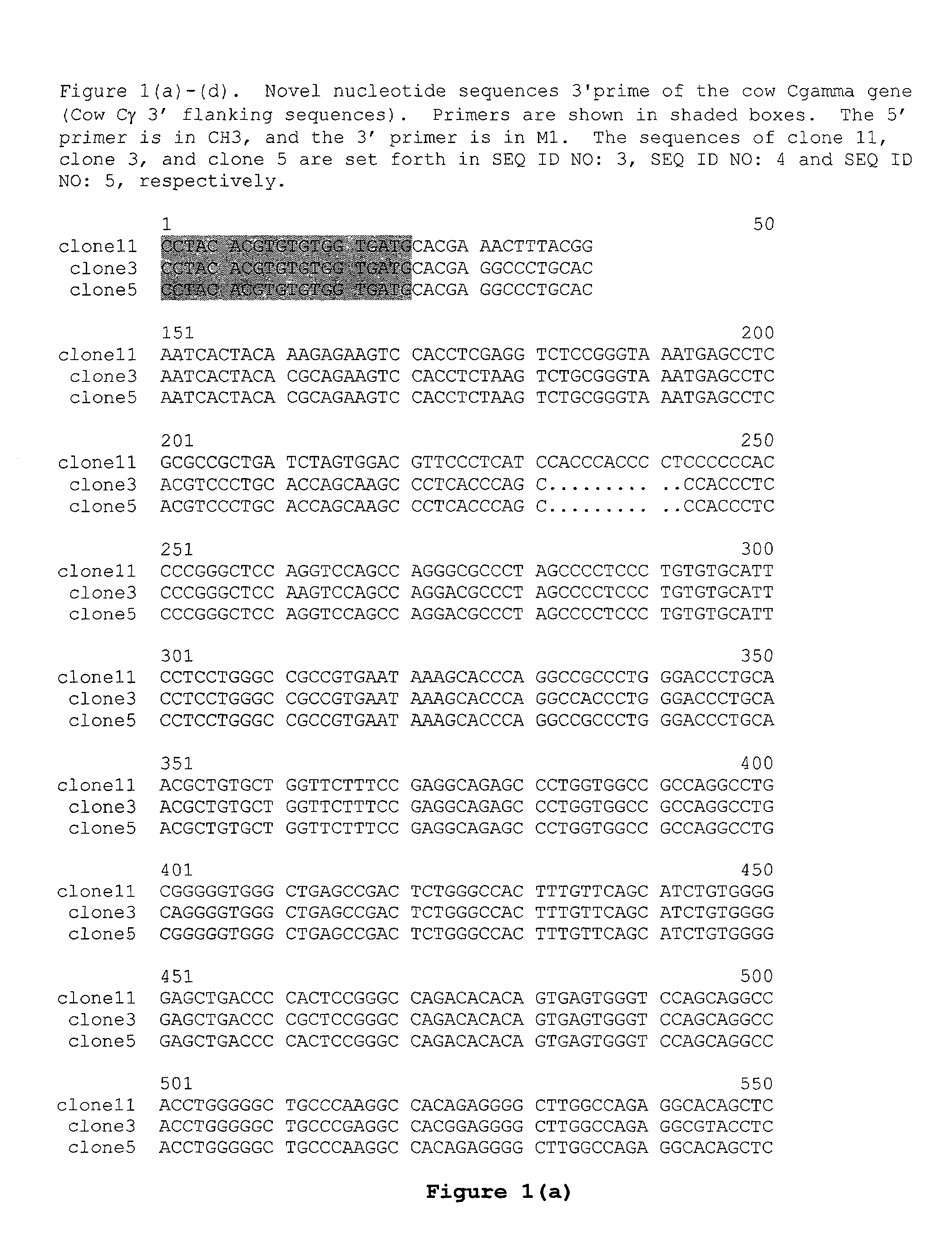
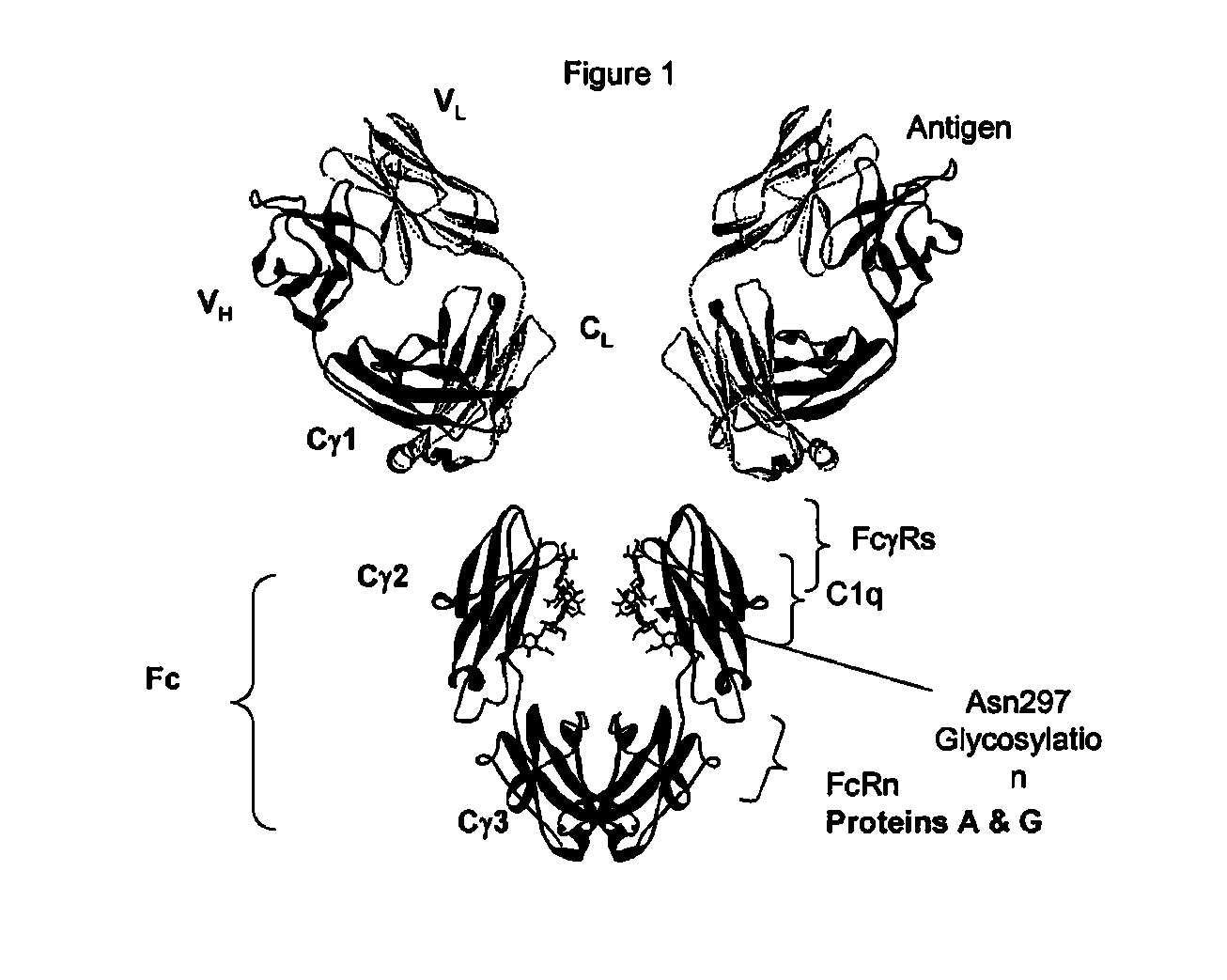
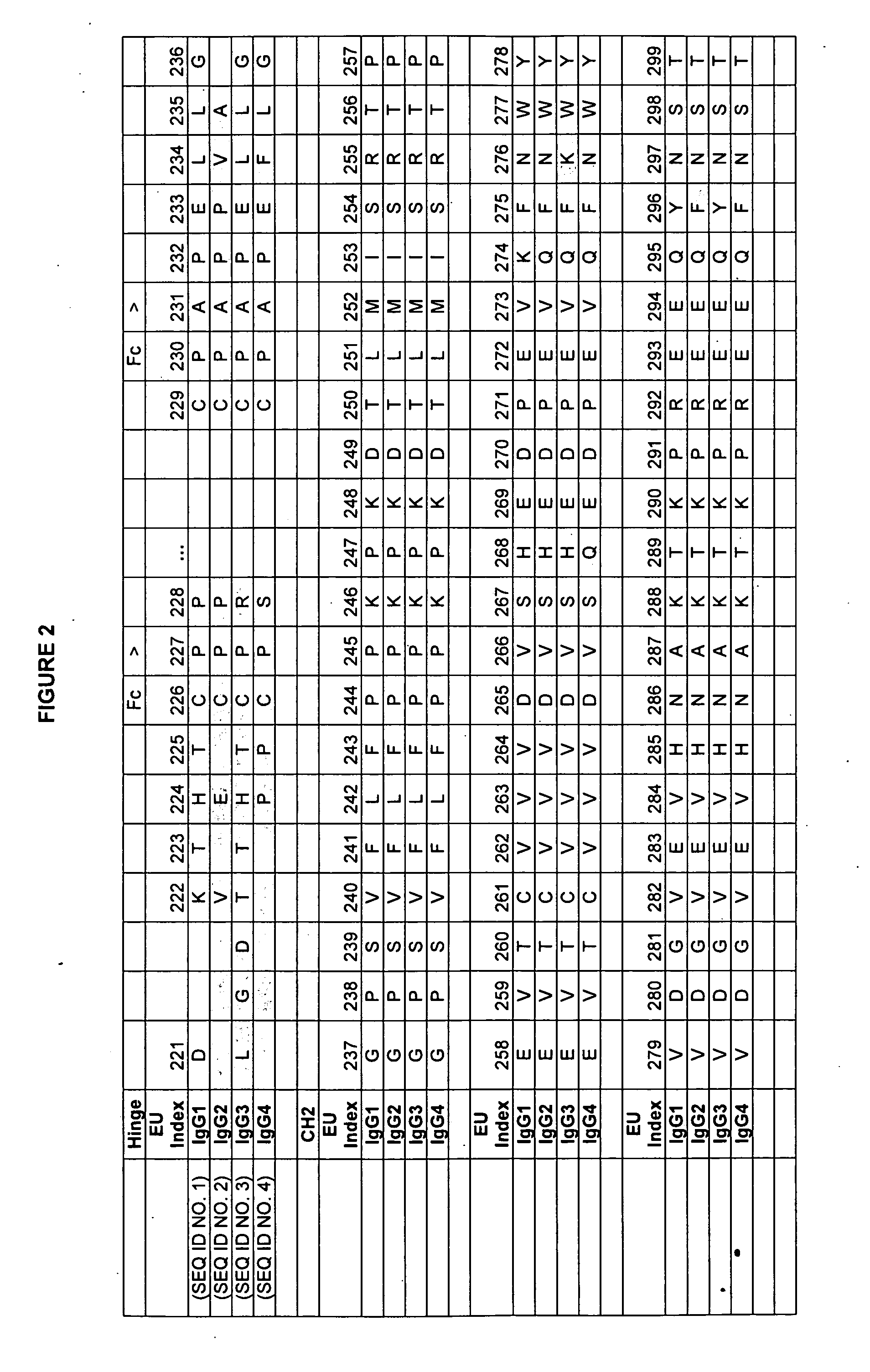
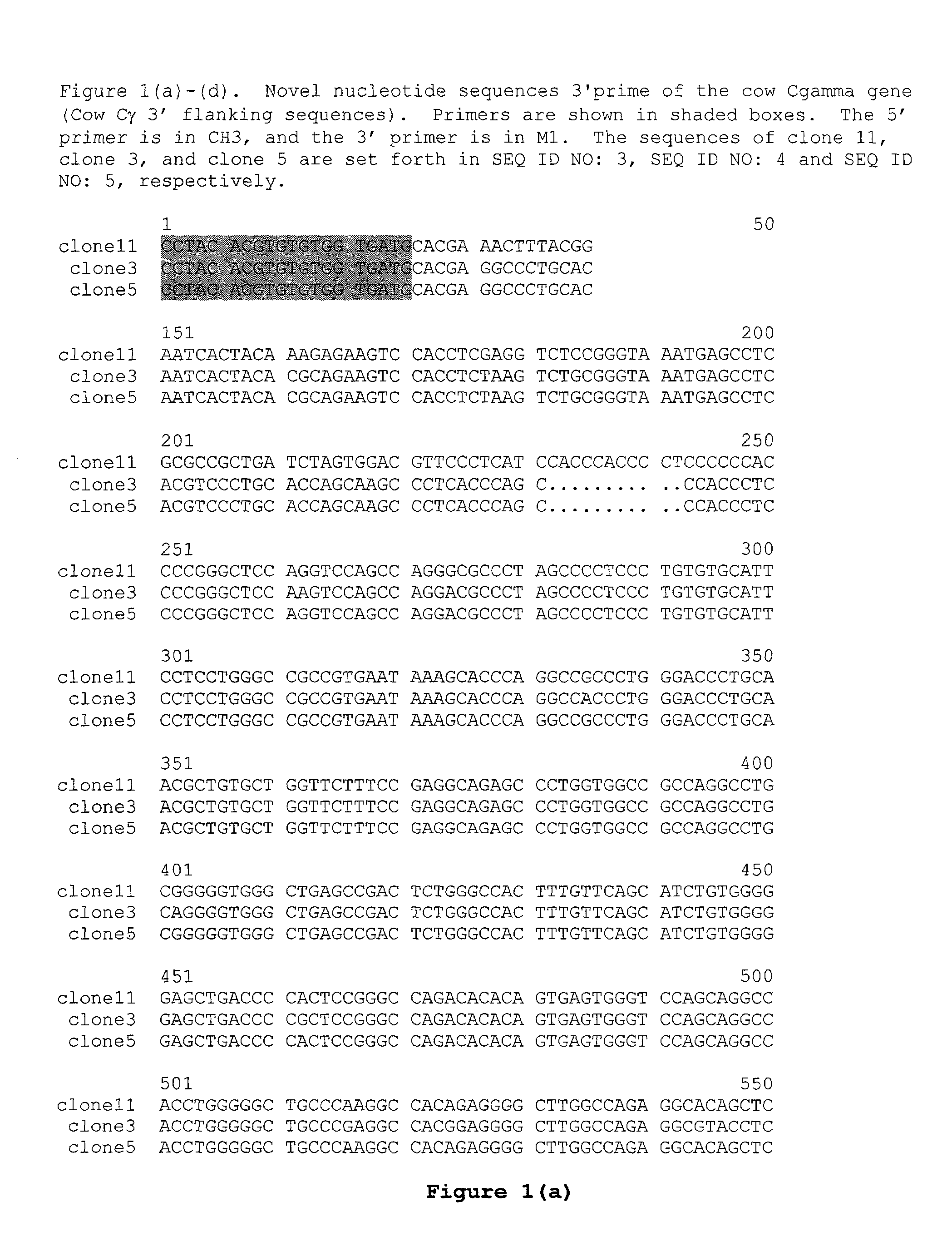
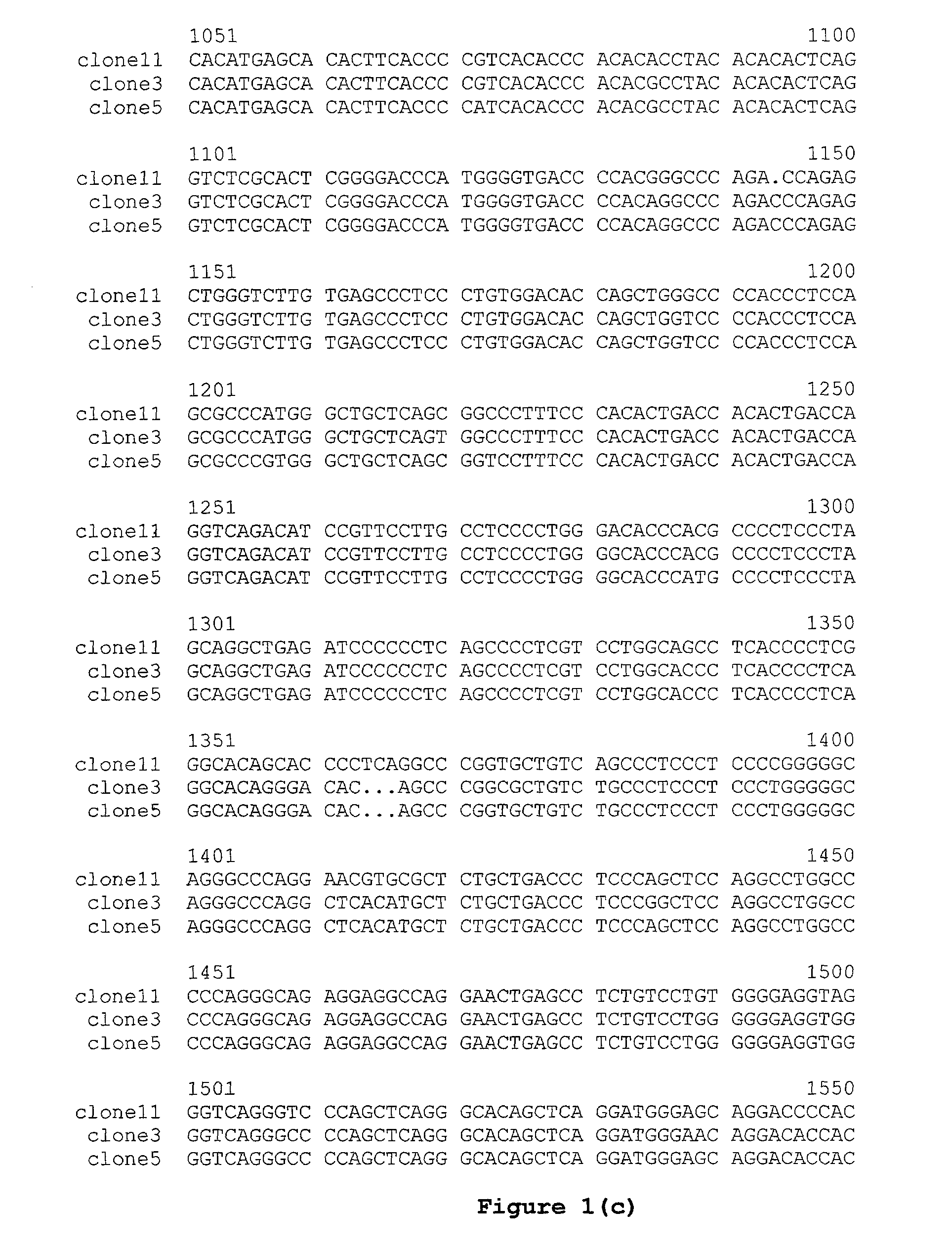
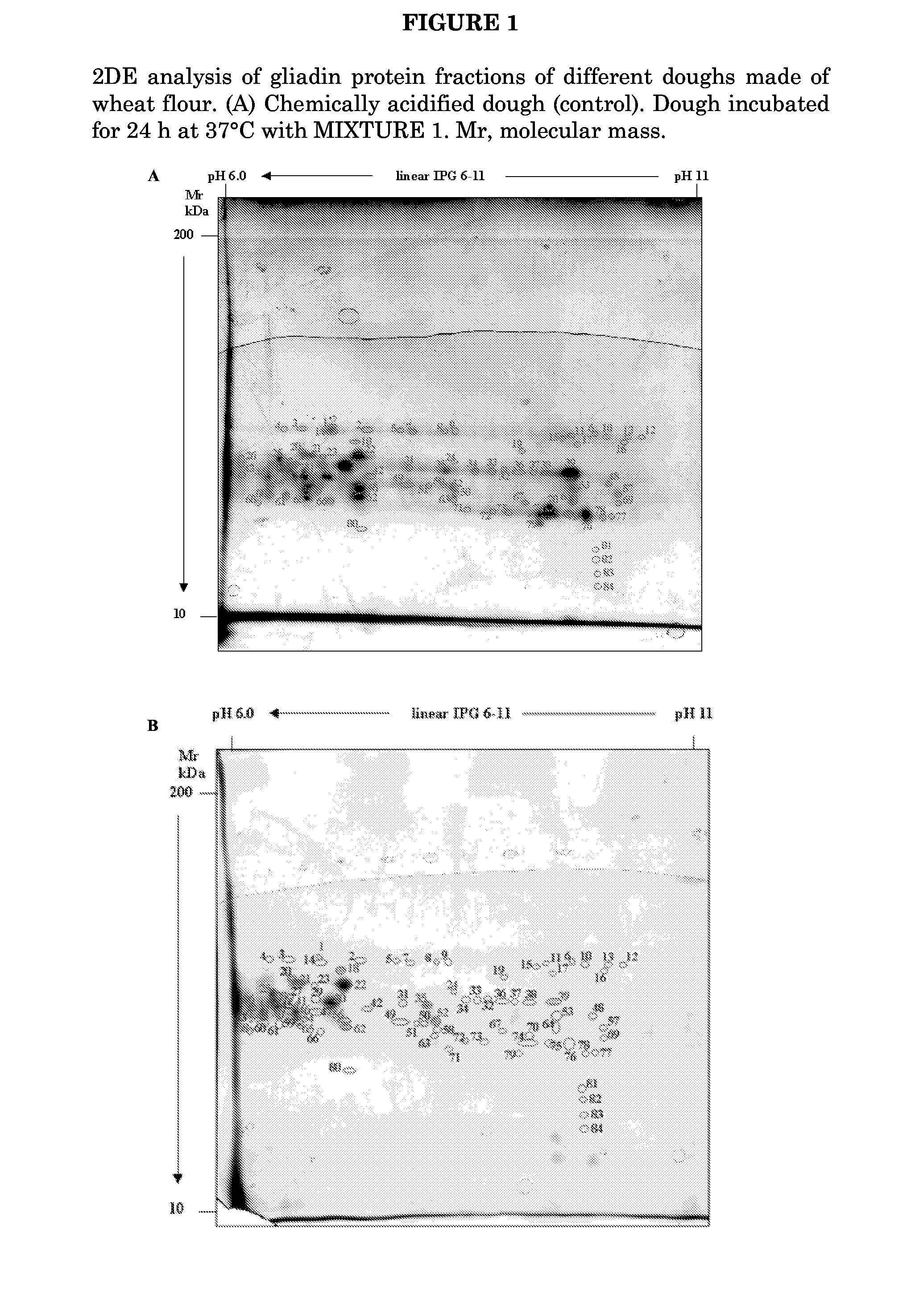
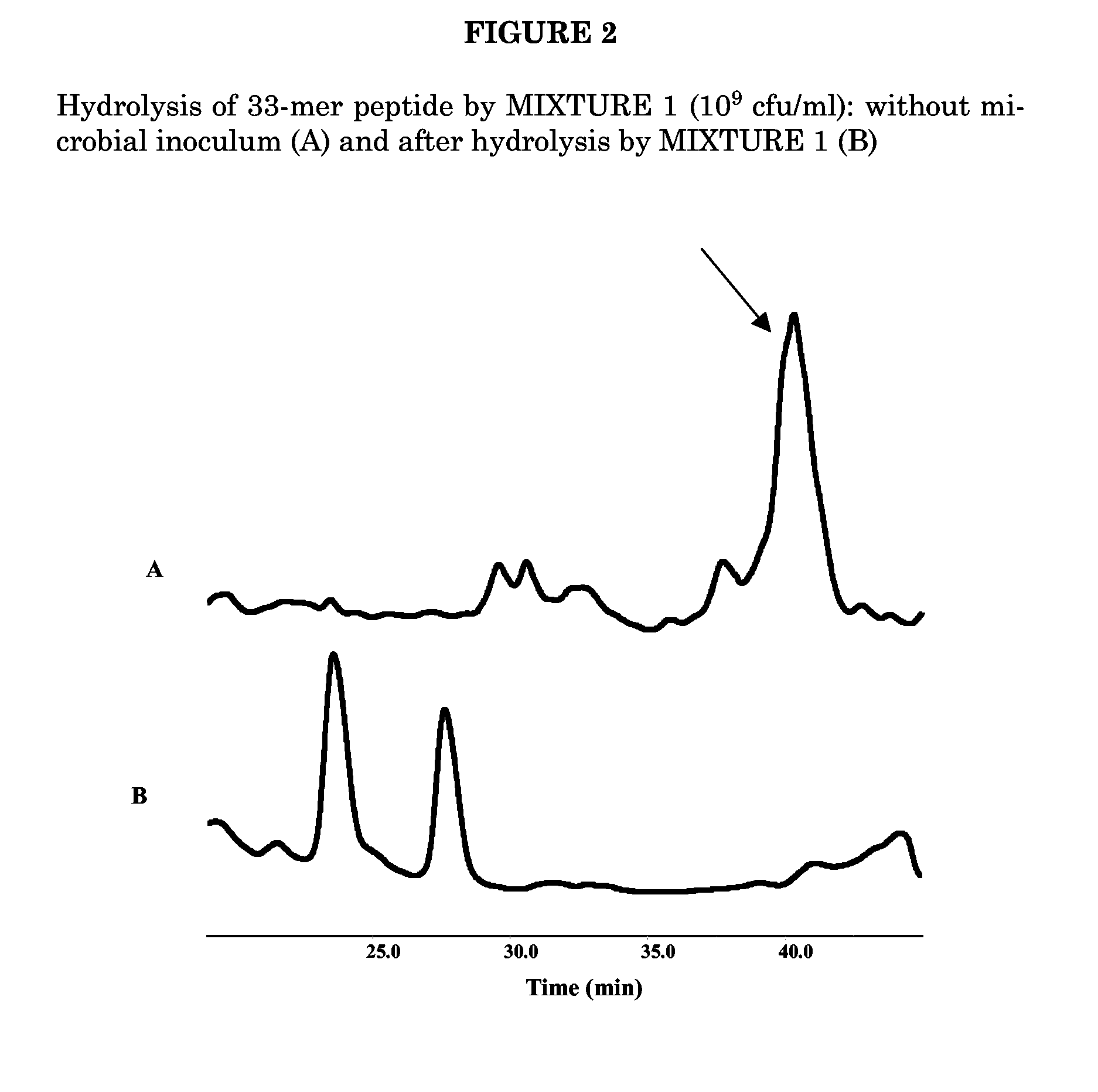
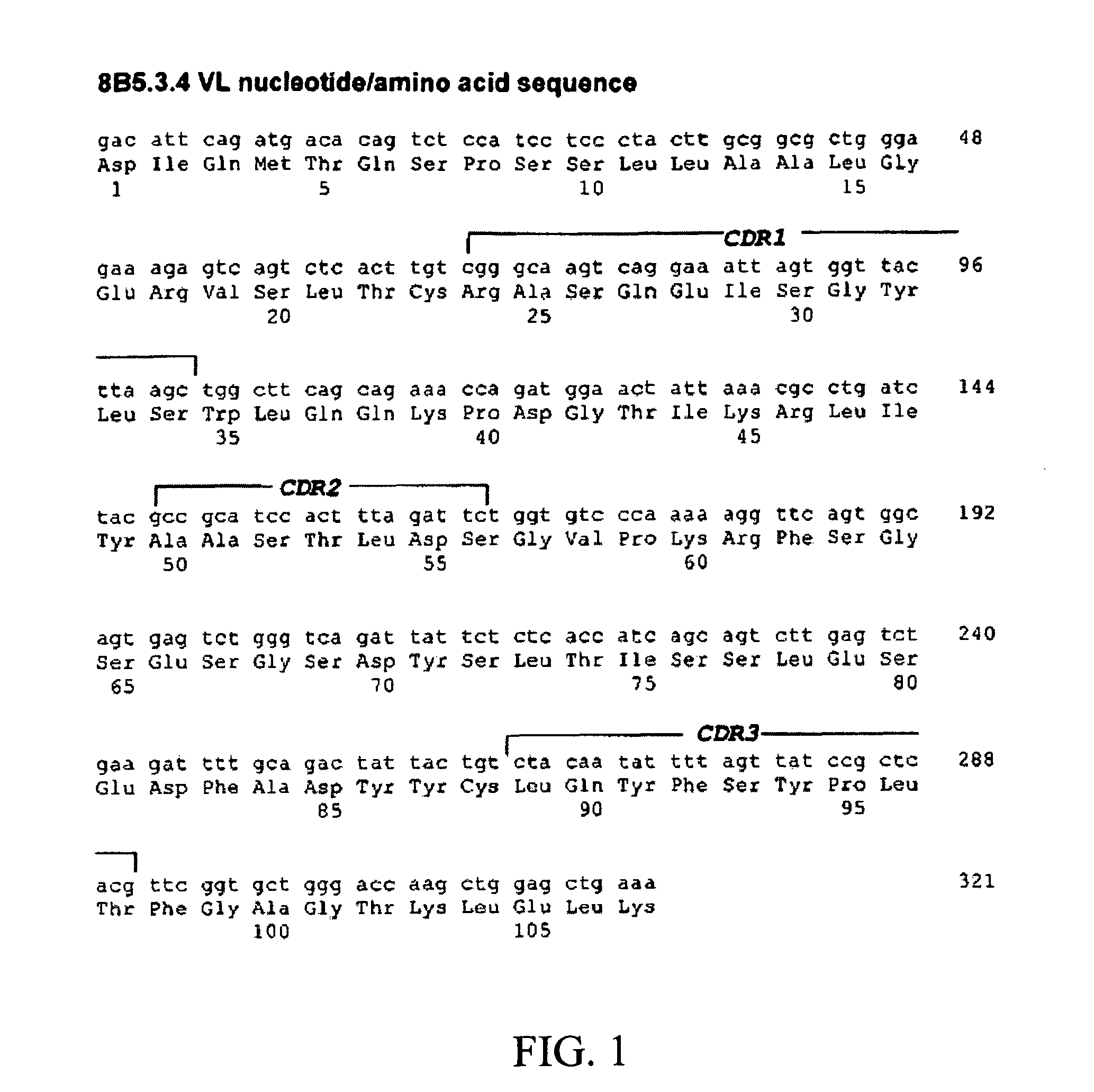
Patents
Literature
2799 results about "Globulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The globulins are a family of globular proteins that have the higher molecular weights than albumins and are insoluble in pure water but dissolve in dilute salt solutions. Some globulins are produced in the liver, while others are made by the immune system. Globulins, albumins, and fibrinogen are the major blood proteins. The normal concentration of globulins in human blood is about 2.6-3.5 g/dL.
Synthetic antibody phage libraries
InactiveUS20050079574A1High-quality target binding characteristicGenerate efficientlyAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeterologousIntravenous gammaglobulin
The invention provides immunoglobulin polypeptides comprising variant amino acids in CDRs of antibody variable domains. In one embodiment, the polypeptide is a variable domain of a monobody and has a variant CDRH3 region. These polypeptides provide a source of great sequence diversity that can be used as a source for identifying novel antigen binding polypeptides. The invention also provides these polypeptides as fusion polypeptides to heterologous polypeptides such as at least a portion of phage or viral coat proteins, tags and linkers. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
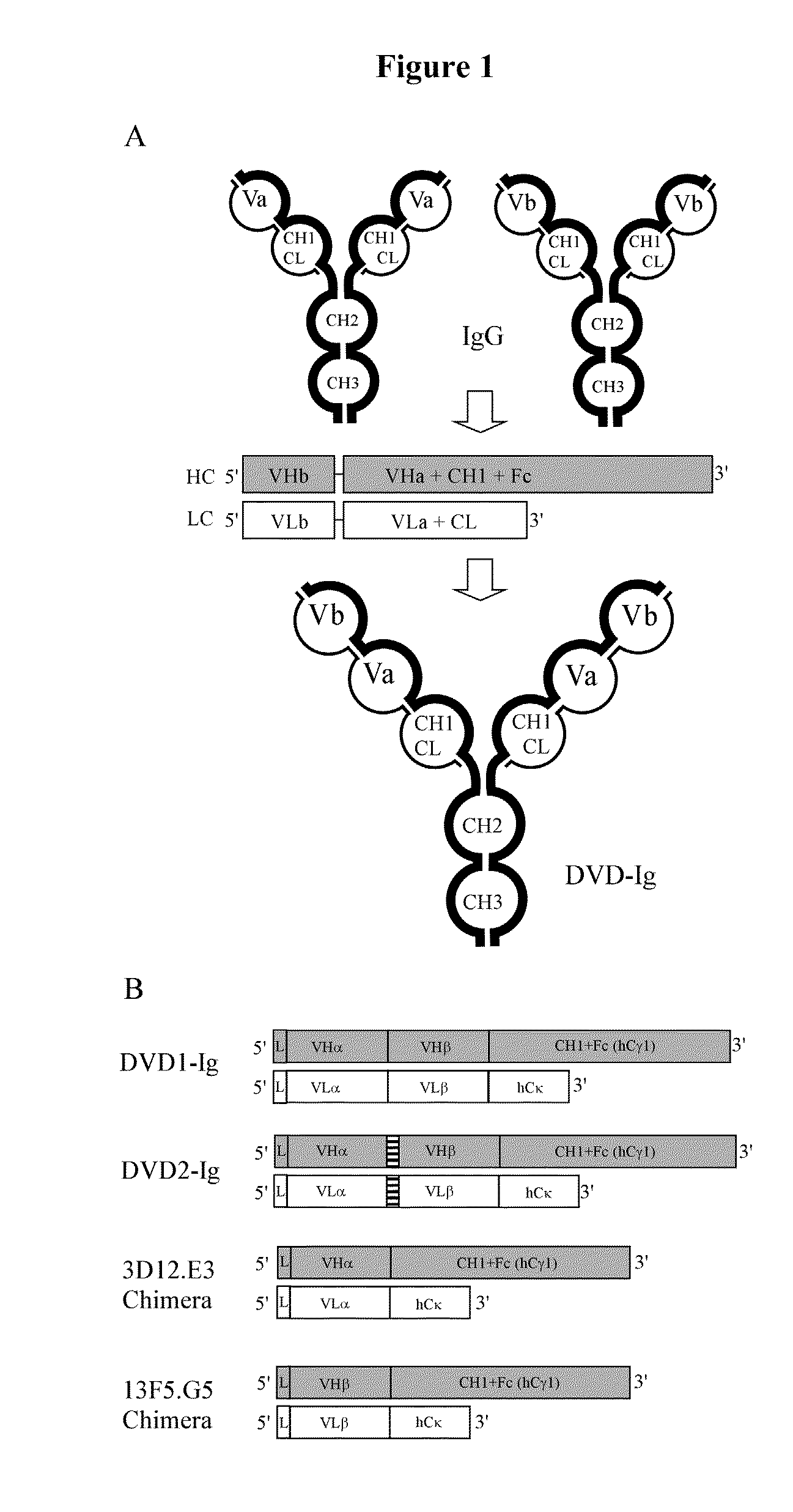
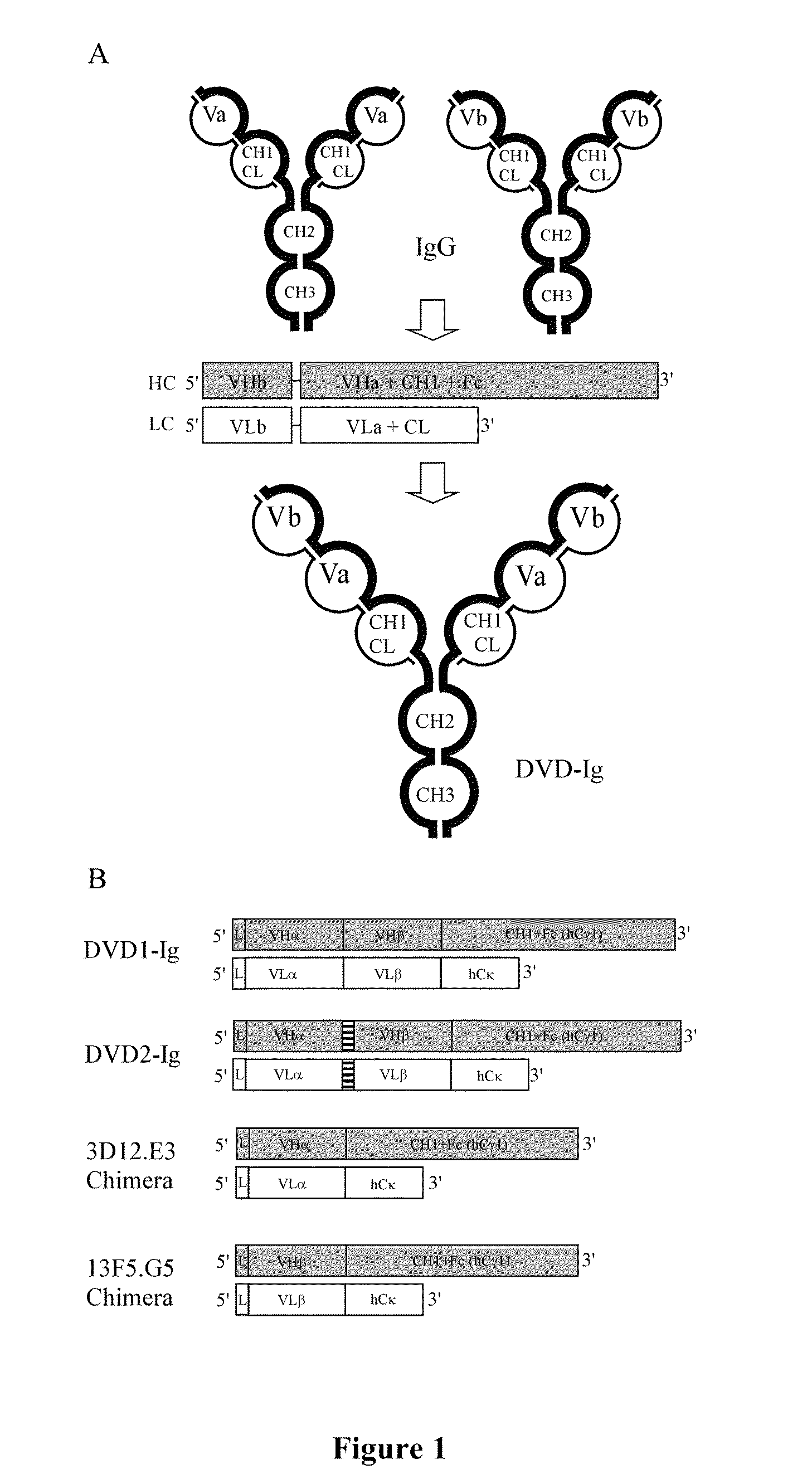
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
Readily Isolated Bispecific Antibodies with Native Immunoglobulin Format
ActiveUS20100331527A1Improve abilitiesReduces and eliminates bindingHybrid immunoglobulinsSerum immunoglobulinsImmunoglobulin heavy chainHeavy chain
A bispecific antibody format providing ease of isolation is provided, comprising immunoglobulin heavy chain variable domains that are differentially modified in the CH3 domain, wherein the differential modifications are non-immunogenic or substantially non-immunogenic with respect to the CH3 modifications, and at least one of the modifications results in a differential affinity for the bispecific antibody for an affinity reagent such as Protein A, and the bispecific antibody is isolable from a disrupted cell, from medium, or from a mixture of antibodies based on its affinity for Protein A.
Owner:REGENERON PHARM INC
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20030190311A1High affinityExtended half-lifeCompounds screening/testingFungiIntravenous gammaglobulinIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
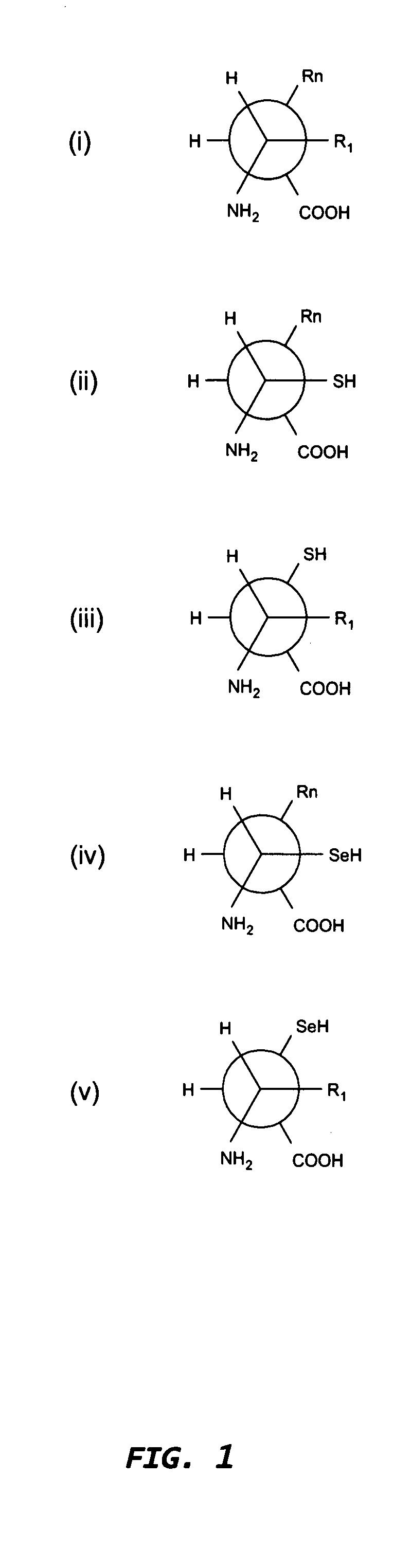
Increasing antibody affinity by altering glycosylation of immunoglobulin variable region
InactiveUS6933368B2Enhanced antigen binding propertyAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody AffinitiesImmunoglobulin Variable Region
The present invention provides methods for producing mutationally-altered immunoglobulins and compositions containing such mutationally-altered immunoglobulins, wherein the mutationally-altered immunoglobulins have at least one mutation that alters the pattern of glycosylation in a variable region and thereby modifies the affinity of the immunoglobulin for a preselected antigen. The methods and compositions of the invention provide immunoglobulins that possess increased affinity for antigen. Such glycosylation-altered immunoglobulins are suitable for diagnostic and therapeutic applications.
Owner:FACET BIOTECH CORP
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
Binding domain-immunoglobulin fusion proteins
InactiveUS20050175614A1Reduced ability to dimerizeHybrid immunoglobulinsAntipyreticCrystallographyAntigen
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
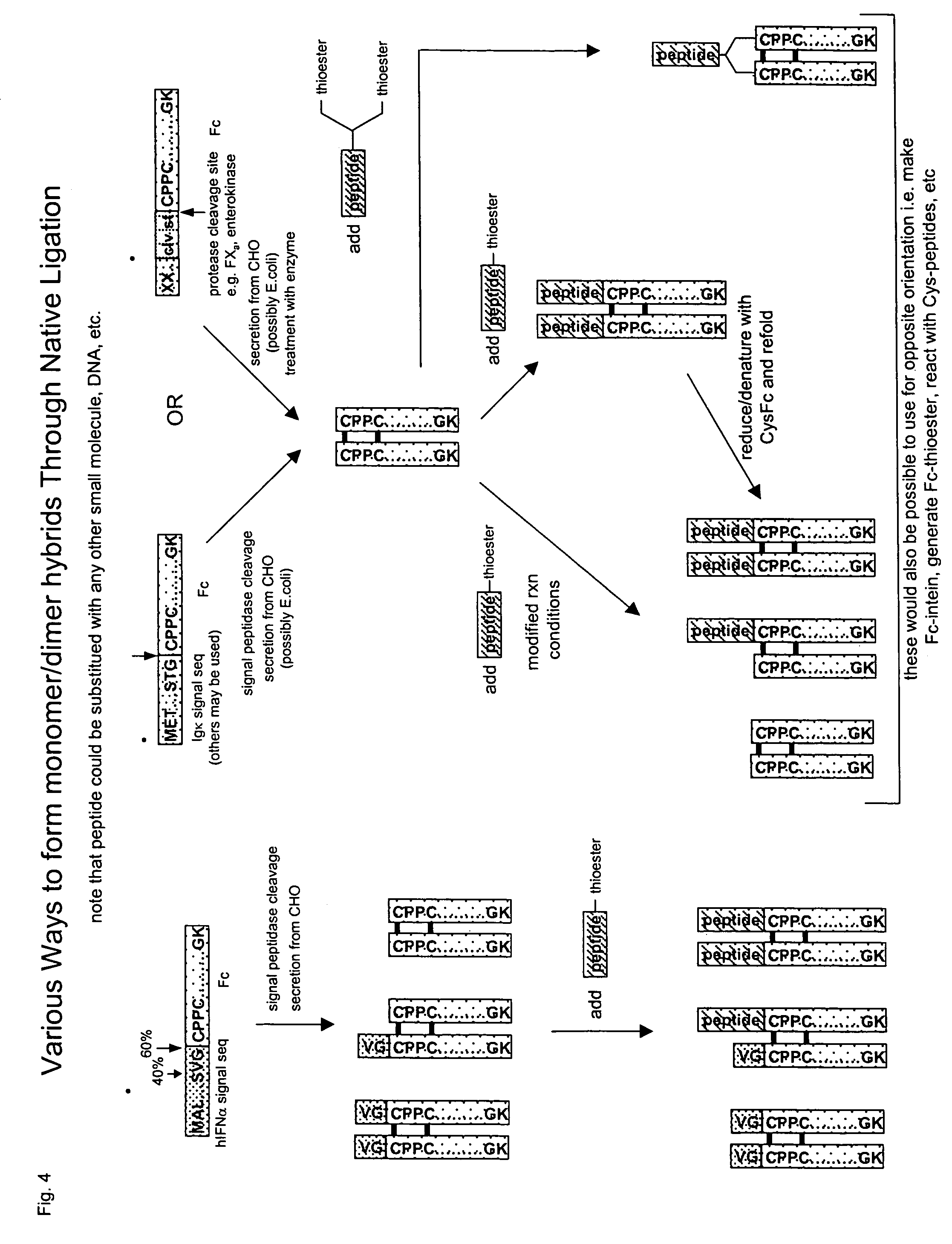
Methods for chemically synthesizing immunoglobulin chimeric proteins
ActiveUS7381408B2Prolong lifeEasy to purifyHydrolasesPeptide/protein ingredientsChimera ProteinBiochemistry
The invention provides methods of chemically synthesizing chimeric proteins comprising at least a portion of an immunoglobulin constant region and a biologically active molecule.
Owner:BIOVERATIV THERAPEUTICS INC
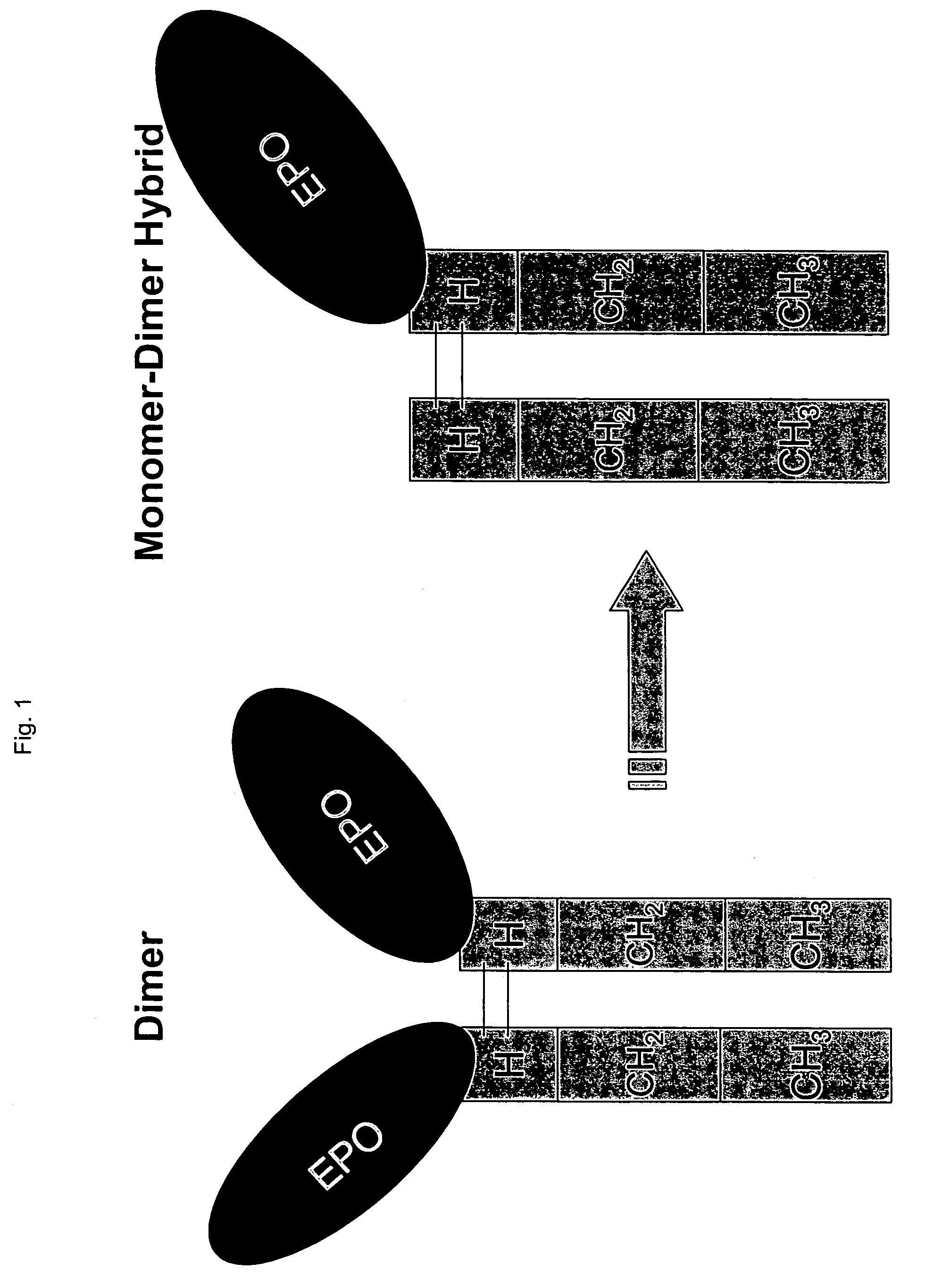
Hybrid immunoglobulins with moving parts
Hybrid immunoglobulins containing moving parts are provided as well as related compositions and methods of use and methods of production. In addition, analogous genetic devices are provided as well as related compositions and methods of use and methods of production.
Owner:BIOMOLECULAR HLDG LLC
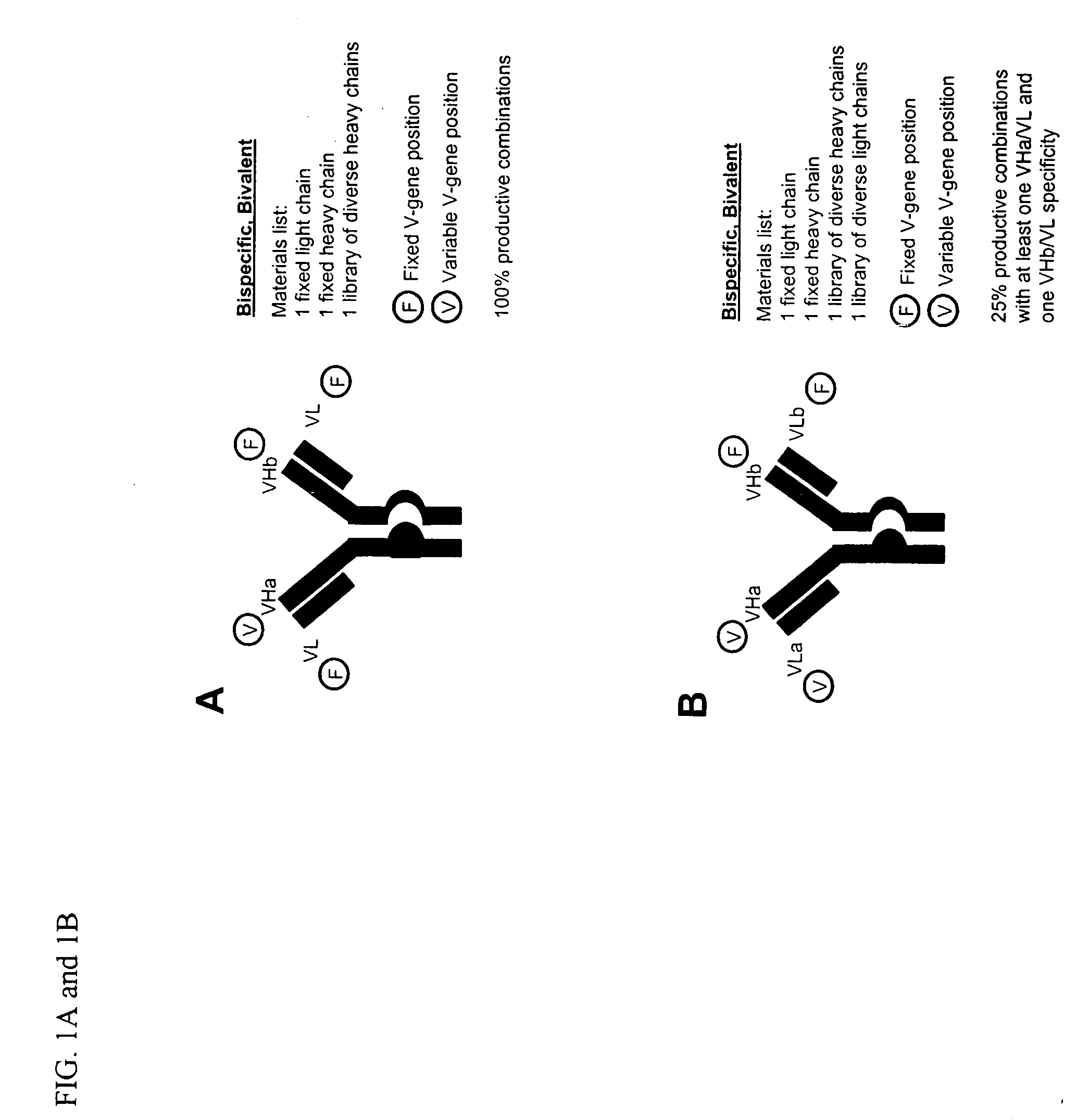
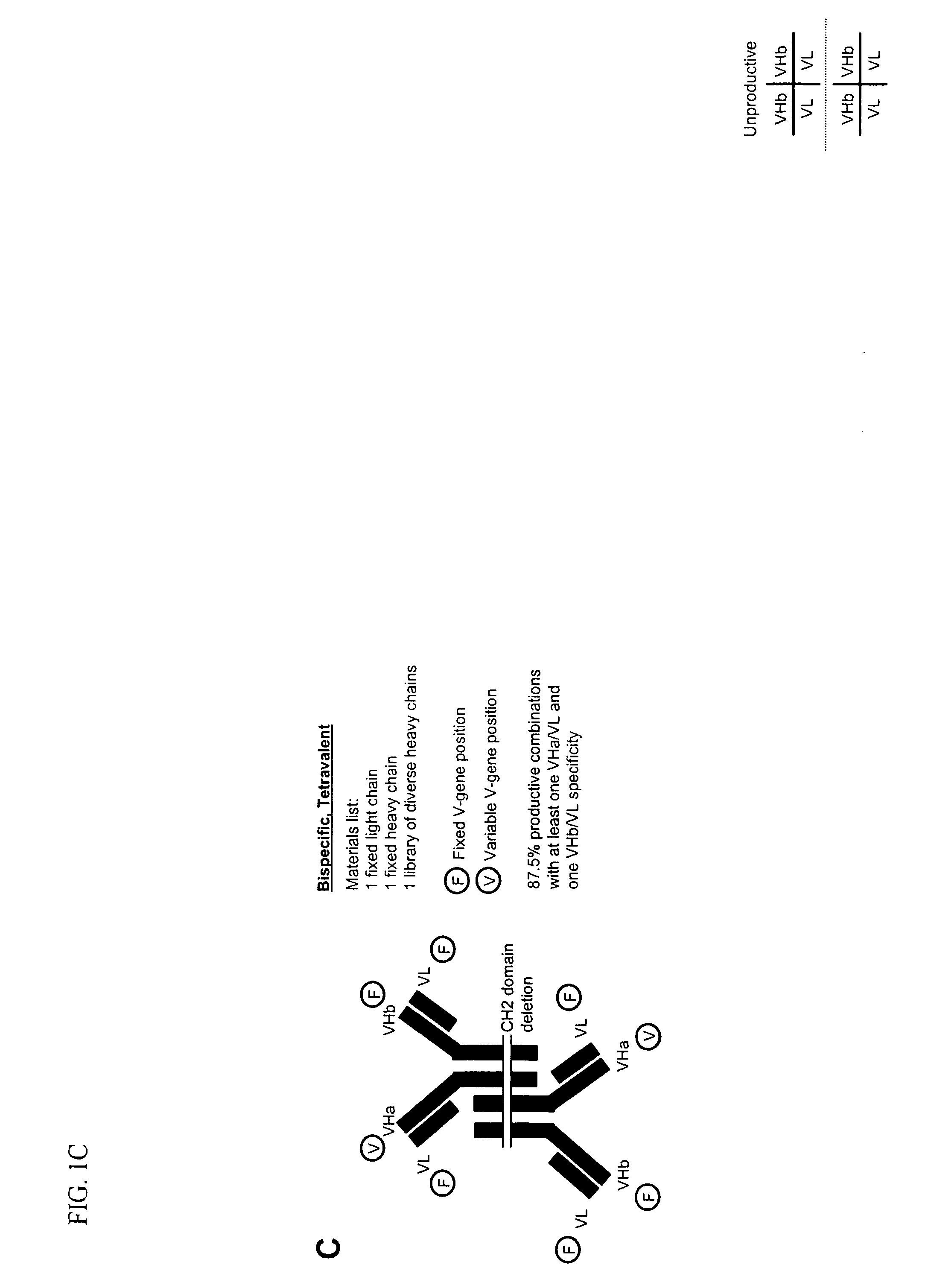
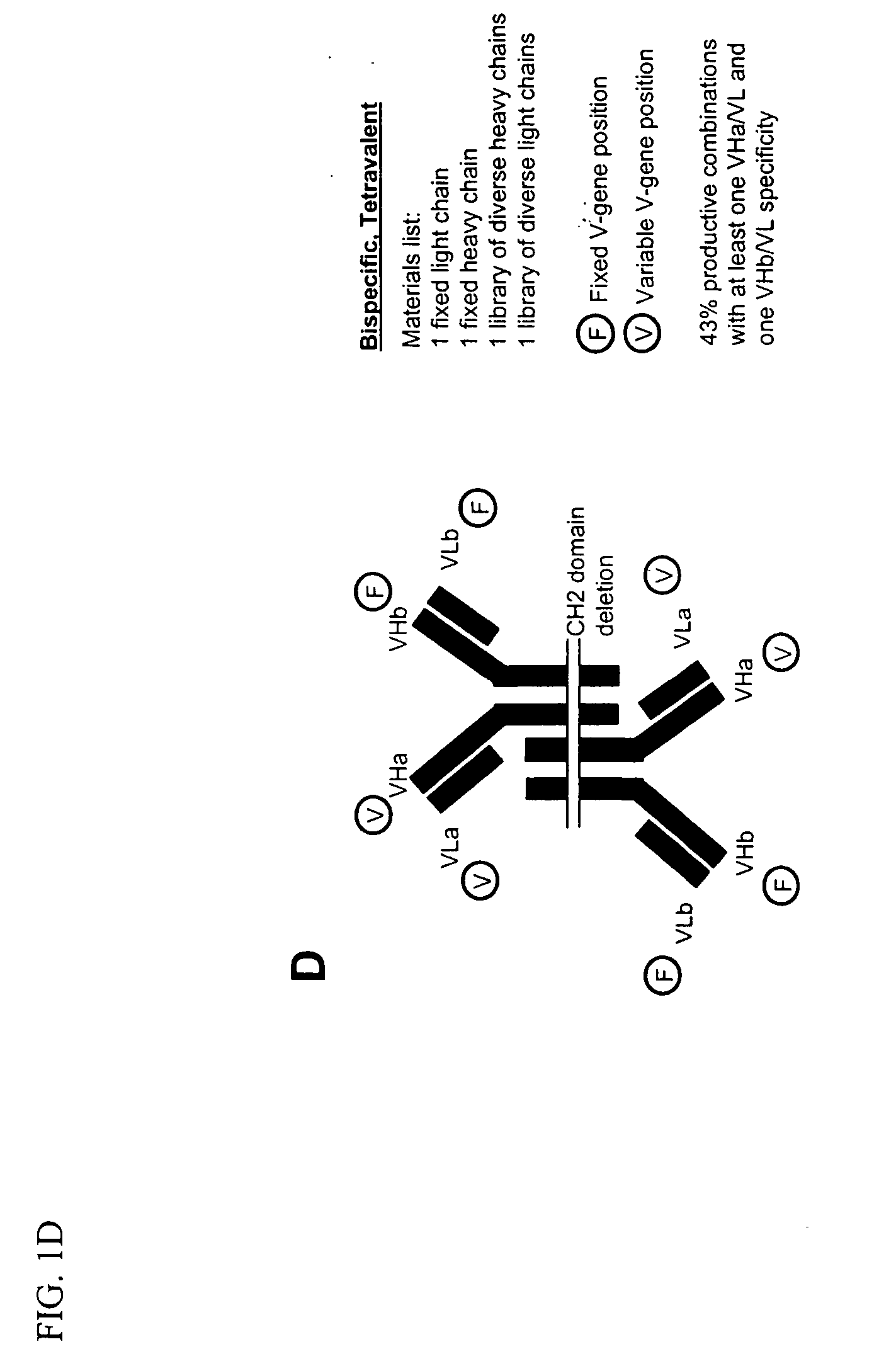
Methods for producing and identifying multispecific antibodies
The present invention relates to a high efficiency method of expressing multispecific antibodies in eukaryotic cells. The invention is further drawn to a method of producing immunoglobulin heavy and light chain libraries, particularly using the trimolecular recombination method, for expression in eukaryotic cells. The invention further provides methods of selecting and screening for multispecific antibodies, and antigen-binding fragments thereof. The invention also provides kits for producing, screening and selecting multispecific antibodies. Finally, the invention provides multispecific antibodies, and antigen-binding fragments thereof, produced by the methods provided herein.
Owner:VACCINEX
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Method to screen phage display libraries with different ligands
InactiveUS20040038291A2Immunoglobulin superfamilyLibrary screeningPolyphageImmunoglobulin superfamily
Abstract of the Disclosure The present invention relates to methods for selecting repertoires of polypeptides using generic and target ligands. In particular, the invention relates to a library comprising a repertoire of polypeptides of the immunoglobulin superfamily, wherein the members of the repertoire have a known main chain conformation.
Owner:DORMANTIS LTD
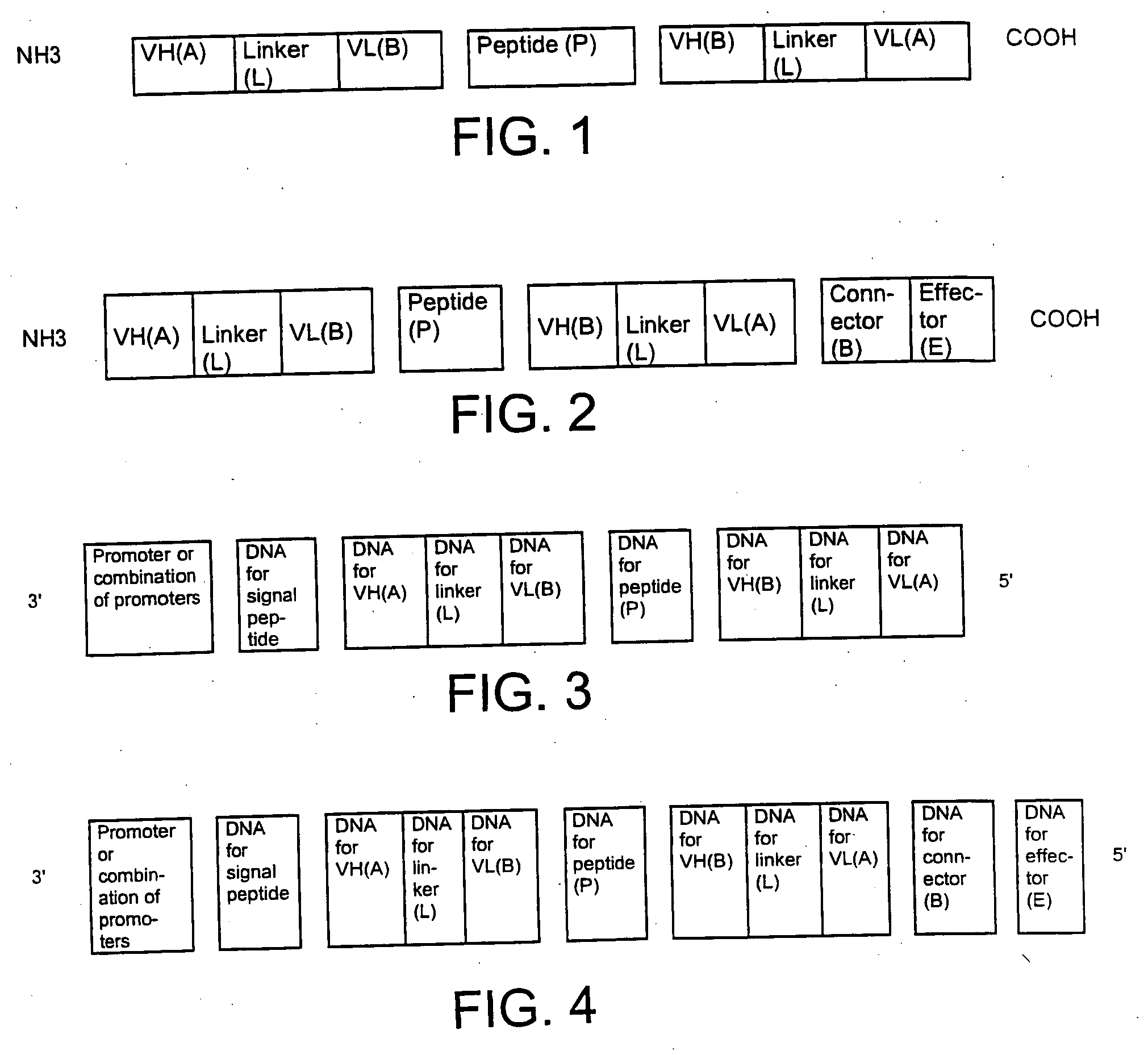
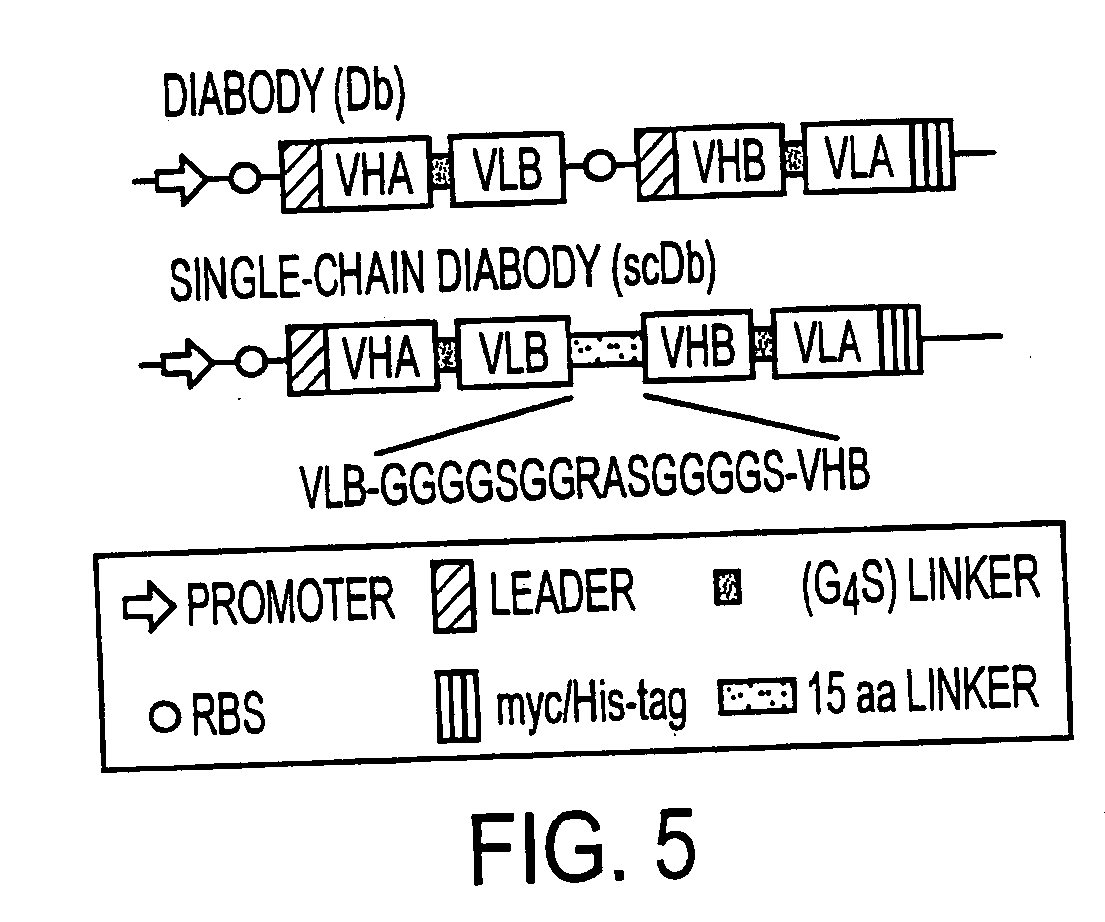
Single-chain multiple antigen-binding molecule, its preparation and use
InactiveUS20050004352A1Reduce dissociationLess complexOrganic active ingredientsFungiAntigen bindingVariable domain
The present invention relates to a single-chain, multiple antigen-binding molecule with diverse variable domains of a heavy and of a light chain of an immunoglobulin, which are connected in the form of a VH-VL construct, which are in turn connected together via a peptide, and to the preparation and use thereof as pharmaceutical or diagnostic aid.
Owner:AFFITECH RESEARCH AS
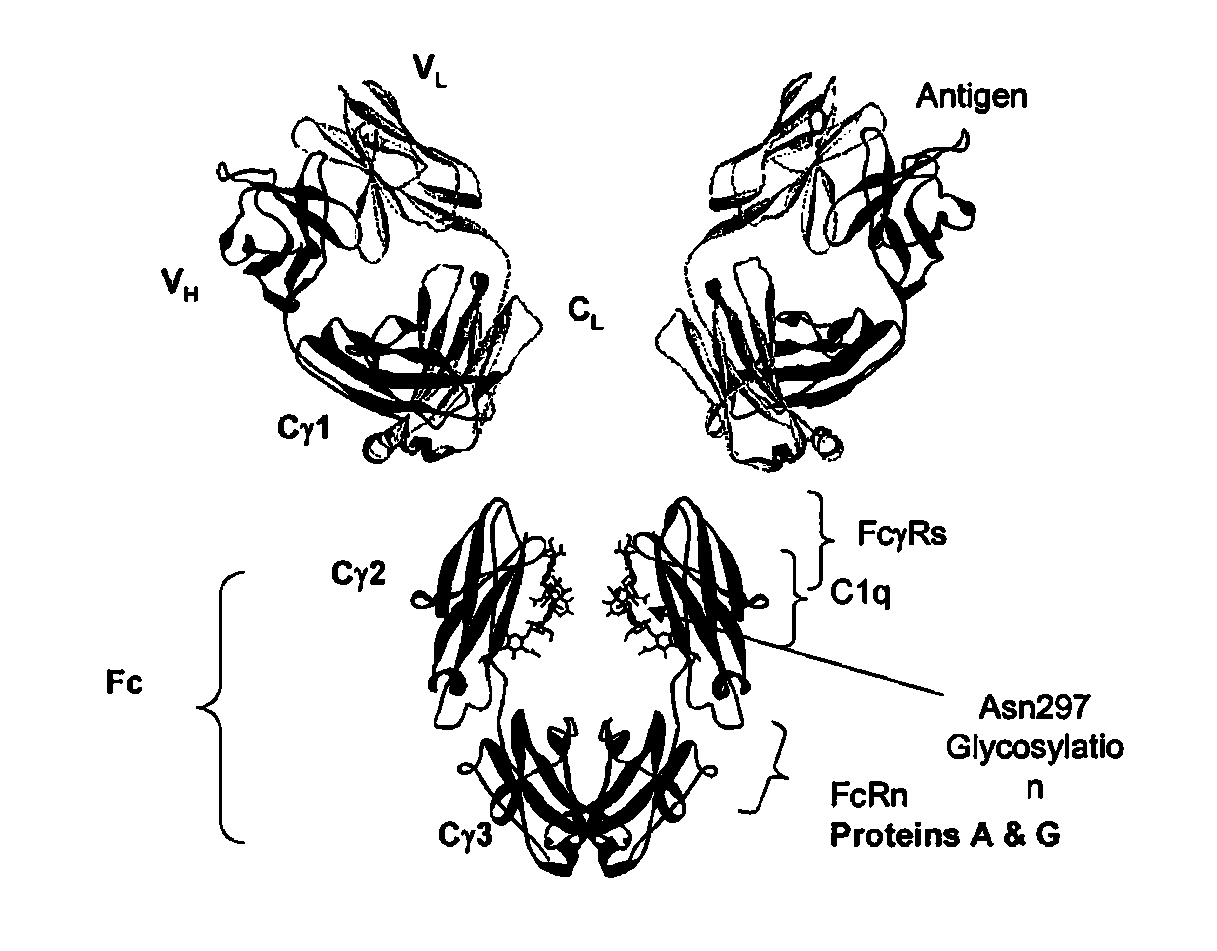
Fc VARIANTS WITH ALTERED BINDING TO FcRn
ActiveUS20090041770A1Extended half-lifeImmunoglobulins against growth factorsAntibody ingredientsTherapeutic intentCancer research
The present application relates to optimized IgG immunoglobulin variants, engineering methods for their generation, and their application, particularly for therapeutic purposes.
Owner:XENCOR
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Heterodimer Binding Proteins and Uses Thereof
InactiveUS20130129723A1Animal cellsFused cellsImmunoglobulin Joining RegionImmunoglobulin light chain
The present disclosure provides polypeptide heterodimers formed between two different single chain fusion polypeptides via natural heterodimerization of an immunoglobulin CH1 region and an immunoglobulin light chain constant region (CL). The polypeptide heterodimer comprises two or more binding domains that specifically bind one or more targets (e.g., a receptor). In addition, both chains of the heterodimer further comprise an Fc region portion. The present disclosure also provides nucleic acids, vectors, host cells and methods for making polypeptide heterodimers as well as methods for using such polypeptide heterodimers, such as in directing T cell activation, inhibiting solid malignancy growth, and treating autoimmune or inflammatory conditions.
Owner:EMERGENT PRODUCTS DEVELOPMENT SEATTLE LLC
Isolation of proteins
InactiveUS20050176122A1Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
Owner:UPFRONT CHROMATOGRAPHY
Transgenic animals expressing chimeric antibodies for use in preparing human antibodies
ActiveUS7910798B2Improved trafficking developmentStrengthen associationSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsTransgenesisIn vivo
The invention provides transgene constructs for expressing chimeric antibodies, and transgenic non-human host animals carrying such constructs, wherein the chimeric antibodies comprise human variable regions and constant regions of the non-human transgenic host animal. The presence of immunoglobulin constant regions of the host animal allows for generation of improved antibodies in such transgenic host animals. Subsequently, the chimeric antibodies can be readily converted to fully human antibodies using recombinant DNA techniques. Thus, the invention provides compositions and methods for generating human antibodies in which chimeric antibodies raised in vivo in transgenic mice are used as intermediates and then converted to fully human antibodies in vitro.
Owner:ER SQUIBB & SONS INC
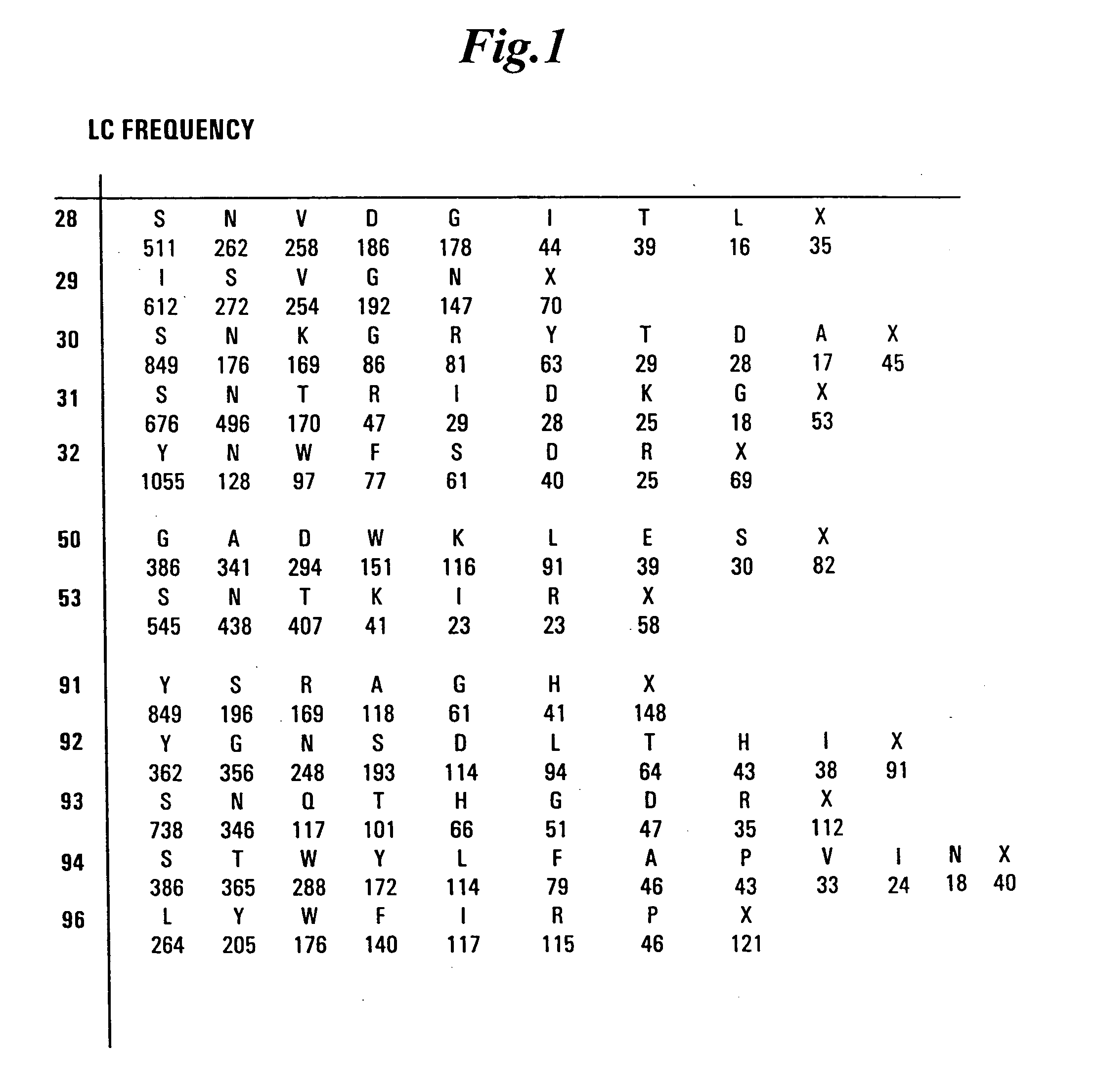
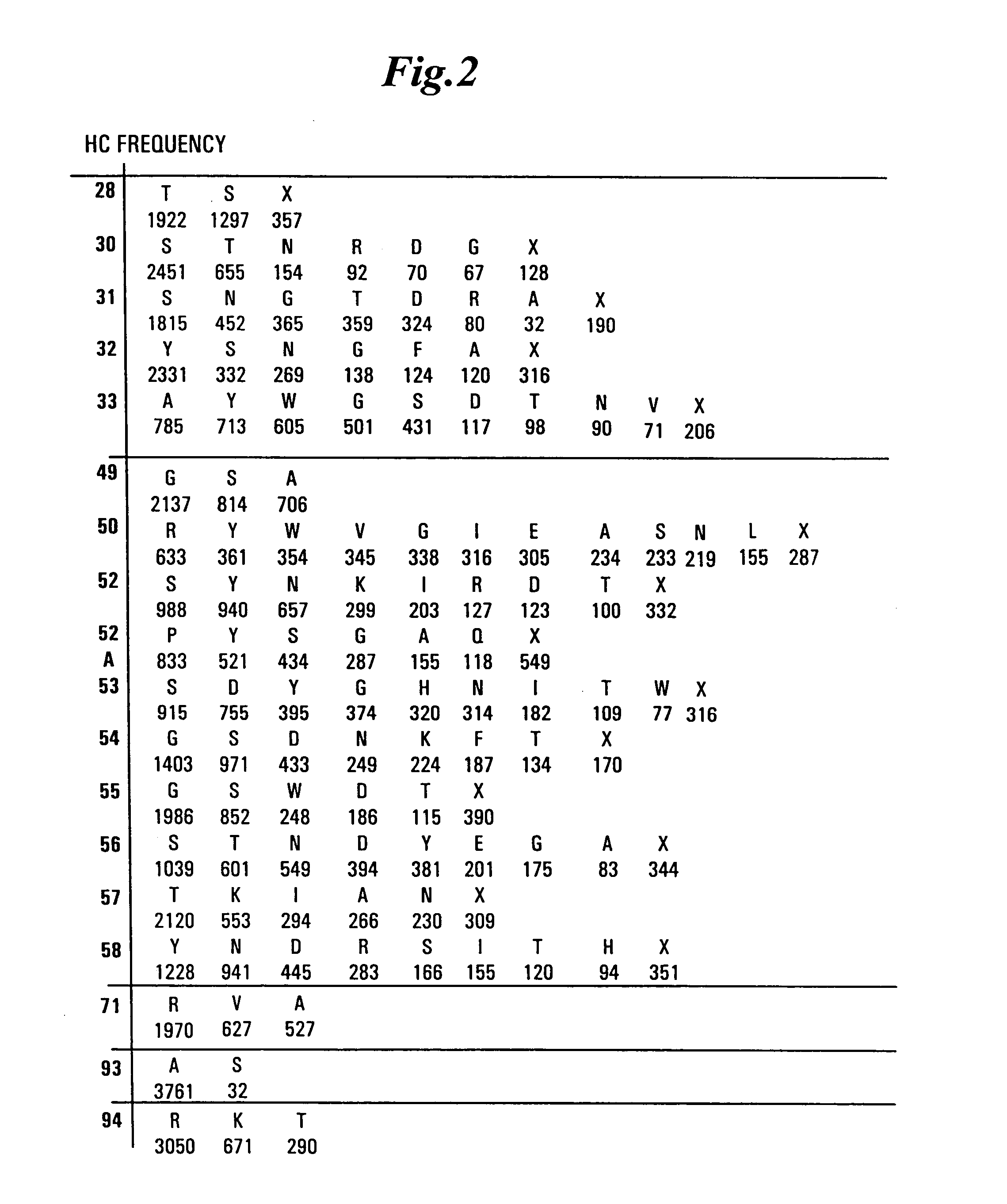
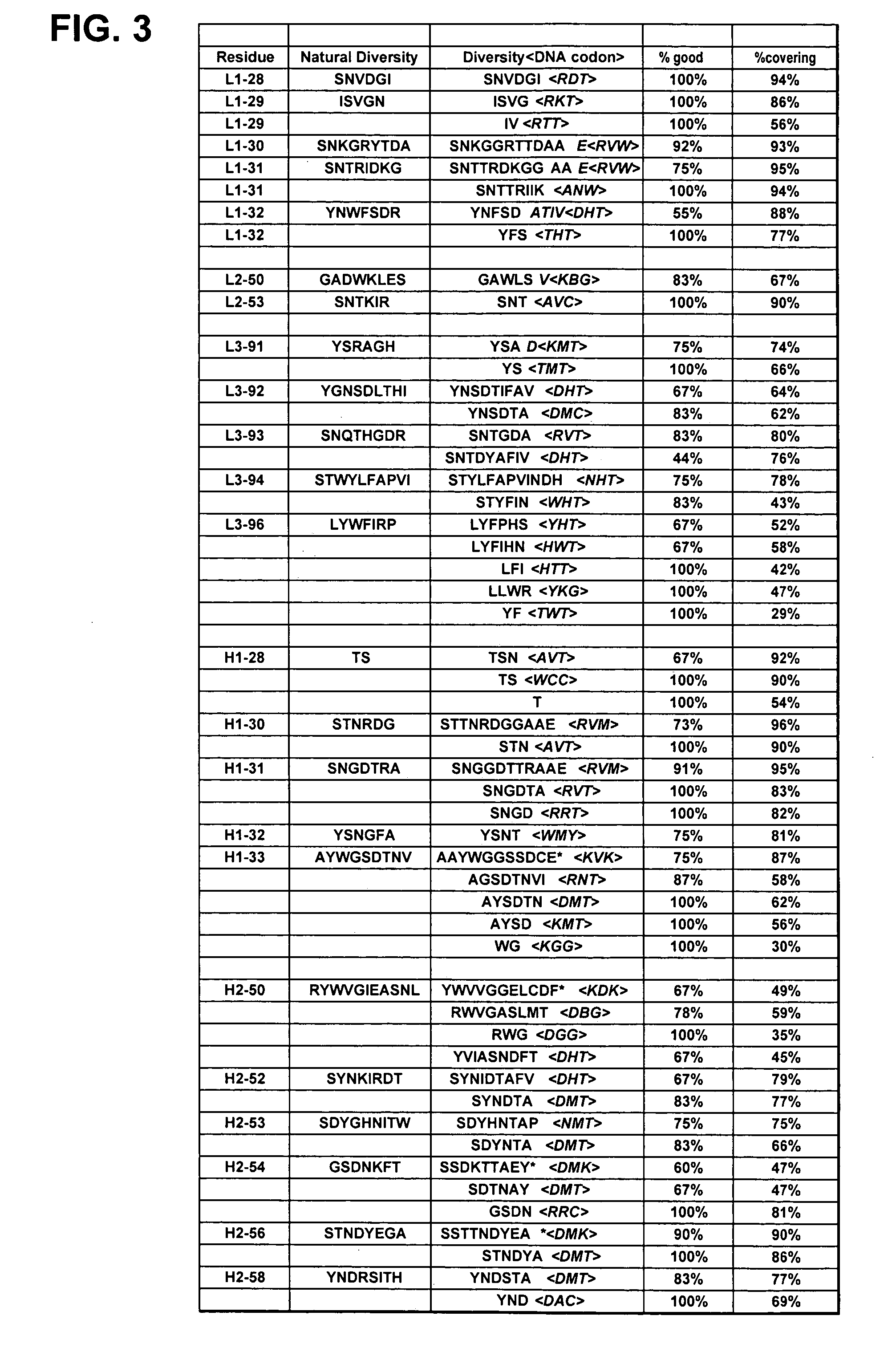
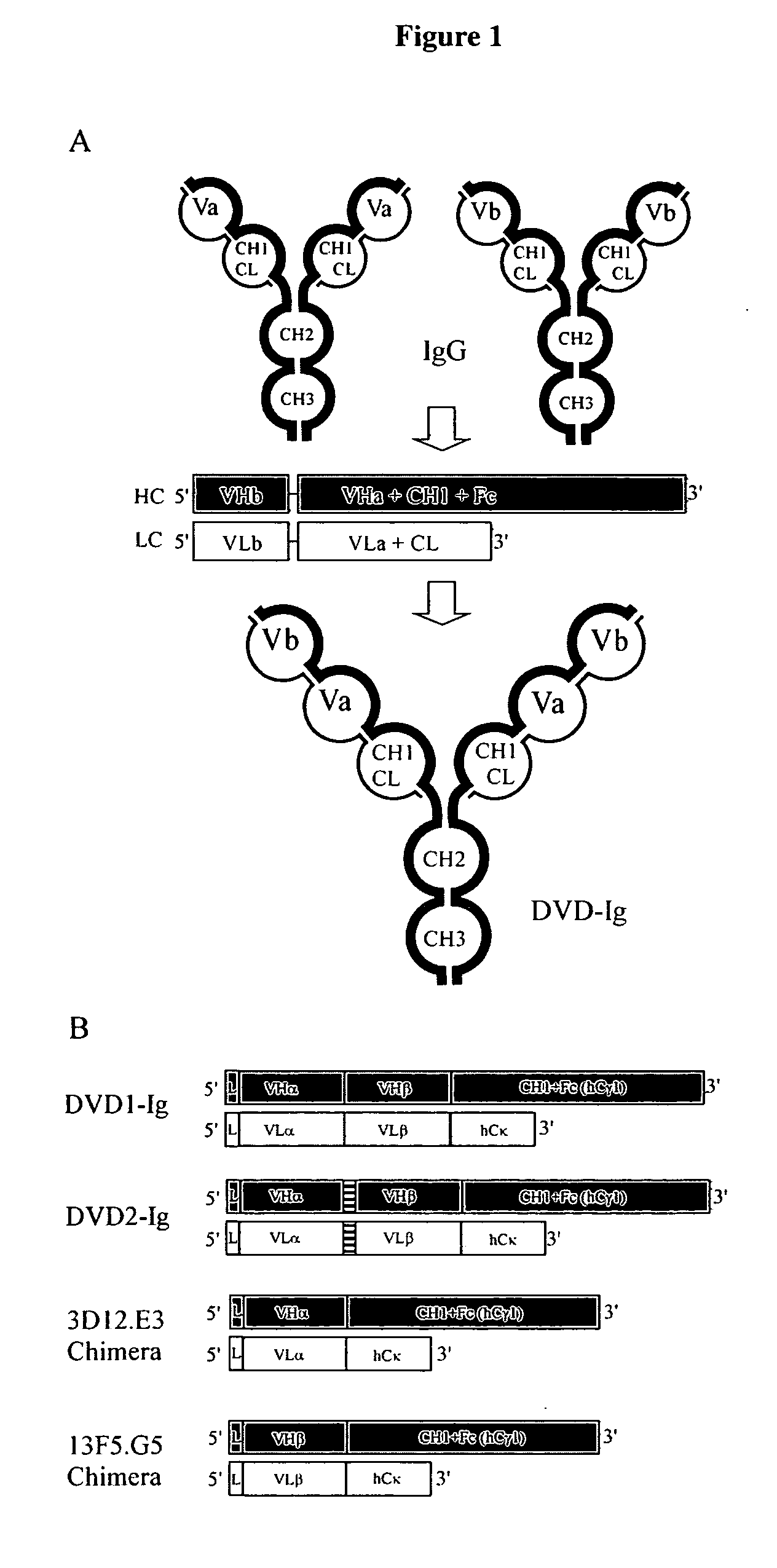
Dual Variable Domain Immunoglobulins and Uses Thereof
The present invention relates to engineered multivalent and multispecific binding proteins, methods of making, and specifically to their uses in the prevention, diagnosis, and / or treatment of disease.
Owner:ABBVIE INC
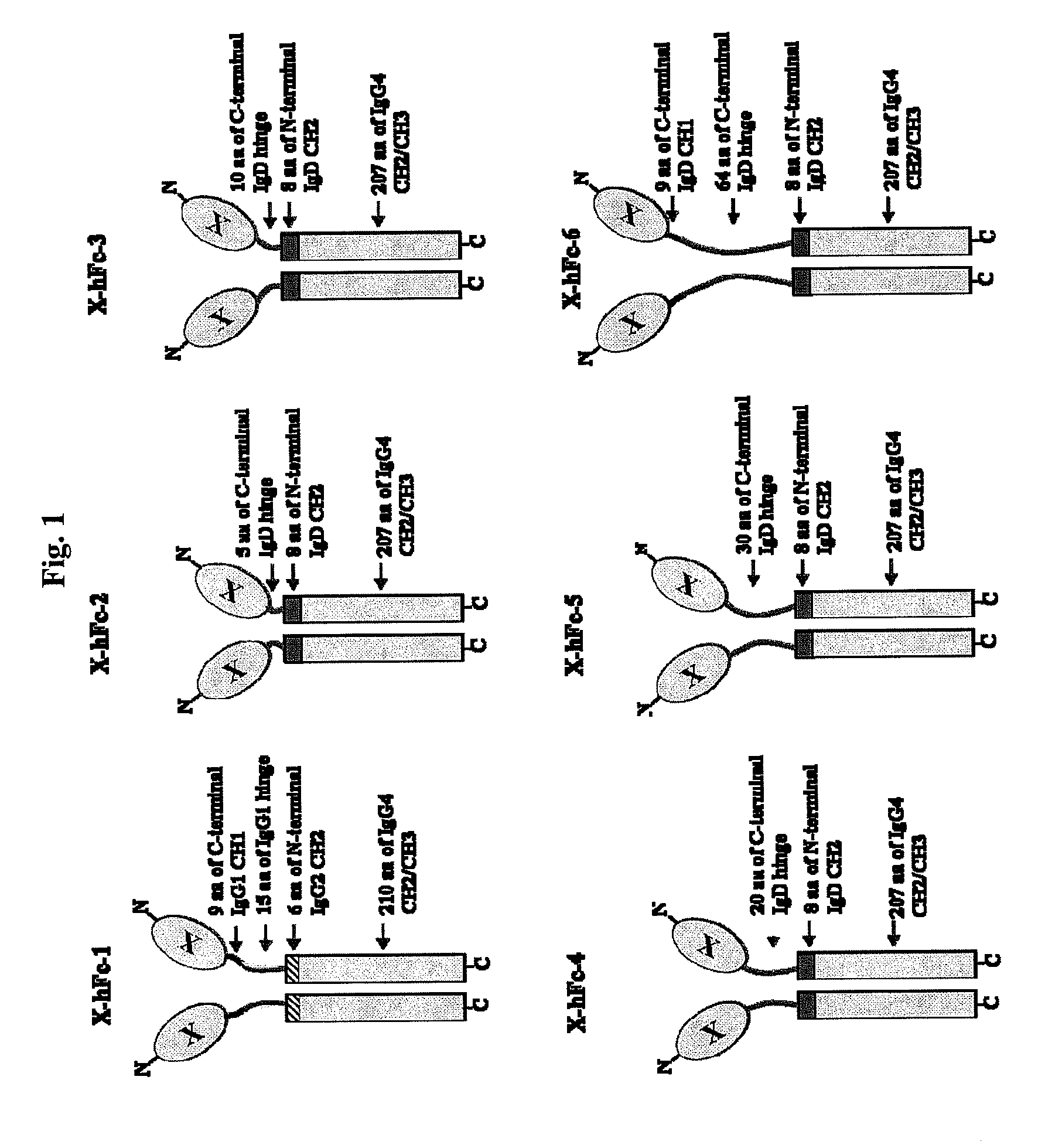
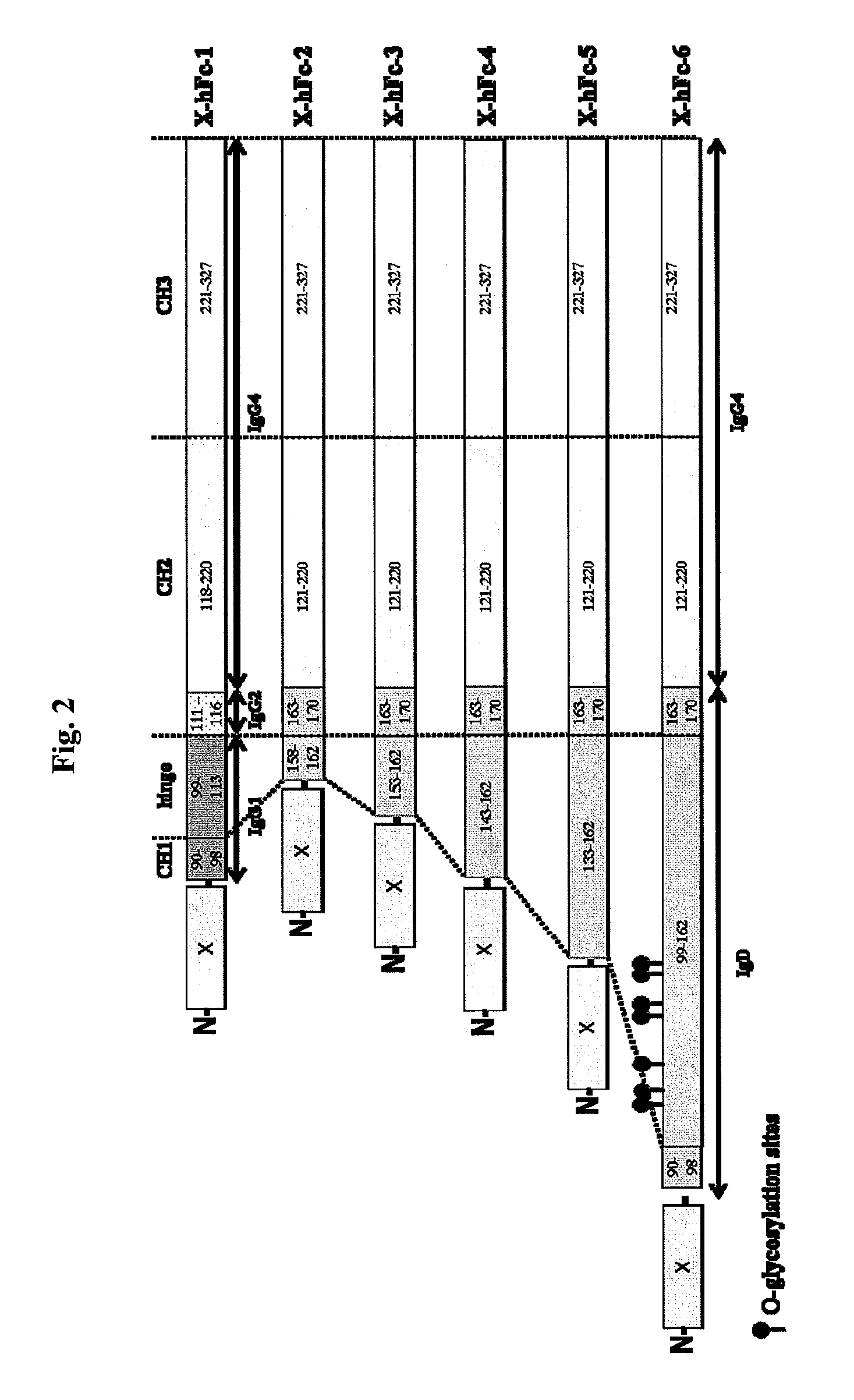
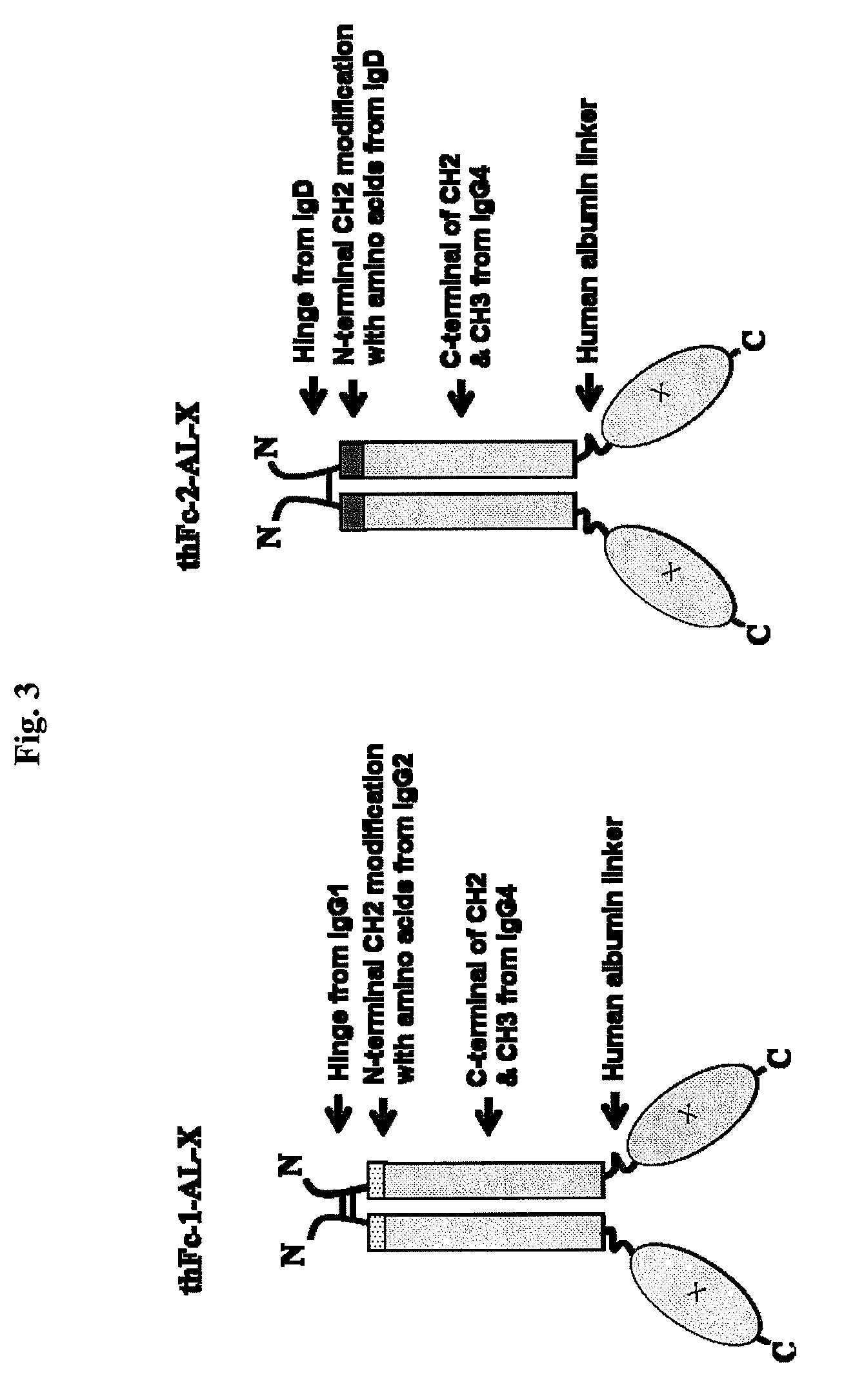
Immunoglobulin fusion proteins
ActiveUS7867491B2Extended half-lifeImprove expression levelPeptide/protein ingredientsAntipyreticFc domainBioactive molecules
Disclosed are fusion proteins comprising a biologically active molecule and an immunoglobulin (Ig) Fc domain which is linked to the biologically active molecule. The Fc domain is a hybrid human Fc domain of (i) IgG1, IgG2 or IgG4 or (ii) IgG4 and IgD. The hybrid Fc is useful as a carrier of biologically active molecules.
Owner:POSTECH ACADEMY IND FOUND OF POHANG UNIV OF SCI & TECH POSTECH +1
Mixture of at Least 6 Species of Lactic Acid Bacteria and/or Bifidobacteria in the Manufacture of Sourdough
A mixture of at least 6 species of lactic acid bacteria and / or Bifidobacteria is disclosed for use in bakery and medical field. The preferred mixture comprises Streptococcus thermophilus, Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus. Said mixture is useful for a sourdough, a leavening composition. Baked goods and other food products obtained therefrom are disclosed. These goods have low or no gluten content and are suitable for the integration of the diet of a subject suffering from celiac disease, for decreasing the risk of allergies due to wheat flour albumins and globulins, for the treatment of schizophrenic symptoms, in the preparation of products for enteric diet.
Owner:VSL PHARMA INC
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS8217147B2High affinityLow affinityAntibody mimetics/scaffoldsImmunoglobulinsDiseaseTherapeutic antibody
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcγRIIIA and / or FcγRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcγR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
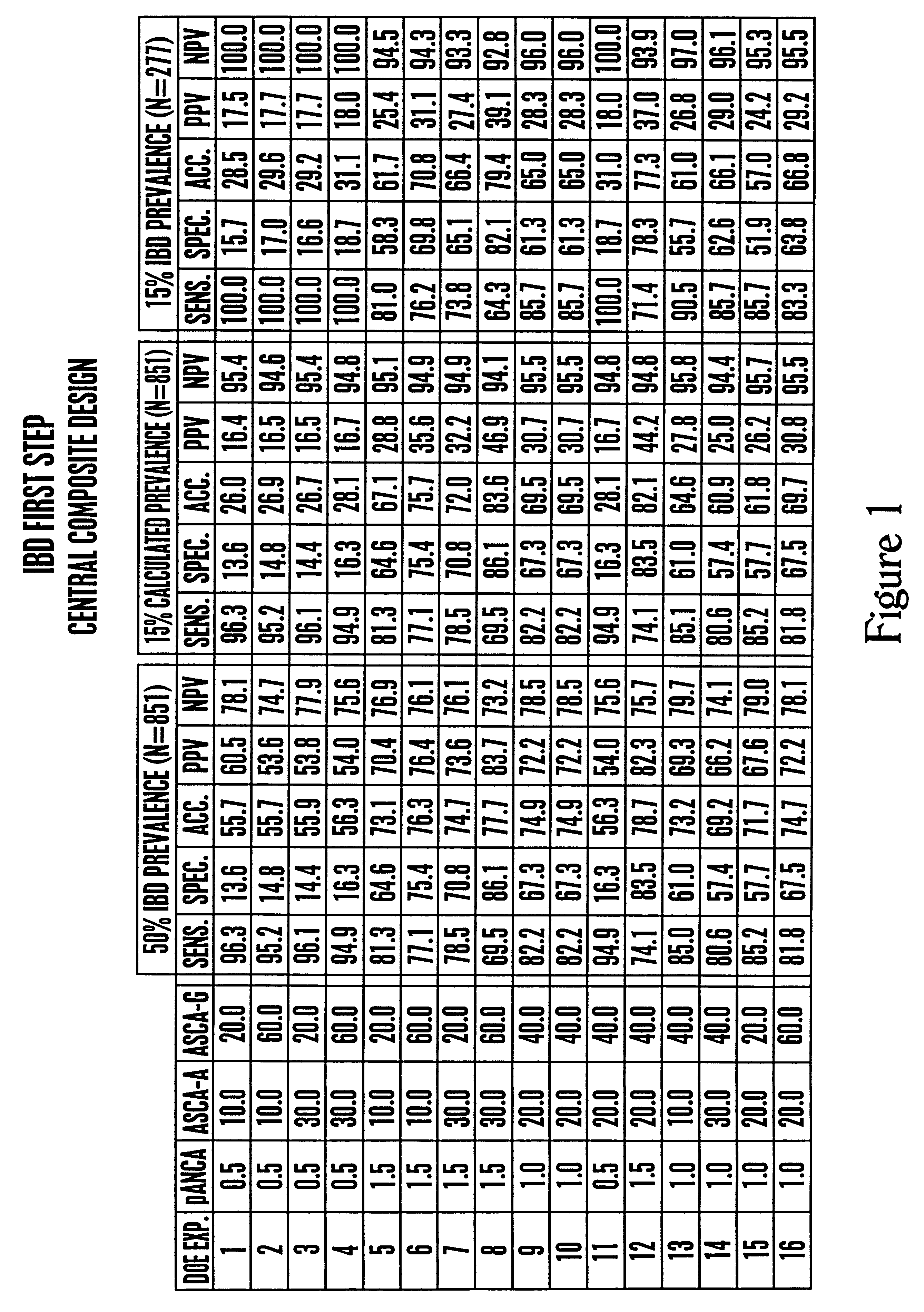
Inflammatory bowel disease first step assay system
The present invention provides a highly sensitive method of diagnosing inflammatory bowel disease (IBD) in an individual. The method includes the steps of isolating a sample from the individual; determining by non-histological means whether the sample is positive for anti-neutrophil cytoplasmic antibodies (ANCA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin A (ASCA-IgA); determining whether the sample is positive for anti-Saccharomyces cerevisiae immunoglobulin G (ASCA-IgG); and diagnosing the individual as having IBD when the sample is positive for ANCA, ASCA-IgA or ASCA-IgG, and diagnosing the individual as not having IBD when the sample is negative for ANCA, ASCA-IgA and ASCA-IgG, provided that the method does not include histological analysis of neutrophils.
Owner:PROMETHEUS LAB +1
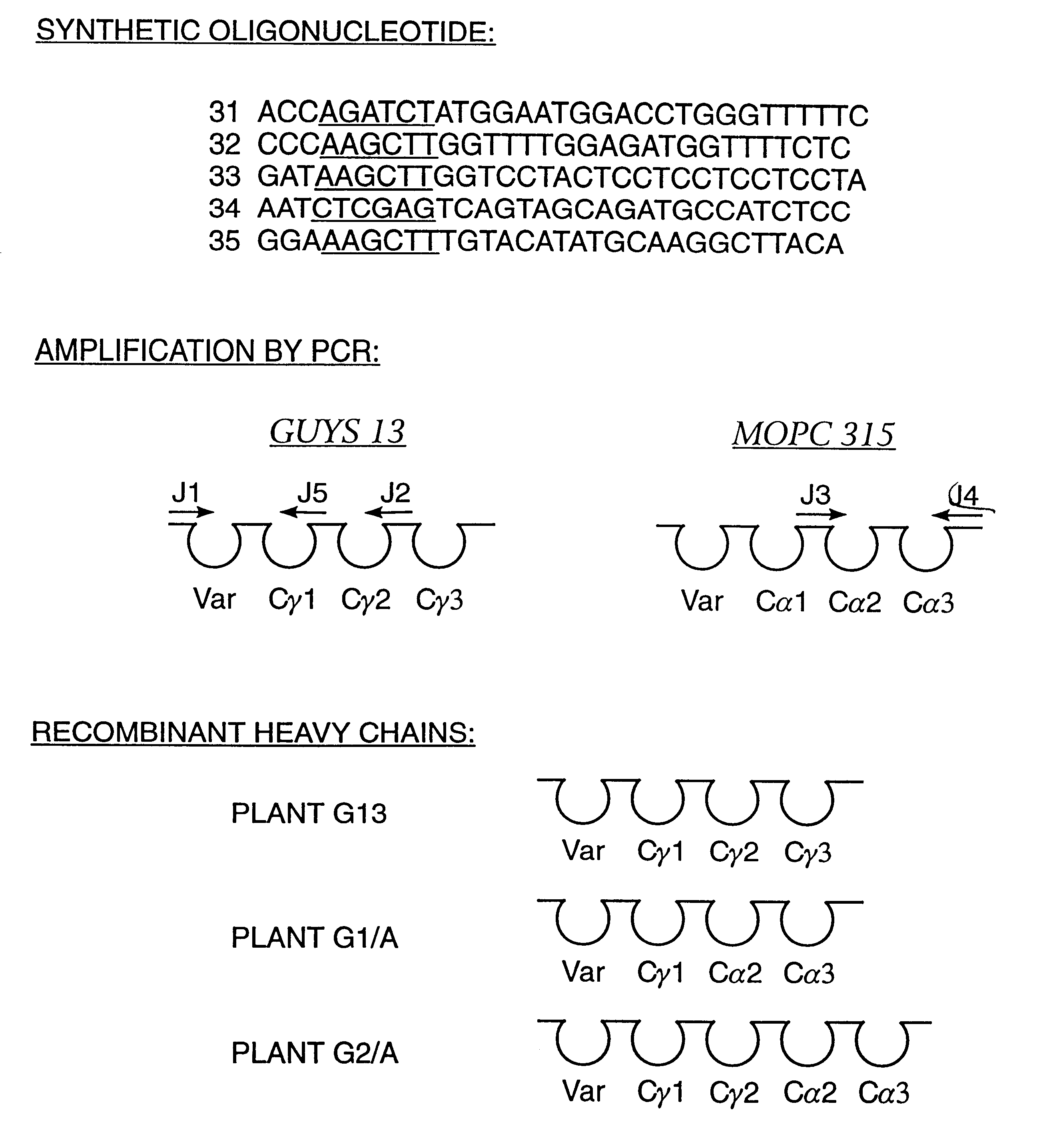
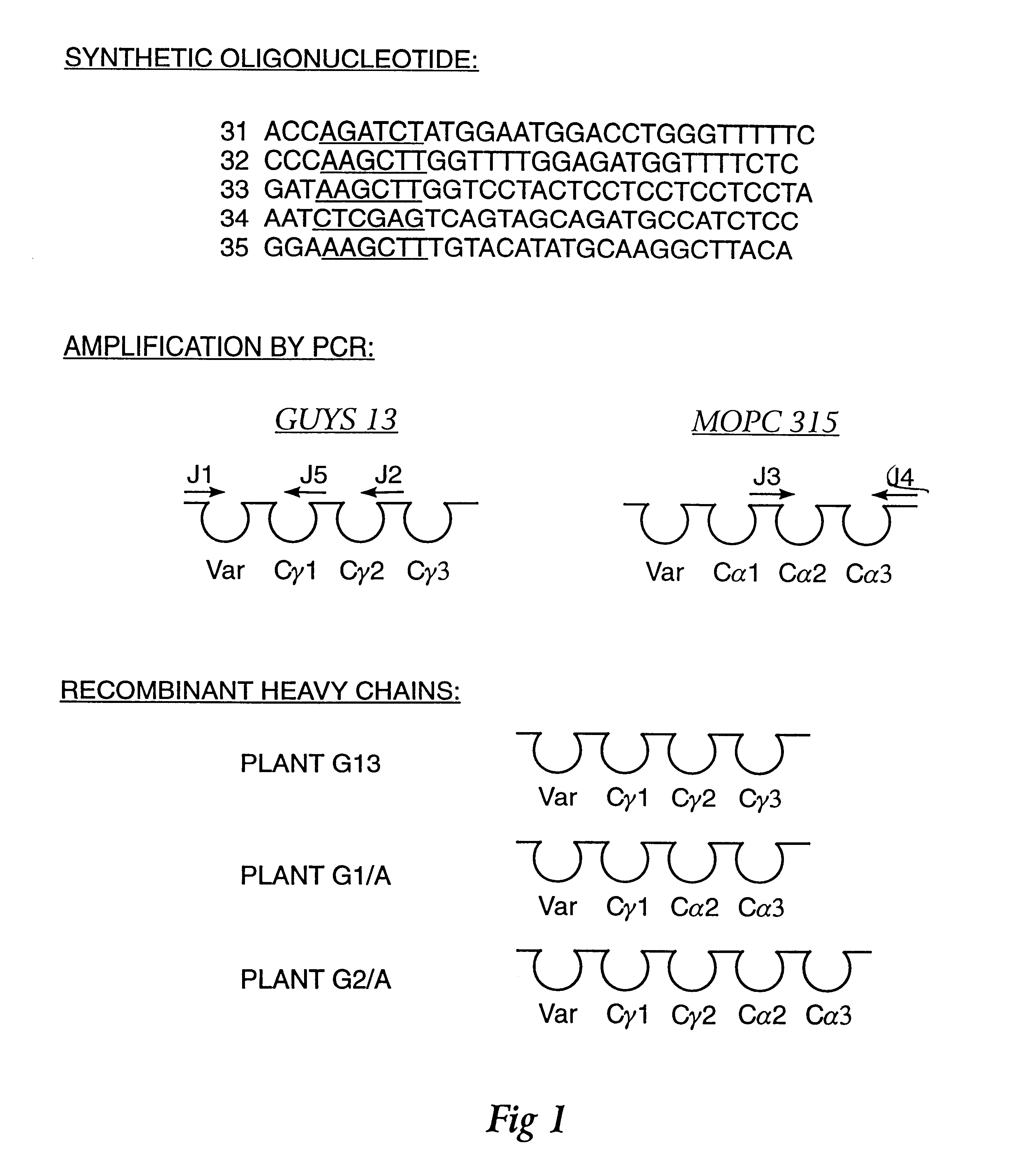
Method for producing immunoglobulins containing protection proteins in plants and their use
The immunoglobulins of the present invention are useful therapeutic immunoglobulins against mucosal pathogens such as S. mutans. The immunoglobulins contain a protection protein that protects the immunoglobulins in the mucosal environment.The invention also includes the greatly improved method of producing immunoglobulins in plants by producing the protection protein in the same cell as the other components of the immunoglobulins. The components of the immunoglobulin are assembled at a much improved efficiency. The method of the invention allows the assembly and high efficiency production of such complex molecules.The invention also contemplates the production of immunoglobulins containing protection proteins in a variety of cells, including plant cells, that can be selected for useful additional properties. The use of immunoglobulins containing protection proteins as therapeutic antibodies against mucosal and other pathogens is also contemplated.
Owner:UNTD MED & DENT SCHLS OF GUYS & ST THOMASS HOSP
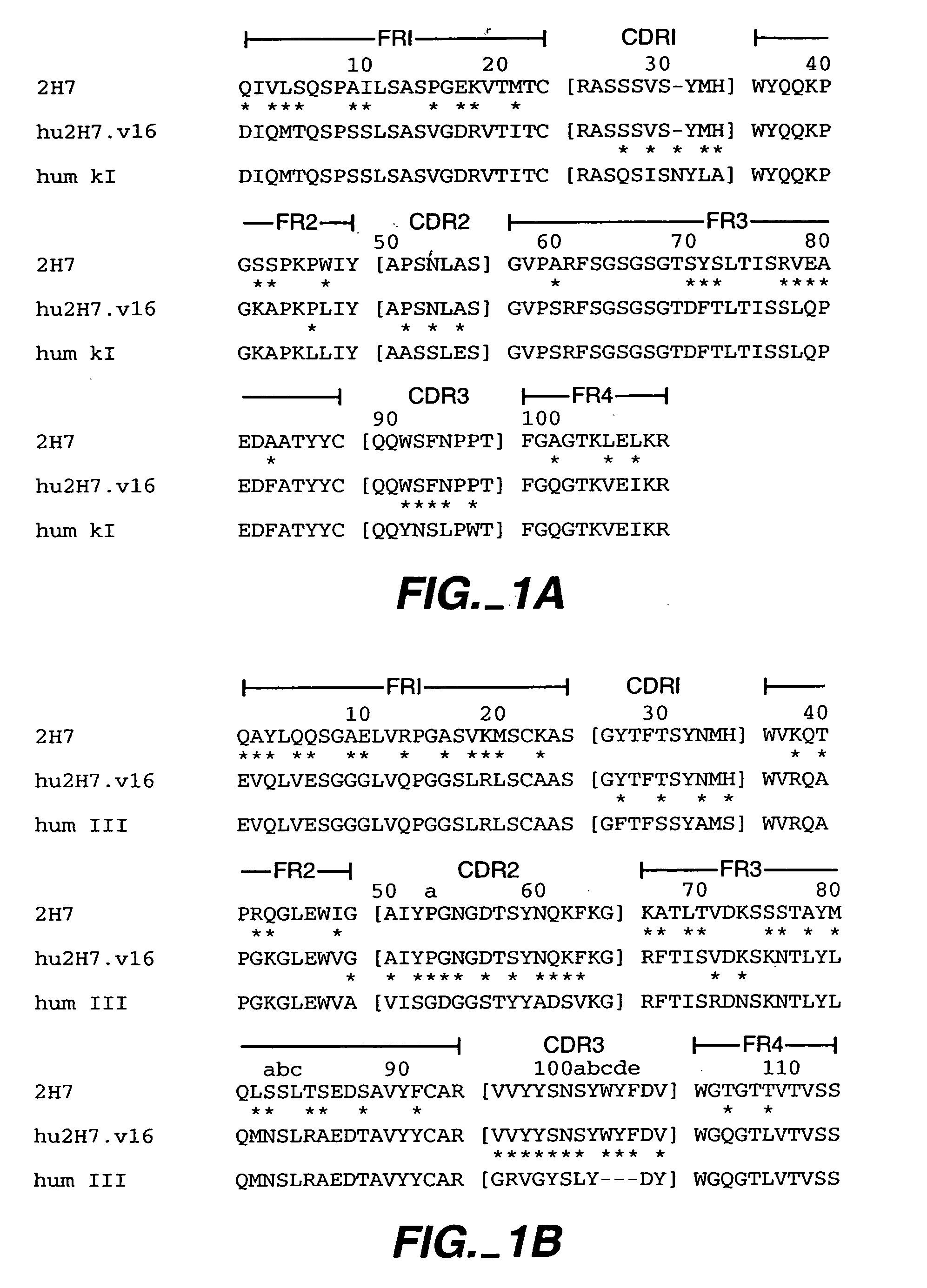
Immunoglobulin variants and uses thereof
InactiveUS20060024300A1Enhanced effector functionImprove stabilitySenses disorderNervous disorderDiseaseAutoimmune disease
Owner:GENENTECH INC
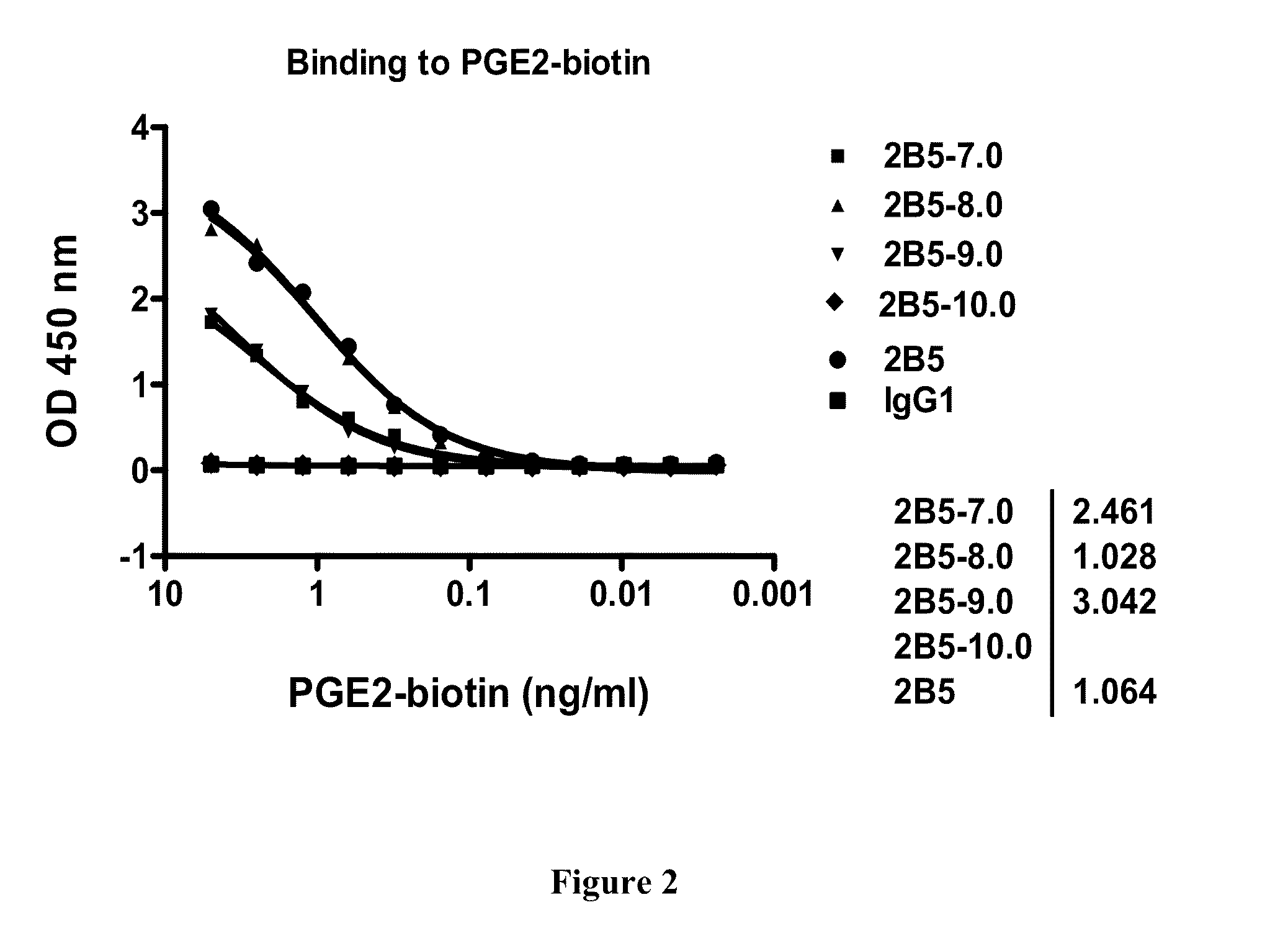
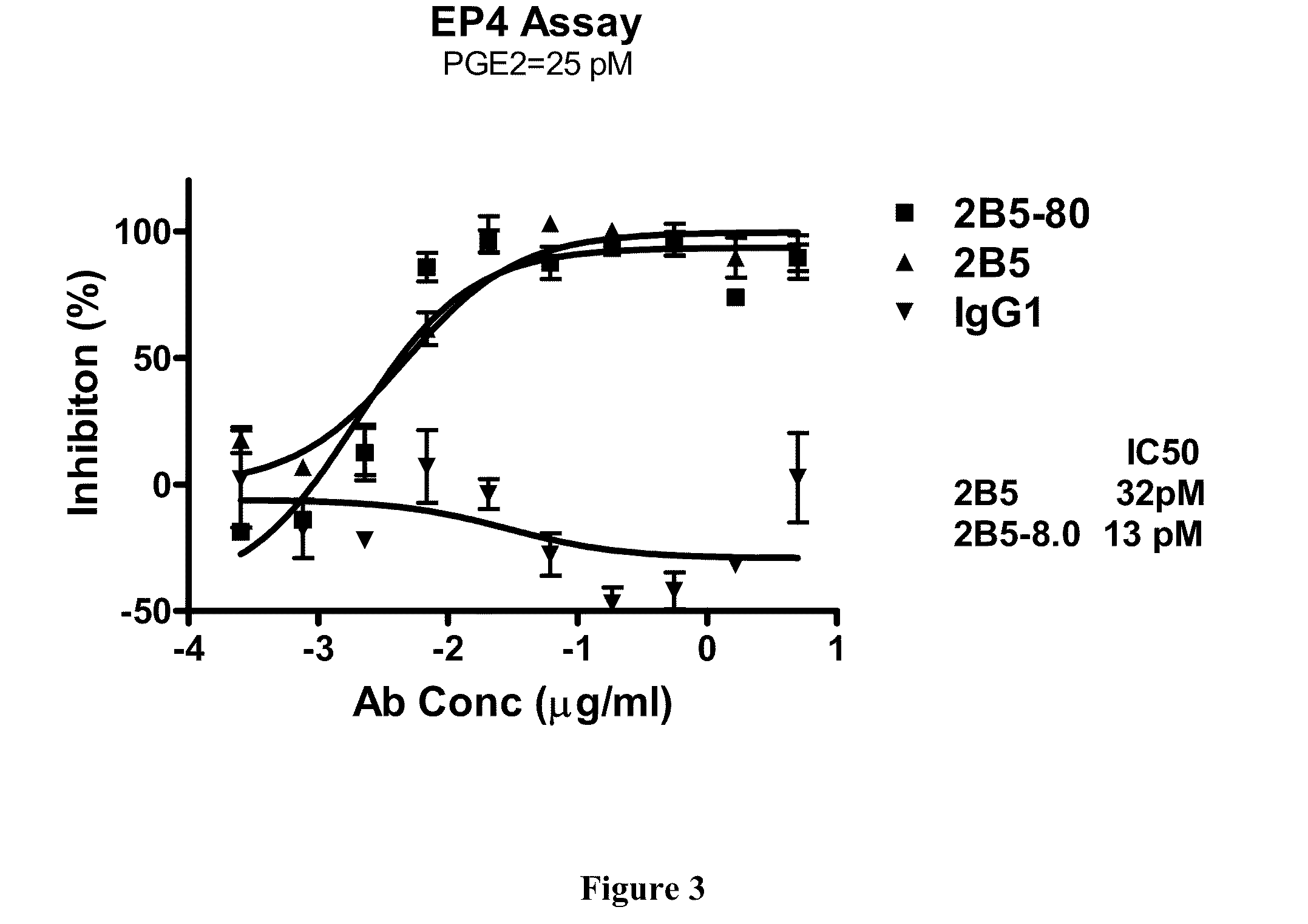
Prostaglandin E2 dual variable domain immunoglobulins and uses thereof
The present invention relates to engineered multivalent and multispecific binding proteins, methods of making, and specifically to their uses in the prevention, diagnosis, and / or treatment of disease.
Owner:ABBVIE INC
Pharmaceutically acceptable FN3 polypeptides for human treatments
InactiveUS20060246059A1Optimal folding and stability and solubilityImproved biophysical propertyBacteriaPeptide/protein ingredientsWAS PROTEINBiology
Disclosed herein are proteins that include an immunoglobulin fold and that can be used as scaffolds. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
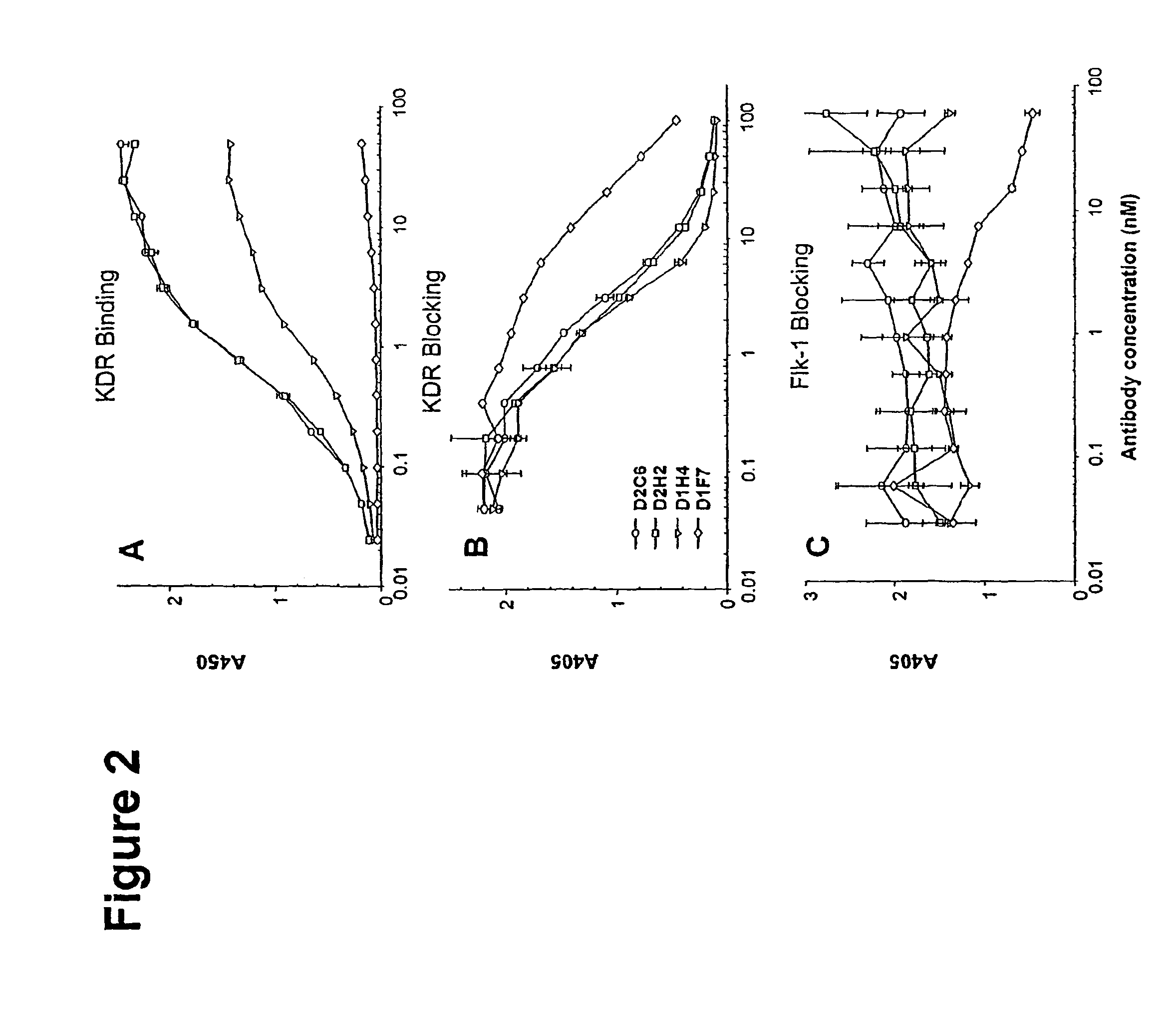
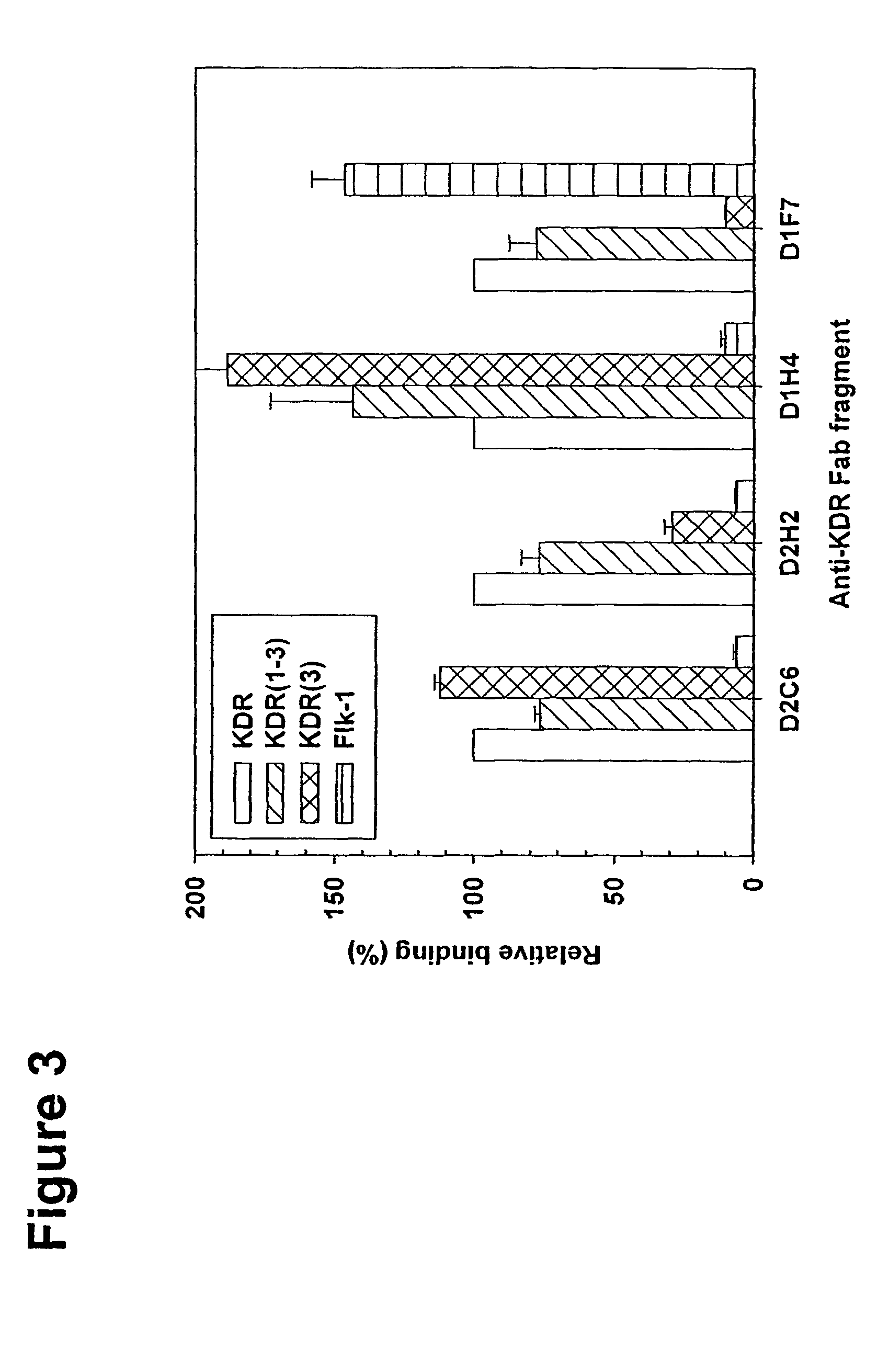
Human antibodies specific to KDR and uses thereof
The invention provides an antibodies that bind to KDR with an affinity comparable to or higher than human VEGF, and that neutralizes activation of KDR. Antibodies include whole immunoglobulins, monovalent Fabs and single chain antibodies, multivalent single chain antibodies, diabodies, triabodies, and single domain antibodies. The invention further provides nucleic acids and host cells that encode and express these antibodies. The invention further provides a method of neutralizing the activation of KDR, a method of inhibiting angiogenesis in a mammal and a method of inhibiting tumor growth in a mammal.
Owner:IMCLONE SYSTEMS
Mutated immunoglobulin-binding protein
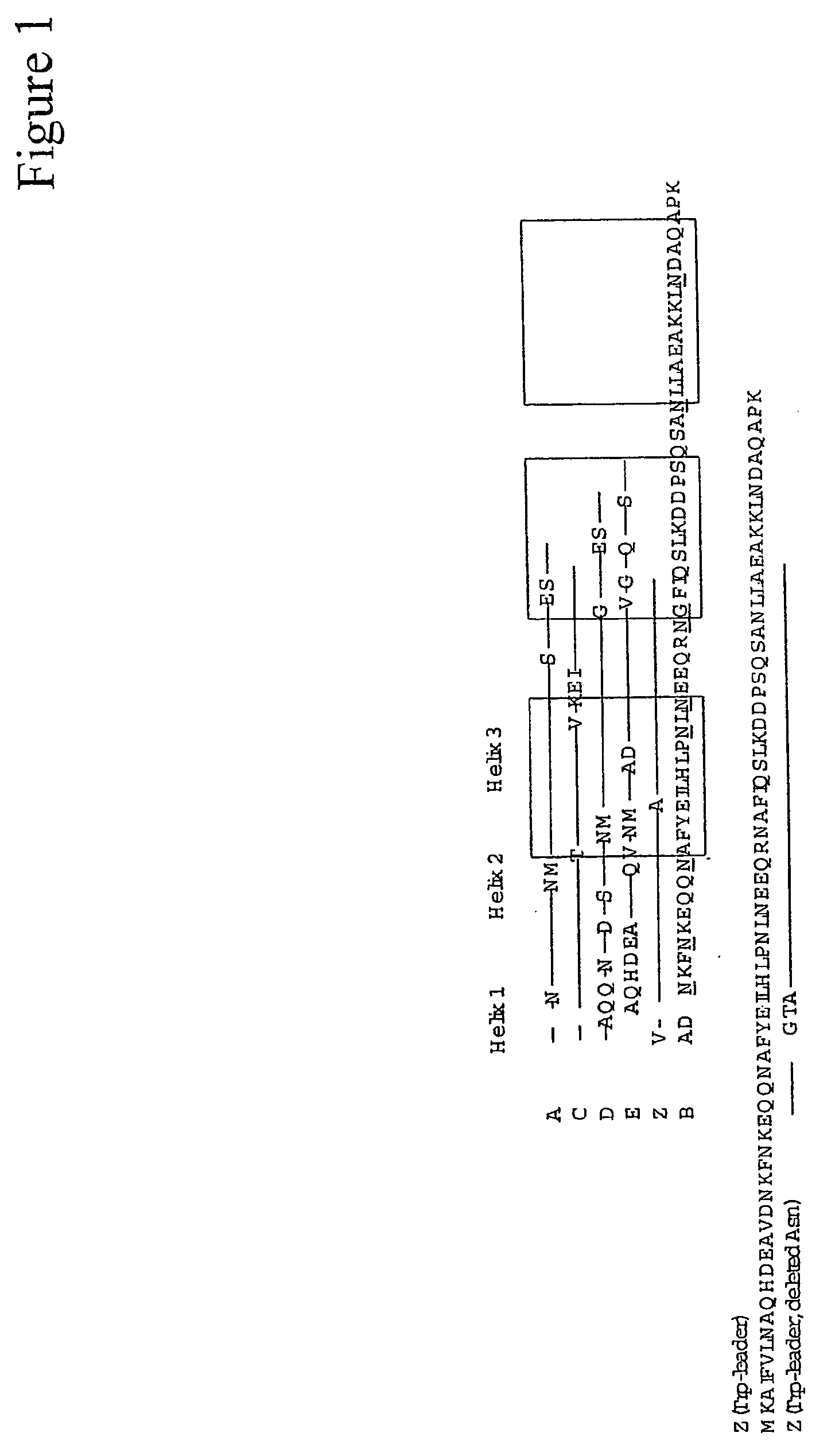
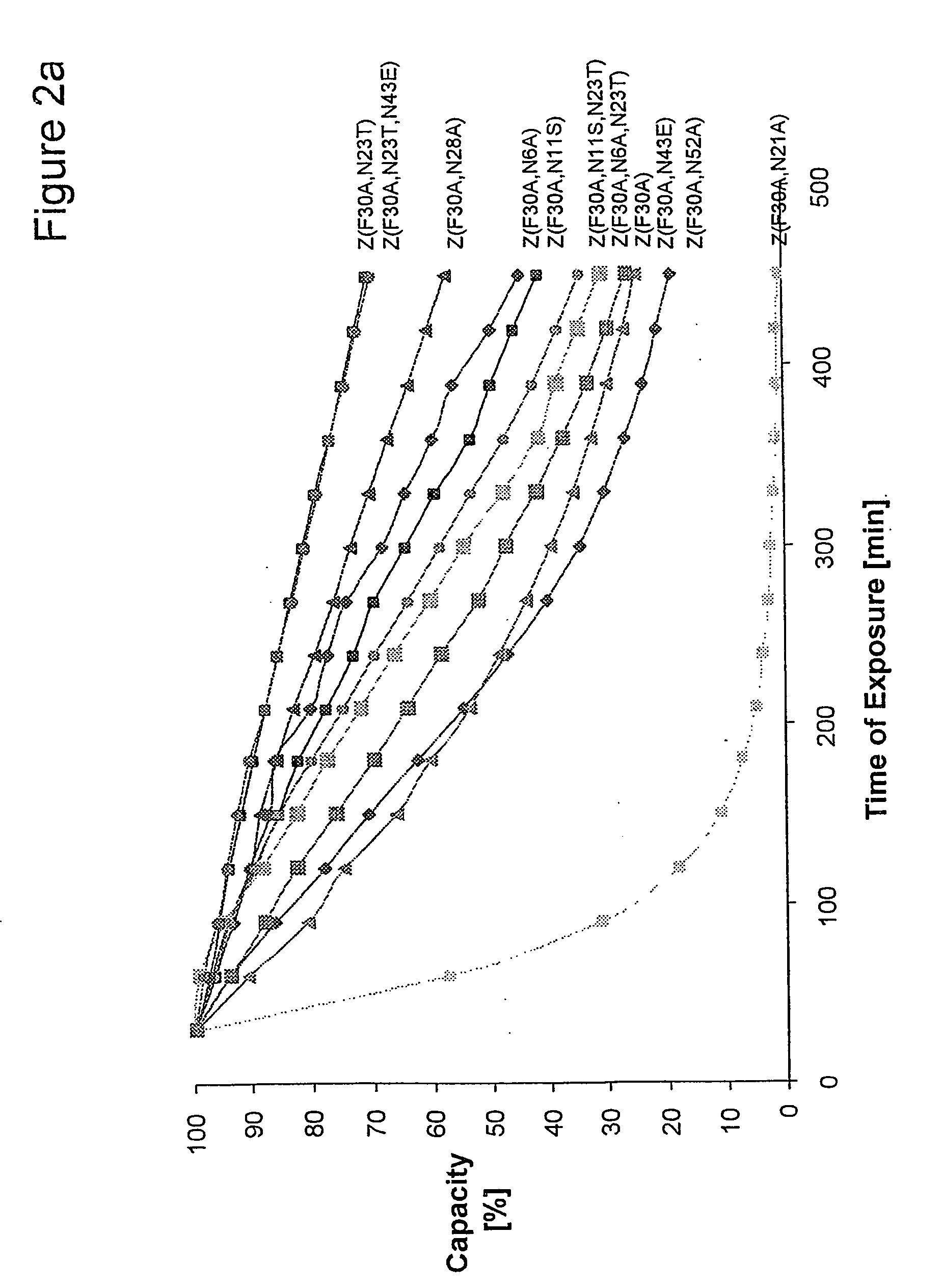
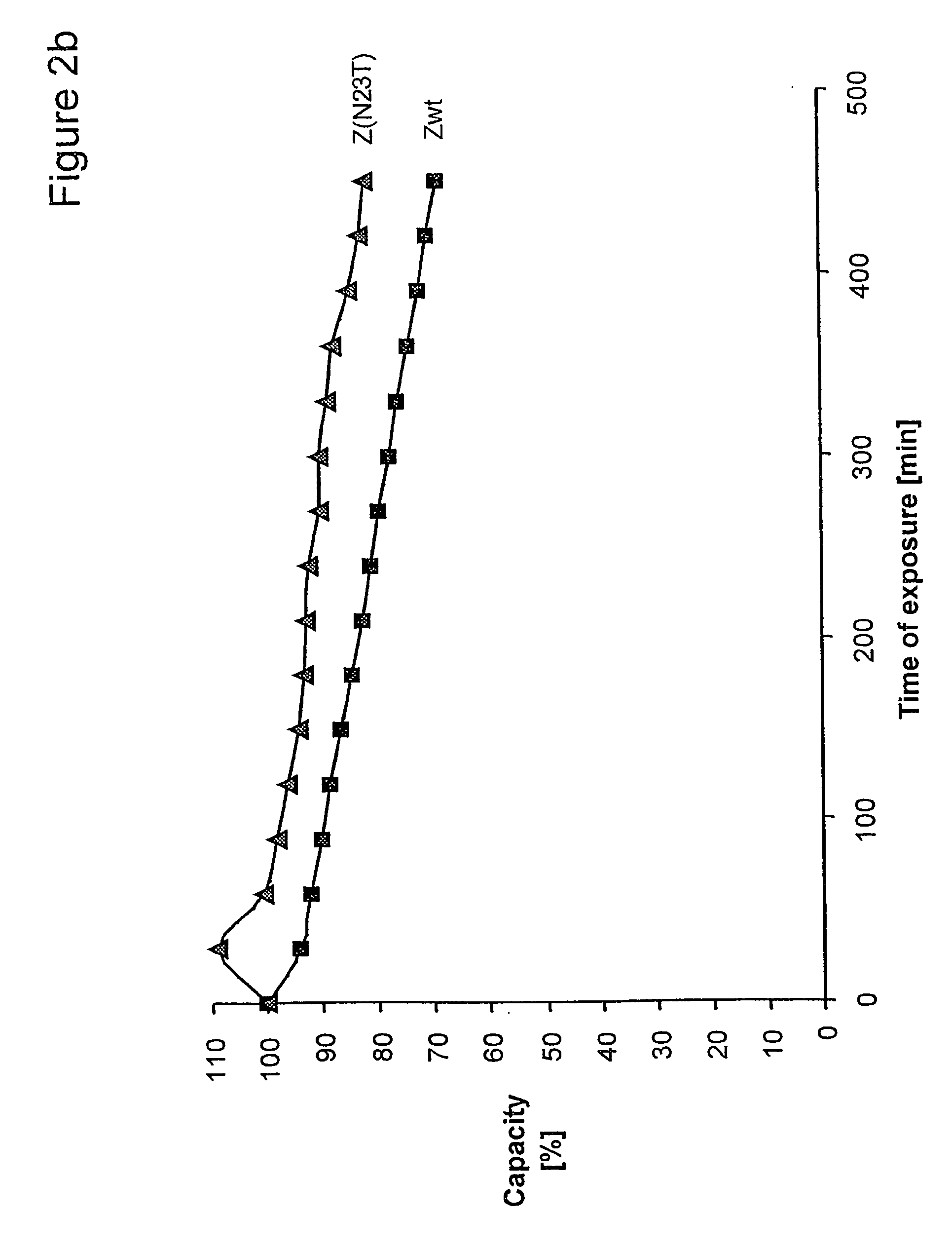
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com