Patents
Literature
160 results about "Peritonitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inflammation of peritonium, the membrane that lines the inner abdominal wall and encloses organs within the abdomen.
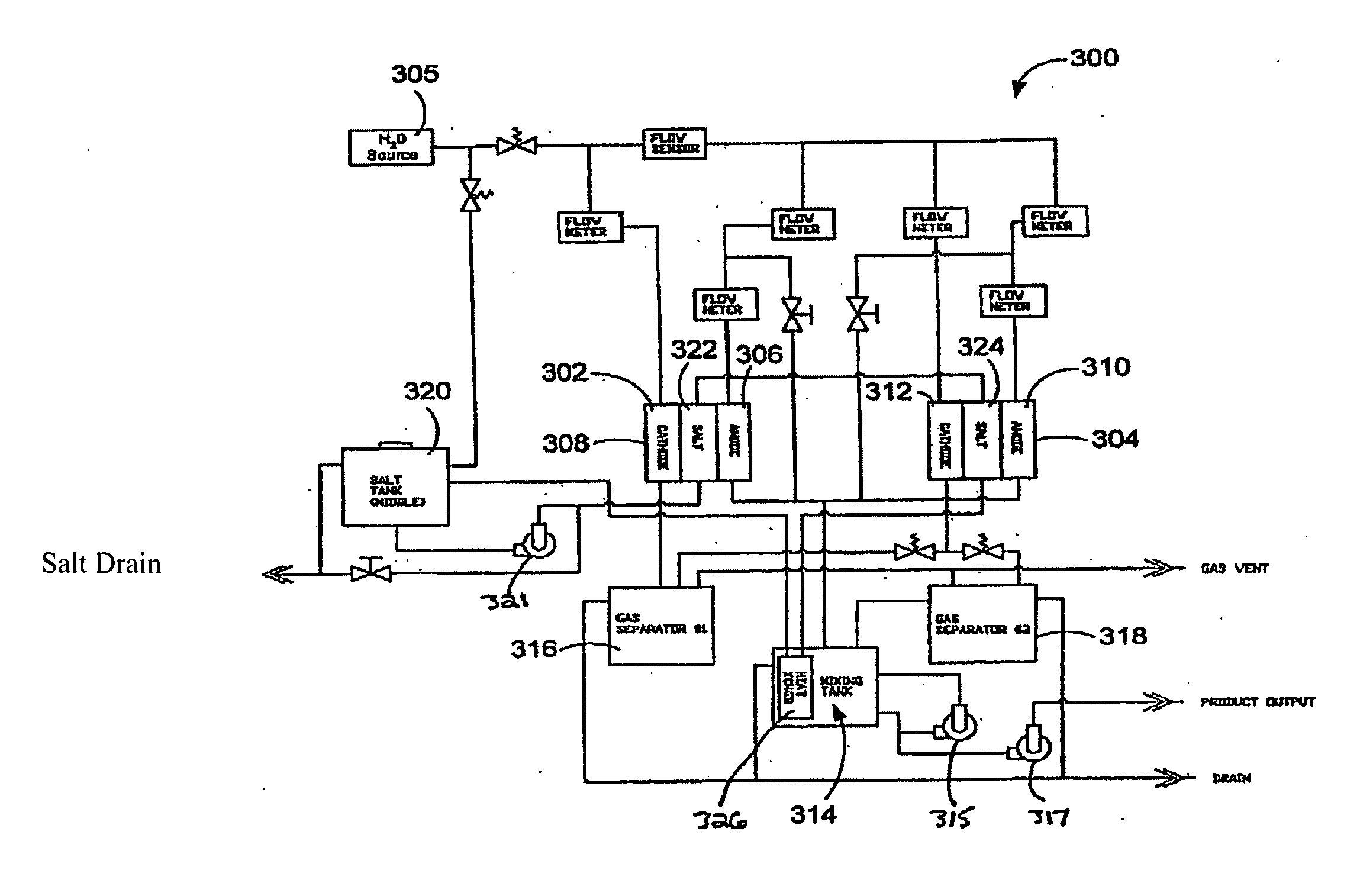
Methods of treating or preventing peritonitis with oxidative reductive potential water solution
ActiveUS20070173755A1Inhibition of secretionReduce bacterial loadAntibacterial agentsBiocideMedicinePeritoneal Hemorrhage
Provided is a method for treating or preventing peritonitis by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers and can be administered in conjunction with one or more additional therapeutic agents. Further provided is a method for preventing peritoneal hemorrhage, adhesions and abscesses.
Owner:SONOMA PHARMA INC
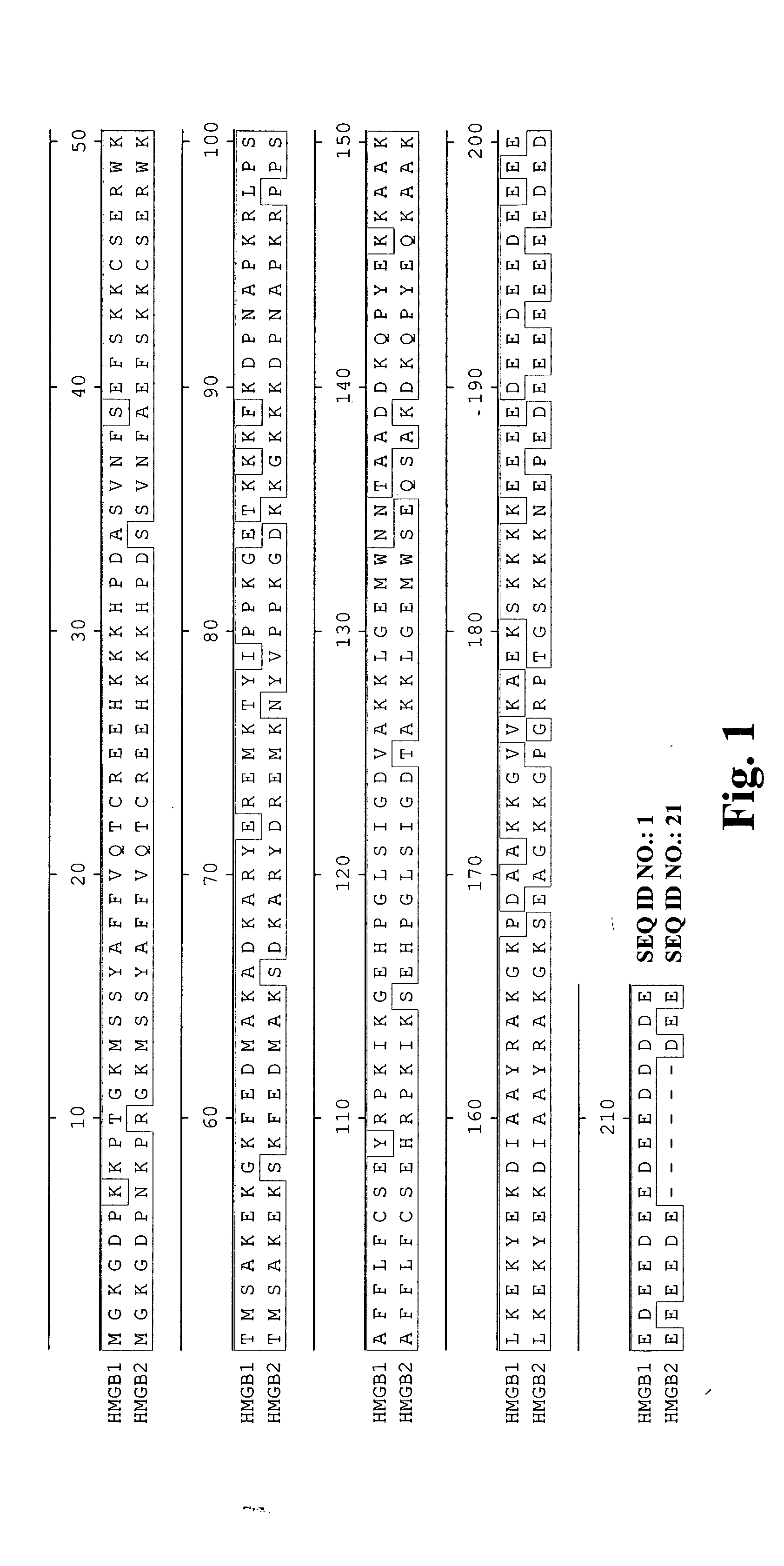
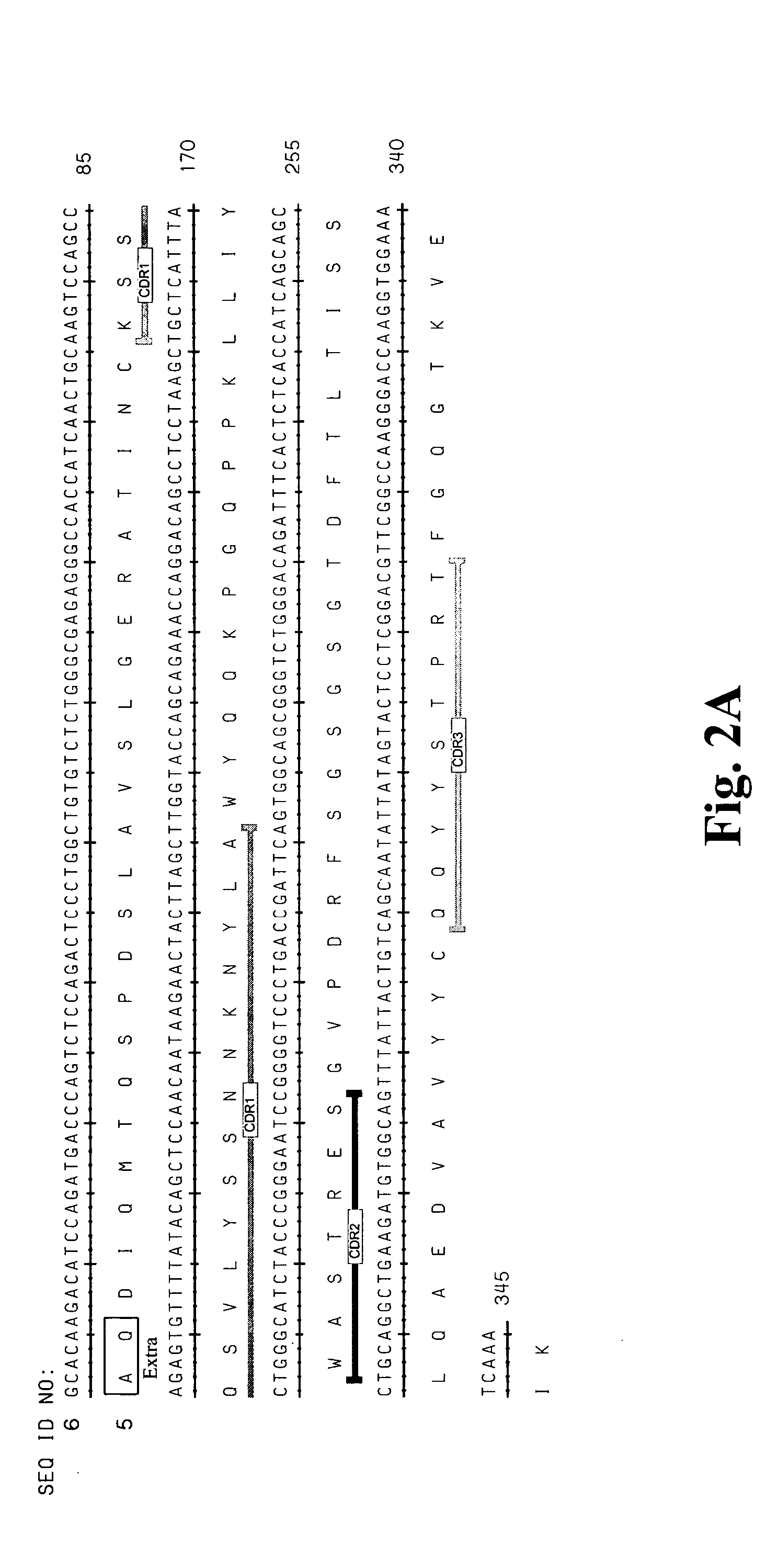
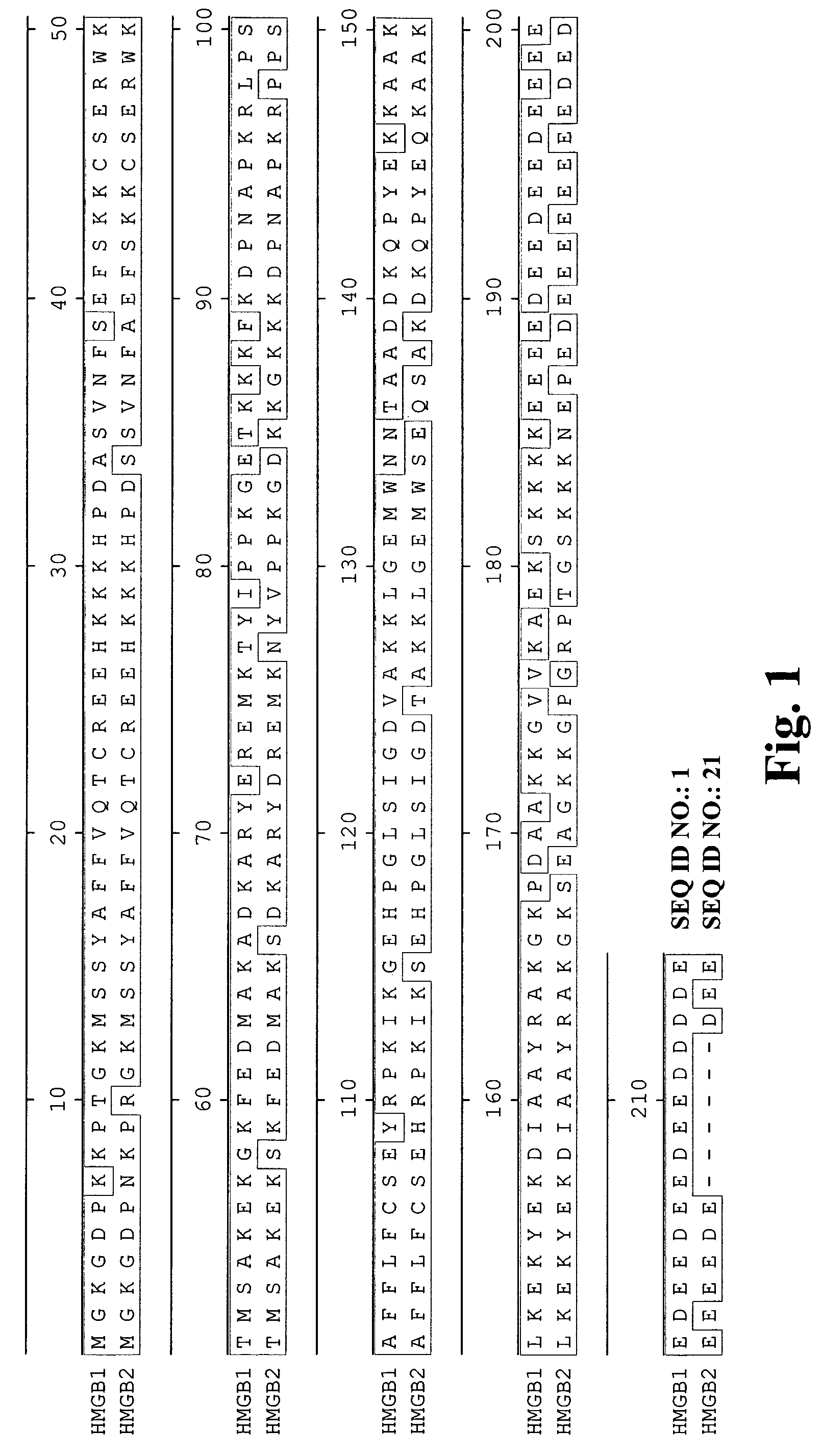
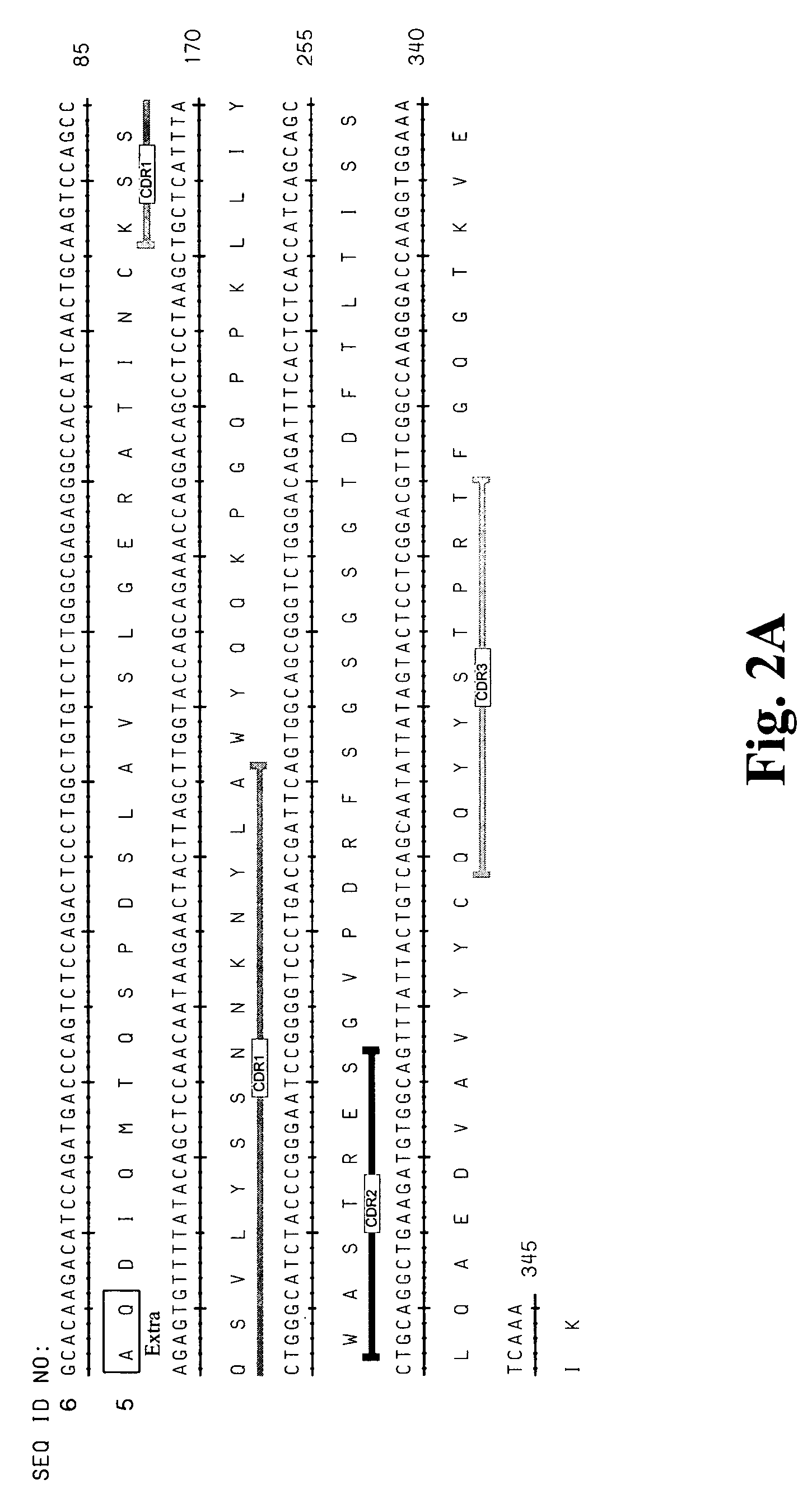
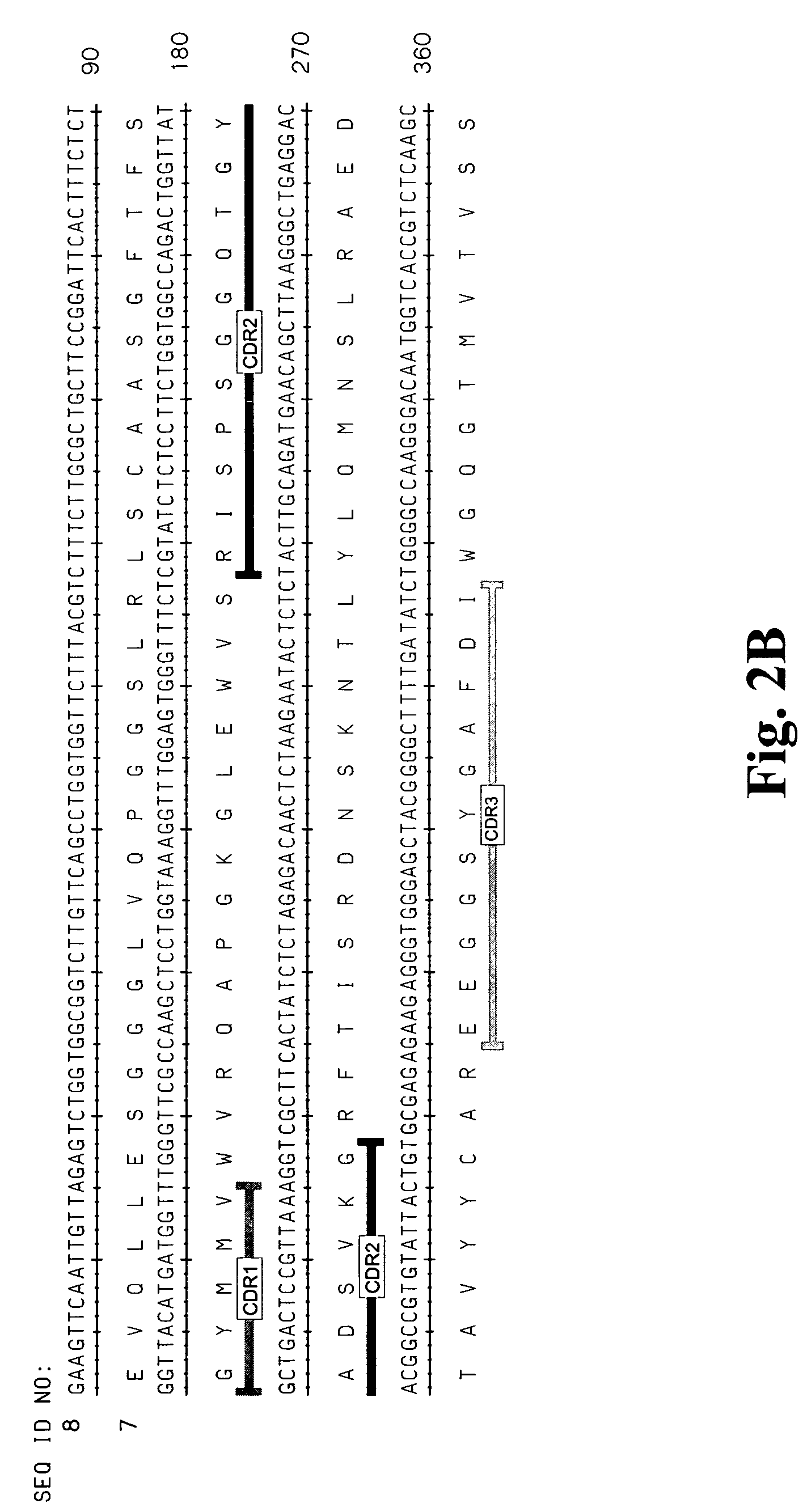
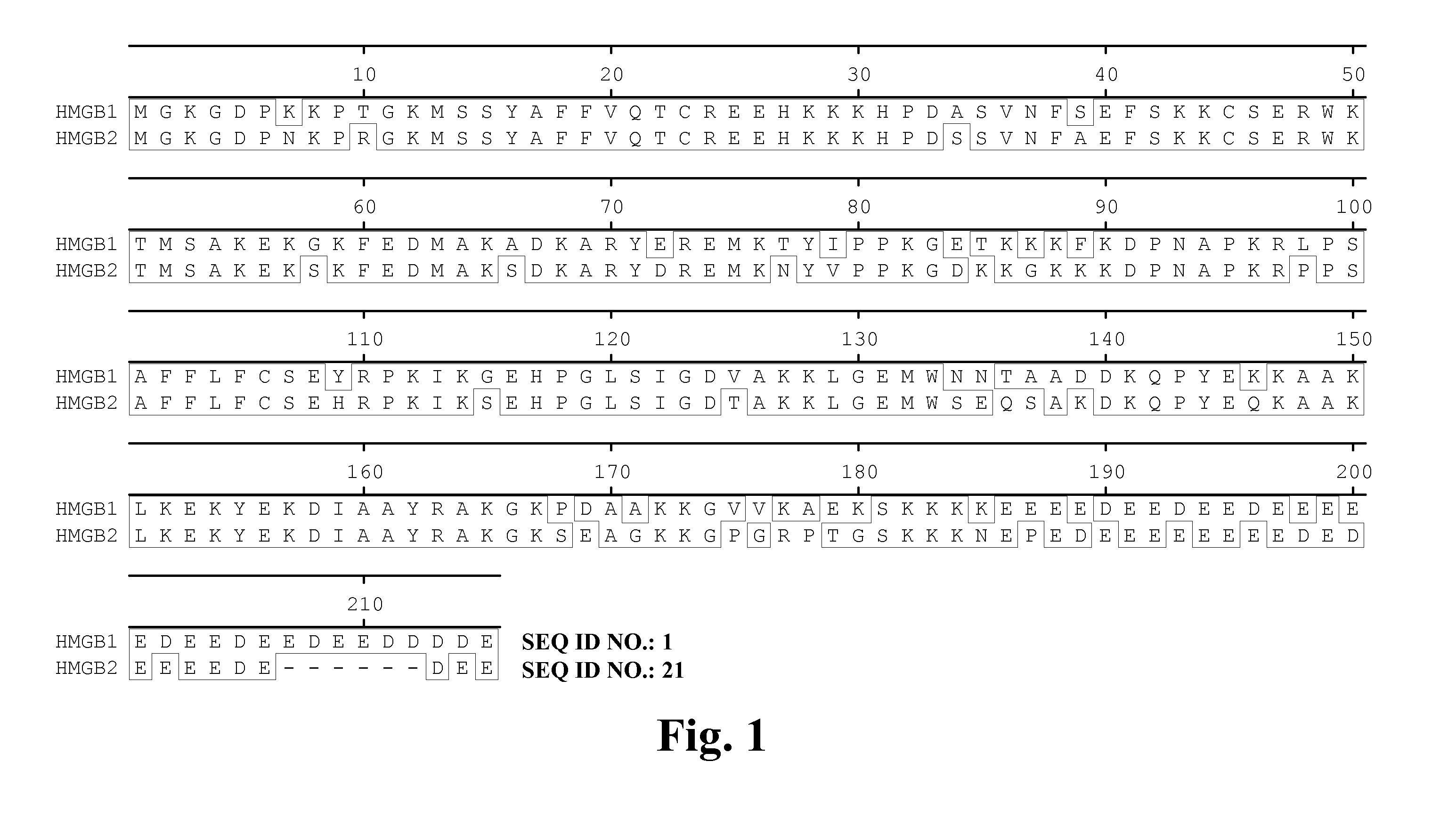
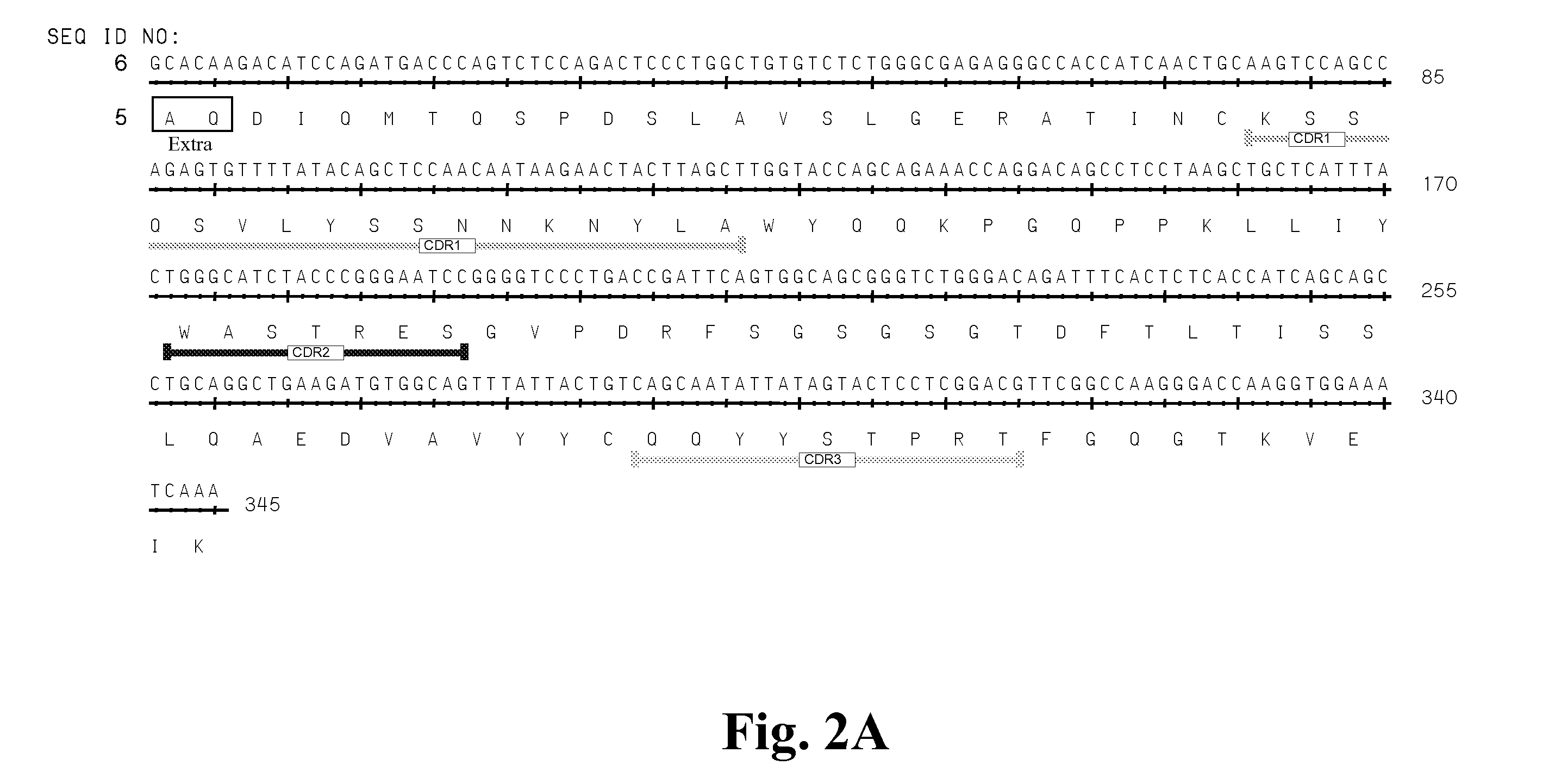
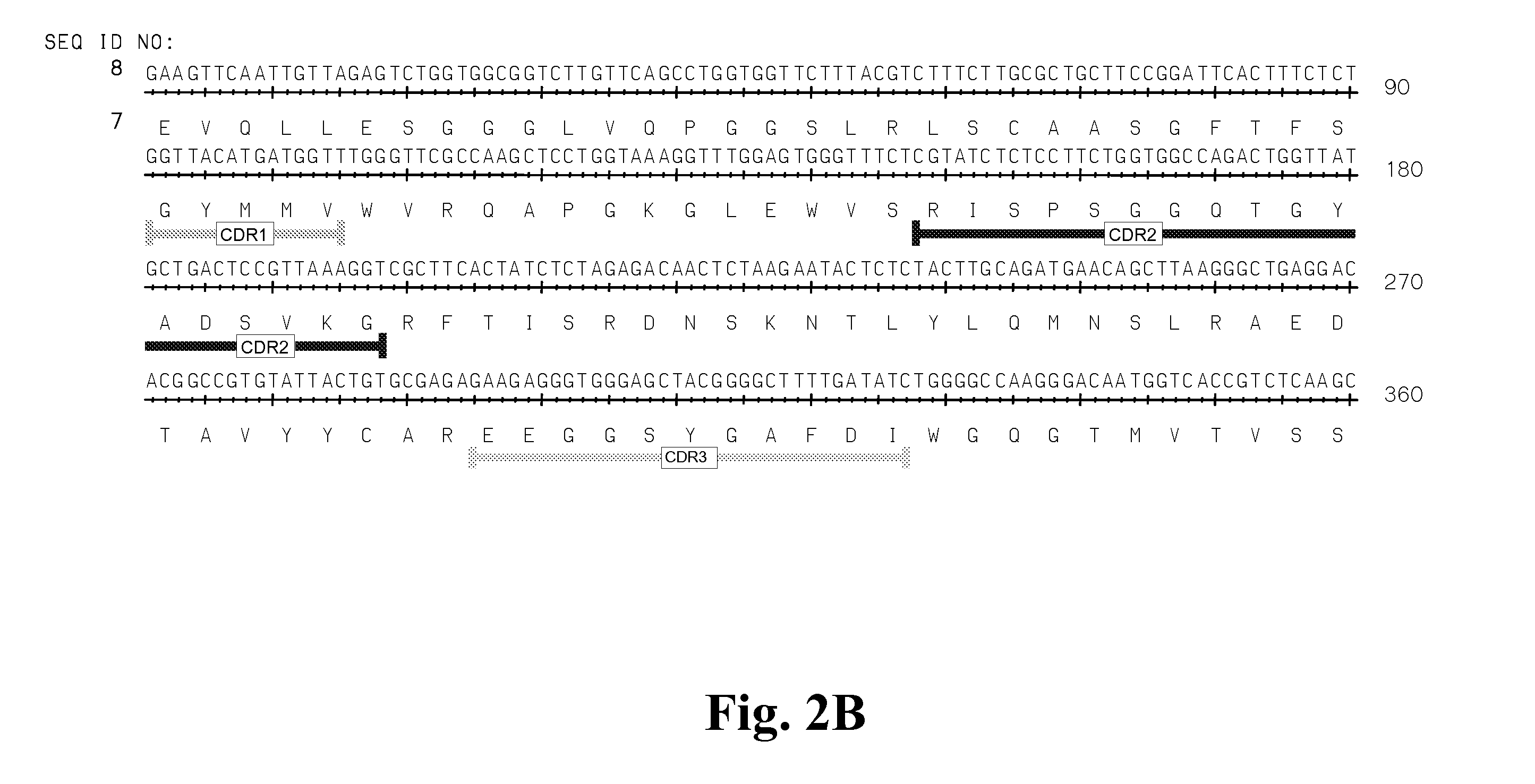
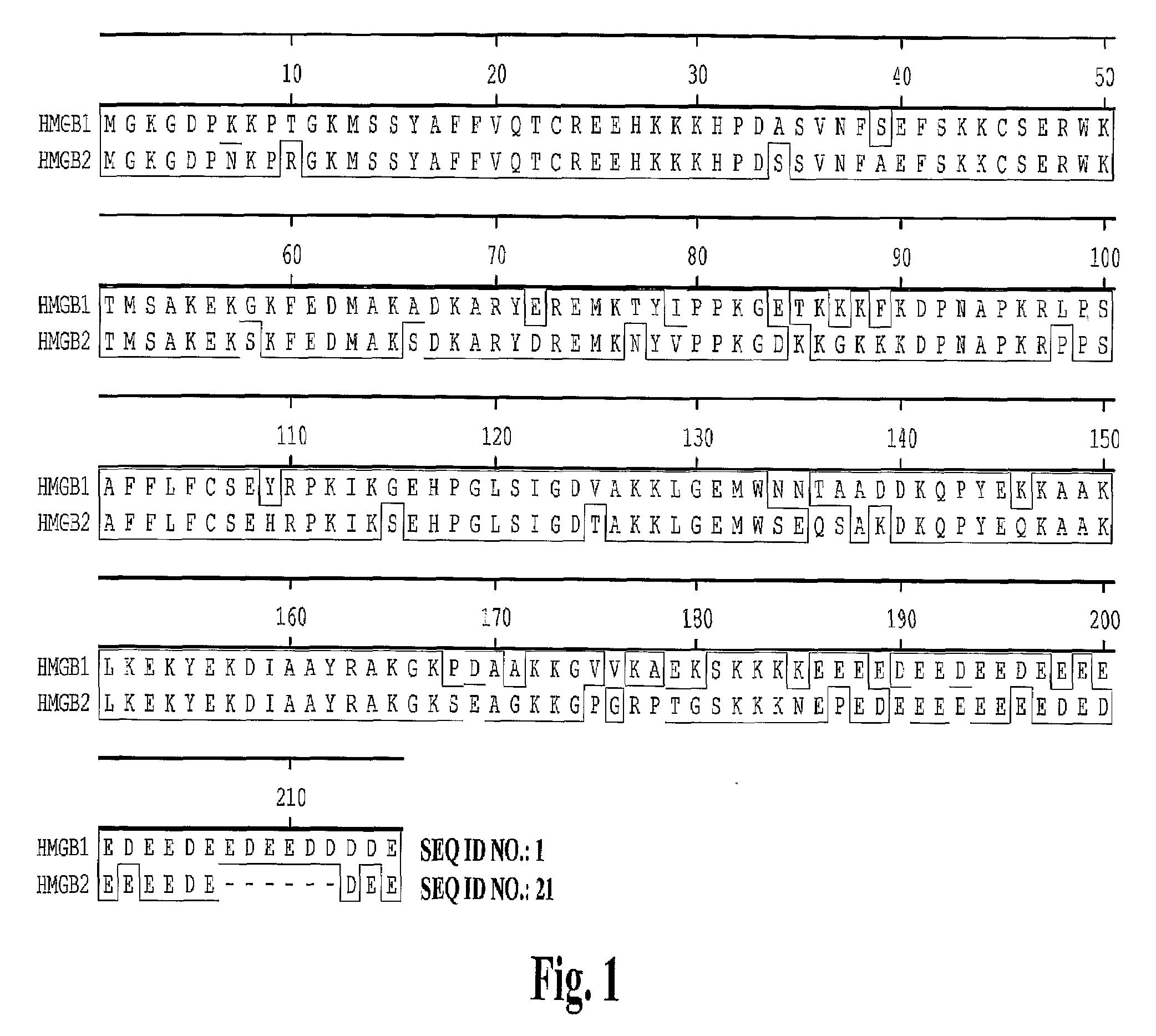
High affinity antibodies against HMGB1 and methods of use thereof
InactiveUS20060099207A1Reduce bone loss and/or cartilage damageAntibacterial agentsAntibody mimetics/scaffoldsReperfusion injuryAllograft rejection
Compositions and methods are disclosed for inhibiting the release of a proinflammatory cytokine from a vertebrate cell, and for inhibiting an inflammatory cytokine cascade in a patient. The compositions comprise, for example, high affinity antibodies that specifically bind HMG1 and antigenic fragments thereof. The high affinity antibodies of the present invention and pharmaceutical compositions comprising the same are useful for many purposes, for example, as therapeutics against a wide range of inflammatory diseases and disorders such as sepsis, rheumatoid arthritis, peritonitis, Crohn's disease, reperfusion injury, septicemia, endotoxic shock, cystic fibrosis, endocarditis, psoriasis, psoriatic arthritis, arthritis, anaphylactic shock, organ ischemia, reperfusion injury, and allograft rejection. In addition, the high affinity antibodies of the present inventions are useful as diagnostic antibodies.
Owner:MEDIMMUNE LLC
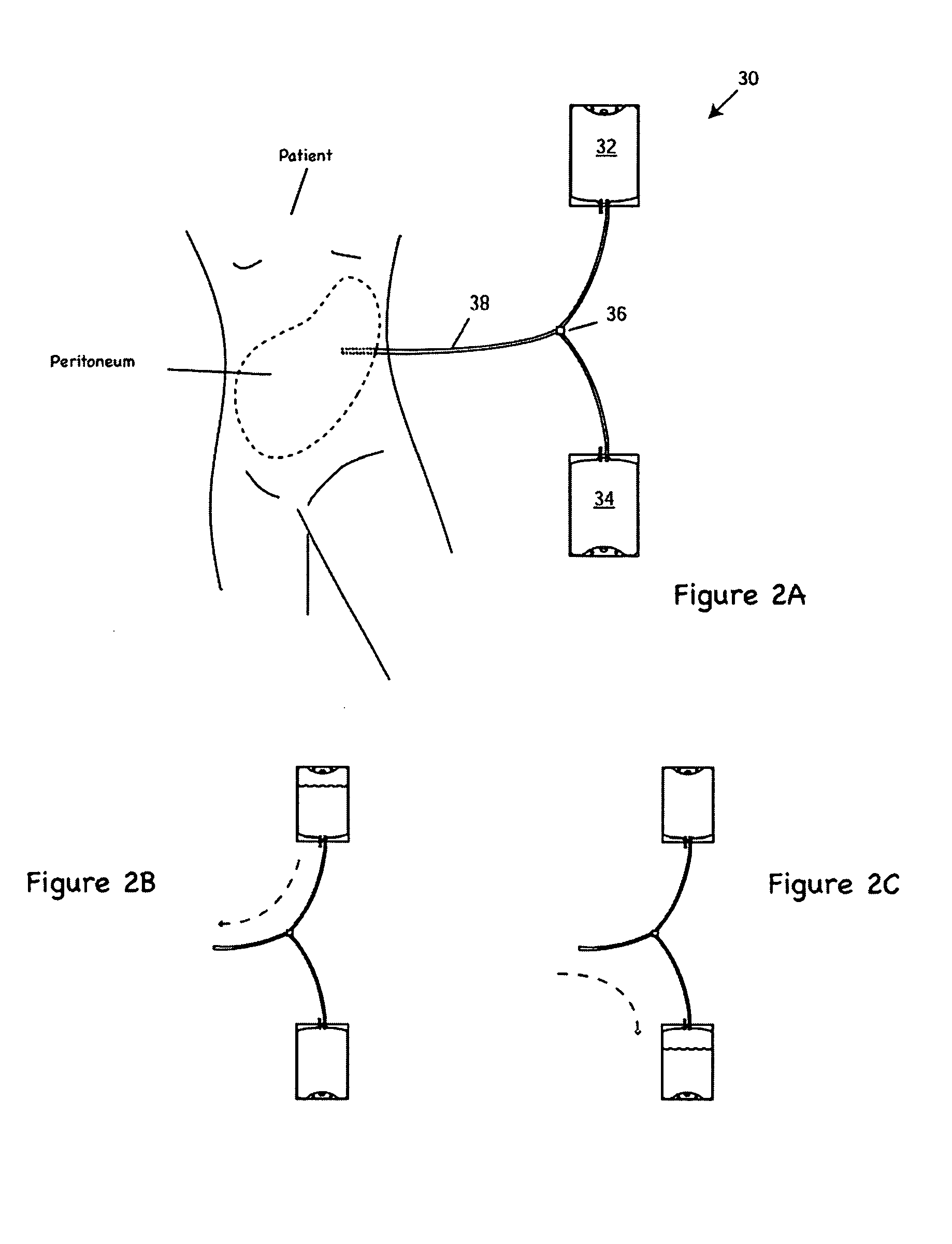
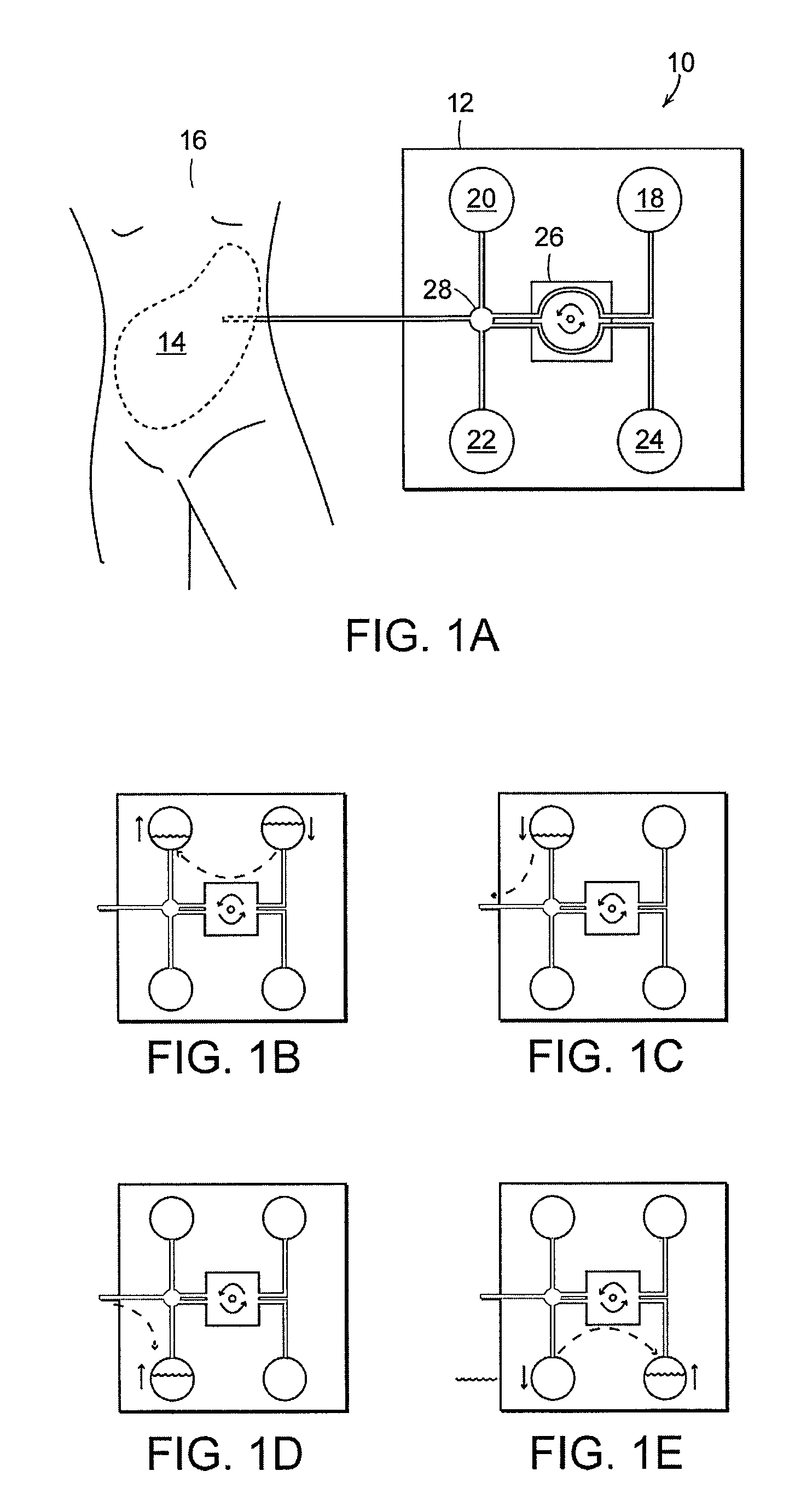
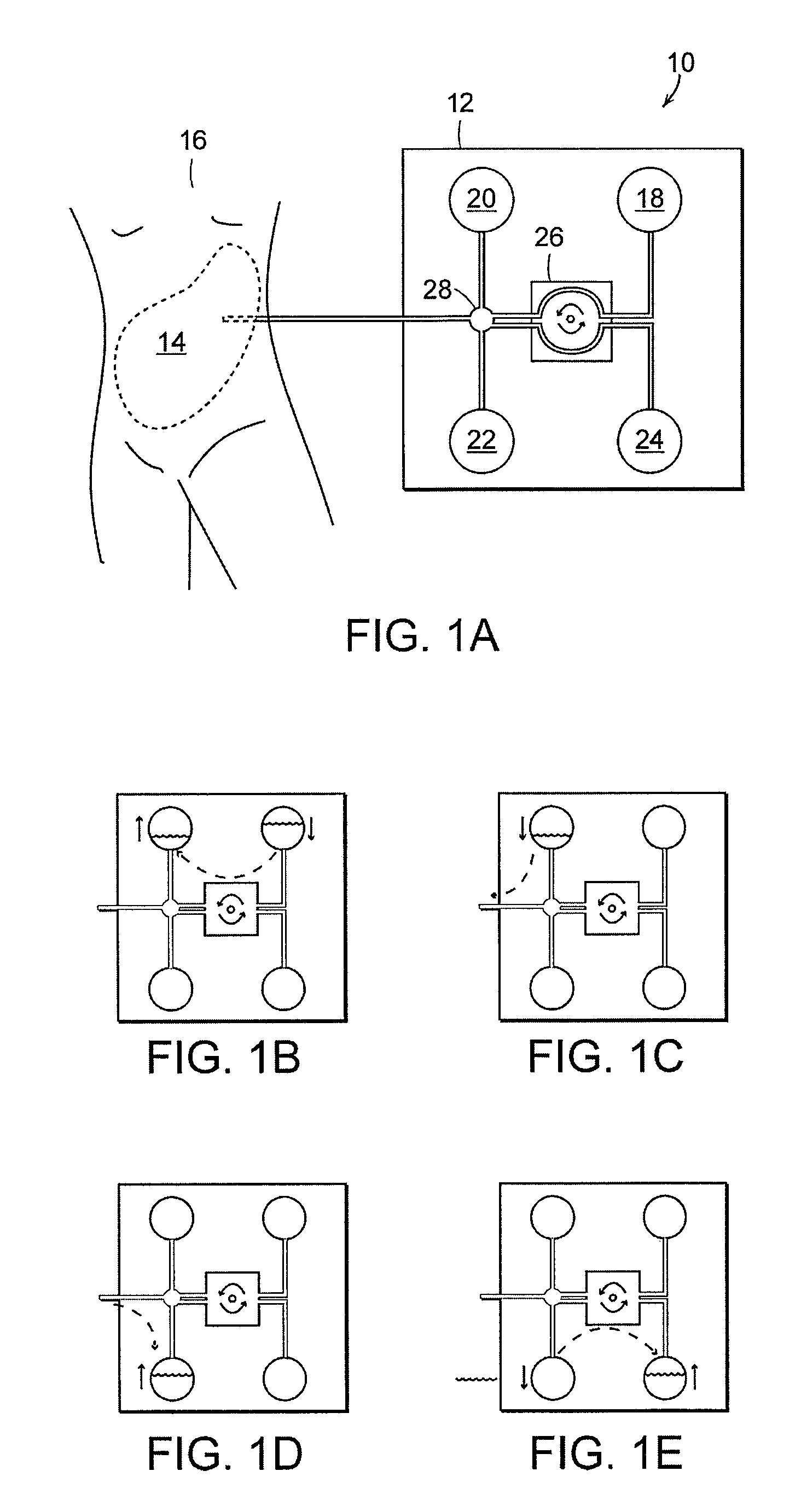
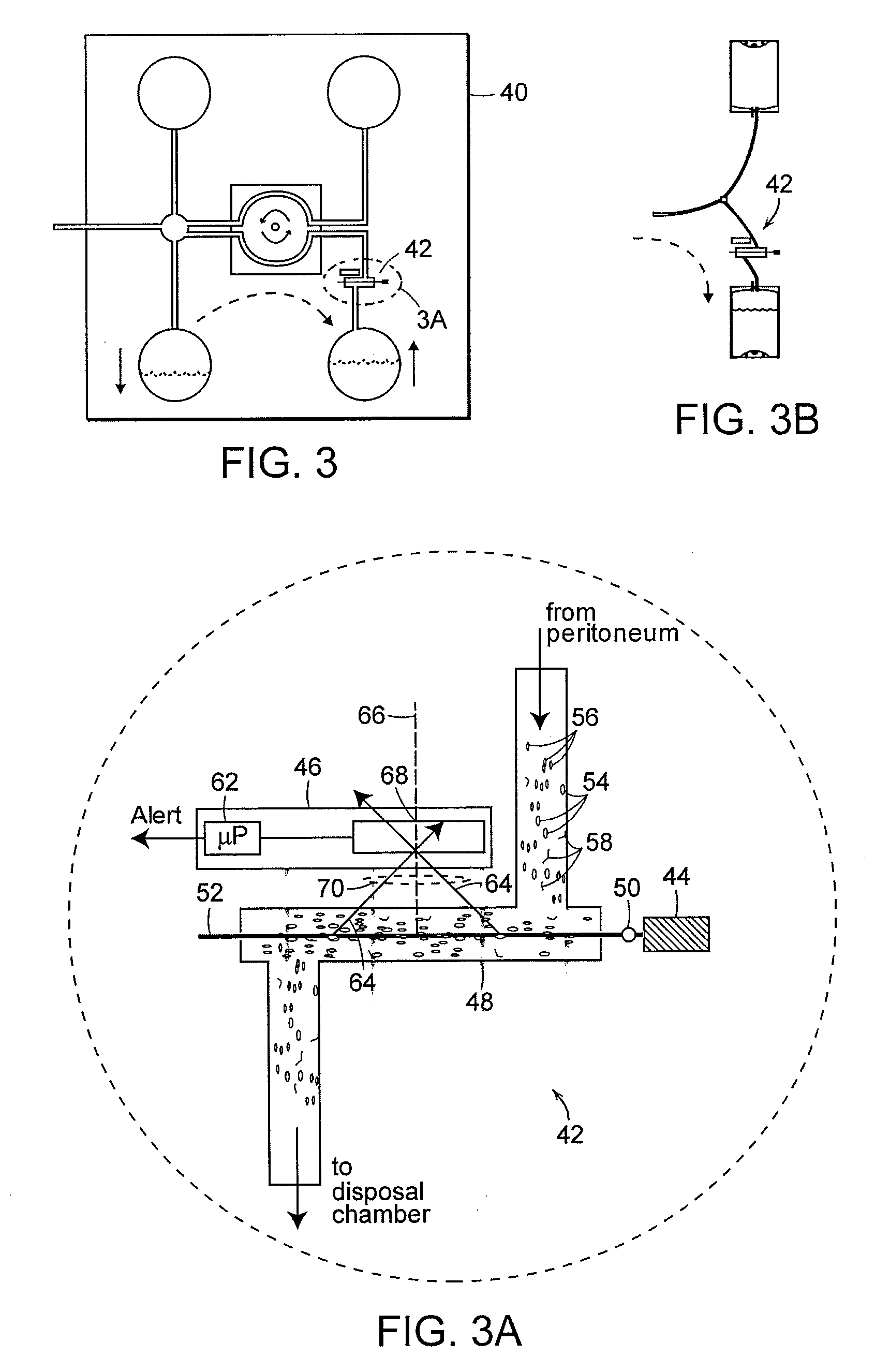
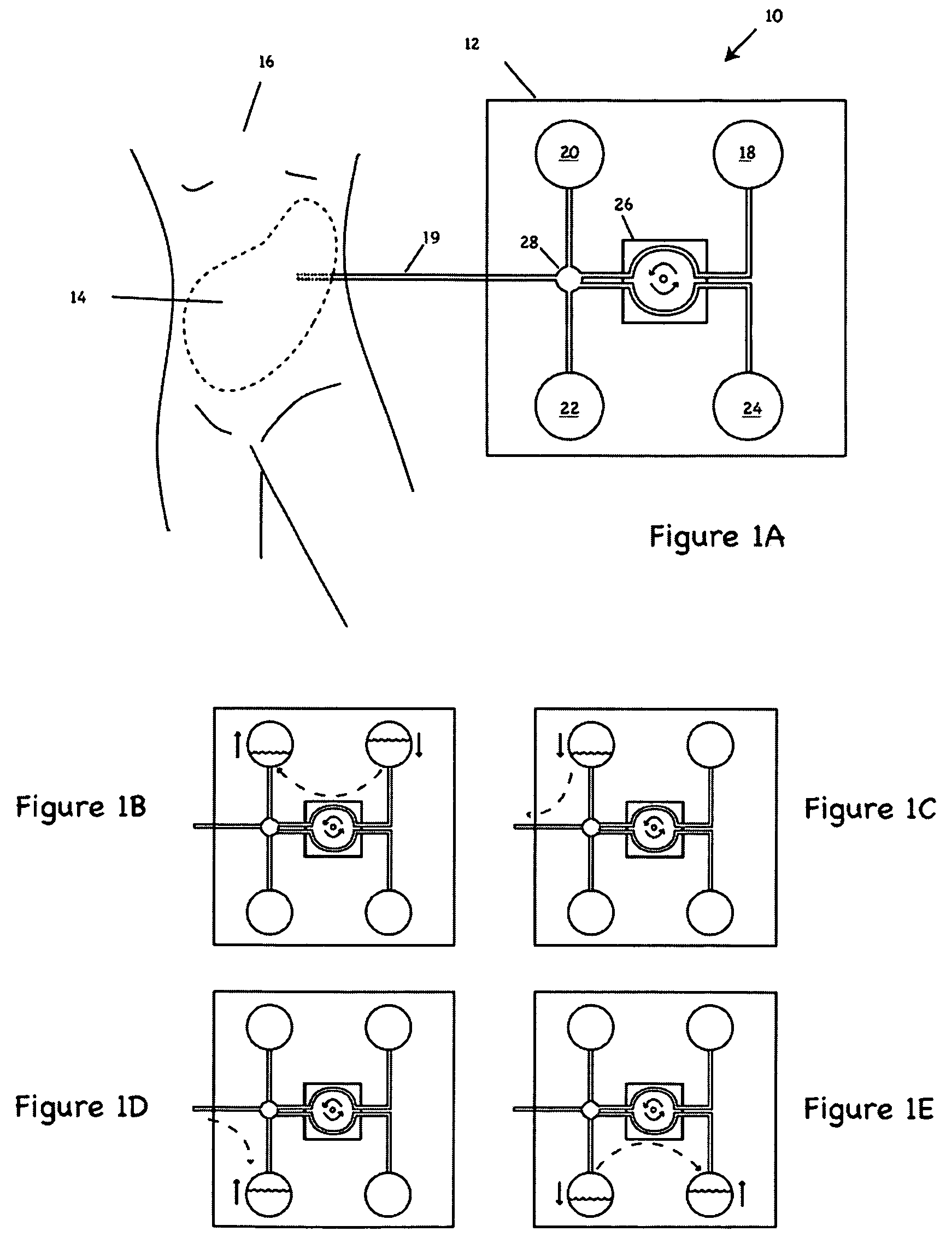
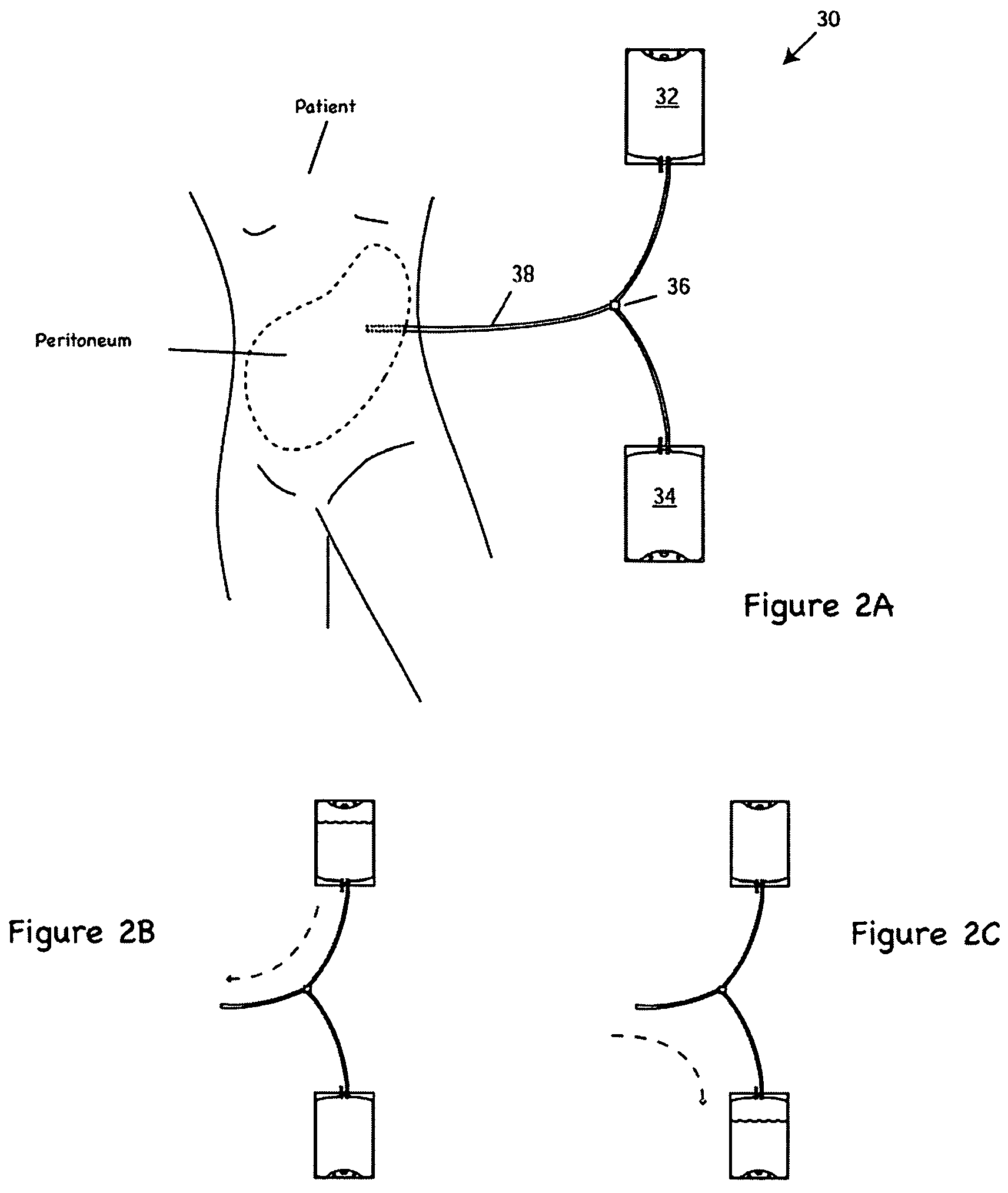
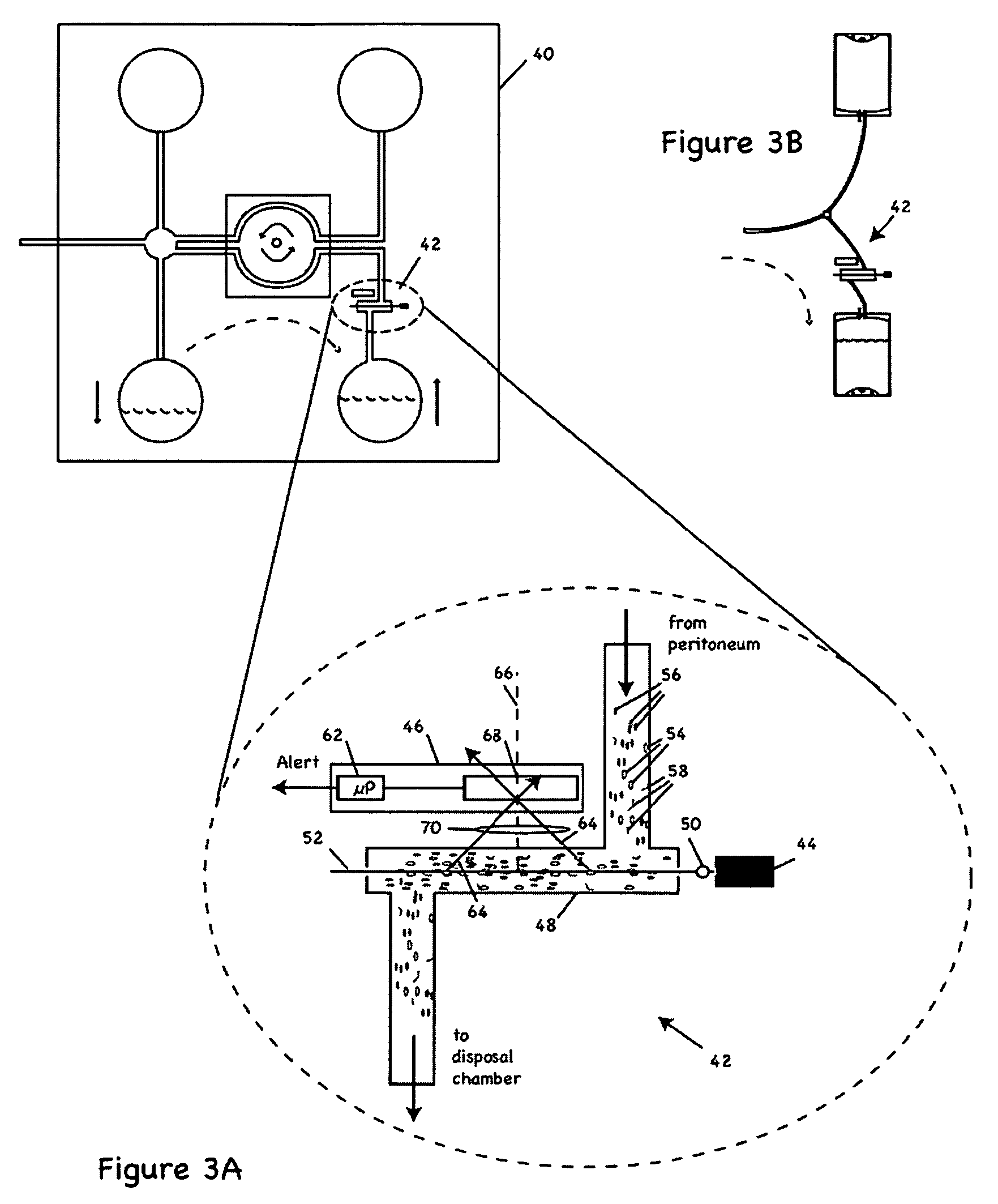
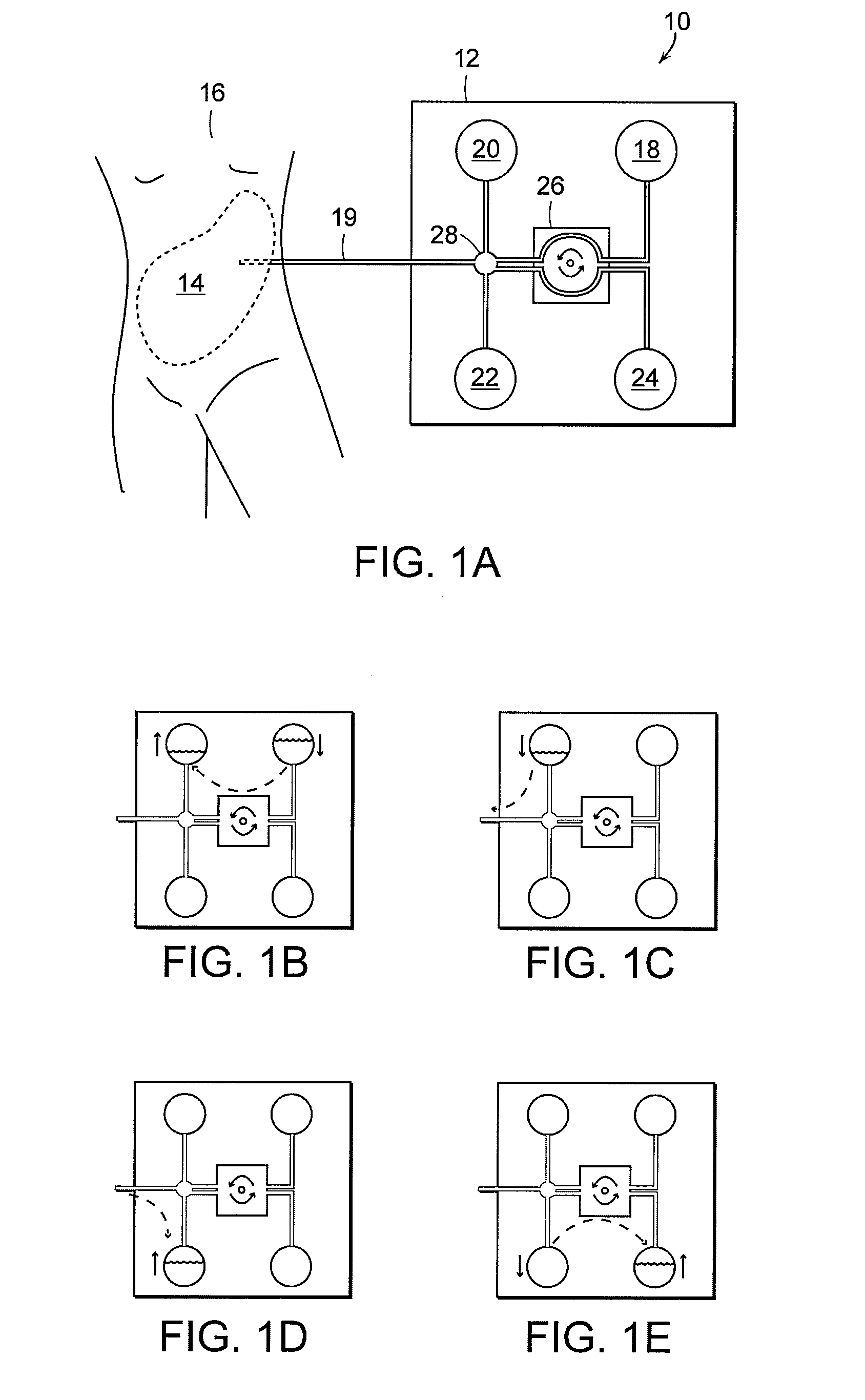
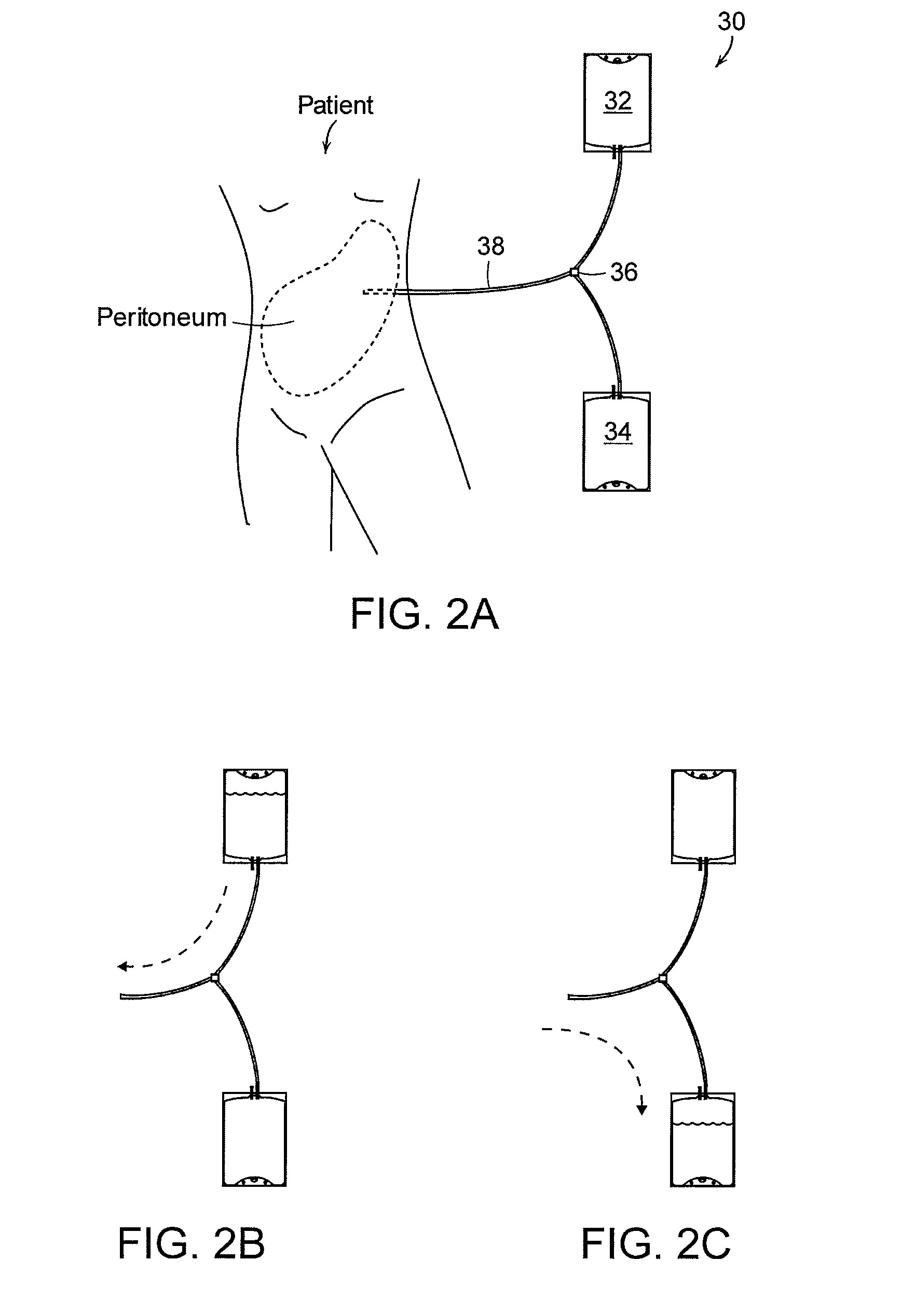
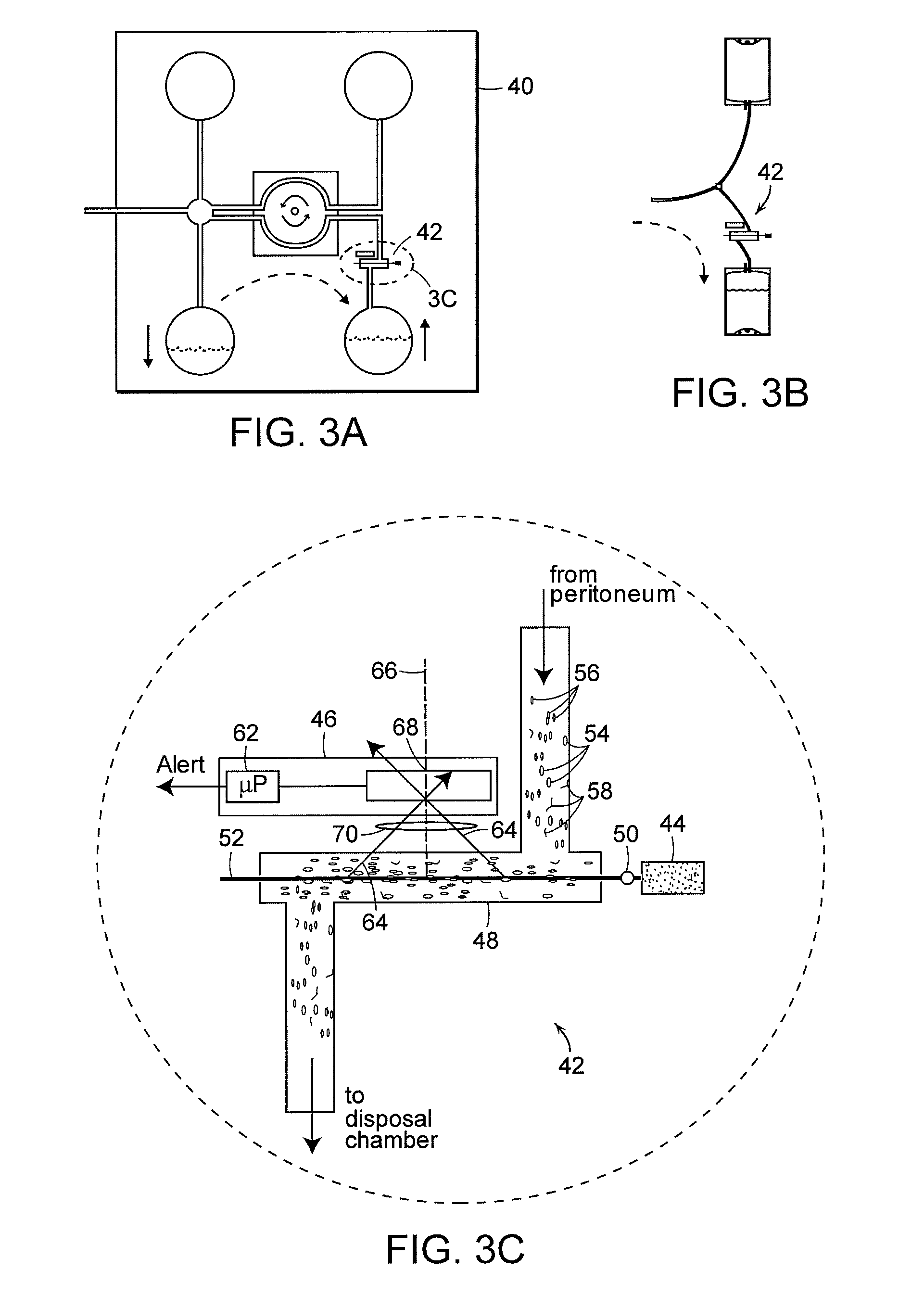
Apparatus and methods for early stage peritonitis detection including self-cleaning effluent chamber
ActiveUS20080183127A1Easy to cleanIncreased turbulenceDiagnostics using lightMedical devicesImage resolutionEngineering
The invention provides, inter alia, automated medical methods and apparatus that test PD effluent in a flow path (e.g., with an APD system or CAPD setup) to detect, for example, the onset of peritonitis, based on optical characteristics of the effluent resolved at cellular scales of distance. For example, according to one aspect of the invention, an APD machine includes, in an effluent flow path, apparatus for early stage peritonitis detection comprising an illumination source and a detector. The source is arranged to illuminate peritoneal effluent in a chamber that forms part of the flow path, and the detector is arranged to detect illuminant scattered by the effluent. The detector detects that reflected or scattered illuminant at a cellular scale of resolution, e.g., on a scale such that separate cellular-sized biological (or other) components in the effluent can be distinguished from one another based on scattering events detected by the detector. According to aspects of the invention, the chamber can utilize a deflector to effect turbulent flow for purposes of cleaning biological and other materials from the chamber walls.
Owner:FRESENIUS MEDICAL CARE HLDG INC
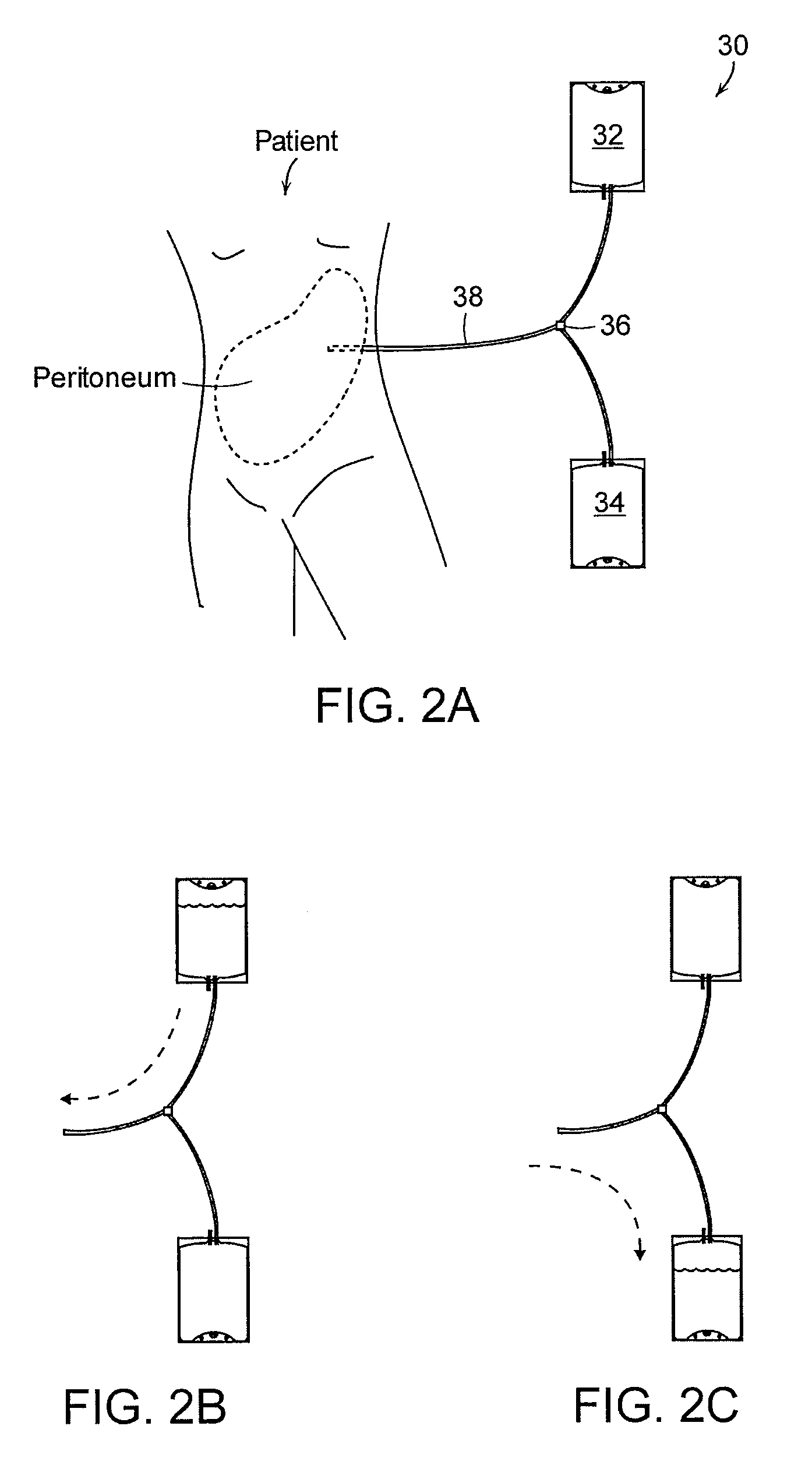
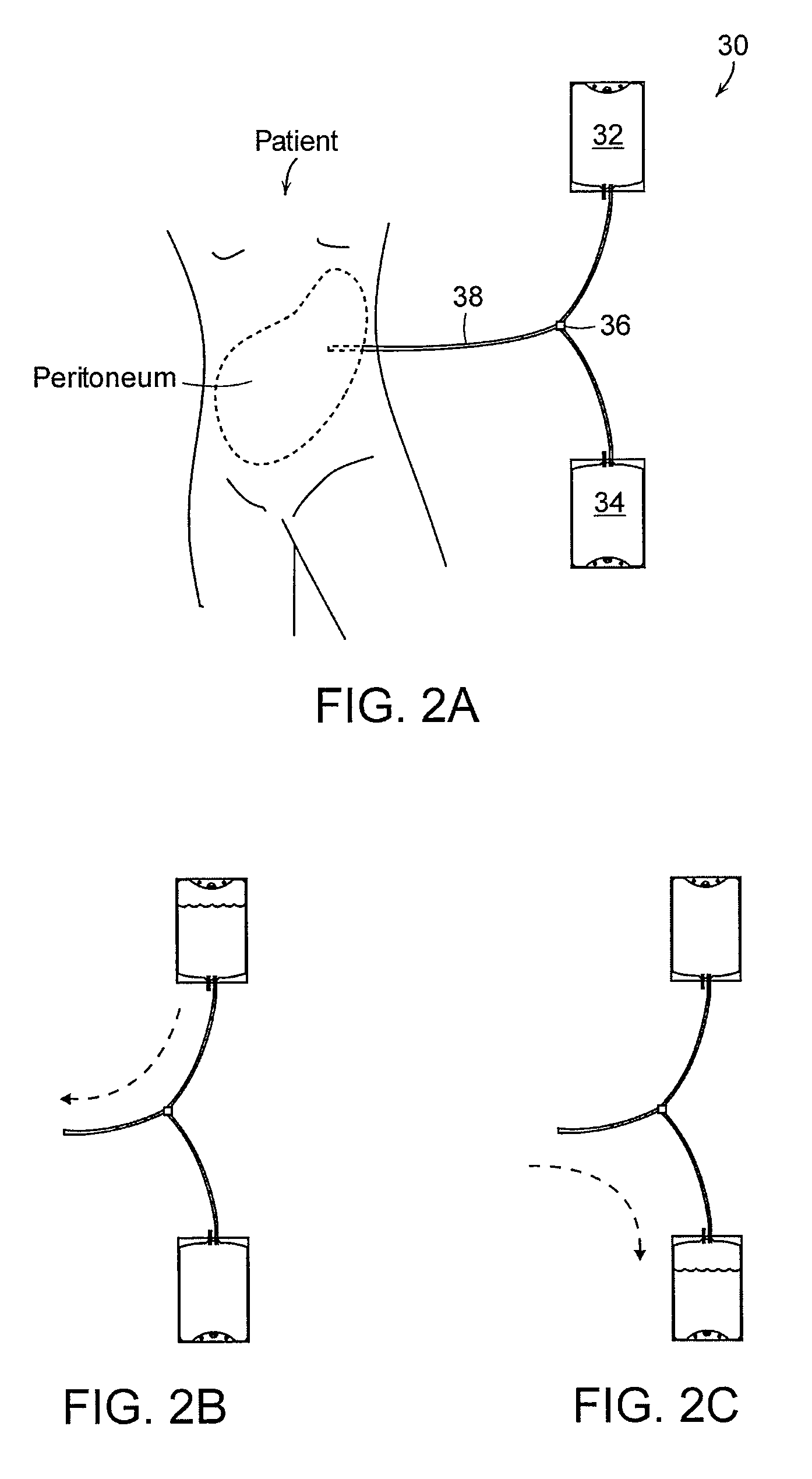
Apparatus and methods for early stage peritonitis detection and for in vivo testing of bodily fluid
InactiveUS20080183126A1Easy to cleanIncreased turbulenceDiagnostics using lightMedical devicesFiberIn vivo
The invention provides, inter alia, automated medical methods and apparatus that test PD effluent in a flow path (e.g., with an APD system or CAPD setup) to detect, for example, the onset of peritonitis, based on optical characteristics of the effluent resolved at cellular scales of distance. For example, according to one aspect of the invention, an APD machine includes, in an effluent flow path, apparatus for early stage peritonitis detection comprising an illumination source and a detector. The source is arranged to illuminate peritoneal effluent in a chamber that forms part of the flow path, and the detector is arranged to detect illuminant scattered by the effluent. The detector detects that reflected or scattered illuminant at a cellular scale of resolution, e.g., on a scale such that separate cellular-sized biological (or other) components in the effluent can be distinguished from one another based on scattering events detected by the detector. Other aspects of the invention provide automated medical testing methods and apparatus that detect the onset of peritonitis and other bodily conditions by testing fluids in the body in vivo, e.g., the patient's peritoneum. Such apparatus and methods utilize a first fiber optic bundle to carry illuminant from a source of the type described above into a bodily organ or cavity, and a second fiber optic bundle to carry illuminant scattered by fluid in that organ or cavity to a detector as described above.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Short peptides useful for treatment of ischemia/reperfusion injury and other tissue damage conditions associated with nitric oxide and its reactive species
ActiveUS20080182797A1Avoid tissue damageLevel of protectionNervous disorderTetrapeptide ingredientsPregnancyAllograft rejection
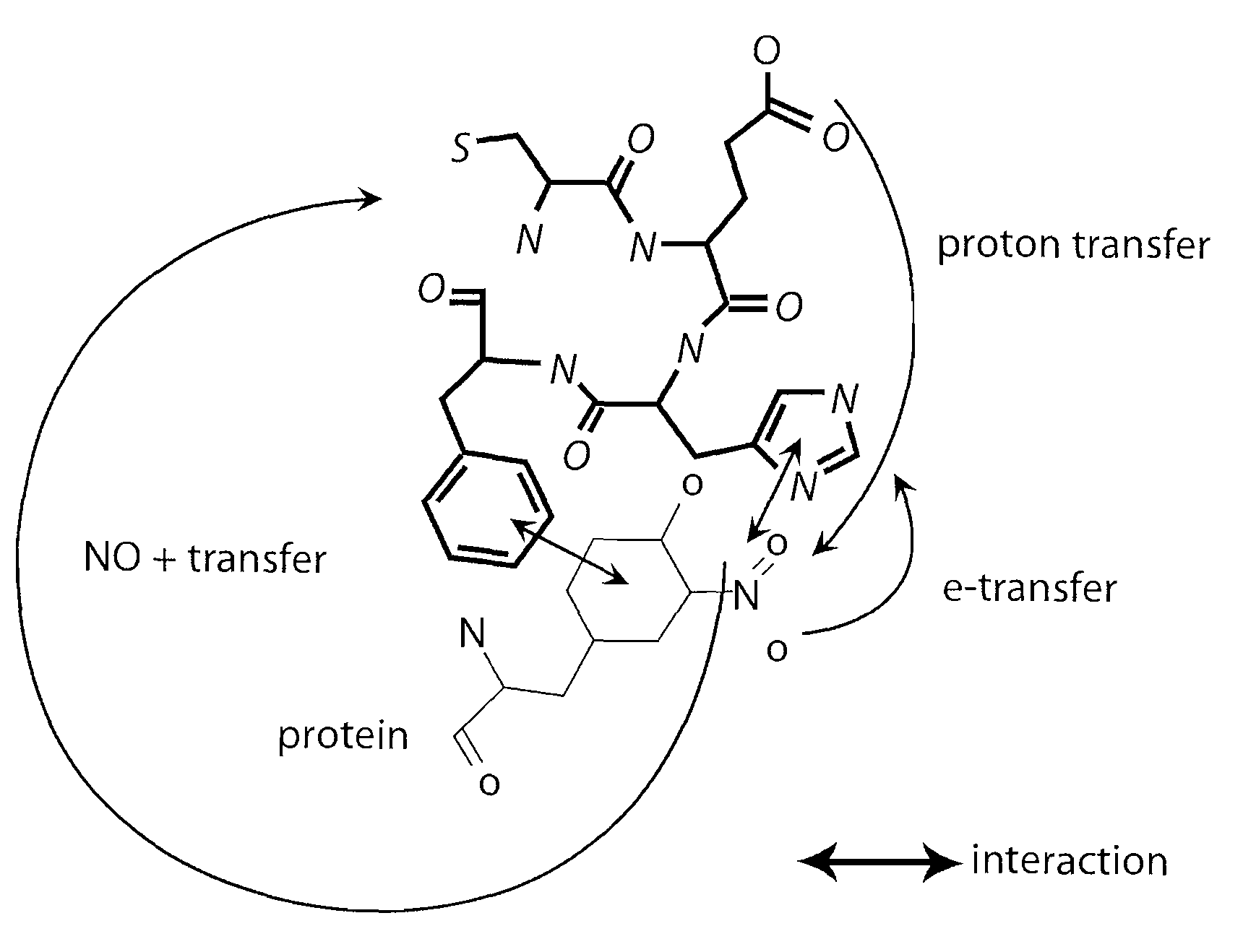
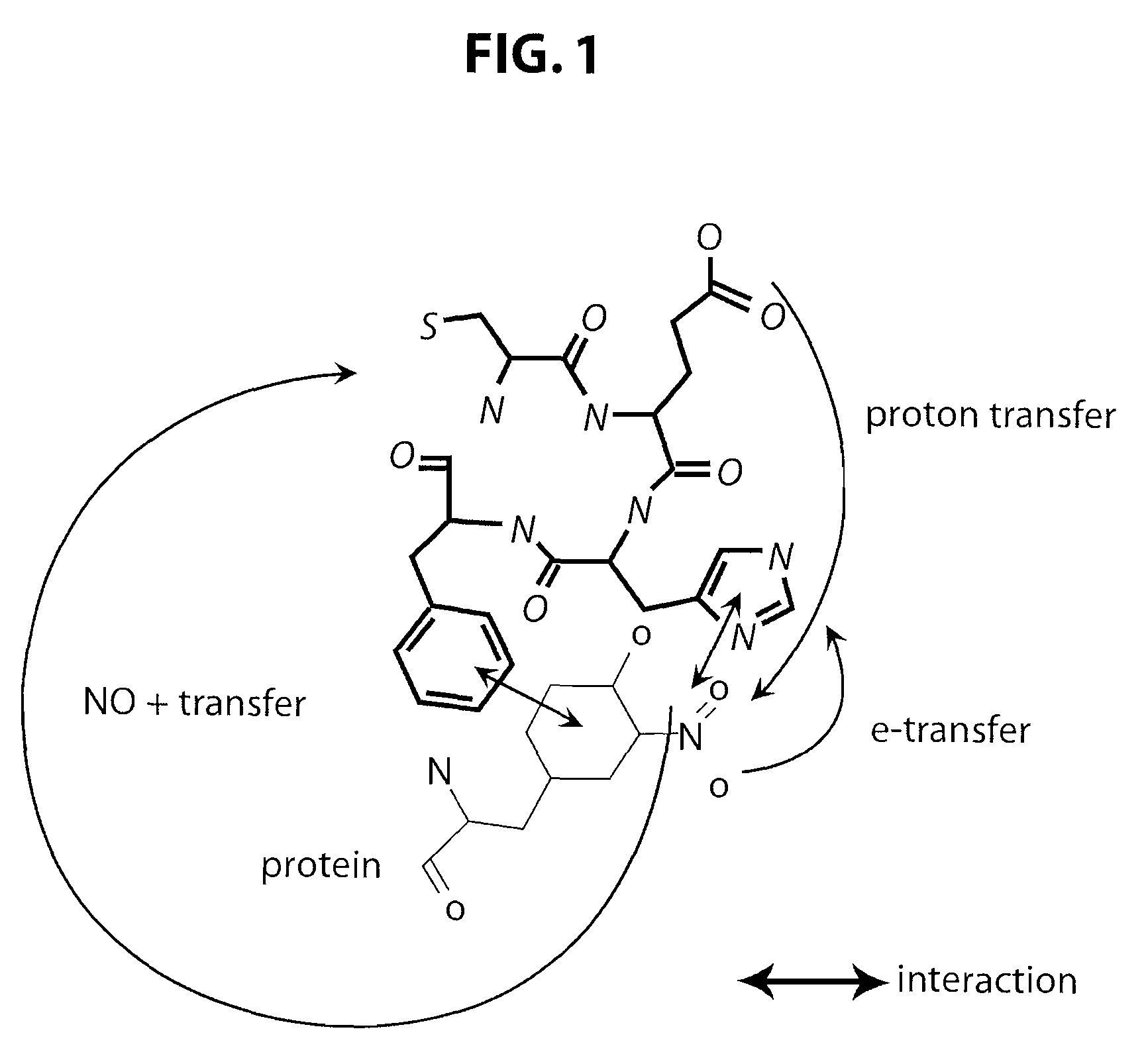
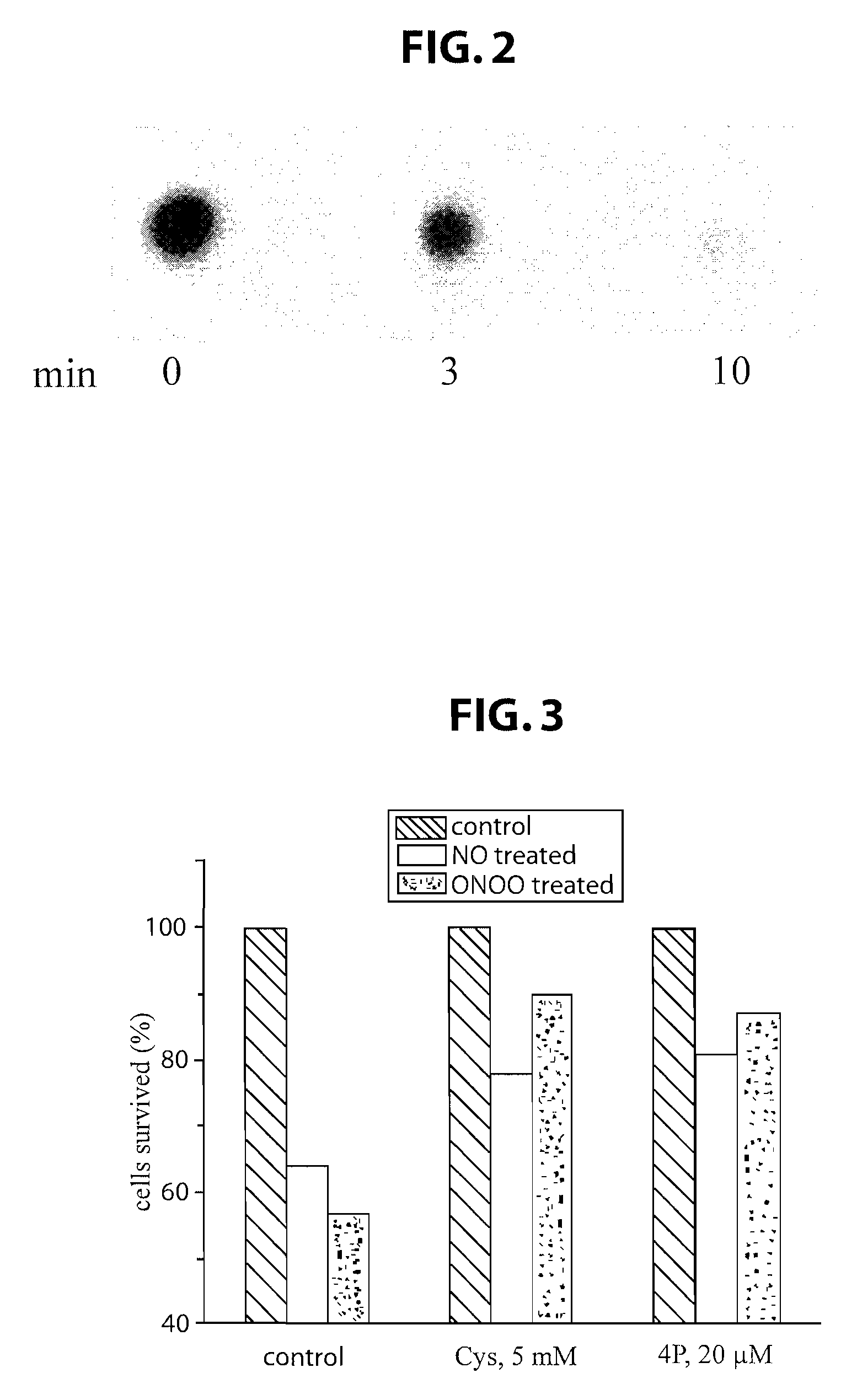
This invention discloses isolated short peptides comprising the amino acid sequence Cys-Glu-Phe-His (CEFH) and analogs thereof as well as compositions comprising CEFH peptides and analogs thereof. The CEFH peptides disclosed herein are effective in mediating the denitration of 3-nitrotyrosines (3-NT) in cellular proteins thereby preventing tissue damage associated with excess nitric oxide (NO) and its reactive species. The CEFH peptides disclosed herein are useful in the treatment of ischemia / reperfusion (I / R) injury of various tissues (e.g., I / R injury of heart muscle associated with heart attack or cardiac surgery, I / R injury of brain tissue associated with stroke, I / R injury of liver tissue, skeletal muscles, etc.), septic shock, anaphylactic shock, neurodegenerative diseases (e.g., Alzheimer's and Parkinson's diseases), neuronal injury, atherosclerosis, diabetes, multiple sclerosis, autoimmune uveitis, pulmonary fibrosis, oobliterative bronchiolitis, bronchopulmonary dysplasia (BPD), amyotrophic lateral sclerosis (ALS), sepsis, inflammatory bowel disease, arthritis, allograft rejection, autoimmune myocarditis, myocardial inflammation, pulmonary granulomatous inflammation, influenza- or HSV-induced pneumonia, chronic cerebral vasospasm, allergic encephalomyelitis, central nervous system (CNS) inflammation, Heliobacterium pylori gastritis, necrotizing entrerocolitis, celliac disease, peritonitis, early prosthesis failure, inclusion body myositis, preeclamptic pregnancies, skin lesions with anaphylactoid purpura, nephrosclerosis, ileitis, leishmaniasis, cancer, and related disorders.
Owner:NEW YORK UNIVERSITY
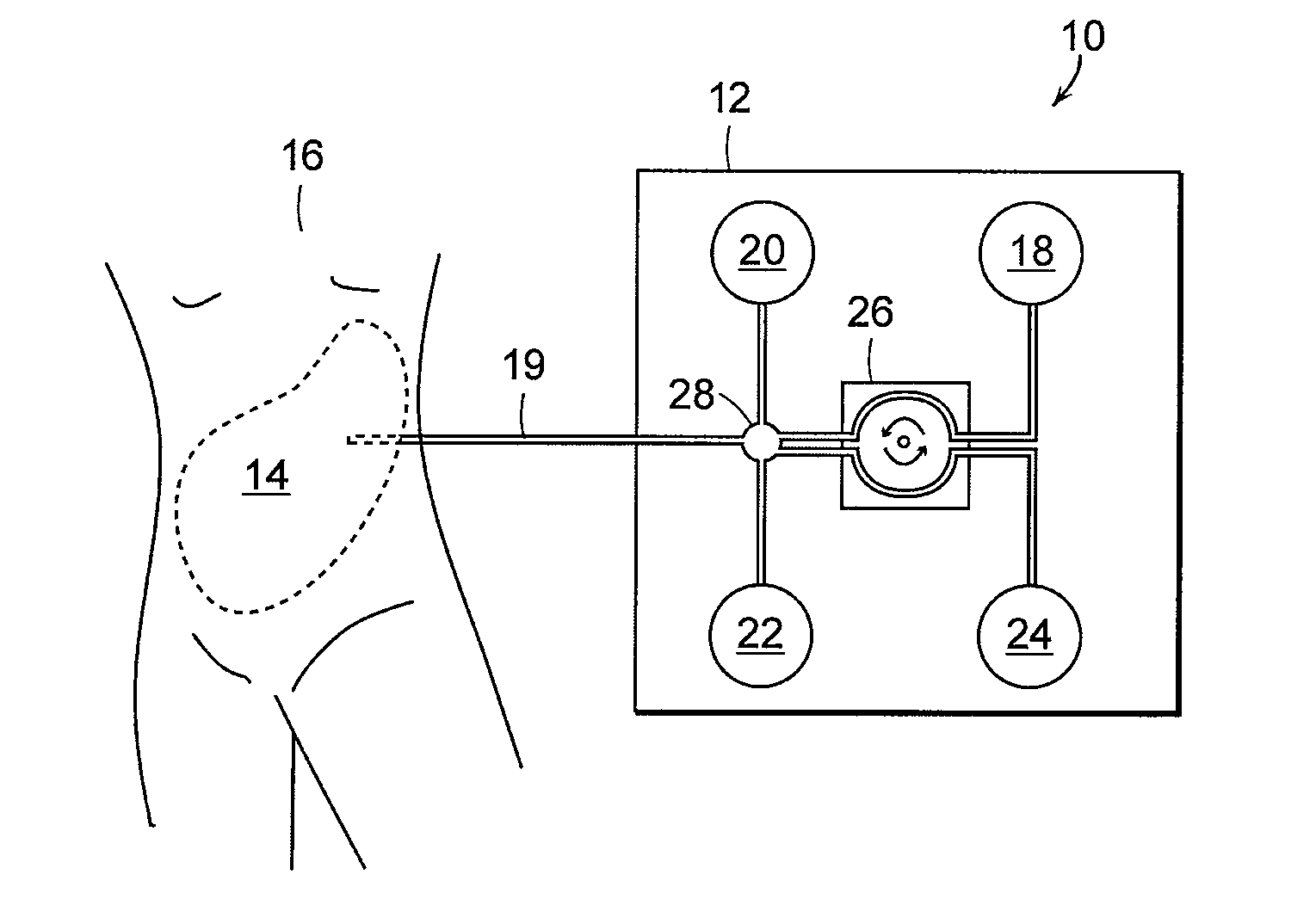
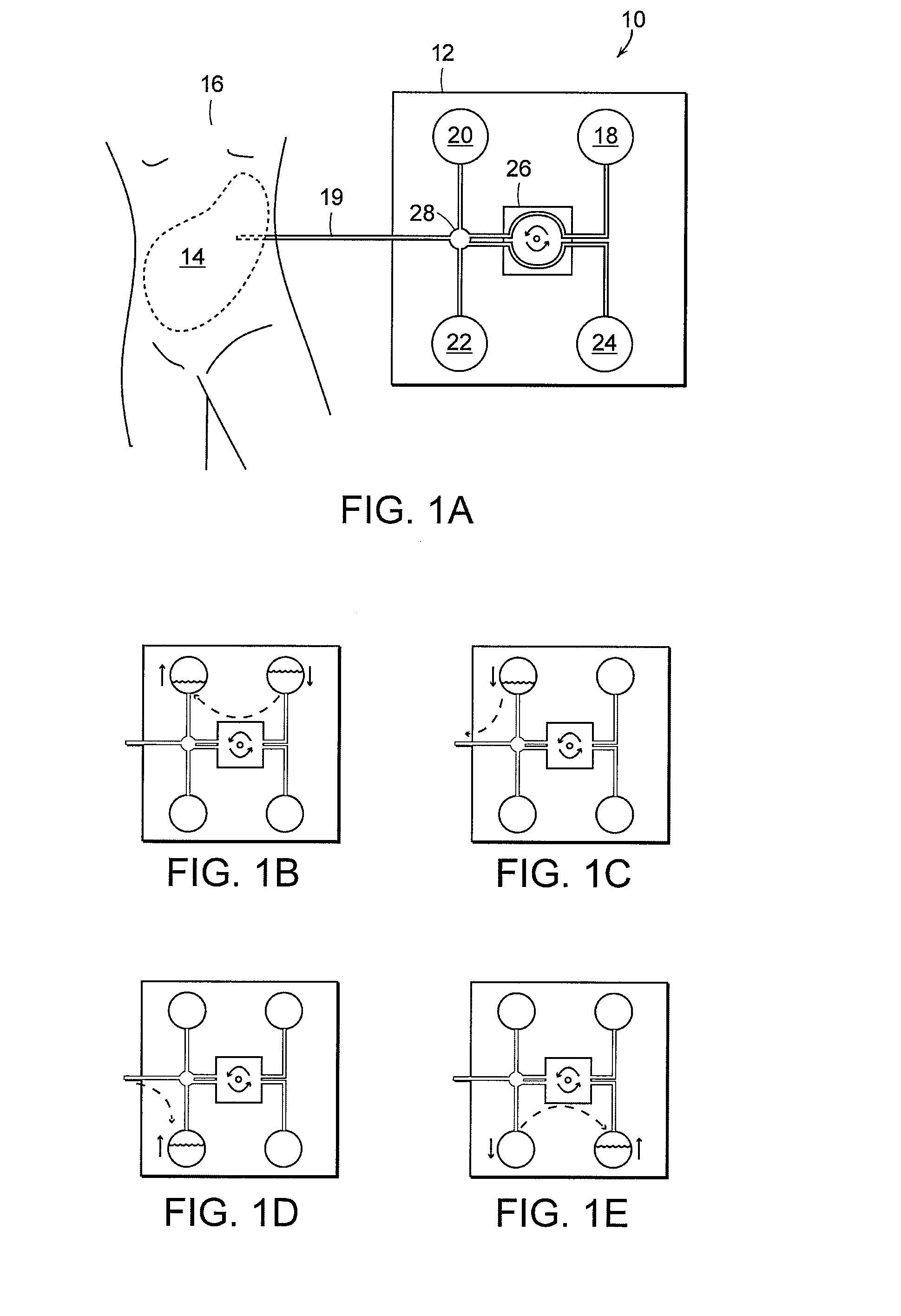
Early stage peritonitis detection apparatus and methods
The invention provides, inter alia, automated medical methods and apparatus that test PD effluent in a flow path (e.g., with an APD system or CAPD setup) to detect, for example, the onset of peritonitis, based on optical characteristics of the effluent resolved at cellular scales of distance. For example, according to one aspect of the invention, an APD machine includes, in an effluent flow path, apparatus for early stage peritonitis detection comprising an illumination source and a detector. The source is arranged to illuminate peritoneal effluent in a chamber that forms part of the flow path, and the detector is arranged to detect illuminant scattered by the effluent. The detector detects that reflected or scattered illuminant at a cellular scale of resolution, e.g., on a scale such that separate cellular-sized biological (or other) components in the effluent can be distinguished from one another based on scattering events detected by the detector.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Lauroyl arginine ethyl ester derivative and application thereof as animal antibacterial agent
ActiveCN108976151AGood antibacterial effectDelay drug resistanceAntibacterial agentsOrganic active ingredientsEscherichia coliSide effect
The invention relates to a novel veterinary antibacterial agent and in particular relates to a lauroyl arginine ethyl ester ion pair compound derivative which is prepared from lauroyl arginine ethyl ester LAE and an organic acid through reactions. As a livestock aquatic animal antibacterial agent, the derivative is capable of preventing and treating duckling riemerella anatipestifer diseases caused by riemerella anatipestifer, is capable of treating peritonitis diseases caused by escherichia coli and staphylococcus aureus, is capable of preventing and treating fish septicemia caused by aeromonas sobria, and has remarkably improved prevention and treatment effects when being compared with a conventional LAE veterinary antibacterial agent. The derivative can be completely degraded in human bodies and animal bodies and is small in toxic and side effect, environment drug resistance bacteria caused by residual antibacterial agents discharged into the environment can be avoided, and small adverse environment influence can be caused while an effective antibacterial effect is achieved.
Owner:EAST CHINA NORMAL UNIV
Phage therapy against Pseudomonas aeruginosa
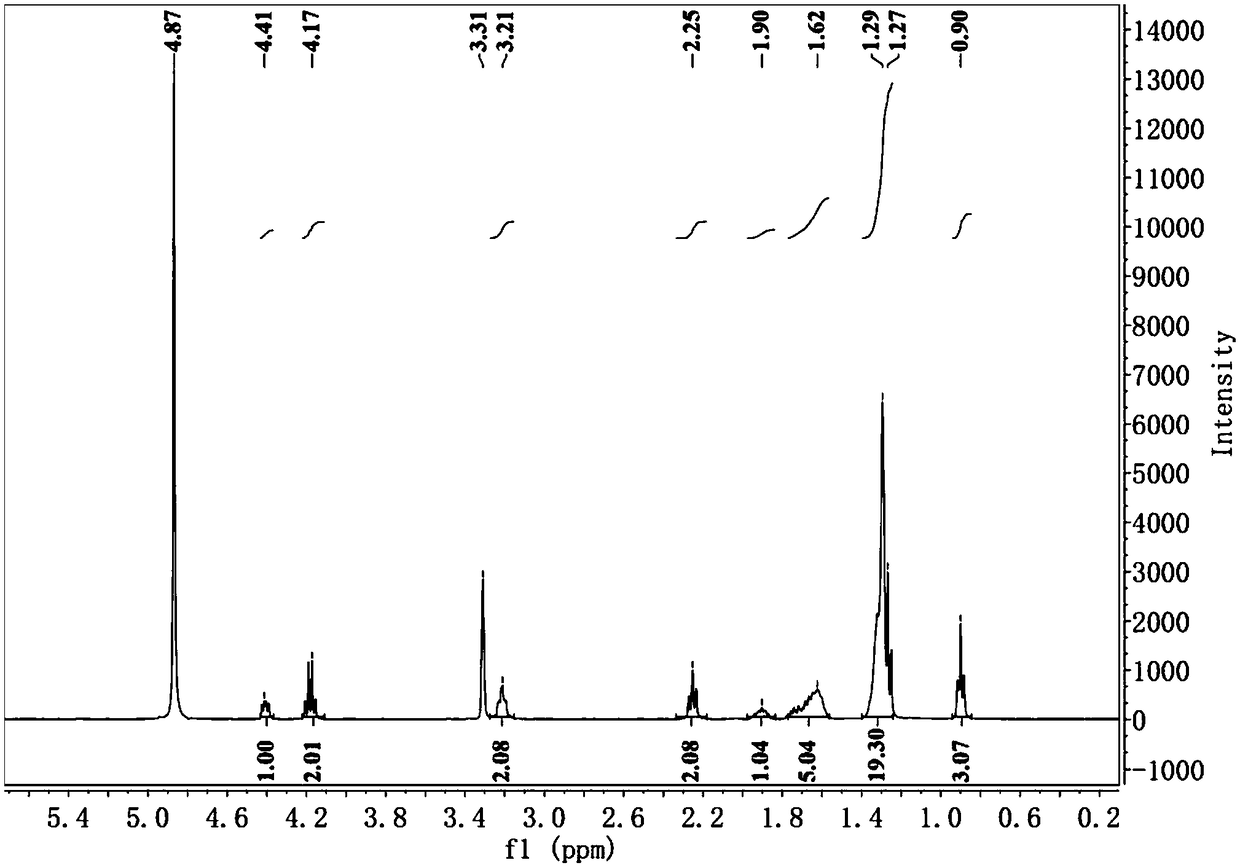
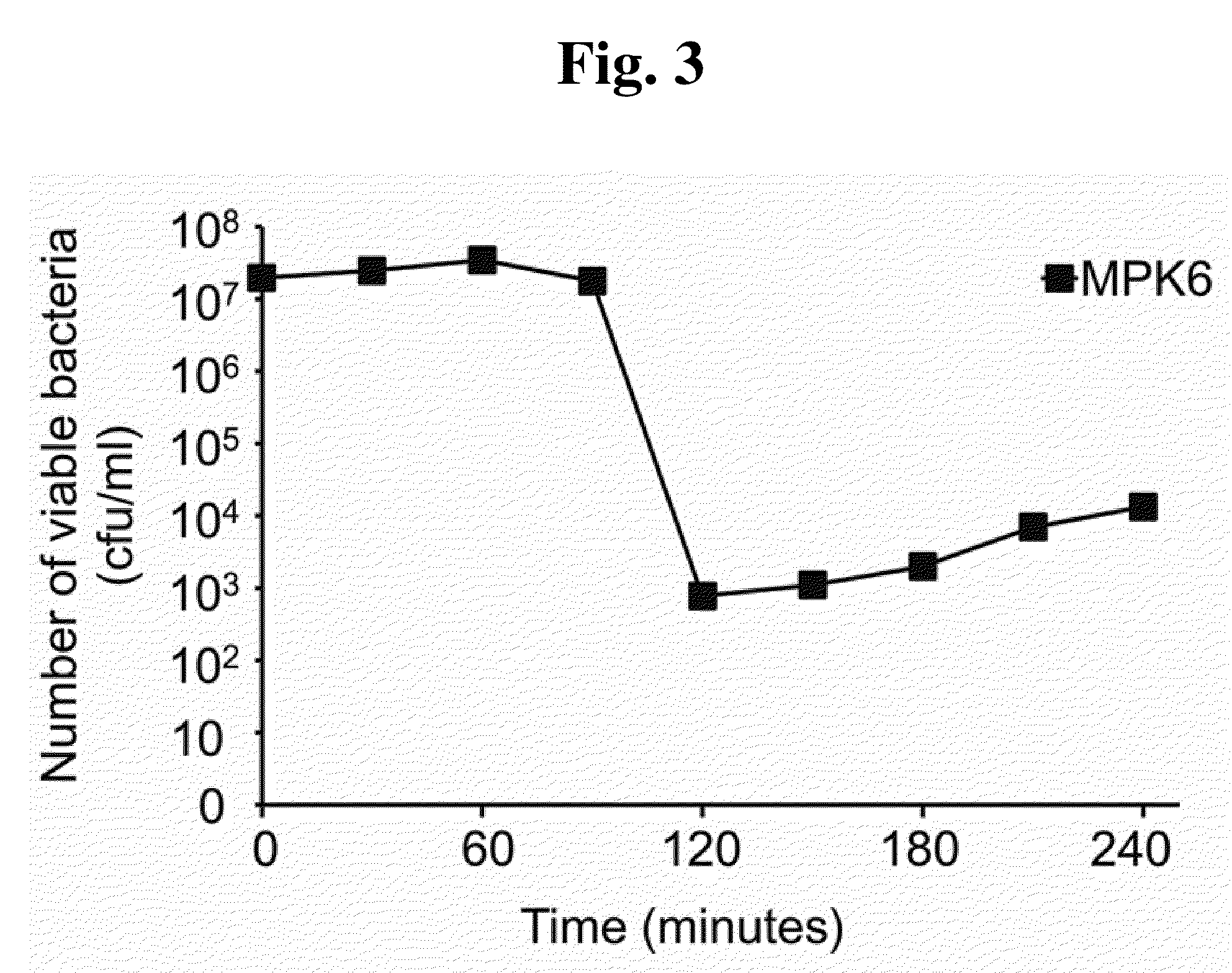
This invention relates to a bacteriophage MPK6 (deposit number: KCCM 11044P) having a lytic activity to Pseudomonas aeruginosa, or a progeny bacteriophage thereof having a RFLP (Restriction fragment length polymorphism) DNA profile substantially equivalent to the bacteriophage MPK6. The present invention provides a bacteriophage MPK6 or a progeny bacteriophage thereof capable of treating a Pseudomonas aeruginosa infection disease, and suggests an anti-bacterial activity of MPK6 and its progeny bacteriophage using a mammalian and non-mammalian infection model. According to the present invention, the present bacteriophage MPK6 or progeny bacteriophage thereof represents very effective efficacy on treatment of P. aeruginosa-induced peritonitis-sepsis.
Owner:IND UNIV COOP FOUND SOGANG UNIV
Early stage peritonitis detection apparatus and methods
Owner:FRESENIUS MEDICAL CARE HLDG INC
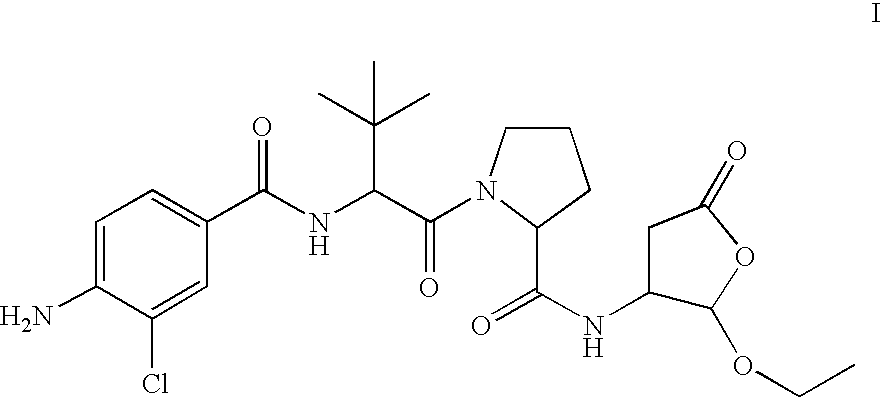
Prodrug of an ice inhibitor
This invention describes an ICE inhibitor prodrug (I) having good bioavailabilityCompound I is useful for treating IL-1 mediated diseases such as rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, ulcerative colitis, inflammatory peritonitis, septic shock, pancreatitis, traumatic brain injury, organ transplant rejection, osteoarthritis, asthma, psoriasis, Alzheimer's disease, myocardial infarction, congestive heart failure, Huntington's disease, atherosclerosis, atopic dermatitis, leukemias and related disorders, myelodysplastic syndrome, uveitis or multiple myeloma.
Owner:VERTEX PHARMA INC
Nucleoside compound and application thereof in treatment of feline infectious peritonitis
The invention relates to a nucleoside compound and application of the nucleoside compound in treatment of feline infectious peritonitis. The compound belongs to a GS-441524 analogue. The compound has a very small toxic effect on normal cells of cats, and has an obvious inhibition effect on feline coronavirus. The compound can be used for preparing a feline coronavirus inhibitor, is used for treating feline infectious peritonitis, has the advantages of good curative effect, high safety and the like, and is very good in water solubility and easier to absorb.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
High affinity antibodies against HMGB1 and methods of use thereof
InactiveUS7585504B2Reduce bone loss and/or cartilage damageAntibacterial agentsAntibody mimetics/scaffoldsReperfusion injuryAllograft rejection
Compositions and methods are disclosed for inhibiting the release of a proinflammatory cytokine from a vertebrate cell, and for inhibiting an inflammatory cytokine cascade in a patient. The compositions comprise, for example, high affinity antibodies that specifically bind HMG1 and antigenic fragments thereof. The high affinity antibodies of the present invention and pharmaceutical compositions comprising the same are useful for many purposes, for example, as therapeutics against a wide range of inflammatory diseases and disorders such as sepsis, rheumatoid arthritis, peritonitis, Crohn's disease, reperfusion injury, septicemia, endotoxic shock, cystic fibrosis, endocarditis, psoriasis, psoriatic arthritis, arthritis, anaphylactic shock, organ ischemia, reperfusion injury, and allograft rejection. In addition, the high affinity antibodies of the present inventions are useful as diagnostic antibodies.
Owner:MEDIMMUNE LLC
High Affinity Antibodies Against HMGB1 and Methods Of Use Thereof
InactiveUS20100061987A1Reduce bone loss and/or cartilage damageAntibacterial agentsAntipyreticAntiendomysial antibodiesAllograft rejection
Owner:MEDIMMUNE LLC
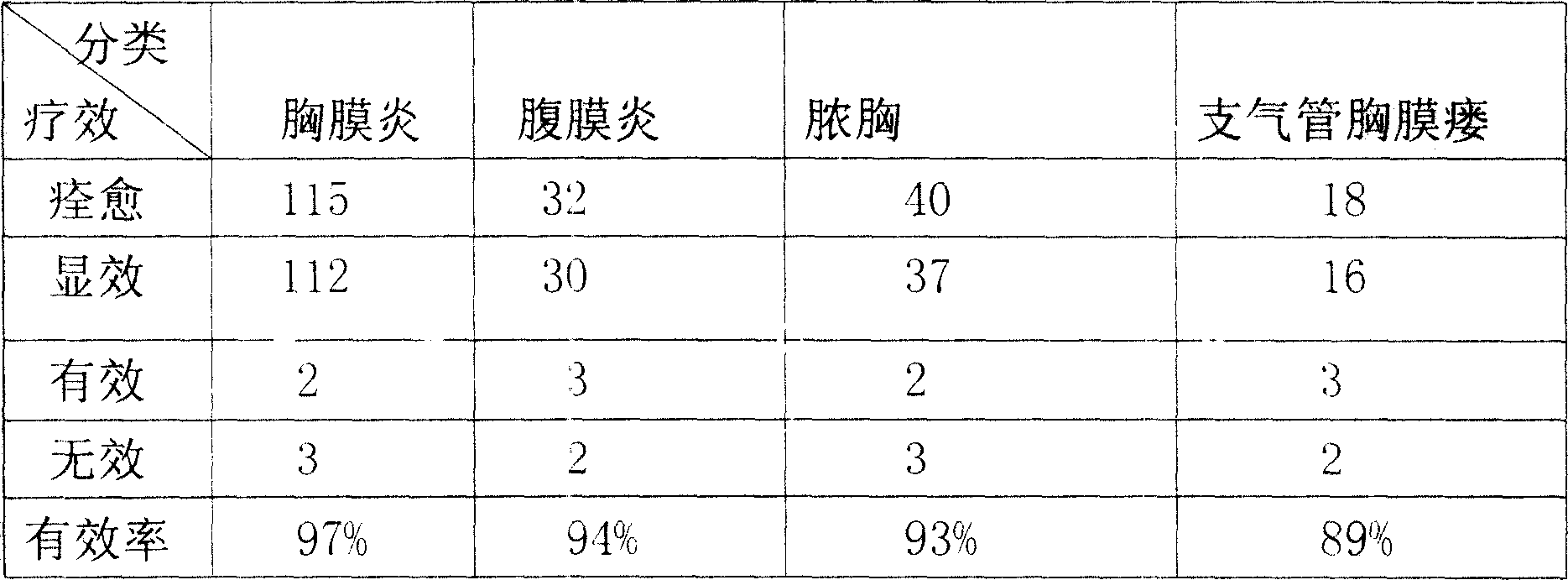
Chest-clearing tuberculosis-dispelling pill for treating tuberculous pleurisy, peritonitis, empyema and bronchopleural fistula
ActiveCN101129904AGood treatment effectRaw materials, a wide range of drug sourcesAntibacterial agentsPlant ingredientsMedicinal herbsBronchopleural fistula
The invention discloses a Chinese medicinal pill preparation for treating tuberculosis pleuritis, peritonitis, empyema and broncho-pleural fistula, which is prepared from 26 kinds of Chinese medicinal herbs including notoginseng, lily bulb, Sichuan fritillary bulb, lepidium seed, selfheal and weeping forsythia through steps of disintegrating, sieving, mixing homogenously, making water pills, and drying at low temperature.
Owner:张立梅
Apparatus and methods for early stage peritonitis detection including self-cleaning effluent chamber
ActiveUS8728023B2Easy to cleanIncreased turbulenceSemi-permeable membranesElectrotherapyImage resolutionEngineering
The invention provides, inter alia, automated medical methods and apparatus that test PD effluent in a flow path (e.g., with an APD system or CAPD setup) to detect, for example, the onset of peritonitis, based on optical characteristics of the effluent resolved at cellular scales of distance. For example, according to one aspect of the invention, an APD machine includes, in an effluent flow path, apparatus for early stage peritonitis detection comprising an illumination source and a detector. The source is arranged to illuminate peritoneal effluent in a chamber that forms part of the flow path, and the detector is arranged to detect illuminant scattered by the effluent. The detector detects that reflected or scattered illuminant at a cellular scale of resolution, e.g., on a scale such that separate cellular-sized biological (or other) components in the effluent can be distinguished from one another based on scattering events detected by the detector. According to aspects of the invention, the chamber can utilize a deflector to effect turbulent flow for purposes of cleaning biological and other materials from the chamber walls.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Apparatus and methods for early stage peritonitis detection and for in vivo testing of bodily fluid
InactiveUS8777891B2Easy to cleanIncreased turbulenceSemi-permeable membranesElectrotherapyFiberIn vivo
The invention provides, inter alia, automated medical methods and apparatus that test PD effluent in a flow path (e.g., with an APD system or CAPD setup) to detect, for example, the onset of peritonitis, based on optical characteristics of the effluent resolved at cellular scales of distance. For example, according to one aspect of the invention, an APD machine includes, in an effluent flow path, apparatus for early stage peritonitis detection comprising an illumination source and a detector. The source is arranged to illuminate peritoneal effluent in a chamber that forms part of the flow path, and the detector is arranged to detect illuminant scattered by the effluent. The detector detects that reflected or scattered illuminant at a cellular scale of resolution, e.g., on a scale such that separate cellular-sized biological (or other) components in the effluent can be distinguished from one another based on scattering events detected by the detector. Other aspects of the invention provide automated medical testing methods and apparatus that detect the onset of peritonitis and other bodily conditions by testing fluids in the body in vivo, e.g., the patient's peritoneum. Such apparatus and methods utilize a first fiber optic bundle to carry illuminant from a source of the type described above into a bodily organ or cavity, and a second fiber optic bundle to carry illuminant scattered by fluid in that organ or cavity to a detector as described above.
Owner:FRESENIUS MEDICAL CARE HLDG INC
High affinity antibodies against hmgb1 and methods of use thereof
InactiveUS20090169546A1Useful in treatmentReduce hyperostosisAntipyreticMicrobiological testing/measurementReperfusion injurySpinal cord
Compositions and methods are disclosed for inhibiting the release of a proinflammatory cytokine from a vertebrate cell, and for inhibiting an inflammatory cytokine cascade in a patient. The compositions comprise, for example, high affinity antibodies that specifically bind HMG1 and antigenic fragments thereof. The high affinity antibodies of the present invention and pharmaceutical compositions comprising the same are useful for many purposes, for example, as therapeutics against a wide range of inflammatory diseases and disorders such as sepsis, rheumatoid arthritis, peritonitis, Crohn s disease, reperfusion injury, septicemia, endotoxic shock, cystic fibrosis, endocarditis, psoriasis, psoriatic arthritis, arthritis, anaphylactic shock, organ ischemia, reperfusion injury, and allograft rejection. In addition, the high affinity antibodies of the present inventions are useful as diagnostic antibodies.
Owner:CORNERSTONE THERAPEUTICS +1
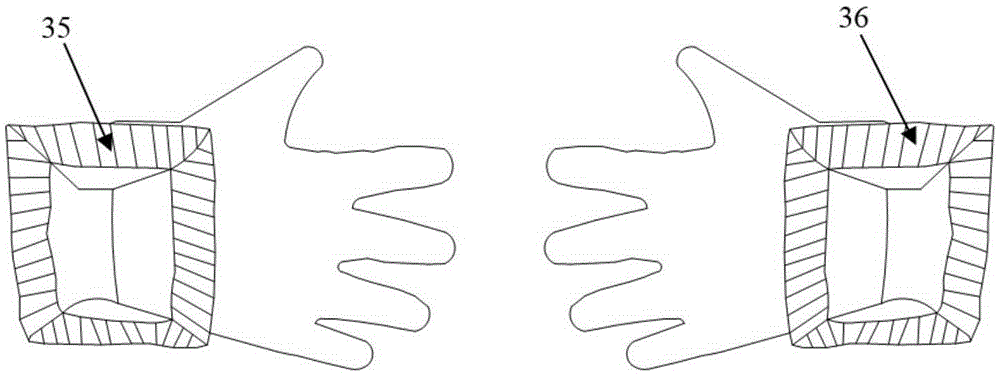
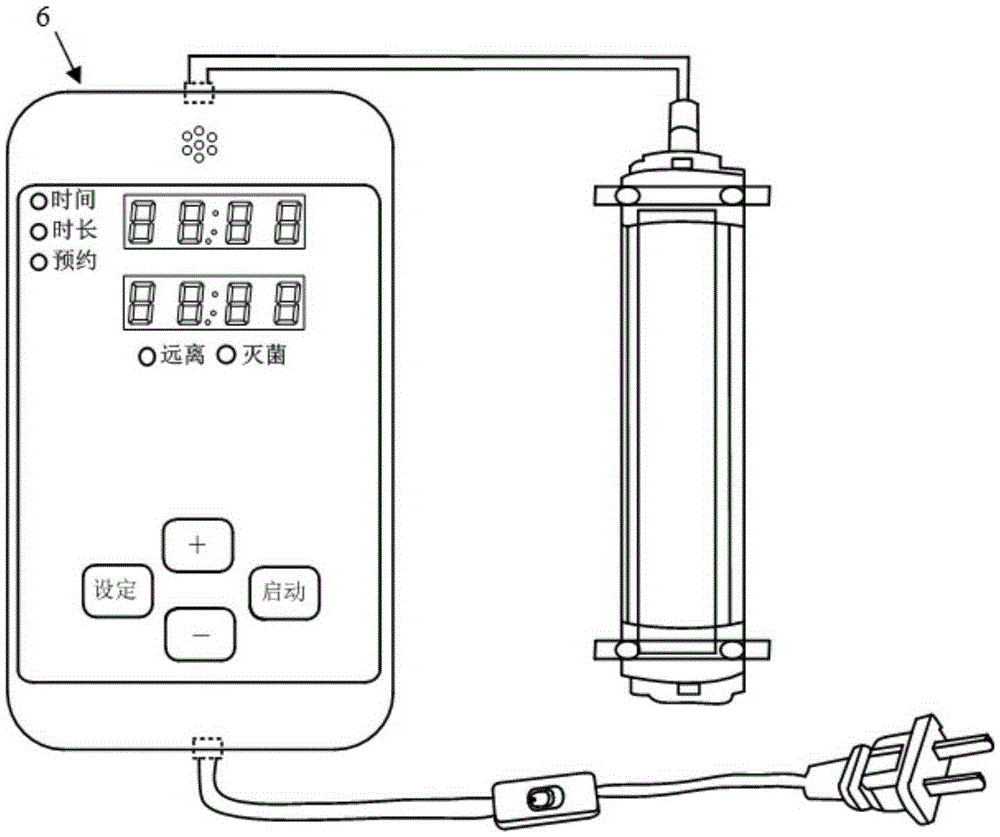
Ultraviolet disinfection box for peritoneal dialysis
InactiveCN105343909AConvenient for going outImprove the quality of lifeLavatory sanitoryRadiationButt jointUltraviolet radiation
The invention discloses an ultraviolet disinfection box for peritoneal dialysis. The ultraviolet disinfection box comprises a box main body, a lifting handle, a reflective film, an iodophor cap storage bag, a movable box cover, a transparent observing window, a protective cover, an infusion pipeline inserting hole, operating gloves, an anti-dust cover, a fixed clamping sheet, a lamp tube bracket, an ultraviolet lamp tube and a connecting wiring harness. According to the ultraviolet disinfection box for peritoneal dialysis provided by the invention, a patient of peritoneal dialysis can operate the gloves to achieve the butt joint of a peritoneal dialysis liquid catheter in a sealed space which is sterilized under ultraviolet radiation, so that peritonitis, which is caused in the case that external bacteria, viruses and dust enter the peritoneal dialysis liquid catheter, is effectively avoided; and the ultraviolet disinfection box for peritoneal dialysis has the characteristics of being low in cost, good in sterilizing efficiency, and being convenient to observe, operate and carry.
Owner:马发财
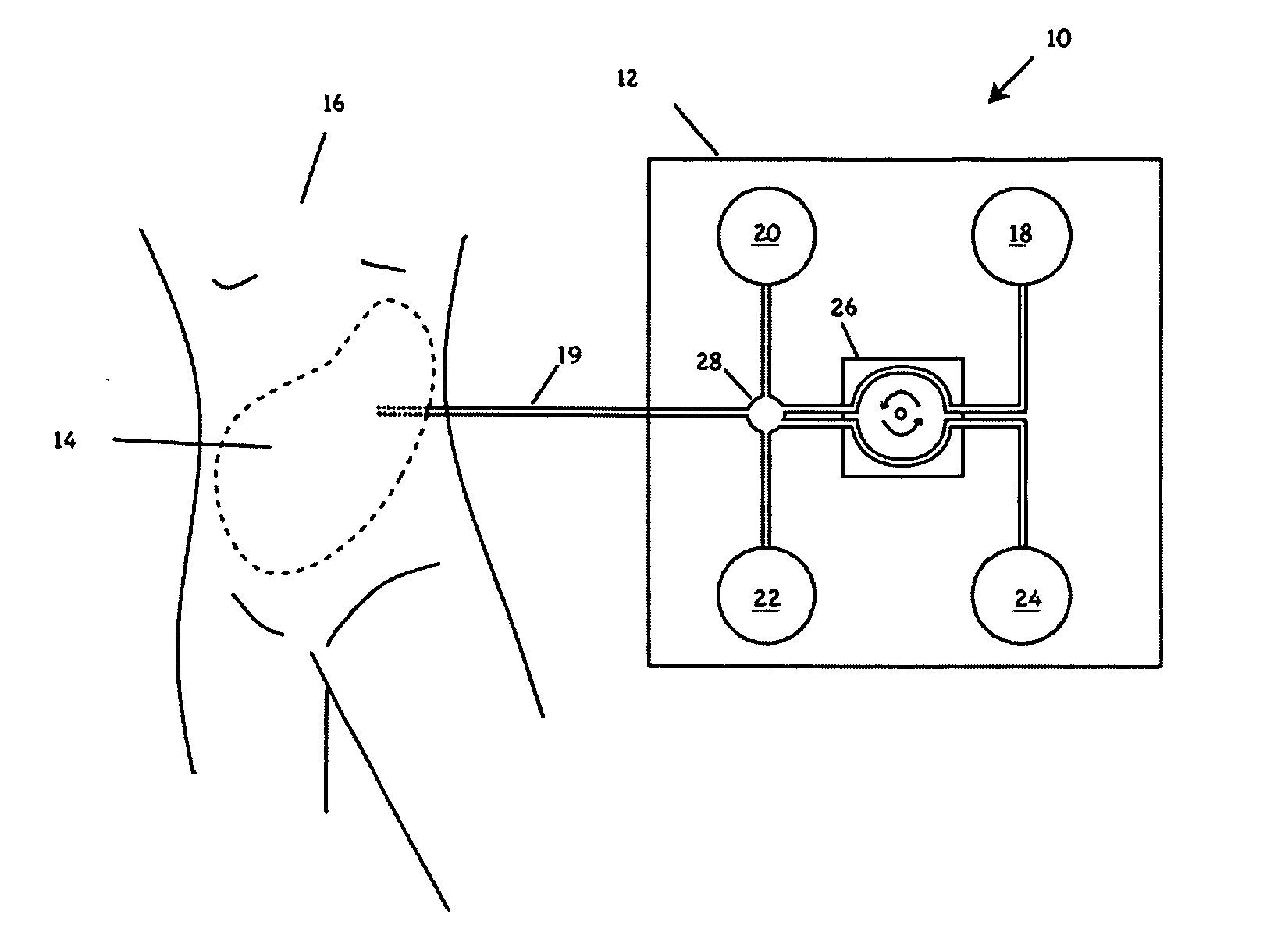
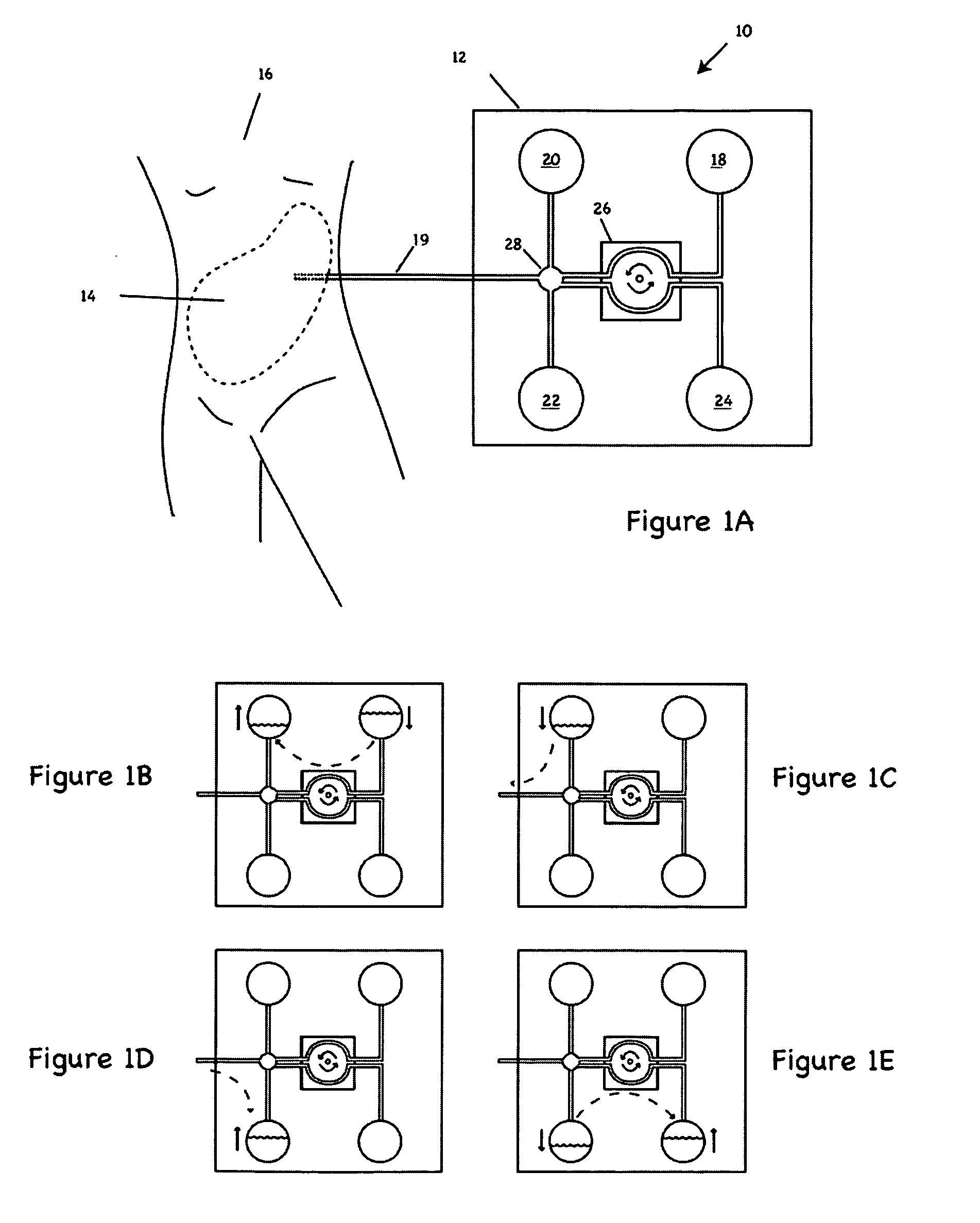
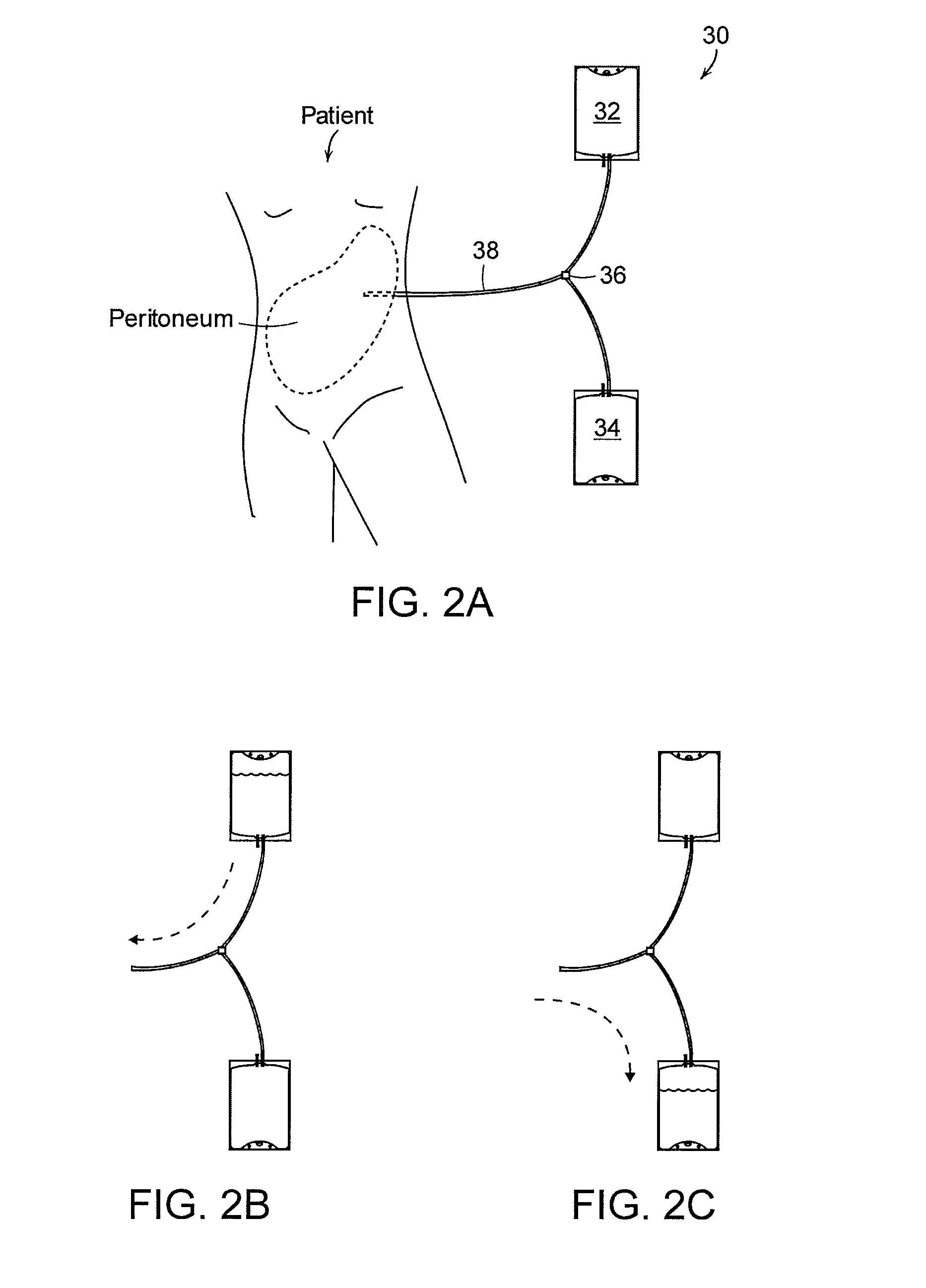
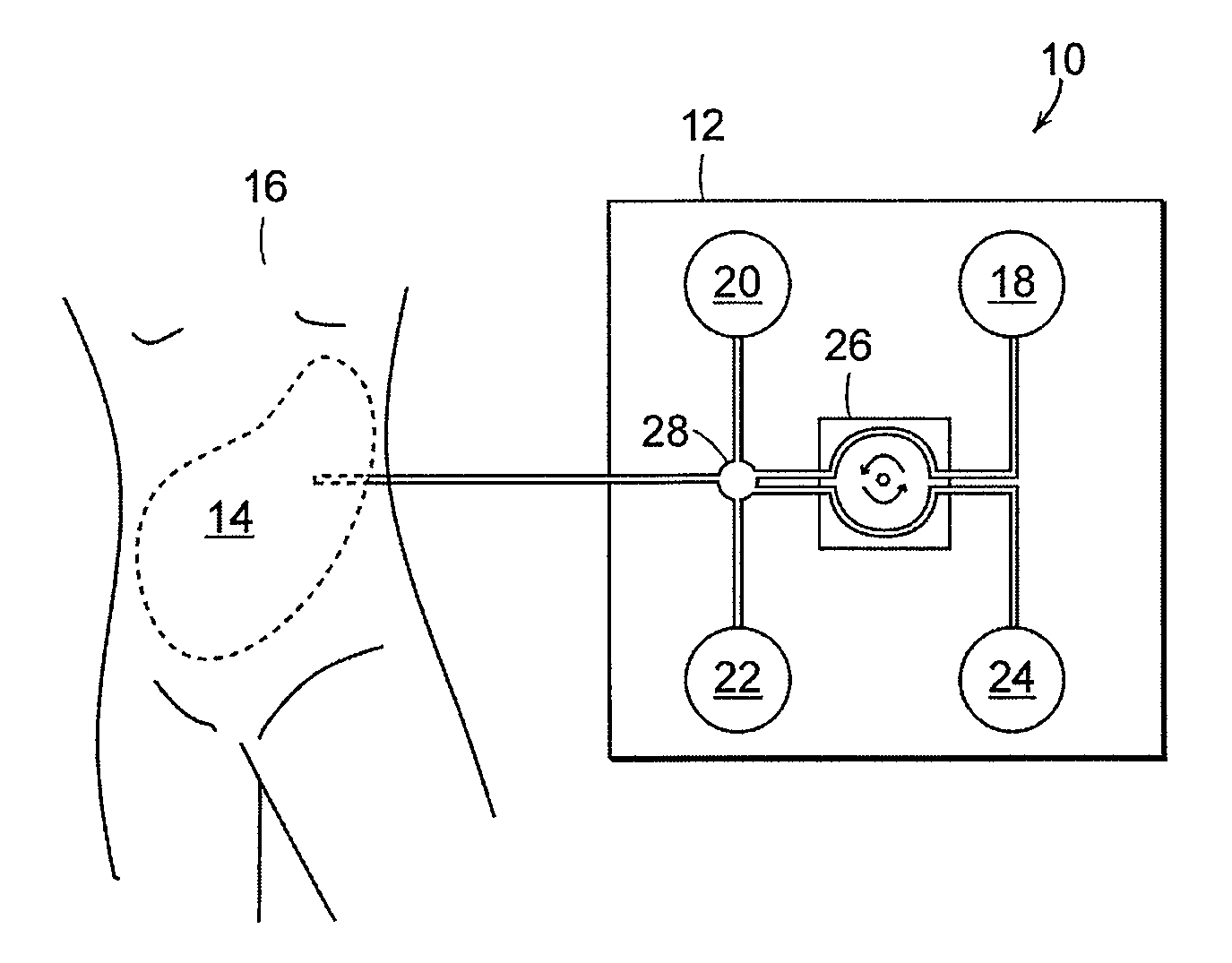
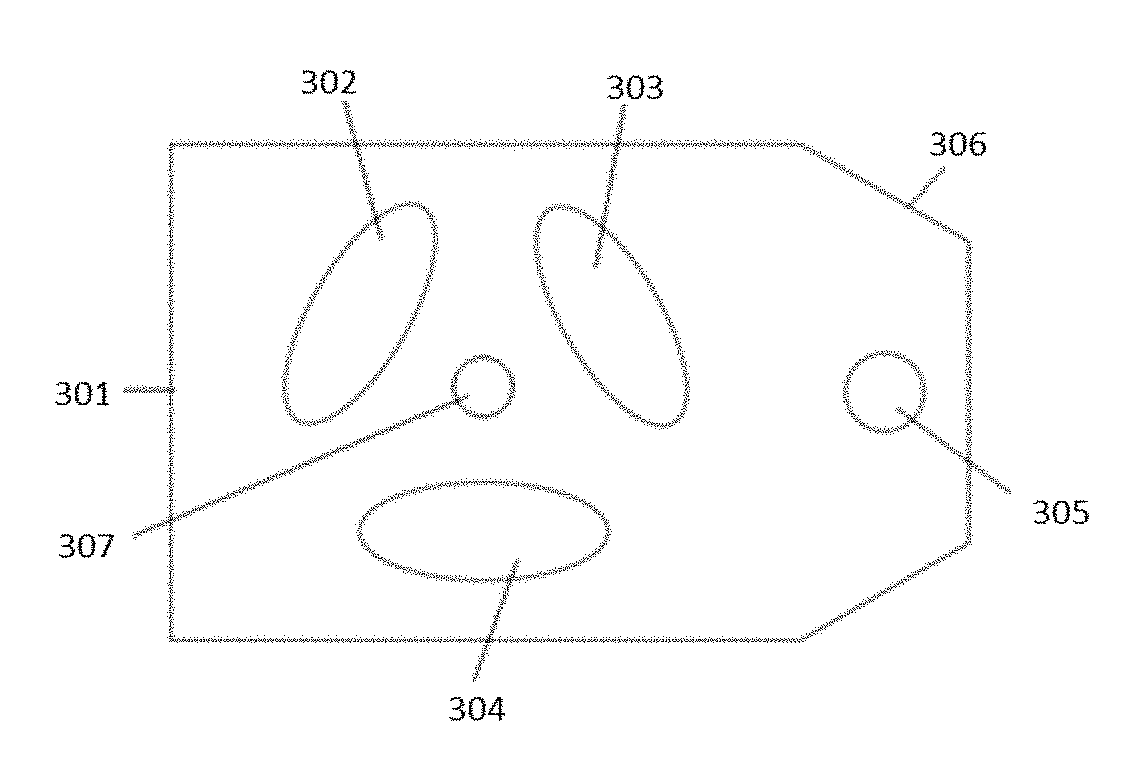
Peritoneal dialysis fluid testing system
ActiveUS20180193546A1Material analysis by observing effect on chemical indicatorMedical devicesPeritoneal dialysis fluidEngineering
The invention relates to a testing system and related methods for detecting peritonitis or infection in peritoneal dialysate removed from a patient. The testing system can include a fluid sensor apparatus in a fluid line of a peritoneal dialysis cycler through which spent peritoneal dialysate can be pumped. The fluid sensor apparatus can detect one or more markers associated with peritonitis or infection.
Owner:MOZARC MEDICAL US LLC
Use of Mycobacterial Mannosylated Lipoglycans Peptide Mimotopes For Treating Inflammation
InactiveUS20080318868A1Antibacterial agentsUltrasonic/sonic/infrasonic diagnosticsAntigen bindingAllergic asthma
The present invention is based on the discovery that biological peptide-based mimotopes of mannose-containing cell-wall compounds of Mycobacterium tuberculosis, specifically, ManLAM, have an anti-inflammatory effect and immunoregulator effect in animal models of inflammation. Such models include animal models of allergic peritonitis, allergic asthma and septic shock model (mice injected with LPS) and in Crohn's disease model (TNBS-induced colitis). Thus, the present invention concerns the use a molecule, particularly, an amino acid based molecule for the production of a pharmaceutical composition for the treatment of an inflammatory condition, the amino acid comprising one or more peptides characterized in that it can bind to ManLAM binding antibodies; and / or it can elicit an immune response in a subject inoculated therewith, giving rise to production of ManLAM-binding antibodies. The invention also provides pharmaceutical compositions for treating inflammatory conditions and comprising the amino acid-based molecule, as well as to methods of treatment of such conditions by administering to a subject an amount of the amino-acid based molecules.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Feline infectious peritonitis vaccine
InactiveUS6096535ASsRNA viruses positive-senseGenetic material ingredientsNucleotide sequencingPlasmid
The invention comprises the nucleotide sequences comprising the FIPV S gene, or a fragment of this gene, which are modified in at least one of the antigenic regions A1 and A2 which are involved in enhancement, as well as the use of these sequences for the expression of modified proteins, and for the construction of recombinant viruses or expression plasmids, and the use of the recombinant viruses as vaccines against feline infectious peritonitis, the use of the expression plasmids as immunizing composition by direct injection of the said plasmids into cats, and the use of the modified proteins as vaccine.
Owner:MERIAL SAS
Application of holomycin in inhibition of activation of NLRP3 inflammasome
ActiveCN108815158AInhibit bindingInhibition formationAntibacterial agentsOrganic active ingredientsDiseaseIn vivo
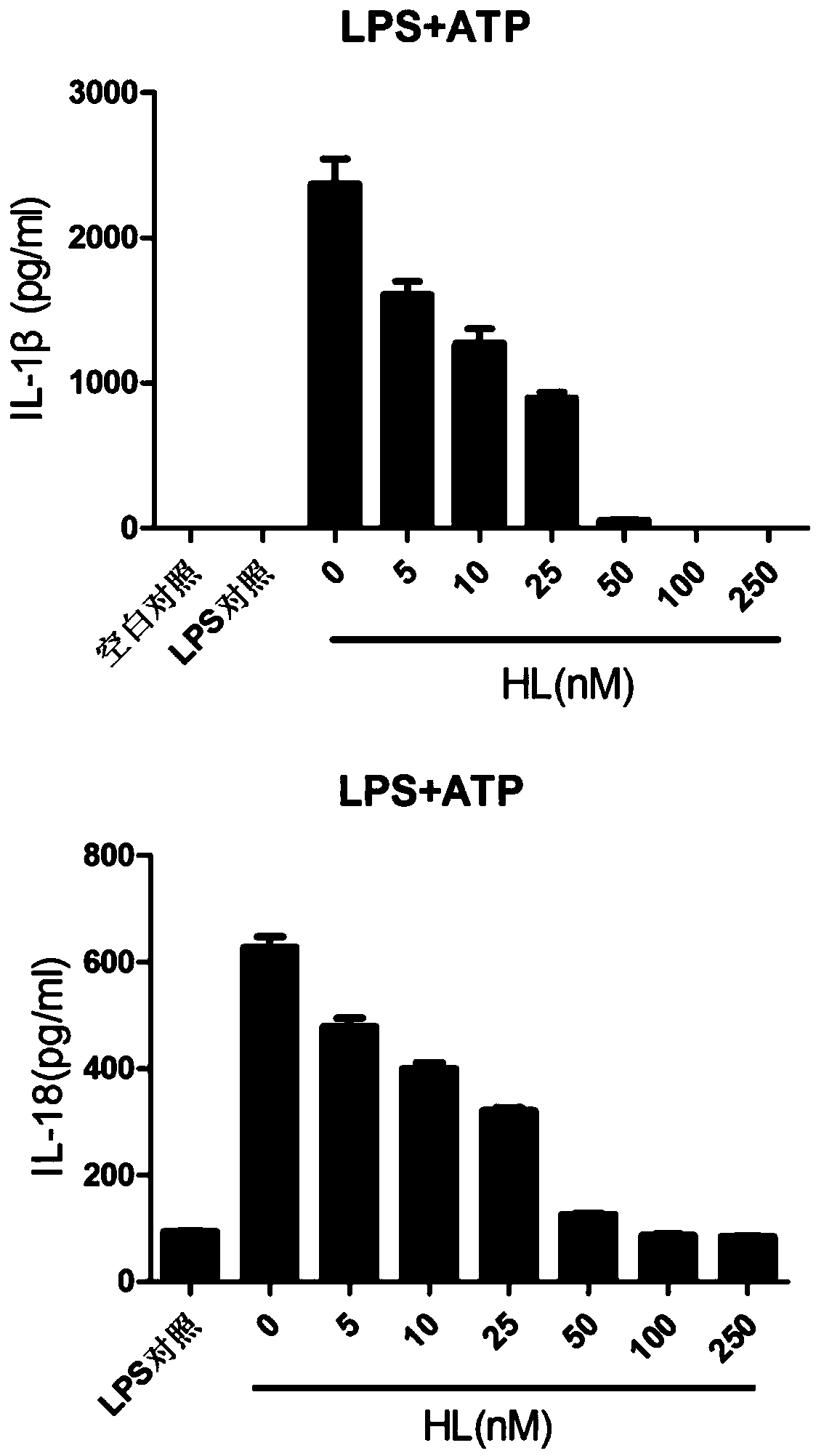
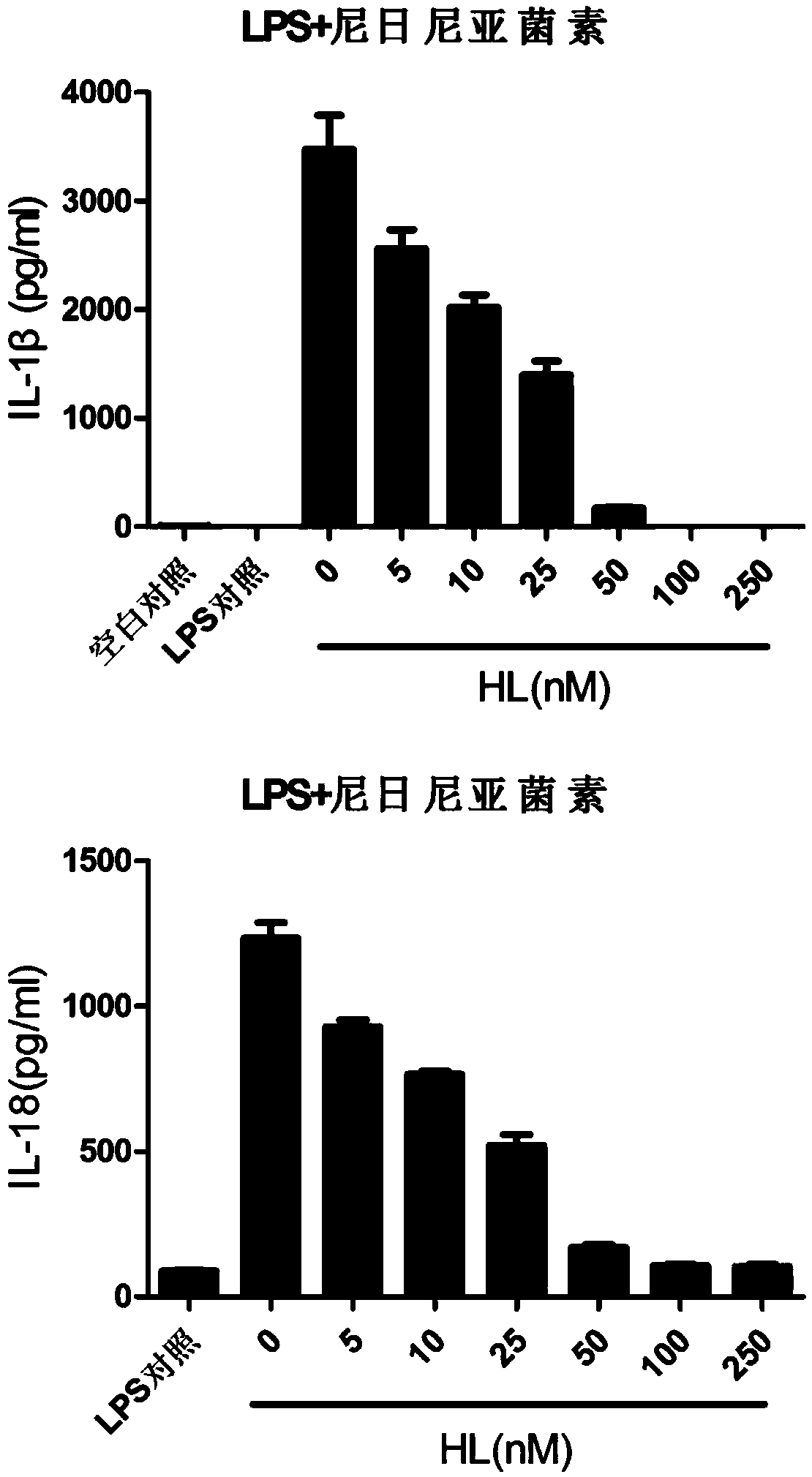
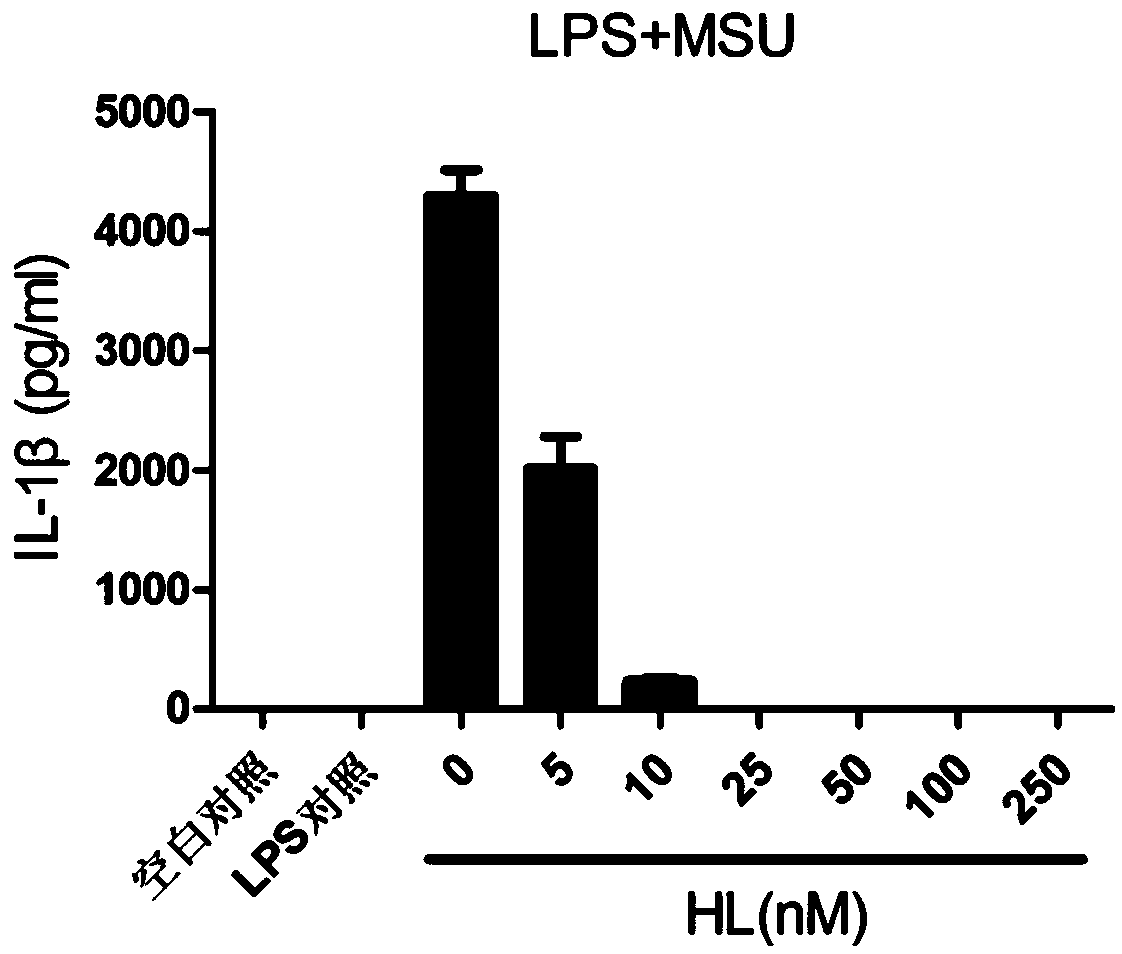
The invention relates to the field of biology and medicine and discloses an application of holomycin in inhibition of the activation of an NLRP3 inflammasome. The holomycin can inhibit NLRP3 protein self-deubiquitination under agonist stimulation, binding of NLRP3 to an ASC protein, formation of ASC spots and activation of Caspase-1, and finally inhibit IL-1 beta and IL-18 secretion. The holomycincan inhibit monosodium urate-induced peritonitis in vivo. Therefore, holomycin can be used a specific drug for treatment on NLRP3 abnormal activation-related diseases.
Owner:ACADEMY OF MILITARY MEDICAL SCI
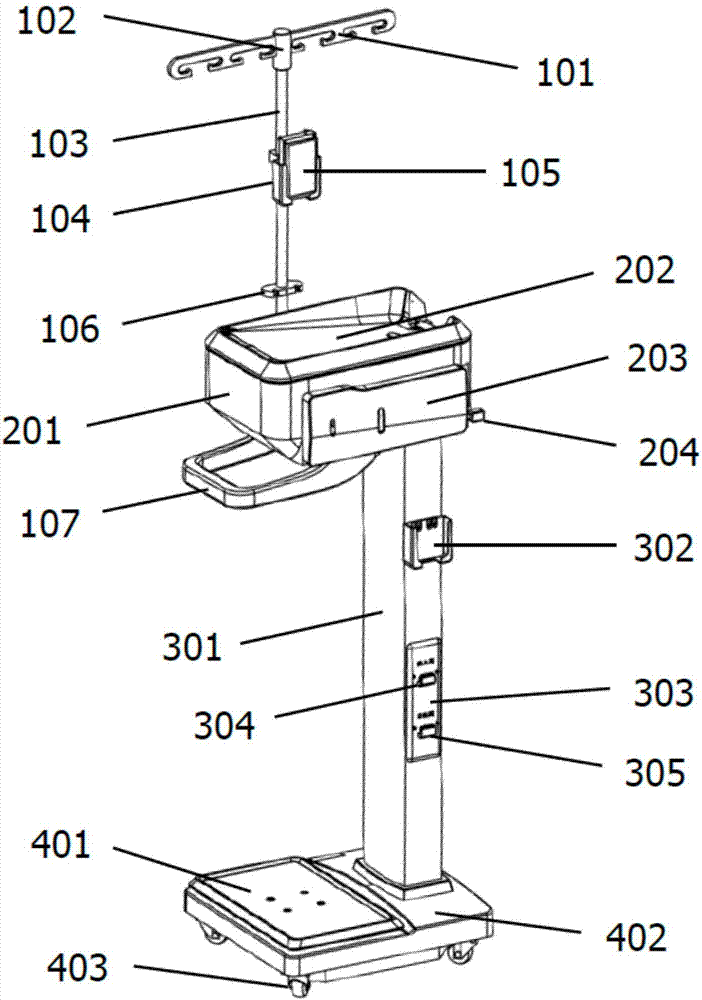
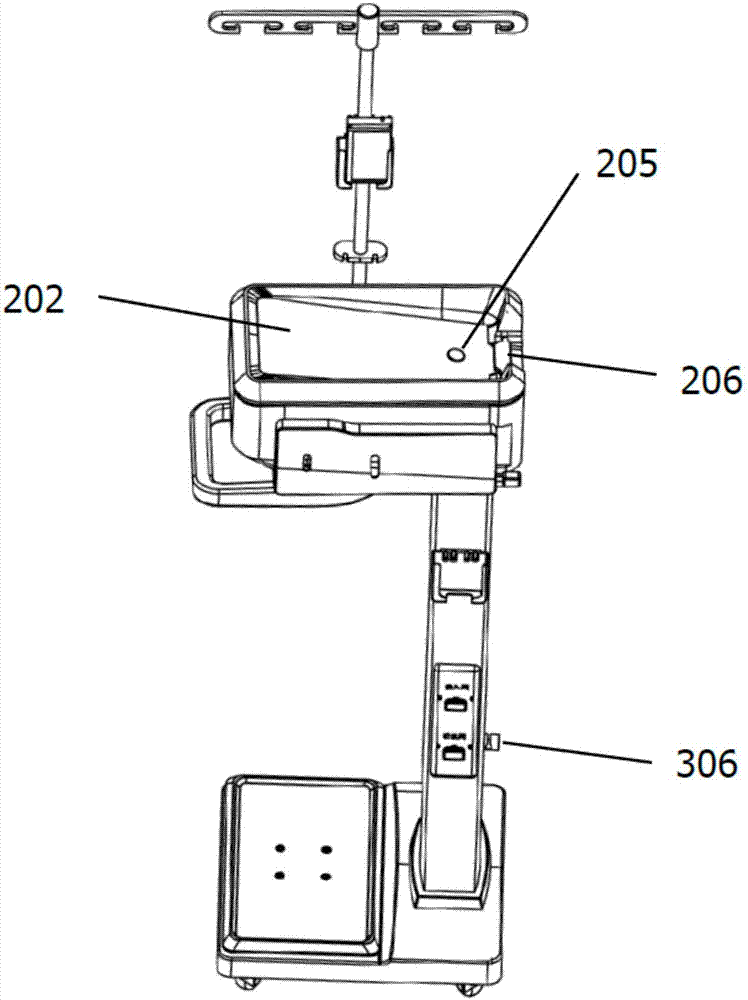
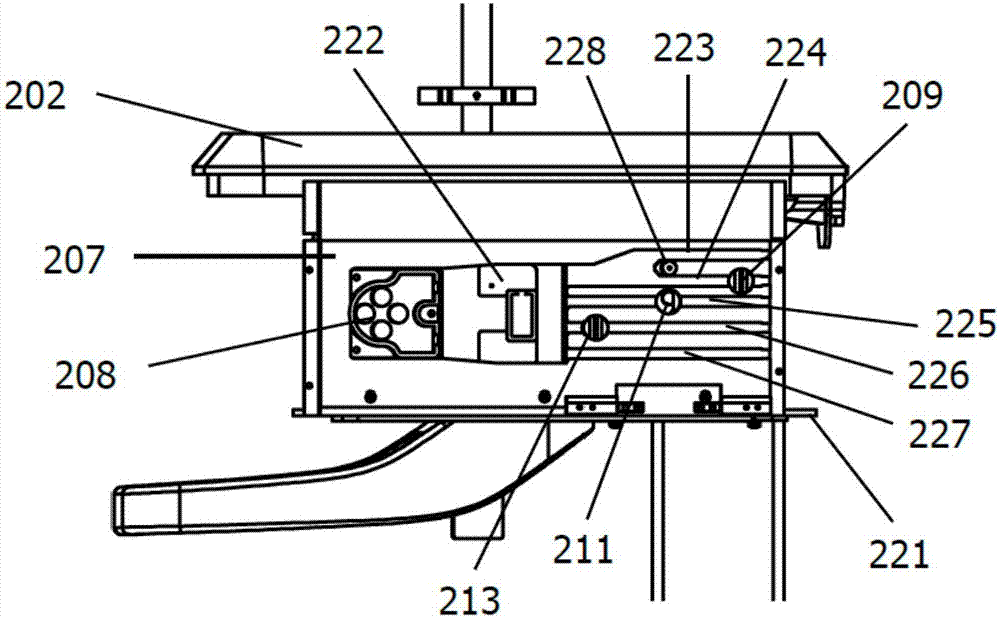
Peritoneal dialysis device and using method thereof
InactiveCN107441577AReduce pollutionEasy to operatePeritoneal dialysisMedical equipmentAbdominal cavity
The invention relates to the technical field of medical equipment, in particular to a peritoneal dialysis device and a using method thereof. The peritoneal dialysis device comprises an infusion rack assembly, a main machine body assembly, a stand column assembly and a bottom table assembly which are successively connected from top to bottom. The invention provides the peritoneal dialysis device and the using method thereof. Exchange of dialysate in a peritoneal dialysis process is finished by machinery, operation is simple relatively. The peritoneal dialysis is superior to the traditional peritoneal dialysis, the degree of automation is high, the chance of peritoneal contamination is reduced, and the probability of occurrence of peritonitis of a patient who uses the peritoneal dialysis device is lower than the probability of occurrence of peritonitis of a patient who uses the traditional peritoneal dialysis.
Owner:王泽义
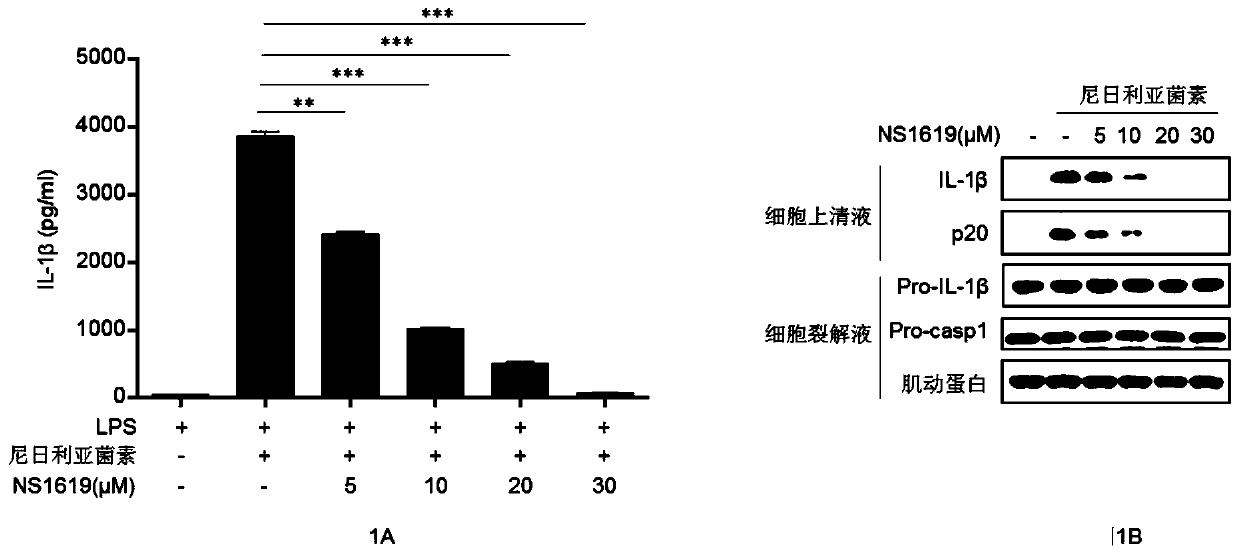
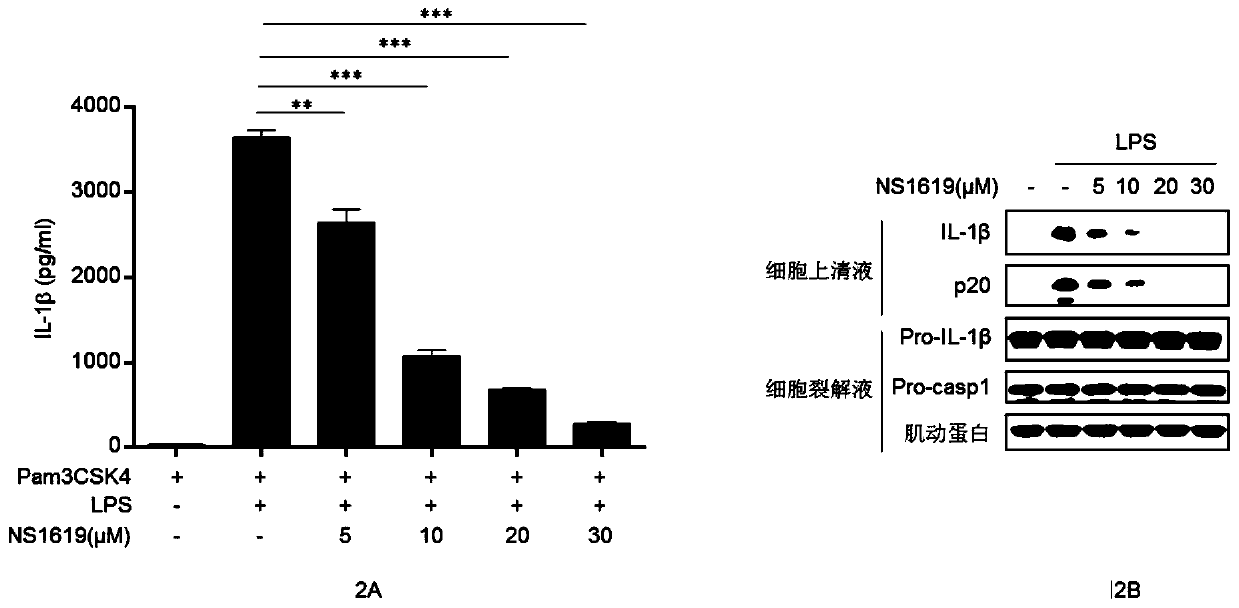
Application of NS1619 for inhibiting activation of NLRP3 inflammatory corpuscles
ActiveCN110575453AInhibition of activationInhibition of shear activationAntibacterial agentsOrganic active ingredientsAgonistBiological activation
The invention belongs to the field of biological medicines. The invention discloses application of NS1619 for inhibiting activation of NLRP3 inflammatory corpuscles. An experiment finds that the NS1619 can inhibit autooligomerization of a NLRP3 protein, thus the activation of the NLRP3 inflammatory corpuscles is specifically inhibited, and maturation and secretion of inflammatory factors IL-1 betaand IL-18 are inhibited. At the same time, the NS1619 can further alleviate peritonitis in vivo induced by NLRP3 inflammatory corpuscle agonist MSU. Therefore, according to application of NS1619 forinhibiting activation of NLRP3 inflammatory corpuscles, the latest insights are provided for the NS1619 to treat the NLRP3 inflammatory corpuscles related inflammatory diseases and preparing drugs fortreatment of related diseases.
Owner:UNIV OF SCI & TECH OF CHINA
Methods of preventing peritonitis by administering lactic acid bacterium
InactiveUS7217414B2Positive influenceReduce in quantityAntibacterial agentsBiocideLactobacillusBacteroides
The present invention relates to the use of lactic acid bacteria capable of adhering to the mucosa of the intestine and especially colonizing it for the prevention of peritonitis. In particular, the present invention relates to the use of such lactic acid bacteria for the prevention of peritonitis caused by cirrhosis of the liver. Specifically, the present invention relates to a method for preventing peritonitis in a patient in need of such prevention. This method includes administering to the patient a lactic acid bacterium that is capable of adhering to the intestine's mucosa and essentially colonizing it for the preparation of an ingestable carrier. The invention also relates to a peritonitis preventing composition of a lactic acid bacterium that is capable of adhering to the intestine's mucosa and essentially colonizing it for the preparation of an ingestable carrier. The carrier is preferably a food or pharmaceutical composition.
Owner:NESTEC SA
Phage Therapy Against Pseudomonas Aeruginosa
This invention relates to a bacteriophage MPK6 (deposit number: KCCM 11044P) having a lytic activity to Pseudomonas aeruginosa, or a progeny bacteriophage thereof having a RFLP (Restriction fragment length polymorphism) DNA profile substantially equivalent to the bacteriophage MPK6. The present invention provides a bacteriophage MPK6 or a progeny bacteriophage thereof capable of treating a Pseudomonas aeruginosa infection disease, and suggests an anti-bacterial activity of MPK6 and its progeny bacteriophage using a mammalian and non-mammalian infection model. According to the present invention, the present bacteriophage MPK6 or progeny bacteriophage thereof represents very effective efficacy on treatment of P. aeruginosa-induced peritonitis-sepsis.
Owner:IND UNIV COOP FOUND SOGANG UNIV
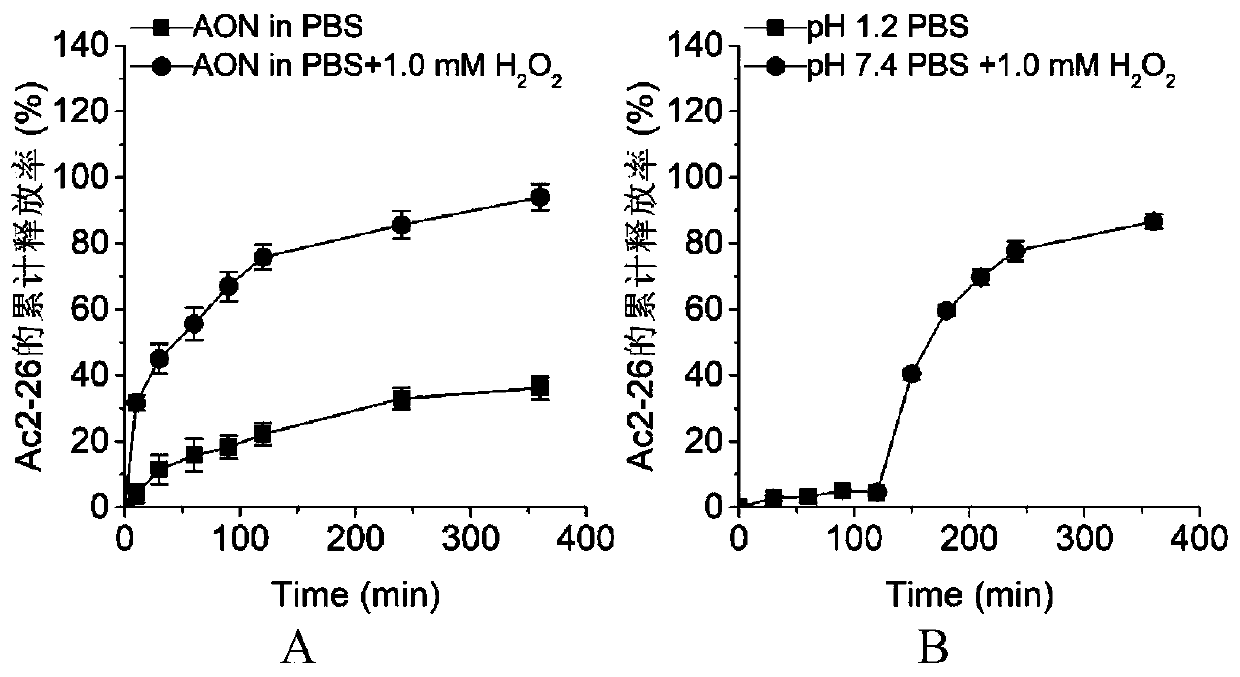
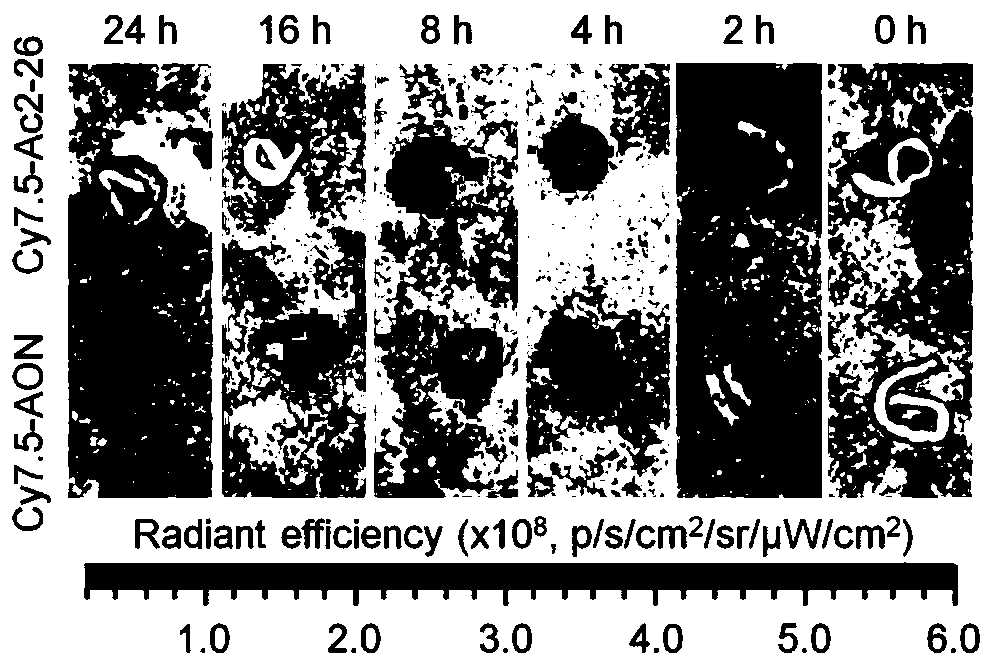
Anti-inflammatory polypeptide nano drug and preparation method thereof
InactiveCN110302176AEasy to synthesizeGood in vivo and in vitro safetyPeptide/protein ingredientsAntipyreticPolyethylene glycolPhospholipid
The invention discloses an anti-inflammatory polypeptide nano drug and a preparation method thereof. The anti-inflammatory polypeptide nano drug comprises anti-inflammatory polypeptides, a pH or reactive oxygen responsive material, phospholipids and polyethylene glycol-distearoyl phosphatidylethanolamine, wherein the anti-inflammatory polypeptide comprises the active N-terminal peptide Ac2-26 of annexin A1 and the tissue protective polypeptide ARA290; the mass ratio of the anti-inflammatory polypeptide to the pH or active oxygen responsive material is 0.01:1 and 2:1, the mass ratio of polyethylene glycol-distearoyl phosphatidylethanolamine to the pH or reactive oxygen responsive material is 0.02:1 and 2.5:1, and the mass ratio of polyethylene glycol-distearoyl phosphatidylethanolamine to phospholipid is 0.01:1 and 1:0.01. The drug can be used in preparation of preventive and therapeutic drugs for acute and chronic inflammation-related diseases. An administration method includes oral administration, intravenous injection, subcutaneous injection, intramuscular injection, and any combination of the above MODEs, and the product has obvious prevention and treatment effects on acute andchronic inflammation-related diseases such as inflammatory bowel disease, peritonitis and atherosclerosis.
Owner:ARMY MEDICAL UNIV
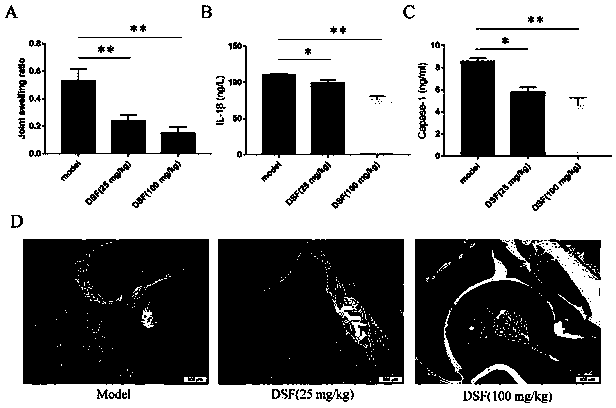
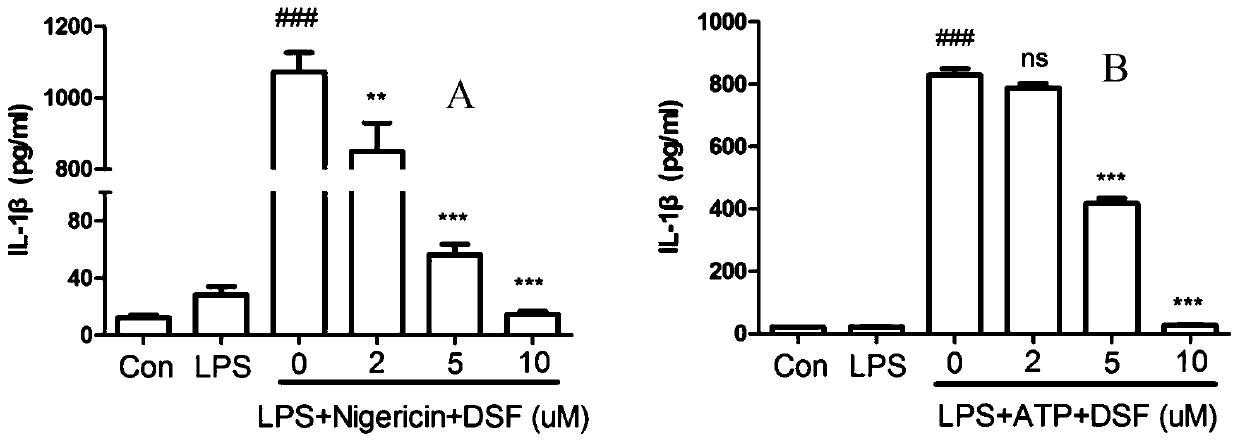
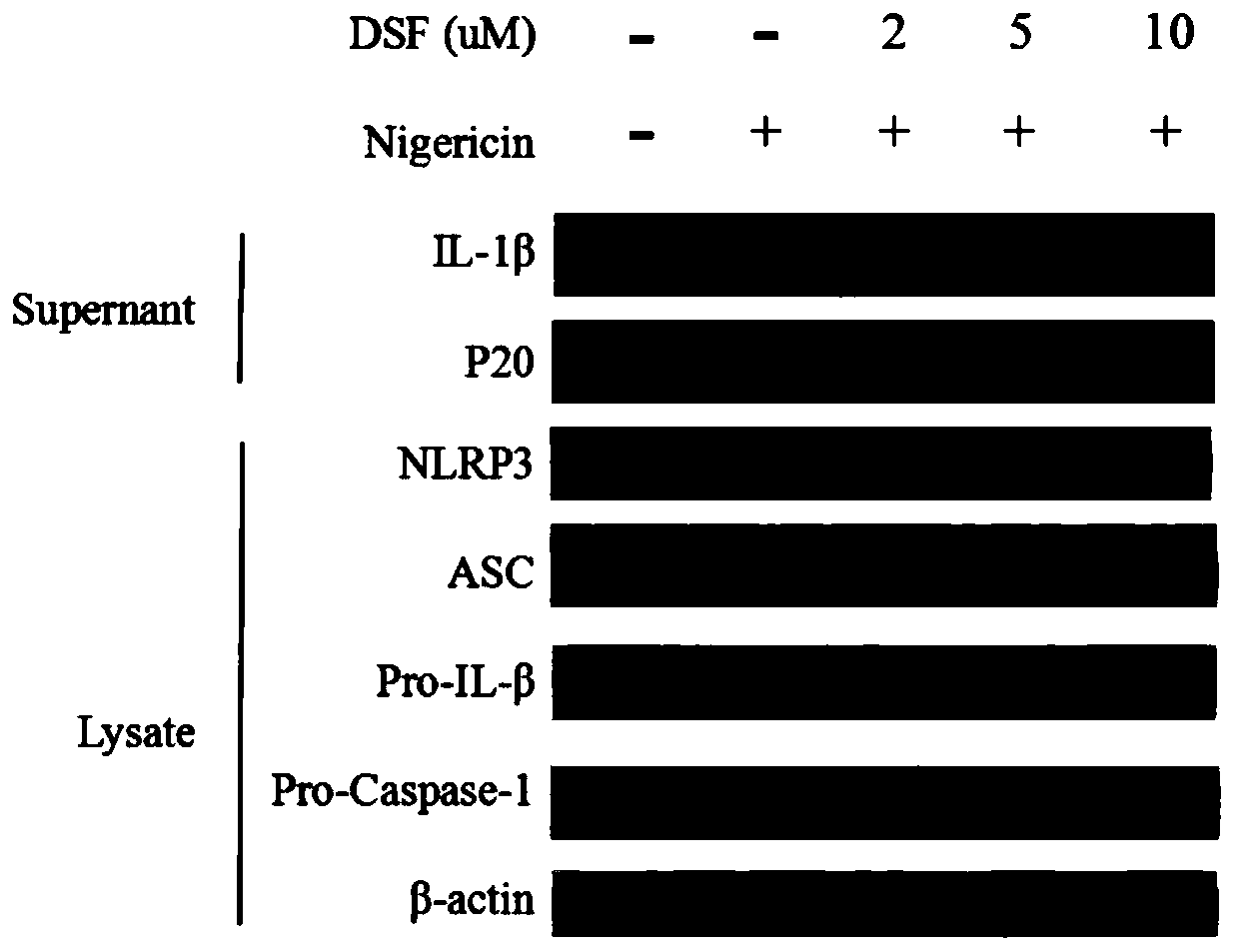
Application of disulfiram in preparing drugs for preventing and treating diseases related to NLRP3 inflammasome
InactiveCN110917182AEnhance pharmacological effectsAim to quit drinkingOrganic active ingredientsAntipyreticDiseasePharmaceutical drug
The invention relates to application of disulfiram in preparing drugs for preventing and treating diseases related to NLRP3 inflammasome. During research, phenomena are found that the disulfiram can effectively inhibit activation of the NLRP3 inflammasome and inhibit maturation and secretion of an inflammatory activation signal molecule Caspase-1P20 and an inflammatory cytokine IL-1[beta], accordingly has good prevention and treatment effects on the diseases related to the NLRP3 inflammasome, and especially has significant prevention and treatment effects on peritonitis and gouty arthritis.
Owner:GUANGZHOU MEDICAL UNIV
Soluble powder for treating infection of domestic bird caused by sensitive bacteria
InactiveCN101467998APromote oral absorptionImprove securityAntibacterial agentsPowder deliveryPerihepatitisEscherichia coli
The invention relates to soluble powder for treating sensitive bacterial infections of poultry, comprising sodium ampicillin, sulbactam sodium and auxiliary materials. As an antibiotic medicine, the inventive medicament is mainly used to treat body infectious diseases such as perihepatitis, pericarditis, salpingitis, vitelline peritonitis, air sacculitis, enteritis, diarrhea and the like caused by sensitive bacteria such as escherichia coli, salmonella and the like. The sodium ampicillin and sulbactam sodium have compatibility when used together. The soluble powder is used to treat body infectious diseases caused by sensitive bacteria, has good absorption and relatively high safety when orally taken, and is suitable for being popularized in animal husbandry.
Owner:TIANJIN RINGPU BIO TECH
Chinese medicinal granule with clearing heat and detoxication, blood-nourishing and dysentery-stopping efficacy
The invention relates to a compound Chinese traditional medicine granules with the efficacies of clearing away heat and toxic material, nourishing the blood and stopping dysentery, including nine Chinese traditional medicines of radix astragali, common andrographis herb, evodia rutaecarpa, rhubarb, sophora flavescens, angelica dahurica, dandelion, Chinese bulbul and liquorice. The invention is mainly used for treating enteritis type and sepsis type colibacillosis. The invention is applicable to the symptoms of avian pericarditis, perihepatitis, peritonitis and chick egg yolk malabsorption, etc. The invention has the efficacies of clearing away heat and toxic material, nourishing the blood and stopping dysentery. By considering the short avian genital tract and the special physiological structure of inability to digest lignocellulose, the medicine is prepared into soluble granules which can quickly be dissolved into drinking water which is absorbed by sick chickens, so as to achieve the purpose of quick effect.
Owner:TIANJIN RINGPU BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com