Patents
Literature
364 results about "Caspase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death (including apoptosis, pyroptosis and necroptosis) and inflammation. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 11 or 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions.
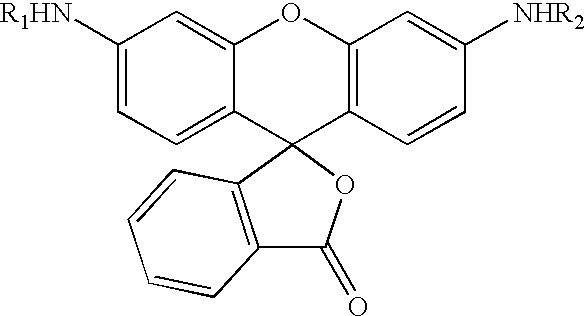
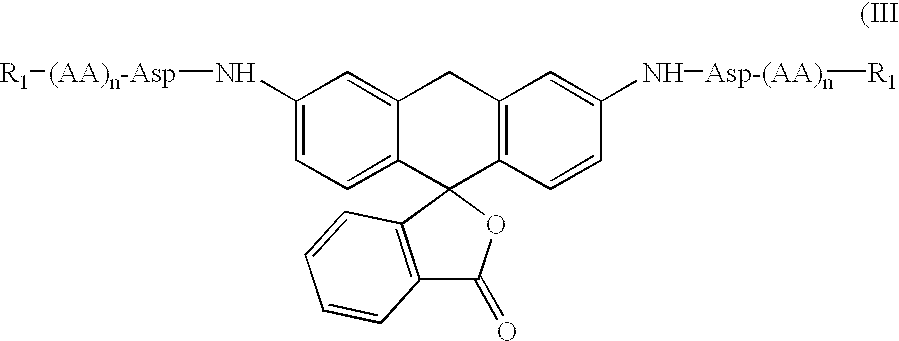
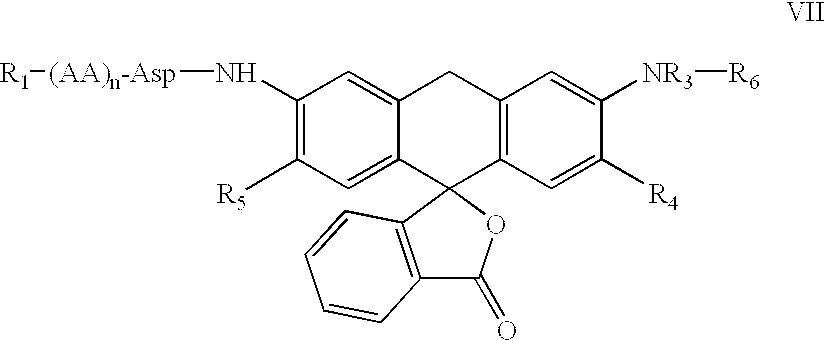
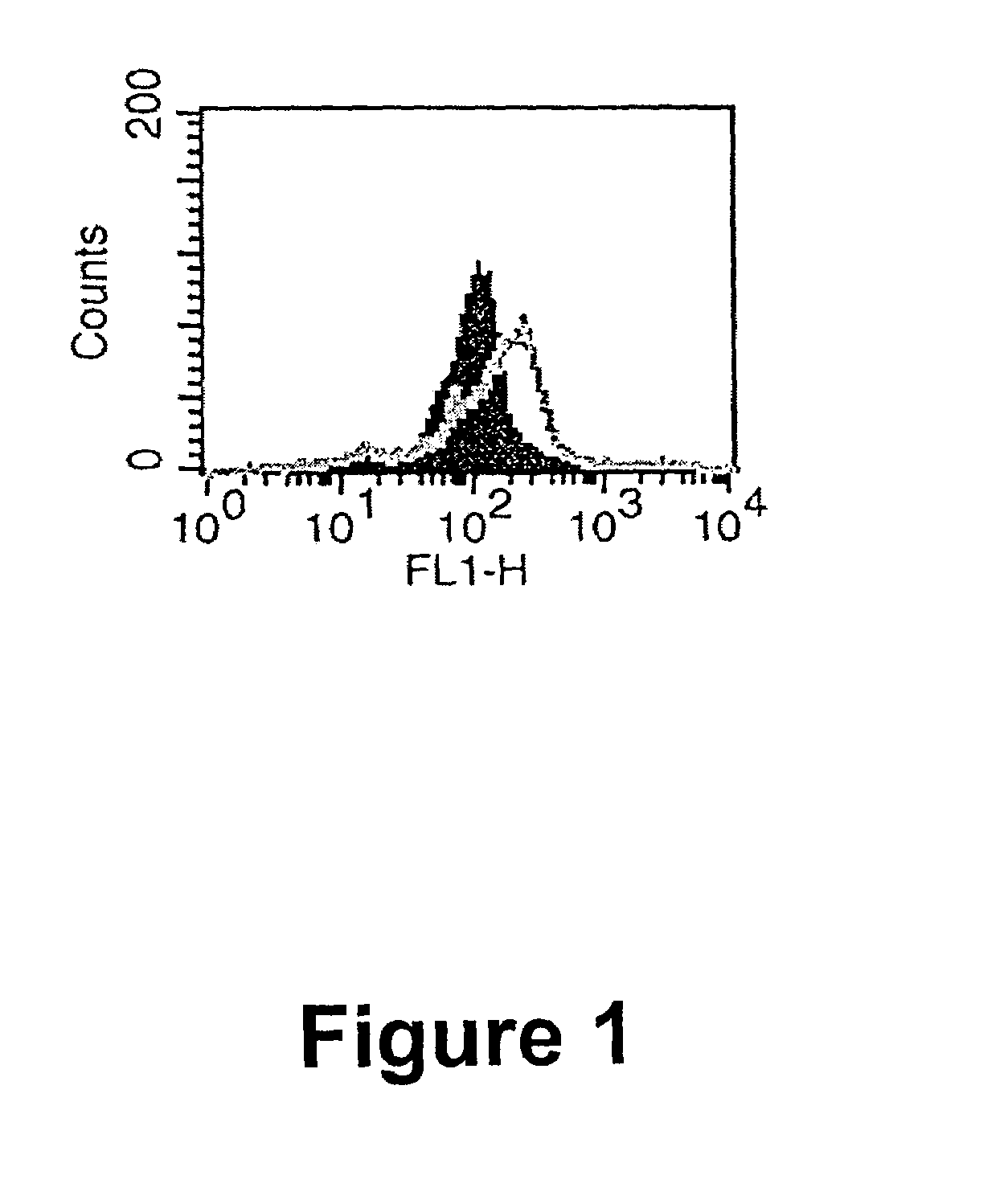
Fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for caspases and other enzymes and the use thereof
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
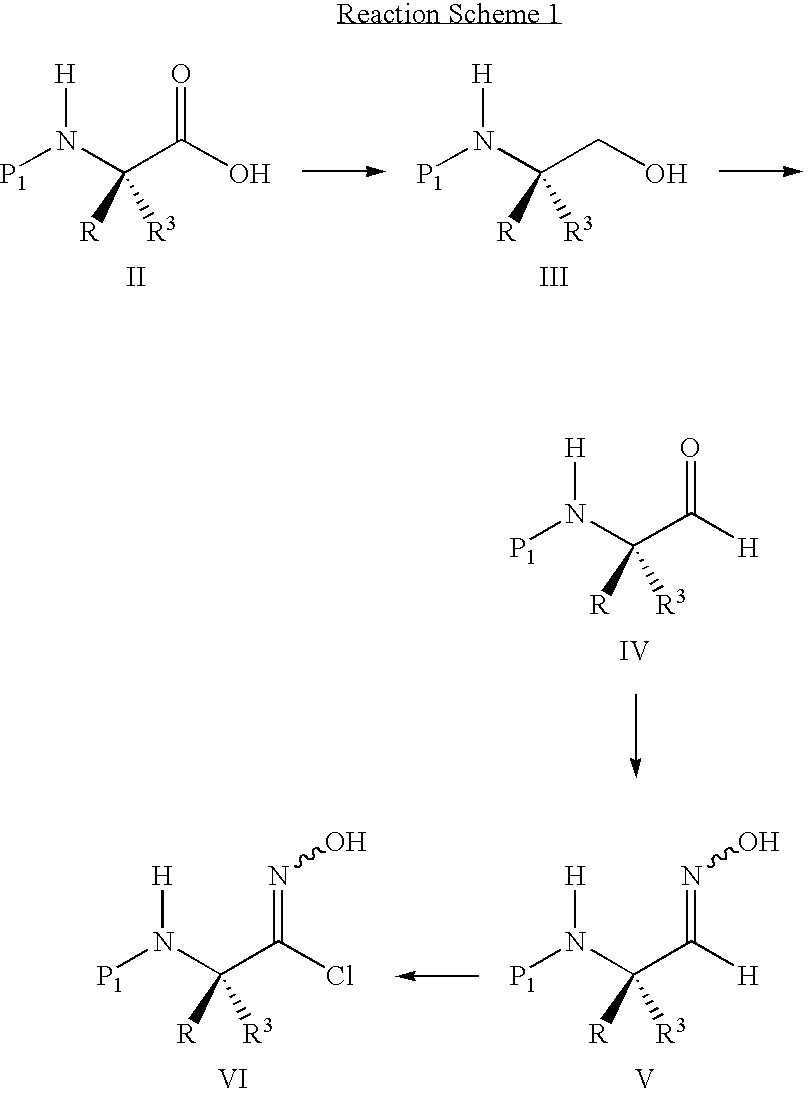
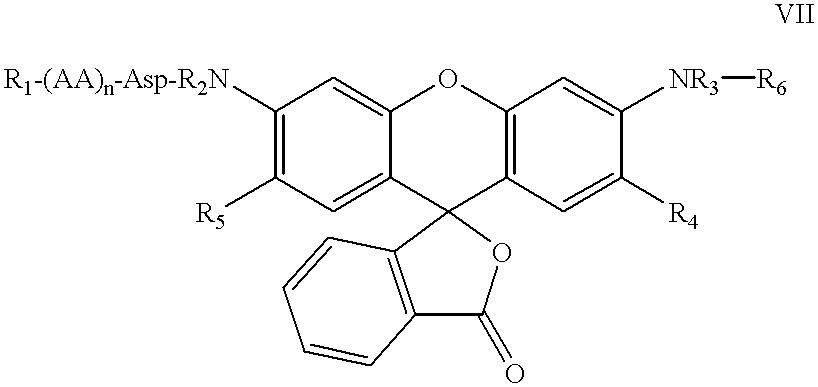
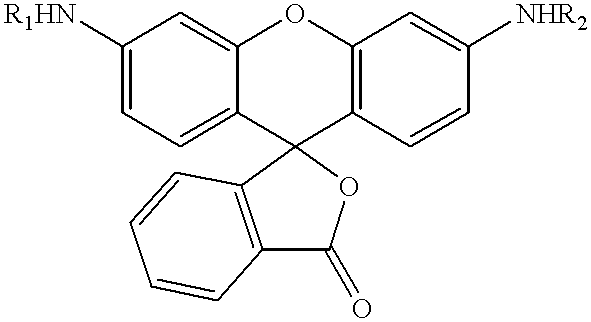
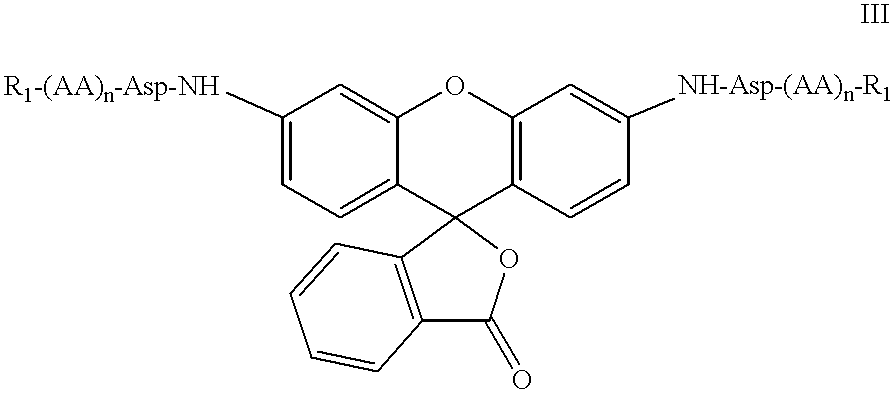
Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof
The present invention is directed to substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs thereof, represented by the Formula I: wherein Ar1, Ar3, A, B and D are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
Fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for capsases and other enzymes and the use thereof
InactiveUS6342611B1Organic chemistryMicrobiological testing/measurementScreening proceduresApoptosis
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Fluorescence dyes and their applications for whole-cell fluorescence screening assays for caspases, peptidases, proteases and other enzymes and the use thereof
InactiveUS6248904B1Microbiological testing/measurementBiological testingScreening proceduresCancer cell
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Methods and compositions for the diagnosis of diseases of the aorta
InactiveUS20070224643A1Facilitate patient treatmentConvenient treatmentDiagnosticsSurgeryAortic dissectionSmooth Muscle Myosins
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to the use of biomarkers, either individually or in combinations with one another to rule in or out diseases of the aorta and its branches, most particularly aortic aneurysm and / or aortic dissection, and for risk stratification in such conditions. Preferred markers include one or more of creatine kinase-BB (CK-BB), creatine kinase-MB (CK-MB), acidic calponin, basic calponin, B-type natriuretic peptide (BNP), NT-proBNP, proBNP, BNP79-108, BNP3-108, caldesmon, caspase-3, D-dimer, soluble elastin fragments, endothelial cell-selective adhesion molecule (ESAM), fibrillin-1, heart-type fatty acid binding protein, MMP-9, myeloperoxidase, myoglobin, smooth muscle myosin, smooth muscle myosin heavy chain, TIMP-1, free cardiac troponin I, complexed cardiac troponin I, free and complexed cardiac troponin I, free cardiac troponin T, complexed cardiac troponin T, and free and complexed cardiac troponin T, and preferred assays are configured to detect these markers.
Owner:BIOSITE INC
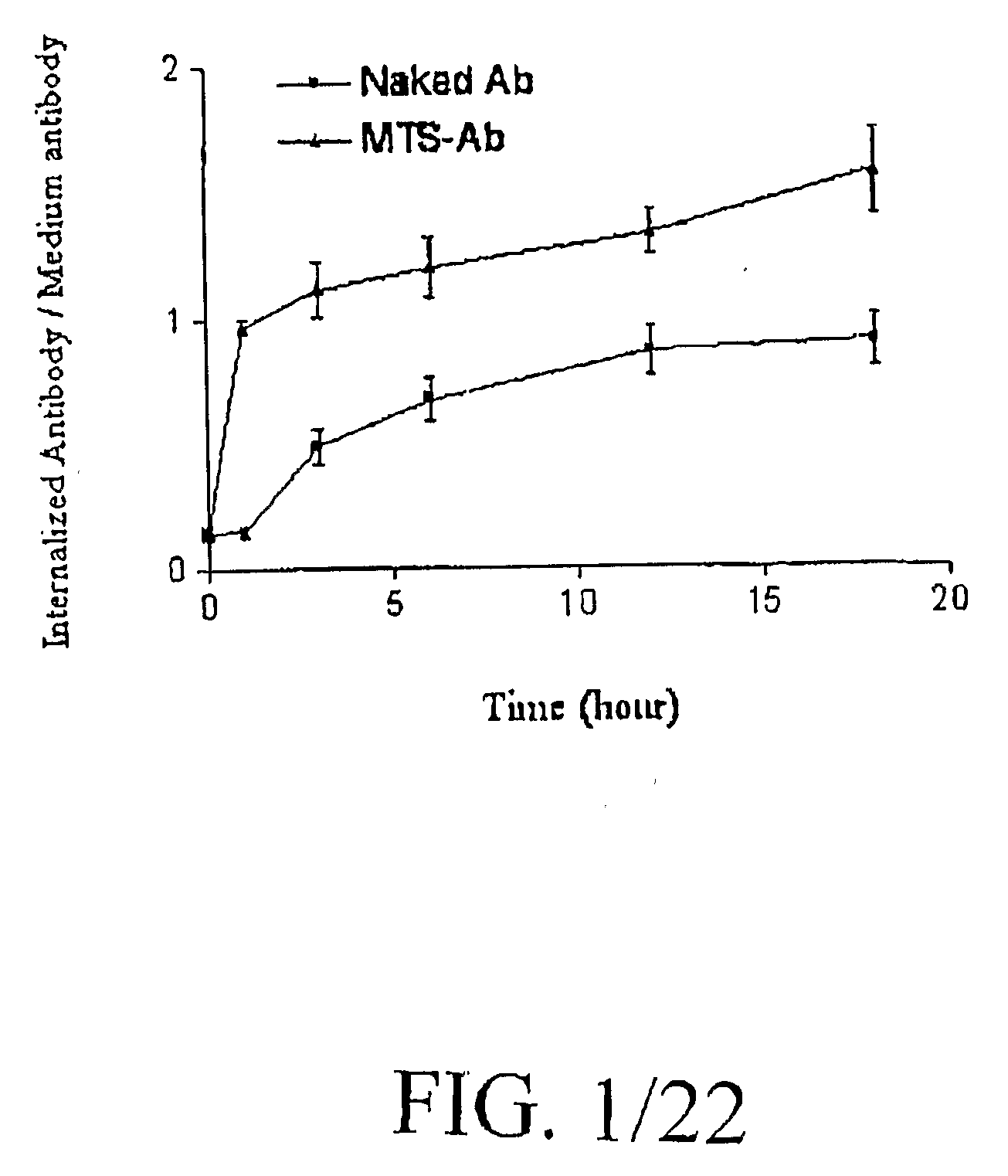
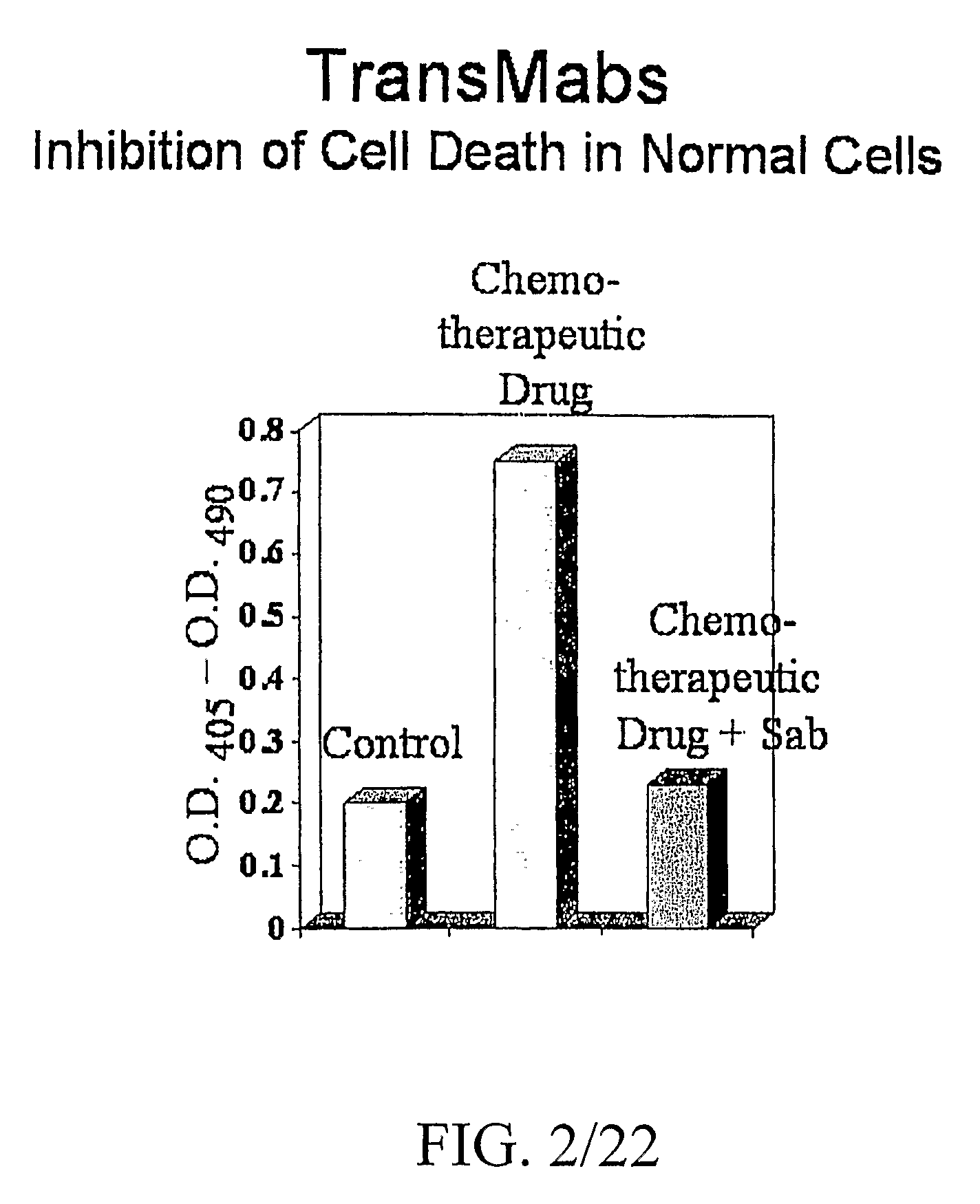
Superantibody synthesis and use in detection, prevention and treatment of disease
InactiveUS20090208418A1Facilitate its translocationFunction increaseUltrasonic/sonic/infrasonic diagnosticsSurgeryPurineApoptosis
Superantibodies having enhanced autophilic, catalytic, and / or membrane-penetrating properties are prepared by affinity-based conjugation of a photoactivatable organic molecule to a target immunoglobulin. The photoactivatable organic molecule bears a chromophoric aromatic hydrocarbon moiety, which has affinity for the immunoglobulin. Upon photolysis, the organic molecule is covalently linked to the immunoglobulin. A preferred organic molecule is a peptide and a preferred aromatic hydrocarbon moiety is a tryptophan residue. The photoactivatable organic molecule need not bear a purine, pyrimidine or azido group to effect binding to the immunoglobulin and / or photoactivation. The superantibodies can enhance the potency and expand the targeting range of target antibodies. Autophilic superantibodies can promote apoptosis of target cells and / or enhance therapeutic efficacies in the treatment of patients with diseases or disorders responsive to antibody therapy. Exemplary of such diseases are atherosclerosis and cardiovascular disease. Membrane-penetrating superantibodies can prevent apoptosis by binding to intracellular anti-caspase signal proteins. Compositions containing the superantibodies, as well as methods of making and using them, are disclosed.
Owner:INNEXUS BIOTECHNOLOGY INT LTD
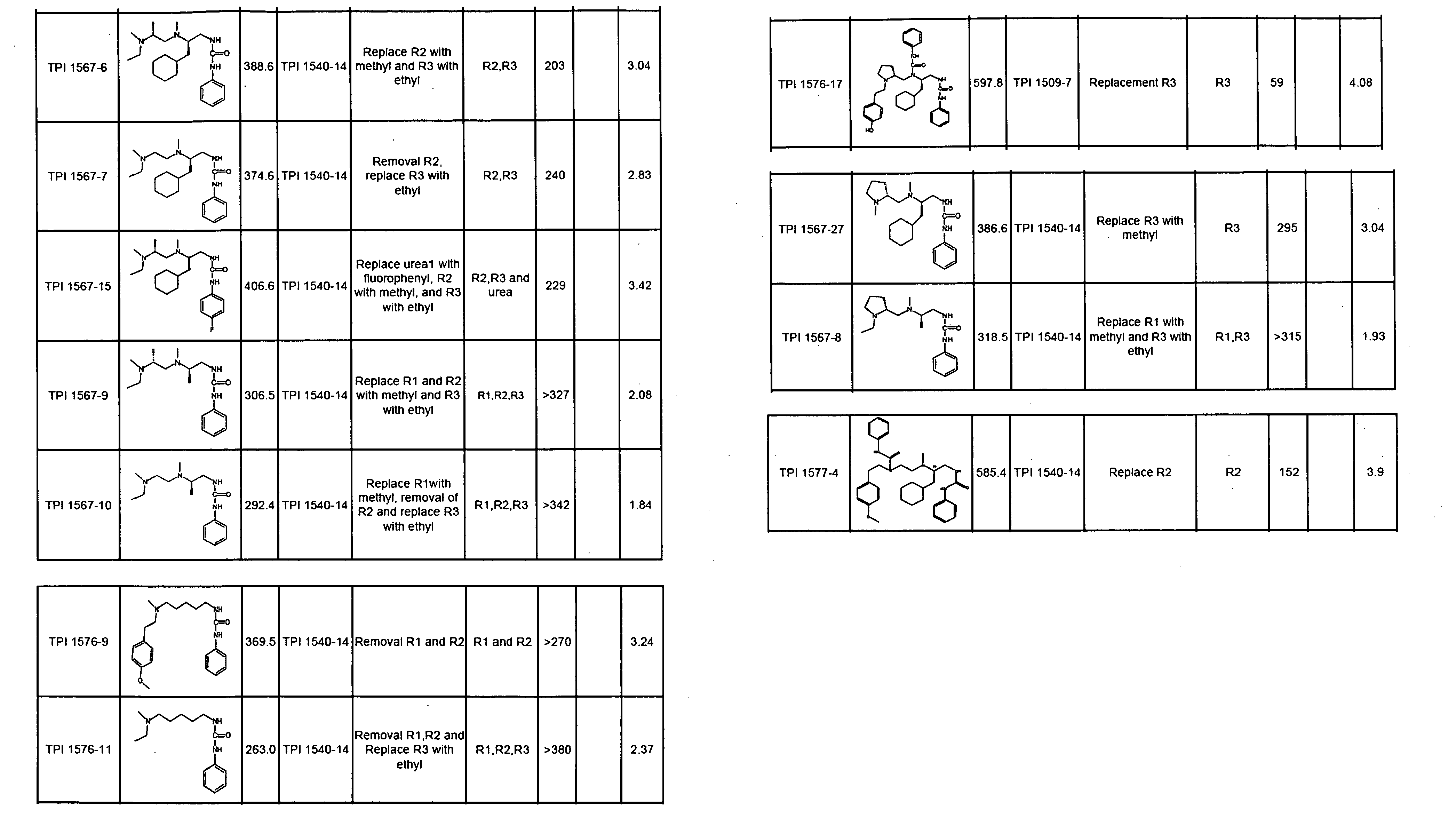
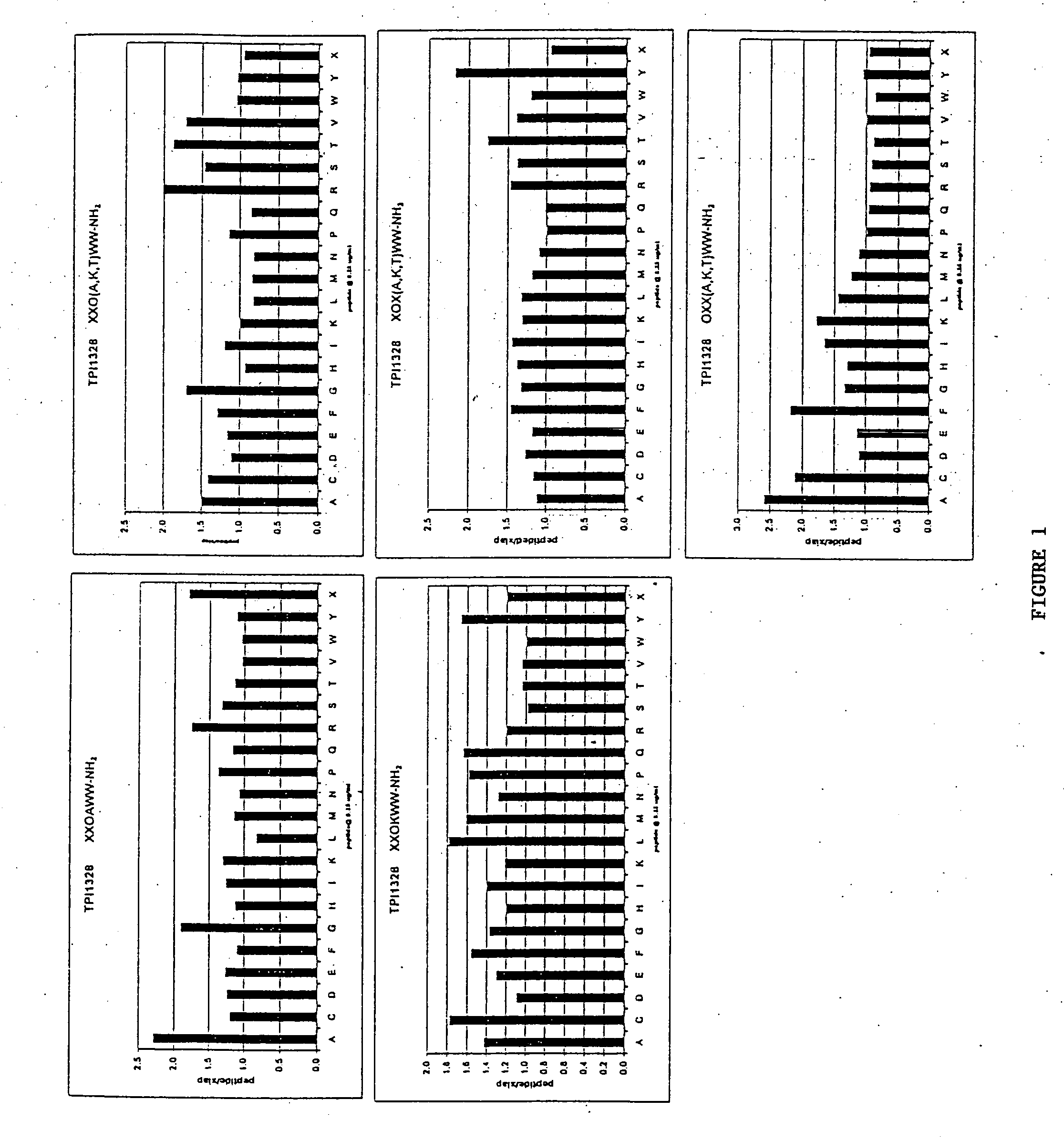
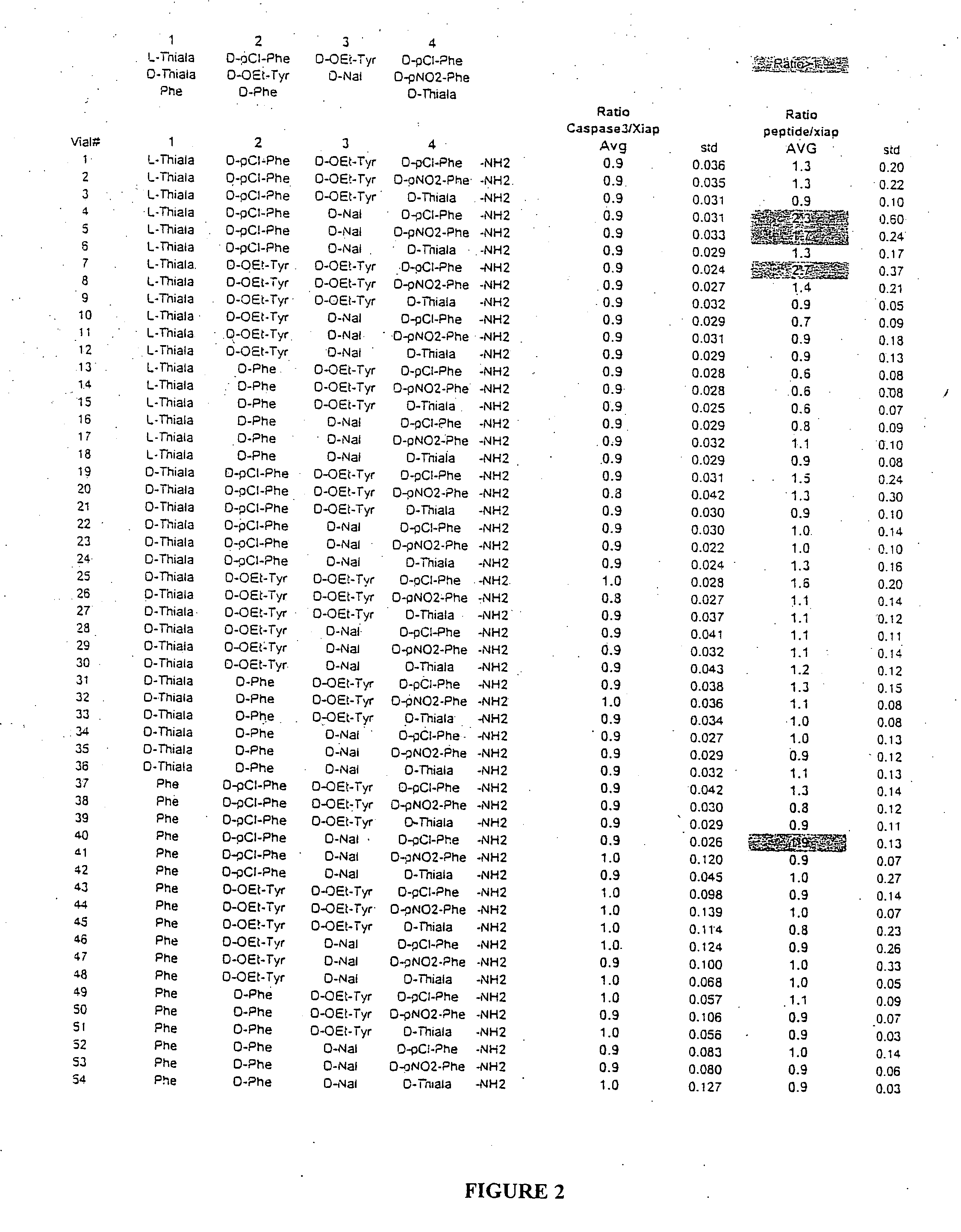
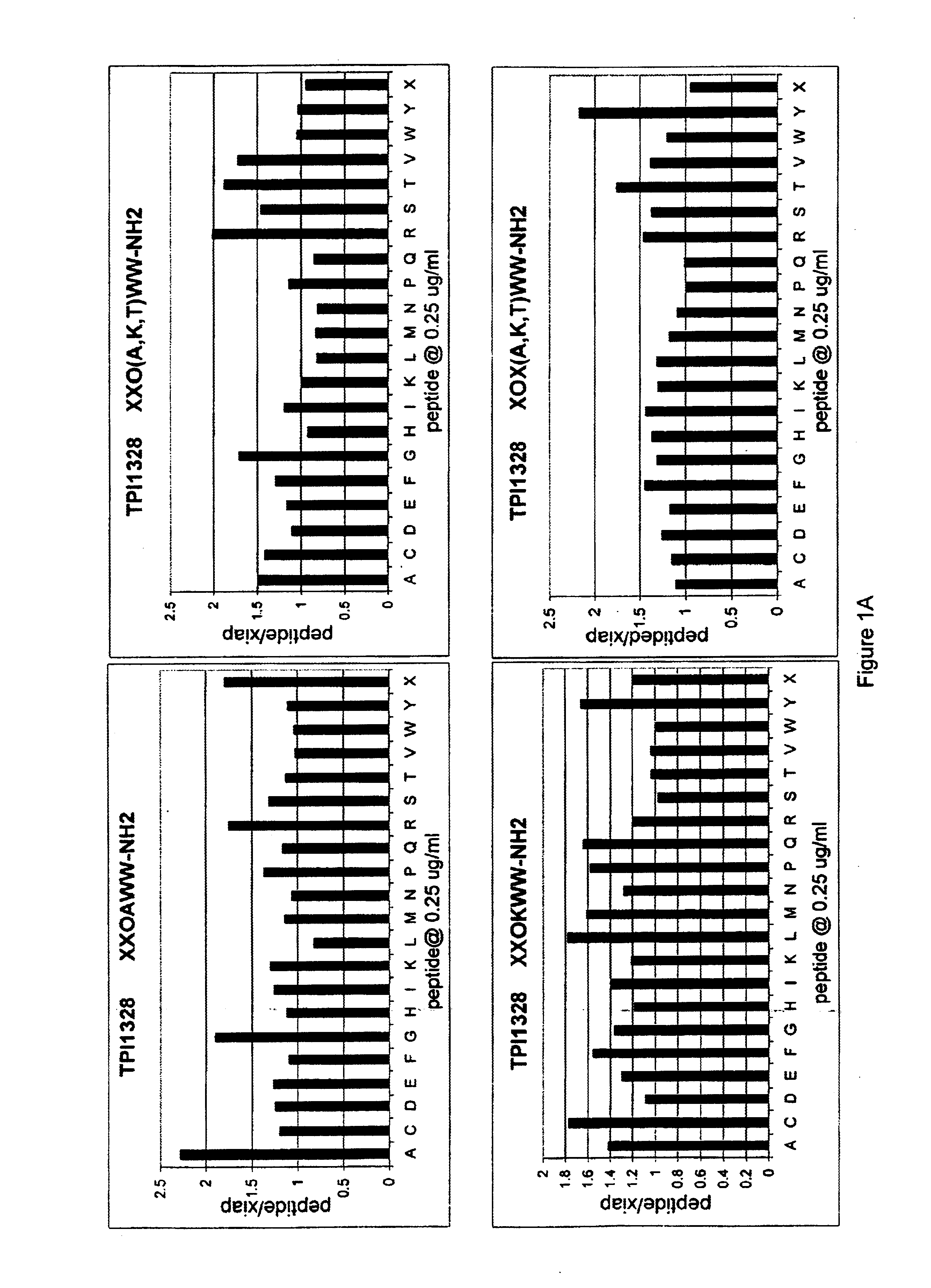
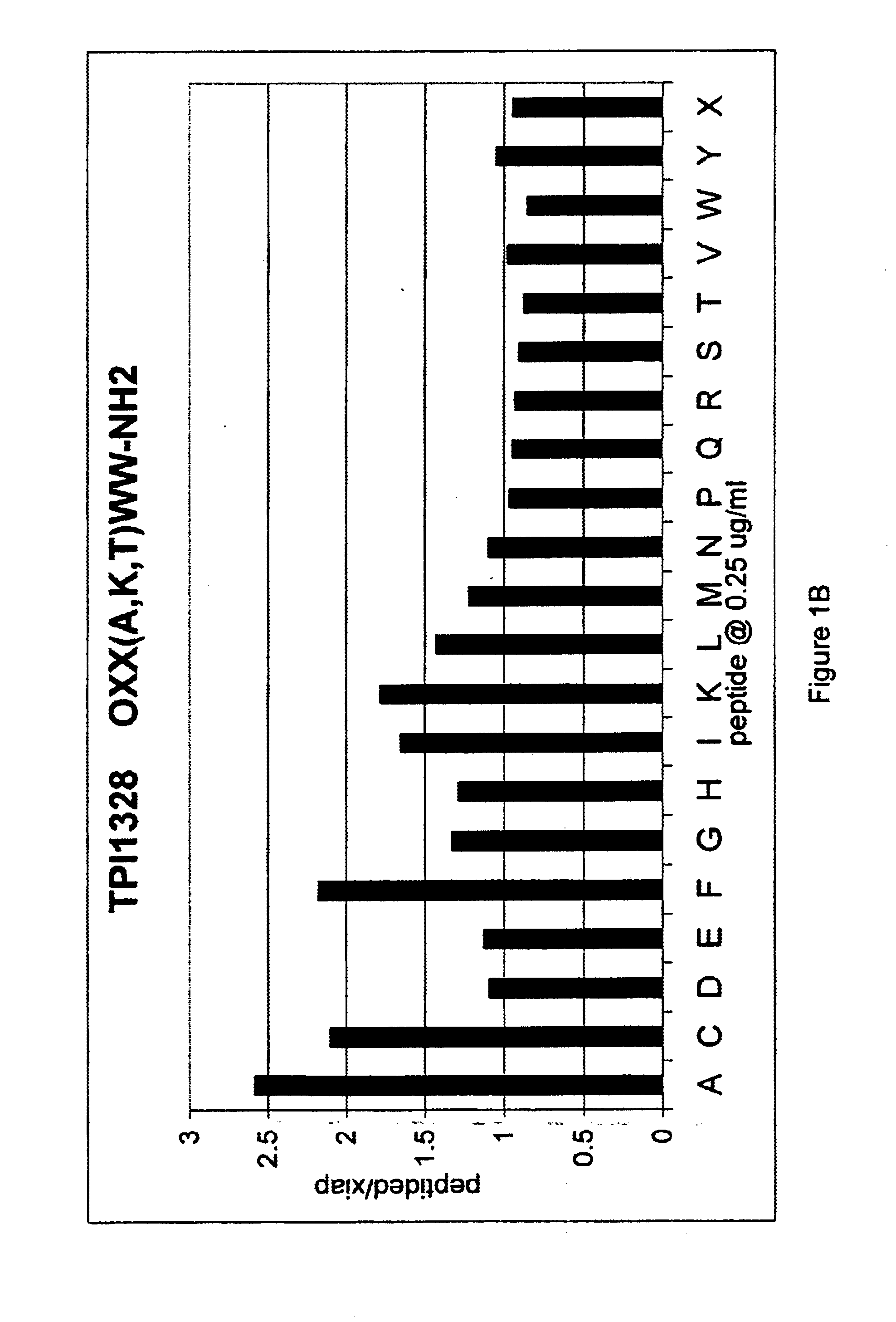
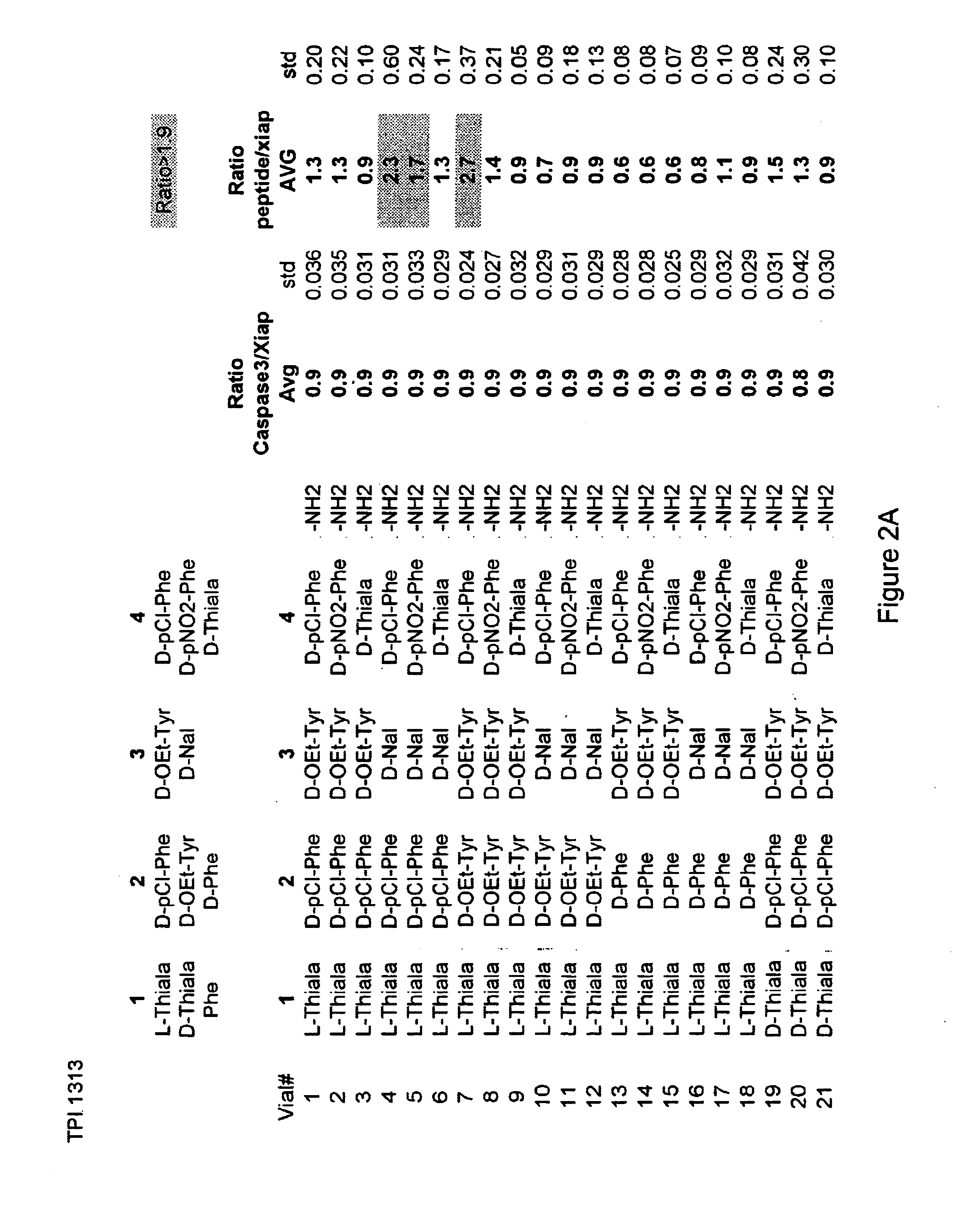
Methods and composition for derepressions of IAP-inhibited caspase
InactiveUS20060258581A1Promote apoptosisReduce severityCompound screeningApoptosis detectionDerepressionCaspase
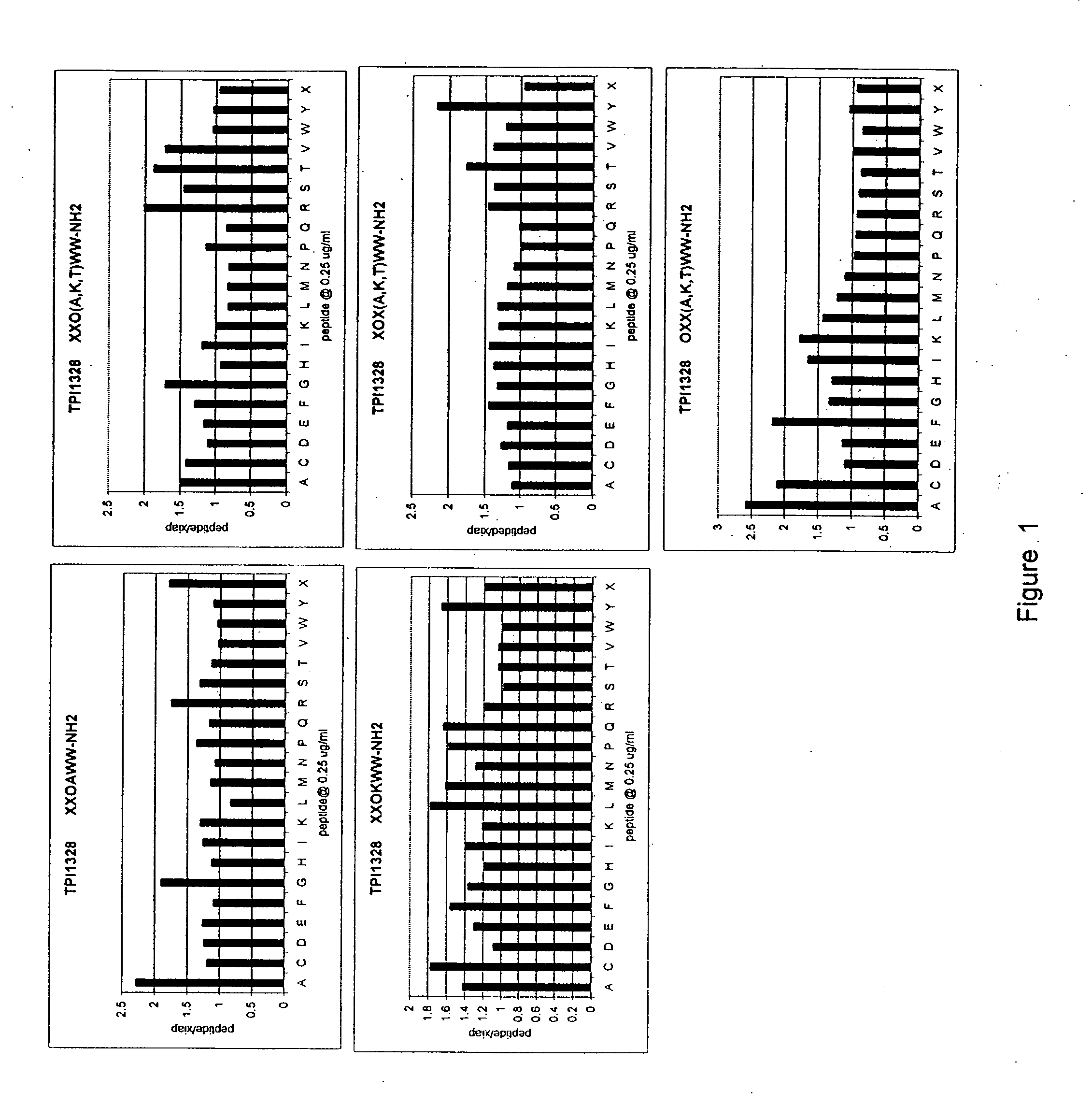
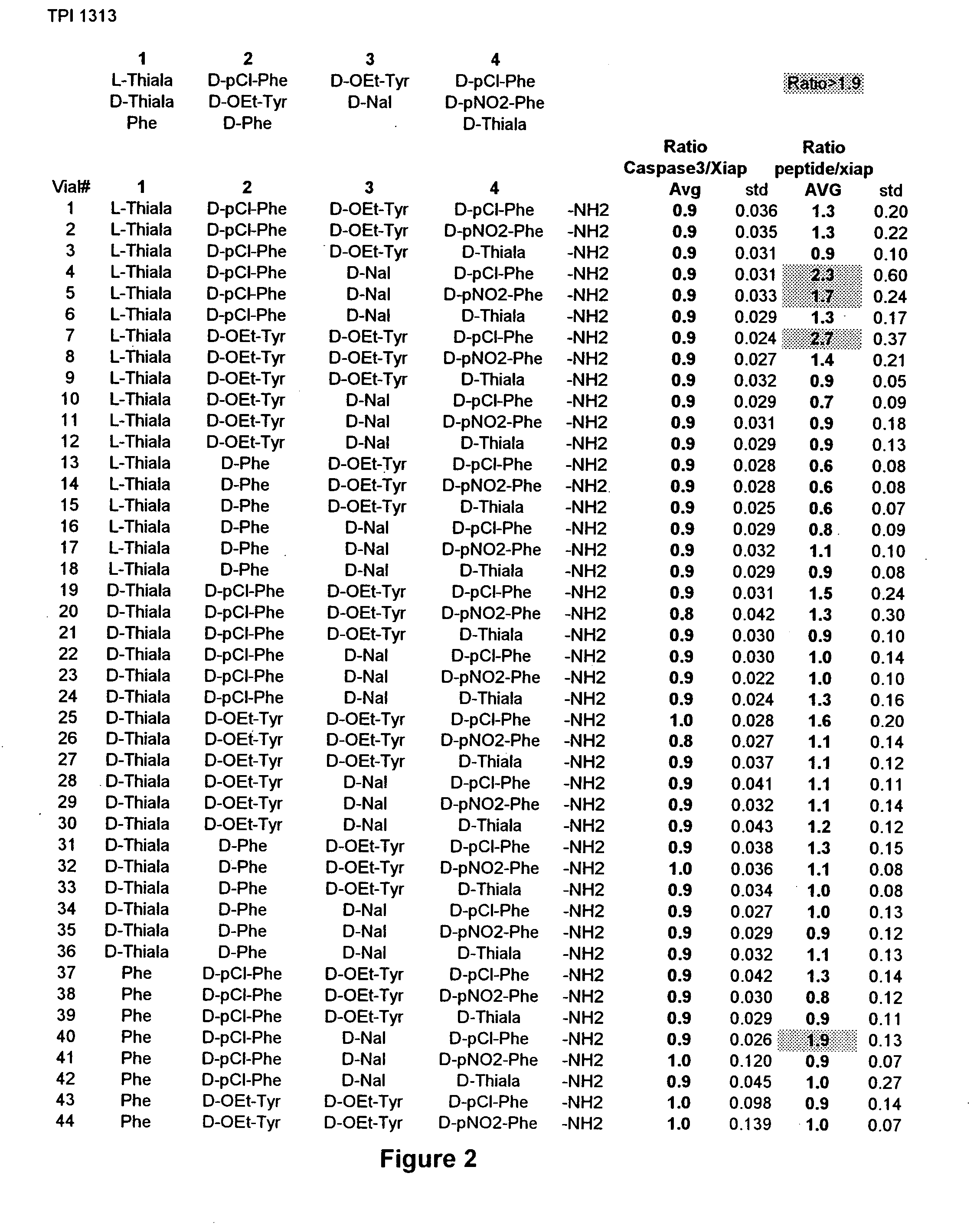
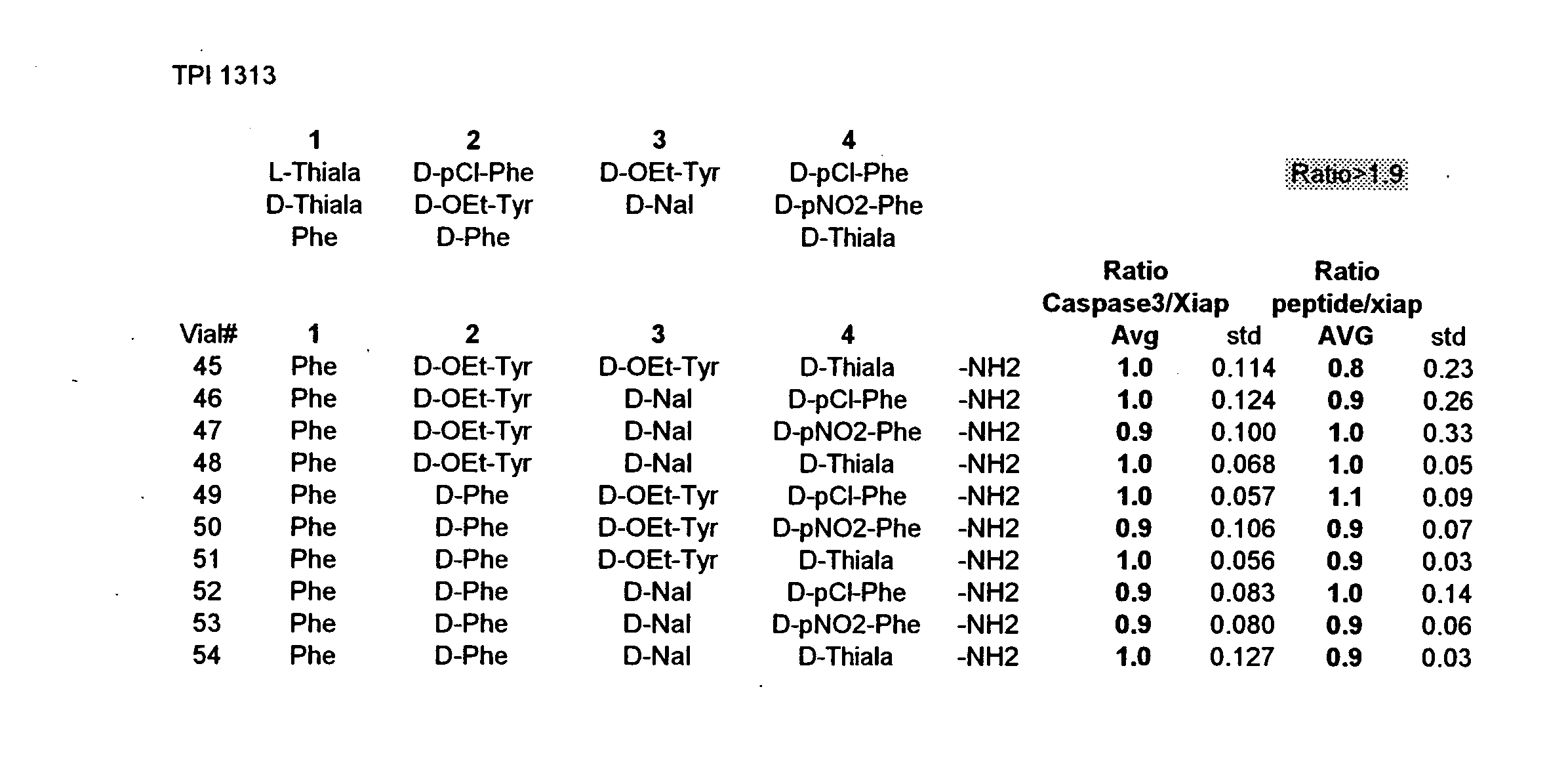
The invention provides isolated agents having a core peptide selected from the group consisting of Core peptides 5 through 39 and 42 through 55, wherein the agent derepresses an IAP-inhibited caspase. Also provided is an isolated agent having a core structure selected from any of the structures shown in FIGS. 5, 9, 10, 14B, 21-24 and 48, a core structure selected from the group of TPI 759, TPI 882, TPI 914 or TPI 927; and a core structure from a library selected from TPI 1391, TPI 1349, TPI 1396, TPI 1509, TPI 1540, TPI 1400, TPI 792, TPI 1332, TPI 1567, TPI 1576 and TPI 1577, wherein the agent derepresses an IAP-inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase.
Owner:THE BURNHAM INST
Methods and compositions for derepression of IAP-inhibited caspase
InactiveUS6911426B2Promote apoptosisReduce severityHydrolasesPeptide/protein ingredientsDerepressionApoptosis
The invention provides isolated agents having a core peptide selected from the group consisting of Core peptides 5 through 39 and 42 through 55, wherein the agent derepresses an IAP-inhibited caspase. Also provided is an isolated agent having a core structure selected from any of the structures shown in FIGS. 5, 9, 10, 14B, and 21-24 wherein said agent derepresses an IAP-inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase. The method consists of contacting an IAP-inhibited caspase with an effective amount of an agent to derepress an IAP-inhibited caspase, the agent having a core motif selected from the group consisting of a core peptide having a sequence set forth in any of Core peptides 4 through 39 and 42 through 55, and a core structure selected from the group consisting of TPI759, TPI882, TPI914 or TPI927. The methods of the invention also can be used for promoting apoptosis in a cell and for reducing the severity of a pathology characterized by reduced levels of apoptosis. Methods for identifying agents that derepress an IAP-inhibited caspase are also provided.
Owner:TORREY PINES INST FOR MOLECULAR STUDIES +1
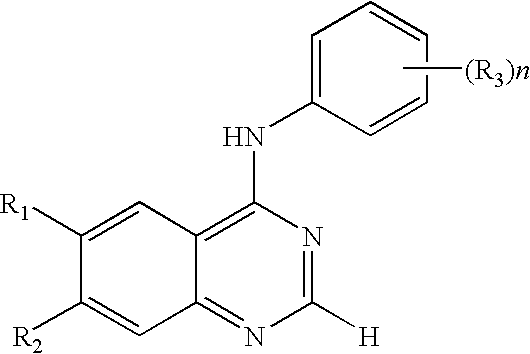
Compounds and therapeutical use thereof
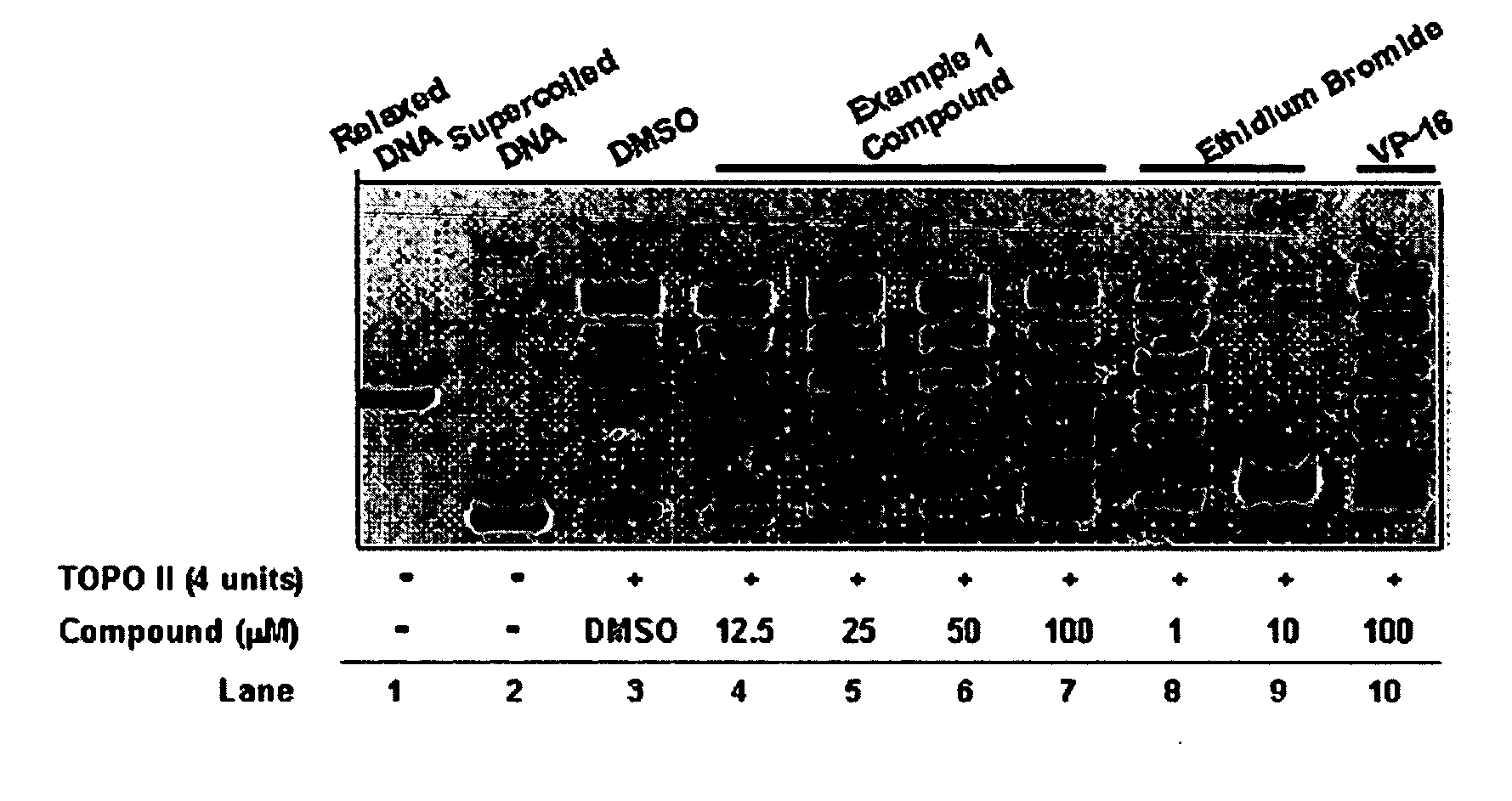
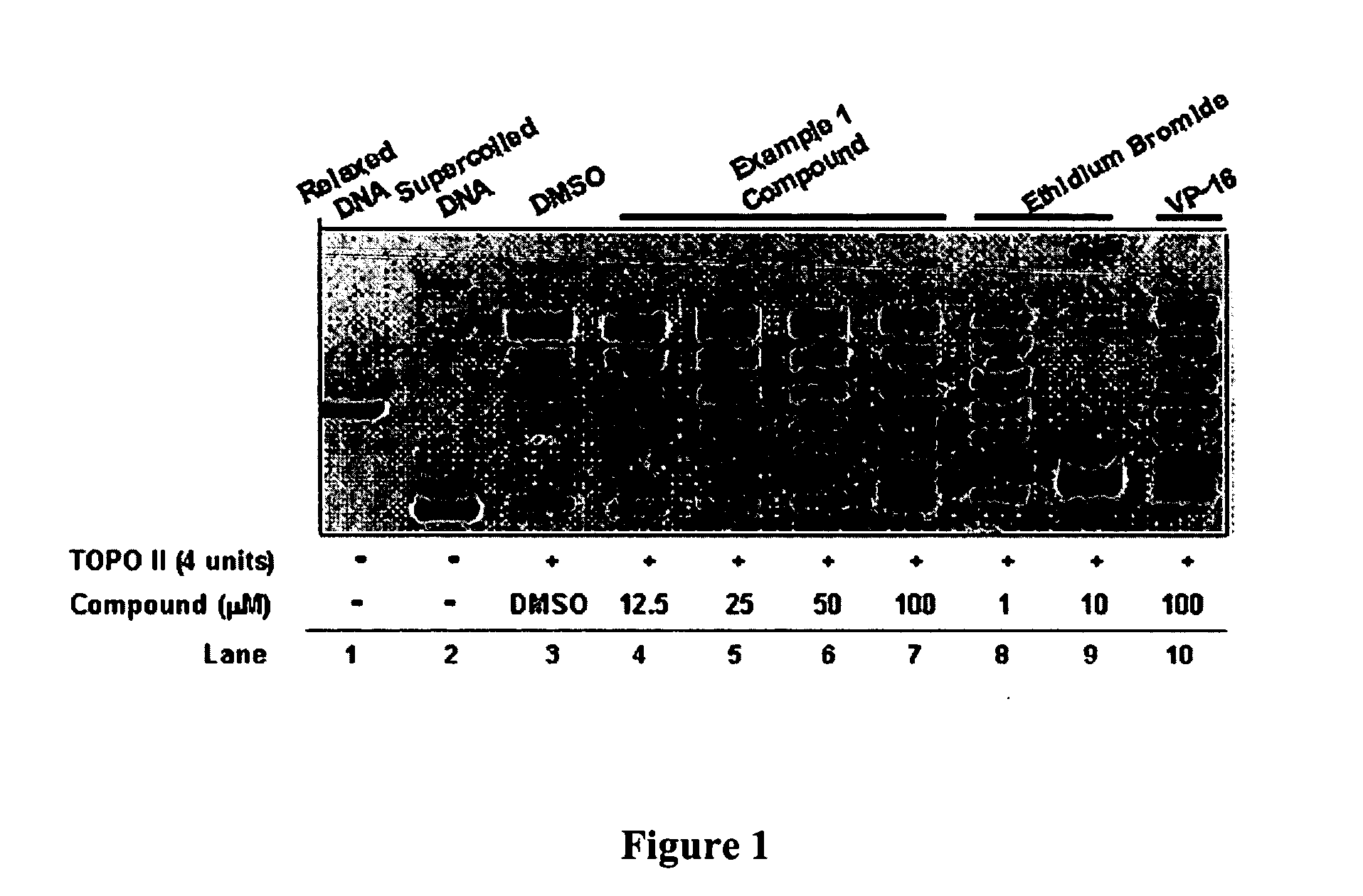
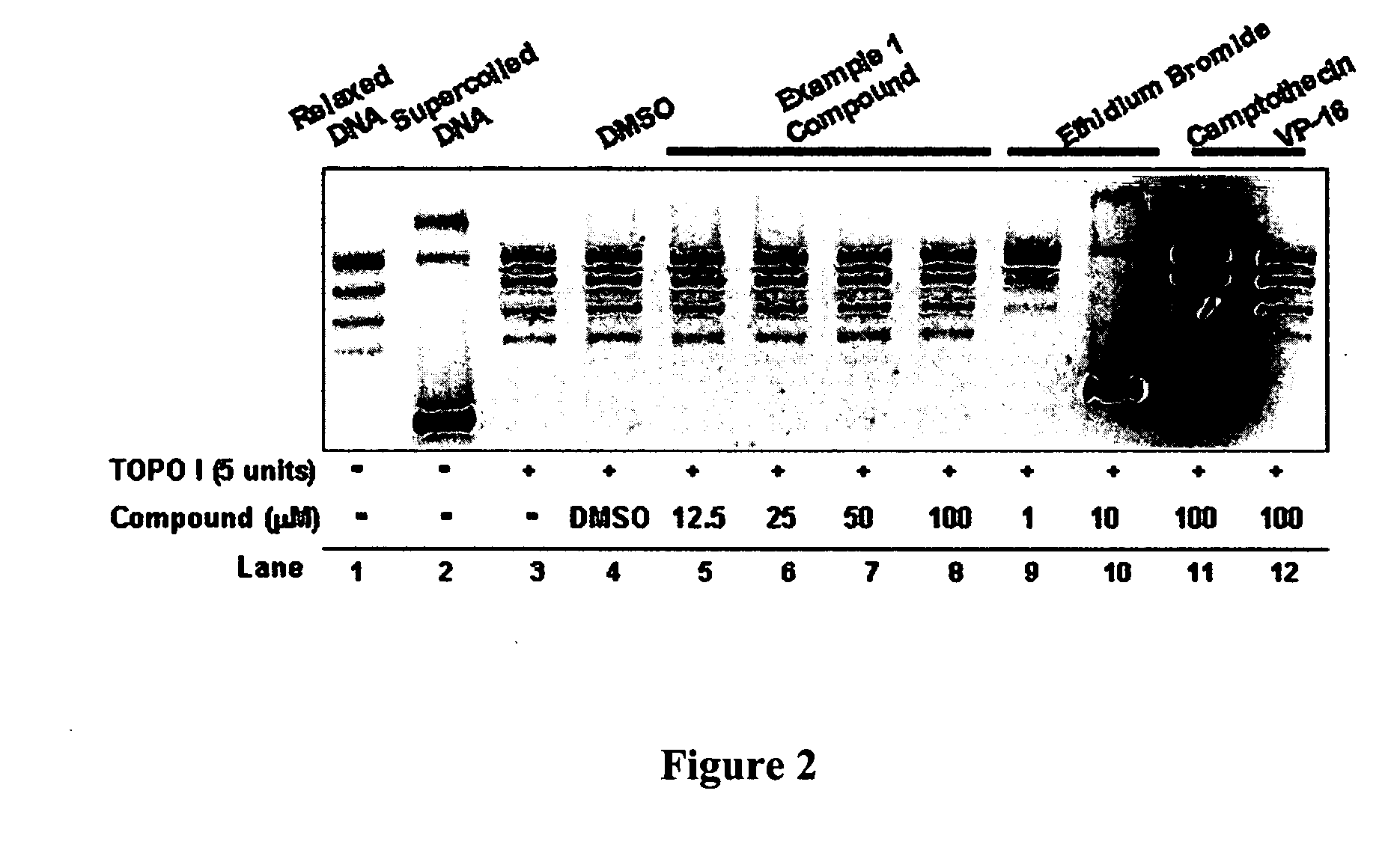
InactiveUS20050137213A1Inhibiting topoisomeraseBiocideOrganic active ingredientsApoptosisAbnormal cell
Disclosed are 4-arylamino-quinazolines and analogs thereof effective as activators of caspases and inducers of apoptosis. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC +1
Bioluminescent protease assay
ActiveUS7148030B2High detection sensitivityLeveling precisionMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementProteinase activityCaspase
Owner:PROMEGA CORP
Methods and compositions for derepression of IAP-inhibited caspase
InactiveUS20070003535A1Promote apoptosisReduce severityPeptide/protein ingredientsImmunological disordersChemical structureDerepression
The invention provides isolated agents having novel chemical structures and possessing superior activity as derepressors of IAP inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase. The invention further provides assay methods employing labeled compounds of the invention, especially fluorescent labeled compounds.
Owner:THE BURNHAM INST
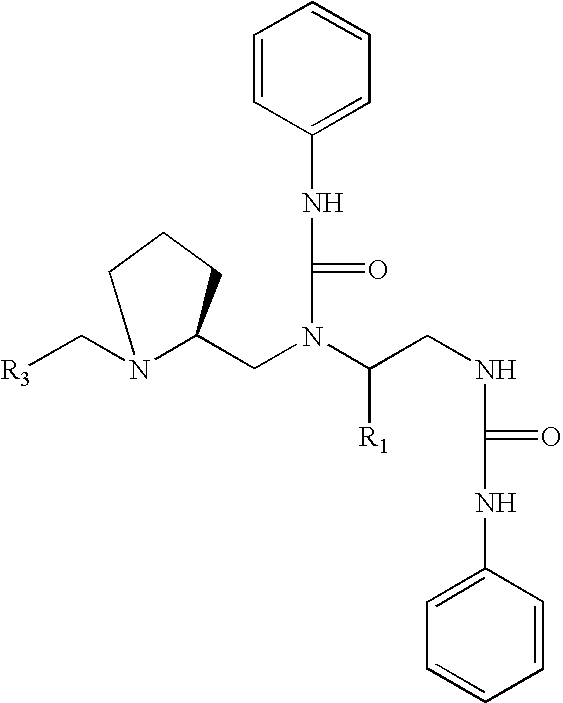
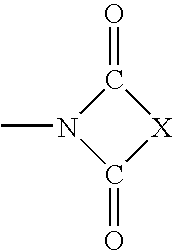
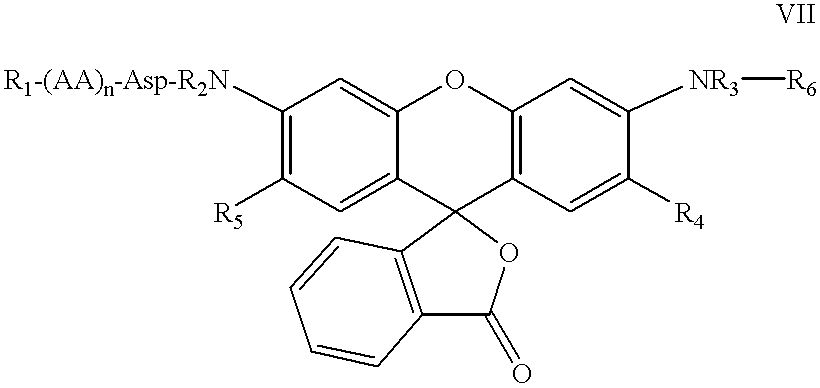
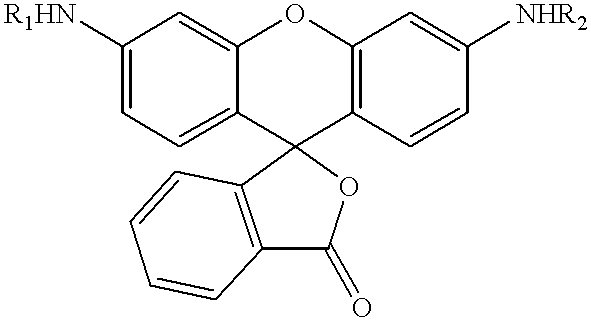
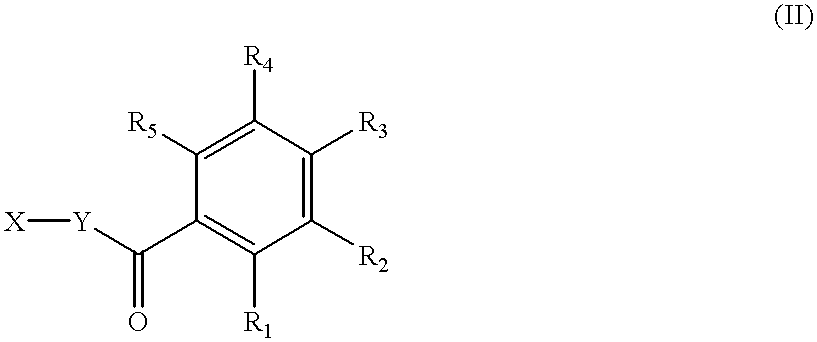
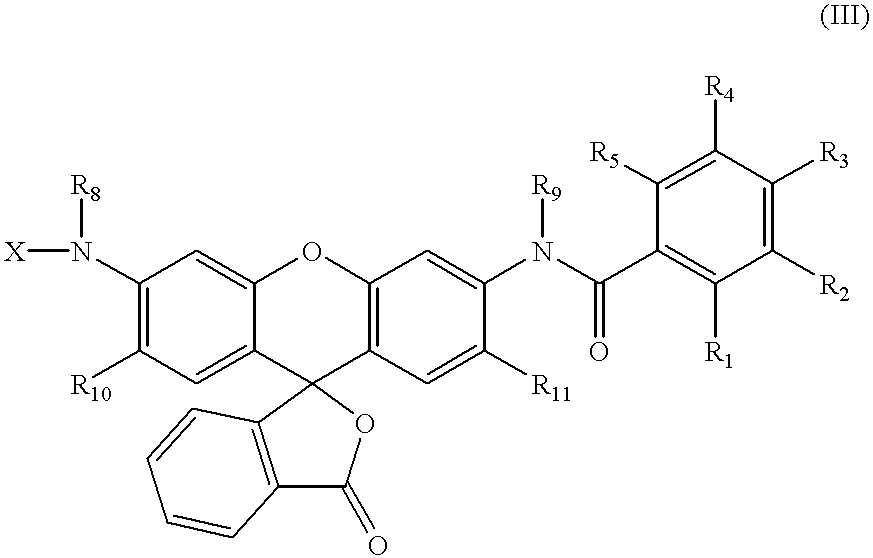
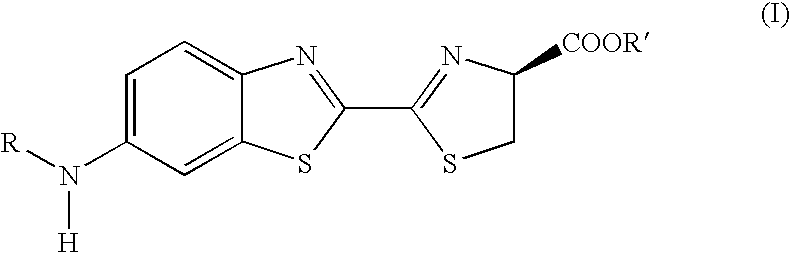
3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof
Disclosed are 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs thereof, represented by the Formula I:wherein Ar1, R2, A, B and D are defined herein. The present invention relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
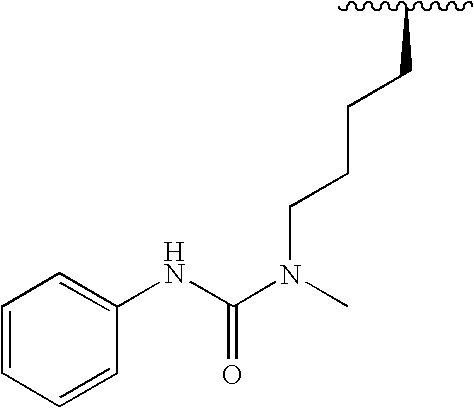
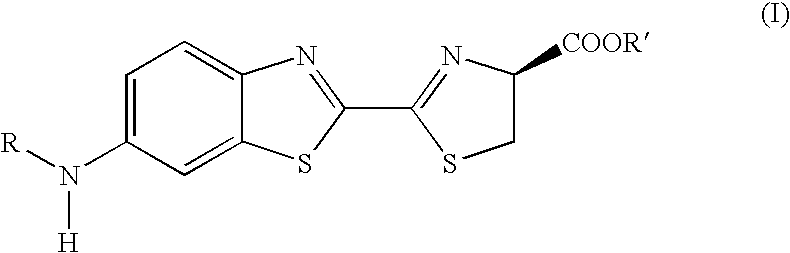
3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof
Disclosed are 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs thereof, represented by the Formula I:wherein Ar1, R2, A, B and D are defined herein. The present invention relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
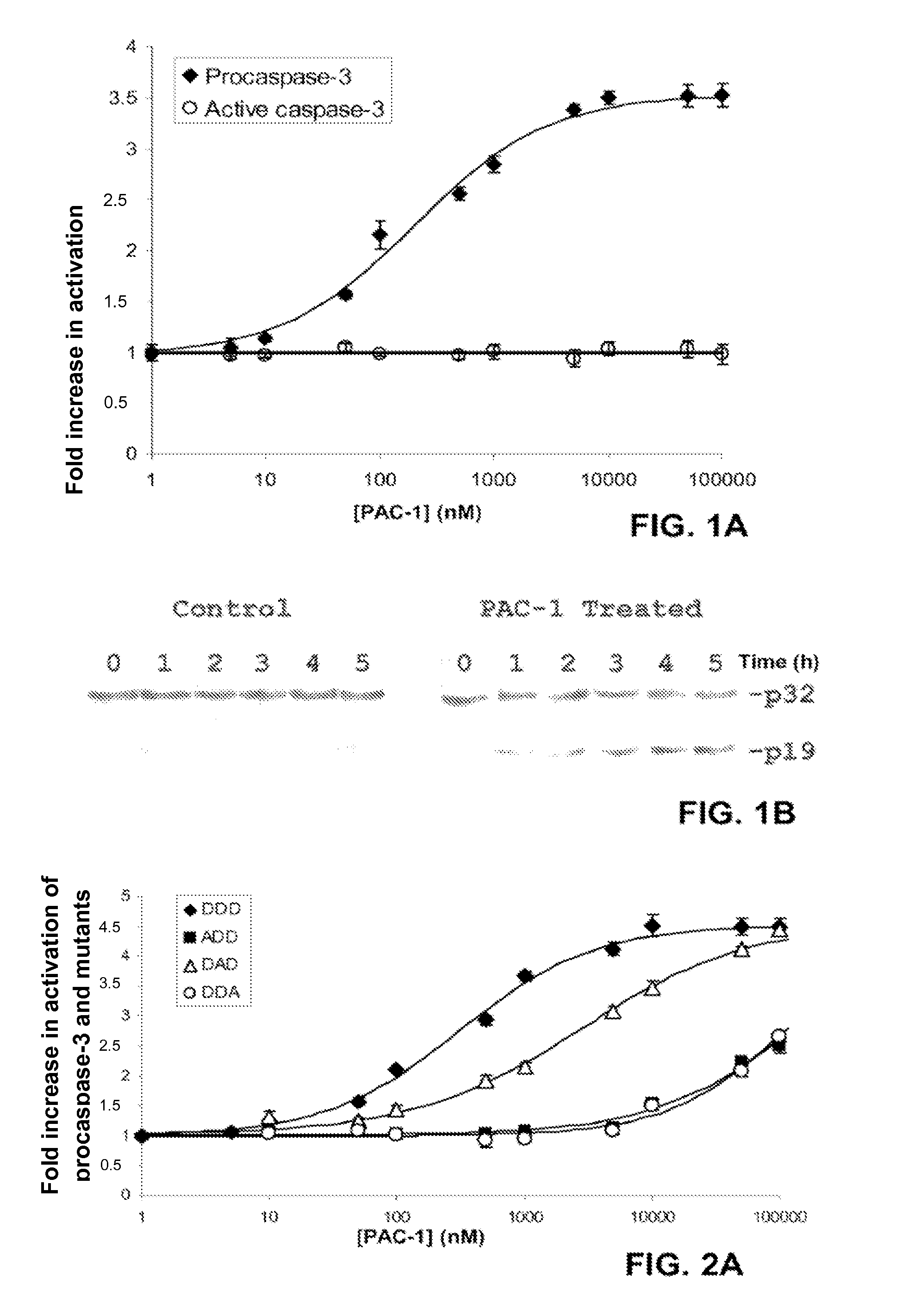
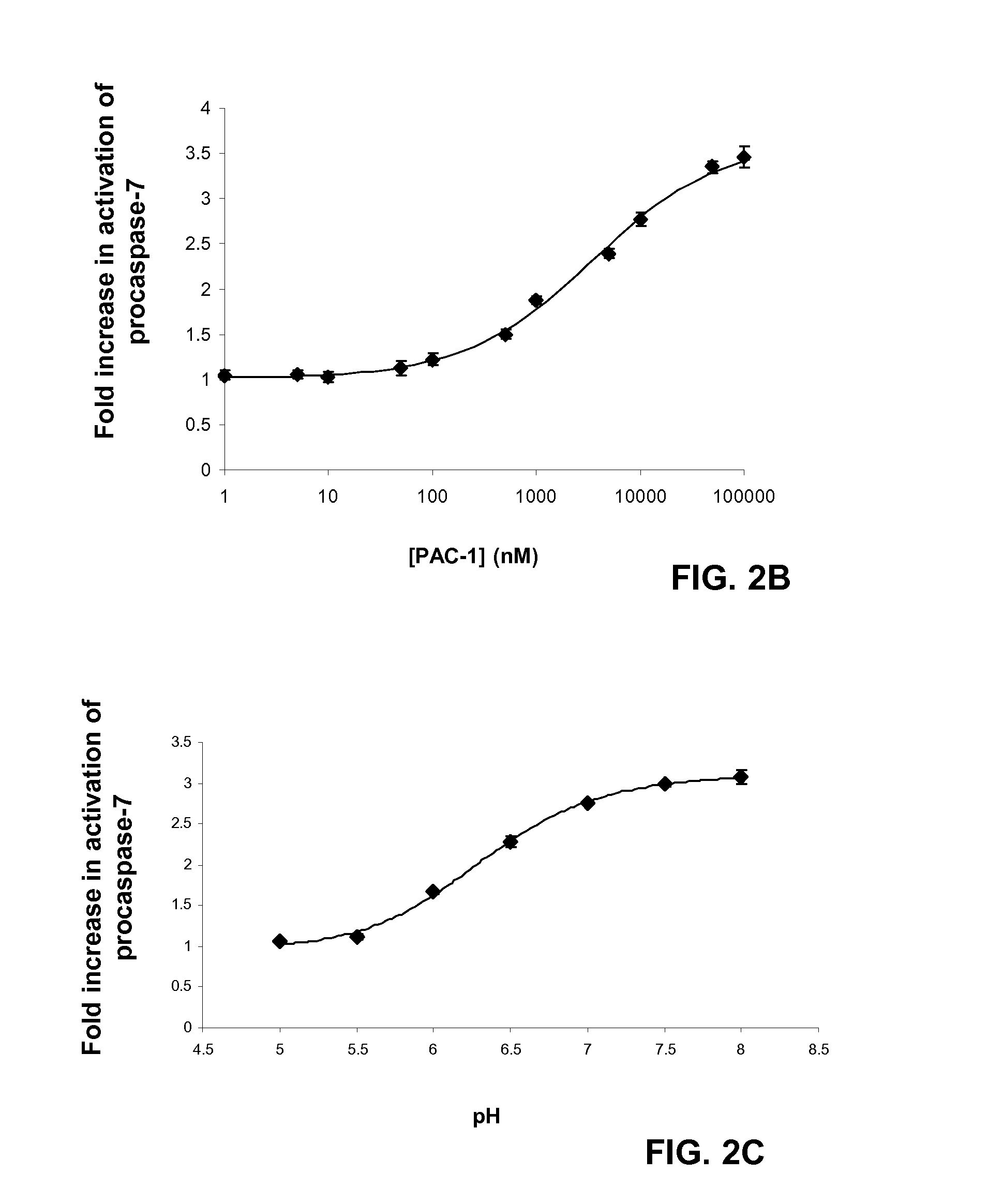
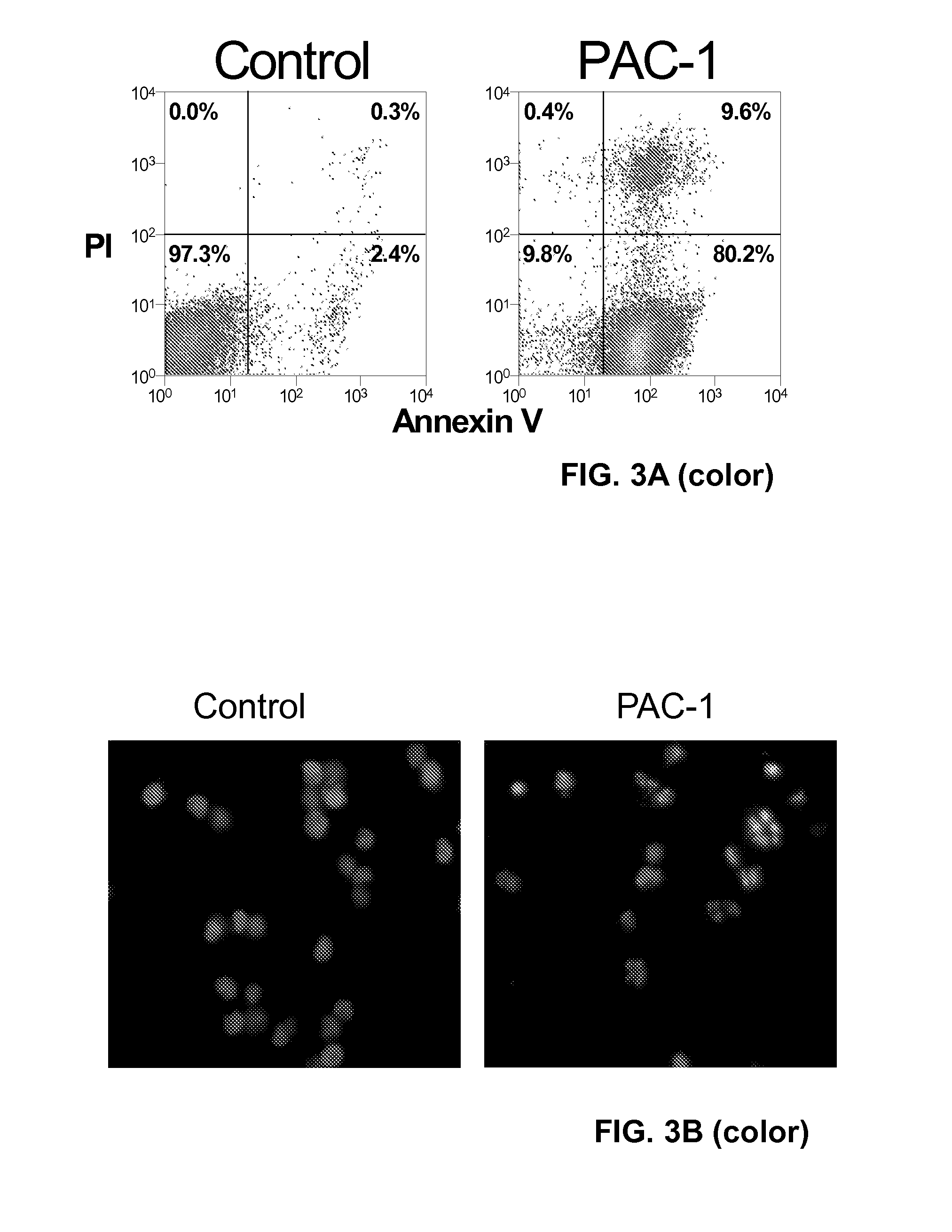
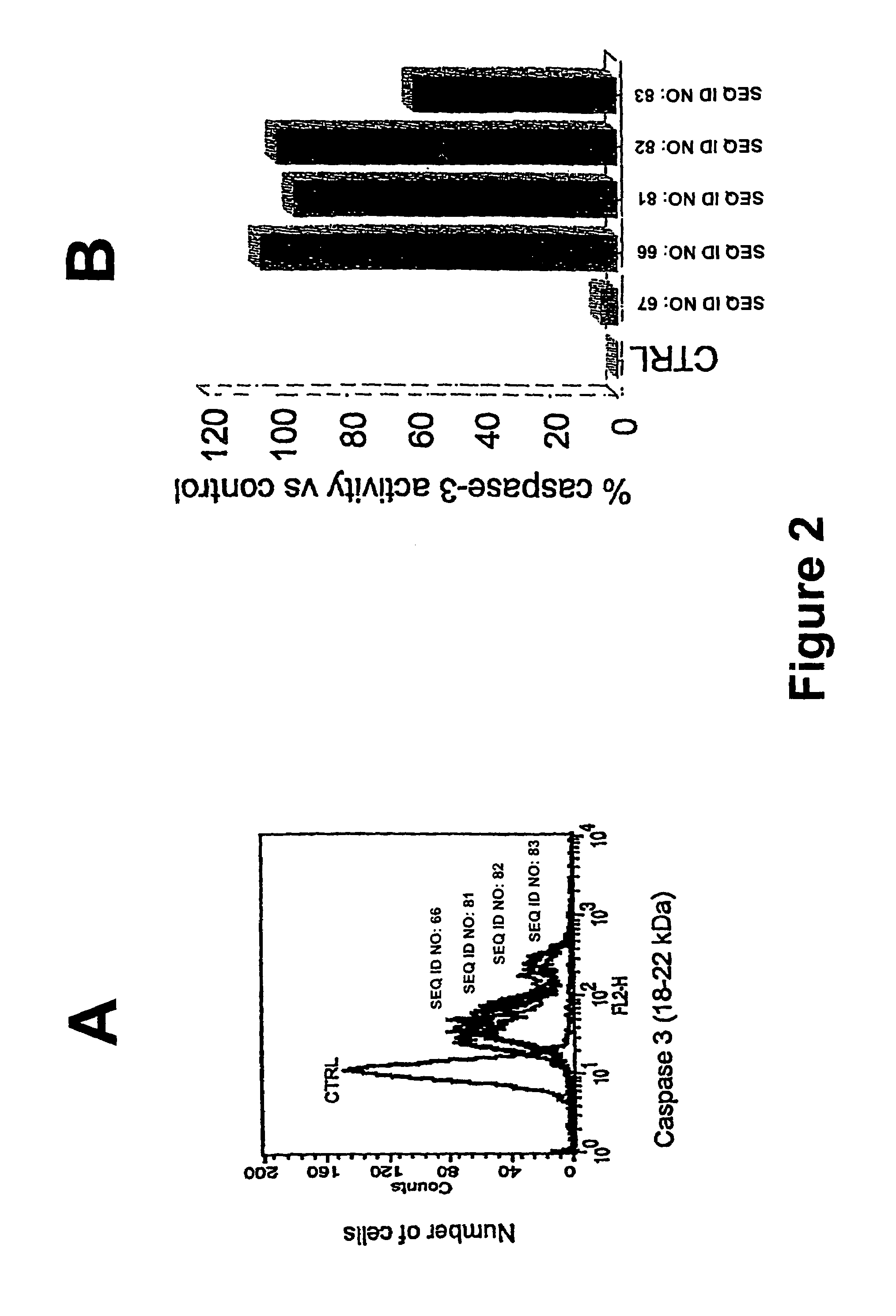
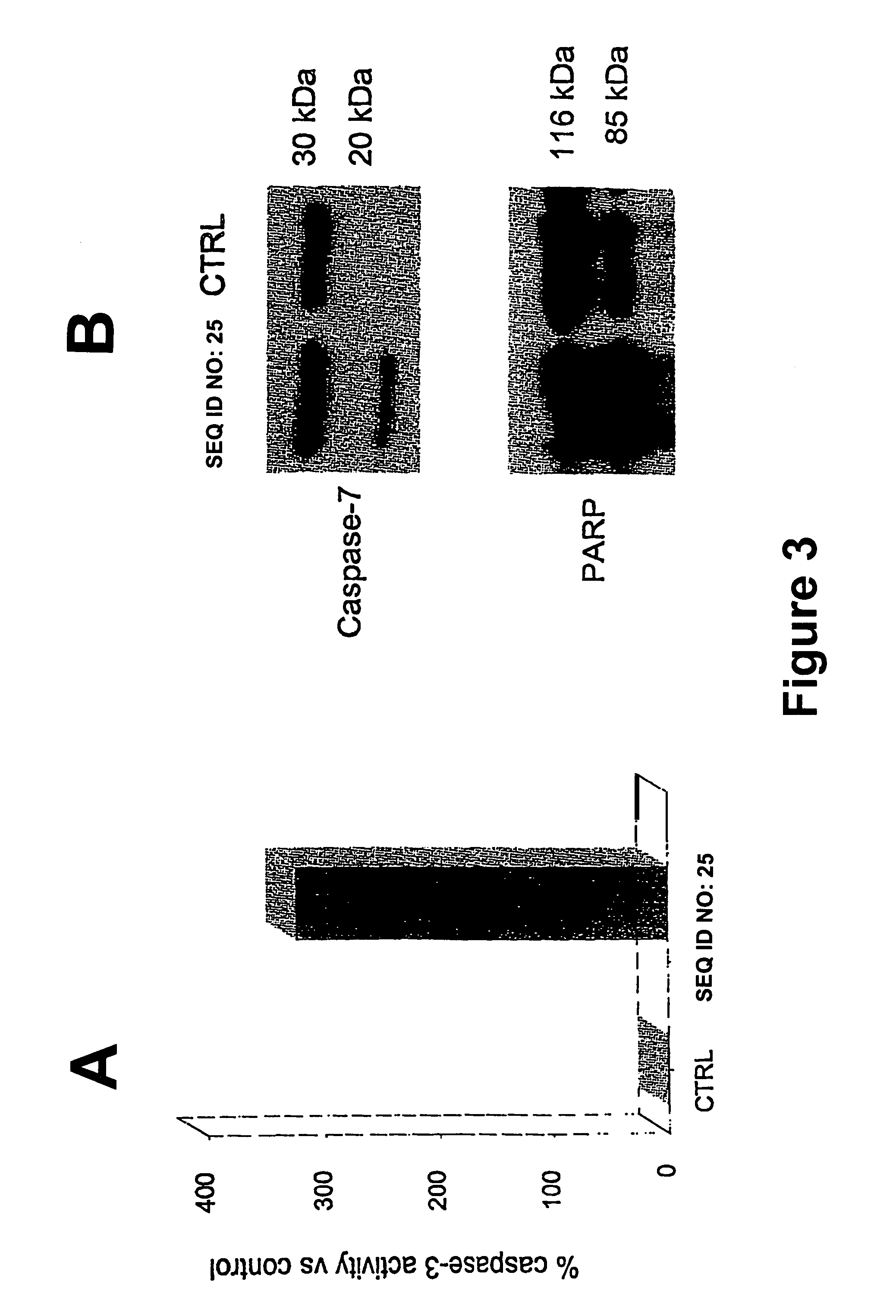
Compositions and Methods Including Cell Death Inducers and Procaspase Activation
ActiveUS20110257398A1Low toxicityEffective compoundOrganic chemistryAntineoplastic agentsCancer cellCaspase
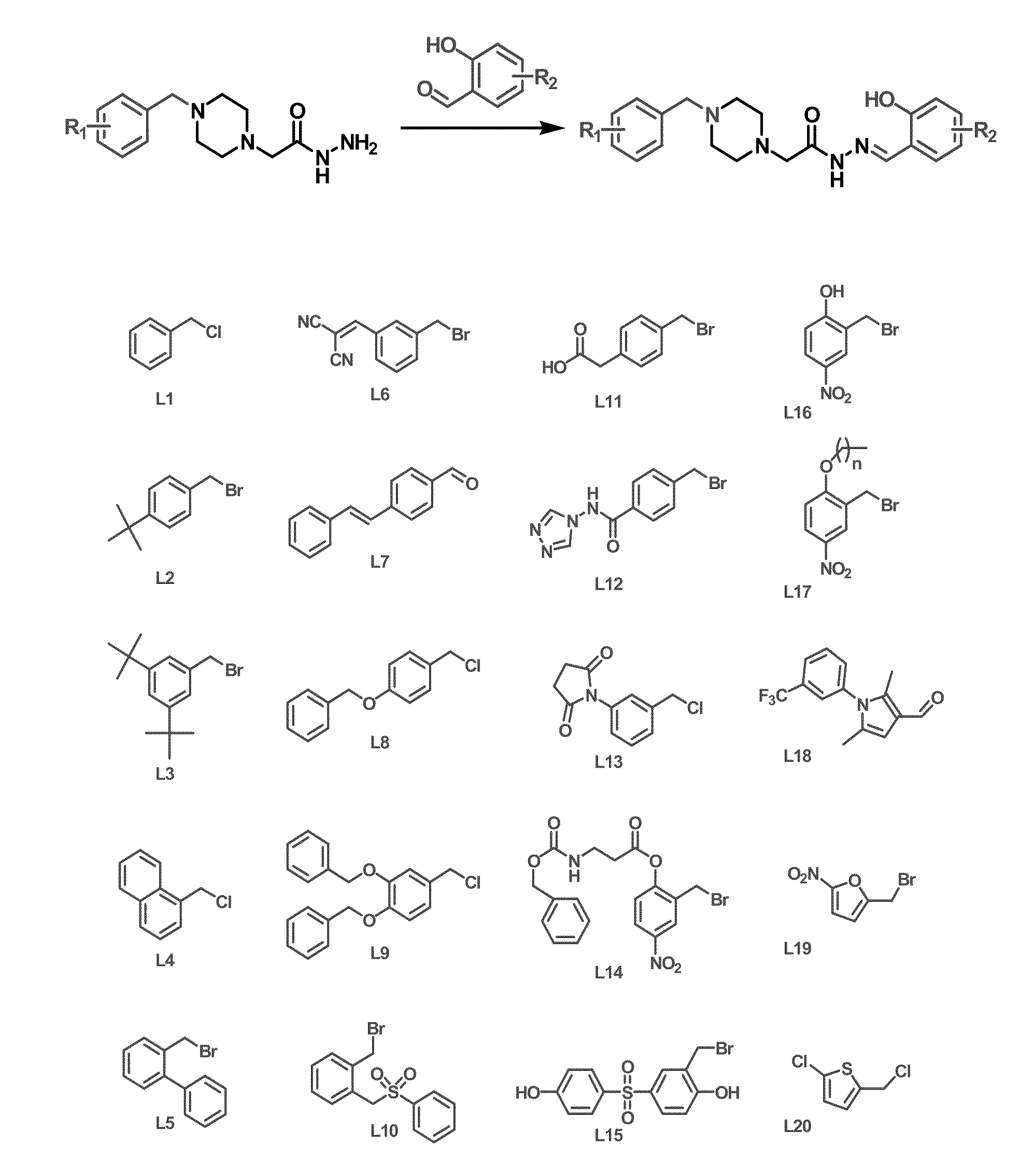
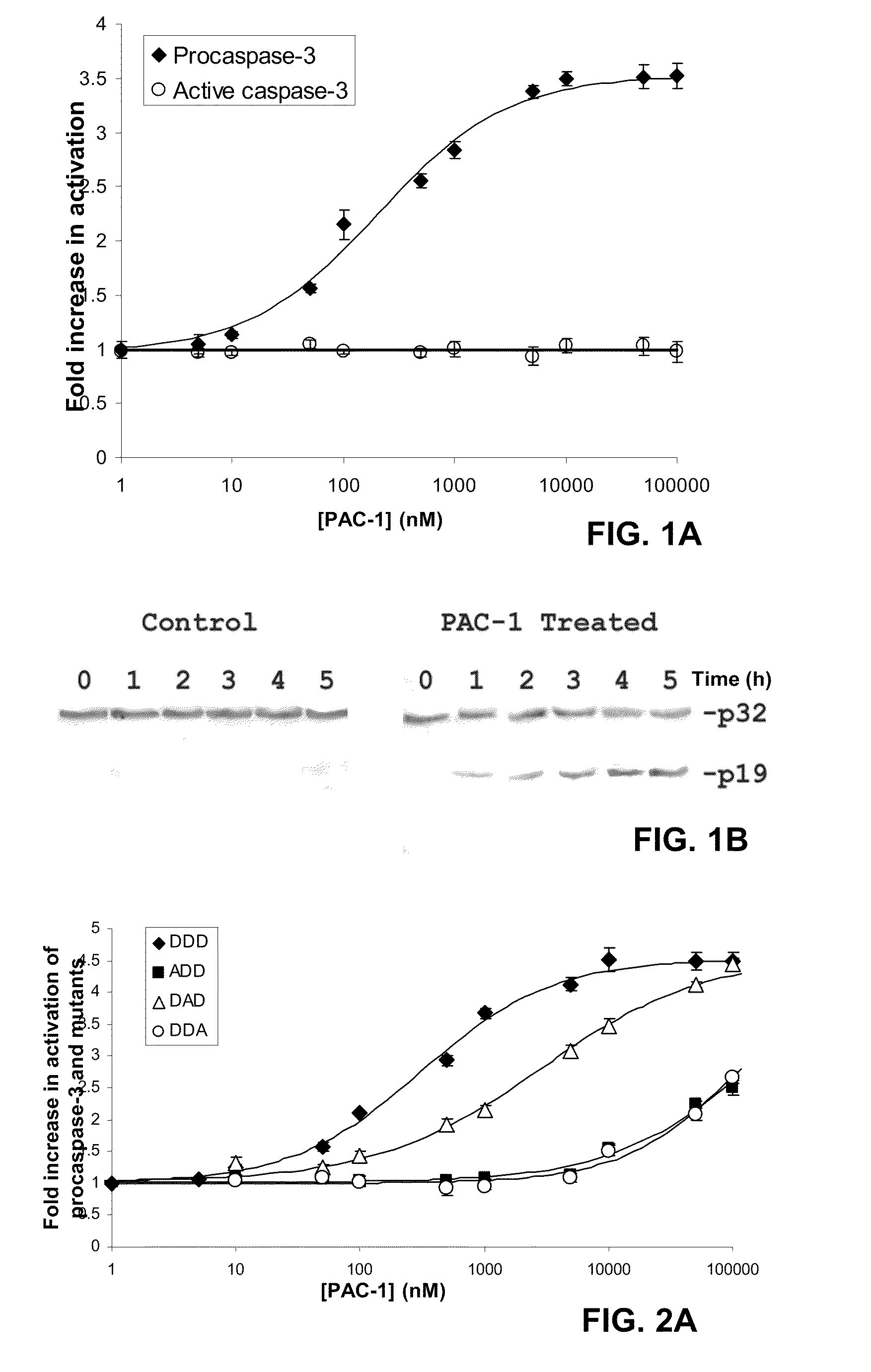
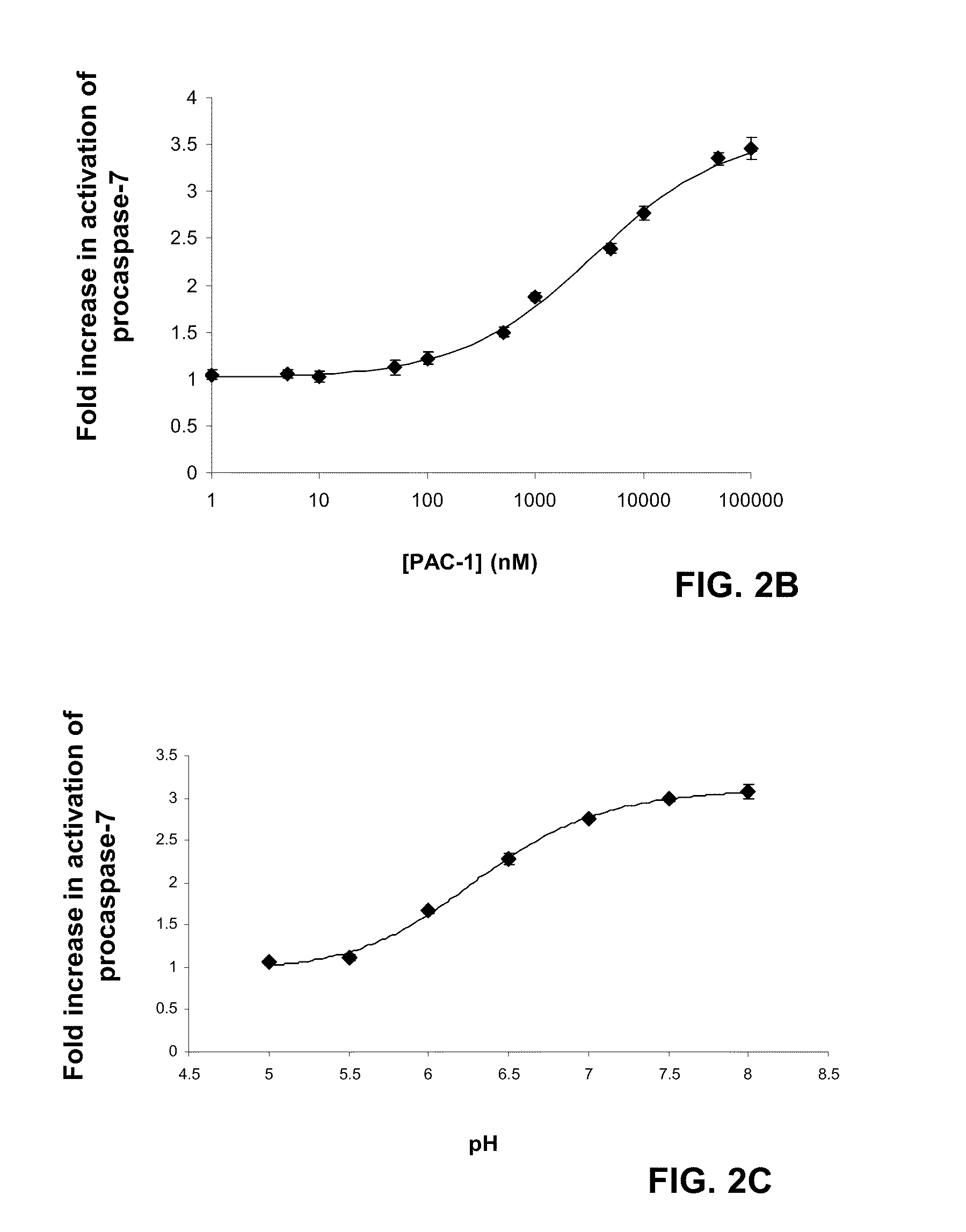
Compositions and methods are disclosed in embodiments relating to induction of cell death such as in cancer cells. Compounds and related methods for synthesis and use thereof, including the use of compounds in therapy for the treatment of cancer and selective induction of apoptosis in cells are disclosed. Compounds are disclosed in connection with modification of procaspases such as procaspase-3. In embodiments, compositions are capable of activation of procaspase-3.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
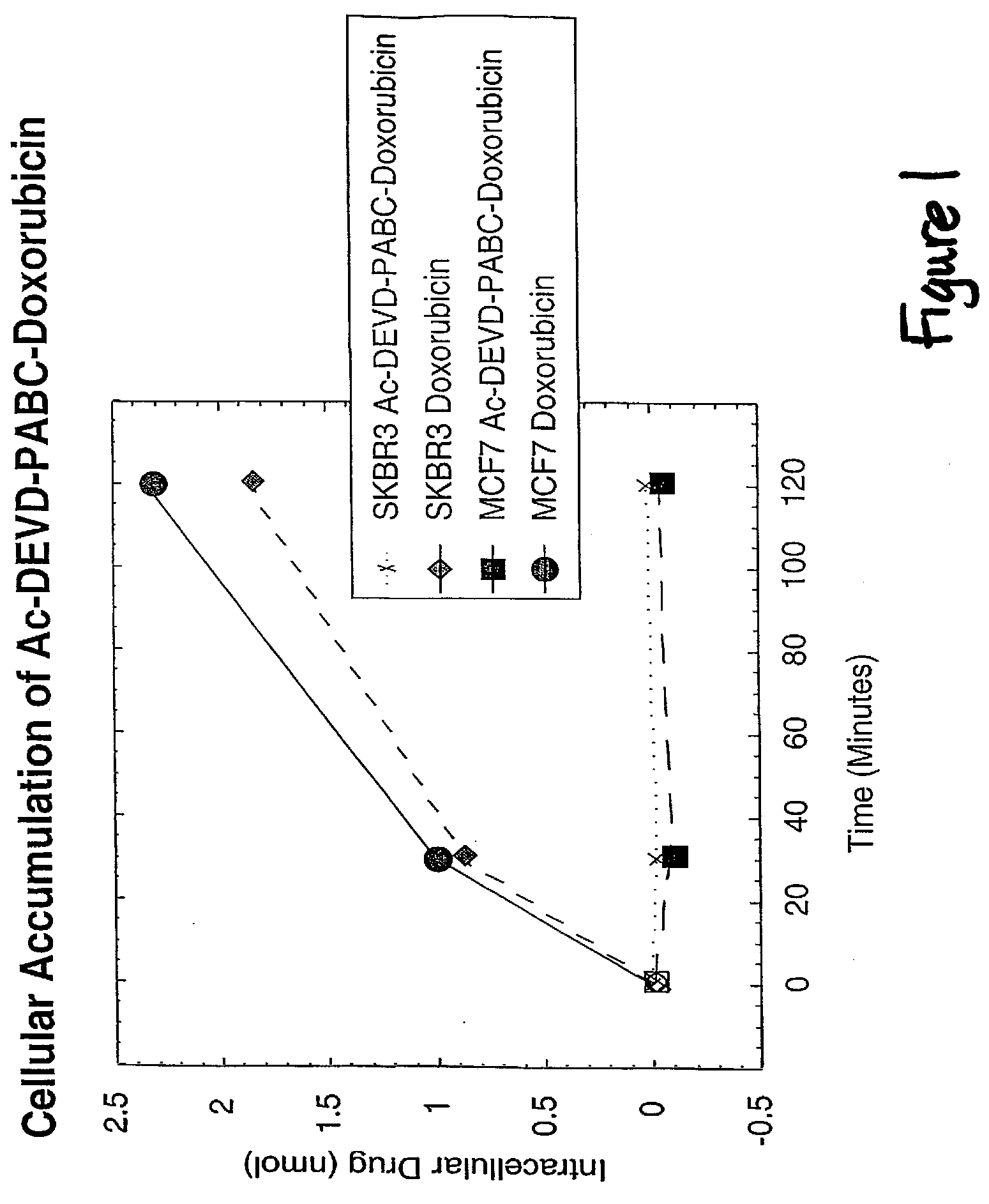
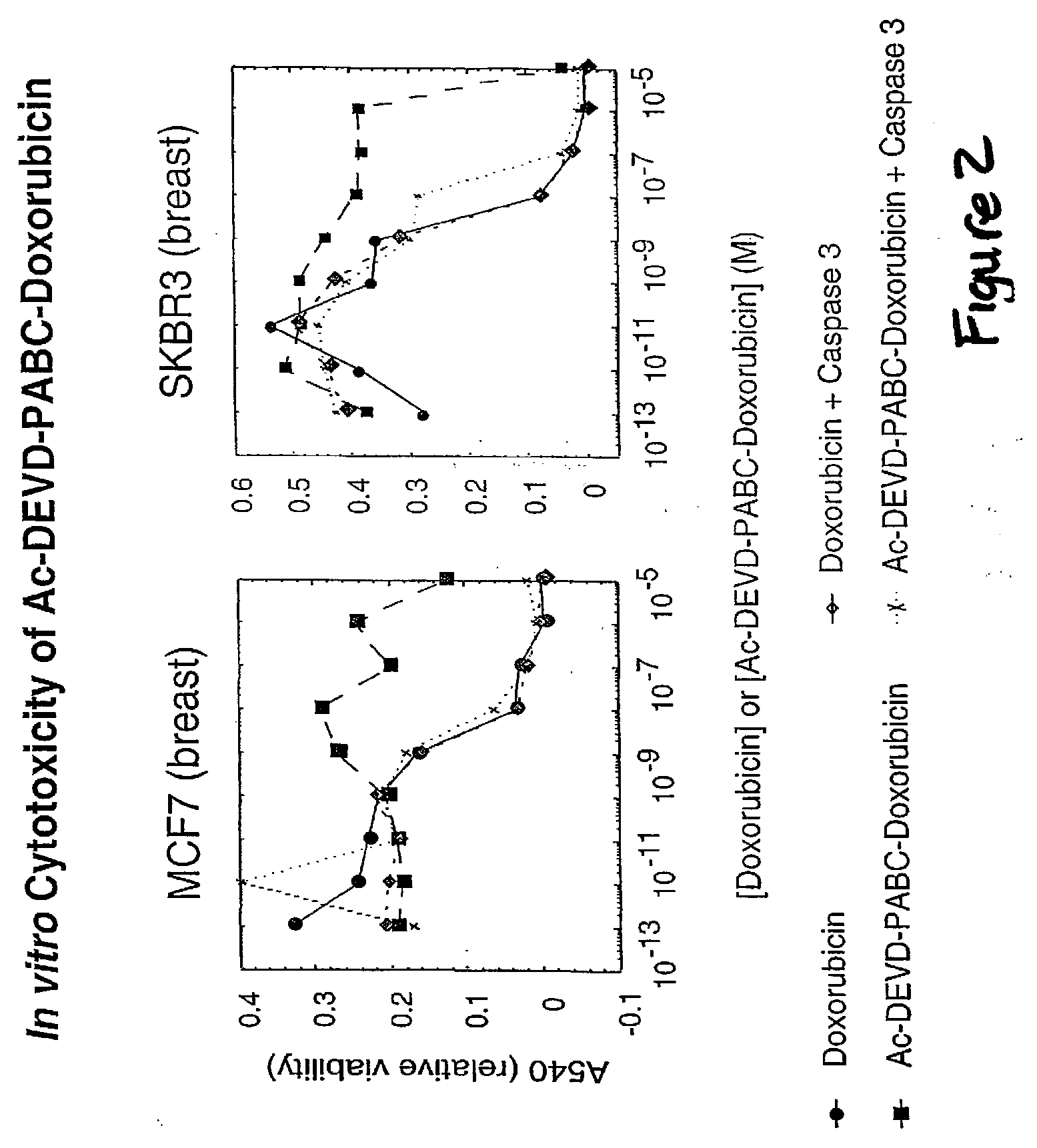
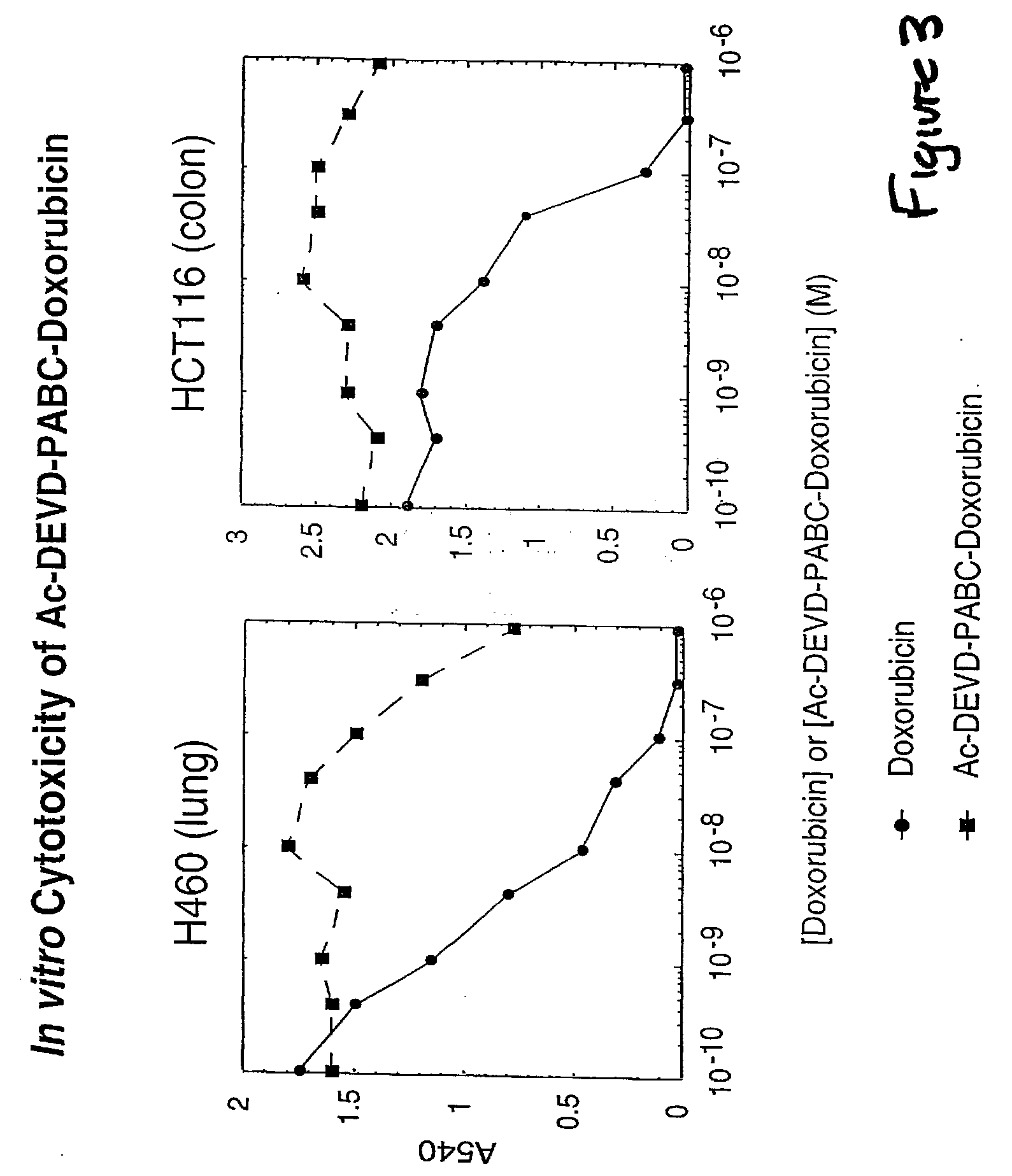
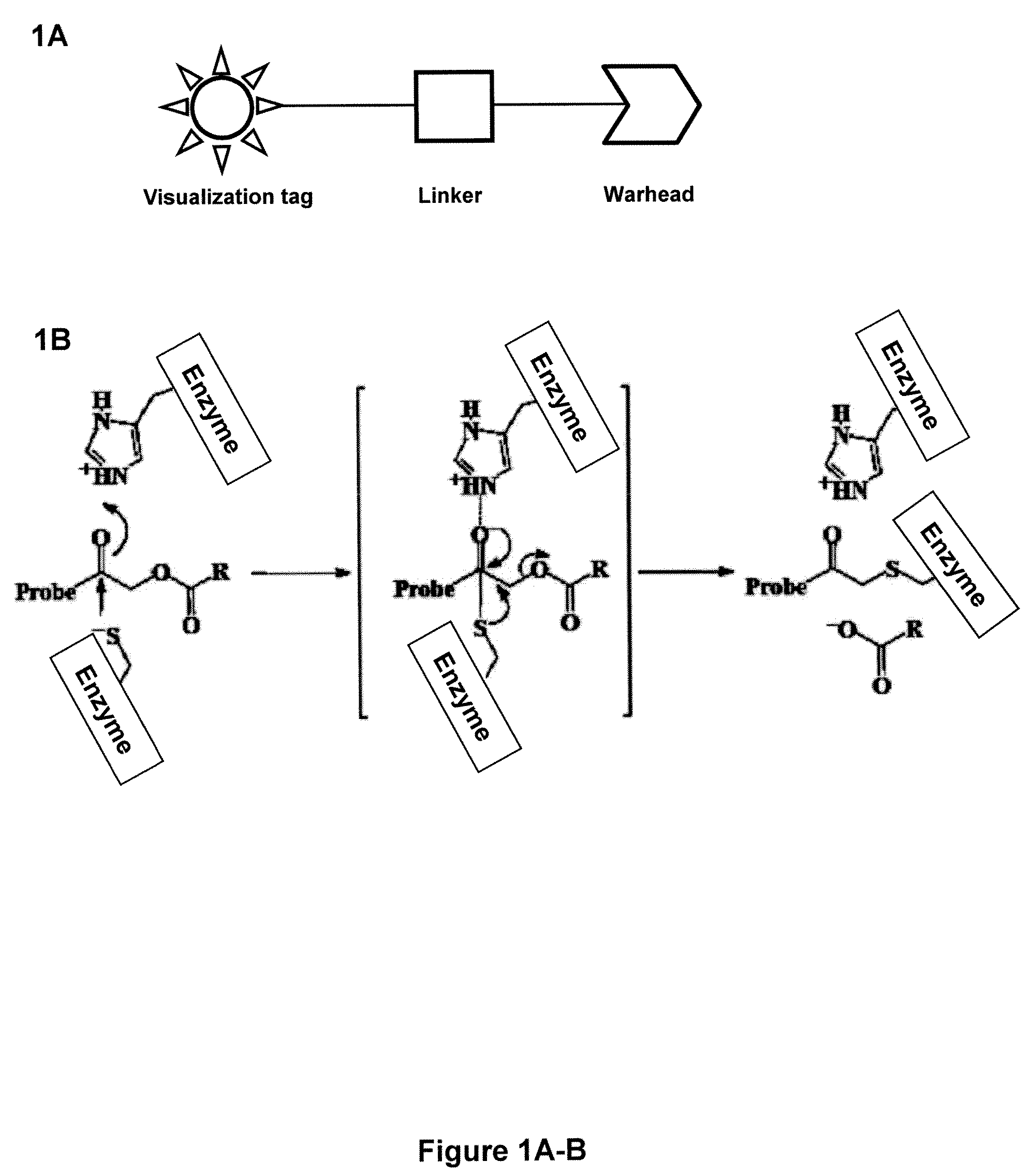
Caspase Activated Prodrugs Therapy
The invention provides novel method for the localized delivery of pharmaceutical agents by the administration of a caspase conjugate that targets a cell type of interest and the additional administration of a pro-agent that is locally converted, in the presence of the caspase, to an active agent. The invention further provides novel tageting agents comprising a caspase as well as novel prodrugs comprising a caspase cleavable prodrug moiety. The invention also provides pharmaceutical compositions as well as methods of treatment comprising the caspase conjugates and prodrugs of the invention
Owner:GENENTECH INC
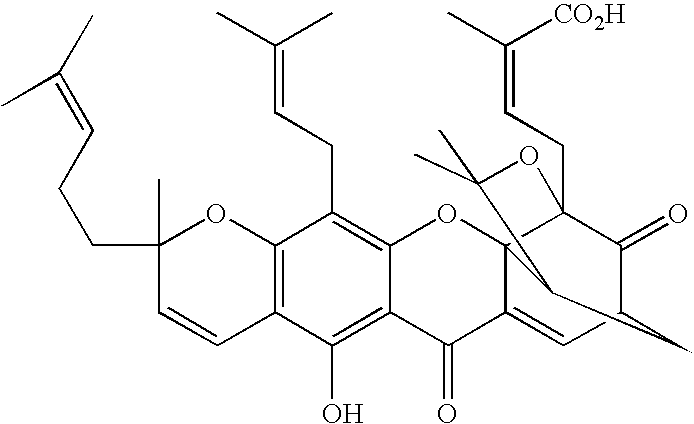
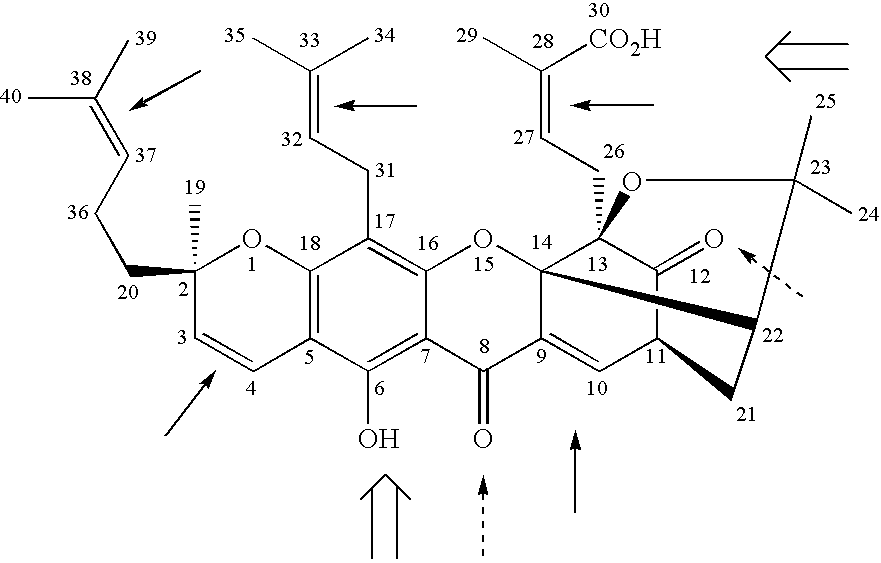
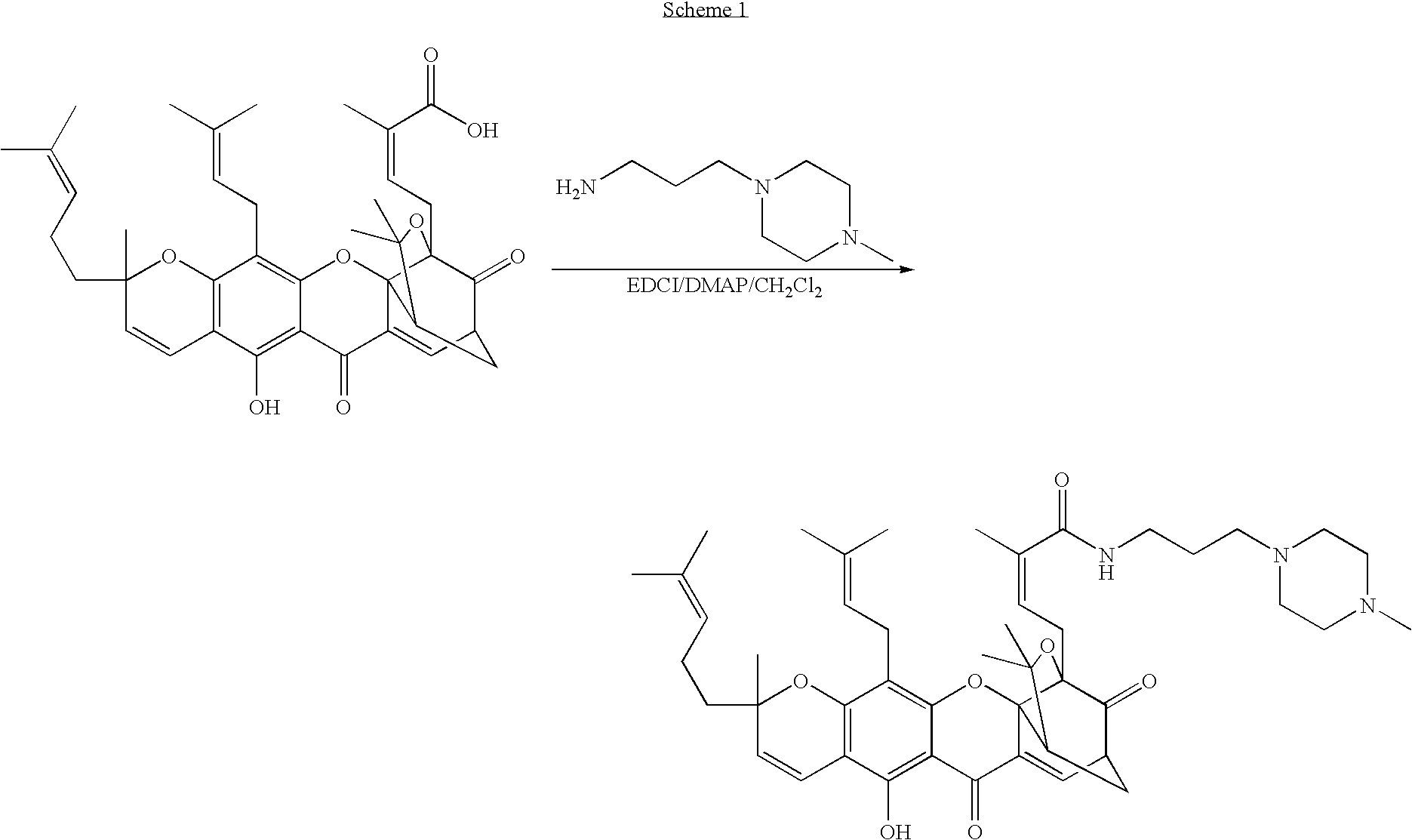
Derivatives of gambogic acid and analogs as activators of caspases and inducers of apoptosis
The present invention is directed to novel derivatives of gambogic acid and analogs thereof. The present invention also relates to the discovery that novel derivatives of gambogic acid are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
Probes for In Vivo Targeting of Active Cysteine Proteases
ActiveUS20090252677A1Stable labelingSufficient half-lifeRadioactive preparation carriersX-ray constrast preparationsActive enzymeHalf-life
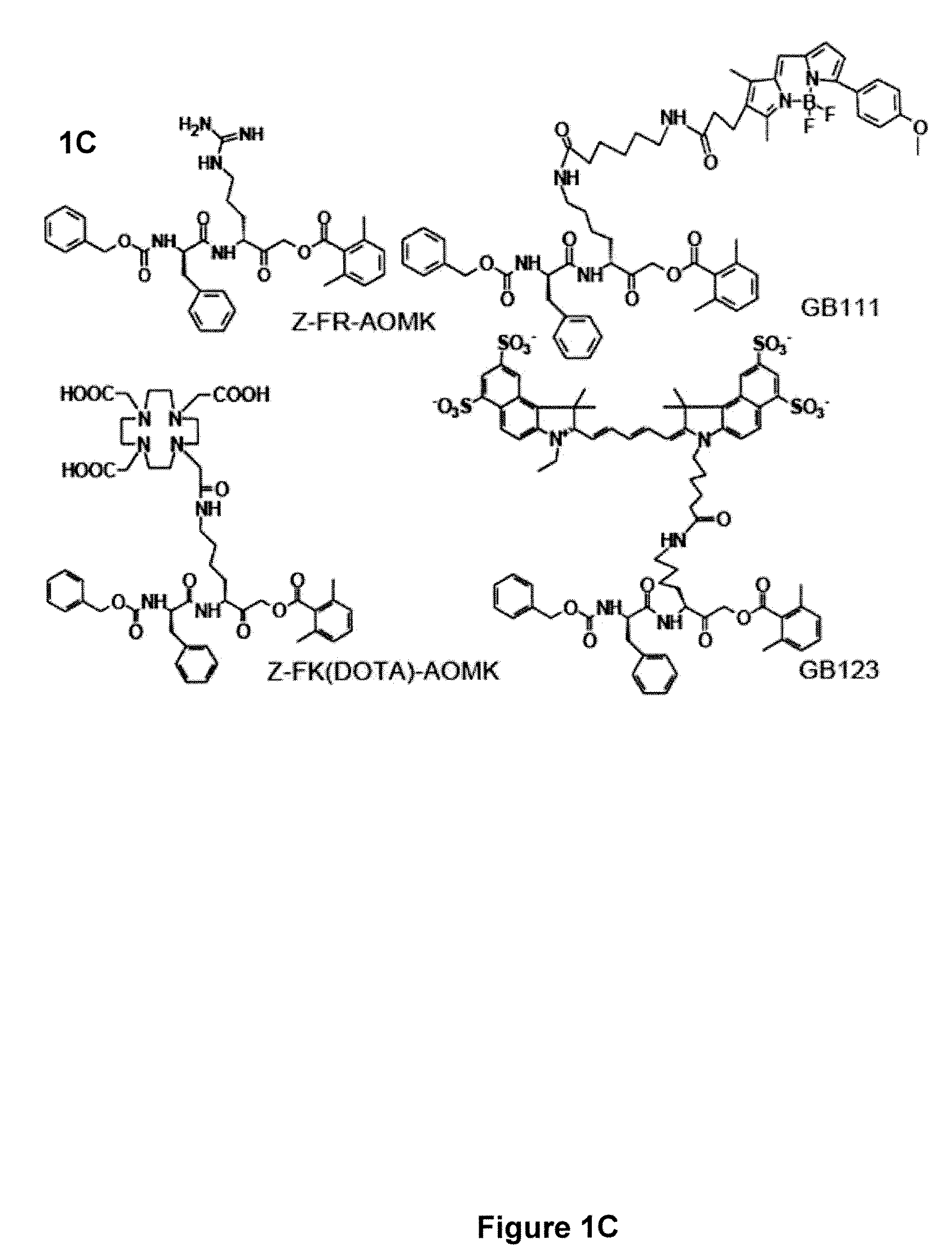
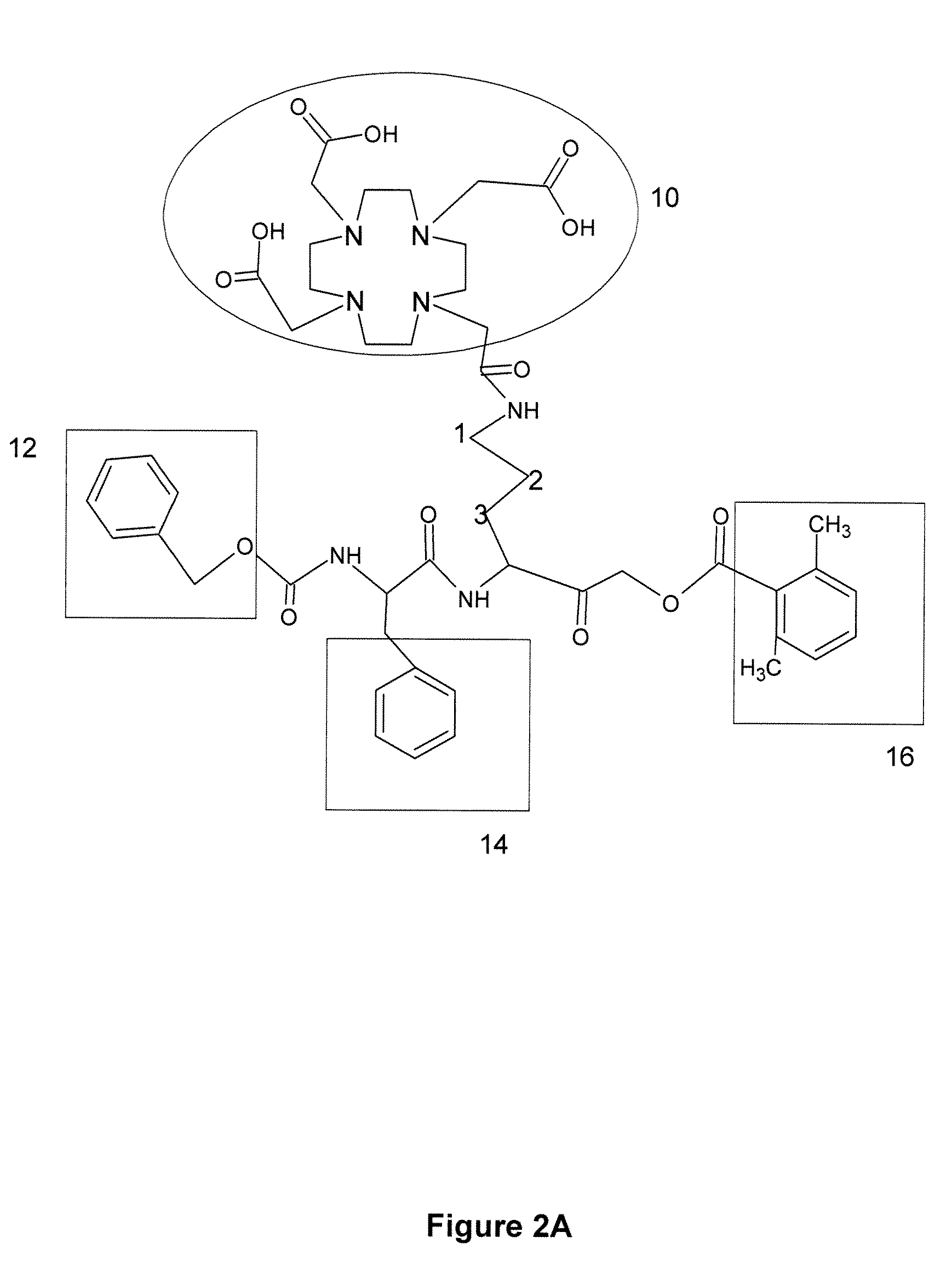
Activity-based probes, which are specific for certain active cysteine proteases (caspase, cathepsin and legumain) and carry radioactive labels, are disclosed. The present probes comprise an acyloxymethyketone (AOMK) “warhead” that binds only to active enzyme. The probes further comprise peptide-like structure that targets the probe to a specific cysteine protease or protease family, and a radiolabel on the probe, which is bound to the targeted enzyme. It has been found that the present probes are stable in vivo and give specific target images distinguishable over background. The preferred probes are labeled with a positron-emitting agent such as 64Cu, 125I (SPECT) and 99mTc (PET). The probes show in vivo half-life and stability well suited for imaging.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Novel fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for caspases and other enzymes and the use thereof
InactiveUS20020150885A1Microbiological testing/measurementChemiluminescene/bioluminescenceScreening proceduresApoptosis
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
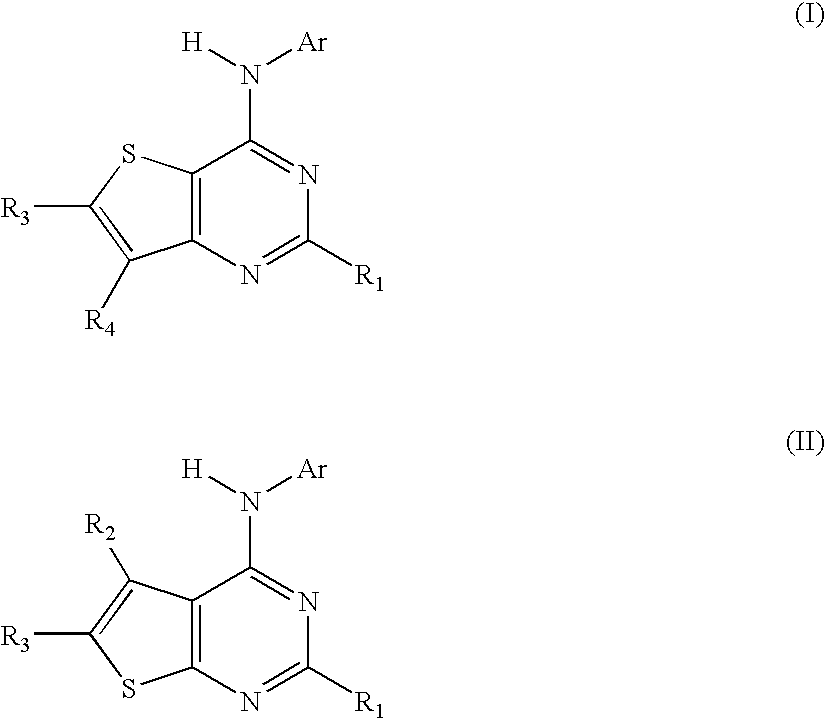
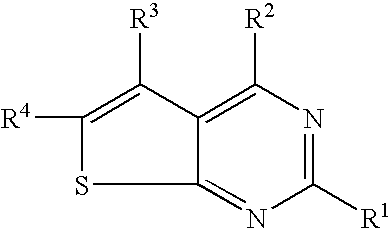
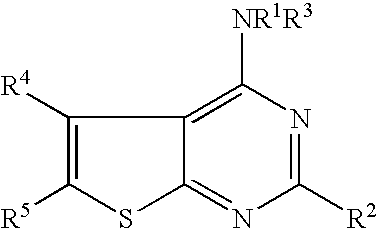
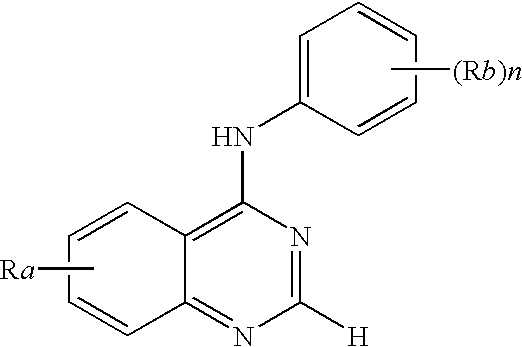
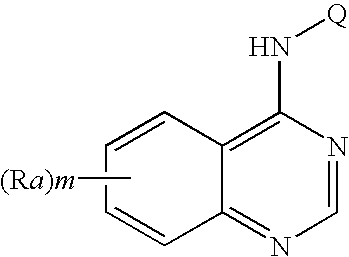
N-aryl-thienopyrimidin-4-amines and analogs as activators of caspases and inducers of apoptosis and the use thereof
Disclosed are N-aryl-thienopyrimidin-4-amines and analogs thereof, represented by the Formulae I-II: wherein Ar and R1-R4 are defined herein. The present invention relates to the discovery that compounds having Formulae I-II are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
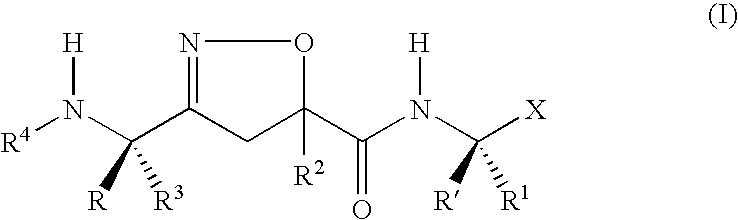
Isoxazoline derivative and a process for the preparation thereof
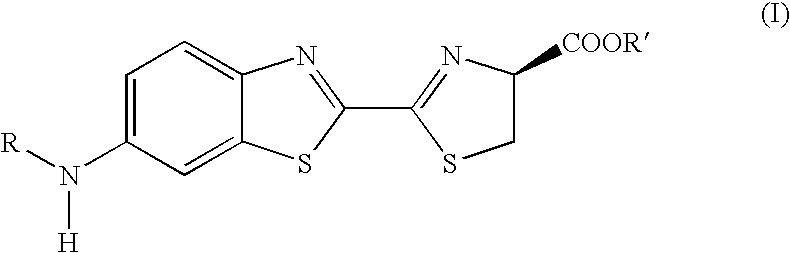
The present invention provides to an isoxazoline derivative of formula (I), the pharmaceutically acceptable salts, esters and stereochemically isomeric forms thereof, and the use of the derivative in inhibiting the activity of caspases. The present invention also provides a pharmaceutical composition for preventing inflammation and apoptosis which comprise the isoxazoline derivative, pharmaceutically acceptable salts, esters and stereochemically isomeric forms thereof and the process for preparing the same. The derivative according to the present invention can be effectively used in treating diseases due to caspases, such as, for example the disease in which cells are abnormally died, dementia, cerebral stroke, AIDS, diabetes, gastric ulcer, hepatic injury by hepatitis, sepsis, organ transplantation rejection reaction and anti-inflammation.
Owner:LG CHEM INVESTMENT LTD
Methods and compositions for derepression of IAP-inhibited caspase
InactiveUS20060211627A1Promote apoptosisReduce severityOrganic chemistryTripeptide ingredientsChemical structureDerepression
The invention provides isolated agents having novel chemical structures and possessing superior activity as derepressors of IAP inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase. The invention further provides assay methods employing labeled compounds of the invention, especially fluorescent labeled compounds.
Owner:TORREY PINES INST FOR MOLECULAR STUDIES +1
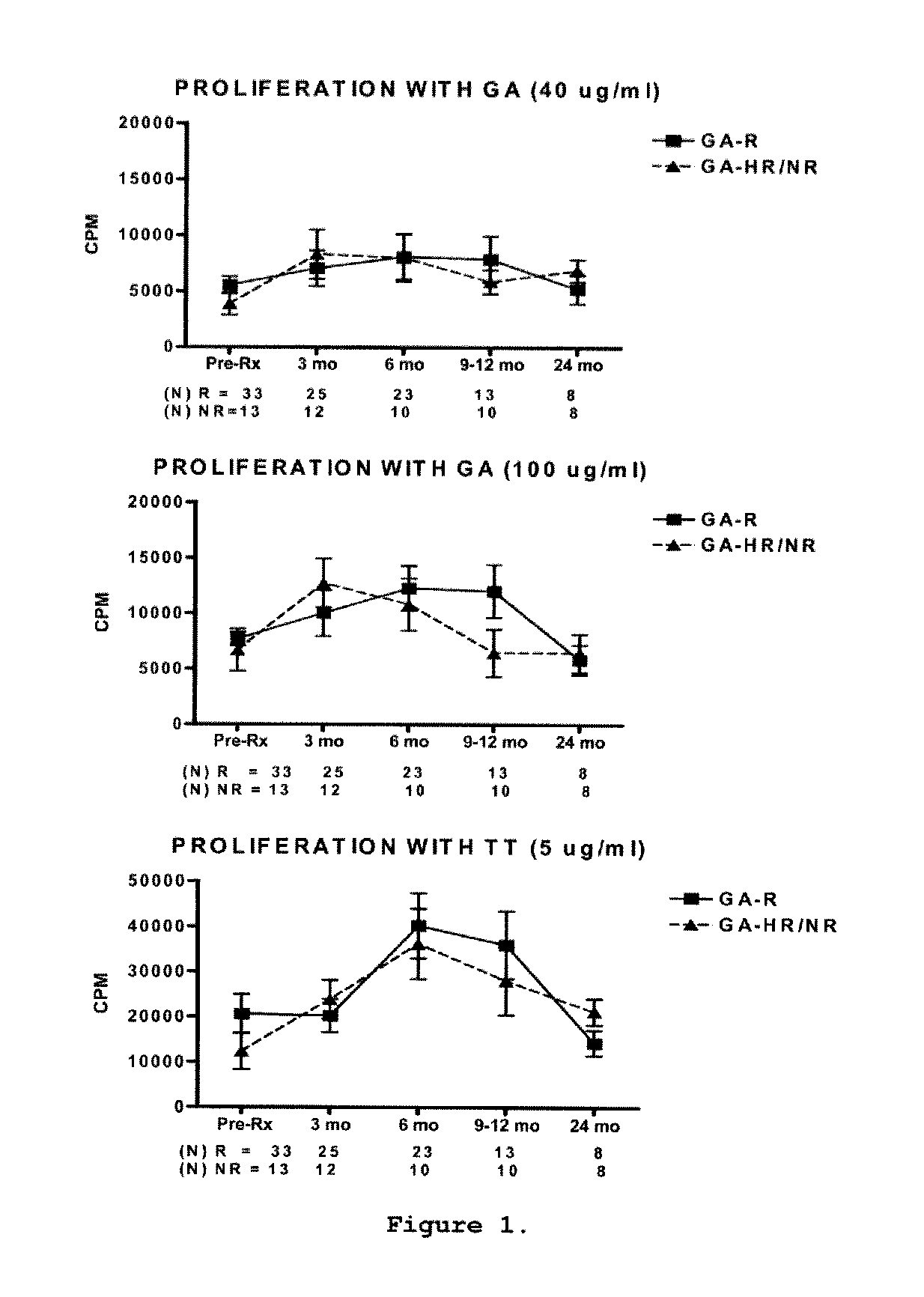
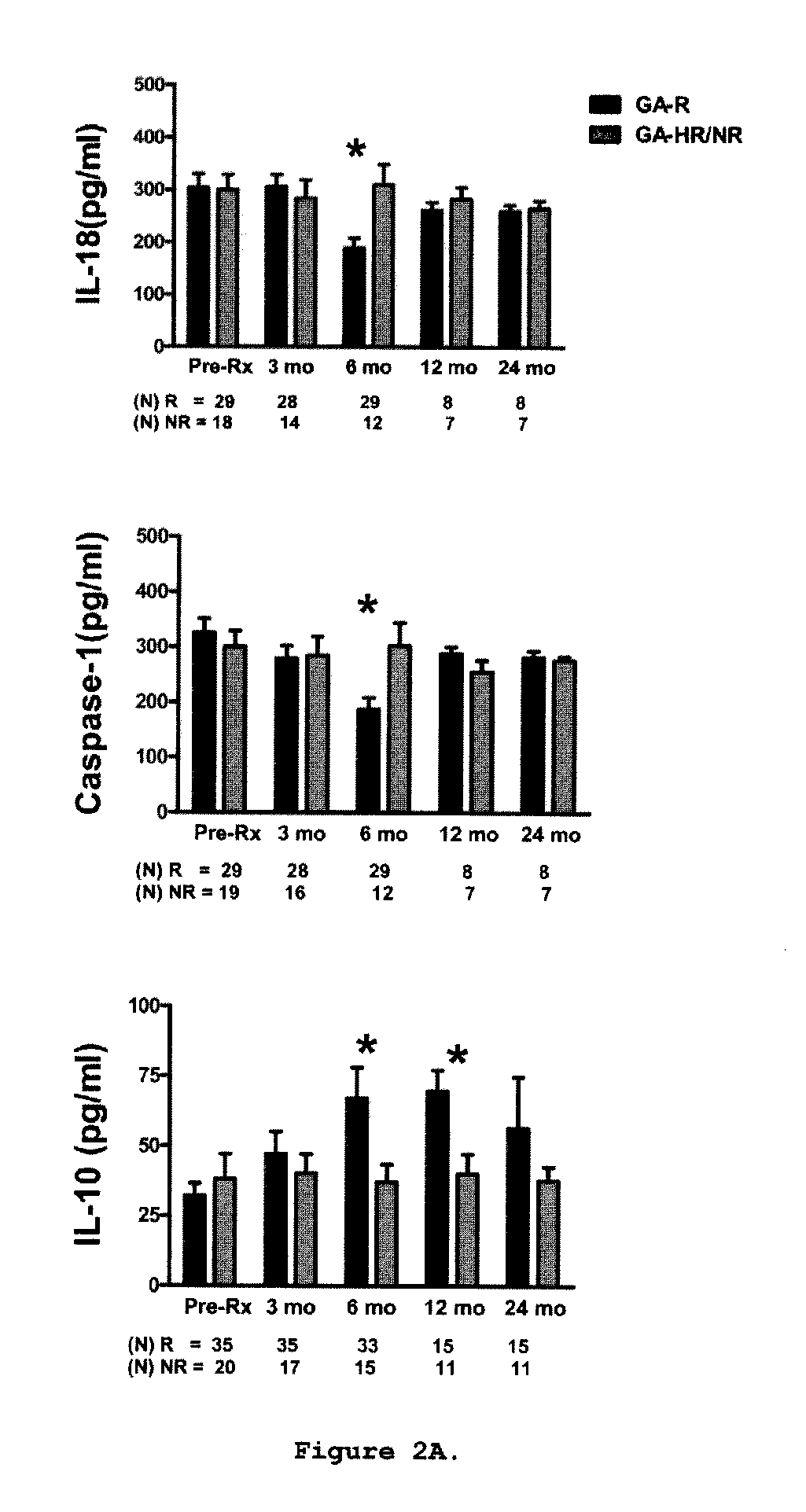
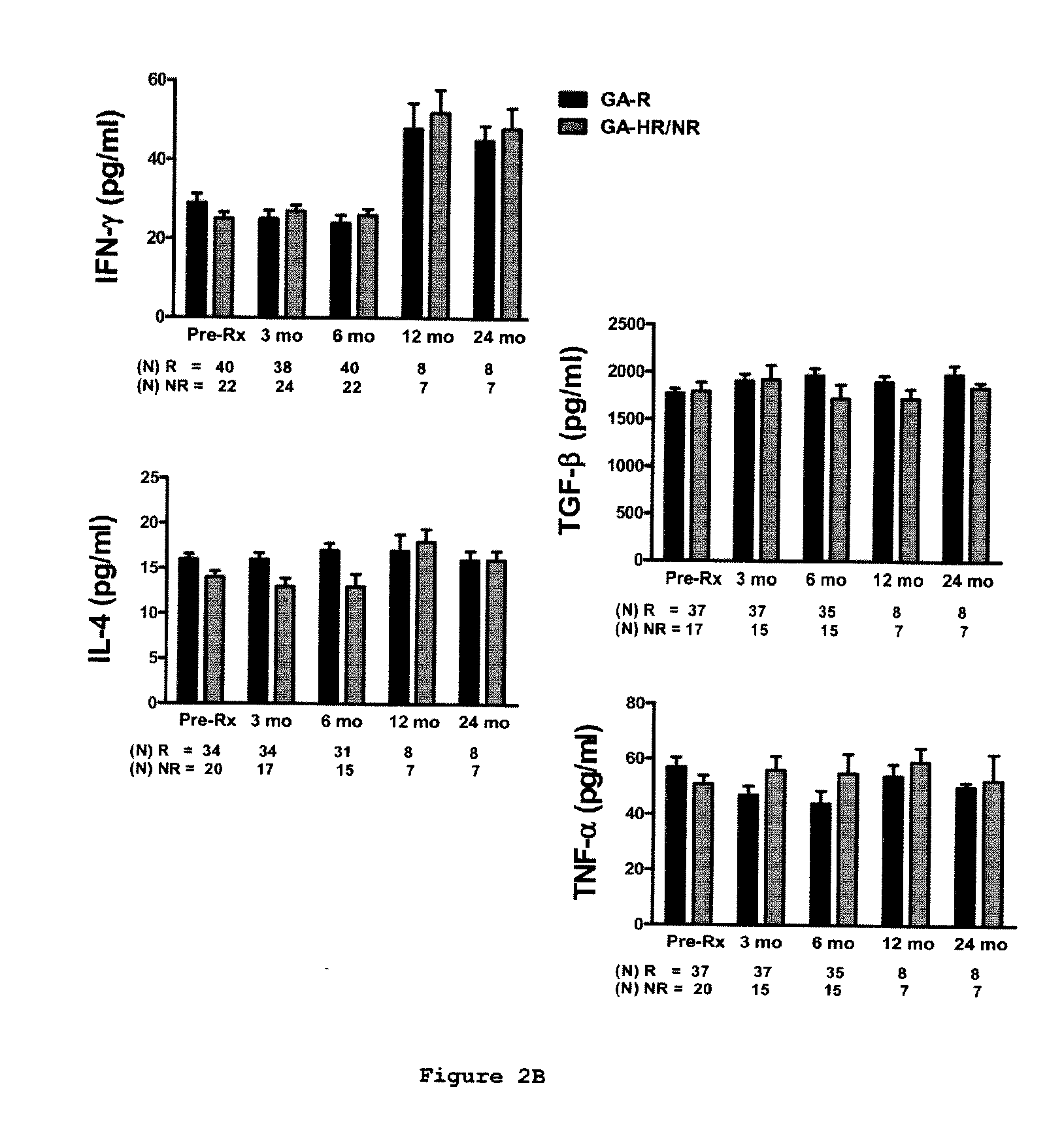
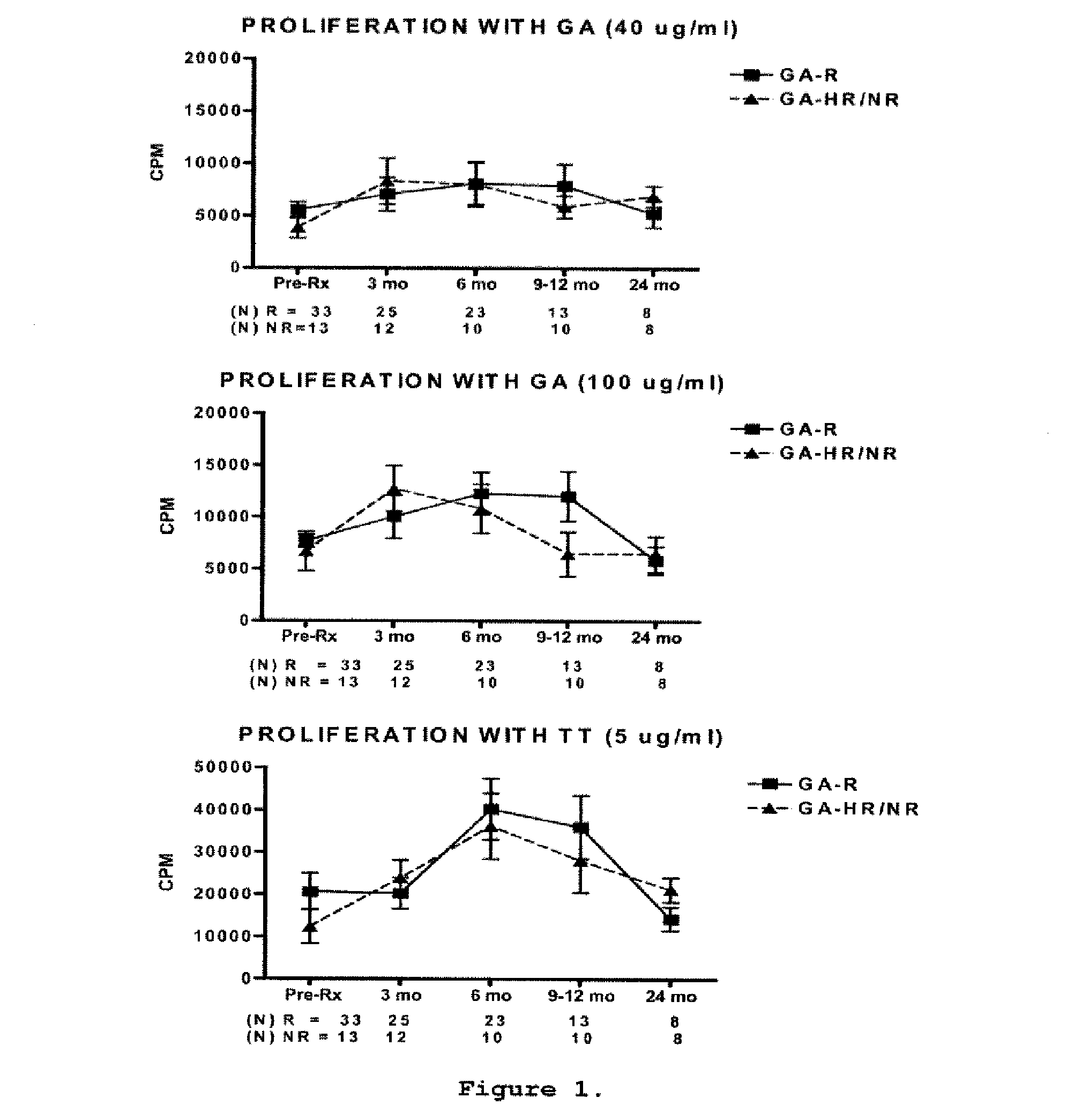
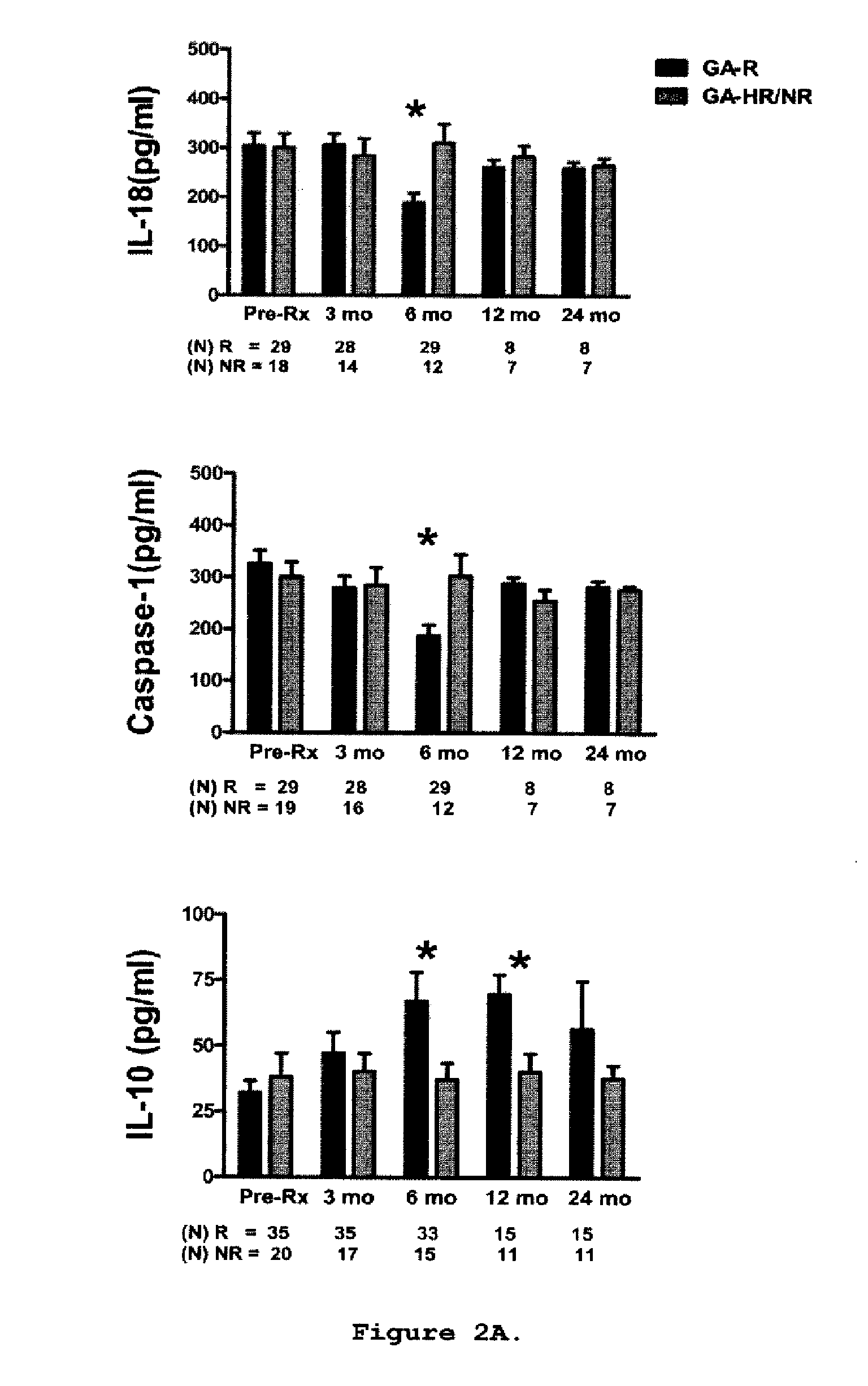
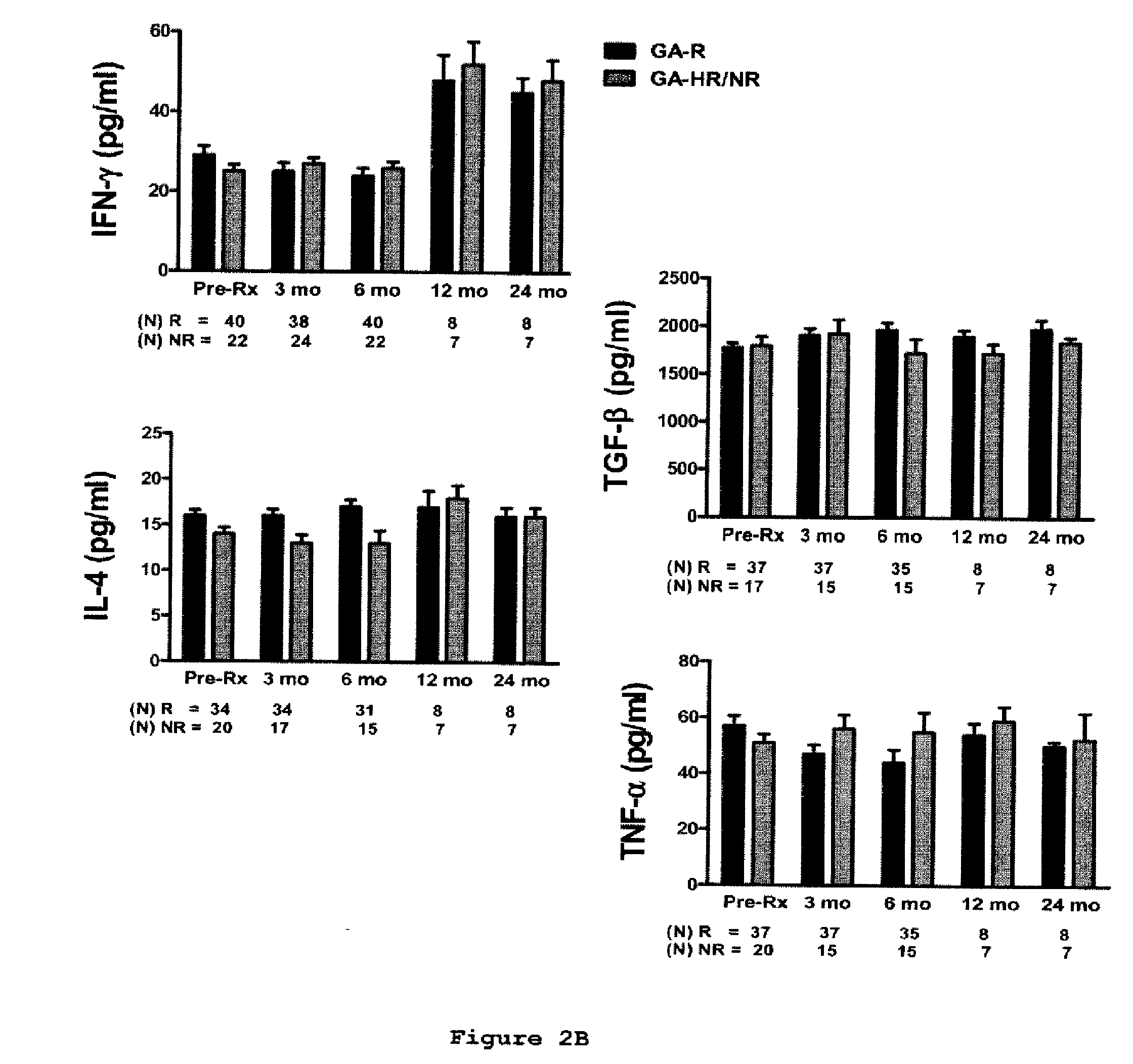
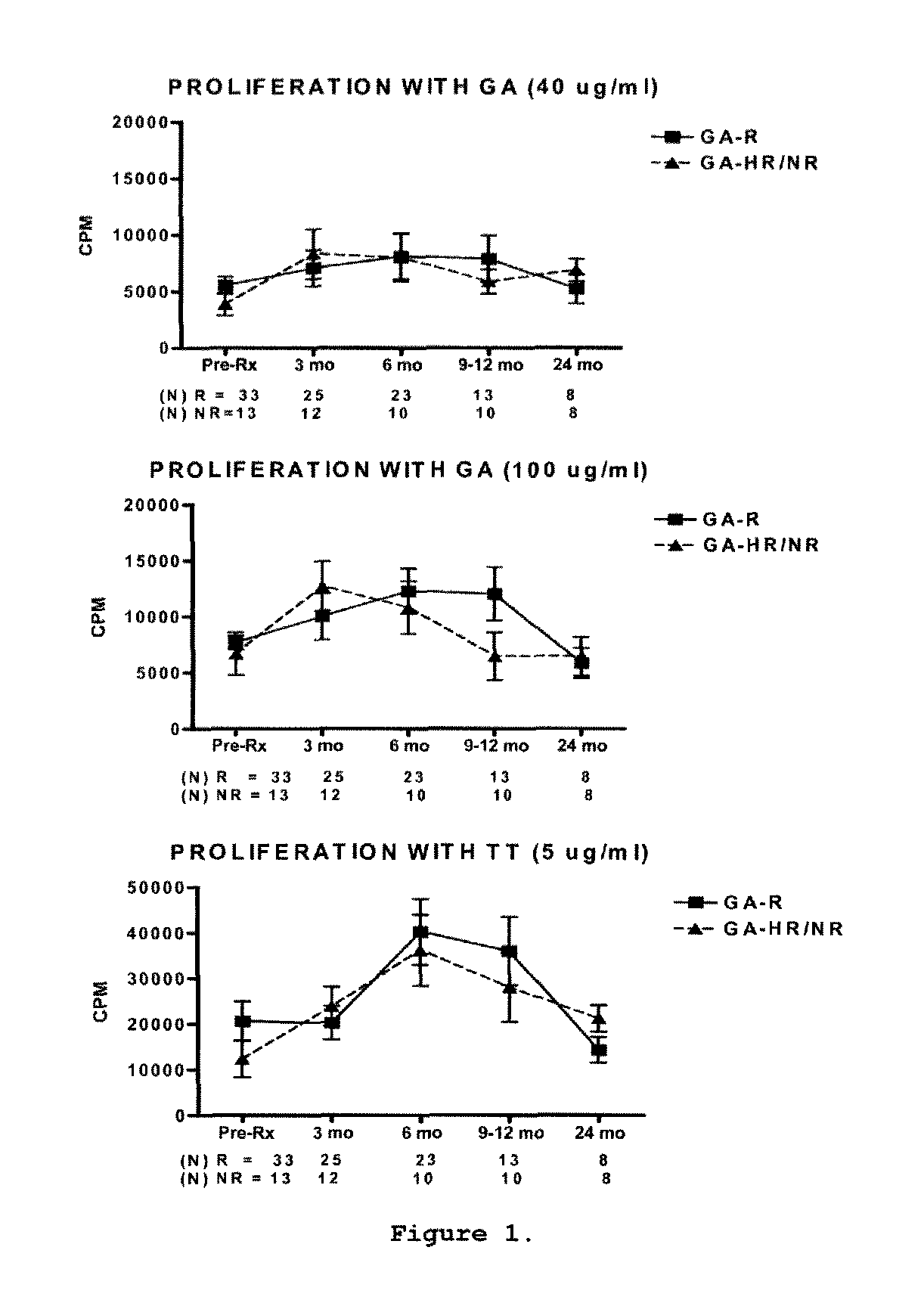
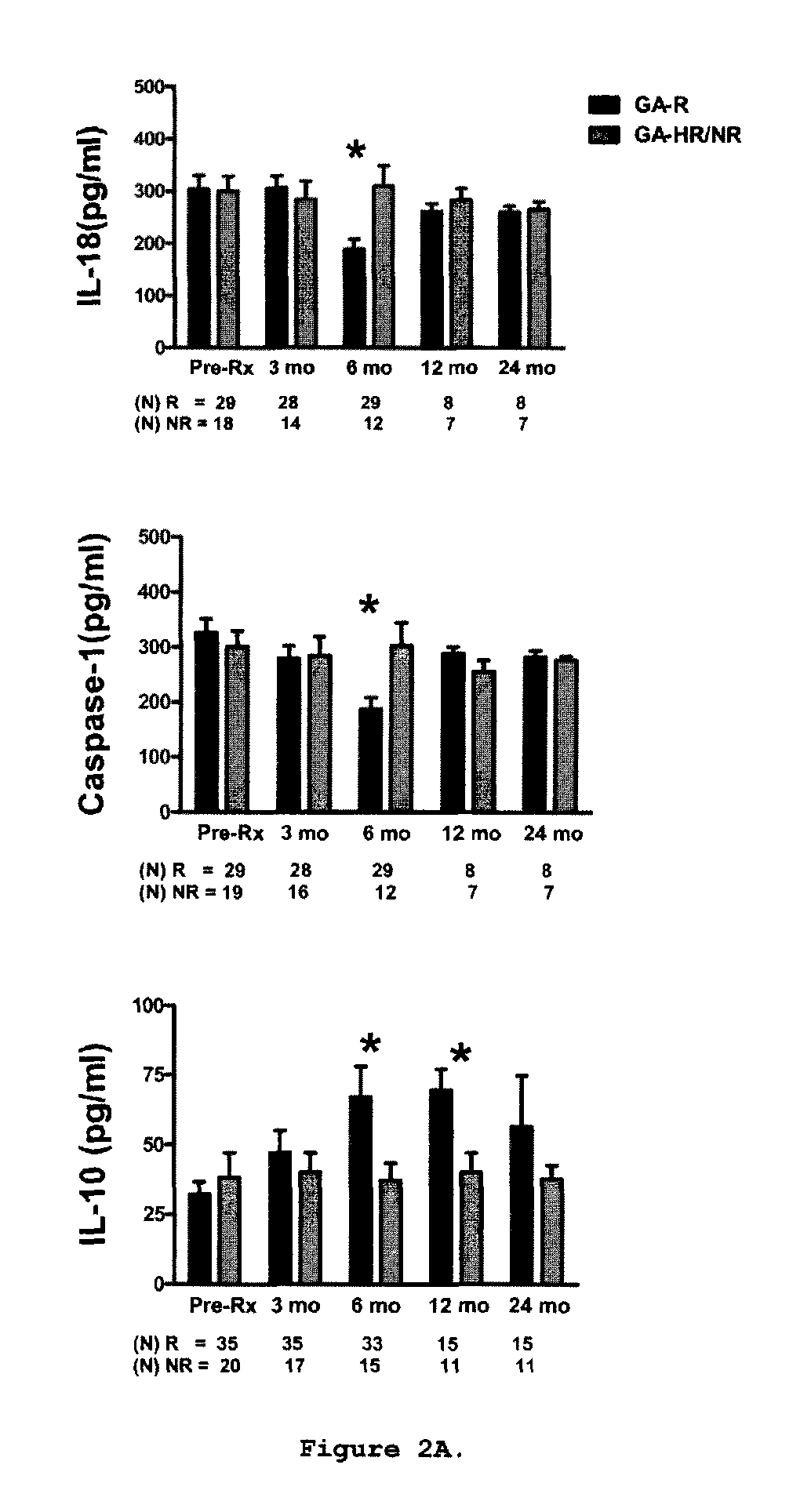
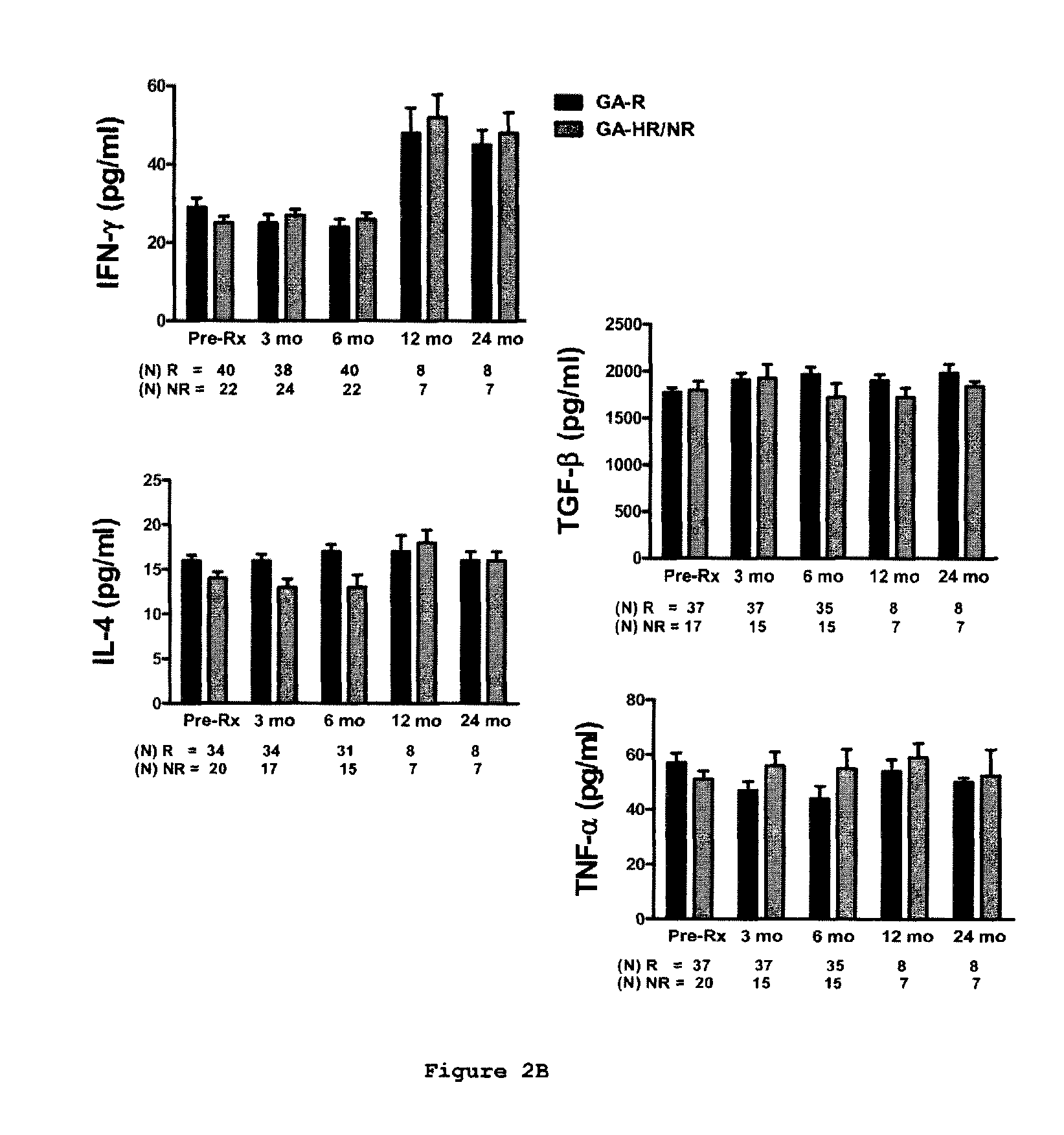
Predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
Compounds and therapeutical use thereof
Disclosed are 4-arylamino-quinazolines and analogs thereof effective as activators of caspases and inducers of apoptosis. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC +1
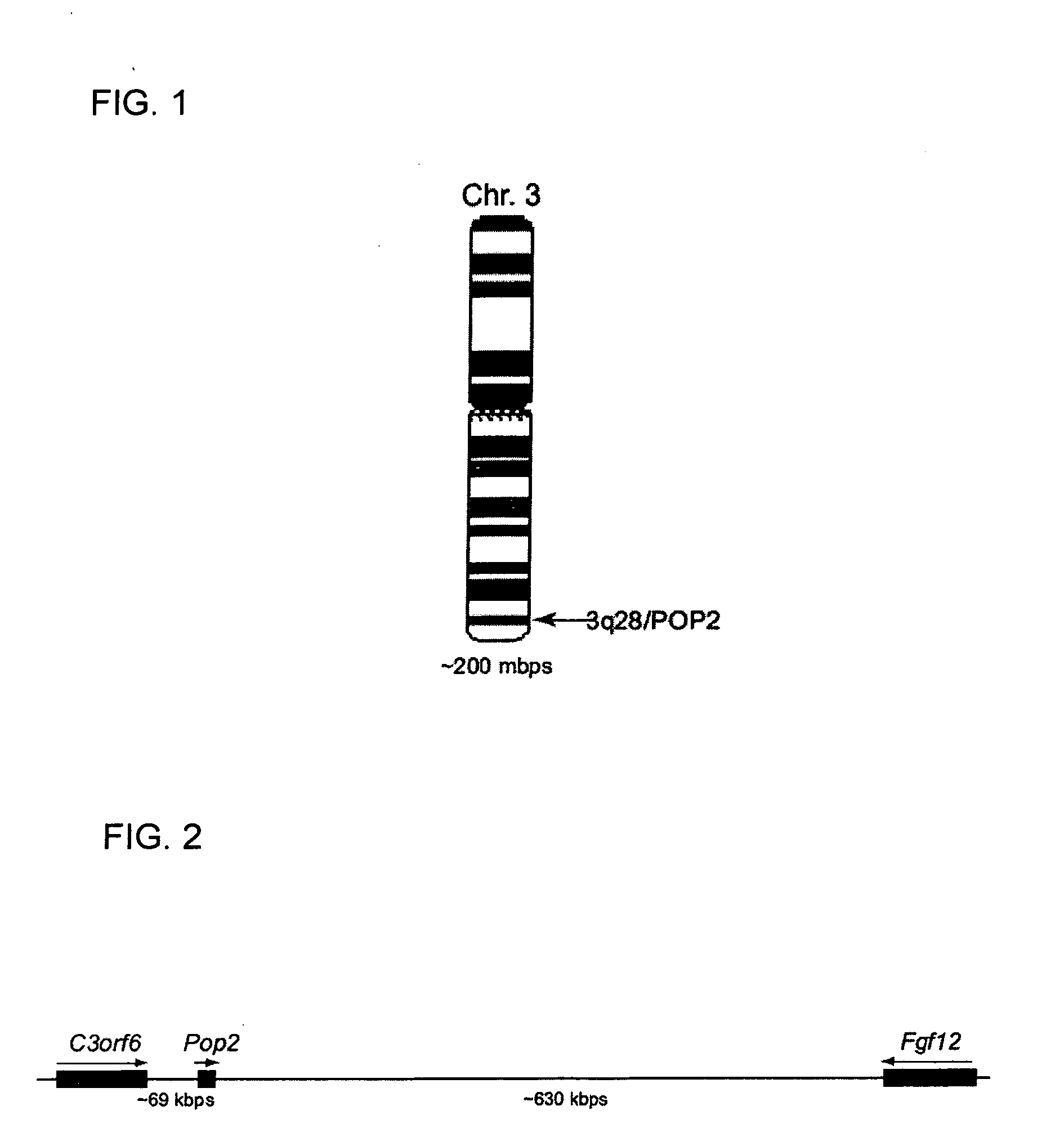
POP2: NFkB - Inhibiting Polypeptides, Nucleic Acids and Methods of Use
This invention provides a novel pyrin-only protein (POP2), polypeptides, nucleic acids encoding them and methods for making and using them. The polypeptides of this invention have nuclear factor-κB (NF-κB) modulating activity. NF-κB is pivotal for transactivation of cell-cycle regulatory, cytokine and adhesion molecule genes and is dysregulated in many cancers, neurodegenerative disorders, and inflammatory diseases. Proteins with Pyrin and / or caspase recruitment (CARD) domains have roles in apoptosis, innate immunity, and inflammation. Many pyrin domain proteins modulate NF-KB activity as well as participate in assembling both the perinuclear “apoptotic speck” and the pro-IL1β / IL-18 converting inflammasome complex. ‘Pyrin-only’ proteins are attractive as negative regulators of pyrin domain-mediated functions and one such protein, POP1, has been reported. We teach a second Pyrin-only protein (POP2). POP2 is a 294 nt single exon gene located on human chromosome 3 encoding a 97 amino acid protein with sequence and predicted structural similarity to other pyrin domains. Highly similar to pyrin domains in CATERPILLER (CLR, NLR, NALP) family proteins, POP2 is less like the prototypic Pyrin and ASC pyrin domains. POP2 is expressed principally in peripheral blood leukocytes and displays both cytoplasmic and nuclear expression patterns in transfected cells. TNFα-stimulated and p65 (RelA) induced NF-KB-dependent gene transcription is inhibited by POP2 in vitro by a mechanism involving changes in NF-κB nuclear import or distribution. While colocalizing with ASC in perinuclear specks, POP2 also inhibits the formation of specks by the CLR protein CIAS1 / NALP3. Together these observations indicate that POP2 is a negative regulator of NF-KB activity that may influence the assembly of pyrin-domain dependent complexes.
Owner:UNIV OF SOUTH FLORIDA
Compositions and methods including cell death inducers and procaspase activation
Compositions and methods are disclosed in embodiments relating to induction of cell death such as in cancer cells. Compounds and related methods for synthesis and use thereof, including the use of compounds in therapy for the treatment of cancer and selective induction of apoptosis in cells are disclosed. Compounds are disclosed in connection with modification of procaspases such as procaspase-3. In embodiments, compositions are capable of activation of procaspase-3.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Therapeutically useful synthetic oligonucleotides
InactiveUS7157436B2Stimulates IL- productionStimulates IL-1 productionBiocideAntipyreticCancer cellCaspase
The present invention provides a composition and method comprising a 2–20 base 3′-OH, 5′-OH synthetic oligonucleotide (sequence) selected from the group consisting of (GxTy)n, (TyGx)n, a(GxTy)n, a(TyGx)n, (GxTy)nb, (TyGx)nb, a(GxTy)nb, a(TyGx)nb, wherein x and y is an integer between 1 and 7, n is an integer between 1 and 12, a and b are one or more As, Cs, Gs or Ts and wherein the sequence induces a response selected from the group consisting of induction of cell cycle arrest, inhibition of proliferation, activation of caspases and induction of apoptosis in cancer cells and production of cytokines by immune system cells.
Owner:BIONICHE LIFE SCI
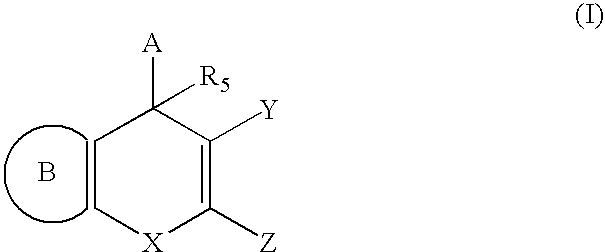
Substituted 4H-chromene and analogs as activators of caspases and inducers of apoptosis and the use thereof
InactiveUS6906203B1Treating preventing amelioratingOrganic chemistryAntipyreticInducerApoptosis inducer
The present invention is directed to substituted 4H-chromene and analogs thereof, represented by the general Formula I: wherein A, B, X, Y, Z and R5 are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
InactiveUS20140322158A1Organic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
Application of IAP inhibitor and oncolytic virus in preparation of antitumor drug
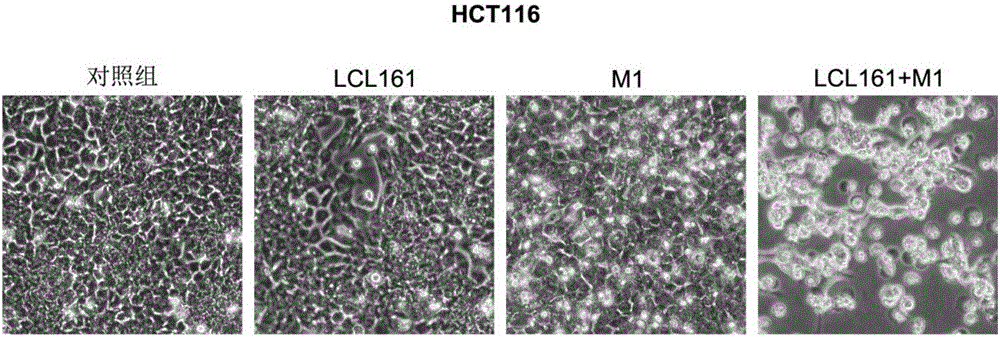
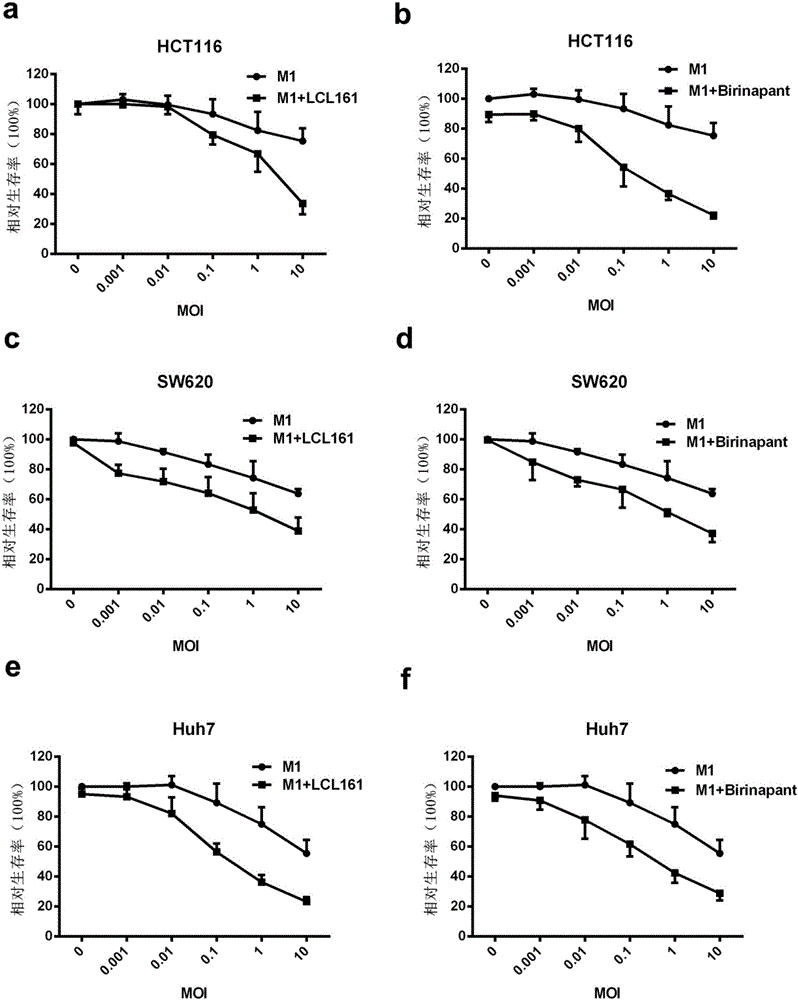
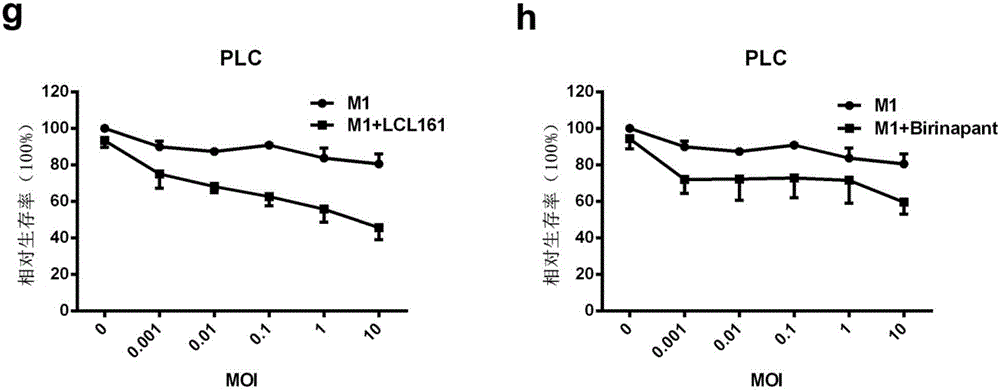
The invention belongs to the field of biological medicines and relates to an application of combination of a Caspase activator and an oncolytic virus in preparation of an antitumor drug. The condition that the antitumor effect of the oncolytic virus can be improved with the Caspase activator is discovered for the first time, the combination of the Caspase activator and the oncolytic virus has a quite high synergistic effect, and an effective therapeutic schedule is provided for oncotherapy with low drug sensitivity.
Owner:GUANGZHOU VIROTECH PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
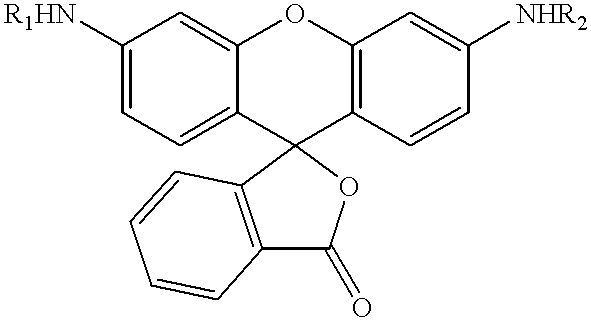
![Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/768e38c5-0ca4-463d-aa96-15225b7f63ef/US07041685-20060509-C00001.png)
![Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/768e38c5-0ca4-463d-aa96-15225b7f63ef/US07041685-20060509-C00002.png)
![Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/768e38c5-0ca4-463d-aa96-15225b7f63ef/US07041685-20060509-C00003.png)
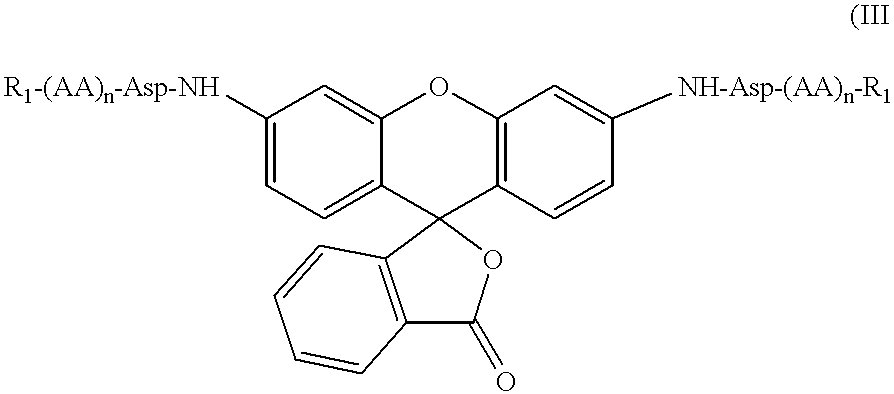
![3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/48af0cf9-bcee-443c-b772-e23fd87dddd1/US07317029-20080108-C00001.png)
![3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/48af0cf9-bcee-443c-b772-e23fd87dddd1/US07317029-20080108-C00002.png)
![3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/48af0cf9-bcee-443c-b772-e23fd87dddd1/US07317029-20080108-C00003.png)
![3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/439e030b-234e-4cd7-9537-32fb6c0d143d/US07144876-20061205-C00001.png)
![3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/439e030b-234e-4cd7-9537-32fb6c0d143d/US07144876-20061205-C00002.png)
![3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/439e030b-234e-4cd7-9537-32fb6c0d143d/US07144876-20061205-C00003.png)