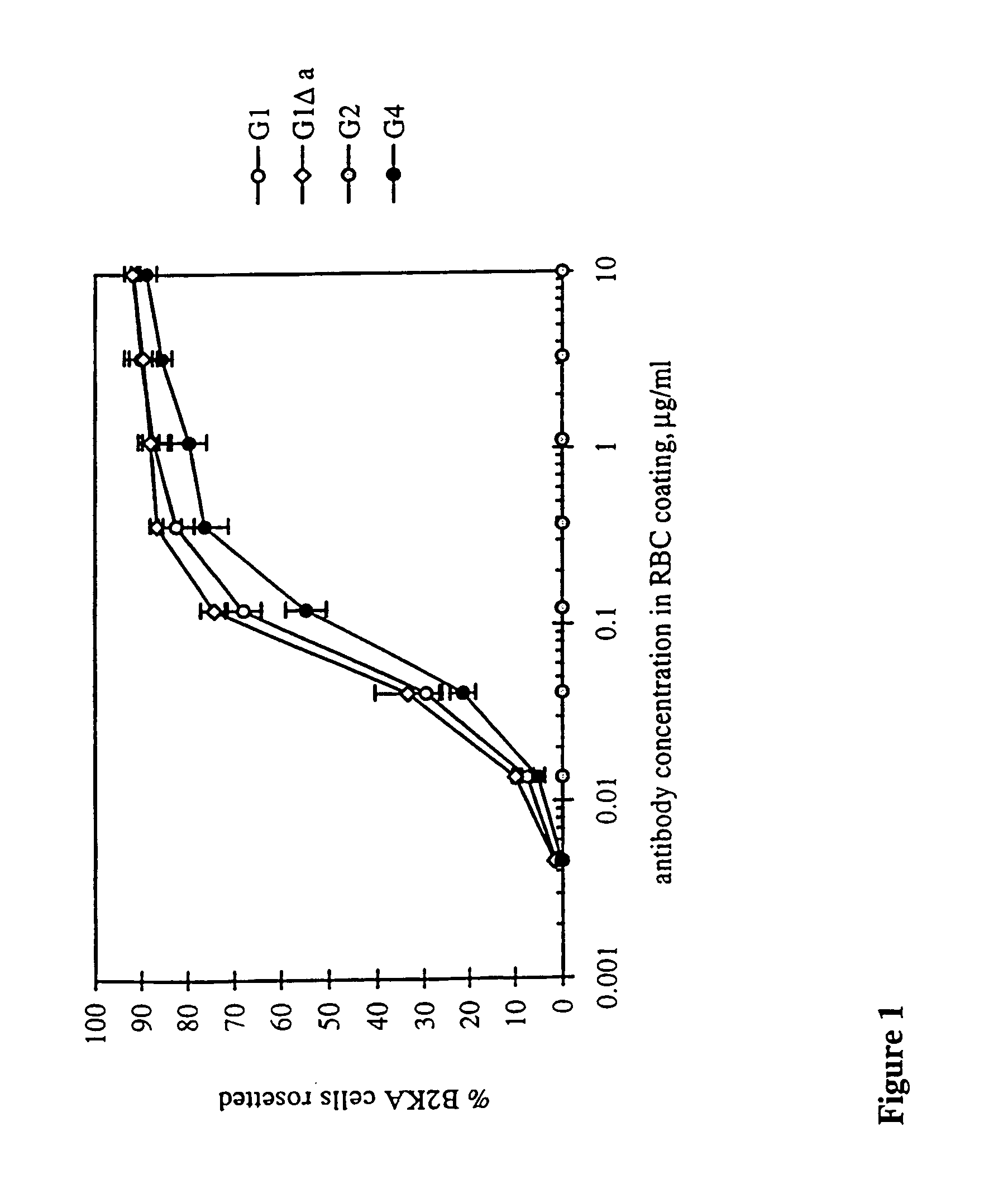
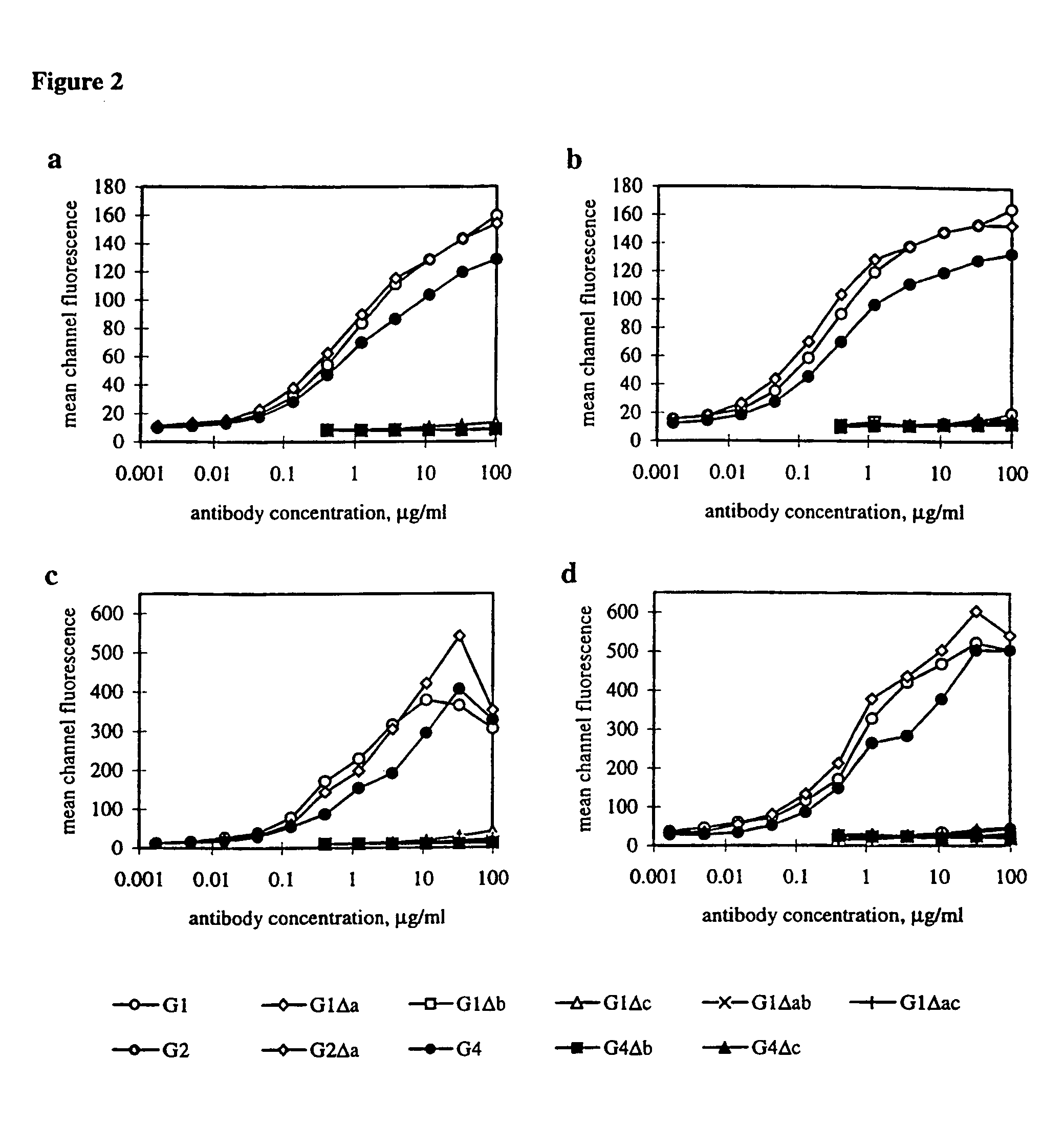
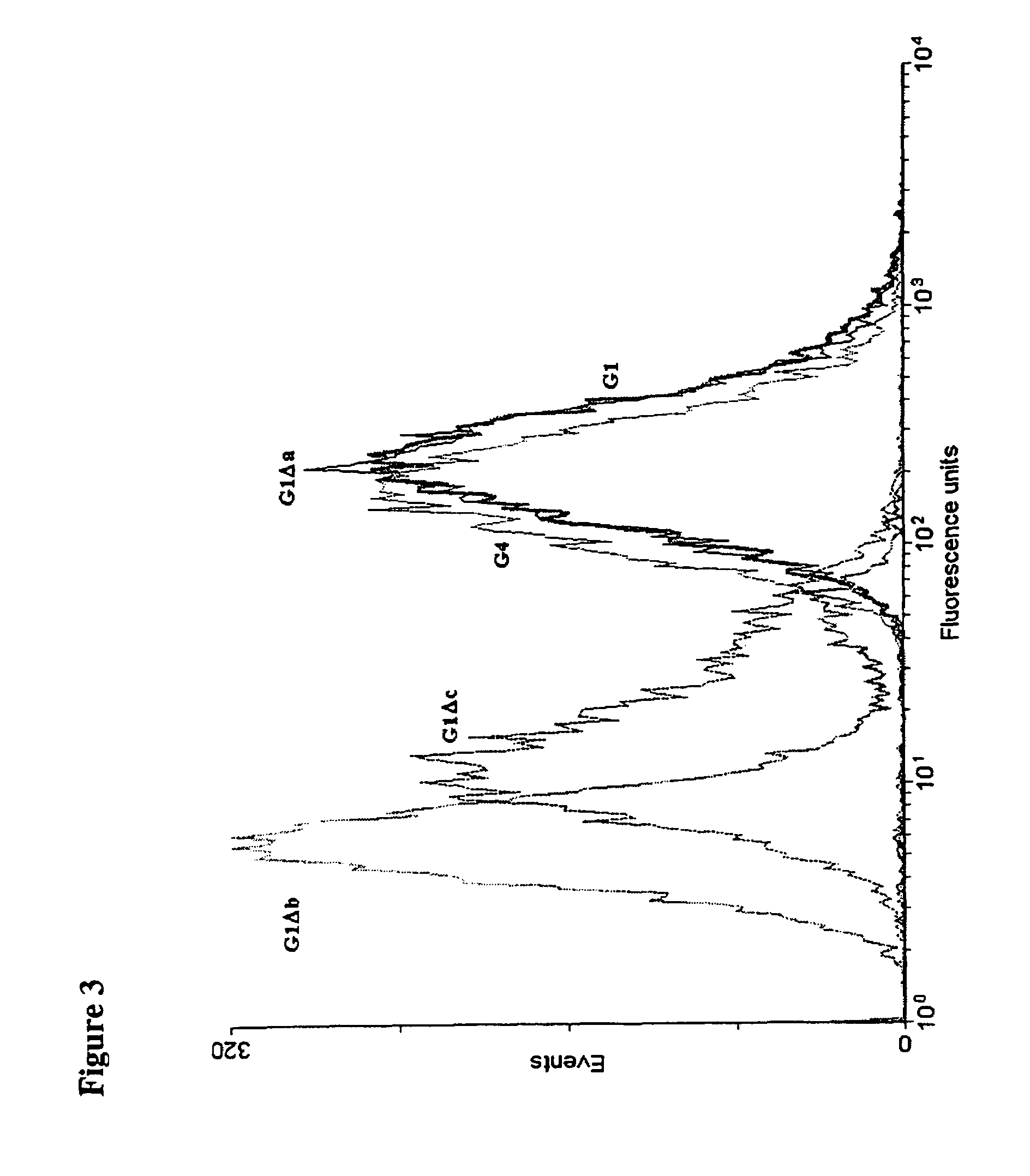
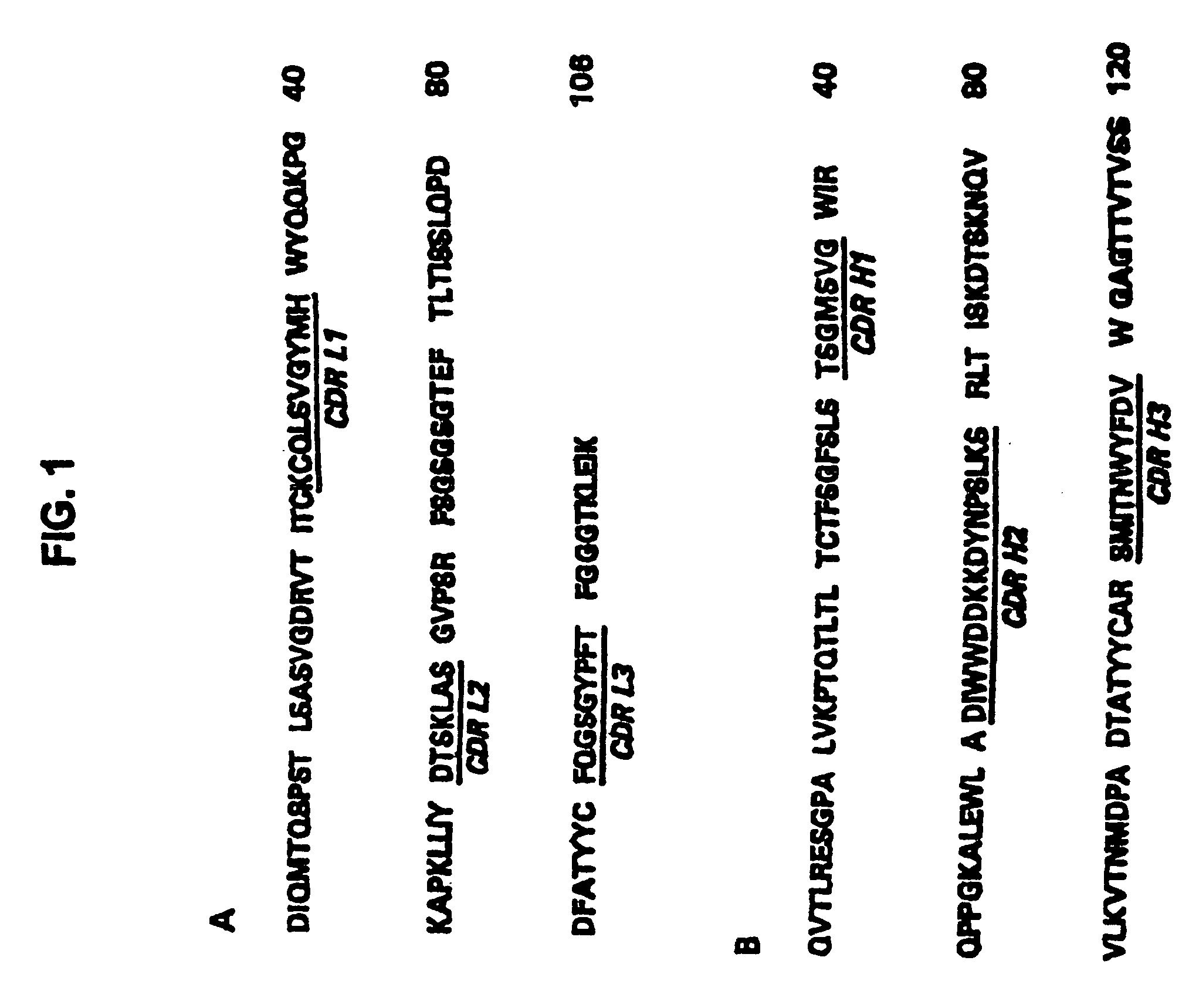
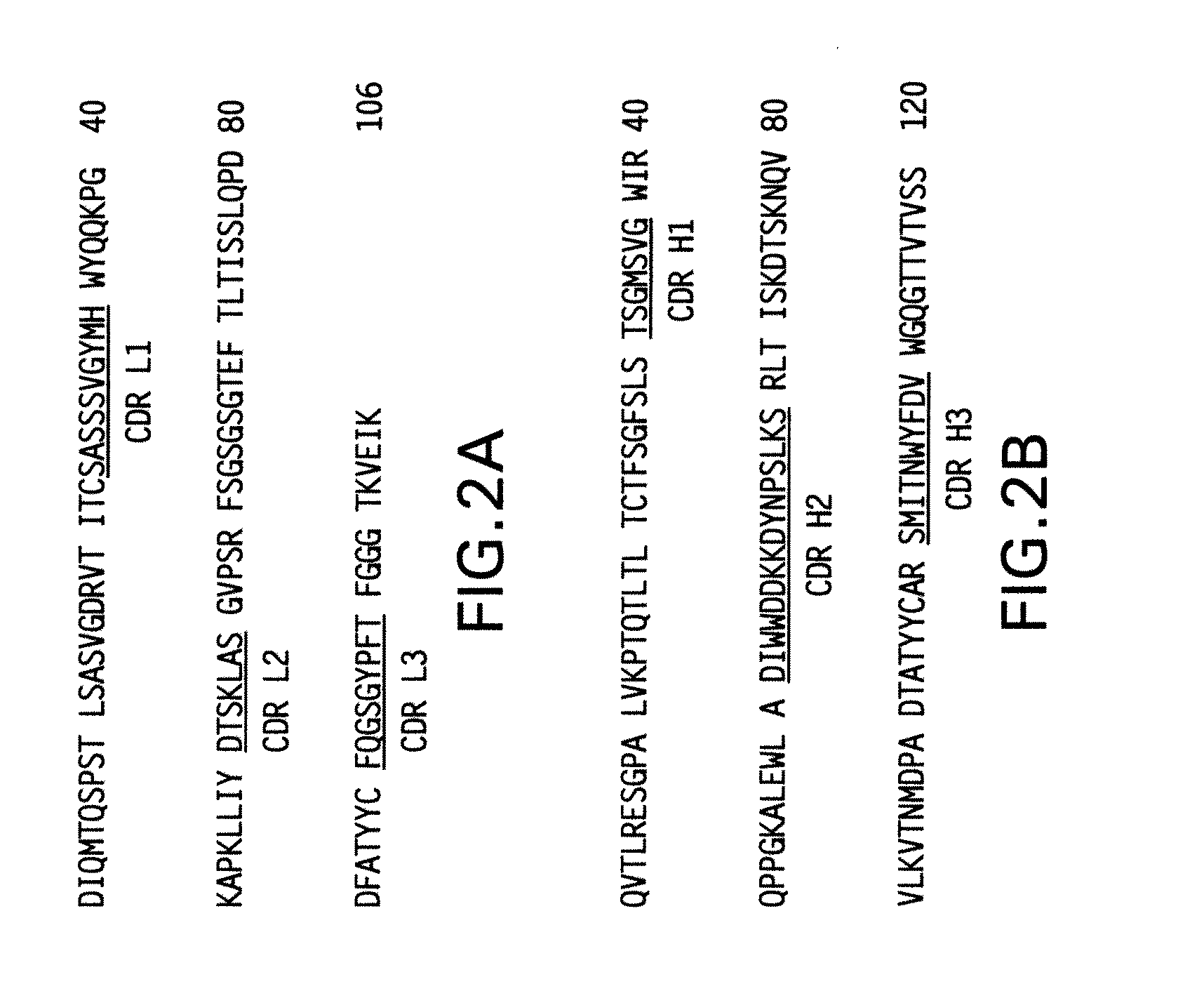
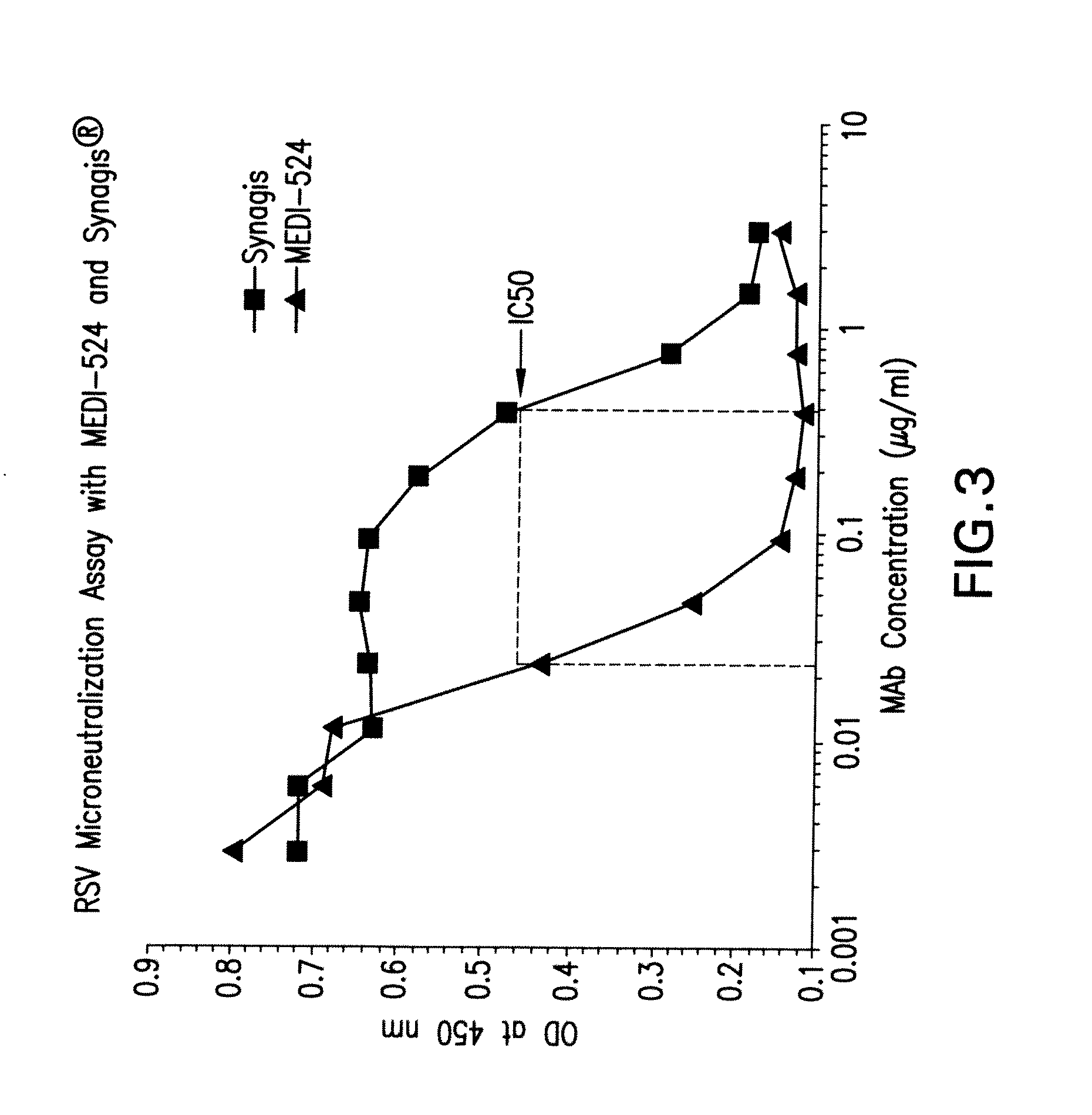
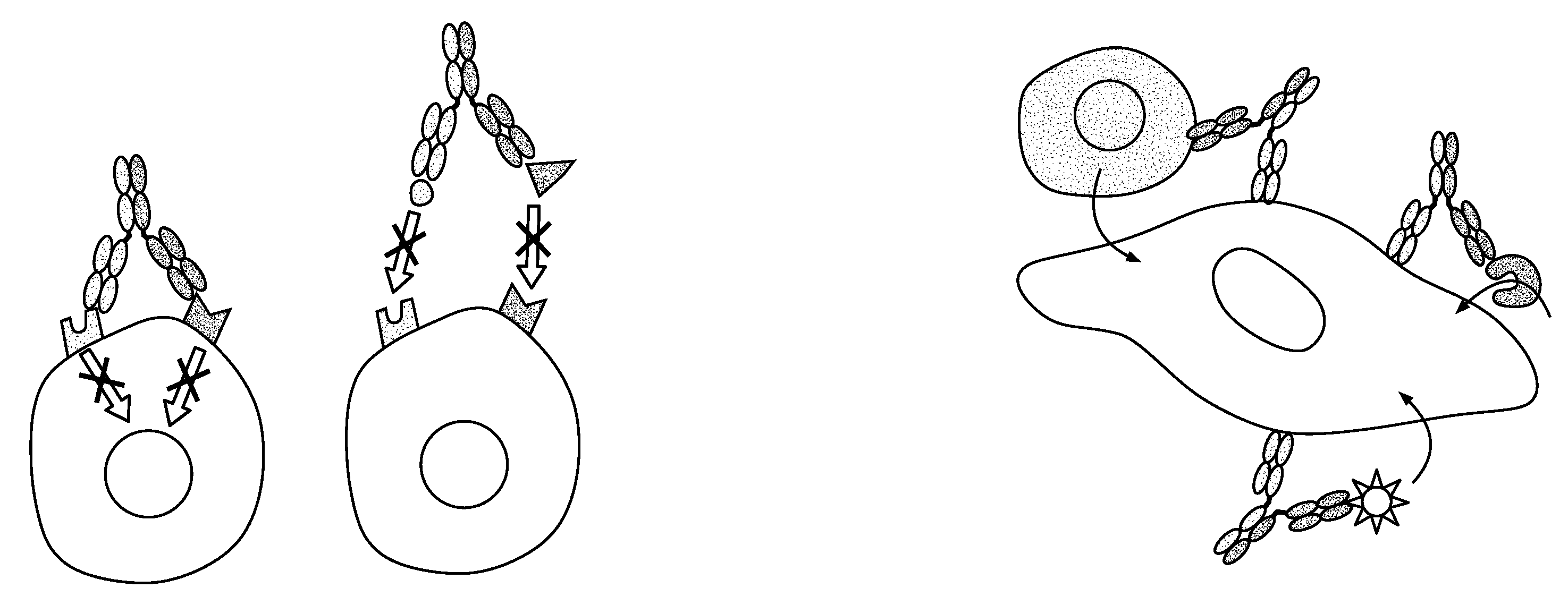Patents
Literature
105 results about "Constant domain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20030190311A1High affinityExtended half-lifeCompounds screening/testingFungiIntravenous gammaglobulinIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Production of Bispecific Antibodies
InactiveUS20090182127A1Reduce interactionImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsConstant domainHeavy chain
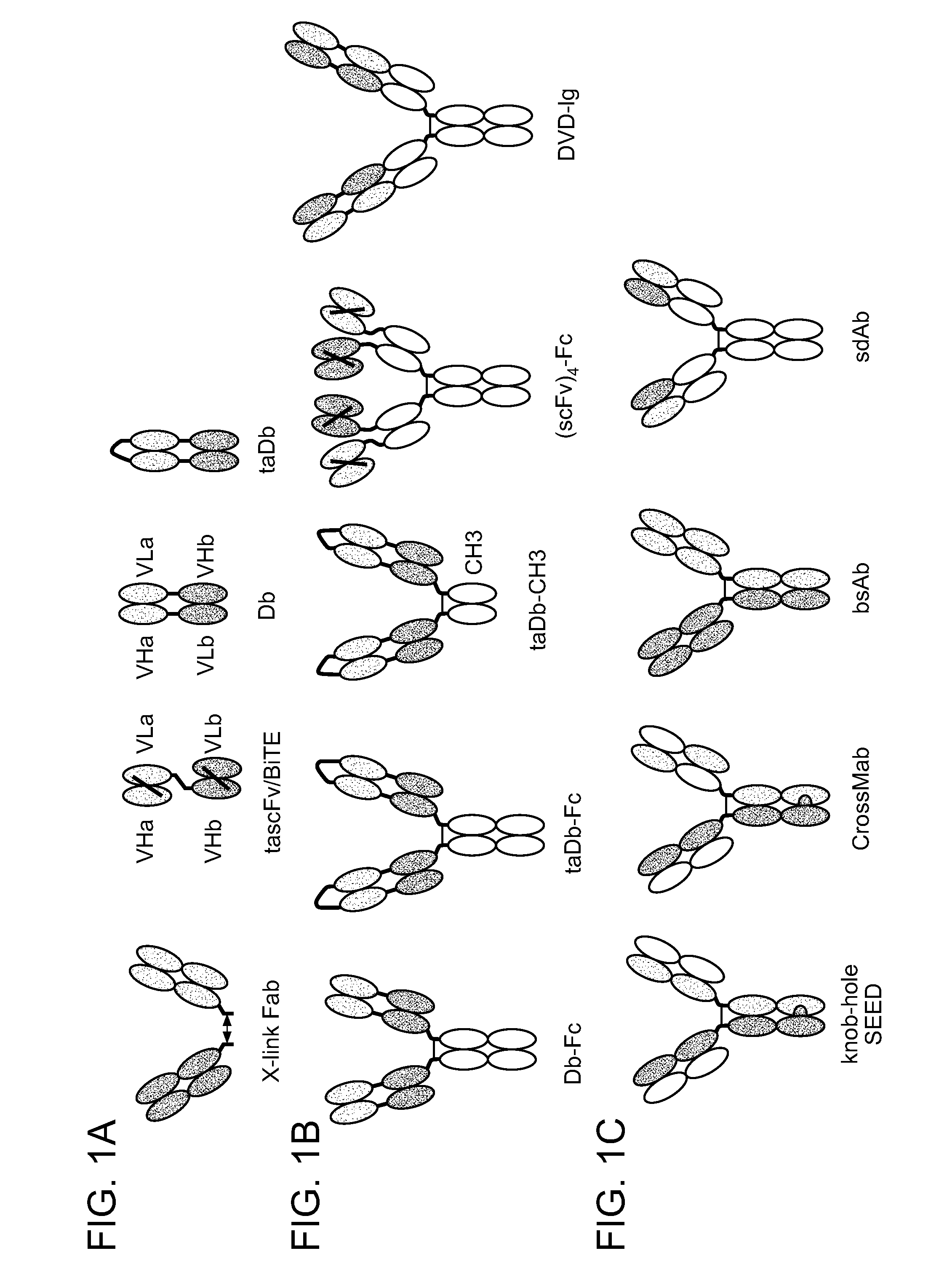
Bispecific antibodies comprising (a) a first light-heavy chain pair having specificity for a first target and a sufficient number of substitutions in its heavy chain constant domain with respect to a corresponding wild-type antibody of the same isotype to significantly reduce the formation of first heavy chain-first heavy chain dimers and (b) a second light-heavy chain pair comprising a heavy chain having a sequence that is complementary to the sequence of the first pair heavy chain sequence with respect to the formation of intramolecular ionic interactions, wherein the first pair or second pair comprises a substitution in the light chain and complementary substitution in the heavy chain that reduces the ability of the light chain to interact with the heavy chain of the other light chain-heavy chain pair are provided. Methods of producing such antibodies in one or more cells also are provided.
Owner:NOVO NORDISK AS
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:MEDIMMUNE LLC
Variant IgG3 Rituxan and therapeutic use thereof
InactiveUS20020128448A1Prevent and reduce proliferation of cellReduce and prevent proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Antigen binding
Monoclonal anti-human CD20 antigen binding antibodies containing human IgG3 constant domains are provided. These antibodies possess effector functions that render them well suited for use in therapeutic methods, especially treatments wherein inhibition of B cell function or B cell number is therapeutically desirable.
Owner:BIOGEN INC
Antibodies comprising chimeric constant domains
ActiveUS20140243504A1Reduced effector functionAnimal cellsHybrid immunoglobulinsFc(alpha) receptorFc receptor
Antibodies, antigen-binding proteins and Fc-fusion proteins that comprise recombinant polypeptides containing a chimeric heavy chain constant region sequence are provided that bind to certain Fc receptors however have reduced effector functions. Methods of making constructs for expression of such chimeric Fc-containing antibodies, antigen-binding proteins and Fc-fusion proteins in cell systems, and methods of producing and isolating the chimeric Fc-containing proteins are provided.
Owner:REGENERON PHARM INC
Heterodimeric proteins
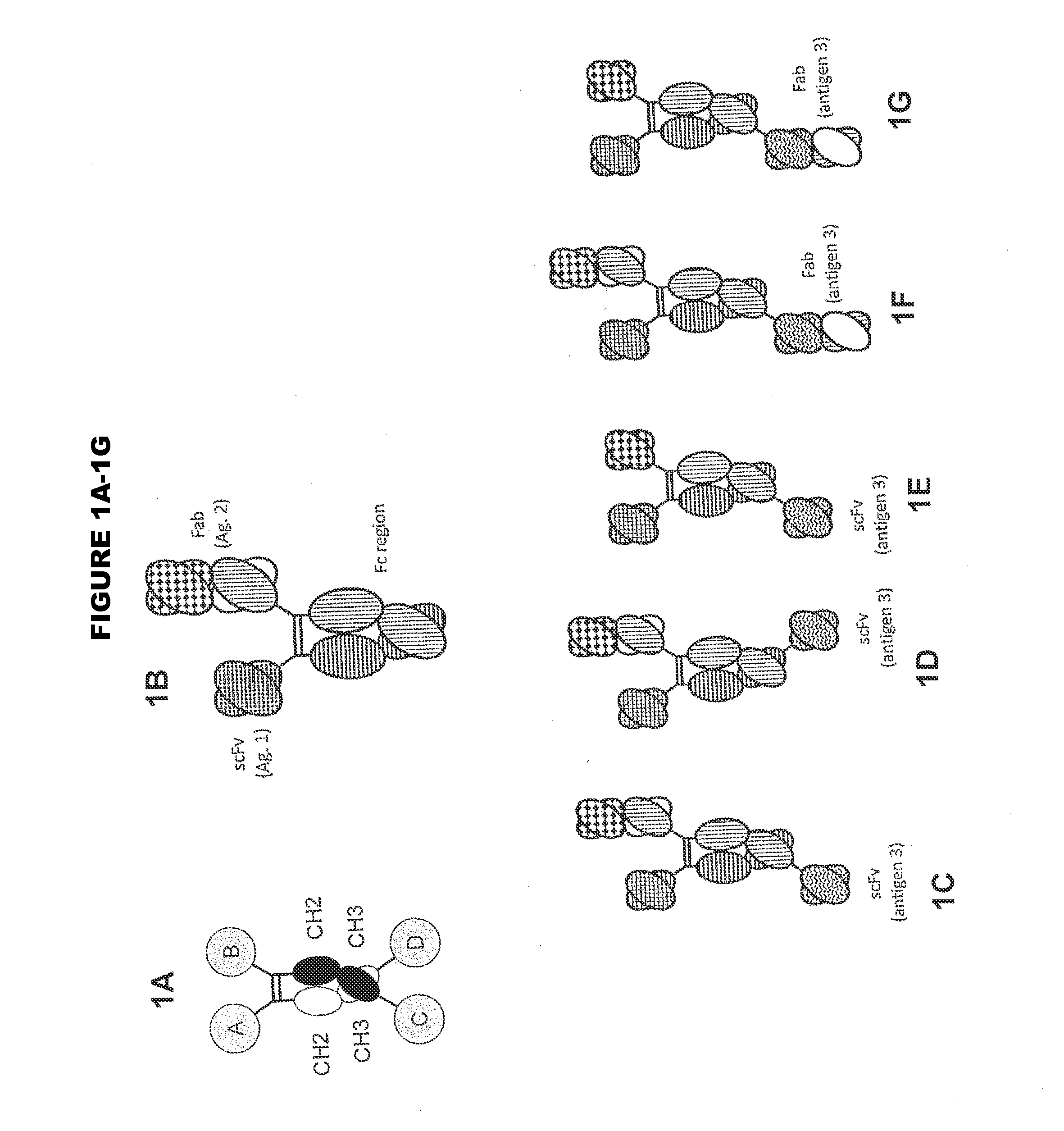
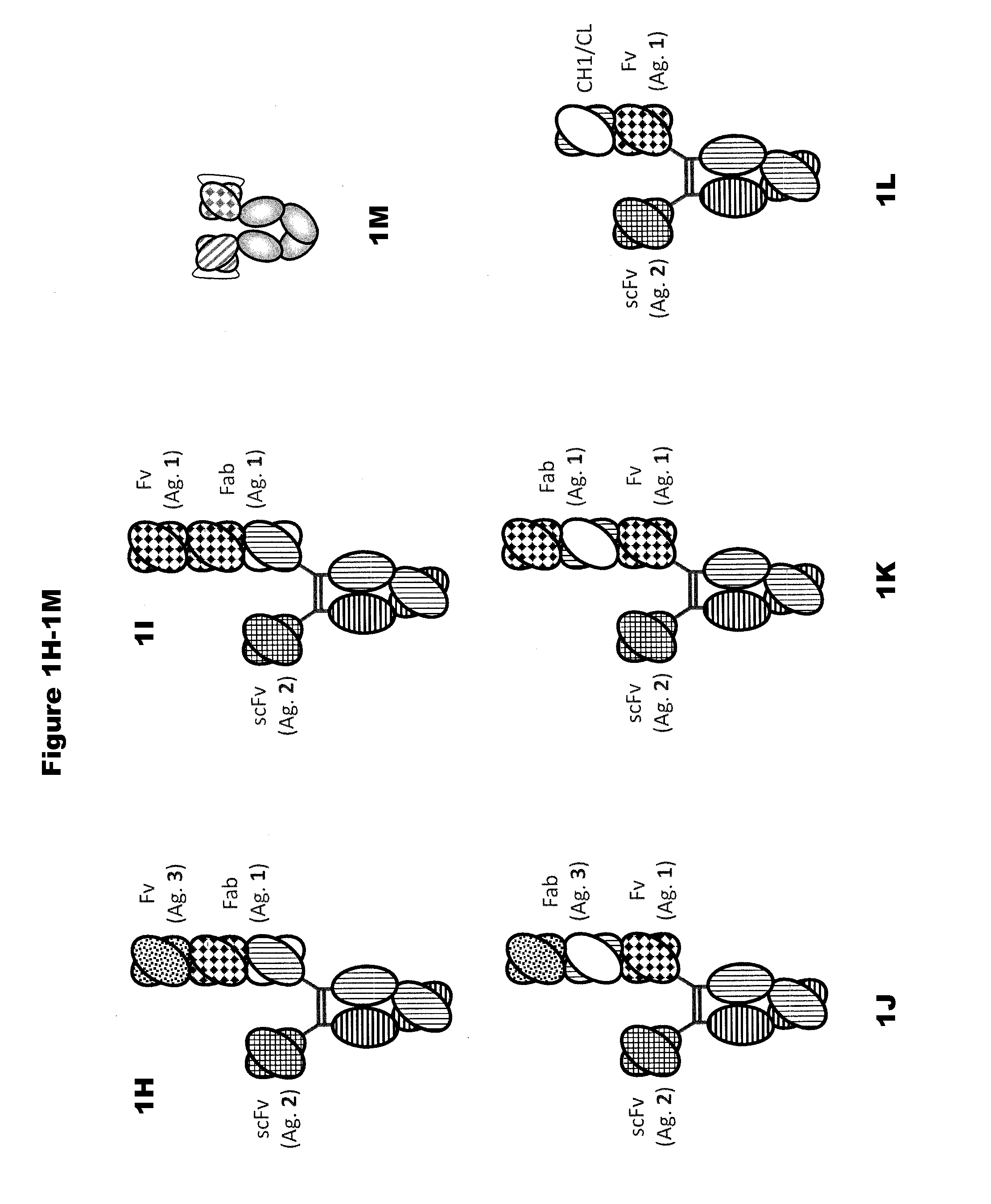
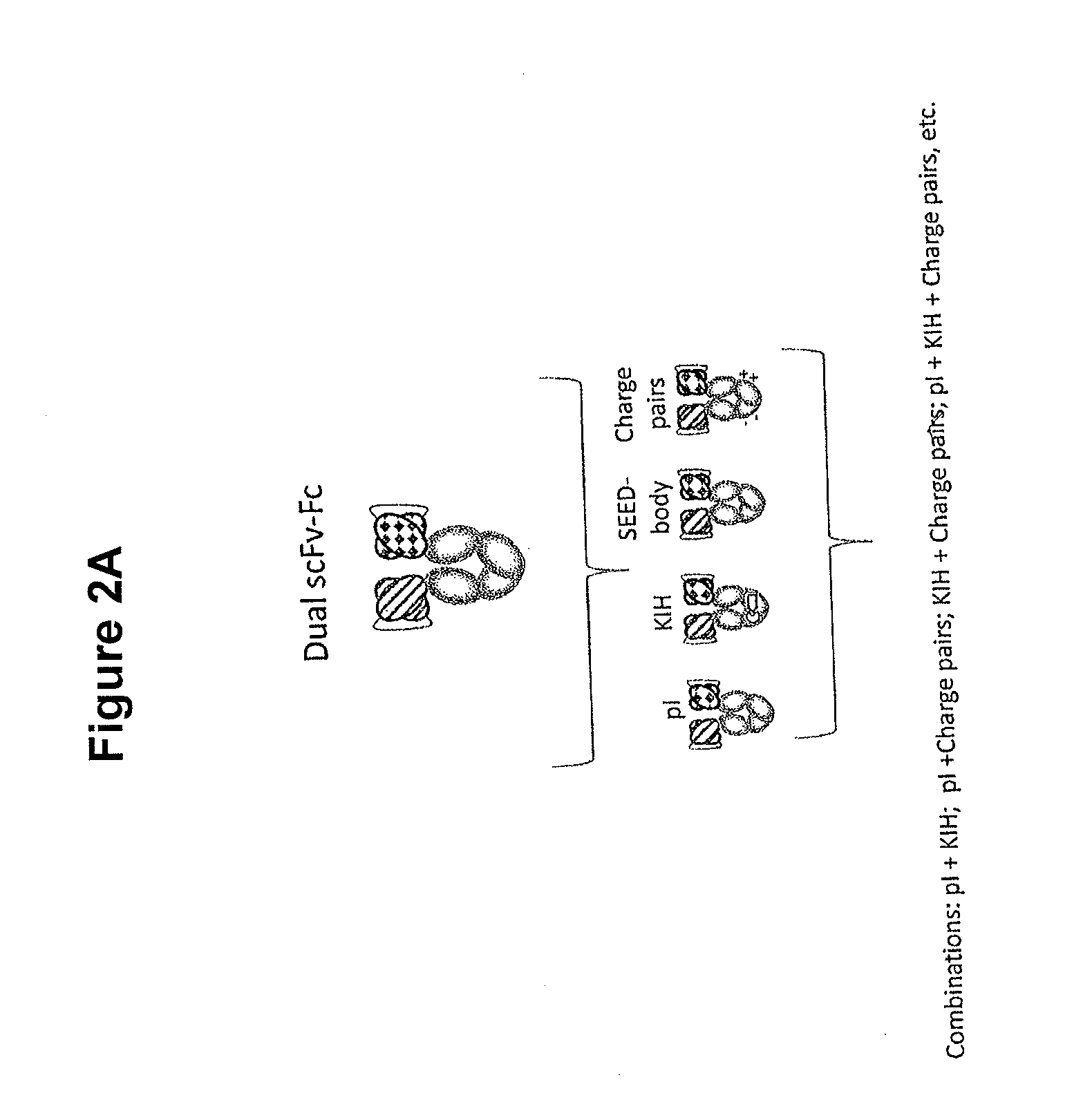
In one aspect, the present invention provides heterodimeric antibodies comprising a first monomer comprising a first heavy chain constant domain comprising a first variant Fc domain and a first antigen binding domain and a second monomer comprising a second heavy chain constant domain comprising a second variant Fc domain and a second antigen binding domain. In an additional aspect the heterodimeric antibody comprises a first monomer comprising a heavy chain comprising a first Fc domain and a single chain Fv region (scFv) that binds a first antigen, wherein the scFv comprises a charged scFv linker. The heterodimeric antibody further comprises a second monomer comprising a first heavy chain comprising a second Fc domain and a first variable heavy chain and a first light chain.
Owner:XENCOR INC
Immunoglobulin Constructs Comprising Selective Pairing of the Light and Heavy Chains
InactiveUS20140072581A1Promote formationImmunoglobulins against blood coagulation factorsSenses disorderHeavy chainSingle-Chain Fv
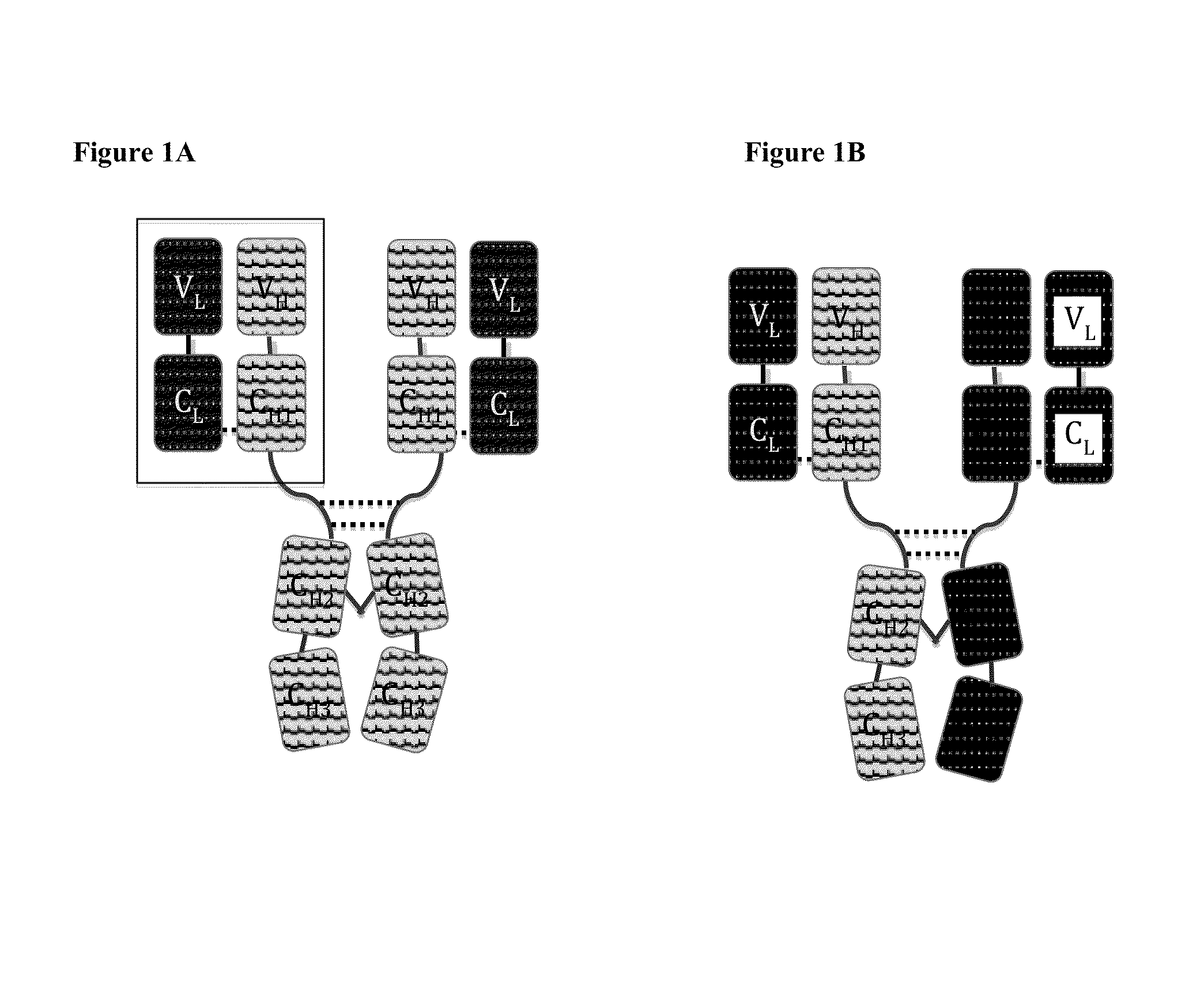
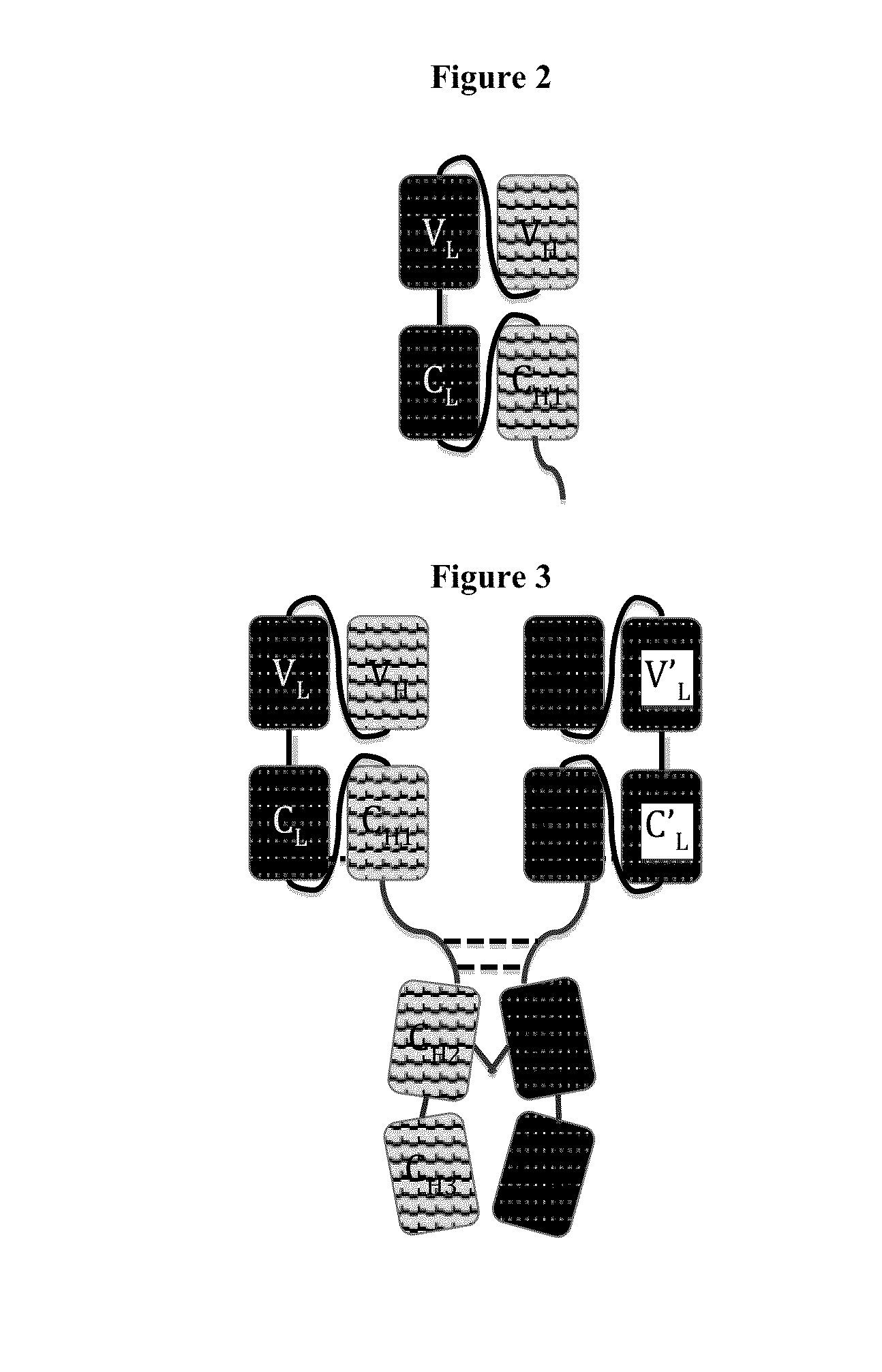
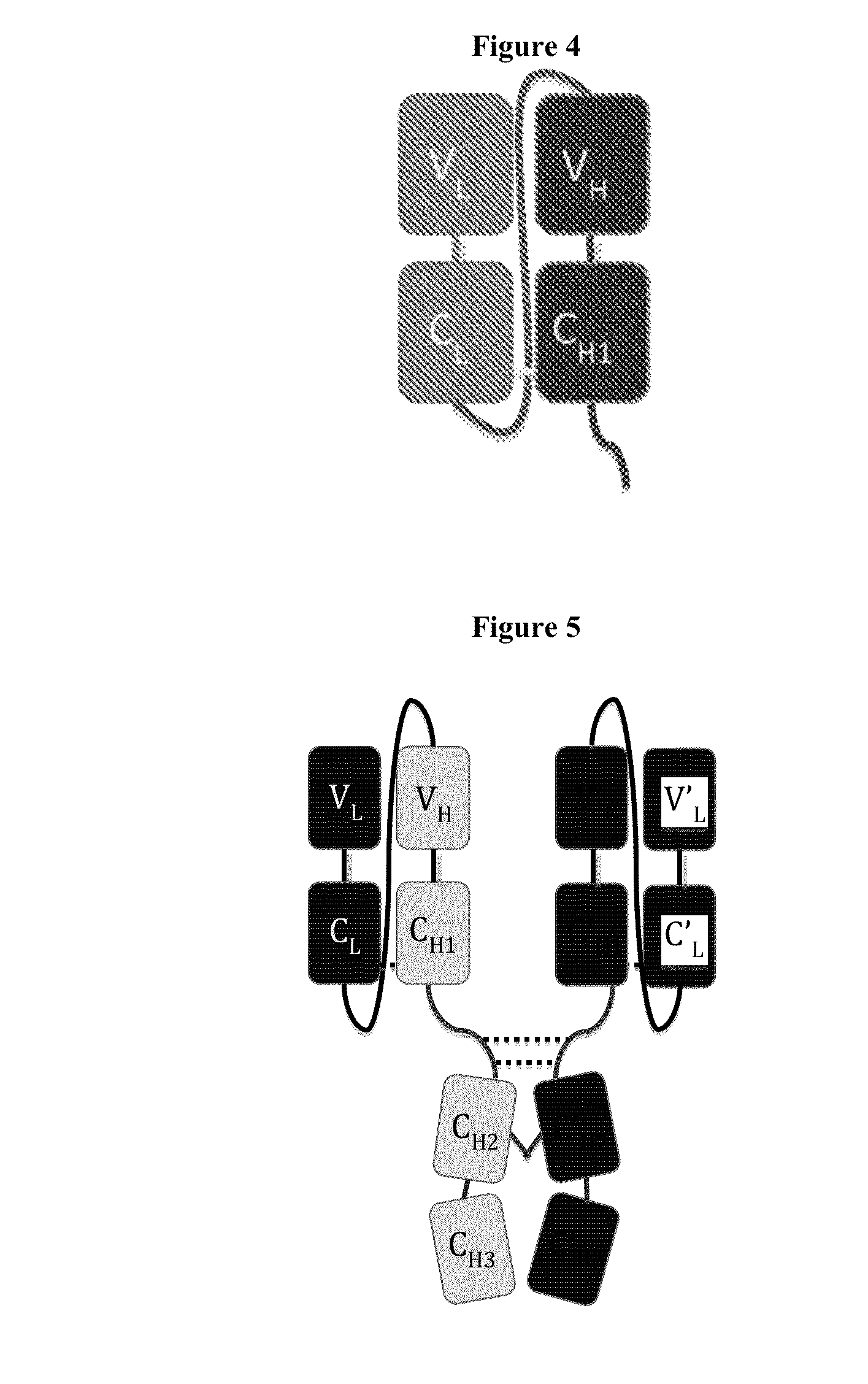
Disclosed herein is an isolated immunoglobulin construct comprising a first monomeric polypeptide comprising a first single chain Fv polypeptide connected to a first constant domain polypeptide; and a second monomeric polypeptide comprising a second single chain Fv polypeptide, connected to a second constant domain polypeptide; each said constant domain polypeptide comprising at least one each of a CL domain, a CH1 domain, a CH2 domain and a CH3 domain or fragments, variants or derivatives thereof; and wherein said first and second constant domain polypeptide form a Fc region.
Owner:ZYMEWORKS INC
Stabilized glycoproteins
ActiveUS20040191265A1Increased in half lifeGood storage stabilitySnake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainGlycoprotein i
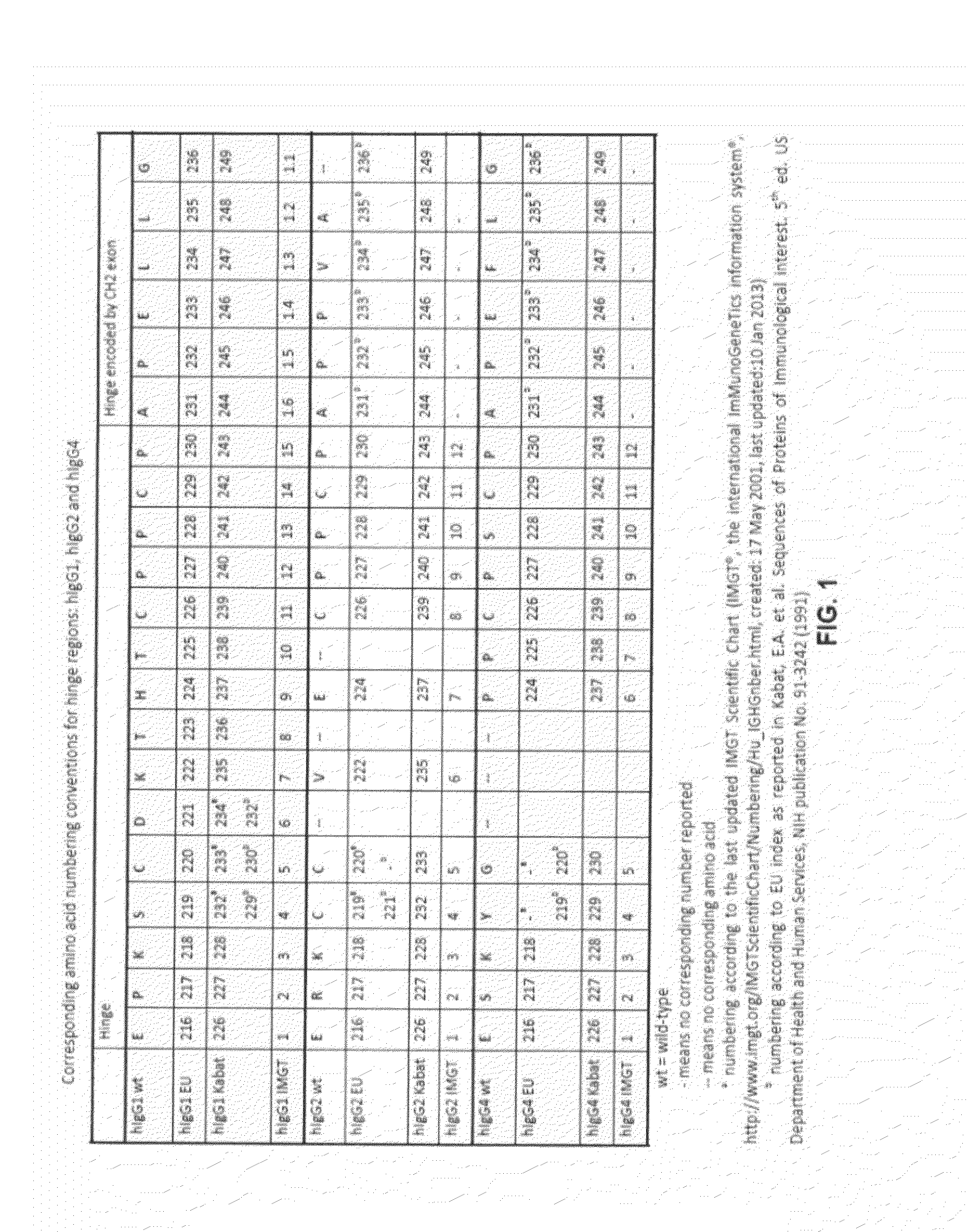
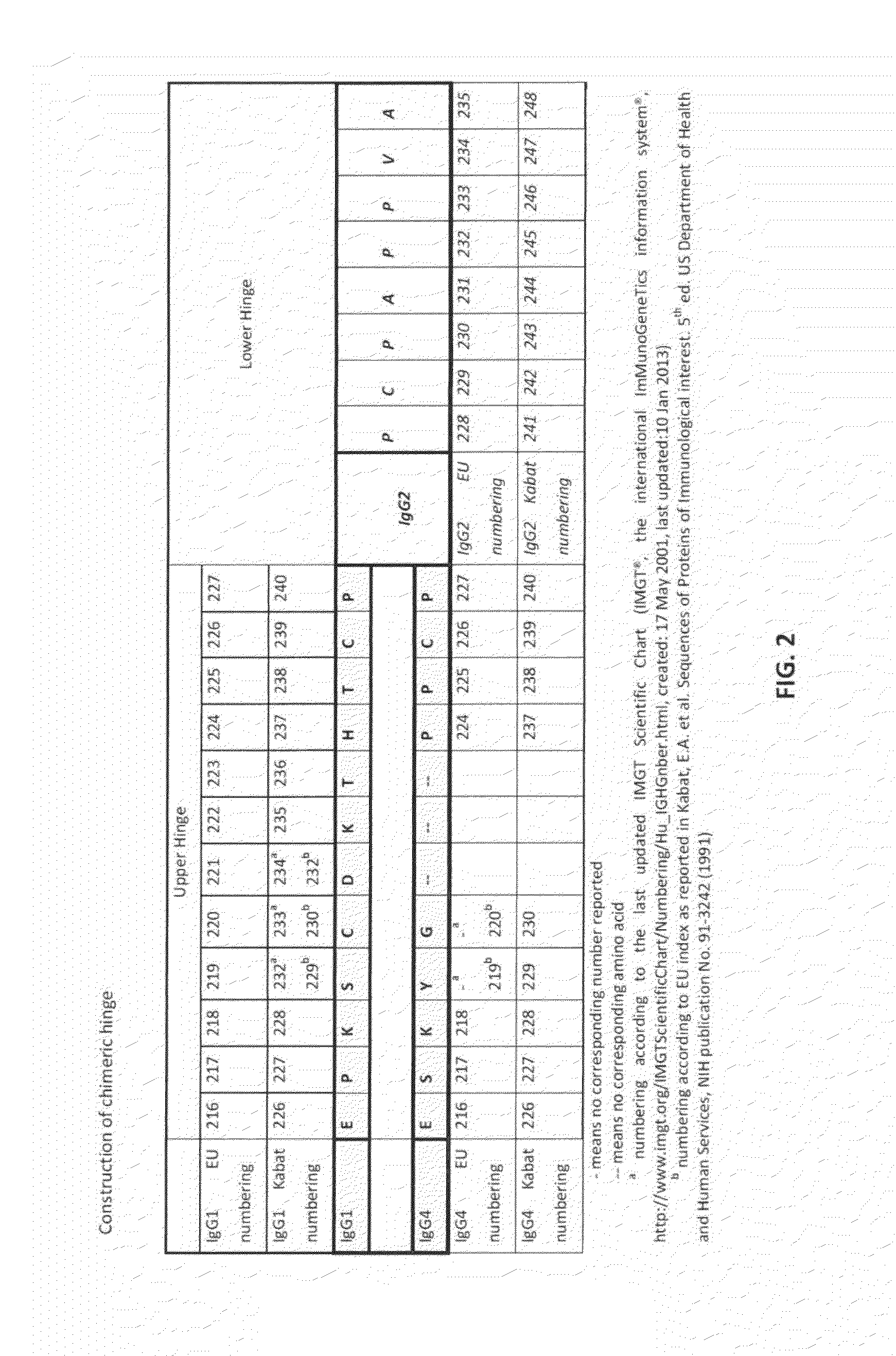
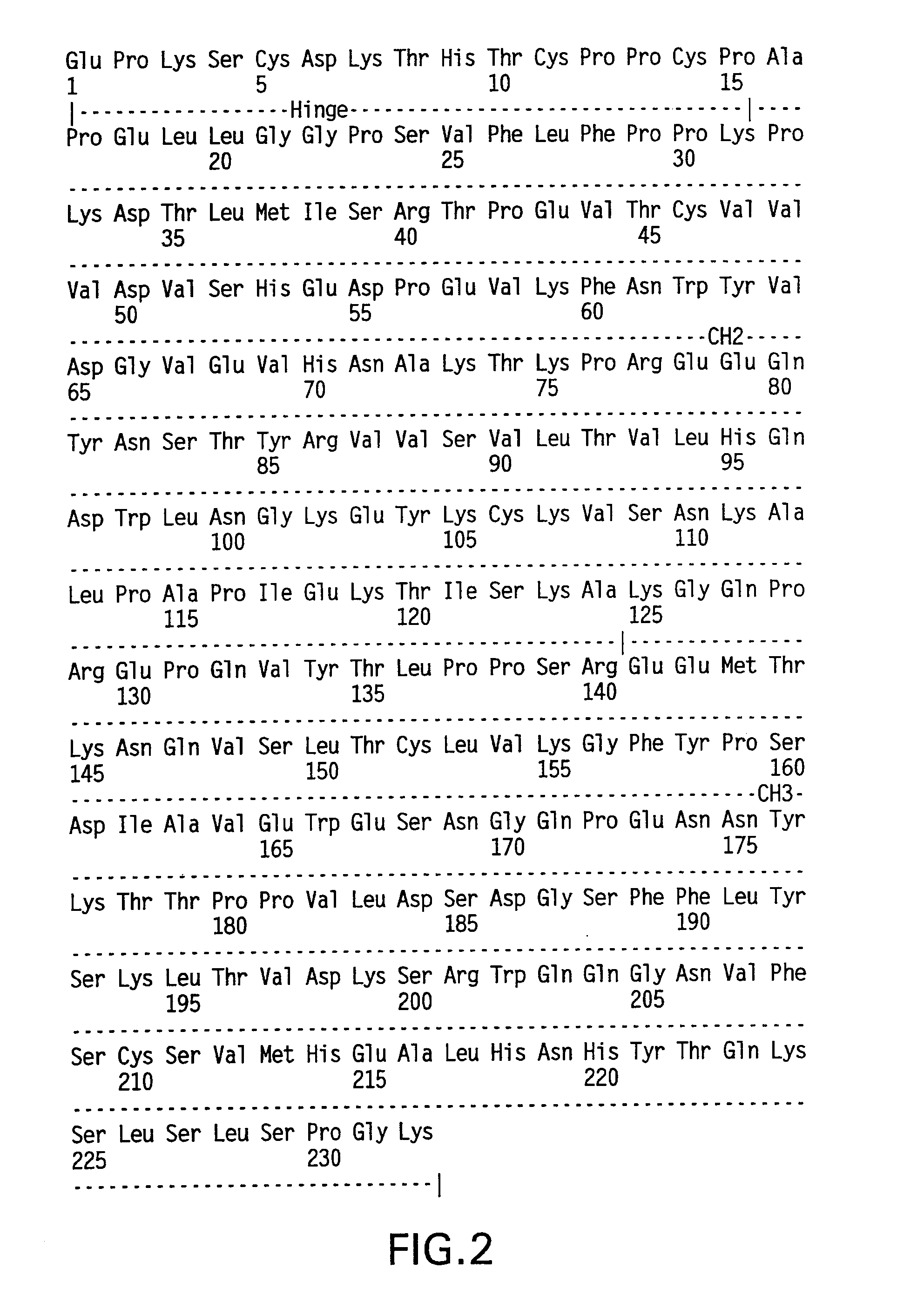
The present invention provides stabilized immunoglobulin molecules that have increased storage stability and / or in vivo half-lives due to the mutation of one or more amino acids that would otherwise render the immunoglobulin molecules susceptible to degradation. In a preferred embodiment, the stabilized immunoglobulins of the invention have mutations at the heavy chain constant domain hinge region. Such stabilized immunoglobulin molecules, i.e., immunoglobulin molecules with increased storage stability have one or more of the following advantages they are more readily transported and / storable for longer periods and / or less stringent conditions than non-stabilized counterparts; that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such stabilized molecules.
Owner:MEDIMMUNE LLC
Antibodies comprising chimeric constant domains
ActiveUS9359437B2Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsFc(alpha) receptorFc receptor
Owner:REGENERON PHARM INC
Identification and engineering of antibodies with variant heavy chains and methods of using same
InactiveUS20090098124A1Optimize therapeutic antibody functionalityAltered affinitySugar derivativesImmunoglobulins against animals/humansTherapeutic antibodyHeavy chain
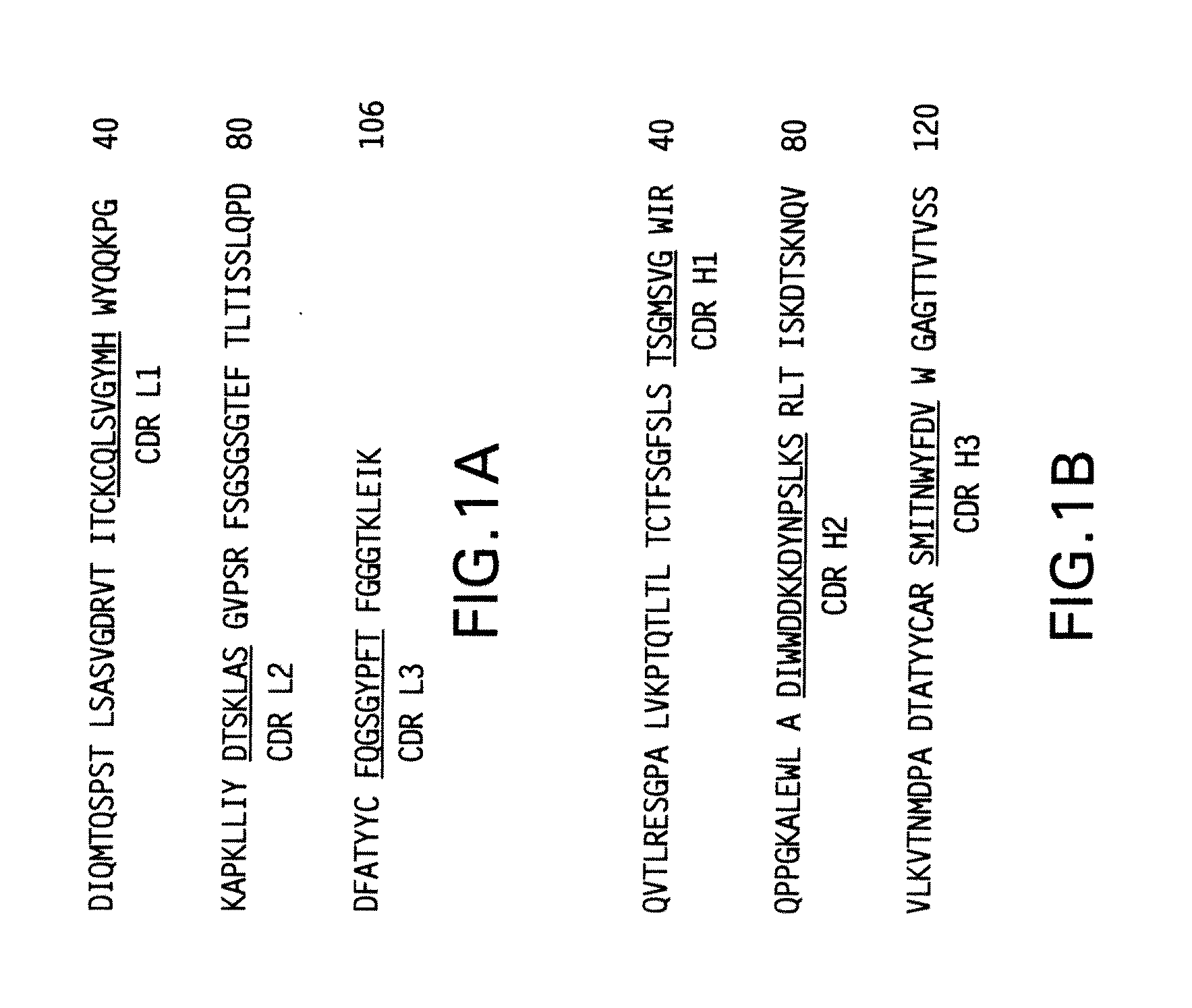
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant heavy chain, which variant heavy chain comprises constant domains from more than one IgG isotype. The variant heavy chain of the invention may further comprise at least one amino acid modification relative to the parental heavy chain, such that the Fc region of said variant heavy chain binds an FcγR with an altered affinity relative to a comparable molecule comprising the wild-type heavy cahin. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcγR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
Binding molecules derived from immunoglobulins which do not trigger complement mediated lysis
InactiveUS7597889B1Maximizing numberIncreasing therapeutic potentialOrganic active ingredientsImmunoglobulins against blood group antigensHeavy chainImmunoglobulin IgE
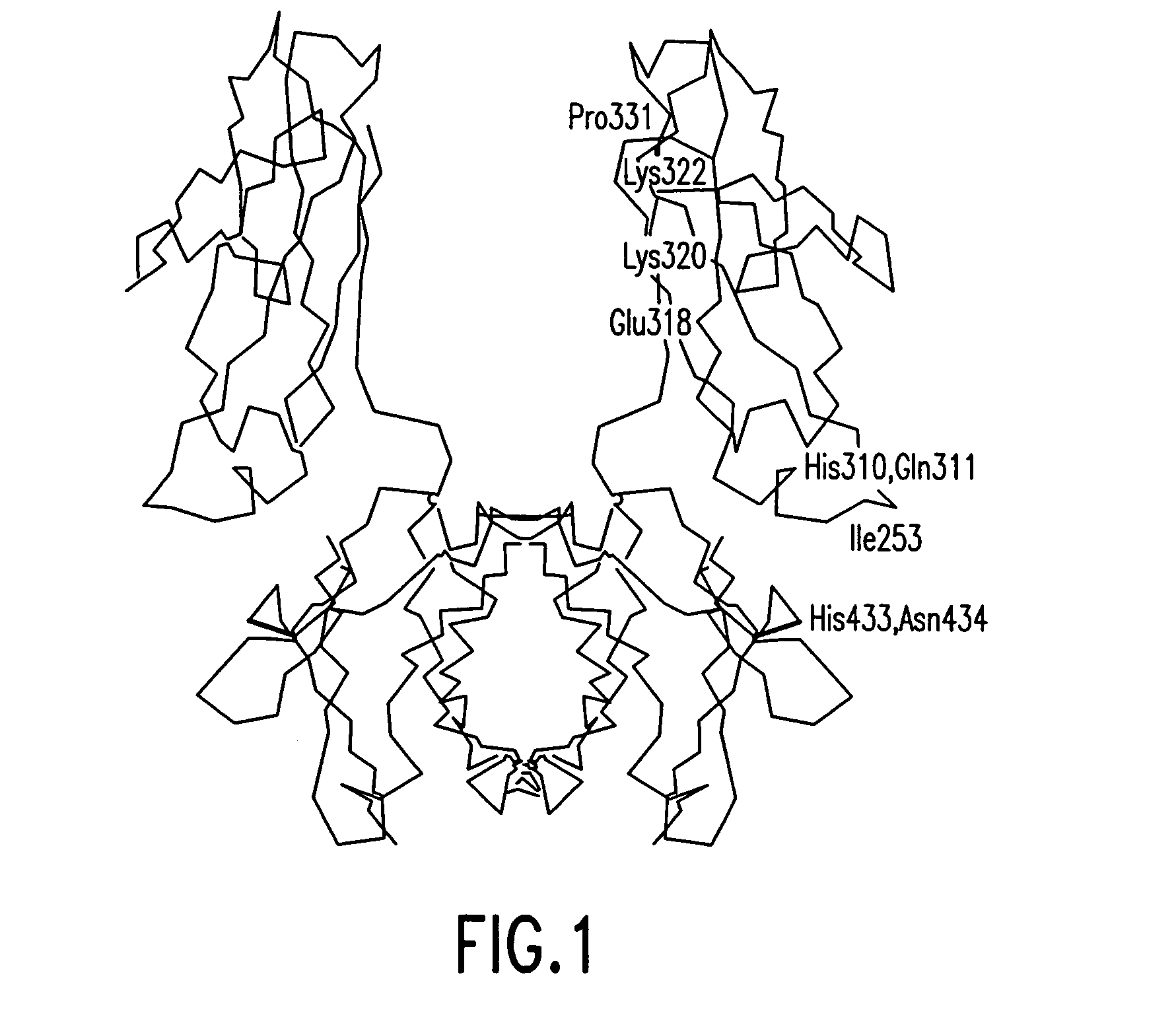
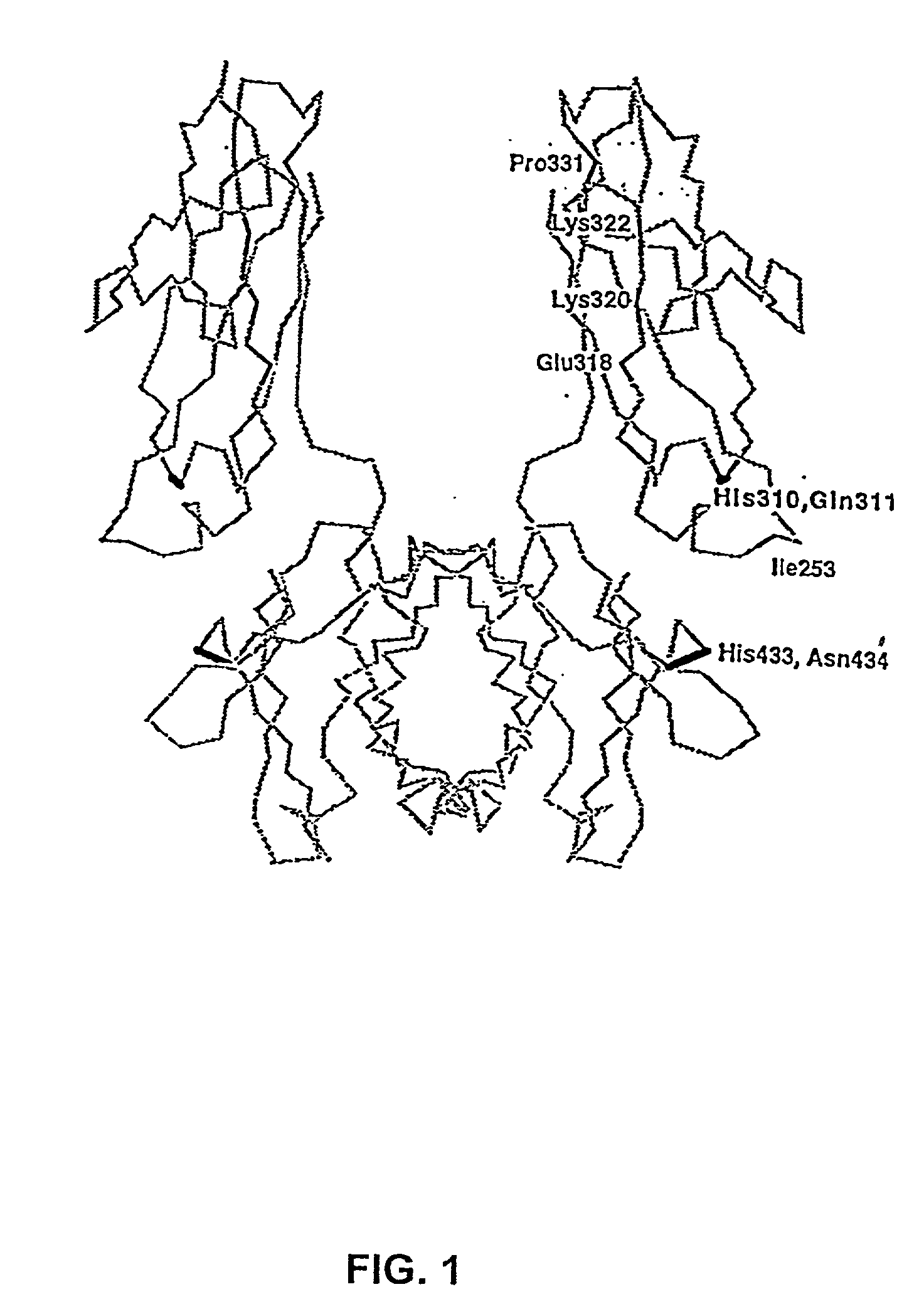
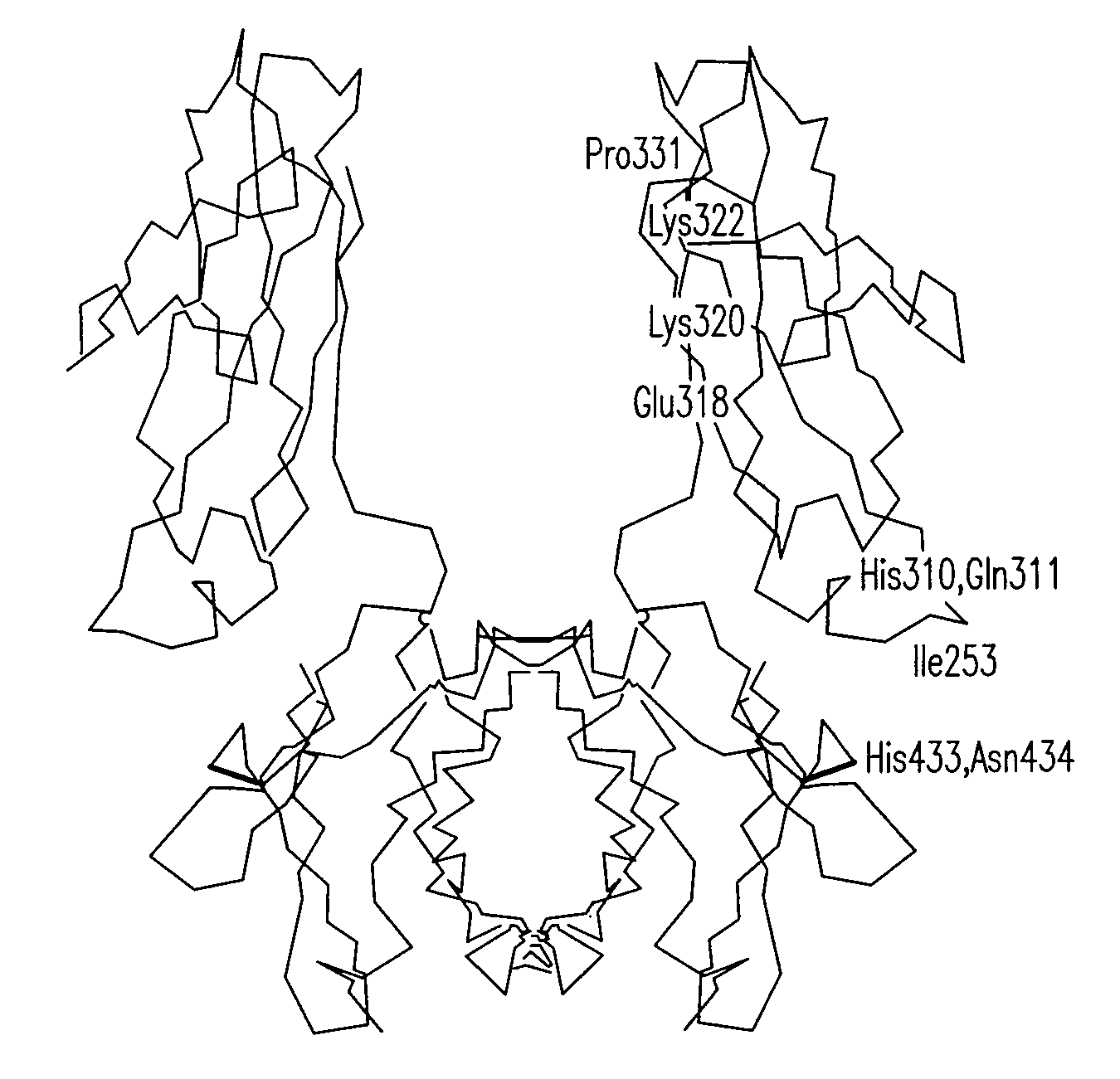
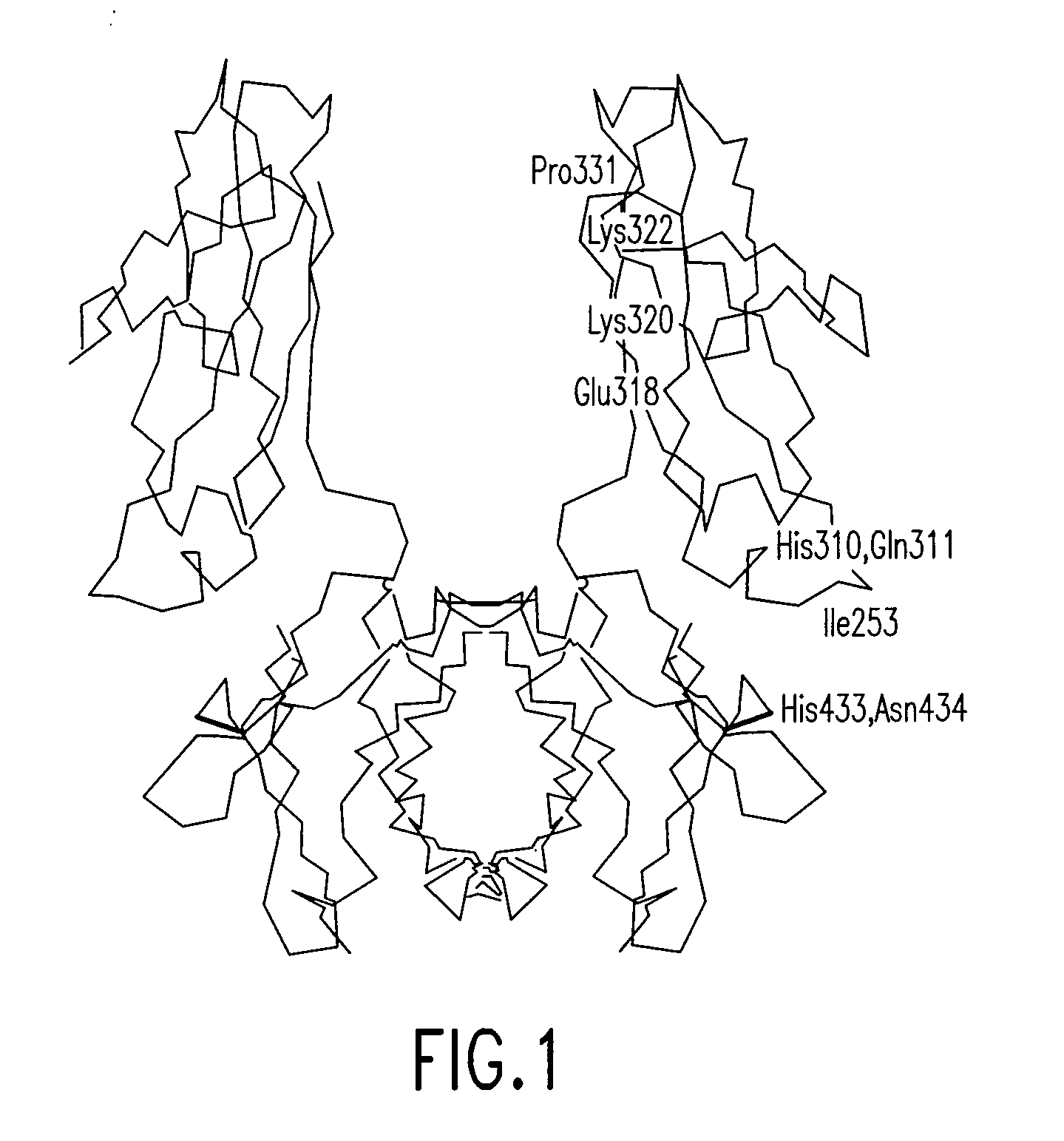
Disclosed are binding molecules which are recombinant polypeptides containing: (i) a binding domain capable of binding a target molecule, and (ii) an effector domain having an amino acid sequence substantially homologous to all or part of a constant domain of a human immunoglobulin heavy chain; characterized in that the binding molecule is capable of binding the target molecule without triggering significant complement dependent lysis, or cell mediated destruction of the target, and more preferably wherein the effector domain is capable of specifically binding FcRn and / or FcγRIIb. These are generally based on chimeric domains which are derived from two or more human immunoglobulin heavy chain CH2 domains domains. In preferred embodiments the regions 233-236, and 327-331, are modified, as are further residues to render the molecule null allotypic. Also disclosed are nucleic acids, host cells, production processes and materials, and uses. Pharmaceutical preparations are also disclosed.
Owner:CAMBRIDGE ENTERPRISE LTD
Methods of preventing and treating RSV infections and related conditions
InactiveUS20060115485A1High affinityEfficient reductionAntibacterial agentsSenses disorderAntigenHalf-life
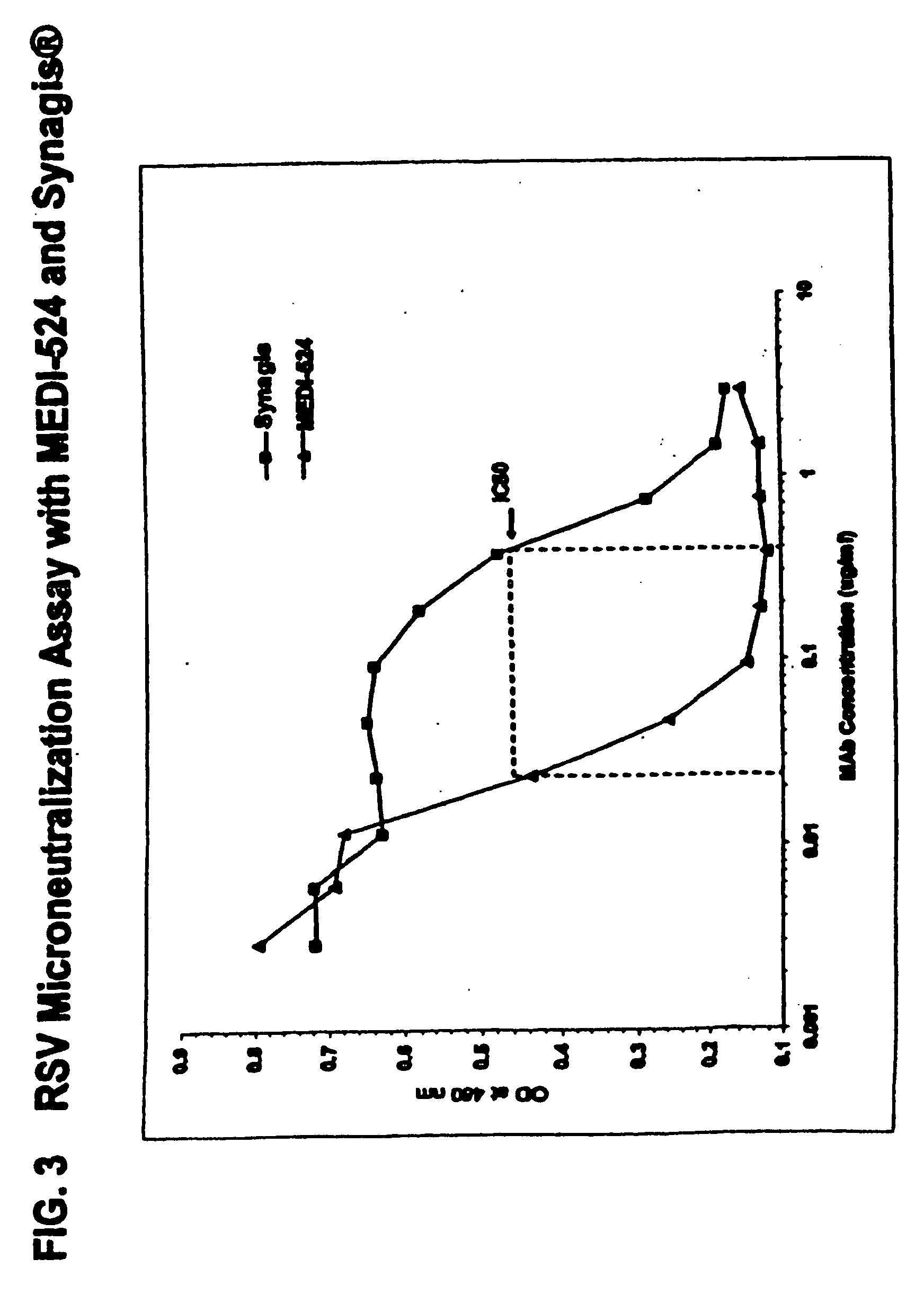
The present invention provides methods for preventing, managing, treating and / or ameliorating a Respiratory Syncytial Virus (RSV) infection (e.g., acute RSV disease, or a RSV upper respiratory tract infection (URI) and / or lower respiratory tract infection (LRI)), otitis media (preferably, stemming from, caused by or associated with a RSV infection, such as a RSV URI and / or LRI), and / or a symptom or respiratory condition relating thereto (e.g., asthma, wheezing, and / or reactive airway disease (RAD)) in a subject, comprising administering to said human an effective amount of one or more antibodies that immunospecifically bind to one or more RSV antigens with a high affinity and / or high avidity. In some embodiments, one or more antibodies comprise a modified IgG constant domain, or FcRn-binding fragment thereof resulting in longer in vivo serum half-life. In particular embodiments the methods of the invention comprising administering to subject an effective amount of one or more modified antibodies that immunospecifically bind to one or more RSV antigens with an association rate (kon) of at least 2×105 M−1s−1 and a dissociation rate (koff) of less than 5×10−4 s−1.
Owner:MEDIMMUNE LLC
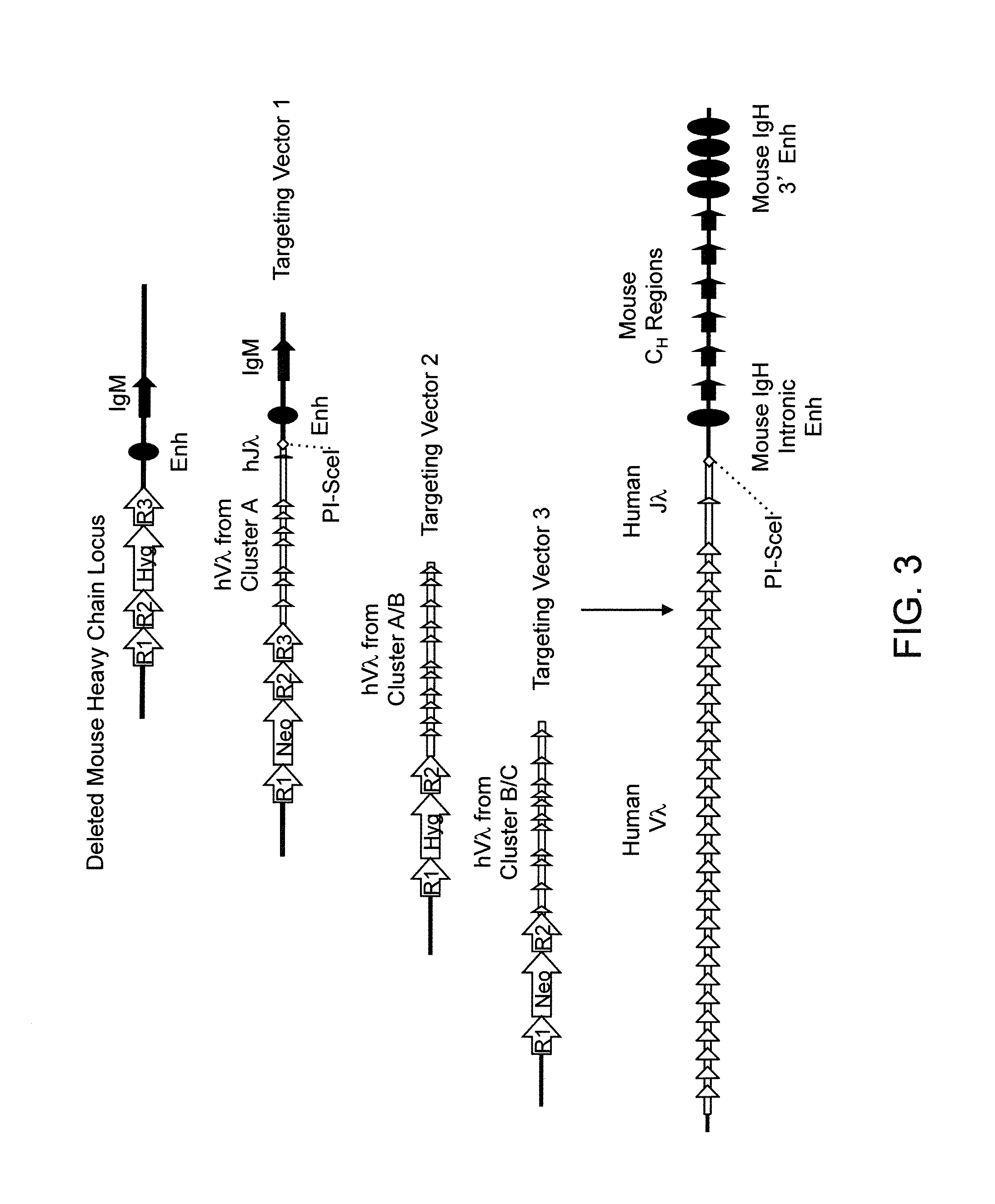
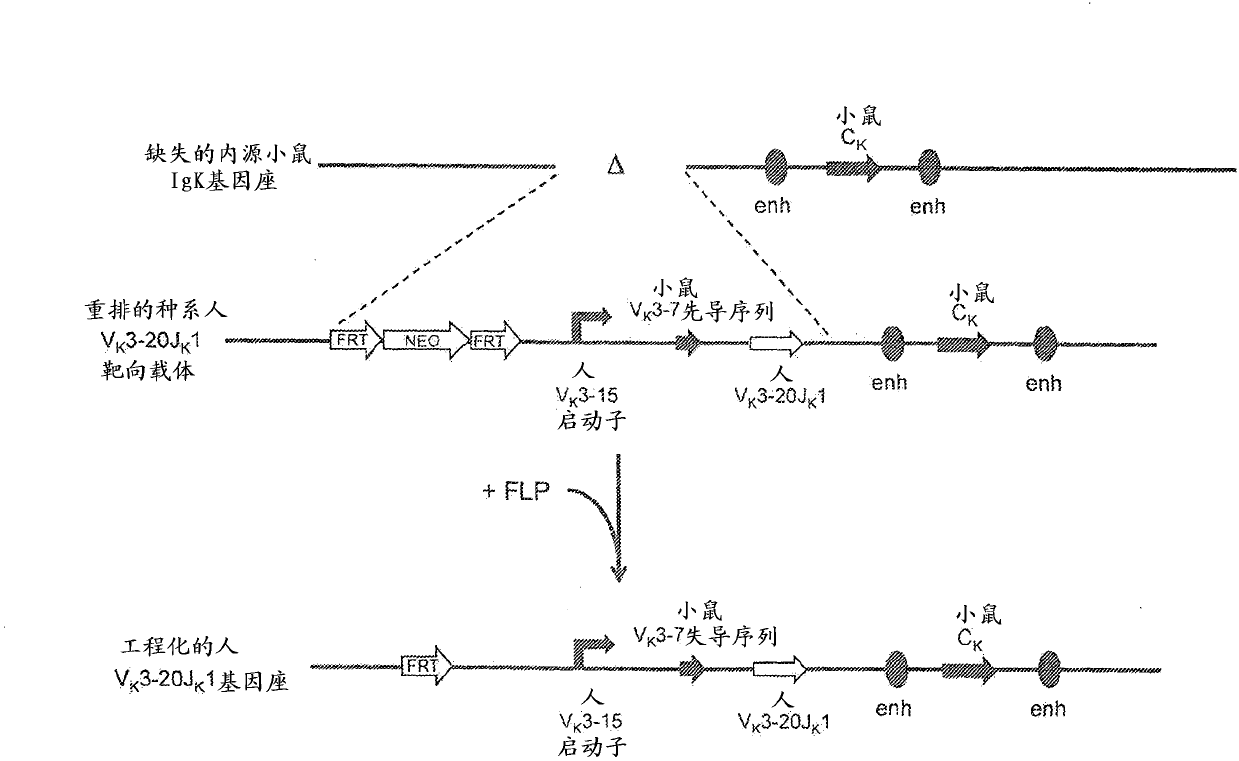
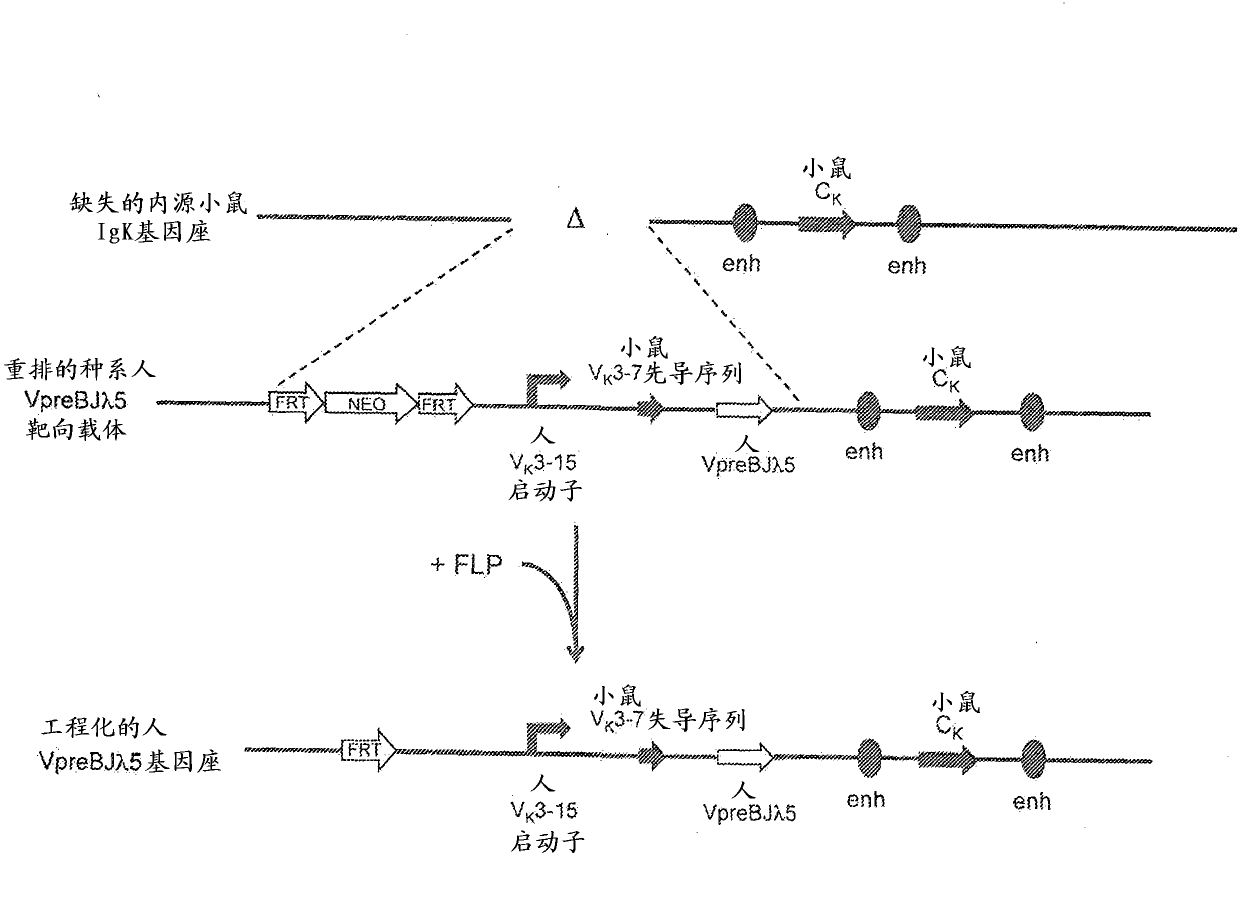
Humanized Rodents that Express Heavy Chain Containing VL Domains
InactiveUS20130212719A1Reduced fertilityImprove fertilityAnimal cellsHybrid immunoglobulinsGenetic MaterialsVariable domain
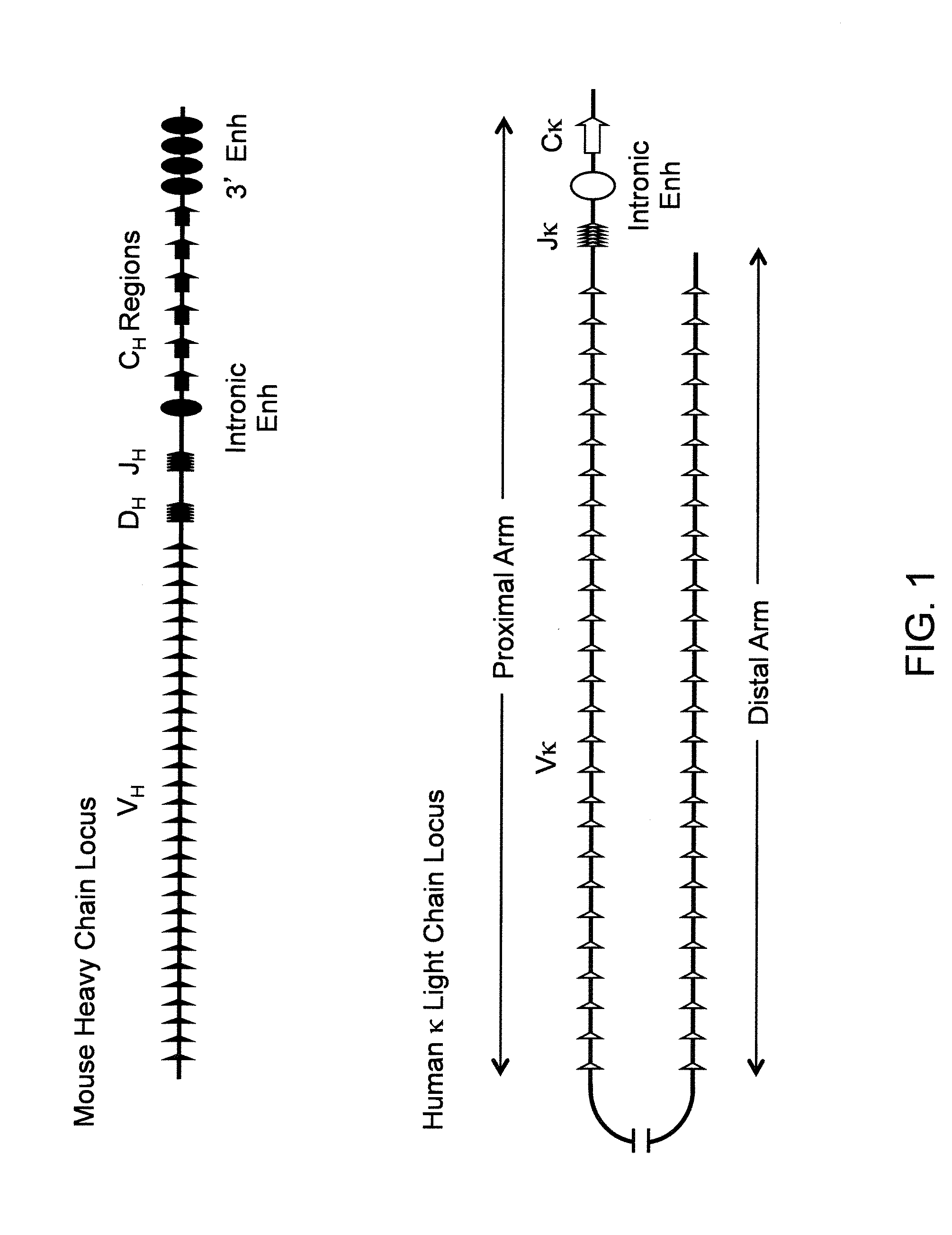
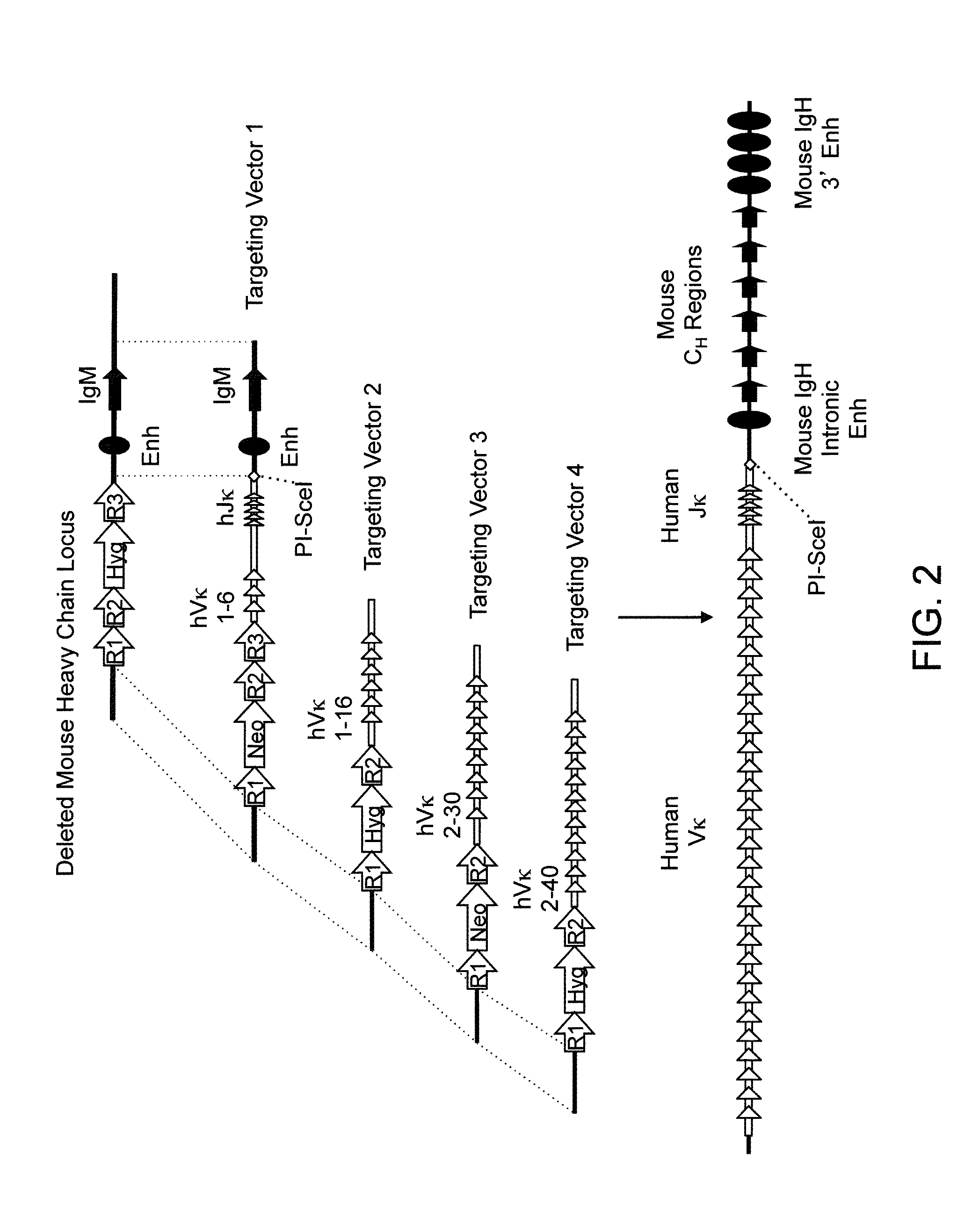
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a rodent (e.g., a mouse), wherein the non-human animals rearrange human immunoglobulin light chain gene segments in the context of heavy chain constant regions and express immunoglobulin-like molecules comprising human immunoglobulin light chain variable domains fused to heavy chain constant domains that are cognate with human immunoglobulin light chain variable domains fused to light chain constant domains.
Owner:REGENERON PHARM INC
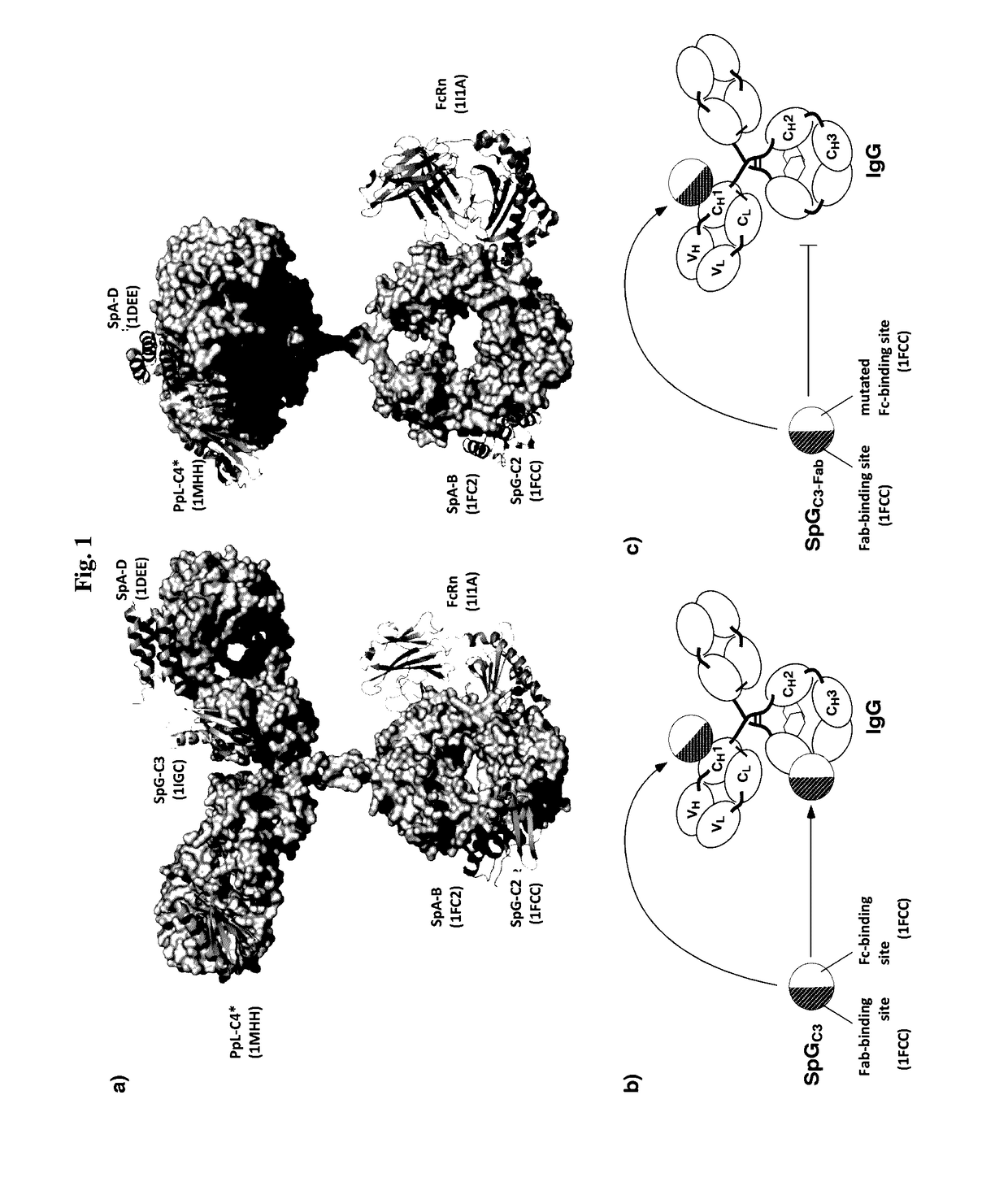
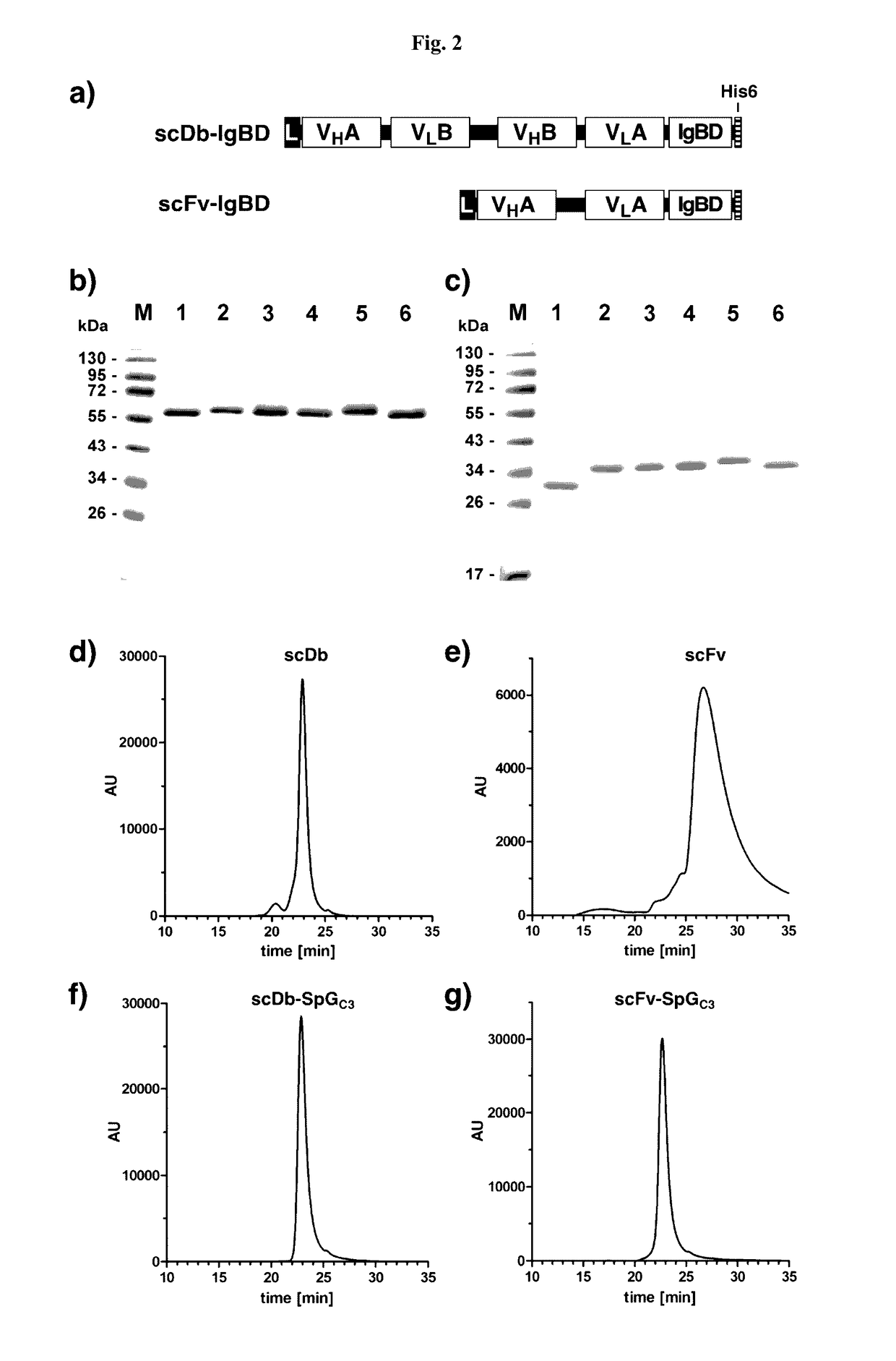
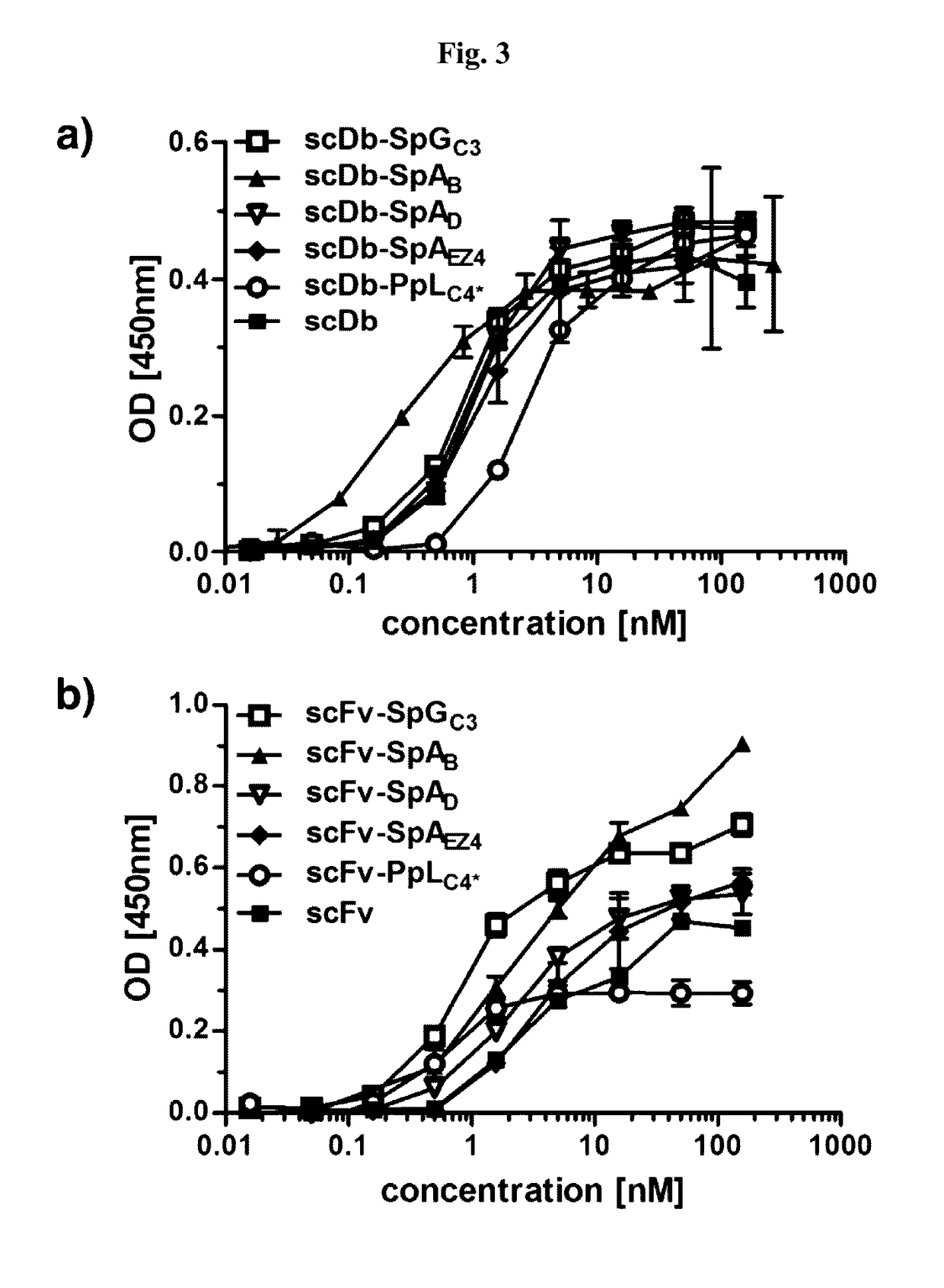
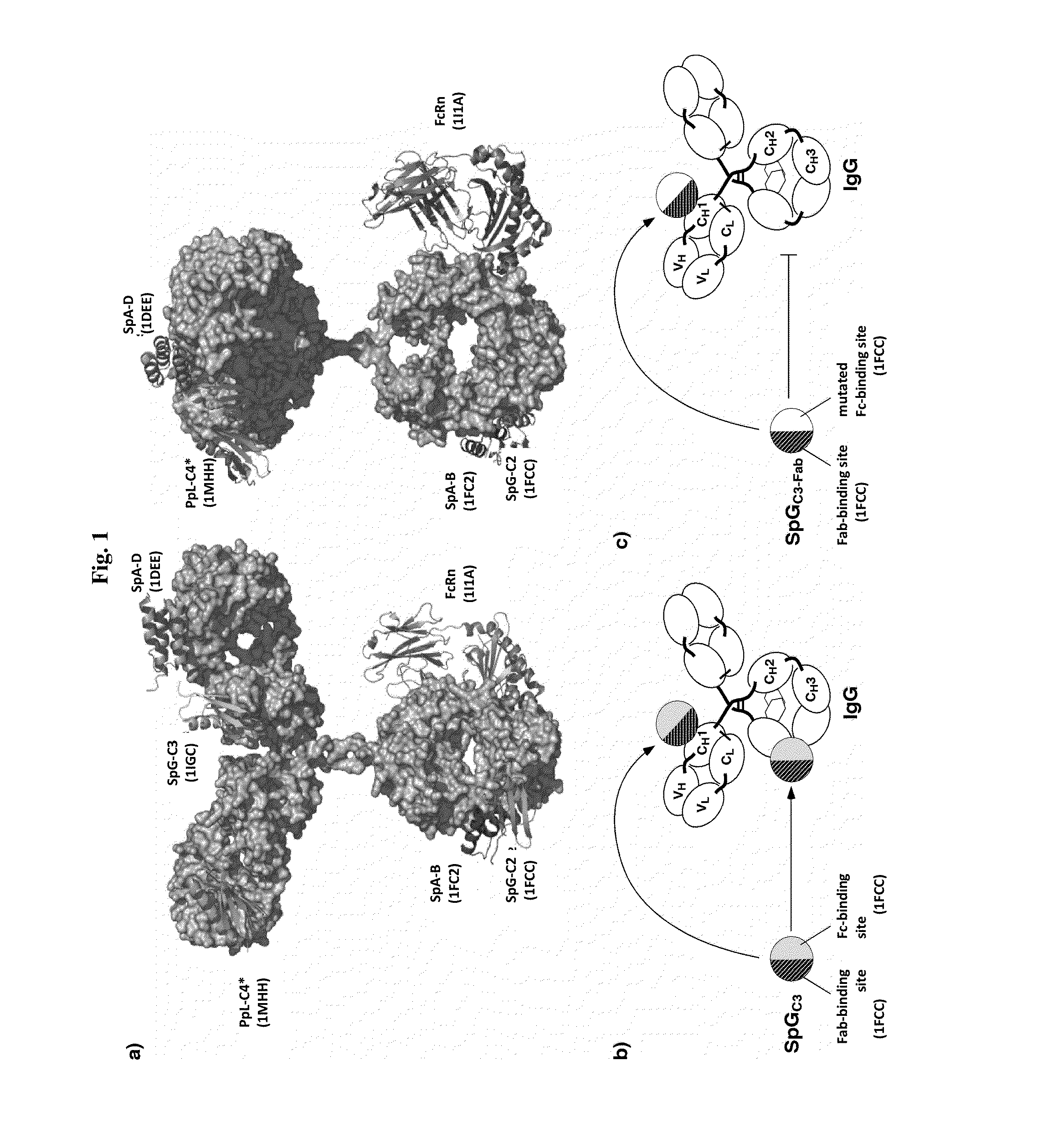
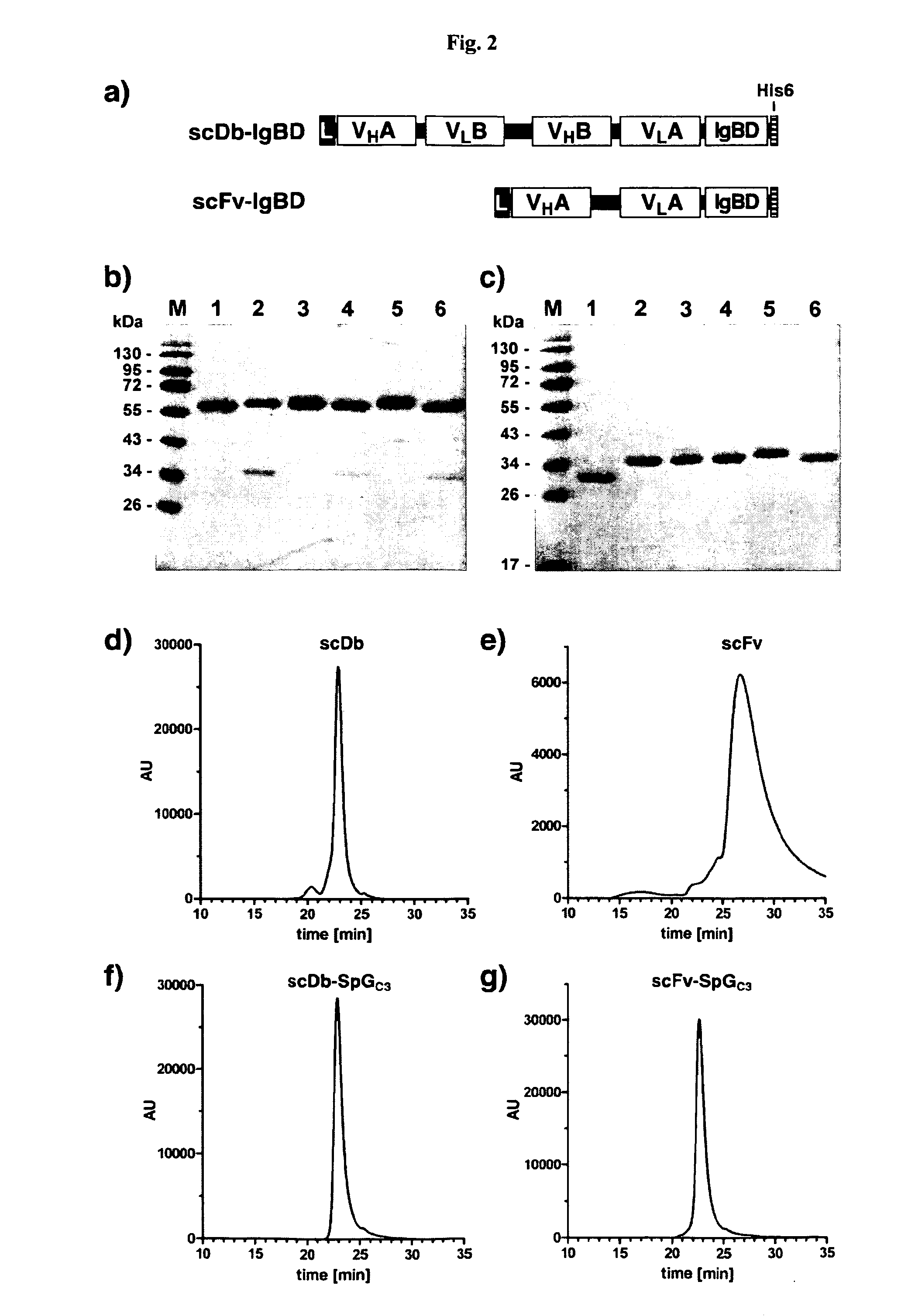
Serum half-life extension using igbd
InactiveUS20170145062A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igeHeavy chain
Owner:UNIV STUTTGART
Method of generating single vl domain antibodies in transgenic animals
InactiveUS20110123527A1Antibody mimetics/scaffoldsFermentationSingle-Chain AntibodiesVariable domain
The present invention describes methods of generating single VL domain antibodies, including chimeric single chain antibodies that comprise of a variable region of a human immunoglobulin κ or λ light chain and a non-human constant region. The non-human constant region is devoid of a first constant domain CH1, and the variable region is devoid of a heavy chain variable domain.
Owner:ABLEXIS LLC
Serum half-life extension using igbd
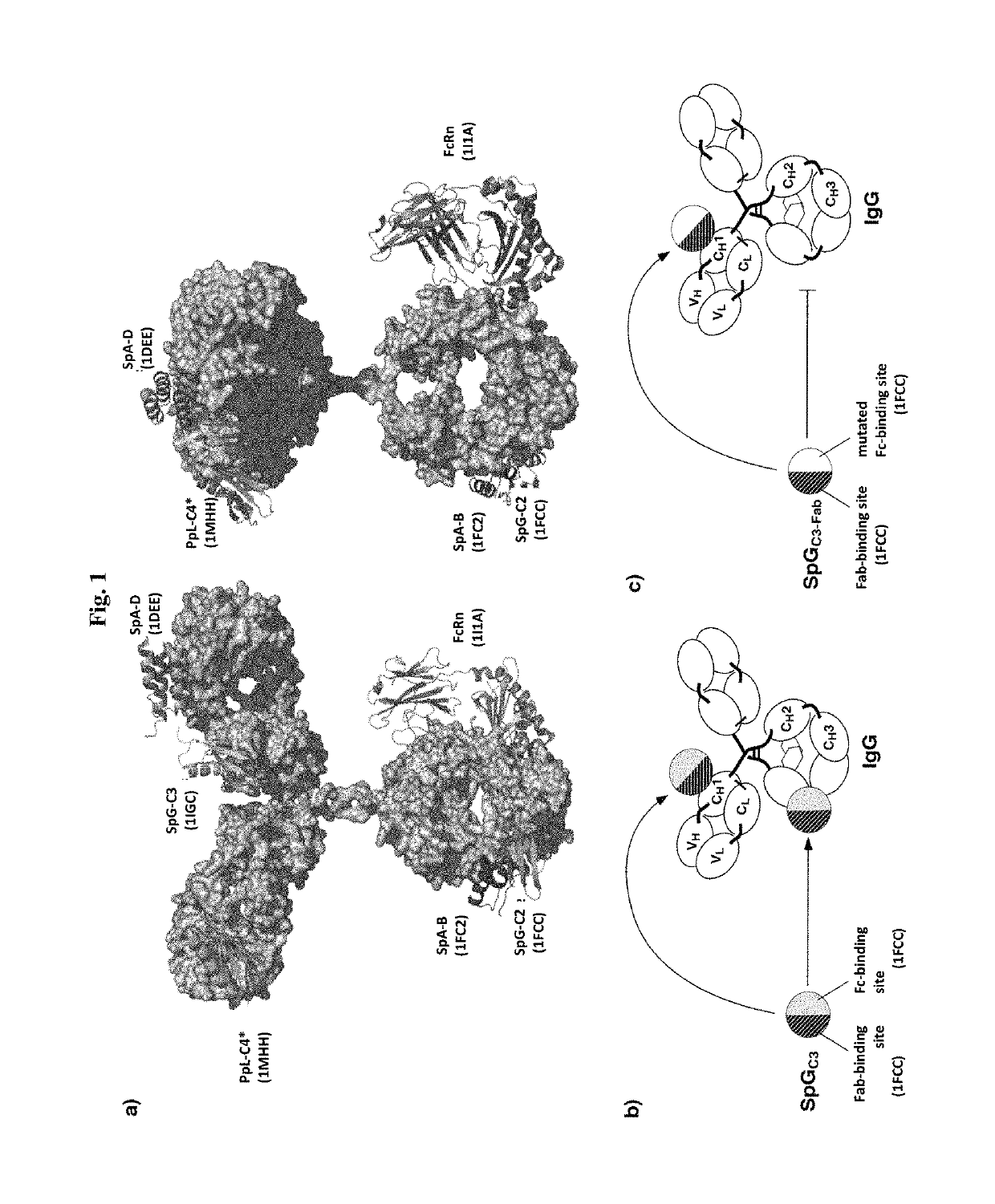
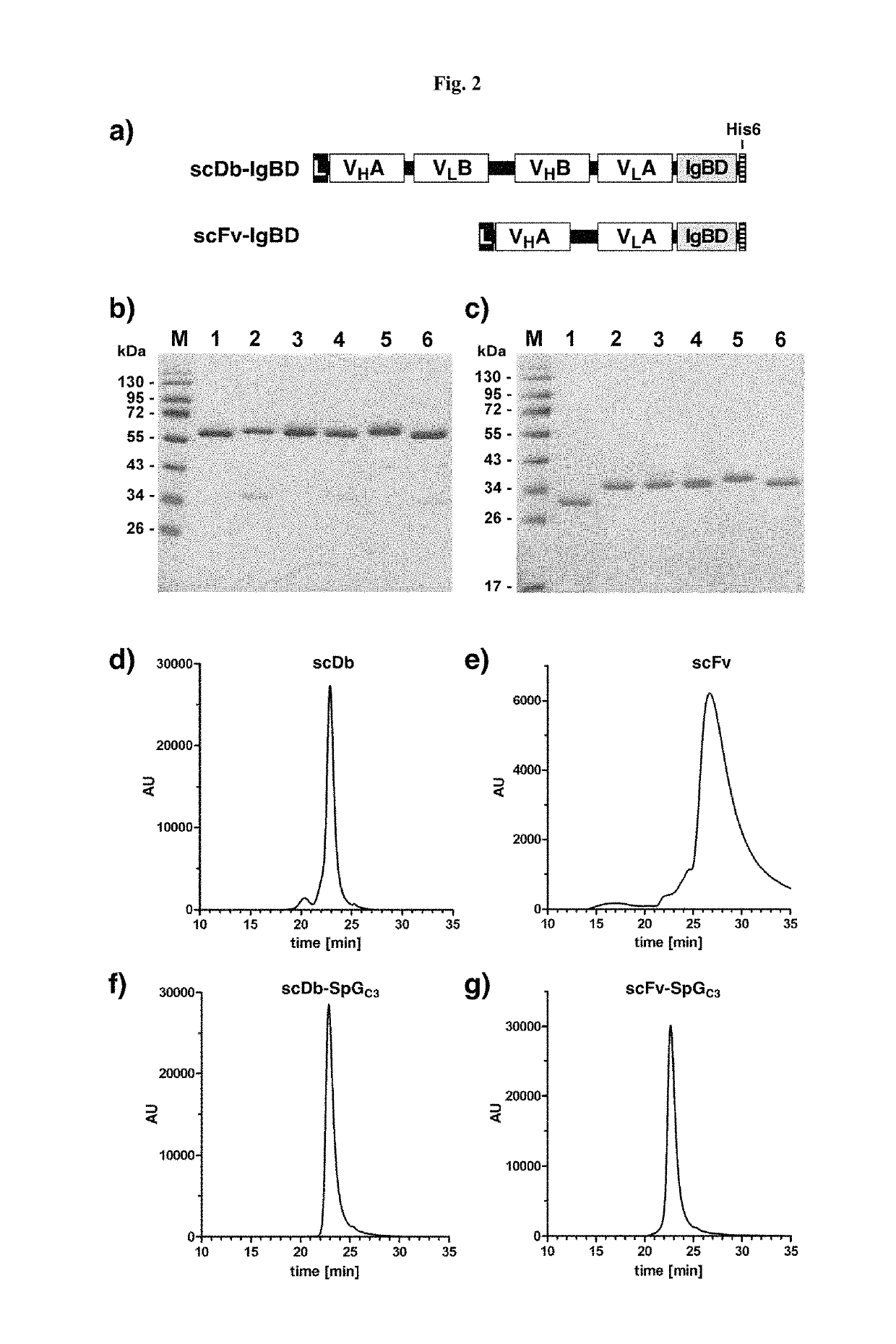
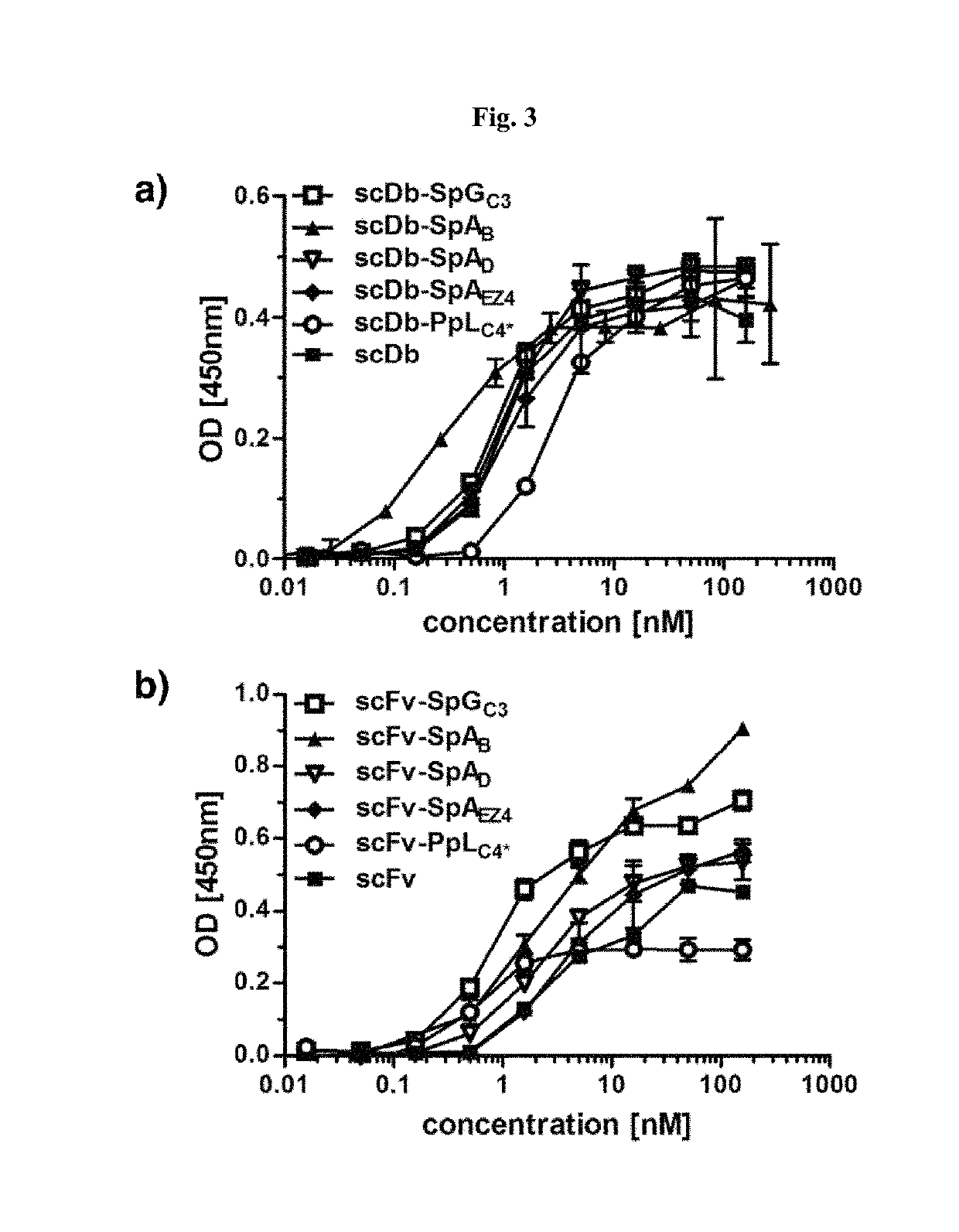
The present invention relates to complexes comprising (i) an immunoglobulin (Ig) binding moiety and (ii) a pharmaceutically active moiety, wherein the Ig binding moiety specifically binds to the constant domain 1 of the heavy chain (CH1) of an Ig molecule and their use for therapy and prophylaxis.
Owner:UNIV STUTTGART
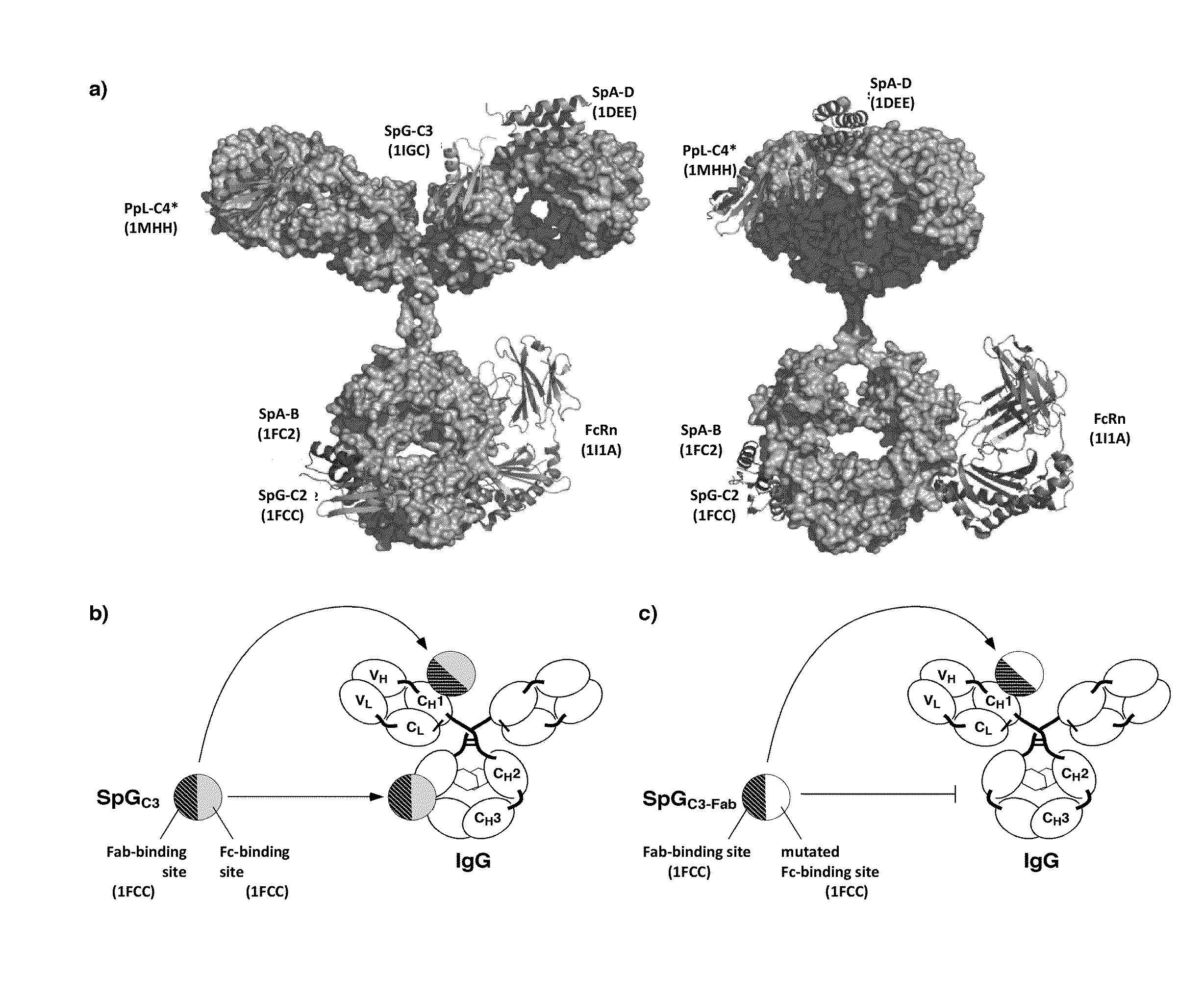
Methods of Purifying Antibodies
ActiveUS20130317200A1Rapid and efficient separationFavorable manufacturing characteristicHybrid immunoglobulinsFermentationHeavy chainBinding site
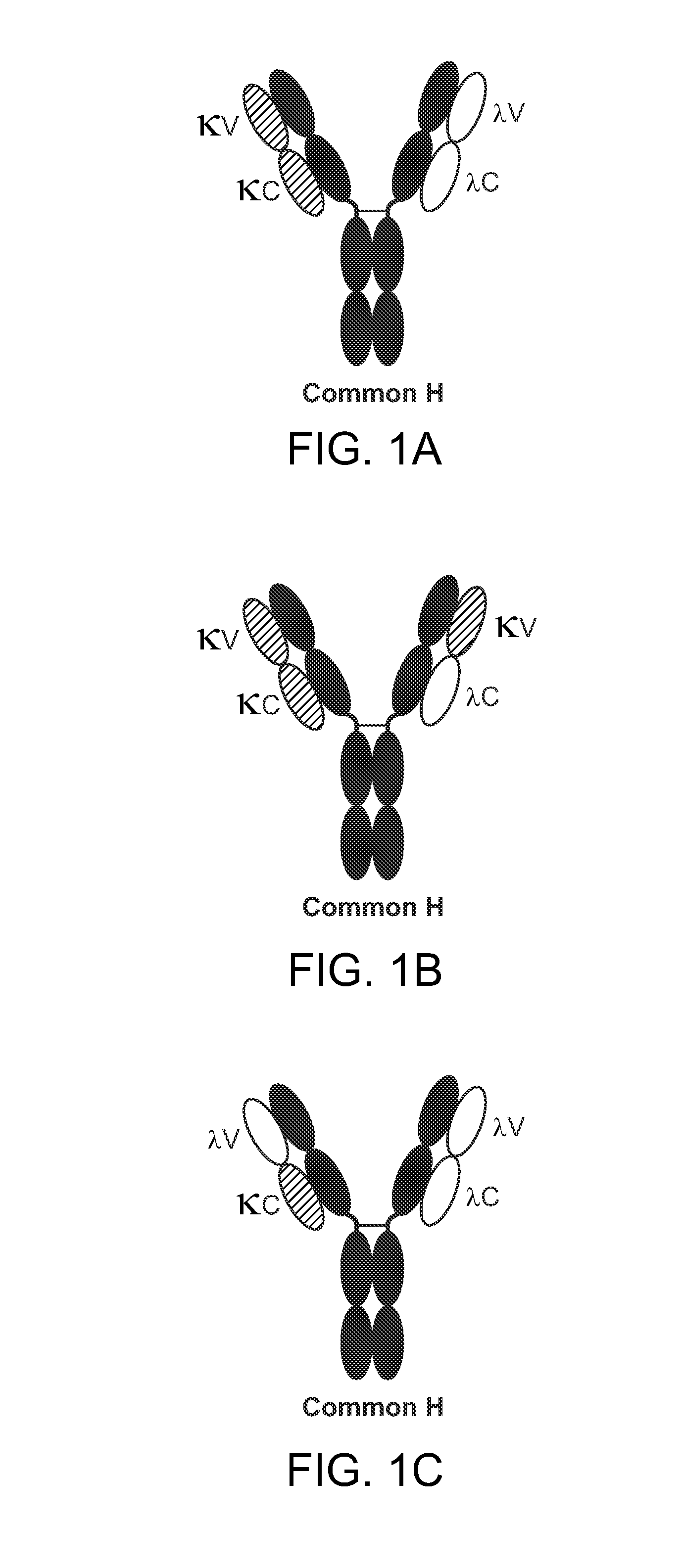
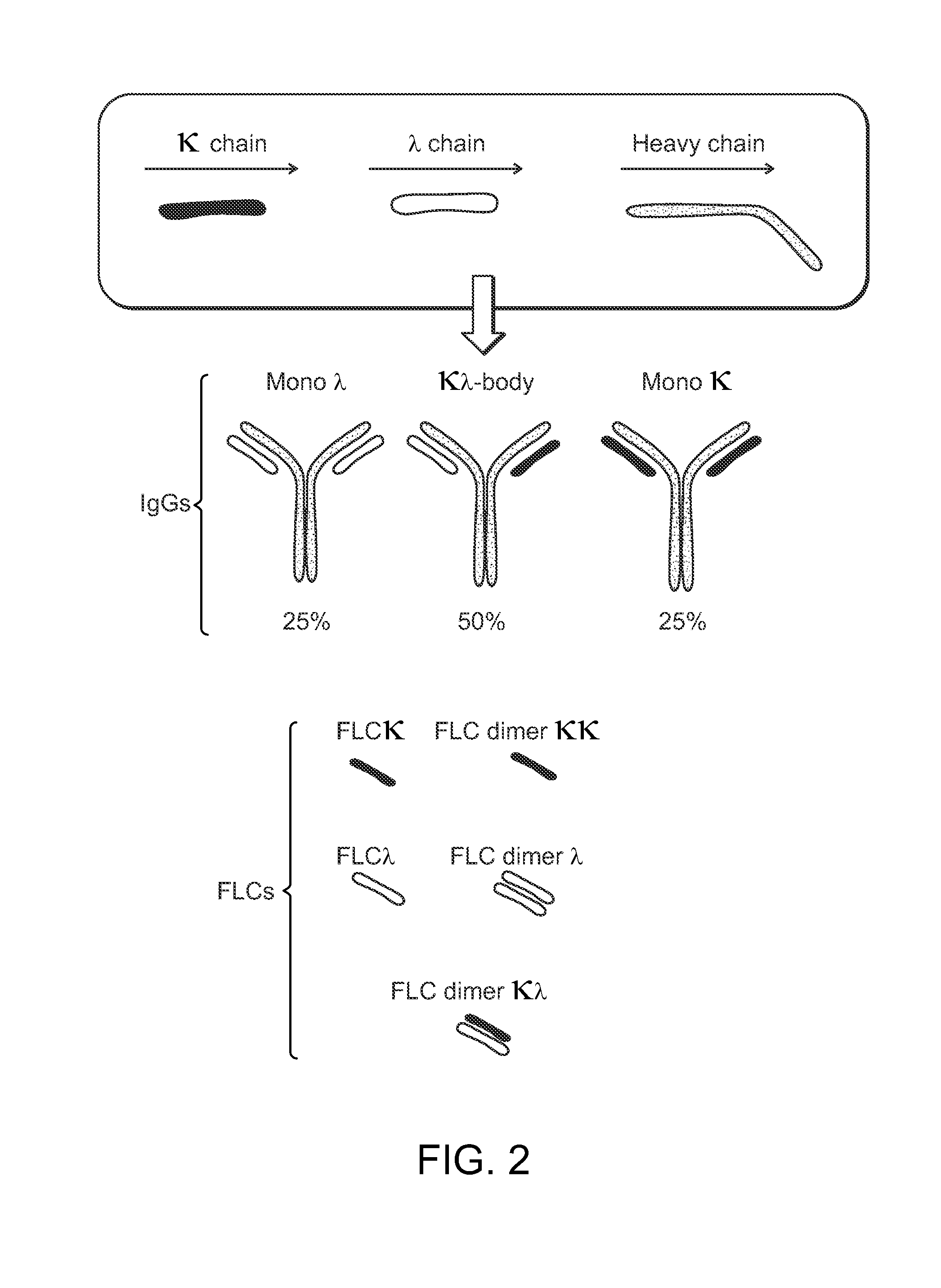
The invention provides methods of purifying antibodies using various antibody-specific purification media to rapidly and efficiently separate mixtures of antibodies, antibody fragments and / or antibody components to isolate a desired antibody product from the mixture. The invention relates to the purification of bispecific monoclonal antibodies carrying a different specificity for each binding site of the immunoglobulin molecule, e.g., antibodies composed of a single heavy chain and two different light chains, one containing a Kappa constant domain and the other a Lambda constant domain, including antibodies of different specificities that share a common heavy chain. The invention also provides the methods of efficiently purifying intact antibodies by separating the intact antibody from non-intact antibodies including free light chains.
Owner:NOVIMMUNE
Methods of purifying bispecific antibodies
ActiveUS20160264685A1Reduce concentrationFavorable manufacturing characteristicHybrid immunoglobulinsPeptide preparation methodsHeavy chainBinding site
The invention relates to the purification of bispecific antibodies carrying a different specificity for each binding site of the immunoglobulin molecule from a mixture of monospecific antibodies. The bispecific antibodies are composed of a single heavy chain and two different light chains, one containing a Kappa constant domain and the other a Lambda constant domain. This invention in particular relates to the isolation of these bispecific antibodies from mixtures that contain monospecific antibodies having two Kappa light chains or portions thereof and monospecific antibodies having two Lambda light chains or portions thereof. The invention also provides the methods of efficiently purifying these bispecific antibodies.
Owner:NOVIMMUNE
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20080181887A1High affinityExtended half-lifeAntibody mimetics/scaffoldsAntibody ingredientsHalf-lifeIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:MEDIMMUNE LLC
Methods of preventing and treating rsv infections and related conditions
Owner:MEDIMMUNE LLC
Immunoglobulin libraries
Methods and compositions for the screening and isolation of ligand-binding polypeptides, such as antibodies. In some aspects, methods of the invention enable the isolation of intact soluble antibodies comprising a constant domain. Screening methods that employ genetic packages such as bacteria and bacteriophages enable high through-put identification of ligand binding molecules.
Owner:RES DEVMENT FOUND
Fusion Protein Comprising an Fc Receptor Binding Polypeptide and an Antigenic Polypeptide for Mediating an Immune Response
InactiveUS20090186025A1Prevent and treat diseaseStrong pathogenicityAntibacterial agentsVirusesFc(alpha) receptorDisease
The present invention provides a fusion protein comprising an Fc receptor binding polypeptide and an antigenic polypeptide. The fusion peptide may further comprise a linker sequence or hinge portion which joins the Fc receptor biding polypeptide and the antigenic polypeptide. The Fc receptor binding polypeptide typically comprises the CH2 constant domain of a human IgG immunoglobulin. The antigenic polypeptide can be any polypeptide which induces an immune response. Administration of the fusion protein to a subject results in a cytotoxic T lymphocyte response being induced against the antigenic polypeptide provided within the fusion protein. The invention further extends to methods for the treatment of a disease condition in a subject using the fusion proteins of the invention.
Owner:IMMUNOBIOLOGY LTD
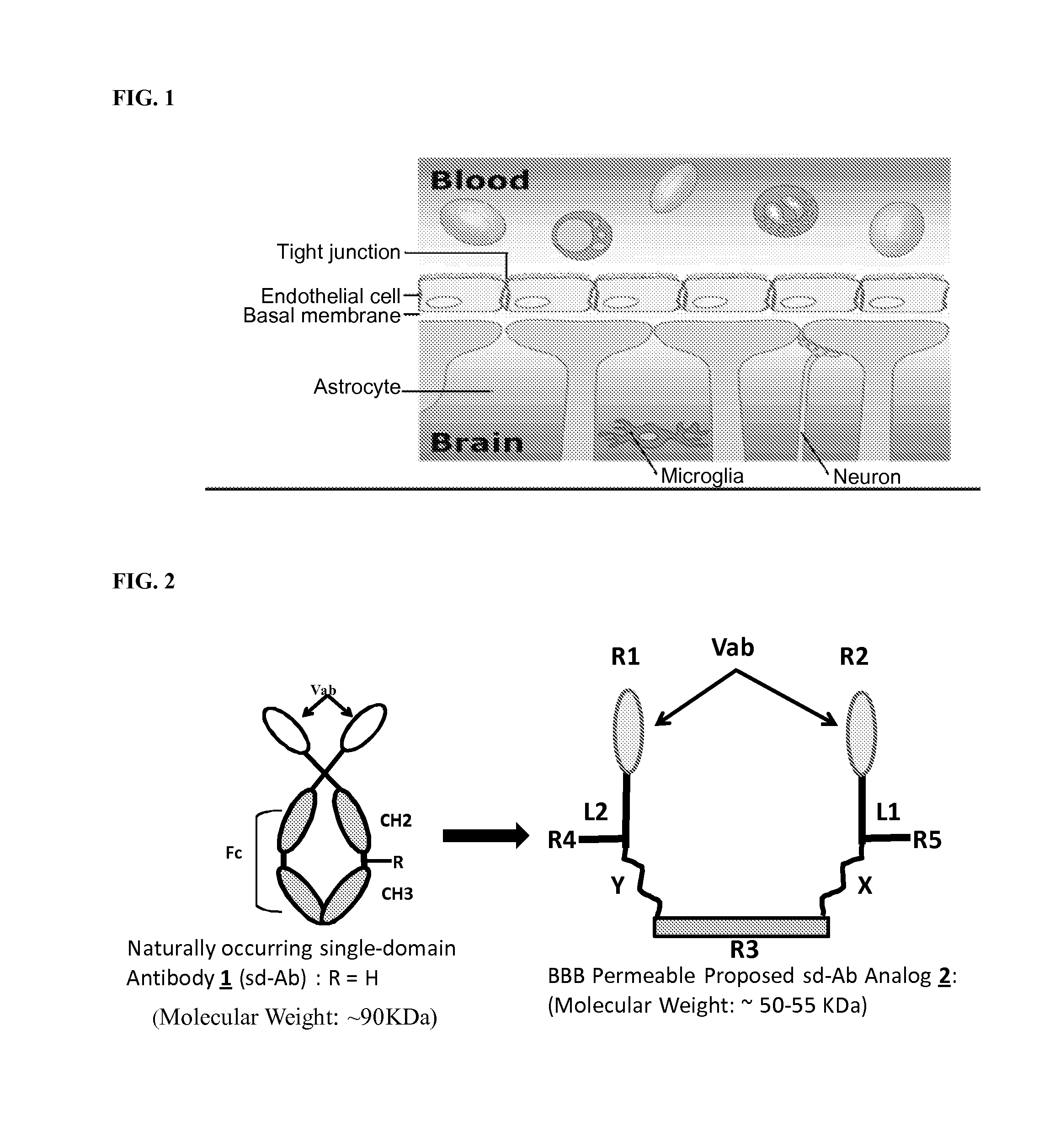
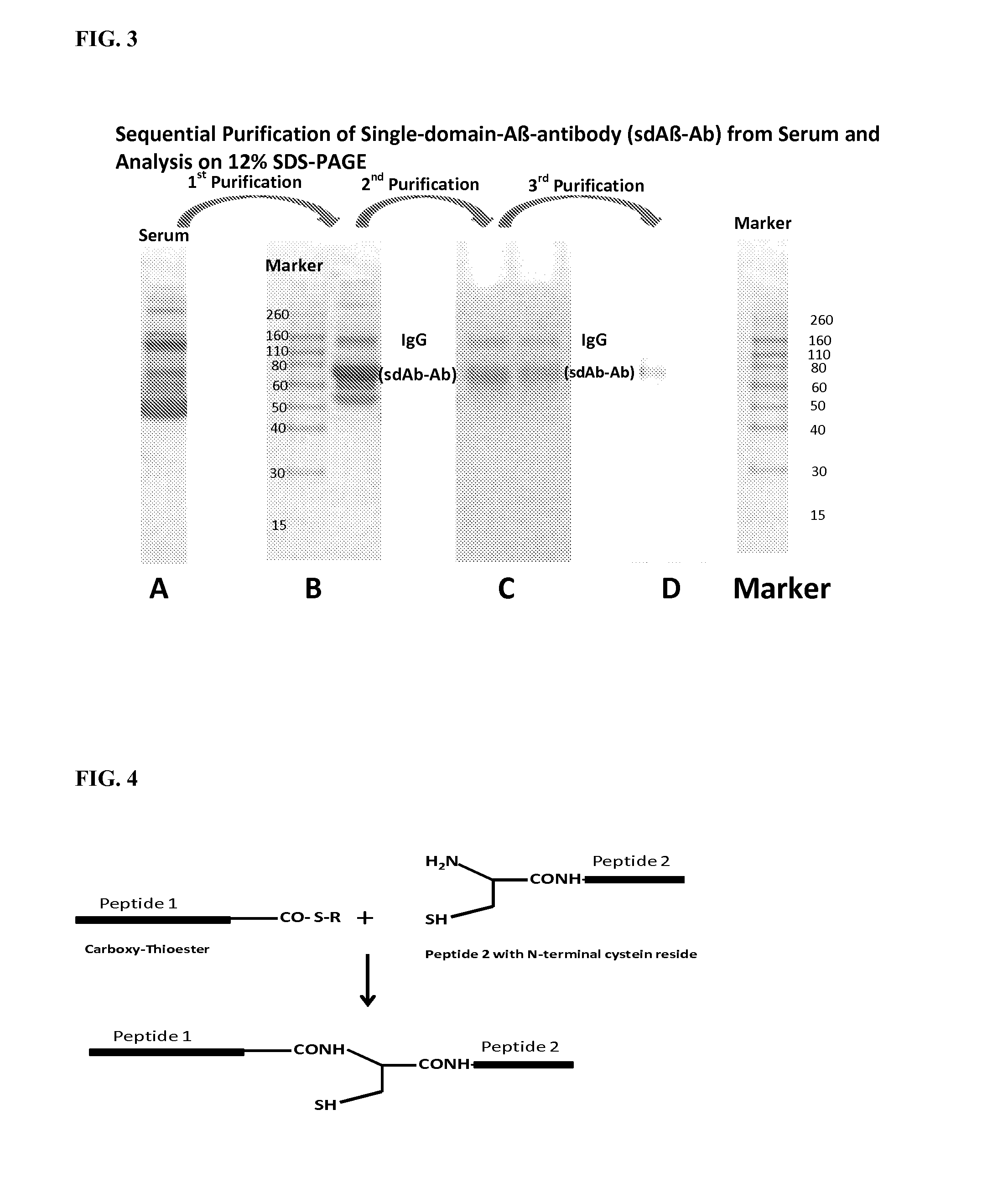
Blood-brain barrier permeable peptide compositions
ActiveUS20130177568A1Immunoglobulins against animals/humansPharmaceutical delivery mechanismAmyloid betaCamelid
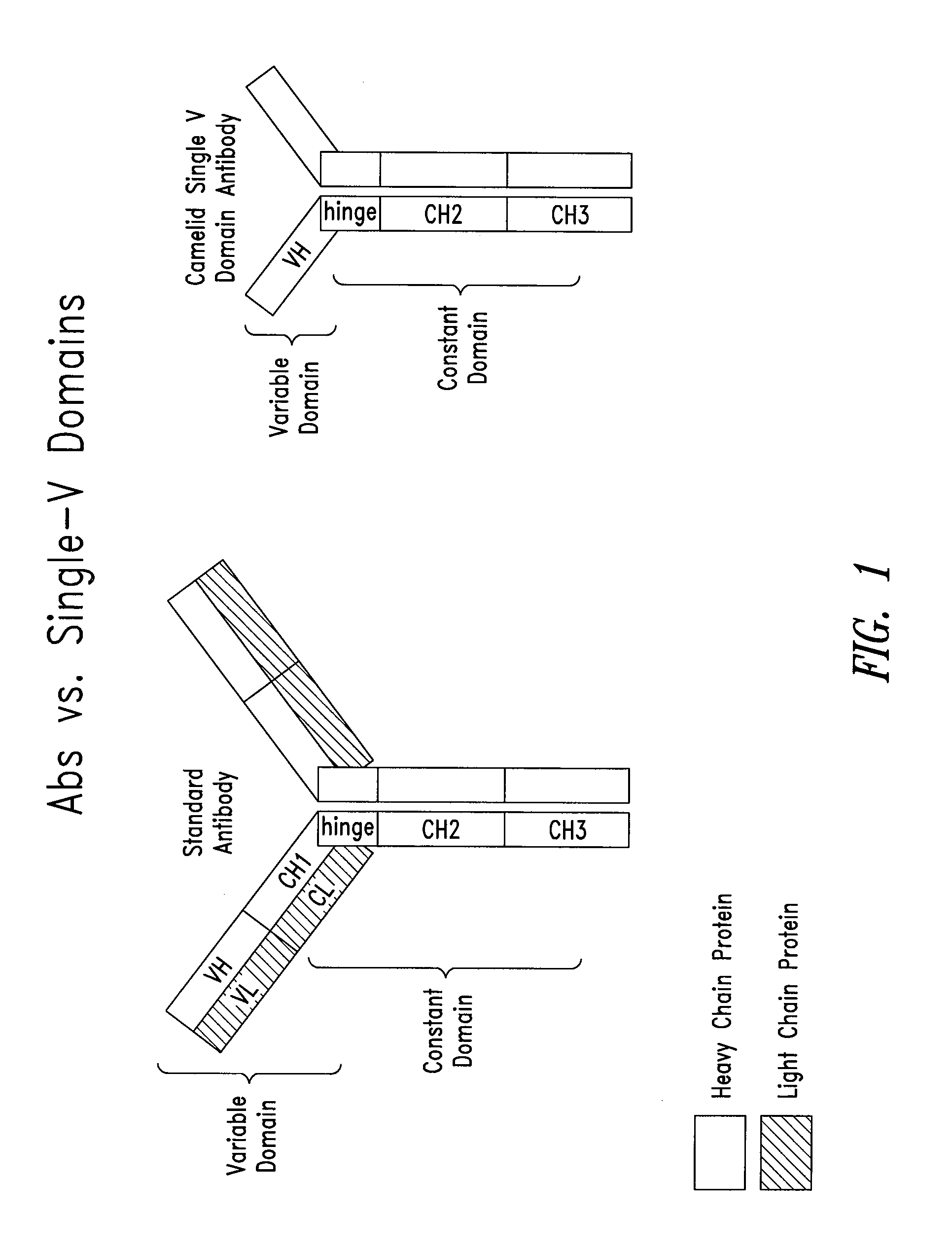
Blood-brain barrier permeable peptide compositions that contain variable antigen binding domains from camelid and / or shark heavy-chain only single-domain antibodies are described. The variable antigen binding domains of the peptide compositions bind to therapeutic and diagnostic biomarkers in the central nervous system, such as the amyloid-beta peptide biomarker for Alzheimer's disease. The peptide compositions contain constant domains from human IgG, camelid IgG, and / or shark IgNAR. The peptide compositions include heavy-chain only single-domain antibodies and compositions with one or more variable antigen binding domain bound to one or more constant domains.
Owner:ICB INT
Common light chain mouse
A genetically modified mouse is provided, wherein the mouse is incapable of rearranging and expressing an endogenous mouse immunoglobulin light chain variable sequence, wherein the mouse expresses only one or two human light chain variable domains encoded by human immunoglobulin sequences operably linked to the mouse kappa (K) constant gene at the endogenous mouse K locus, wherein the mouse expresses a reverse chimeric antibody having a light chain variable domain derived from one of only two human light chain variable region gene segments and a mouse K constant domain,. and a human heavy chain variable domain and a mouse heavy chain constant domain, from an endogenous mouse heavy chain locus. Bispecific epitope-binding proteins that are fully human are provided, comprising two different heavy chains that associate with an identical light chain that comprises a variable domain derived from one of two different human light chain variable region gene segments.
Owner:REGENERON PHARM INC
Variant IgG3 RITUXAN and therapeutic uses thereof
InactiveUS20060121028A1Prevent and reduce proliferation of cellReduce and prevent proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Antigen binding
Monoclonal anti-human CD20 antigen binding antibodies containing human IgG3 constant domains are provided. These antibodies possess effector functions that render them well suited for use in therapeutic methods, especially treatments wherein inhibition of B cell function or B cell number is therapeutically desirable.
Owner:BIOGEN INC
Methods For The Generation Of Multispecific And Multivalent Antibodies
ActiveUS20140179547A1Favorable manufacturing characteristicMaximize productionHybrid immunoglobulinsDNA preparationHeavy chainBispecific monoclonal antibody
Owner:NOVIMMUNE
Immunoglobulin libraries
Methods and compositions for the screening and isolation of ligand-binding polypeptides, such as antibodies. In some aspects, methods of the invention enable the isolation of intact soluble antibodies comprising a constant domain. Screening methods that employ genetic packages such as bacteria and bacteriophages enable high through-put identification of ligand binding molecules.
Owner:RES DEVMENT FOUND
Serum half-life extension using IgBD
InactiveUS10428120B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igeHeavy chain
The present invention relates to complexes comprising (i) an immunoglobulin (Ig) binding moiety and (ii) a pharmaceutically active moiety, wherein the Ig binding moiety specifically binds to the constant domain 1 of the heavy chain (CH1) of an Ig molecule and their use for therapy and prophylaxis.
Owner:UNIV STUTTGART
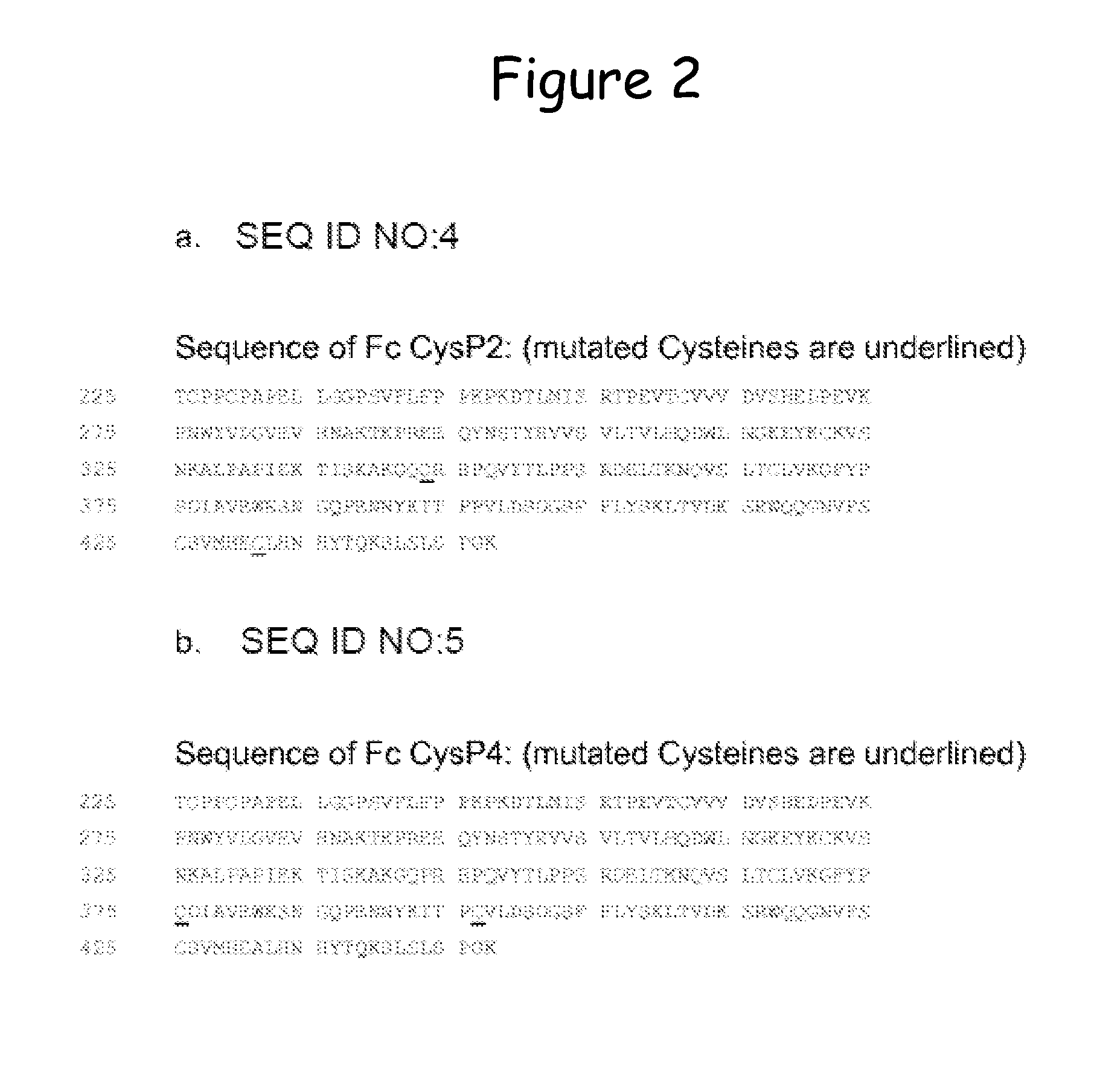
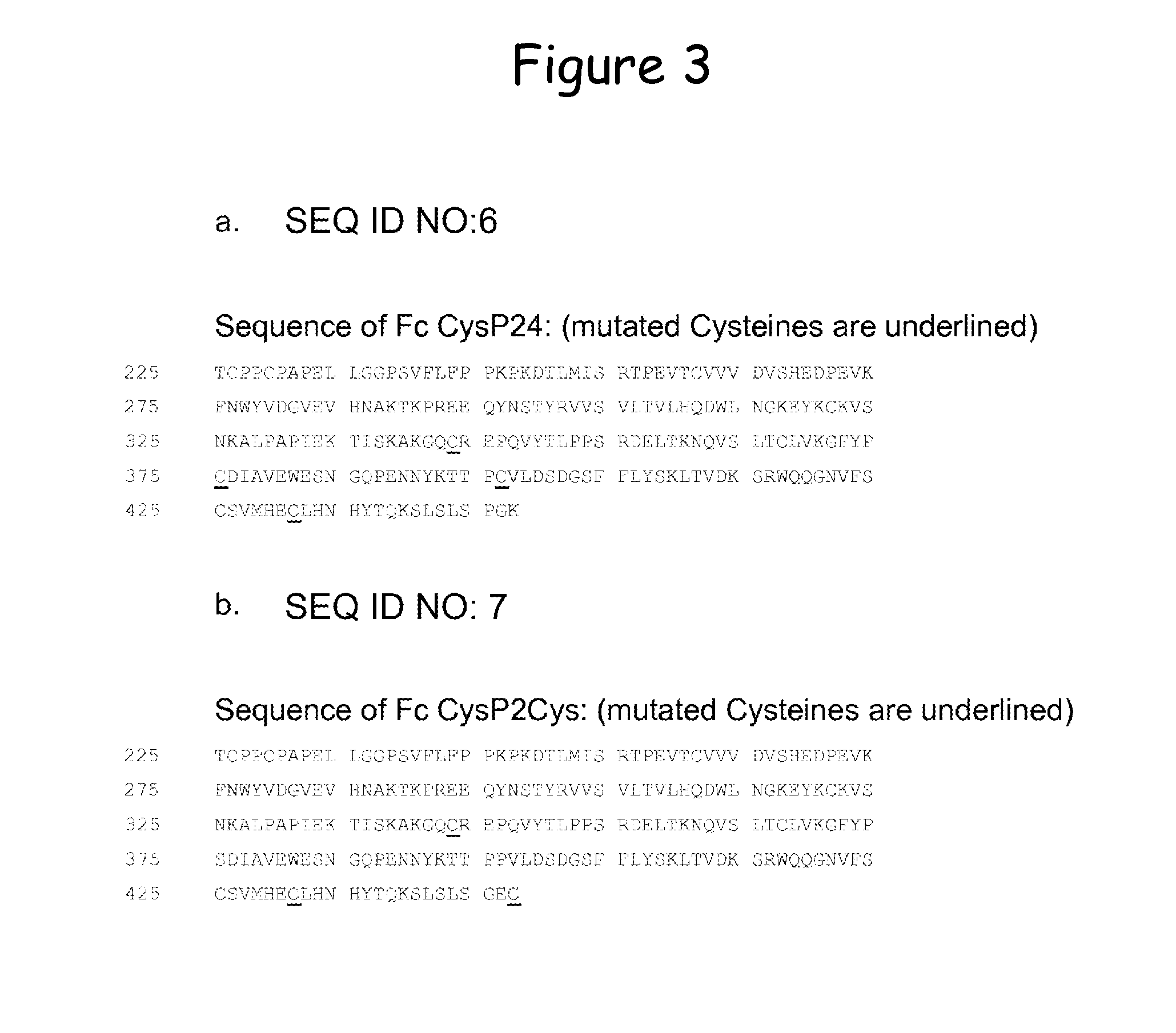
Stabilized immunoglobulin constant domains
InactiveUS20120094874A1Improve thermal stabilityImprove antigen-bindingPeptide librariesImmunoglobulinsInter-domainAntibody
The invention refers to a multidomain modular antibody comprising at least one constant antibody domain, which is mutated to form an artificial disulfide bridge by introducing at least one Cys residue into the amino acid sequence through mutagenesis of said constant domain to obtain an intra-domain or inter-domain disulfide bridge within the framework region, libraries based on such antibodies and methods of producing.
Owner:F STAR BIOTECHNOLOGISCHE FORSCHUNGS & ENTWICKLUNGS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com