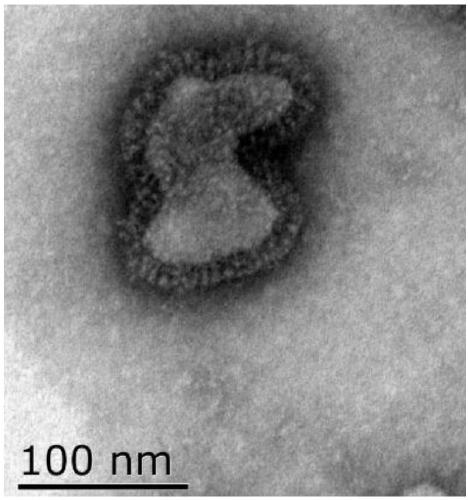
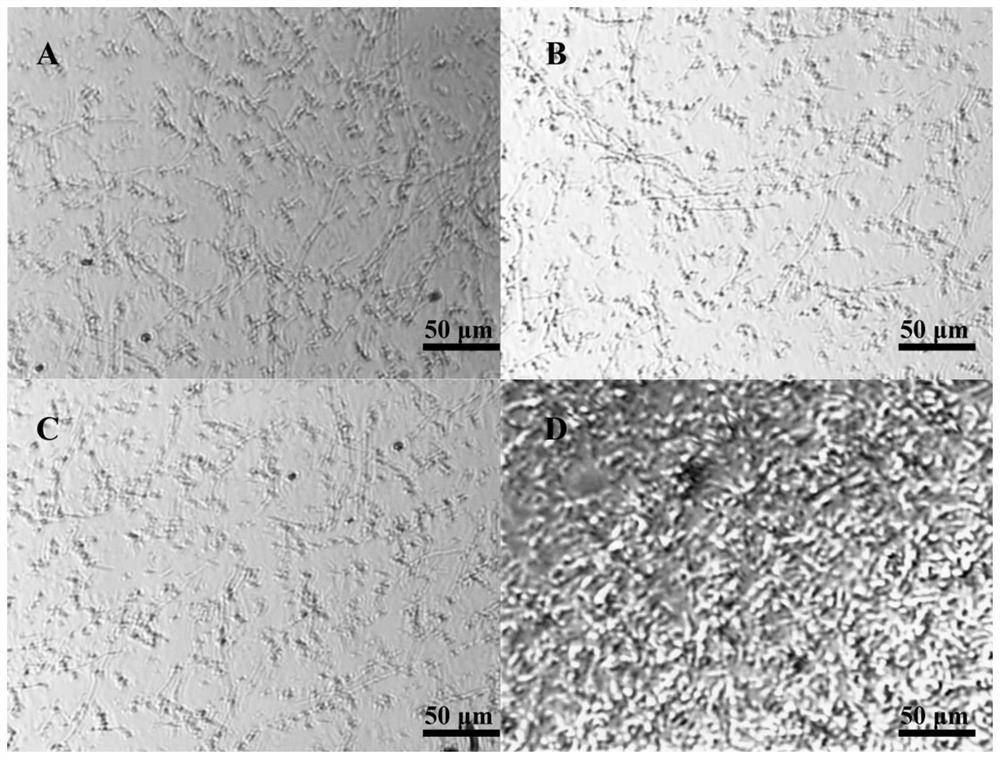
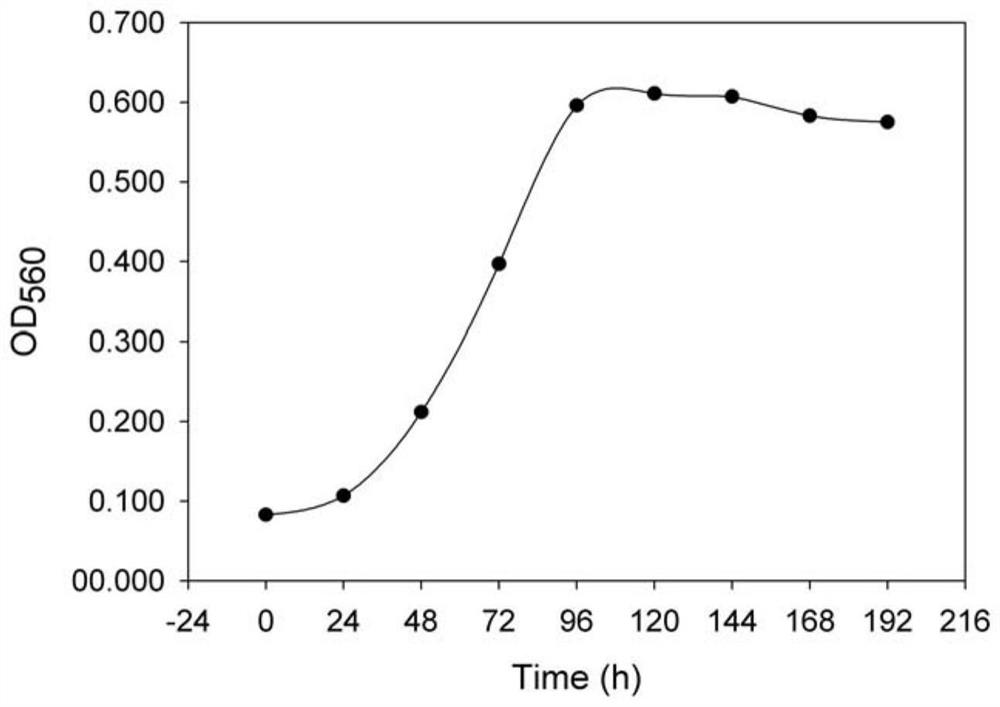
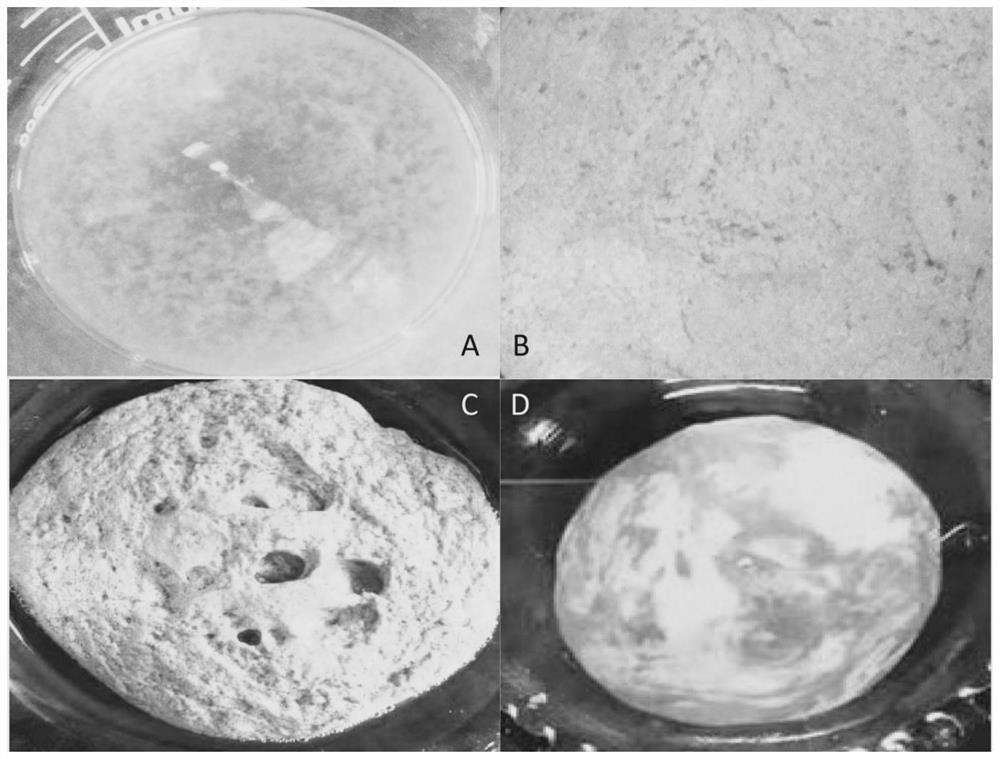
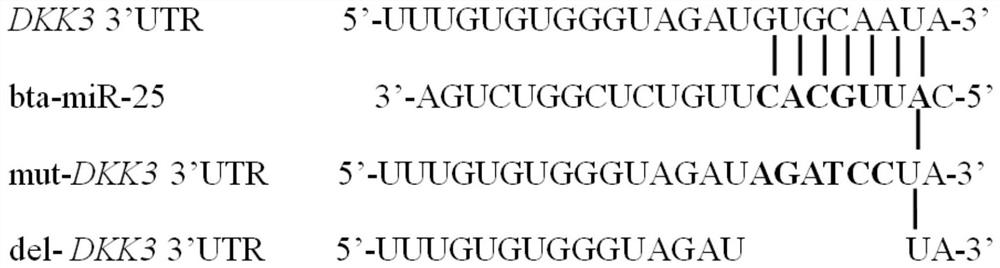Patents
Literature
30 results about "Bovine kidney" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bovine cystitis is an inflammation of the urinary bladder of cattle that may ascend the ureters to cause infection of the kidneys (pyelonephritis).
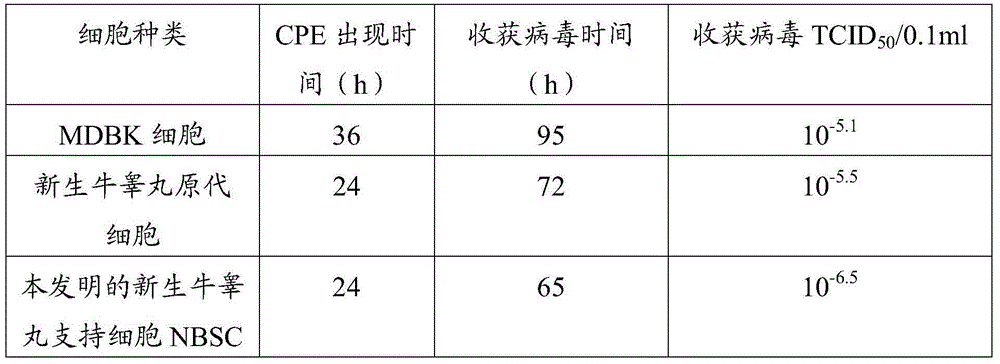
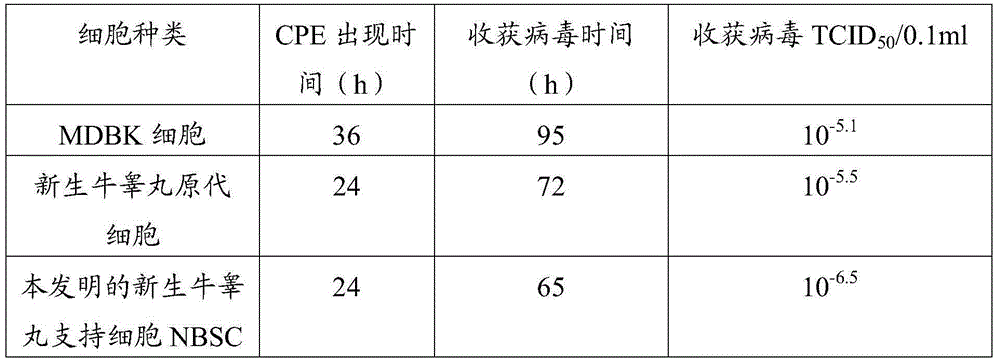
Cell line used for separation culture and multiplication of Orf virus (ORFV) as well as preparation method and application of cell line
ActiveCN103952377AAvoid the risk of carrying other pathogensQuality improvementMicroorganism based processesVertebrate cellsImpetigo contagiosaTiter
The invention discloses a cell line used for separation culture and multiplication of an Orf virus (ORFV) as well as a preparation method and application of the cell line. The cell line disclosed by the invention is a newly born bovine testis supported cell line obtained by separating and purifying newly born bovine testis primary culture cells; research results show that the cell line is more sensitive to inoculation of contagious ovine ecthyma virus, virus titer multiplied by utilizing the cell line is about 1 virus titer higher than that of newly born bovine testis primary culture cells and 1.9 virus titer higher than that of a madin-darby bovine kidney (MDBK) cell line. ORFV is separated by utilizing the cell line, uniformity and stability of virus can be guaranteed, and a risk that the separated viruses carry other pathogens as the primary culture cells are utilized for multiple times can be prevented; besides, the passage cell line is utilized for preparing a vaccine, a production process of the vaccine can be simplified, a production cycle is shortened, production cost is reduced, and stable quality of the prepared vaccine is also guaranteed, so that the cell line has a certain practical significance on large-scale production of the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of andrographolide C15 substitution derivative in manufacturing anti-hepatitis drug
ActiveCN102600129ATo clarify the anti-BVDV activity in vitroOrganic active ingredientsDigestive systemBovine Viral Diarrhea VirusesCytopathic effect
The invention discloses the application of an andrographolide C15 substitution derivative in manufacturing an anti-hepatitis drug, which belongs to the technical field of pharmaceutical chemistry. According to the invention, MDBK (mardin-darby bovine kidney cells) and BVDV (bovine viral diarrhea virus) are utilized to study the inhibitory action of the compound on cytopathic effect caused by the virus through an external screening model. Through the screening of a large number of andrographolide derivatives, the compound with a structure of general formula 1 is found to significantly inhibit MDBK cytopathic effect caused by the BVDV; the andrographolide C15 substitution derivative has high efficiency and low toxicity, thereby being used for manufacturing medicaments for treating and preventing hepatitis and having good prospect in development and application. The general formula 1 is shown in the description.
Owner:ZHENGZHOU UNIV
Recombinant porcine alpha interferon and application thereof in preparing medicines for treating Porcine cytomegalovirus (PCMV)
InactiveCN102796758APeptide/protein ingredientsMicroorganism based processesInfected cellProtein target
The invention discloses a recombinant porcine alpha interferon, prepared by the following methods: A1, synthesizing an artificially modified porcine alpha interferon; A2, constructing a recombinant eukaryotic expression vector pGAPZ alpha-IFN alpha; and A3, highly expressing IFN alpha by an eukaryotic cell yeast expression system. The invention is characterized by taking a supernatant to purifying the porcine alpha interferon which is cultured by a lot of engineering bacteria and expressed with a chromatography column, then collecting a target protein eluate, filtering through a 0.22 mum microfiltration membrane, then determining the activity by cytopathic inhibition, and calculating the potency unit according to 50% pathology; wherein the cell used for determination is Madin-Darby bovine kidney (MDBK), and vesicular stomatitis virus (VSV) is used for attacking the virus. According to the invention, the recombinant porcine alpha interferon obtained by purification is applied in controlling PCMV, and experiments prove that the recombinant porcine alpha interferon has obvious protection effect on PCMV infected cells. The route of administration of the porcine alpha interferon is injection or mucous membrane administration, and the dosage form comprises injection or nasal drops.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
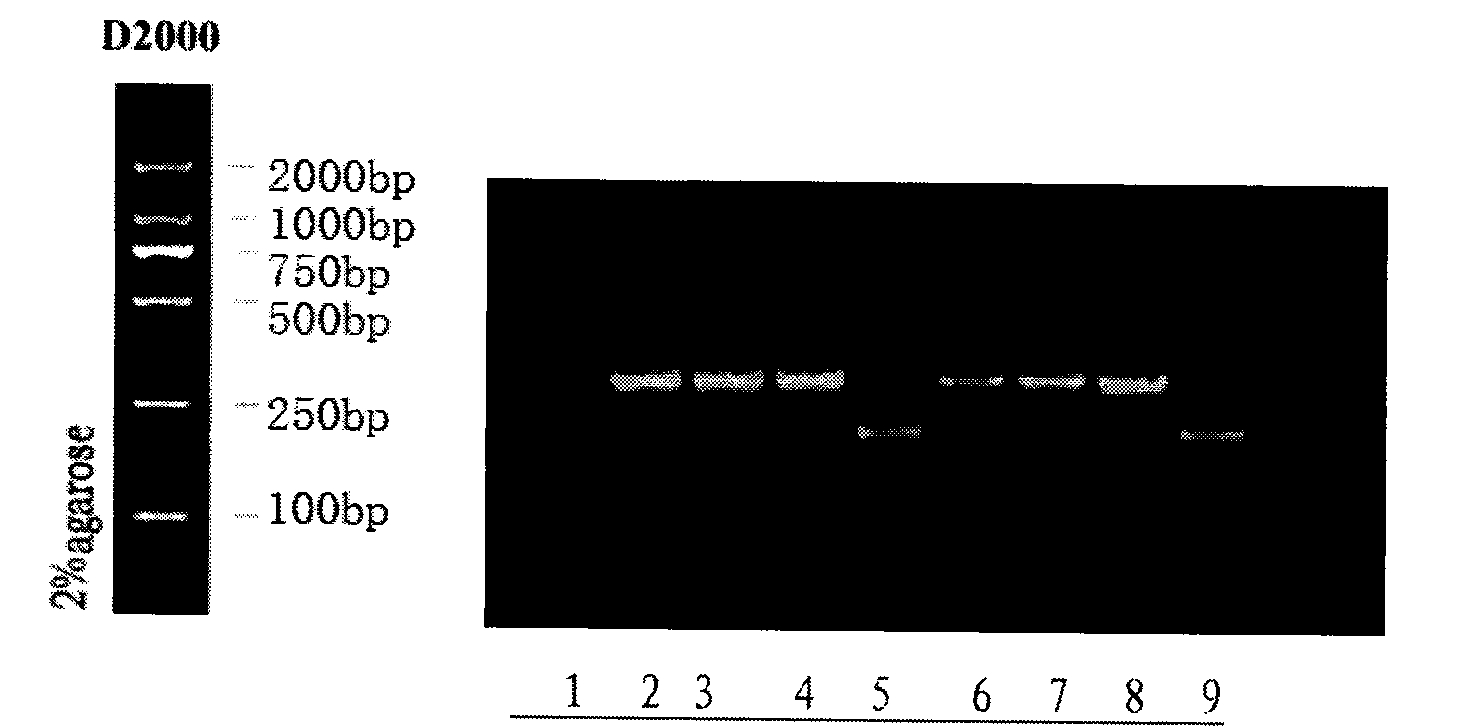
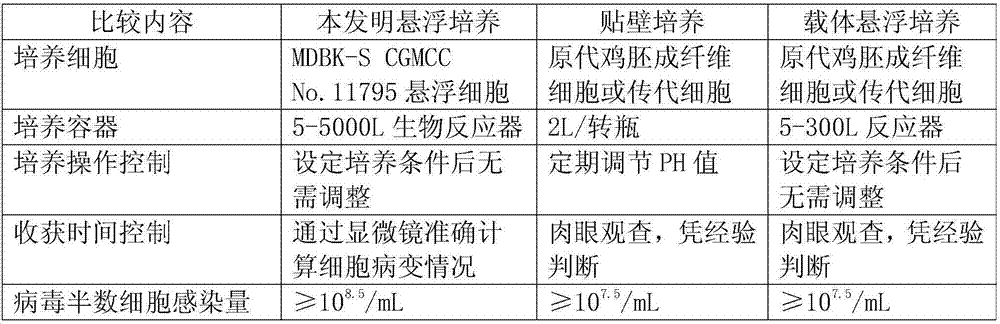
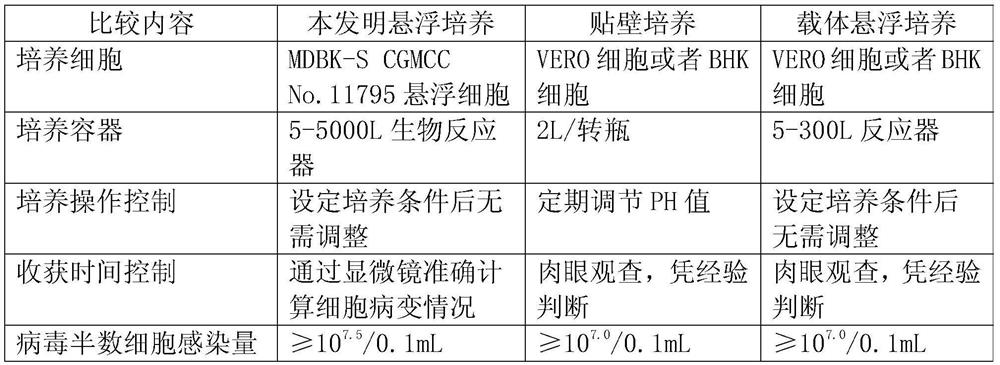
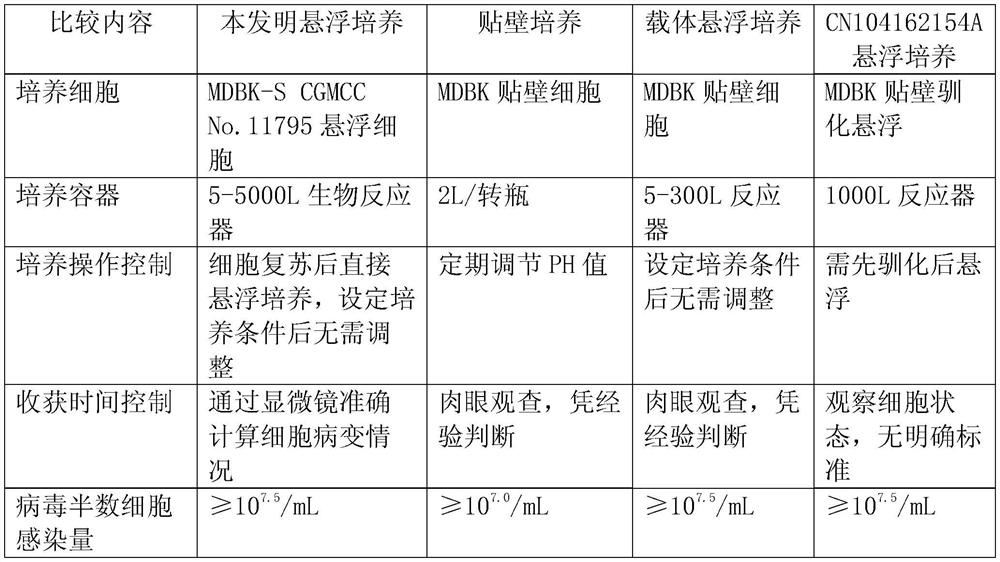
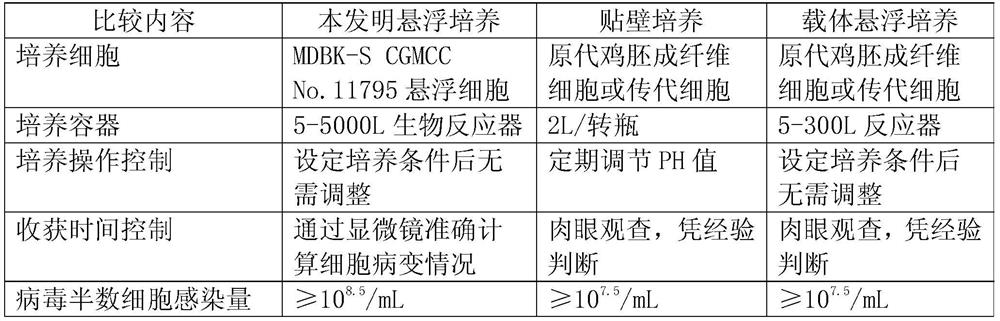
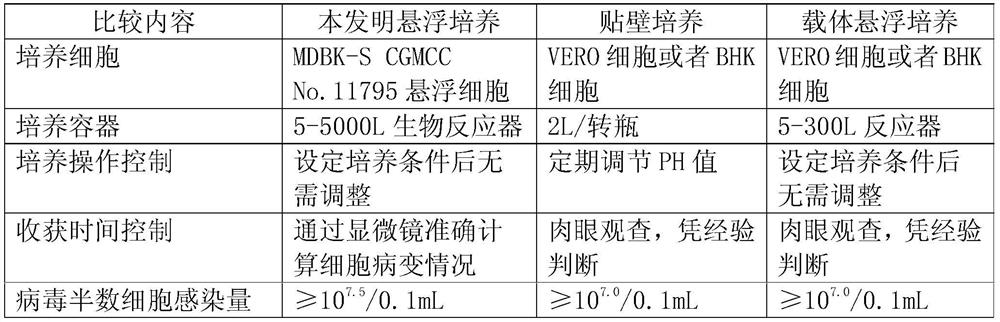
Application of bovine kidney cells capable of being subjected to suspension culture to virus culture and vaccine production
ActiveCN107201333AEliminate repetitive domestication workThe production scale-up process is simpleSsRNA viruses negative-senseSsRNA viruses positive-senseLiquid ChangeVaccine Production
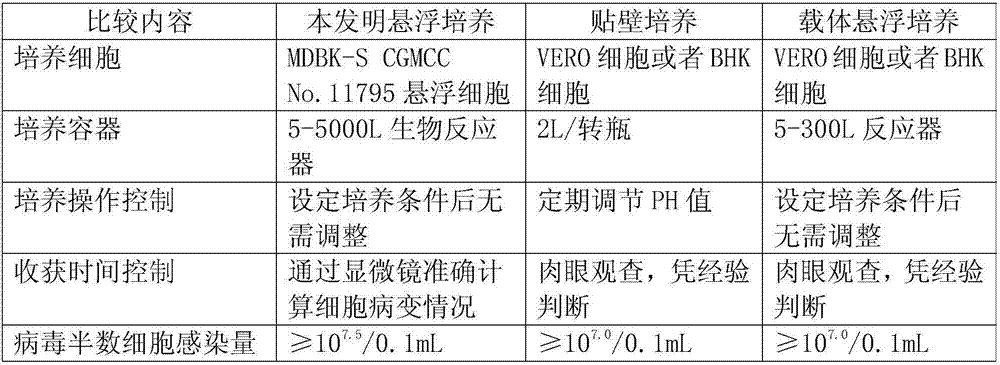
The invention discloses application of bovine kidney cells capable of being subjected to suspension culture to virus culture and vaccine production. The application has the advantages that a large quantity of manual operation for cell digestion, liquid change, frequent seed separation and the like can be omitted by bovine kidney cell (MDBK) suspension culture methods, the suspension culture methods are simple, convenient and speedy, processes can be automated by the aid of reactor culture, the cell yield of the unit volume can be increased, and the technical difficult problem of difference between adherent culture cell batches can be solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Infectious bovine rhinotracheitis virus strain and preparation method and application thereof
ActiveCN102337250AEasy to operateHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCytopathic effectFluorescence
The invention discloses an infectious bovine rhinotracheitis virus strain and a preparation method and application thereof. A wild strain of infectious bovine rhinotracheitis virus is found. The preparation method comprises the following steps of: firstly, identifying a sample by a PCR (Polymerase Chain Reaction) and a fluorescence PCR; then adding bovine serum which is detected to be free of IBRV (Infectious Bovine Rhinotracheitis Virus) to MDBK (Madindarby Bovine Kidney) cells by means of a culture medium, cultivating for a certain period of time so as to grow single-layer cells; after inoculating the single-layer cells with the processed sample, observing the cytopathic effect; after taking a pathological cell sample and identifying the pathological cell to be positive by the PCR, measuring TCID50 (Tissue Culture Infective Dose) as 106.2, and carrying out the virus neutralization test, wherein the TCID is 27; then inoculating the sample into the single layer cells; and when 70 percent to 80 percent of cells undergo the cytopathic effect and storing the cells into a refrigerator at a temperature of -40 DEG C. The infectious bovine rhinotracheitis virus strain and the preparation method disclosed by the invention can be applied to preparation of an engineered vaccine for preventing the infectious bovine rhinotracheitis.
Owner:湖南普简生物科技有限公司
Method for constructing swine-borne BVDV-22 strain infectious cDNA (complementary deoxyribonucleic acid) clone and application thereof
InactiveCN106318975ASsRNA viruses positive-senseMicroorganism based processesBovine Viral Diarrhea VirusesT7 RNA polymerase
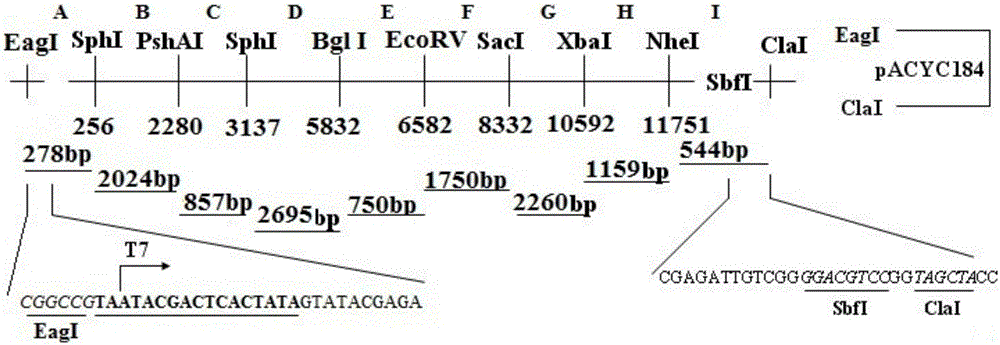
The invention belongs to the technical field of veterinary biological products, and particularly relates to swine-borne BVDV-2 strain infectious cDNA (complementary deoxyribonucleic acid) clone and application thereof. The swine-borne BVDV-2 strain infectious cDNA clone contains swine-borne BVDV-2 seed virus full-length cDNA, T7 RNA (ribonucleic acid) polymerase promoters are inserted in 5' terminals of the swine-borne BVDV-2 seed virus full-length cDNA, and SbfI restriction enzymes are inserted in 3' terminals of the swine-borne BVDV-2 seed virus full-length cDNA. The invention further discloses a plasmid pASH28 with the swine-borne BVDV-2 strain infectious cDNA. The plasmid is linearized and then is subjected to in-vitro transcription to obtain RNA, MDBK (Madin-Darby bovine kidney) cells are transfected by the plasmid, and bovine viral diarrhea viruses can be successfully rescued. The swine-borne BVDV-2 strain infectious cDNA clone, the application and the plasmid have the advantage that the swine-born BVDV-2 strain infectious cDNA clone can be applied to research on functional difference between different animal-borne BVDV-2 proteins and also can be used for genetically modifying and preparing high-titer attenuation BVDV vaccine.
Owner:YANGZHOU UNIV
Sika deer mucosal disease homologous inactivated vaccine and preparation method thereof
InactiveCN104174017AEmulsification procedure is simpleModerate viscosityViral antigen ingredientsAntiviralsMucosal diseaseCytopathic effect
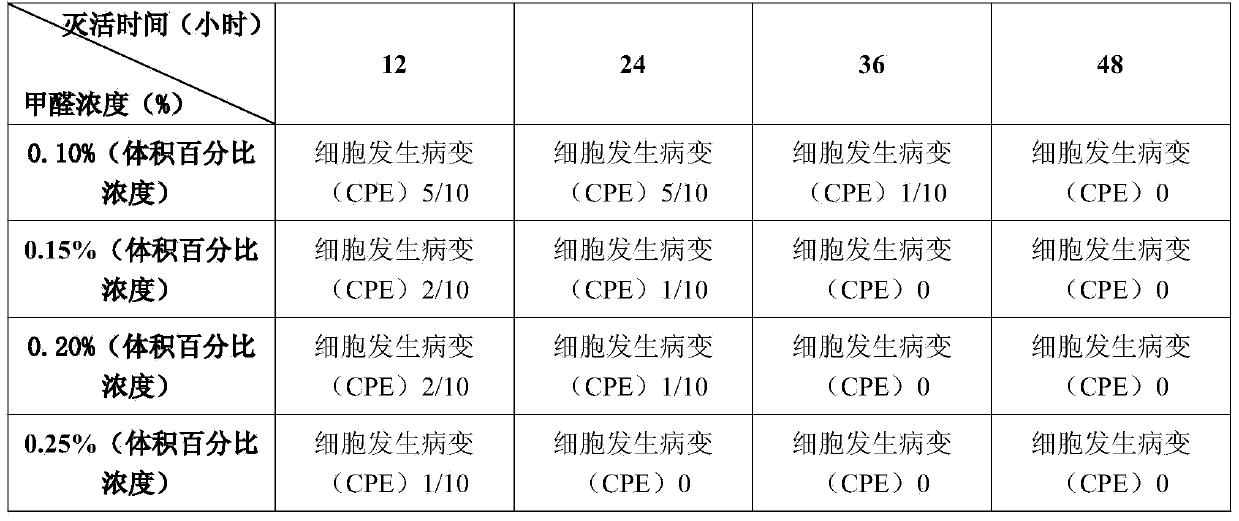
The invention discloses a sika deer mucosal disease homologous inactivated vaccine and a preparation method thereof. The preparation method comprises the following steps: performing inoculated culture on deer source BVDV (bovine viral diarrhea / virus) to the third to sixth generations in stable cell flasks with MDBK (madin-darby bovine kidney) cells fully spread in a single layer, selecting cell flasks which are cultured for 72-144 hours and generate a cytopathic effect (CPE), repeatedly freezing and thawing after pathological change, collecting viral liquid, adding formaldehyde into the viral liquid for inactivating, mixing the inactivated viral liquid with an oil agent consisting of white oil and sorbitan, and adding aluminum stearate to obtain the sika deer mucosal disease homologous inactivated vaccine.
Owner:JILIN UNIV
Caprine herpesvirus I vaccine strain and application thereof
ActiveCN107893056AEpidemic preventionReduce morbidityMicroorganism based processesAntiviralsOil adjuvantPathogenicity
The invention provides a caprine herpesvirus I vaccine strain and an application thereof, and relates to the field of biological veterinary products. The caprine herpesvirus I vaccine strain is a caprine herpesvirus I JSHA1405 strain, which is preserved with preservation number of CCTCC NO:V201745. Virus liquid is obtained through multiplication of the virus (caprine herpesvirus I) by virtue of mardin-darby bovine kidney cells. The invention also provides an application fot eh vaccine strain in preparing caprine herpesvirus I vaccines. The caprine herpesvirus I JSHA1405 strain provided by theinvention is relatively strong in pathogenicity and is good in immunogenicity. An oil adjuvant inactivated vaccine, which is prepared by the caprine herpesvirus I JSHA1405 strain, is safe and reliable; goats, which are immunized, can generate an antibody at a relatively high level, and the duration of the antibody can reach 6 months; in addition, an excellent protective effect can be taken on homologous virus seed toxicity attack; morbidity in the immunized goats is obviously reduced; and the prevalence of the caprine herpesvirus I can be effectively prevented.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Caprine parainfluenza virus type 3 JS14-2 strain and application thereof
ActiveCN108949700AImproving immunogenicityEpidemic preventionSsRNA viruses negative-senseMicrobiological testing/measurementAdjuvantImmunogenicity
The invention belongs to the field of veterinary biological products and discloses a caprine parainfluenza virus type 3 JS14-2 strain and an application thereof in preparation of inactivated vaccines.The inactivated vaccines are prepared and obtained by the steps of: separating and purifying to obtain the caprine parainfluenza virus type 3 JS14-2 strain, carrying out centrifugation and formaldehyde inactivation on virus liquid and adding an adjuvant for emulsification. The invention provides a virus strain which can be used for preparing caprine parainfluenza virus type 3 inactivated vaccines. The strain belongs to domestic prevalent strains, and is strong in virulence and good in antigenicity; the strain has better growth characteristic on MDBK (Madin-Darby Bovine Kidney) cells, and canobtain stable high titer (more than or equal to 107 TCID50 / mL, and the hemagglutination valence is more than or equal to 28); the vaccine preparation method is perfect, and the immunogenicity and theimmune protective effect are good; the used immunizing dosage is low, the safety is high, the immune duration is long, the production and use cost is greatly saved, and the benefit for effectively controlling the caprine parainfluenza virus type 3 in clinical practices is achieved.
Owner:JIANGSU ACAD OF AGRI SCI
Anti-IBRV single-chain antibody and preparation method and application thereof
InactiveCN107312087ASmall molecular weightEasy constructionImmunoglobulins against virusesAntiviralsEscherichia coliWAS PROTEIN
The invention provides an anti-IBRV single-chain antibody which is protein consisting of a heavy chain variable region shown as SEQ ID NO.1, a light chain variable region shown as SEQ ID NO.2 and a connecting peptide for connecting the two regions and having the characteristic of resisting an infectious bovine rhinotracheitis peplos gD protein antibody. The invention also provides a preparation method of the single-chain antibody. The single-chain antibody can be specifically combined with IBRV and can be also specifically combined with IBV gD protein in an escherichia coli expression product. An indirect immunofluorescence assay shows that the single-chain antibody can identify the IBRV infected in the bovine kidney cell MDBK, and the single-chain antibody has the good application value when applied to infectious bovine rhinotracheitis detection and development of treatment preparations.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
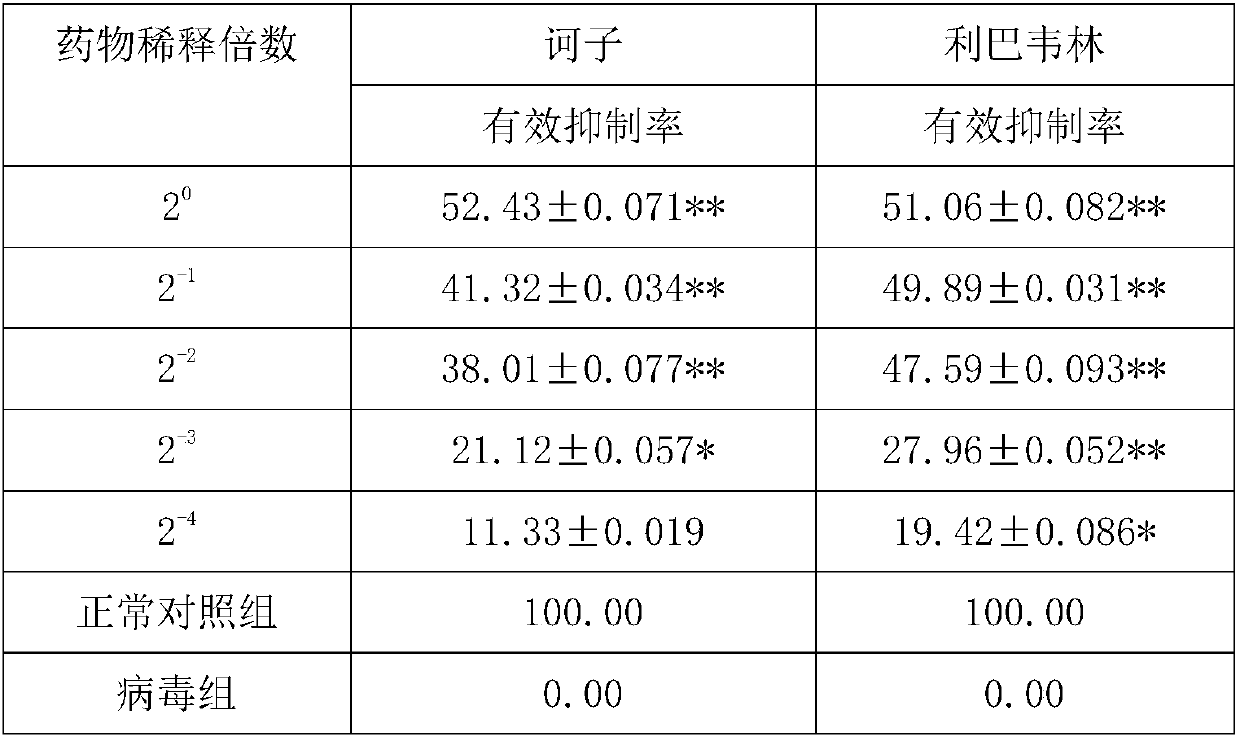
Application of fructus chebulae in preparing medicine for inhibiting and killing BVDV (bovine viral diarrhea virus)
ActiveCN107854499ABetter than adsorptionBetter than replication blockAntiviralsPlant ingredientsDiseaseBovine Viral Diarrhea Viruses
The invention discloses application of fructus chebulae in preparing a medicine for inhibiting and killing BVDV (bovine viral diarrhea virus). The application of the fructus chebulae in preparing themedicine for inhibiting and killing the BVDV has the beneficial effects that the Tibetan medicine is used as a study object, and the screened fructus chebulae water extract has certain inhibition on the BVDV in a MDBK(Madin-darby-Bovine-Kidney) model; the direct killing function on viruses is better than both the adsorption blocking function and the duplicating blocking function, and a foundationis laid for the preventing and controlling of BVD diseases and the study and development of medicines; the novel pharmacologic action of the fructus chebulae is found, the fructus chebulae can be usedas a candidate medicine for preventing and controlling the BVDV and / or clearing the infection of BVDV, and the fructus chebulae can be singly used or jointly used.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Method of screening MDBK (Madin-Darby Bovine Kidney) cell lines sensitive to bovine viral diarrhea/mucosal viruses
ActiveCN105734007AShorten the timeEasy to operateCell dissociation methodsBiological material analysisMucosal diseaseTiter
The invention discloses a method of screening MDBK (Madin-Darby Bovine Kidney) cell lines sensitive to bovine viral diarrhea / mucosal viruses. The method includes: performing mono-cloning on MDBK cells; inoculating bovine viral diarrhea / mucosal viruses of two biotypes (cytopathic and noncytopathic); adopting indirect immunofluorescence technology to measure viral titer, and determining the cell lines with the viral titer higher than 106TCID50 / ml as the MDBK cell lines sensitive to the bovine viral diarrhea / mucosal viruses. The MDBK cell lines screened by the method are sensitive to the bovine viral diarrhea / mucosal viruses of the two biotypes, and the method can be directly applied in research and production of bovine viral diarrhea / mucosal disease vaccines.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Health wine and preparation method thereof
ActiveCN101984035BImprove sexual functionChange the tasteNervous disorderDigestive systemPapaya familyOyster
The invention discloses a health wine and a preparation method thereof, wherein the health wine comprises the following raw materials: bovine kidney, sheep kidney, penis et testis serpentis, cinnamon, polygonatum, raspberry, licorice, clove, cordyceps sinensis, yam, oyster, papaya, Chinese dates, wolfberry fruit, arillus longan, honey, crystal sugar and 30-degree Daqu spirit. The preparation method is as follows: cleaning the bovine kidney, sheep kidney, penis et testis serpentis, cinnamon, polygonatum, raspberry, licorice, clove, cordyceps sinensis, yam, oyster, papaya, Chinese dates, wolfberry fruit and arillus longan, adding water for leaching, filtering to separate filtrate liquor from filter residue, then repeatedly leaching twice, mixing filtrates obtained at the three times, heating and concentrating to obtain extract, mixing the extract, refined honey, refined crystal sugar and 30-degree Daqu spirit and evenly stirring, sealing and standing for one month, then taking out, filtering to remove impurities, and finally filling to obtain the finished product. The health wine has the efficacies of invigorating premodial energy, tonifying kidney yang, enriching essence and blood,supplementing blood, nourishing yin, and nourishing blood to tranquillize mind.
Owner:安徽方敏医药科技股份有限公司
Goat herpes virus type I vaccine strain and its application
ActiveCN107893056BEpidemic preventionReduce morbidityMicroorganism based processesAntiviralsOil adjuvantPathogenicity
The invention provides a caprine herpesvirus I vaccine strain and an application thereof, and relates to the field of biological veterinary products. The caprine herpesvirus I vaccine strain is a caprine herpesvirus I JSHA1405 strain, which is preserved with preservation number of CCTCC NO:V201745. Virus liquid is obtained through multiplication of the virus (caprine herpesvirus I) by virtue of mardin-darby bovine kidney cells. The invention also provides an application fot eh vaccine strain in preparing caprine herpesvirus I vaccines. The caprine herpesvirus I JSHA1405 strain provided by theinvention is relatively strong in pathogenicity and is good in immunogenicity. An oil adjuvant inactivated vaccine, which is prepared by the caprine herpesvirus I JSHA1405 strain, is safe and reliable; goats, which are immunized, can generate an antibody at a relatively high level, and the duration of the antibody can reach 6 months; in addition, an excellent protective effect can be taken on homologous virus seed toxicity attack; morbidity in the immunized goats is obviously reduced; and the prevalence of the caprine herpesvirus I can be effectively prevented.
Owner:JIANGSU ACAD OF AGRI SCI
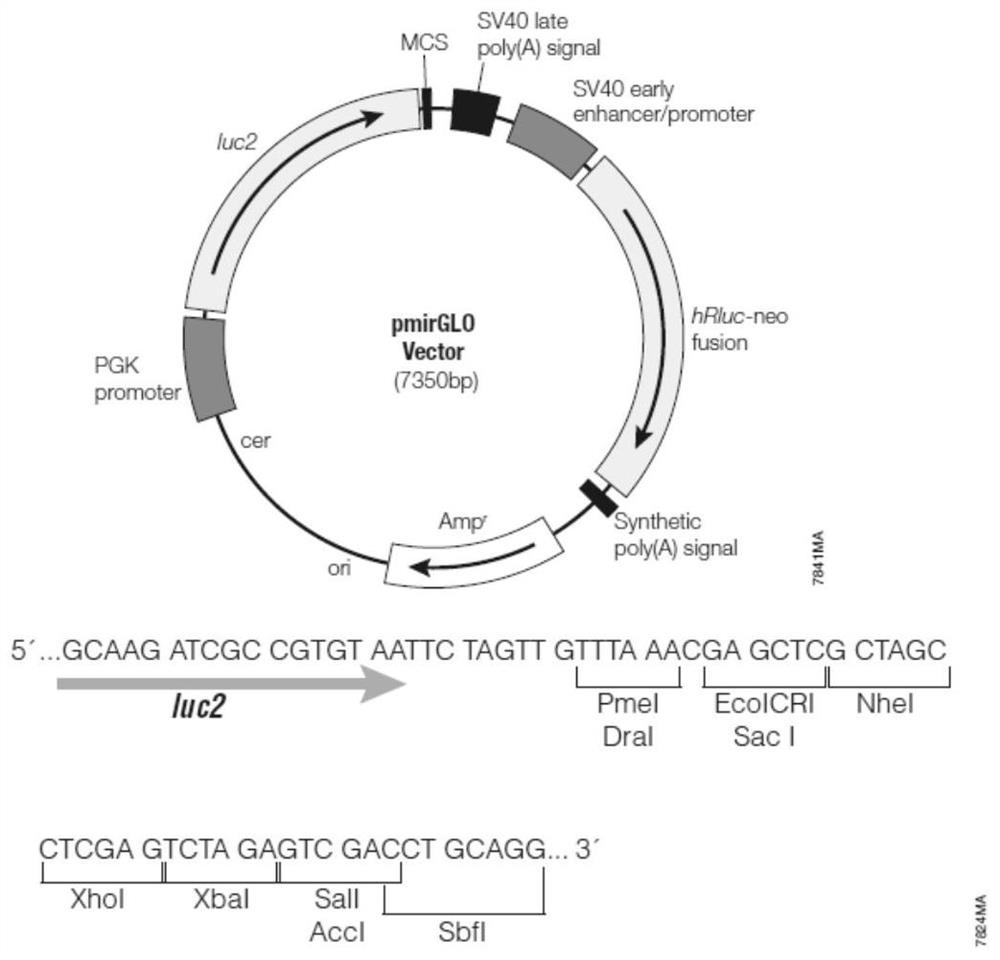
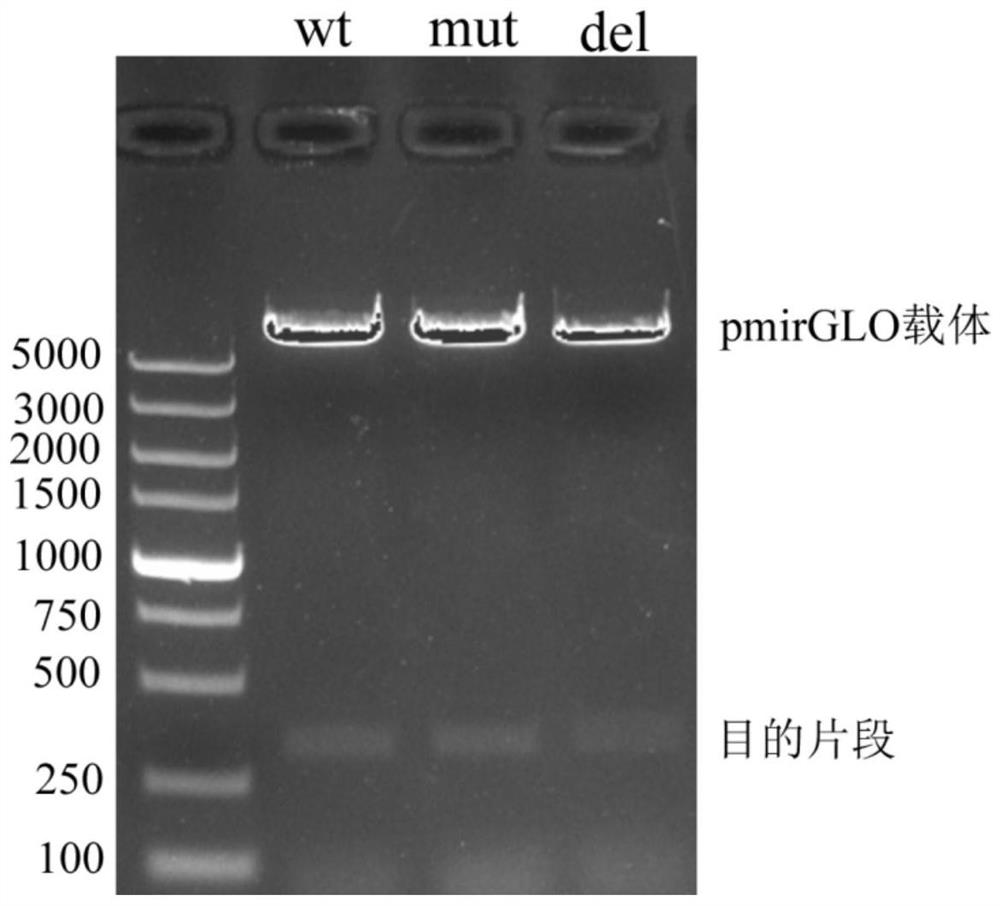
A plasmid containing beef cattle dkk3 gene 3'utr sequence and dual luciferase reporter gene and its application
ActiveCN113322278BHigh sensitivityHigh speedMicrobiological testing/measurementPeptidesDigestionLuciferases
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
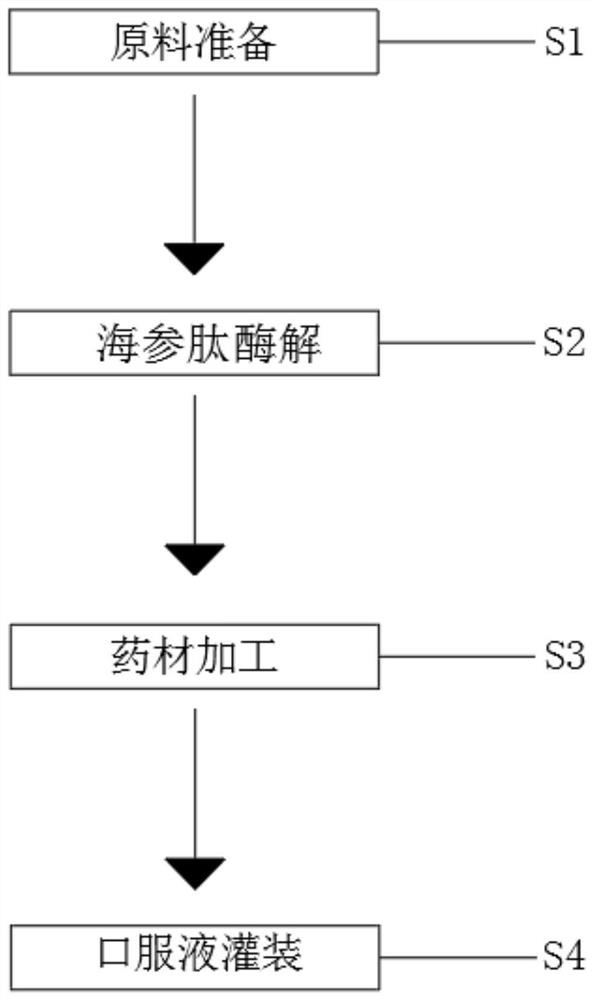
Oral liquid sea cucumber cordyceps peptide
PendingCN114377108APreserve activityPromote absorptionNervous disorderHydrolysed protein ingredientsBiotechnologyMedicinal herbs
The invention relates to the technical field of health-care products, and discloses oral liquid sea cucumber and cordyceps sinensis peptide, which is prepared from the following raw materials in percentage by weight: 25 to 35 percent of sea cucumber, 7 to 13 percent of fructus lycii, 3 to 7 percent of bovine kidney, 3 to 7 percent of radix ginseng, 3 to 7 percent of radix ophiopogonis, 0.5 to 2 percent of pericarpium citri reticulatae, 0.1 to 0.5 percent of xylooligosaccharide, 0.15 to 0.05 percent of sodium carboxymethyl cellulose, 0.15 to 0.05 percent of potassium sorbate, 0.1 to 0.3 percent of cordyceps sinensis and the balance of water. The traditional Chinese medicine formula has the advantages that the traditional Chinese medicine formula can tonify qi and yin, the traditional Chinese medicine liquid is used for nourishing, the activity of sea cucumber peptide can be well preserved, the sea cucumber peptide can be absorbed within one minute on an empty stomach, the sea cucumber peptide can be directly absorbed into blood and tonify the kidney without digestion, high-quality protein which is higher in quality and easier to absorb is provided for a human body, and the traditional Chinese medicine composition is more reasonably and effectively utilized; according to the formula, the sea cucumber peptide is processed and produced by adopting a biological enzymolysis technology, and other medicinal materials are processed and produced by adopting a multi-stage separation and high-pressure extraction technology.
Owner:华医联华(广东省)中医药研究所
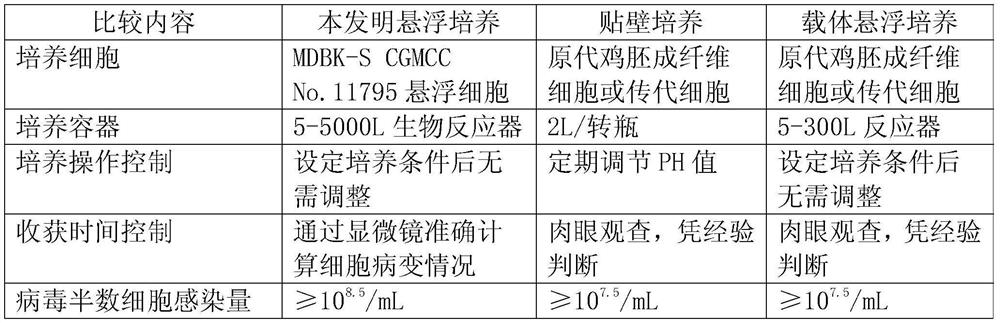
Application of Suspension Cultured Bovine Kidney Cells in Virus Culture and Vaccine Production
ActiveCN107201333BEliminate repetitive domestication workThe production scale-up process is simpleSsRNA viruses negative-senseSsRNA viruses positive-senseVaccine ProductionCultured cell
The invention discloses the application of bovine kidney cells capable of suspension culture in virus culture and vaccine preparation. The bovine kidney cell MDBK suspension culture method of the present invention saves a large number of manual operations such as cell digestion, liquid replacement, frequent sorting, etc., the culture method is simple and fast, and the reactor culture is used to realize process automation, which not only improves the cell yield per unit volume , It also solves the technical problem of differences between batches of traditional adherent cultured cells.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Application of andrographolide C15 substitution derivative in manufacturing anti-hepatitis drug
ActiveCN102600129BTo clarify the anti-BVDV activity in vitroOrganic active ingredientsDigestive systemCytopathic effectBovine Viral Diarrhea Viruses
The invention discloses the application of an andrographolide C15 substitution derivative in manufacturing an anti-hepatitis drug, which belongs to the technical field of pharmaceutical chemistry. According to the invention, MDBK (mardin-darby bovine kidney cells) and BVDV (bovine viral diarrhea virus) are utilized to study the inhibitory action of the compound on cytopathic effect caused by the virus through an external screening model. Through the screening of a large number of andrographolide derivatives, the compound with a structure of general formula 1 is found to significantly inhibit MDBK cytopathic effect caused by the BVDV; the andrographolide C15 substitution derivative has high efficiency and low toxicity, thereby being used for manufacturing medicaments for treating and preventing hepatitis and having good prospect in development and application. The general formula 1 is shown in the description.
Owner:ZHENGZHOU UNIV
Type I interferon receptor gene knockout bovine kidney cell line as well as construction method and application thereof
PendingCN114874991AThe construction method is simple and fastLow costSsRNA viruses negative-senseSsRNA viruses positive-senseBovine parainfluenza virusBovine Viral Diarrhea Viruses
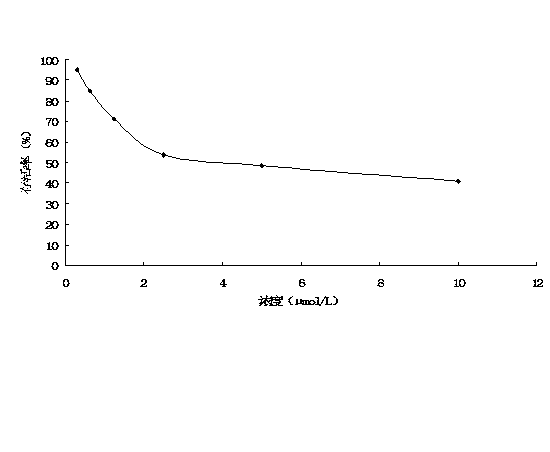
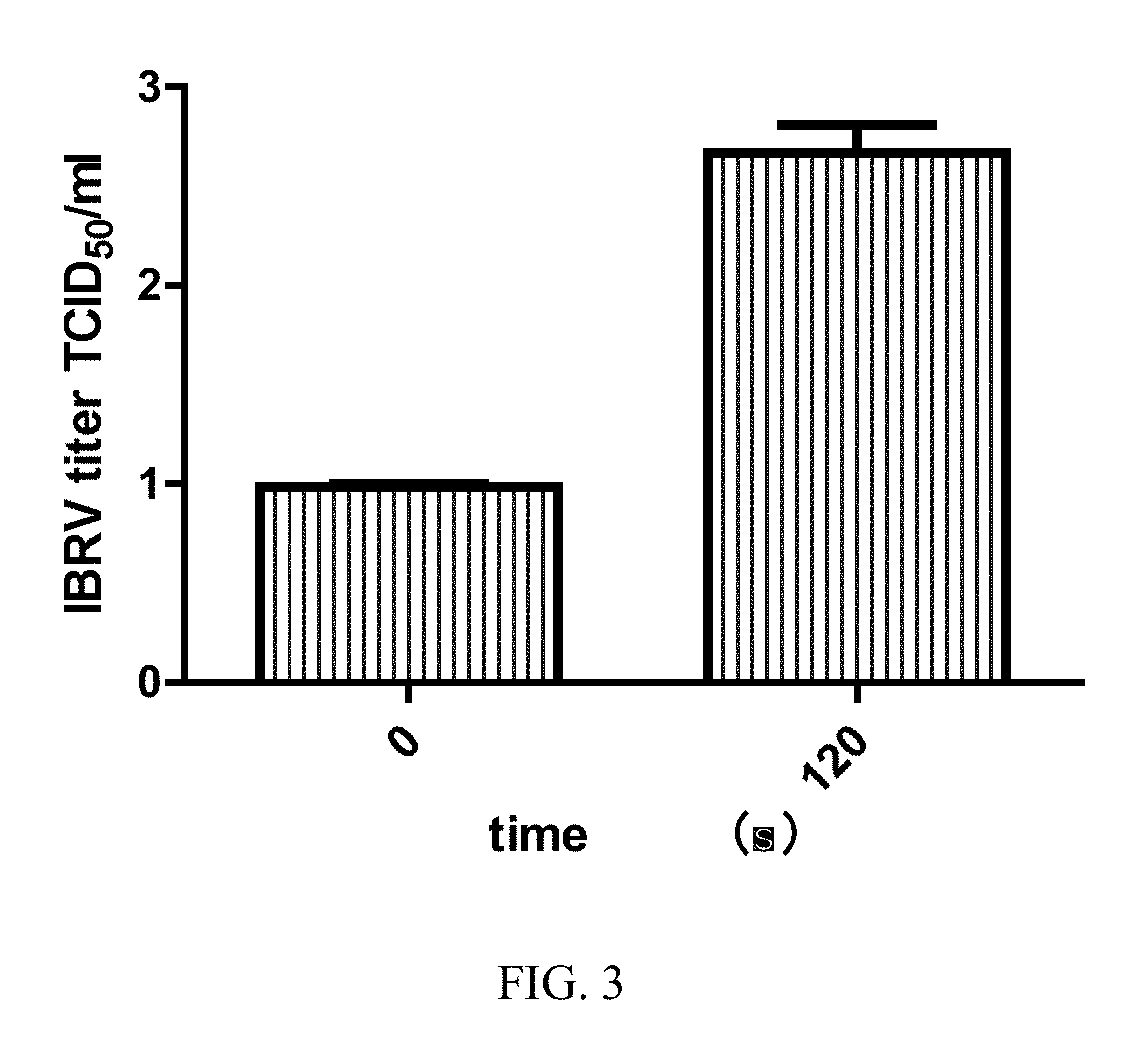
The invention discloses an I-type interferon receptor (IFNAR) gene knockout bovine kidney cell line, which is obtained by carrying out gene editing on bovine kidney cells by utilizing a CRISPR / Cas9 gene editing technology and carrying out monoclonal selection. Phenotype verification shows that after the IFNAR1 knockout bovine kidney cell line does not express IFNAR1 and is infected with a bovine viral diarrhea virus (BVDV), an infectious bovine rhinotracheitis virus (IBRV) and a 3-type bovine parainfluenza virus (BPIV-3), the virus titer can be remarkably improved, so that the IFNAR1 knockout bovine kidney cell line can be used for separating and identifying bovine-derived virus clinical strains and improving the virus proliferation efficiency.
Owner:HUAZHONG AGRI UNIV
A method for screening mdbk cell lines susceptible to bovine viral diarrhea/mucosal virus
ActiveCN105734007BShorten the timeEasy to operateCell dissociation methodsBiological material analysisMucosal diseaseEngineering
The invention discloses a method of screening MDBK (Madin-Darby Bovine Kidney) cell lines sensitive to bovine viral diarrhea / mucosal viruses. The method includes: performing mono-cloning on MDBK cells; inoculating bovine viral diarrhea / mucosal viruses of two biotypes (cytopathic and noncytopathic); adopting indirect immunofluorescence technology to measure viral titer, and determining the cell lines with the viral titer higher than 106TCID50 / ml as the MDBK cell lines sensitive to the bovine viral diarrhea / mucosal viruses. The MDBK cell lines screened by the method are sensitive to the bovine viral diarrhea / mucosal viruses of the two biotypes, and the method can be directly applied in research and production of bovine viral diarrhea / mucosal disease vaccines.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Mdbk irf3/irf7 knock out mutant cell and its use for vaccine production
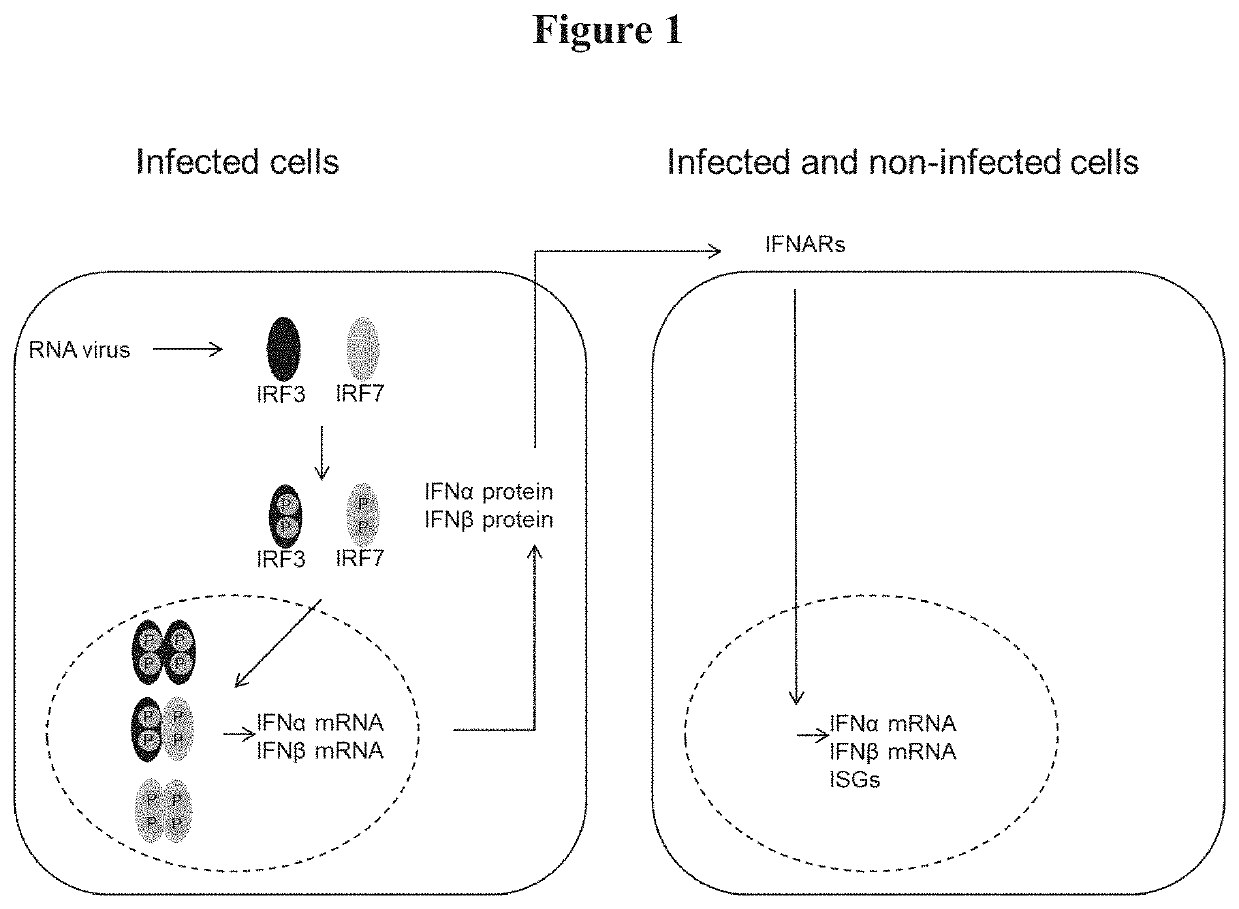
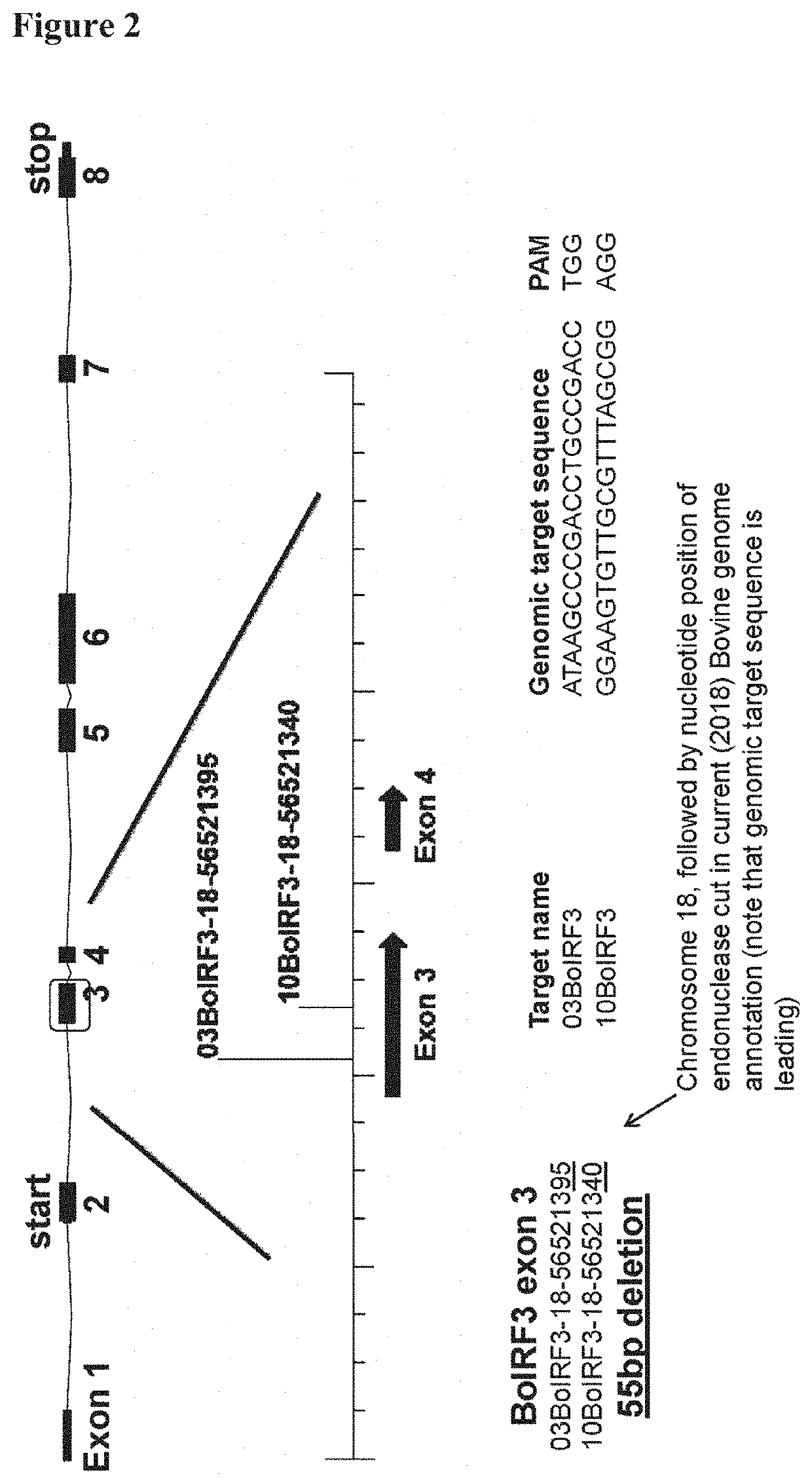
PendingUS20220315953A1Increase virus yieldStably and permanently inactivateSsRNA viruses negative-senseGenetically modified cellsVaccine ProductionTGE VACCINE
The present invention pertains to a Madin-Darby bovine kidney (MDBK) cell, wherein the interferon regulatory factors (IRF) IRF3 and / or IRF7 encoding genes are functionally inactivated. The invention also pertains to a cell culture comprising the MDBK cell, use of the MDBK cell culture, a method for the production of a virus using the cell and a vaccine prepared by using the cell.
Owner:INTERVET INC
Porcine BVDV (Bovine Viral Diarrhea Virus) recombinant virus for expressing porcine epidemic diarrhea virus (PEDV) S genes and application
InactiveCN108611329ASsRNA viruses positive-senseMicroorganism based processesBovine Viral Diarrhea VirusesViral vector
The invention discloses a porcine BVDV (Bovine Viral Diarrhea Virus) recombinant virus for expressing porcine epidemic diarrhea virus (PEDV) S genes and the application thereof. A neutralization antigenic epitope sequence of the PEDV S genes is inserted into a terminal N of a C nucleocapsid protein gene of porcine BVDV. A recombinant infectious cloning plasmid is obtained. The plasmid is linearized, subjected to in vitro transcription to obtain RNA and transfected with MDBK (Madin-Darby Bovine Kidney) cells, so that the porcine BVDV recombinant virus for expressing the PEDV S proteins can be successfully rescued. The porcine BVDV recombinant virus is capable of expressing a PEDV S foreign protein, and is simultaneously used for controlling the PEDV and BVDV. The porcine BVDV infectious clone provides suitable insertion sites for other exogenous genes, can serve as a recombinant viral vector, and provides an effective technology platform for researches such as subsequent in-vitro modification of RNA, insertion of exogenous genes, preparation of recombinant viruses and the like.
Owner:SHANGHAI ACAD OF AGRI SCI
A method for constructing recombinant goat pox virus expressing empty capsid of foot-and-mouth disease virus
InactiveCN103343110BReduce apoptosis-inducing effectPreserve splicing activityViruses/bacteriophagesVector-based foreign material introductionProtein precursorVaccinia
The invention relates to a construction method of recombinant coatpox virus used for expressing foot and mouth disease virus empty capsid. The method comprises the following steps of: constructing transfer plasmids on the basis of forward and reverse homologous arms; inserting a foot and mouth disease virus protein precursor gene P1-2A, a vaccinia virus back-to-back promoter P7.5-P11, a hypoxanthine-guanine phosphoribosyltransferase gene, a foot and mouth disease virus internal ribosome entry site sequence and a foot and mouth disease virus 3C protease 9-site mutant gene to the transfer plasmids in sequence; co-transfecting an MDBK (Madin-darby Bovine Kidney) cell through the transfer plasmids and the coatpox virus; and then inserting an exogenous gene between the forward and reverse homologous arms in a homologous recombination way. By adopting the construction method, the screened recombinant coatpox virus can exist stably and can be used for expressing the foot and mouth disease virus empty capsid.
Owner:新疆维吾尔自治区畜牧科学院兽医研究所
Method for promoting replication of bovine rhinotracheitis viruses using cold atmospheric plasma
ActiveUS20190330598A1Improve the level ofImproving vaccine production efficiencyViral antigen ingredientsGenetically modified cellsVaccine ProductionCo incubation
A method for promoting replication of infectious bovine rhinotracheitis viruses using cold atmospheric plasma, including: irradiating a medium for Madin-Darby bovine kidney cells with a cold atmospheric plasma generator; adding the irradiated medium to the Madin-Darby bovine kidney cells; and adding infectious bovine rhinotracheitis viruses for incubation. The time required for treatment is 2 min allowing for a simple and rapid operation. The plasma is used for the indirect treatment of cells with a uniform process and a controllable intensity. The replication of the infectious bovine rhinotracheitis viruses in the Madin-Darby bovine kidney cells is significantly promoted by co-incubation in the treated DMEM for 1 hour, so that the high levels of infectious bovine rhinotracheitis viruses obtained can be used for vaccine production after inactivation, improving the vaccine production efficiency.
Owner:JIANGSU DAISYFEN BIOTECHNOLOGY CO LTD +1
A cell line for isolation, culture and propagation of sheep infectious pustular virus and its preparation method and application
ActiveCN103952377BAvoid the risk of carrying other pathogensQuality improvementMicroorganism based processesAntiviralsImpetigo contagiosaBovine kidney
The invention discloses a cell line used for separation culture and multiplication of an Orf virus (ORFV) as well as a preparation method and application of the cell line. The cell line disclosed by the invention is a newly born bovine testis supported cell line obtained by separating and purifying newly born bovine testis primary culture cells; research results show that the cell line is more sensitive to inoculation of contagious ovine ecthyma virus, virus titer multiplied by utilizing the cell line is about 1 virus titer higher than that of newly born bovine testis primary culture cells and 1.9 virus titer higher than that of a madin-darby bovine kidney (MDBK) cell line. ORFV is separated by utilizing the cell line, uniformity and stability of virus can be guaranteed, and a risk that the separated viruses carry other pathogens as the primary culture cells are utilized for multiple times can be prevented; besides, the passage cell line is utilized for preparing a vaccine, a production process of the vaccine can be simplified, a production cycle is shortened, production cost is reduced, and stable quality of the prepared vaccine is also guaranteed, so that the cell line has a certain practical significance on large-scale production of the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Bovine kidney cells capable of suspension culture and suspension culture method and application thereof
ActiveCN107201334BIncrease productionSolve technical problems with differencesSsRNA viruses negative-senseSsRNA viruses positive-senseVirologic responseCultured cell
Owner:JINYUBAOLING BIO PHARMA CO LTD
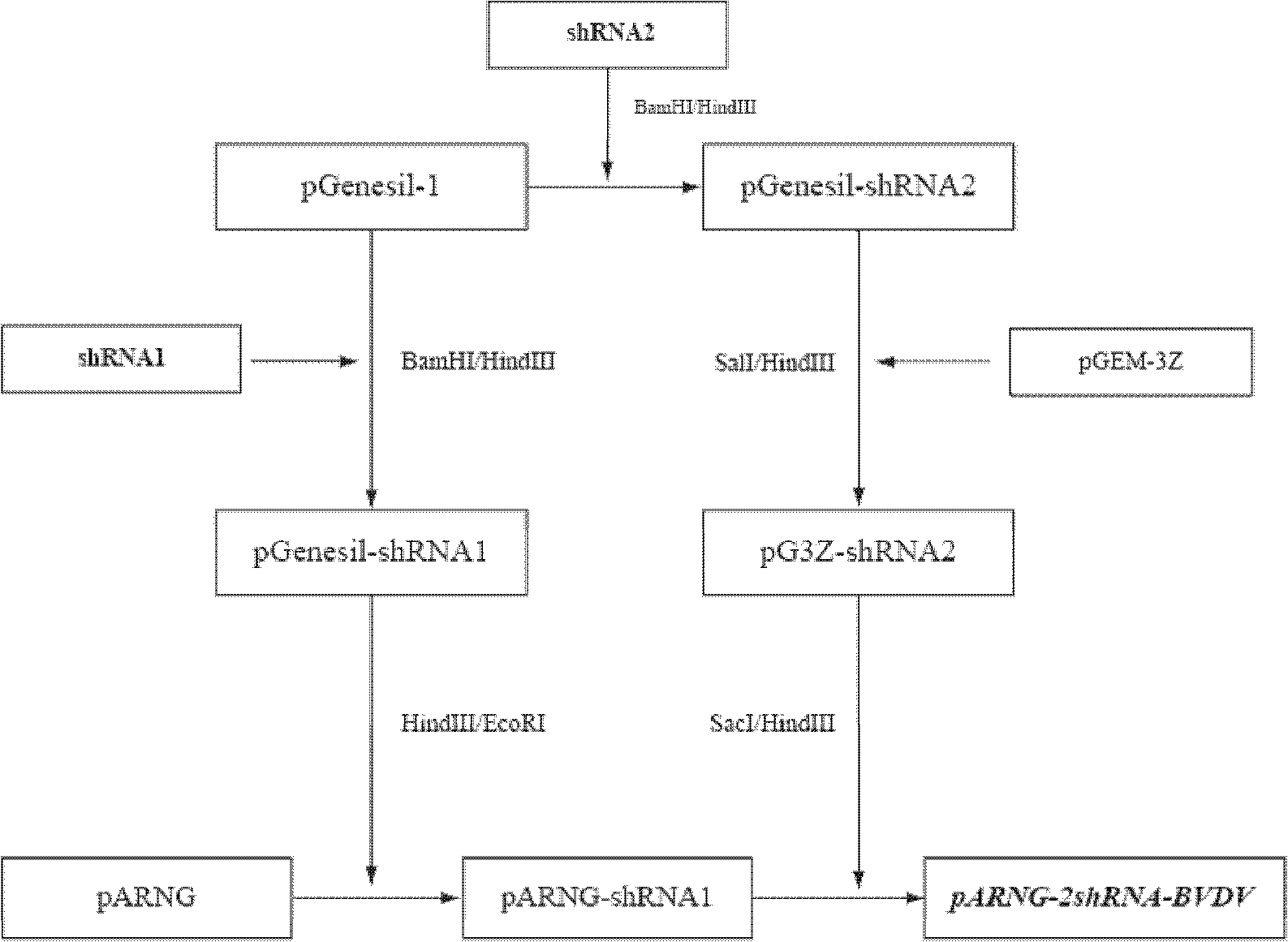
ShRNA (short hairpin Ribonucleic Acid) expression vector for interfering BVDV (Bovine Viral Diarrhea Virus) copying and constructed recombinant cell
InactiveCN102628045AReduce RNA levelsReduce outputVector-based foreign material introductionForeign genetic material cellsBovine Viral Diarrhea VirusesReprogramming
The invention discloses a shRNA (short hairpin Ribonucleic Acid) expression vector for interfering BVDV (Bovine Viral Diarrhea Virus) copying and a constructed recombinant cell. Different shRNA fragments are designed specific to different regions of a BVDV. SiRNA (small interfering Ribonucleic Acid) is formed by transcribing shRNA in a host cell and shearing, and acts on different regions of a virus RNA to interfere the copying and expression of viruses. BVDV cell surface receptors exists in bovine kidney cells, testicle cells and the like, so that BVDV is most sensitive to fetal bovine kidney cells and testicle cells, a complete transgenic cloned cattle individual is formed by performing induced rearrangement on a fetal bovine fibroblast serving as a somatic cell nuclear transplanting donor cell, and BVDV sensitive organs such as kidney and the like have the capabilities of suppressing virus expression.
Owner:NORTHWEST A & F UNIV +1
A kind of anti-ibrv single-chain antibody, its preparation method and application
InactiveCN107312087BSmall molecular weightEasy to buildImmunoglobulins against virusesAntiviralsEscherichia coliSingle-Chain Antibodies
The invention provides an anti-IBRV single-chain antibody which is protein consisting of a heavy chain variable region shown as SEQ ID NO.1, a light chain variable region shown as SEQ ID NO.2 and a connecting peptide for connecting the two regions and having the characteristic of resisting an infectious bovine rhinotracheitis peplos gD protein antibody. The invention also provides a preparation method of the single-chain antibody. The single-chain antibody can be specifically combined with IBRV and can be also specifically combined with IBV gD protein in an escherichia coli expression product. An indirect immunofluorescence assay shows that the single-chain antibody can identify the IBRV infected in the bovine kidney cell MDBK, and the single-chain antibody has the good application value when applied to infectious bovine rhinotracheitis detection and development of treatment preparations.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Infectious bovine rhinotracheitis virus strain and preparation method and application thereof
ActiveCN102337250BEasy to operateHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCytopathic effectFluorescence
The invention discloses an infectious bovine rhinotracheitis virus strain and a preparation method and application thereof. A wild strain of infectious bovine rhinotracheitis virus is found. The preparation method comprises the following steps of: firstly, identifying a sample by a PCR (Polymerase Chain Reaction) and a fluorescence PCR; then adding bovine serum which is detected to be free of IBRV (Infectious Bovine Rhinotracheitis Virus) to MDBK (Madindarby Bovine Kidney) cells by means of a culture medium, cultivating for a certain period of time so as to grow single-layer cells; after inoculating the single-layer cells with the processed sample, observing the cytopathic effect; after taking a pathological cell sample and identifying the pathological cell to be positive by the PCR, measuring TCID50 (Tissue Culture Infective Dose) as 106.2, and carrying out the virus neutralization test, wherein the TCID is 27; then inoculating the sample into the single layer cells; and when 70 percent to 80 percent of cells undergo the cytopathic effect and storing the cells into a refrigerator at a temperature of -40 DEG C. The infectious bovine rhinotracheitis virus strain and the preparation method disclosed by the invention can be applied to preparation of an engineered vaccine for preventing the infectious bovine rhinotracheitis.
Owner:湖南普简生物科技有限公司
Ox-bile-tolerant bezoar transformation bacterium screening culture medium, preparation method and application
ActiveCN114703109AMeet nutritional needs for reproductionBacteriaMicroorganism separationBiotechnologyBile Juice
The invention relates to a culture medium for screening microorganisms, in particular to a culture medium for separating efficient bezoar transforming bacteria resistant to oxgall, a preparation method and application. The formula of the culture medium comprises the following components: relative to the amount of 1000.0 ml of a solvent, the culture medium further comprises 100.0 to 300.0 g of a bovine liver extract, 100.0 to 300.0 g of a bovine kidney extract, 5.0 to 15.0 g of peptone, 3.0 to 5.0 g of glucose, 3.0 to 5.0 g of NaCl, 3.0 to 5.0 g of disodium hydrogen phosphate and 100.0 to 600.0 ml of ox bile, 10.0 to 30.0 g of agar is added on the basis of the solid culture medium, and the pH value is adjusted to 6.8 to 7.5. The strain separated and screened by using the culture medium disclosed by the invention has the characteristics of being capable of tolerating ox bile for a long time and high in bezoar conversion efficiency.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com