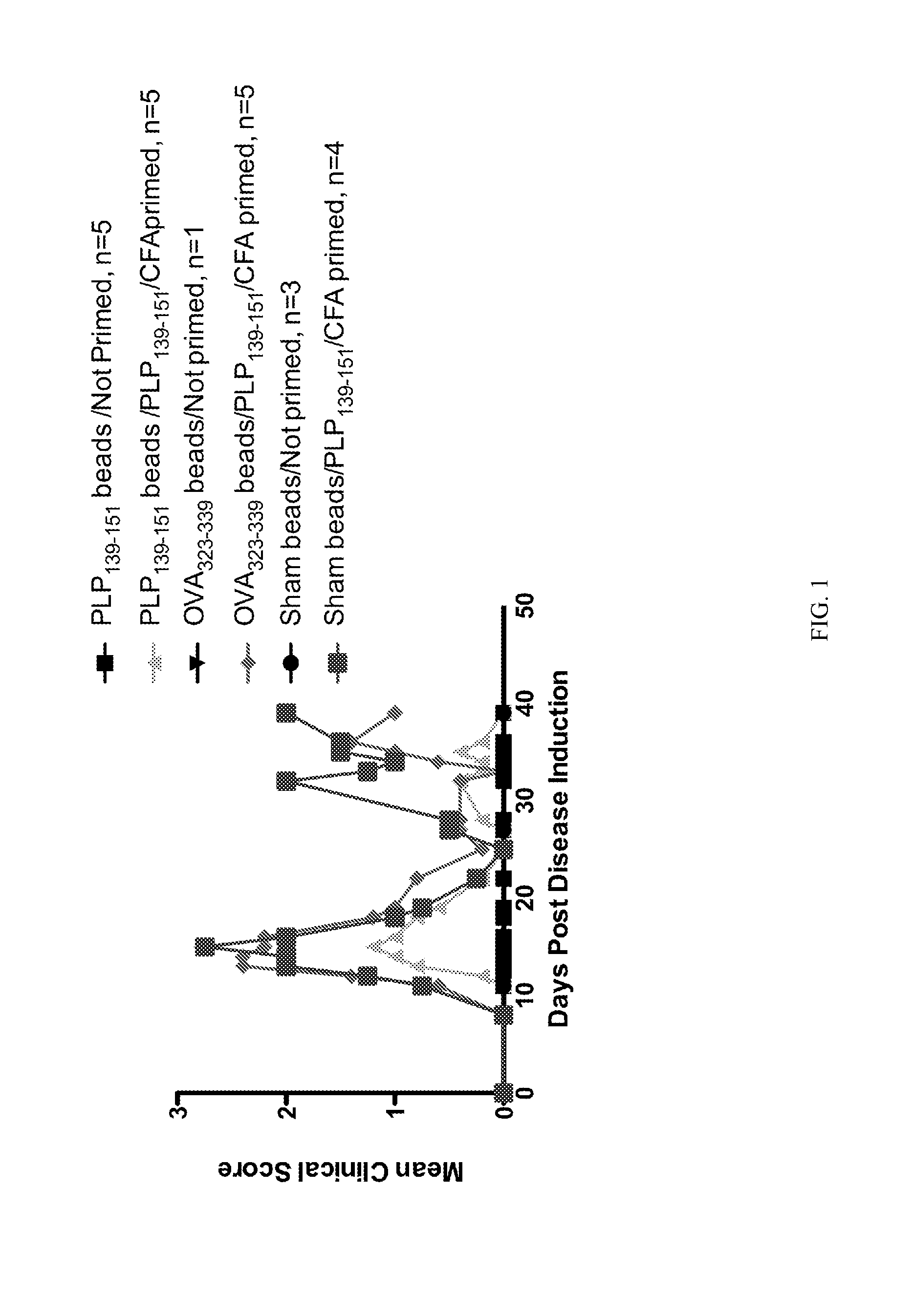
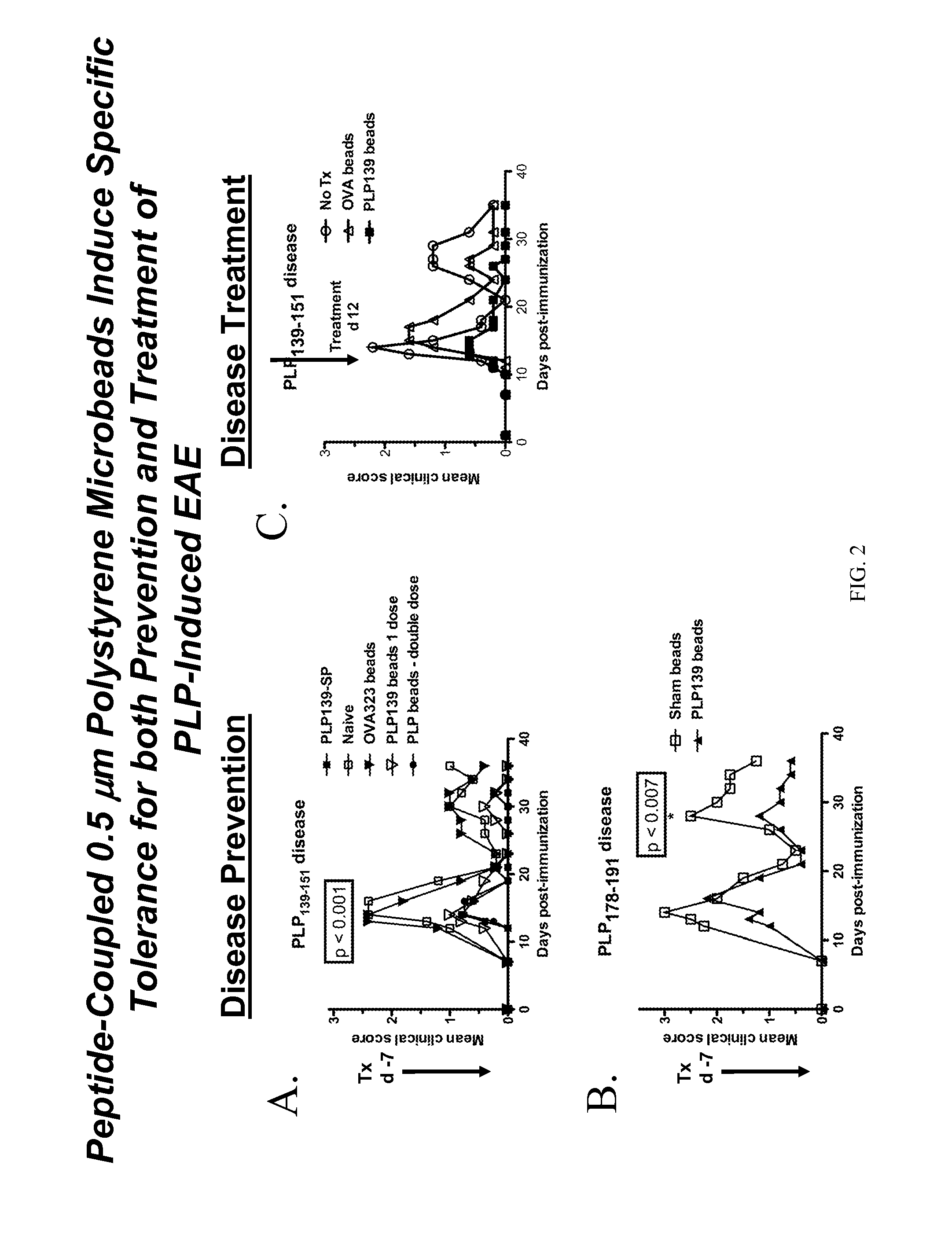
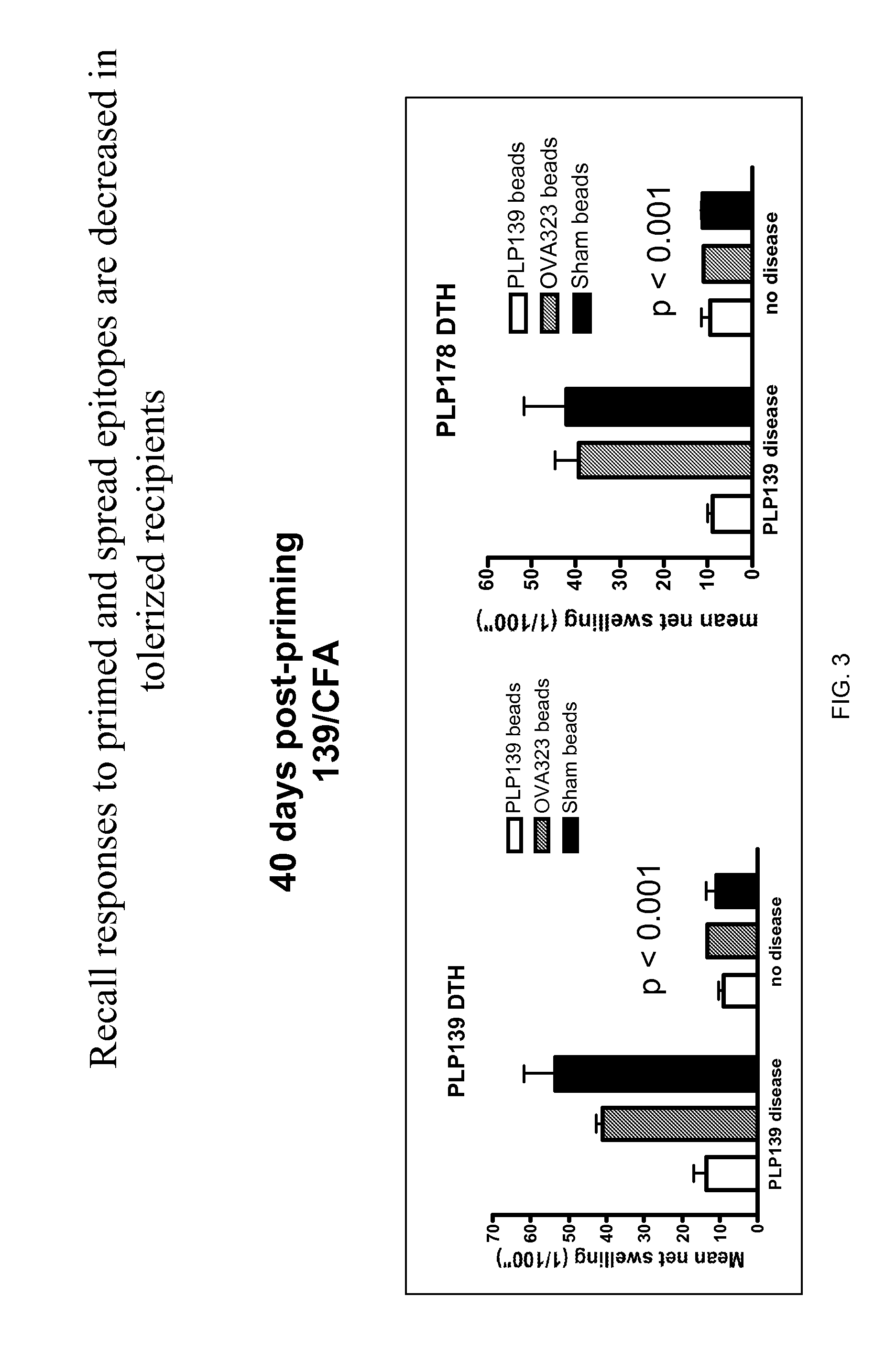
Patents
Literature
436 results about "Immune tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune tolerance, or immunological tolerance, or immunotolerance, is a state of unresponsiveness of the immune system to substances or tissue that have the capacity to elicit an immune response in given organism. It is induced by prior exposure to that specific antigen and contrasts with conventional immune-mediated elimination of foreign antigens (see Immune response). Tolerance is classified into central tolerance or peripheral tolerance depending on where the state is originally induced—in the thymus and bone marrow (central) or in other tissues and lymph nodes (peripheral). The mechanisms by which these forms of tolerance are established are distinct, but the resulting effect is similar.
Compositions and Methods for Targeted Immunomodulatory Antibodies and Fusion Proteins
ActiveUS20130039911A1Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antobodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antobodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immuno-suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β)) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By conteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for induction of antigen-specific tolerance
InactiveUS20120076831A1Efficiently inducing long-term immune tolerancePowder deliveryNervous disorderDiseaseTransplant rejection
The present invention utilizes carrier particles to present antigen peptides and proteins to the immune system in such a way as to induce antigen specific tolerance. The carrier particle is designed in order to trigger an immune tolerance effect. The invention is useful for treatment of immune related disorders such as autoimmune disease, transplant rejection and allergic reactions.
Owner:MILLER STEPHEN +4
Compositions and methods for targeted immunomodulatory antibodies and fusion proteins
ActiveUS8993524B2Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antibodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antibodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immune suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By counteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
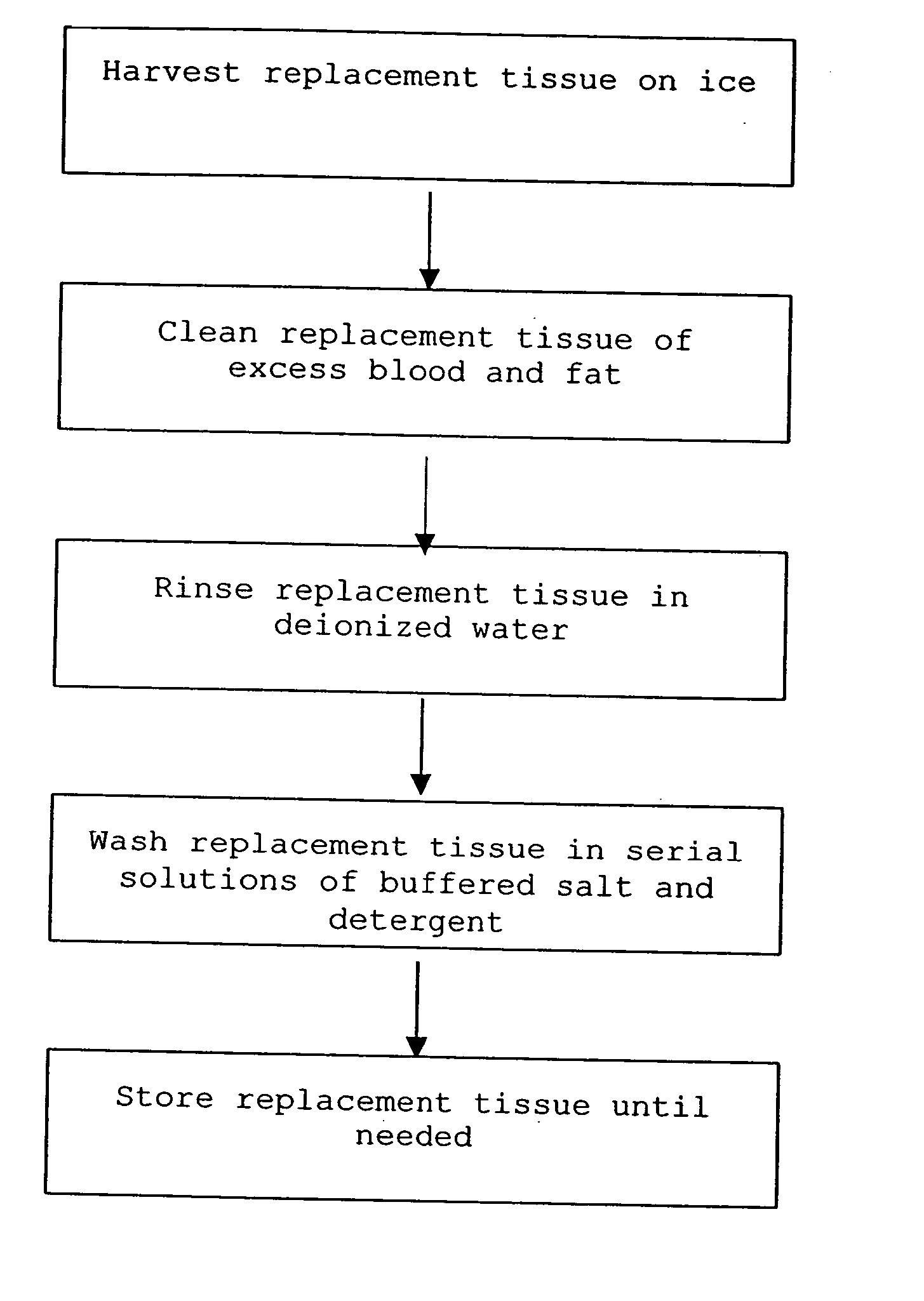
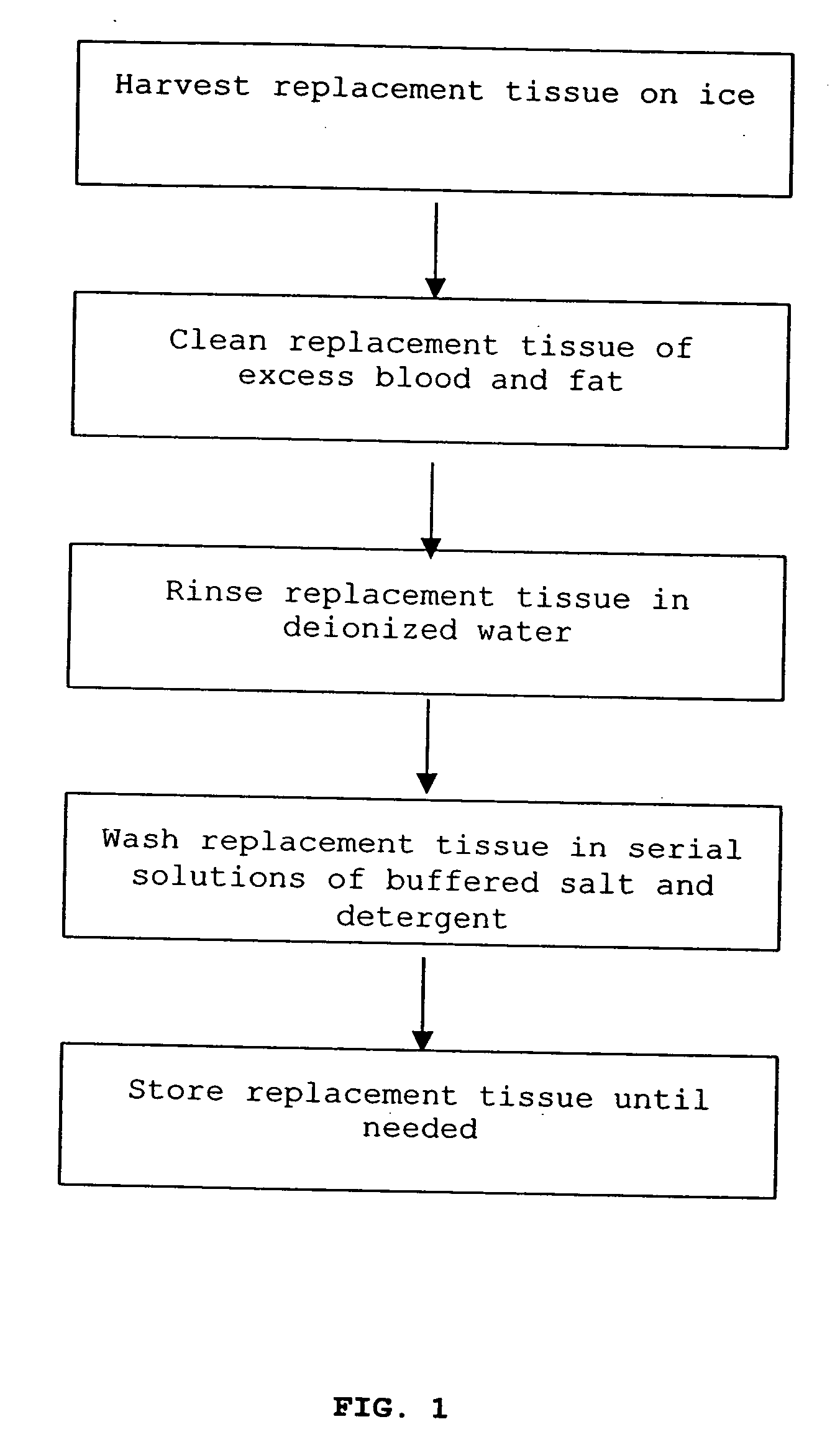
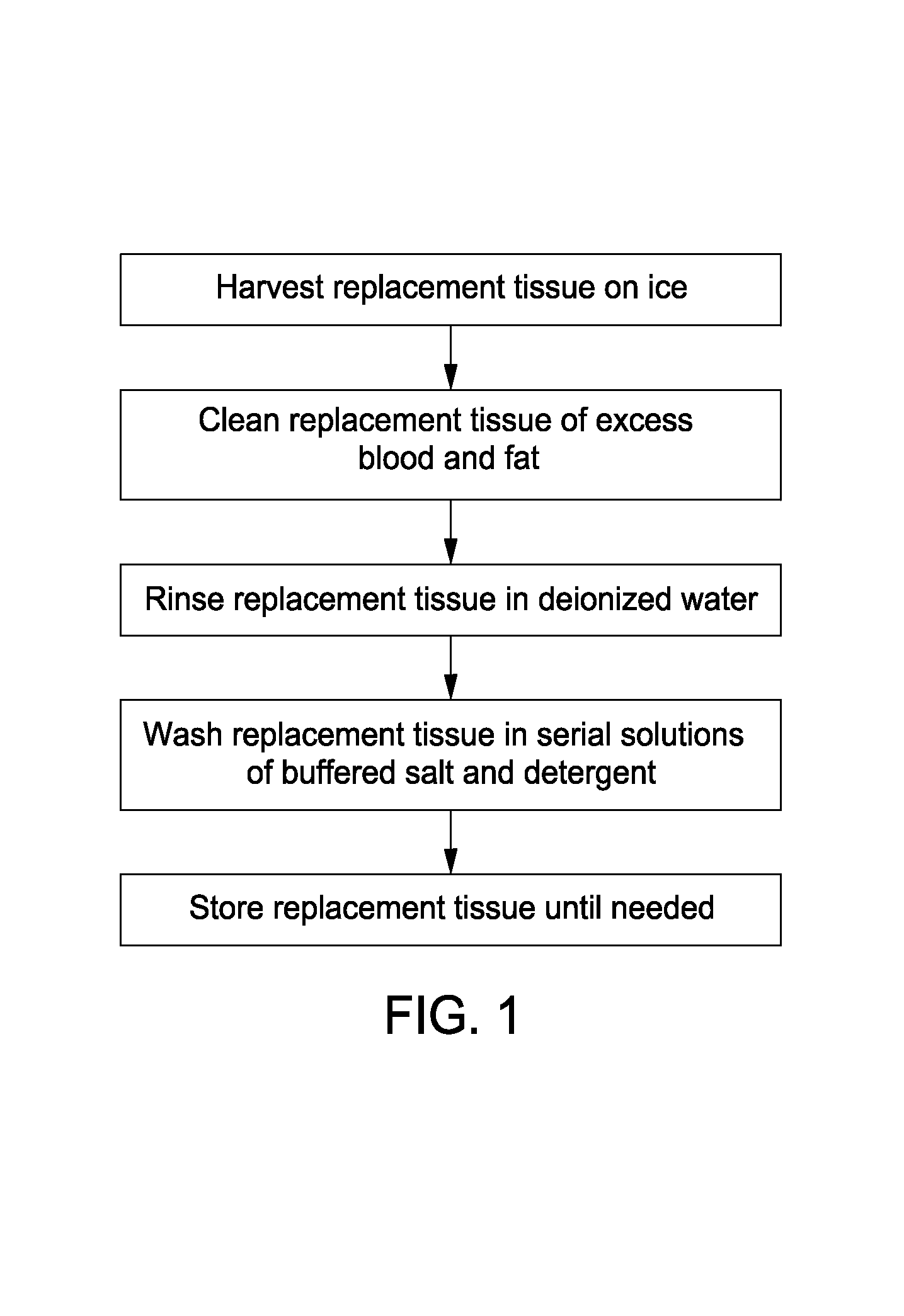
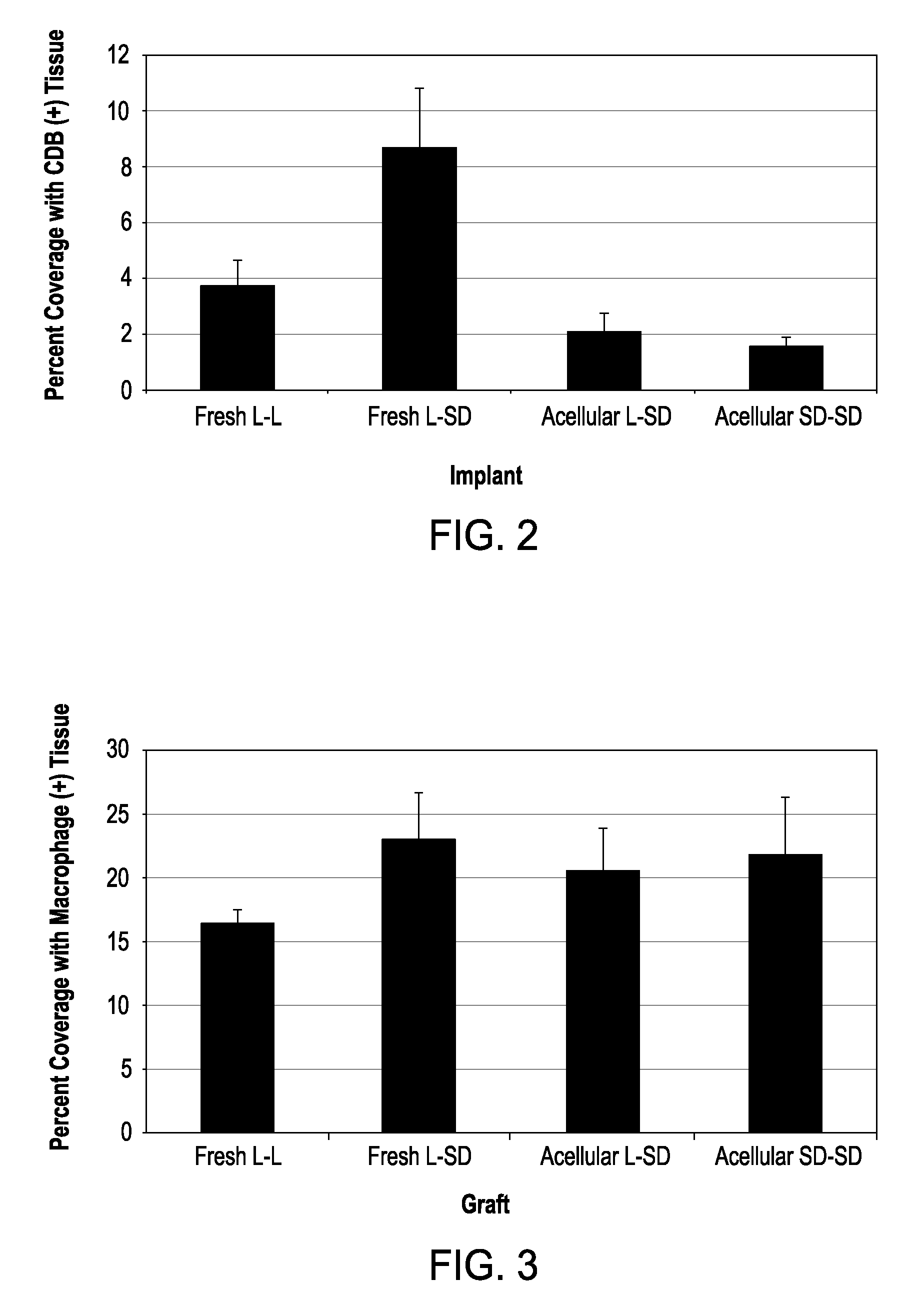
Cell-free tissue replacement for tissue engineering
InactiveUS20050043819A1Less remodelingPrevent penetrationPowder deliveryTissue regenerationCell freeNerve graft
The present invention is a natural, cell-free tissue replacement that does not require difficult or extensive preparation made by washing tissue replacement in a solution including one or more sulfobetaines and an anionic surface-active detergent and washing the tissue replacement in serial solutions of the buffered salt to remove excess detergent. The natural, cell-free tissue replacement may be a nerve graft that supports axonal regeneration, guides the axons toward the distal nerve end and / or is immunologically tolerated. Other forms of the invention are a composition and kit prepared by the method of making a native, cell-free tissue replacement. The present invention may be modified for use in diagnostic, therapeutic, and prophylactic applications.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
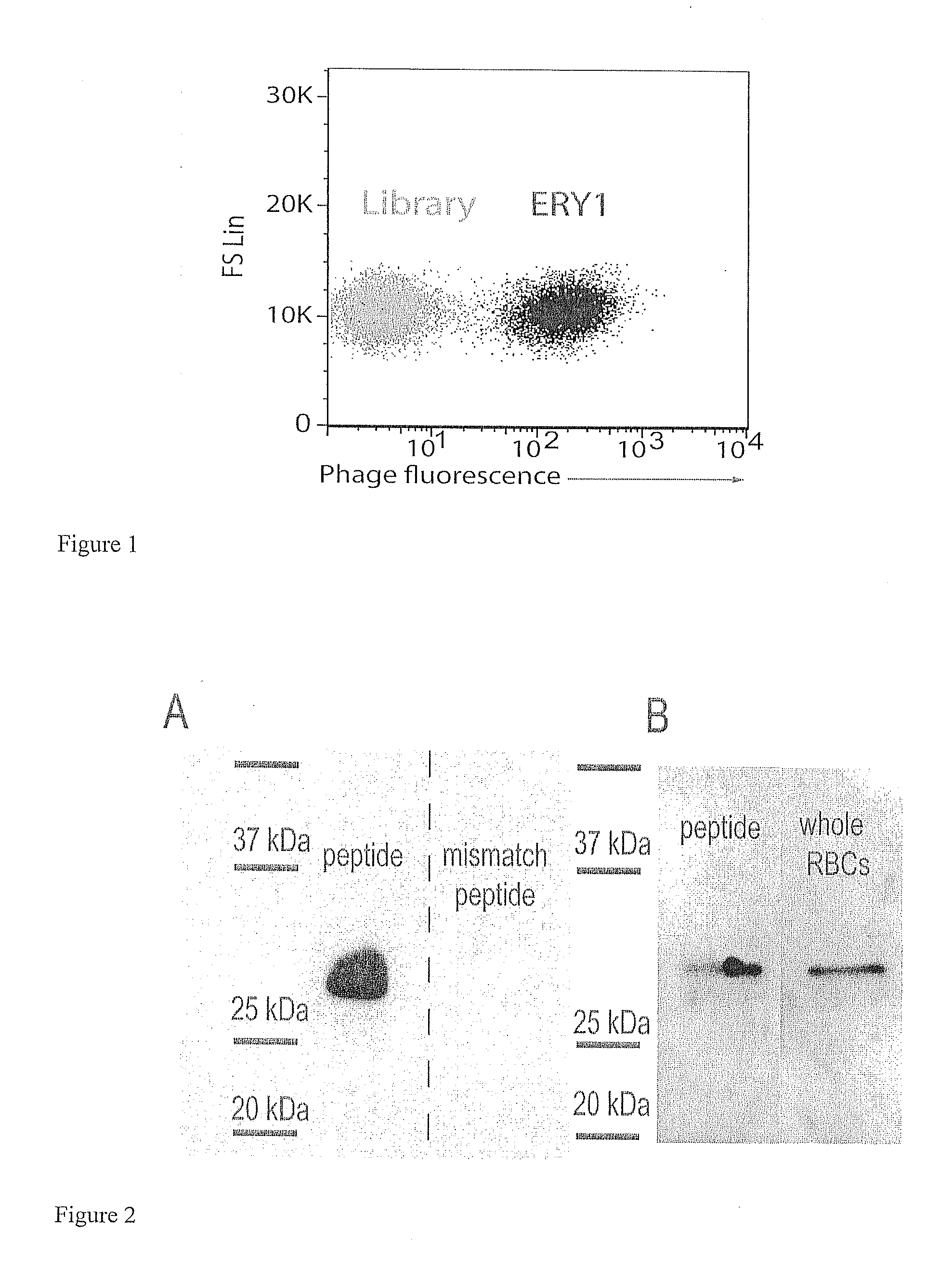
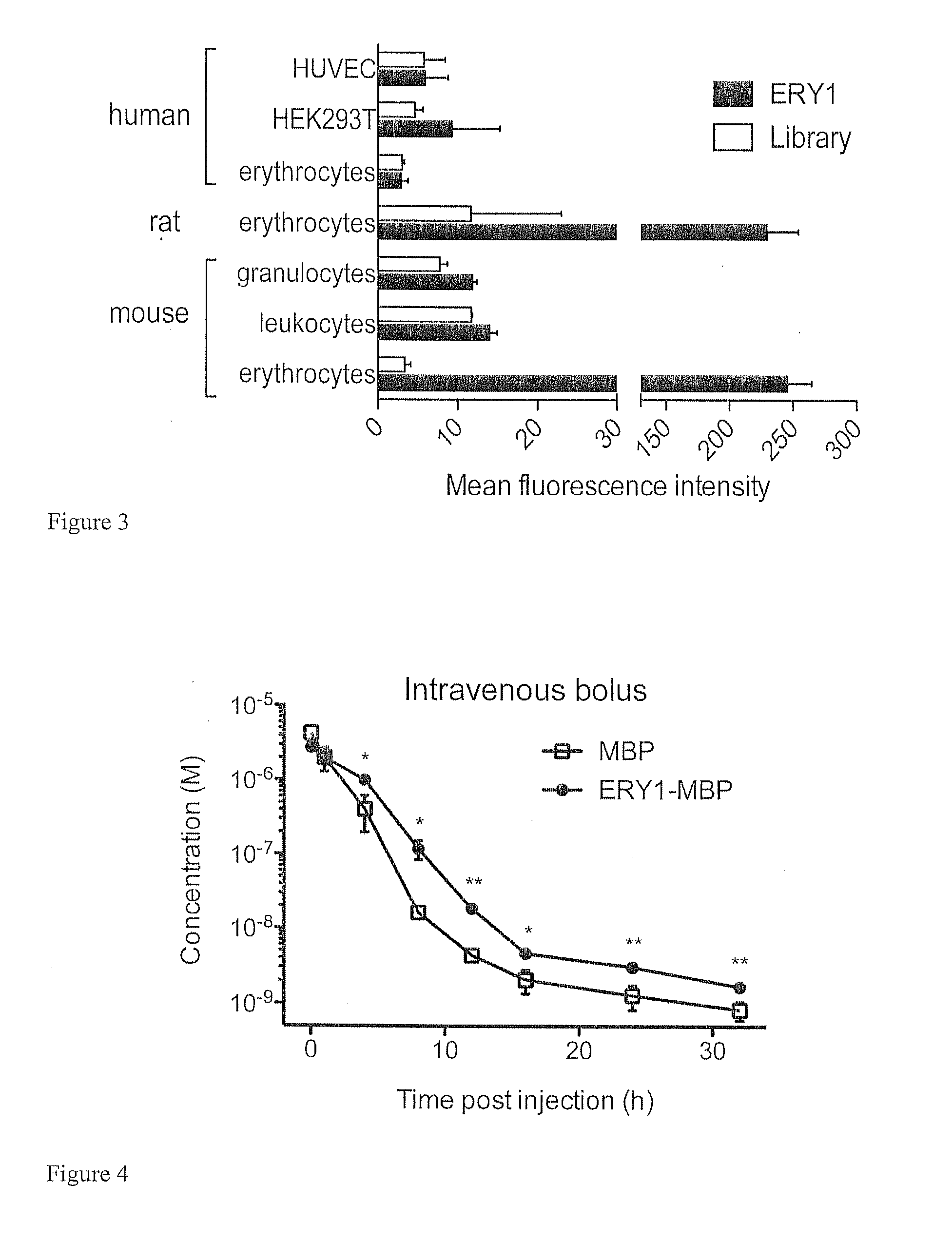
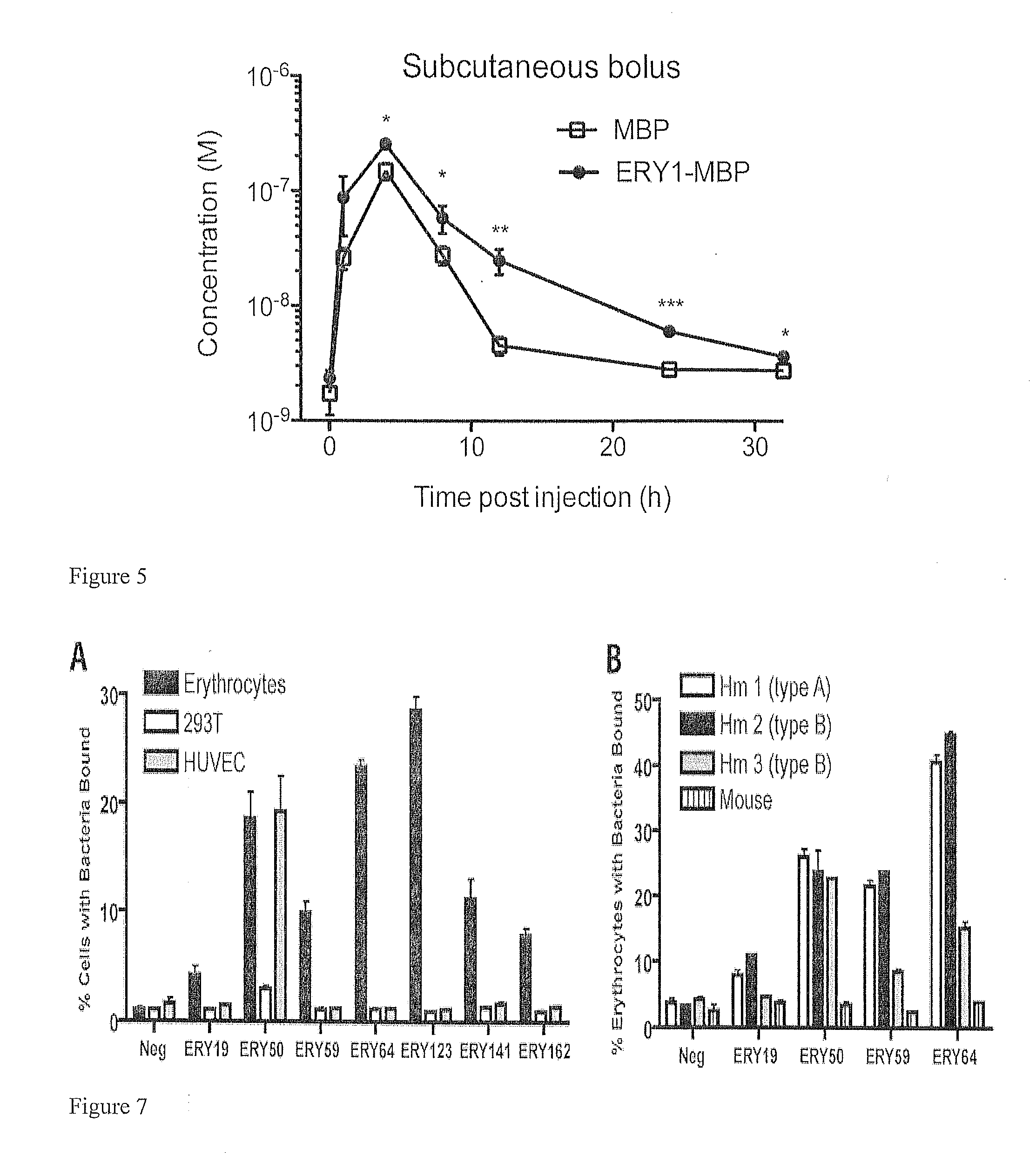
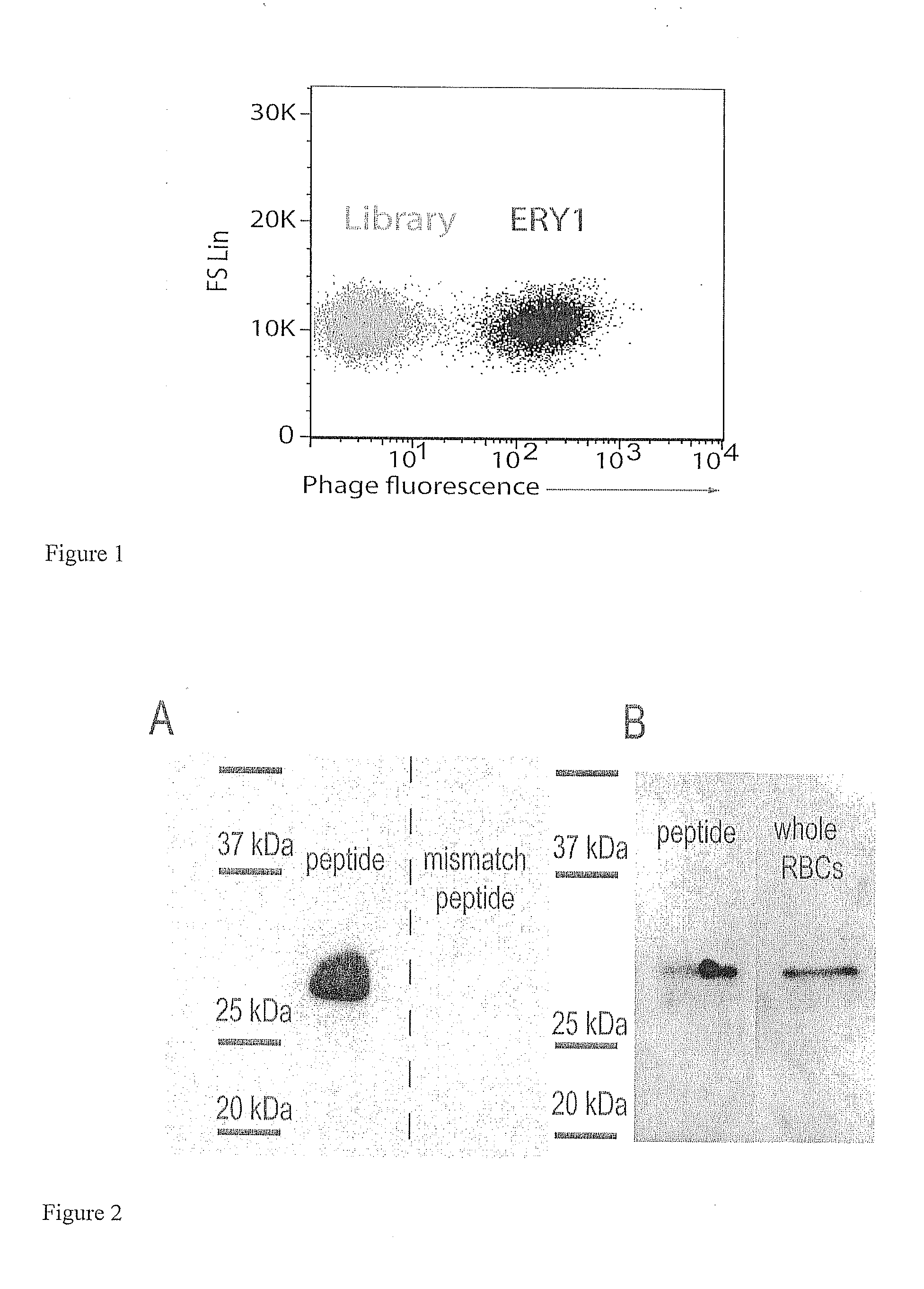
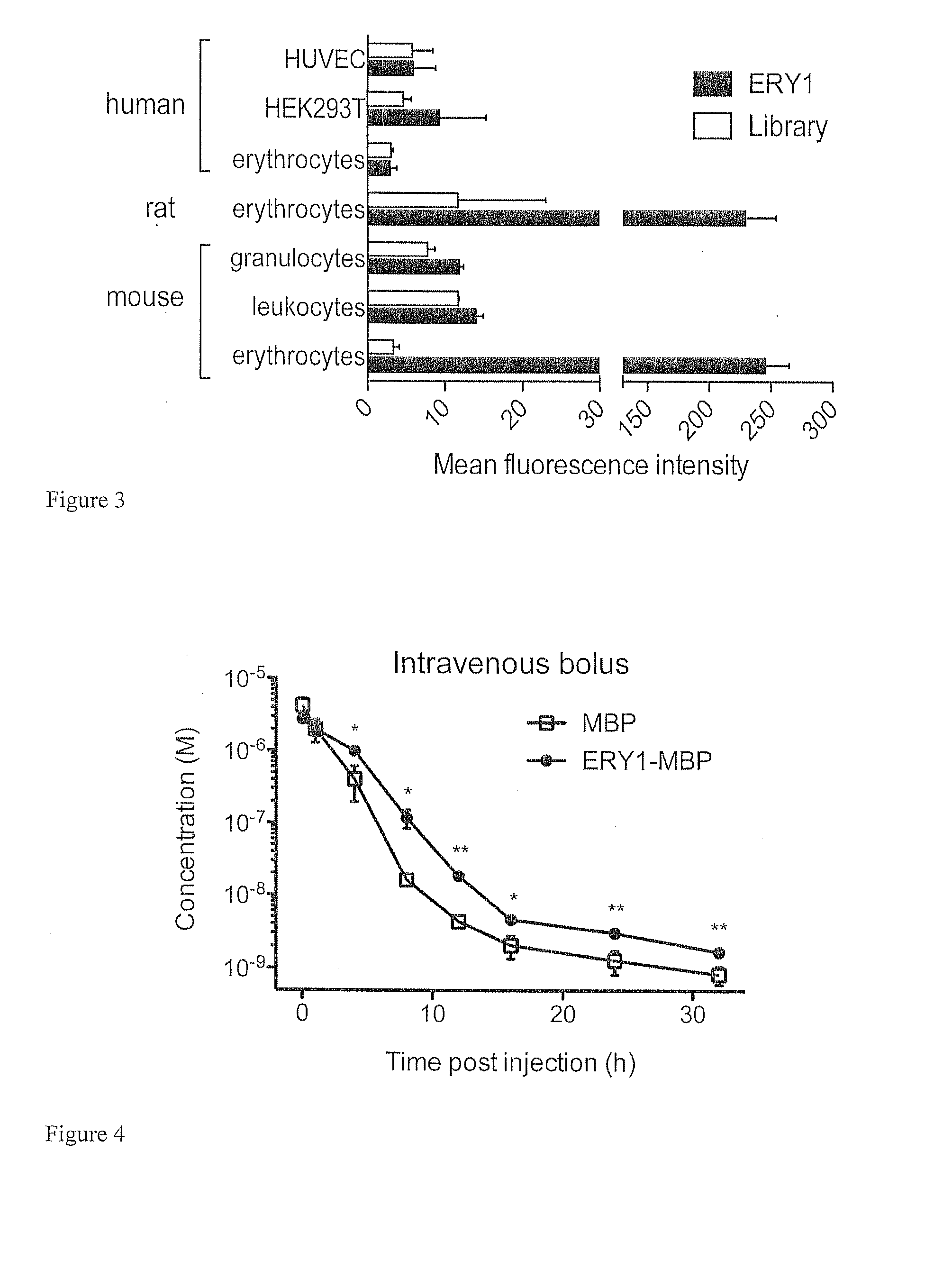
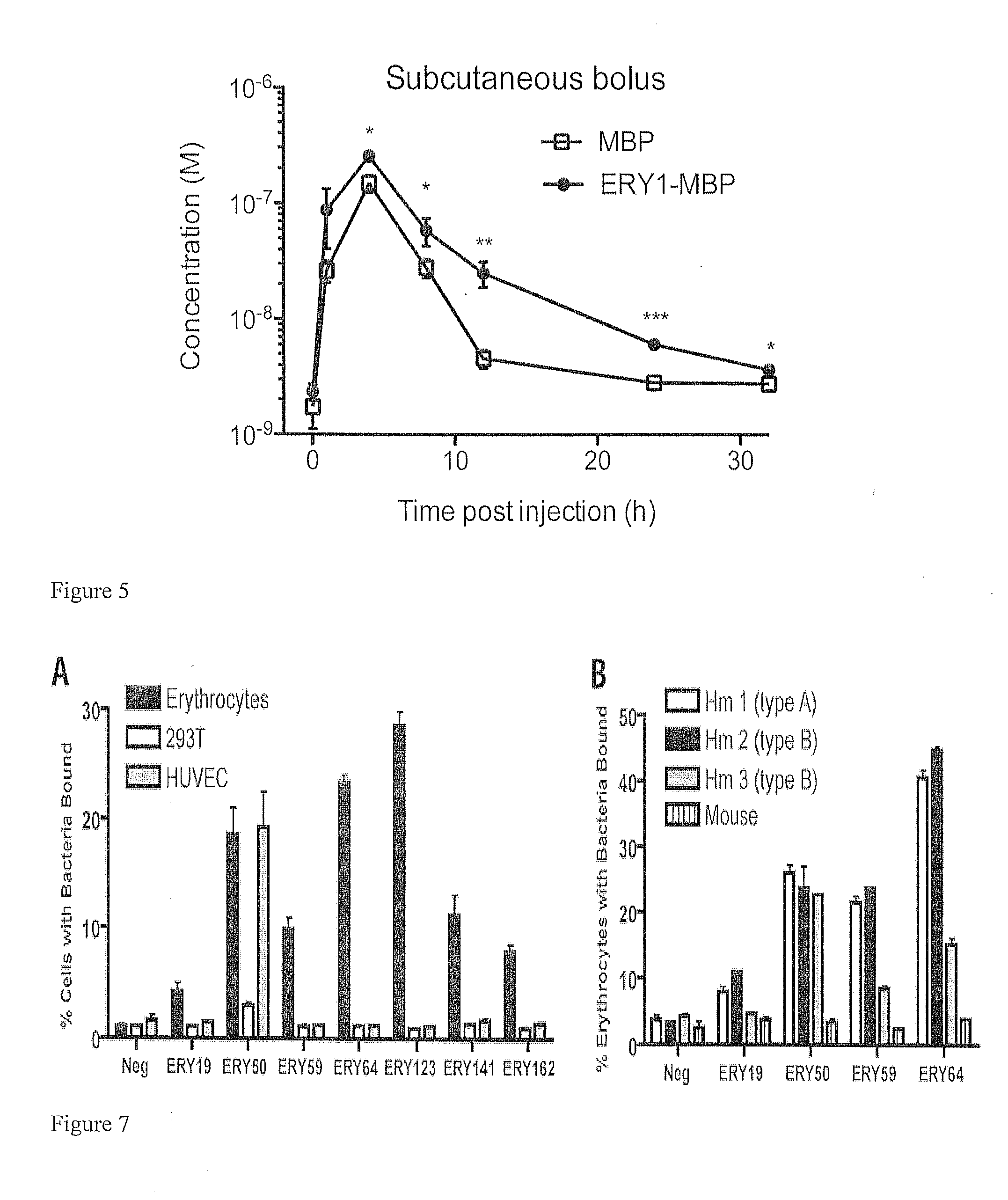
Erythrocyte-binding therapeutics
ActiveUS20120039989A1Convenient treatmentPeptide/protein ingredientsAntibody mimetics/scaffoldsErythrocyte bindingImmune tolerance
Peptides that specifically bind erythrocytes are described. These are provided as peptidic ligands having sequences that specifically bind, or as antibodies or fragments thereof that provide specific binding, to erythrocytes. The peptides may be prepared as molecular fusions with therapeutic agents, tolerizing antigens, or targeting peptides. Immunotolerance may be created by use of the fusions and choice of an antigen on a substance for which tolerance is desired. Fusions with targeting peptides direct the fusions to the target, for instance a tumor, where the erythrocyte-binding ligands reduce or entirely eliminate blood flow to the tumor by recruiting erythrocytes to the target.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Erythrocyte-binding therapeutics
ActiveUS20120178139A1Blockage of blood supplySimple processHydrolasesPeptide/protein ingredientsErythrocyte bindingImmune tolerance
Peptides that specifically bind erythrocytes are described. These are provided as peptidic ligands having sequences that specifically bind, or as antibodies or fragments thereof that provide specific binding, to erythrocytes. The peptides may be prepared as molecular fusions with therapeutic agents, tolerizing antigens, or targeting peptides. Immunotolerance may be created by use of the fusions and choice of an antigen on a substance for which tolerance is desired.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
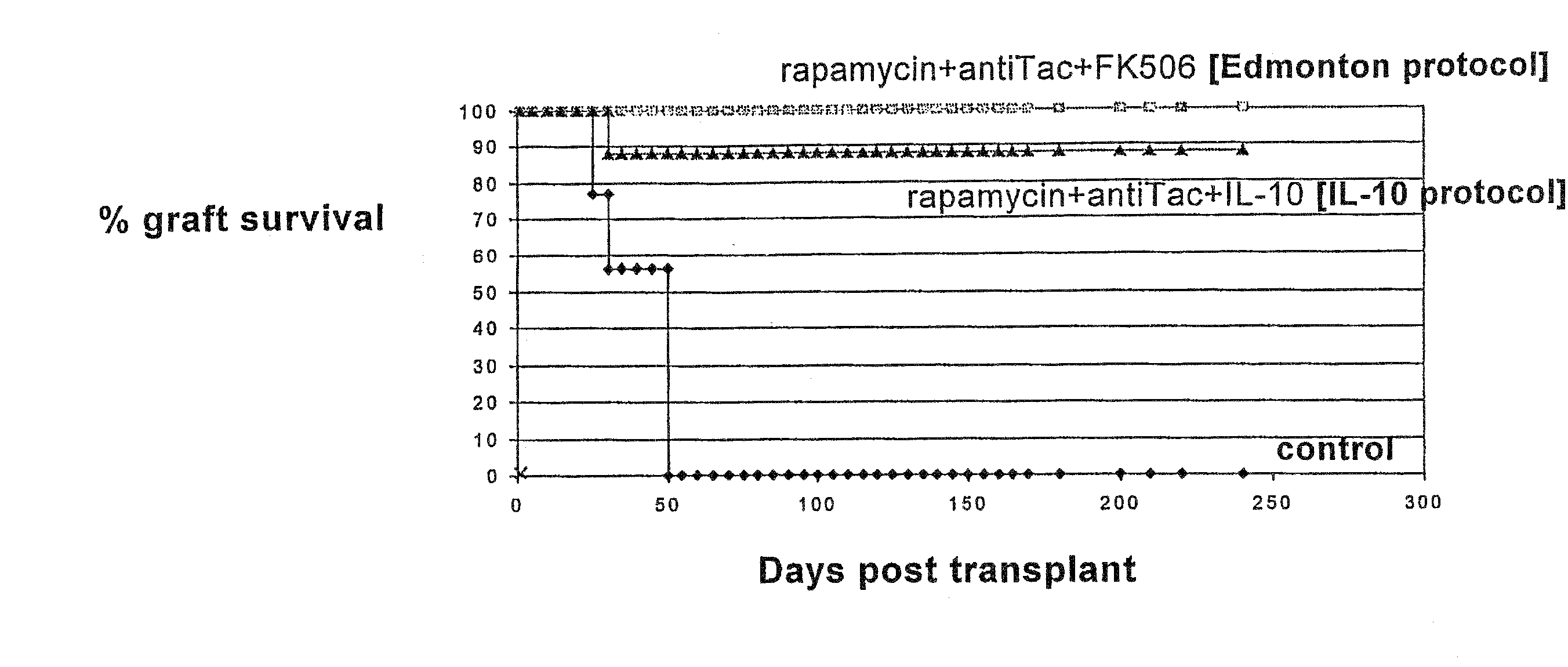
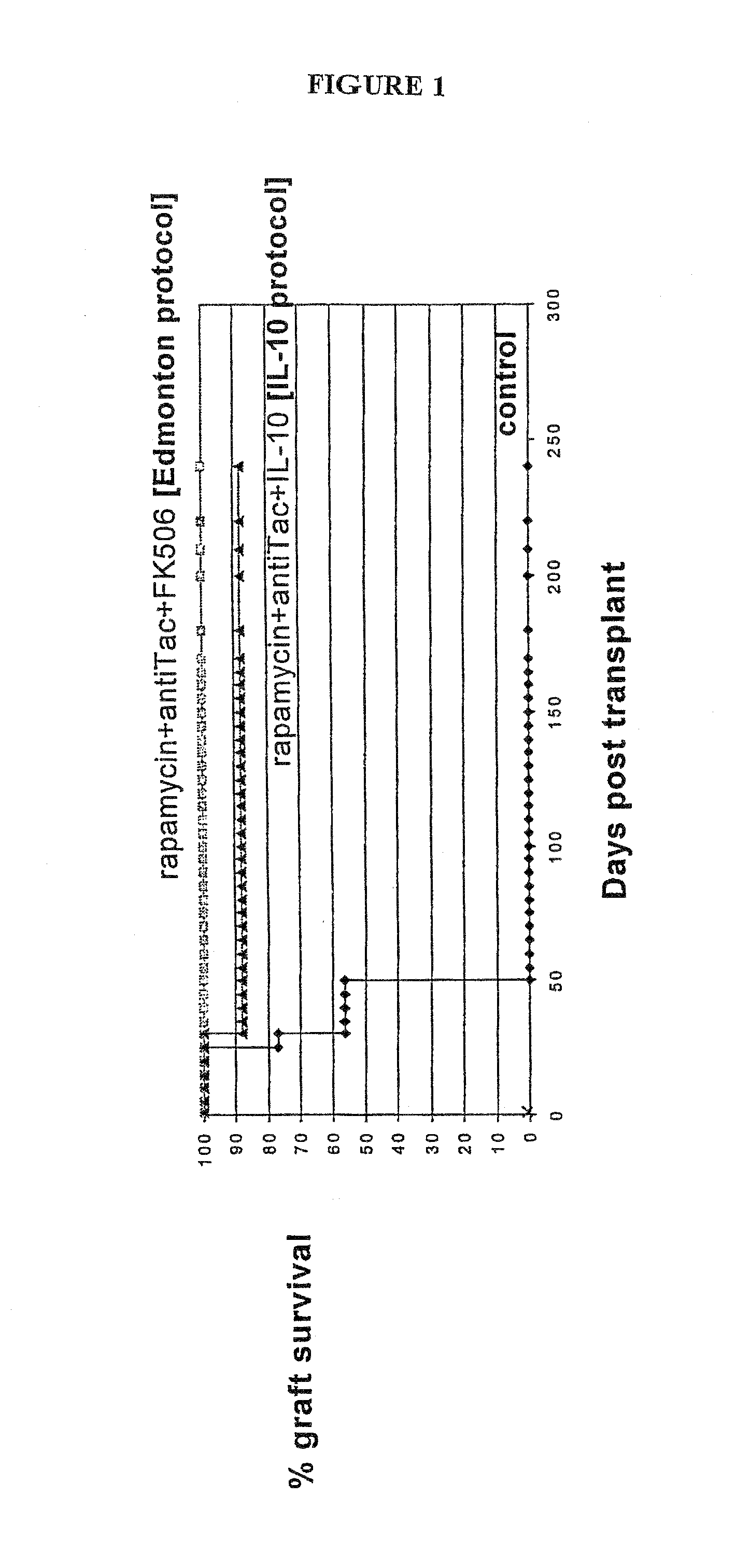
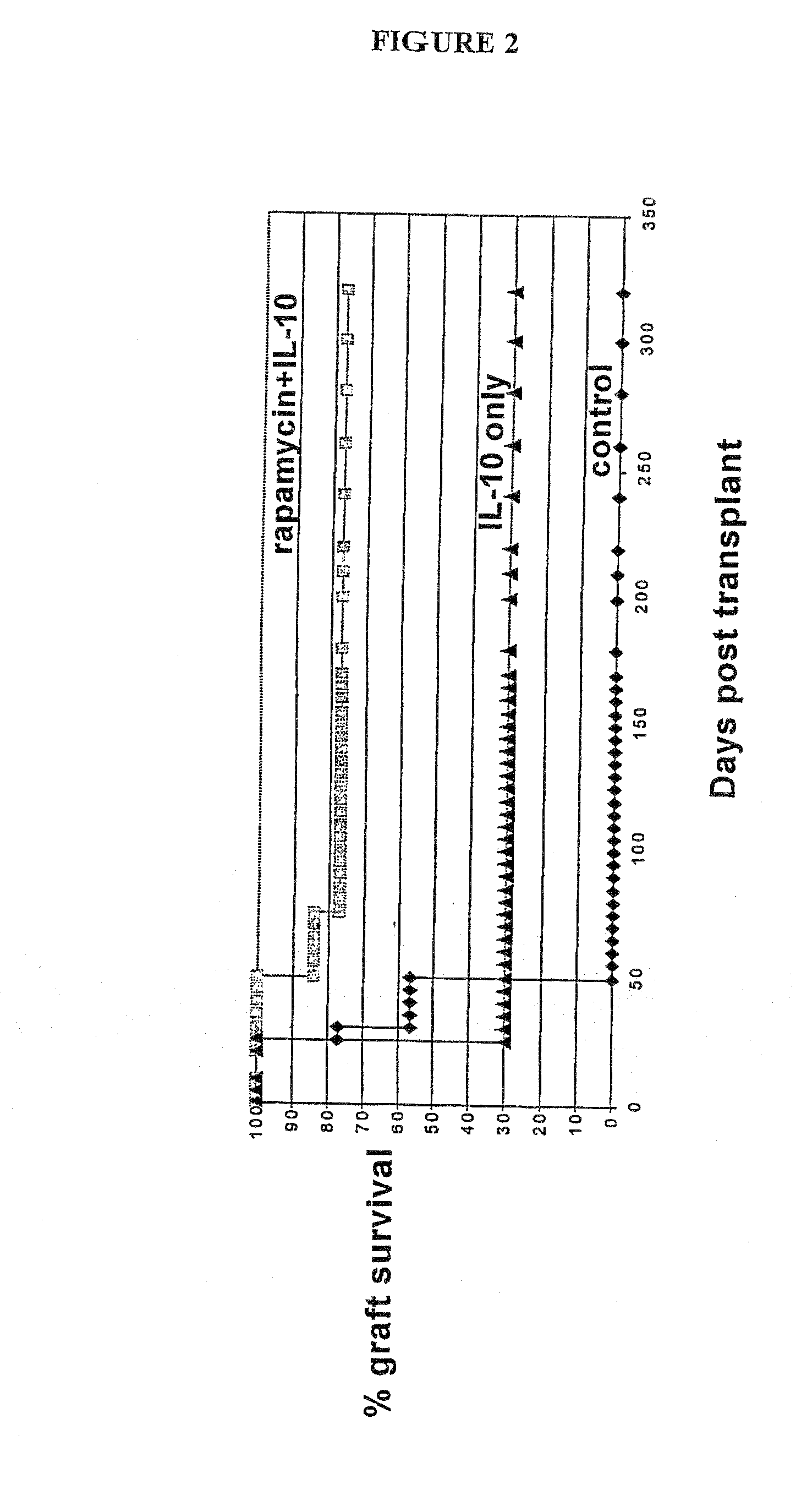
Rapamycin and il-10 for the treatment of immune diseases
ActiveUS20080069797A1Improve bioavailabilityImprove efficacyBiocideNervous disorderT cellImmune tolerance
The invention discloses a combined preparation containing IL-10 and rapamycin, able to induce immunosuppression and antigen-specific immune tolerance, and the use thereof in the treatment of diseases involving an excessive, dysfunctional or uncontrolled immune responses mediated by T cells.
Owner:TR1X INC
Targeted/immunomodulatory fusion proteins and methods for making same
ActiveUS20130287802A1Effective compositionEfficient methodBacteriaPeptide/protein ingredientsCancer cellNucleotide
The present invention relates generally to the field of generating fusion proteins to be used in cancer therapy, and more specifically, to nucleotide sequences encoding the fusion proteins, wherein the chimeric fusion proteins comprises at least one targeting moiety and at least one immunomodulatory moiety that counteracts the immune tolerance of cancer cells.
Owner:BIOCON LTD
Surrogate tolerogenesis for the development of tolerance to xenografts
InactiveUS6060049AIncrease differentiationIncreased proliferationBiocideGenetic material ingredientsHematopoietic cellTolerability
PCT No. PCT / US94 / 05844 Sec. 371 Date Jun. 6, 1995 Sec. 102(e) Date Jun. 6, 1995 PCT Filed May 24, 1994 PCT Pub. No. WO94 / 27622 PCT Pub. Date Dec. 8, 1993This invention provides a method for developing immune tolerance in xenogeneic organ graft recipients, in which lympho-hematopoietic cells from an intended organ graft recipient are differentiated within a xenogeneic surrogate, such as a fetal animal. After birth of the surrogate, the matured lympho-hematopoietic cells containing antigen specific regulatory cells, including suppressor cells, veto cells, select B cells, anti-idiotype antibodies, and other related factors responsible for antigen specific tolerance in a surrogate animal are reintroduced into the intended organ graft recipient, in conjunction with an organ transplant or a tissue transplant from the xenograft surrogate. The invention also provides an organ graft repopulated with cells from the intended organ graft recipient produced in a surrogate animal.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Mutant ctla4 gene transfected t cell and composition including same for anticancer immunotherapy
ActiveUS20140242049A1Enhance luciferase activityMaximize the effectBiocideAntibody mimetics/scaffoldsSide effectCancer cell
A transformed T-cell for T-cell therapy, and a composition including the same for anticancer immunotherapy. More particularly, the transformed T-cell is characterized by the transfection of a gene for coding a chimera protein. The T-cell, to which the gene for coding the chimera protein is transected, may improve the therapeutic effects induced by immune tolerance of cancer cells, and furthermore maximize anti-cancer effects by activating signal transduction to induce the activation of T-cells. Also, the disclosure allows treatments that minimize side effects such as the development of autoimmune diseases due to systematic T-cell activation.
Owner:NAT CANCER CENT
Fusion protein for treating diabetes, and its preparing method and use
InactiveCN1935846AImprove efficacyEasy to separate and purifyOrganic active ingredientsMetabolism disorderHalf-lifeFactor ii
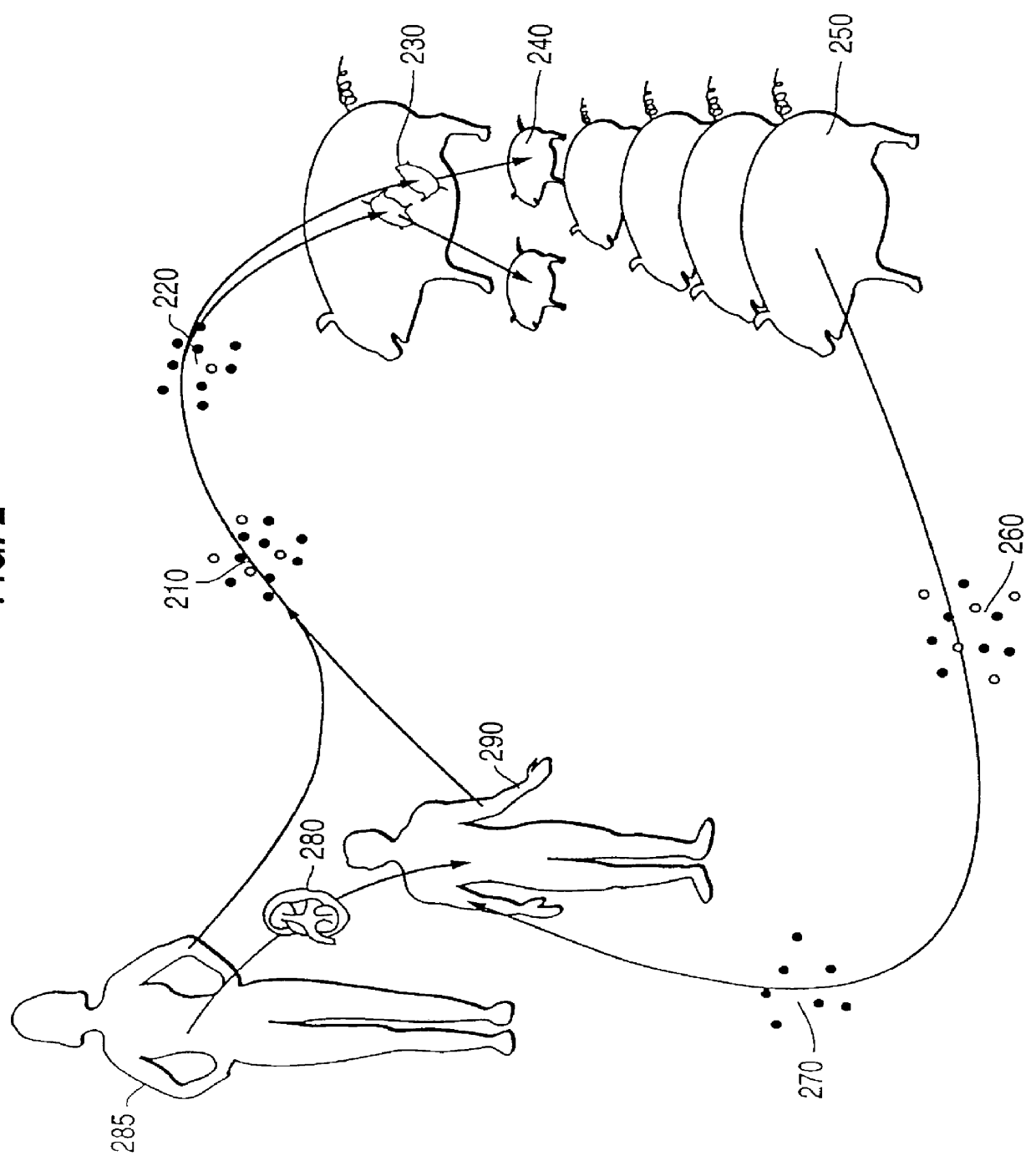
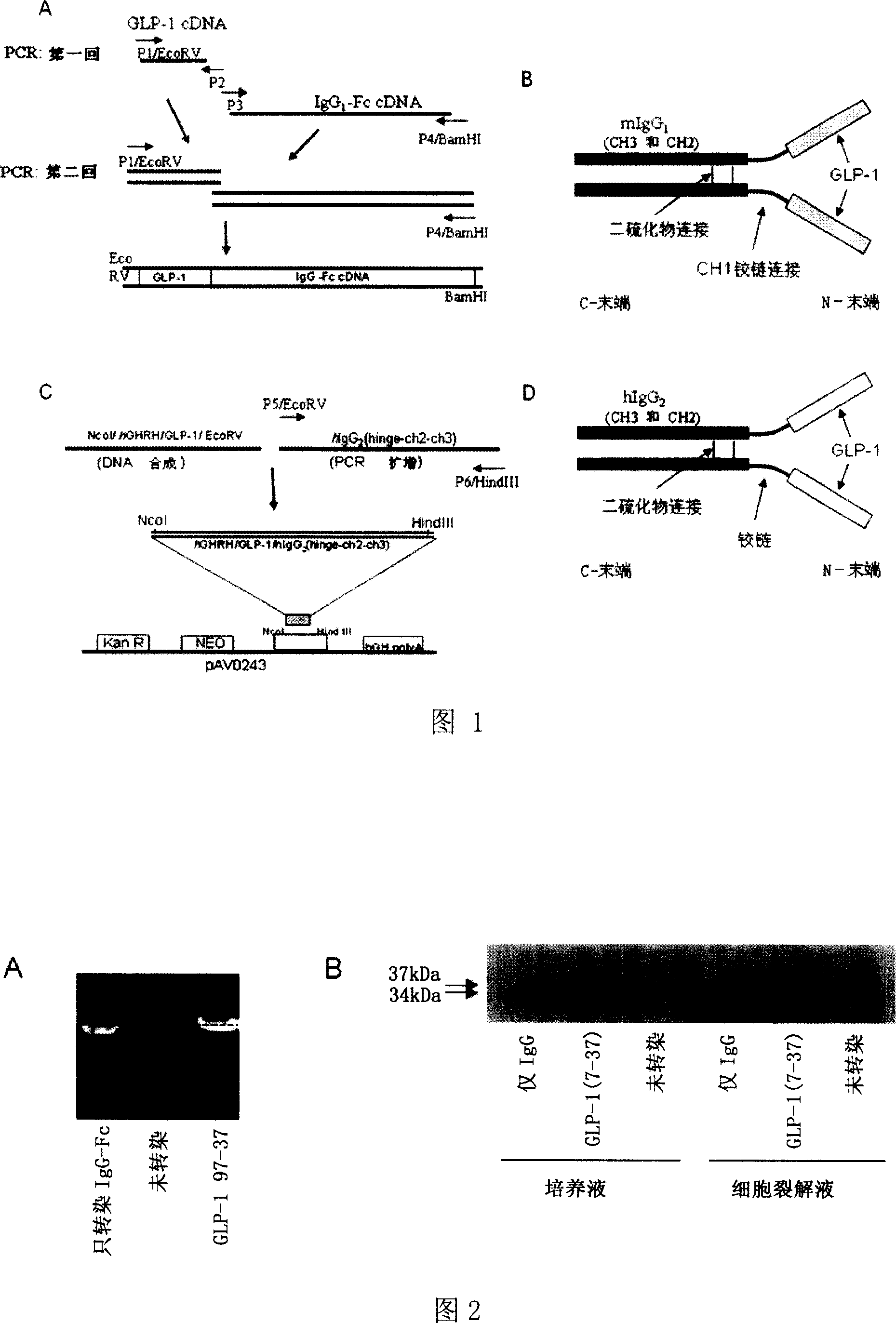
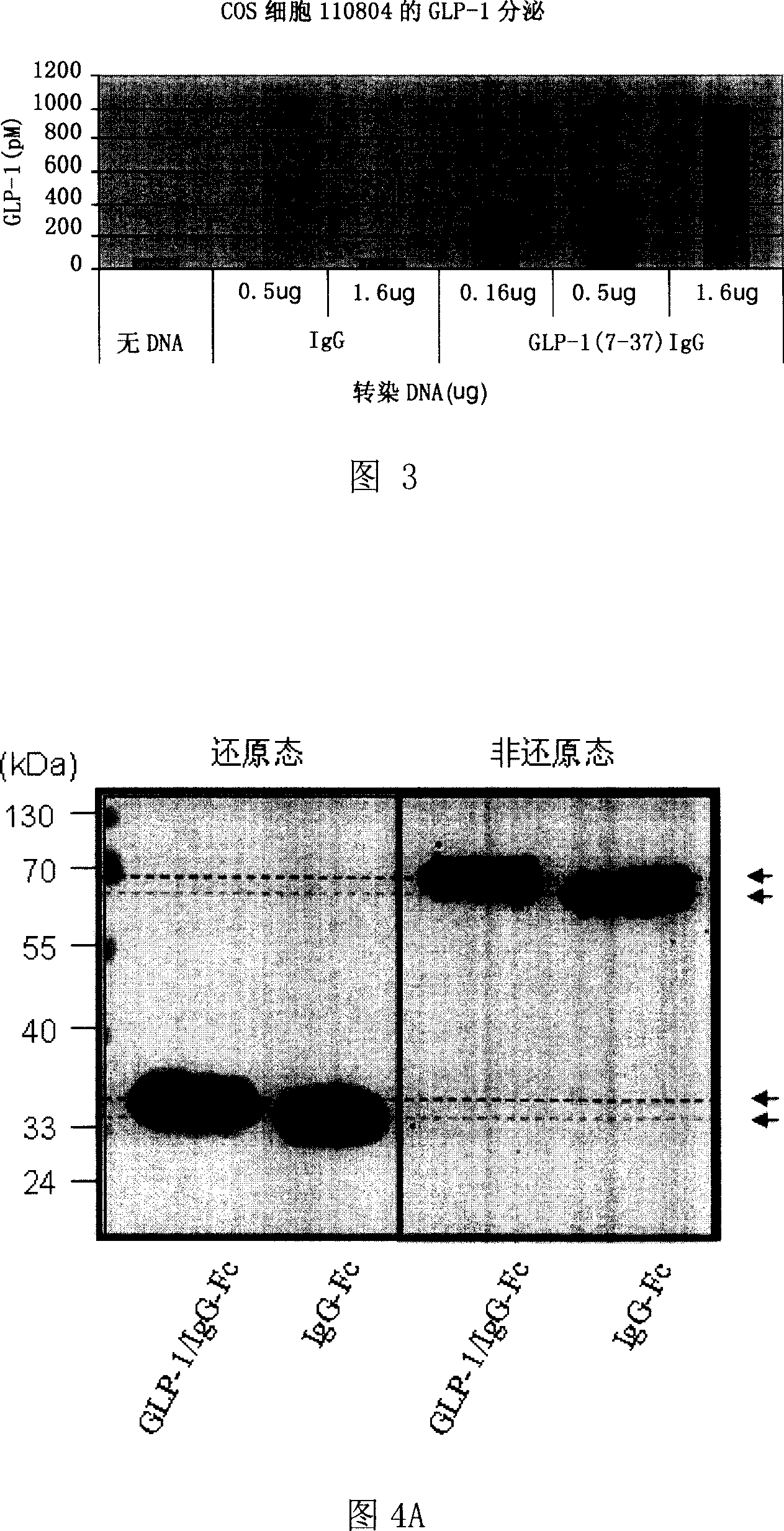
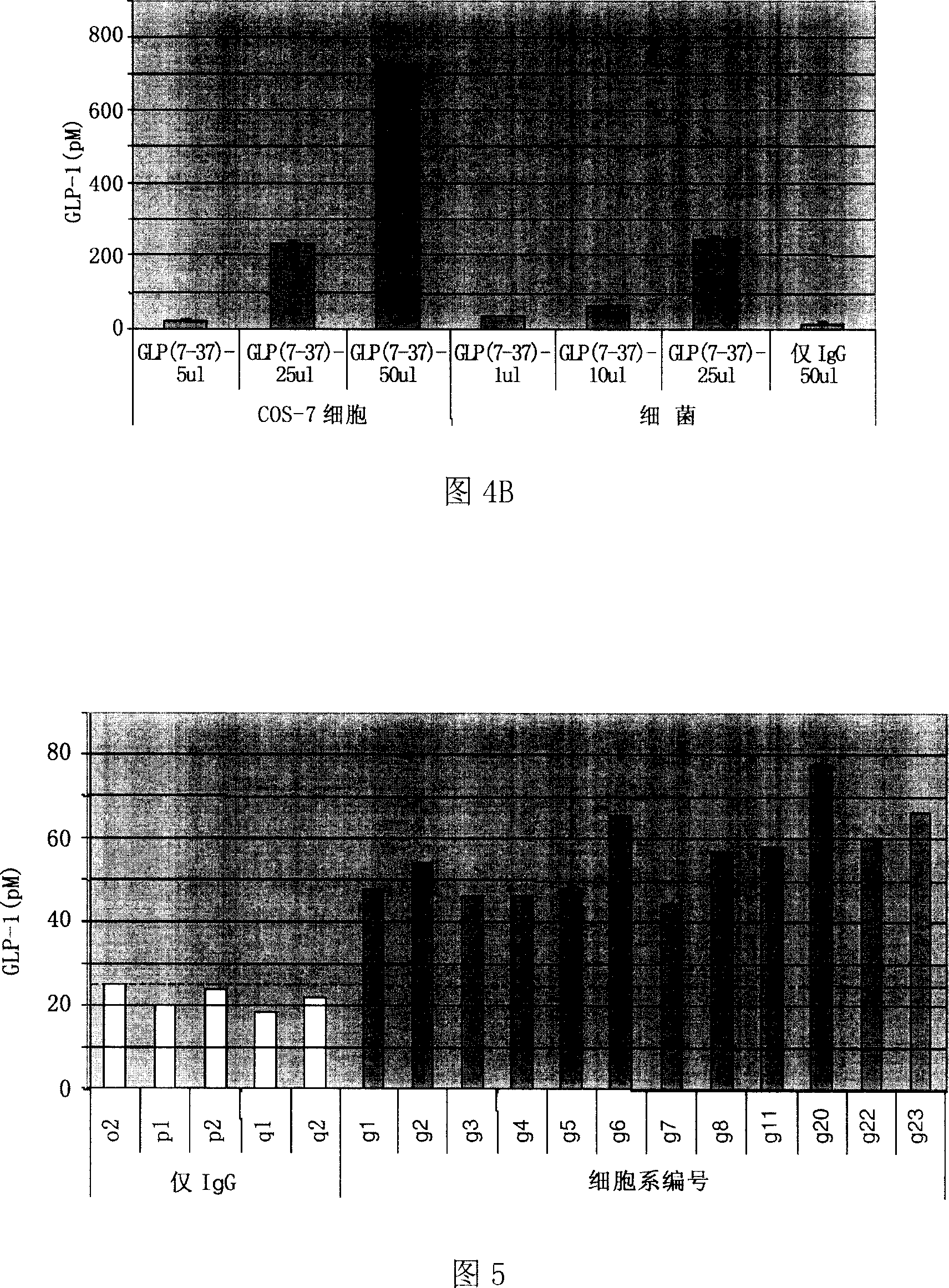
The invention relates to fusion protein used to prevent and cure I type and II type diabetes and its preparation method and application. It supplies the fusion protein which is formed by glucagon analogy peptide GLP-1, GLP-1 mutant and IgG-Fc, its analogy factor lizard salivary gland polypeptide extendin-4 and IgG-Fc, and the application of the above fusion protein and its DNA used in diabetes prevention and therapy. The fusion protein can increase not only GLP-1 effect, but also the affinity and immunological tolerance of ligand, which is excreted with the form of homogeneity dimmer to improve polypeptide drug effect, overcomes the defect of the GLP-1 for short half-life, and simplifies purification process.
Owner:王庆华 +1
Formative agent of protein complex
InactiveUS20020119946A1Keep for a long timePromote resultsBiocidePeptide/protein ingredientsCartilage cellsCuticle
The present invention proposes formative agent of protein complex, in which a polyphenol is useful component, and the agent is useful as gene complex, cell adhesion inhibitor or immune tolerogen. The polyphenol of forming the agent is selected from catechin group consisting of epigallocatechin-gallate, tannic acids, or proanto-dianisidine, a protein of the protein complex is selected from proteins consisting of animal proteins composed of polypeptide chain of peptide-combined amino acids, vegetative proteins, nucleus proteins, glycogen proteins, lipo-proteins and metal proteins, the gene complex comprises by compositing genes by polyphenol catechins in order to introduce genes to cells of animals or human bodies, a cell composed of the cell adhesion inhibitor is selected from cells consisting of an animal cell including a stem cell, skin cell, mucosa cell, hepatocyte, islet cell, neural cell, cartilage cell, endothelial cell, epidermal cell, osteocyte or muscle cell isolated from human or animal organism, or sperm, ovum or fertilized egg of domestic animals or fishes and a tissue or an organ for transplantation of the immune tolerogen is selected from the tissue or the organ consisting of skin, blood vessel, cornea, kidney, heart, liver, umbilical cord, bowels, nerve, lung, placenta or pancreas.
Owner:BERTELSMANN MUSIC GROUP
Cell-free tissue replacement for tissue engineering
InactiveUS7402319B2Prevent penetrationLoss of functionPowder deliveryTissue regenerationCell freeNerve graft
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and compositions for induction or promotion of immune tolerance
InactiveUS20060193869A1Suppressing autoimmune responseSuppresses autoimmune responsePeptide/protein ingredientsGenetic material ingredientsImmune tolerancePolynucleotide
The invention provides non-immunostimulatory polynucleotide antigen conjugates and methods for treating unwanted immune reactions in individuals using the non-immunostimulatory polynucleotide antigen conjugates.
Owner:DYNAVAX TECH CORP
Mesenchymal stem cell-mediated autologous dendritic cells with increased immunosuppression
InactiveUS20100055076A1Inhibition of secretionPromote secretionBiocideMammal material medical ingredientsDiseaseMesenchymal stem cell
Owner:JW CREAGENE
Method and pharmaceutical composition for the treatment of multiple sclerosis
InactiveUS20030166589A1Peptide/protein ingredientsGenetic material ingredientsDiseaseAutoimmune responses
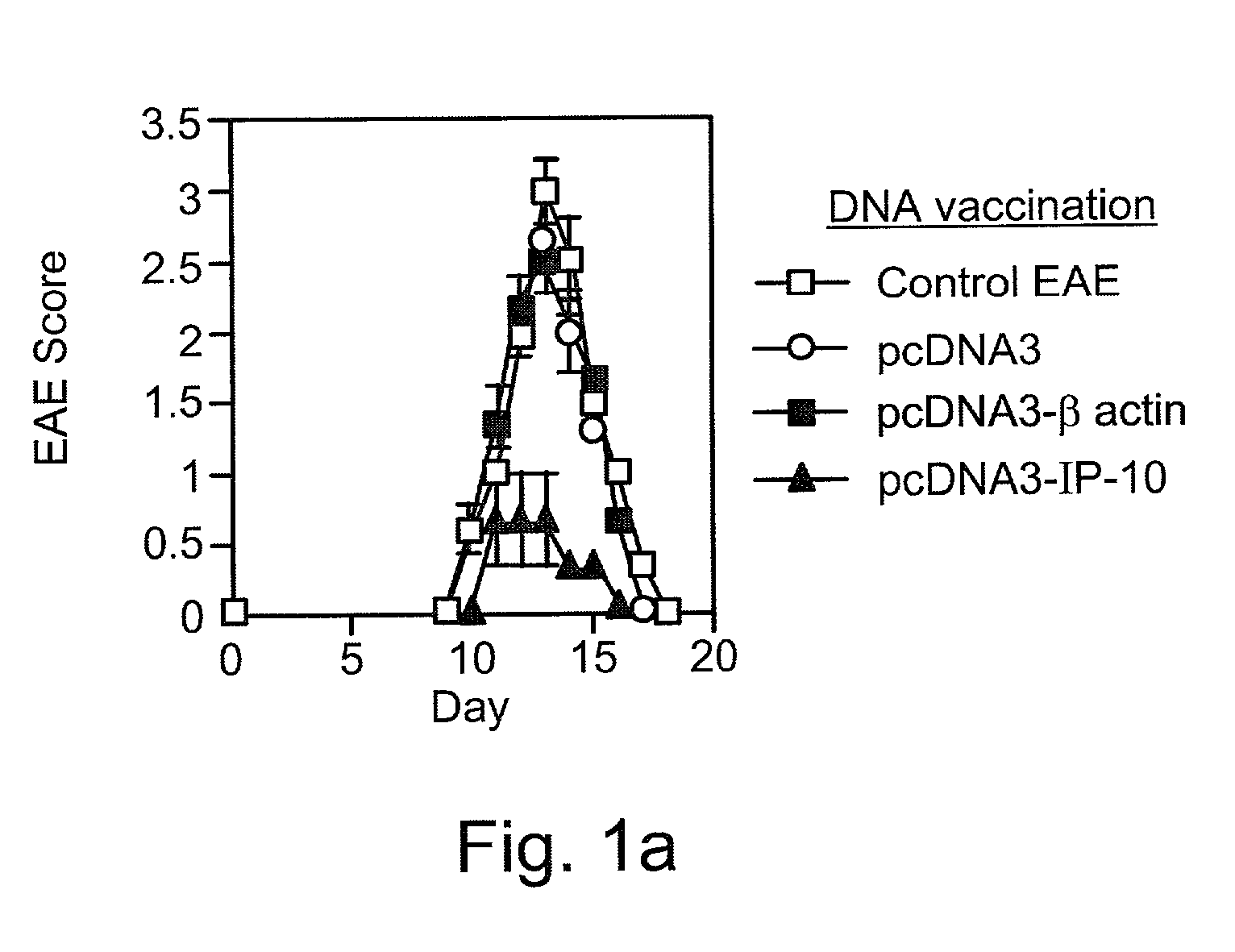
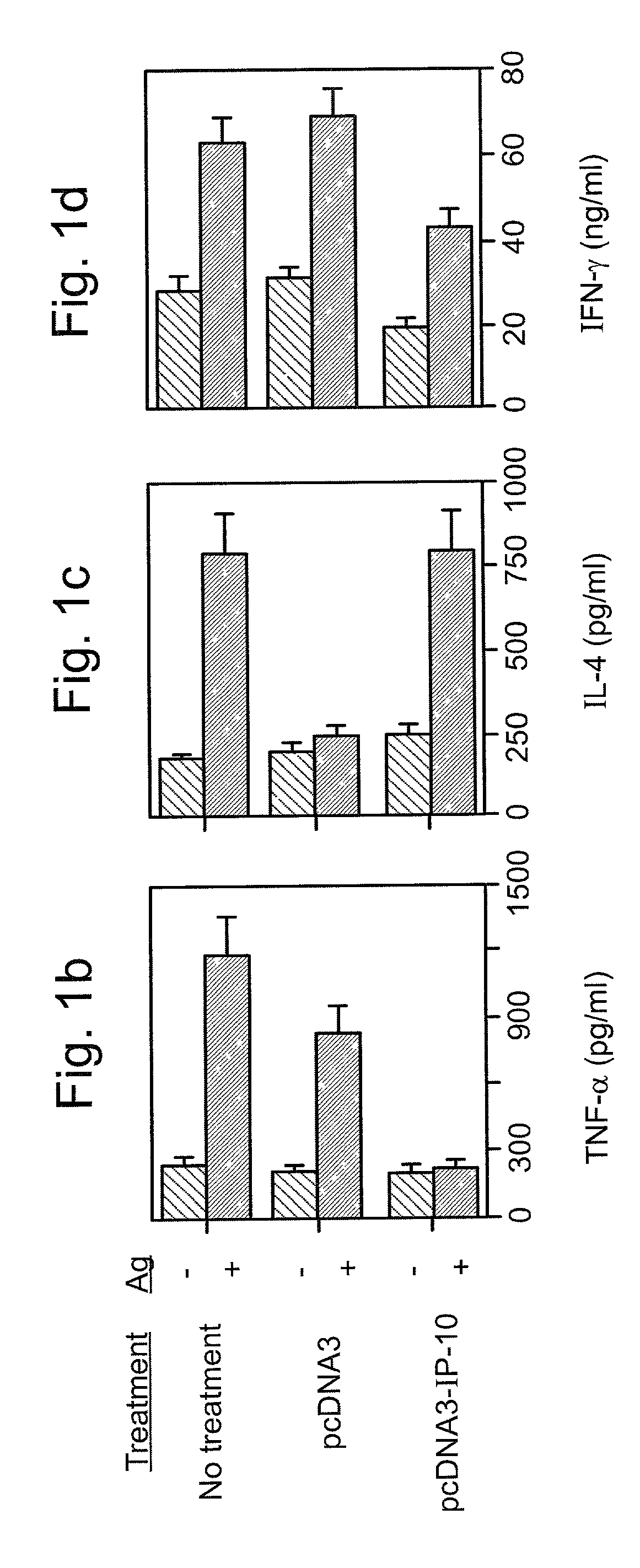
Methods and pharmaceutical compositions are disclosed, effective in breaking-down immunological tolerance the CXC chemokine interferon gamma-inducible protein 10 (IP-10), resulting in the generation of self specific immunity to IP-10, for the treatment of diseases, such as autoimmune diseases, in which IP-10 plays a pivotal role in disease onset and / or progression, e.g., in multiple sclerosis (MS).
Owner:KARIN NATHAN
Induction of antigen specific immunologic tolerance
InactiveUS7485314B2Improve tolerancePromote absorptionBiocideSnake antigen ingredientsEpitopeRegimen
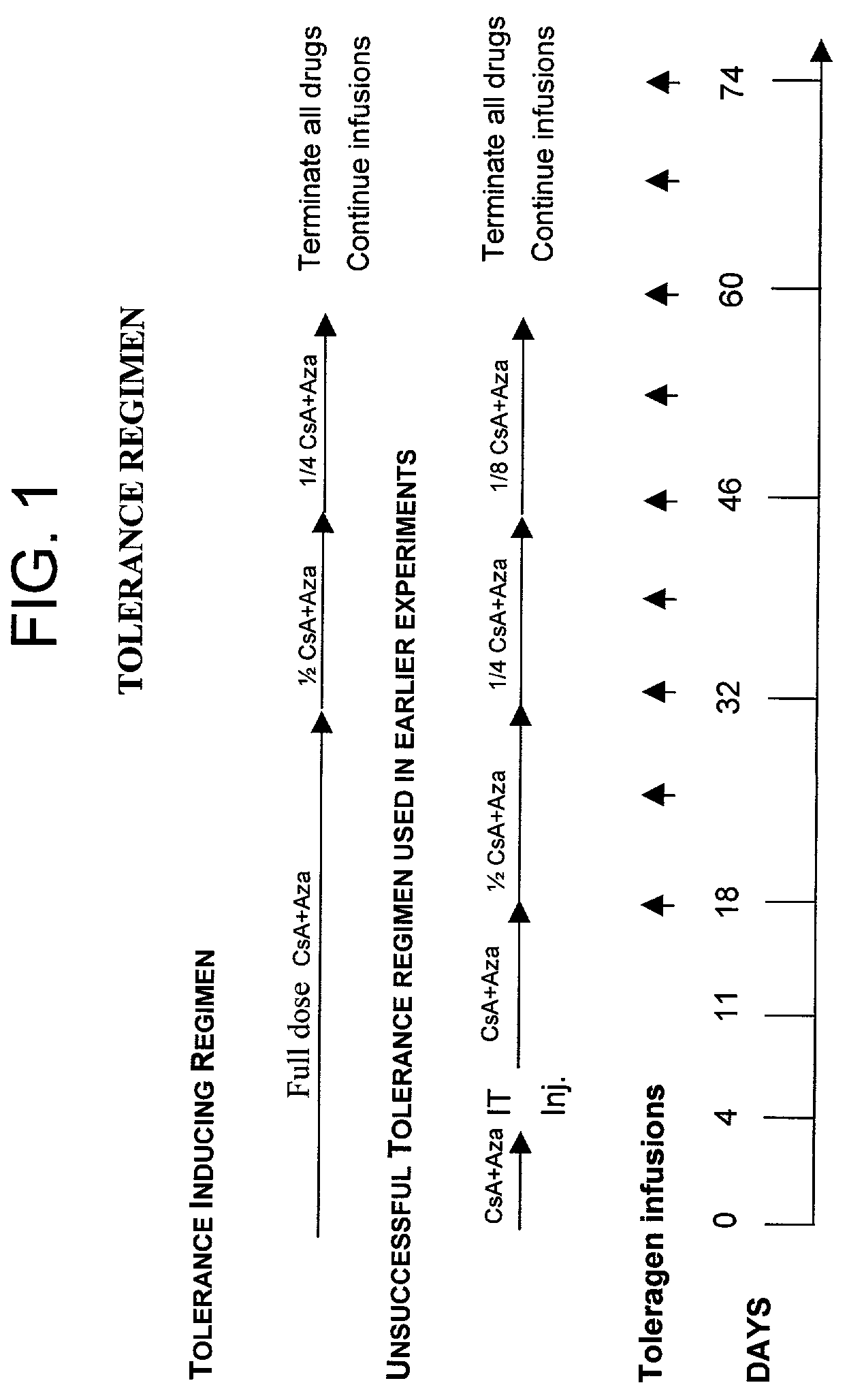
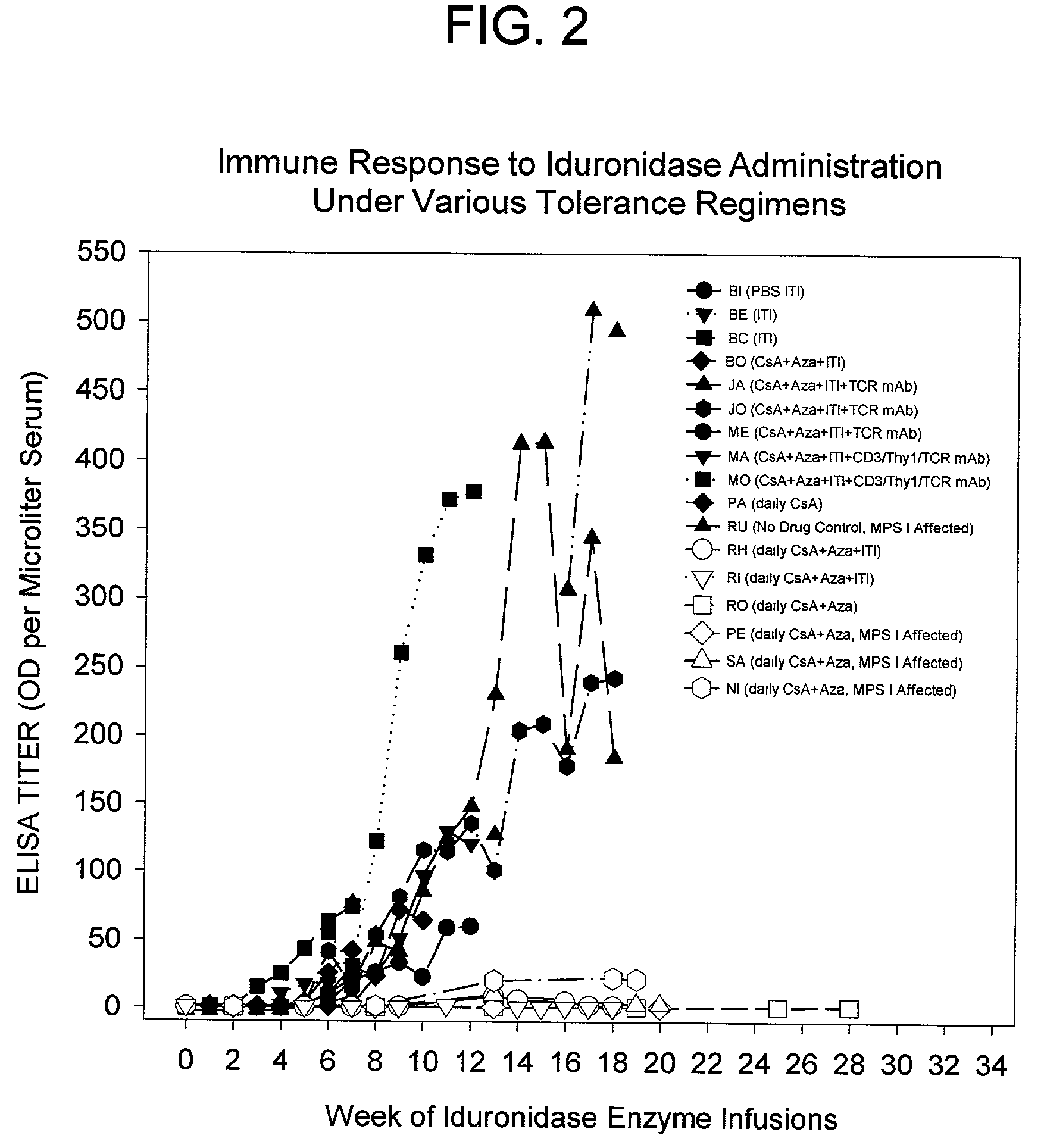
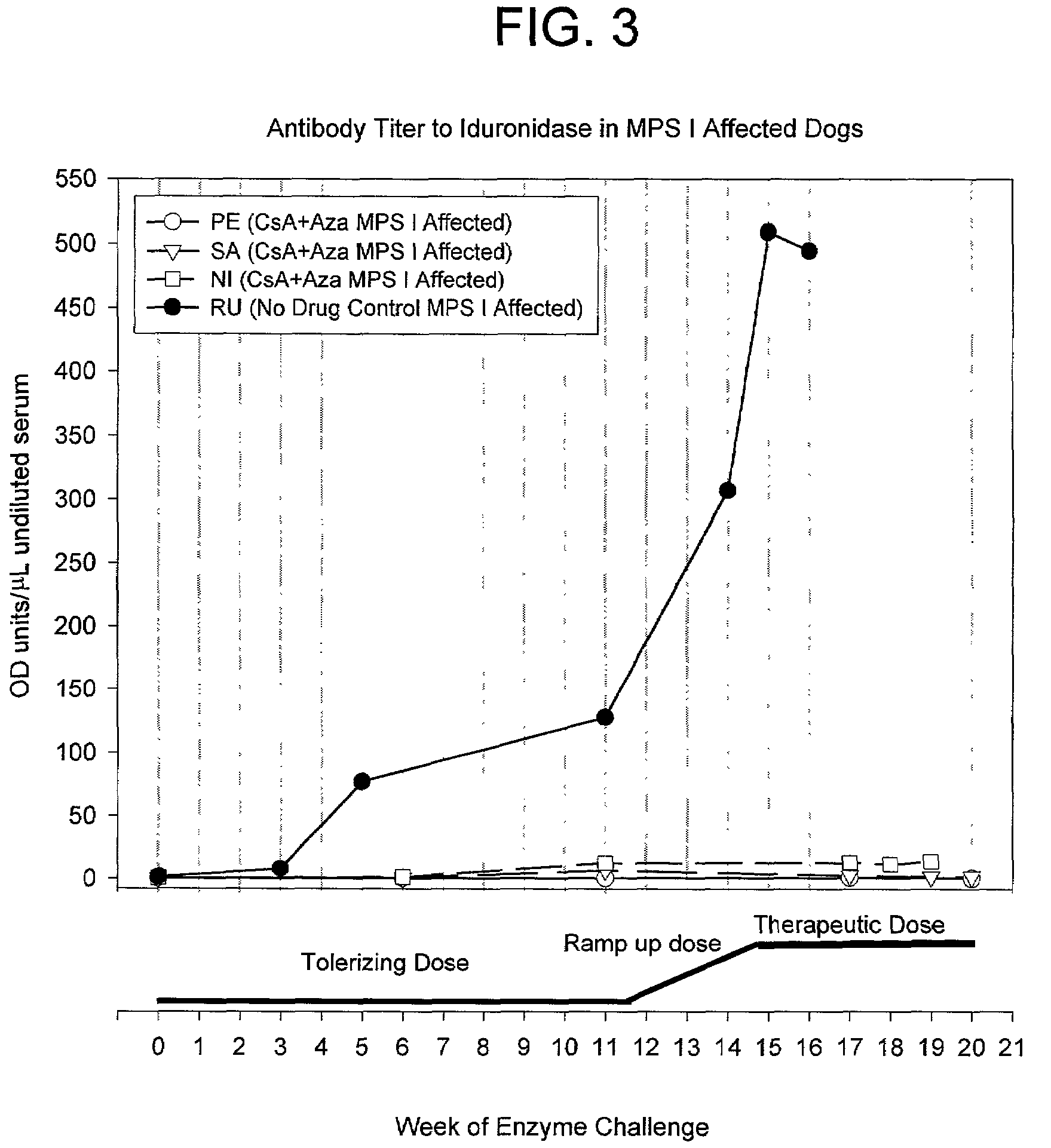
Antigen specific immune tolerance is induced in a mammalian host by administration of a toleragen in combination with a regimen of immunosuppression. The methods optionally include a preceding conditioning period, where immunosuppressive agents are administered in the absence of the toleragen. After the tolerizing regimen, the host is withdrawn from the suppressive agents, but is able to maintain specific immune tolerance to the immunogenic epitopes present on the toleragen. Optimally, the toleragen will have high uptake properties that allow uptake in vivo at low concentrations in a wide variety of tolerizing cell types.
Owner:RES & EDUCATION INST HARBOR - UCLA MEDICAL CENT
Immunological tolerance-inducing agent
InactiveUS6322796B1Reduce and prevent immune responseImprove survivalBacterial antigen ingredientsAllergen ingredientsImmune toleranceHapten
An immunological tolerance-inducing agent comprising a mucosa-binding molecule linked to a specific tolerogen is disclosed. Further, a method of inducing immunological tolerance in an individual against a specific antigen, including hapten, which causes an unwanted immune response in said individual comprising administration by a mucosal route of an immunologically effective amount of an immunological tolerance-inducing agent of the invention to said individual, is described.
Owner:DUOTOL
Immunological tolerance-inducing agent
InactiveUS6365163B1Enhanced couplingEnhance tolerogenic activityBacterial antigen ingredientsPeptide/protein ingredientsMicroorganismImmune tolerance
An agent comprising a mucosa-binding molecule linked to a specific microbial antigen is disclosed. Further, a method of inducing immnunological tolerance in an individual against a specific microbial antigen, including hapten, which causes an unwanted immune response in said individual, comprising administration by a mucosal route of an immunologically effective amount of an immunological tolerance-inducing agent of the invention to said individual, is described.
Owner:DUOTOL
Method of pre-inducing a state of immune tolerance before organ transplantation
InactiveUS6923959B2Delaying occurrenceReduce intensityOrganic active ingredientsBiocideInsulin dependent diabetesDuctal cells
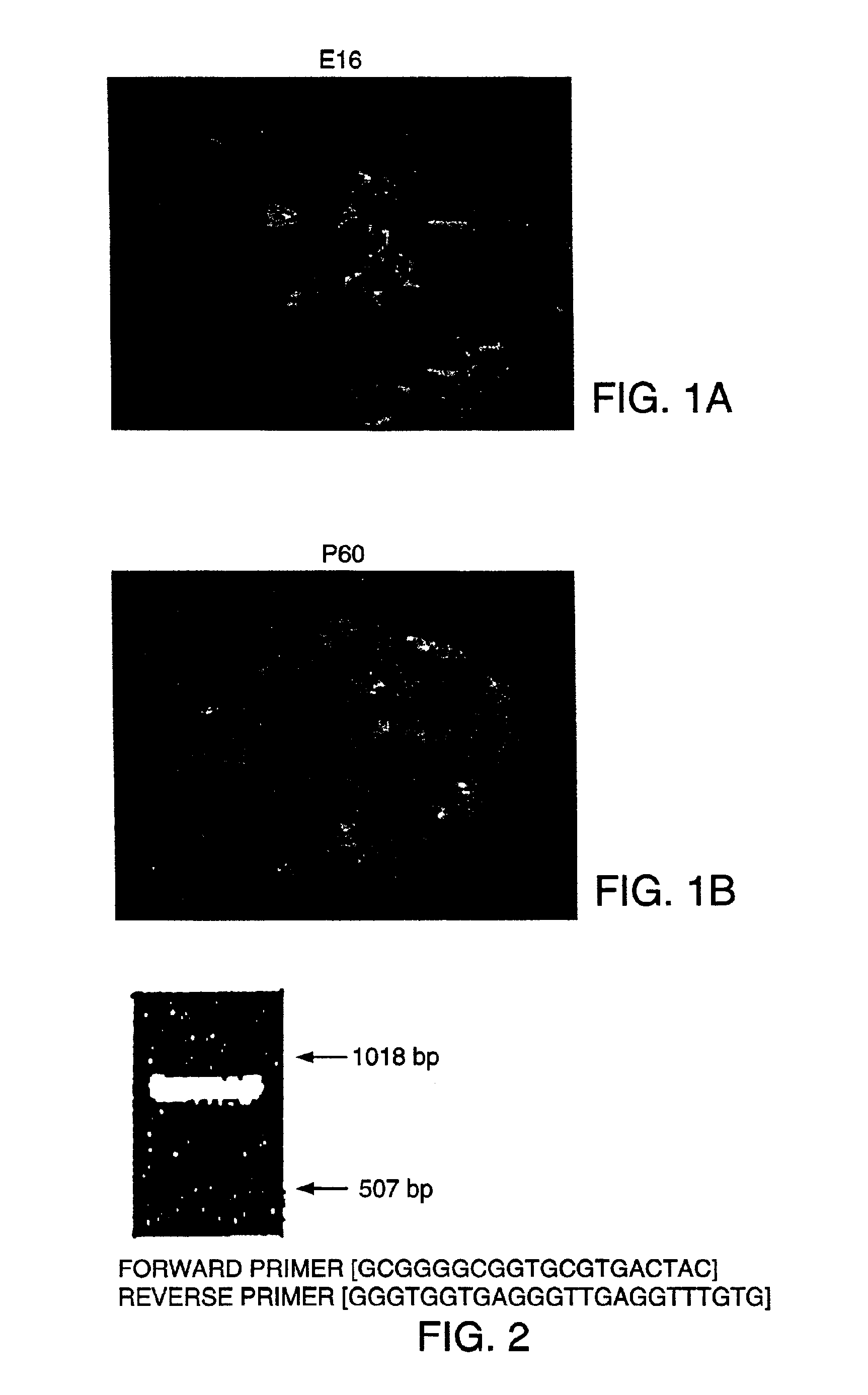
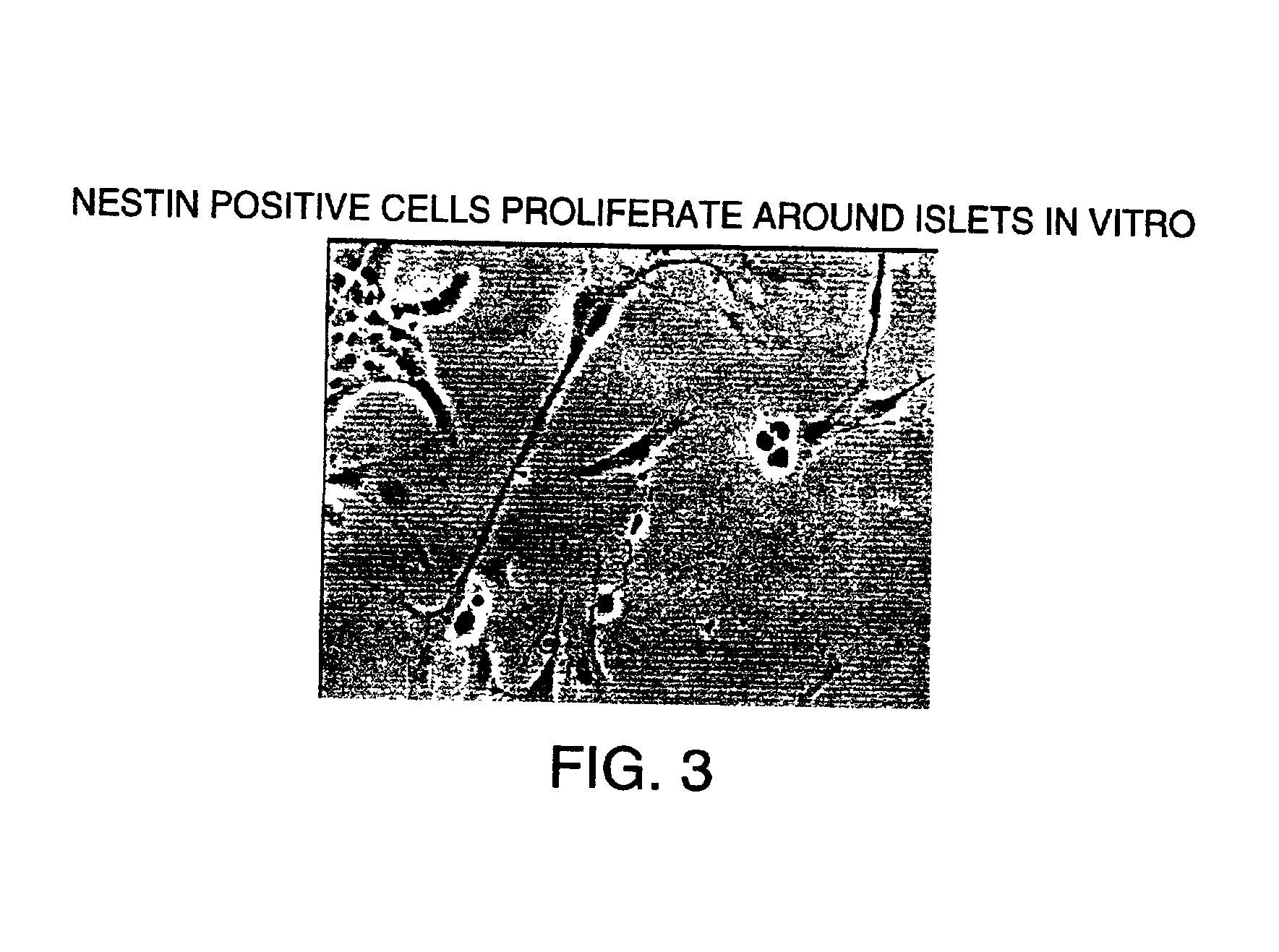
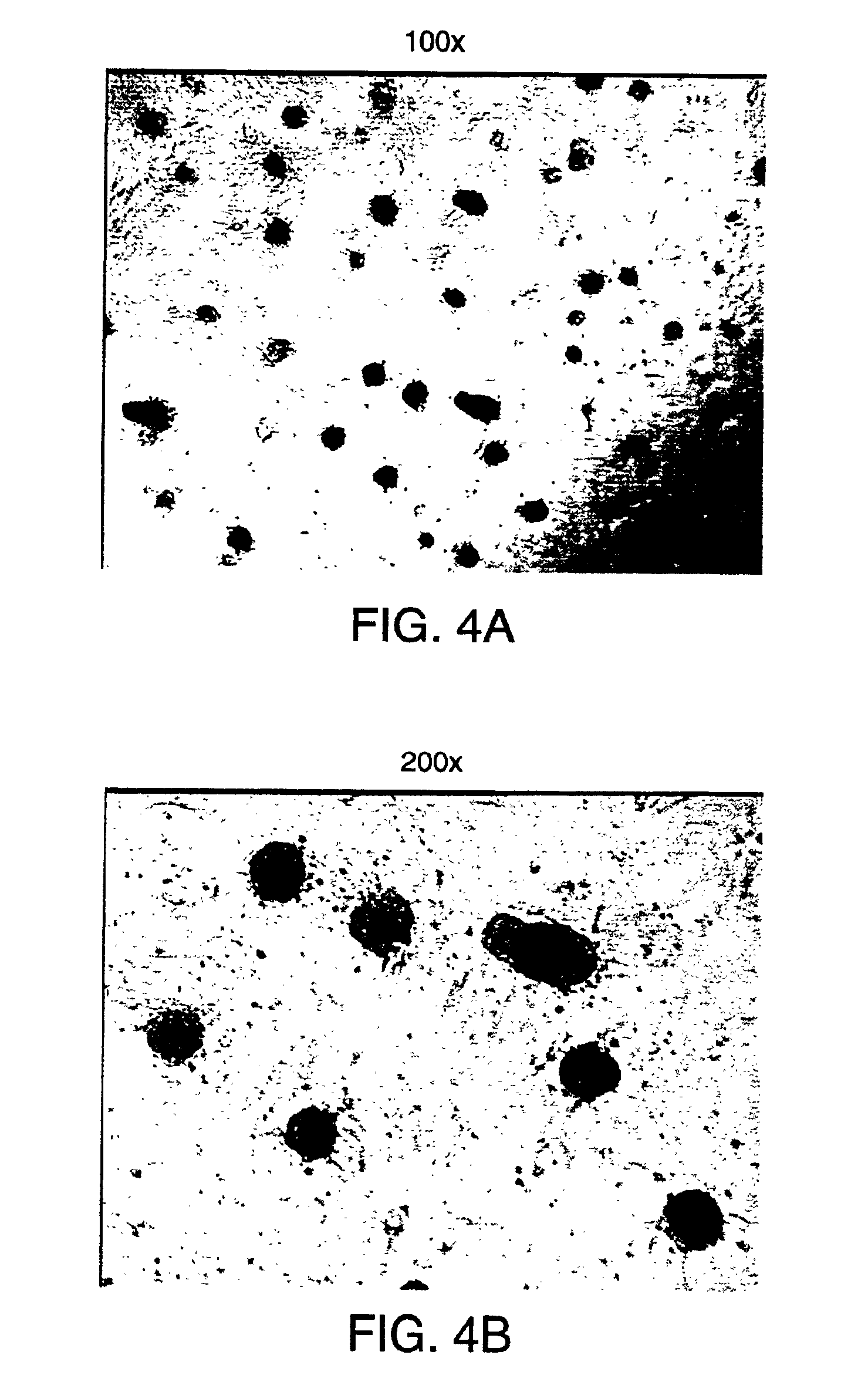
Methods and compositions are described for the treatment of type I insulin-dependent diabetes mellitus and other conditions using newly identified stem cells that are capable of differentiation into a variety of pancreatic islet cells, including insulin-producing beta cells, as well as hepatocytes. Nestin has been identified as a molecular marker for pancreatic stem cells, while cytokeratin-19 serves as a marker for a distinct class of islet ductal cells. Methods are described whereby nestin-positive stem cells can be isolated from pancreatic islets and cultured to obtain further stem cells or pseudo-islet like structures. Methods for ex vivo differentiation of the pancreatic stem cells are disclosed. Methods are described whereby pancreatic stem cells can be isolated, expanded, and transplanted into a patient in need thereof, either allogeneically, isogeneically or xenogenically, to provide replacement for lost or damaged insulin-secreting cells or other cells.
Owner:THE GENERAL HOSPITAL CORP
Human interleukin-2 (IL-2)/Fc fusion protein
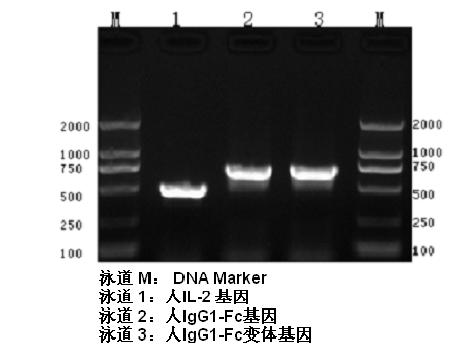
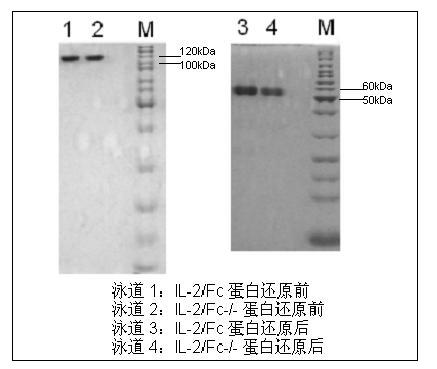
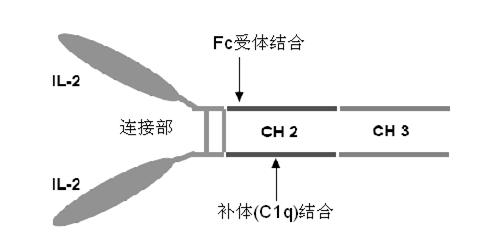
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
Compositions and methods for inducing immune tolerance
The present invention provides liposomal compositions for inducing immune tolerance. The compounds typically comprise a liposome displaying a specific antigen and also a binding moiety for a sialic acid binding Ig-like lectin (Siglec) expressed on B cells. The invention also provides methods for inducing tolerance to a protein or polypeptide antigen (e.g., a protein antigen) in a subject. The methods involve administering to the subject a pharmaceutical composition that co-presents both the antigen and a glycan ligand for a Siglec expressed on B lymphocytes.
Owner:THE SCRIPPS RES INST
Food anaphylactogen specificity IgG4 antibody ELISA detection kit and preparation method thereof
The invention discloses an ELISA detection kit of food allergen specificity IgG4 antibody and preparation method thereof, using enzyme target rod coated by purified food allergen protein. The kit comprises: enzyme target rod coated by food allergen protein, human IgG2 titer, enzymic-lableled antibody, TMB substrate developing liquid, sample diluent, concentrated cleaning solution and termination reaction liquid. The specificity IgG4 antibodies in sample serum are bonded with the corresponding antigens fixed on the micropore surface of the enzyme target rod and the enzymic-lableled antibodies identify and bond with the IgG4 to form antigen-IgG4-antibody-enzyme composite. The absorbency is measured by the development reaction between the enzyme and substrate and the specificity IgG4 antibody concentration in sample serum is measured by standard curve. The allergen specificity IgG4 antibody concentration in sample serum is used as biology index of exposure state of food allergen and for judging the patient immune deviation and immune tolerance and monitoring the immunotherapy effectiveness of special food selected by patients.
Owner:杭州浙大生科生物技术有限公司
Methods of reducing immunogenicity against factor viii in individuals undergoing factor viii therapy
ActiveUS20140370035A1Improve immune toleranceReduce morbidityFactor VIINervous disorderFactor iiImmune tolerance
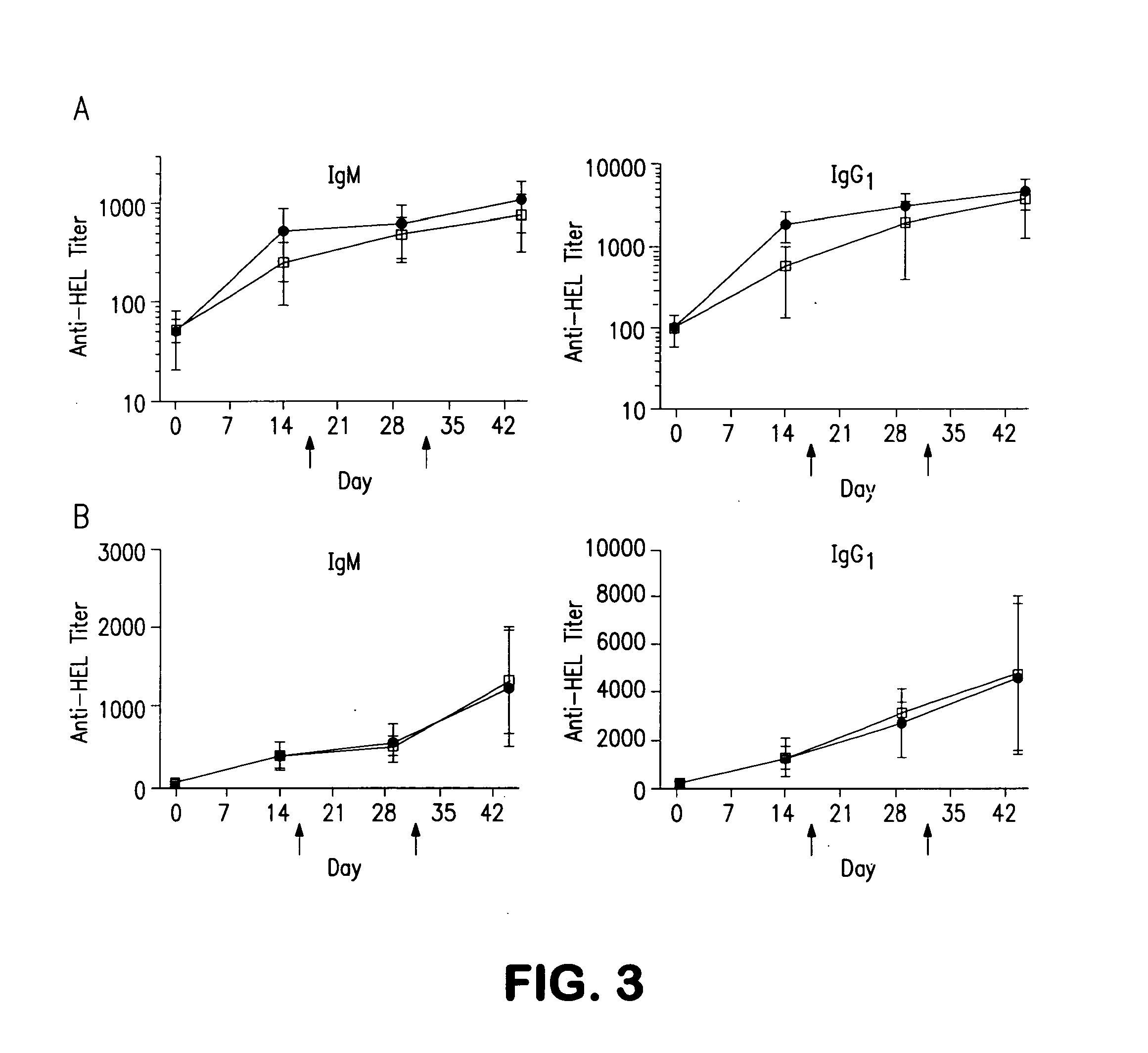
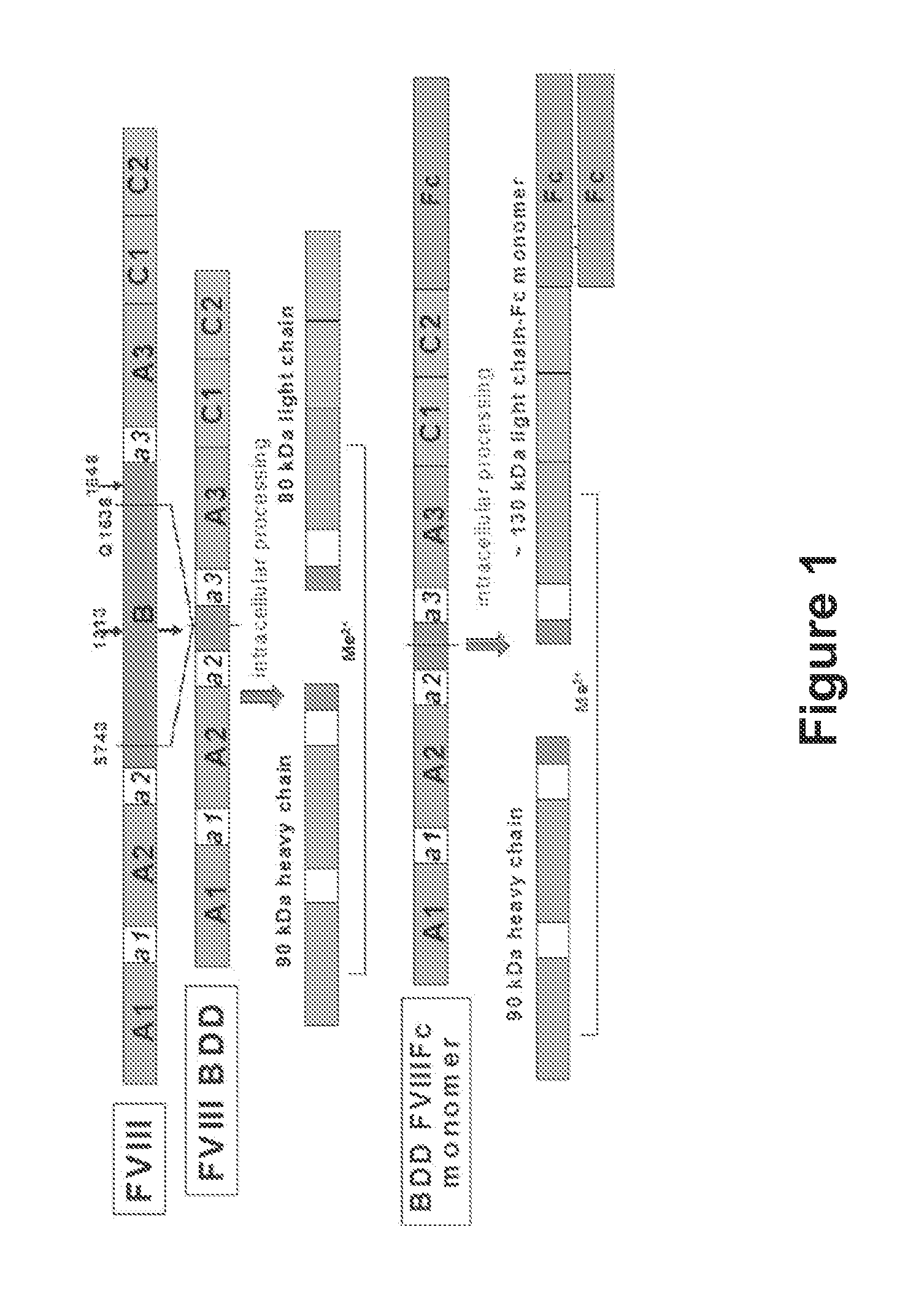
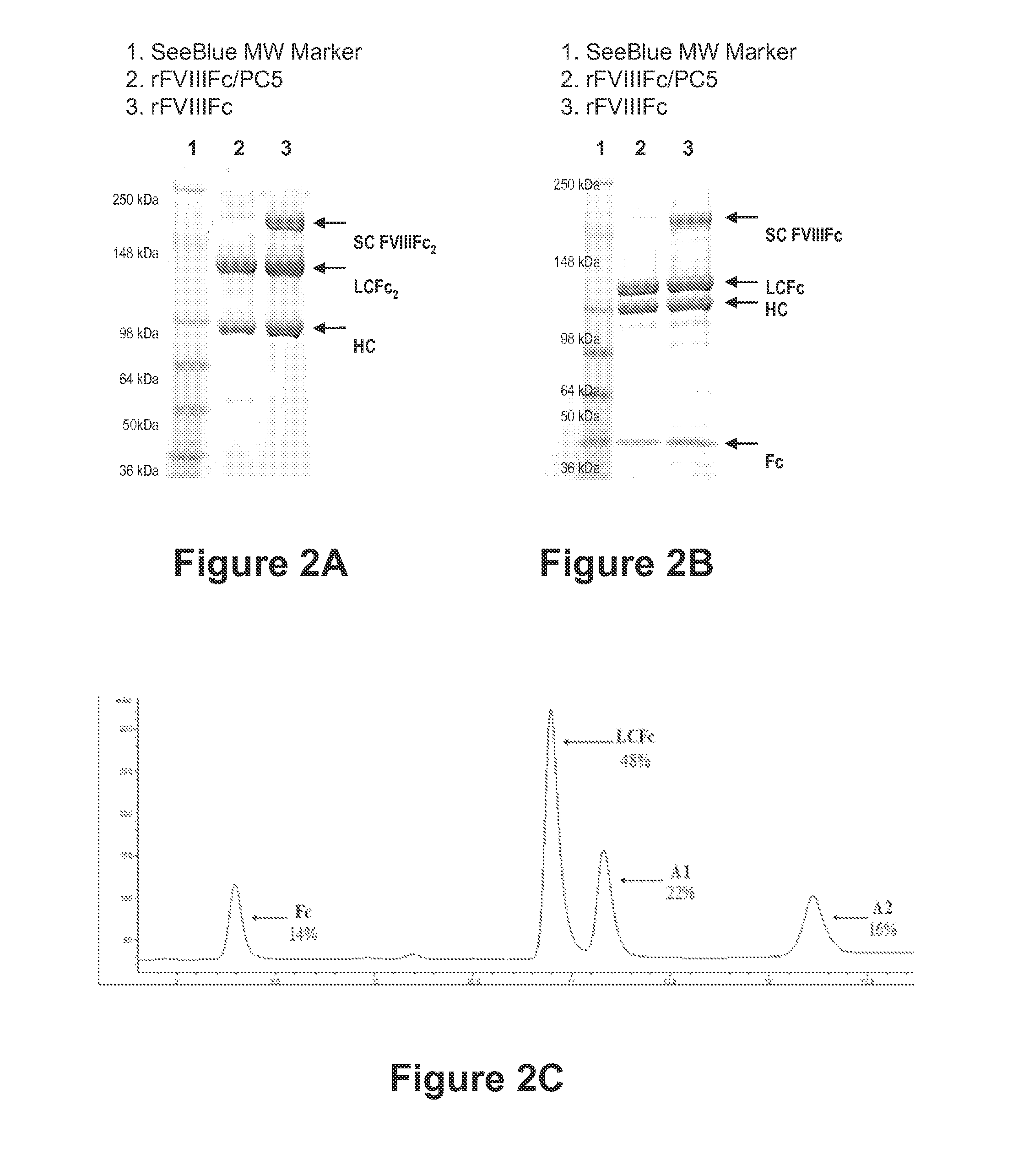
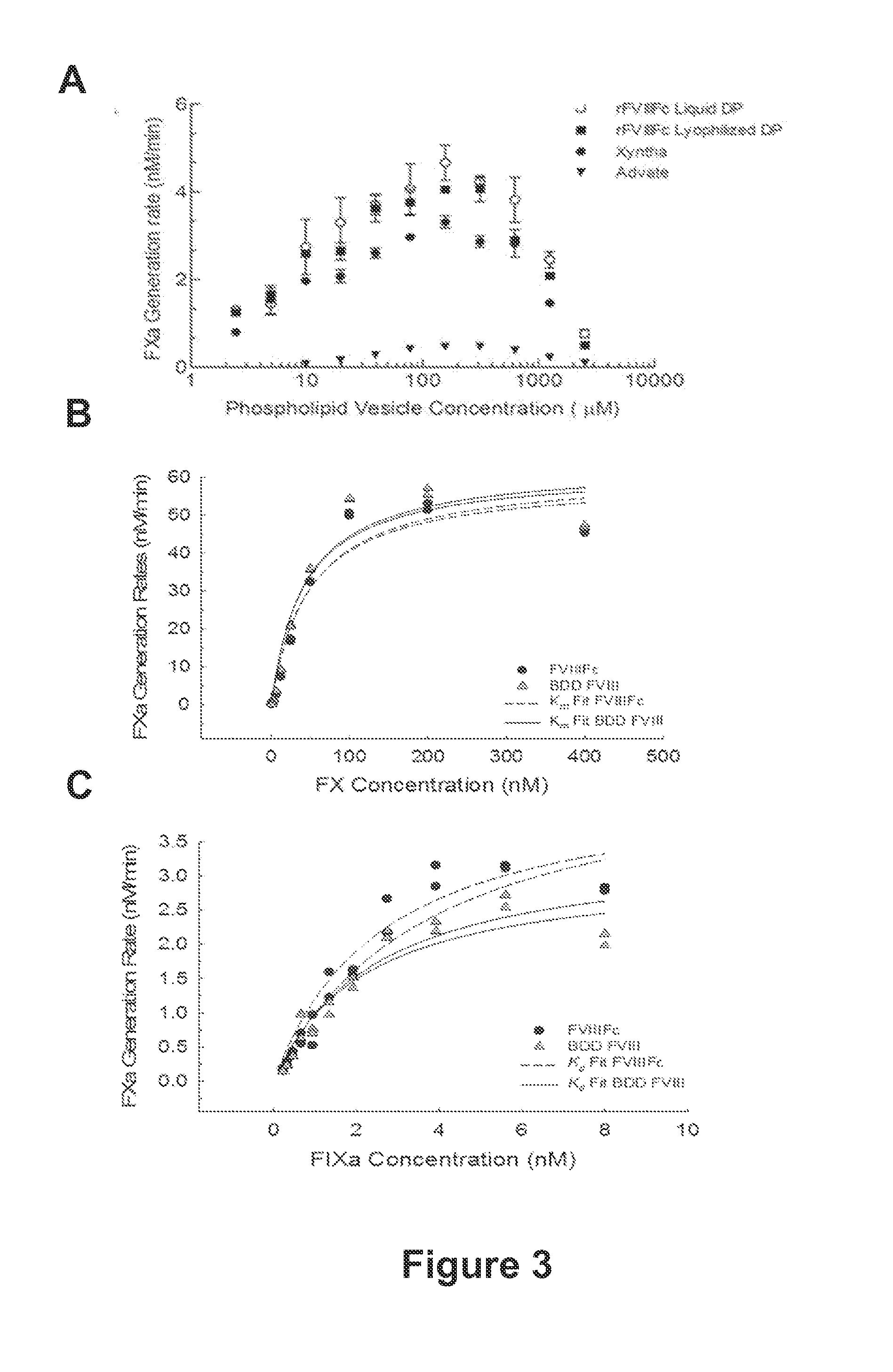
The present disclosure provides methods of administering chimeric and hybrid Factor VIII (FVIII) polypeptides comprising FVIII and Fc to subjects at risk of developing inhibitory FVIII immune responses, including anti-FVIII antibodies and / or cell-mediated immunity. The administration is sufficient to promote coagulation and to induce immune tolerance to FVIII. The chimeric polypeptide can comprise full-length FVIII or a FVIII polypeptide containing a deletion, e.g., a full or partial deletion of the B domain.
Owner:PUGET SOUND BLOOD CENT +1
Method for Inducing Immune Tolerance Using Viable Polymer-Modified Allogeneic Leukocytes
ActiveUS20140017218A1Induce anergyInduce toleranceBiocidePharmaceutical delivery mechanismRegulatory T cellTreatment effect
This invention relates to cellular-based therapies for increasing the level of regulatory T cells (Treg) and / or decreasing the level of pro-inflammatory T cells (Th17) to induce anergy or immune tolerance. To provide these therapeutic effects, two allogeneic leukocyte populations are contacted (in vivo, in vitro or ex vivo) and one of these leukocyte population is modified to bear on its surface a low-immunogenic biocompatible polymer so as to prevent pro-inflammatory allo-recognition. Cellular-based preparations and processes for achieving cellular therapy are also provided.
Owner:CANADIAN BLOOD SERVICES
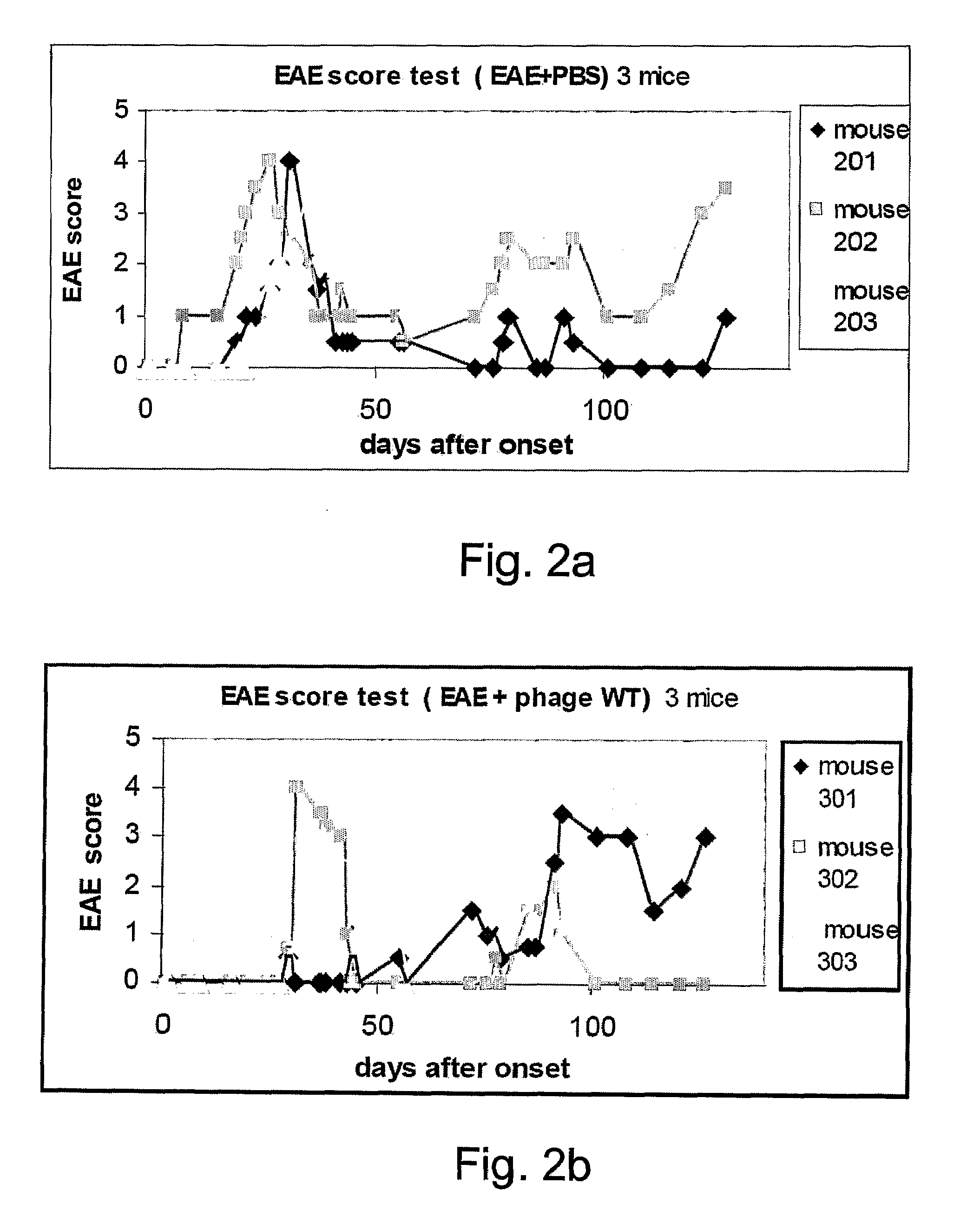
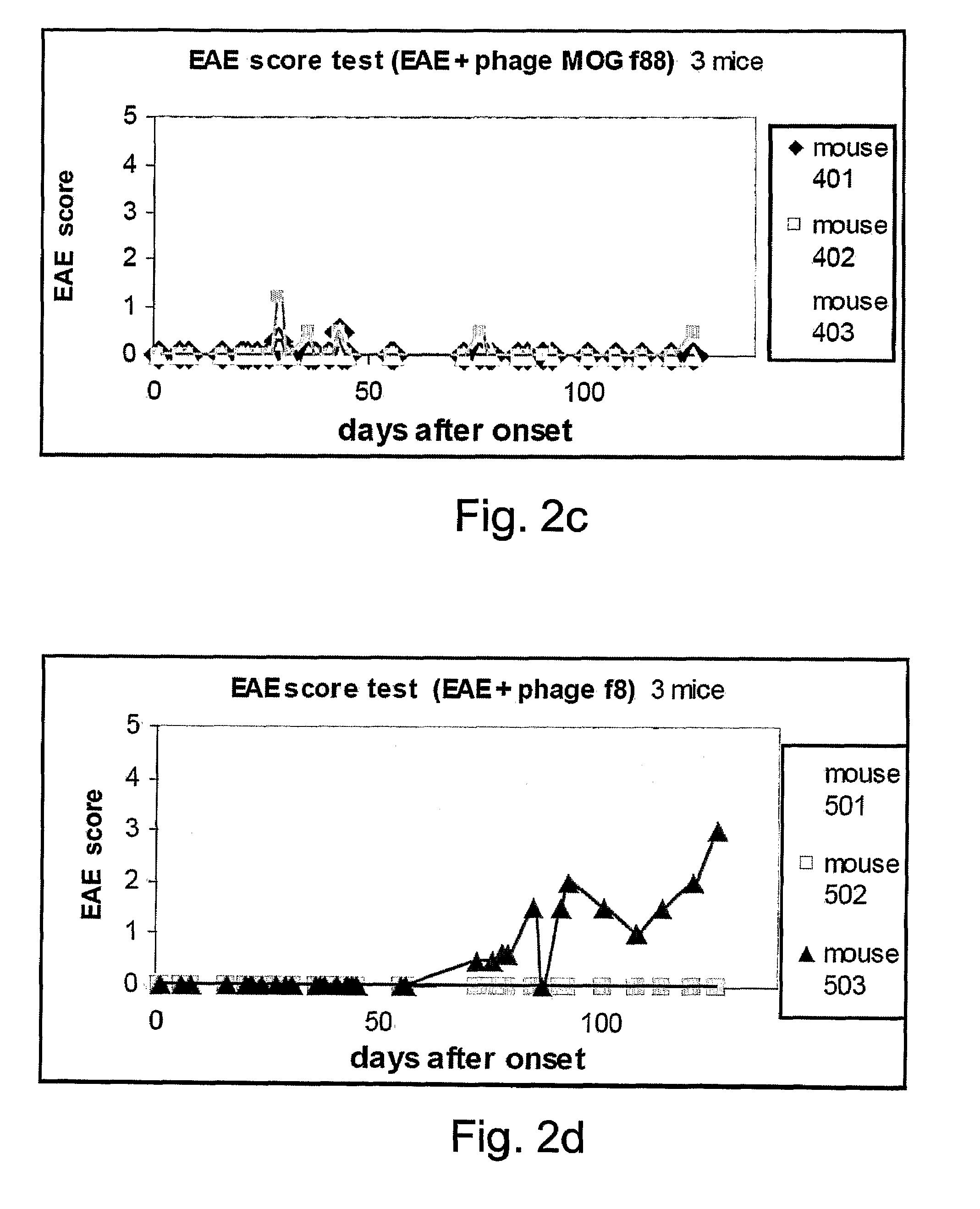
Viral display vehicles for treating multiple sclerosis
Provided are viral display vehicles which display multiple sclerosis associated antigens on the surface thereof for induction of immune tolerance to autoantigens such as MOG. Also provided are methods and pharmaceutical compositions for treating multiple sclerosis using the viral display vehicles of the present invention.
Owner:RAMOT AT TEL AVIV UNIV LTD
Targeted/immunomodulatory fusion proteins
ActiveUS8815247B2Reducing the ability of cancer cells to avoid an immune responseImprove anti-tumor efficacyPeptide/protein ingredientsAntibody mimetics/scaffoldsCancer cellNucleotide
The present invention relates generally to the field of generating fusion proteins to be used in cancer therapy, and more specifically, to nucleotide sequences encoding the fusion proteins, wherein the chimeric fusion proteins comprises at least one targeting moiety and at least one immunomodulatory moiety that counteracts the immune tolerance of cancer cells.
Owner:BIOCON LTD
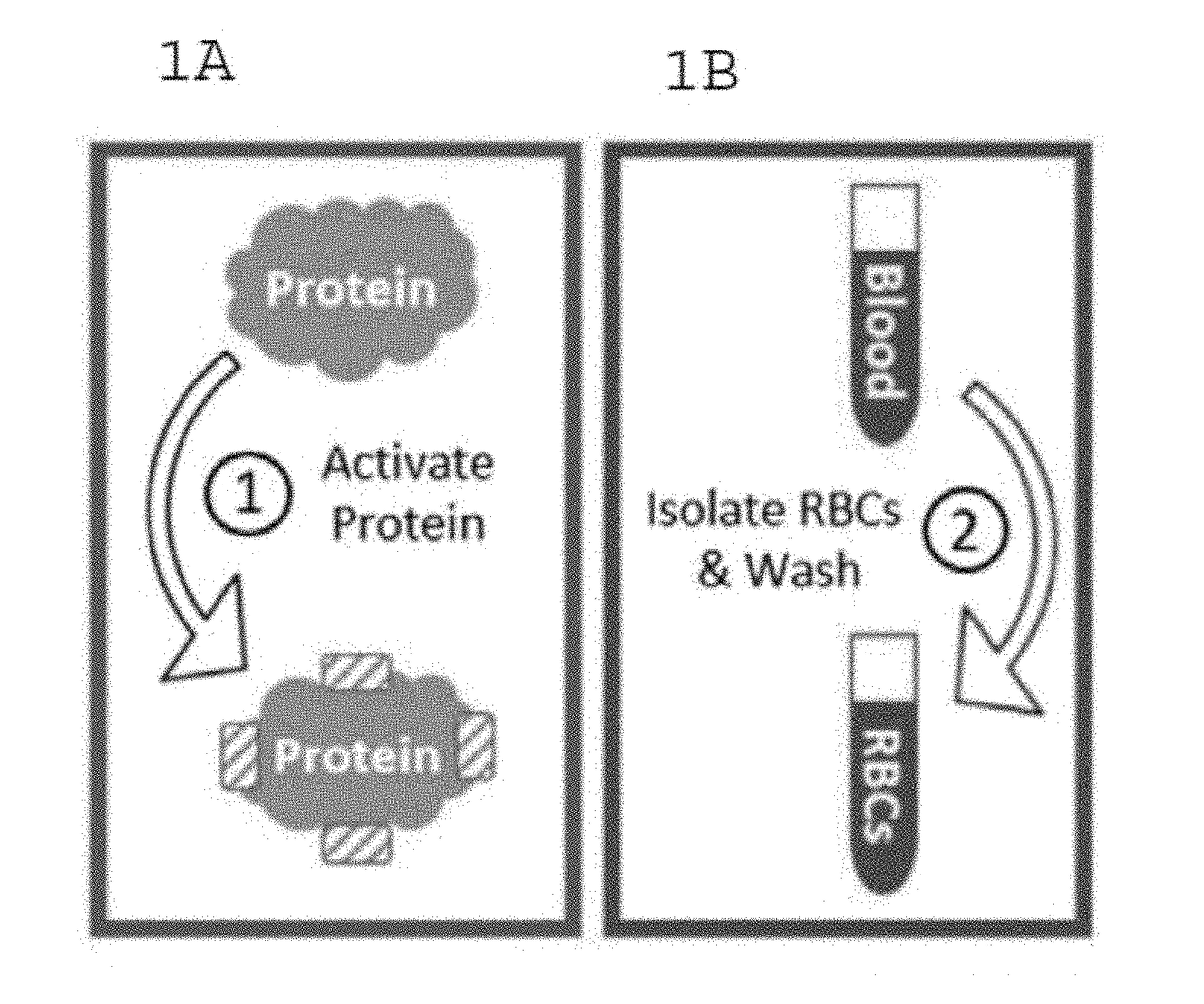
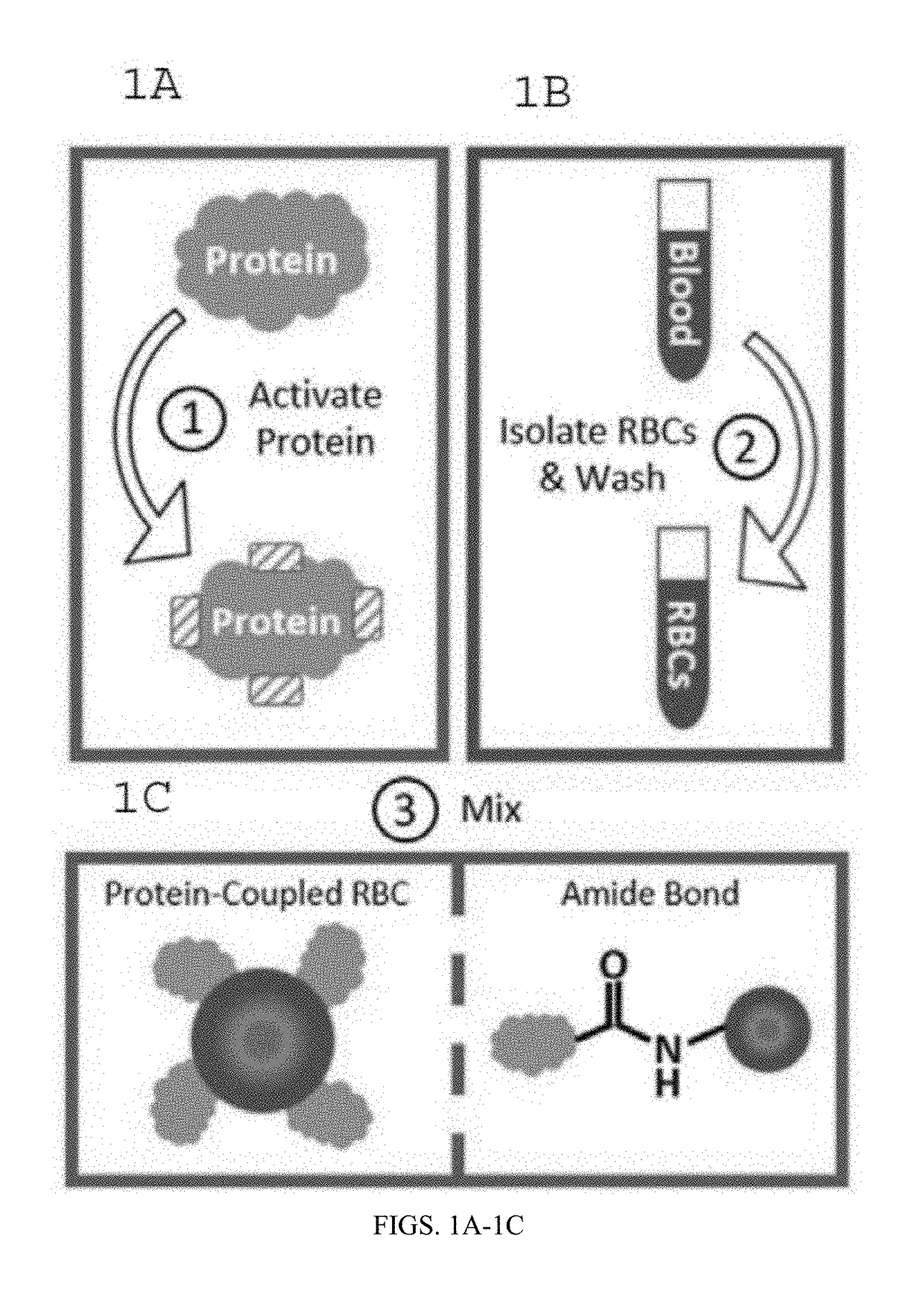
Protein-Coupled Red Blood Cell Compositions and Methods of Their Use
InactiveUS20170326213A1Extended half-lifeLimited half-lifePowder deliveryPharmaceutical non-active ingredientsN-hydroxysulfosuccinimideConjugated protein
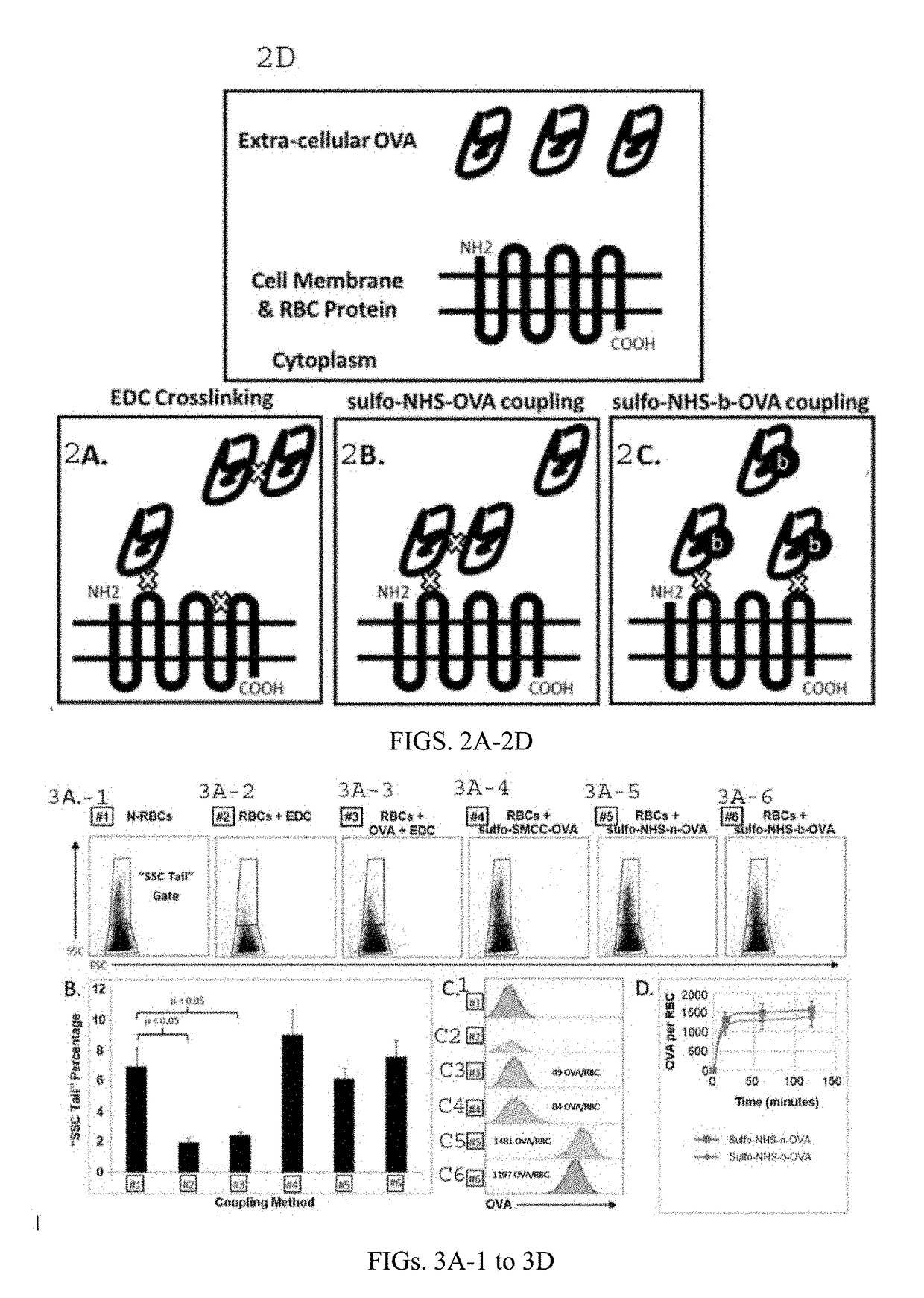
Methods and compositions for using N-hydroxysuccinimide and N-hydroxysulfosuccinimide to covalently couple protein(s) to the surface of red blood cells universally, rapidly and conveniently are provided. In one embodiment, the compositions promote immune tolerance in a subject. One embodiment provides autologous or allogenic red blood cells having whole protein(s) of interests conjugated to proteins on or within the plasma membrane of the red blood cells, wherein the conjugated proteins display at least one antigen to which immune tolerance is desired. The proteins are conjugated to the RBCs using carbodiimide chemistry. In a preferred embodiment, the whole proteins are conjugated using EDC in combination with NHS or sulfo-NHS.
Owner:AUGUSTA UNIV RES INST INC
Methods and compositions for obtaining hematopoietic stem cells derived from embryonic stem cells and uses thereof
InactiveUS20050221482A1Good conditionMammal material medical ingredientsArtificial cell constructsAutoimmune conditionAutoimmune disease
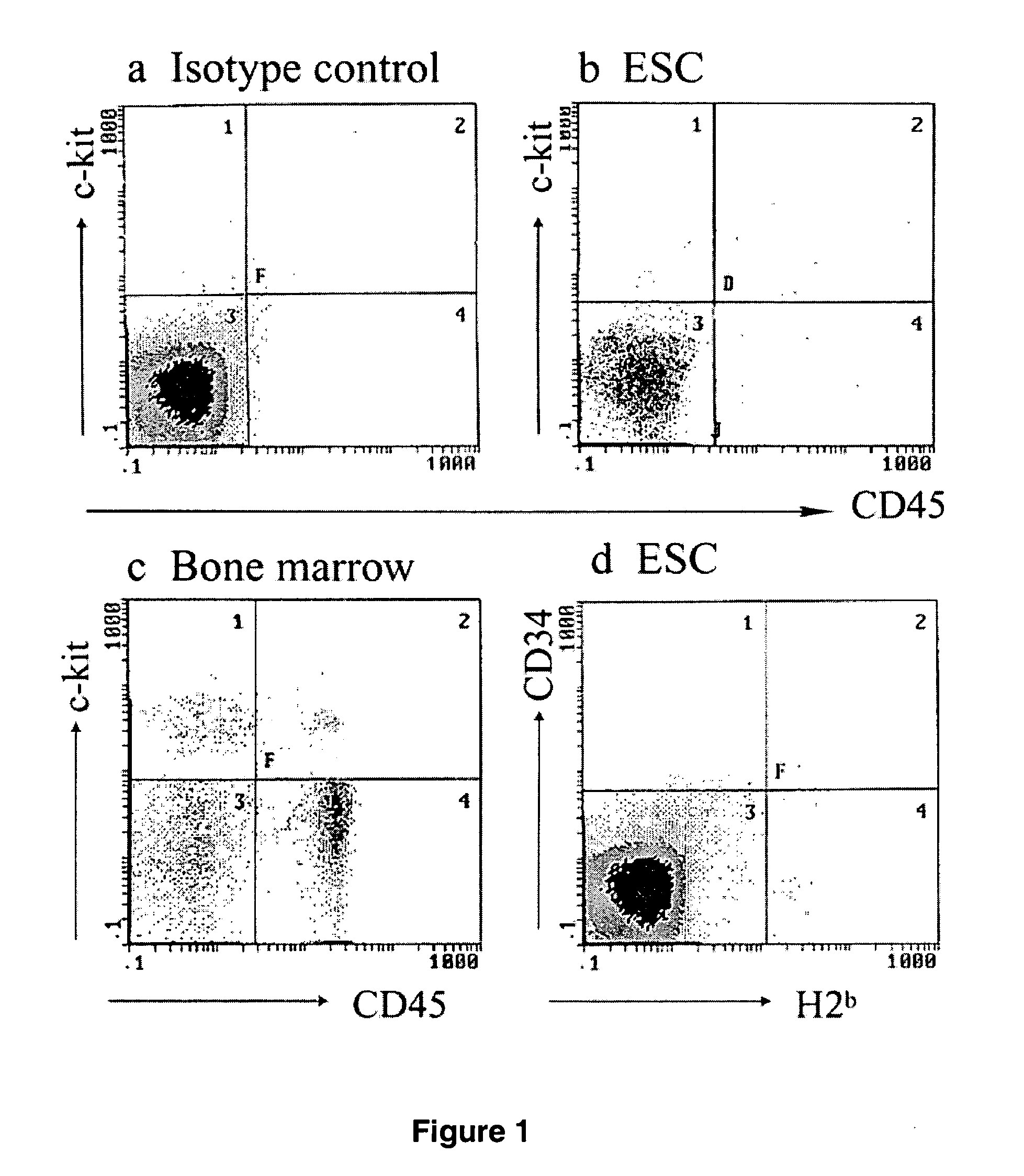
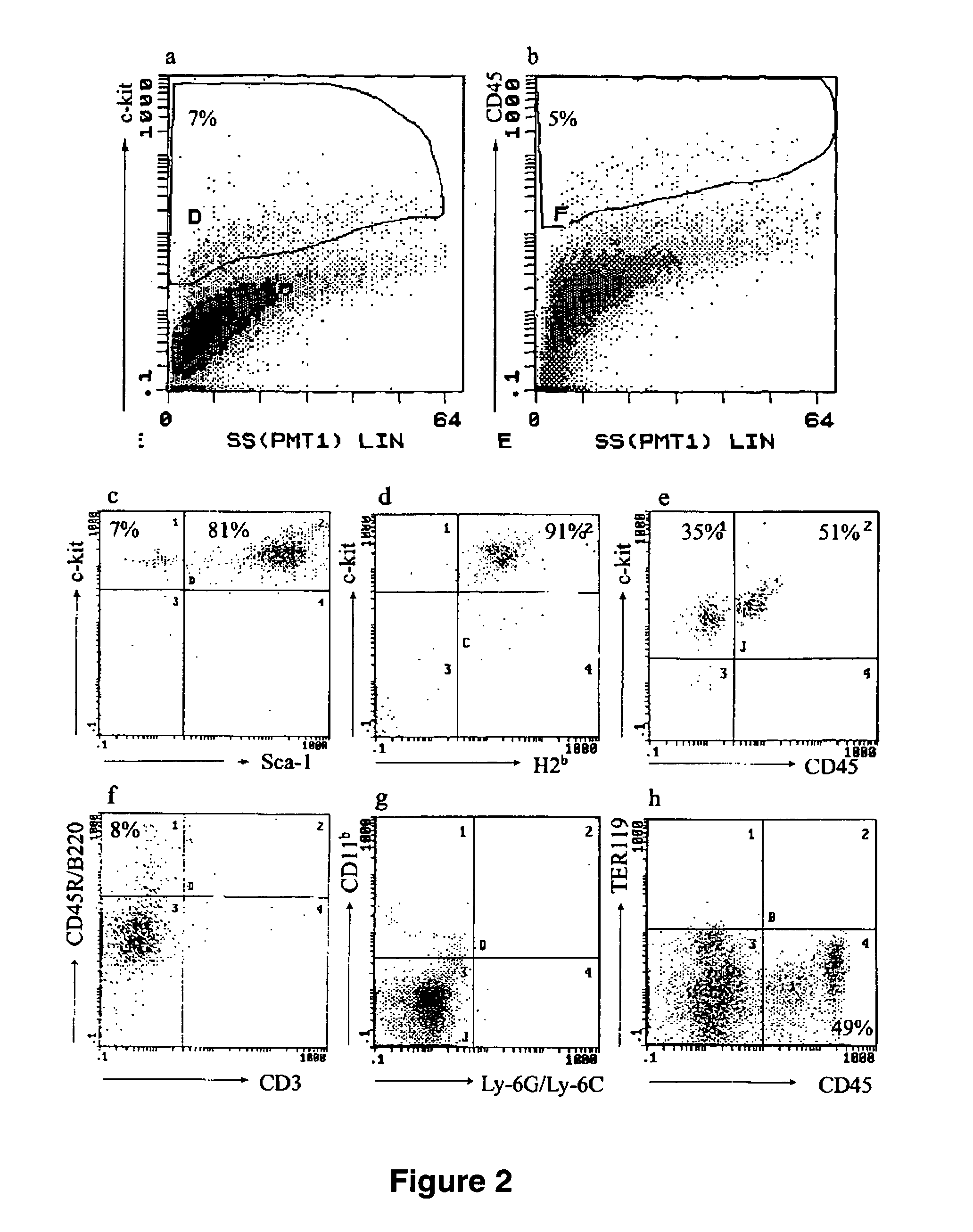
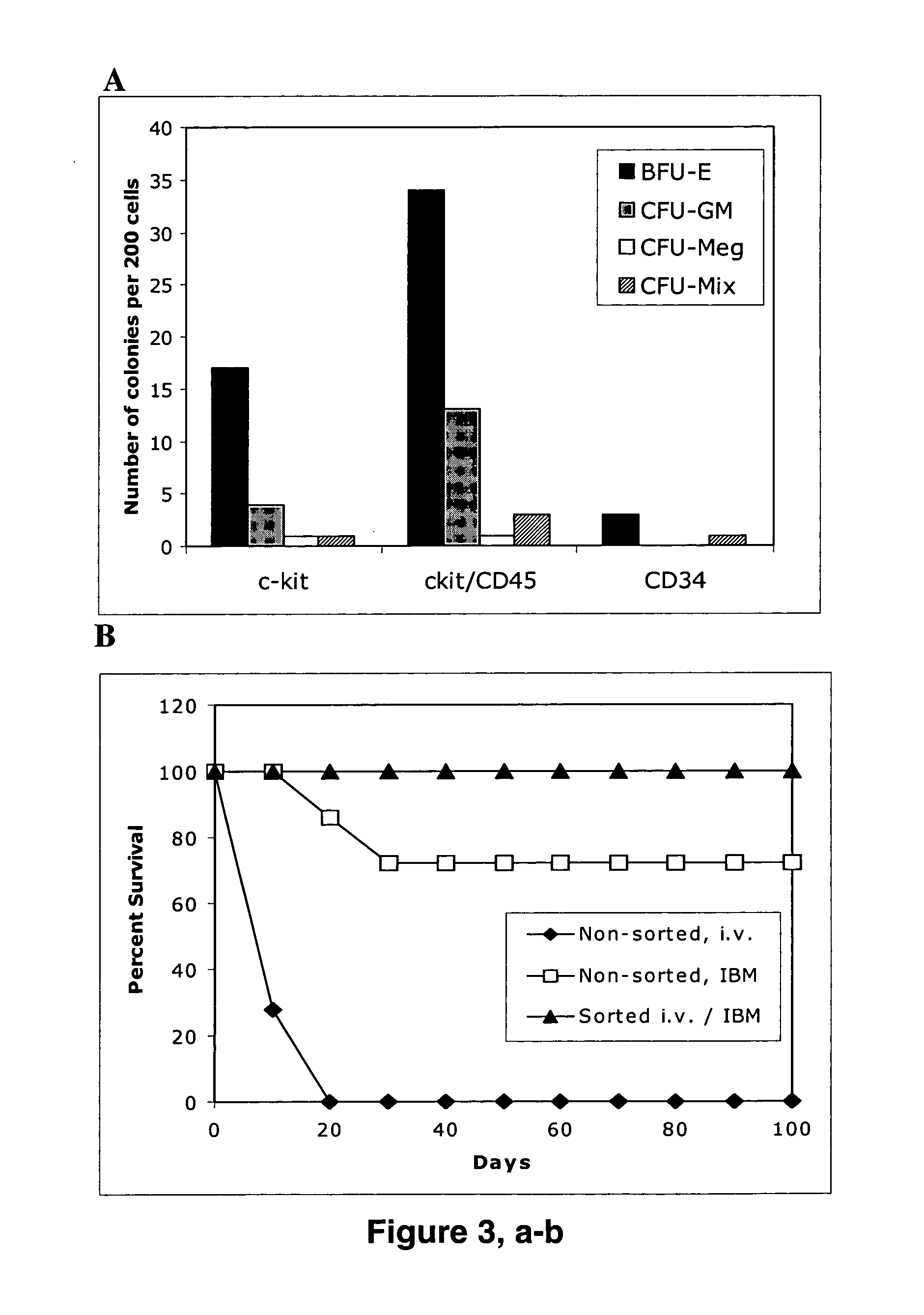
The present invention provides an isolated population of adult hematopoietic stem cells (HSC) derived from embryonic stem cells (ESC) induced to differentiate in vitro by culturing ESCs in a medium with stem cell factor, interleukin (IL)-3, and IL-6. HSC of immunophenotype c-kit+ or c-kit+ / CD45+ from this population are isolated and injected either intra bone marrow or intravenously into myeloablated recipient individuals. This method allows for the establishment of banks of allogeneic ESC-derived adult stem cells for treatments of autoimmune diseases, immune deficiencies and induction of immunotolerance during organ transplantation. These allogeneic ESC-derived adult hematopoietic stem cells (HSC) may be used in reconstituting bone marrow, without the development of teratomas or graft versus host disease, despite crossing histocompatibility barriers. Additionally, allogeneic ESC-derived HSC can be used to prevent the development of autoimmune diseases or organ rejection during transplantation.
Owner:NEWLINK GENETICS +1
Antisense oligomers and methods for inducing immune tolerance and immunosuppression
InactiveUS20050234002A1Reduce capacityIncrease productionOrganic active ingredientsBiocideExtracellularDendritic cell
A method and composition for inducing human dendritic cells to a condition of reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10 is disclosed. A population of dendritic cells is exposed to a substantially uncharged antisense compound containing 12-40 subunits and a base sequence effective to hybridize to an expression-sensitive region of a preprocessed or processed human CD86 transcript identified, in its processed form, by SEQ ID NO:33, to form a duplex structure between said compound and transcript having a Tm of at least 45° C. Formation of the duplex blocks expression of full-length CD86 in said cells, which in turn leads to reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10.
Owner:AVI BIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com