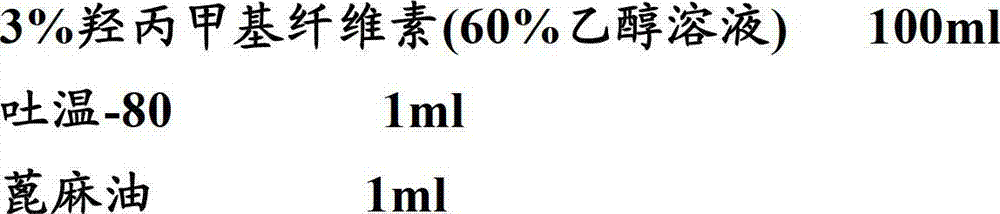Patents
Literature
94 results about "Oxycodone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to help relieve moderate to severe pain.
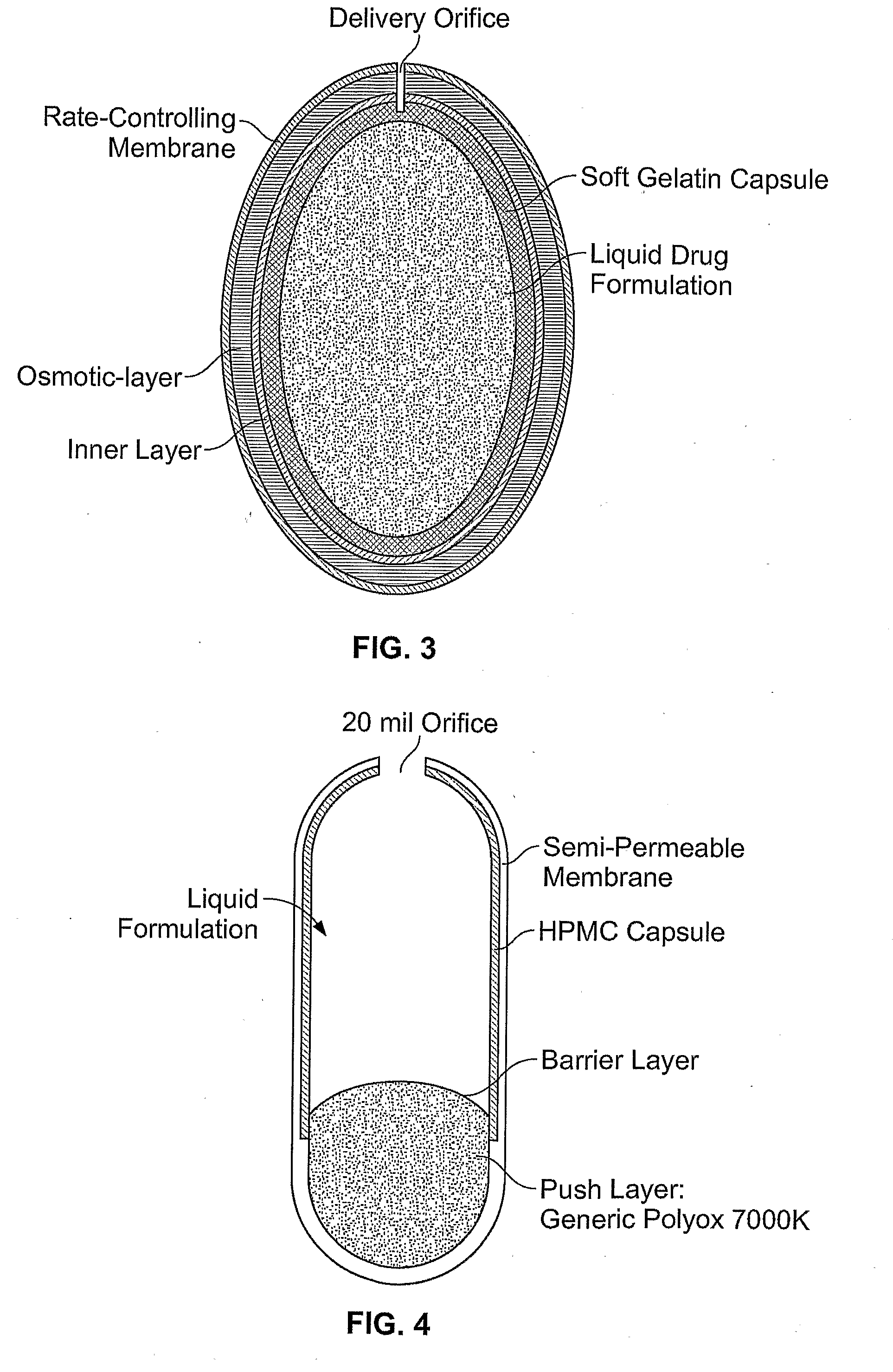
Once-a-day, oral, controlled-release, oxycodone dosage forms
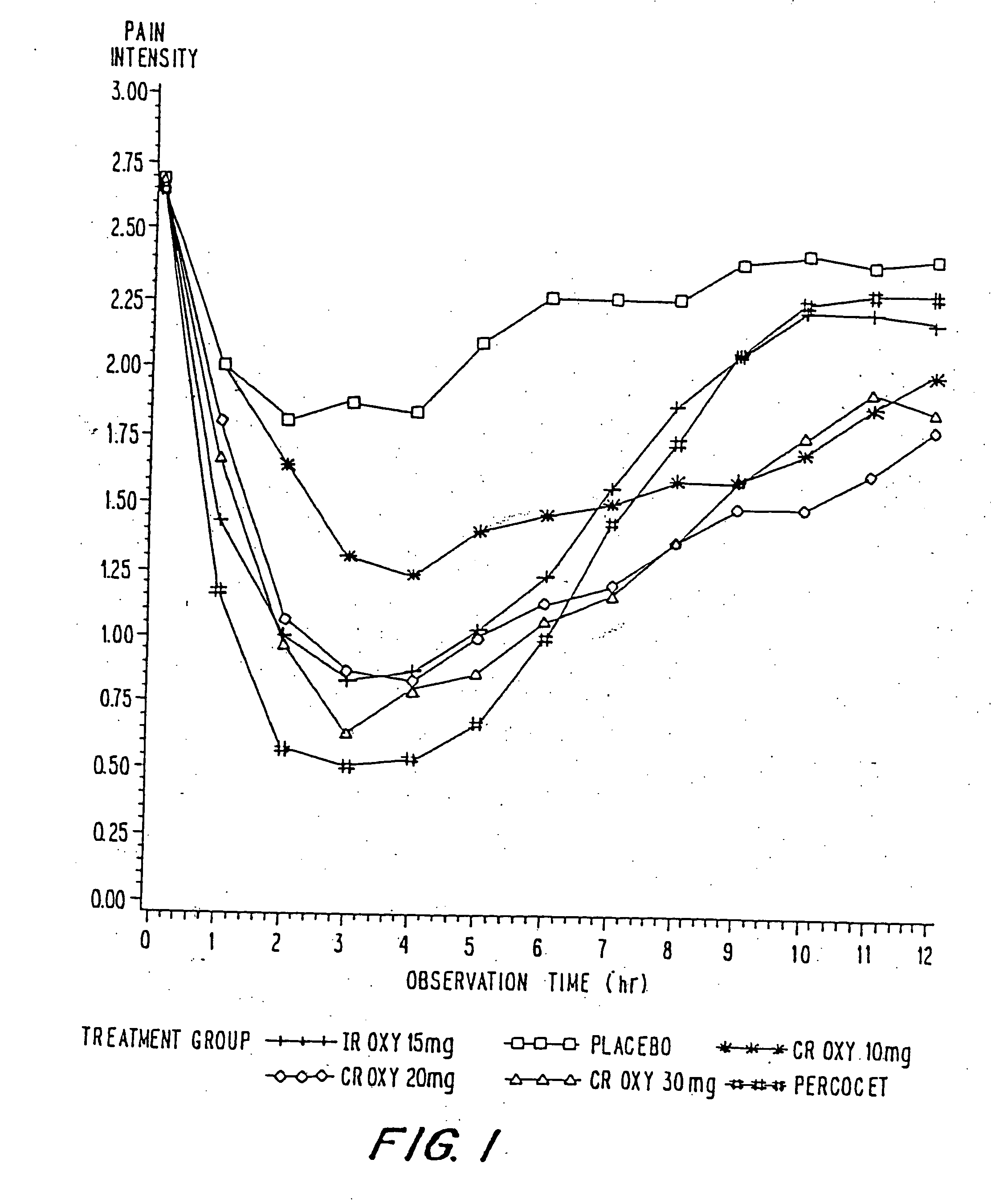
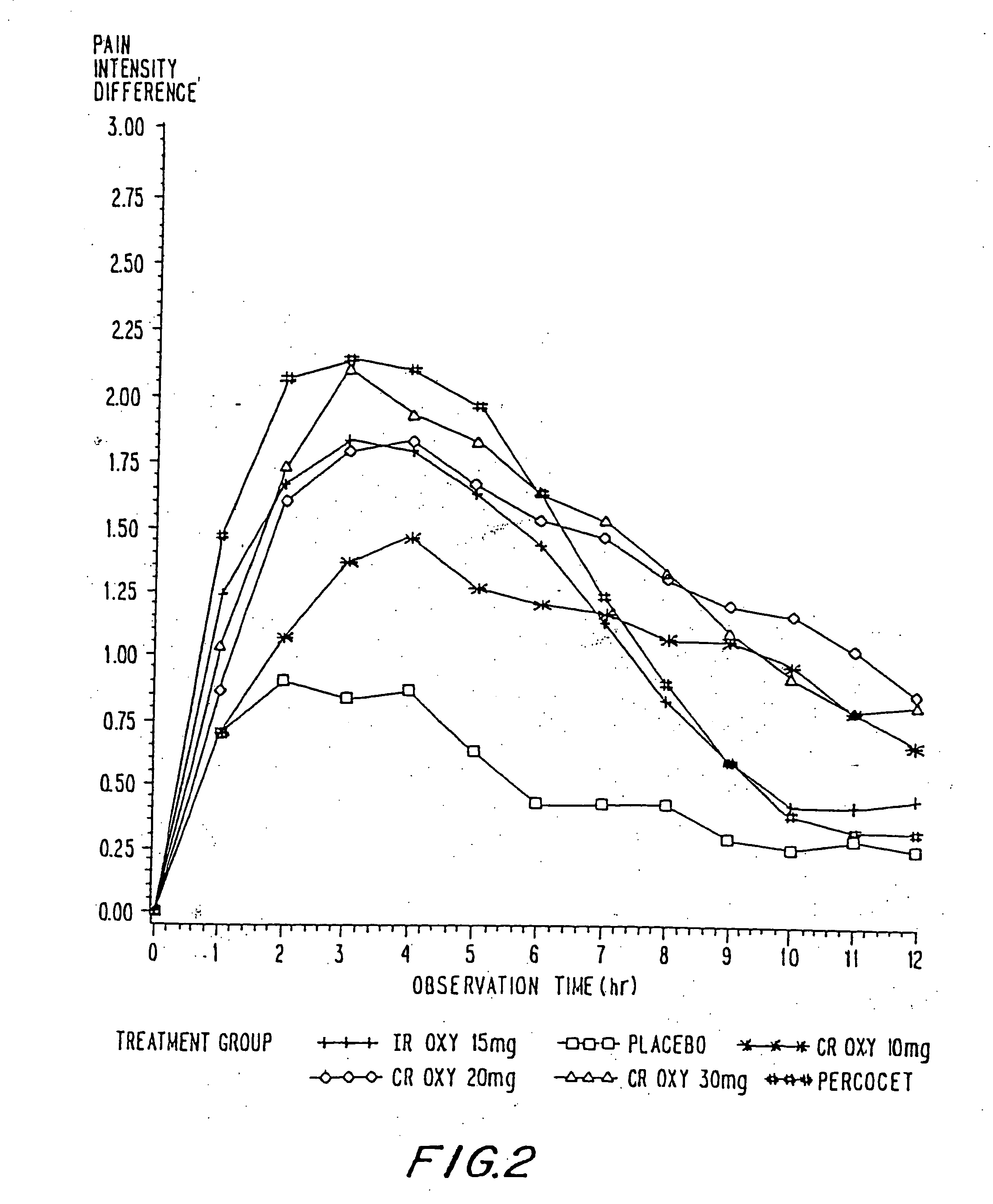
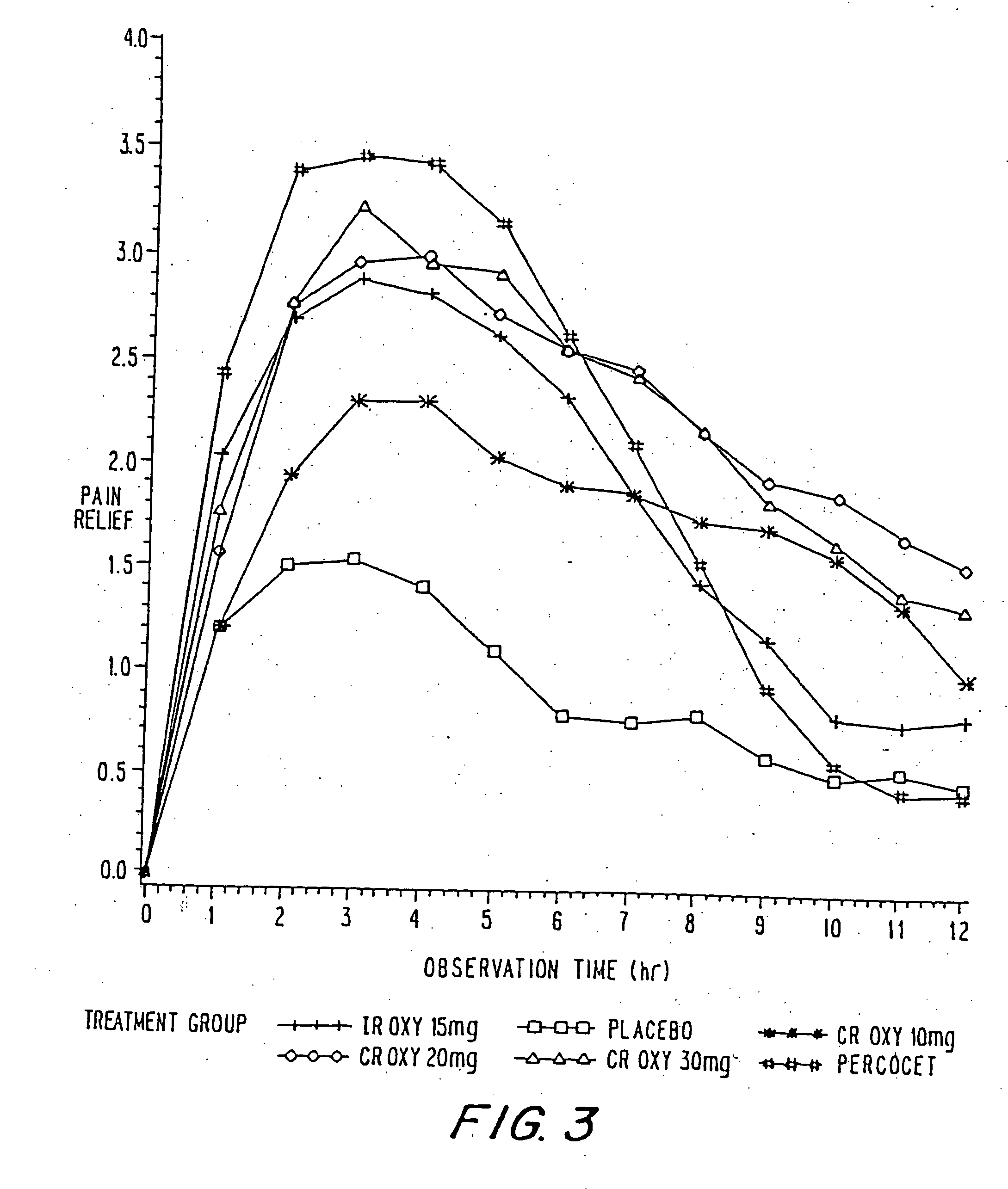
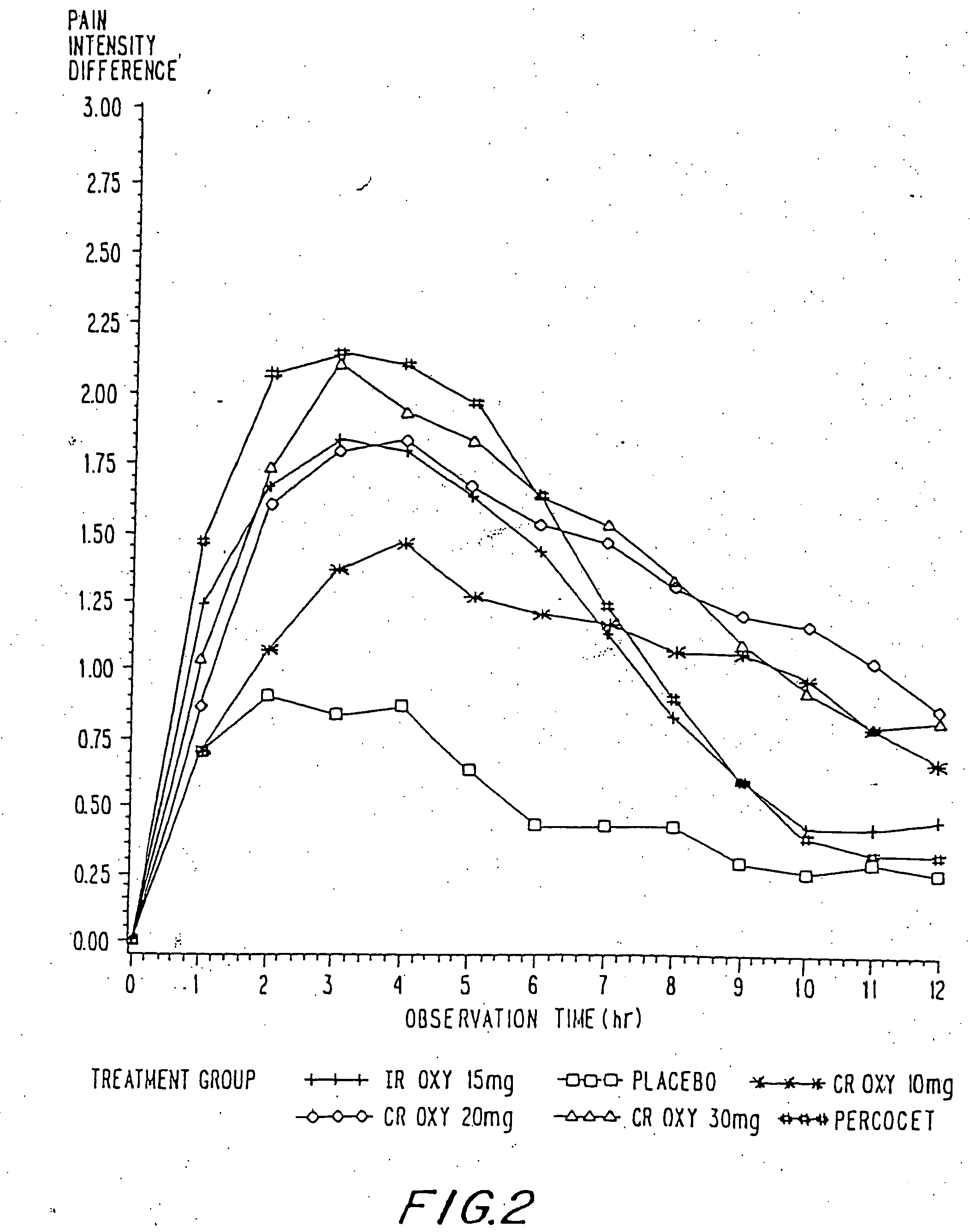
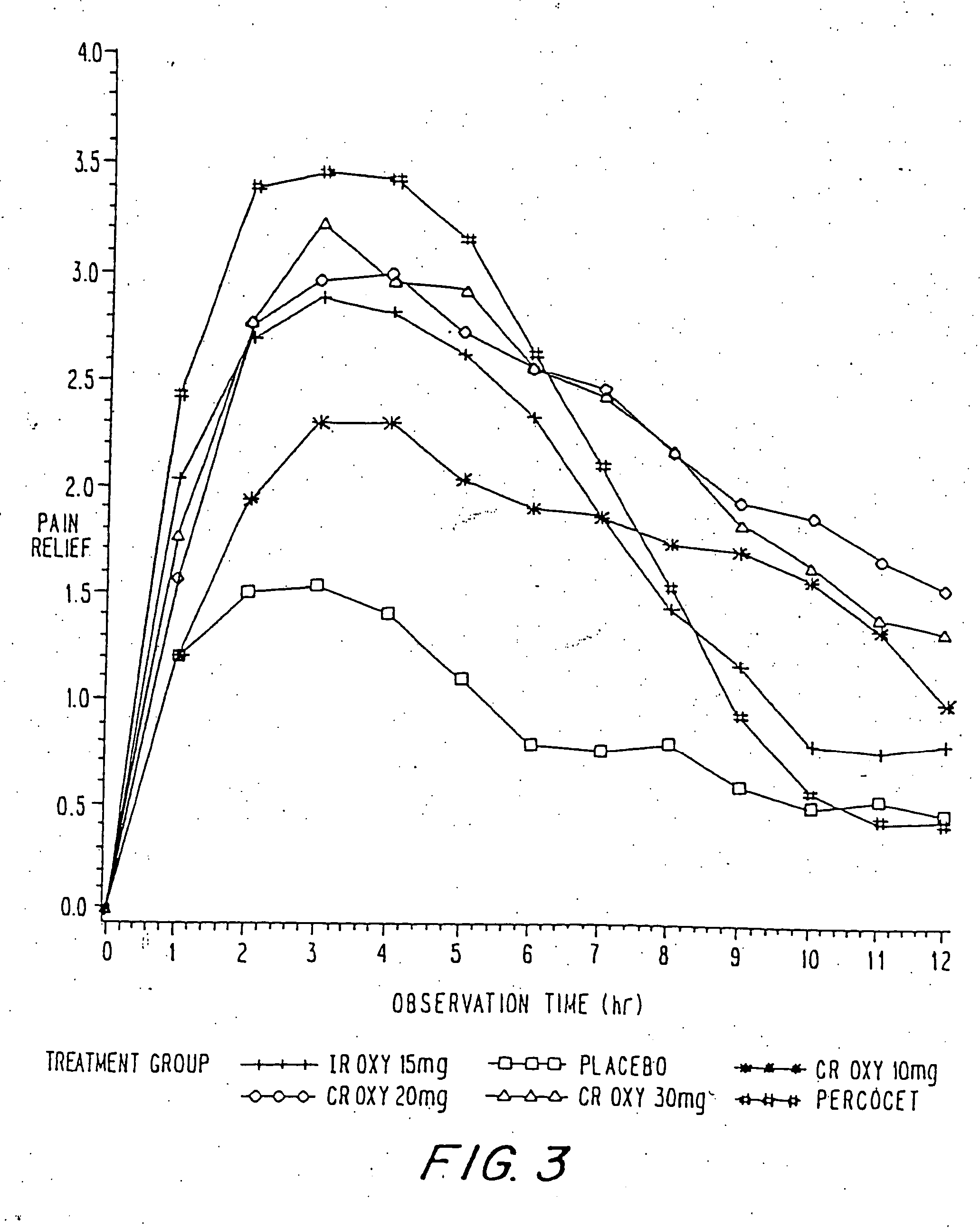
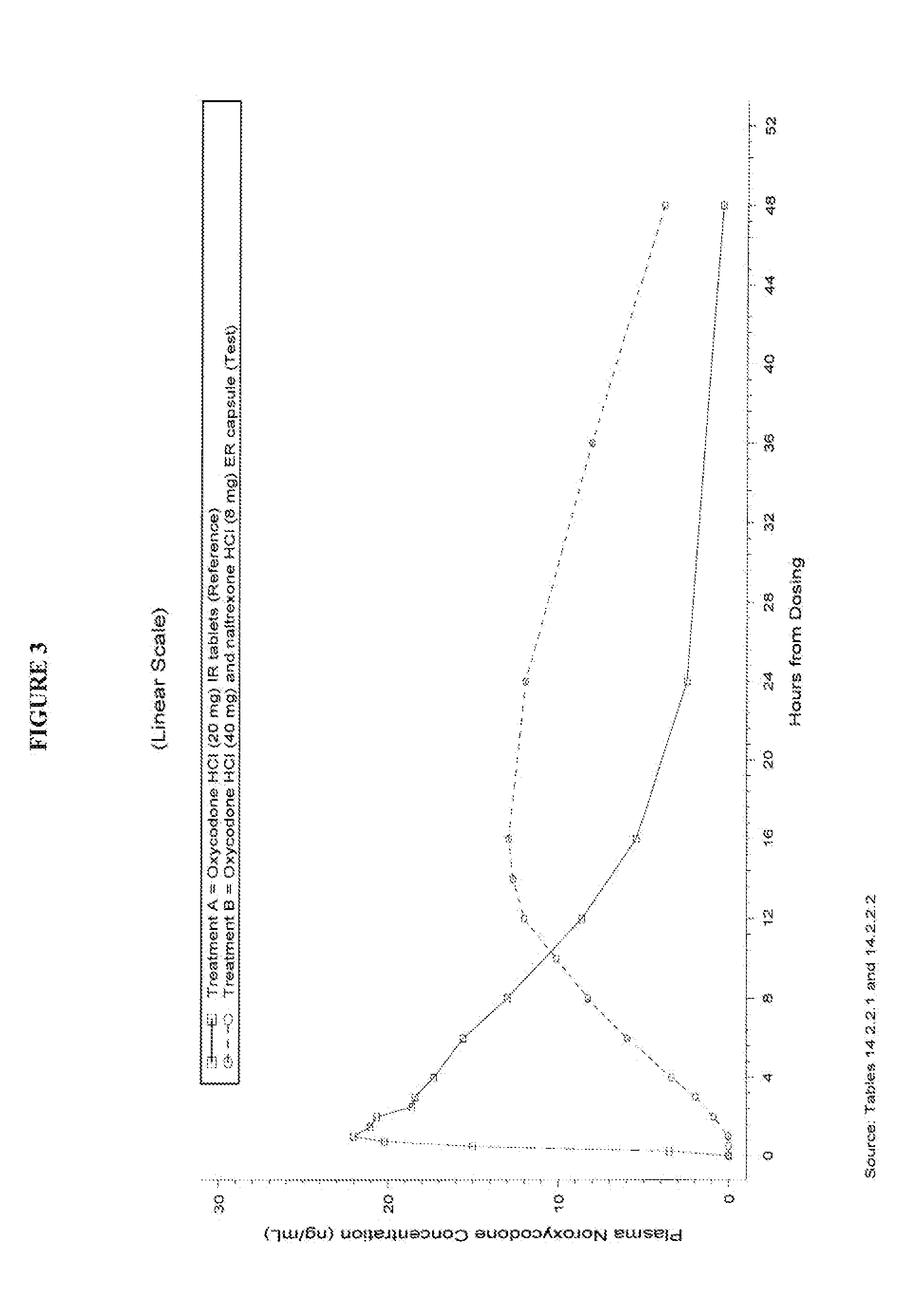
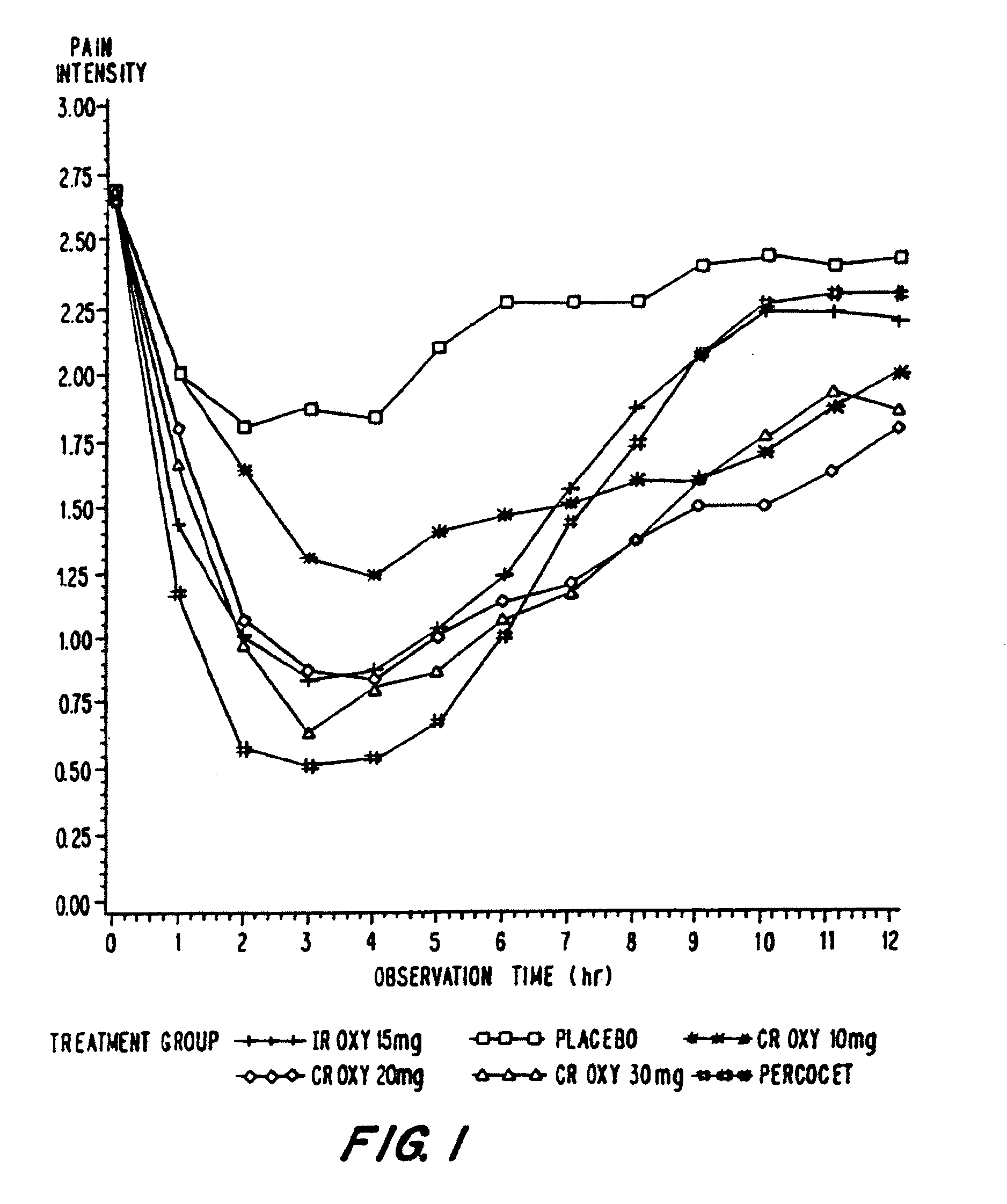
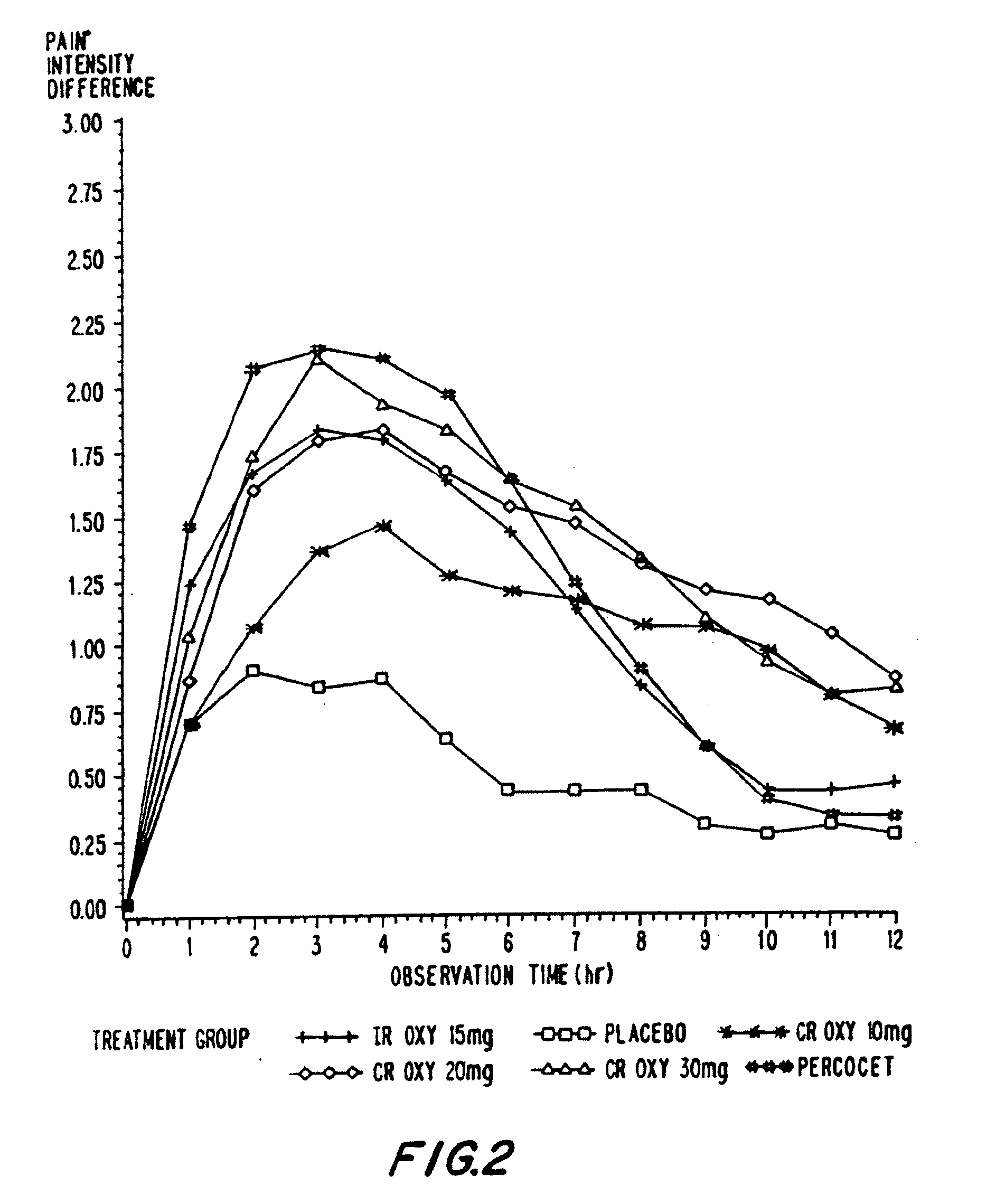
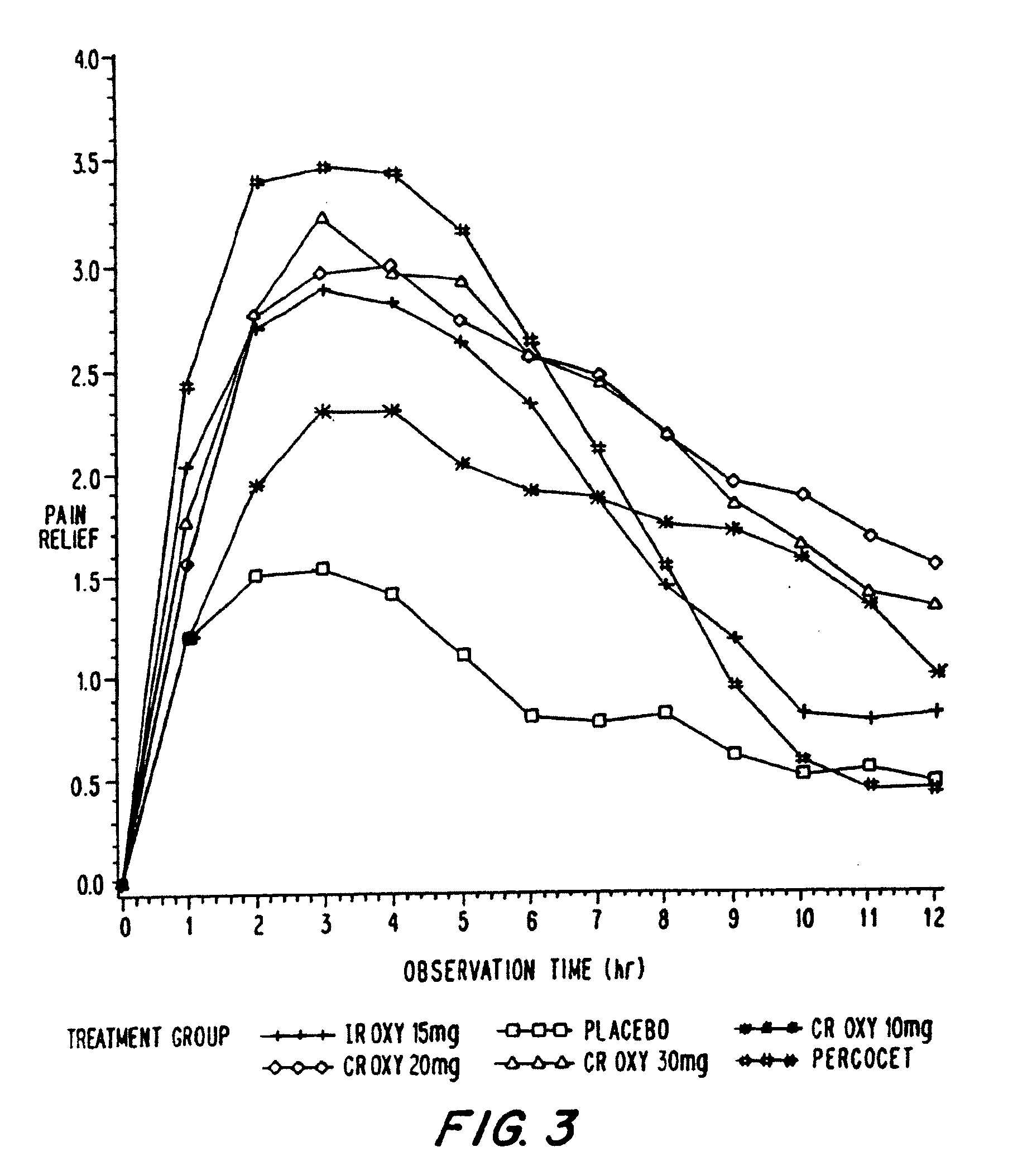
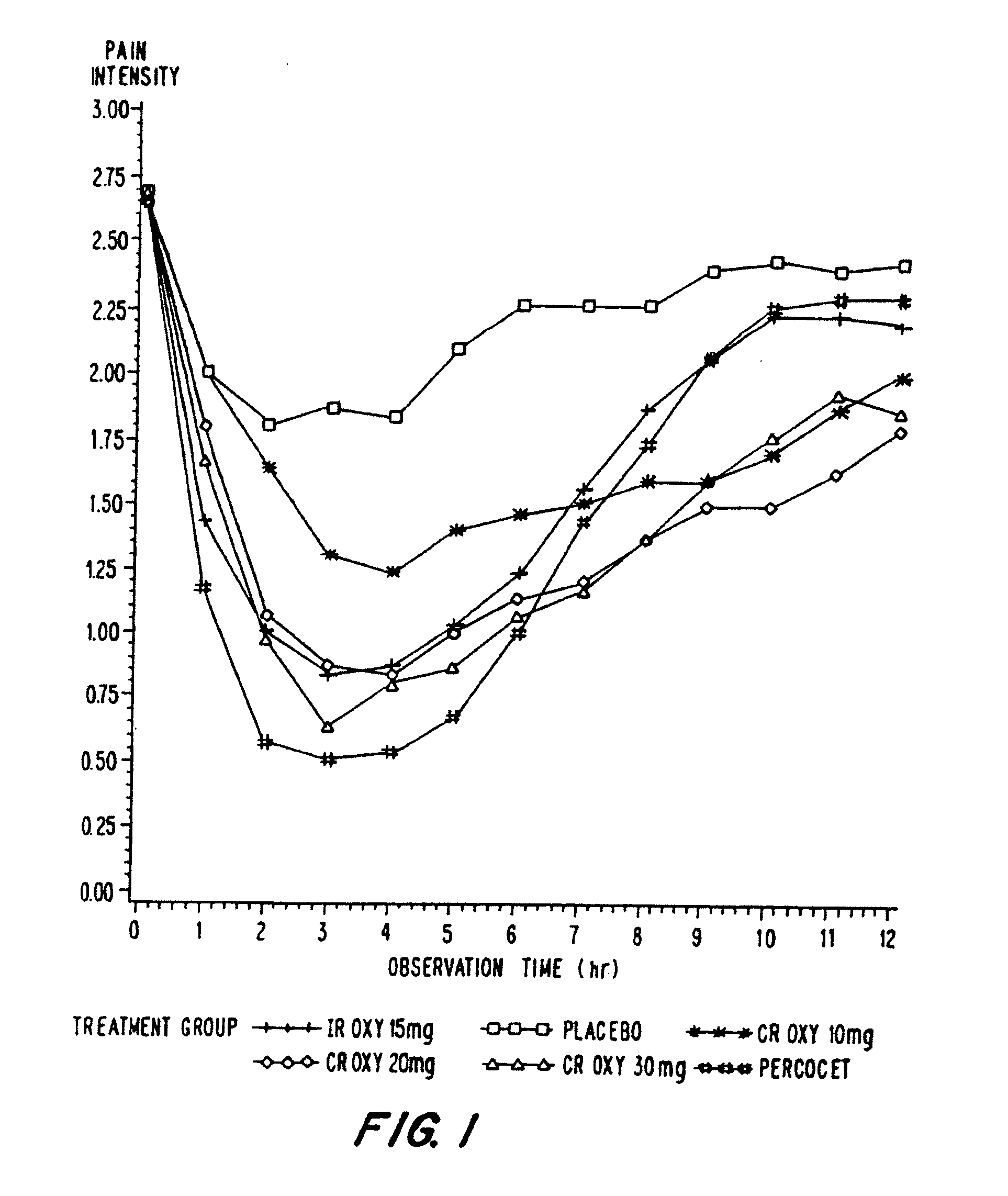
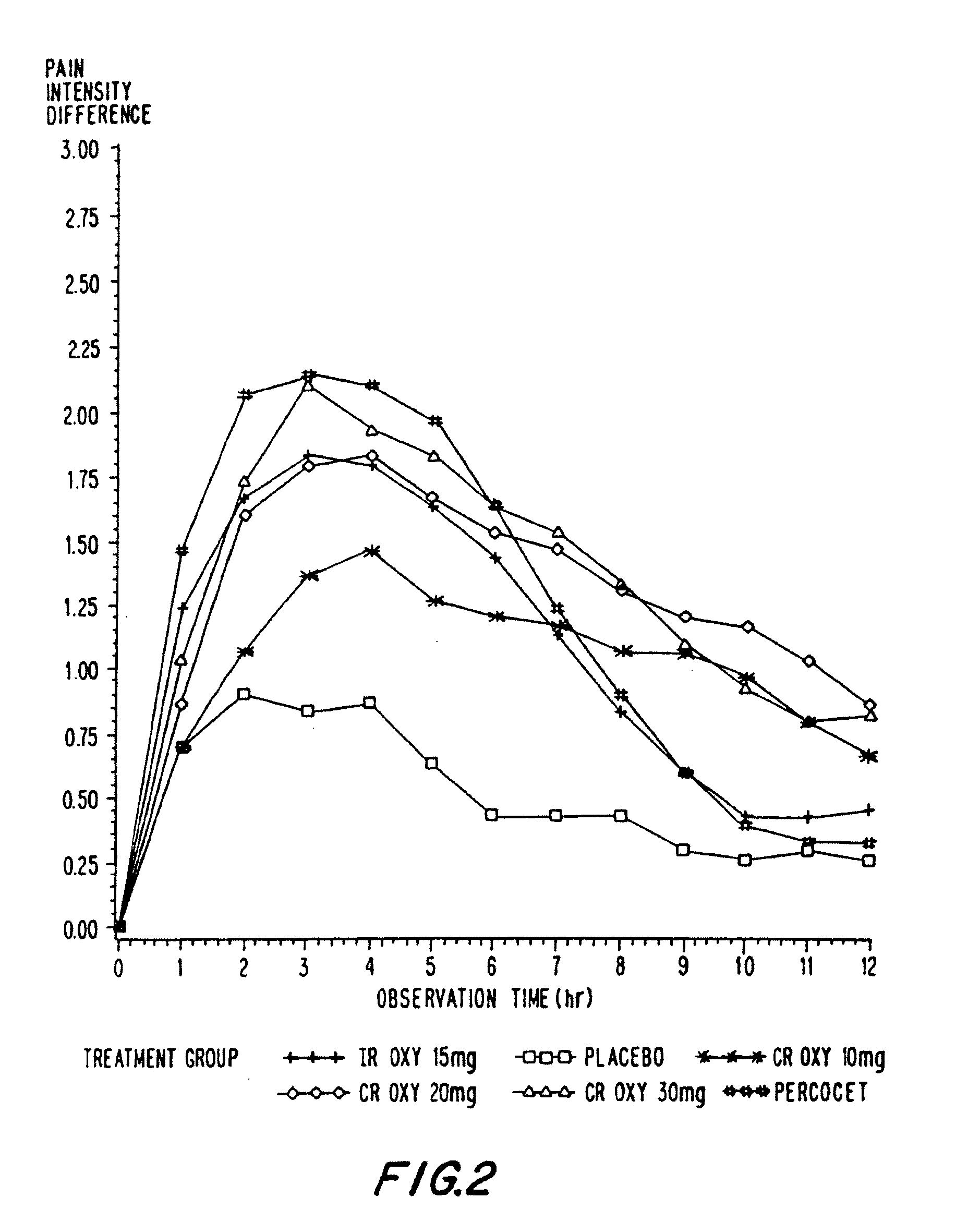
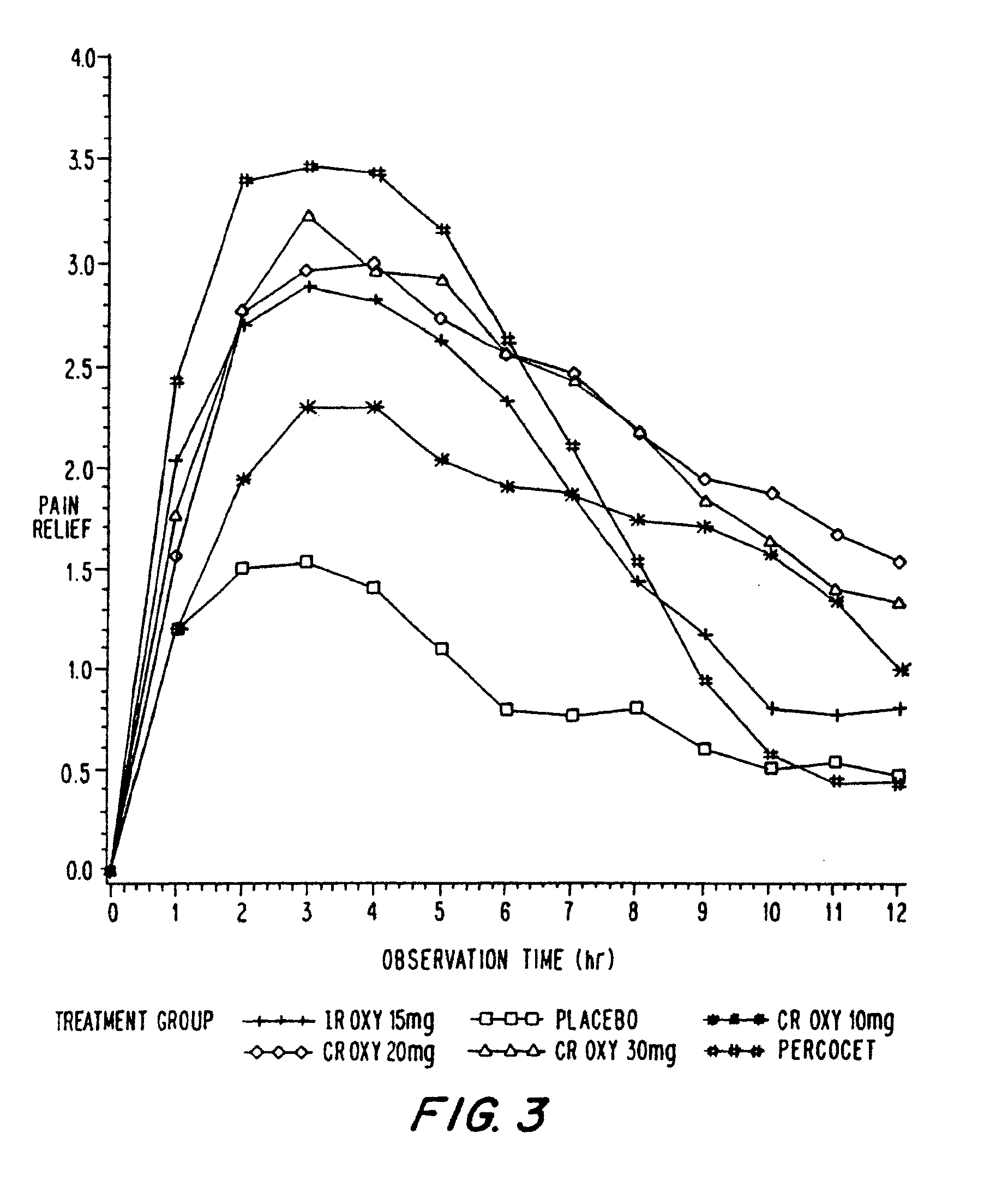
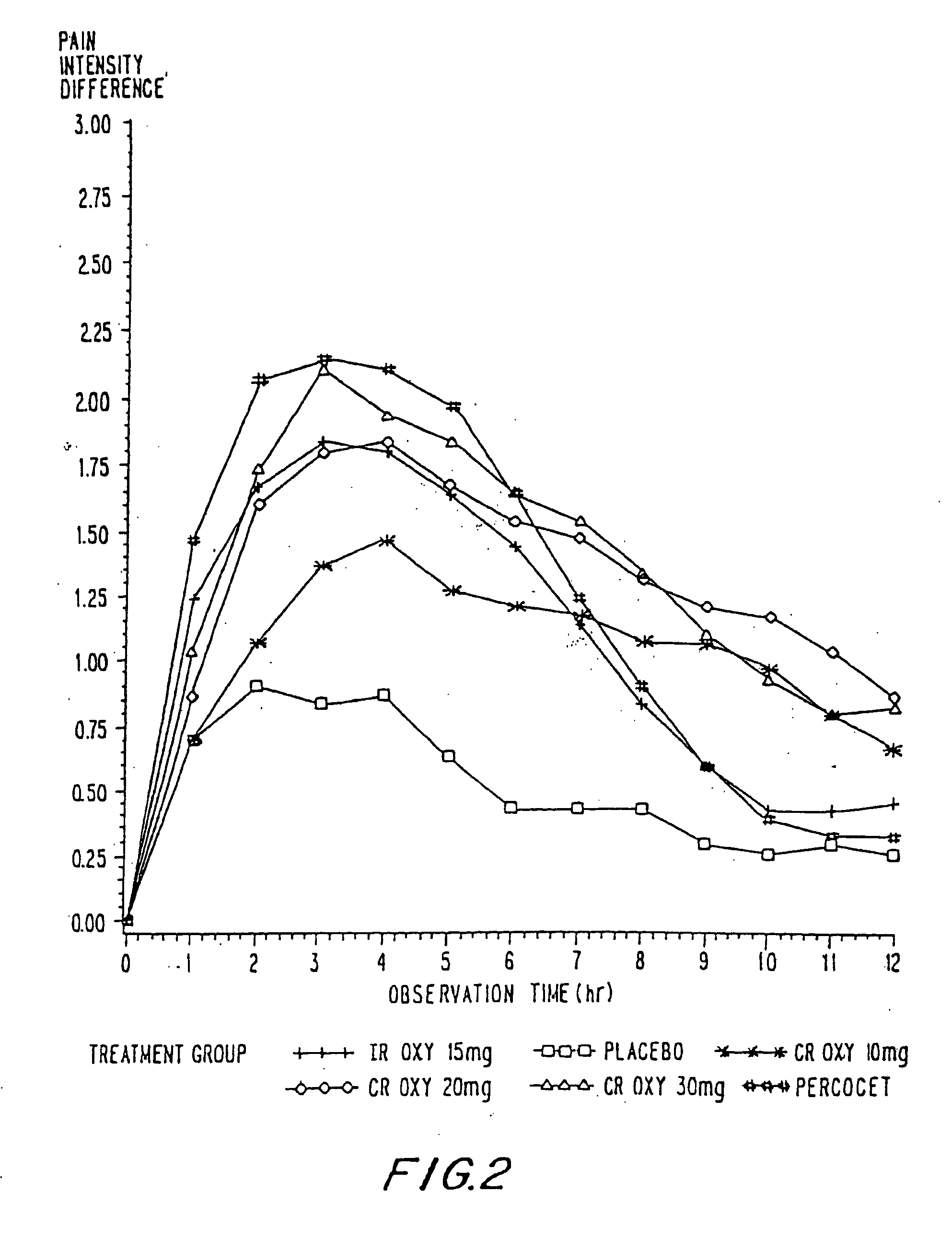
Oxycodone formulations are provided which produce substantially flat in vivo steady state plasma profiles. Tolerance levels associated with such profiles and tolerance levels associated with biphasic profiles are shown not to be statistically different. The substantially flat in vivo steady state plasma profiles are produced by dosage forms having substantially zero order in vitro release profiles. Such release profiles produce low single dose in vivo Cmax levels which can reduce the probability of adverse side effects.
Owner:ALZA CORP
Multiparticulates
InactiveUS20080260815A1Reduce adhesionReduce the required powerOrganic active ingredientsPowder deliveryActive agentExcipient
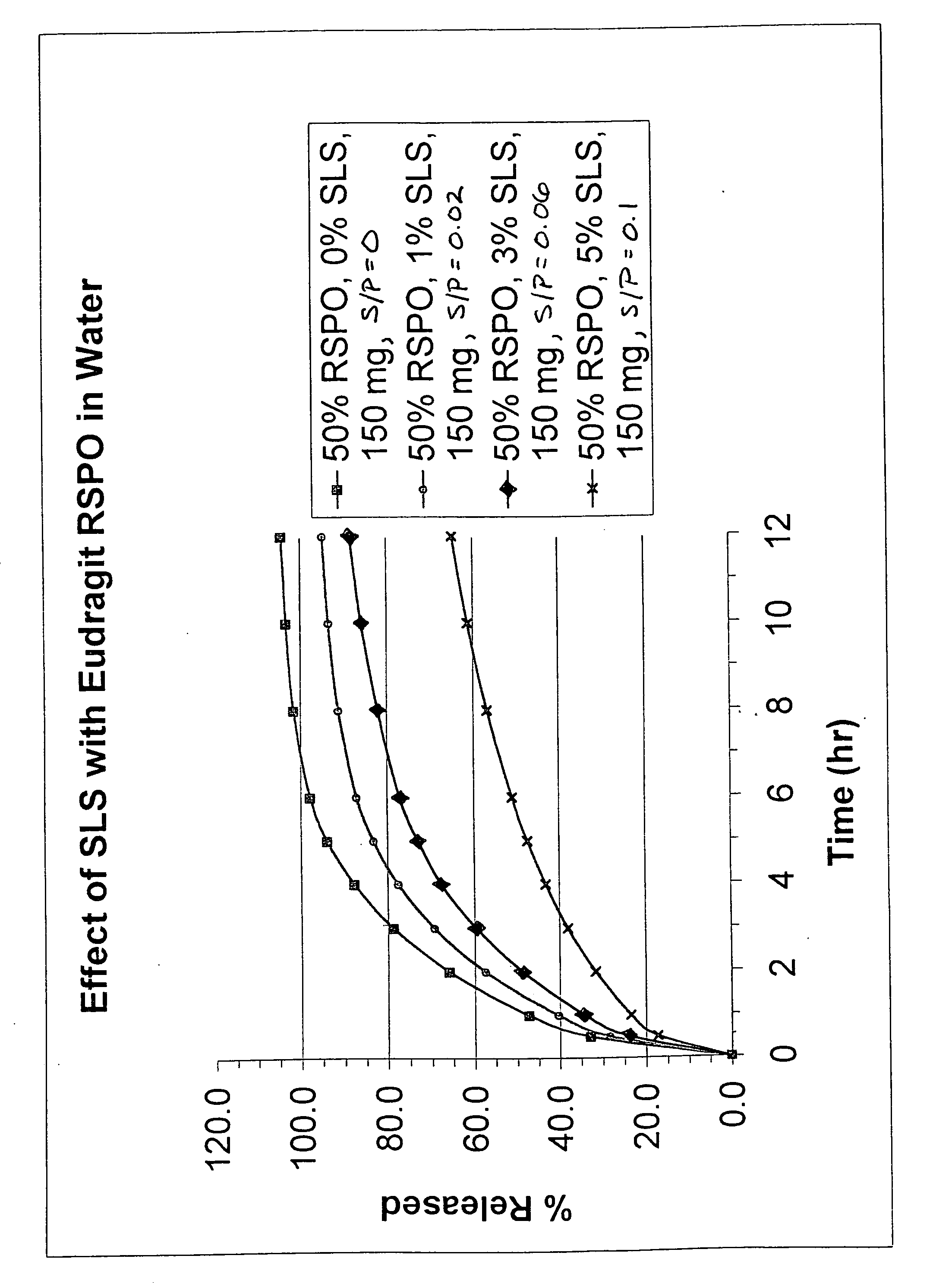
Extrusion of a mix containing a pharmaceutically active agent can be achieved using a plasticising excipient in an amount sufficient to act as plasticiser and also act as lubricant, thereby avoiding the need for inclusion of a lubricant. The invention provides multiparticulates with controlled release properties, substantially free of lubricant. The present invention is preferably directed to extruded multiparticulates containing an opioid such as oxycodone, an ammonium methacrylate copolymer such as Eudragit® RSPO, a plasticising excipient such as preferably stearyl alcohol and a water permeability modifier such as preferably Eudragit® RLPO. The obtained multiparticulates show a release rate profile which is pH-independent.
Owner:EURO-CELTIQUE SA
Dosage Form Containing Oxycodone and Naloxone
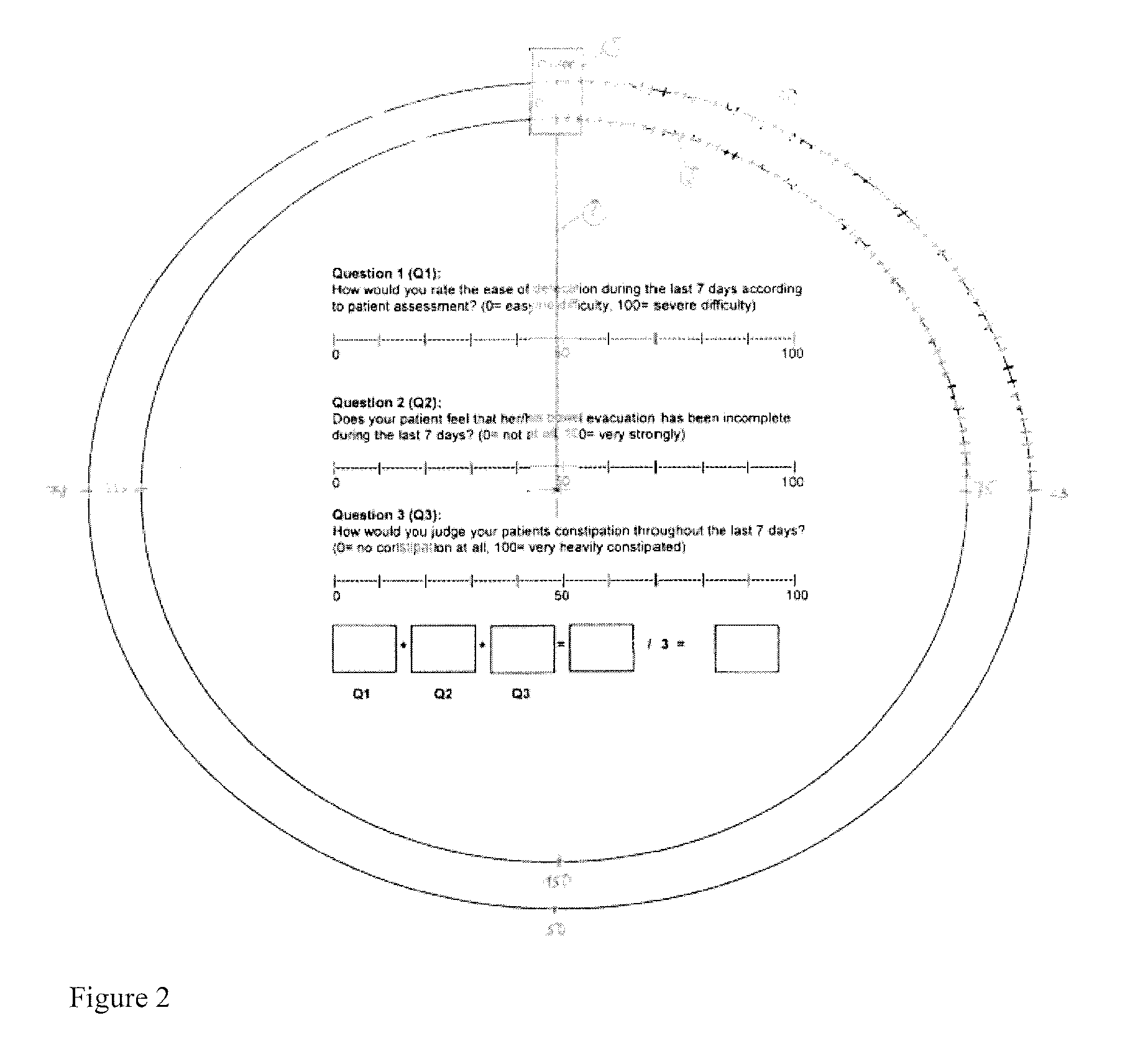
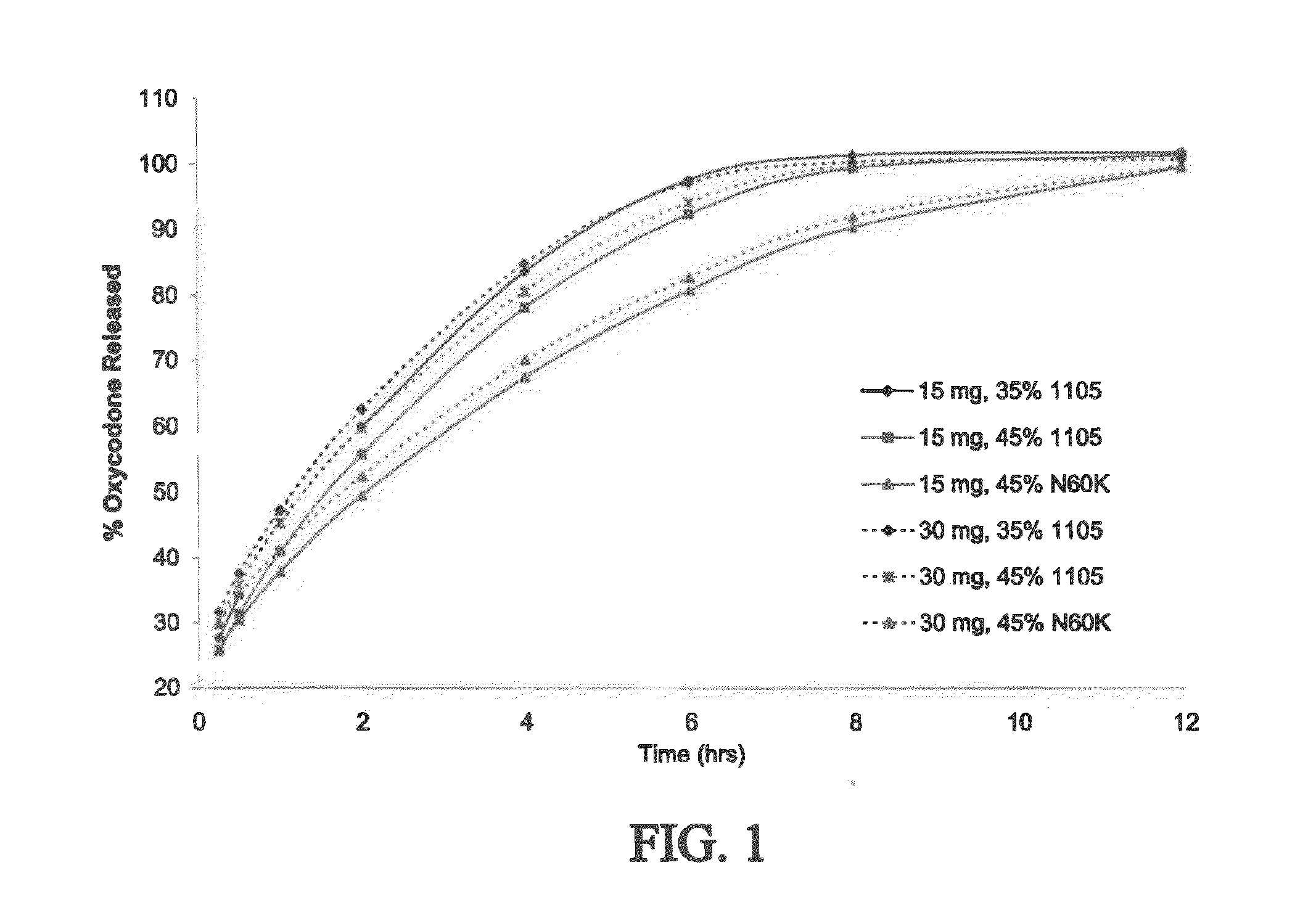
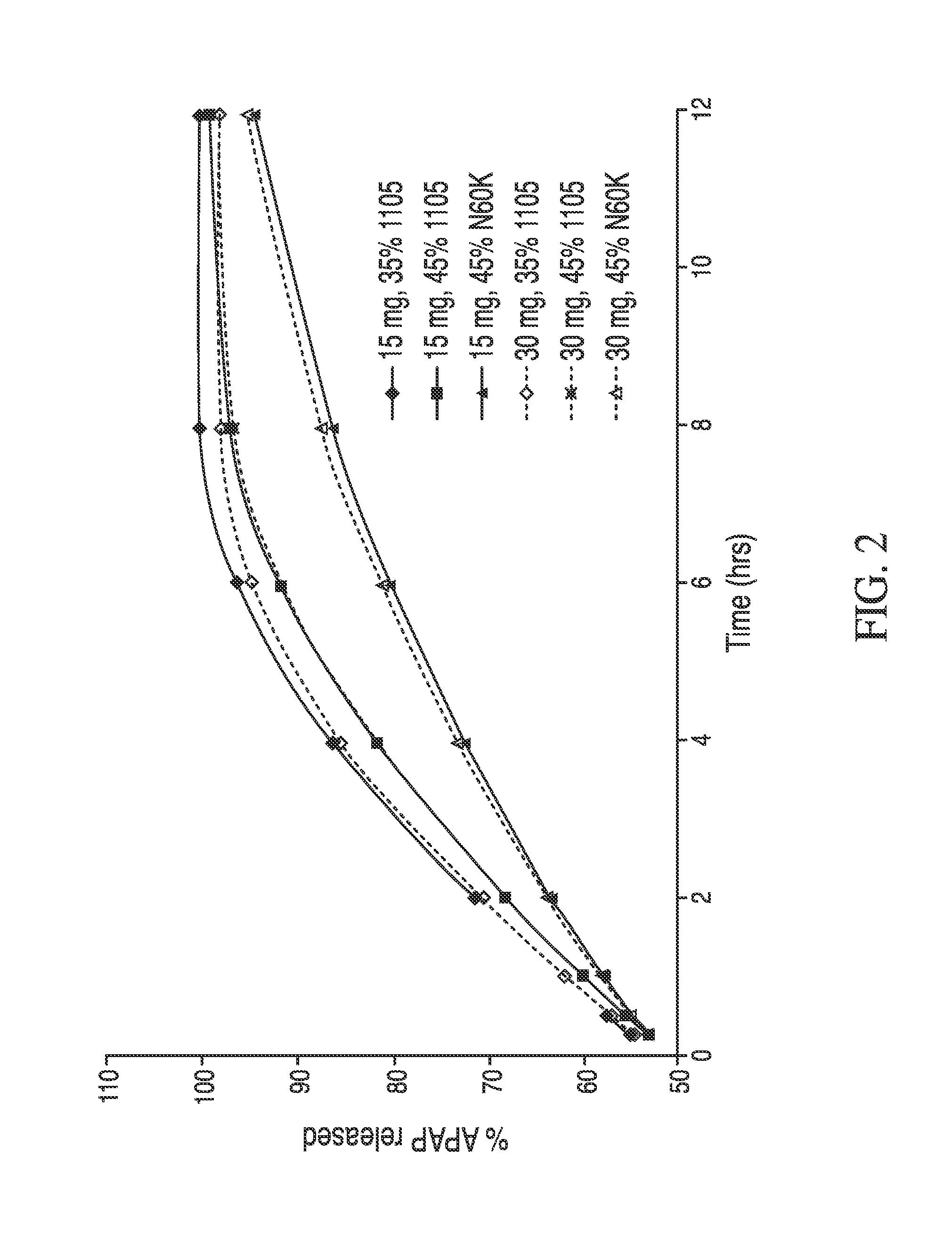
The present invention concerns a dosage form comprising oxycodone and naloxone which is characterized by specific in vivo parameters such as tmax, Cmax, AUCt value, mean bowel function score and / or duration of analgesic efficacy.
Owner:PURDUE PHARMA LP
Tamper resistant lipid-based oral dosage form for opioid agonists
A tamper resistant drug delivery system made of at least one lipid, at least one gelling agent and at least one drug active, such as oxycodone, where the system gels rapidly in the presence of water or a solution containing water, and the drug active releases into the digestive system, wherein the weight ratio of gelling agent to lipid is less than 1:1.4.
Owner:SHEARKERSHMAN LAB
Analgesic combination of tramadol and meloxicam
InactiveUS20080050427A1Reduce concentrationEfficient managementBiocidePowder deliveryMeloxicamPharmaceutical drug
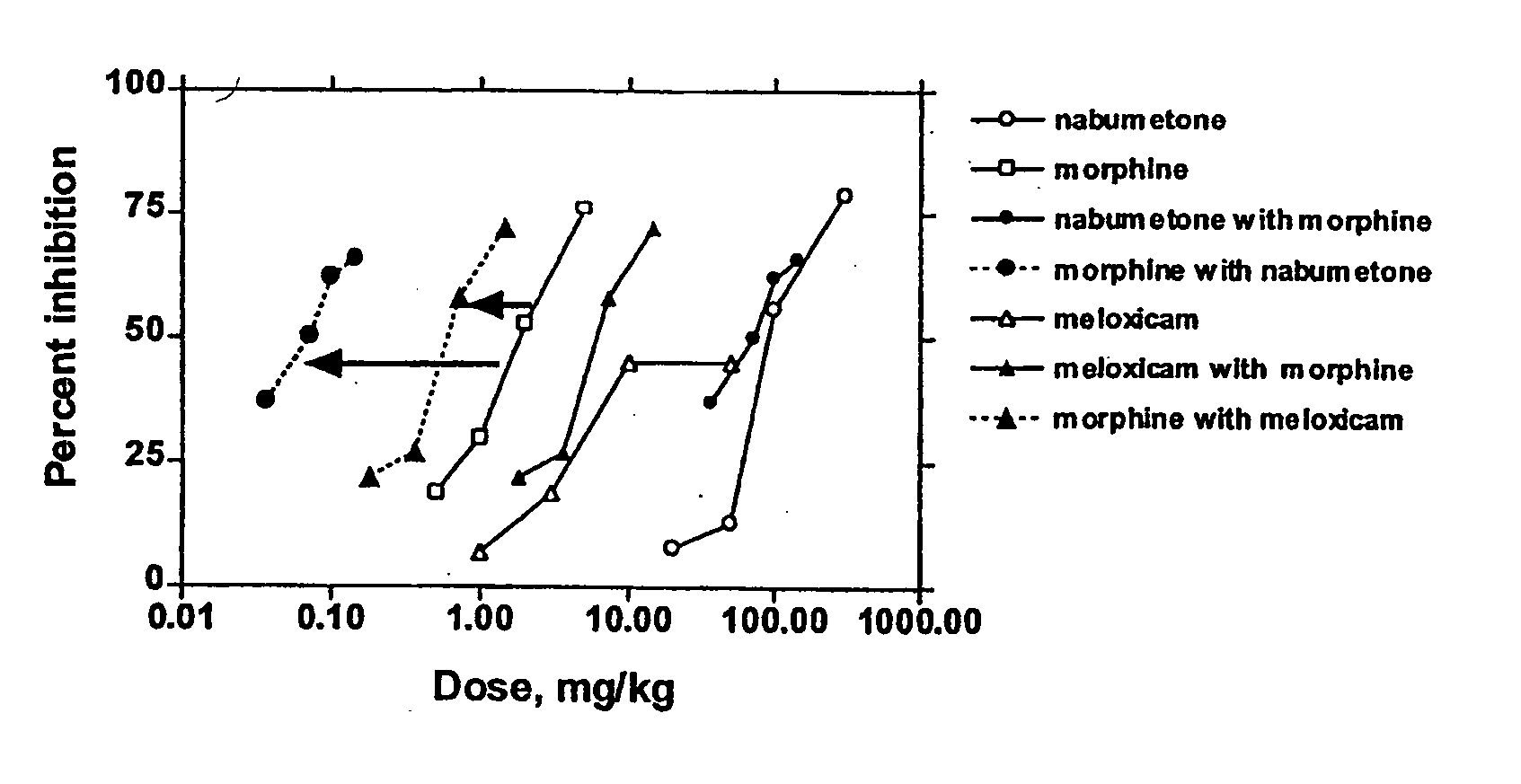
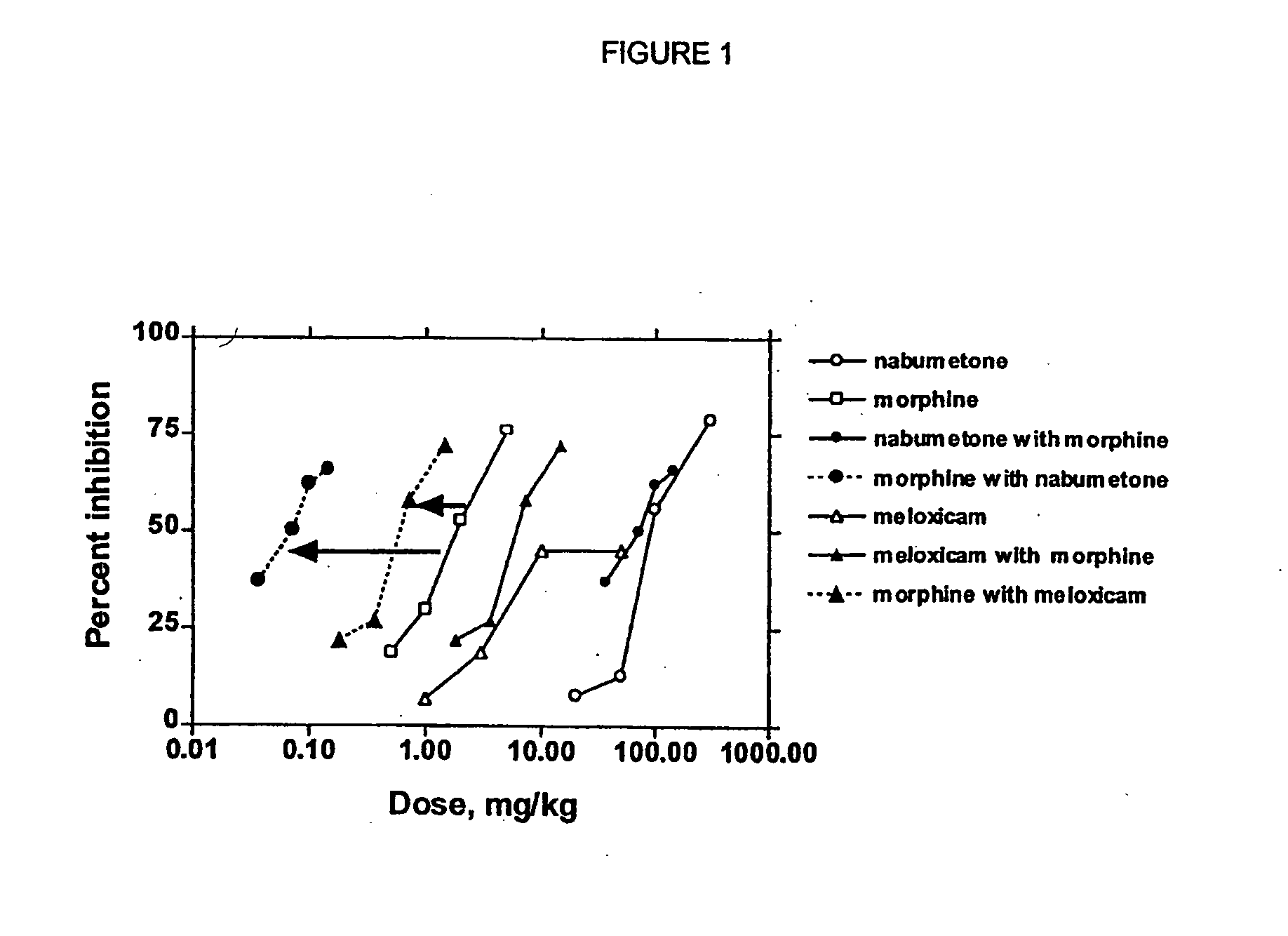
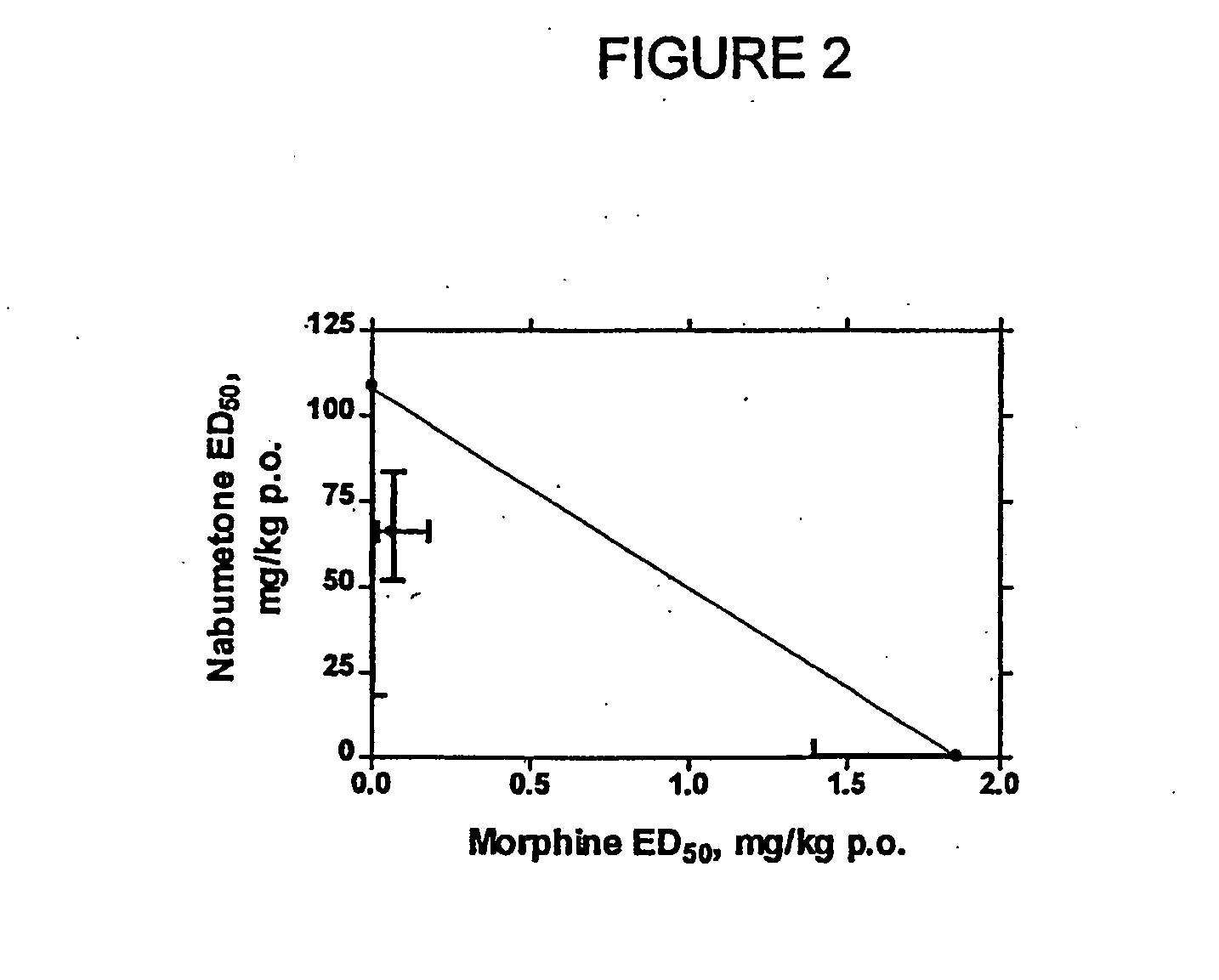
Disclosed is a pharmaceutical composition, comprising a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof, said combination in an amount sufficient to provide an analgesic effect in a human patient. Also disclosed is a method of effectively treating pain in humans or other mammals, comprising administering to the patient a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof such that the dosing interval of the meloxicam overlaps with the dosing interval of the oxycodone, said combination in an amount sufficient to provide an analgesic effect in a human patient.
Owner:PURDUE PHARMA LP
Granule and orally disintegrating tablet comprising oxycodone
The present invention relates to granules comprising oxycodone, as well as to orally disintegrating tablets including same and optionally acetaminophen.
Owner:ETHYPHARM SA
Compositions Comprising An Opioid And An Additional Active Pharmaceutical Ingredient For Rapid Onset And Extended Duration Of Analgesia That May Be Administered Without Regard To Food
The present disclosure provides pharmaceutical compositions comprising an opioid and an additional active pharmaceutical ingredient, wherein the composition exhibits gastric retentive properties which are achieved by a combination of a physical property of the composition and release of the opioid, wherein upon administration to a subject, the composition has at least one pharmacokinetic parameter that differs by less than about 30% when the subject is in a fasted state as compared to a fed state. The present disclosure further provides pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides reduced abuse potential.
Owner:MALLINCKRODT INC
Methods of Reducing Alcohol-Induced Dose Dumping for Opioid Sustained Release Oral Dosage Forms
Disclosed are methods of sustained release administration of opioids, including but not limited to hydromorphone and oxycodone, that exhibit improved properties with respect to co-ingestion with aqueous alcohol.
Owner:ALZA CORP
Novel Opiate Reduction Utilizing Catalytic Hydrogen Transfer Reaction
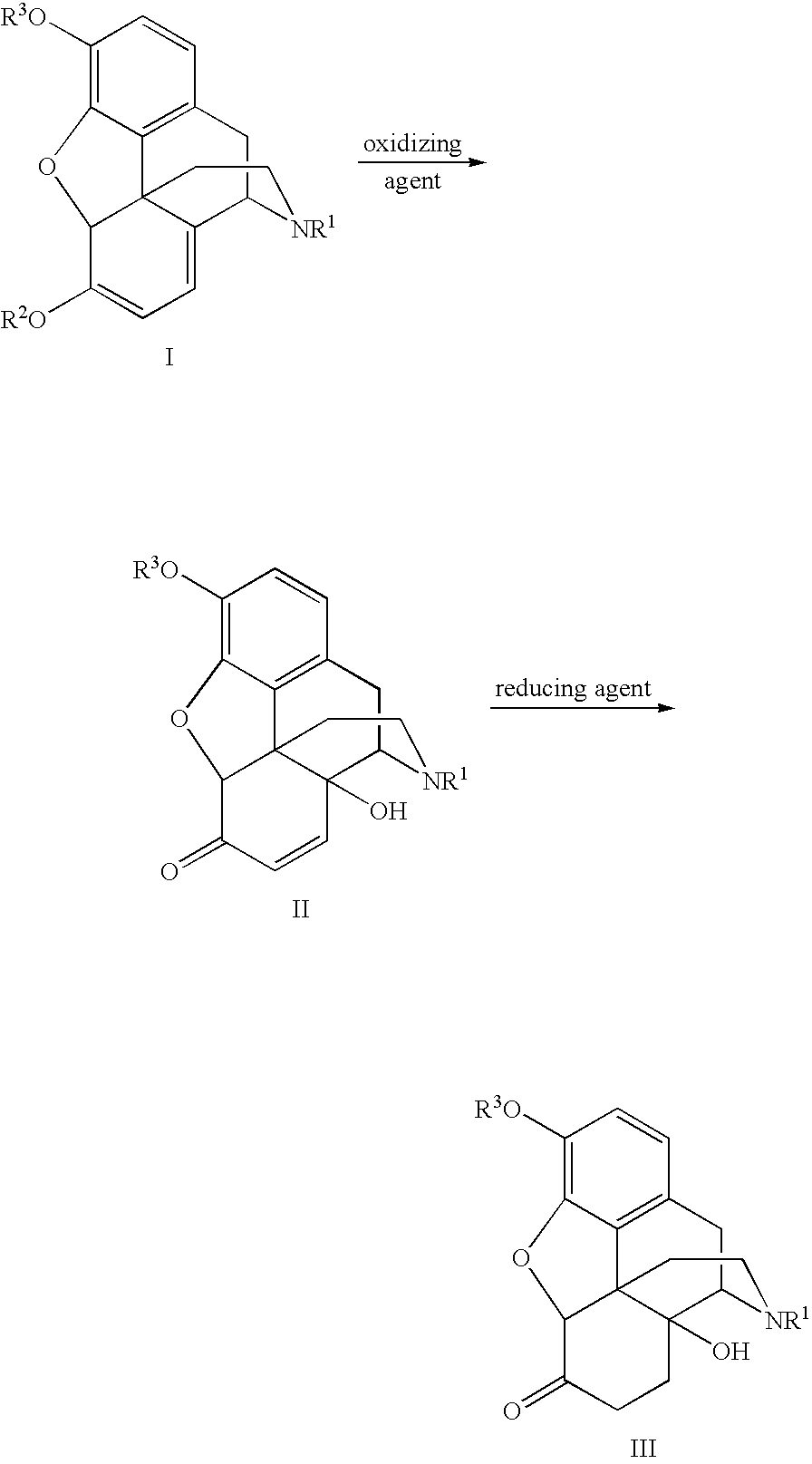
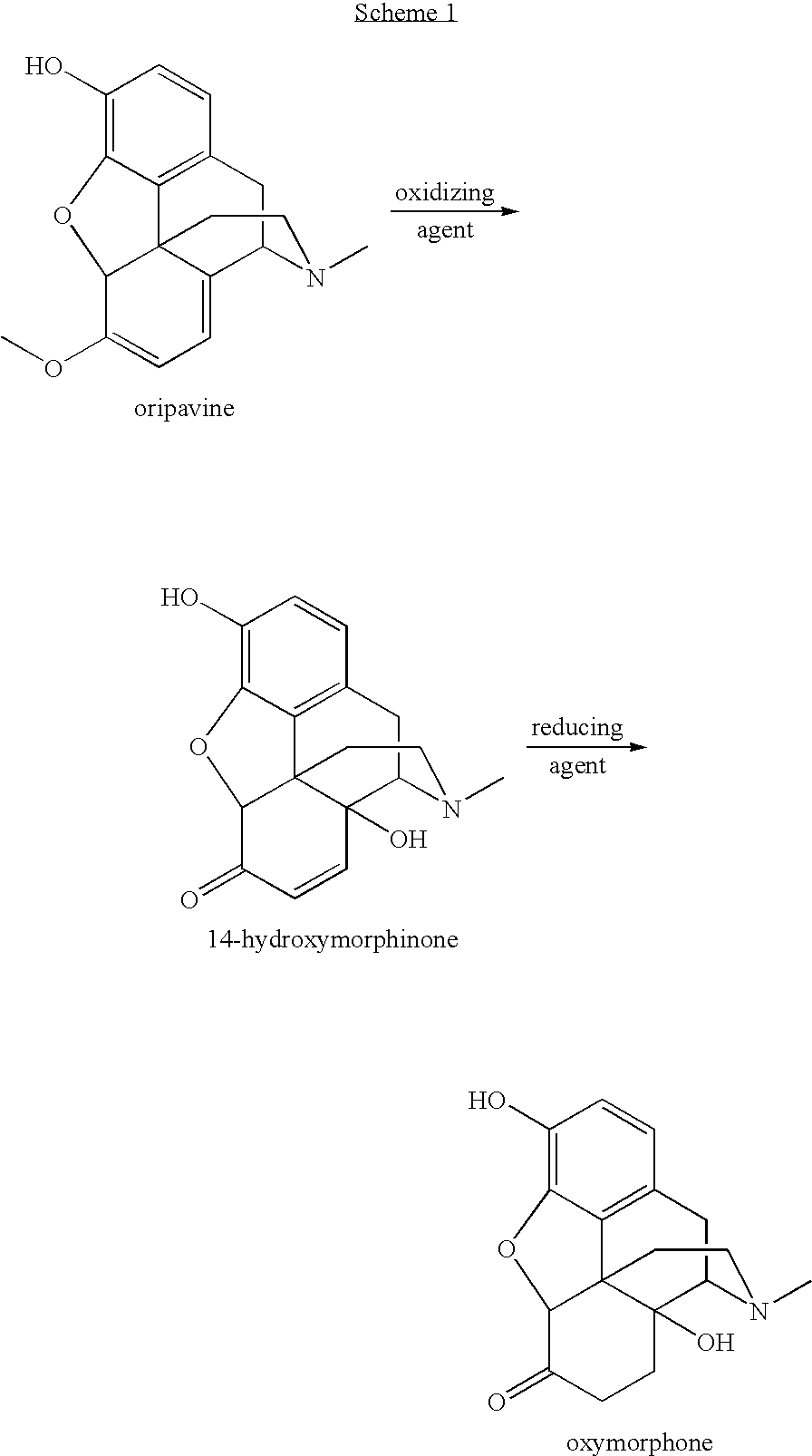
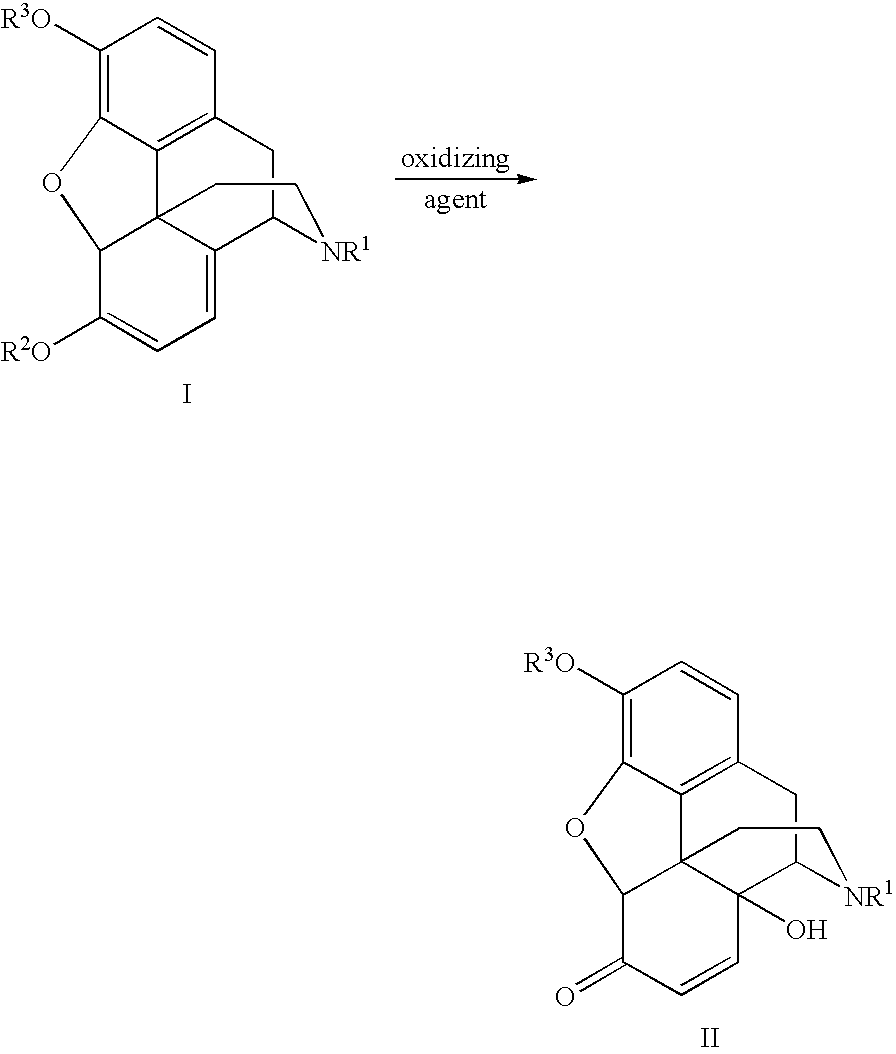
An improved opiate synthesis scheme is provided. An improvement to the oxidation of oripavine and oripavine derivatives comprises the in-situ formation of the peroxacids required to oxidize the oripavine and oripavine derivatives to form an intermediate. An improvement to the reduction of the intermediate to form oxycodone and oxycodone derivatives comprises reduction utilizing a hydrogen transfer reagent. These improvements allow the production of oxycodone and oxycodone derivatives without isolation of the intermediate, providing a one-pot synthesis method.
Owner:SPECGX LLC
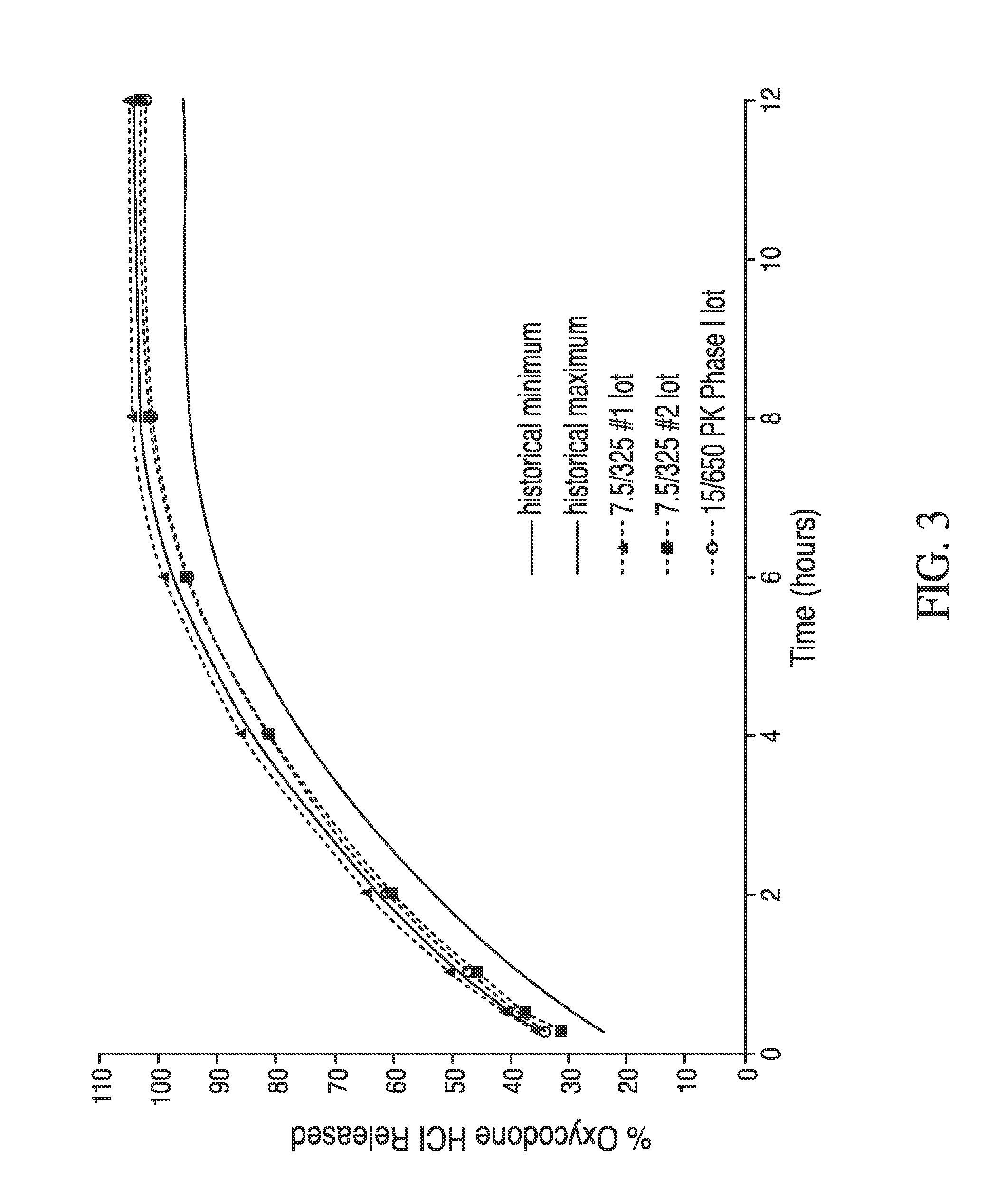
Controlled release oxycodone compositions
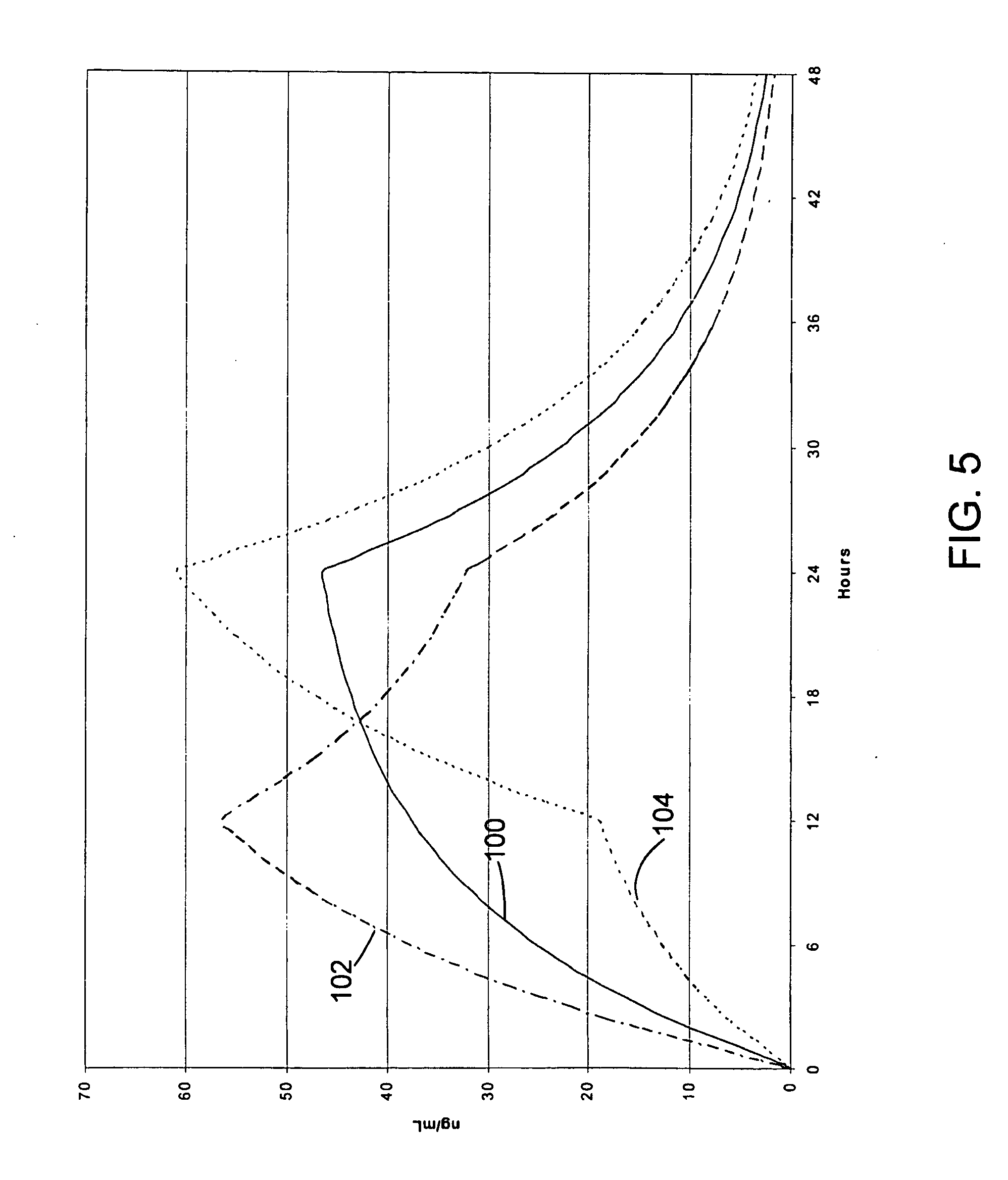
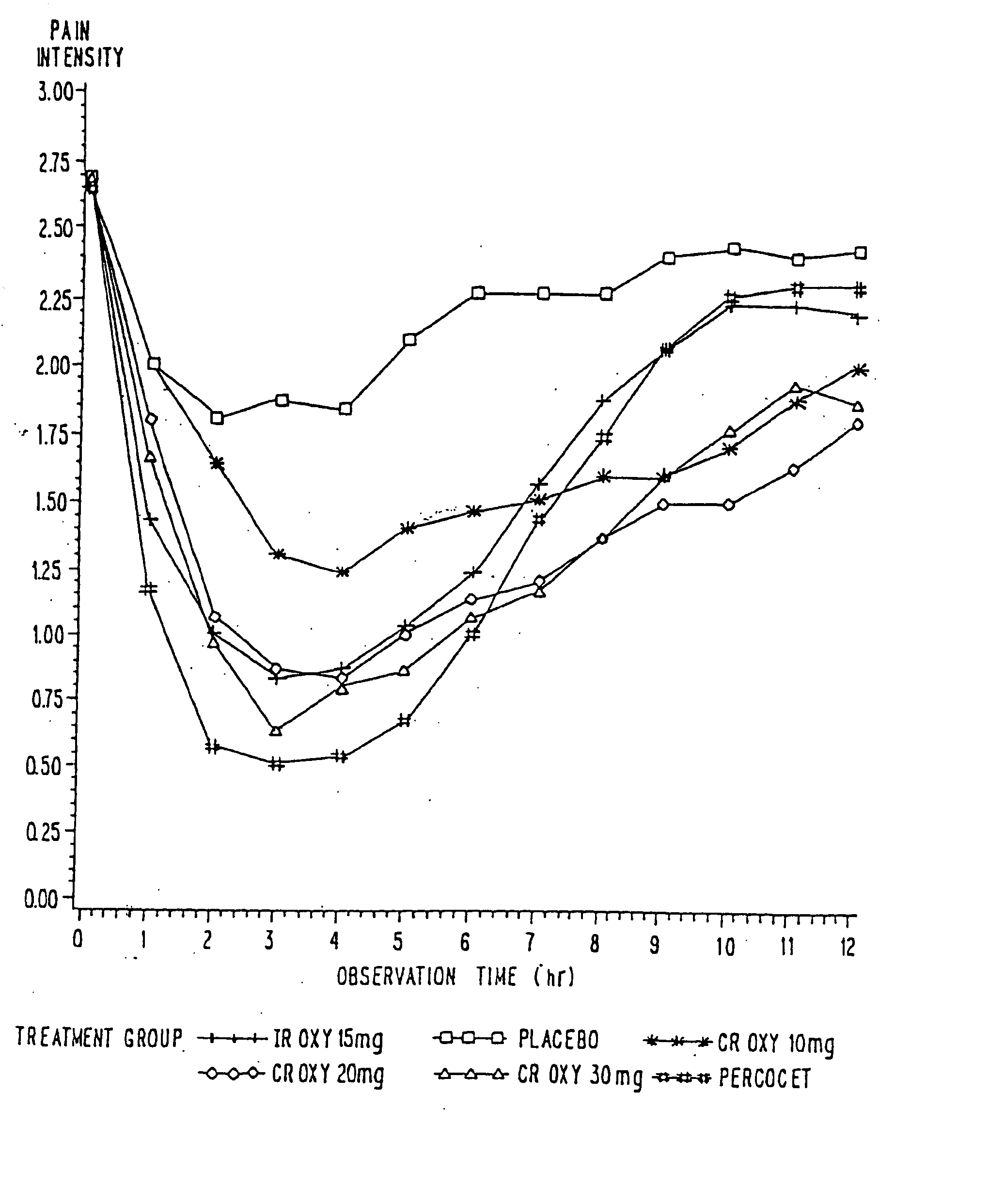
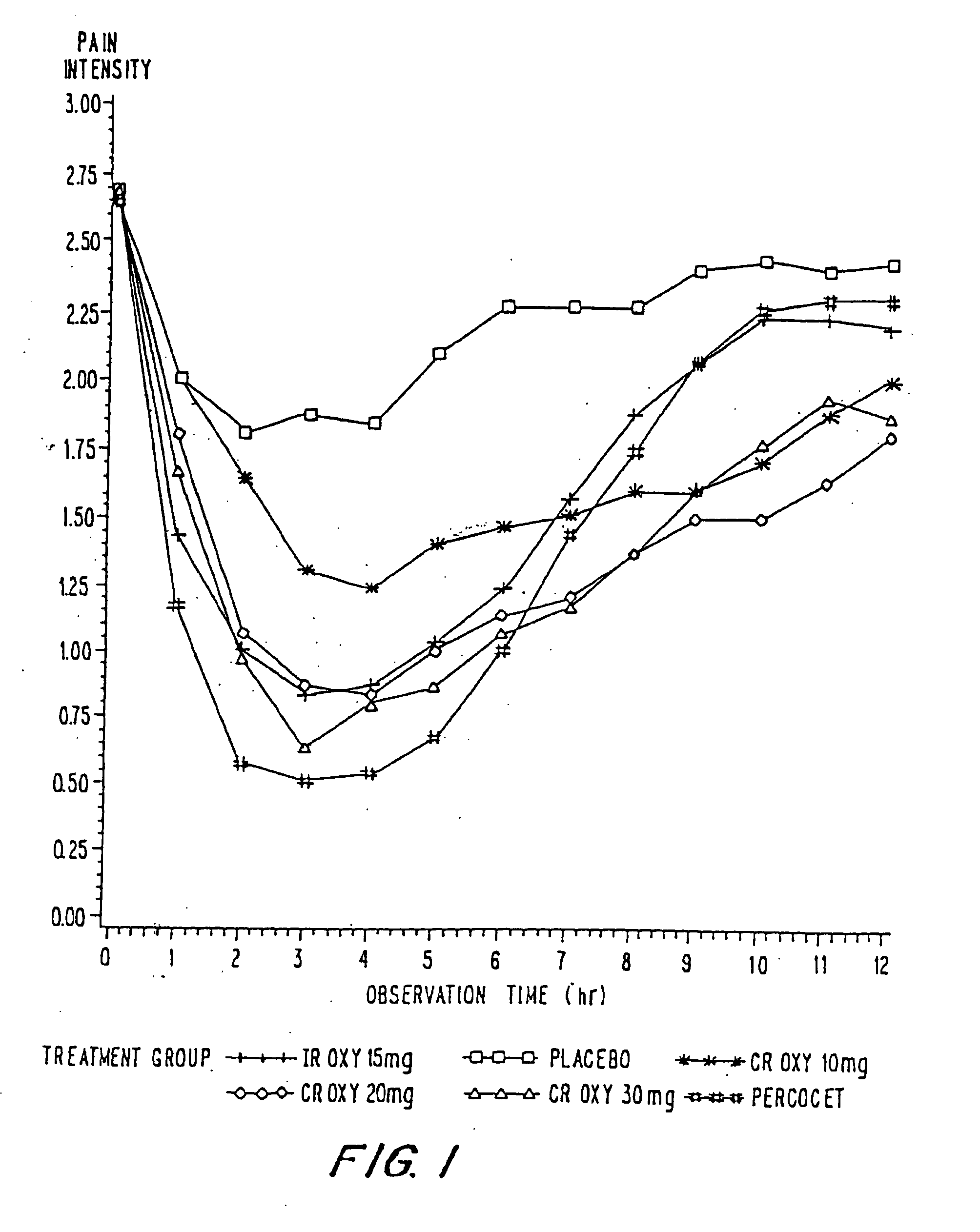
InactiveUS20060057210A1Improve efficiencyQuality improvementBiocidePowder deliveryControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Pharmaceutical combinations of oxycodone and naloxone
InactiveUS7943173B2Reducing abuse potential of dosage formLess abuse potentialPowder deliveryOrganic active ingredientsPharmacologyOxycodone
Owner:PURDUE PHARMA LP
Controlled release oxycodone compositions
InactiveUS20060099255A1Improve efficiency and qualityOrganic active ingredientsNervous disorderControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:OSHLACK BENJAMIN +3
Dosage form containing oxycodone and naloxone
InactiveUS20110172259A1Quick effectEliminate the effects ofBiocideNervous disorderIn vivoBowel function
Owner:PURDUE PHARMA LP
Dual opioid pain therapy
InactiveUS20090291975A1Eliminate side effectsGood analgesic effectBiocideDispersion deliveryPain therapySide effect
Provided are pharmaceutical compositions and methods for the alleviation of pain in a patient with optimal ratios of morphine and oxycodone that provide superior analgesic efficacy and lower incidence of adverse side effects compared to morphine and oxycodone alone. The pharmaceutical compositions comprise morphine and oxycodone, or pharmaceutically acceptable salts thereof, in ratios of about 3 to 2 to about 1 to 2, morphine to oxycodone by weight.
Owner:QRXPHARMA
Compositions comprising enzyme-cleavable oxycodone prodrug
The embodiments provide Compound KC-7, N-1-[(S)-2-(oxycodone-6-enol-carbonyl-methyl-amino)-2-carbonyl-sarcosine-ethyl amine]-arginine-glycine-acetate, or acceptable salts, solvates, and hydrates thereof. The present disclosure also provides compositions, and their methods of use, where the \compositions comprise a prodrug, Compound KC-7, that provides controlled release of oxycodone. Such compositions can optionally provide a trypsin inhibitor that interacts with the enzyme that mediates the controlled release of oxycodone from the prodrug so as to attenuate enzymatic cleavage of the prodrug.
Owner:SIGNATURE THERAPEUTICS
Combination composition comprising oxycodone and acetaminophen for rapid onset and extended duration of analgesia
The present disclosure provides an extended release pharmaceutical composition comprising oxycodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
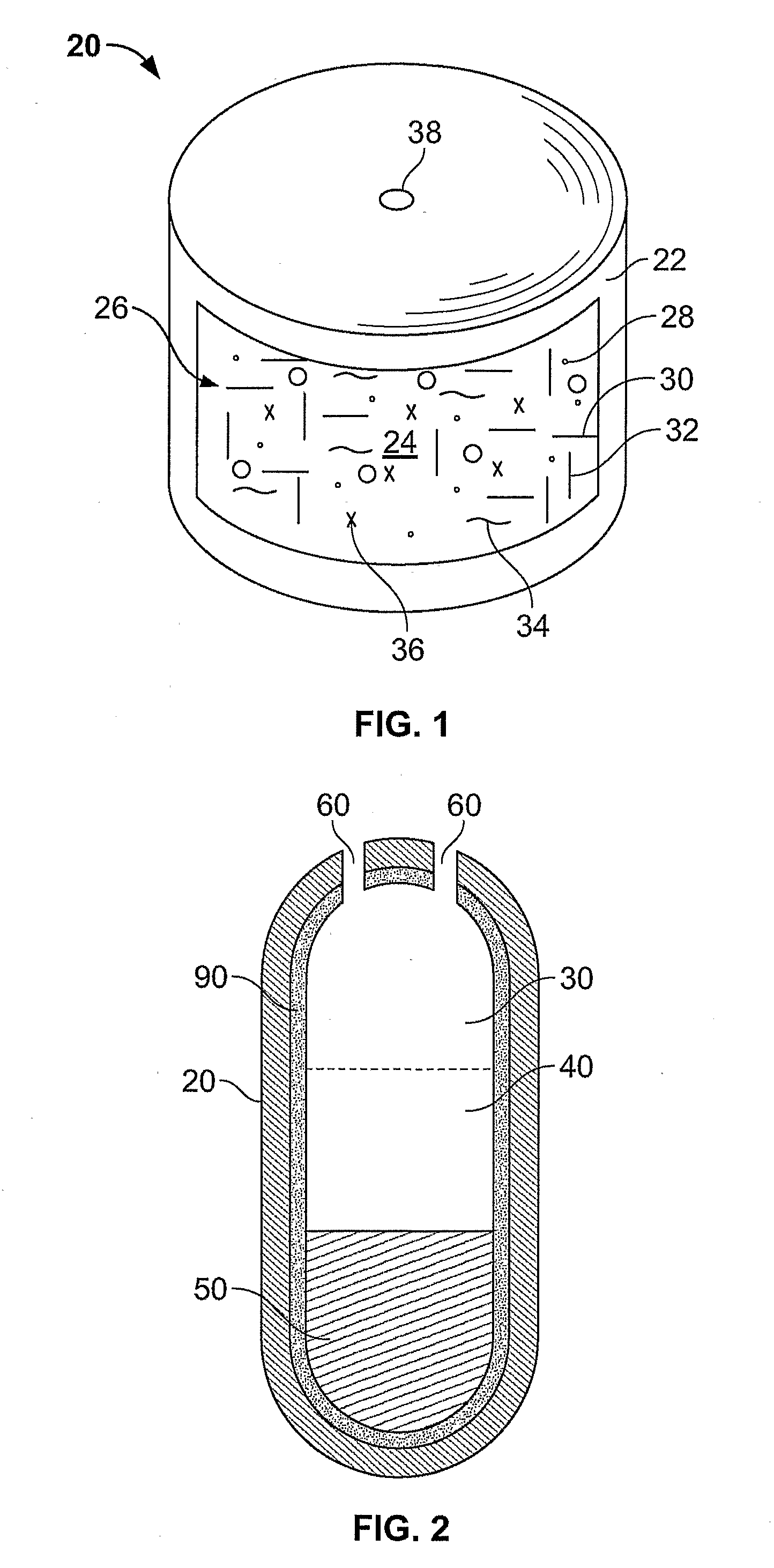
Pharmaceutical Composition Comprising Opioid Agonist And Sequestered Antagonist
This invention pertains to pharmaceutical composition comprising a plurality of multi-layered beads having an oxycodone layer and a sequestering subunit comprising a naltrexone and a blocking agent, in particular pharmaceutical compositions comprising a higher level of naltrexone, and related compositions and methods of use, such as in the prevention of abuse of a therapeutic agent. The compositions of the present invention also have a long Tmax for oxycodone release and a flatter release profile of oxycodone over time.
Owner:WILSON EDWARD S
Alcohol-resistant drug formulations
ActiveUS20200330393A1Eliminate side effectsNervous disorderGranular deliverySleep arousalParacetamolum
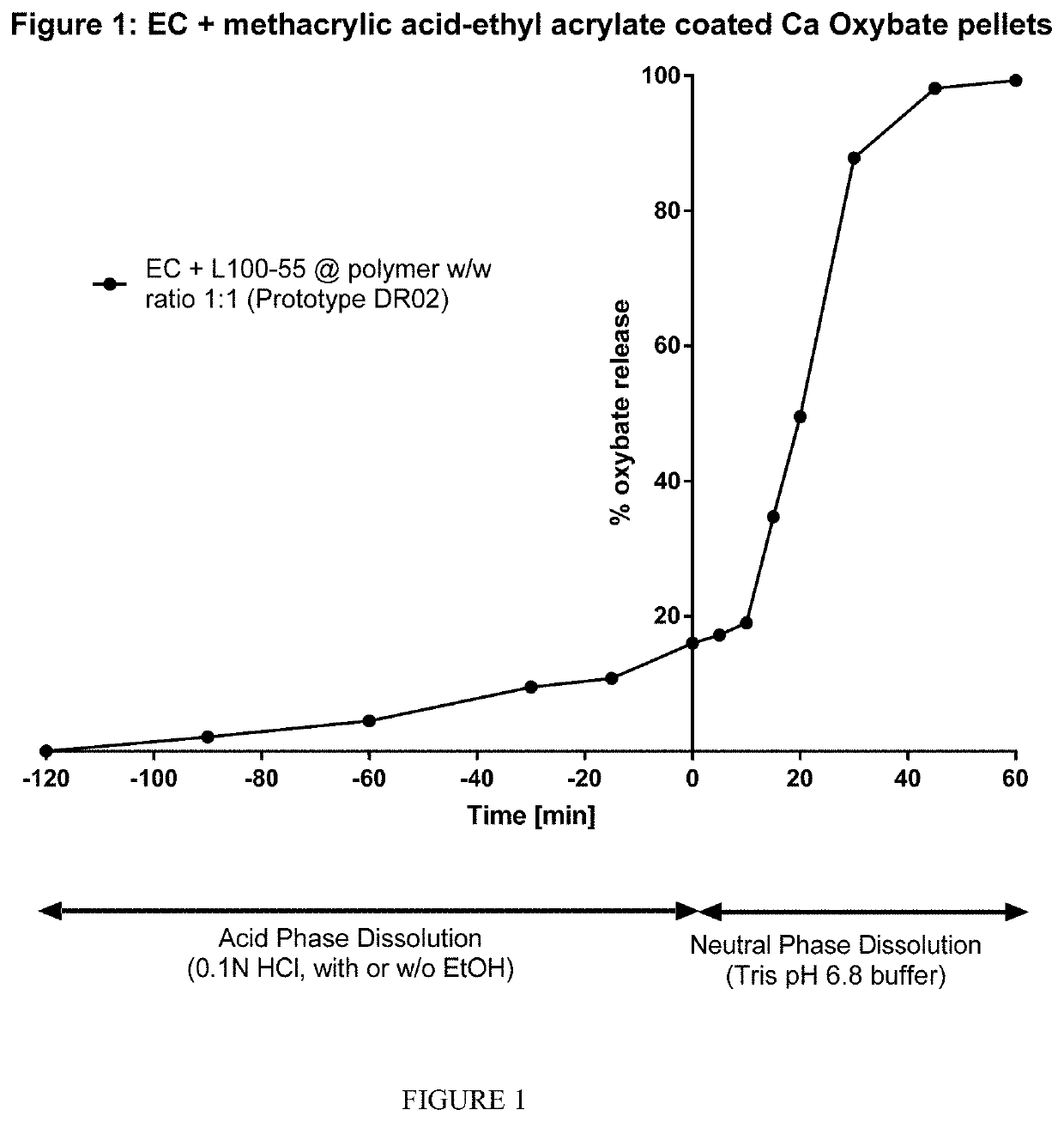
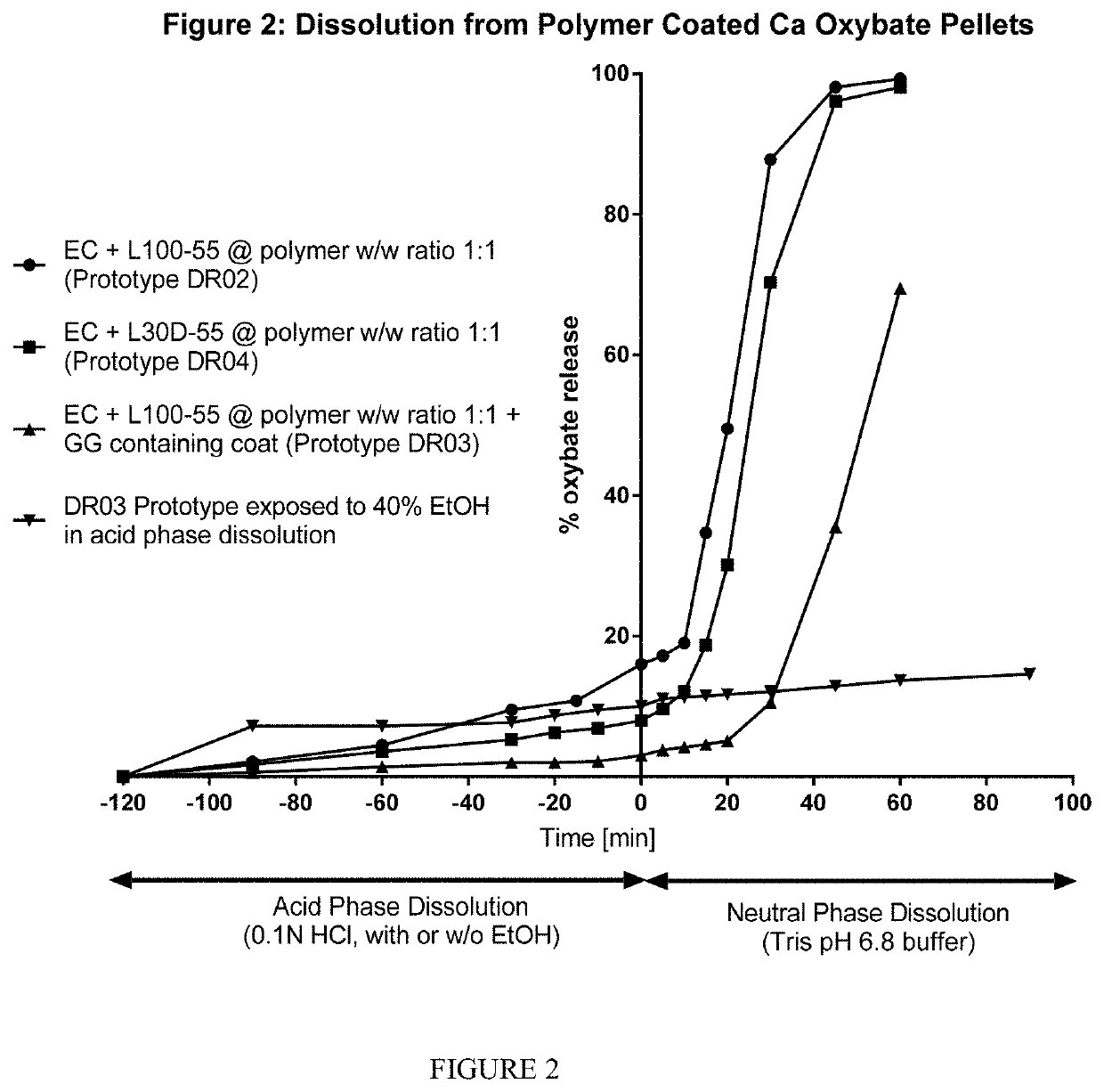
The invention relates to modified release oral formulations of therapeutic agents, including gamma hydroxybutyrate (GHB), paracetamol, codeine or oxycodone, which are resistant to alcohol induced dose dumping. Provided are formulations that have improved resistance to rapid release of the active ingredient in the presence of increasing amounts of alcohol. Also provided are formulations that can reduce or prevent the release of the active ingredient following exposure to alcohol-containing media. The invention also relates to methods of making the formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Controlled release oxycodone compositions
InactiveUS20100092570A1Improve efficiency and qualityLow variabilityBiocidePowder deliveryControl releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +1
Controlled release oxycodone compositions
InactiveUS20100034876A1Improve efficiency and qualityBiocidePowder deliveryControlled releaseMedicine
Owner:PURDUE PHARMA LP +1
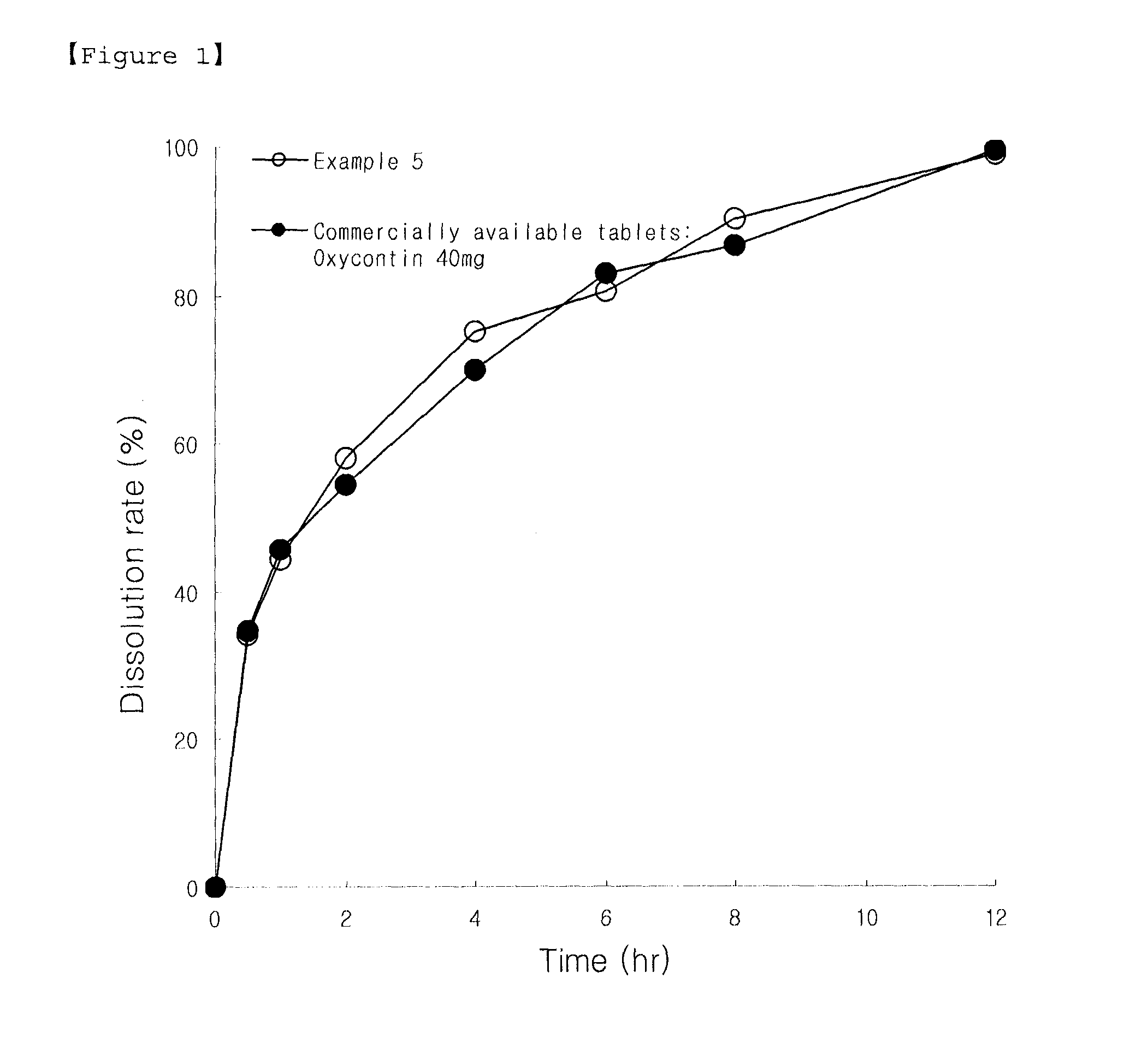
Sustained Release Solid Formulations and Methods of Manufacturing the Same
InactiveUS20110008424A1Easy to compressGood sustained release effectBiocidePowder deliveryWaxWater insoluble
Disclosed are a sustained release solid formulation comprising a drug, for example, oxycodone or its pharmaceutically acceptable salt, in a water-insoluble matrix, which comprises a wax type excipient and copovidone, and thus, has increased compressibility and fluidity and reduced adhesiveness, and a method of preparing the same.
Owner:HANA PHARM CO LTD
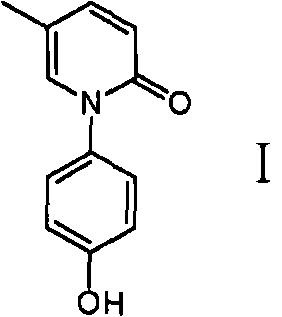
Method for preparing oxycodone
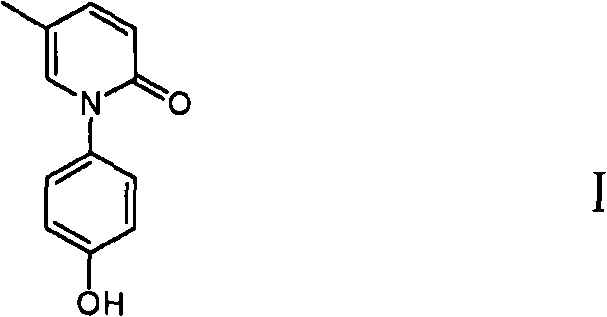
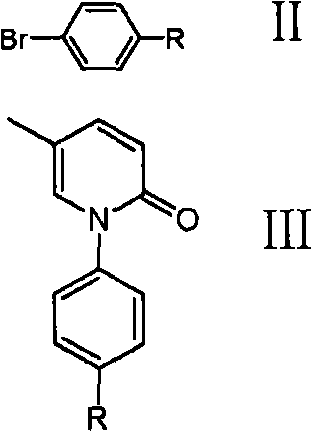
ActiveCN101723883AEasy to operateLow costOrganic chemistryDigestive systemEtherCombinatorial chemistry
The invention discloses a method for preparing a compound shown in a formula I. The method comprises the following steps: (1) mixing 5-methylpyridone and a compound shown in a formula II to obtain a compound shown in a formula III; and (2) reacting the compound shown in the formula III and a dehydroxylation protection reagent to obtain the compound shown in the formula I, wherein R is ether group.
Owner:BEIJING CONTINENT PHARM CO LTD
Controlled release oxycodone compositions
InactiveUS20070275065A1Improve efficiencyQuality improvementBiocidePill deliveryControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately. 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean,of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour): administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Sustained release oxycodone composition with acrylic polymer and surfactant
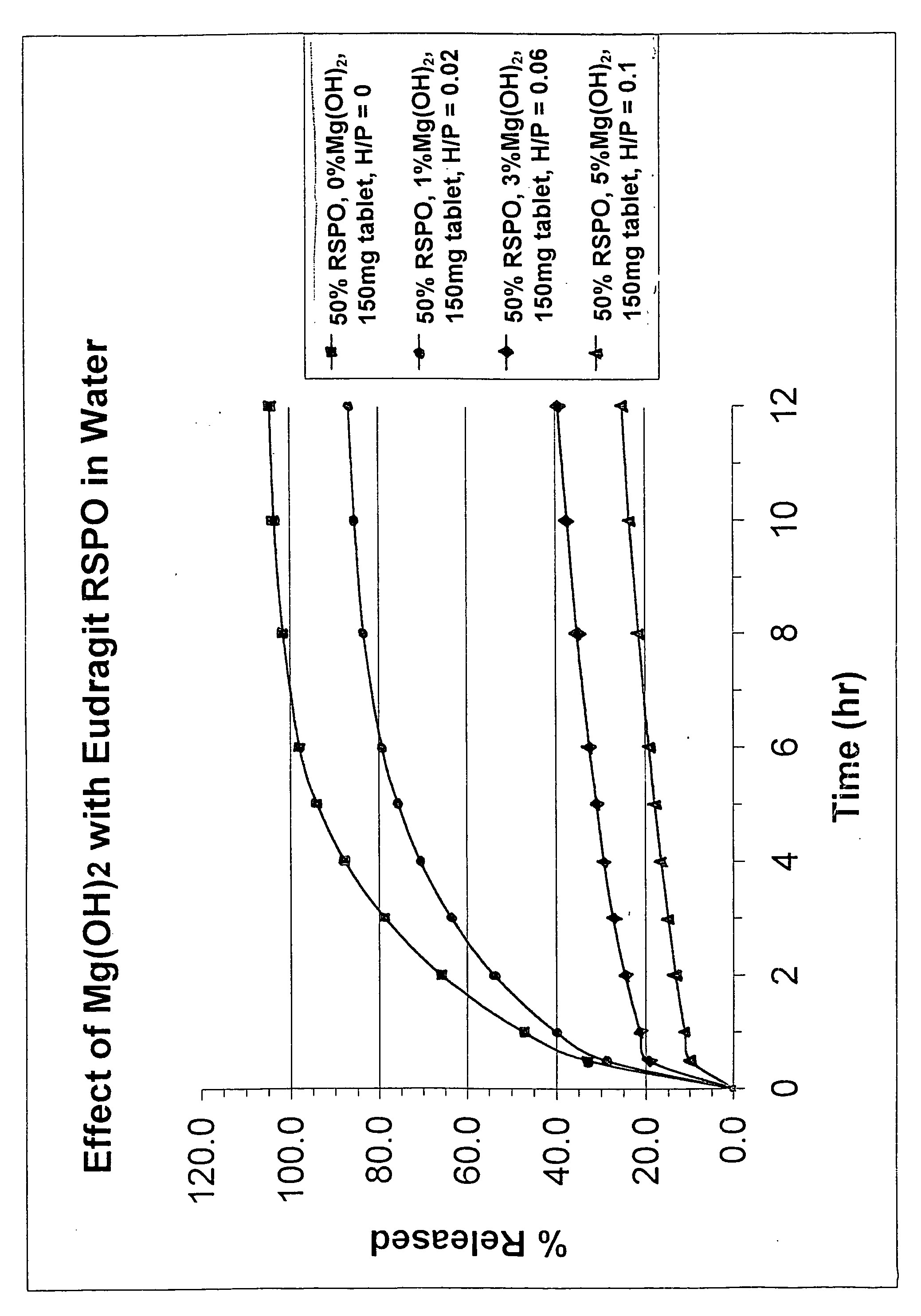
The invention is a controlled release composition comprising a therapeutic amount of an active ingredient in a controlled release matrix. The matrix comprises a combination of a pharmaceutically acceptable acrylic polymer and a metal hydroxide. The amount of surfactant, relative to a given amount of acrylic polymer, is selected for and corresponds to a predetermined release rate for said active ingredient. The compound is preferably used to provide controlled release dosage of oxycodone through a matrix of ammonio methacrylic polymer and sodium lauryl sulfate.
Owner:ENDO PHARMA INC
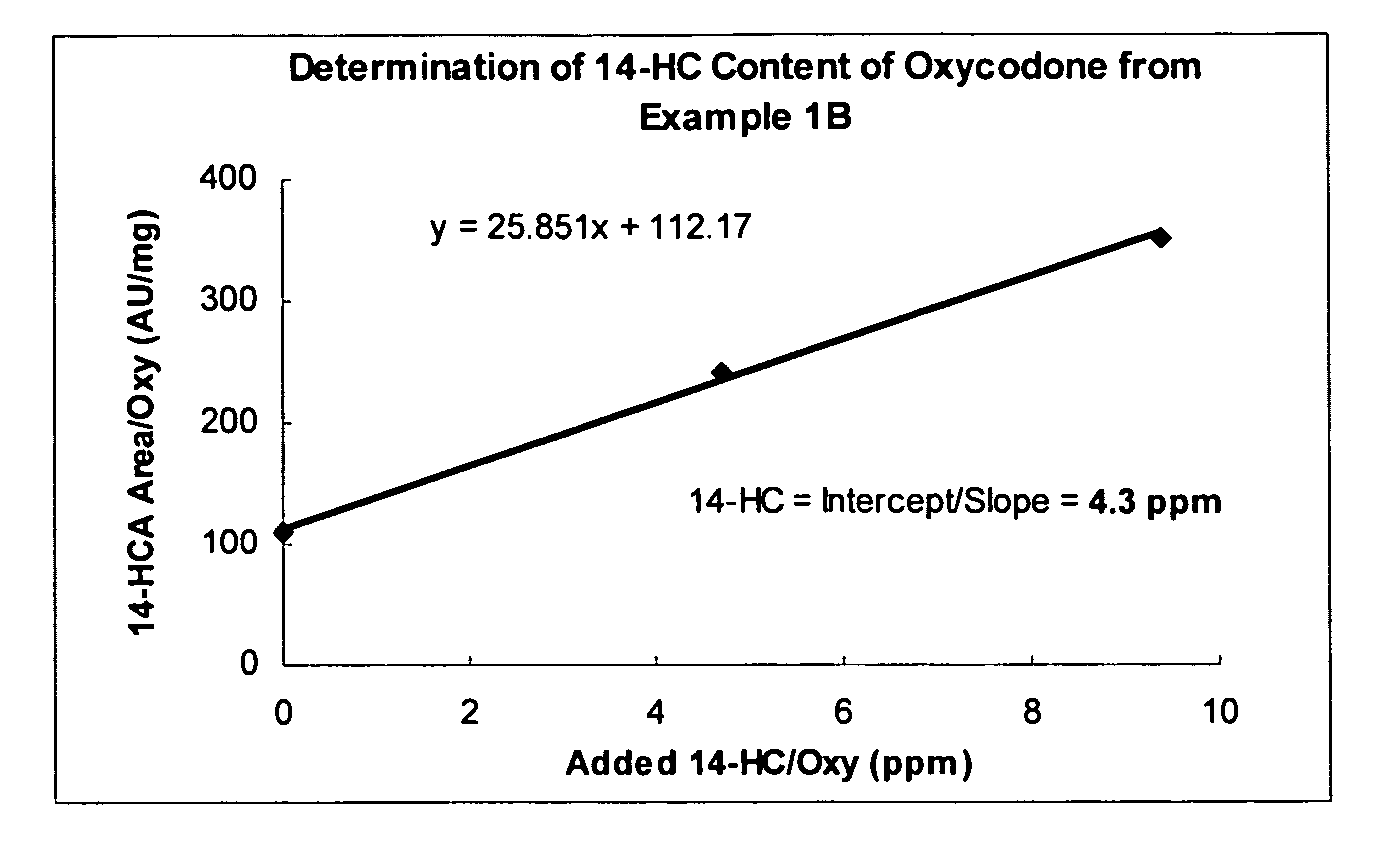
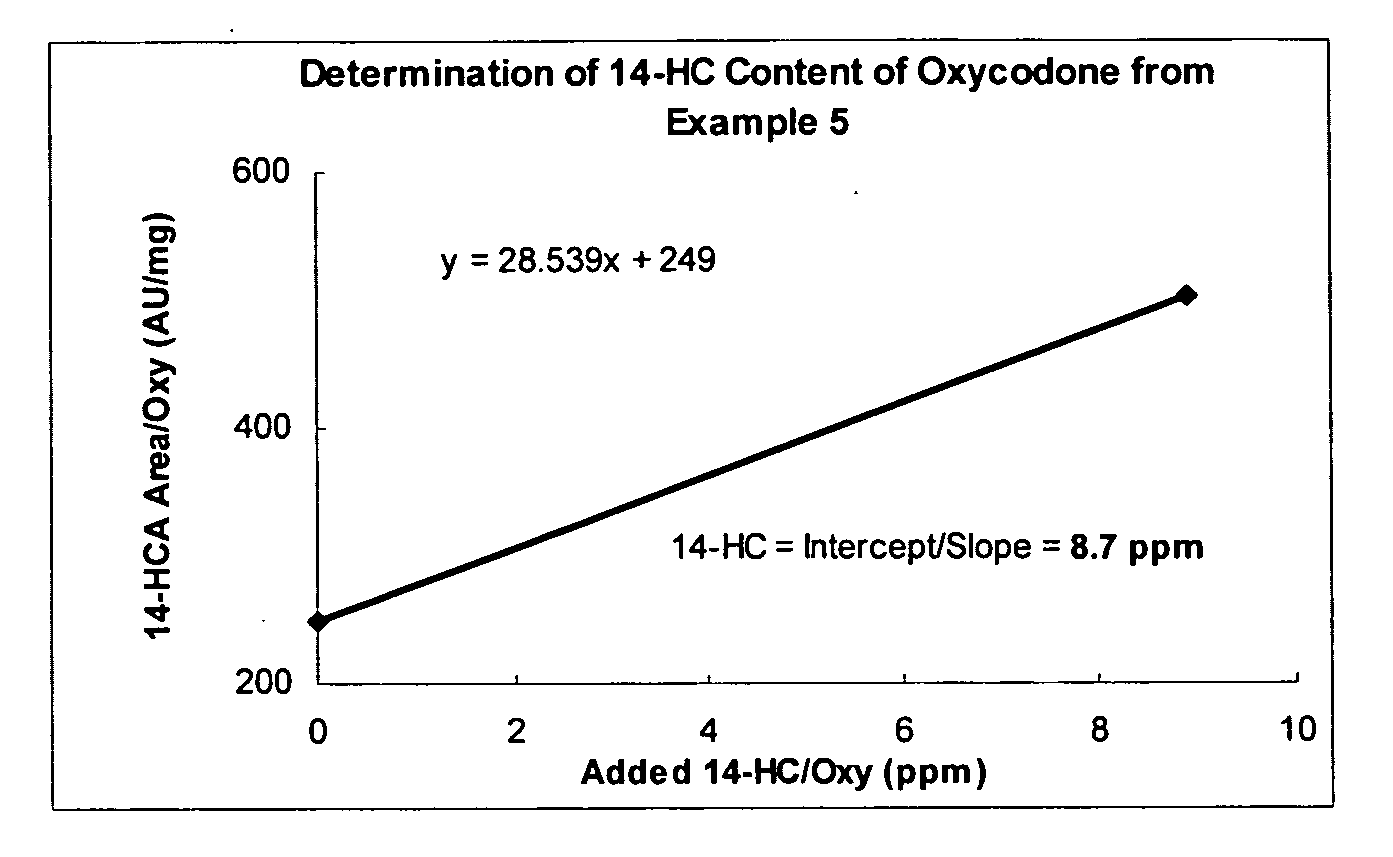
Process for reducing contaminating Michael acceptor levels in oxycodone and other compositions
InactiveUS20070149559A1Low water solubilityReduce solubilityBiocideNervous disorderThiolCompound (substance)
The present invention relates to processes for removal of Michael acceptors from certain compositions wherein the composition is treated with a thiol-containing compound under conditions sufficient to remove Michael acceptors and the resulting thiol-Michael adducts. Certain embodiments of the present invention enable quantification and / or removal of Michael acceptors and / or Michael acceptor precursors.
Owner:CONTROLLED CHEM INC
Pharmaceutical composition containing lappaconitine and oxycodone
ActiveCN103040829AReduce Dependency PotentialReduce dosageOrganic active ingredientsNervous disorderSolventOxycodone
The invention relates to a pharmaceutical composition containing lappaconitine and oxycodone for treating pain. The pharmaceutical composition contains a treatment effective quantity of lappaconitine or a pharmaceutically acceptable salt thereof or a solvate thereof, a treatment effective quantity of oxycodone or a pharmaceutically acceptable salt thereof and an optional pharmaceutically acceptable carrier. The invention further relates to a use of the composition containing the treatment effective quantity of lappaconitine or the pharmaceutically acceptable salt thereof or the solvate thereof and the treatment effective quantity of oxycodone or the pharmaceutically acceptable salt thereof in the preparation of medicines for relieving pain. The pharmaceutical composition and the use, disclosed by the invention, have the beneficial effects as described in the specification.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
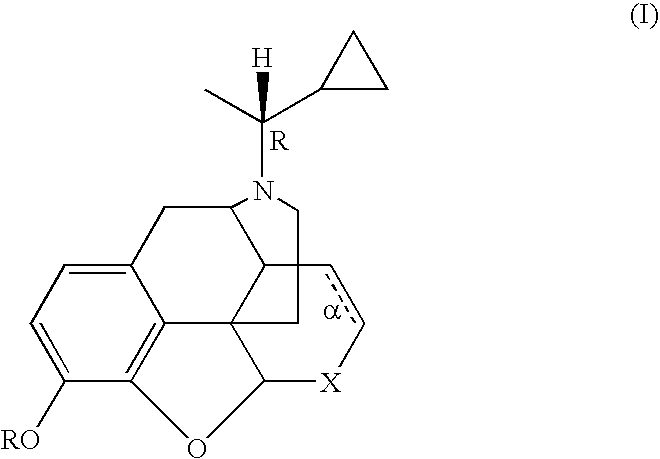
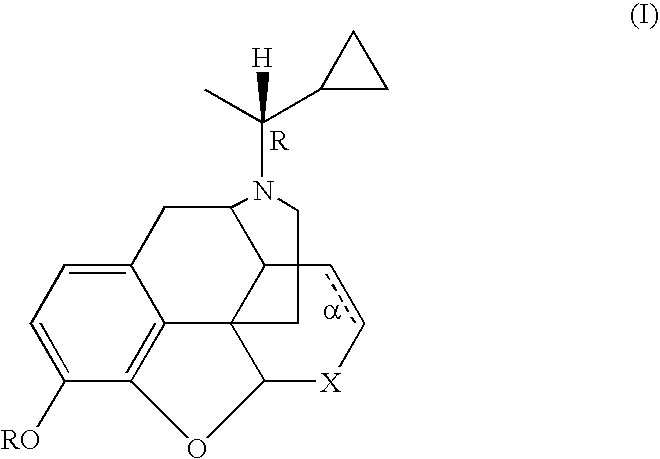
Treatment of chemical dependency with substantially nonaddicting normorphine and norcodeine derivatives
A method is provided for treating a drug-dependent individual so as to effect withdrawal from a drug of abuse, e.g., an opiate such as heroin or oxycodone, a stimulant such as cocaine, or alcohol. The method involves substitution therapy wherein a substantially nonaddicting normorphine or norcodeine derivative is substituted for the drug of abuse. The active agent has the structure of formula (I) wherein: R is H, alkyl, or acyl; X is CH(OR′) or C═O, wherein R′ is H or acyl; α is an optional double bond, with the proviso that when α is present, then X is necessarily CH(OH), or an acid addition salt thereof, wherein preferred such agents are in a stereoisomerically pure form that corresponds to that of N-[(1R)-1-cyclopropylethyl]-normorphine (1) which melts at approximately 188° C.-189° C.
Owner:LAWSON JOHN A
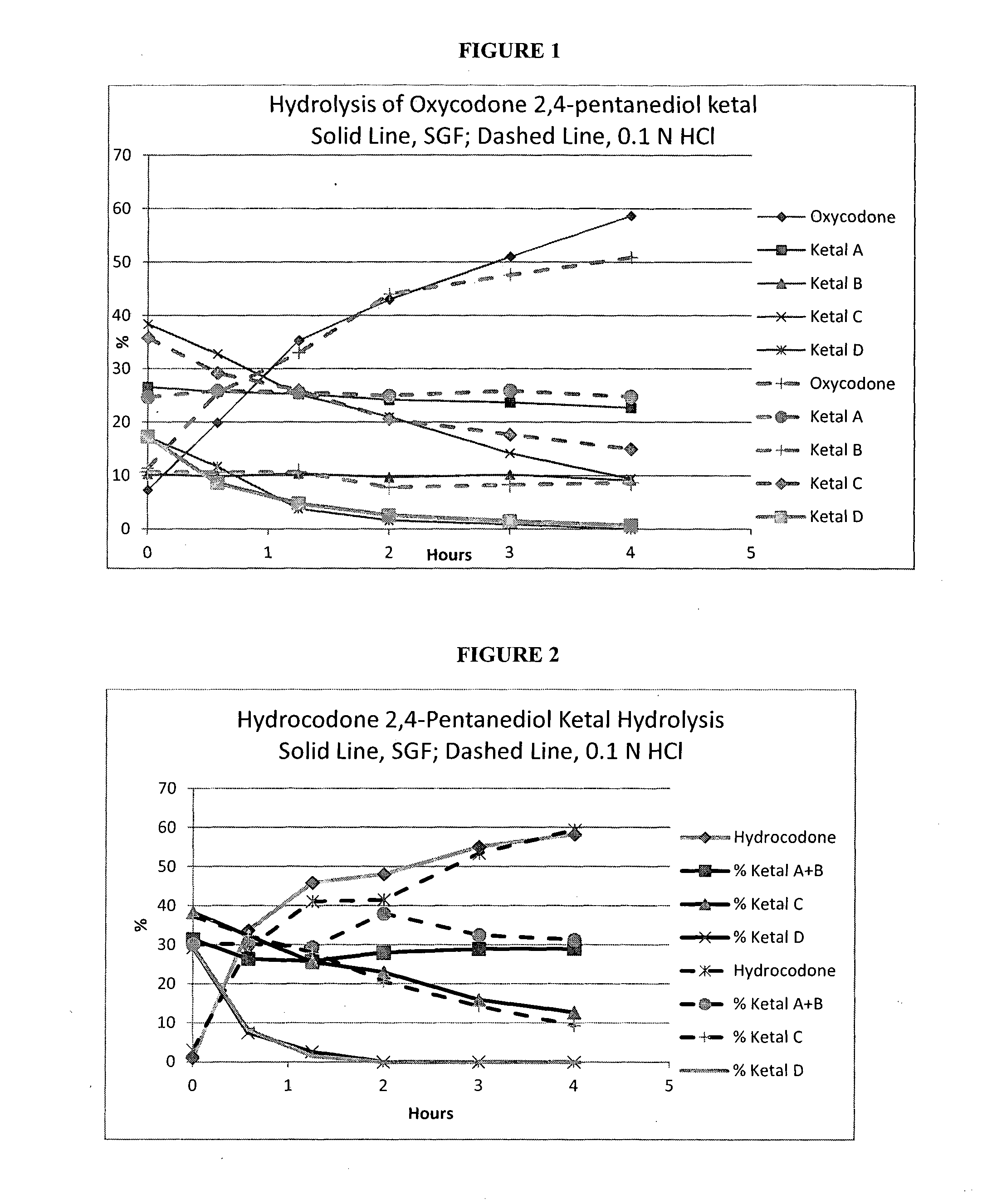
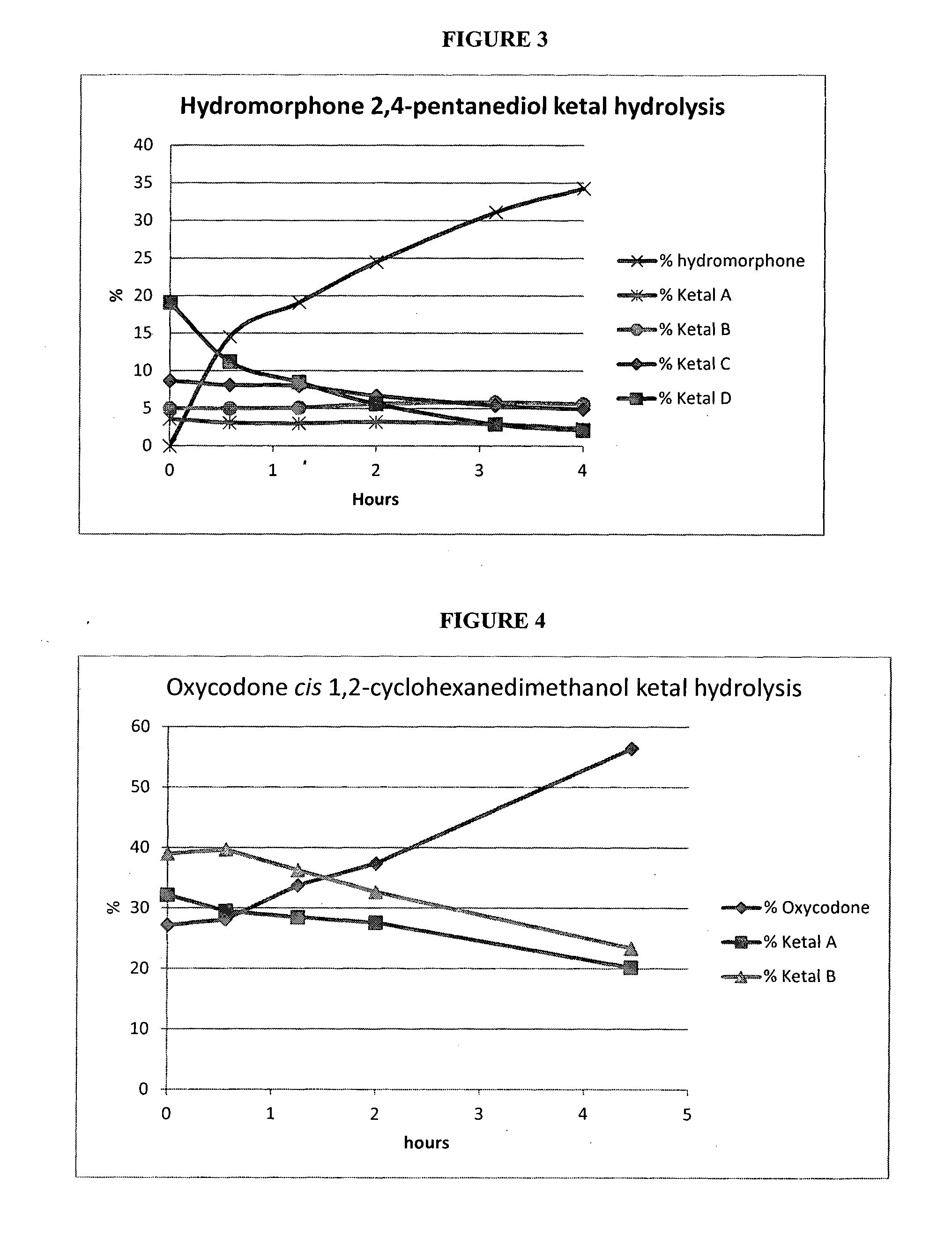
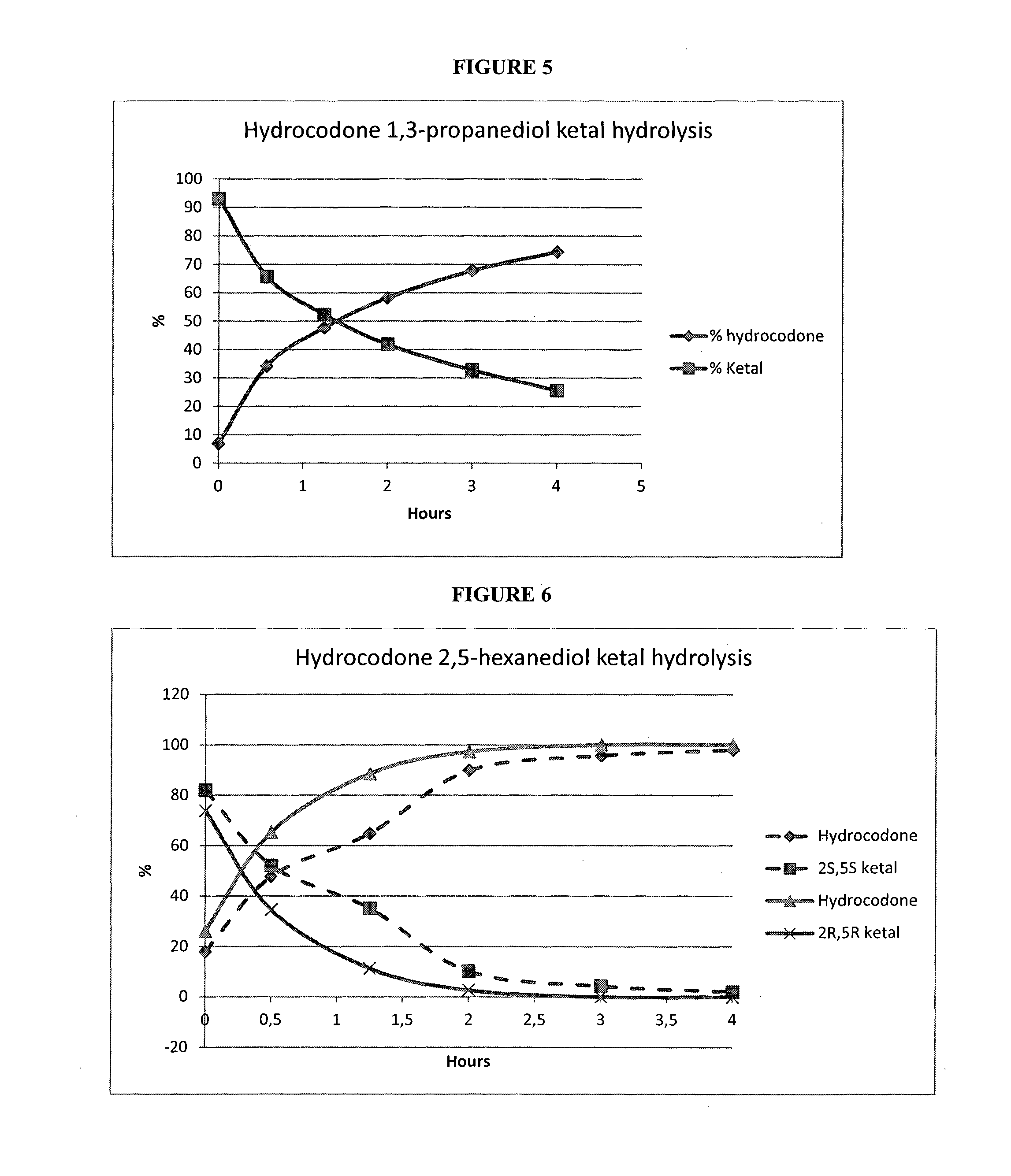
Opioid ketal compounds and uses thereof
InactiveUS20160244459A1Treating and ameliorating and preventing painAvoid painOrganic active ingredientsNervous disorderMedicinal chemistryCarbon atom
This invention relates to opioid ketal compounds of Formula (I), Formula (II), or Formula (III): or a pharmaceutically acceptable salts thereof, wherein R1 is H or CH3, R2 is H or OH, n is 0, 1, 2 or 3, R3 and R4 are independently H or optionally substituted C1-C4 alkyl, or when n is 0, then R3 and R4 and the carbon atoms to which they are attached together form six, or seven membered ring, which is optionally mono or disubstituted by C1-C4 alkyl. The invention also relates to oxycodone ketal compounds of Formula (IV) or (V): or a pharmaceutically acceptable salts thereof. The invention also relates to the use of such compounds for the treatment, prevention, or amelioration of pain.
Owner:NORDBOTICS INC
Sustained release oxycodone composition with acrylic polymer and metal hydroxide
The invention is a controlled release composition comprising a therapeutic amount of an active ingredient in a controlled release matrix. The matrix comprises a combination of a pharmaceutically acceptable acrylic polymer and a metal hydroxide. The amount of metal hydroxide, relative to a given amount of acrylic polymer, is selected for and corresponds to a pre-determined release rate for said active ingredient. The compound is preferably used to provide controlled release dosage of oxycodone through a matrix of ammonio methacrylic polymer and magnesium hydroxide.
Owner:ENDO PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com