Patents
Literature
567 results about "Foot-and-mouth disease virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foot-and-mouth disease virus (FMDV) is the pathogen that causes foot-and-mouth disease. It is a picornavirus, the prototypical member of the genus Aphthovirus. The disease, which causes vesicles (blisters) in the mouth and feet of bovids, suids, ovids, caprids and other cloven-hoofed animals is highly infectious and a major plague of animal farming.
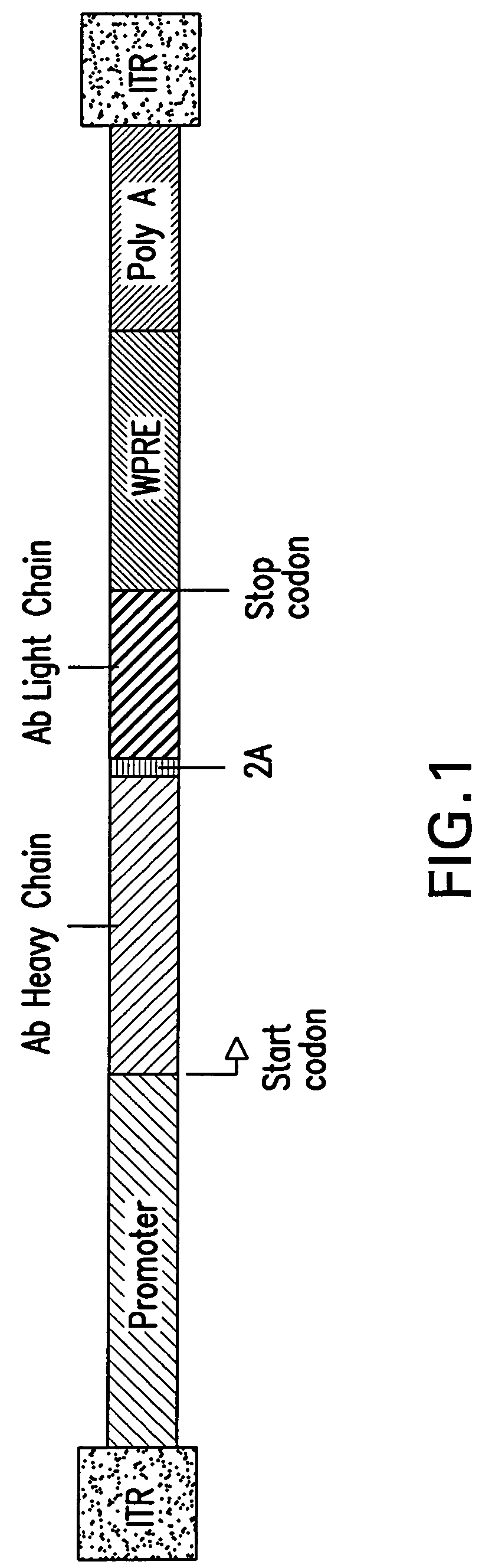
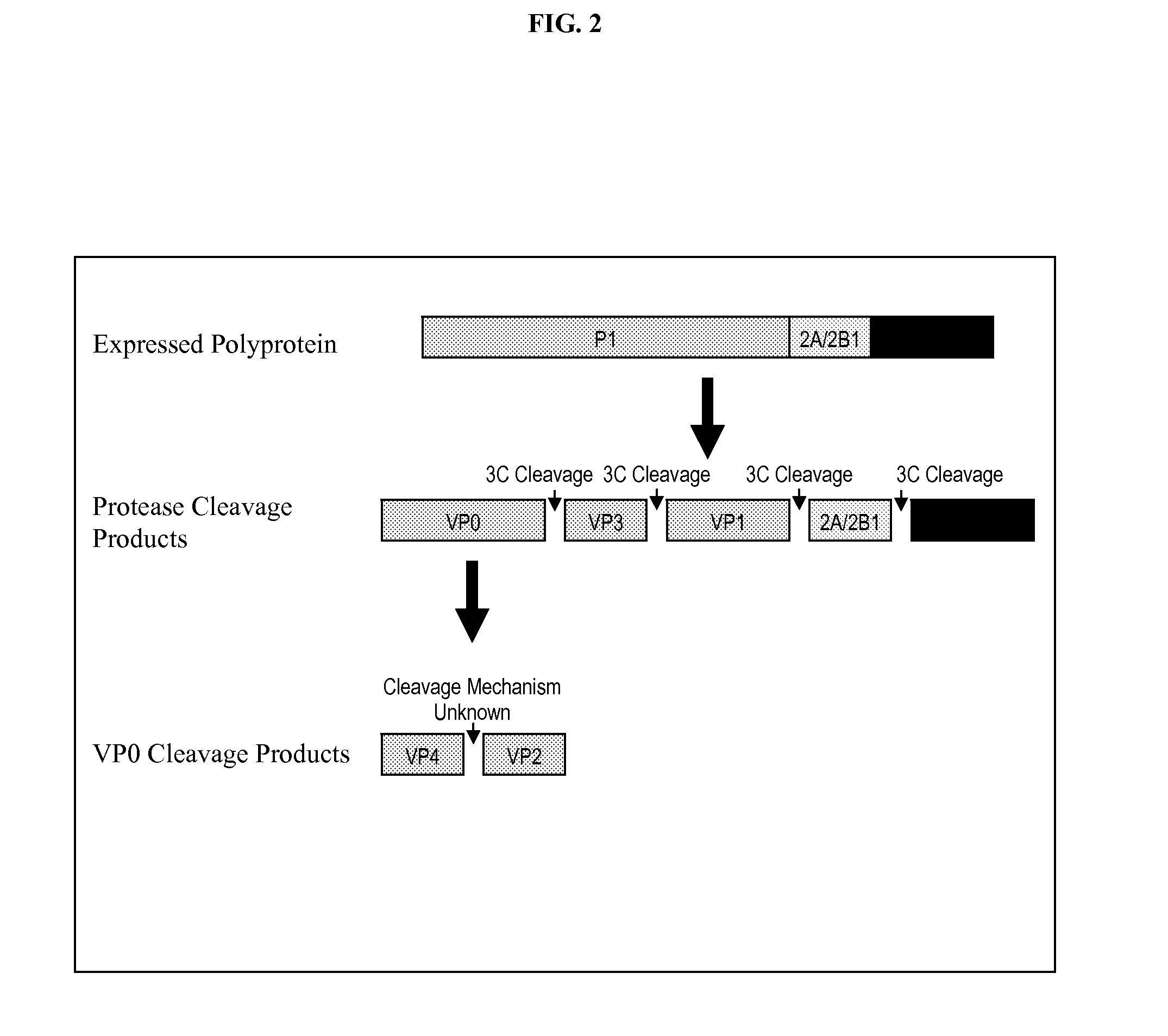
Compositions and methods for generating multiple polypeptides from a single vector using a virus derived peptide cleavage site, and uses thereof
Single vector constructs for expression of a functional antibody molecule are described. The vectors have a self-processing cleavage site between two heterologous DNA coding sequences allowing for expression of two coding sequences using a single promoter. Exemplary vector constructs comprise a foot and mouth disease virus (FMDV) 2A sequence. The vector constructs can be used in methods relating to antibody delivery and therapy and in the production of a biologically active antibody or fragment thereof.
Owner:BIOSANTE PHARMA
Monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and method for detecting nonstructural protein (NSP) antibody of foot-and-mouth disease virus (FMDV)
The invention discloses a monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and a method for detecting the nonstructural protein (NSP) antibody of a foot-and-mouth disease virus (FMDV) (FMD NSP B-ELISA); the kit comprises ELISA reaction plates, serum diluent, 25 times concentrated detergent, substrate solution, 100* concentrated ELISA detecting antibody, stop buffer, positive control serum and negative control serum; the ELISA reaction plates are two 96-pore high-affinity ELISA reaction plates, firstly 6* groups of amino acid monoclonal antibody or NSP 2C polyclonal antibody, and then FMDV 3ABC or 2C3AB NSP which is expressed by pronucleus and is provided with 6* groups of amino acid labels is captured through the monoclonal antibody or the polyclonal antibody; and compared with other similar kits, the method has higher coincidence rate and higher positive serum detection rate, and is applicable to detecting the serum of cattle, sheep, pigs and other susceptible animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Foot-and-mouth disease virus capsid protein tandem coexpressions and virus-like particle preparation method
ActiveCN104404074AHigh activityNatural binding activityBacteriaInactivation/attenuationEscherichia coliVirus-like particle
The invention relates to escherichia coli-derived single-plasmid-tandem soluble coexpression foot-and-mouth disease virus capsid proteins VP0 (which is a VP4 and VP2 fusion gene), VP1 and VP3, and a foot-and-mouth disease virus capsid protein virus-like particle preparation method. Foot-and-mouth disease virus capsid protein virus-like particles can be used for preparation of a foot-and-mouth disease vaccine. According to the method, a plurality of aspects of escherichia coli-derived soluble coexpression foot-and-mouth disease virus capsid protein are studied, by comprehensive use of tandem coexpression and SUMO(suggested upper merged ontology) technology with a tag for soluble coexpression of the foot-and-mouth disease virus capsid proteins VP0 (which is the VP4 and VP2 fusion gene), VP1 and VP3, the ultimate objective protein accounts for about 20% of total bacterial protein, and the foot-and-mouth disease virus capsid proteins obtained by purification can be successfully assembled into the virus like particles.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Method for detection of foot-and-mouth disease virus with chromatographic strip test
InactiveUS20080280296A1Improve detection efficiencyQuick stepsMicrobiological testing/measurementReverse transcriptasePoint-of-care testing
The present invention discloses a method for detection of foot-and-mouth disease virus with chromatographic strip test. Firstly, the nucleic acid sequence of FMDV NSPs is set up, the nucleic acid sequence is amplified by the reverse transcriptase polymerase chain reaction (RT-PCR) method, the recombinant vector is constructed and performed through a prokaryotic system to transform and express the recombinant protein, and the purified recombinant protein is mass produced. Design principles of the method are based on immunoassay and chromatographic analysis. The advantages are easy and simple to handle, no need of elaborate equipment, only one drop of body fluid is required to quickly complete the qualitative test in 10-20 minutes, and operating with a portable POCT (Point of care testing) instrument to complete the quantitative detection within 40-50 minutes.
Owner:NAT INST FOR ANIMAL HEALTH COUNCIL AGRI EXECUTIVE YUAN
Foot and mouth disease virus-like particle, preparation method and application thereof
ActiveCN101914501AImproving immunogenicityImprove biological activityInactivation/attenuationAntiviralsEnzyme digestionStructural protein
The invention discloses an Asia I type foot and mouth disease virus-like particle, a preparation method and an application thereof. The Asian I type foot and mouth disease virus-like particle comprises structural proteins of VP0, VP3 and VP1 of Asia I type foot and mouth disease virus, wherein the gene sequence of VP0 is shown in SEQ 1, the gene sequence of VP3 is shown in SEQ 3, and the gene sequence of VP1 is shown in SEQ 2. The preparation method of the Asian I type foot and mouth disease virus-like particle has the following steps: performing amplification to obtain the VP0, VP3 and VP1, performing enzyme digestion to obtain a recombinant expression vector, performing enzyme digestion on fusion protein by small ubiquitin-like modifier (SUMO), and carrying out in-vitro assembling to obtain the foot and mouth disease virus-like particle.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for preparing monoclonal antibody resisting O-type foot and mouth disease virus and antibody and use
InactiveCN1900115AHigh serotype specificityHighly serotype specificPeptide preparation methodsImmunoglobulinsMonoclonal antibody preparationVirology
The present invention discloses a kind of monoclonal antibody for detecting type O foot and mouth disease virus and its preparation process and use.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Development of a Marker Foot and Mouth Disease Virus Vaccine Candidate That is Attenuated in the Natural Host
ActiveUS20120315295A1Easy to replaceQuick changeSsRNA viruses positive-senseSugar derivativesSerotypeViral Vaccine
We have generated novel molecularly marked FMDV A24LL3DYR and A24LL3BPVKV3DYR vaccine candidates. The mutant viruses contain a deletion of the leader coding region (LL) rendering the virus attenuated in vivo and negative antigenic markers introduced in one or both of the viral non-structural 3Dpol and 3B proteins. The vaccine platform includes unique restriction endonuclease sites for easy swapping of capsid proteins for different FMDV subtypes and serotypes. The mutant viruses produced no signs of FMD and no shedding of virulent virus in cattle. No clinical signs of disease or fever were observed and no transmission to in-contact animals was detected in pigs inoculated with live A24LL3DYR. Cattle immunized with chemically inactivated vaccine candidates showed an efficacy comparable to a polyvalent commercial FMDV vaccine. These vaccine candidates used in conjunction with a cELISA provide a suitable target for DIVA companion tests.
Owner:US SEC AGRI
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
Porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as preparation method and application thereof
InactiveCN103122353AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliShuttle vector
The invention discloses porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as a preparation method and application thereof. Sequences of VP0, VP1 and VP3 genes are artificially synthesized by referring to an FMDV (Foot And Mouth Disease Virus) O-type epidemic strain gene sequence; the VP0, VP1 and VP3 genes are connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHmVP013 is obtained. The baculovirus transfer vector pFBDPHmVP013 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant shuttle vector Bacmid; the shuttle vetcor Bacmid is transferred with a sf9 cell, and the recombinant baculovirus QP-Ac-FVLP is obtained by collecting the cell supernatant. The recombinant baculovirus can be used for efficiently expressing FMDVVP0, Vp1 and Vp3 proteins and forming virus-like particles. And the virus-like particles are used for preparing subunit vaccine, so that the organism is induced to generate specific immunity response after the mouse is immunized.
Owner:HUAZHONG AGRI UNIV
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
O type foot and mouth disease virus-like particle and preparation method thereof and application
ActiveCN106479986AHigh expressionImprove assembly efficiencySsRNA viruses positive-senseViral antigen ingredientsAnimal testingStructural protein
The invention discloses an O type foot and mouth disease virus-like particle and a preparation method of the O type foot and mouth disease virus-like particle and an application. The O type foot and mouth disease virus-like particle is formed by assembling structural protein VP0, VP1 and optimized OP3 structural proteins of O type foot and mouth disease virus, wherein the gene sequence of the VP1 is shown as SEQ ID NO.1; the gene sequence of the VP0 is shown as SEQ ID NO.2; the gene sequence of the optimized VP3 is shown as SEQ ID NO.3. The invention tries to perform artificially missing on a part of section of the structural protein VP3 of O type foot and mouth disease virus; the result shows that the protein expression amount of the VP3 gene after the artificial missing is improved by 20% in comparison to that before mutation; the assembling efficiency of the virus-like particle is also improved by 15%; moreover, the animal test result shows that the immunogenicity thereof is good and free from significant difference with VLPs obtained through unmissed VP3 assembling. The invention provides a new technical manner for the research of the foot and mouth disease vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Avipox recombinants expressing foot and mouth disease virus genes
ActiveUS7527960B2SsRNA viruses positive-senseViral antigen ingredientsGene productProtective immunity
The present invention relates to modified poxviral vectors and to methods of making and using the same. In particular, the invention relates to recombinant avipox that expresses gene products of foot and mouth disease virus (FMDV), and to compositions or vaccines that elicit immune responses directed to FMDV gene products and which can confer protective immunity against infection by FMDV.
Owner:MERIAL INC
Honeysuckle composition for resisting virus diseases of pigs
The invention discloses a honeysuckle composition for resisting virus diseases of pigs, and relates to a feed additive or Chinese veterinary medicine special for preventing and controlling the virus diseases of the pigs and capable of promoting the growth of the pigs. The composition consists of the following components: 30 to 60 portions of honeysuckle extract, 5 to 20 portions of Chinese thorowax root extract, 5 to 20 portions of baical skullcap root P.E, 5 to 20 portions of dandelion P.E, and 10 to 25 portions of licorice extract. The composition is Chinese medicinal powder of which the granularity is between 200 and 300 meshes and which is yellow or brown-yellow, and has the following content of the active components: 5 to 20 percent of chlorogenic acid, 2 to 10 percent of baicalin and5 to 15 percent of glycyrrhizic acid. When used, the composition is mixed with a feed in a ratio of 0.02-0.2 percent and then is fed to animals. The composition has the effect of preventing and controlling highly pathogenic blue ear pig disease, porcine rotavirus disease, influenza, infectious bronchitis and the like, and virus diseases caused by porcine circovirus PCV, foot-and-mouth disease virus and the like, and can effectively promote the growth of the live pigs.
Owner:新乡博凯生物技术有限公司
Method for expanding antigen spectrum of foot-and-mouth disease vaccine strain by reverse genetic operation and preparation method of vaccine
ActiveCN101948811AHigh protection rateBroad antigen spectrumVirus peptidesMicroorganism based processesImmune effectsSoutheast asia
The invention relates to a method for expanding the antigen spectrum of a foot-and-mouth disease vaccine strain by reverse genetic operation and a preparation method of a vaccine. The amino acid sequence of the VP3 and VP1 structural proteins of the foot-and-mouth disease virus strain of the invention is represented by the amino acid residues from a position 304 to a position 736 in SEQ ID No.4. Experiments show that the vaccine prepared from the mutant virus strain obtained by the invention can resist porcine epidemic viruses of China O / TL / Taiwan / 97 lineage, Pan-Asia O / China / 99 lineage and Southeast Asia Myanmar O / GS / 2010 / 98 lineage, has a characteristic of wide antigen spectrum, can immunize pigs and obviously improve the rate of protection against foot-and-mouth disease viruses which are of the same type and have antigenicity difference, achieves an immune effect of cross protection, and is expected to play an important role in the prevention and control of foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Emulsion breaking method for aftosa oil emulsion inactivated vaccine
InactiveCN102380232AEasy to separateHigh recovery rateNon-miscible liquid separationAntigenDemulsifier
The invention relates to an emulsion breaking method for an aftosa oil emulsion inactivated vaccine used in an aftosa vaccine quality detection technology. The method comprises the following steps of: fully and uniformly mixing an emulsion breaking agent with an aftosa oil emulsion inactivated vaccine; standing; separating oil from water; and distributing aftosa virus particles into a water phase, wherein the emulsion breaking agent is selected from any of benzyl alcohol, n-amyl alcohol, chloroform and hexyl alcohol serving as organic solvents. Due to the adoption of the method, the completeness of aftosa virus particles 146S is not broken basically, emulsion is broken rapidly and simply, the antigen 146S recovery rate is high, and the stability is high; and the method is particularly suitable to be taken as a method for monitoring the quality of commercial aftosa vaccines for aftosa vaccine producing enterprises and the national quality test departments.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombined cattle O type foot and mouth disease virus amalgamation protein vaccine
InactiveCN101293098ASecurity advantageAntiviralsAntibody medical ingredientsNucleotide sequencingGenetic engineering
The invention provides a recombinant foot-and-mouth disease virus fusion protein vaccine designed on the basis of O type foot-and-mouth disease virus. Experiments prove that the vaccine of the invention which is produced by applying the genetic engineering technology has good effects for the prevention and treatment of foot-and-mouth disease virus infection. The invention provides an amino acid sequence of the recombinant foot-and-mouth disease virus fusion protein vaccine, a nucleotide sequence thereof and a research on the prevention of foot-and-mouth disease virus infection.
Owner:BEIJING HYDVAX BIOTECH +1
Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
InactiveCN108107220AAdequate responseIncrease binding areaBiological testingBiotin-streptavidin complexSorbent
The invention discloses a kit for quantitative detection on an O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles. The kit consists of O type foot-and-mouth disease virus antibody negative serum, O type foot-and-mouth disease virus antibody positive serum, VP1 coating magnetic beads, a biotinylation goat-anti-pig antibody, a streptavidin marking fluorescent substance, a cleaning solution and an enhancing solution. The magnetic beads used in the kit have relatively large binding areas, so that the detection range is greatly increased, the reaction time is shortened, and the sensitivity is improved. The kit has a relatively wide stimulation spectrum and a relatively narrow emitting spectrum, the cost can be reduced, and the sensitivity can be improved; compared with a conventional fluorescent substance, the kit is relatively wide in detection range and relatively good in specificity. Due to adoption of a streptavidin-biotin signal amplification system, the detection sensitivity is further improved, and the kit is relatively high in sensitivity when being compared with ELISA (Enzyme-Linked Immuno Sorbent Assay) and chemiluminiscence. Together with a full-automatic detector, on-site automatic operation can be achieved, one or more samples can be simultaneously detected, and the kit is simple, convenient and rapid to operate and low in price.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Dual real-time fluorescence quantitative PCR detection kit for foot-and-mouth disease and Seneca valley virus
InactiveCN107326100ARapid differential testImprove throughputMicrobiological testing/measurementMicroorganism based processesFluorescenceRapid identification
The invention provides a combination of a primer and a probe used for dual real-time fluorescence quantitative PCR detection of the foot-and-mouth disease virus and the Seneca valley virus and a detection kit. The sequences of the combination of the primer and the probe are respectively shown in the SEQ ID NO:1-7. The invention further provides a non-diagnostic-purpose dual real-time fluorescence quantitative PCR detection method for the foot-and-mouth disease virus and the Seneca valley virus. The three serotype foot-and-mouth disease viruses of O, A and AsiaI and the Seneca valley virus are rapidly identified and detected at the same time in the same reaction, the detection is finished within two hours, and the primer, the probe and the detection method have the advantages of rapidness, specificity, sensitivity and high throughput, and meet the requirements on large-batch rapid identification and detection of the three serotype foot-and-mouth disease viruses of O, A and AsiaI and the Seneca valley virus.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Method for determining components and estimating anti-gen content of foot-and-mouth disease vaccine
ActiveCN105467138AEasy to operateStrong specificityBiological material analysisBiological testingSerum igeAgricultural science
The invention provides a method for determining components and estimating the anti-gen content of a foot-and-mouth disease vaccine. The method comprises the following steps: (1) performing emulsion resolving on a foot-and-mouth disease vaccine to separate out an antigen phase; (2) concentrating the antigen phase; (3) using purified foot-and-mouth disease virus Asia 1-type, O-type and A-type 146S antigens to immunize animals and prepare detection serums; (4) diluting the concentrated antigens by different multiples and respectively performing immunodiffusion tests on the detection serums; (5) judging the components of the foot-and-mouth disease vaccine according to precipitation lines, that is, whether the vaccine is an O-type univalent vaccine, an O-type + A-type bivalent vaccine or an O-type + Asia 1-type + A-type trivalent vaccine; (6) estimating the content of the antigen in a detected vaccine sample according to the highest antigen dilution multiple at which the precipitation lines appear. The method can not only identify the components of the foot-and-mouth disease vaccine but also estimate the contents of all the components, and is simple to operate, strong in specificity, good in stability and suitable for basic-level application.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Foot and mouth disease virus non-structural protein antibody enzyme-linked immunodetection kit
ActiveCN103864906AStrong specificityHigh sensitivitySsRNA viruses positive-senseVirus peptidesAntigen epitopeAntigen
The invention discloses a foot and mouth disease virus non-structural protein antibody enzyme-linked immunodetection kit. The kit comprises a foot and mouth disease virus non-structural protein 3B antigen epitope peptide-coated polymerase chain reaction plate and an enzyme-labeled antibody, wherein the foot and mouth disease virus non-structural protein 3B antigen epitope peptide is a polypeptide shown as a sequence 1 in a sequence table. The kit adopts a chemical synthesis non-structural protein 3B antigen peptide coated reaction plate and is small in antigen amount, high in sensitivity and high in specificity, and whether the foot and mouth disease virus infection exists can be efficiently detected. The kit is high in specificity, sensitive and high-efficiency and has good market prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN105567871AShorten test timeLow reaction temperatureMicrobiological testing/measurementMicroorganism based processesBiologyDifferential diagnosis
The invention discloses an RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting a high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof. The kit comprises a pair of primers and a probe, the sequences of the primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe is shown as SEQ ID NO.3. It is proved through experiments that the kit can detect adverse effects of the high-pathogenicity porcine reproductive and respiratory syndrome virus (HP-PRRSV), a hog cholera virus, a C-type porcine reproductive and respiratory syndrome virus, a porcine circovirus type II, a porcine pseudorabies virus and a foot and mouth disease virus in a specificity mode. It is proved through experiments that the kit can detect out templates of at least 70 copies at the temperature of 40 DEG C on the condition of 20 min amplification, and the conformity between the kit and RT-qPCR is high. This shows that the kit can detect HP-PRRSV fast, efficiently and sensitively and provides an effective technological means for differential diagnosis of HP-PRRSV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for preparing purified foot-and-mouth disease vaccine
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com