Sika deer mucosal disease homologous inactivated vaccine and preparation method thereof
A technology for inactivated vaccines and mucosal diseases, which is applied in the direction of antiviral agents, virus antigen components, emulsion delivery, etc., can solve the problems of no immunity, etc., and achieve the effect of simple emulsification procedure and suitable viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, sika deer mucosal disease homologous inactivated vaccine and preparation method thereof
[0021] In order to safely and specifically prevent the viral infectious disease of sika deer mucosal disease, a homologous inactivated vaccine was developed. Its preparation method is as follows:
[0022] 1. Preparation of sika deer mucosal disease virus solution
[0023] 1. Materials
[0024] Sika deer-derived bovine viral diarrhea strain CC-CRB01 (isolated and identified by the Laboratory of Infectious Diseases, College of Veterinary Medicine, Jilin University) is the second-generation basic virus, with a virulence of 10 5.5 TCID 50 / 0.1mL
[0025] Determination of CC-CRB01 Strain Virus Content
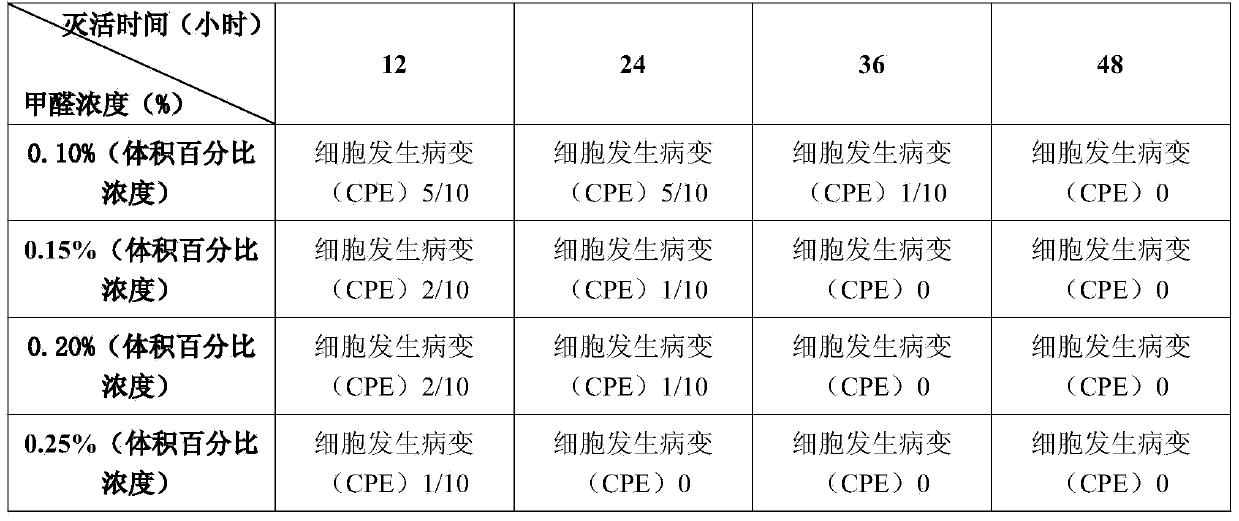
[0026] Dilute the virus sample 10 times, and inoculate it on the MDBK cells that have grown into a single layer, repeat 4 wells for each dilution, and use the well directly added to the maintenance solution as a blank control, 37 ° C, 5% CO 2 After culturing for 1 h, ...
Embodiment 2
[0090] Embodiment 2, the detection of the effect of the homologous inactivated vaccine of sika deer mucosa disease
[0091] 1. Field trial report of homologous inactivated vaccine against sika deer mucosal disease
[0092] After completing the laboratory test of the inactivated vaccine, field tests were carried out with four batches of qualified laboratory products to observe the safety and efficacy of the vaccine under production conditions.
[0093] 1. Materials
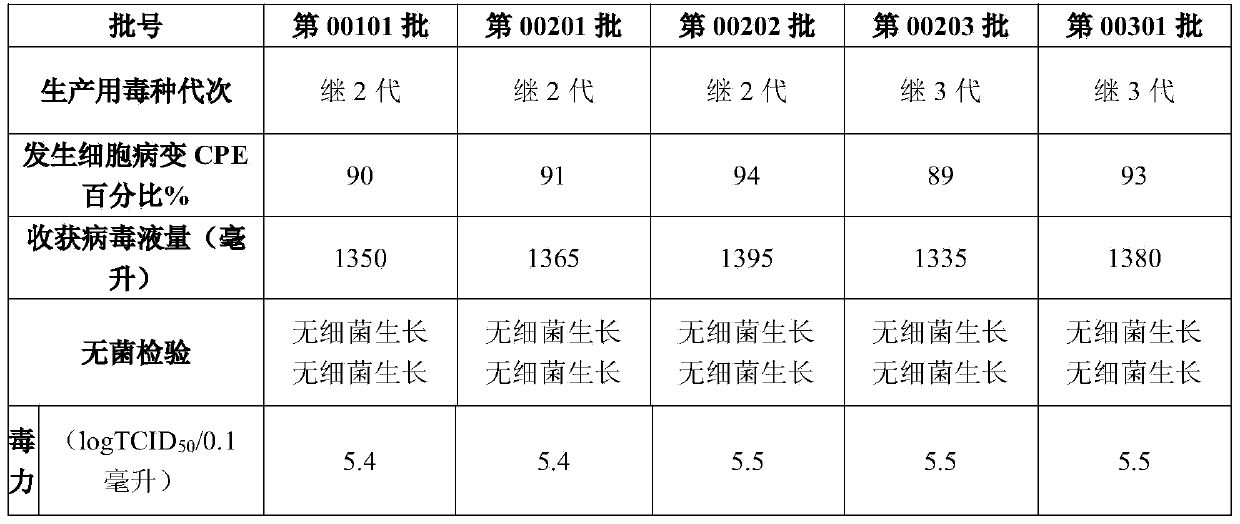
[0094] Vaccine: the homologous inactivated vaccine of sika deer mucosal disease with batch numbers 00201, 00202, 00203, and 00301 prepared in Example 1.
[0095] Immune doe: select 4 test sites are:
[0096]
[0097] Clinical examination before immunization Observe the performance and remove the diseased deer.
[0098] 2. Field efficacy test
[0099] 1) Immunization: Batch numbers 00201, 00202, 00203, and 00301 sika deer mucosal disease homologous inactivated vaccines prepared in Example 1 were intramuscular...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com