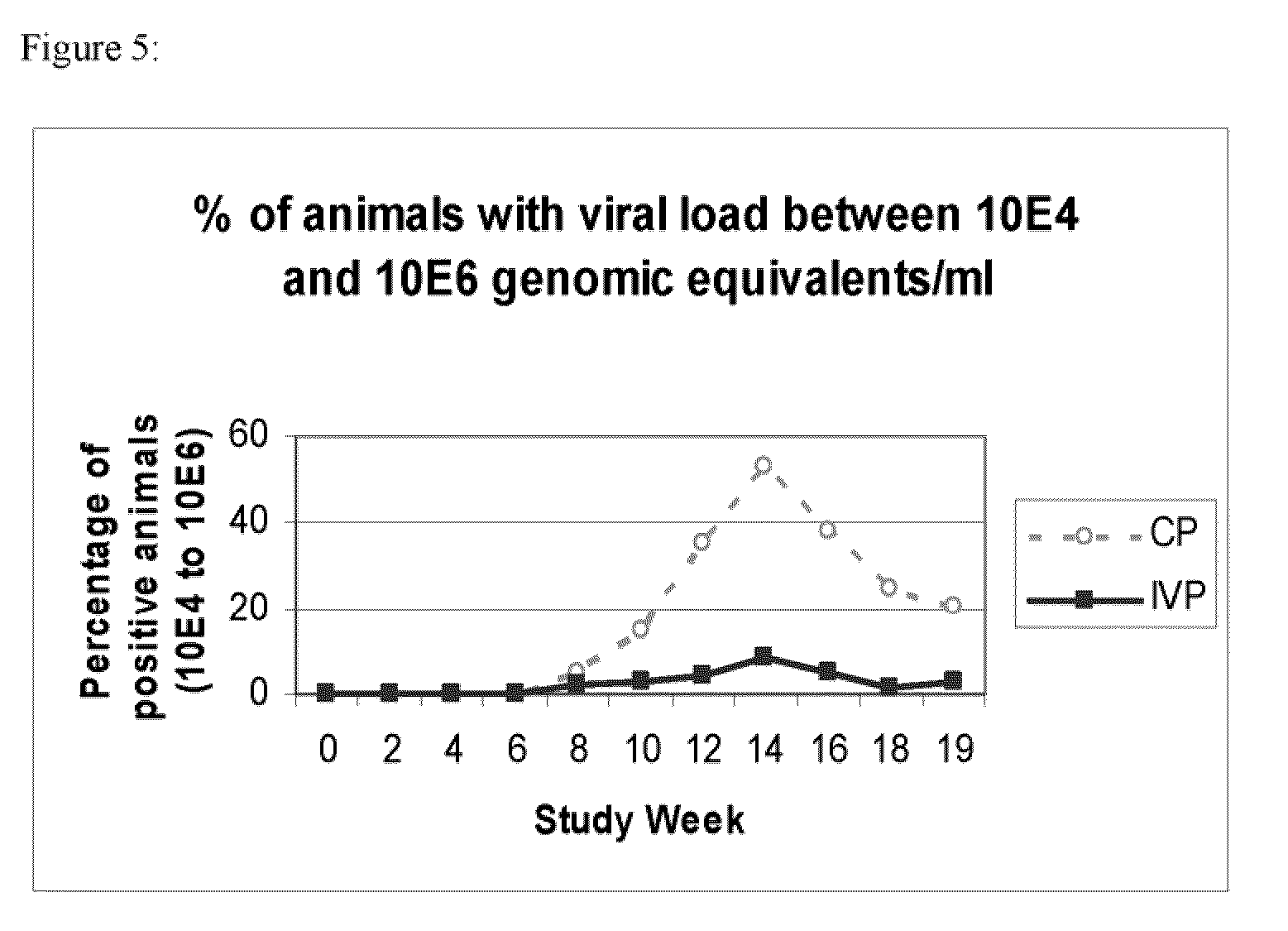
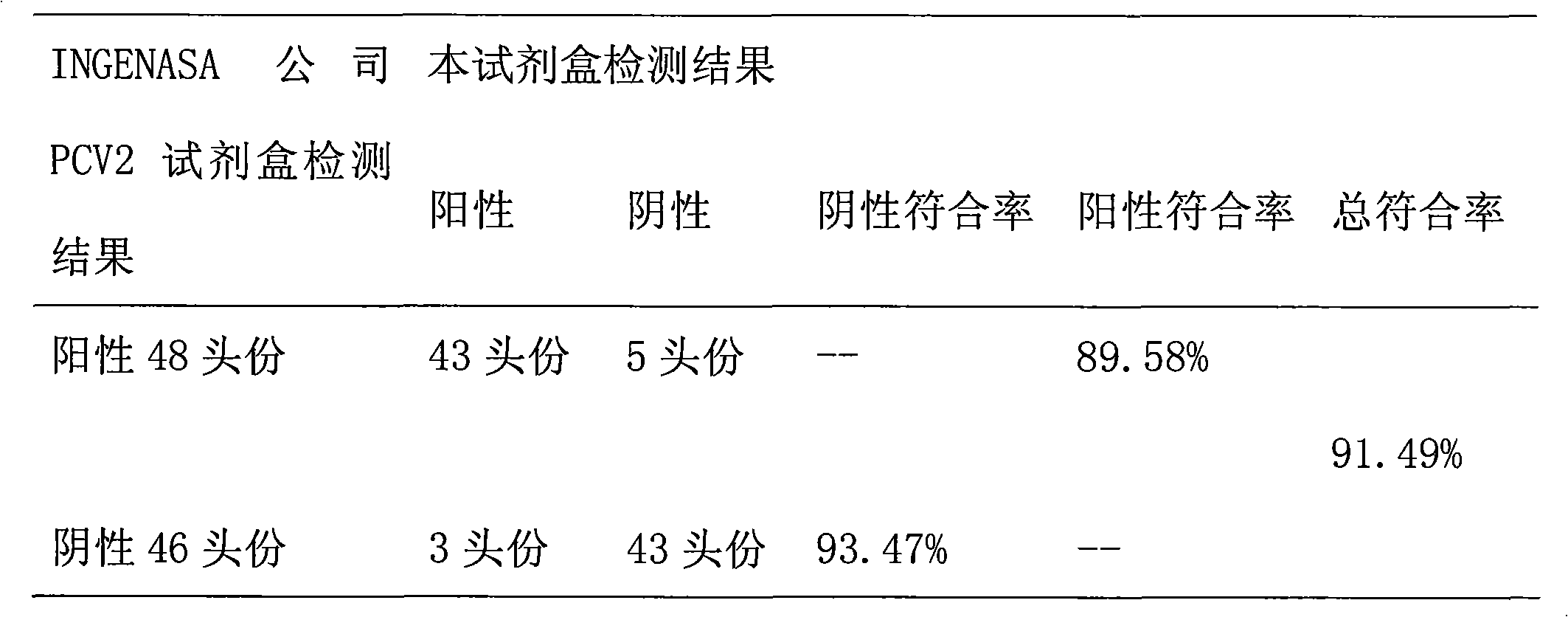
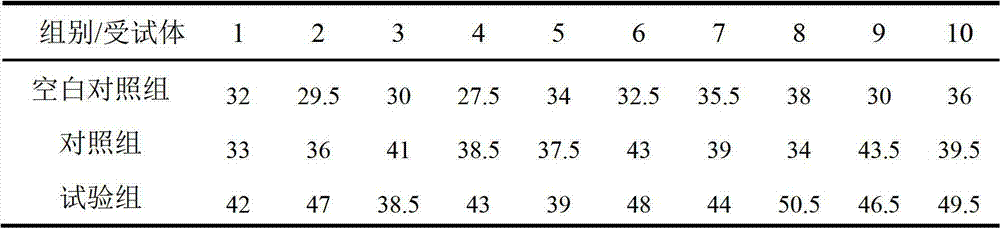
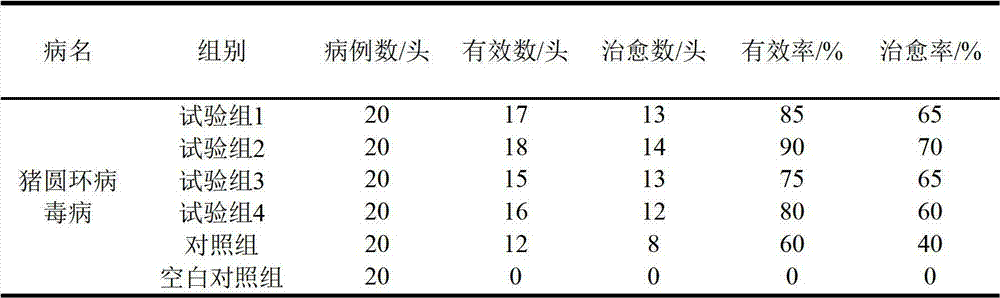
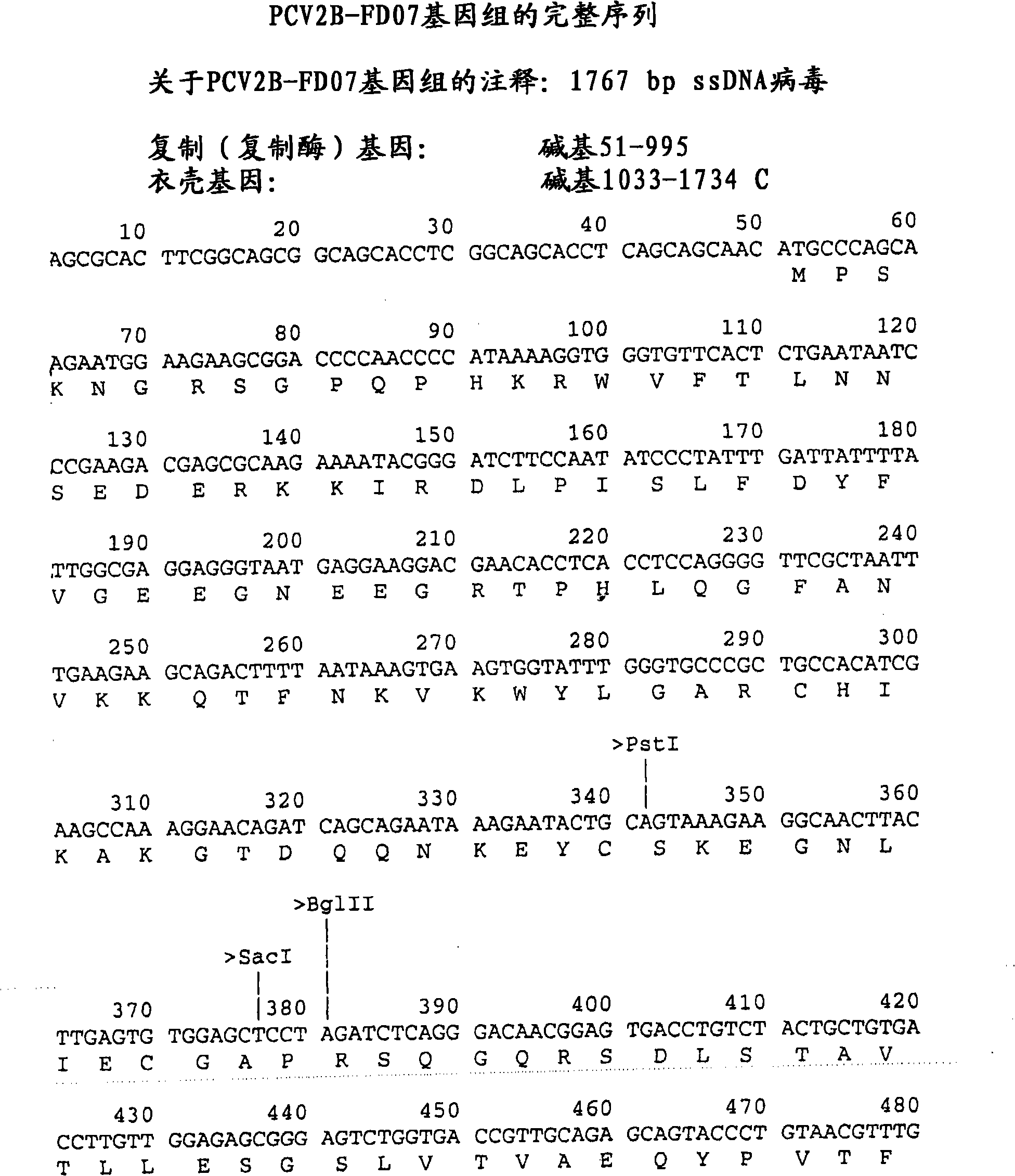
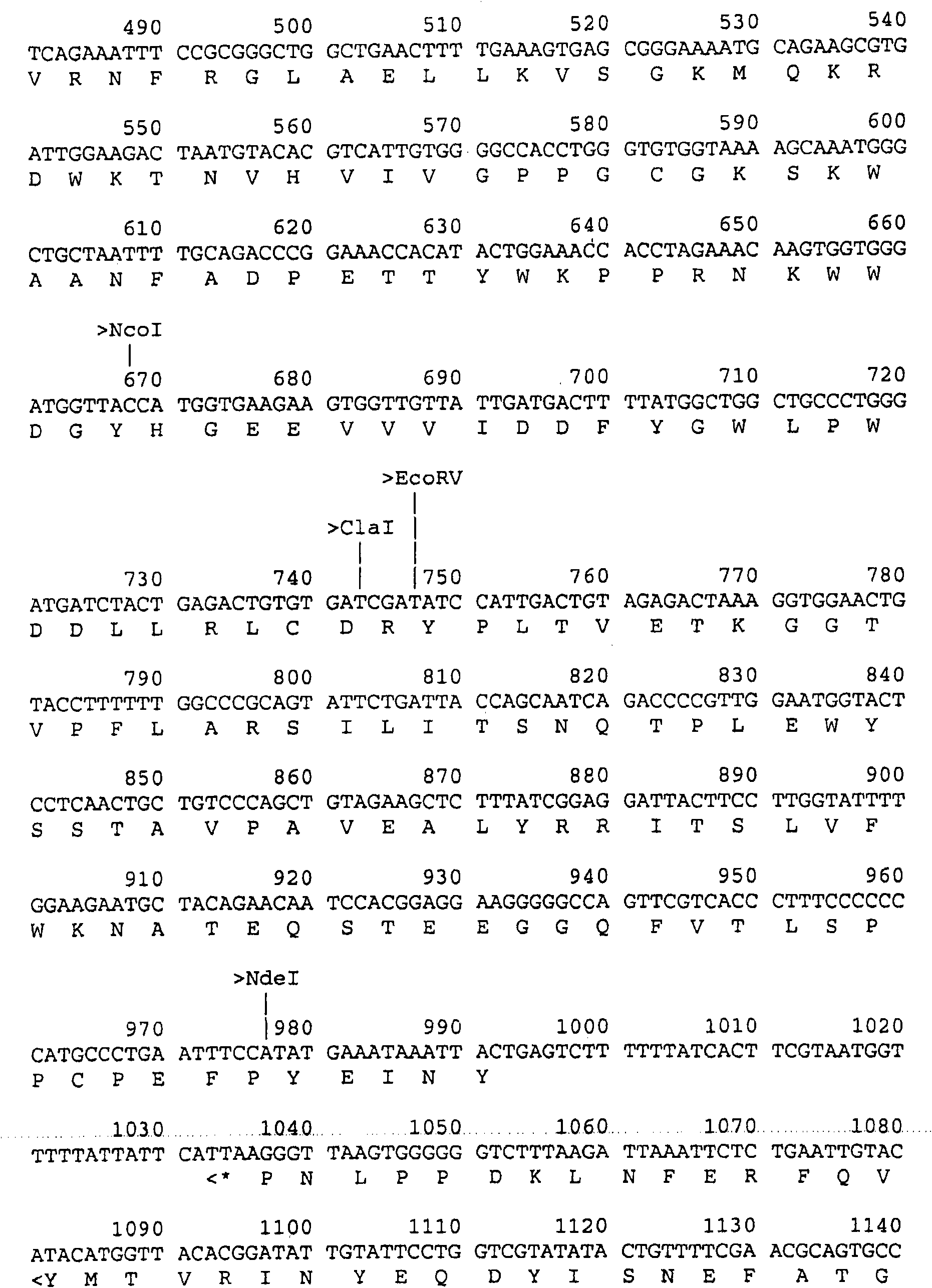
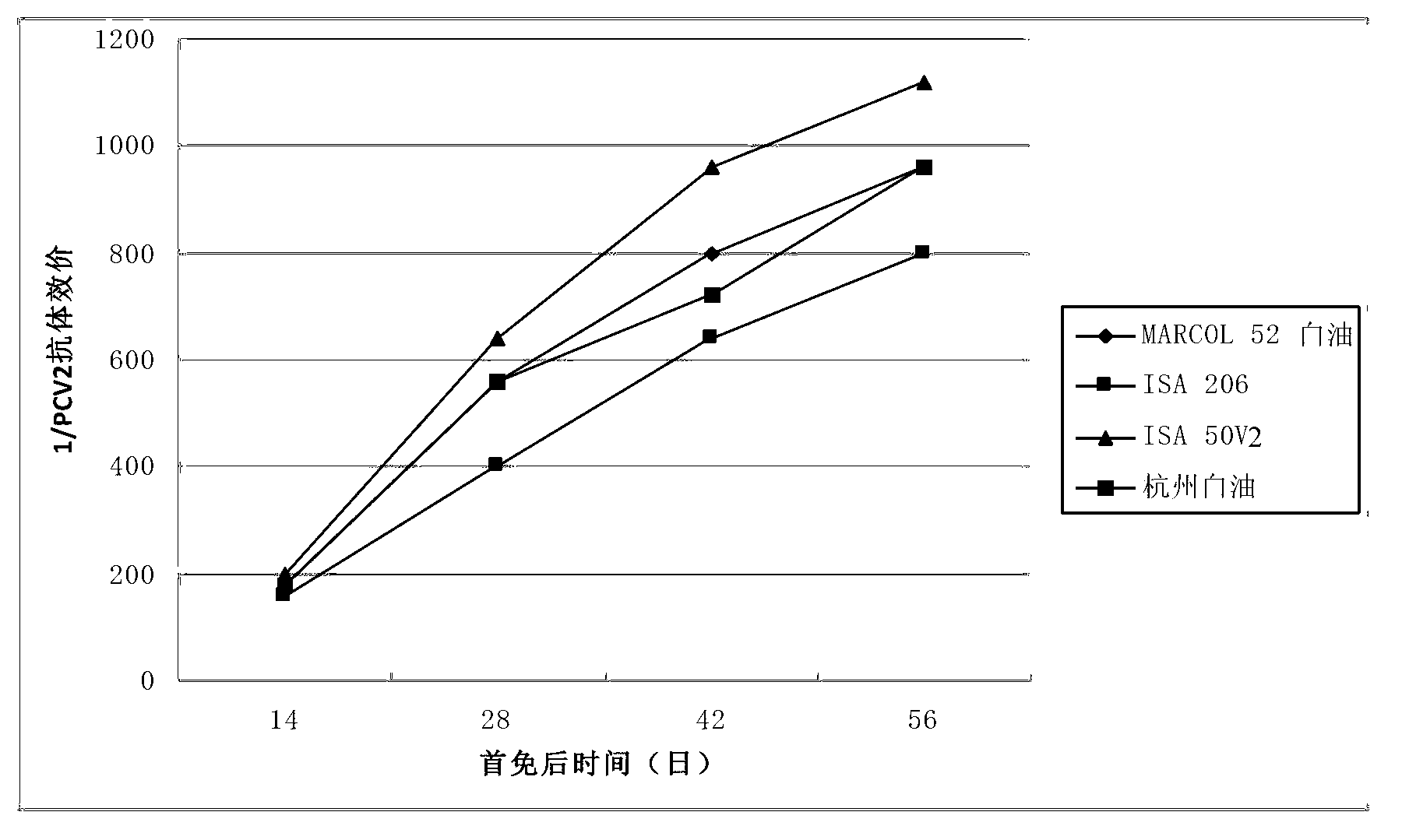
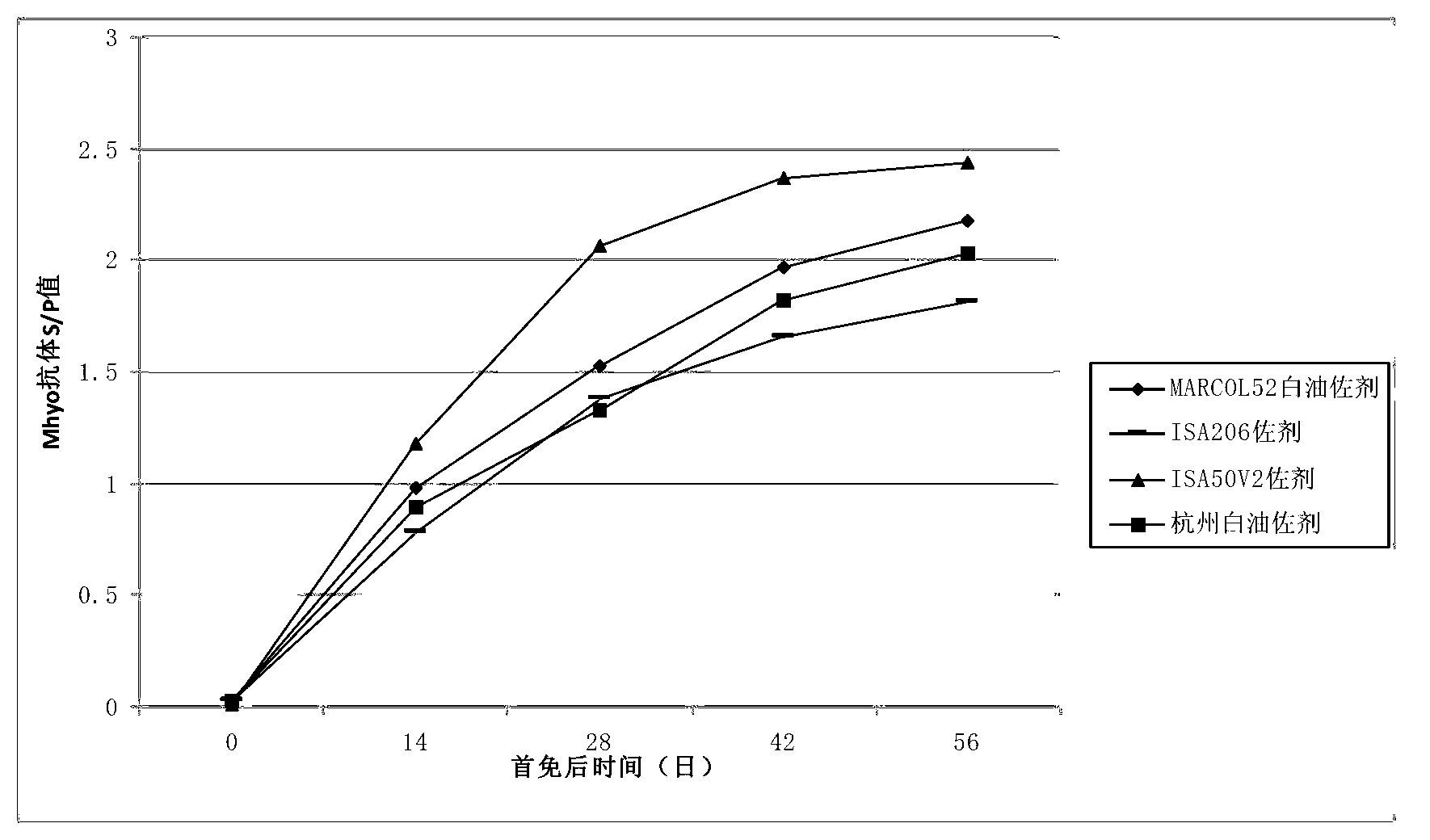
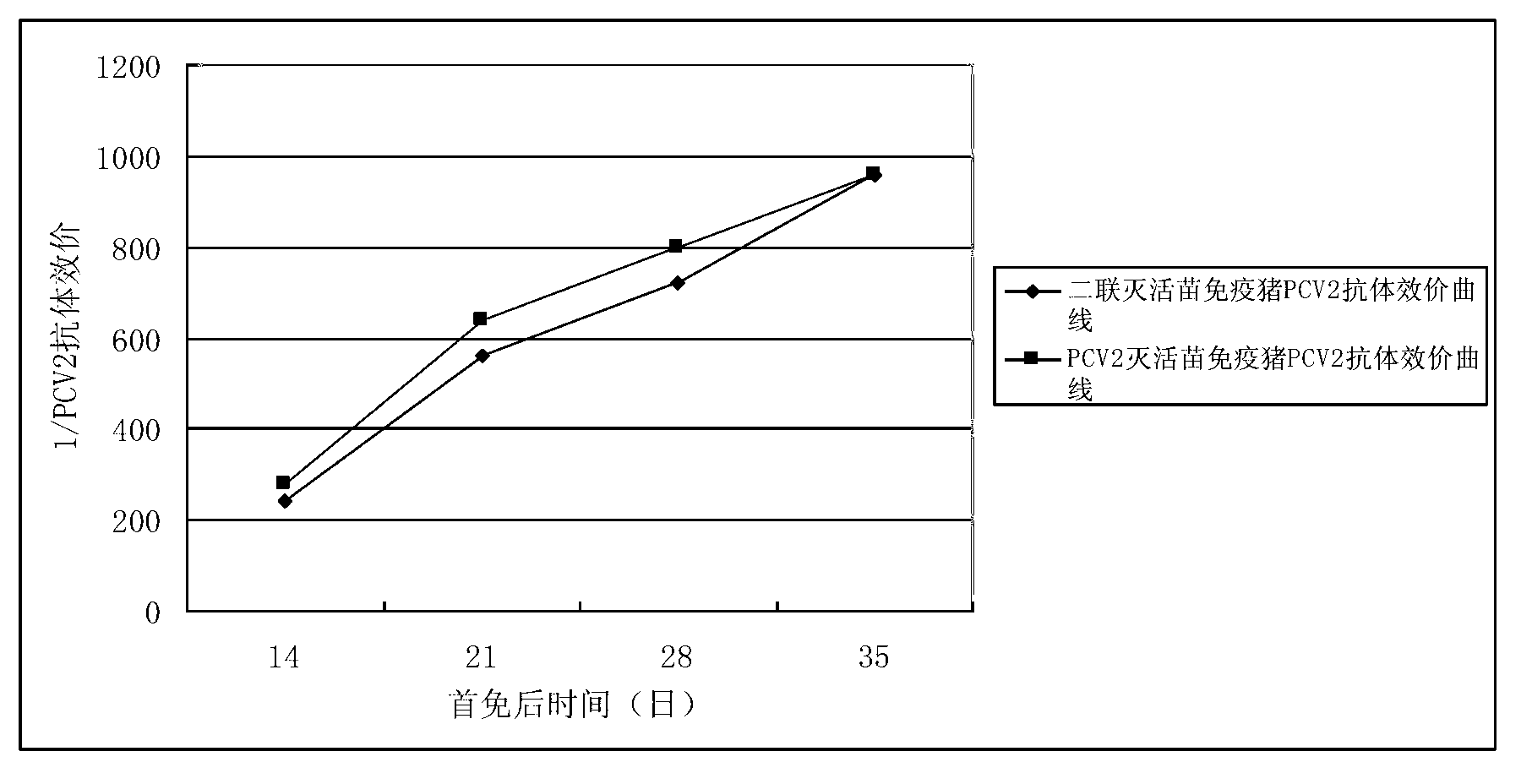
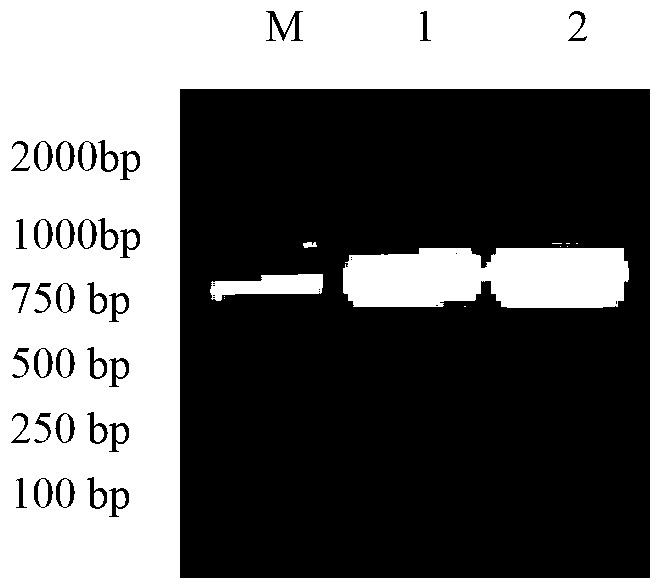
Patents
Literature
492 results about "Porcine circovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
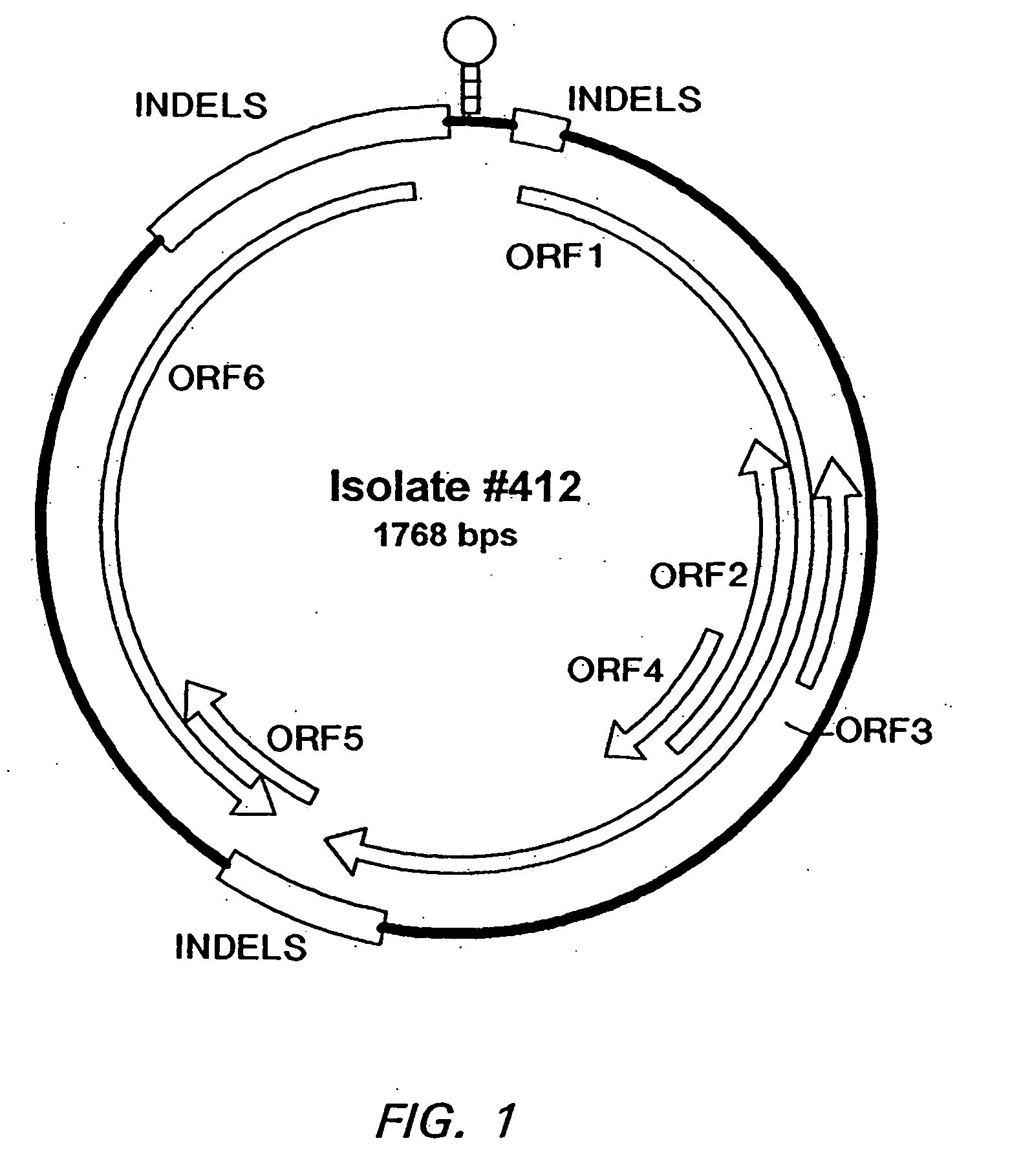
Porcine circovirus (PCV) is a group of single-stranded DNA viruses (class II), that is nonenveloped with an unsegmented circular genome. The viral capsid is icosahedral and approximately 17 nm in diameter. PCV is a member of the virus family Circoviridae.
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
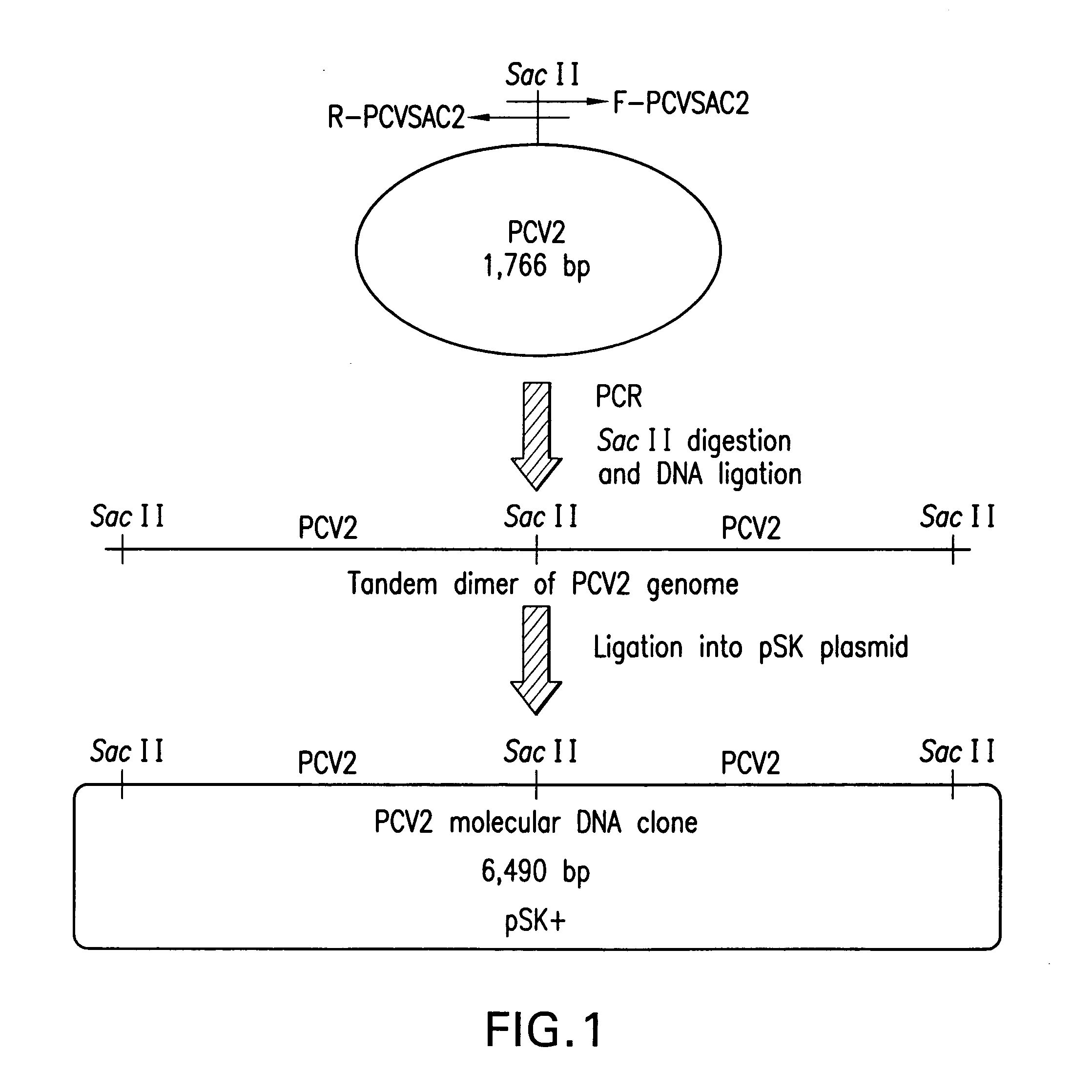
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease
InactiveCN101695342APrevention and treatment of asthmaPrevention and treatment of contagious pleuropneumoniaFood processingAnimal feeding stuffMedicinal herbsBaical Skullcap Root
The invention provides a Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease. The swine feed additive consists of 37 Chinese medicinal herbs, namely, gypsum, rehmannia root, rhinoceros horn, golden thread, cape jasmine fruit, tree peony bark, baical skullcap root, red paeony root, figwort root, common anemarrhena rhizome, forsythia suspensa , platycodon root, liquorice root, common lophatherum herb, amur corktree bark, honeysuckle flower, Chinese pulsatilla root, indigowoad root, heartleaf houttuynia herb, astragalus, szechwon tangshan root, hawkthorn fruit, medicated leaven, barley sprout, radish seed, chicken's gizzard -membrane, Chinese thorowax root, common andrographis herb, philippine violet herb, tuber fleeceflower root, massa medicata fermentata fujianensis, cyrtomium rhizome, tung leaf, tangerine peel, white paeony root, pine needle and indigowoad leaf through scientific compatibility. The feed additive is added into swine feed in the proportion; under the condition of not using any vaccine, the feed additive can effectively prevent and cure severe mixed flu symptoms, infection and other syndromes caused by swine respiratory disease, asthma, contagious pleuropneumonia, swine virus mixed flu, high swine fever, porcine circovirus, swine fever, flu, pseudorabies, salmonellosis, bacillosis, streptococcus, erysipelas, paratyphoid, eperythrozoon, toxoplasm and other multi-pathogeny and provides genuine green food for the market.
Owner:孟祥合
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
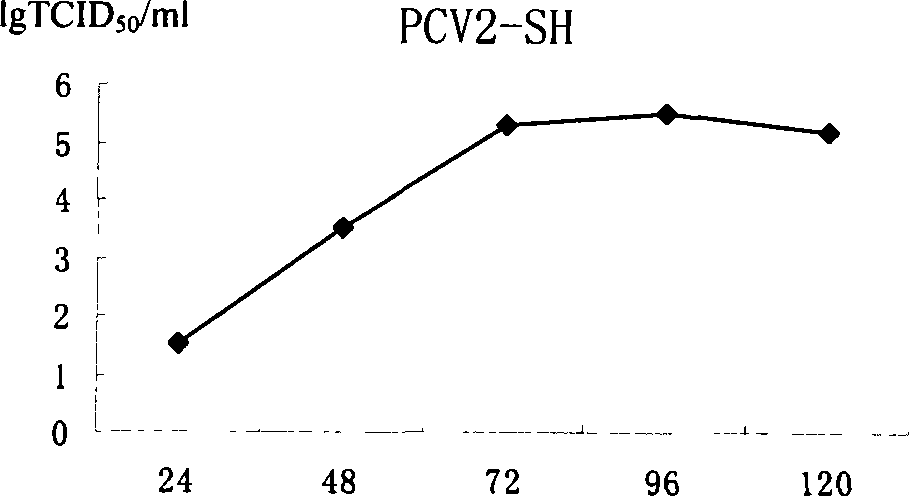
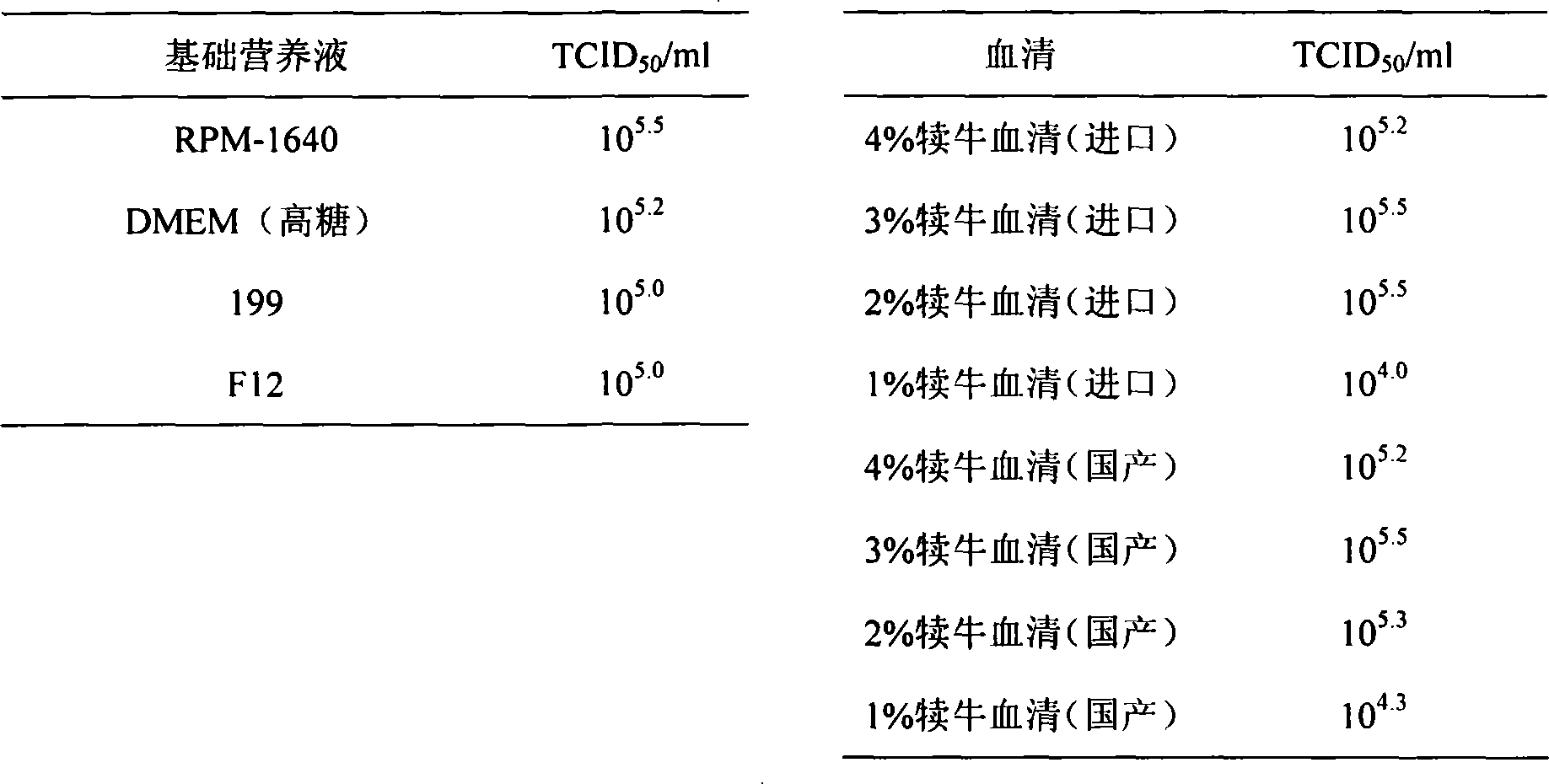
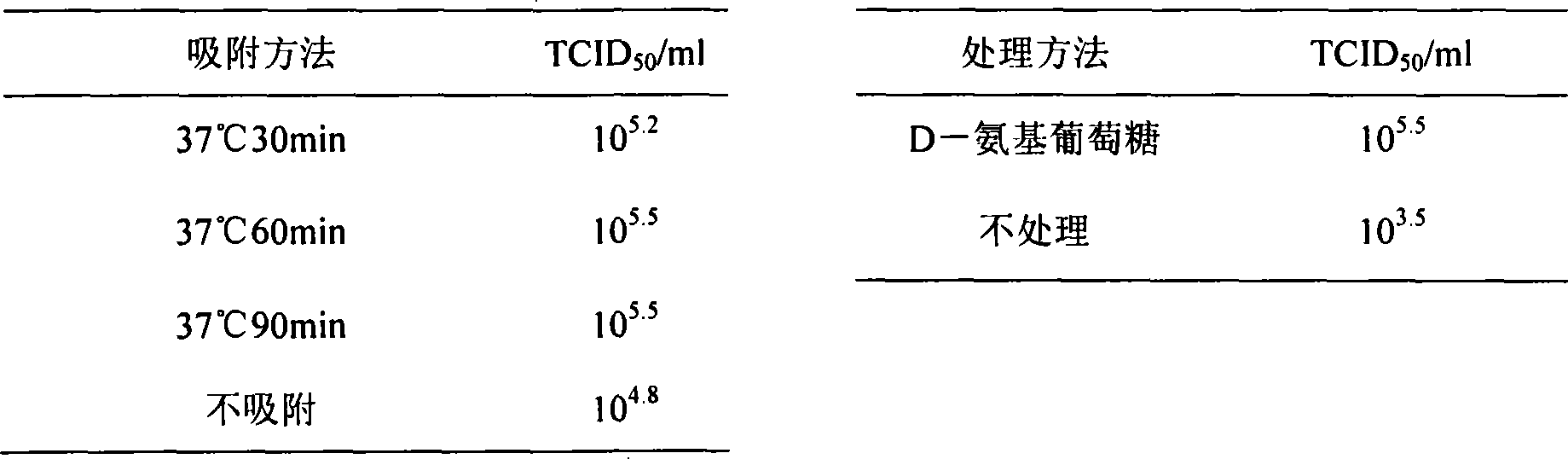
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
Use of a pcv2 immunogenic composition for lessening clinical symptoms in pigs
InactiveUS20080181910A1Reduces circovirus loadImprove the level ofViral antigen ingredientsDigestive systemClinical manifestationPorcine circovirus
The present invention relates to the use of an immunogenic composition that comprises a porcine circovirus type 2 (PCV2) antigen for treatment of several clinical manifestations (diseases). Preferably, the clinical manifestations are associated with a PCV2 infection. Preferably, they include lymphadenopathy, lymphoid depletion and / or multinucleated / giant histiocytes. Moreover, the clinical symptoms include lymphadenopathy in combination with one or a multiple of the following symptoms in pigs: (1) interstitial pneumonia with interlobular edema, (2) cutaneous pallor or icterus, (3) mottled atrophic livers, (4) gastric ulcers, (5) nephritis and (6) reproductive disorders, e.g. abortion, stillbirths, mummies, etc. Furthermore the clinical symptoms include Pia like lesions, normally known to be associated with Lawsonia intracellularis infections.
Owner:BOEHRINGER INGELHEM VETMADICA INC
Prevention of myocarditis, abortion and intrauterine infection associated with porcine circovirus-2
InactiveUS7211379B2Peptide/protein ingredientsGenetic material ingredientsLegally induced abortionViral carditis
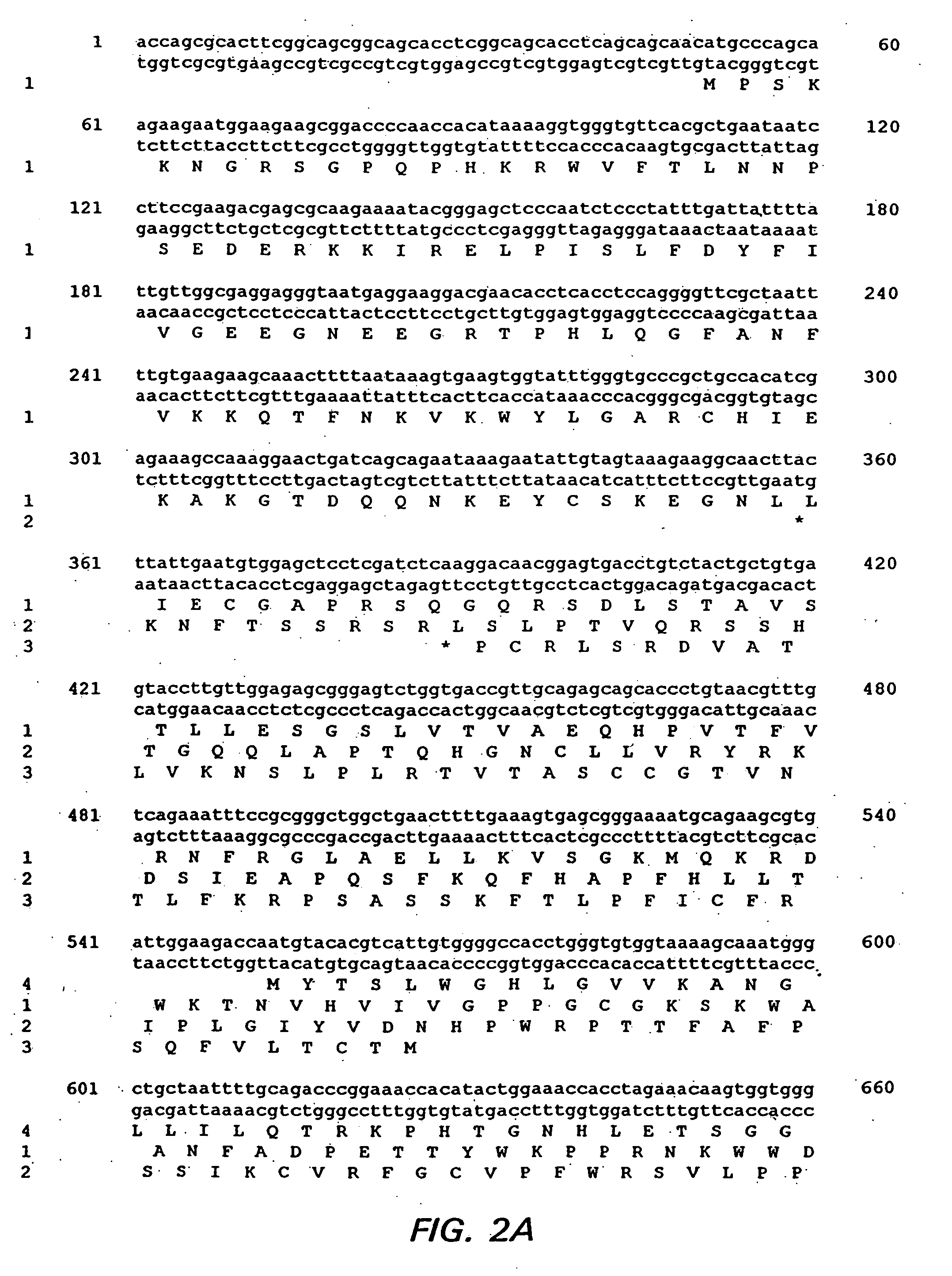
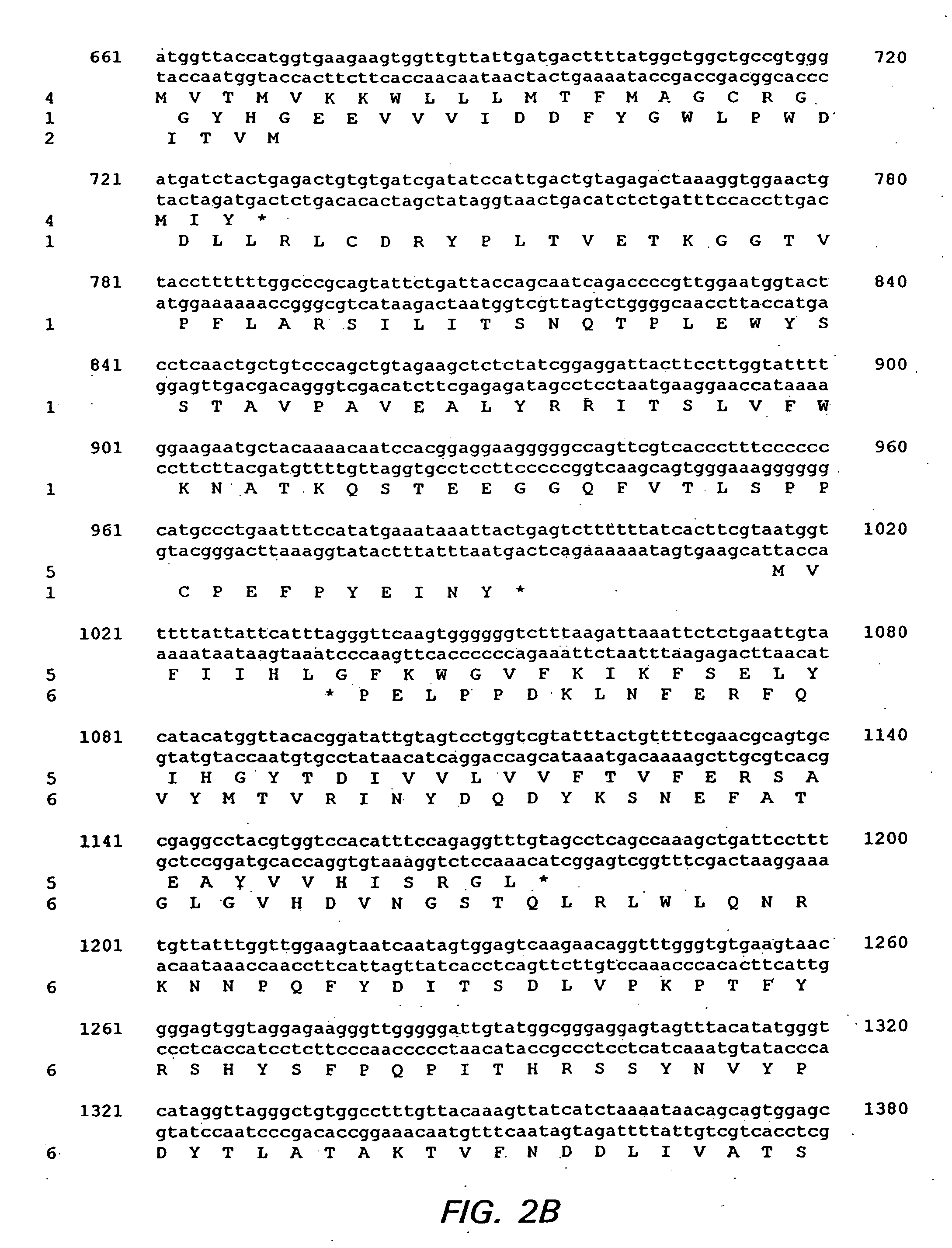
Provided is a method for reducing viral load of porcine circovirus type 2 (PCV-2) in a pig by inducing an immune response against PCV-2 through the administration of an immunogenic composition comprising a PCV-2 antigen. A preferred antigen is a vector containing a PCV-2 nucleotide sequence. In a particularly preferred embodiment, the PCV-2 nucleotide sequence is ORF4, ORF13, or ORF4 and ORF13. In some embodiments, the immunogenic composition includes one or more additional pig pathogens.
Owner:MERIAL SAS +1
Use of a pcv2 immunogenic composition for lessening clinical symptoms in pigs
ActiveUS20080261887A1Reduce severityReduce morbidityPeptide/protein ingredientsAntiviralsDiseaseClinical manifestation
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Culturing circular ssDNA viruses for the production of vaccines
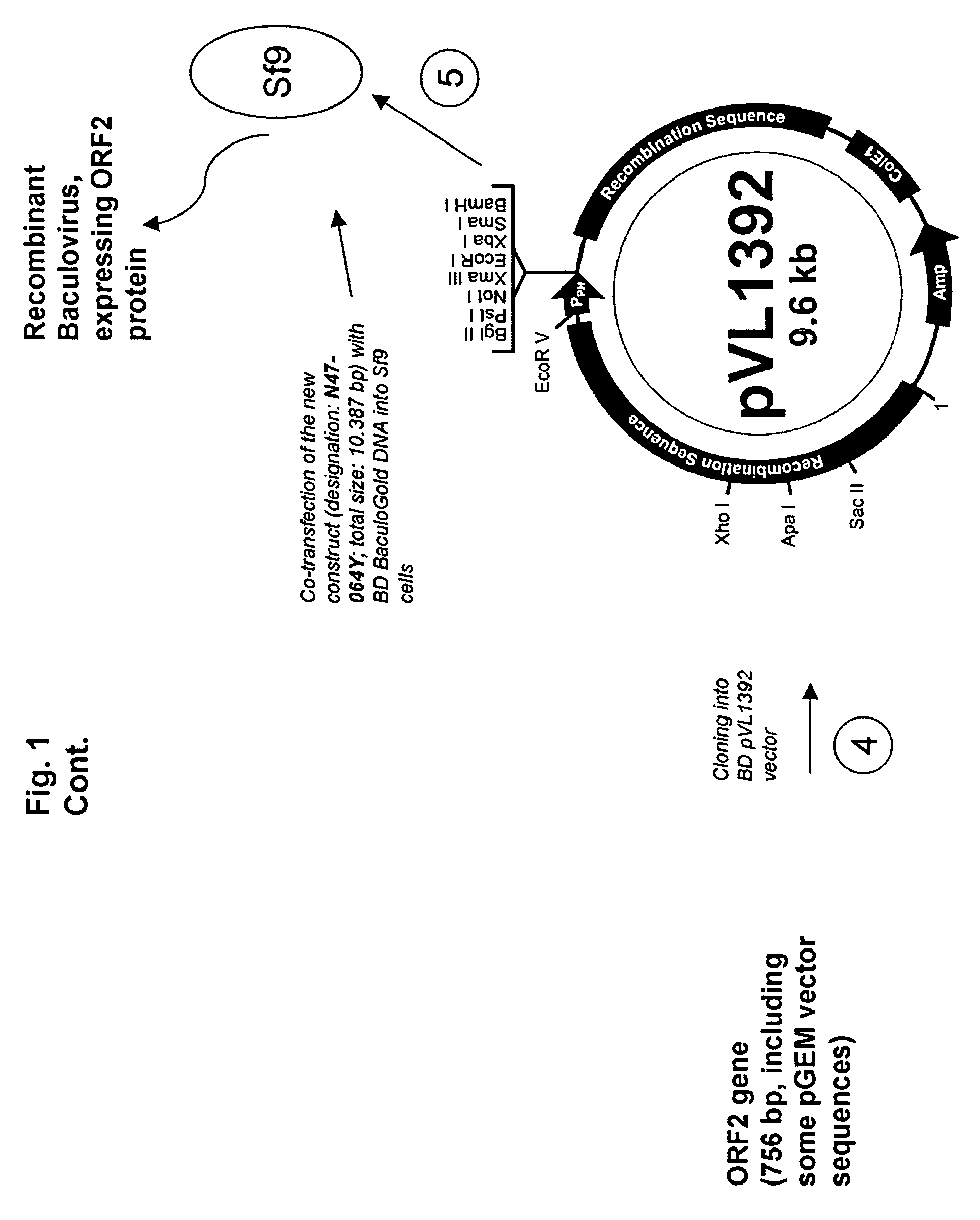
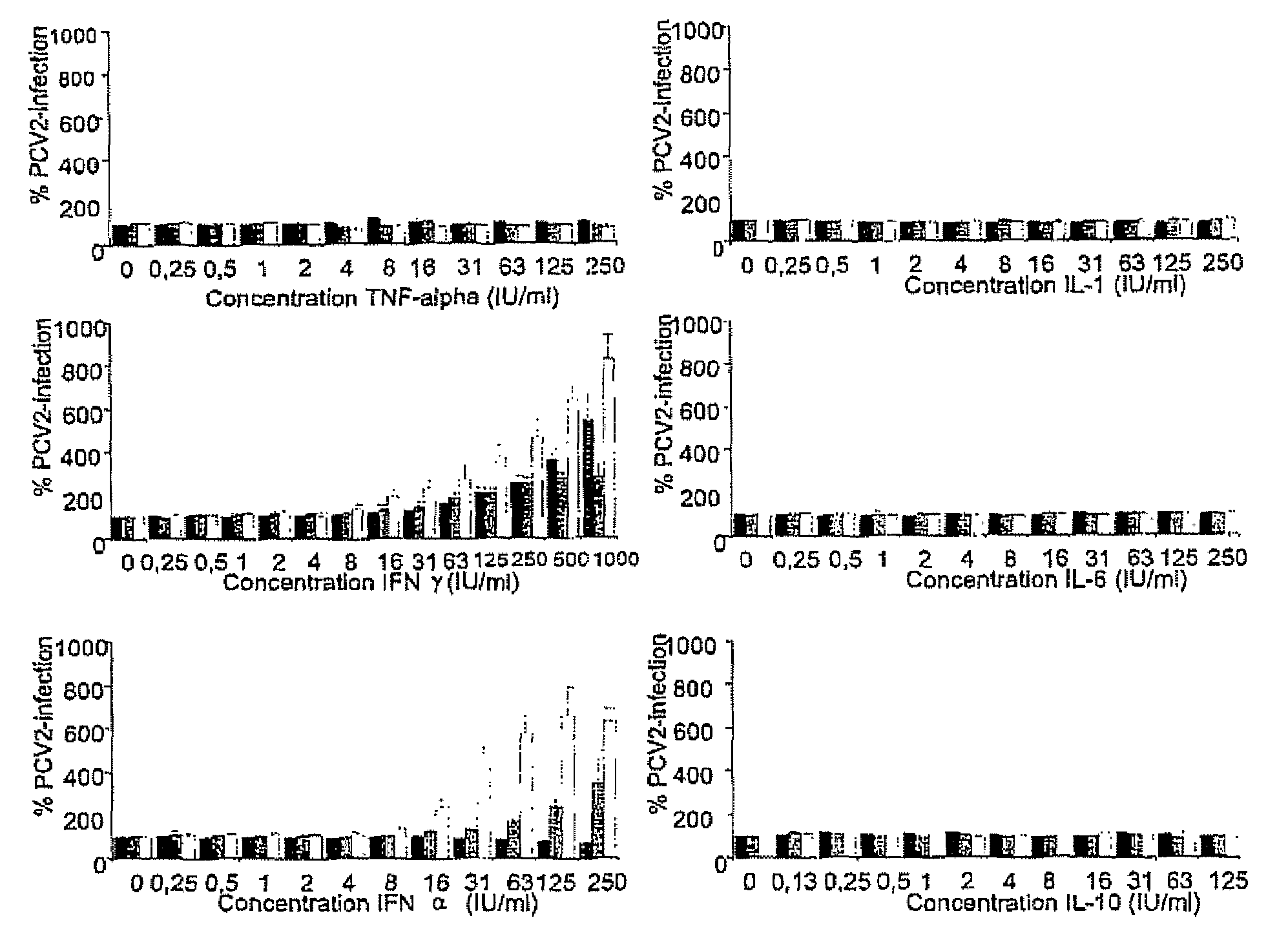
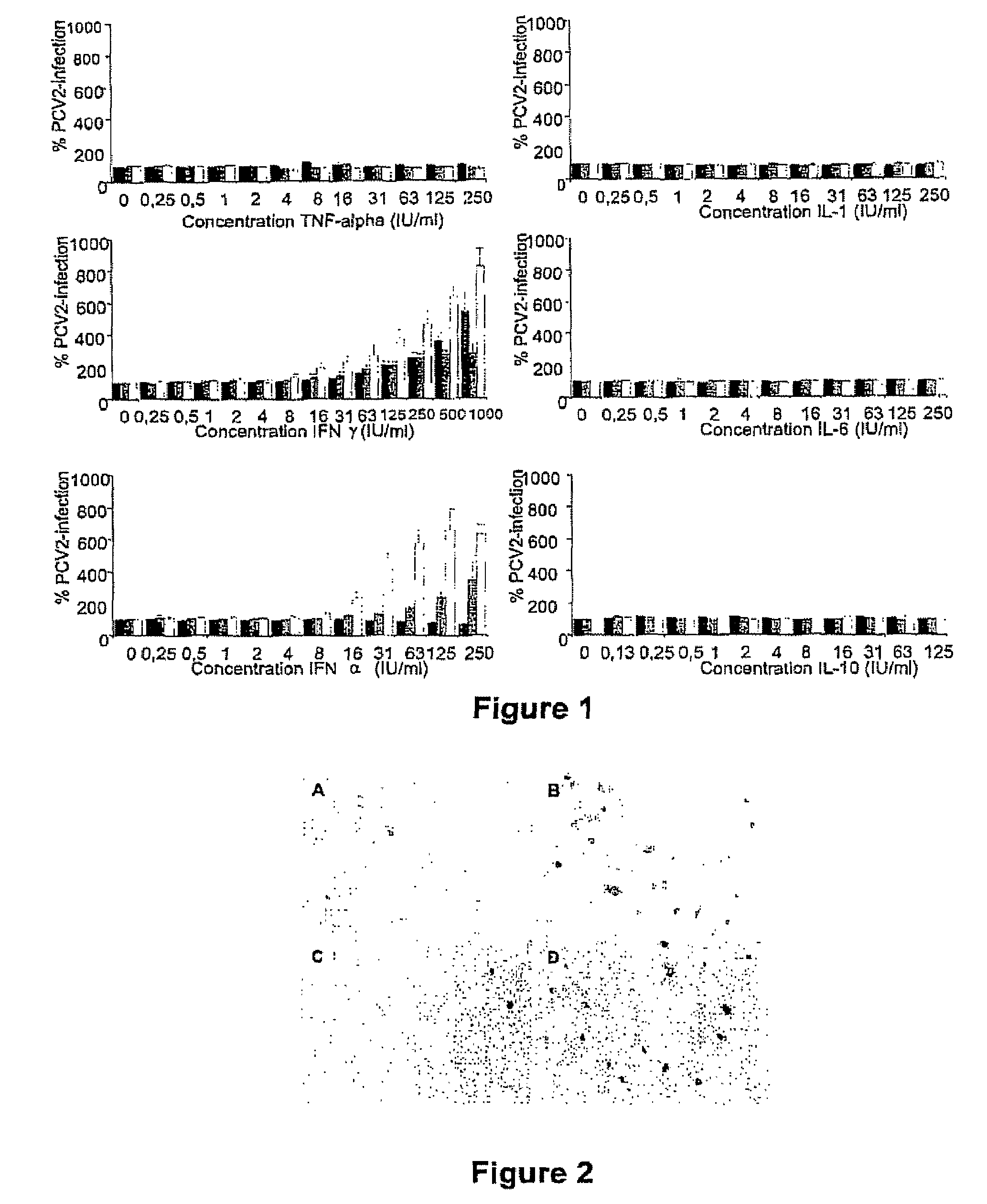
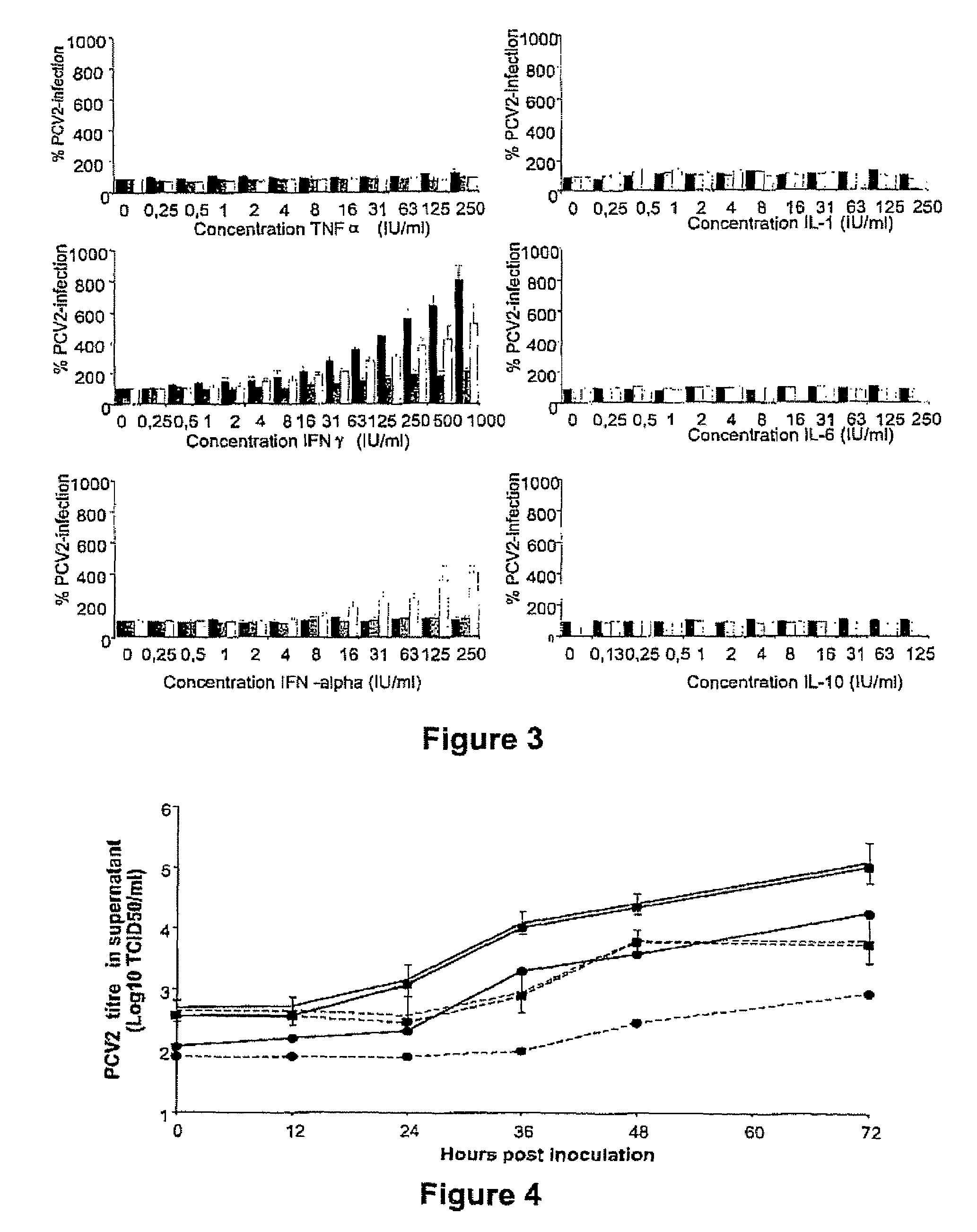
InactiveUS7300785B2Increase virus titresIncreased virus titresViral antigen ingredientsMicrobiological testing/measurementSsDNA virusesInterferon alpha
The present invention relates to the use of interferon in the in vitro cultivation of animal circular ssDNA virus such as Porcine Circovirus 2 or human TT virus in an animal cell line. Increased titres of animal circular ssDNA virus are obtained by addition of interferons or agents which ensure the production of endogenous interferons by said cell line.
Owner:UNIV GENT
Postweaning multisystemic wasting syndrome and porcine circovirus from pigs
InactiveUS20060002952A1Peptide/protein ingredientsGenetic material ingredientsPorcine circovirusPolynucleotide
The cloning of a novel PCVII viral genome is described as is expression of proteins derived from the PCVII genome. These proteins can be used in vaccine compositions for the prevention and treatment of PCVII infections, as well as in diagnostic methods for determining the presence of PCVII infections in a vertebrate subject. Polynucleotides derived from the viral genome can be used as diagnostic primers and probes.
Owner:MERIAL SAS
PCV2 immunogenic compositions and methods of producing such compositions
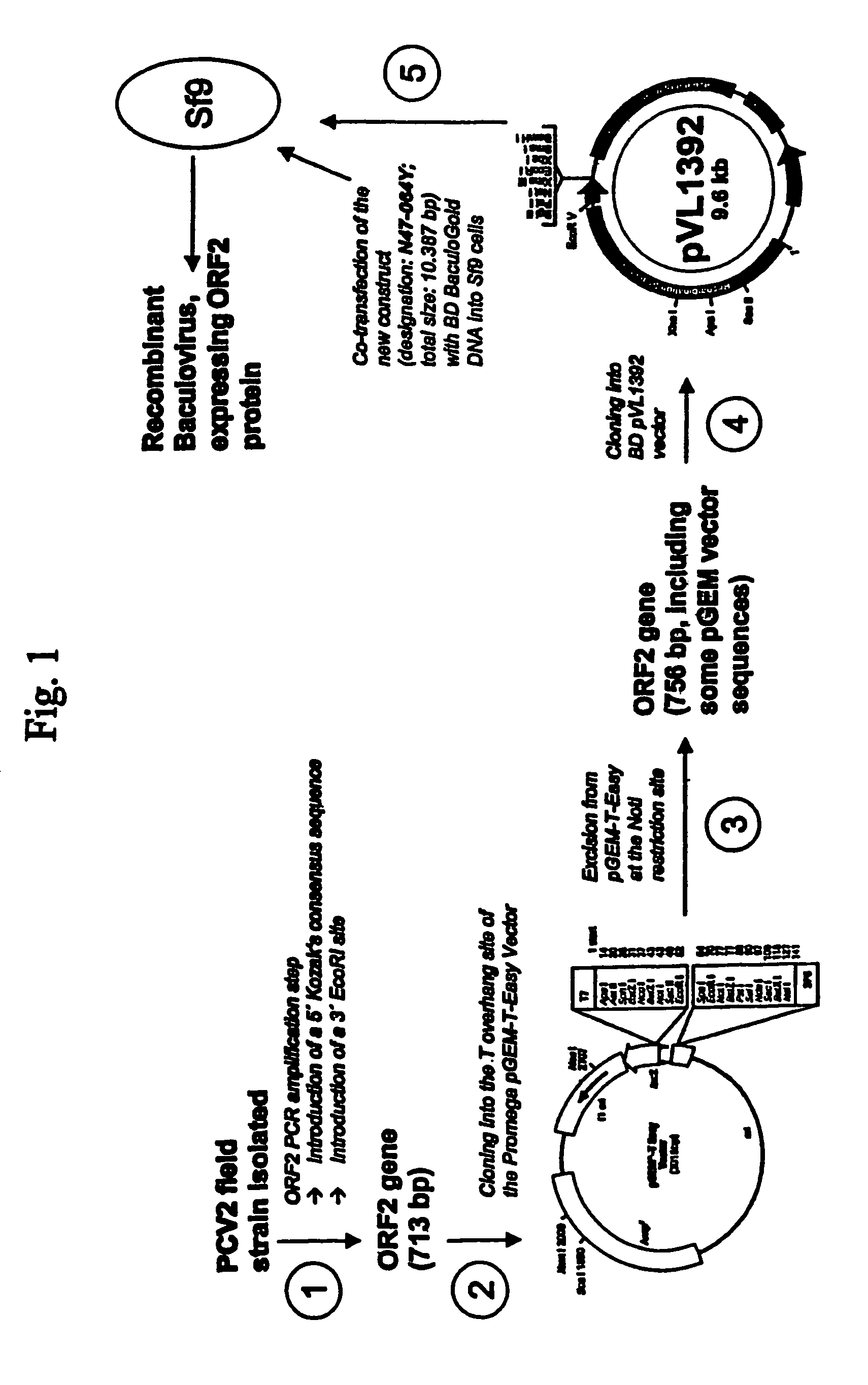
ActiveUS7700285B1Reduce the impactReduce severityViral antigen ingredientsMicrobiological testing/measurementOpen reading frameCell culture media
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Treatment of PRDC in pigs
The present invention relates to the use of an immunogenic composition comprising a porcine circovirus type 2 (PCV2) antigen for the prevention and treatment, including a reduction in the severity of, duration of, and manifestations of, porcine respiratory disease complex (PRDC) in animals, preferably in pigs.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Methods and compositions in the treatment of porcine circoviral infection
InactiveUS20100184016A1Viral antigen ingredientsMicrobiological testing/measurementMammalPorcine circovirus
The invention relates generally to the field of virology. More particularly, the present invention relates to methods of diagnosing, prognosis, treatment and prevention of porcine circoviral infection in mammals, in particular of porcine circovirus type 2 (PCV2).Methods of using a nucleic acid(s) and / or a protein(s), which are immunogenic in said mammal, and antibodies immunospecific for said protein(s), to treat, diagnose and / or prevent said porcine circoviral infection, are provided for by the present invention.
Owner:UNIV GENT
Prevention and treatment of sub-clinical PCVD
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
ELISA kit for detecting porcine circovirus antibody II
ActiveCN101629956ASimilar sensitivityStrong specificityMicroorganism based processesBiological testingSerum igeElisa kit
The invention relates to an ELISA kit for detecting porcine circovirus antibodies II, wherein, the kit is developed according to the double antigen sandwich ELISA principle. The ELISA kit comprises a prepacked ELISA antigen plate bar, a cleaning solution, 100 times concentrated enzyme labeled antigen, an enzyme labeled dilution, a zymolyte coloration, a stopping solution, standard PCV2 negative sera, and standard PCV2 positive sera. The kit replaces enzyme labeled anti-antibodies with the enzyme labeled antigen. The enzyme labeled antigen can not combine with antibodies absorbed with ELSA plate without specificity. Meanwhile, the serum specimen to be detected does not need to be pre-diluted, and the serum specimen can be directly mixed with the enzyme labeled antigen with working concentration to be directly used for determination. The ELISA kit has the advantages of simple operation and shorter detecting time.
Owner:北京金诺百泰生物技术有限公司
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Pcv2 immunogenic compositions and methods of producing such compositions
ActiveUS20090016992A1Reduce the impactReduce severityBiocideSsRNA viruses positive-senseOpen reading frameCell culture media
An improved method for recovering the protein expressed by open reading frame 2 from porcine circovirus type 2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time-consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine Circovirus Type 2 (PCV2), Immunogenic Composition Containing the Same, Test Kit, and Application Thereof
ActiveUS20120164170A1Improve immunityReduce severityAntibacterial agentsVirus peptidesPorcine circovirusImmunogenicity
The invention relates to a novel porcine circovirus type 2 (PCV2) strain. The invention also relates to immunogenic compositions containing the novel PCV2 strain, PCV2 test kits, and applications of the novel PCV2 strain.
Owner:VIRBAC H K TRADING LTD
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
Porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triple virus-like particle vaccine and its preparation method
The purpose of the invention is to disclose a porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triplex virus-like particle vaccine and its preparation method. The triple virus-like particle vaccine (Triple VLP vaccine) of the invention contains VLP which is composed of PCV-2 major structural protein CAP protein, PPV VP2 protein epitope and PRRSV Gp5 protein epitope. It is proved by experiment that the vaccine can stimulate good double cellular and humoral immune response. It is shown by pharmacodynamic test that after immunization of different animal groups, the vaccine of injection, nose drops and water forms prepared by VLP antigen formed by the method with or without adjuvants can safely and effectively prevent the infection of PCV-2, PPV and PRRSV. The invention provides an ideal vaccine for the security of sows, piglets and fattening pigs to effectively prevent mixed infection of PCV-2, PPV and PRRSV.
Owner:CHONGQING UNIV
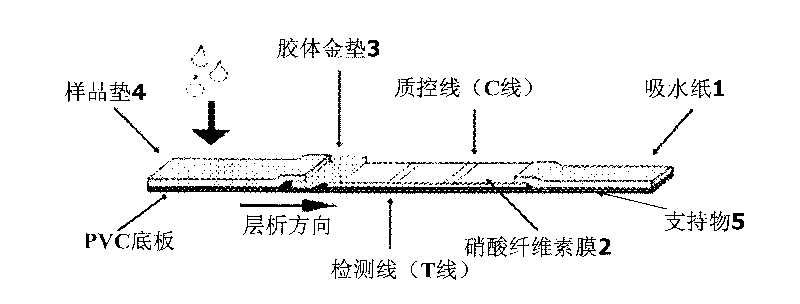
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as preparation method and applications thereof
ActiveCN103033622AImprove expression efficiencyImproving immunogenicityMicroorganism based processesGenetic engineeringSorbentMonoclonal antibody
The invention relates to a PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as a preparation method and applications thereof, wherein the detection kit comprises an elisa plate of a polyclonal antibody of peridium anti-PCV2-Cap (nucleocapsid) protein, seal liquids, sample diluent, an antigen standard product, a second antibody of a monoclonal antibody of HRP marked anti-PCV2-Cap protein, a concentrated washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution, wherein the antigen standard product is purified reconstructed PCV2-Cap protein. The specificity of the kit provided by the invention achieves 100%, and the sensitivity is as high as 4ng / ml, and the kit can be used for swinery PCV2 antigen detection and PCV2 vaccine product quantitative detection.
Owner:WUHAN CHOPPER BIOLOGY
Honeysuckle composition for resisting virus diseases of pigs
The invention discloses a honeysuckle composition for resisting virus diseases of pigs, and relates to a feed additive or Chinese veterinary medicine special for preventing and controlling the virus diseases of the pigs and capable of promoting the growth of the pigs. The composition consists of the following components: 30 to 60 portions of honeysuckle extract, 5 to 20 portions of Chinese thorowax root extract, 5 to 20 portions of baical skullcap root P.E, 5 to 20 portions of dandelion P.E, and 10 to 25 portions of licorice extract. The composition is Chinese medicinal powder of which the granularity is between 200 and 300 meshes and which is yellow or brown-yellow, and has the following content of the active components: 5 to 20 percent of chlorogenic acid, 2 to 10 percent of baicalin and5 to 15 percent of glycyrrhizic acid. When used, the composition is mixed with a feed in a ratio of 0.02-0.2 percent and then is fed to animals. The composition has the effect of preventing and controlling highly pathogenic blue ear pig disease, porcine rotavirus disease, influenza, infectious bronchitis and the like, and virus diseases caused by porcine circovirus PCV, foot-and-mouth disease virus and the like, and can effectively promote the growth of the live pigs.
Owner:新乡博凯生物技术有限公司
Porcine circovirus type 2 recombinant cap protein and subunit vaccine
ActiveCN102174086AImprove securityNot pathogenicBacteriaViral antigen ingredientsAntigenEscherichia coli
The invention belongs to the field of molecular biology, and discloses a porcine circovirus type 2 recombinant cap protein and a subunit vaccine. The porcine circovirus type 2 cap protein expressed by recombinant Escherichia coli is obtained by steps of cloning a porcine circovirus type 2 cap protein in a nuclear localization signal area of which the N terminal is cut and which is rich in arginine into a prokaryotic expression vector to obtain a recombinant expression vector, transfecting the recombinant expression vector into Escherichia coli BL21(DE3), and expressing by using the recombinant Escherichia coli BL21(DE3). Tests prove that the constructed recombinant strain expresses a foreign protein stably. When the subunit vaccine is prepared from the expressed recombinant protein, an antigen has high purity and safety, does not have pathogenicity on animals such as pigs and the like, and passes safety evaluation easily.
Owner:NANJING AGRICULTURAL UNIVERSITY
Chinese medicine composition for prevention and control of porcine circovirus disease and preparation method thereof
The invention discloses a Chinese medicine composition for prevention and control of porcine circovirus disease and a preparation method thereof, and belongs to the field of traditional Chinese medicine for livestock. The Chinese medicine composition is composed of twenty traditional Chinese medicines comprising honeysuckle flower, bupleurum, Herba Houttuyniae, Radix Isatidis, Radix Astragali, licorice, fructus forsythiae, wolfberry fruit, dried tangerine peel, baikal skullcap root, epimedium, bighead atractylodes rhizome, cucumber, rhizoma atractylodis, Folium Isatidis, Polyporus umbellatus, angelica, Codonopsis pilosula, Poria and Rhizoma Coptidis. An injection is prepared by steps of crushing, decoction, enzymolysis, flocculation precipitation, concentration, filtration, sealing and sterilization. The pure natural herbs adopted by the invention are safe, reliable and without toxic or side effects; compatibility of the medicines is in line with the principle of assistant and guide; and the composition is safe, and has no toxic or side effects, and the efficacies of clearing heat, removing toxicity, resisting bacteria, relieving inflammation, and enhancing immunity of the body. The prepared injection has high bioavailability and stable traits, can effectively enhance immunity of the body, and has good treatment effect on porcine circovirus disease.
Owner:ZHENGZHOU HOUYI PHARMA
Methods and compositions for immunizing pigs against porcine circovirus
The present invention relates to the isolation and identification of two new strains of type 2B porcine circovirus. These two new strains of porcine circovirus may be used for the preparation of vaccine or immunogenic compositions for immunizing pigs against postweaning multisystemic wasting syndrome (PMWS). Accordingly, the invention provides methods for eliciting a protective immune response against a pathogenic porcine circovirus by administering to a pig an immunogenically effective amount of a type 2B porcine circovirus vaccine or immunogenic composition comprising at least one of the porcine circoviruses having a nucleic acid sequence as set forth in SEQ ID NOs: 1 or 2, or at least one protein from at least one of the two new type 2B strains of porcine circovirus as described herein. The invention further relates to protection of a pig from any one or more of the symptoms or sequelae associated with PMWS.
Owner:WYETH LLC
2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
InactiveCN102517331AQuick responseHigh activityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a 2 type subunit vaccine for a porcine circovirus as well as a preparation method and application thereof. A recombinant bacilliform virus contains double promoters (a polyhedrin promoter and a P10 promoter), a coding gene of a Cap protein with double copying can be expressed, and the expression efficiency of the protein is obviously enhanced; moreover, the Cap protein expressed by an inserted foreign gene does not contain an excess sequence, virus-like particles (VLPs) can be effectively formed, and the immunogenicity of an expressed protein is enhanced; furthermore, a produced antigen has high content; and according to the 2 type subunit vaccine for the porcine circovirus, which is disclosed by the invention, the productivity ratio and the quality of a viral protein of the 2 type subunit vaccine for the porcine circovirus are obviously enhanced, and a prepared vaccine composition has the advantages of stable and persistent immune effect, high safety and the like.
Owner:WUHAN CHOPPER BIOLOGY
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
ActiveCN103263666AImprove protectionEffective protectionAntibacterial agentsBacteriaOil adjuvantMental state
The invention discloses a duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The duplex inactivated vaccine comprises an inactivated porcine circovirus type 2 antigen, inactivated mycoplasma hyopneumoniae and an oil adjuvant, wherein the mycoplasma hyopneumoniae is of DJ-166 strains and has the preservation number No.4545 in China general microbiological culture collection center. The porcine duplex inactivated vaccine has an obvious technical effect on prevention of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The safety test shows that the single dosage of the vaccine, the repetition of the single dosage and an overdosing amount of inoculation against test animals are safe, the test animals have normal body temperature and mental states, and the clinical symptoms are avoided; and the efficacy test shows that the duplex inactivated vaccine has a good protection function of virulently attacking the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae, so that the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +3
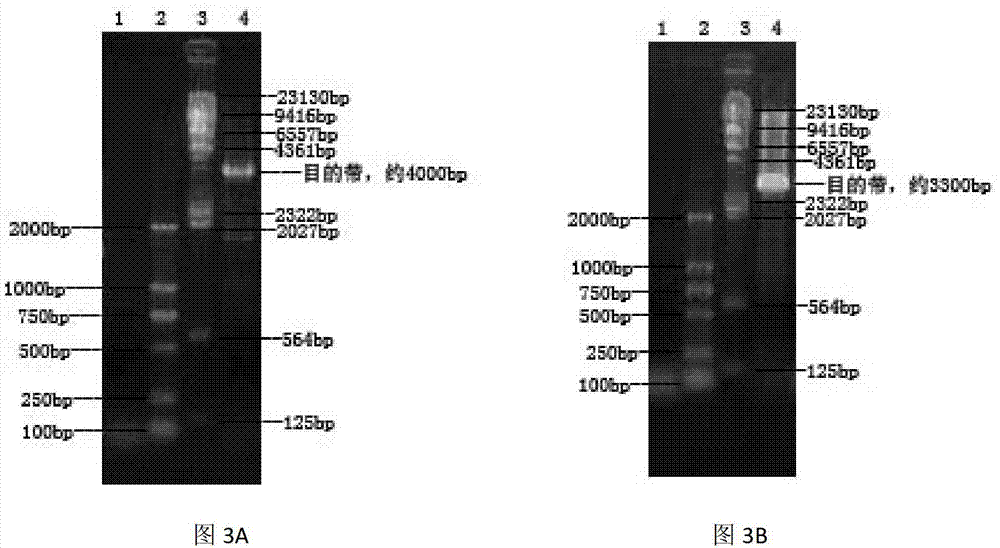
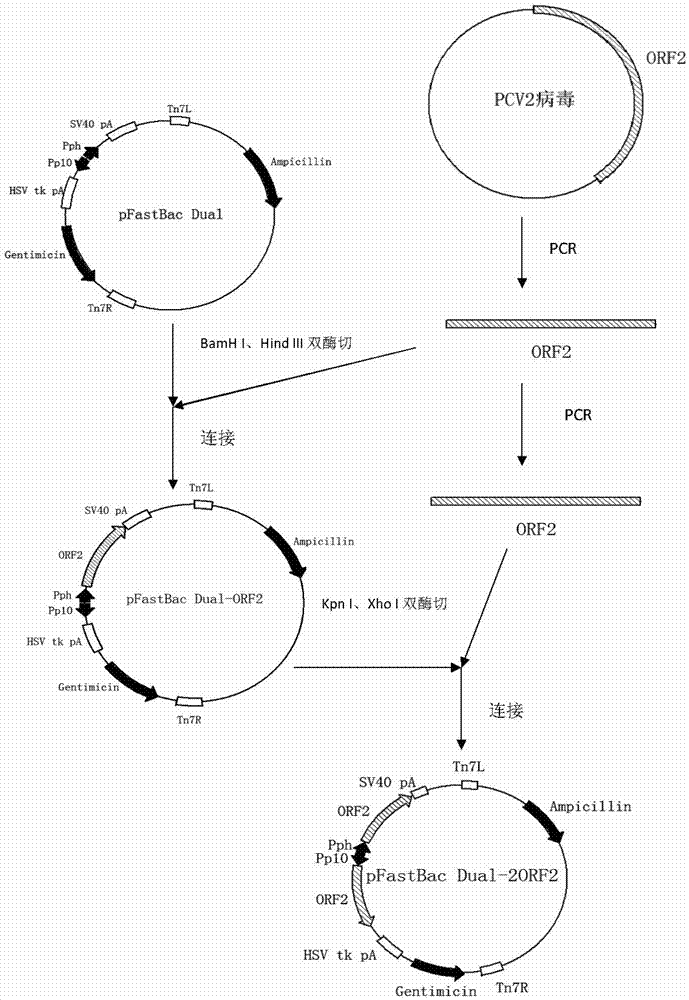
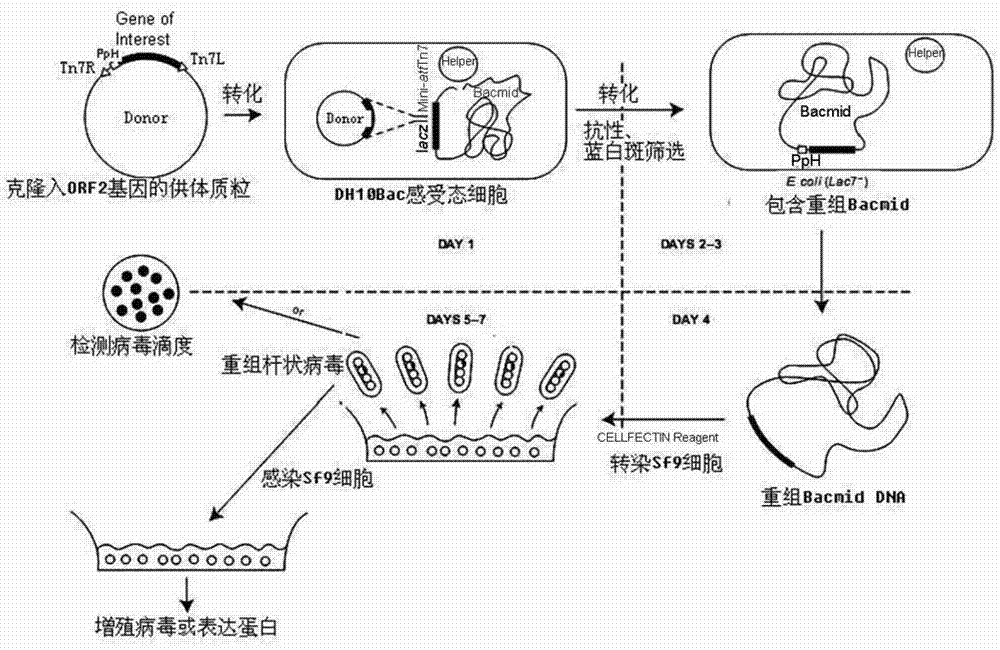
Method for expression of PCV 2 Cap protein by pFast Bac Dual baculovirus
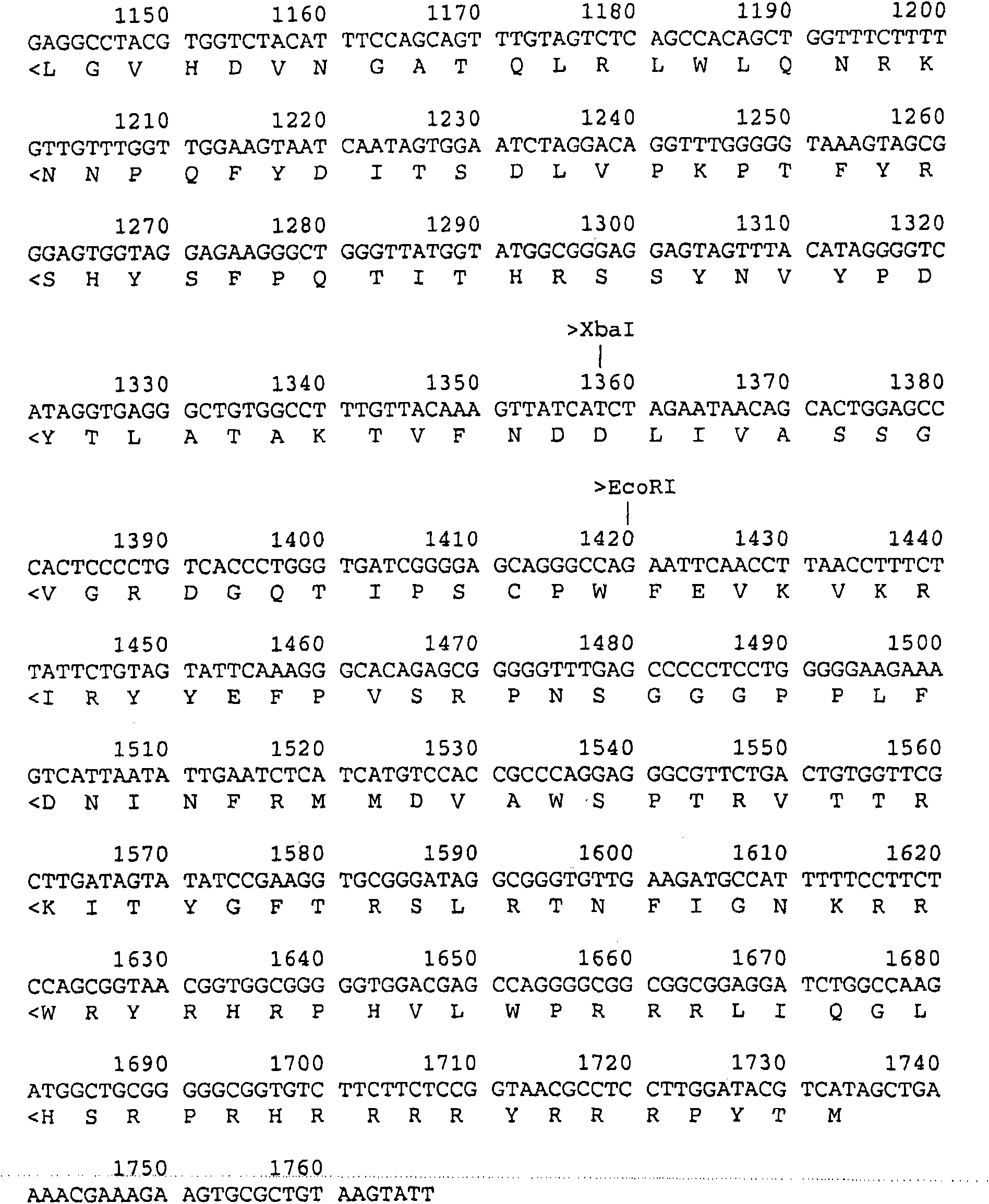
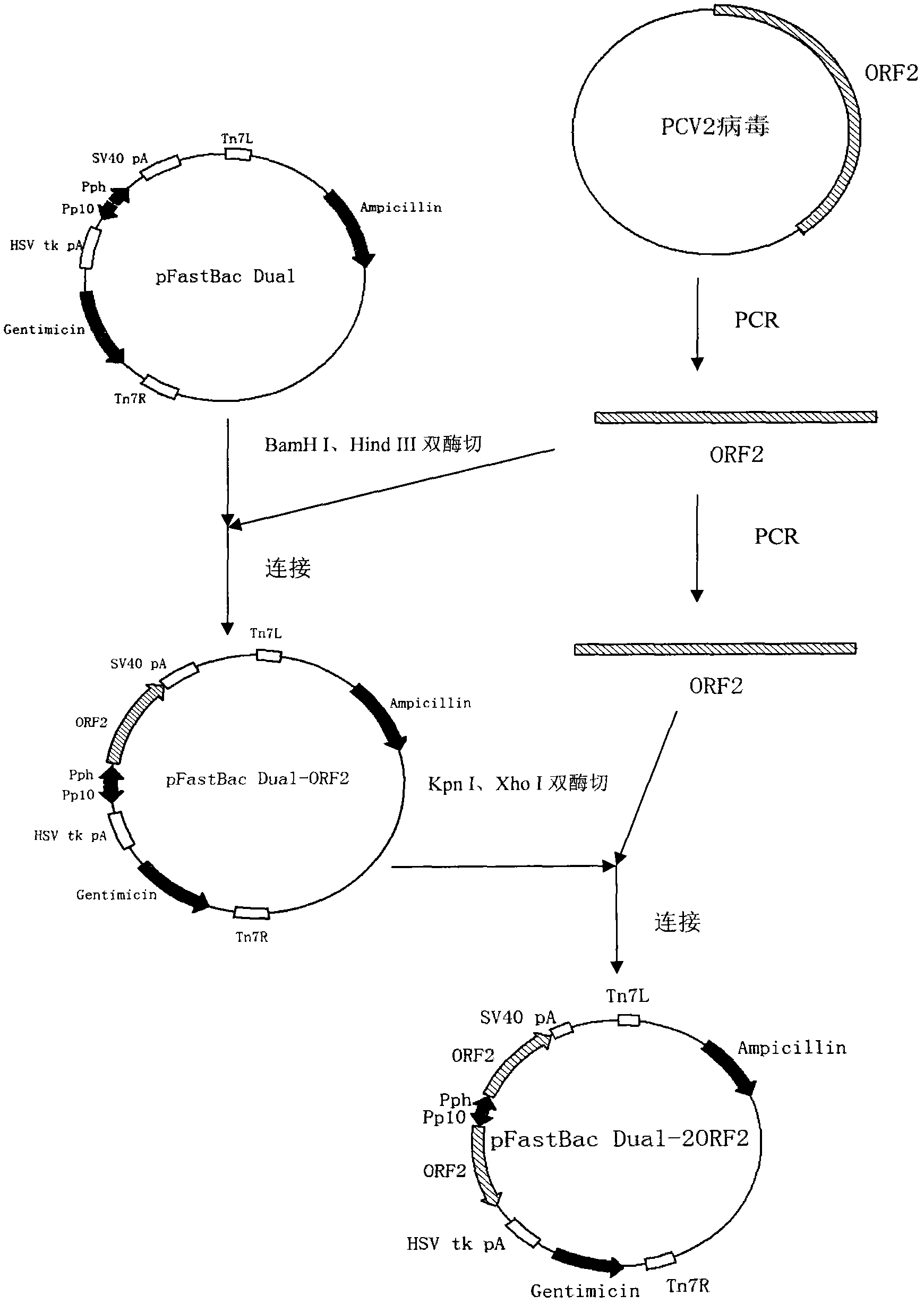
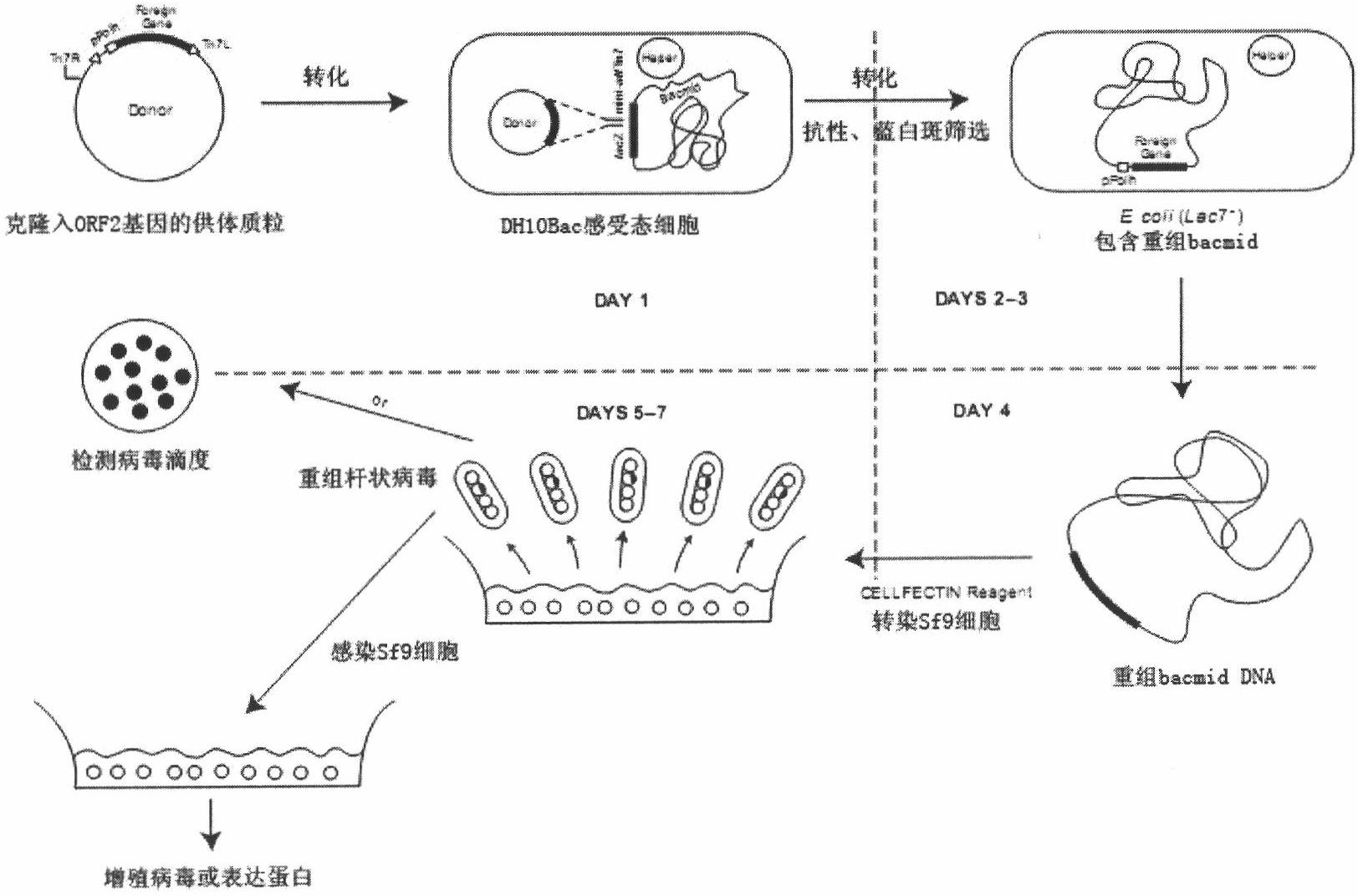
InactiveCN102839195AEfficient expressionHighly efficient expression systemMicroorganism based processesFermentationEscherichia coliShuttle vector
The invention discloses a method for expression of a PCV 2 (Porcine circovirus type2) Cap protein by a pFast Bac Dual baculovirus. The method comprises the following steps of: amplifying a gene fragment of an encoded PCV 2 Cap protein with a His tag; connecting the gene fragment to a pFast Bac Dual plasmid so as to obtain a recombinant transfer plasmid pFast Bac-p10-ORF2-pH-ORF2; transforming Escherichia coli DH10Bac with the recombinant transfer plasmid, and carrying out blue-white selection to obtain a recombinant shuttle vector Bac-p10-ORF2-pH-ORF2; transfecting an insect cell with the recombinant shuttle vector so as to obtain a recombinant baculovirus Ac.Dual-Cap; and poisoning the insect cell with the recombinant baculovirus, performing cultivation, then collecting the insect cell, and purifying an expression product so as to obtain the recombinant Cap protein. The method disclosed in the invention solves the problem of low expression level of the Cap protein in eukaryotic cells. The recombinant Cap protein in the invention is designed with a His tag, thus being beneficial to the follow-up purification. And the recombinant Cap protein has biological activity superior to that of a Cap protein expressed by a prokaryotic expression system, thus being applicable to establishment of epidemiological diagnosis methods and reseach as well as development of PCV2 subunit vaccines.
Owner:SOUTH CHINA AGRI UNIV
Recombination virus particles for expressing 2-typed porcine circovirus nucleocapsid protein Cap gene
InactiveCN101289658ANon-pathogenicImprove replication efficiencyViral antigen ingredientsAntiviralsAntigenShuttle vector
The invention relates to construction and application of recombinant nucleocapsids of Cap genes of porcine circovirus expression type 2 nucleocapsid protein, belonging to the genetic engineering bacterin field. C-terminal gene fragments of porcine parvovirus (PPV) VP2 genes are cloned into a type 5 adenovirus shuttle vector of the human beings, and recombinant adenovirus rAd-deltaVP2 is obtained; deltaVP2 proteins are expressed successfully and highly efficiently and can be self-assembled into the nucleocapsids [PPV:VLPs]; the PPV VP2 nucleocapsids are used as antigen transport vectors and 165 to 200 sites of amino acid (deltaCap) genes of the porcine circovirus type 2 (PCV2) nucleocapsid proteins (Cap) are embedded into an N-terminal (deltaVP2) of the PPV VP2, and then recombinant adenovirus rAd-deltaCap-deltaVP2 is obtained; embedded VP2 (deltaCap-deltaVP2) proteins are expressed successfully and highly efficiently and can be self-assembled into nucleocapsids [PPV:VLP(PCV2)]. The invention also relates to application of the recombinant virus and recombinant PPV VP2 nucleocapsids of the expression Cap genes of the recombinant virus in the aspects of bacterin immunity and so on.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
High-prolificacy porcine circovirus type-2 strain and application thereof
ActiveCN102787100AImprove reproductive performanceGood immune protectionViral antigen ingredientsMicroorganism based processesNucleotideMicrobiology
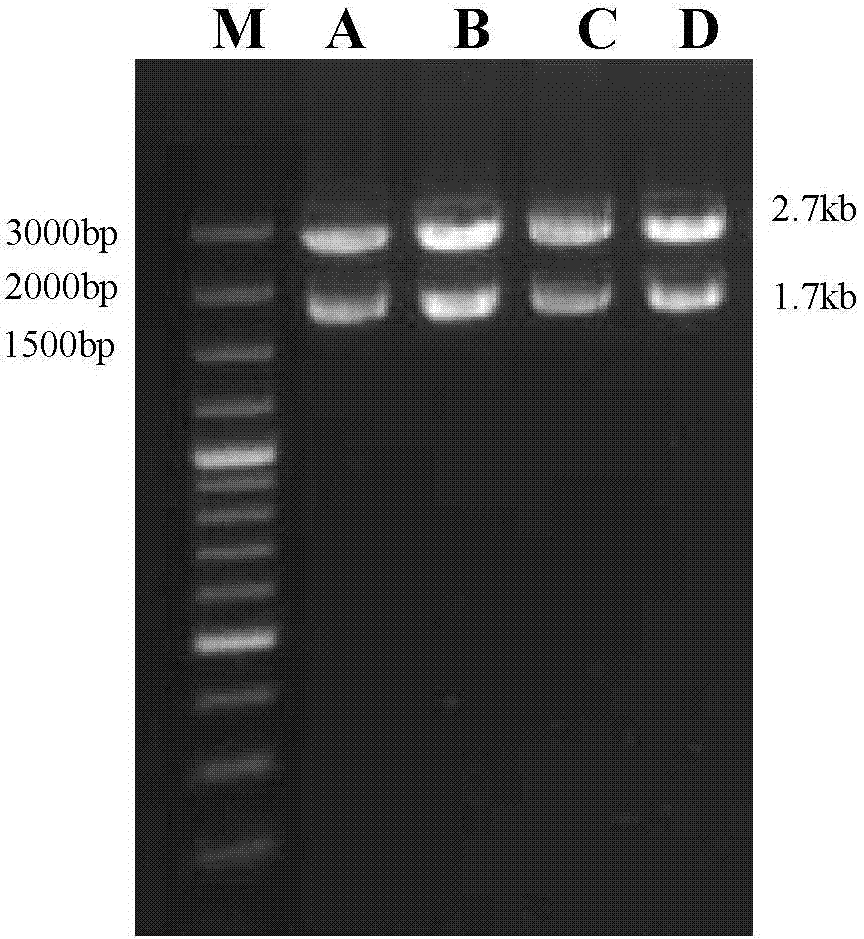
The invention discloses a high-prolificacy porcine circovirus type-2 strain and application thereof. The porcine circovirus type-2 strain is named as PCV2-ZJ / H strain, the preservation number of the strain is CGMCC No.6391, the gene sequence of the strain is shown as SEQ ID NO:1, and nucleotide at a 1376 locus in the nucleotide sequence of a genome of the strain is absent. The porcine circovirus type-2 strain has high prolificacy, the virus titer (TCID50) in PK-15 cells reaches 107.6 / mL and is more than 10 times higher than that (106.3 / mL) of a parent virus, and a vaccine prepared with the strain has high immune protection capacity.
Owner:ZHEJIANG UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com