Patents
Literature
280 results about "Cell activation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Activate a cell at the start of the range, then press the SHIFT key. With the SHIFT key still selected, use the left, right, up or down arrow keys on the keyboard, to increase or decrease the selected range, by one cell at a time. Activate a cell at the start of the range, then press the SHIFT and CTRL keys.
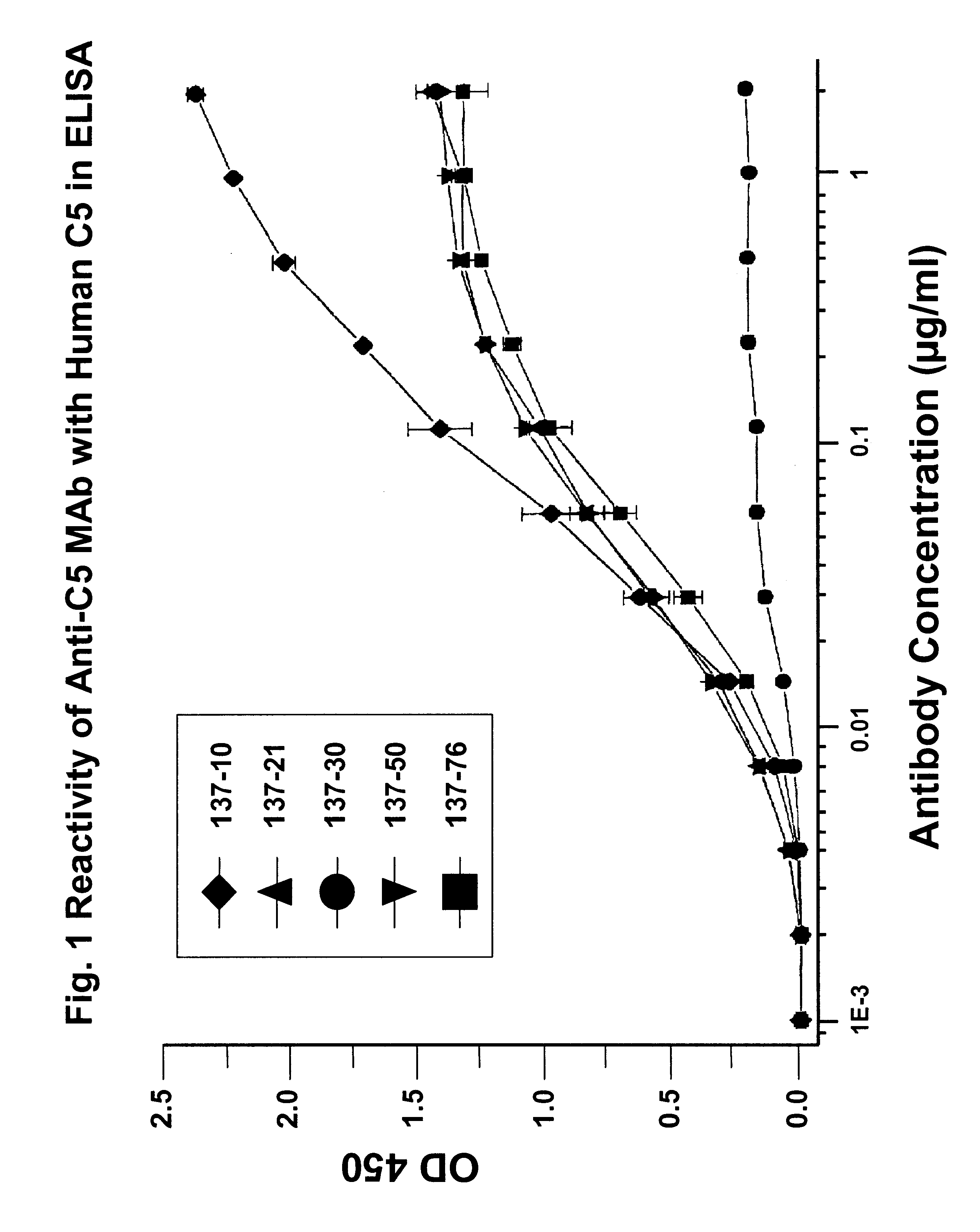
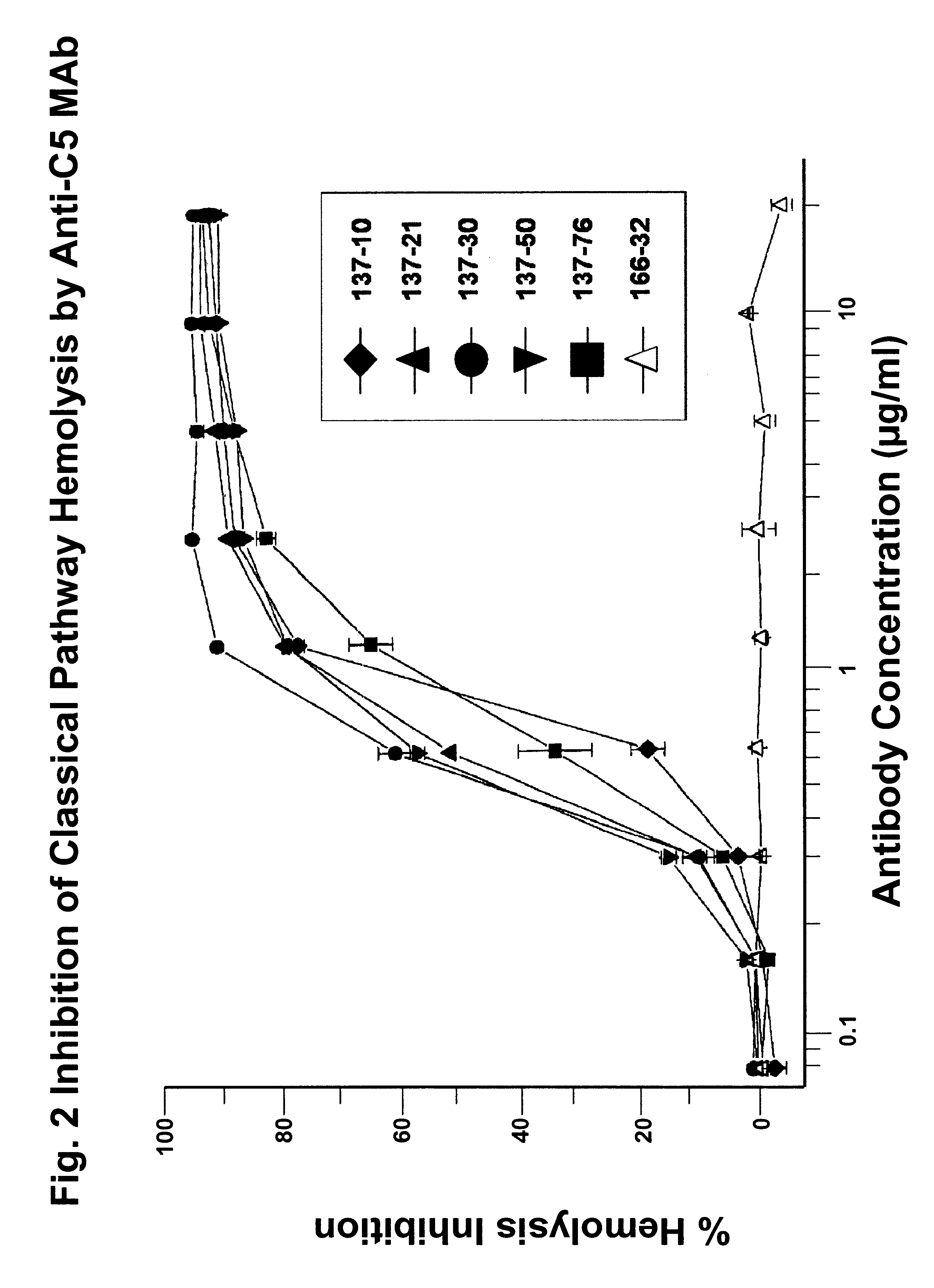
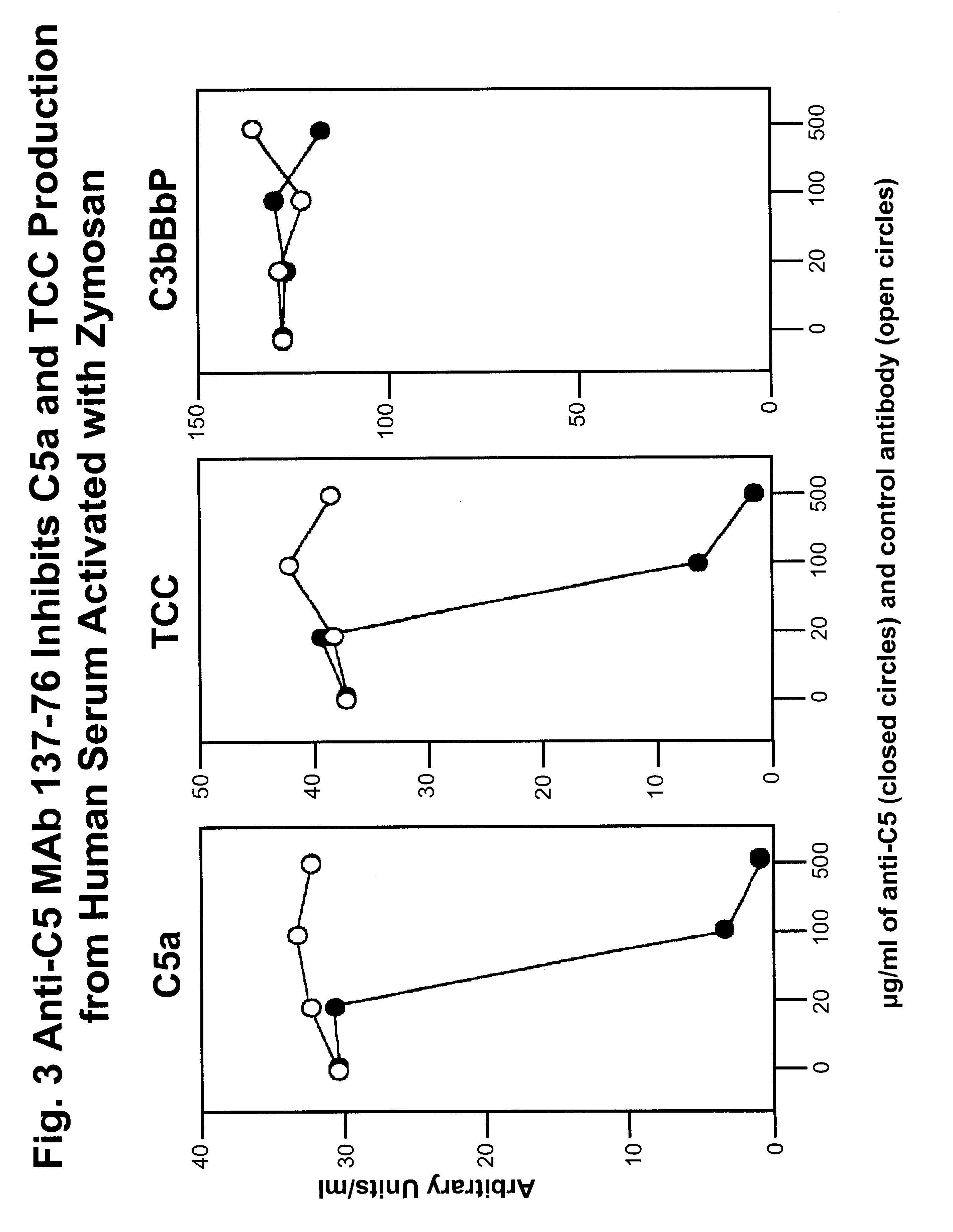
Anti-C5 monoclonal antibodies
InactiveUS6534058B2High affinityMaximizing characteristicImmunoglobulins against blood coagulation factorsAnimal cellsAcute vascular rejectionOligonucleotide
The invention relates to C5 inhibitors, which inhibit type II endothelial cell activation, wherein the inhibition is manifested by the suppression of E-selectin. These inhibitors are useful in treatment of delayed xenograft rejection or acute vascular rejection. The inhibitors include antibody molecules, as well as homologues, analogues and modified or derived forms thereof, including immunoglobulin fragments like Fab, F(ab')2 and Fv, small molecules, including peptides, oligonucleotides, peptidomimetics and organic compounds. Examples of monoclonal antibodies, which bind to and inhibit C5, were generated and are designated MAb 137-76 and MAb 137-30.
Owner:GENENTECH INC
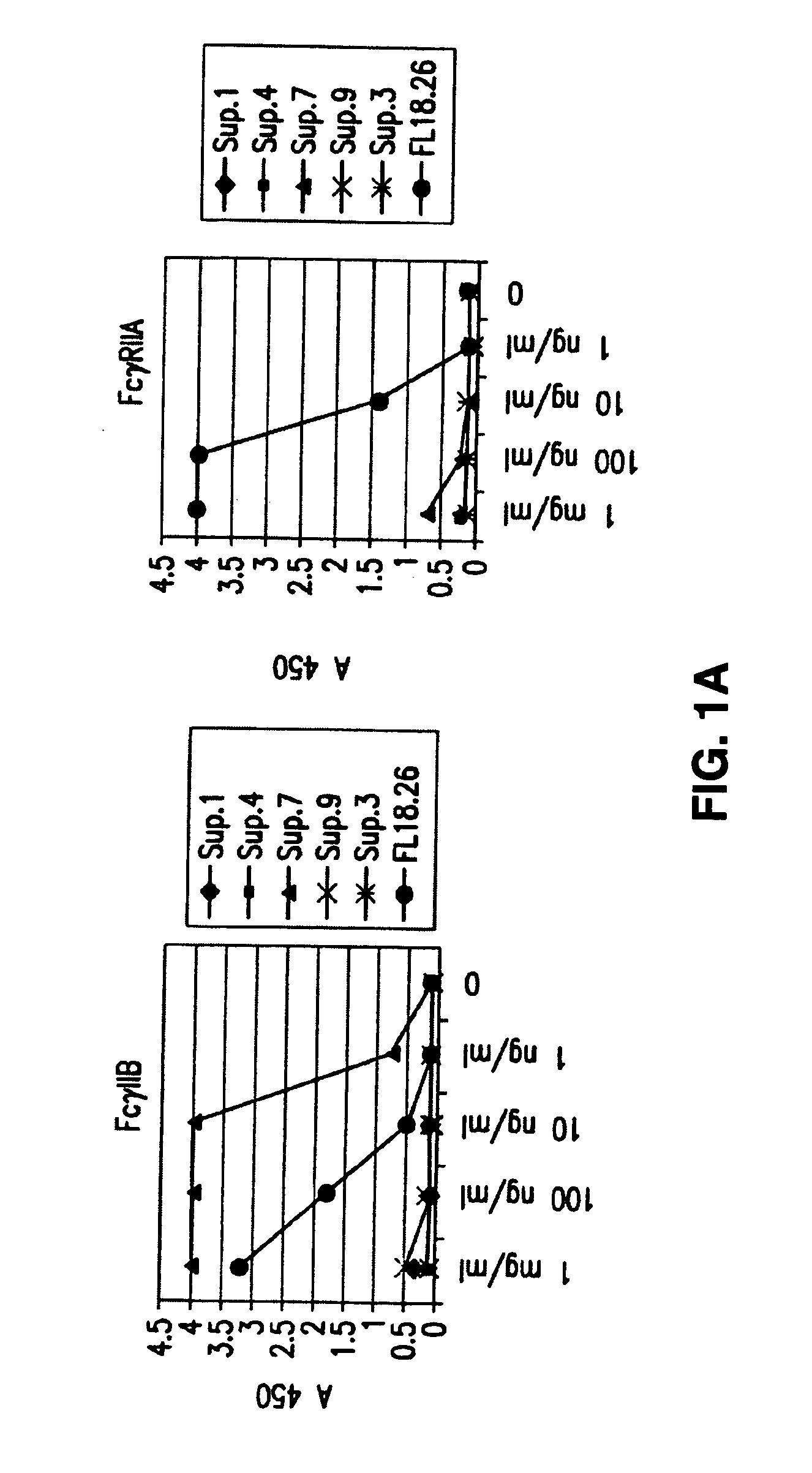
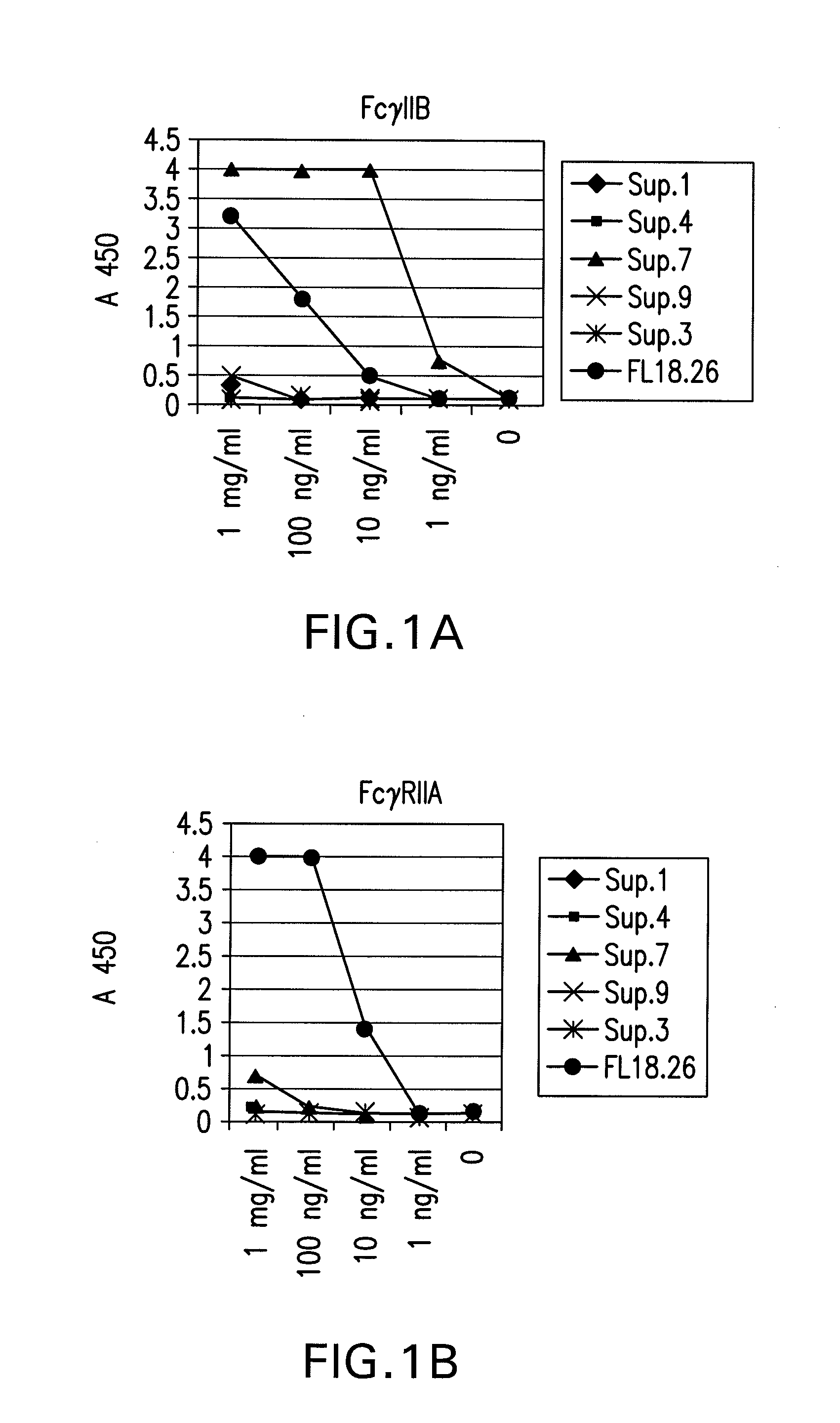
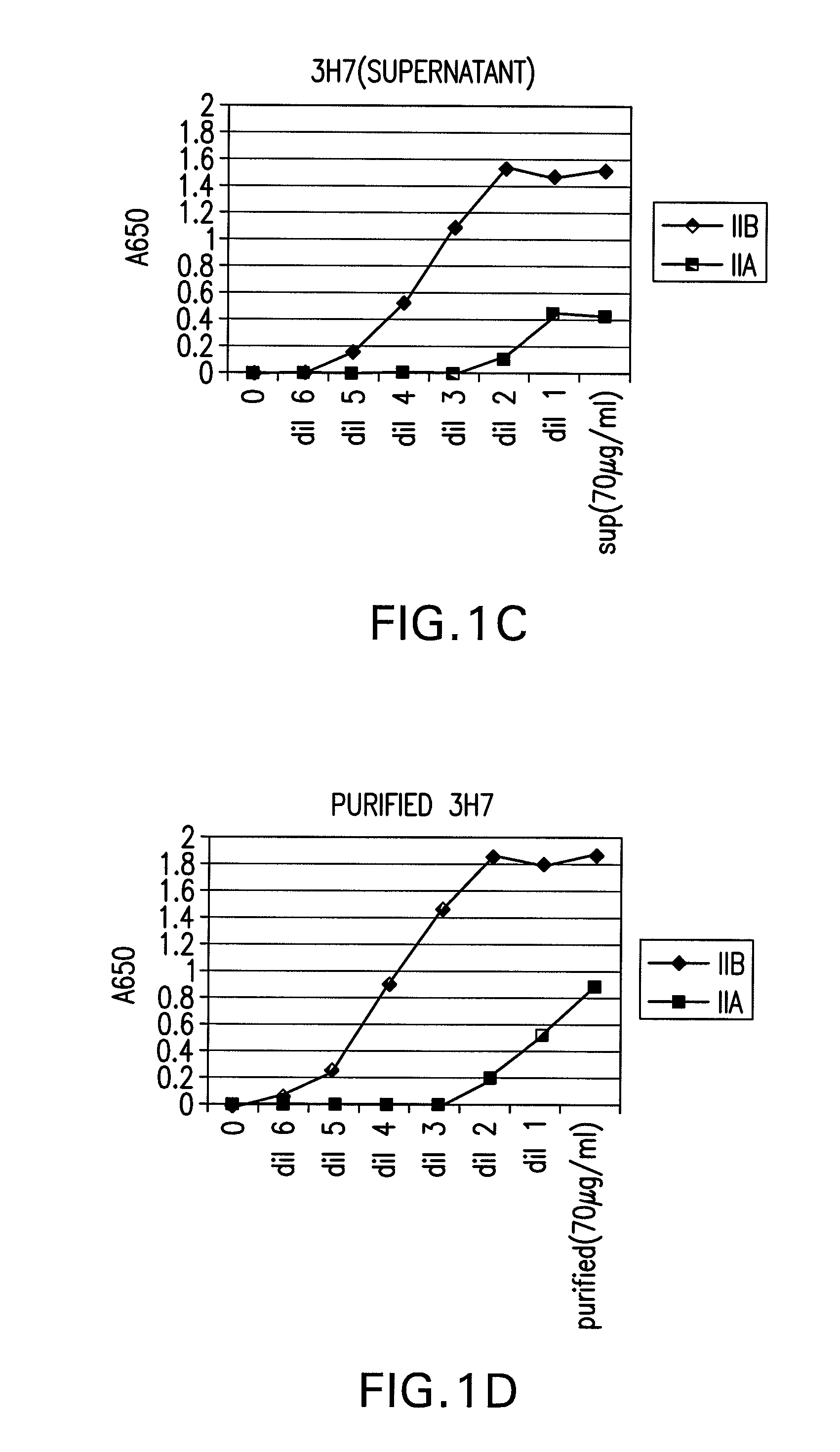
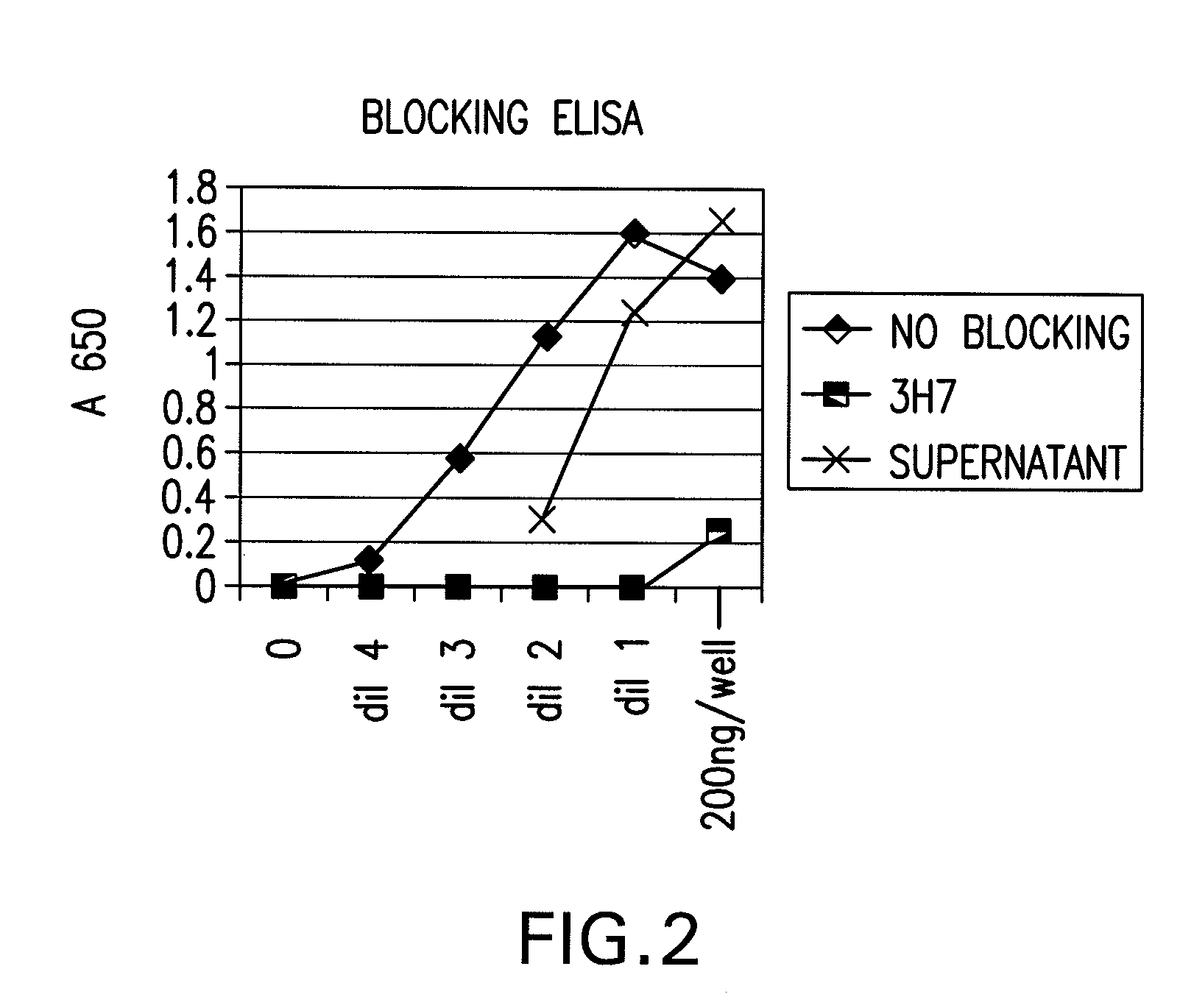
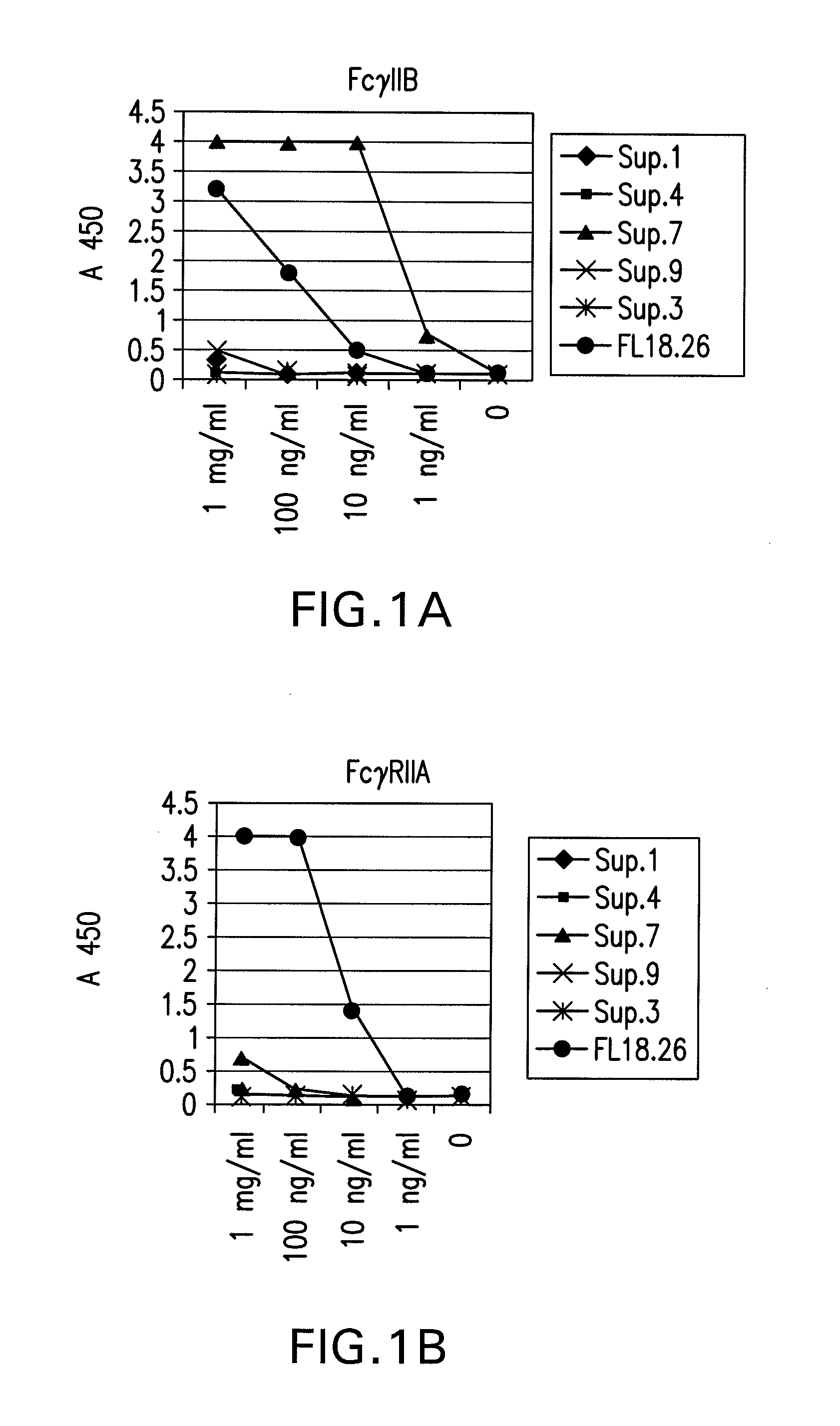
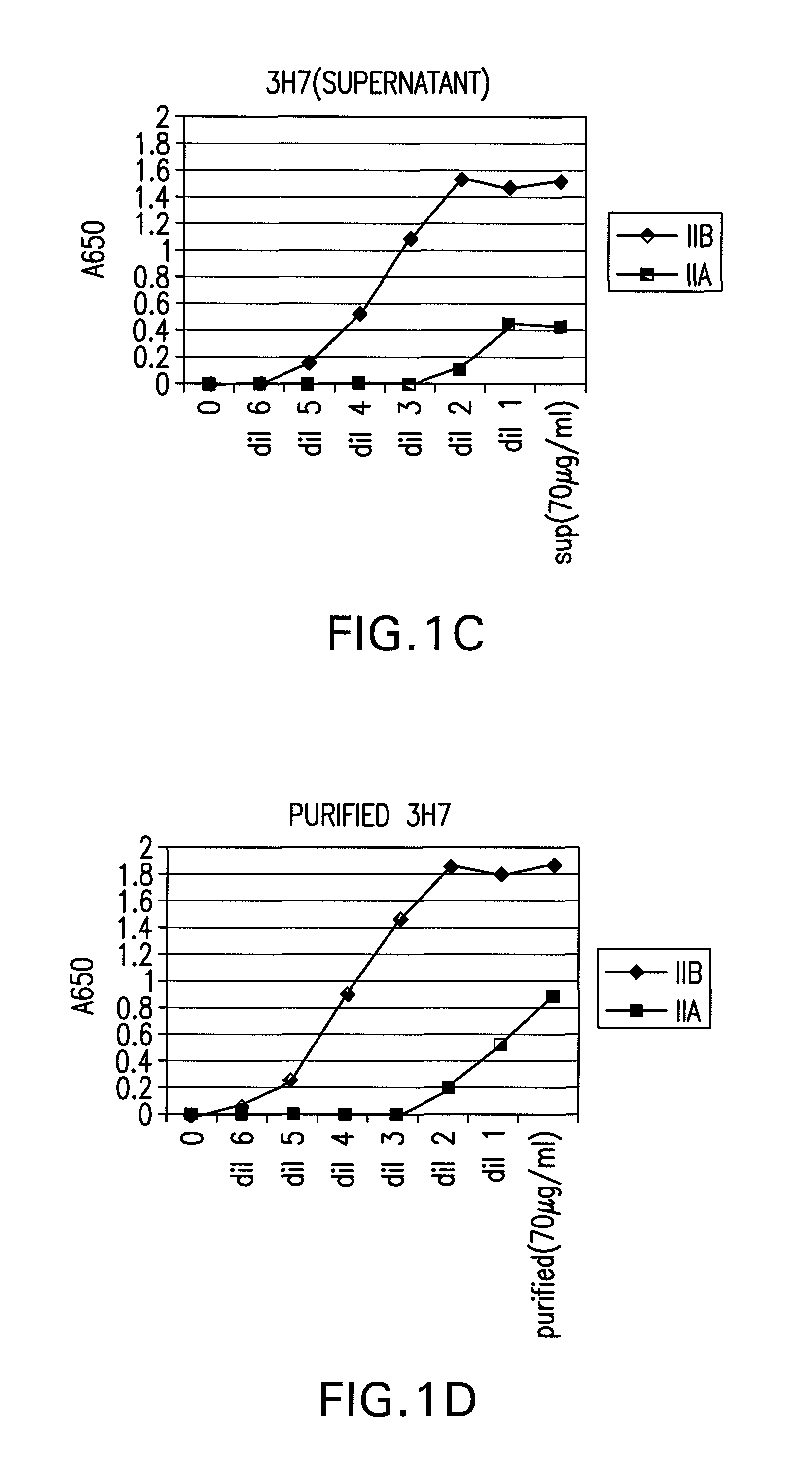
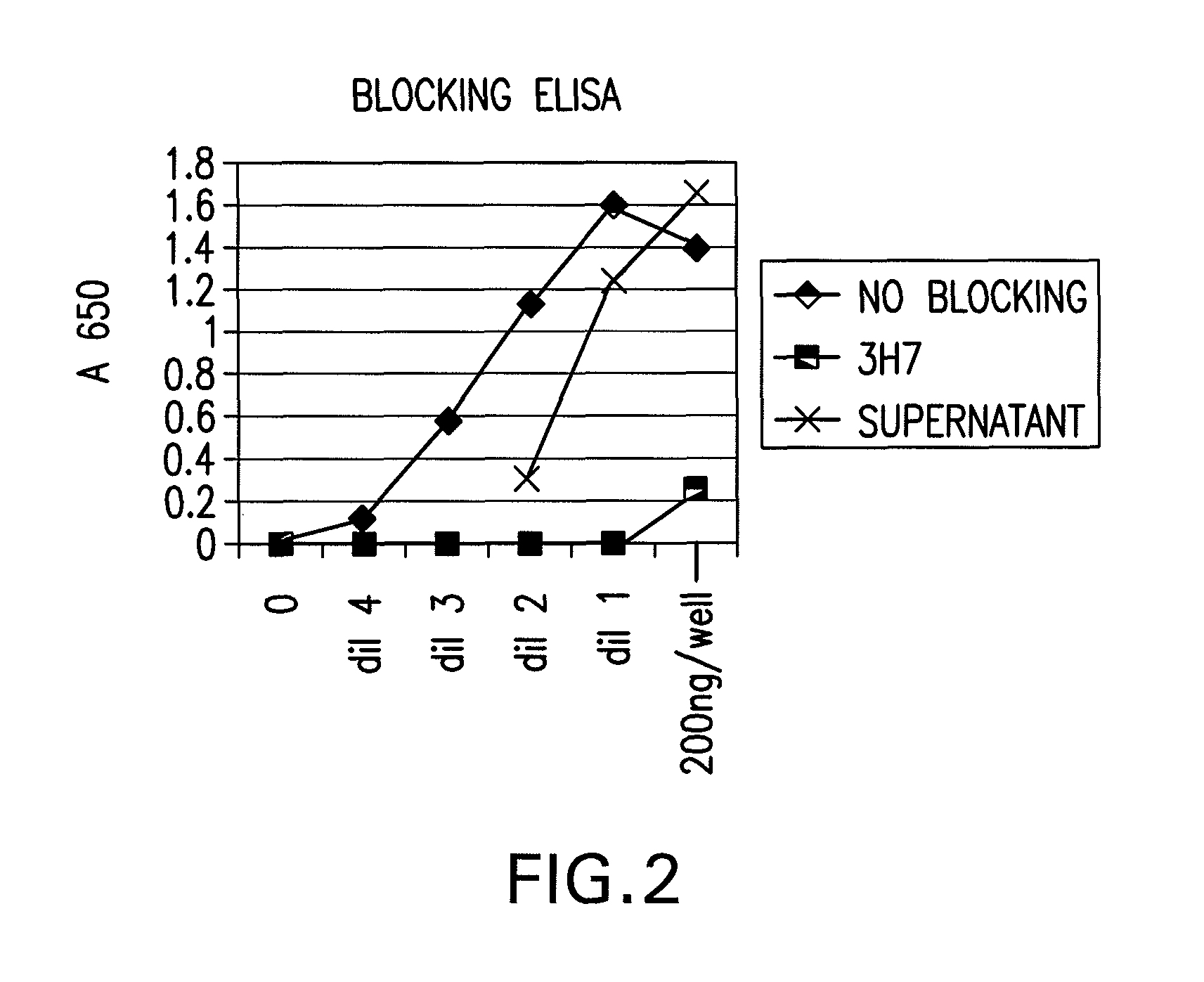
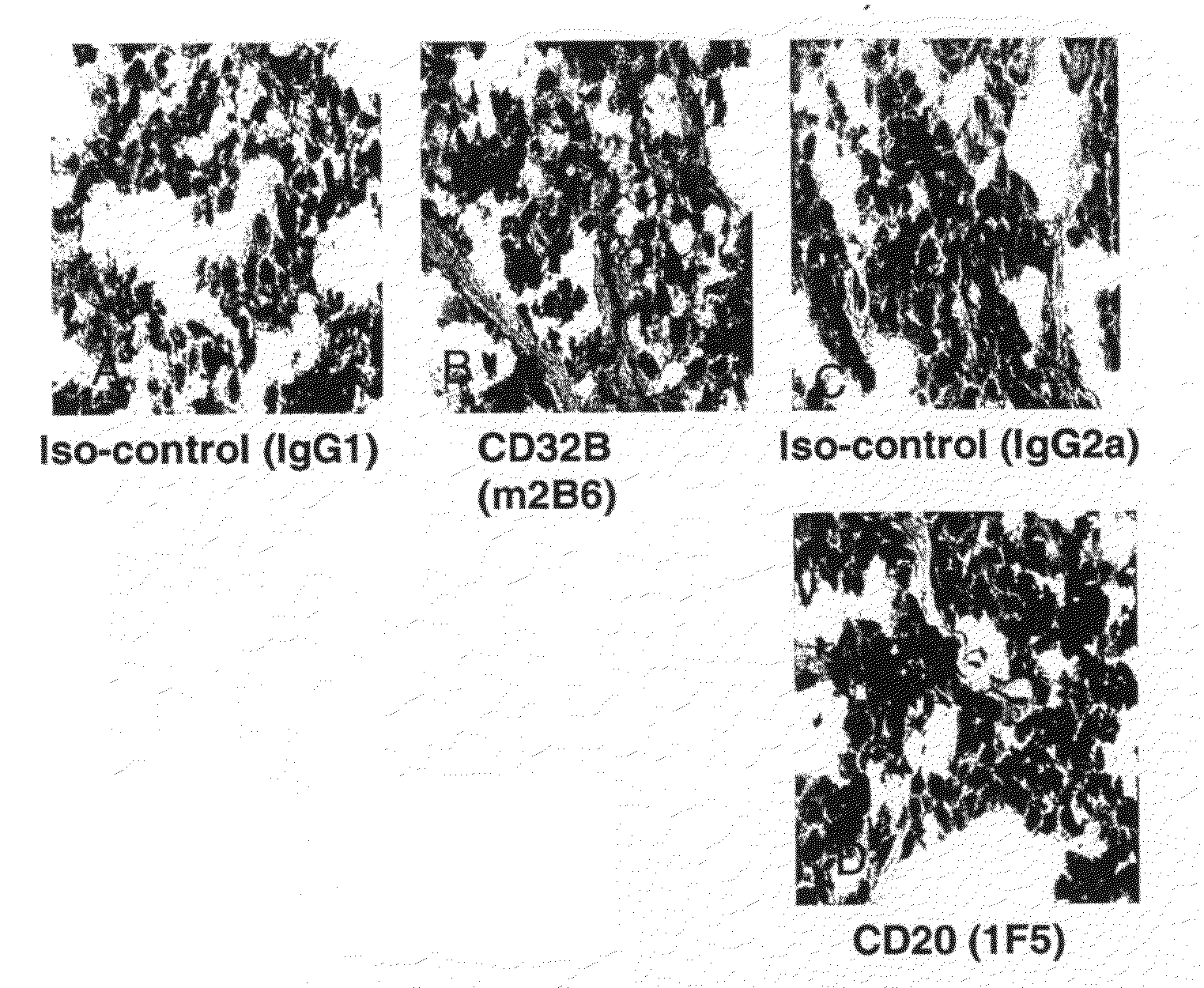
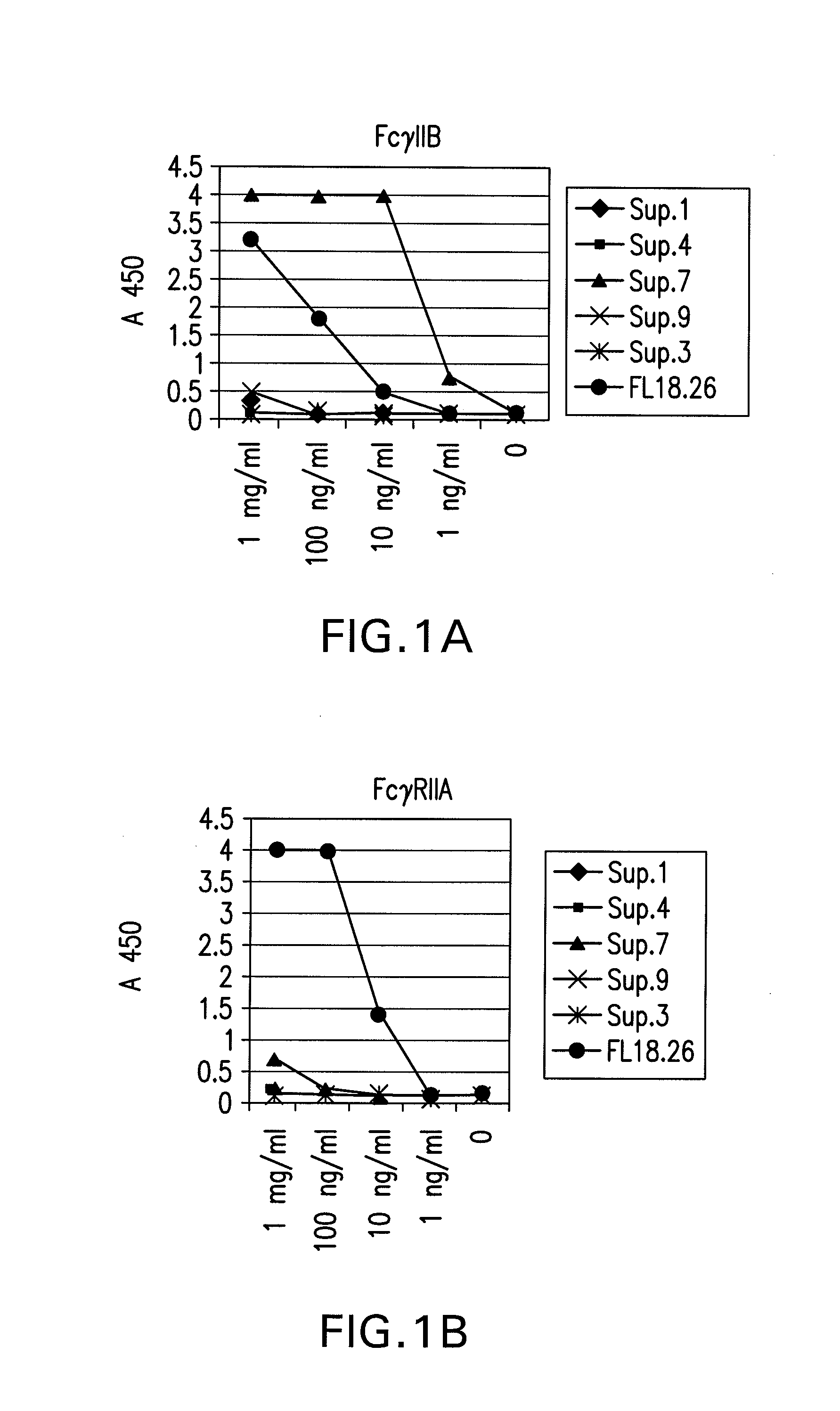
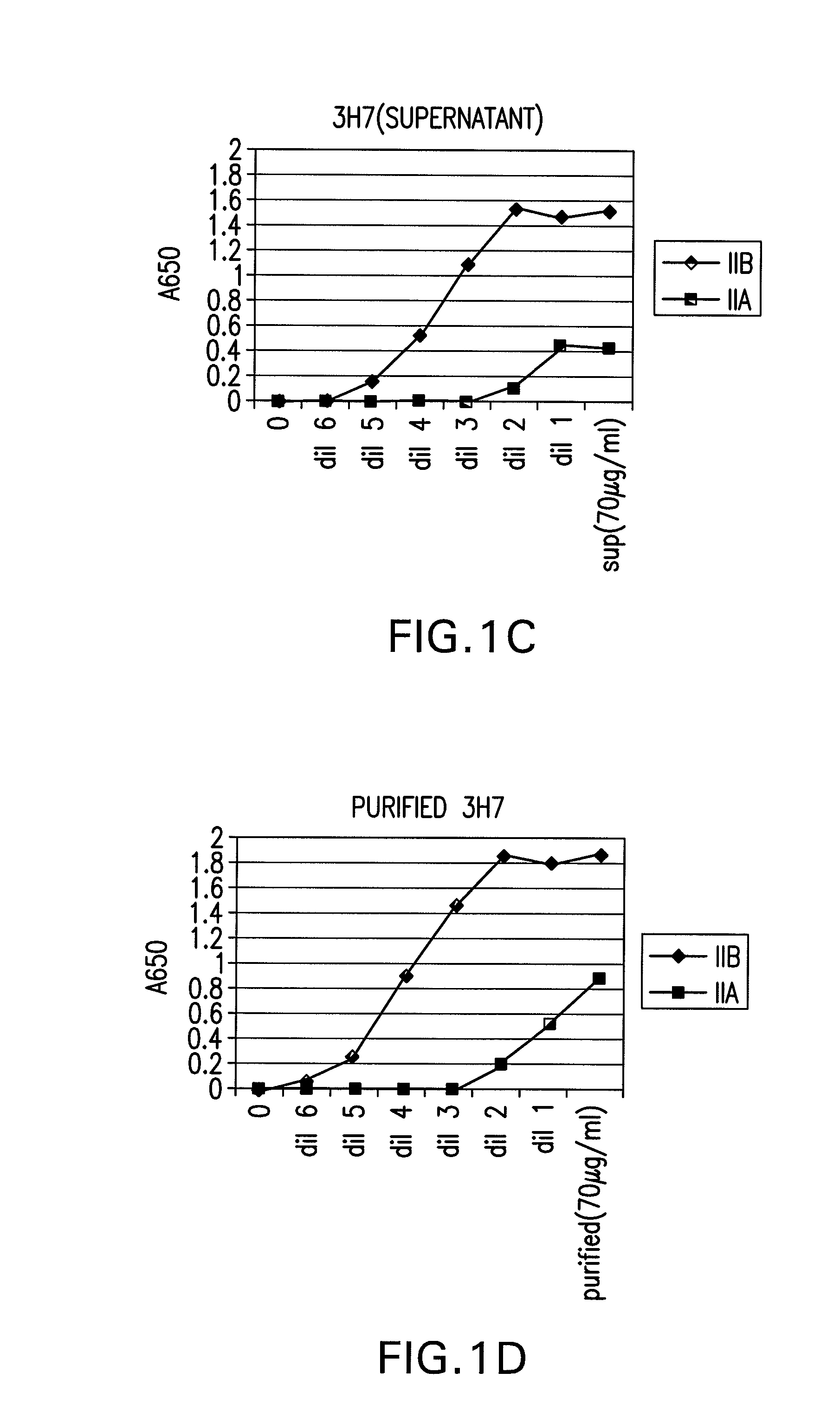
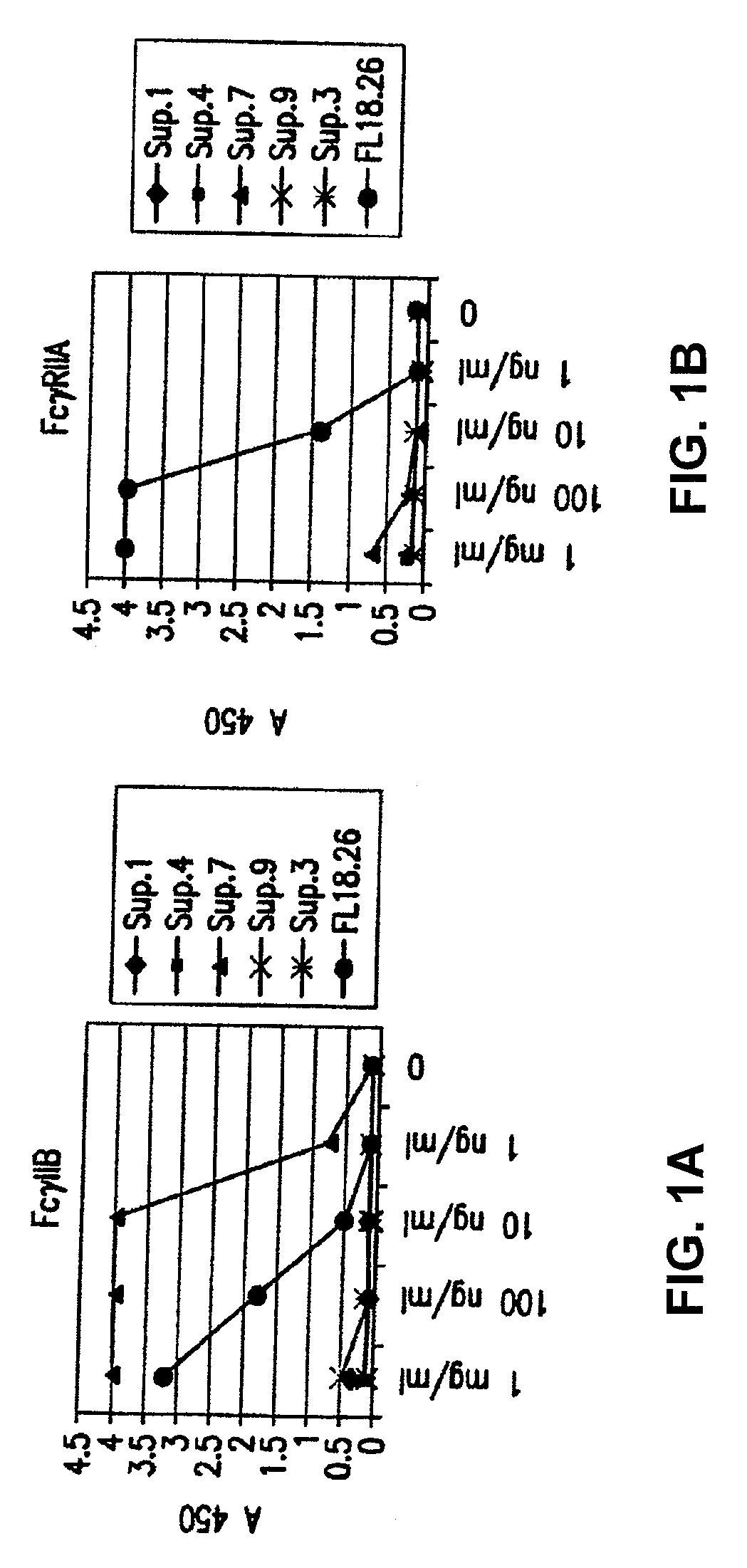
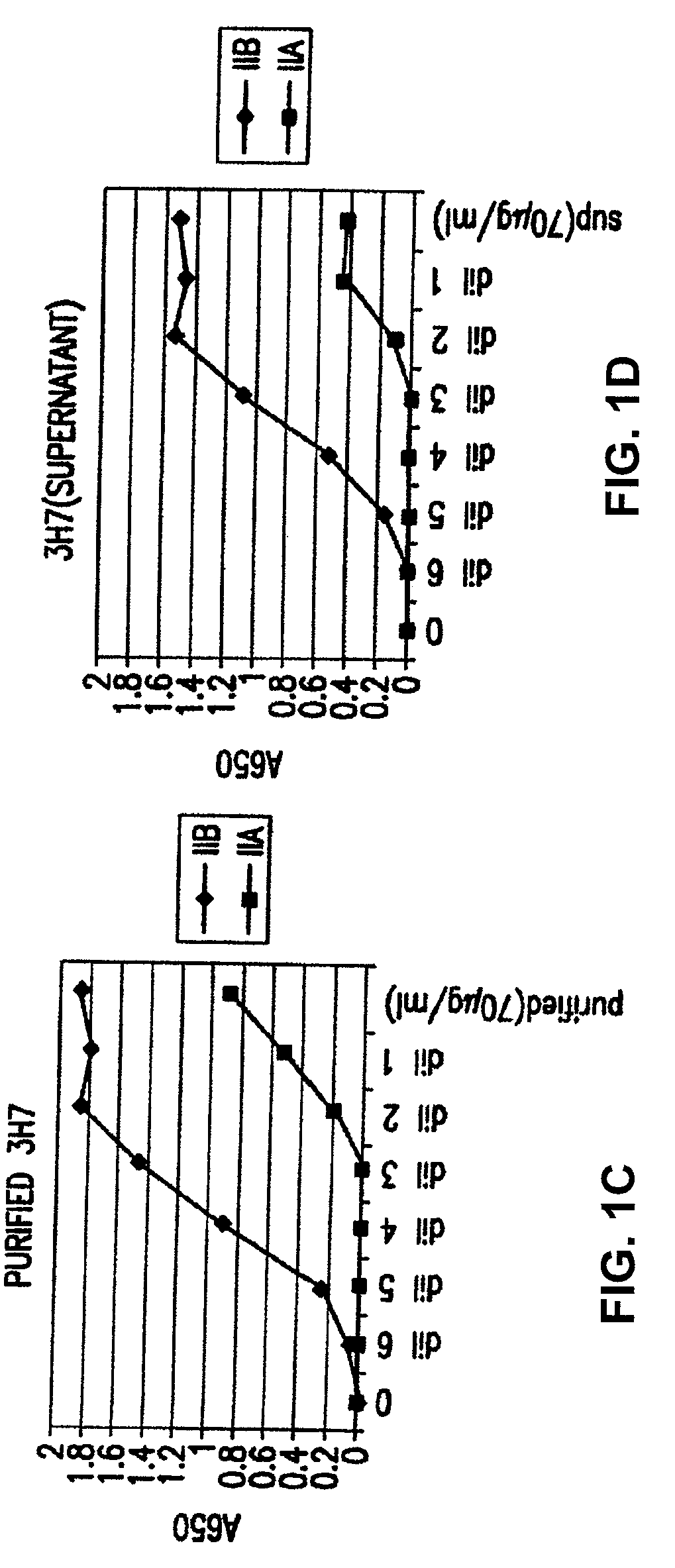
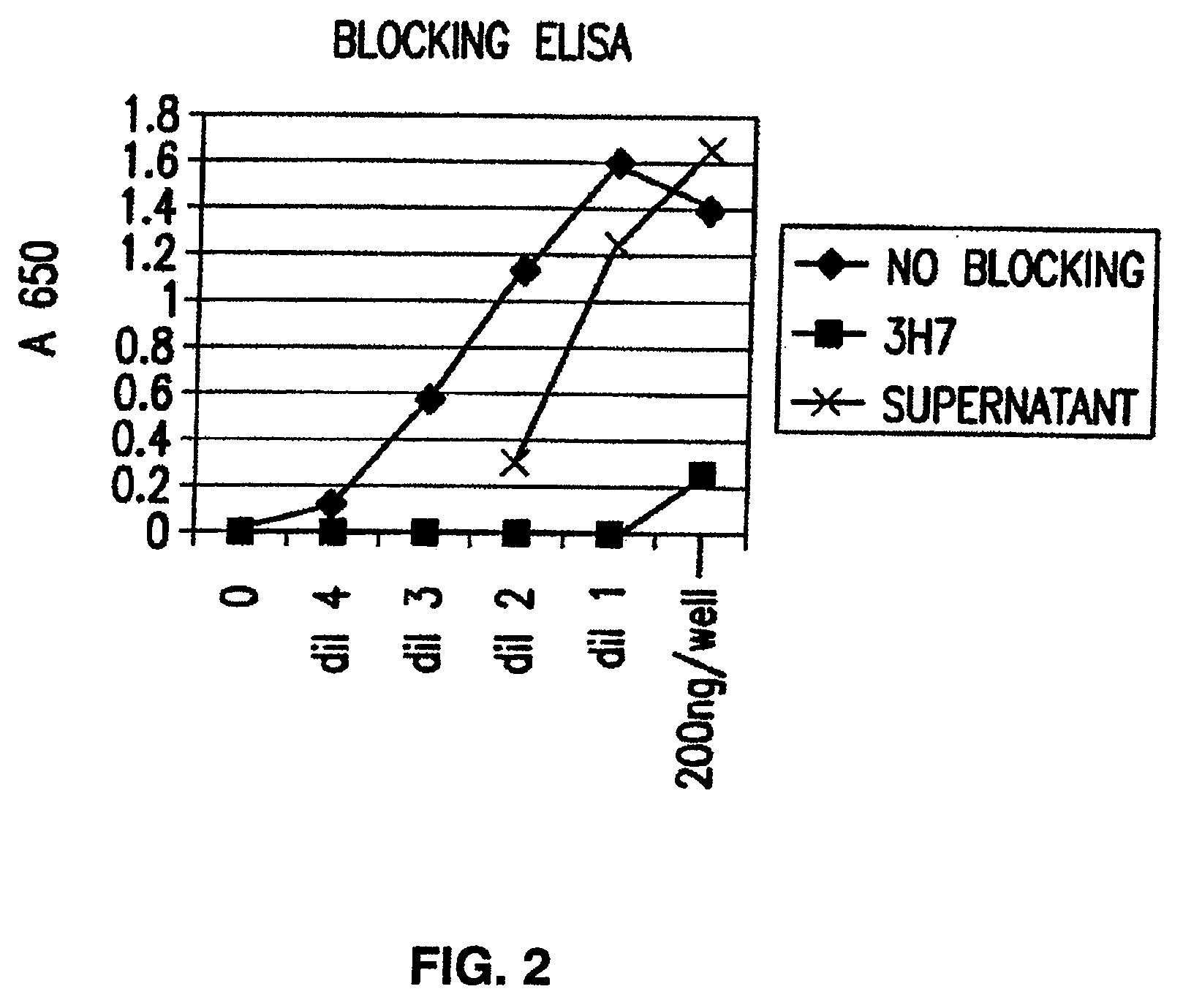
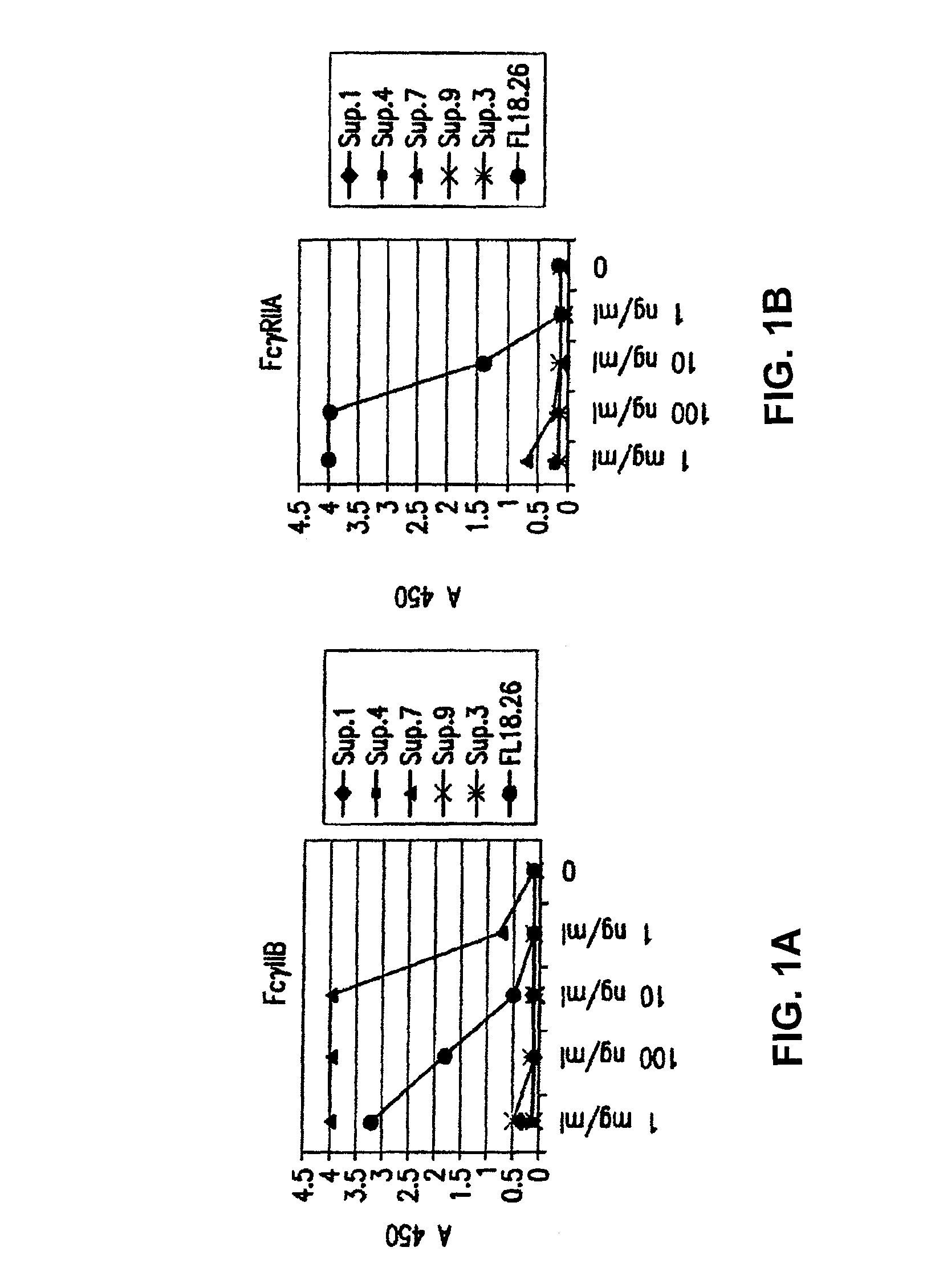
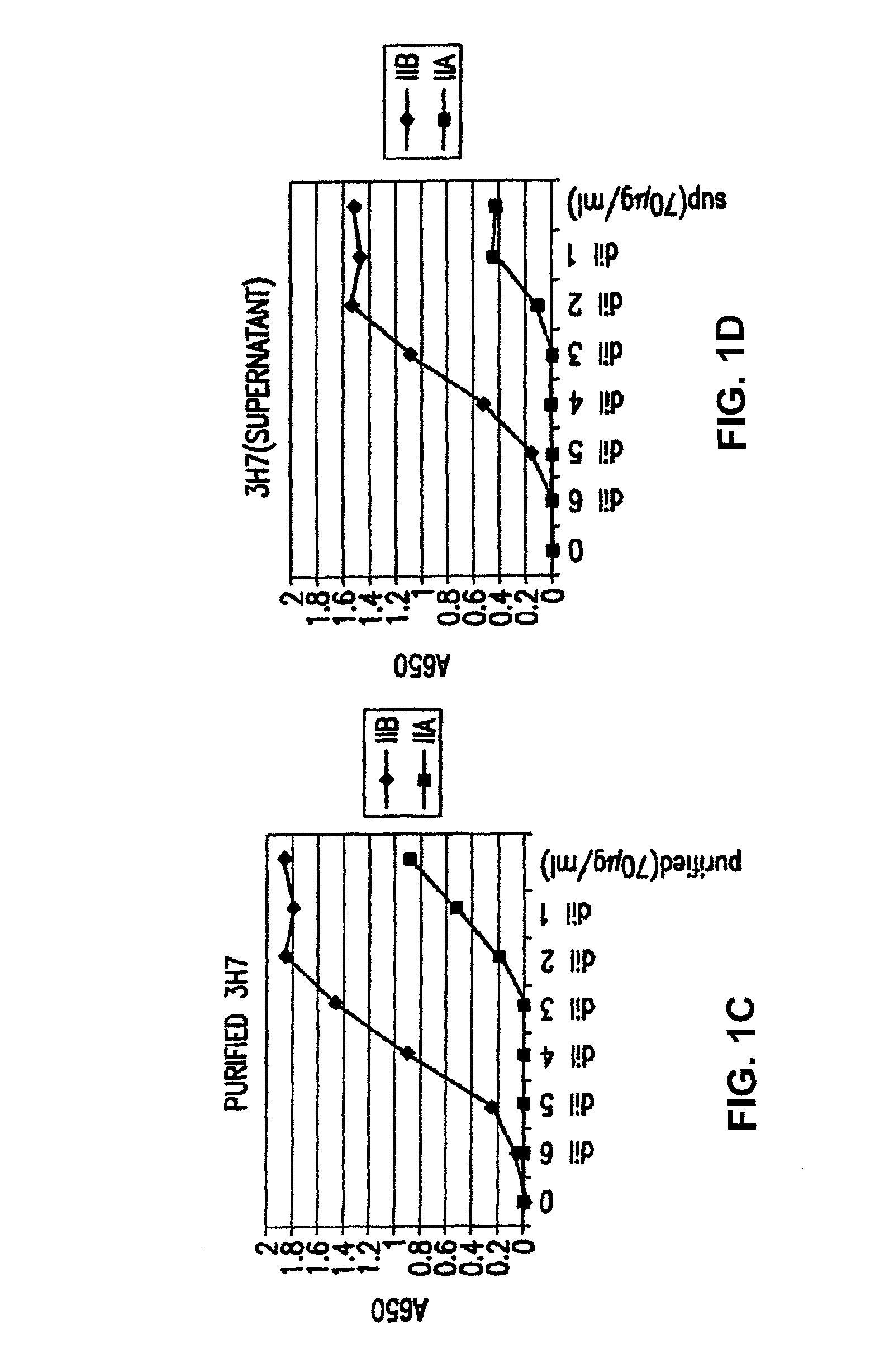
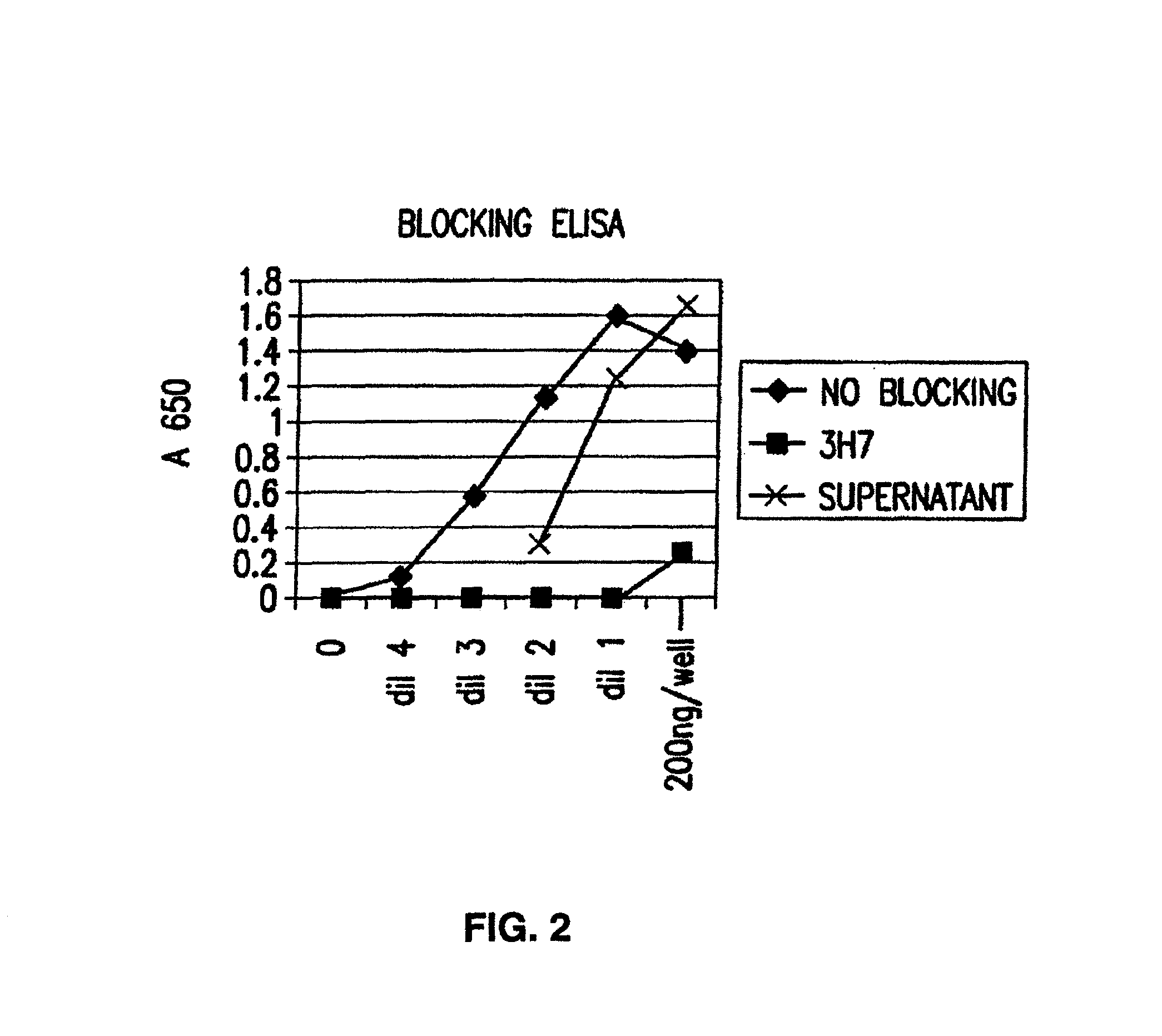
FcgammaRIIB-specific antibodies and methods of use thereof
InactiveUS20060177439A1Good curative effectConvenient treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind the extracellular domain of FcγRIIB, particularly human FcγRIIB, and block the Fc binding site of human FcγRIIB. The invention provides methods of treating cancer and / or regulating immune complex mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
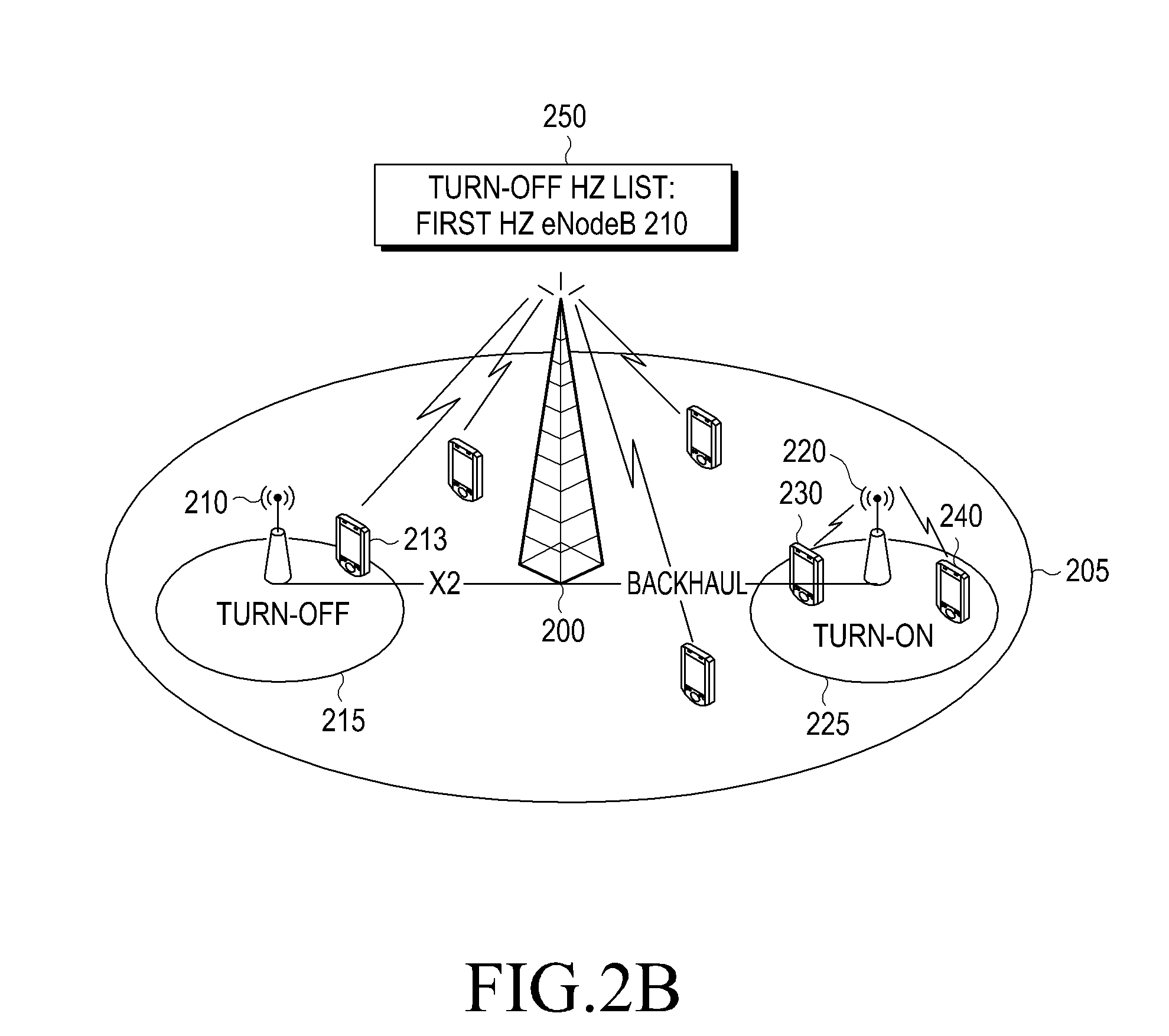
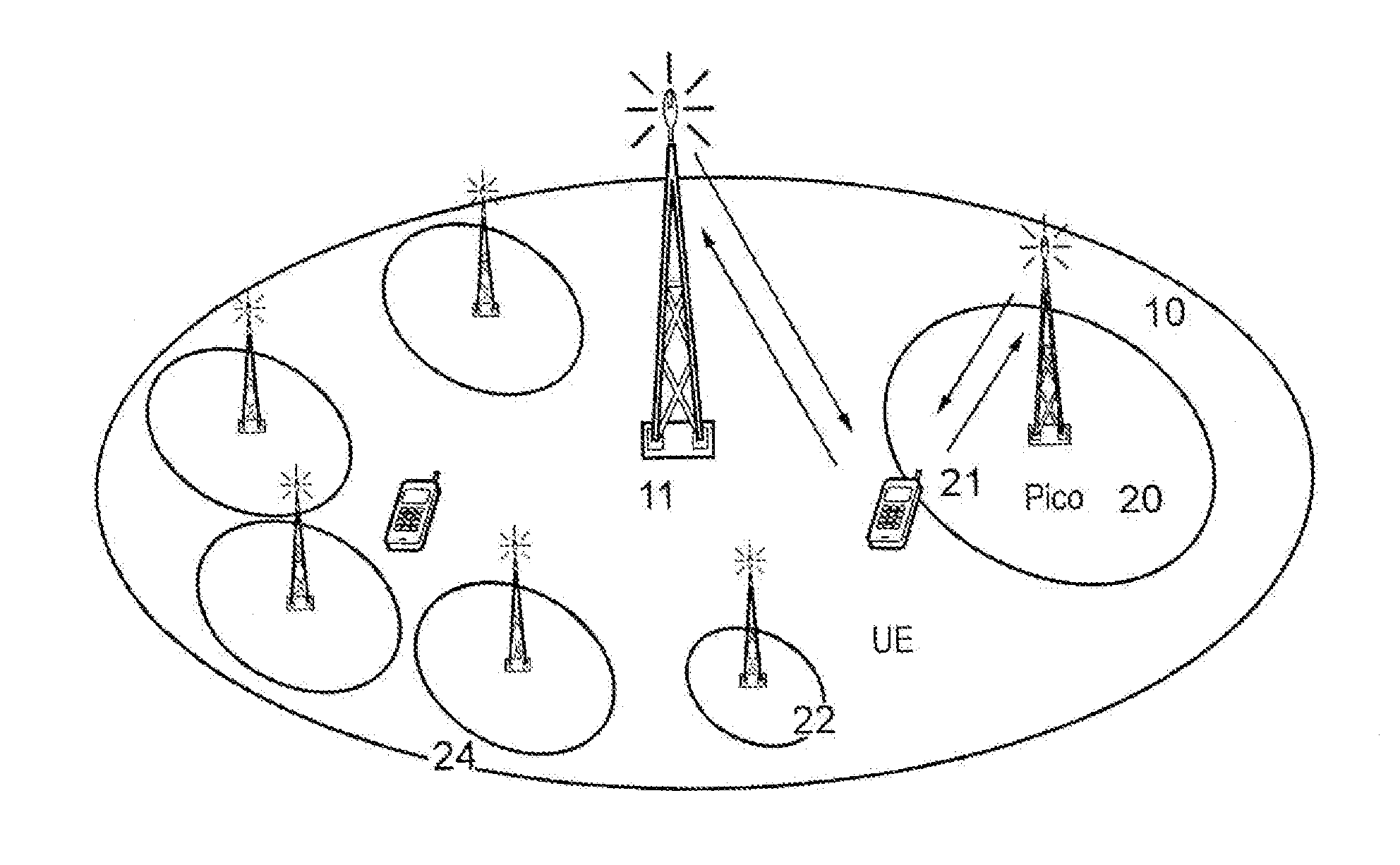
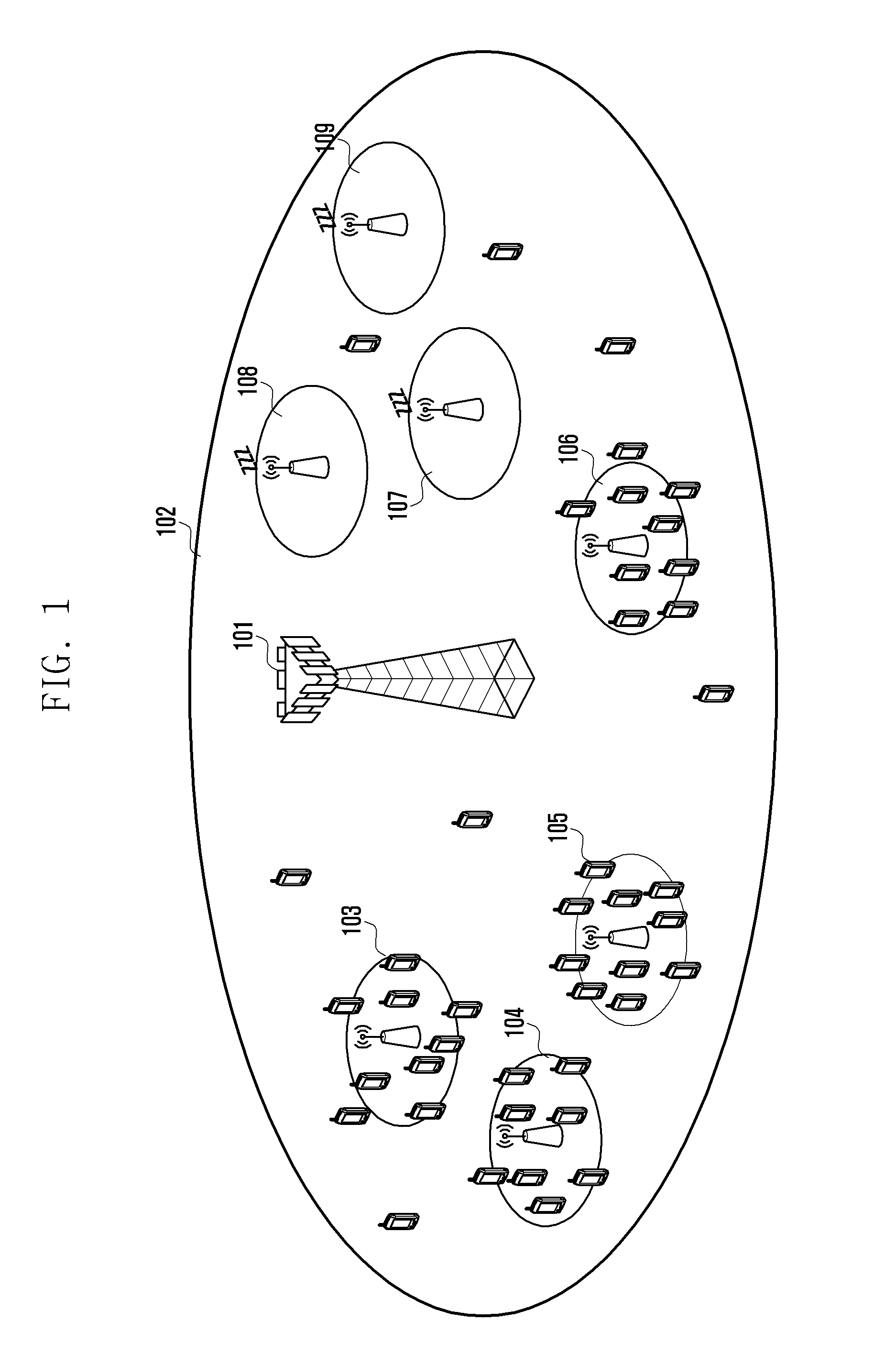
Method for reducing power consumption of base station in wireless communication system
ActiveUS20110170466A1Reduce power consumptionReducing power consumption by an upper base stationPower managementEnergy efficient ICTCell activationCommunications system
A method for reducing power consumption by a base station in a wireless communication system is provided. The method includes transmitting a cell activation request, by an upper base station including a base station that has cell coverage including cell coverage of lower base stations, instructing to perform a turn-on operation to lower base stations in an energy saving mode, receiving a Radio Resource Control (RRC) measurement report from a User Equipment (UE), and transmitting a cell deactivation request instructing to perform a turn-off operation for entry into the energy saving mode to at least one turn-off target base station, determined according to the RRC measurement report. The at least one turn-off target base station includes at least one of base stations performing the turn-on operation according to the cell activation request, and the lower base stations includes base stations included in the cell coverage of the upper base station.
Owner:SAMSUNG ELECTRONICS CO LTD
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Compositions and methods for regulating lymphocyte activation
InactiveUS20020155604A1Increased proliferationHigh affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsCell activationCell Surface Antigens
The present invention relates to regulation of lymphocyte activation. In particular, it relates to compositions and methods for regulating lymphocyte activation by selectively binding multiple cell surface antigens expressed by the same lymphocyte.
Owner:CYCLACEL PHARMA
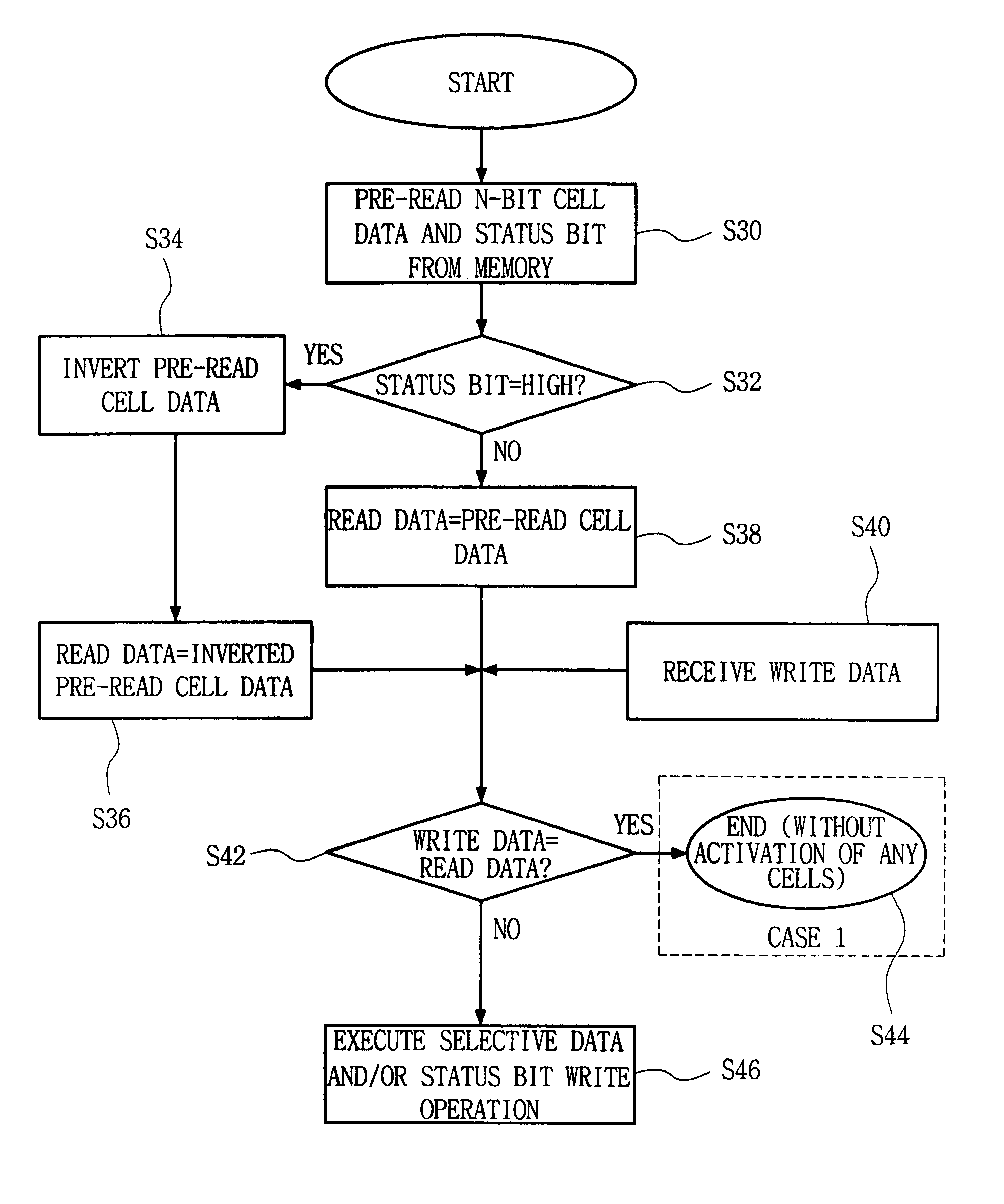
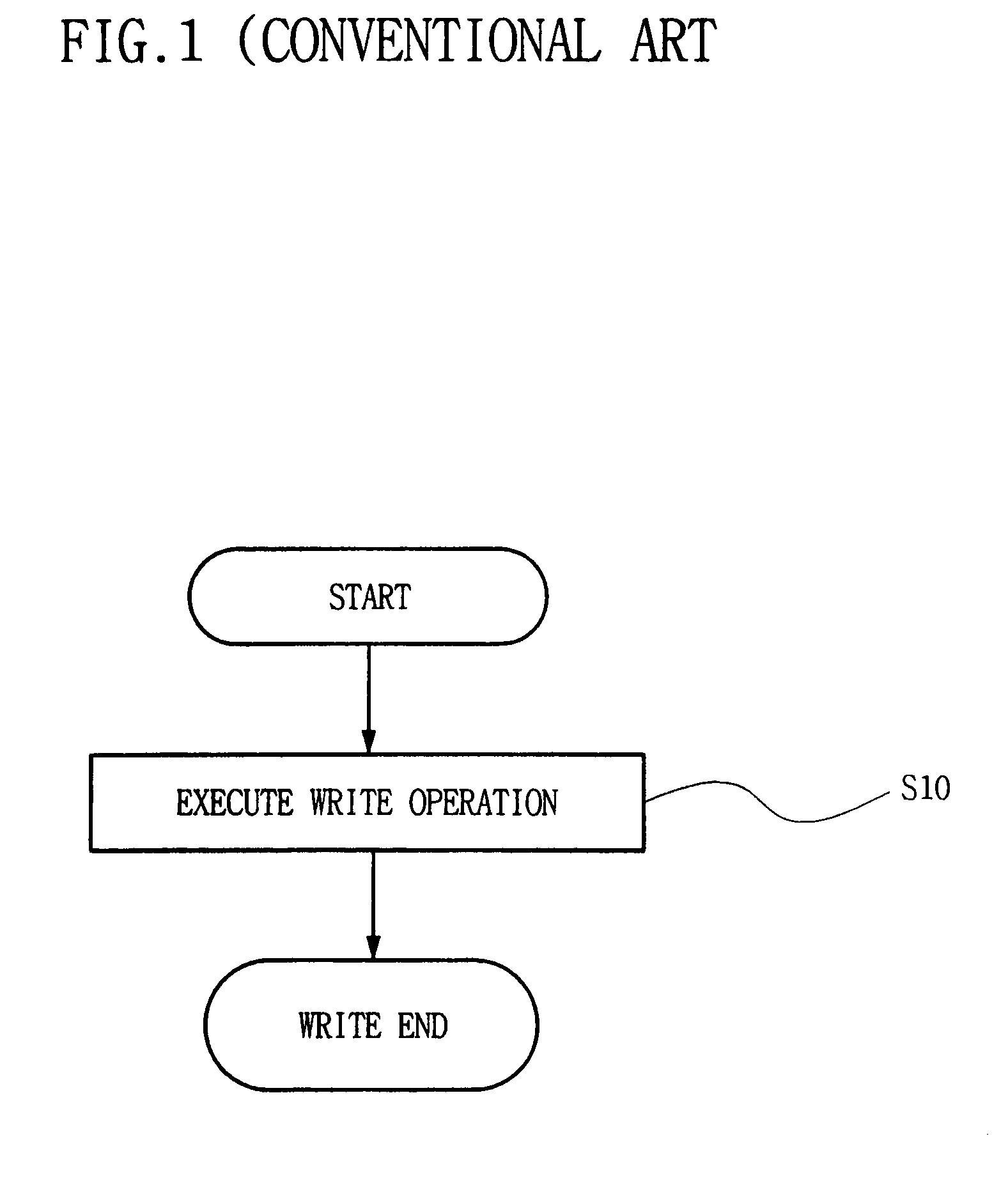
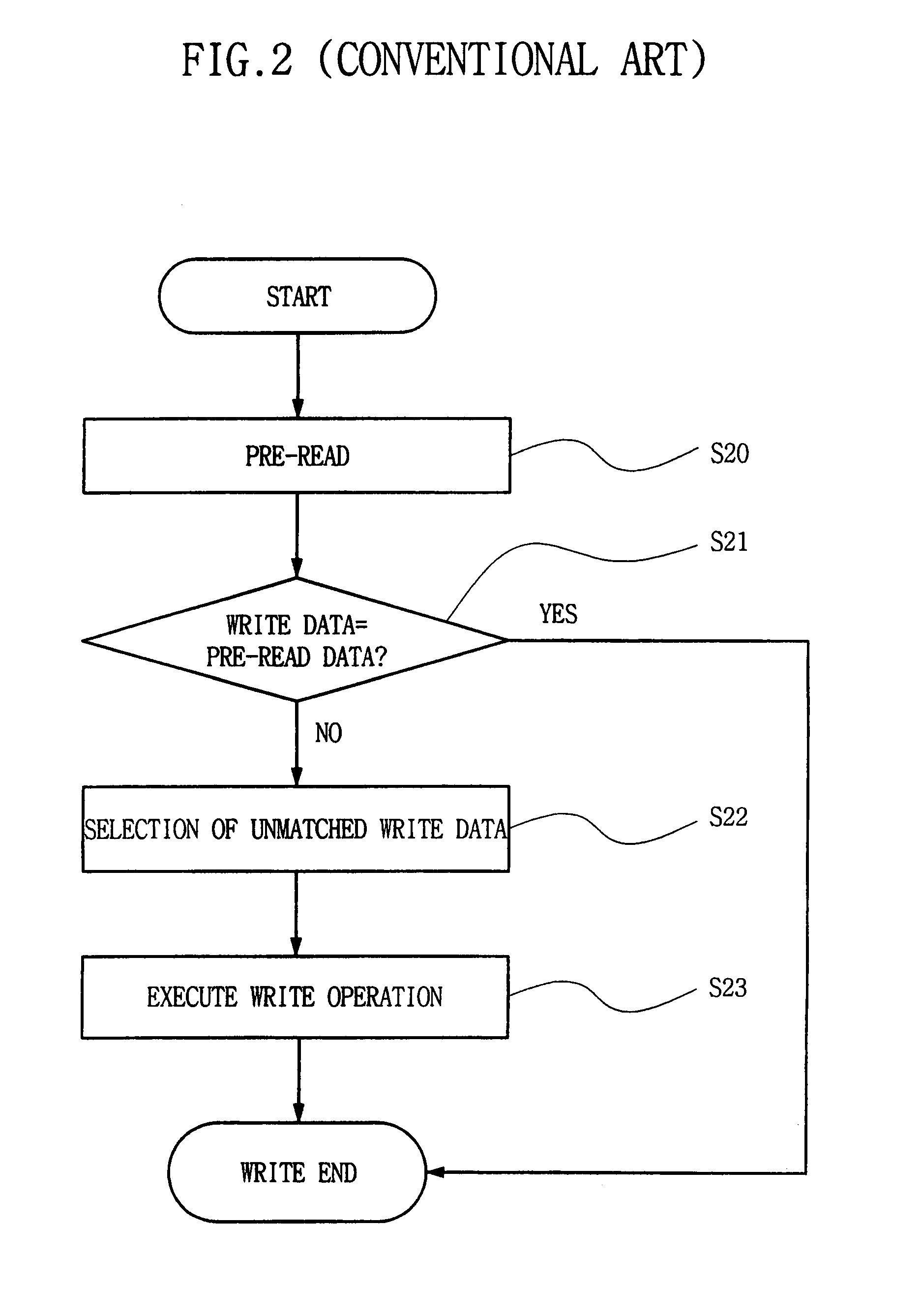
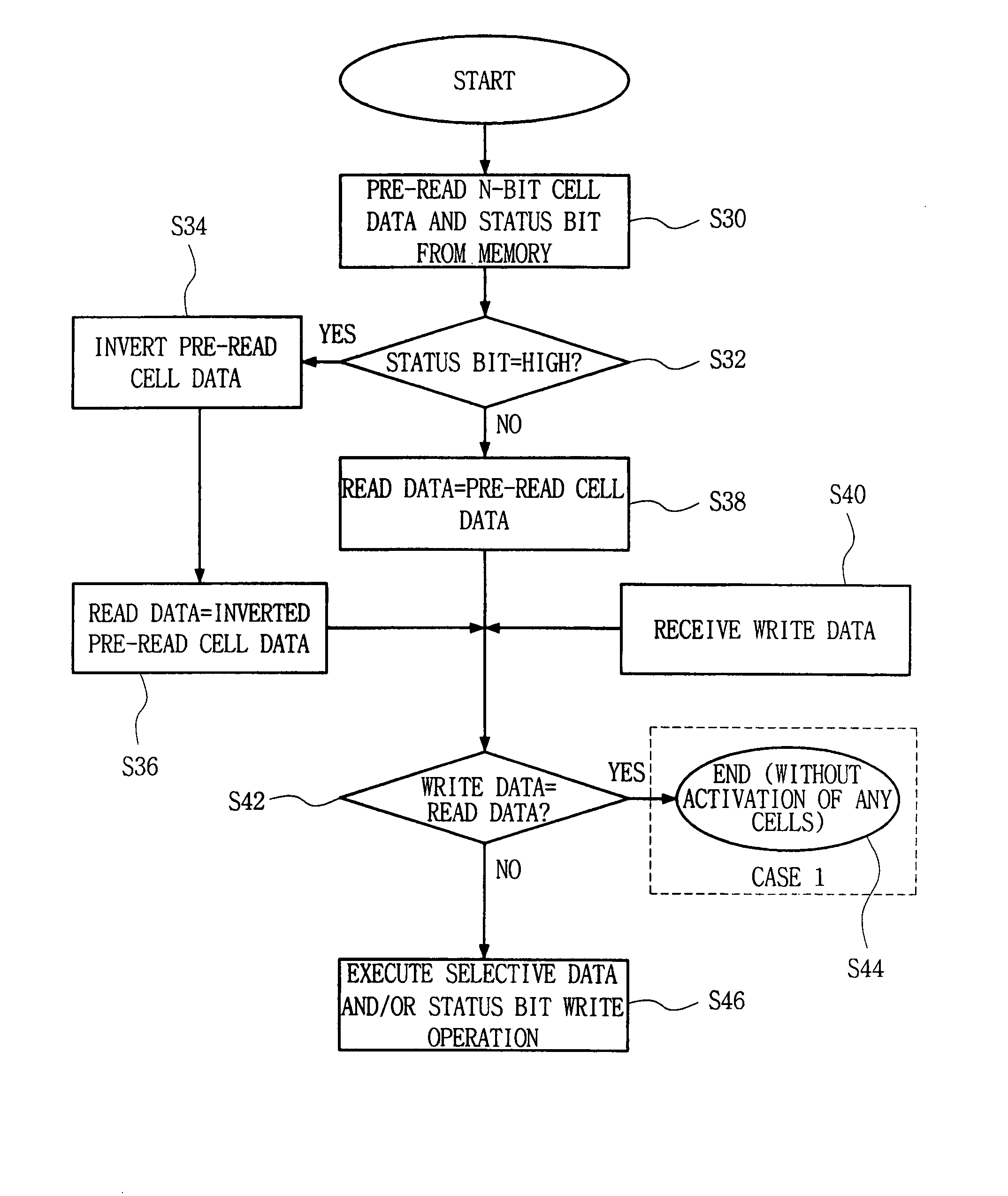
Semiconductor memory device and method for reducing cell activation during write operations
ActiveUS7542356B2Reduce the amount requiredActivated lessRead-only memoriesDigital storageDevice typeCell activation
Owner:SAMSUNG ELECTRONICS CO LTD
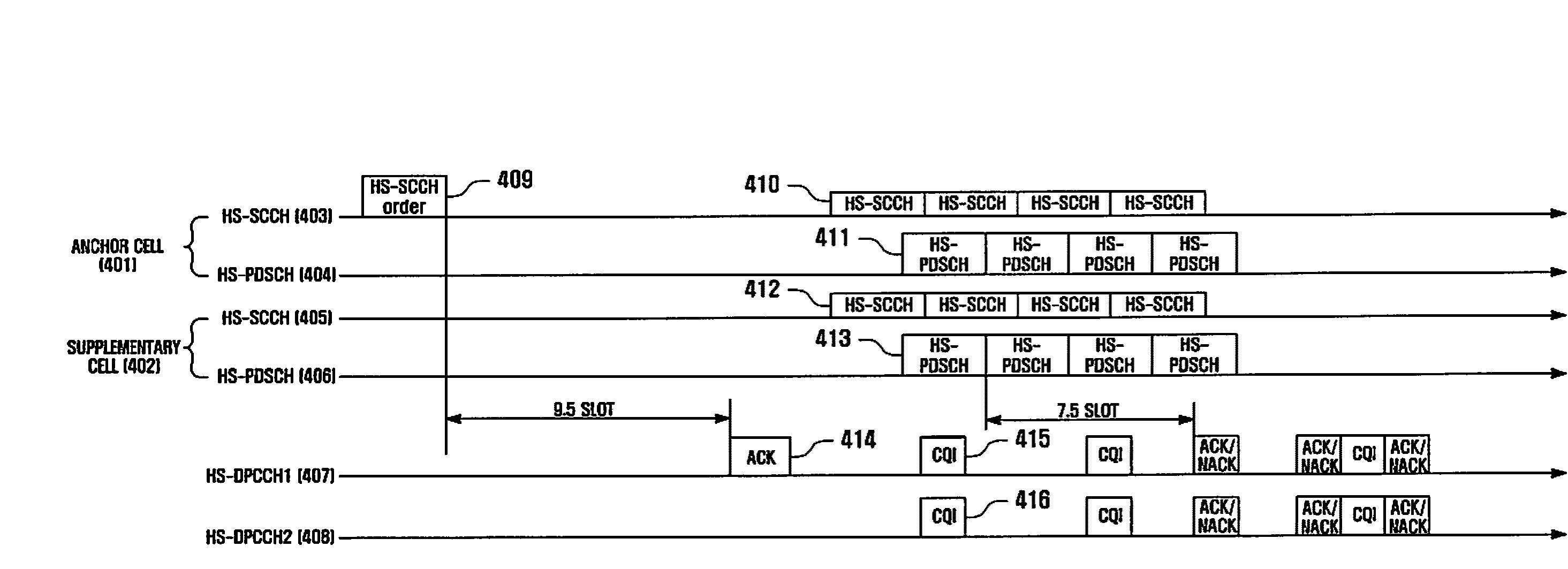
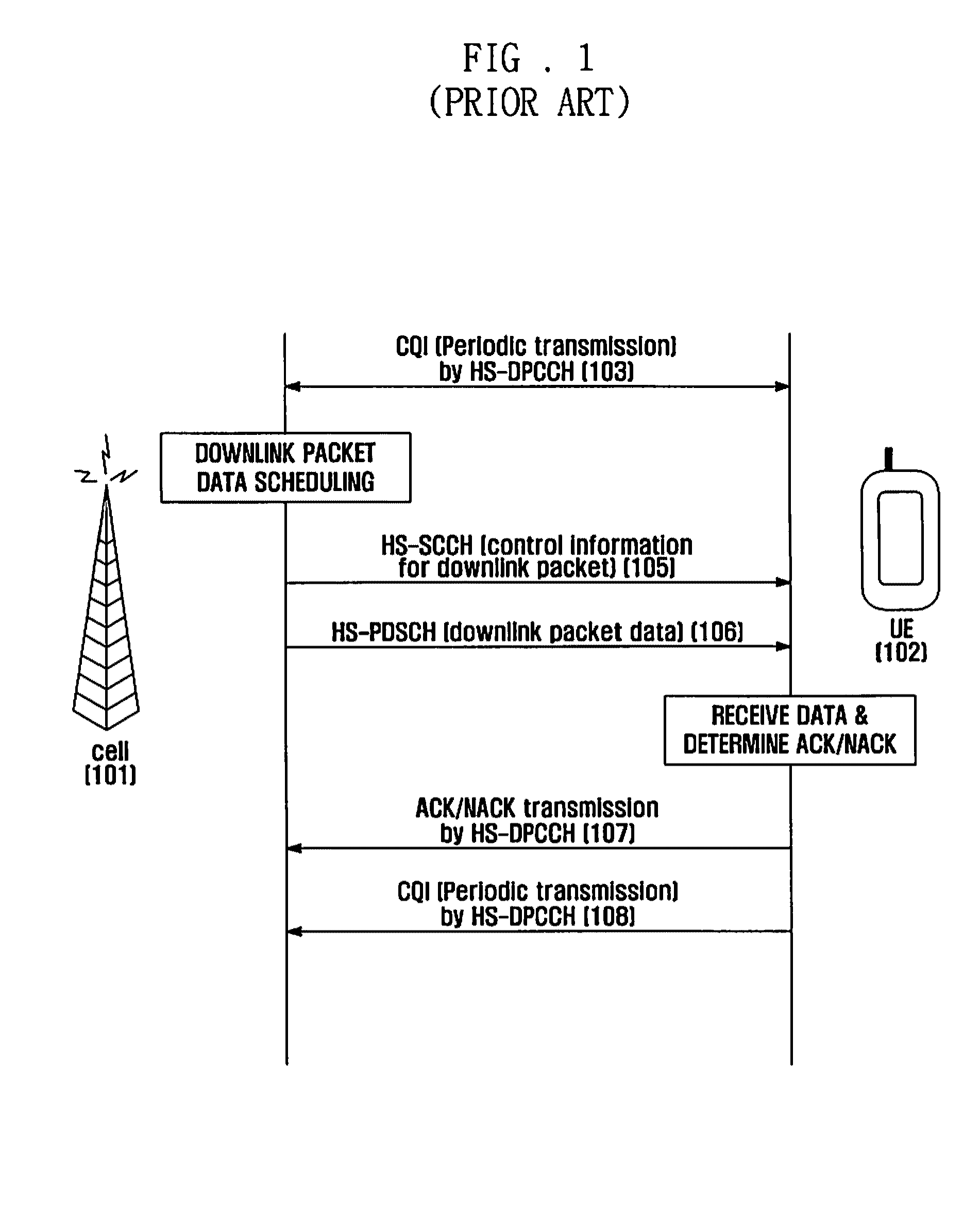
Method and apparatus for dynamically activating and deactivating a supplementary cell for a wcdma system
InactiveUS20100034176A1Efficient workEfficient managementEnergy efficient ICTRadio transmissionCell activationUplink transmission
A method and apparatus for dynamically activating and deactivating a supplementary cell in Dual-Cell HSDPA service of a Universal Mobile Communication Service (UMTS) system. In a supplementary cell activation and deactivation method, a user equipment receives a supplementary cell activation command from a base station; compares an uplink transmission power with a predetermined threshold value; transmits a supplementary cell activation reply, when the uplink transmission power is equal to or greater than the threshold value; and transmits a supplementary cell deactivation reply, when the uplink transmission power is less than the threshold value.
Owner:SAMSUNG ELECTRONICS CO LTD
FcγRIIB specific antibodies and methods of use thereof
InactiveUS8946387B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Stem cell mediated treg activation/expansion for therapeutic immune modulation
ActiveUS20080159998A1High activityInduce upregulationBiocideGenetic material ingredientsAntigenT cell
Disclosed are cells, methods of modulating cells, and therapeutic uses of the cells for the immune modulation of mammals in need thereof. Immune modulation including alteration of cytokine profile, cytotoxic activity, antibody production and inflammatory states is achieved through the administration of various cell types that have been unmanipulated or manipulated in order to endow specific biological activity. Cellular subsets and administration of the subsets in combination with various agents are also provided. One embodiment teaches the previously unknown finding that adipose tissue derived mononuclear cells contain T cells with immune regulatory properties that alone or synergistically with various stem cells induce immune modulation upon administration. Another embodiment is the finding that stimulation of stem cell activation results in stem cell secondary activation of immune modulatory cells, one type which is T regulatory cells (Tregs). One specific embodiment involves extraction of a heterogenous stem cell pool, which contains T regulatory cells, treatment in culture of the population with agents known to stimulate stem cell activation, then subsequent extraction and administration of the purified Tregs. Other embodiments include expansion of Tregs in the presence of antigen in order to generate anti-specific Tregs.
Owner:XON CELLS
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090076251A1Strong therapeutic activityEnhancing antibody-mediated effector functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Cell activation and deactivation in heterogeneous networks
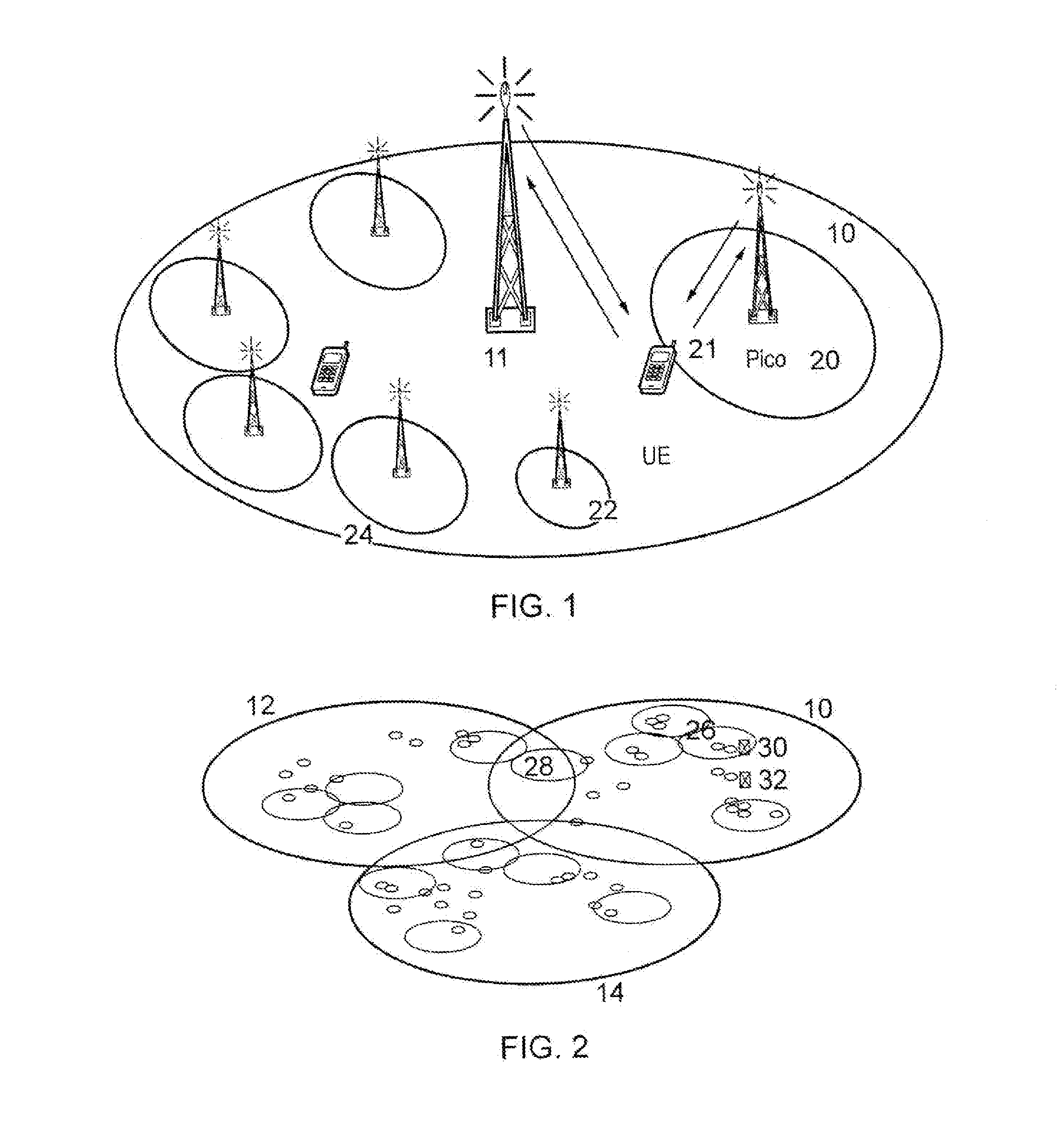
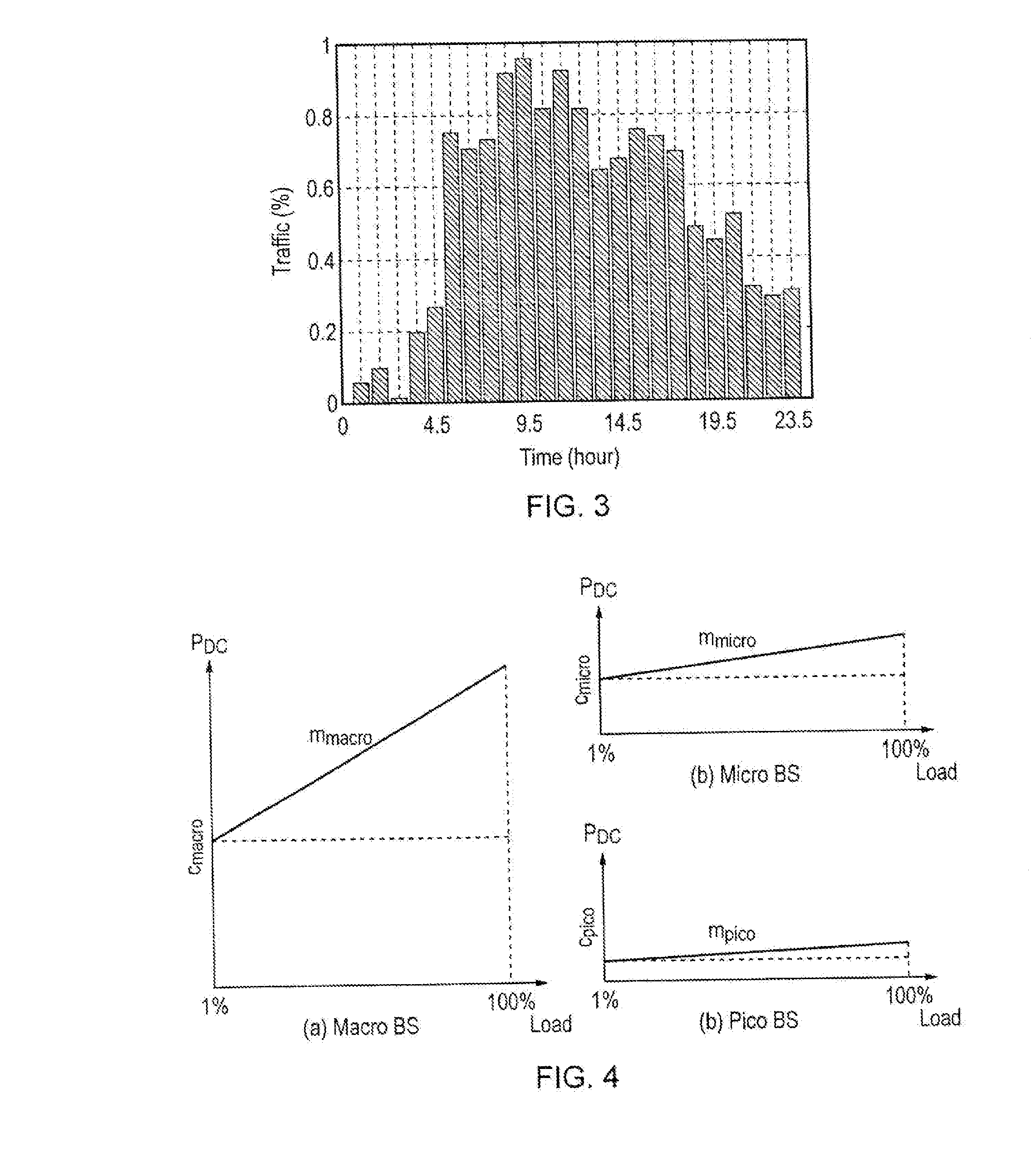
InactiveUS20130310048A1Improve energy efficiencyPower managementHigh level techniquesCell activationEngineering
An algorithm which considers the individual power consumption profiles for different types of base stations in a heterogeneous cellular network, and determines which base stations can be switched OFF in line with the traffic demand. The algorithm predicts (302) a spatially-accurate network load; calculates (304) the best serving BS for each load region; investigates (308-318) toads on Pico cells with respect to a threshold, and ranks (320) candidate Pico cells to be switched OFF in order of least power efficiency and most resource efficiency. Pico cells determined to remain on are fully loaded (322) allowing more candidates to be switched OFF (324). The algorithm optimizes the energy efficiency of the heterogeneous, cellular network, enabling the activation of the minimum number of base stations to support the predicted traffic demand for a given time period and allowing significant energy savings for the operator.
Owner:FUJITSU LTD
Anticoagulant fusion protein anchored to cell membrane
InactiveUS6423316B1Prolong clotting timeGood curative effectFungiVirusesCell membraneBlood coagulations
The invention relates to the inhibition of blood coagulation, especially during organ rejection, and in particular the inhibition of delayed vascular rejection. The invention provides anticoagulant proteins which are anchored to cell membranes. The anticoagulant function preferably provided by heparin, antithrombin, hirudin, TFPI, tick anticoagulant peptide, or a snake venom factor. These anticoagulant proteins are preferably prevented from being constitutively expressed at the cell surface. In particular, expression at the cell surface is regulated according to cell activation, for instance by targeting the protein to a suitable secretory granule. Expression of these proteins renders cells, tissues and organs less vulnerable to rejection after transplantation (e.g. after xenotransplantation).
Owner:IMPERIAL INNOVATIONS LTD
Semiconductor memory device and method for reducing cell activation during write operations
ActiveUS20080101131A1Reduce the amount requiredActivated lessRead-only memoriesDigital storageDevice typeCell activation
Owner:SAMSUNG ELECTRONICS CO LTD
Light guide comb
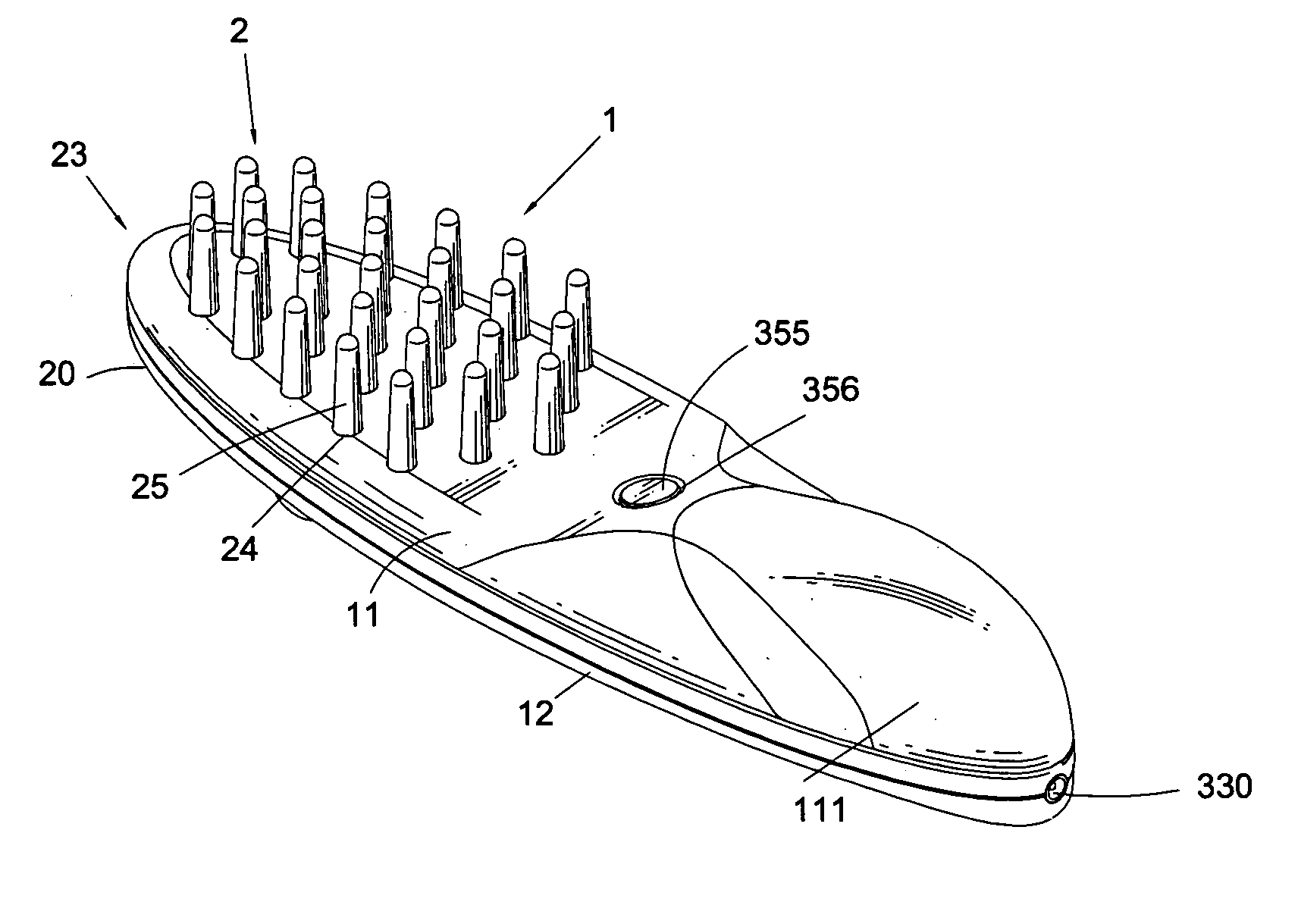
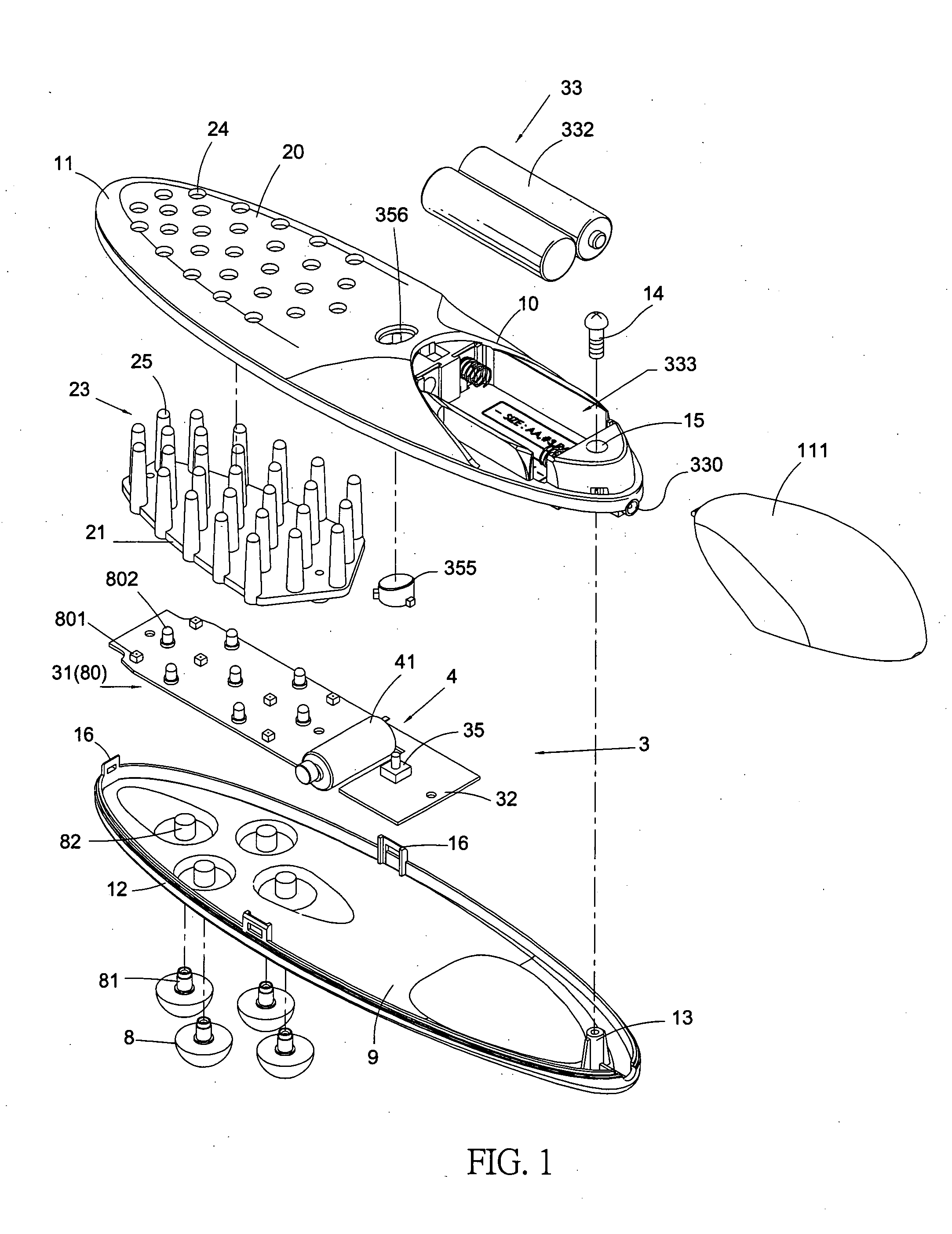
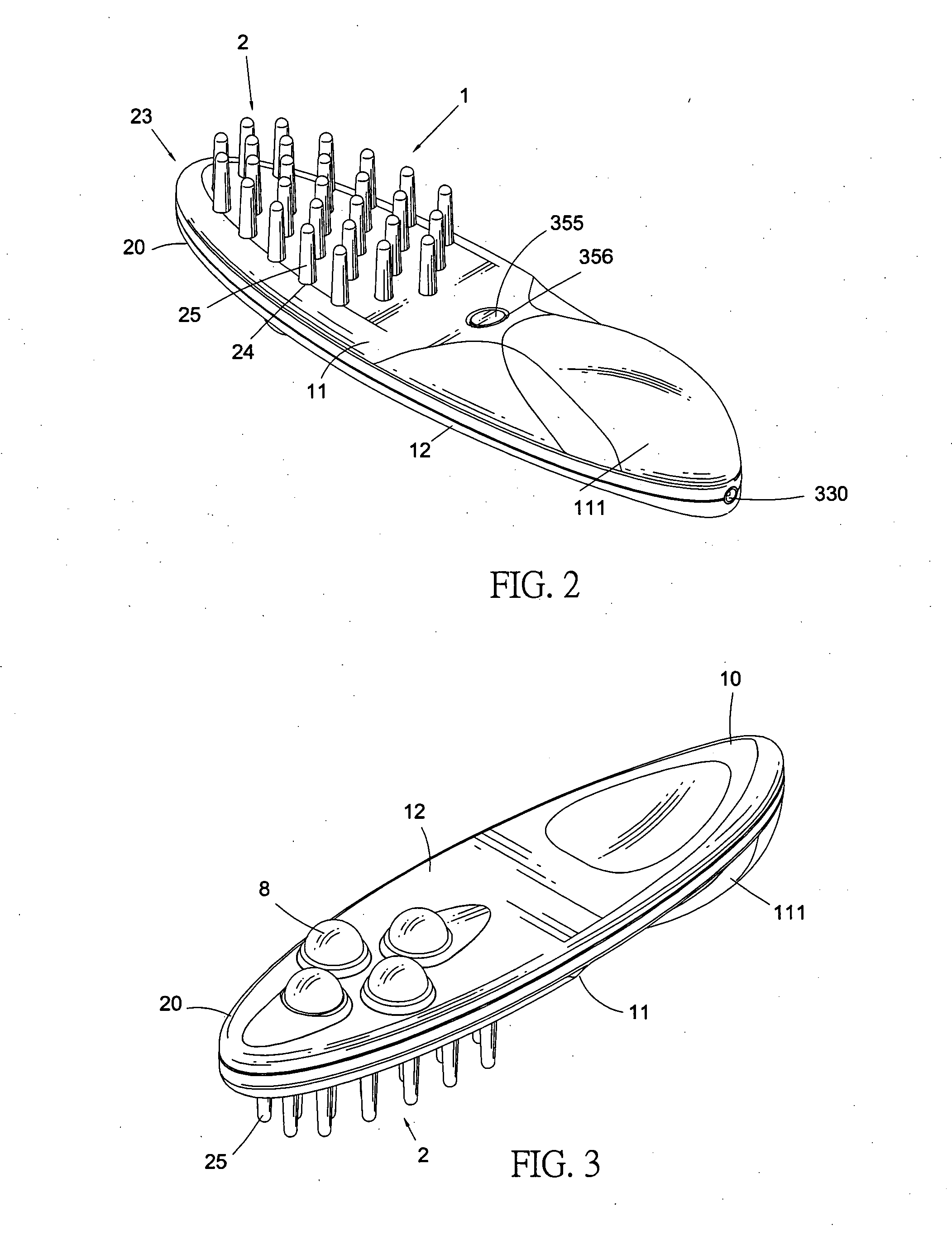
InactiveUS20070149900A1Synergistic effectPromotes hair follicle activationChiropractic devicesEye exercisersCell activationLight guide
A light guide comb that produces a direct massage effect on the head of a user, and, moreover, uses light rays to promote cell activation of the head, cortical layer and acupuncture points, while simultaneously stimulating hair follicles to promote the health and growth of hair. The light guide comb includes an upper shell, a lower shell, a compartment, a light stimulation device and a light guide. A plurality of guide holes are defined in the upper shell to correspond to a plurality of guide pins configured on the light guide. Light rays produced by the light stimulation device disposed within the compartment are propagated through the plurality of guide pins and transmitted outward. A plurality of massage knobs are disposed on a comb head of the lower shell to provide a massage surface area greater than that of the plurality of guide pins.
Owner:LIN CHE WEN
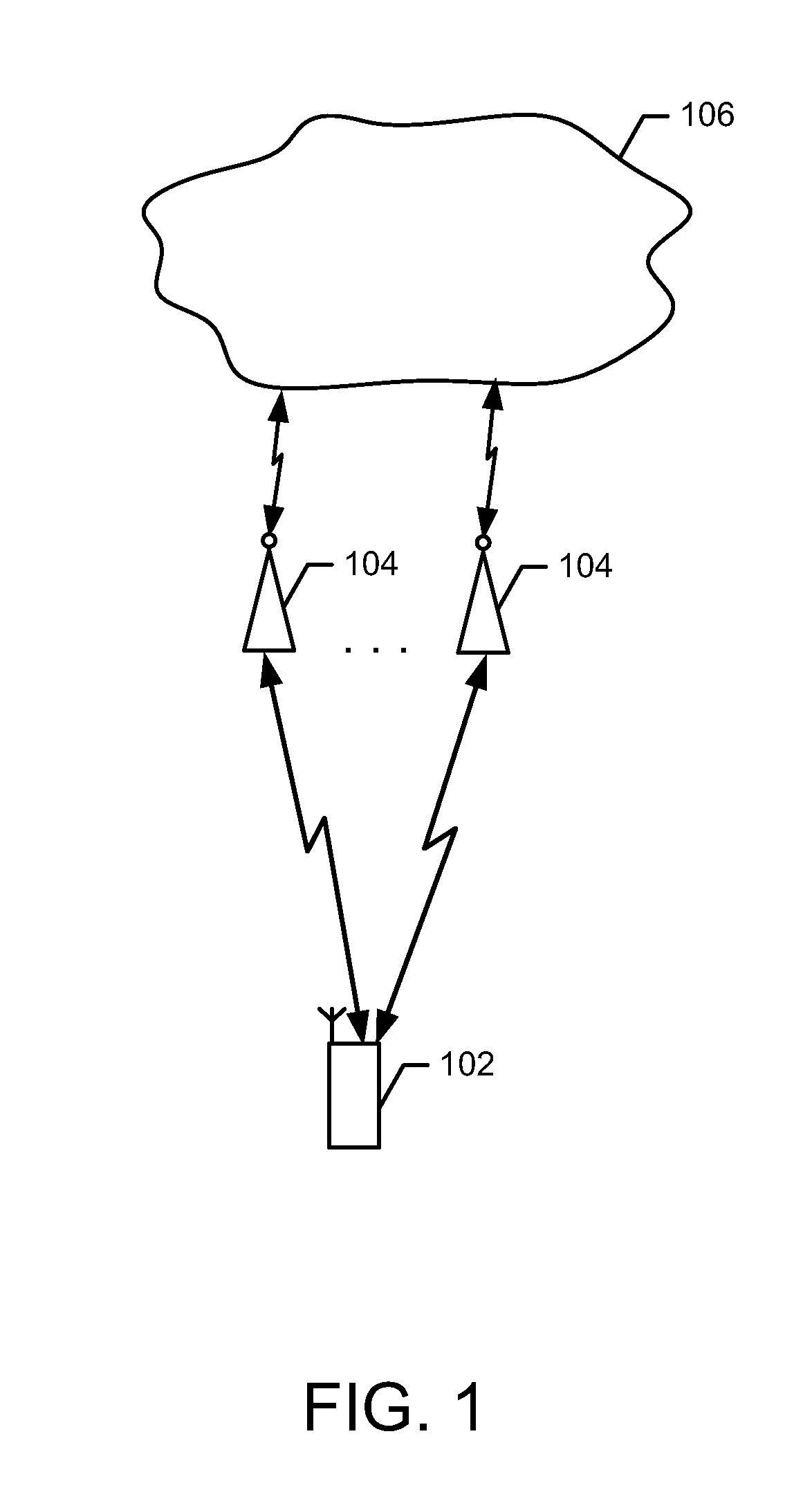
Method and apparatus for enhanced secondary cell activation and deactivation
A method, apparatus and computer program product are provided in order to enhance network notification of secondary cell activation. In a first embodiment, the method includes receiving a secondary cell activation command, activating a secondary cell, and transmitting a PHR indicating activation of the secondary cell. In a second embodiment, the method includes receiving a secondary cell activation command, activating a secondary cell, and transmitting a signal indicating an expected time period until activation of the secondary cell. In a third embodiment, the method may optimize implicit SCell deactivation by receiving a secondary cell activation command, activating a secondary cell, receiving a PDCCH order for an uplink grant or a downlink assignment, and starting a secondary cell deactivation timer associated with the secondary cell in response to receiving the PDCCH order. A corresponding apparatus and computer program product are also provided.
Owner:AVAGO TECH INT SALES PTE LTD
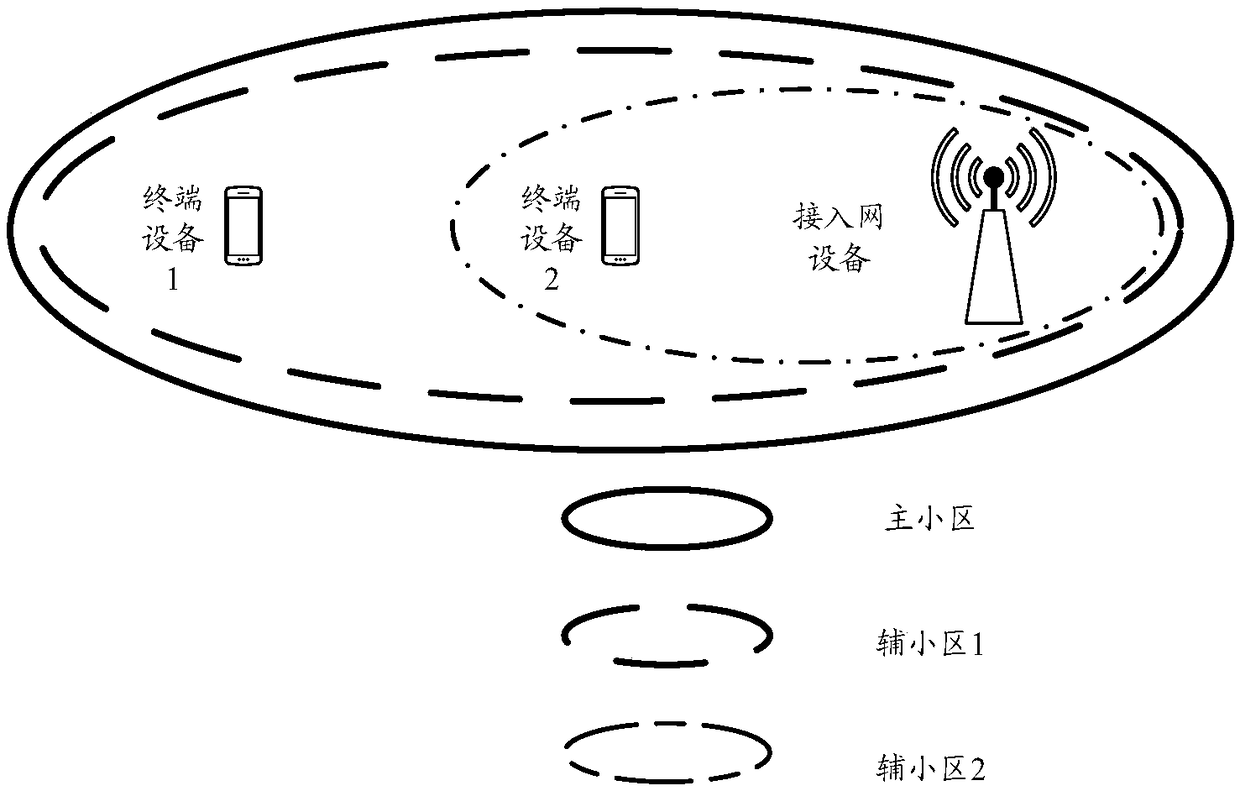
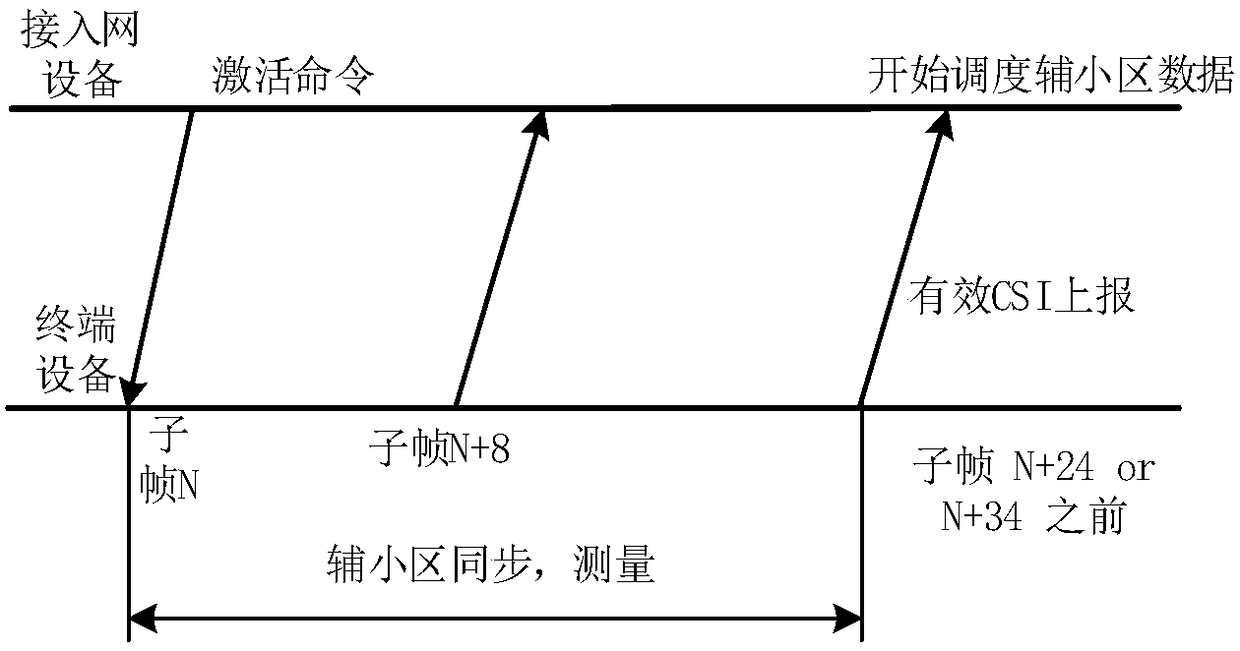
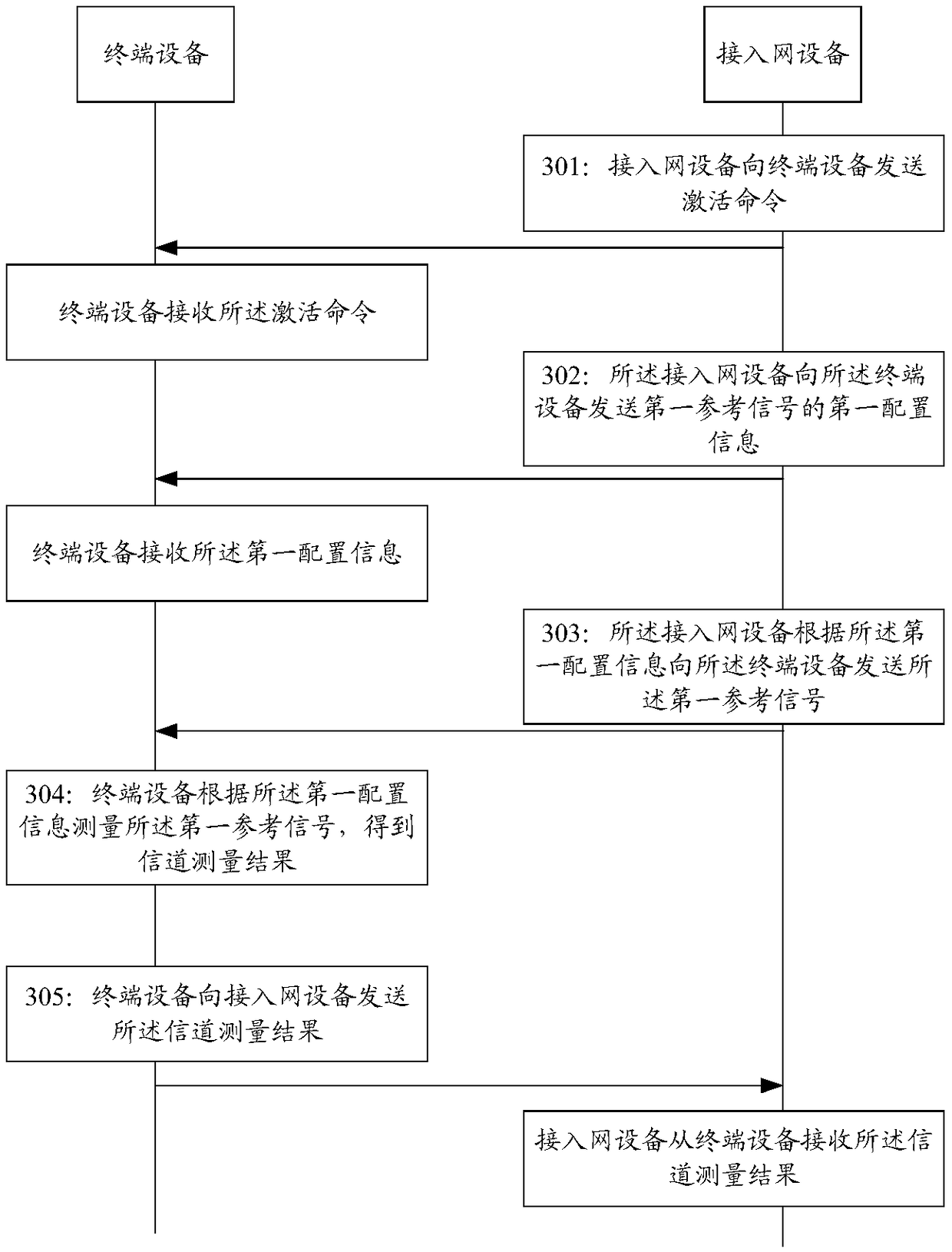
Secondary cell activation method, access network device, communication device, and system
ActiveCN109391986AIncrease flexibilityImprove activation efficiencyTransmission path divisionPilot signal allocationAccess networkCell activation
The embodiment of the present invention provides a secondary cell activation method, a wireless access network device, a terminal device, and a communication system, and belongs to the field of communication. The method includes the steps that the access network device sends an activation command to the terminal device, wherein the activation command is used to indicate that a secondary cell is activated; the access network device sends the first configuration information of a first reference signal to the terminal device; the access network device sends a first reference signal to the terminal device according to the first configuration information, wherein the first reference signal is used to obtain a channel measurement result of the secondary cell. The secondary cell activation methodprovided by the embodiment of the present invention can provide a flexible and efficient secondary cell activation mechanism, and improves the activation efficiency of the secondary cell.
Owner:HUAWEI TECH CO LTD
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
ActiveUS20090092610A1Enhance immune responseImprove responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind the extracellular domain of FcγRIIB, particularly human FcγRIIB, and block the Fc binding site of human FcγRIIB. The invention provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Bradykinin analogs as selective inhibitors of cell activation
InactiveUS6982249B1Suppression problemInhibits platelet aggregationHormone peptidesPeptide/protein ingredientsCell activationKinin
The present invention provides bradykinin peptide analogs, compositions, and methods of inhibiting thrombin-induced platelet and other cell activation. The bradykinin analogs comprise single or multiple peptide segments. The invention also provides a method for identifying compounds that selectively inhibit thrombin-induced platelet and other cell activation.
Owner:MICHIGAN UNIV OF THE RGT
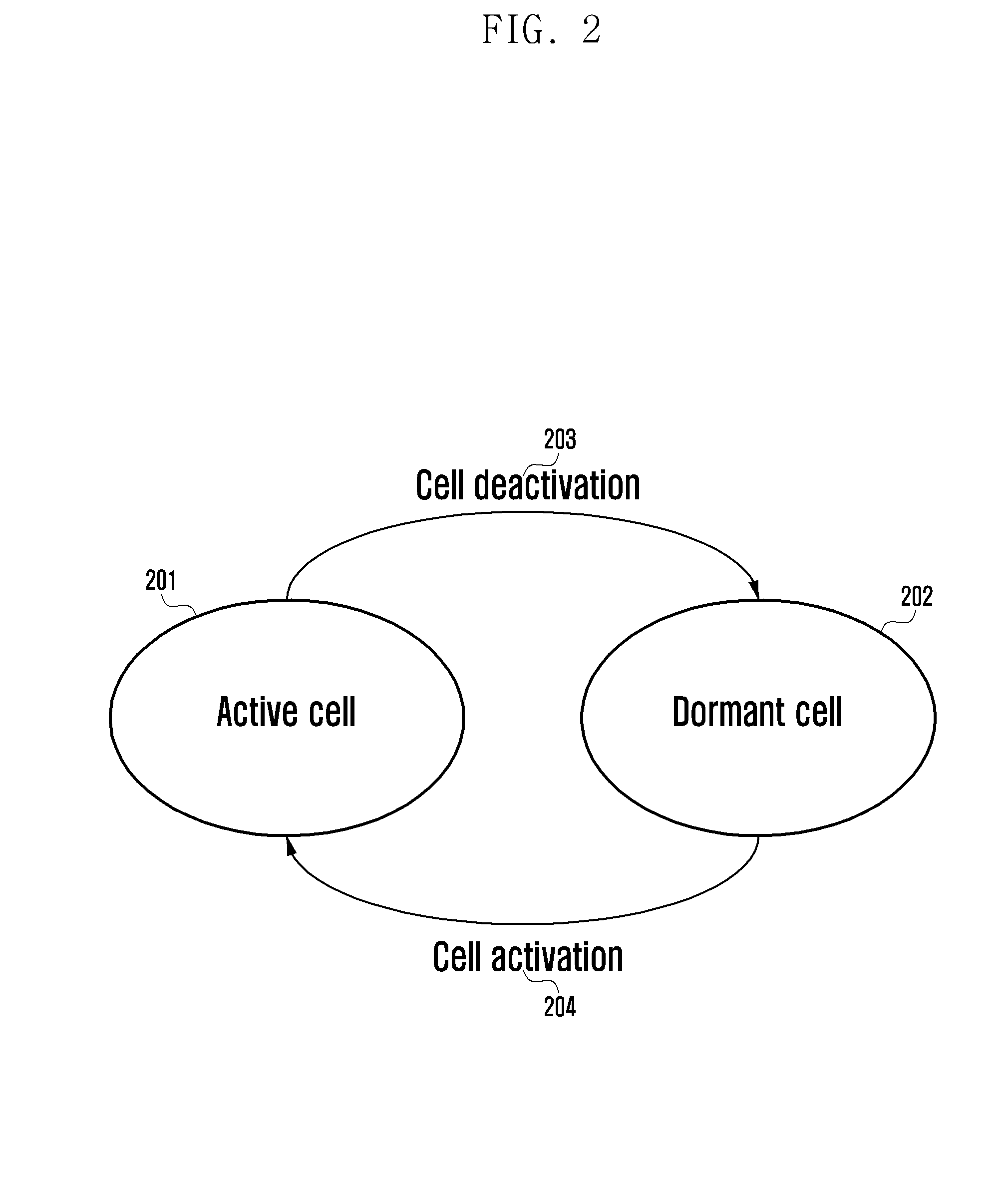
Method and apparatus for controlling operation state of base station in wireless communication system
ActiveUS20140134993A1Efficiently transitionSupporting the state transition procedurePower managementAssess restrictionCommunications systemCell activation
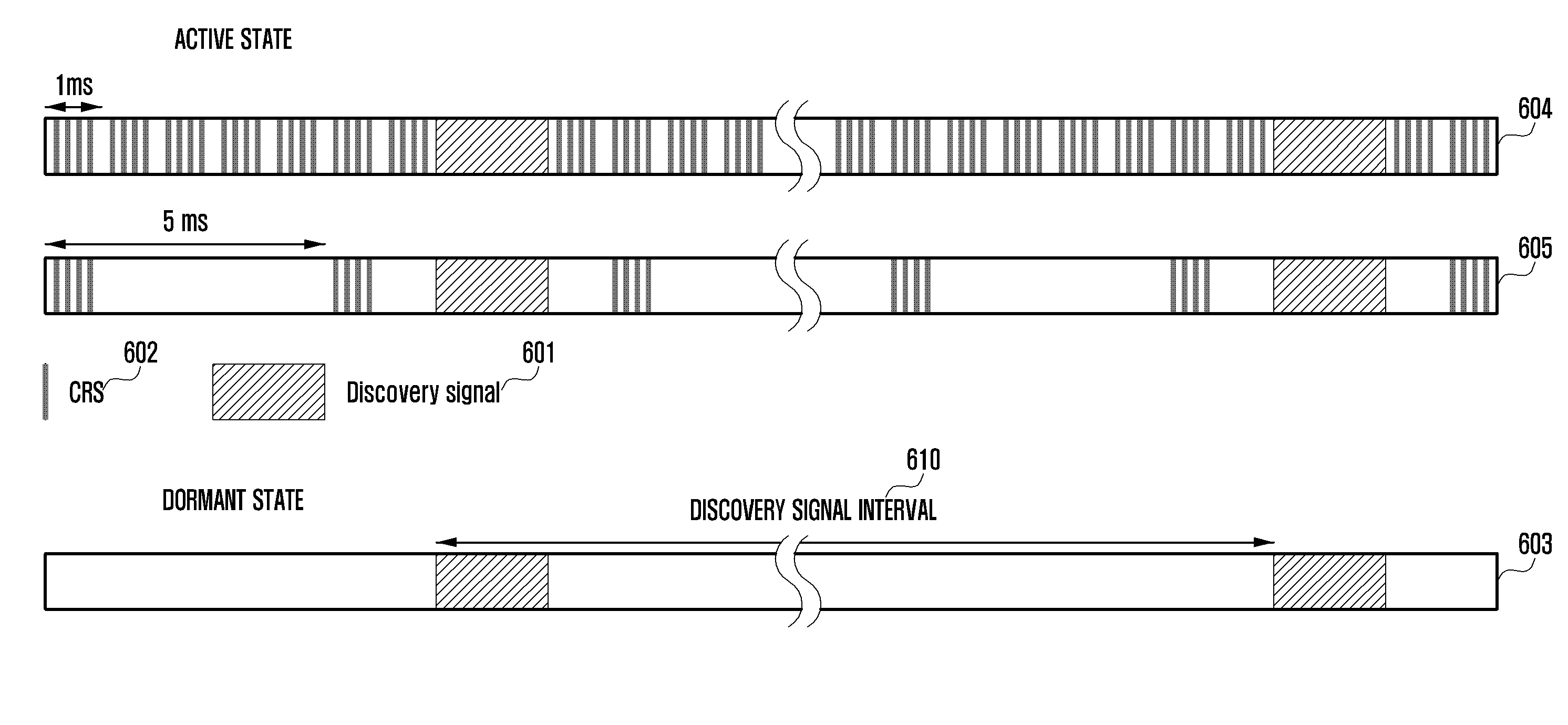
Methods and apparatus are provided for controlling transition of an operation state of a cell in a wireless communication system. The cell includes a transceiver configured to transmit and receive signals to and from a terminal and another cell. The cell also includes a controller configured to transition an operation state of the cell from an active state to a dormant state, transmit a discovery signal, determine whether a cell activation signal is received from a node that controls the cell, and transition the operation state of the cell from the dormant state to the active state when the cell activation signal is received.
Owner:SAMSUNG ELECTRONICS CO LTD
Compounds and therapies for the prevention of vascular and non-vascular pathologies
The invention provides a method of treating a mammal having, or at risk of, an indication associated with a TGF-beta deficiency comprising administering one or more agents that is effective to elevate the level of TGF-beta. The invention also provides novel compounds that elevate TGF-beta levels, as well as pharmaceutical compositions comprising compounds that elevate TGF-beta levels, and methods for detecting diseases associated with endothelial cell activation.
Owner:UAB RES FOUND +1
Establishing method of immortal AM cell line
InactiveCN101798581AGuaranteed stable expressionConsistent with epithelial featuresFermentationGenetic engineeringTelomeraseCell activation
The invention provides an establishing method of an immortal AM cell line, which establishes the immortal AM cell line by constructing a retrovirus carrier to infect cell activation telomerase. The establishing method comprises the following processes: constructing the pLXSNneo-hTERT retrovirus carrier, and carrying out AM cell primary culture and AM cell immune cell chemical identification to establish a stable and reliable immortal AM cell line. The cell line meets the features of epithelial cells, can ensure the stable expression of a target gene and provides a valuable in-vitro model for researching AM development and invasion features.
Owner:陶谦
Methods and compositions for inhibiting CD14 mediated cell activation
This invention provides hybridoma cell lines producing monoclonal antibodies which inhibit CD14 mediated cell activation. Monoclonal antibodies produced by these cell lines also are provided. The antibodies are useful for the detection of the presence of cell surface and soluble CD14 in a sample. Chimeric and CDR grafted antibodies generated from the above monoclonal antibodies are further provided. Pharmaceutical compositions containing the above biological compositions are provided. These are useful to treat and prevent disorders with CD14 mediated cell activation, such as sepsis.
Owner:THE SCRIPPS RES INST
Microbe inactivation processing device and cell activation processing device
ActiveUS20190192708A1Preventing and inhibiting damageReduced Power RequirementsOptical filtersElectrical/wave energy microorganism treatmentCell activationUltraviolet lights
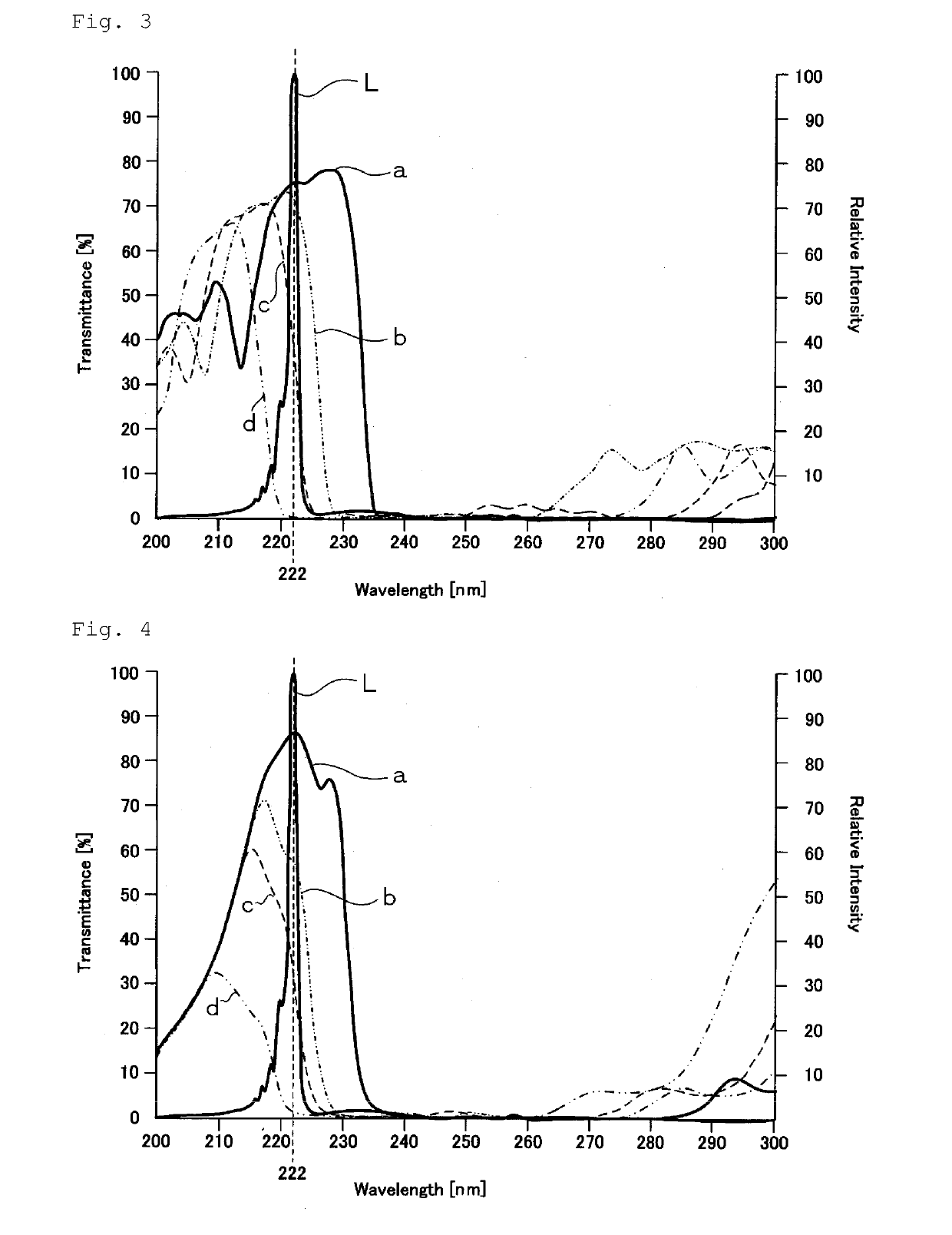
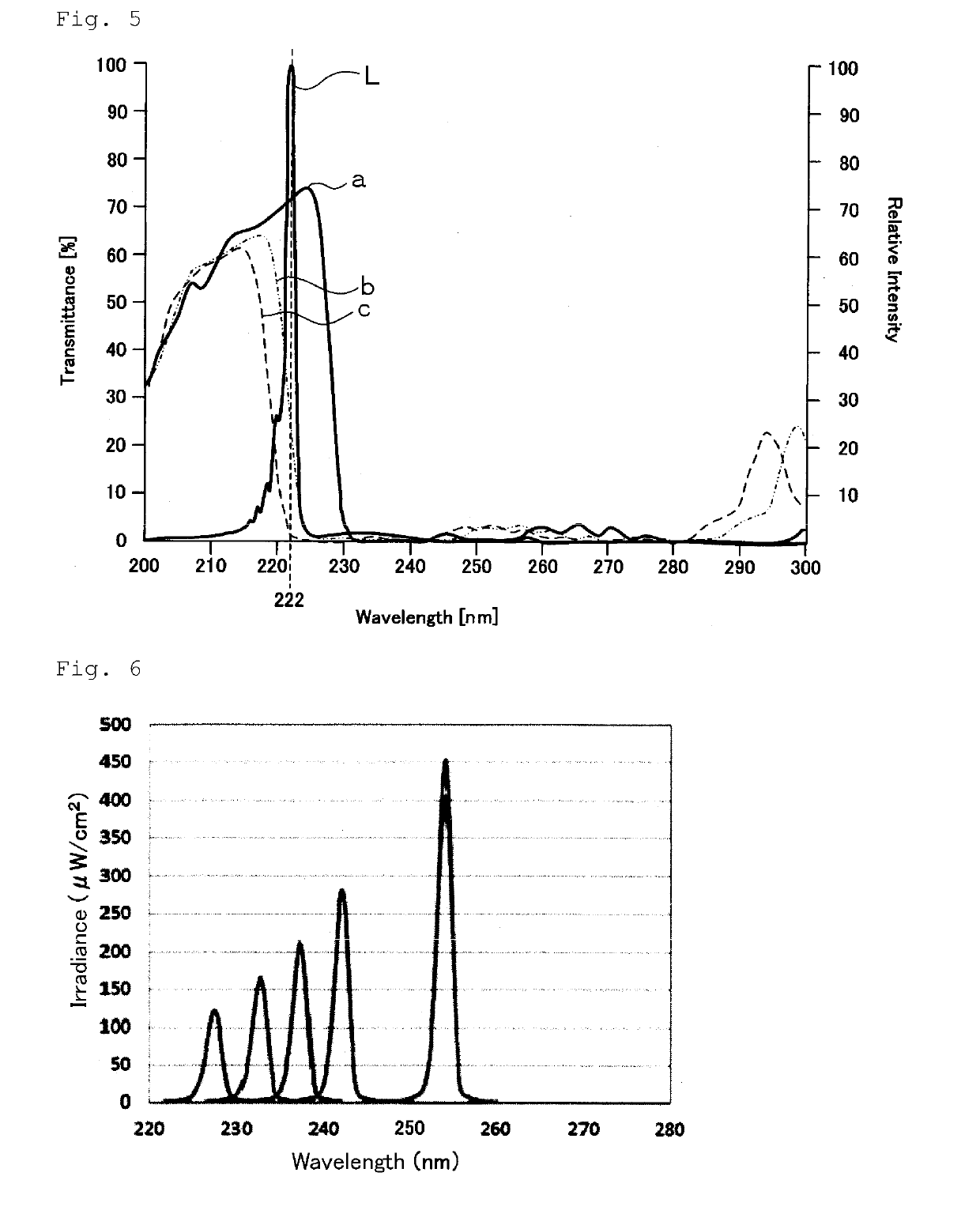
Disclosed are a microbe inactivation processing device that can perform inactivation processing of microbes, while damage to human body cells is prevented or inhibited, with an efficient use of light emitted from a light source and the obtainment of a large effective irradiation area. Also provided are a cell activation processing device that can reliably activate target cells with high efficiency. The microbe inactivation processing device includes: a light source configured to emit light having a wavelength within a wavelength range of 190 nm to 237 nm; a power supply unit configured to supply electric power to the light source; a control unit configured to control the power supply unit; and an optical filter. The microbe inactivation processing device is configured to perform inactivation processing of a target microbe by applying the light emitted from the light source through the optical filter. When the light emitted from the light source is incident at an incident angle of 0°, the optical filter transmits at least a part of ultraviolet light having a wavelength within a range of not lower than 190 nm and not more than 230 nm, and transmits at least a part of ultraviolet light having a wavelength within a range of more than 230 nm and not more than 237 nm, and the optical filter blocks transmission of ultraviolet light having a wavelength out of a wavelength range of not lower than 190 nm and not more than 237 nm.
Owner:USHIO DENKI KK
Far infrared heat insulation wind coat
InactiveCN102835748AGood warmth retentionIncreased durabilityOvergarmentsYarnCell activationEngineering
The invention discloses a far infrared heat insulation wind coat, which comprises an inner layer and an outer layer, wherein the inner layer is formed by mercerized cloth through mixed weaving, the outer layer is formed by cotton and hemp through mixed weaving, a heat insulation sandwich layer is arranged between the inner layer and the outer layer, the heat insulation sandwich layer is a far infrared heat insulation plus material, and the inner layer, the outer layer and the sandwich layer are closed through adopting zippers. The far infrared heat insulation wind coat has the advantages that the affinity with the skin of the human body is good, a good health care function is realized, the infrared heat insulation sandwich layer has the excellent heat insulation function, the cell activation function can also be realized, old and dead cells can be discharged, or the regeneration capability is given to the old and dead cells, the cell energy can be enhanced, and the effects of enhancing the functions and the activity of the cells and the like are realized. The far infrared heat insulation wind coat has the advantages that the heat insulation effect is excellent, the durability is high, and good heat insulation performance is still realized after the washing for many times.
Owner:KUSN ZHOUSHI HUIHONG GARMENT FACTORY
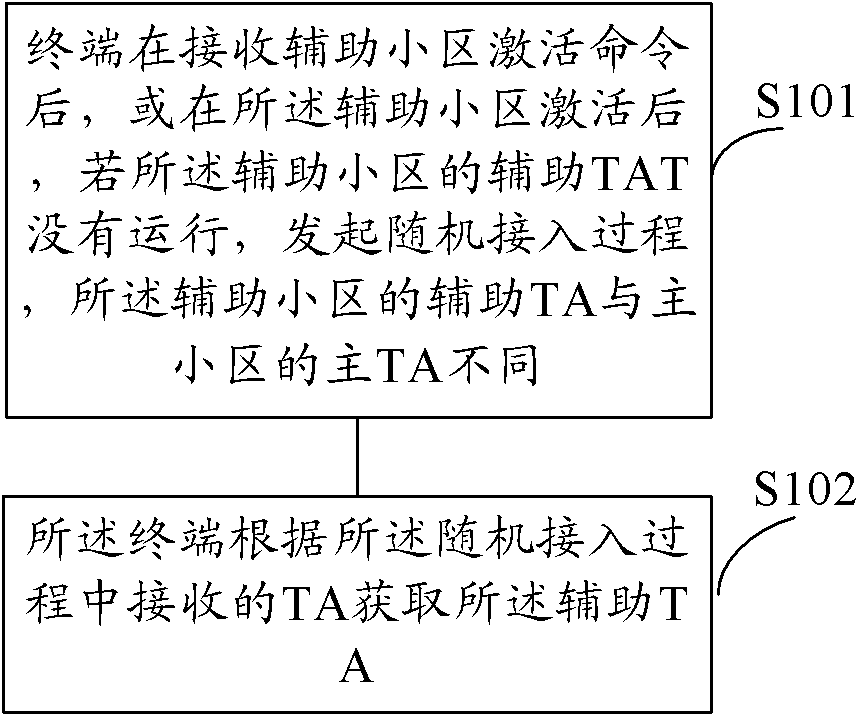
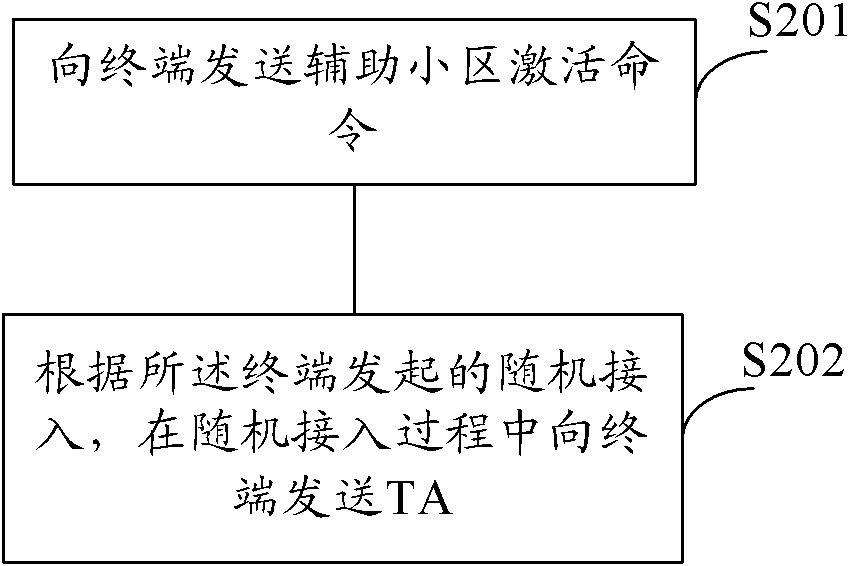
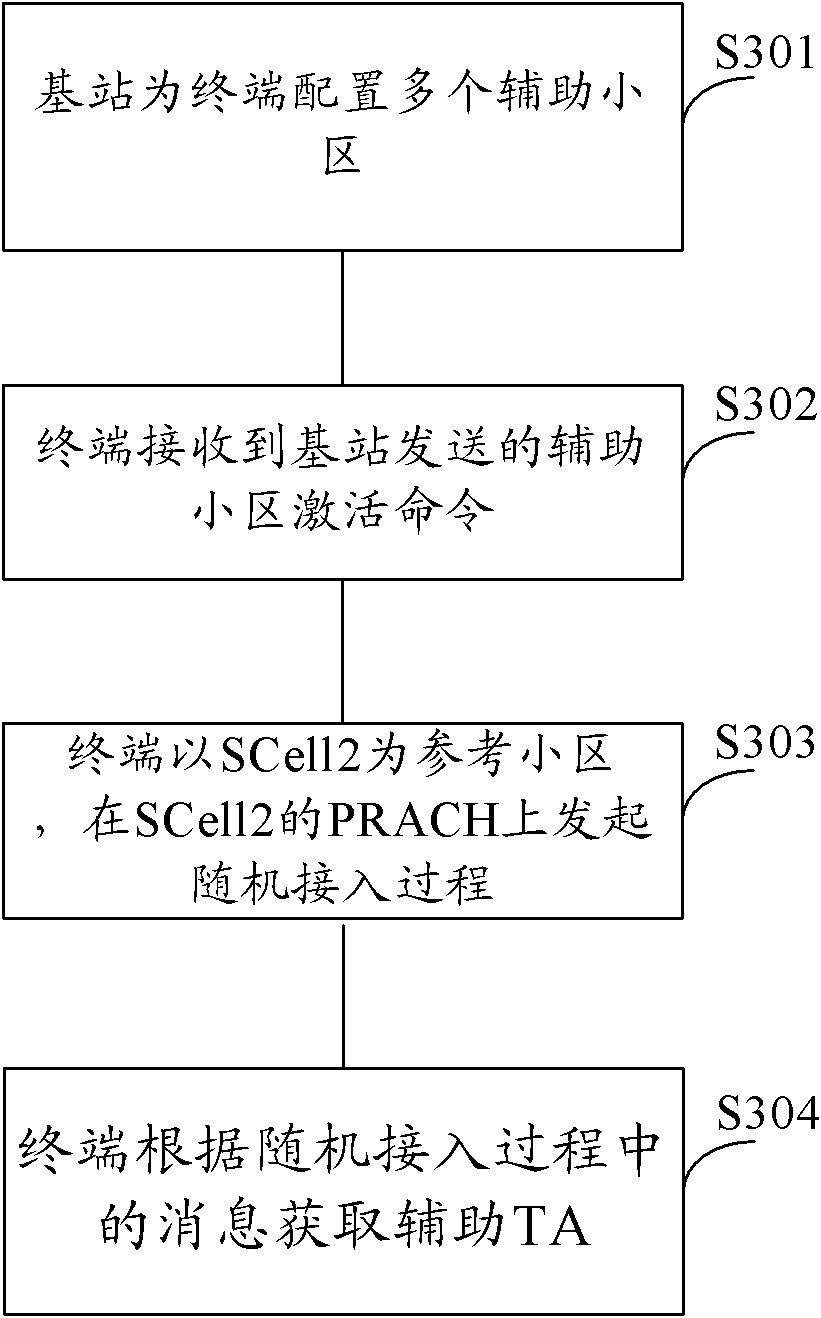
Method for acquiring secondary timing advance and equipment
The embodiments of the invention provide a method for acquiring secondary timing advance (TA) and equipment. The method comprises the following steps that: after a terminal receives a secondary cell activation command, or after the secondary cell is activated, if a secondary time alignment timer (TAT) of the secondary cell is not operated, the terminal initiates an random access process, wherein the secondary TA of the secondary cell is different from primary TA of a primary cell; and the terminal obtains the secondary TA according to the received TA during the random access process. According to method for acquiring secondary TA and equipment provided in the embodiments of the invention, after the secondary cell activation command emitted by a base station is received or the secondary cell is activated, the terminal initiates the random access process so as to obtain the secondary TA, thereby saving signaling cost and system resources.
Owner:HUAWEI TECH CO LTD
Compositions and methods for treating disease states associated with activated t cells and/or b cells
Disclosed are combination therapies and related compositions that may contain one or more of a p53 potentiating agent, a DNA-damaging agent, an agent that inhibits cell cycle check point, and a pharmaceutically acceptable carrier. Also disclosed are methods of using such compositions for the treatment of conditions related to T cell and / or B cell activation in subjects in need of such treatment.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Method for improving activation efficiency of fuel cell
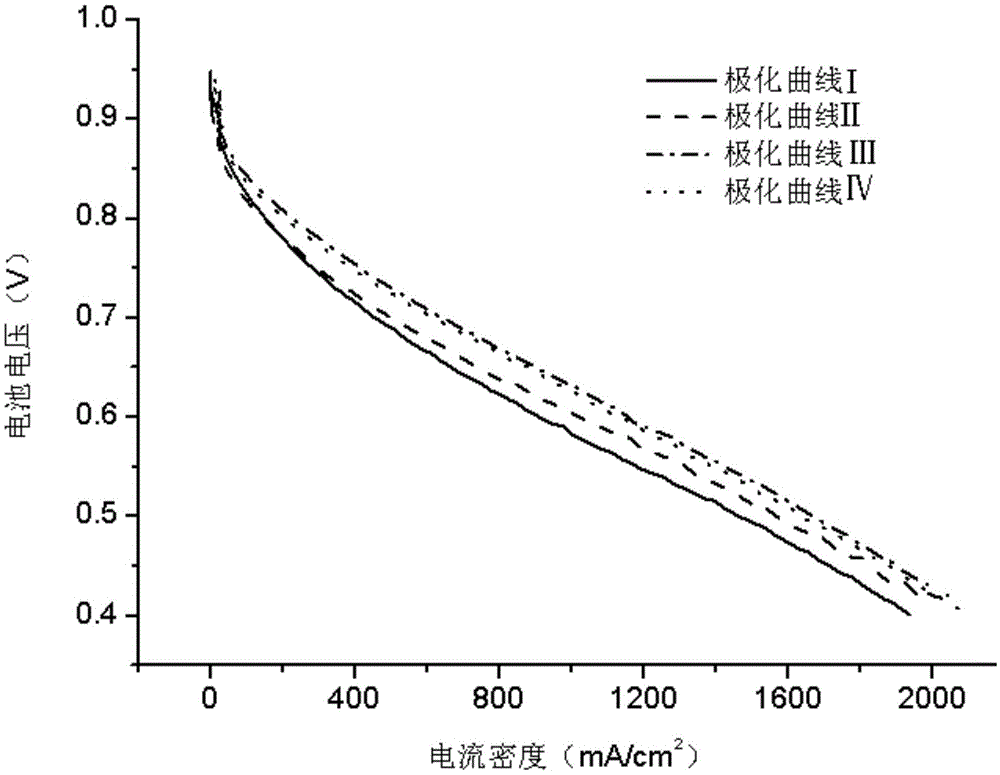
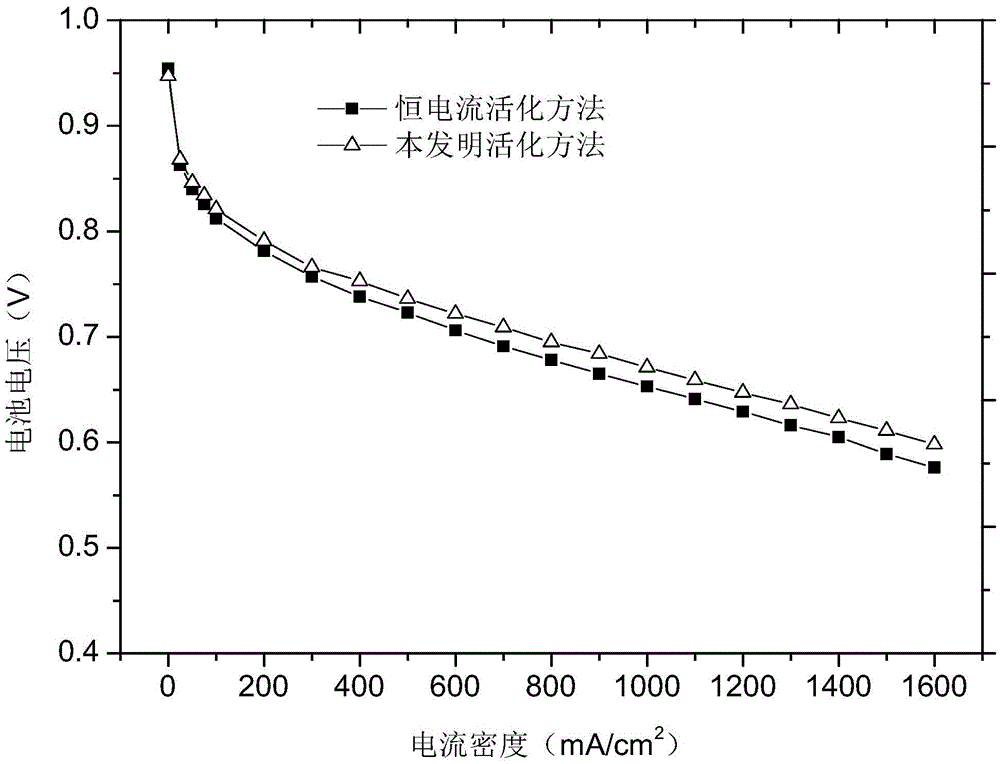
ActiveCN105552405AFully activatedThe activation process is controllableFuel cellsActivation methodFuel cells
The invention provides a method for improving the activation efficiency of a fuel cell. The method comprises the following steps: under a normal operation of the cell, testing a cell polarization curve before activation by a voltage scanning method; carrying out forced discharge treatment on the cell under nitrogen for a cathode instead of air; testing the cell polarization curve by the voltage scanning method; introducing air at a low stoichiometric ratio for constant-voltage activation treatment for twice, and testing each cell polarization curve by the voltage scanning method after each activation treatment; and judging whether activation is finished or not according to the deviation degrees of two polarization curves. The method has the beneficial effects that with quick testing of the cell polarization curves by the voltage scanning method as a characterization method for the cell activation degree, the activation process is controllable; the treatment method employing the nitrogen for the cathode instead of air is beneficial to eliminating surface impurities of a catalyst and opening a closed hole and a semi-closed hole in a catalyst layer; the mass transfer ability is strengthened; and the activation method is simple to operate, short in activation time, low in fuel consumption, high in activation degree and controllable in activation process.
Owner:SUNRISE POWER CO LTD
Flow control implementation method and device based on the output queue
ActiveCN101035067AEasy to implementTake up less resourcesError prevention/detection by using return channelStore-and-forward switching systemsCell activationCell chip
This invention relates to a method and flow control devices based on output queue based on the control devices. This invention mainly includes: Based on the cell output ports on the cell line and from different angles to achieve statistical count queue scheduling and flow control. In such a system, flow control and queue management is separate; Queue Management referred directly applied cell activation, the flow control is not mentioned directly dependent on Queue Management in cell survey results, but through the cell's priority, output ports, the source of cell chips, and other point of view, using different combinations of cell counting statistics, and on this basis to achieve flow control. Accordingly, the present invention can participate in the realization of the queue scheduling less number of simple, easy-to-manage, cells scheduling easy to be realized.
Owner:HUAWEI TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com