contact with the transported fluid, and an external surface which is destined to have a grounded galvanic contact. The joint is inserted in the discharge fluid drainage line of a dialyzer filter, in an apparatus for intensive treatment of acute renal insufficiency, for eliminating ECG artefacts due to functioning of peristaltic pumps in the apparatus.
Owner:GAMBRO LUNDIA AB
Compositions and methods for augmenting kidney function
InactiveUS20050074442A1Promote healthy intestinal microenvironmentReduce decreaseBiocideOrganic active ingredientsNitrogenous waste productRenal function
Owner:KIBOW BIOTECH
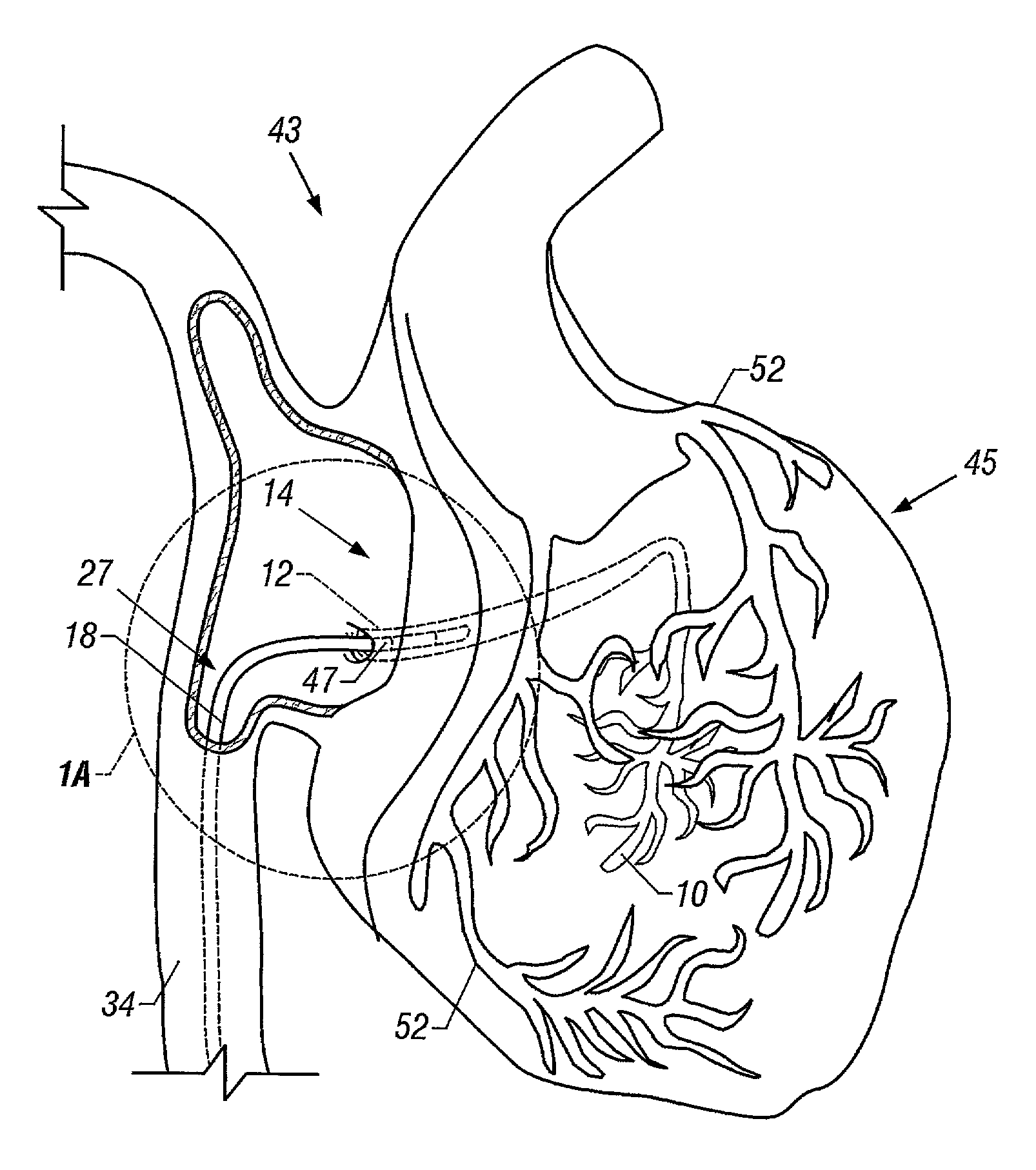
Method and apparatus to remove substances from vessels of the heart and other parts of the body to minimize or avoid renal or other harm or dysfunction
To accomplish isolation and removal of a substance from a vasculature, a catheter is employed to occlude a vessel of the vasculature. The substance is thus isolated in the vasculature and can be removed. In this way, the substance is removed before entering other parts of the circulatory system. This method is applicable to removal of contrast from the coronary sinus shortly after injection of the coronary arteries with the contrast. The method substantially minimizes or avoids renal dysfunction caused by angiographic procedures in which contrast must be injected. Such angiographic procedures are often performed during intervention procedures. This method substantially prevents circulation of the contrast to the kidneys where it could otherwise cause renal dysfunction or failure. The apparatus for implementation of the method is also disclosed.
Owner:CATHAROS MEDICAL SYST
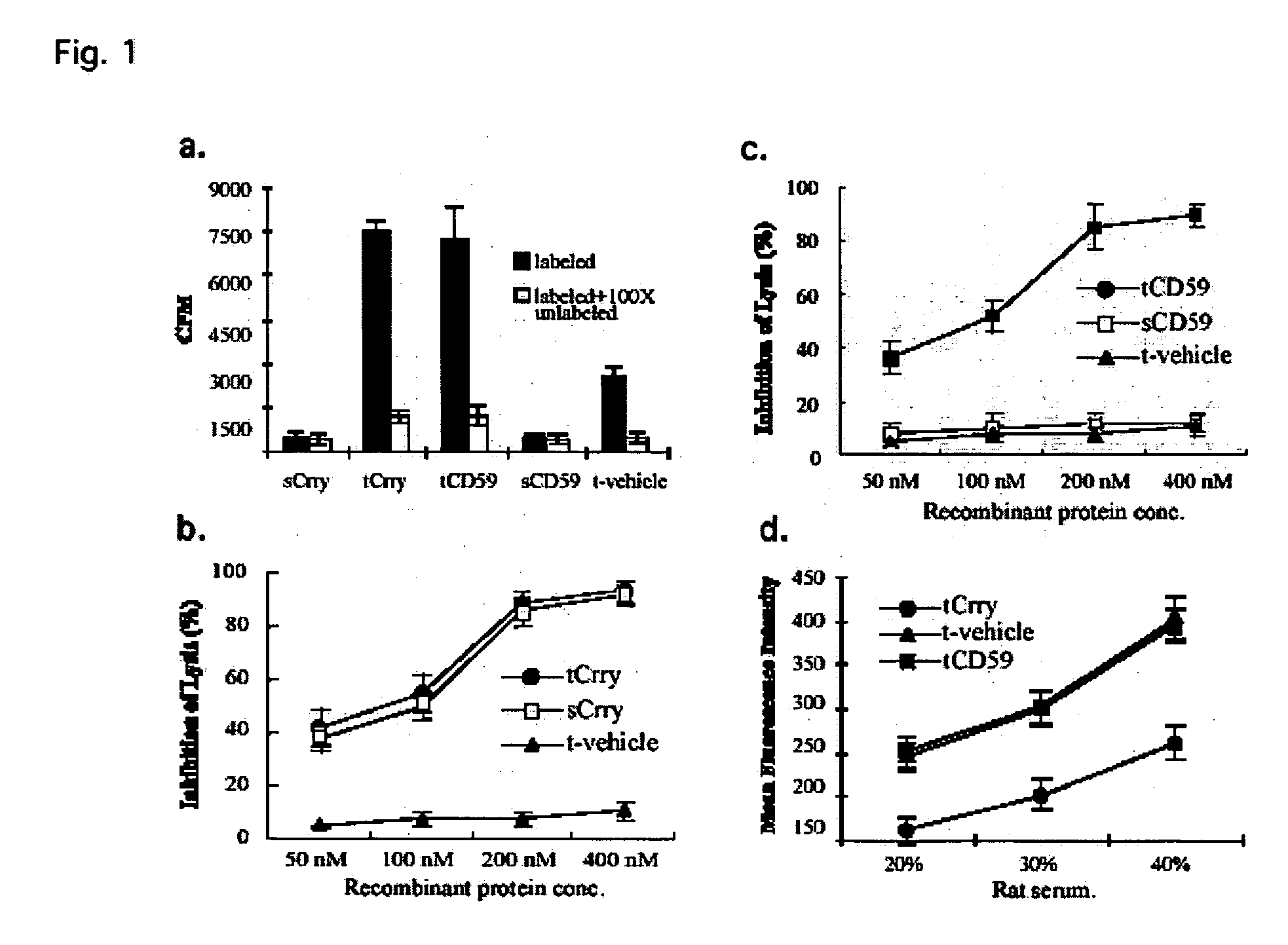
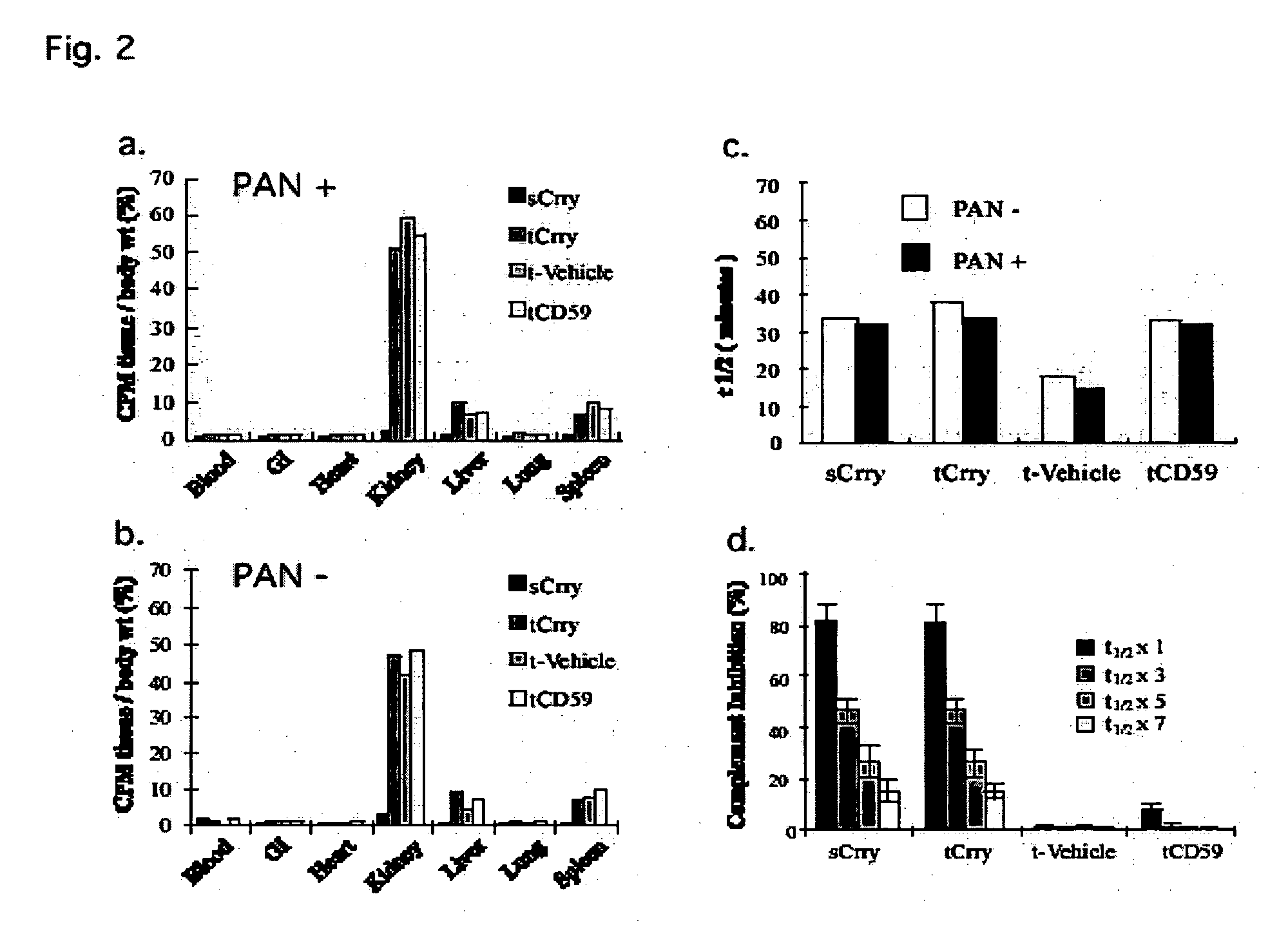
Tissue targeted complement modulators
InactiveUS20050265995A1Cell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsEpitheliumWhole body
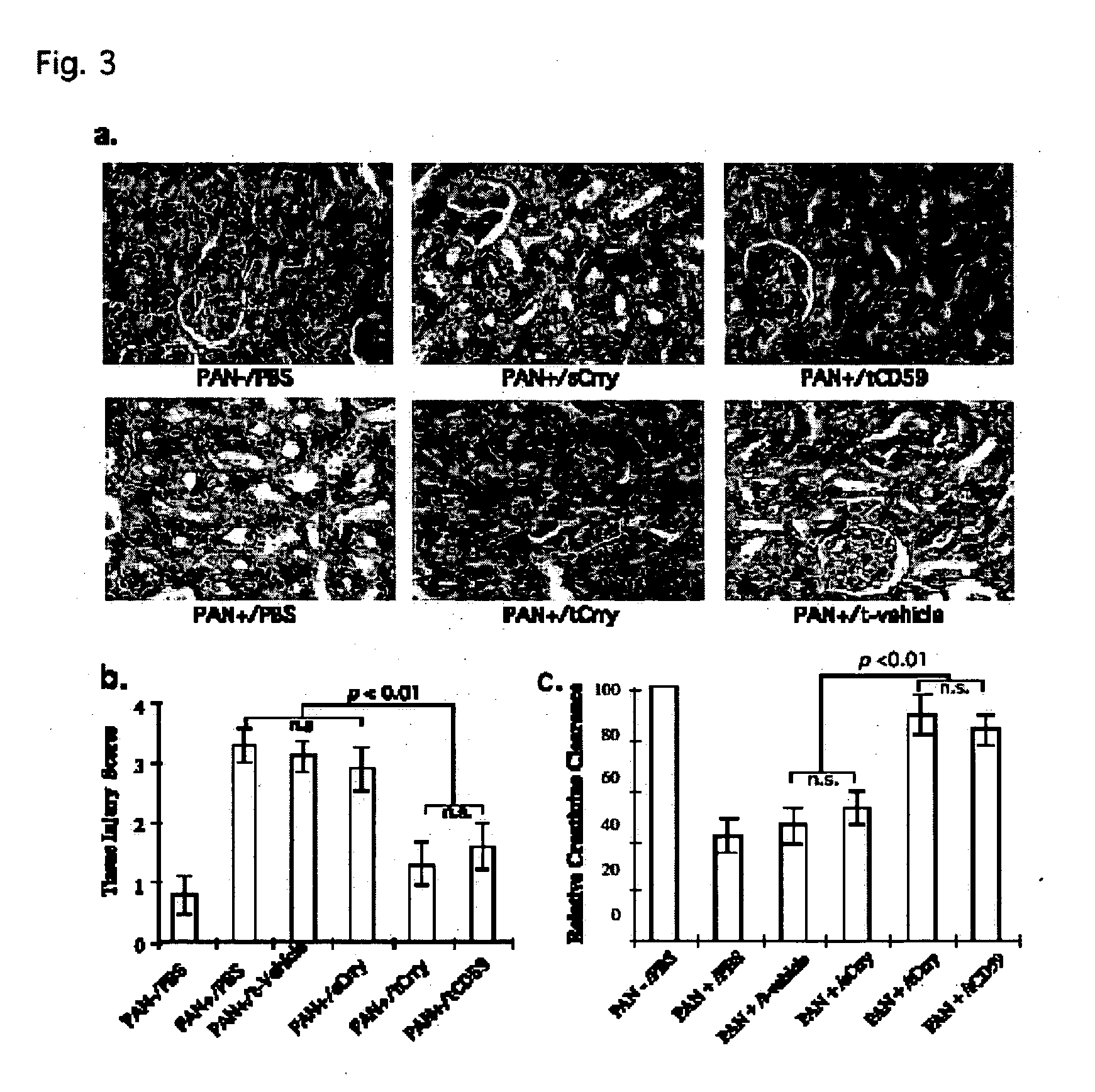
Systemic suppression of the complement system has been shown to be effective to treat inflammatory disease, yet at the potential cost of compromising host defense and immune homeostasis. Herein disclosed are methods for antigen-specific targeting of complement inhibitors that show that complement inhibitors targeted to the proximal tubular epithelium protect against tubulointerstitial injury and renal dysfunction in a rat model of nephrosis. It is shown that appropriate targeting of a systemically administered complement inhibitor to a site of disease markedy enhances efficacy and obviates the need to systemically inhibit complement. Additionally, it is shown by specifically inhibiting the terminal pathway of complement, that the membrane attack complex (MAC) plays a key role in proteinuria-induced tubulointerstitial injury, thus establishing the MAC as a valid target for pharmacological intervention in proteinuric disorders. The disclosed are compositions can be used in methods of treating pathogenic diseases and inflammatory conditions by modulating the complement system.
Owner:UNIVERSITY OF CHICAGO +1
Stem-cell, precursor cell, or target cell-based treatment of multiorgan failure and renal dysfunction
Methods for the treatment of acute renal failure, multi-organ failure, early dysfunction of kidney transplant, chronic renal failure, organ dysfunction, and wound healing are provided. The methods include delivering a therapeutic amount of hematopoietic stem cells, non-hematopoietic, mesenchymal stem cells, hemangioblasts, and pre-differentiated cells to a patient in need thereof.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Inhalation of nitric oxide for treating respiratory diseases
InactiveUS20150034084A1Effected safelyRespiratorsInorganic active ingredientsLiver and kidneyVascular endothelium
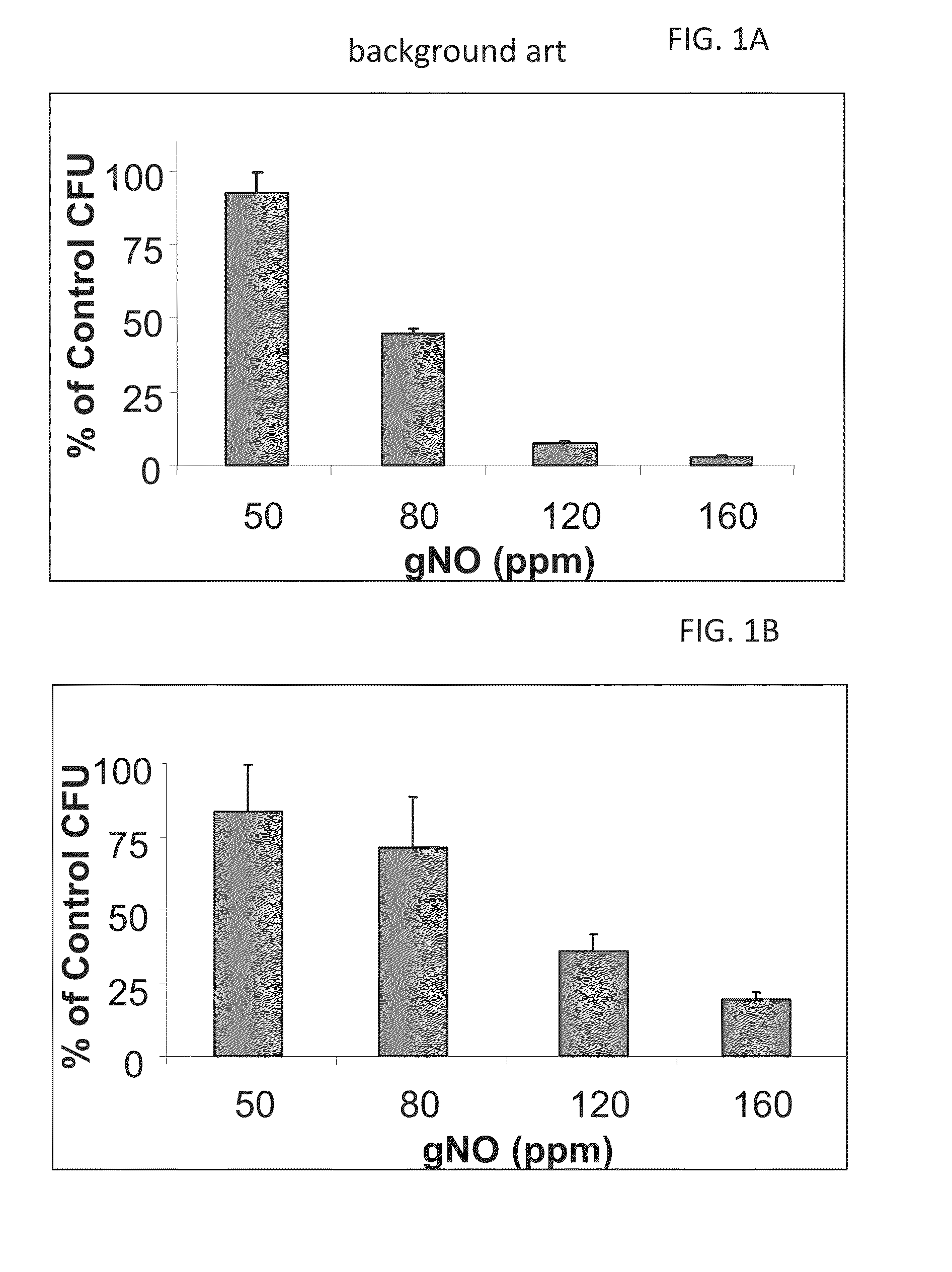
A method of treating a human subject which is effected by intermittent inhalation of gaseous nitric oxide at a concentration of at least 160 ppm is disclosed. The method can be utilized for treating a human subject suffering from, or prone to suffer from, a disease or disorder that is manifested in the respiratory tract, or from a disease or disorder that can be treated via the respiratory tract. The disclosed method can be effected while monitoring one or more of on-site and off-site parameters such as vital signs, methemoglobin levels, pulmonary function parameters, blood chemistry and hematological parameters, blood coagulation parameters, inflammatory marker levels, liver and kidney function parameters and vascular endothelial activation parameters, such that no substantial deviation from a baseline in seen in one or more of the monitored parameters.
Owner:ADVANCED INHILATION THERAPIES AIT LTD
Method for artificially culturing paecilomyces cicadae and application of culturing product thereof
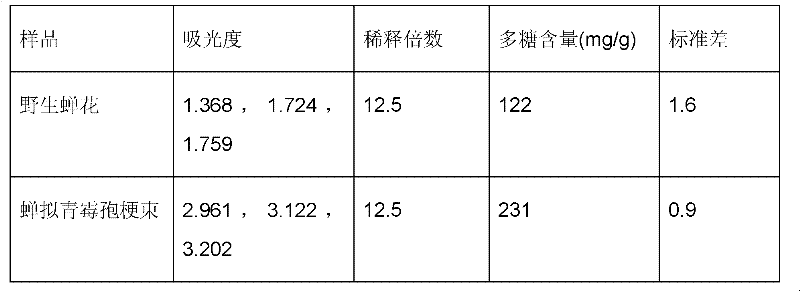
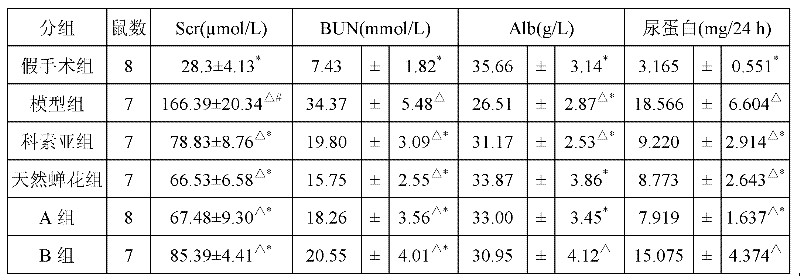
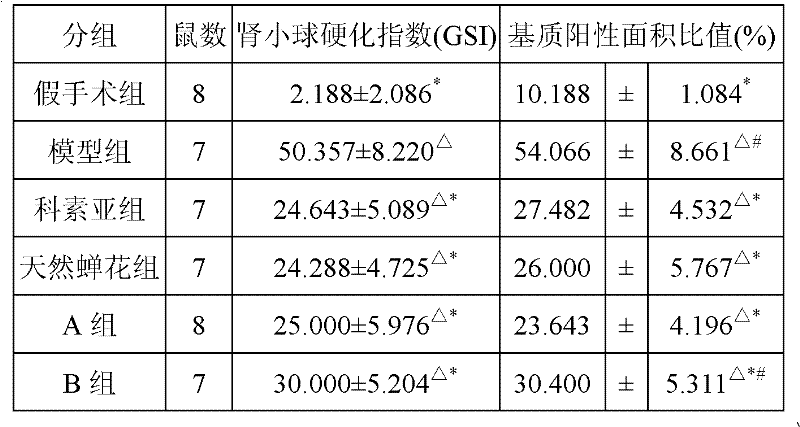
ActiveCN102242070AA Simple Method for Artificially Cultivating Paecilomyces cicadaeReduce manufacturing costCosmetic preparationsSenses disorderSucroseSaccharum
The invention discloses a method for artificially culturing paecilomyces cicadae and application of a culturing product thereof. The method for artificially culturing paecilomyces cicadae in large scales comprises the steps of: preparing strains, dosing and packing into a box, sterilizing, inoculating, solid-fermenting, collecting and the like. The paecilomyces cicadae is cultured by utilizing grains, such as corn flour, bran, wheat, barley, rice, millet, broomcorn and the like and culture mediums of bagasse, cane sugar, shell powder, silkworm chrysalis meal and potassium nitrate. The raw materials are obtained from local resources, a large amount of production cost is saved, the culturing period is shortened, and cordyceps sobolifera obtained by culturing has the advantages of high quality and favorable stability and the like. A culture obtained by the invention can be used for preparing foods, health-care products, drugs and cosmetics with functions of fighting tumor, regulating immunity, reducing blood sugar, blood fat and blood pressure, improving eye sight, resisting radiation, dispelling heat and easing pains, calming and hypnotizing, nourishing and strengthening, improving kidney function and the like.
Owner:ZHEJIANG BIOASIA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com