Patents
Literature
1193 results about "Pregnancy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pregnancy, also known as gestation, is the time during which one or more offspring develops inside a woman. A multiple pregnancy involves more than one offspring, such as with twins. Pregnancy can occur by sexual intercourse or assisted reproductive technology. A pregnancy may end in a live birth, abortion, or miscarriage, though access to safe abortion care varies globally. Childbirth typically occurs around 40 weeks from the start of the last menstrual period (LMP). This is just over nine months, where each month averages 31 days. When measured from fertilization it is about 38 weeks. An embryo is the developing offspring during the first eight weeks following fertilization, after which, the term fetus is used until birth. Symptoms of early pregnancy may include missed periods, tender breasts, nausea and vomiting, hunger, and frequent urination. Pregnancy may be confirmed with a pregnancy test.
Non-invasive detection of fetal genetic traits
InactiveUS20050164241A1Facilitates non-invasive detectionComponent separationOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains ≦500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of <500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising ≦500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
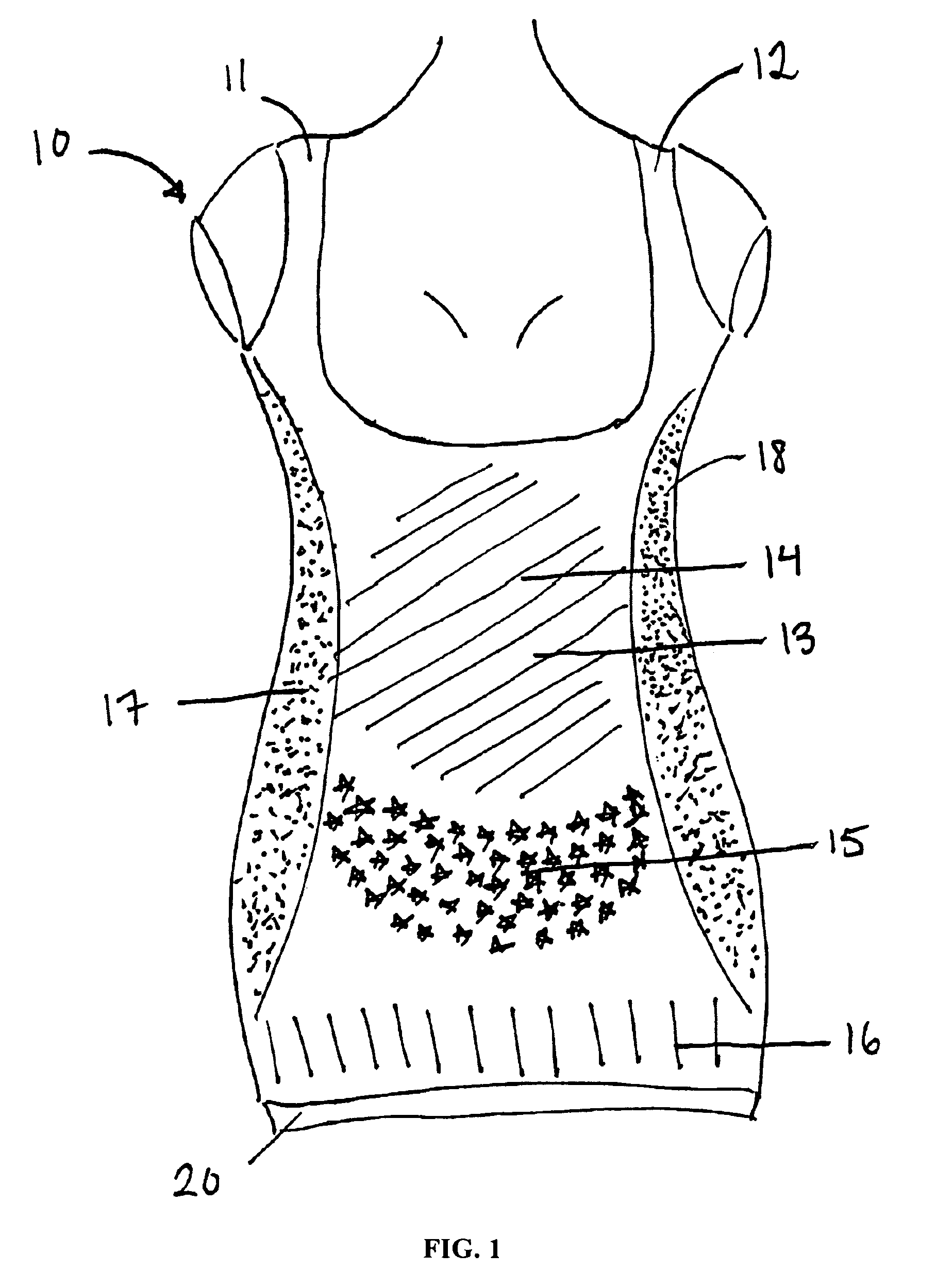
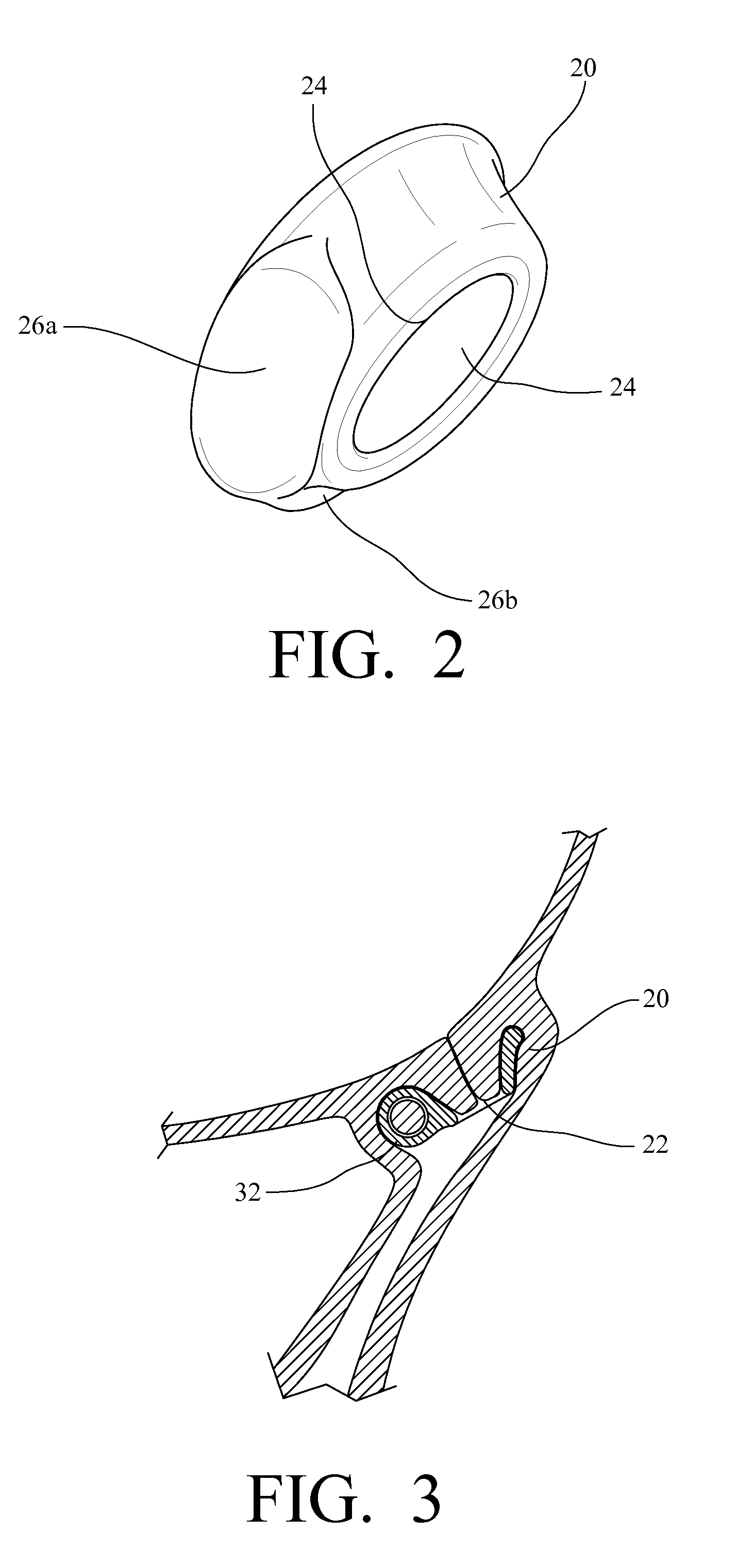
Mastopexy stabilization apparatus and method
An apparatus and method for mastopexy surgeries correcting a ptosis condition caused by tissue stretching, in the breast as a result of pregnancy, time, aging, and the effects of gravity and athletic activity provide an implant having homogeneously formed connectors extending from inside an implant wall for anchoring to the chest wall or chest muscles of a patient. Embedded reinforcements and anchoring tabs or sutures may be readily oriented along a rib or other defining physiological location in order to provide immediate, permanent, and symmetric installation of implants in a mastopexy reconstruction.
Owner:SMITH LANE FIELDING +1
Non-invasive detection of fetal genetic traits
ActiveUS20080071076A1Facilitates non-invasive detectionSugar derivativesOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains .Itoreq.500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of .Itoreq.500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising .Itoreq.500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
Methods for reducing the occurrence of preterm delivery and other pregnancy-related conditions
The present invention relates to methods and kits for reducing the occurrence of preterm delivery and other pregnancy-related conditions in pregnant female subjects exhibiting one or more risk factors for preterm delivery and other pregnancy-related conditions. For example, the present invention relates to methods for reducing the occurrence of preterm delivery in a pregnant female subject having no history of preterm delivery and exhibiting one or more risk factors for preterm delivery (e.g., smoking during pregnancy). The methods and kits provide for the administration of a steroid hormone to the pregnant female subject.
Owner:LUMARA HEALTH IP
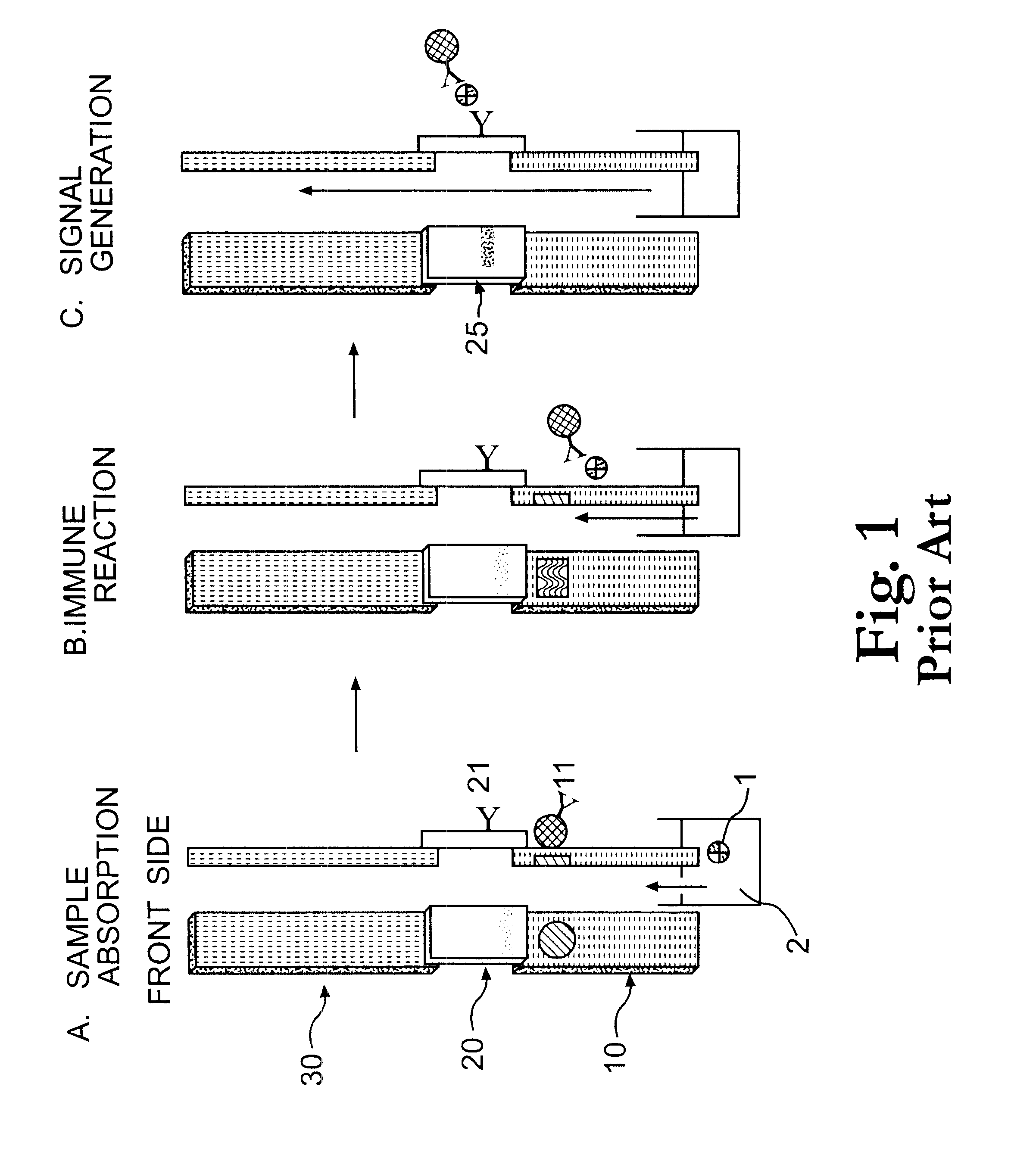
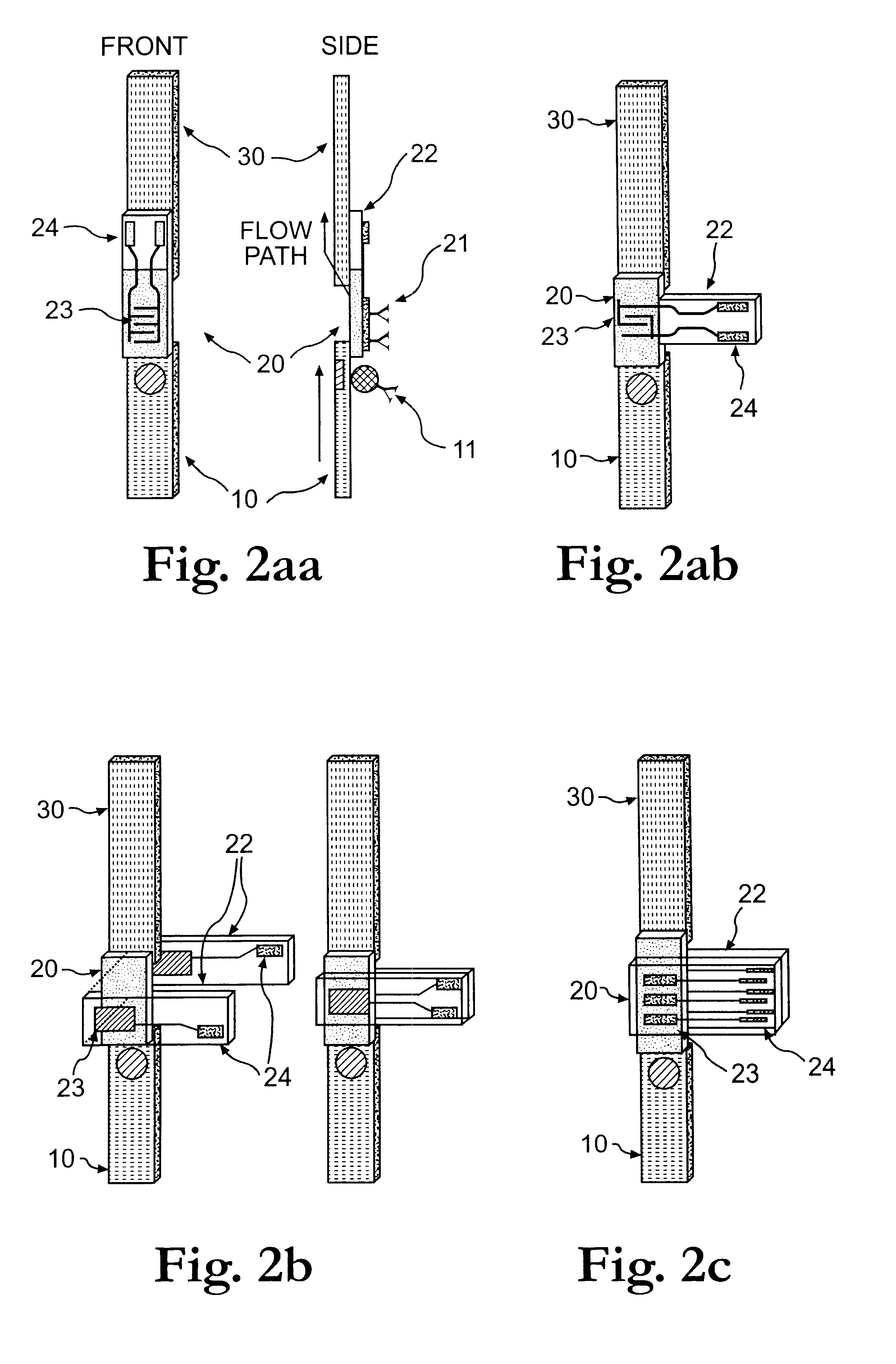
Electrochemical membrane strip biosensor
The present invention is directed to the development of a biosensor based on the immuno-chromatographic method that can provide an assay speed and convenience required for point-of-care (the doctor's office and emergency room) testing or home-version diagnosis. Though certain physical symptoms, such as pregnancy and ovulation, or bacterial infection may be identified by a qualitative analysis for the presence of indicating substances, most analytes for clinical investigation demand their concentrations known in specimens. Therefore, the inventors of the present invention have developed a novel biosensor by combining the immuno-chromatographic method and the electric conductivity detection technology so that on-site quantitative determination at the points of care or at home may be carried out.
Owner:BIODIGIT LAB CORP
Systems, Devices, and Methods for Tracking Abdominal Orientation and Activity
ActiveUS20160374608A1Systemic blood oxygen concentrationIncreased riskDiagnostics using lightHealth-index calculationCumulative riskCardiology
The disclosed apparatus, systems and methods relate to tracking abdominal orientation and activity for purposes of preventing or treating conditions of pregnancy or other types of medical conditions. In certain specific embodiments, the system, device, or method relates to identifying abdominal orientation risk values, calculating and updating a cumulative risk value, comparing the cumulative risk value to a threshold, and outputting a warning when the cumulative risk value crosses the threshold.
Owner:SMART HUMAN DYNAMICS
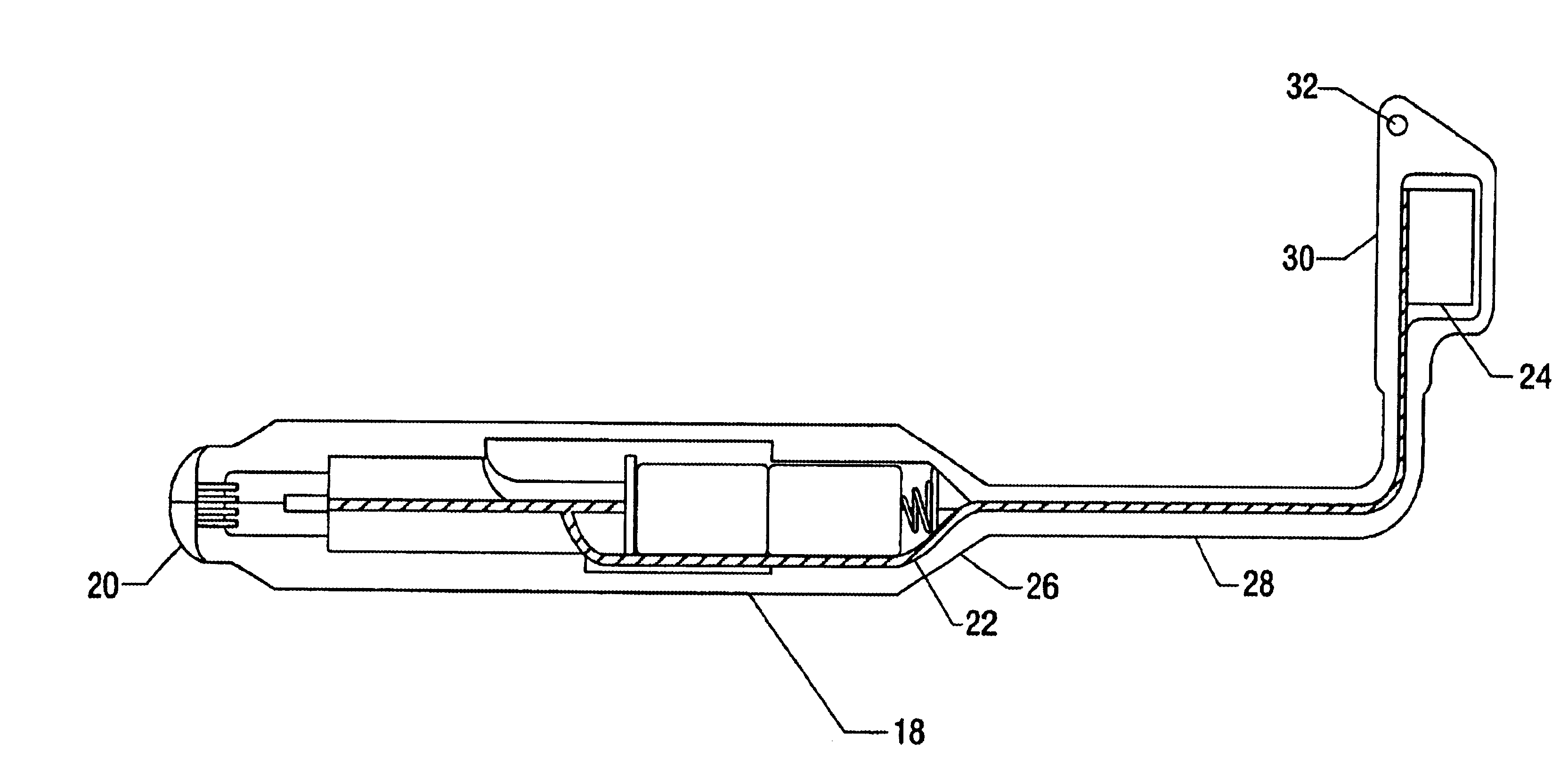
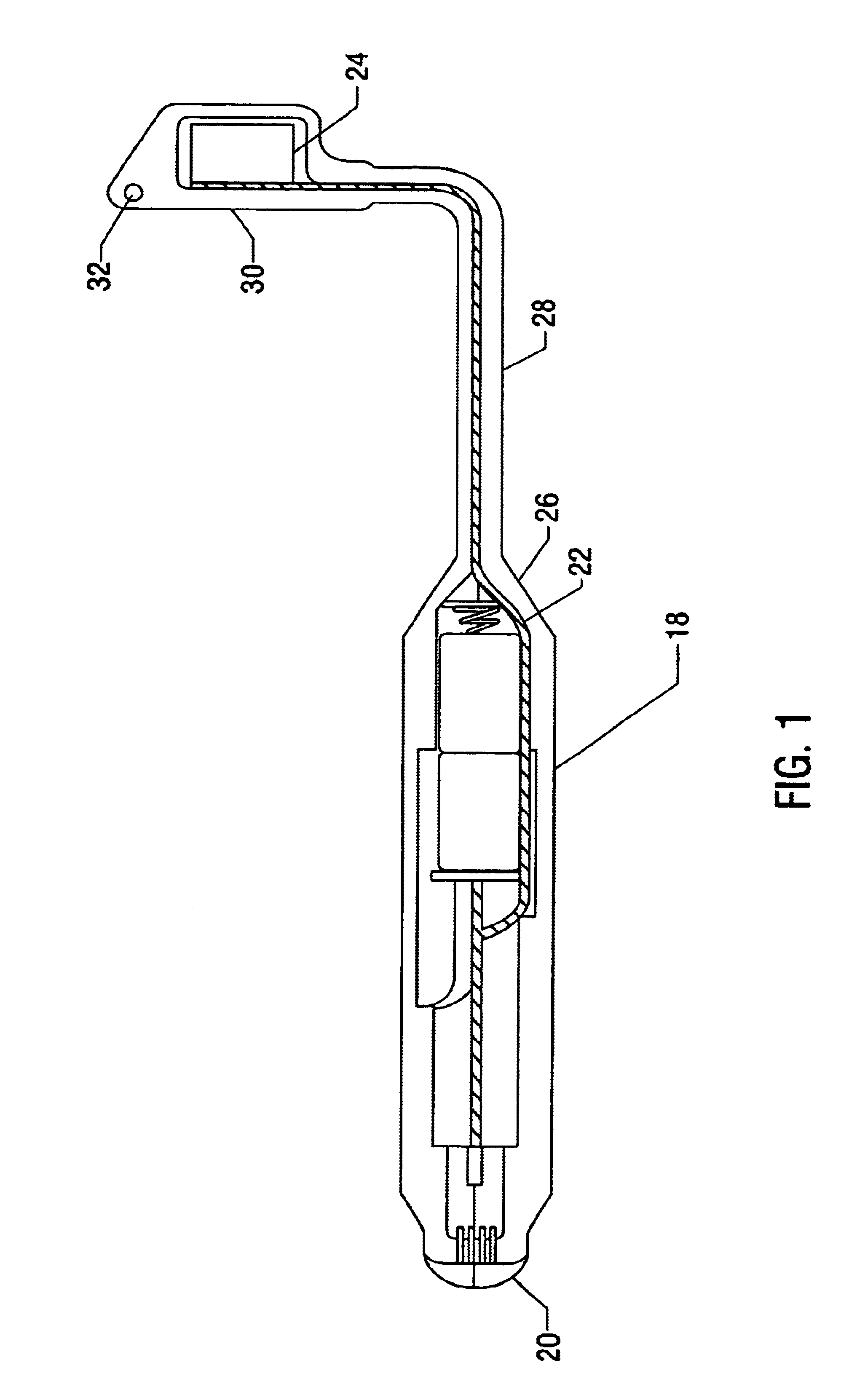
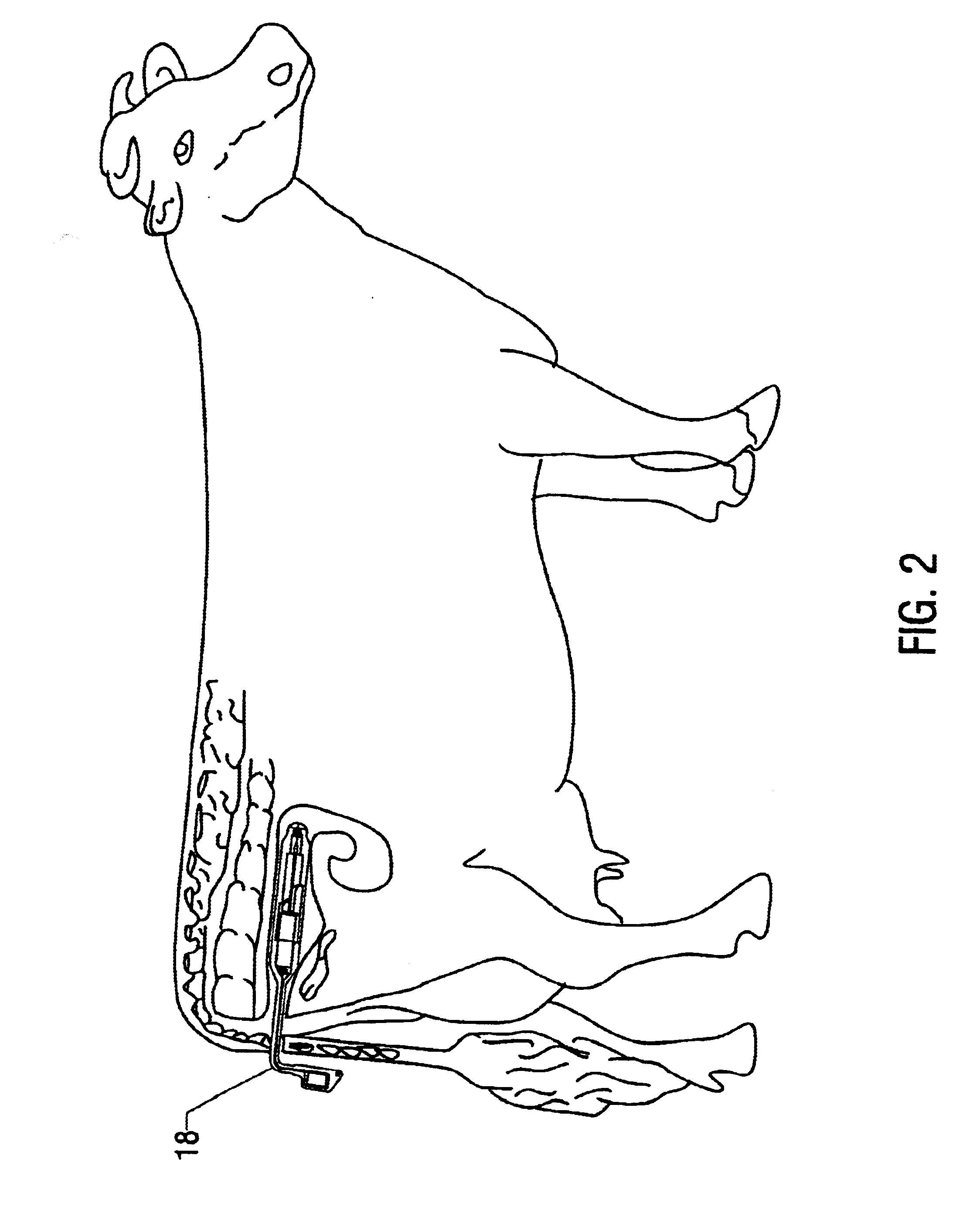
Apparatus and method for detection of estrus and/or non-pregnancy
The present invention is directed to devices for detection estrus-state and / or non-pregnancy in mammals and methods for using the same. The device and / or methods may be of particular use in cattle. The devices disclosed include at least a housing of a size, shape, and outer surface quality permitting it to be securely maintained within a mammals vagina. The housing has at least one interior space for installation of instrument(s) for measuring, intra-vaginally, at least on indicia of estrus (heat) or non-pregnancy, converting the resulting measurement into a signal or signals (electronic or otherwise). The device also comprises a signal transmitting means and, optionally, a remote receiving station capable of interpreting the signal(s).
Owner:MONSANTO TECH LLC
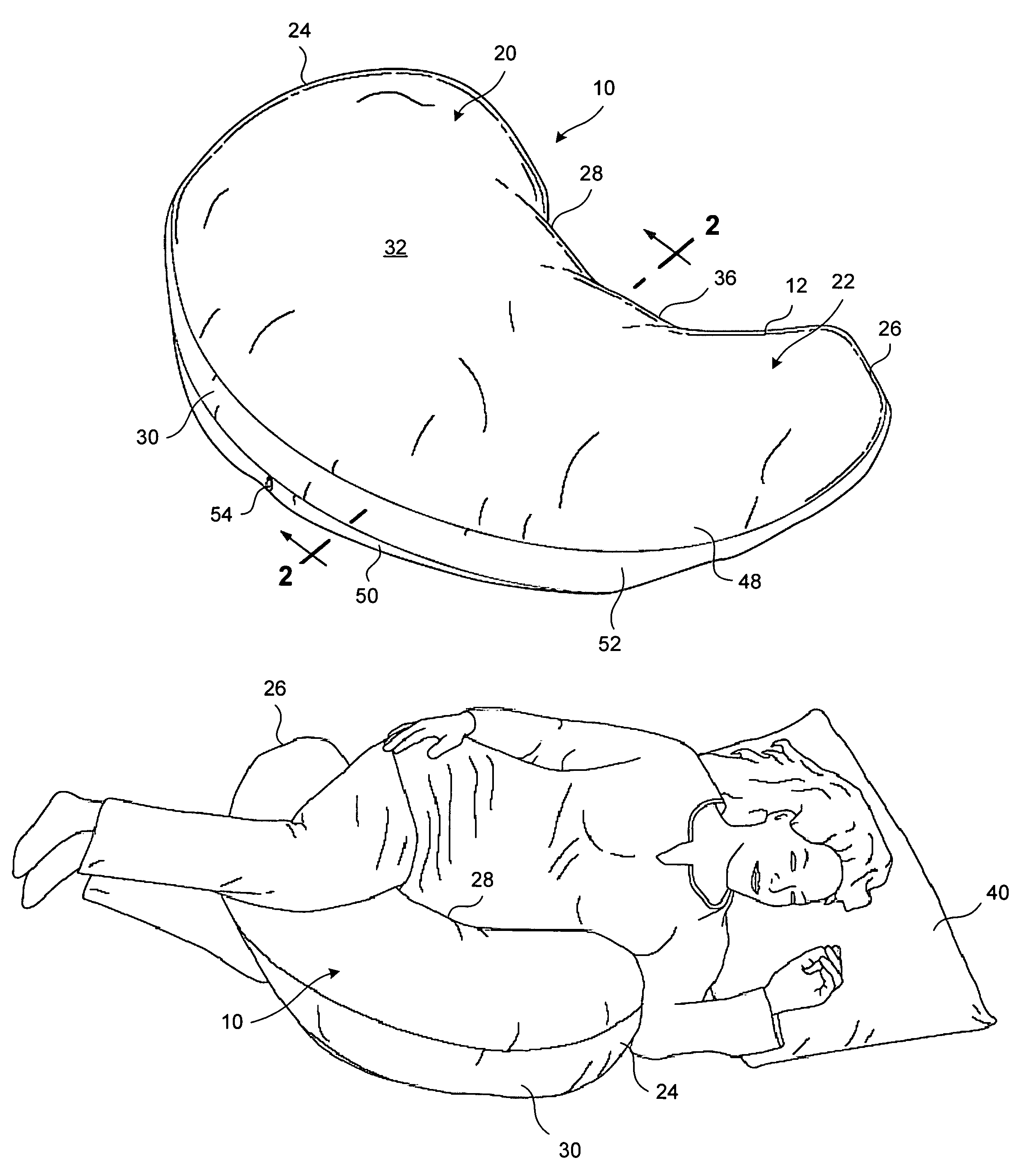
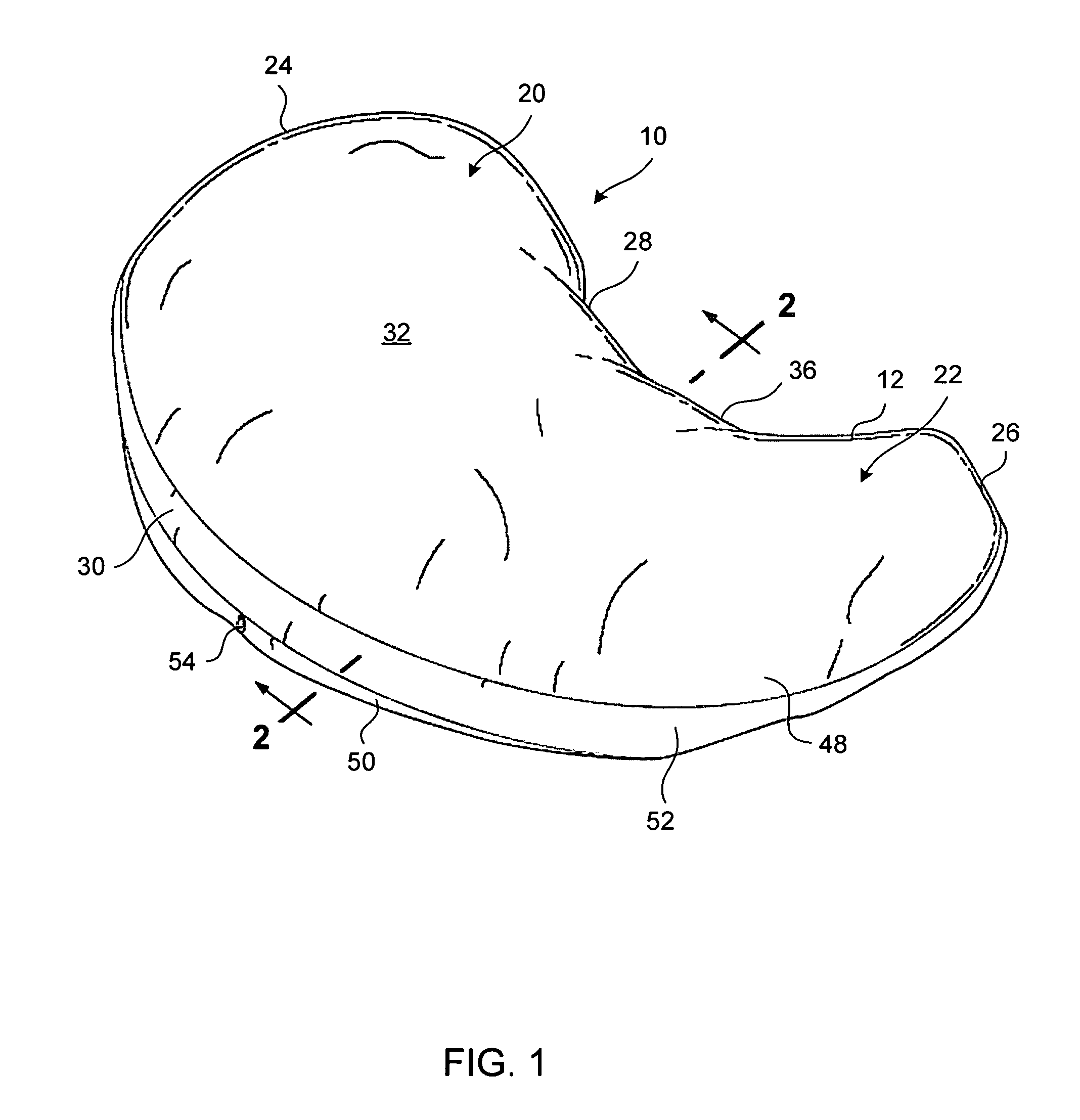
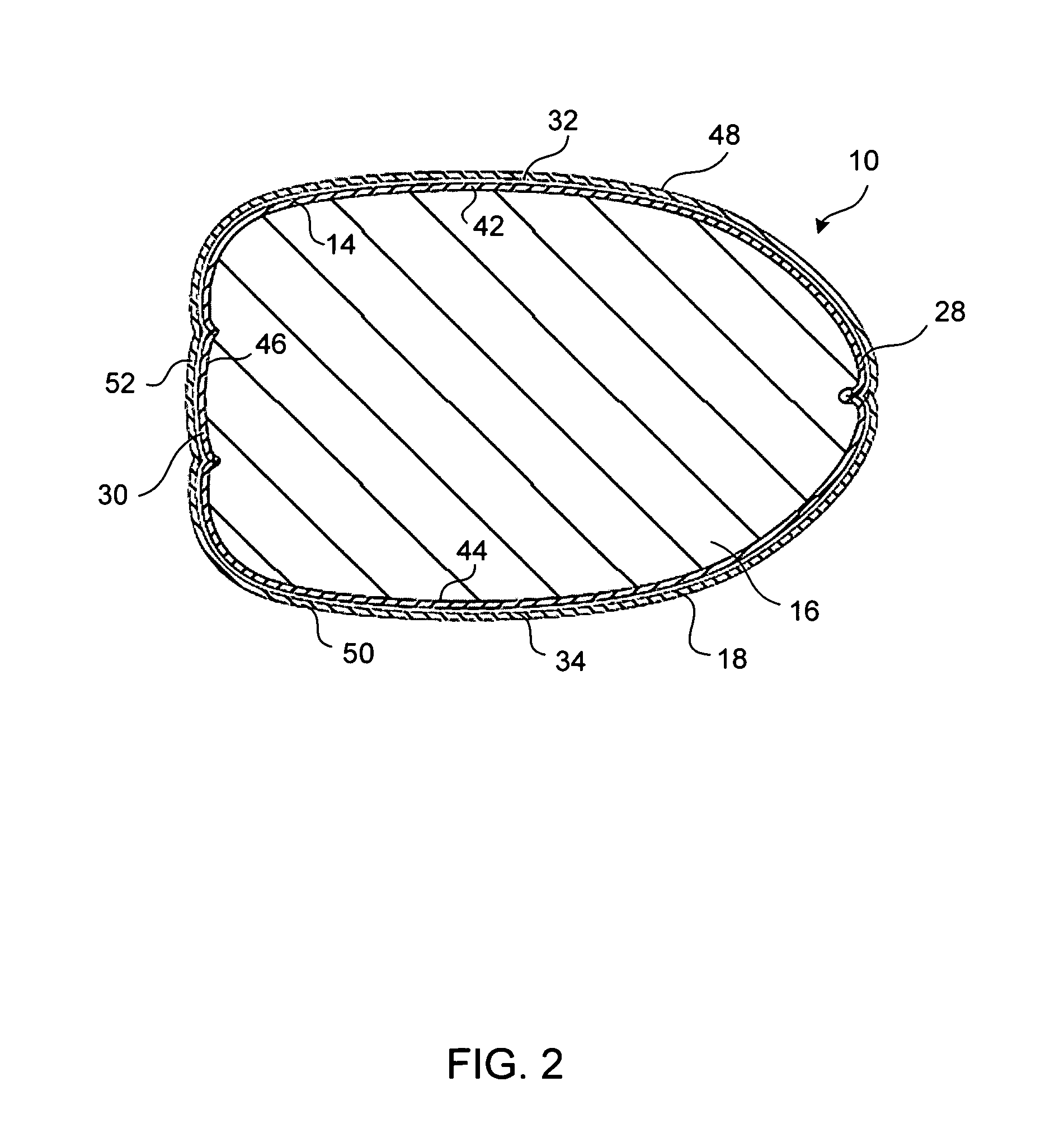
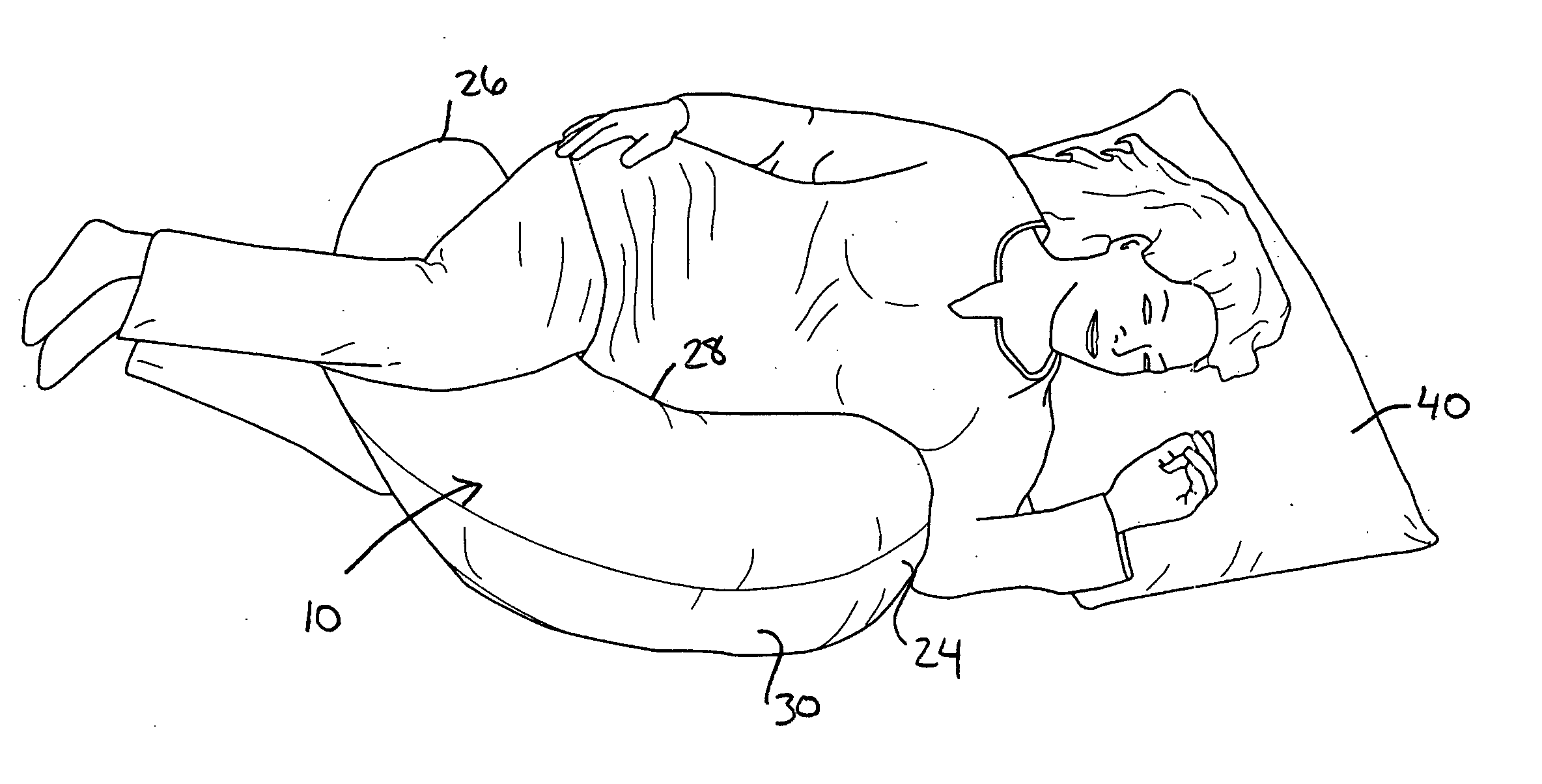
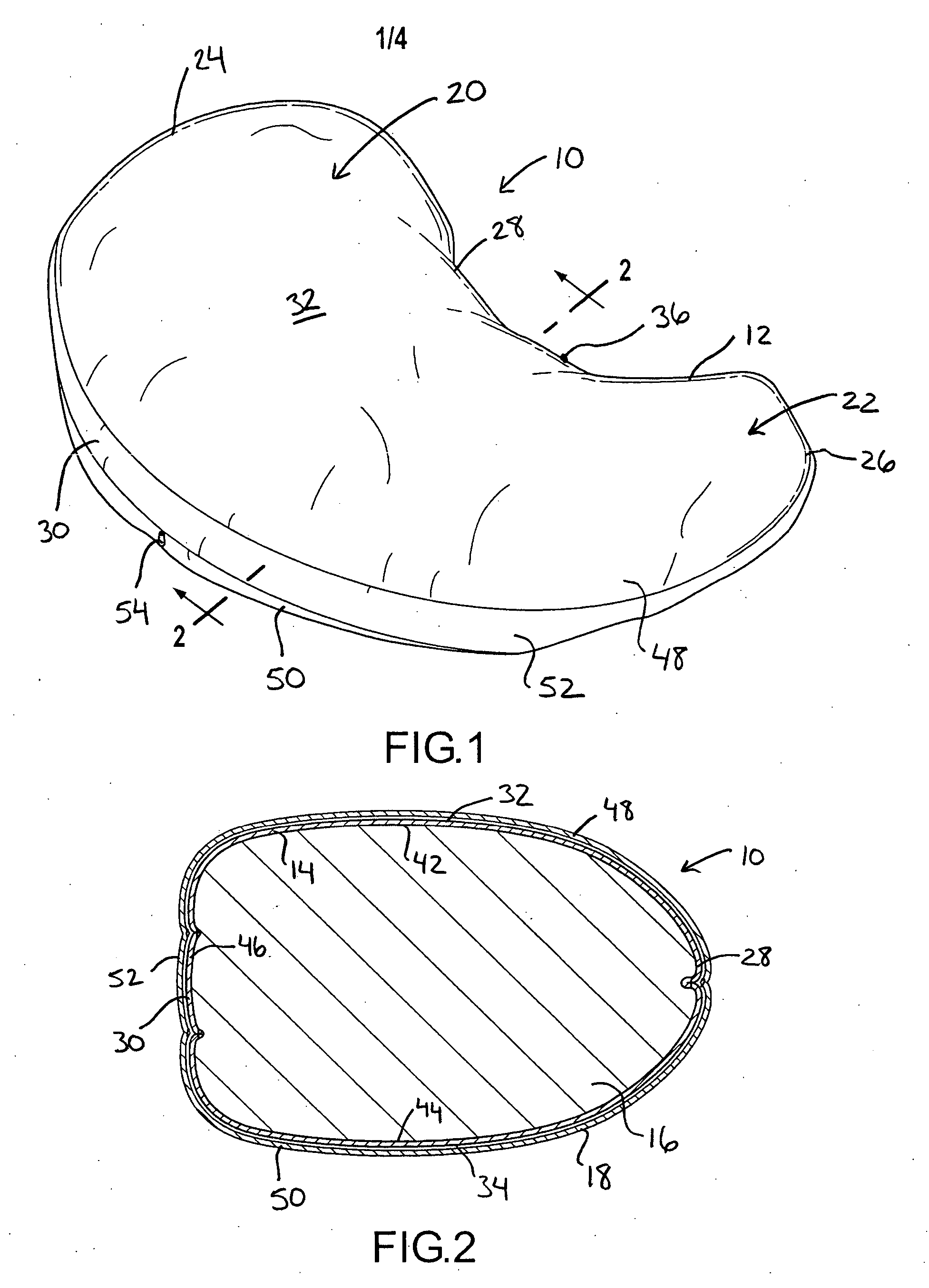
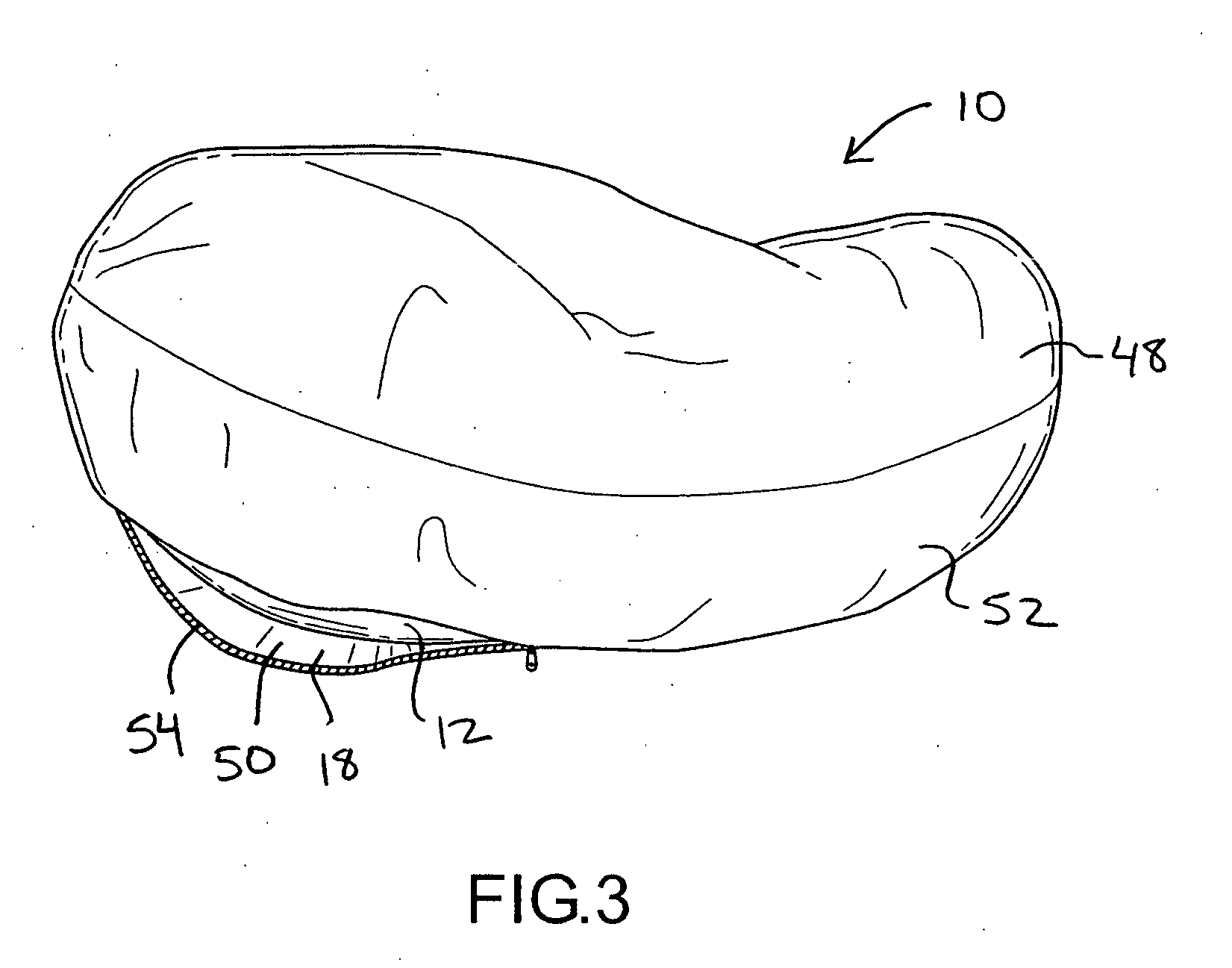
Pregnancy support pillow
In one embodiment, a pillow comprises a curved pillow body having a top region and a bottom region. The top region is larger in size than the bottom region. Also, the bottom region is adapted to be placed between a user's lets while the user is lying on her side while the top region conforms to the user's stomach and chest.
Owner:THE BOPPY CO LLC
Inactivated Zika virus vaccine
ActiveCN105749268AEase the epidemicReduce the burden onSsRNA viruses positive-senseViral antigen ingredientsZika virusSide effect
The invention provides an inactivated Zika virus vaccine. The inactivated Zika virus vaccine is obtained by: performing ultrafiltration and concentration on Zika virus liquid after inactivation, centrifuging the concentrated virus liquid by adopting a sucrose density zone, performing ion exchange and concentration sterilization on a centrifugal product to obtain a Zika virus vaccine stock solution, diluting the vaccine stock solution until the total protein content is not more than 20mu g / ml, and adding an adjuvant to obtain a vaccine semi-finished product. The method for preparing the vaccine provided by the invention is simple, convenient and easy to operate, the cost is saved, the produced vaccine is suitable for Asian people, a unit dose of the Zika virus liquid is high in immunogenicity, the content of hybrid protein is low, the side effect after injection is small, and the safety is high, so that the vaccine is suitable for vaccination of fertile women before pregnancy, can avoid newborn Brazil microcephaly caused by infection of Zika virus, and is significant in social value and market efficiency.
Owner:SINOVAC RES & DEV
Electro active elastic compression bandage
InactiveUS7868221B2Low mobilityEasy to moveBlood stagnation preventionElectrotherapyPregnancyActive support
This invention relates to an elastic bandage for supporting a body extremity such as a leg. Such bandages are used to overcome problems with fluid retention and swelling in the legs, occurring as a consequence of varicose veins, vascular incompetence, pregnancy, etc. It is a task of this invention to supply an active support for a body extremity such as a leg, which can be used by a person underneath the clothes and will not reduce the mobility of the patient. This task is solved in that an elastic bandage comprises an elastic layer for surrounding a body extremity to exert compressive force on the extremity, the bandage being, at least partly, formed by elastomeric actuation elements, whereby electrical control of the compressive force is possible, and where the control is due to a signal from some sensing system.
Owner:DANFOSS AS
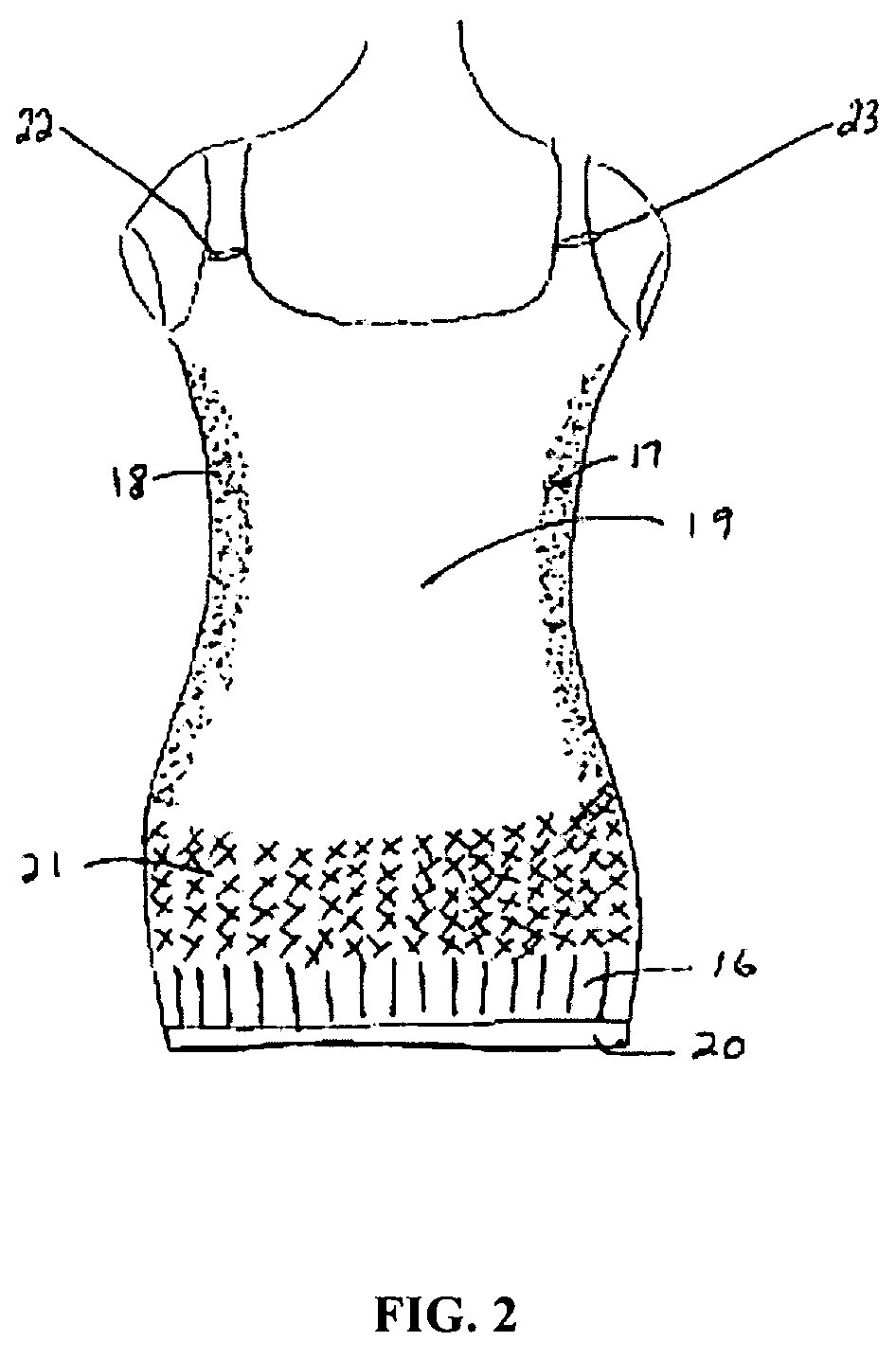
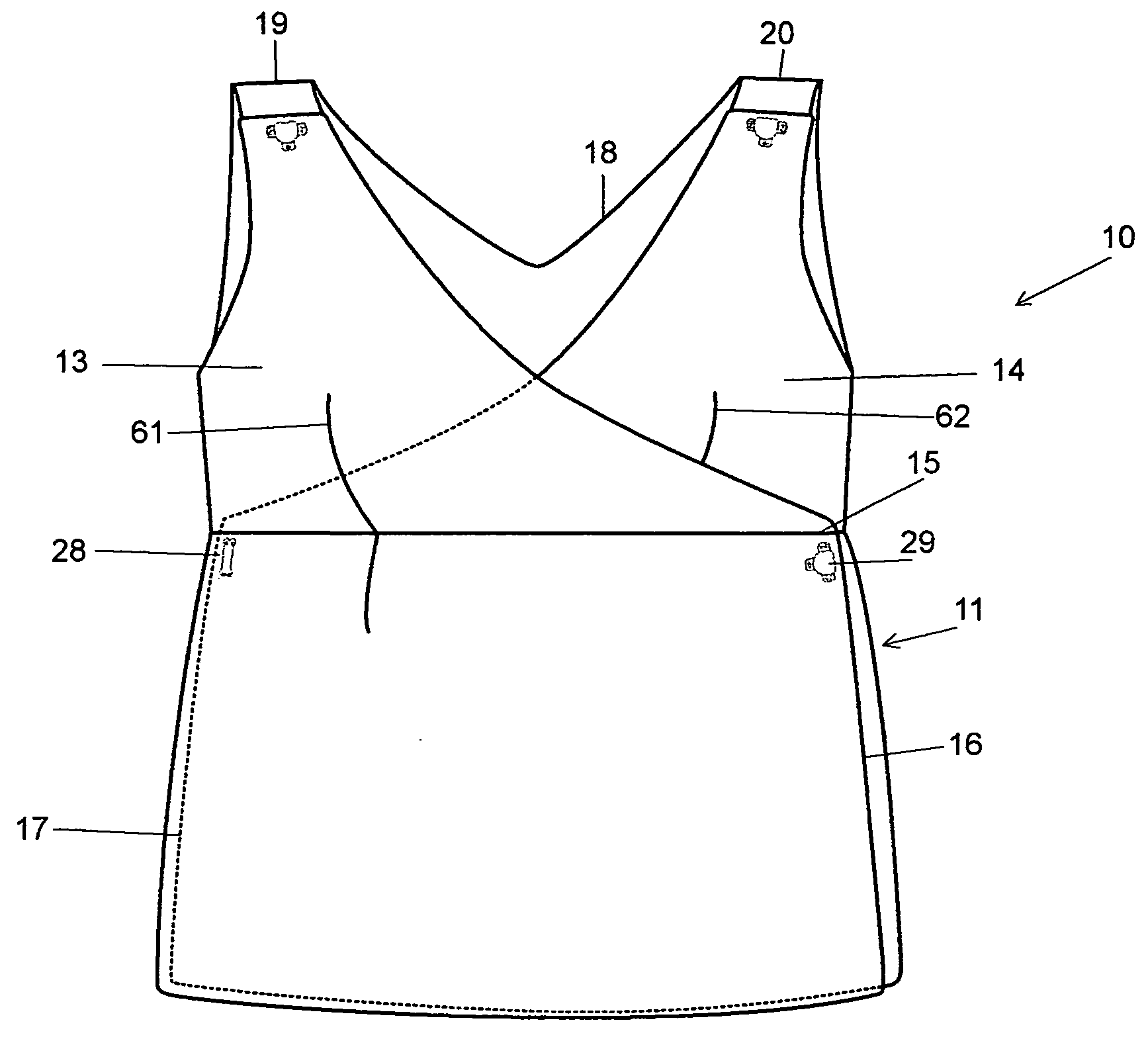
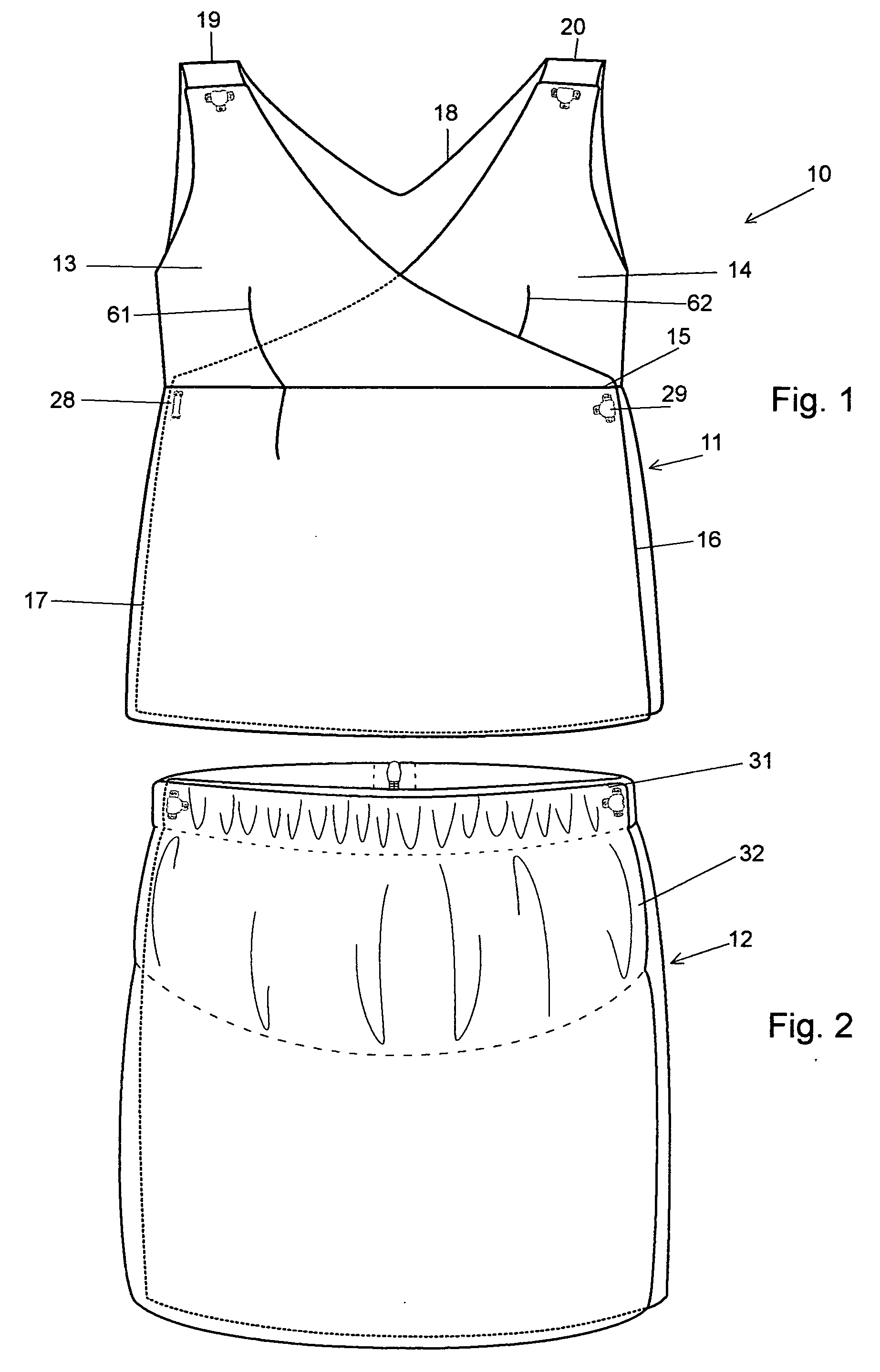
Full torso maternity garment
A maternity garment, which provides mild support to shape a woman's body and to act as a suspender for bottoms such as pants, shorts, or skirts that may be ill fitting due to body changes as a result of pregnancy, wherein the preferred garment has at least one shoulder strap, a belly panel, a hip band, two side panels, and a back panel, and is made of high performance fabric with varying degrees of compression.
Owner:BLANQI
Segregated early weaning method of piglet
ActiveCN101406166AReduce feeding costsIncrease the number of births per yearFood processingAnimal feeding stuffPig farmsMaternal antibody
The invention discloses a method for segregated early weaning of piglets, which comprises the steps of medicine adding, segregation, early transfer and the feeding of early weaning by piglet feed, wherein the medicine adding is performed before and after the delivery of a sow, the advanced medicine adding is used to kill off microorganisms which is expected to be killed, and the medicine adding is also performed from the birth of a suckling piglet to the weaning; the segregation adopts that the parturient sow is transferred into a clean farrowing house for delivery from the pregnancy, and the piglet at the age of between 10 and 14 days is transferred into a clean and comfortable breeding house from the farrowing house; when the level of a maternal antibody of the piglet at the age of between 10 and 14 days is in higher state, the piglet is moved away from the sow and is transferred into a cleaner environment; and the piglet feed for segregated early weaning adopts four stages to feed. The piglet has early weaning segregation time, high survival rate, and quick growth speed, the segregated early weaning of the piglet can save feeding cost of the sow, improve the yearly parity number of the sow, improve the utilization rate of the farrowing house, and reduce the probability that the piglet is infected with sow diseases, and a segregated early weaning technology of the piglet is suitable for the application of modern pig farms.
Owner:MUYUAN FOODS CO LTD
Human and mouse targeting peptides identified by phage display
The present invention concerns methods and compositions for in vivo and in vitro targeting. A large number of targeting peptides directed towards human organs, tissues or cell types are disclosed. The peptides are of use for targeted delivery of therapeutic agents, including but not limited to gene therapy vectors. A novel class of gene therapy vectors is disclosed. Certain of the disclosed peptides have therapeutic use for inhibiting angiogenesis, inhibiting tumor growth, inducing apoptosis, inhibiting pregnancy or inducing weight loss. Methods of identifying novel targeting peptides in humans, as well as identifying endogenous receptor-ligand pairs are disclosed. Methods of identifying novel infectious agents that are causal for human disease states are also disclosed. A novel mechanism for inducing apoptosis is further disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Short peptides useful for treatment of ischemia/reperfusion injury and other tissue damage conditions associated with nitric oxide and its reactive species
ActiveUS20080182797A1Avoid tissue damageLevel of protectionNervous disorderTetrapeptide ingredientsPregnancyAllograft rejection
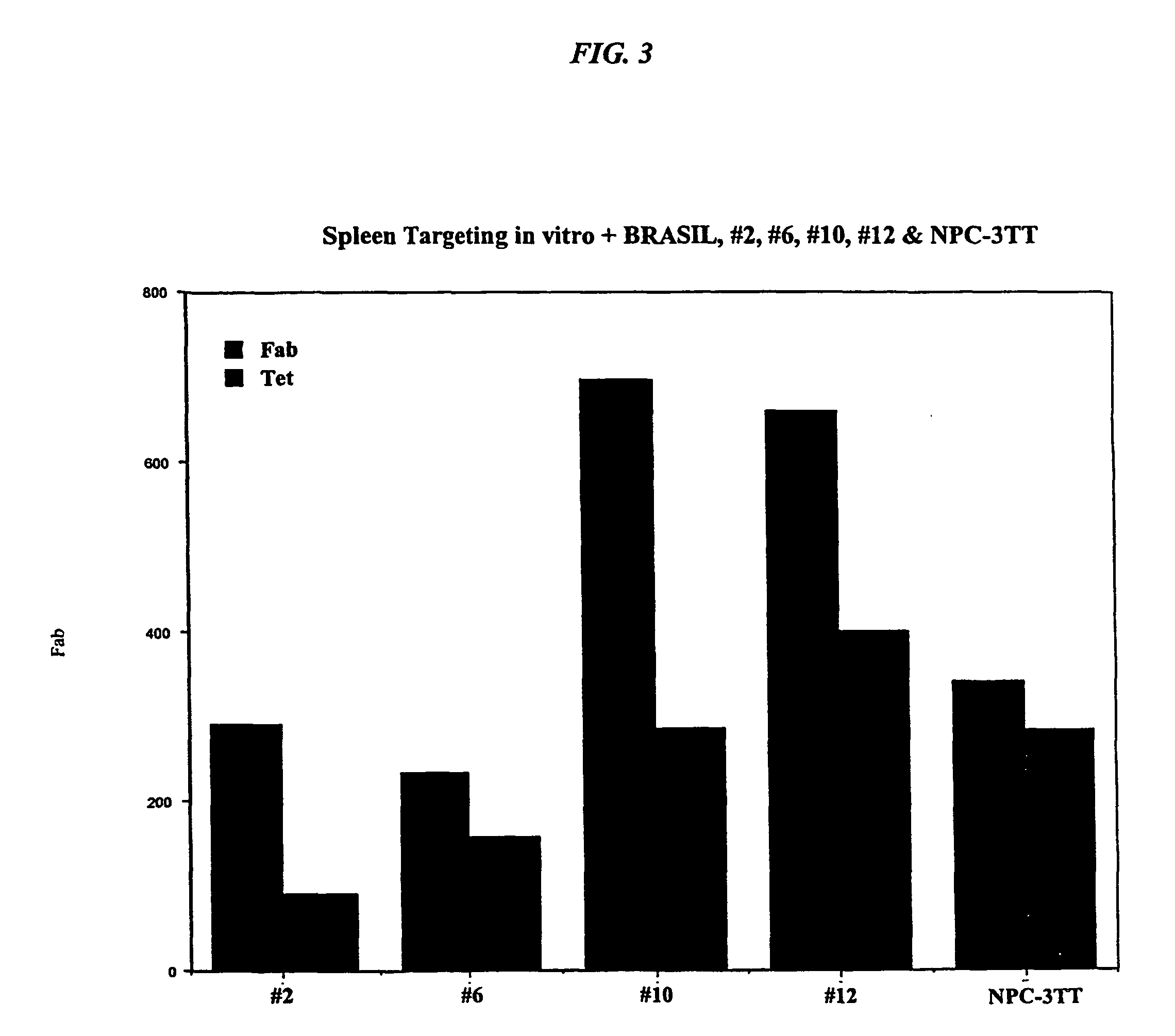
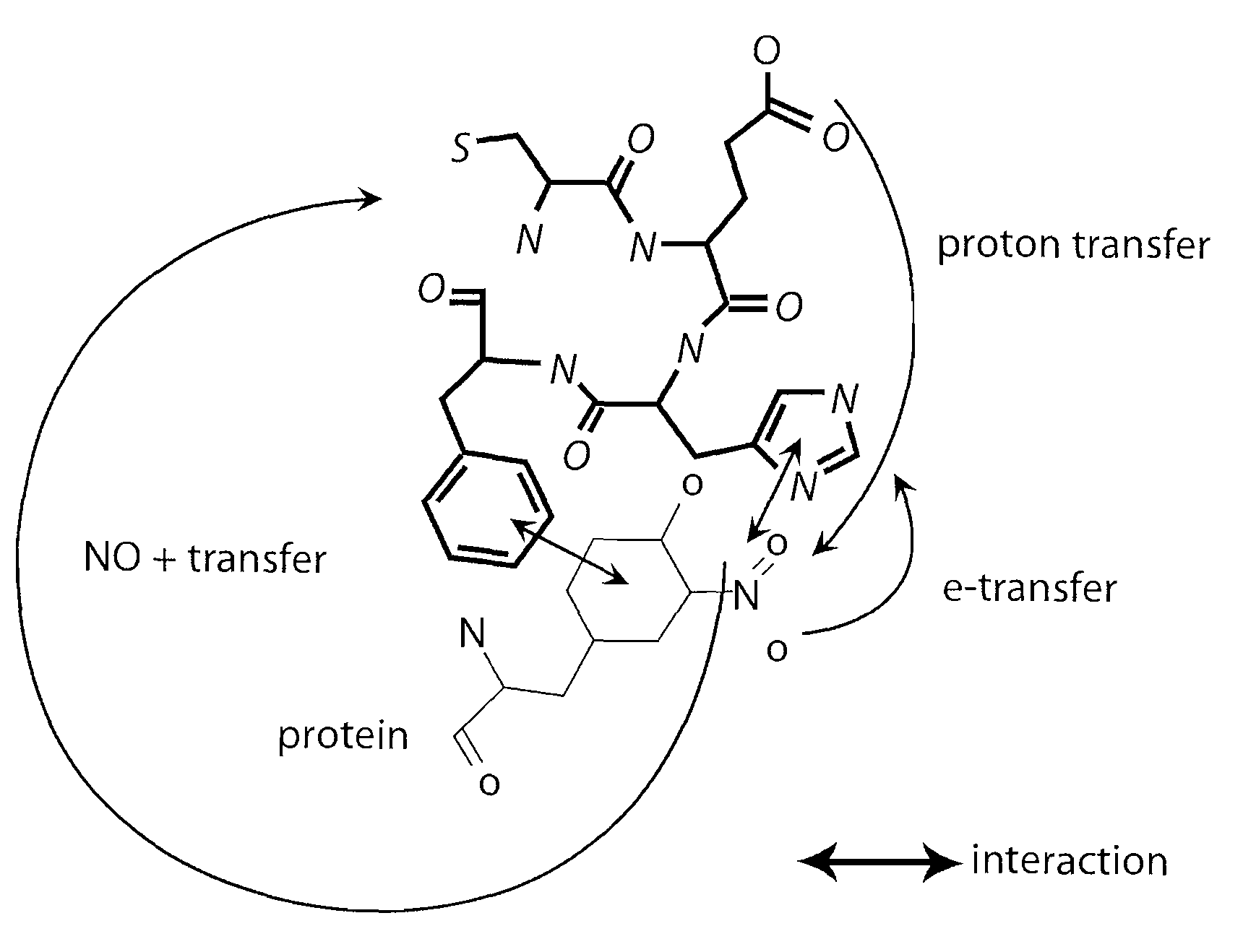
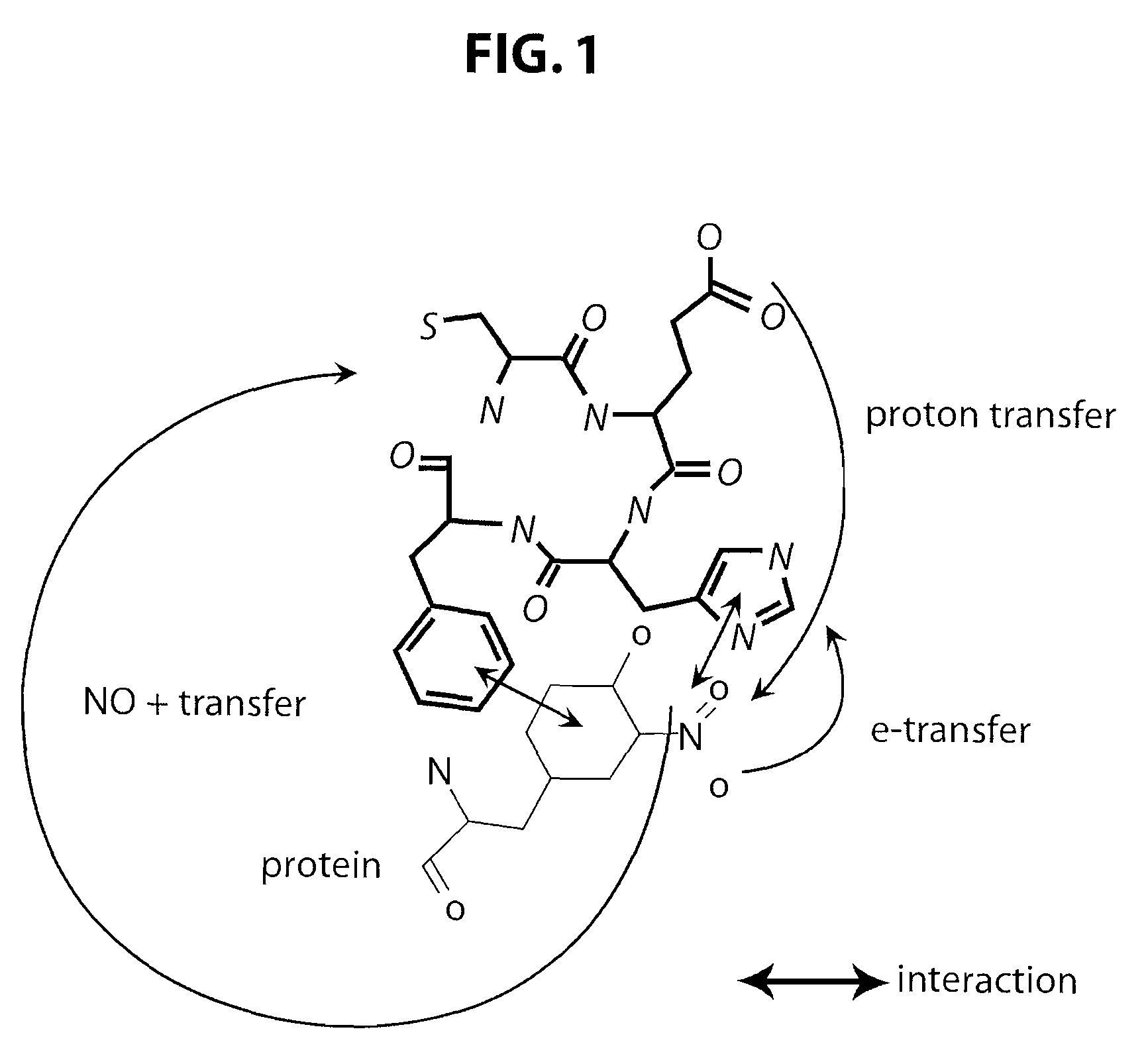
This invention discloses isolated short peptides comprising the amino acid sequence Cys-Glu-Phe-His (CEFH) and analogs thereof as well as compositions comprising CEFH peptides and analogs thereof. The CEFH peptides disclosed herein are effective in mediating the denitration of 3-nitrotyrosines (3-NT) in cellular proteins thereby preventing tissue damage associated with excess nitric oxide (NO) and its reactive species. The CEFH peptides disclosed herein are useful in the treatment of ischemia / reperfusion (I / R) injury of various tissues (e.g., I / R injury of heart muscle associated with heart attack or cardiac surgery, I / R injury of brain tissue associated with stroke, I / R injury of liver tissue, skeletal muscles, etc.), septic shock, anaphylactic shock, neurodegenerative diseases (e.g., Alzheimer's and Parkinson's diseases), neuronal injury, atherosclerosis, diabetes, multiple sclerosis, autoimmune uveitis, pulmonary fibrosis, oobliterative bronchiolitis, bronchopulmonary dysplasia (BPD), amyotrophic lateral sclerosis (ALS), sepsis, inflammatory bowel disease, arthritis, allograft rejection, autoimmune myocarditis, myocardial inflammation, pulmonary granulomatous inflammation, influenza- or HSV-induced pneumonia, chronic cerebral vasospasm, allergic encephalomyelitis, central nervous system (CNS) inflammation, Heliobacterium pylori gastritis, necrotizing entrerocolitis, celliac disease, peritonitis, early prosthesis failure, inclusion body myositis, preeclamptic pregnancies, skin lesions with anaphylactoid purpura, nephrosclerosis, ileitis, leishmaniasis, cancer, and related disorders.
Owner:NEW YORK UNIVERSITY
Electro active elastic compression bandage
InactiveUS20060079824A1Low mobilityEasy to moveBlood stagnation preventionElectrotherapyElastomerPhysical medicine and rehabilitation
This invention relates to an elastic bandage for supporting a body extremity such as a leg. Such bandages are used to overcome problems with fluid retention and swelling in the legs, occurring as a consequence of varicose veins, vascular incompetence, pregnancy, etc. It is a task of this invention to supply an active support for a body extremity such as a leg, which can be used by a person underneath the clothes and will not reduce the mobility of the patient. This task is solved in that an elastic bandage comprises an elastic layer for surrounding a body extremity to exert compressive force on the extremity, the bandage being, at least partly, formed by elastomeric actuation elements, whereby electrical control of the compressive force is possible, and where the control is due to a signal from some sensing system.
Owner:DANFOSS AS
Devices for alleviating back strain and back pain
InactiveUS7819831B2Relieve back pressureDegree of avoidanceRestraining devicesStretcherBody axisPregnancy
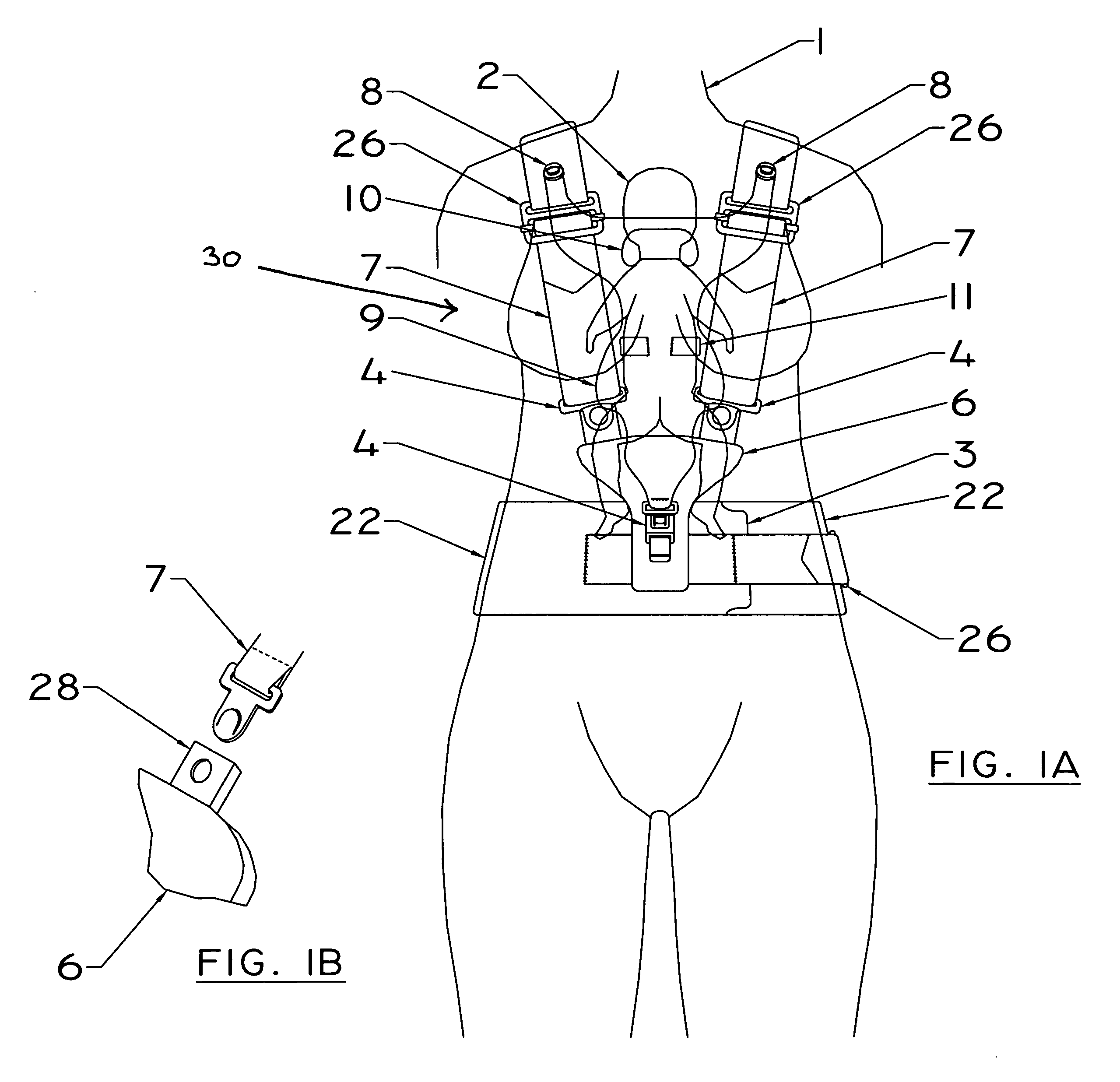
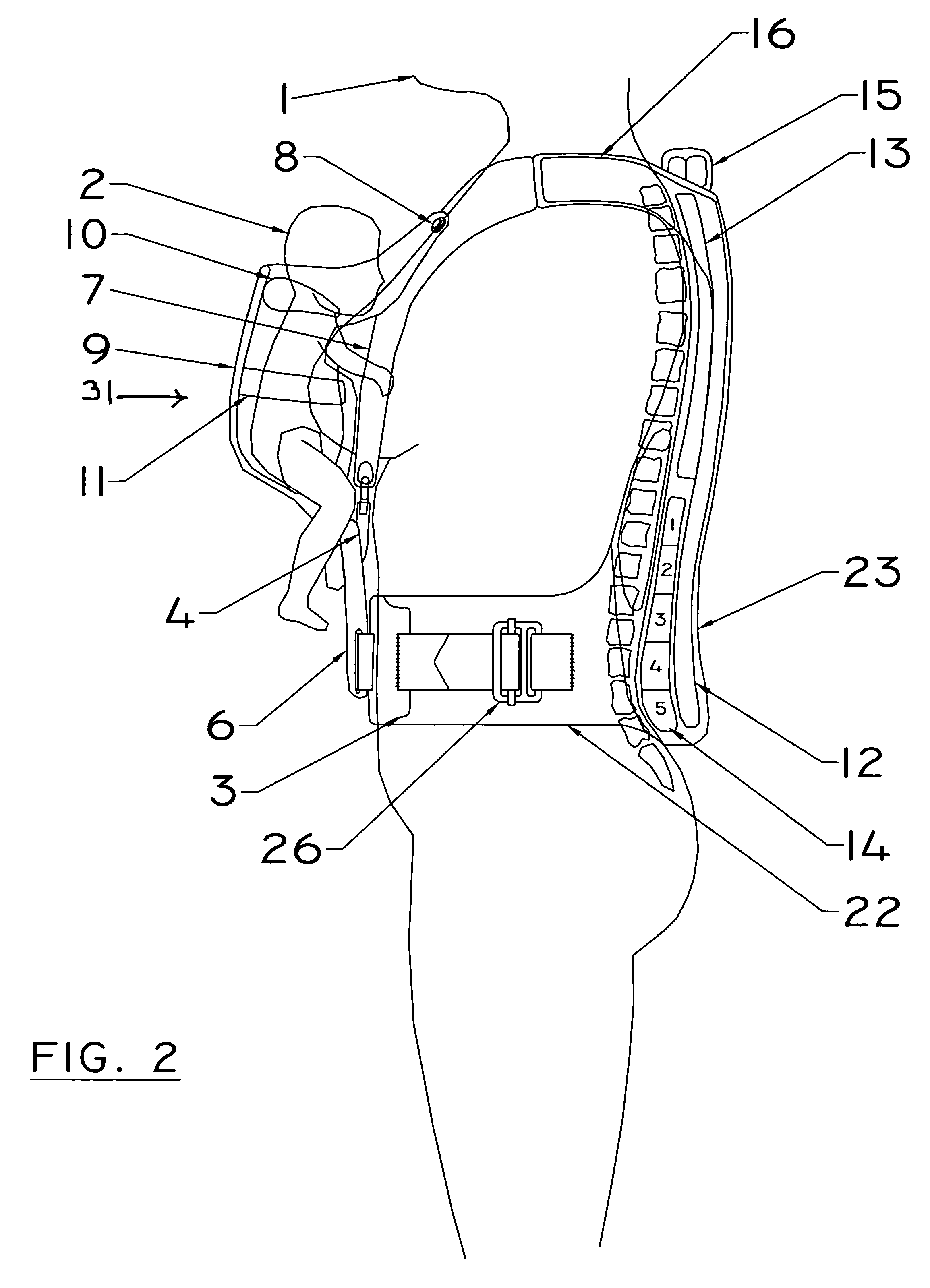
A device for relieving back strain for a user who is supporting a substantial weight which is off the user's vertical body axis at his or her front side. The device alleviates the aforementioned difficulties by utilizing the otherwise detrimental forces generated by the off-axis weight to press a rigid plate behind and adjacent to the spine of the device user against the user's spine. This rigid plate is preferably contoured to mirror the shape of the human spine. The device in one embodiment is used as a baby carrier. In another it is used with an abdominal support during pregnancy. In a third embodiment it provides support for the breasts of large breasted women.
Owner:DELLANNO RONALD P
Sow feed and breeding mode thereof
ActiveCN103461742AImprove immunityReduce laborFood processingAnimal feeding stuffAnimal sciencePregnancy
The invention discloses a sow feed and a breeding mode. The sow feed includes a pregnant sow feed and a lactating sow feed. By adoption of the feed, the immunity of sows can be effectively improved, the birth process and the estrus time are shortened, meanwhile, the weight of piglets can be increased, the possibility of yellow-white dysentery of piglets is reduced, and the survival rate of piglets is high. The breeding mode comprises the following steps: (1) five bags of pregnant sow feed are fed to a sow from the first day of the pregnancy to the 94th day of the pregnancy, wherein the specification of the feed is 40kg each bag; (2) five bags of lactating sow feed are fed to the sow from the 95th day of the pregnancy to the next mating, wherein the specification of the feed is 40kg each bag; (3) three bottles of injection liquid are injected to the sow during the delivery process for midwifery and healthcare. The mode is scientific, reasonable, healthy and efficient, not only is the feed used reasonably, but also the sow healthcare is helped, and furthermore, the immunity of the sow is improved.
Owner:廉江双胞胎饲料有限公司
Nutritional preparations
InactiveUS20060217385A1Promote maturityReduce and alleviate fetusBiocideAnimal repellantsPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives—all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Minimally invasive surgical stabilization devices and methods
The various embodiments of the present inventions provide stabilization devices and methods for use of the stabilization devices with minimally invasive gynecological procedures such as methods of preventing pregnancy by inserting intrafallopian contraceptive devices into the fallopian tubes.
Owner:BAYER HEALTHCARE LLC
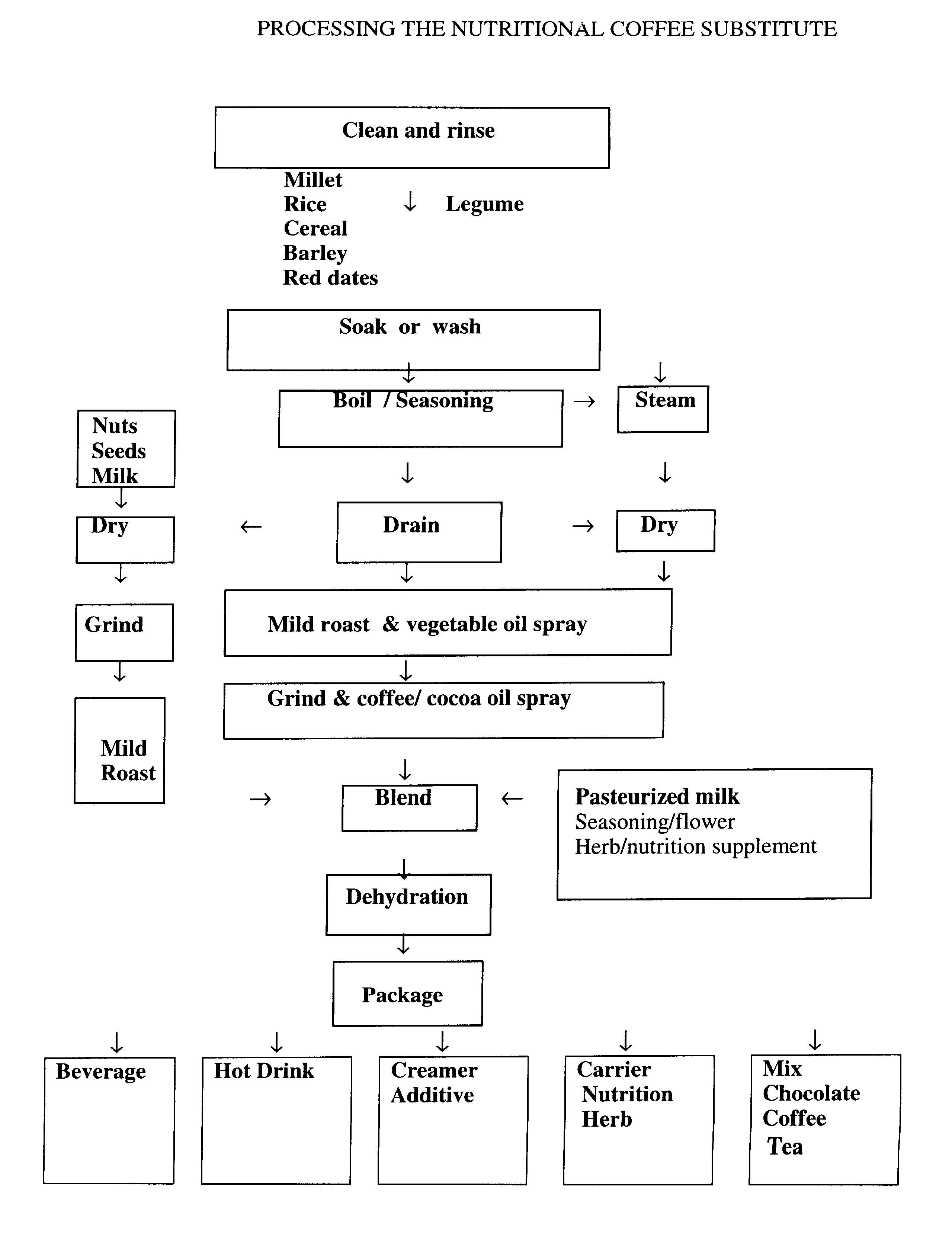
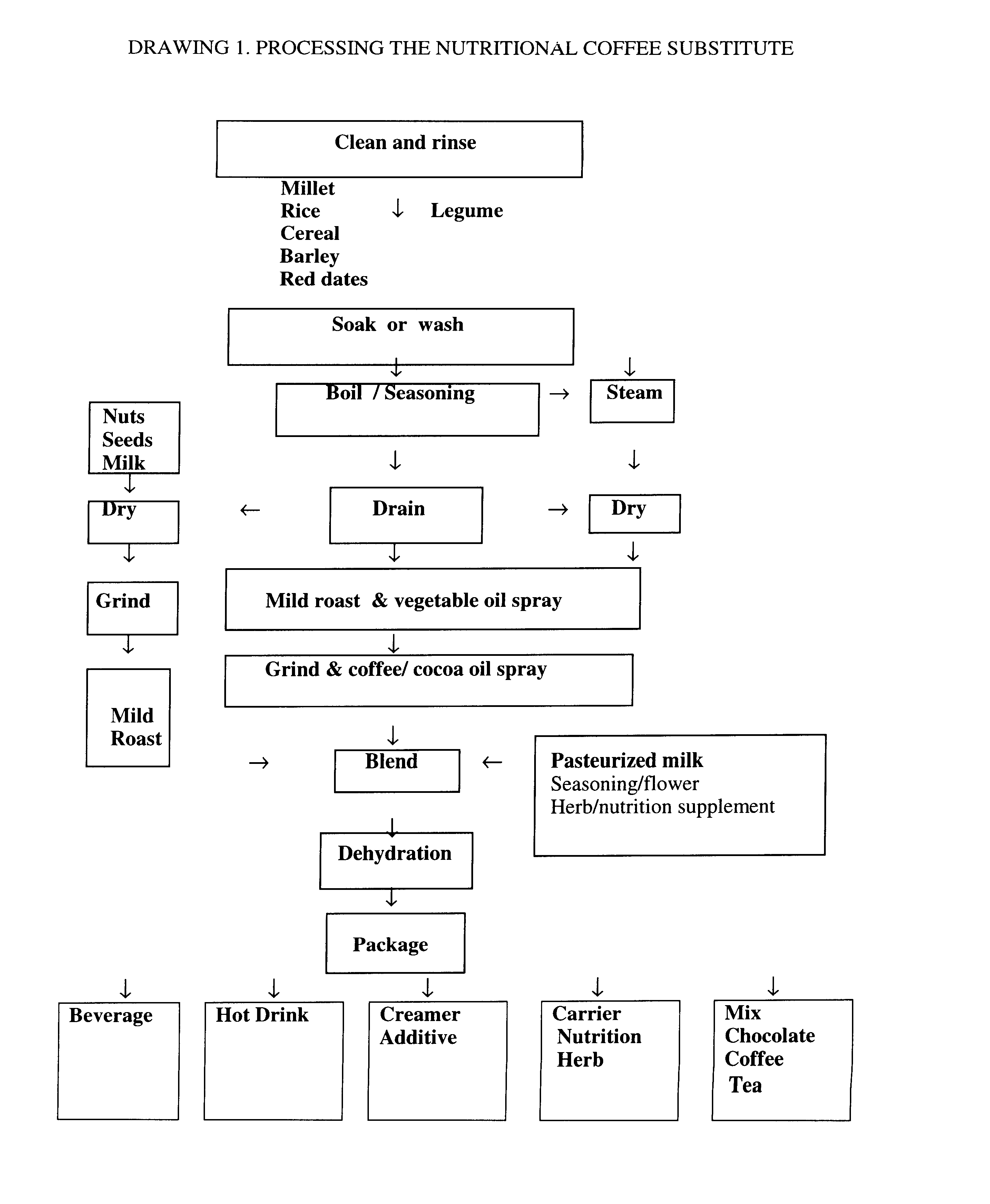
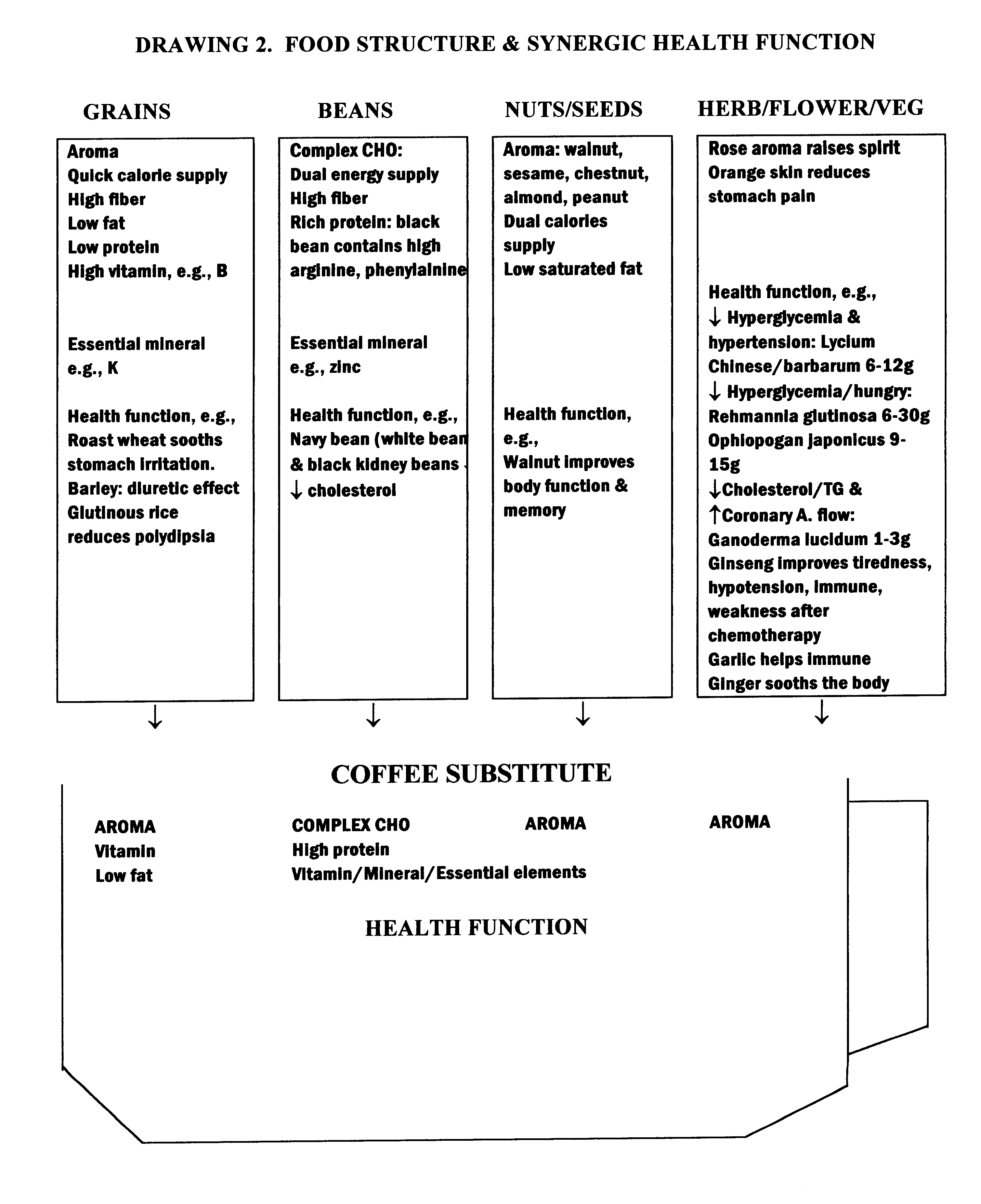
Coffee substitute
InactiveUS6171635B2Sufficient supplyEasy to acceptNatural extract food ingredientsFood preparationGastric irritationNutrition supplementation
A coffee-type beverage base is prepared by light roast method under 200° C. and originated from grain and legume. This coffee substitute has a pleasant aroma, and can be used as a carrier of nutritional supplement or herb therapy as well as an additive of coffee, tea, or chocolate. This novel drink is especially suitable for individuals who suffer from conditions making them coffee intolerant, e.g., pregnancy, or those who suffer form hypoglycemia, hypertension, arrhythmia, insomnia, or gastric irritation.
Owner:ZHAO IRIS G
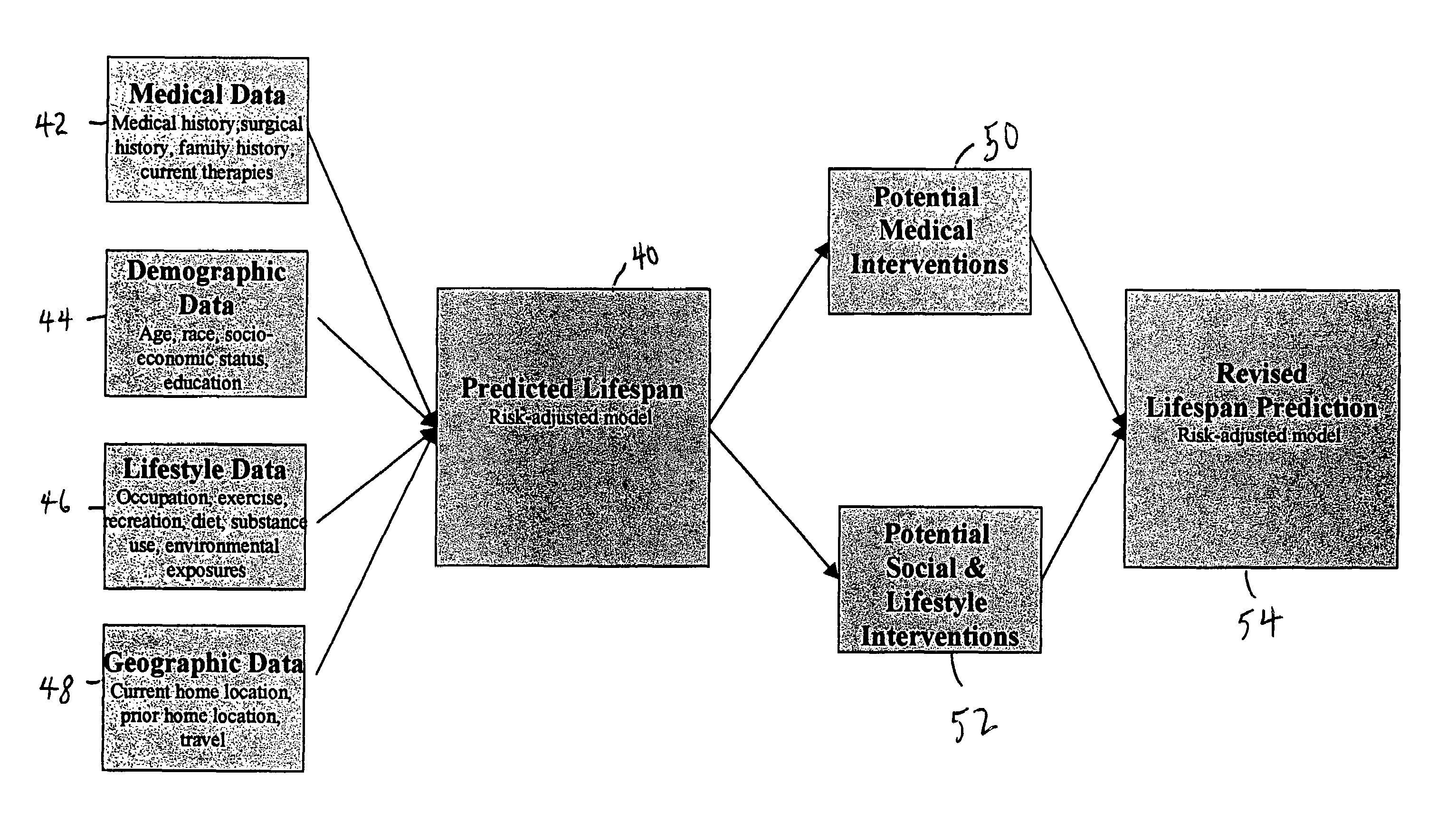
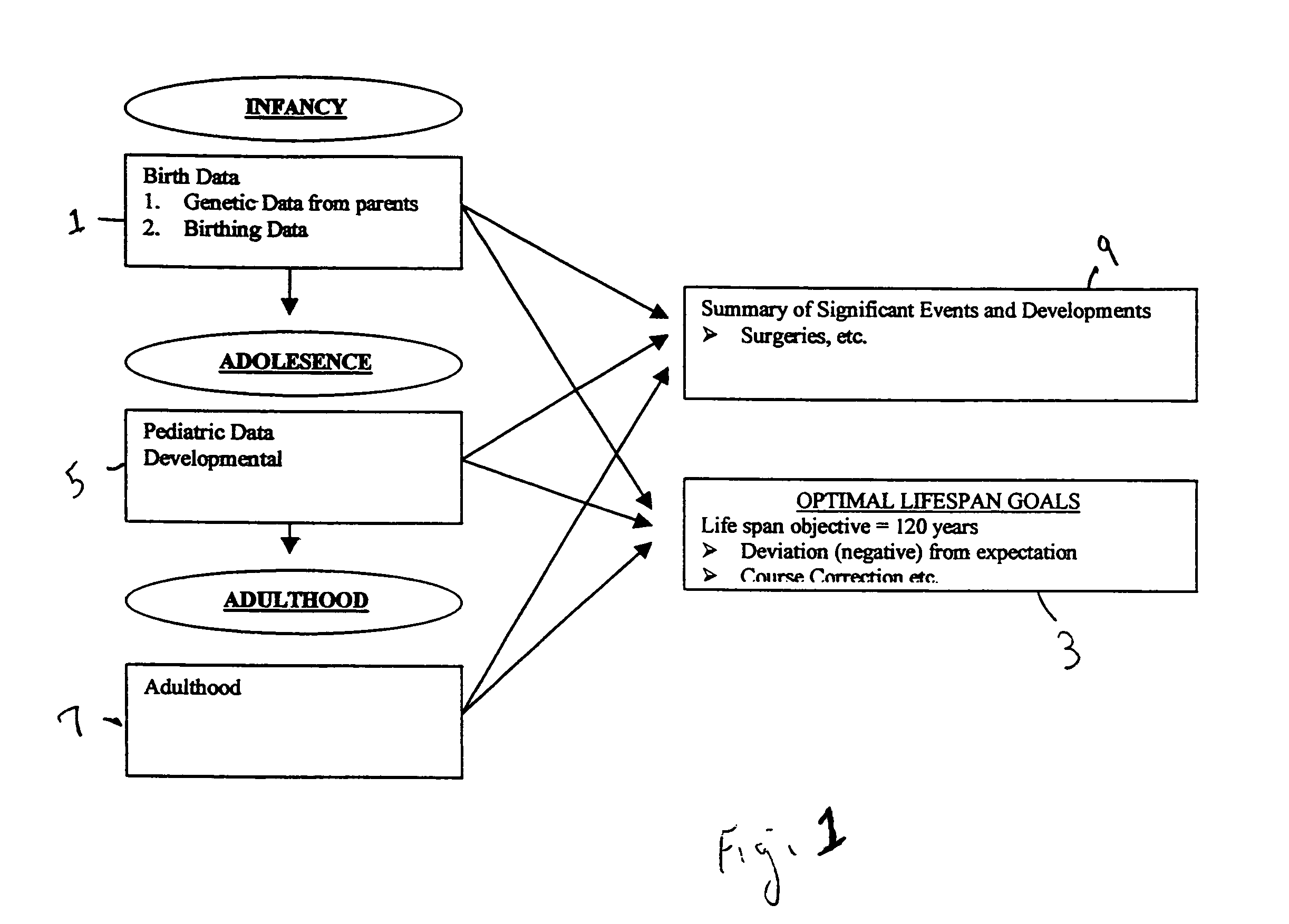
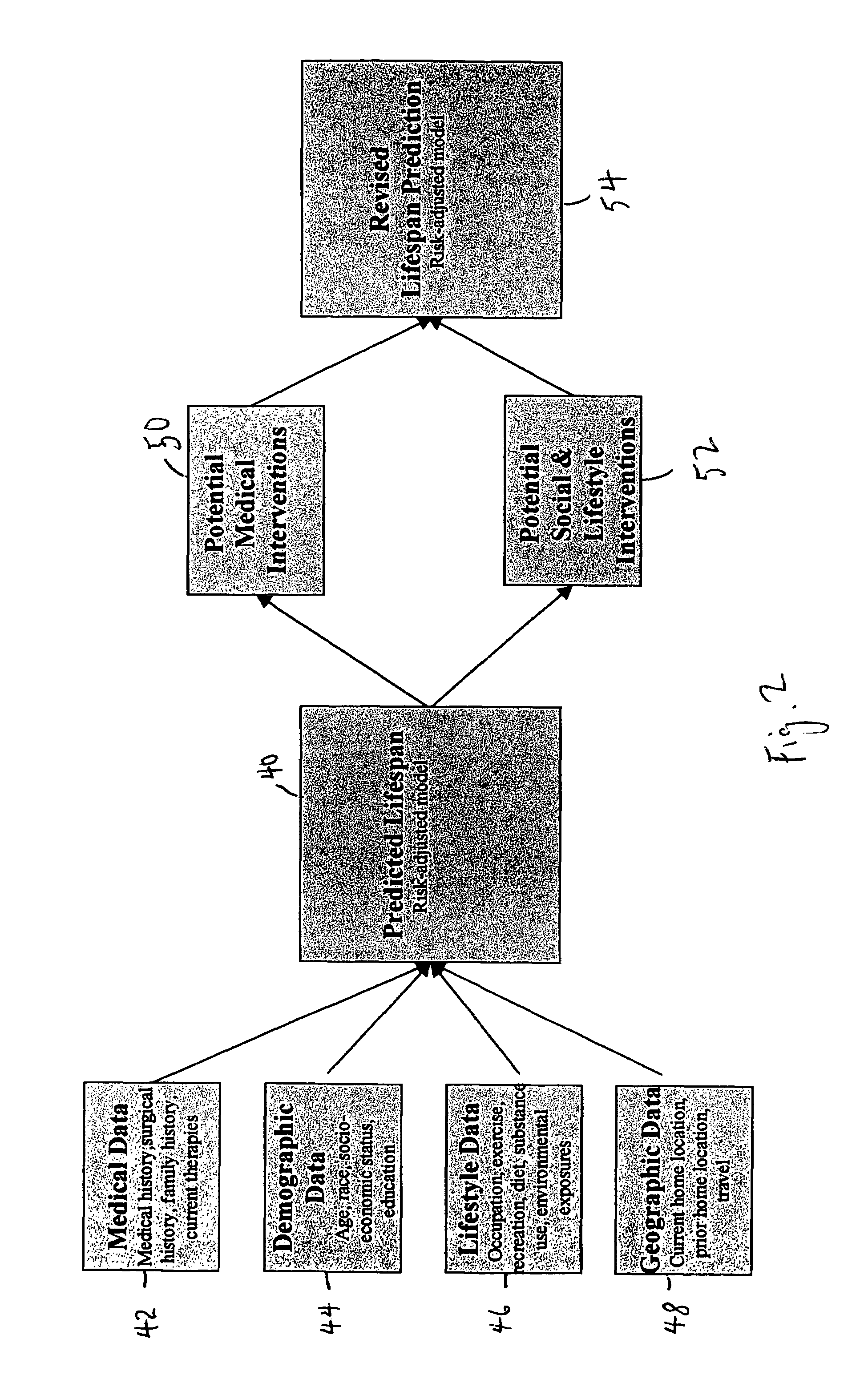
Health and life expectancy management system
A life expectancy management system which comprises: a storage means which is capable of storing data, such as genetic data, birth data, lifestyle data, pediatric health data, and adulthood health data; a means for altering the data based upon the occurrence of at least one event selected from the group consisting of: chronic and routine health events, emergency health events, pregnancy data and medical advancements; and a prediction modeling logic which provides a predetermined life expectancy that can be reduced by deviations from expectations which are calculated from the data and altered or adjusted data. Optionally, a means for providing recommended goals based upon the life expectancy predicted and the predetermined life expectancy.
Owner:LIFESPAN INTERACTIVE MEDICAL INFORMATION MANAGEMENT
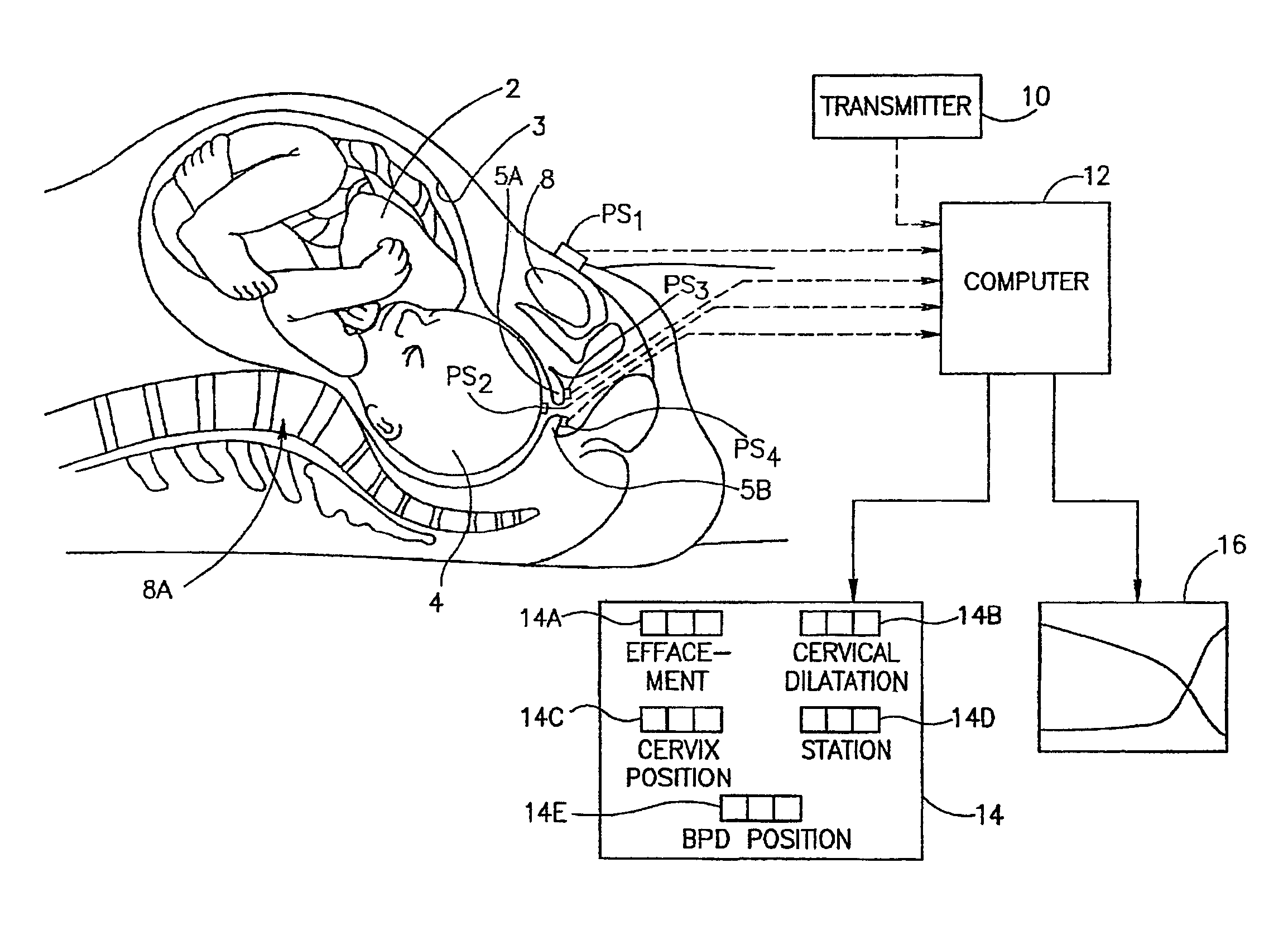
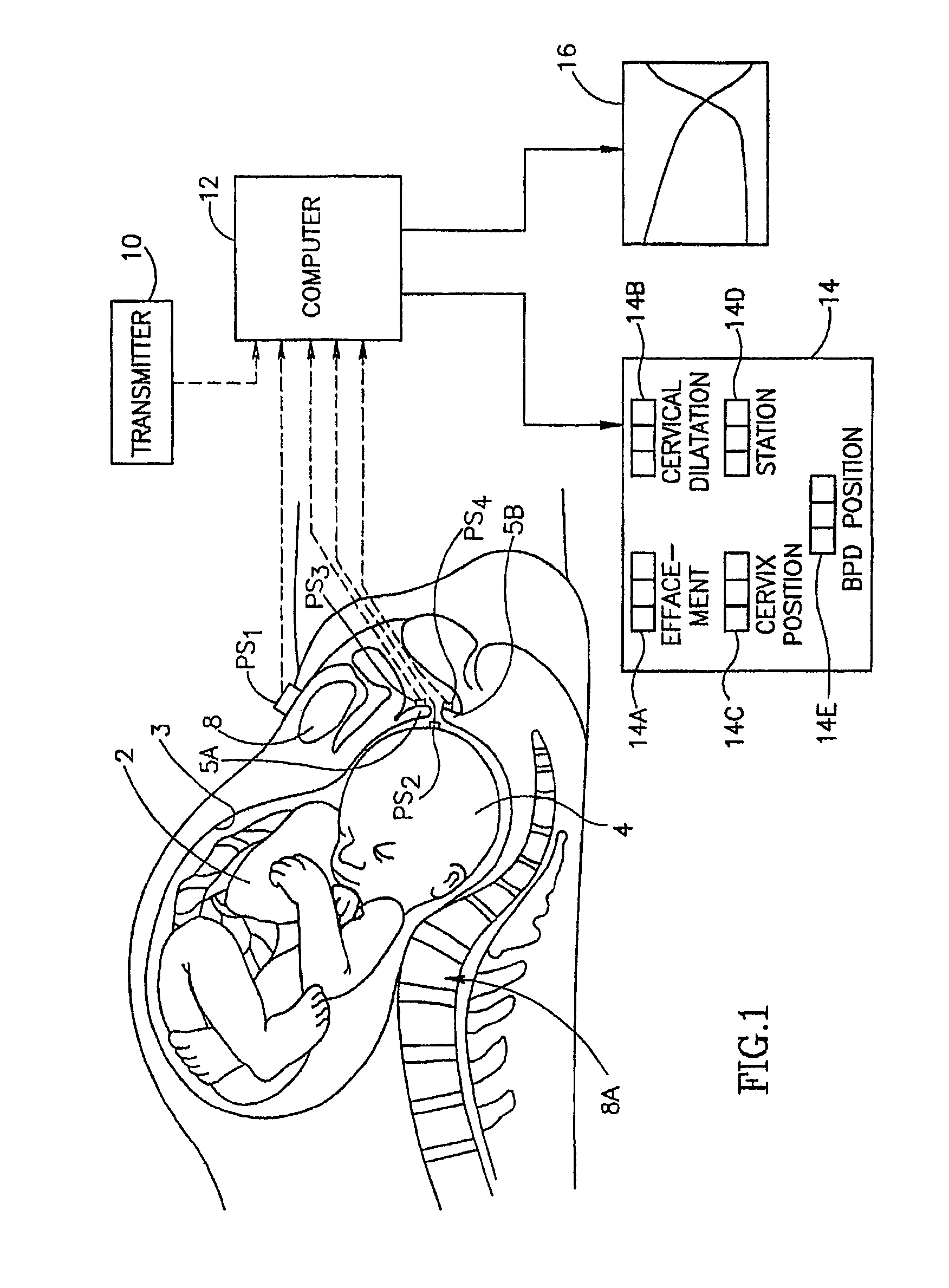
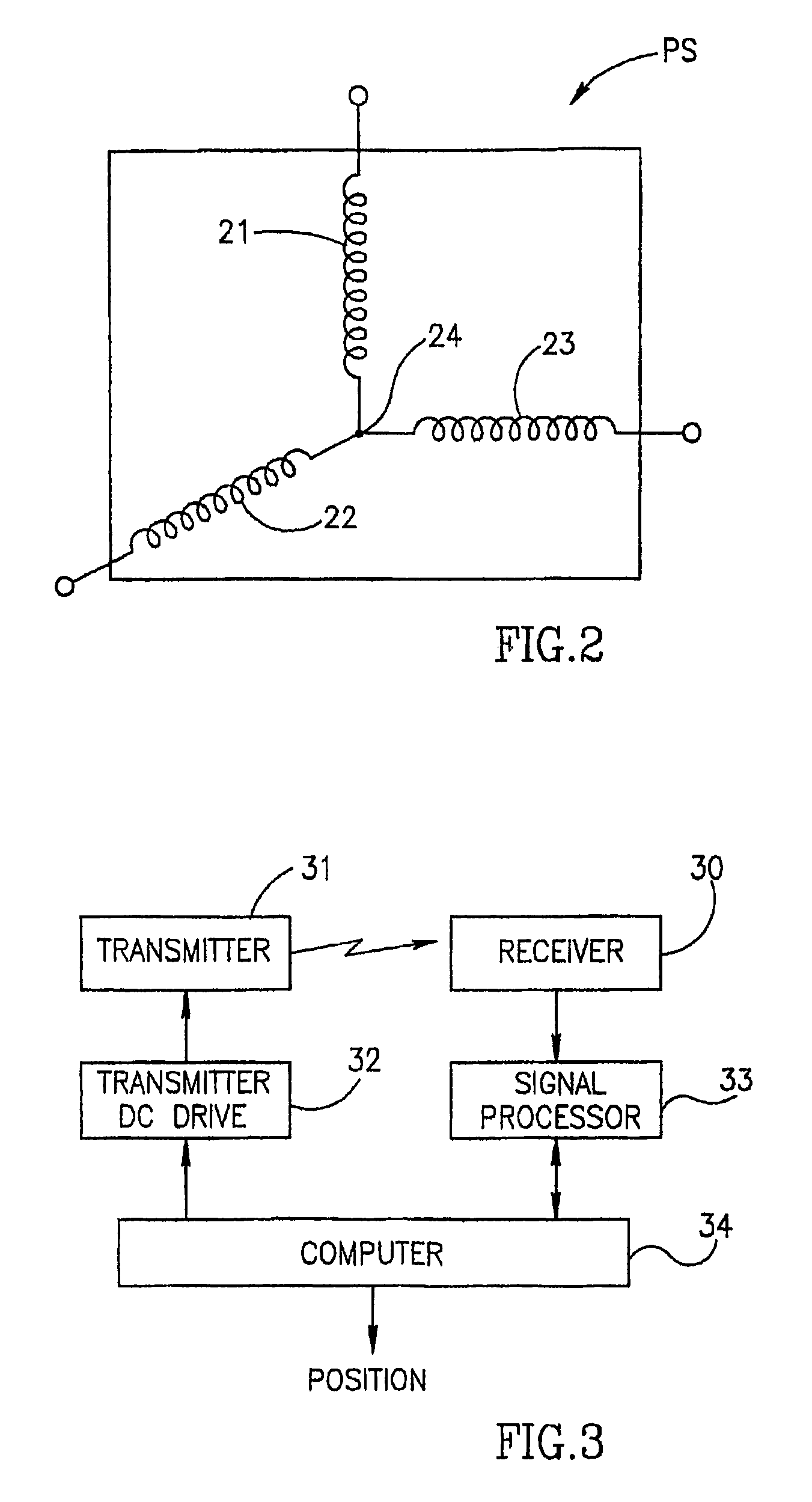
Method and apparatus for monitoring labor parameter
A method for early detection of a pregnancy complication, including touching a position sensor to a point on a fetal presenting part of a fetus in a mother, and capturing a position of the position sensor, touching the position sensor to a set of points on the mother and capturing the position of the position sensor at each point, and detecting a pregnancy complication sign based upon a predefined criterion for said pregnancy complication. Methods and apparatus are provided for identifying the BPD pattern in an ultrasound image, for determining characteristics of body parts outside of a pelvic region, for BPD reconstruction and for an adapter for a position sensor are also described among other embodiments.
Owner:TRIG MEDICAL
Full torso maternity garment
A maternity garment, which provides mild support to shape a woman's body and to act as a suspender for bottoms such as pants, shorts, or skirts that may be ill fitting due to body changes as a result of pregnancy, wherein the preferred garment has at least one shoulder strap, a belly panel, a hip band, two side panels, and a back panel, and is made of high performance fabric with varying degrees of compression.
Owner:BLANQI
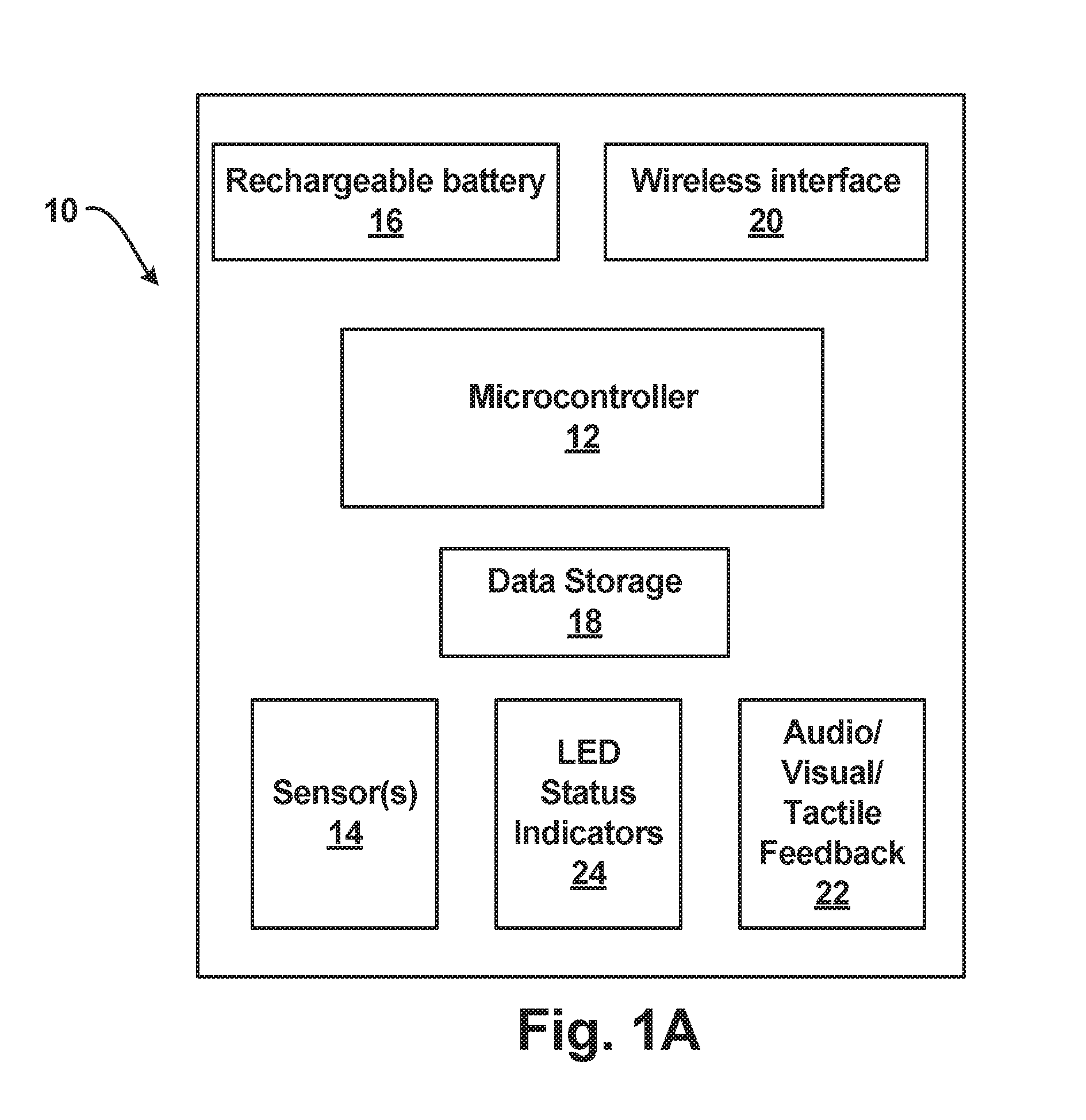
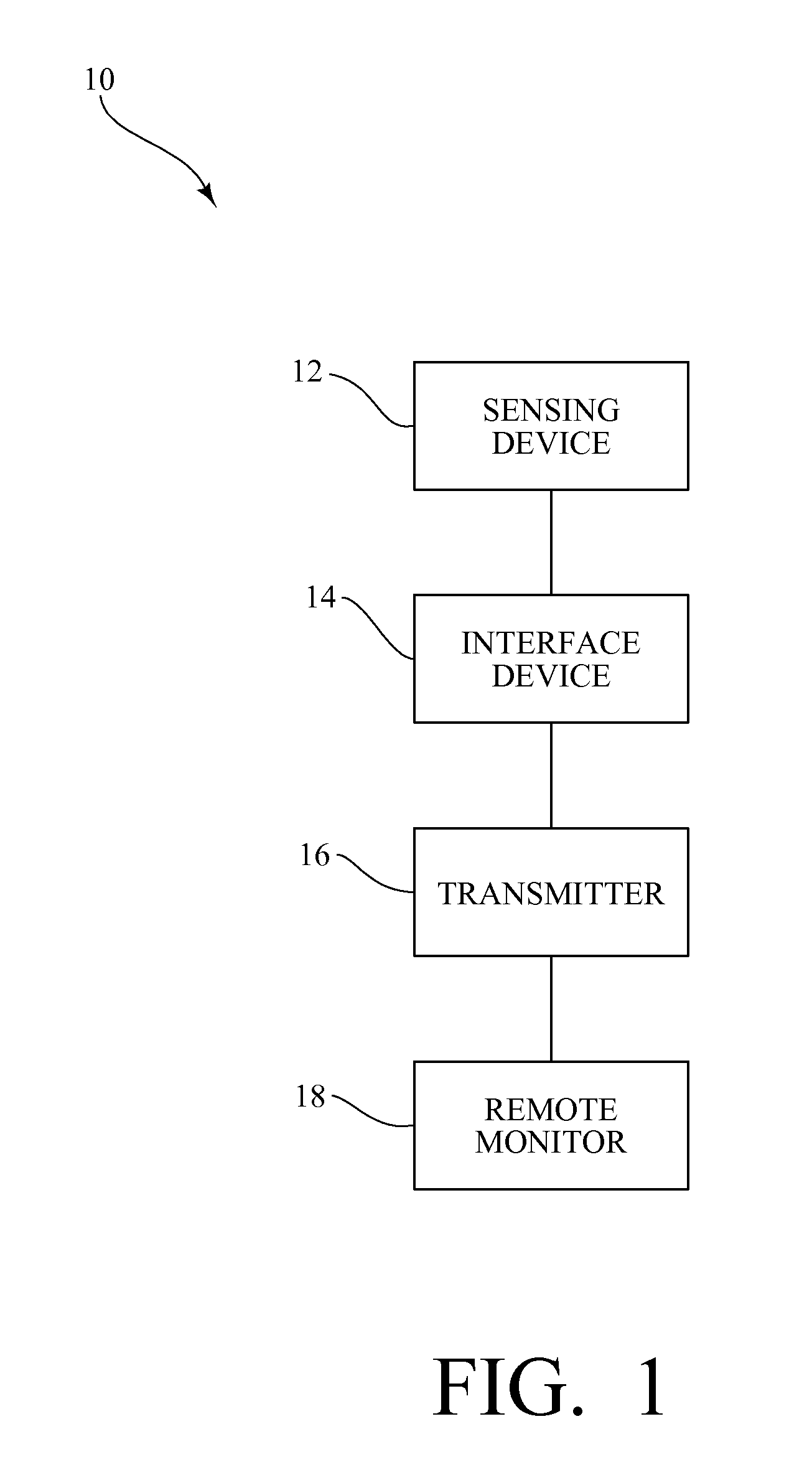
System for monitoring pregnancy in mammals
InactiveUS20140073879A1Prevent free movementEasy to adaptAnimal reproductionSensorsObstetricsPregnancy
A system is disclosed for long term, continuous, monitoring of pregnant mammals particularly to detect the onset of preterm labor. For example, the system enables communication between sensors and a collection unit for transmittal to a remote unit for display and monitoring of the collected data by a clinician while the individual is ambulatory and not in the presence of the clinician.
Owner:MB DEVICE
Quinoline derivatives, their production and use
The present compounds are intermediates for the preparation of quinoline derivatives and compositions having gonadotropin-releasing hormone antagonistic activity useful as propylactics or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, uterine or cervical cancer, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; are effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a male or female contraceptive, as an ovulation-inducing agent; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; and are useful for modulating estrous cycles in animals in the field of animal husbandry, as agents for improving the quality of edible meat or promoting the growth of animals, and as agents for promoting spawning in fish.
Owner:TAKEDA PHARMA CO LTD
Nutritional preparations
InactiveUS20060217386A1Promote maturityReduce and alleviate fetusBiocideSkeletal disorderPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives-all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Herd management technology
ActiveUS20080110406A1Practical and reliable and accurate and efficientSimple methodAnimal reproductionSurgeryPregnancyEstrus Detection
A method of determining breeding status in animals by (a) determining a breeding event (cyclicity, mounting, pregnancy, open) time, (b) providing an estrus or other breeding event detection apparatus, (c) affixing the estrus detection apparatus at a predetermined location on a female animal at a predetermined application time prior to the breeding event time, and (d) monitoring the estrus detection apparatus for activation thereof. An indicator apparatus is provided for use in indicating when an animal is in estrus. The apparatus is configured to be affixed to the rump of an animal to detect and indicate when the animal has been mounted and, thus, when the animal is in estrus. The apparatus is affixed to the animal by an adhesive layer. Layered on the top surface of the adhesive layer is an indicator layer that is in turn covered with a floodcoat layer. The floodcoat layer is adapted for removal upon the mounting of the first animal by a second animal. Removal of the floodcoat layer exposes the indicator layer indicating that the first animal has been mounted, thereby indicating that the first animal is in heat. The floodcoat layer is highly visible and may be seen from a distance.
Owner:ANDERSON MARK
Pregnancy support pillow
In one embodiment, a pillow comprises a curved pillow body having a top region and a bottom region. The top region is larger in size than the bottom region. Also, the bottom region is adapted to be placed between a user's lets while the user is lying on her side while the top region conforms to the user's stomach and chest.
Owner:THE BOPPY CO LLC
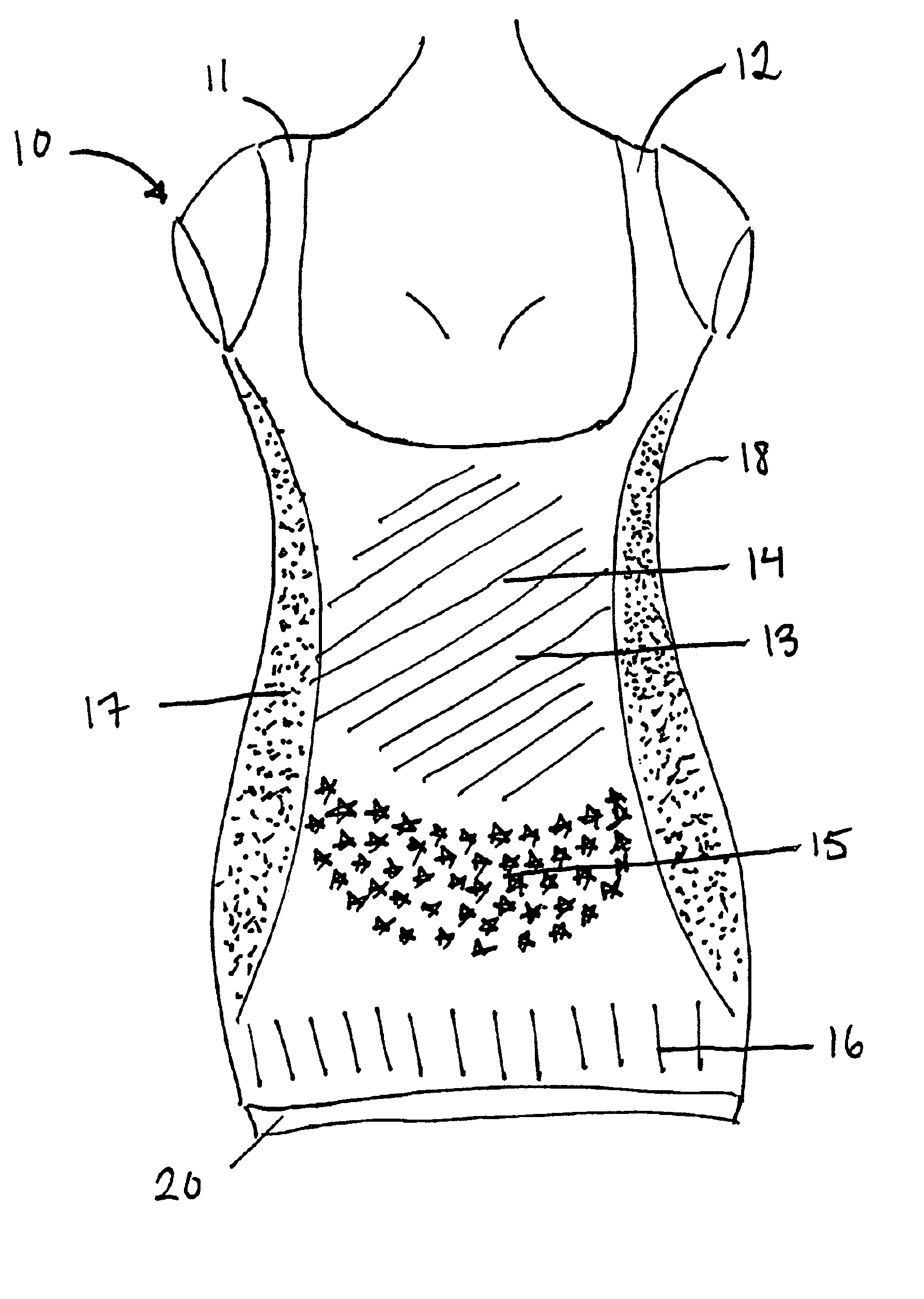
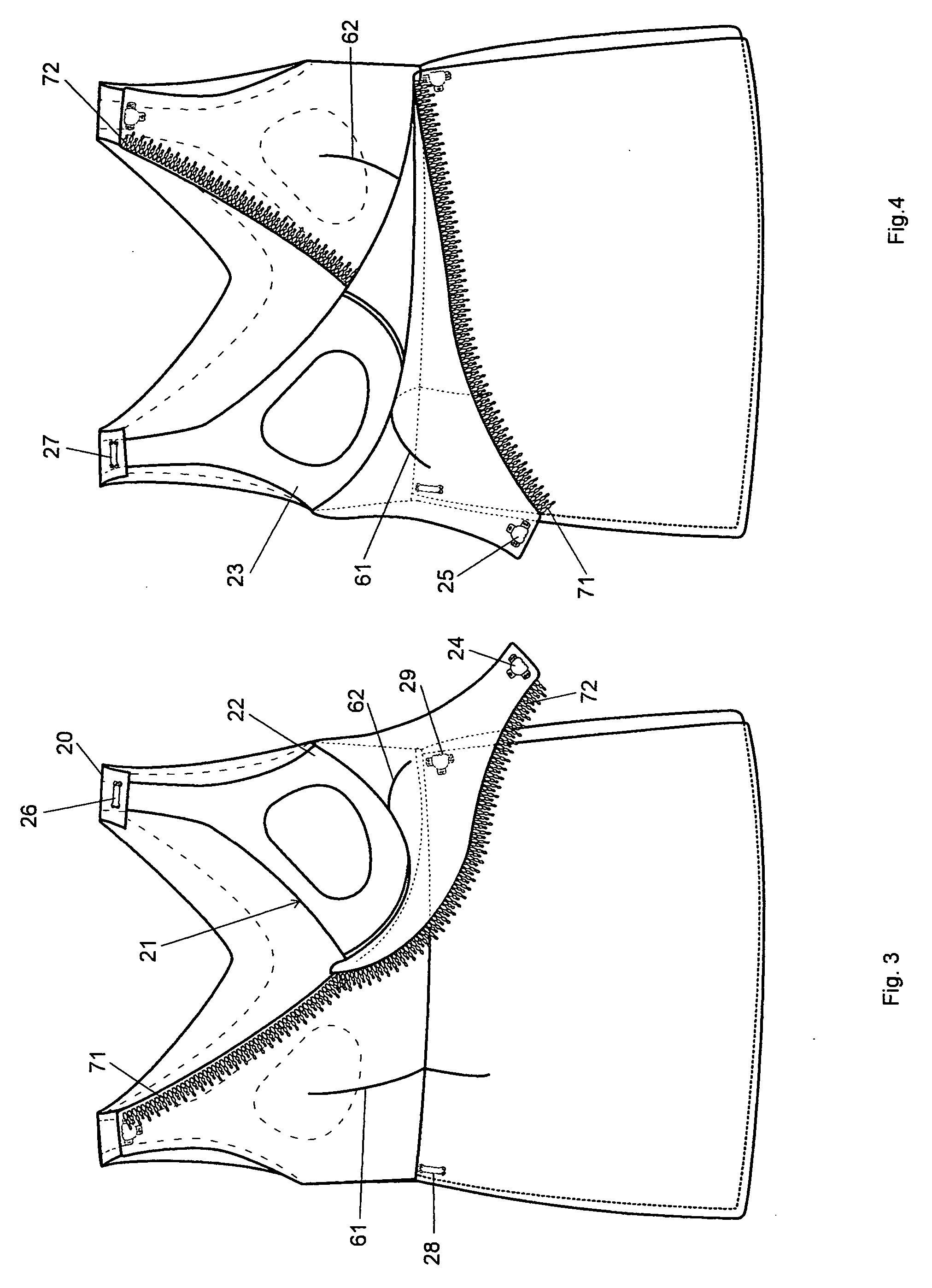
Labor and delivery outfit
InactiveUS20070271675A1Improve mental statusImprove comfortMaternity clothingProtective garmentLeft breastPregnancy
A labor and delivery outfit designed to provide function, comfort and dignity, comprises: a sleeveless, wrap shirt having an upper portion that includes a right nursing flap, and a left nursing flap, a first lower portion extending from the right nursing flap, a second lower portion extending from the left nursing flap, a back having a right strap, a left strap, and overlapping right rear lower portion and left rear lower portion defining an opening therebetween; a bra, preferably a built-in, supporting, nursing bra having a first section extending between the right strap and the first lower portion and including a right breast nursing cup, and having a second section extending between the left strap and the second lower portion and including a right breast nursing cup, and, a left breast nursing cup, releasable fasteners for fastening the right nursing flap to the right strap, thereby covering the right nursing cup, and releasable fasteners for fastening the left nursing flap to the left strap, thereby covering the left nursing cup; and, a wrap skirt having an elastic waist, a built-in pregnancy panel, and, a rear zipper. The nursing flaps of the shirt in closed position may form a V-neck. The back may be shaped in a V. A seam is provided between the upper portion and lower portions. Releasable fasteners are provided for fastening the rear lower portions to the upper portion of the shirt, thereby providing access to the spine.
Owner:ERACA JENNIFER A
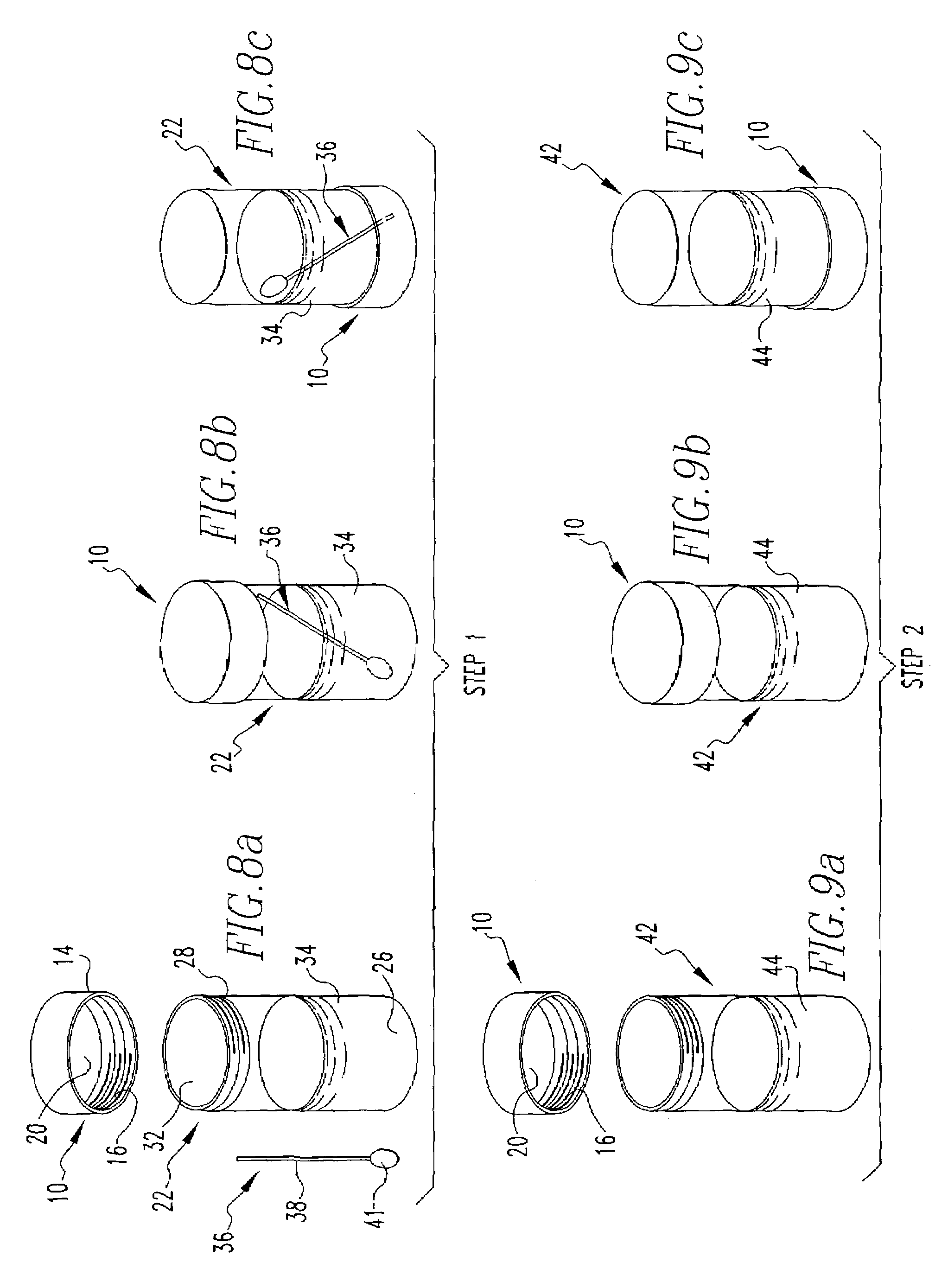
Pregnancy and sex identification test based on saliva or other bodily fluids
InactiveUS7410807B2Accurate and easy and inexpensiveAccurate and easy and inexpensive meanAnimal reproductionBiological testingPregnancyHormones regulation
A method of testing an animal for pregnancy or sex identification comprising the steps of first, providing a first vessel containing a liquid and having a removable surface wherein said removable surface is at least partially coated with an antibody and then introducing a bodily fluid from the female animal into said first vessel so that said bodily fluid contacts the liquid and then manipulating the first vessel so that the liquid contacts the antibody. Then, a second vessel containing a reporter hormone solution is provided and the removable surface from the first vessel is displaced to the second vessel and manipulating the second vessel so that the reporter hormone solution contacts the removable surface. Then, a third vessel containing an indicating solution which has an appearance which is related to the amount of the reporter hormone contacted is provided, and the removable surface is displaced from the second vessel to the third vessel. The third vessel is manipulated so that the indicating solution contacts the removable surface. Then, a determination is made regarding the pregnancy or sex of the animal based on the appearance of the indicating solution.
Owner:DREAM MAKERS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com