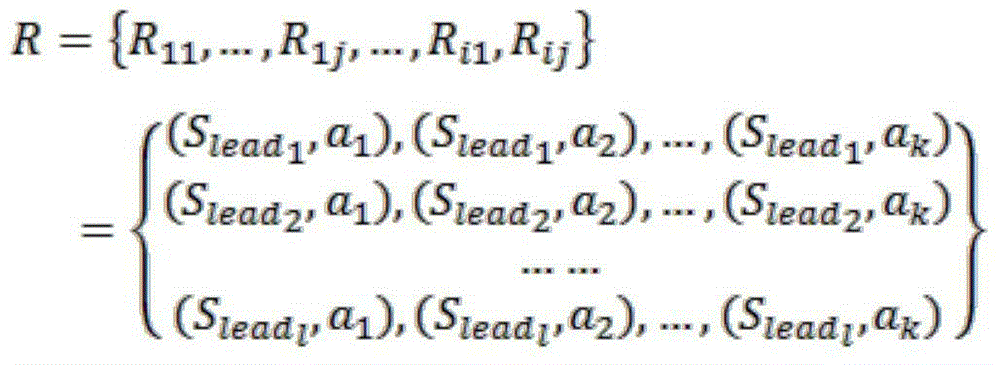Patents
Literature
93 results about "Early onset" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
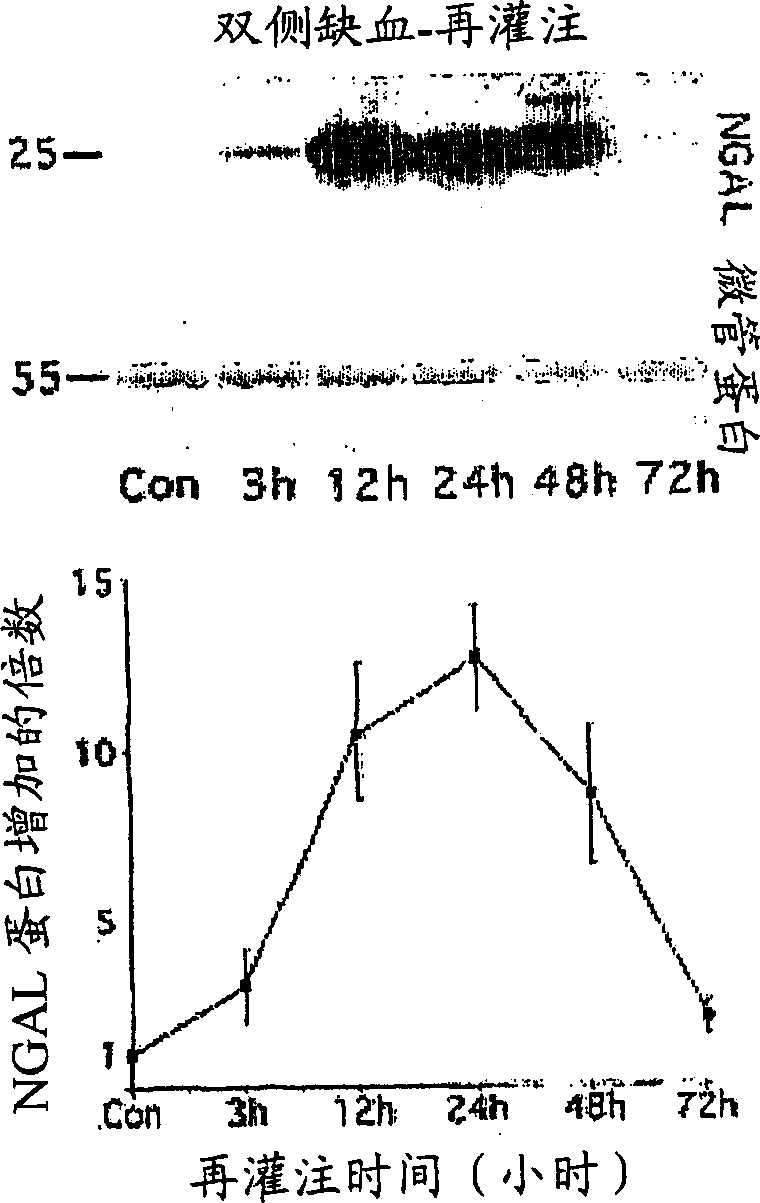
Method for the early detection of renal injury
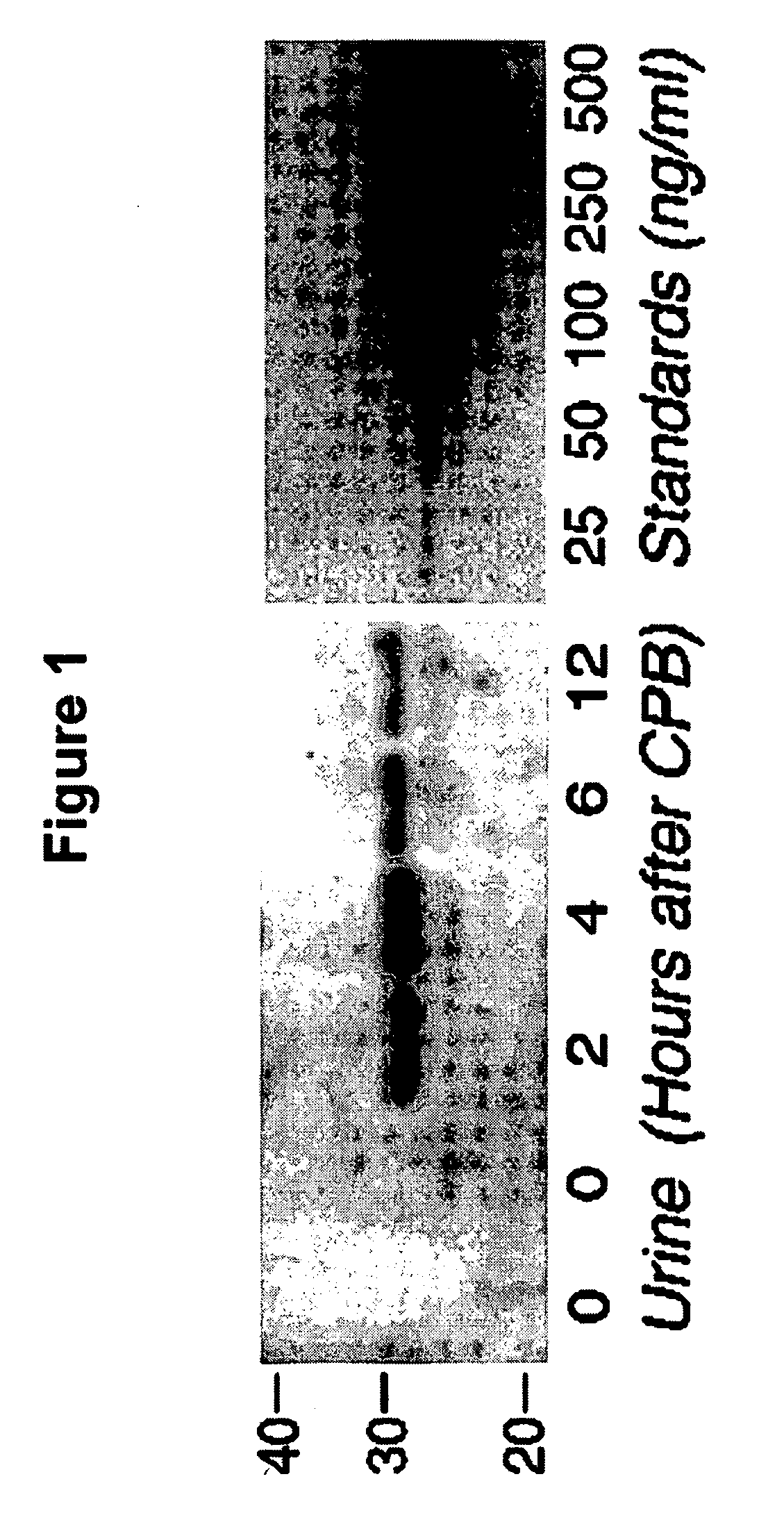
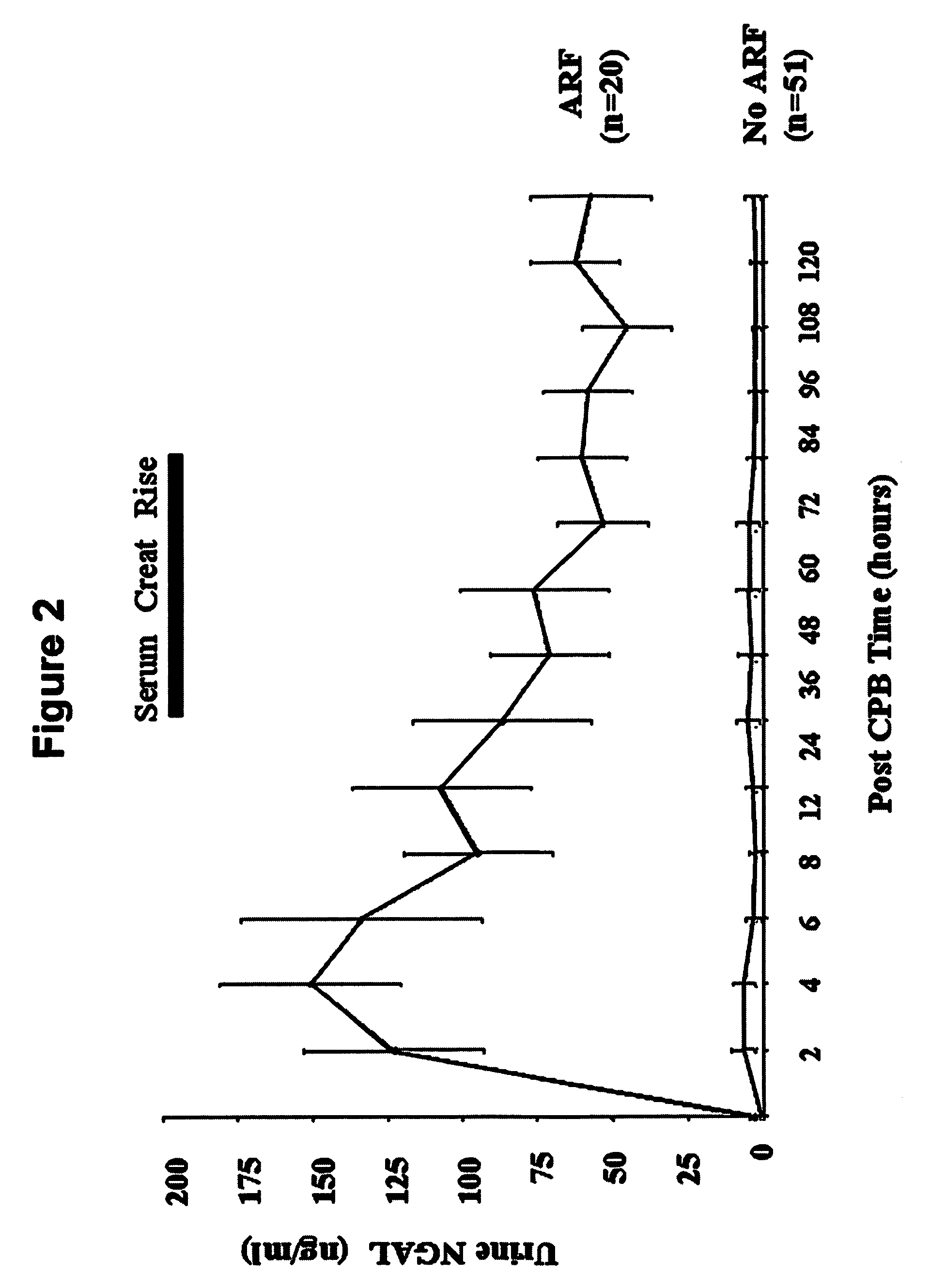
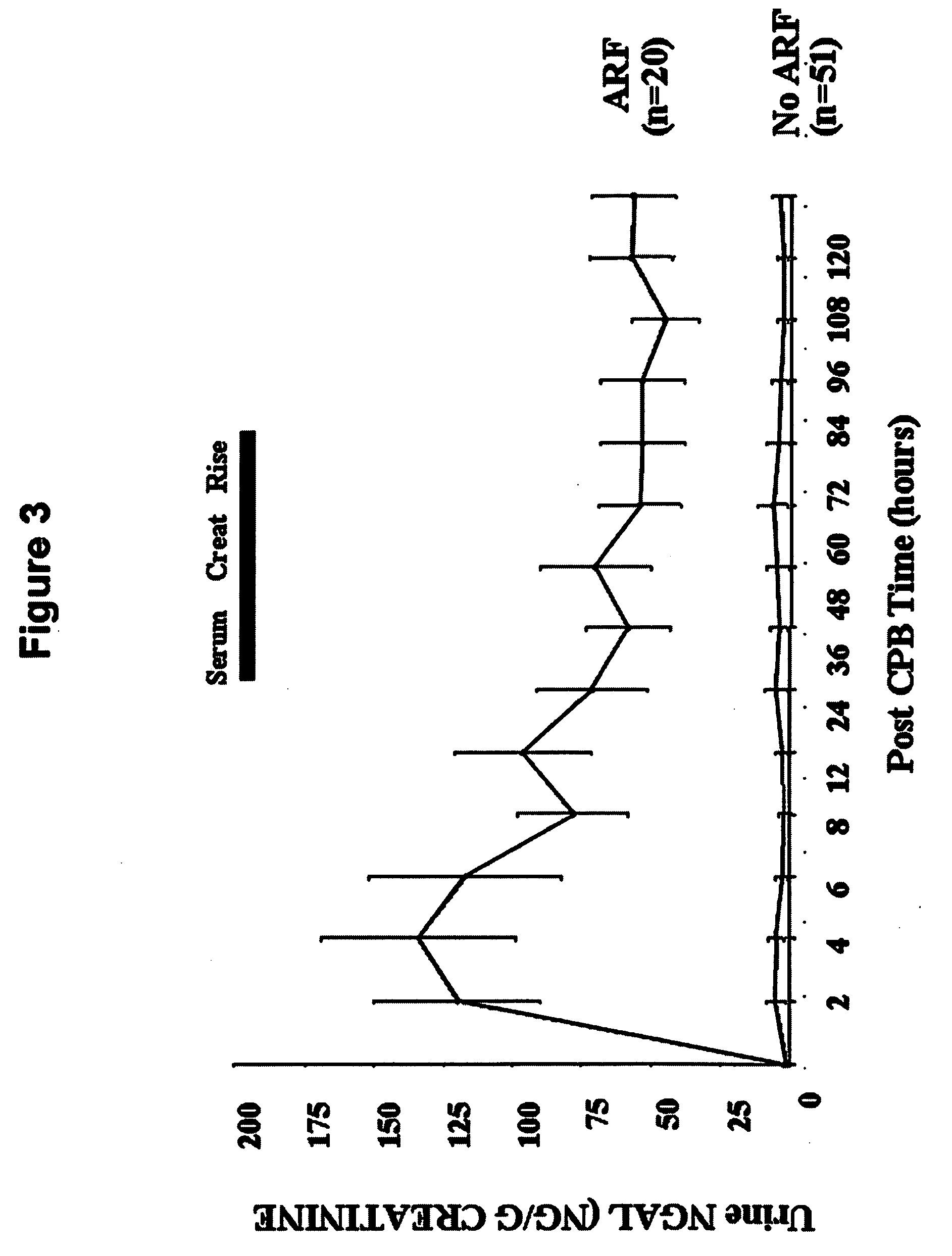
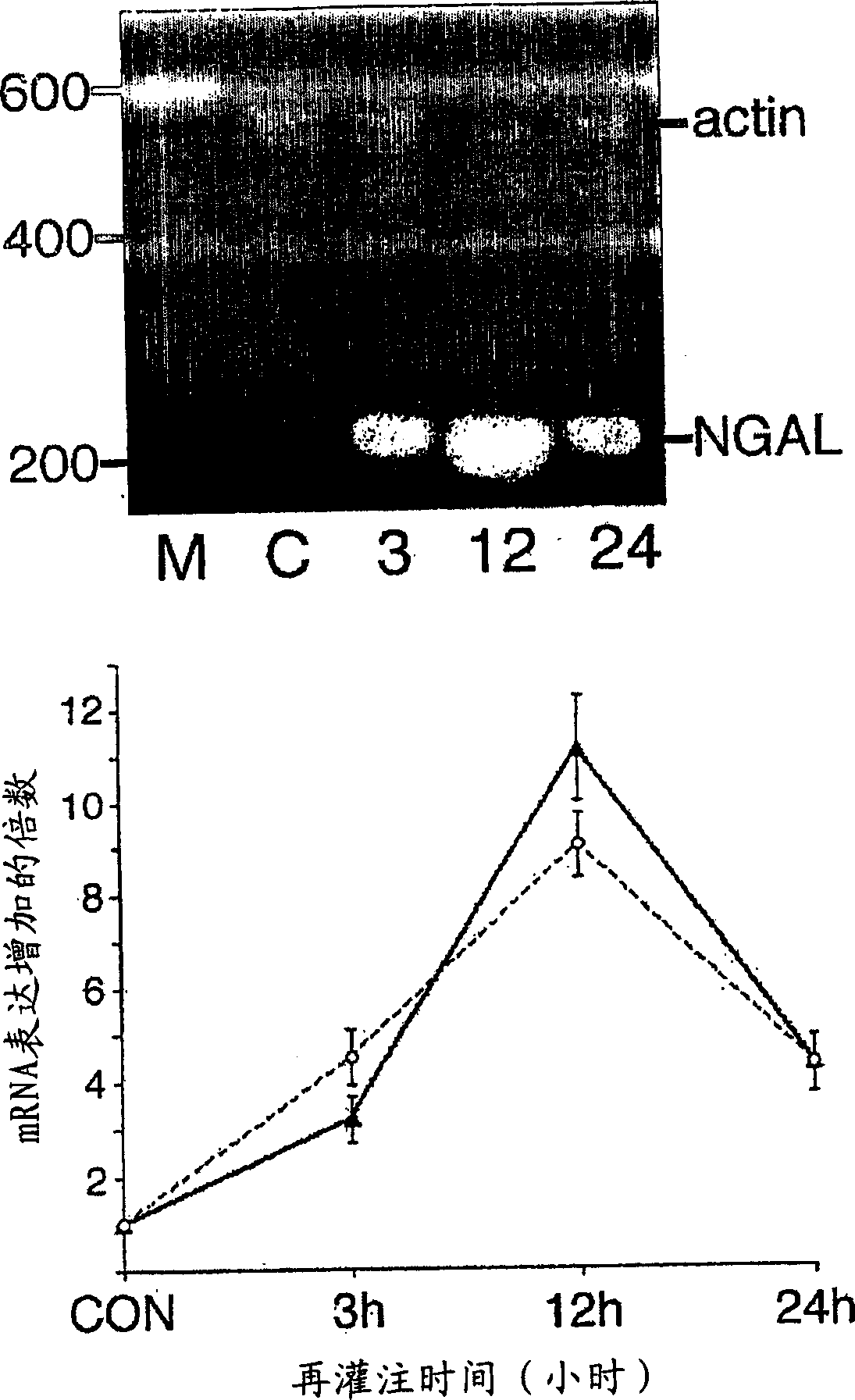
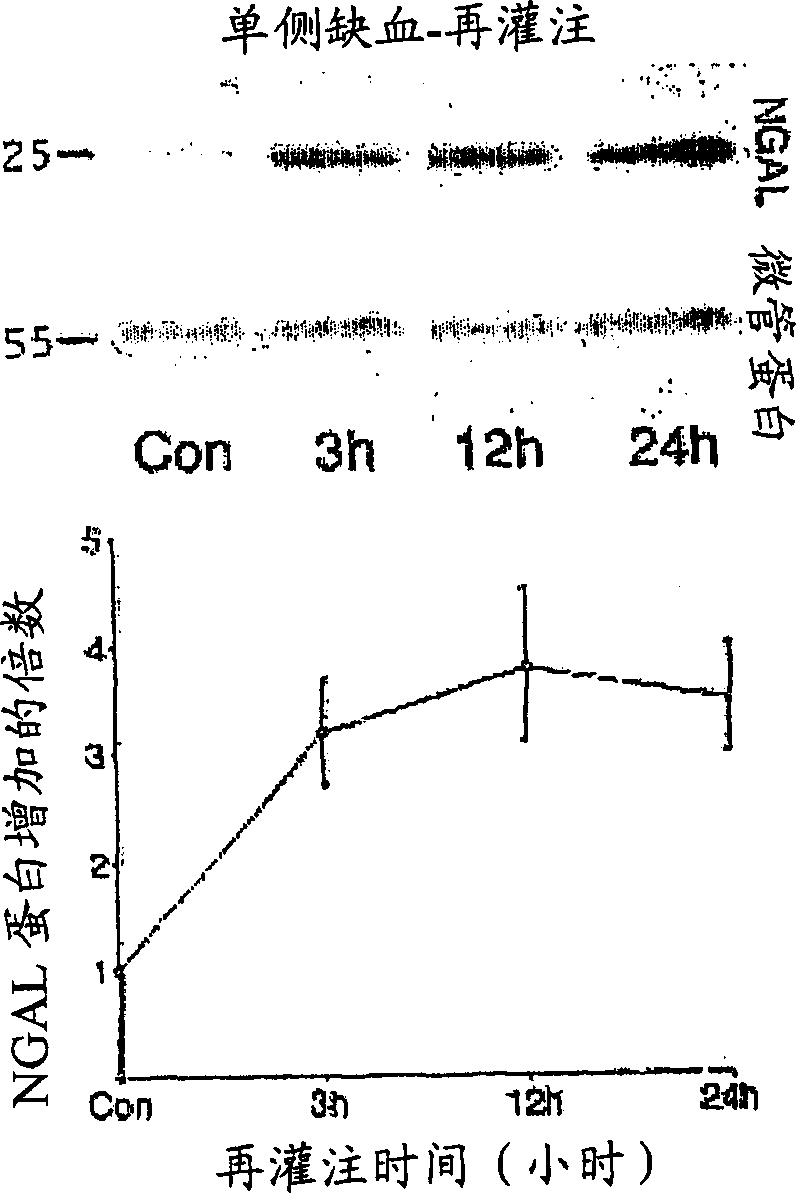
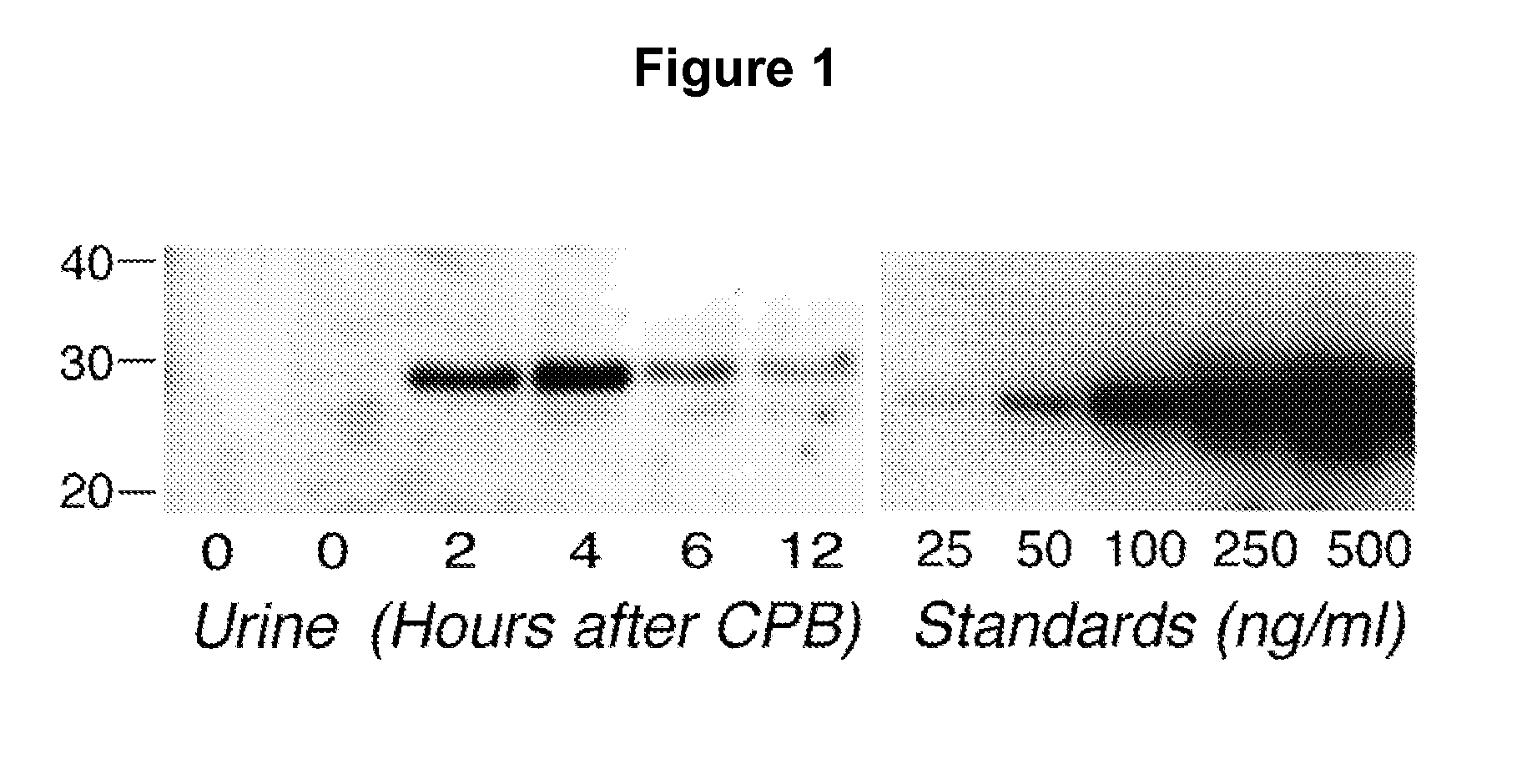
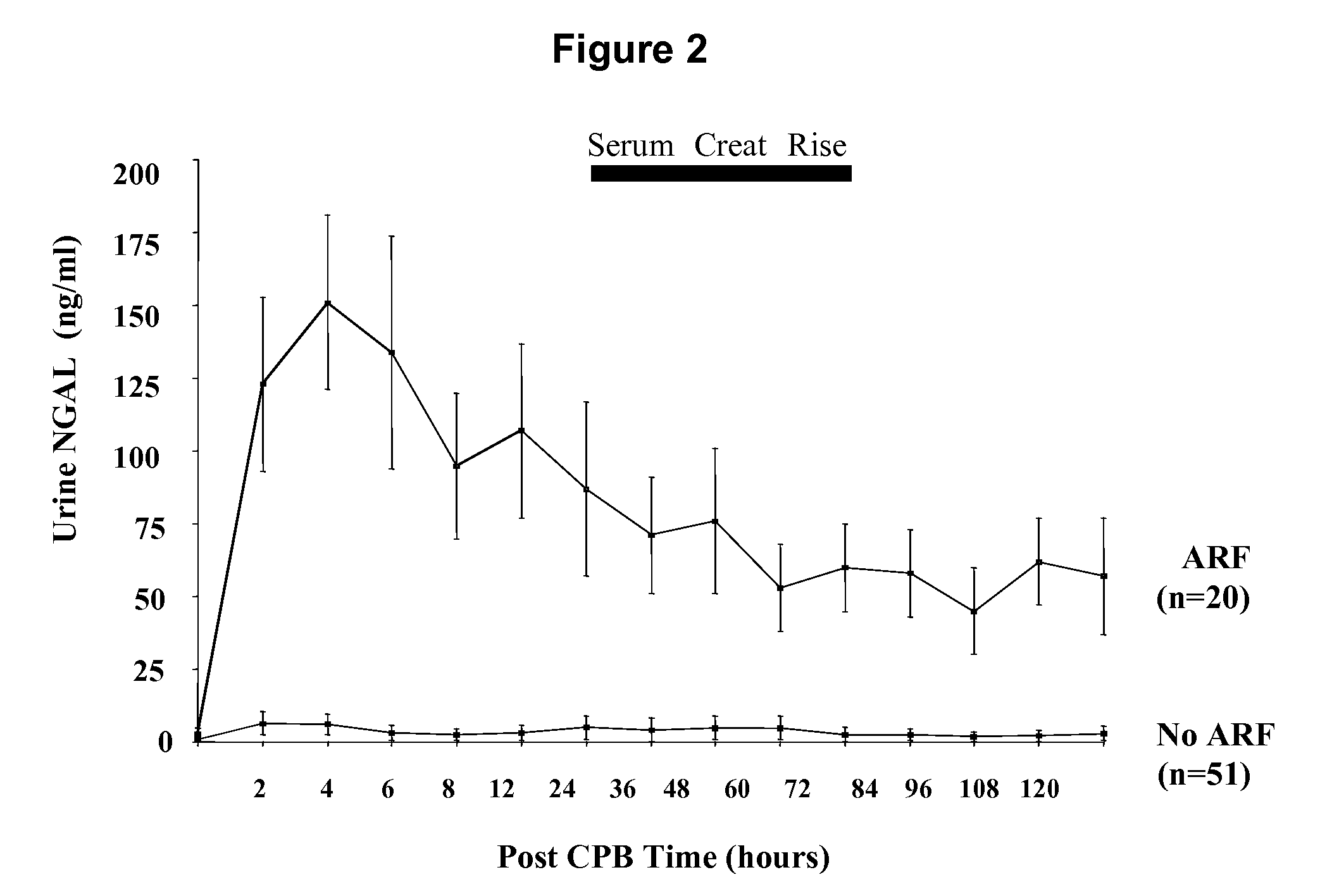
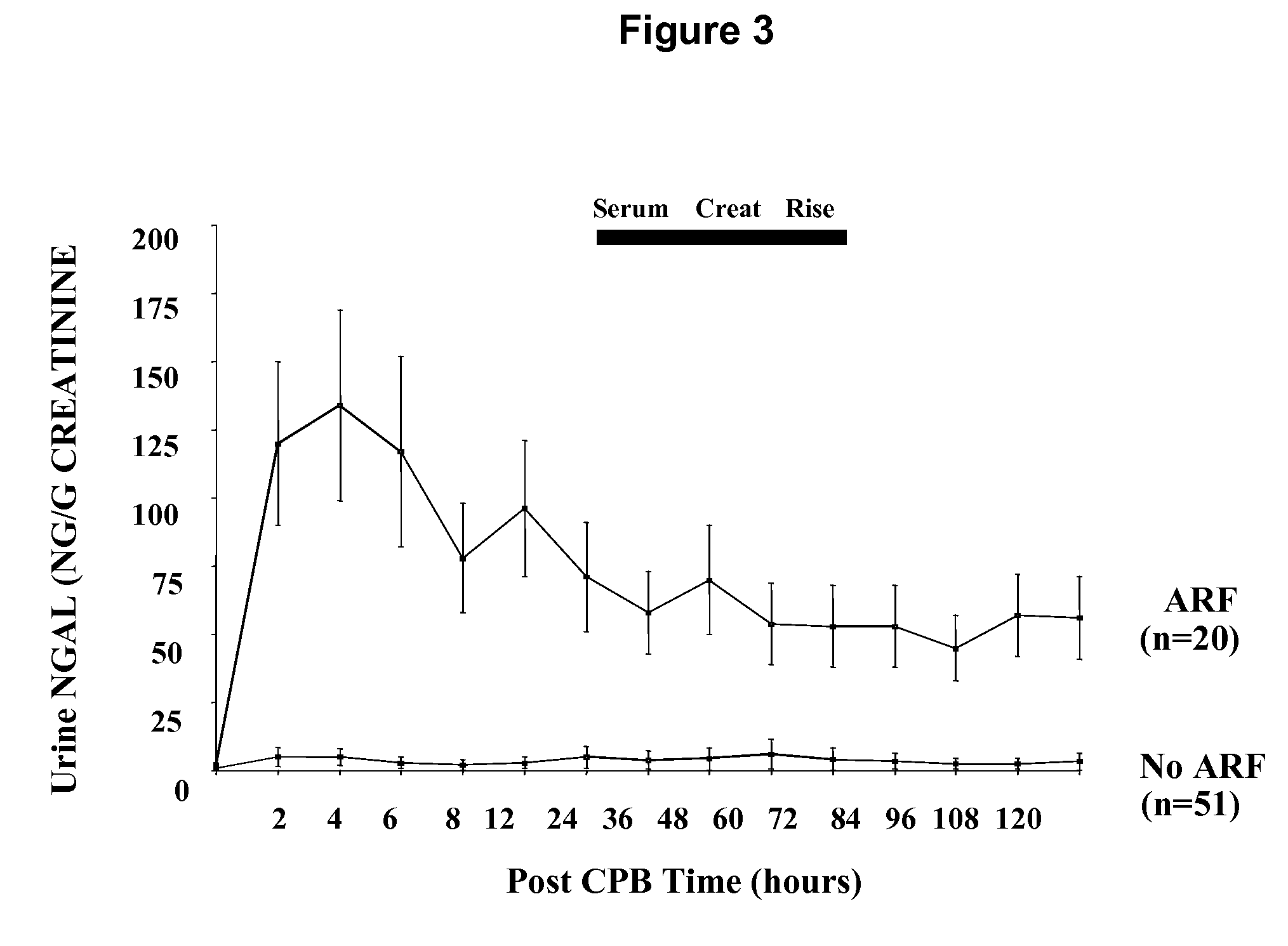
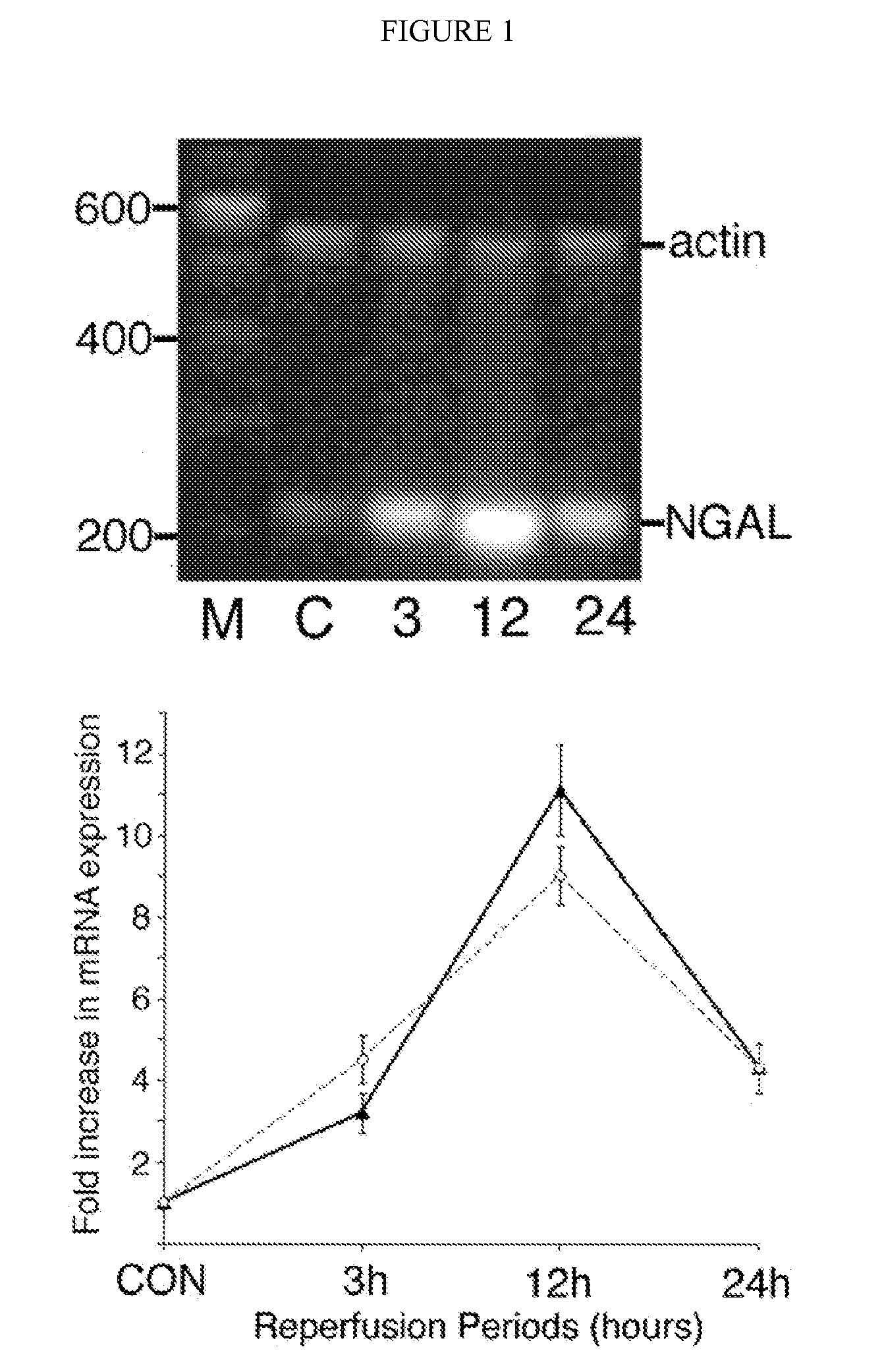
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Method and apparatus for improving personnel safety and performance using logged and real-time vital sign monitoring
InactiveUS20120010488A1Good effectSafe and efficientSensorsBlood characterising devicesOccupational workEmergency medicine
Group (including, without limitation, occupational work forces) safety and wellness monitoring utilizes baseline physiology testing and vital sign monitoring and sampling technologies. Aerobic capacity of individual members of a group is determined through initial baseline testing which results in an individual health risk assessment. Thereafter non-invasively observed and monitored during incremental work and exercise is commenced. Subsequent data collected is used for advance identification of personnel at risk of injury, such as during unexpected vital sign elevations that can signal early onset of fatigue and heat stress / dehydration prior to injury.
Owner:HENRY BARRY J +1
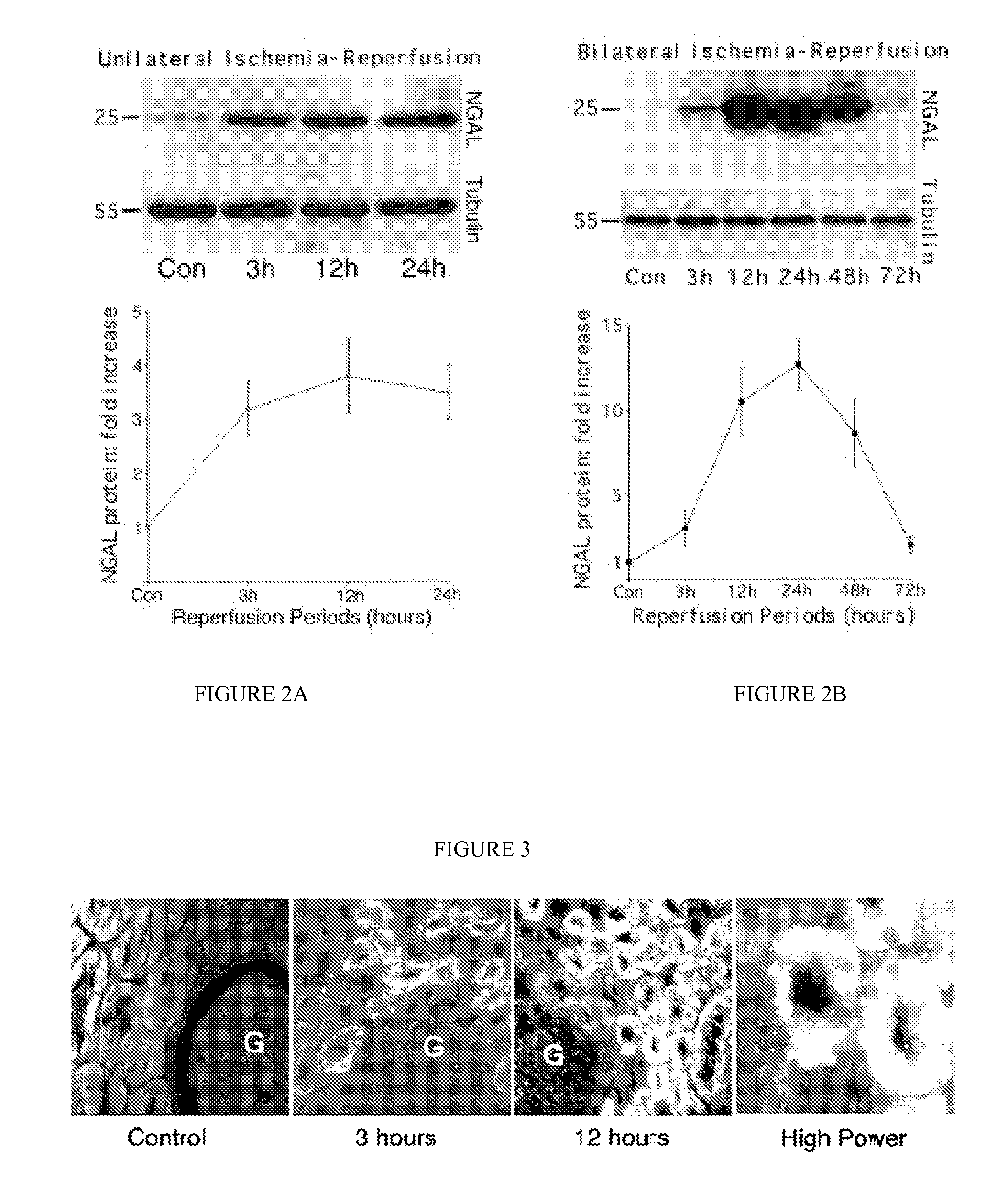
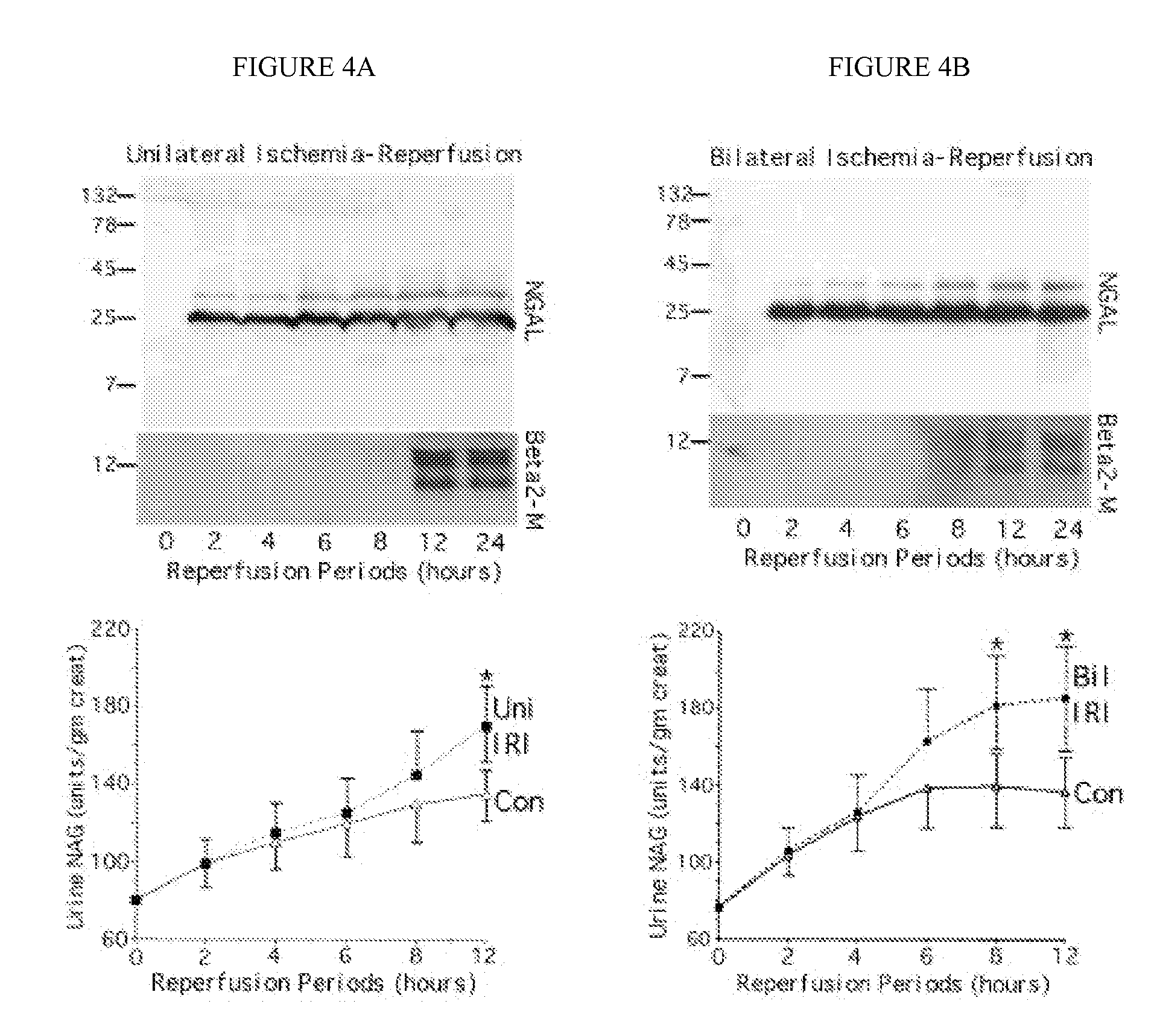
A method and kit for detecting the early onset of renal tubular cell injury
An early-onset method and kit for detecting renal tubular cell injury, using NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and thus readily detected in urine following tubular cell injury. NGAL protein expression was mainly detected in the proximal tubule cells, which resembled a secreted protein with a punctate distribution in the cytoplasm. Apparent NGAL in urine correlates with the amount and duration of renal ischemia and nephrotoxicemia and is a diagnostic feature of tubular cell injury and renal failure. NGAL detection is also a useful marker for monitoring nephrotoxic side effects of drugs or other therapeutic agents.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Deposition sensor based on differential heat flux measurement
An apparatus and method for the monitoring and measurement of chemical and / or biological deposition in heat exchangers and other fluid processing vessels. The new and original sensing system includes at least two hollow fluid vessels conductively mounted across a constant heat transfer path. Thin film heat flux sensors are attached to a heat transfer surface of the vessels in order to measure changes in differential heat flux that occur when deposition begins to accumulate in the vessel. In this way, it is shown that differential heat flux measurements can be used to detect and measure the early onset of chemical and / or biological deposition.
Owner:BL TECH INC
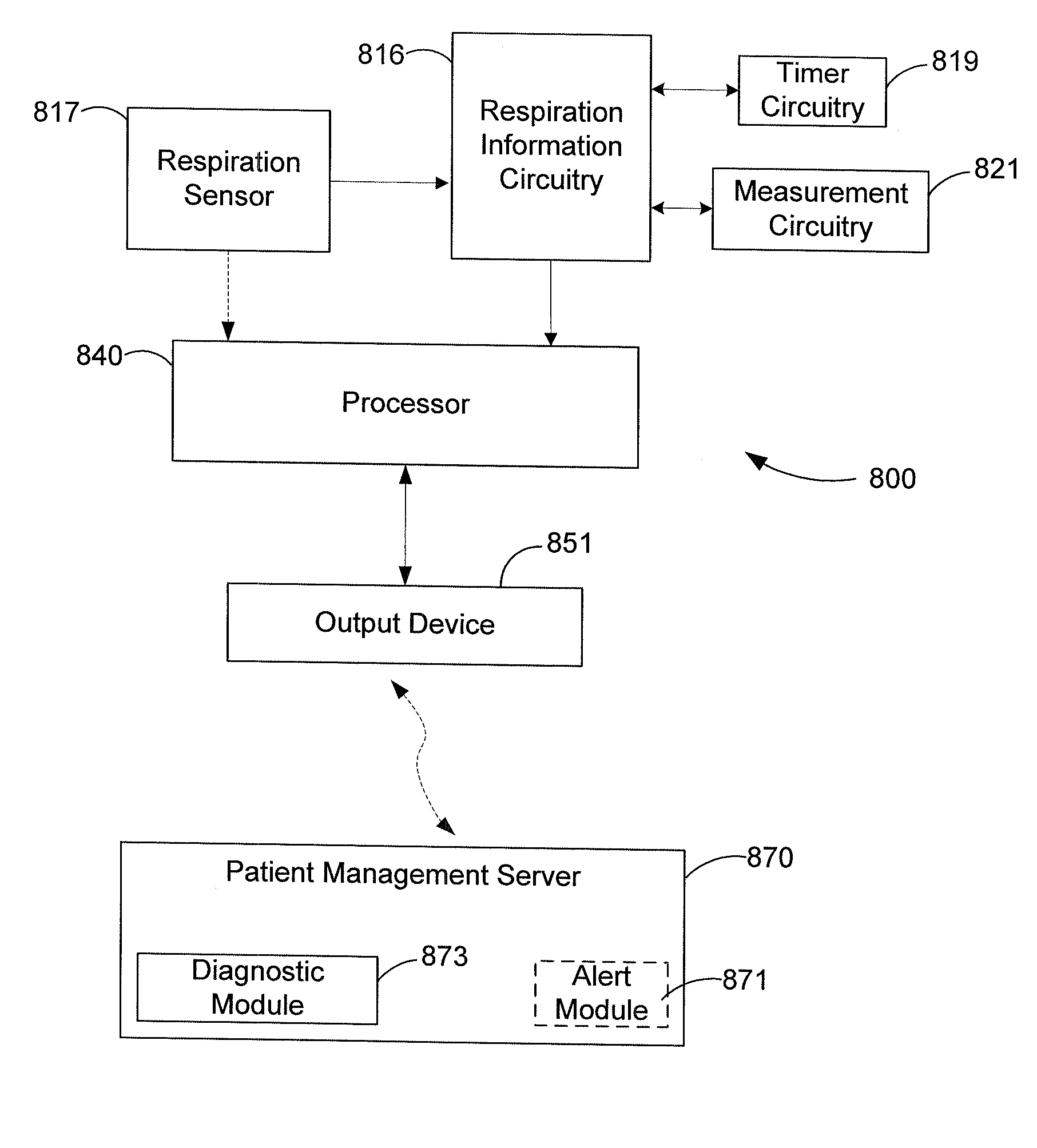
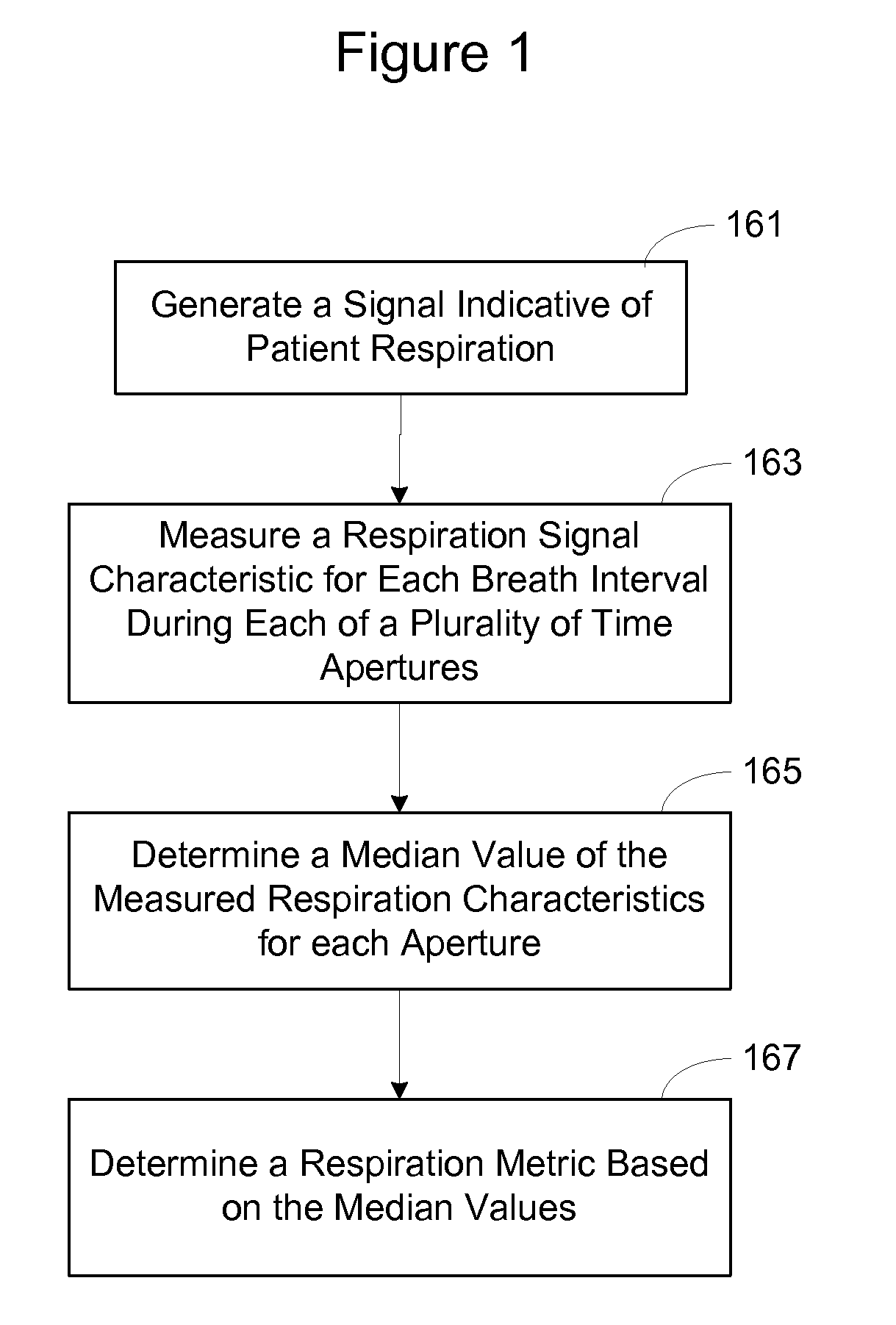
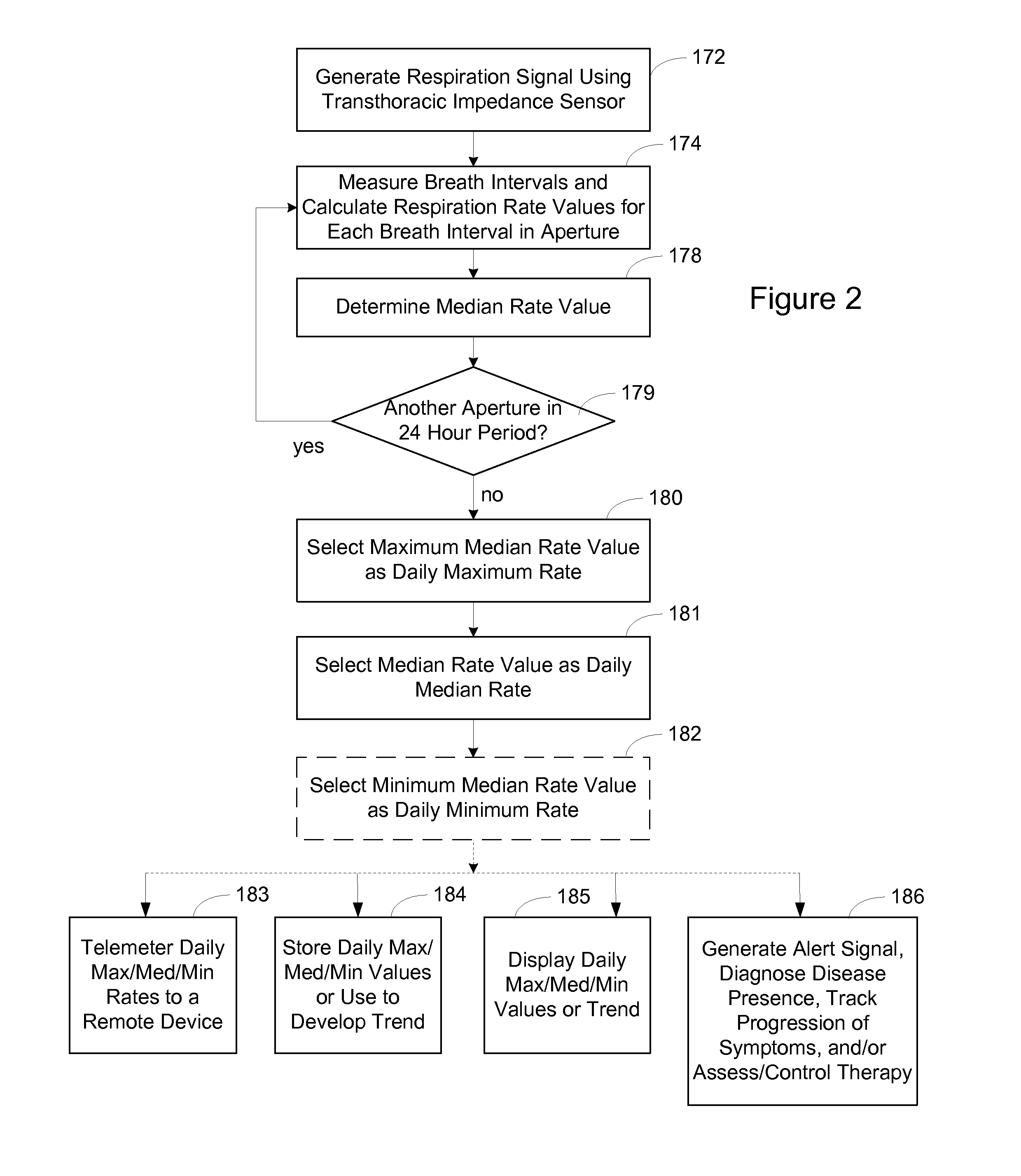
Respiration Rate Trending for Detecting Early Onset of Worsening Heart Failure
Patient respiration is sensed from which respiration measurements are made, including a median respiration rate (MedRR) and a maximum respiration rate (MaxRR). Determinations are made as to whether an abnormality exists in MedRR and in MaxRR. An output indicative of the patient's tachypnea status is generated in response to determining the abnormality in MedRR and MaxRR.
Owner:CARDIAC PACEMAKERS INC
Noninvasive Measurement and Identification of Biomarkers in Disease State
ActiveUS20090104596A1Quick measurementQuickly and reliably and inexpensively identify disease stateMicrobiological testing/measurementDisease diagnosisArginineTyrosine
The invention is methods and related kits for diagnosing a disease state of cachexia by measuring biomarker profiles from a biological sample. Rapid measurement of early onset or progression of the disease in a subject is determined by measuring biomarker levels from the subject and optionally comparing the biomarker levels to a standard biomarker profile or metabolome phase portrait for the disease. The biomarkers measured in the assay and related kit for cachexia progression include biomarkers selected from the group consisting of lactate, citrate, formate, acetoacetate, 3-hydroxy butrate, alanine, glutamine, glutamate, valine, isoleucine leucine, thrionine, lysine, arginine, tyrosine, phenyl alanine, histidine and tryptophan.
Owner:WISCONSIN ALUMNI RES FOUND
Deposition sensor based on differential heat flux measurement
ActiveUS20050105583A1Thermometer detailsMaterial thermal conductivityEarly onsetCompound (substance)
An apparatus and method for the monitoring and measurement of chemical and / or biological deposition in heat exchangers and other fluid processing vessels. The new and original sensing system includes at least two hollow fluid vessels conductively mounted across a constant heat transfer path. Thin film heat flux sensors are attached to a heat transfer surface of the vessels in order to measure changes in differential heat flux that occur when deposition begins to accumulate in the vessel. In this way, it is shown that differential heat flux measurements can be used to detect and measure the early onset of chemical and / or biological deposition.
Owner:BL TECH INC
Methods of diagnosing early-onset menopause
InactiveUS6730476B1Effectively prescribeAvoid developmentAntipyreticGenetic material ingredientsEarly onsetMedicine
A method of predicting whether a subject is predisposed to developing early-onset menopause is provided. The method involves genotyping a patient at the IL-1 gene loci.
Owner:INTERLEUKIN GENETICS
Devices, systems and methods for bioimpedance measurement of cervical tissue and methods for diagnosis and treatment of human cervix
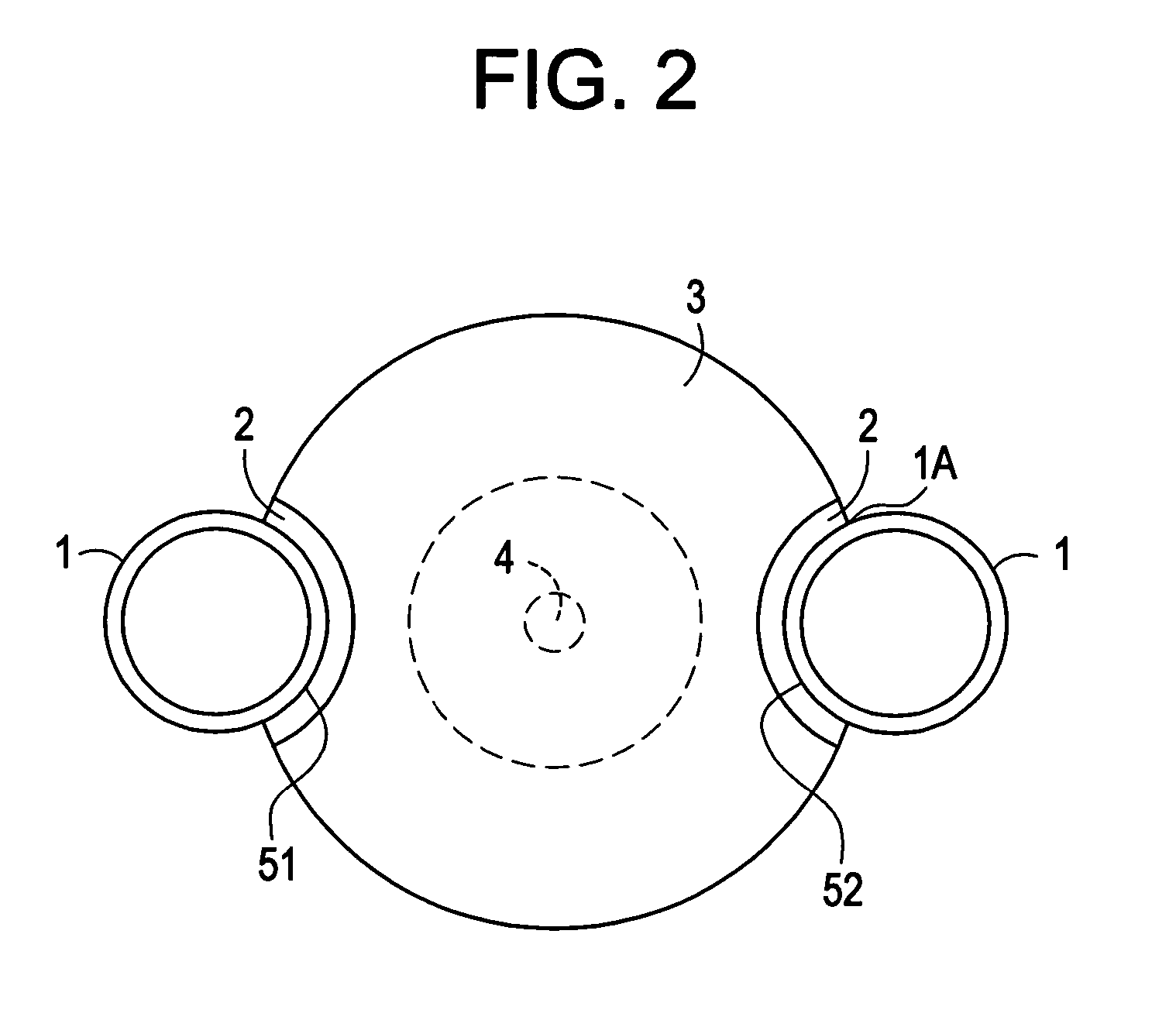
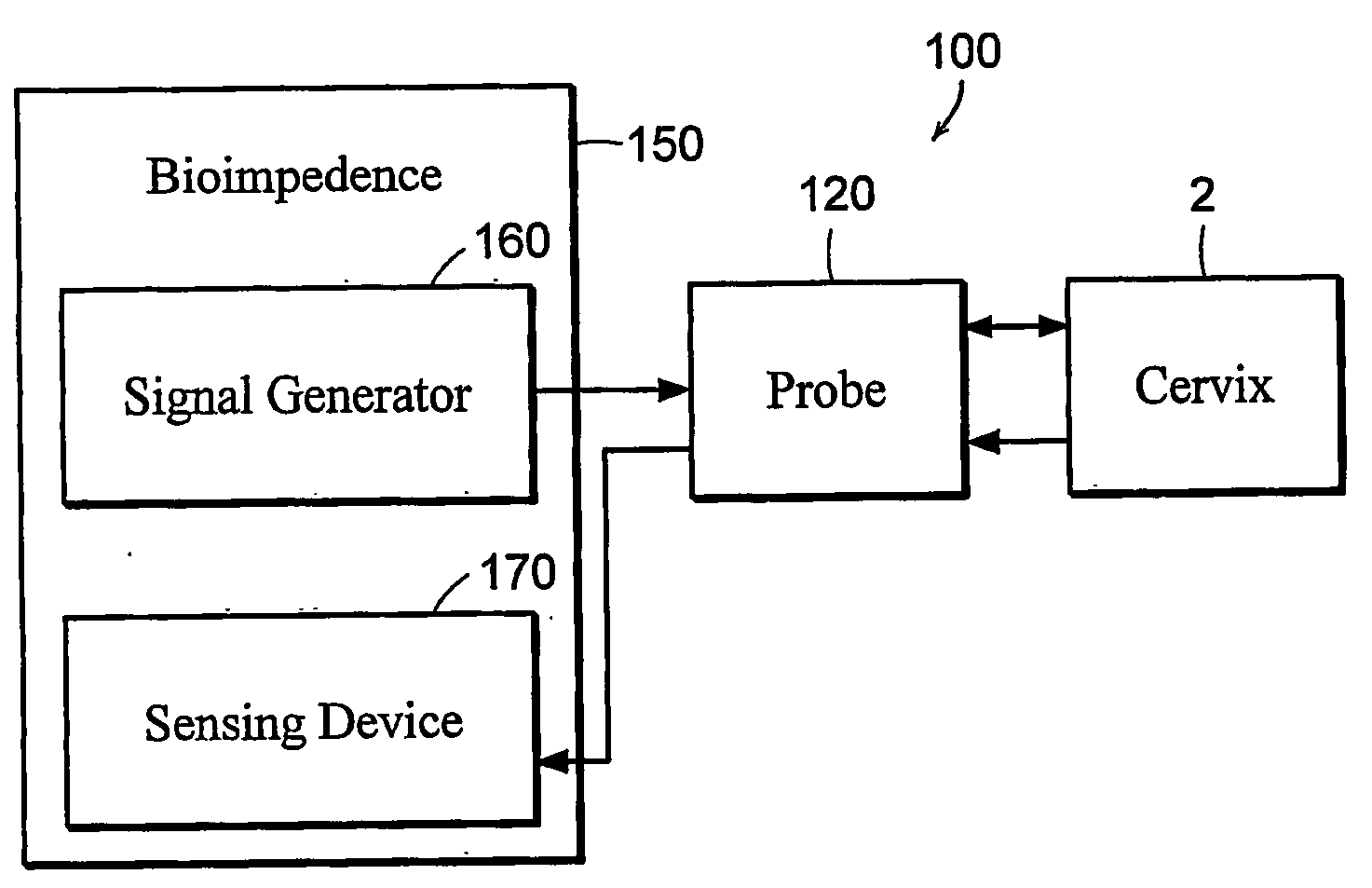
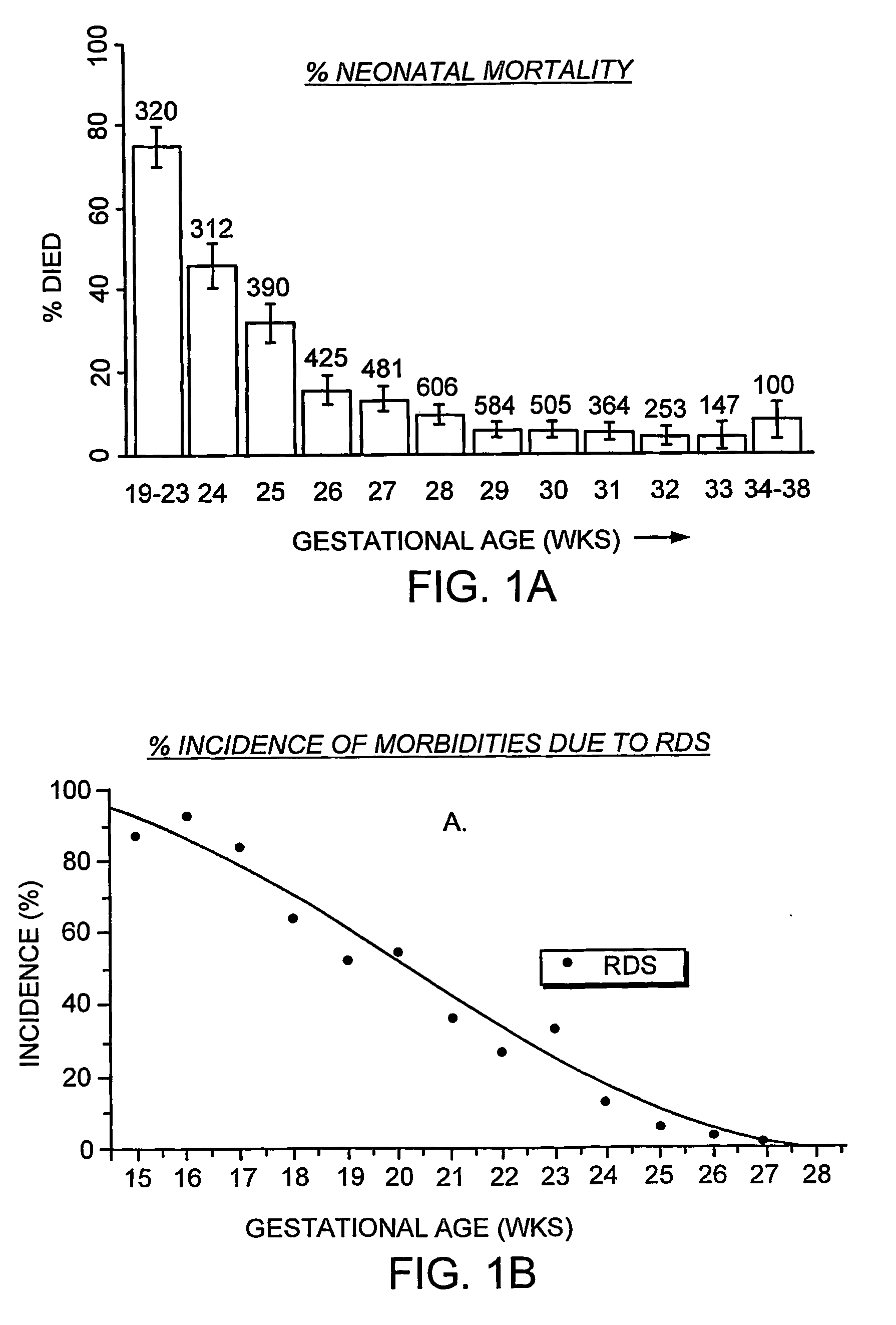
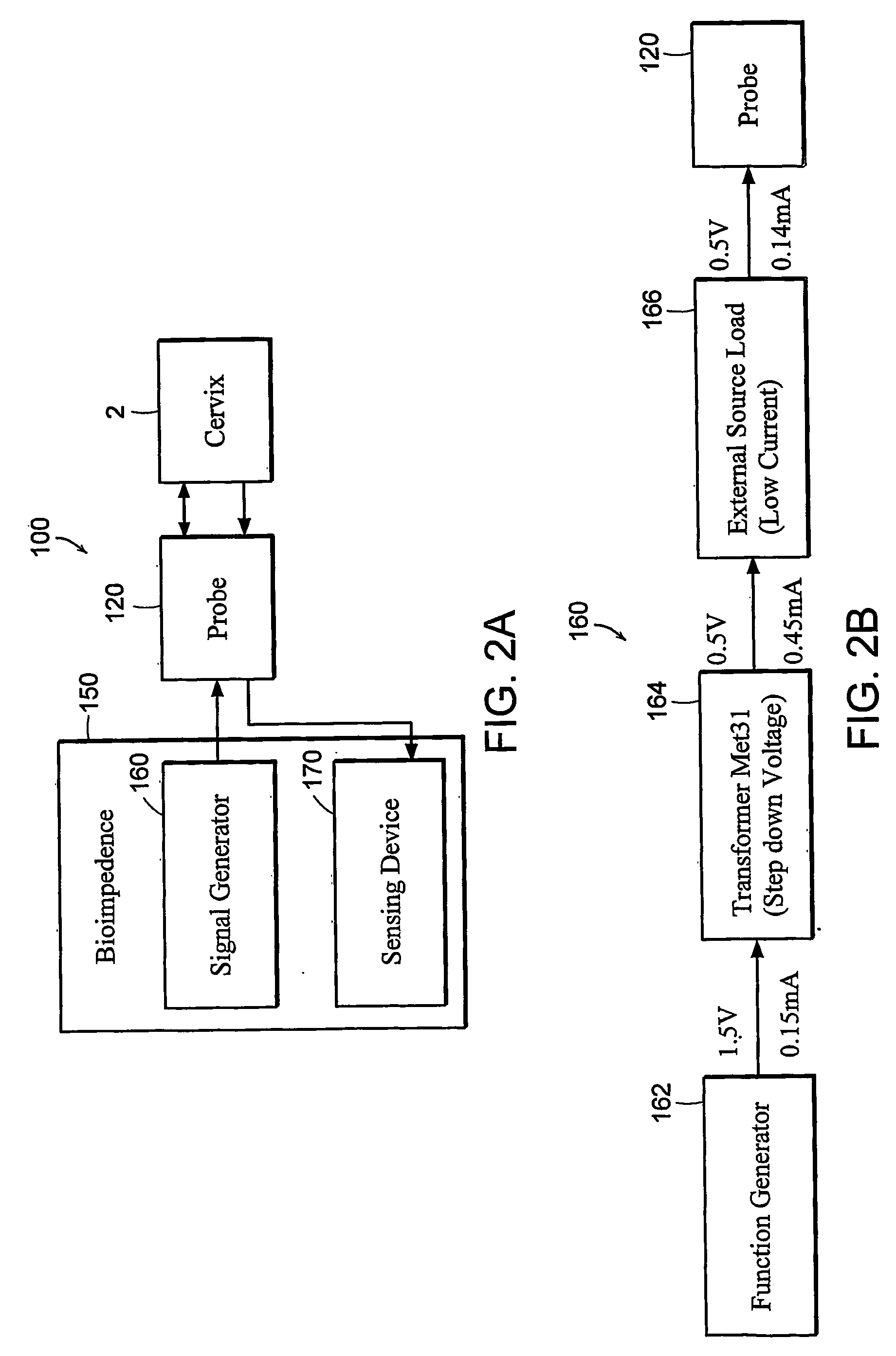
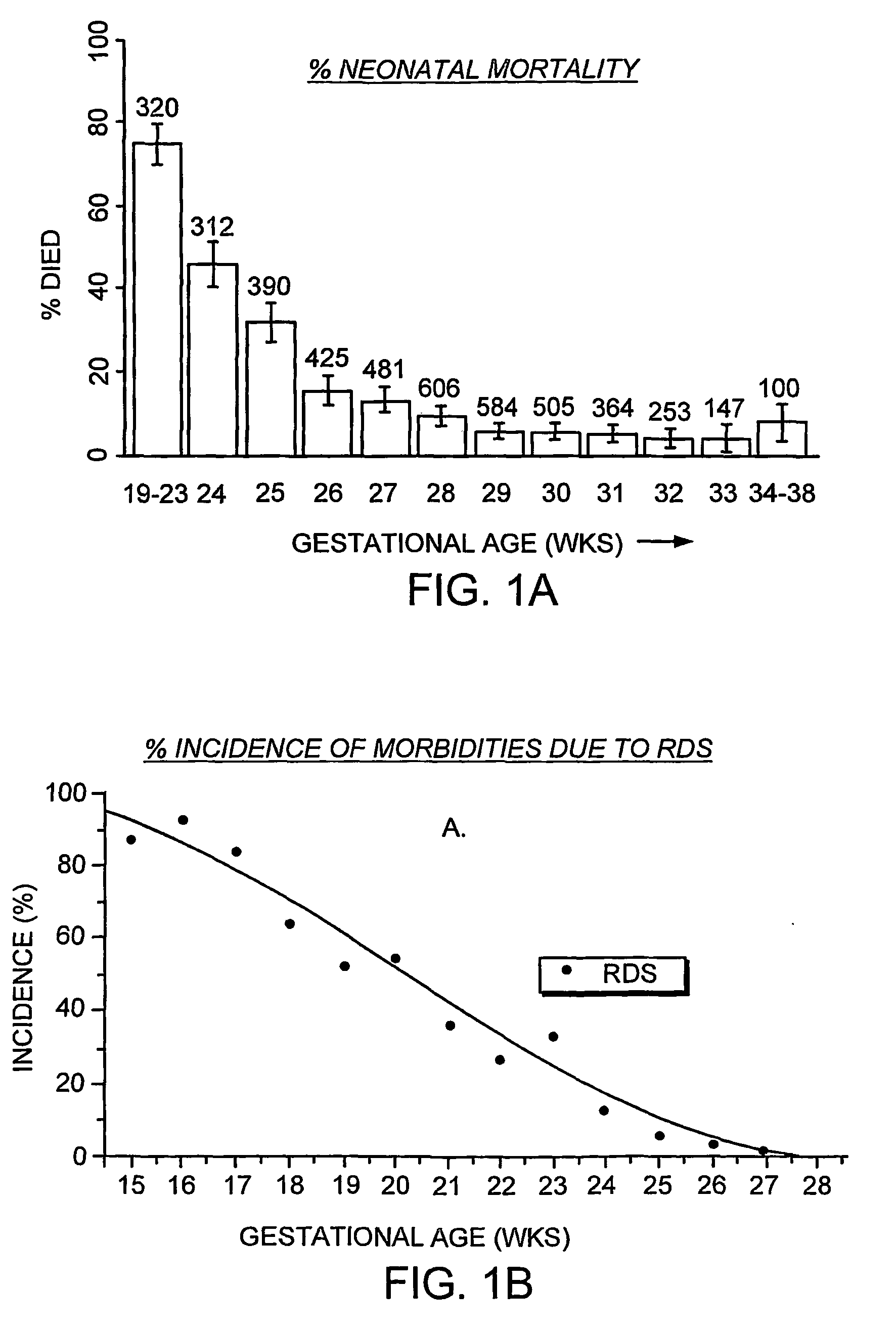
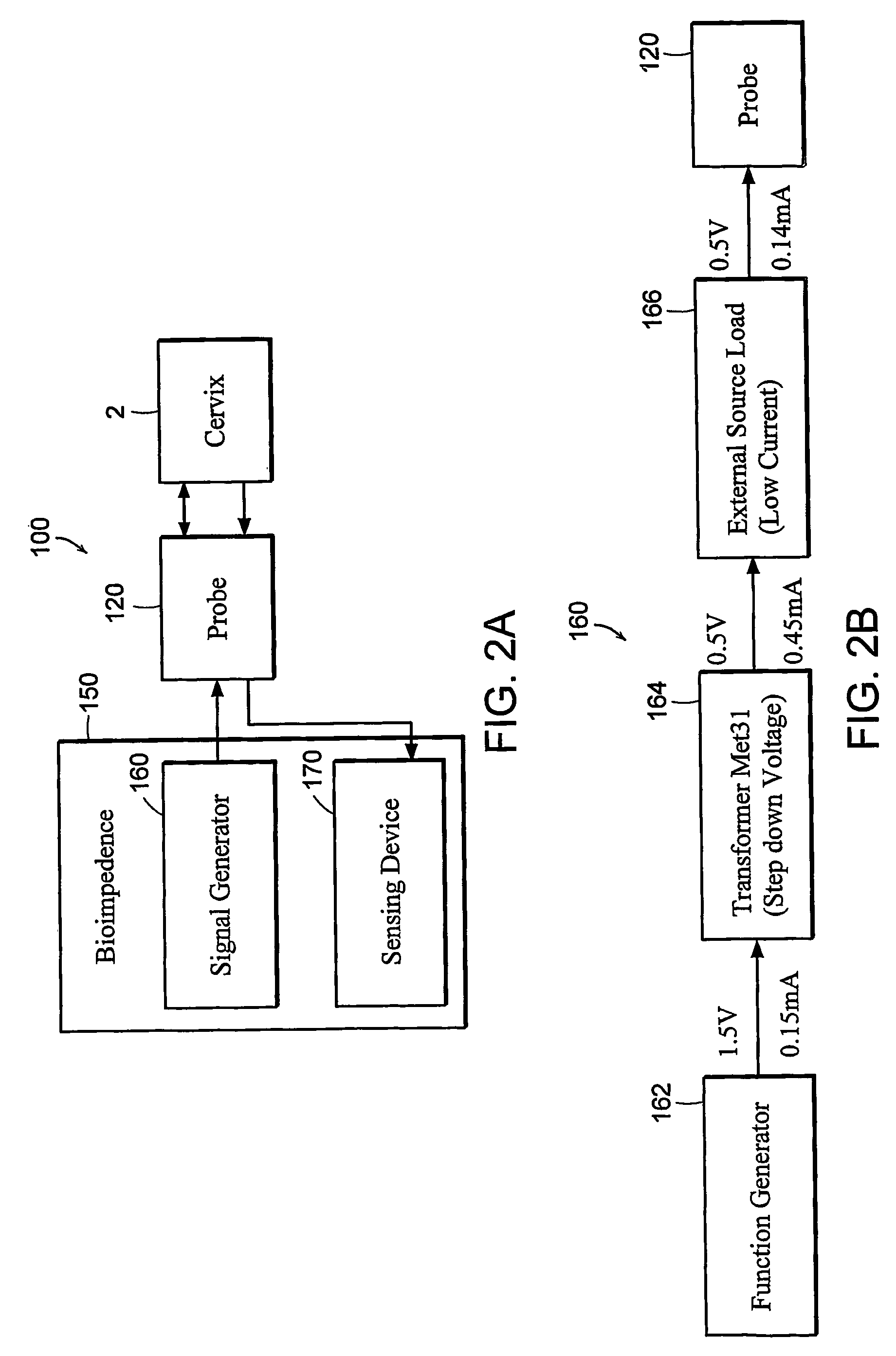
Featured are apparatuses for measuring bioimpendence of tissues of the cervix, more specifically the mammalian cervix. Also featured are methods for examining the tissues of the cervix for clinical or diagnostic purposes such as during routine gynecological examinations to determine early onset of labor in pregnant patients or to assess such tissues for the presence of abnormalities such as cancerous lesions in both pregnant and non-pregnant women. Also featured are methods for treating onset of early or pre-term labor that embody such devices, apparatuses and methods of the present invention. Also featured are systems embodying such devices, apparatuses and / or methods, where such systems preferably are configured to provide diagnostic and / or clinical information to further assist the diagnostician or clinician in diagnosing and / or examining pregnant or non-pregnant patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for the early detection of renal injury
InactiveUS20090142774A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseSide effect
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
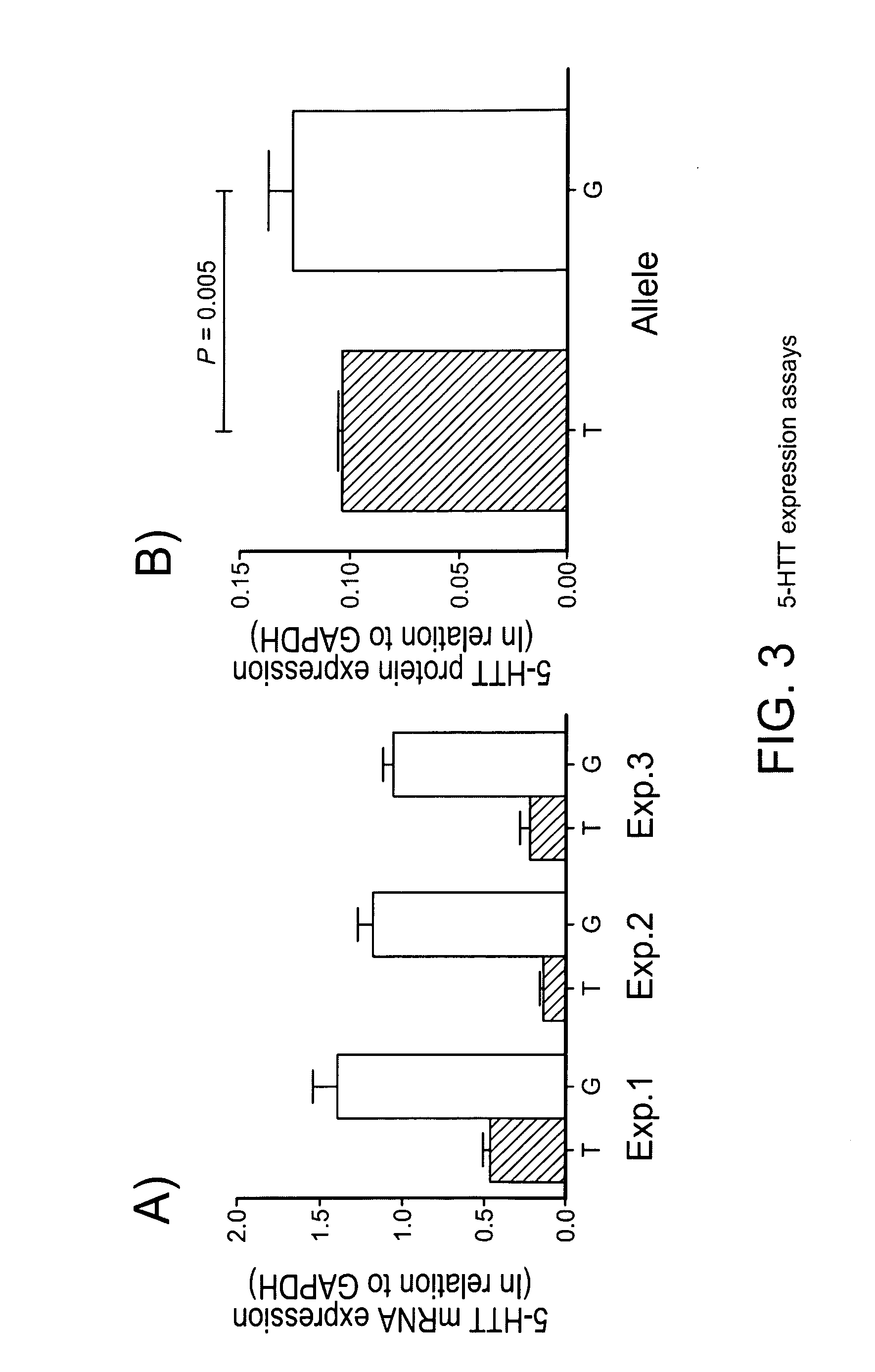
Serotonin transporter gene and treament of alcoholism
ActiveUS20110112159A1Physical improvementImproving psychological sequelaOrganic active ingredientsBiocideDiseaseAlcoholisms
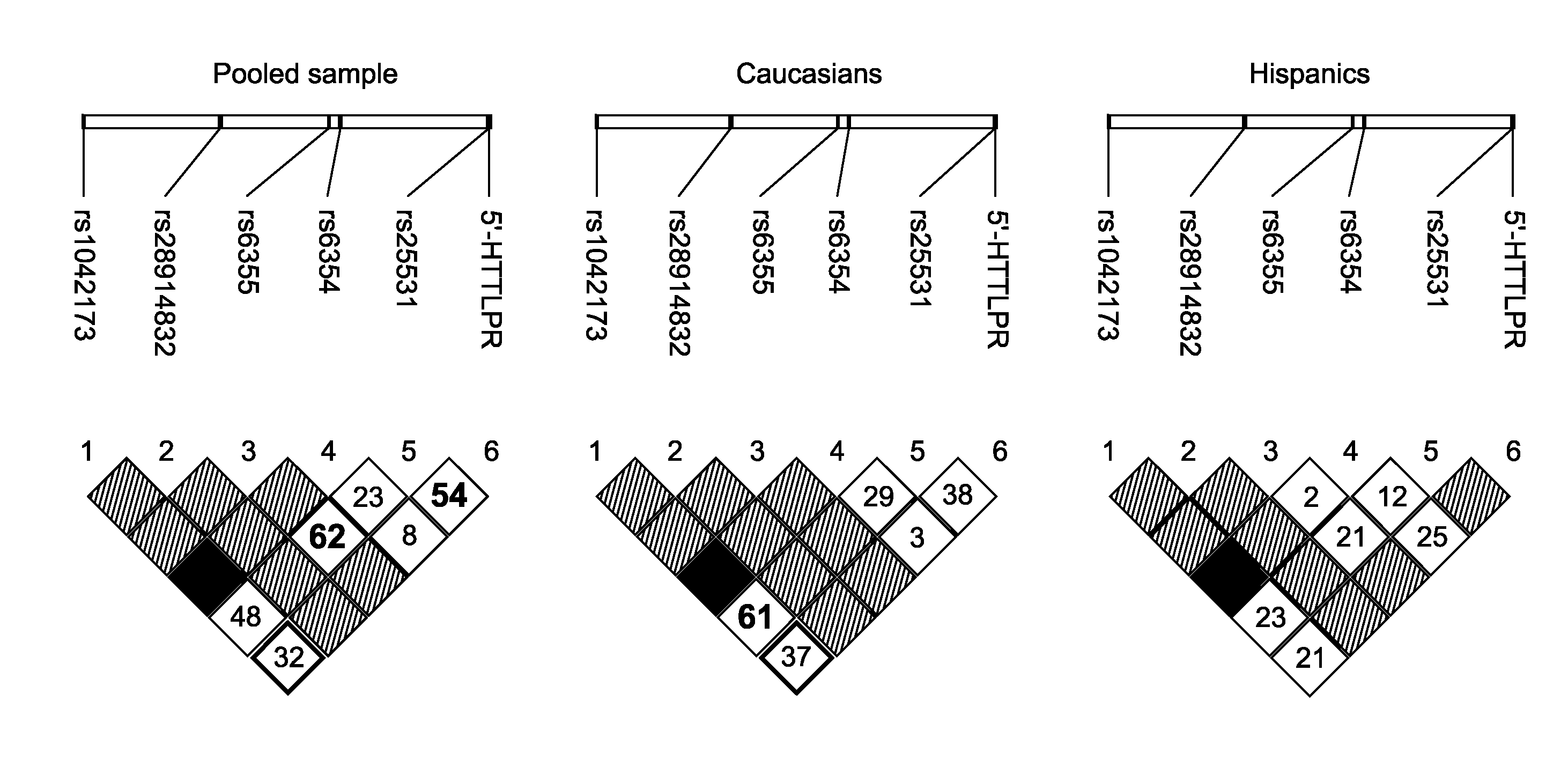
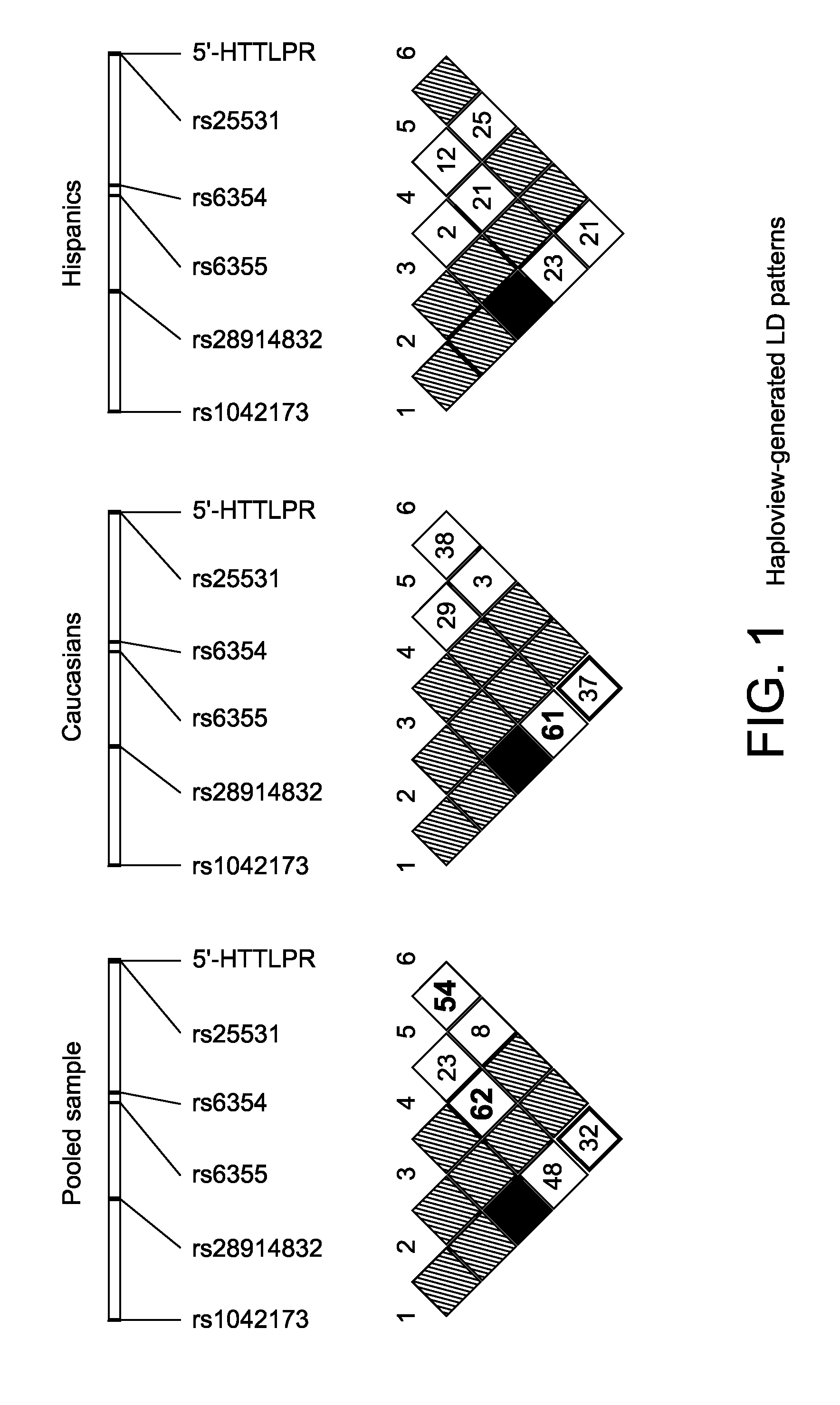
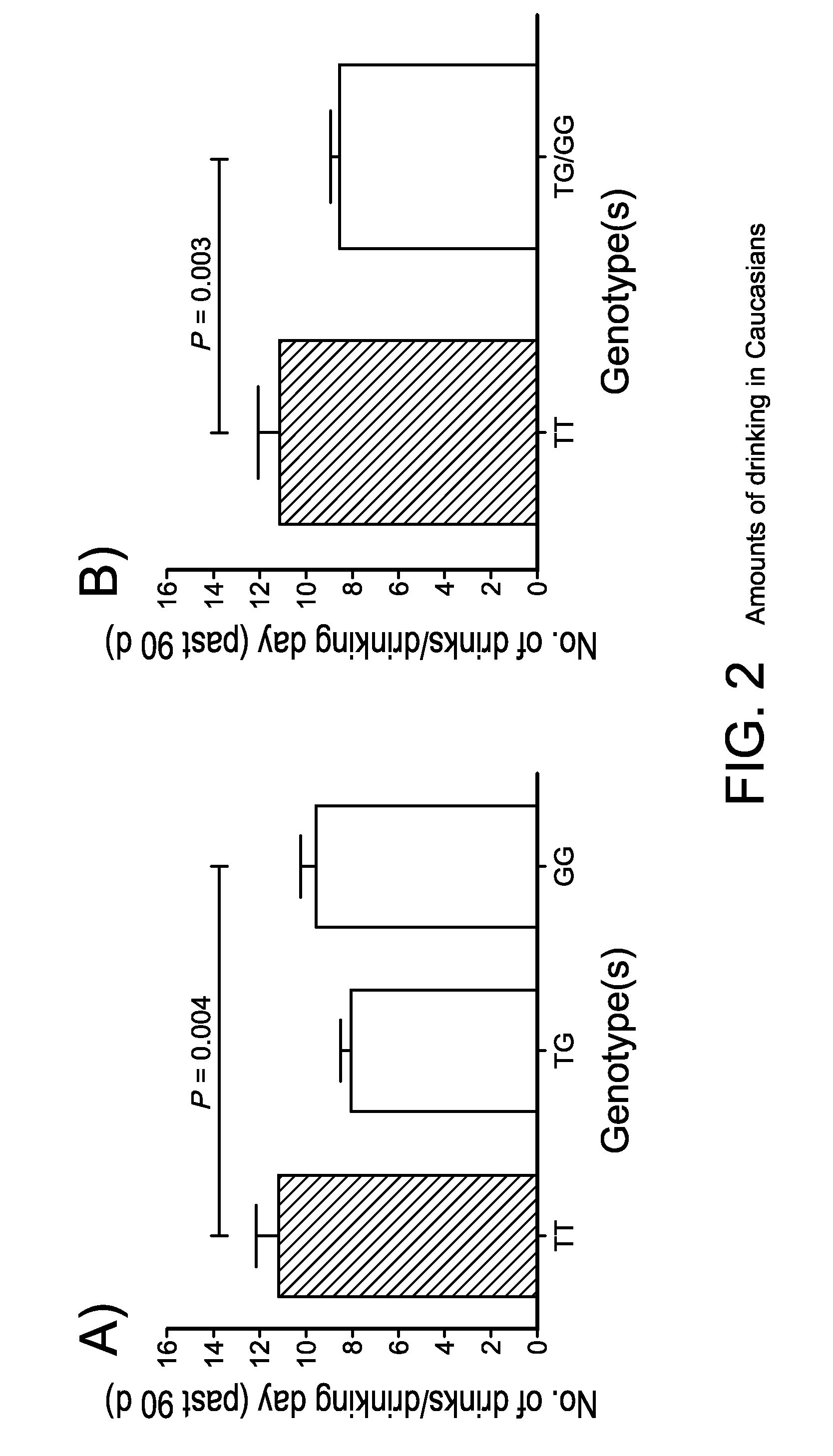
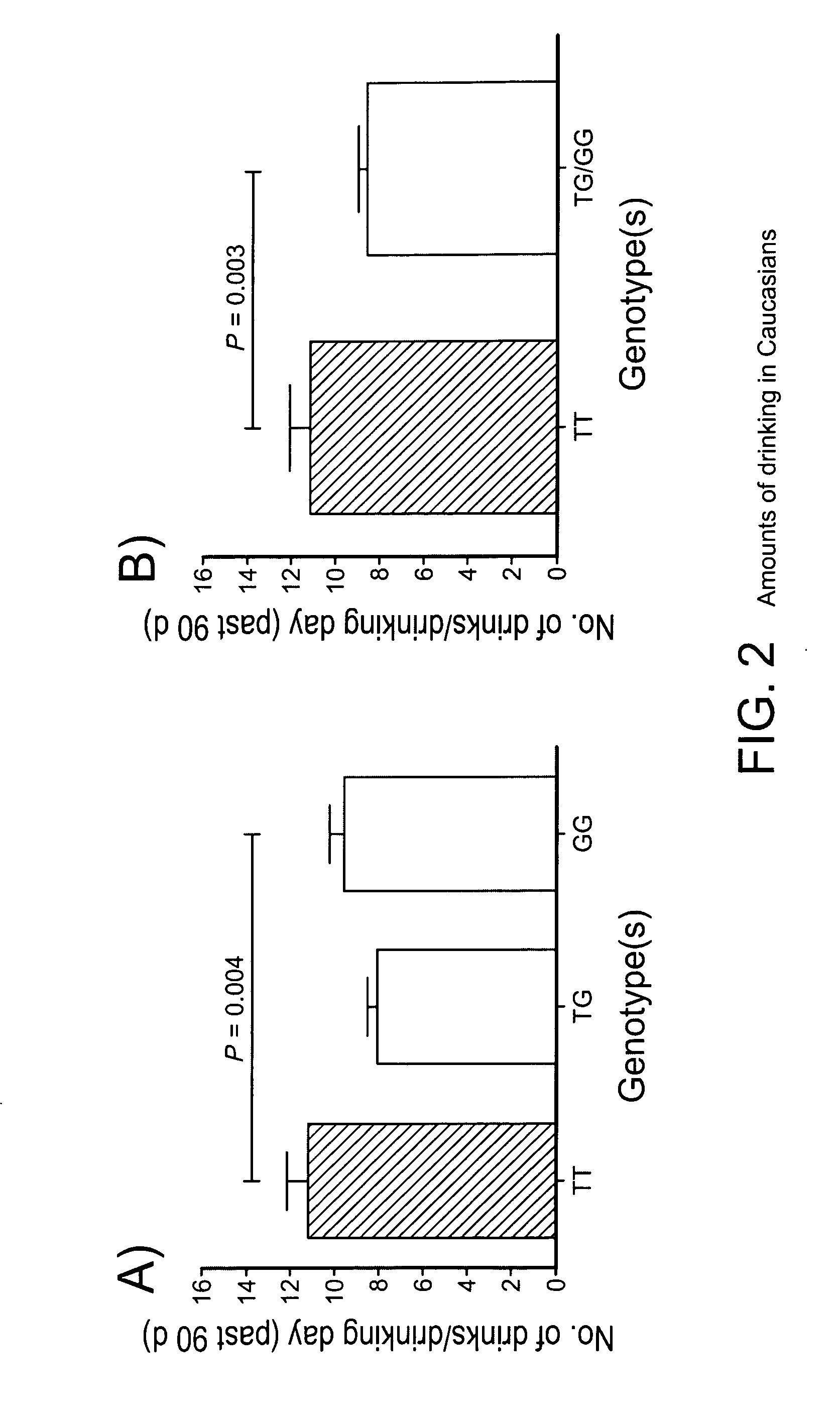
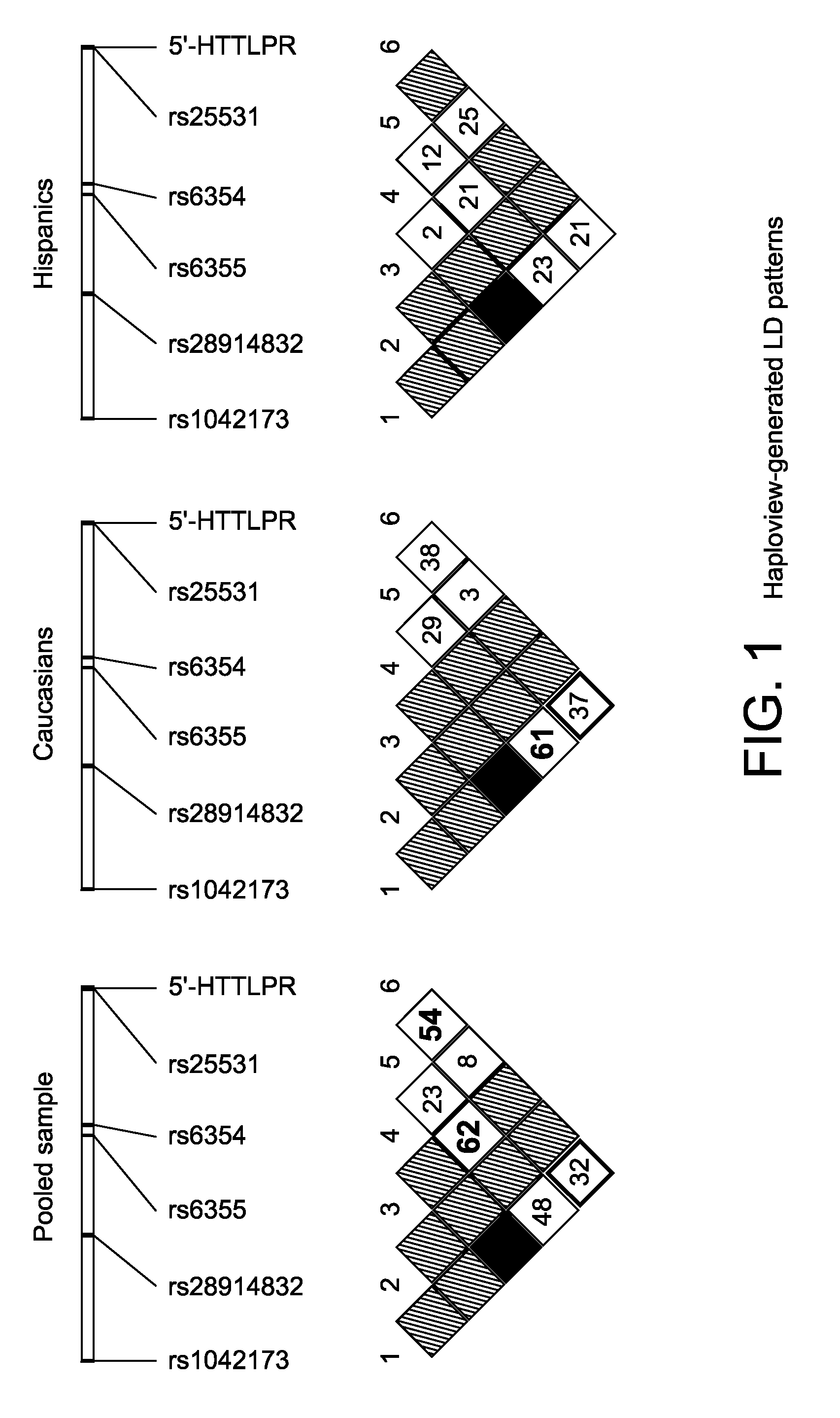
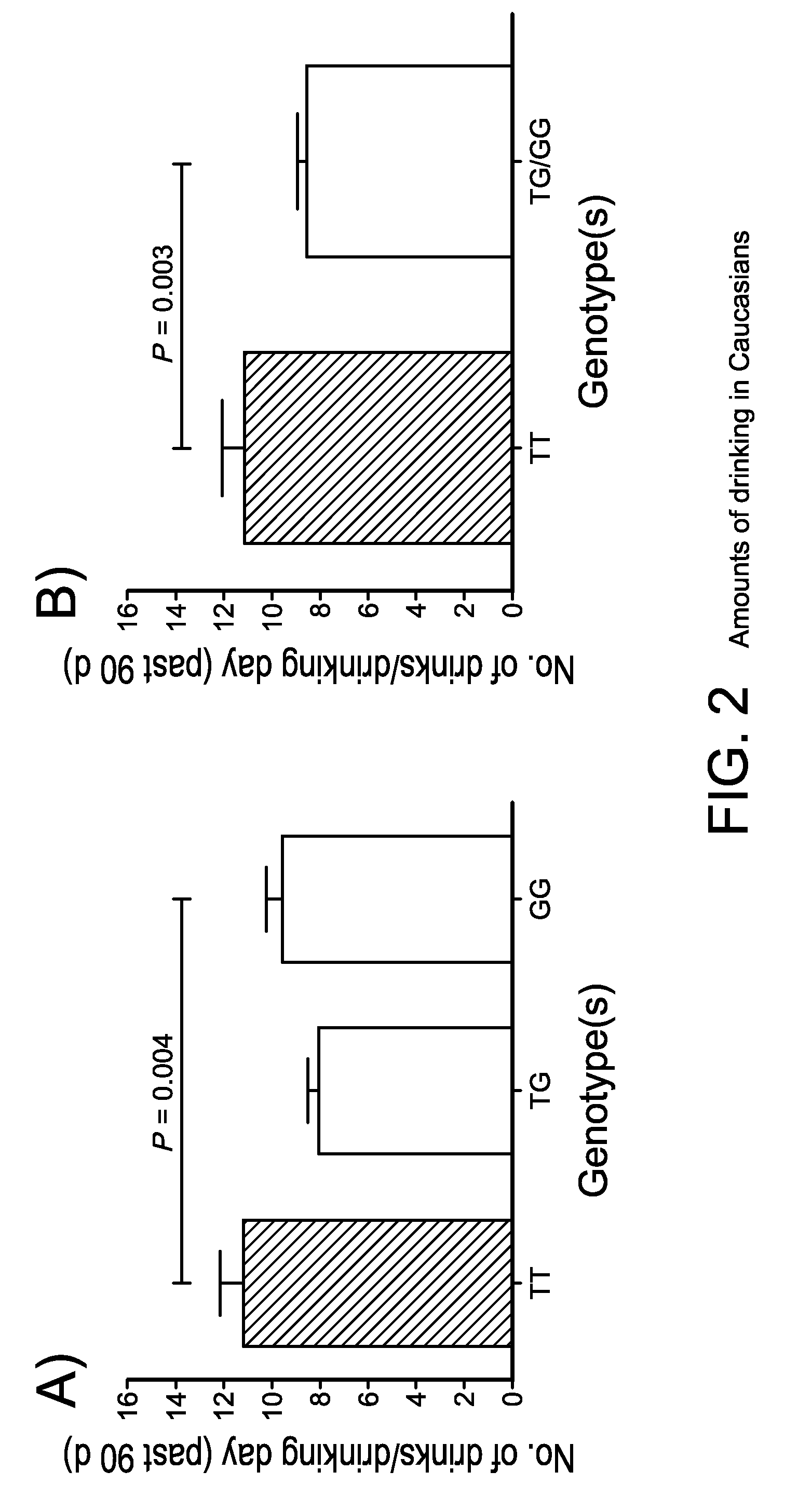
The gene responsible for encoding SERT has a functional polymorphism at the 5′-regulatory promoter region, which results in two forms, long (L) and short (S). The LL-genotype is hypothesized to play a key role in the early onset of alcohol use. The present invention discloses the differences in treatment and diagnosis based on the L or short genotypes as well as on a single nucleotide polymorphism of the SERT gene, the 3′ UTR SNP rs 1042173. The present invention demonstrates the efficacy of using the drug ondansetron and similar drugs for treatment based on variations in the polymorphisms of the SERT gene as well as methods for diagnosing susceptibility to abuse of alcohol and other addiction-related diseases and disorders.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Method for estimating vehicle import behavior on high-grade road bottleneck zone on-ramp
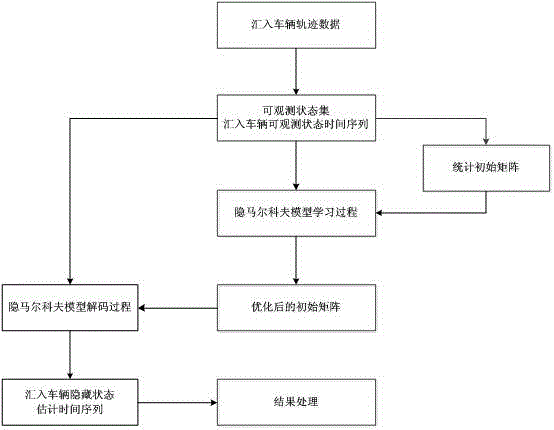
The invention discloses a method for estimating vehicle import behavior on a high-grade road (highway and city expressway) bottleneck zone on-ramp. The method uses a hidden Markov model to estimate decision conditions of a driver in an import process. The method comprises five steps of a data preparation stage, a data preprocessing stage, a model learning stage, a model decoding stage, and a result processing stage. The method can estimate states of a vehicle at different time before importing to a high-grade road main line. An estimated state time sequence can reflect the decision process of a driver. The method analyzes driver state transition spatial distribution conditions, so as to find out frequent places and zones where the drivers have lane changing intention. Therefore, the method provides basis for designing and developing an efficient on-ramp bottleneck zone lane-changing assistance system, and provides important reference for measure design for preventing early-onset failure of high-grade road on-ramp bottleneck.
Owner:TONGJI UNIV
Optical biomodule for detection of diseases at an early onset
An optical biomodule for detecting a disease specific biomarker(s), utilizing enhanced fluorescence emission (due to integration of a three-dimensional (3-D) protruded structure (s)) in a fluidic container / zero-mode waveguide, upon chemical binding of a disease specific biomarker(s) with its corresponding disease specific biomarker binder(s) (e.g., an aptamer(s)) is disclosed.
Owner:MAZED MOHAMMAD A
Markers for detection of complications resulting from in utero encounters
ActiveUS20120021442A1Unreliable resultMore targeted therapyDisease diagnosisNeonatal sepsisTraumatic intraventricular hemorrhage
Described herein are biomarkers, such as protein biomarkers, which are diagnostic of and predictive for complications that result from an in utero encounter, such as an infection by the fetus, that can lead to premature birth (PTB). The biomarkers can be used to identify fetuses and newborns at risk for complications of PTB, such as (Early Onset Neonatal Sepsis) EONS, intra-ventricular hemorrhage (IVH) and other poor outcomes.
Owner:YALE UNIV
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20090181407A1Organic active ingredientsMicrobiological testing/measurementSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20090123941A1Bioreactor/fermenter combinationsOrganic active ingredientsSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Devices, systems and methods for bioimpedance measurement of cervical tissue and methods for diagnosis and treatment of human cervix
Featured are apparatuses for measuring bioimpendence of tissues of the cervix, more specifically the mammalian cervix. Also featured are methods for examining the tissues of the cervix for clinical or diagnostic purposes such as during routine gynecological examinations to determine early onset of labor in pregnant patients or to assess such tissues for the presence of abnormalities such as cancerous lesions in both pregnant and non-pregnant women. Also featured are methods for treating onset of early or pre-term labor that embody such devices, apparatuses and methods of the present invention. Also featured are systems embodying such devices, apparatuses and / or methods, where such systems preferably are configured to provide diagnostic and / or clinical information to further assist the diagnostician or clinician in diagnosing and / or examining pregnant or non-pregnant patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Serotonin transporter gene and treatment of alcoholism
InactiveUS20120115149A1Physical improvementImproving psychological sequelaOrganic active ingredientsMicrobiological testing/measurementAlcoholismsNucleotide
The gene (SLC6A4) responsible for encoding the serotonin transporter (SERT) has a functional polymorphism at the 5′-regulatory promoter region, which results in two forms, long (L) and short (S). The LL-genotype is hypothesized to play a key role in the early onset of alcohol use. The present invention discloses the differences in treatment and diagnosis based on the L or short genotypes as well as on a single nucleotide polymorphism of the SERT gene, the 3′ UTR SNP rs1042173. The present invention demonstrates the efficacy of using the drug ondansetron and similar drugs for treatment based on diagnosing variations in the polymorphisms of the SERT gene and expression and activity of the SERT gene, as well as methods for diagnosing susceptibility to abuse of alcohol and other addiction-related diseases and disorders, for monitoring treatment and / or abuse (addictive behavior), and for determining which treatment should be used.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
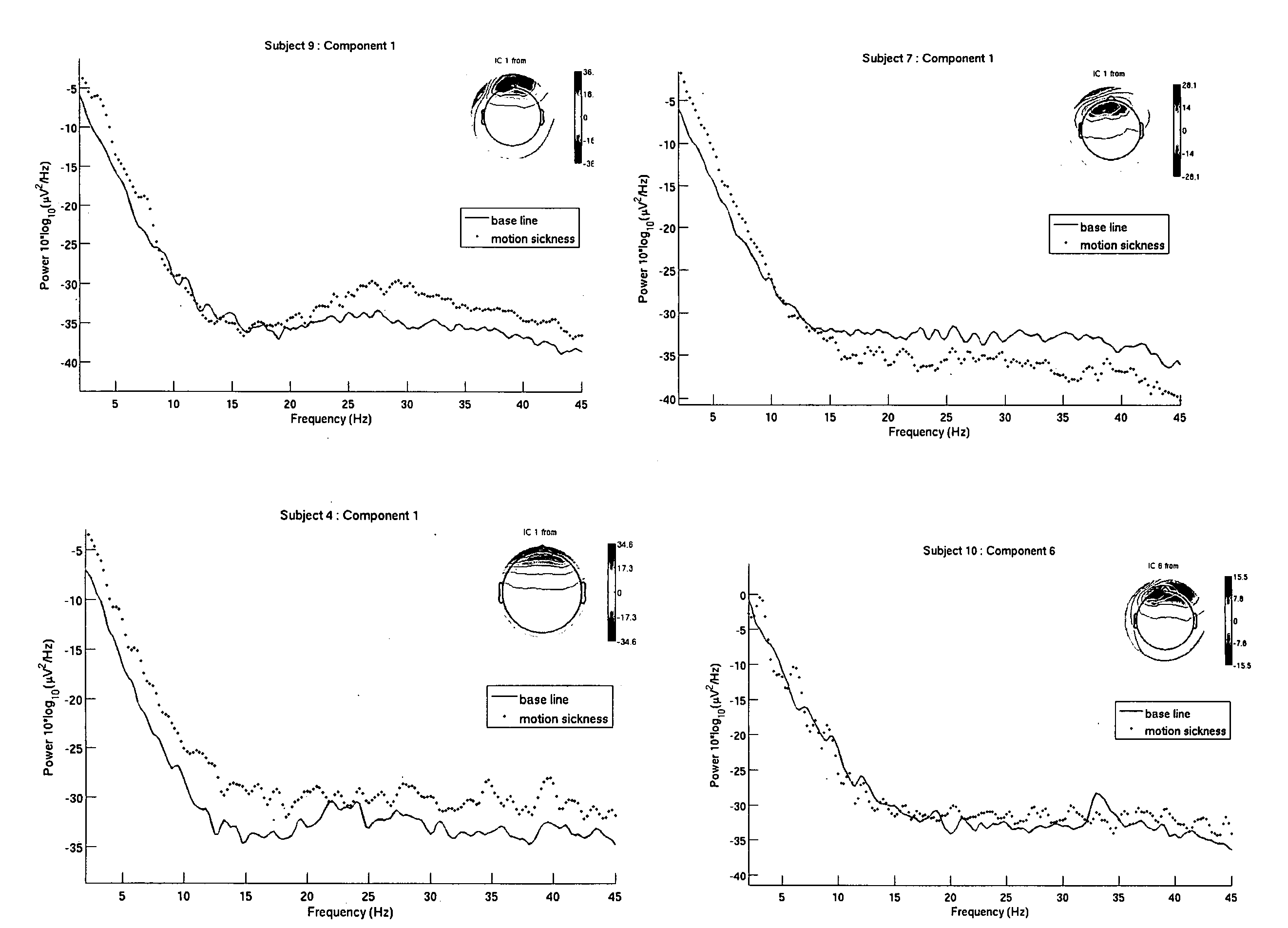
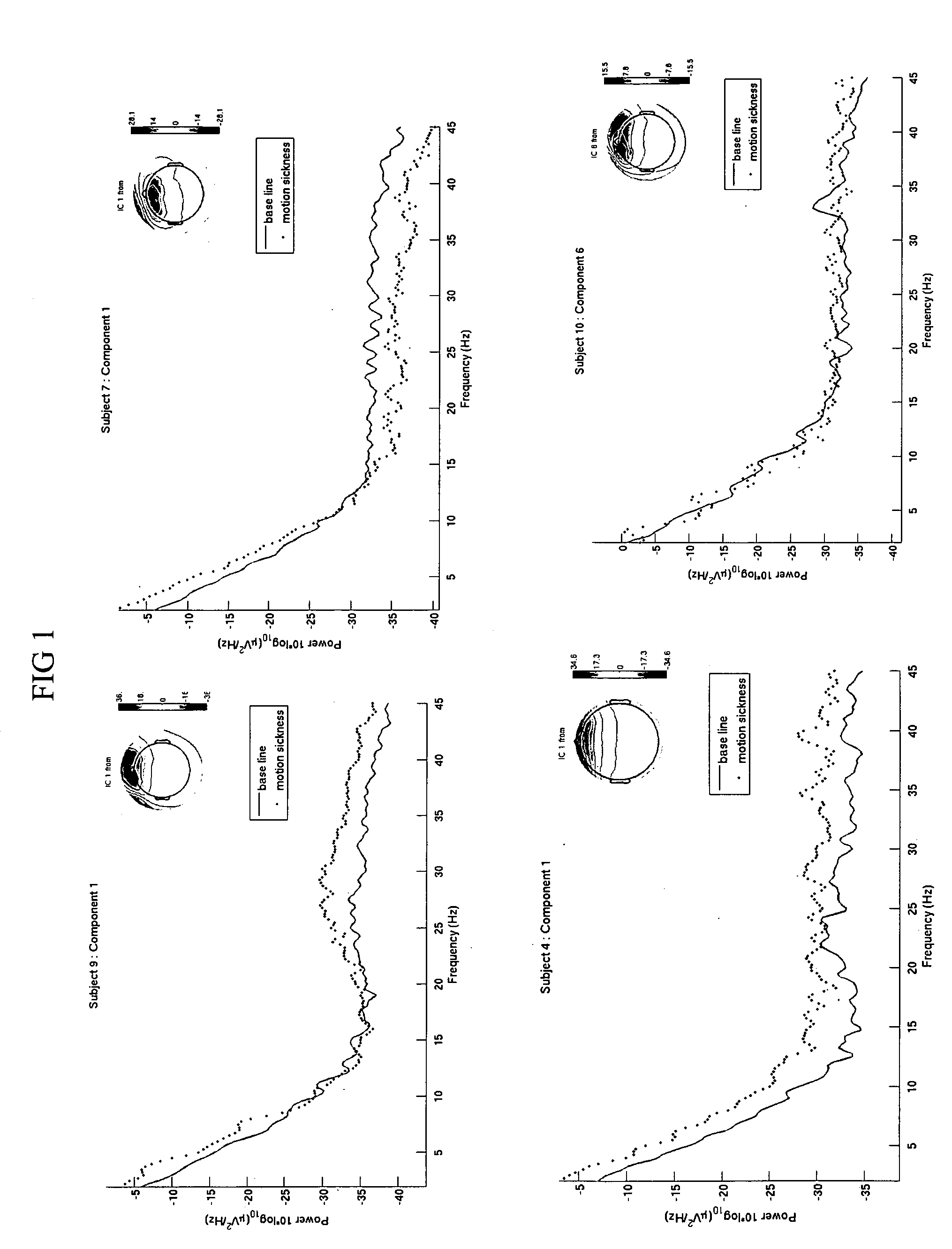
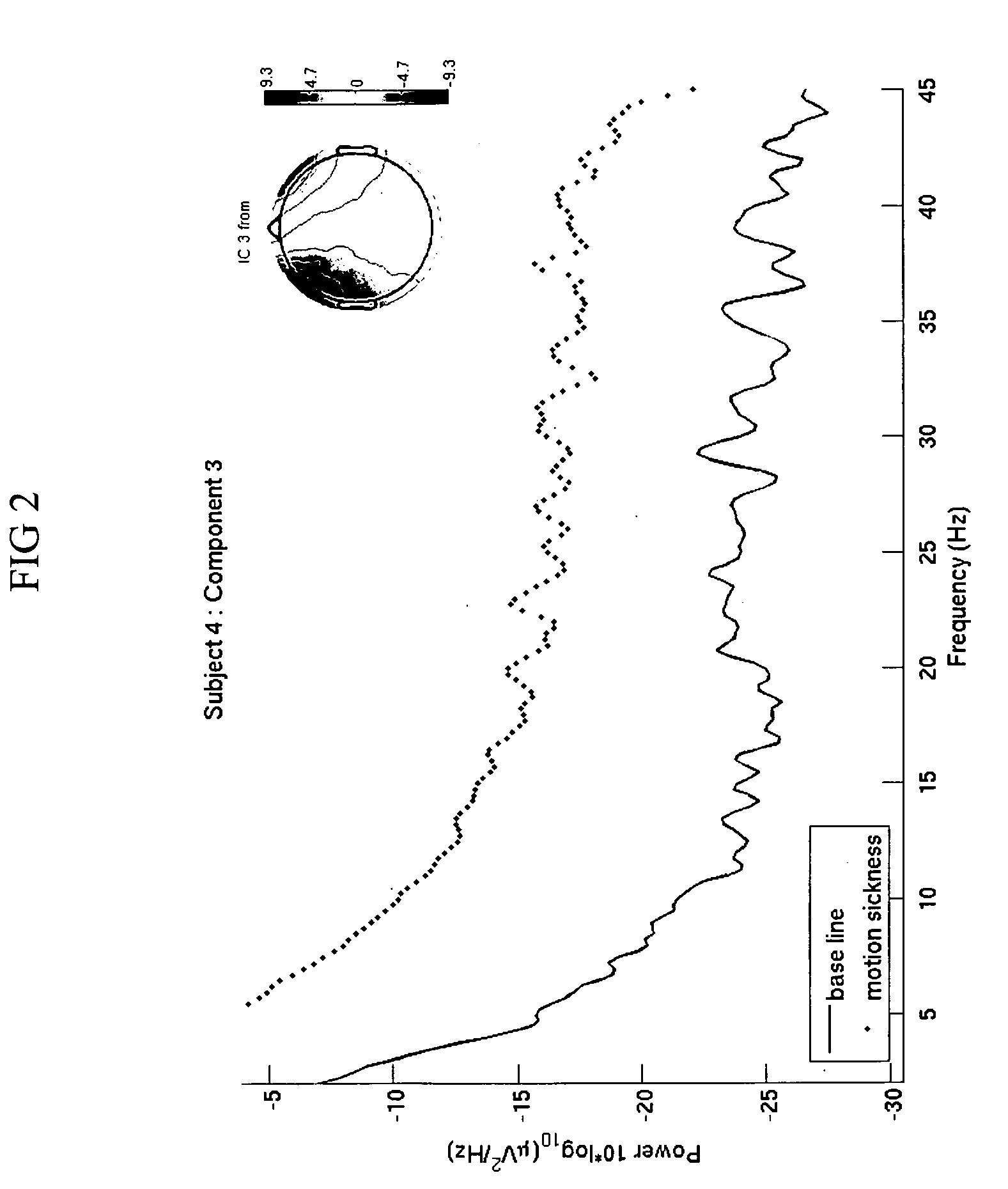
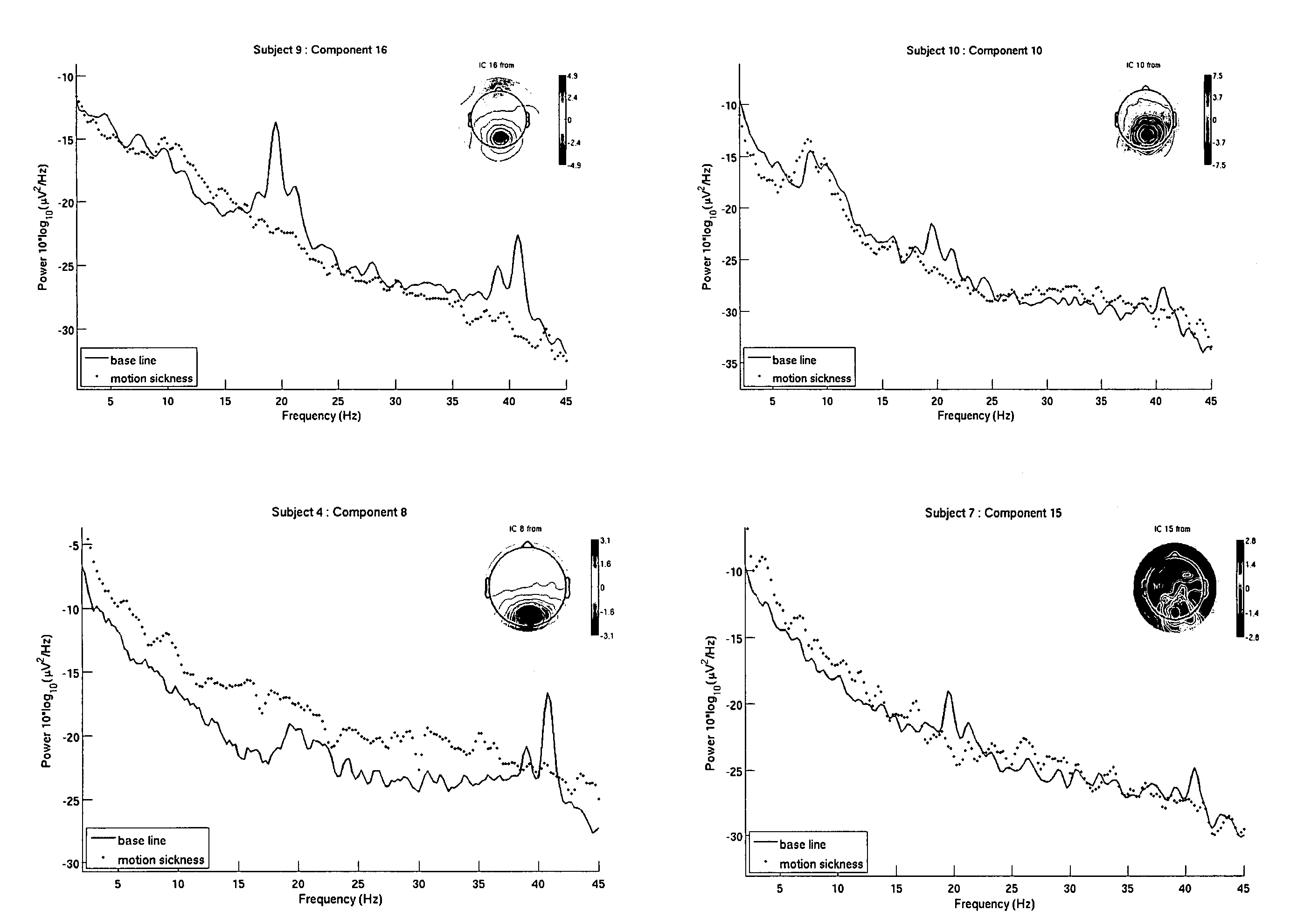
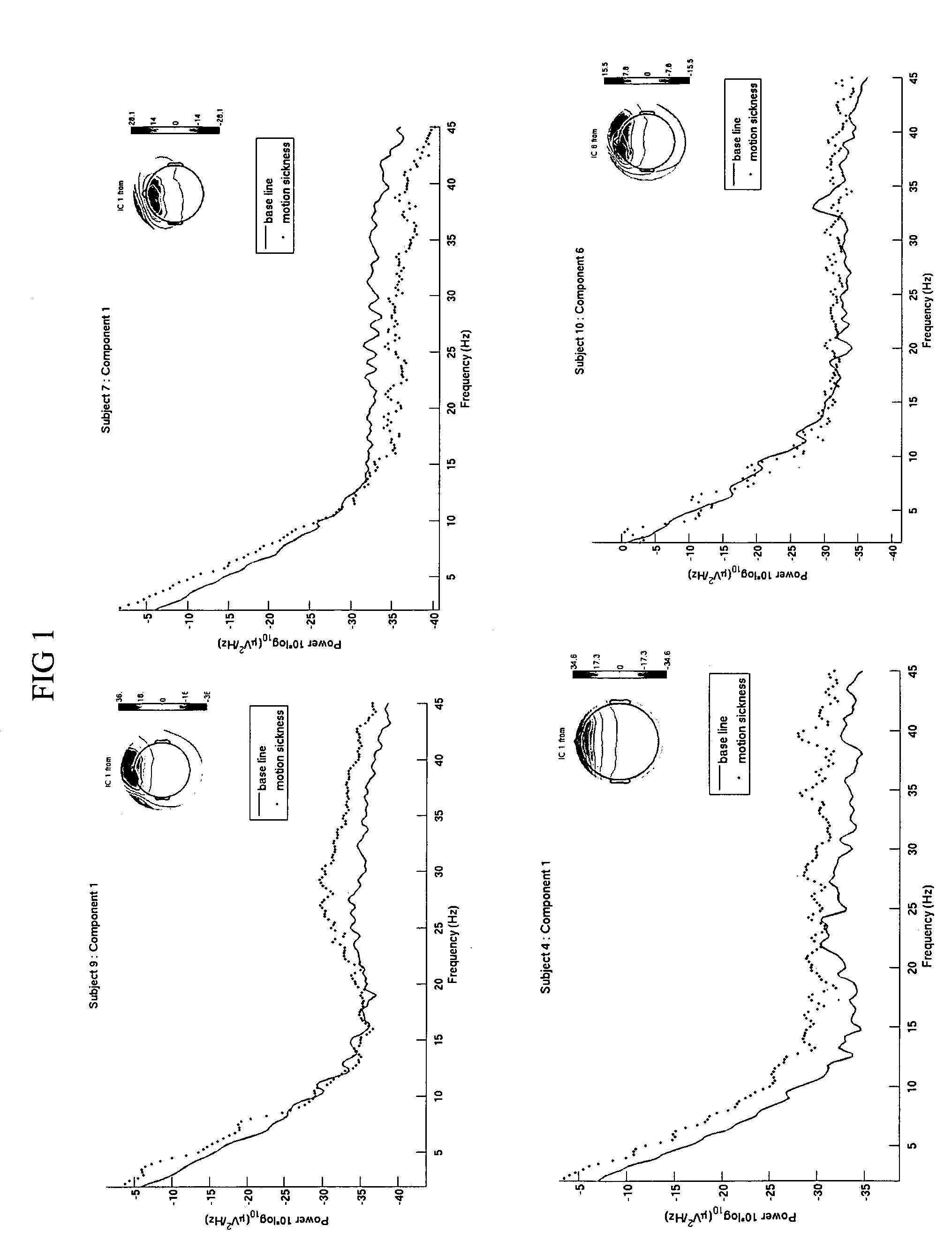
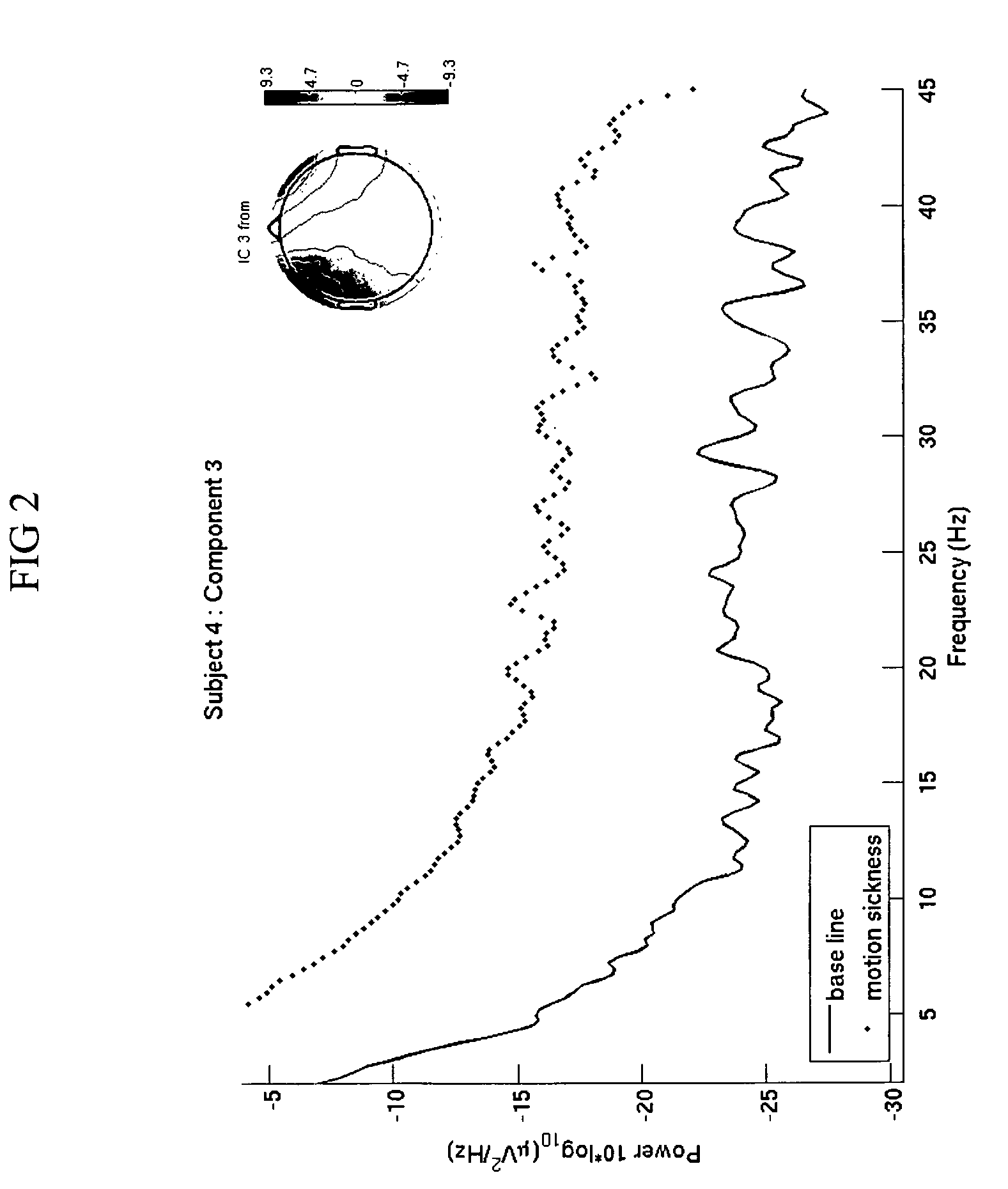
Use of EEG to measure cerebral changes during computer-based motion sickness-inducing tasks
The invention relates to a method of determining early onset of motion sickness by brain imaging. The method discloses an objective means of determining the onset of motion sickness by evaluating a specific region of the brain. The method can also be utilized in evaluating the predisposition toward motion sickness in workers in occupations prone to motion sickness.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
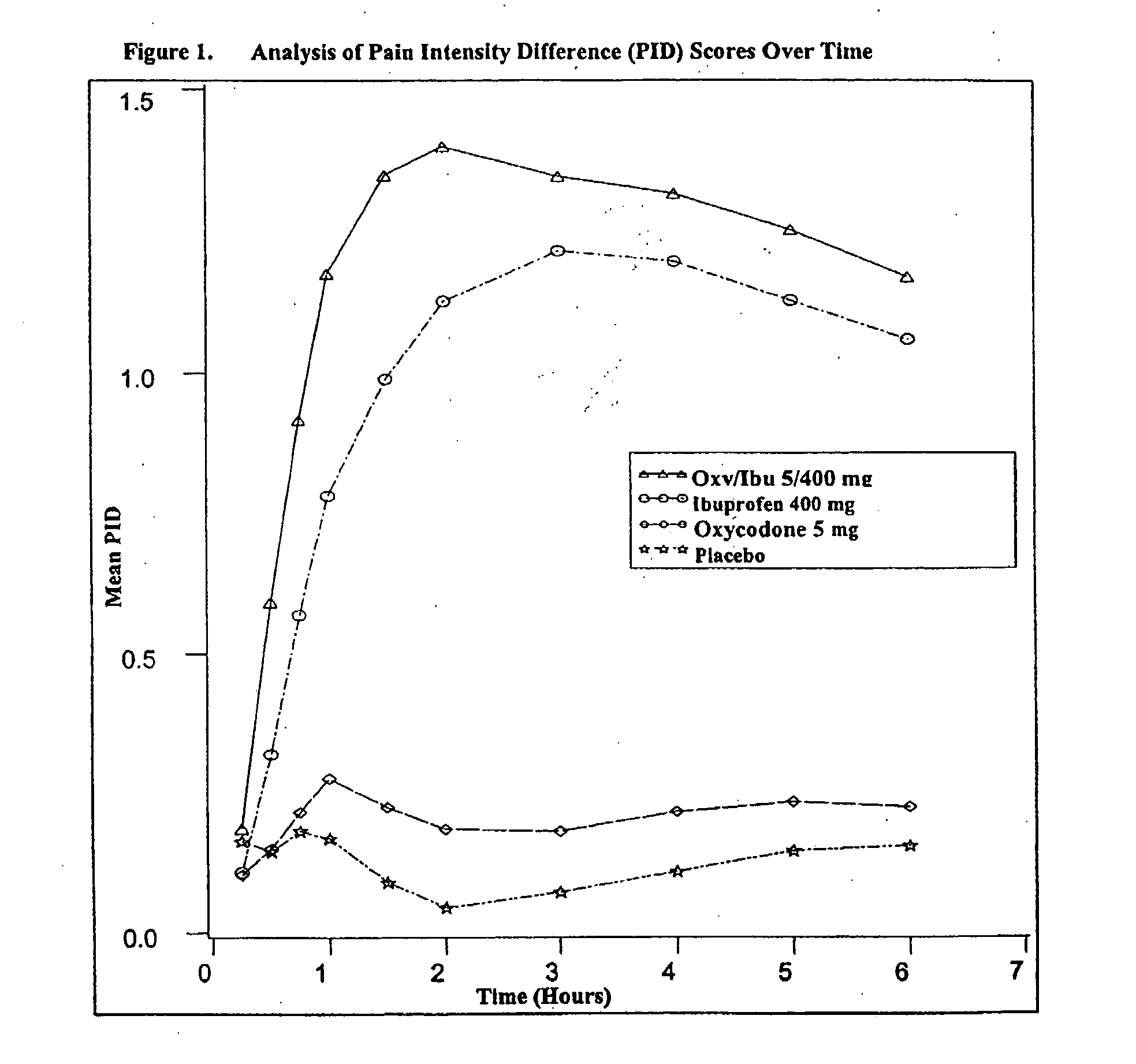
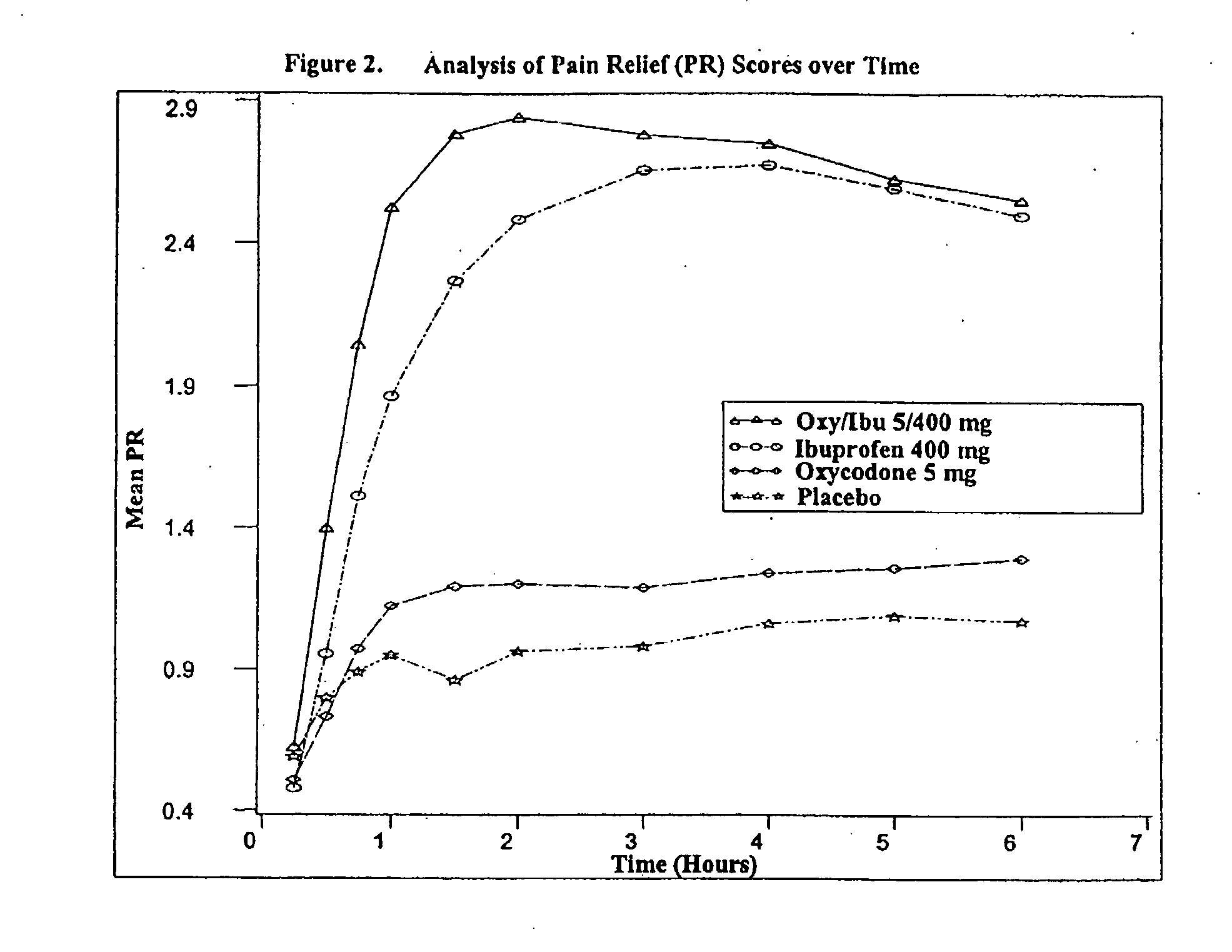
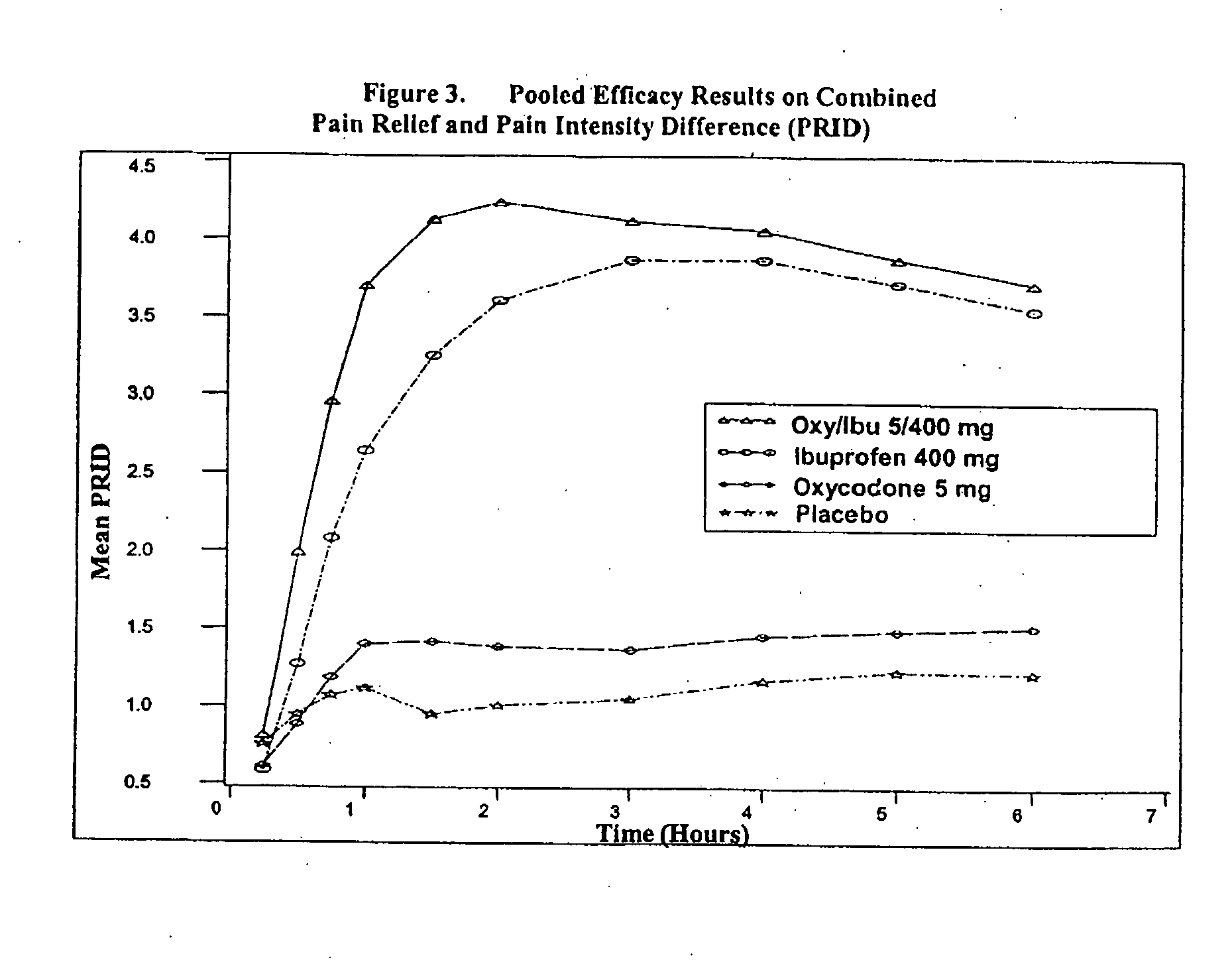
Method of treating acute pain with a unitary dosage form comprising ibuprofen and oxycodone
InactiveUS20050059690A1Reduce the amount of solutionBiocideNervous disorderDental surgeryEarly onset
The present invention is a method of achieving fast onset of pain relief for acute pain in a patient in need thereof comprising orally administering a unitary formulation (or oral dosage form) containing an effective analgesic amount of (a) oxycodone or a pharmaceutically acceptable salt thereof and (b) ibuprofen or a pharmaceutically acceptable salt thereof. Preferably, the unitary formulation contains (a) oxycodone or a pharmaceutically acceptable salt thereof and (b) ibuprofen or a pharmaceutically acceptable salt thereof at a weight ratio of from about 1:20 to about 1:100 and more preferably about 1:40 to about 1:80, based on the weights of molar equivalents of oxycodone hydrochloride and ibuprofen, respectively. Preferably, an amount of oxycodone and ibuprofen effective to provide partial or complete pain relief within 30 minutes is administered. More preferably, the amount is sufficient to provide partial or complete pain relief within 25 minutes. It has been discovered that administration of an oral dosage form containing both oxycodone and ibuprofen provides earlier onset of pain relief than administration of either active ingredient alone. Moreover, the earlier onset of pain relief may be attributable at least in part to administration of a single dosage form containing both active ingredients as opposed to administering oxycodone and ibuprofen in separate oral dosage forms (i.e., administration of a first dosage form containing oxycodone and a second dosage form containing ibuprofen). The method of the present invention is particularly useful for treating acute postoperative pain, including, but not limited to, moderate and / or severe acute postoperative pain (such as that resulting from dental surgery).
Owner:FOREST LABORATORIES
Use of EEG to measure cerebral changes during computer-based motion sickness-inducing tasks
The invention relates to a method of determining early onset of motion sickness by brain imaging. The method discloses an objective means of determining the onset of motion sickness by evaluating a specific region of the brain. The method can also be utilized in evaluating the predisposition toward motion sickness in workers in occupations prone to motion sickness.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Method and apparatus for detecting endometriosis
A vaginal probe includes a metal detection sensor for detecting the presence of ferrous-laden endometriotic tissues. The probe is electrically connected to external electronic circuitry for interpreting the readings taken by the sensor and for providing a qualitative or quantitative measurement of the amount of endometriotic tissues present. The probe is inserted in either the vagina or peritoneal cavity of a patient to detect the location and the relative amounts of iron or ferrous-laden tissues in the pelvic area of the patient. Using the probe and monitoring the readings over time as part of pelvic exams assists in determining the early onset or progression of endometriosis and / or the response to one or more therapies being provided to the patient to treat the disease.
Owner:JARRELL JOHN F
Biomarker for the estimation of acute renal disorder and prognosis of the disorder, and use of the biomarker
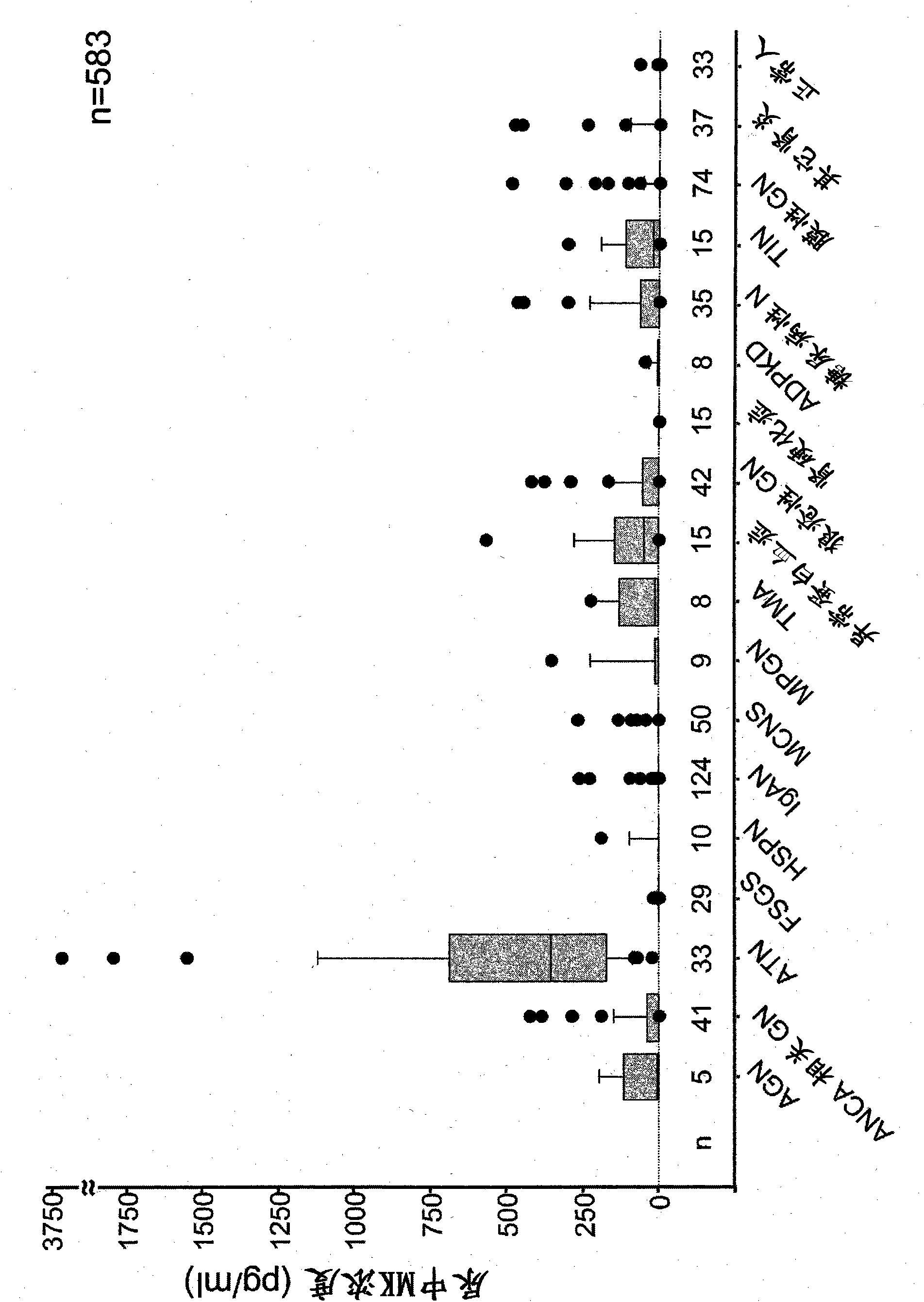
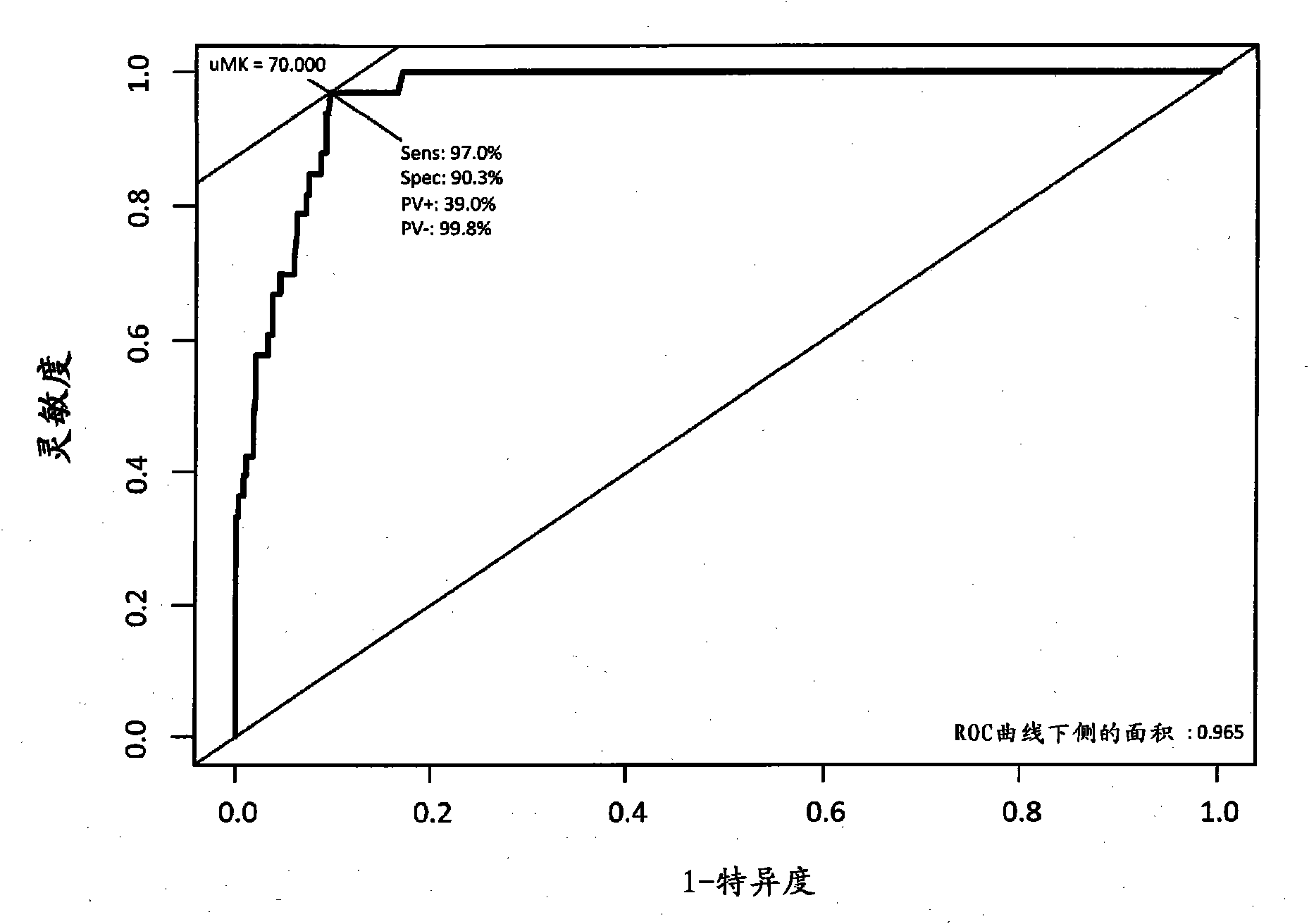
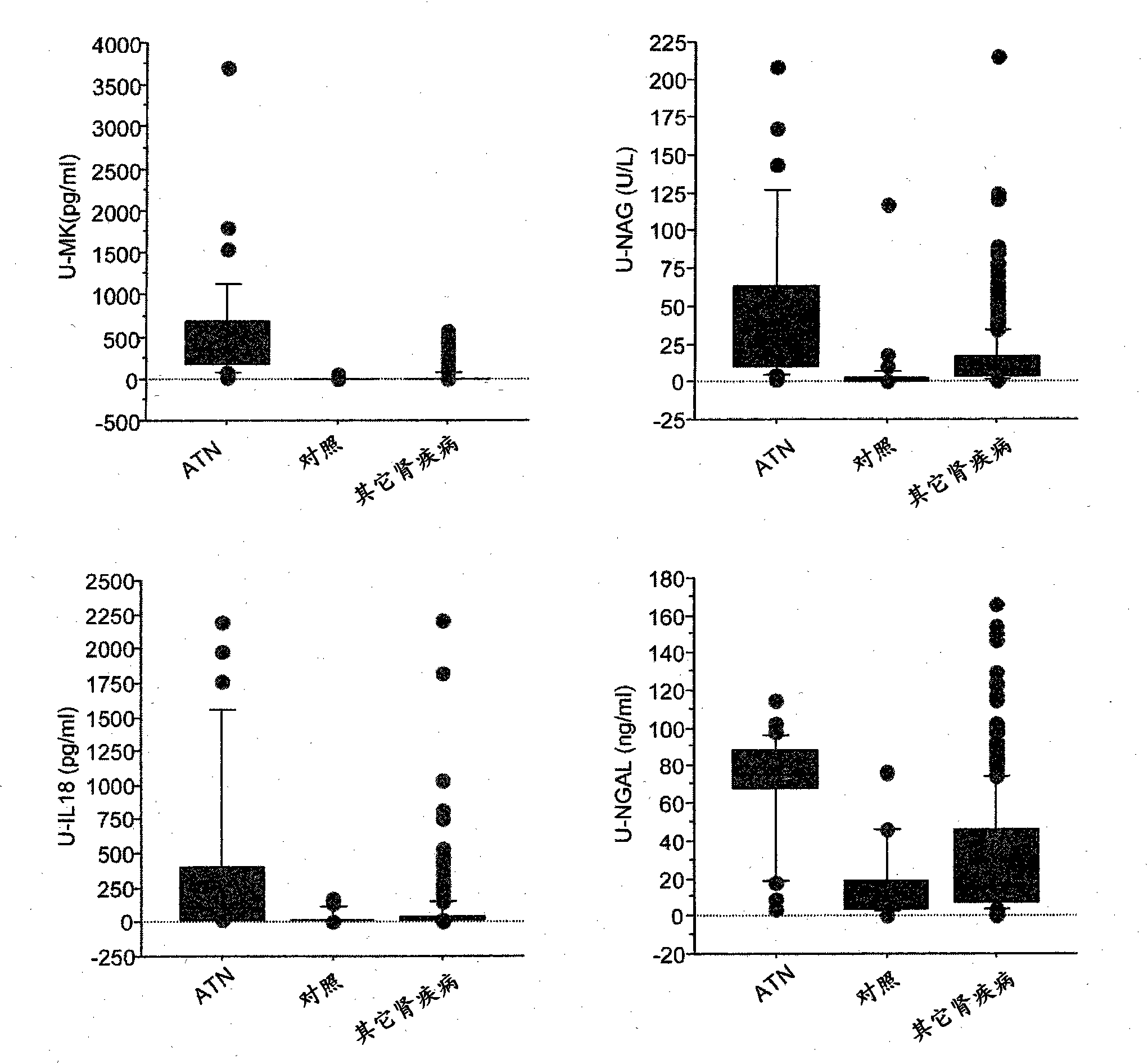
Disclosed is a novel biomarker which is useful for the prediction of early onset of acute renal disorder, the estimation of prognosis associated with a renal function, and the diagnosis of acute renal disorder. Also disclosed is use of the novel biomarker. A midkine is used as the biomarker. The determination of the possibility of the onset of acute renal disorder, the estimation of prognosis associated with a renal function or the diagnosis of acute renal disorder can be achieved based on the results of the detection of a midkine in urine.
Owner:NAGOYA UNIVERSITY
Traditional Chinese medicine composition for treating psoriasis
ActiveCN105213971ALess medicinalReduce complicationsDermatological disorderPlant ingredientsPsoriasis patientLife quality
The invention discloses a traditional Chinese medicine composition for treating psoriasis. The traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 5-15 parts of radix paeoniae rubra, 3-9 parts of rhizoma curcumae, 10-20 parts of glabrous sarcandra herb, 10-20 parts of rhizoma smilacis glabrae and 5-15 parts of dark plum. The traditional Chinese medicine composition has the following benefits: the treating effect can be achieved by virtue of a relatively small dose, the toxicity is remarkably reduced, the traditional Chinese medicine composition takes effect relatively fast, and recurrence can be delayed; the traditional Chinese medicine has a more outstanding effect on common psoriasis (PASI 50 can reach 60%); especially for an early-onset psoriasis patient, such complications as cardiovascular events, psoriasis arfhropathica and diabetes can be reduced (for a patient who takes the traditional Chinese medicine composition, the complication rate can be lowered by 20%); therefore, the traditional Chinese medicine composition can remarkably improve the life quality of the psoriasis patients, and has a better effect.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Diagnostic compositions and treatment methods for conditions involving trophoblast cell death, differentiation, invasion and/or cell fusion and turnover
InactiveUS20090246773A1Reduce deathReduce differentiationCompound screeningApoptosis detectionFactor iiProtein C
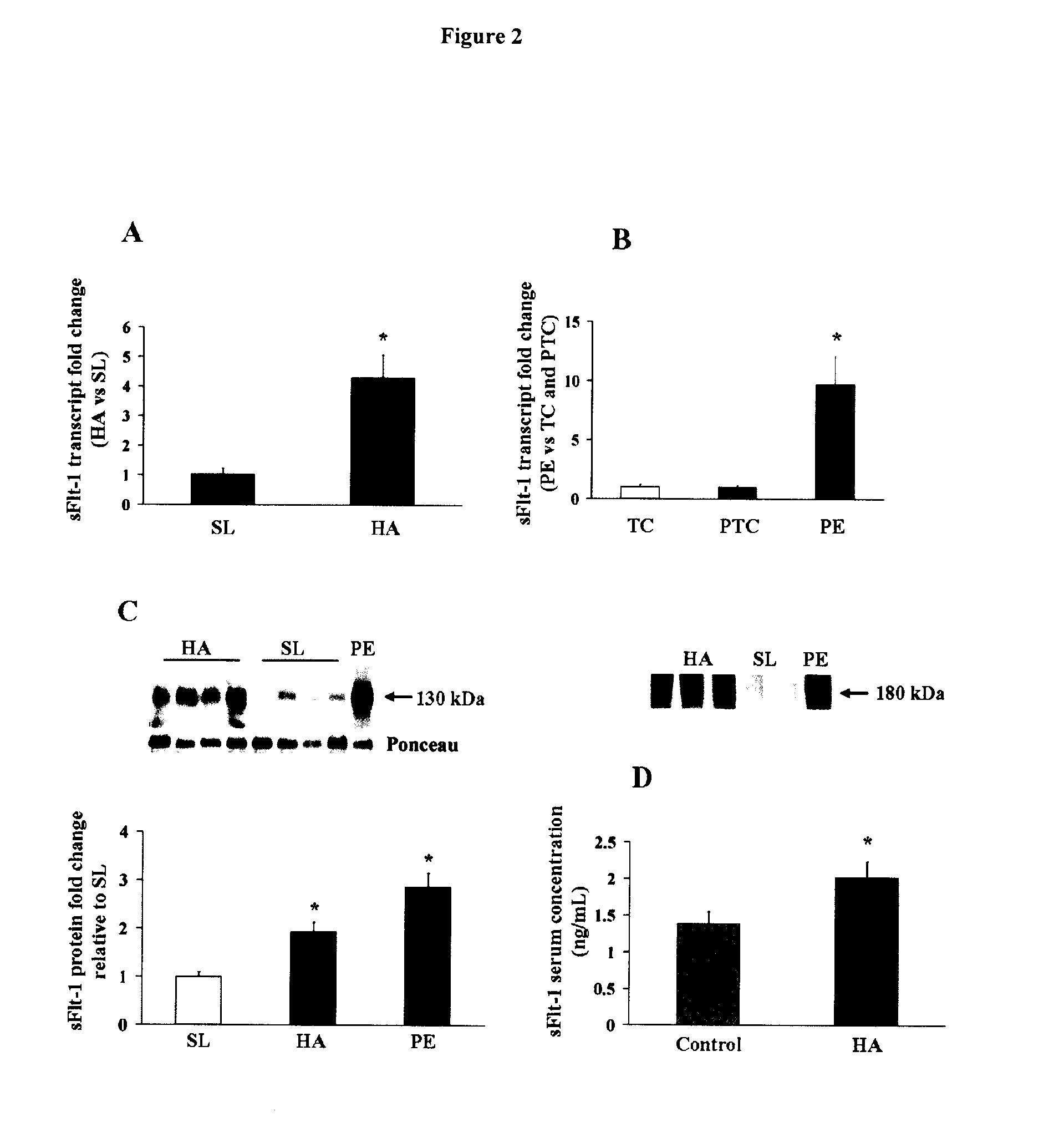
The invention provides a method for diagnosing in a subject a condition requiring modulation of or involving trophoblast cell death, differentiation, invasion, and / or cell fusion and turnover, comprising detecting HIF Iα and the factors which modulate or are modulated by this protein, specifically TGFβ3, sFLT, VEGF, SMAD2, 3 and 7, MtdP, MtdL, MclI 1, MclIc, VHL, SiahI, Siah2, ENG, and PHD. The invention also provides a method for diagnosing or distinguishing in a subject a specific condition requiring modulation of or involving trophoblast cell death, differentiation, invasion, and / or cell fusion and turnover, in particular early onset severe preeclampsia (EPE), late onset preeclampsia (LPE) and mtre-uterme growth restriction (IUGR) comprising detecting HLF Iα and the factors which modulate or are modulated by this protein as defined above.
Owner:MOUNT SINAI HOSPITAL
Serotonin transporter gene and treatment of alcoholism
ActiveUS8697361B2Long durationHigher paroxetine binding (density of SERT)Organic active ingredientsBiocideAlcoholismsNucleotide
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
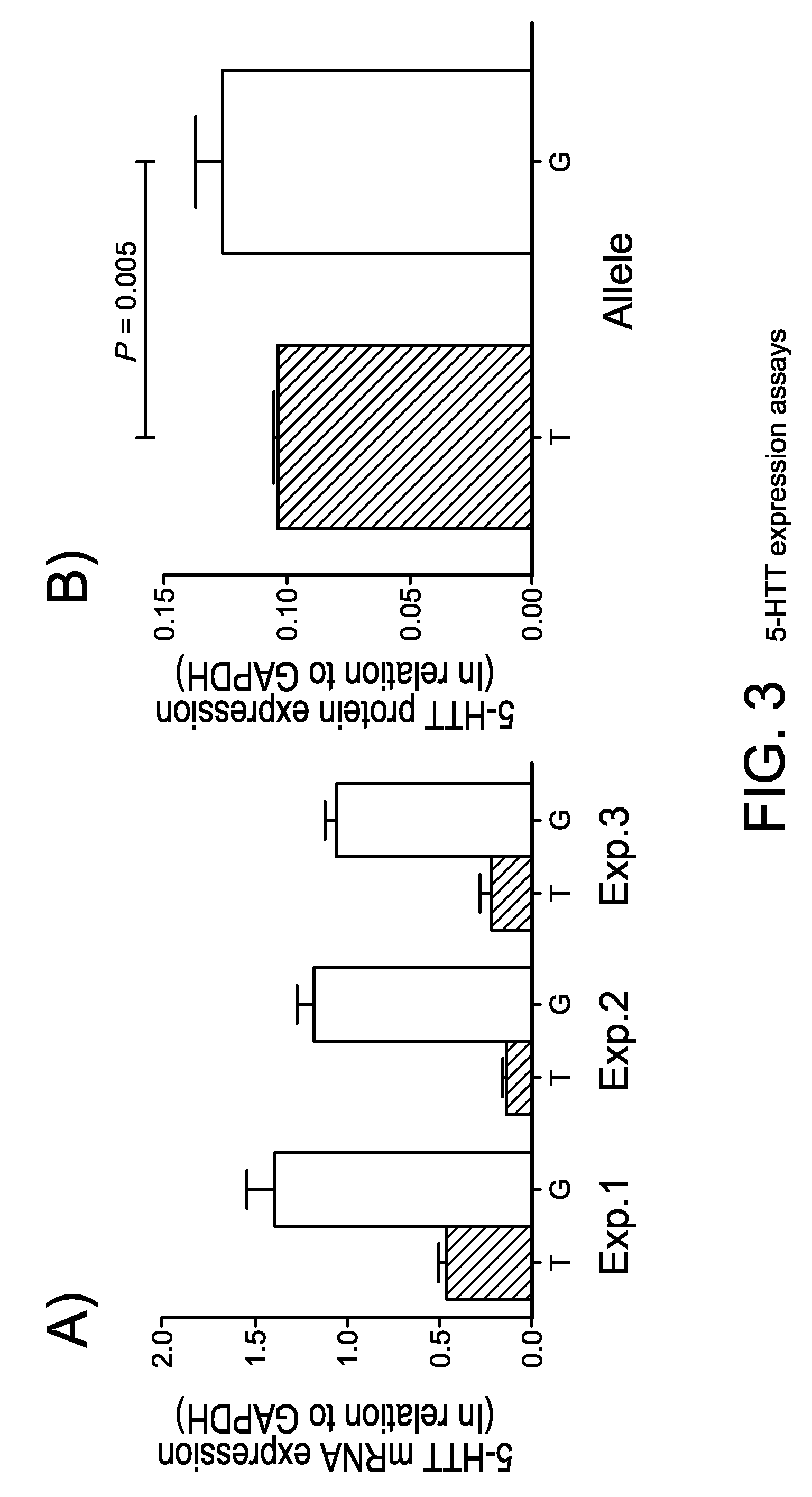
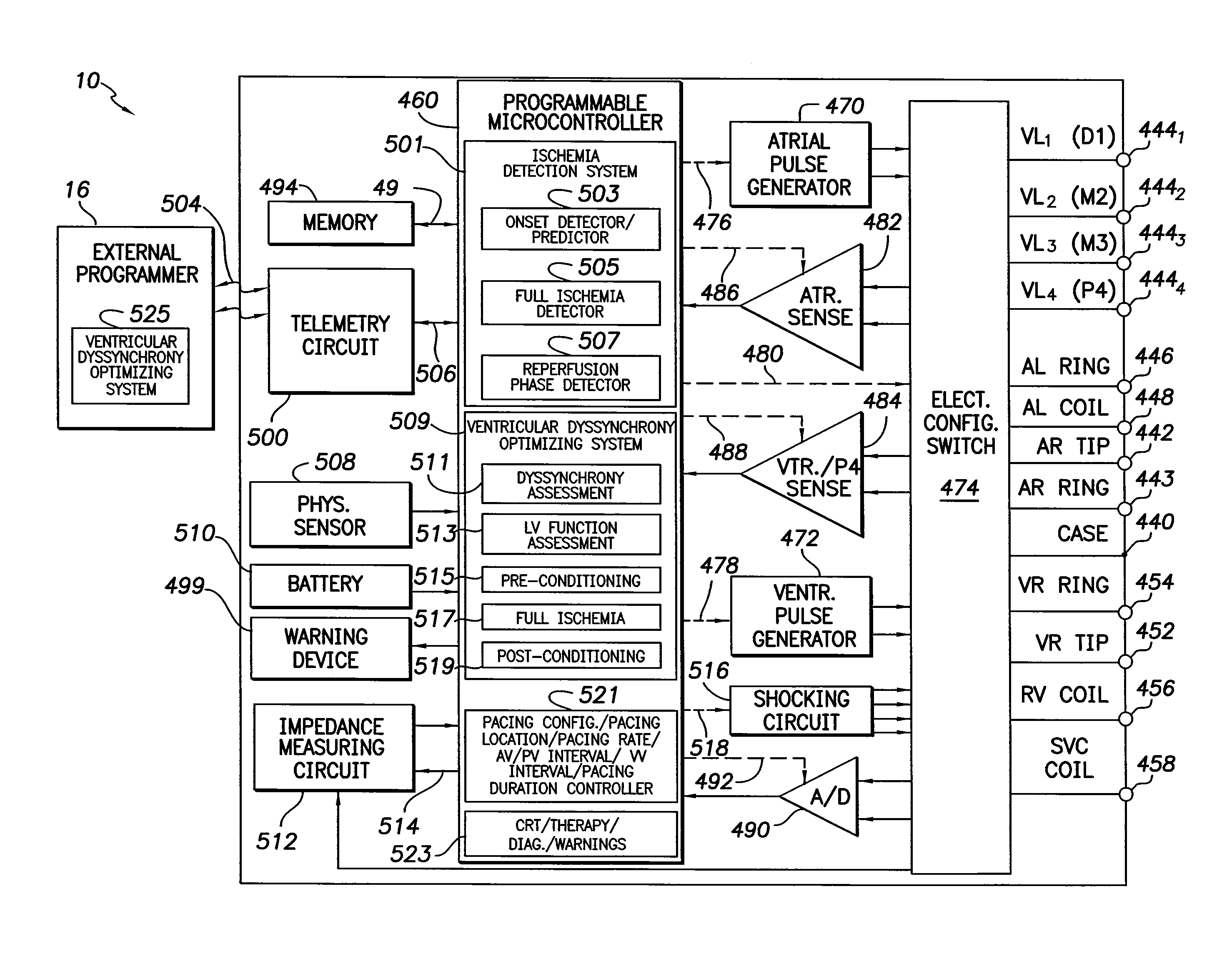
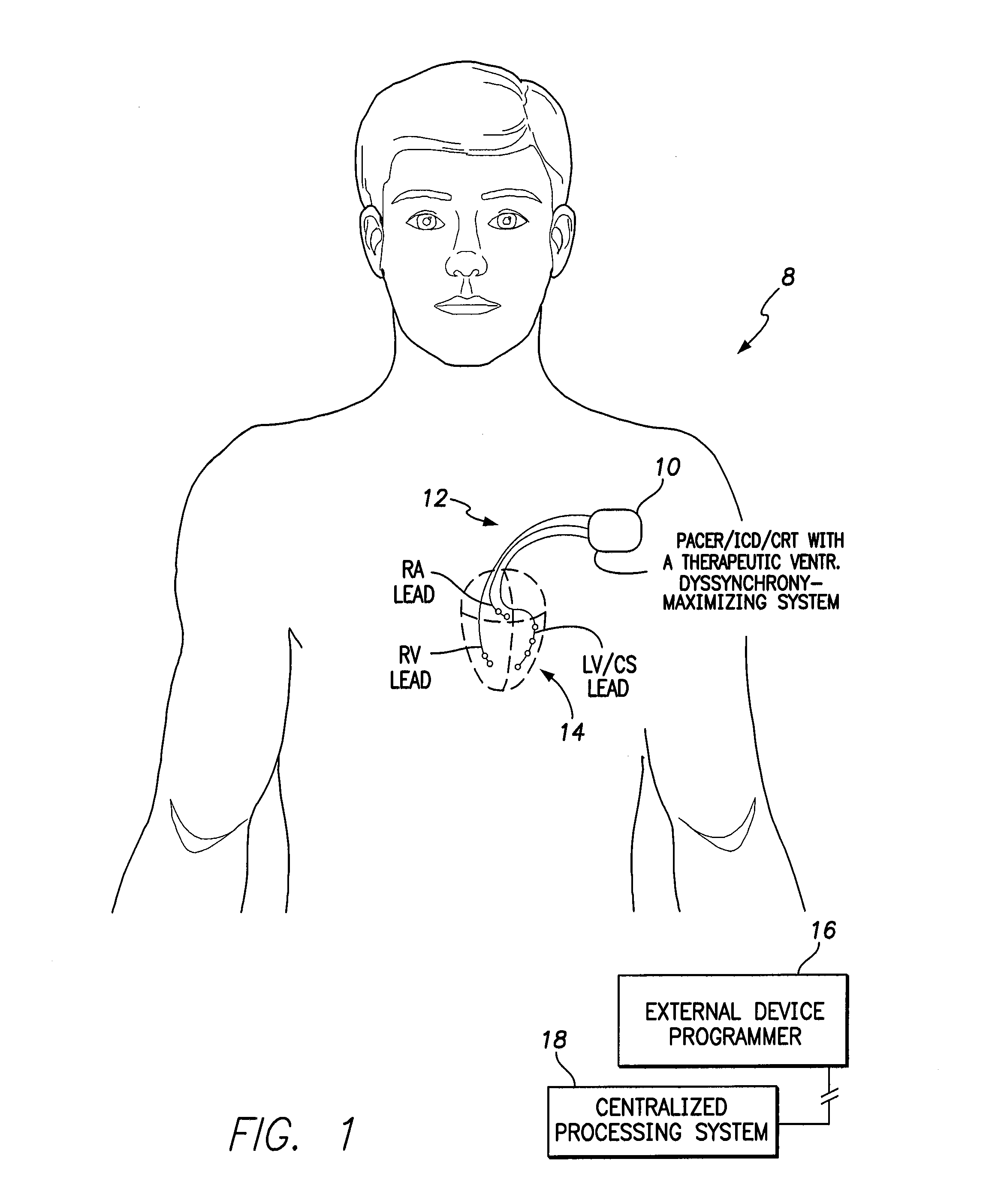
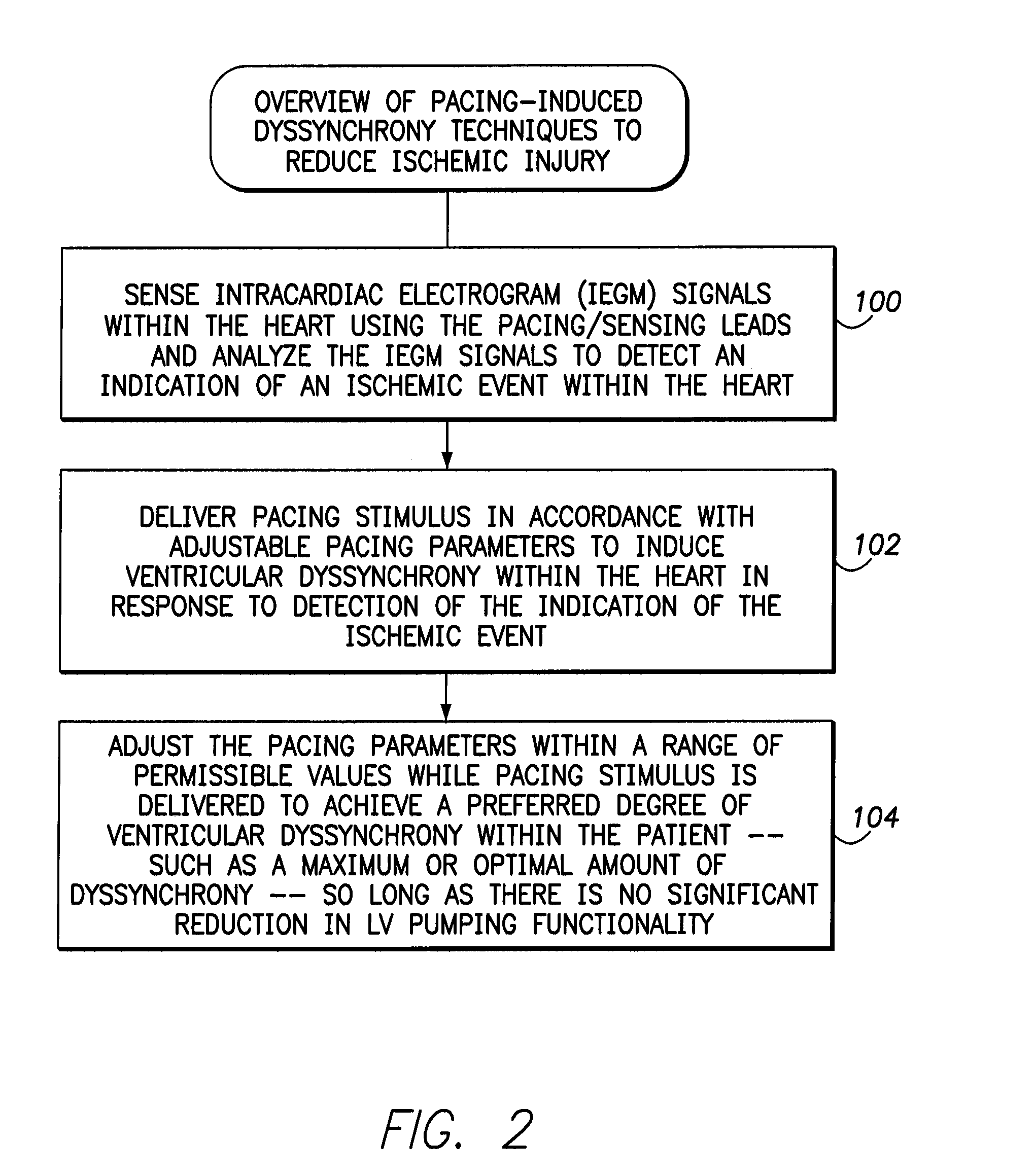
Systems and methods for controlling pacing induced dyssynchrony to reduce ischemic injury using an implantable medical device
InactiveUS20130204312A1No significant drop in LV pumping functionalityAvoid adjustmentHeart stimulatorsLeft ventricular sizeEarly onset
Techniques are provided for use by an implantable medical device for optimizing the amount of ventricular dyssynchrony induced within a patient during protective pacing. In one example, the device analyzes intracardiac electrogram signals to detect an ischemic event within the heart. The device then delivers pacing stimulus in accordance with adjustable pacing parameters to induce ventricular dyssynchrony within the heart and adjusts the pacing parameters within a range of permissible values to achieve a preferred degree of ventricular dyssynchrony within the patient, so long as there is no significant reduction in left ventricular pumping functionality. Preferably, the pacing parameters are adjusted to maximize or otherwise optimize the degree of dyssynchrony induced within the patient. If a significant reduction in LV pumping functionality is detected, the dyssynchrony-inducing pacing is preferably suspended to avoid any deterioration in the condition of the heart. Techniques for detecting early onset of ischemia are also disclosed.
Owner:PACESETTER INC
Methods for the treatment of a feeding disorder with onset during neonate development using an agonist of the oxytocin receptor
ActiveUS8853158B2Good curative effectLow toxicityNervous disorderPeptide/protein ingredientsEarly onsetAgonist
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
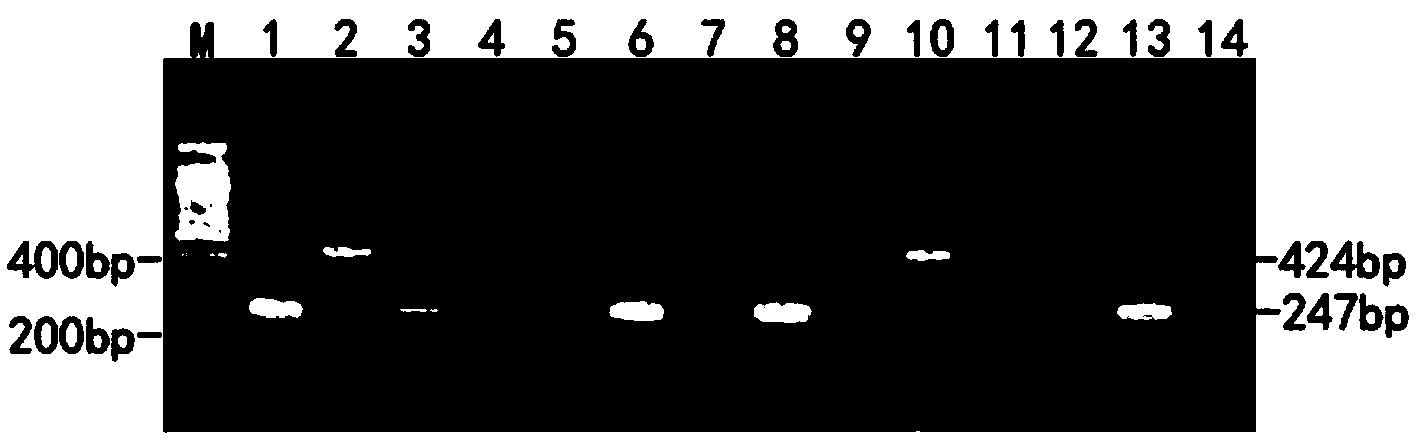
Double RT-PCR kit for newcastle disease virus and avian pneumovirus, and application thereof
ActiveCN103820575AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesEarly onsetNewcastle disease virus NDV
The invention discloses a double RT-PCR kit for newcastle disease virus (NDV) and avian pneumovirus (APV), and an application thereof. The kit contains a set of primer pairs for determining or assistantly determining NDV and APV: a primer pair 1 (sequence 1 and sequence 2) and a primer pair (sequence 3 and sequence 4). Experiments show that two pathogens of NDV and APV can be detected and determined at the same time by only one-time PCR. The kit has the advantages of high specificity, high sensitivity, low cost and high efficiency. The kit can directly determine amplification results by using different amplification fragment lengths in primer design; and the kit is simple and convenient, intuitive and practical. The kit can simultaneously detect 10 pg of NDV RNA and 10 pg of APV RNA at least. Sensitivity of the kit provides rapid and accurate diagnosis results for early onset caused by the two viruses. The kit has great significance for preventing and controlling the two diseases, and has significant realistic significance for sustainable and healthy development of poultry industry.
Owner:GUANGXI VETERINARY RES INST
Tetrazole compounds for reducing uric acid
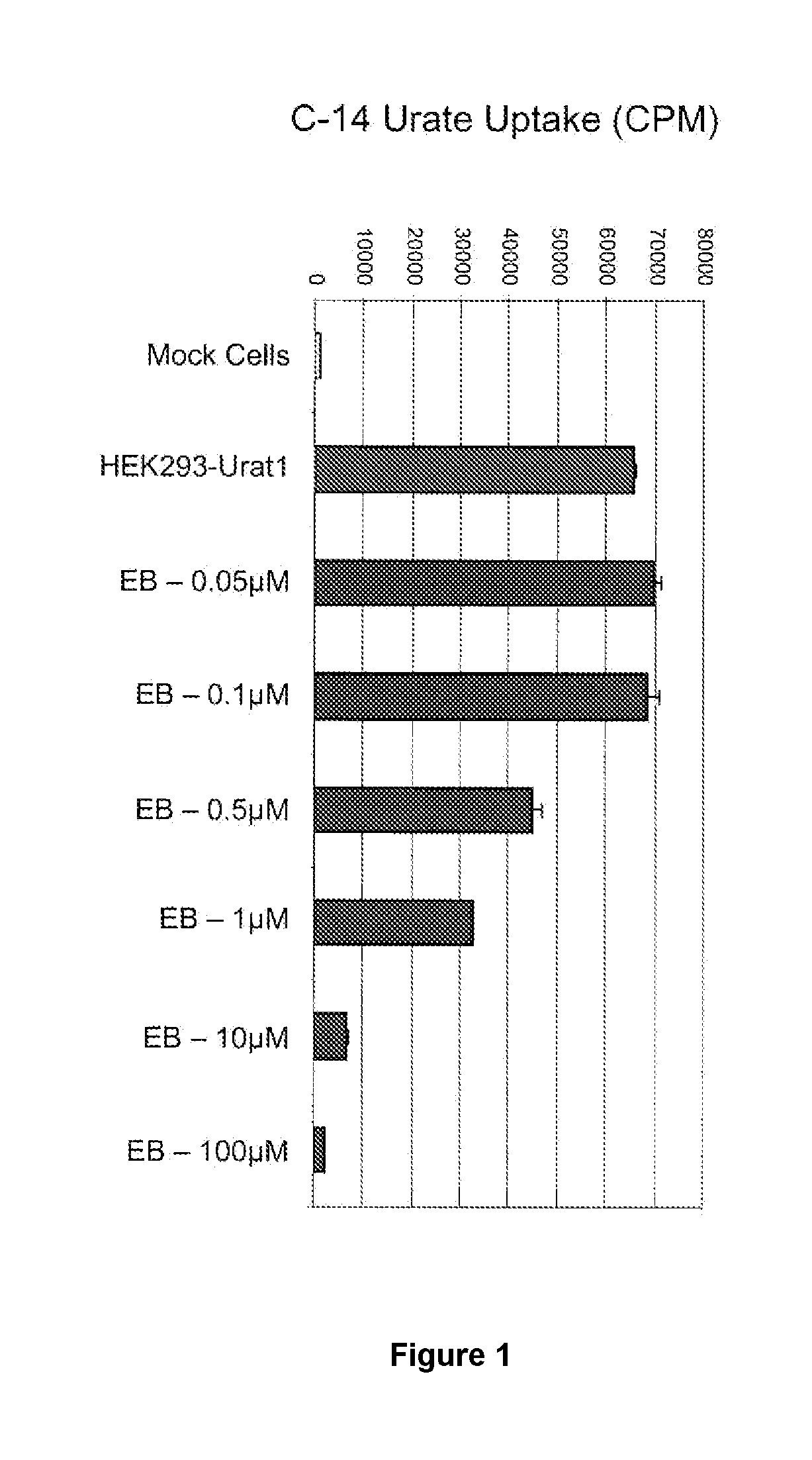
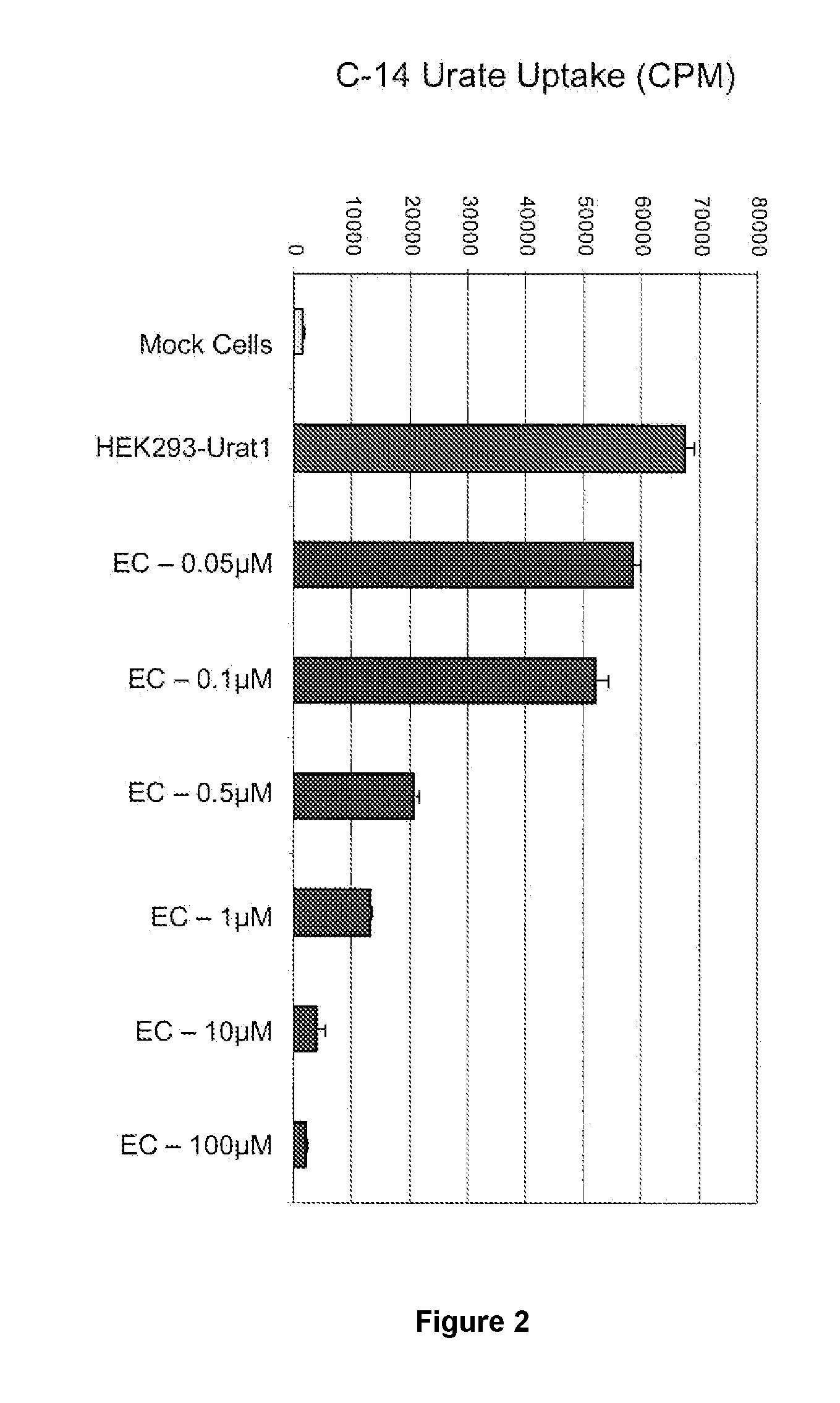
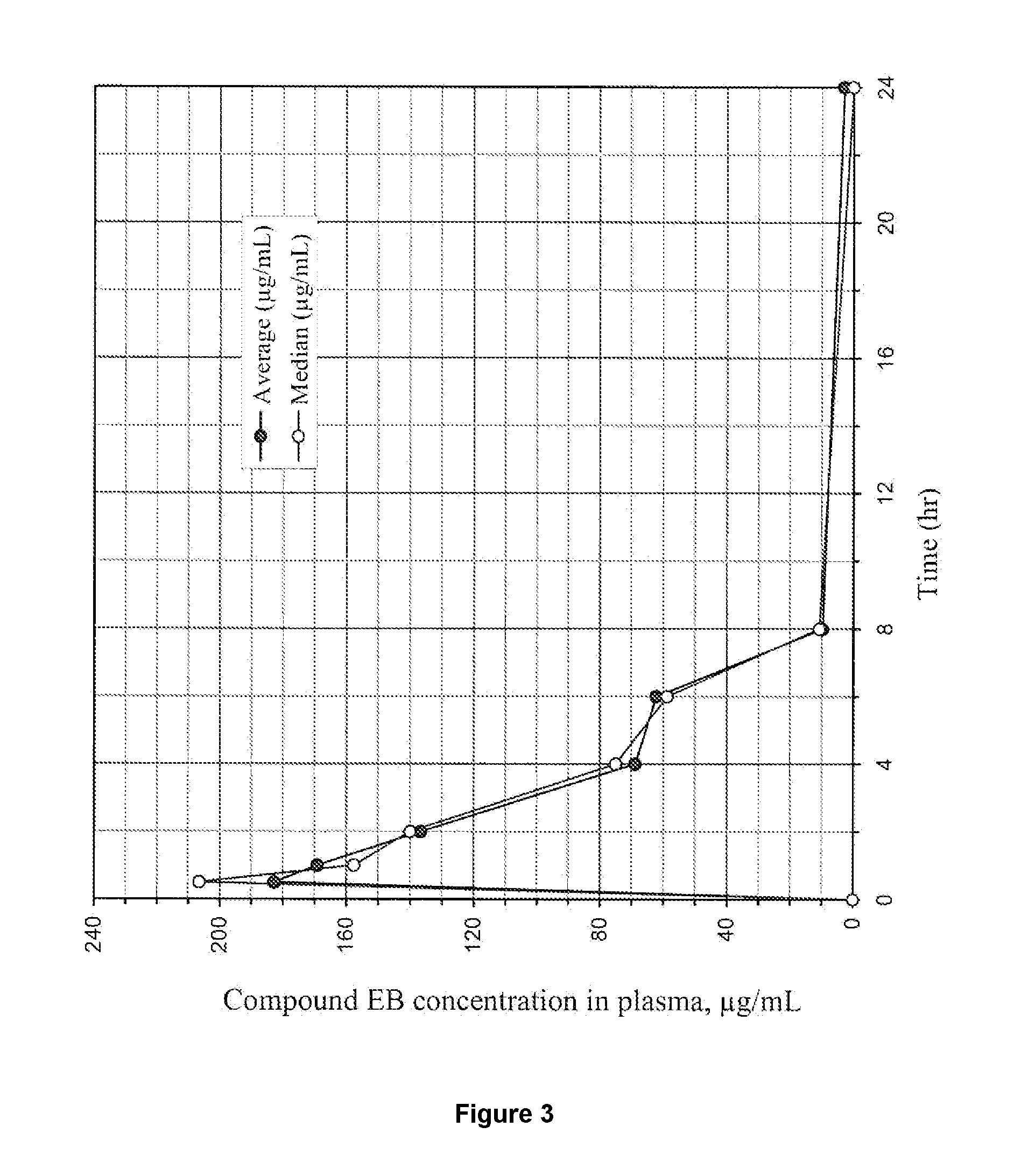
Uric acid in mammalian subjects is reduced and excretion of uric acid is increased by administering a compound of Formula I. The uric acid-lowering effects of the compounds of this invention are used to treat or prevent a variety of conditions including gout, hyperuricemia, elevated levels of uric acid that do not meet the levels customarily justifying a diagnosis of hyperuricemia, renal dysfunction, kidney stones, cardiovascular disease, risk for developing cardiovascular disease, tumor-lysis syndrome, cognitive impairment, early-onset essential hypertension, and Plasmodium falciparum-induced inflammation. In Formula 1, x is 1 or 2: y is O, 1, 2 or 3; and R1 is selected from the group consisting of hydrogen, alkyl having 1 or 2 carbon atoms, hydroxy, alkoxy having 1 or 2 carbon atoms, fluoro, chloro, bromo, and amino. A is phenyl unsubstituted or substituted by one, two or three groups selected from the group consisting of halo, alkyl having 1 or 2 carbon atoms, perfluoromethyL alkoxy having 1 or 2 carbon atoms, and perfluoromethoxy; or cycloalkyl having from 3 to 6 ring atoms wherein the cycloalky! is unsubstituted or one one two ring carbons are independently mono-substituted by methyl or ethyl; or a 5 or 6 membered heleraromatic ring having 1 or 2 ring heteroatoms selected from N, S and O and the heteroaromatic ring is covalently bound to the remainder of the compound by a ring carbon.
Owner:WELLSTAT THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com