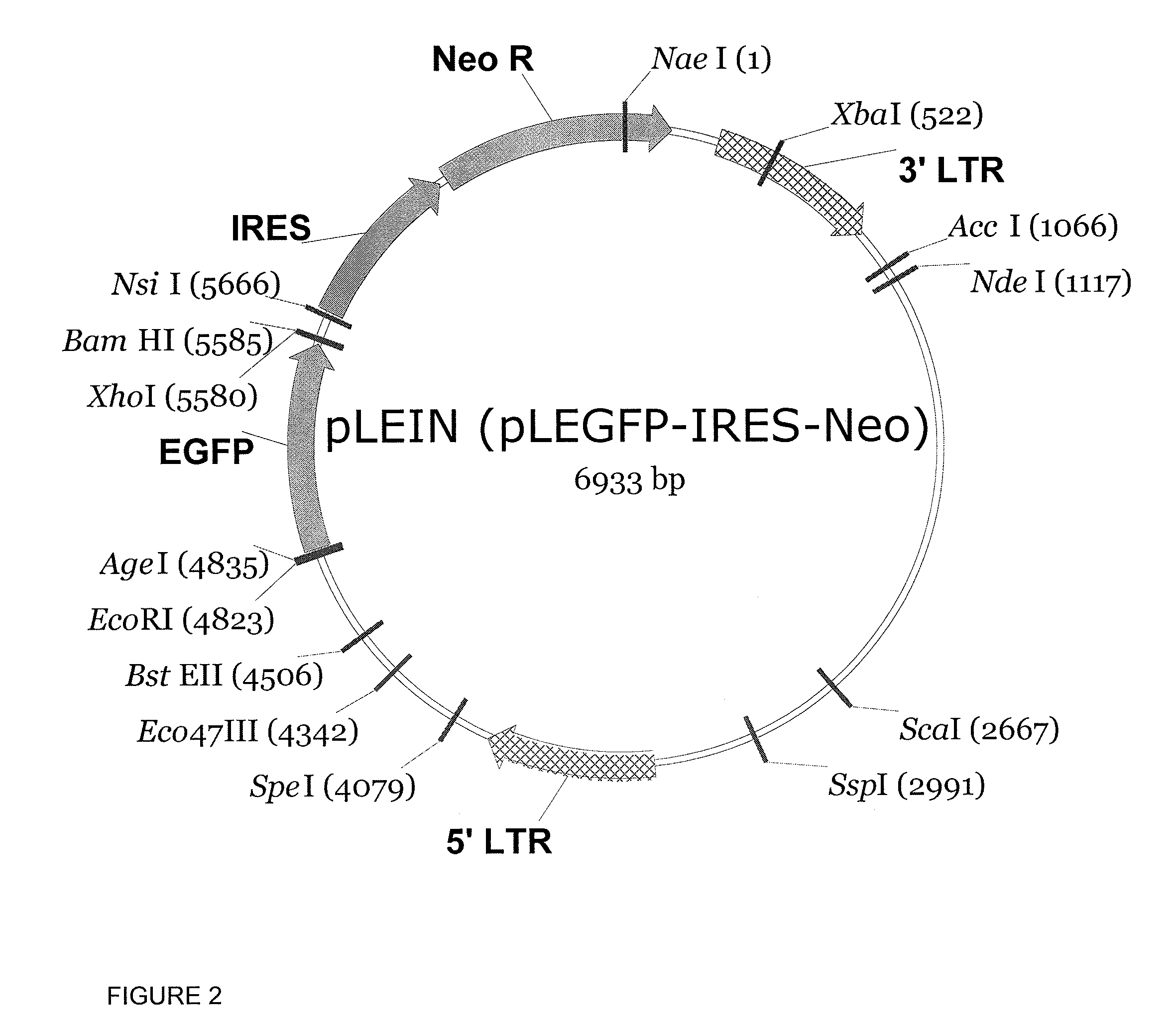
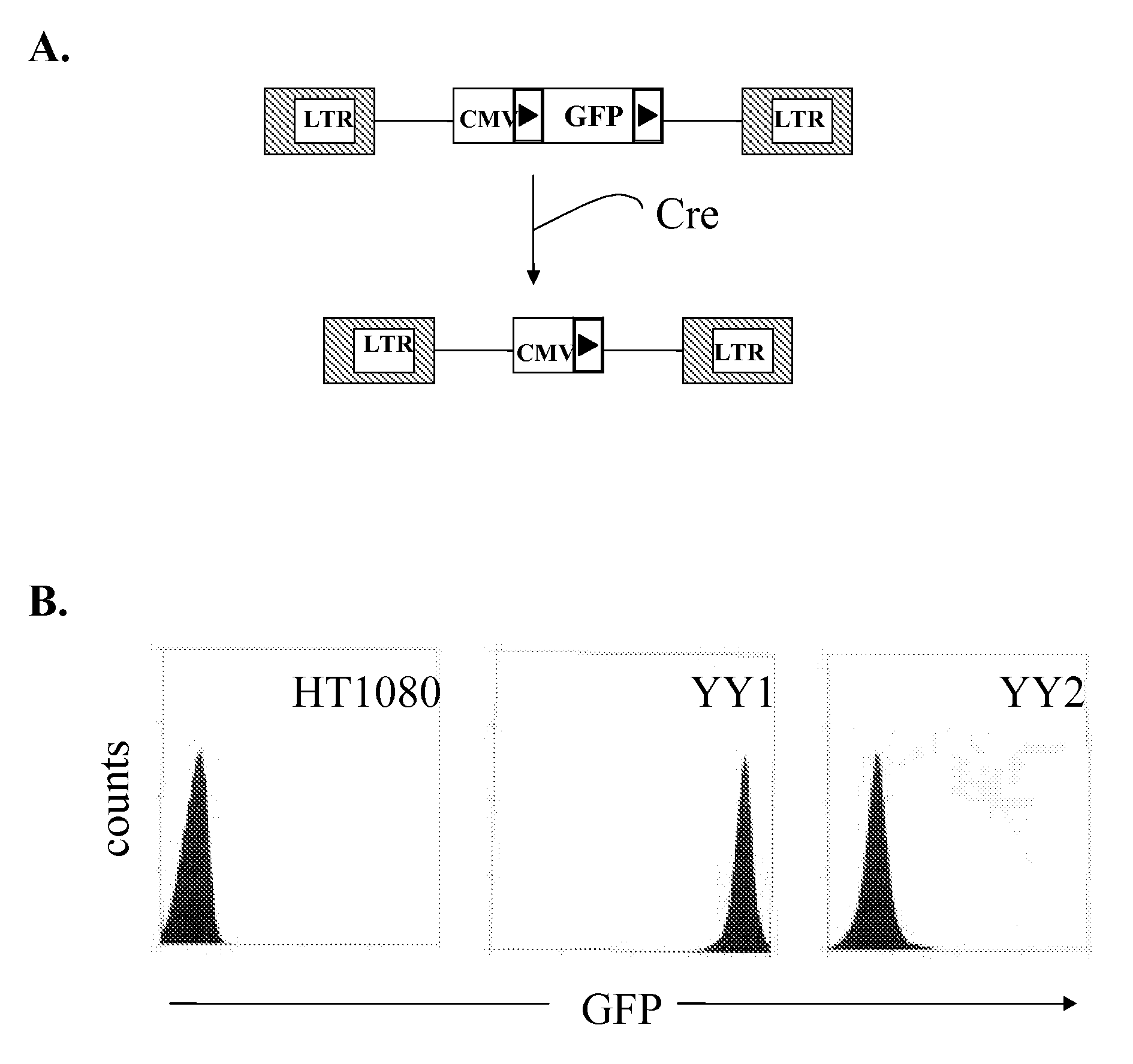
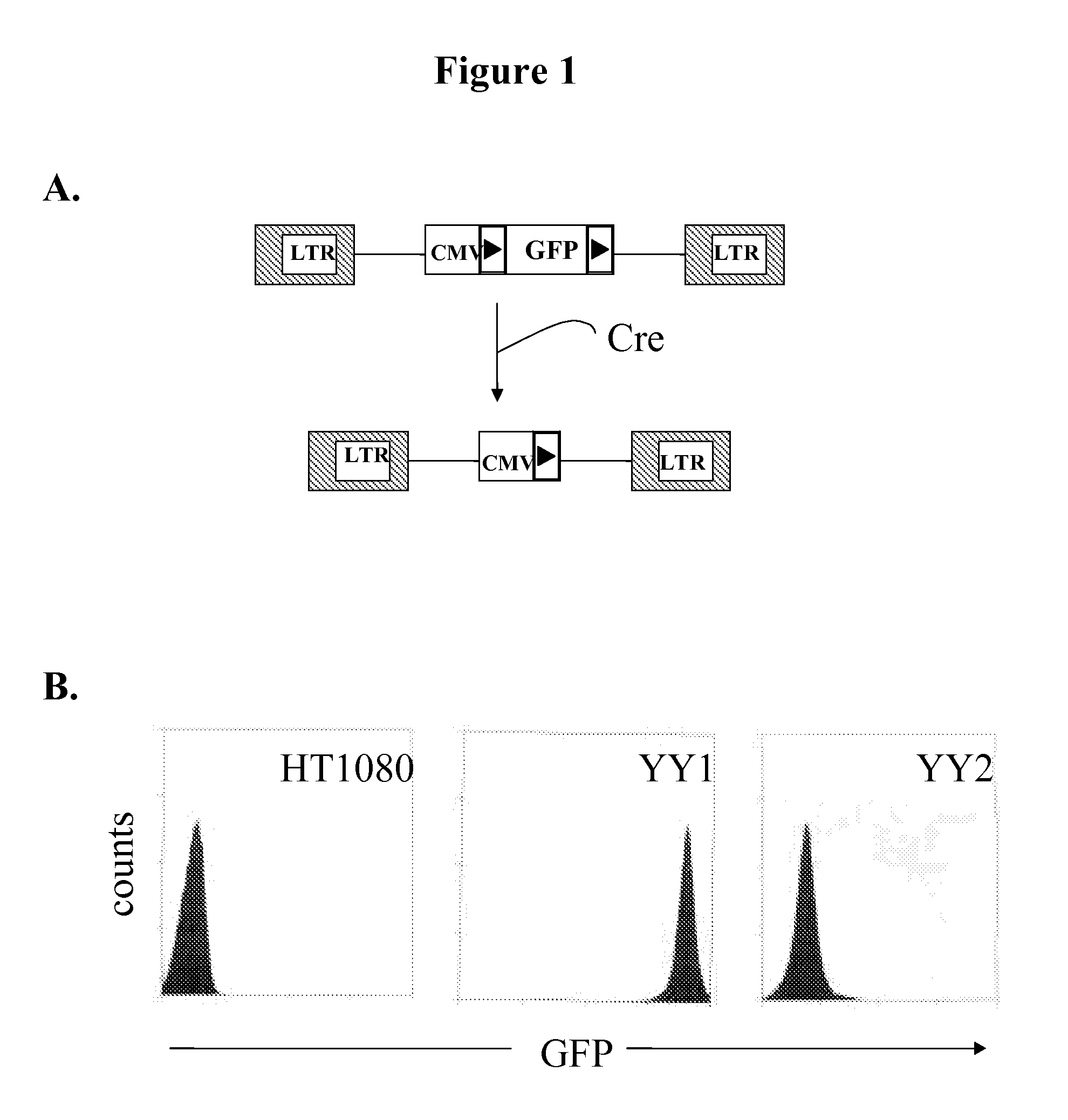
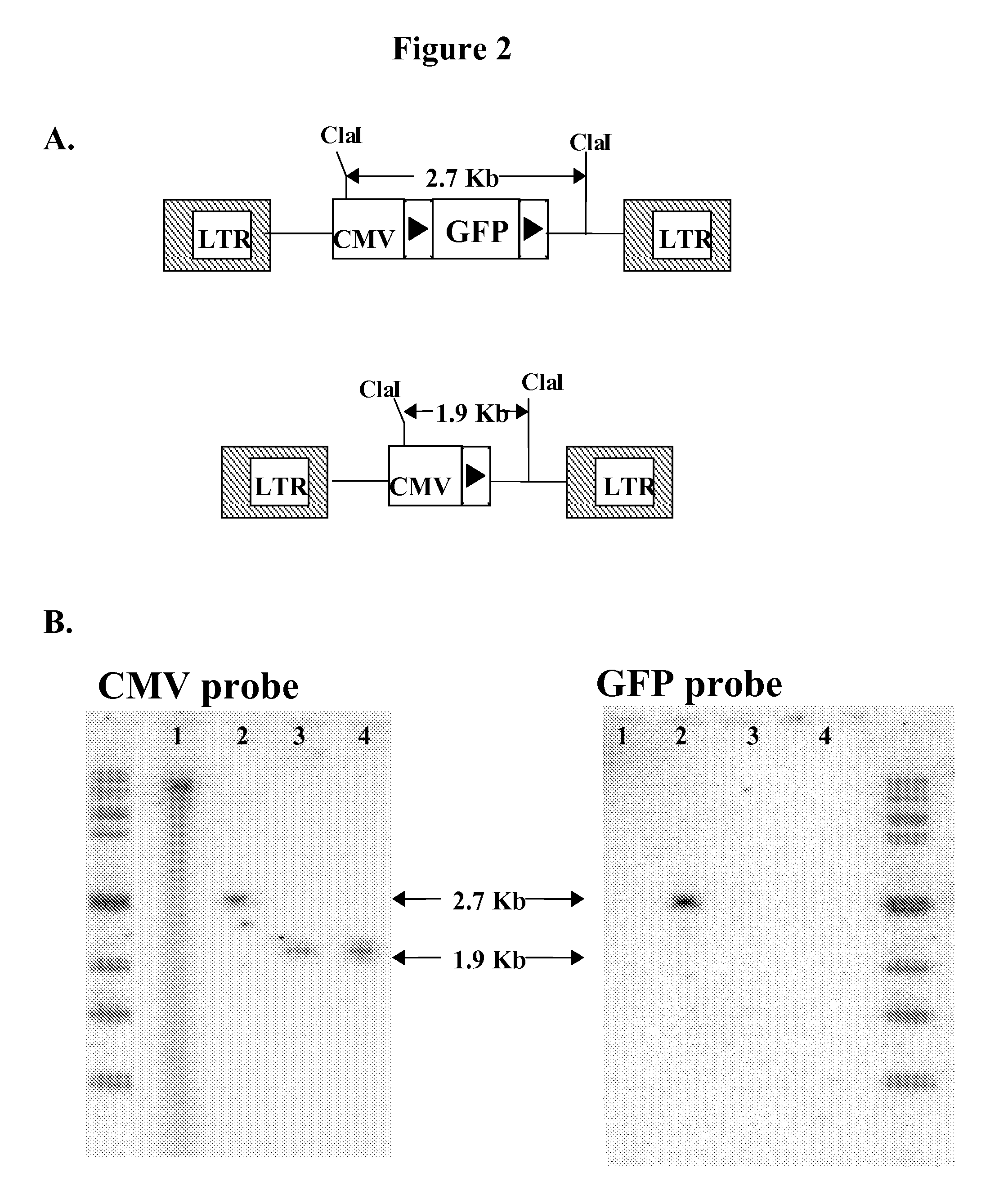
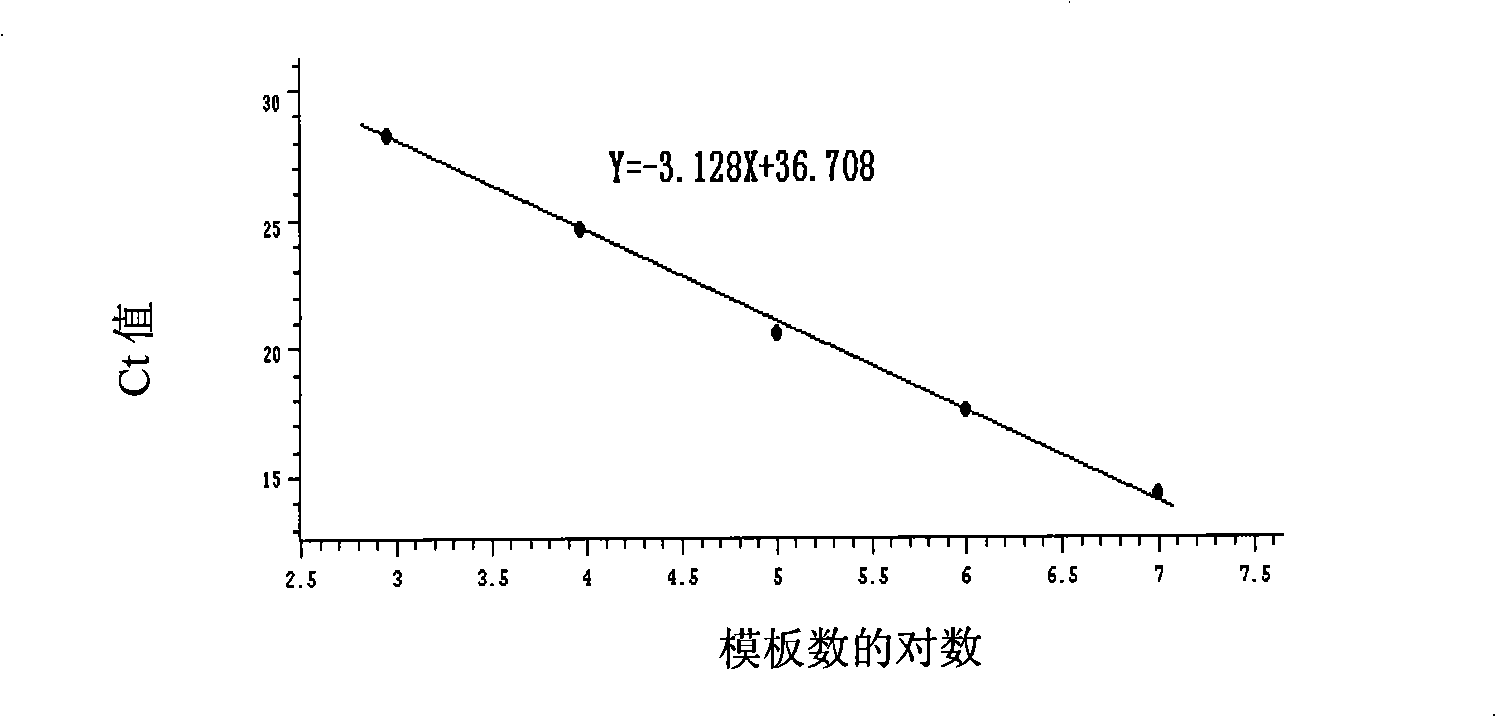
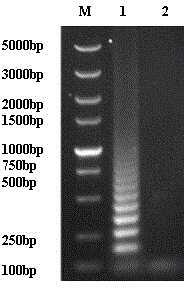
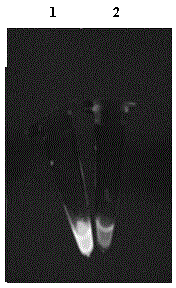
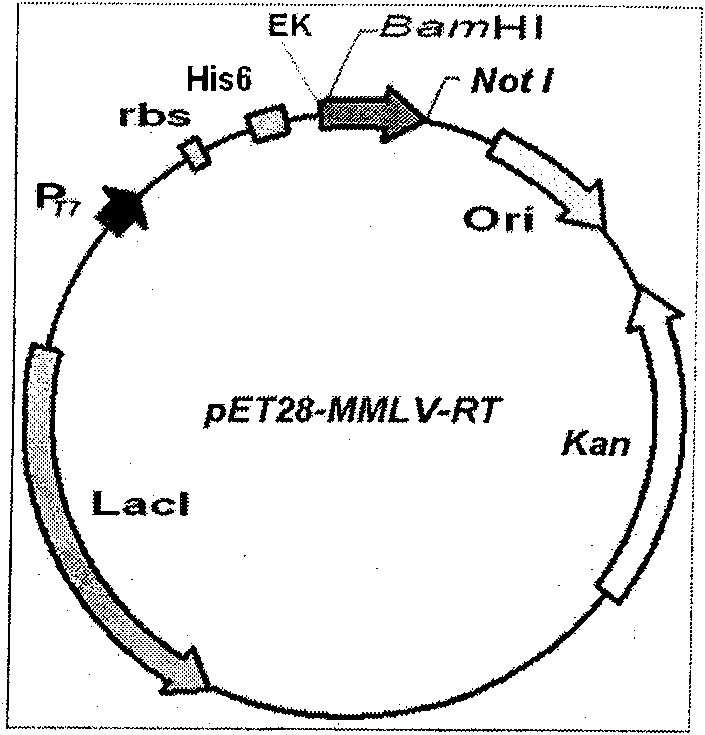
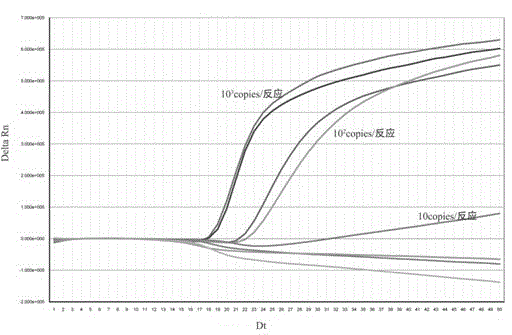
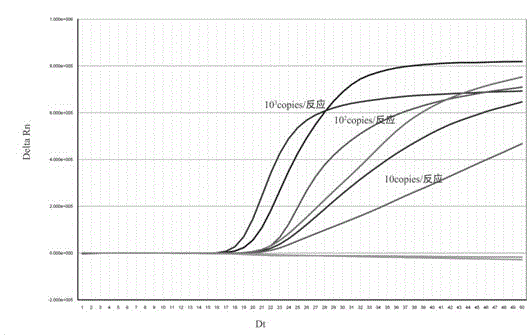
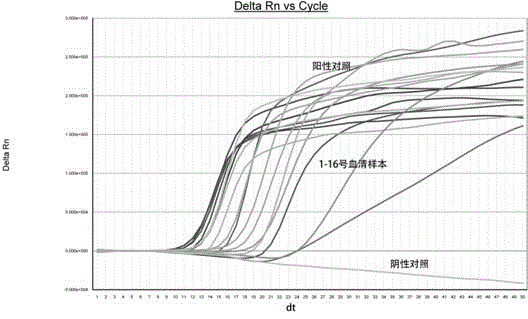
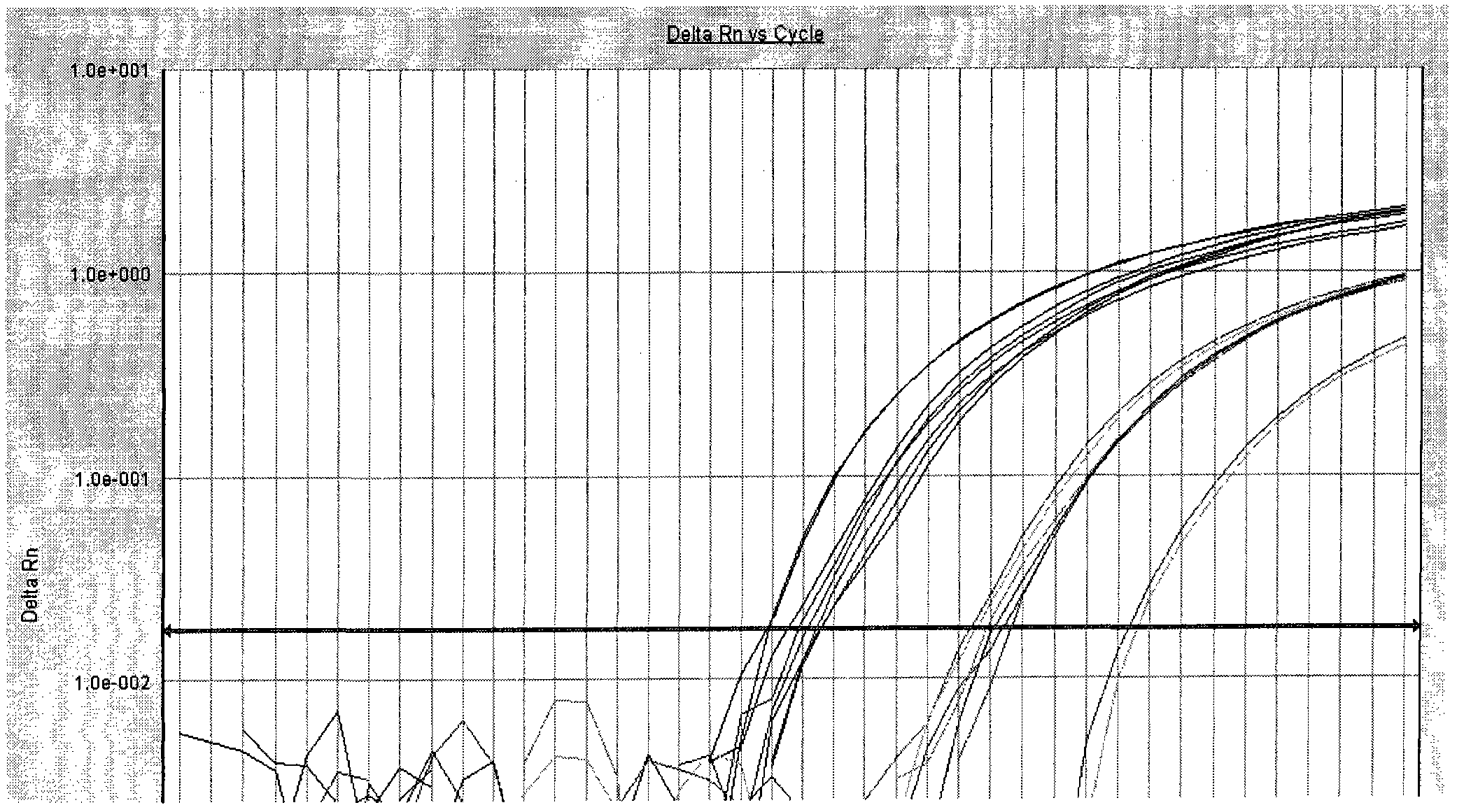
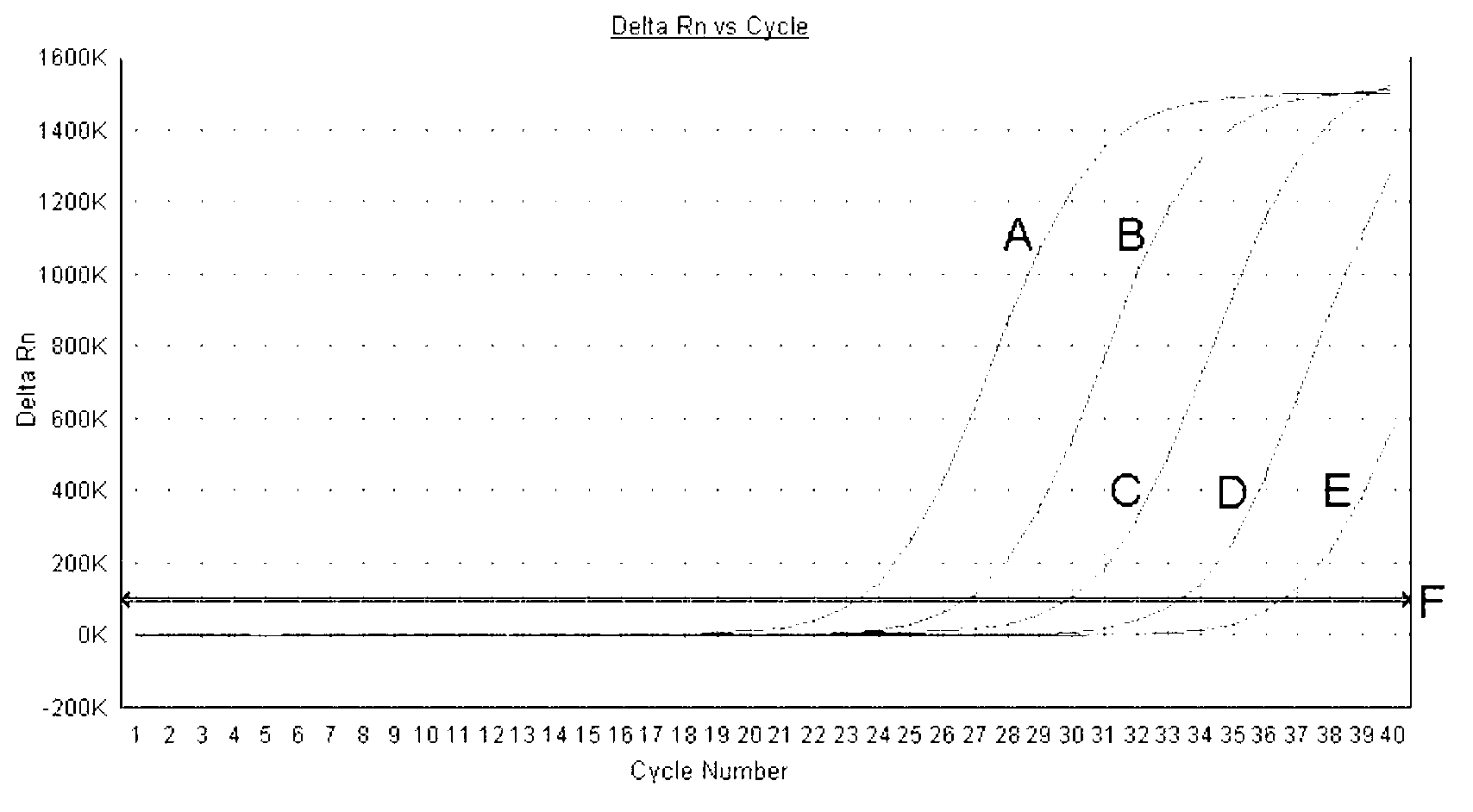
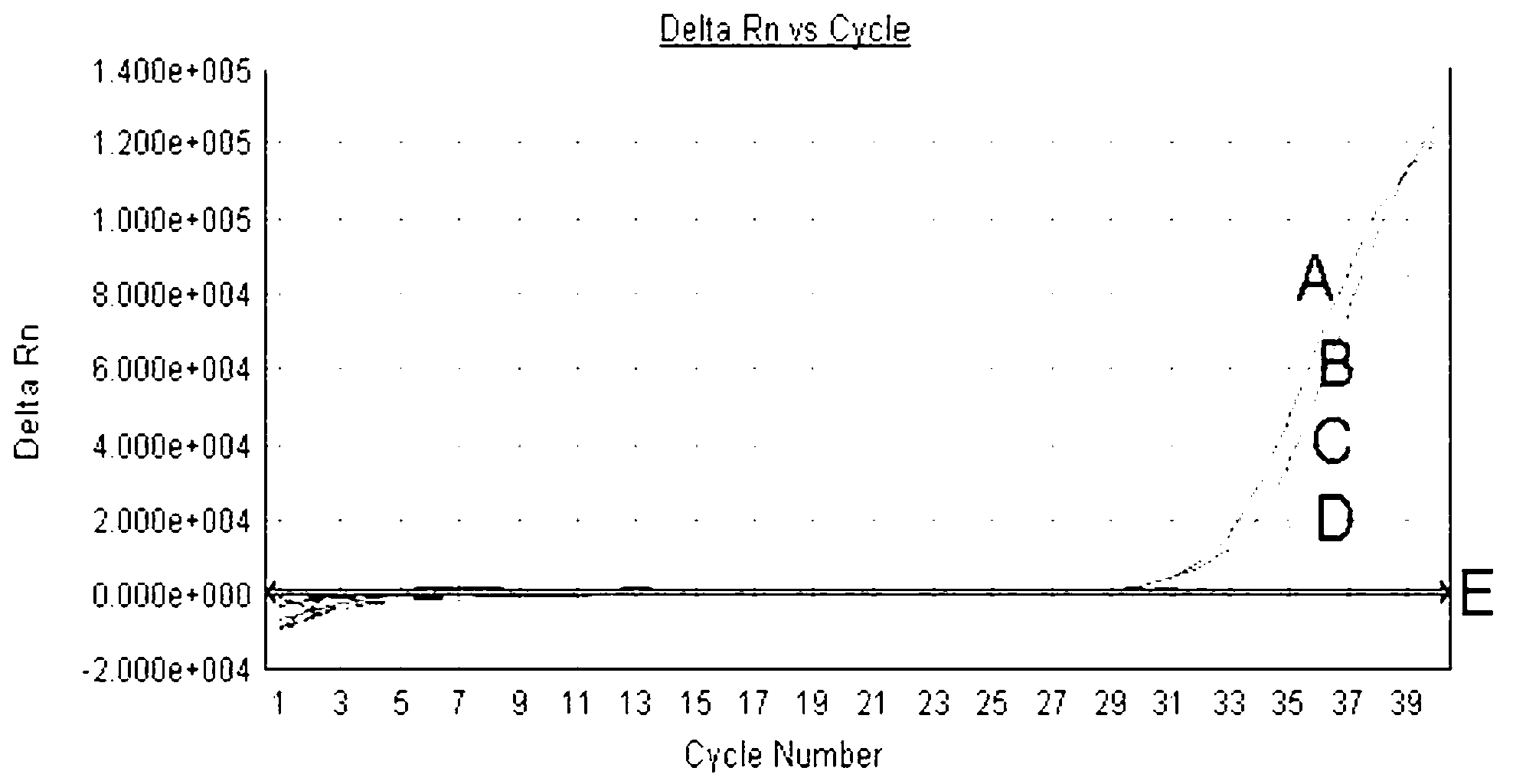
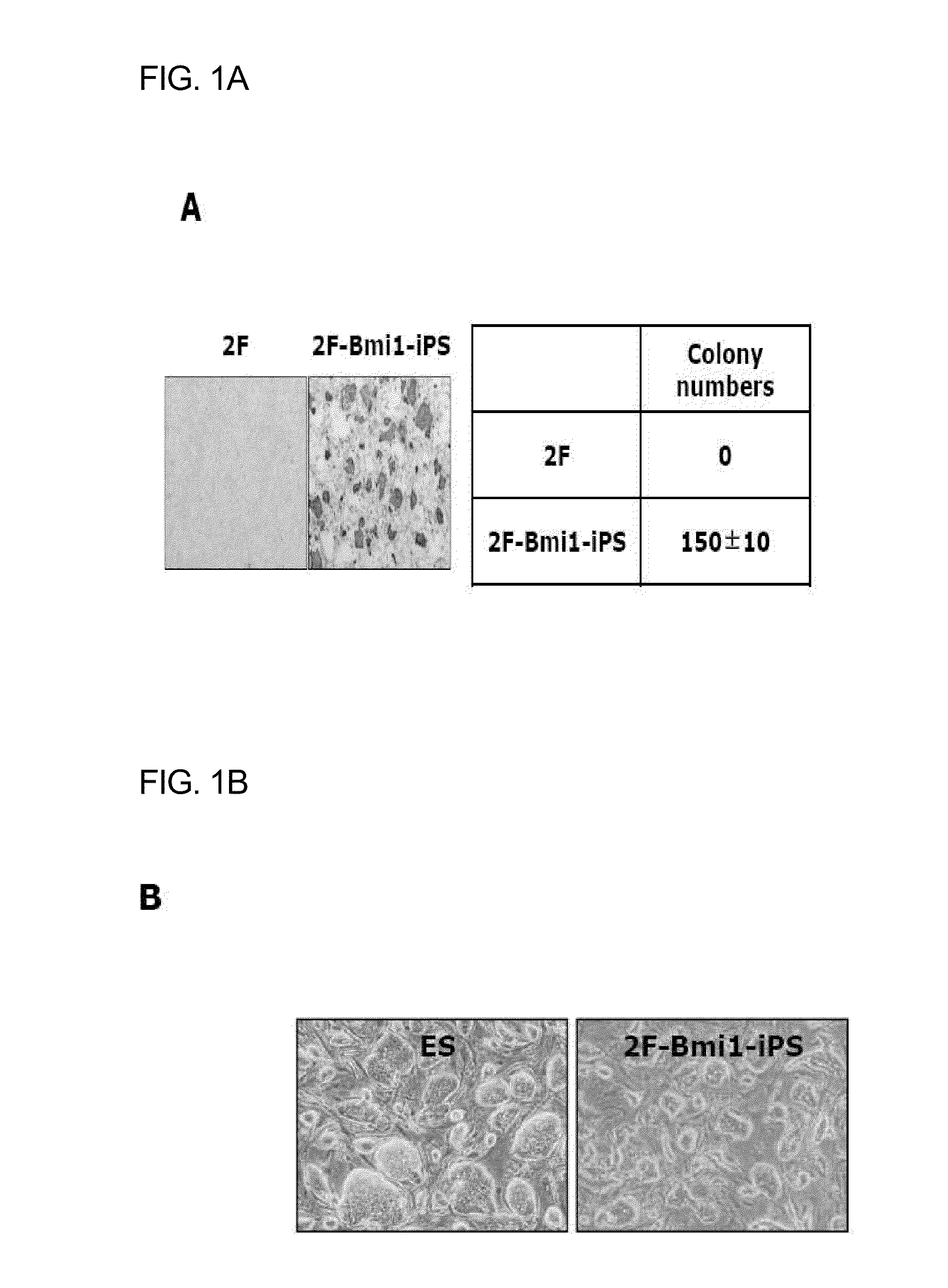
Patents
Literature
77 results about "Murine leukemia virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
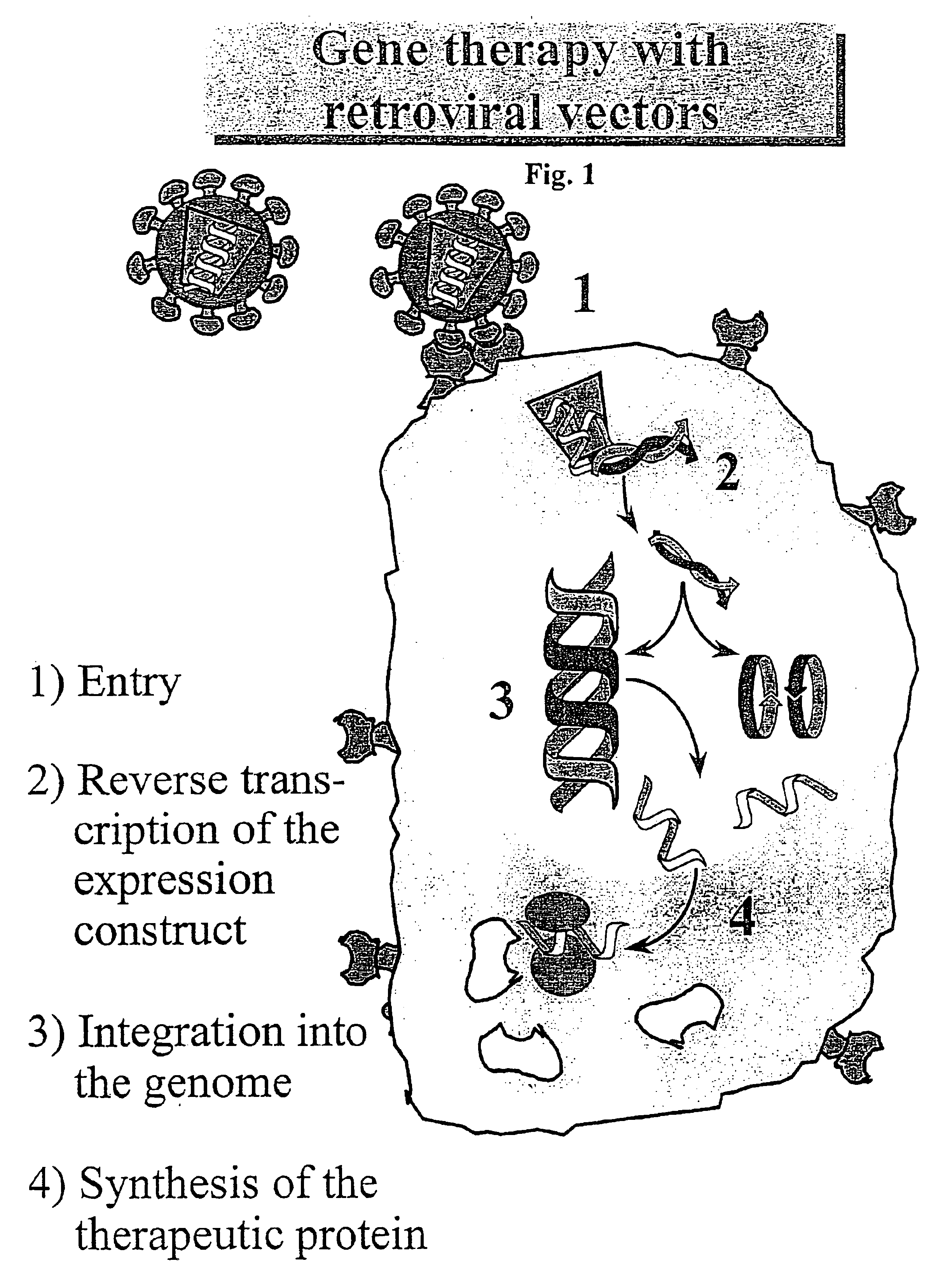
The murine leukemia viruses (MLVs or MuLVs) are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.
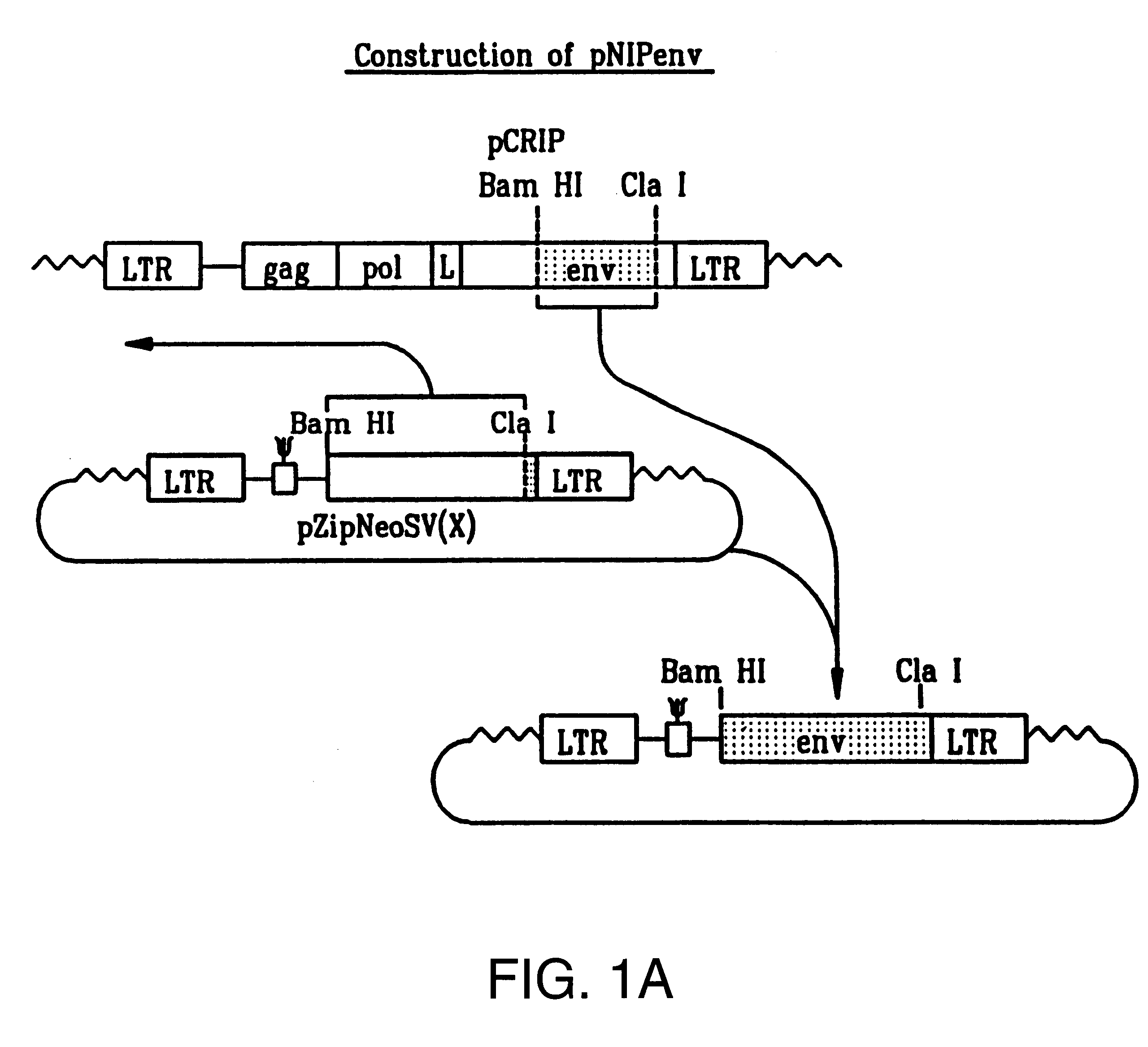
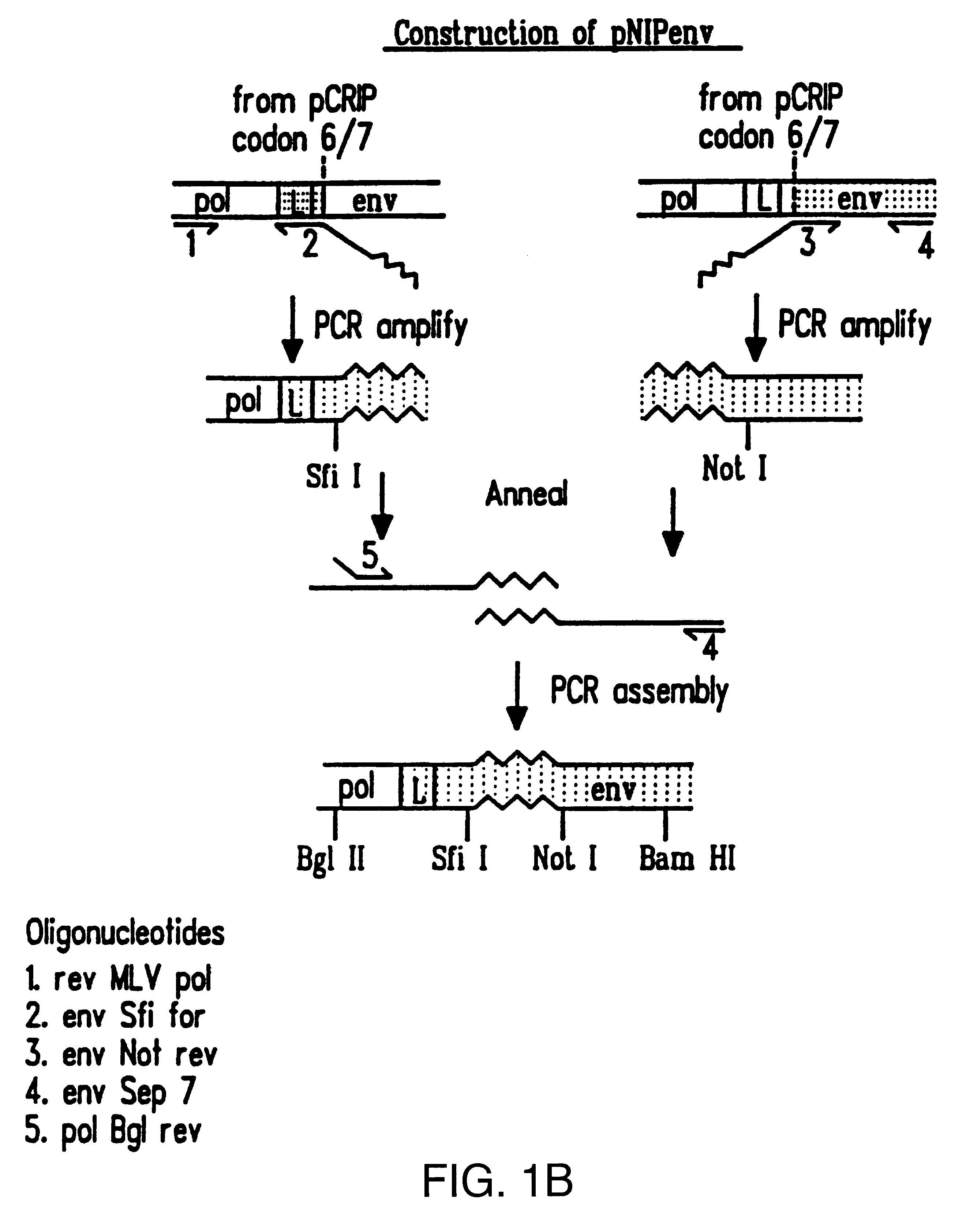
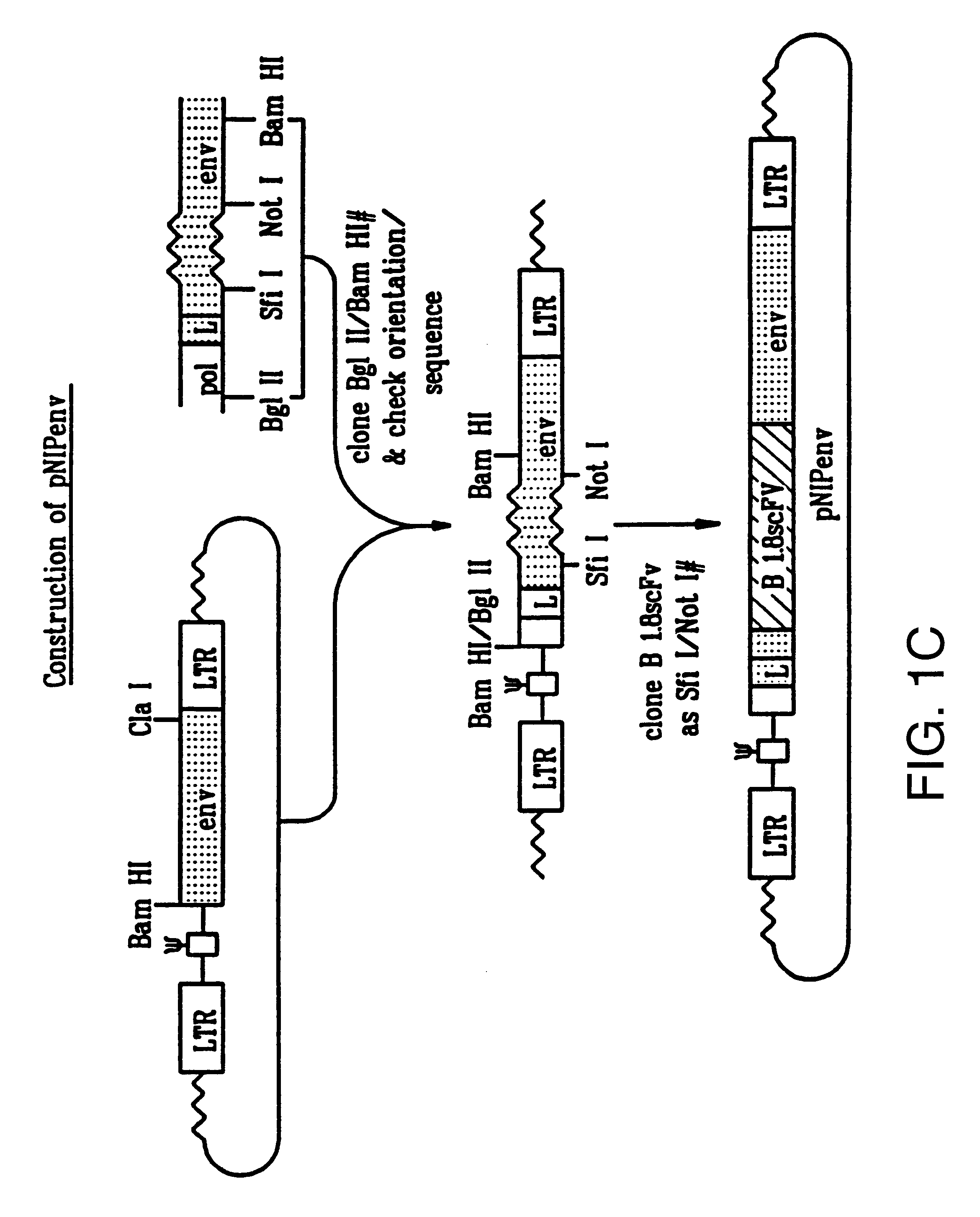
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
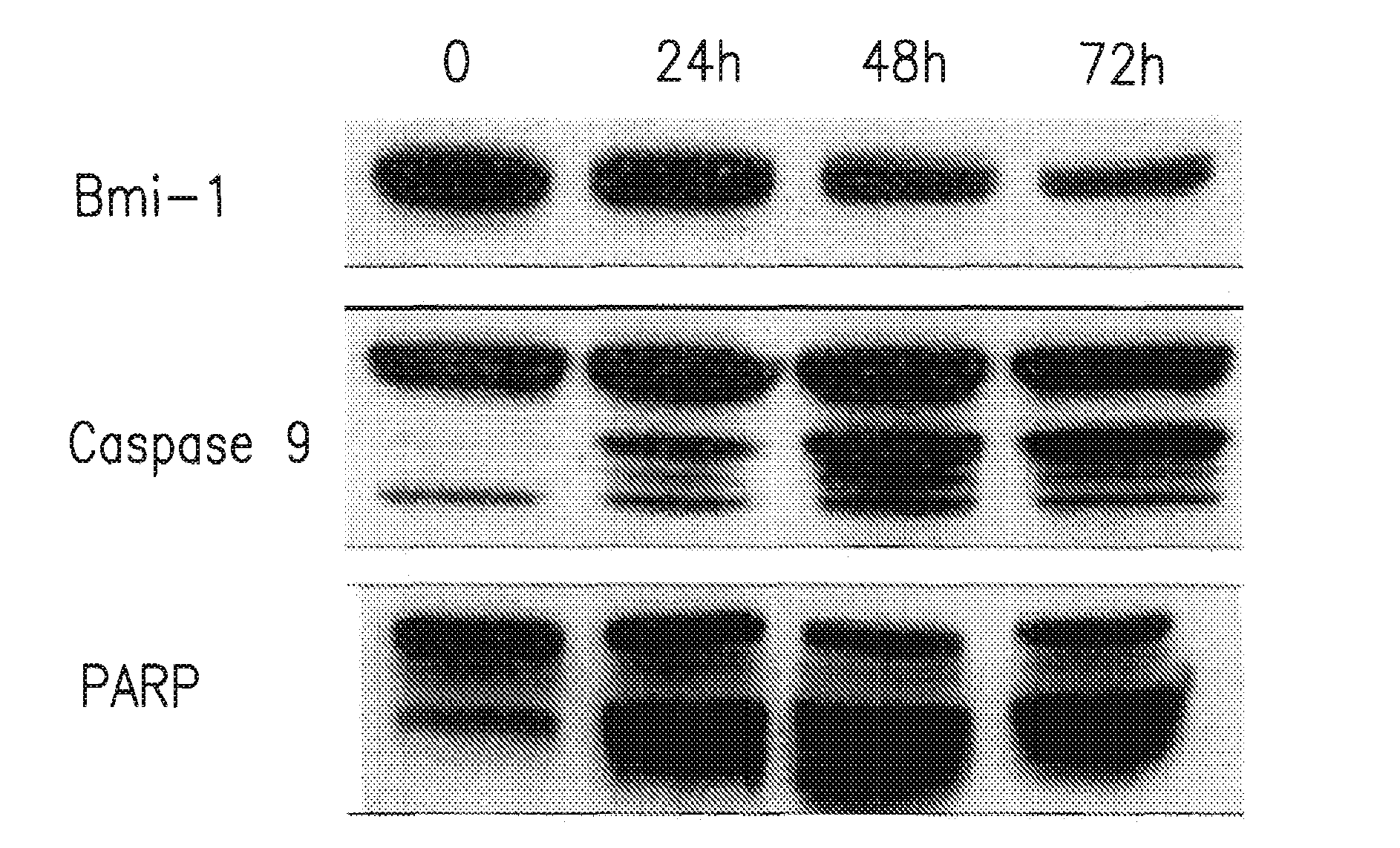
Bmi-1 protein expression modulators
The compounds, pharmaceutical compositions, and methods of using such compounds or compositions thereof described herein are useful for treating a disease modulated by B-cell specific Moloney murine leukemia virus integration site 1 (Bmi-1) protein expression.
Owner:PTC THERAPEUTICS INC
Momlv-based pseudovirion packaging cell line
The present invention discloses Moloney murine leukemia virus (MoMLV)-based viral packaging cell line for the production of anti-viral vaccines. The invention also includes methods of making, administering and formulating pseudovirions and replicon deficient viral particles of the invention and methods of inducing immunity.
Owner:BIOPROTECTION SYST
Novel lentiviral vectors for site-specific gene insertion
ActiveUS20080200663A1Easy to reorganizeSugar derivativesGenetic material ingredientsGene deliveryTherapy Trial
Murine leukemia virus (MLV) and lentivirus vectors have been used previously to deliver genes to hematopoietic stem cells (HSCs) in human gene therapy trials. However, these vectors integrate randomly into the host genome, leading to disruption or inactivation of vital host genes. The present invention discloses a novel lentiviral vector system that overcomes this problem by integrating into a host genome in a site-specific manner.
Owner:CITY OF HOPE
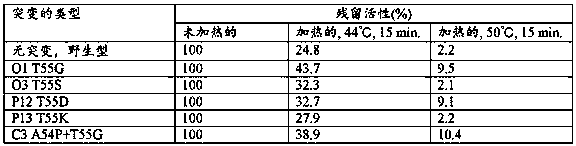
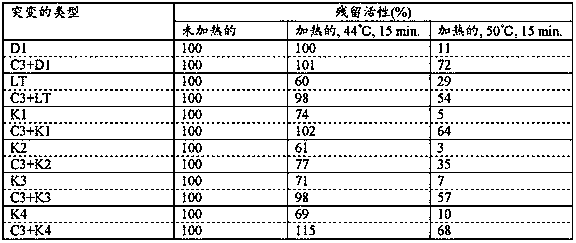
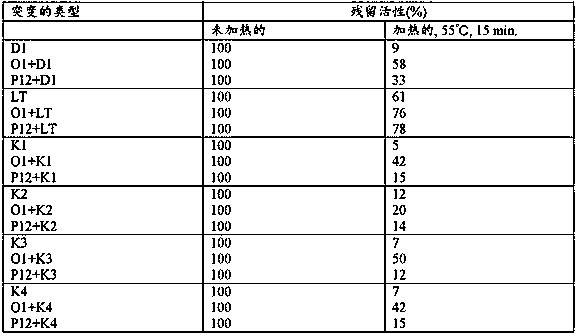
Heat-resistant reverse transcriptase mutant
Provided are: a reverse transcriptase mutant including an amino acid mutation at a position corresponding to position 55 of the amino acid sequence of wild-type reverse transcriptase derived from the Moloney murine leukemia virus, wherein the reverse transcriptase mutant is characterized in that the amino acid mutation is a substitution from threonine to another amino acid, and the other amino acid is selected from the group consisting of amino acids having a nonpolar aliphatic side chain and amino acids having a polar acidic functional group side chain; a nucleic acid that encodes the mutant; a method for producing the mutant and the nucleic acid that encodes the mutant; a method for synthesizing cDNA in which the mutant is used; and a composition and kit including the mutant.
Owner:TAKARA HOLDINGS
Retroviral vectors, methods for their preparation and their use for gene transfer into CD4-positive cells
InactiveUS6902929B1Improve efficiencyEasy constructionBiocideGenetic material ingredientsCell specificImmunodeficiency virus
The invention relates to the production and use of retroviral vectors for cell specific gene transfer, specially to a production method of retroviral vectors containing capsid particles of murine leukemia virus (MLV) and envelope proteins of human immunodeficiency vises (HIV) or simian immunodeficiency viruses (SIV). Said vectors can be used for gene transfer in selected cell types, specially in CD4-positive mammal cells.
Owner:BUNDESREPUBLIK DEUTLAND LETZTVERTRETEN DURCH DEN PRASIDENTEN DES PAUL EHRLICH INSTITUTS
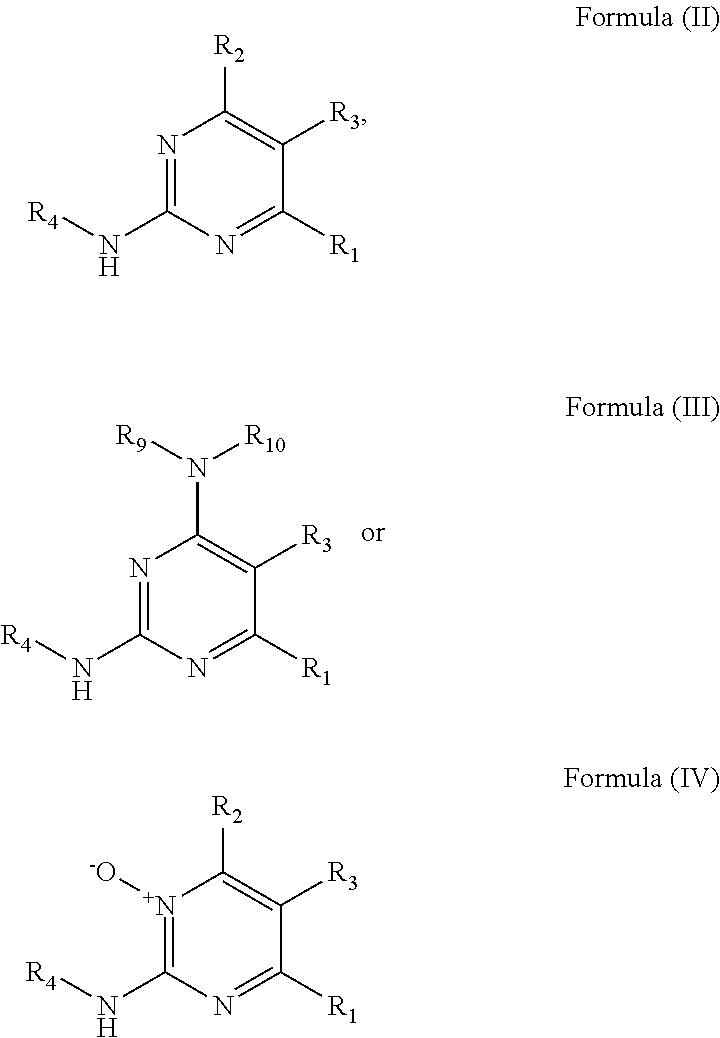
Substituted reverse pyrimidine bmi-1 inhibitors
Amine substituted reverse pyrimidine compounds and forms thereof that inhibit the function and reduce the level of B-cell specific Moloney murine leukemia virus integration site 1 (Bmi-1) protein and methods for their use to inhibit Bmi-1 function and reduce the level of Bmi-1 to treat a cancer mediated by Bmi-1 are described herein.
Owner:PTC THERAPEUTICS INC
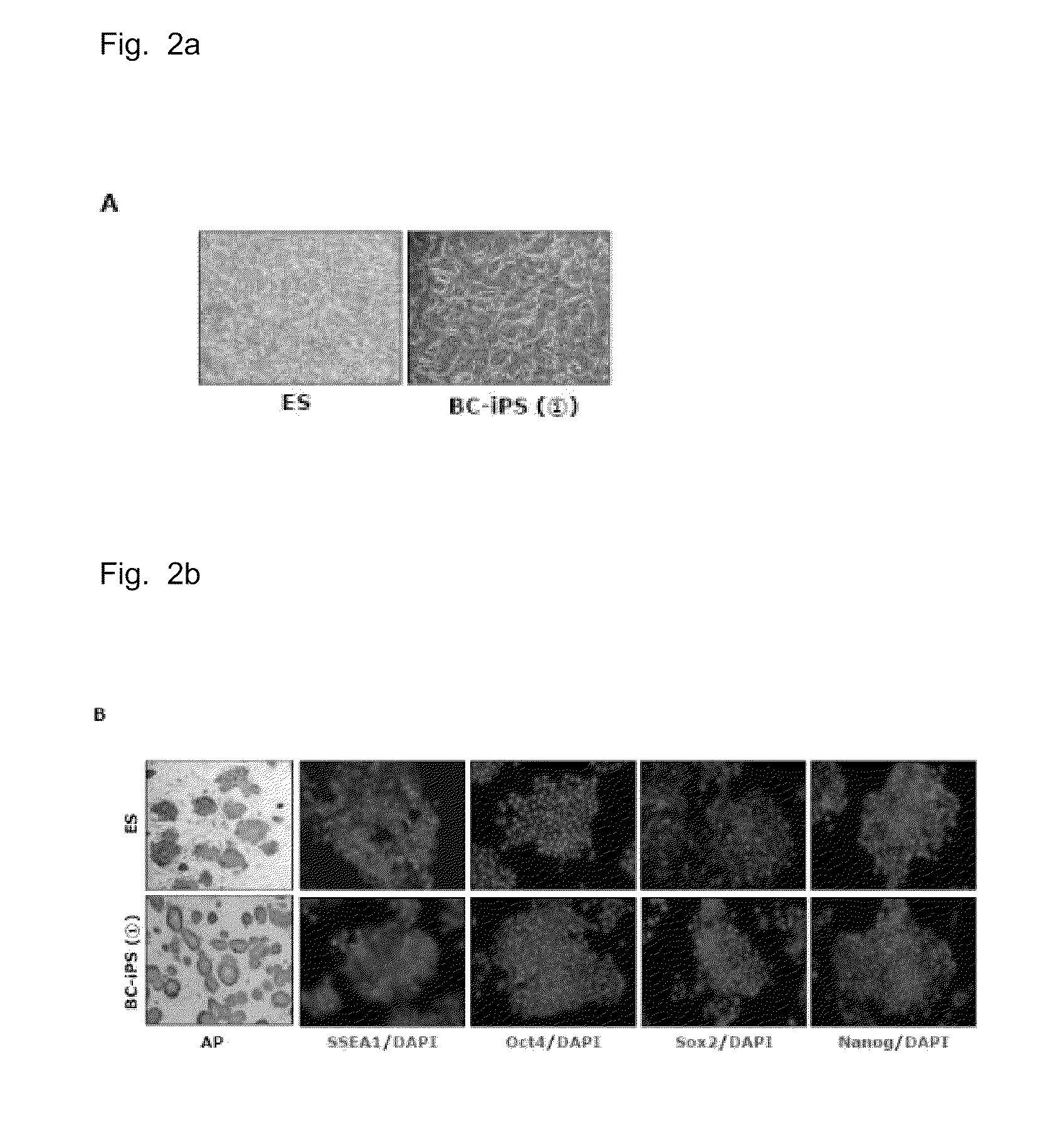
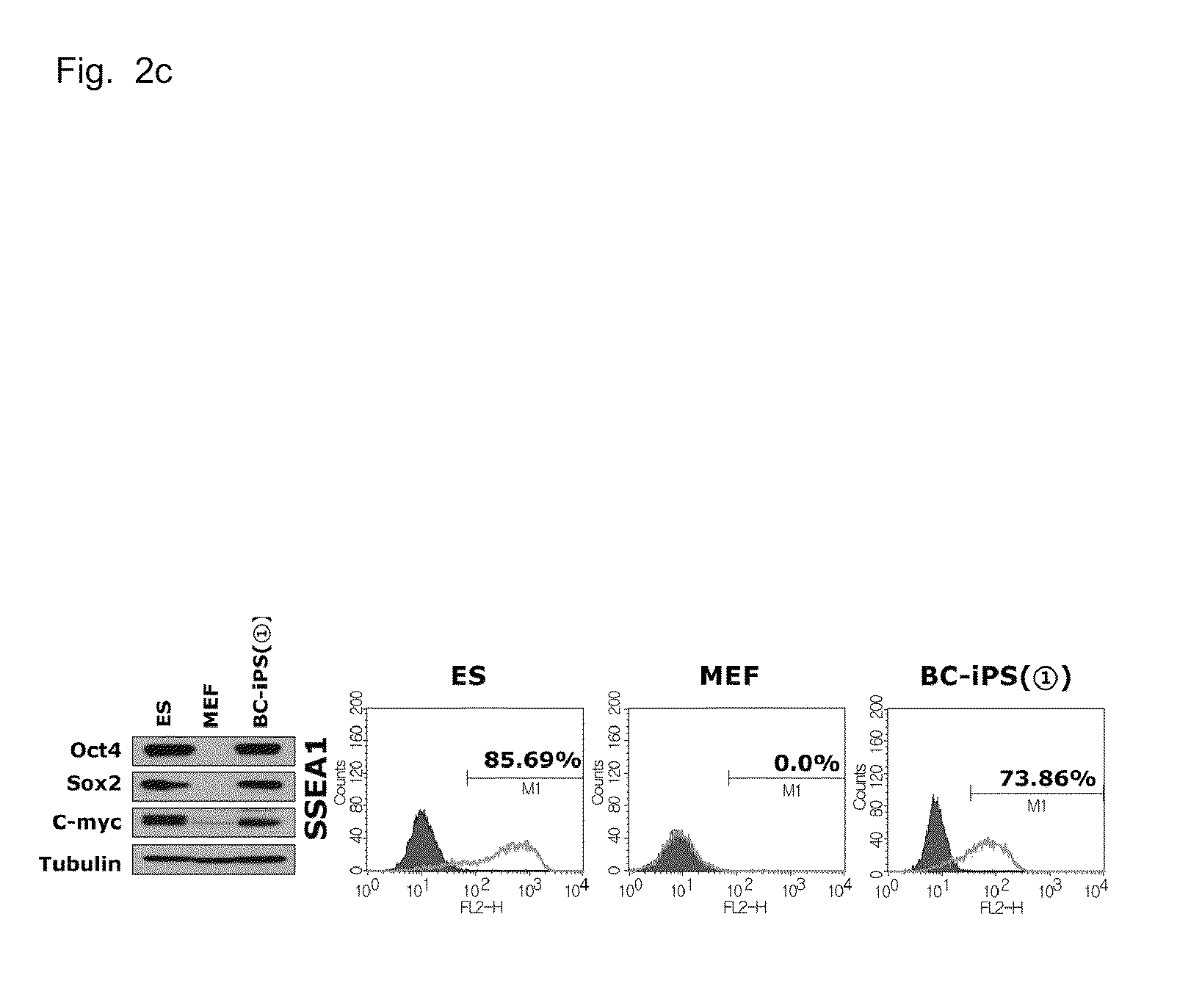
COMPOSITION FOR REPROGRAMMING SOMATIC CELLS TO GENERATE INDUCED PLURIPOTENT STEM CELLS, COMPRISING Bmi1 AND LOW MOLECULAR WEIGHT SUBSTANCE, AND METHOD FOR GENERATING INDUCED PLURIPOTENT STEM CELLS USING THE SAME
Provided is a composition for reprogramming somatic cells to generate embryonic stem cell-like cells, comprising: a) a Bmi1 (B cell-specific Moloney murine leukemia virus integration site 1) protein or a nucleic acid molecule encoding the Bmi1 protein; and b) at least one low molecular weight substance selected from the group consisting of a set of a MEK / ERK (mitogen-activated protein kinase / extracellular regulated kinase) inhibitor and a GSK (glycogen synthase kinase) inhibitor, a set of a G9a HMTase (G9a histone methyltransferase) inhibitor and a DMNT (DNA methyltransferase) inhibitor, and a histone deacetylase inhibitor. Also, a method is provided for reprogramming somatic cells to generate embryonic stem cell-like cells using the composition. In addition to reducing the number of the reprogramming factors conventionally needed, the composition and method allow the generation of pluripotent embryonic stem cell-like cells which have high potential in the cell therapy of various diseases.
Owner:STEMLAB
Novel lentiviral vectors for site-specific gene insertion
ActiveUS20050266565A1Easy to reorganizeGenetic material ingredientsDepsipeptidesTherapy TrialVector system
Murine leukemia virus (MLV) and lentivirus vectors have been used previously to deliver genes to hematopoietic stem cells (HSCs) in human gene therapy trials. However, these vectors integrate randomly into the host genome, leading to disruption or inactivation of vital host genes. The present invention discloses a novel lentiviral vector system that overcomes this problem by integrating into a host genome in a site-specific manner.
Owner:CITY OF HOPE
Composition and method for generating induced pluripotent stem cells using the same
The present invention relates to a composition and a method for generating induced pluripotent stem cells using the same Provided is a composition for reprogramming somatic cells to generate embryonic stem cell-like cells, comprising: a) a Bmi1 (B cell-specific Moloney murine leukemia virus integration site 1) protein or a nucleic acid molecule encoding the Bmi1 protein; and b) at least one low molecular weight substance selected from the group consisting of a set of a MEK / ERK (mitogen-activated protein kinase / extracellular regulated kinase) inhibitor and a GSK (glycogen synthase kinase) inhibitor, a set of a G9a HMTase (G9a histone methyltransferase) inhibitor and a DMNT (DNA methyltransferase) inhibitor, and a histone deacetylase inhibitor. Also, a method is provided for reprogramming somatic cells to generate embryonic stem cell-like cells using the composition.
Owner:STEMLAB
Avian influenza H7N9 virus RT-PCR (reverse transcription-polymerase chain reaction) detecting kit and detecting method
InactiveCN103276109AStrong specificityMeet the needs of prevention and control in a timely mannerMicrobiological testing/measurementMicroorganism based processesFluorescenceReverse transcriptase
The invention relates to an avian influenza H7N9 virus RT-PCR (reverse transcription-polymerase chain reaction) detecting kit and a detecting method, and aims at providing the detecting kit which has the characteristics of convenience in use and accuracy in detection, and the detecting method which has the characteristics of high accuracy, simplicity and convenience in detection. The technical scheme is as follows: the avian influenza H7N9 virus fluorescence-quantitative RT-PCR detecting kit comprises deoxynucleotide triphosphate, MgCl2, an RT-PCR buffer solution, an avian influenza H7N9 virogene standard product, an RNA (Ribonucleic Acid) enzyme inhibitor, an MMLV (Moloney Murine Leukemia Virus) reverse transcriptase and a DNA (Deoxyribonucleic Acid) polymerase, and is characterized in that the detecting kit also comprises an upstream primer, a downstream primer and a specific probe. The avian influenza H7N9 virus fluorescence-quantitative RT-PCR detecting method comprises the following steps of: (1) extracting RNA of a sample to be detected; (2) carrying out RT-PCR reaction; and (3) carrying out fluorescence detection on the RT-PCR reaction product.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Seroconversion assays for detecting xenotropic murine leukemia virus-related virus
Methods of detecting, diagnosing, monitoring or managing an XMRV-related disease such as an XMRV-related neuroimmune disease such as chronic fatigue syndrome or an XMRV-related lymphoma such as mantle cell lymphoma in a subject are disclosed. These methods comprise determining presence, absence or quantity of antibodies against XMRV in a sample from a subject.
Owner:WHITTEMORE PETERSON INST FOR NEURO IMMUNE DISEASE
High efficiency retroviral vector which contains genetically engineered cellular non-coding sequence harboring splicing acceptor
InactiveUS7049143B2Risk of missingSafe and highly efficientVectorsBacteriaEngineered geneticFhit gene
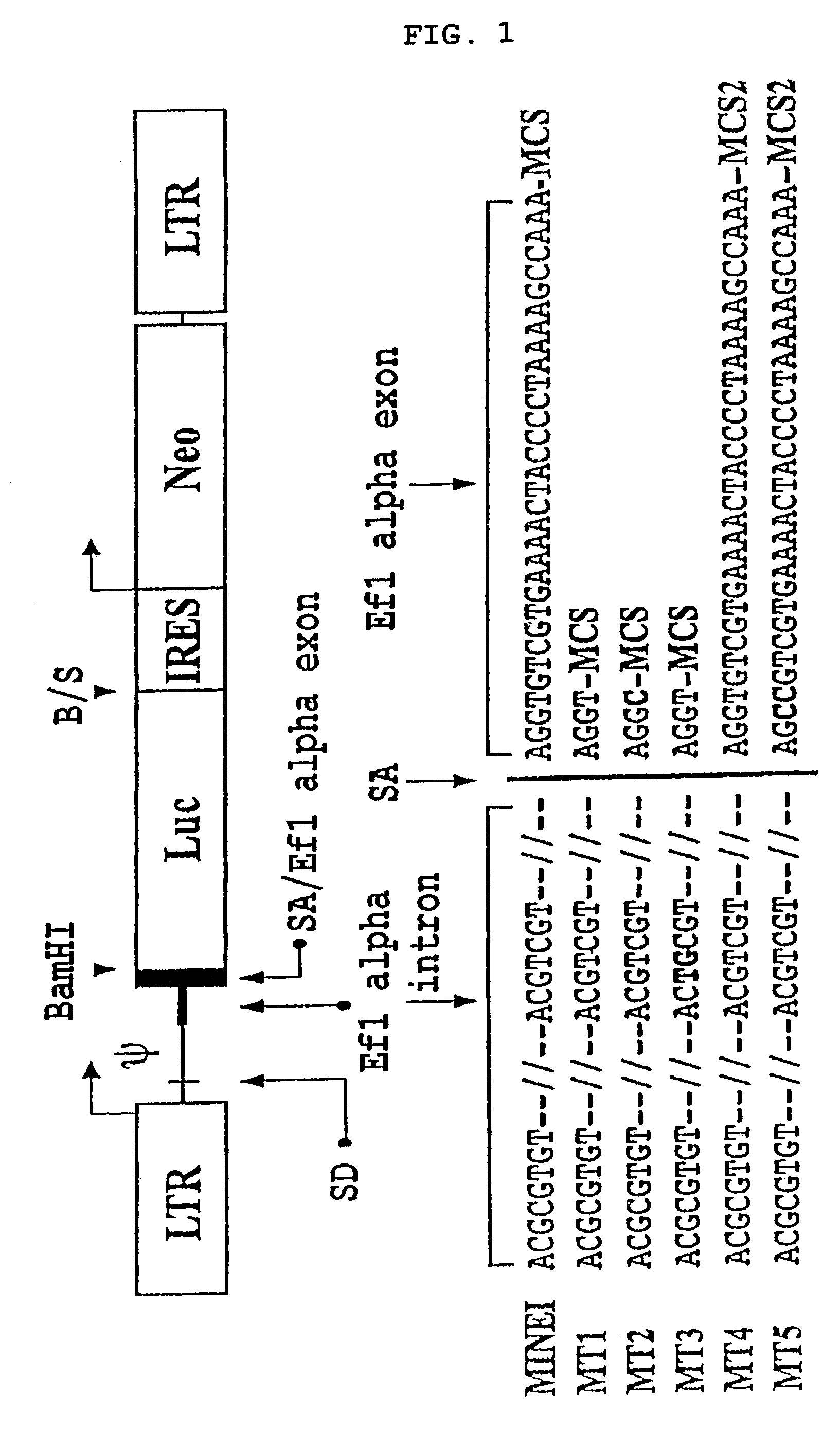
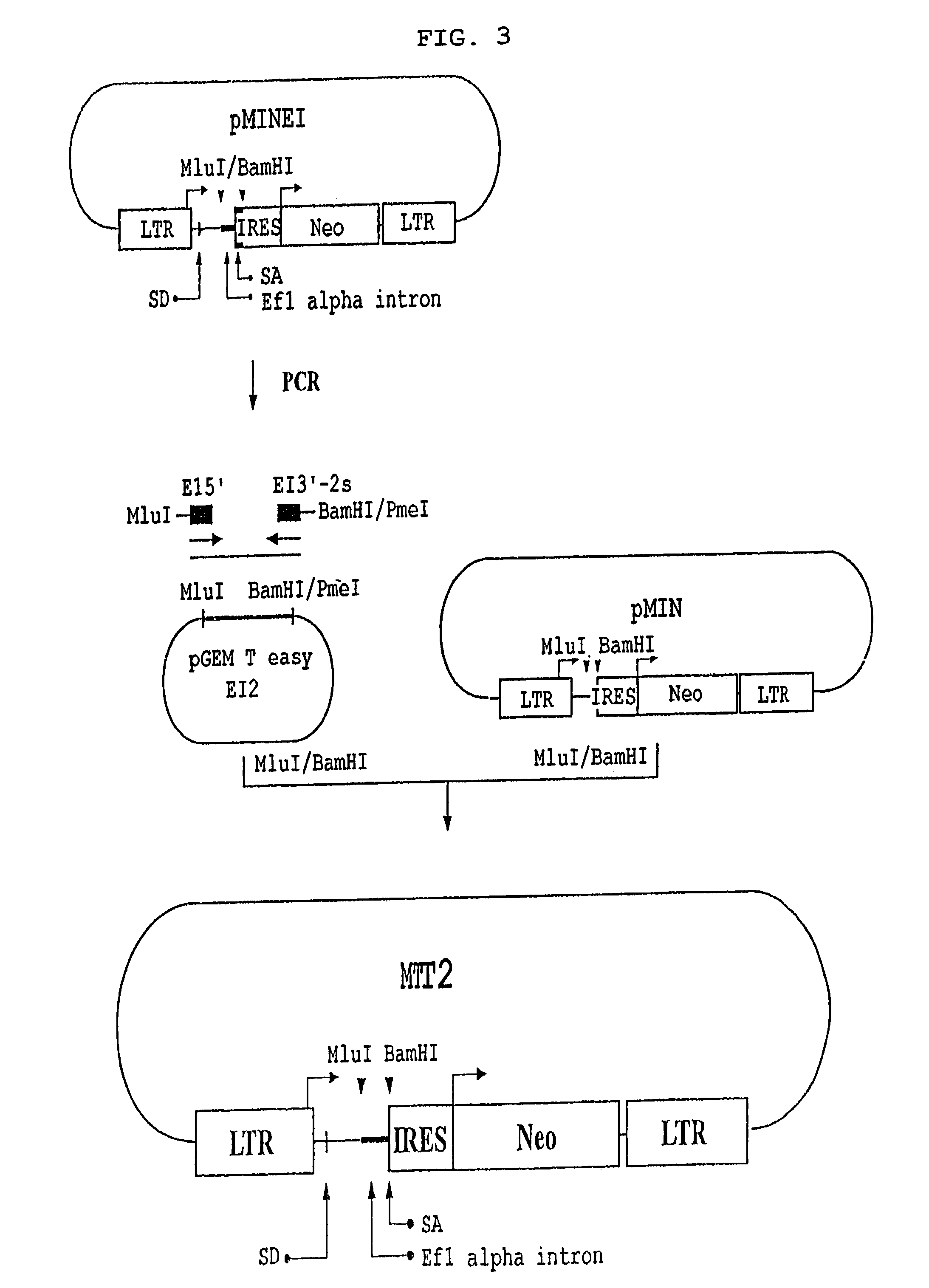
The present invention provides a safe and highly efficient retroviral vector derived from the MLV (murine leukemia virus) for use in gene therapy, which lacks viral coding sequences but contains the genetically engineered EF Iα non-coding sequence harboring splicing acceptor.
Owner:VIROMED CO LTD
Method for preparing visual gene detection reagent based on G4DNAzyme coloration
ActiveCN102041318AThe detection process is fastThe detection method is accurate and stableMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementClassical swine fever virus CSFVReverse transcriptase
The invention relates to a method for preparing a visual classical swine fever virus (CSFV) gene detection reagent based on G4DNAzyme coloration. The method comprises the following steps: selecting cells infected with viruses, after freeze thawing and lysing, adopting a gene extraction kit for extracting a target gene and obtaining cDNA after carrying out reverse transcription and inactivation onMoloney murine leukemia virus (MMLV) reverse transcriptase; adding the cDNA to an asymmetric polymerase chain reaction (PCR) system and obtaining an asymmetric PCR product through degeneration, annealing and extension amplification for 50-100 cycles; and adding upstream and downstream probes and the asymmetric PCR product to G4DNAzyme coloration reaction buffer, and after degeneration and annealing, adding Hemin, ATBS and H2O2 to carry out coloration reaction and observing whether macroscopic green color appears, thus judging whether CSFV infection exists. The method has the beneficial effects of high detection speed, accuracy, stability, good repeatability, simple and easy-to-operate detection steps, capability of directly observing the coloration reaction with naked eyes, intuitionisticresults and low cost.
Owner:巨星农牧有限公司 +1
Dual fluorescence quantification RT-PCR detection kit and application thereof
ActiveCN102230023AIncreased biosecurity riskPerfecting flu testingMicrobiological testing/measurementFluorescence/phosphorescenceSequence analysisReverse transcriptase
The invention provides a dual fluorescence quantification RT-PCR (reverse transcription-polymerase chain reaction) detection kit, comprising a deoxynucleoside triphosphate mixture, MgCl2, an RNA enzyme inhibitor, a Moloney murine leukemia virus reverse transcriptase, a DNA polymerase, a influenza virus standard and a reference substance. Based on sequence analysis of present pervasive A H1N1 influenza virus, the invention provides a multiple fluorescence quantification PCR molecular biology gene diagnosis method and a diagnostic kit which are rapid, specific, accurate and sensitive. In addition, one reaction tube can simultaneously detect and differentiate an influenza A virus or an influenza B virus in 2h, so as to improve influenza detection.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
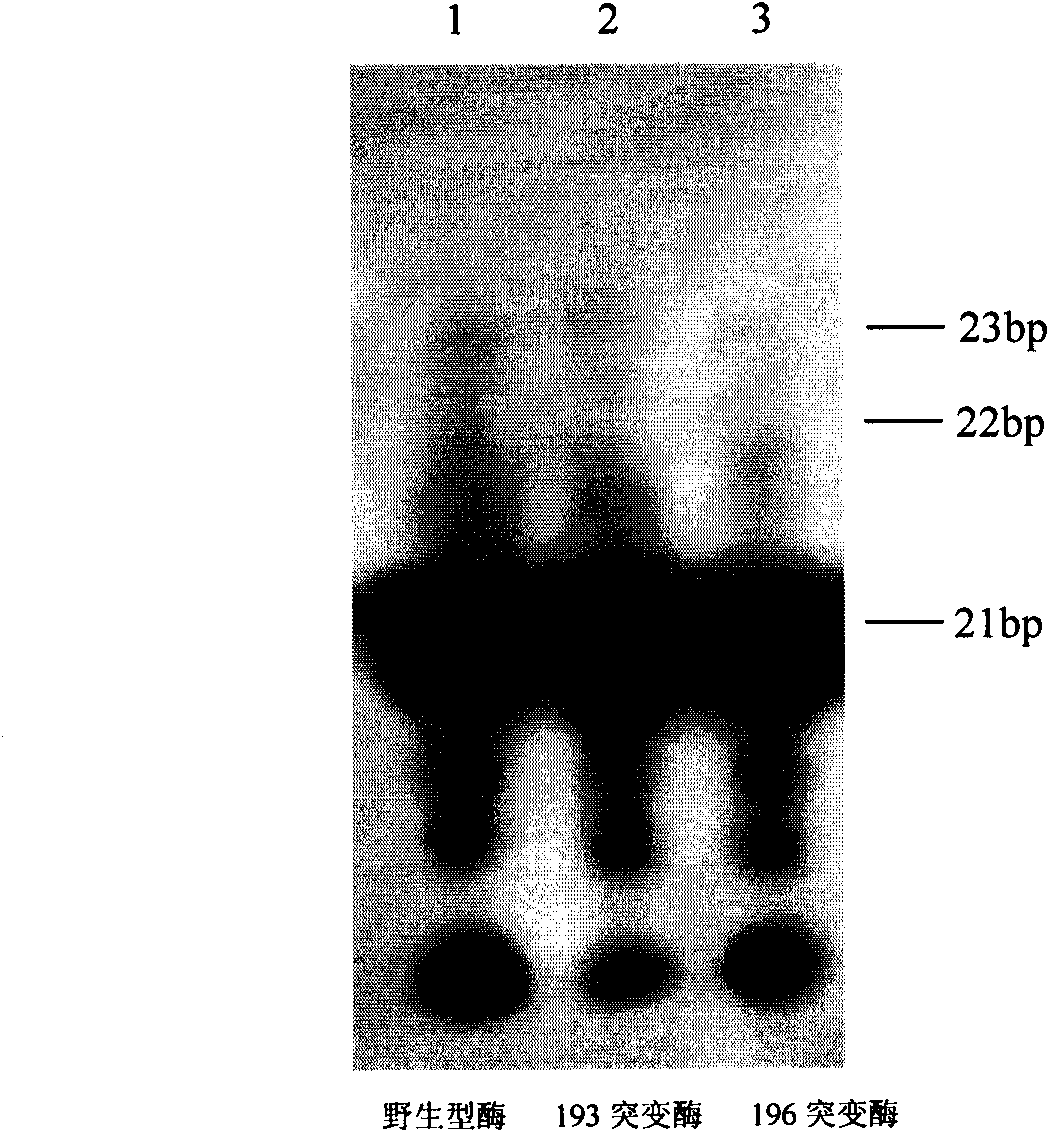
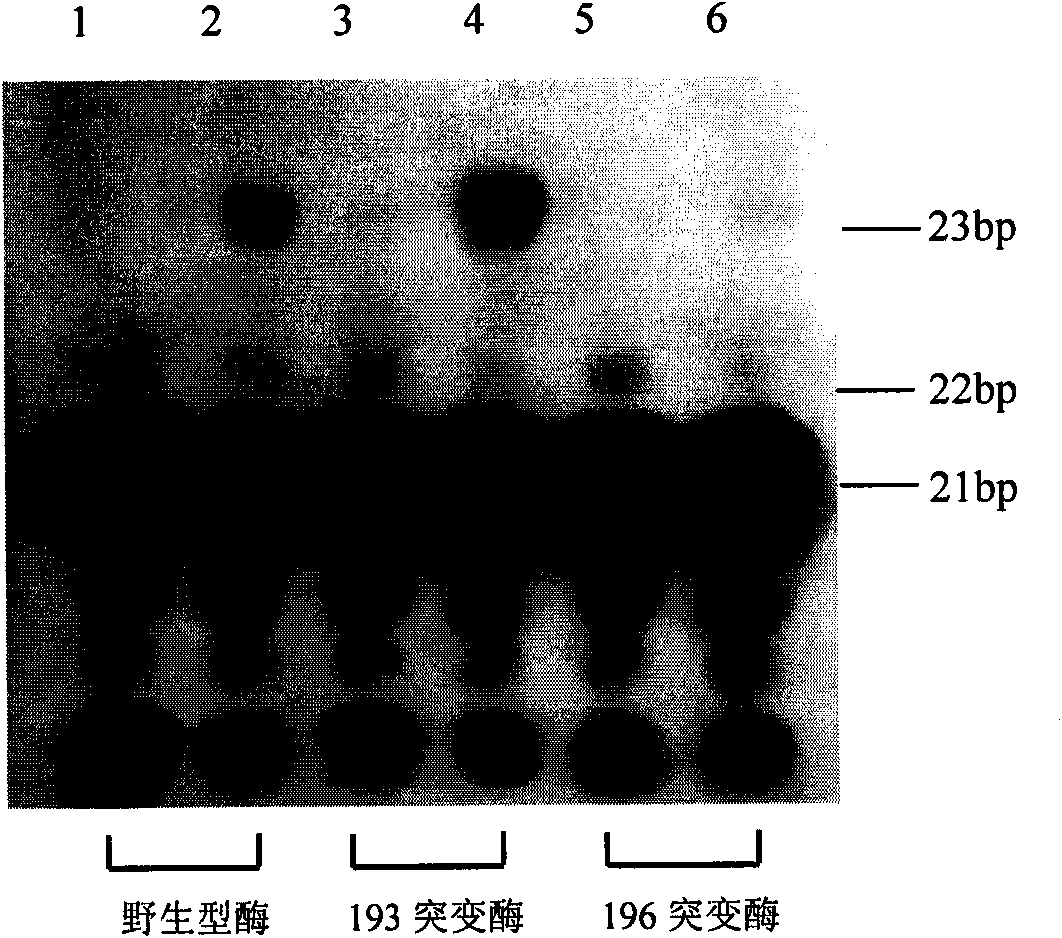
Moloney murine leukemia virus reverse transcriptase mutant as well as expression method and application thereof
The invention provides a moloney murine leukemia virus reverse transcriptase mutant as well as an expression method and application thereof. The mutant is a protein formed by substituting a praline residue at the 196th site of the moloney murine leukemia virus reverse transcriptase from the N end by an alanine residue. The expression method of the moloney murine leukemia virus reverse transcriptase comprises the steps of transforming an expression vector containing the coding gene of the moloney murine leukemia virus reverse transcriptase into Escherichia coli, culturing positive clones and expressing to obtain the moloney murine leukemia virus reverse transcriptase mutant. The mutant can be applied to RNA synthesis, and the reverse transcriptase mutant obtained from reconstructing the moloney murine leukemia virus reverse transcriptase has a fidelity function.
Owner:GUANGZHOU HUAYIN MEDICINE SCI & TECH +1
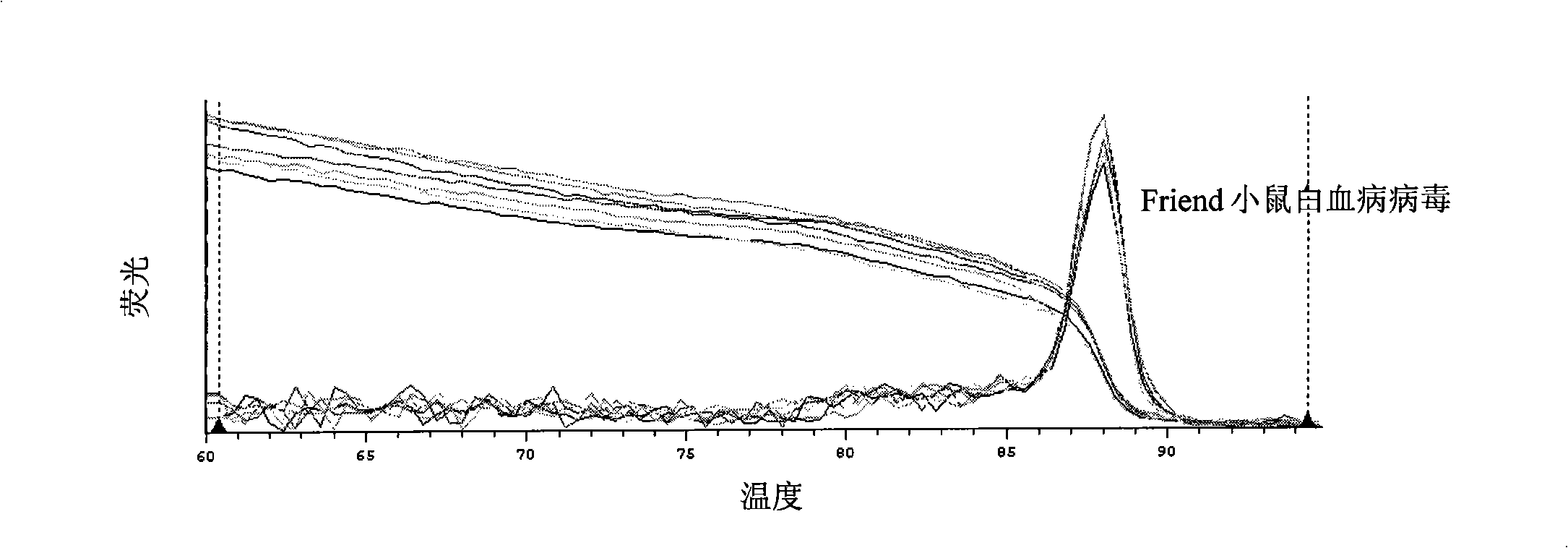
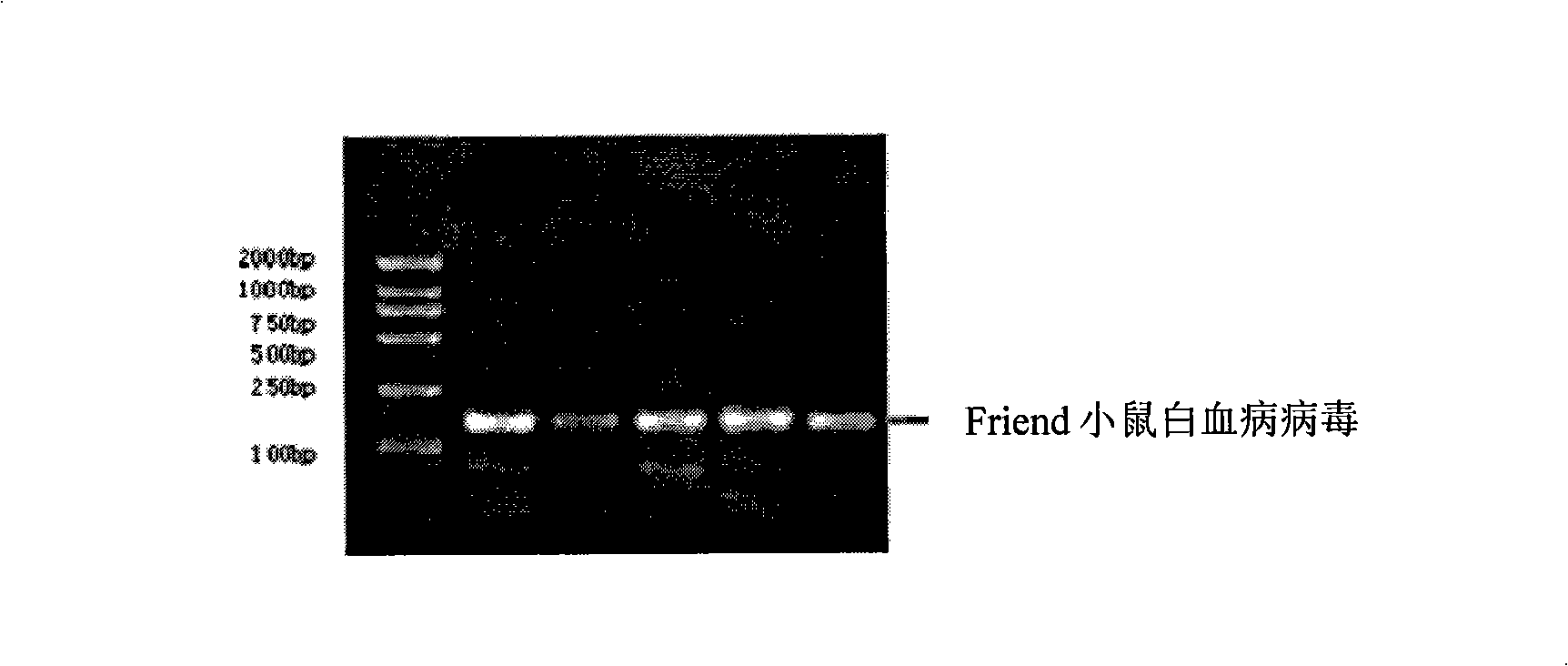
Standard article and method for detecting carry quantity of leucovirus
The invention discloses a standard product which is used for detecting the viral load of Friend murine leukemia, and a method which detects the viral load of the Friend murine leukemia by a real-time fluorescent quantitative polymerase chain reaction method (Real-Time RT-PCR method) by using the standard product. The detection process includes the steps of the extraction and content measurement of leukovirus nucleic acid (RNA), the obtaining of the nucleic acid segment by amplifying reverse transcription by using a primer, the detection of the real-time fluorescent quantitative polymerase chain reaction (Real-time PCR), and the like. Compared with conventional PCR detection methods, the method of the invention, which detects the viral load of the Friend murine leukemia, has better specificity. The detected genetic amplified products are target genetic products to be detected. The method has a better linear relationship and is suitable for being applied to the detection of the viral load of the Friend murine leukemia (Fr. MuLV).
Owner:崔晓兰
LAMP (Loop-Mediated Isothermal Amplification) detection method of PRV (Porcine Rotavirus) inverse transcription and application
InactiveCN104694670AImprove efficiencyIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseUltraviolet
The invention discloses an LAMP (Loop-Mediated Isothermal Amplification) detection method of PRV (Porcine Rotavirus) inverse transcription and an application. The detection method comprises the following steps: (1) building a RT-LAMP reaction system, and setting a negative control at the same time, wherein the RT-LAMP reaction system is 25 [mu] L and comprises 2 [mu] L of template, 0.5 [mu]L of 0.8 [mu]M FIP primer, 0.5 [mu]L of 0.8 [mu]M BIP primer, 0.25 [mu]L of 0.2 [mu]M of F3 primer, 0.25 [mu]L of 0.2 [mu]M of B3primer,2[mu]L of dNTP (Diethyl-Nitrophenyl Thiophosphate), 5 [mu]M of MgSO4 (Magnesium Sulfate), 8UBst of DNA polymerase and 1*ThermoPol Buffer, and 1[mu]L of MLV (Murine Leukemia Virus) reverse transcriptase, and supplementing the volume to 25 [mu]L by sterile water; and the negative control template is sterile water; (2) after reacting for 20-60min at 61-65 DEG C in a constant temperature water bath boiler, terminating for 20 min at 80 DEG C, carrying out AGE (Agarose Gel Electrophoresis) identification on an amplification product or adding 2 [mu]L of SYBR Green dye, and observing a result in an UV lamp (Ultraviolet Lamp) or by naked eyes. According to the LAMP detection method provided by the invention, a convenient, fast and accurate molecular biological diagnosis method can be provided for the clinical diagnosis and epidemiological investigation of the PRV.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
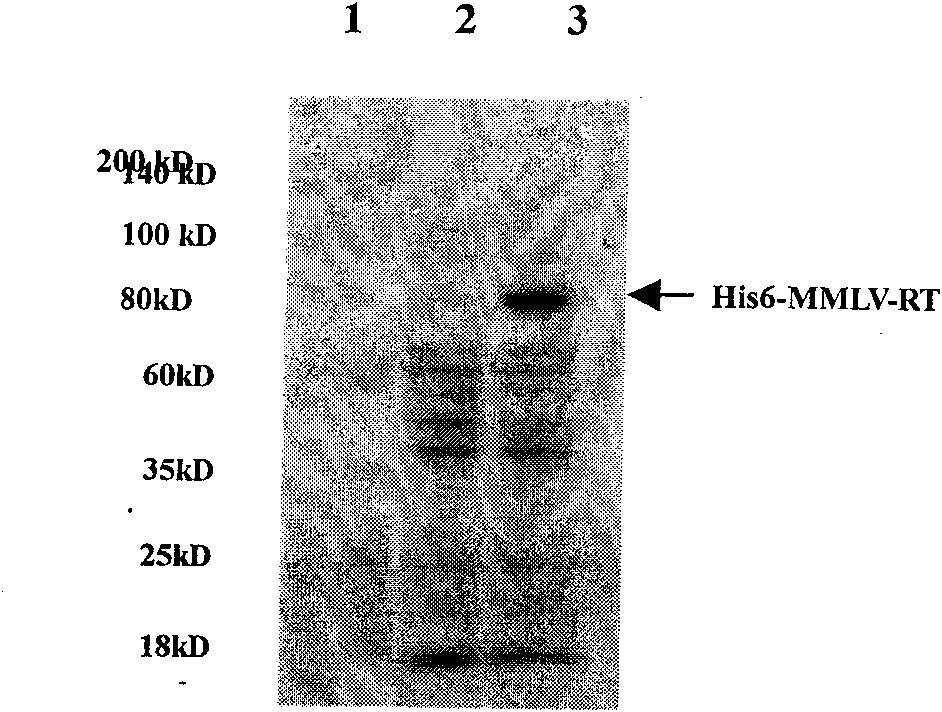
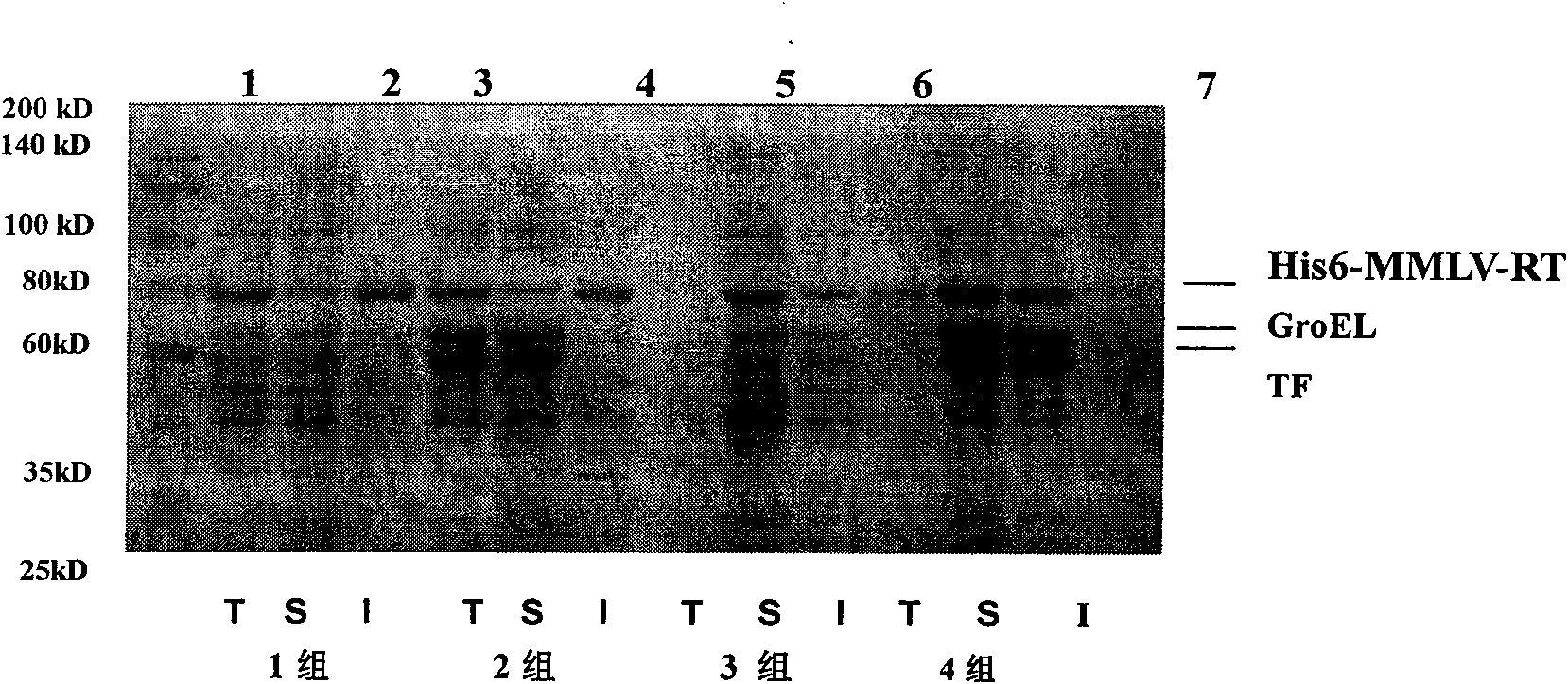
Soluble expression and purification method for recombinant murine leukemia virus reverse transcriptase (MMLV-RT) in colon bacillus
InactiveCN101684474AImprove solubilityEasy to purifyMicroorganism based processesEnzymesRestriction Enzyme Cut SiteSolubility
The invention relates to an expression and purification of murine leukemia virus reverse transcriptase (MMLV-RT), in particular to a soluble expression and a purification method for recombinant murineleukemia virus reverse transcriptase (MMLV-RT), which belongs to the field of biochemistry. MMLV-RT genes and enterokinase restriction enzyme cutting sites are cloned in a pET28a vector through vector construction; the MMLV-RT and molecular chaperones are co-expressed at the low temperature; the expression amount of protein is improved to 15 percent; and the solubility of the protein is 10 timesthat of the protein expressed by a conventional method.
Owner:孙启明 +1
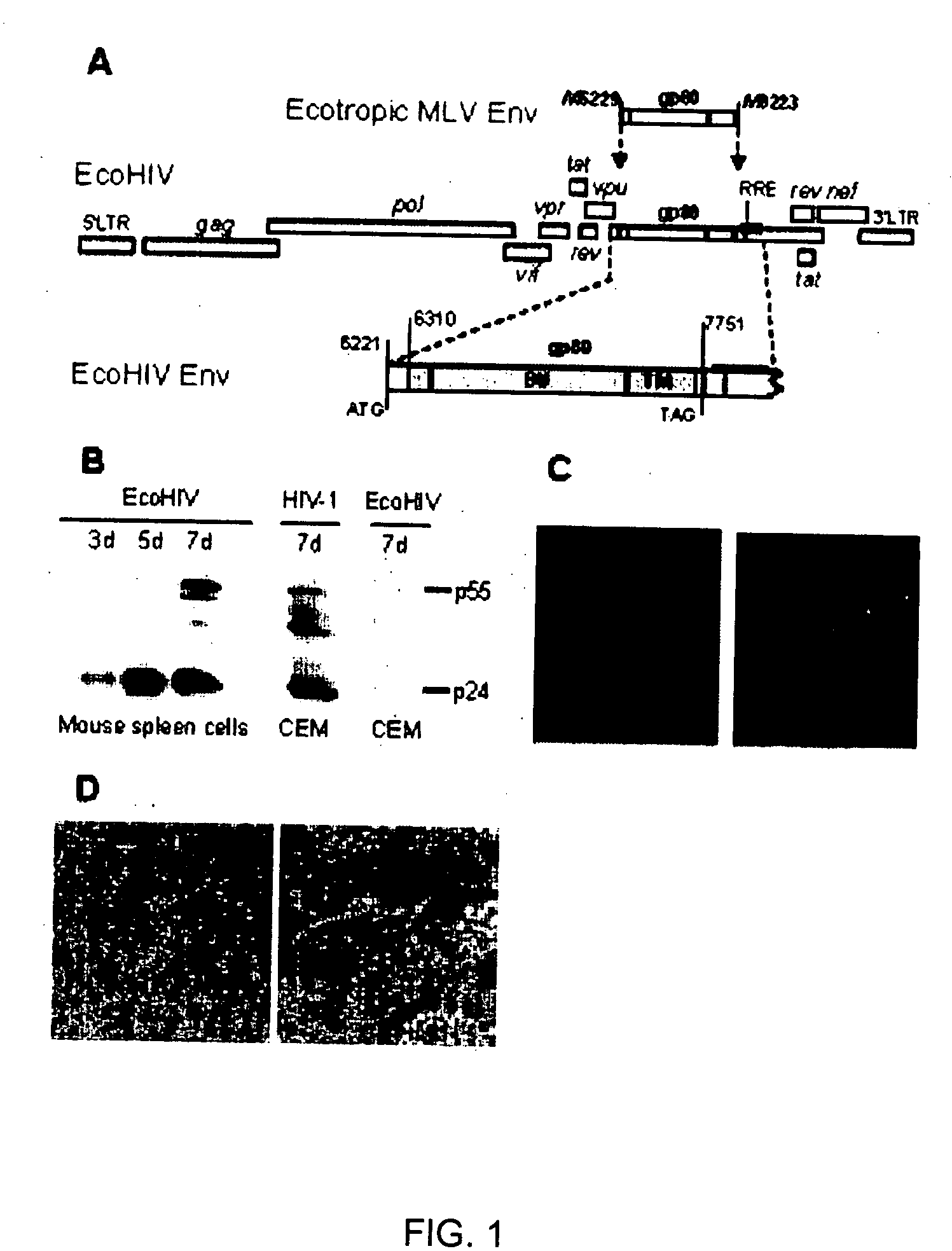
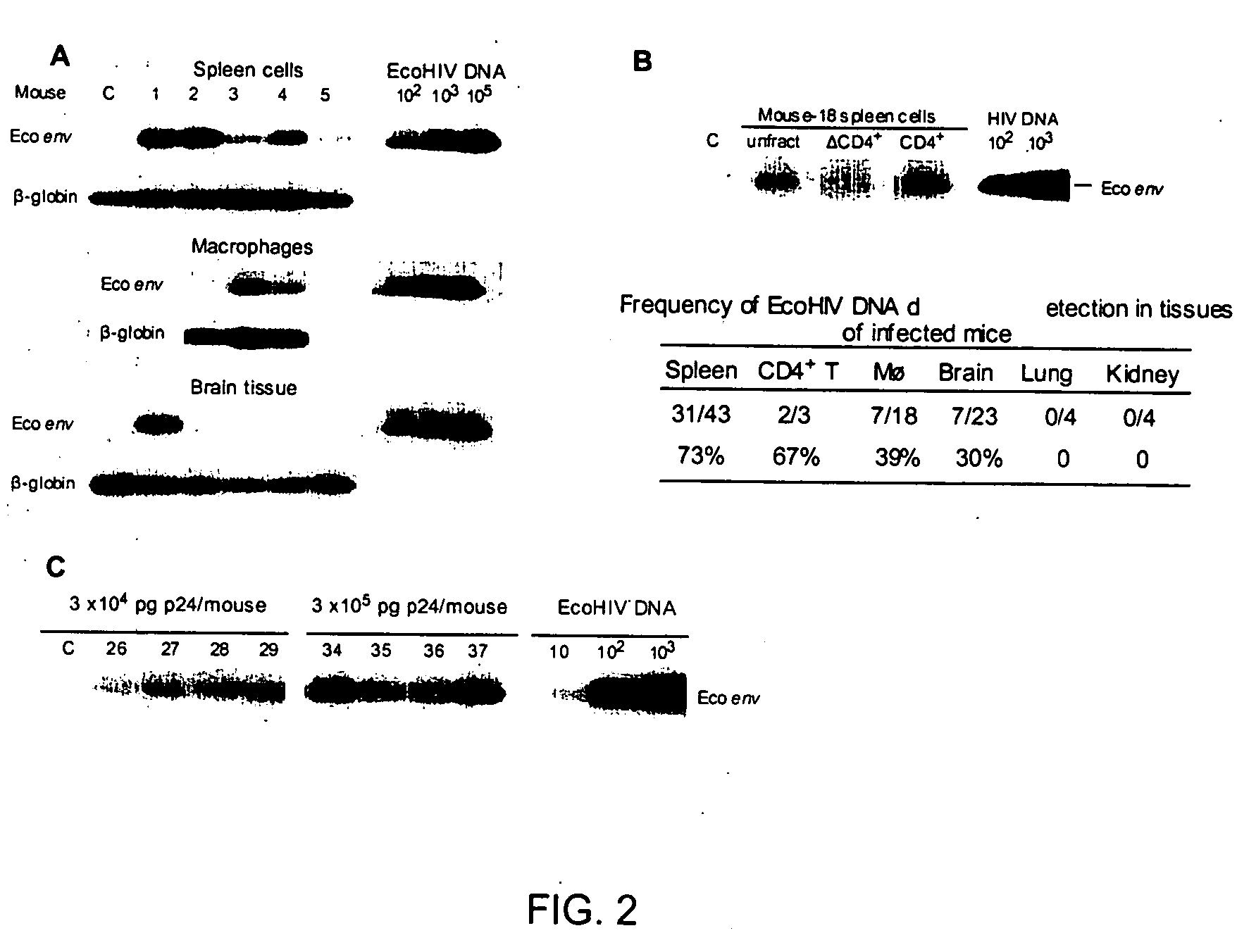
Development of a murine model of HIV-1 infection on the basis of construction of EcoHIV, a chimeric, molecular clone of human immunodeficiency virus type 1 and ecotropic moloney murine leukemia virus competent to infect murine cells and mice
The present invention provides a chimeric HIV-1 construct, EcoHIV, capable of replication in a rodent cell. The invention also provides a convenient and safe rodent model of HIV-1 infection and AIDS. Methods for producing a rodent model of HIV-1 infection are also provided. Additionally, the invention provides the means to test immunogenic compositions or pharmaceutical interventions effective in preventing infection, reducing viral load, or reducing disease symptoms in a subject.
Owner:POTASH MARY +1
Method for detecting nucleic acid of porcine reproductive and respiratory syndrome virus in one step
InactiveCN102181580AThe solution is not easy to saveSolve transportation problemsMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseCentral laboratory
The invention relates to a method for detecting nucleic acid of a porcine reproductive and respiratory syndrome (PRRS) virus by one step, which comprises the following steps of: collecting, processing and detecting samples, wherein in the collecting step, an animal blood sample is dripped into a full type approval (FTA) card sample area; and the detecting steps comprises the: (1) designing specific primers and a probe for general type PRRS virus, and marking carboxyfluorescein (FAM) and tetramethyl rhodamine (TAMARA) fluorescent groups on the probe; and (2) preparing and optimizing a detection system and a reaction condition, wherein a reaction system comprises 25 or 50 microliters of trihydroxymethyl aminomethane-hydrogen chloride (HCl) (the pH value is between 7.8 and 9.0), 0.1 to 0.5 micro mol of upstream primer and 0.1 to 0.5 micro mol of downstream primer, 100 to 400 micro mols of deoxynucleotide mixture, 0.1 to 0.5 micro mol of probe, 100 to 300 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase, 1 to 5 U of thermostable deoxyribonucleic acid (DNA) polymerase, 4 to 8 mols of Mg<2+->, 300 to 500 nano mol of homogenized reference dyes ROX, and 1 to 15 microliters of sample which is re-suspended in trihydroxymethyl aminomethane-ethylene diamine tetraacetic acid (EDTA) buffer solution and is added before each time of reaction. In the method, the sample collection is easy and convenient, so that an FTA card containing s ribonucleic acid (RNA) sample can be posted to any one central laboratory to be detected according to a form of regular mails; and the pollution risks are reduced, and the detection sensitivity is improved.
Owner:湖南农安生物技术有限公司
Abl1 inhibitor for treating and preventing ocular neovascularisation
InactiveUS20170027936A1Inhibit angiogenesisInhibit cell migrationOrganic active ingredientsSenses disorderViral OncogeneVEGF receptors
The present invention relates to an Abelson murine leukaemia viral oncogene homolog 1 (ABL1) inhibitor for use in the treatment of ocular neovascularisation associated with a non-cancerous condition. The invention also relates to the use of an ABL1 inhibitor as a complementary therapy with VEGF or VEGF receptor inhibitor treatment and provides pharmaceutical compos itions and kits comprising one or both inhibitors.
Owner:UCL BUSINESS PLC
Composition for reprogramming somatic cells to generate induced pluripotent stem cells, comprising Bmi1 and low molecular weight substance, and method for generating induced pluripotent stem cells using the same
Provided is a composition for reprogramming somatic cells to generate embryonic stem cell-like cells, comprising: a) a Bmi1 (B cell-specific Moloney murine leukemia virus integration site 1) protein or a nucleic acid molecule encoding the Bmi1 protein; and b) at least one low molecular weight substance selected from the group consisting of a set of a MEK / ERK (mitogen-activated protein kinase / extracellular regulated kinase) inhibitor and a GSK (glycogen synthase kinase) inhibitor, a set of a G9a HMTase (G9a histone methyltransferase) inhibitor and a DMNT (DNA methyltransferase) inhibitor, and a histone deacetylase inhibitor. Also, a method is provided for reprogramming somatic cells to generate embryonic stem cell-like cells using the composition. In addition to reducing the number of the reprogramming factors conventionally needed, the composition and method allow the generation of pluripotent embryonic stem cell-like cells which have high potential in the cell therapy of various diseases.
Owner:STEMLAB
Substituted pyrimidine bmi-1 inhibitors
Amine substituted pyrimidine compounds and forms thereof that inhibit the function and reduce the level of B-cell specific Moloney murine leukemia virus integration site 1 (Bmi-1) protein and methods for their use to inhibit Bmi-1 function and reduce the level of Bmi-1 to treat a cancer mediated by Bmi-1 are described herein.
Owner:PTC THERAPEUTICS INC
Avian influenza virus H7 type RT-PCR (reverses transcription-polymerase chain reaction) detecting kit and detecting method
InactiveCN103276108AStrong specificityMeet the needs of prevention and controlMicrobiological testing/measurementMicroorganism based processesFluorescenceReverse transcriptase
The invention relates to an avian influenza virus H7 type RT-PCR (reverses transcription-polymerase chain reaction) detecting kit and a detecting method, and aims at providing the detecting kit which has the characteristics of convenience in use and accuracy in detection, and the detecting method which has the characteristics of high accuracy, simplicity and convenience in detection. The technical scheme is as follows: the avian influenza virus H7 type fluorescence-quantitative RT-PCR detecting kit comprises deoxynucleotide triphosphate, MgCl2, an RT-PCR buffer solution, an avian influenza virus H7 gene standard product, an RNA (Ribonucleic Acid) enzyme inhibitor, an MMLV (Moloney Murine Leukemia Virus) reverse transcriptase and a DNA (Deoxyribonucleic Acid) polymerase, and is characterized in that the fluorescence-quantitative RT-PCR detecting kit also comprises an upstream primer, a downstream primer and a specific probe. The avian influenza virus H7 type fluorescence-quantitative RT-PCR detecting method comprises the following steps of: (1) extracting RNA of a sample; (2) carrying out RT-PCR reaction; and (3) carrying out fluorescence detection on the RT-PCR reaction product.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
HBV (hepatitis B virus) real-time fluorescent nucleic acid isothermal amplification detection kit
ActiveCN105525036AAccurate measurementRapid determinationMicrobiological testing/measurementMicroorganism based processesHepatitis B immunizationReverse transcriptase
The invention discloses an HBV (hepatitis B virus) real-time fluorescent nucleic acid isothermal amplification detection kit, comprising a capture probe, an HBV primer, a primer T7 and a primer nT7, an HBV detection probe, an M-MLV (Moloney Murine Leukemia Virus) reverse transcriptase, polymerase T7RNA and the like reagents. A method of the invention enables high-specificity high-sensitivity low-pollution quick nucleic acid amplification detection for serum or plasma samples containing hepatitis B virus, has the advantages of high detection efficiency and high accuracy, can detect A-I 9 genotypes of HBV and has a promising application prospect.
Owner:SHANGHAI RENDU BIOTECH
Technology for detecting tiny RNA155 (ribonucleic acid 155) relative content of T cells to reflect individual immunity state
InactiveCN102168132AReduce processing timeIncrease usageMicrobiological testing/measurementReverse transcriptasePolymerase L
The invention discloses a stem-loop primer and a plurality of mating amplified primers, a kit and an assessment form, which can be used for carrying out quantitive detection on miR-155 (microRNA 155). The kit consists of a reagent A: 50mu mL of reverse transcription stem-loop primer mixed liquor; a reagent B: an M-MuLV (Moloney Murine Leukemia Virus) reverse transcriptase; a reagent C: a reverse transcription buffer solution; a reagent D: 100mu mL of universal primer; a reagent E: a miR-155specific primer; a reagent F: a U6 specific primer; a reagent G: Taq DNA (deoxyribose nucleic acid) polymerase; and a reagent H: a 2*PCR (Polymerase Chain Reaction) buffer solution. The kit provided by the invention can be used for carrying out relatively quantitive analysis on the miR-155 segments of different types of T cells, and assessing individual immunity states.
Owner:陈必成 +2
Substituted reverse pyrimidine bmi-1 inhibitors
Amine substituted reverse pyrimidine compounds and forms thereof that inhibit the function and reduce the level of B-cell specific Moloney murine leukemia virus integration site 1 (Bmi-1) protein and methods for their use to inhibit Bmi-1 function and reduce the level of Bmi-1 to treat a cancer mediated by Bmi-1 are described herein.
Owner:PTC THERAPEUTICS INC
Detection kit and detection method of reverse transcription polymerase chain reaction (RT-PCR) of avian influenza virus N9
ActiveCN103255233AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceReverse transcriptase
The invention relates to a detection kit and a detection method of reverse transcription polymerase chain reaction (RT-PCR) of an avian influenza virus N9, and aims at providing the detection kit and the detection method, wherein the provided detection kit has the characteristics of being convenient to use and accurate to detect; and the provided method has the characteristics of being high in detection accuracy, and simple and convenient to detecting process. The technical scheme is as follows: the detection kit of fluorescent quantitative RT-PCR of the avian influenza virus N9 comprises deoxy triphosphate nucleoside, MgCl2, RT-PCR buffer solution, an avian influenza virus N9 genetic standard, a ribonucleic acid (RNA) enzyme inhibitor, moloney murine leukemia virus (MMLV) reverse transcriptase and deoxyribonucleic acid (DNA) polymerase. The detection kit of fluorescent quantitative RT-PCR is characterized by also comprising an upstream primer, a downstream primer and a specific probe. The detection method of fluorescent quantitative RT-PCR of the avian influenza virus N9 is carried out according to the following steps of: (1) extracting the RNA; (2) carrying out RT-PCR; and (3) carrying out fluorescence detection on an RT-PCR reaction product.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com