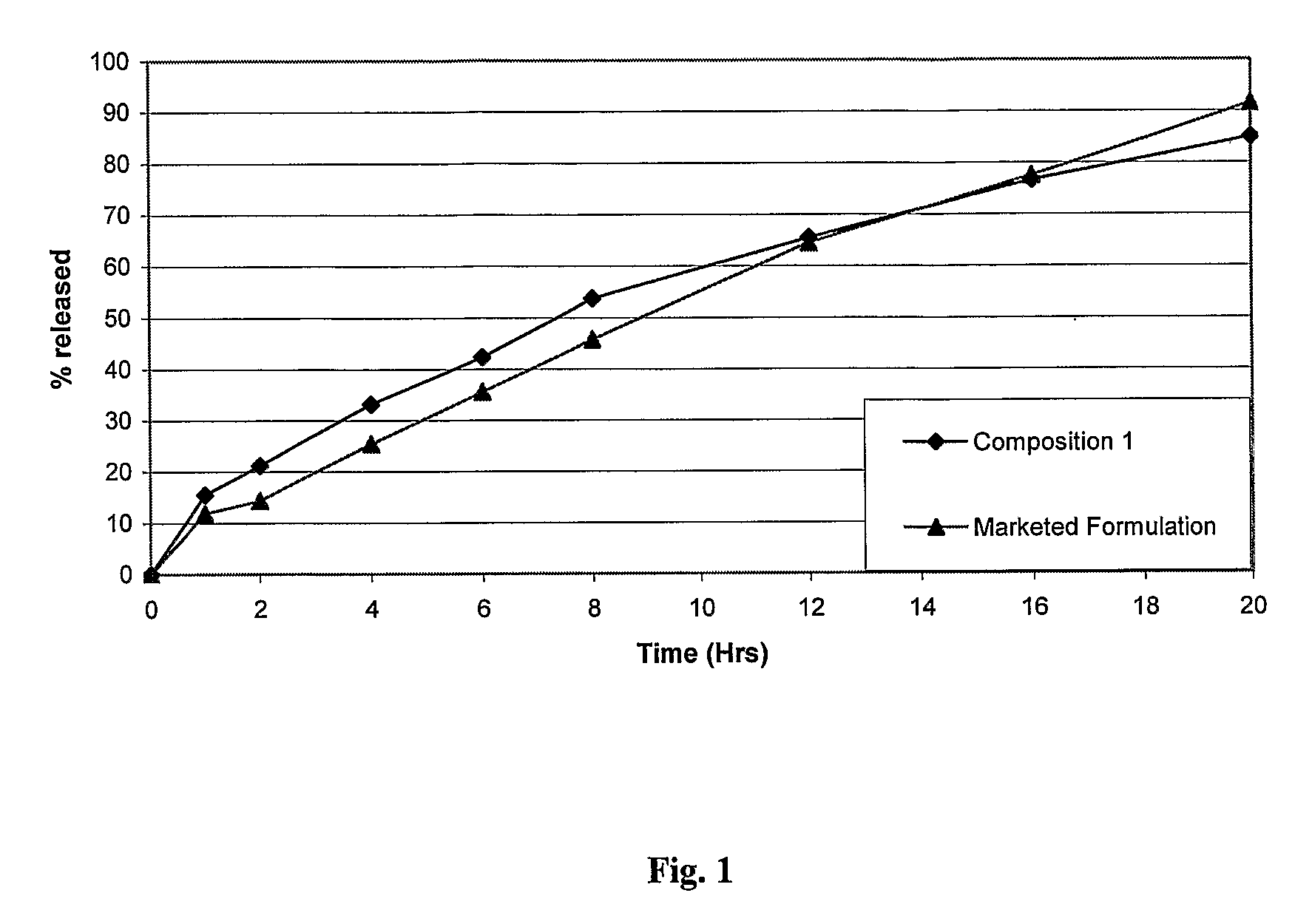
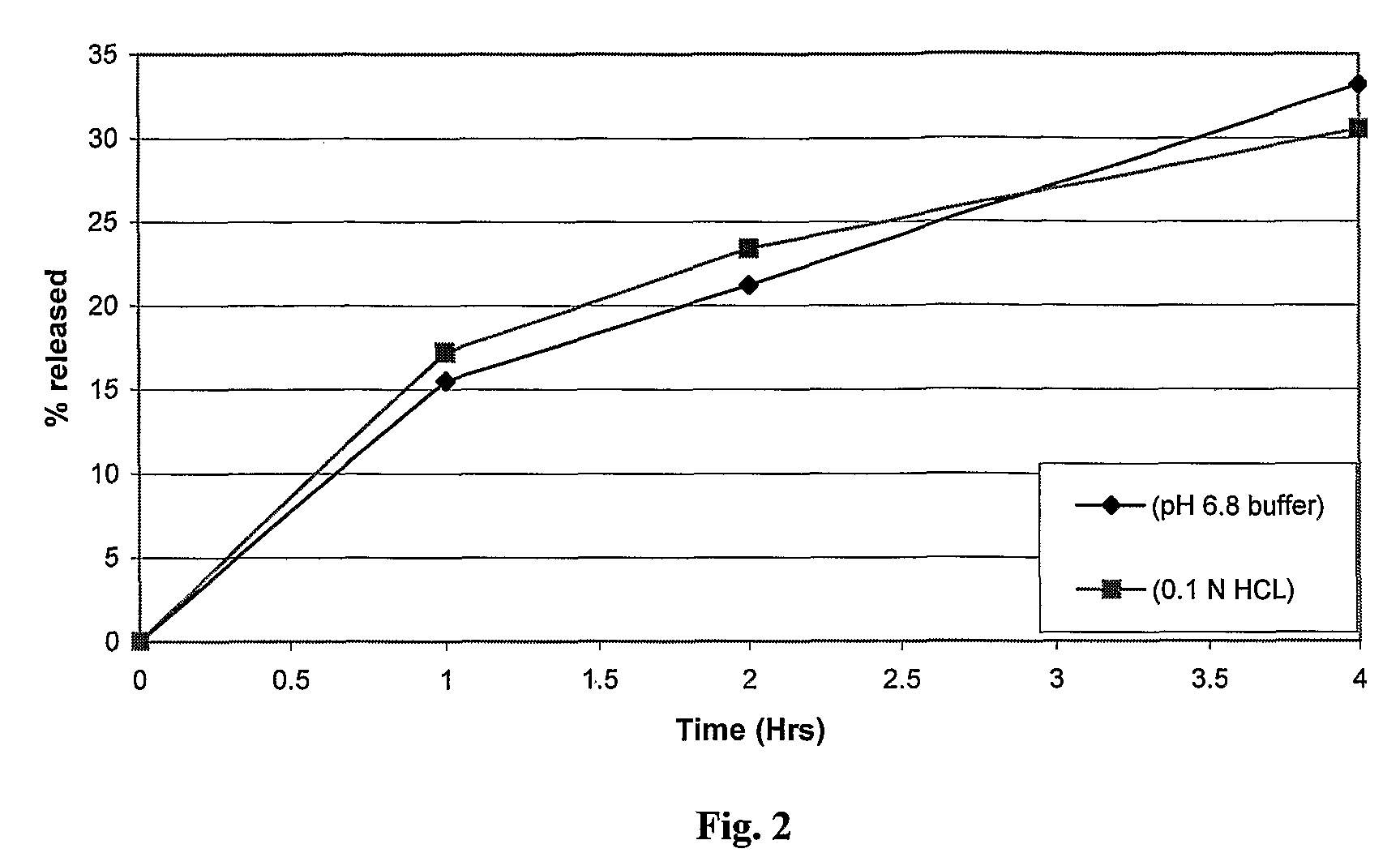
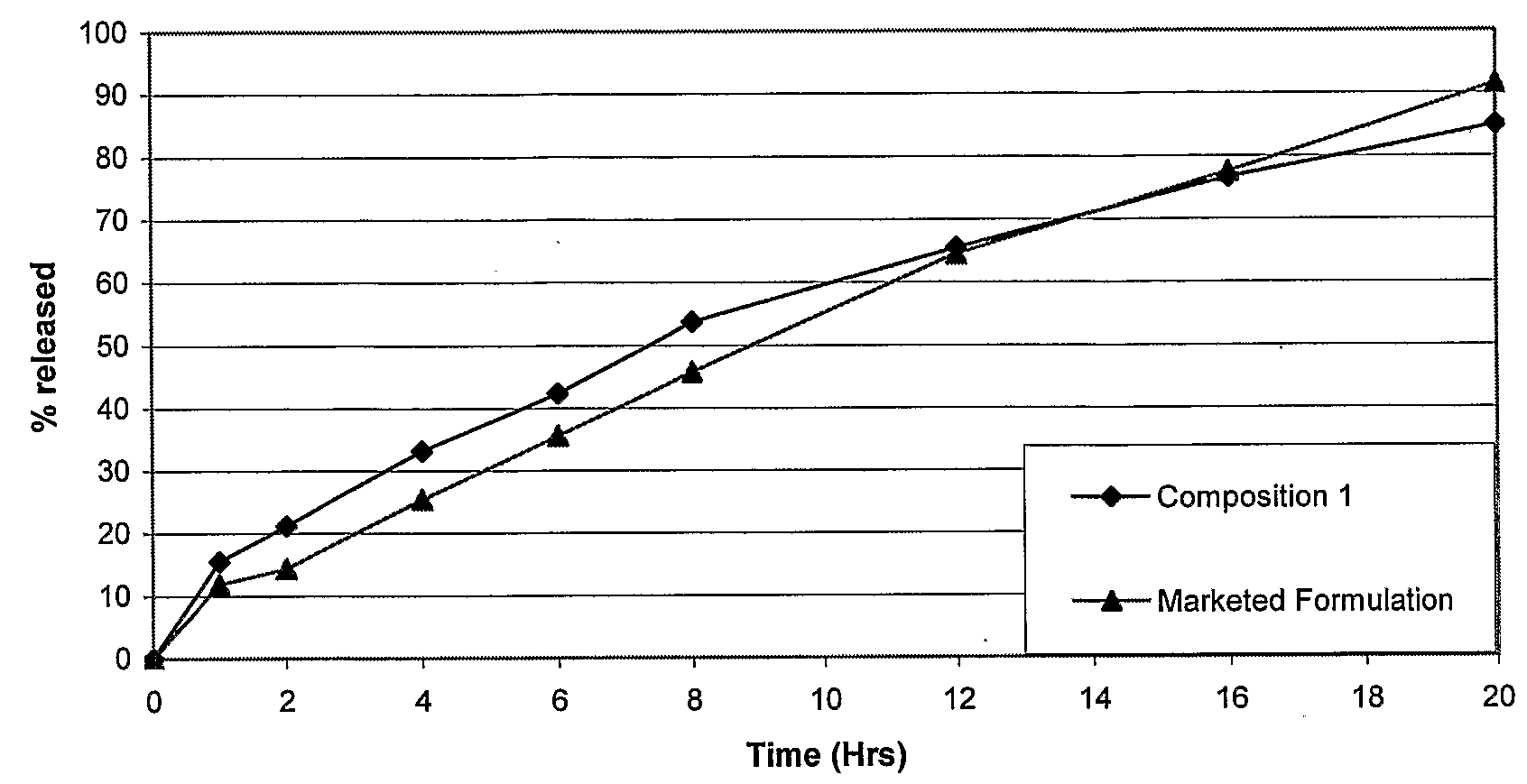
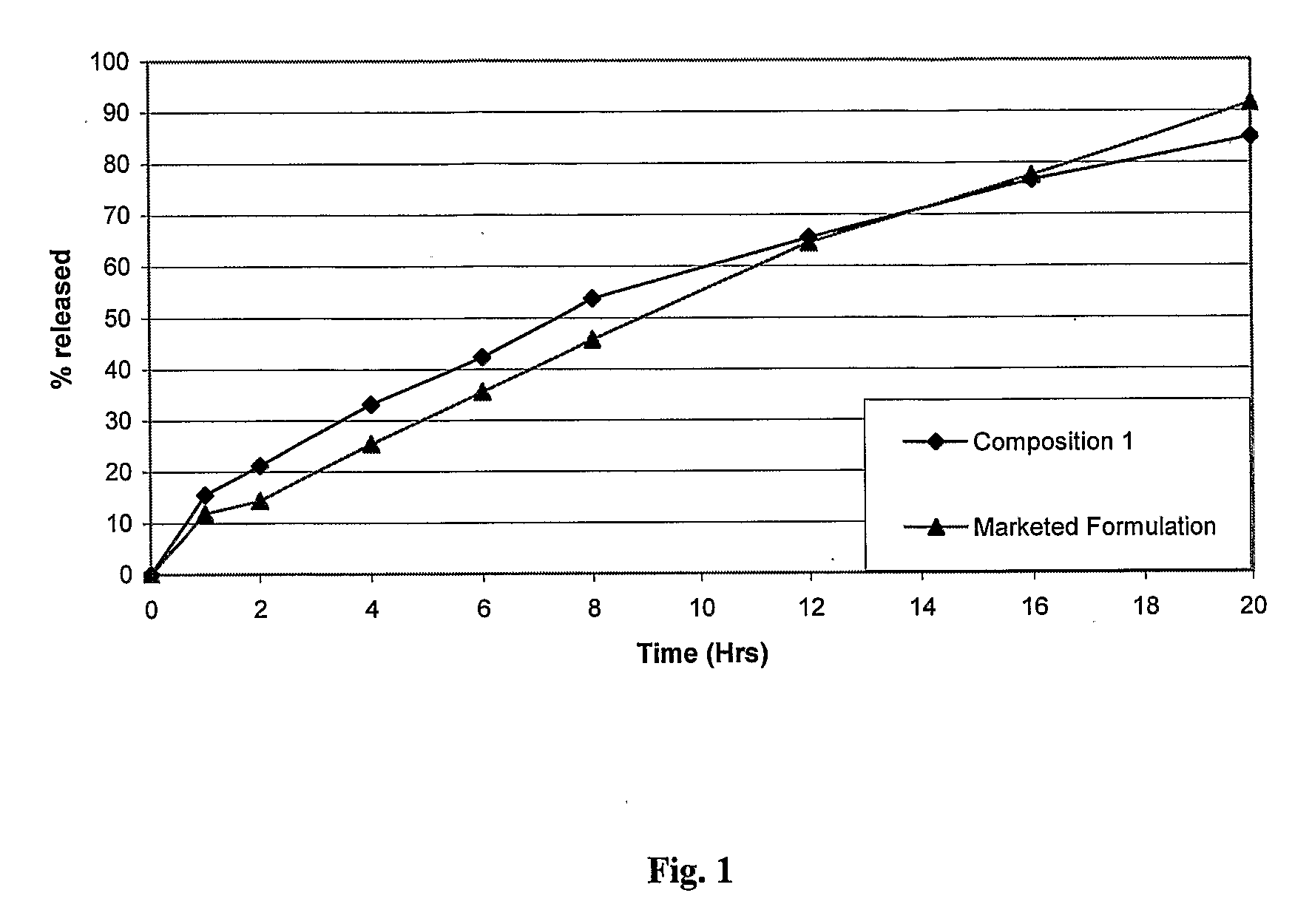
Patents
Literature
71 results about "Ph independent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multiparticulates
InactiveUS20080260815A1Reduce adhesionReduce the required powerOrganic active ingredientsPowder deliveryActive agentExcipient
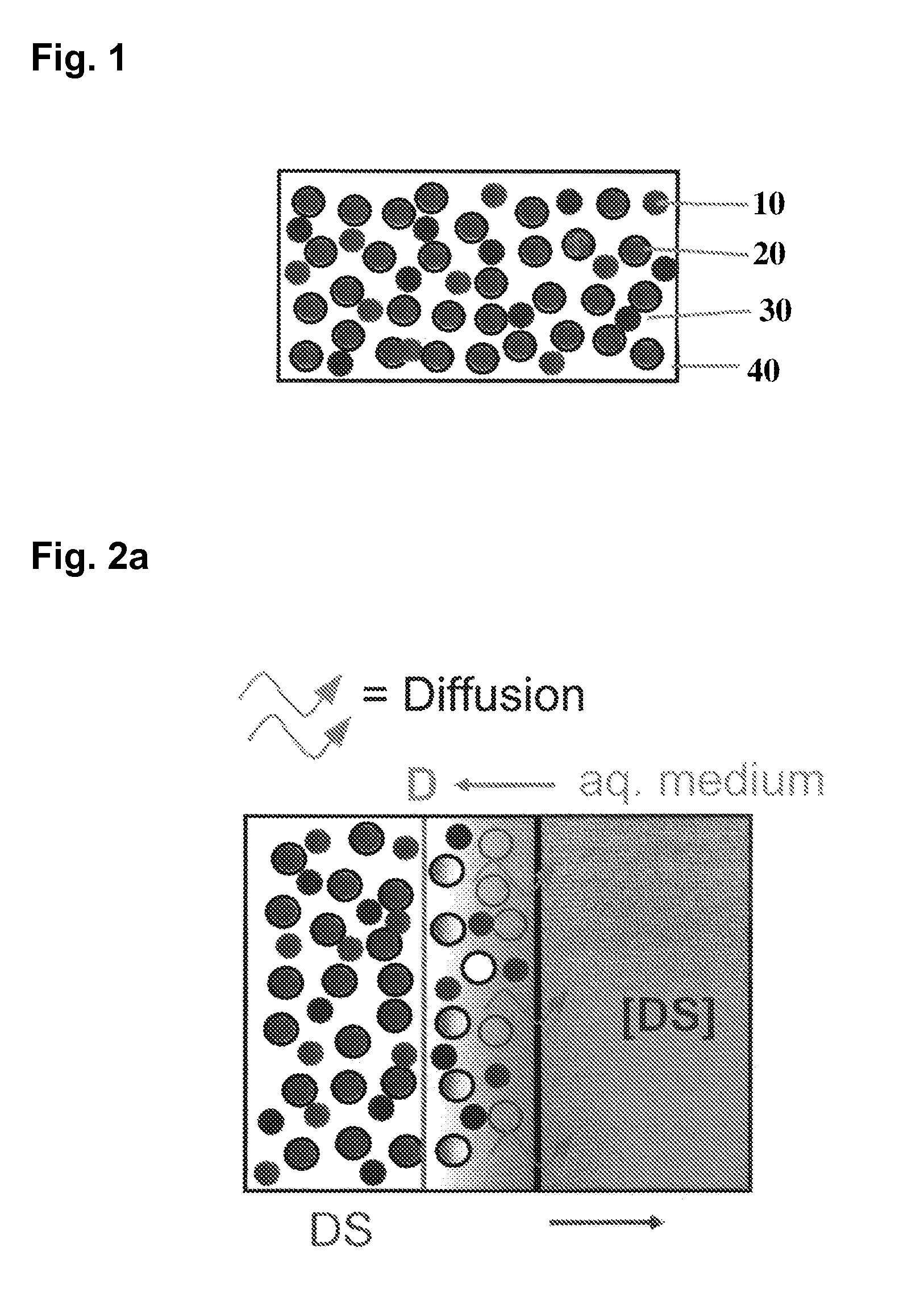
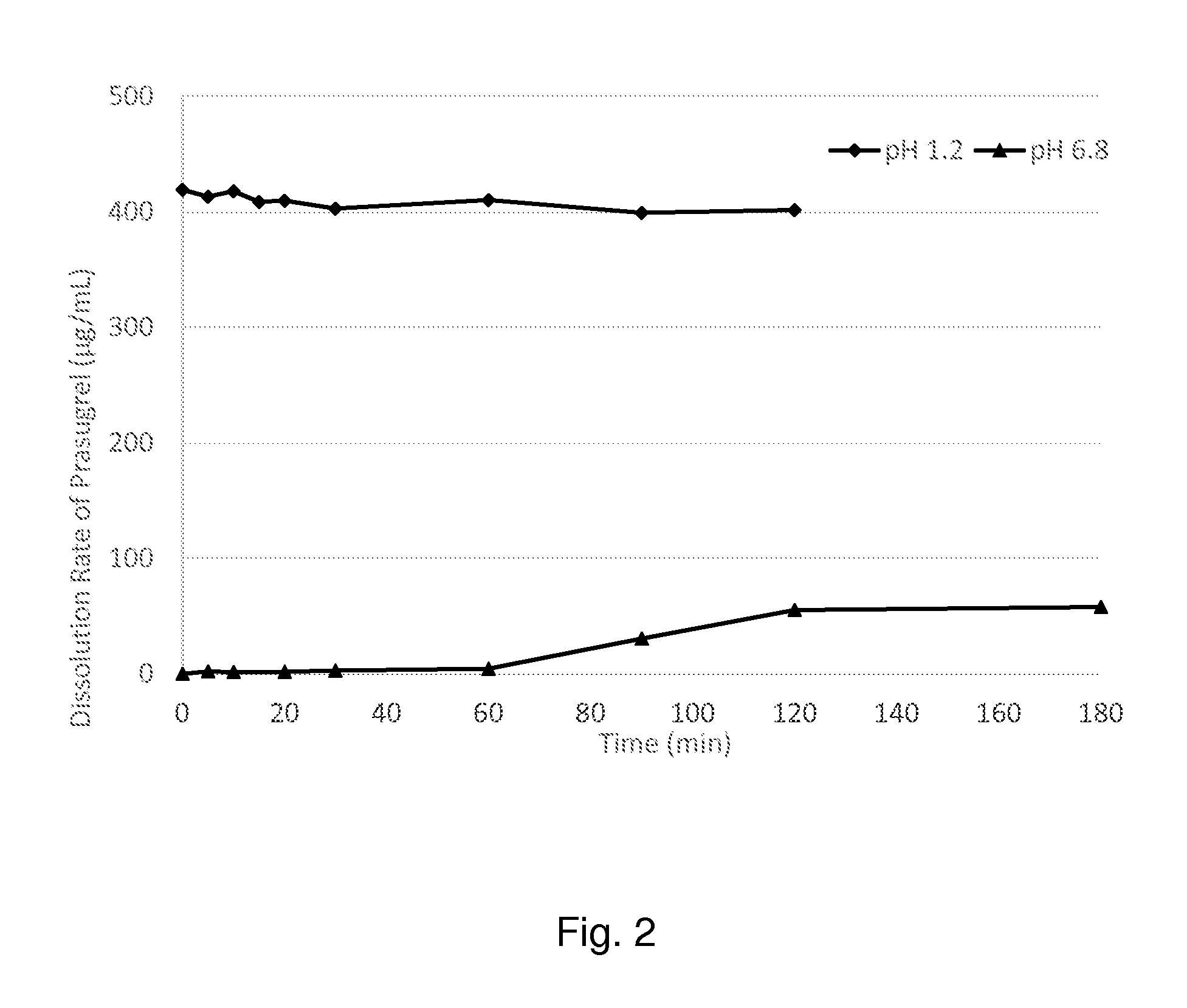
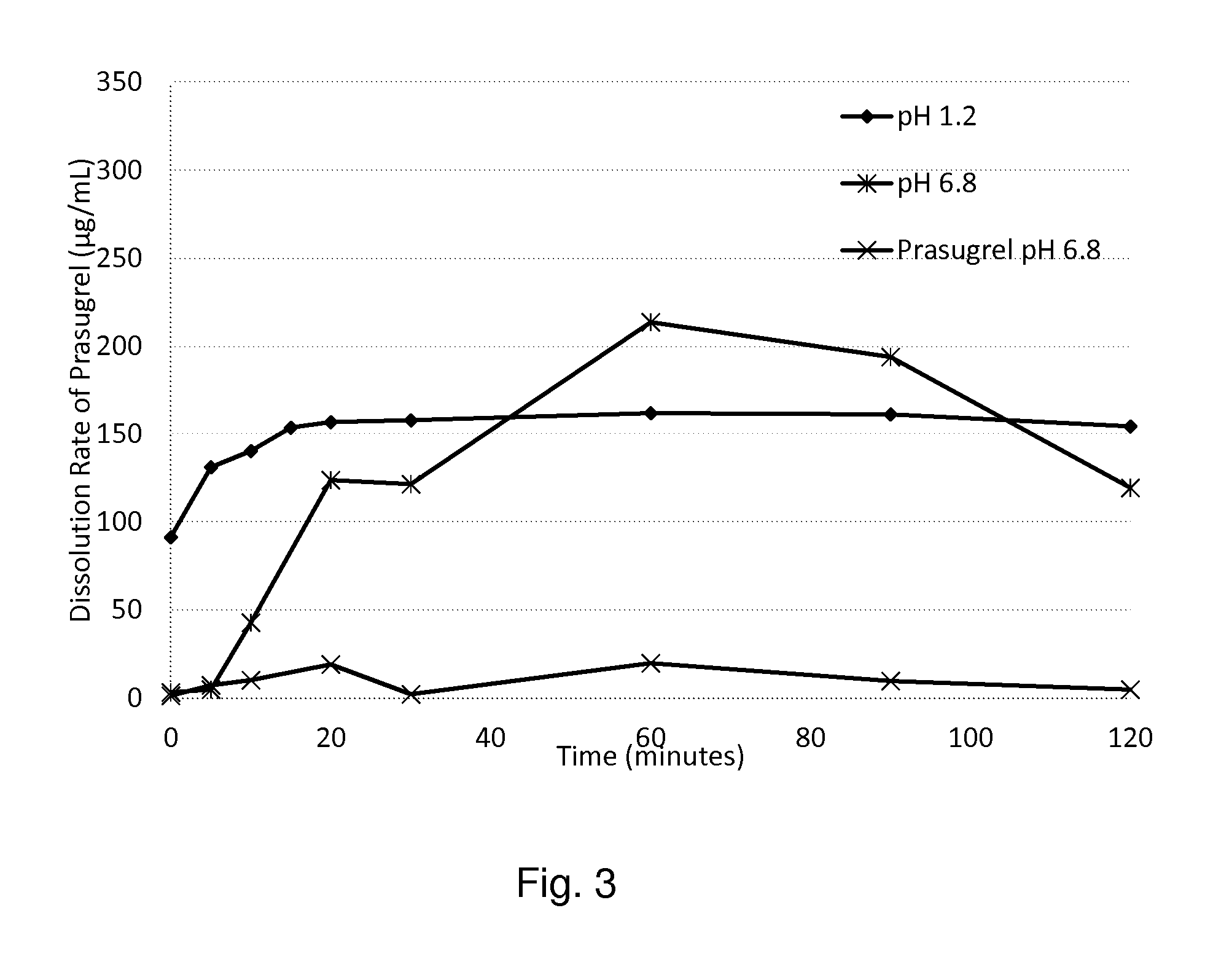
Extrusion of a mix containing a pharmaceutically active agent can be achieved using a plasticising excipient in an amount sufficient to act as plasticiser and also act as lubricant, thereby avoiding the need for inclusion of a lubricant. The invention provides multiparticulates with controlled release properties, substantially free of lubricant. The present invention is preferably directed to extruded multiparticulates containing an opioid such as oxycodone, an ammonium methacrylate copolymer such as Eudragit® RSPO, a plasticising excipient such as preferably stearyl alcohol and a water permeability modifier such as preferably Eudragit® RLPO. The obtained multiparticulates show a release rate profile which is pH-independent.
Owner:EURO-CELTIQUE SA
Controlled release system and method for manufacturing the same
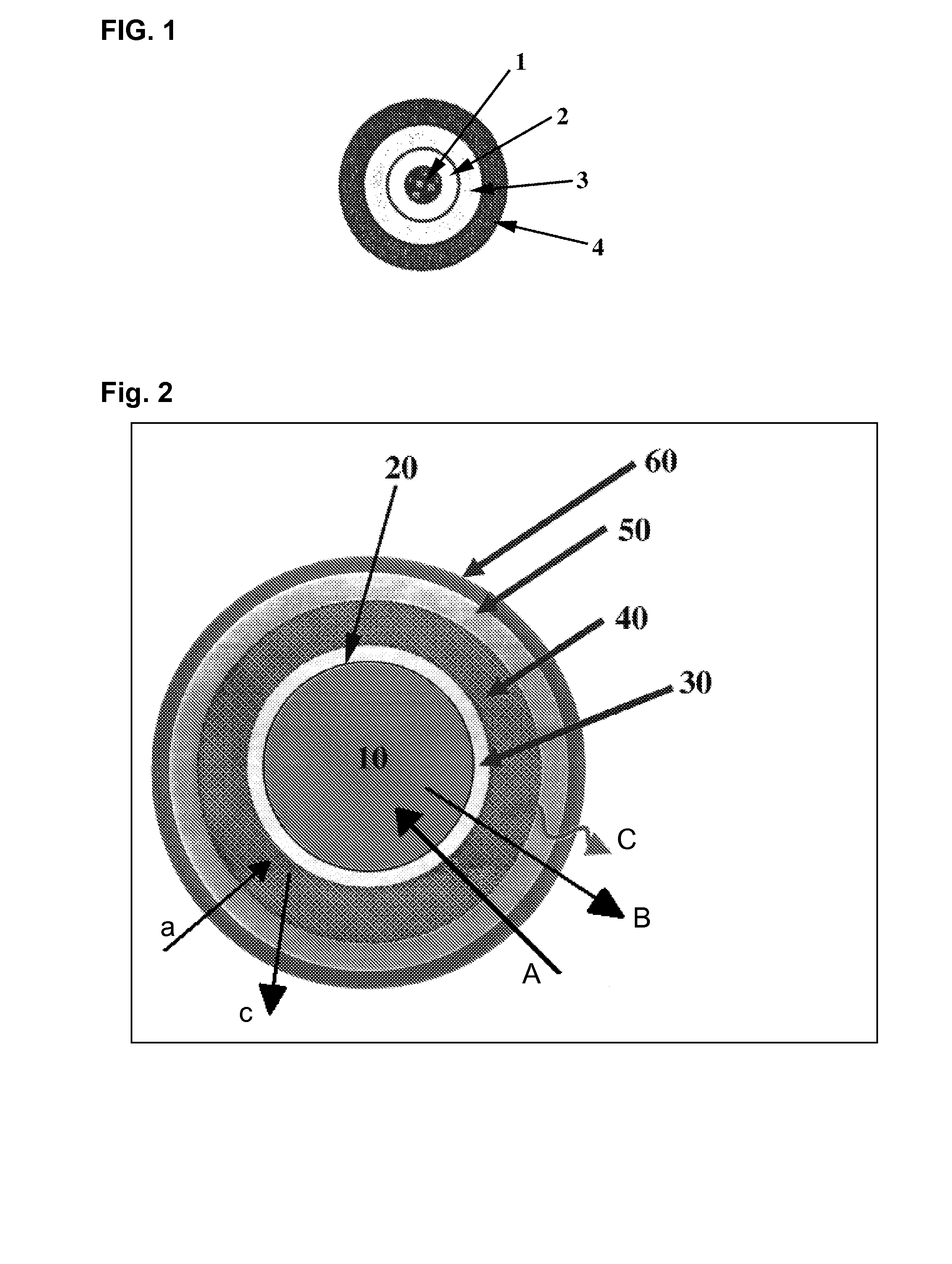
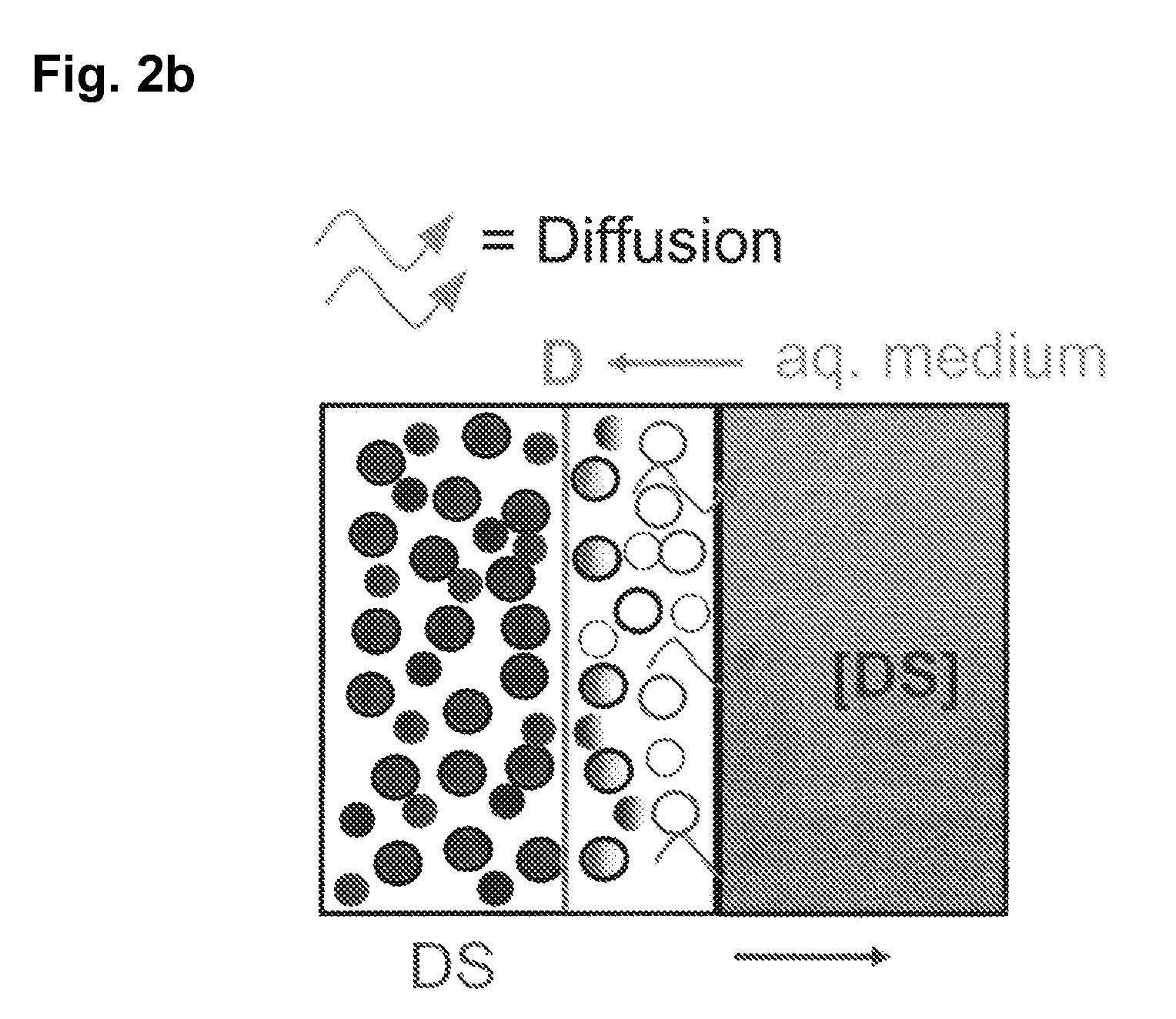
The invention is directed to a pharmaceutical controlled release system for administration, particularly oral administration, of active substances with pH-dependent solubilities, comprising a) a core material containing or consisting of one or more pharmaceutically acceptable pH modifiers; b) optionally an insulating layer, c) a first layer containing or consisting of one or more pharmaceutically acceptable water-insoluble polymers; d) a second layer containing or consisting of at least one active substance having a pH-dependent solubility; e) a third layer containing or consisting of one or more pharmaceutically acceptable polymers having anionic or no ionic groups; and f) optionally a fourth layer, preferably in form of an outer coating layer. It is provided a pH-independent release profile of active substances having pH-dependent solubilities in vitro and vivo.
Owner:BOEHRINGER INGELHEIM INT GMBH
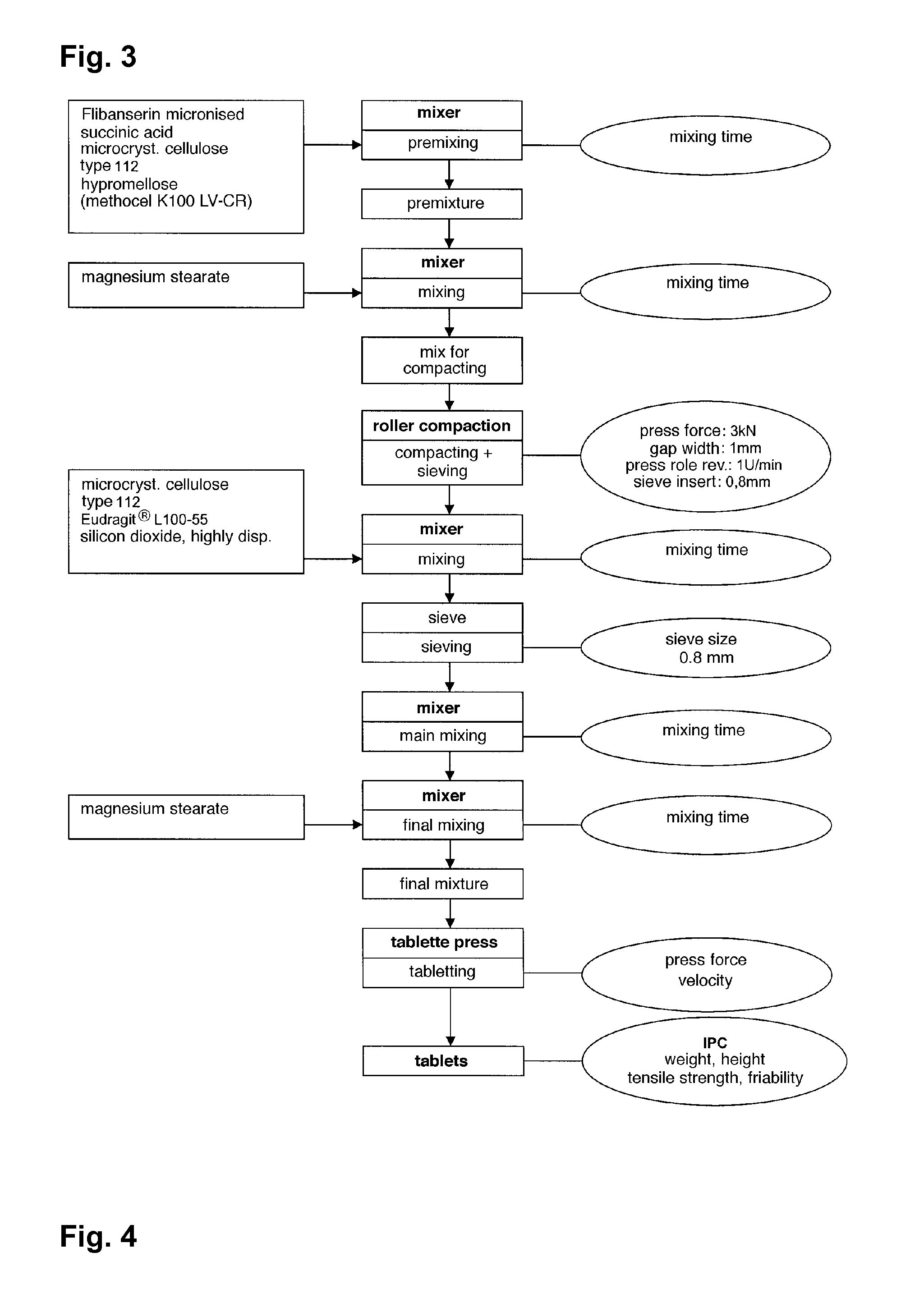
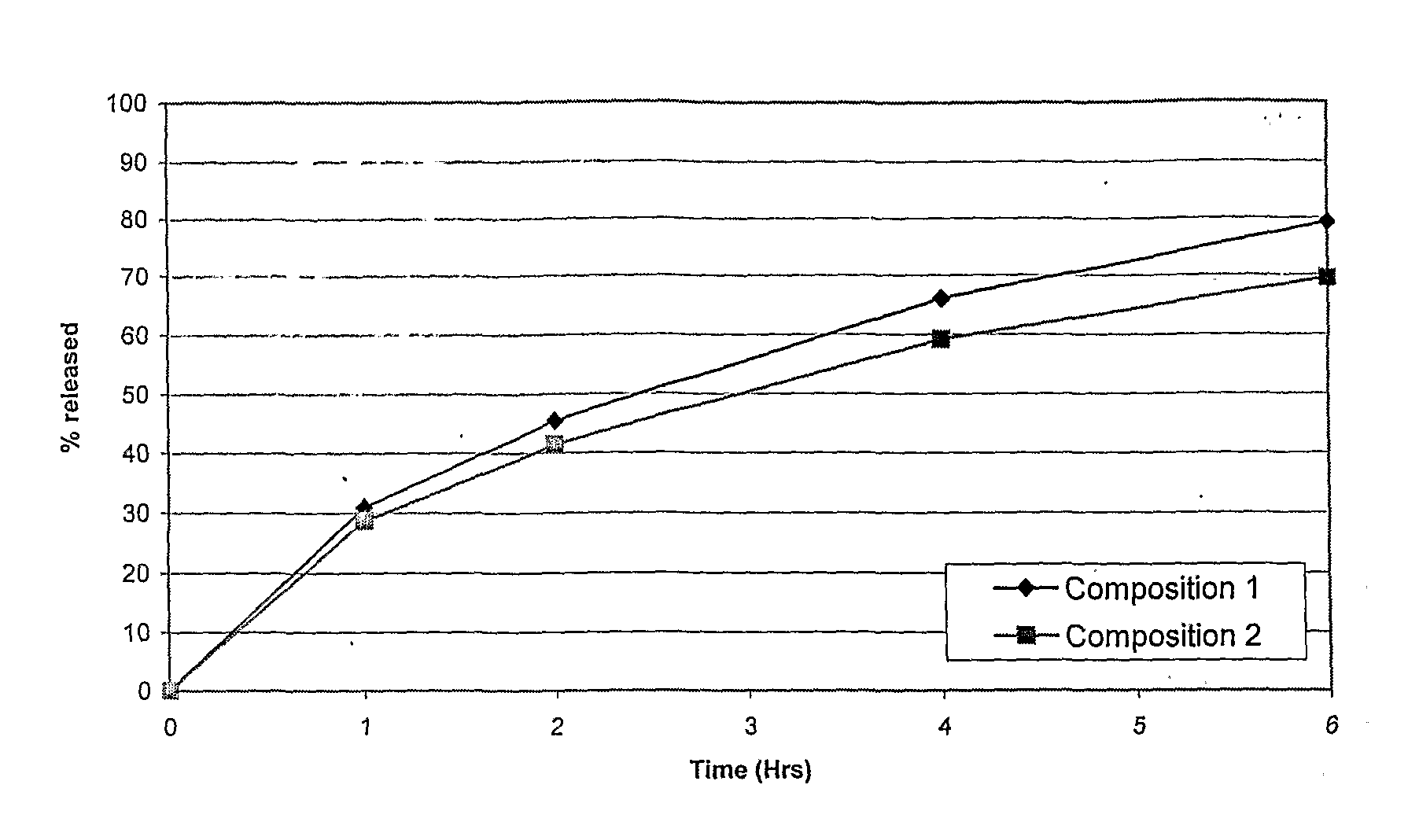
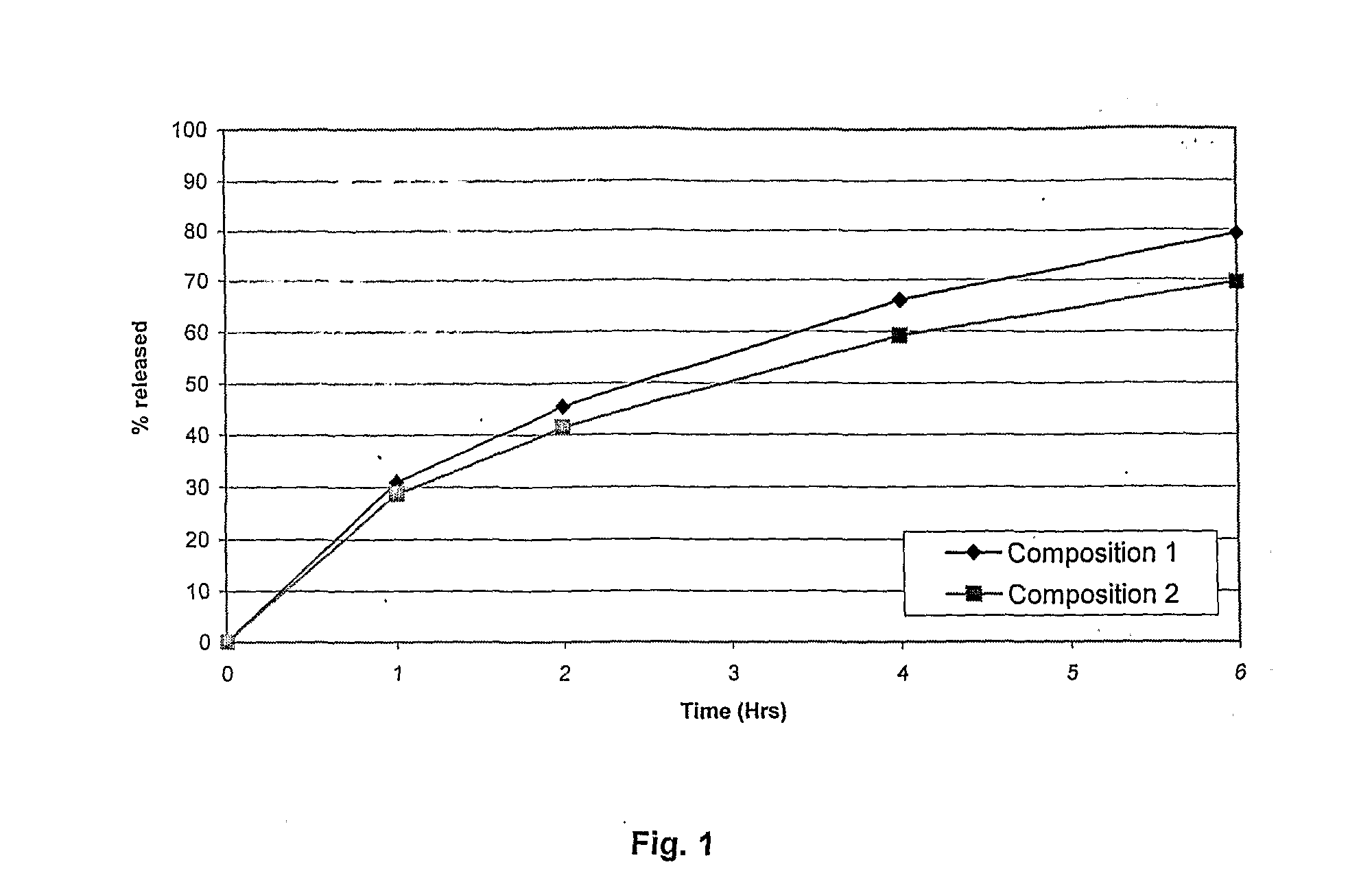
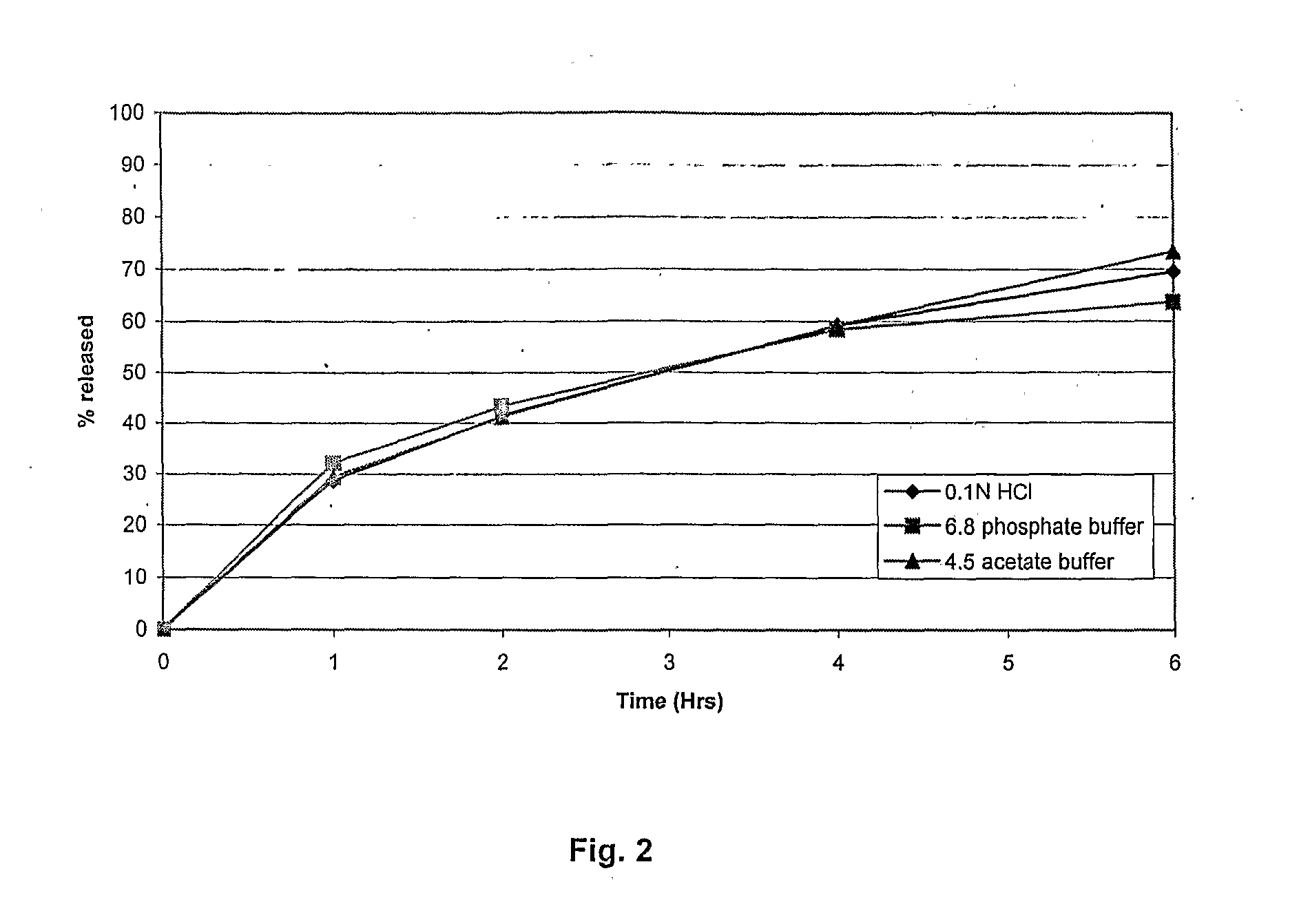
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
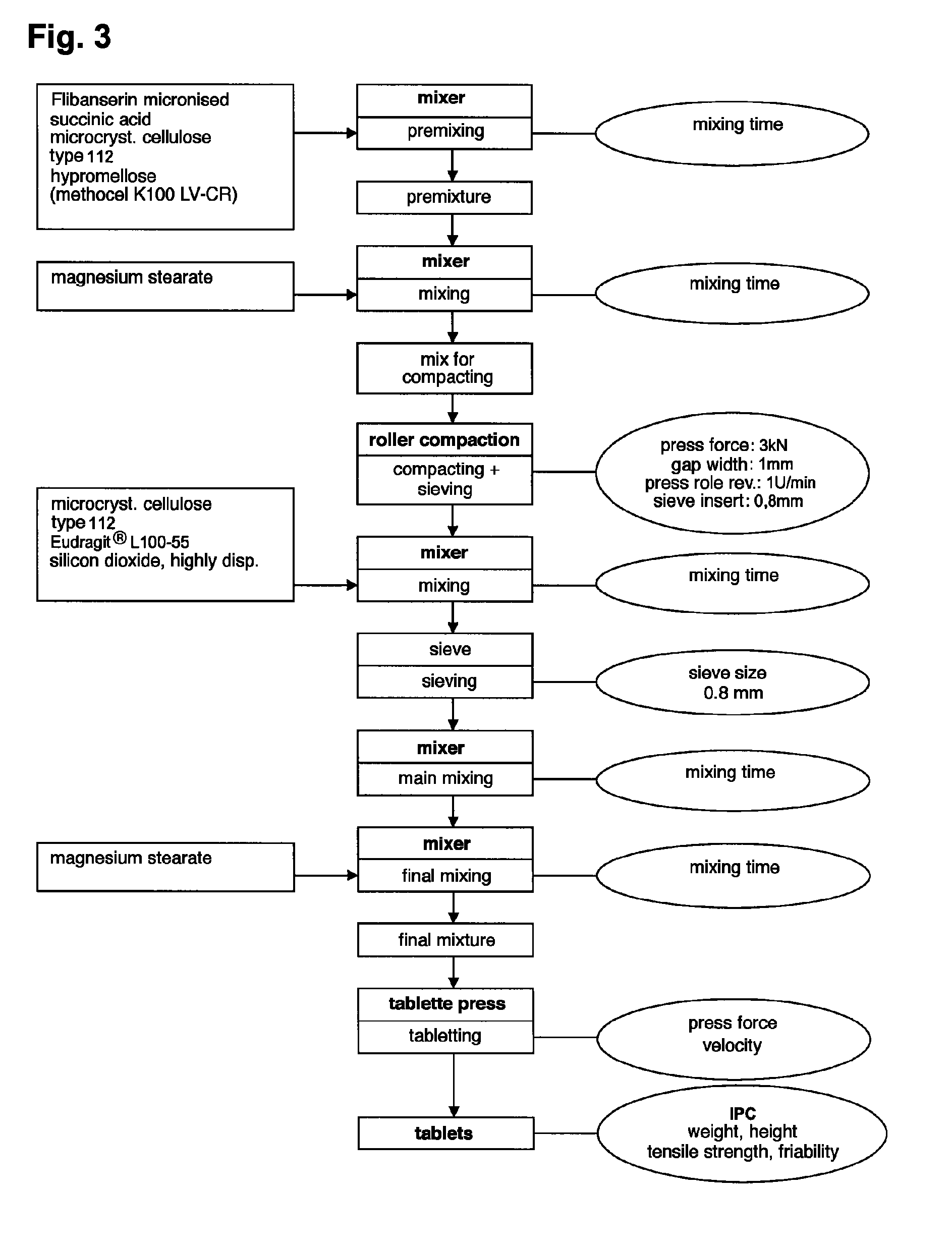
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
Multilayer tablets containing thiazolidinedione and biguanides and methods for producing them
InactiveUS20060057202A1Facilitated releaseEfficient compressionBiocideOrganic active ingredientsAcute hyperglycaemiaImmediate release
A novel patient-convenient, cost effective pharmaceutical composition, comprising of thiazolidinediones and biguanide for controlling hyperglycemia manufactured as multilayer tablet and its process of manufacturing, for immediate release of thiazolidinediones or thiazolidinediones and biguanide and prolonged release of the biguanide only, the tablet comprising of minimum two layers wherein one outer layer comprises of a mixture of excipients and thiazolidinediones or thiazolidinediones and biguanide allowing immediate release of thiazolidinediones or thiazolidinediones and biguanide respectively and the other layer arranged in contact with the immediate release layer which comprises of a novel composition of excipients and a minimum one or more non-biodegradable, inert polymer(s) and the biguanide allowing pH independent prolonged release of the biguanide up to a period of 8-12 hours. The tablets are for once a day dosing. The tablets may optionally be film coated or enrobed by soft gelatin ribbons for additional protection against oxidation, photodegradation, identification, ease of swallowing, taste masking and for aesthetic appeal without altering the dissolution profile.
Owner:INVENTIA HEALTHCARE LTD
Controlled Release Formulation
InactiveUS20080248107A1Reduced initial burst releaseGood physical propertiesPowder deliveryBiocideSolubilityActive agent
The present invention provides a controlled release formulation comprising an therapeutically effective amount of pharmacologically active substance having high water solubility, at least one non-polymeric release retardant, and at least one pH independent non-swelling release retarding polymer. The said dosage form provides controlled release of the active agent with reduced initial burst release.
Owner:RUBICON RES PTY LTD
Immediate release pH-independent solid dosage form of cisapride
InactiveUS6030988APatient compliance is goodIncrease in motilityBiocideDigestive systemCITRATE ESTERImmediate release
The present invention concerns oral dosage forms of some particular salts of cisapride, more particularly cisapride-(L)-tartrate, cisapride-(D)-tartrate, cisapride-sulfate, or cisapride citrate, which avoid drugfood interaction and which allow comedication of agents that increase the pH of the stomach. The invention particularly relates to solid oral dosage forms suitable for rapid dissolution. The present invention also concerns tablets which can be prepared via direct compression.
Owner:JANSSEN PHARMA NV
Coating compositions for bitterness inhibition
InactiveUS7294347B2Mask bitternessImprove palatabilityPowder deliveryInksPH-sensitive polymersPh independent
The present invention discloses coating compositions with taste masking property, comprising a blend of pH sensitive polymers and optionally a pH independent polymer or a blend of the pH sensitive polymer and pH independent polymer used for taste masking of highly bitter drugs. The pH sensitive polymers used comprise the acid soluble polymers and the enteric polymers. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said coating compositions is disclosed. The concomitant use of the polymers inhibits the release of the bitter drug at the pH of saliva. The said coating compositions deliver substantial amount of the bitter drug immediately with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Self-emulsifying drug delivery system for improving bioavailability of insoluble medicine, and application thereof
ActiveCN105535979AGood water solubilityIncrease dissolution rateOrganic active ingredientsCapsule deliverySolubilityOil phase
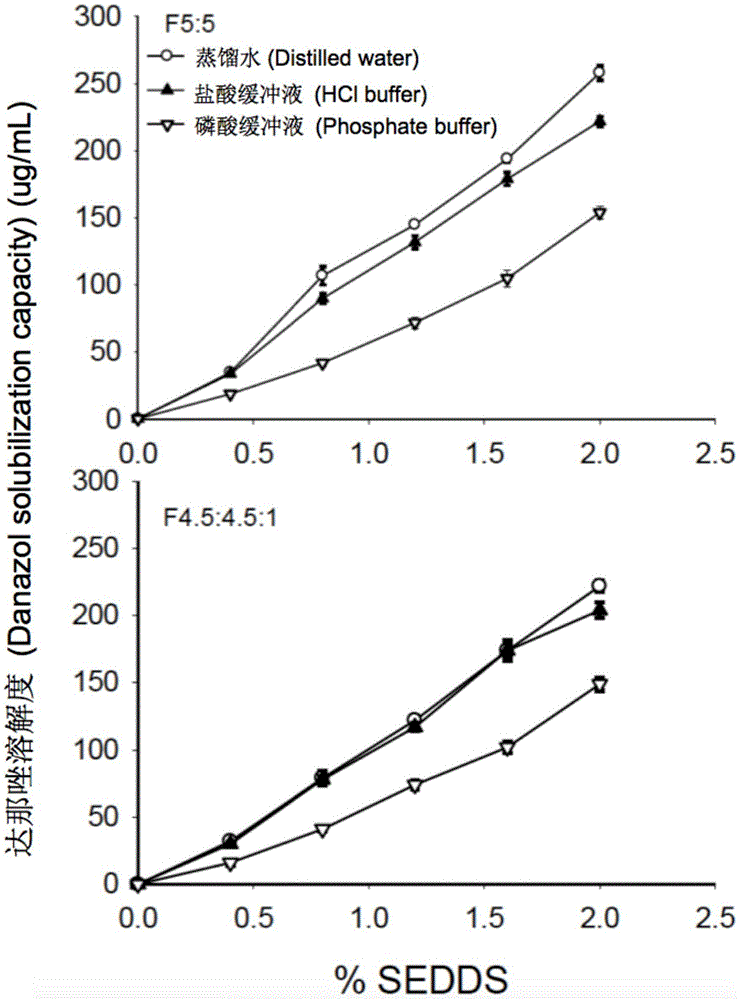
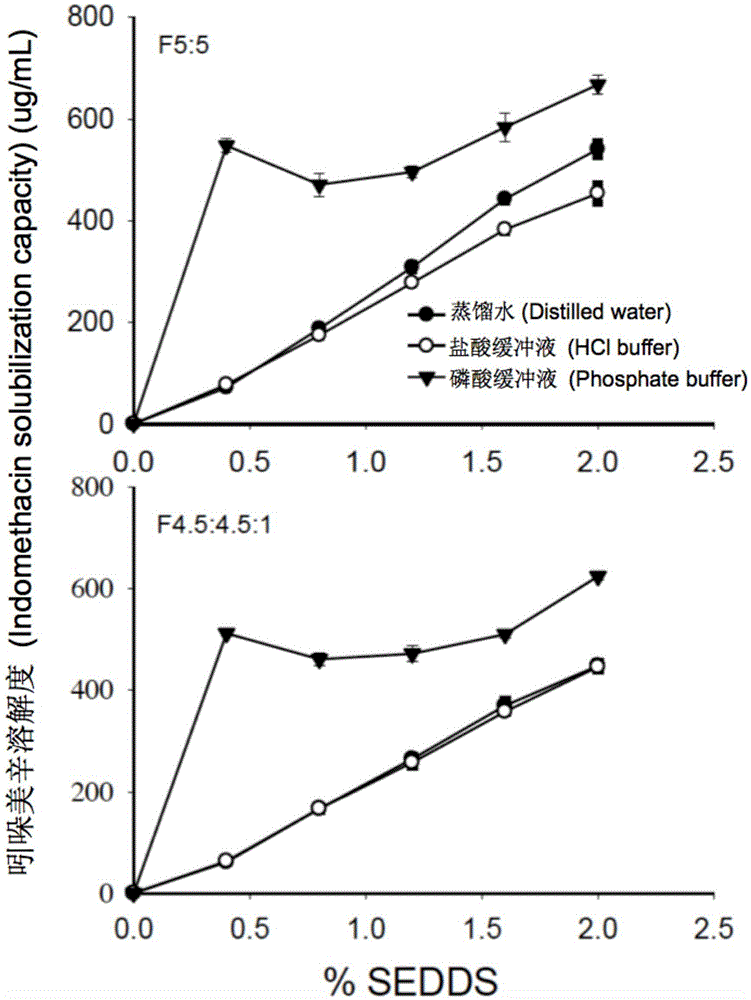
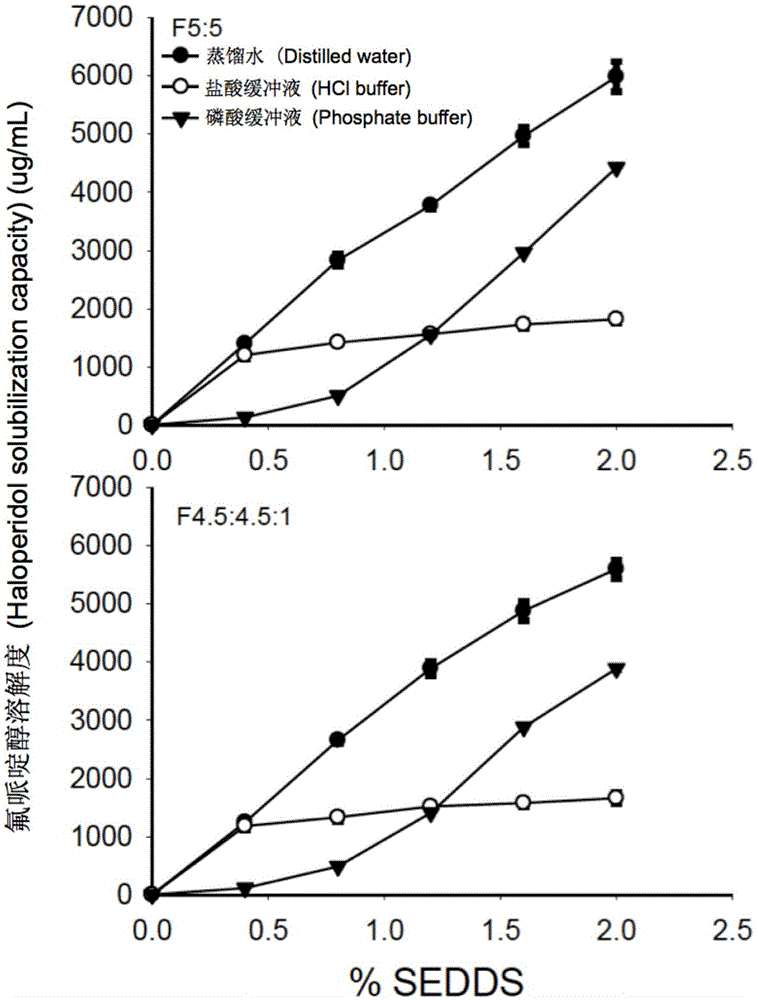
The invention provides a novel self-emulsifying drug delivery system (SEDDS). An SEDDS carrier material comprises a surfactant and an oil phase containing Capmul MCMs and medium chain fatty acids, is suitable for loading pH-dependent (weakly acidic and weakly alkaline) and a pH-independent (neutral) insoluble medicines, greatly improves the solubility of the medicines to realize optimum bioavailability, and has important application values in the development of preparations of the insoluble medicines.
Owner:李素华
Coated fine particles containing drug for intrabuccally fast disintegrating tablet
The present invention makes it possible to provide drug-containing coated microparticles for quick-disintegrating oral tablets wherein microparticles containing a drug with an unpleasant taste are coated with a film composed of (1) a pH-independent water-insoluble polymer accounting for 60% or more but less than 80% of the film and (2) a pH-independent water-soluble substance accounting for more than 20% to 40% or less of the film, these microparticles being characterized in that the rate of dissolution of the drug from the drug-containing microparticles is 0% to 10% in one minute and 80% to 100% in 30 minutes, and the average particle diameter is 350 μm or less, in order to realize sufficient control of oral drug dissolution and fast gastrointestinal drug dissolution.
Owner:ASTELLAS PHARMA INC
Sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
Dermatological/cosmetic gels comprising at least one retinoid and benzoyl peroxide
ActiveUS20080181963A1Improve physical stabilityGood chemical stabilityBiocideCosmetic preparationsRetinoidBenzoyl peroxide
Owner:GALDERMA RES & DEV SNC
Novel sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
Process for release of biologically active species
ActiveUS20090192205A1Improve bioavailabilityPromote absorptionCosmetic preparationsBiocidePorosityIn vivo
A process for the release of a biologically active species comprising the steps of:providing a mesoporous oxide-based material having structural order and at least one level of porosity;fixing or immobilizing said biologically active species in said ordered mesoporous oxide; andproviding said ordered mesoporous oxide with said fixed or immobilized biologically active species in vivo thereby realizing intraluminally induced substantially pH-independent supersaturation of said biologically active species resulting in enhanced transepithelial transport; wherein said biologically active species is a poorly soluble therapeutic drug classified as belonging to Class II or Class IV of the Biopharmaceutical Classification System and said ordered mesoporous oxide has a pore size in the range of 4 to 14 nm.
Owner:FORMAC PHARMA
Methods and formulations for making pharmaceutical compositions containing bupropion
InactiveUS20060099260A1Organic active ingredientsNervous disorderPh independentPharmaceutical formulation
Embodiments of the invention generally provide pharmaceutical drug compositions, methods of preparing oral drug compositions, such as extended release dosage compositions, and methods for treating antidepressant or smoking cessation. In one aspect, the invention provides a pharmaceutical formulation comprising a core, including bupropion and its salt derivatives, and a coating. The coating may include from about 5% to about 99% by weight of the coating of a pharmaceutically acceptable pH-independent polymer. The coating may further include from about 0.001% to about 30% by weight of the coating of a surfactant. In another aspect, the invention provides methods for preparing and administering a pharmaceutical composition in oral dosage form, such as a tablet.
Owner:BIOKEY
Proton pump inhibitor formulations, and methods of preparing and using such formulations
InactiveUS20050232992A1Organic active ingredientsDigestive systemPh independentPharmaceutical formulation
Pharmaceutical formulation comprising at least one proton pump inhibitor structured and arranged to provide an initial pH independent time-based delayed-release, and a subsequent extended-release of the at least one proton pump inhibitor.
Owner:AGI THERAPEUTICS RES
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS8545886B2Reduce solubilitySufficiently slow releasePowder deliveryNervous disorderExtended release tabletsOral medication
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting ofa) flibanserin or a pharmaceutically acceptable derivative thereof as active substance;b) one or more pharmaceutically acceptable pH-dependent polymers;c) one or more pharmaceutically acceptable pH-independent polymers;d) one or more pharmaceutically acceptable acids; ande) optionally one or more additives.The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical Compositions For Poorly Water-Soluble Compounds
InactiveUS20150182457A1Reduced food effectMinimizing pH sensitivityPowder deliveryBiocideSolubilityPH-sensitive polymers
This present invention is concerned with novel solid dispersion pharmaceutical compositions for preparation of composition which is comprised of a compound with poor water solubility (a weakly basic, neutral and / or non-ionizable, or a weakly acidic compound), water-soluble polymer(s), pH-sensitive polymer(s) (either enteric polymer or gastric-soluble polymer that is soluble at gastric fluid and insoluble at intestine pH range such as Eudragit E), and / or pharmaceutical acceptable surfactant(s) that would improve the solubility / dissolution of the compound in aqueous media of both low and neutral pHs and provide a relative pH-independent dissolution profile.
Owner:ASCENDIA PHARMA
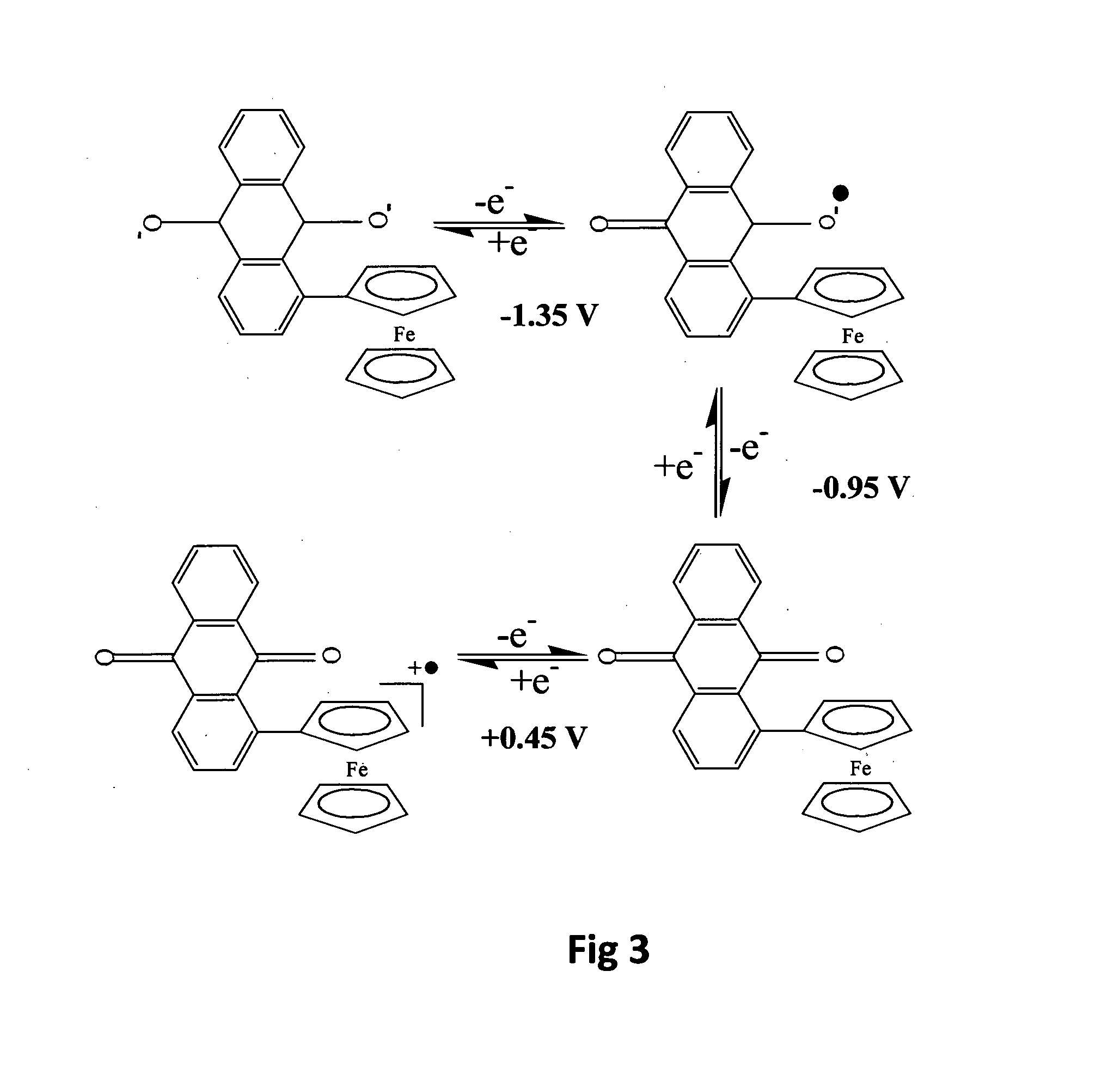
Electrochemical sensor utilising a dual redox system contained within a single molecule
ActiveUS20110162977A1Weather/light/corrosion resistanceVolume/mass flow measurementElectrochemical gas sensorChemical compound
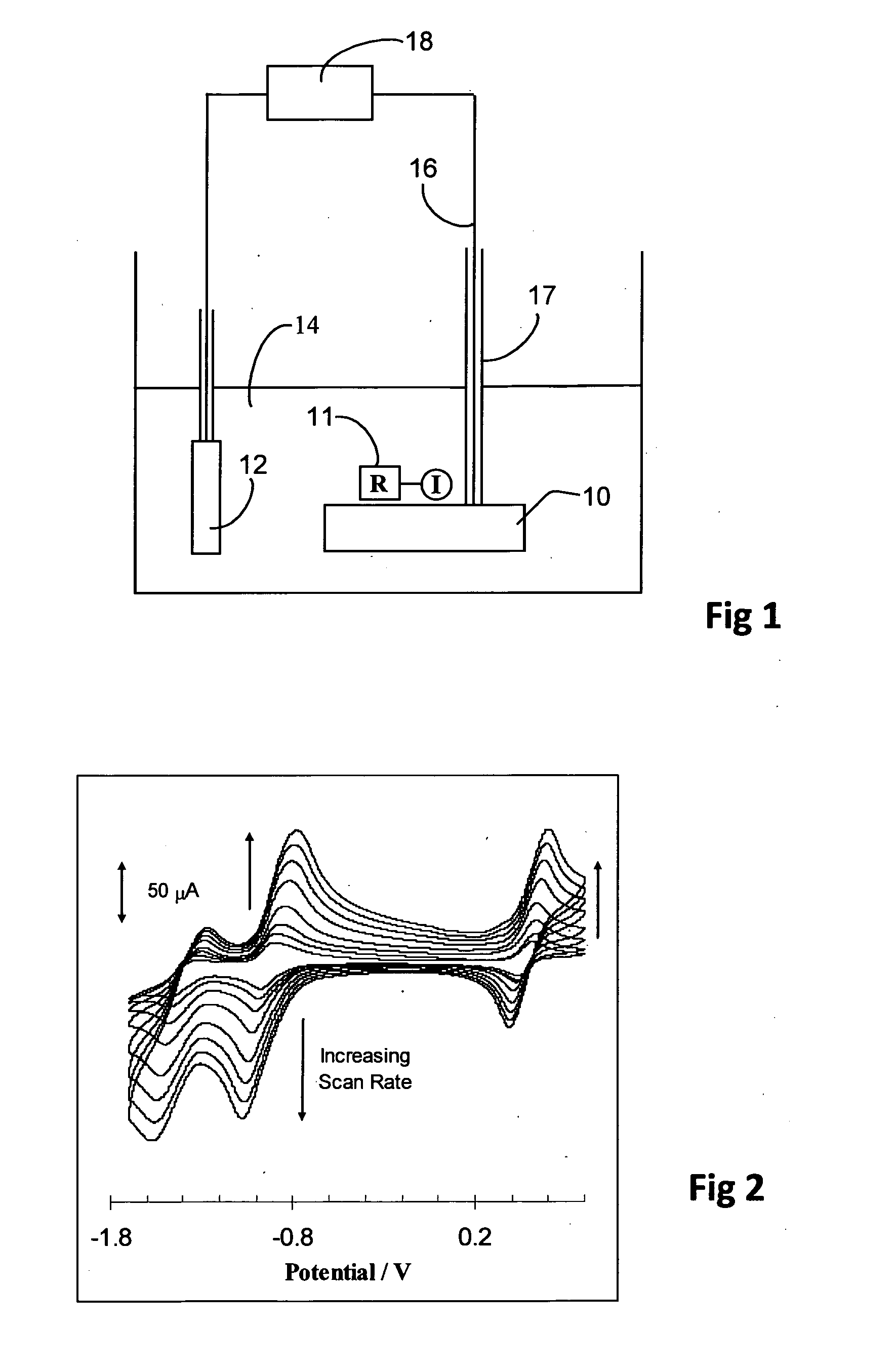
An electrochemical sensor utilises a chemical compound which is not a macromolecule but rather is a single chemical compound of determinate structure, incorporating two redox systems which differ in their response to a species to be detected. In one form, one redox system displays a voltammetric wave which is pH dependent while another displays a voltammetric wave which is pH independent and acts as an internal reference. The sensor comprises a solid substrate, which may be carbonaceous, on which the compound is immobilized. The sensor may be incorporated into a tool to be suspended in a wellbore.
Owner:SCHLUMBERGER TECH CORP
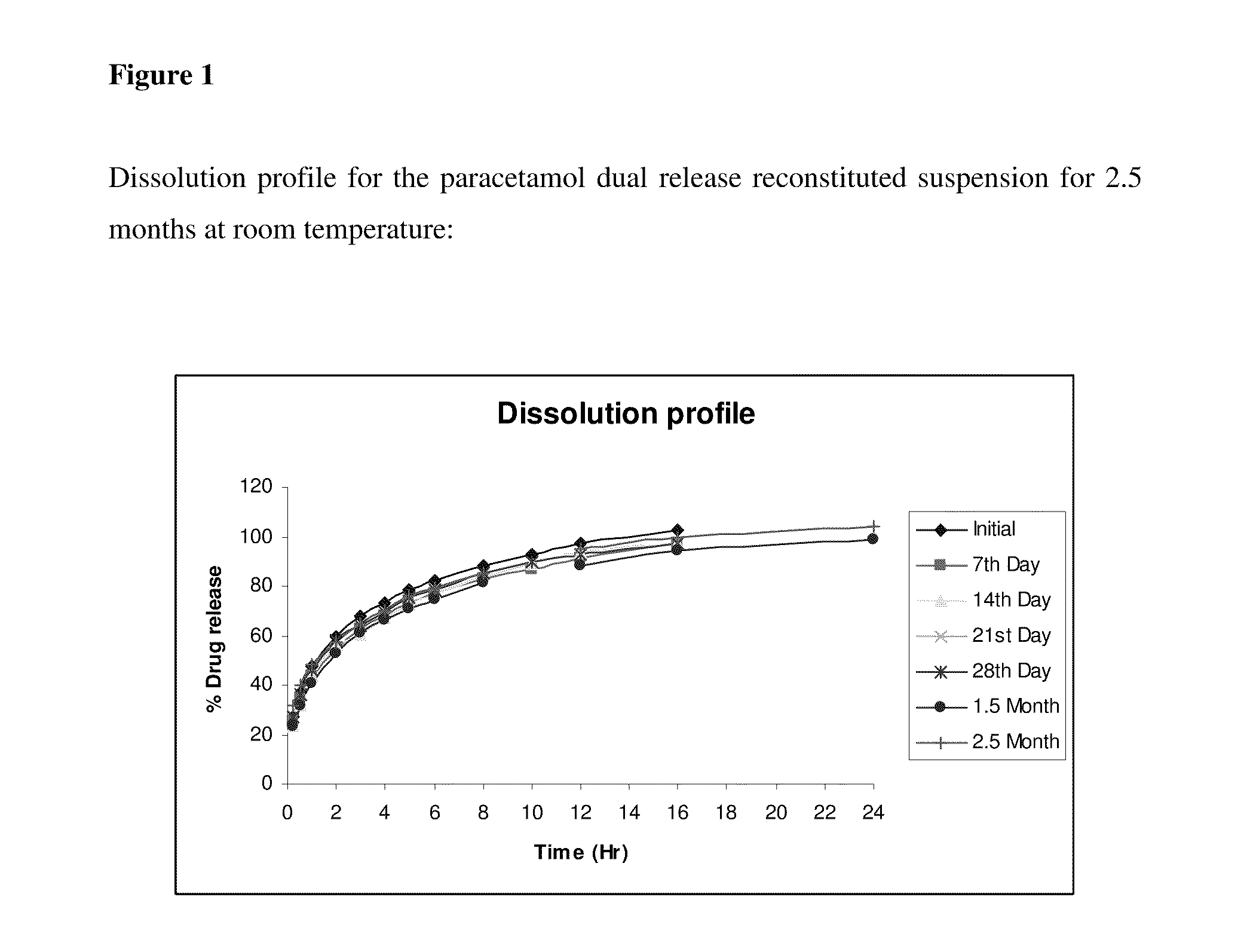
Dual-release pharmaceutical suspension
InactiveUS20110268808A1Stable dissolution profileExtended shelf lifeAntibacterial agentsBiocideDual releaseImmediate release
Orally deliverable dual-release pharmaceutical suspensions, having a first portion comprising an immediate release form of the active in the solution form or granules or suspended form in the vehicle / medium preferably in the solution form and a second portion comprising a sustained-release form of active in the form of microgranules / microparticles suspended in the immediate release fraction of the solulabilised active agent which comprise a core and at least one coat suitable for liquid dosage forms for the administration of the active ingredients, wherein the core comprises at least one active agent(s) or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof; optionally at least one water insoluble, and optionally one or more pharmaceutically acceptable excipient(s); and at least one coat comprising at least one pH independent water-insoluble polymer(s) along with one or more pharmaceutically acceptable excipient(s). This coated microparticles and solution of the active agent in the vehicle ensures a dual release profile i.e. immediate release profile as well as predetermined sustained release profile of the active agent and also ensures maintenance of said release profile over time. The present invention can be administered either in the form of ready to use suspension or in the form of powder ready for reconstitution. Further, this invention provides process of preparation of such suspensions and method of using them.
Owner:PANACEA BIOTEC
Coated tablet with zero-order of near zero-order release kinetics
ActiveUS20070020331A1Release rate is suppressedHigh molecular weightCoatingsDrageesCellulosePolyethylene oxide
Tablets for the controlled release of an active ingredient in a zero-order or near zero-order fashion are provided. The tablet includes a core and a coating. The core includes at least one active pharmaceutical agent and a polyethylene oxide with a molecular weight of between about 1,000,000 and 10,000,000, preferably between about 4,000,000 and 8,000,000. The core material is optionally, but preferably, coated with a cellulosic material. The active pharmaceutical agent can be hydrophilic, hydrophobic, or amphiphilic. When the active pharmaceutical agent is a hydrophilic agent, it is preferred that the coating is a relatively hydrophobic cellulose, such as ethylcellulose or propylcellulose. However, if the tablet is uncoated, it can provide a near-zero-order release rate rather than a zero-order release rate. When the active pharmaceutical agent is a hydrophobic or amphiphilic agent, the hydrophilic polymeric carrier is the same as in the first embodiment, the coating is a relatively more hydrophilic cellulose. The release rate for the active pharmaceutical agent can be controlled by adjusting the thickness of the coating, and, optionally, by adjusting the concentration of the polymeric excipients, as well as certain non-polymeric excipients which may optionally be present. An advantage of using relatively high molecular weight polyethylene oxide is that the release is pH independent, unlike where ionic polymers such as polyacrylic acids are used. Further, active pharmaceutical agents including functional groups that might react with such polymers can be used without an adverse reaction between the active agent and the polymer.
Owner:CATALENT GREENVILLE INC +1
Multiparticulates
InactiveUS9259872B2Reduce adhesionReduce the required powerOrganic active ingredientsCosmetic preparationsActive agentExcipient
Extrusion of a mix containing a pharmaceutically active agent can be achieved using a plasticizing excipient in an amount sufficient to act as plasticizer and also act as lubricant, thereby avoiding the need for inclusion of a lubricant. The invention provides multiparticulates with controlled release properties, substantially free of lubricant. The present invention is preferably directed to extruded multiparticulates containing an opioid such as oxycodone, an ammonium methacrylate copolymer such as Eudragit® RSPO, a plasticizing excipient such as preferably stearyl alcohol and a water permeability modifier such as preferably Eudragit® RLPO. The obtained multiparticulates show a release rate profile which is pH-independent.
Owner:EURO-CELTIQUE SA
Enteric coatings for orally ingestible compositions
A suspendible enteric coating composition for encasing orally ingestible articles wherein the enteric coating composition comprises a pH-dependent polymer selected from a group containing alginates and alginic acids, a pH-independent water insoluble polymer selected from the group comprising ethylcellulose and ethylcellulose-containing compositions, and a plasticizer selected from the group containing triethyl citrate, glycerin, propylene glycol, triacetin, acetylated monoglycerides, dibutyl sebacate, polyethylene glycols, sorbitals, middle chain triglycerides and combinations thereof. A three step method for providing a stable outer enteric coating on an ingestable item comprising a first step of encasing the item with a suspension comprising a mixture of at least a sugar and a microcrystalline cellulose, a second step of then encasing the item with a suspension comprising a mixture of a film-forming polymer and a plasticizer, and a third step of finally encasing the item with the enteric coating composition.
Owner:VIVA PHARMA
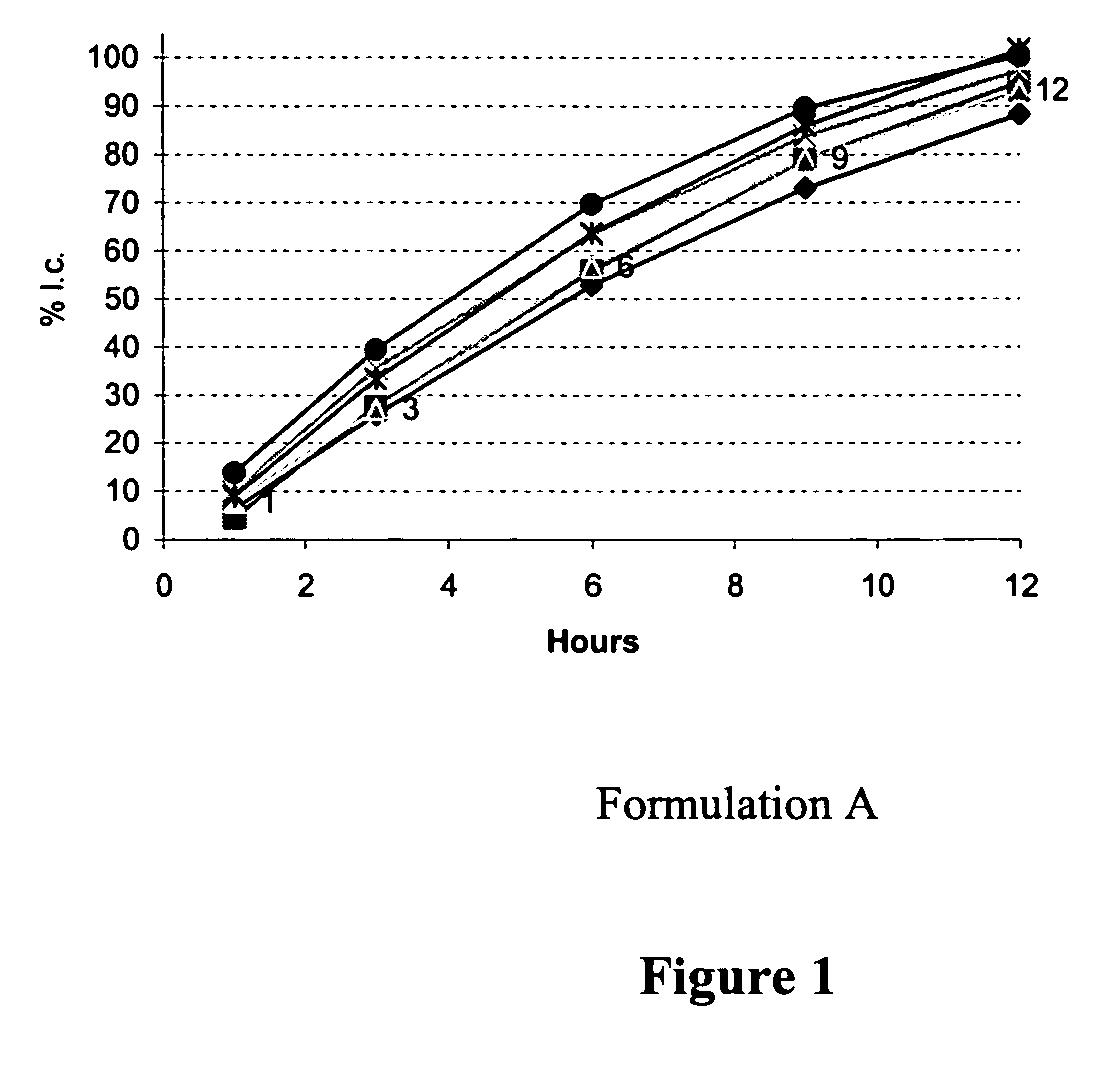
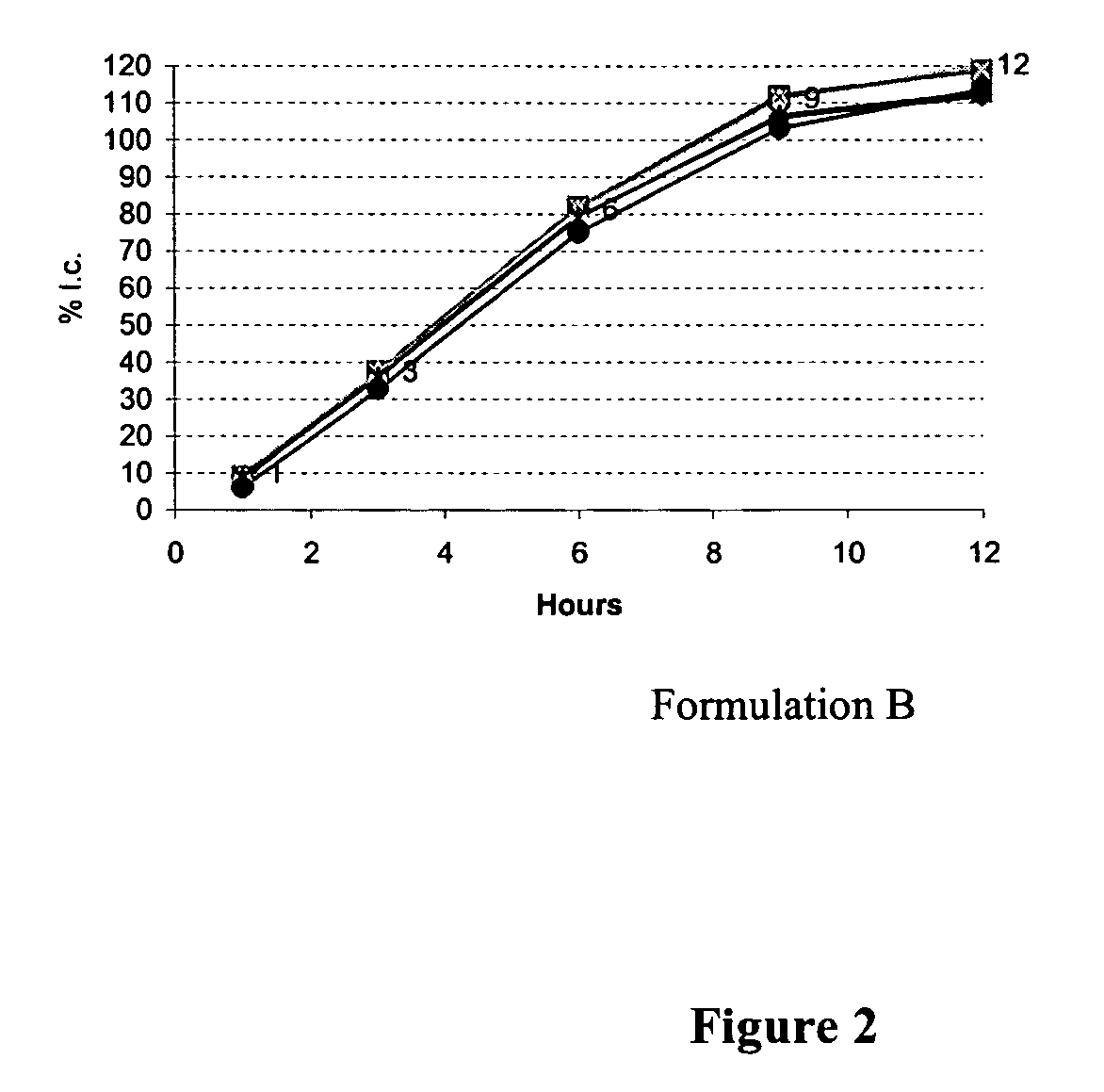
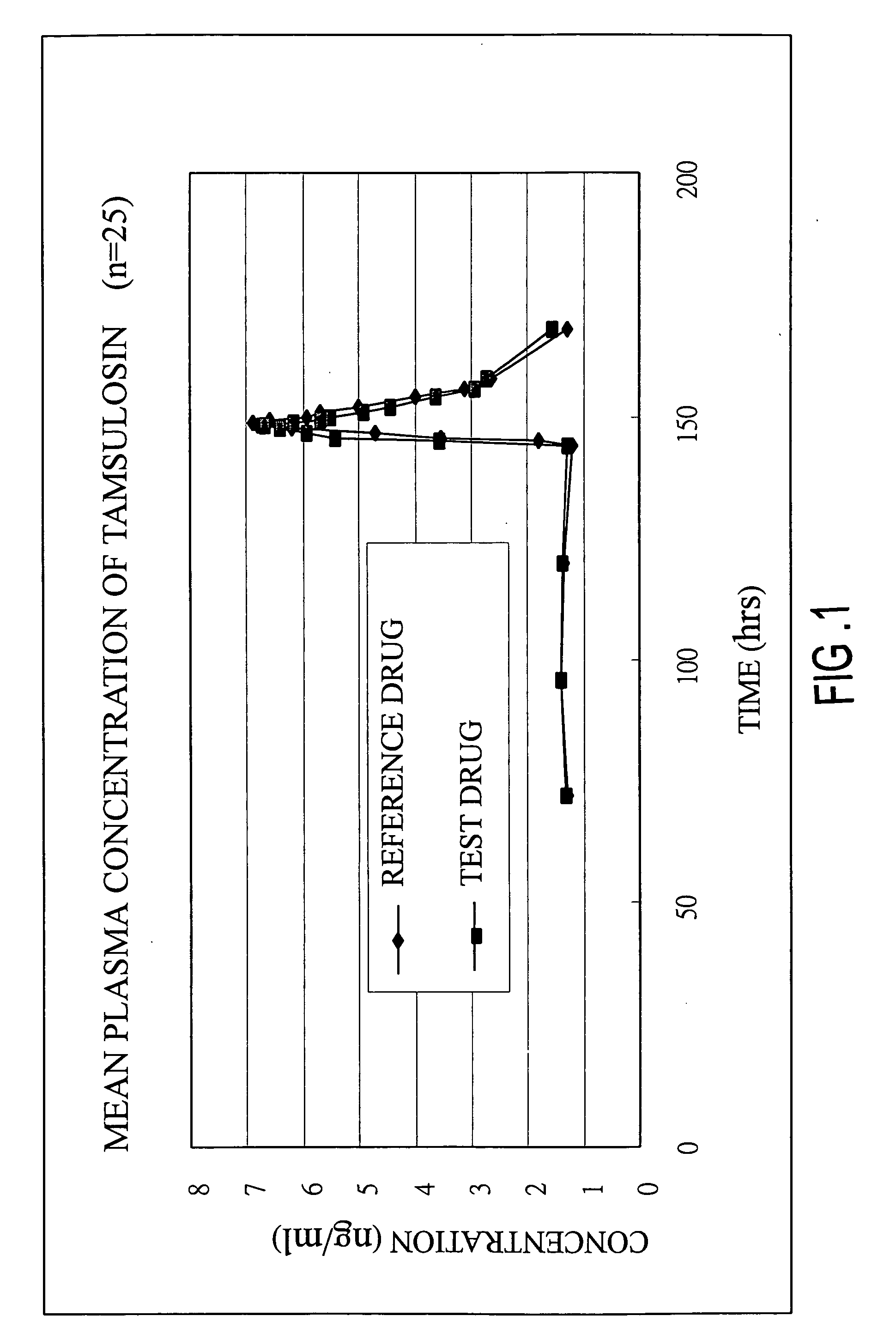
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract.
Owner:STANDARD CHEM PHARM
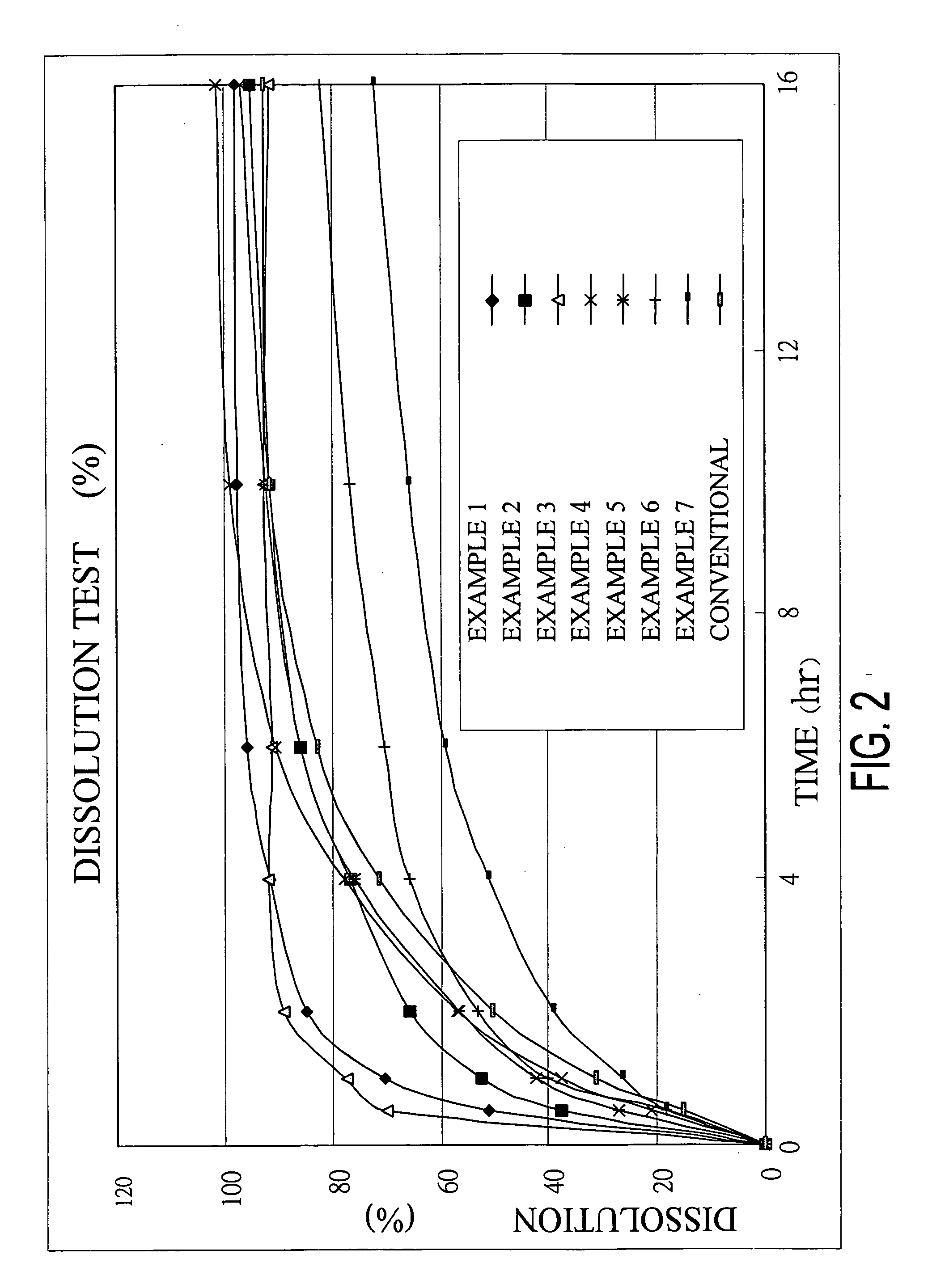
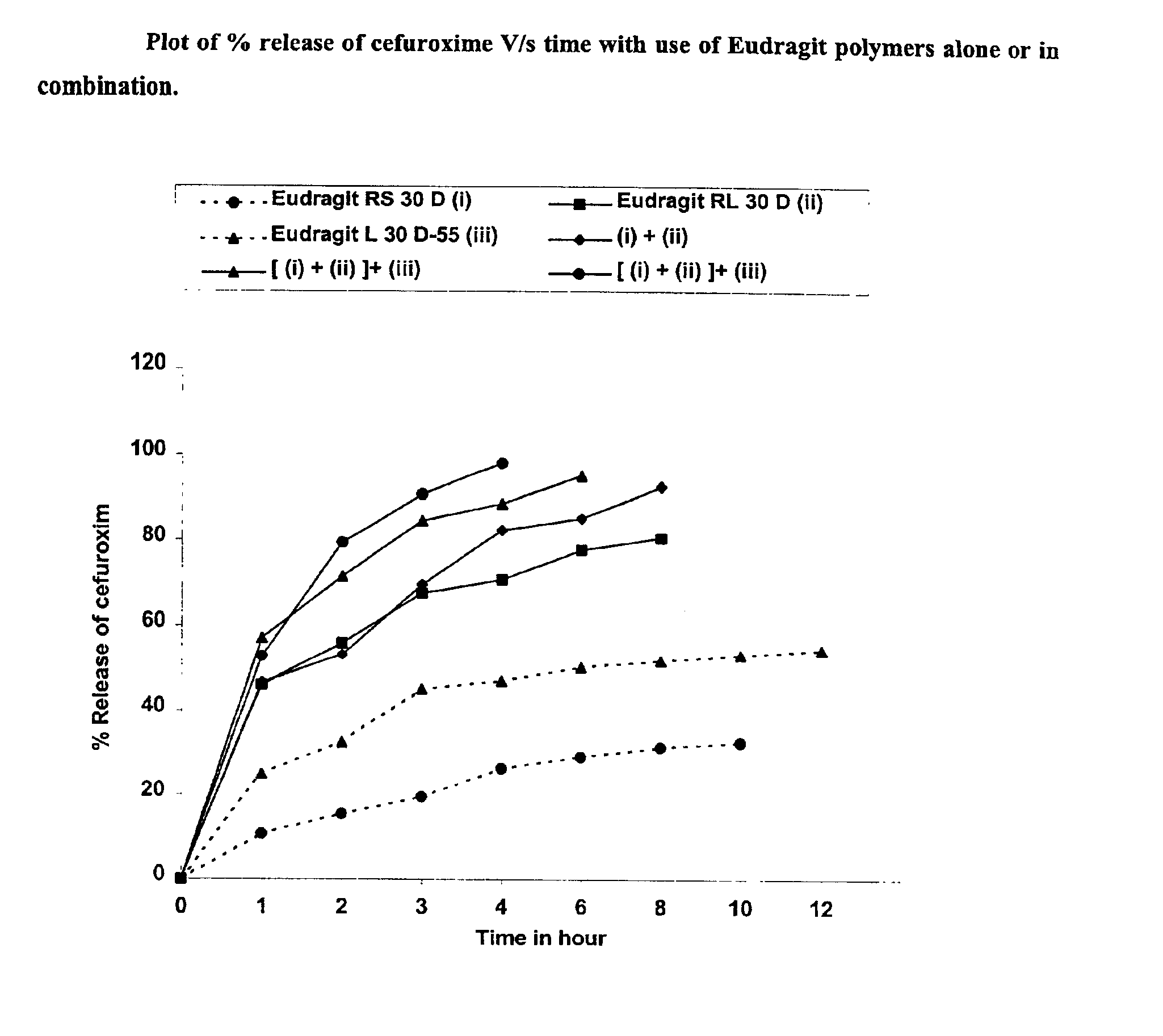
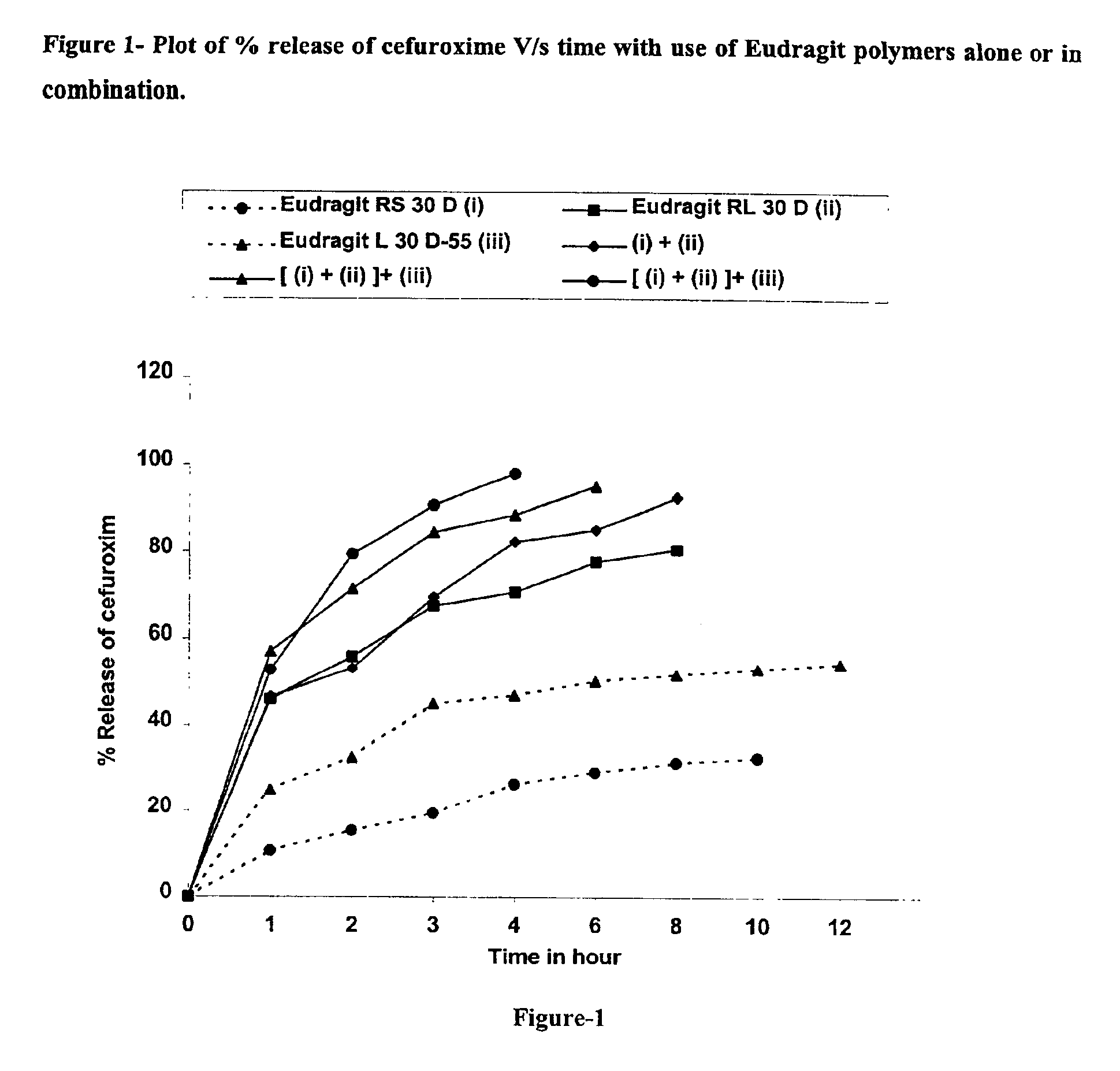
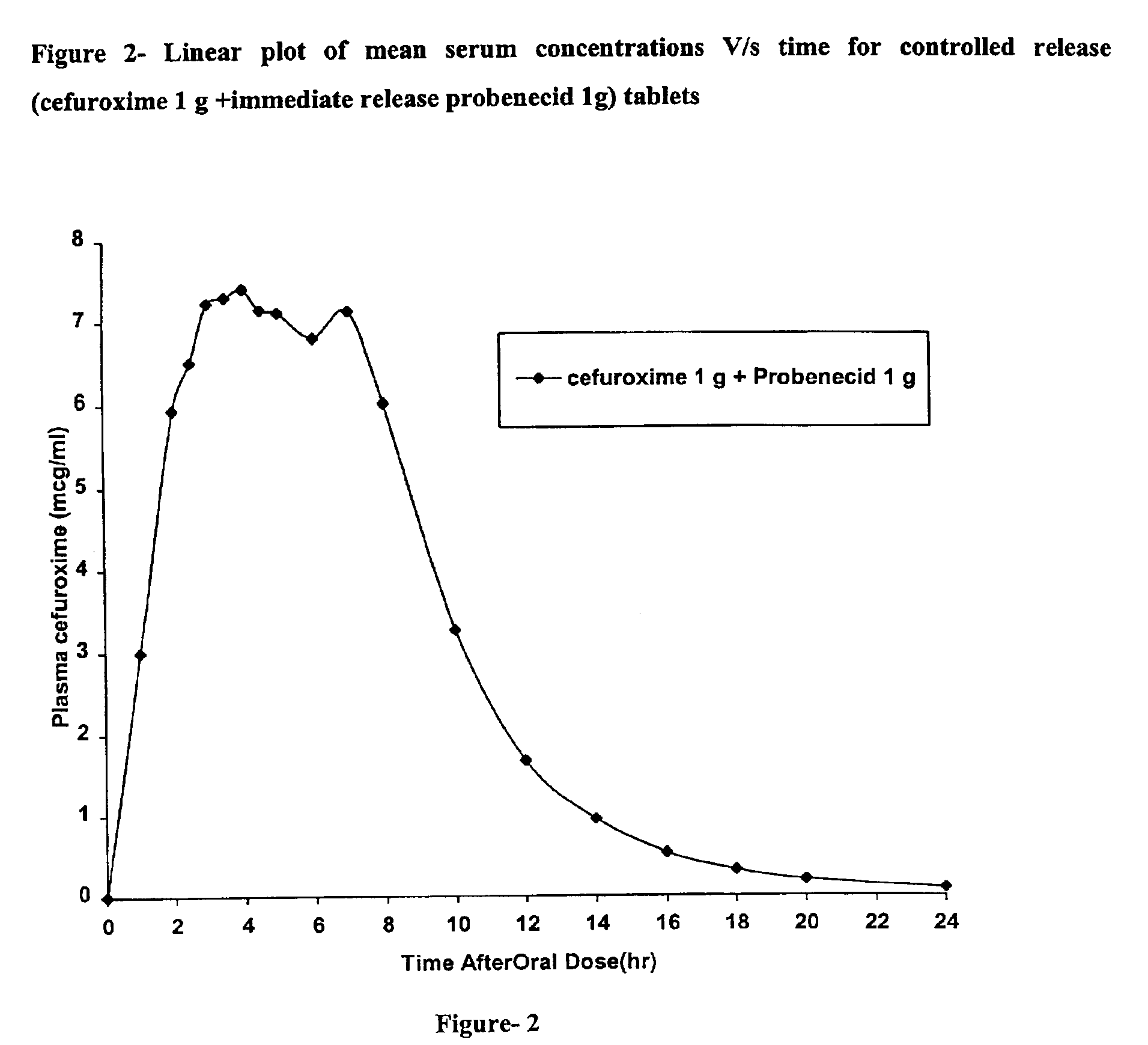
Rapidly disintegrating sustained release cefuroxime axetil composition
InactiveUS6932981B2Increased blood levelsGood curative effectPharmaceutical non-active ingredientsMicrocapsulesMethacrylateControlled release
A fast disintegrating controlled release oral composition comprising a core material containing cefuroxime axetil present as controlled release form, the cefuroxime axetil being provided with an outer coating of a copolymer selected from aqueous dispersions of enteric methacrylic acid and methacrylic acid esters anionic copolymers having carboxyl group as the functional group or mixtures thereof and an inner coating of a sustained-release copolymer selected from aqueous dispersions of acrylate and methacrylate pH independent copolymers having quaternary ammonium group as a functional group or mixtures thereof, and optionally probenecid. Additionally, the coating composition may contain plasticizers. The composition is suitable for once daily administration.
Owner:LUPIN LABORATORIES LTD
Pharmaceutical compositions of metabotropic glutamate 5 receptor (MGLU5) antagonists
Pharmaceutical compositions of metabotropic glutamate 5 receptor (mGlu5) antagonists or a pharmacologically acceptable salt thereof are disclosed. The compositions contain the therapeutic active compound with non-ionic polymer and ionic polymer, binder and fillers in either matrix pellet, matrix tablet or coated pellets. The compositions provide a pH-independent in vitro release profile with NMT 70% in one hour, NMT 85% in 4 hour, and NLT 80% in 8 hours. The compositions are useful for the treatment of CNS disorders, such as Treatment-Resistant Depression (TRD) and Fragile X Syndrome.
Owner:CHATTERJI ASHISH +5
Extended-release dosage form
Provided are pharmaceutical formulations comprising sustained release particles each having an inner core bead comprising an active pharmaceutical ingredient an intermediate coating substantially surrounding the inner core bead, and an outer coating substantially surrounding the intermediate coating comprising a pH independent polymer. Also provided is a pharmaceutical formulation comprising two bead populations wherein each of the first and second bead populations have a different drug release profile. Also provided is a method of preparing an extended release dosage composition comprising one or more bead populations.
Owner:MYLAN PHARMA INC
Modified release pharmaceutical composition and a process of making the same
The present invention refers to a modified release pharmaceutical composition comprising an in-situ gelling agent (=0.5 % w / w) and a gellation facilitating agent (e.g. calcium sulfate) in an amount of 2-17.5 % w / w. Additionally, the composition contains a release rate controlling polymer such as an acrylate and optionally a pH independent rate controlling polymer such as HPMC. A preferred active agent is mycophenolate mofetil. A process of making the above described composition is also disclosed.
Owner:PANACEA BIOTEC
Sustained release tamsulosin formulations
A sustained release tamsulosin formulation contains tamsulosin, a hydrophobic polymer, a microsphere forming agent and a diluent. The hydrophobic polymers include pH-dependent and pH-independent polymers are used as the release-modulating agent to control the dissolution profile of tamsulosin formulation so that the formulation releases tamsulosin slowly and continuously as the formulation passed through the stomach and gastrointestinal tract. The present invention further relates to a method for preparing the sustained release tamsulosin formulation.
Owner:STANDARD CHEM & PHARMA
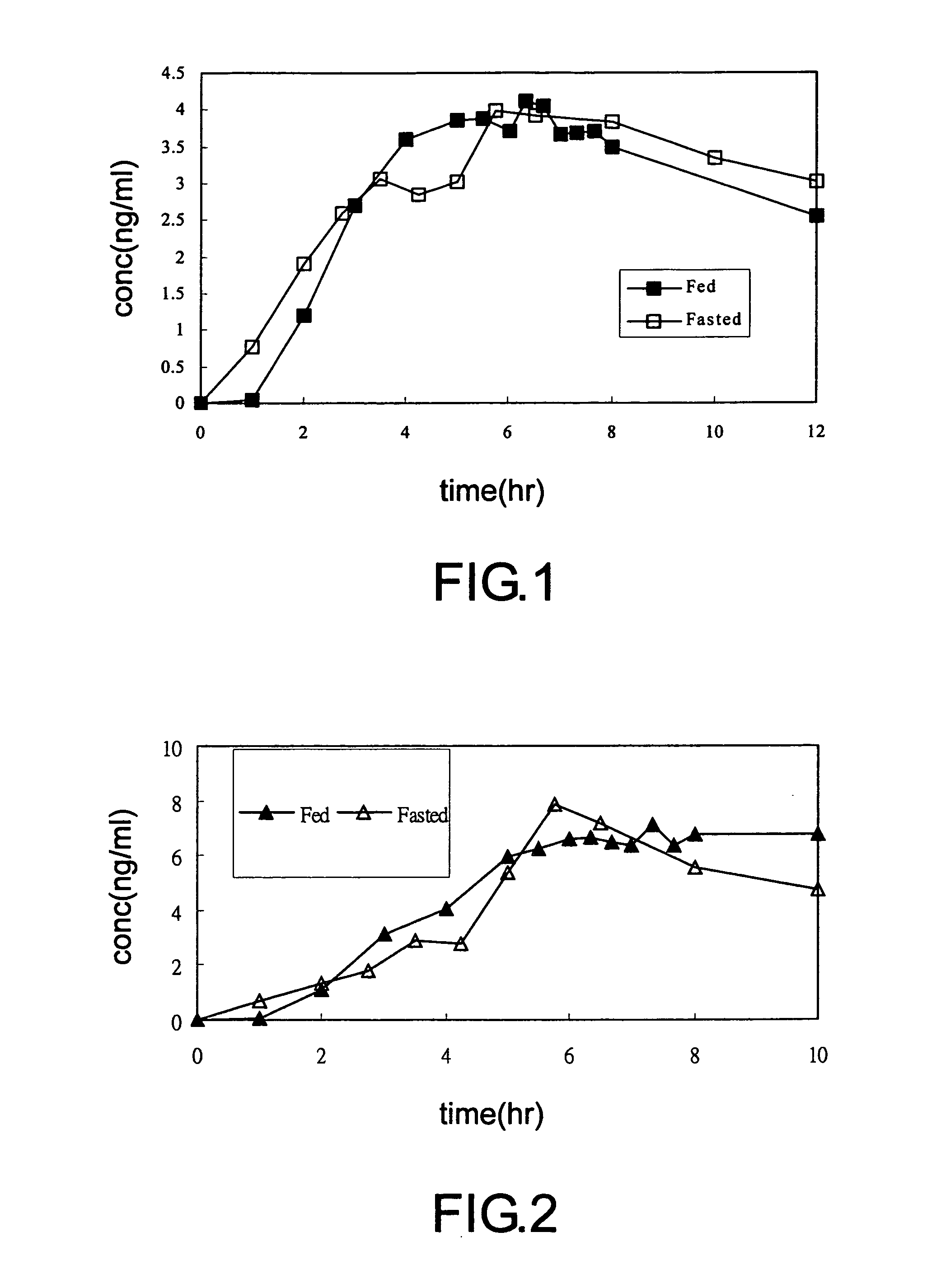
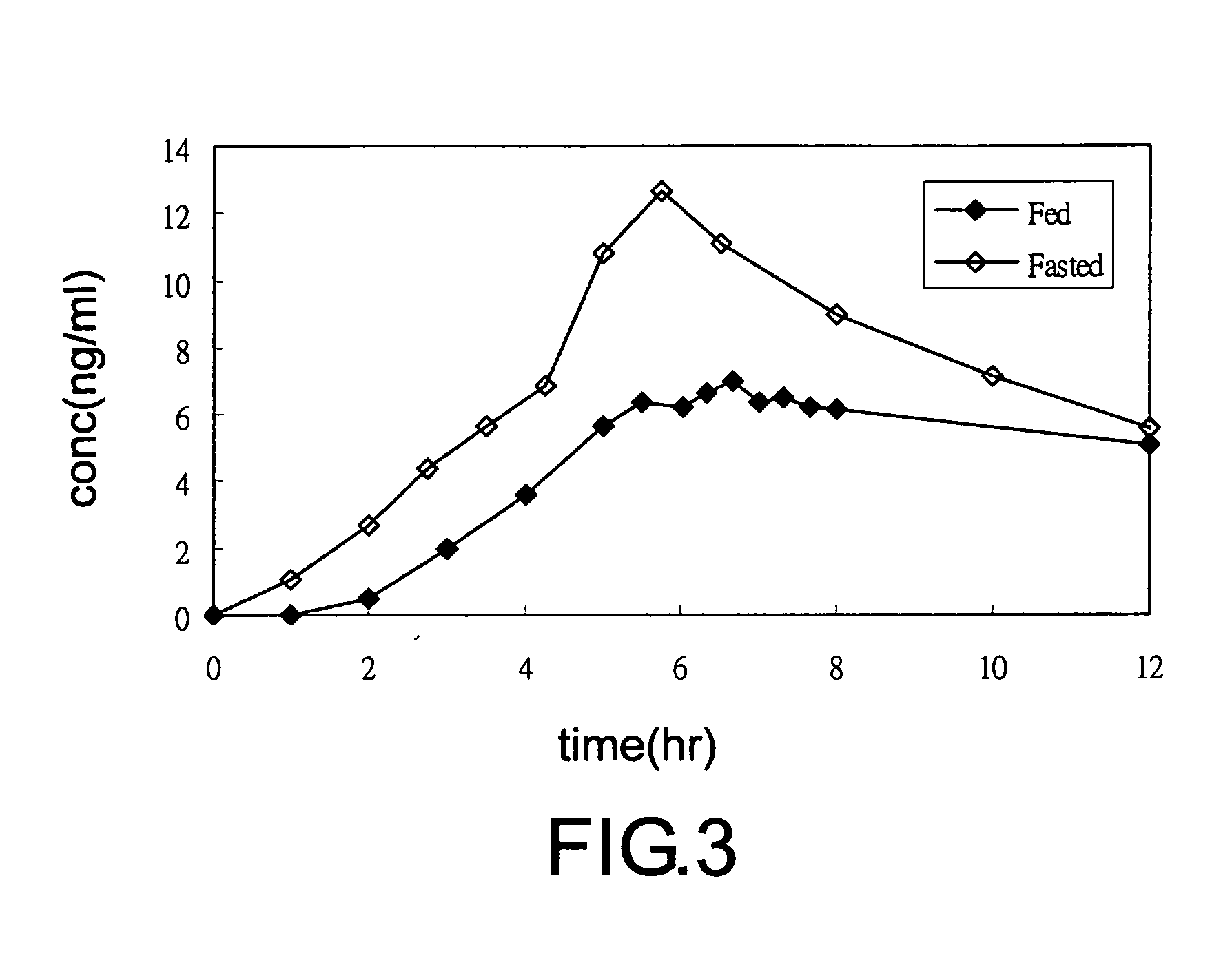
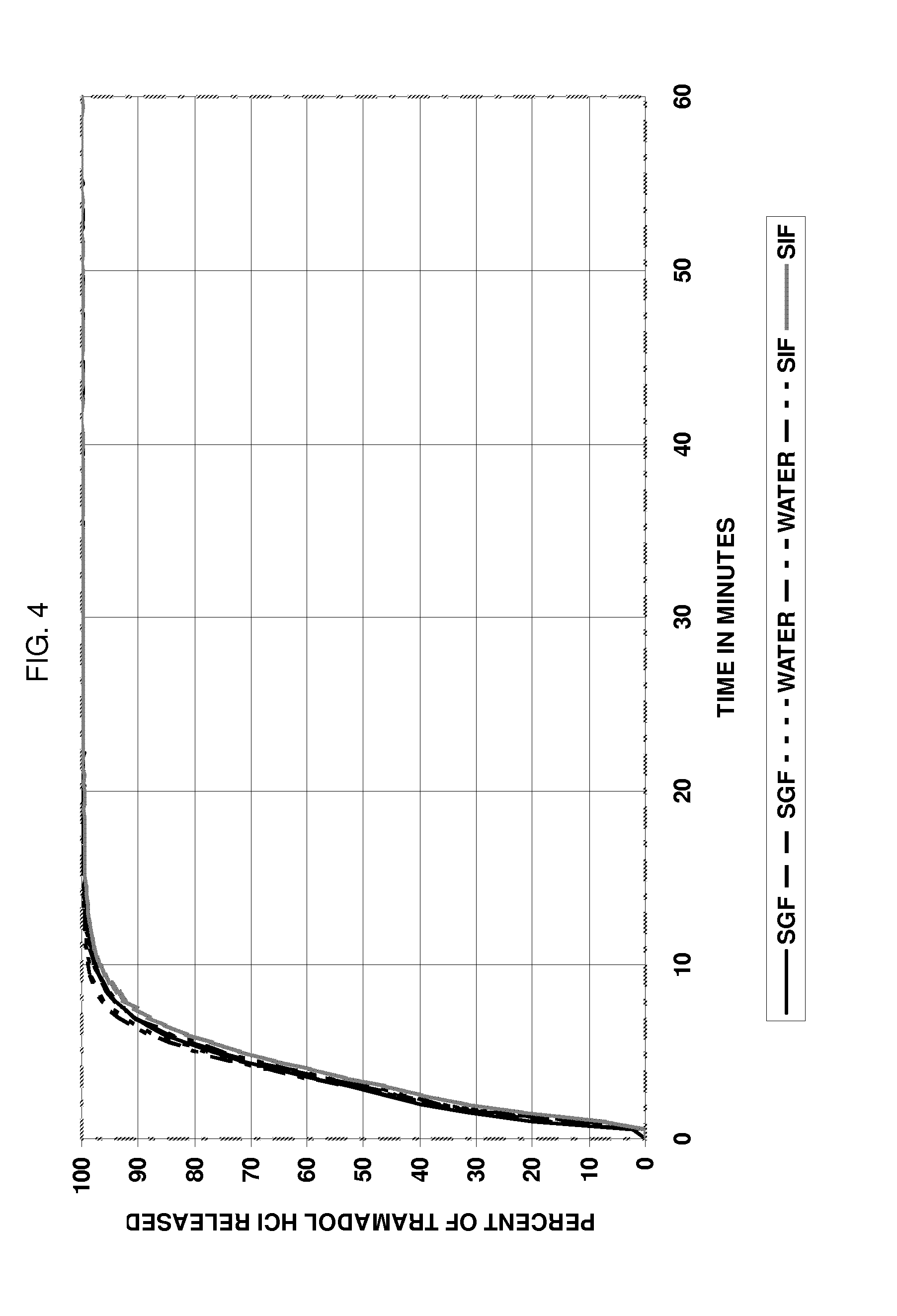
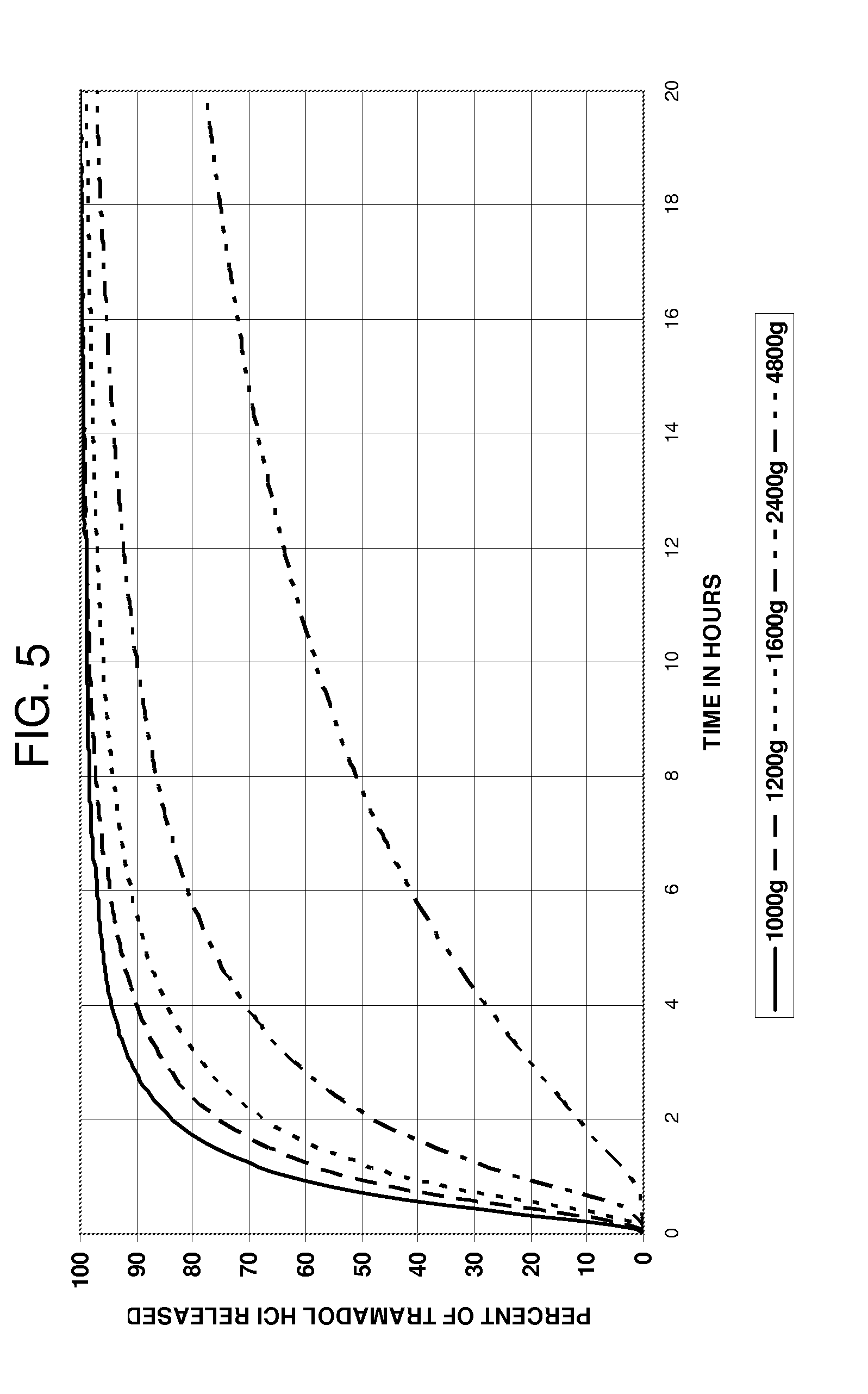
Multiparticulate formulation having tramadol in immediate and controlled release form
InactiveUS20100047343A1Reduced food-effectBiocideOrganic active ingredientsControlled releaseOral medication
A multi-particulate pharmaceutical composition of rapid release particles and controlled release particles comprising tramadol or a salt thereof is provided. The composition provides a rapid and controlled release of tramadol or a salt thereof in a substantially pH independent manner after oral administration to a subject. The composition can be included in a capsule, caplet, sachet, or other solid dosage form adapted to retain and then release solid pharmaceutical compositions.
Owner:UNIVERSITY OF KANSAS
Extended release film-coated capsules
Pharmaceutical formulations, preferably in the form of softgel capsules or hard-shell capsules, exhibit extended release through the use of a coating comprising a water-insoluble polymer and a pH-independent pore former. Extended release from softgel capsules and hard-shell capsules can be achieved without the use of lipid-based semi-solid or solid materials.
Owner:R P SCHERER TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com