DNA antibody constructs and method of using same
A technology for synthesizing antibodies and nucleic acid sequences, which can be used in the fields of antibodies, DNA/RNA vaccination, and antibody medical components. It can solve the problems of short-term stability of antibody preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0368] Construction of a high expression system for in vivo immunoglobulin (Ig) production. Specifically, the Ig heavy and light chain sequences are modified to increase in vivo expression of a fully assembled Ig molecule comprising 2 heavy and 2 light chain polypeptides. Constructs of gp120IgG-heavy and light chain molecules were generated and inserted individually into pVAX1 vector (Life Technologies, Carlsbad, CA). This antibody has defined properties that allow its use in characterization studies as described below. Several modifications can be included when generating constructs to optimize Ig expression in vivo. Optimization included codon optimization and introduction of kozak sequence (GCCACC). The nucleotide sequences of the optimized constructs of the heavy chain and light chain of Ig are shown in SEQ ID NO:6 and SEQ ID NO:7 respectively (respectively figure 1 and 2 )middle. exist figure 1 and 2 In , the BamHI (GGATCC) and XhoI (CTCGAG) restriction enzyme site...
Embodiment 2
[0373] Materials and methods used in Examples 3-7
[0374] cells and reagents. 293T and TZM-Bl cells were maintained in Dulbecco's Modified Eagle's medium (DMEM; Gibco-Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS) and antibiotics, and in Subculture after confluence. Recombinant HIV-1 p24 and gp120Env (rgp120) proteins were obtained from ProteinScience Inc., and peroxidase-conjugated streptavidin was from Jackson Laboratory. Listed cell lines and other reagents were obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
[0375] Animal and protein and plasmid administration and delivery. Female BALB / c mice (8 weeks old) were purchased from TaconicFarms (Germantown, NY). For these administrations, 25 μg of plasmid DNA (pVax1 or pHIV-1 Env-Fab) in a volume of 50 μl was injected intramuscularly (IM), followed by the MID-EP system ( Inovio Pharmaceuticals, Blue Bell, PA) for EP-mediated enhanced delivery. The pulse parameter...
Embodiment 3
[0384] Generation of anti-HIV-1 Env-Fab expression constructs
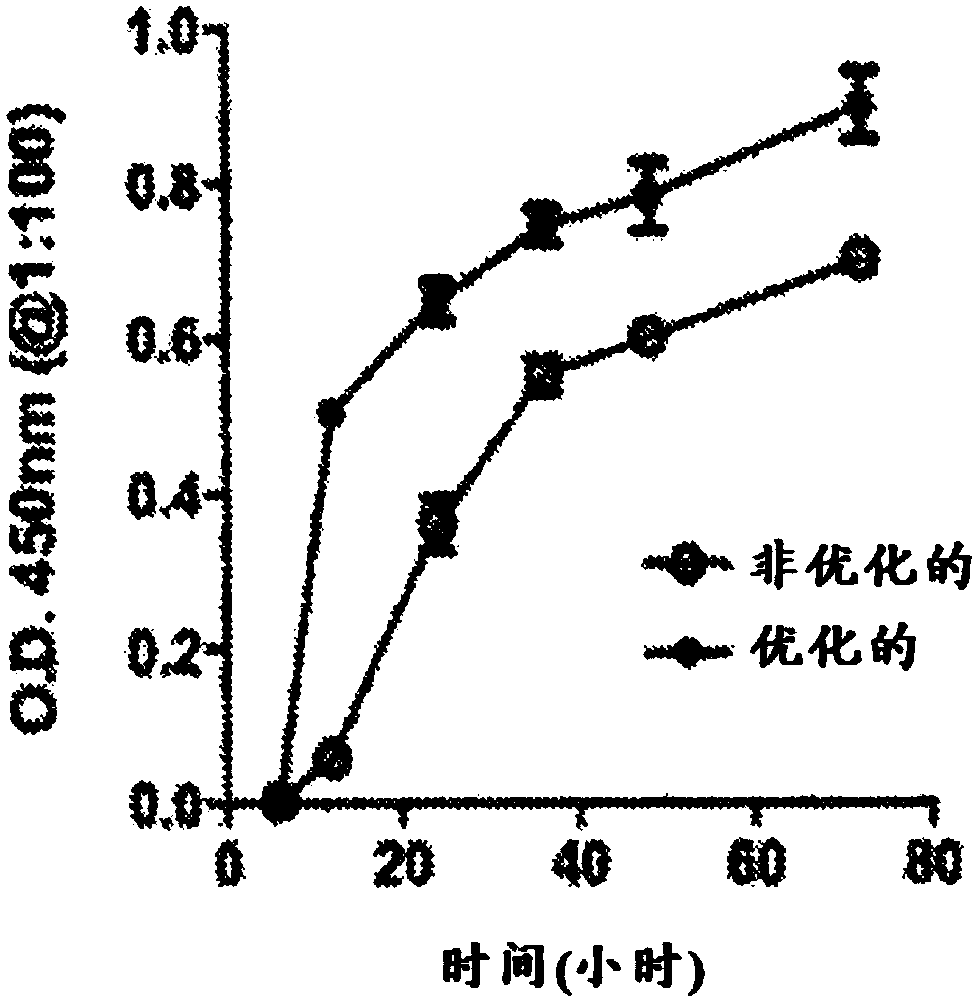
[0385]cDNA encoding the anti-HIV-1 envelope VH and VL-Ig (immunoglobulin) chains of broadly neutralizing human mAbVRC01 was obtained from the VRC (Vaccine Research Center, NIH) by the NIHAIDS Research and Reference Reagent Program and subsequently cloned into the pVax1 vector. Several modifications as specified in Example 2 above were incorporated into the expression vector to maximize and optimize the production of biologically active Ig molecules. Specifically, these modifications include codon and RNA optimization and stabilization, enhanced leader utility, plasmid production at high concentrations, and in vivo plasmid delivery facilitated by EP. Placement of the resulting construct under the control of the immediate early promoter from human cytomegalovirus (CMV) is important for proper and efficient expression in mammalian cells and tissues. Schematic maps of the constructs used in this study are shown in Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com