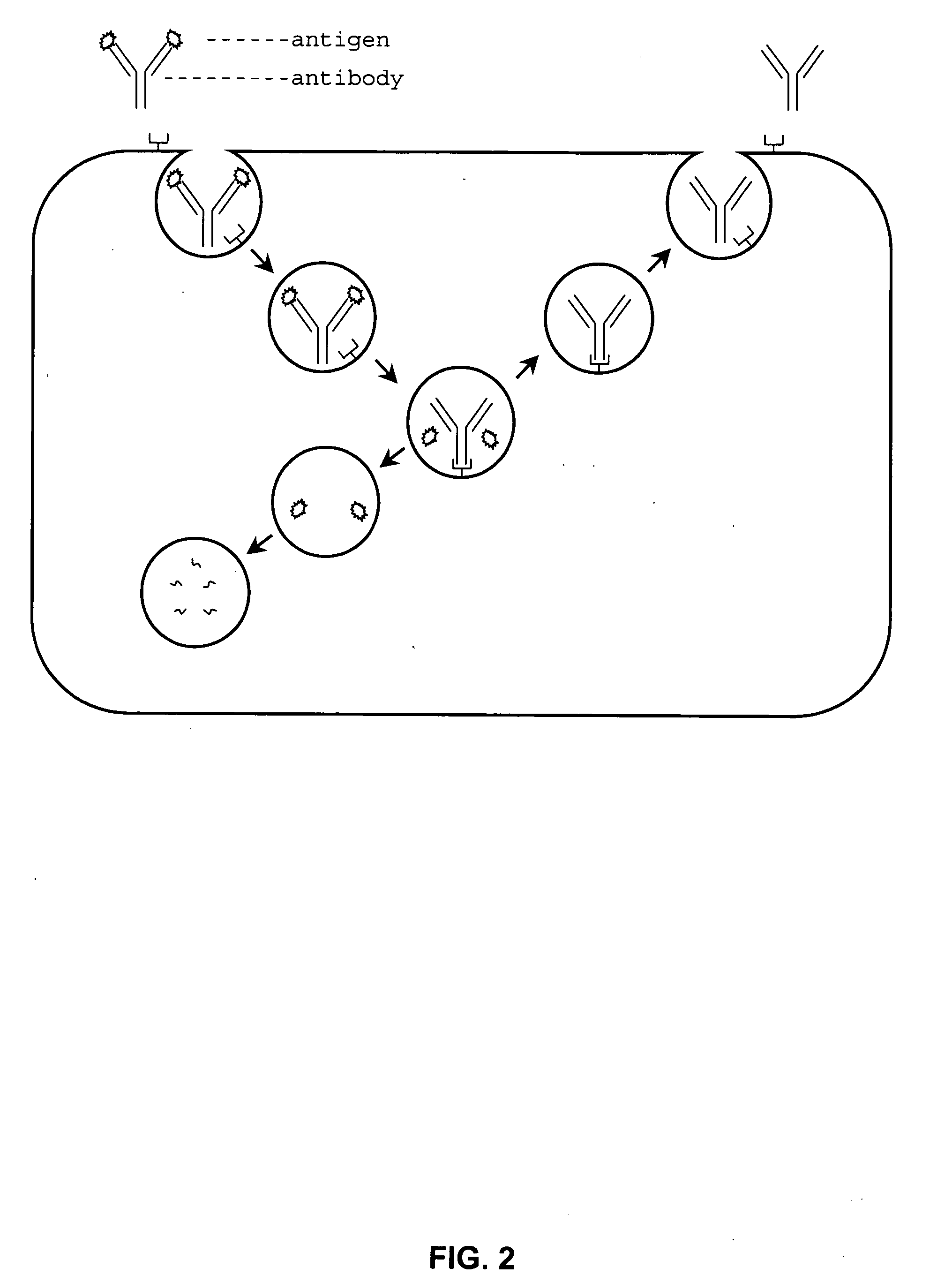
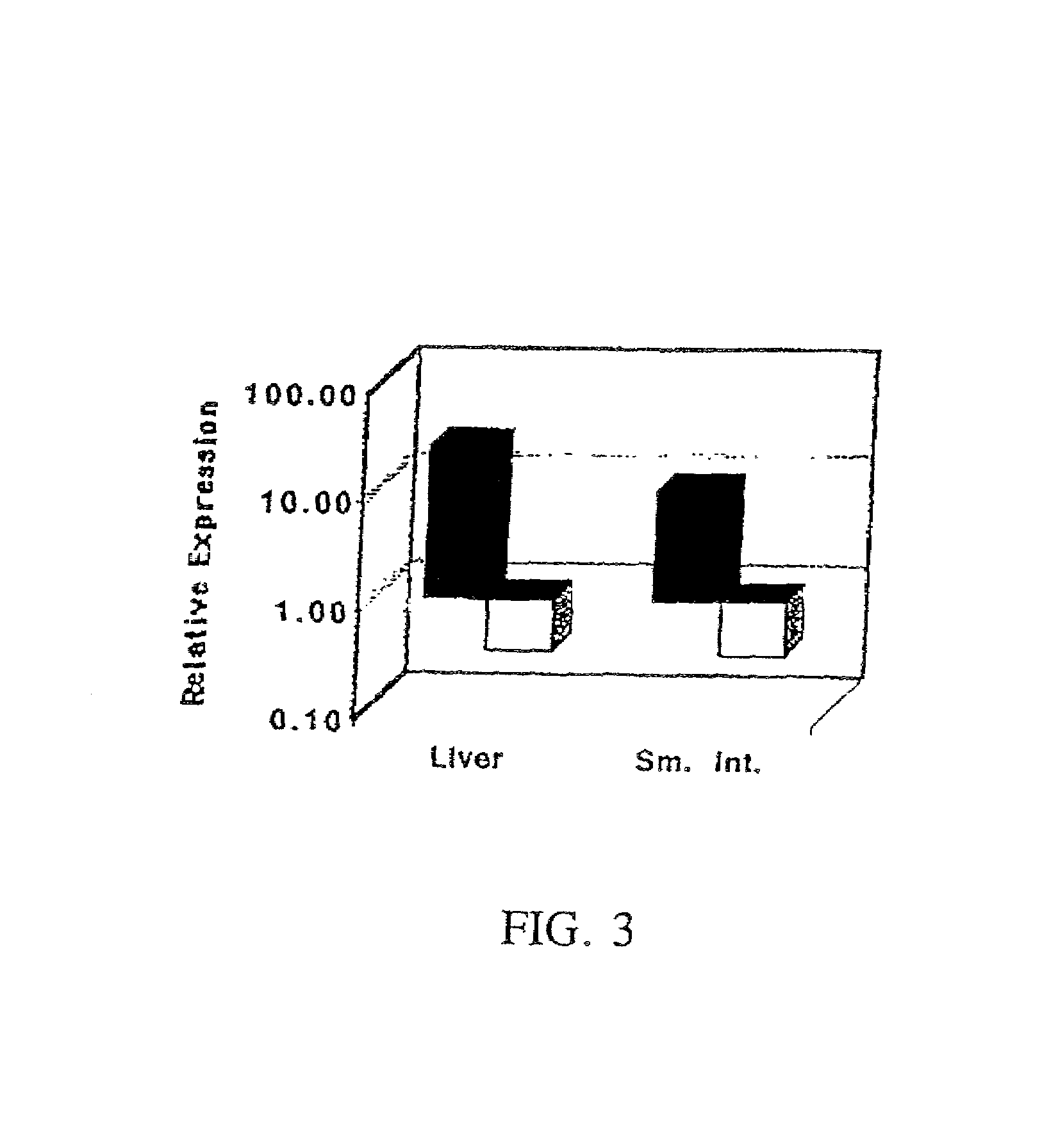
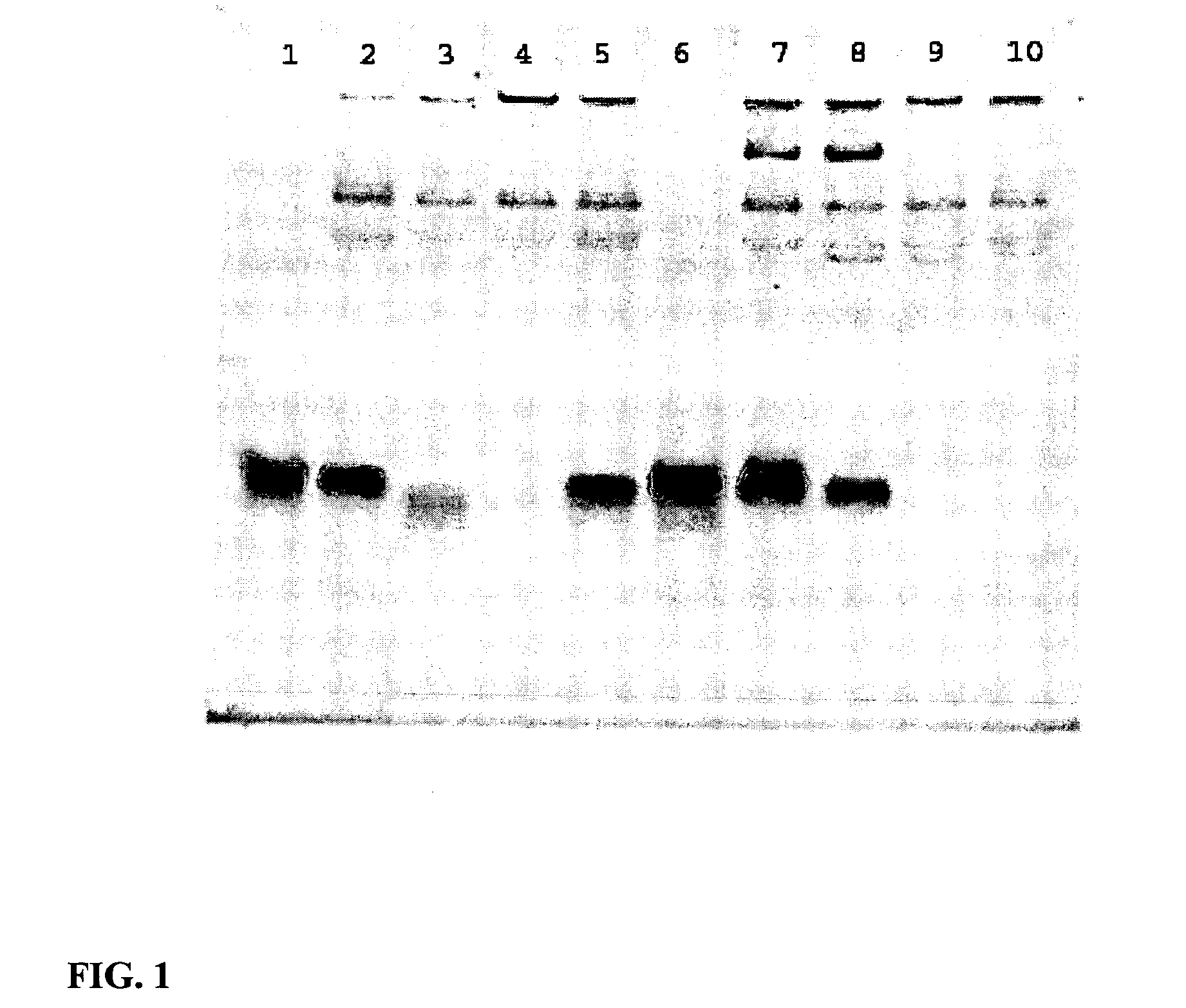
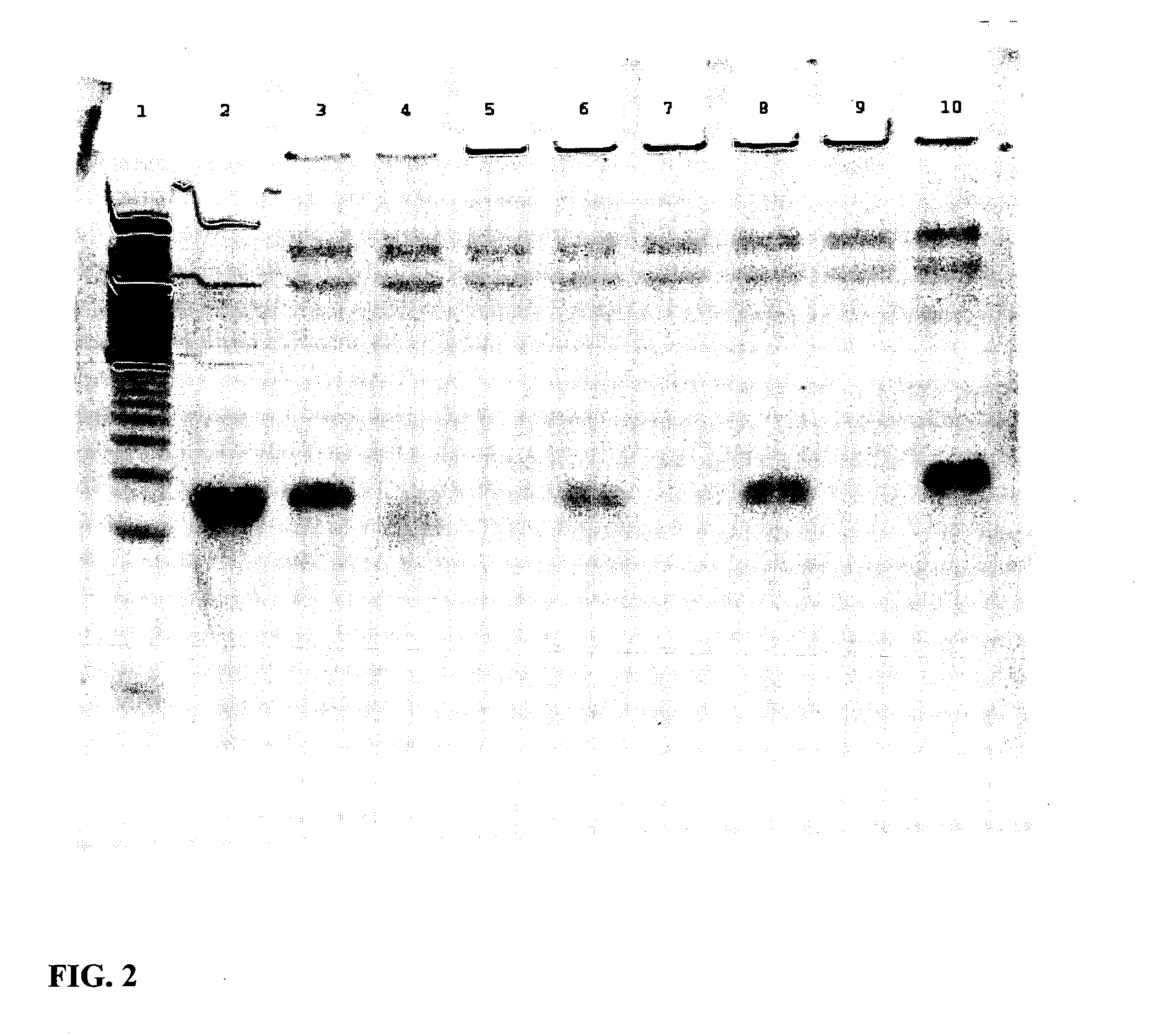
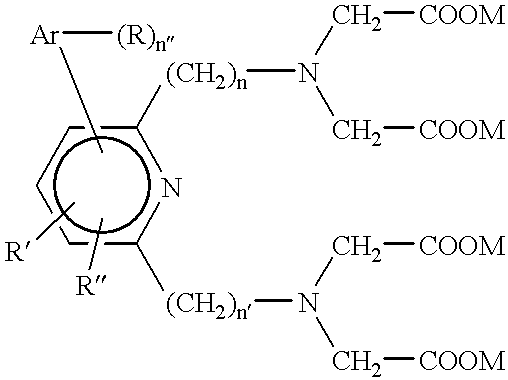
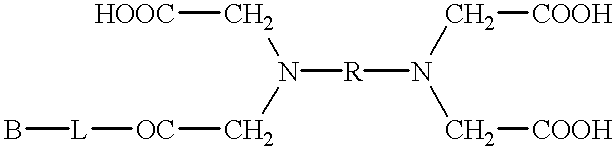
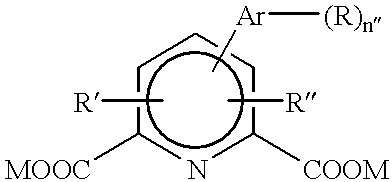
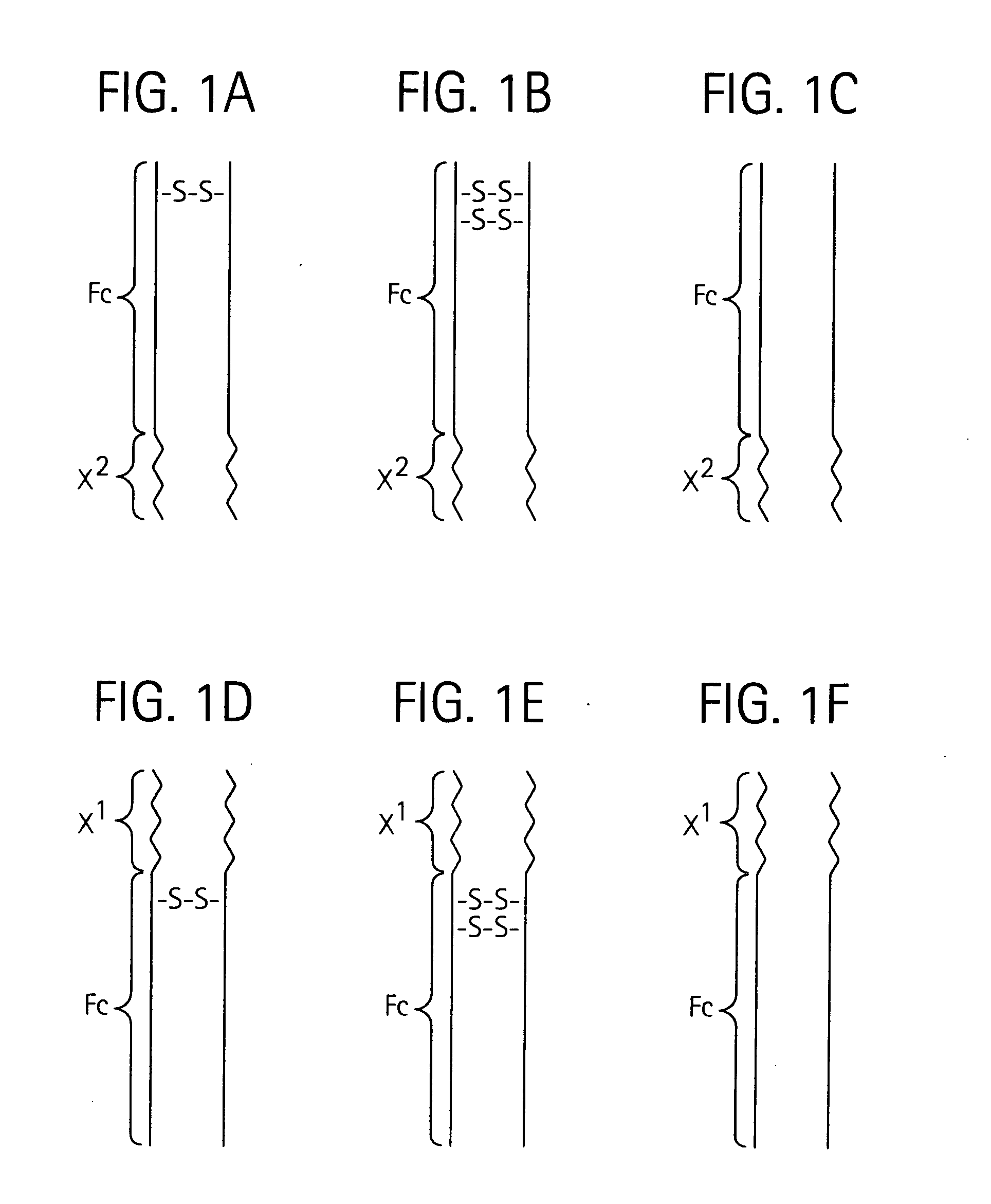
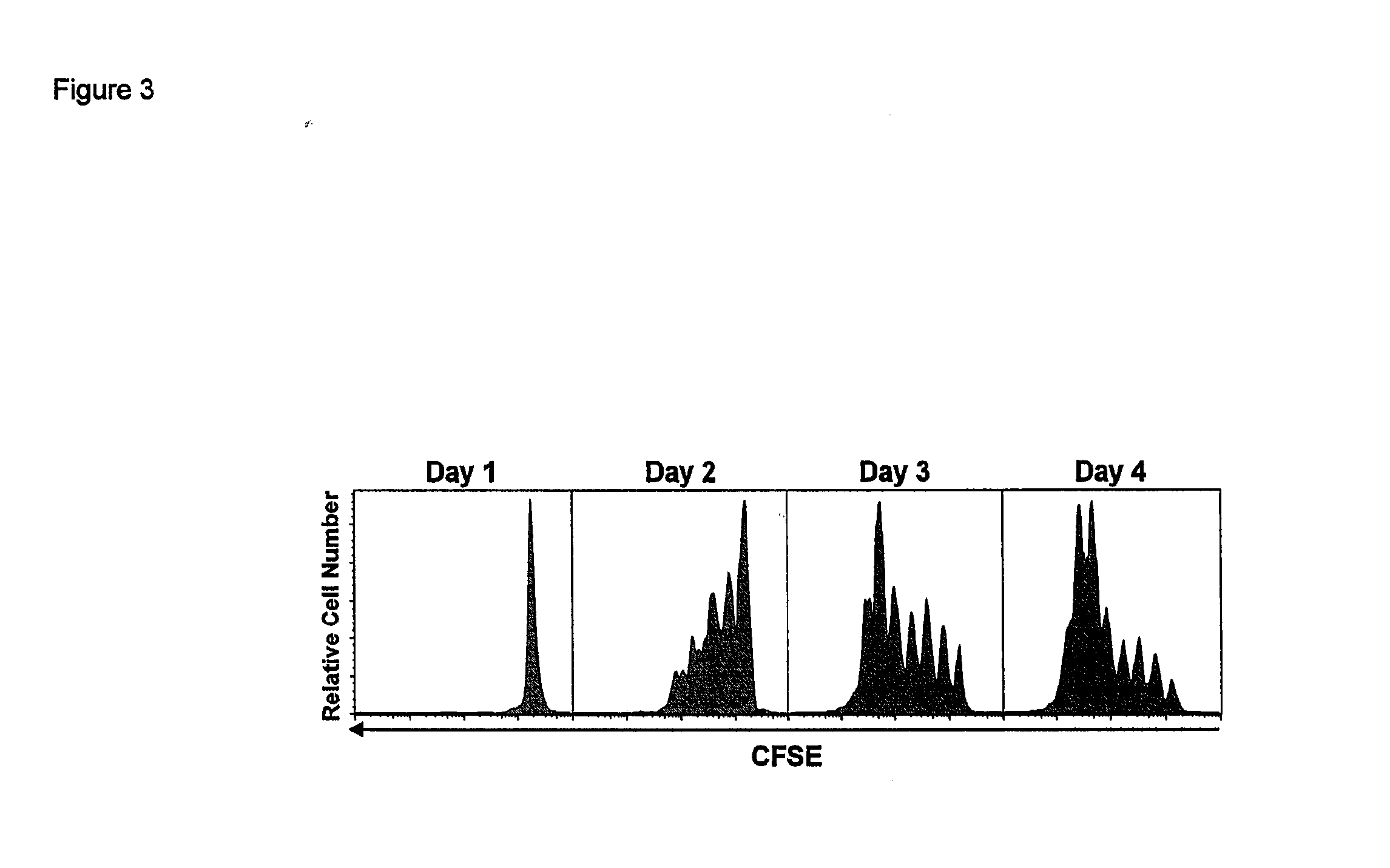
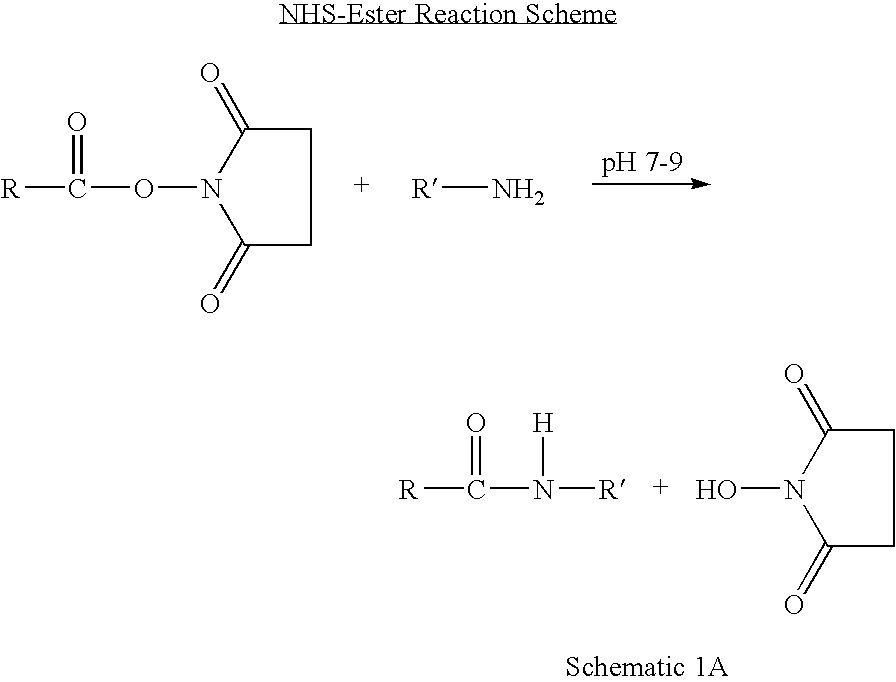
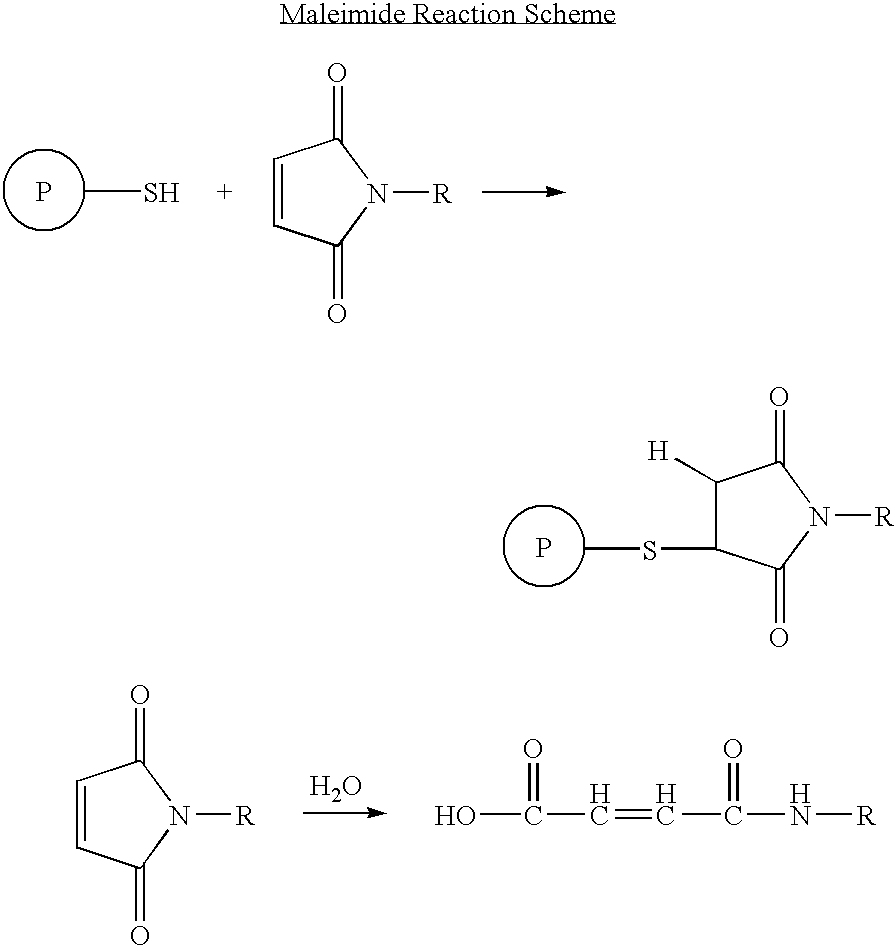
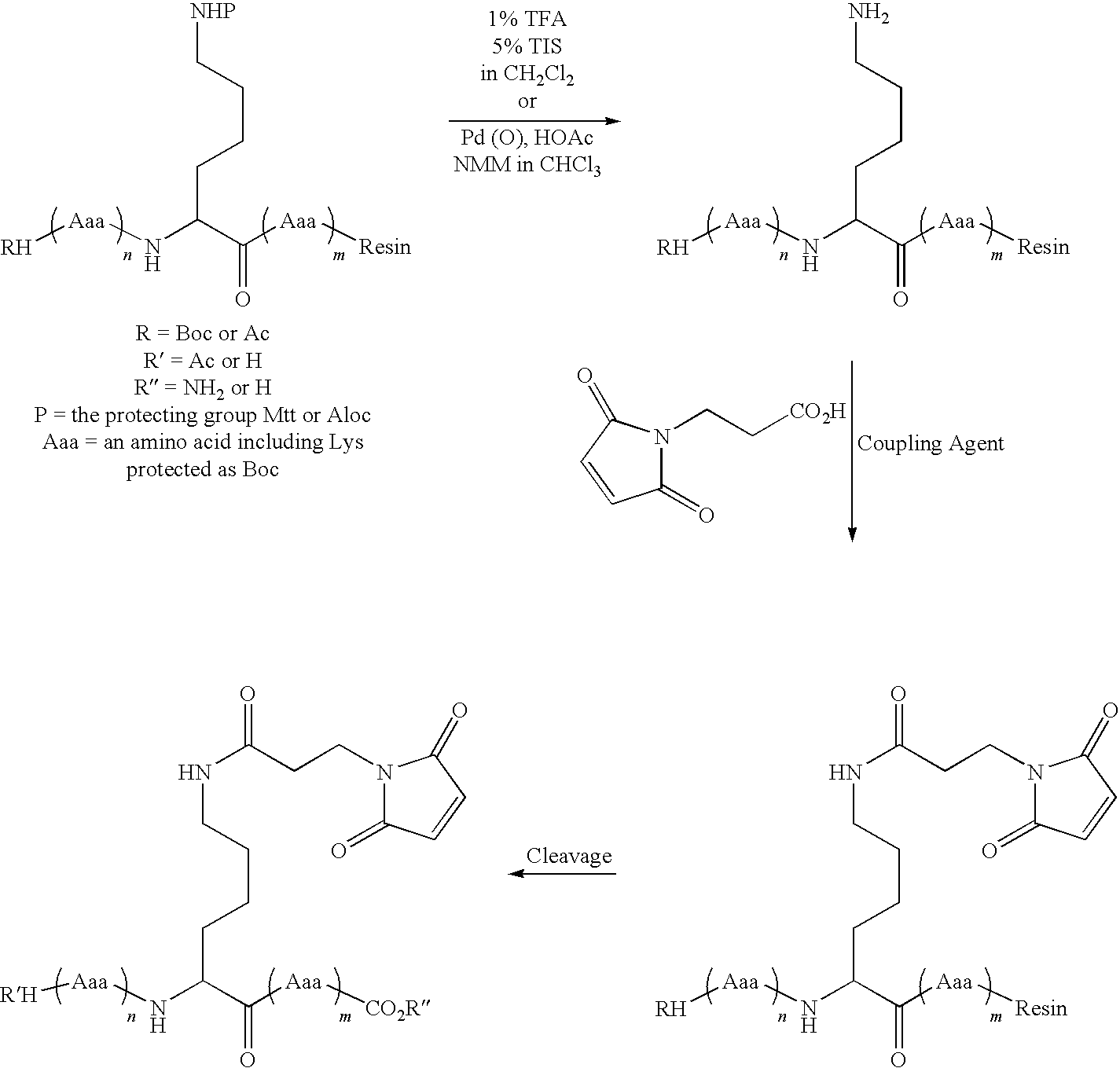
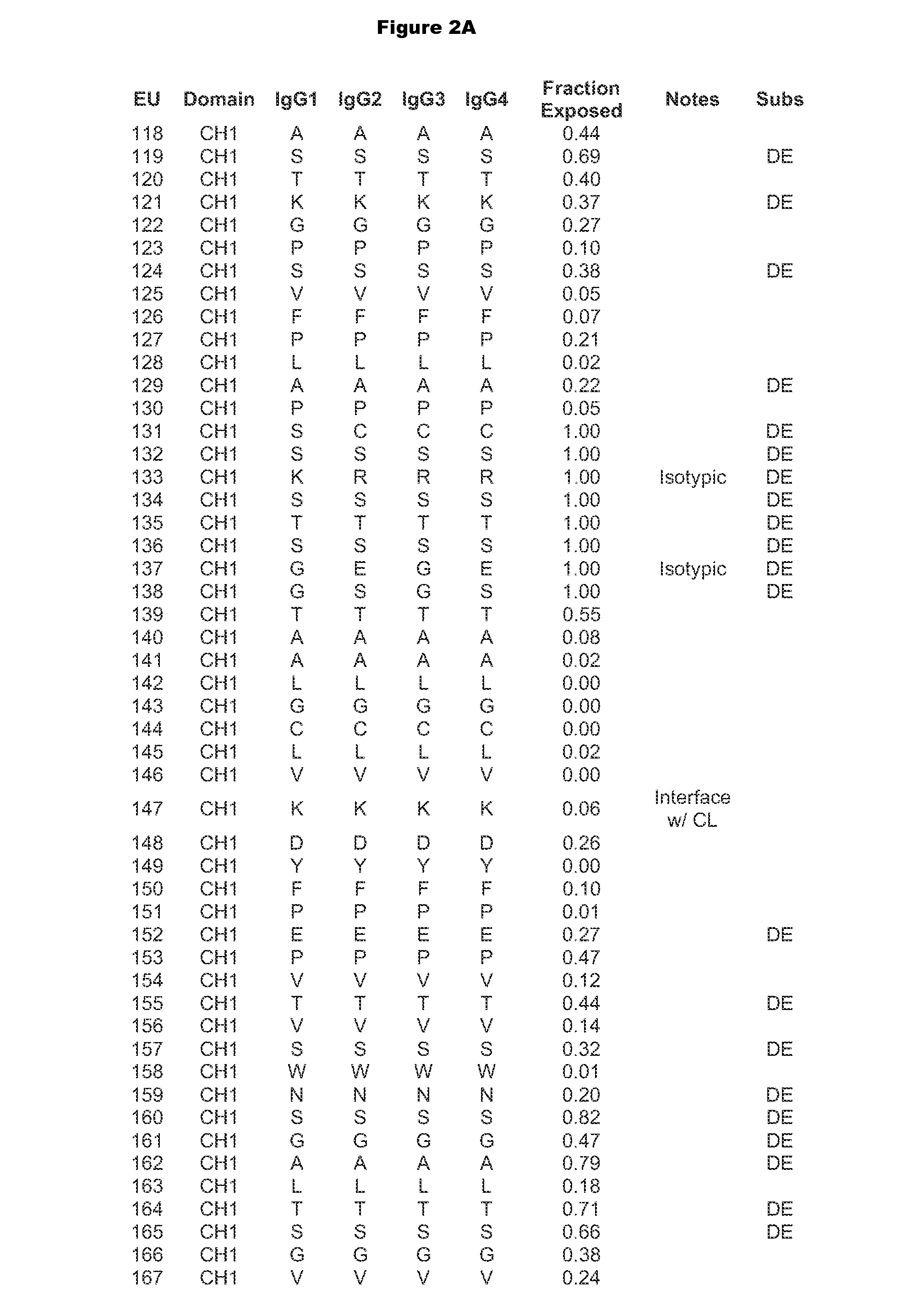
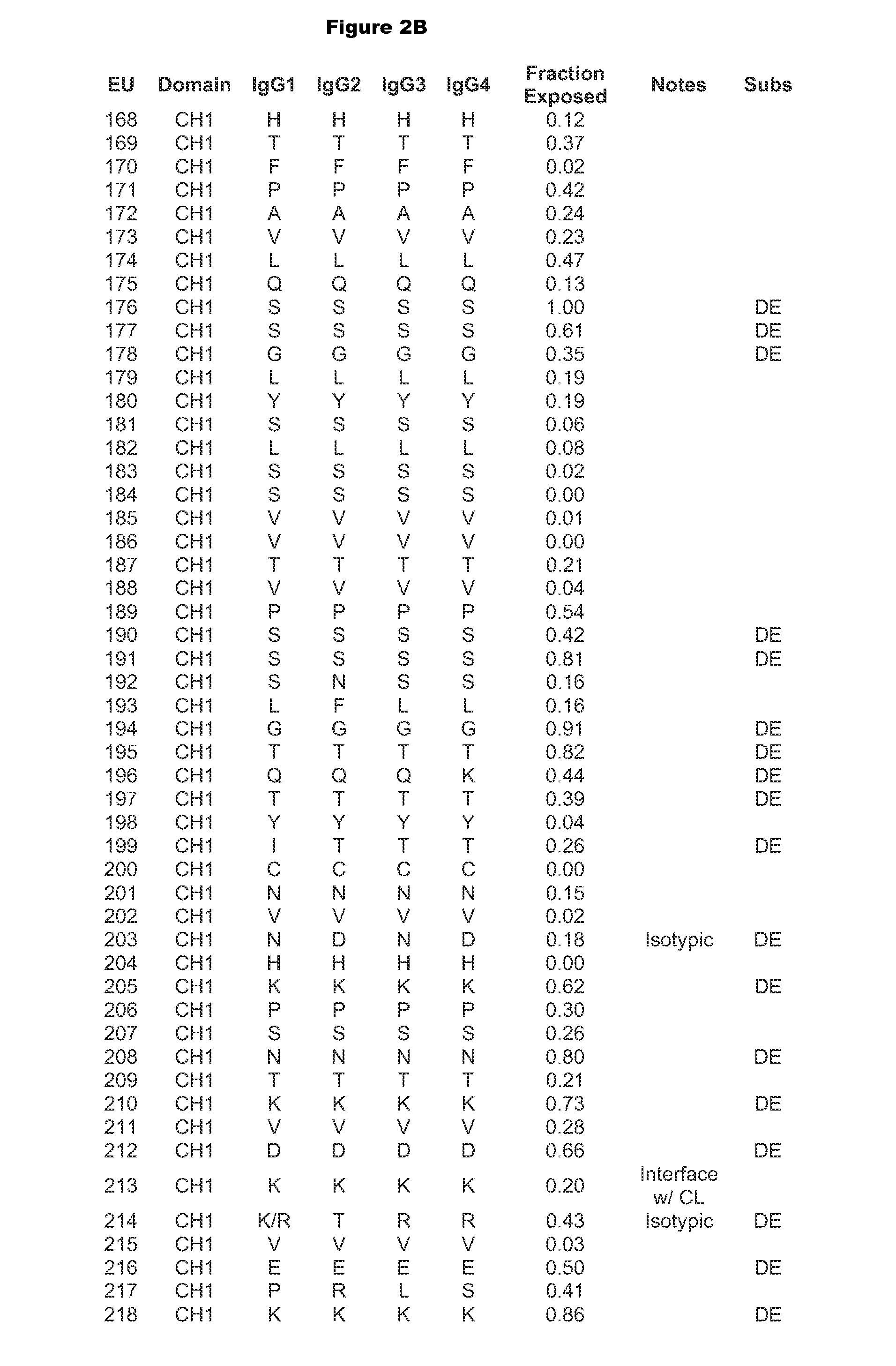
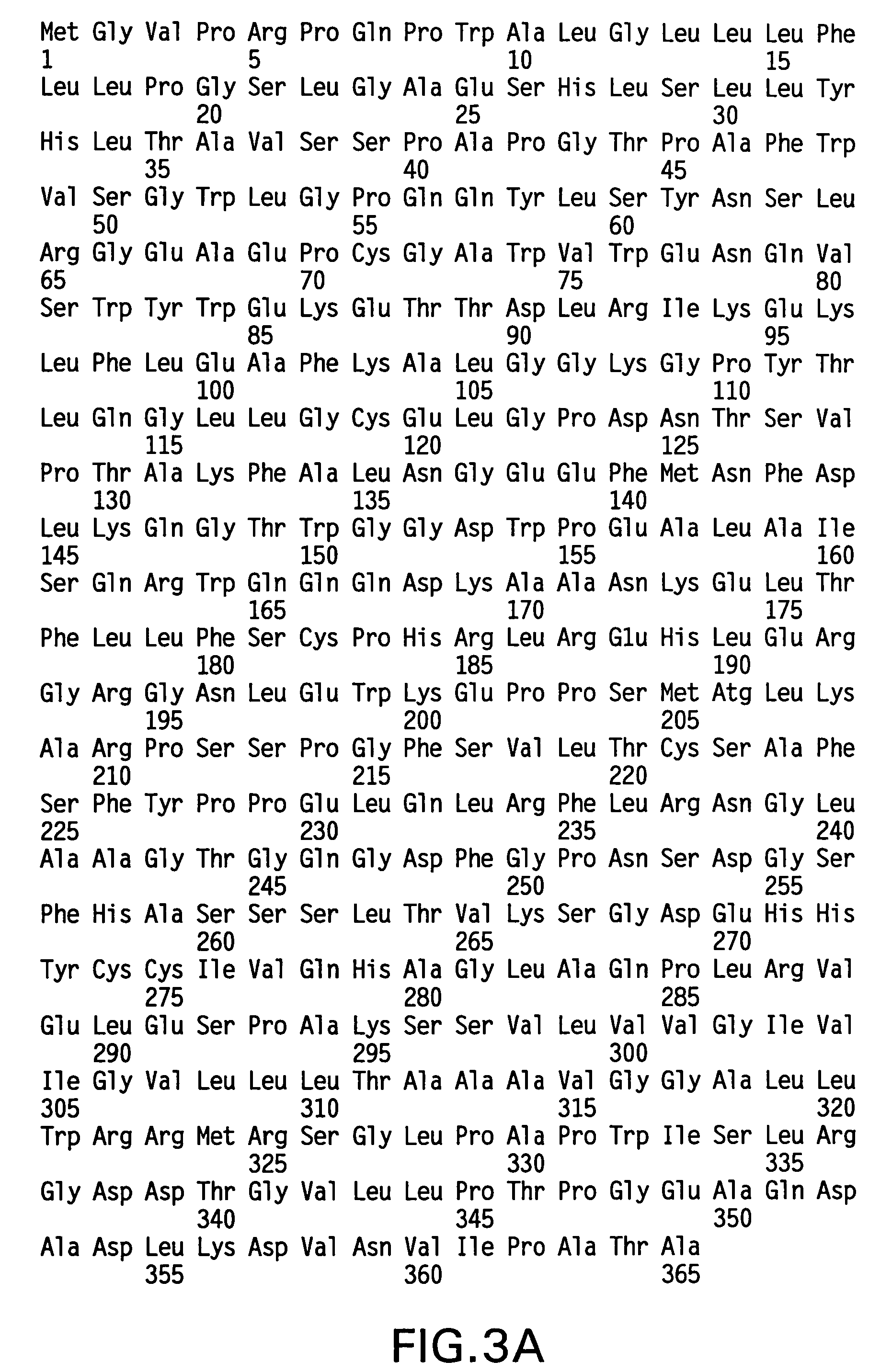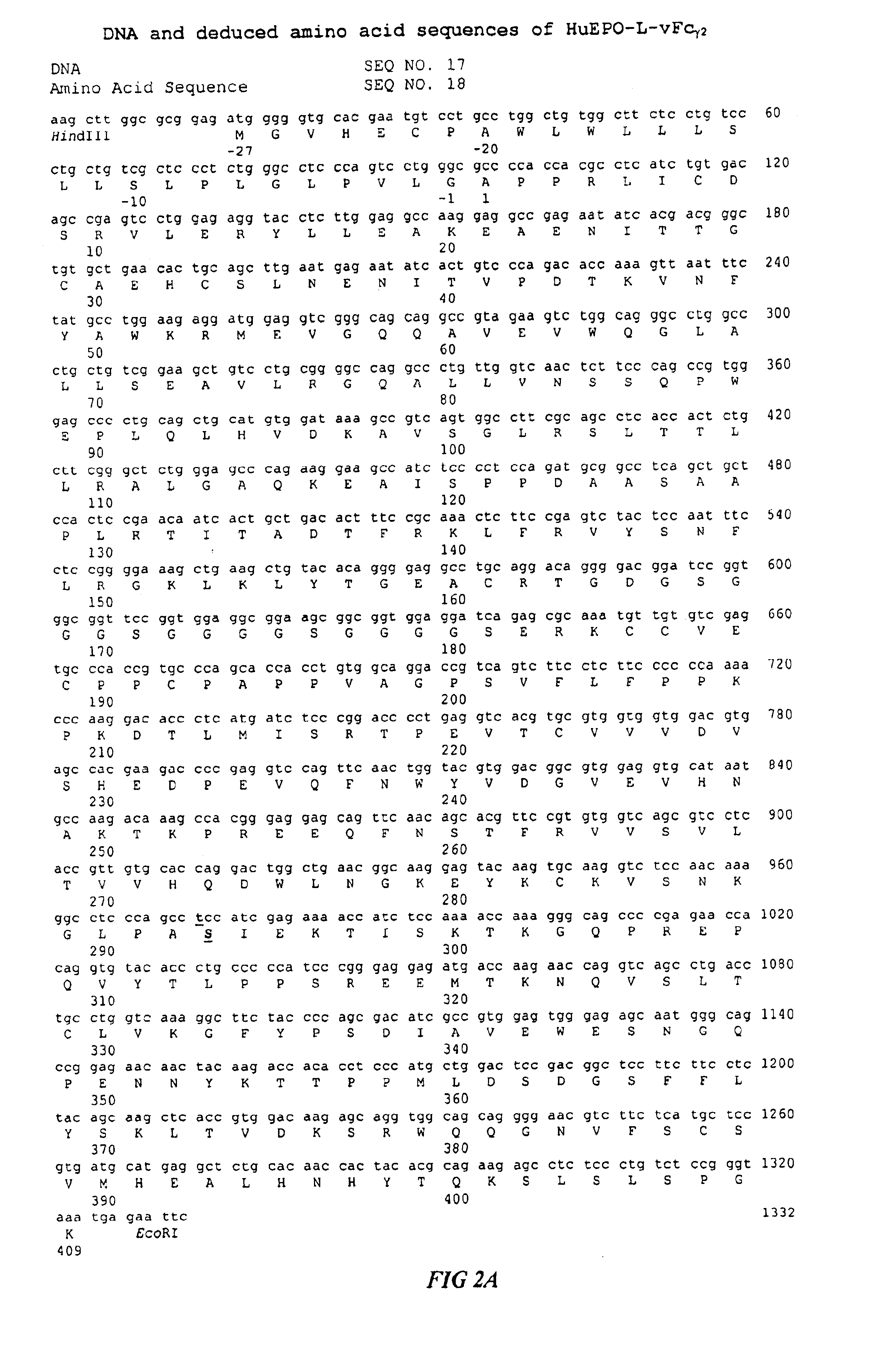Patents
Literature
2616 results about "Half-life" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Half-life (symbol t1⁄2) is the time required for a quantity to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo, or how long stable atoms survive, radioactive decay. The term is also used more generally to characterize any type of exponential or non-exponential decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life is doubling time.
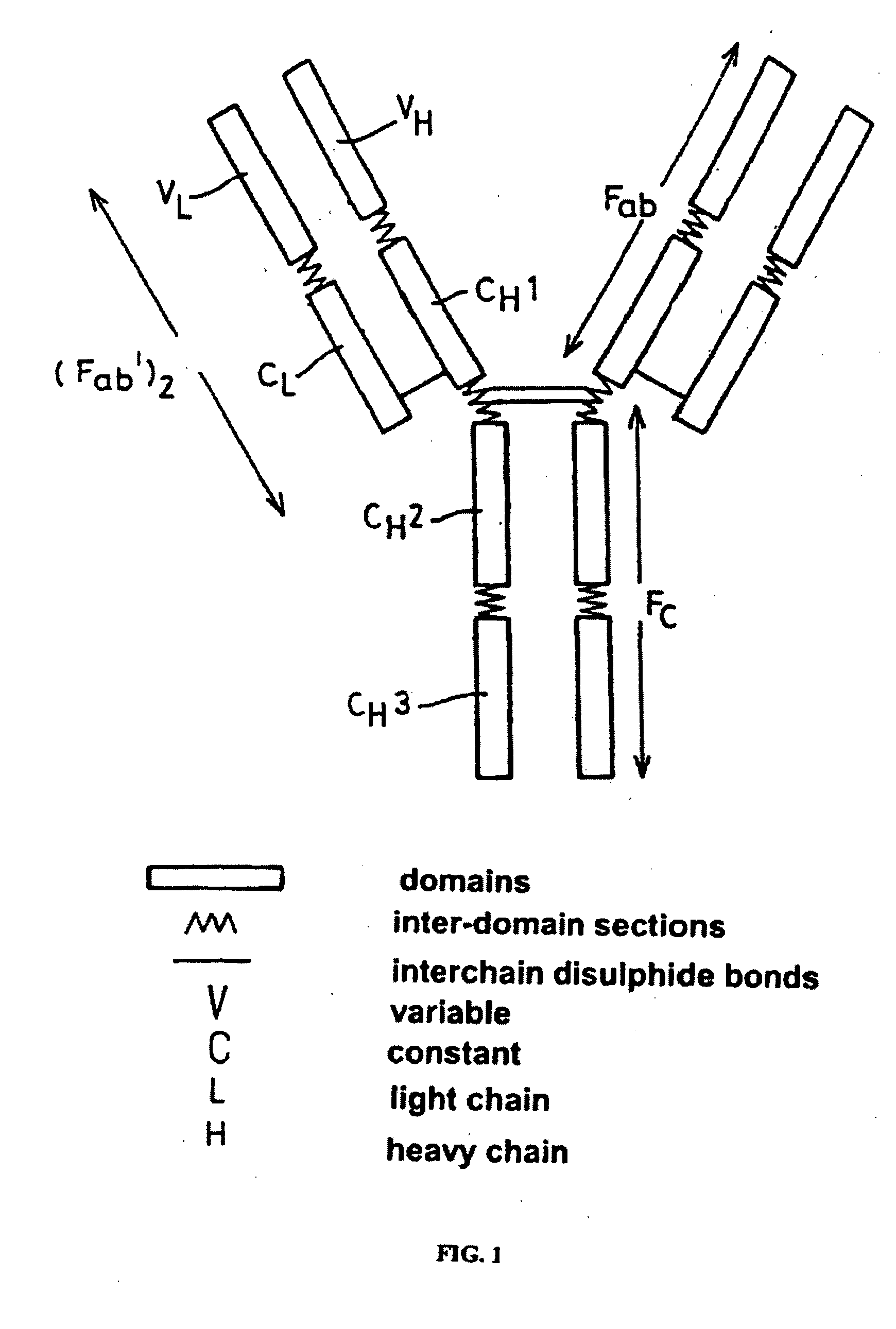
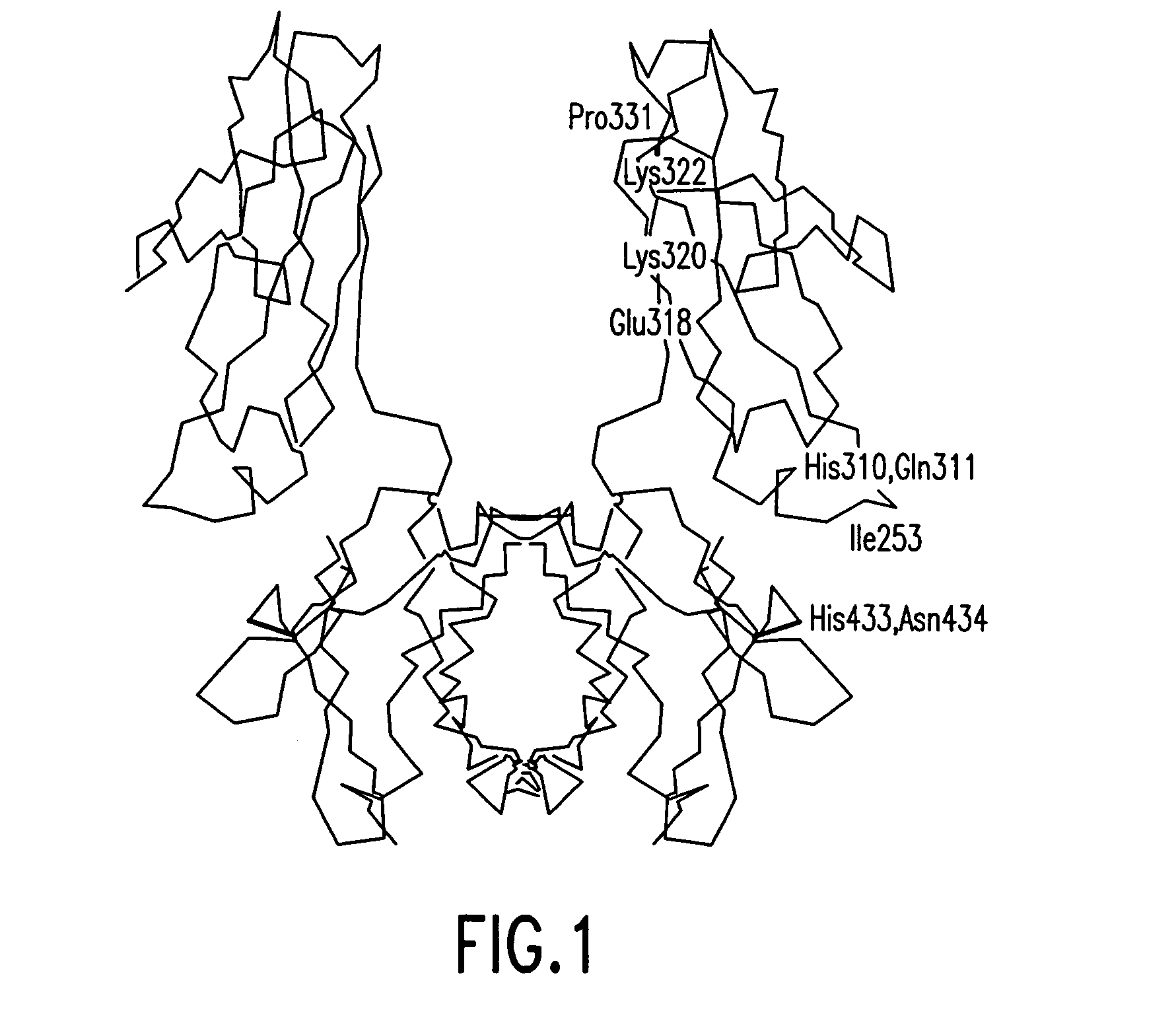
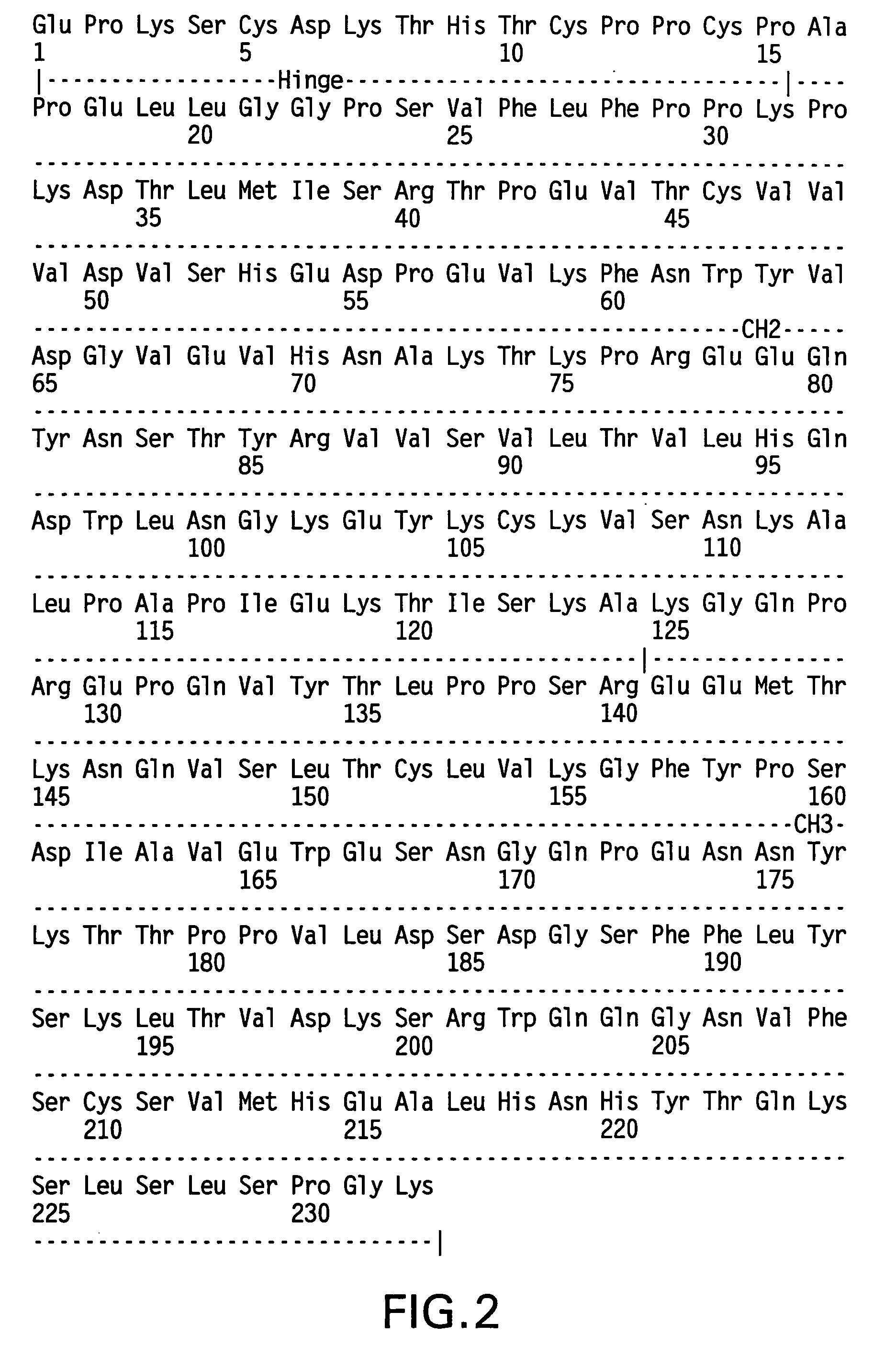
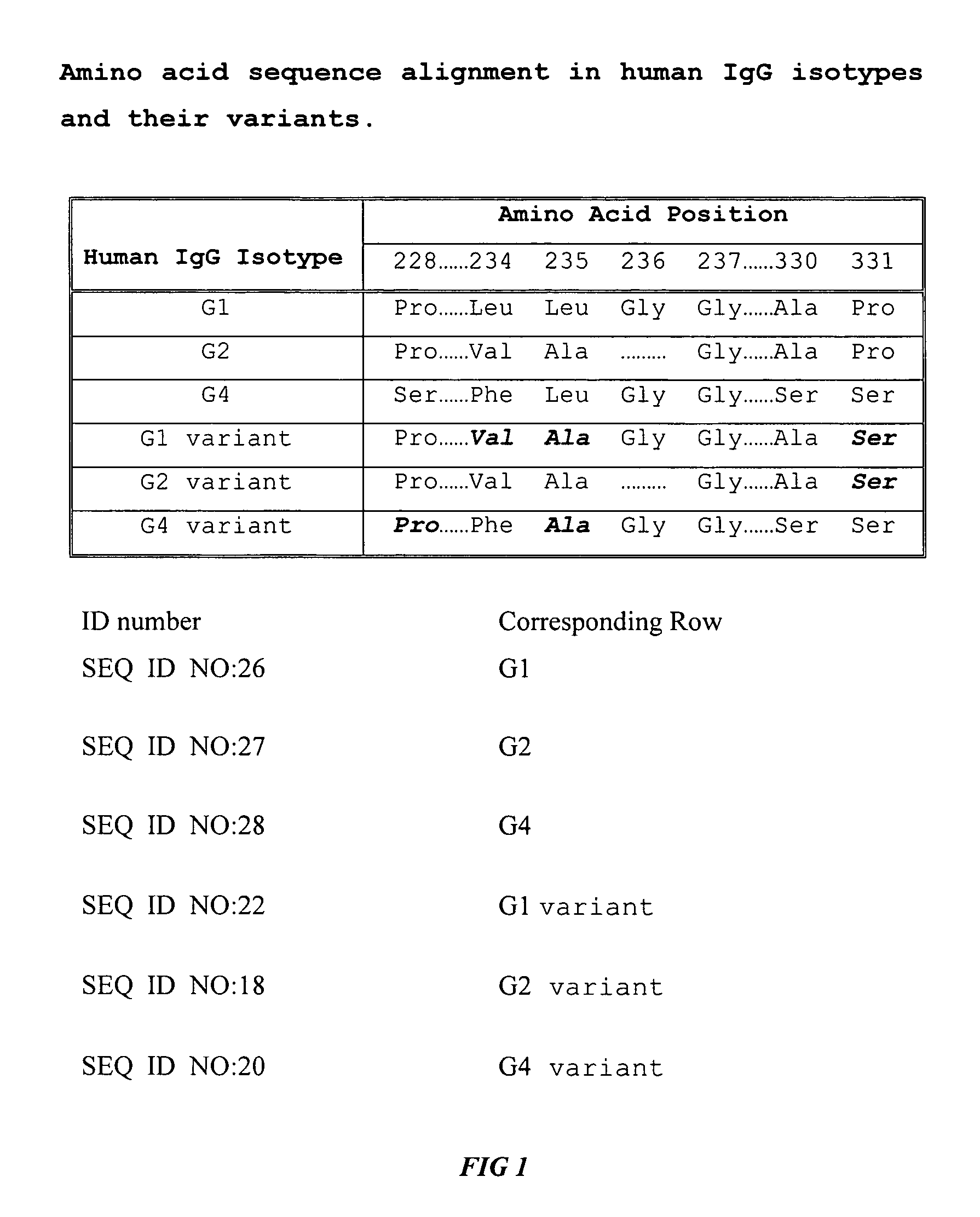
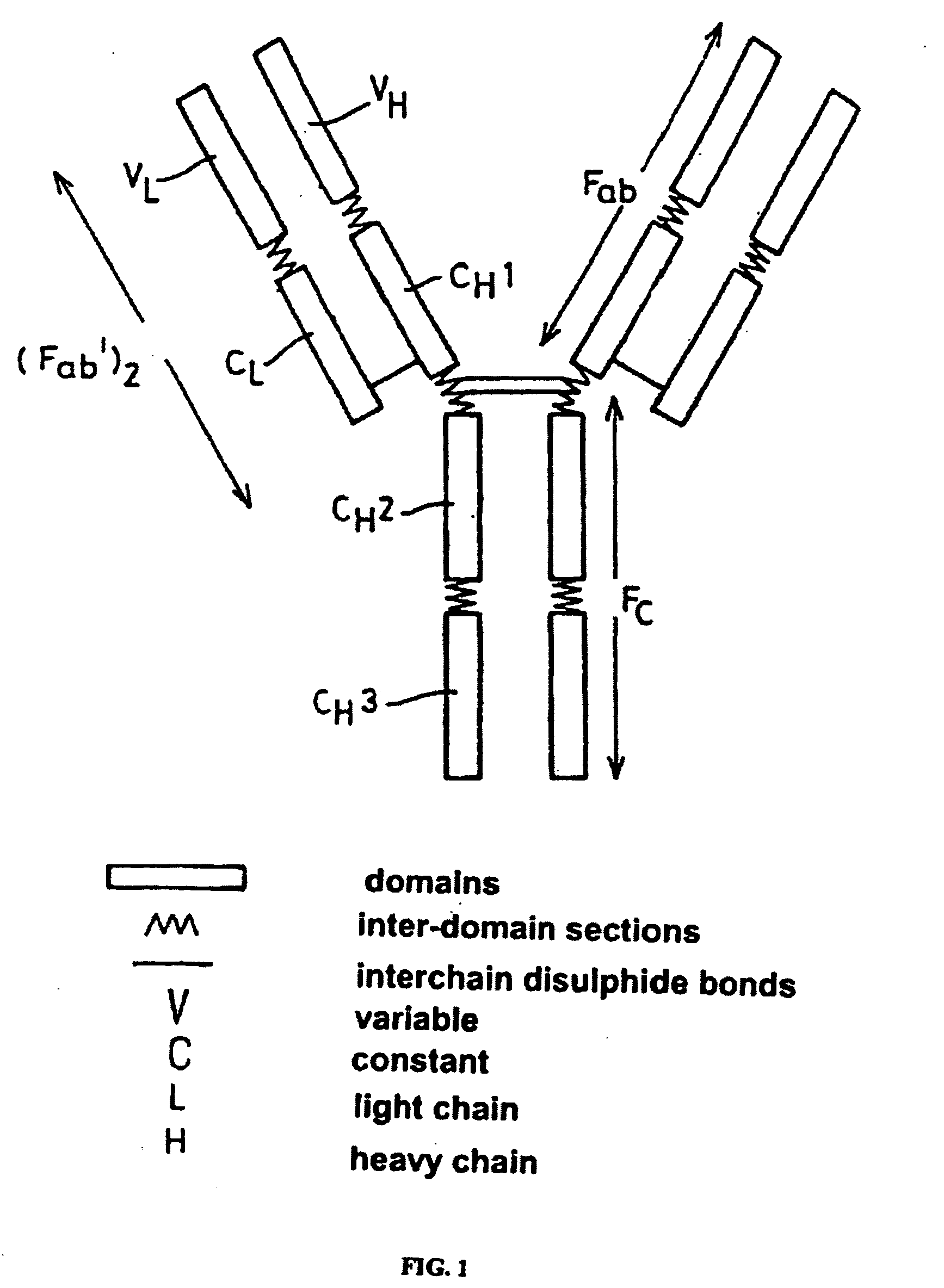
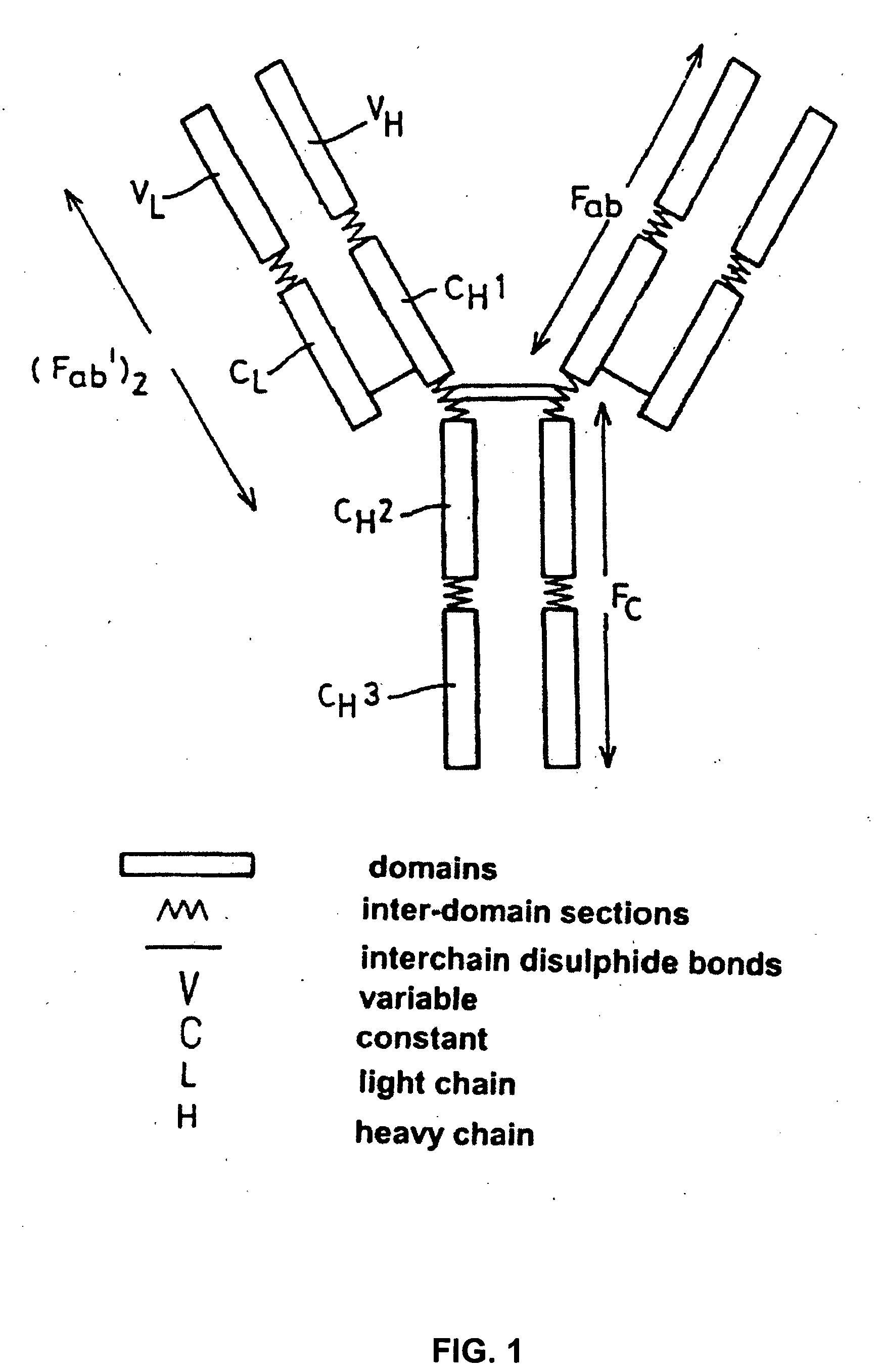
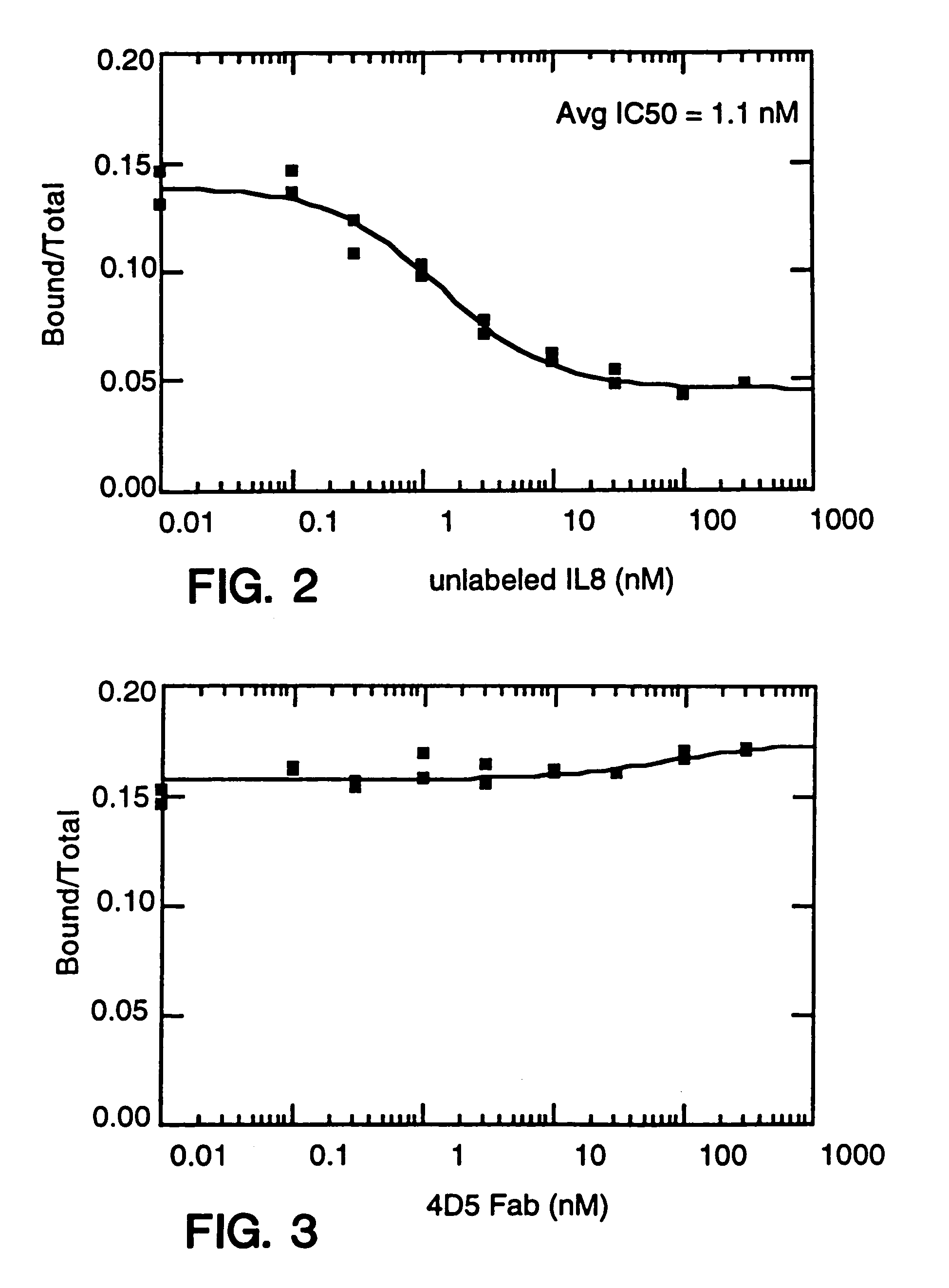
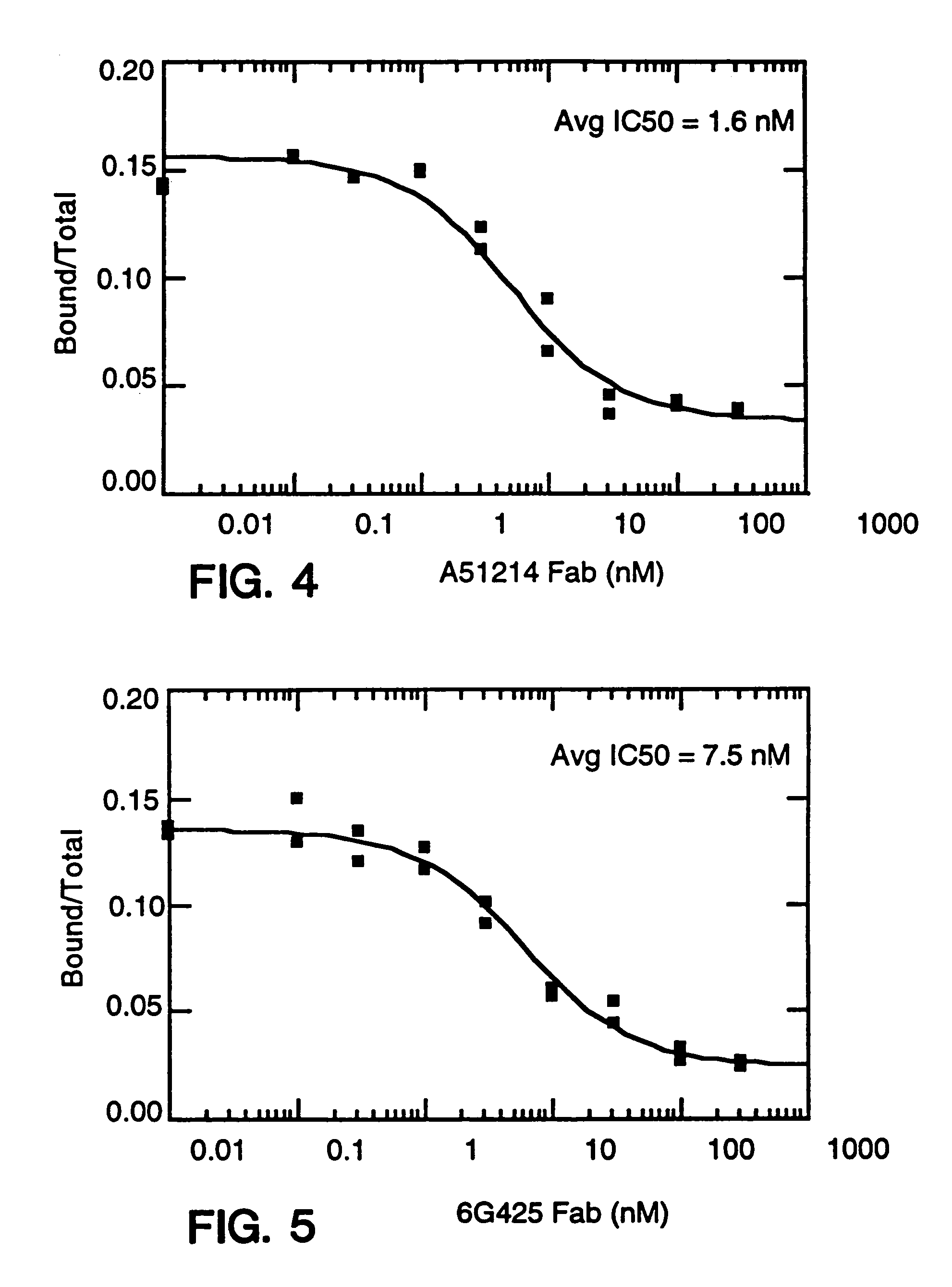
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050014934A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesHalf-lifeAntibody
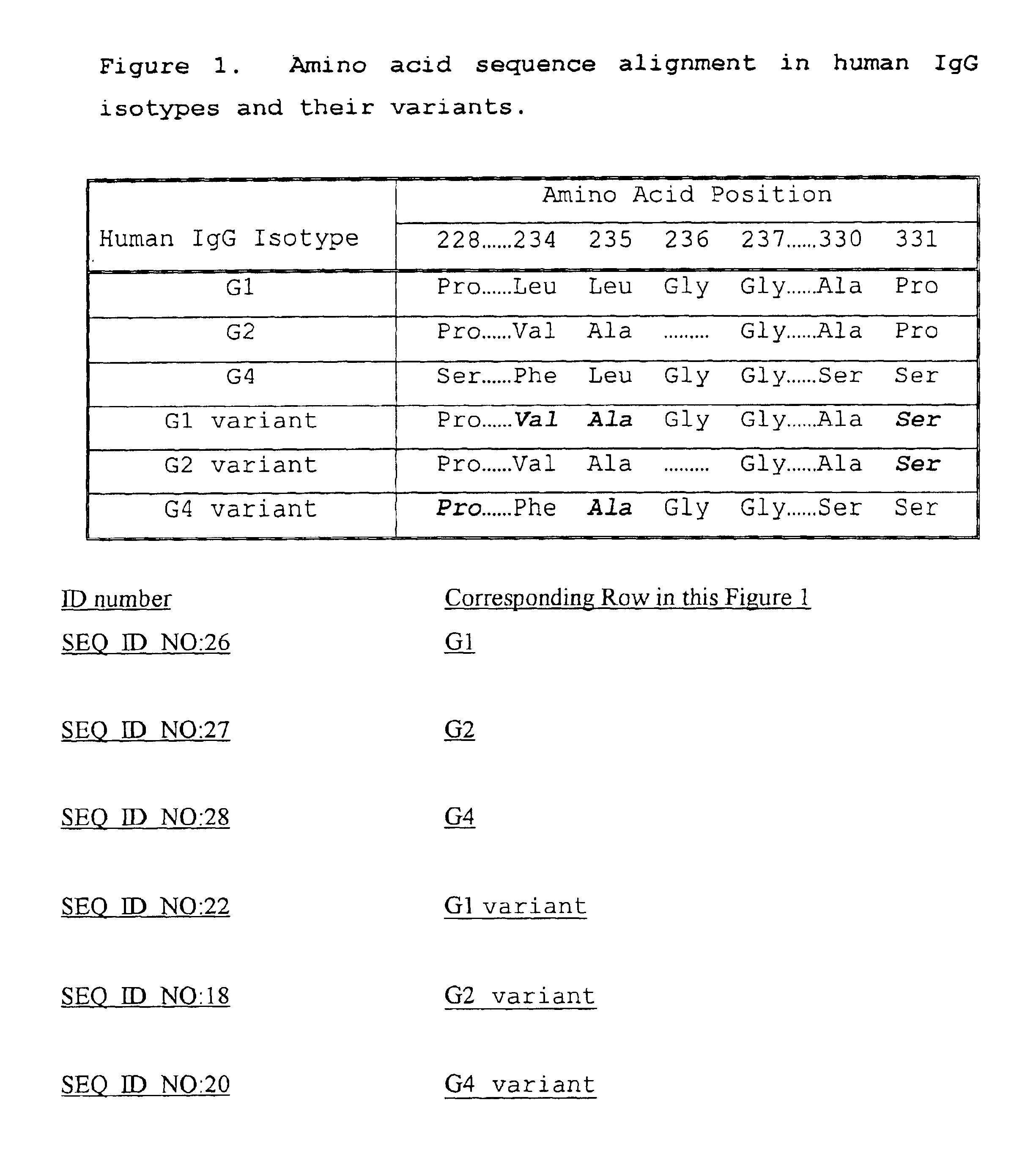
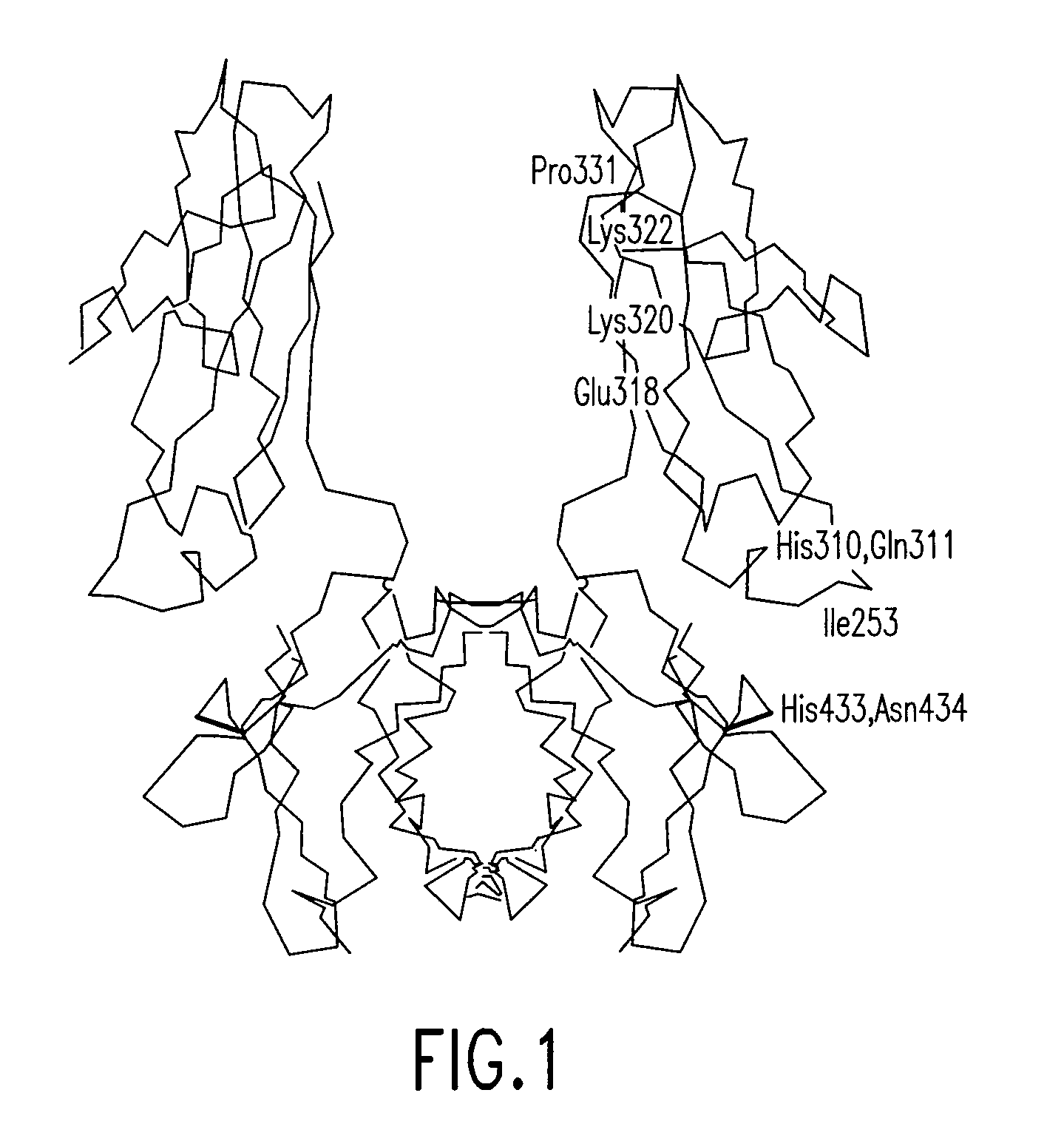
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
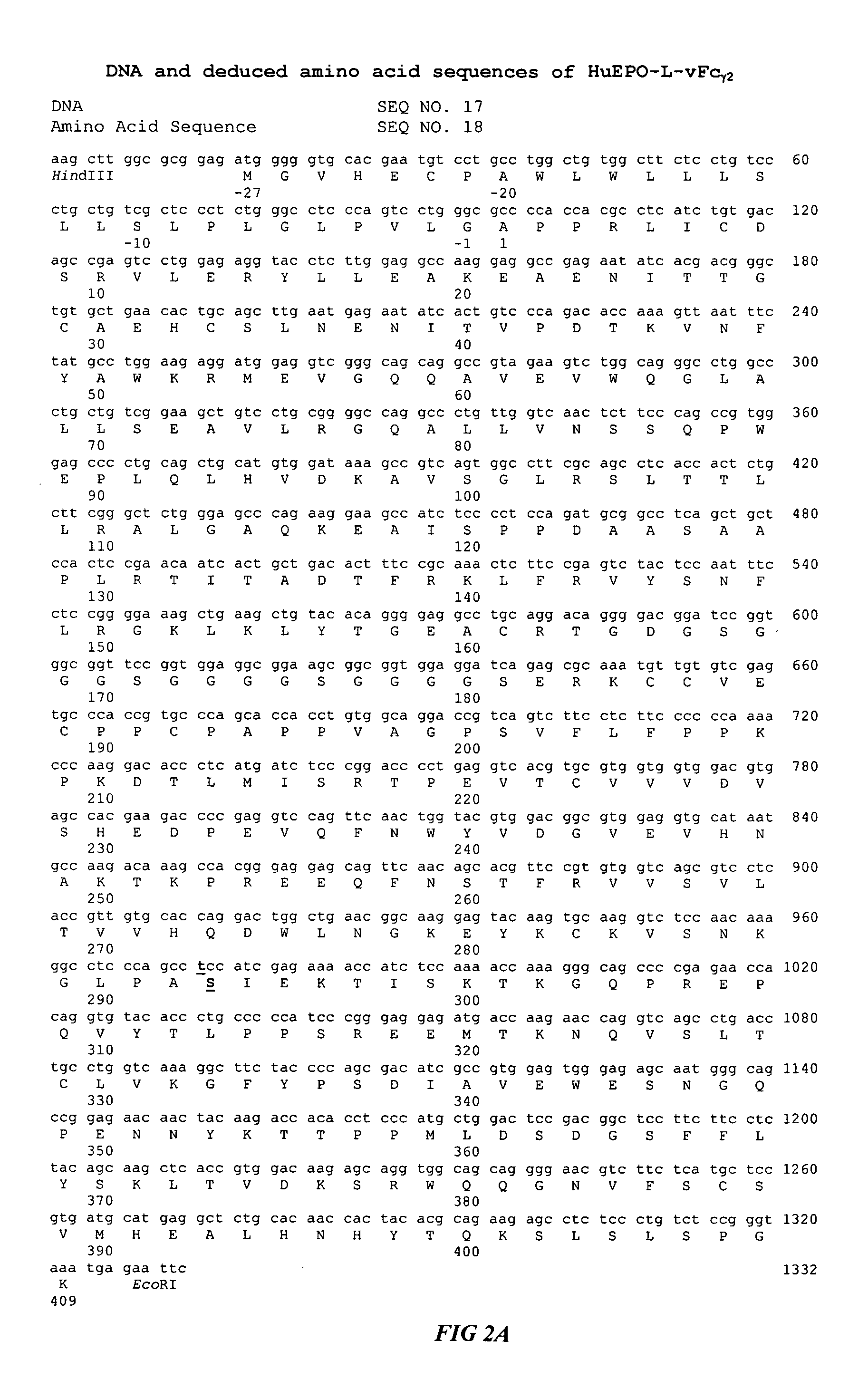
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:MEDIMMUNE LLC
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
Pseudo-antibody constructs
InactiveUS20030211078A1Reduce productionInhibit synthesisOrganic active ingredientsBiocideHalf-lifeIn vivo
This invention relates to novel pharmaceutically useful compositions that bind to a biological molecule, having improved circulatory half-life, increased avidity, increased affinity, or multifunctionality, and methods of use thereof. The present invention provides a pseudo-antibody comprising an organic moiety covalenty coupled to at least two target-binding moieties, wherein the target-binding moieties are selected from the group consisting of a protein, a peptide, a peptidomimetic, and a non-peptide molecule that binds to a specific targeted biological molecule. The pseudo-antibody of the present invention may affect a specific ligand in vitro, in situ and / or in vivo. The pseudo-antibodies of the present invention can be used to measure or effect in an cell, tissue, organ or animal (including humans), to diagnose, monitor, modulate, treat, alleviate, help prevent the incidence of, or reduce the symptoms of, at least one condition.
Owner:CENTOCOR
Novel recombinant proteins with N-terminal free thiol
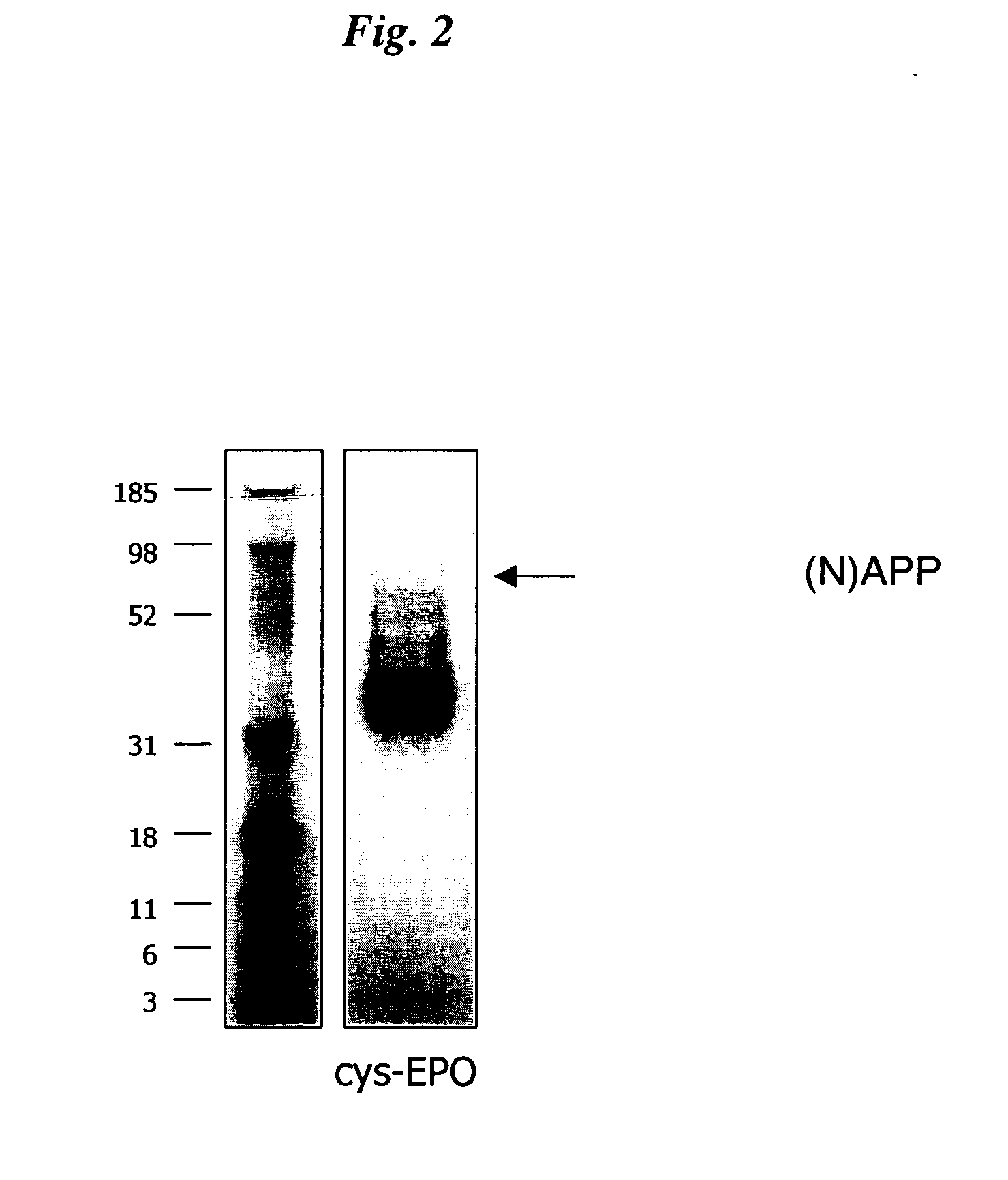
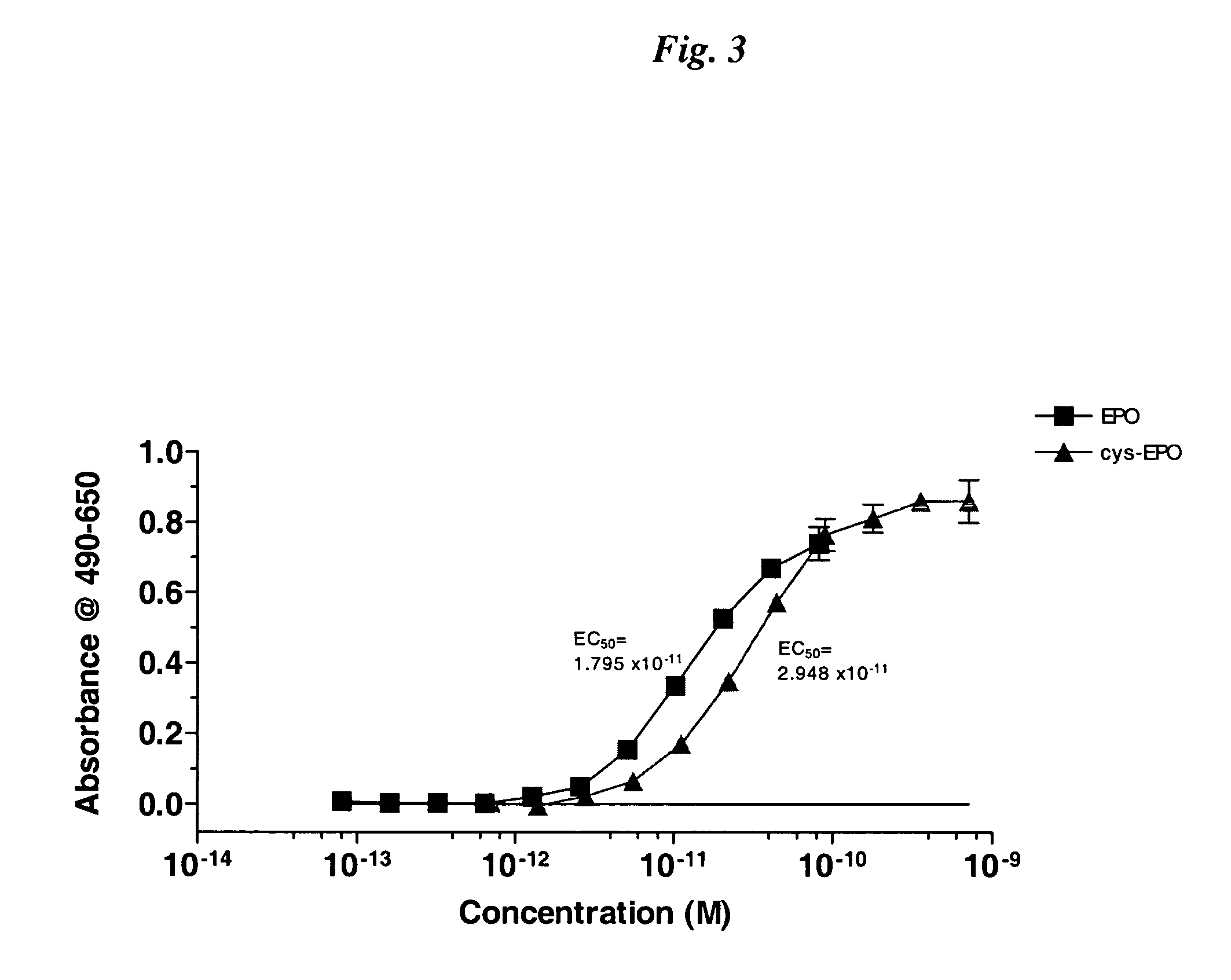
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
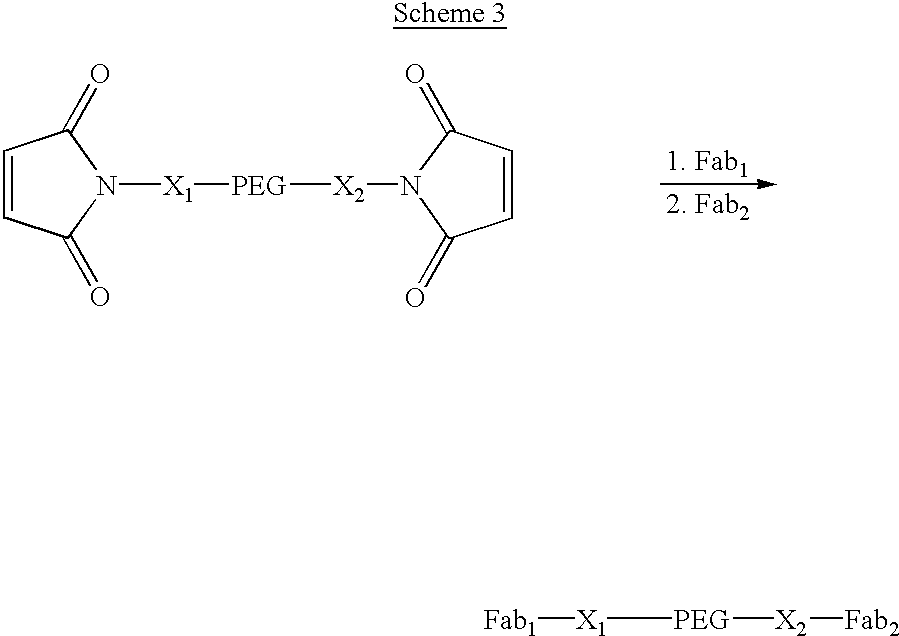
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
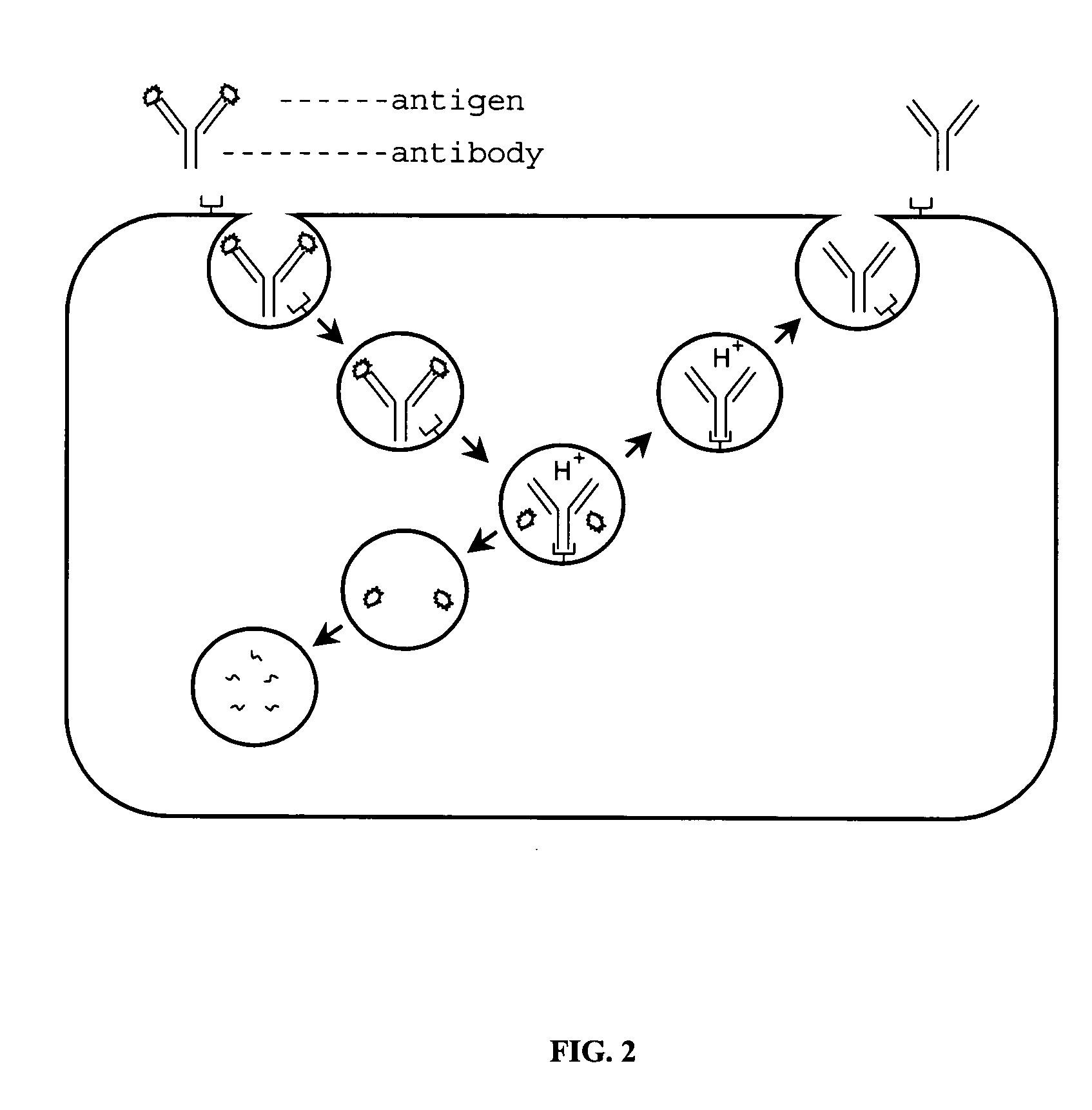
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
InactiveUS20110111406A1Pharmacokinetics of the antigen-binding molecule can be improvedGood effectSsRNA viruses negative-senseCompound screeningHalf-lifeAntigen binding
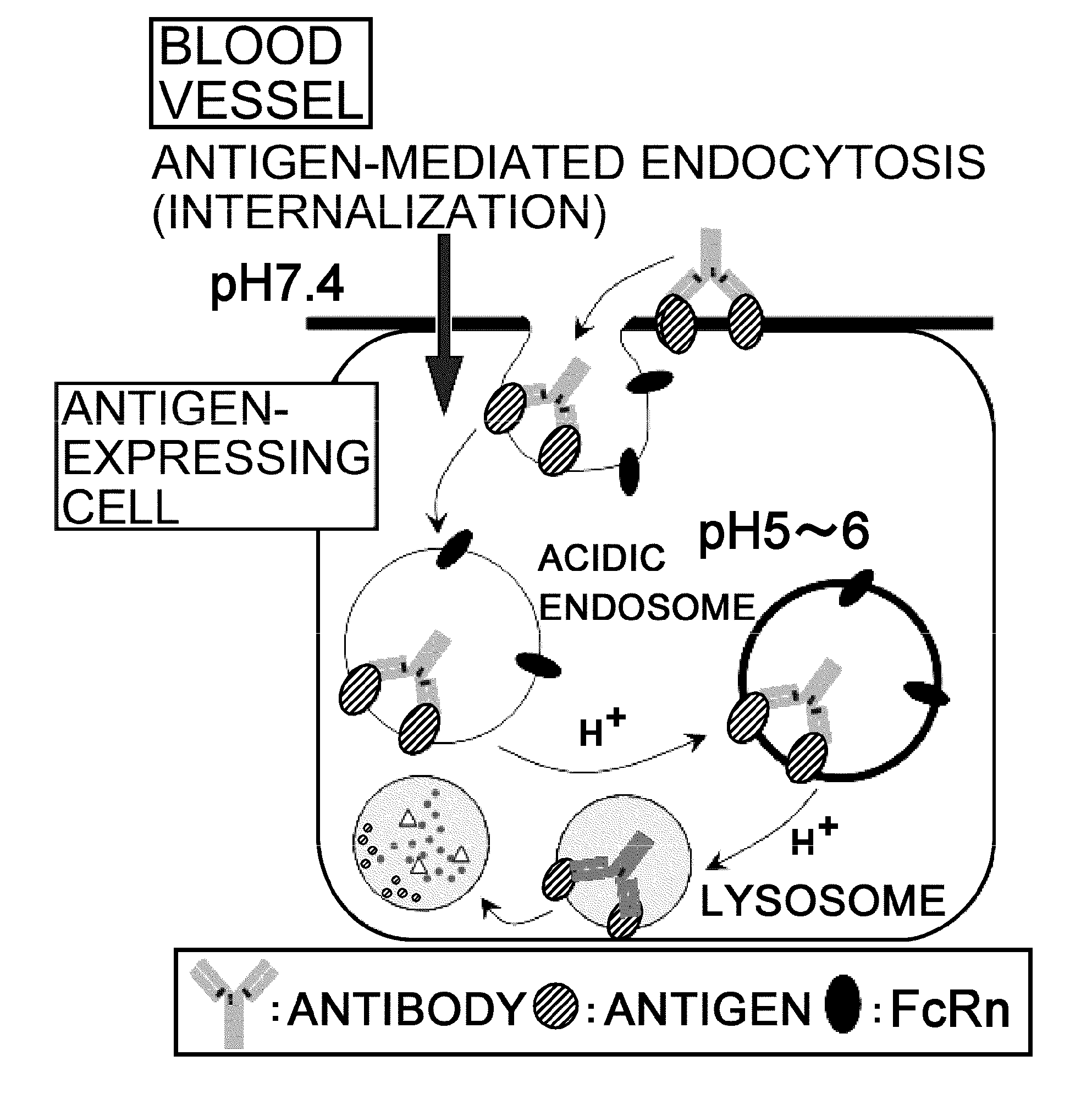
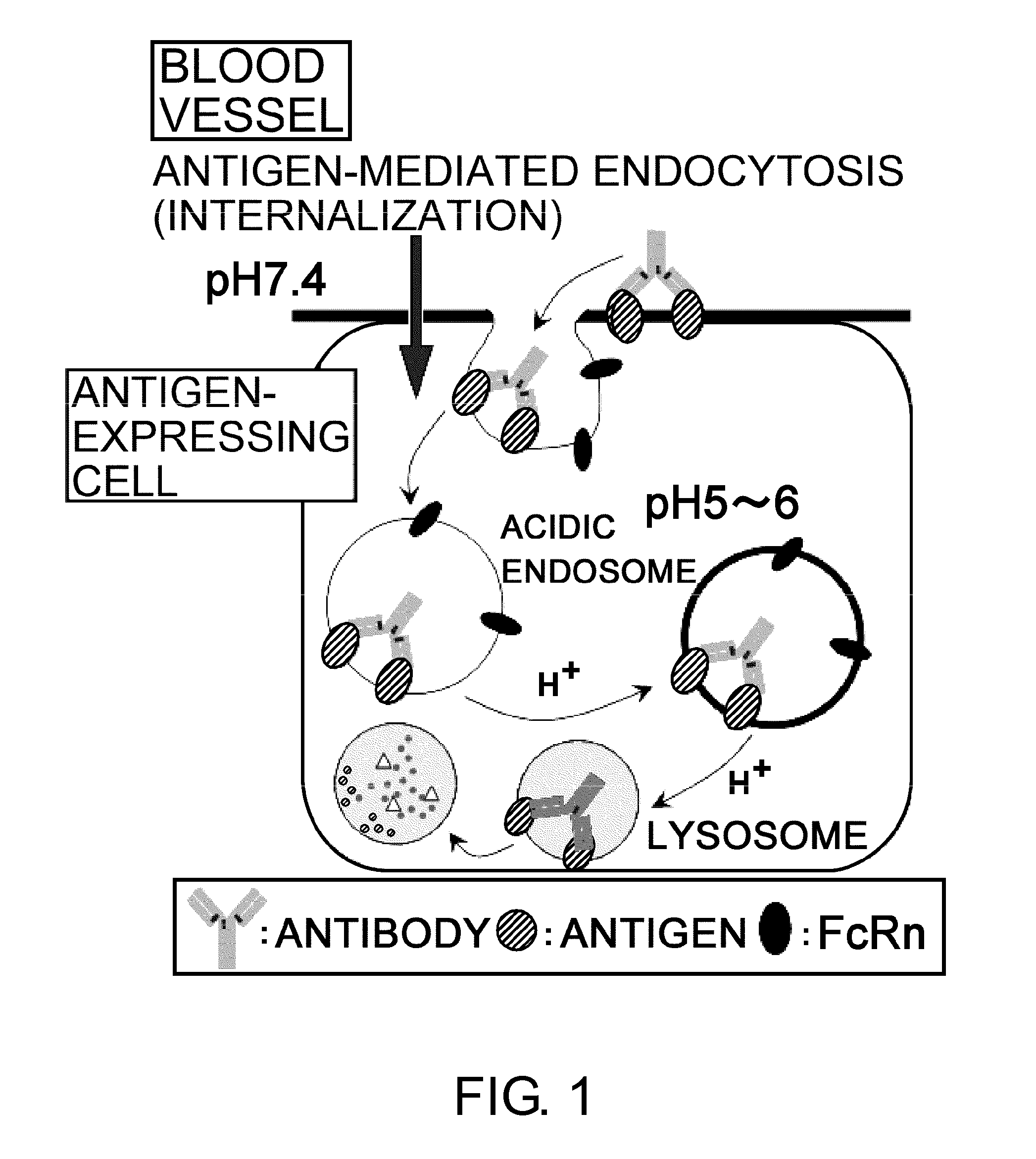
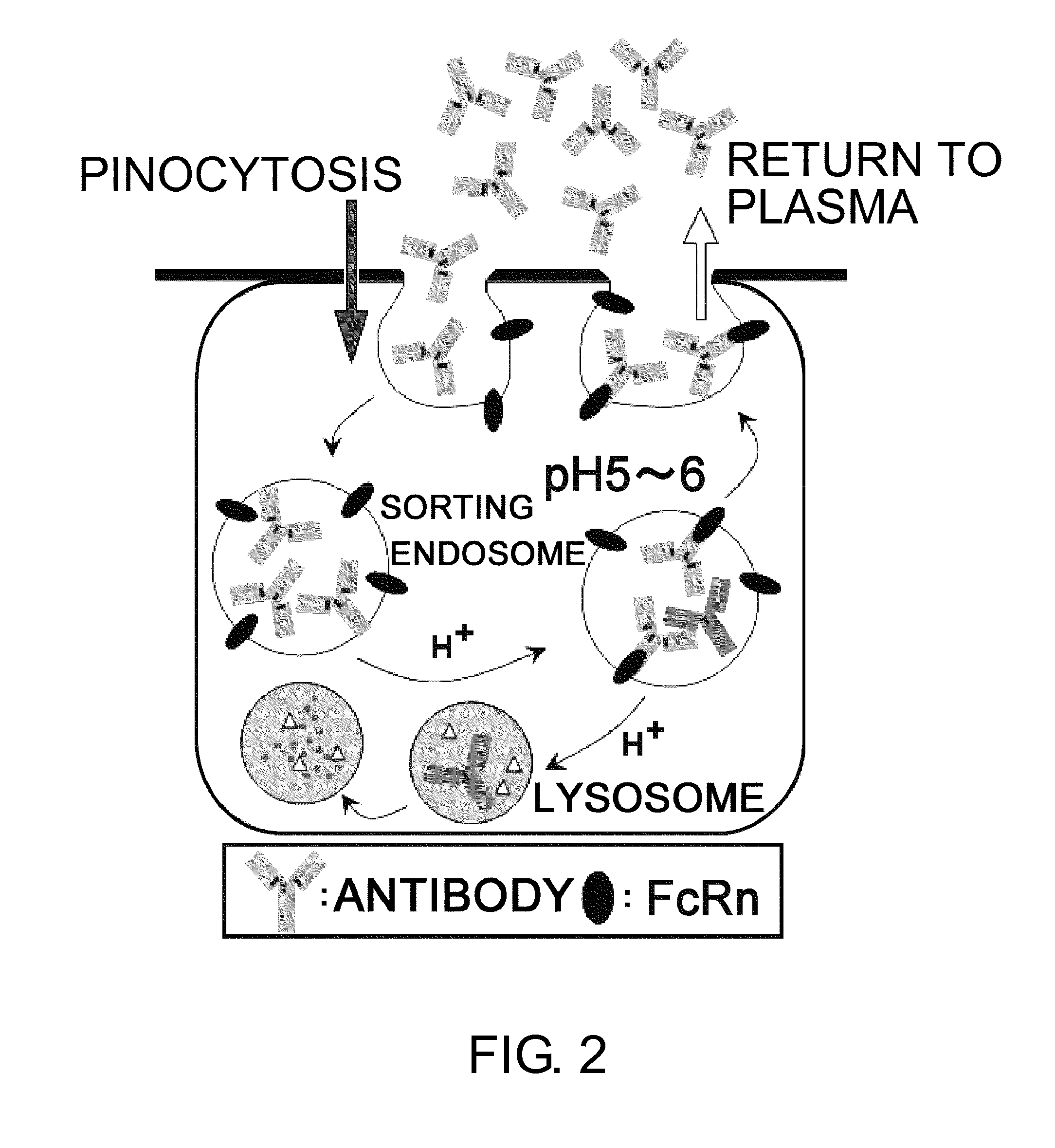
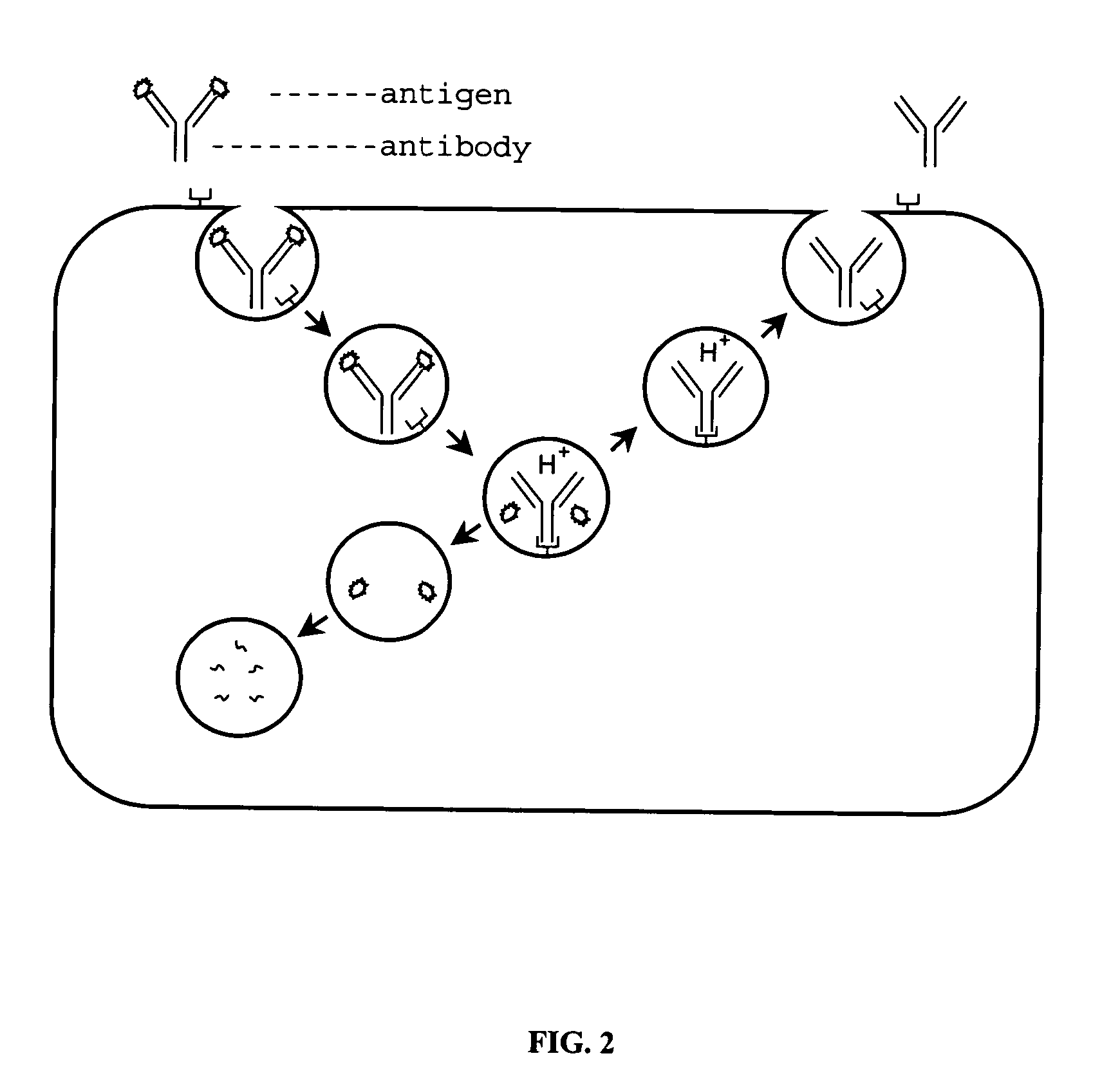
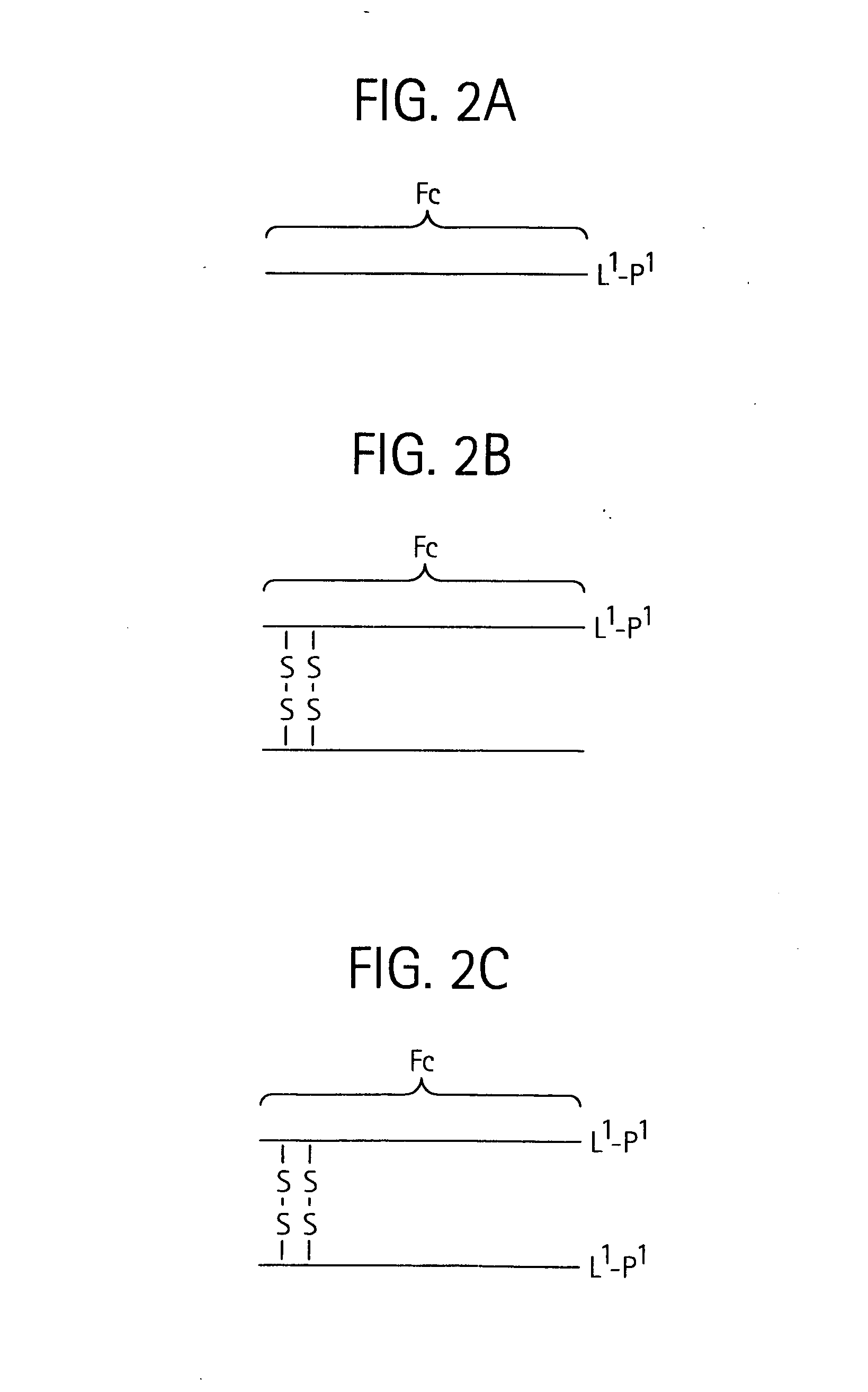
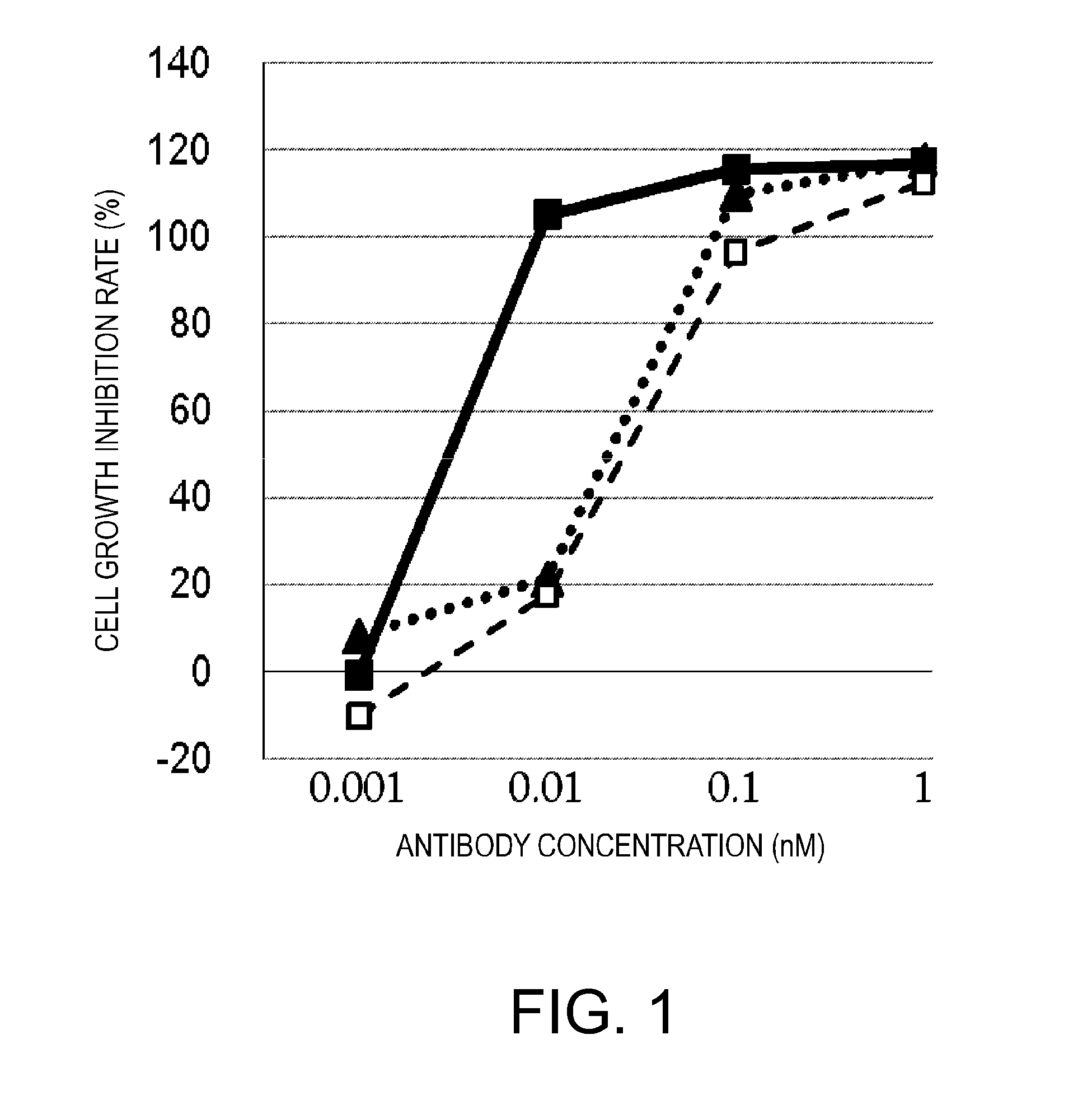
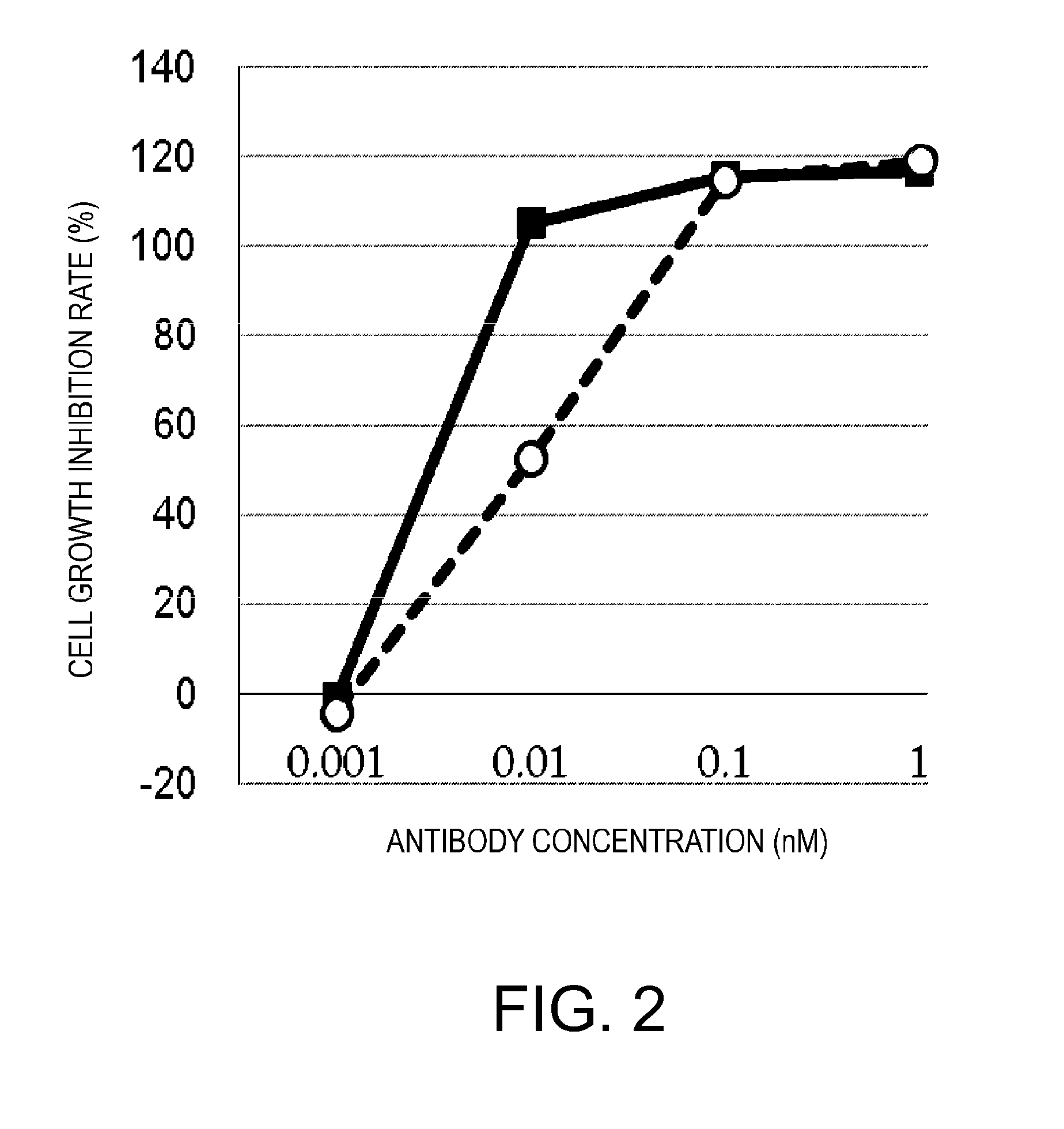
The present inventors discovered that antibodies having weaker antigen-binding activity at the early endosomal pH in comparison with that at the pH of plasma are capable of binding to multiple antigen molecules with a single antibody molecule, have long half-lives in plasma, and have improved durations of time in which they can bind to antigen.
Owner:CHUGAI PHARMA CO LTD
Generation of modified molecules with increased serum half-lives
InactiveUS20020142374A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsSerum igeHalf-life
In accordance with the present invention, there are provided methods for the extension of serum half-lives of proteinaceous molecules, particularly antibody molecules, and compositions of molecules modified in accordance with the methods of the invention. In accordance with a first aspect of the present invention, there is provided a method of modifying the half-life of an antibody through providing an antibody containing an FcRn binding domain or the genes encoding such antibody and physically linking the antibody or the antibody as encoded to a second FcRn binding domain. In accordance with a second aspect of the present invention, there is provided a molecule that contains at least two distinct FcRn binding moieties.
Owner:ABQENIX INC
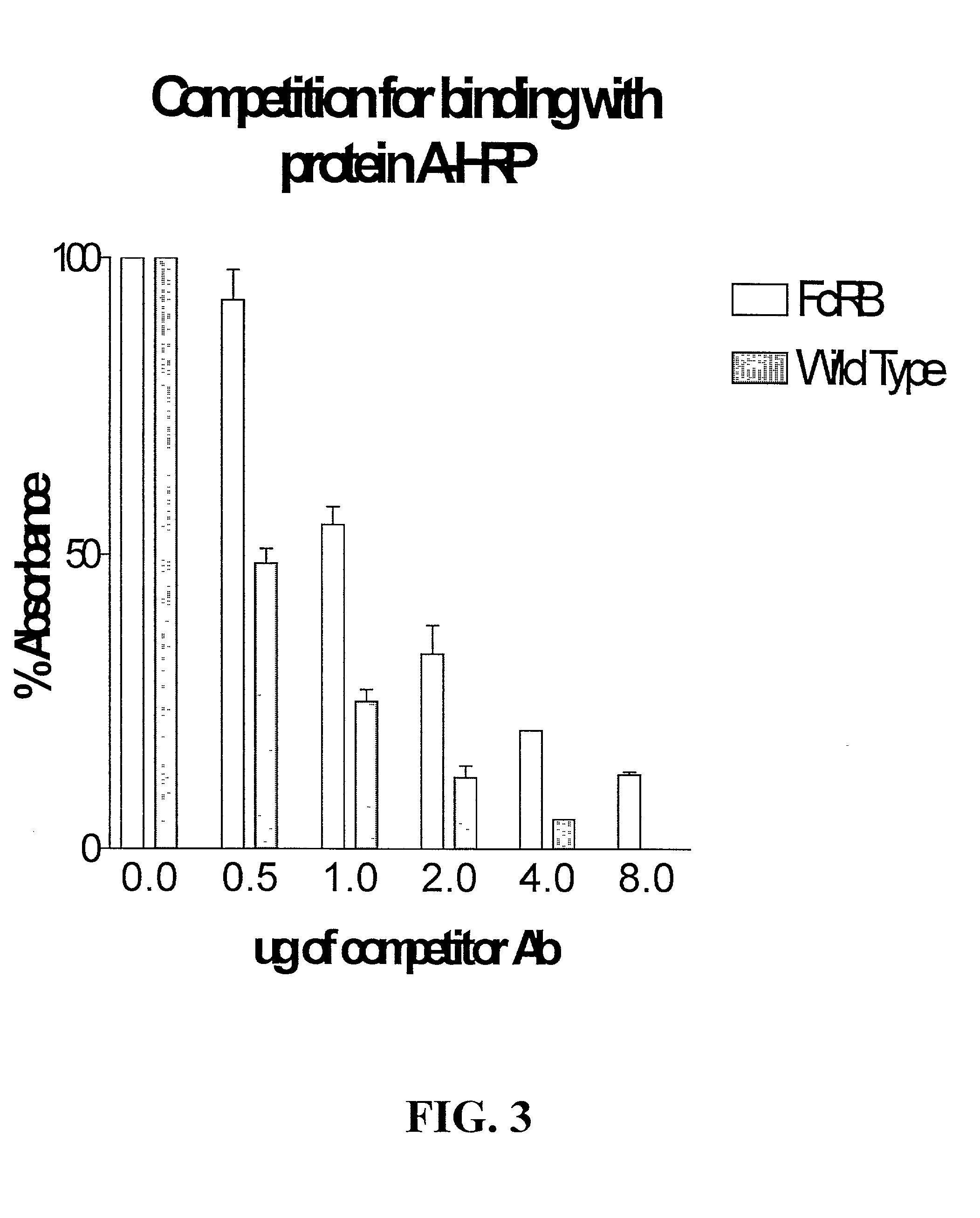
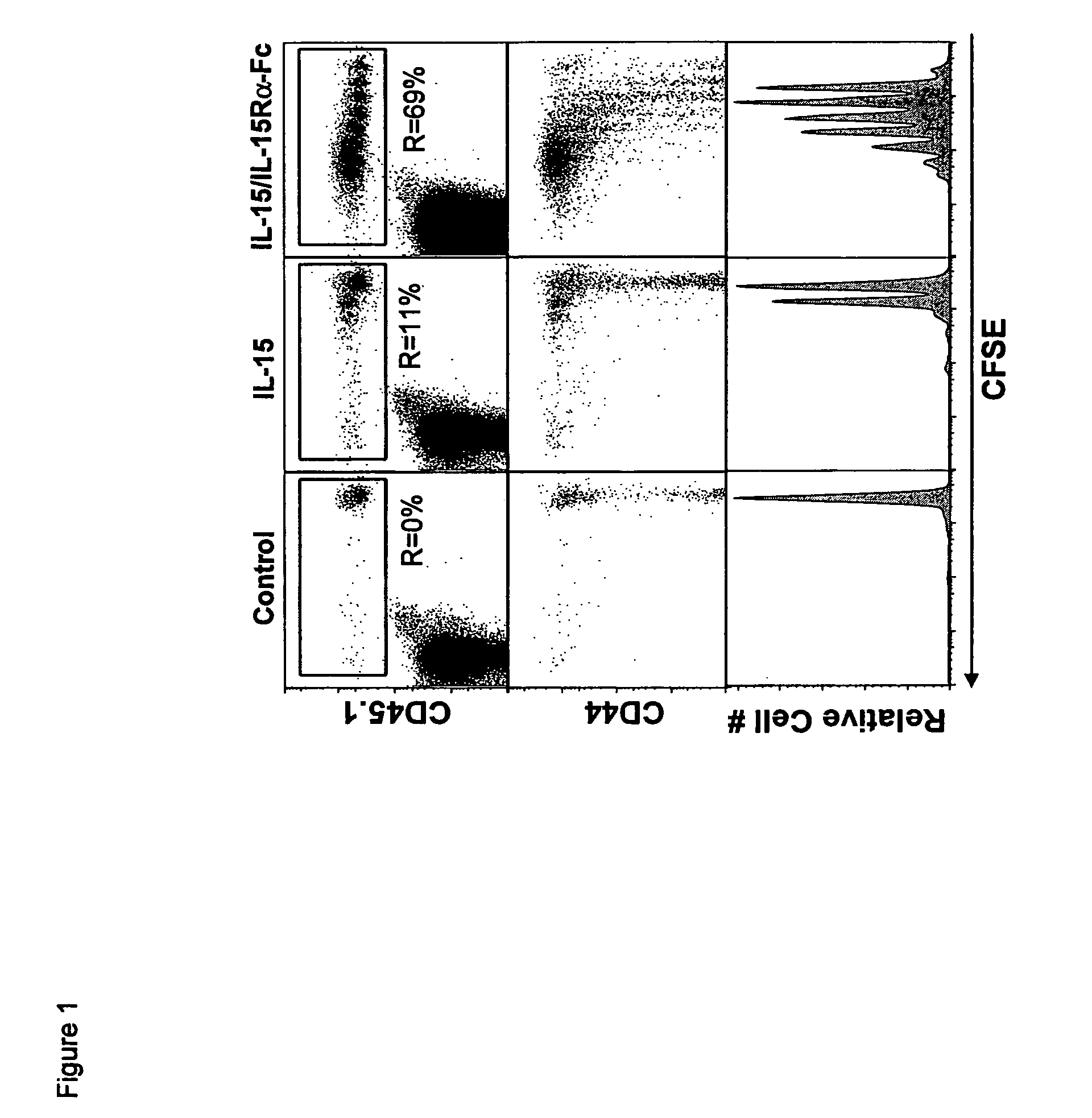
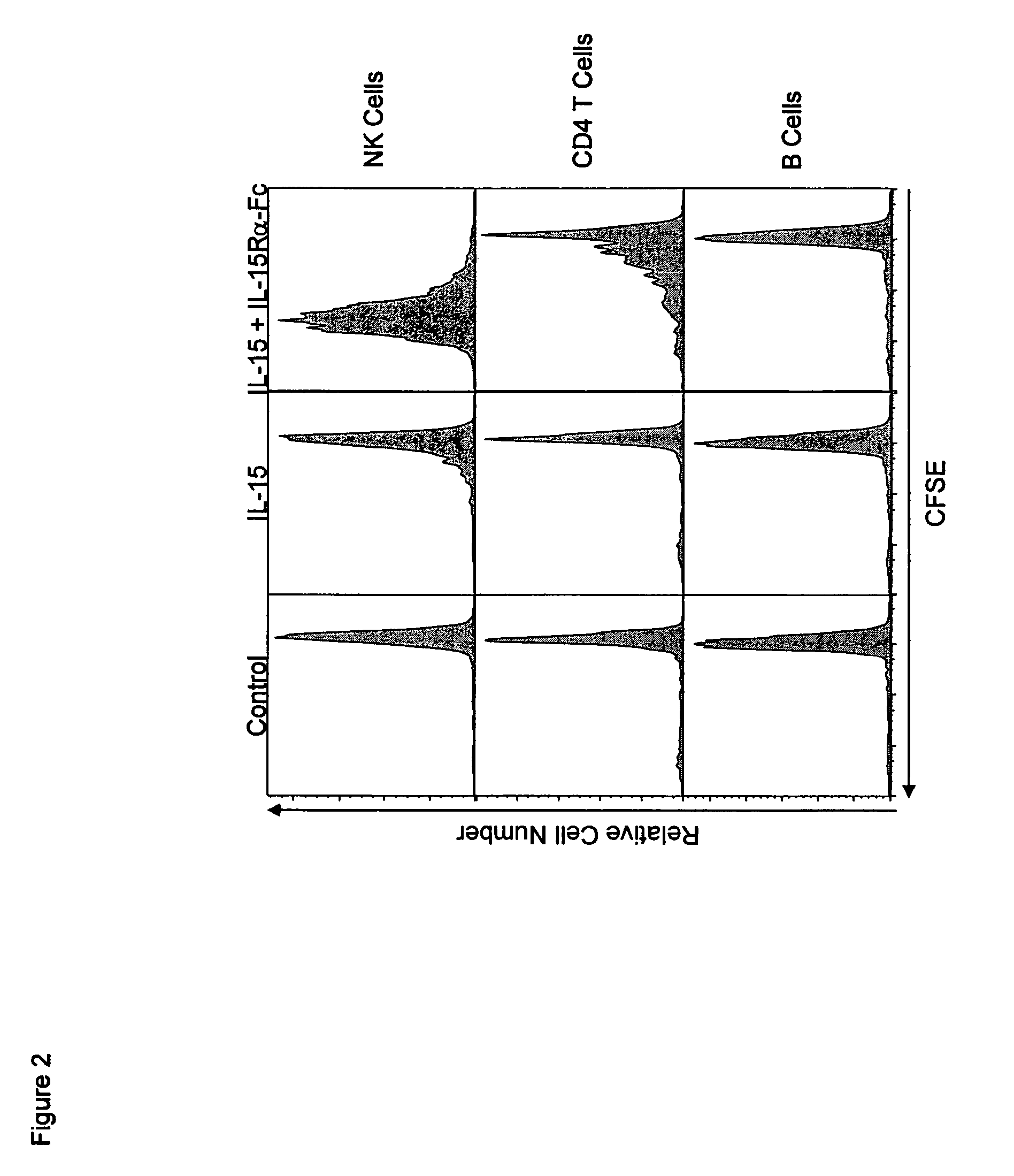
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
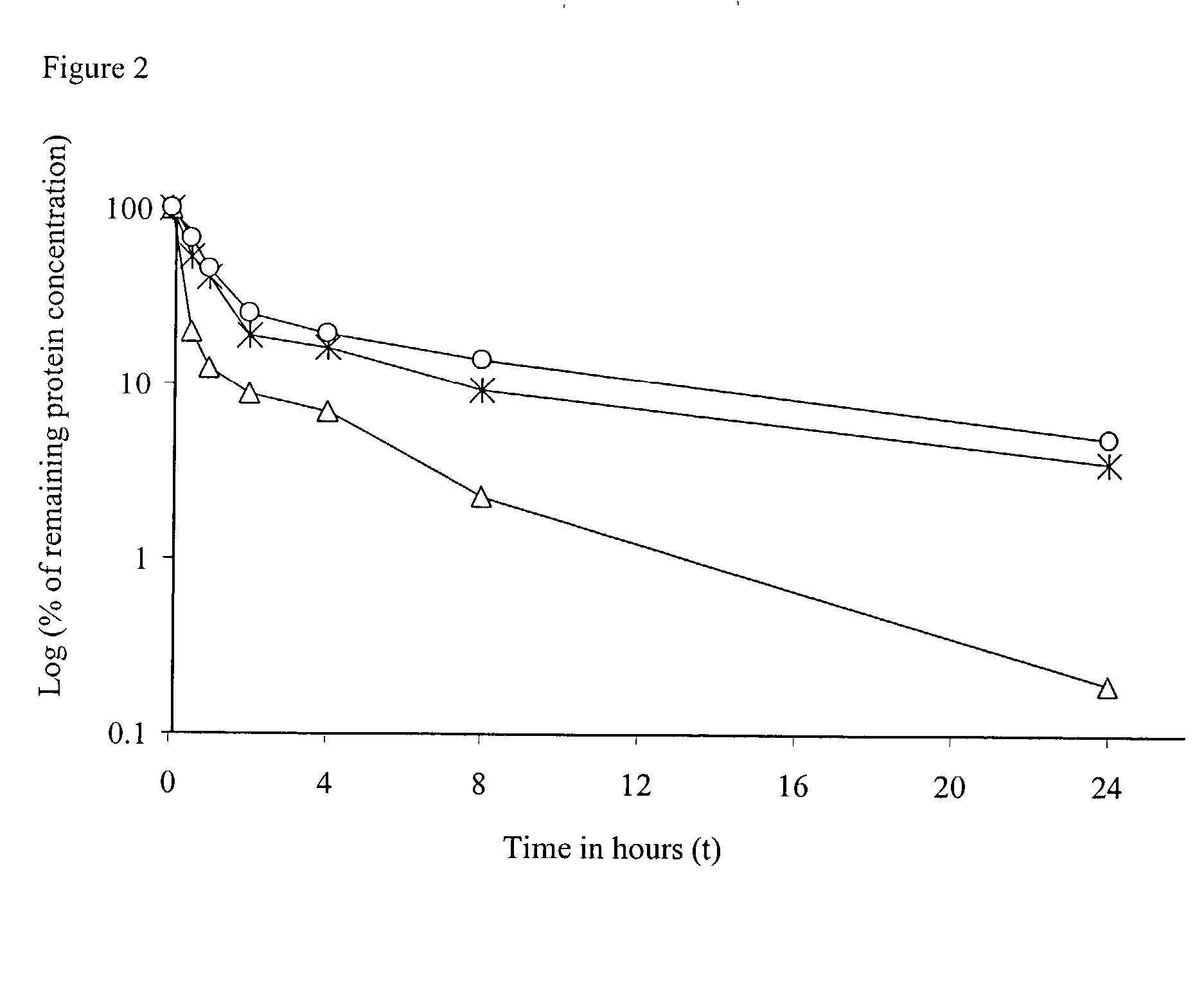
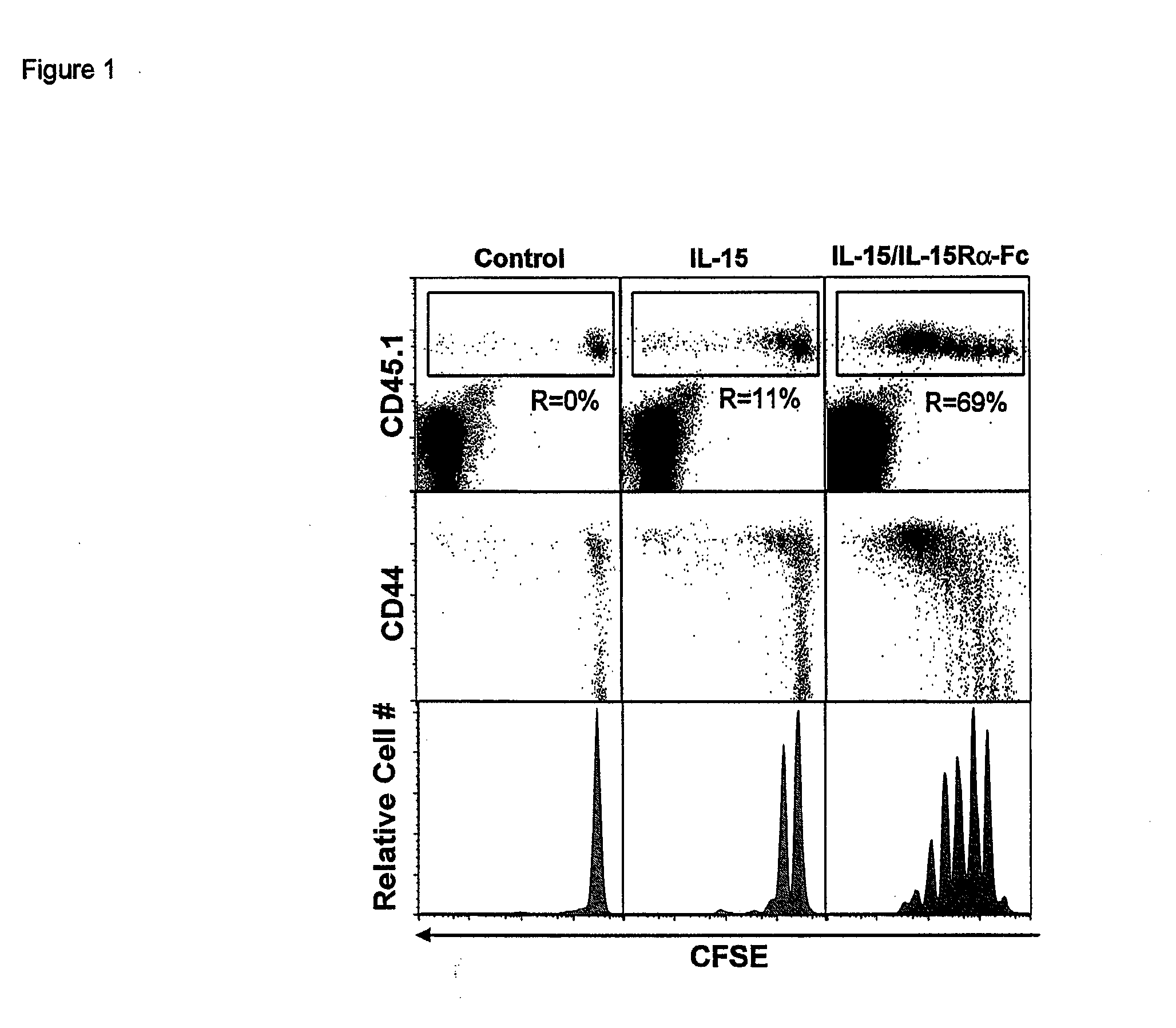
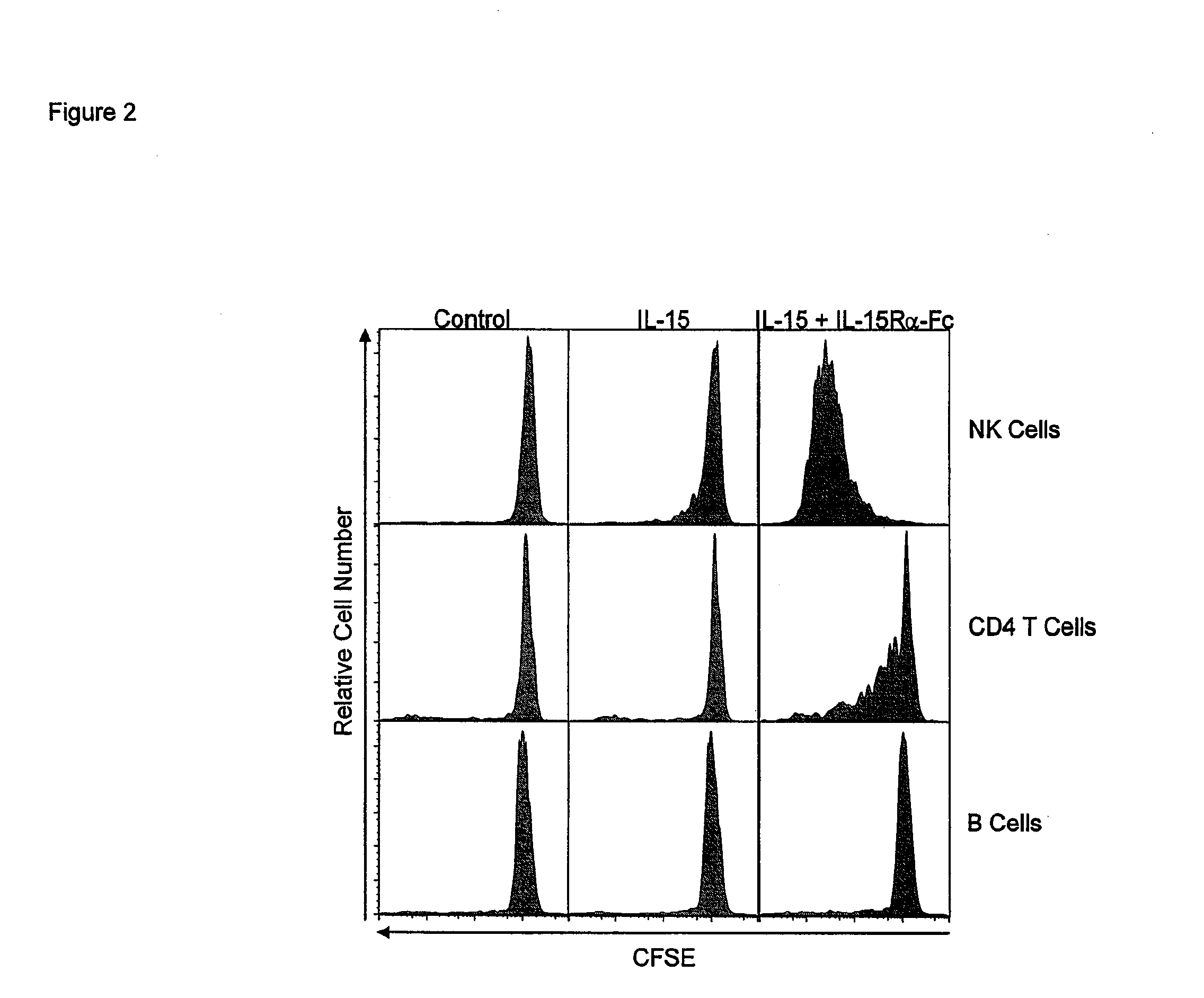
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Serum albumin binding proteins with long half-lives
InactiveUS20070269422A1Increased serum half-lifeReduce dosing frequencyOrganic active ingredientsPeptide/protein ingredientsSerum igePrimate
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides. Particularly, the amino acid sequences and compounds of the present invention bind to or otherwise associate with serum albumin in such a way that, when the amino acid sequence or compound is bound to or otherwise associated with a serum albumin molecule in a primate, it exhibits a serum half-life of at least 50% of the natural half-life of serum albumin in said primate.
Owner:ABLYNX NV
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Antibodies with modified isoelectric points
ActiveUS20120028304A1Increased serum half-lifeMinimize the possibilityAnimal cellsSugar derivativesHalf-lifeBlood plasma
The invention relates generally to compositions and methods for altering the isoelectric point of an antibody, and in some cases, resulting in improved plasma pharmacokinetics, e.g. increased serum half-life in vivo.
Owner:XENCOR
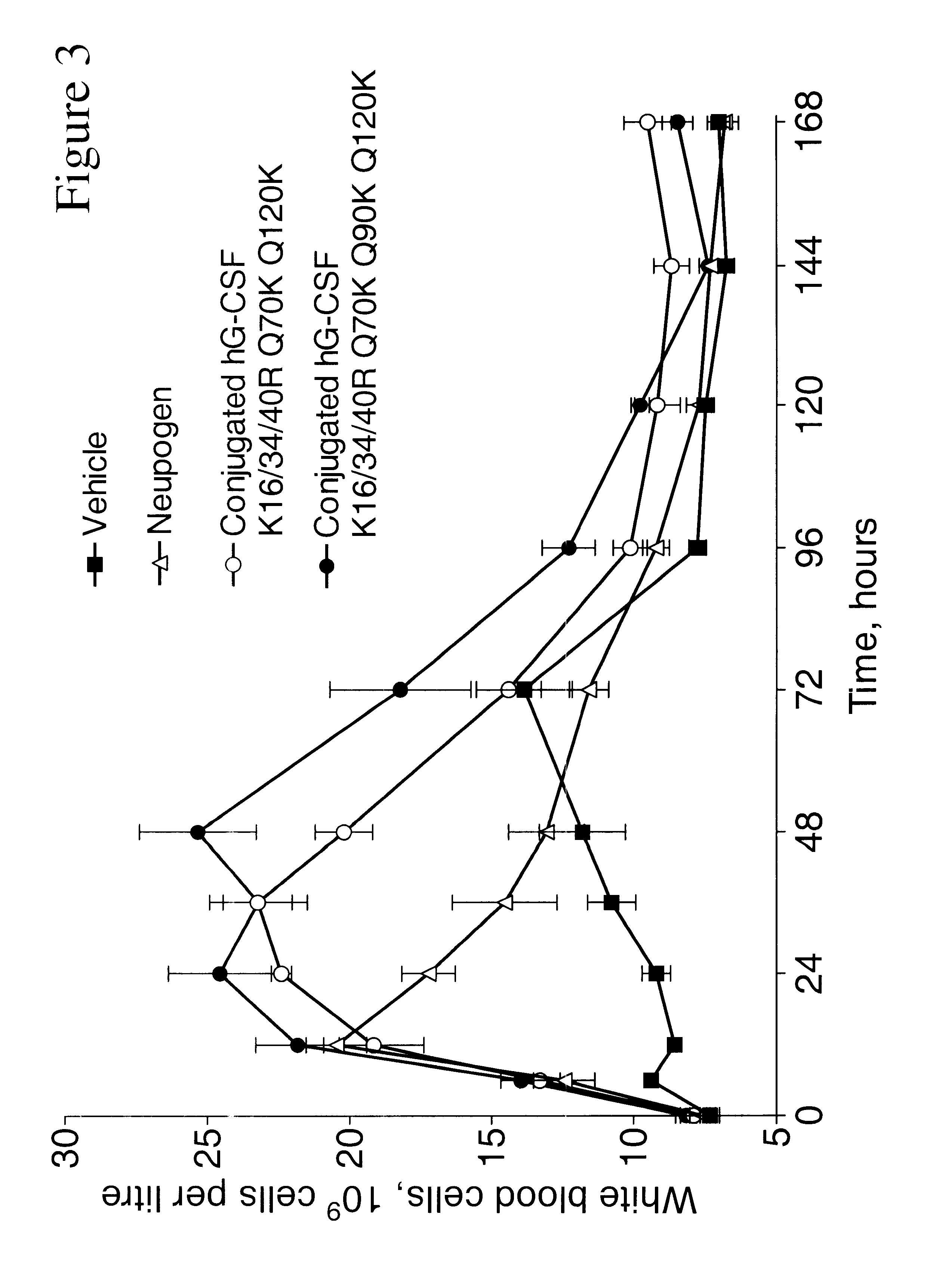
G-CSF conjugates
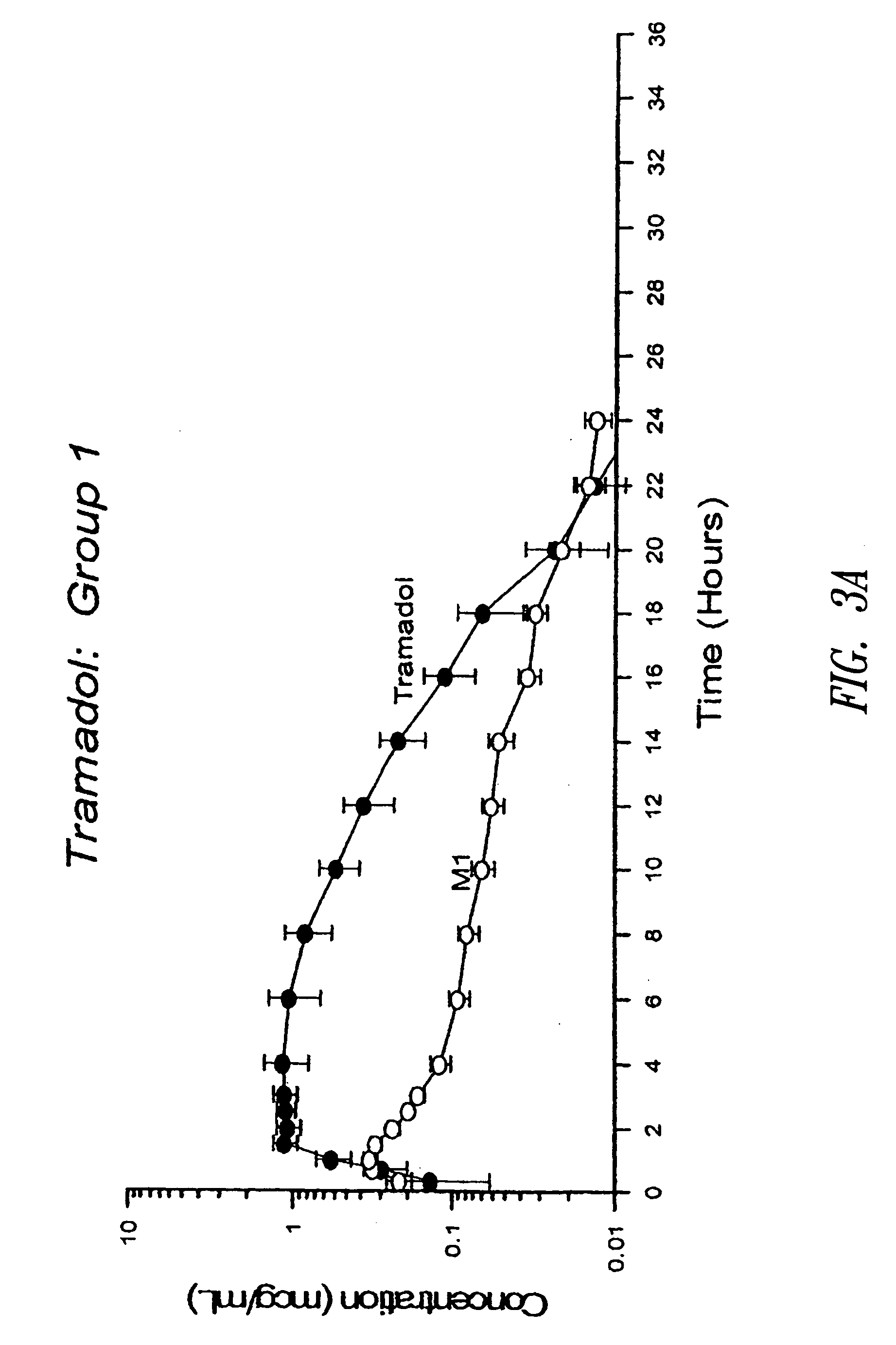
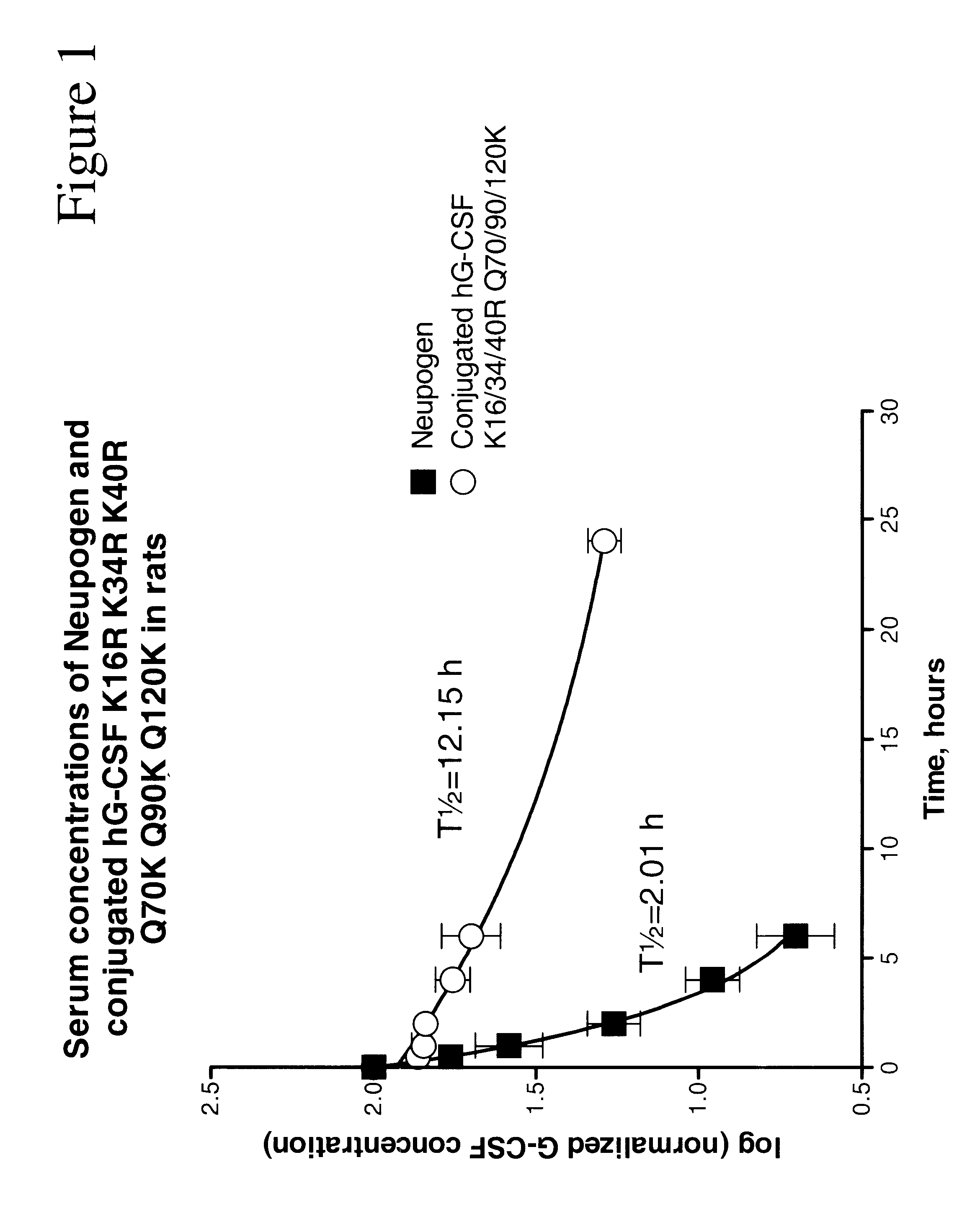
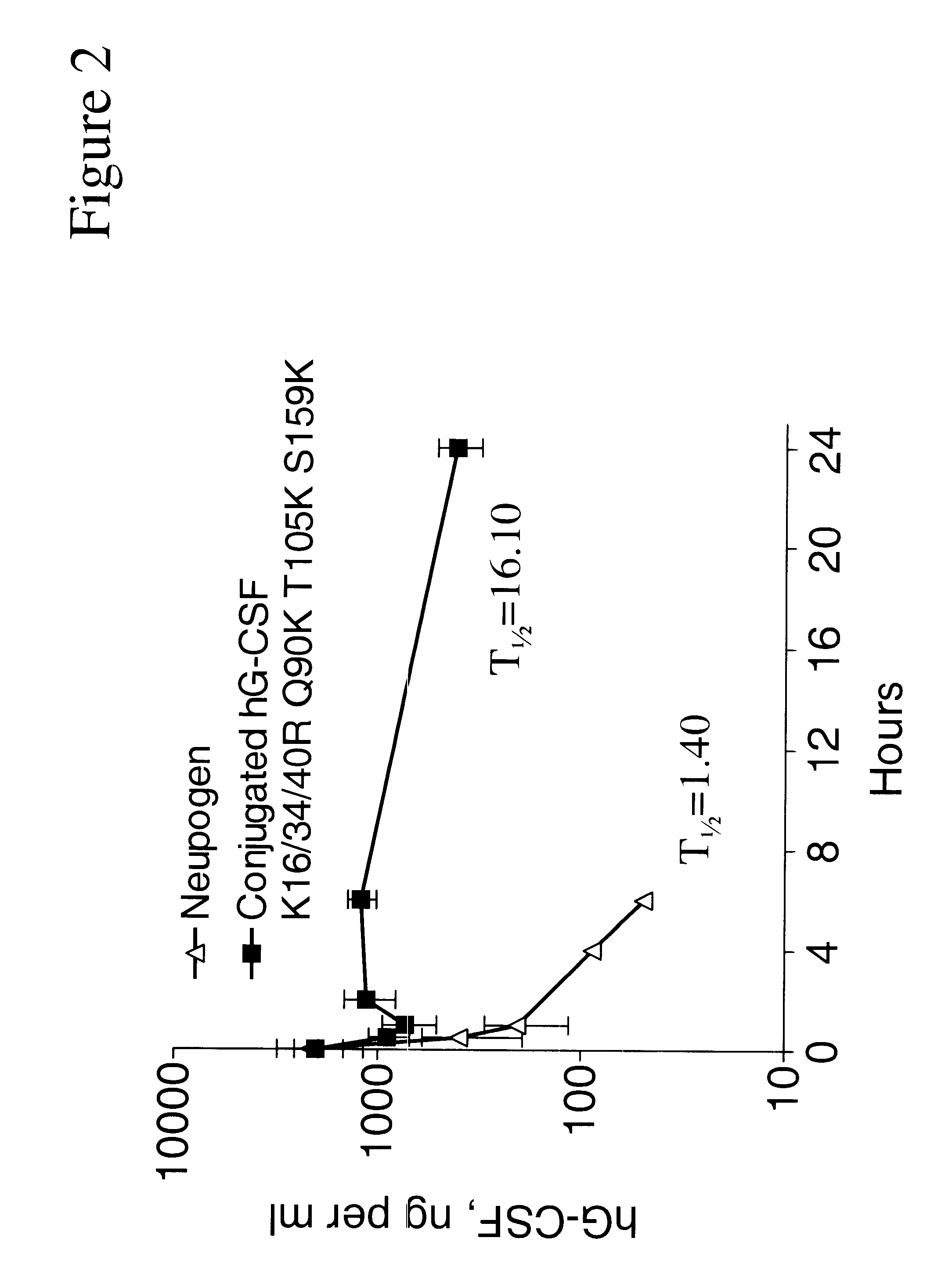
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
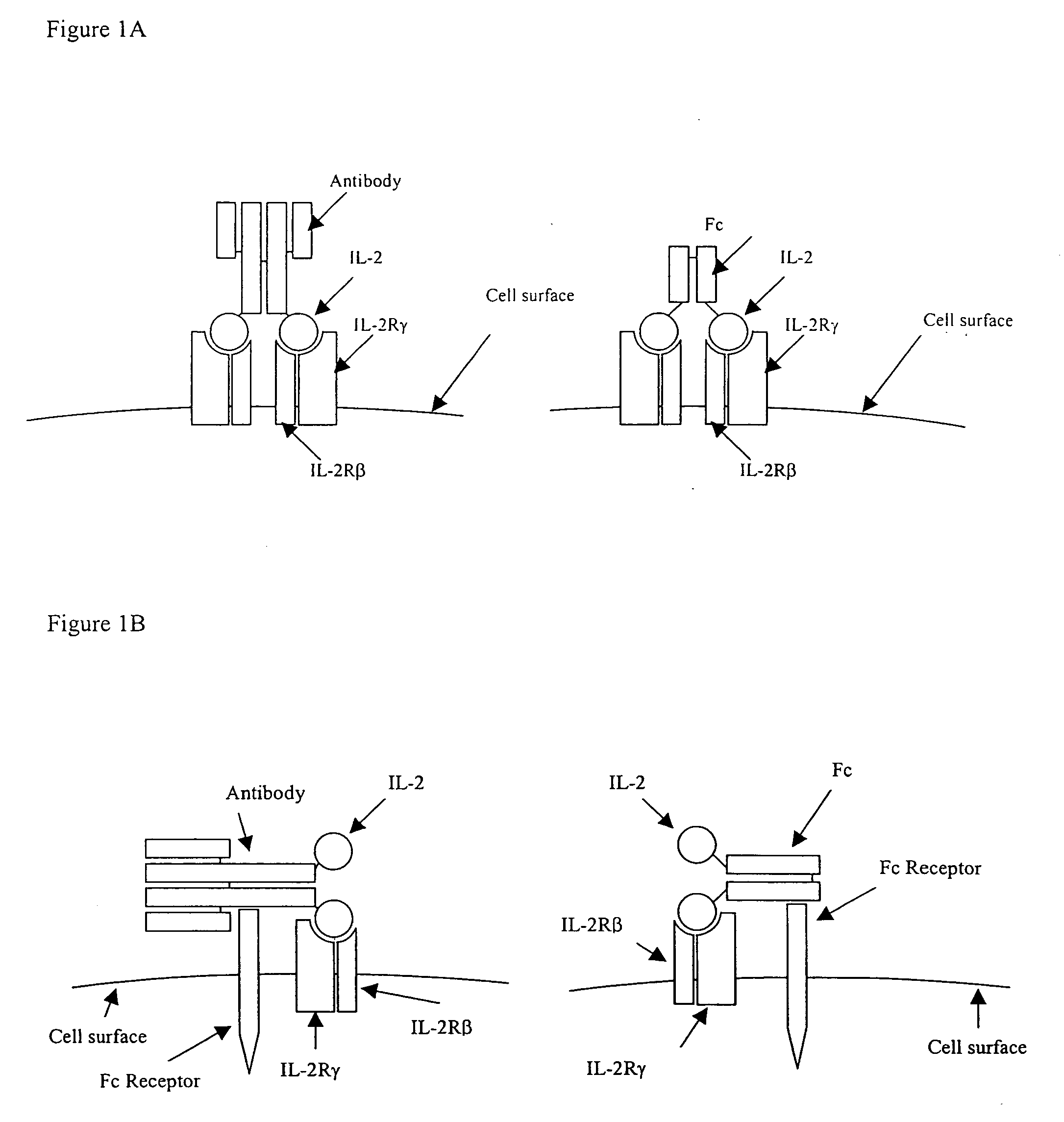
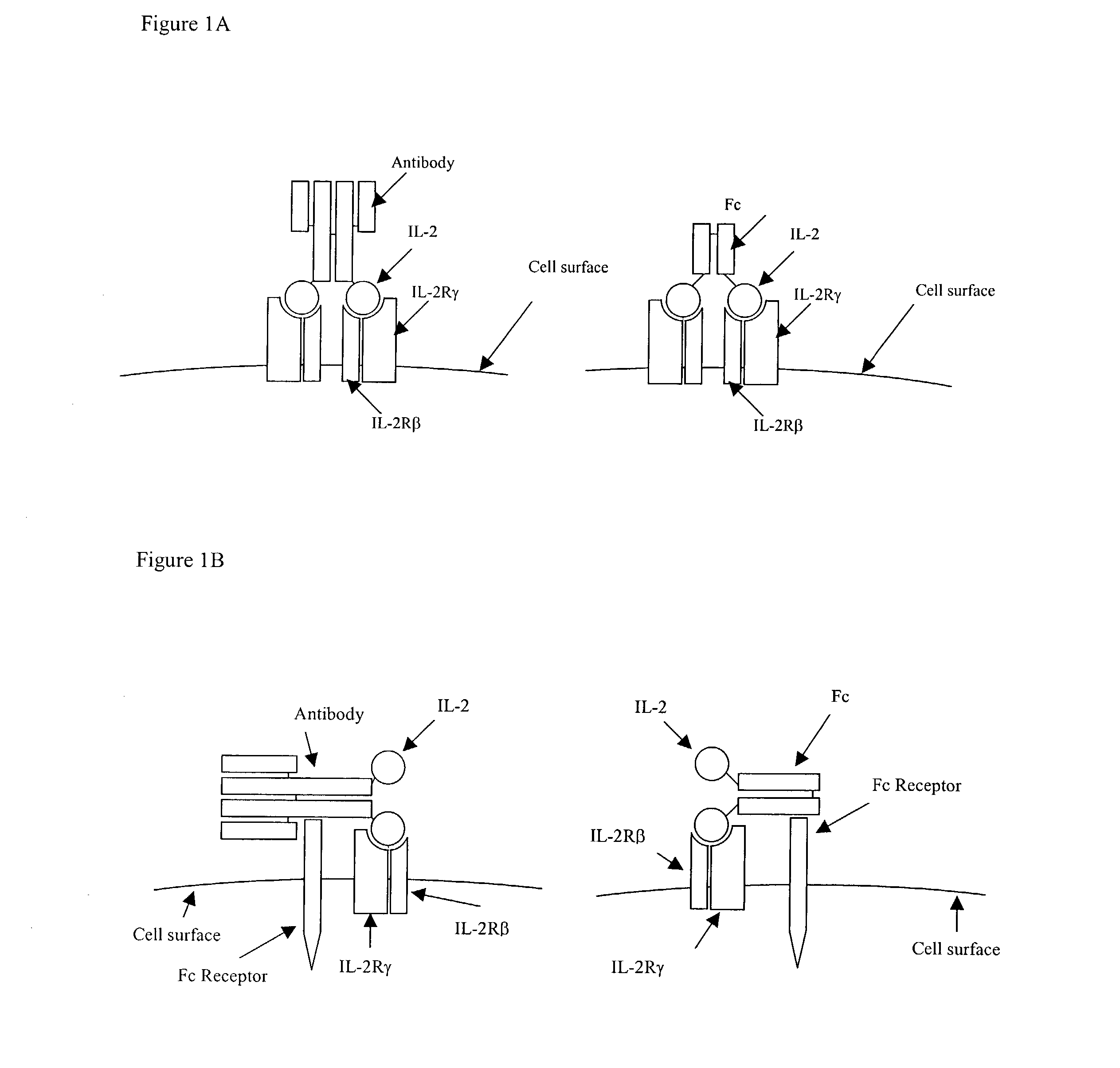
IL-2 fusion proteins with modulated selectivity
The invention provides cytokine fusion proteins with an increased therapeutic index, and methods to increase the therapeutic index of such fusion proteins. The fusion proteins of the invention are able to bind to more than one type of cytokine receptor expressed on cells and also bind to more than one cell type. In addition, the fusion proteins of the invention exhibit a longer circulating half-life in a patient's body than the corresponding naturally occurring cytokine.
Owner:MERCK PATENT GMBH
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050032114A1Reducing FcRn binding affinityReduced half-lifeAnimal cellsSugar derivativesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050276799A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
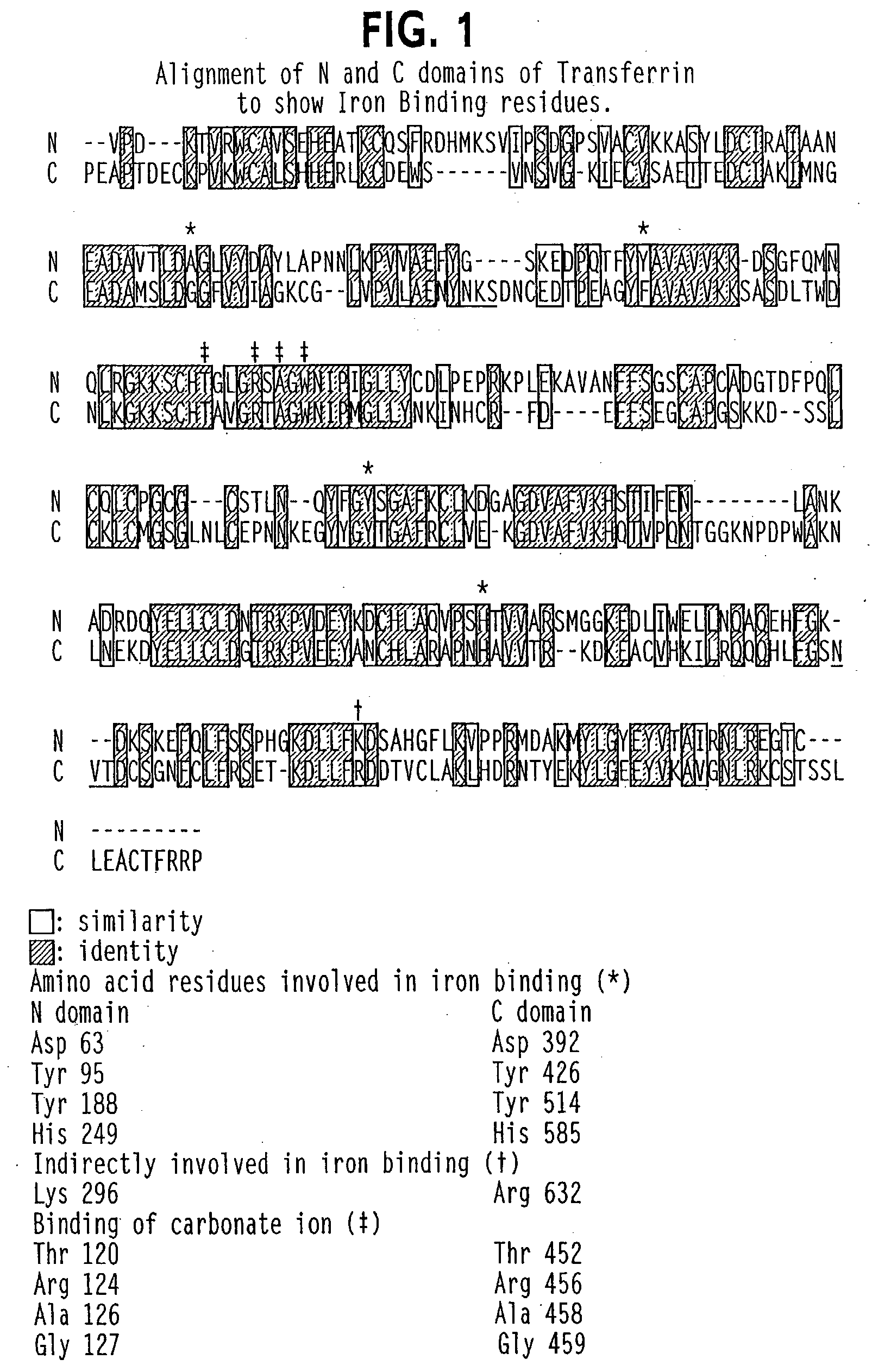
Modified transferrin fusion proteins
InactiveUS7176278B2Increased serum half-lifeImprove bioavailabilityAntibody mimetics/scaffoldsVirus peptidesDiseaseSerum ige
The present invention discloses fusion proteins comprising transferrin, lactoferrin or melanotransferrin fused to glucagon-like peptide 1 (GLP-1). In one embodiment of the invention, the fusion protein displays increased serum half-life as compared to a GLP-1 peptide in an unfused state. The invention includes a pharmaceutical composition comprising the GLP-1 fusion protein of the invention and a carrier. The fusion protein of the invention can be administered to a subject for treatment of diseases or conditions treatable by GLP-1, including, but not limited to, diabetes, obesity, congestive heart failure and inflammatory bowel syndrome.
Owner:BIOREXIS TECH INC
Enhancing the circulating half-life of antibody-based fusion proteins
InactiveUS7091321B2Increased serum half-lifeHigh affinityCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsLymphatic SpreadHalf-life
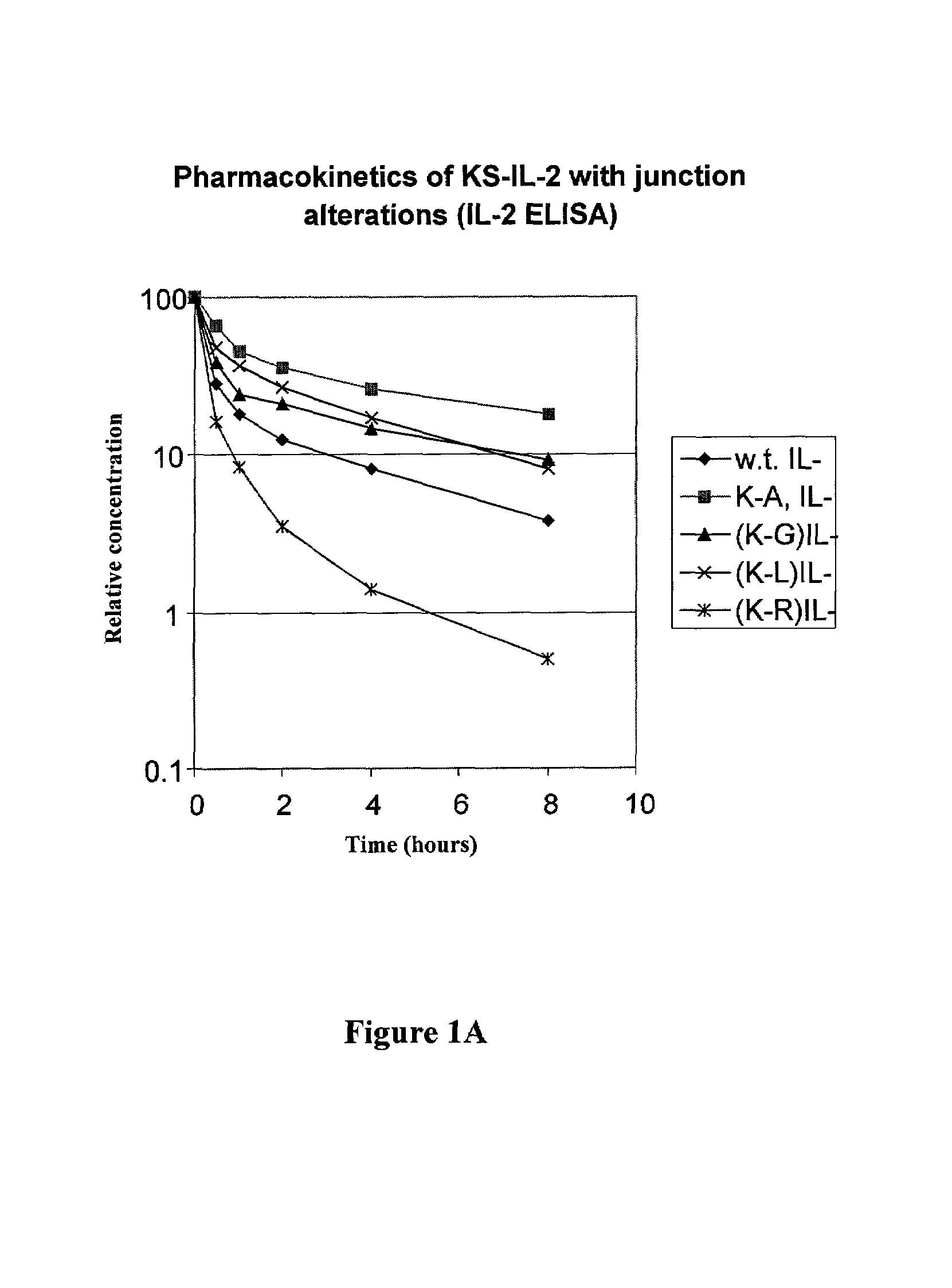
Disclosed are compositions and methods for enhancing the circulating half-life of antibody-based fusion proteins. Disclosed methods and compositions rely on altering the amino acid sequence of the junction region between the antibody moiety and the fused protein moiety in an antibody-based fusion protein. An antibody-based fusion protein with an altered amino acid sequence in the junction region has a greater circulating half-life when administered to a mammal. Disclosed methods and compositions are particularly useful for reducing tumor size and metastasis in a mammal.
Owner:MERCK PATENT GMBH
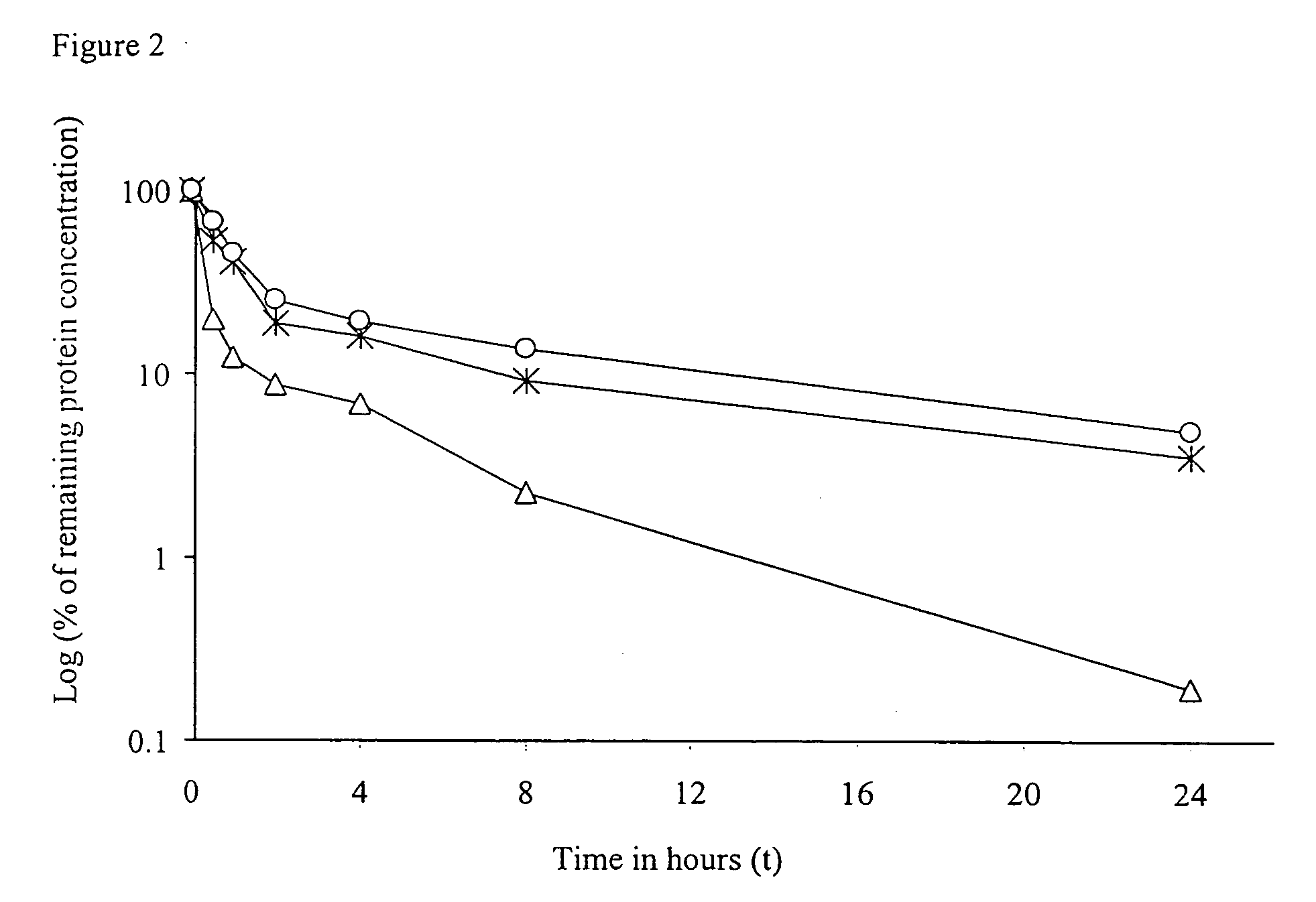
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
FcRn-based therapeutics for the treatment of auto-immune disorders
InactiveUS6992234B2Increased catabolismShort livedAntibody ingredientsDepsipeptidesDiseaseTransgenic knockout
Owner:JACKSON LAB THE
Inhibitor nucleic acids
InactiveUS20050256071A1Low melting pointQuality improvementOrganic active ingredientsNervous disorderAptamerHalf-life
The present invention provides methods and compositions for attenuating expression of a target gene in vivo. In general, the method includes administering RNAi constructs (such as small-interfering RNAs (i.e., siRNAs) that are targeted to particular mRNA sequences, or nucleic acid material that can produce siRNAs in a cell), in an amount sufficient to attenuate expression of a target gene by an RNA interference mechanism. In particular, the RNAi constructs may include one or more modifications to improve serum stability, cellular uptake and / or to avoid non-specific effect. In certain embodiments, the RNAi constructs contain an aptamer portion. The aptamer may bind to human serum albumin to improve serum half life. The aptamer may also bind to a cell surface protein that improves uptake of the construct.
Owner:CALIFORNIA INST OF TECH
Homogeneous fluorassay methods employing fluorescent background rejection and water-soluble rare earth metal chelates
InactiveUS6242268B1High binding constantNon-metal conductorsGlass making apparatusHalf-lifeMetal chelate
Homogeneous assays for determining quantitatively the extent of a specific binding reaction can be carried out effectively on very dilute solutions using measurements of fluorescence if a fluorescence measurement scheme that is capable of rejecting short-lived background fluorescence is employed and if the fluorescent group being measured has the following properties: a. the group being measured must be a rare earth metal chelate complex combination; b. the chelate must be water-soluble; c. the complex combination must also be stable in extremely dilute aqueous solutions, that is, the measured chelate must have at least one ligand having a metal-to-ligand binding constant of at least about 1013M-1 or greater and it must have a fluorescent emission that is long-lived compared to the longest decay lifetime of ambient substances and have a half life of from 0.01 to 50 msec.
Owner:EG&G WALLAC
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Cytotoxicity-inducing therapeutic agent
ActiveUS20140112914A1Lessen the burden on the bodyHighly convenientBacteriaAntibody mimetics/scaffoldsHalf-lifeCytotoxicity
By replacing the antigen-binding domain, the present inventors discovered novel polypeptide complexes that retain BiTE's strong anti-tumor activity and excellent safety properties, as well as have long half-life in blood and can damage various different target cells.
Owner:CHUGAI PHARMA CO LTD
IL-2 fusion proteins with modulated selectivity
The invention provides cytokine fusion proteins with an increased therapeutic index, and methods to increase the therapeutic index of such fusion proteins. The fusion proteins of the invention are able to bind to more than one type of cytokine receptor expressed on cells and also bind to more than one cell type. In addition, the fusion proteins of the invention exhibit a longer circulating half-life in a patient's body than the corresponding naturally occurring cytokine.
Owner:MERCK PATENT GMBH
Compositions and Methods for Immunomodulation in an Organism
ActiveUS20120177598A1Long half-lifeGood treatment effectPolypeptide with localisation/targeting motifPeptide/protein ingredientsVaccinationHalf-life
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Modified therapeutic peptides with extended half-lives in vivo
A method for protecting a peptide from peptidase activity in vivo, the peptide being composed of between 2 and 50 amino acids and having a C-terminus and an N-terminus and a C-terminus amino acid and an N-terminus amino acid is described. In the first step of the method, the peptide is modified by attaching a reactive group to the C-terminus amino acid, to the N-terminus amino acid, or to an amino acid located between the N-terminus and the C-terminus, such that the modified peptide is capable of forming a covalent bond in vivo with a reactive functionality on a blood component. In the next step, a covalent bond is formed between the reactive group and a reactive functionality on a blood component to form a peptide-blood component conjugate, thereby protecting said peptide from peptidase activity. The final step of the method involves the analyzing of the stability of the peptide-blood component conjugate to assess the protection of the peptide from peptidase activity.
Owner:CONJUCHEM
Antibodies with modified isoelectric points
ActiveUS8637641B2Increased serum half-lifeMinimize the possibilityAnimal cellsSugar derivativesSerum igeHalf-life
The invention relates generally to compositions and methods for altering the isoelectric point of an antibody, and in some cases, resulting in improved plasma pharmacokinetics, e.g. increased serum half-life in vivo.
Owner:XENCOR INC
Pegylated factor VIII
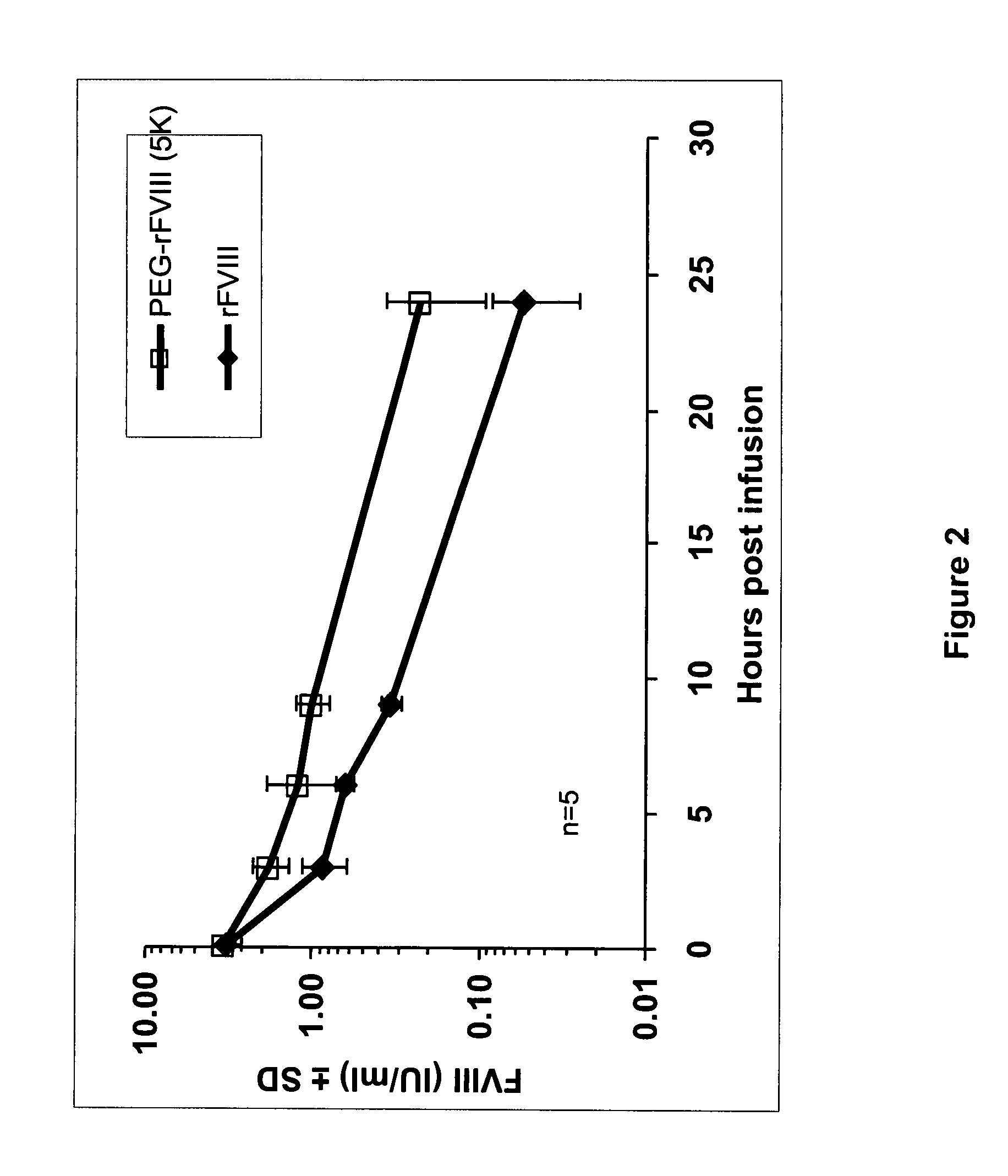
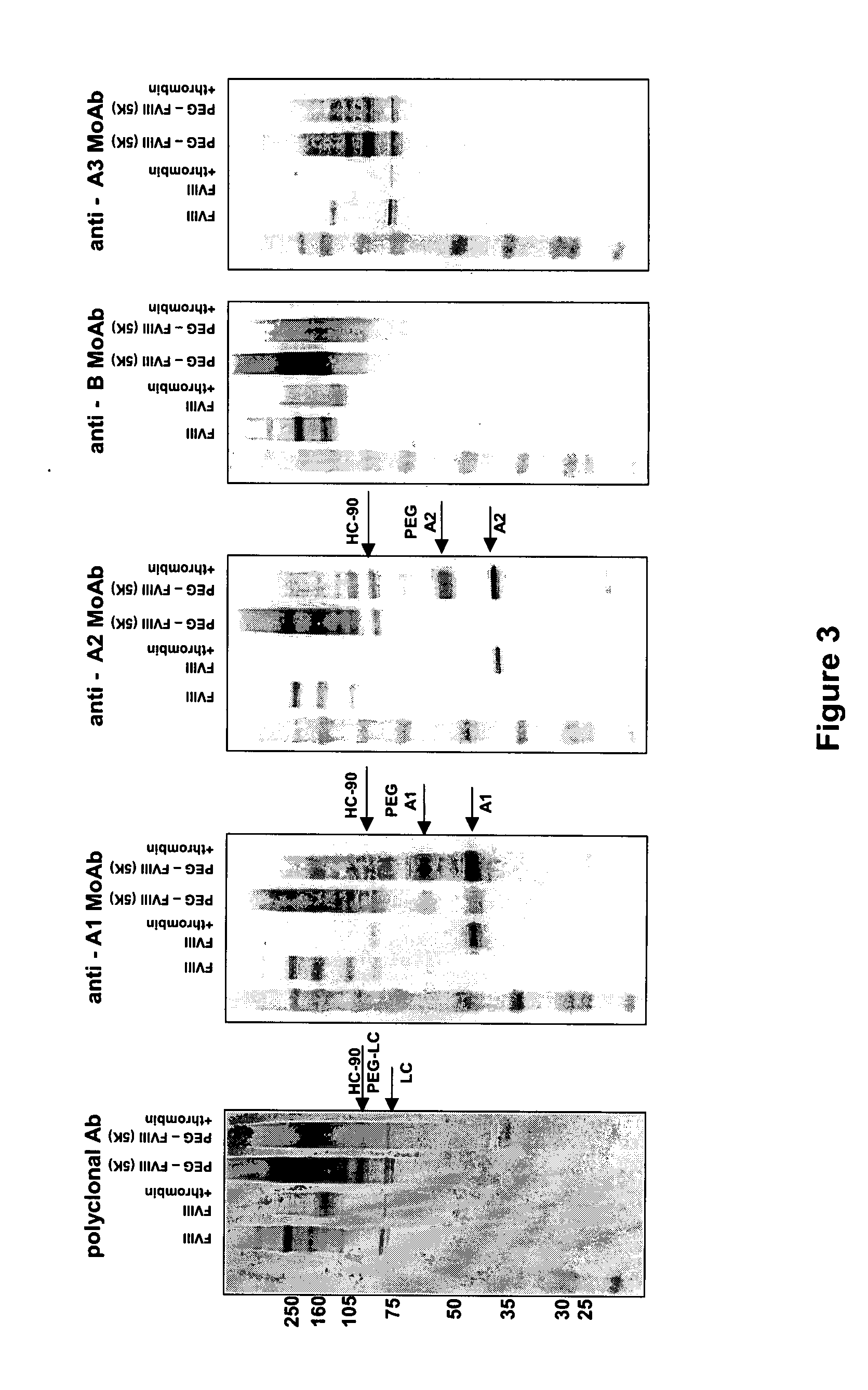
The invention is a proteinaceous construct comprising a Factor VIII molecule having at least a portion of the B domain intact, which is conjugated to a water-soluble polymer such as polyethylene glycol having a molecular weight of greater than 10,000 Daltons. The construct has a biological activity of at least 80% of the biological activity of native Factor VIII, and the in vivo half-life of the construct is increased by at least 1.5 fold as compared to the in vivo half-life of native factor FVIII.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com