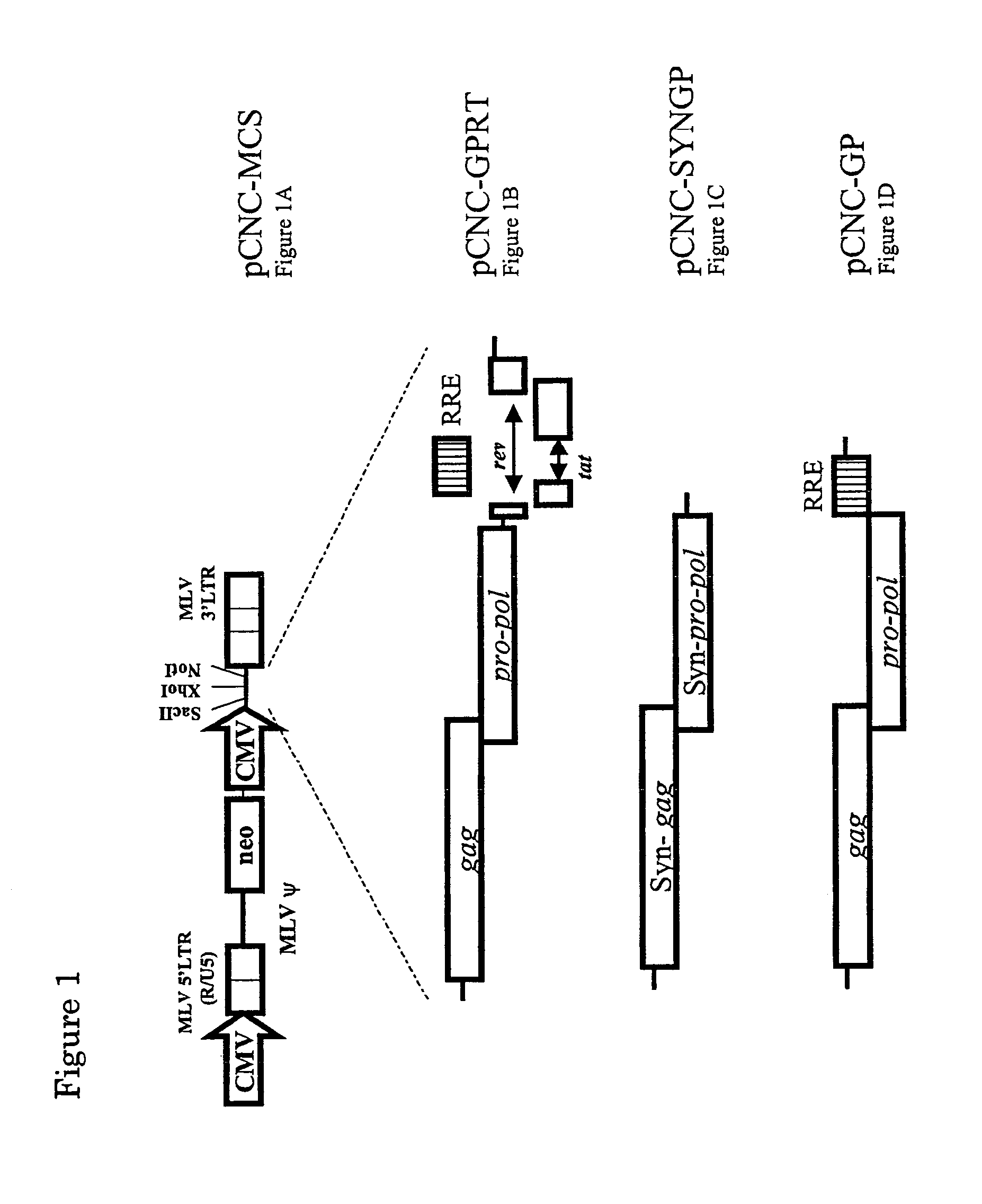
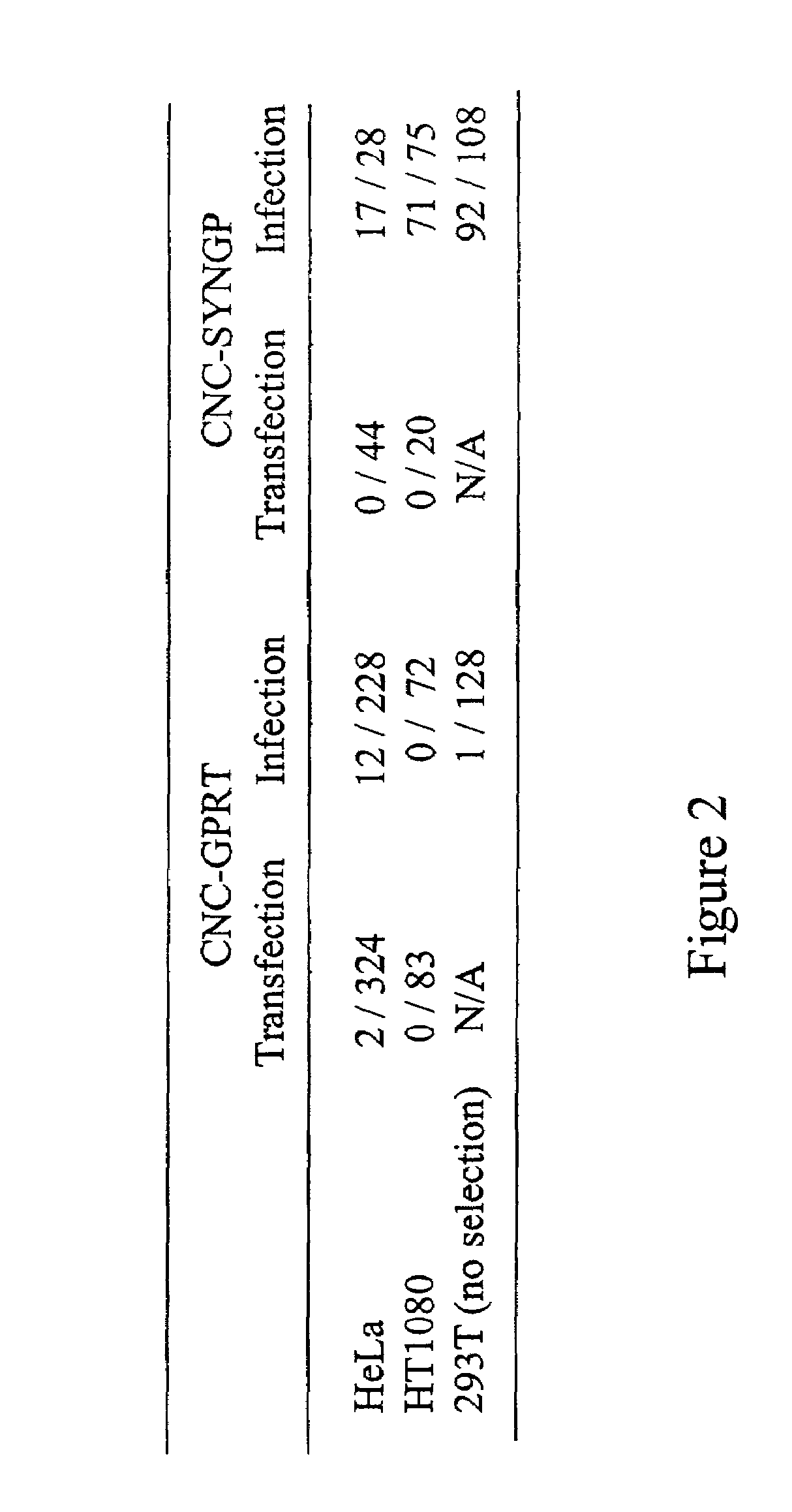
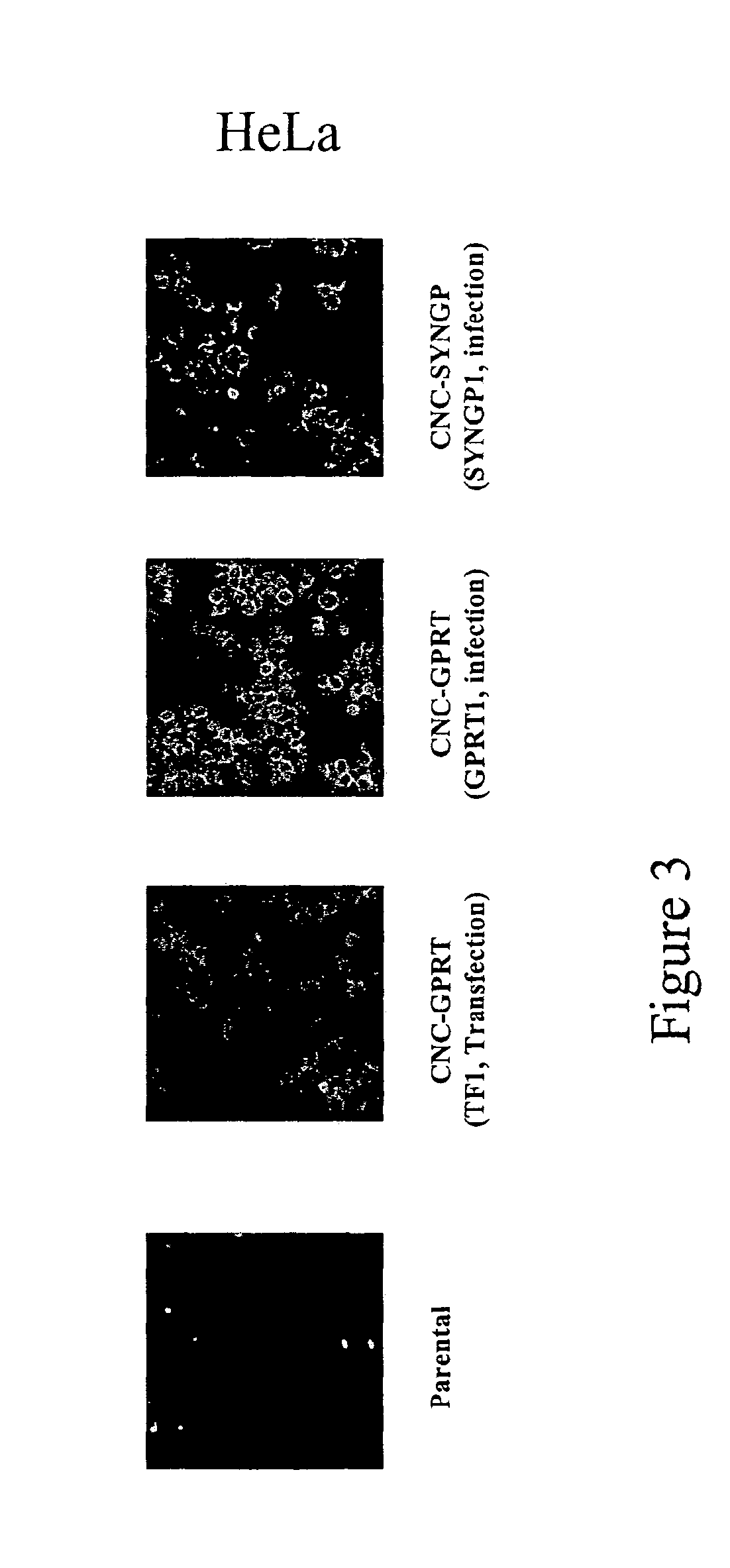
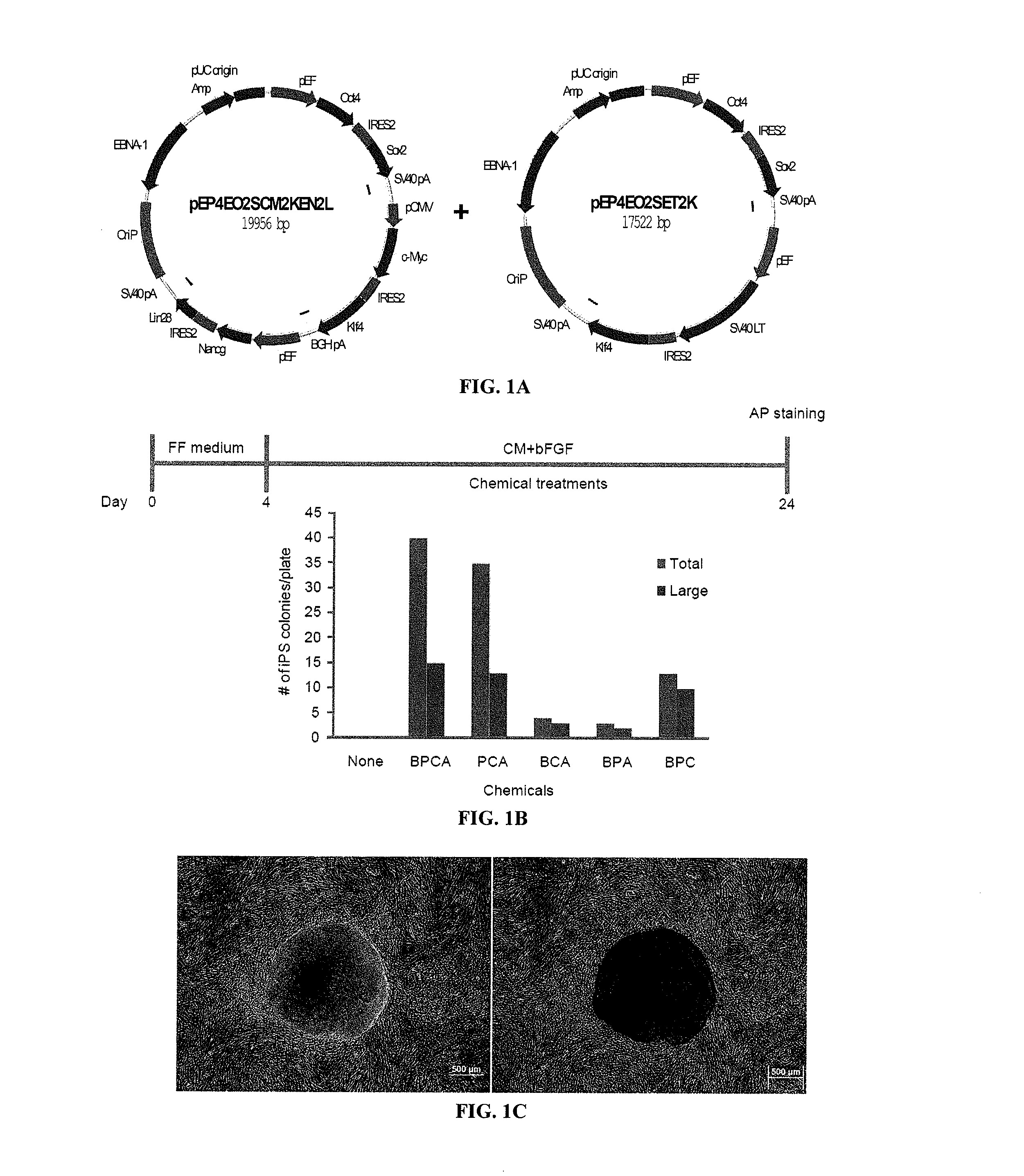
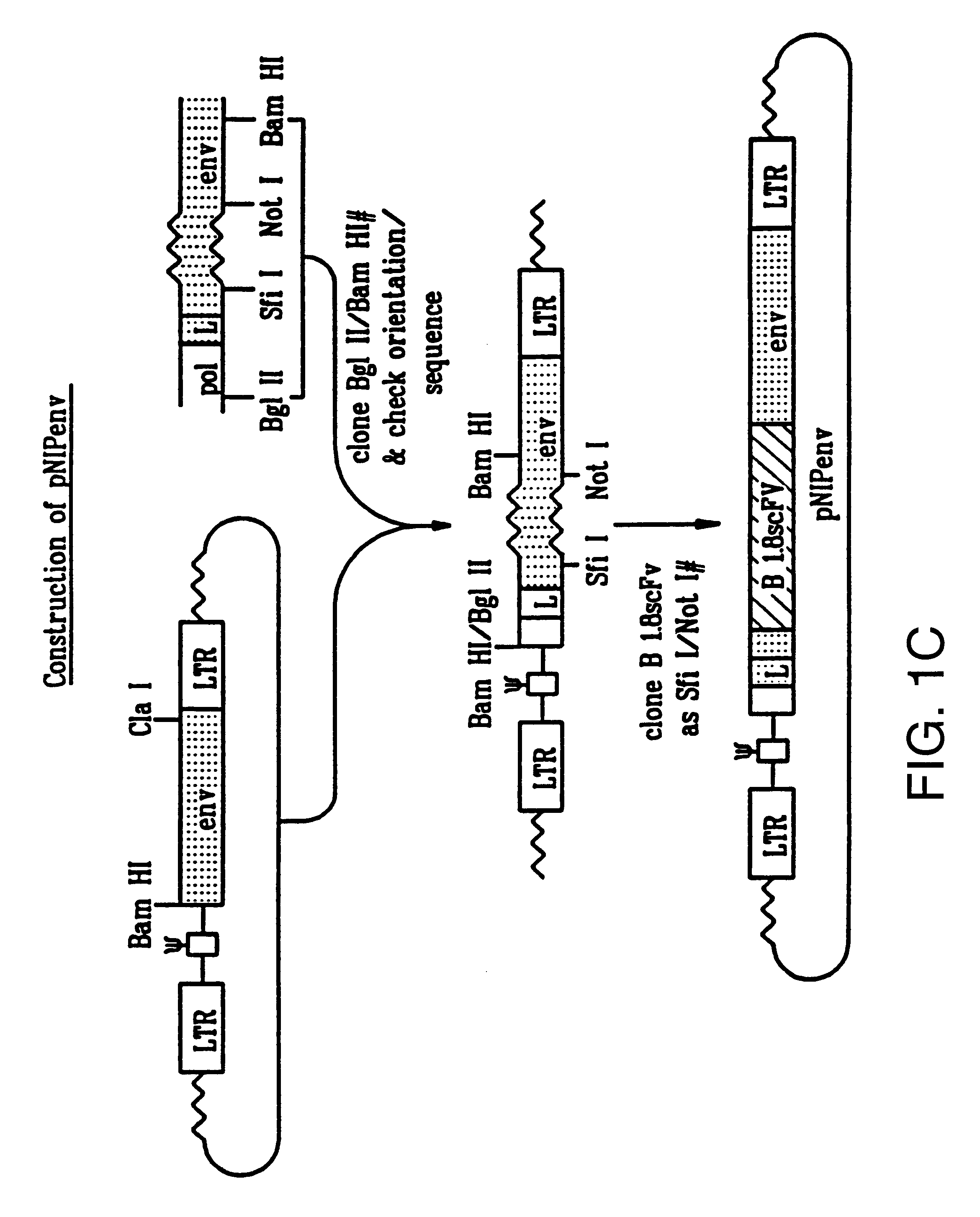
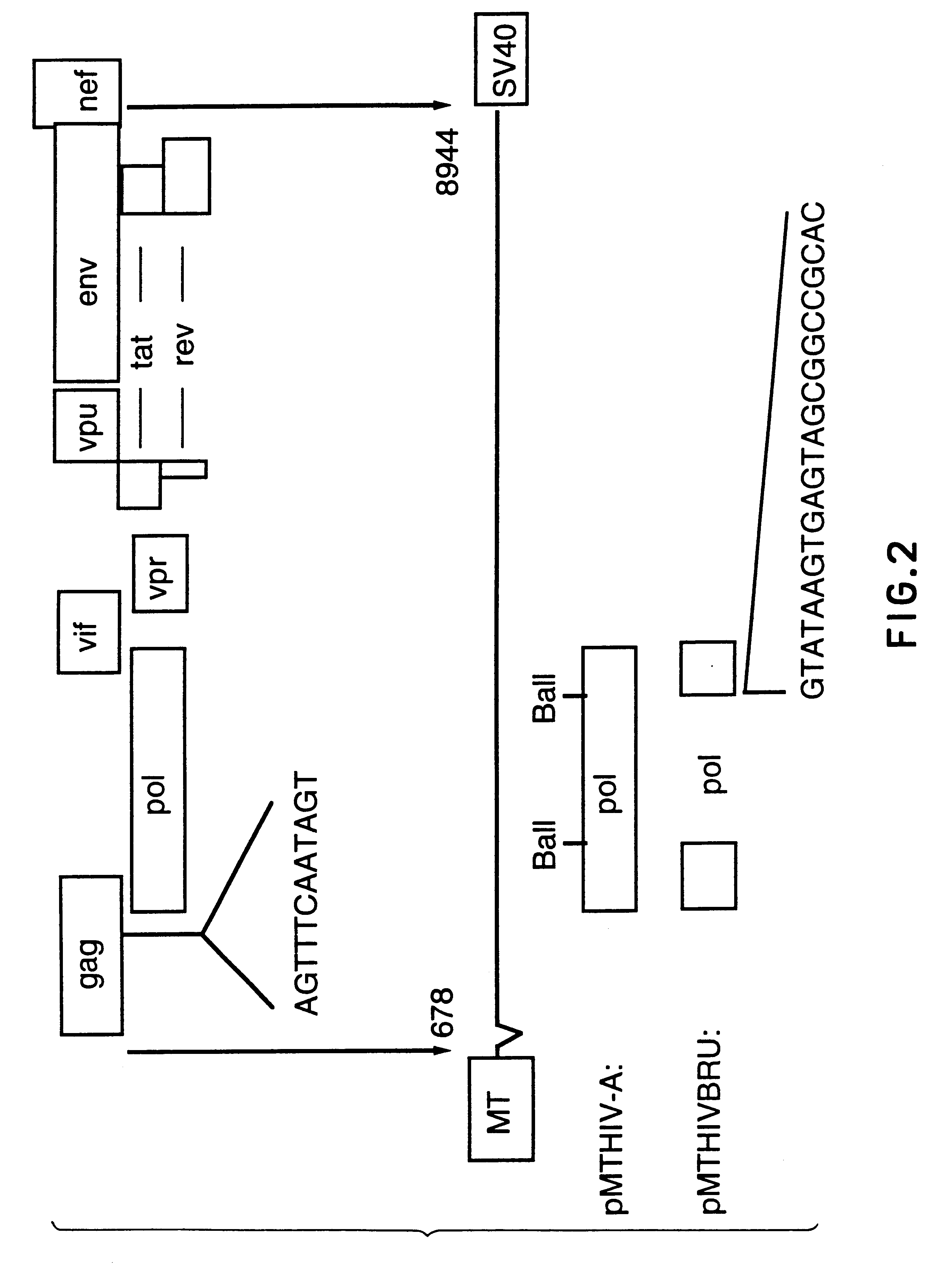
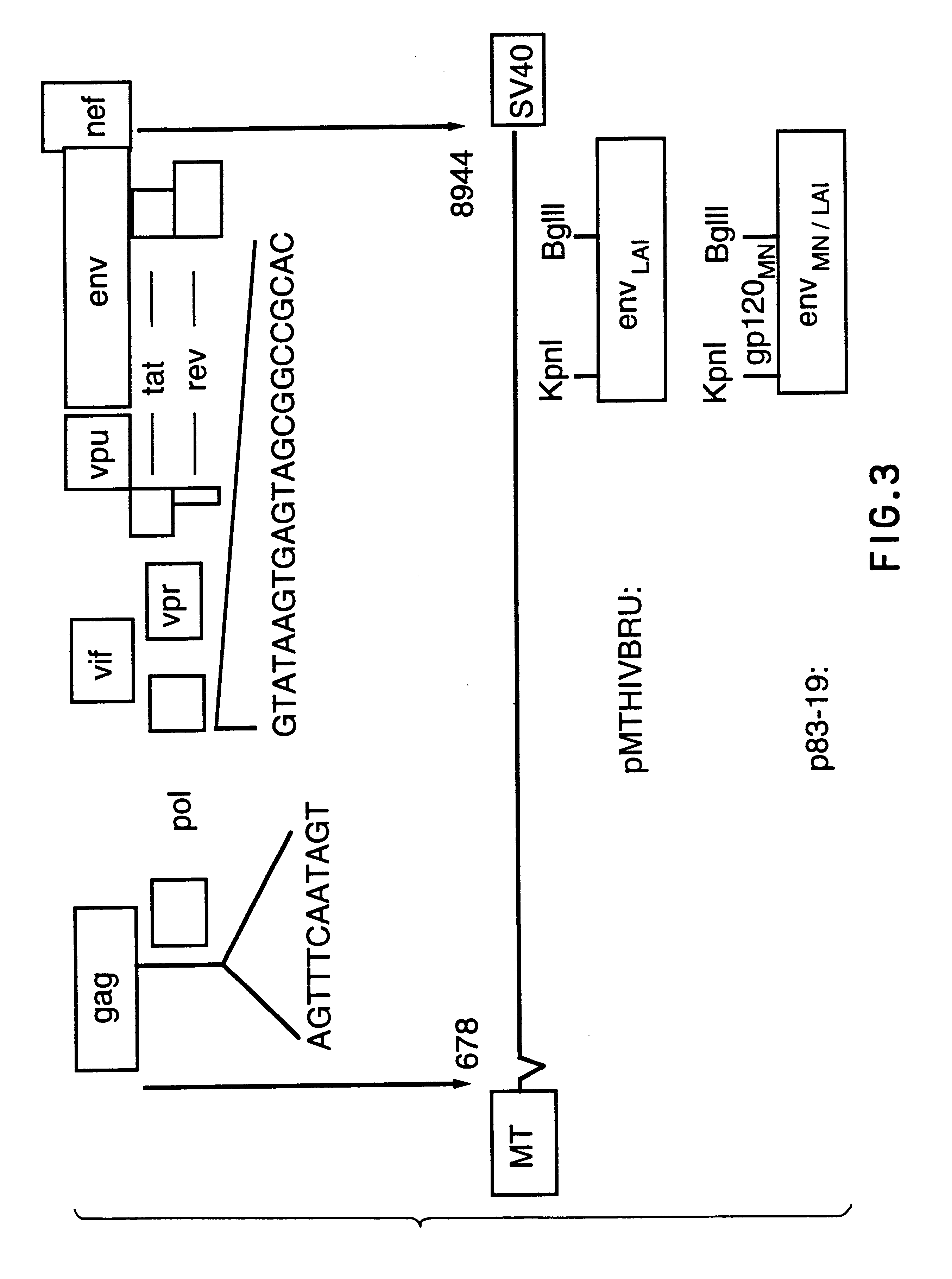
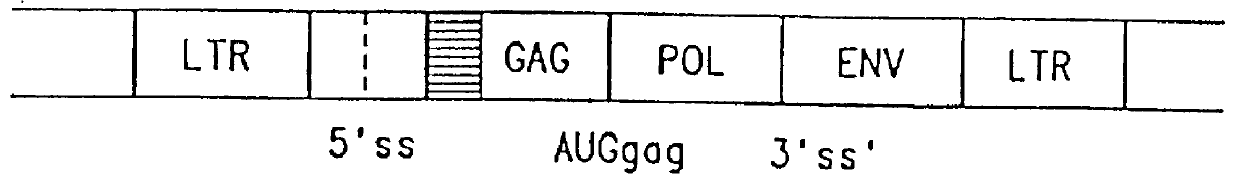
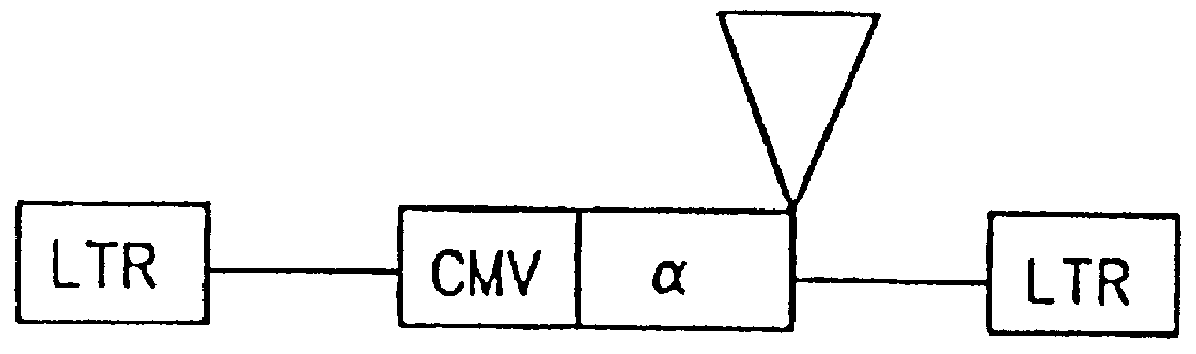
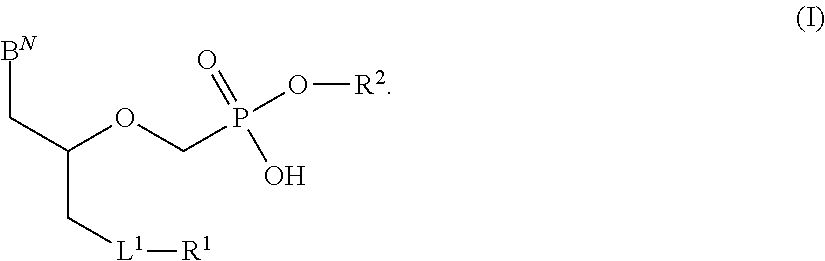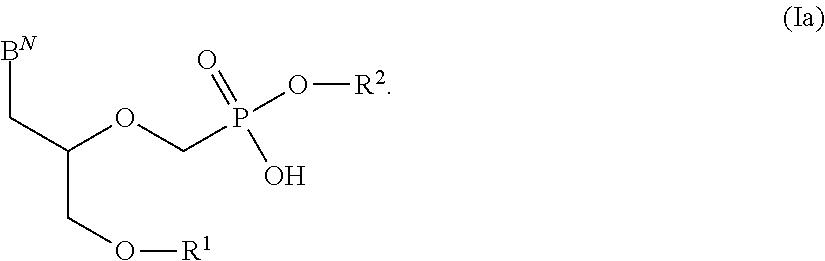Patents
Literature
610 results about "Retrovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A retrovirus is a type of RNA virus that inserts a copy of its genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. It is difficult to detect the virus until it has infected the host. At that point, the infection will persist indefinitely.
Vector system
InactiveUS7259015B2Eliminate the effects ofInhibition of activationFungiNervous disorderVector systemTyrosine
Provided are retroviral vector genomes and vector systems comprising the genomes. In particular, a retroviral vector genome comprising two or more NOIs, operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and, optionally, Aromatic Amino Acid Dopa Decarboxylase are provided.
Owner:OXFORD BIOMEDICA (UK) LTD
Vector system
InactiveUS20070025970A1Eliminate the effects ofInhibition of activationBiocideFungiVector systemTyrosine hydroxylase
The present invention relates to retroviral vector genomes and to vector systems comprising such genomes. In particular the present invention relates to a retroviral vector genome comprising two or more NOIs operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and optionally Aromatic Amino Acid Dopa Decarboxylase.
Owner:OXFORD BIOMEDICA (UK) LTD
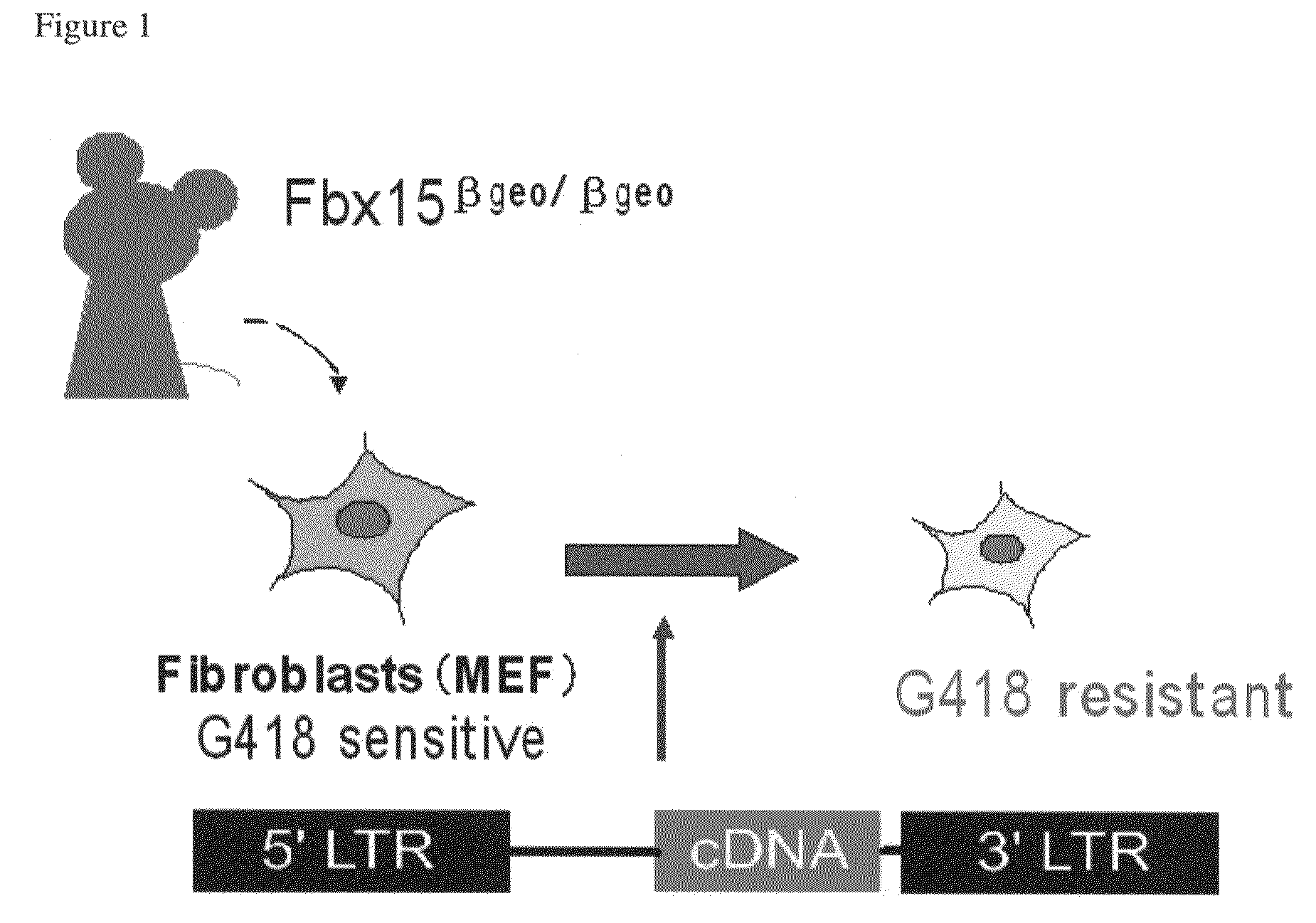
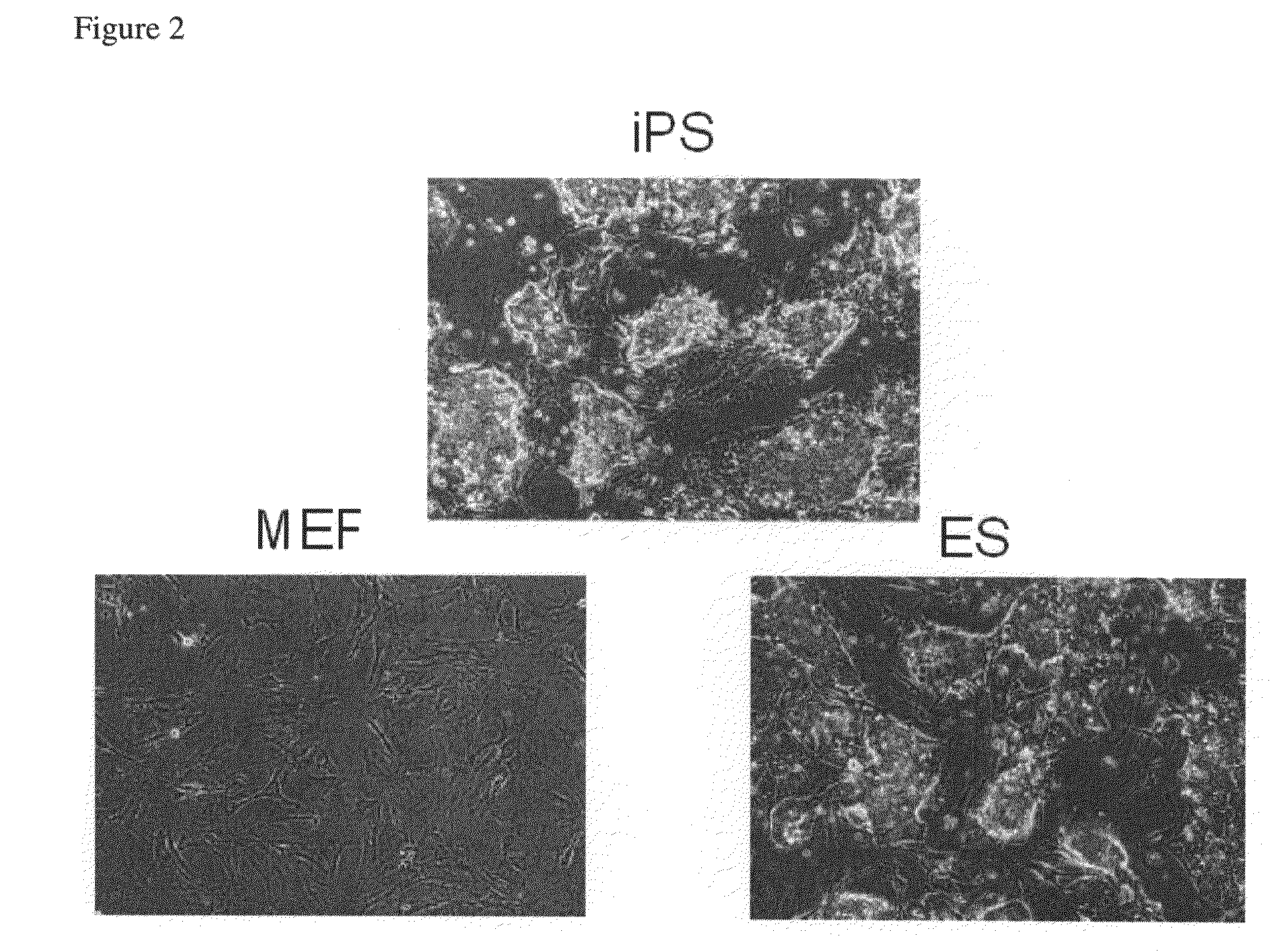
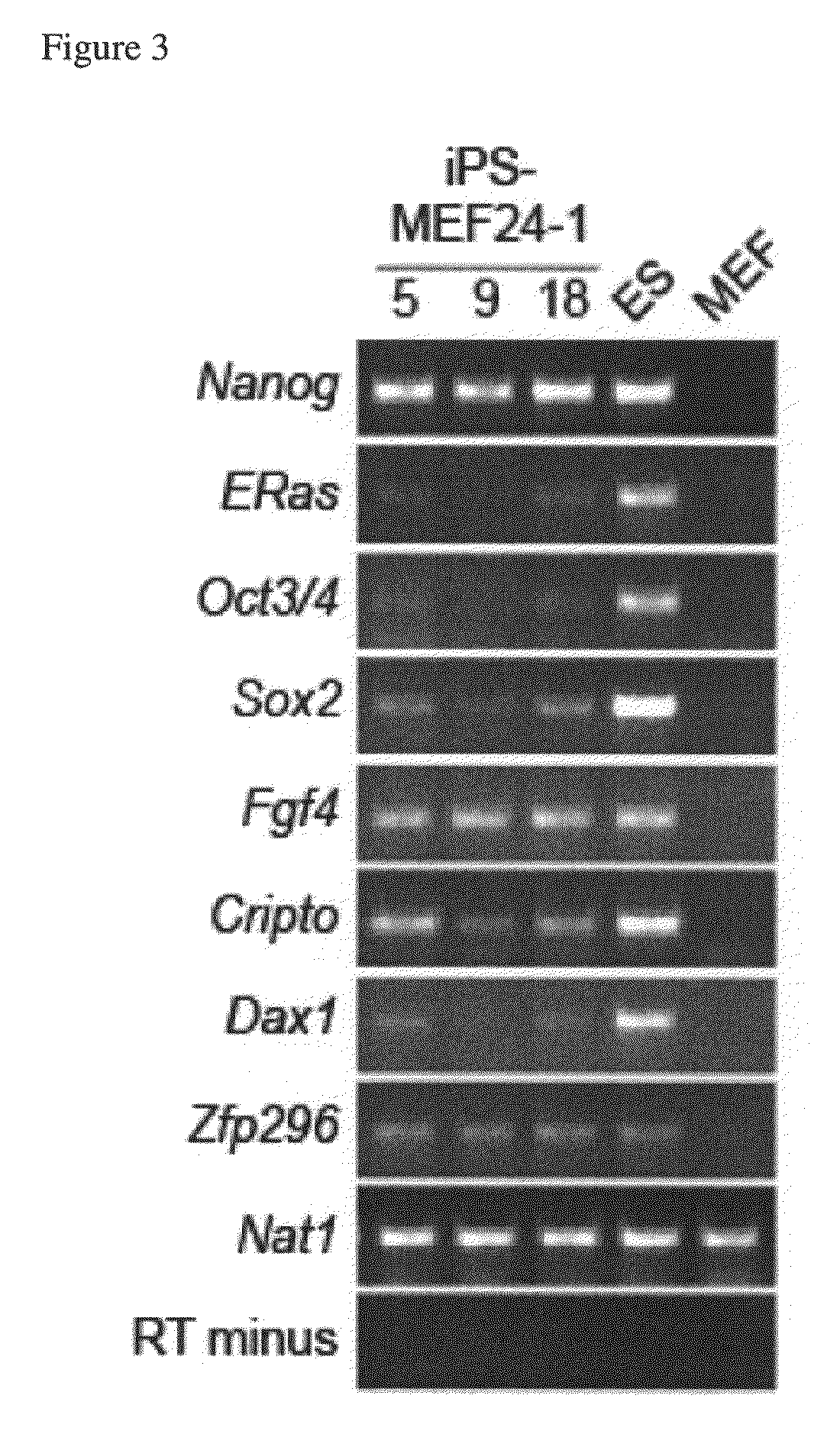
Somatic cell reprogramming by retroviral vectors encoding Oct3/4. Klf4, c-Myc and Sox2
ActiveUS8129187B2Easy to prepareEffective isolationGenetically modified cellsArtificial cell constructsNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
Method for expression of small antiviral RNA molecules within a cell
ActiveUS20030059944A1Prevents sequenceHigh expressionOrganic active ingredientsPeptide/protein ingredientsGeneticsViral life cycle
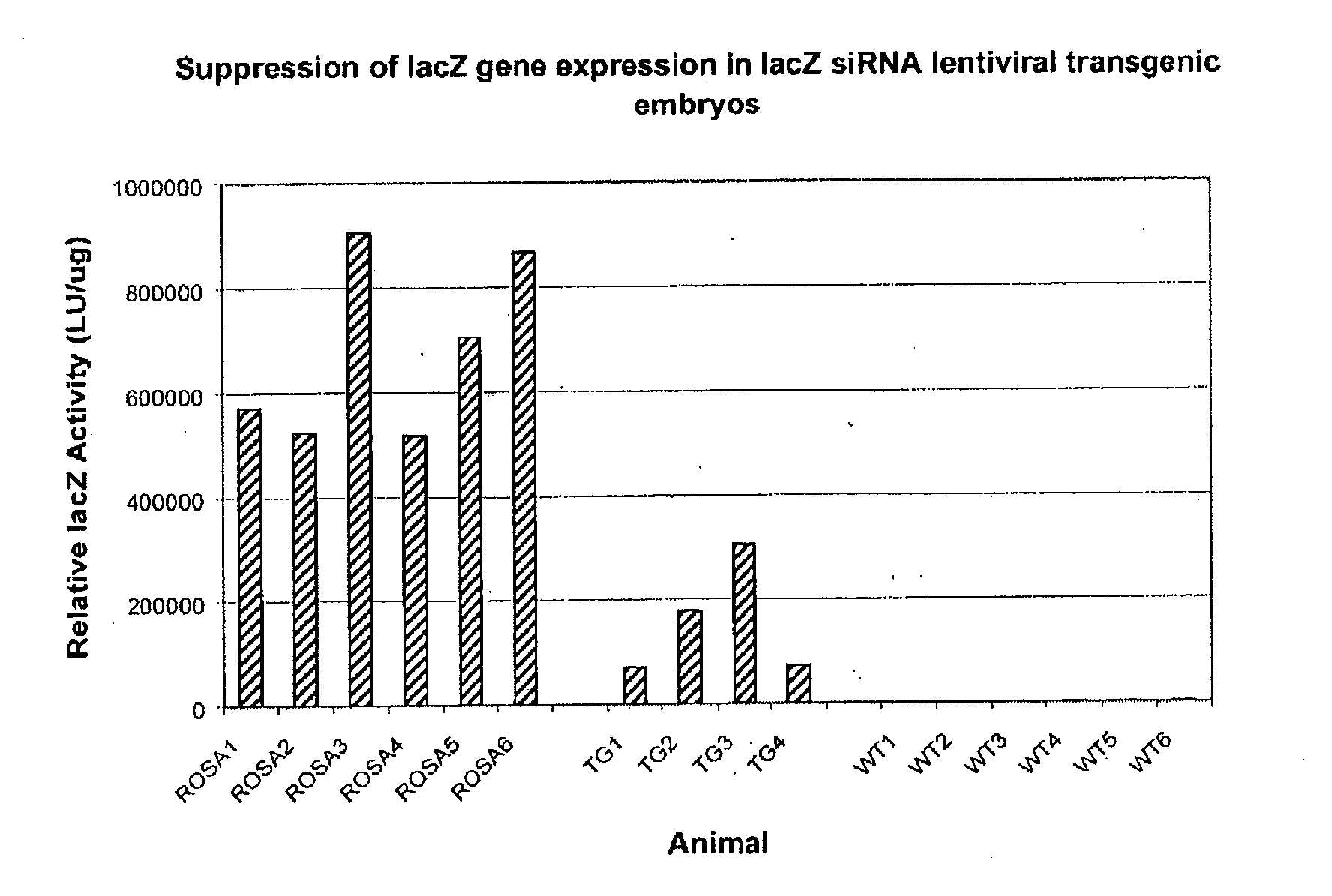
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV.
Owner:CALIFORNIA INST OF TECH
Retroviral vector
InactiveUS7351585B2High titerKeep for a long timeGenetic material ingredientsArtificial cell constructsNucleotideRetrovirus
Provided herein is a retroviral vector comprising, and capable of expressing, a nucleotide of interest (NOI), wherein the NOI encodes an RNA or protein which is harmful to a cell.
Owner:OXFORD BIOMEDICA (UK) LTD
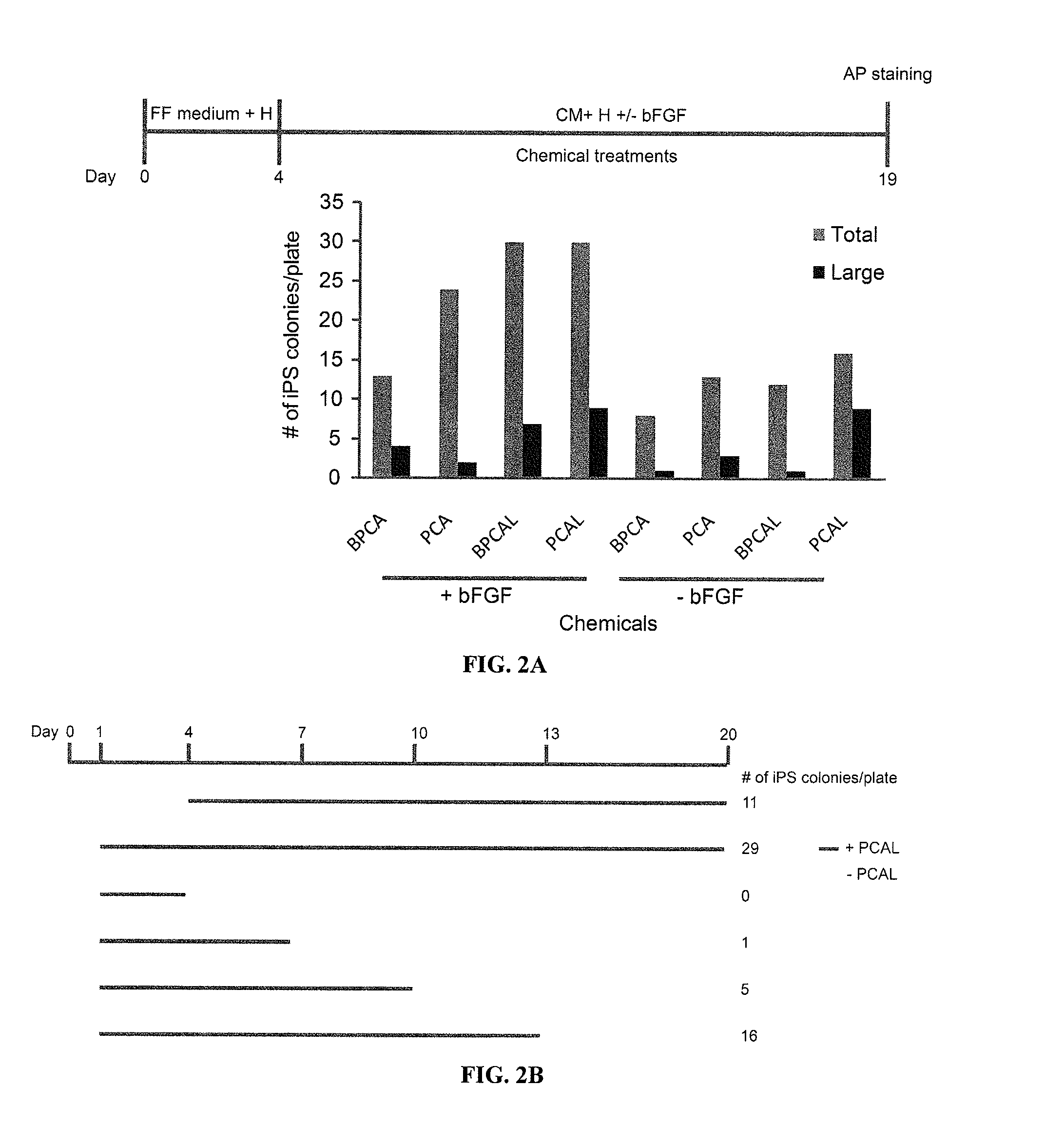
Episomal reprogramming with chemicals
ActiveUS20110104125A1Easy to adaptHigh reprogramming efficiencyBiocideNervous disorderVector elementViral vector
Methods and composition of induction of pluripotent stem cells are disclosed. For example, in certain aspects methods for generating essentially vector-free induced pluripotent stem cells with cell signaling regulators are described. Furthermore, certain aspects of the invention provide novel compositions comprising induced pluripotent stem cells essentially free of exogenous retroviral vector elements in the presence of a medium comprising signaling inhibitors. In certain aspects, feeder-free episomal reprogramming methods may be provided.
Owner:FUJIFILM CELLULAR DYNAMICS INC
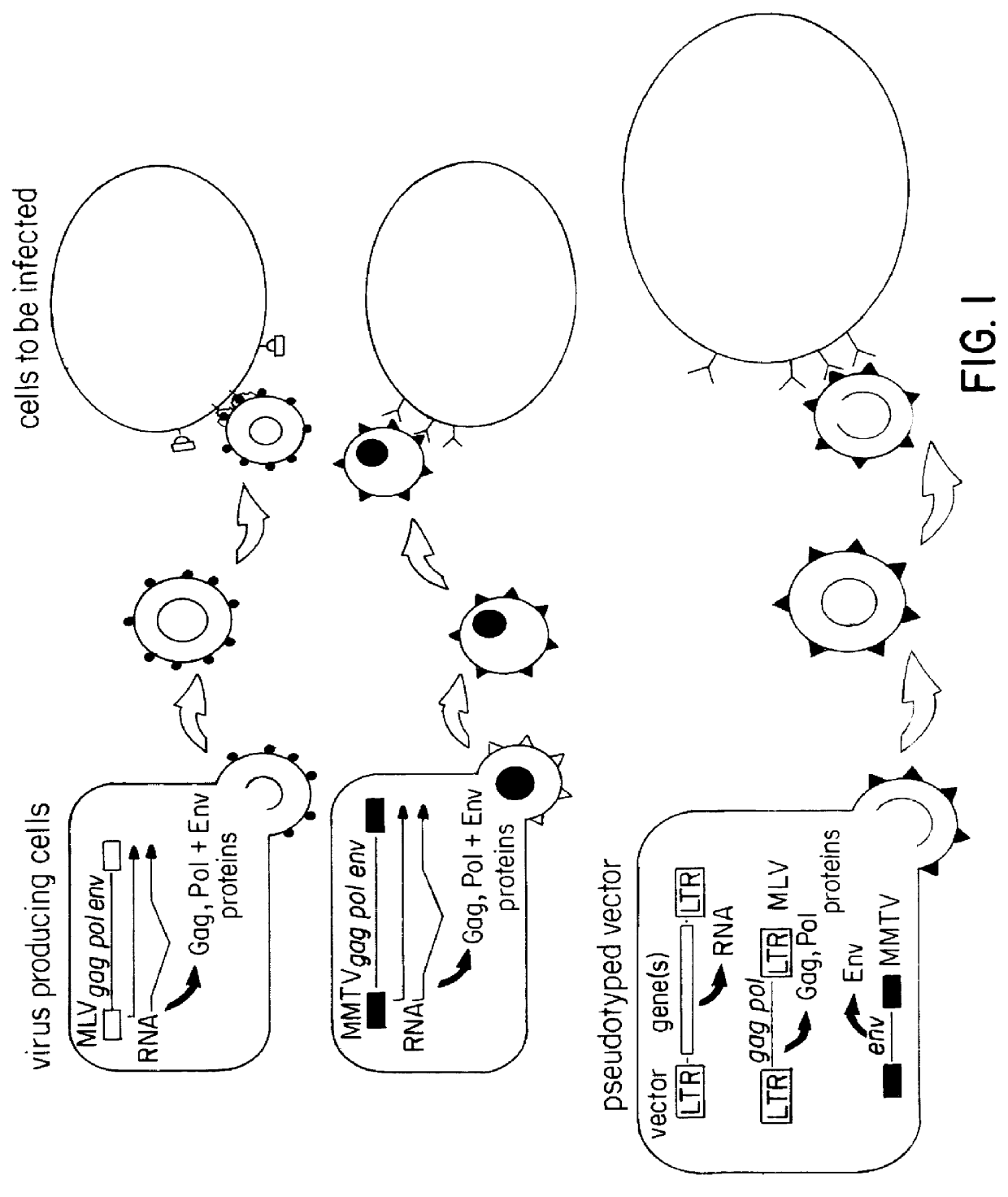
Pseudotyped retroviral particles
InactiveUS6117681AEasy to insertReduce the possibilityAnimal cellsGenetic material ingredientsVector systemRetrovirus
The present invention relates to a retroviral vector system comprising a packaging cell line that synthesize the core and enzymatic proteins of MLV virus from one or more gag and pol containing constructs and the envelope of MMTV virus from the same or an independent env containing construct, and to pseudotyped retroviral particles produced by culturing said retroviral vector system transfected with a MLV based retroviral vector, and isolation of said pseudotyped retroviral particles.
Owner:F GES FUR UMWELT & GESUNDHEIT GMBH +1
Human immunodeficiency virus type 1 nucleic acids devoid of long terminal repeats capable of encoding for non-infectious, immunogenic, retrovirus-like particles
InactiveUS6080408AImprove efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in diagnosis.
Owner:CONNAUGHT LAB
Targeted gene discovery
InactiveUS6139833AEnhancing general accessibilityRaise the possibilityBiocideBacteriaGenomic DNAIn vivo
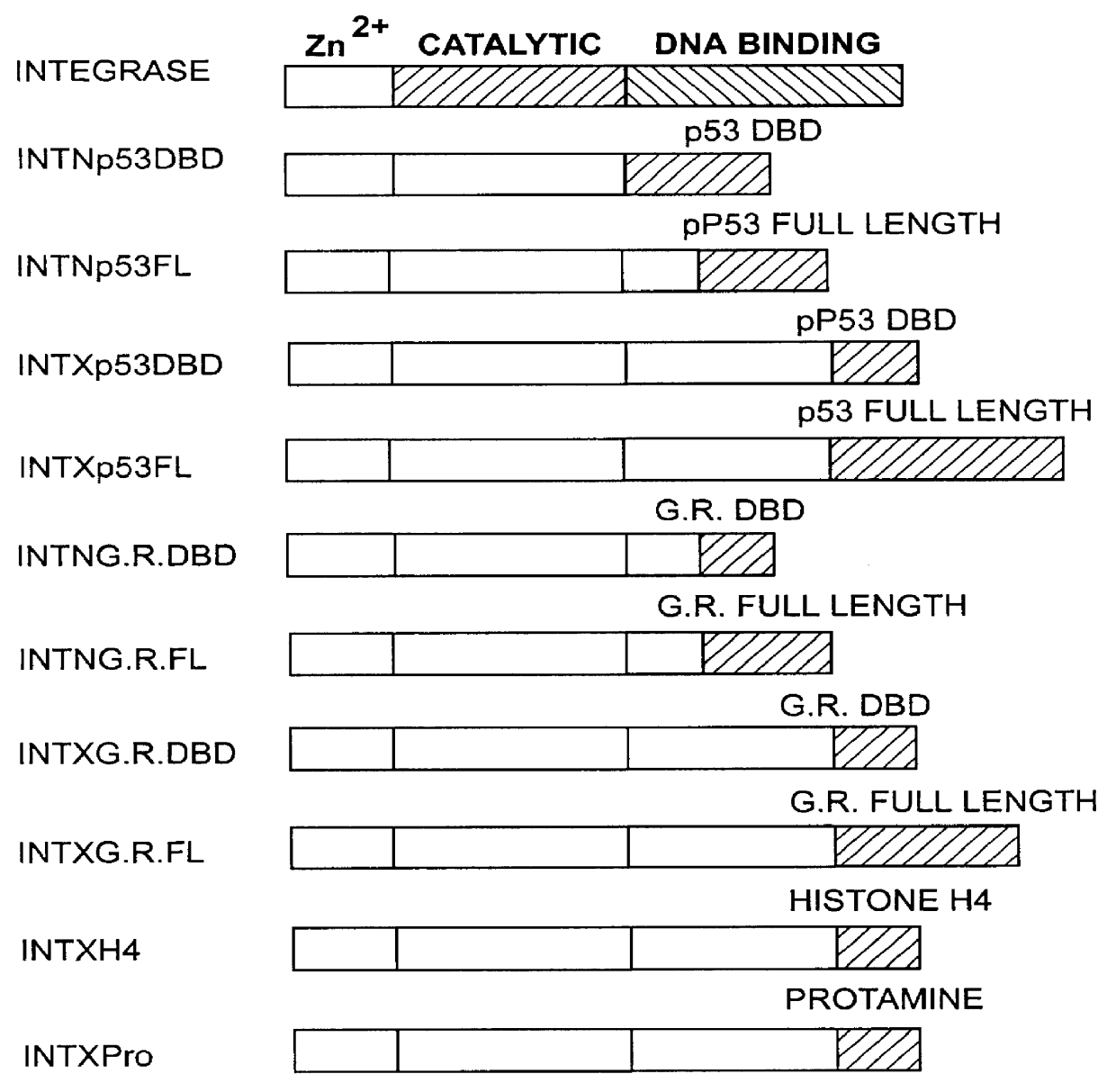
The present invention describes a comprehensive system for gene discovery using retrovirus that have been engineered to exhibit increased accessibility to genomic DNA, or to mutate and identify the chromosomal target sequences of DNA binding proteins. The strategy employs the combination of retroviral integrase / DNA binding protein fusion constructs and gene-trapping methodologies. This novel technology provides the ability to establish proviral integration at any location within the genome. In addition, it allows for the generation of a collection of eukaryotic cells in which each cell contains a mutation in a target gene or sequence for a known DNA binding protein which also allow for rapid in vivo functional analysis. Sequence information obtained for genes identified using the described methods identify a collection of eukaryotic genes related by, or directly or indirectly regulated by, a given DNA binding protein.
Owner:LEXICON PHARM INC
Endogenous retroviruses up-regulated in prostate cancer
InactiveUS7776523B2High sensitivityEasy to implementNervous disorderPeptide/protein ingredientsHuman endogenous retrovirus HERV-KProstate cancer screening
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Methods and compositions for rna-guided treatment of HIV infection
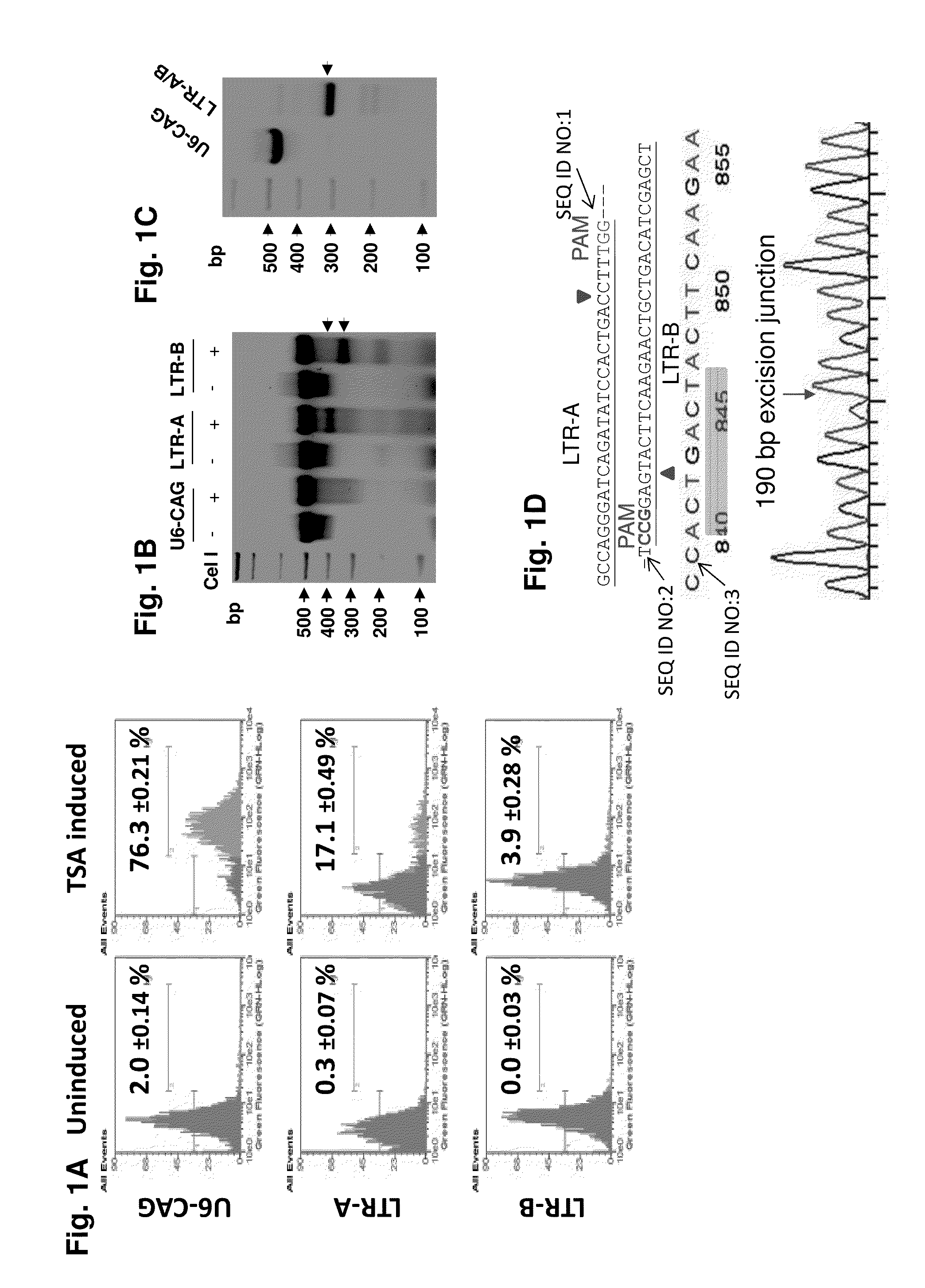
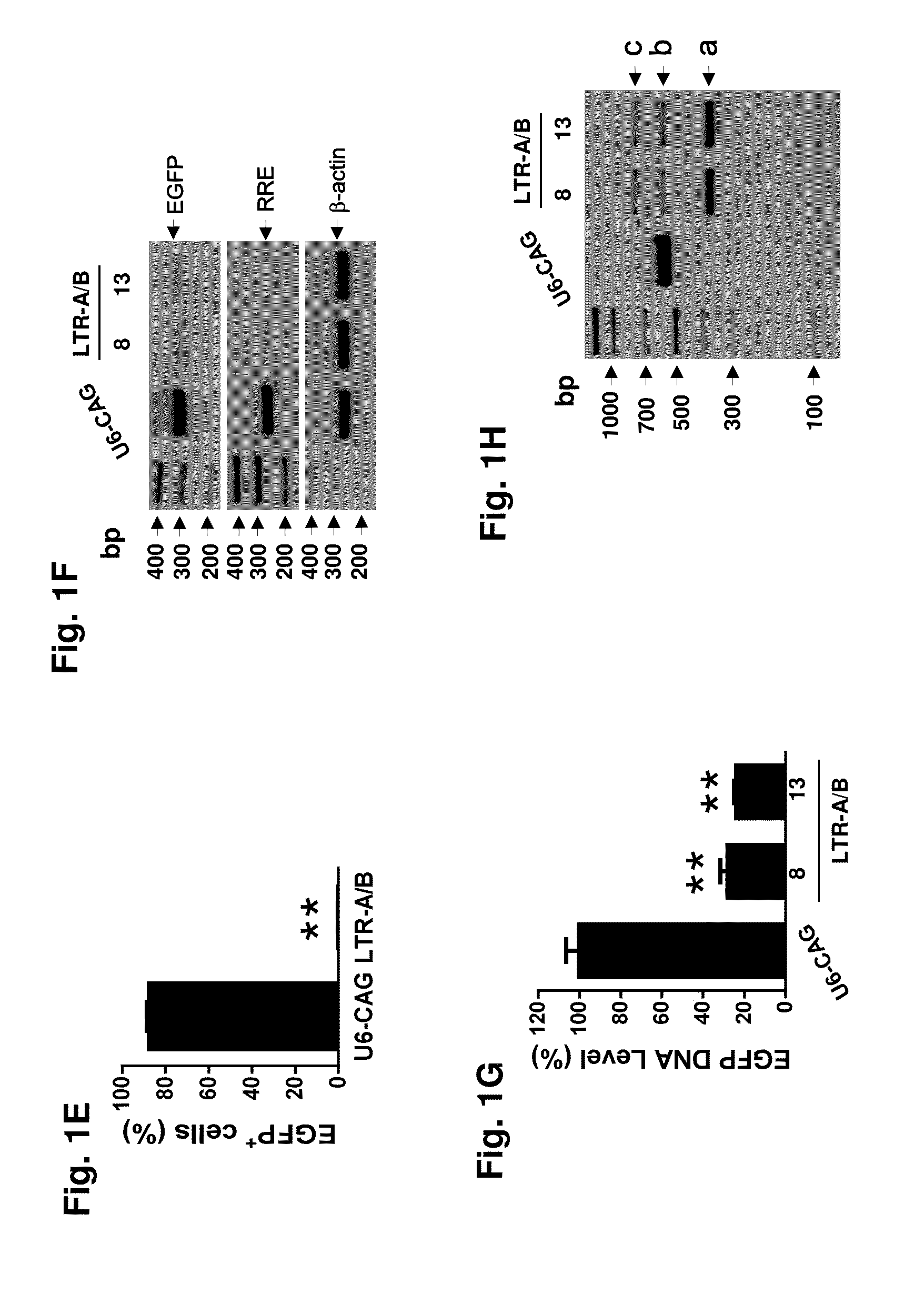
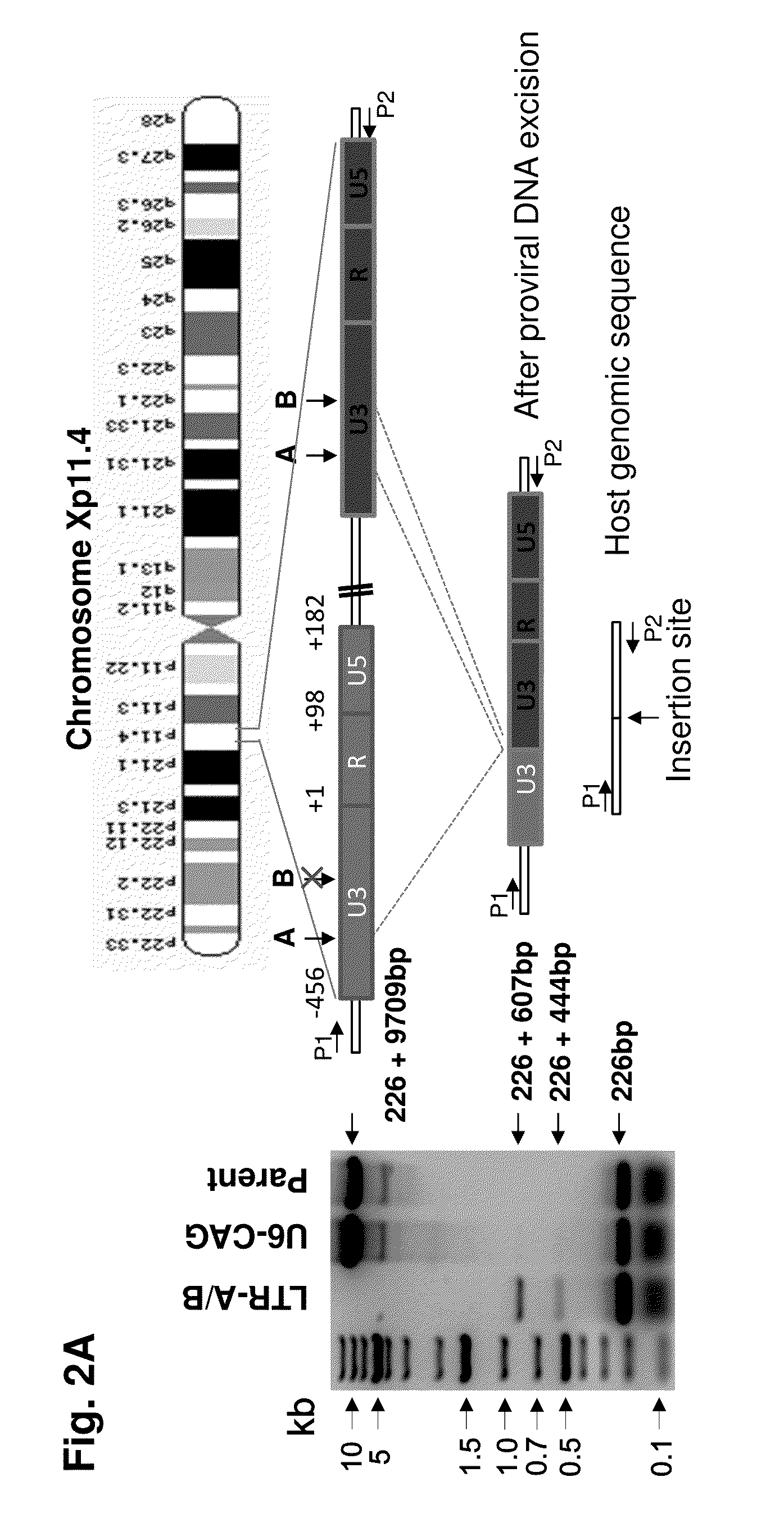
A method of inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus by treating the host cell with a composition comprising a Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)-associated endonuclease, and two or more different guide RNAs (gRNAs), wherein each of the at least two gRNAs is complementary to a different target nucleic acid sequence in a long terminal repeat (LTR) in the proviral DNA, and inactivating the proviral DNA. A composition for use in inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus including isolated nucleic acid sequences comprising a CRISPR-associated endonuclease and a guide RNA, wherein the guide RNA is complementary to a target sequence in a human immunodeficiency virus.
Owner:TEMPLE UNIVERSITY
Hybrid adeno-retroviral vector for the transfection of cells
InactiveUS7052904B2BiocideGenetic material ingredientsViral Reverse TranscriptionNucleic acid sequencing
An adenovirus, including adenoviral capsid proteins, and a replication-defective adenoviral vector that includes a 5′ retroviral LTR nucleic acid sequence, a 3′ retroviral LTR nucleic acid sequence, a nucleic acid sequence encoding a portion of a retroviral envelope protein adjacent to either the 5′ LTR or the 3′ LTR nucleic acid sequence, a retroviral packaging sequence and a nucleic acid sequence encoding a transgene located between the 5′ LTR and the 3′ LTR is provided. Host cells infected with this adenovirus are also provided. An adenoviral vector is provided that includes an adenoviral polynucleotide sequence comprising a nucleic acid encoding a transgene, a retroviral packaging signal, a 5′ and a 3′ retroviral LTR, and a portion of a retroviral envelope polypeptide, wherein the adenoviral polynucleotide sequence does not encode one or more of E1, E3 or E4. A method for transforming a cell is also provided using a virus or a vector of the invention, as is a method for introducing a transgene into a cell that is not able to produce viral particles with a single viral vector. A method is also provided for preventing or treating disorder in a subject using the adenoviral vectors of the invention. A pharmaceutical composition is also provided that includes an adenoviral vector of the invention and a pharmaceutically acceptable carrier.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
Method for expression of small antiviral RNA molecules within a cell
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV. In a further aspect, the invention provides methods for producing siRNA encoding lentivirus where the siRNA activity may interfere with the lentiviral life cycle.
Owner:CALIFORNIA INST OF TECH +1
Targeting Pseudotyped Retroviral Vectors
InactiveUS20080227736A1Reduce natural tropismStrong specificityNervous disorderSsRNA viruses positive-senseCell type specificViral vector
Owner:RGT UNIV OF CALIFORNIA
Antigenically-marked non-infectious retrovirus-like particles
InactiveUS6291157B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:CONNAUGHT LAB
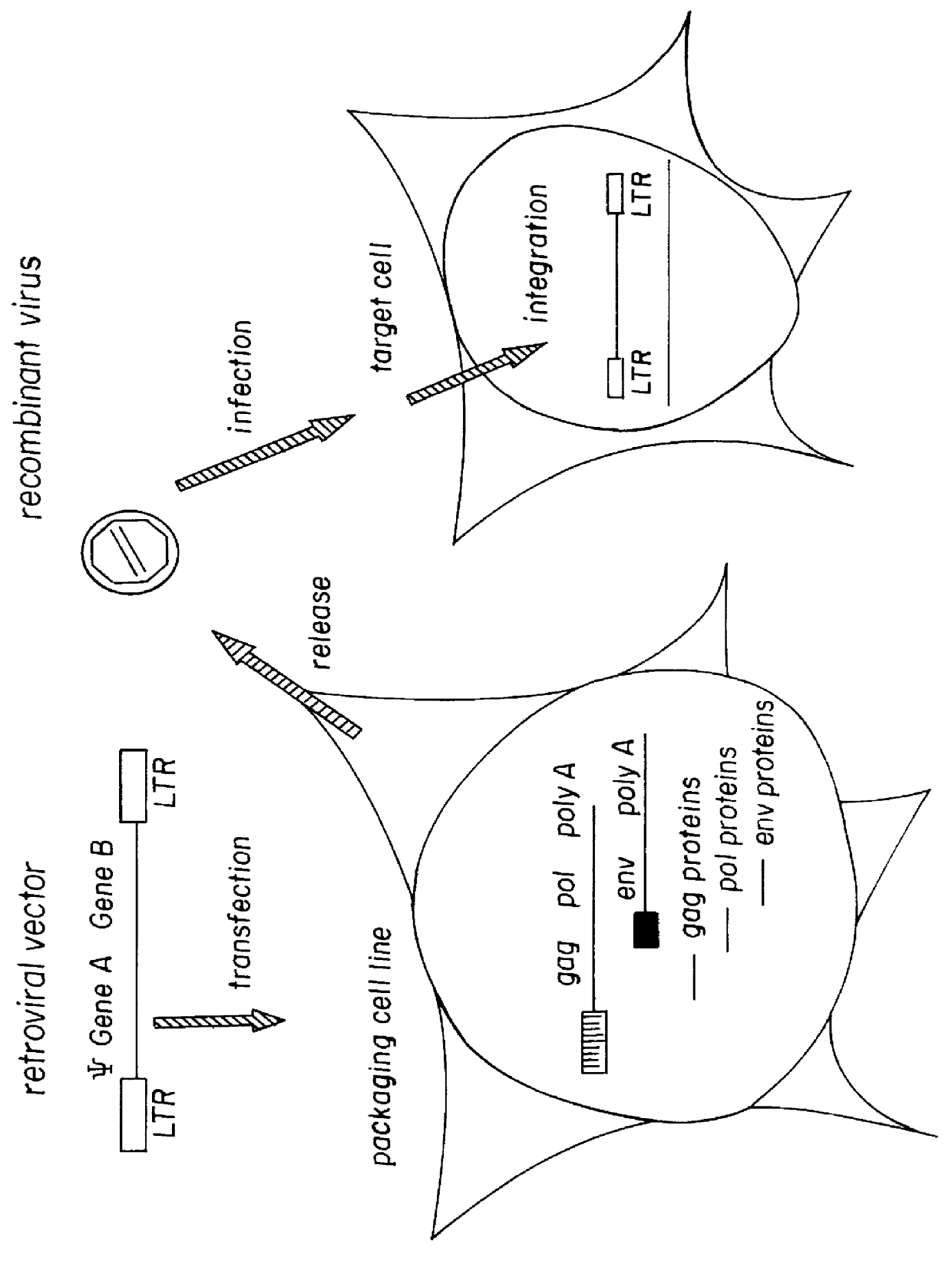
Retroviral gene therapy vectors and therapeutic methods based thereon
Retroviral vectors are disclosed which include an insertion site for genes of interest and are capable of expressing high levels of the protein derived from the genes of interest in a wide variety of transfected cell types. Also disclosed are retroviral vectors lacking a selectable marker, thus rendering them suitable for human gene therapy in the treatment of a variety of disease states without the co-expression of a marker product, such as an antibiotic. These retroviral vectors are especially suited for use in certain packaging cell lines.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES +1
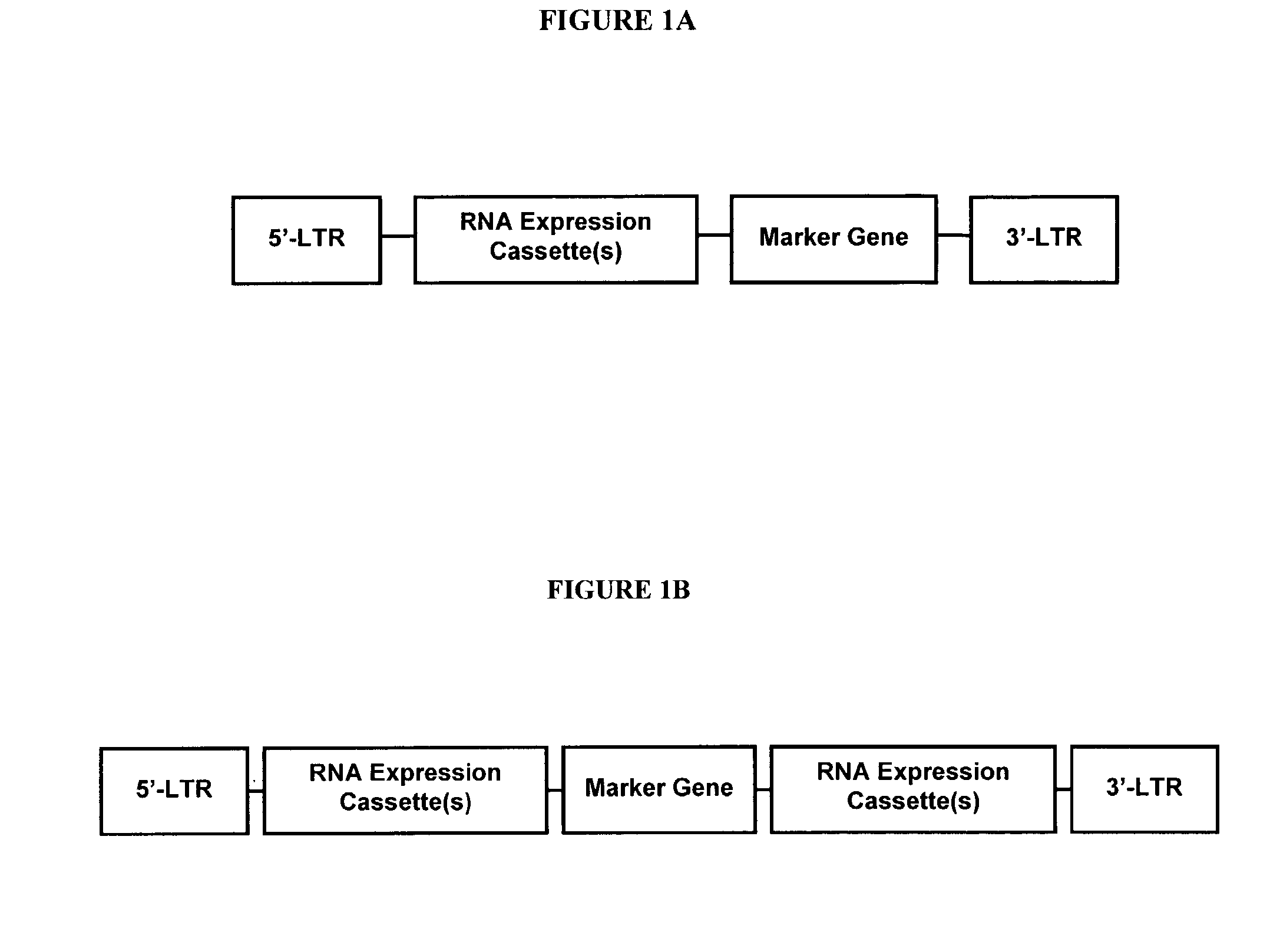
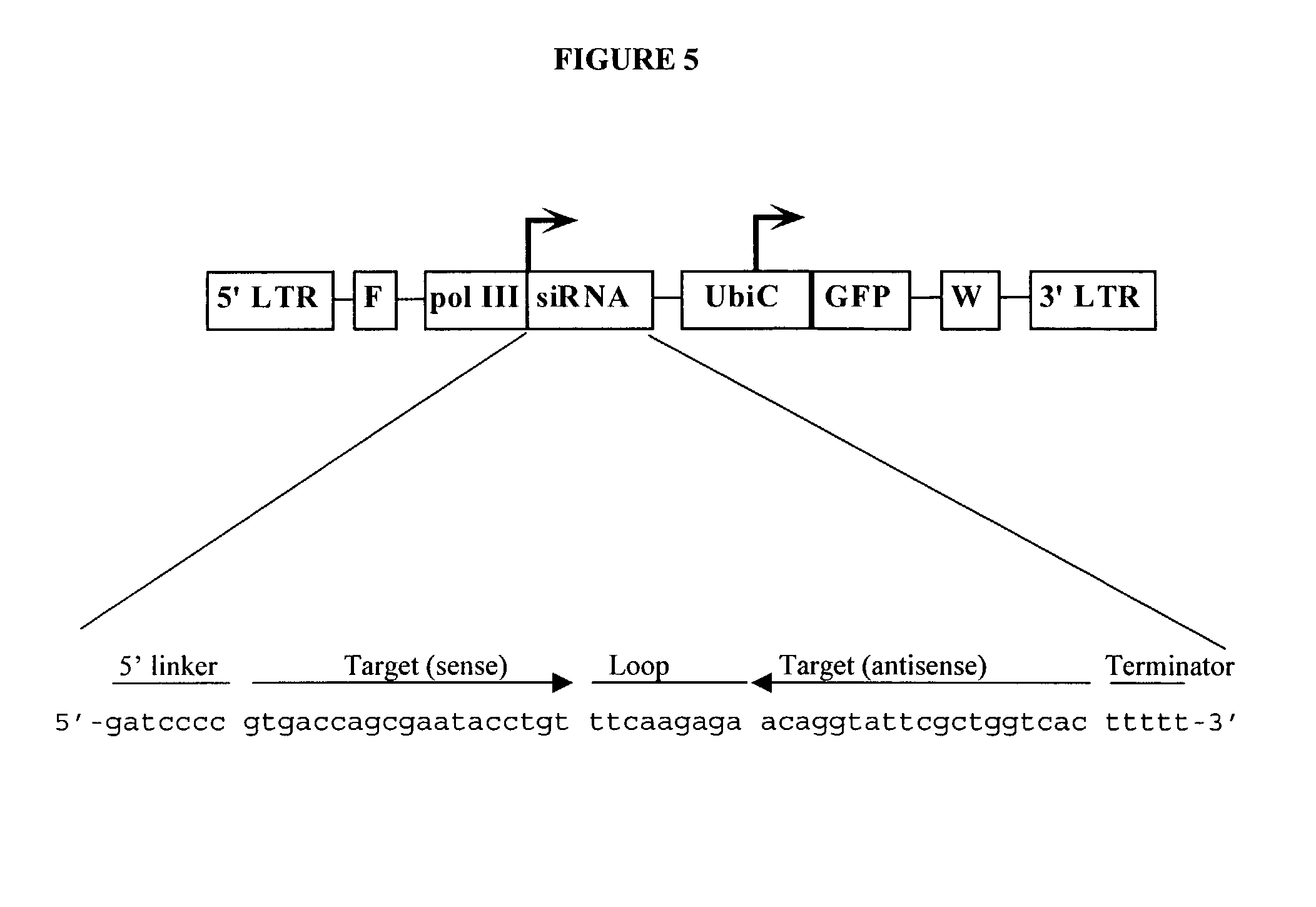
Method for expression of small antiviral RNA molecules with reduced cytotoxicity within a cell
InactiveUS20080003682A1Inhibiting RNA interferenceSsRNA viruses negative-senseOrganic active ingredientsRetrovirusRNA molecule
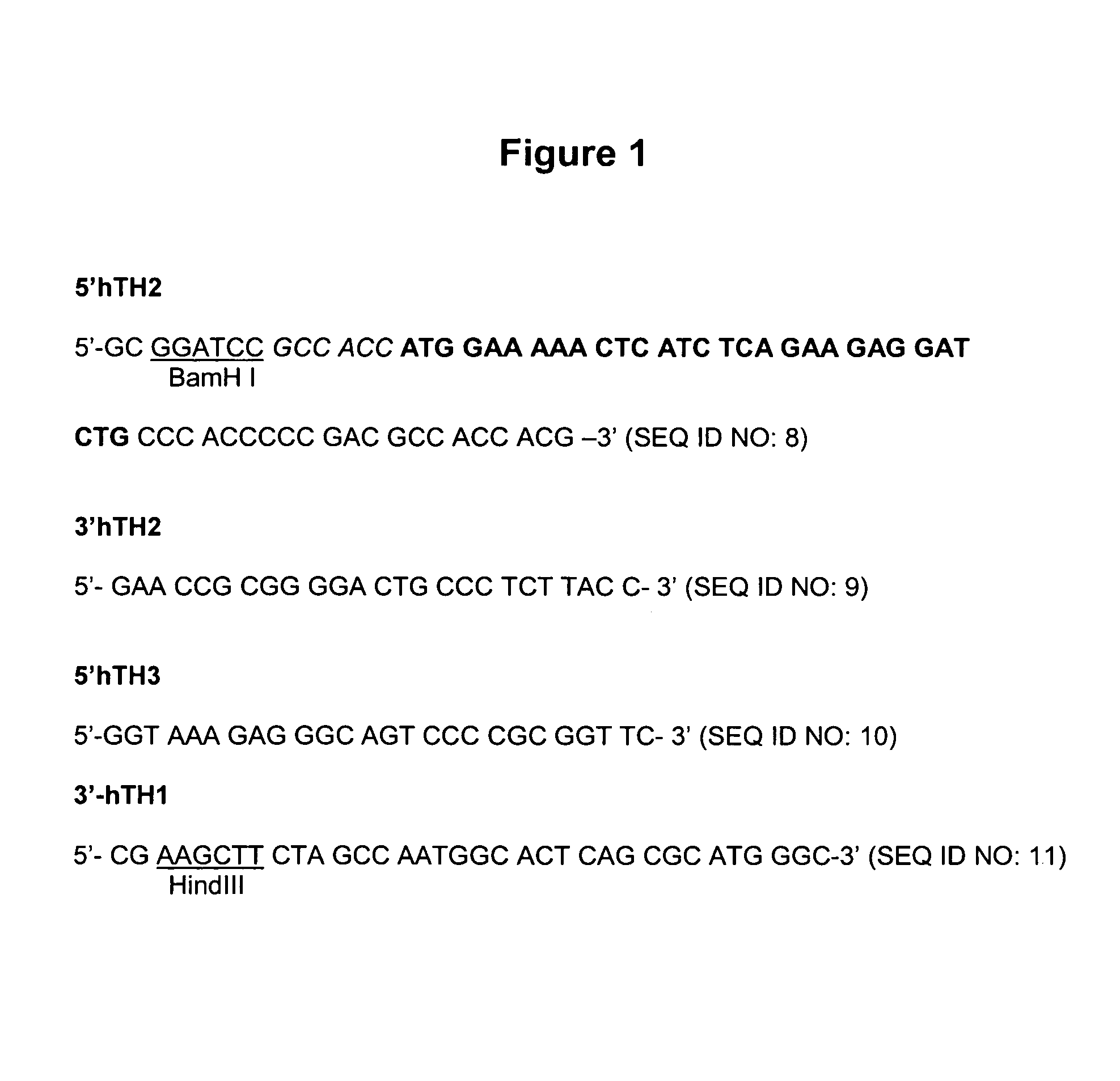
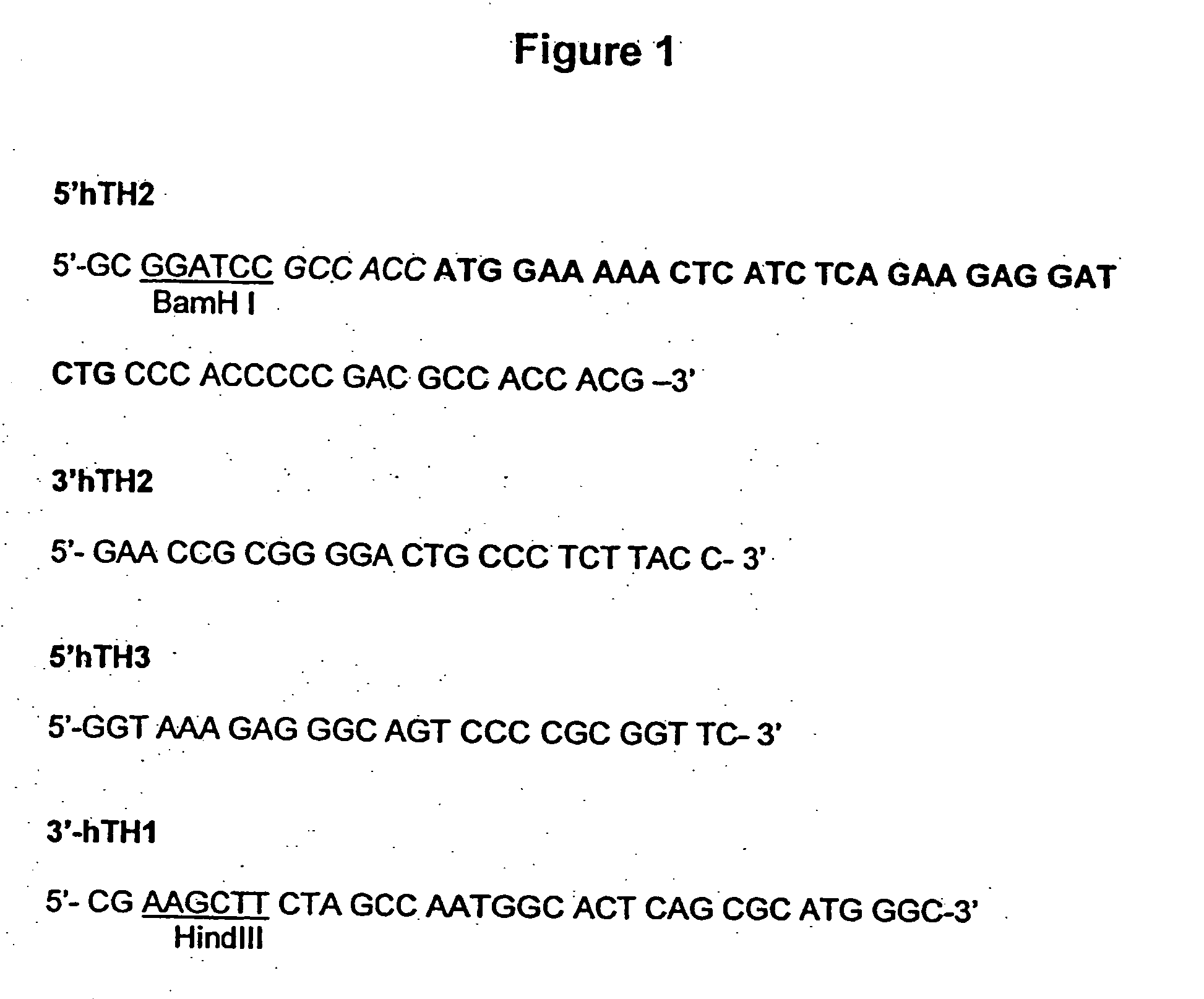
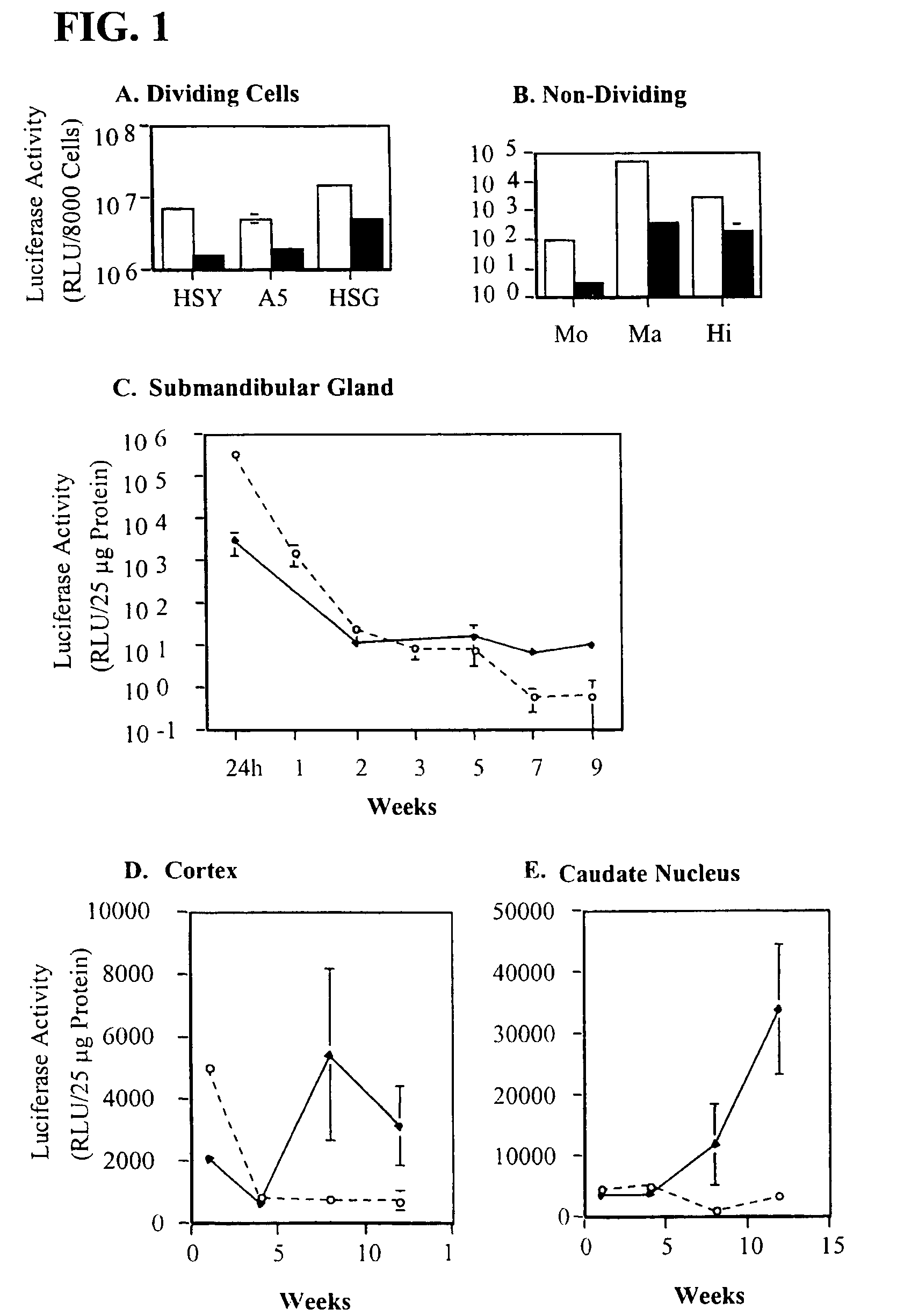
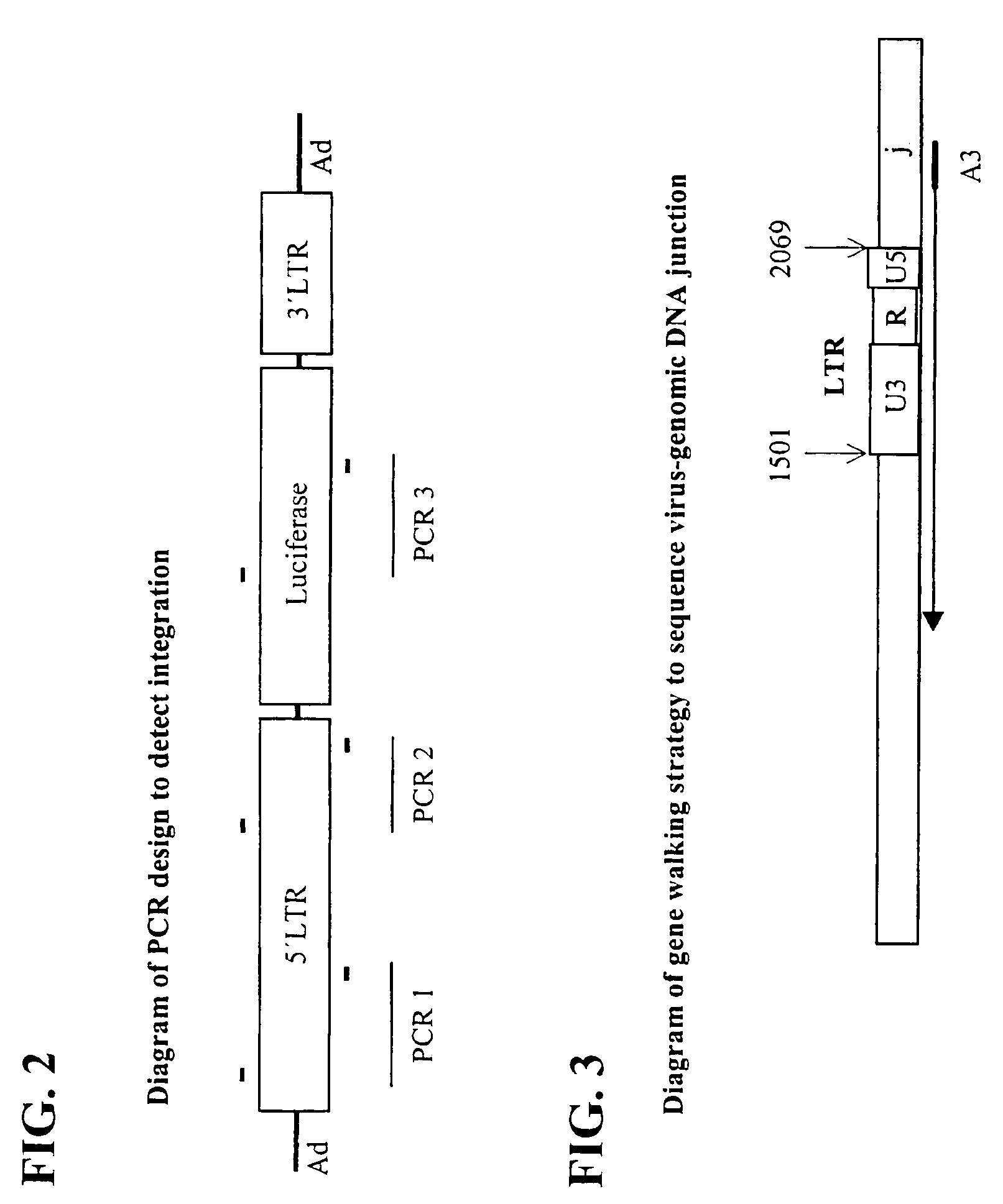
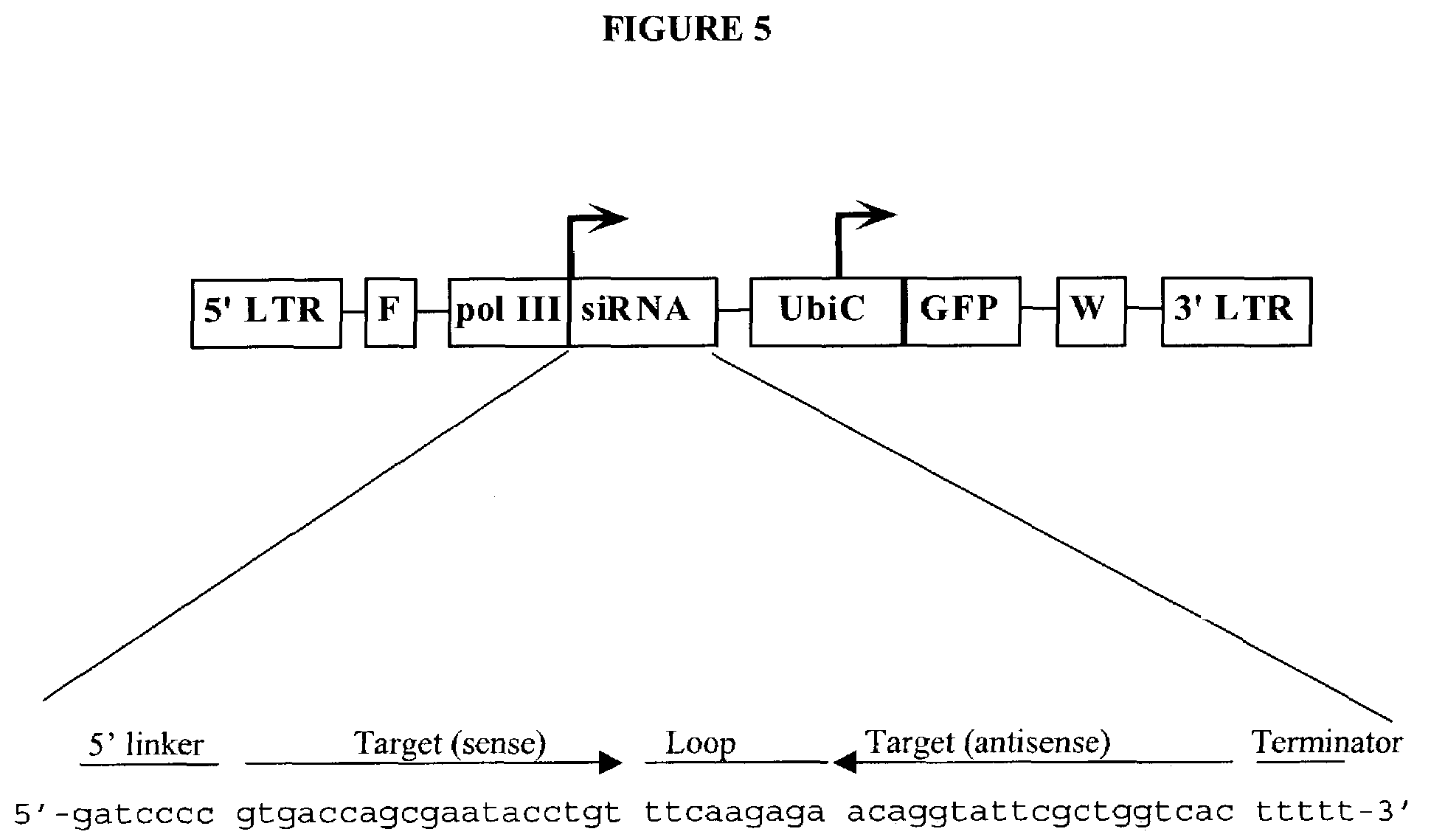
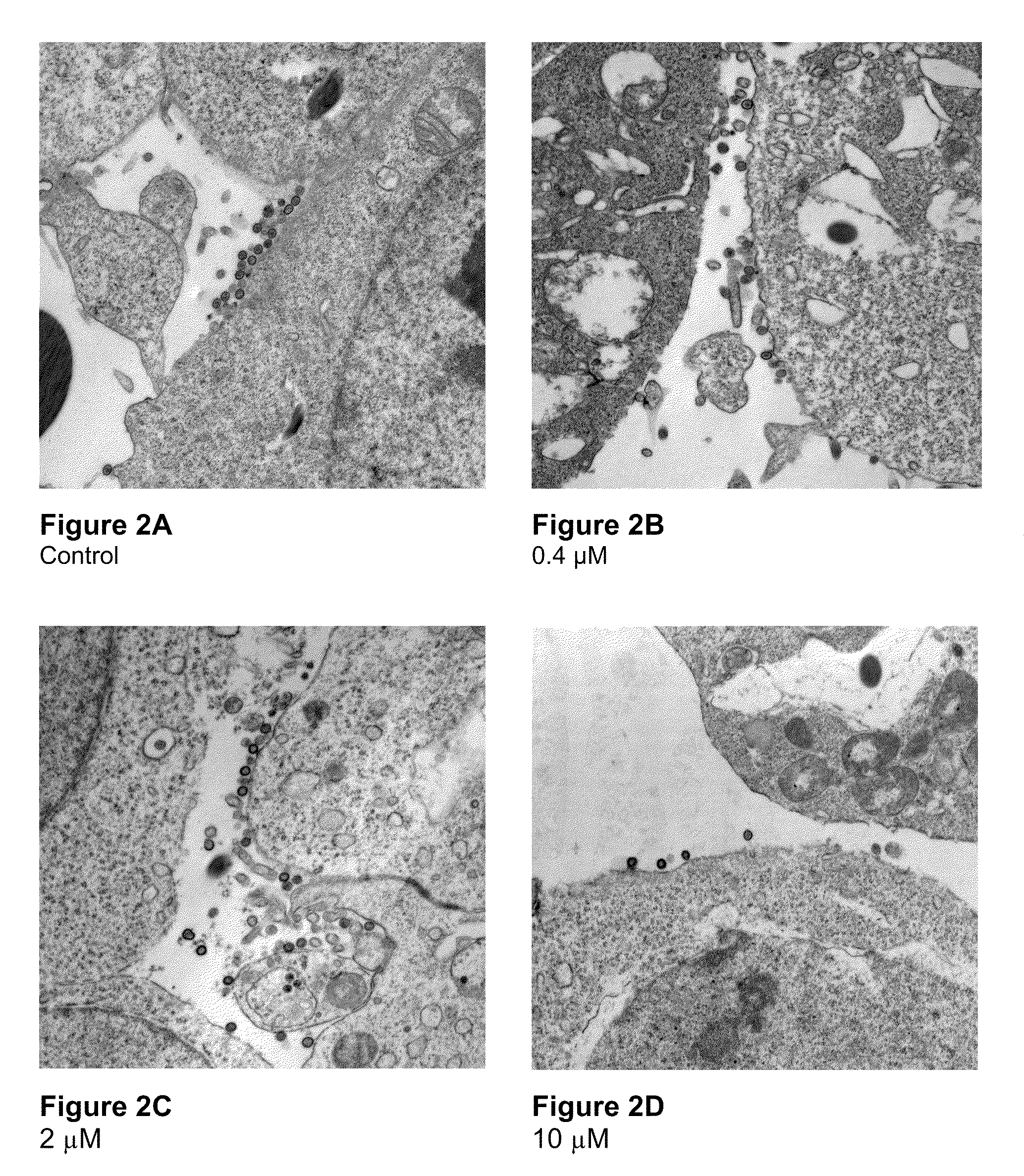
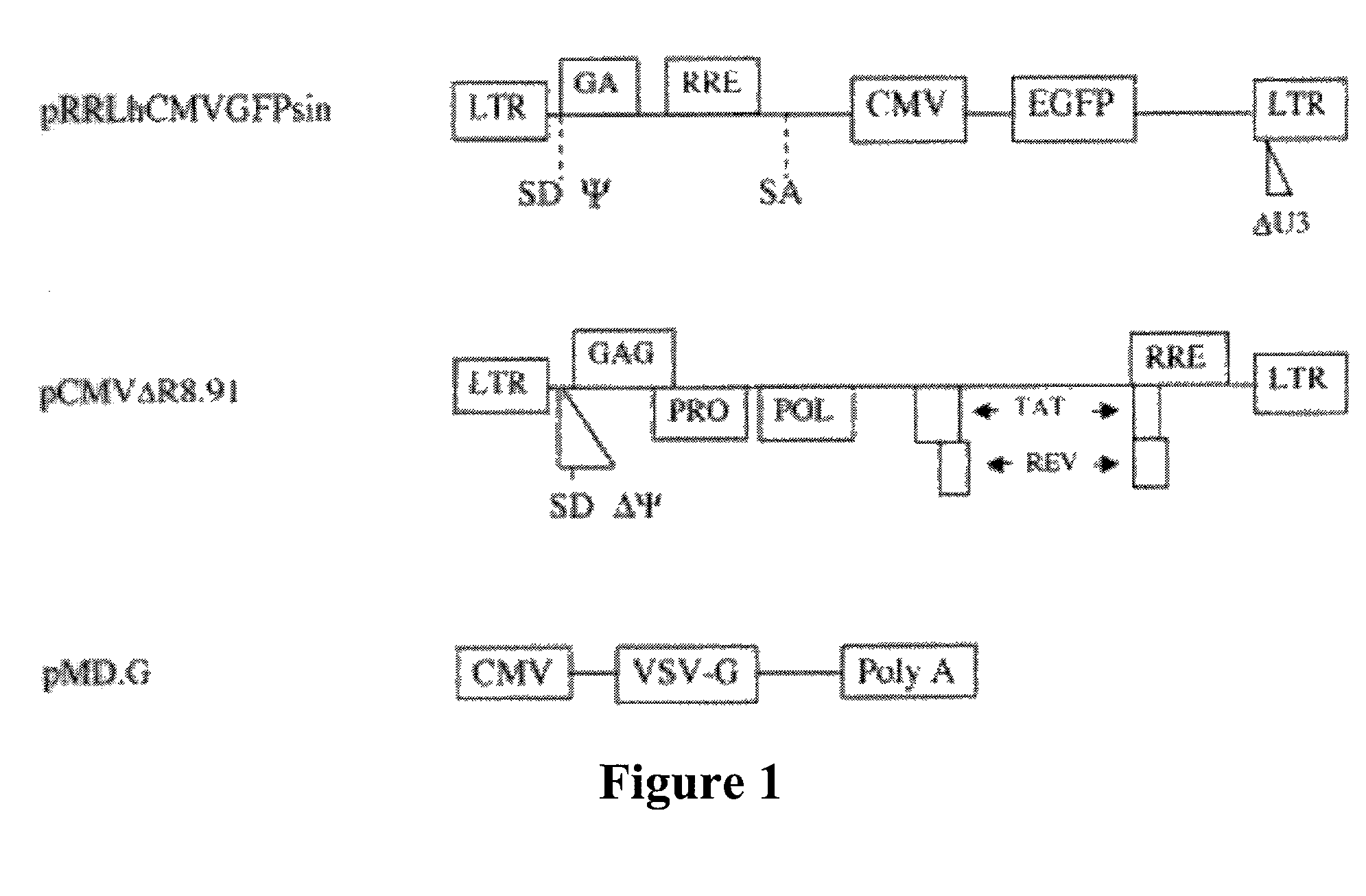
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector (FIG. 1A). Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell. In a further aspect, the invention provides methods for producing siRNA encoding lentivirus where the siRNA activity may interfere with the lentiviral life cycle. In yet a further aspect, the invention provides methods for expression of a small RNA molecule within a cell, such as an siRNA capable of downregulating CCR5, wherein expression of the small RNA molecule is relatively non-cytotoxic to the cell. The invention also includes small RNA molecules, such as an siRNA capable of downregulating CCR5, that are relatively non-cytotoxic to cells.
Owner:CALIFORNIA INST OF TECH
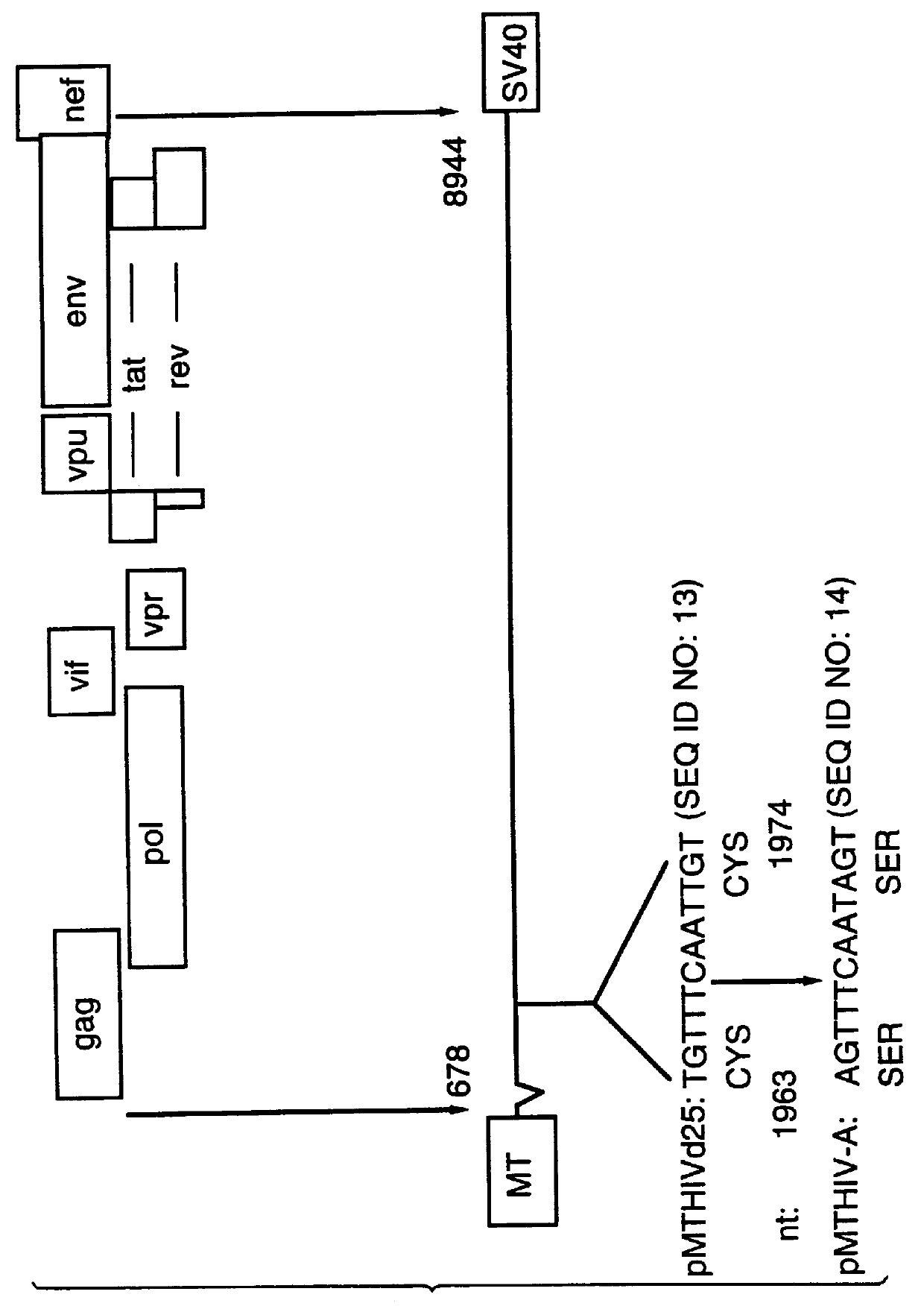
ALVAC/FIV constructs
InactiveUS7255862B1Improve security levelViral antigen ingredientsGenetic material ingredientsFeline immunodeficiency virusHeterologous
Recombinants containing and expressing lentivirus, retrovirus or immunodeficiency virus DNA and methods for making and using the same are disclosed and claimed. In an exemplified embodiment, attenuated recombinant viruses containing DNA encoding a feline immunodeficiency virus epitope such as an antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinants can be NYVAC or ALVAC recombinants. The DNA can encode at least one of: Env, Gag, Pol, or combinations thereof such as Gag and Pol or protease or Env, Gag and Pol or protease. The recombinants and gene products therefrom and antibodies generated by them have several preventive, therapeutic and diagnostic uses. DNA from the recombinants are useful as probes or, for generating PCR primers or for immunization. The immunogenicity and protective efficacy of immunization protocols involving ALVAC-FIV and priming with a recombinant canarypox virus ALVAC-FIV vaccine followed by a booster immunization with inactivated FIV-infected celled vaccine (ICV) was evaluated against FIV challenge in cats and the protocol was shown to effectively induce FIV-specific protective immune responses. Further, it was found that immunized cats were fully protected from an initial challenge with a slightly heterologous FIV strain (50CID50) and were partially protected from a second challenge with a distinctly heterologous FIV strain (75CID50) given eight months after the initial challenge without any intervening booster.
Owner:VIROGENETICS
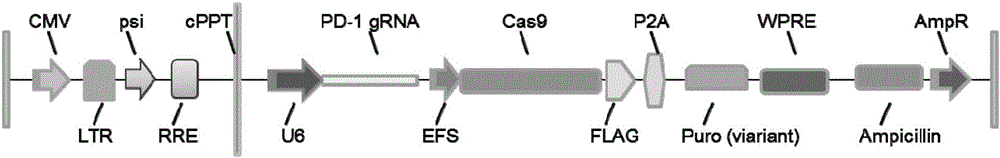
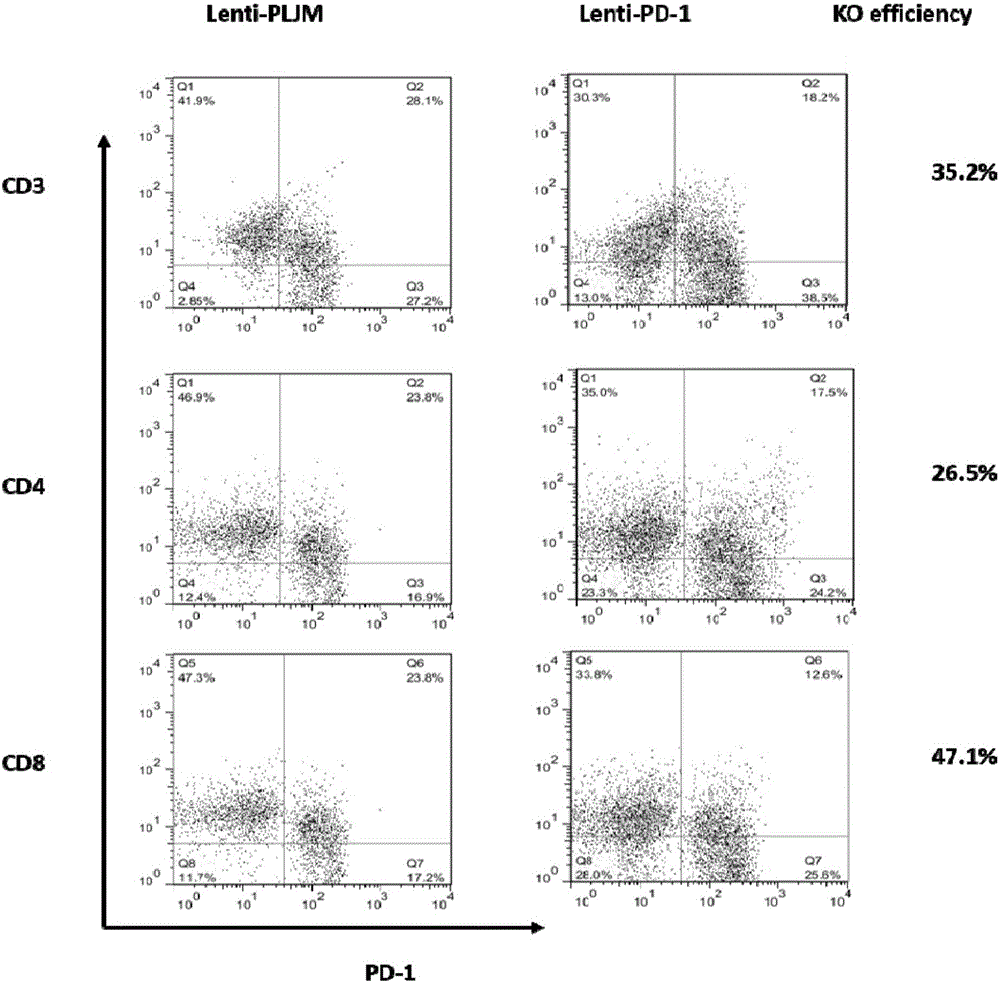
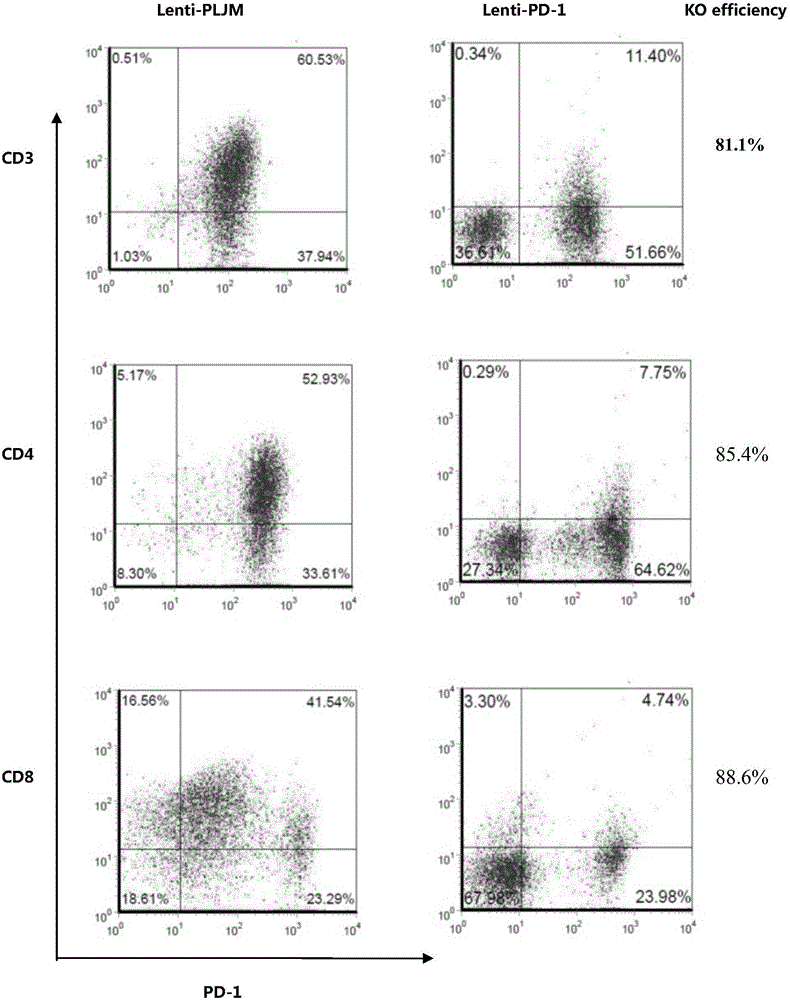
PD-1 gene recombinant virus plasmid, construction thereof, recombinant retrovirus Lenti-PD-1-Puro and packaging and application of recombinant retrovirus Lenti-PD-1-Puro
ActiveCN105671083AStrong specificityRelease the suppressed stateCell receptors/surface-antigens/surface-determinantsUnknown materialsT cellViral infection
The invention discloses a PD-1 gene recombinant virus. The collection number of the recombinant virus Lenti-PD-1-Puro is CCTCC No:V201601. According to the PD-1 gene recombinant virus, PD-1gRNA sequences are cloned into a retrovirus plasmid Lenti-CRISPR / Cas9-Puro, and a PD-1 recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro is obtained; then the PD-1 recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro, a plasmid pSPAX and pMD2.G are jointly transfected by 293T cells, and packaging of the recombinant virus Lenti-PD-1-Puro is completed. After T cells of peripheral blood of tumor patients are infected by the PD-1 recombinant virus, PD-1 on the T cells is successfully knocked out, the inhibitory state of the T cells of the tumor patients is relieved, the capacity of attacking tumor cells of the T cells embellished by the PD-1 recombinant virus is recovered accordingly, and the effect of immune cell treating is achieved.
Owner:安徽柯顿生物科技有限公司
Methods and systems for detection and isolation of a nucleotide sequence
InactiveUS20050053942A1Microbiological testing/measurementDNA preparationPolyadenylationRna degradation
A method for isolating nucleic acid molecules having a repeating nucleotide sequence or a homopolymeric nucleotide sequence, e.g. a poly A stretch, is described. In particular, the method uses oligomeric capture probes spiked with various amounts of locked nucleic acid (LNA). The invention further describes methods for the isolation of RNA molecules, for example polyadenylated mRNA molecules, which overcome the problems of rapid RNA degradation during isolation and analysis of such nucleic acid molecules. This is of major clinical and diagnostic importance, especially when dealing with RNA viruses, such as retroviruses or when analyzing rare or low-abundant mRNAs or mRNAs from biopsies or tissues enriched with RNases.
Owner:EXIQON AS
Tailor-made pluripotent stem cell and use of the same
InactiveUS20080003560A1Lower Level RequirementsTransplantation rejection reaction could be significantly reducedGenetically modified cellsHybrid cell preparationEgg cellSomatic cell
An object of the present invention is to efficiently establish cells, tissues, and organs capable of serving as donors for treating diseases, without eliciting immune rejection reactions, without starting with an egg cell. This object was achieved by providing a pluripotent stem cell having a desired genome. The cell was produced by treating with a reprogramming agent, producing a fusion cell of an MHC deficient stem cell with a somatic cell, or after producing a fusion cell of a stem cell with a somatic cell, removing a gene derived from the stem cell by performing genetic manipulation with a retrovirus.
Owner:REPROCELL
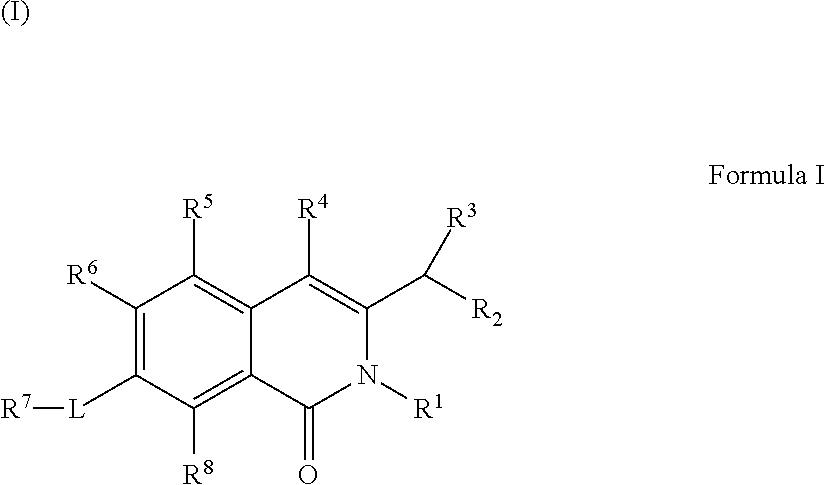
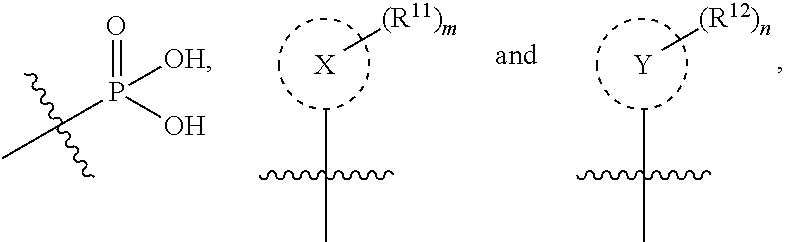
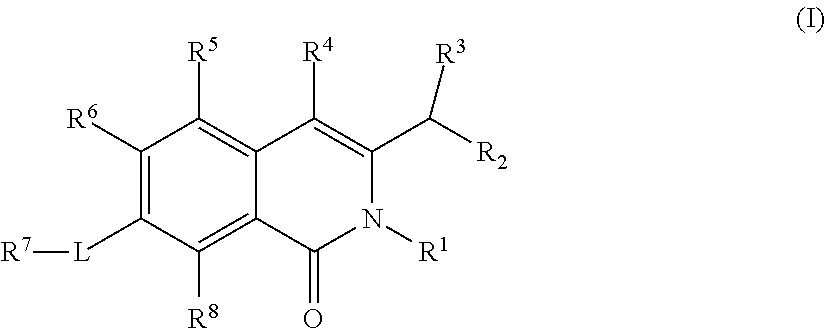
Isoquinoline Compounds And Methods For Treating HIV
Provided are compounds and pharmaceutically acceptable salts thereof, their pharmaceutical compositions, their methods of preparation, and their use for treating viral infections mediated by a member of the retrovirus family of viruses such as the Human Immunodeficiency Virus (HIV).
Owner:VIIV HEALTHCARE UK LTD
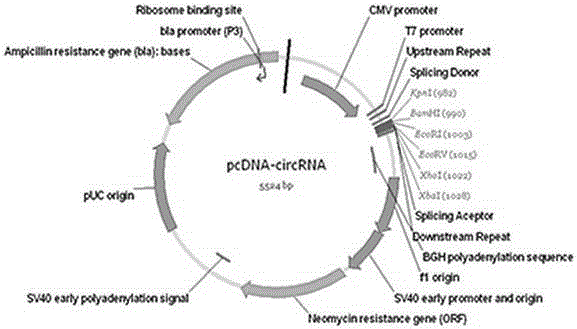
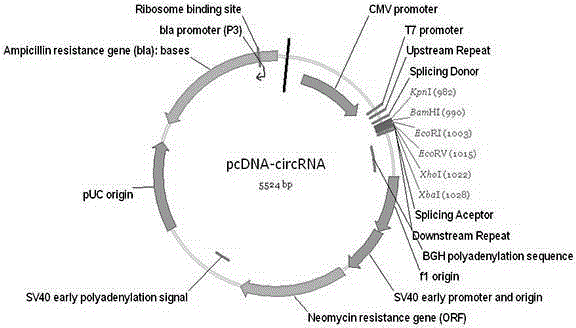
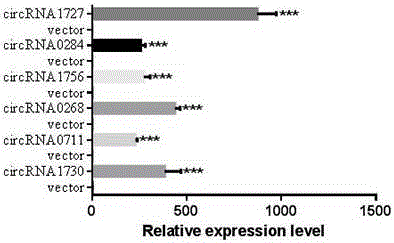
DNA (deoxyribonucleic acid) sequence used for circular RNA (ribonucleic acid) expression, expression vector and applications of DNA sequence and expression vector
ActiveCN105176981AGood expression vectorSimple and fast operationFermentationVector-based foreign material introductionDiseaseRna expression
The invention provides a DNA (deoxyribonucleic acid) sequence used for circular RNA (ribonucleic acid) expression and a vector containing the DNA sequence used for circular RNA expression. The vector is inserted in a eukaryotic expression vector, a lentiviral vector, an adenovirus vector or a retroviral vector to form a series of special expression vectors for circRNA. The DNA sequence and the corresponding expression vector have the best effects, are simple and convenient to operate and are stable in results and efficient in expression (the circRNA expression level is increased by more than a hundredfold). The sequence and the corresponding vector can be widely applied to expression of various circRNA, provide a powerful research tool for the functions and mechanisms of circRNA and provide theoretical support for research and development of further determination of circRNA molecules as novel markers and disease treatment targets.
Owner:GUANGZHOU FOREVERGEN HEALTH TECH CO LTD
Therapeutic retroviral vectors for gene therapy
InactiveUS7901671B2Maintaining therapeutic levelImprove the level ofBiocidePeptide/protein ingredientsHematopoietic cellRed blood cell
Owner:MASSACHUSETTS INST OF TECH
Phosphonates with reduced toxicity for treatment of viral infections
There are provided, inter alia, acyclic nucleoside phosphonate compounds having reduced toxicity and enhanced antiviral activity, and pharmaceutically accepted salts and solvates thereof. There are also provided methods of using the disclosed compounds for inhibiting viral RNA-dependent RNA polymerase, inhibiting viral reverse transcriptase, inhibiting replication of virus, including hepatitis C virus or a human retrovirus, and treating a subject infected with a virus, including hepatitis C virus or a human retrovirus.
Owner:RGT UNIV OF CALIFORNIA
Small Molecule Inhibitors of Retroviral Assembly and Maturation
Chemical compounds that disrupt retroviral assembly and maturation are presented herein. More particularly, this disclosure provides small molecule compounds that disrupt the formation and maturation of virus particles and methods of using such small molecules to treat HIV-1 infection.
Owner:NEW YORK BLOOD CENT
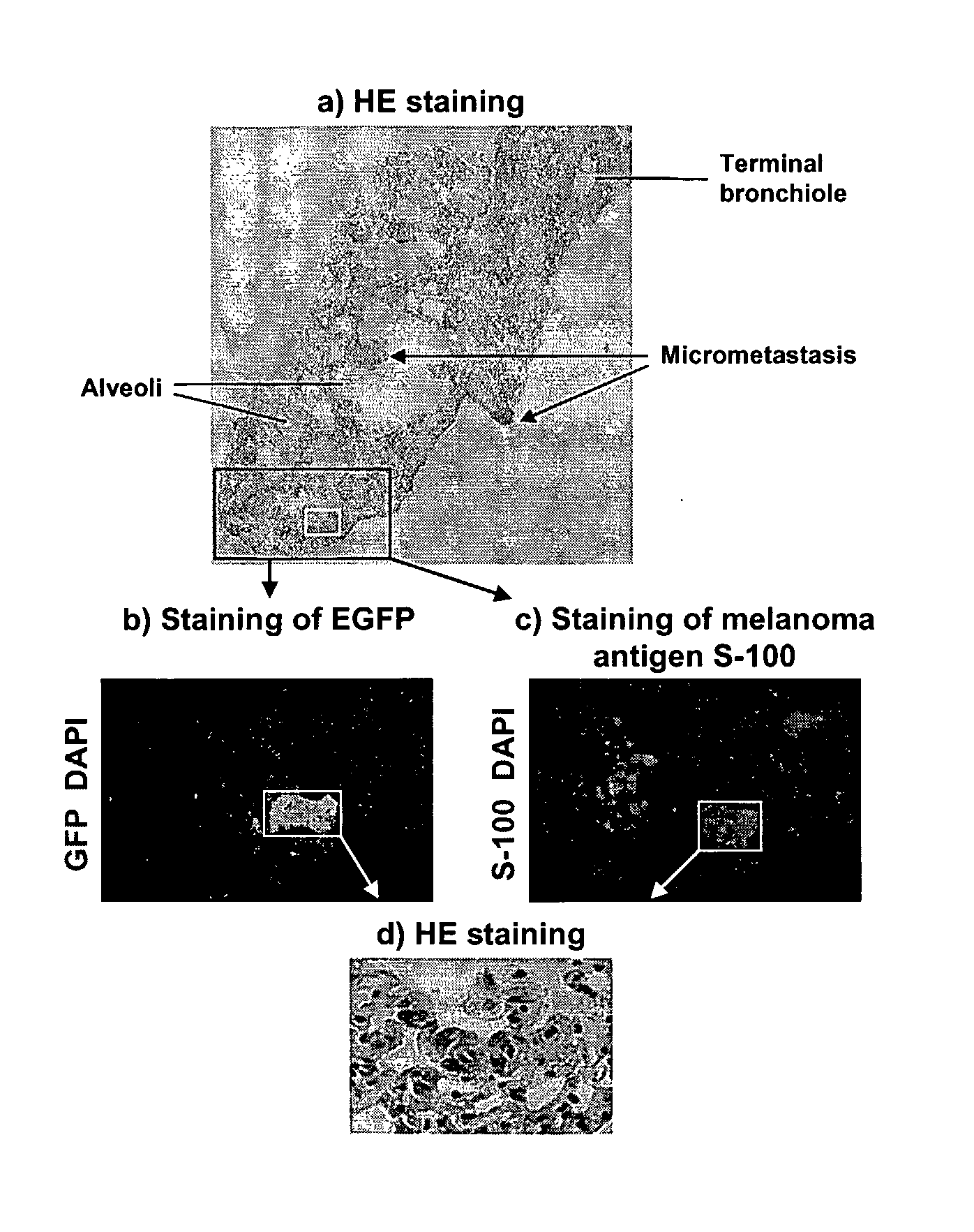
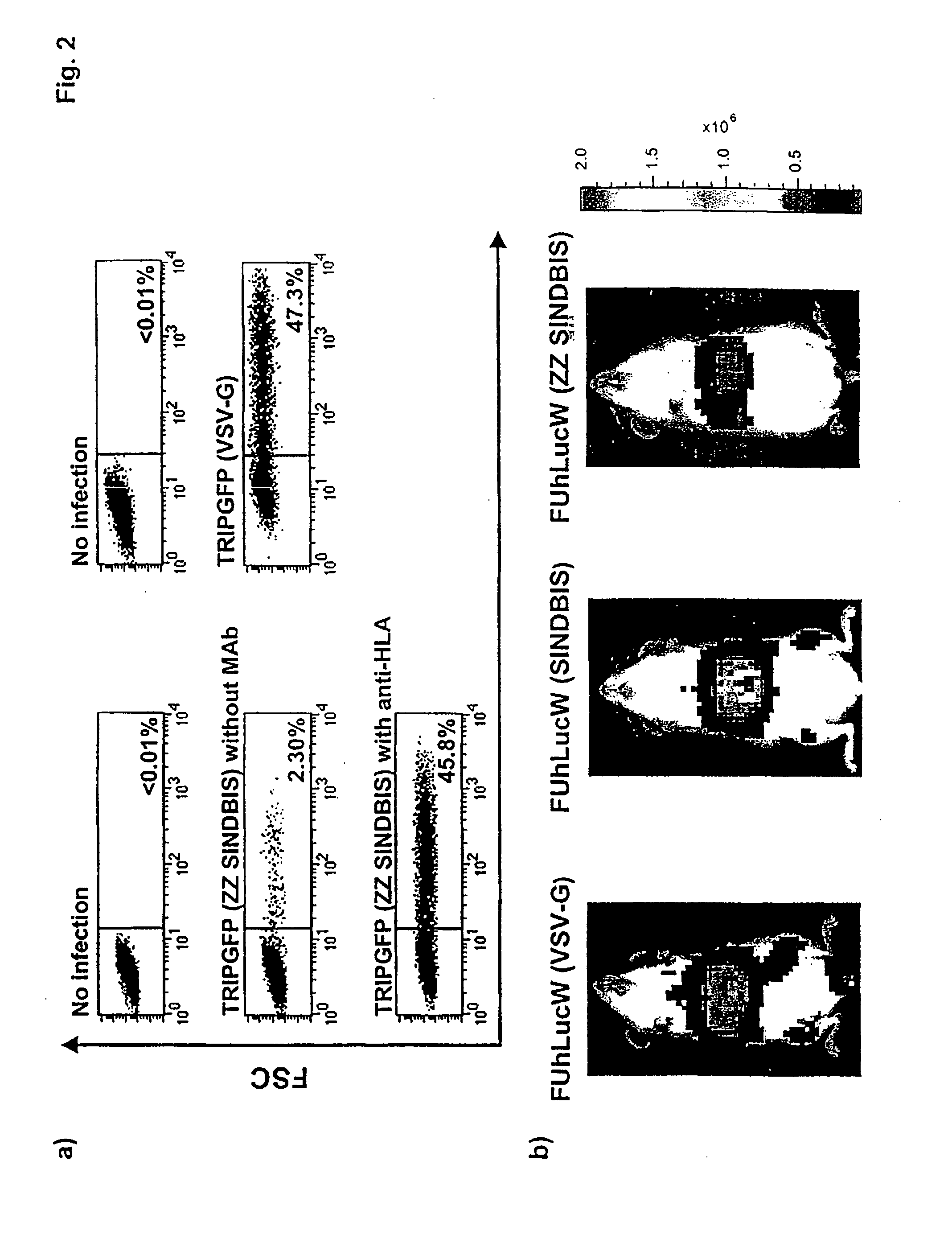
Lentivirus vectors for gene transfer to alveolar epithelial cells
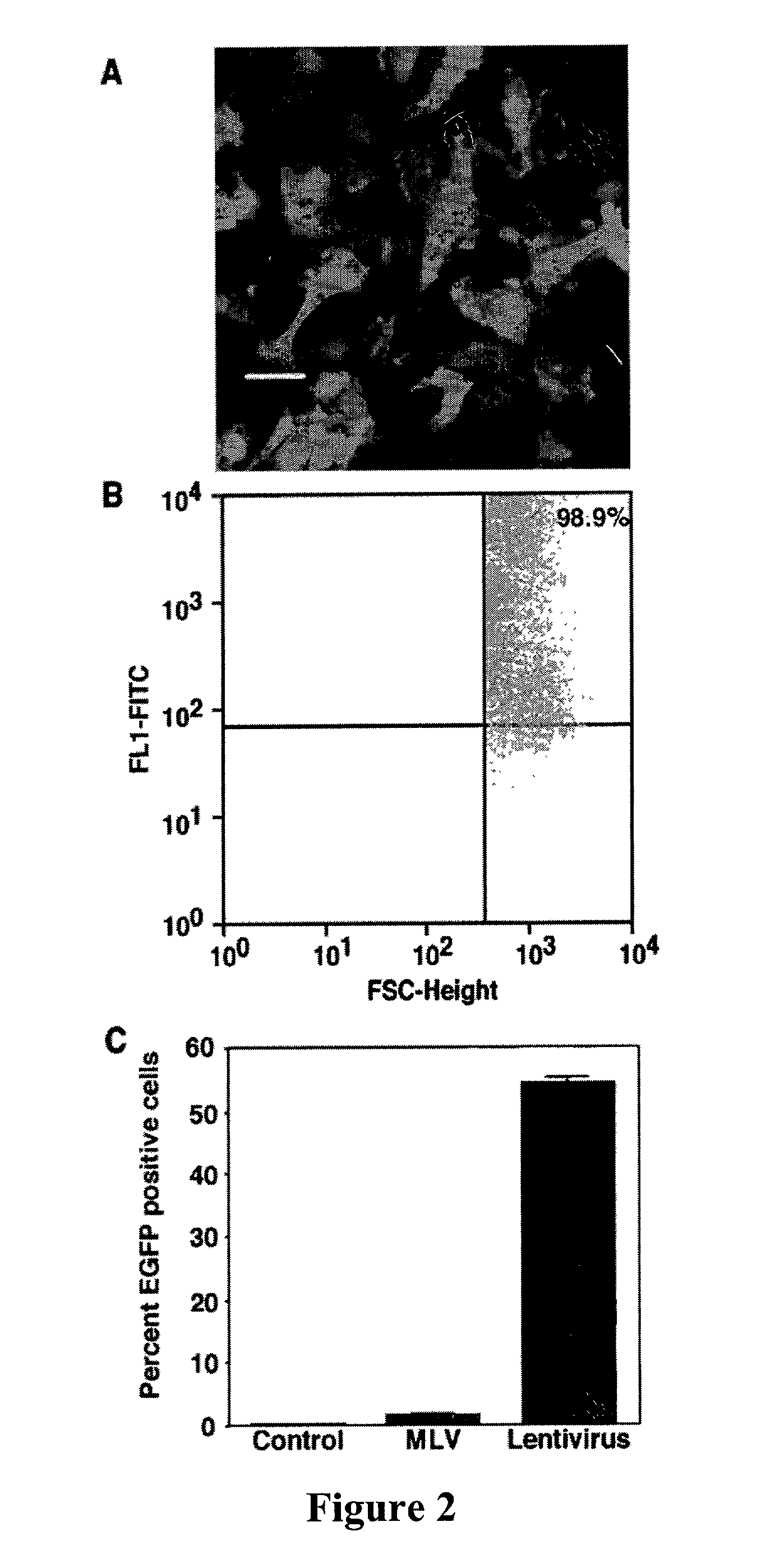
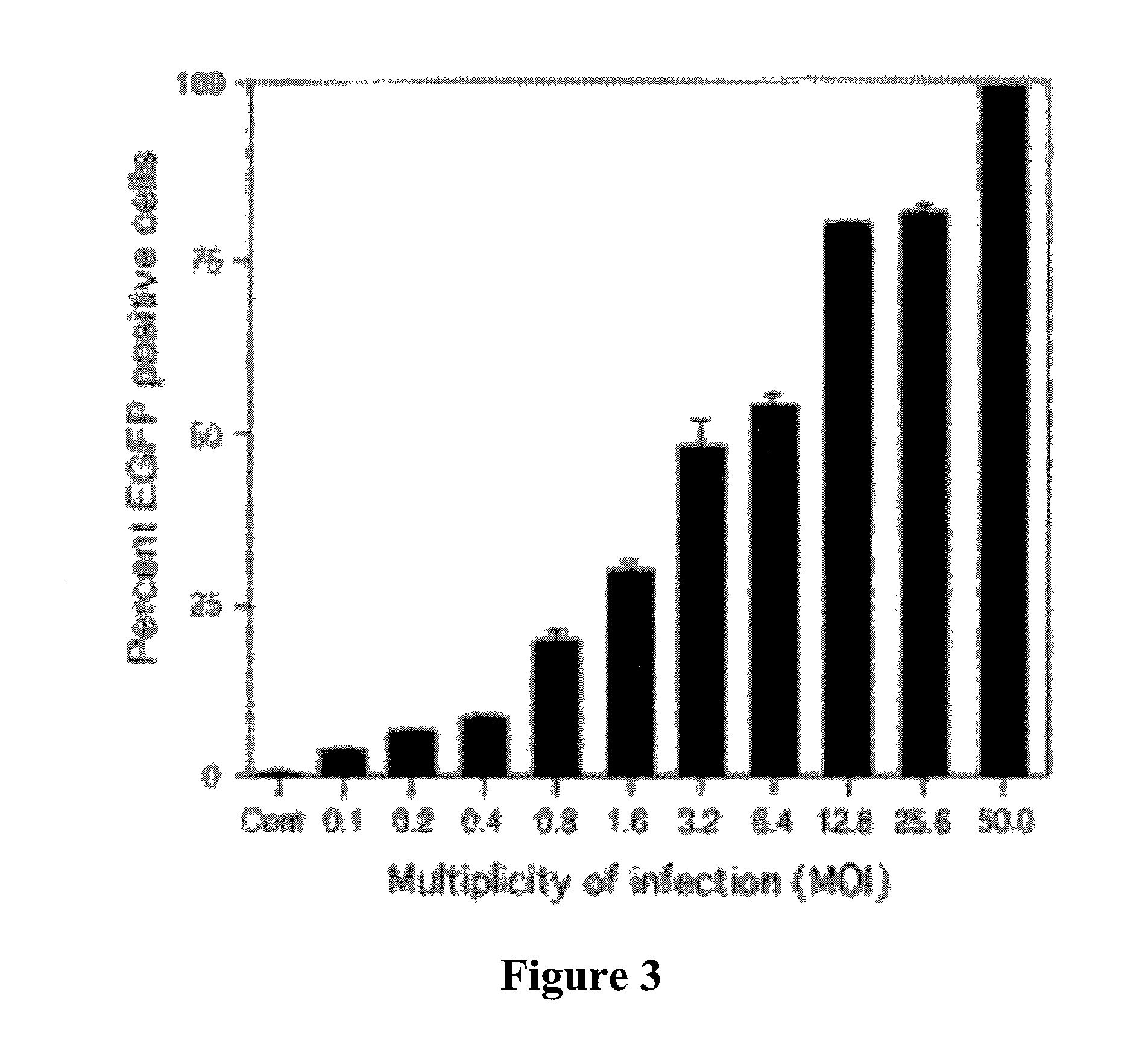
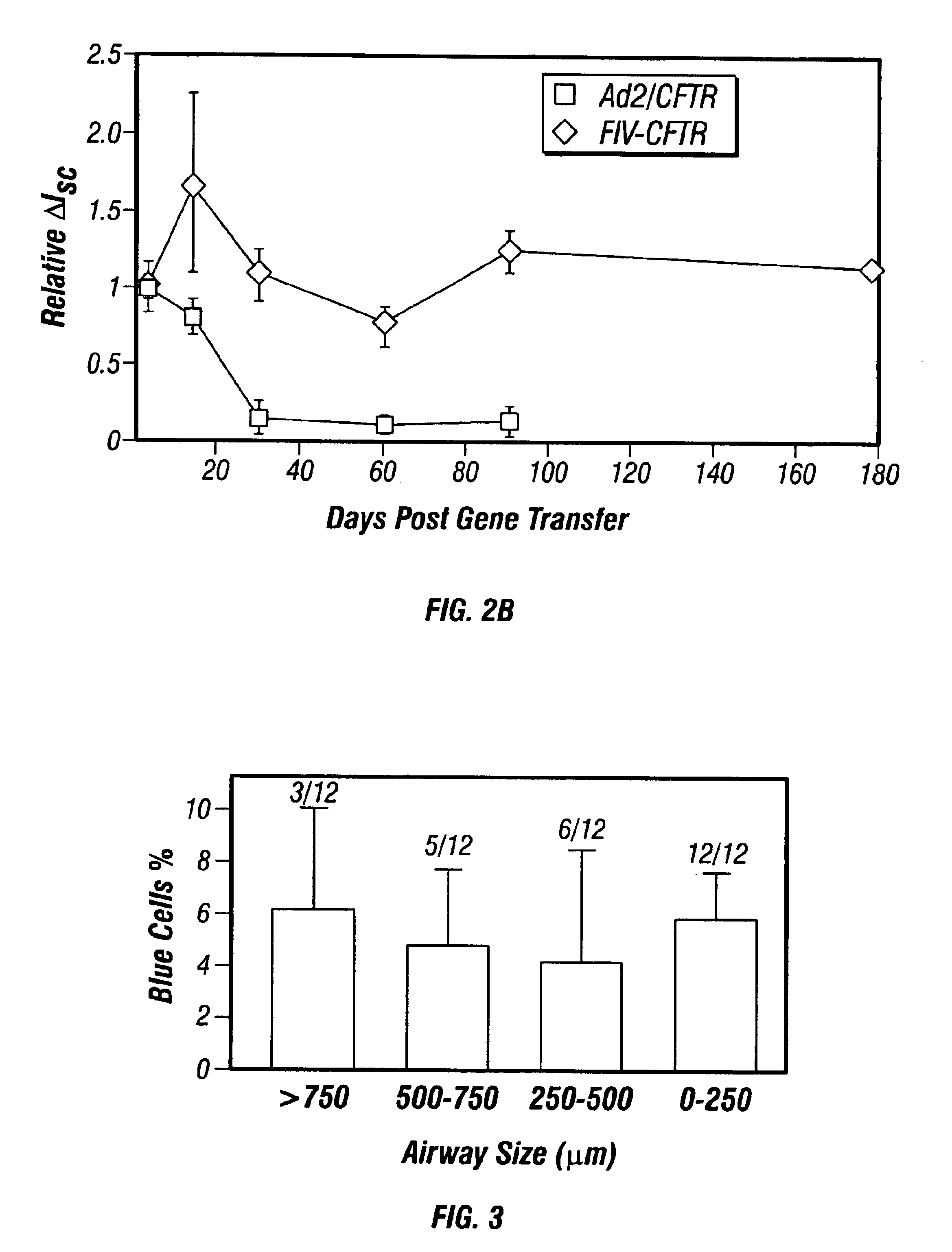
The present invention demonstrates that VSV-G-pseudotyped lentivirus vectors efficiently transduce AEC in primary culture and in vivo with transduction favored by virus application from the apical side. Transduction efficiency in AEC increased with increasing MOI and greatly exceeded that achieved with a similarly pseudotyped MLV retrovirus vector. The present invention also demonstrates the successful in vivo transfer of genes through lentivirus vector transduction. Mammals injected with lentivirus vector via the trachea expressed the reporter protein in alveolar epithelial cells within 48 to 72 hours after infection.
Owner:UNIV OF SOUTHERN CALIFORNIA
Methods and compositions for increasing the infectivity of gene transfer vectors
InactiveUS6855549B1Increased susceptibilityImprove breathabilityPowder deliveryBiocideGene transferCystic fibrosis lungs
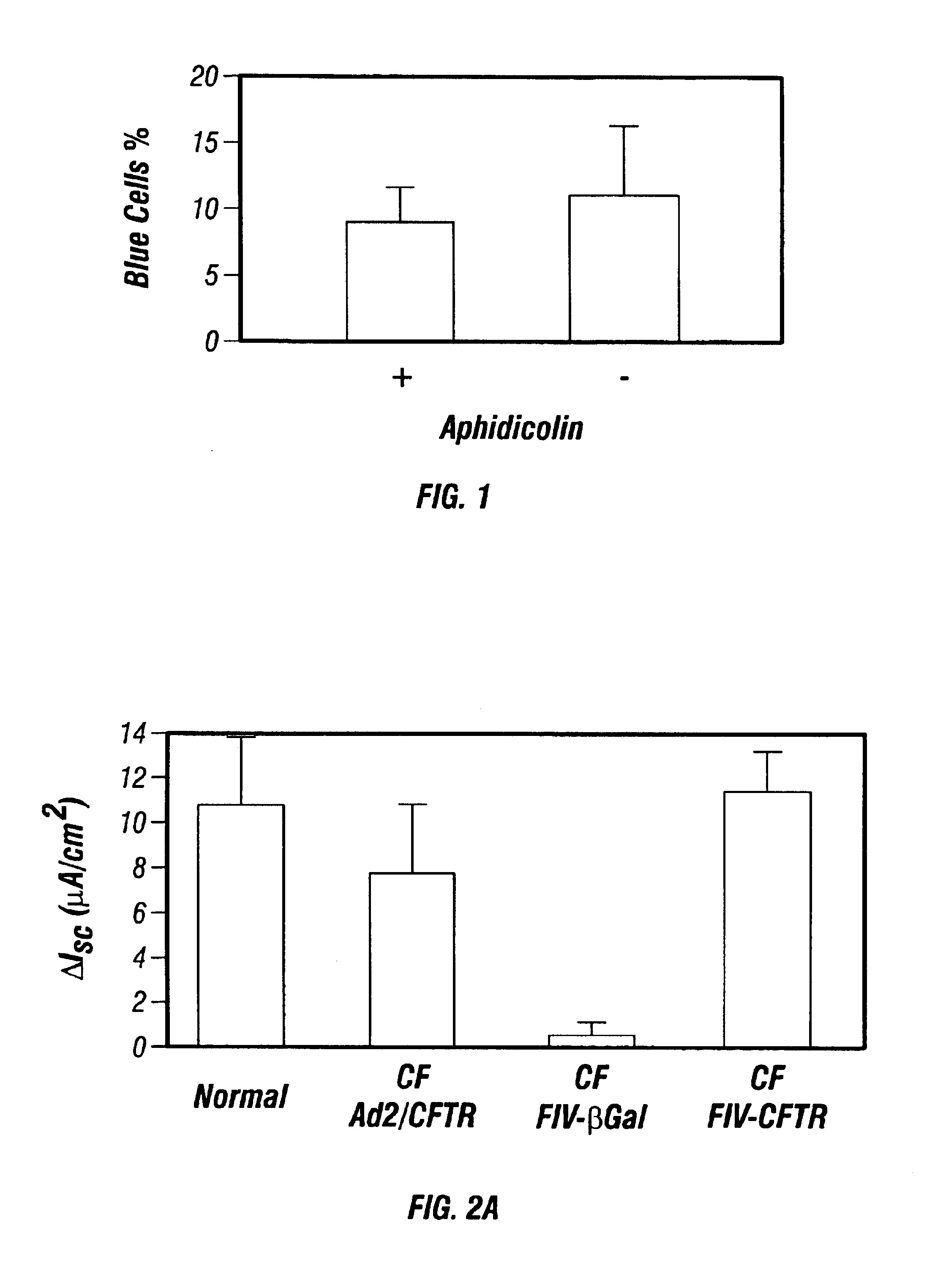
The present invention involves methods and compositions for increasing the susceptibility of target cells to transduction by gene transfer vectors. Specifically, it is proposed that increasing intracellular permeability in epithelial tissue increases the percentage of input vector that will transduce that target tissue. Specific examples show that receptors for retrovirus are preferentially accessible on the basolateral surface of airway epithelia, and permeabilizing such tissues results in greater infection with retrovirus. This has important implications in gene therapy, for example, to treat cystic fibrosis with the CFTR gene.
Owner:CHIRON CORP +1
Method of preventing virus: cell fusion by inhibiting the function of the fusion initiation region in rna viruses having class i membrane fusogenic envelope proteins
ActiveUS20060280754A1Prevent and inhibit infectionSsRNA viruses negative-senseSsRNA viruses positive-senseCell membraneDisease cause
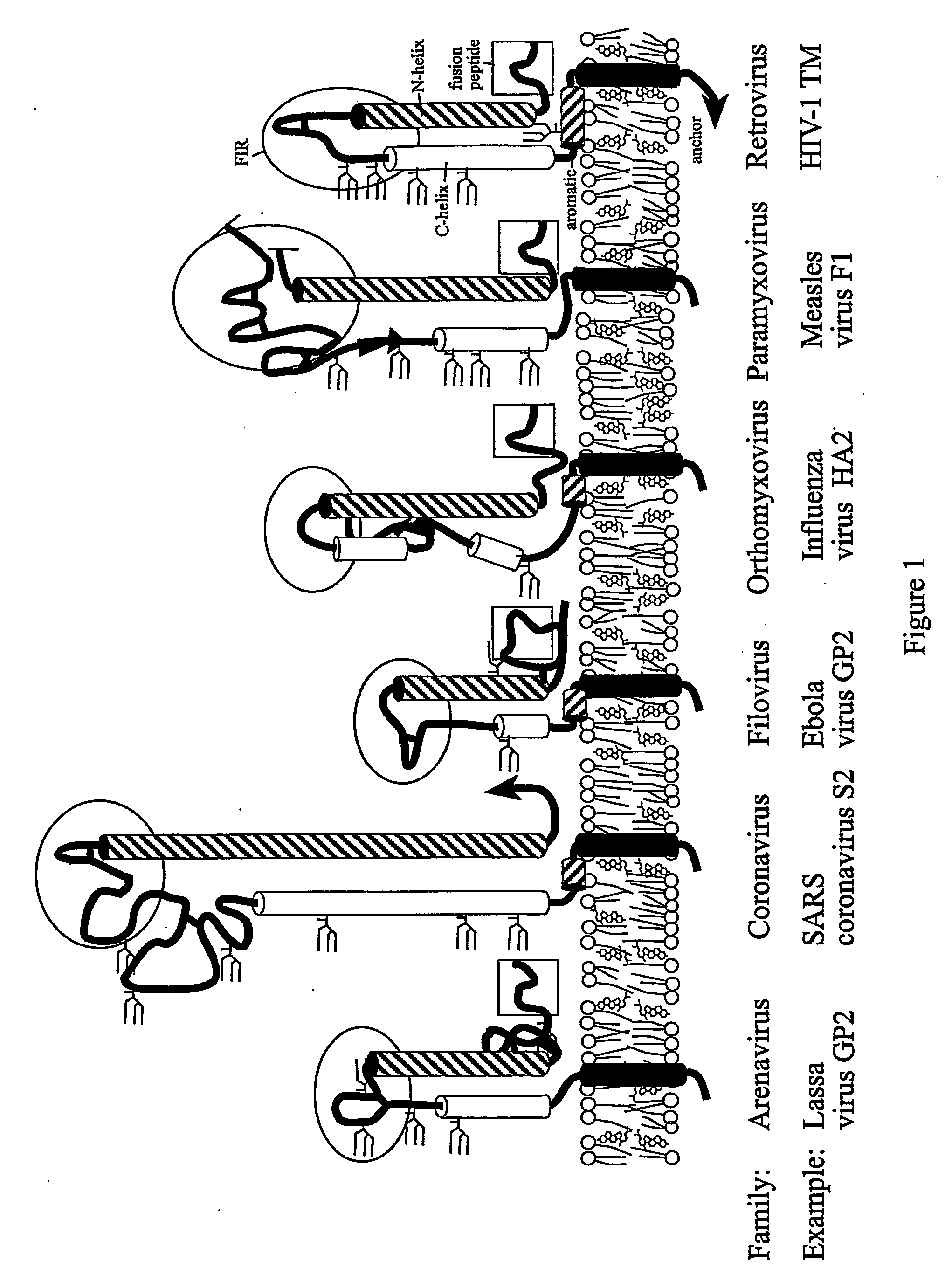
The present invention relates to a method of preventing or inhibiting viral infection of a cell and / or fusion between the envelope of a virus and the membranes of a cell targeted by the virus (thereby preventing delivery of the viral genome into the cell cytoplasm, a step required for. viral infection). The present invention particularly relates to the families of RNA viruses, including the arenaviruses, coronaviruses, filoviruses, orthomyxoviruses, paramyxoviruses, and retroviruses, having Class I membrane fusion proteins as the fusion proteins that mediate this fusion process. The present invention provides for a method of identifying a conserved motif or domain called the fusion initiation region (FIR) in these viruses. The present invention further provides for methods of preventing infection by such viruses, by interfering with their FIR. The present invention further provides for methods of treatment and prophylaxis of diseases induced by such viruses.
Owner:TULANE EDUCATIONAL FUND THE ADMINISTRATORS OF THE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com