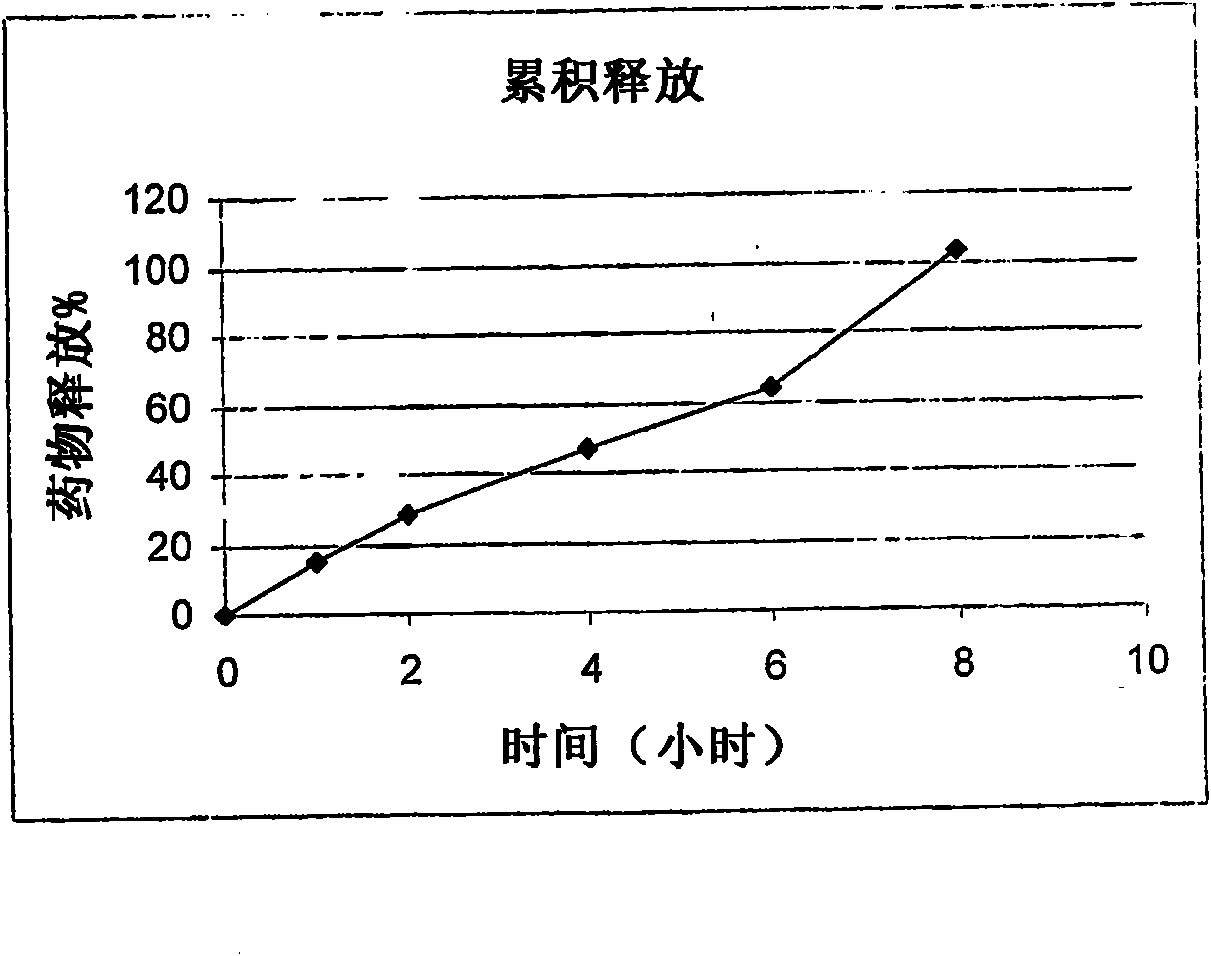
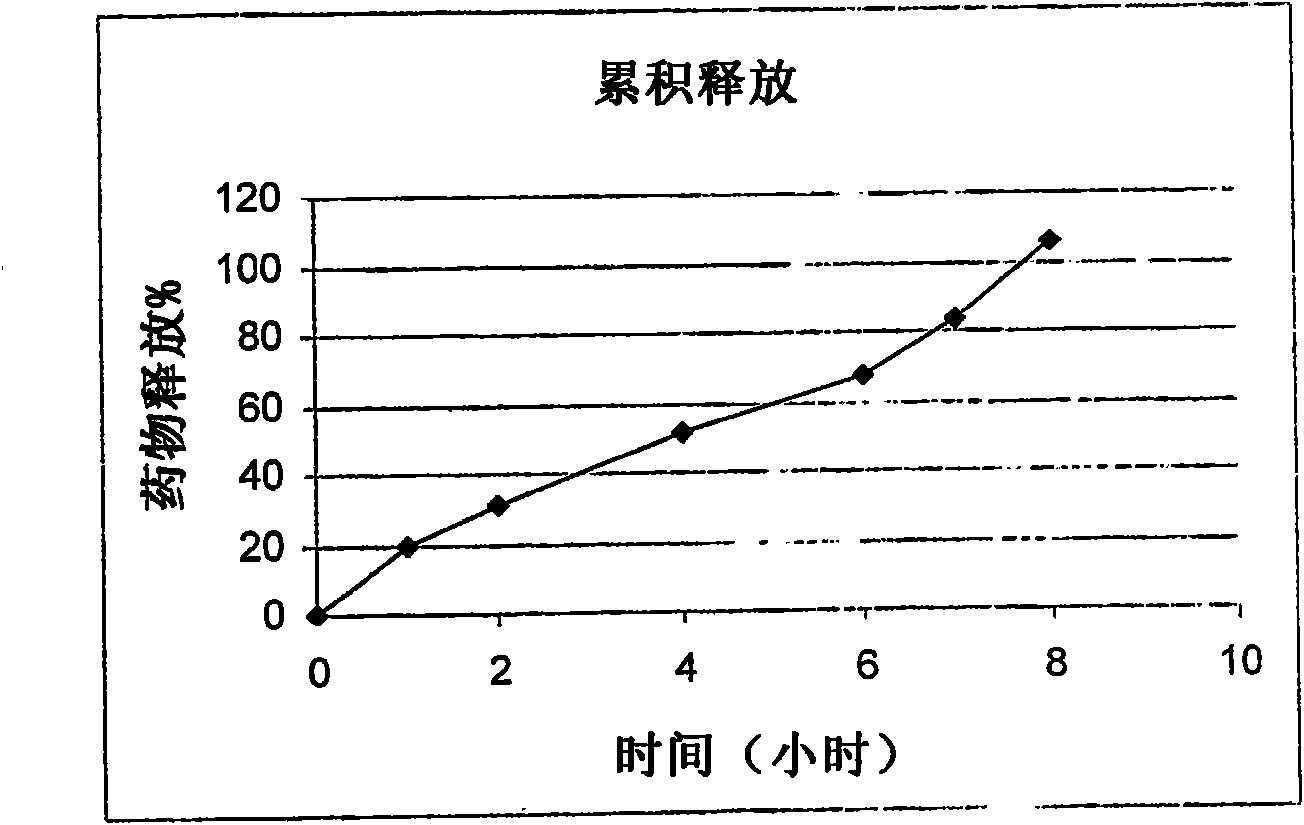
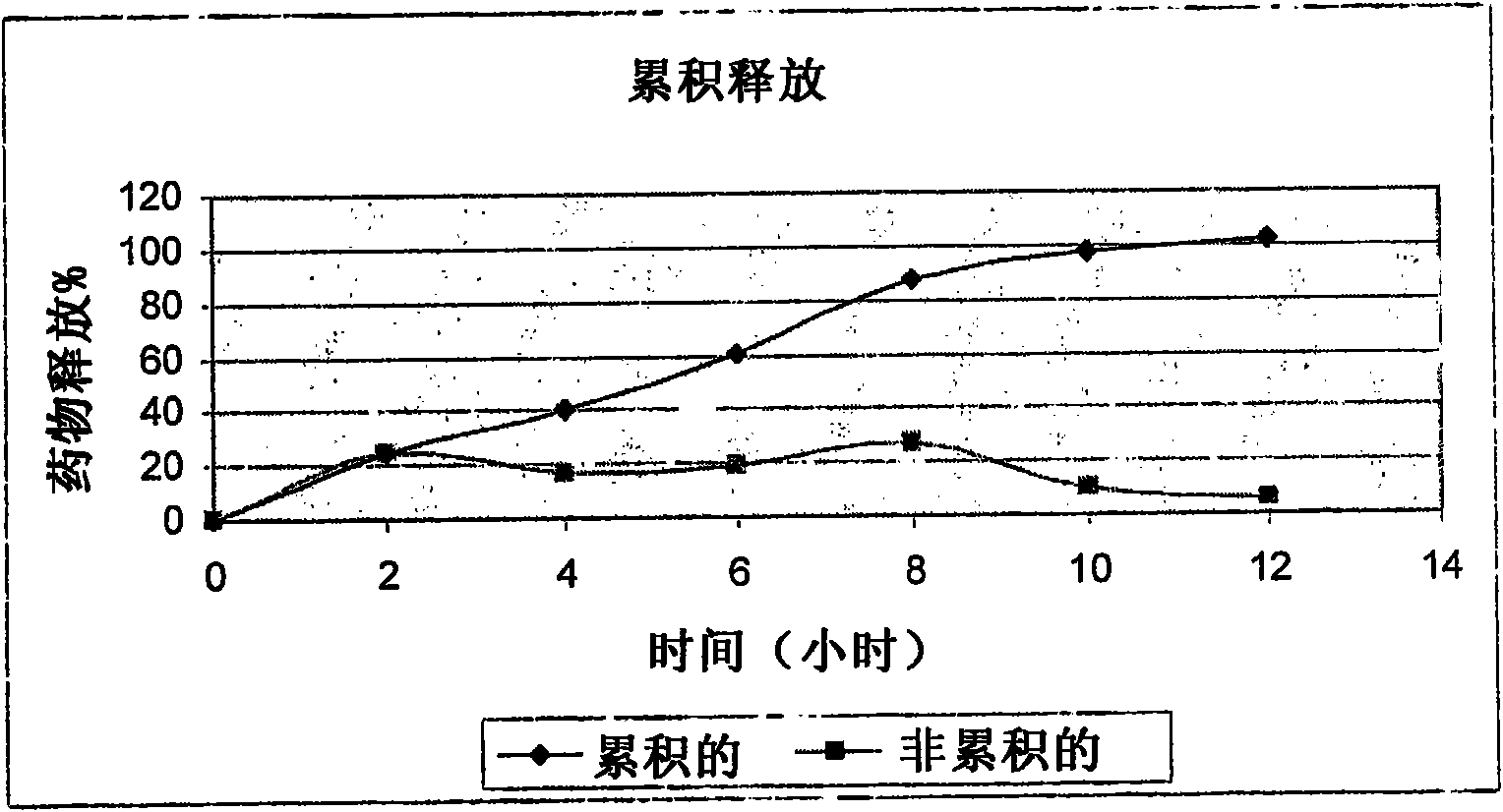
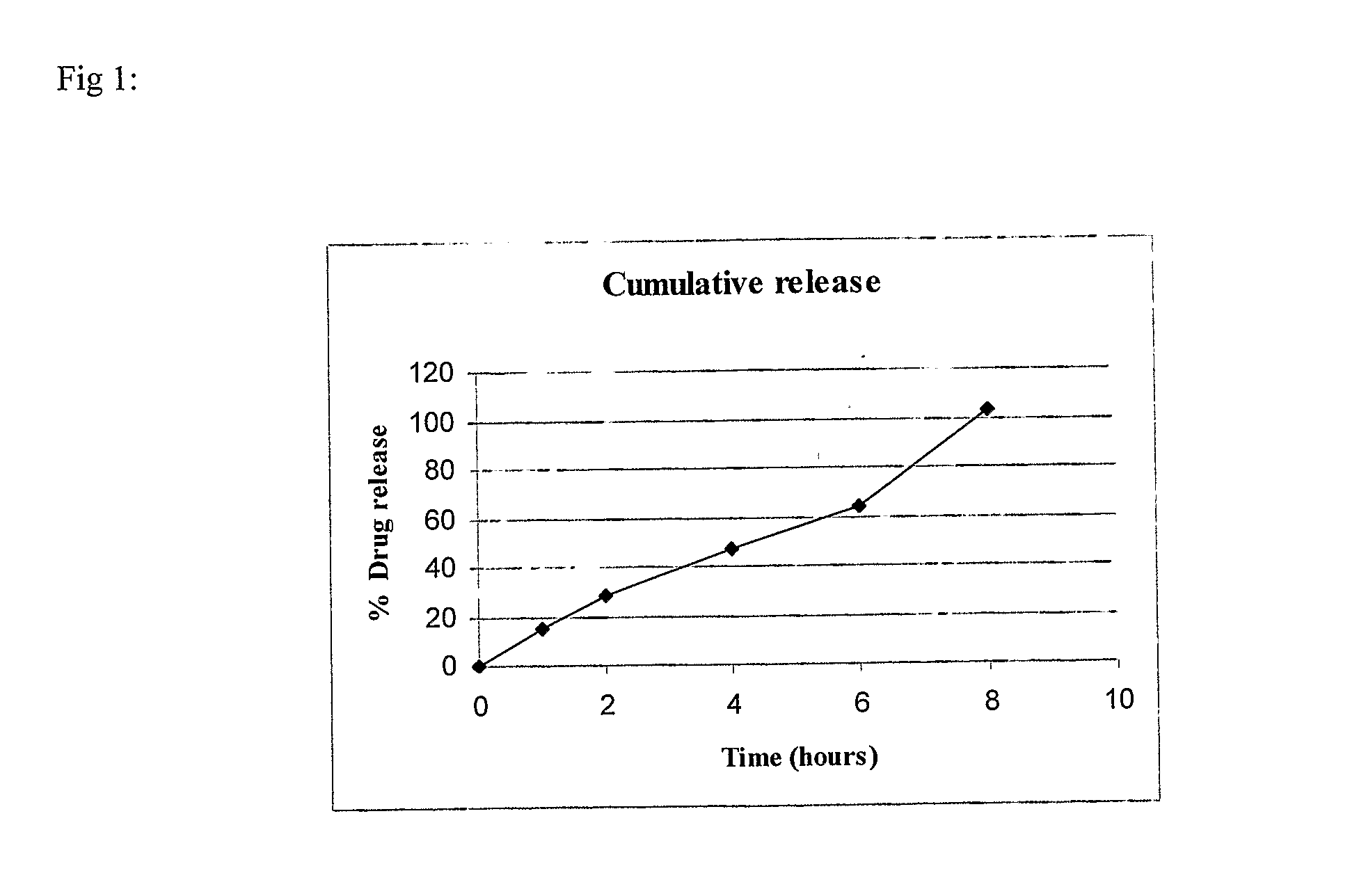
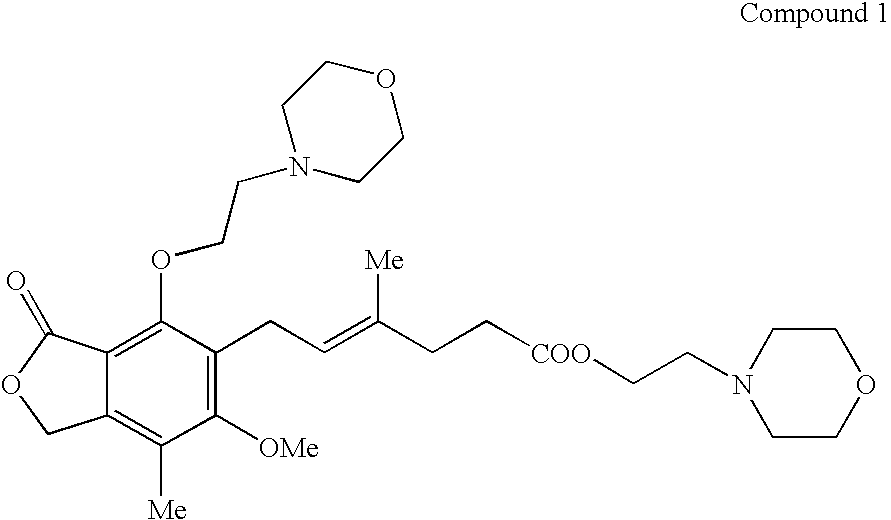
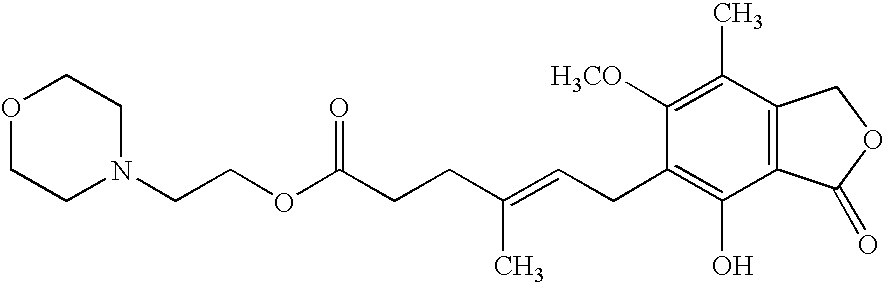
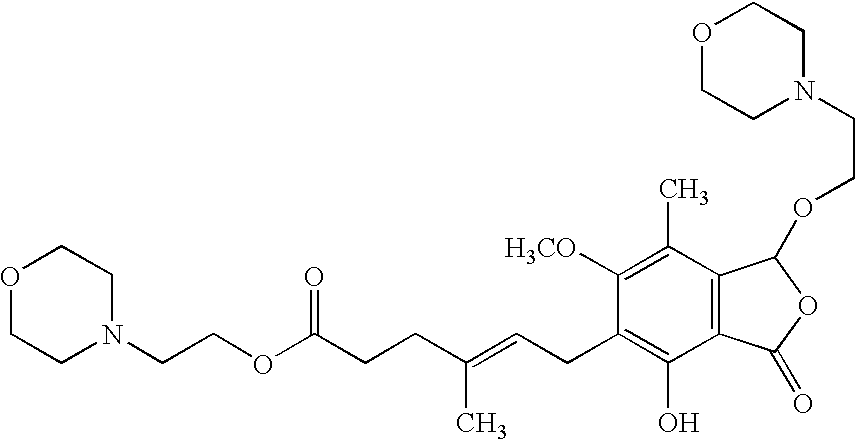
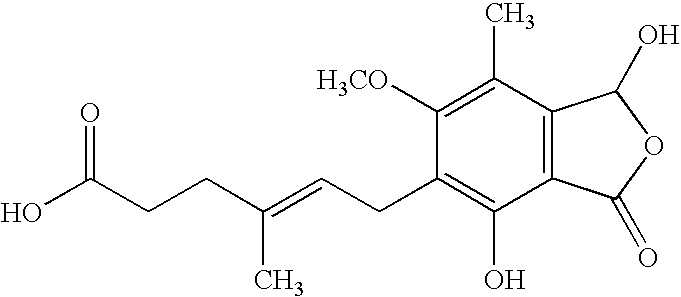
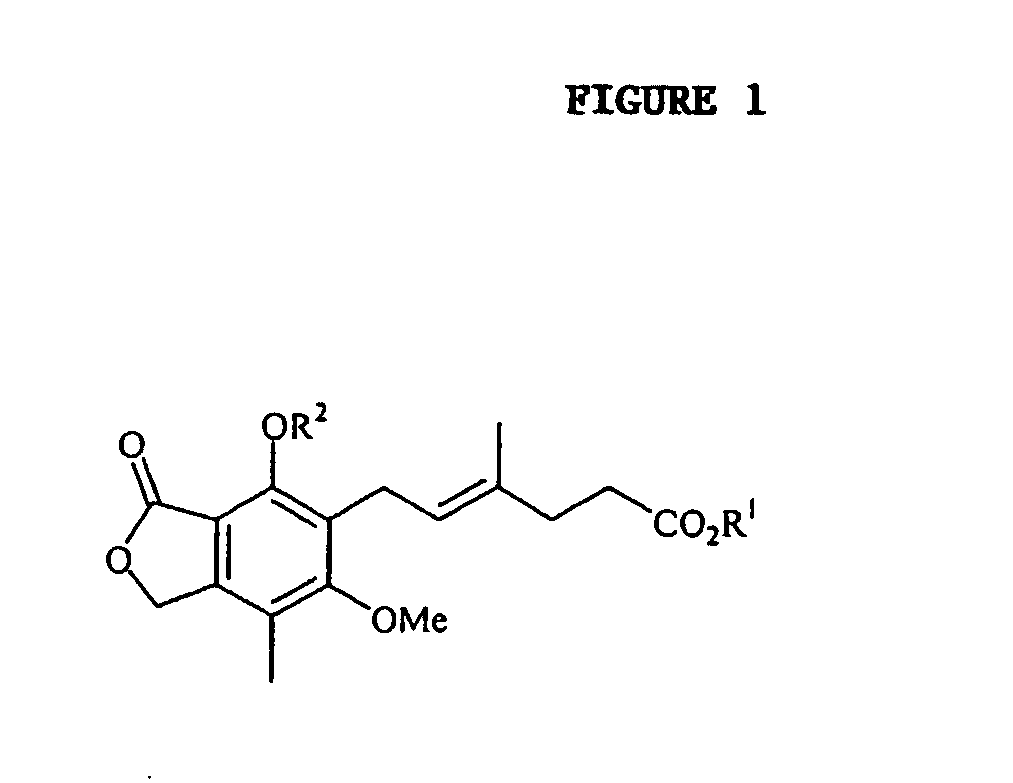
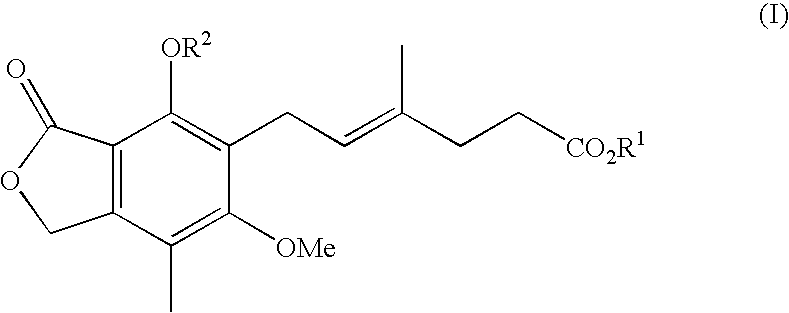
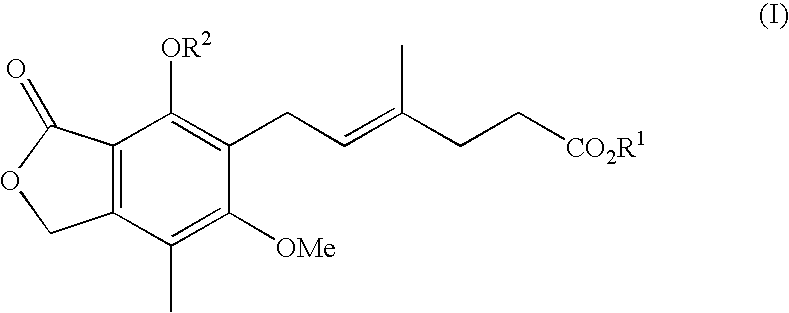
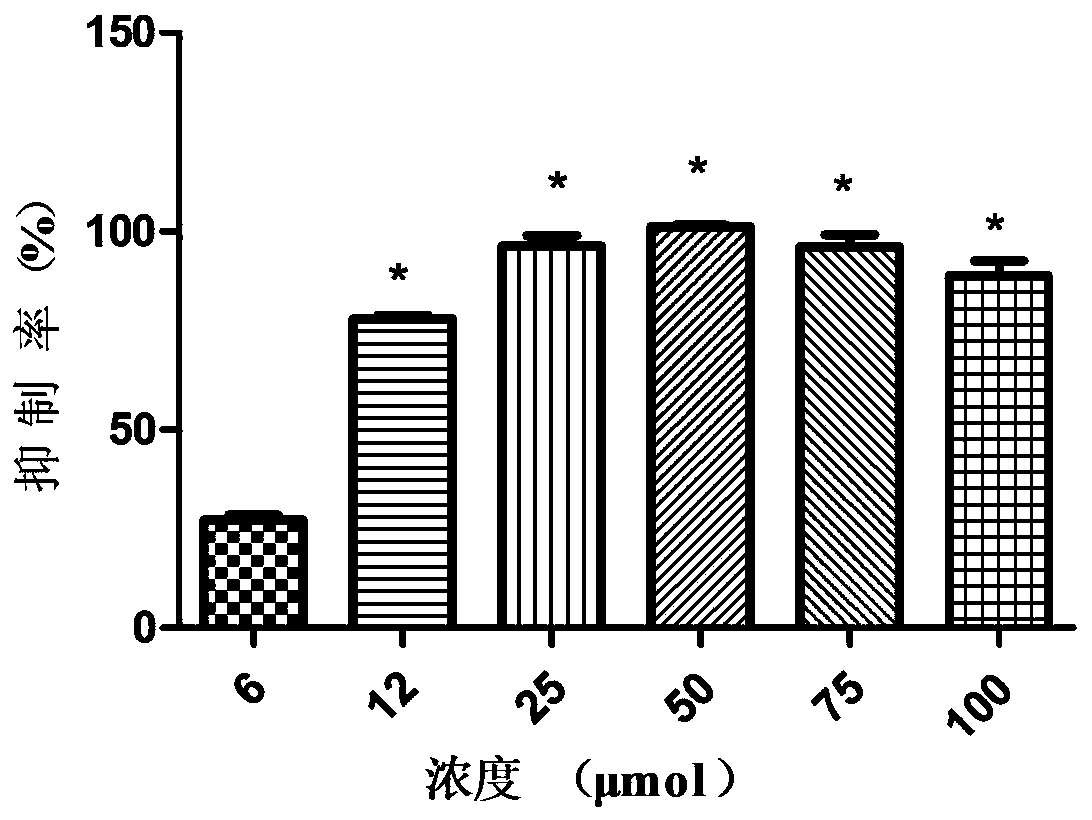
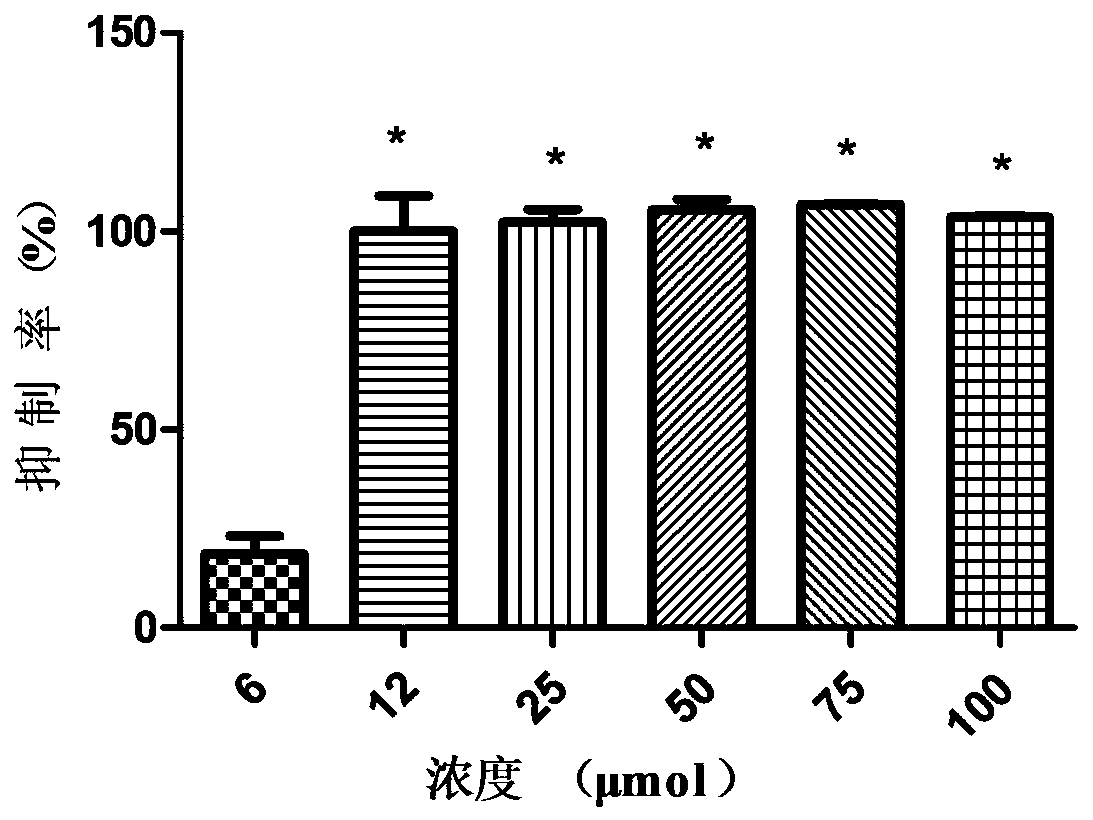
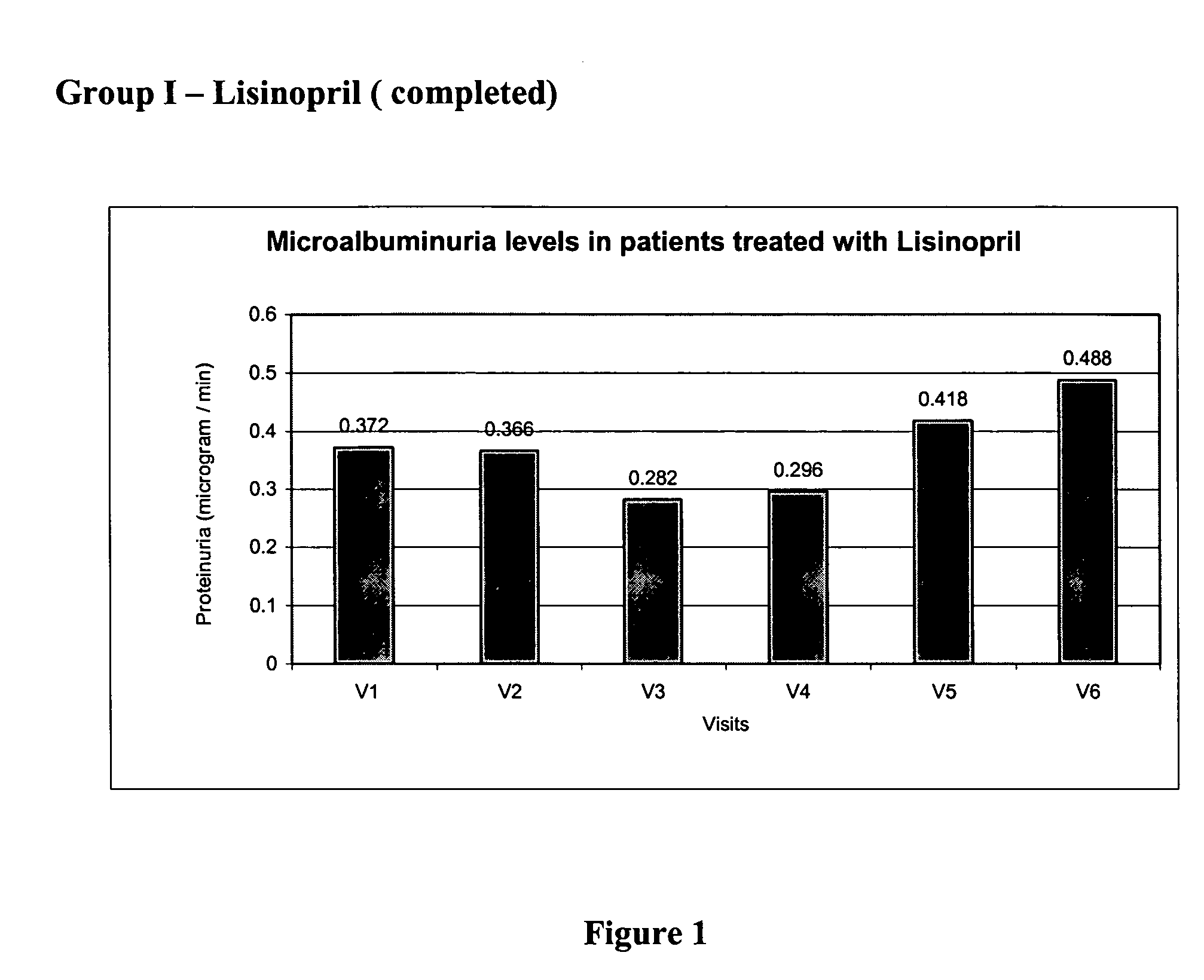
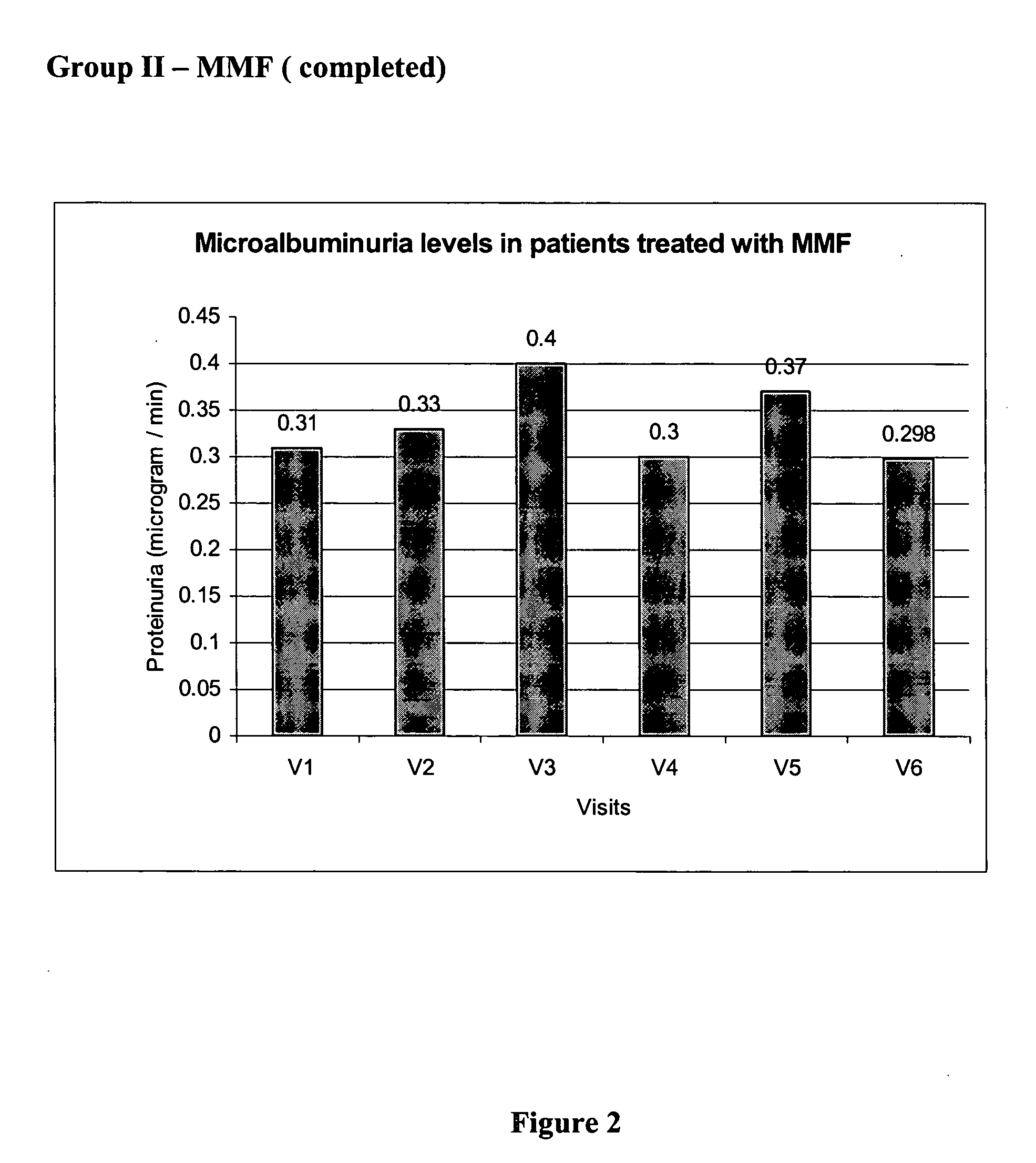
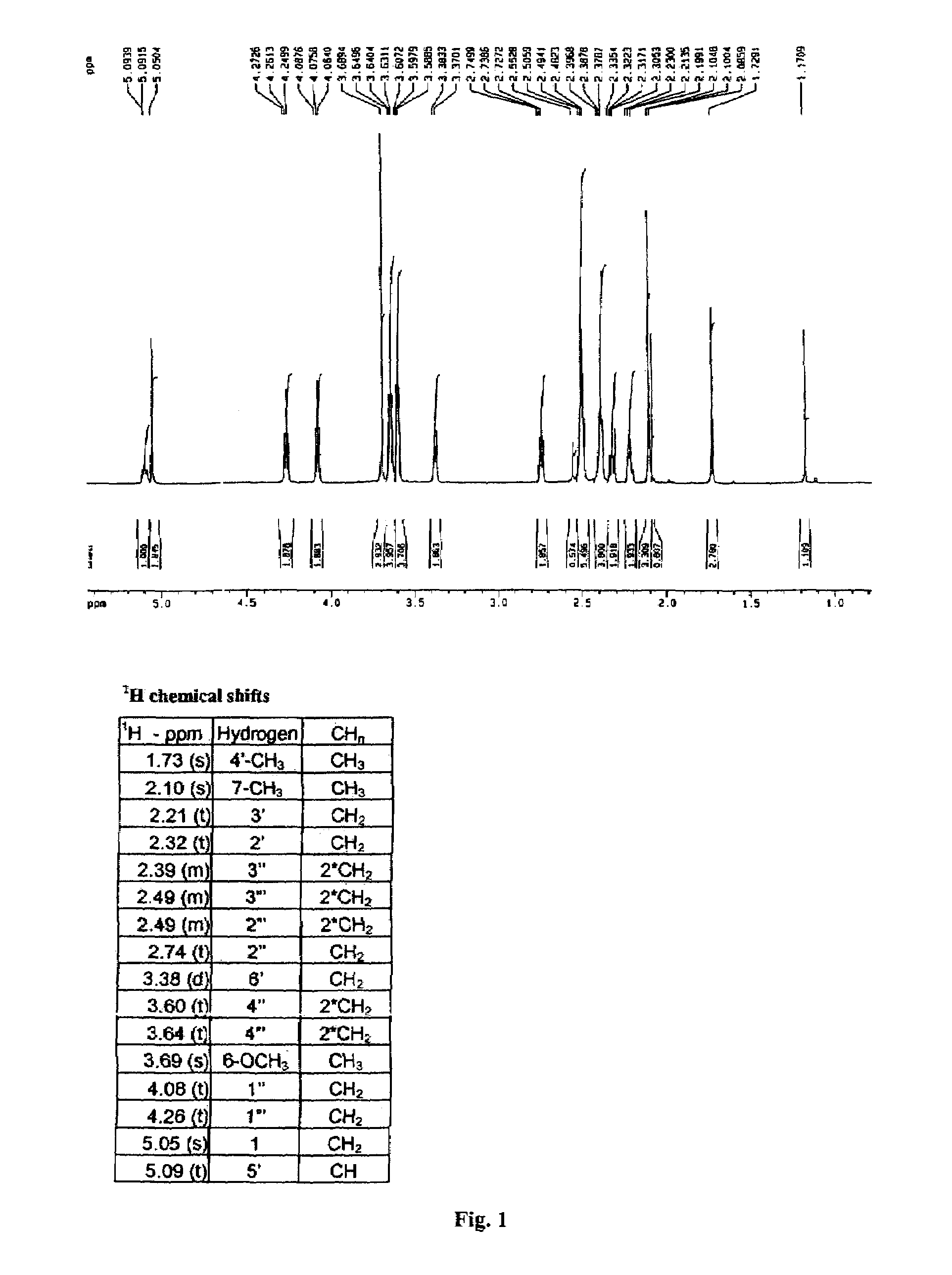
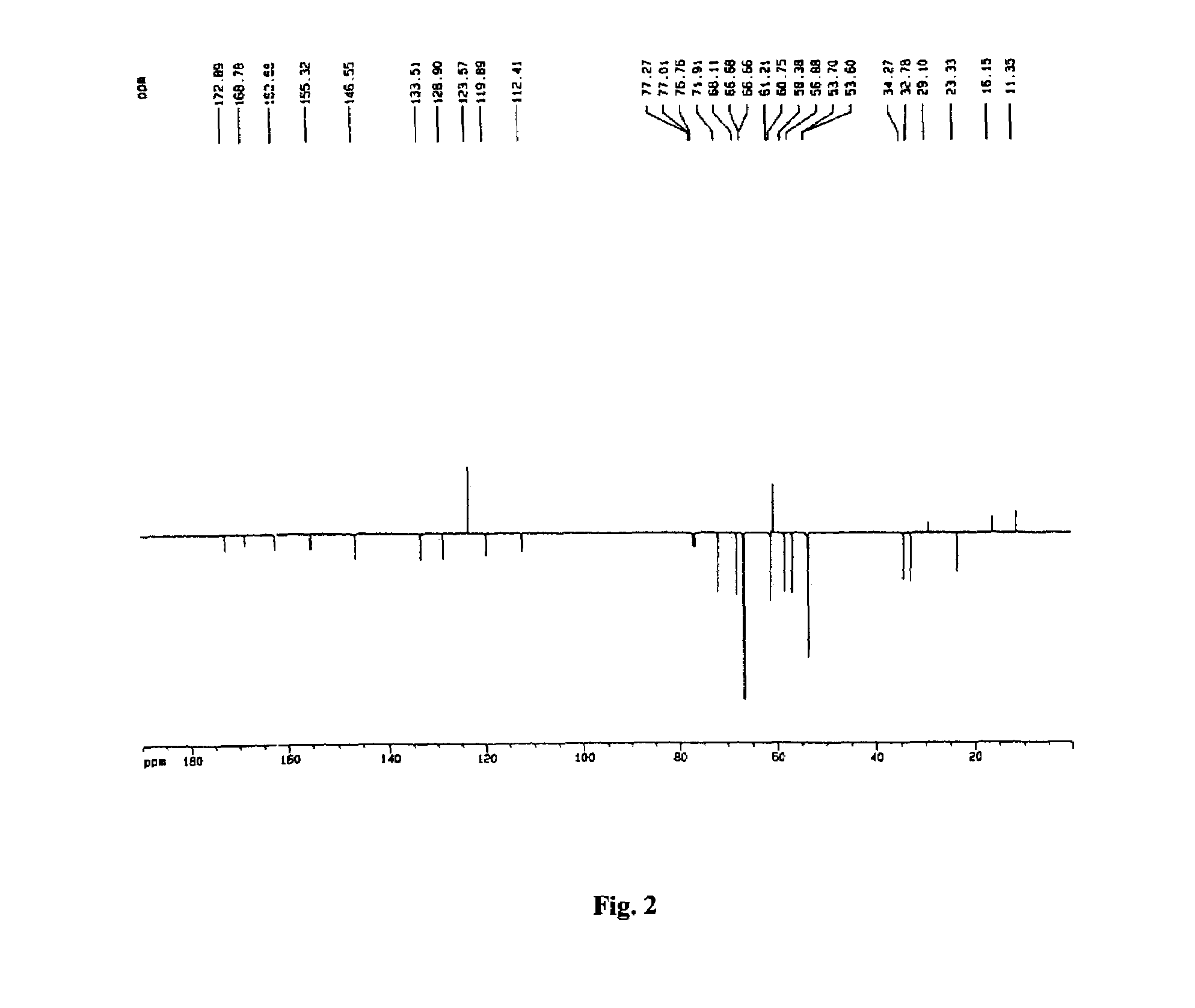
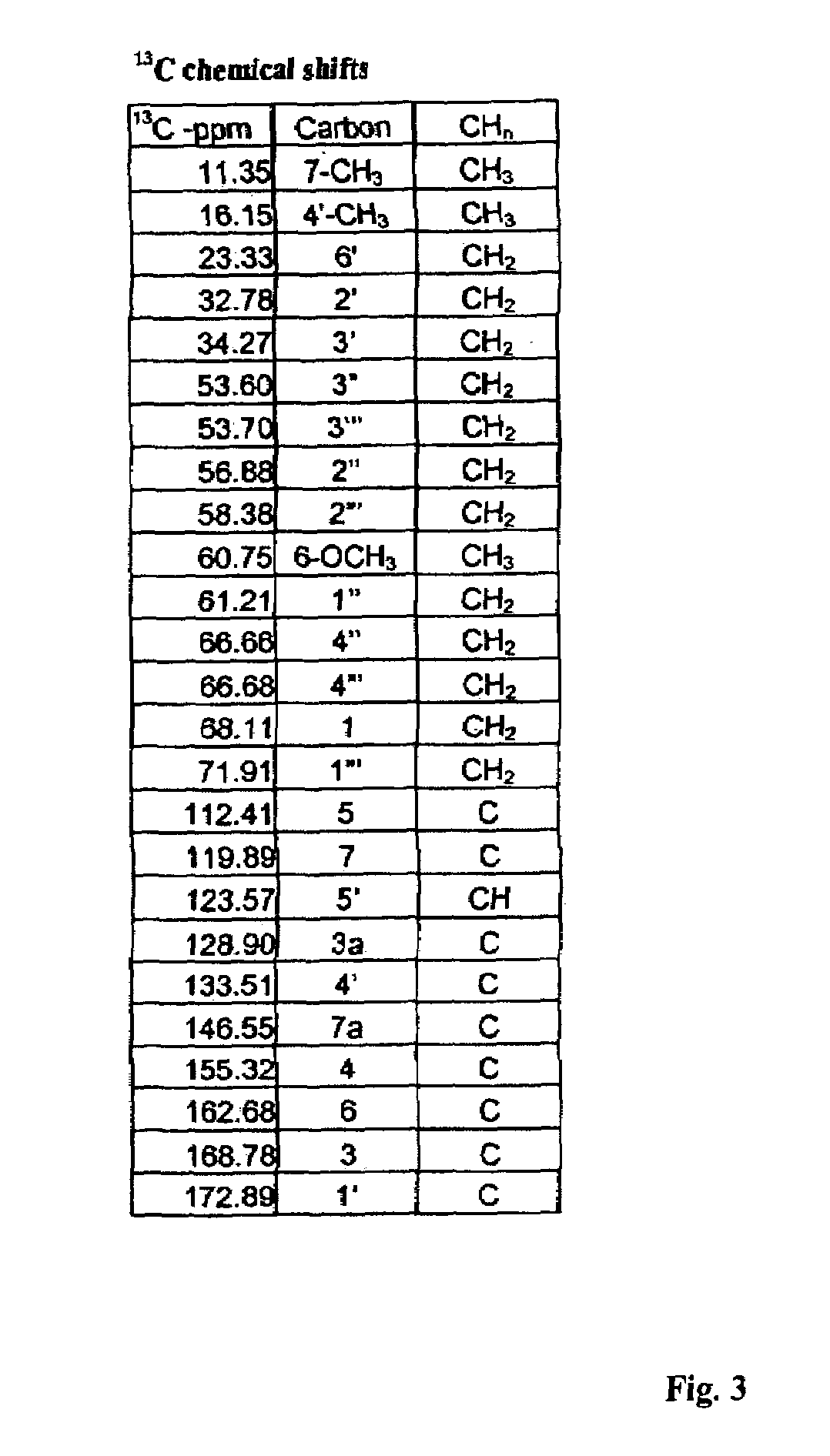
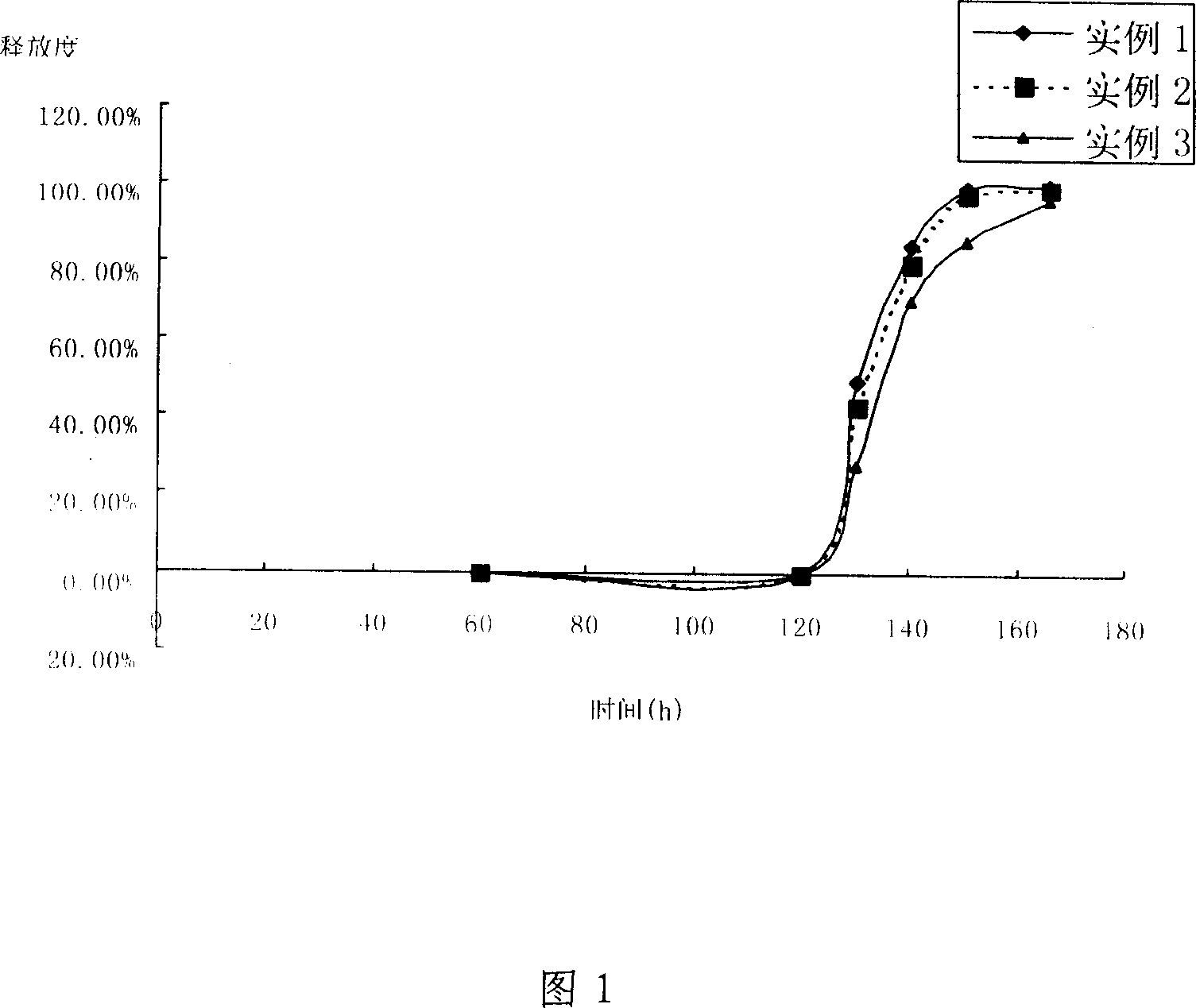
Patents
Literature
69 results about "Mycophenolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
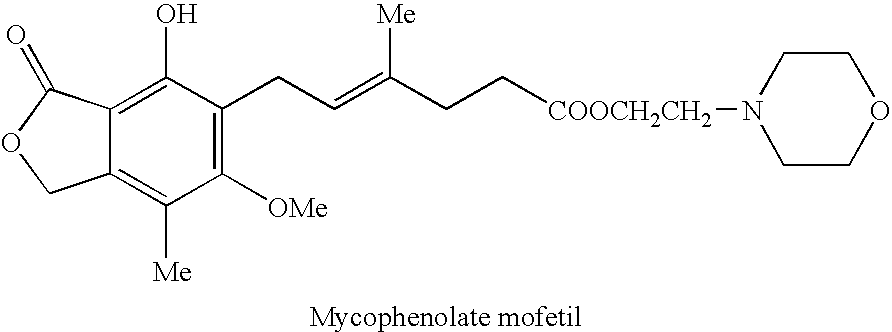
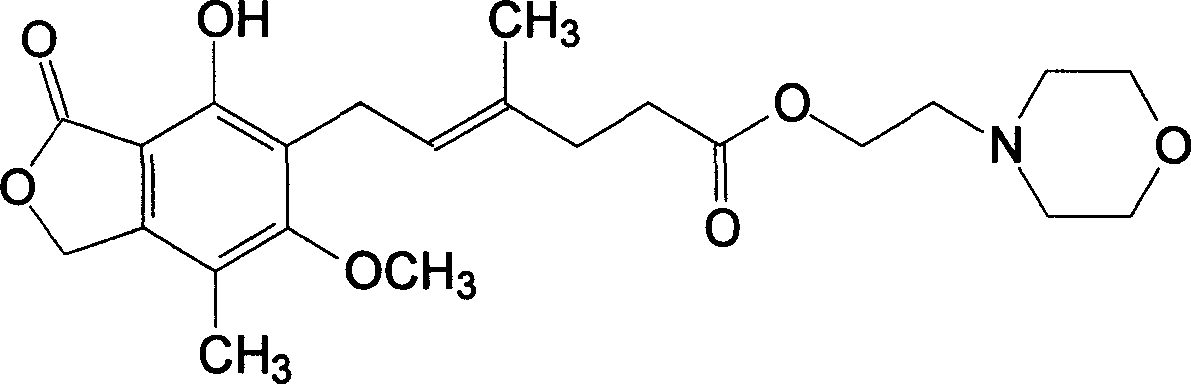
The morpholinoethyl ester of mycophenolic acid (MPA). As an immunosuppressive agent in vivo, the active metabolite mycophenolate reversibly inhibits inosine 5'-monophosphate dehydrogenase (IMPDH), an enzyme involved in the de novo synthesis of guanine nucleotides, thereby retarding T-cell and B-cell proliferation. MPA displays high lymphocyte specificity and cytotoxicity because lymphocyte metabolism is highly dependent on both salvage and de novo synthesis of guanine nucleotides. (NCI04)
Modified release pharmaceutical composition and a process of making the same
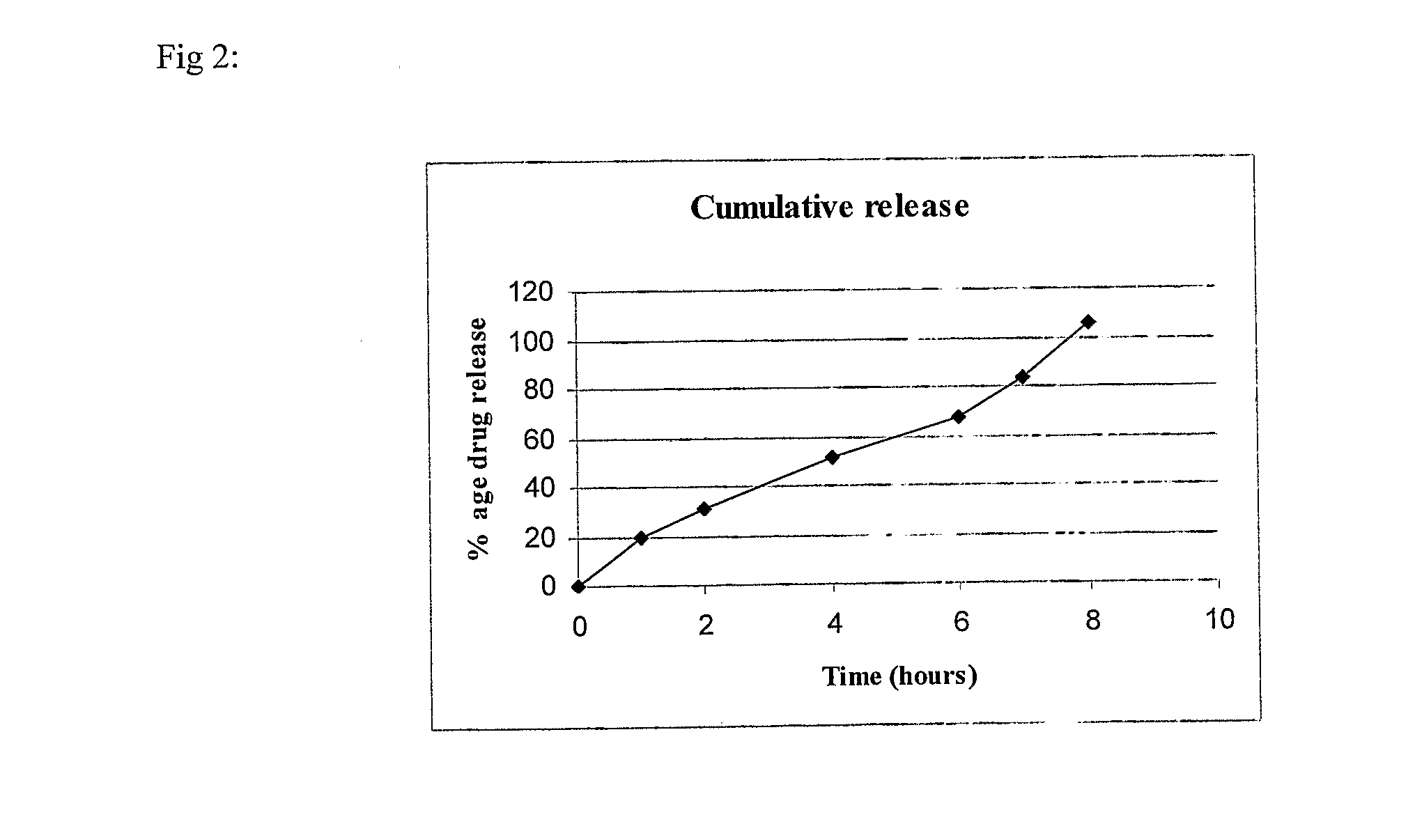
The present invention refers to a modified release pharmaceutical composition comprising an in-situ gelling agent (=0,5 % w / w) and a gellation facilitating agent (e.g. calcium sulfate) in an amount of 2-17,5 % w / w. Additionally, the composition contains a release rate controlling polymer such as an acrylate and optionally a pH independent rate controlling polymer such as HPMC. A preferred active agent is mycophenolate mofetil. A process of making the above described composition is also disclosed.
Owner:PANACEA BIOTEC
Modified release pharmaceutical compositions comprising mycophenolate and processes thereof
InactiveCN101969931AMaintain therapeutic effectEasy to solveOrganic active ingredientsPill deliveryDrugs levelsActive agent
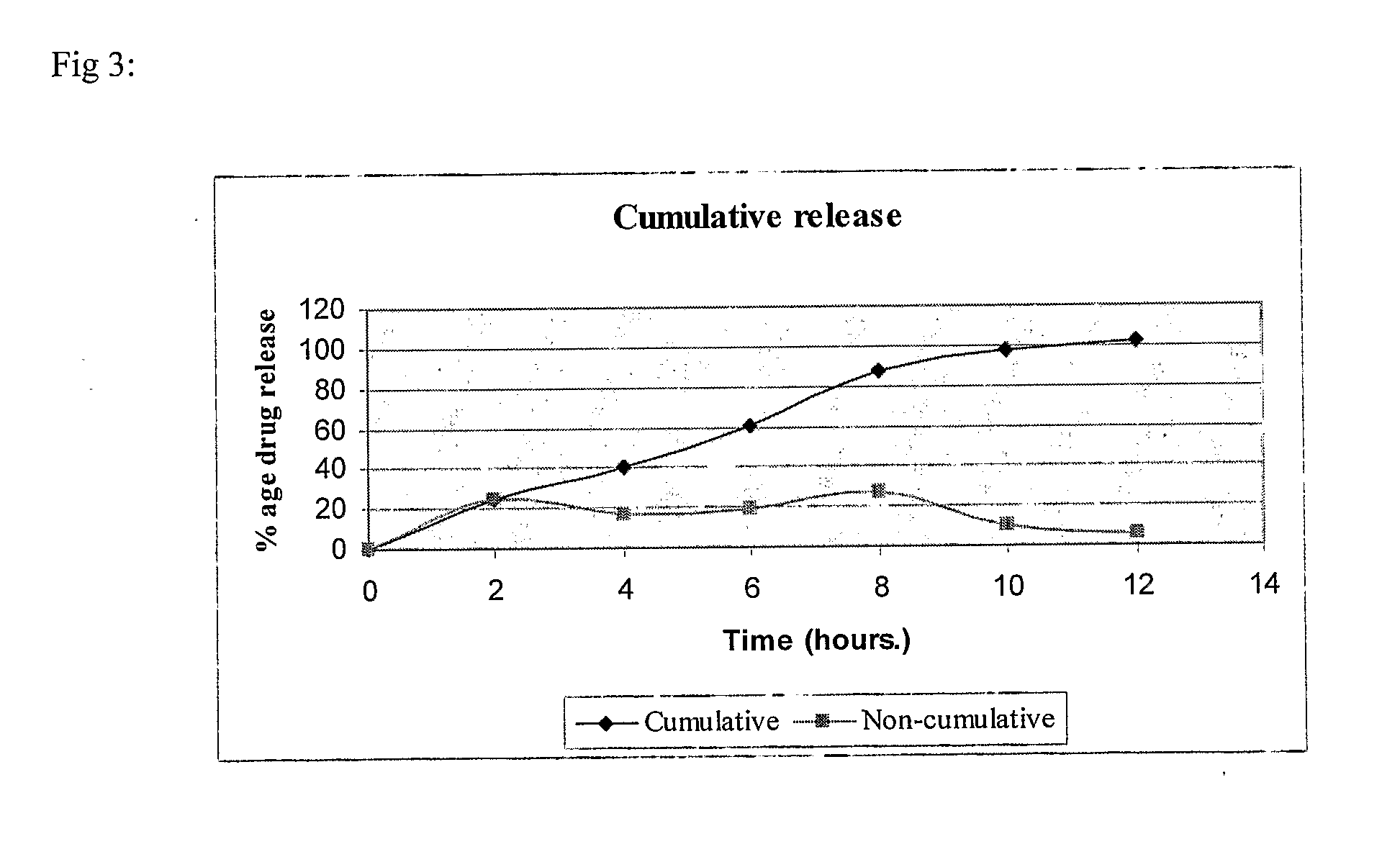
Modified release pharmaceutical compositions comprising mycophenolate as the active agent or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof, wherein the said composition exhibits a biphasic release profile when subjected to in- vitro dissolution and / or upon administration in- vivo are provided. The composition provides a drug release in a manner such that the drug levels are maintained above the therapeutically effective concentration (EC) constantly for an extended duration of time. Further, the difference between the maximum plasma concentration of the drug (Cmax) and the minimum plasma concentration of the drug (Cmjn), and in turn the flux defined as ((Cmax - Cmjn) / Cavg) is minimal. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such compositions.
Owner:PANACEA BIOTEC
Modified release pharmaceutical compositions comprising mycophenolate and processes thereof
InactiveUS20110008426A1Easy and cost-effectiveImprove the level ofBiocidePowder deliveryDrugs levelsActive agent
Modified release pharmaceutical compositions comprising mycophenolate as the active agent or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof, wherein the said composition exhibits a biphasic release profile when subjected to in-vitro dissolution and / or upon administration in-vivo are provided. The composition provides a drug release in a manner such that the drug levels are maintained above the therapeutically effective concentration (EC) constantly for an extended duration of time. Further, the difference between the maximum plasma concentration of the drug (Cmax) and the minimum plasma concentration of the drug (Cmjn), and in turn the flux defined as ((Cmax−Cmjn) / Cavg) is minimal. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such compositions.
Owner:PANACEA BIOTEC
Active constituent of curcuma longa for inhibiting and killing plant pathogenic fungi and its preparation and application
InactiveCN101053332AHigh activityWide variety of sourcesBiocideFungicidesVerticillium speciesAdditive ingredient
The present invention discloses an inhibiting and killing plant pathogenic fungi active constituent of curcuma longa and its preparation and application. The active component is extract extracted by ethanol or methanol from curcuma longa or fine extract extracted further by n-hexane based on the extract extracted by ethanol or methanol. The inhibiting and killing plant pathogenic fungi active constituent of curcuma longa can be prepared inhibiting and killing plant pathogenic fungi and mushroom pathogenic fungi preparation, especially prepared preparation for inhibiting and killing phorna wasabiae Yokogi, sclerotinia sclerotiorum, tomato botrytis cinerea, verticillium, wheat phytoalexin, edible fungi mycogone perniciosa, olive green mycophenolate or P. pallidum. The preparation formulations can be water solution, soluble powder, wettable powder, thick suspending agent and granule. The active components disclosed by this present invention has extensive source of raw materials, no-toxic, easy degradation features, can be developed a non-pollution botanical inhibiting and killing fungi preparation.
Owner:SICHUAN UNIV
Process for preparing mycophenolate mofetil
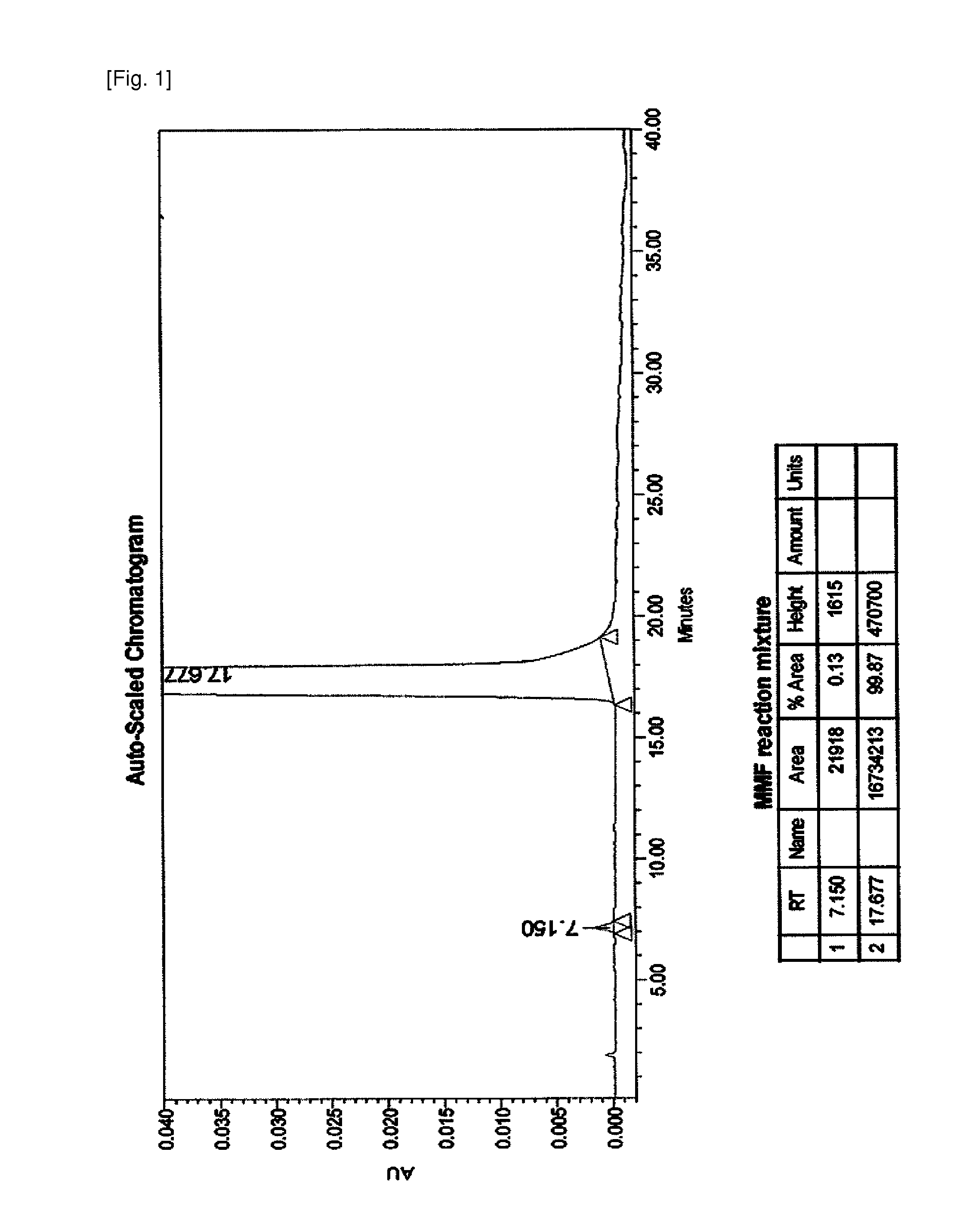
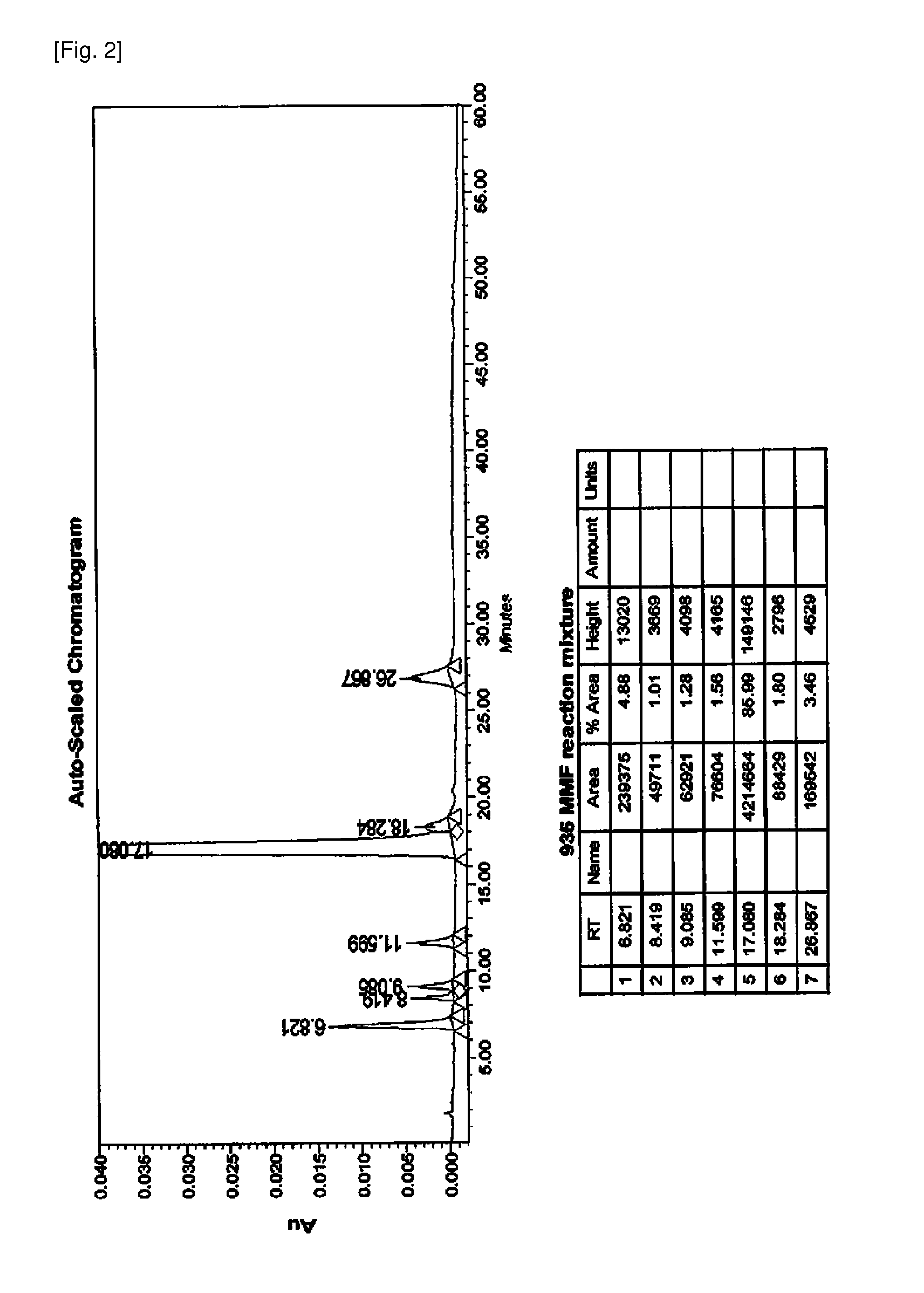
The present invention relates to an improved method of manufacturing mycophenolate mofetil. More particularly, the present invention relates to a method of manufacturing mycophenolate mofetil with high purity comprising : a) converting mycophenolate to an amine salt by reacting with an amine base; and b) reacting the resultant with a halogenating agent and 2-morpholinoethanol continuously.
Owner:CHONGKUNDANG BIO
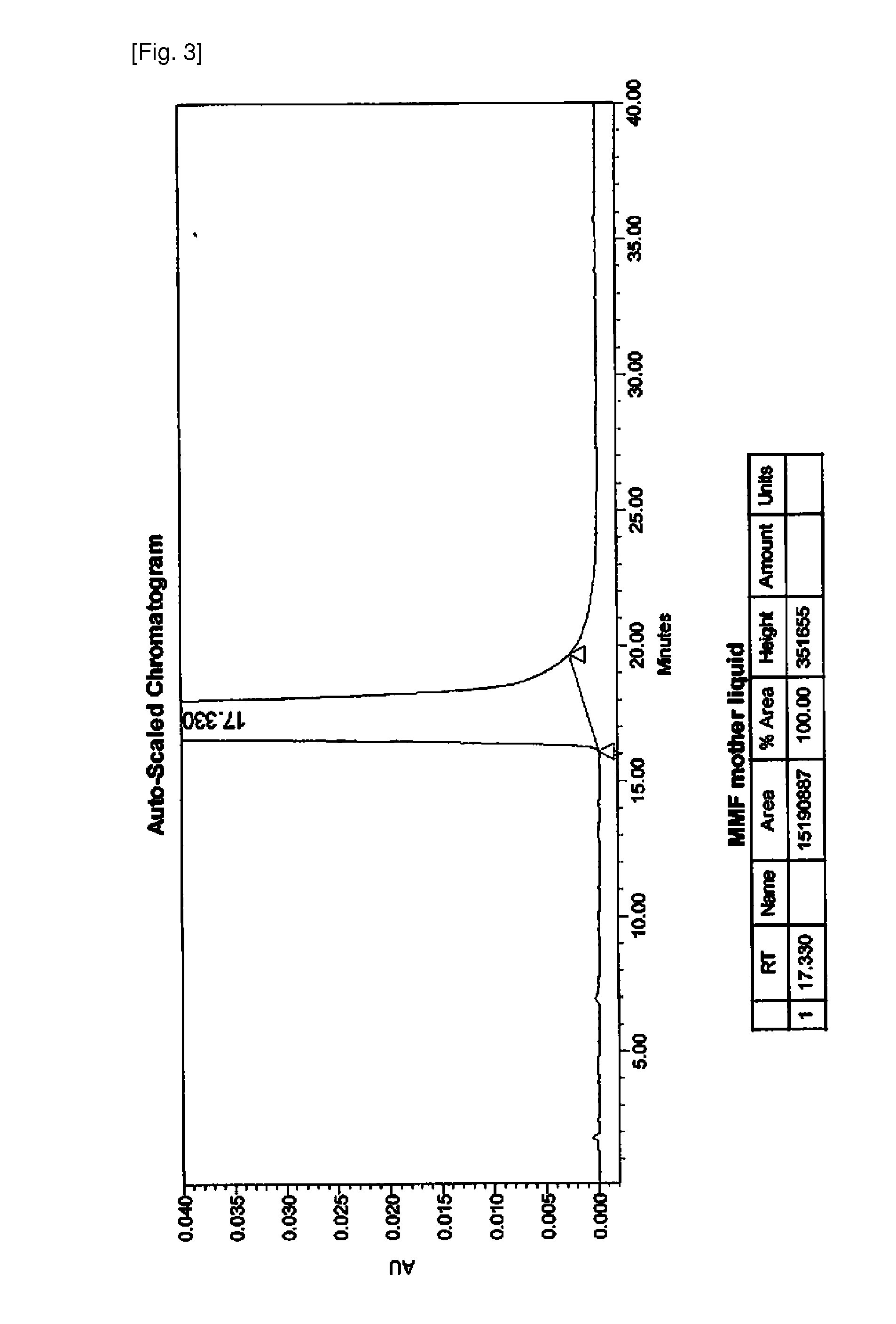
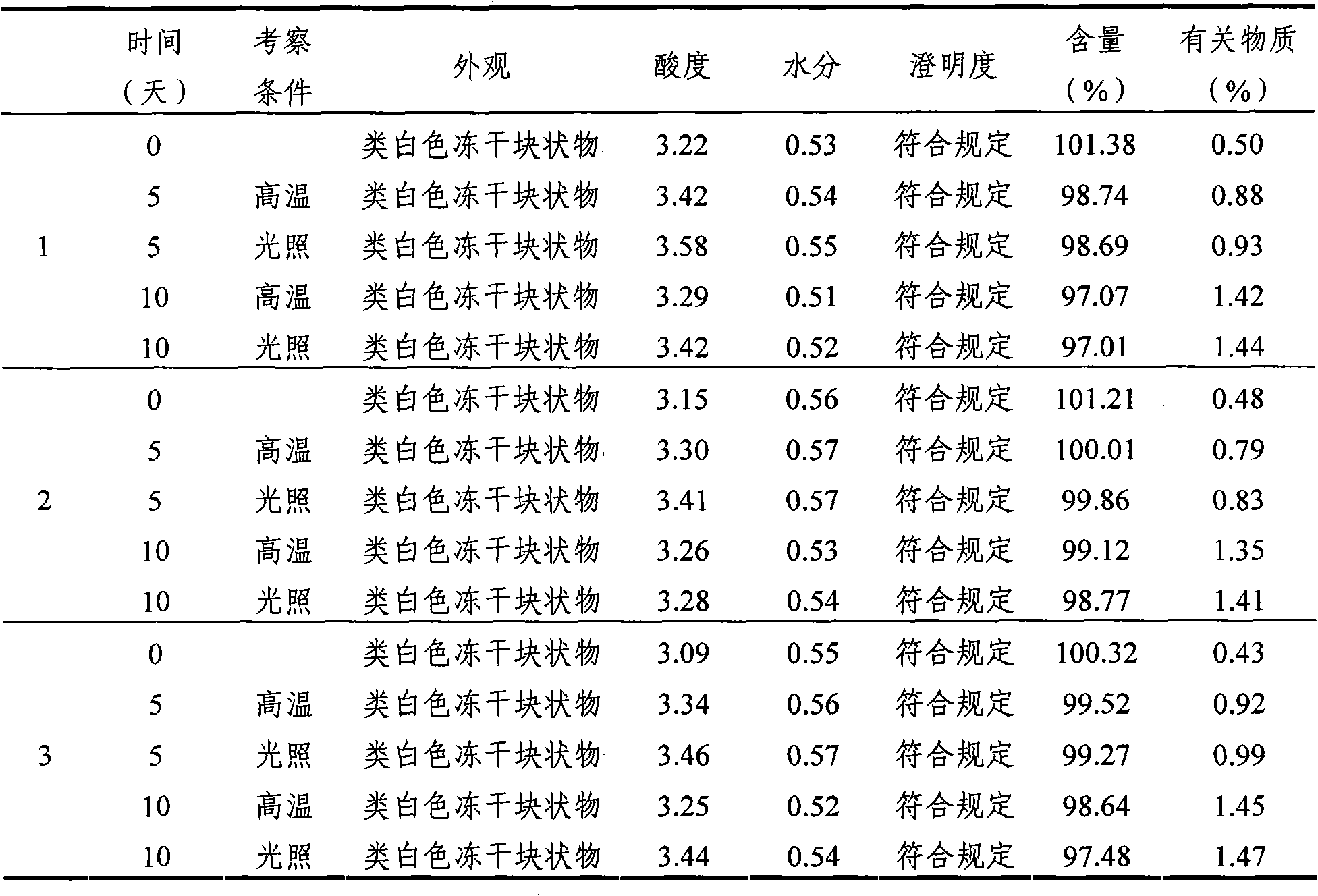
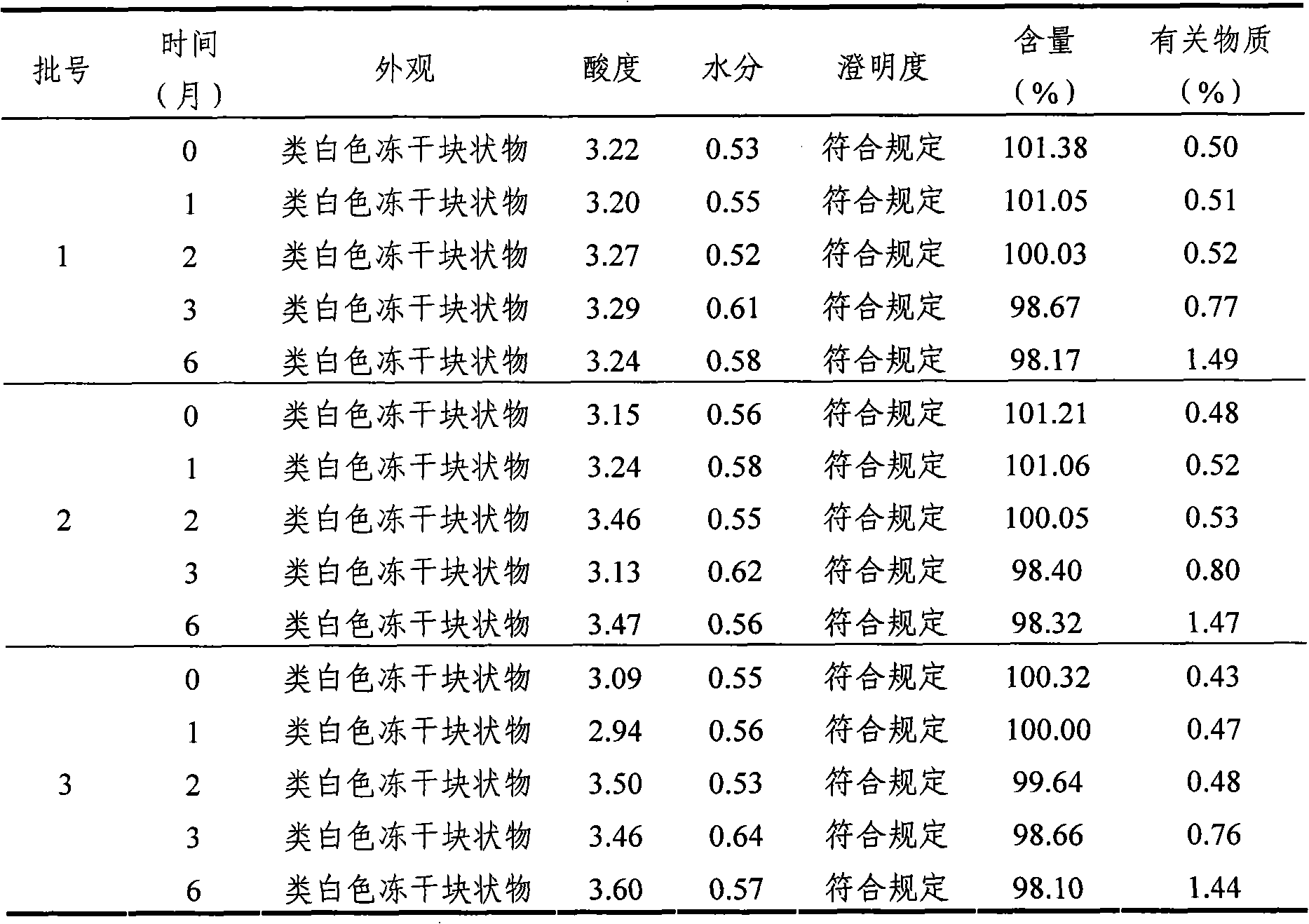
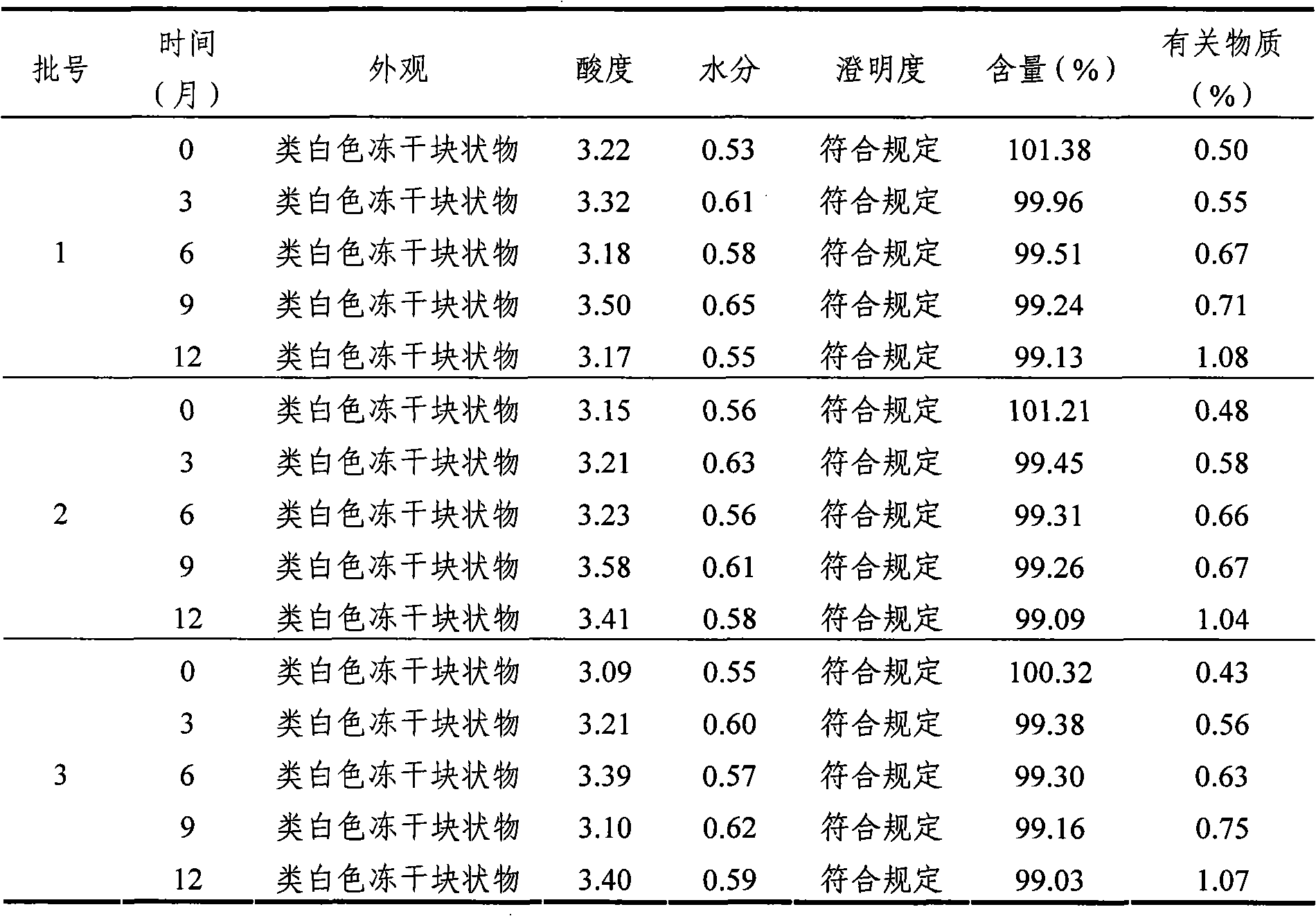
Mycophenolate mofetil lyophilized powder injection for injection and preparation method thereof
InactiveCN101953807ALittle hemolysisHigh physiological toleranceOrganic active ingredientsPowder deliveryHemolysisSide effect
The invention provides a mycophenolate mofetil lyophilized powder injection for injection. The injection is prepared from mycophenolate mofetil serving as an active ingredient and polyethylene glycol-12-hydroxy stearate serving as a solubilizing agent in a weight ratio of 250-1,250:100-500. The invention also provides a method for preparing the mycophenolate mofetil lyophilized powder injection for injection. The mycophenolate mofetil lyophilized powder injection for injection has the advantages of simple and reasonable prescription, stable quality, convenient use, capabilities of preventing adverse reactions such as nausea, vomit, diarrhea and the like caused by oral preparations and the like and preventing toxic or side effects such as drop of blood pressure, hemolysis and the like caused by solubilizing agents such as Twain 80 and the like, high safety and good application prospect.
Owner:SHANXI PUDE PHARMA CO LTD
Mycophenolate mofetil capsule and preparation method thereof
InactiveCN106389379AReduce intensityLow densityOrganic active ingredientsSkeletal disorderIrritationAdhesive
The invention provides a mycophenolate mofetil capsule and a preparation method thereof. The mycophenolate mofetil capsule comprises, by weight, 1100-1400 parts of mycophenolate mofetil, 150-170 parts of filler and 75-110 parts of pharmaceutical acceptable auxiliary materials; the filler is selectively prepared from a mixture of one or more optional proportions of pregelatinized starch, corn starch or lactose; the pharmaceutical acceptable auxiliary materials include adhesive, disintegrant and lubricant. A fluidized-bed granulation technology is adopted, the process is simplified, time is saved, labor intensity is low, obtained particles are porous soft particles, low density and low strength are achieved, the particles are evenly distributed in particle size, and good fluxility is achieved; the mycophenolate mofetil capsule has the advantages of high disintegration speed, good absorptivity, convenience in taking, small in intestinal irritation, stable in long-term storage quality and the like; the advantages of both simpleness in production equipment for ordinary capsules and convenience in package storage and transportation as well as carrying are achieved, and the mycophenolate mofetil capsule is convenient for patients to take.
Owner:WUXI FORTUNE PHARMA
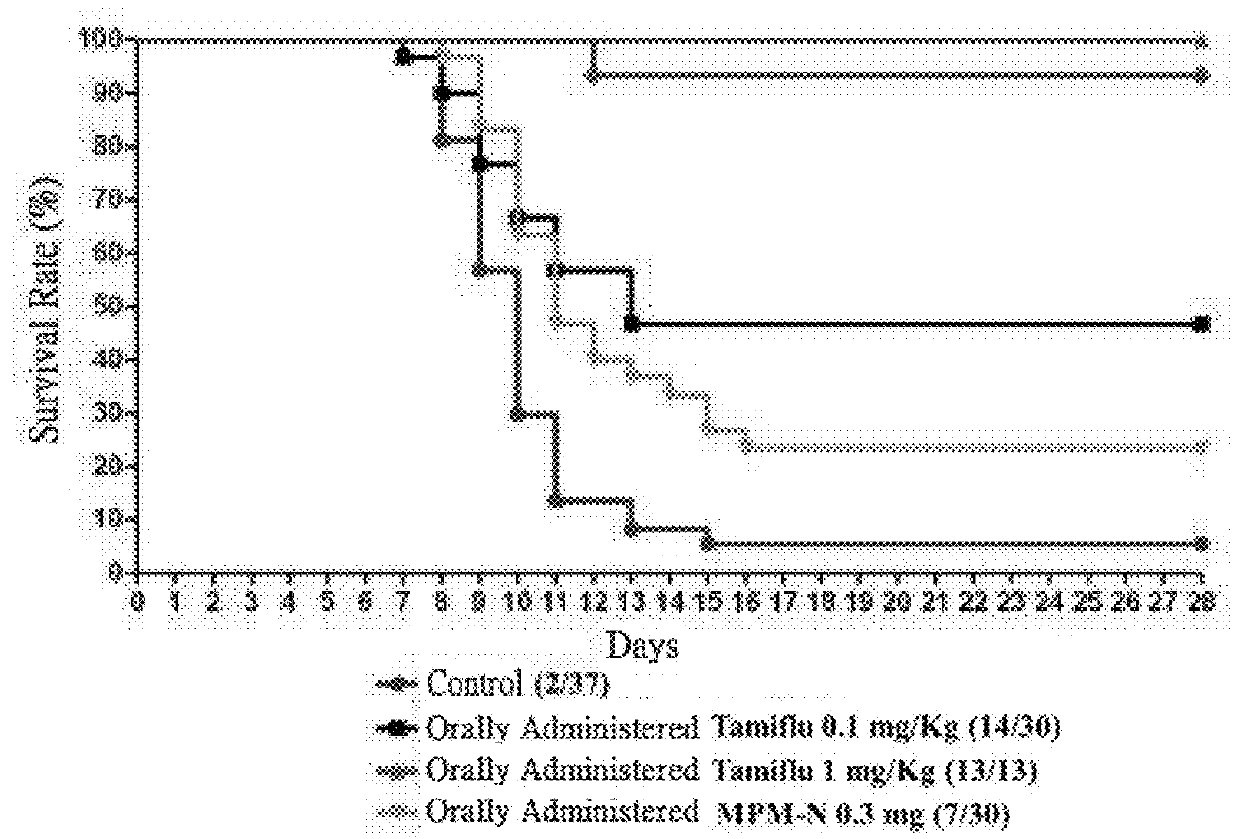
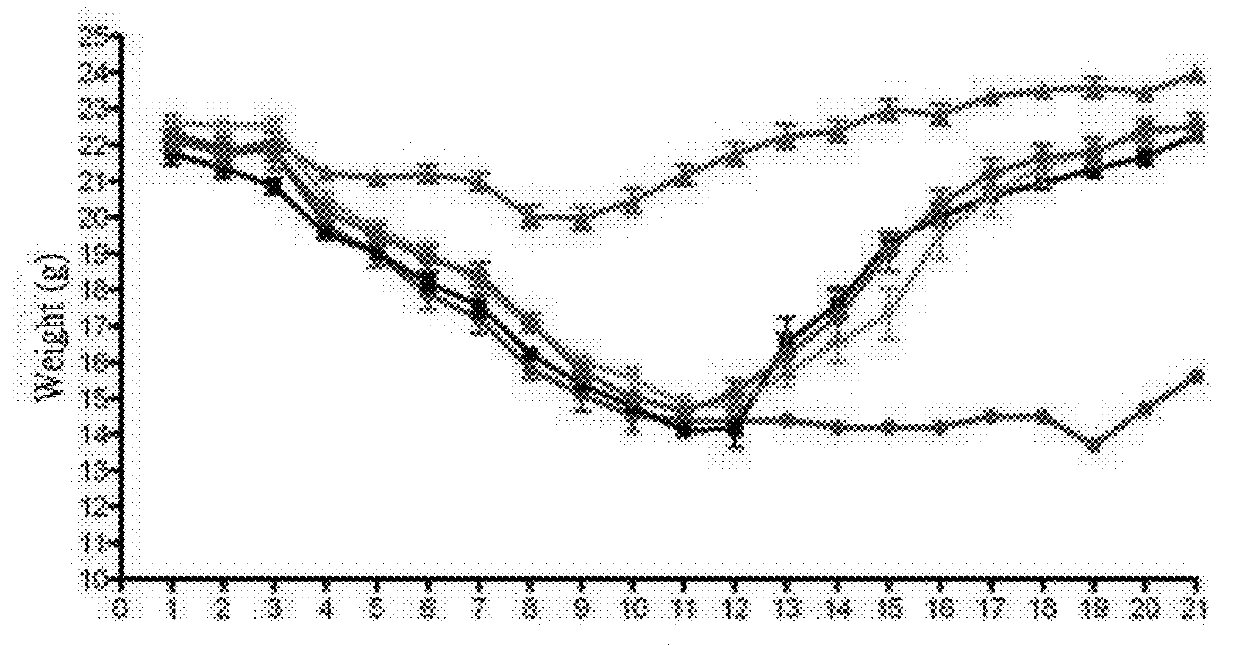
Usage of mycophenolate mofetil or salt thereof in preparing drug for resisting against influenza virus
The present invention relates to a use of mycophenolate mofetil or a pharmaceutically acceptable salt thereof in the manufacture of a medicament against influenza virus. The present invention also relates to a use of mycophenolate mofetil or a pharmaceutically acceptable salt thereof in the manufacture of a medicament against drug-resistant influenza virus strains. The present invention is further related to a method for treating influenza in a subject, comprising administering to said subject an effective amount of mycophenolate mofetil or a pharmaceutically acceptable salt thereof.
Owner:GIANT FORCE TECH
Mycophenolate mofetil impurity
InactiveUS20050282806A1Organic active ingredientsOrganic compounds purification/separation/stabilisationMycophenolateImpurity
Provided is an impurity of mycophenolate mofetil, processes for its preparation and its use as a reference.
Owner:TEVA PHARM USA INC
Method for the purification of mycophenolate mofetil
The present invention provides a method for the preparation of mycophenolate mofetil wherein mycophenolic acid or an amine salt of mycophenolic acid is esterified with 2-morpholinoethanol, the resulting mixture is extracted into water at a pH-value between 1.0 and 3.0, and mycophenolate mofetil is back-extracted in a water-immiscible solvent at a pH-value between 3.0 and 5.0.
Owner:DSM SINOCHEM PHARMA NETHERLANDS
Method of mycophenolate mofetil preparation
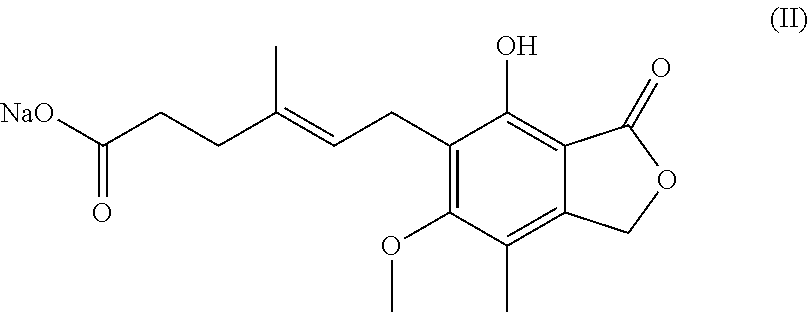
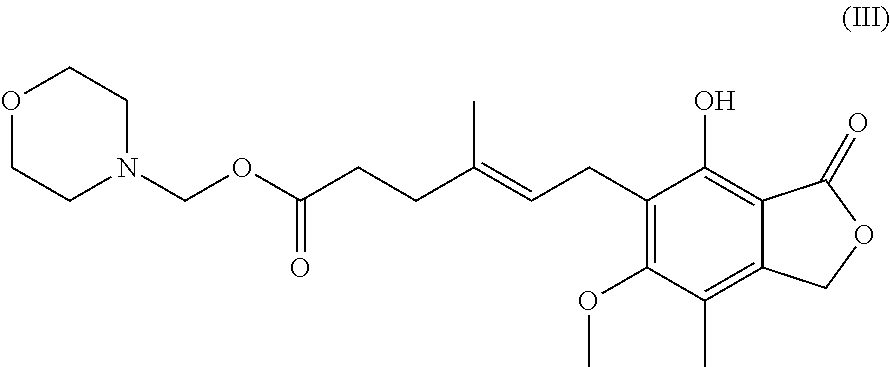
Synthesis of mycophenolate mofetil (1), where R1=2-(-morpholinyl)ethyl and R2=hydrogen atom, includes reaction of mycophenolic acid with 4-(2-hydroxyethyl)morpholine in a suitable solvent under azeotropic separation of water.
Owner:IVAX PHARMA
Application of mycophenolate mofetil in preparing medicine for preventing or treating foot-and-mouth disease virus infection
InactiveCN109745327ALow toxicityProlong survival timeOrganic active ingredientsAntiviralsMycophenolateFoot-and-mouth disease virus
The invention relates to application of mycophenolate mofetil in preparing a medicine for preventing or treating the foot-and-mouth disease virus infection, and belongs to the technical field of veterinary medicines. The By means of the application, the efficient, safe and quality-controllable foot-and-mouth disease virus medicine can be provided for further controlling the spreading of the foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
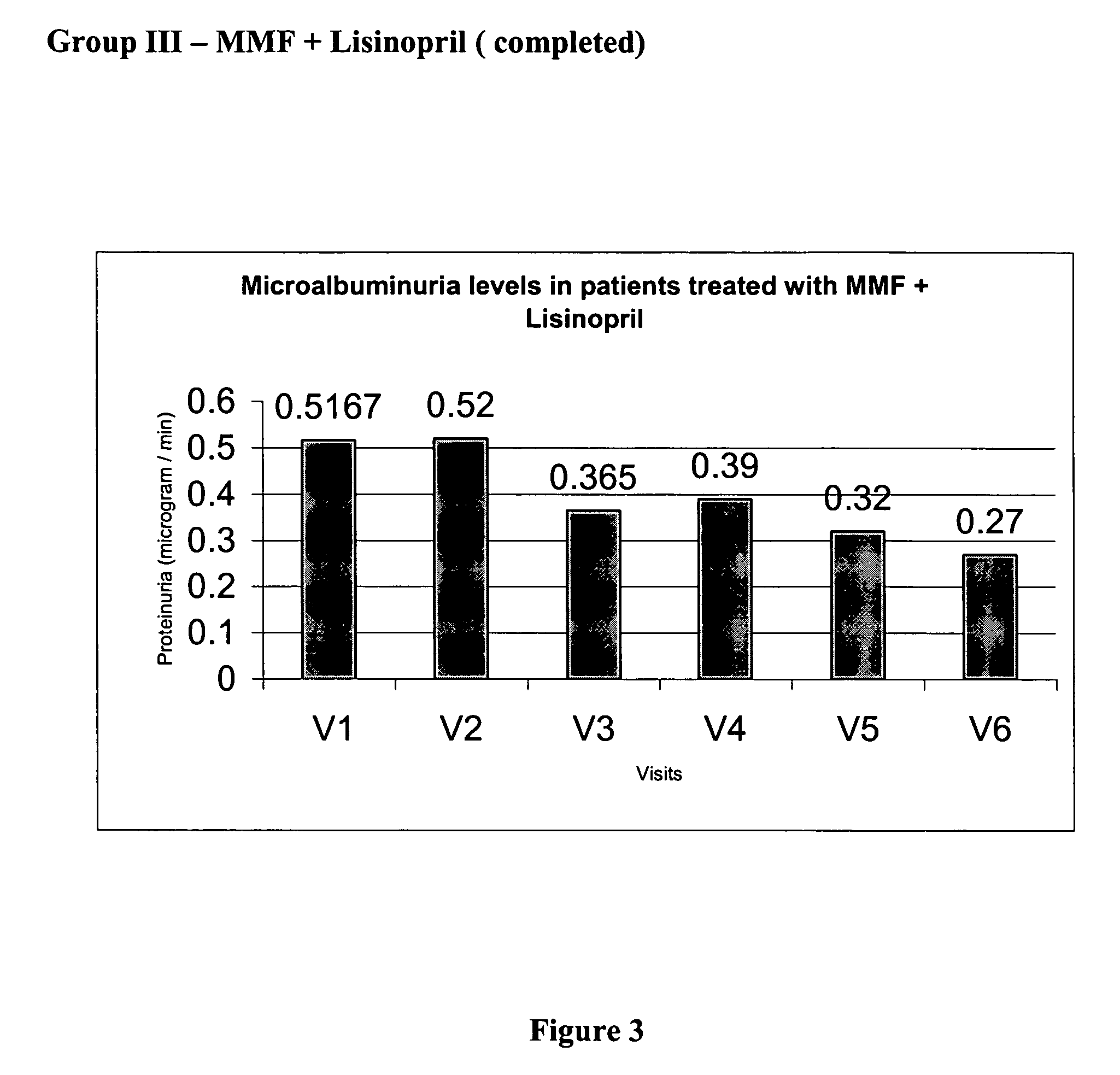
Mycophenolate mofetil in diabetic nephropathy
The present invention relates to a method of treating diabetic nephropathy with a combination of an immunosuppressive agent and an ACE inhibitor.
Owner:CLINIGENE INT PRIVATE
Compound preparation for treating immunological rejection after organ transplantation and preparation method thereof
InactiveCN107669737ALittle side effectsAvoid adverse reactionsOrganic active ingredientsGranular deliverySide effectLymph fluid
The invention provides a compound preparation for treating immunological rejection after organ transplantation and a preparation method thereof. The compound preparation comprises the following components in parts by weight: 25-35 parts of mycophenolate, 8-10 parts of red sage root, 6-8 parts of Ligusticum wallichii, 3-4 parts of Chinese angelica, 3-4 parts of radix astragali, 1-2 parts of thundergod vine, 1-2 parts of safflower, 6-8 parts of a filler, 3-5 parts of an adhesive, 6-8 parts of a disintegrating agent, 2-3 parts of a diluent, 1-2 parts of a flow aid, 0.5-1 part of a lubricant, and6-8 parts of an enteric coating material. The compound preparation is composed of the enteric delayed-release mycophenolate and a gastric-dissolved quick-release traditional Chinese medicine extractproduct, the compound preparation can avoid adverse reaction of gastrointestinal tract, and can obviously reduce the side effect due to long-term administration of mycophenolate in the compound preparation extracted through the traditional Chinese medicine extract product, so that the probability of skin cancer, lymph tumour and other immunosuppression-related pathology can be obviously reduced.
Owner:WUXI FORTUNE PHARMA
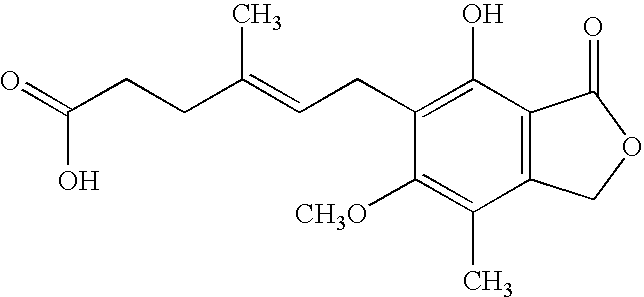
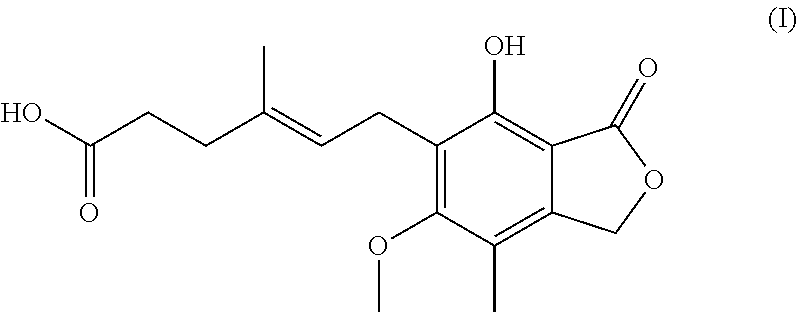
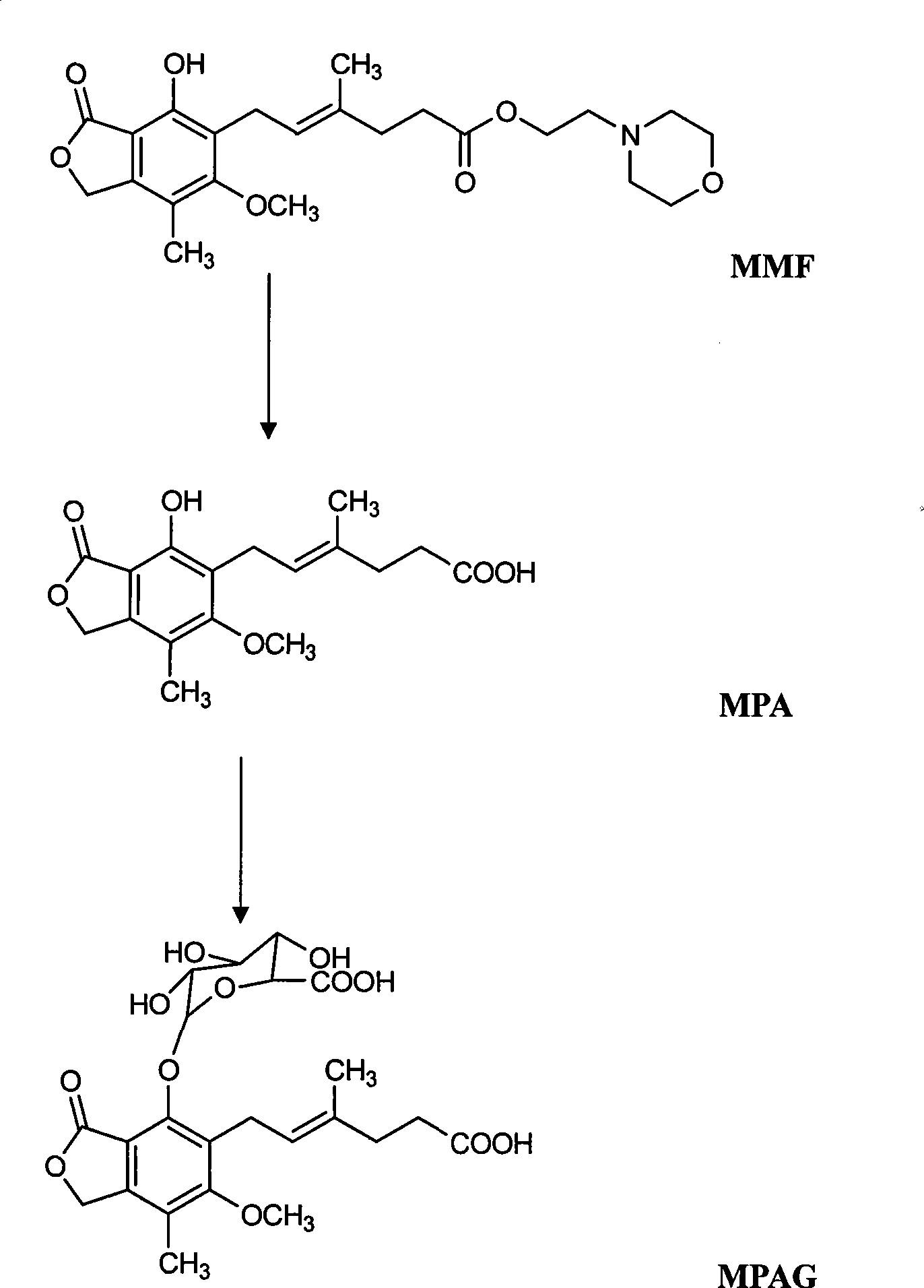
Process for preparation of mycophenolic acid, its salt and ester derivatives
InactiveUS20110166347A1Speed up the conversion processOrganic chemistryFermentationMedicinal chemistryMycophenolate
The present invention discloses an isolation and purification process for mycophenolic acid obtained from the fermentation process. Invention further discloses preparation of sodium salt of mycophenolic acid and mycophenolate mofetil from mycophenolic acid.
Owner:IPCA LAB LTD
Mycophenolate mofetil dispersible tablet
ActiveCN104382873APiece weight smallDisintegrates quicklyOrganic active ingredientsPill deliverySulfateDissolution
The invention discloses a mycophenolate mofetil dispersible tablet. The tablet consists of mycophenolate mofetil, calcium sulfate, glycerin monostearate, a disintegrating agent and an adhesive. Compared with the prior art, the mycophenolate mofetil dispersible tablet prepared by the invention is small in tablet weight and rapid in dissolution and disintegration and does not need lots of disintegrating agents, the disintegrated granules can smoothly pass through a No.2 sieve, and the mycophenolate mofetil dispersible tablet is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Method for preparing mycophenolate mofetil
The invention discloses a method for preparing mycophenolate mofetil, which comprises the following steps of: using dibutyl ether as a solvent, performing complete reaction between MPA and 2-hydroxyethyl morpholine under inert gas protection and constant boiling water conditions so as to obtain a crude product of the mycophenolate mofetil; and using an ionic liquid as a chelating agent to decolor and purify, seeding the mycophenolate mofetil, and crystallizing a mixed solvent to obtain high-purity mycophenolate mofetil. The method has the advantages of short reaction time, high MPM purity, high reaction yield, low generation cost and the like, and has a wide industrial application prospect, and the problem of the color of a final product is solved.
Owner:CHONGQING UNIV OF TECH
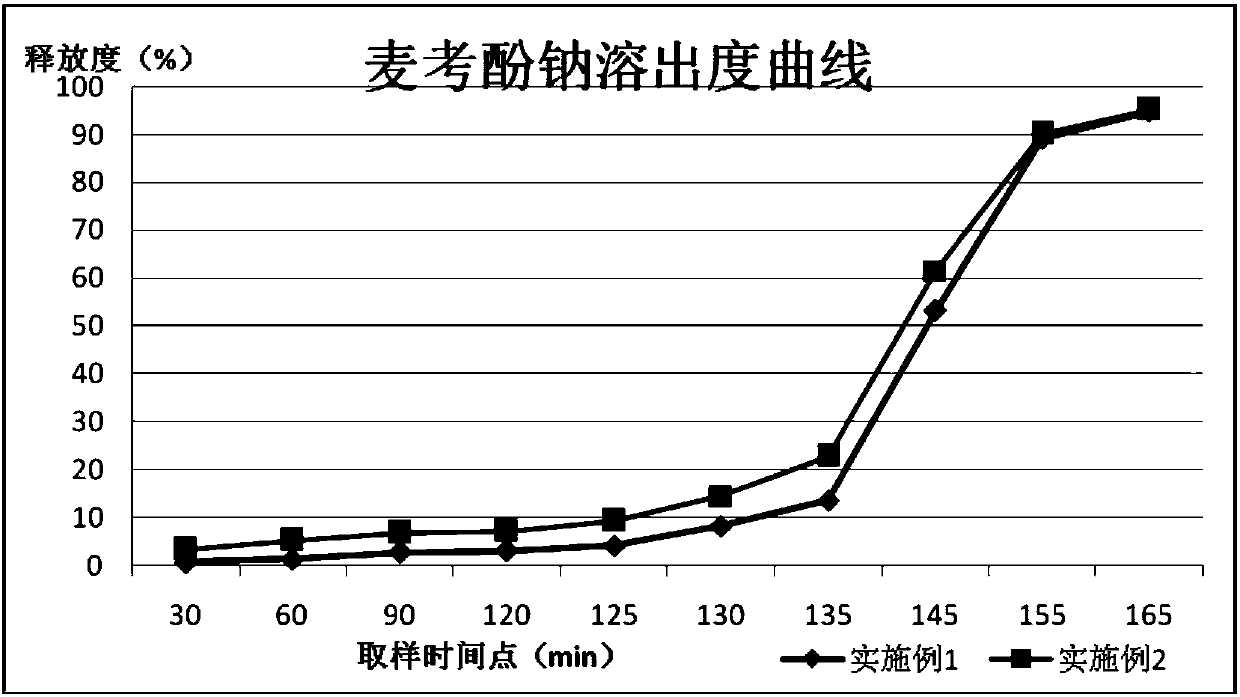
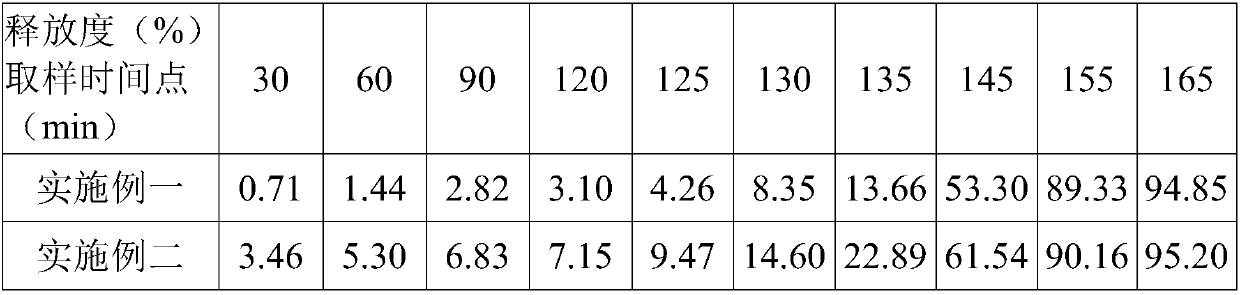
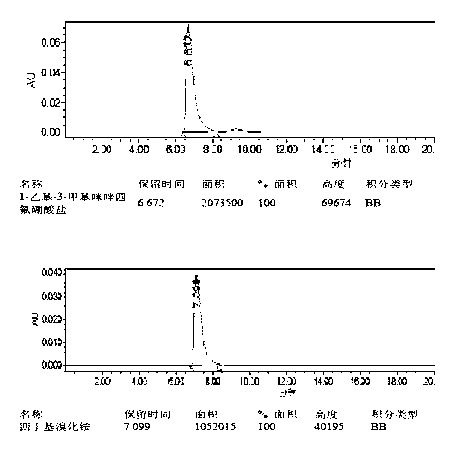
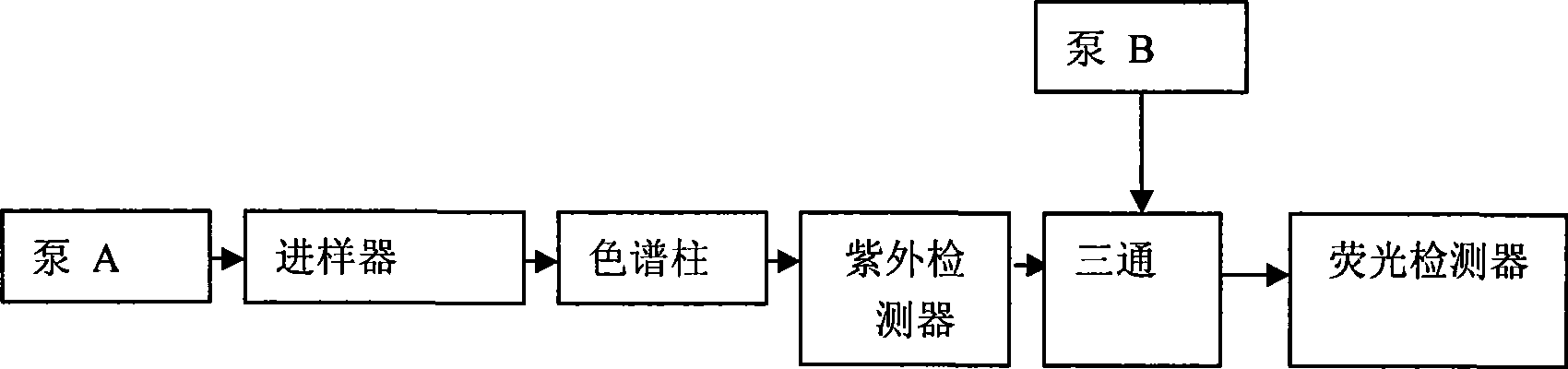
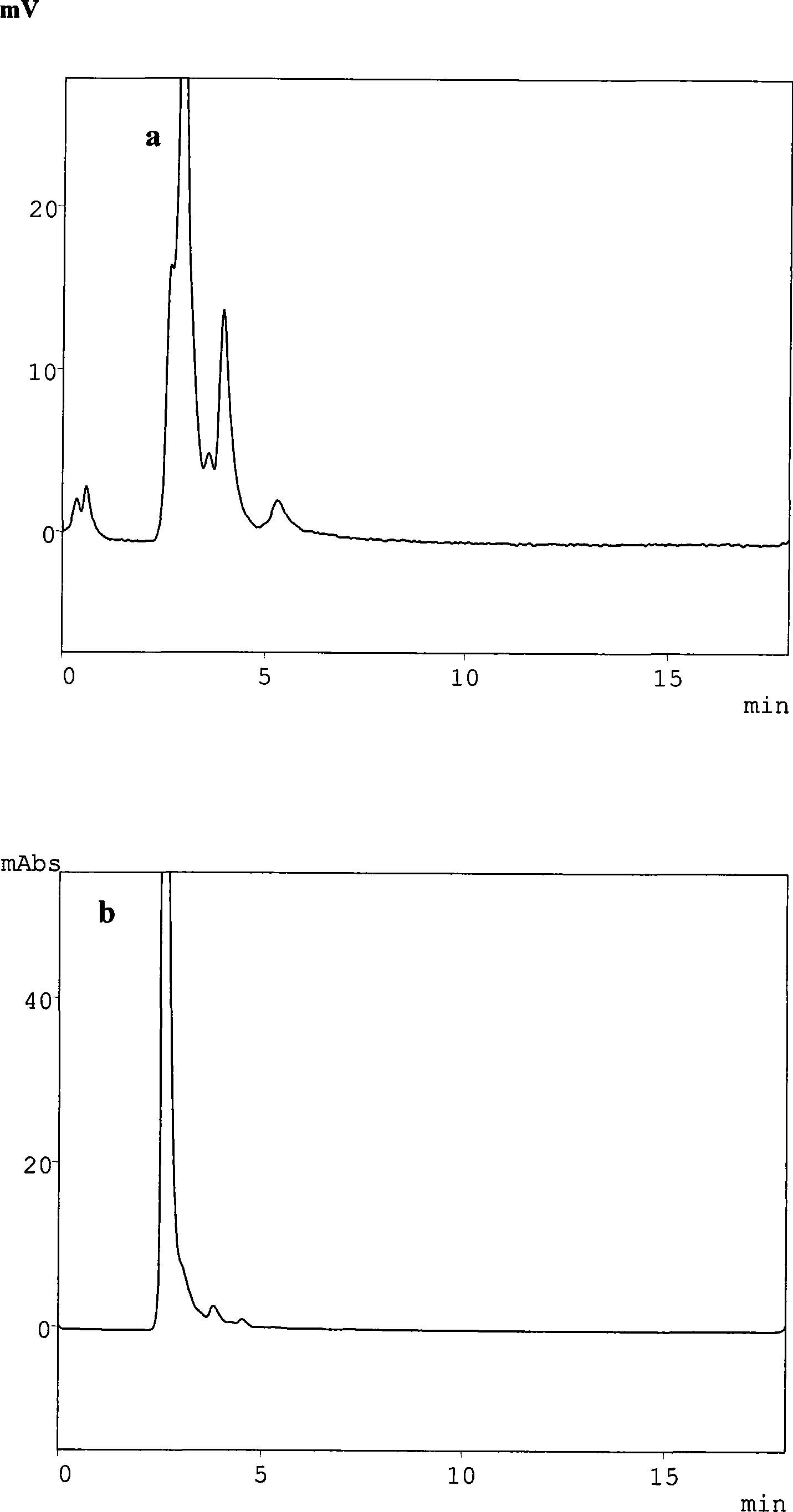
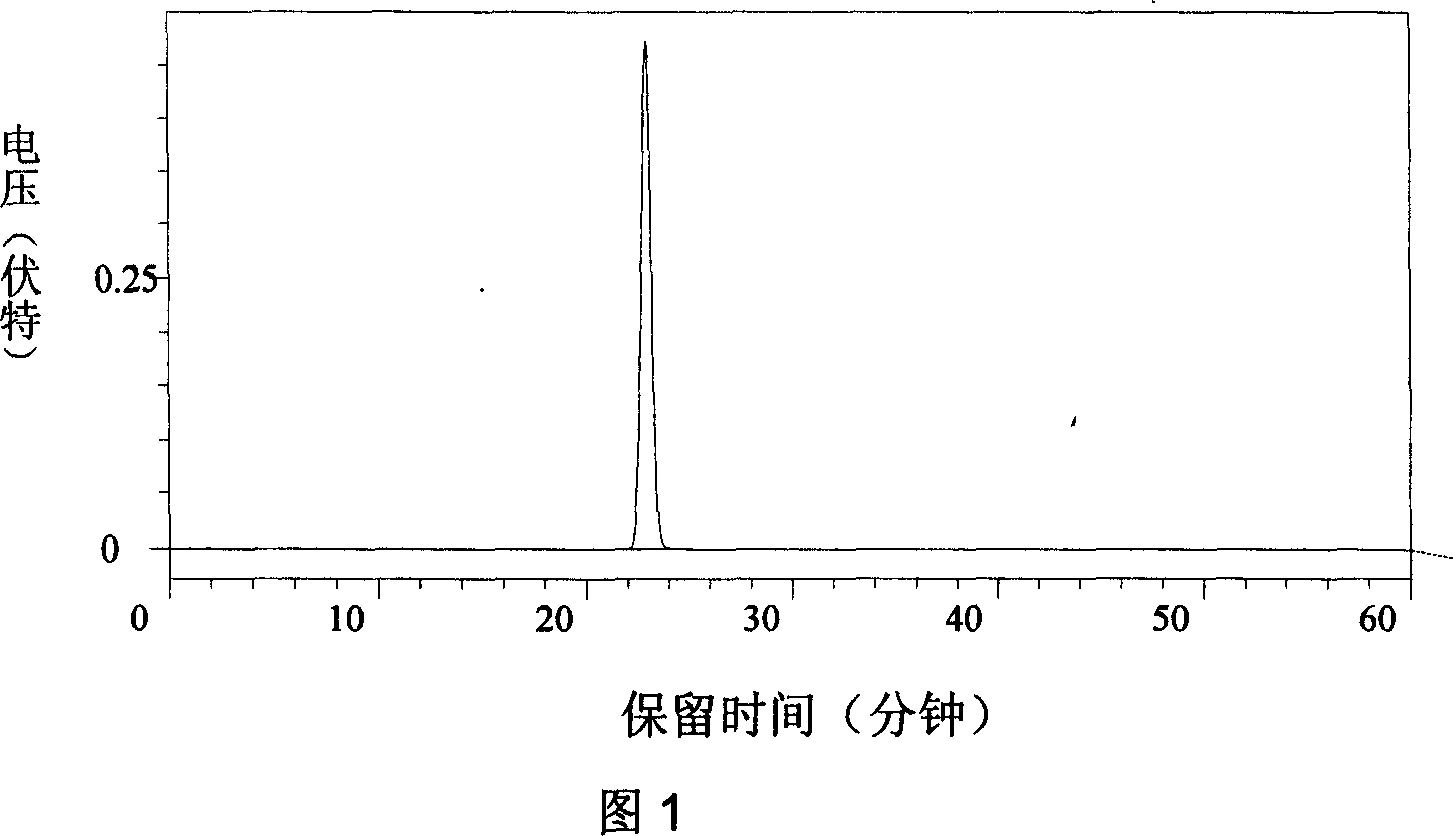
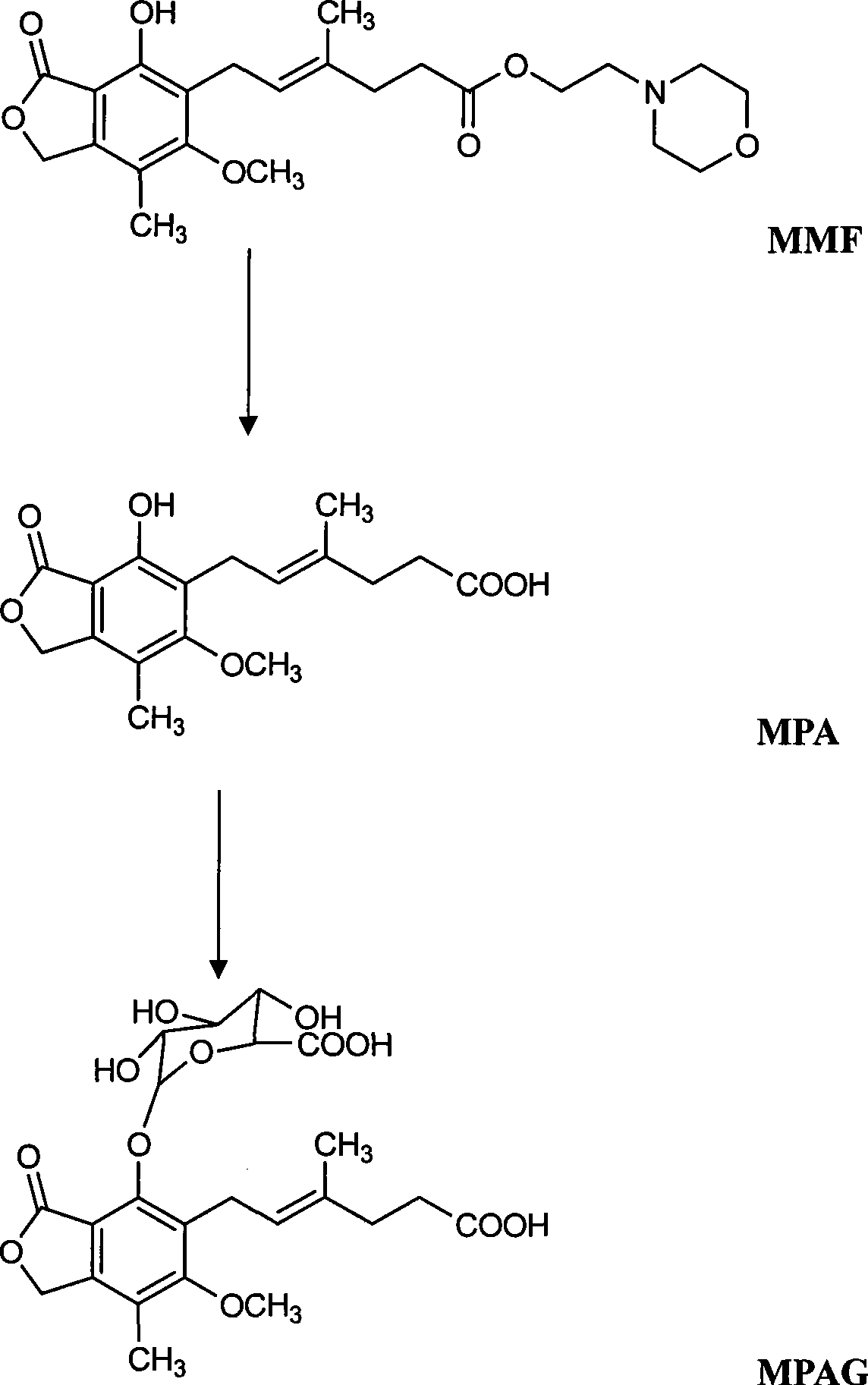
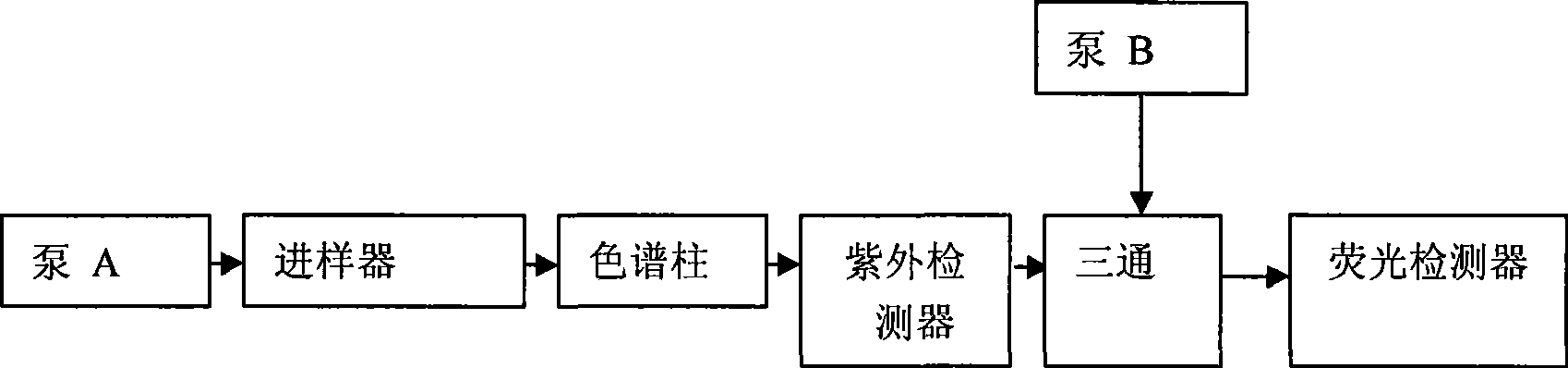
Method for simultaneously determining mycophenolic acid ester, mycophenolic acid and metabolite thereof in human blood plasma
InactiveCN101419224AAccurate methodSimple and fast operationComponent separationBiological testingMetaboliteFluorescence
The invention belongs to the field of medical test, and relates to a method for analyzing and measuring in vivo drugs, in particular to a method for measuring mycophenolate mofetil, mycophenolic acid and metabolites thereof in human plasma simultaneously. After the pretreatment of a sample to be measured, the method makes use of the characteristic of strong fluorescence absorption of the mycophenolate mofetil and the mycophenolic acid under an alkaline condition, performs derivatization after the analysis of chromatographic column separation, and detects with a fluorescence detector. The method can greatly improve the sensitivity for detecting the mycophenolate mofetil and the mycophenolic acid. The method has the advantages of few samples, simple pretreatment, rapidness, sensitivity, short analysis cycle and low cost, no needs of expensive equipment or reagents, and is suitable for clinical routine monitoring and pharmacokinetic study.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Mycophenolate mofetil impurity
InactiveUS7358247B2Organic active ingredientsOrganic compounds purification/separation/stabilisationImpurityMycophenolate
Provided is an impurity of mycophenolate mofetil, processes for its preparation and its use as a reference.
Owner:TEVA PHARM USA INC
Improved enteric medicine composition with mycophenolate
The present invention relates to mycophenolic acid, and is especially a kind of improved medicine composition of mycophenolate with enteric coating. The medicine composition includes pharmaceutically acceptable mycophenolate with enteric coating, and the enteric coating includes poly(acetic vinyl phthalate) or acetic succinic hydroxypropyl methyl cellulose. The improved medicine composition of the present invention has excellent medicine releasing property.
Owner:海南澳合医药有限公司
Compound preparation for treating rejection reaction after organ transplanting
InactiveCN107582583AReduce the risk of infectionImprove medication safetyOrganic active ingredientsCapsule deliveryCordycepsAdhesive
The invention provides a compound preparation for treating rejection reaction after organ transplanting. The compound preparation is prepared from the following components in parts by weight: 50 to 85parts of mycophenolate mofetil, 3 to 6 parts of radix angelicae sinensis, 3 to 6 parts of cordyceps, 8 to 20 parts of filling agent, 3 to 8 parts of adhesive, 2 to 10 parts of disintegrating agent, and 0.5 to 2 parts of lubricant. The compound preparation for treating the rejection reaction after organ transplanting has the advantages that the infection risk after operation is obviously decreased; more importantly, the inducing risk of lymph cancers, skin cancers and other malicious tumors due to taking of immunity inhibitors which takes the mycophenolate mofetil as the main component for a long time can be obviously decreased, so that the safety in medicine taking is improved.
Owner:WUXI FORTUNE PHARMA
Stable mycophenolate mofetil for injection, and preparation method thereof
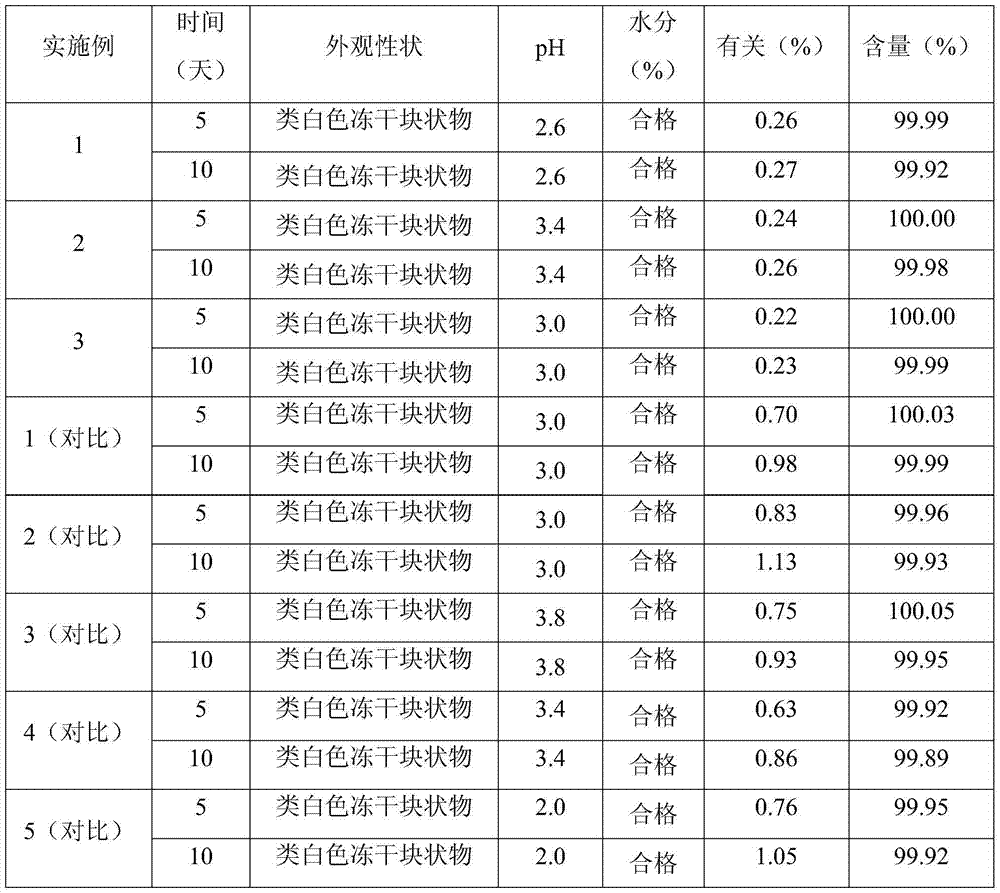
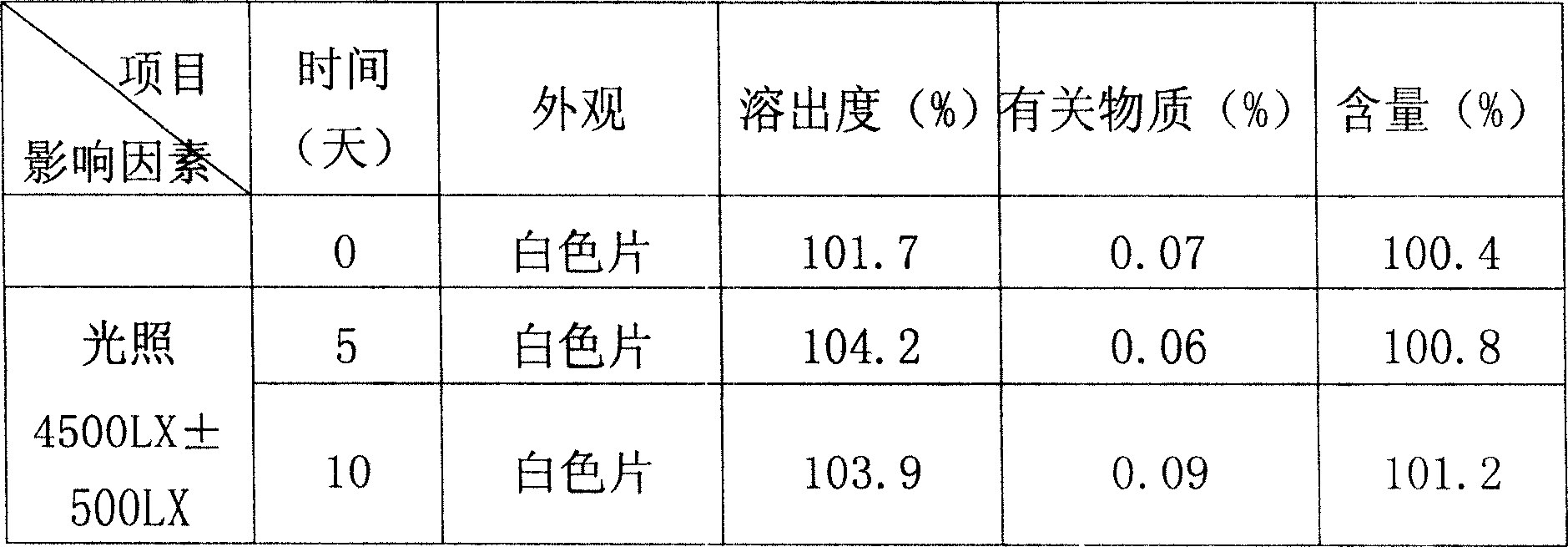
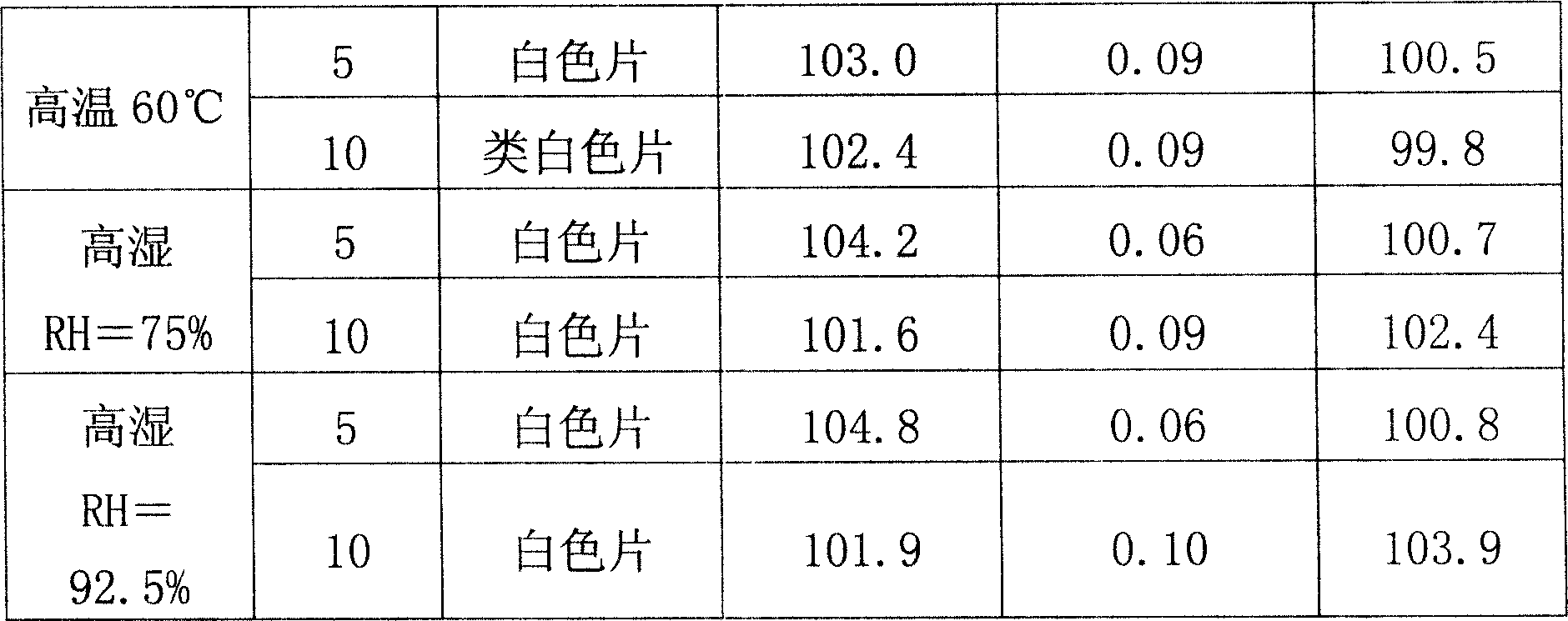
ActiveCN106943359AAvoid safety hazardsSimple processPowder deliveryOrganic active ingredientsFreeze-dryingTherapeutic effect
The present invention relates to a stable mycophenolate mofetil freeze-drying composition for injection, wherein the composition is prepared from mycophenolate mofetil, citric acid, polysorbate 80, disodium tetraborate, a pH value adjuster, and water for injection. The preparation method comprises: adding a prescription amount of mycophenolate mofetil, citric acid and polysorbate 80 to a proper amount of water for injection, uniformly stirring and mixing, adjusting the pH value of the solution to 2.6-3.4 with 1 M hydrochloric acid, adding a prescription amount of disodium tetraborate, carrying out volume metering to achieve the total amount, stirring the solution until achieving a colorless and clear liquid, filtering, filling, carrying out freeze drying, and carrying out visual inspection so as to obtain the finished product. According to the present invention, the product is stable, such that the safety risk caused by the treatment effect reducing and the impurity increasing due to the drug degradation can be avoided; and the method has characteristics of simple process and simple operation, and is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
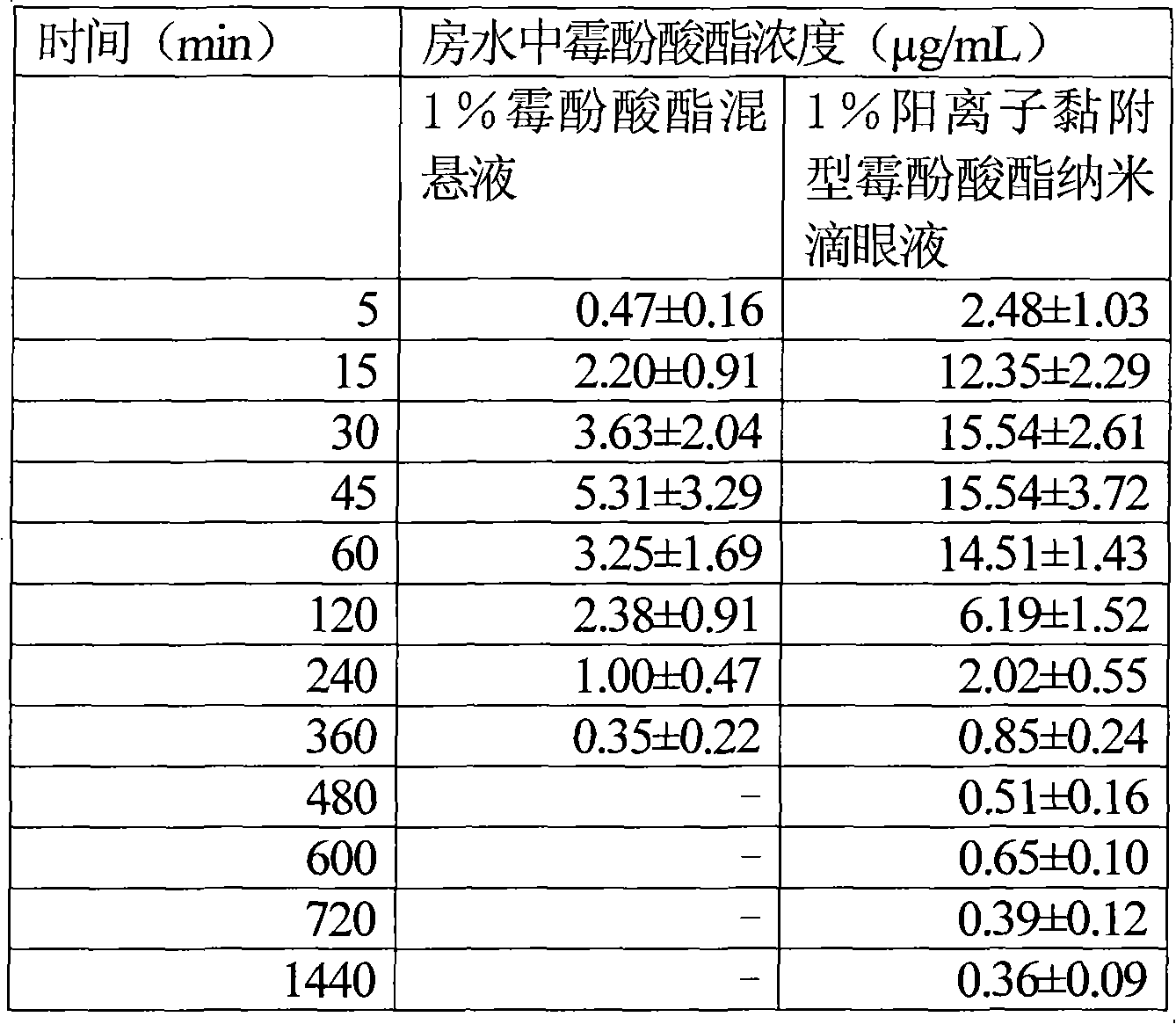
Cationic adhesion type mycophenolate mofetil nano eye drop
InactiveCN101590015AImprove solubilityIncreased corneal resorption featuresOrganic active ingredientsSenses disorderSolubilityPhospholipid
The invention relates to a cationic adhesion type mycophenolate mofetil nano eye drop, which comprises mycophenolate mofetil of which the mass percentage concentration is 1 percent, proper amount of osmotic pressure modifying agent and pH modifying agent. The eye drop is characterized in that the eye drop also comprises chitosan, Pluronic F-68 and phospholipid, wherein the mass percentage concentration of the phospholipids in the eye drop is between 0.2 and 2 percent; the mass percentage concentration of the Pluronic F-68 in the eye drop is between 0.5 and 4 percent; the mass percentage concentration of the chitosan in the eye drop is between 0.5 and 4 percent; the molecular weight of the chitosan is between 5,000 and 300,000; the deacetylation degree is more than 85 percent; and the grain diameter of the mycophenolate mofetil is between 10 and 700 nm. The eye drop has the characteristics that: the mycophenolate mofetil with nanometer grain diameter improves the medicament solubility, the safety and the effectiveness; mycophenolate mofetil medicament particles are charged with positive charges so as to be favorable for improving the cornea absorption of the medicament, improving the medicament effect, prolonging the medicament duration, and greatly improving the cornea adhesion.
Owner:SHANDONG EYE INST
Method for purifying and decolorizing mycophenolate mofetil
The invention provides a method for purifying and decolorizing mycophenolate mofetil. The method for purifying and decolorizing mycophenolate mofetil comprises the following steps of dissolving mycophenolate mofetil reaction raw products by utilizing a mixed solvent; carrying out continuous decolorizing and purifying by enabling the solution to pass through a device containing a decolorizing medium; eluting collected liquid, removing residual mycophenolic acid, dewatering, concentrating and crystallizing to obtain purified and decolorized mycophenolate mofetil; and flushing 3-4 volumes of the device containing the decolorizing medium by utilizing acetone, and flushing two volumes by utilizing the mixed solvent. According to the method for purifying and decolorizing mycophenolate mofetil, disclosed by the invention, the process is simple, the industrial continuous production can be carried out, the yield is high, the product purity is high, and the prepared products are white samples so that problems of purity and appearance color of the products are solved.
Owner:FUJIAN KERUI PHARMA
Medicinal composition containing mycophonolate mofetil and its preparation method
InactiveCN101006986APromote dissolutionEasy to takeOrganic active ingredientsPill deliveryDifficult swallowingPharmacology
The invention relates to a medicinal composition containing mycophenolate mofetil, which can be prepared into dispersible tablets, thereby overcoming the shortcomings of difficult swallowing and weak medicine compliance existed in the conventional dose forms, as a result, the patients can administrate the medicament more easily. The invention relates to the process for preparing compositions containing mycophenolate mofetil.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Prepn process of Mycophenolate mofetil
The present invention discloses preparation process of Mycopenolate mofetil. The preparation process includes the following steps: dissolving mycopenolic acid in tetrahydrofuran inside a reactor with reflux cooler through stirring; adding morpholine, alcohol and catalyst magnesia and / or zinc oxide; heating and refluxing; filtering to eliminate catalyst and decompression evaporating to eliminate organic solvent and obtain crude Mycopenolate mofetil product. The preparation process is simple, and has low reaction temperature, less produced impurity, conversion rate as high as 85 % and product purity not lower than 99.7 %, and easy industrial application.
Owner:LIVZON NEW NORTH RIVER PHARMA
Method for simultaneously determining mycophenolic acid ester, mycophenolic acid and metabolite thereof in human urine
InactiveCN101419225ASimple and fast operationHigh sensitivityComponent separationBiological testingMetaboliteFluorescence
The invention belongs to the field of medical test, and relates to a method for analyzing and measuring in vivo drugs, in particular to a method for measuring mycophenolate mofetil, mycophenolic acid and metabolites thereof in urine simultaneously. After the pretreatment of a sample to be measured, the method makes use of the characteristic of strong fluorescence absorption of the mycophenolate mofetil and the mycophenolic acid under an alkaline condition, performs derivatization after the analysis of chromatographic column separation, and detects with a fluorescence detector. The method can greatly improve the sensitivity for detecting the mycophenolate mofetil and the mycophenolic acid. The method has the advantages of few samples, simple pretreatment, rapidness, sensitivity, no needs of expensive equipment or reagents, short analysis cycle and low cost, and is suitable for clinical routine monitoring and pharmacokinetic study.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Pharmaceutical composition containing mycophenolate mofetil
InactiveCN109364108ALower doseSmall toxicityOrganic active ingredientsAntinoxious agentsAdhesiveSolvent
The invention discloses a pharmaceutical composition containing mycophenolate mofetil. The pharmaceutical composition is prepared from the following components in parts by weight: 5-80 parts of the mycophenolate mofetil, 5-50 parts of a second- type of therapeutic agent, 5-30 parts of a filler, 0.5-10 parts of an adhesive, 0.1-10 parts of a disintegrating agent, 0.5-5 parts of a lubricating agentand 1-20 parts of solubilizer. According to the pharmaceutical composition containing the mycophenolate mofetil, the application dosage of the mycophenolate mofetil is reduceddecreased, the toxic andside effects are reduced, and meanwhile, the pharmaceutical composition has the synergistic effect on efficacy of different drugs.
Owner:WUXI FORTUNE PHARMA
Mycophenolate mofetil impurity
InactiveCN1906184AOrganic compounds purification/separation/stabilisationCarboxylic acid ester formation/introductionEthyl esterMycophenolate
Provided is an impurity of mycophenolate mofetil, processes for its preparation and its use as a reference.
Owner:TEVA GYOGYSZERGYAR RESVENYTARSASAG
Mycophenolate mofetil capsule and preparation method thereof
PendingCN111450074APromote dissolutionUniform particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseFluidized bed drying
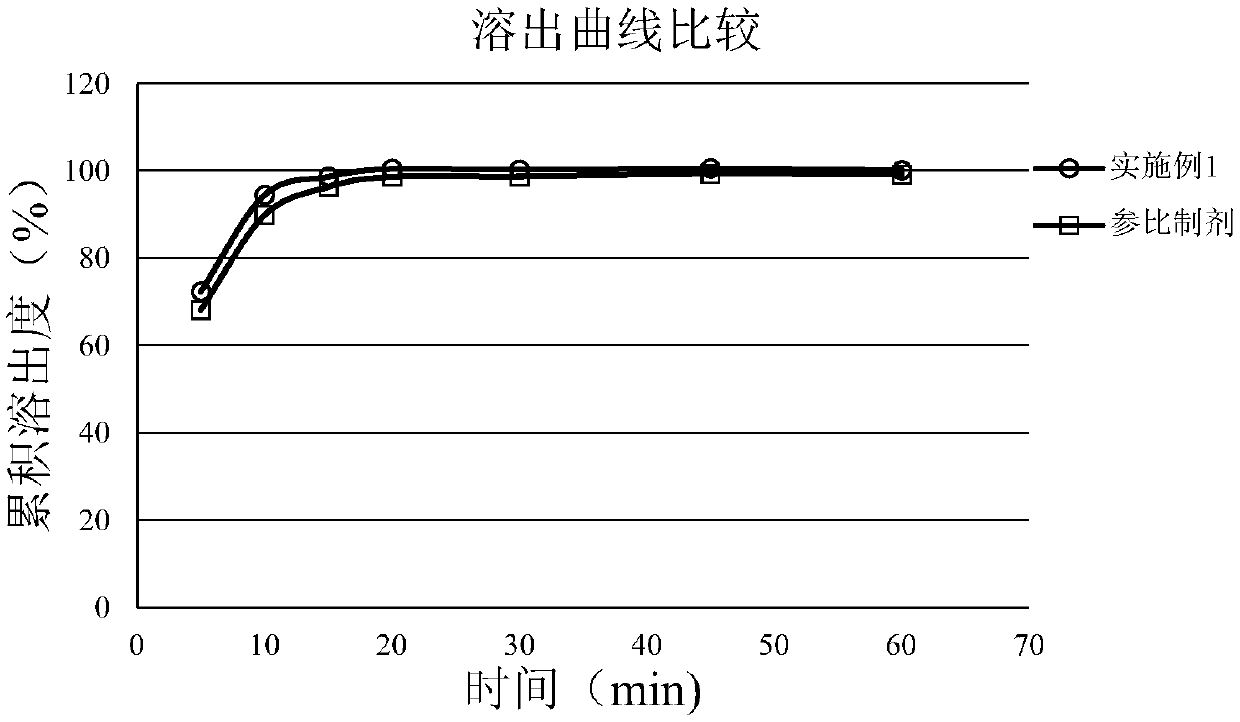
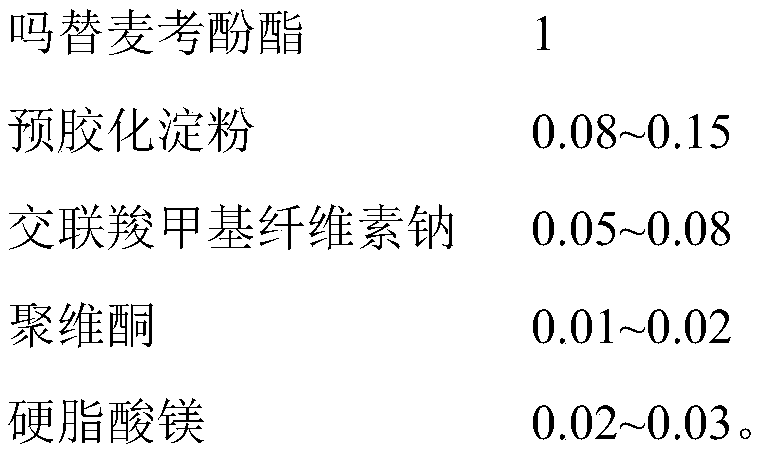
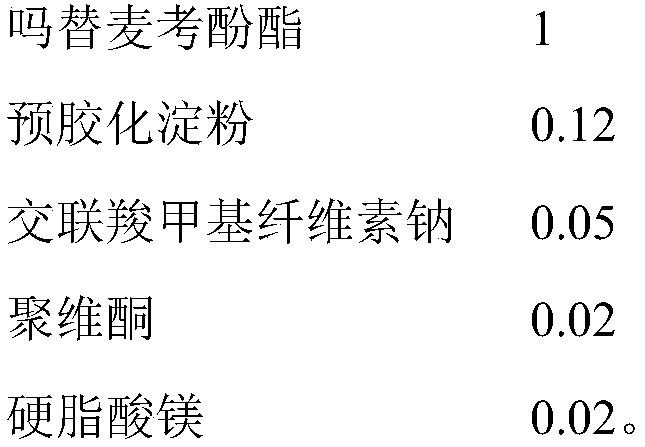
The invention discloses a mycophenolate mofetil capsule and a preparation method thereof. The mycophenolate mofetil capsule contains mycophenolate mofetil, pregelatinized starch, croscarmellose sodium, povidone and magnesium stearate, wherein the weight ratio of the mycophenolate mofetil to the pregelatinized starch to the croscarmellose sodium to the povidone to the magnesium stearate is 1: (0.08-0.15): (0.05-0.08): (0.01-0.02): (0.02-0.03). The mycophenolate mofetil capsule provided by the invention is rapid in dissolution, the dissolution rate is consistent with that of a reference preparation, and related substances are stable and controllable in the preparation acceleration process; and moreover, the provided preparation method is simple in process, and particles obtained by fluidizedbed drying are uniform in granularity and high in flowability and are suitable for large-scale production requirements.
Owner:JIANGSU SIMCERE PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com