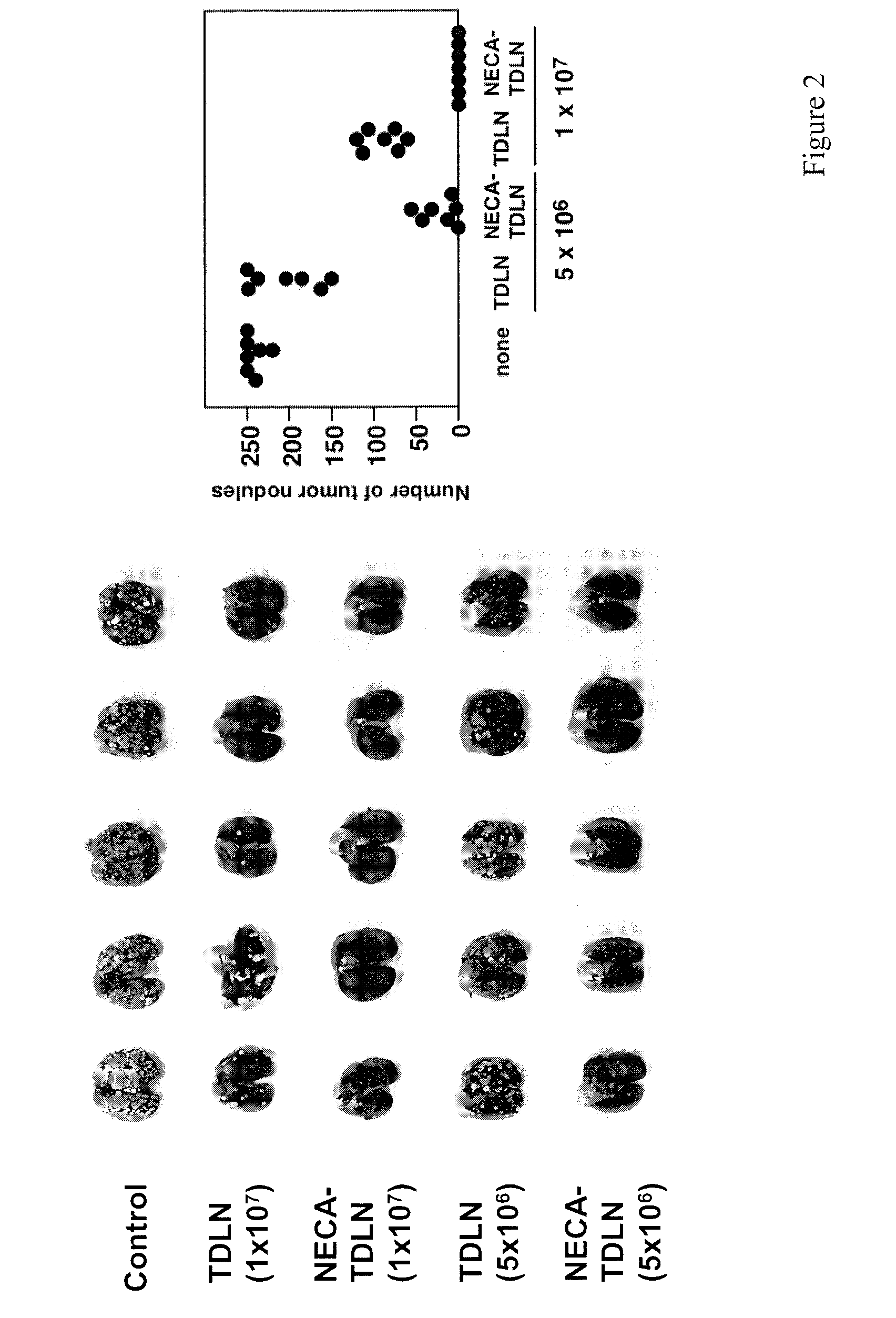
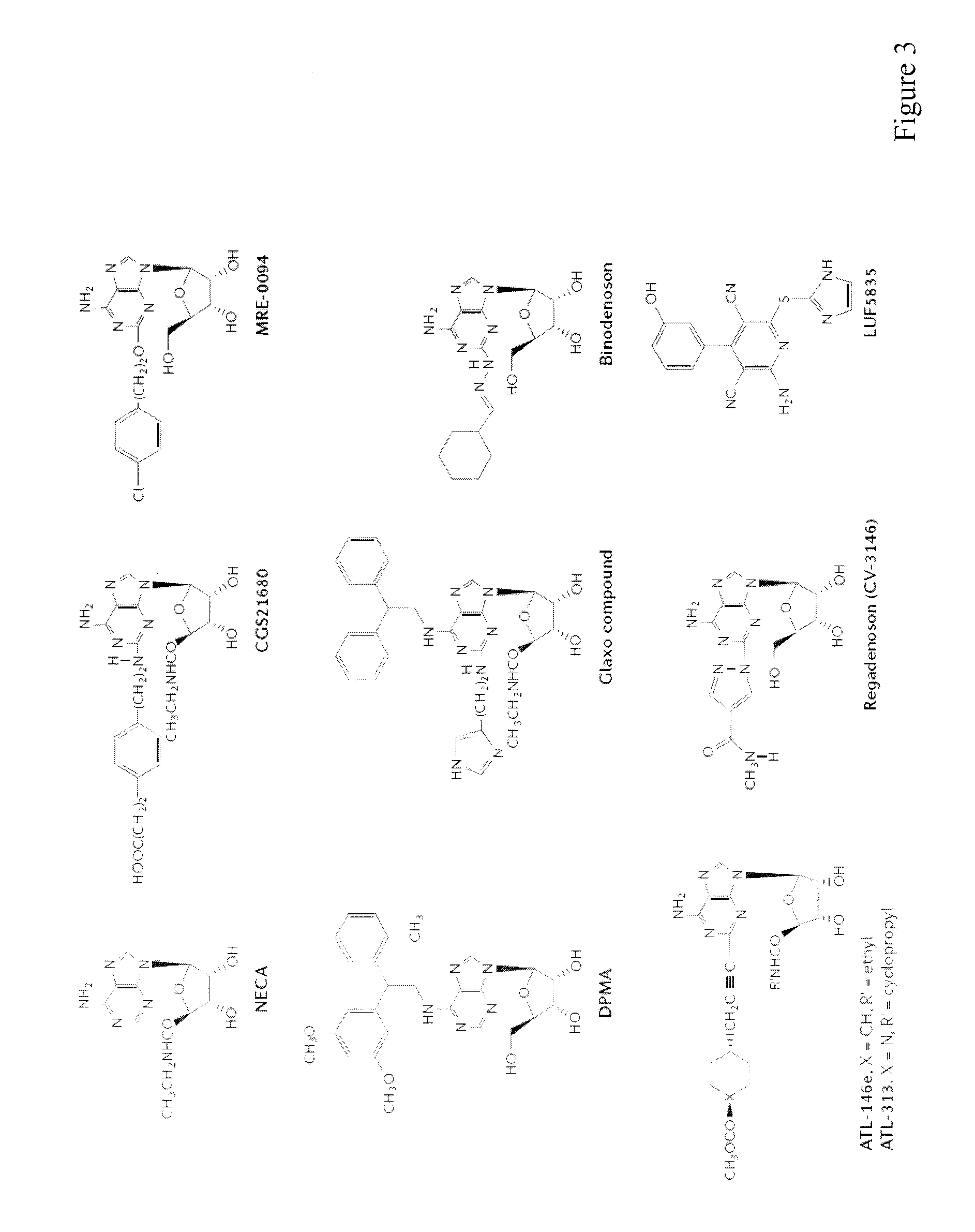
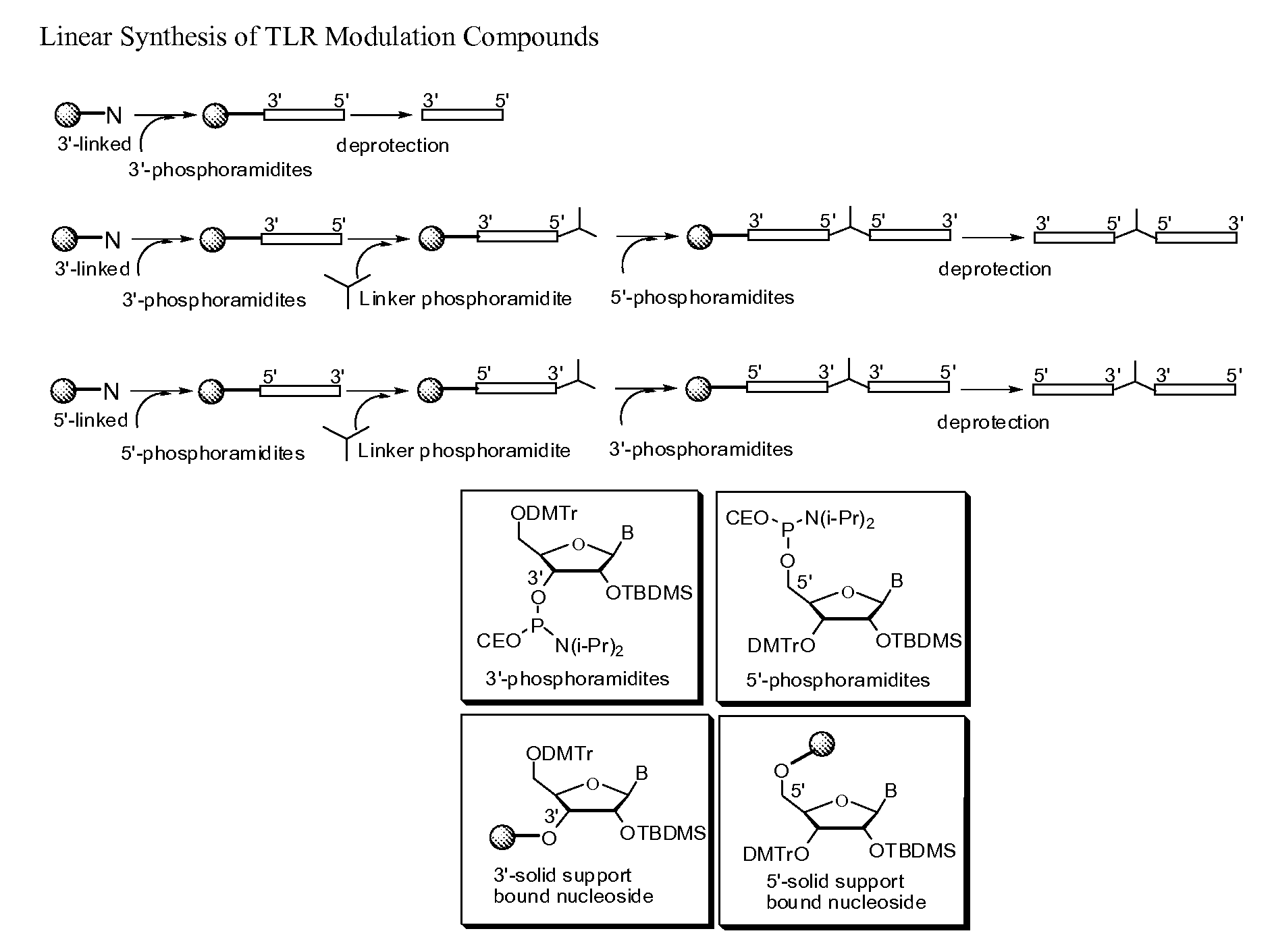
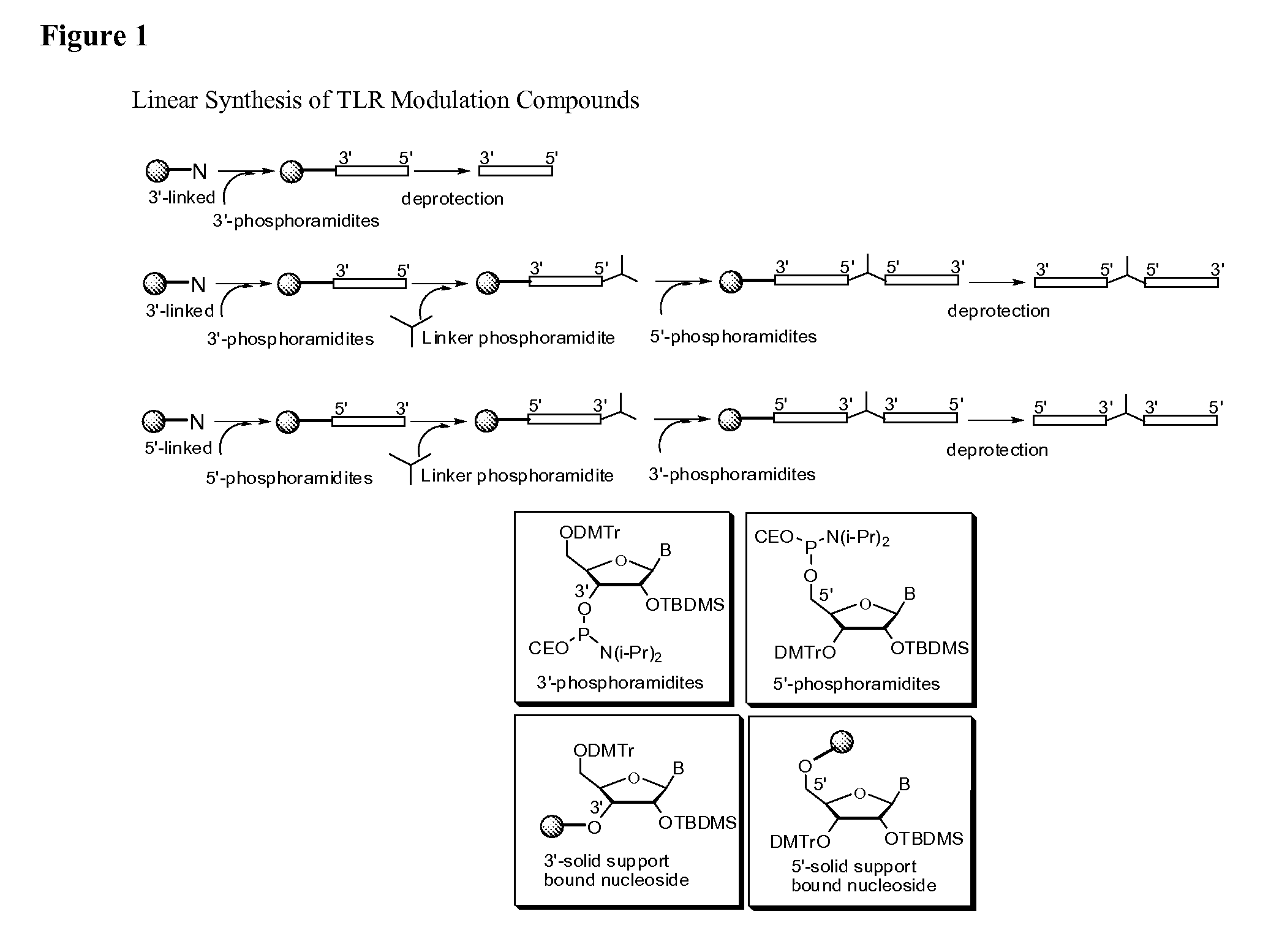
Patents
Literature
458 results about "Immunodeficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunodeficiency is a state in which the immune system's ability to fight infectious disease and cancer is compromised or entirely absent. Most cases of immunodeficiency are acquired ("secondary") due to extrinsic factors that affect the patient's immune system. Examples of these extrinsic factors include HIV infection, extremes of age, and environmental factors, such as nutrition. In the clinical setting, the immunosuppression by some drugs, such as steroids, can be either an adverse effect or the intended purpose of the treatment. Examples of such use is in organ transplant surgery as an anti-rejection measure and in patients suffering from an overactive immune system, as in autoimmune diseases. Some people are born with intrinsic defects in their immune system, or primary immunodeficiency. A person who has an immunodeficiency of any kind is said to be immunocompromised. An immunocompromised person may be particularly vulnerable to opportunistic infections, in addition to normal infections that could affect everyone. Immunodeficiency also decreases cancer immunosurveillance, in which the immune system scans the body's cells and kills neoplastic ones.
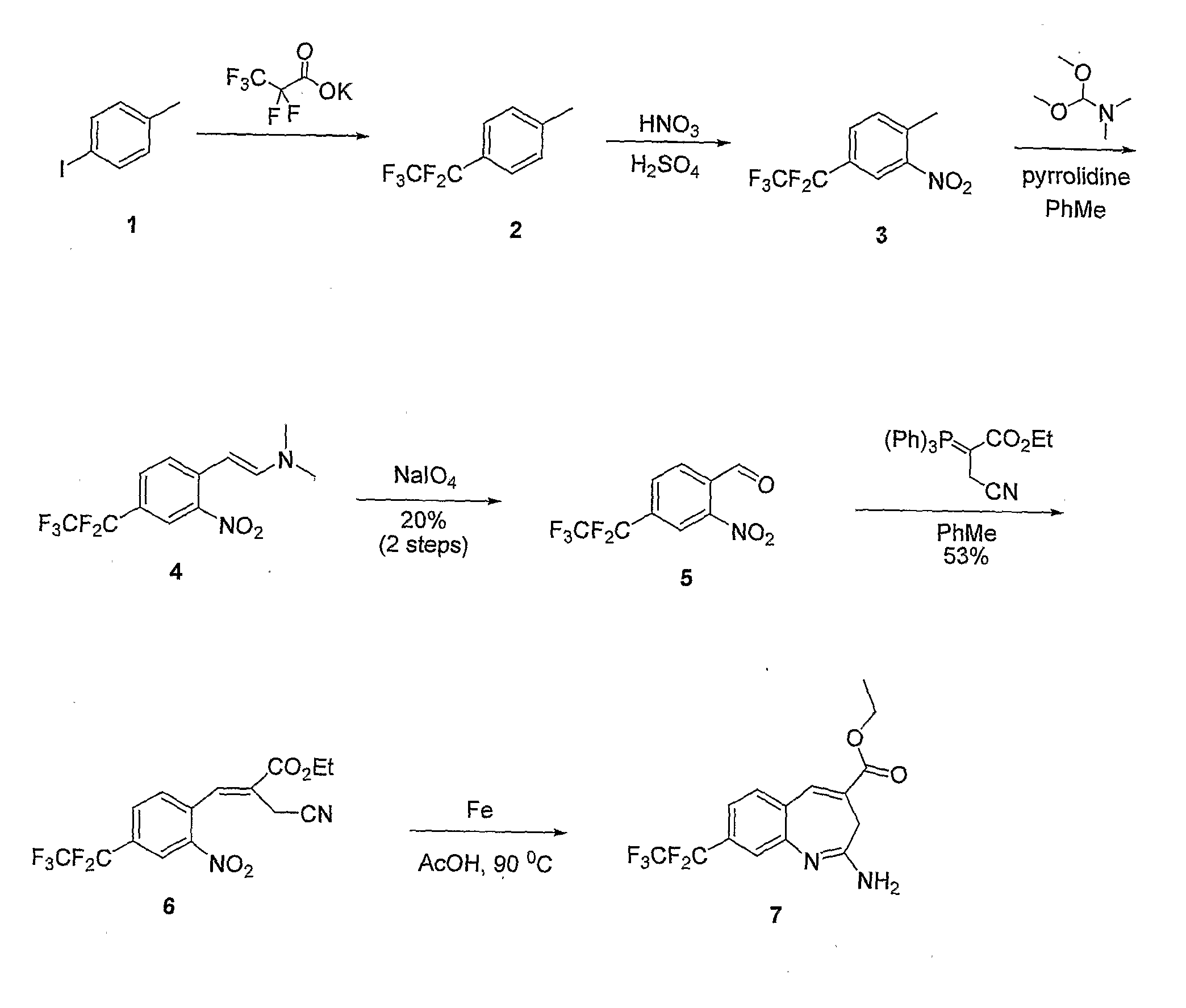
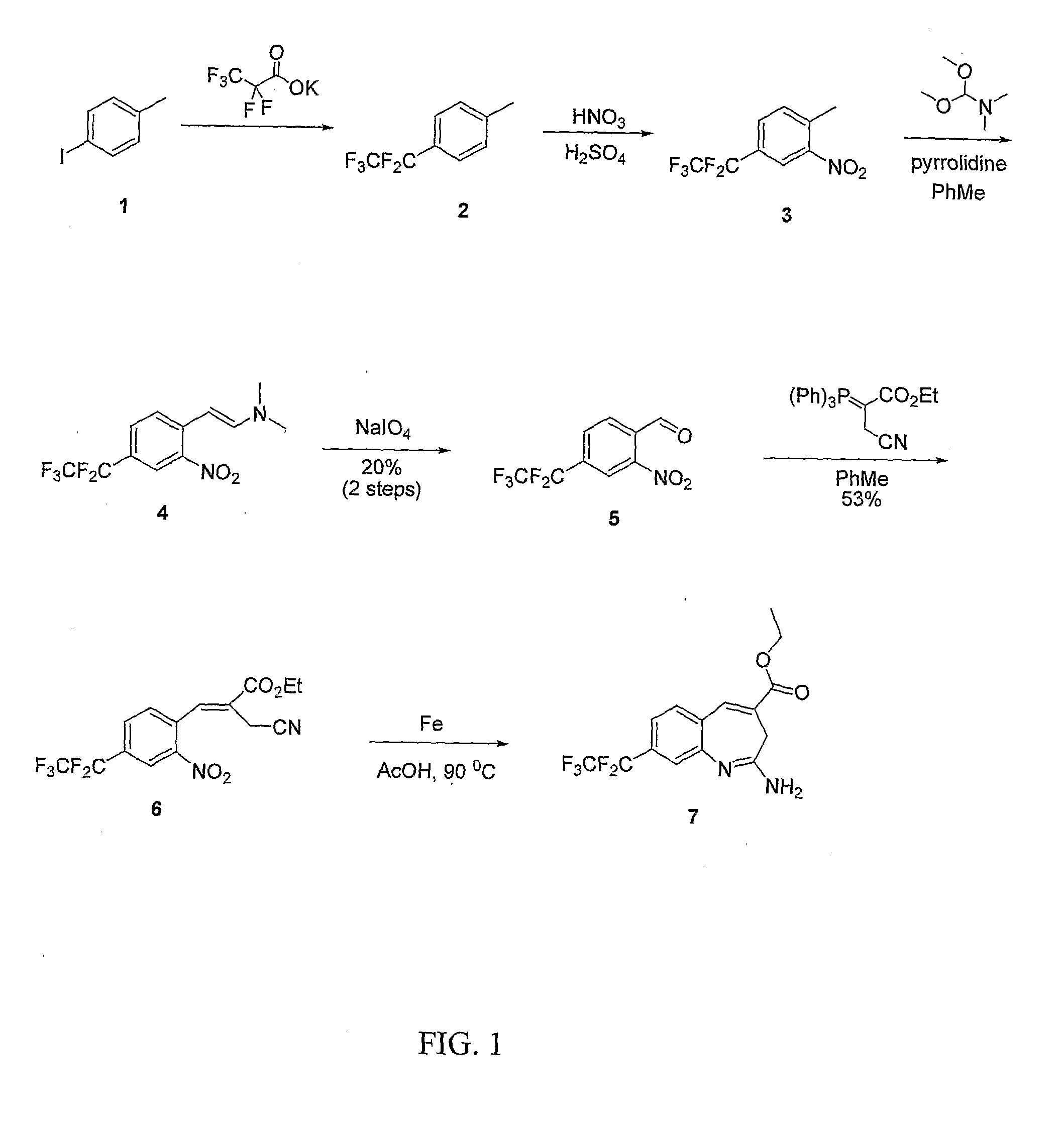
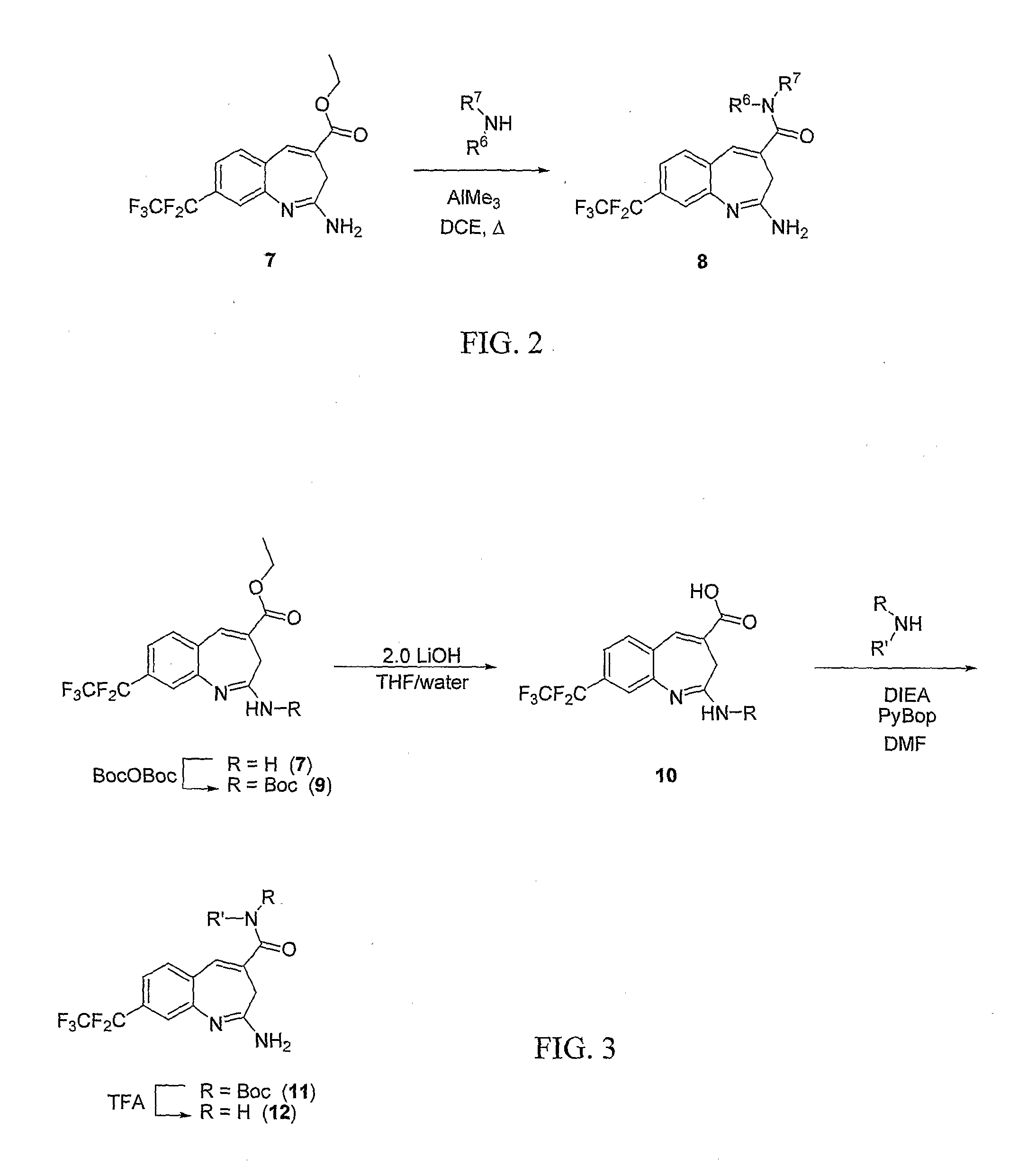
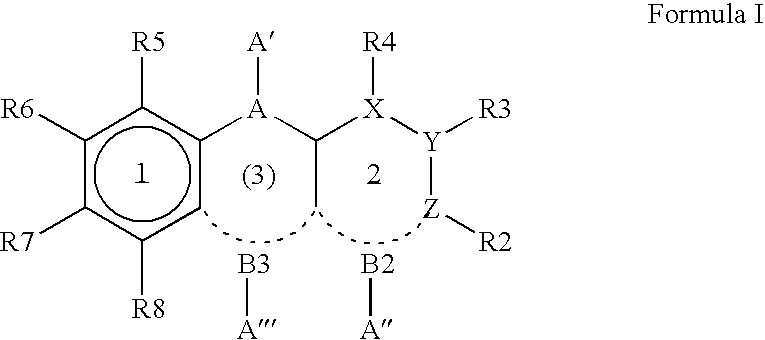
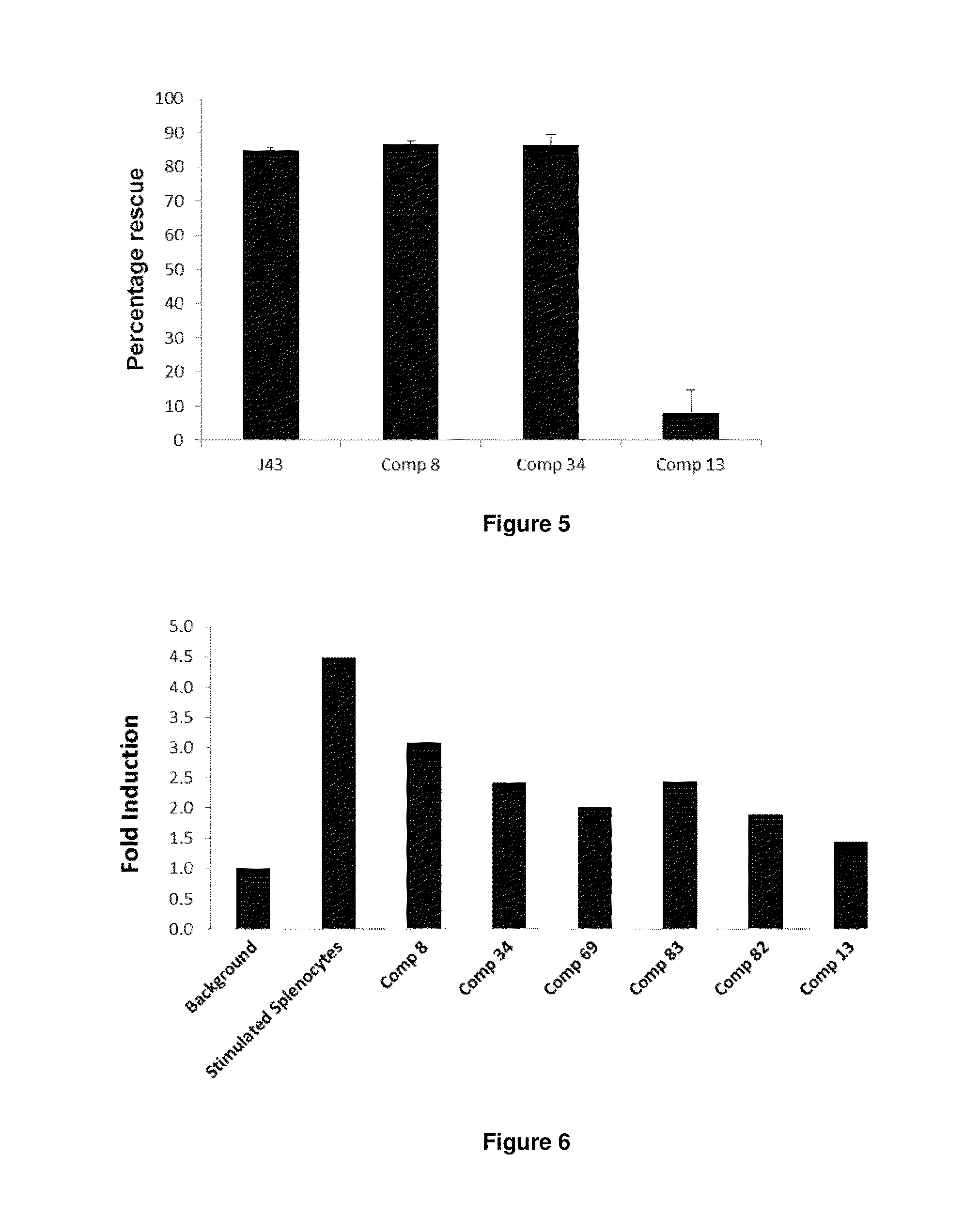
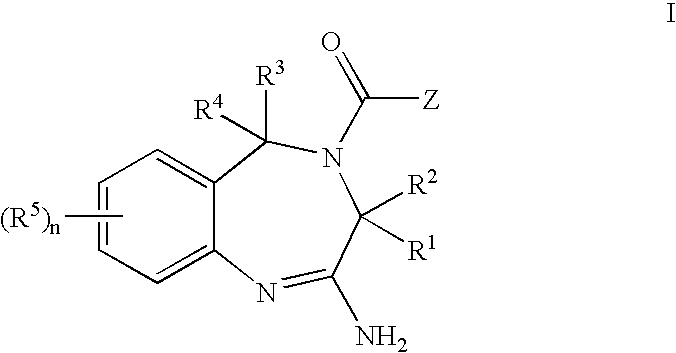
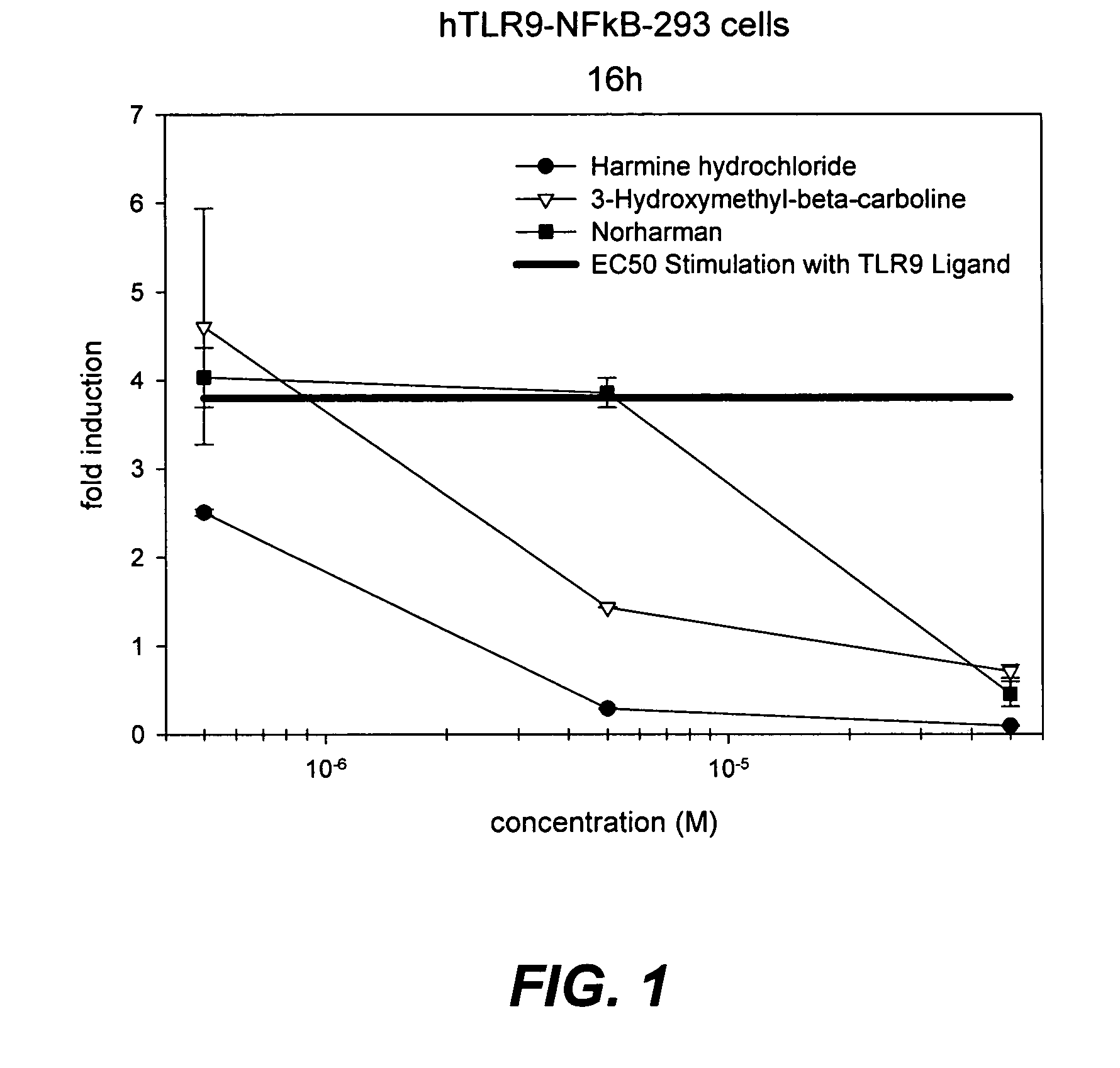
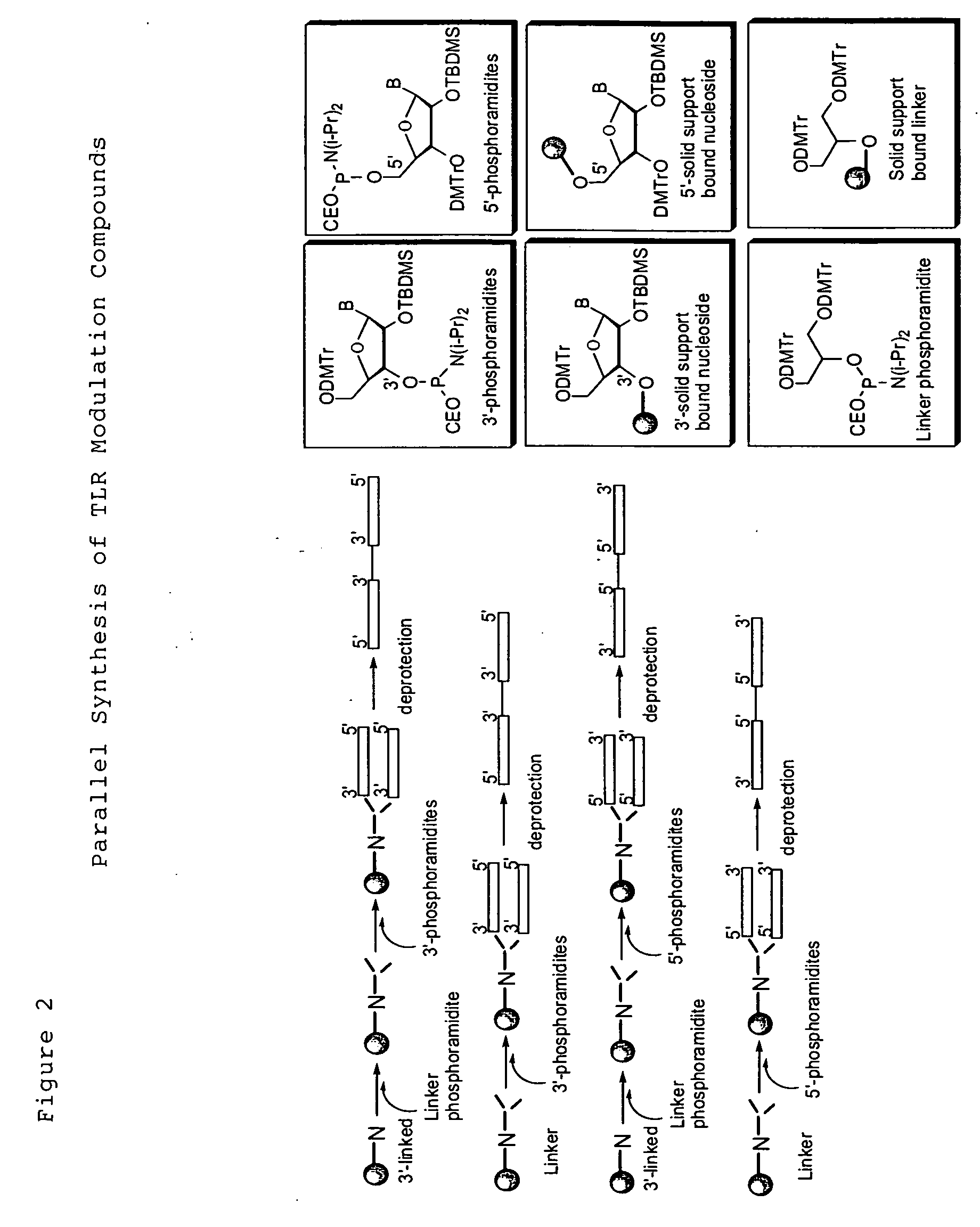
8-Substituted Benzoazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
Small molecule toll-like receptor (TLR) antagonists
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Owner:COLEY PHARMA GMBH +1
Immunosuppression modulating compounds
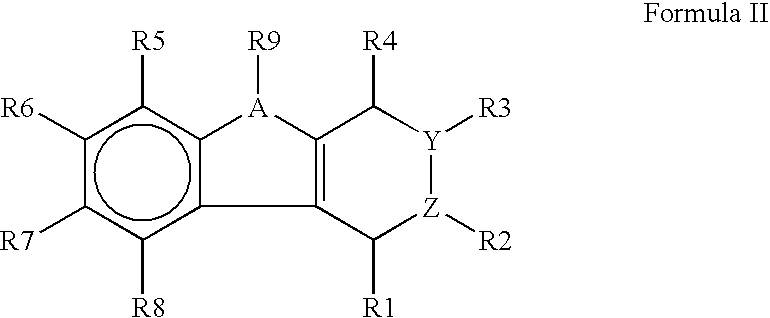
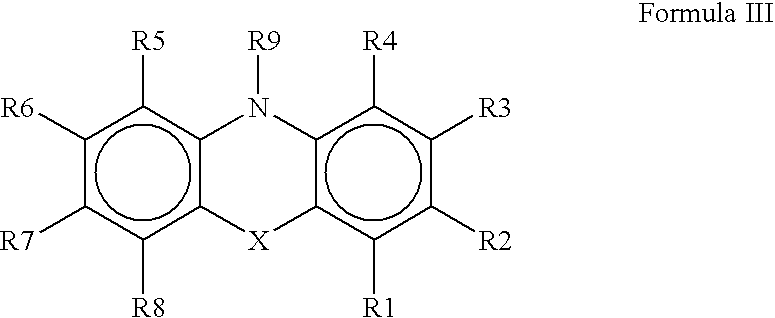
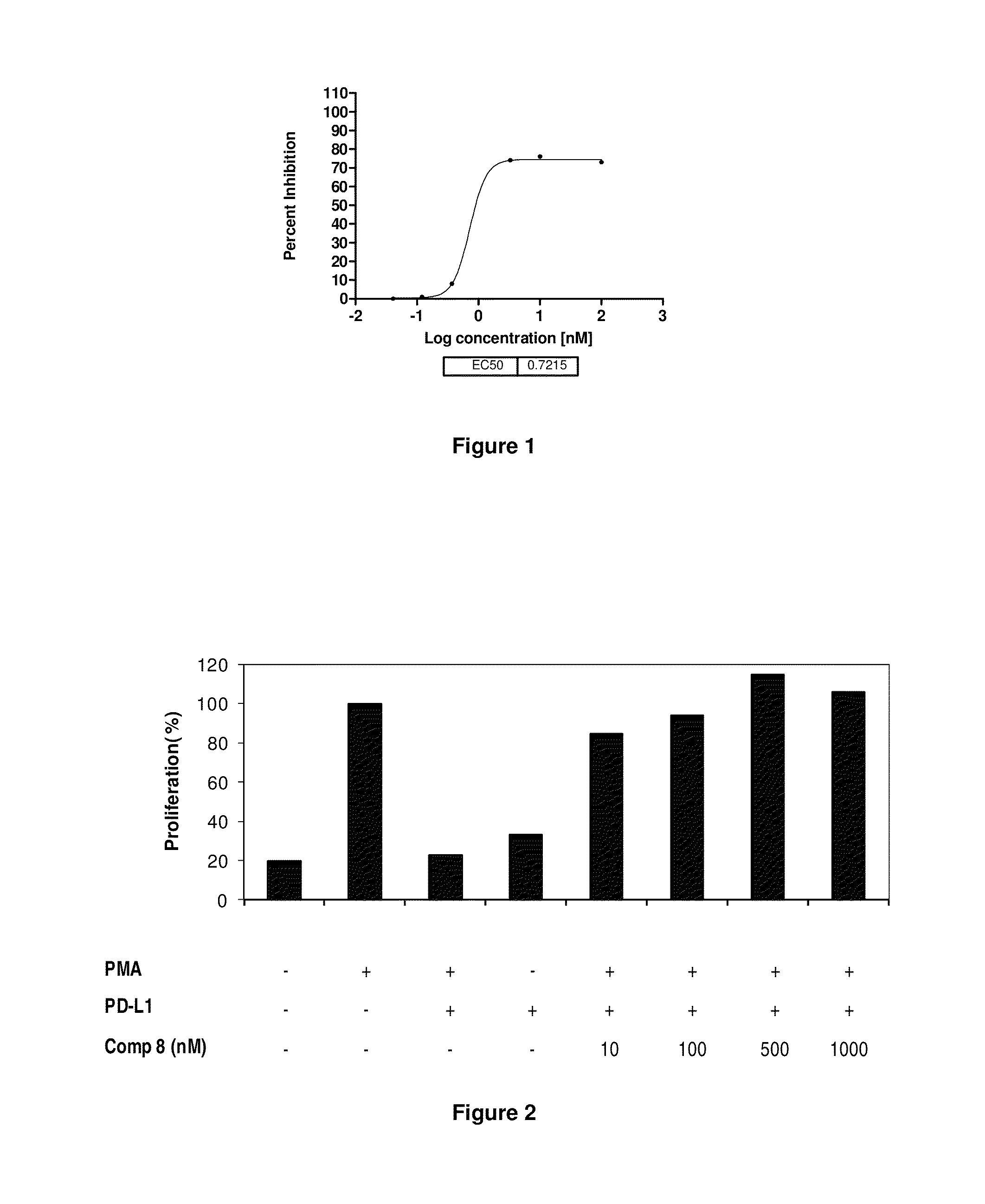
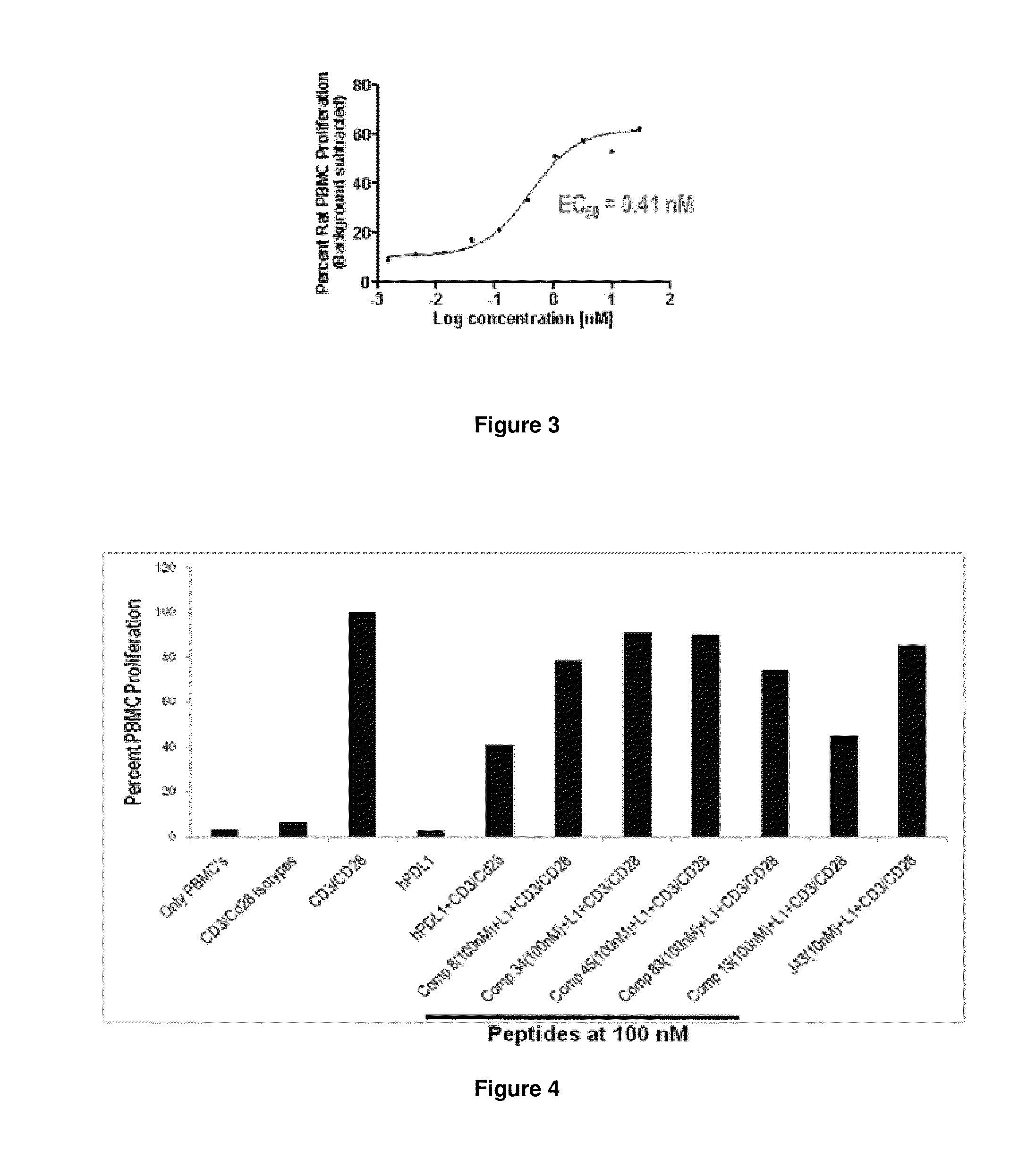
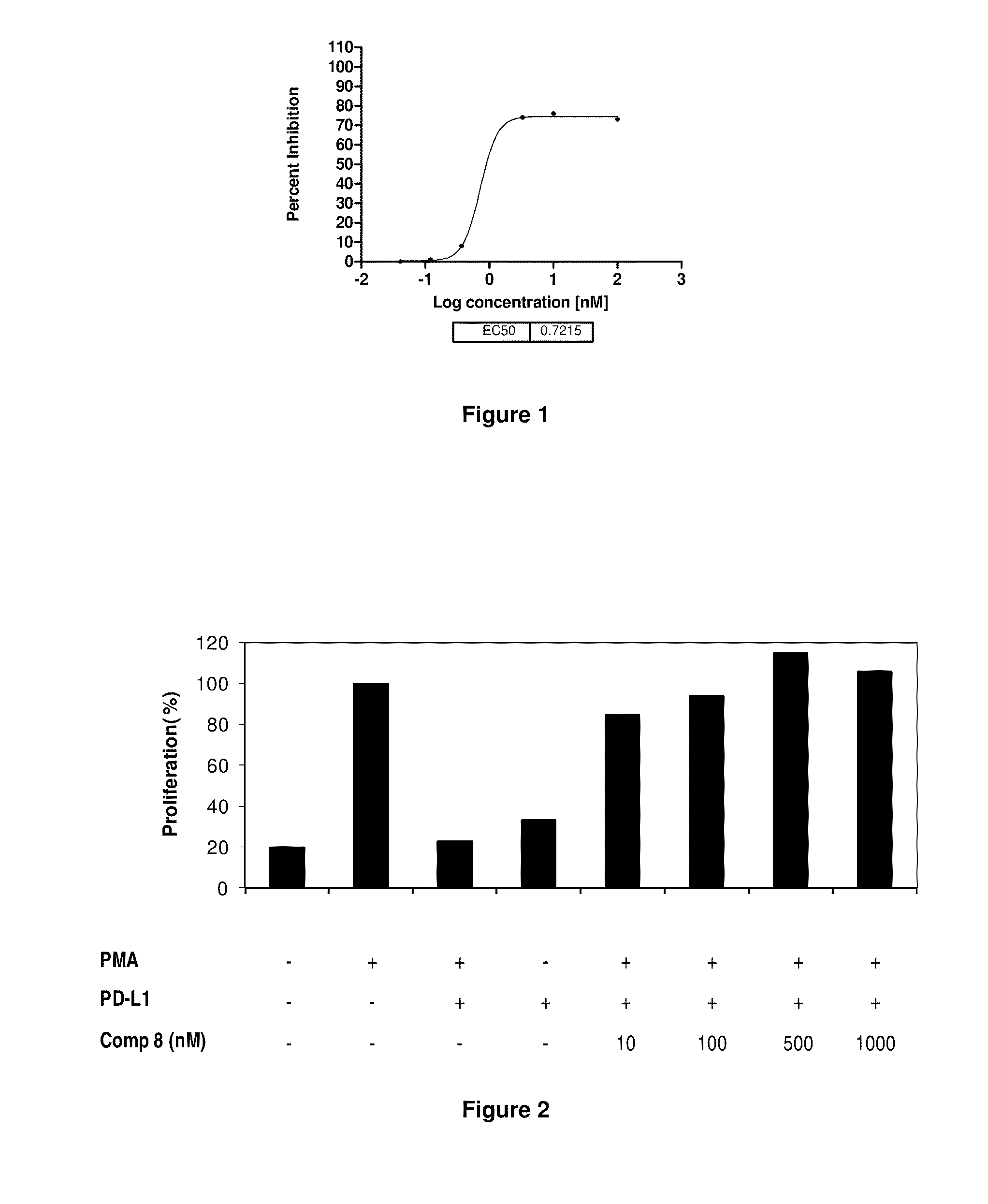
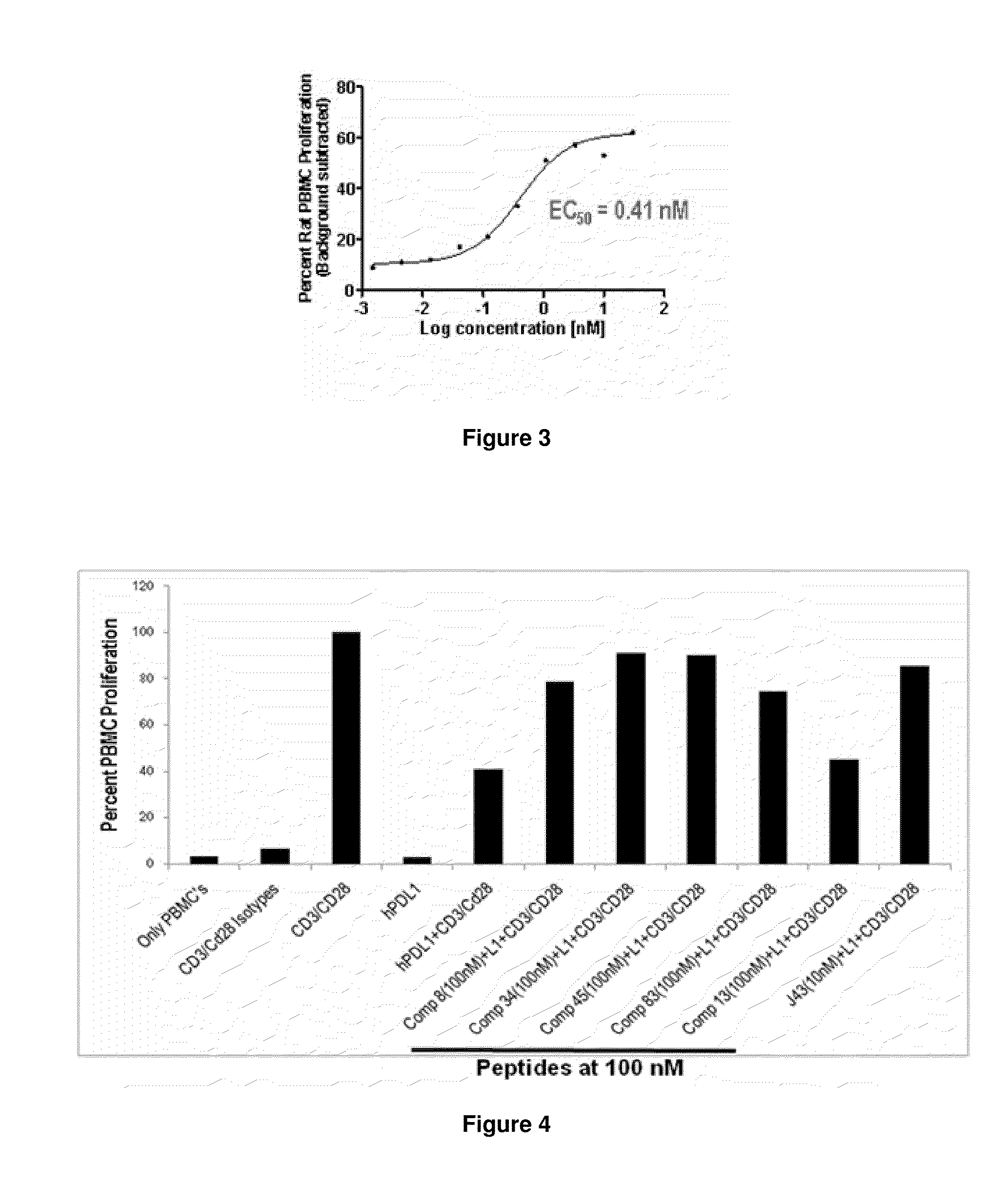
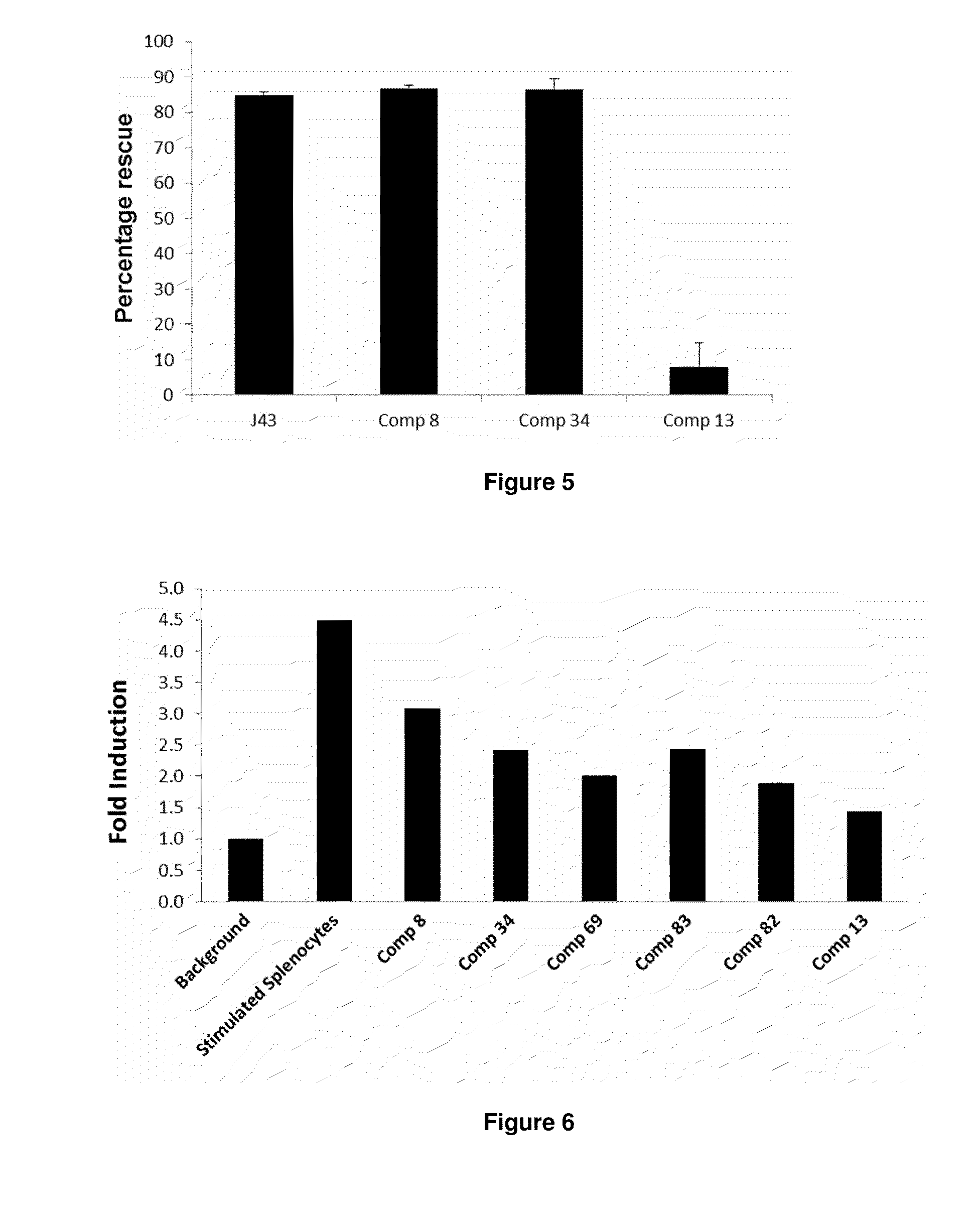
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Aminodiazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation signaling through the Toll-like receptor TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
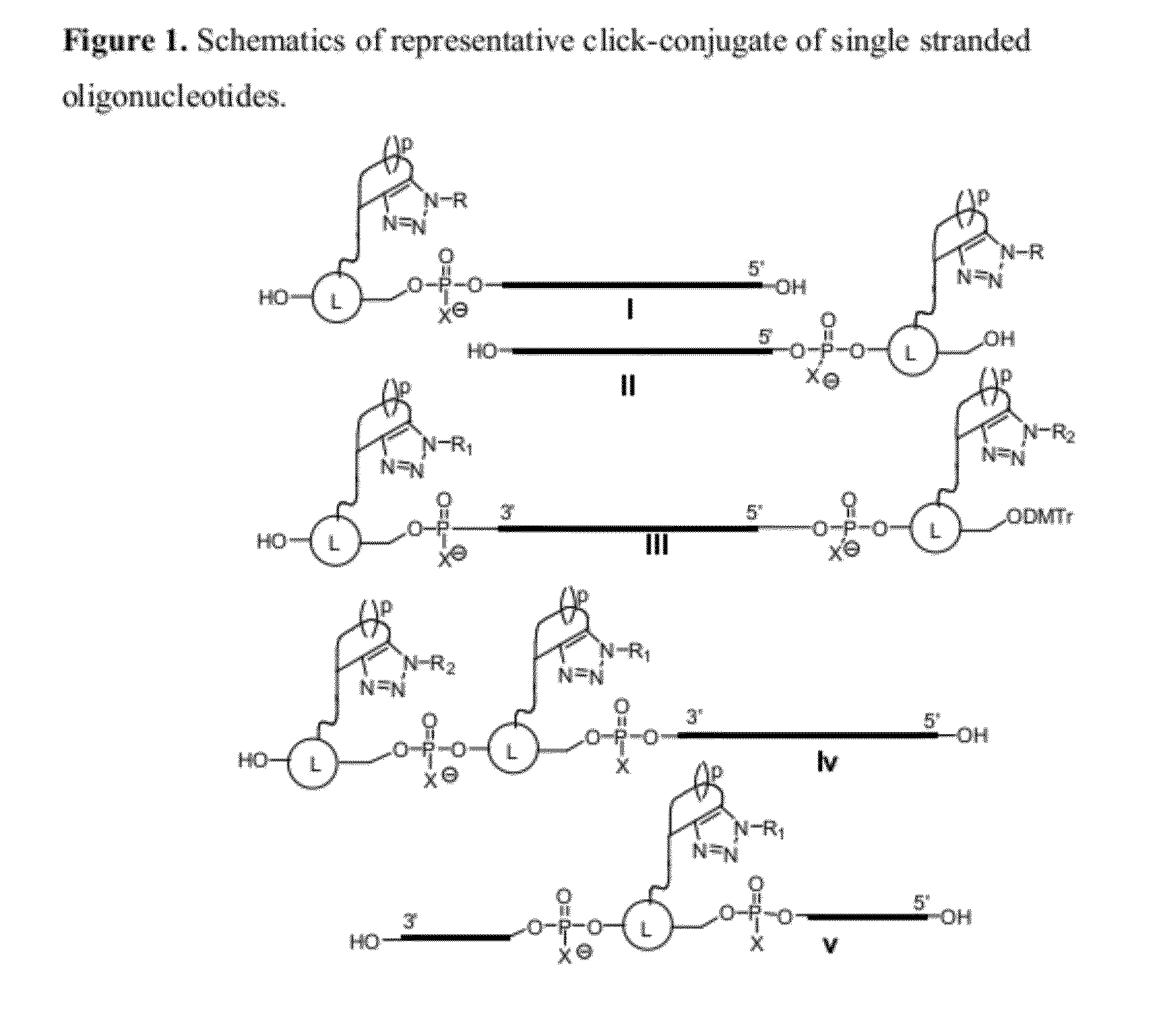
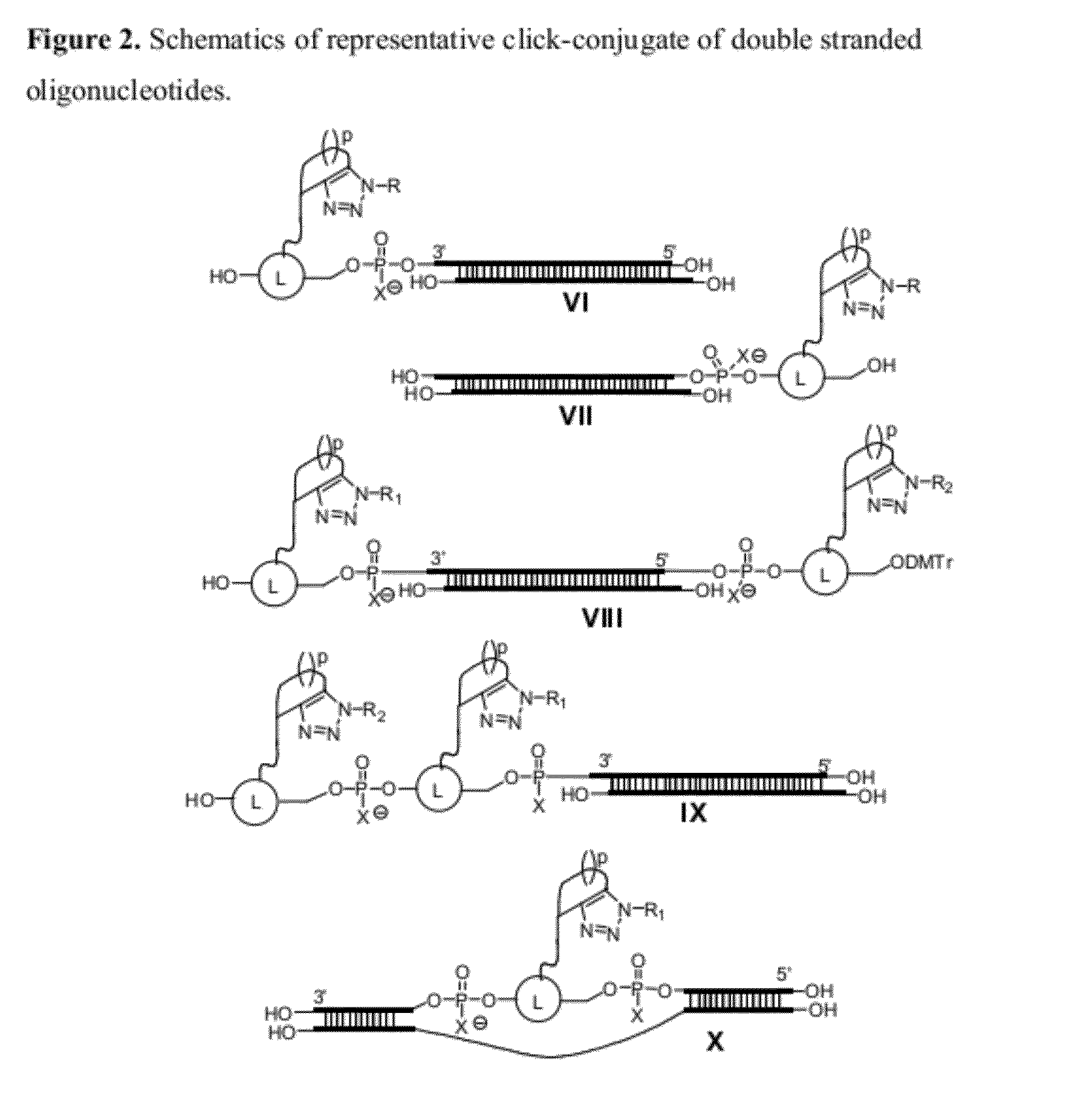
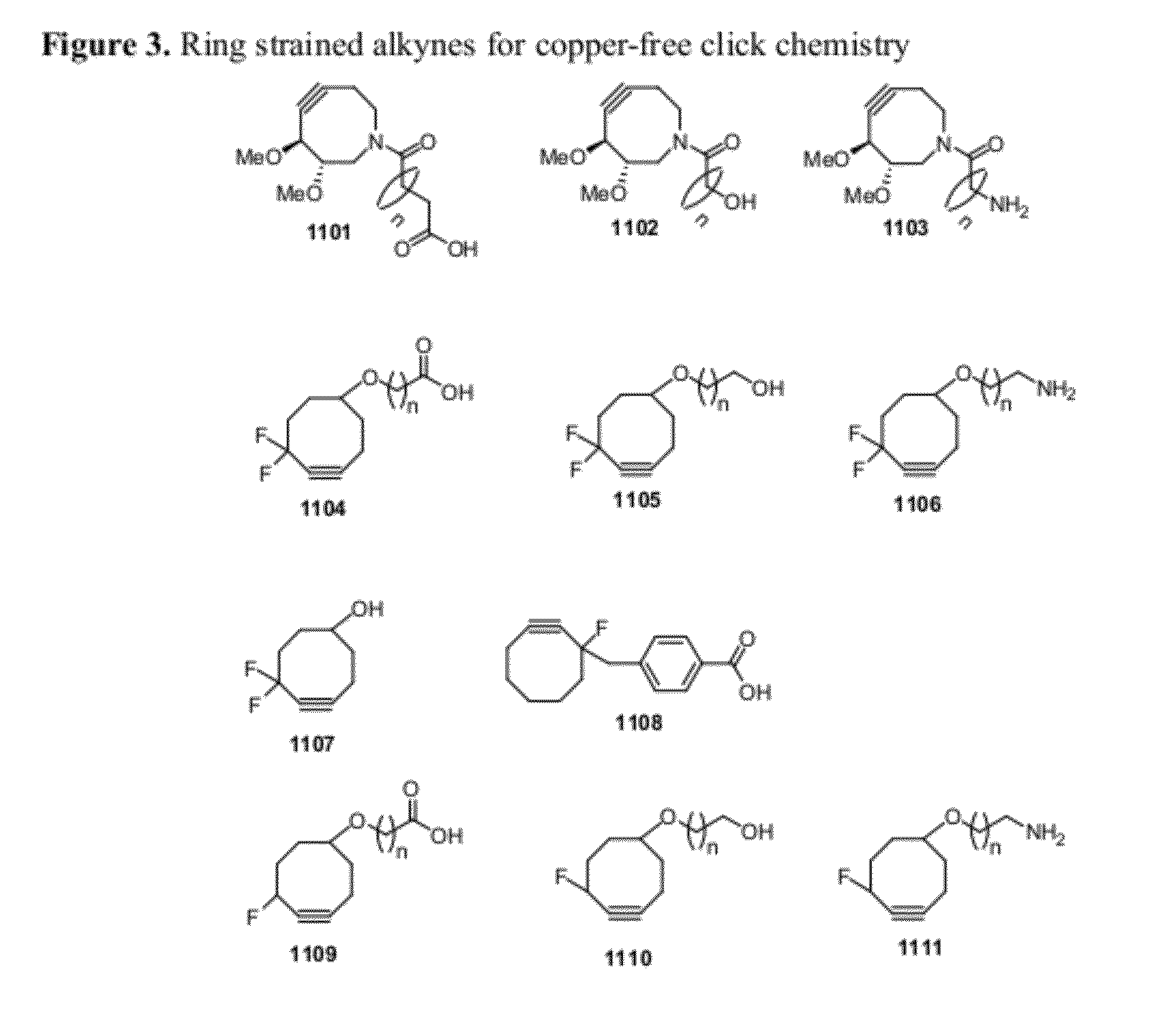
Chemical modifications of monomers and oligonucleotides with cycloaddition
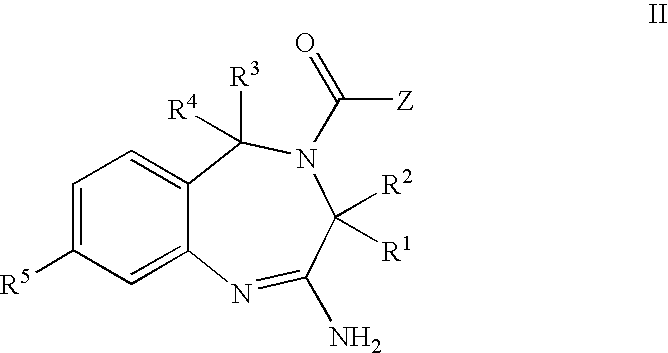
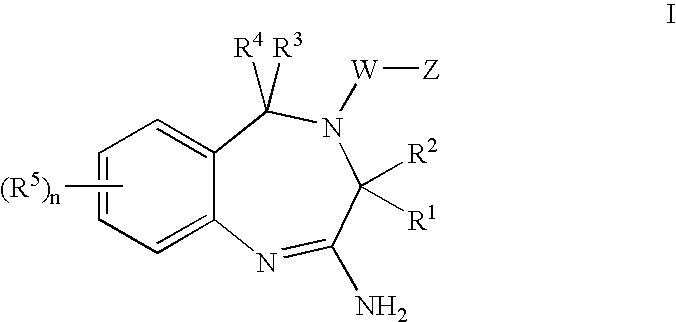
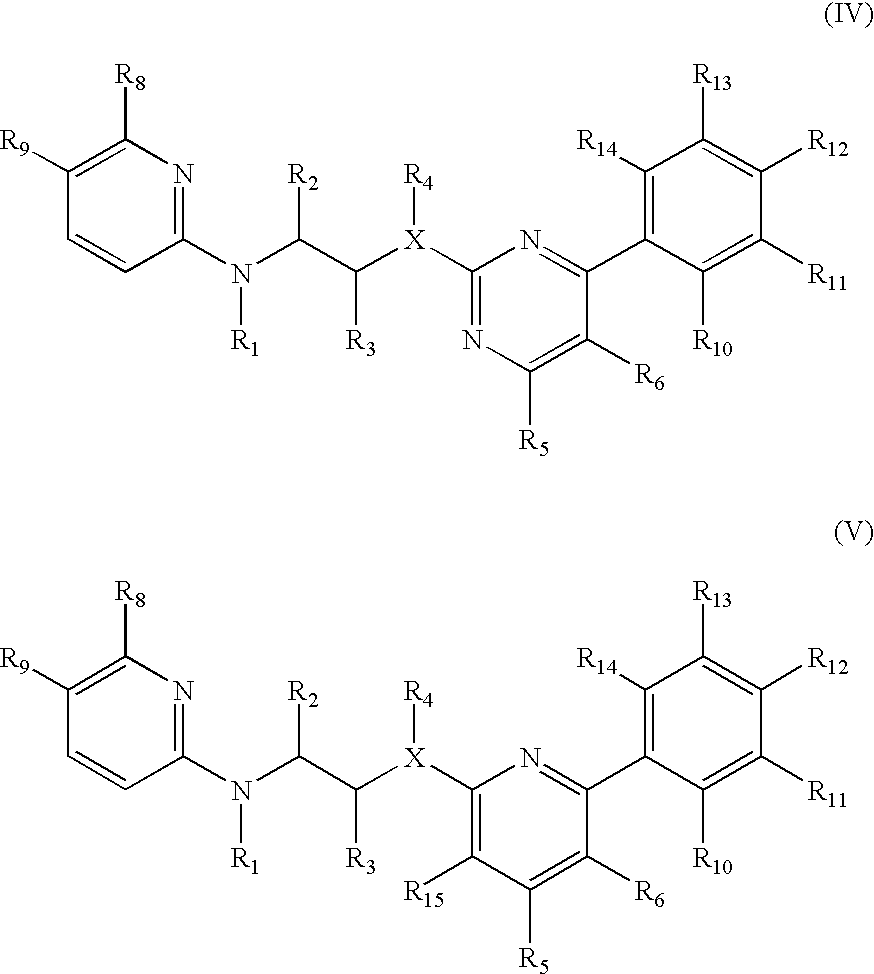
The invention features compounds of formula I or II:In one embodiment, the invention relates compounds and processes for conjugating ligand to oligonucleotide. The invention further relates to methods for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, cancers, allergies, autoimmune diseases, immunodeficiencies and immunosuppression.
Owner:ALNYLAM PHARMA INC
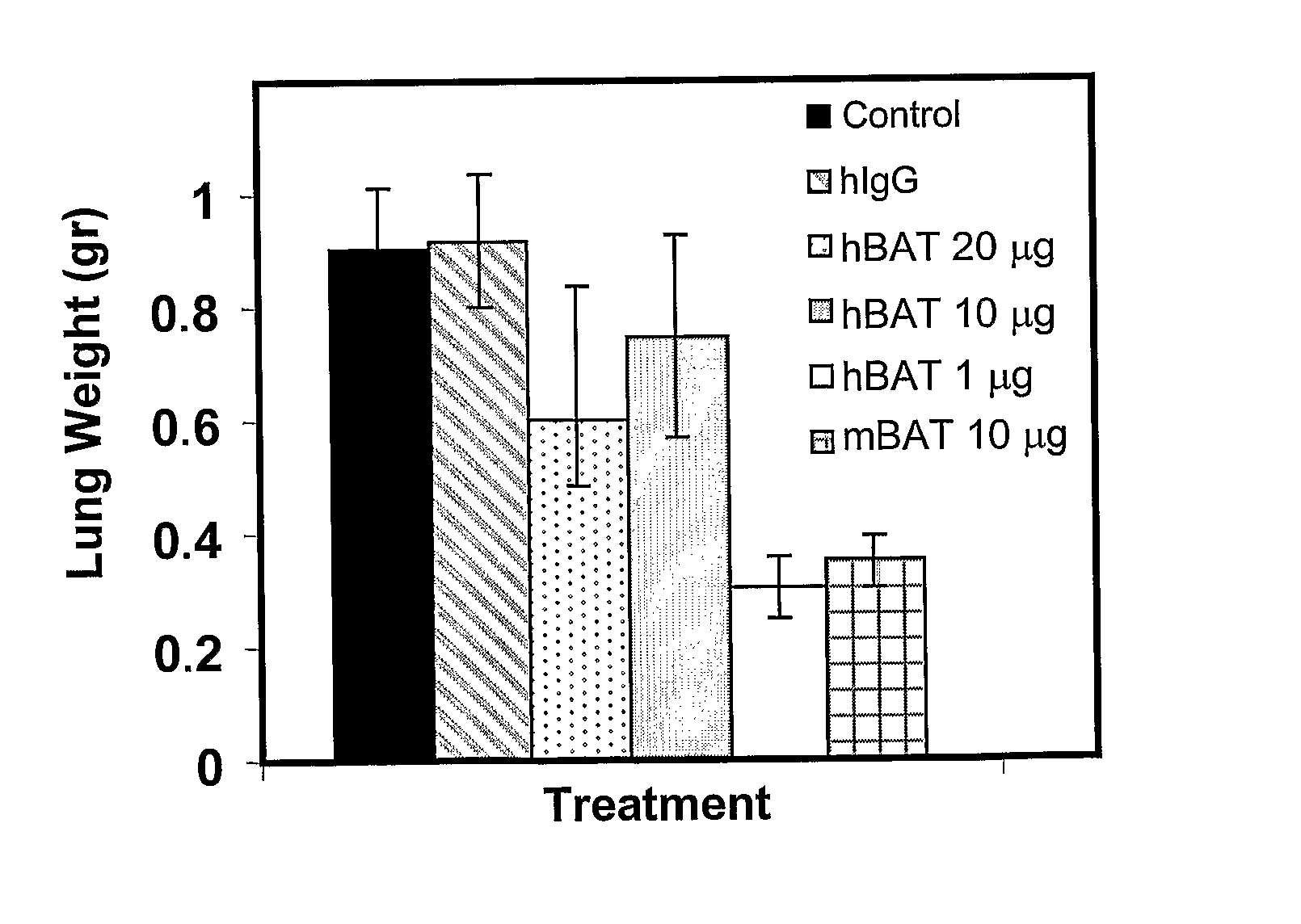
Humanized immunomodulatory monoclonal antibodies for the treatment of neoplastic disease or immunodeficiency
InactiveUS20080025980A1Induces proliferation and activationAvoids adverse immunogenic responseHybrid immunoglobulinsAntiviralsDiseaseAntiendomysial antibodies
Owner:CURETECH LTD +1
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
Immunosuppression modulating compounds
InactiveUS20110318373A1Reduce the binding forceAntibacterial agentsBiocideDiseaseSignalling pathways
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Small molecule toll-like receptor (TLR) antagonists
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Owner:COLEY PHARMA GMBH +1
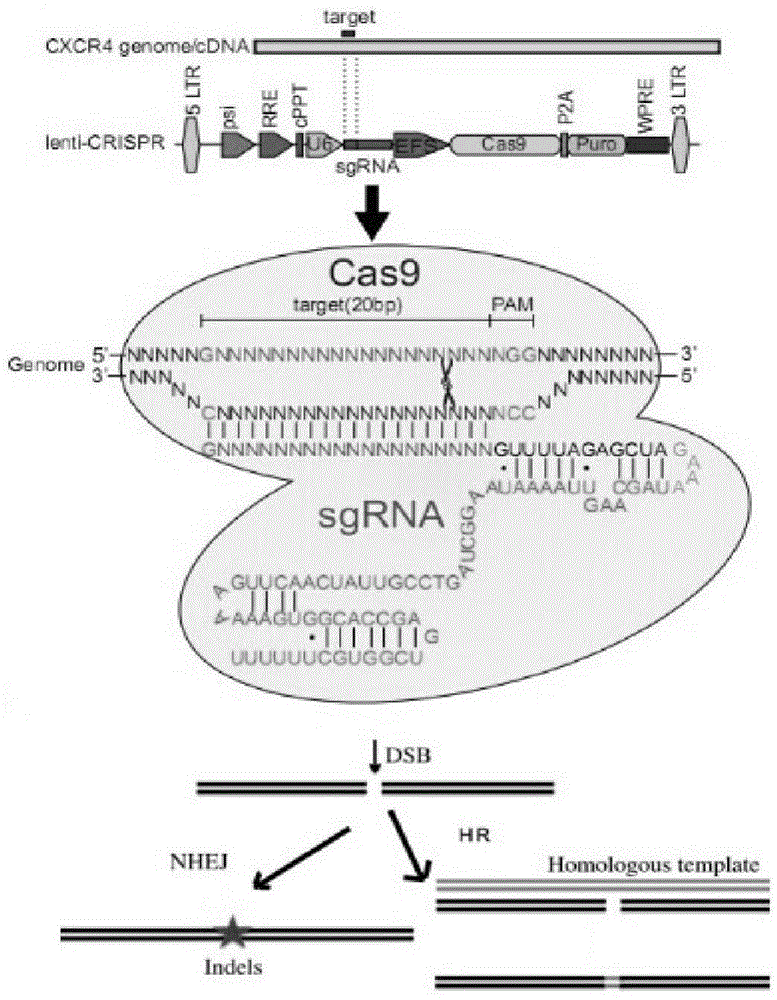
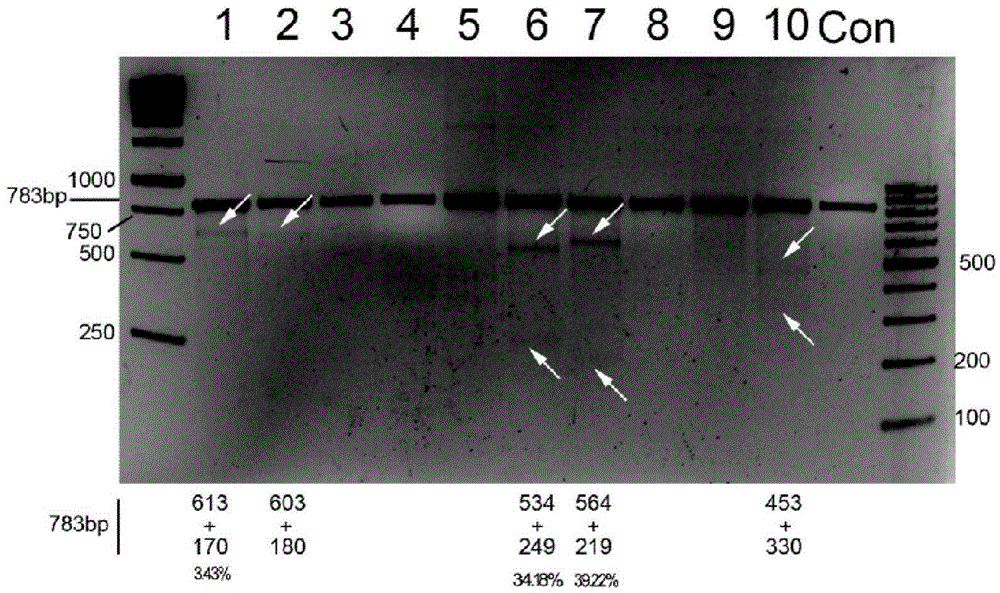
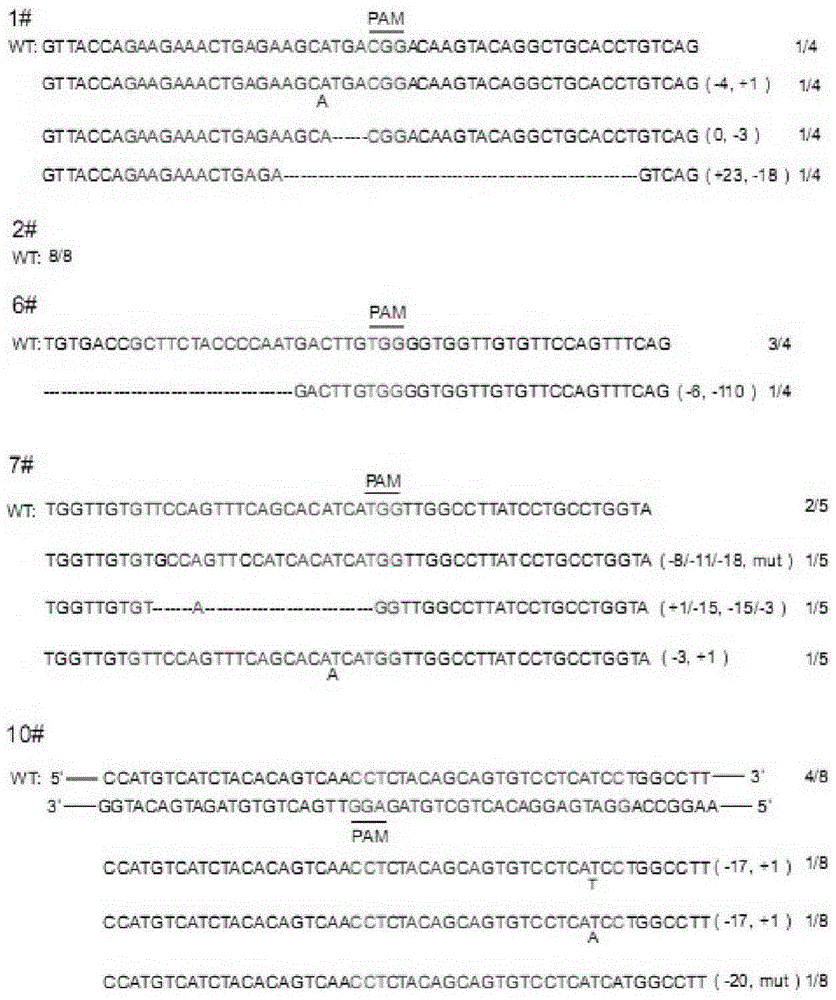
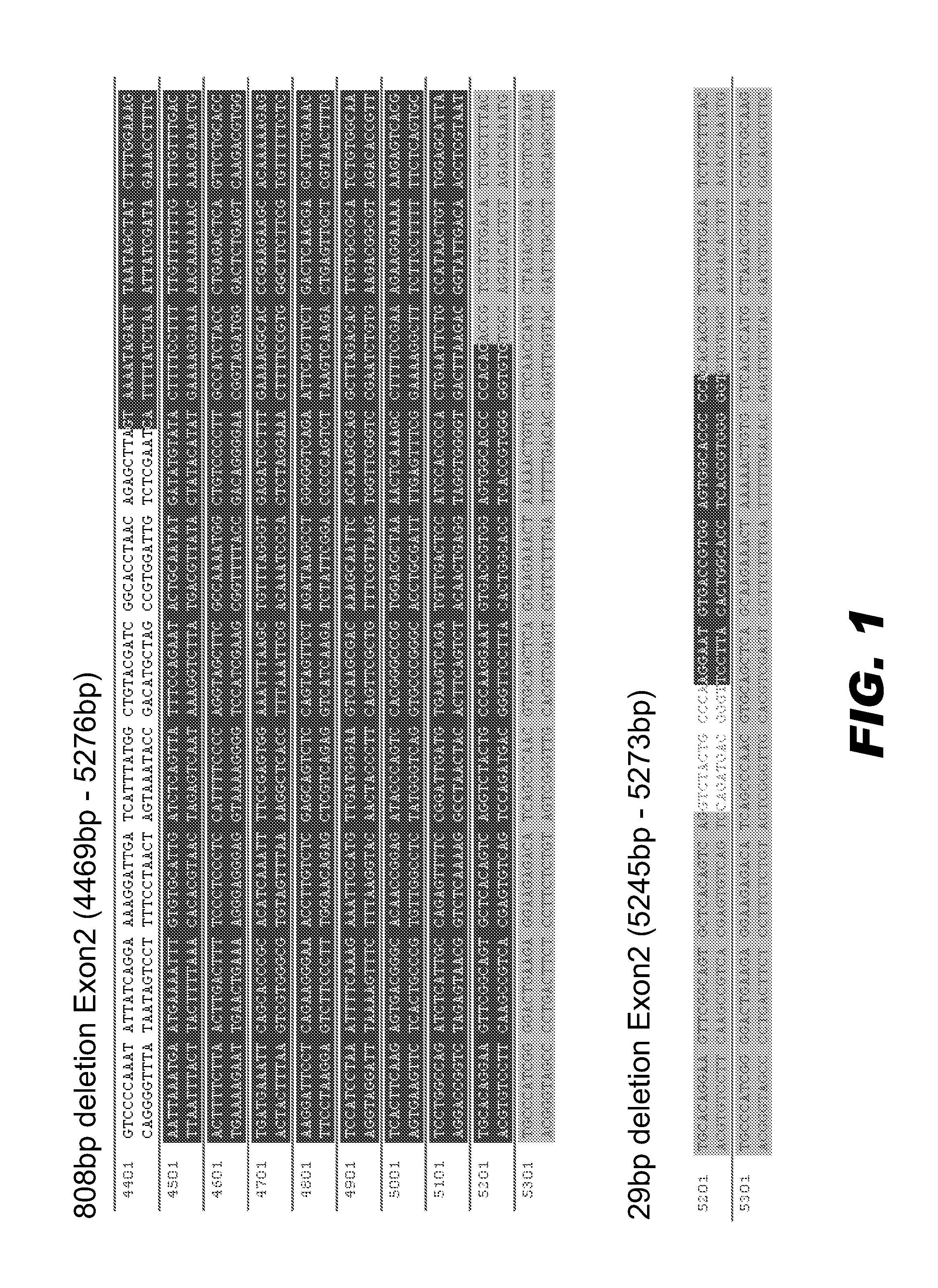
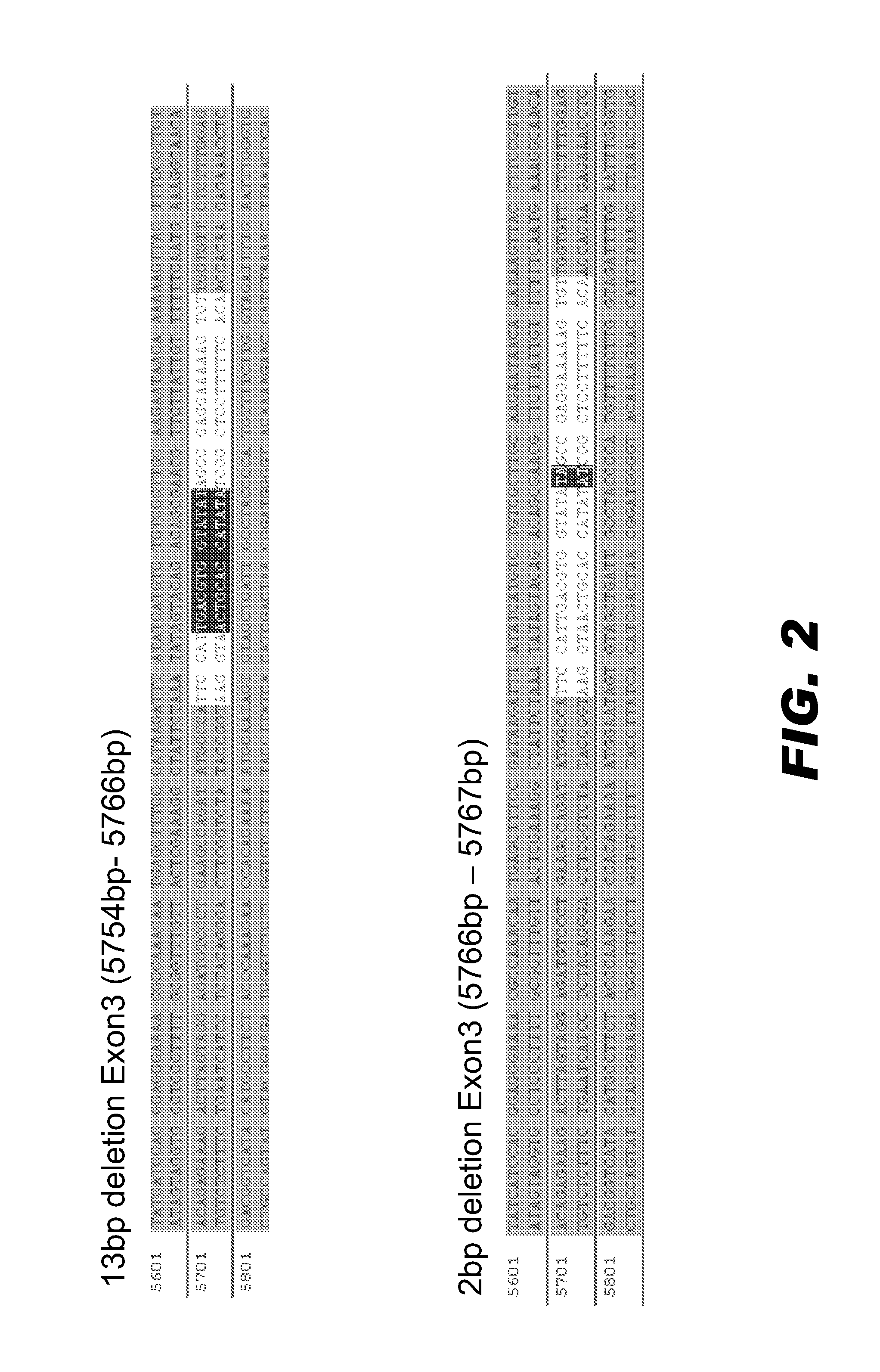
CRISPR/Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and lentivirus of CRISPR/Cas9 recombinant lentiviral vector
ActiveCN104480144AAvoid or delay intrusionInhibit the spread of infectionGenetic material ingredientsAntiviralsEnzyme digestionCXCR4
The invention belongs to the field of pharmaceutical and biological engineering, and relates to a CRISPR / Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and a lentivirus of the CRISPR / Cas9 recombinant lentiviral vector. The recombinant lentiviral vector is prepared by carrying out enzyme digestion on a lentiviral vectorlentiCRISPR by BsmBI and connecting into a BsmBI cohesive end-containing CXCR4 specific target sequence to recombine; the obtained CRISPR / Cas9 recombinant lentiviral vector is capable of mutating gene sequences at four different loci of ahuman immunodeficiency virusco-receptor CXCR4 and themutatuin rate is high and up to 25-75%. The cells transformed by the recombinant lentiviral vector cannot be infected by the human immunodeficiency virus. Compared with theRNAi-Knockdown, ZFN and TALEN technologies, the method has higher efficiency of suppressing the human immunodeficiency virus replication; the system is rapid to construct, simple and low in cost, is capable of preventing the invasion of the human immunodeficiency virus and is suitable for human immunodeficiency virus gene therapy.
Owner:WUHAN UNIV
Genome editing of immunodeficiency genes in animals
The present invention provides genetically modified animals and cells comprising edited chromosomal sequences encoding immunodeficiency proteins. In particular, the animals or cells are generated using a zinc finger nuclease-mediated editing process. Also provided are methods of assessing the effects of agents in genetically modified animals and cells comprising edited chromosomal sequences encoding immunodeficiency proteins.
Owner:SIGMA ALDRICH CO LLC
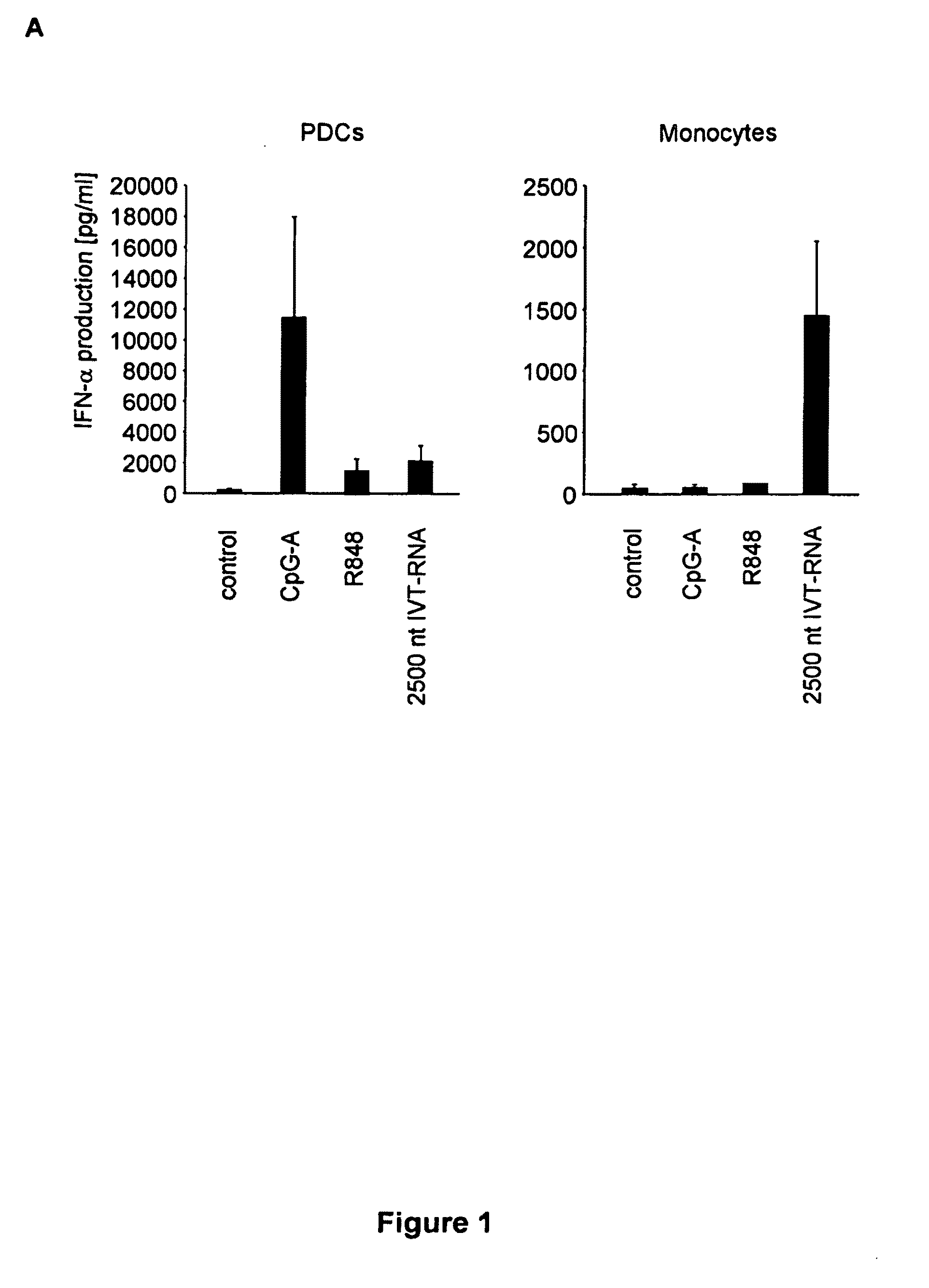
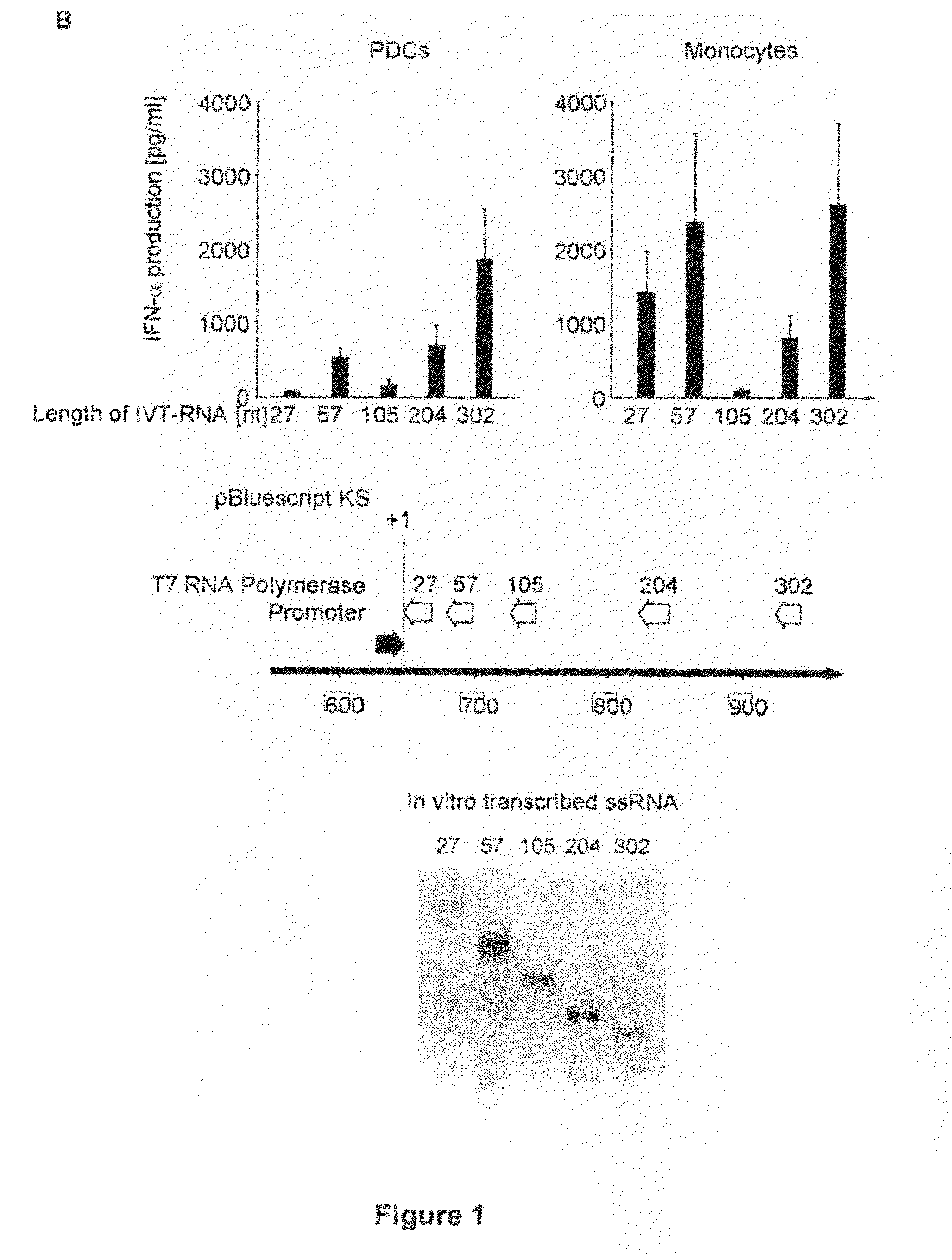
Structure and use of 5' phosphate oligonucleotides
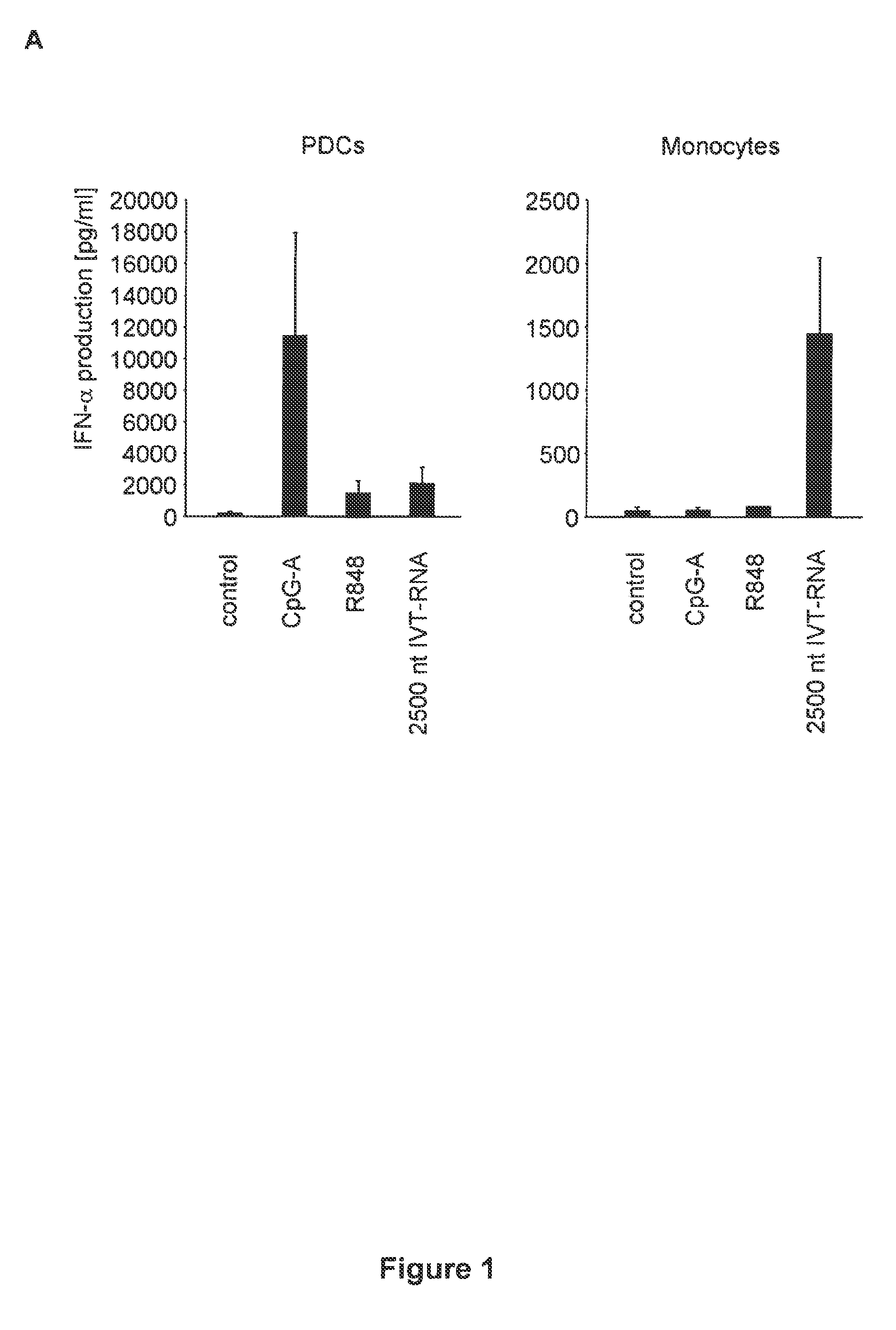
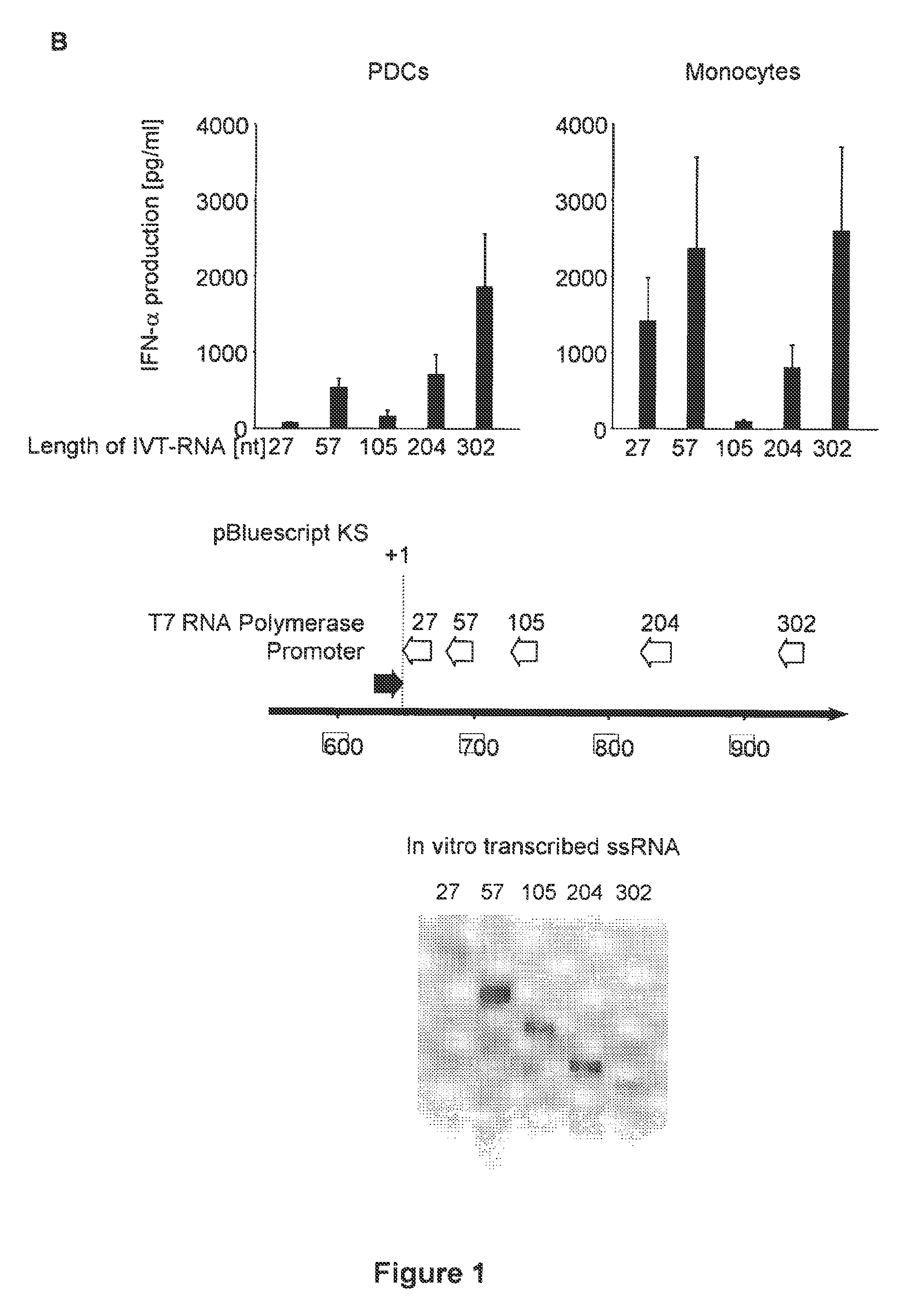
Oligonucleotides bearing free, uncapped 5′ phosphate group(s) are recognized by RIG-I, leading to the induction of type I IFN, IL-18 and IL-1β production. Bacterial RNA also induces type I IFN production. 5′ phosphate oligonucleotides and bacterial RNA can be used for inducing an anti-viral response or an anti-bacterial response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in vitro and in vivo and for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, tumors, allergies, autoimmune diseases, immunodeficiencies and immunosuppression. Single-stranded 5′ triphosphate RNA can be used for inducing an anti-viral response, an anti-bacterial response, or an anti-tumor response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in a target cell-specific manner.
Owner:UNIVERSITY OF BONN
Human monoclonal antibody binding to human cytomegalovirus and its antigen binding portion
The present invention aims to provide a human monoclonal antibody and an antigen binding portion of a human monoclonal antibody with higher affinity and neutralizing capacity to the human cytomegalovirus (HCMV), a virus which causes various diseases in situations where immunodeficiencies are present. The current invention provides an anti-human cytomegalovirus (HCMV) monoclonal antibody which is a human monoclonal antibody capable of binding to HCMV and neutralizing bioactivity of the HCMV, and which may be further characterized as possessing a light chain (L chain) comprising an amino acid sequence of SEQ ID. NO. 1, and has a heavy chain (H chain) comprising an amino acid sequence of SEQ ID NO. 2.
Owner:EVEC +1
Inhibitors of glycogen synthase kinase 3
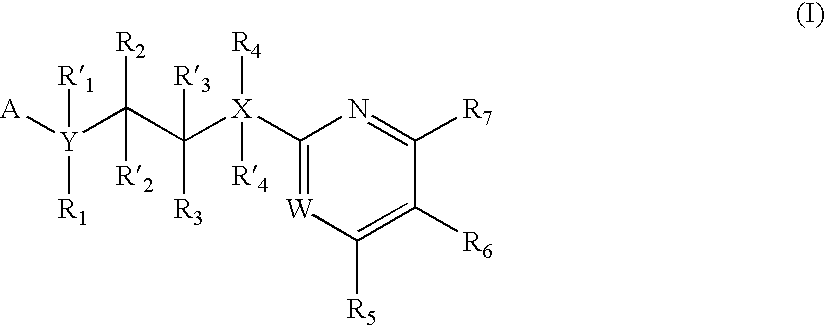
New pyrimidine or pyridine based compounds, compositions and methods of inhibiting the activity of glycogen synthase kinase (GSK3) in vitro and of treatment of GSK3 mediated disorders in vivo are provided. The methods, compounds and compositions of the invention may be employed alone, or in combination with other pharmacologically active agents in the treatment of disorders mediated by GSK3 activity, such as in the treatment of diabetes, Alzheimer's disease and other neurodegenerative disorders, obesity, atherosclerotic cardiovascular disease, essential hypertension, polycystic ovary syndrome, syndrome X, ischemia, traumatic brain injury, bipolar disorder, immunodeficiency or cancer.
Owner:CHIRON CORP
Methods for treating viral infection using il-28 and il-29 cysteine mutants
InactiveUS20080075693A1Prolonged Circulatory Half-LifeLow immunogenicityPeptide/protein ingredientsAntipyreticInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Inhibitors of glycogen synthase kinase 3
Owner:CHIRON CORP
Genetically modified non-human animals and methods of use thereof
ActiveUS20140134662A1Reduce rateReduced viabilityCompounds screening/testingThrombopoietinDiseaseHematopoietic cell
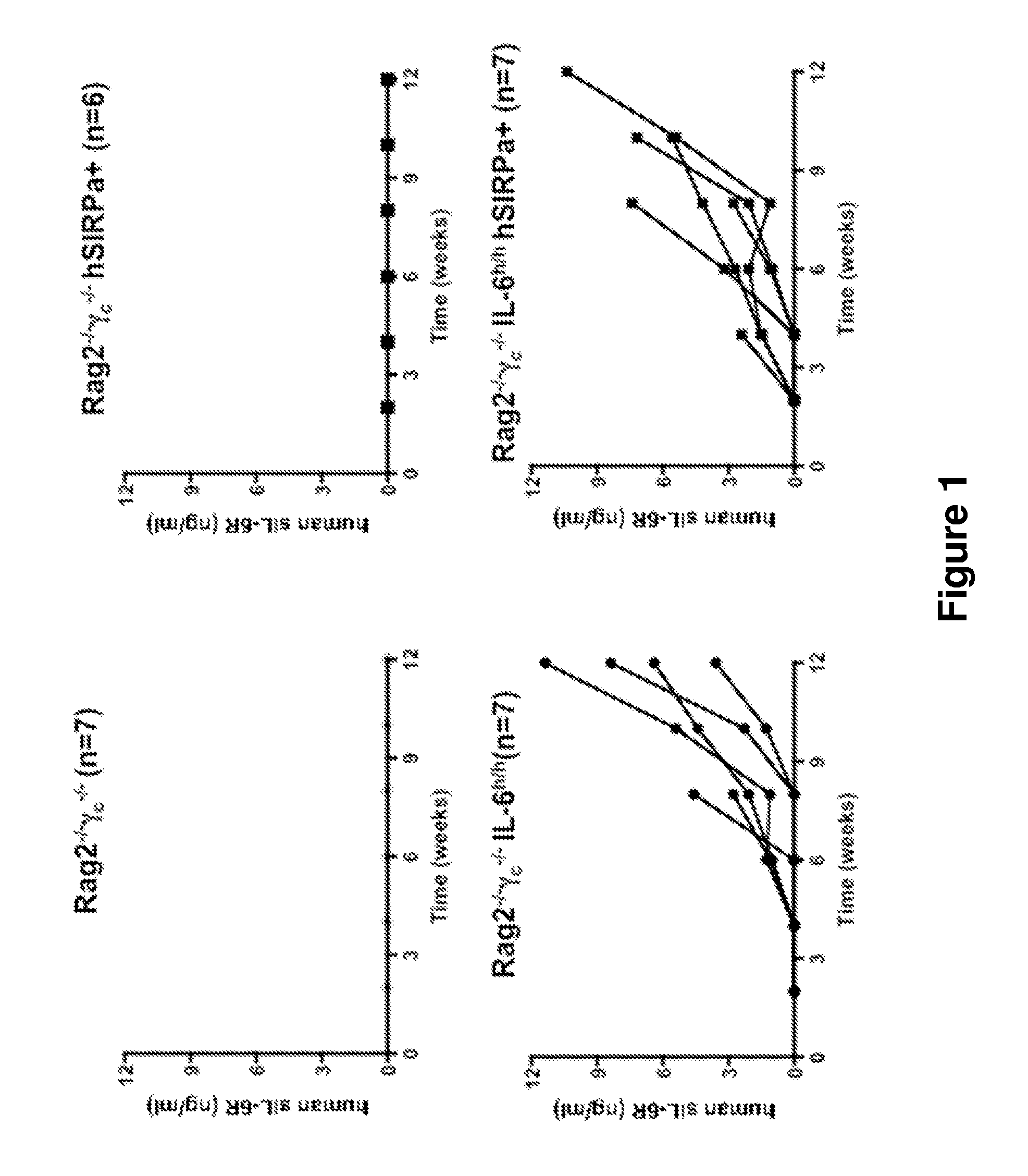
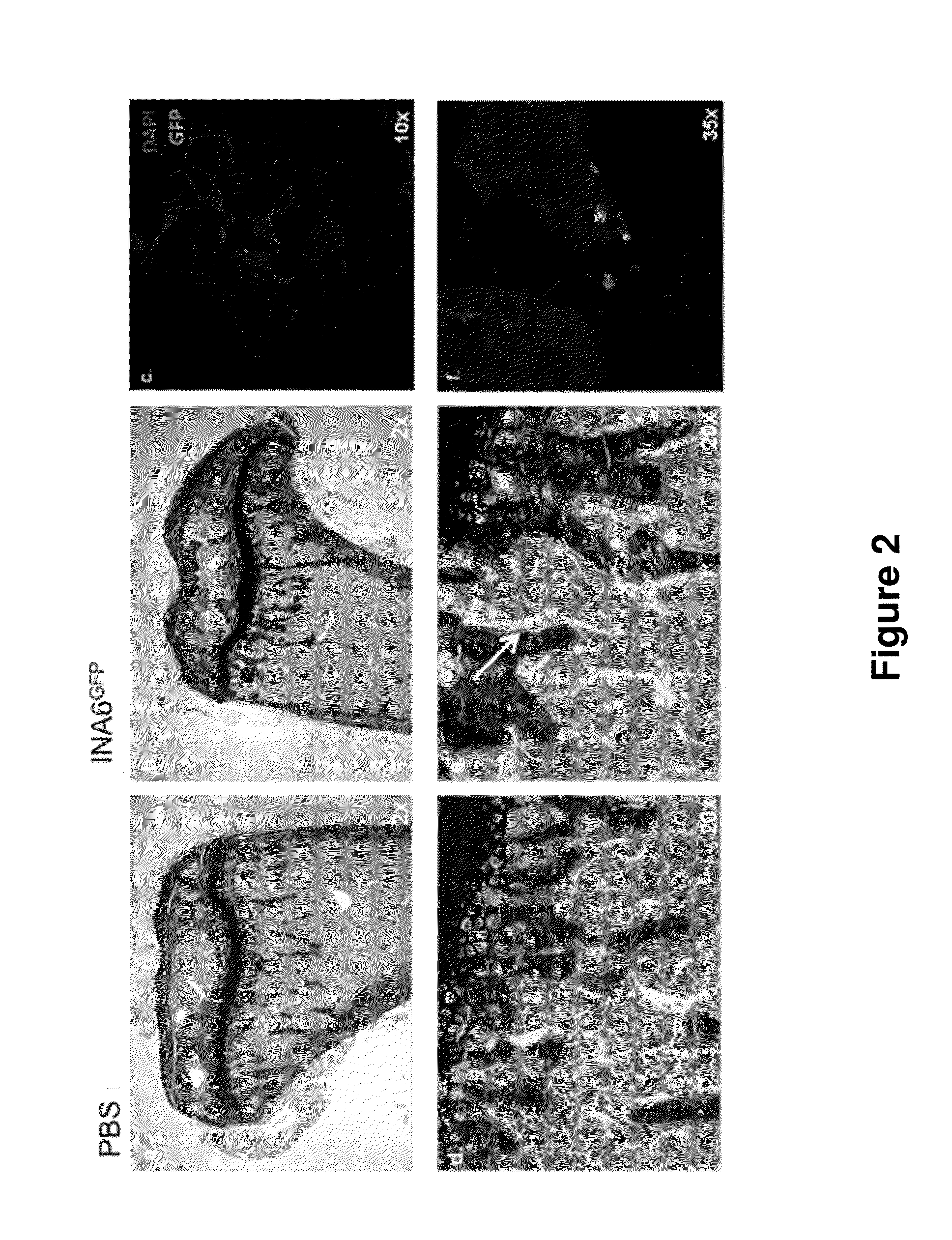
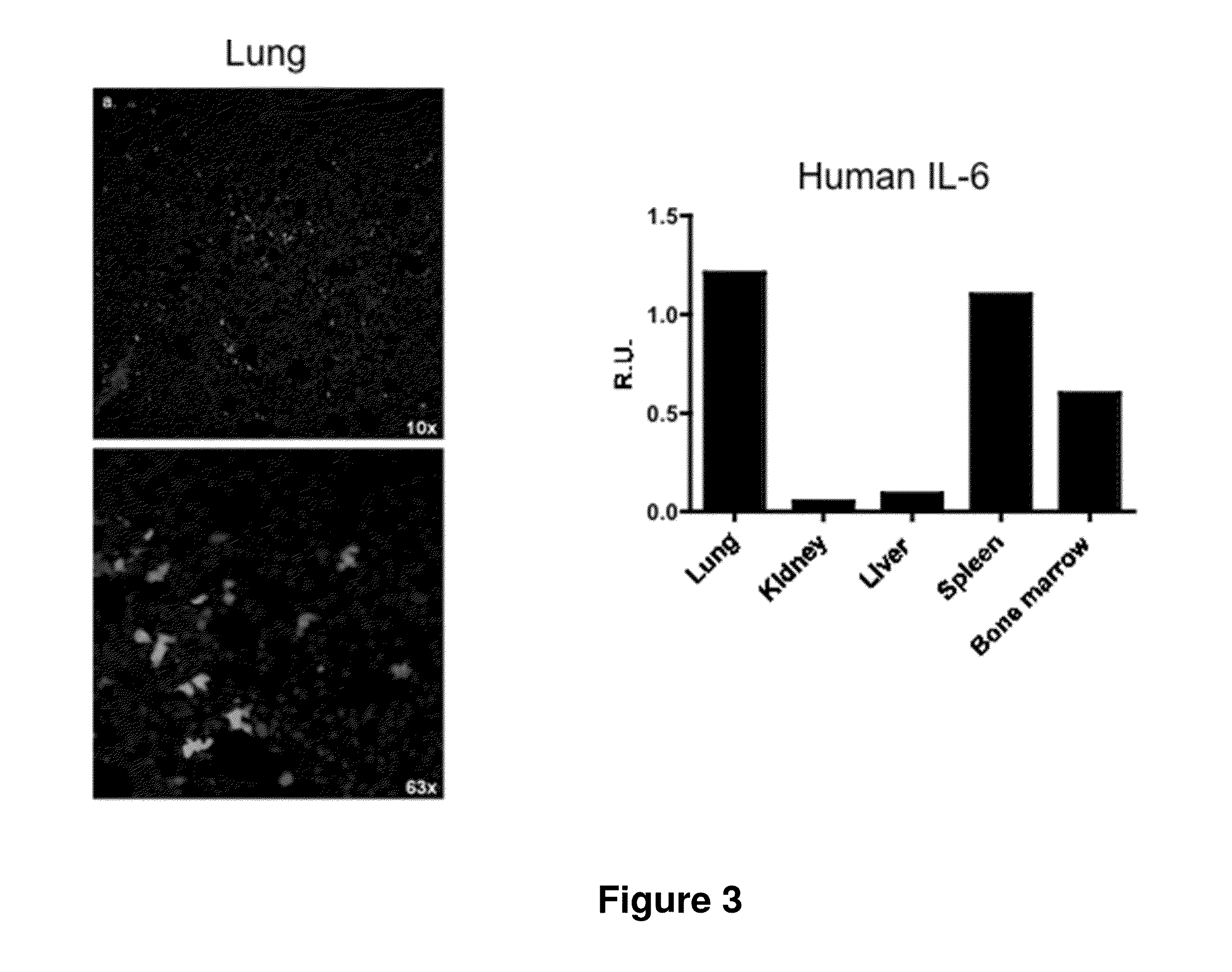
Genetically modified non-human animals are provided that may be used to model human hematopoietic cell development, function, or disease. The genetically modified non-human animals comprise a nucleic acid encoding human IL-6 operably linked to an IL-6 promoter. In some instances, the genetically modified non-human animal expressing human IL-6 also expresses at least one of human M-CSF, human IL-3, human GM-CSF, human SIRPa or human TPO. In some instances, the genetically modified non-human animal is immunodeficient. In some such instances, the genetically modified non-human animal is engrafted with healthy or diseased human hematopoietic cells. Also provided are methods for using the subject genetically modified non-human animals in modeling human hematopoietic cell development, function, and / or disease, as well as reagents and kits thereof that find use in making the subject genetically modified non-human animals and / or practicing the subject methods.
Owner:REGENERON PHARM INC +3
Patient-specific stem cell lines derived from human parthenogenetic blastocysts
InactiveUS20080299091A1Increase alkaline phosphatase activityHigh levelBiocideSenses disorderTelomeraseOocyte donor
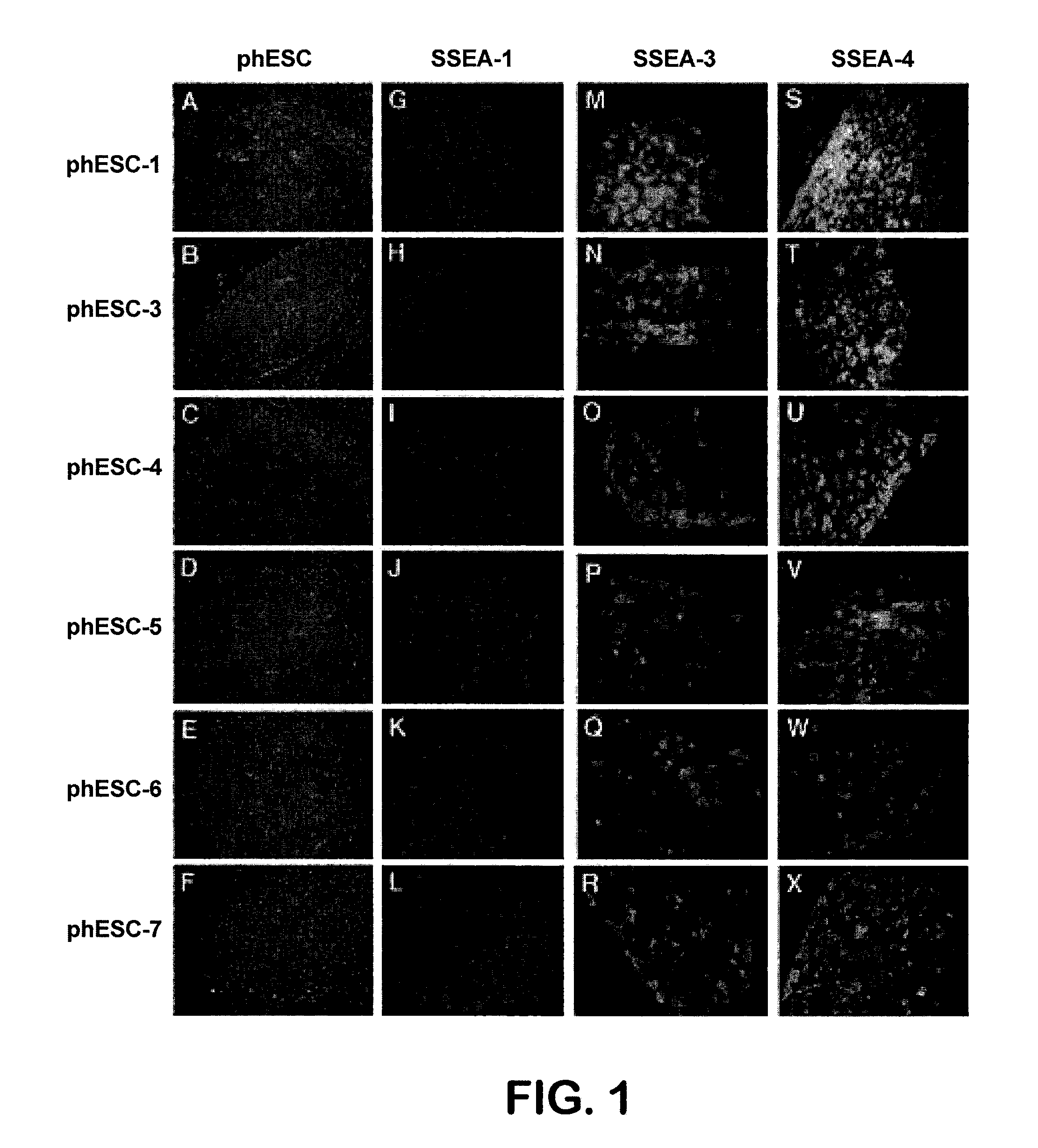
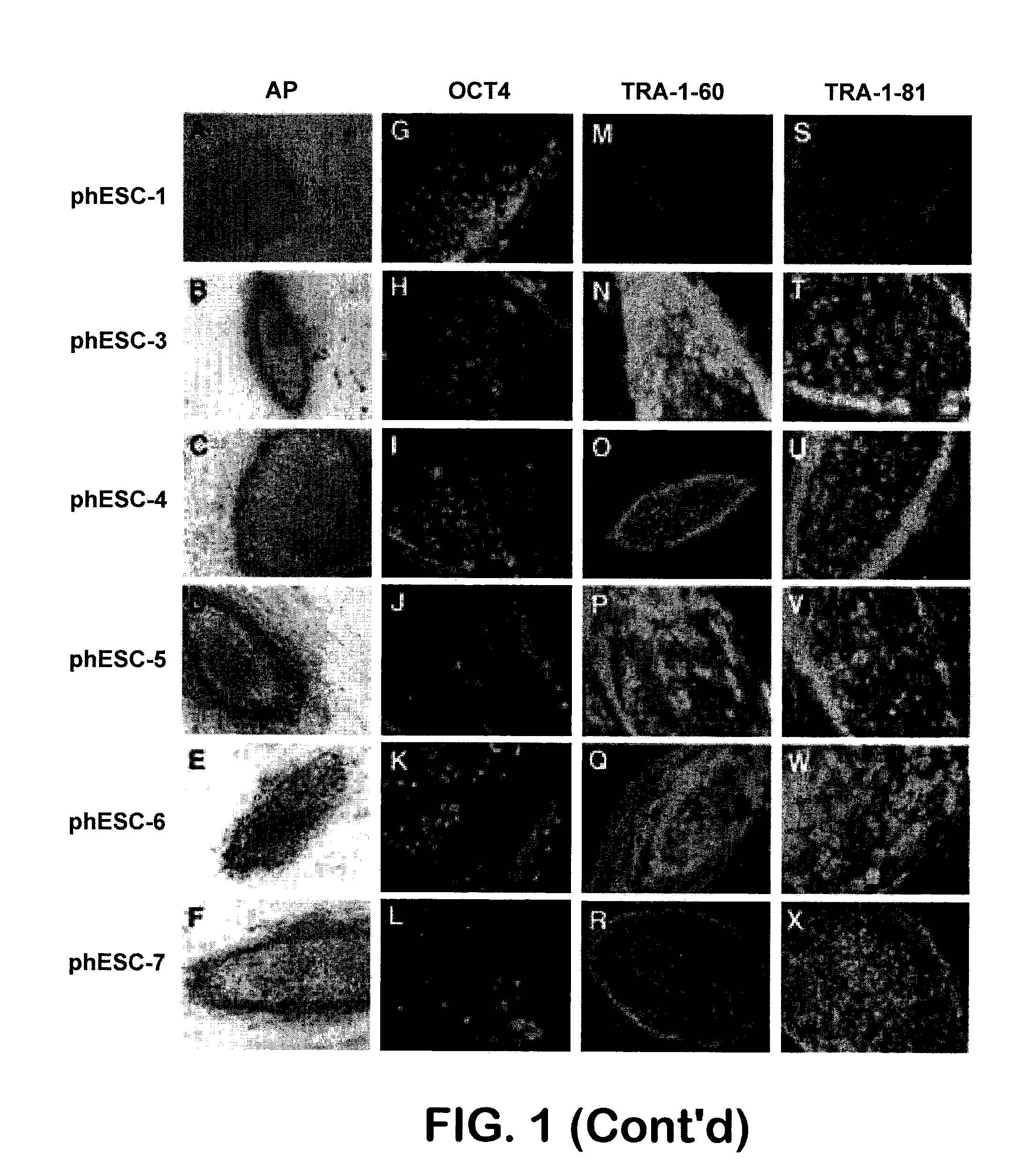
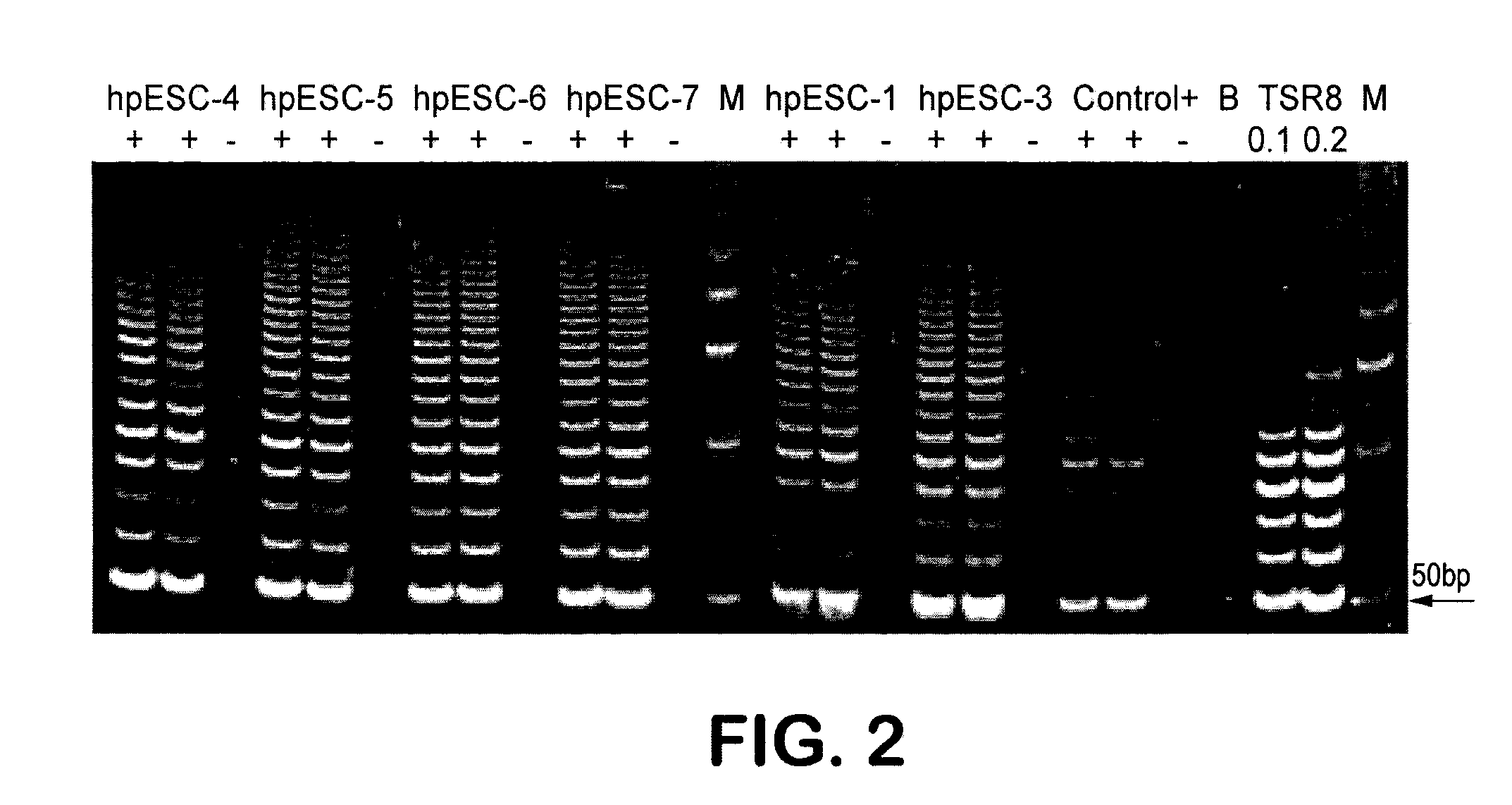
Methods are disclosed for generating HLA homozygous parthenogenetic human stem cell (hpSC-Hhom) lines from both HLA homozygous and HLA heterozygous donors. These hpSC-Hhom lines demonstrate typical human embryonic stem cell morphology, expressing appropriate stem cell markers and possessing high levels of alkaline phosphatase and telomerase activity. Additionally, injection of these cell lines into immunodeficient animals leads to teratoma formation. Furthermore, in the case of HLA heterozygous donors, the hpSC-Hhom lines inherit the haplotype from only one of the donor's parents. SNP data analysis suggests that hpSC-Hhom lines derived from HLA heterozygous oocyte donors are homozygous throughout the genome as assessed by single-nucleotide polymorphism (SNP) analysis. The protocol as disclosed minimizes the use of animal-derived components, which makes the stem cells more practical for clinical application.
Owner:INT STEM CELL CORP
Therapeutic compounds for immunomodulation
The present invention provides Immunosuppressive compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signalling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides pharmaceutical compositions comprising the Immunosuppressive peptide compounds or modified peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of PD-1 or PD-L1 as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Method of preparing adenosine-resistant anti-tumor T lymphocytes for adoptive immunotherapy
In the past, adoptive immunotherapy often failed because the transferred immune cells were inactive in vivo. This disclosure provides a method of producing immune cells that are highly active in vivo. The immune cells may be expanded in vitro in the presence of an adenosine receptor agonist or an antisense nucleic acid that downregulates expression of an adenosine receptor, for example. The immune cells may be tumor-infiltrating lymphocytes (TIL), cytotoxic T lymphocytes (CTL), natural killer (NK) cells, or lymphokine-activated killer (LAK) cells, for example. The methods described herein may be used to treat a number of diseases including cancer, infectious diseases, and immunodeficiencies.
Owner:NORTHEASTERN UNIV
Toll like receptor modulators
ActiveUS20090053148A1Prevent diseaseLower immune responseSenses disorderNervous disorderInflammatory Bowel DiseasesAutoimmune responses
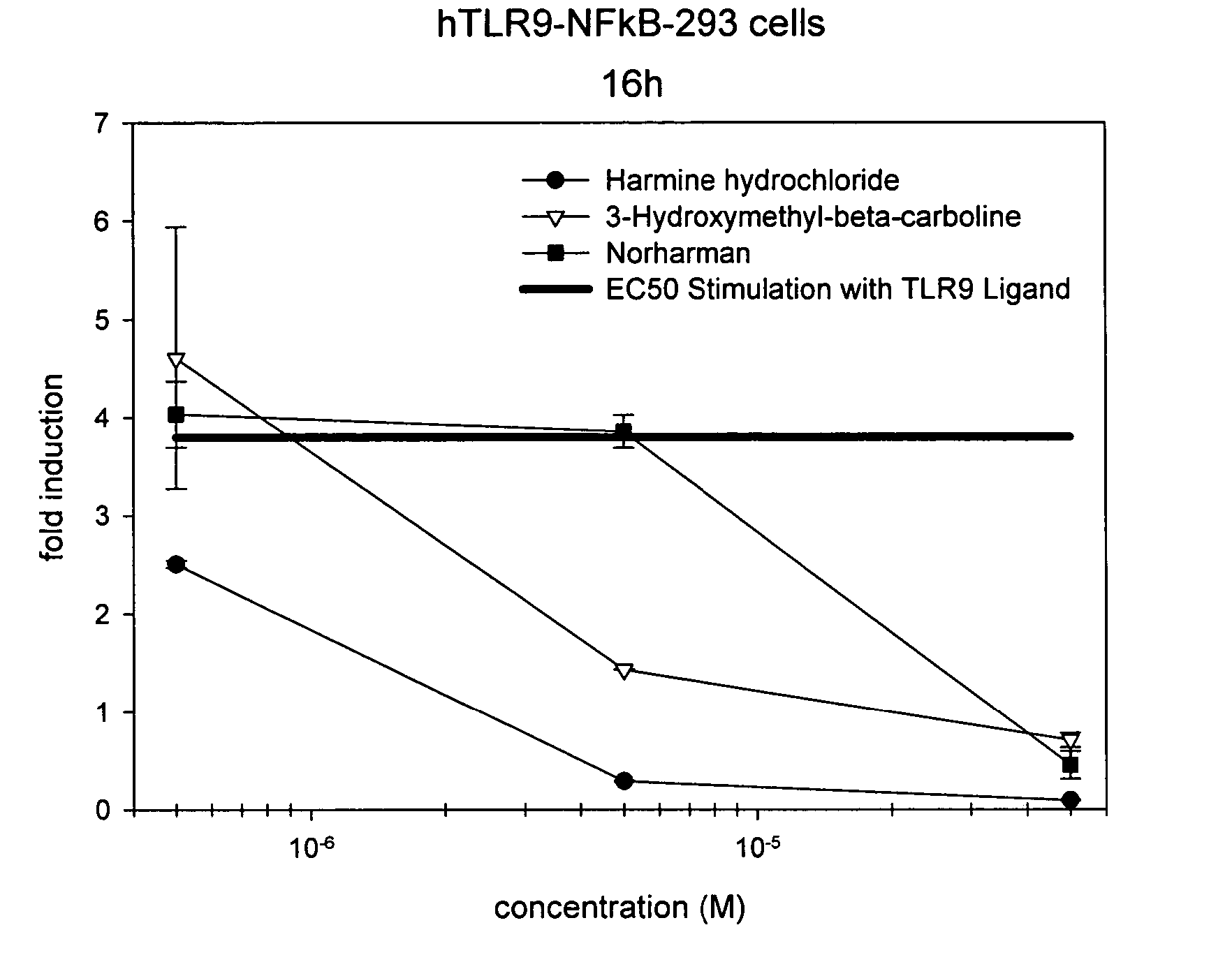
The invention relates to TLR9 antagonist compounds and their therapeutic or prophylactic use. The invention provides novel immune regulatory oligonucleotides and immunomers as antagonist of TLRs and methods of use thereof. These immune regulatory oligonucleotides have unique sequences that suppress, without completely ablating, TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. The methods may have use in the prevention and treatment of autoimmunity, inflammation, inflammatory bowel disease, lupus, allergy, asthma, infection, sepsis, cancer and immunodeficiency.
Owner:IDERA PHARMA INC
Therapeutic uses of milk mineral fortified food products
Food products fortified with a therapeutically effective amount of milk mineral are administered for the treatment of high blood pressure, stroke, obesity, kidney stones, colon cancer, breast cancer, head and neck tumors, premenstrual syndrome, postpartum depression, hypertensive disorders of pregnancy, Type-2 diabetes, depression, asthma, inflammatory bowel disease, attention deficit disorder, migraine headaches, kidney disease, hypercholesterolaemia, congestive heart failure, or immune deficiency.
Owner:GLANBIA FOODS
Compositions and methods for eliminating undesired subpopulations of T cells in patients with immunological defects related to autoimmunity and organ or hematopoietic stem cell transplantation
InactiveUS20050084967A1Reduce riskReduce severityMicrobiological testing/measurementArtificial cell constructsAutoimmune responsesHematopoietic stem cell transplantation
The present invention relates generally to methods for stimulating T cells, and more particularly, to methods to eliminate undesired (e.g. autoreactive, alloreactive, pathogenic) subpopulations of T cells from a mixed population of T cells, thereby restoring the normal immune repertoire of said T cells. The present invention also relates to compositions of cells, including stimulated T cells having restored immune repertoire and uses thereof.
Owner:LIFE TECH CORP
Immuno-reactive peptide CTL epitopes of human cytomegalovirus
The invention provides a plurality of peptides (and immunologically functional variants thereof) which are immunogenic epitopes recognized by CD8<+> class I MHC restricted cytotoxic T-lymphocytes of patients harboring latent human cytomegalovirus (HCMV) infection. The peptides are capable of activating CTLs and CTLps in the absence of active viral replication, and thus are useful for eliciting a cellular immune response against HCMV by normal and immunodeficient subjects. Peptide and lipopeptide vaccines, with and without adjuvants, also are disclosed. Cellular vaccines comprising the peptides form a further embodiment of this invention.
Owner:CITY OF HOPE
Structure and use of 5'phosphate oligonucleotides
InactiveUS20100178272A1Antibacterial agentsOrganic active ingredientsImmunologic disordersAutoimmune condition
Owner:KLINISCHE PHARMAKOLOGIE
Health food for improving immunity and preparation method thereof
ActiveCN102415568AEnhance immune functionEnhance humoral immune functionFood preparationBiotechnologyDisease
The invention discloses a health food for improving immunity and a preparation method thereof. The health food is prepared from the following raw materials in part by weight: 1 to 3 parts of dendrobium officinale, 2 to 6 parts of American ginseng and 6 to 18 parts of Chinese wolfberry. Pharmacological experiments prove that: the health food has an obvious effect of promoting an immune system, canobviously improve a phagocytic index of macrophage of immunosuppressed mice and increase coefficients of immune organs in nonspecifc immune experiments, can improve the conversion rate of lymphocytes, improve the level of immunoglobulin IgG, IgA and IgM in serum of the immunosuppressed mice and assist in conditioning immunodeficiency disease, and diseases such as weakened immune system, low resistance and the like.
Owner:常熟雷允上制药有限公司
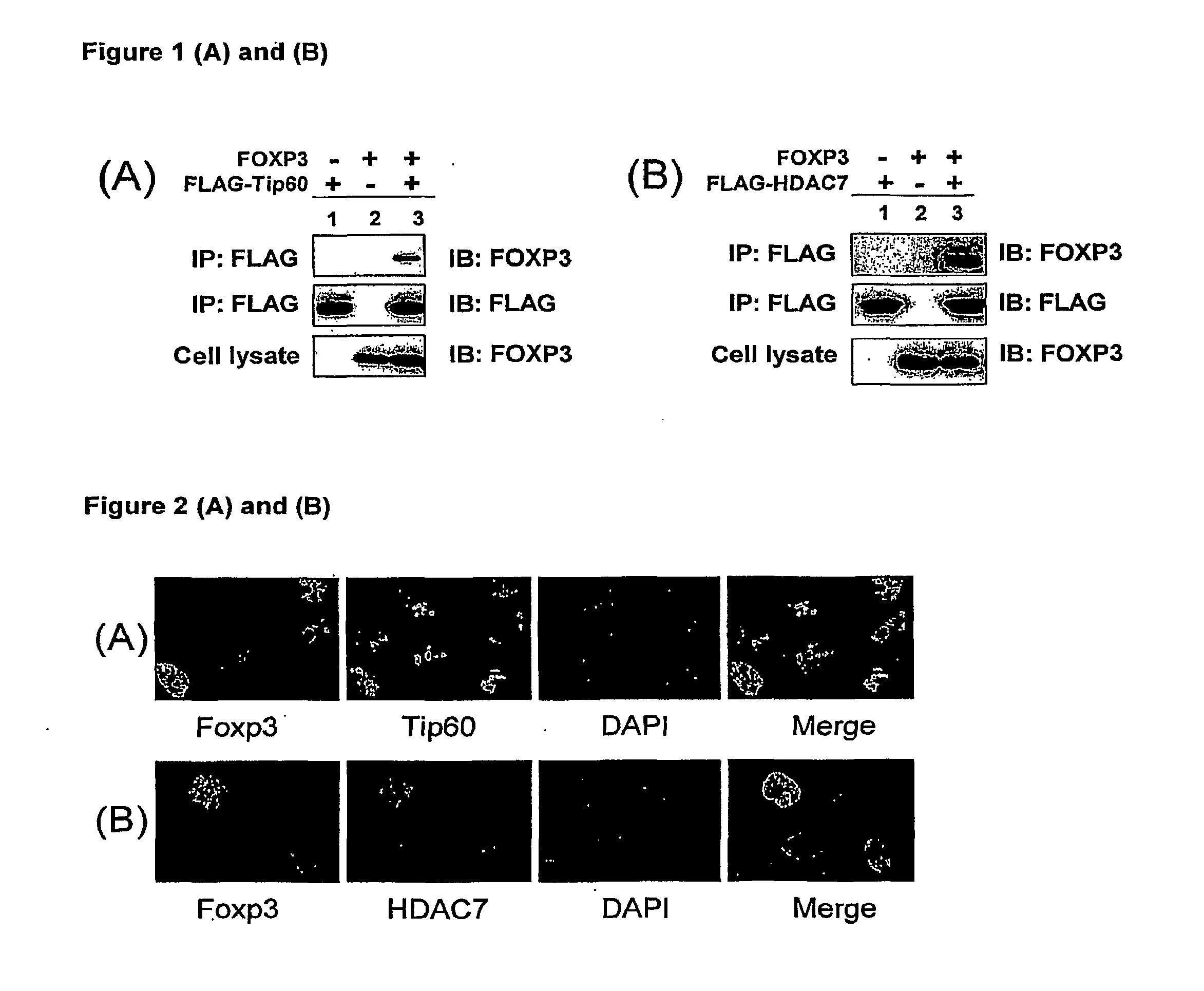
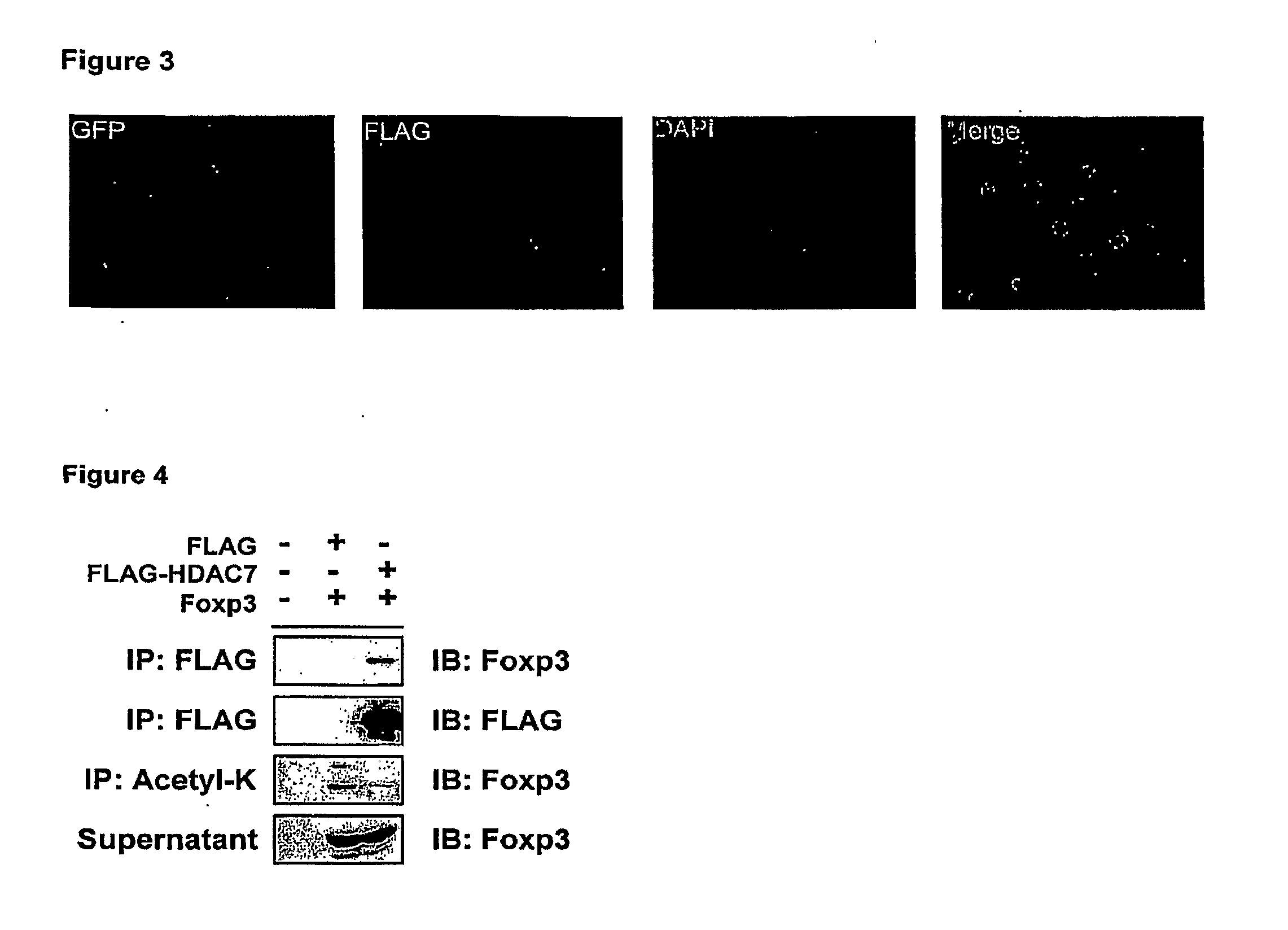
Compositions and methods for modulation of suppressor t cell activation
InactiveUS20100061984A1Relieve symptomsIncreased acetylation levelsCompounds screening/testingAntibacterial agentsAntigenImmunologic disorders
Methods of treating autoimmune disorders, coronary artery disease, allergy symptoms, allograft rejection sepsis / toxic shock are disclosed. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3 in combination with a T suppressor stimulus and / or an antigen. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3. Some methods comprise administering soluble GITR or antibodies that bind to GITR ligand. Methods of treating cancer, infectious diseases, and immune deficiency are also disclosed as are vaccination methods. The methods comprise administering one or more regulatory compositions to inactivate the T suppressor cells by reducing the acetylation level and / or protein level of FOXP3. Improved vaccines and vaccination methods are disclosed. Methods of identifying compounds that are useful to modulate acetylation level and / or protein level of FOXP3 and treat diseases are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Humanized immunomodulatory monoclonal antibodies for the treatment of neoplastic disease or immunodeficiency
InactiveUS20050180969A1Induces proliferation and activationAvoids adverse immunogenic responseAnimal cellsHybrid immunoglobulinsDiseaseAntiendomysial antibodies
The present invention provides to a humanized monoclonal antibody having immunostimulatory effects. This antibody binds specifically to B lymphoblastoid cells, induces proliferation and activation of peripheral blood lymphocytes, and is capable of eliciting an anti-tumor effect upon administration to subjects suffering from cancer.
Owner:CURETECH LTD +1
Amplifying, freezing and storing and recovering method of activated lymphocyte with CD3+CD8+as major
InactiveCN102839153ASolve the problem of multiple blood collectionHigh purityDead animal preservationBlood/immune system cellsPatient needT lymphocyte
The invention discloses culturing, freezing and recovering methods of an activated lymphocyte with CD3+CD8+as major, which can solve problems that a patient needs carrying out blood sampling for many times caused by continuously utilizing the activated lymphocyte with CD3+CD8+as major. The method comprises the following steps of: (1) contacting the extracted lymphocyte of the peripheral blood with IL-2, IL-15, an anti-CD3 antibody and an anti-CD28 antibody, so as to amplify the activated lymphocyte with CD3+CD8+as major; (2) freezing and storing the activated lymphocyte; and (3) recovering the activated lymphocyte. The activated lymphocyte cultured via the method disclosed by the invention has clear components, and comprises few CD4+CD25+Treg cells and more CD8+T lymphocyte; feedback time and frequency of the activated lymphocyte can be adjusted according to other treatments for a patient, such as a radiotherapy or a chemotherapy, so that diseases, such as tumor, infectious diseases and immunodeficiency can be treated well.
Owner:JINAN TAISHENG BIOLOGICAL TECH CO LTD
Medicinal composition and use thereof
InactiveCN1451426AImprove sexual functionImprove securityUnknown materialsSexual disorderSide effectCitrulline
A medical composition for treating sexual disfunction, impotence, immunodeficiency, chronic fatigue, and hepal and renal disfunction is prepared from ornithin and citrulline or their salts, jujube, ginkgo seed and ginseng. Its advantages are high curative effect and no toxic by-effect.
Owner:ZHEJIANG ADAMERCK BIOPHARMLABS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com