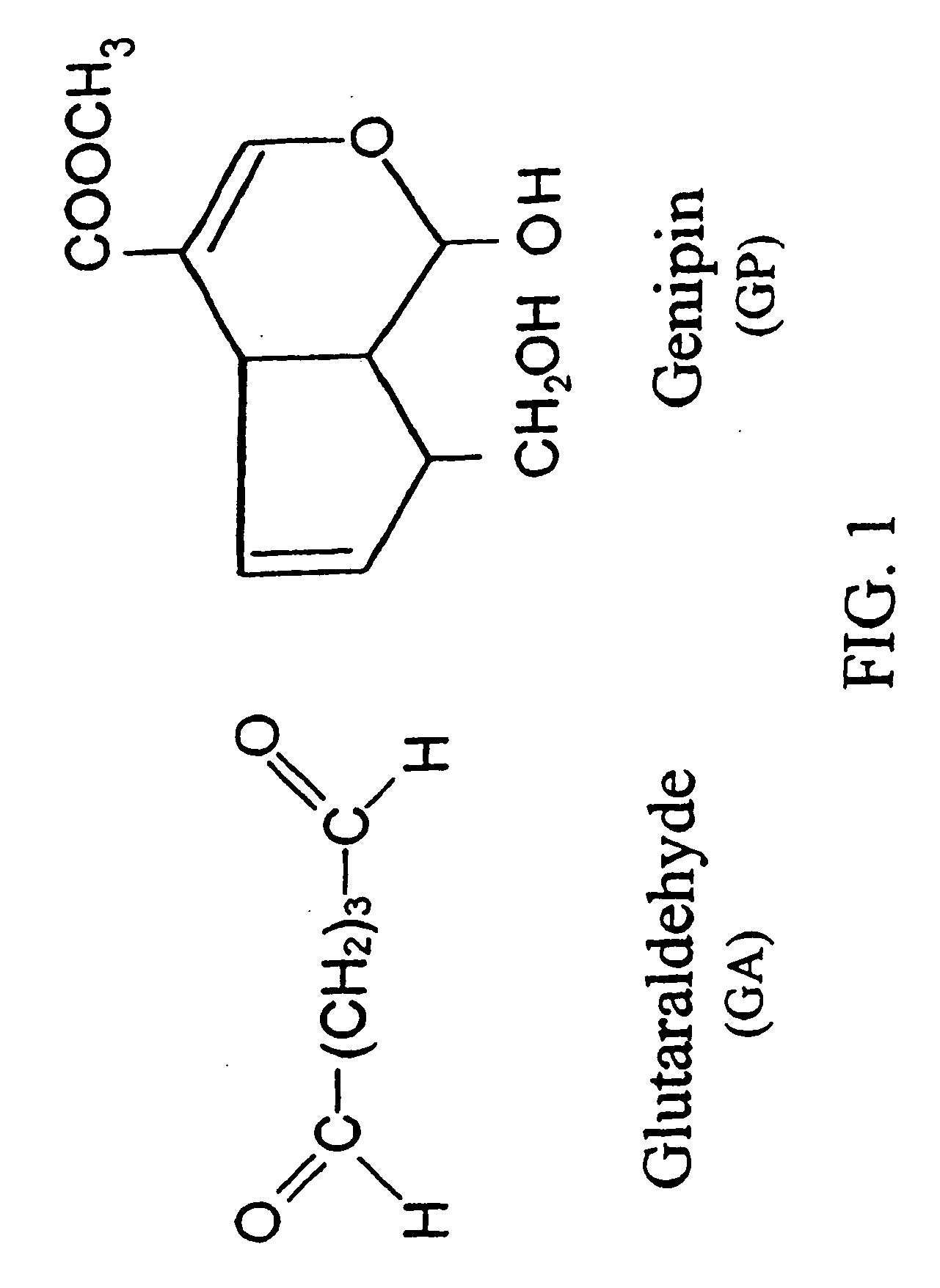
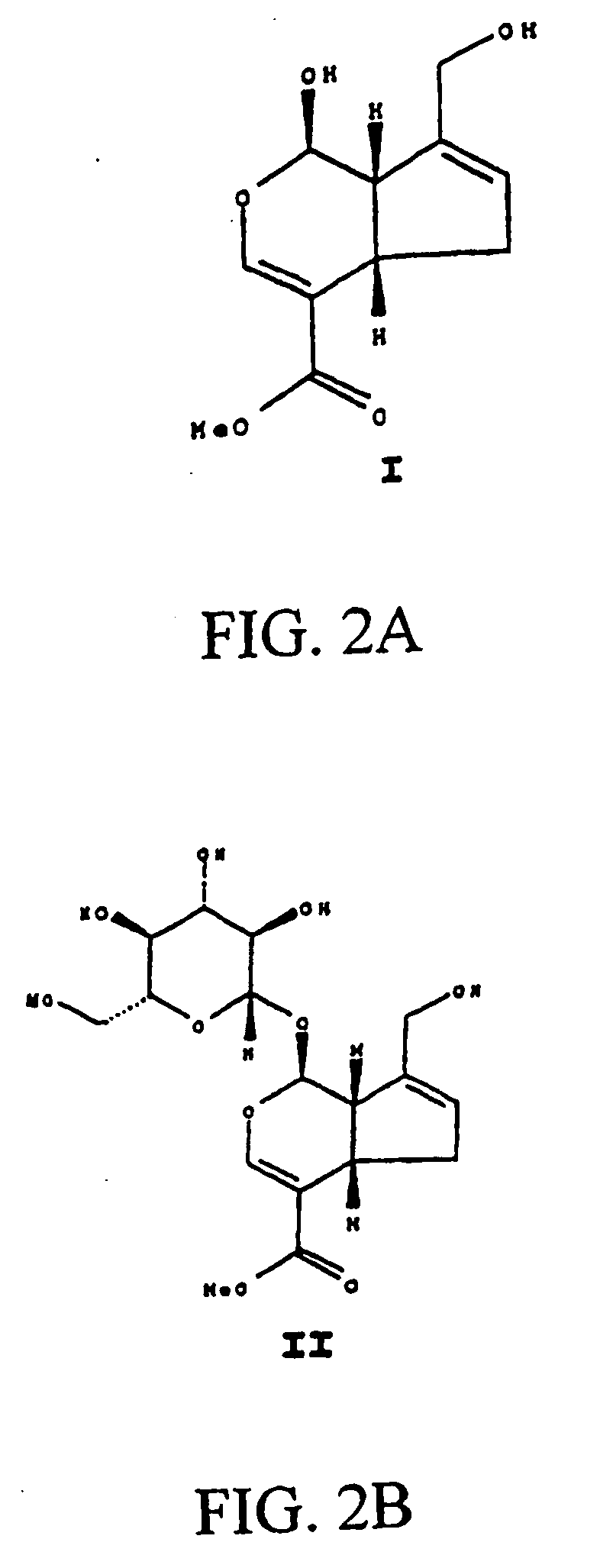
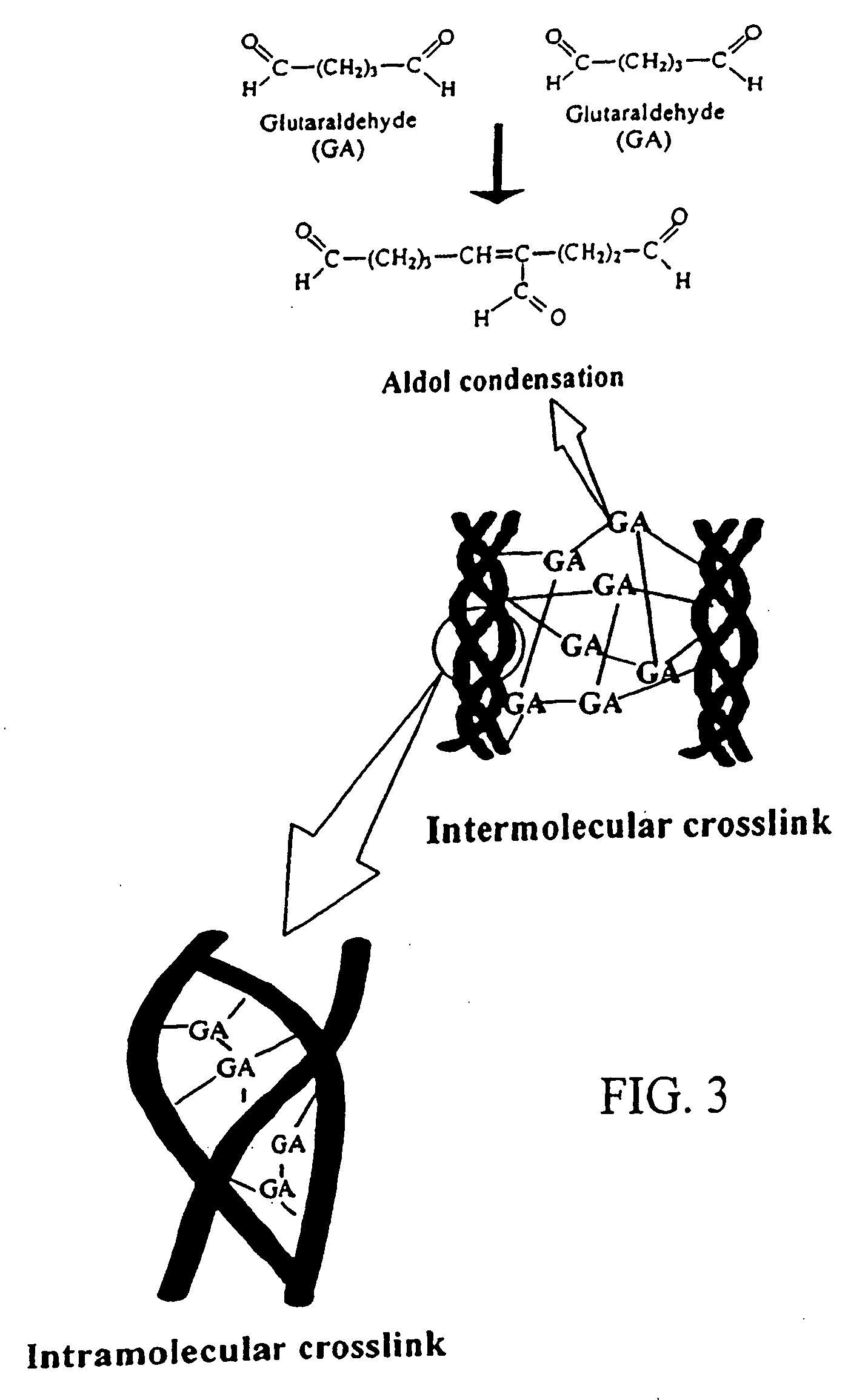
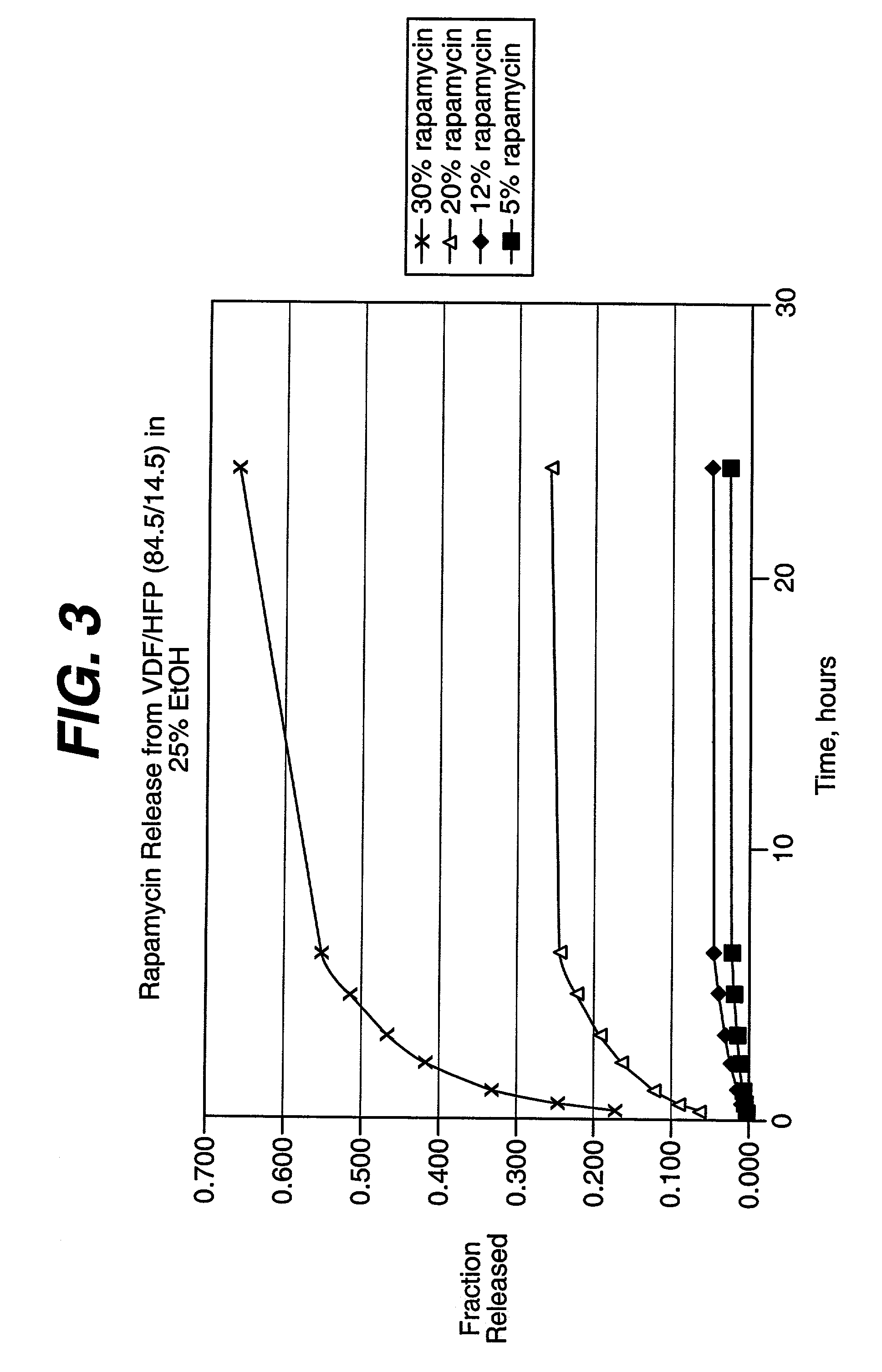
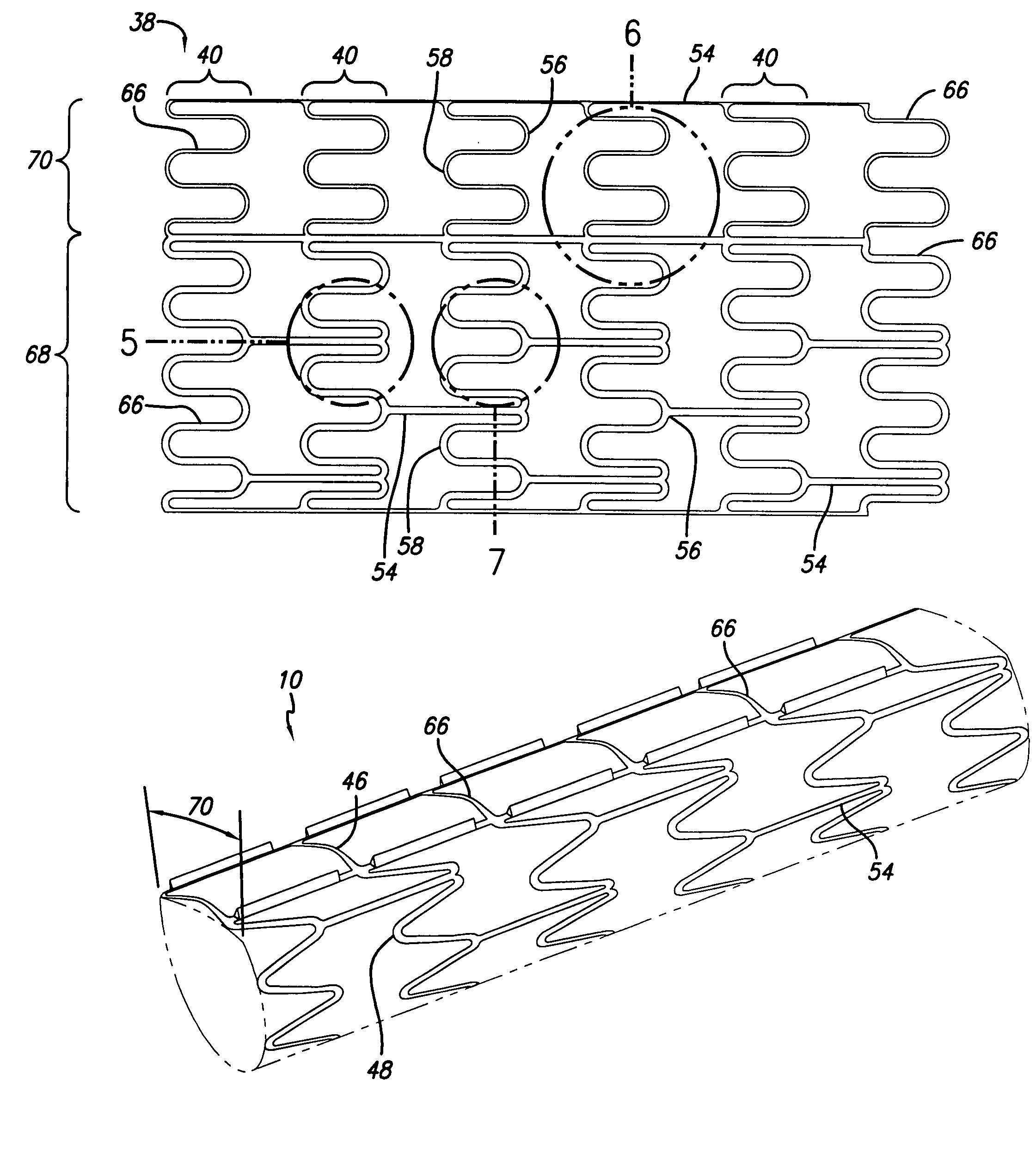
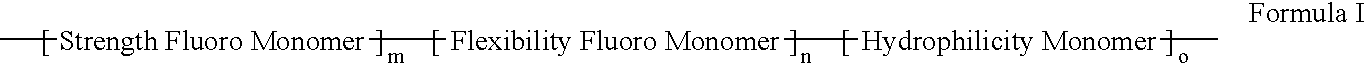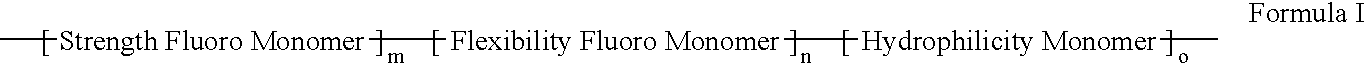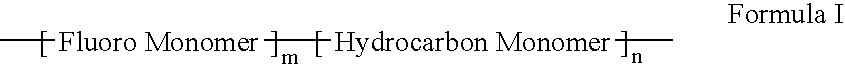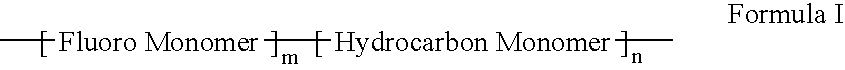Patents
Literature
276 results about "Vulnerable plaque" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
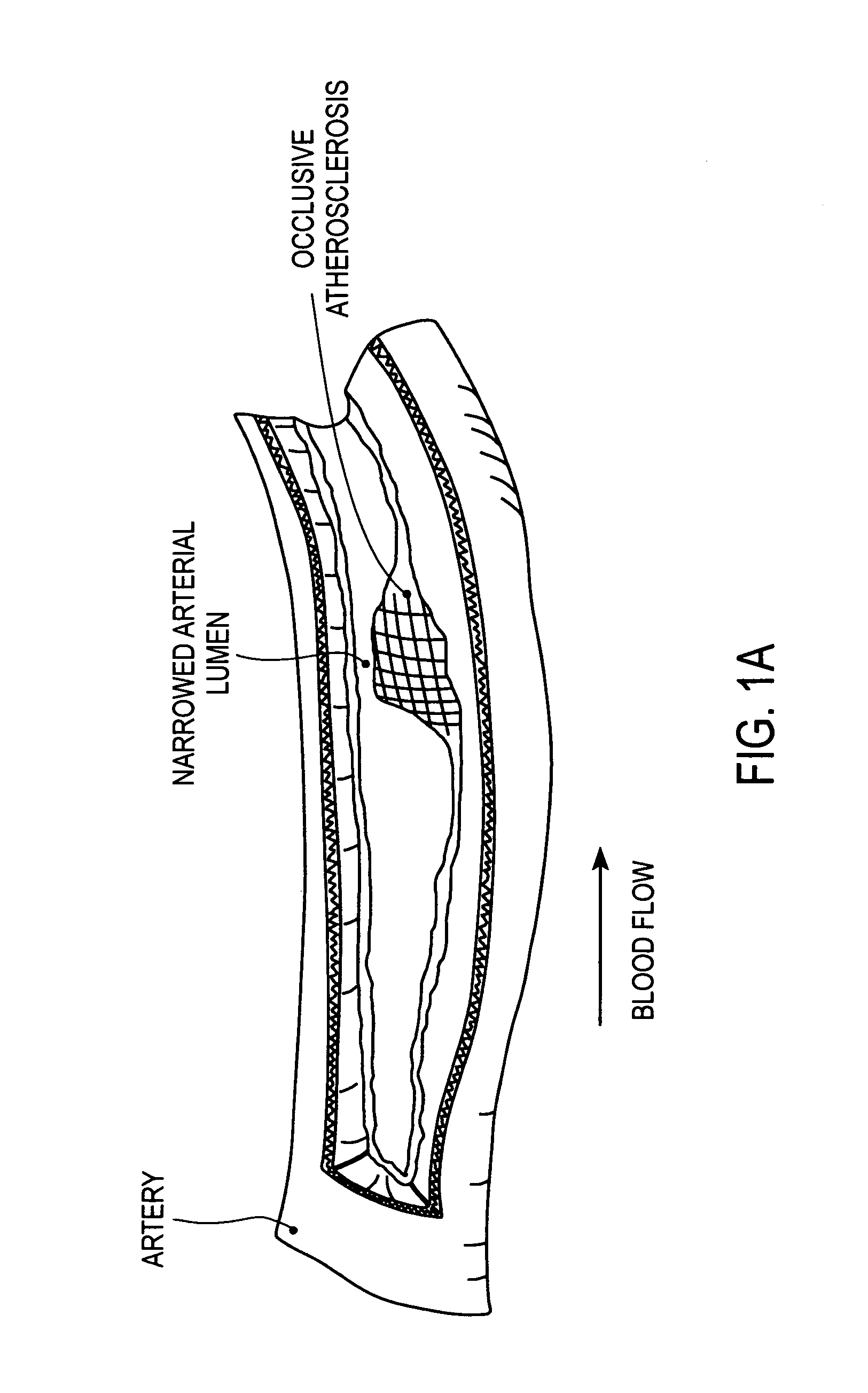
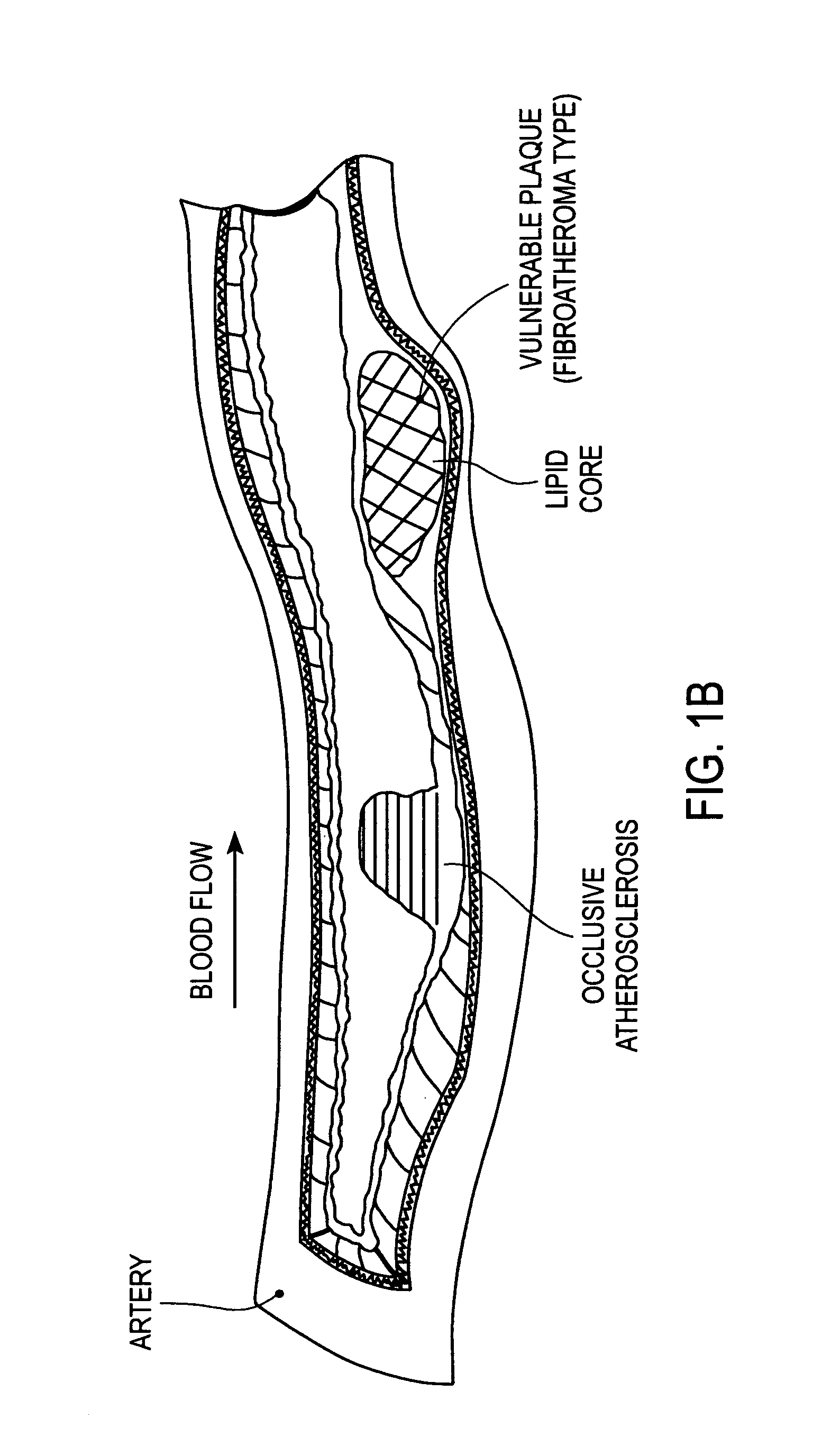
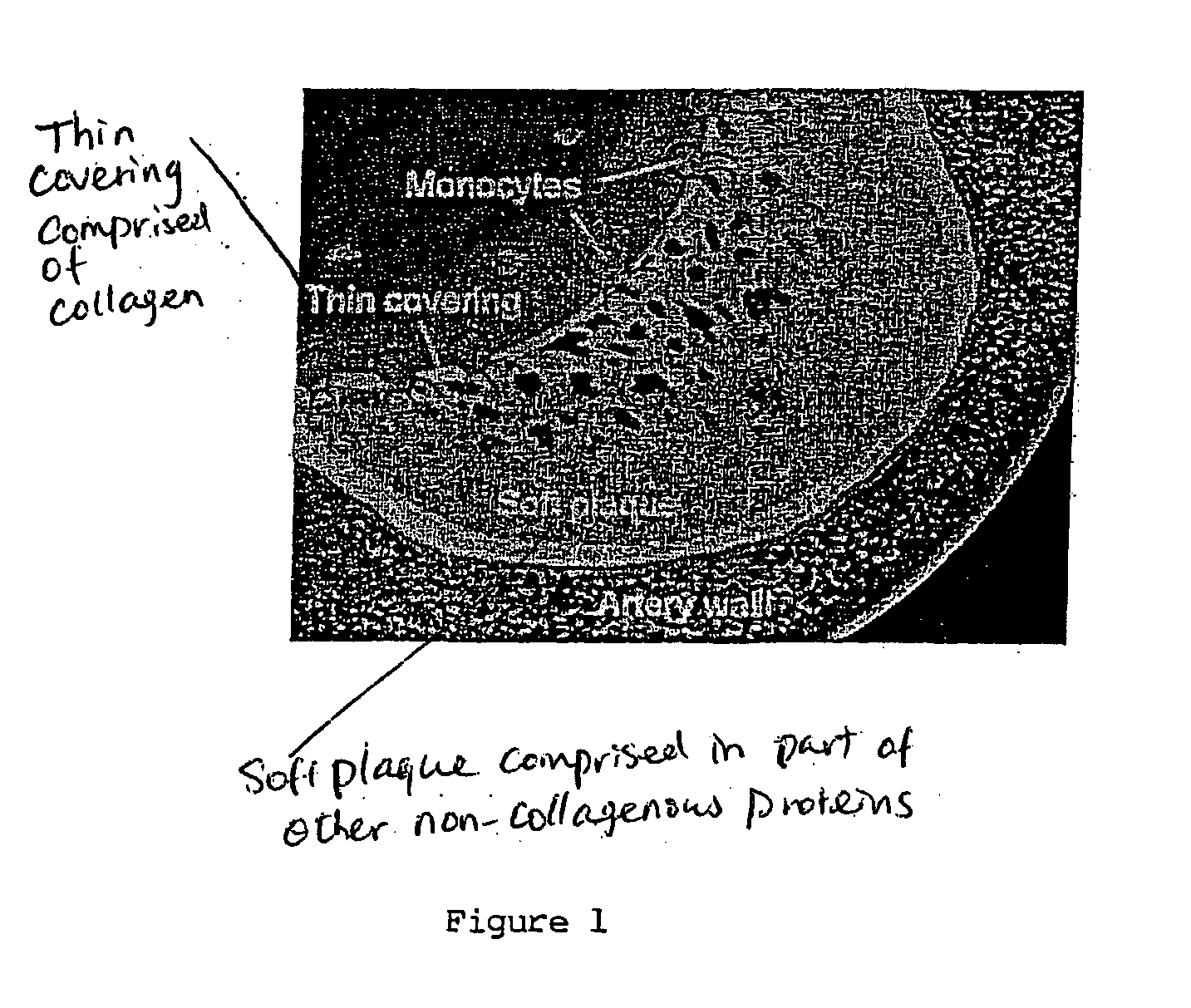
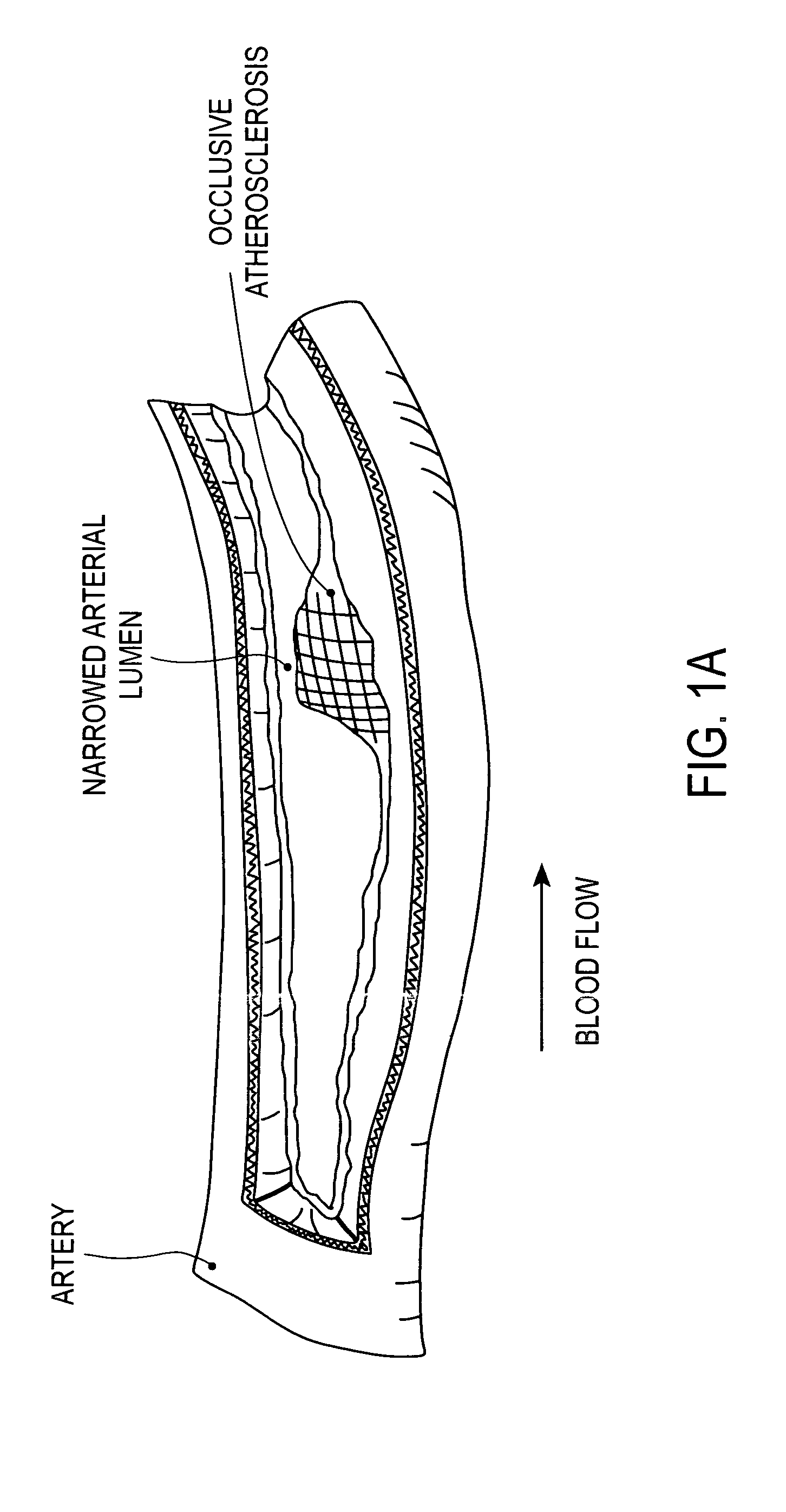
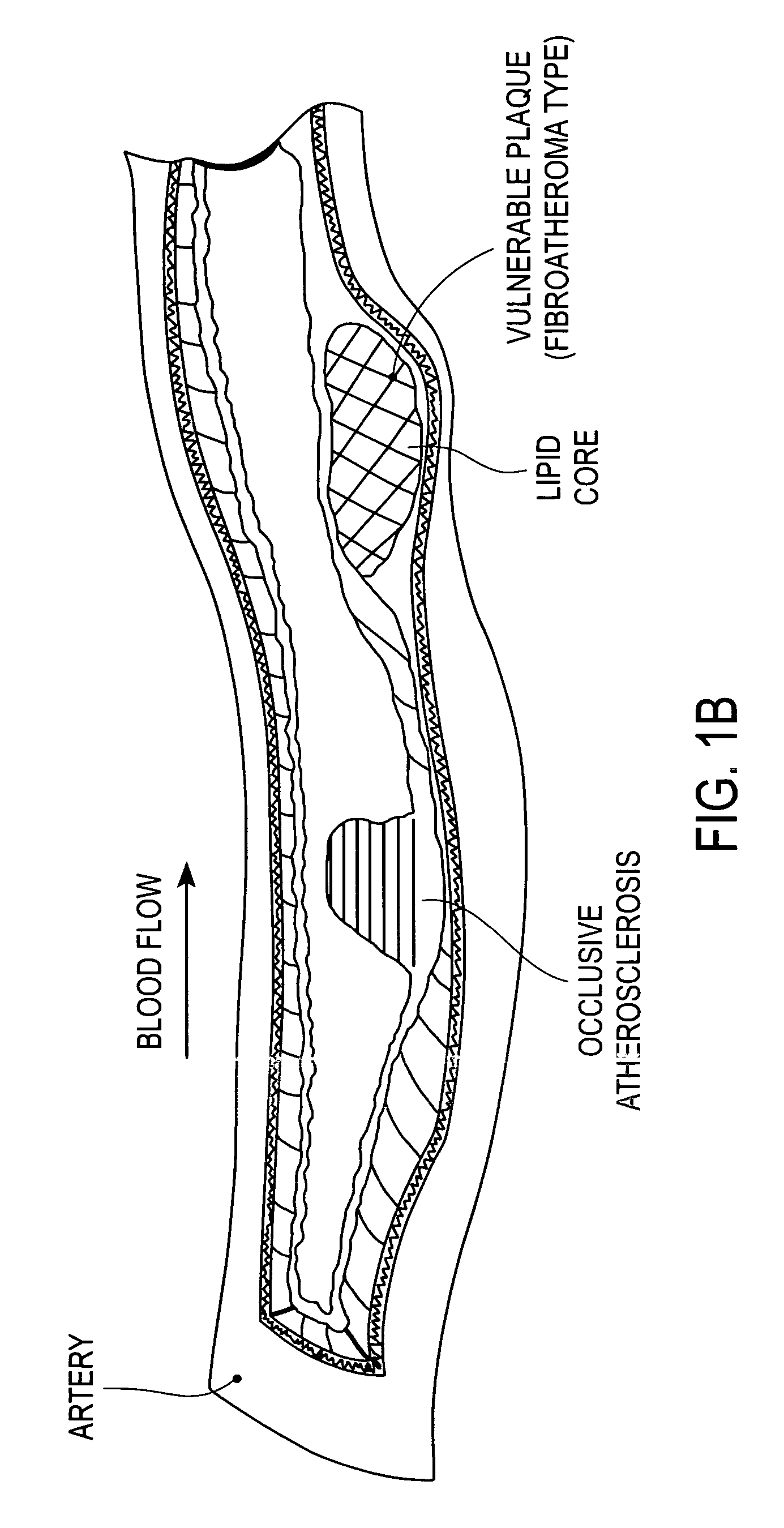
A vulnerable plaque is a kind of atheromatous plaque – a collection of white blood cells (primarily macrophages) and lipids (including cholesterol) in the wall of an artery – that is particularly unstable and prone to produce sudden major problems such as a heart attack or stroke.
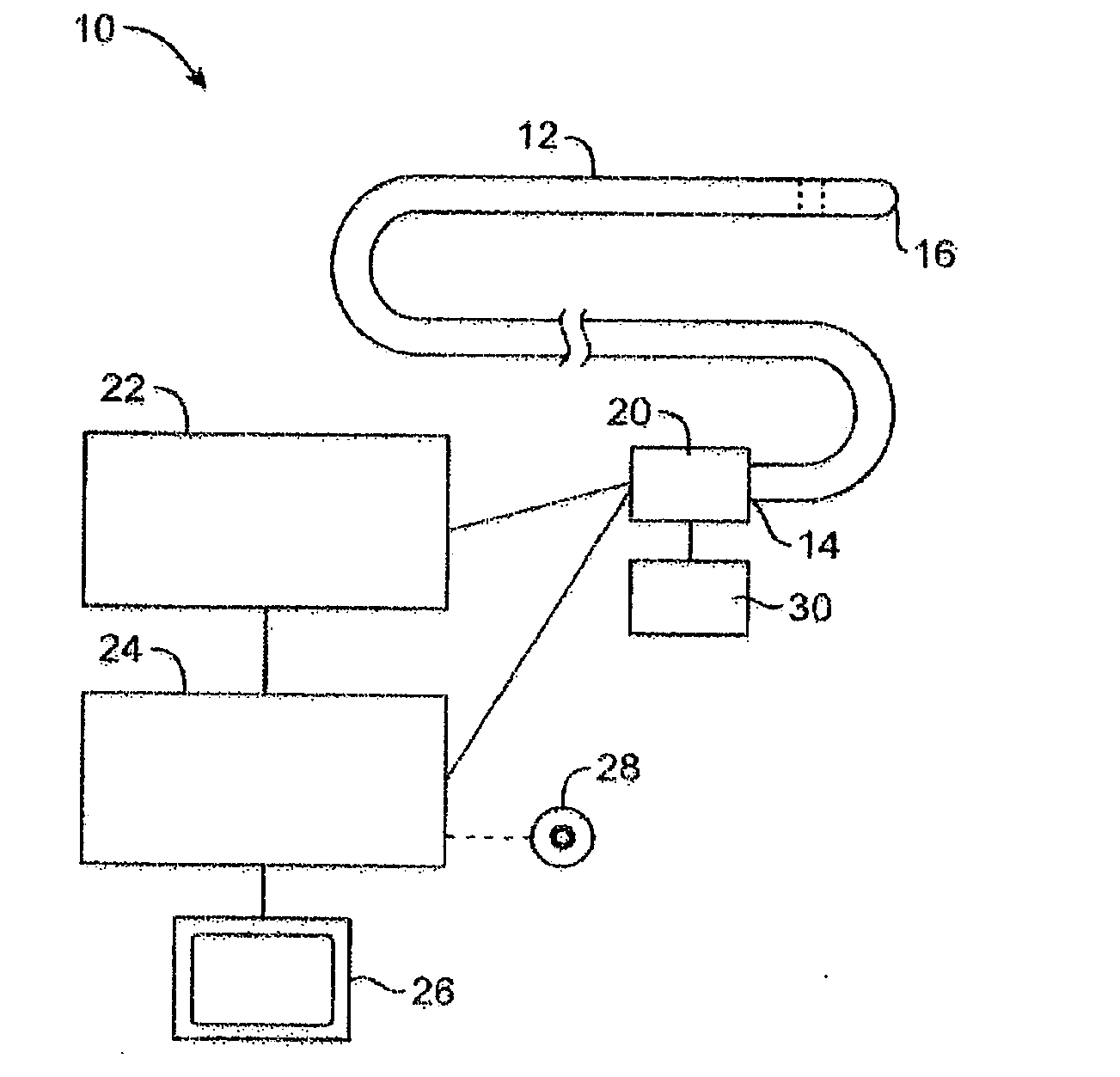
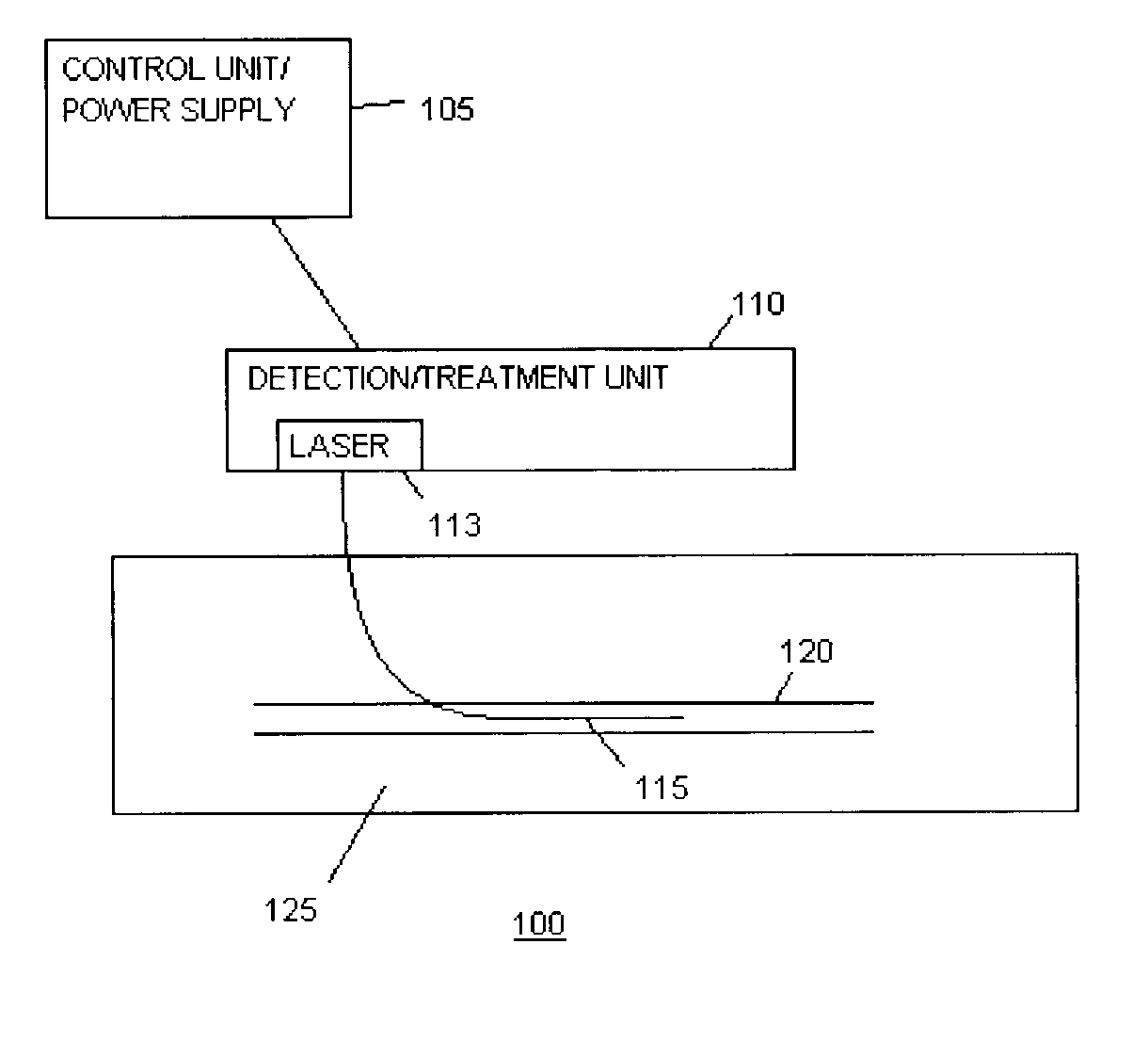
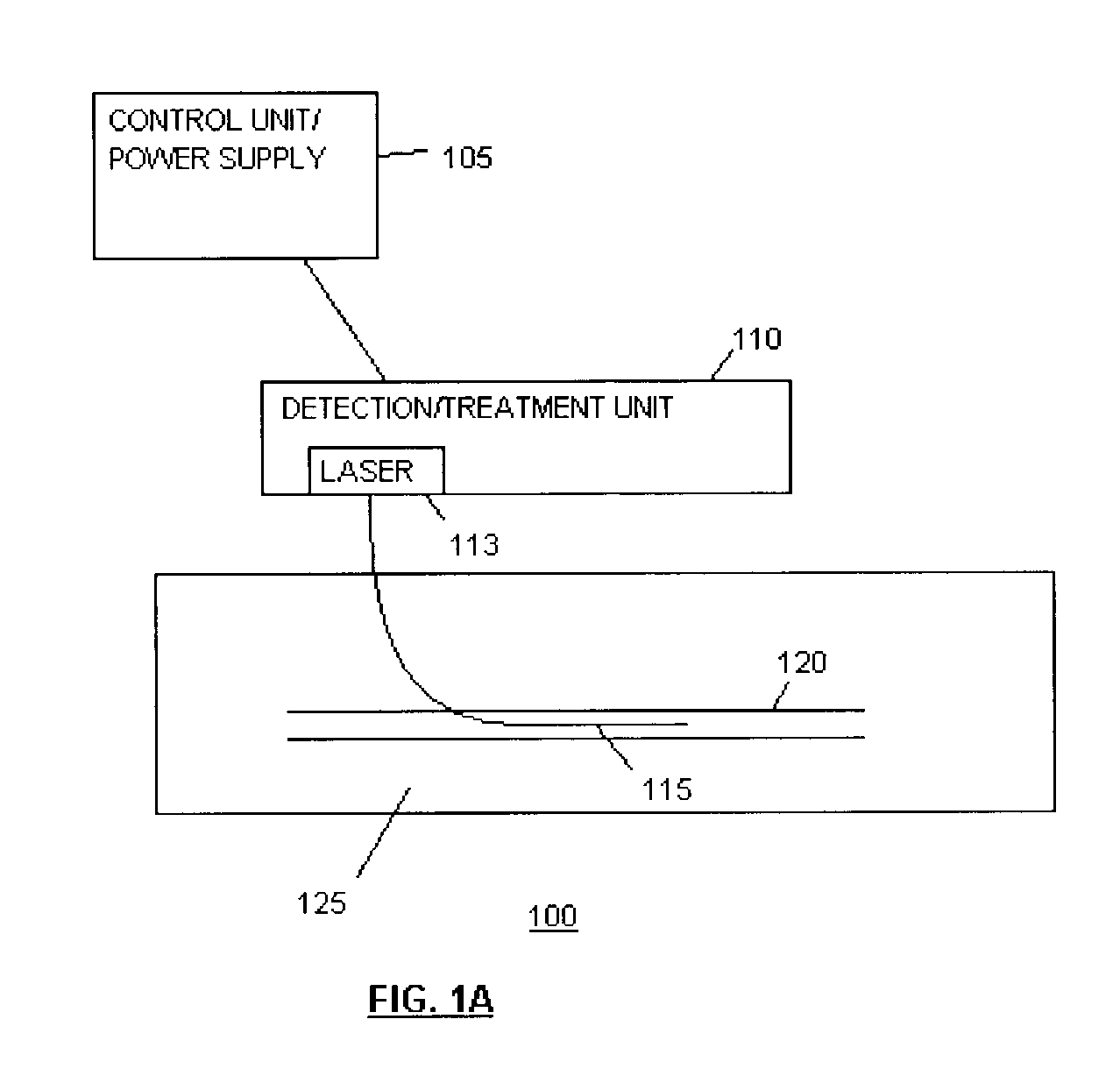
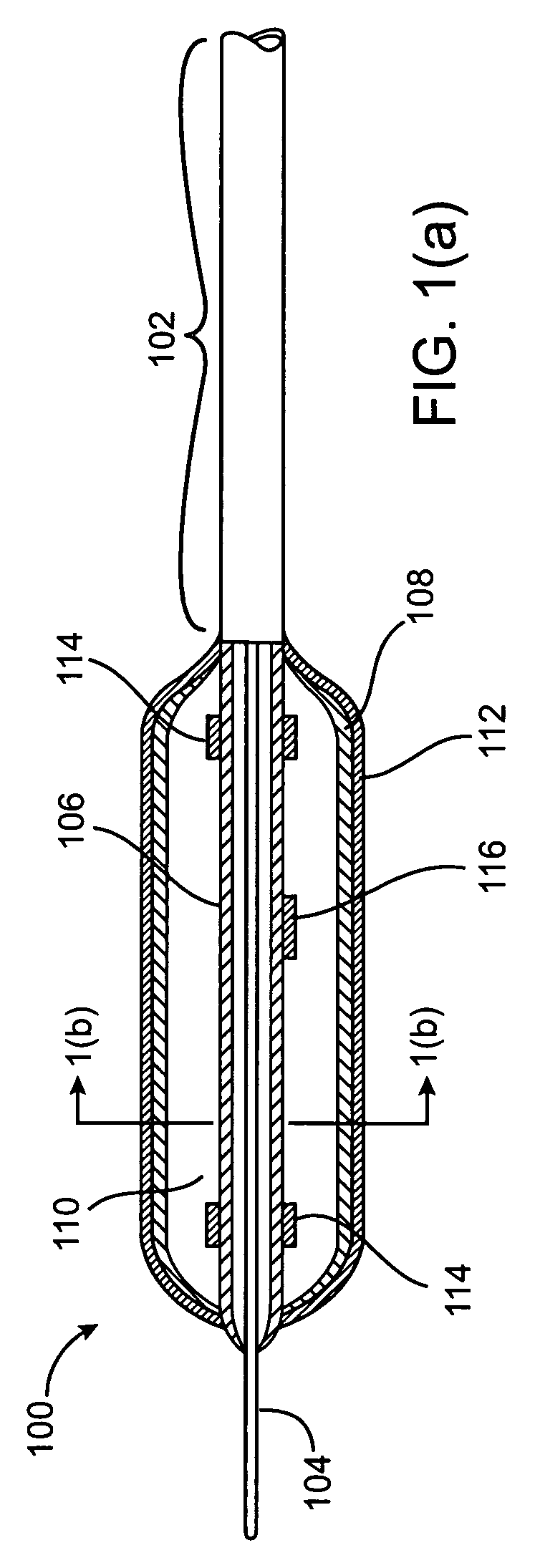
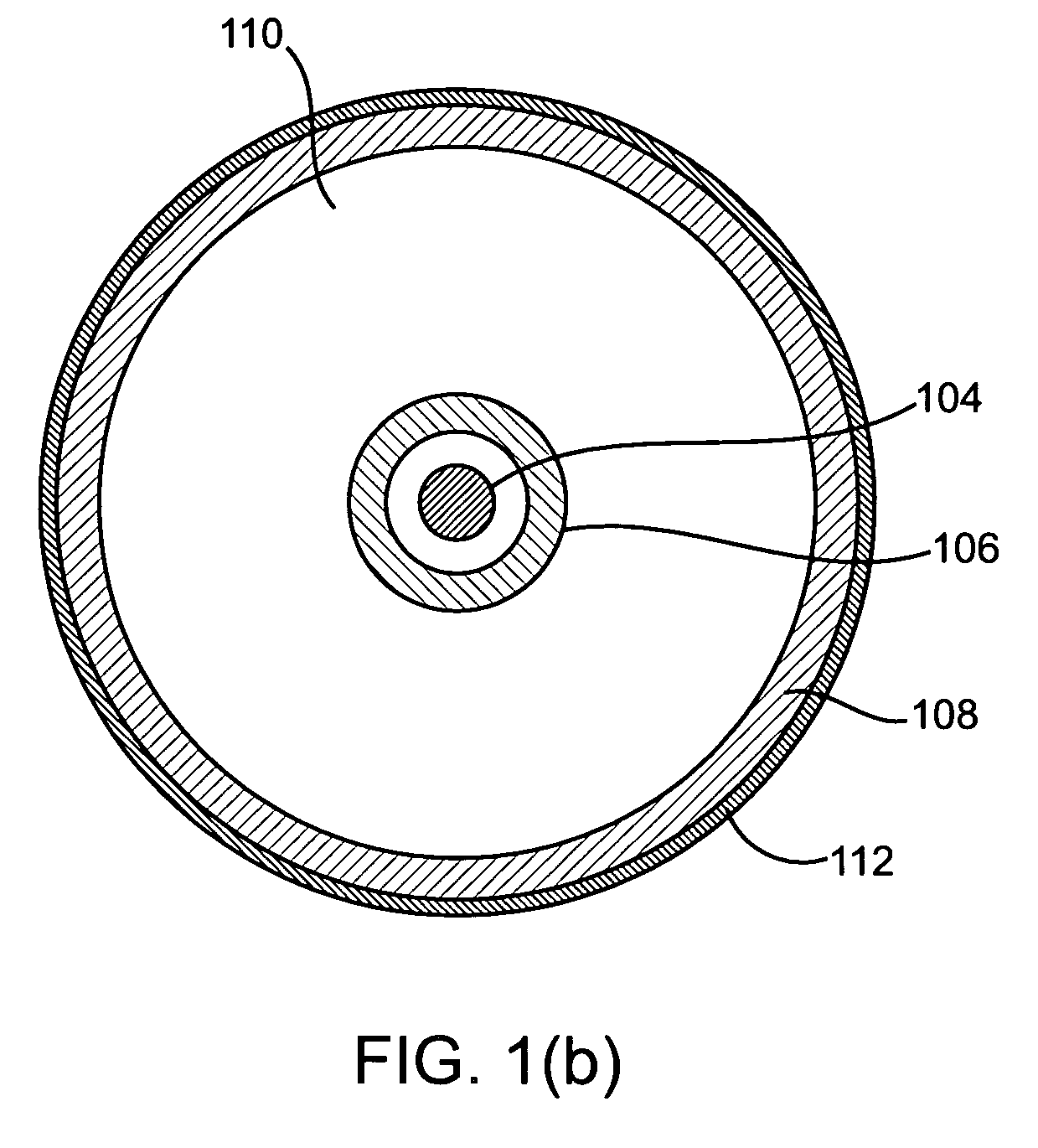
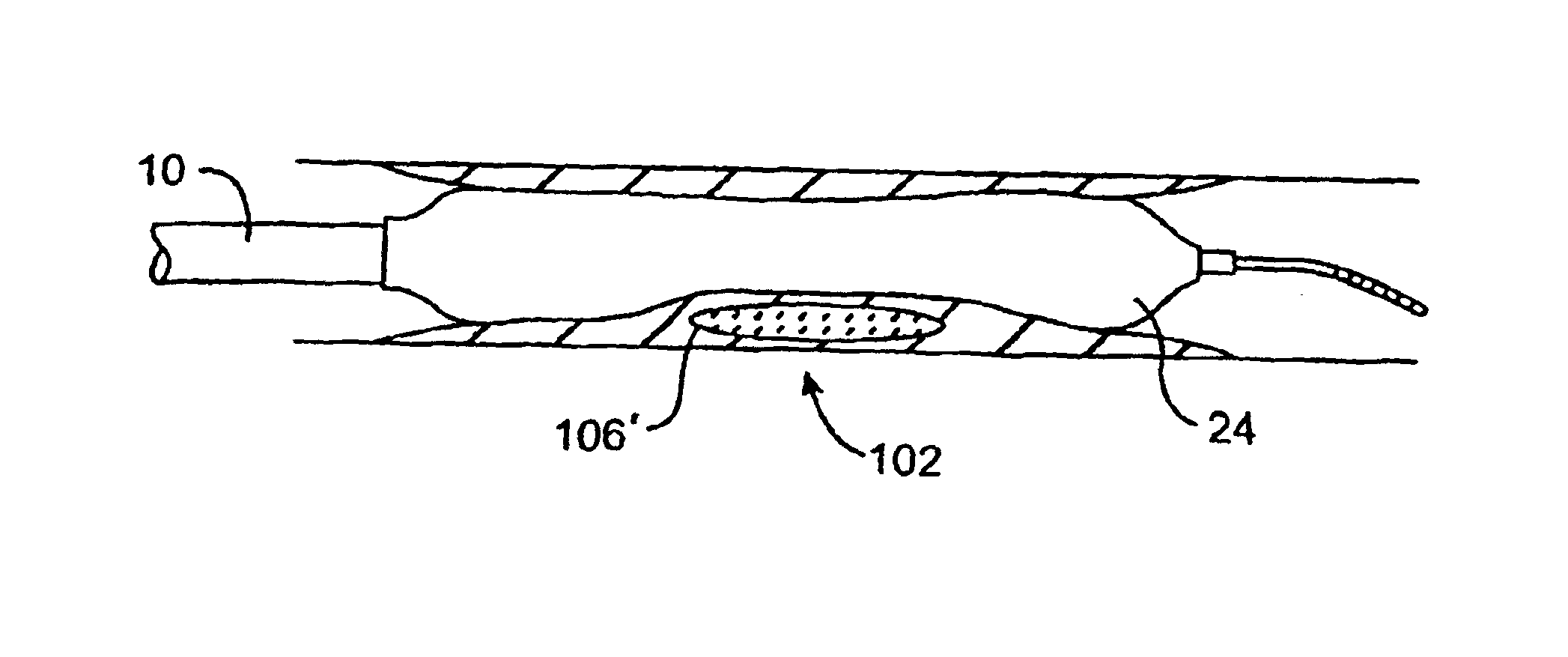
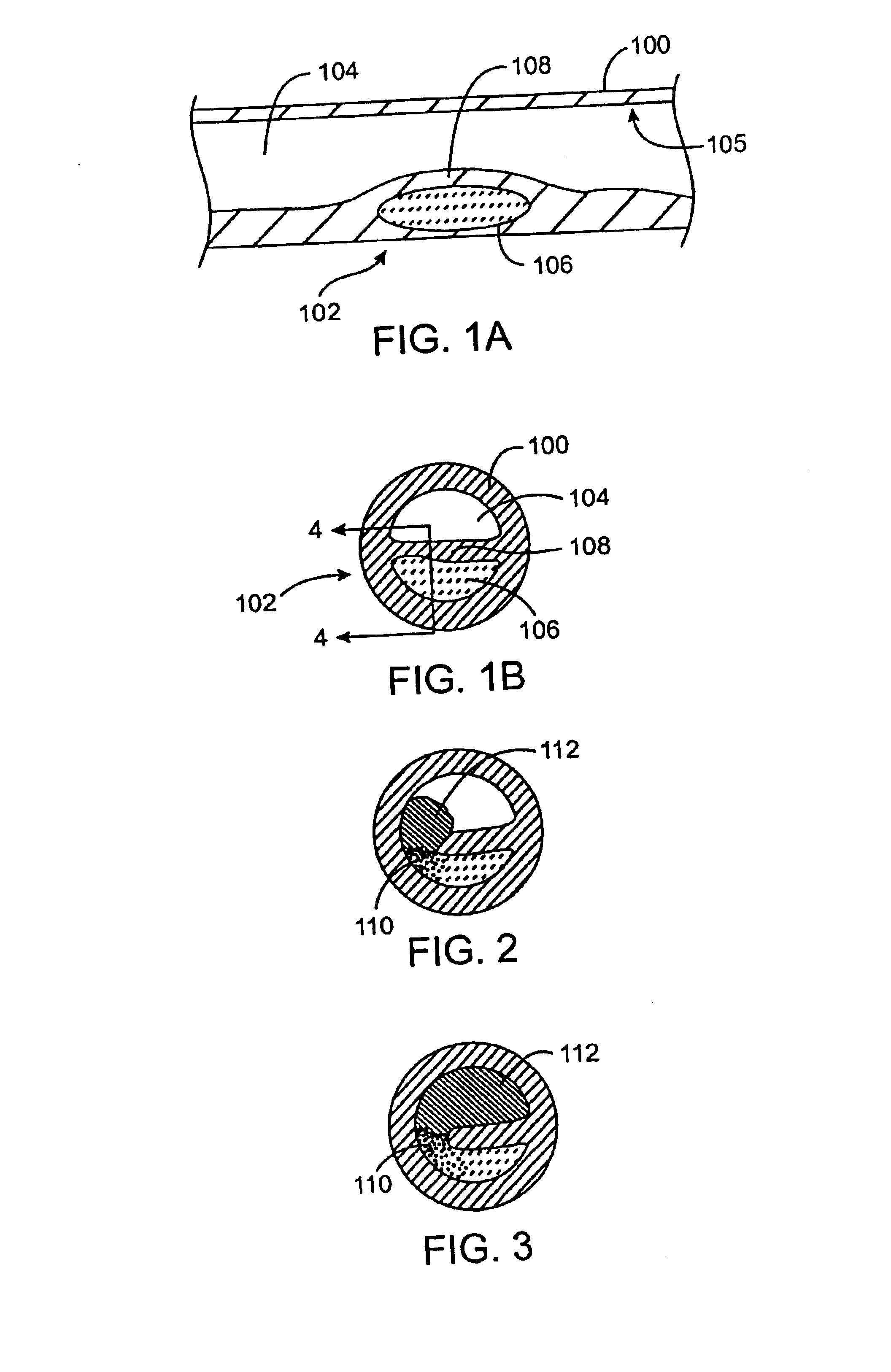
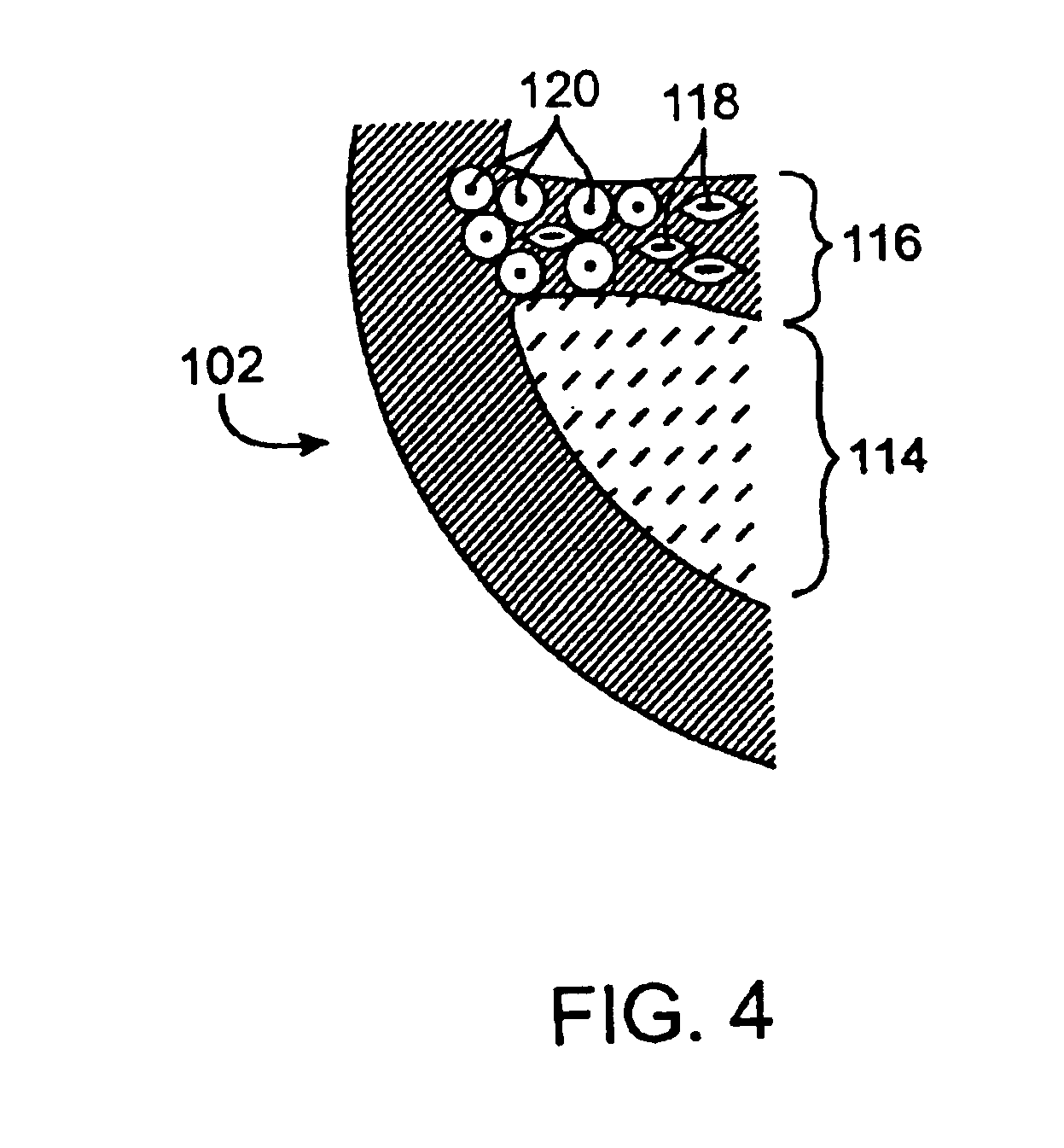
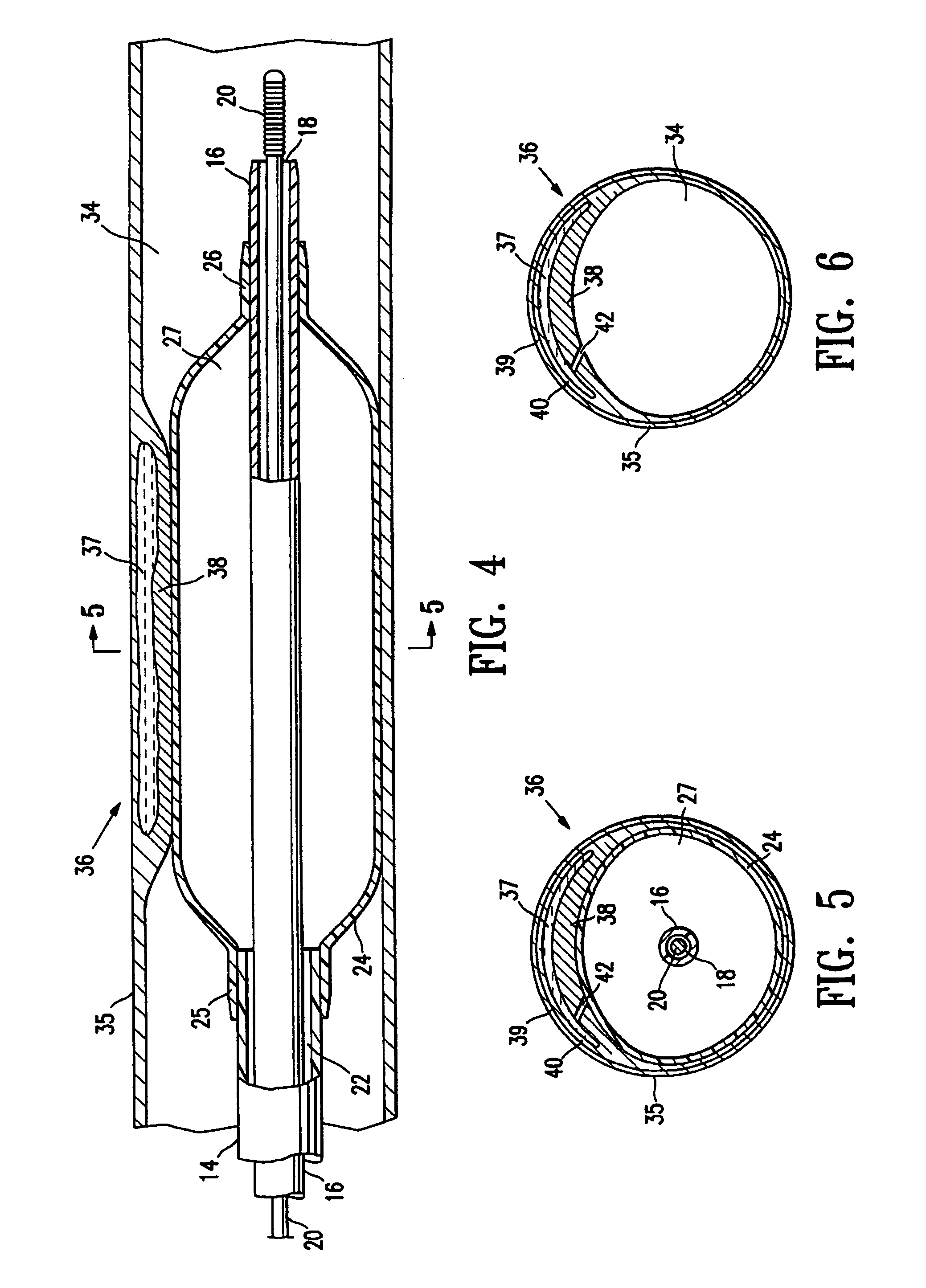
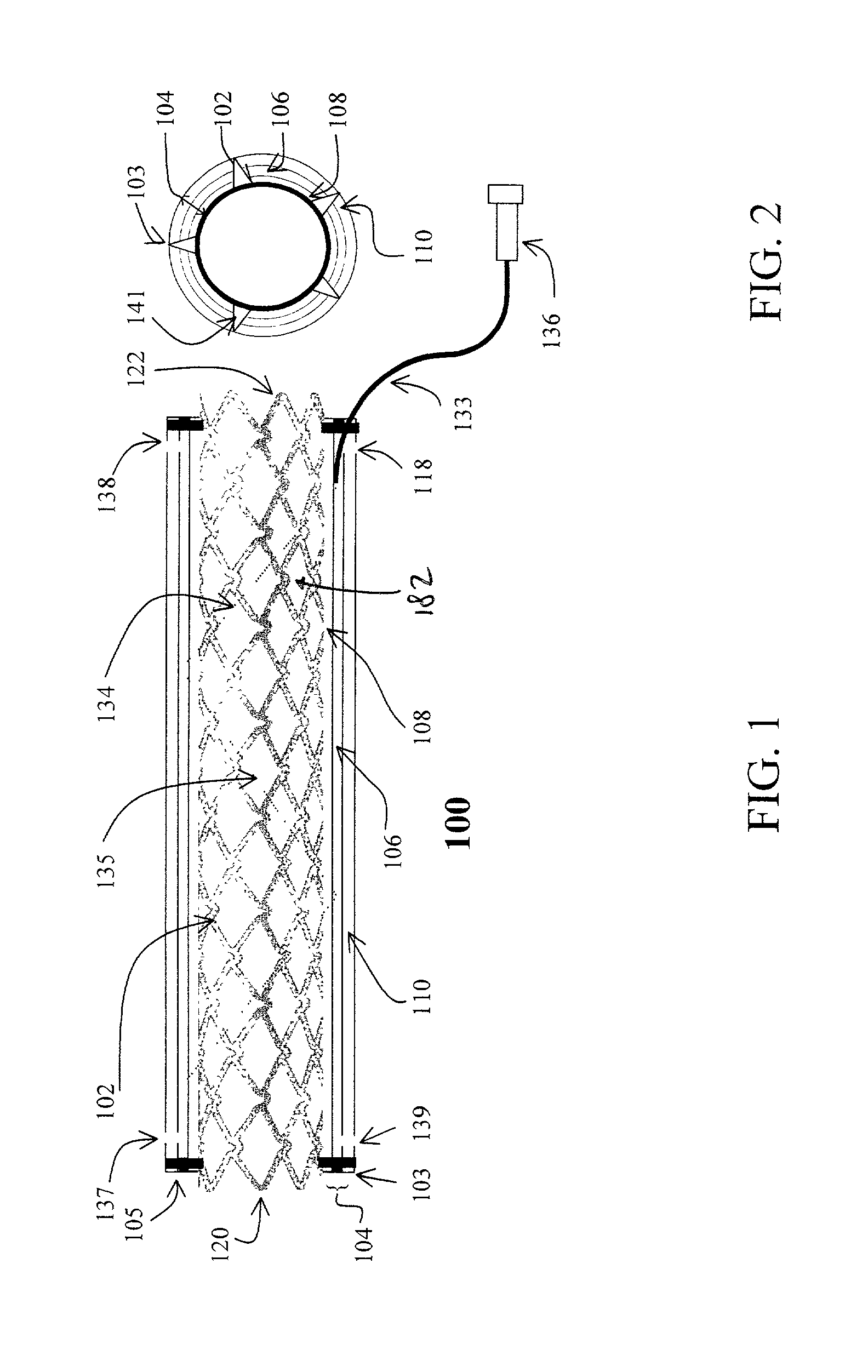
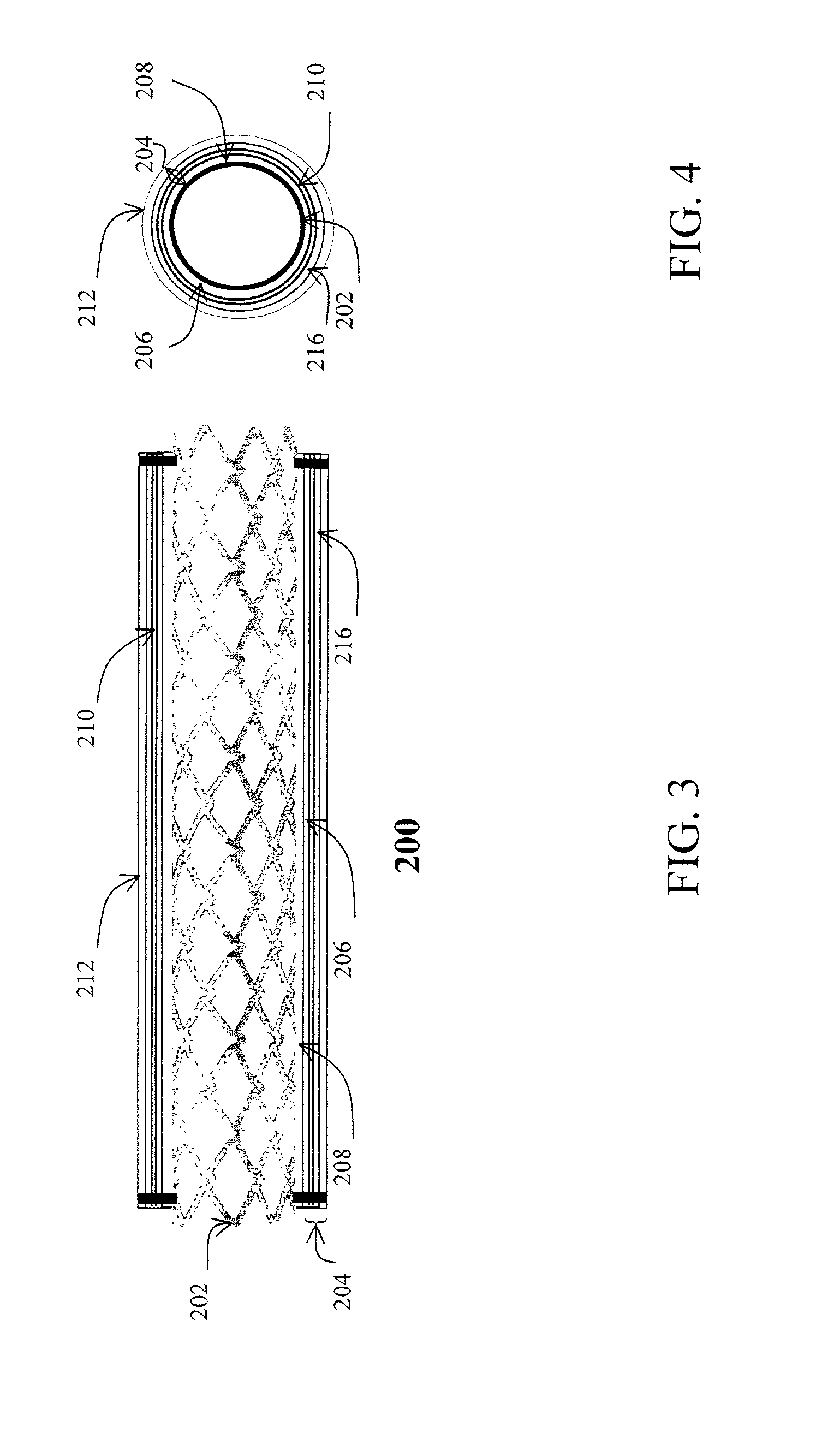
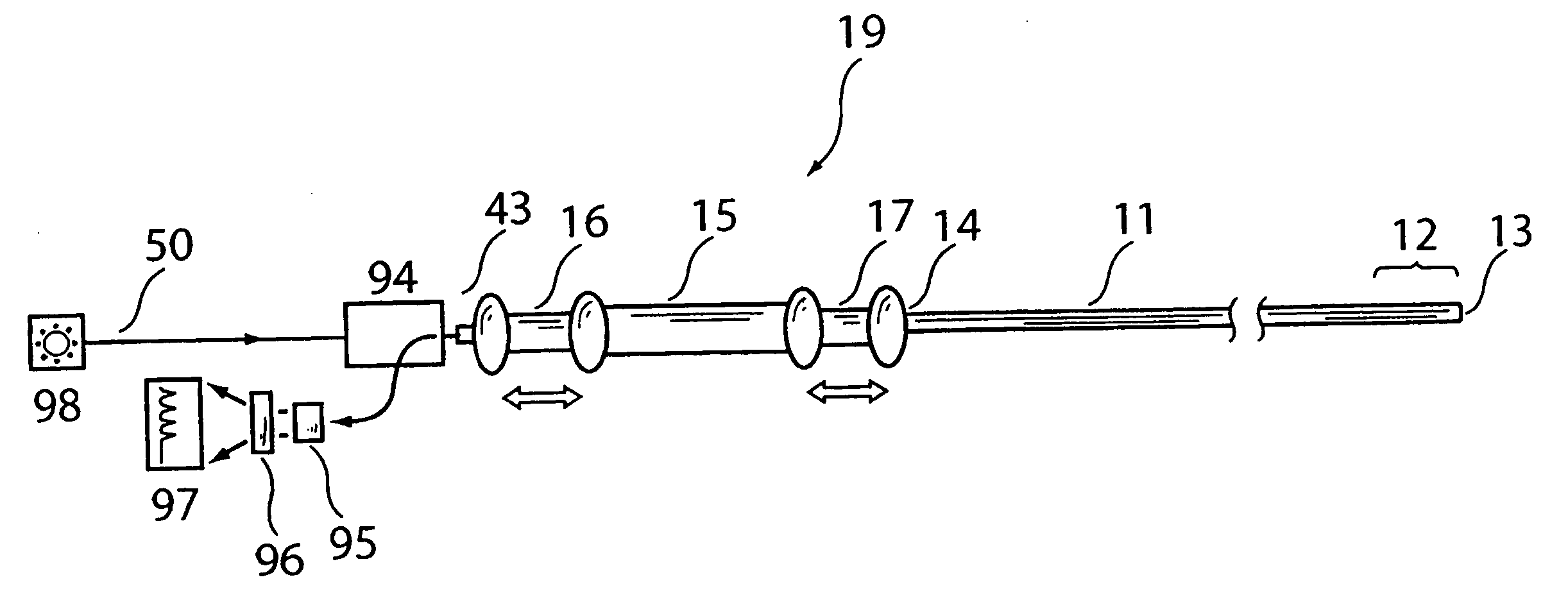
Imaging and eccentric atherosclerotic material laser remodeling and/or ablation catheter
Devices, systems, and methods for treating atherosclerotic lesions and other disease states, particularly for treatment of vulnerable plaques, can incorporate optical coherence tomography or other imaging techniques which allow a structure and location of an eccentric plaque to be characterized. Remodeling and / or ablative laser energy can then be selectively and automatically directed to the appropriate plaque structures, often without imposing mechanical trauma to the entire circumference of the lumen wall.
Owner:VESSIX VASCULAR
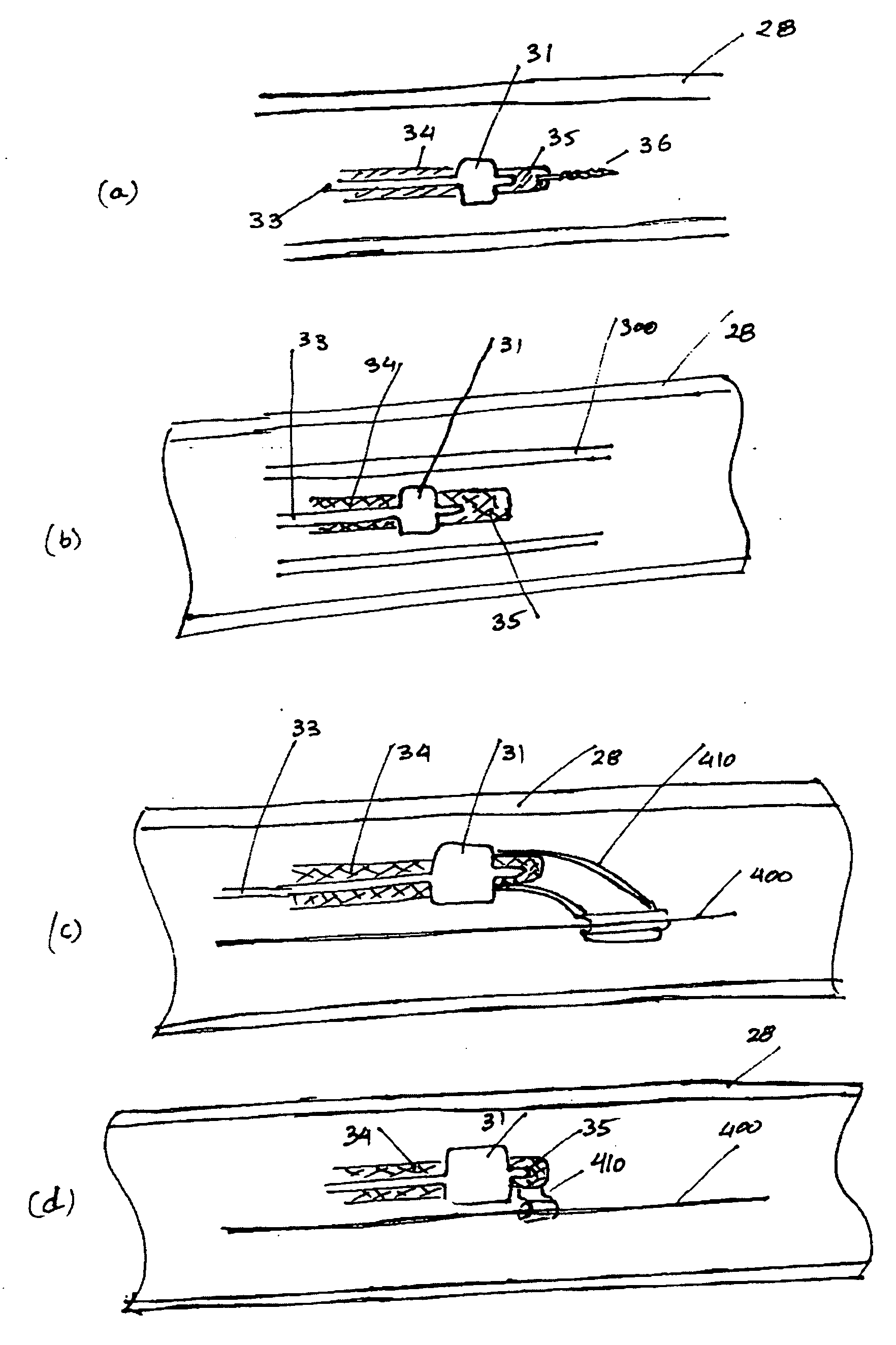
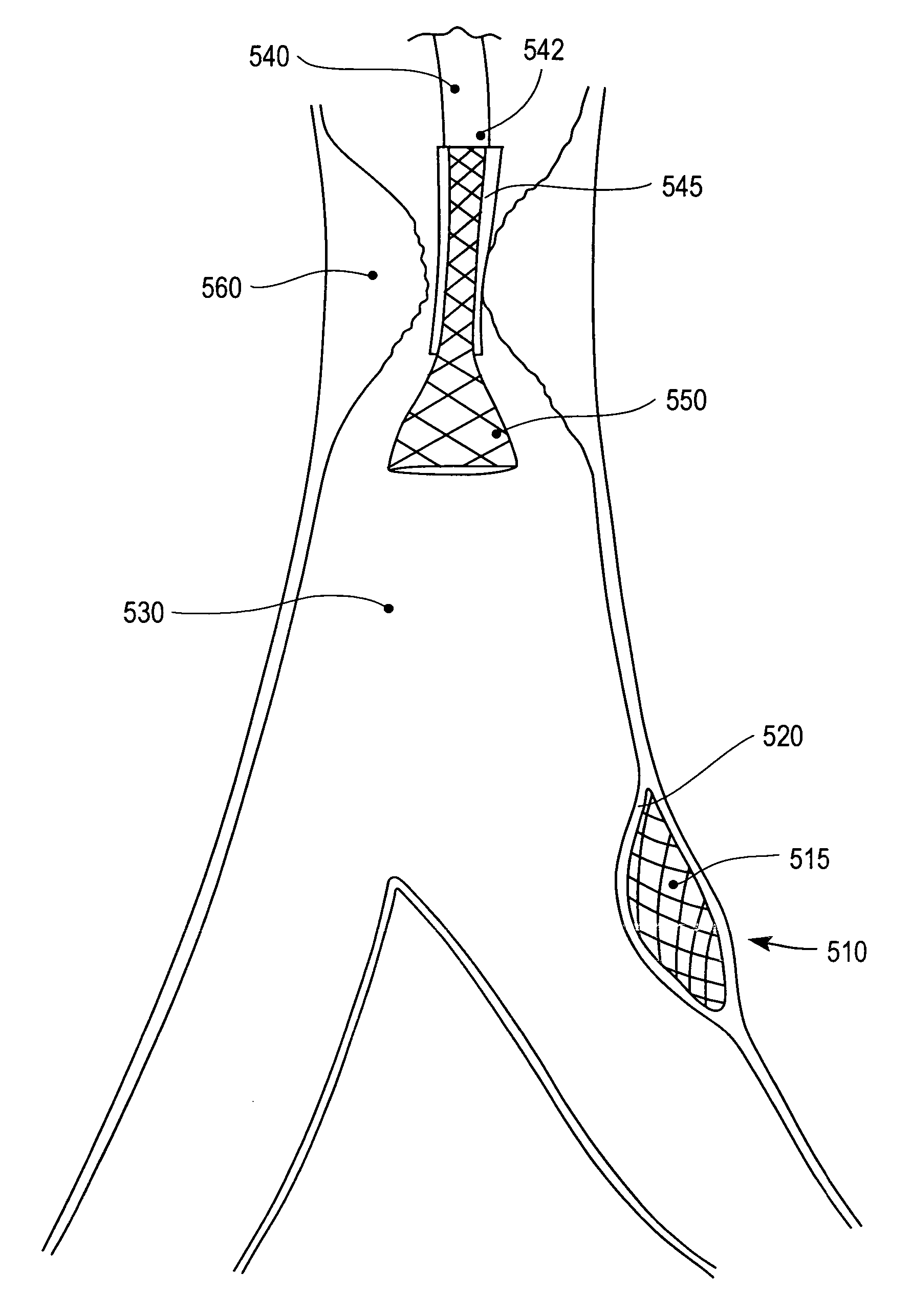
Method and apparatus for treating vulnerable plaque
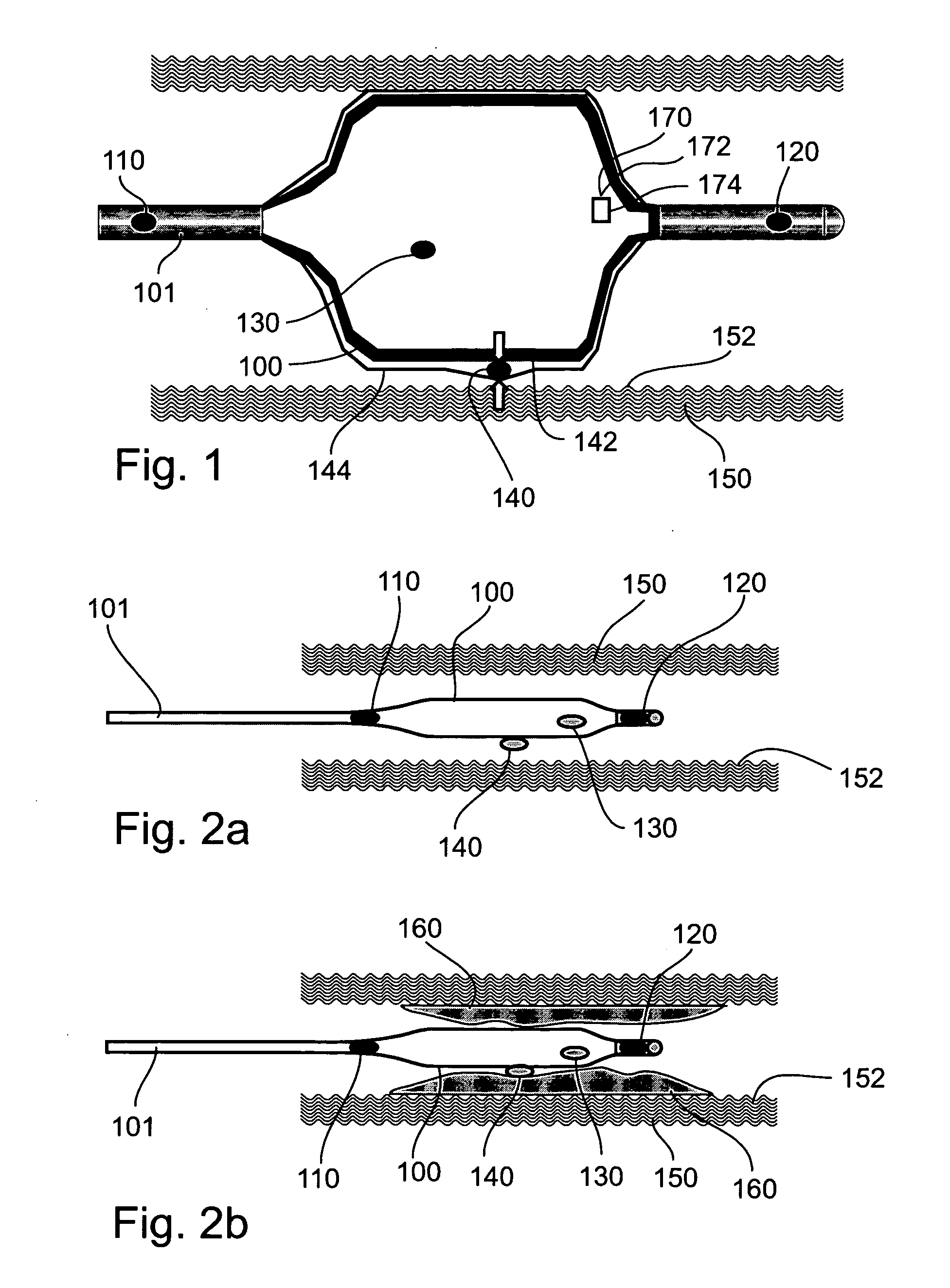
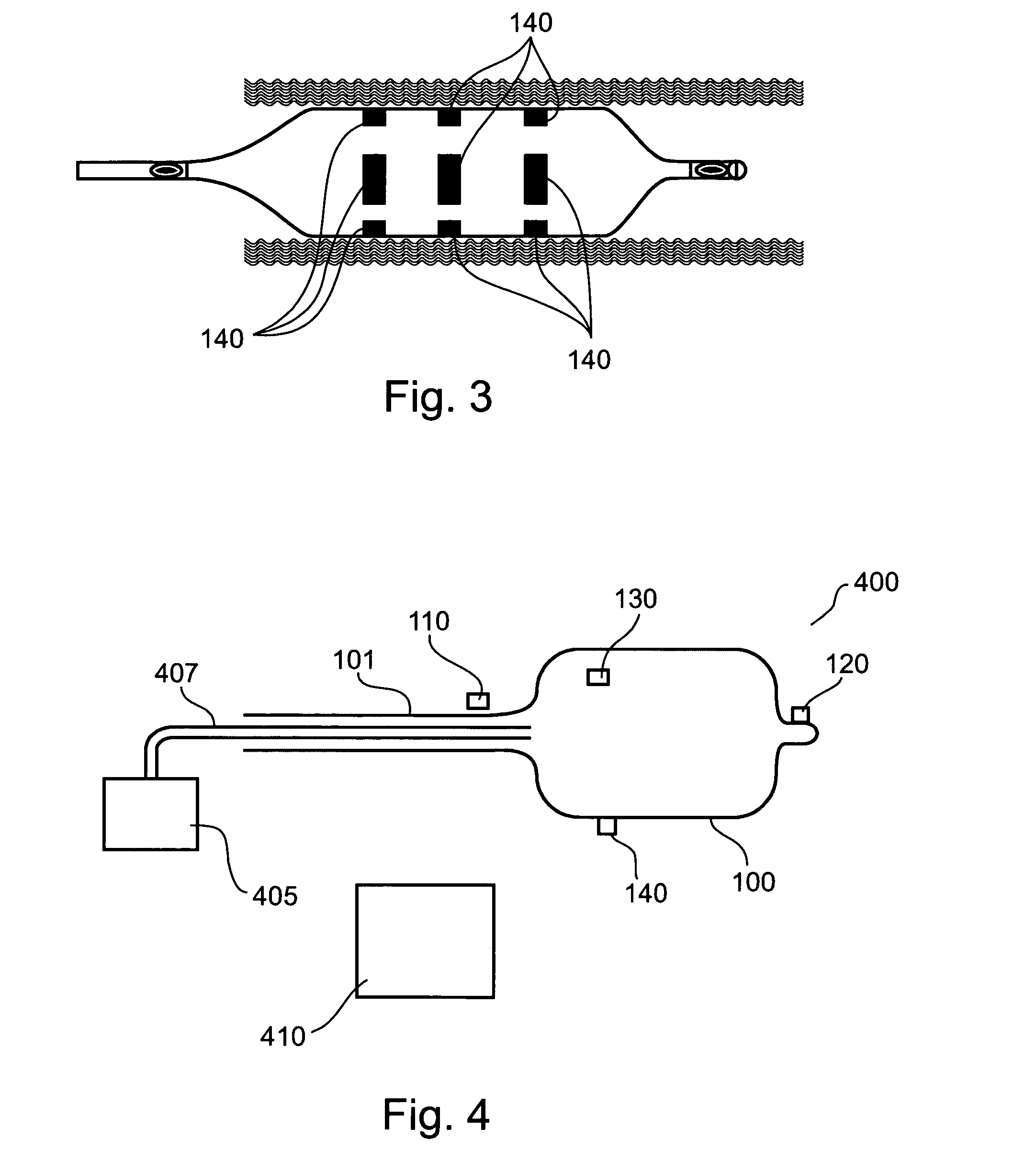
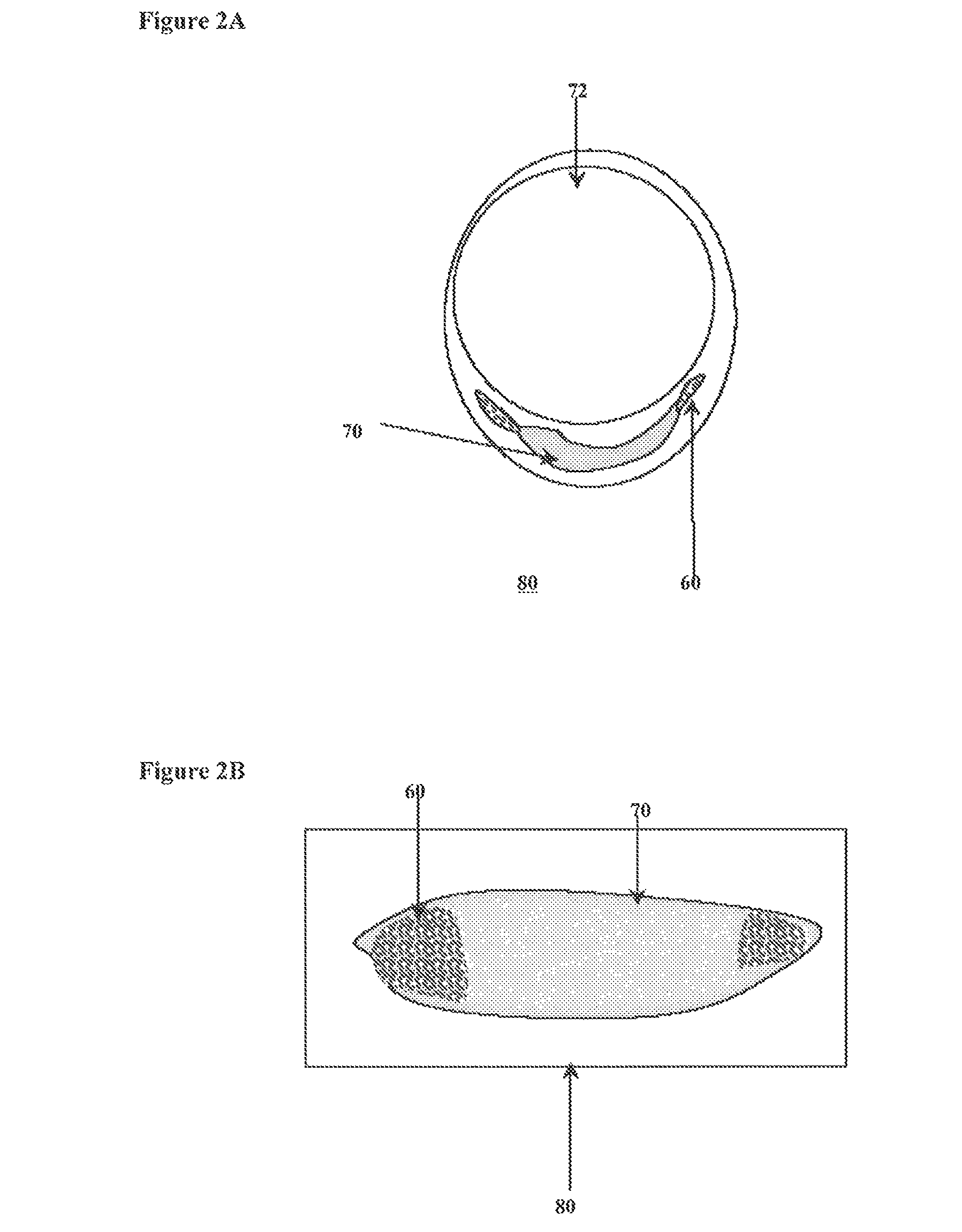
An apparatus and method to treat vulnerable plaque. In one embodiment, the apparatus has an elongated catheter body adapted for insertion in a body lumen, with a drug delivery device attached near a distal portion of the elongated body. The drug delivery device is configured to deliver a biologically active agent to stabilize a vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
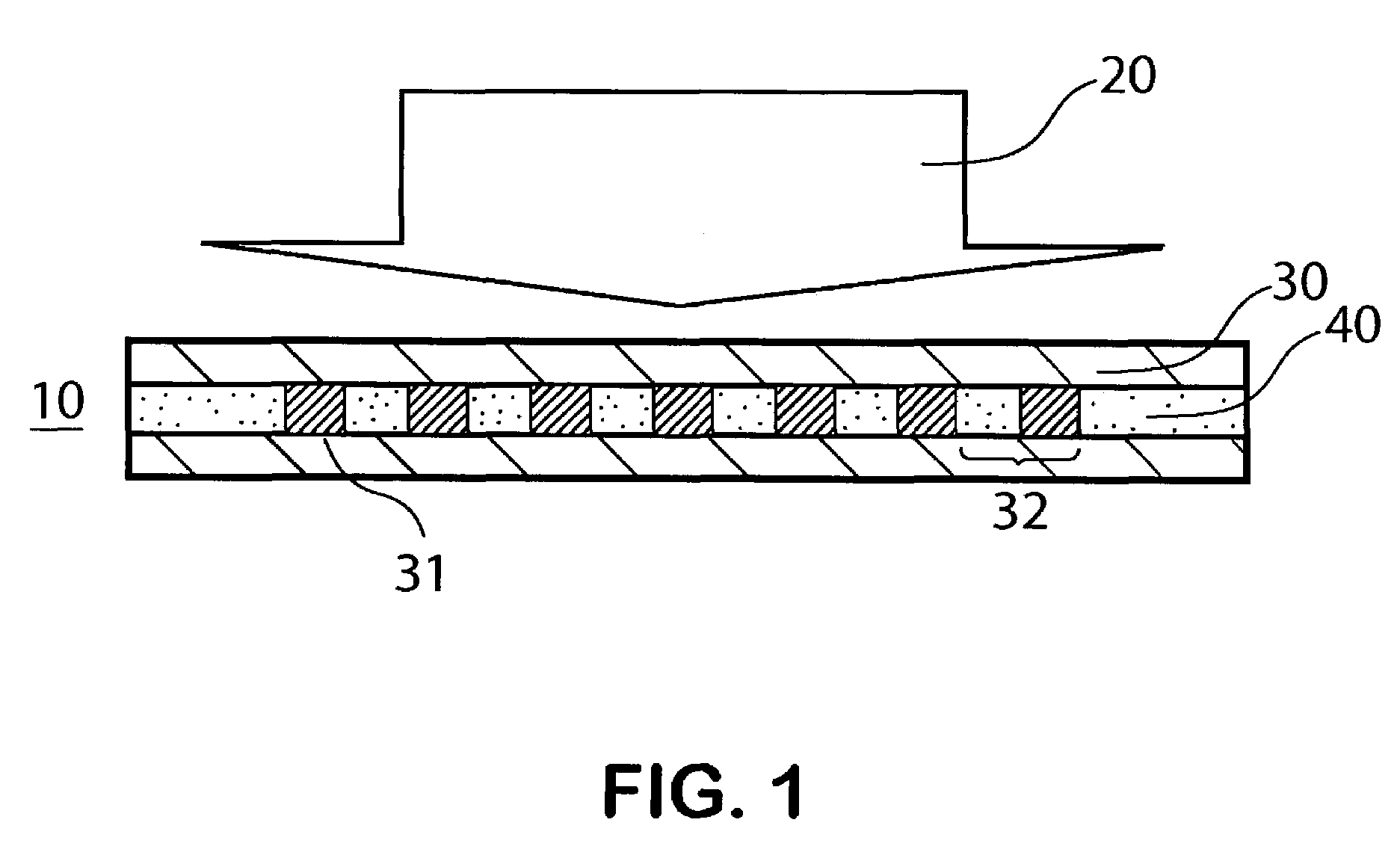
Methods for modulating thermal and mechanical properties of coatings on implantable devices
InactiveUS20050245637A1Narrow molecular weight distributionHigh molecular weightStentsSurgical adhesivesVulnerable plaquePercent Diameter Stenosis
Methods for modulating and enhancing thermal and mechanical properties and biocompatibilities of coatings on implantable devices are disclosed. Implantable devices containing the enhanced thermal and mechanical properties and biocompatibilities are also described. The implantable devices can be used to treat a medical condition such as vulnerable plaque or restenosis.
Owner:ABBOTT CARDIOVASCULAR
Methods and devices for detection and therapy of atheromatous plaque
InactiveUS20030082105A1Easy to detectHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsVulnerable plaqueFluorescence
The present invention relates to devices for detection and therapy of active atheromatous plaque and / or thin-capped fibro-atheroma ("vulnerable plaque"), using selectively targeted fluorescent, radiolabeled, or fluorescent and radiolabeled compositions. The present invention further relates to methods and devices for detection and theraphy of active atheromatous plaques and / or vulnerable plaques, using selectively targeted beta-emitting compositions, optionally comprising fluorescent compositions.
Owner:THE GENERAL HOSPITAL CORP
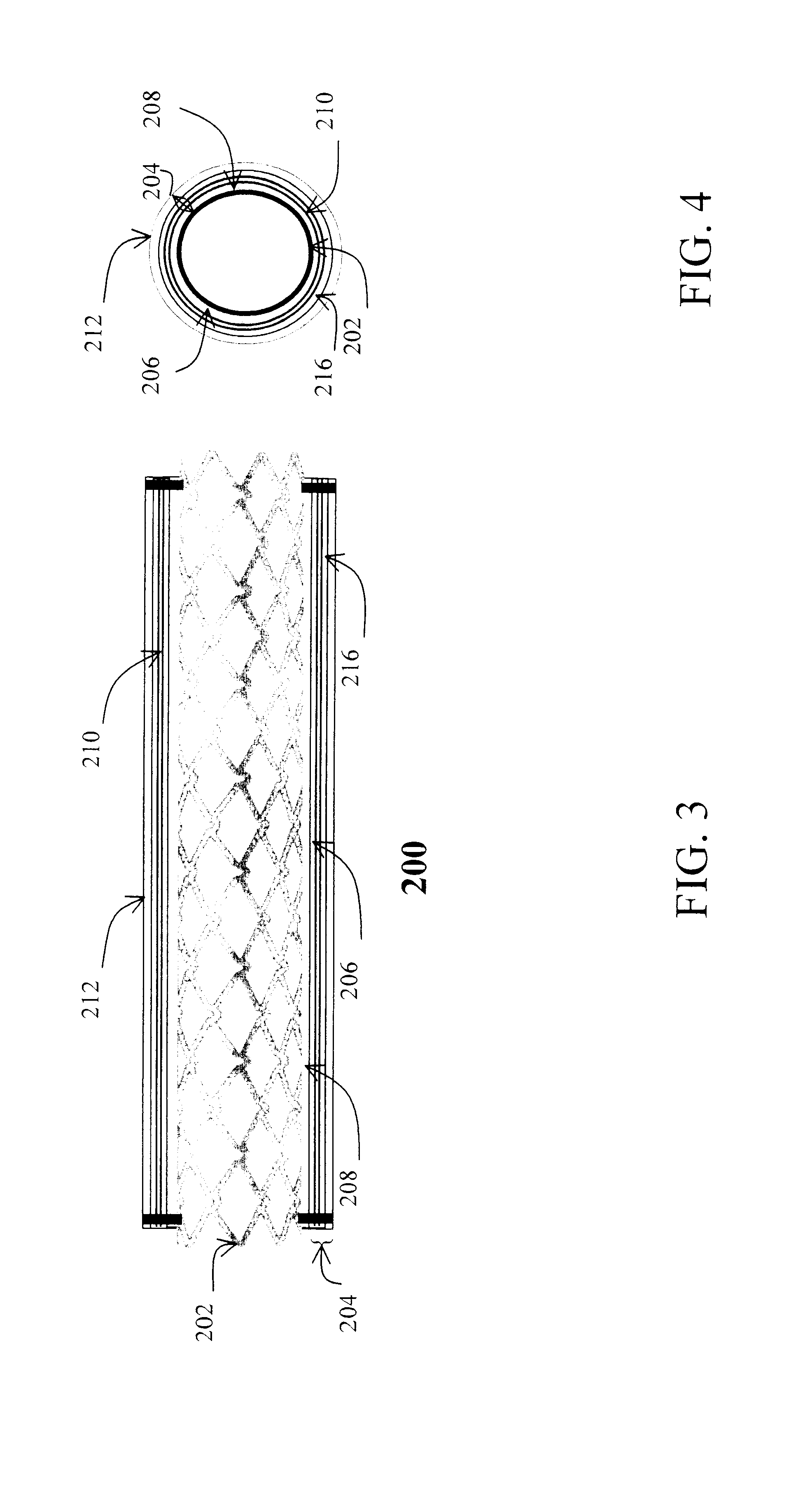
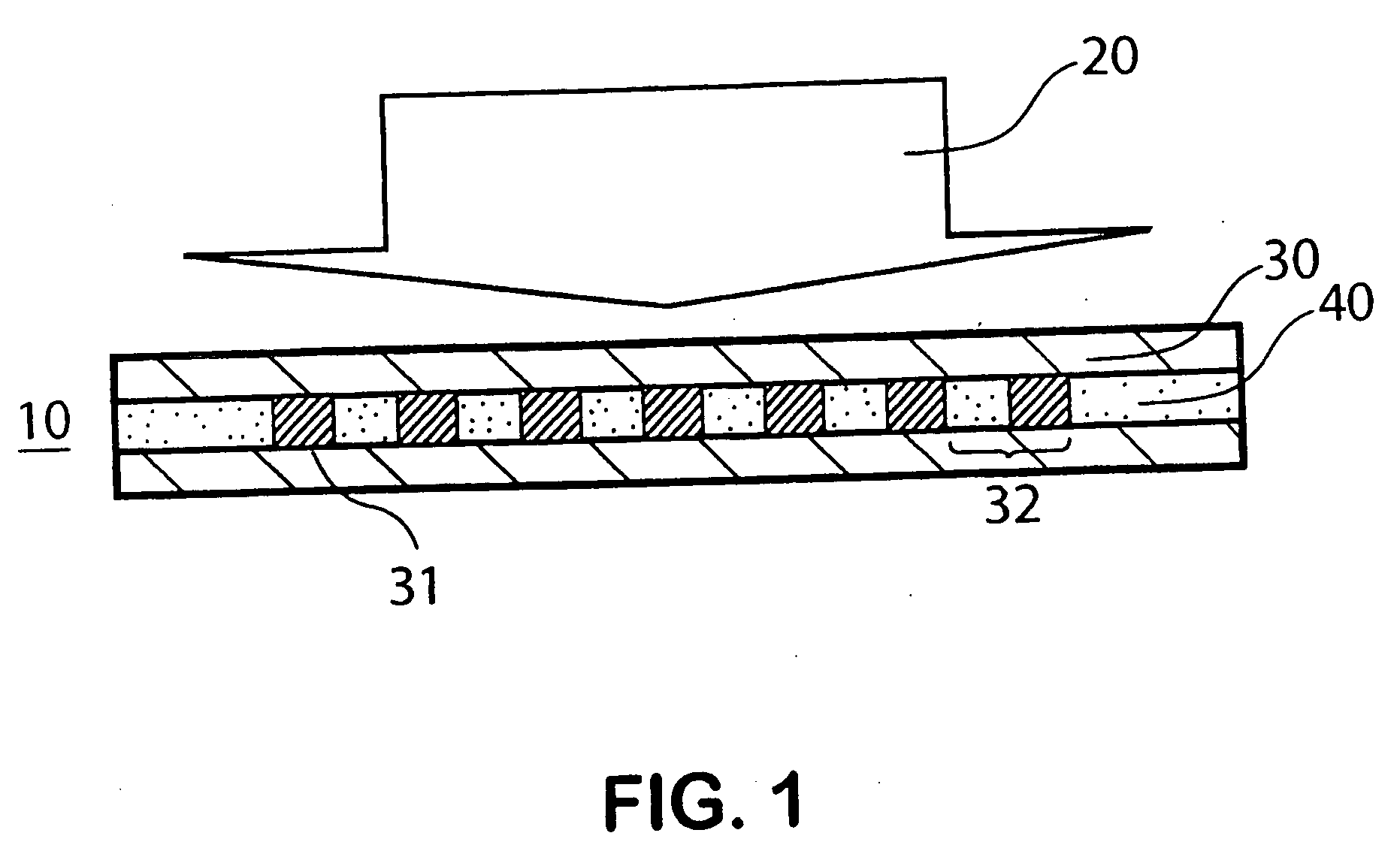
Drug-eluting biodegradable stent
The present invention relates to a drug-loaded biodegradable stent and methods for treating vulnerable plaques of a patient comprising a plurality of layers or zones, each layer or zone comprising its own specific biodegradation rate and its specific drug loading characteristics. In one embodiment, the layers and zones are configured and arranged, in combination, radially, circumferentially and longitudinally.
Owner:GP MEDICAL
Method and apparatus for detecting and treating vulnerable plaques
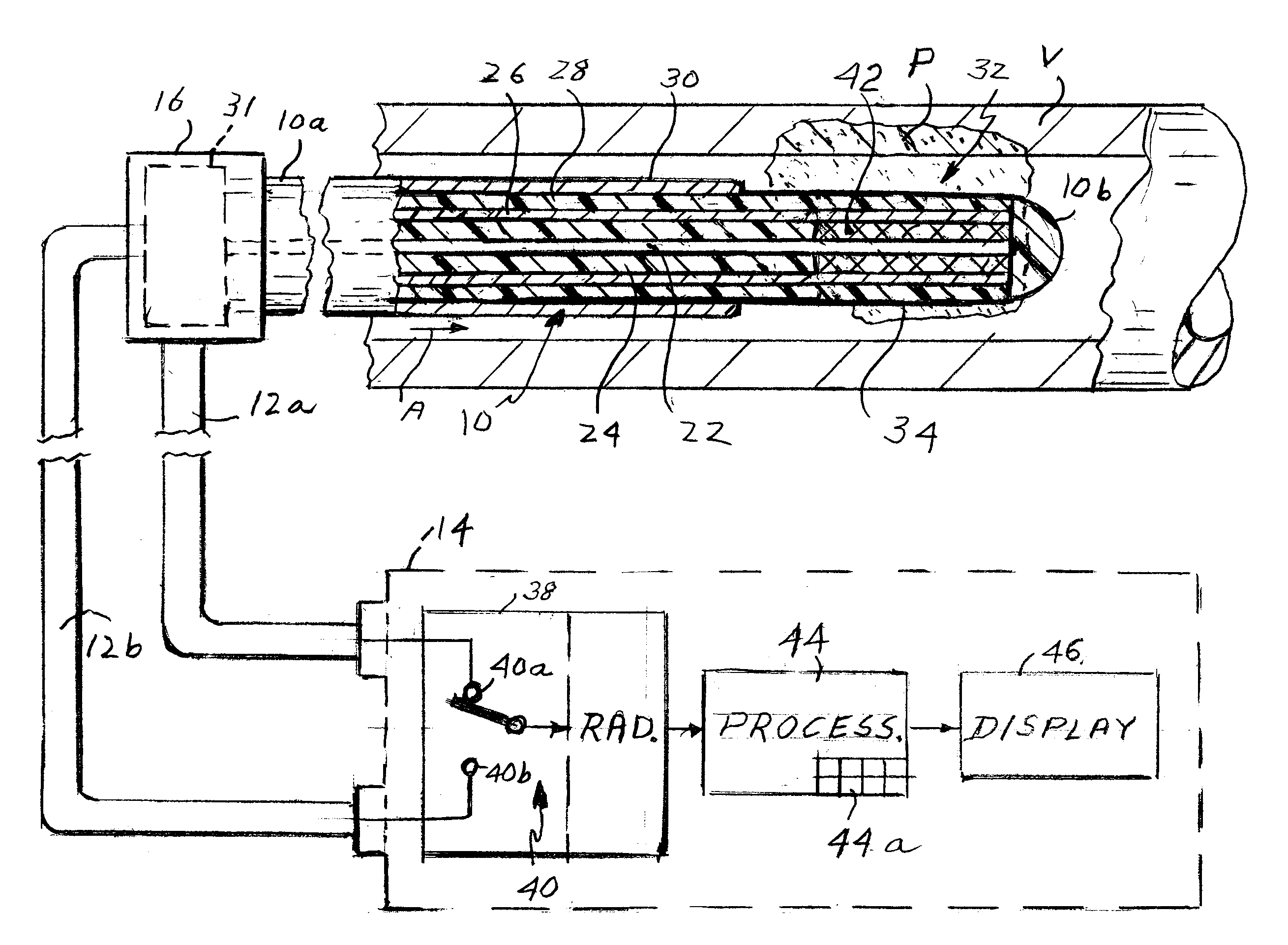
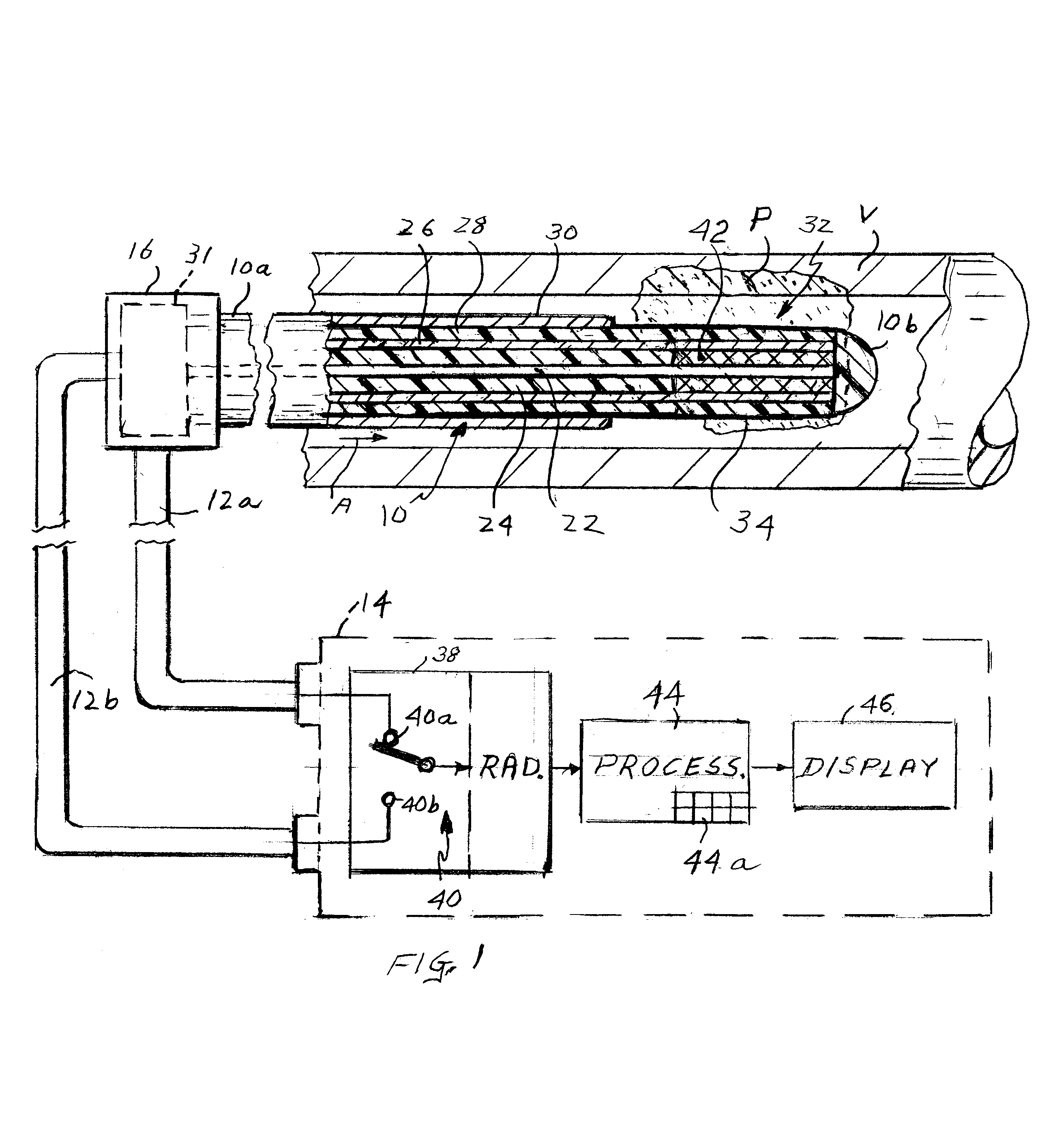
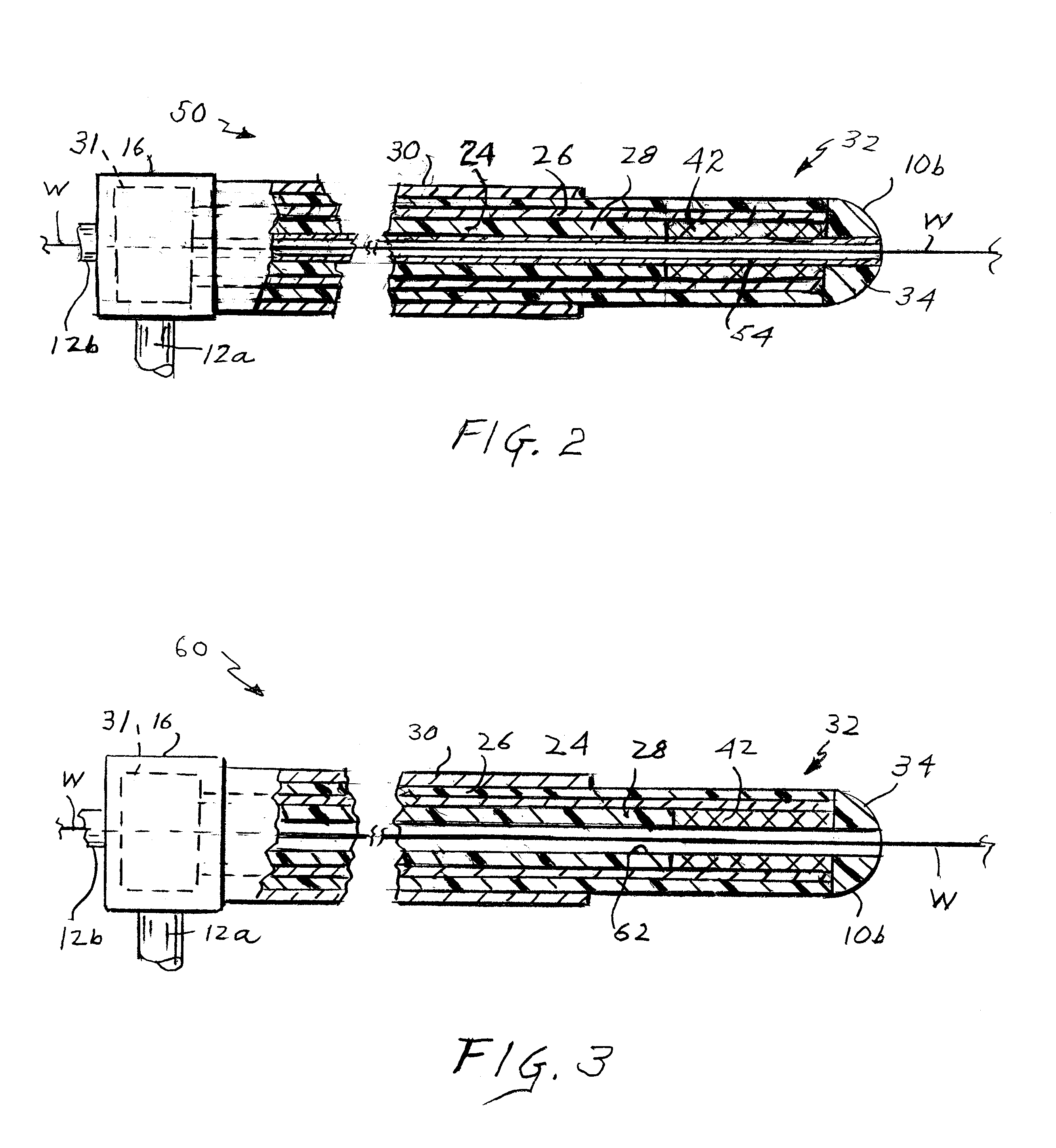
Apparatus for detecting vulnerable plaques embedded in the wall of a patient's blood vessel includes an intravascular catheter containing a microwave antenna, an extra-corporeal radiometer having a signal input, a reference input and an output, a cable for electrically connecting the antenna to the signal input, and a device for applying an indication of the patient's normal tissue temperature to the reference input so that when the catheter is moved along the vessel, the locations of the vulnerable plaques are reflected in a signal from the output as thermal anomalies due to the higher emissivity of the vulnerable plaques as compared to the normal tissue. A second embodiment of the apparatus has two coaxial antennas in the catheter serving two radiometers. One measures the temperature at locations in the vessel wall, the other measures the temperature at the surface. By subtracting the two signals, the locations of vulnerable plaque may be visualized. The apparatus employs a special diplexer for separating the signals from the two antennas and a method of detecting and possibly destroying the plaques is also disclosed.
Owner:CORAL SAND BEACH LLC
Optical thermal mapping for detecting vulnerable plaque
InactiveUS7004911B1Easy to operateSimple, real-timeCatheterDiagnostic recording/measuringFiberGrating
An optical thermal device and methods for monitoring temperature and detection of a vulnerable plaque of a patient comprising an elongate tubular element comprising at least one optical fiber; the fiber having at least one optical grating along an axis of the fiber; and a light source having a light beam that is coupled into the at least one optical fiber; wherein the optical grating reflects a certain wavelength or intensity of the light beam, the certain wavelength or intensity of the reflected light beam being correlated to the temperature of the tissue regions.
Owner:MAXWELL SENSORS +1
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Temperature mapping balloon
ActiveUS7081096B2Reliable detectionEndoscopesDiagnostic recording/measuringTemperature mappingVulnerable plaque
The temperature mapping balloon of the present invention provides a device for locating inflammation and increased metabolic activity associated with conditions such as vulnerable plaque, by mapping temperature of a body lumen, such as an artery or blood vessel. The temperature mapping balloon comprises a balloon with a thermal mapping coating disposed on the inside or outside of the balloon. The thermal mapping coating can be a thermochromic coating that changes color in response to temperature or a sensor coating comprising a plurality of temperature sensors.
Owner:MEDTRONIC VASCULAR INC
Electromagnetic photonic catheter for reducing restenosis
InactiveUS6962584B1Reduce spreadImprove scalabilitySurgical instrument detailsCatheterPhotonicsPercent Diameter Stenosis
The method of vascular treatment for restenosis or vulnerable plaque after an invasive procedure, such as for example angioplasty, stenting with or without drug coating, or drug delivery, comprises: inserting a catheter or hollow guide wire to the treatment location; delivering light through the catheter in the wavelength range of about 700–2500 nm; and moving the light to treat the affected region.
Owner:STONE GREGG W +5
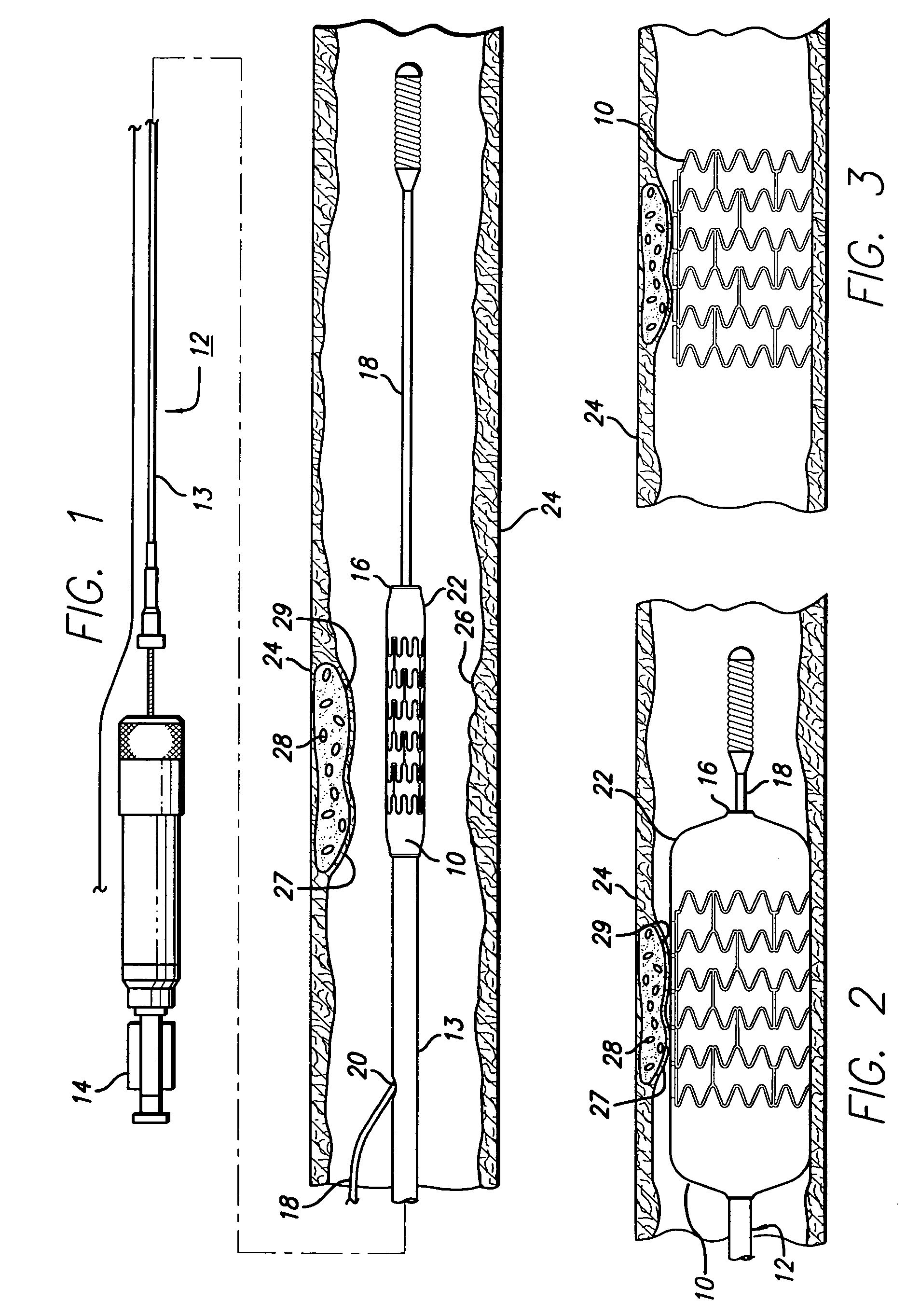
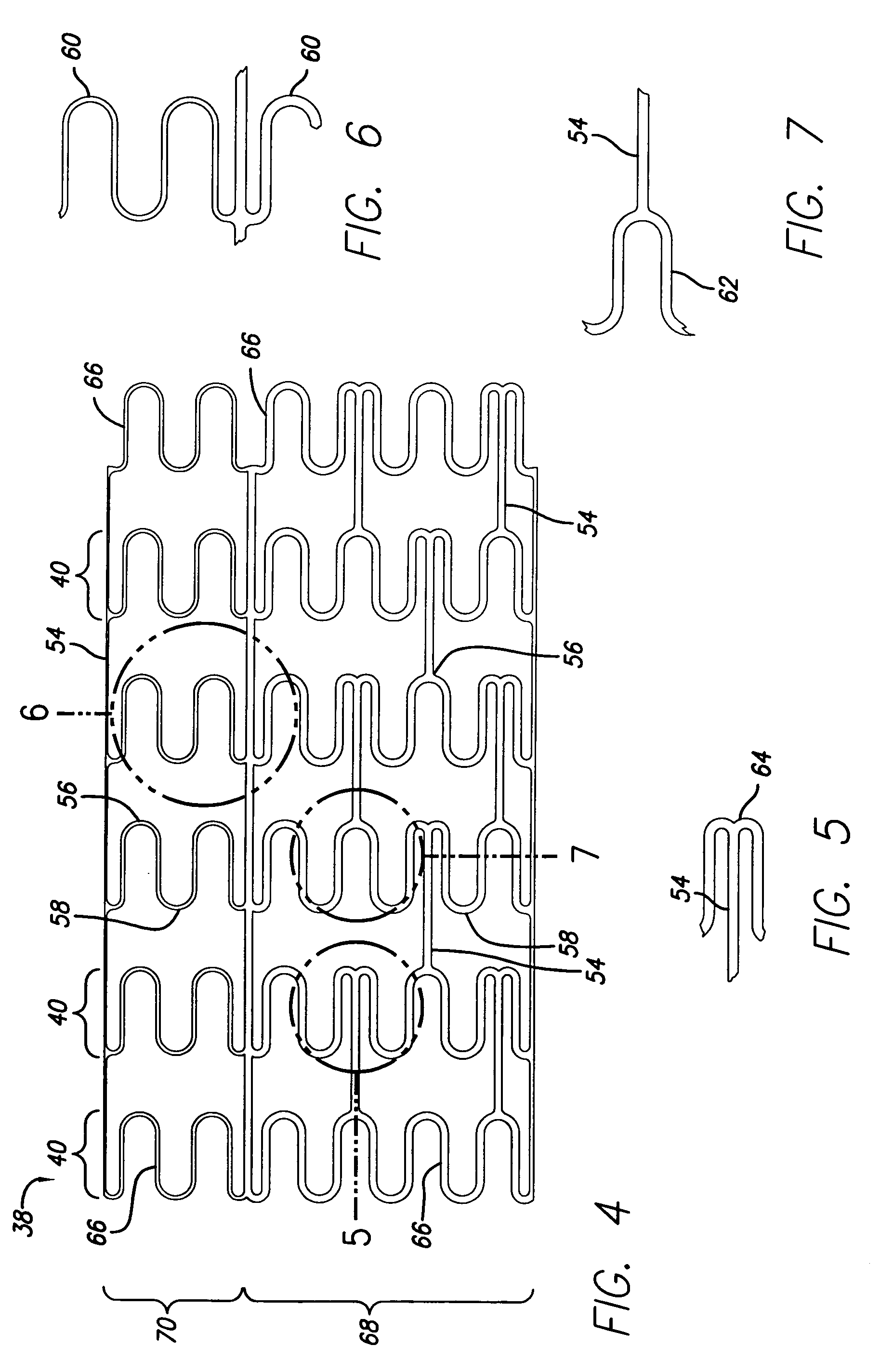
Methods and devices for treating vulnerable plaque
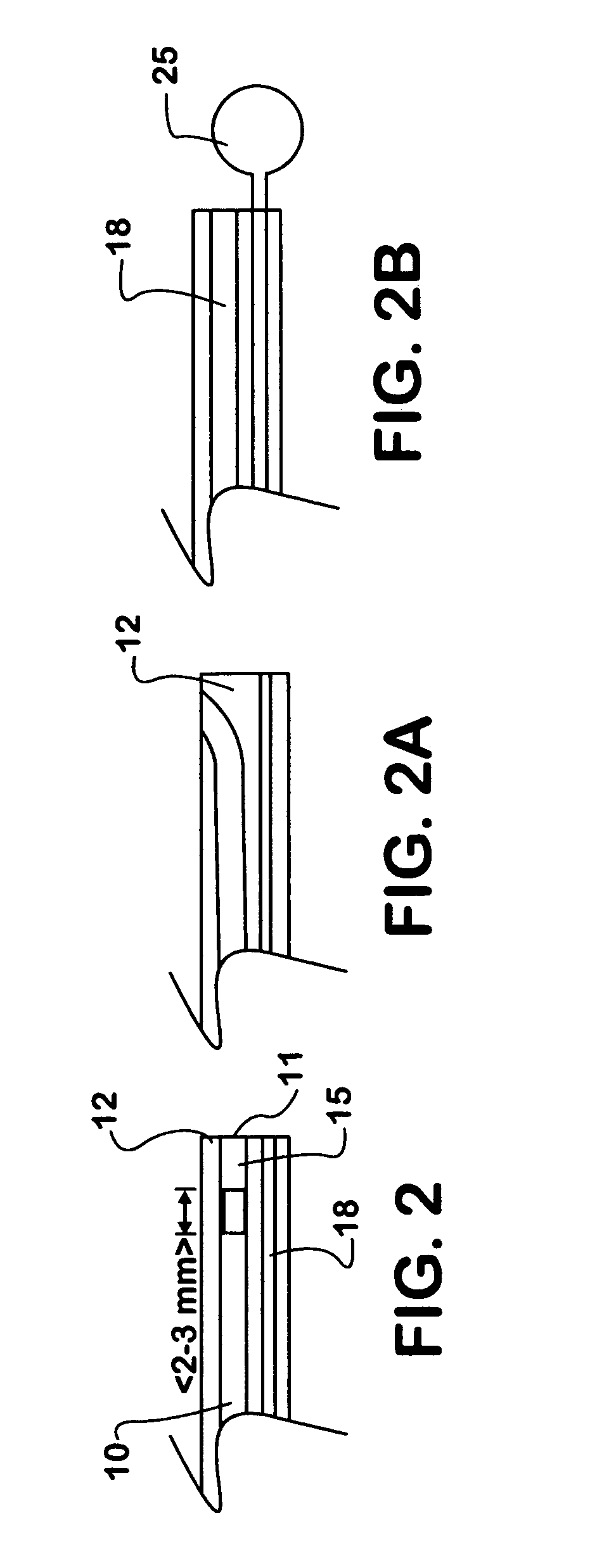
ActiveUS20070123839A1Reduce the possibility of leaksControlled diffusionStentsElectrotherapyCoronary arteriesVulnerable plaque
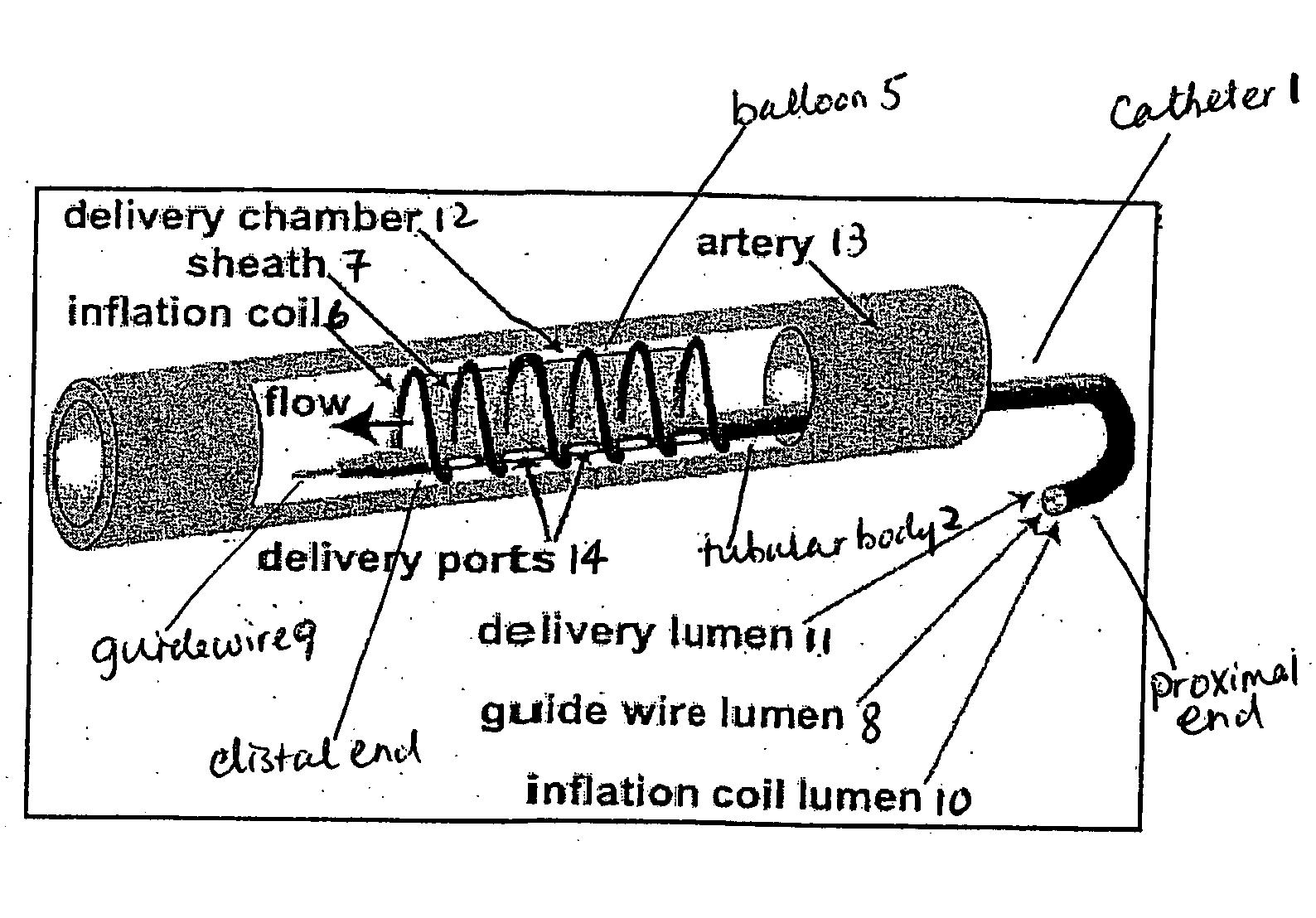
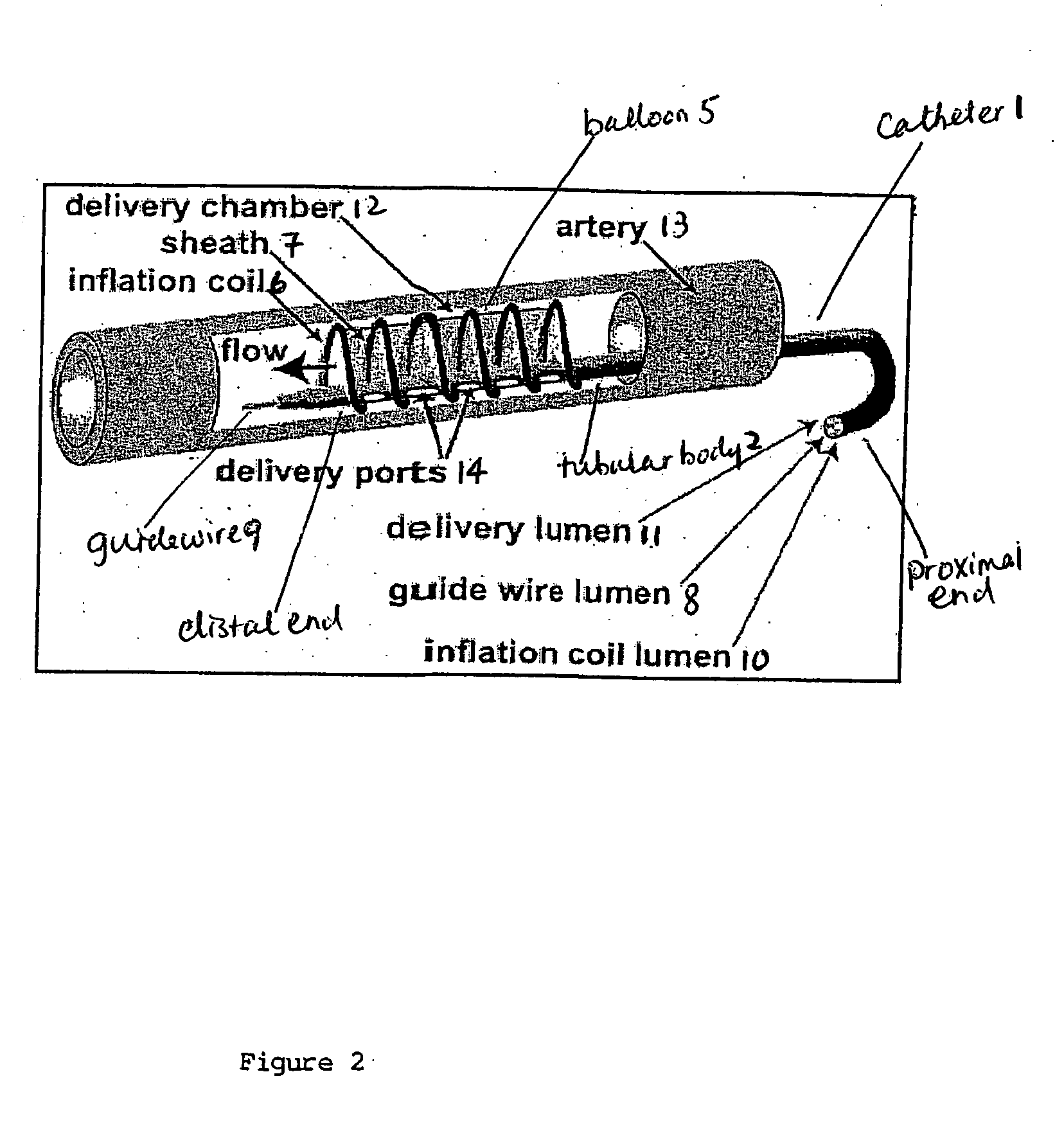
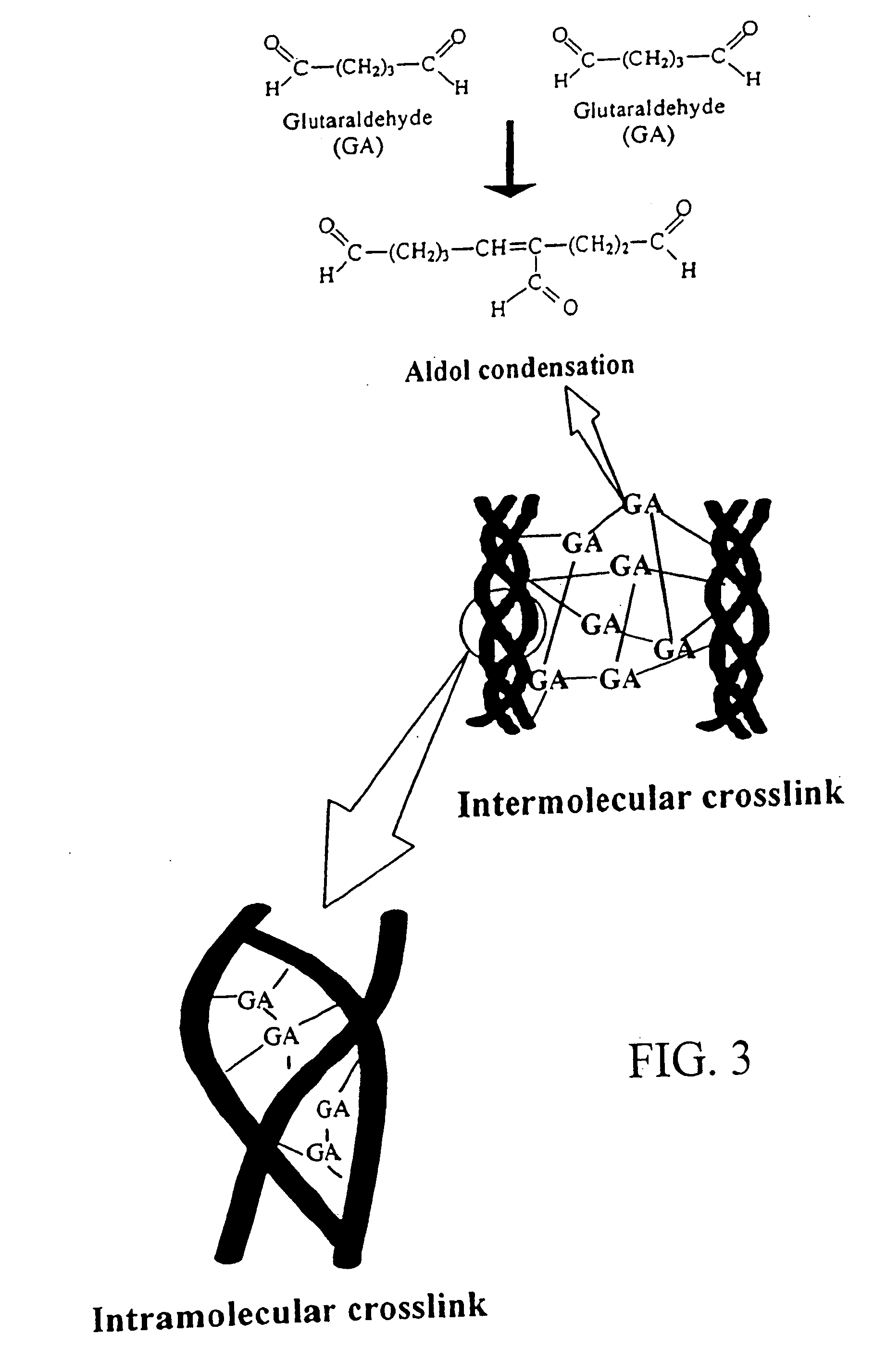
This invention provides methods, devices and kits for treating coronary artery disease, particularly “soft” or vulnerable plaque. The invention is based on the local delivery of crosslinking means to crosslink the collagen covering the plaque and other proteins that may be present at the site to stabilize the plaque and prevent leakage of thrombogenic material into the lumen of the coronary artery. In certain embodiments, catheters are employed to achieve local delivery of a crosslinking agent or ultraviolet light.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Cryotherapy method for detecting and treating vulnerable plaque
InactiveUS6955174B2Keep openDifferentiation of vulnerableLaproscopesEndoscopesVulnerable plaqueRadiology
Methods, apparatus, and kits detect and / or treat vulnerable plaque of a blood vessel. A temperature differential can be sensed along a lumen surface with temperature sensors on a balloon filled with warm gas. Treatment methods include controlled and safe cryogenic cooling of vulnerable plaque to inhibit release of retained fluid within the vulnerable plaque so as to inhibit acute coronary syndrome and to help maintain patency of a body lumen.
Owner:BOSTON SCI SCIMED INC
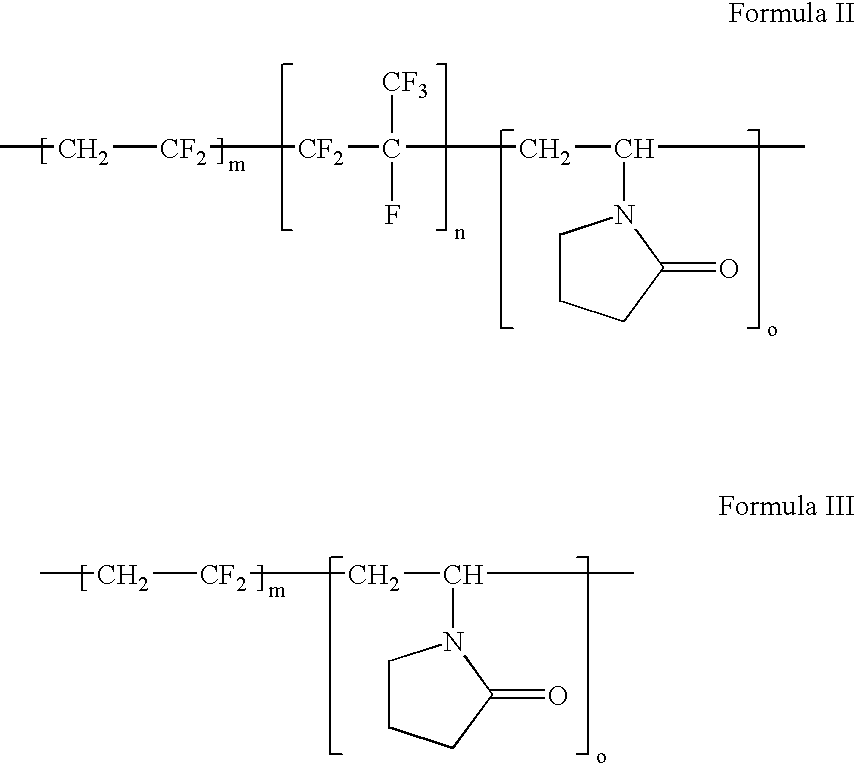
Polymers of fluorinated monomers and hydrophilic monomers
ActiveUS20060047095A1Improve propertiesProvide flexibilityFibre treatmentSurgeryDiseasePolymer science
A polymer of fluorinated monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains a polymer of fluorinated monomers and another biocompatible polymer. The polymer of fluorinated monomers or polymer blend described herein and optionally a bioactive agent can form a coating on an implantable device such as a drug-delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Stent having cover with drug delivery capability
A prosthesis has a stent, and a cover that covers a portion of the stent. The cover has at least two layers of materials that can define at least one chamber therebetween, with a drug loaded into the at least one chamber by a dug dispersing element. The cover can be provided inside the luminal walls of the stent, or the stent can be retained in the at least one chamber. The cover can also be deployed to treat vulnerable plaque.
Owner:YANG JUN
Radio-frequency device for passivation of vascular plaque and method of using same
InactiveUS20060089638A1Less-proneLittle or no deleterious effects on the surrounding patient tissueSurgical instrument detailsHuman bodyVulnerable plaque
Disclosed herein is a minimally invasive, radio-frequency device and a method for local and regional vascular therapy, more particularly for passivation of atherosclerotic, inflammatory, and / or vulnerable plaque in blood vessels. Radio-frequency devices of the type described herein constitute an important, inexpensive, disposable, minimally invasive approach for passivation or removal of plaques in various parts of the human body, and, as such, have cardiological applications, such as the treatment of coronary atherosclerosis, as well as other applications, such as the treatment occluded blood vessels in the legs and extremities.
Owner:CARMEL YUVAL +2
Coated medical devices for the treatment of vulnerable plaque
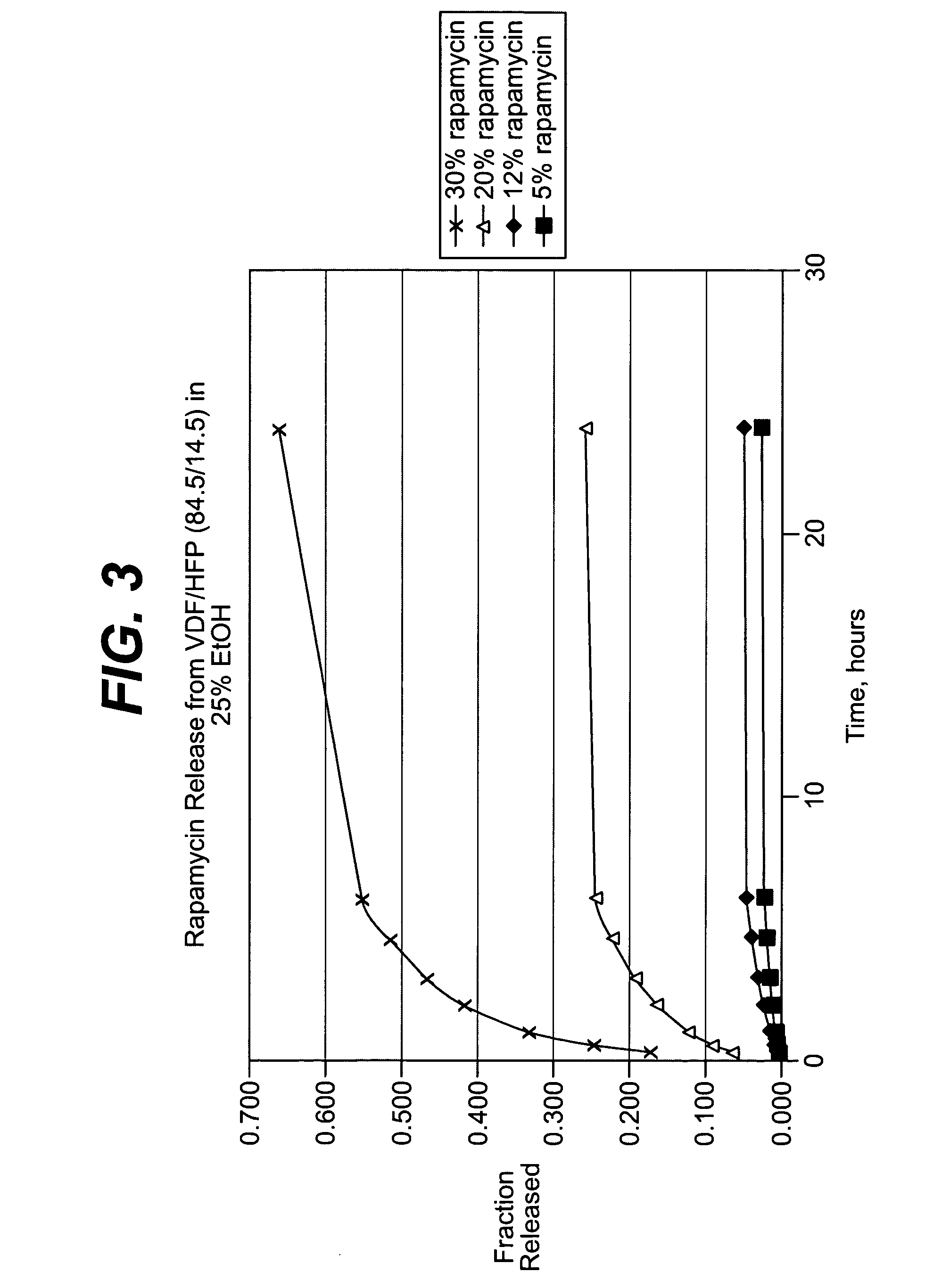
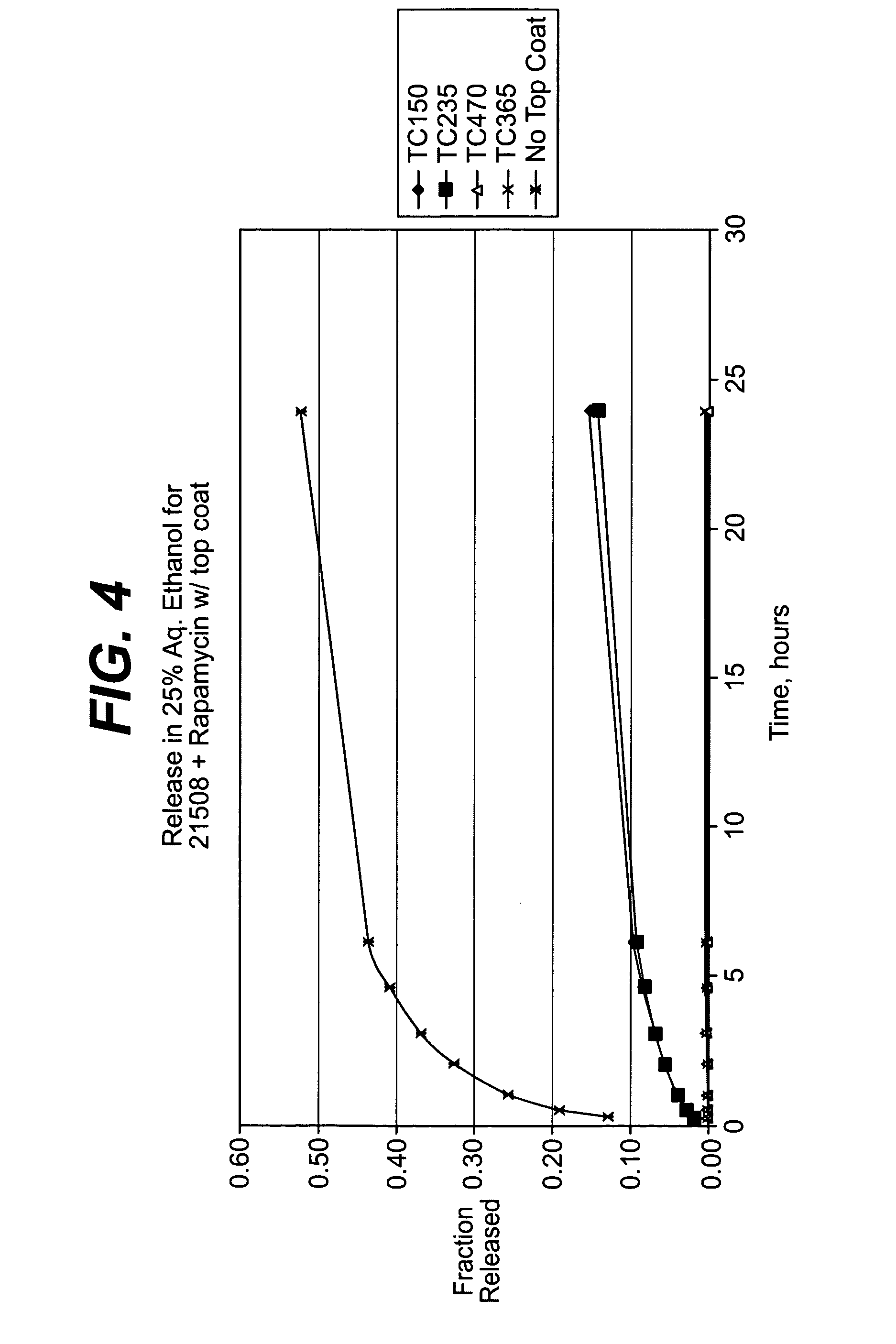
InactiveUS7195640B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesPercent Diameter StenosisCoating
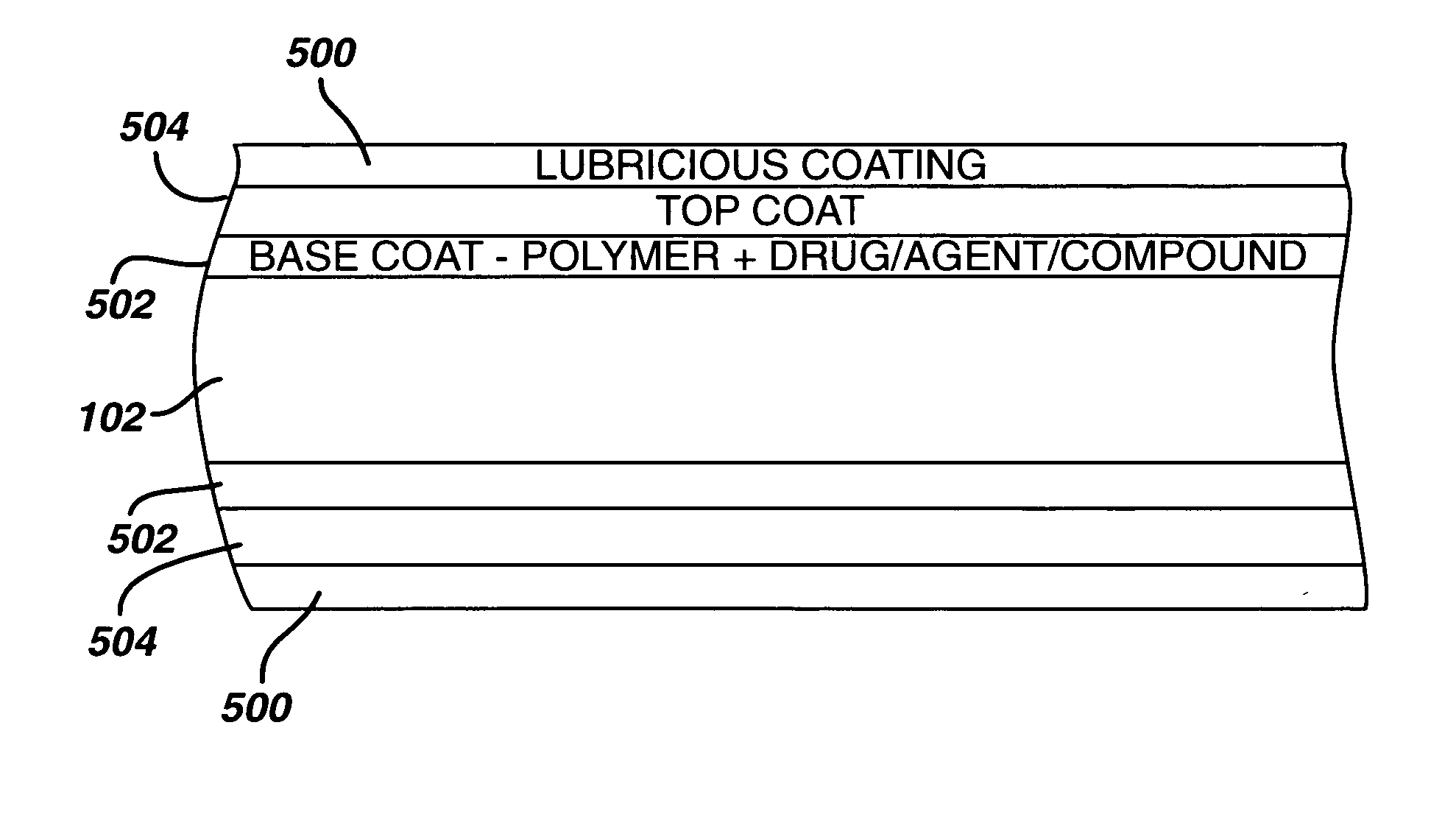
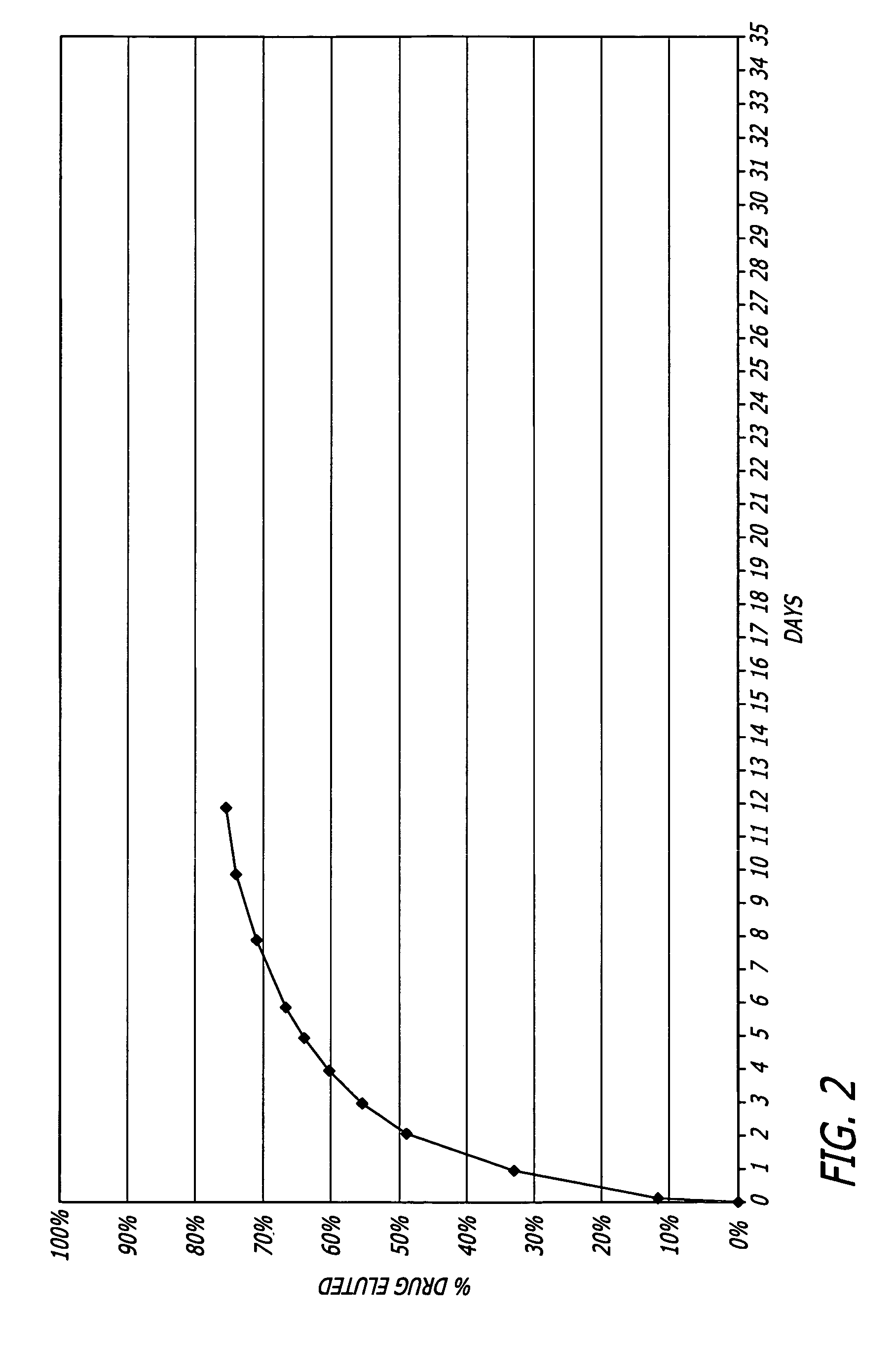
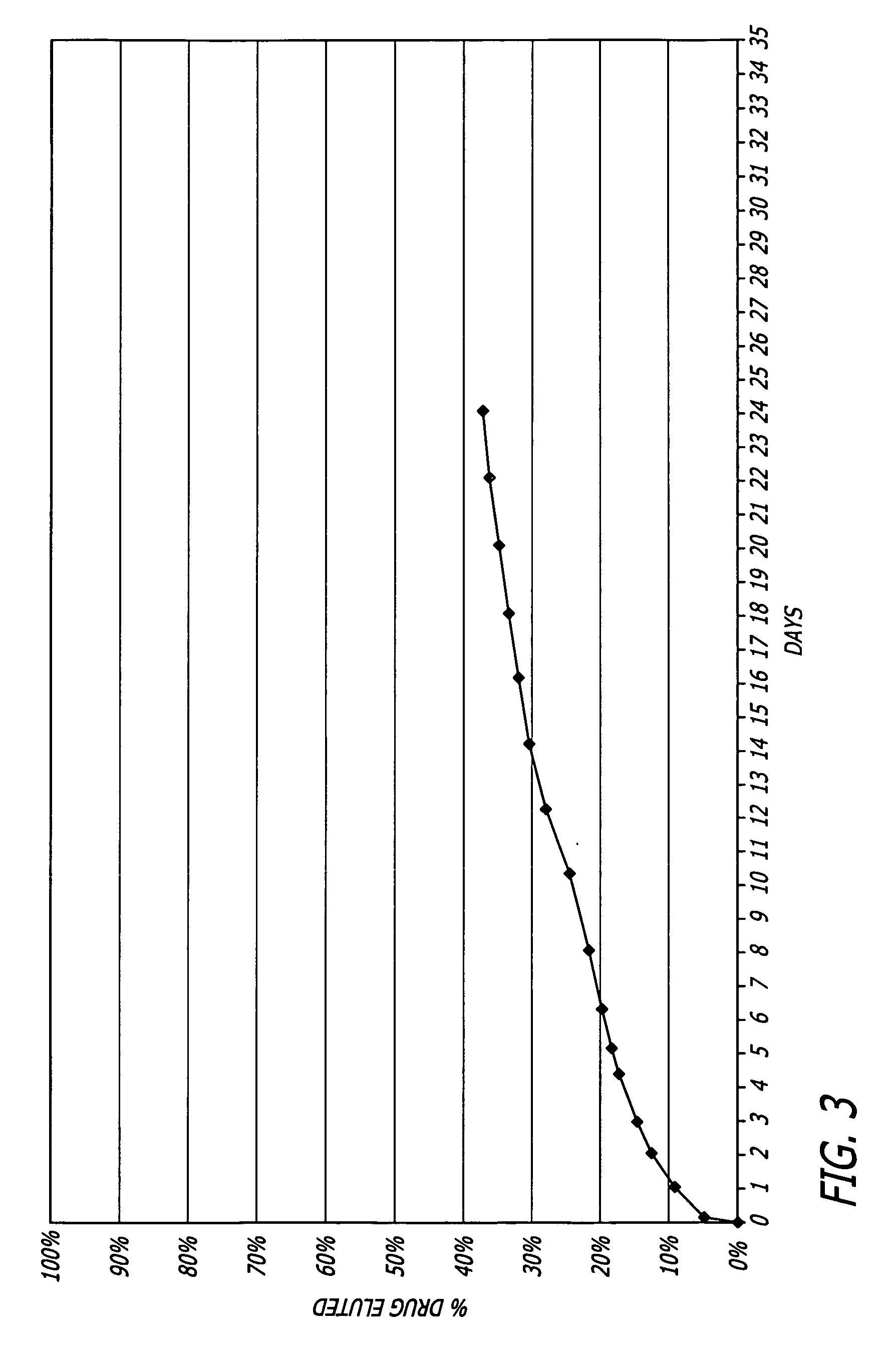
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. In addition to reducing or substantially eliminating a biological organism's reaction to the introduction of the medical device to the organism, the medical device in combination with one or more therapeutic drugs, agents and / or compounds may be utilized to treat various vascular diseases, for example, restenosis and vulnerable plaque. In the case of vulnerable plaque, one or more drugs, agents or compounds may be utilized to treat the various aspects of vulnerable plaque and these drugs, agents and / or compounds may be released with a given release profile for the most effective treatment. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH +1
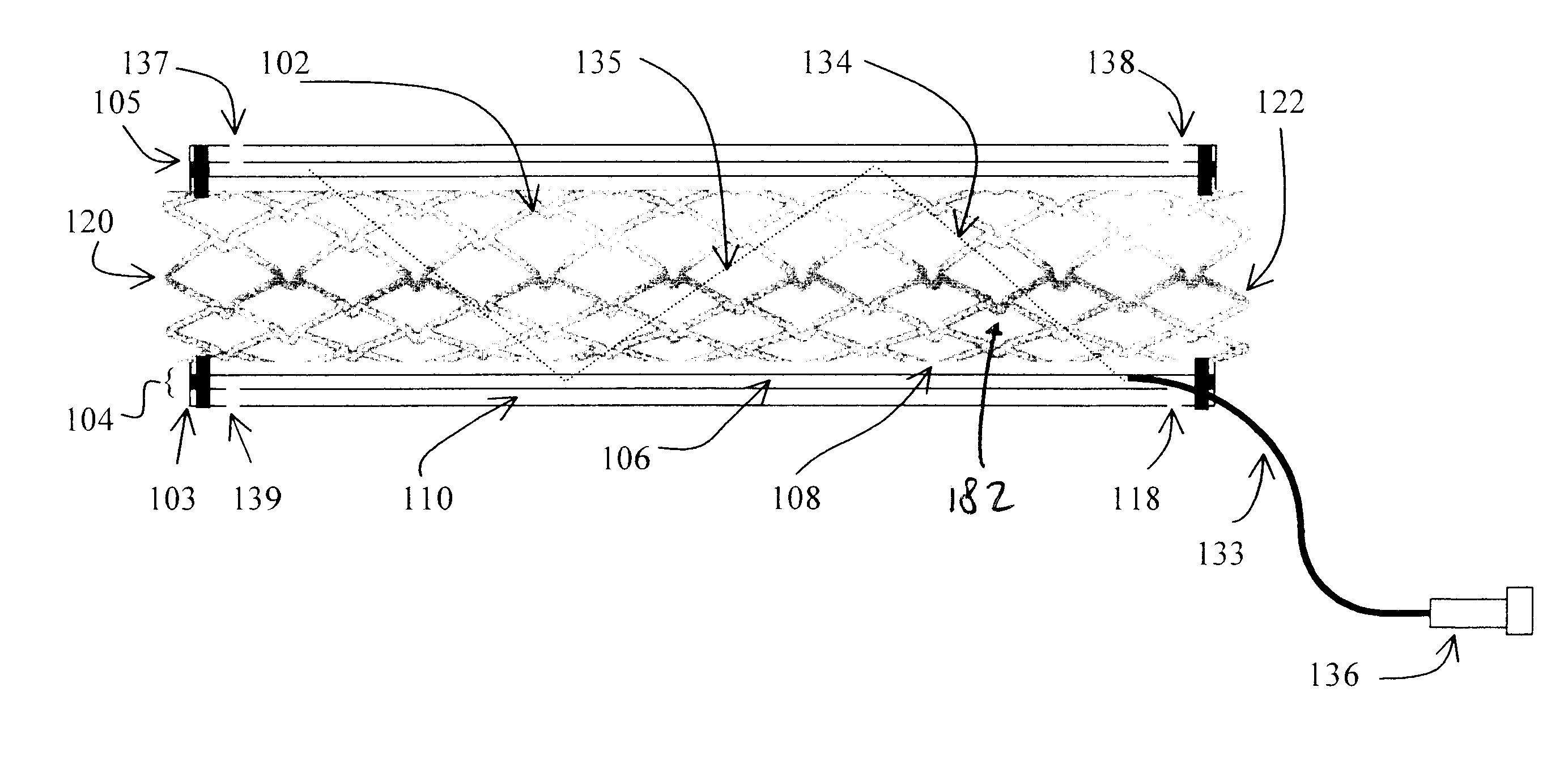
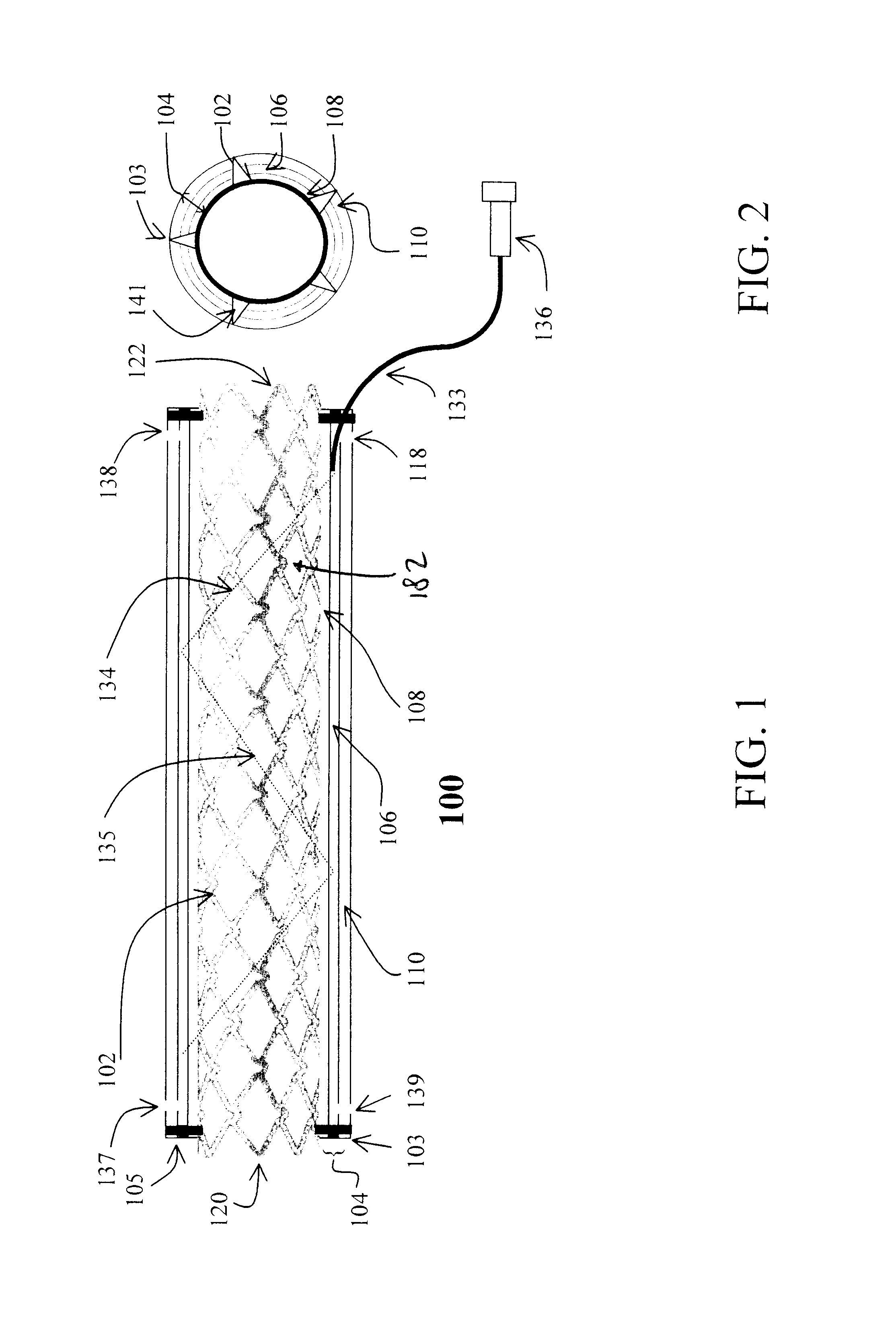
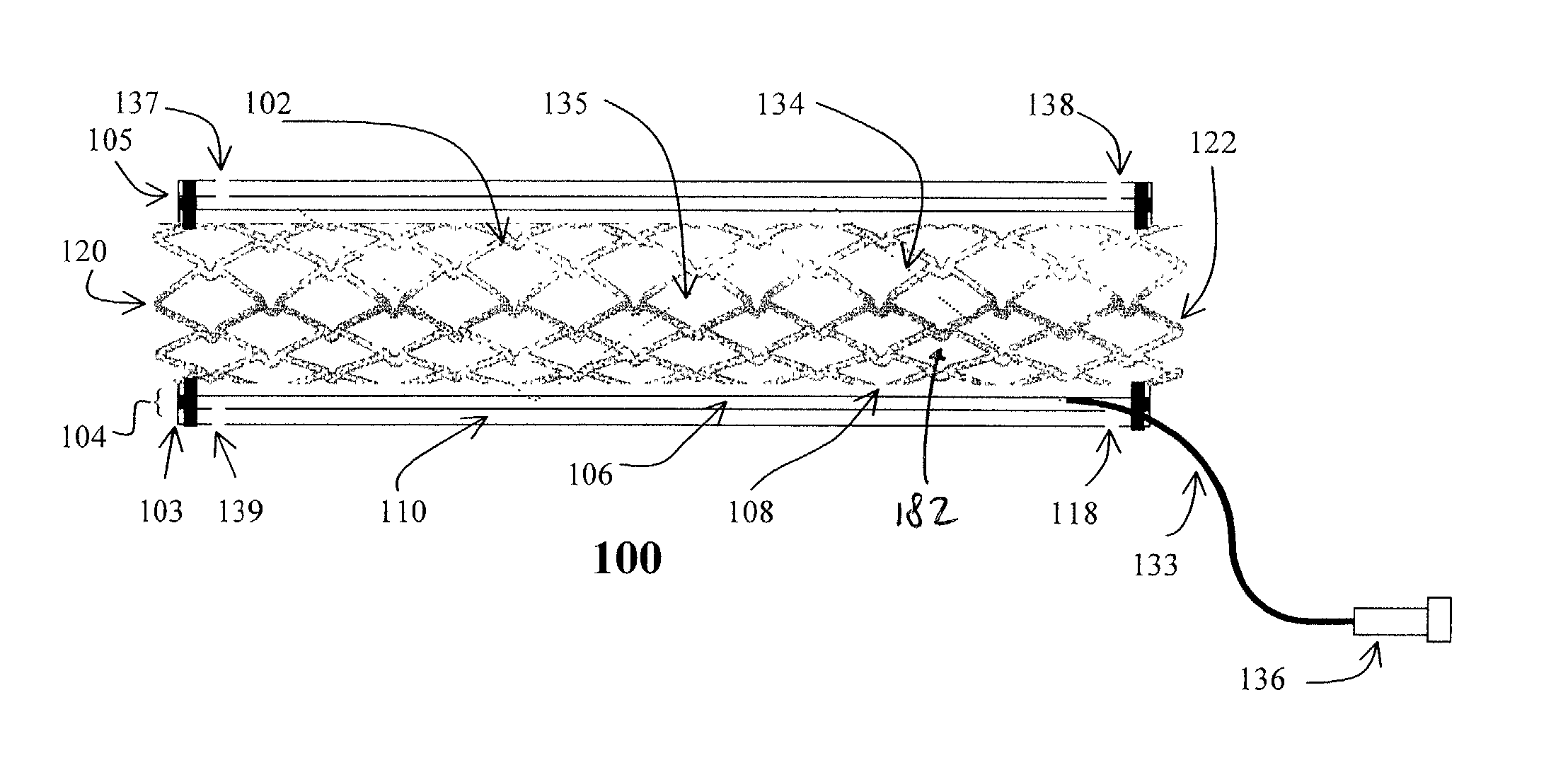
Stent with anchors to prevent vulnerable plaque rupture during deployment
InactiveUS7258697B1Reducing fibrous cap stressPrevent high expansion forceStentsBlood vesselsVulnerable plaqueFibrous cap
A stent for implantation in a body lumen for protecting from rupture a fibrous cap in order to treat vulnerable plaque. One embodiment of the stent achieves staged expansion through stronger and weaker circumferential regions, and includes optional anchors positioned at the circumferential transition between the stronger and weaker regions. During the first stage expansion, the weaker region expands moving the anchors laterally apart. The anchors straddle the fibrous cap and embed into the vessel wall. The second stage expansion of the stent exerts gentler stresses by the weaker region against the fibrous cap while the stronger region exerts greater stresses on the remainder of the vessel wall to open the vessel.
Owner:ABBOTT CARDIOVASCULAR
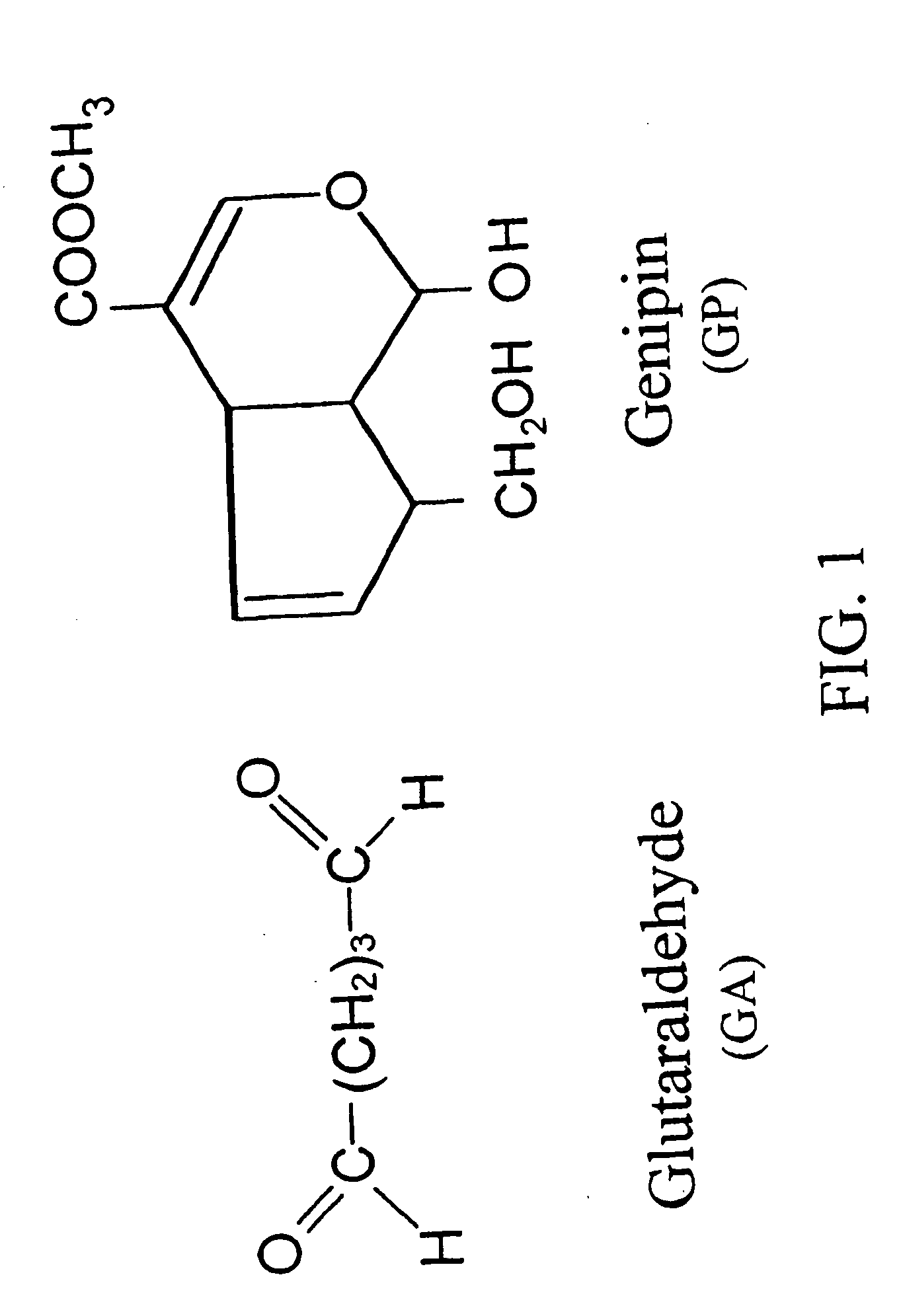
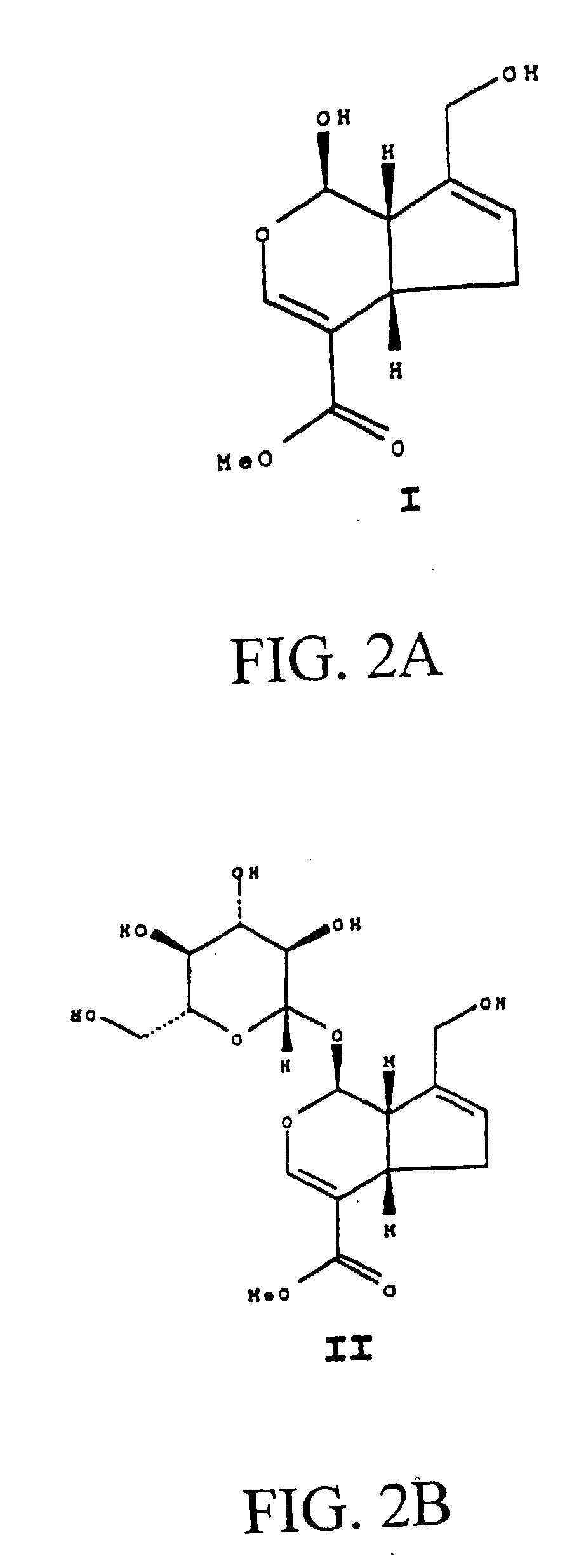
Drug-eluting stent having collagen drug carrier chemically treated with genipin
InactiveUS20050123582A1Reduce releaseAntibacterial agentsOrganic active ingredientsVulnerable plaqueInsertion stent
A method for treating vulnerable plaques of a patient, comprising: providing a biodegradable stent comprising a first supporting zone made of a first biodegradable material, wherein the supporting zone comprises at least a portion of continuous circumference of the stent; and a second therapeutic zone made of a second biodegradable material, wherein the therapeutic zone comprises at least one bioactive agent; delivering the biodegradable stent to the vulnerable plaques; orienting the therapeutic zone at about the luminal surface of the vulnerable plaque; and releasing the at least one bioactive agent for treating the vulnerable plaques.
Owner:GP MEDICAL
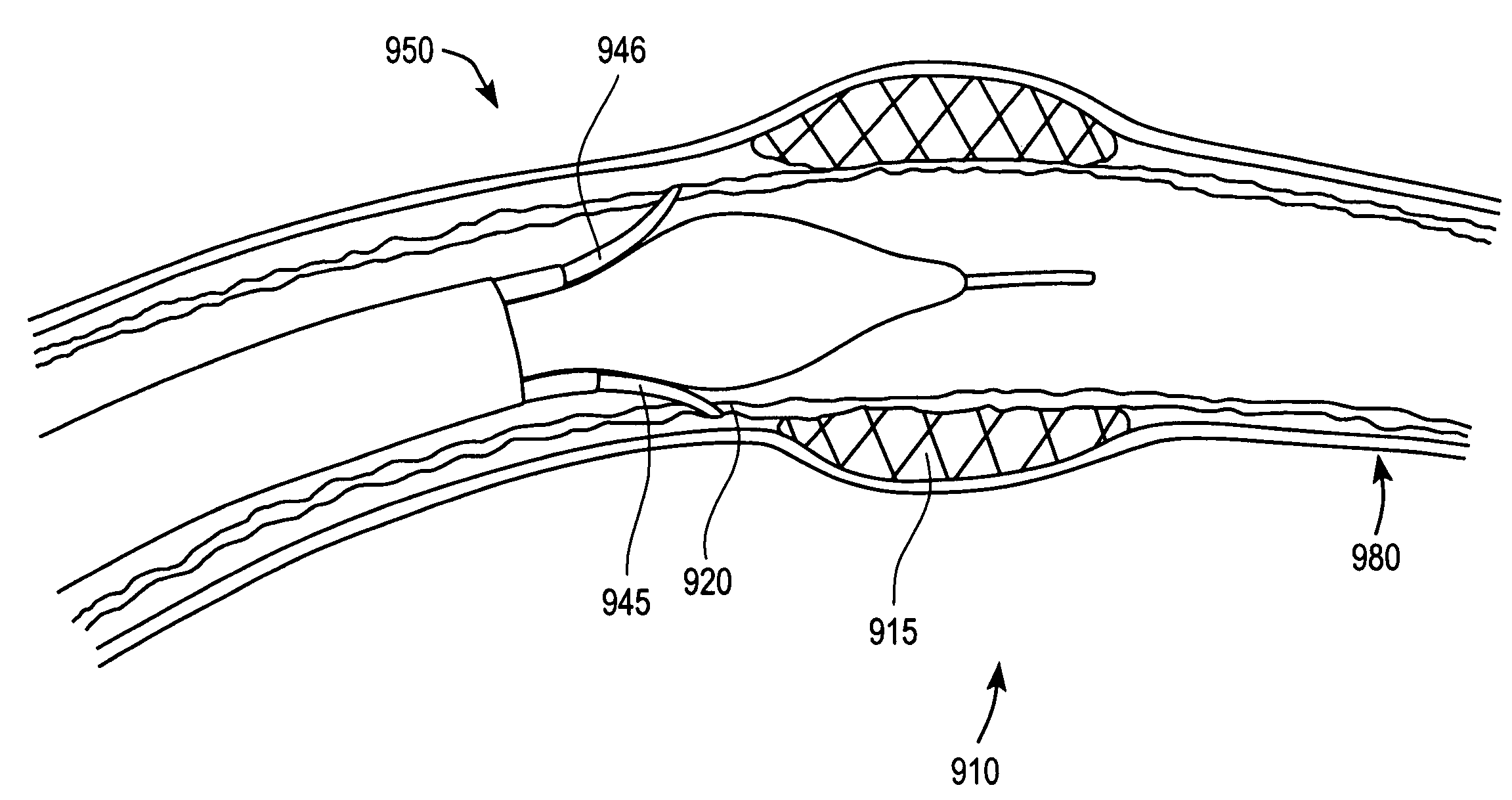
Device, system, and method for detecting, localizing, and characterizing plaque-induced stenosis of a blood vessel
InactiveUS20060106321A1Easy constructionSimple in useOrgan movement/changes detectionPerson identificationVulnerable plaqueCompressibility
The present invention is of system, device, and method for detection, localization, and characterization of plaque-induced stenosis of a blood vessel. More particularly, the present invention relates to a balloon catheter having an expandable balloon insertable into a blood vessel, which balloon comprises a plurality of pressure sensors operable to detect stenosis of the vessel, and further operable to report degrees of compressibility of stenotic regions of plaque within the vessel, thereby distinguishing between standard and vulnerable plaque.
Owner:GALIL MEDICAL
Device for the delivery of a cardioprotective agent to ischemic reperfused myocardium
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Implantable medical devices may be coated or otherwise have affixed thereto agents for healing ischemic tissue.
Owner:WYETH LLC
Method of treating vulnerable plaque
InactiveUS6972024B1Minimizing avoiding damageIncrease synthesisStentsEar treatmentSelf limitingVulnerable plaque
A method of treating vulnerable plaque comprising intentionally damaging or rupturing the vulnerable plaque using a wingless balloon which is inflated from a wingless unexpanded diameter to a limited expanded diameter. This process produces significant increase in ECM synthesis at the site of the damage or rupture. As a result, the method strengthens the vulnerable plaque while minimizing or avoiding damage to the surrounding wall of the body lumen or damaging a stable plaque mistakenly believed to be a vulnerable plaque. The method of the invention is particularly useful in treating a fibroatheroma type of vulnerable plaque. In one embodiment, the balloon is self-limiting such that it expands compliantly at initial inflation pressures, and above nominal pressure it expands noncompliantly. In an alternative embodiment, the balloon is inflated using a diameter-limiting device, such as a device which limits the inflation pressure or the volume of inflation fluid in the balloon.
Owner:ABBOTT CARDIOVASCULAR
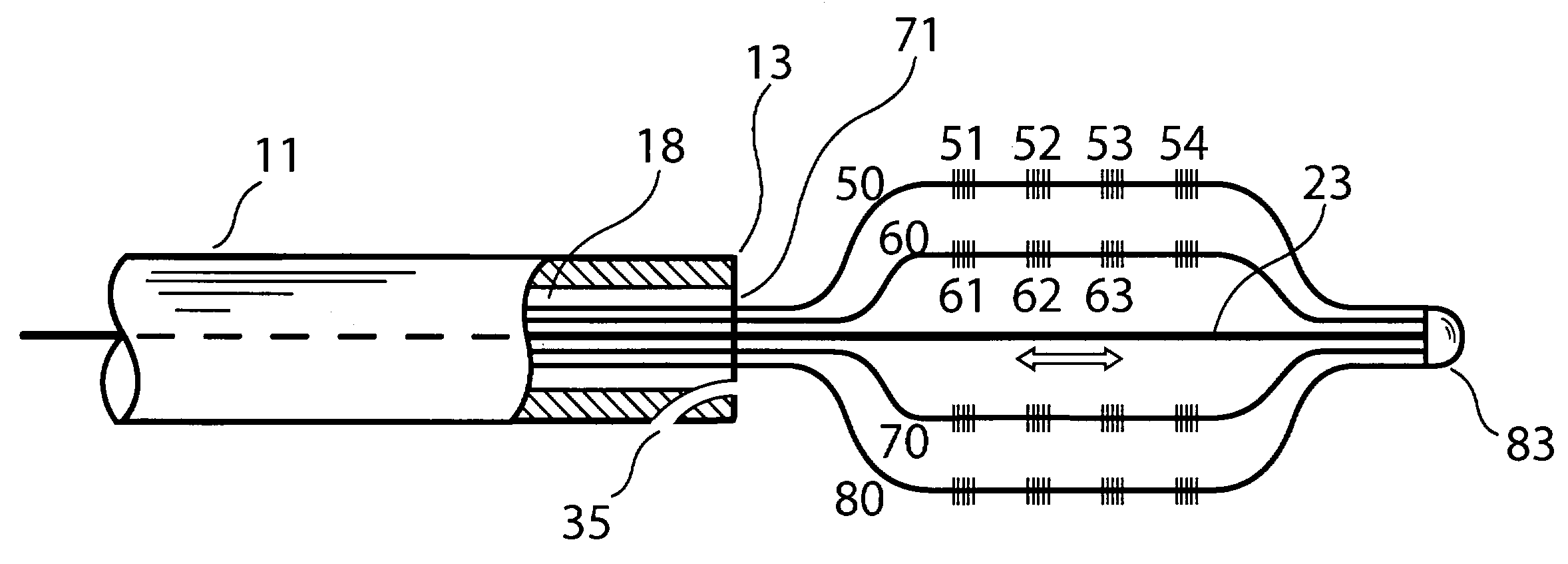
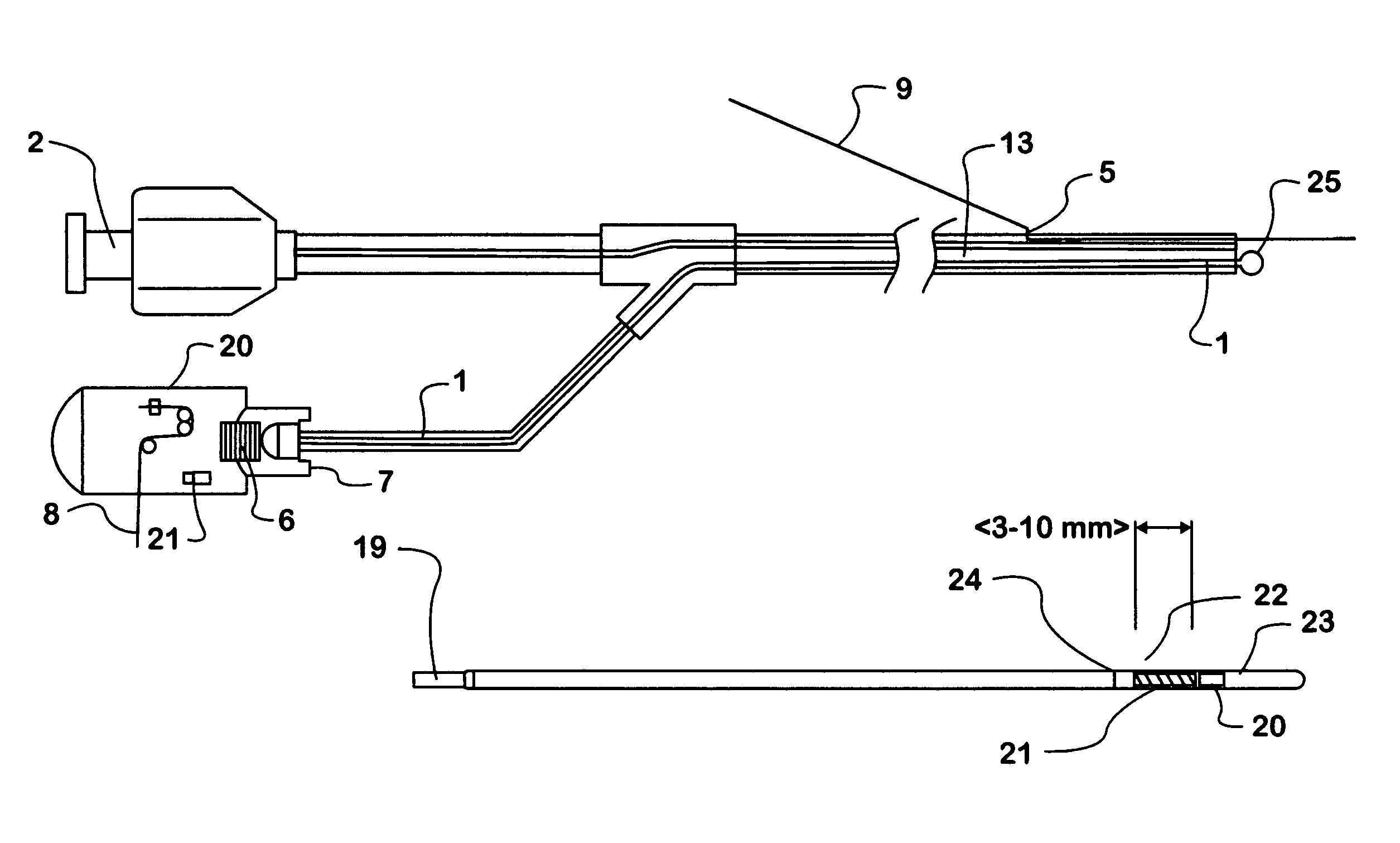
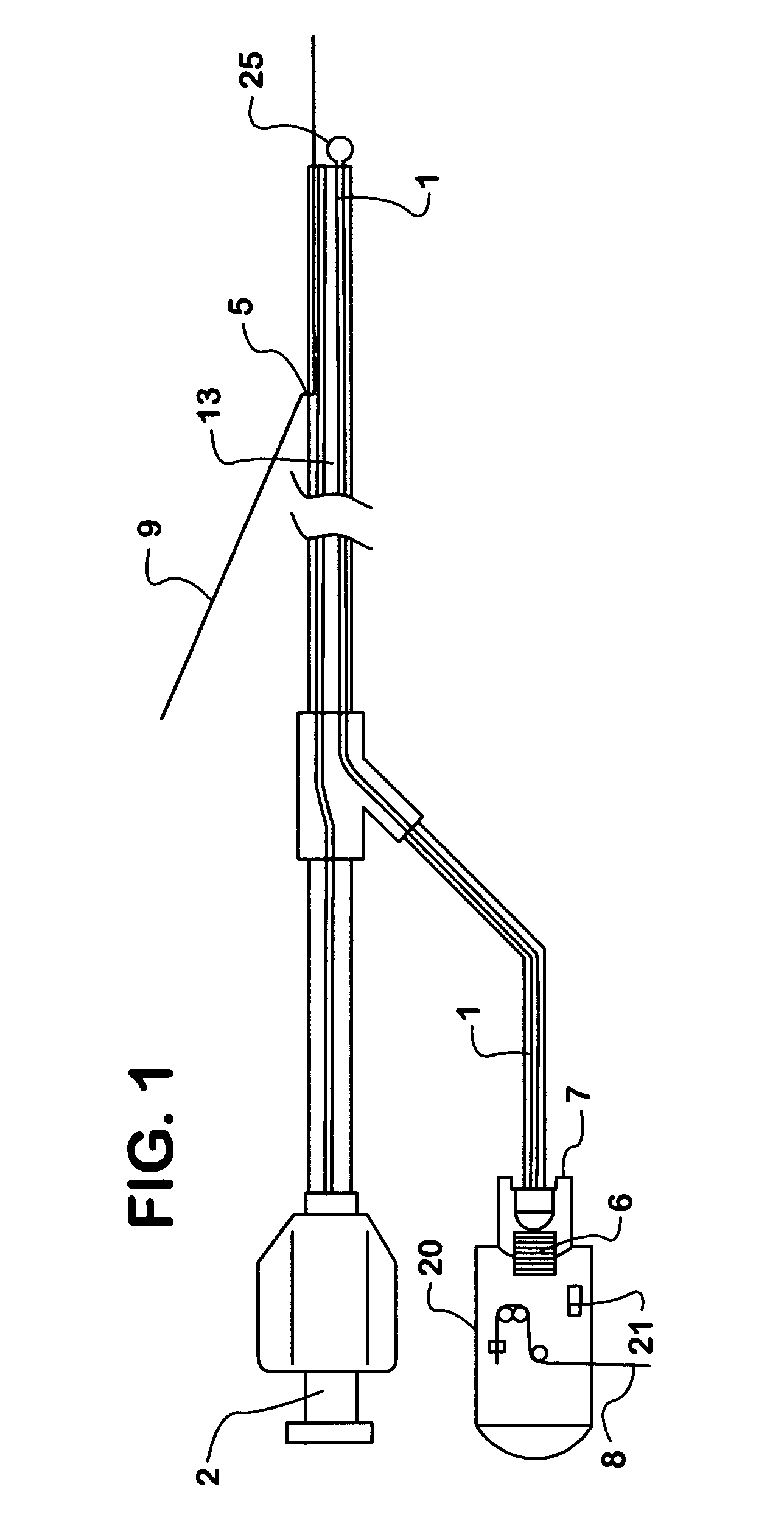
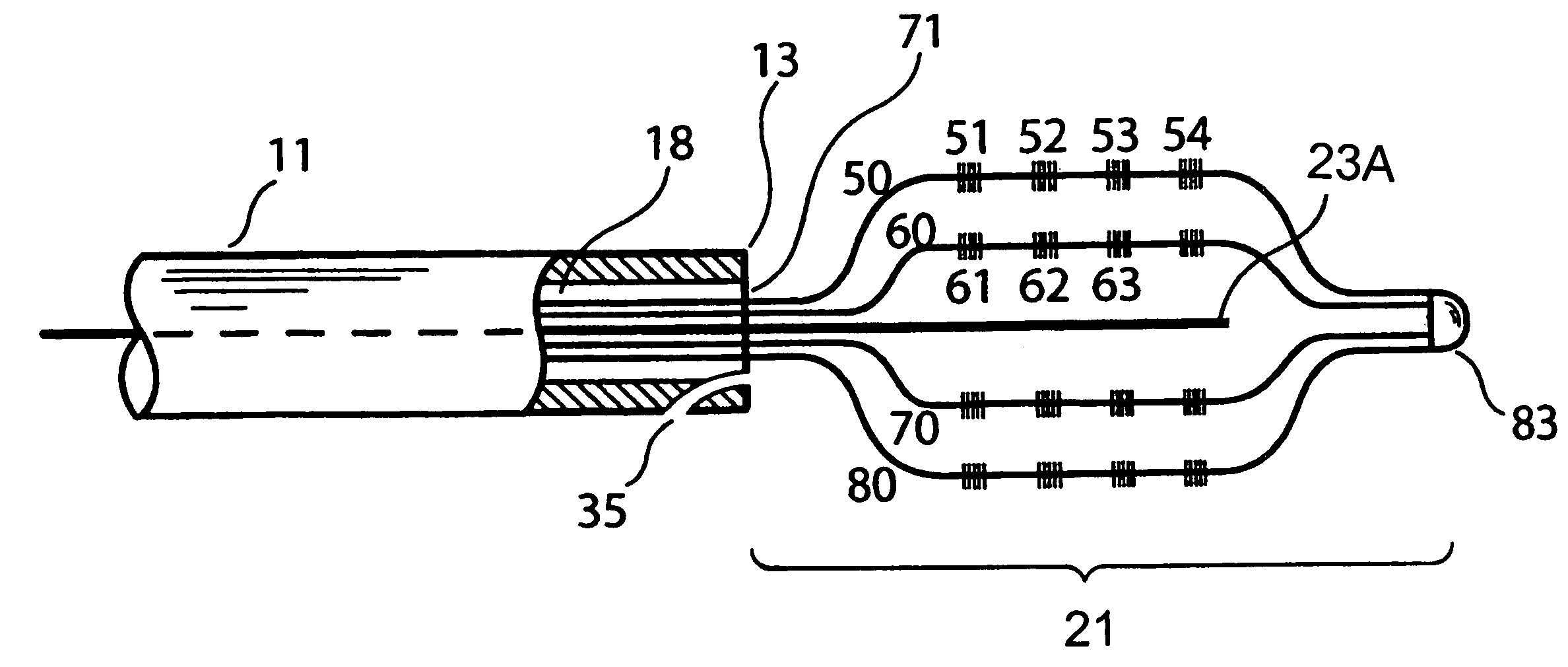
Optical apparatus for detecting and treating vulnerable plaque
InactiveUS7153299B1Easy to operateSimple, real-timeDiagnosticsSurgical instrument detailsFiberPhotodynamic therapy
An optical apparatus and methods for monitoring temperature enabling detection and treatment of a vulnerable plaque of a patient comprising an elongate catheter, a plurality of outer optical fibers deployably disposed within the lumen of the catheter and suitably expandable in an outwardly radial manner configured for forming a basket shape, the outer fibers having at least one optical grating along an axis of the fiber wherein the at least one optical grating reflects a certain wavelength or intensity of the light beam, the certain wavelength or intensity of the reflected light beam being correlated to known temperature, and a longitudinal middle optical fiber emitting a light energy suitable for photodynamic therapy.
Owner:MAXWELL SENSORS +1
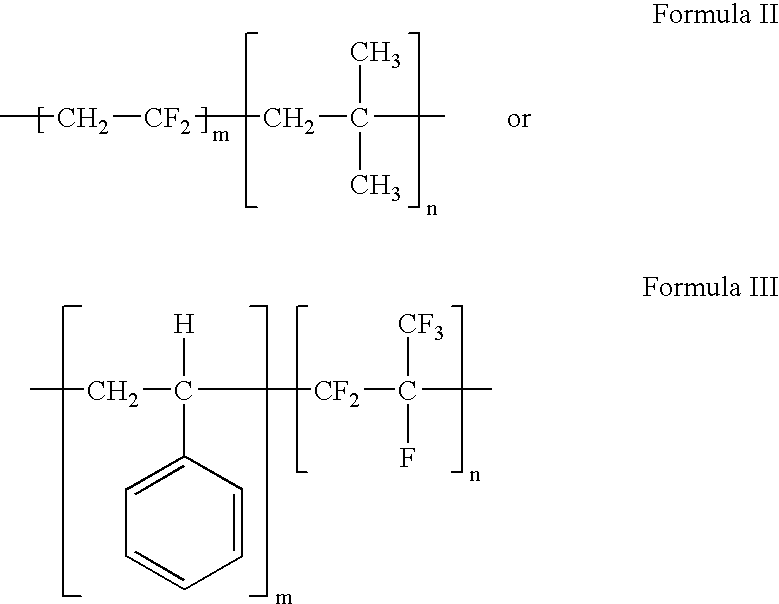
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS20060134165A1Provide mechanical strengthGive flexibilityStentsSurgeryAbnormal tissue growthPolymer science
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Stent having cover with drug delivery capability
InactiveUS20020062147A1Minimizes prevents ingrowthEffective localized drug deliveryStentsSurgeryVulnerable plaqueInsertion stent
A prosthesis has a stent, and a cover that covers a portion of the stent. The cover has at least two layers of materials that can define at least one chamber therebetween, with a drug loaded into the at least one chamber by a dug dispersing element. The cover can be provided inside the luminal walls of the stent, or the stent can be retained in the at least one chamber. The cover can also be deployed to treat vulnerable plaque.
Owner:YANG JUN
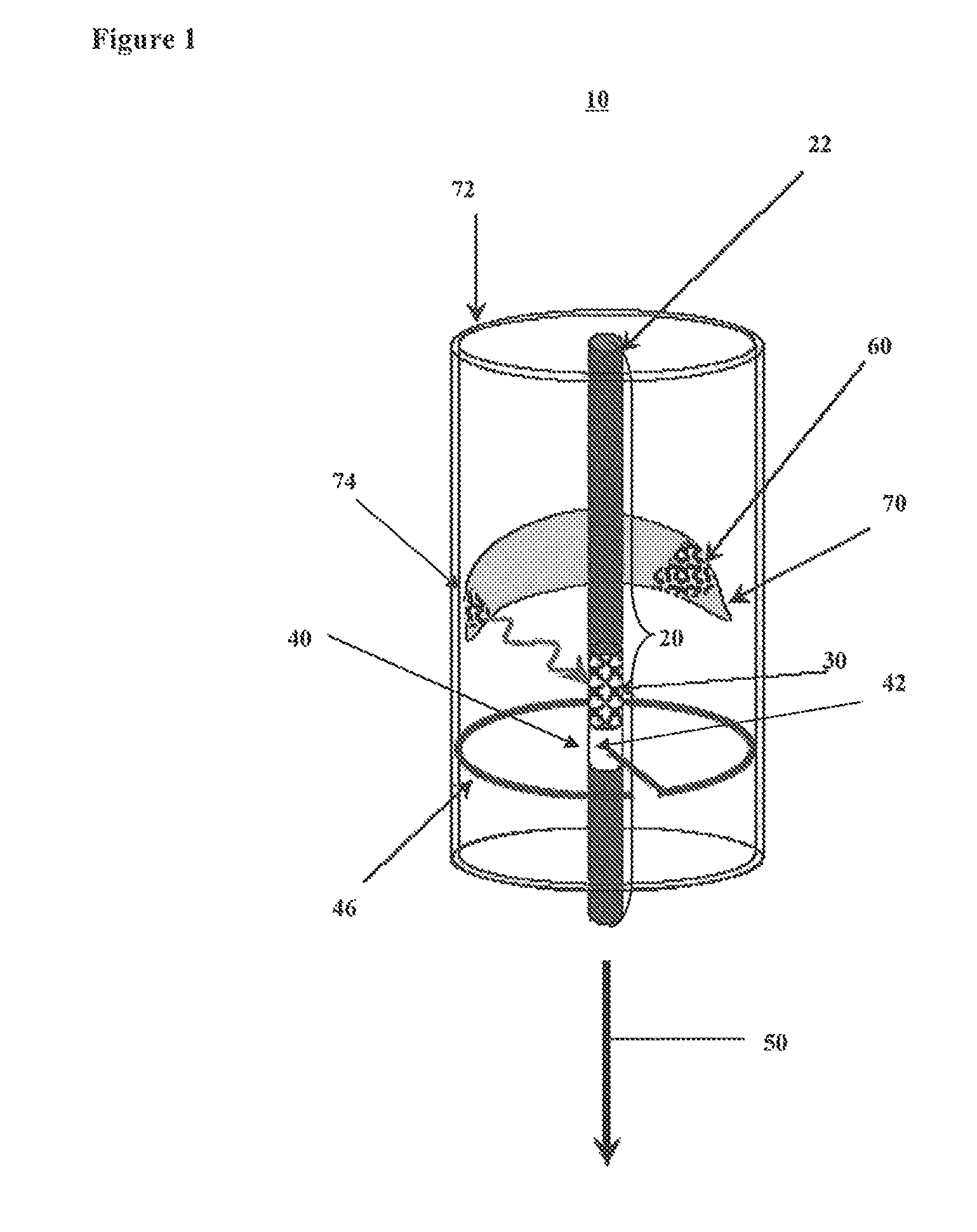
Method and apparatus to identify vulnerable plaques with thermal wave imaging of heated nanoparticles
Owner:BOARD OF REGENTS THE TEXAS UNIV SYST +1
Nitric oxide-releasing polymers derived from modified polymers
InactiveUS20070053952A1Treat and inhibit restenosisSurgerySynthetic polymeric active ingredientsPolymer modifiedVulnerable plaque
Modified polyimines and derivatives thereof suitable as implantable medical devices and coatings therefore are provided. Specifically, implantable medical devices and / or coatings comprise amphiphilic polymers derived from modified polyimines. The medical devices and coatings of the present invention can also be used for in situ nitric oxide release / controlled release drug delivery and are useful for treating or preventing medical conditions such as restenosis, aneurysms and vulnerable plaque.
Owner:MEDTRONIC VASCULAR INC
Method and apparatus for treating vulnerable plaque
Various apparatuses and methods are described to treat vulnerable plaque with the use of combination drug therapy. In one embodiment, the apparatus has an elongated catheter body adapted for insertion in a body lumen, with a drug delivery device attached near a distal portion of the elongated body. The drug delivery device is configured to deliver a therapeutic or biologically active agent to stabilize a vulnerable plaque. In another embodiment, a drug eluting stent is delivered and deployed in conjunction with a second apparatus that delivers drug in pressurized retrograde perfusion. Still another embodiment uses a uniquely shaped balloon to deploy a stent while utilizing a drug infusion needle to inject drug into the vessel wall through a hollow guide wire.
Owner:ABBOTT CARDIOVASCULAR
Optical apparatus for detecting and treating vulnerable plaque
InactiveUS20050075704A1Simple, real-timeCatheterSurgical instrument detailsFiberPhotodynamic therapy
An optical apparatus and methods for monitoring temperature enabling detection and treatment of a vulnerable plaque of a patient comprising an elongate catheter, a plurality of outer optical fibers deployably disposed within the lumen of the catheter and suitably expandable in an outwardly radial manner configured for forming a basket shape, the outer fibers having at least one optical grating along an axis of the fiber wherein the at least one optical grating reflects a certain wavelength or intensity of the light beam, the certain wavelength or intensity of the reflected light beam being correlated to known temperature, and a longitudinal middle optical fiber emitting a light energy suitable for photodynamic therapy.
Owner:MAXWELL SENSORS +1
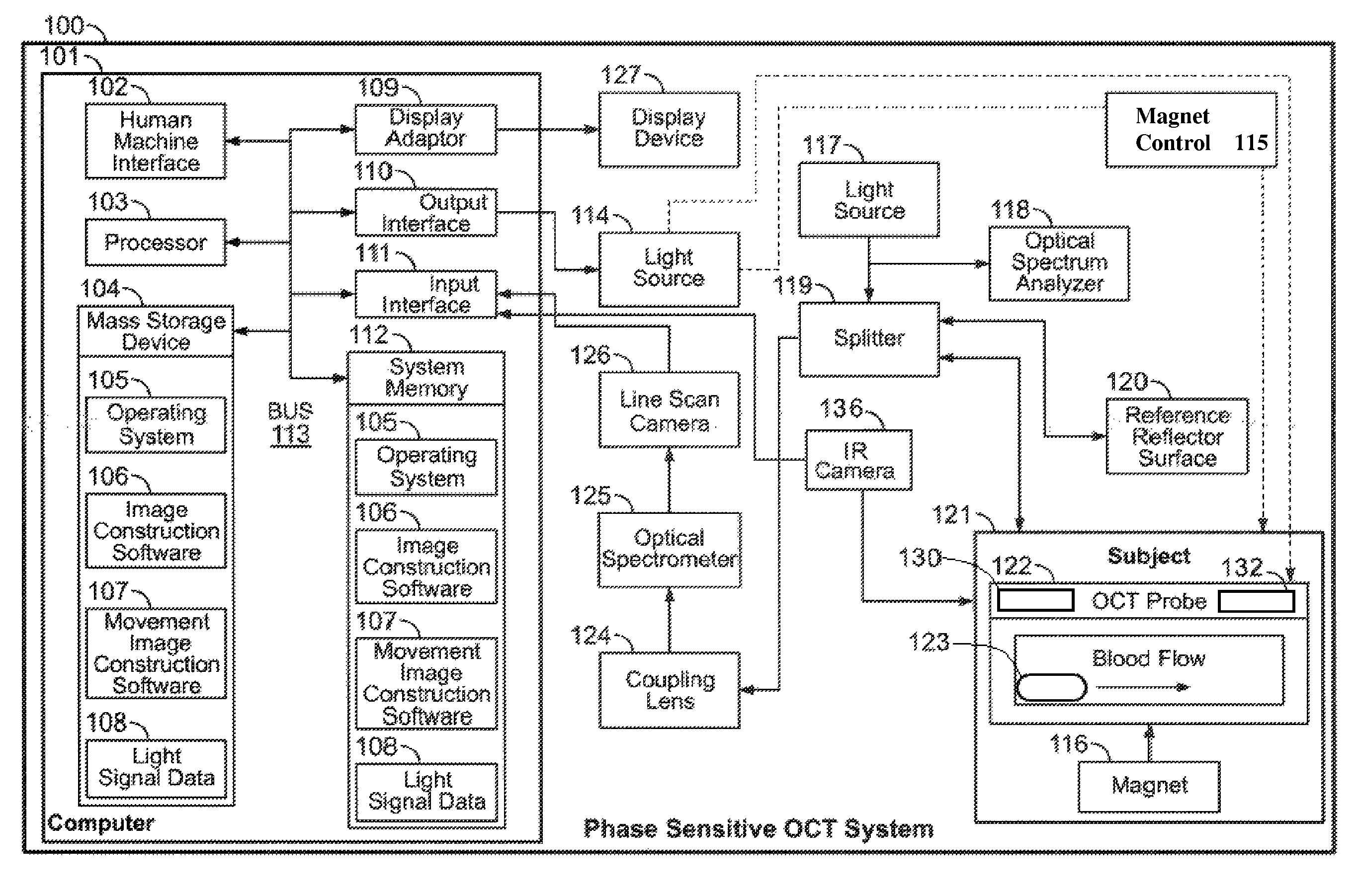
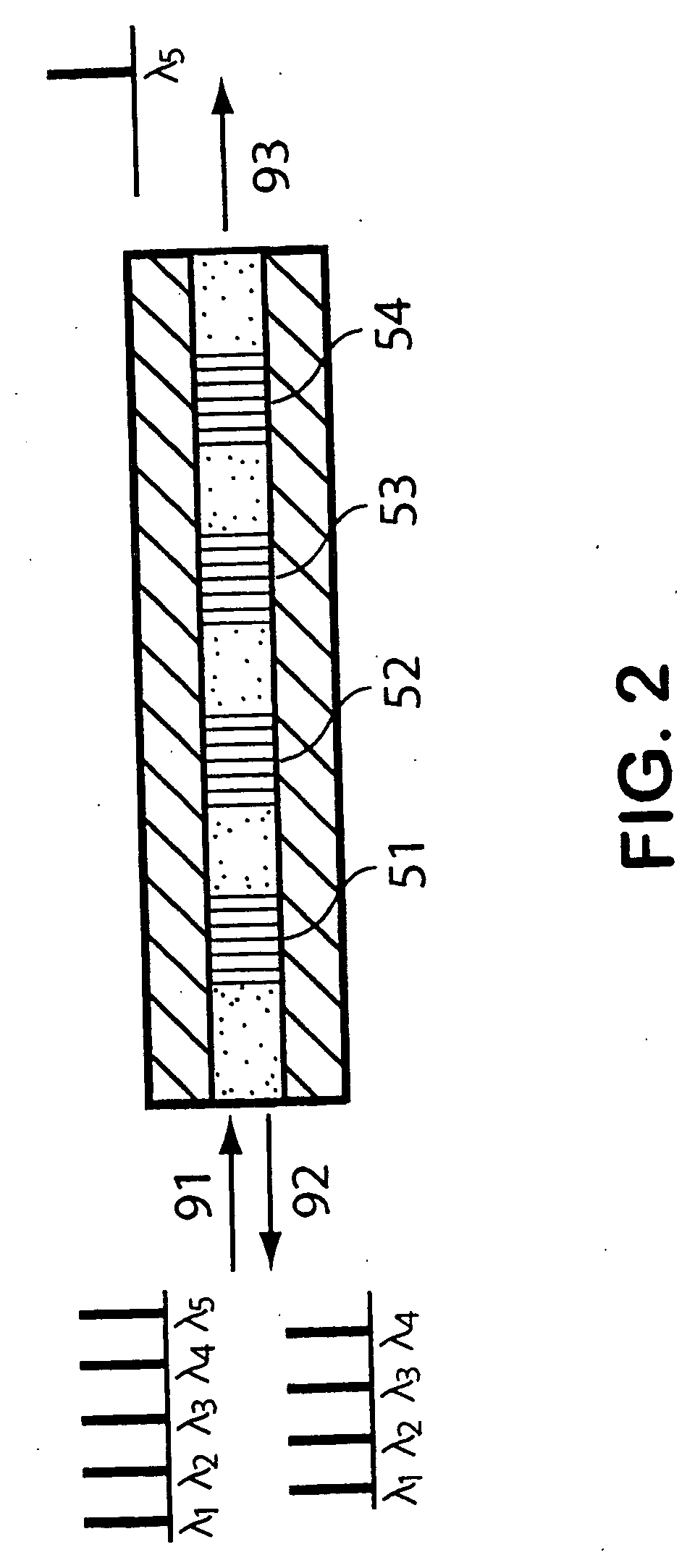
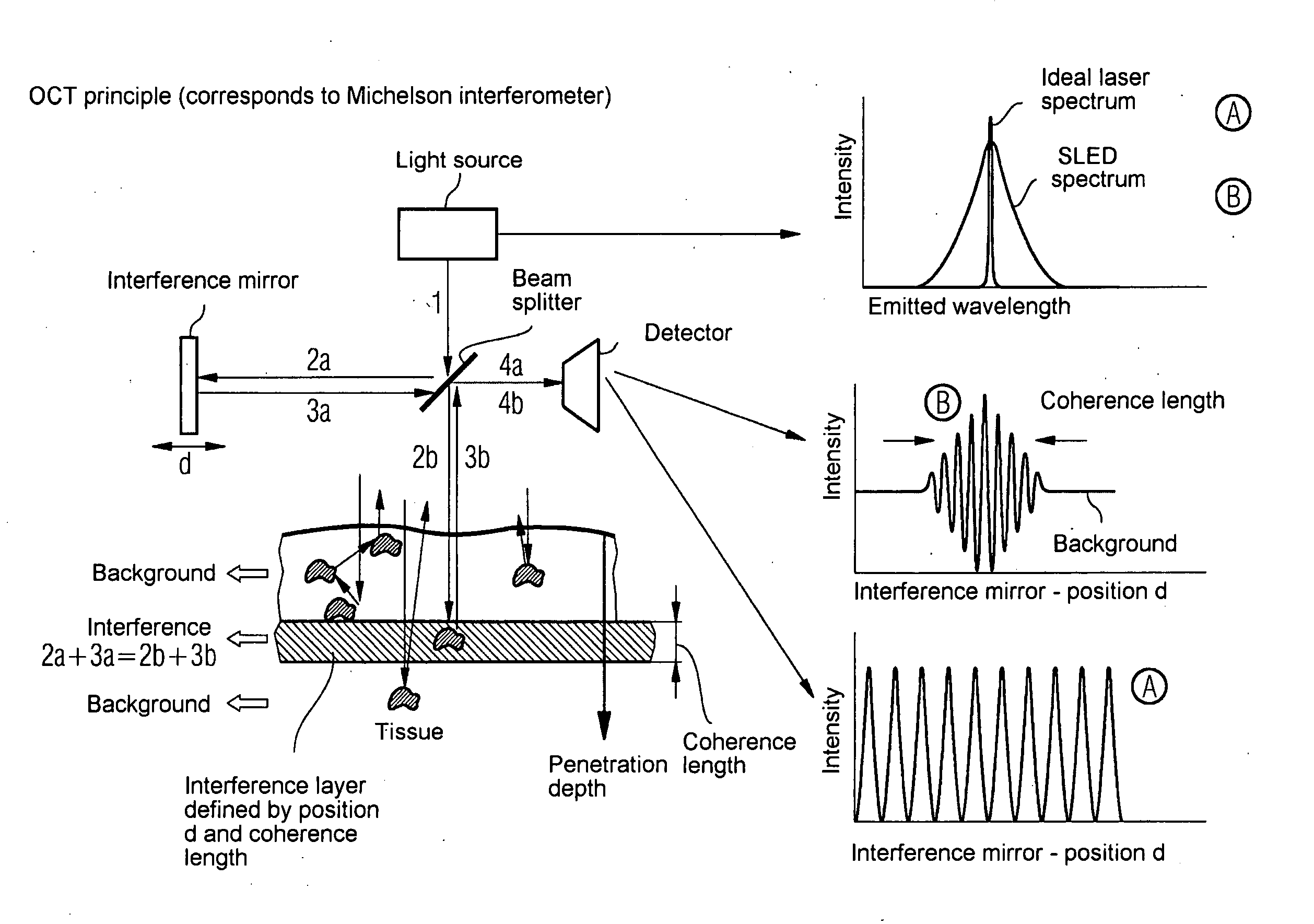
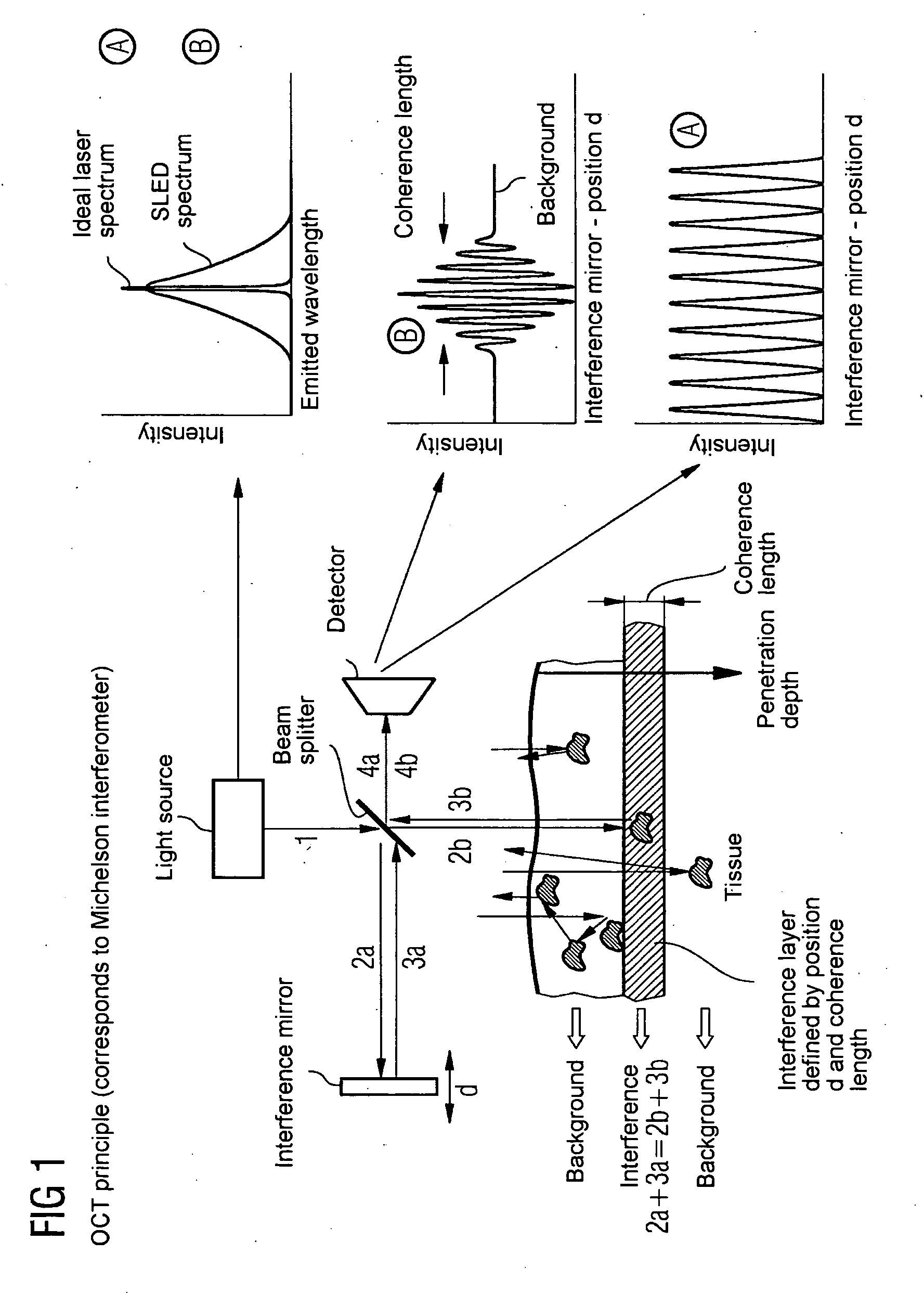
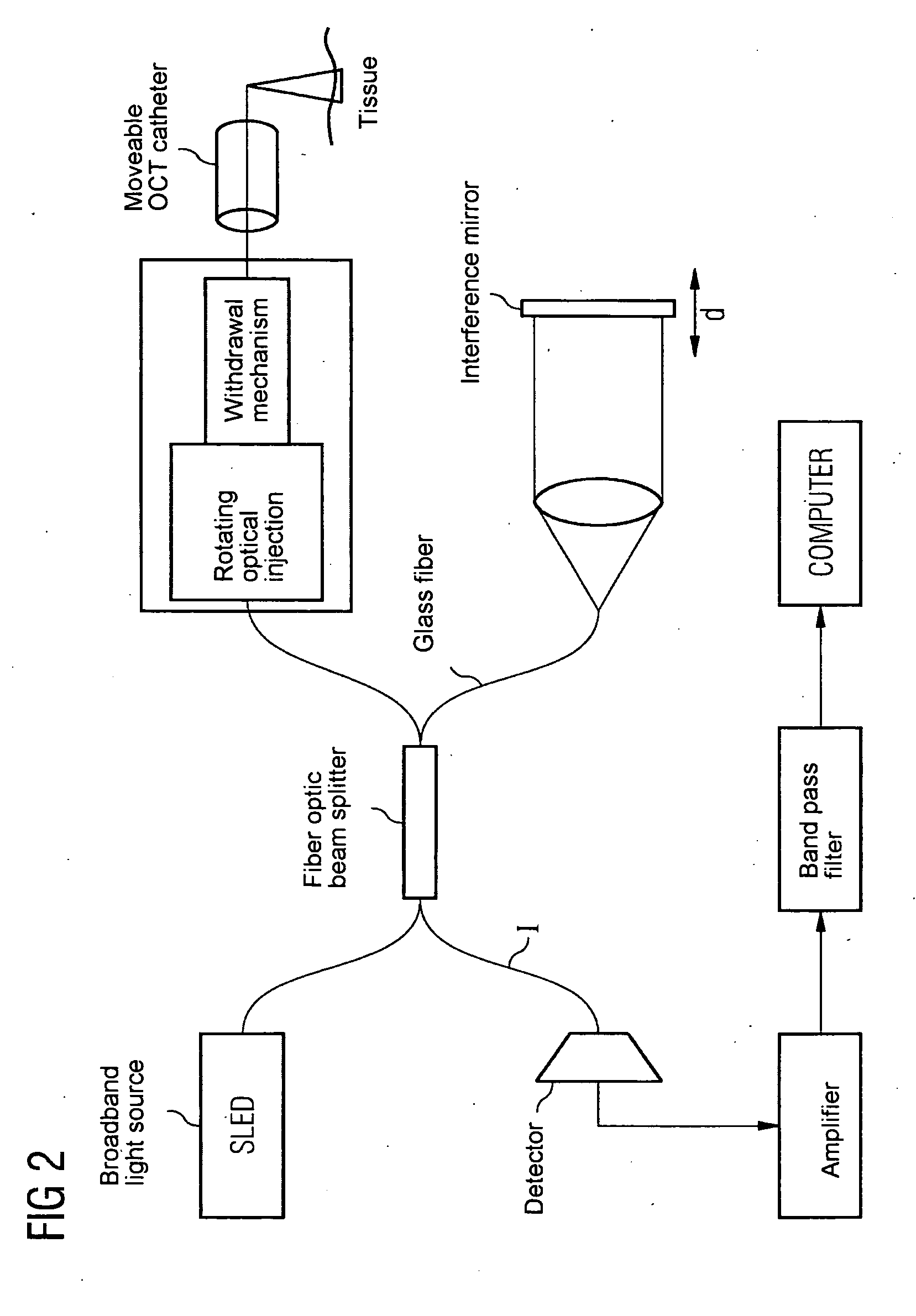
Oct-based imaging method
InactiveUS20070038125A1Function increaseSharp contrastSolid-state devicesNanomedicineVulnerable plaqueContrast medium
The present invention relates to an imaging method with an OCT catheter for visualizing molecular functional processes in vulnerable plaques of a blood vessel of the blood vessel system of a patient, with OCT images of the contrast medium-marked vulnerable plaque being generated during continuously controlled movement of the light-emitting and light-absorbing OCT catheter head along the vulnerable plaque after the intravascular injection of a contrast medium into the blood vessel system and after the intravascular insertion of an imaging OCT catheter into the blood vessel comprising the vulnerable plaque.
Owner:SIEMENS AG
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com