Patents
Literature
197 results about "Bovine Viral Diarrhea Viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
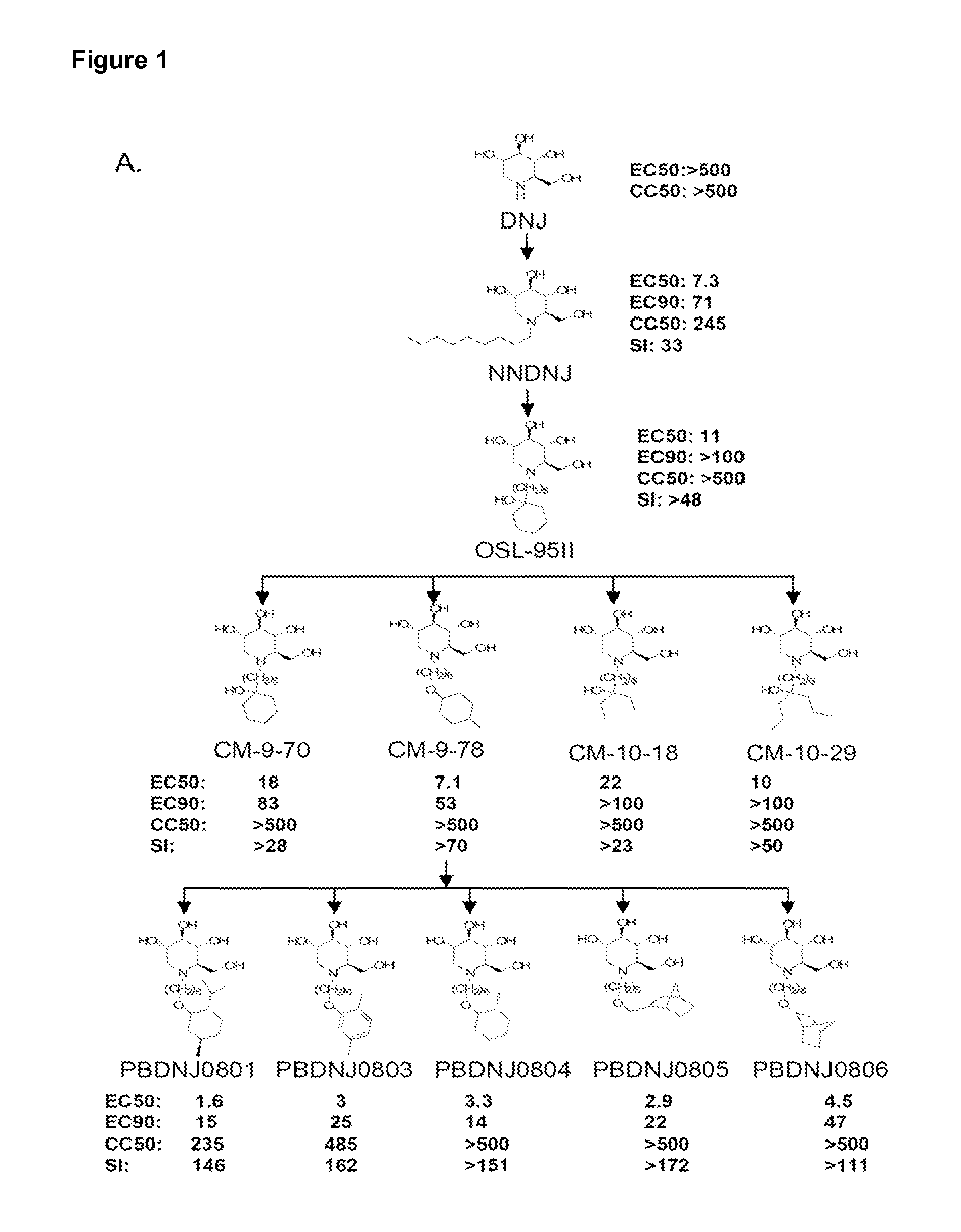
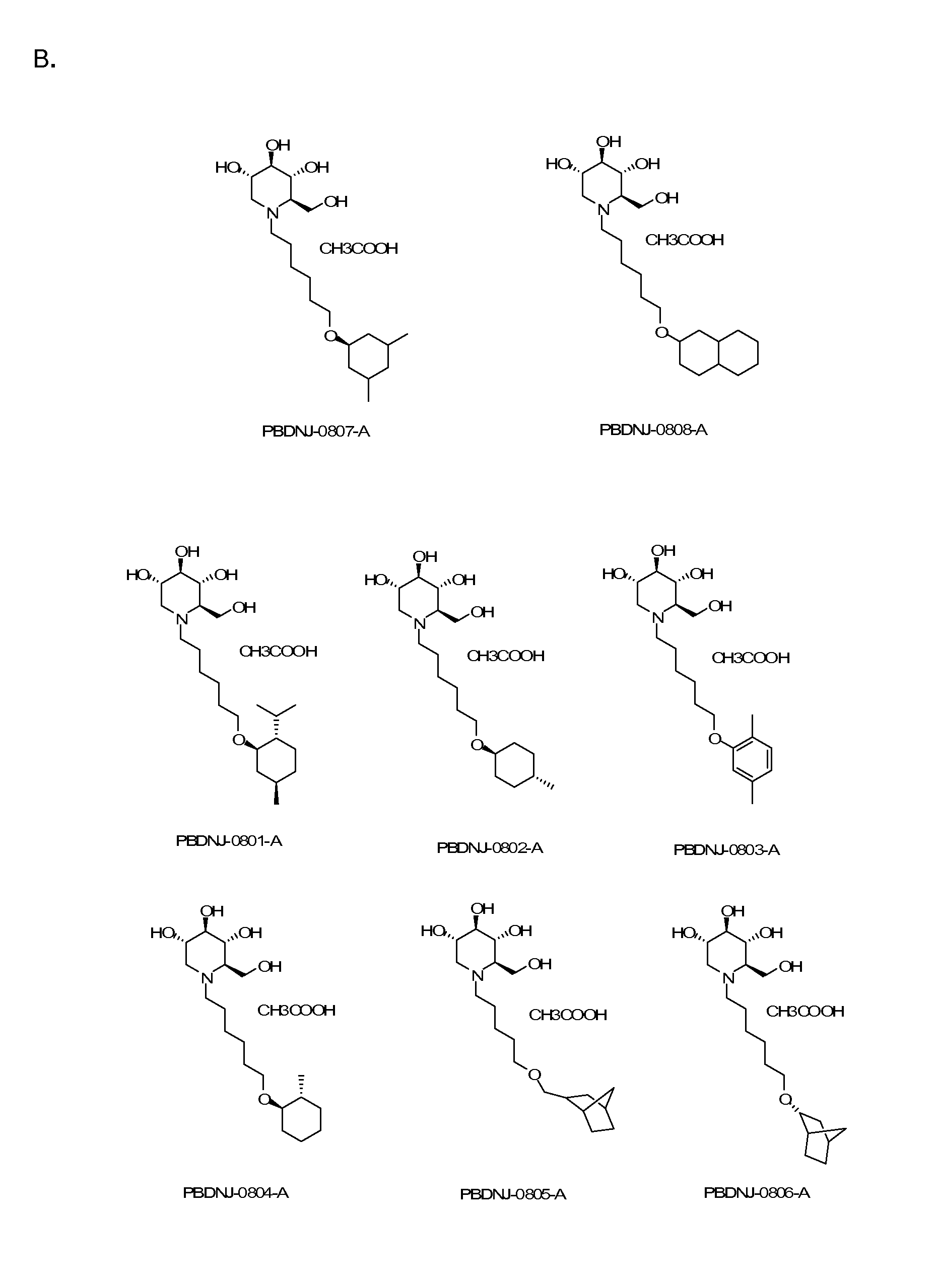
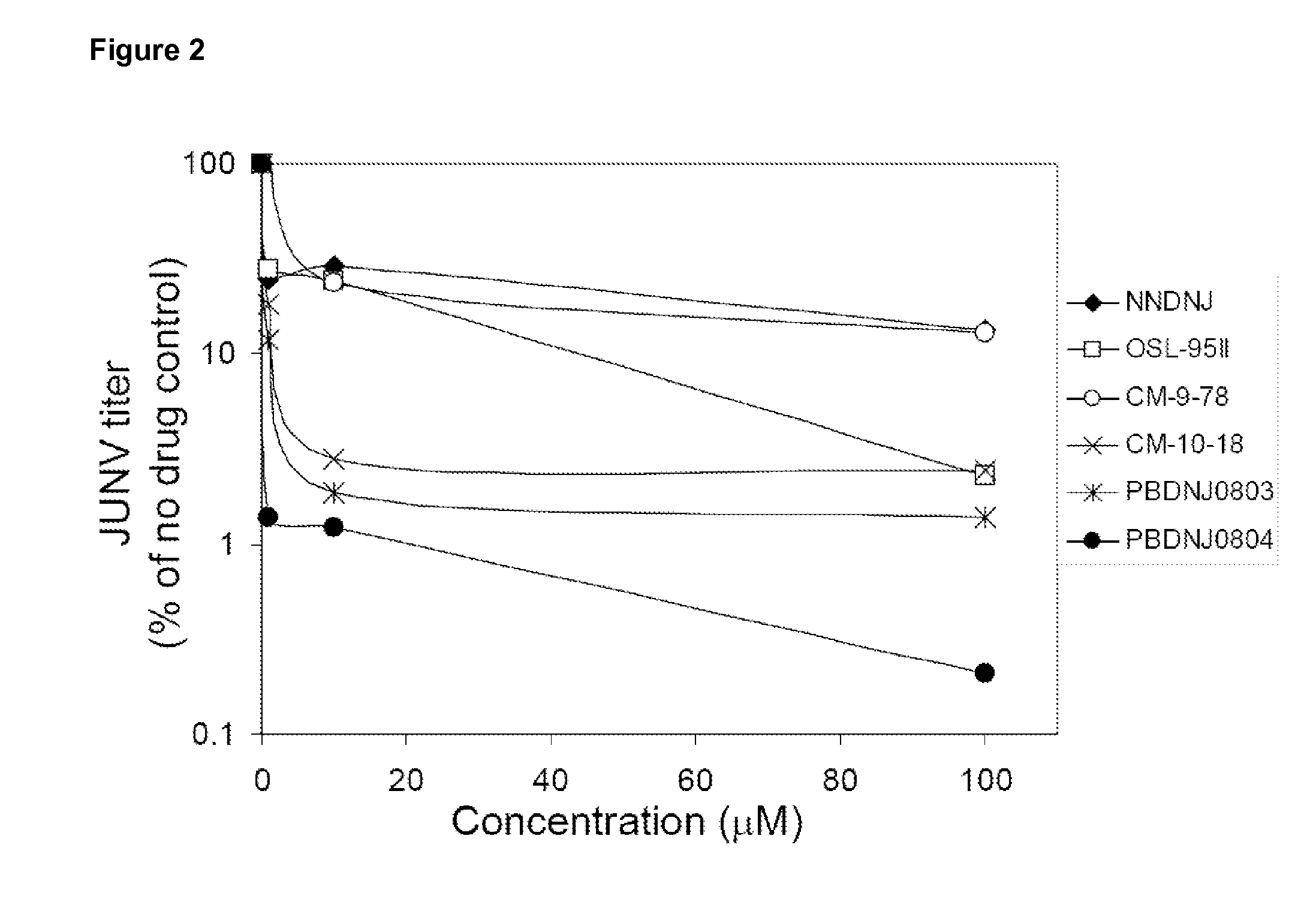
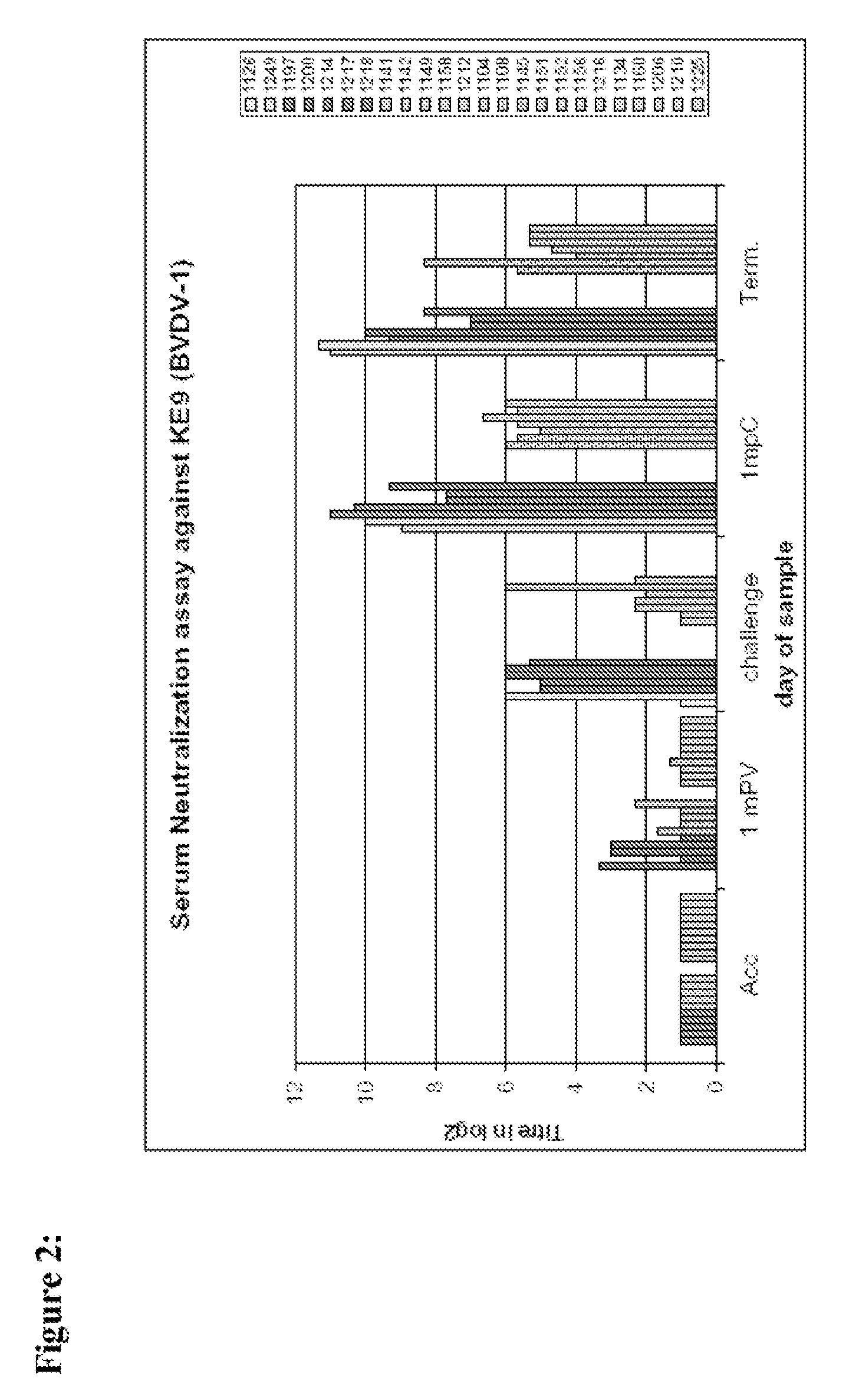
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
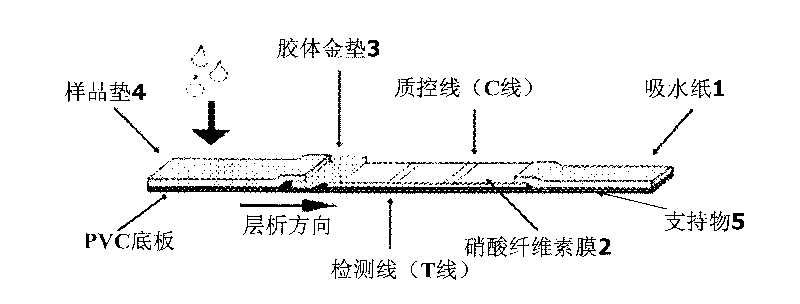
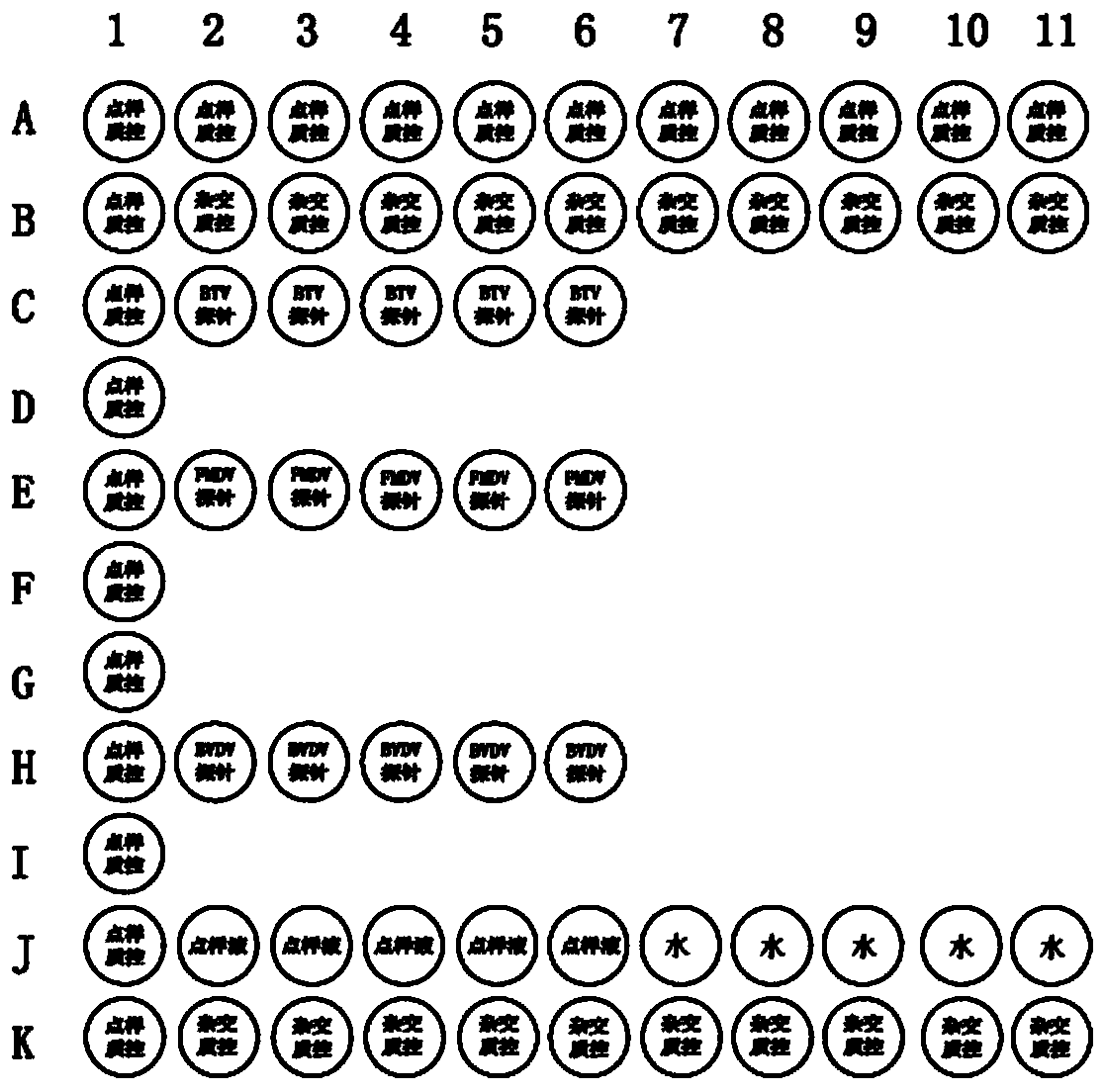
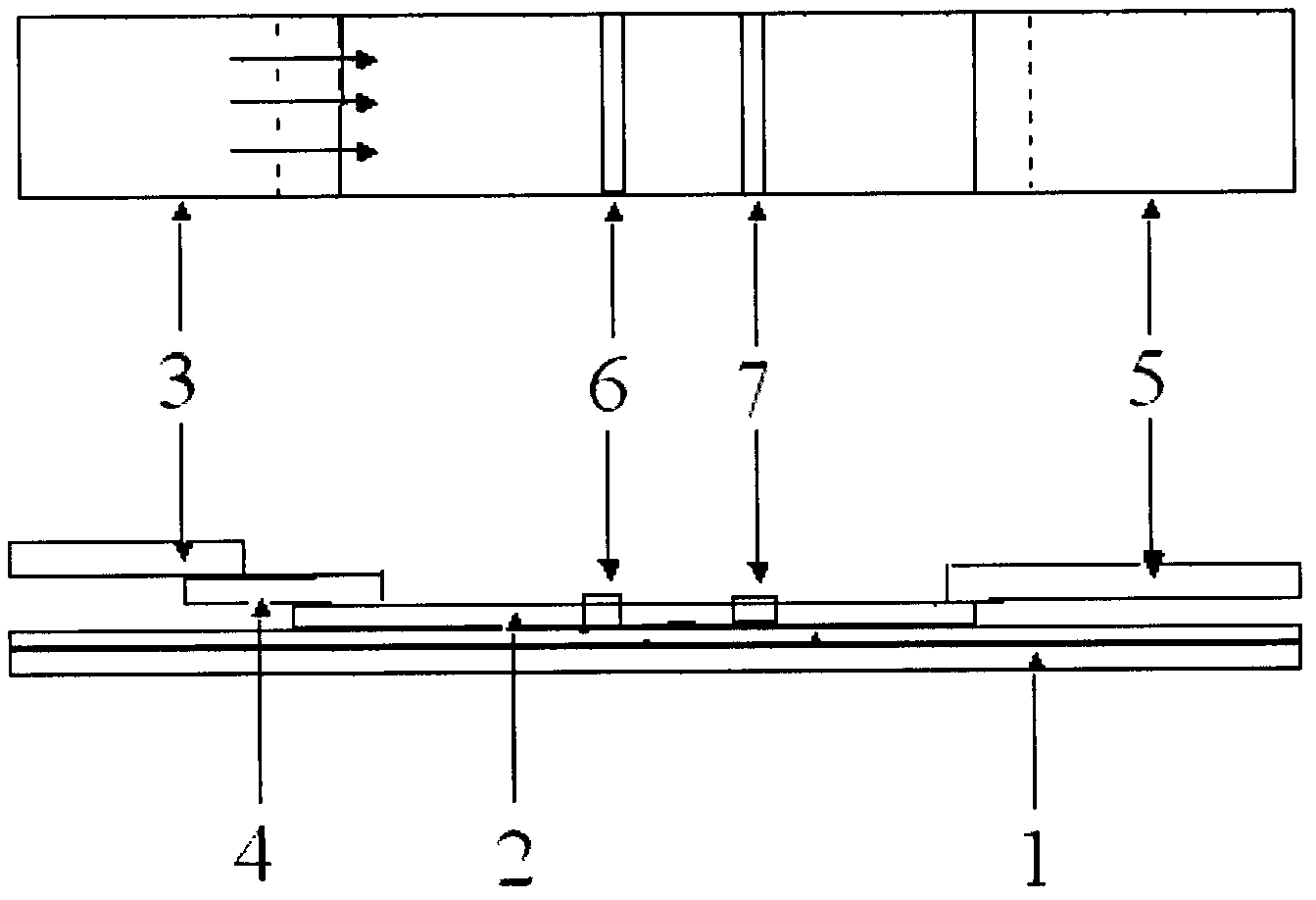
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Combination vaccine comprising an attenuated bovine viral diarrhea virus
InactiveUS20090068223A1SsRNA viruses negative-senseSsRNA viruses positive-senseBovine Viral Diarrhea VirusesActive component
The present invention relates to combination vaccines for the prophylaxis and treatment of microbiological infections in cattle which comprise an attenuated bovine viral diarrhea virus (BVDV) for the prophylaxis and treatment of BVDV caused infections, and a further immunological active component for the prophylaxis and treatment of microbiological infections other than BVDV.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
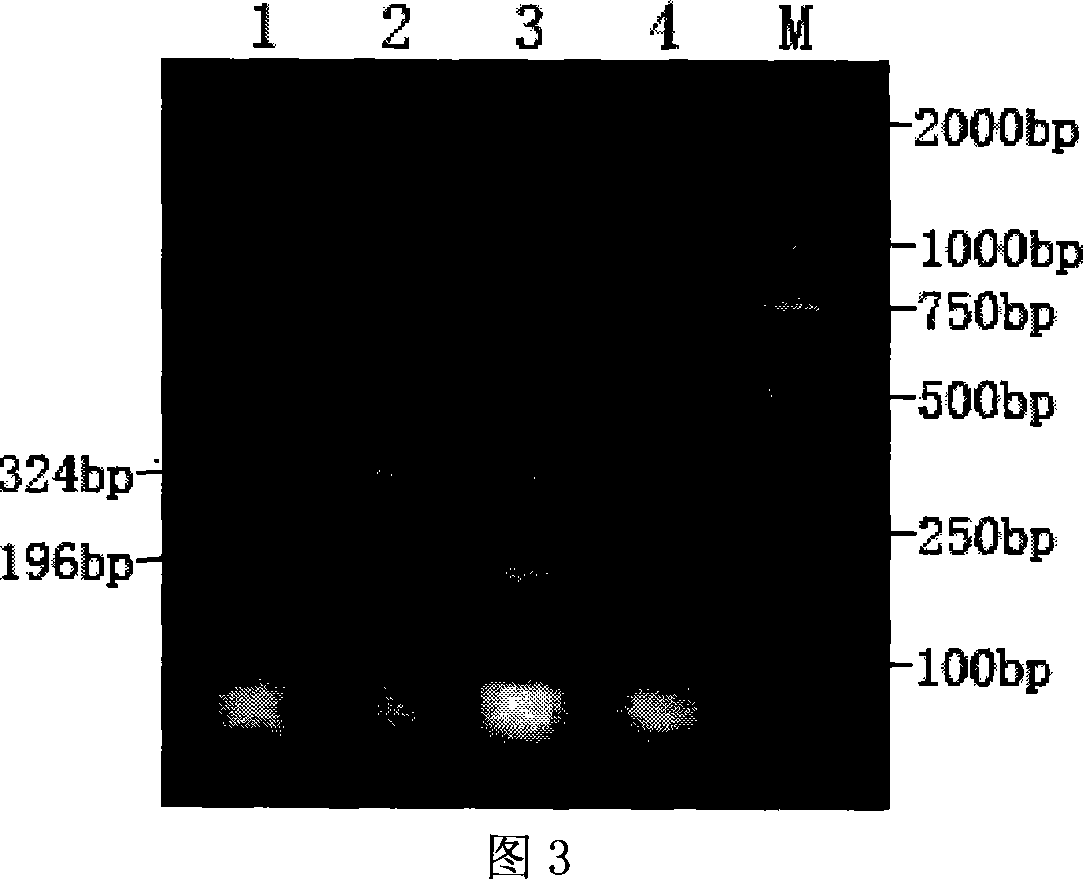
Multiplex PCR (polymerase chain reaction) primer, probe and gene chip for detecting bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus
InactiveCN103695566AImprove throughputShorten diagnostic timeMicrobiological testing/measurementDNA/RNA fragmentationForward primerMultiplex
The invention relates to a multiplex PCR (polymerase chain reaction) primer, a probe and a gene chip for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus. The multiplex PCR primer and probe have the nucleotide sequences shown by SEQ ID No.1 to SEQ ID and No.9. The gene chip comprises a solid-phase carrier, a sample application quality control probe, a positive hybrid quality control probe and a multiplex PCR primer for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus and the corresponding probe. In the invention, the forward primers of three viruses are marked with fluorescence, a gene chip detection technology carrying three viruses in animal fur is established based on multiplex RT-PCR (reverse transcription-polymerase chain reaction), and the RNA virus in the fur can be sensitively and specifically detected with high flux; the three viruses are screened at the same time in detection once, and the situation that a specific method is required for each virus before is changed, thereby saving the diagnosis time, meeting the needs for quick detection of mass imported / exported fur samples of the exit-entry inspection and quarantine departments and the fur import and export enterprises, and realizing relatively high application values.
Owner:徐超
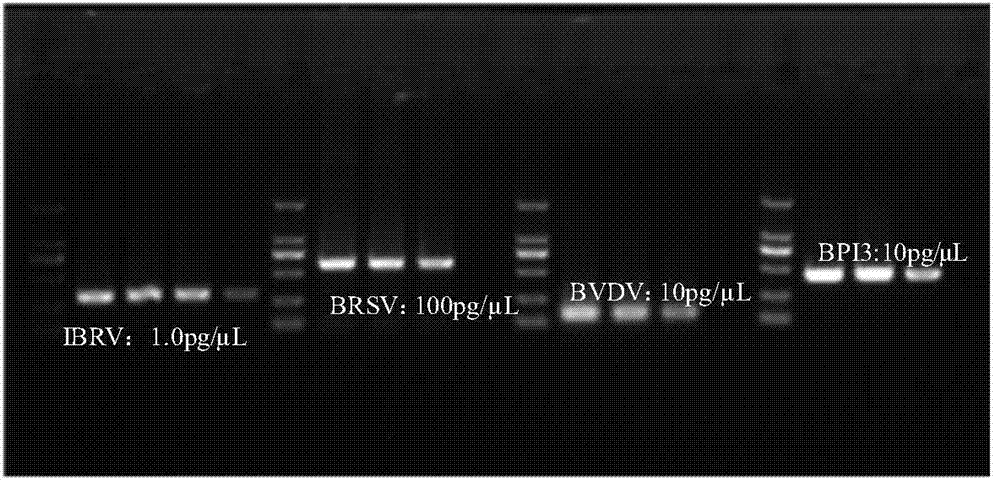
Multiplex-PCR (polymerase chain reaction) detection kit for bovine respiratory disease complex and preparation method thereof
InactiveCN103498009AAccurate detectionImprove featuresMicrobiological testing/measurementPositive controlBovine Viral Diarrhea Viruses
The invention discloses a multiplex-PCR (polymerase chain reaction) detection kit for bovine respiratory disease complex and a preparation method of the multiplex-PCR detection kit and relates to the field of detection of main viruses of bovine respiratory diseases. The problem that four pathogens of the bovine respiratory disease complex cannot be simultaneously and effectively detected in a multiplex-PCR method for detecting the bovine respiratory disease complex is solved. The kit comprises MightyAmp DNA polymerase, a 2xBuffe Mix buffer solution, sterile double distilled water, and four pairs of specific primers for identifying infectious bovine rhinotracheitis virus, bovine respiratory syncytial virus, bovine viral diarrhea virus and bovine parainfluenza 3 virus respectively, and also comprises positive control plasmids of the four viruses. The kit can simultaneously detect nucleic acids containing the four viruses in the same reaction system, is high in specificity and sensitivity and can accurately detect hosts and pathogenetic animals which suffer from invisible infection or continuously take viruses in the group of cattle, infectivity is avoided, the safety is high, the result can be detected in a short time, and time and labor are saved.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
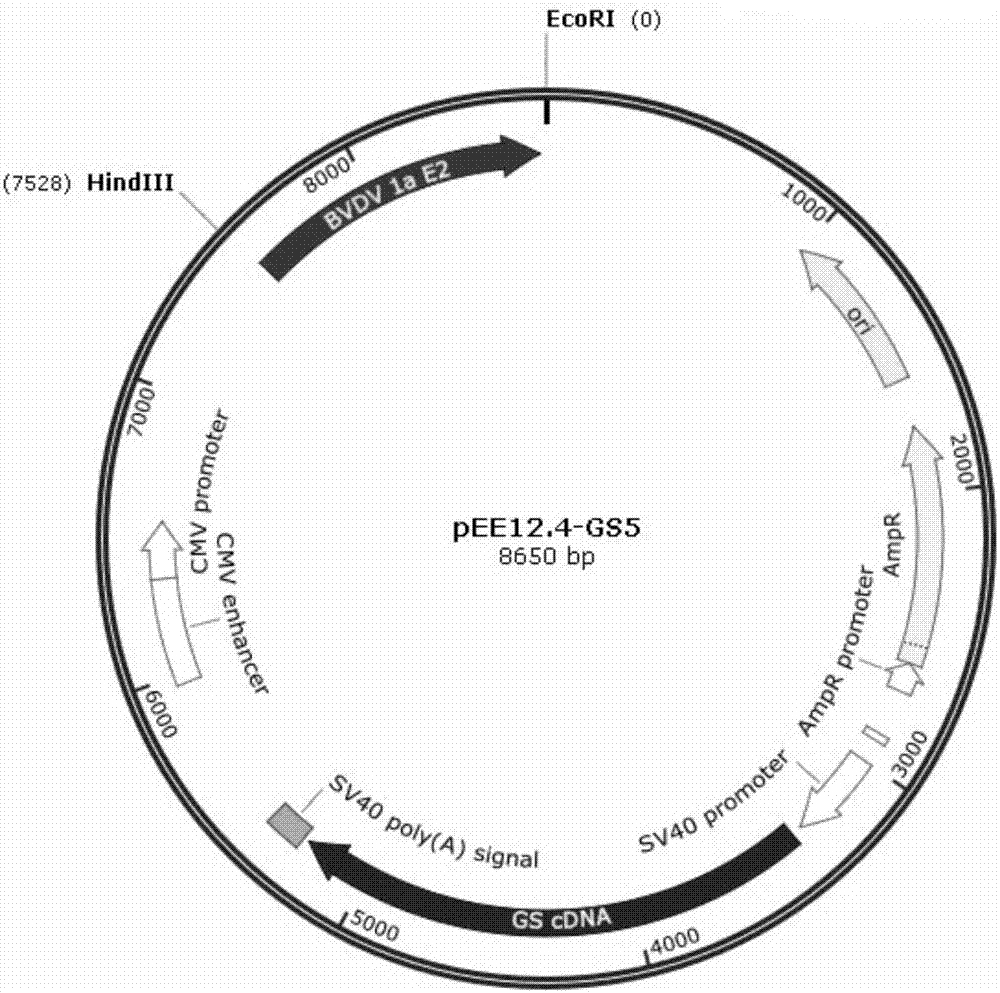
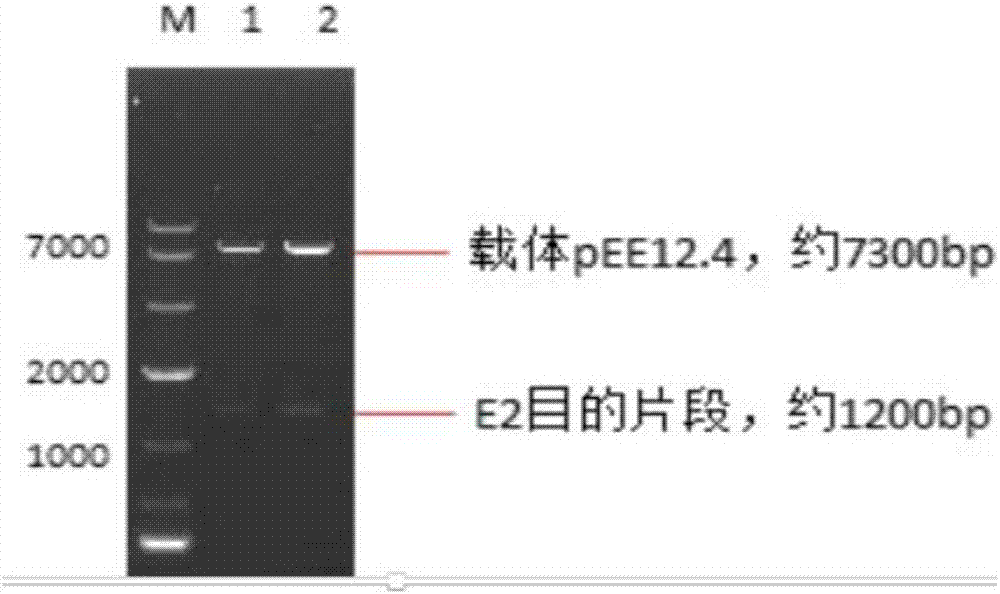
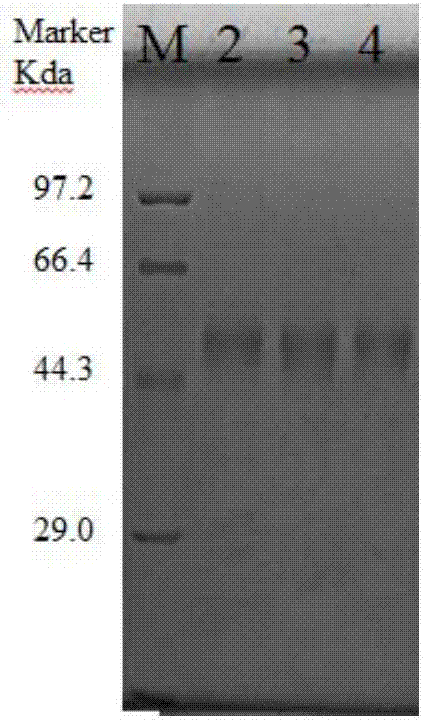
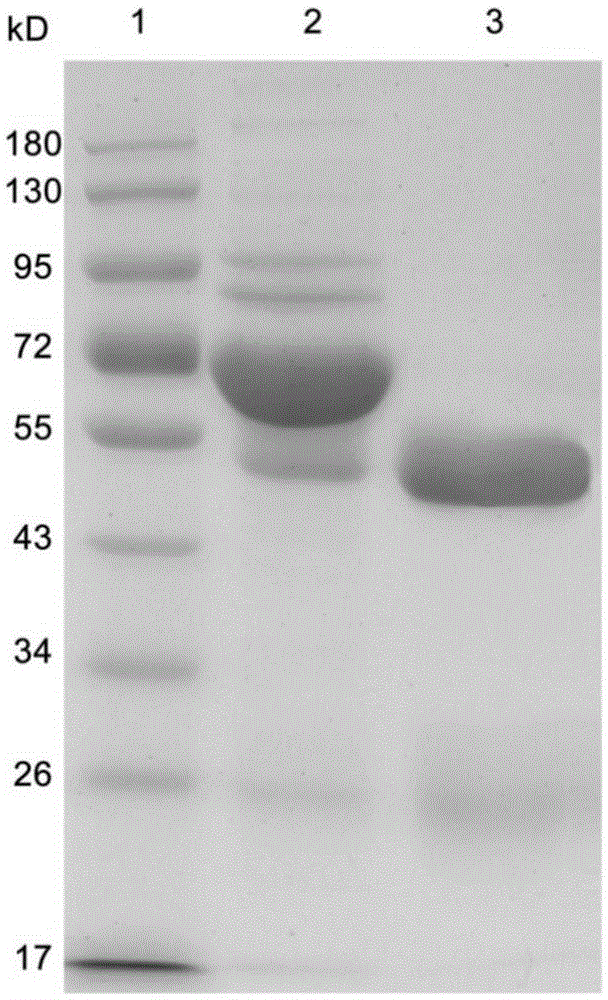
Preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and subunit vaccine and application
ActiveCN107973841AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetBovine Viral Diarrhea Viruses
The invention discloses preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and a subunit vaccine and applications and belongs to the technical fields of animal vaccines and veterinary biologicals. The object of the invention is to provide a preparation method capable of industrially producing the bovine viral diarrhea virus recombinant subunit vaccine ona large scale. The preparation method for the recombinant subunit vaccine, provided by the invention, comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein E2coding gene; 2) transfecting CHO cells, and performing selection, screening and acclimatizing to obtain suspending CHO cell strains, which stably and efficiently express the protein E2; 3) subjectingthe cell strains obtained in the step 2) to fermentation culture, and carrying out purification, so as to obtain recombinant protein E2; and 4) uniformly mixing the recombinant protein E2 and ISA 201VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches upto 500mg / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
Hog cholera virus truncated E2 protein and application of same
ActiveCN108107217APreserve antigenicityReduce manufacturing costBiological testingBovine Viral Diarrhea VirusesBiology
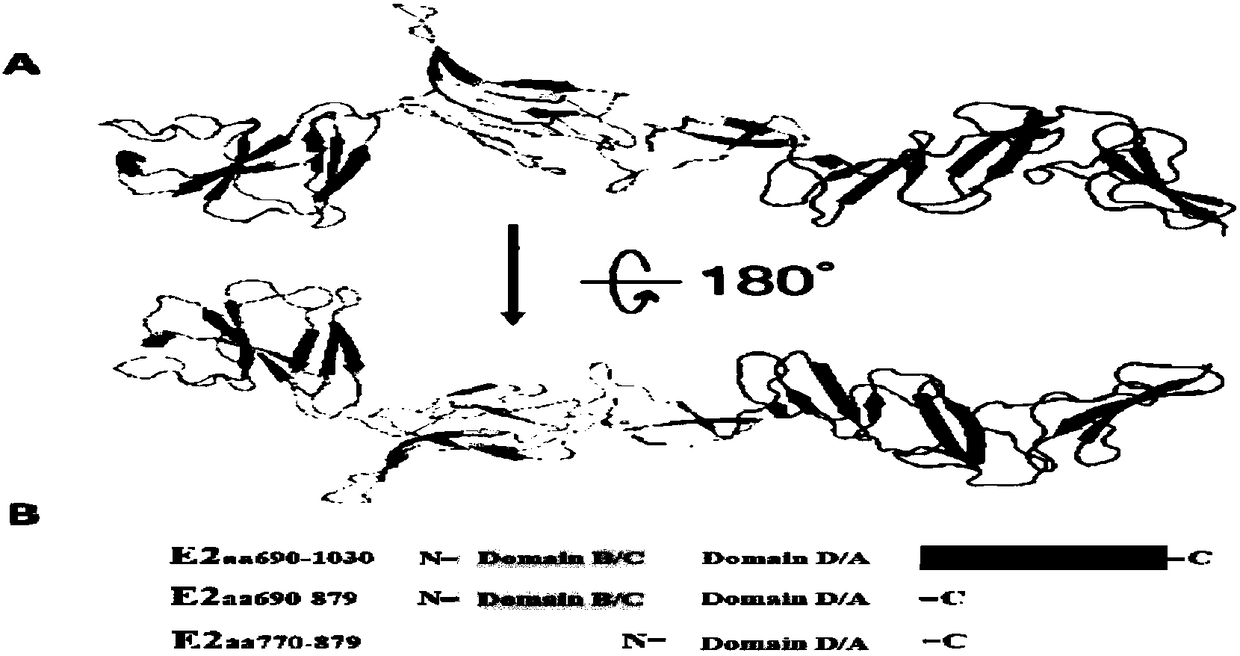
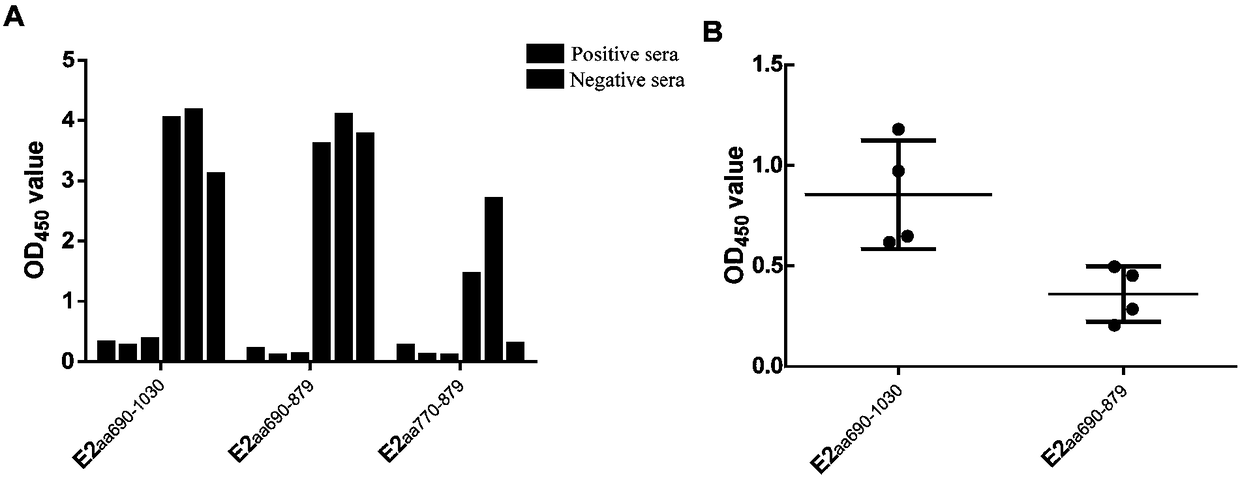
The invention discloses a hog cholera virus truncated E2 protein which is designed on the basis of protein spatial structure, and an application of the same. In the invention, according to the crystalstructure of bovine viral diarrhea virus E2 protein, the spatial structure of the hog cholera virus E2 protein is simulated, and then the hog cholera virus E2 protein is subjected to truncated expression, wherein the amino acid sequence of the truncated protein E2B / C / D / A is represented as the SEQ ID No.1. The truncated protein can maintain the complete antigenicity of the E2 protein, and has no cross reaction with a bovine viral diarrhea virus antibody. The invention further constructs a CHO cell line which stably expresses the truncated protein E2B / C / D / A and is assigned the accession numberof CGMCC No.14722. The invention also discloses an indirect ELISA kit which is used for detection of a hog cholera virus antibody, wherein the enveloped antigen is the hog cholera virus truncated protein E2B / C / D / A. The kit is used for specifically detecting the hog cholera virus antibody with high specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Primer, probe and kit for detecting bovine viral diarrhea virus
InactiveCN104894118AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationForward primerRNA extraction
The invention discloses a primer and a probe combination for detecting bovine viral diarrhea virus by a recombinase polymerase application (RPA) technology. The forward primer sequence is as shown in SEQ ID No.1; the reverse primer sequence is as shown in SEQ ID No.2; and the probe sequence is as shown in SEQ ID No.3. The invention also discloses a kit for bovine viral diarrhea virus detection. When the primer and the probe are used for detecting, a clinical sample only needs the treatment steps of virus RNA extraction, reverse transcription by a one-step method to form cDNA, and isothermal amplification, thermal cycle reaction is not needed, amplification does not need to be performed in a polymerase chain reaction (PCR) instrument, and the result can be displayed clearly on a lateral flow assay test strip, so that the primer and the probe have the advantages of high sensitivity, high specificity, simple reaction procedure, short detection time and the like, and are applicable to quick, accurate, simple and convenient diagnosis and treatment on the diseases in cattle farms.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
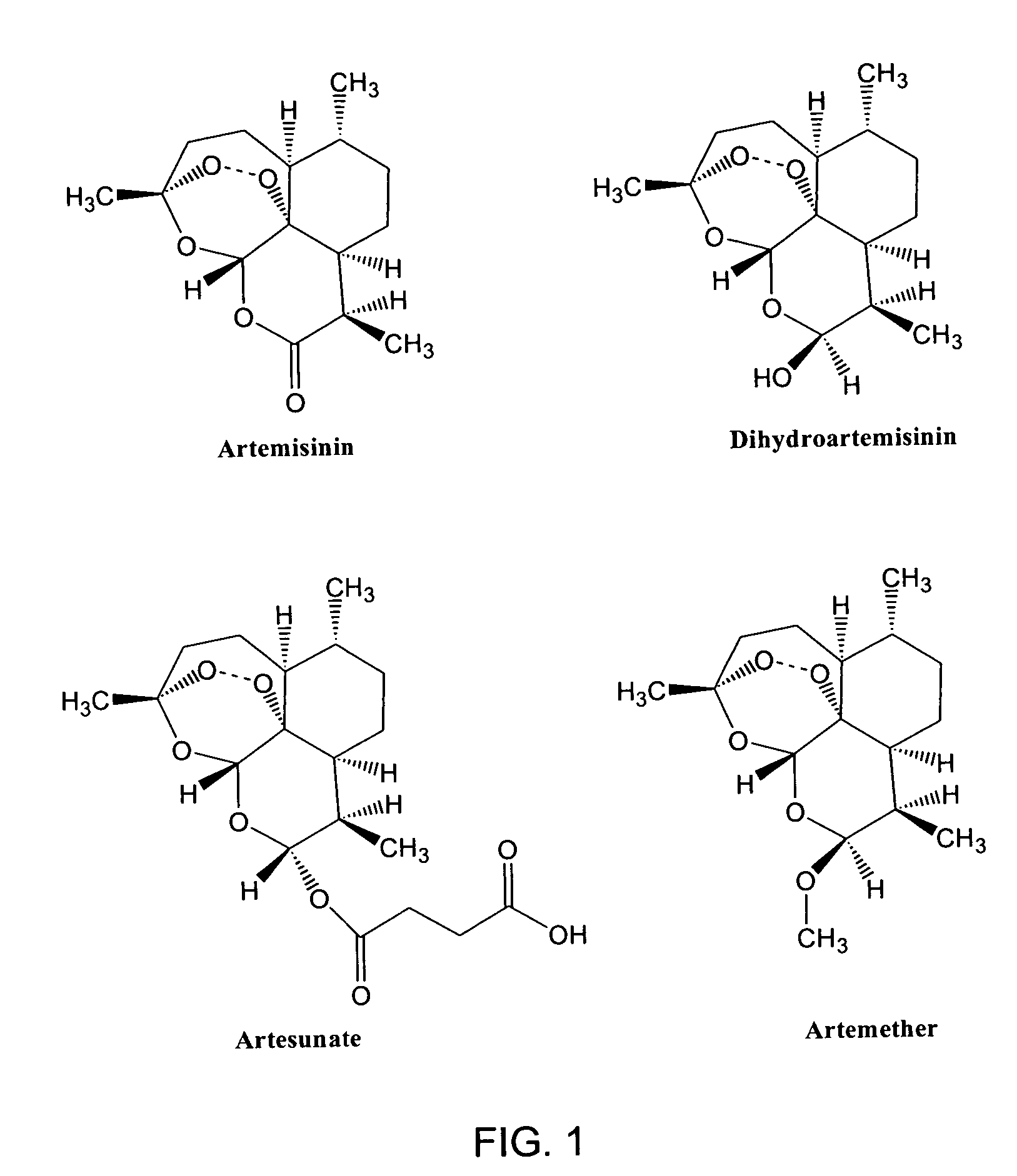
Use of endoperoxides for the treatment of infections caused by flaviviridae, including hepatitis C, bovine viral diarrhea and classical swine fever virus
InactiveUS20050059647A1Useful in treatmentBiocidePeptide/protein ingredientsAbnormal tissue growthBovine Viral Diarrhea Viruses
The use of sesquiterpenes and, in particular sesquiterpene lactone endoperoxides, such as artemisinin and analogs thereof, for the treatment of hepatitis C virus infections. Artemisinin, analogs of artemsisnin and some crude Artemisia extracts were tested in vitro against DNA-viruses, retro-viruses and Flavivirida, (an important family of human and animal RNA pathogens). These compounds were also screened for anti-tumor activity. Strong activity of artemisinin was noticed against the bovine viral diarrhea virus (BVDV). As pestiviruses, such as BVDV, share many similarities with hepatitis C virus (HCV), we can conclude that endoperoxides in general and artemisinin more specificly have efficacy as treatments for hepatitis C viral infections.
Owner:KEMIN FOODS L C
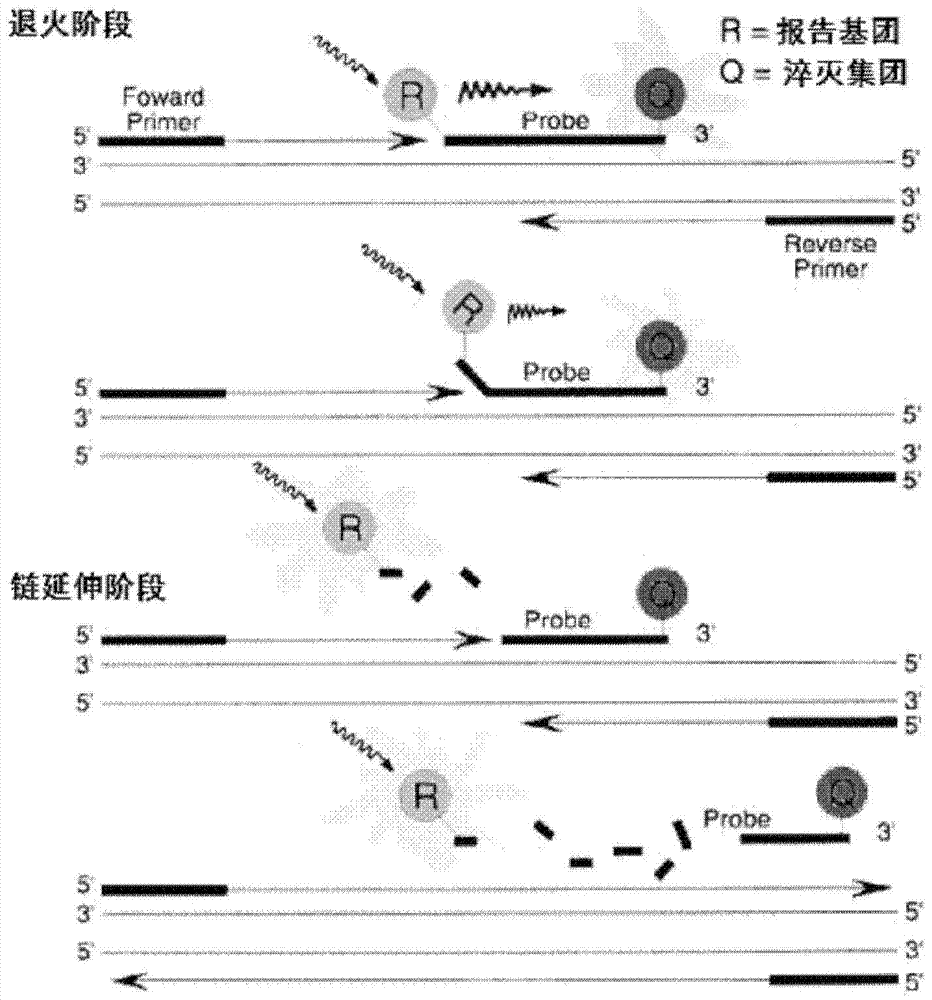
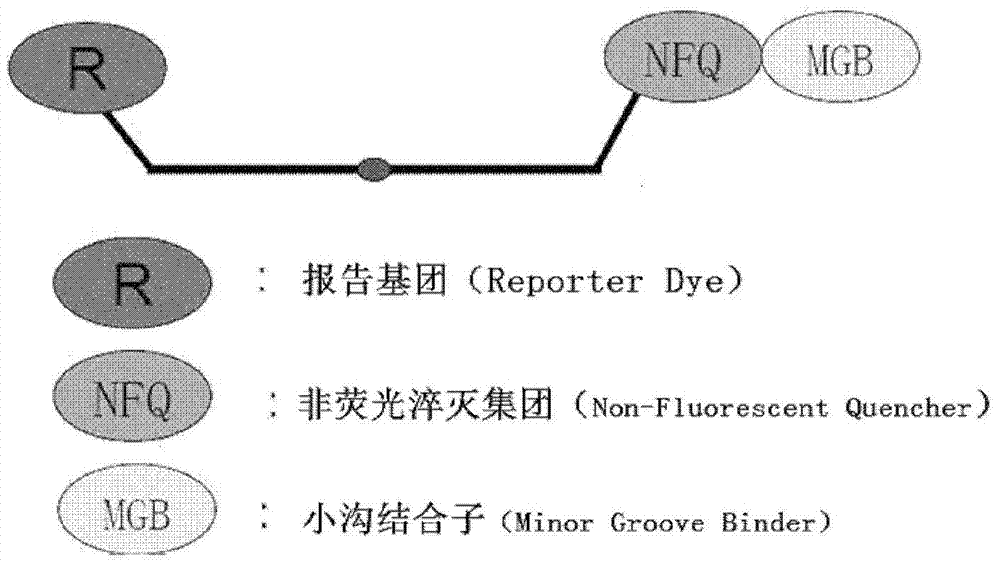
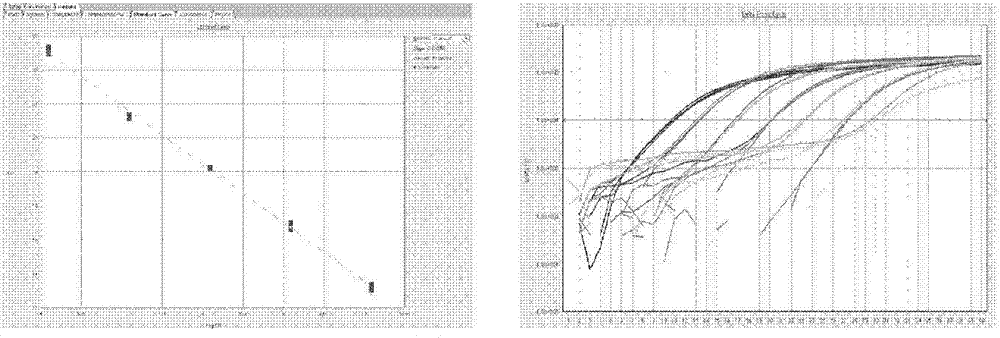
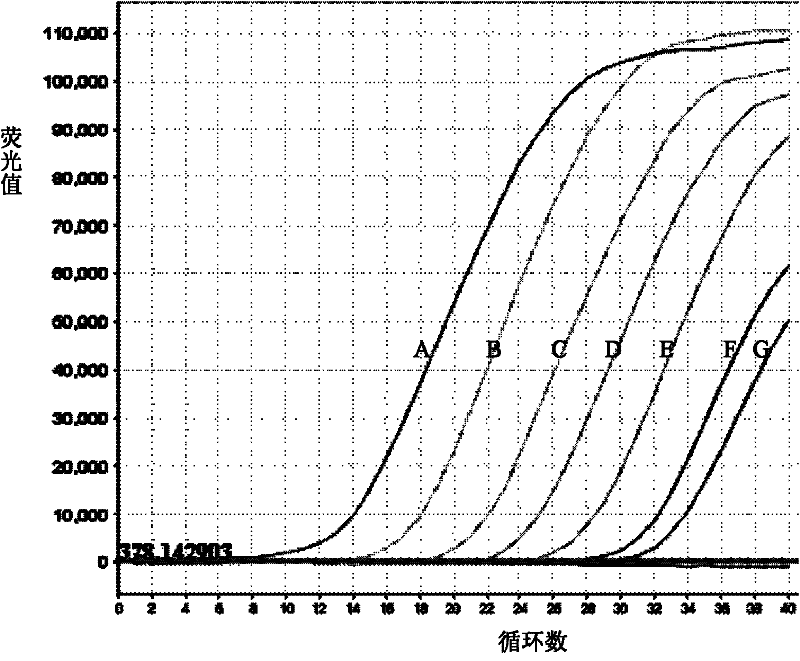
Bovine viral diarrhea virus (BVDV) fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) detection kit
ActiveCN103397107AMicrobiological testing/measurementBovine Viral Diarrhea VirusesReverse transcriptase
The invention relates to a bovine viral diarrhea virus (BVDV) fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) detection kit. According to 21 BVDV-I genome complete sequences, 6 BVDV-II genome complete sequences and 34 CSFV (classical swine fever virus) full-length sequences which are published by GeneBank, one pair of degenerate primers specific to BVDV and one probe are designed in a conserved region of BVDV by utilizing software such as DANMAN and Primer Express3.0. By optimizing reverse transcriptase concentration, DNA (deoxyribonucleic acid) polymerase concentration, upstream and downstream primer concentration and probe concentration, the BVDV fluorescent quantitative RT-PCR detection kit is invented. The BVDV fluorescent quantitative RT-PCR detection kit can specifically detect all the strains of BVDV and has no cross reaction with other pathogens; the BVDV fluorescent quantitative RT-PCR detection kit has good sensitivity which is one order of magnitude higher than that of the conventional RT-PCR; tests on repeatability and stability of 20 quality control samples show that the BVDV fluorescent quantitative RT-PCR detection kit has good repeatability and stability; detection results of 94 clinical samples show that coincidence rate of the method and a BVDV RT-PCR detection kit is 97.9%.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting five cow disease viruses
ActiveCN105002301AGuaranteed sensitivityEnable multiple detectionMicrobiological testing/measurementMicroorganism based processesLeucosisBovine Viral Diarrhea Viruses
The invention discloses a multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses. The multiple-connection probe is shown in sequence tables from SEQ ID NO:1 to SEQ ID NO:10. The primer is shown in sequence tables from SEQ ID NO:11 to SEQ ID NO:12. The primer, the probe and / or the multiple-connection probe amplification detection kit including the primer and the probe can detect the five vital cow disease pathogenies including the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses at the same time, the detection time and cost are saved, and epidemic diseases can be diagnosed in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Dual RT-PCR method and special reagent case for checking cattle rotavirus and cattle virus diarrhea virus simultaneously
InactiveCN101063176ARapid diagnosisImprove featuresMicrobiological testing/measurementBovine Viral Diarrhea VirusesRotavirus RNA
Owner:SHANDONG OX LIVESTOCK BREEDING CO LTD
Bovine viral diarrhea virus strain and application thereof
ActiveCN105296441AIncreased sensitivityImprove convenienceImmunoglobulins against virusesAntiviralsMicroorganismBovine Viral Diarrhea Viruses
The invention relates to a bovine viral diarrhea virus BJ1305 strain, which is named as bovine viral diarrhea virus in a classifying manner, the preservation number is CGMCC NO.11185, the preservation date is September 7, 2015, and the preservation authority is General Microbiology Center of China Committee for Culture Collection of Microorganisms. The strain provided by the invention has the characteristics of being new in a region and epidemic, the immunogenicity is strong, and due to the fact that the train is separated from a Beijing area, products developed by utilizing the strain can specifically aim at prevalent diseases in the Beijing area, which is caused by the bovine viral diarrhea virus.
Owner:CHINA AGRI UNIV
Application of andrographolide C15 substitution derivative in manufacturing anti-hepatitis drug
ActiveCN102600129ATo clarify the anti-BVDV activity in vitroOrganic active ingredientsDigestive systemBovine Viral Diarrhea VirusesCytopathic effect
The invention discloses the application of an andrographolide C15 substitution derivative in manufacturing an anti-hepatitis drug, which belongs to the technical field of pharmaceutical chemistry. According to the invention, MDBK (mardin-darby bovine kidney cells) and BVDV (bovine viral diarrhea virus) are utilized to study the inhibitory action of the compound on cytopathic effect caused by the virus through an external screening model. Through the screening of a large number of andrographolide derivatives, the compound with a structure of general formula 1 is found to significantly inhibit MDBK cytopathic effect caused by the BVDV; the andrographolide C15 substitution derivative has high efficiency and low toxicity, thereby being used for manufacturing medicaments for treating and preventing hepatitis and having good prospect in development and application. The general formula 1 is shown in the description.
Owner:ZHENGZHOU UNIV
BVDV (bovine viral diarrhea virus) internal control typing fluorescent PCR (polymerase chain reaction) detection kit and preparation thereof
InactiveCN103725796AShort detection timeShorten detection timeMicrobiological testing/measurementFluorescence/phosphorescenceBovine Viral Diarrhea VirusesComplementary deoxyribonucleic acid
The invention discloses a BVDV (bovine viral diarrhea virus) internal control typing fluorescent PCR (polymerase chain reaction) detection kit and a preparation thereof. By means of the primer design, a BVDV I, a BVDV II and a PCR template for monitoring internal control are obtained through single PCR amplification, a BVDV internal control typing fluorescent PCR detection system is established through the primer design, PCR amplification and optimization of reaction conditions, the BVDV I and the BVDV II can be independently detected or synchronously detected, and quality monitoring can be performed on the detection result. The prepared kit comprises two parts of a cDNA (complementary deoxyribonucleic acid) synthesis system and an SYBR fluorescent PCR amplification system. The minimum detection limit of the kit to three types of genes is 102 copy, the kit has better specificity and repeatability, the detection quality is ensured, and the kit has a better application value.
Owner:XINJIANG AGRI UNIV +1
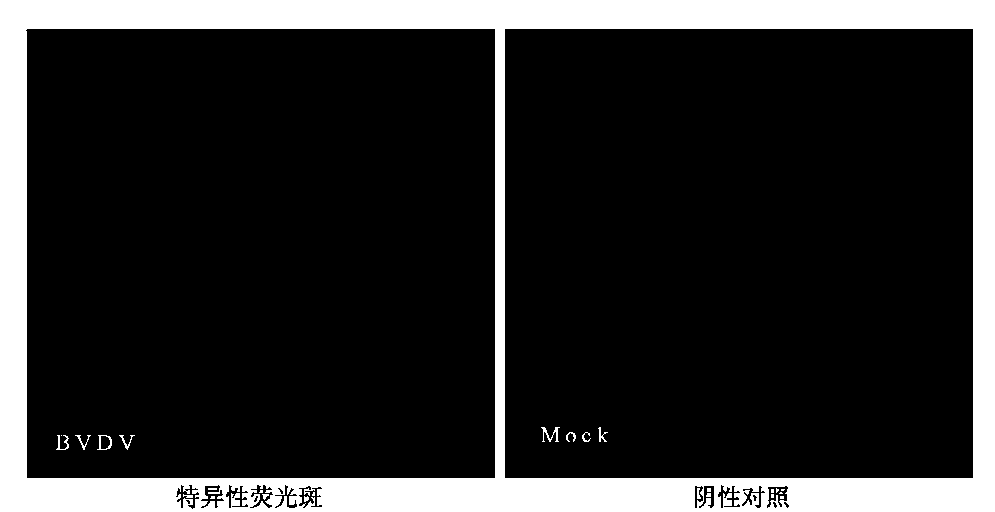
Method for detecting bovine viral diarrhea virus by virtue of indirect immunofluorescence
Owner:成都史纪生物制药有限公司
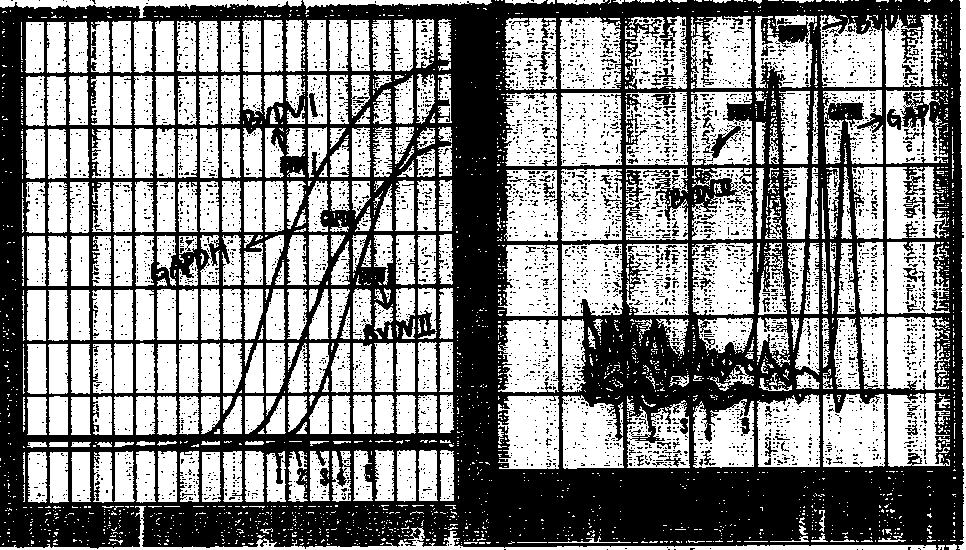
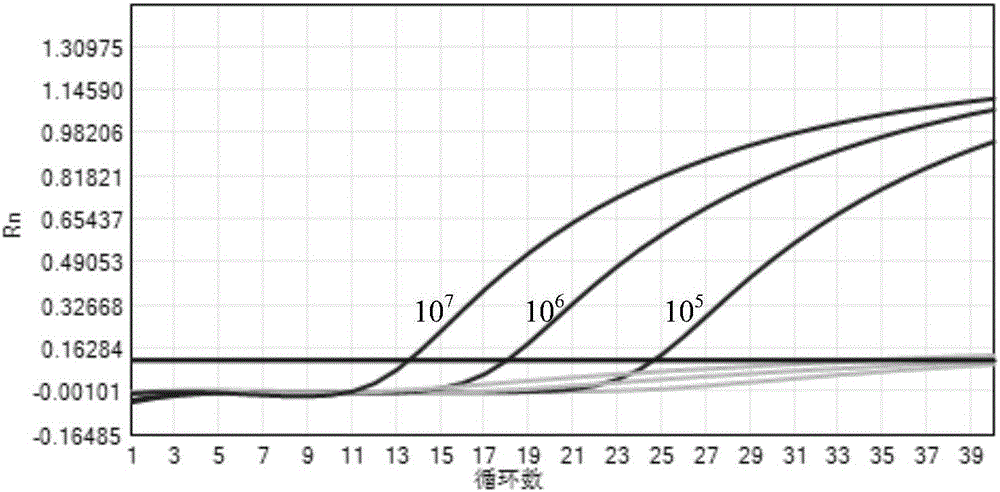
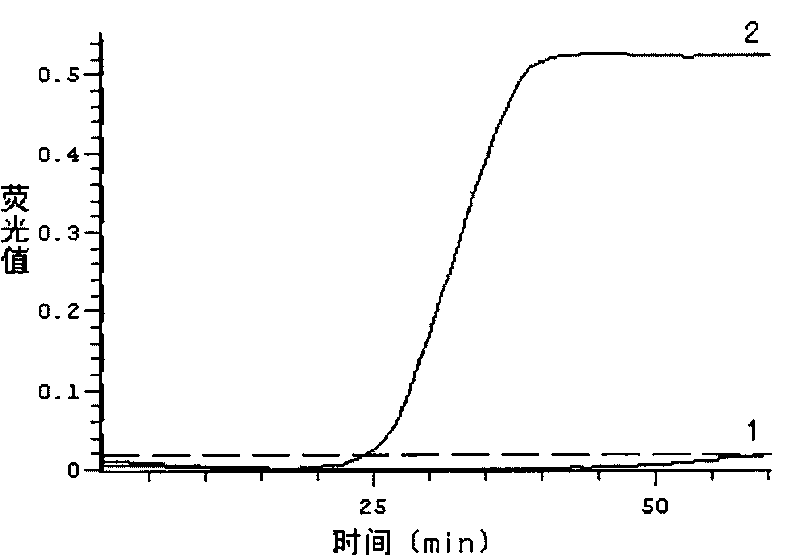
Fluorescent quantitative rt-pcr detection kit for detecting bovine viral diarrhea virus and its application
ActiveCN102268488AImprove accuracyStrong specificityMicrobiological testing/measurementMicroorganism based processesBovine Viral Diarrhea VirusesFluorescence
The invention provides a fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR) detection kit for detecting bovine viral diarrhea virus (BVDV) and application of the kit. The kit is used for the field of clinic and scientific research, including quick quantitative detection of BVDV infection and BVDV pollution monitoring of biological products such as bovine serum, bovine serum albumin and bovine testicle cell derived hog cholera lapinized virus vaccines and the like. The invention also provides a non-diagnostic fluorescence quantitative RT-PCR method for detectingBVDV infection.
Owner:WUHAN CHOPPER BIOLOGY
Infectious bovine viral diarrhea virus
InactiveUS20070015203A1SsRNA viruses positive-senseMicrobiological testing/measurementBovine Viral Diarrhea VirusesAnimal health
The invention belongs to the field of animal health and in particular Bovine Viral Diarrhea Virus (BVDV). The invention provides infectious BVDV clones and methods to produce said BVDV clones. The invention further relates to methods of attenuating said clones, attenuated BVDV clones and vaccines comprising said attenuated clones.
Owner:ELBERS KNUT +3
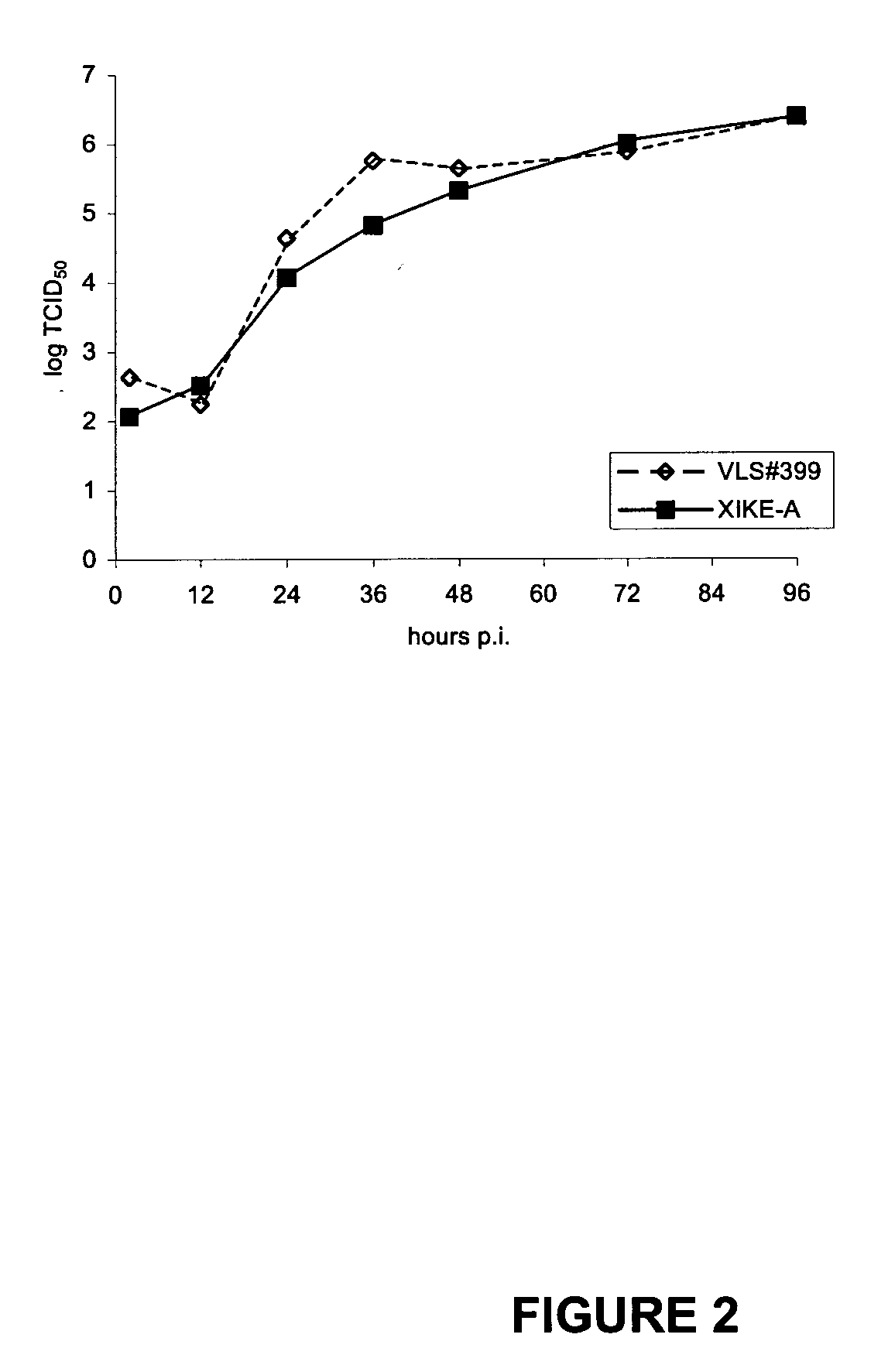
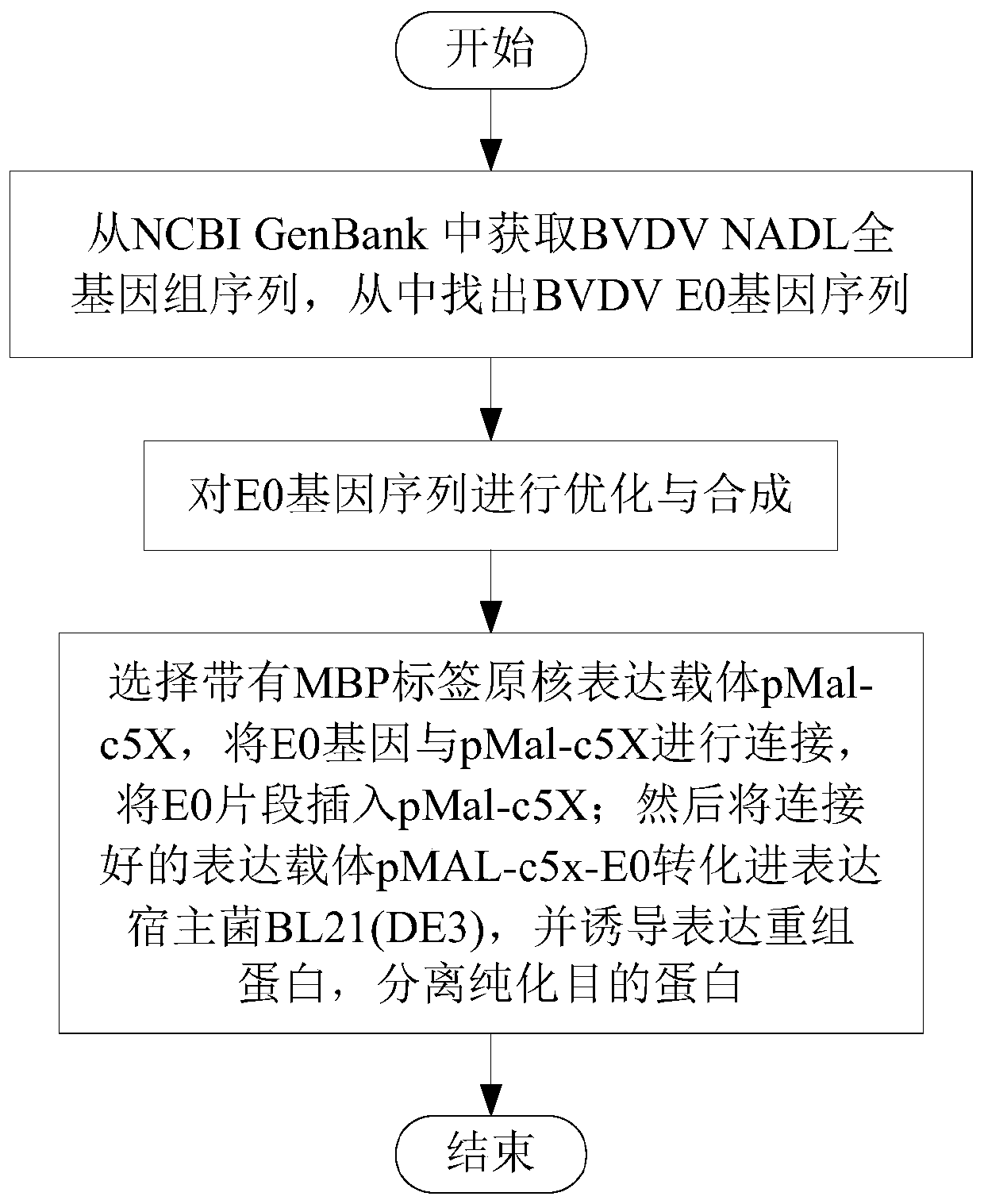
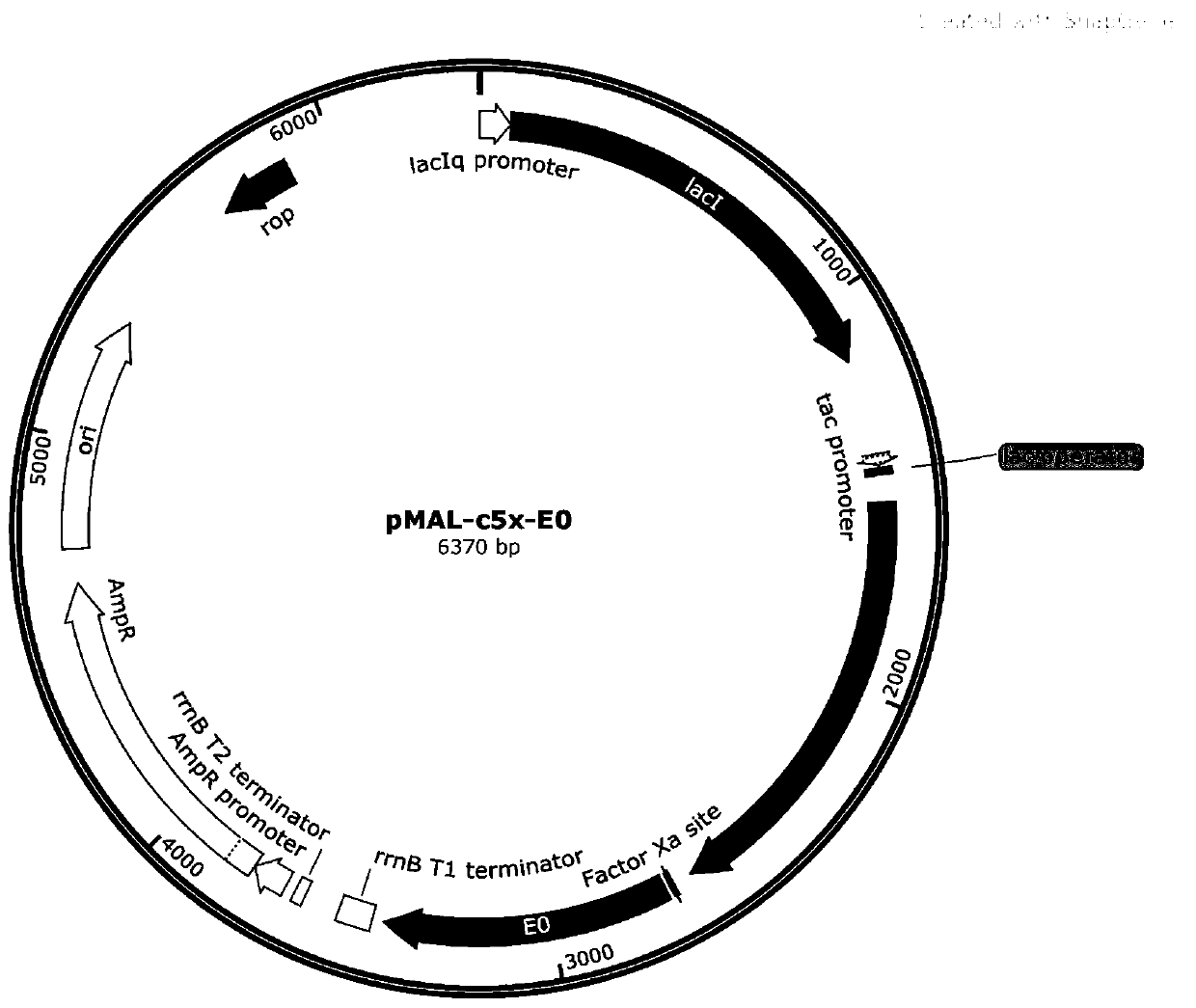
Bovine viral diarrhea virus E0 protein amino acid and preparation method thereof
ActiveCN111057132AEasy to purifyImprove solubilitySsRNA viruses positive-senseVirus peptidesBovine Viral Diarrhea VirusesTarget gene
The invention provides a bovine viral diarrhea virus E0 protein amino acid and a preparation method thereof. The amino acid sequence of the bovine viral diarrhea virus E0 protein amino acid is represented by SEQ ID NO:3. The preparation method comprises the following steps:S1, obtaining a BVDV NADL whole genome sequence from NCBI GenBank, and finding out a BVDV E0 gene sequence from the BVDV NADLwhole genome sequence; S2, optimizing and synthesizing the E0 gene sequence obtained in step S1; and S3, selecting a prokaryotic expression vector pMal-c5X with an MBP label, connecting the E0 gene with the pMal-c5X, inserting an E0 fragment into the pMal-c5X, converting the connected expression vector pMAL-c5x-E0 into an expression host bacterium BL21 (DE3), inducing the expression of the obtained recombinant protein, and separating and purifying the target protein. The target gene E0 is optimized, and is subjected to fusion expression with the MBP tag protein to finally achieve the efficientsoluble expression of the E0 protein, so the bovine viral diarrhea virus E0 protein amino acid has the advantages of high expression yield, good solubility, and convenience in expression protein purification; and the solubility of the Escherichia coli expression protein can be improved during the fusion expression of the foreign protein and the MBP so as to provide convenience for the purification of the expression protein.
Owner:SICHUAN AGRI UNIV
Kit, primer and probe for simultaneously detecting bovine viral diarrhea virus, bovine rotavirus and bovine coronavirus
ActiveCN112094953ASimple and fast operationStrong specificityMicrobiological testing/measurementMicroorganism based processesBovine rotavirusBovine Viral Diarrhea Viruses
The invention relates to the field of virus detection, and particularly relates to a kit, a primer and a probe for simultaneously detecting bovine rotavirus, bovine rotavirus and bovine coronavirus. The nucleotide sequences of the primer and the probe in the kit are shown as SED ID NO: 1-SEQ ID NO: 9. The kit has the technical advantages of being easy and convenient to operate, high in specificity, high in sensitivity, good in repeatability and capable of simultaneously achieving qualitative detection and accurate quantification of BVDV, BRV and BCoV.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Detection method for infectious bovine viral diarrhea virus in aerosol
ActiveCN103981285AReal-time monitoring of epidemicsReal-time monitoring of disseminationMicrobiological testing/measurementDNA/RNA fragmentationBovine Viral Diarrhea VirusesVirus
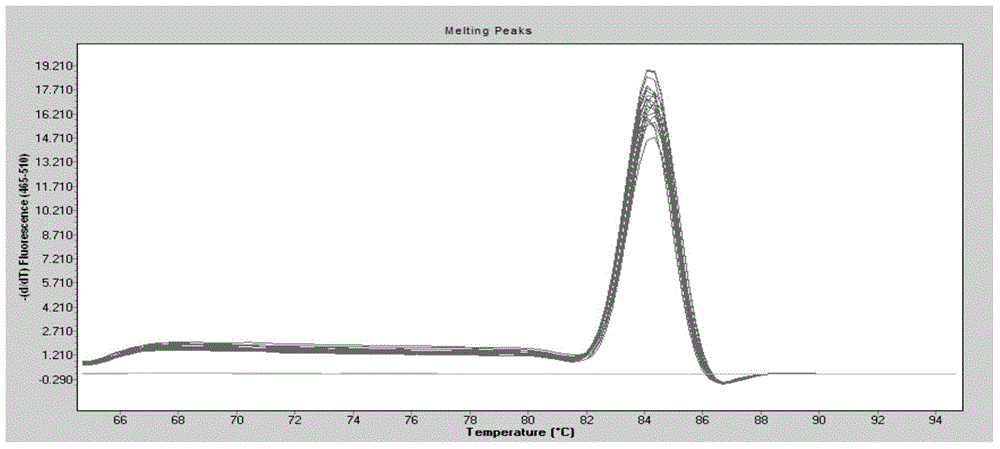
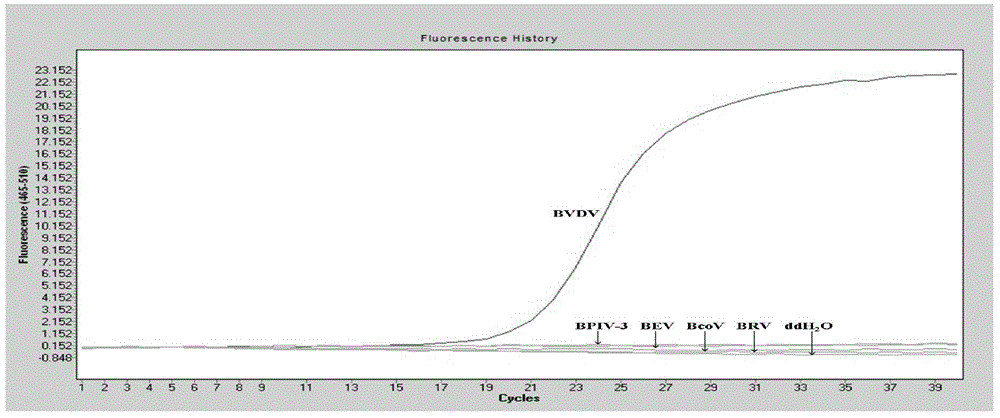
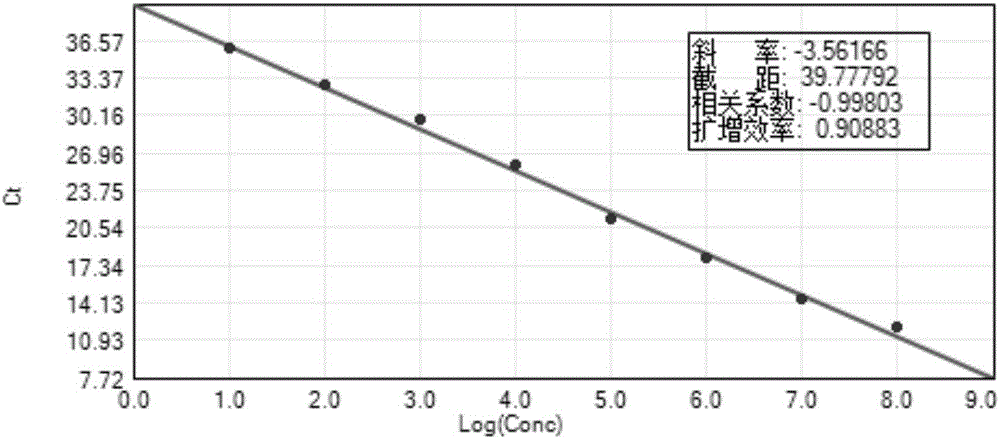
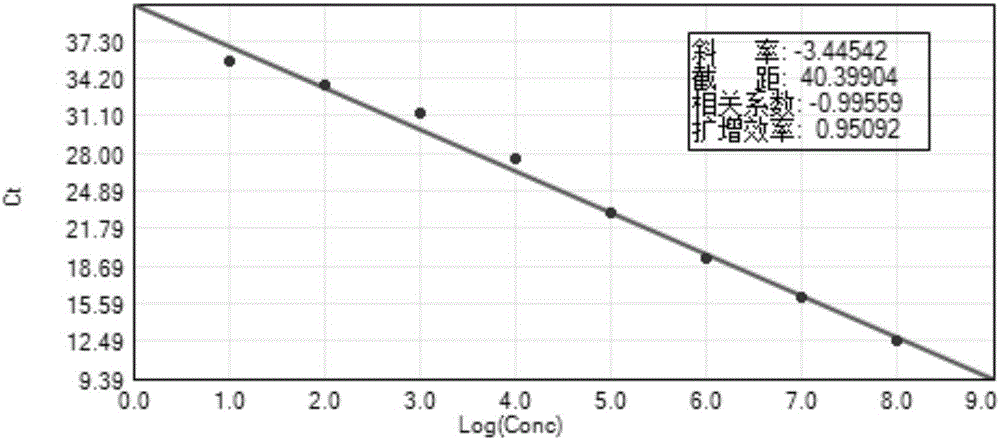
The invention discloses a detection method for the bovine viral diarrhea virus in an aerosol. The method comprises the following steps: (1) acquisition of an aerosol sample; (2) extraction of RNA and cDNA of the virus in the aerosol sample; (3) detection: a step of carrying out PCR amplification; (4) establishment of a standard curve and a melting curve: a step of establishing the standard curve of positive standard plasmid and the melting curve of an amplification system; and (5) judgment: a step of judging whether the aerosol sample contains the bovine viral diarrhea virus. The invention further discloses specific primers (as shown in SEQ ID No. 3 to 8) and a kit (composed of the specific primers, the bovine viral diarrhea virus positive standard plasmid pEASY-T3-B1, a SYBRGreenI real-time fluorescent quantitative PCR reagent and ddH2O). The method provided by the invention is applicable to typing detection of a bovine viral diarrhea virus aerosol sample and to detection of samples like clinical blood, milk and tissue and has wide application prospects.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Dual real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting and identifying wild strain and vaccine strain of CSFV (classical swine fever virus) in swine umbilical cord blood and application of dual real-time fluorescence RT-PCR kit
InactiveCN106435033ANo cross contaminationEfficient detectionMicrobiological testing/measurementBorder disease virusBovine Viral Diarrhea Viruses
The invention discloses a dual real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting and identifying a wild strain and a vaccine strain of a CSFV (classical swine fever virus) in swine umbilical cord blood and an application of the dual real-time fluorescence RT-PCR kit. The kit comprises a pair of primers and two fluorescent probes, wherein the sequences of the pair of primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequences of the two fluorescent probes are shown as SEQ ID NO.3 and SEQ ID NO.4. The kit has the advantages of high specificity, sensitivity and accuracy and excellent repeatability, meanwhile, the wild strain and the vaccine strain of the CSFV can be identified and detected by detecting the same sample once, and the problem of genetic crossover of the CSFV with the bovine viral diarrhea virus and the border disease virus which belong to the same genus can be solved. The kit is applicable to fast identification and detection of the wild strain and the vaccine strain of the CSFV in scientific research and clinical detection, can be used for precisely evaluating and diagnosing CSFV carrying and expelling conditions of sows and latent infection conditions of piglets and can be further used for evaluating effects of CSFV vaccines.
Owner:HUNAN XINNANFANG CULTURE SERVICE CO LTD
Cattle reproductive disease vaccines
InactiveUS20070298053A1Avoid infectionAntibacterial agentsSsRNA viruses negative-senseBovine Viral Diarrhea VirusesVirus type
The present invention relates to combination vaccines and methods for treating or preventing diseases or disorders in an animal caused by infection by Bovine Viral Diarrhea Virus (BVDV) Types 1 and 2, Bovine Herpes Virus Type-1 (BHV-1), Bovine Respiratory Syncytial Virus (BRSV), Parainfluenza Virus (PI3), Campylobacter fetus, Leptospira canicola, Leptospira grippotyphosa, Leptospira hardj-prajitno, Leptospira icterohaemmorrhagiae, Leptospira hardjo-bovis and Leptospira pomona by administering to the animal an effective amount of a combination vaccine. The combination vaccine can be a whole or partial cell inactivated or modified live preparation.
Owner:PFIZER INC
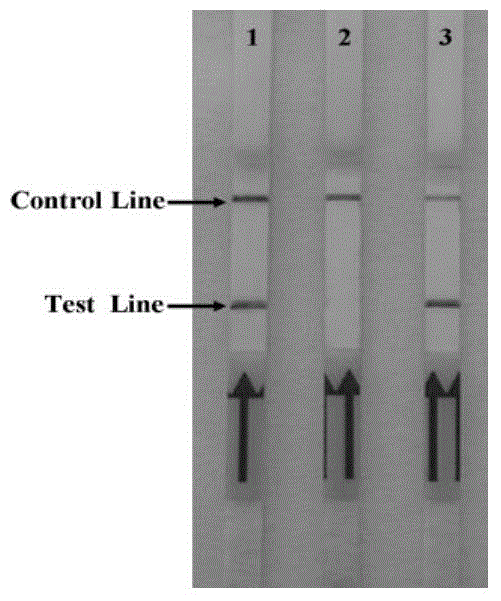
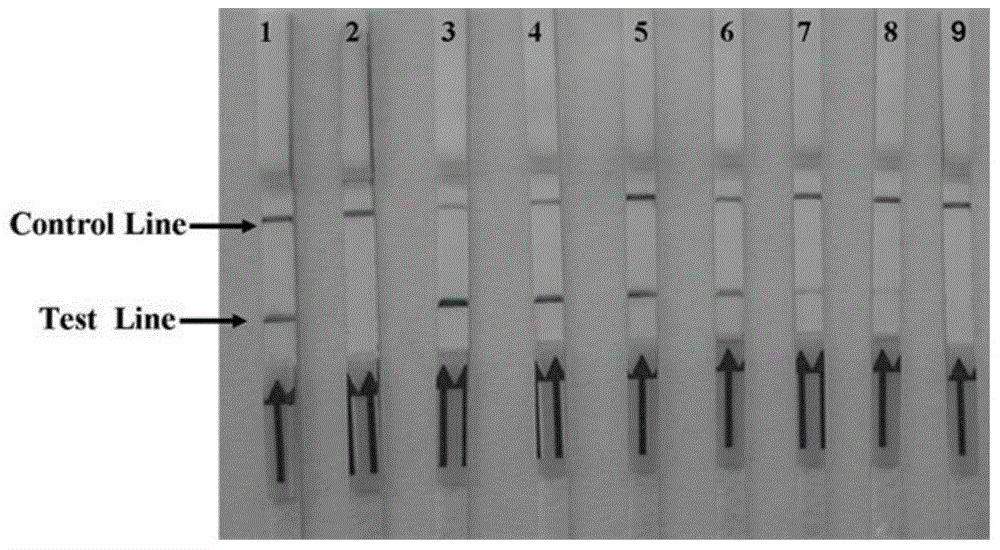
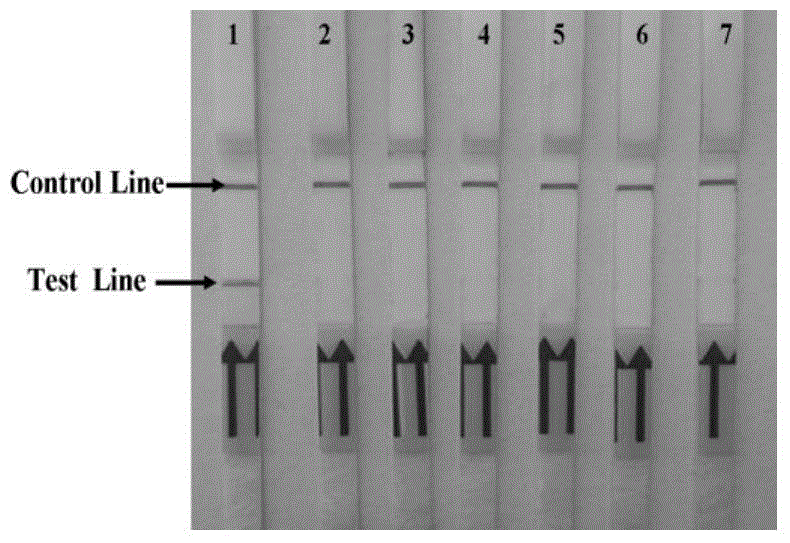
Rapid detection test strip for BVDV (bovine viral diarrhea virus) antibody and preparation method thereof
InactiveCN103197082AQuick checkDetection securityBiological testingBovine Viral Diarrhea VirusesNucleotide
The invention discloses a recombinant antigen for BVDV (bovine viral diarrhea virus) antibody detection and a rapid detection test strip thereof, mainly aiming at a truncated BVDV NS expression antigen for BVDV antibody detection and a rapid detection test strip for detecting BVDV antibodies. A BVDV NS protein amino acid sequence is analyzed by utilizing epitope analysis software; a fragment with the main epitope is taken; a truncated BVDV NS recombinant antigen is obtained according to the corresponding nucleotide sequence design primer through PCR (polymerase chain reaction) amplification, recombination expression carrier construction and prokaryotic expression, and can be used for BVDV antibody detection after being purified. The rapid detection test strip established based on the truncated BVDV NS antigen has the advantages of convenience in operation, rapid detection, no requirement of a special laboratory and equipment and the like, overcomes the limits of the existing detection method, and can be used for rapid detection and serum epidemiological investigation of the BVDV antibodies.
Owner:SHENZHEN AUDAQUE DATA TECH
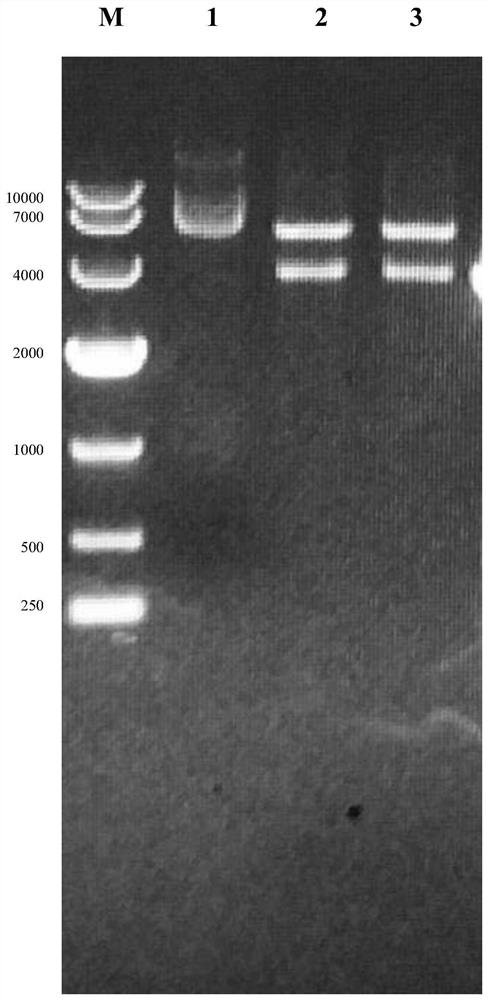
Bovine viral diarrhea virus-like particle and construction method and application thereof
ActiveCN109021081ASafe generationImprove securitySsRNA viruses positive-senseViral antigen ingredientsBaculovirus expressionBovine Viral Diarrhea Viruses
The invention relates to a bovine viral diarrhea virus-like particle and a construction method and application thereof. According to the invention, the bovine viral diarrhea virus C-E0-E1-E2-P7 gene is firstly connected with a baculovirus expression vector, and then a baculovirus expression system is used to safely and efficiently produce bovine viral diarrhea virus-like particles in insect cell bioreactors. The prepared bovine viral diarrhea virus-like particles are similar in morphology to the real virus particles. Compared with the inactivated virus, the virus-like particle immunization caneffectively induce the cellular immune response without the risk of virulence recovery of the attenuated vaccine. Compared with other vaccines, the virus-like particles can more realistically displaythe viral antigen conformation, are easy to recognize by the immune system, and efficiently induce the body to produce neutralizing antibodies, thus filling the blank of such vaccines in China. The preparation method of the invention is suitable for industrial production, and has the advantages of low cost, high safety and the like.
Owner:SHANDONG UNIV
Expression and application of bovine viral diarrhea virus (BVDV) type I E2 protein
InactiveCN105949286AStrong specificityIncreased sensitivityPolypeptide with localisation/targeting motifSsRNA viruses positive-sensePurification methodsBovine Viral Diarrhea Viruses
The invention relates to expression and application of bovine viral diarrhea virus (BVDV) type I E2 protein. The expression and the application have the advantages that (1) an expression and purification method for efficiently obtaining BVDV E2 protein is established and can be used for preparing the pure BVDV E2 protein; (2) a rabbit is immunized by the BVDV E2 protein to prepare an antibody; compared with an antibody prepared by whole-virus immune cattle, the antibody has good specificity and shallow background and is easy to determine; a secondary antibody is marked by FITC (Fluorescein Isothiocyanate) and an indirect fluorescence method is established, so that the sensitivity is better; the operation is simple: a pre-mixed solution is adopted so that operation time is shortened; (3) the pure BVDV E2 protein is used for covering, sealing conditions, blood serum and antibody preserving fluid are optimized, and a BVDV antibody indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) method is established; a BVDV antibody indirect ELISA kit, which is sensitive and low in price, is assembled and a situation of dependence on imported BVDV antibody ELISA kits for a long period can be changed.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method for detecting bovine viral diarrhea viruses as well as preparation method and use method of reverse transcription loop-mediated isothermal amplification (RT-LAMP) reaction kit
InactiveCN101696453AMeet the needs of on-site quarantineEasy to prepareMicrobiological testing/measurementMicroorganism based processesRNA extractionBovine Viral Diarrhea Viruses
The invention provides a method for detecting bovine viral diarrhea viruses as well as a preparation method and a use method of a reverse transcription loop-mediated isothermal amplification (RT-LAMP) reaction kit, relating to a preparation method and a use method of a reaction kit. The invention solves the problems that the traditional method for detecting bovine viral diarrhea viruses has expensive cost, needs professional equipment and professional staff to participate in detection, and is not suitable for the development state of the animal husbandry at the present stage of China. The preparation method comprises the following steps: firstly, designing a primer, preparing RT-LAMP reaction liquid, and then, finishing the preparation. The use method comprises the following steps: adding RNA extract containing bovine viral diarrhea virus samples into the reaction liquid, ungergoing RT-LAMP reaction after mixing, and then, centrifugally detecting; or adding 10,000*SYBR GREEN I, and putting below an ultraviolet lamp for detecting; or carrying out electrophoresis detection; or carrying out insulation reaction detection by adopting a Real-time PCR instrument. The reaction kit has convenient detection, does not need expensive instruments and reduces the cost.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
CRISPR-Cas13 a-based bovine viral diarrhea virus detection method
ActiveCN111979357AQuick checkStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBovine Viral Diarrhea VirusesNucleic acid detection
The invention discloses a primer for detecting bovine viral diarrhea virus, a kit prepared by using the primer and used for detecting BVDV, and a CRISPR-Cas13 a-based bovine viral diarrhea virus detection method. The primer is 5'-GGGGAUUUAGACUACCCCAAAAACGAAGGGGACUAAAACGCCAUCCAACGAACUCACCACUGUUGCU-3'; the kit at least comprises the primer; a sample to be detected is added into the kit for detection. According to the CRISPR-Cas13 a-based bovine viral diarrhea virus detection method, a CRISPR-Cas13a system is successfully applied to nucleic acid detection of the BVDV for the first time, and whether the BVDV is contained or not can be identified only by adding a micro-scale sample. The CRISPR-Cas13 a-based bovine viral diarrhea virus detection method has high specificity and sensitivity. The method provides a wide prospect for early diagnosis of the BVDV.
Owner:SHIHEZI UNIVERSITY
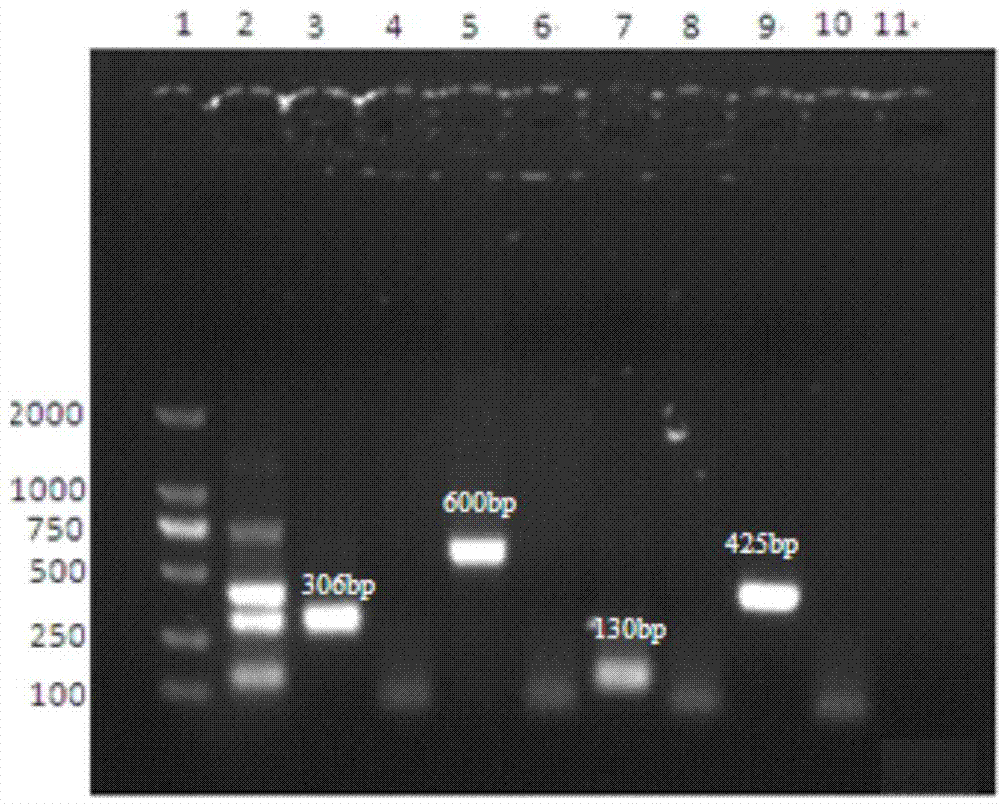
Bovine viral diarrhea virus nano-PCR detection kit and preparation method thereof
InactiveCN107058618AAccurate detectionImprove featuresMicrobiological testing/measurementBovine Viral Diarrhea VirusesNanoparticle
The invention discloses a bovine viral diarrhea virus nano-PCR detection kit and a preparation method thereof, relates to the field of bovine viral diarrhea virus detection, and solves the problems that BVD infection frequently has cross infection and is easily misdiagnosed, and that existing detection methods waste much time and labor. The kit comprises an Mighty Amp enzyme, a 2xBuffer Mix buffer liquid, gold nanoparticle sol, sterile double distilled water, a pair of specific primers and bovine viral diarrhea virus positive reference plasmid; the pair of specific primers include P1(5'-GGTAGCAACAGTGGTGAGTTC-3') and P2(5'-CTCAGGTTAAGATGTGCTGTG-3'). The bovine viral diarrhea virus positive reference plasmid is pMD18-T recombinant plasmid formed by a nucleotide fragment containing 130 basic groups, and the sequence of the plasmid is as shown in SEQ ID NO.2. The kit is high in sensitivity, simple in operation and low in cost.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
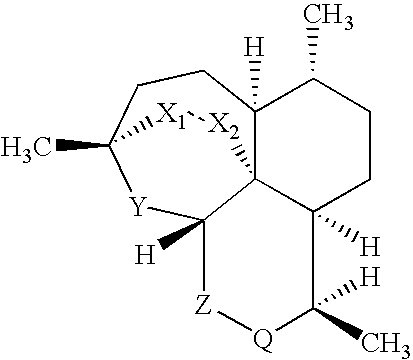
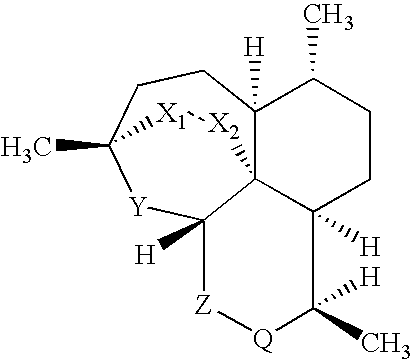
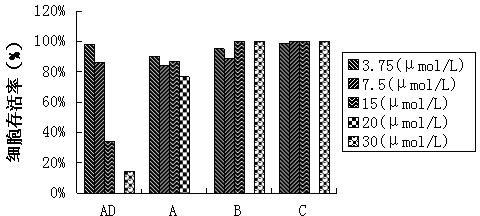
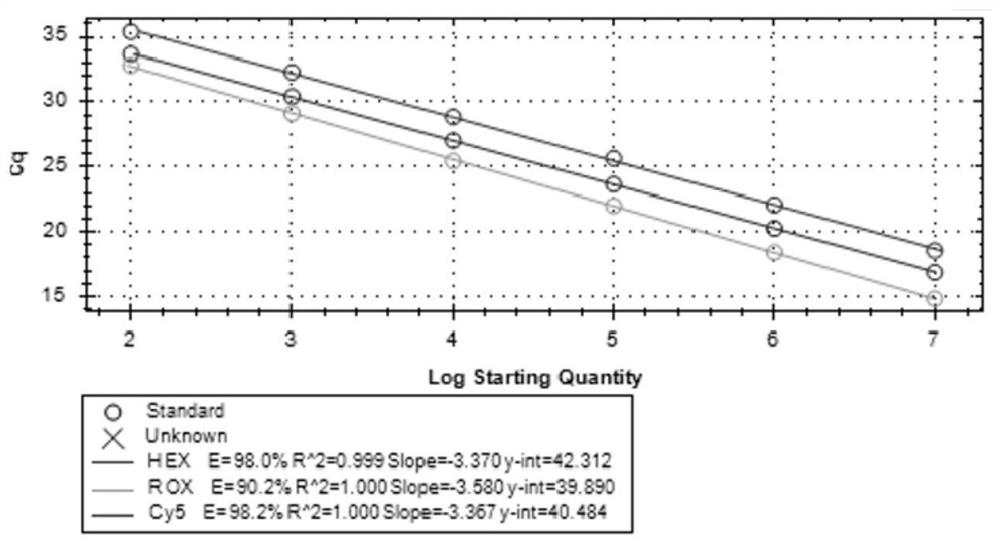
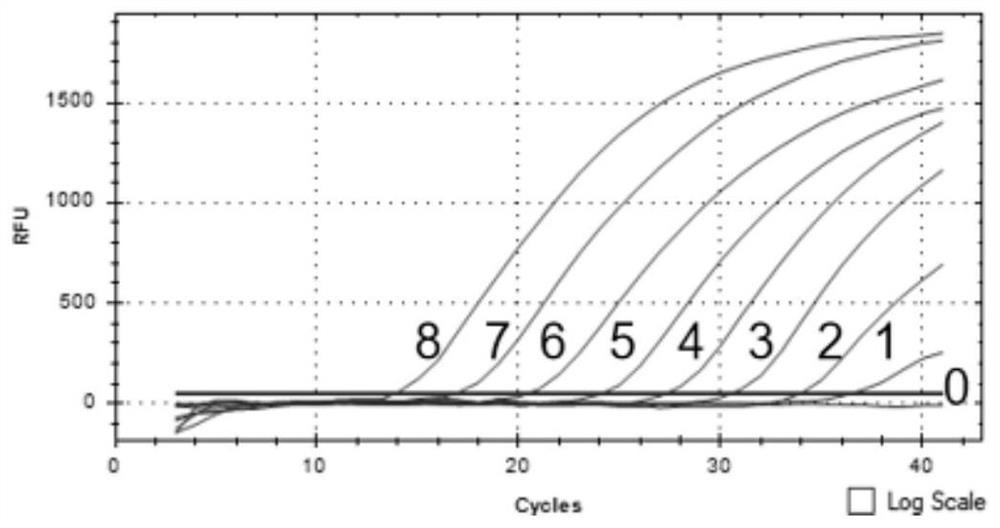
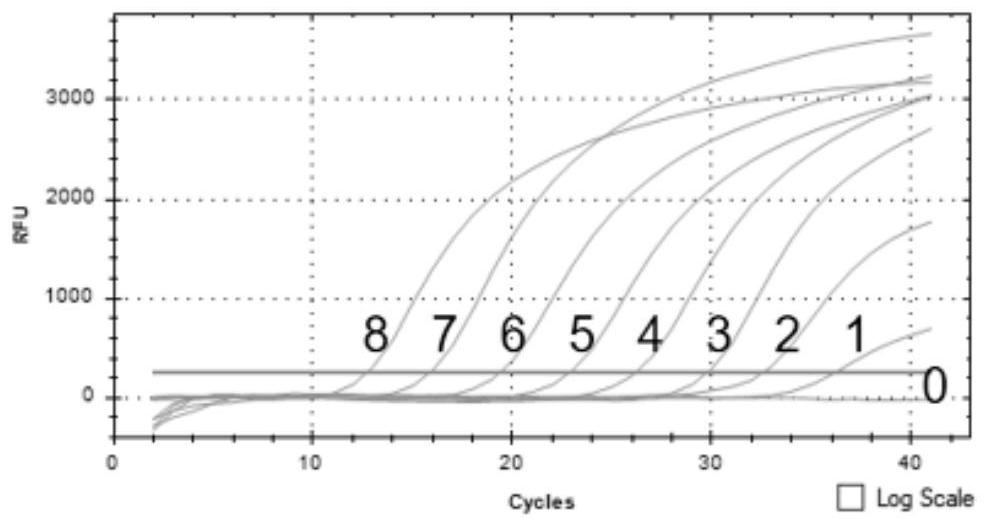
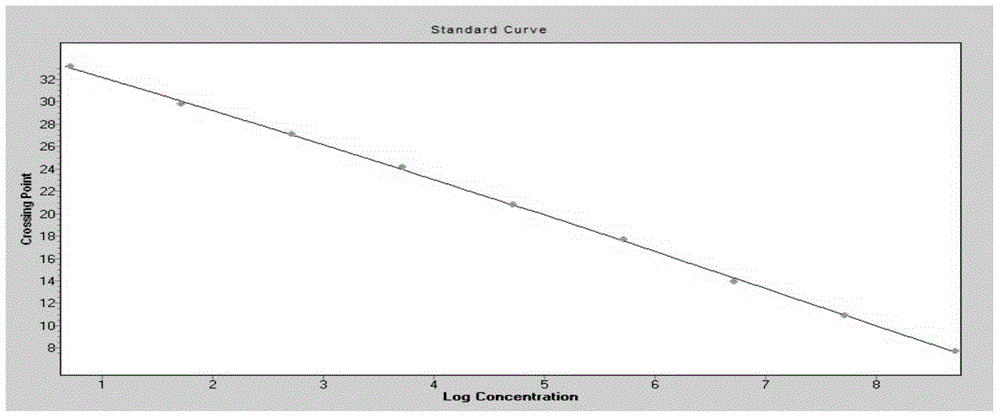
Imidazo [1, 2-a] pyrrolo [3, 2-c] pyridine compounds useful as pestivirus inhibitors
Owner:UNIV CLERMONT AUVERGNE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00001.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00002.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00003.png)