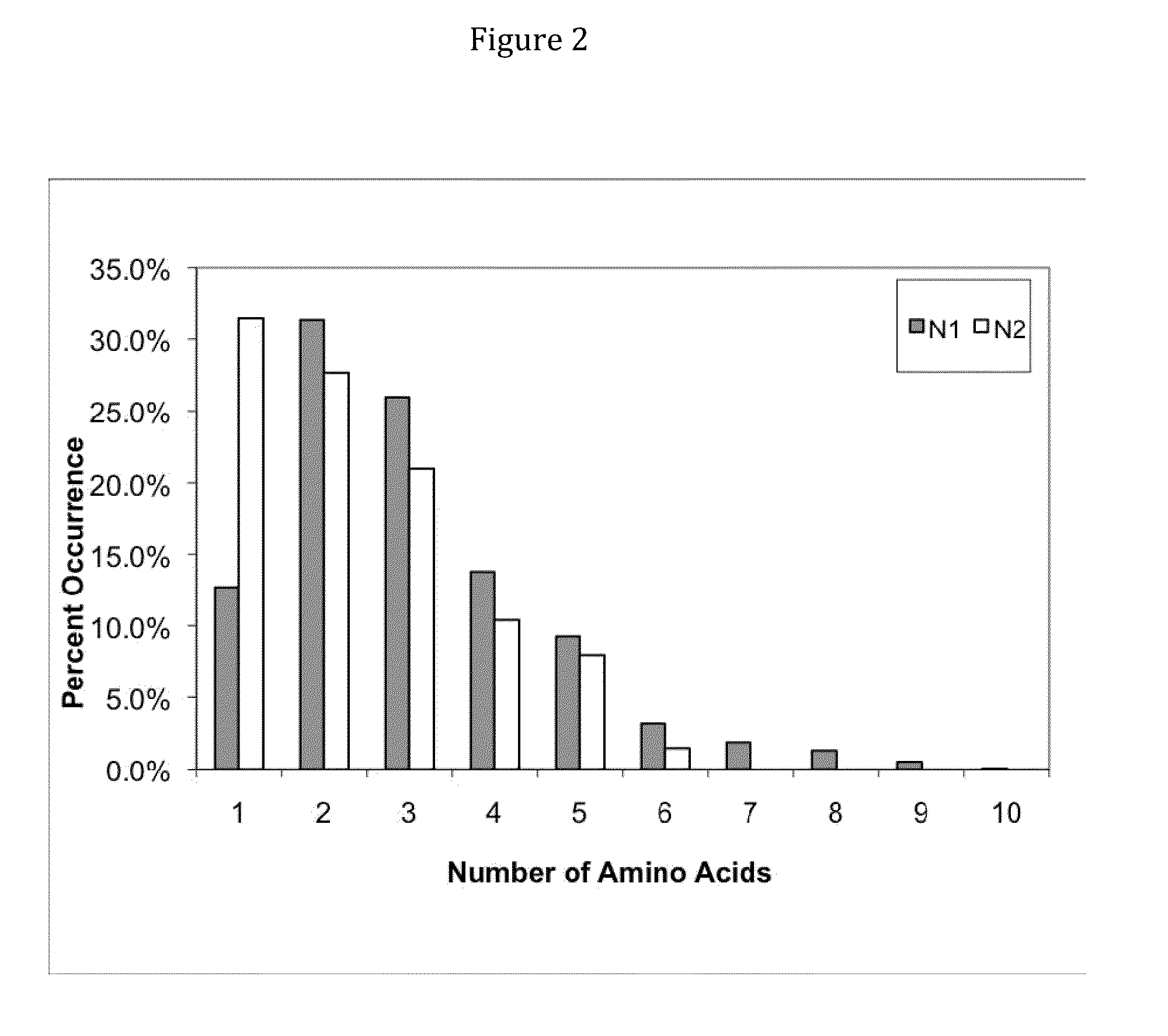
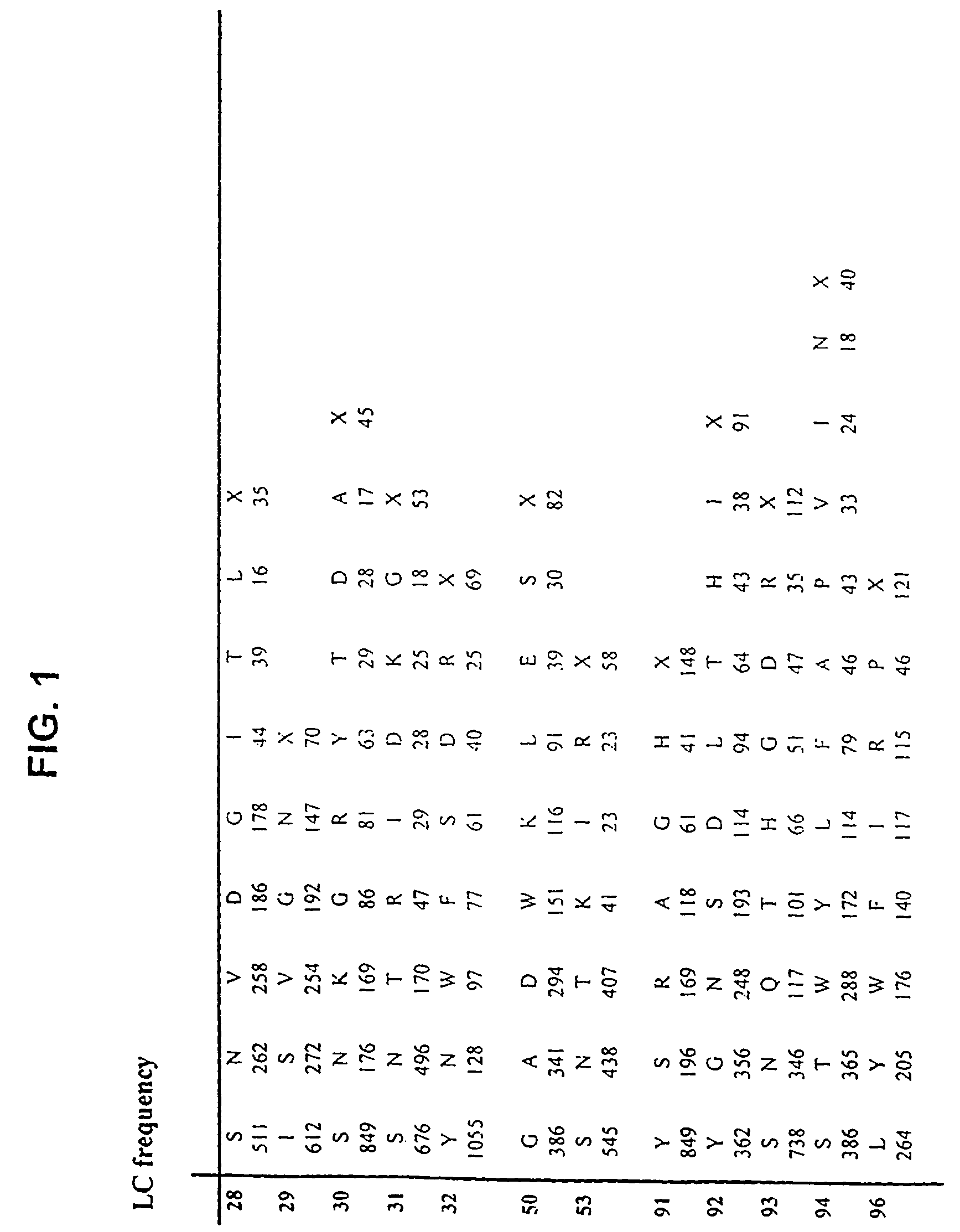
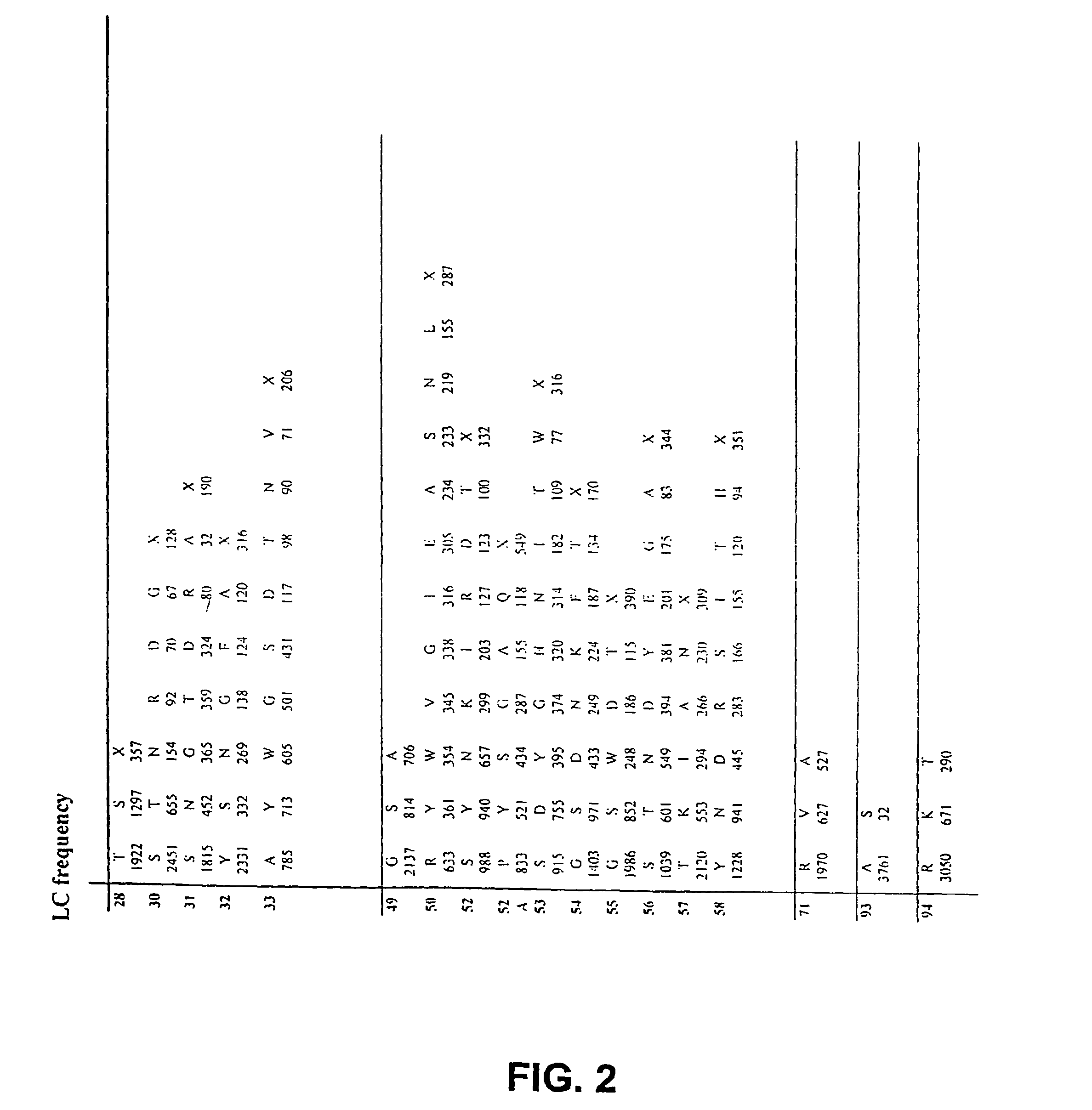
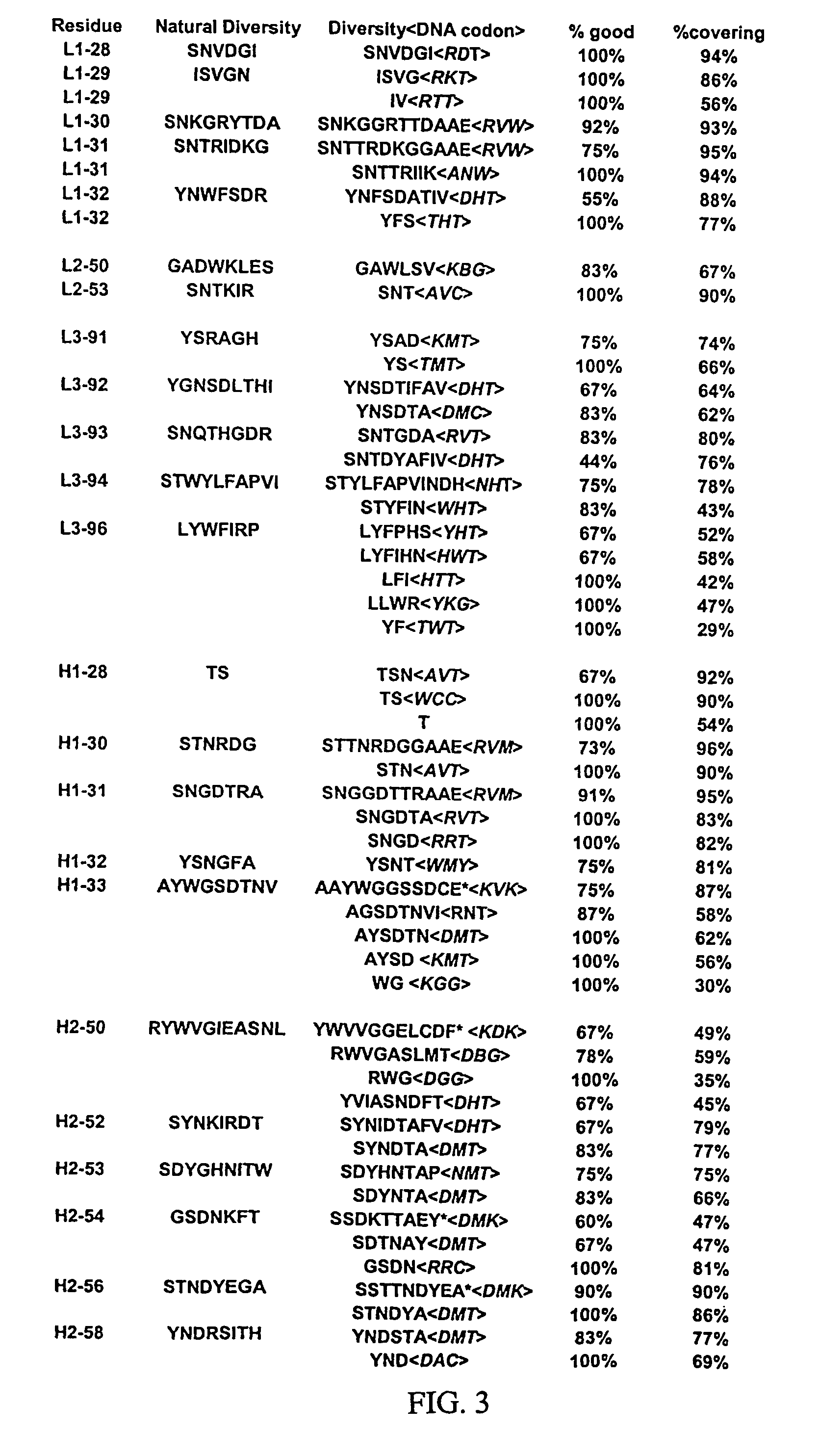
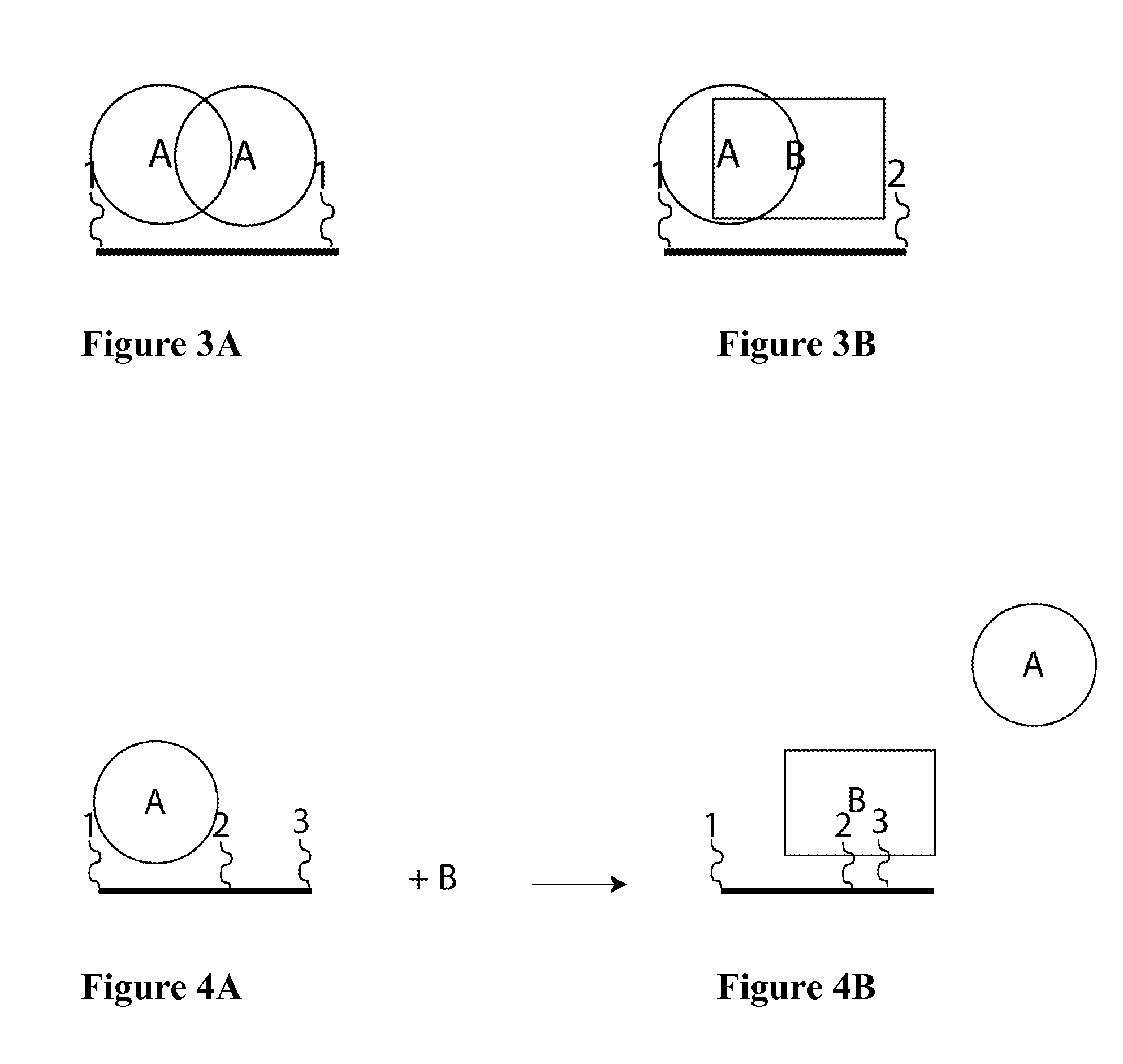
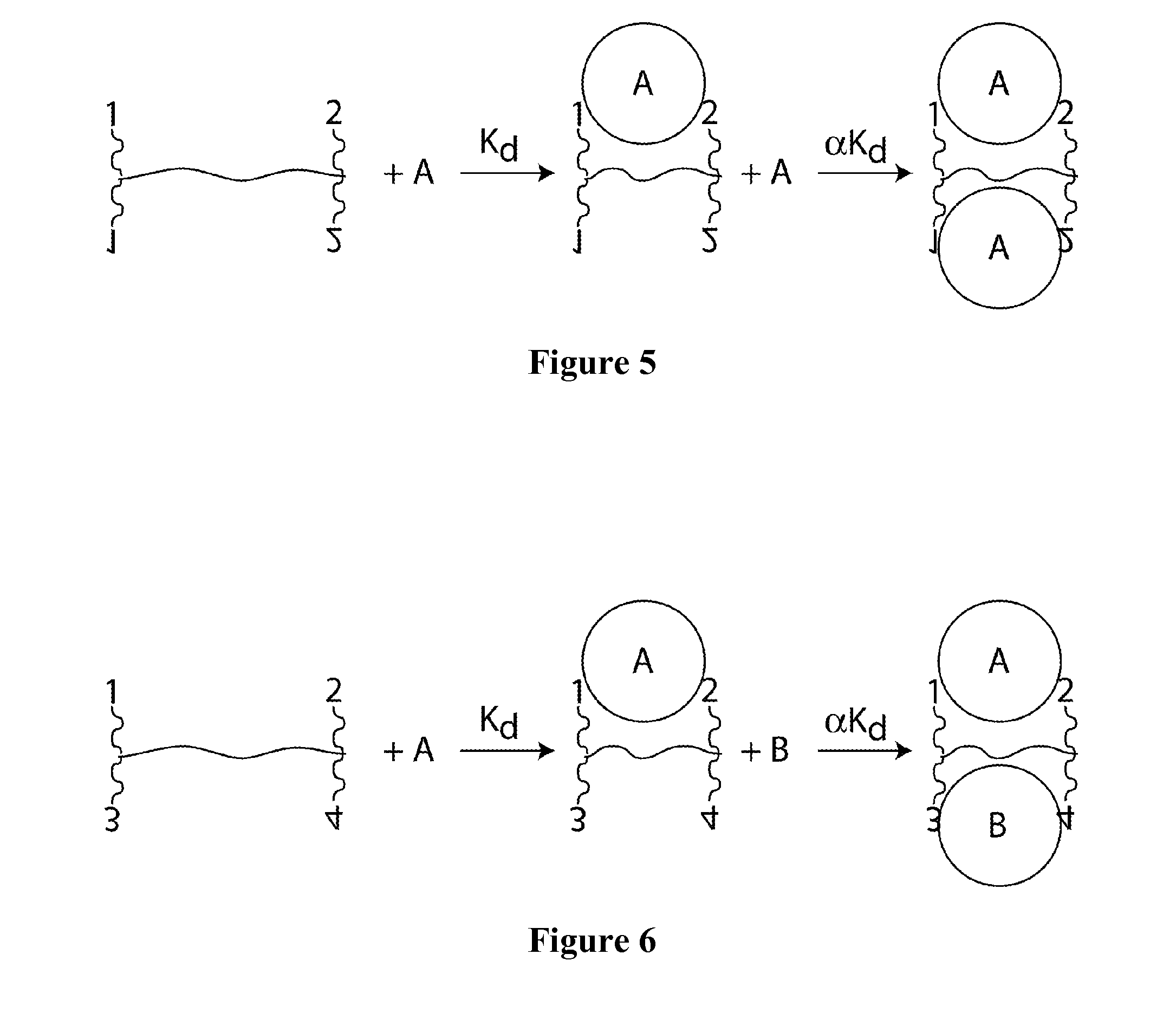
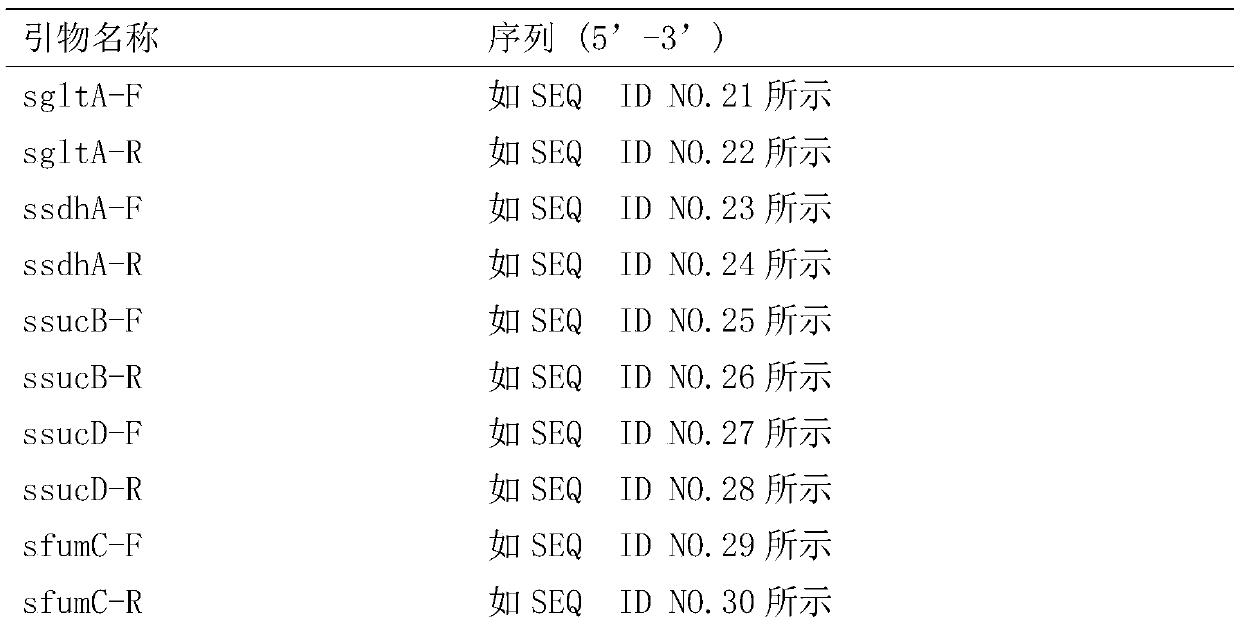
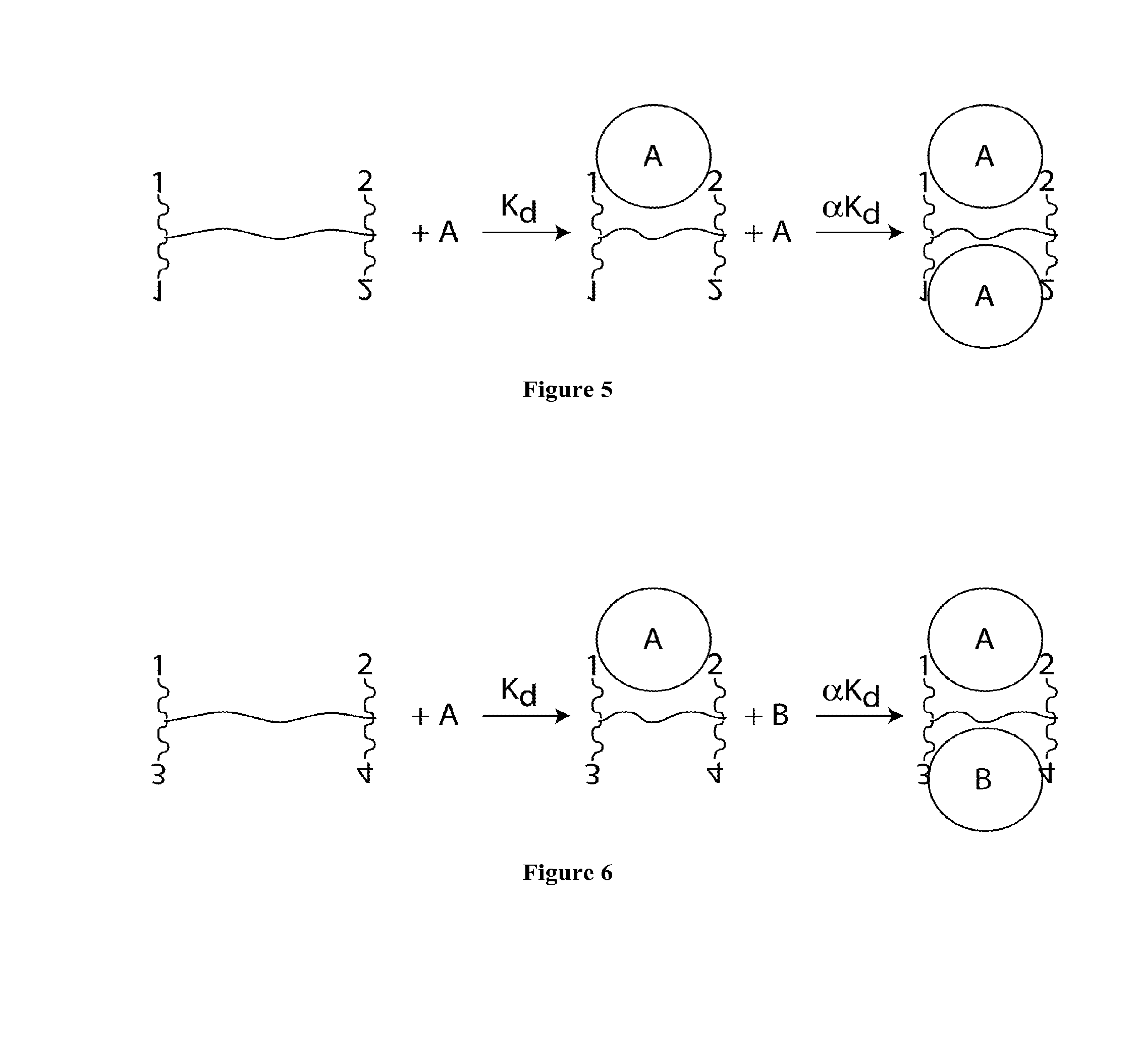Patents
Literature
67 results about "Synthetic antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
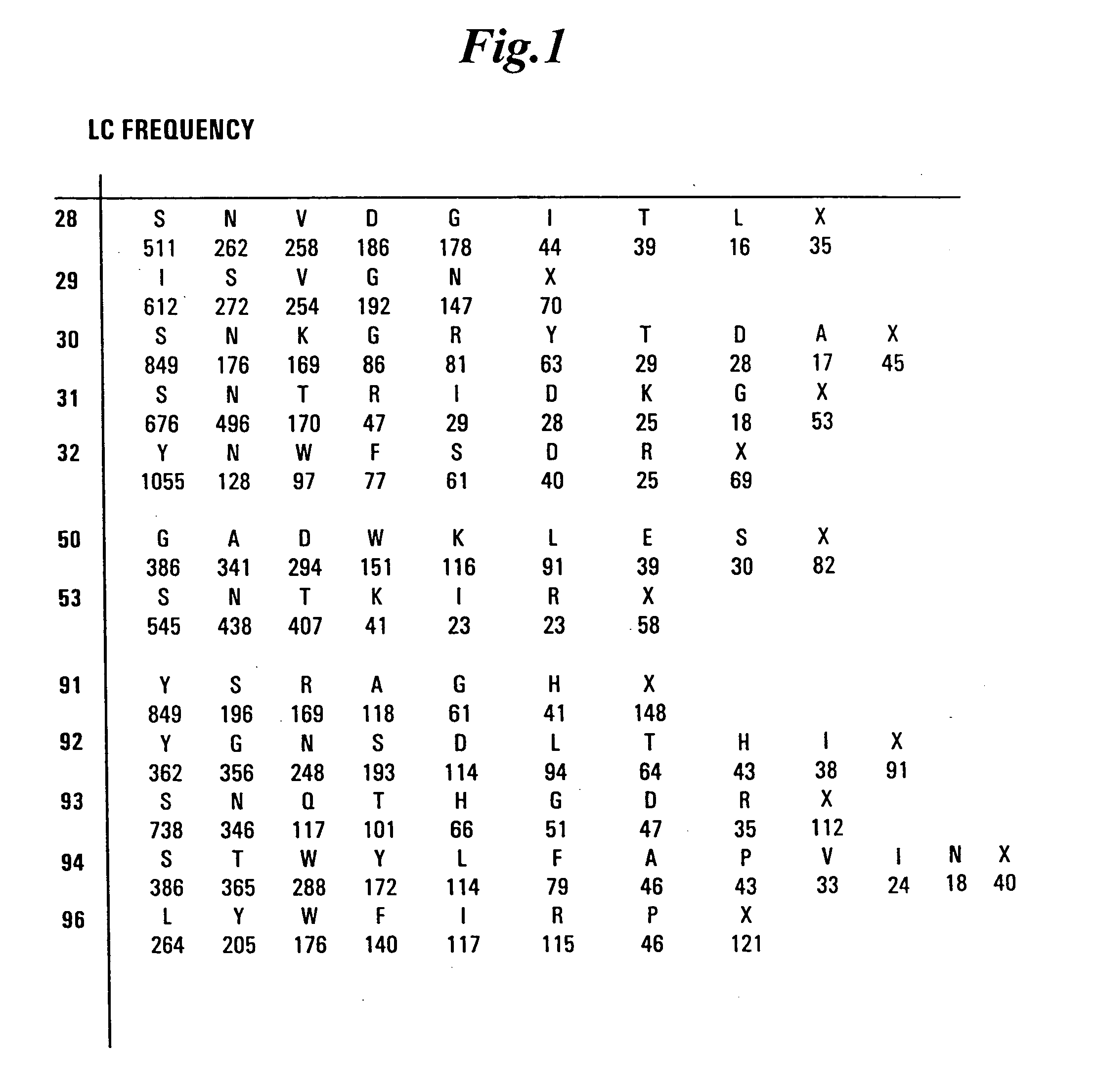
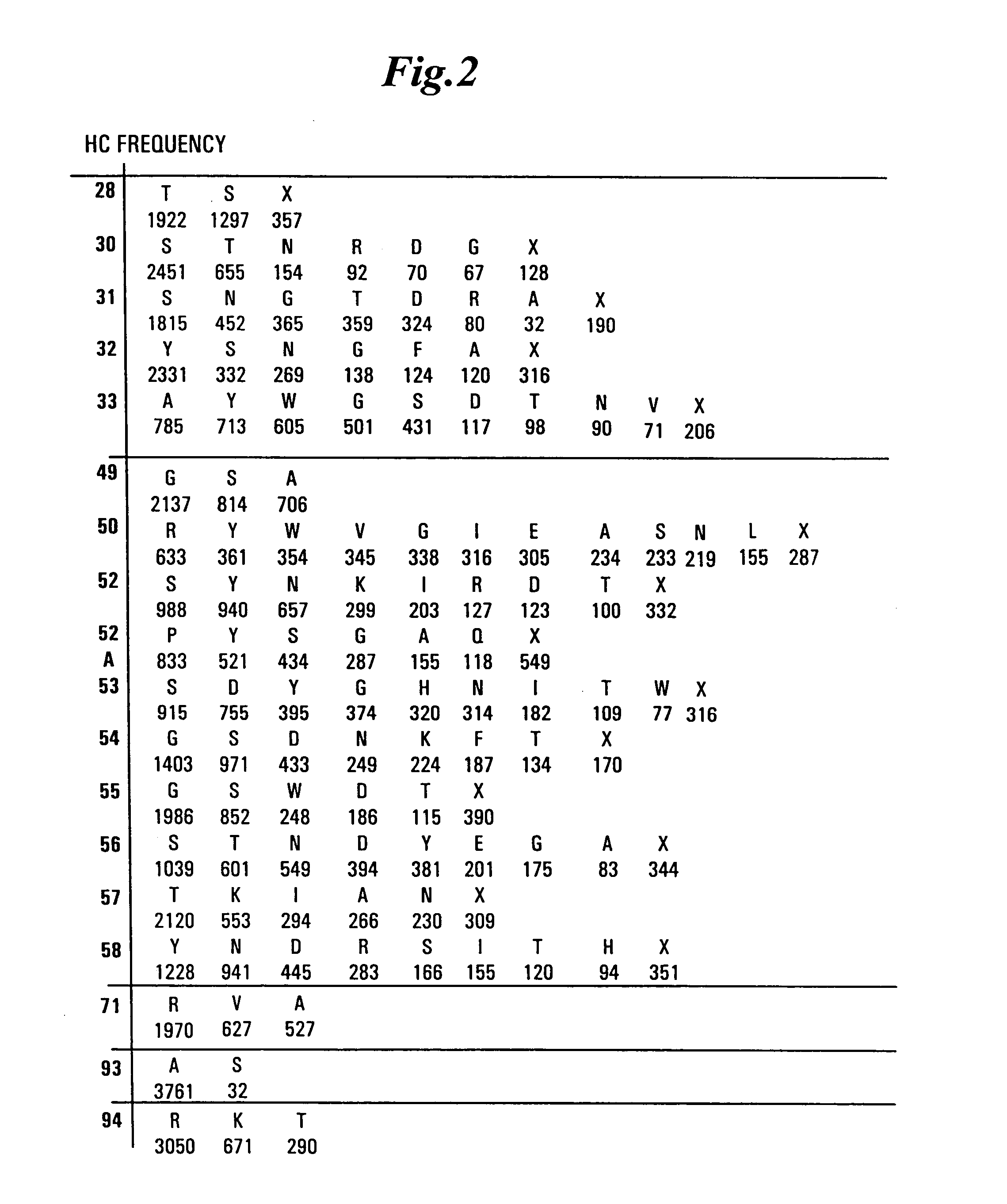
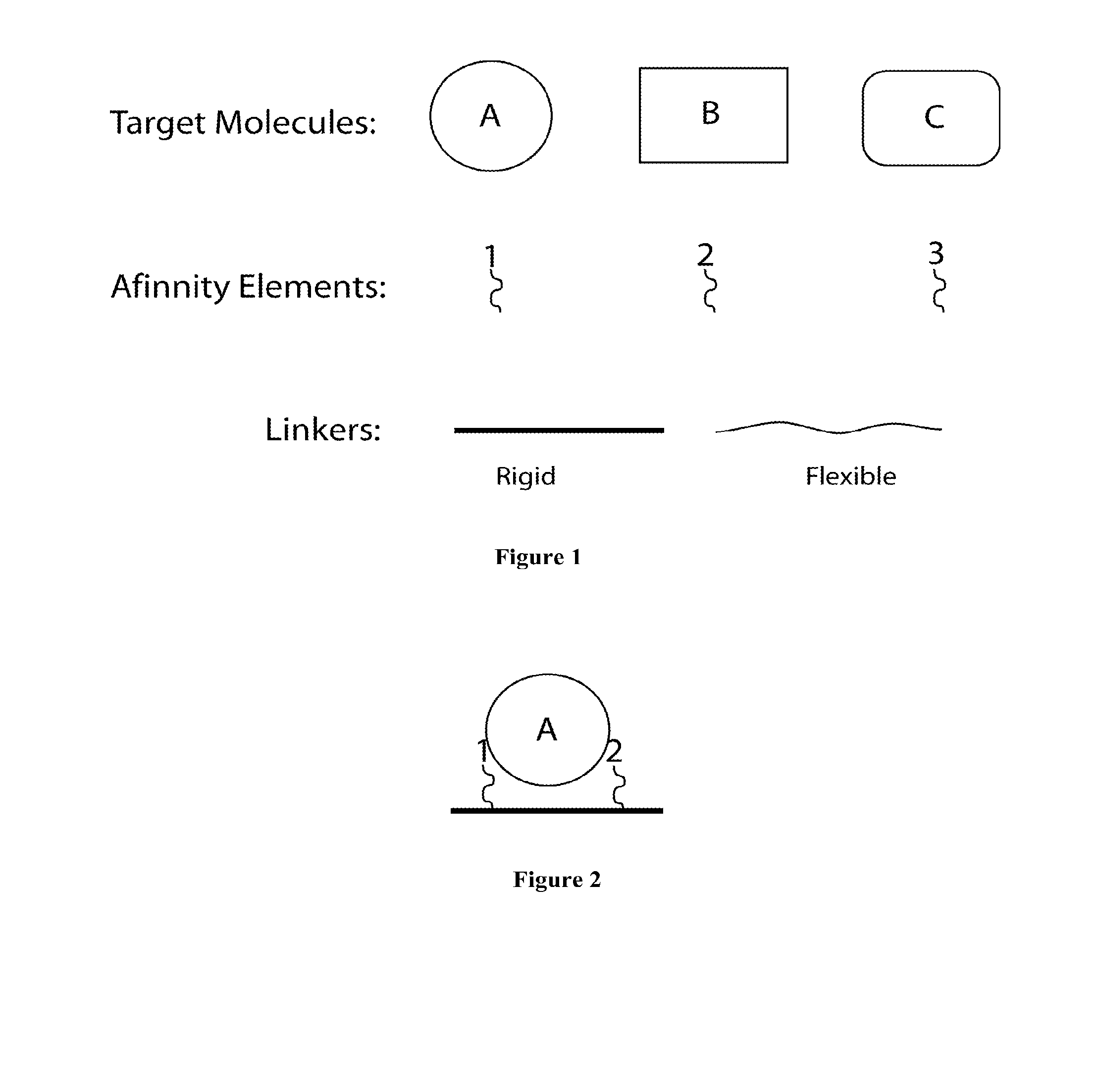
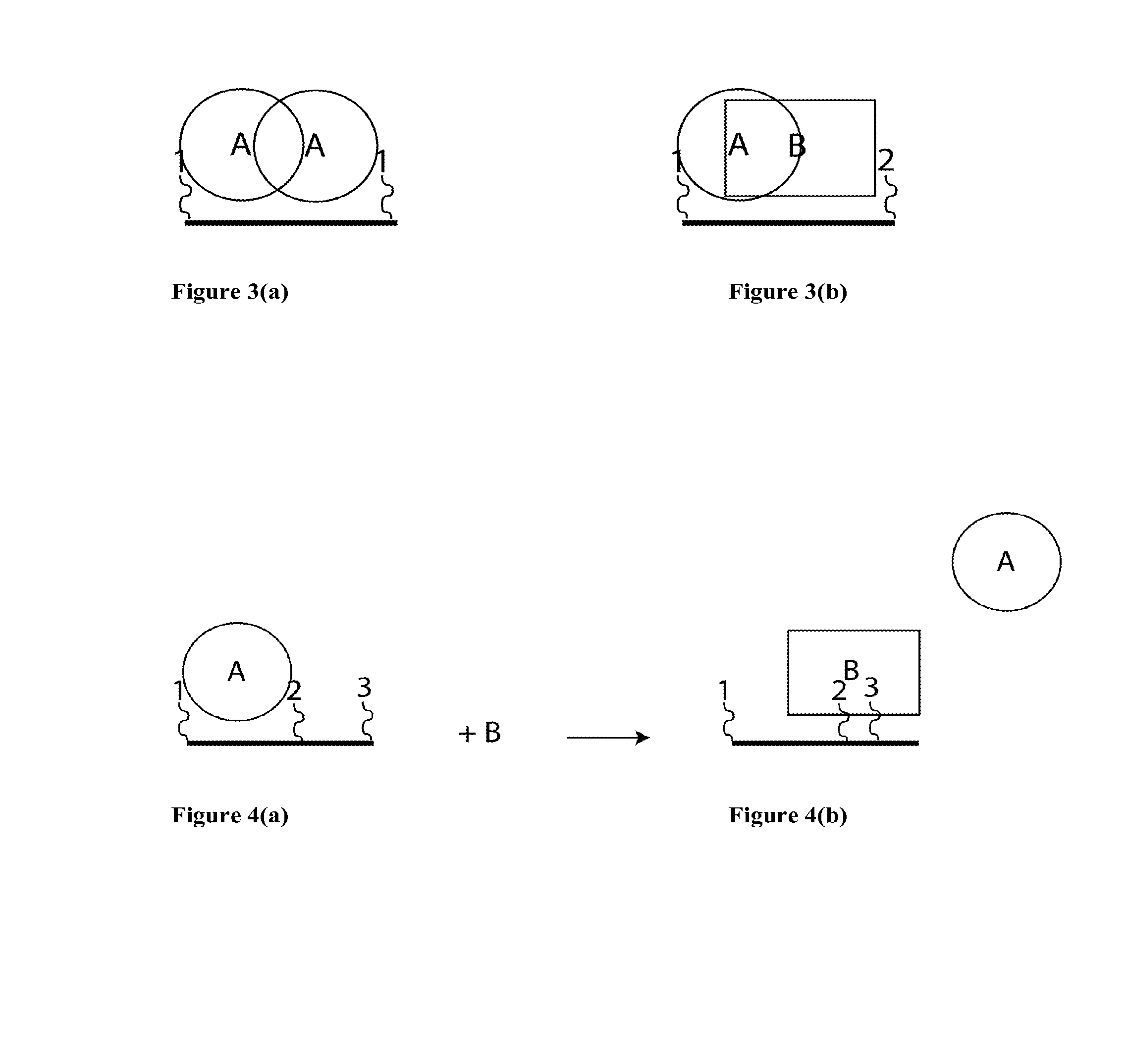
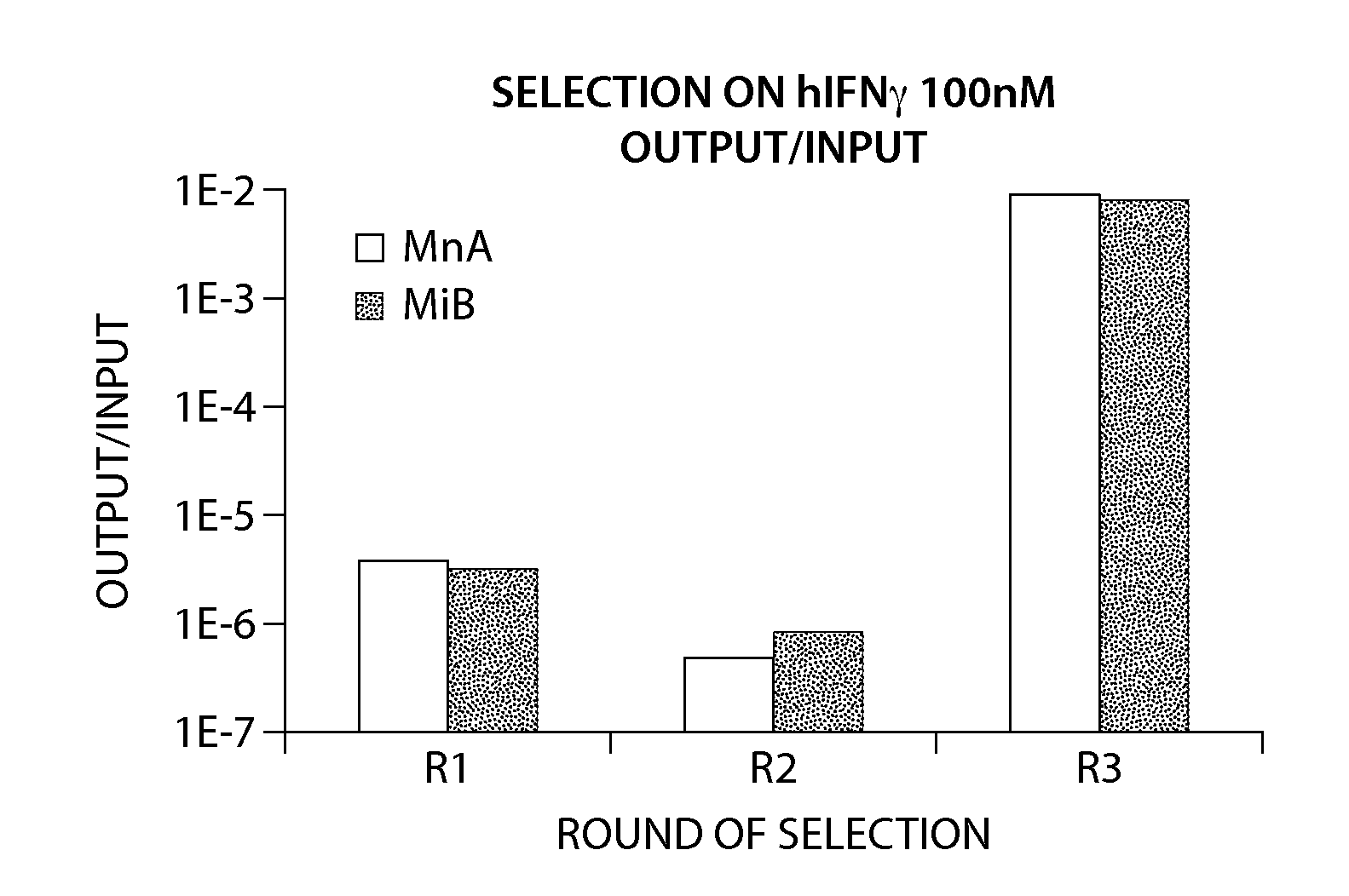
Synthetic antibodies are affinity reagents generated entirely in vitro, thus completely eliminating animals from the production process. Synthetic antibodies include recombinant antibodies, nucleic acid aptamers and non-immunoglobulin protein scaffolds. As a consequence of their in vitro manufacturing method the antigen recognition site of synthetic antibodies can be engineered to any desired target and may extend beyond the typical immune repertoire offered by natural antibodies. Synthetic antibodies are being developed for use in research, diagnostic and therapeutic applications. Synthetic antibodies can be used in all applications where traditional monoclonal or polyclonal antibodies are used and offer many inherent advantages over animal-derived antibodies, including comparatively low production costs, reagent reproducibility and increased affinity, specificity and stability across a range of experimental conditions.
Synthetic antibody phage libraries
InactiveUS20050079574A1High-quality target binding characteristicGenerate efficientlyAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeterologousIntravenous gammaglobulin
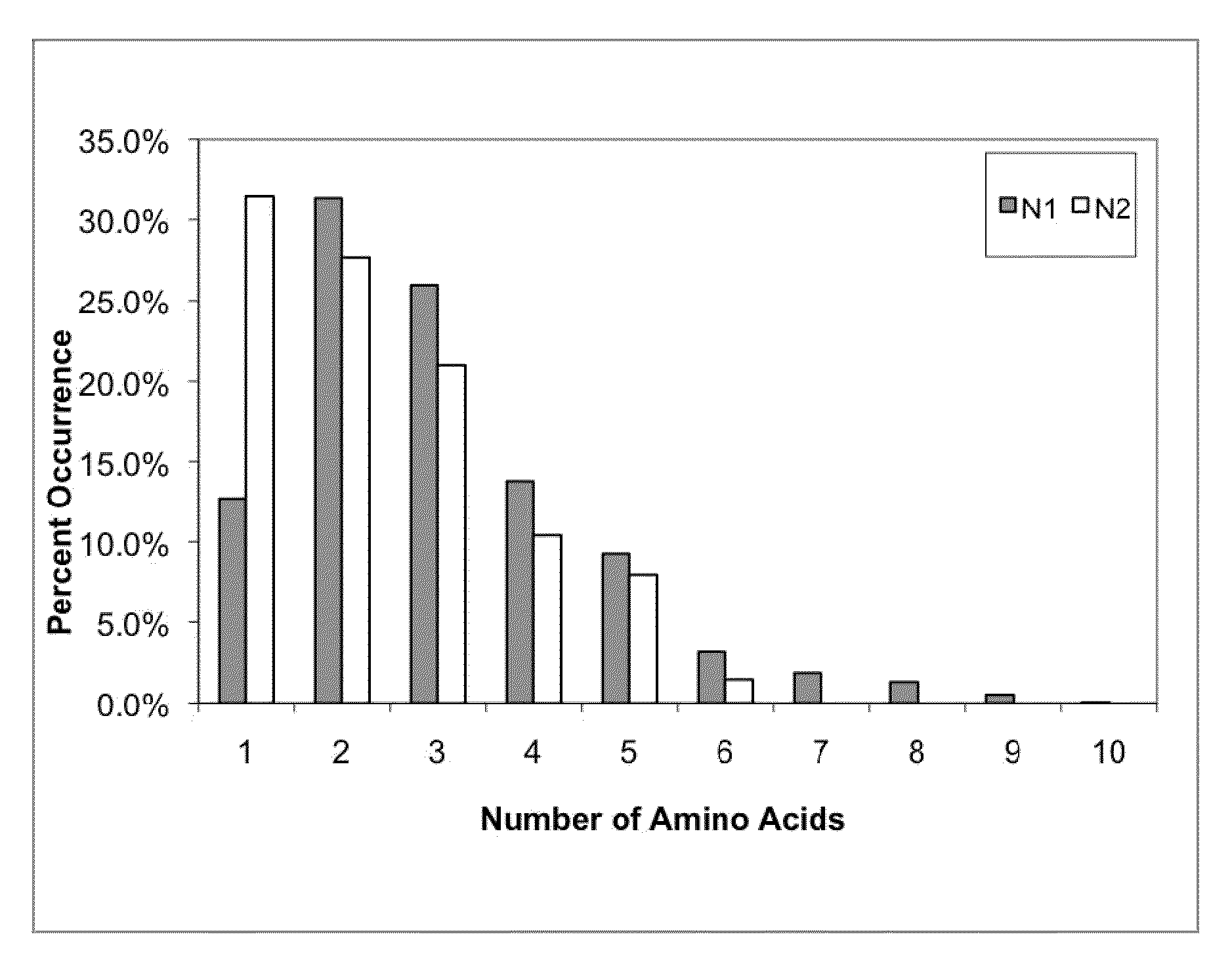
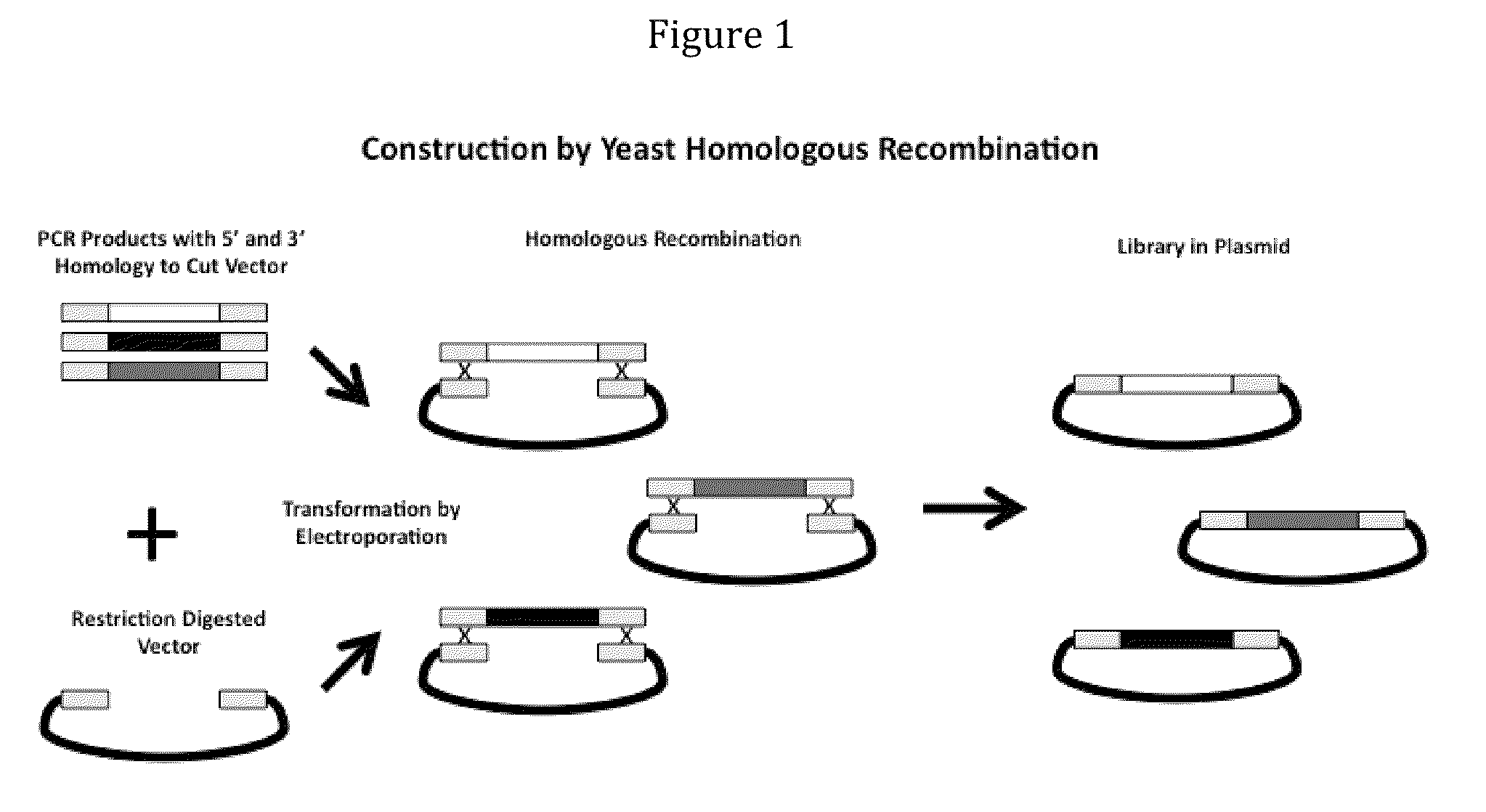
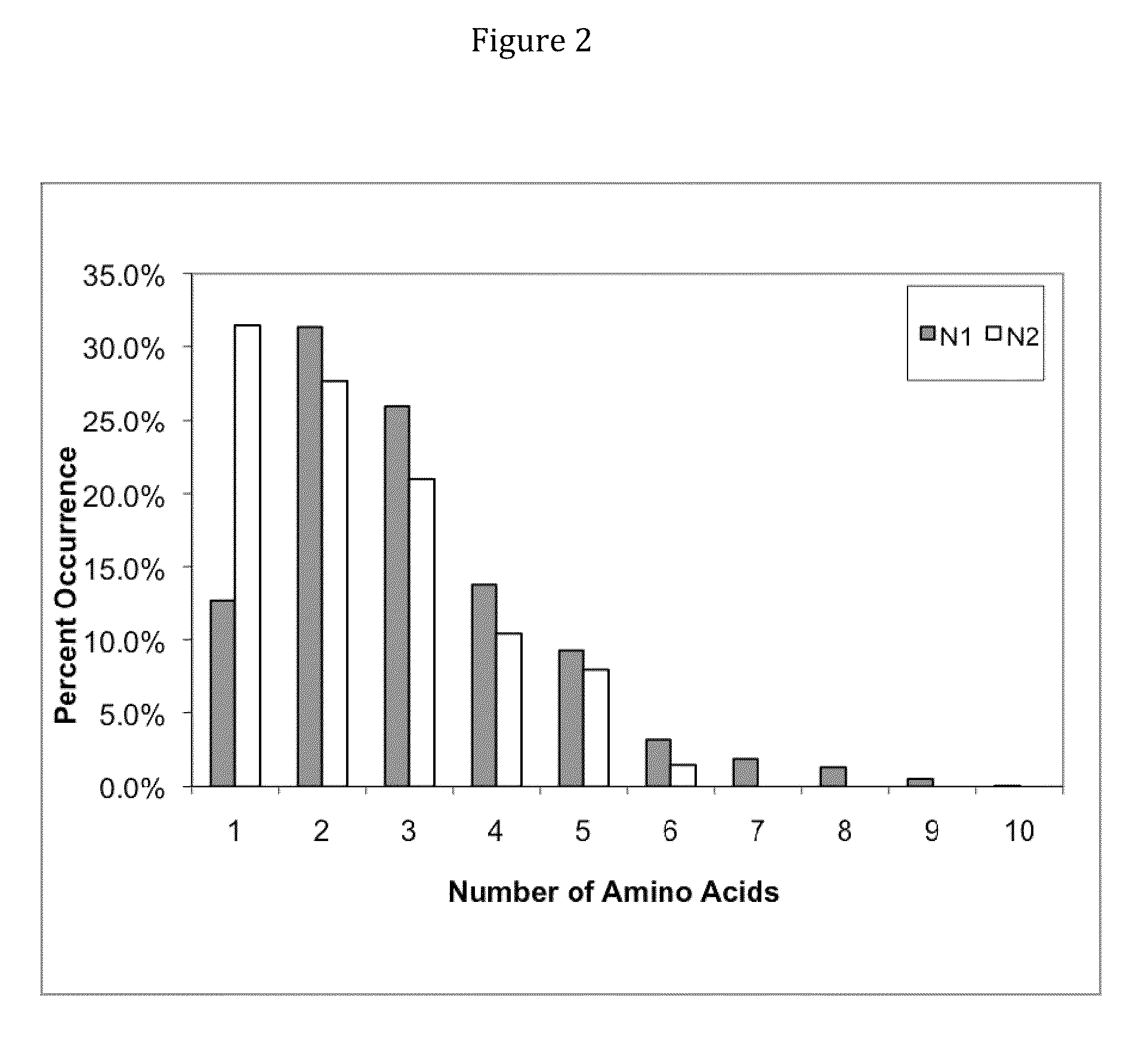
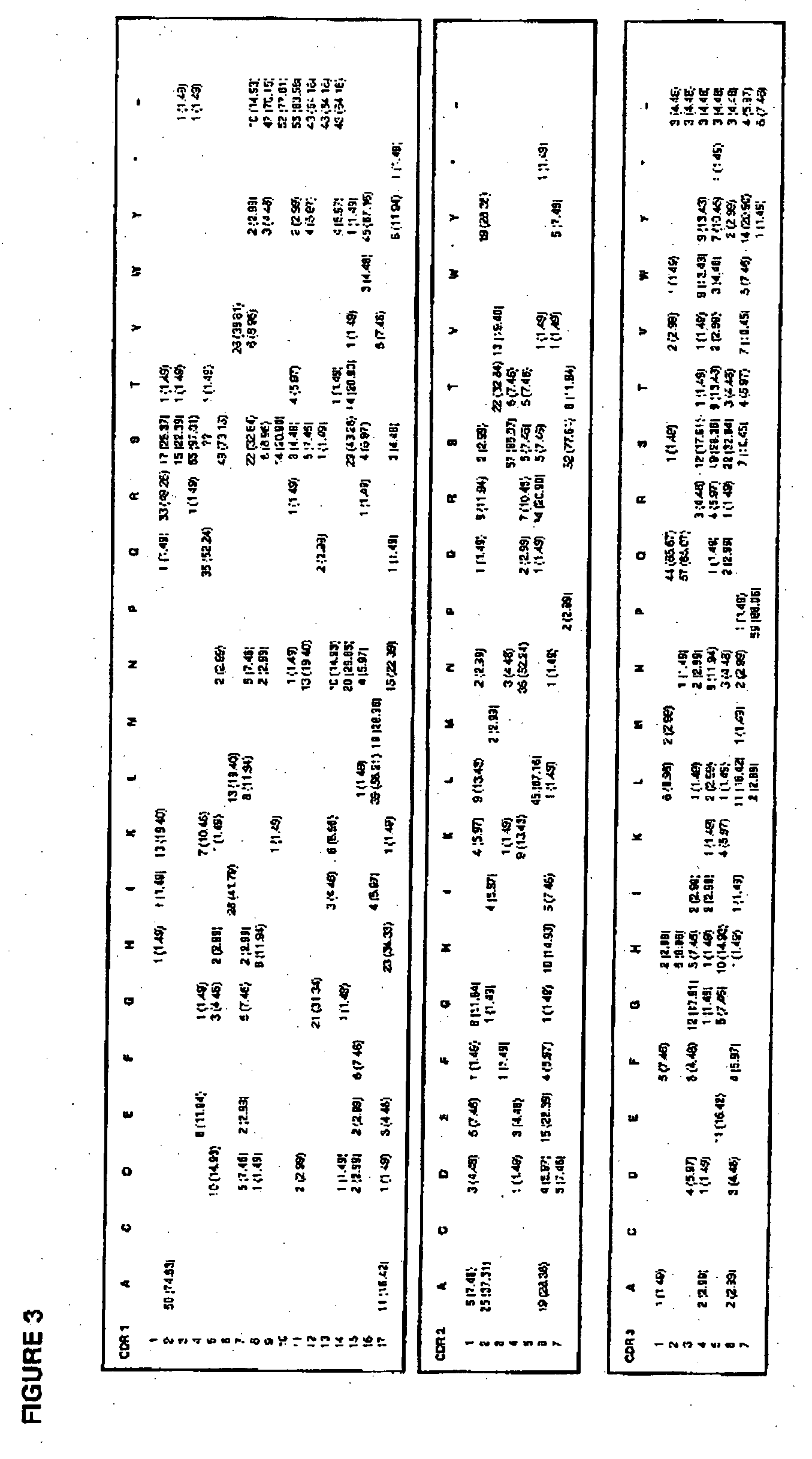
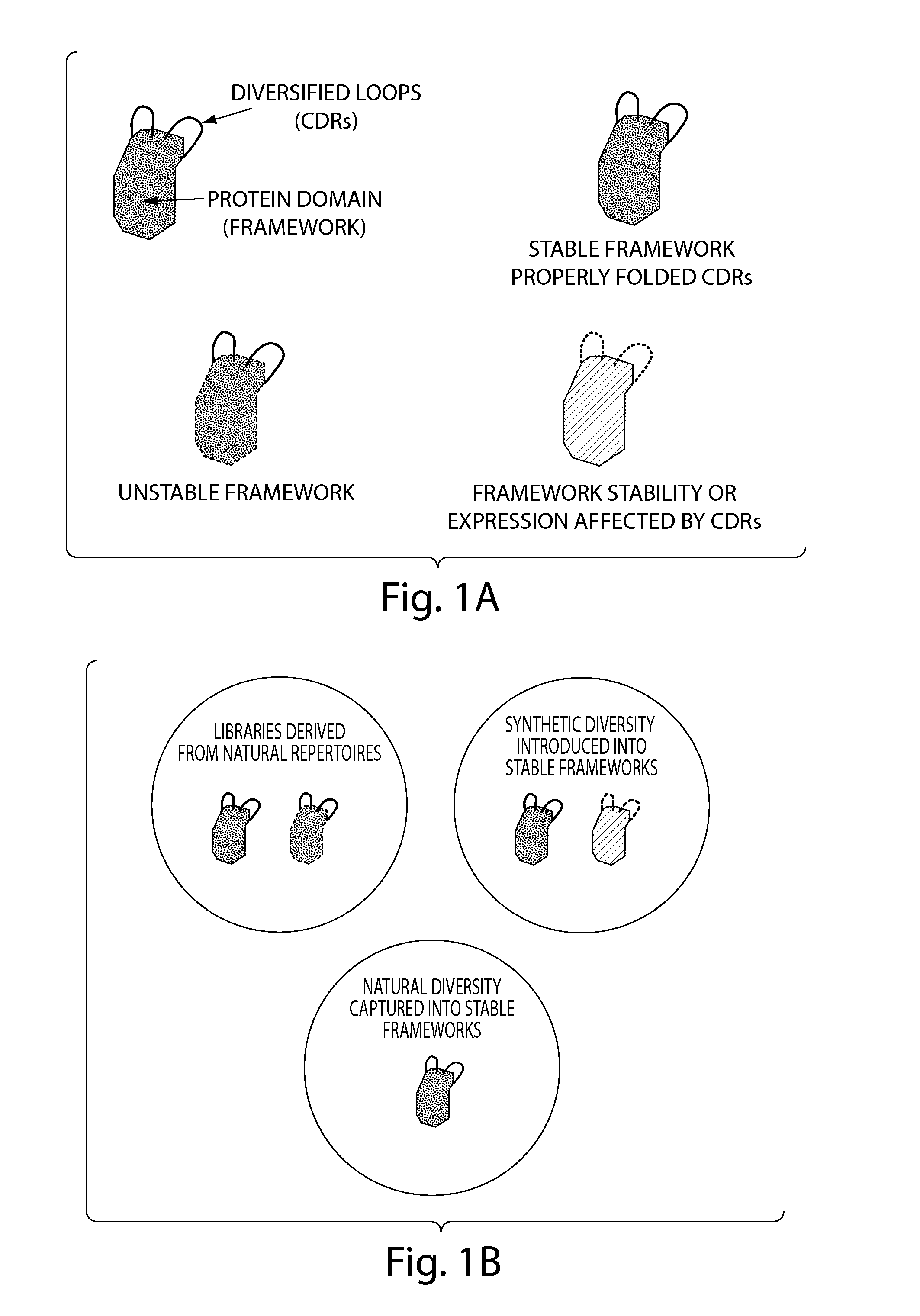
The invention provides immunoglobulin polypeptides comprising variant amino acids in CDRs of antibody variable domains. In one embodiment, the polypeptide is a variable domain of a monobody and has a variant CDRH3 region. These polypeptides provide a source of great sequence diversity that can be used as a source for identifying novel antigen binding polypeptides. The invention also provides these polypeptides as fusion polypeptides to heterologous polypeptides such as at least a portion of phage or viral coat proteins, tags and linkers. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
Antibody
InactiveUS20070274985A1Generate efficientlyEffective isolationAntibody mimetics/scaffoldsImmunoglobulins against animals/humansNatural antibodySingle-Chain Antibodies
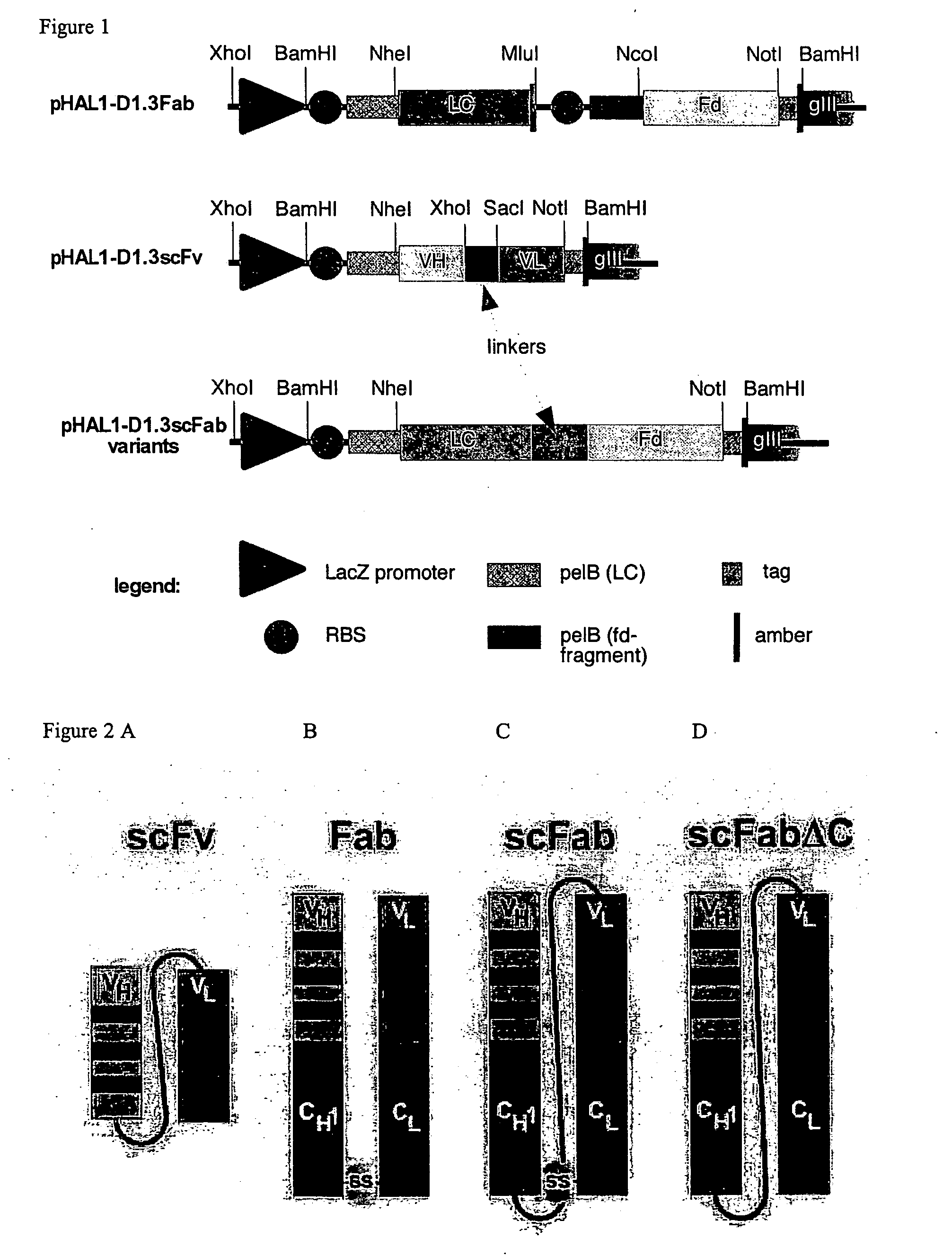
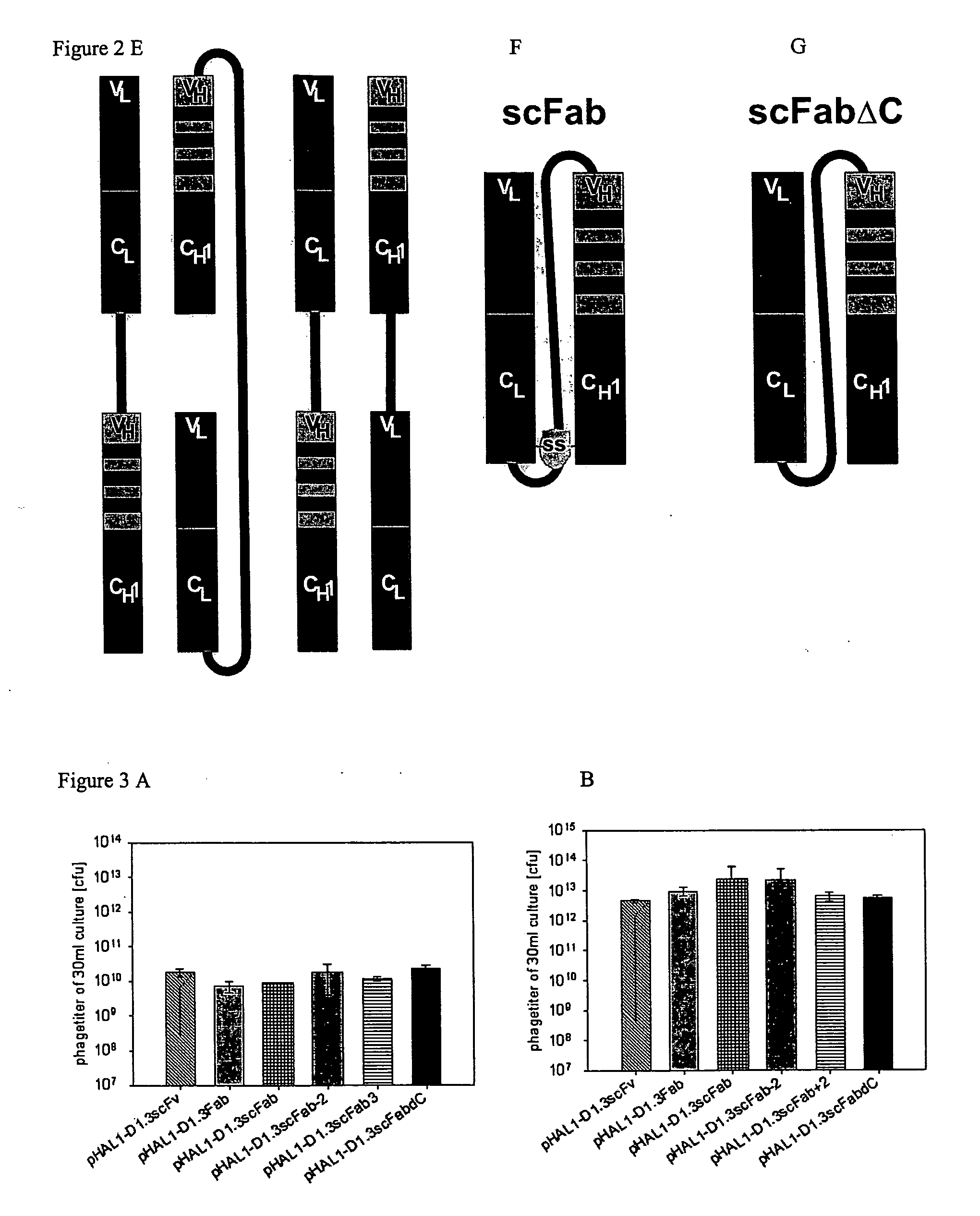
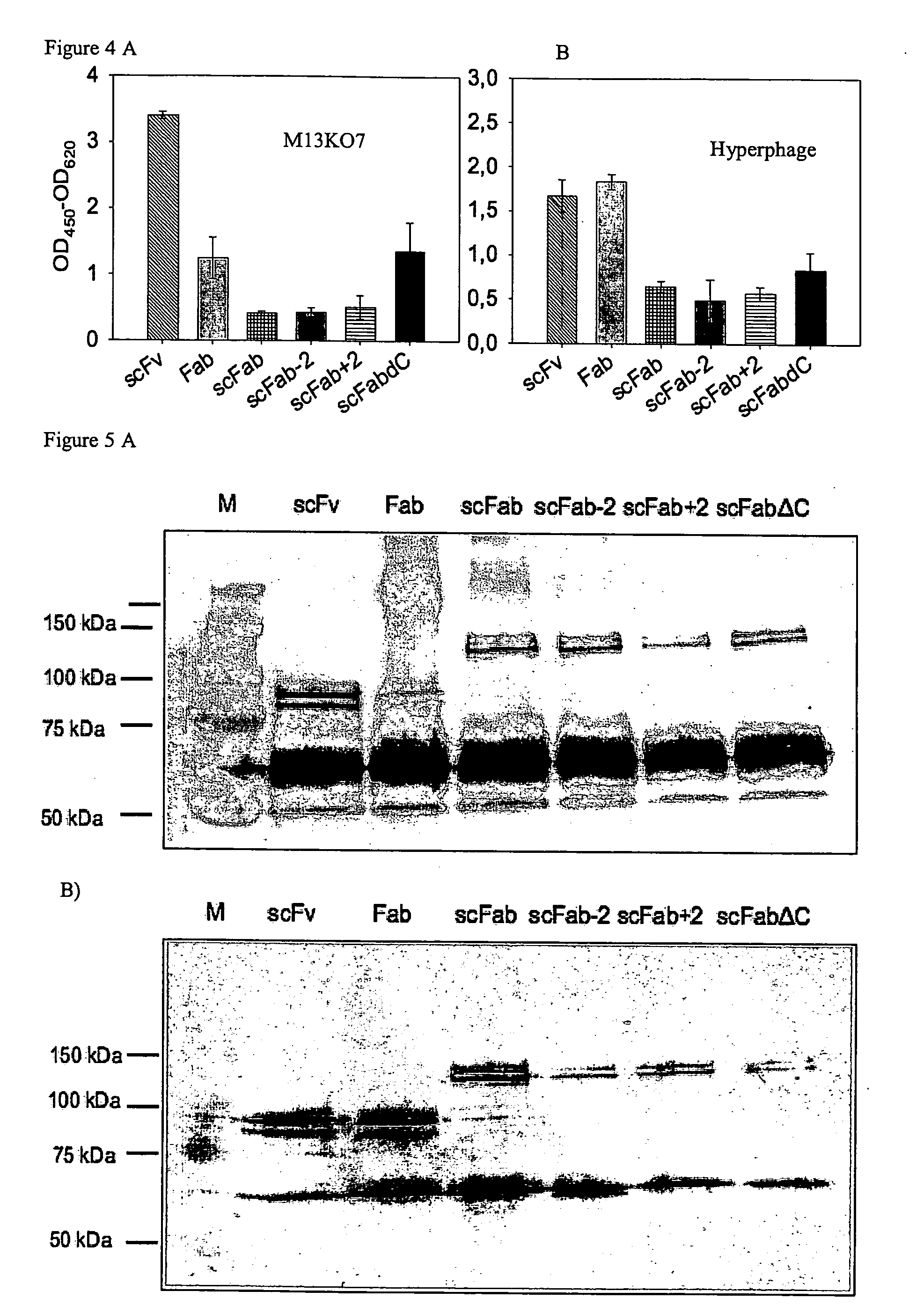
The present invention refers to synthetic antibody molecules which comprise domains from naturally occuring antibodies, e.g. domains derivable from IgG, preferably of human origin, in a novel arrangement. Single chain molecules are provided which are suitable for expression in micro-organisms in their active conformation, which single chain molecules generally comprise a VL domain, a CL domain, and a VH domain, a CH1 domain, linked by a linker arranged between VUCL and VH / CH1. Accordingly, these antibody molecules can be termed single chain Fabs (scFabs). These antibody molecules are single chain proteins, which can also be associated to dimers, including heteromeric antibodies, wherein at least two single chain antibody molecules are associated.
Owner:TECH UNIV BRAUNSCHWEIG
Rationally Designed, Synthetic Antibody Libraries and Uses Therefor
ActiveUS20090181855A1Peptide librariesImmunoglobulins against animals/humansPolynucleotideSynthetic antibody
The present invention overcomes the inadequacies inherent in the known methods for generating libraries of antibody-encoding polynucleotides by specifically designing the libraries with directed sequence and length diversity. The libraries are designed to reflect the preimmune repertoire naturally created by the human immune system and are based on rational design informed by examination of publicly available databases of human antibody sequences.
Owner:ADIMAB LLC
Synthetic antibody phage libraries
The invention provides comprising variant amino acids in CDRs of antibody variable domains. These polypeptides provide a source of great sequence diversity that can be used as a source for identifying novel antigen binding polypeptides. The invention also provides these polypeptides as fusion polypeptides to heterologous polypeptides such as at least a portion of phage or viral coat proteins, tags, and linkers. Libraries comprising a plurality of these polypeptides are also provided. In addition, methods of and compositions for generating and using these polypeptides and libraries are provided.
Owner:GENENTECH INC
Rationally designed, synthetic antibody libraries and uses therefor
ActiveUS8691730B2Peptide librariesImmunoglobulins against animals/humansPolynucleotideSynthetic antibody
The present invention overcomes the inadequacies inherent in the known methods for generating libraries of antibody-encoding polynucleotides by specifically designing the libraries with directed sequence and length diversity. The libraries are designed to reflect the preimmune repertoire naturally created by the human immune system and are based on rational design informed by examination of publicly available databases of human antibody sequences.
Owner:ADIMAB LLC
Antibodies against non functional p2x7 receptor
InactiveUS20100036101A1Immunoglobulins against cell receptors/antigens/surface-determinantsFermentationComplementarity determining regionBinding site
A recombinant or synthetic antibody or fragment thereof, said antibody or fragment thereof including three complementarity determining regions (CDR1L or H, CDR2L or H and CDR3L or H) for forming an antigen binding site that is capable of binding to a non functional P2X7 receptor but not capable of binding to a functional P2X7 receptor.
Owner:BIOSCEPTRE INT
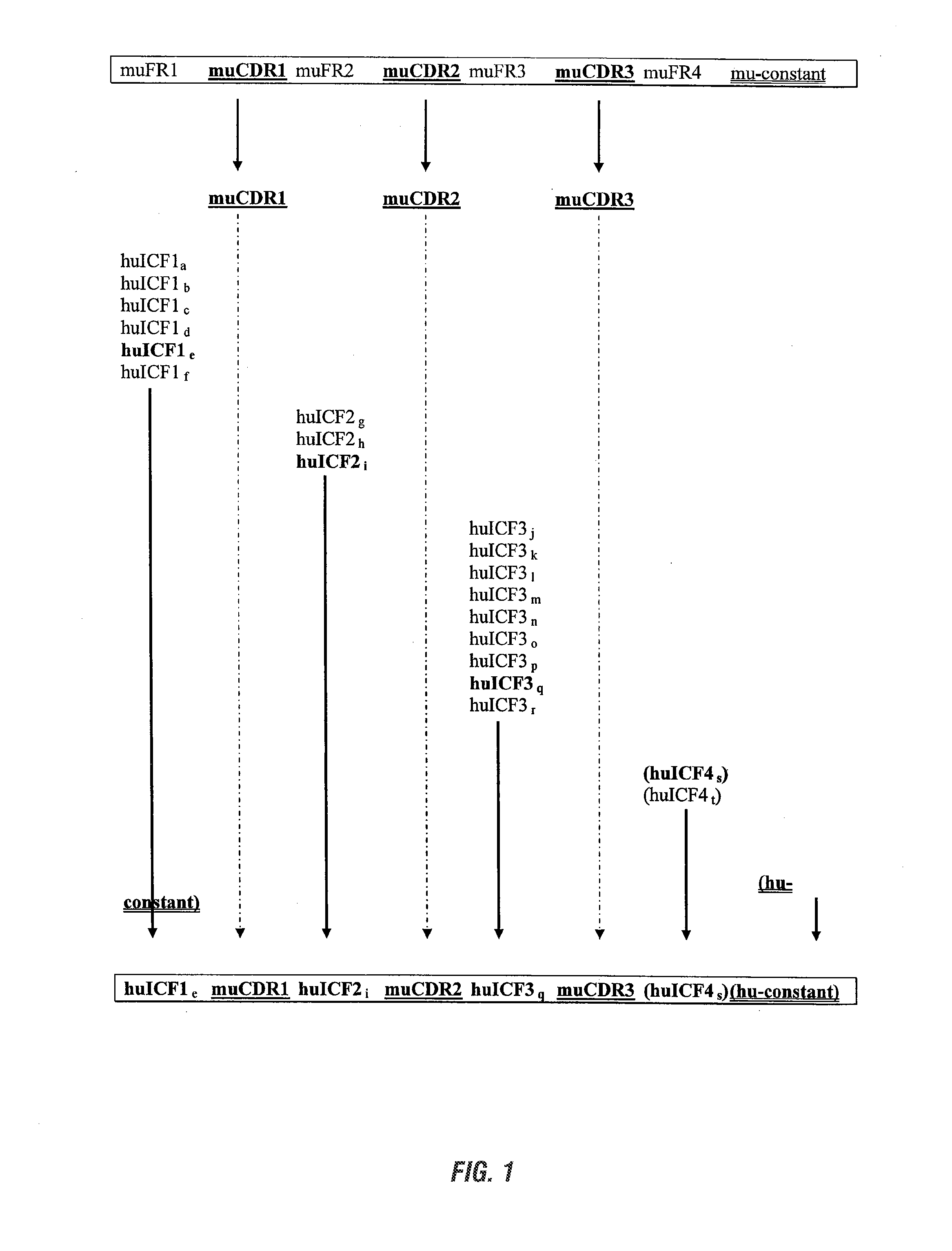
Antibodies and methods for making and using them
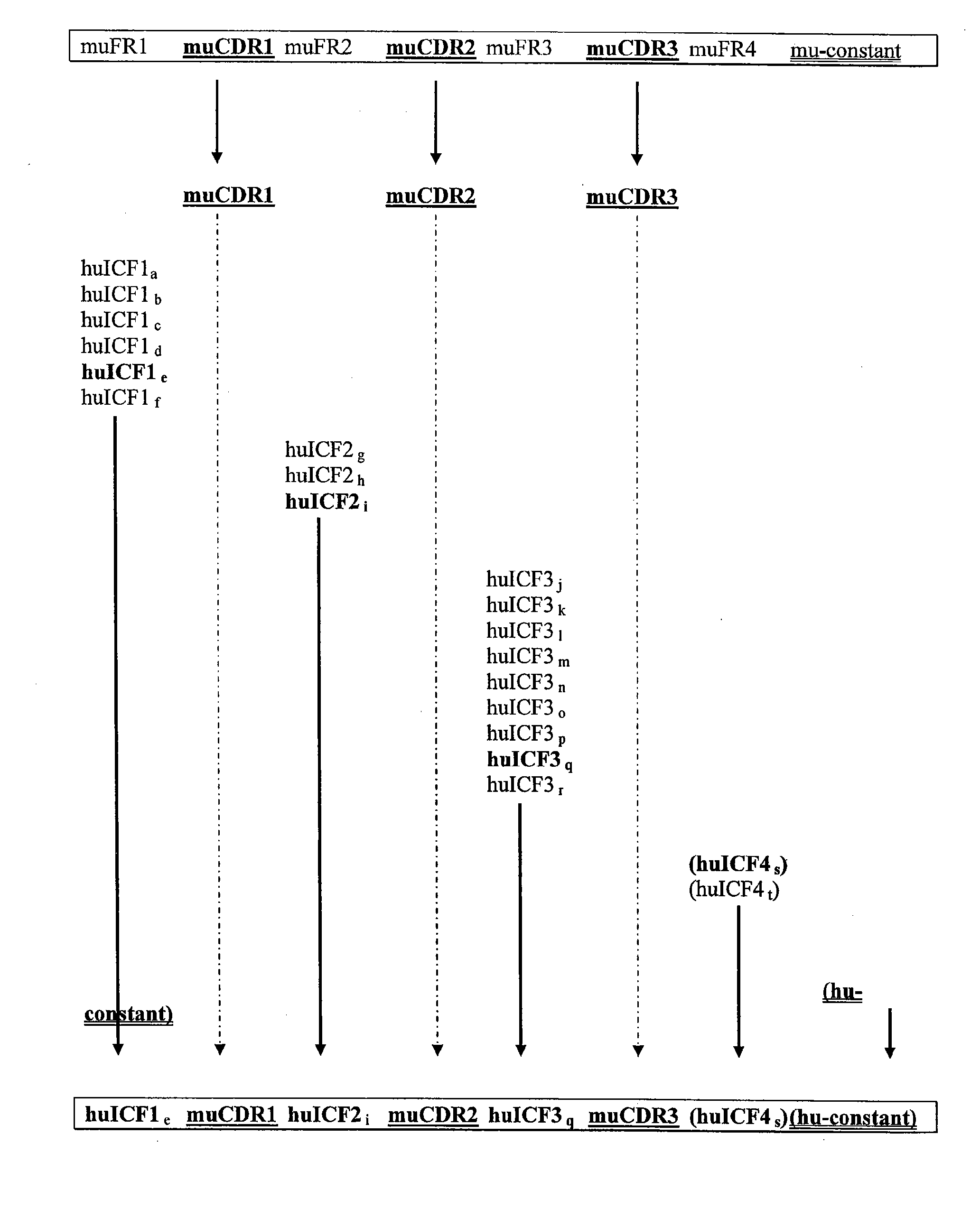
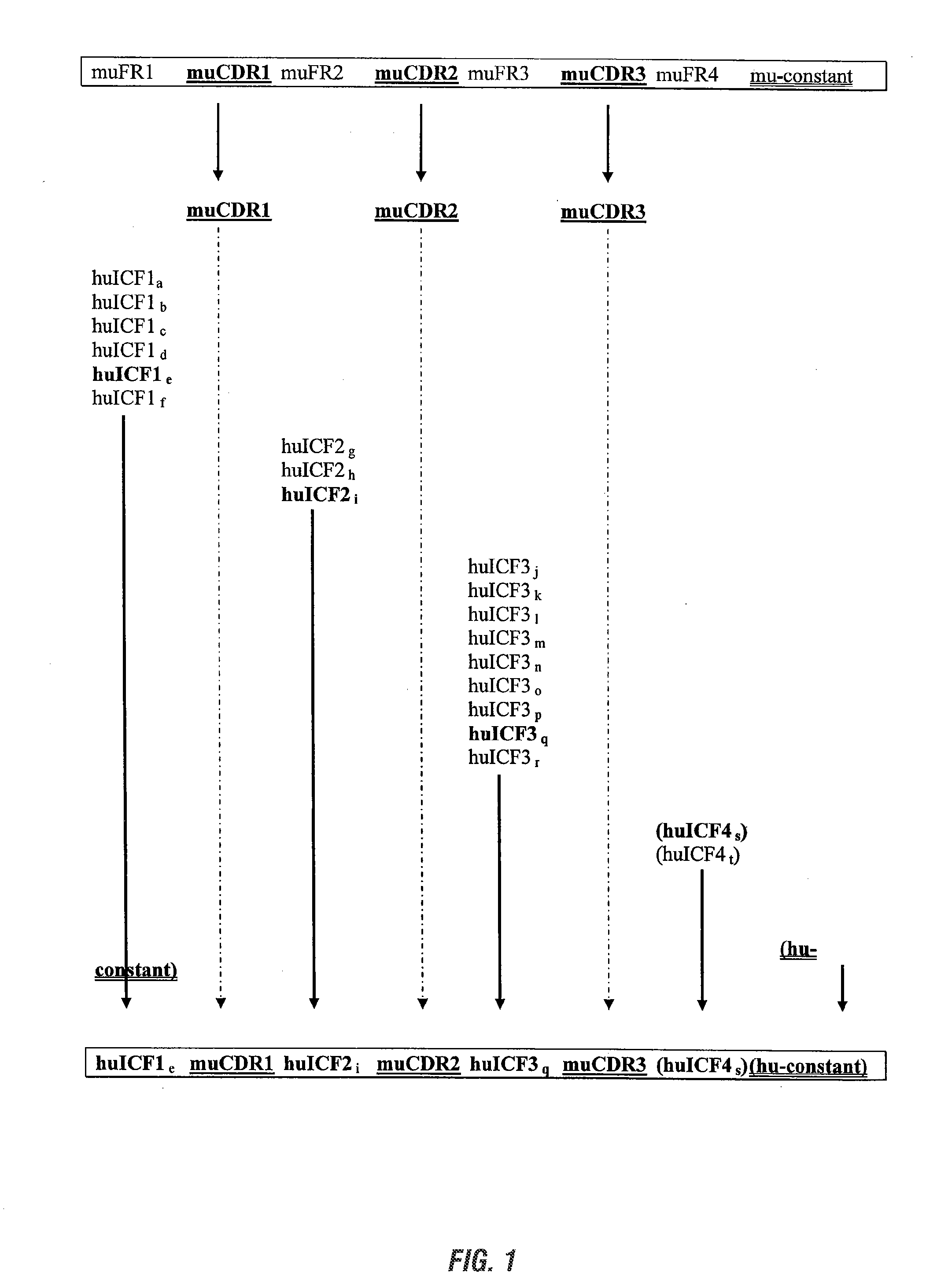
InactiveUS20090285813A1Low immunogenicityNervous disorderAntipyreticSynthetic antibodyFramework region
The invention provides antibodies, including chimeric human antibodies, recombinant antibodies, synthetic anti-bodies, and the nucleic acids encoding them, and methods for making and using these immunoglobulins. The invention provides recombinant and synthetic polypeptide and nucleic acid embodiments of these polypeptides and / or antibodies. The invention also provides polypeptides comprising, or consisting of, consensus human framework regions, or “Independently Consensused Frameworks (ICFs)”, nucleic acids encoding them, and libraries and kits comprising these ICFs and / or antibodies of the invention, individually and in combinatorial libraries and combinations.
Owner:MMRGLOBAL
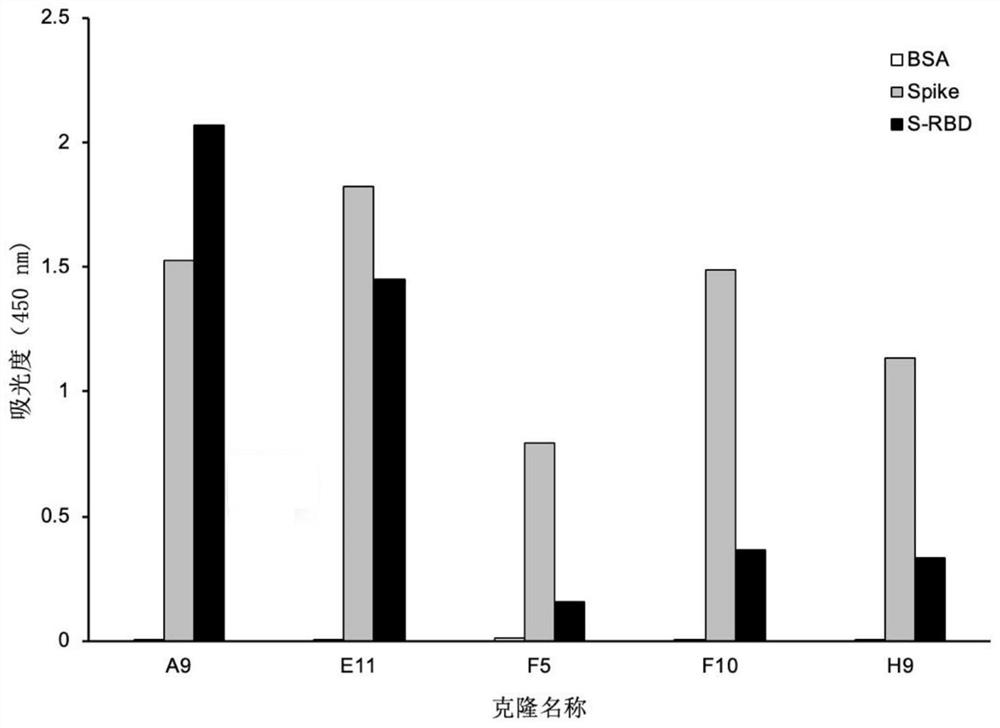
Phage display antibody library and monoclonal antibodies aiming at novel coronavirus SARS-CoV-2 and obtained by panning based on same
ActiveCN111778218ANeutralizes contagionMicroorganism based processesImmunoglobulins against virusesEscherichia coliMonoclonal antibody agent
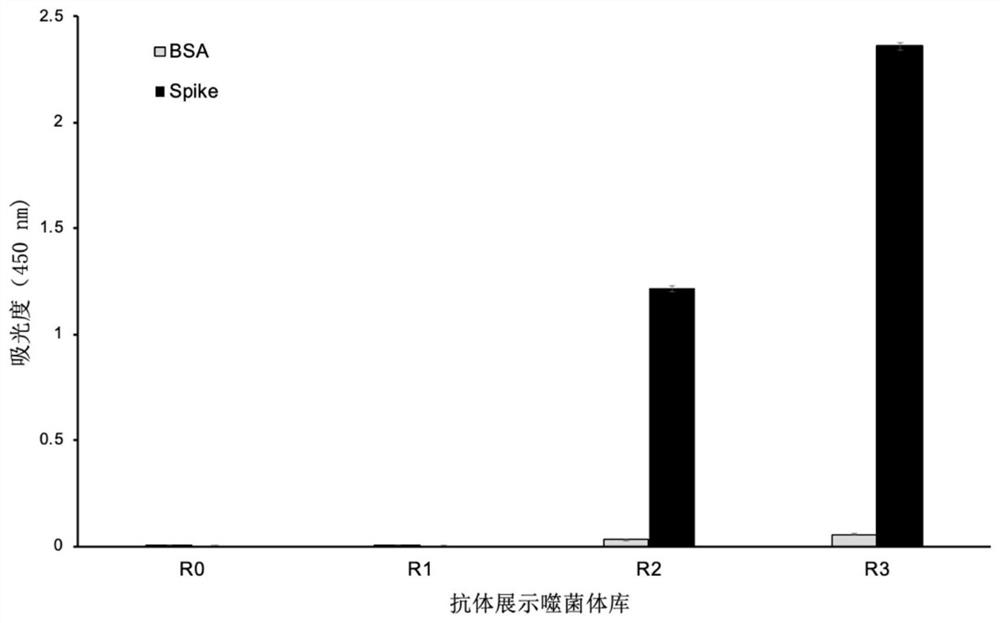
The invention discloses a phage display antibody library and five strains of screened antibodies capable of being combined with S protein of novel coronavirus SARS-CoV-2. Mutation is introduced into an ultra-variable region of an antibody variable region based on synthetic biology and a phage display technology, and a gene is transferred into escherichia coli, so that a synthetic antibody librarycontaining 108 kinds of antibodies is constructed; the phage display antibody library provided by the invention can perform screening to obtain the antibody with the specificity and the detection function, so that powerful resources of biological research and medical diagnosis are expanded; and the five strains of antibodies capable of being combined with the S protein of the novel coronavirus arefurther screened out and can be used for detecting the virus, part of the antibodies can block combination of the virus and cells, and the antibodies have the capacity of neutralizing novel coronavirus infectivity, can be used for preparing a novel coronavirus detection product, preparing a drug for inhibiting the novel coronavirus and preparing a pharmaceutical preparation for preventing or treating diseases caused by the novel coronavirus, and have a wide application prospect.
Owner:山东宽和正生物医药有限公司
Synthetic Antibodies
Owner:ARIZONA STATE UNIVERSITY
Method for screening single chain antibodies of Microcystin-LR and verification thereof
InactiveCN101857866AEasy to filterEasy to identifyImmunoglobulins against fungi/algae/lichensBiological testingEscherichia coliEnzyme digestion
The invention relates to a method for screening single chain antibodies of Microcystin-LR and verification thereof. The method comprises the following steps: carrying out two rounds of affinity screening on the biotinylated Microcystin-LR in a human source synthetic antibody library by using an avidin labeled magnetic bead and a negative screening method; extracting total plasmid DNA from phage colonies produced in the second round, carrying out enzyme digestion with enzyme Sfi I, recycling gel to obtain single chain antibody genes, connecting the single chain antibody genes with soluble expression carrier pAK100CL which is processed by enzyme digestion in the same way, and electrically transforming the connected carrier into colibacillus XL1-Blue to obtain soluble expression single chain antibodies; and verifying the soluble expression single chain antibodies by using a competitive time-resolved fluorescence immune analytical method. The invention has the advantage of quick, simple and convenient screening, and can well expose the Microcystin-LR three-dimensional structure into the incubation system. The verification on the screening result has the advantages of high detection signal and strong anti-interference capacity against stroma.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Synthetic antibodies
InactiveUS20120021967A1Peptide/protein ingredientsLibrary screeningAntiendomysial antibodiesBiochemistry
Owner:ARIZONA STATE UNIVERSITY
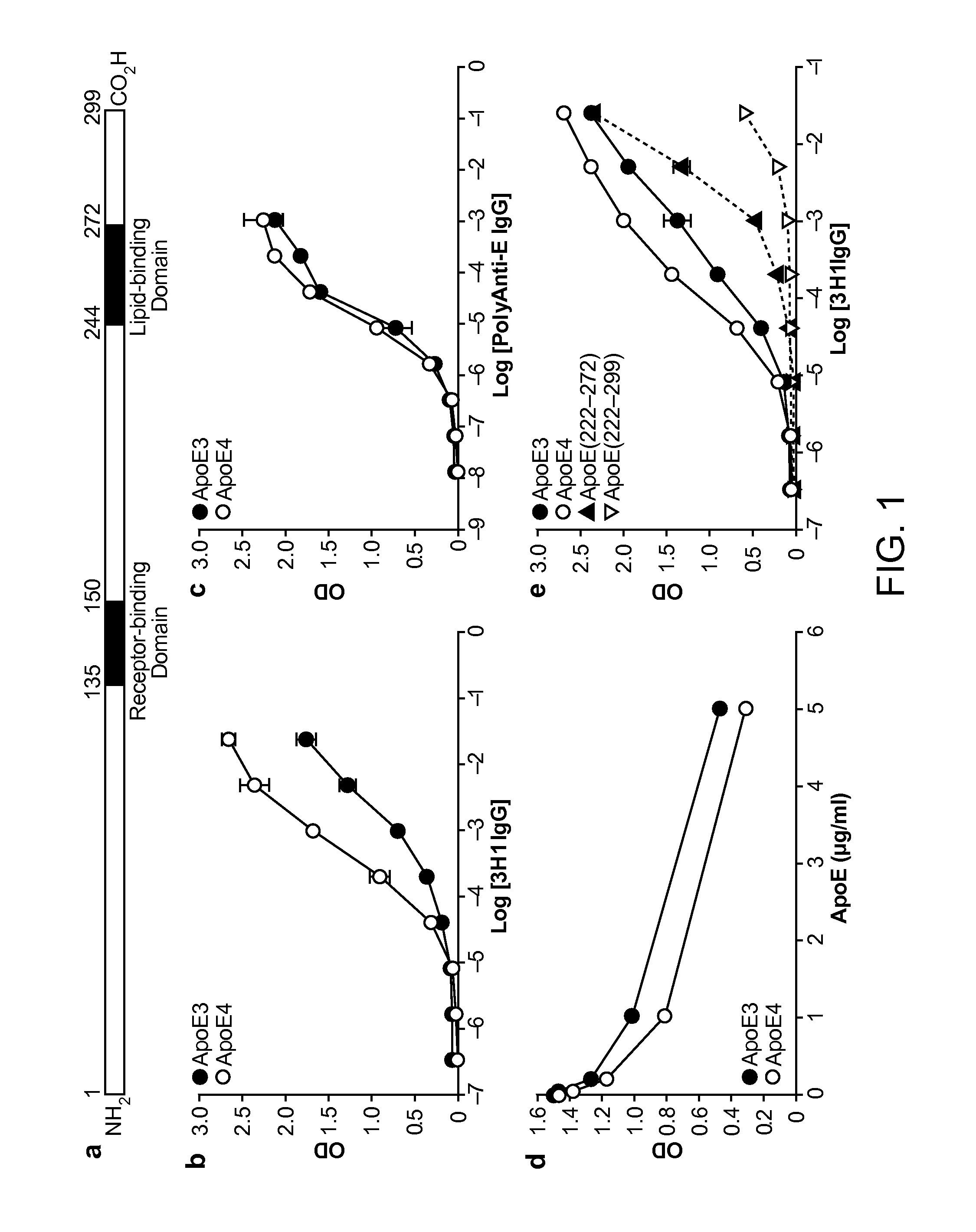
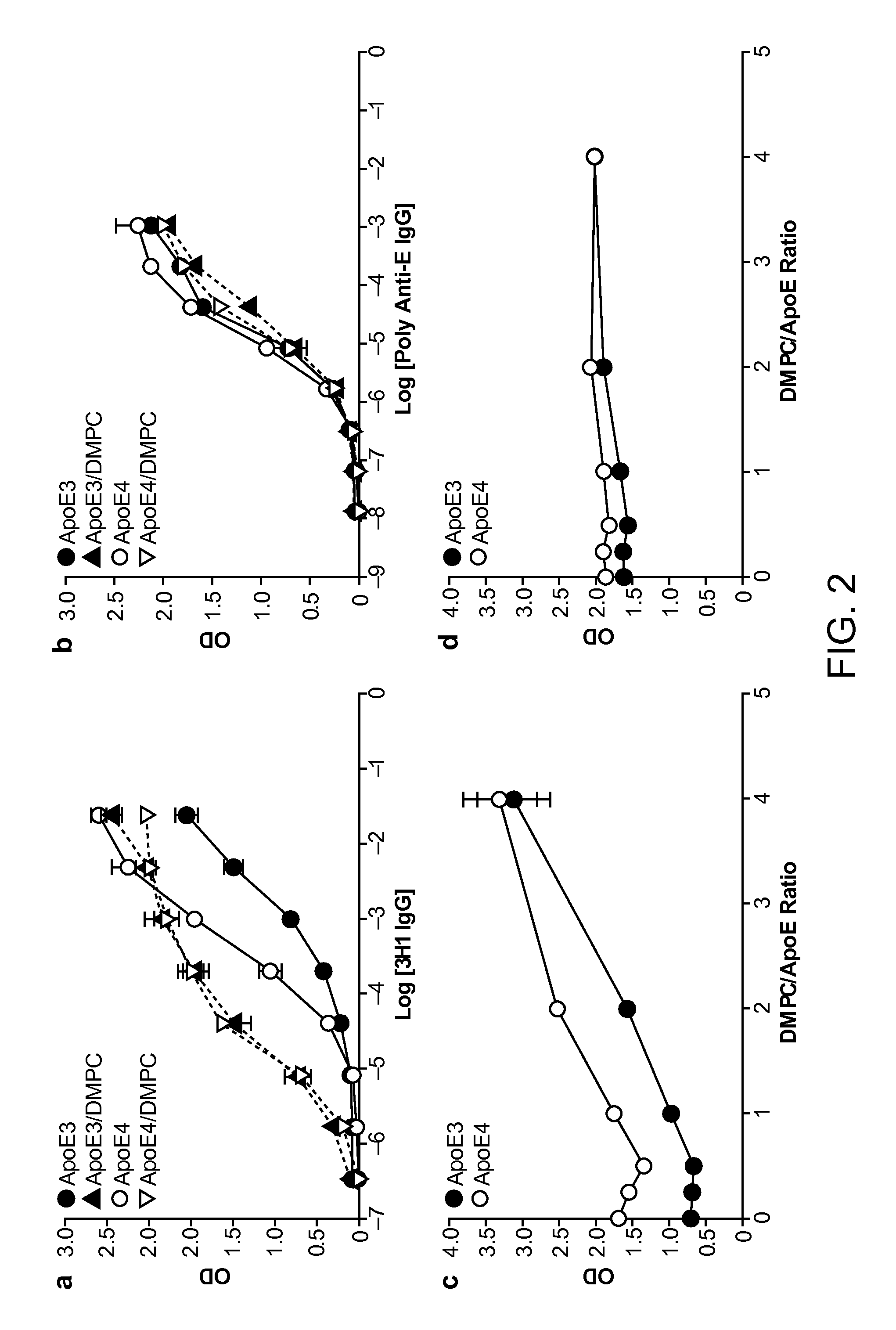
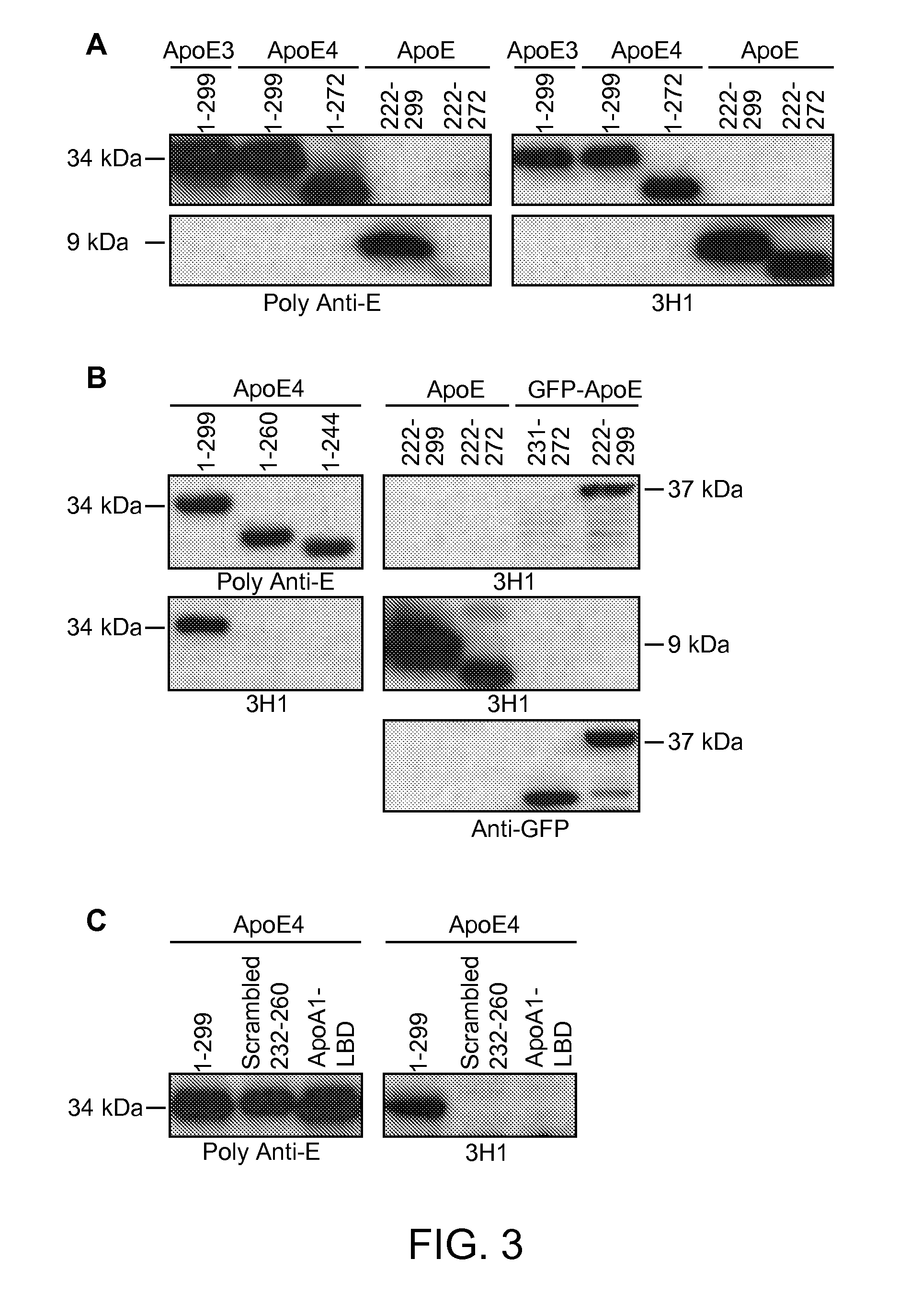
Antibody Specific for Apolipoprotein and Methods of Use Thereof
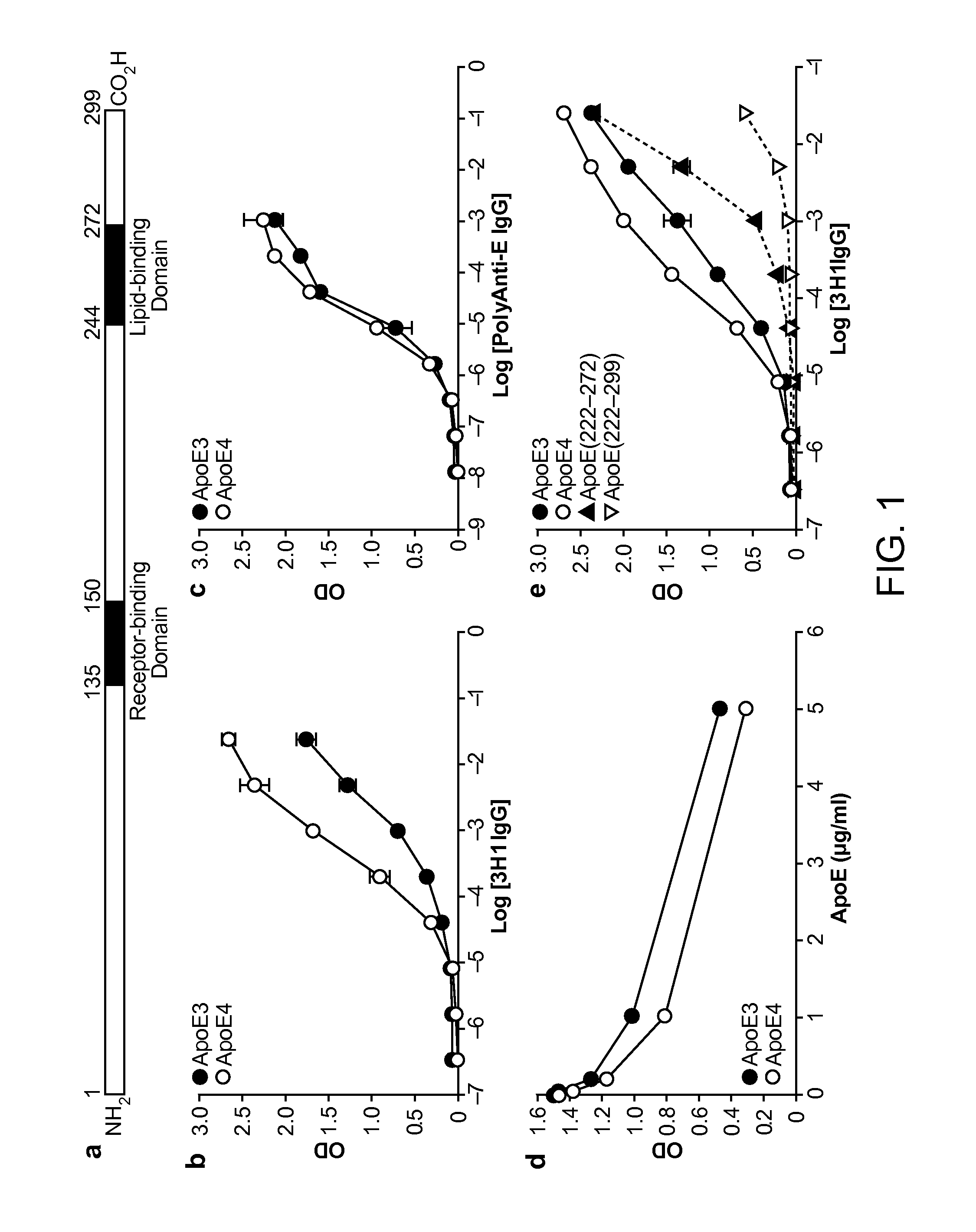
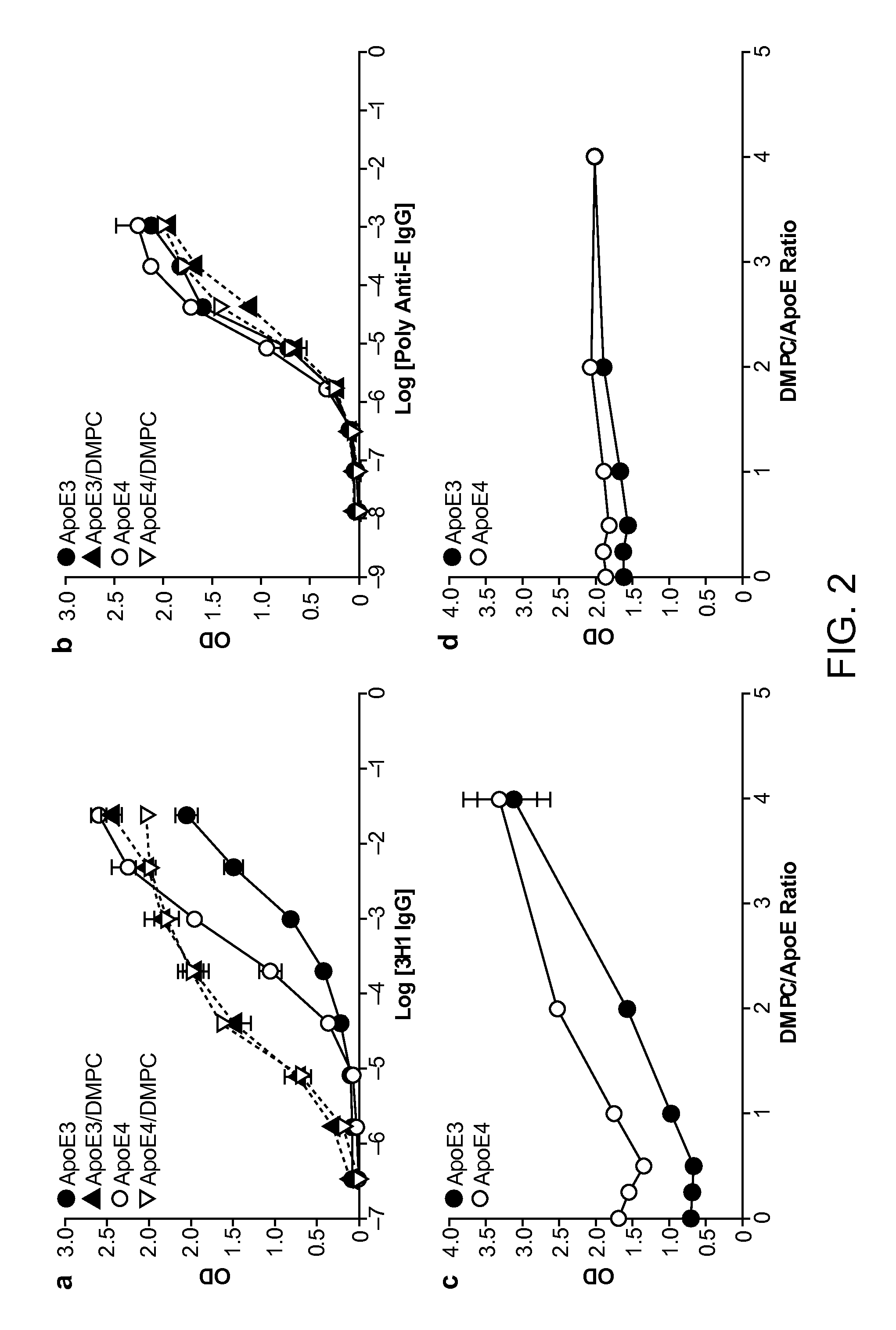
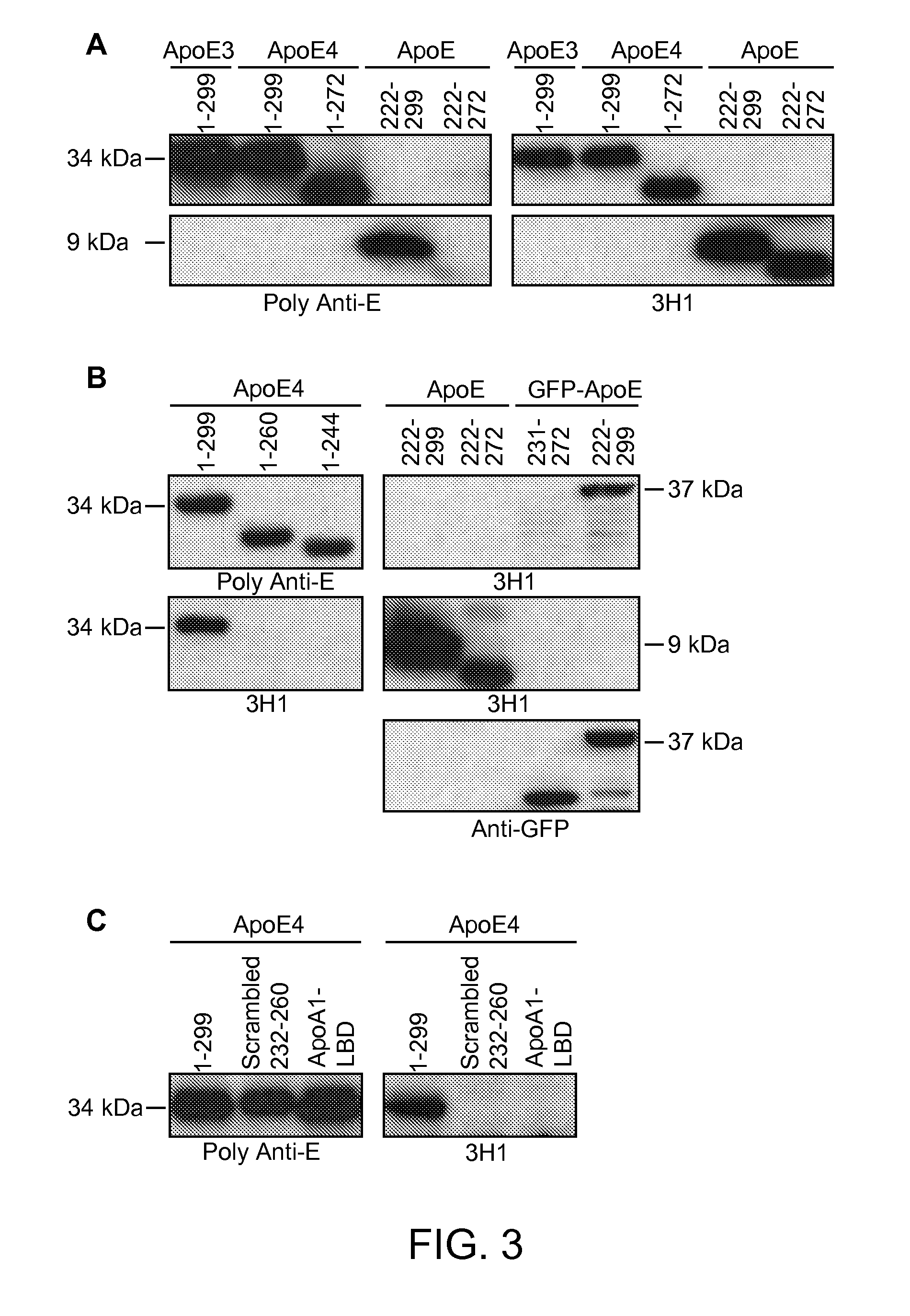
The present disclosure provides synthetic antibodies specific for an epitope present on an apolipoprotein E polypeptide. The antibodies are useful in various treatment, diagnostic, and monitoring applications, which are also provided.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Antibodies and methods for making and using them
The invention provides antibodies, including chimeric human antibodies, recombinant antibodies, synthetic antibodies, and the nucleic acids encoding them, and methods for making and using these immunoglobulins. The invention provides recombinant and synthetic polypeptide and nucleic acid embodiments of these polypeptides and / or antibodies. The invention also provides polypeptides comprising, or consisting of, consensus human framework regions, or “Independently Consensused Frameworks (ICFs)”, nucleic acids encoding them, and libraries and kits comprising these ICFs and / or antibodies of the invention, individually and in combinatorial libraries and combinations.
Owner:MMRGLOBAL
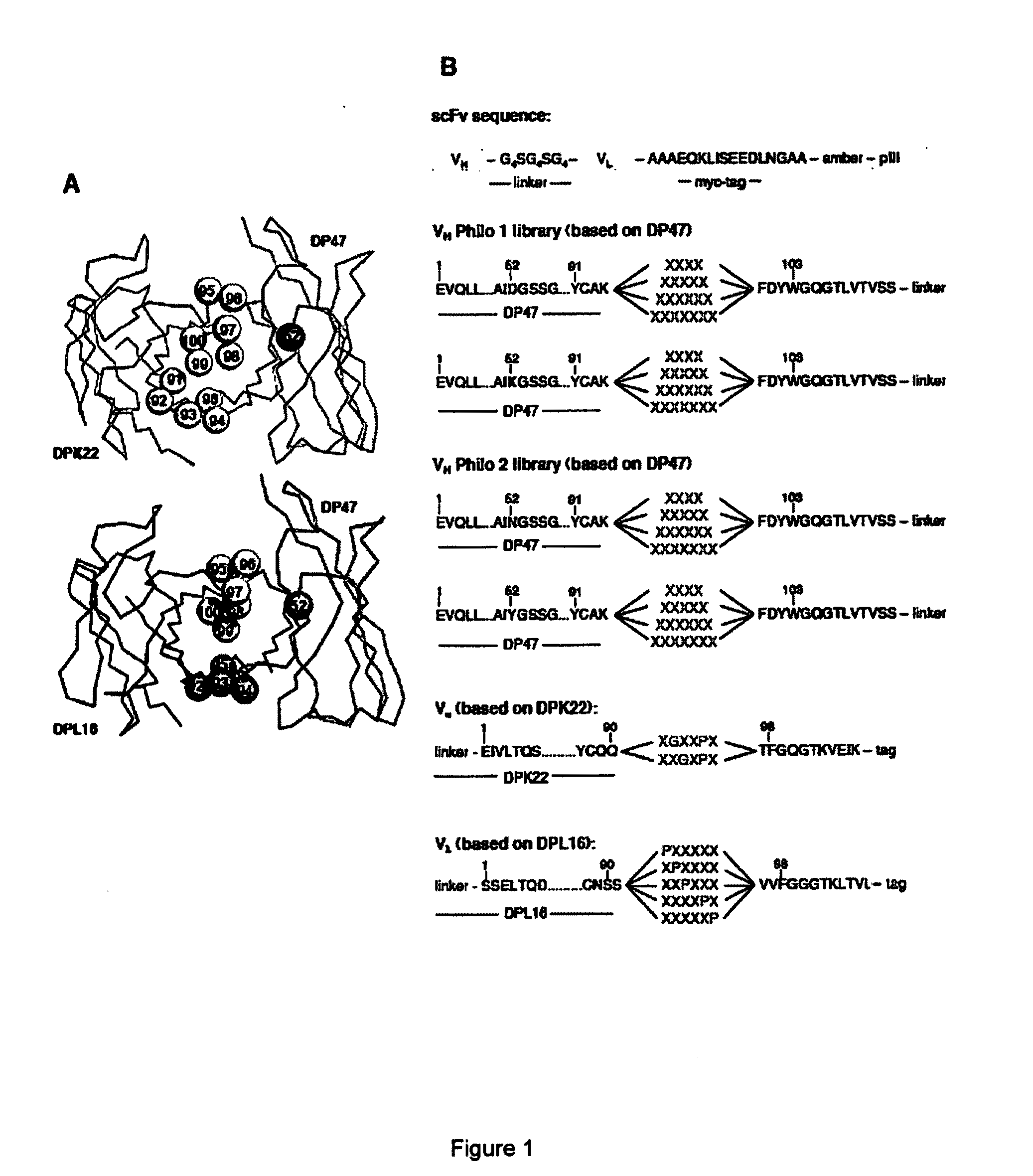
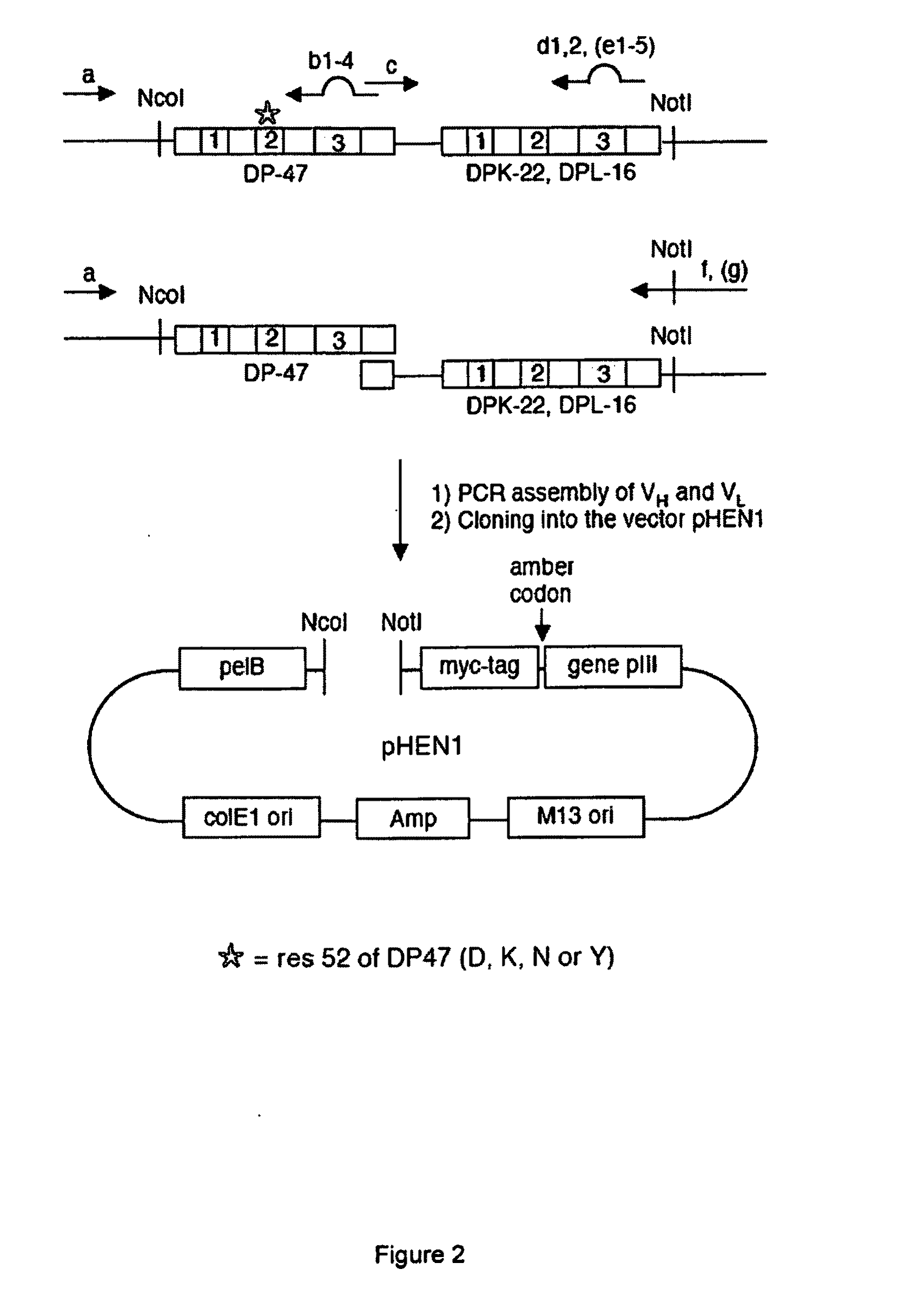
Display Library for Antibody Selection
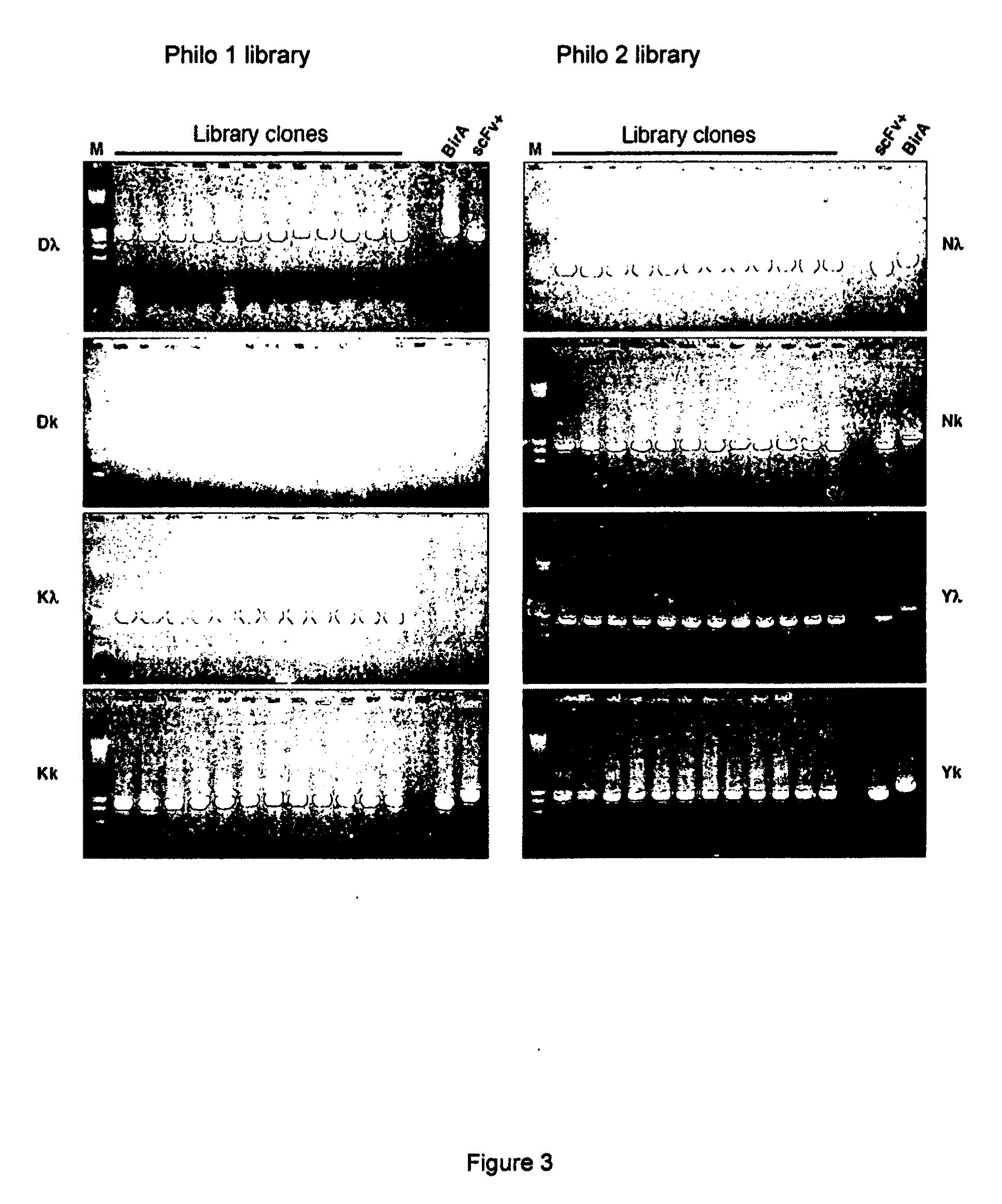
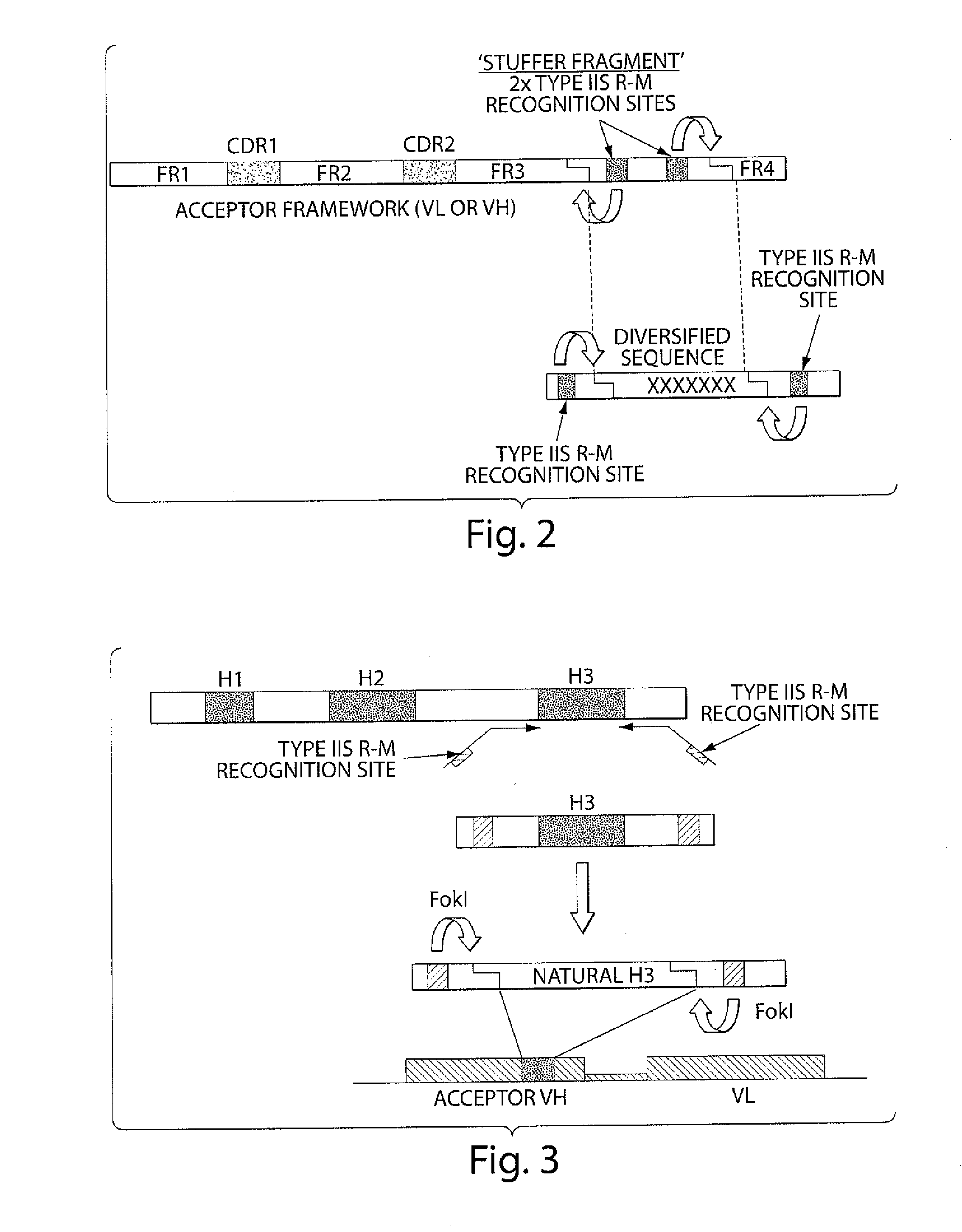
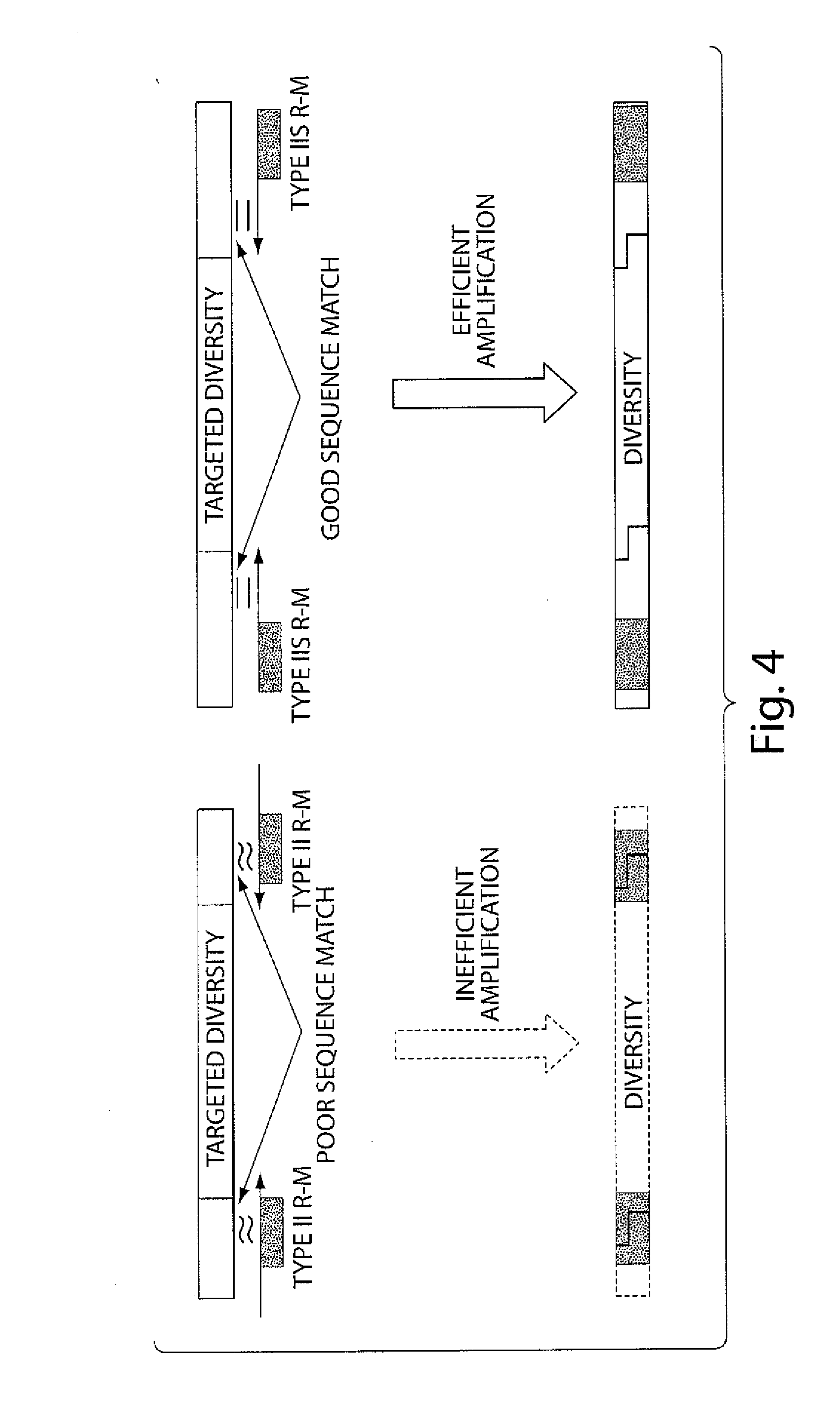
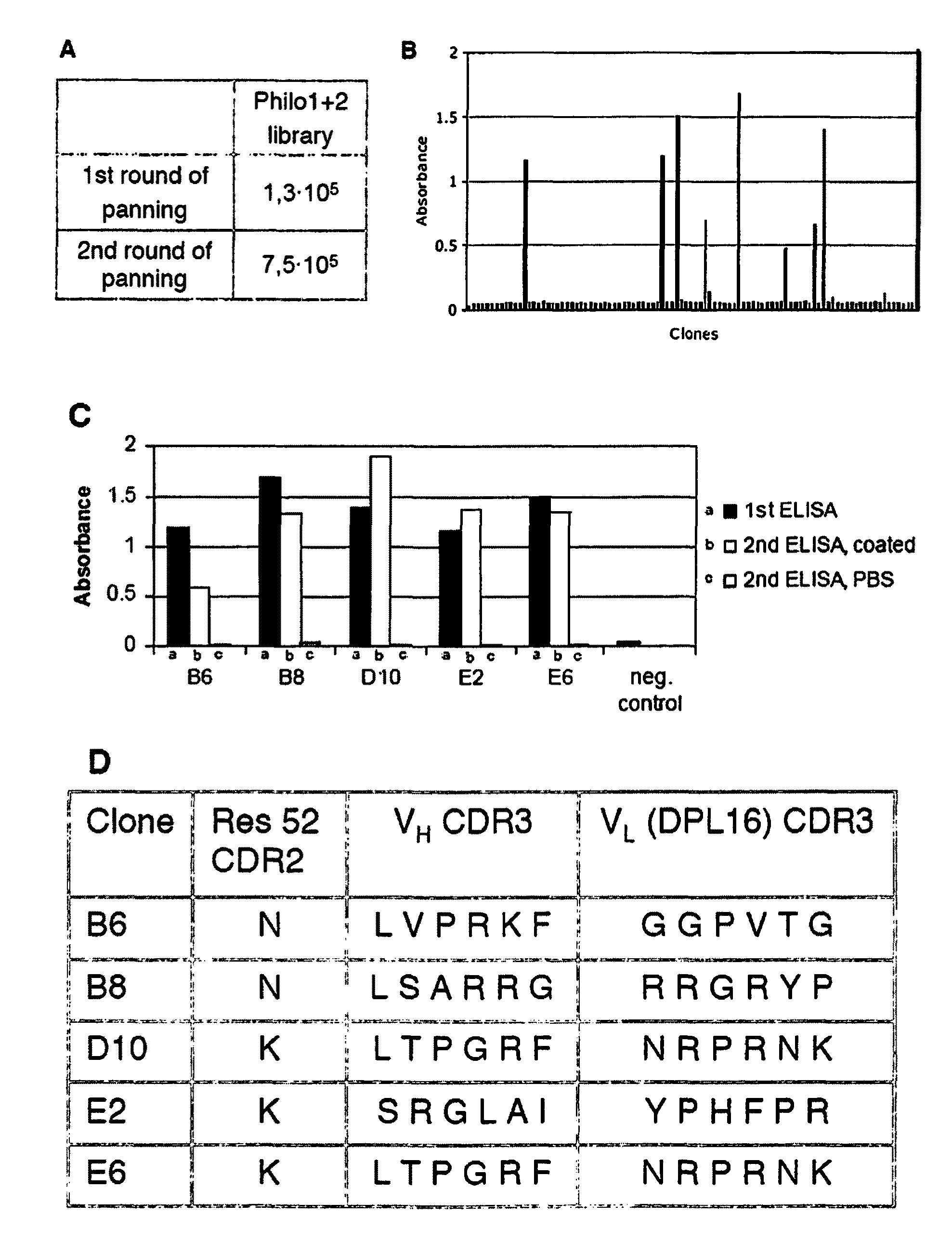
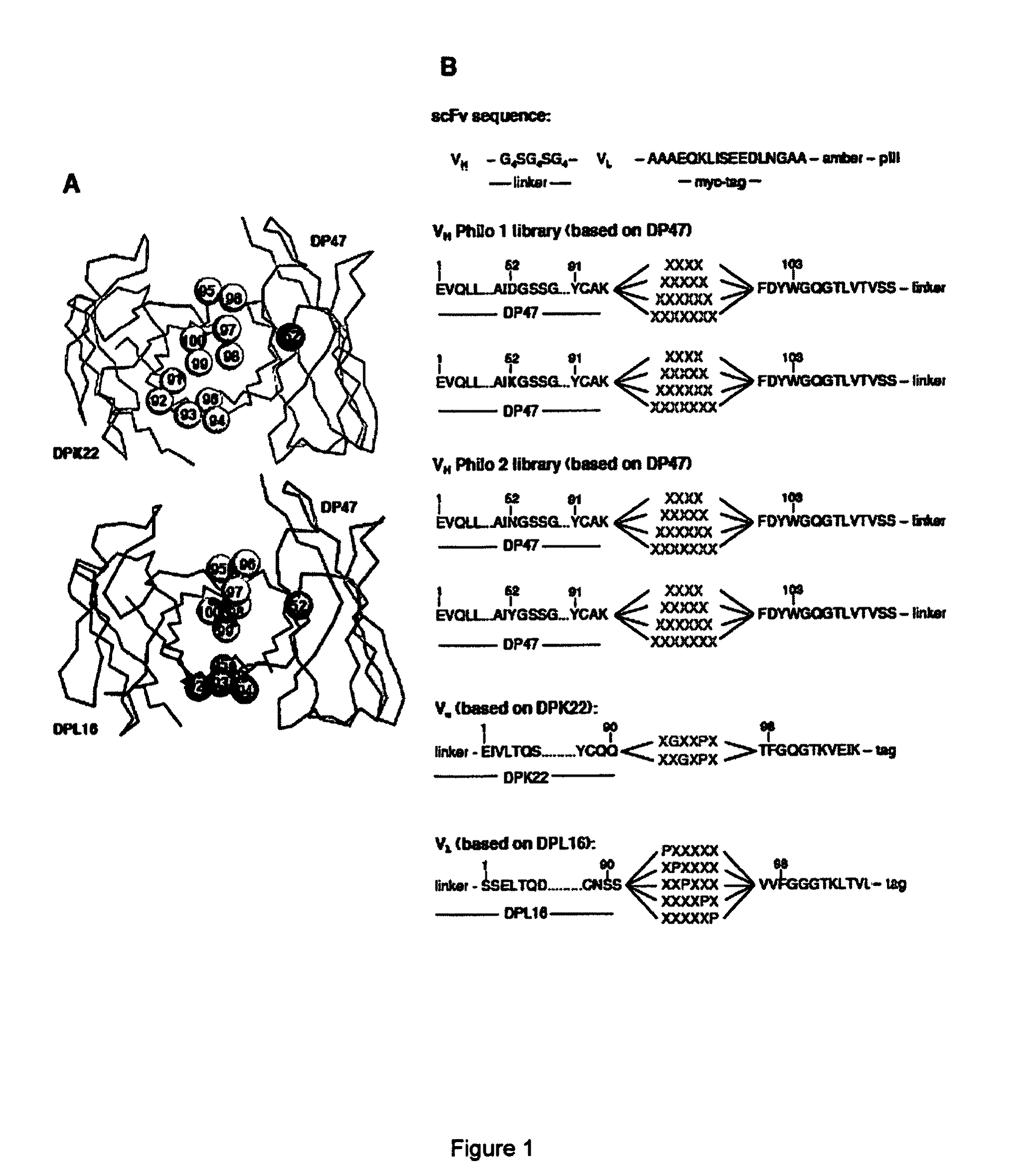
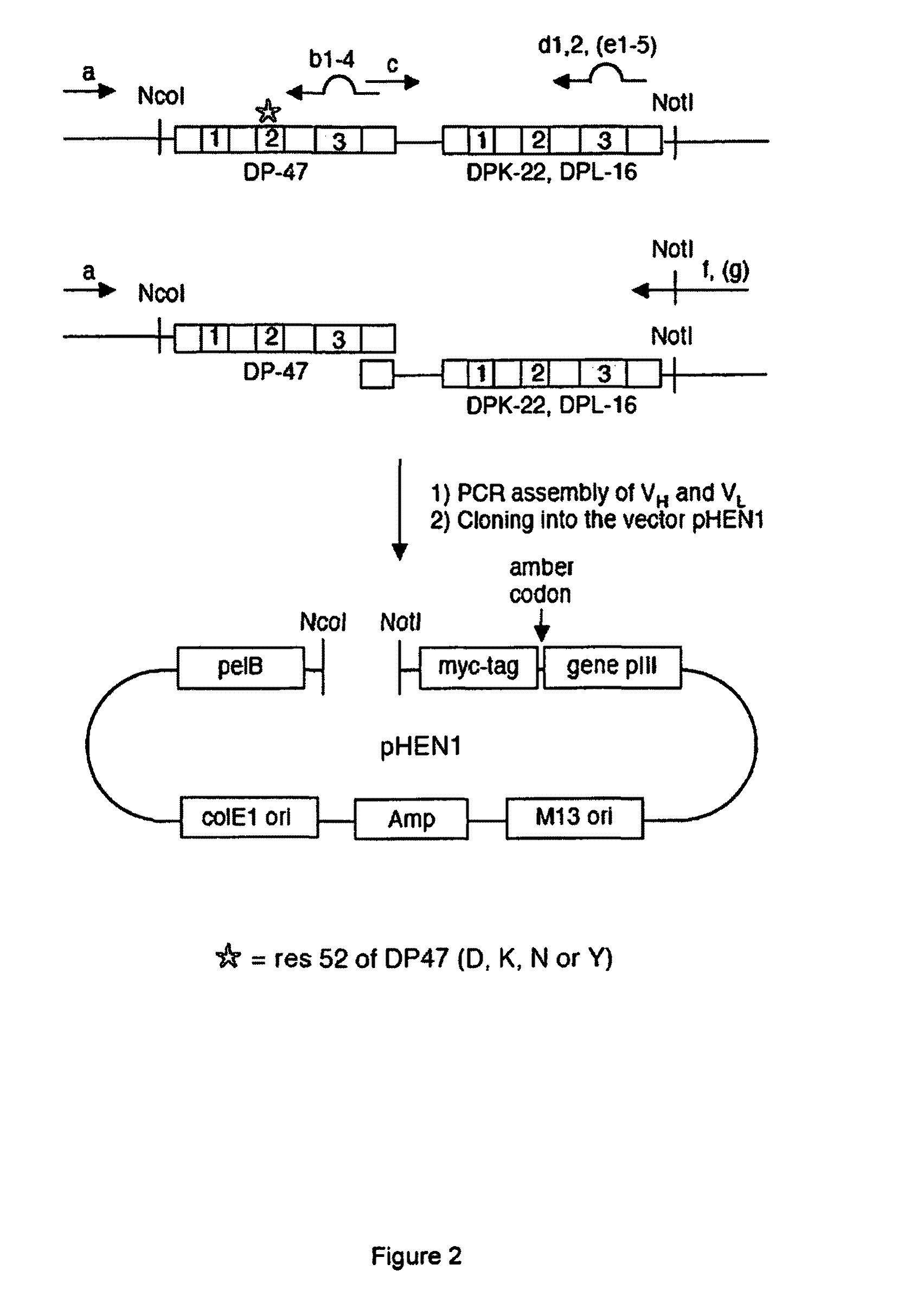
Synthetic antibody display library containing human germline antibody molecules with variation in VH CDR3 and VL CDR3 and at position 52 of VH CDR2, for screening and selection of antibody molecules specific for antigens of interest.
Owner:PHILOCHEM AG
Antibody specific for apolipoprotein and methods of use thereof
The present disclosure provides synthetic antibodies specific for an epitope present on an apolipoprotein E polypeptide. The antibodies are useful in various treatment, diagnostic, and monitoring applications, which are also provided.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Synthetic Antibodies
Owner:ARIZONA STATE UNIVERSITY
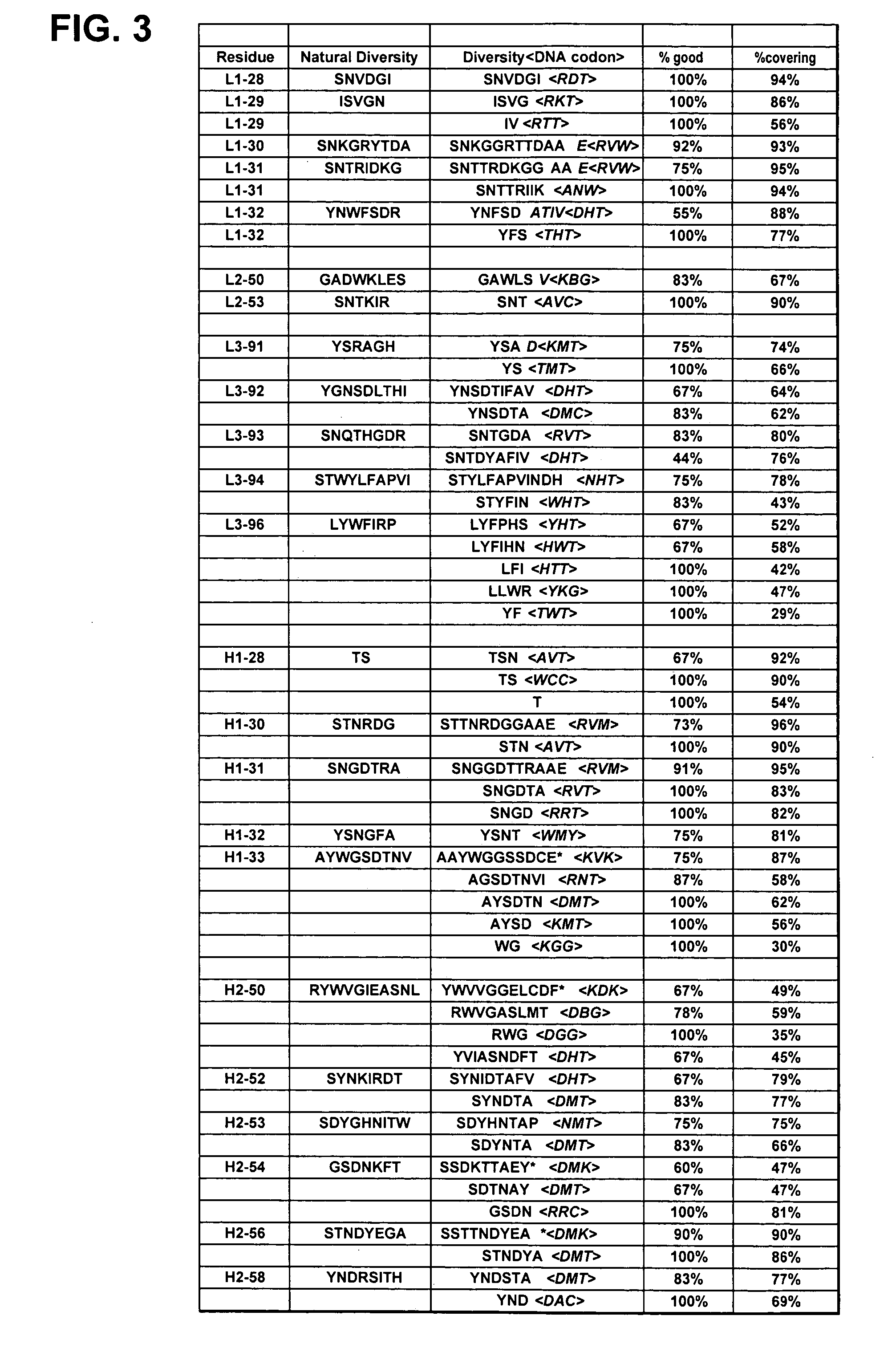
Synthetic Polypeptide Libraries and Methods for Generating Naturally Diversified Polypeptide Variants
ActiveUS20110118149A1High success frequencyAvoid excessive errorImmunoglobulinsFermentationNatural sourceComplementarity determining region
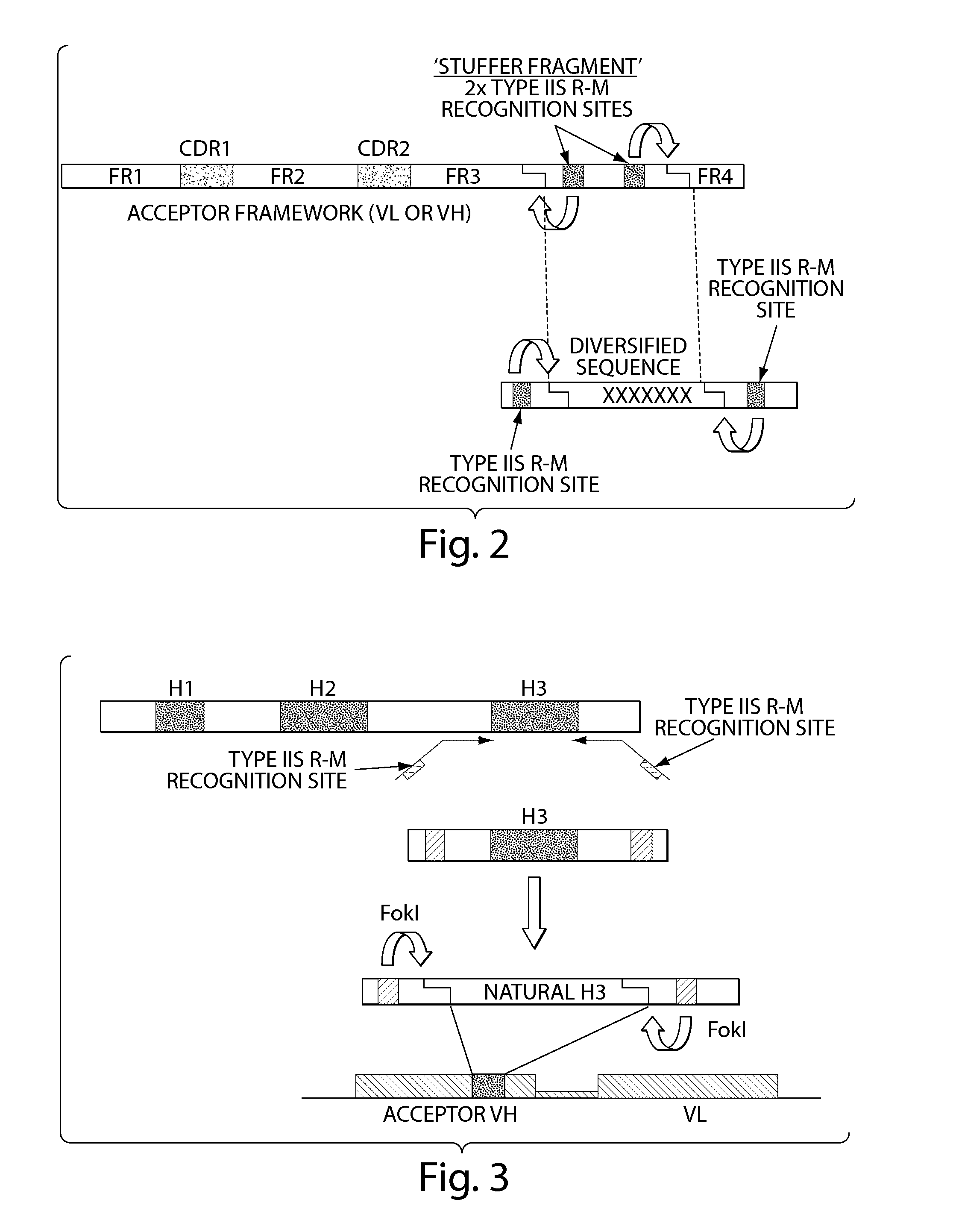
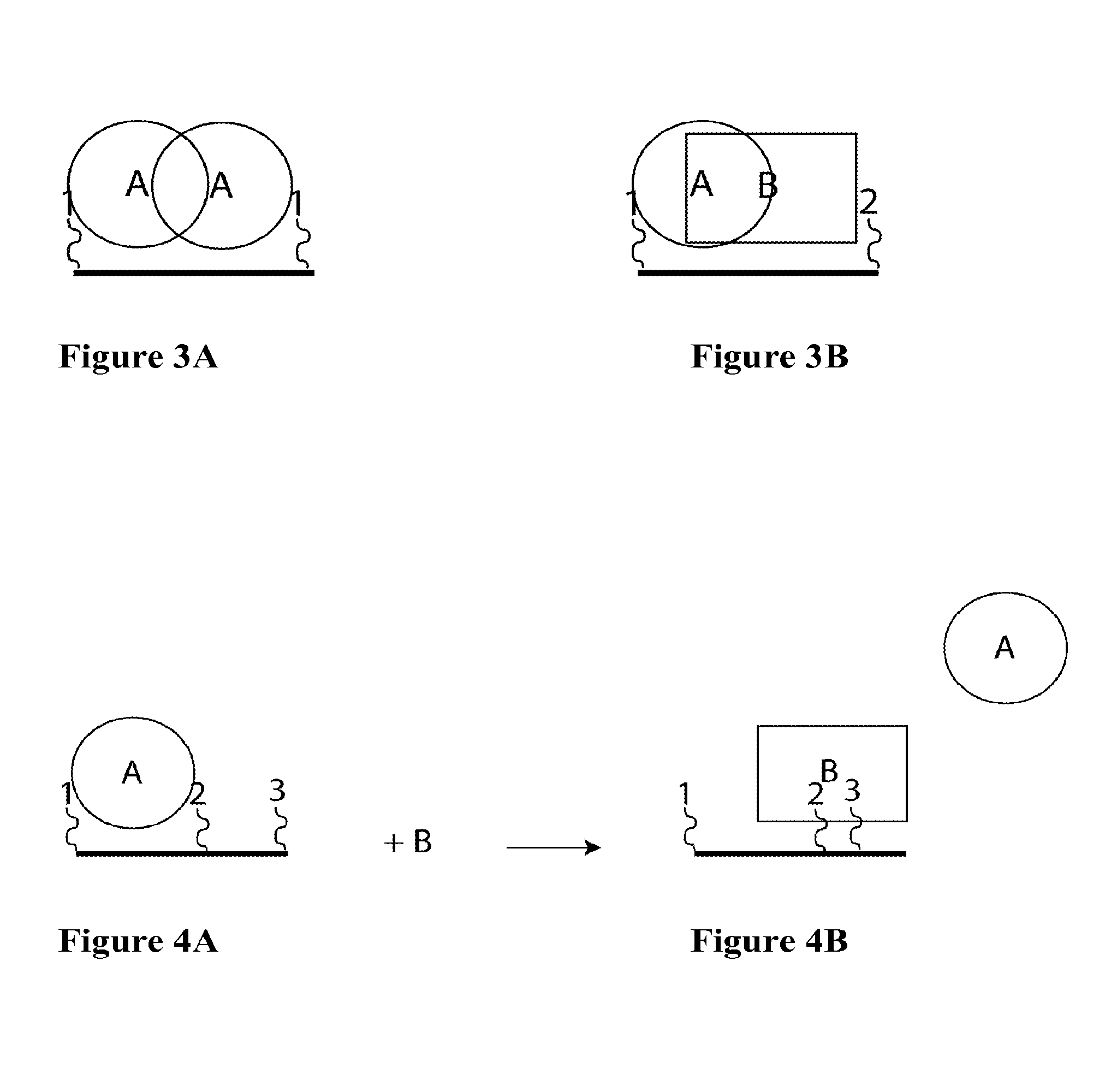
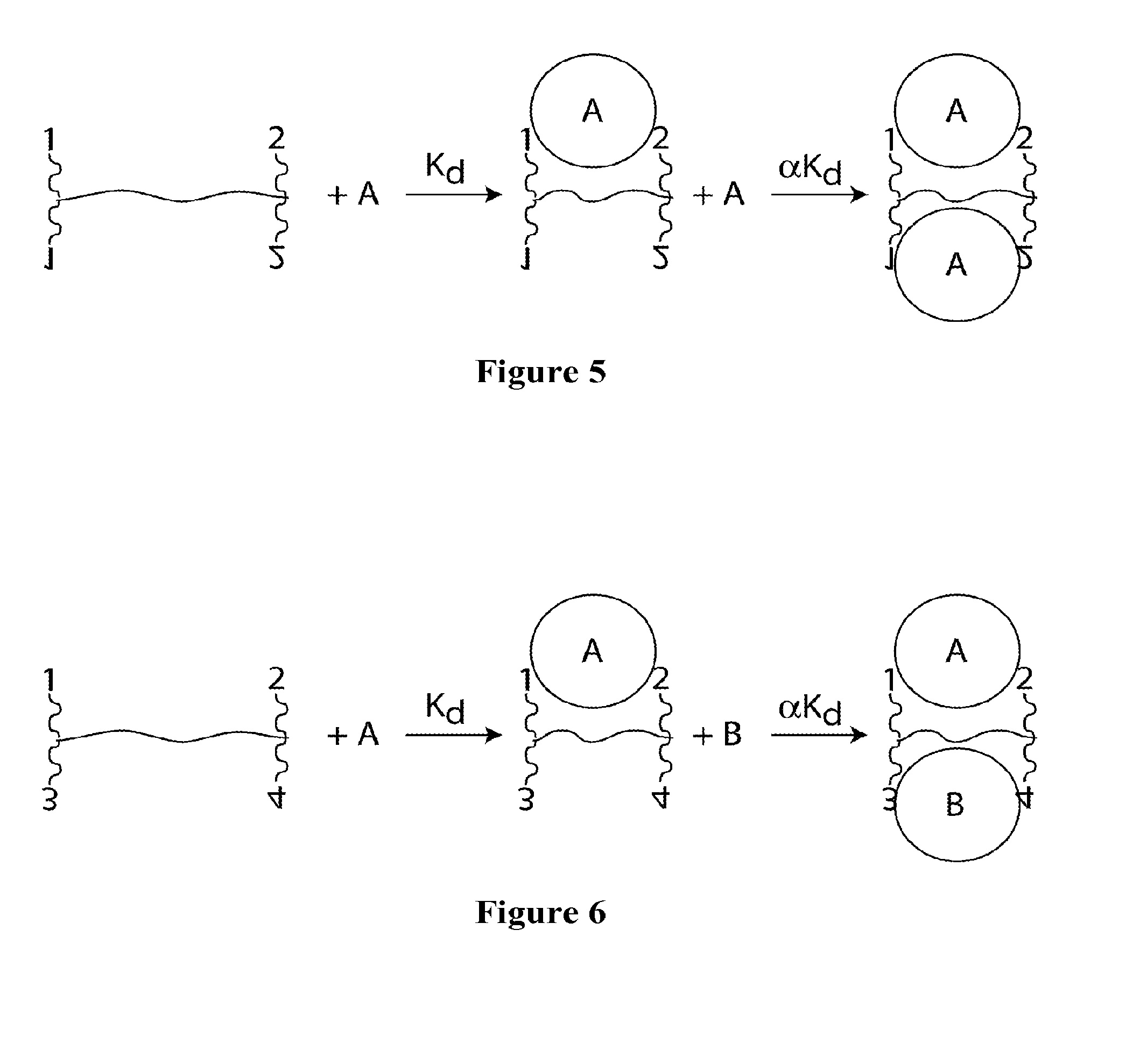
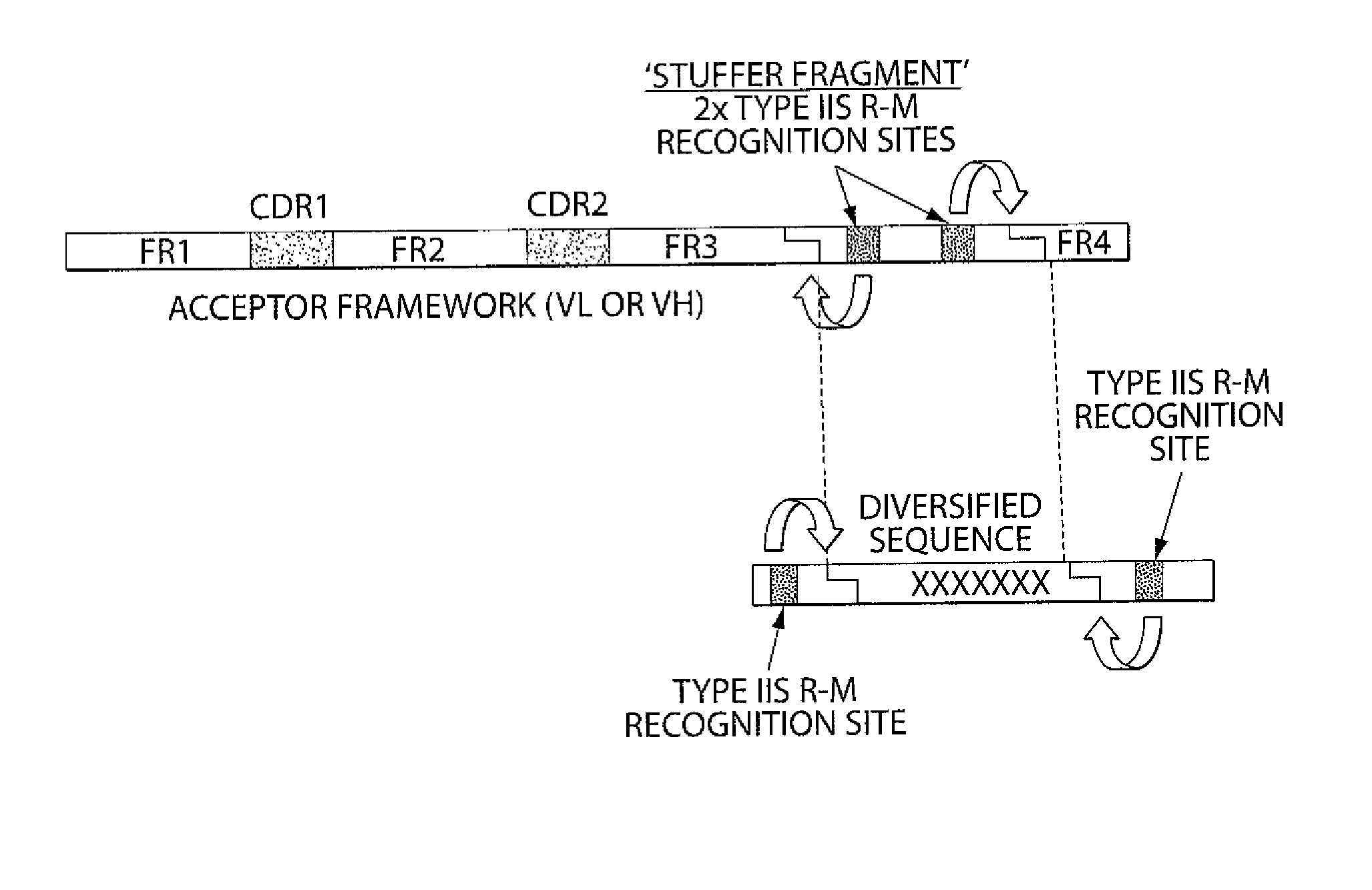
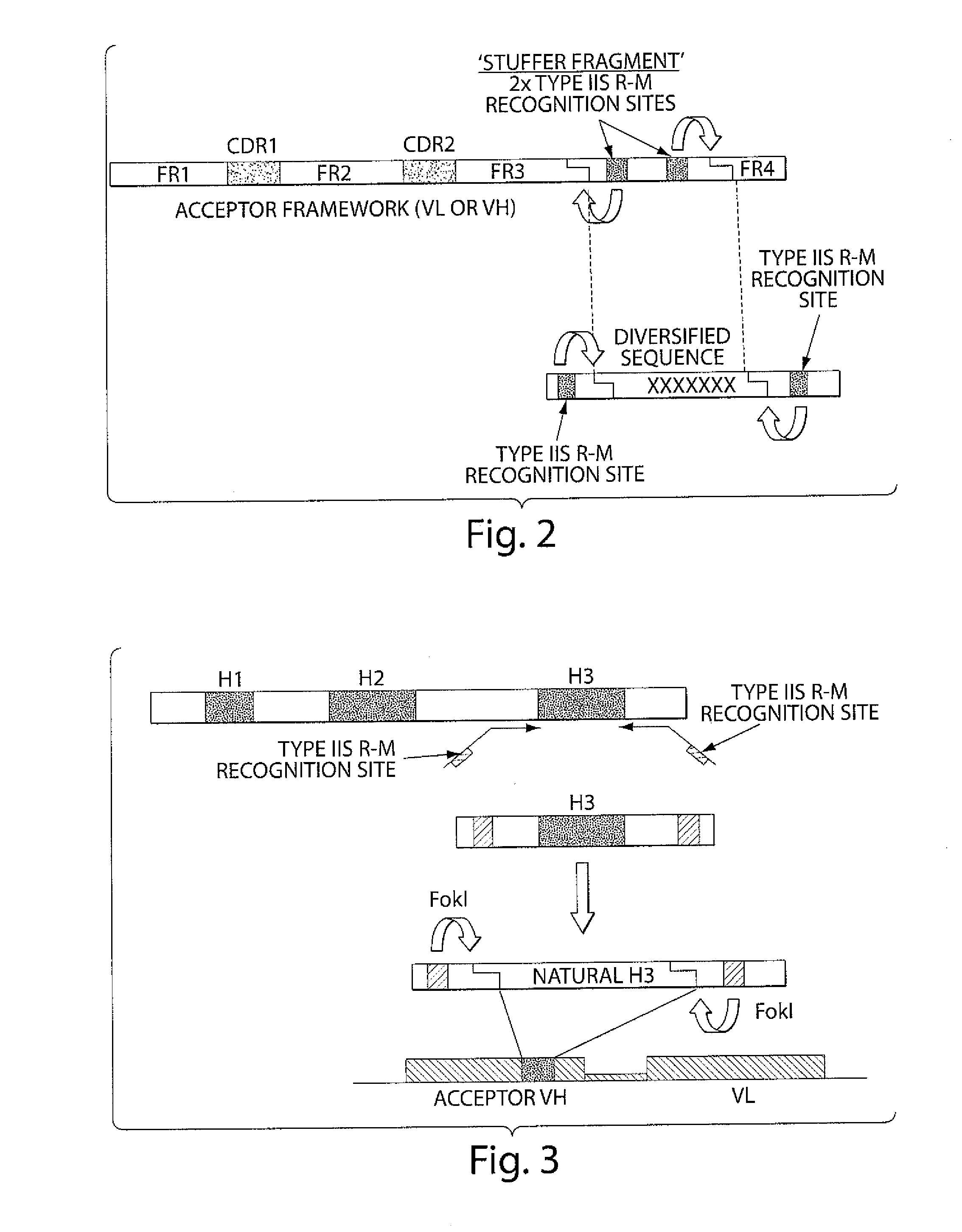
The invention provides compositions and methods for generating libraries of DNA sequences encoding homologous polypeptides, and uses of the libraries to identify naturally diversified polypeptide variants. The invention also provides compositions and methods for generating collections of synthetic antibody fragments in which one or several complementary determining regions (CDR) are replaced by a collection of the corresponding CDR captured from a natural source. The invention further provides compositions and methods for diversifying a portion of a polypeptide by inserting a diversified sequence of synthetic or natural origin without the need for modification of the original polypeptide coding sequence.
Owner:NOVIMMUNE
Method for improving IgG1 antibody yield
ActiveCN110669132AHigh expressionIncrease productionImmunoglobulins against cell receptors/antigens/surface-determinantsVector-based foreign material introductionEscherichia coliHeavy chain
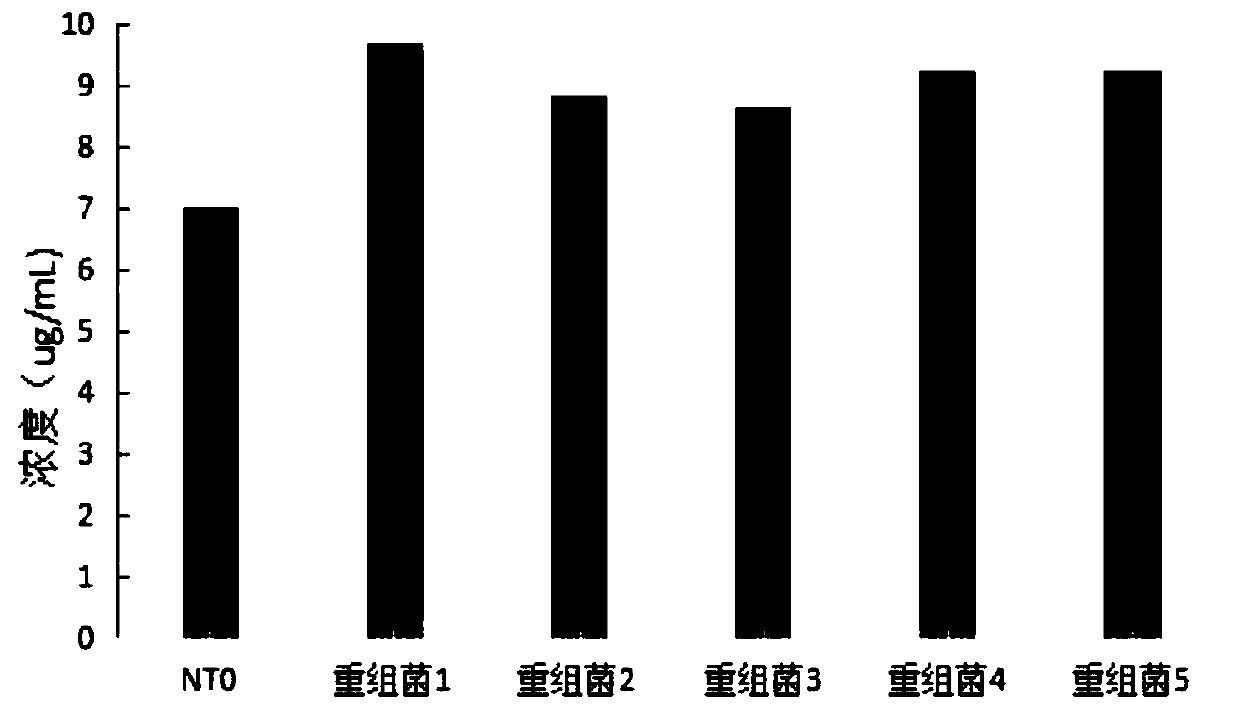
The invention discloses a method for improving IgG1 antibody yield, which comprises the following steps: (1) gene in-vitro synthesis and amplification: obtaining optimized nucleotide sequences of a light chain and a heavy chain of lgG1, and connecting the nucleotide sequences into a nucleotide sequence; (2) connecting the nucleotide sequence to a p2A4 vector to obtain p2A4-HL; (3) designing and assembling sRNA; (4) respectively connecting the sRNA sequence connected to a sRNA receiver to the p3C5 vector; (5) obtaining the recombinant bacterium 1, the recombinant bacterium 2, the recombinant bacterium 3, the recombinant bacterium 4 and the recombinant bacterium 5 by respectively performing introducing into escherichia coli together with p2A4-HL; and (6) respectively fermenting the recombinant bacteria to obtain the high-yield IgG1 antibody. According to the method disclosed by the invention, the expression quantity of the monoclonal antibody in escherichia coli can be increased, relatedenzymes of a tricarboxylic acid circulating metabolic pathway can be inhibited in a targeted manner, and more amino acids for synthesizing redundant enzymes can be used for synthesizing the IgG1 antibody, so that the yield of IgG1 is increased.
Owner:天津大学前沿技术研究院
Cancer neoepitopes
InactiveCN108513593APeptide/protein ingredientsLibrary screeningCellular componentAntiendomysial antibodies
Contemplated compositions and methods are directed to cancer neoepitopes and uses of such neoepitopes, especially to generate synthetic antibodies against neoepitopes that may then be employed in themanufacture of a therapeutic agent. Preferred therapeutic agents will comprise a synthetic antibody against a neoepitope, and most preferably in combination with a cellular or non-cellular component for use as a diagnostic or therapeutic agent.
Owner:NANTOMICS LLC +1
Synthetic antibodies
ActiveUS9863938B2Peptide librariesImmunoglobulins against cell receptors/antigens/surface-determinantsSynthetic antibodyBiology
Owner:ARIZONA STATE UNIVERSITY
Synthetic Antibodies
Owner:ARIZONA BOARD OF REGENTS A BODY OF THE STATE OF ARIZONA ACTING FOR & ON BEHAIF OF ARIZ
DNA antibody constructs and method of using same
The invention relate to DNA antibody constructs and a method of using same. Disclosed herein is a composition including a recombinant nucleic acid sequence that encodes an antibody. Also disclosed herein is a method of generating a synthetic antibody in a subject by administering the composition to the subject. The disclosure also provides a method of preventing and / or treating disease in a subject using said composition and method of generation.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Synthetic Polypeptide Libraries and Methods for Generating Naturally Diversified Polypeptide Variants
ActiveUS20110065610A1High success frequencyAvoid excessive errorImmunoglobulinsVector-based foreign material introductionNatural sourceComplementarity determining region
The invention provides compositions and methods for generating libraries of DNA sequences encoding homologous polypeptides, and uses of the libraries to identify naturally diversified polypeptide variants. The invention also provides compositions and methods for generating collections of synthetic antibody fragments in which one or several complementary determining regions (CDR) are replaced by a collection of the corresponding CDR captured from a natural source. The invention further provides compositions and methods for diversifying a portion of a polypeptide by inserting a diversified sequence of synthetic or natural origin without the need for modification of the original polypeptide coding sequence.
Owner:NOVIMMUNE
Display library for antibody selection
Synthetic antibody display library containing human germline antibody molecules with variation in VH CDR3 and VL CDR3 and at position 52 of VH CDR2, for screening and selection of antibody molecules specific for antigens of interest.
Owner:PHILOCHEM AG
Synthetic polypeptide libraries and methods for generating naturally diversified polypeptide variants
ActiveUS8921281B2Robust introductionIncrease probabilityImmunoglobulinsVector-based foreign material introductionNatural sourceComplementarity determining region
The invention provides compositions and methods for generating libraries of DNA sequences encoding homologous polypeptides, and uses of the libraries to identify naturally diversified polypeptide variants. The invention also provides compositions and methods for generating collections of synthetic antibody fragments in which one or several complementary determining regions (CDR) are replaced by a collection of the corresponding CDR captured from a natural source. The invention further provides compositions and methods for diversifying a portion of a polypeptide by inserting a diversified sequence of synthetic or natural origin without the need for modification of the original polypeptide coding sequence.
Owner:NOVIMMUNE
Anti-bacterial polypeptides and pathogen specific synthetic antibodies
ActiveUS9309298B2Peptide/protein ingredientsFusions for specific cell targetingDisinfectantAnti bacterial
Owner:ARIZONA STATE UNIVERSITY
High-throughput screening of functional antibody fragments, immunoconjugate comprising the same, and adaptor-drug conjugate for screening
ActiveUS20170355750A1Polypeptide with localisation/targeting motifAntibody ingredientsAntigenHigh-Throughput Screening Methods
Disclosed herein are methods for high-throughput screening of a functional antibody fragment for an immunoconjugate that targets a protein antigen. The method combines a phage-displayed synthetic antibody library and high-throughput cytotoxicity screening of non-covalently assembled immunotoxins or cytotoxic drug to identify highly functional synthetic antibody fragments for delivering toxin payloads.
Owner:ACAD SINIC
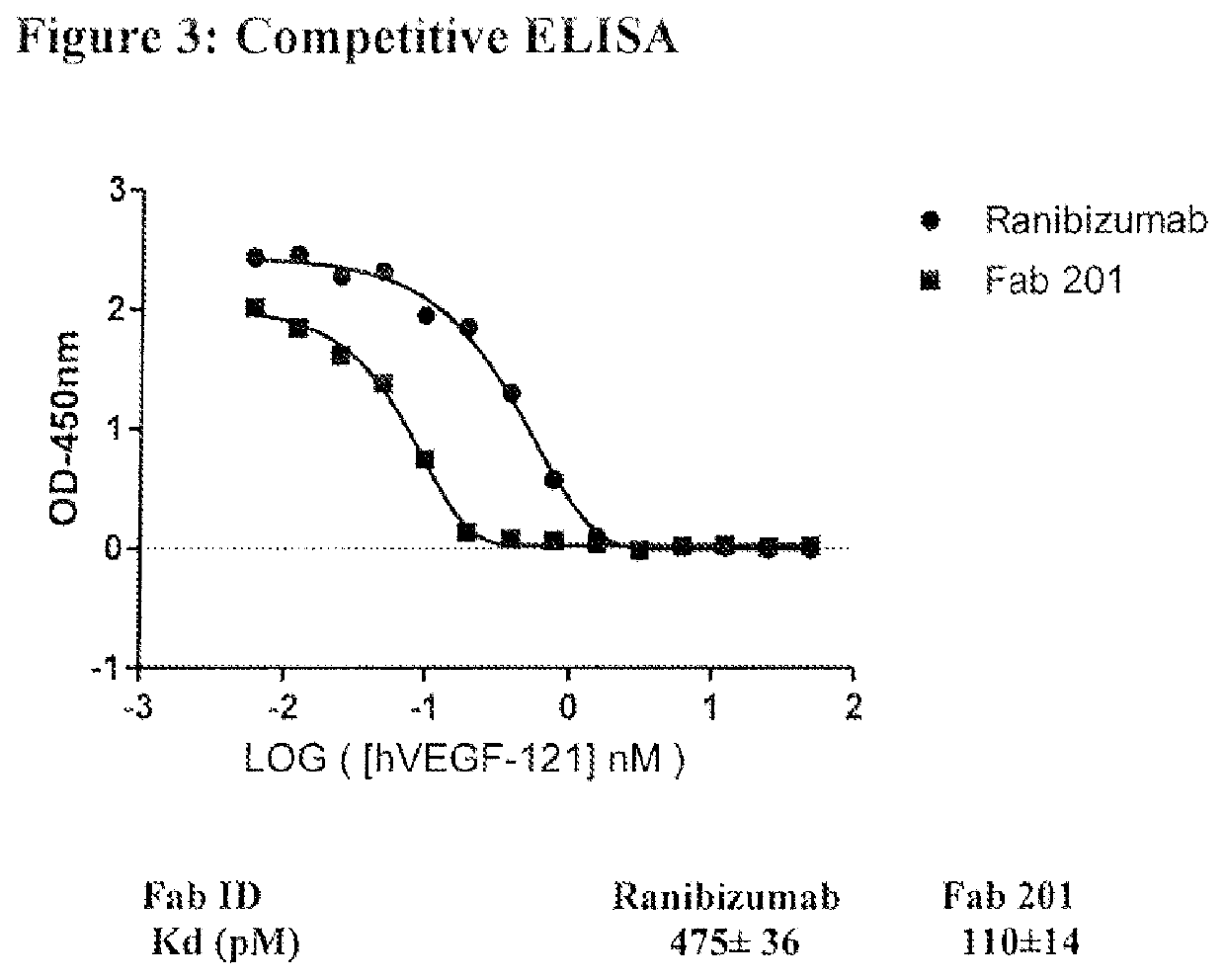
Synthetic antibodies against VEGF and their uses
Owner:THE GOVERNING COUNCIL OF THE UNIV OF TORONTO +1
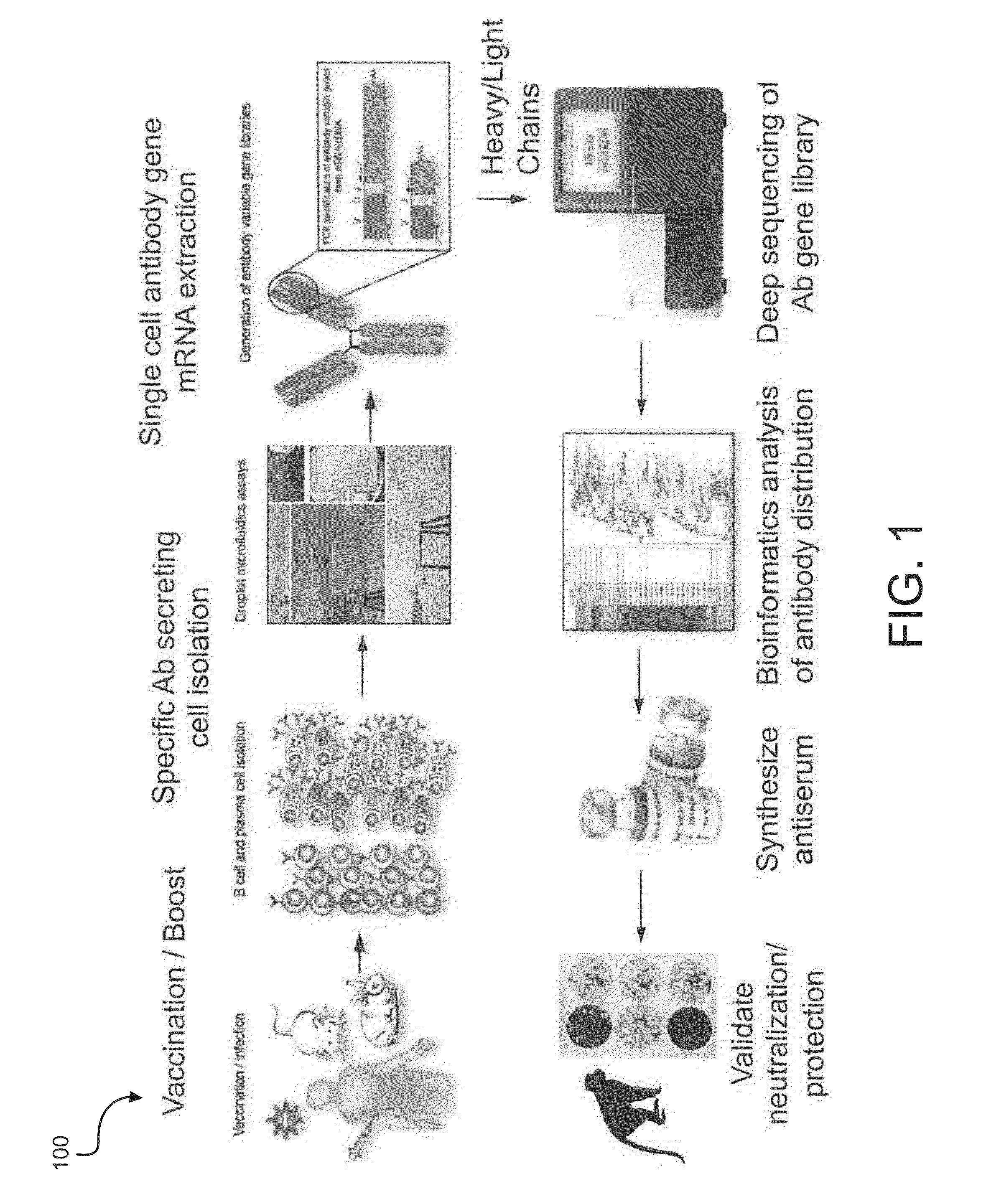
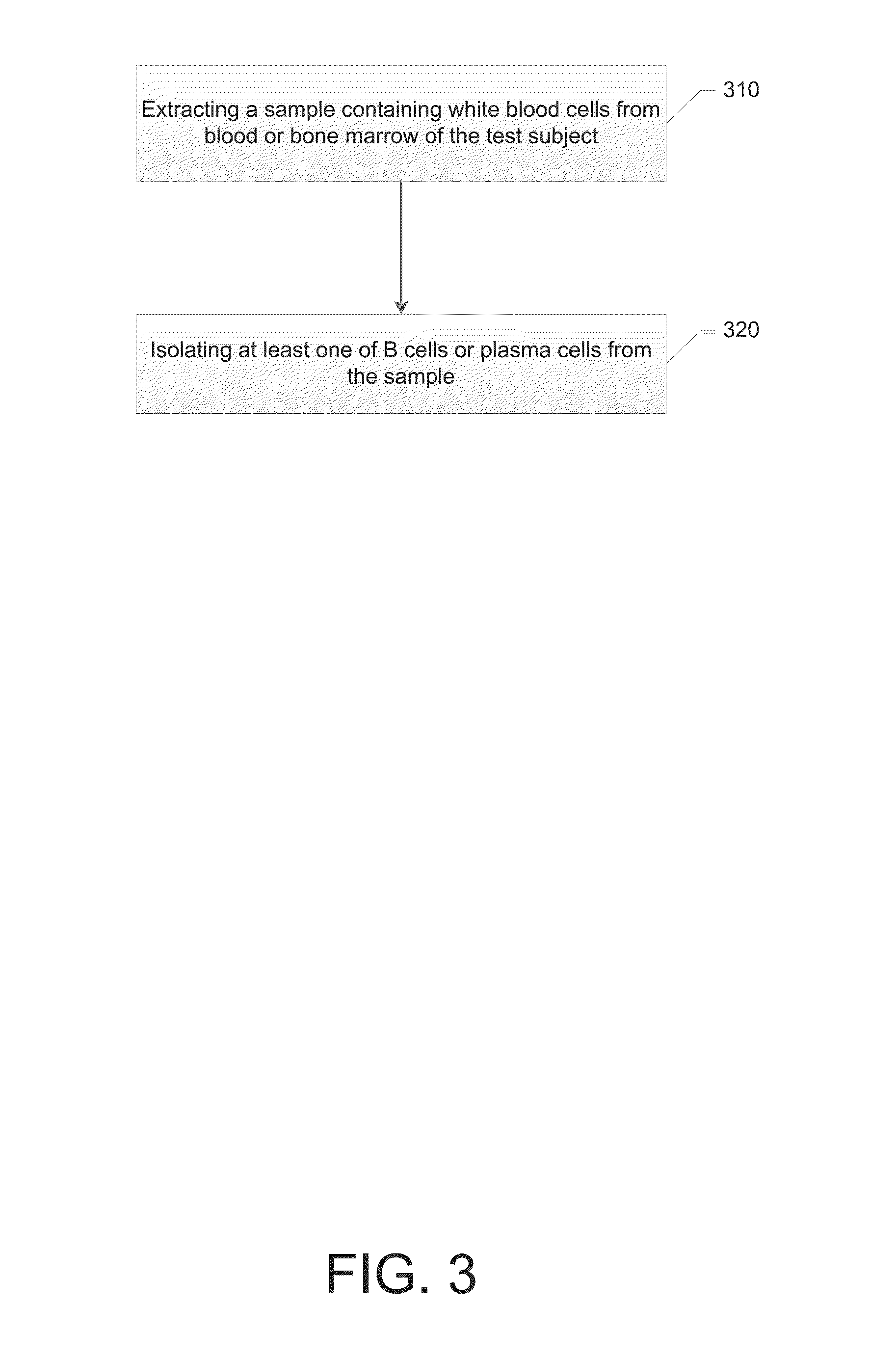
Synthetic antiserum for rapid-turnaround therapies
ActiveUS20160215282A1Reduce the impactImmunoglobulins against bacteriaImmunoglobulins against virusesAntibody typesSecreting cell
A method for synthesizing an antiserum for rapid-turnaround therapies includes collecting antibody-secreting cells from a test subject, wherein the test subject has been exposed to a target biological agent and has produced an antibody response; selecting a subset of the antibody-secreting cells, the subset of the antibody-secreting cells producing antibodies that neutralize the target biological agent; generating variable-region-coding DNA sequences from the antibodies that neutralize the target biological agent; tagging amplicons of the variable-region-coding DNA sequences with unique nucleic acid identifiers to associate the variable-region-coding DNA sequences derived from individual ones of the subset of the antibody-secreting cells; analyzing antibody-type distribution in a natural immune response; synthesizing antibodies from the variable-region-coding DNA sequences to form synthetic antibodies; and mixing the synthetic antibodies in a proportion equal to the antibody-type distribution in the natural immune response to form the antiserum.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Synthetic Antibodies
InactiveUS20190194358A1Carrier-bound/immobilised peptidesBiological testingBiologySynthetic antibody
Owner:ARIZONA STATE UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com