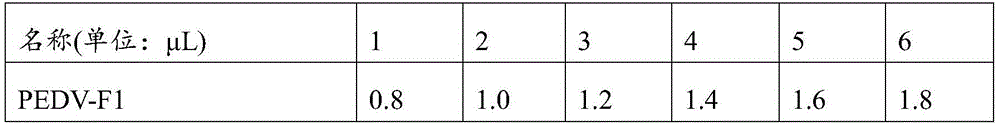
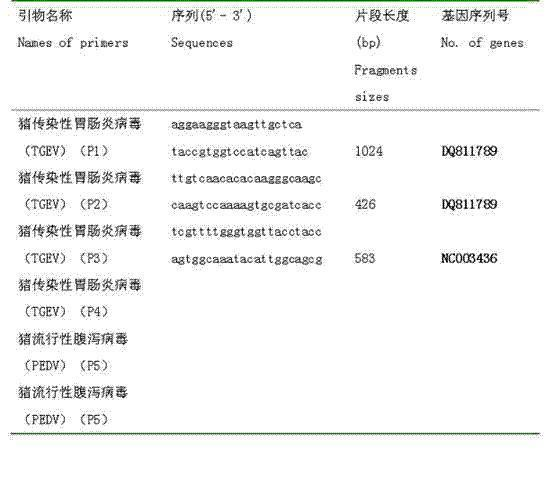
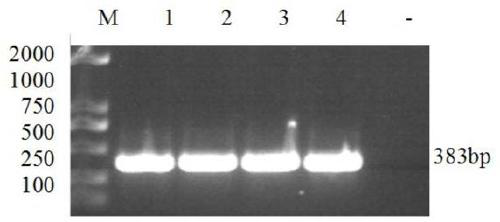
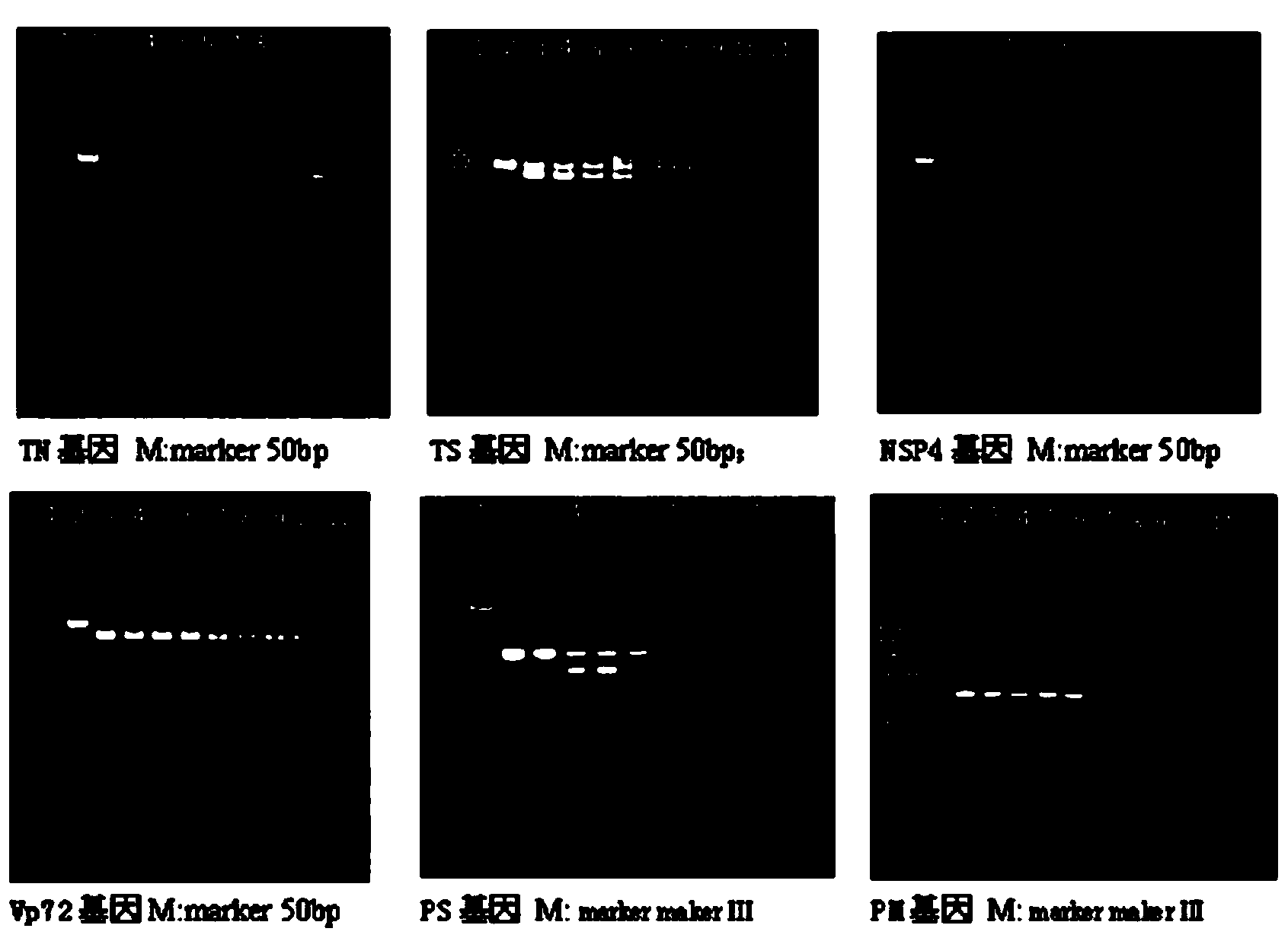
Patents
Literature
110 results about "Transmissible gastroenteritis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transmissible gastroenteritis coronavirus (TGEV) is a virus belonging to the family Coronaviridae, genus Alphacoronavirus, species Alphacoronavirus 1. TGEV are enveloped viruses with a positive-sense single-stranded RNA genome and a helical symmetry.
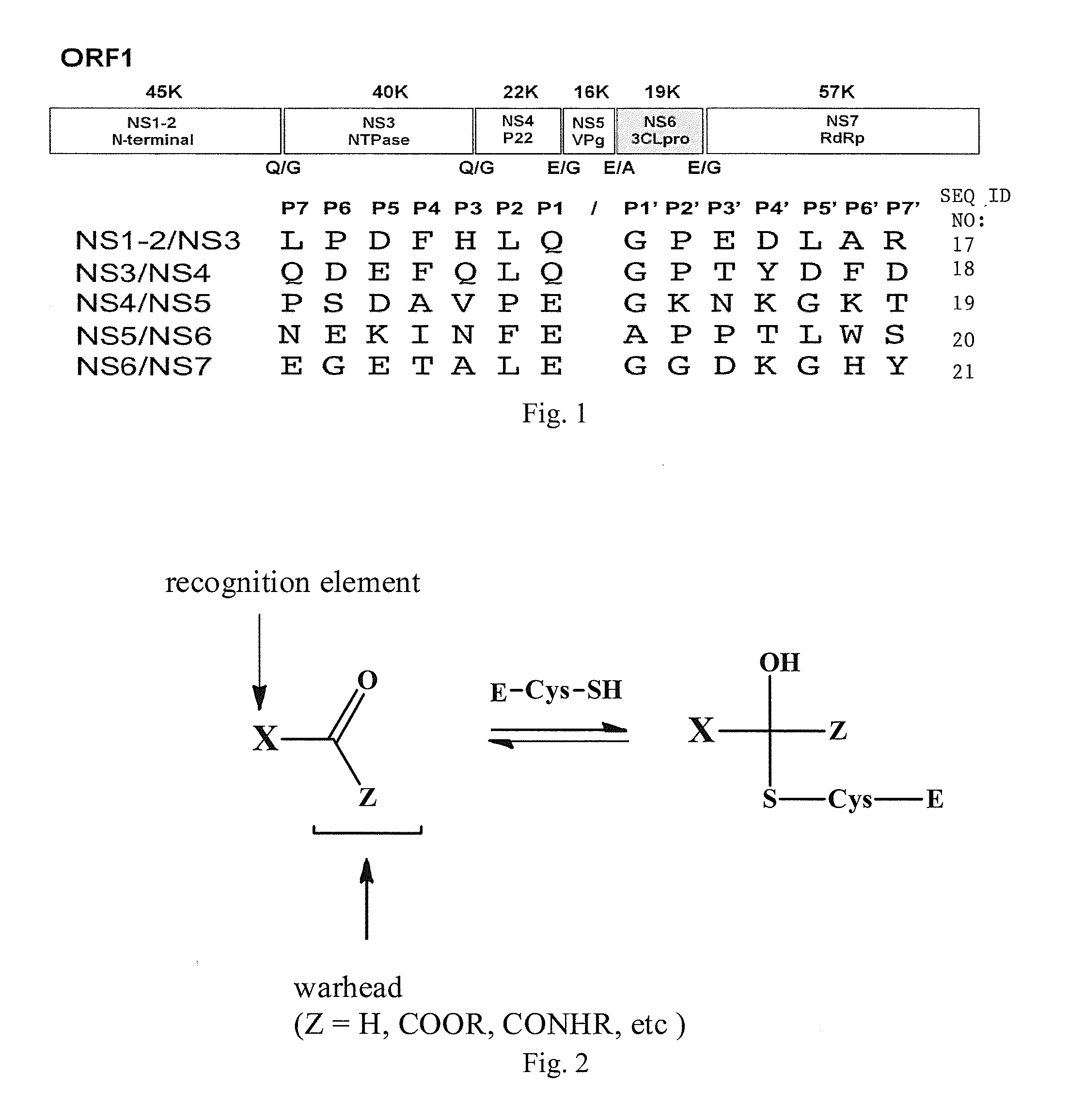
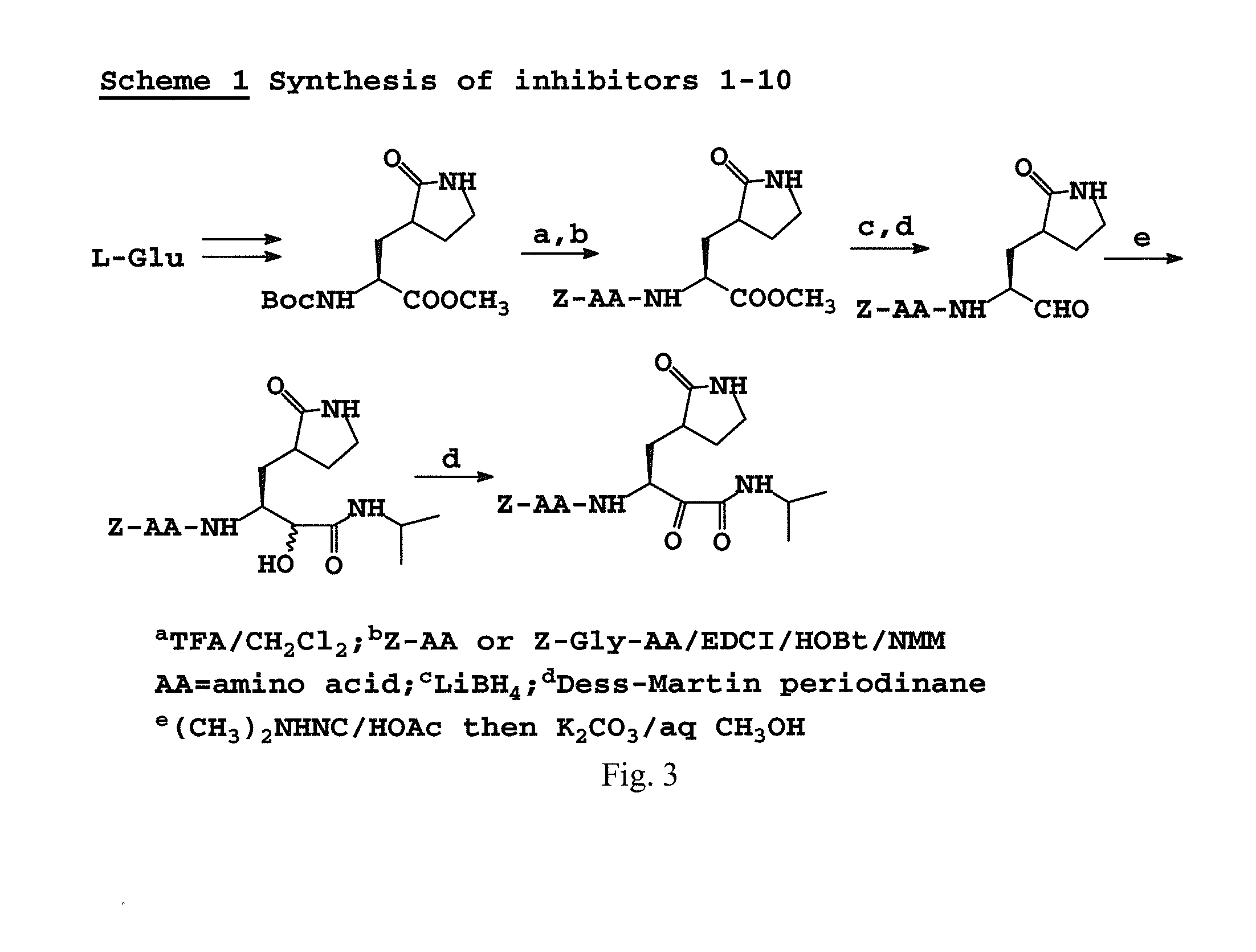
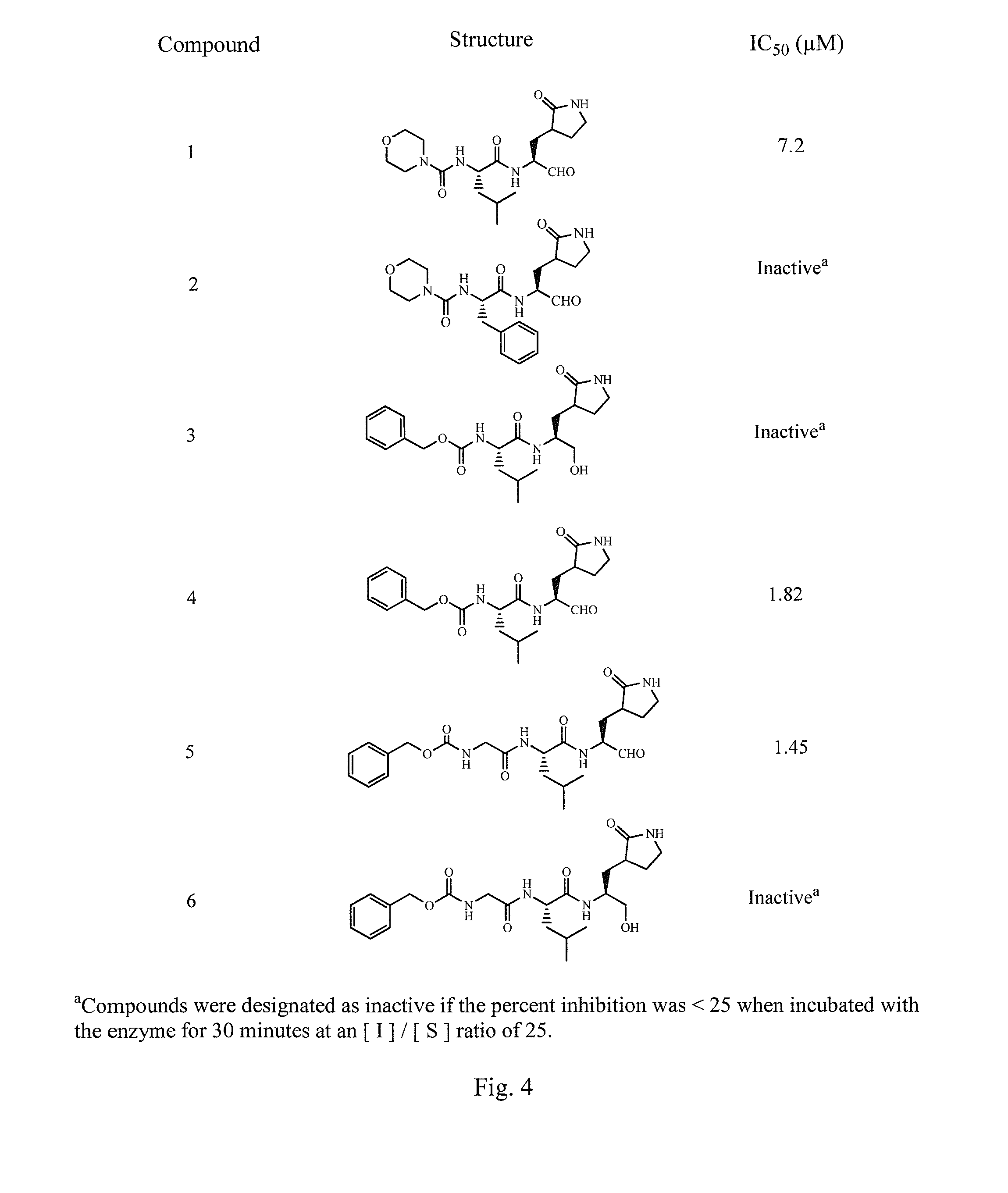
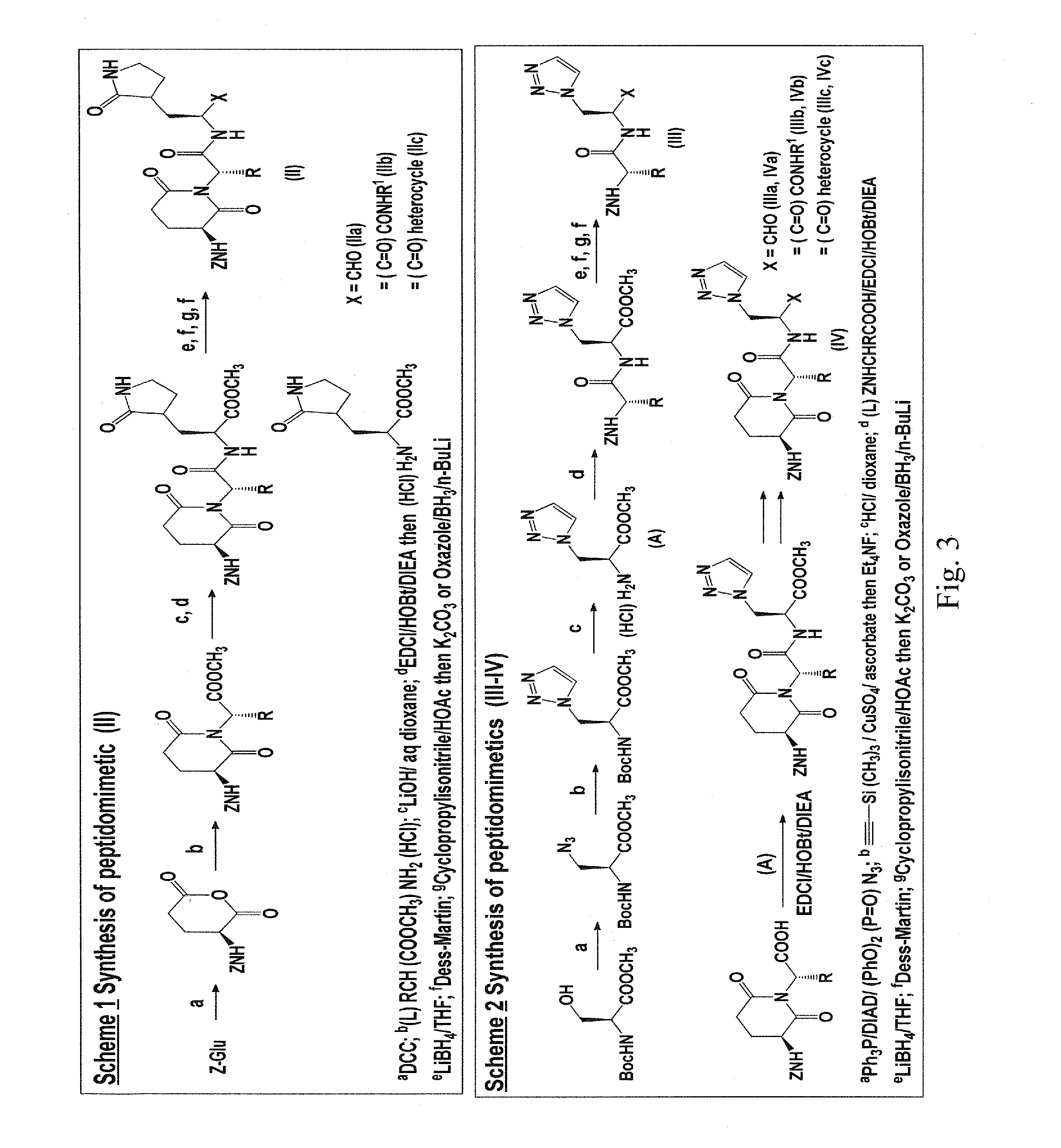
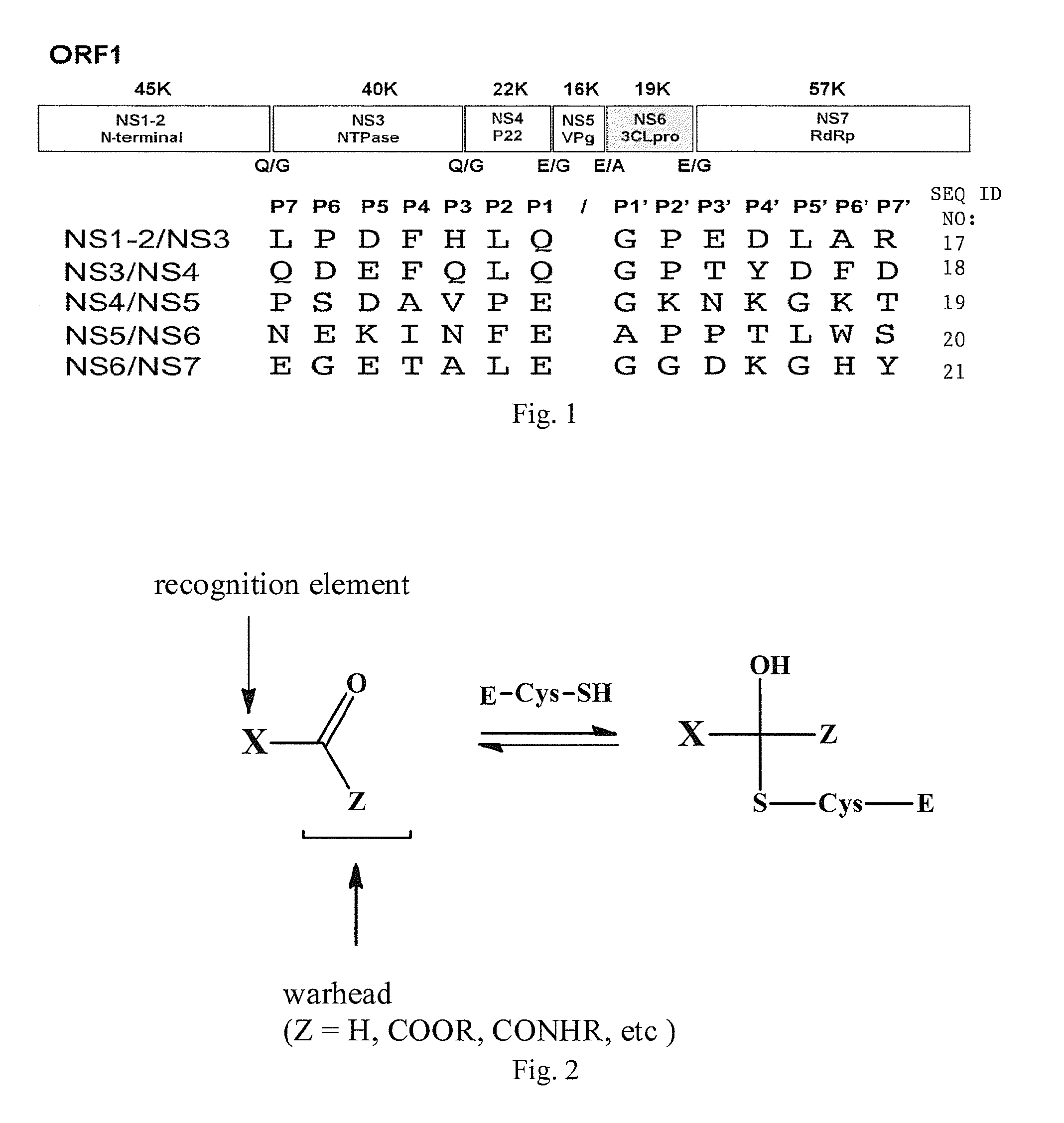
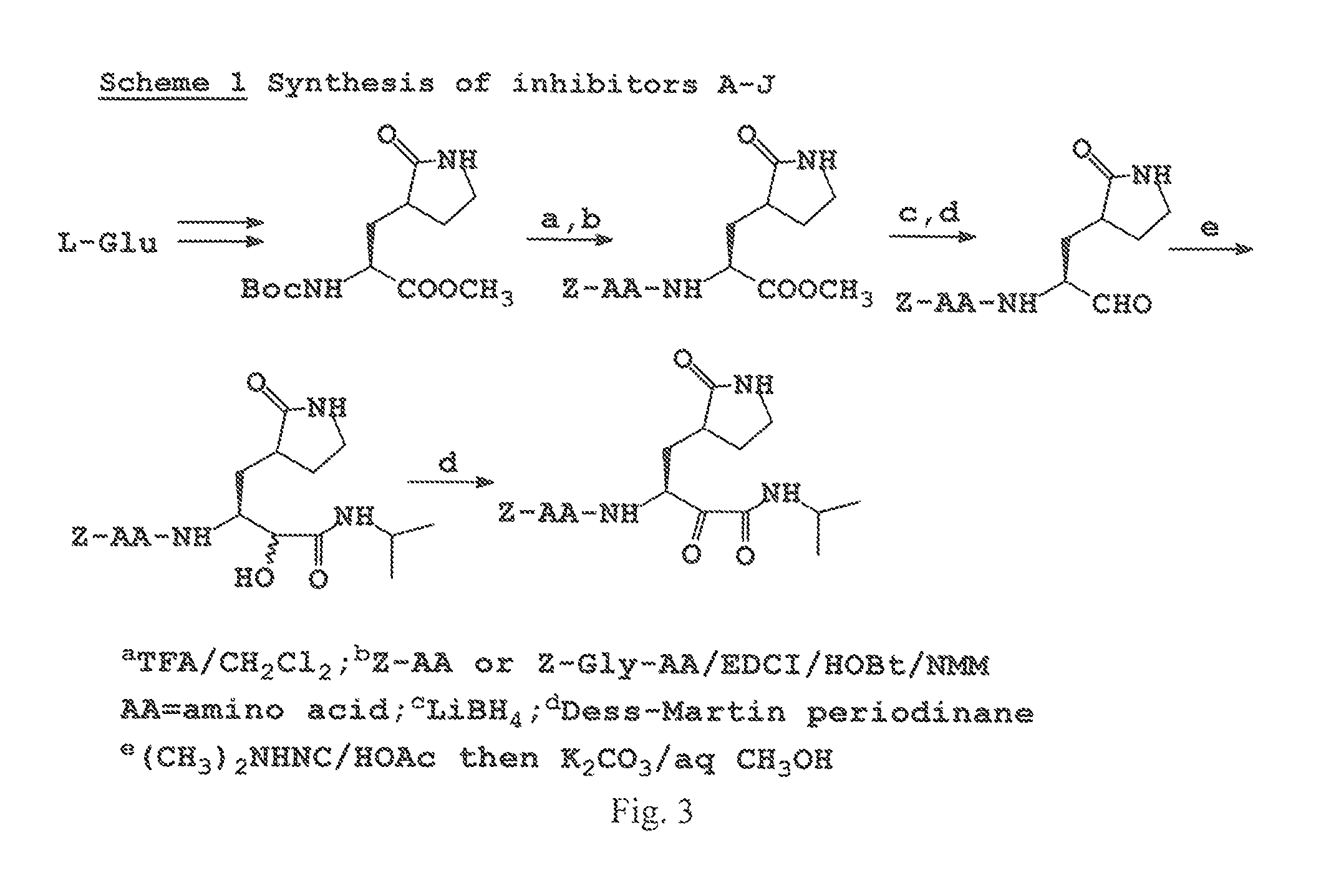
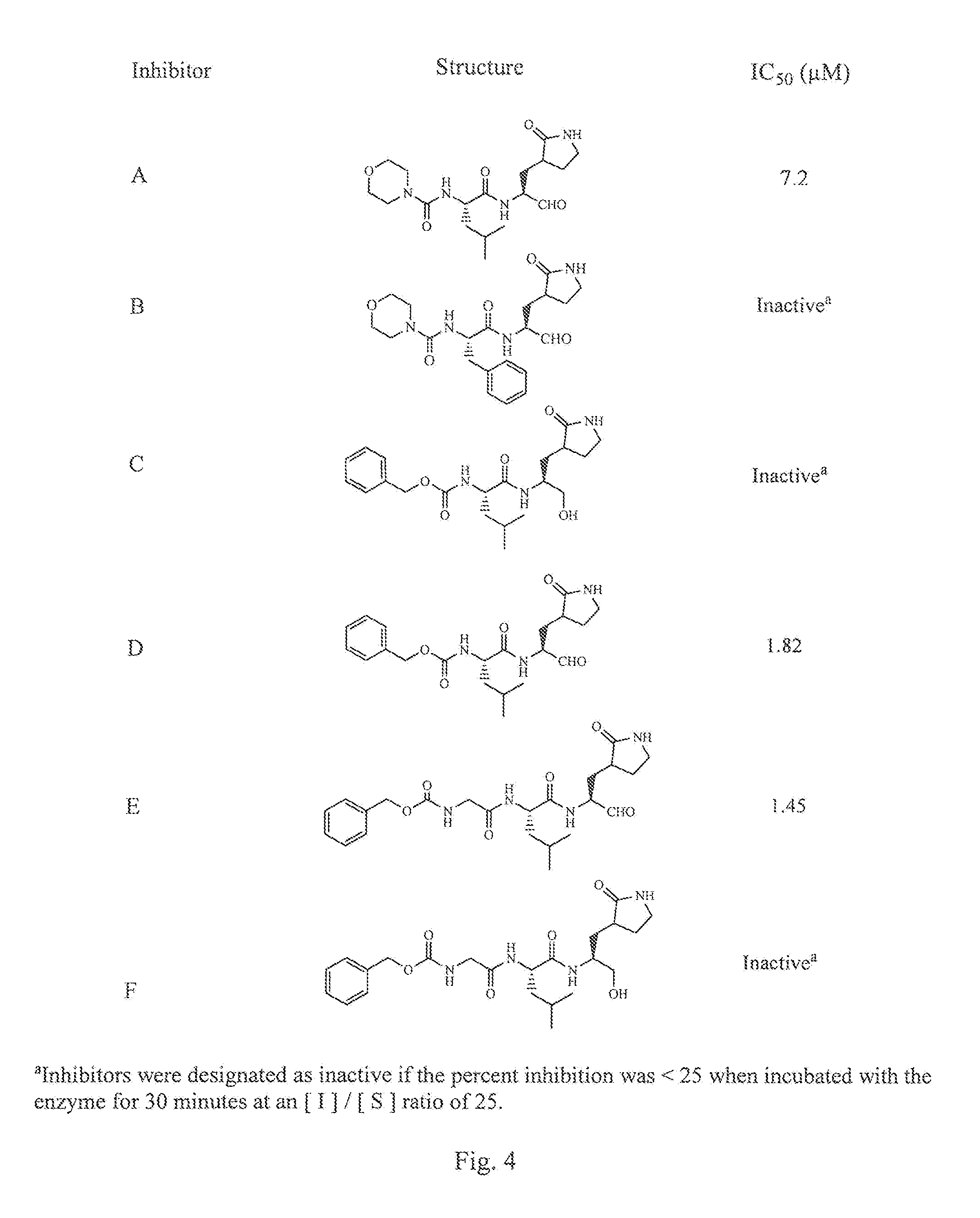
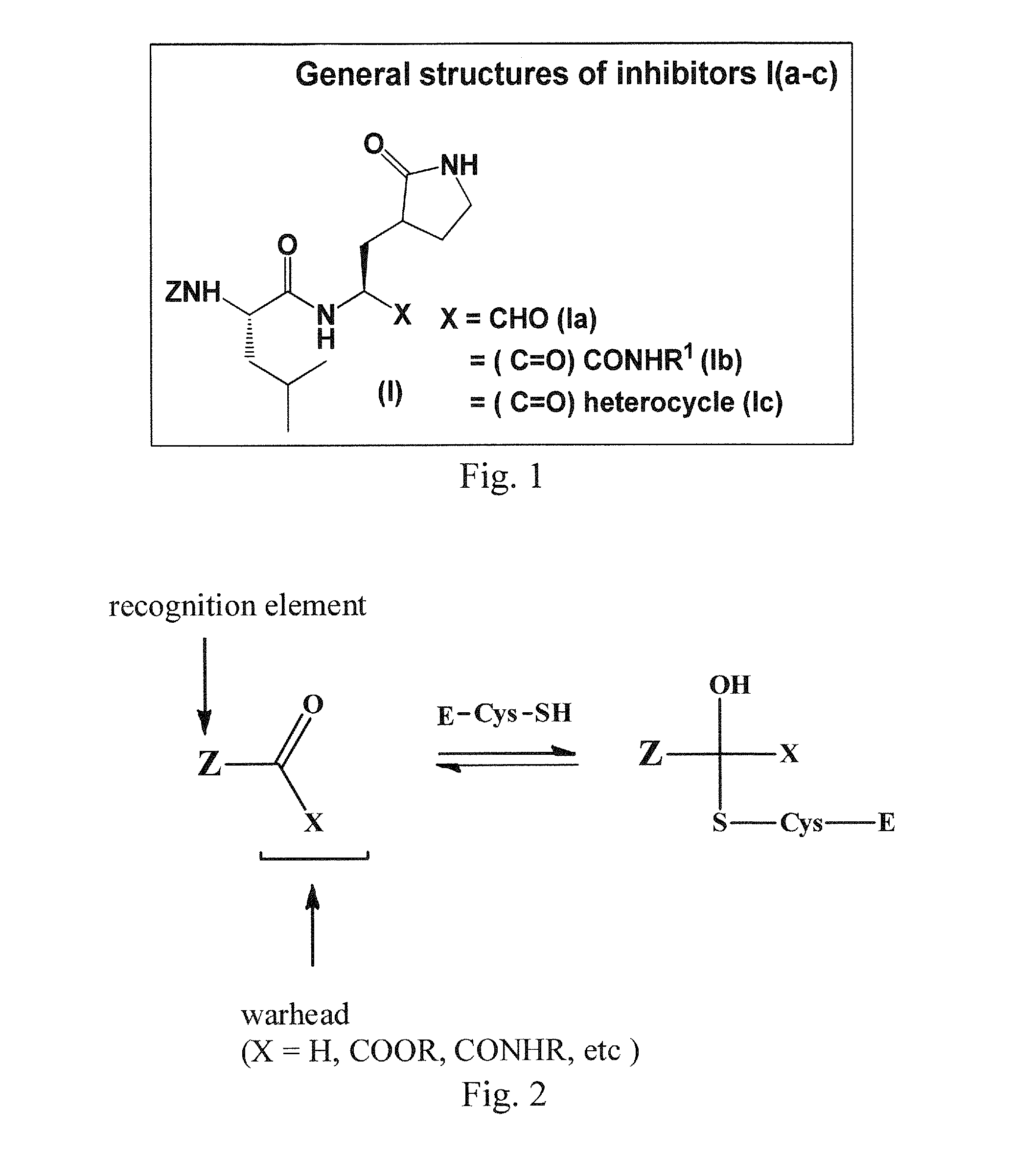
Broad-spectrum antivirals against 3c or 3c-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
ActiveUS20140243341A1Preventing and inhibiting replicationBiocideSsRNA viruses positive-senseEnterovirusDisease
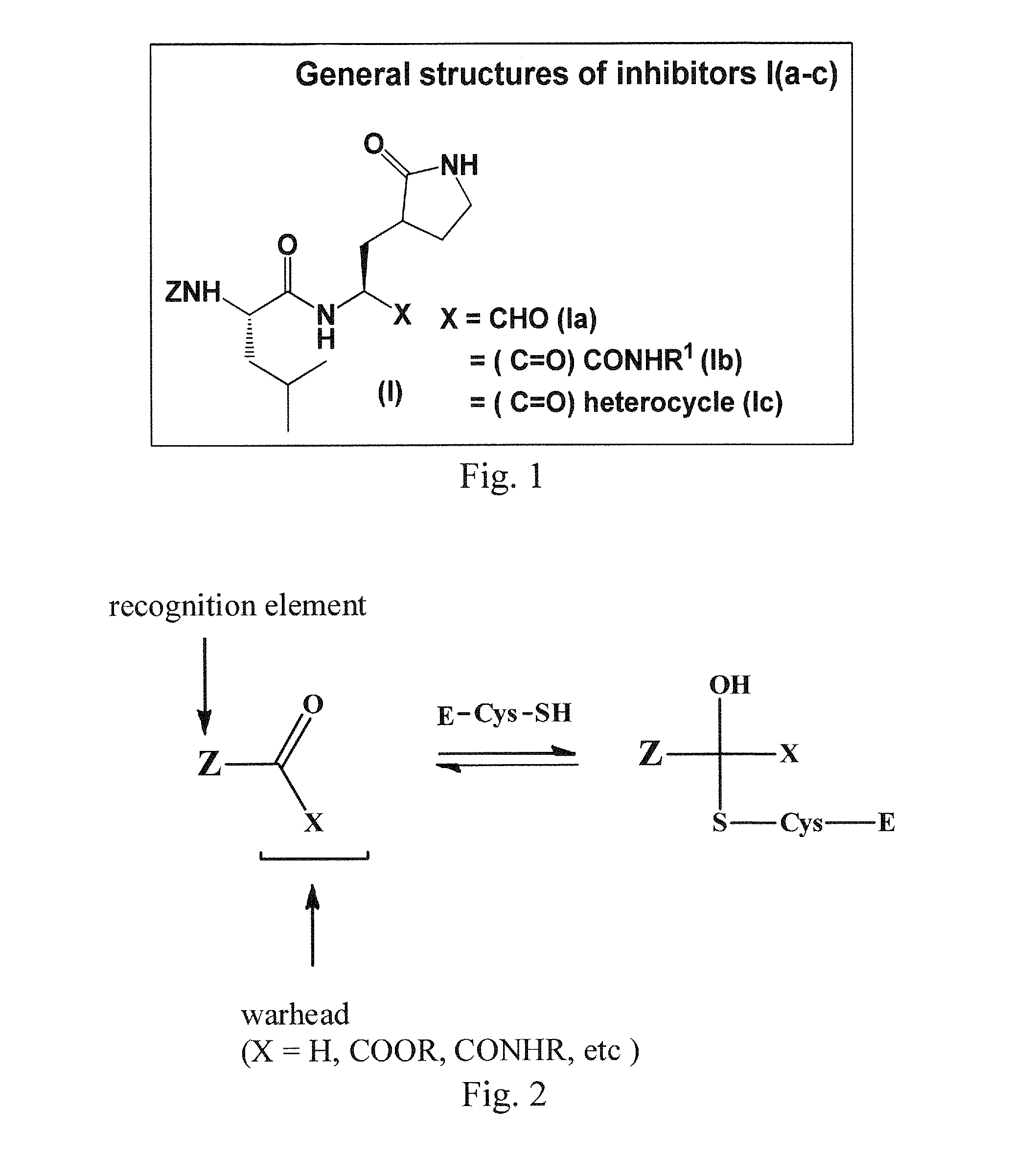
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfite salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
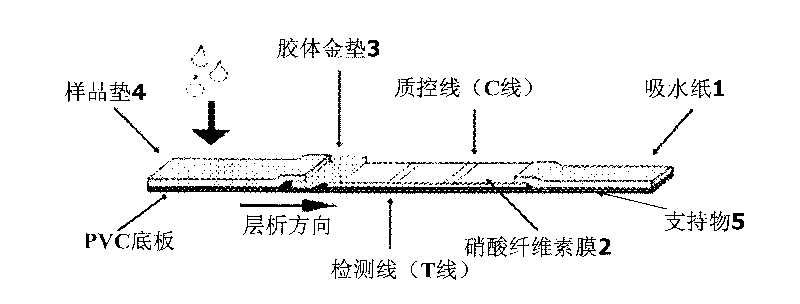
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
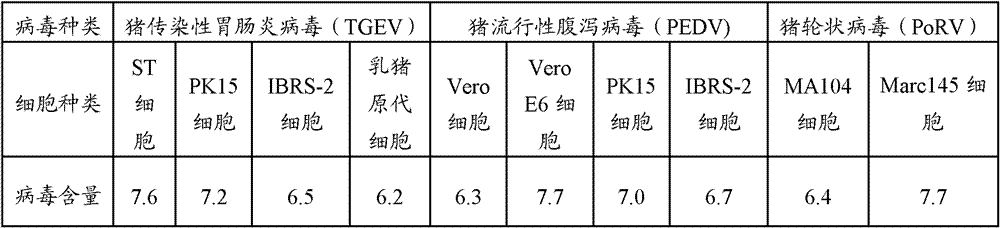
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
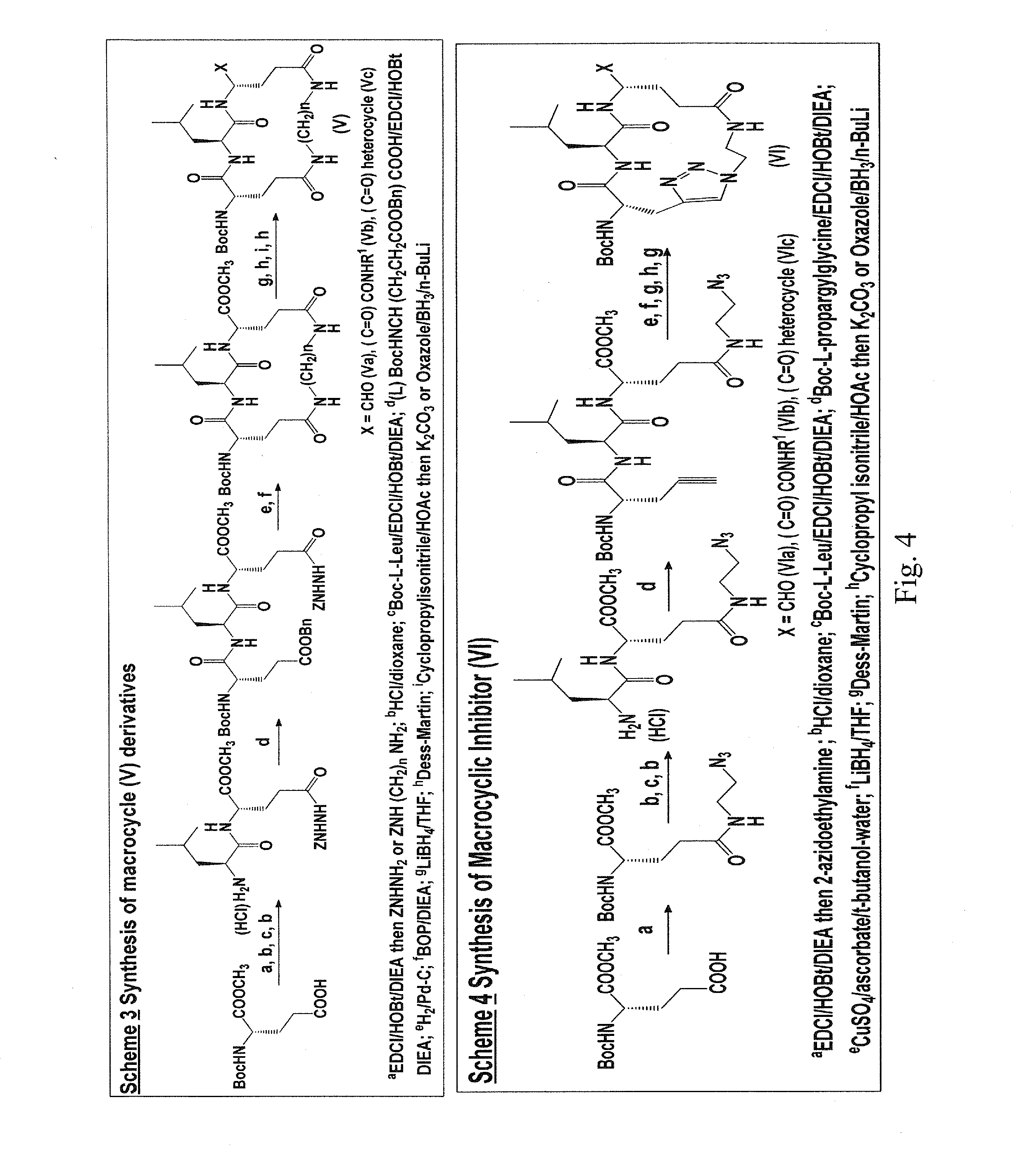
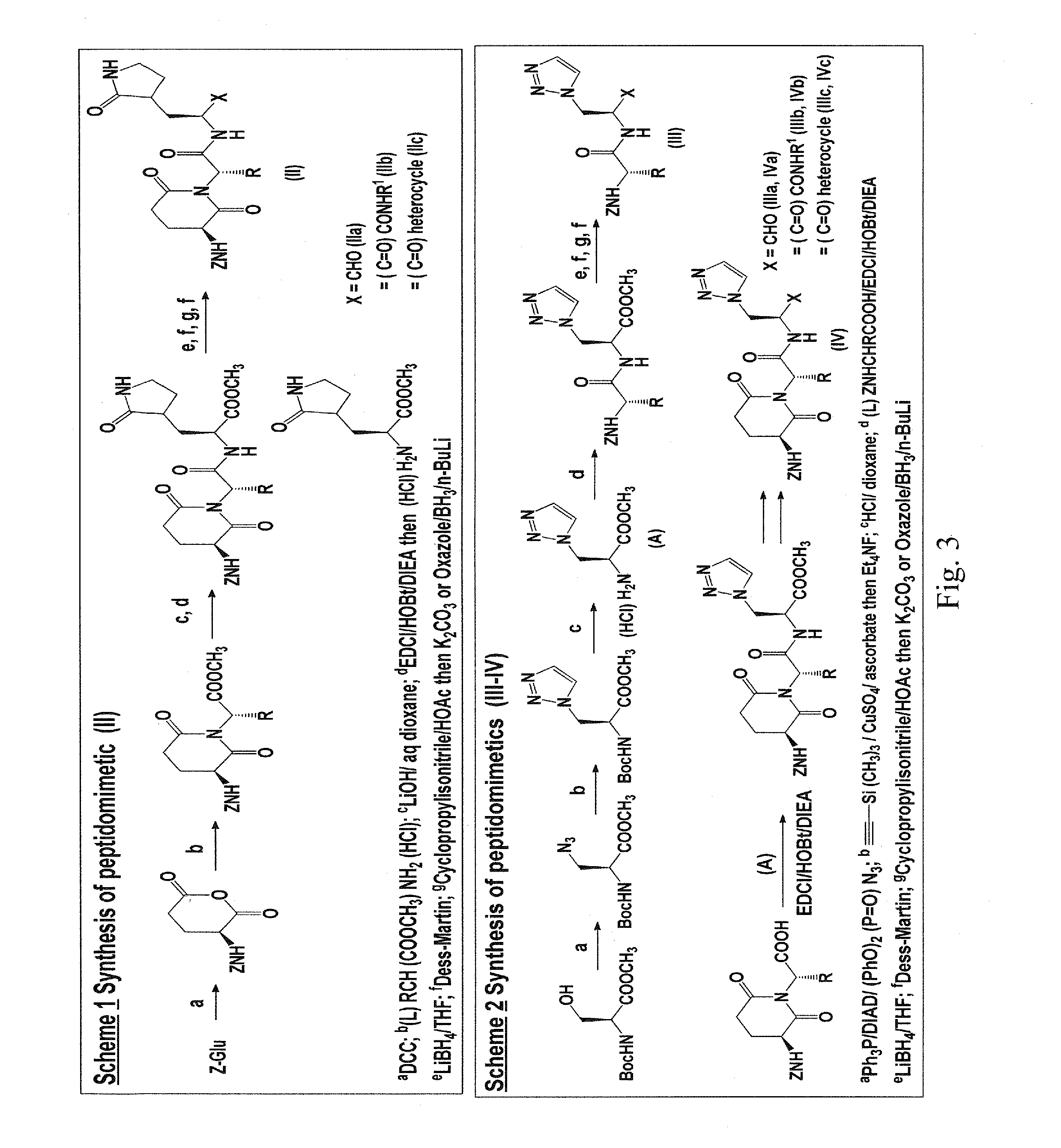
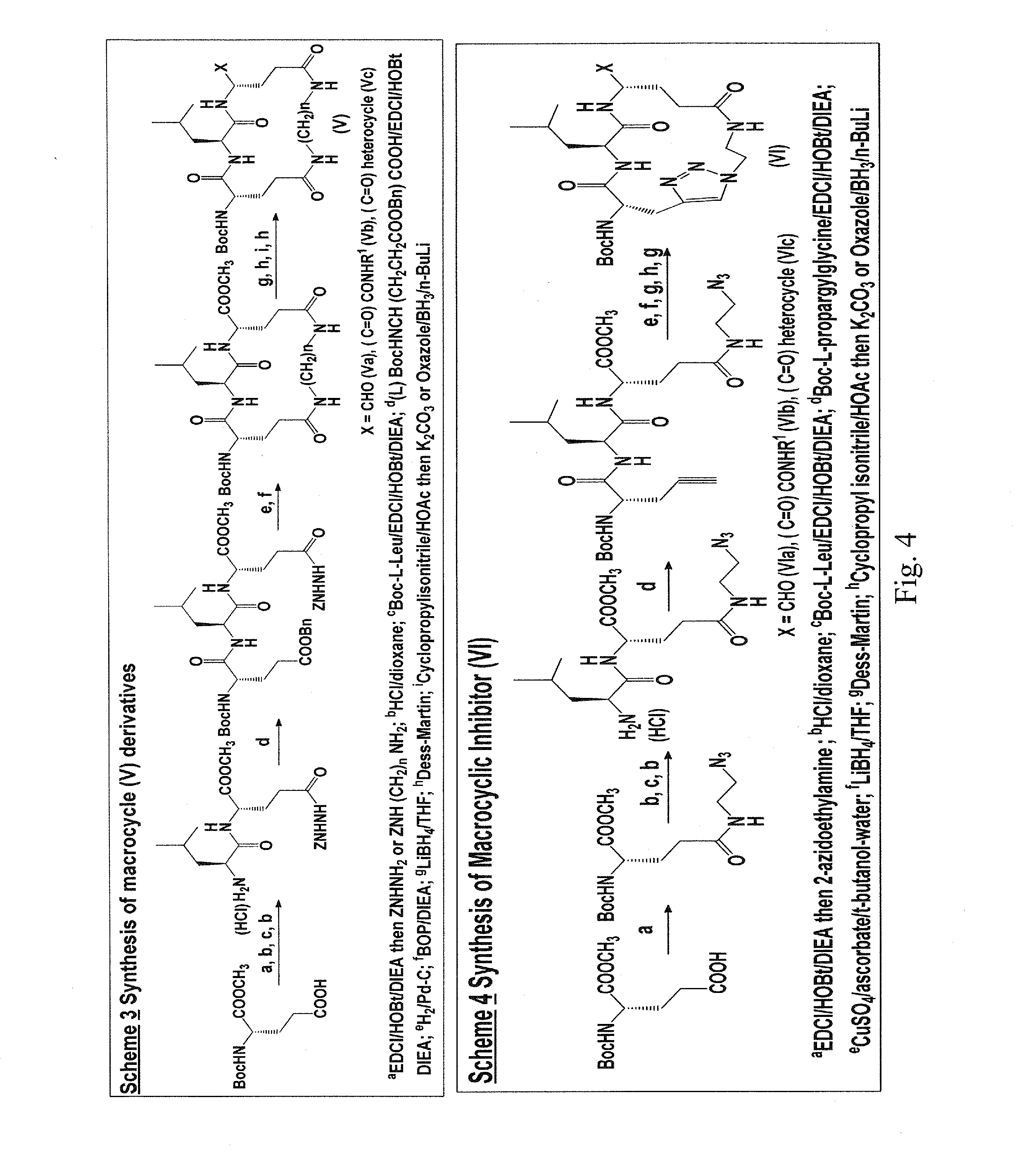
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Broad-spectrum antivirals against 3C or 3C-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfate salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:THE WICHITA STATE UNIV +1
TGEV and PEDV combined live vaccine and preparation method thereof
The invention discloses a combined live vaccine of transmissible gastroenteritis virus of swine (TGEV) and porcine epidemic diarrhea virus (PEDV) and a preparation method thereof. An attenuated swine transmissible gastroenteritis virus HB08 and an attenuated porcine epidemic diarrhea virus ZJ08 which are self-separated, attenuated and stored respectively undergo viral multiplication on ST cells and VeroE6 cells, and seedling and freeze drying are then carried out by adding a freeze-drying protective additive into a virus solution which is qualified after inspected. The two diseases, transmissible gastroenteritis of swine and porcine epidemic diarrhea virus, which are epidemic in clinic at present, can be effectively prevented by the use of the combined live vaccine.
Owner:兆丰华生物科技(南京)有限公司 +3
Molecular kit for rapidly identifying three types of piglet virus diarrhea and application of molecular kit
ActiveCN104611466ANo mutual interferenceLow minimum detectable concentrationMicrobiological testing/measurementMicroorganism based processesRotavirus RNAAstrovirus gastroenteritis
The invention discloses a kit for detecting pig epidemic diarrhea viruses, pig transmissible gastroenteritis viruses and pig rotaviruses. The kit comprises primer pairs shown by SEQ ID NO:1-2, SEQ ID NO:3-4 and SEQ ID NO:5-6 for respectively performing specific amplification on the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The invention also discloses applications of the primer pairs shown by the SEQ ID NO:1-2, the SEQ ID NO:3-4 and the SEQ ID NO:5-6 in preparation of reagents for detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The detection kit disclosed by the invention can be used for accurately and effectively detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses, and is strong in specificity, high in sensitivity, short in time consumption, rapid in detection and good in application prospect.
Owner:SICHUAN AGRI UNIV
Pig transmissible gastroenteritis virus vaccine strain and application thereof
ActiveCN101235363AImprove securitySafe and no side effectsViral/bacteriophage medical ingredientsAntiviralsMicroorganism preservationMicroorganism
The invention discloses a transmissible gastroenteritis virus (H) attenuated vaccine strain and the application. The microorganism preservation number of the attenuated vaccine strain of the invention is CCTCC-V200609. The safety of attenuated strain TGEV of the invention is excellent, which has excellent immune protective rate. The attenuated strain of the invention can be applied in preparing diagnostic reagent for diagnosing transmissible gastroenteritis, and also can be applied in preparing single vaccine or mixed vaccine (active or inactivated vaccine) and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus
ActiveCN104232801AEfficient detectionQuick checkMicrobiological testing/measurementMicroorganism based processesPorcine rotavirusVirology
The invention discloses a kit for detecting a porcine epidemic diarrhea virus, a porcine transmissible gastroenteritis virus and a porcine rotavirus. The gene detection kit disclosed by the invention can accurately and effectively detect the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus, and is strong in specificity, high in sensitivity, short in time consumption, fast in detection and good in application prospects.
Owner:SICHUAN AGRI UNIV
Colloidal gold strip for TGEV antibody and PEDV antibody
ActiveCN103033626AObvious superiorityGuaranteed FeaturesMaterial analysisSerum igeMonoclonal antibody
The invention discloses a test strip for quickly detecting a PEDV (Porcine Epidemic Diarrhea Virus) serum antibody and a TGEV (Transmissible Gastroenteritis Virus) serum antibody and a preparation method of the test strip. The test strip comprises a support layer, a sample loading layer, a gold labeling protein release pad, a detection layer and an absorption layer, wherein a gold labeling protein and a detection line protein of the test strip are an S1 protein of a PEDV and a recombination protein of a TGEV, which are obtained by an efficient prokaryotic expression system; the recombination protein comprises an S protein AD site; and two quality control line proteins are monoclonal antibodies for the two proteins. Compared with the traditional test strip for detecting the PEDV antibody and the TGEV antibody, the test strip is high in specificity and safety and simple to operate, and judges results quickly.
Owner:兆丰华生物科技(南京)有限公司 +3
Lactobacillus reuteri LR-CO21 and application thereof
ActiveCN110734879AImprove adhesionGood colonization effectAntibacterial agentsBacteriaInfectious DisorderImmunity
The invention provides Lactobacillus reuteri LR-CO21 and application thereof and relates to the technical field of probiotics. The preservation number of the Lactobacillus reuteri LR-CO21 in the ChinaGeneral Microbiological Culture Collection Center is CCTCC No.M 2019601. The Lactobacillus reuteri LR-CO21 has good transplanting performance and adhesion in an intestinal tract and is capable of effectively inhibiting TGEV (transmissible gastroenteritis virus) infection and improving the ability and immunity of piglets in resisting virus infection and bacterial infection after transplanting. Inaddition, the invention further provides a method for applying the Lactobacillus reuteri LR-CO21 strain in improving the ability of piglets in resisting infectious diseases.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
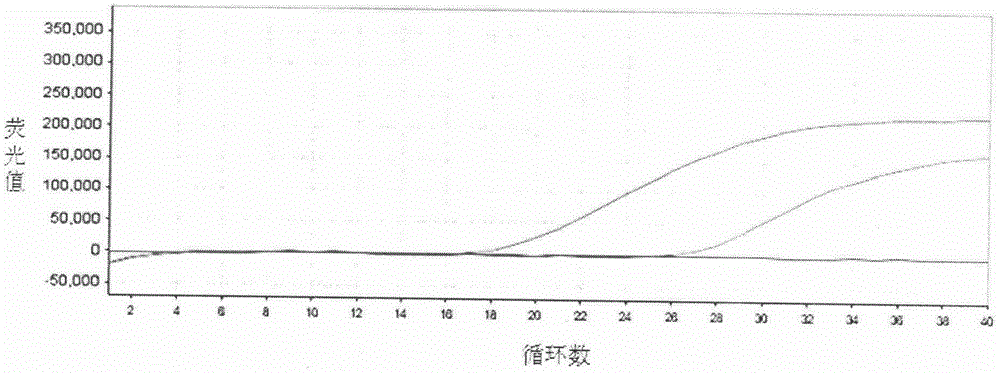
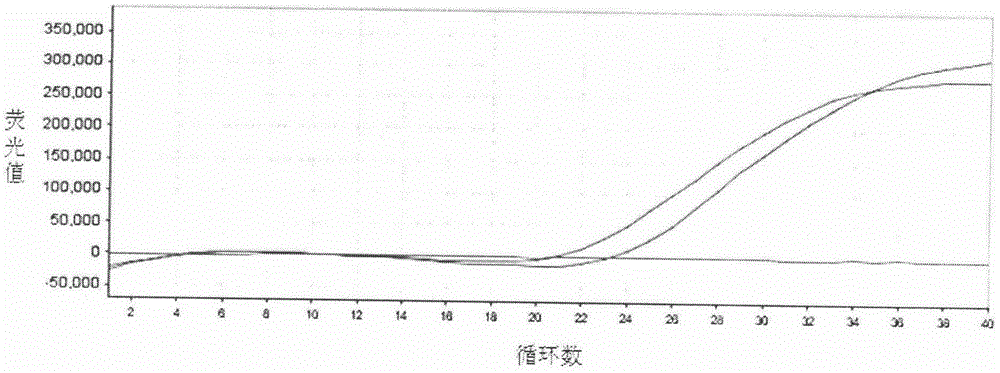
SYBR GreenI real-time quantitative polymerase chain reaction (PCR) detection method for S gene of transmissible gastroenteritis virus of swine
InactiveCN102102132AGood repeatabilityNo cross reactionMicrobiological testing/measurementFluorescence/phosphorescenceTotal rnaTransmissible gastroenteritis virus
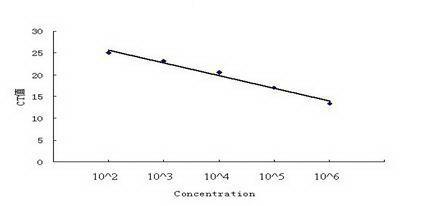
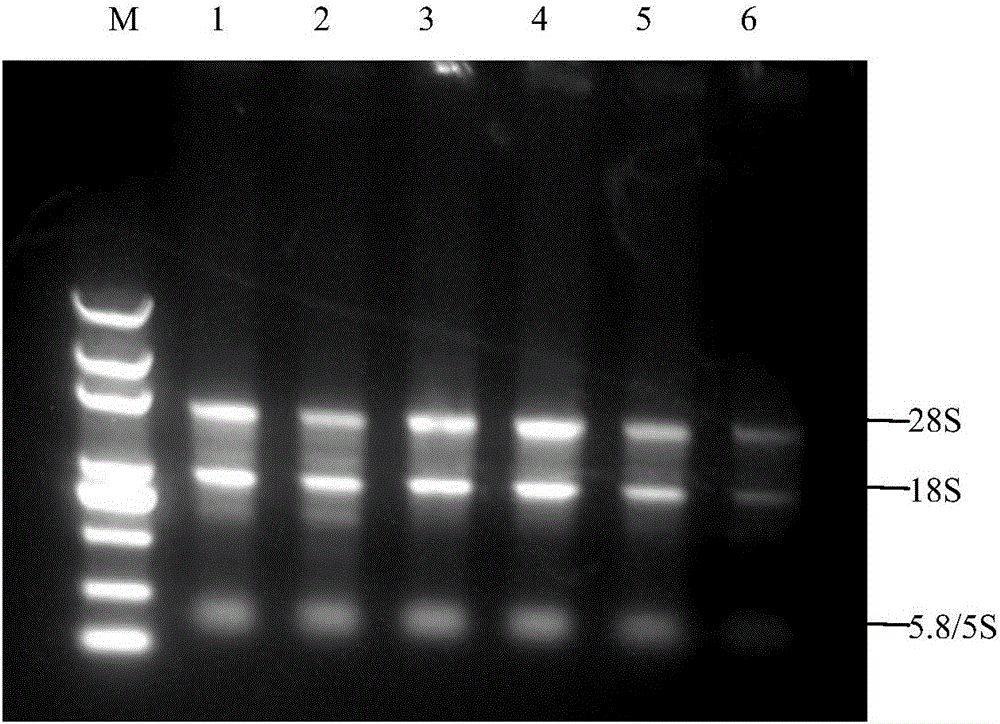
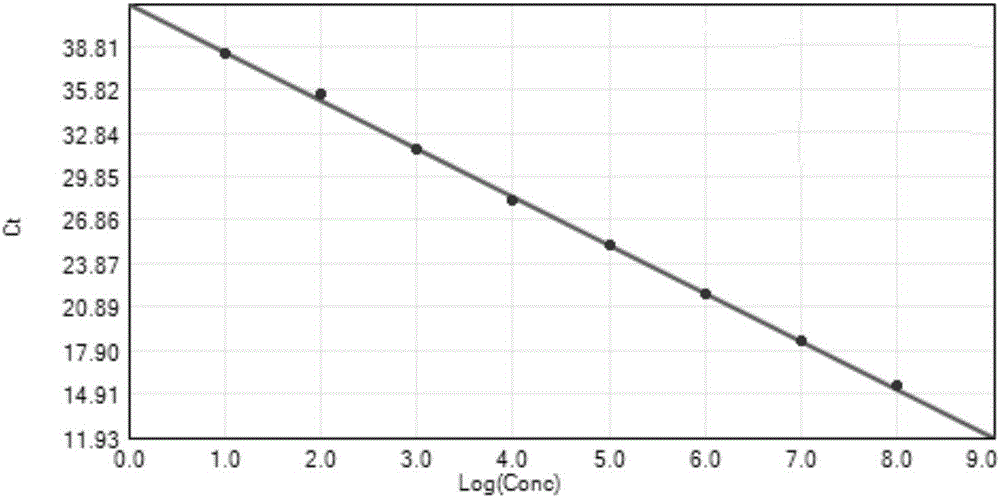
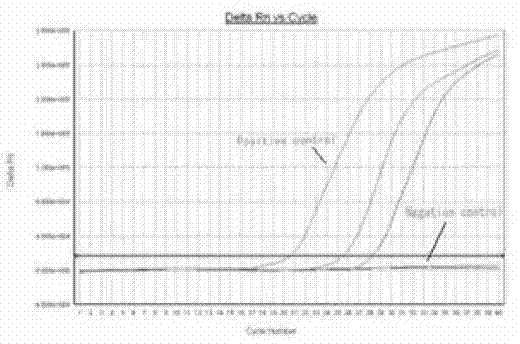
The invention discloses an SYBR GreenI real-time quantitative PCR detection method for the S gene of transmissible gastroenteritis virus of swine, which comprises the following steps: extracting total RNA of viral gastroenteritis; designing primers; performing reverse transcription; performing PCR amplification of a fragment of the S gene of transmissible gastroenteritis virus of swine; cloning and identifying the S gene of transmissible gastroenteritis virus of swine; preparing a standard product template; performing fluorescence quantitative PCR amplification; obtaining a kinetic curve and a standard curve; and performing repeatability, sensibility and specificity tests. In the method of the invention, only a good S type amplification curve is shown, only specific single peak of the product of the amplification appears, and the Tm value of the product is uniform; the detection sensitivity reaches 3 copies / microliter, and the sensitivity is high; the method avoids cross reaction with other viruses and has high specificity; and the concentration of the starting template has a good linear relationship with a Ct value, and the reaction system has high accuracy and high stability.
Owner:ZHENGZHOU HOUYI PHARMA
Production method of transmissible gastroenteritis virus vaccine
ActiveCN103550771AHigh potencyReduce manufacturing costMicroorganism based processesAntiviralsViral VaccineBottle
The invention discloses a production method of a transmissible gastroenteritis virus vaccine. The production method comprises the steps of carrying out subculture on cells for production so as to form monolayer cells; multiplying virus seeds for production, namely inoculating the basic virus seeds to the monolayer cells for culturing; dissociating the monolayer cells into a monolayer cell suspension liquid, and inoculating the cell suspension liquid into a bioreactor for culturing; multiplying vaccine culturing virus liquid, namely inoculating the virus seeds for production to the cells to be cultured after the quantity of the cells reaches 5*106-5*107 unit / ml; and harvesting the virus liquid. The production method has the advantages that the production cost can be greatly lowered, the production cycles are short, each production cycle only lasts for 5-7 days, and compared with that of transmissible gastroenteritis virus generated by an existing spinner bottle culture method, the titer of the transmissible gastroenteritis virus produced by the method is higher; the automation degree is high, few workers are required, the production process is simple and stable, the operation is easy, the yield is high, the occupied area is small, the production scale can be easily and rapidly expanded, and the quality is balanced and stable basically; the environmental pollution is slight and is easy to avoid.
Owner:成都史纪生物制药有限公司
Porcine epizootic diarrhea, transmissible gastroenteritis and porcine deltacoronavirus triple subunit vaccine
ActiveCN107899008AExtended shelf lifeSmall dose of immunizationSsRNA viruses positive-senseViral antigen ingredientsAstrovirus gastroenteritisTransmissible gastroenteritis virus
The invention provides a porcine epizootic diarrhea, transmissible gastroenteritis and porcine deltacoronavirus triple subunit vaccine, which consists of an antigen and a vaccine adjuvant, wherein theantigen includes porcine epizootic diarrhea virus S1 protein which is expressed by an X33-PEDV-S1 strain that preservation number of CGMCC No.14372, transmissible gastroenteritis virus S1 protein which is expressed by an X33-TGEV-S1 strain that preservation number is CGMCC No.14371 and porcine deltacoronavirus S1 protein which is expressed by an X33-PDCoV-S1 strain that preservation number is CGMCC No.14370. The porcine epizootic diarrhea, transmissible gastroenteritis and porcine deltacoronavirus triple subunit vaccine provided by the invention is high in safety and good in immunogenicity, and the triple vaccine, after being applied to immunization, can rapidly generate an antibody and can keep antibody titer at a relatively high level for a long time; the vaccine is long in preservationperiod and low in immunizing dose; the adopted adjuvant is easy to inject; and three major diarrhea-related viral diseases can be prevented and treated by conducting injection once, so that the survival rate of piglets is improved.
Owner:陕西诺威利华生物科技有限公司
Test paper strip for detecting one or more porcine virus diarrhea disease antibody
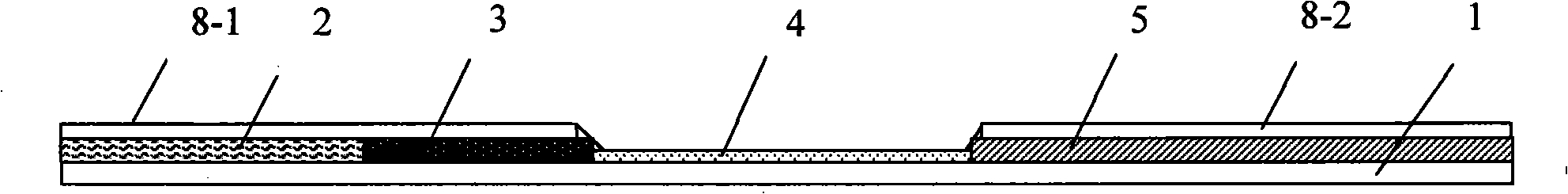
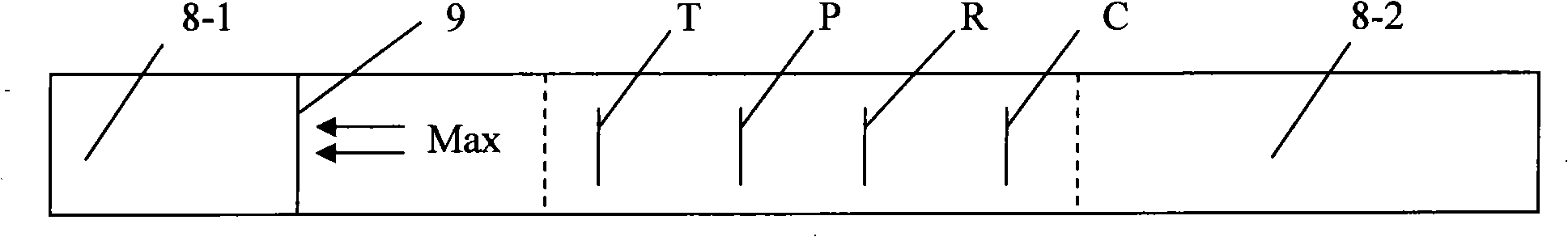
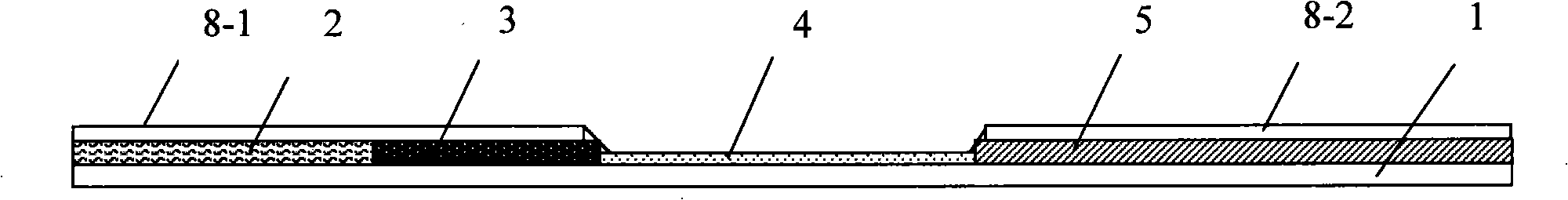
The present invention relates to a test strip which is used for testing a kind of or a plurality of kinds of disease antibodies of porcine virus diarrhea; the test strip comprises a supporting layer and a reaction reagent carrier absorbing layer which is pasted on the supporting layer; the reaction reagent carrier absorbing layer comprises a testing end fiber layer, a fiber layer of absorbing gold-labeled SPA protein or gold-labeled antigen which corresponds to the antigen to be tested, and a cellulose layer, which are arranged at the sample end in sequence, and an absorbent material layer which is positioned at the handle end; the cellulose layer contains one, two or three testing print(s) which are printed by anyone, any two or three of the purified transmissible gastroenteritis virus TGEV solution, the porcine epidemic diarrhea virus PDEV solution and the porcine rotavirus RV, and the cellulose layer also contains the contrast prints which are printed by anyone, any two or three of the anti-SPA protein IgG solution of the sheep or the rabbit or the anti-TGEV, anti-PDEV, anti-RV IgG solution of the sheep or the rabbit; and the invention provides a test strip for testing a kind of or a plurality of kinds of disease antibodies of the porcine virus diarrhea, which has the advantages of accurate and rapid detection, convenient operation and low costs.
Owner:HENAN ACAD OF AGRI SCI
Double microdroplet digital PCR (Polymerase Chain Reaction) absolute quantitative detection kit for porcine epidemic diarrhea virus and transmissible gastroenteritis virus
InactiveCN105543416AImprove efficiencySimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationEpidemiologic surveyTransmissible gastroenteritis virus
The invention provides a specific primer and probe combination for detecting porcine epidemic diarrhea virus (PEDV) and transmissible gastroenteritis virus (TGEV). The specific primer and probe combination comprises two pairs of specific primers and two specific probes used in conjunction with the primers. The invention provides a detection kit or a detection reagent for detecting PEDV and TGEV at the same time. The specific primer and the detection kit or the detection reagent provided by the invention have the advantages of rapidness, sensitivity, specificity and the like, and can lay a foundation for double digital PCR (Polymerase Chain Reaction) absolute quantitative detection of PEDV and TEGV, epidemiological investigation, vaccine usage and the like.
Owner:BEIJING SEAGULL BIOVENTURES & BIOTECH CO LTD
Multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis
InactiveCN102586409AIncreased sensitivityStrong specificityMicrobiological testing/measurementSwine Transmissible GastroenteritisPorcine epidemic diarrhoea virus
The invention relates to a multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis. An antibody is needed to be prepared by an euzymelinked immunosorbent assay, so that the period is longer, and external factors have larger interference on the diagnostic method of the euzymelinked immunosorbent assay. The method comprises the following steps of: designing and synthesizing a transmissible swine gastroenteritis virus gene primer; establishing a multiple reverse transcription polymerase chain reaction diagnostic method of the transmissible swine gastroenteritis viruses and porcine epidemic diarrhea viruses; optimizing concentration of the primer of a bigeminy reverse transcription polymerase chain reaction; optimizing the concentration of magnesium oxide of the bigeminy reverse transcription polymerase chain reaction; optimizing the concentration of triphosphoric acid base deoxynucleotide of the bigeminy reverse transcription polymerase chain reaction; determining the optimal annealing temperature of the bigeminy reverse transcription polymerase chain reaction; testing the sensitivity of the bigeminy reverse transcription polymerase chain reaction; and testing the specificity of the bigeminy reverse transcription polymerase chain reaction. The multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis is suitable for detecting swine transmissible gastroenteritis.
Owner:郑世民
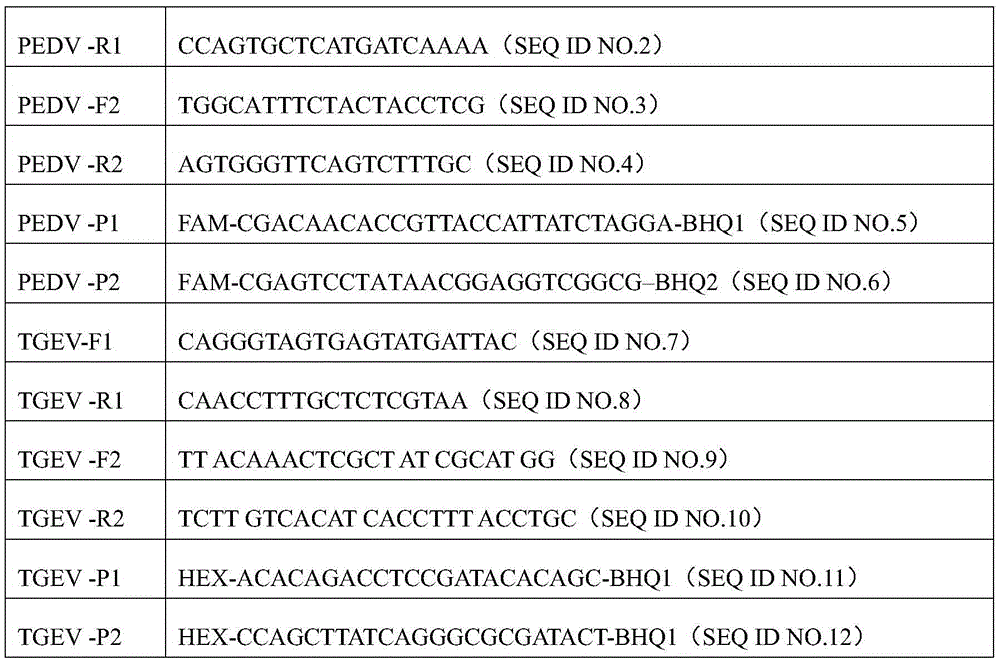
Fourfold RT-PCR detection primer and reagent kit of four porcine epidemic diarrheaviruses
ActiveCN110093461AStrong specificityRepeatableMicrobiological testing/measurementMicroorganism based processesEpidemic diarrheaAstrovirus gastroenteritis
The invention relates to a detection primer and reagent kit of four RT-PCR of porcine epidemic diarrheaviruses, transmissible gastroenteritis of swine, porcine rotaviruses and porcine deltacoronaviruses, and belongs to the technical field of molecular detection. The fourfold RT-PCR detection primer comprises detection primers PEDV F and PEDV R of porcine epidemic diarrhea viruses, detection primers TGEV F and TGEV R of the transmissible gastroenteritis of swine, detection primers PoRV F and PoRV R of the porcine rotaviruses and detection primers PDCoV-F and PDCoV-R of the porcine delta coronaviruses. The primer is high in specificity, has repeatability, is high in sensitivity and is high in clinical reliability.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Gene chip and kit for detecting porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus
ActiveCN104293977AEfficient detectionQuick checkNucleotide librariesMicrobiological testing/measurementPorcine rotavirusTransmissible gastroenteritis virus
The invention discloses a gene chip and kit for detecting porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus. The gene chip disclosed by the invention comprises a solid phase carrier and a probe fixed on the solid phase carrier, wherein the probe comprises any one or two oligonucleotide fragments shown in SEQ ID NO.1 or 2, any one or two oligonucleotide fragments shown in SEQ ID NO.3 or 4, and any one or two of oligonucleotide fragments shown in SEQ ID NO.5 or 6. The kit disclosed by the invention comprises the gene chip and reagents for amplifying genes of porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus. The gene chip and detection kit disclosed by the invention can accurately and effectively detect the porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus and are good in specificity, high in sensitivity, low in time consumption, high in detection speed and good in application prospects.
Owner:SICHUAN AGRI UNIV
Taqman real-time fluorescent PCR kit for detecting pig umbilical cord blood transmissible gastroenteritis virus and application thereof
ActiveCN106435023AWide detection rangeExtensiveMicrobiological testing/measurementFluorescenceQuality control
The invention discloses a Taqman real-time fluorescent PCR kit for detecting pig umbilical cord blood transmissible gastroenteritis virus and application thereof. The kit comprises a pair of primers and a specific fluorescent probe, the sequences of the primers are shown as SEQ ID NO:1 and SEQ ID NO:2, and the sequence of the specific fluorescent probe are shown as SEQ ID NO:3. The Taqman real-time fluorescent PCR kit can specifically detect the pig umbilical cord blood transmissible gastroenteritis virus, and has high sensitivity, strong capacity of resisting disturbance, strong universality and good repeatability. The kit can precisely evaluate and diagnose conditions of carrying and expelling of porcine transmissible gastroenteritis virus of sows as well as infection of piglets, can further implement purification and effect evaluation of pig transmissible gastroenteritis, and has a very high application value. Meanwhile, the kit has a simple and convenient detecting process, needed instruments and equipment are simple, and detecting time is short; the result is relatively reliable, and the kit is not prone to contamination; the kit has a low technological operation demand, and can be generally popularized in the grass root; quality control is relatively easy, and the kit is easy to standardize.
Owner:湖南国测生物科技有限公司
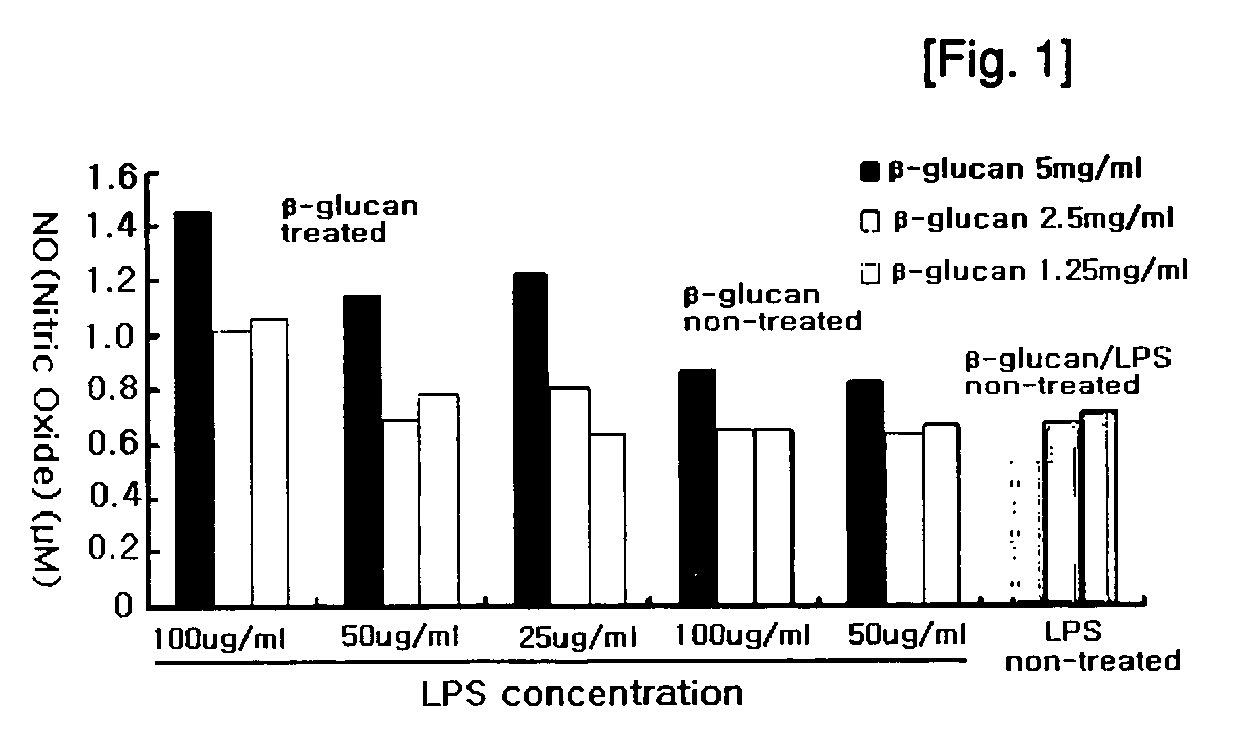
Composition comprising soluble glucan oligomer from saccharomyces cerevisiae is2 inhibiting the swine influenza (SIV) and transmissible gastroenteritis coronavirus (tgev)
The present invention relates to the soluble glucan oligomer having a M.W. ranging from 1,000 to 10,000 prepared by treating insoluble beta-glucan isolated the cell wall of yeast variant IS2 with commercially available beta-glucan hydrolyzing enzymes. The soluble glucan oligomer showed potent efficacy on inhibiting activity of influenza virus and transmissible gastroenteritis coronavirus, therefore, it can be used as the therapeutics or health care food for treating and preventing mammal's diseases caused by the infection of influenza virus and transmissible gastroenteritis coronavirus.
Owner:MOON WON KOOK +3
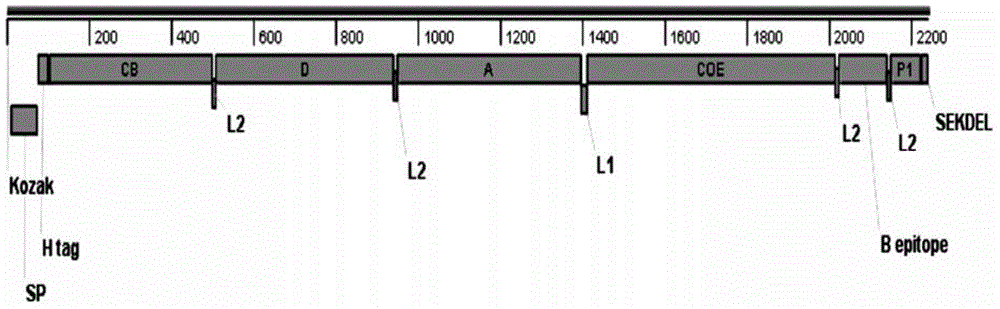
Fusion gene containing transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV) protective antigens as well as encoding protein and application thereof
InactiveCN104805106AHybrid peptidesVector-based foreign material introductionTransmissible gastroenteritis virusGenetically modified maize
The invention discloses a fusion gene containing transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV) protective antigens as well as encoding protein and application of the fusion gene. The base sequence of the fusion gene containing the TGEV and the PEDV protective antigens is shown by SEQ ID No. 1. The protein for encoding the fusion gene has the amino acid sequence shown by SEQ ID No. 2. The fusion gene is introduced into corns, and transgenic corn plants containing the TGEV and the PEDV protective antigens are obtained by screening and indentifying. The transgenic corn vaccine is capable of efficiently expressing the fusion protein containing the TGEV and the PEDV protective antigens, and the expression product has remarkable immunogenicity.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Nucleic acid sequences encoding proteins capable of associating into a virus-like particle
InactiveUS20060165723A1Reduce and eliminate symptomEnhance immune responseSsRNA viruses positive-senseVirus peptidesVirus-like particleNucleic acid sequencing
The present invention relates to nucleic acids comprising: (a) sequences of a replication competent transmissible gastroenteritis virus (TGEV), which sequences encode a TGEV replicase under the control of expression regulatory sequences so that expression of the replicase in a cell containing the nucleic acid will initiate replication of the nucleic acid and thus increase the number of nucleic acids in the cell; and (b) sequences encoding one or more proteins of a different virus wherein the one or more proteins are capable of associating into a virus-like particle (VLP) that does not contain any infectious nucleic acid. The present invention further relates to vectors, virus particles and host cells comprising these nucleic acids as well as their use for the preparation of vaccines, specifically for the preparation of vaccines.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Kit for simultaneously detecting eight pig disease related antibodies and use method of kit
InactiveCN105606802AReduce consumptionIncreased sensitivityMaterial analysis by observing effect on chemical indicatorDiseaseSwine Fever Virus
The invention discloses a kit for simultaneously detecting eight pig disease related antibodies against CSFV (classical swine fever virus), PPV (porcine parvovirus), a PRV (porcine pseudorabies virus), PRRSV (porcine reproductive and respiratory syndrome virus), PCV2 (porcine circovirus), PEDV (porcine epidemic diarrhea virus), TGEV (transmissible gastroenteritis virus) and SIV (swine influenza virus). The kit comprises a reaction card, an ELA reagent, a color developing agent, a stop solution, a concentrated cleaning solution and a sample diluent. The invention further provides a method for simultaneously detecting eight virus related antibodies in vitro by the aid of the kit. The kit can accurately detect a CSFV antibody, a PPV antibody, a PRV antibody, a PRRSV antibody, a PCV2 antibody, a PEDV antibody, a TGEV antibody, an SIV antibody and the like and has the advantages of high sensitivity, high specificity, good accuracy, simplicity and convenience in operation, low consumption of samples, objective judgment of results and the like.
Owner:哈药集团生物疫苗有限公司
Multiple PCR detection primer group and kit for rapidly differentiating PCV1 type from PCV3 type
InactiveCN107653346AIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPorcine rotavirusTransmissible gastroenteritis virus
The invention discloses a multiple PCR detection primer group and kit for rapidly differentiating the PCV1 type from the PCV3 type. The primer group includes primers L1, L2 and L3, wherein L1 represents 5'-ACCAGCGCACTTCGGCAGCGGCAGCA-3', L2 represents 5'-GCATTGTTCACCAGTACCCA-3', and L3 represents 5'-AAGTCCCCTTCCTGCGTCCGCTAATCT-3'. The special kit has very high sensitivity, and minimum detection quantities for the PCV1 type and the PCV3 type are 1*10<-7> ng / mL and 2*10<-7> ng / mL respectively; the specificity is high, and the amplification results of porcine reproductive and respiratory syndromevirus, classical swine fever virus, porcine pseudorabies virus, porcine parvovirus, porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus are negative; the repeatability is good, the same sample is detected for 3 times at an interval of one month, and the same result is obtained; moreover, the coincidence rate can reach 95% or above compared with methods of virus isolation, IFA and the like, and the primer group and the kit can be widely used in clinical typing detection of the PCV1 type and the PCV3 type.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
CSFV detection method utilizing realtime fluorescence quantitative RT-PCR
InactiveCN102206709AReduce pollutionShorten inspection timeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescencePorcine circovirus
The invention discloses a CSFV (classical swine fever virus) detection method utilizing realtime fluorescence quantitative RT-PCR. According to results of singularity and sensitivity teats of a method established in the invention, the method can well distinguish the CSFV from FMDV (Foot And Mouth Disease Virus), TGEV (Transmissible Gastroenteritis Virus), PRRSV (Porcine Respiratory and Reproductive Syndrome Virus) and PCVII( Porcine Circovirus II), and sensitivity can reach an order of magnitude of 10 copy number. In addition, whole reaction process of the method established by the invention is operated in a close pipe, so as to reduce solution to the system at most. Compared with a traditional method, the method in the invention has a substantially shortened detection time, on the premise of guaranteeing detection result. For the whole process, comprising steps of obtaining of sample to be checked, nucleic acid extraction, preparation of fluorescence quantitative RT-PCR reaction system, and appearance of reaction result, can be finished within 2.5 h.
Owner:珠海出入境检验检疫局检验检疫技术中心
Multiplex RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primer probe group for real-time fluorescent quantitative detection of four porcine diarrhea viruses, kit and detection method
PendingCN113462820AThe test result is accurateReal-time quantitative detectionMicrobiological testing/measurementAgainst vector-borne diseasesMultiplexPig farms
The invention discloses a multiplex RT-PCR (reverse transcription-polymerase chain reaction) primer probe group for real-time fluorescent quantitative detection of four porcine epidemic diarrhea viruses. The multiplex RT-PCR primer probe group comprises an upstream primer, a downstream primer and a specific fluorescent probe of a porcine epidemic diarrhea virus M gene, an upstream primer, a downstream primer and a specific fluorescent probe of a swine transmissible gastroenteritis virus S gene; an upstream primer, a downstream primer and a specific fluorescent probe of a porcine rotavirus VP6 gene; and an upstream primer, a downstream primer and a specific fluorescent probe of a porcine D-type coronavirus M gene. The kit assembled on the basis of the primer probe group has the advantages of high sensitivity, high specificity, low pollution and real-time detection, and provides a reliable technology and product for early warning, early diagnosis and prevention and control monitoring of clinical diarrhea virus in a first-line pig farm.
Owner:HEBEI UNIV OF ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com