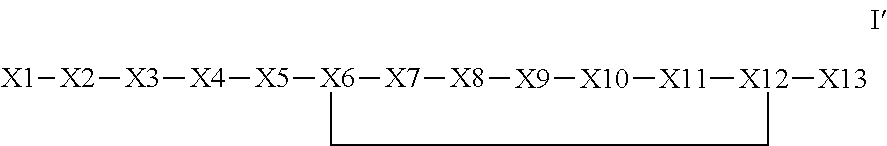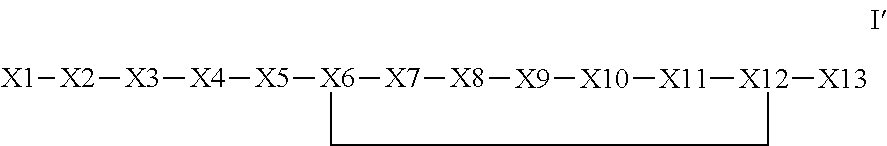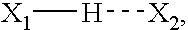Patents
Literature
507 results about "Restenosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
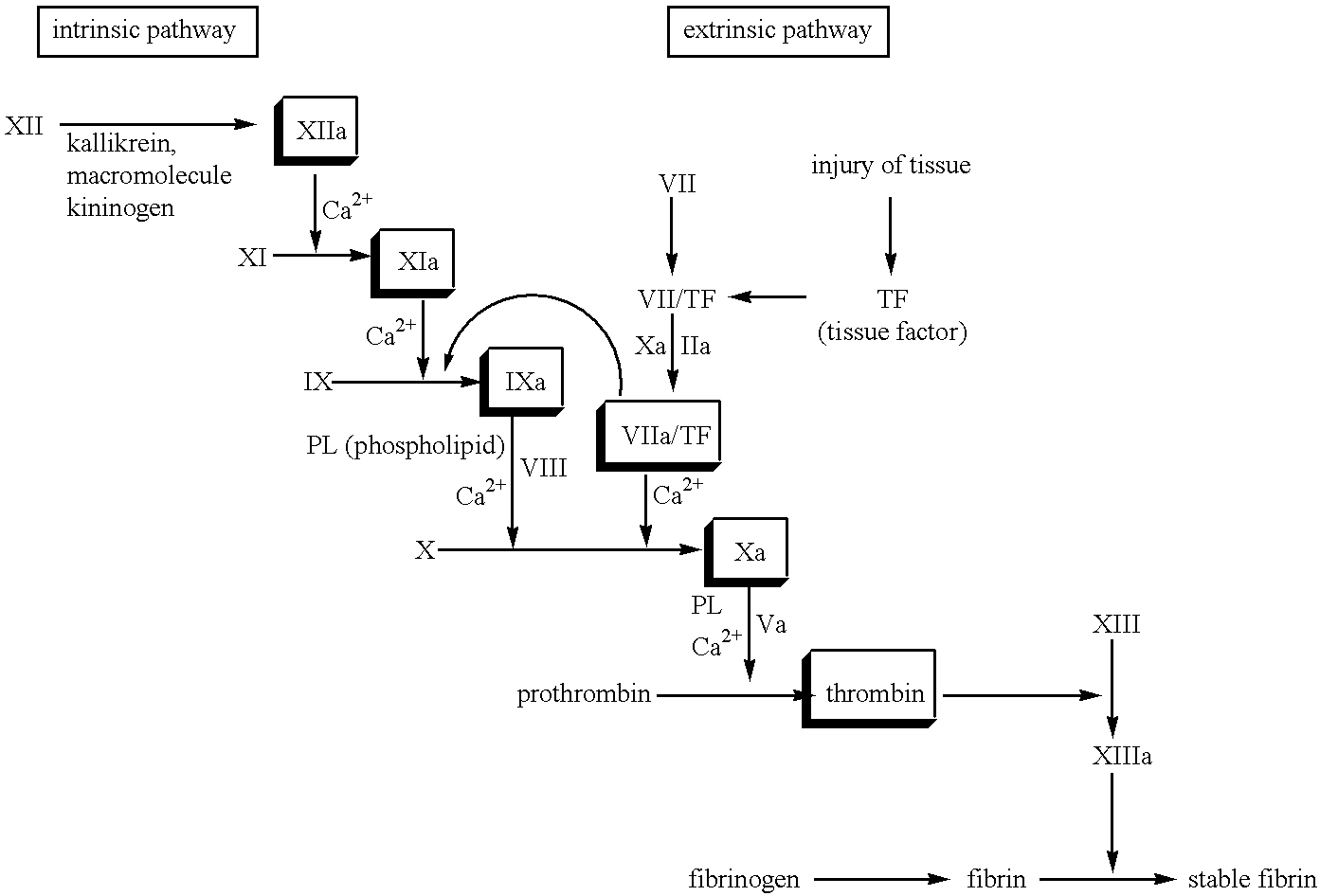
Restenosis is the recurrence of stenosis, a narrowing of a blood vessel, leading to restricted blood flow. Restenosis usually pertains to an artery or other large blood vessel that has become narrowed, received treatment to clear the blockage and subsequently become renarrowed. This is usually restenosis of an artery, or other blood vessel, or possibly a vessel within an organ.
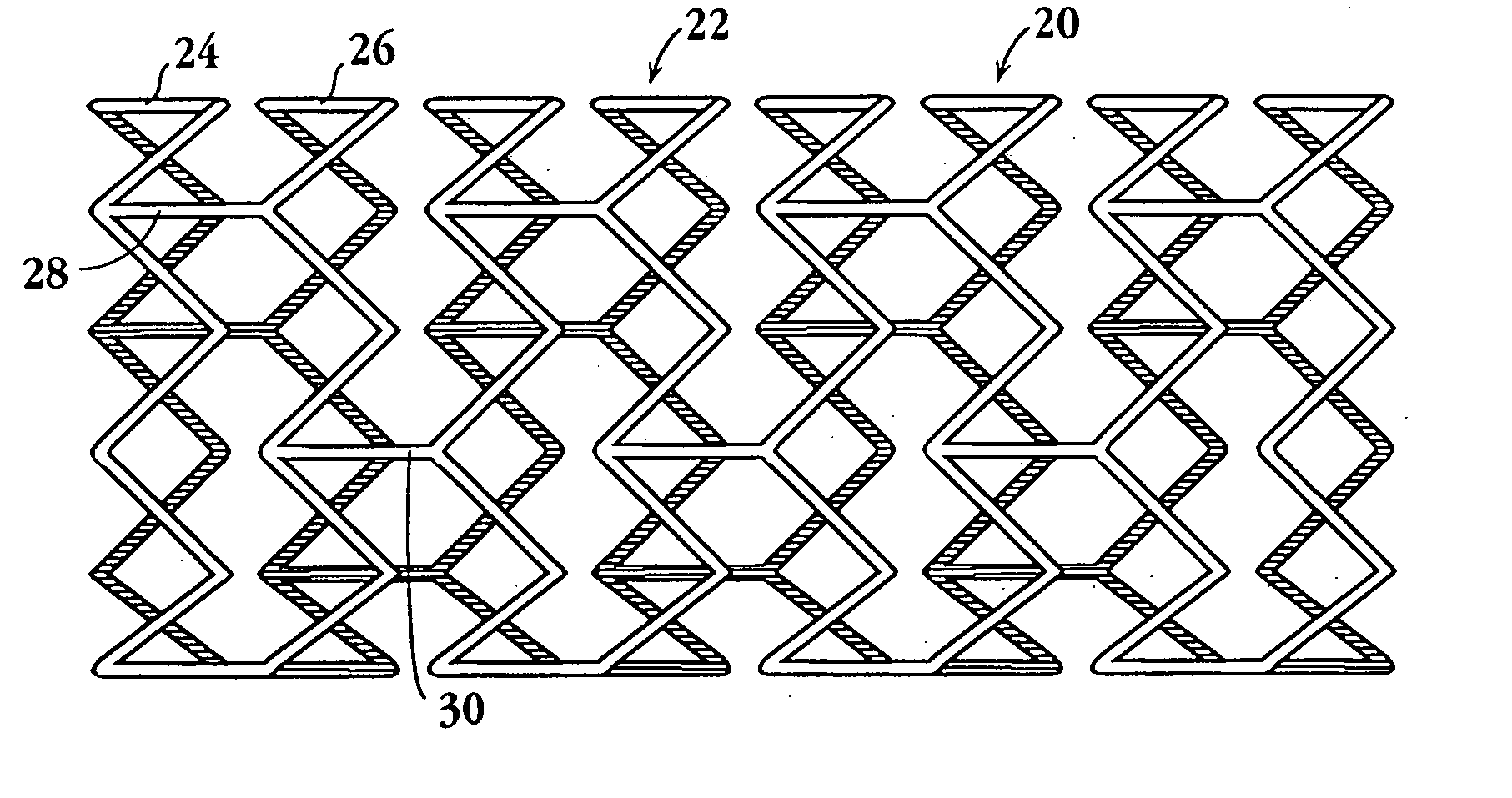
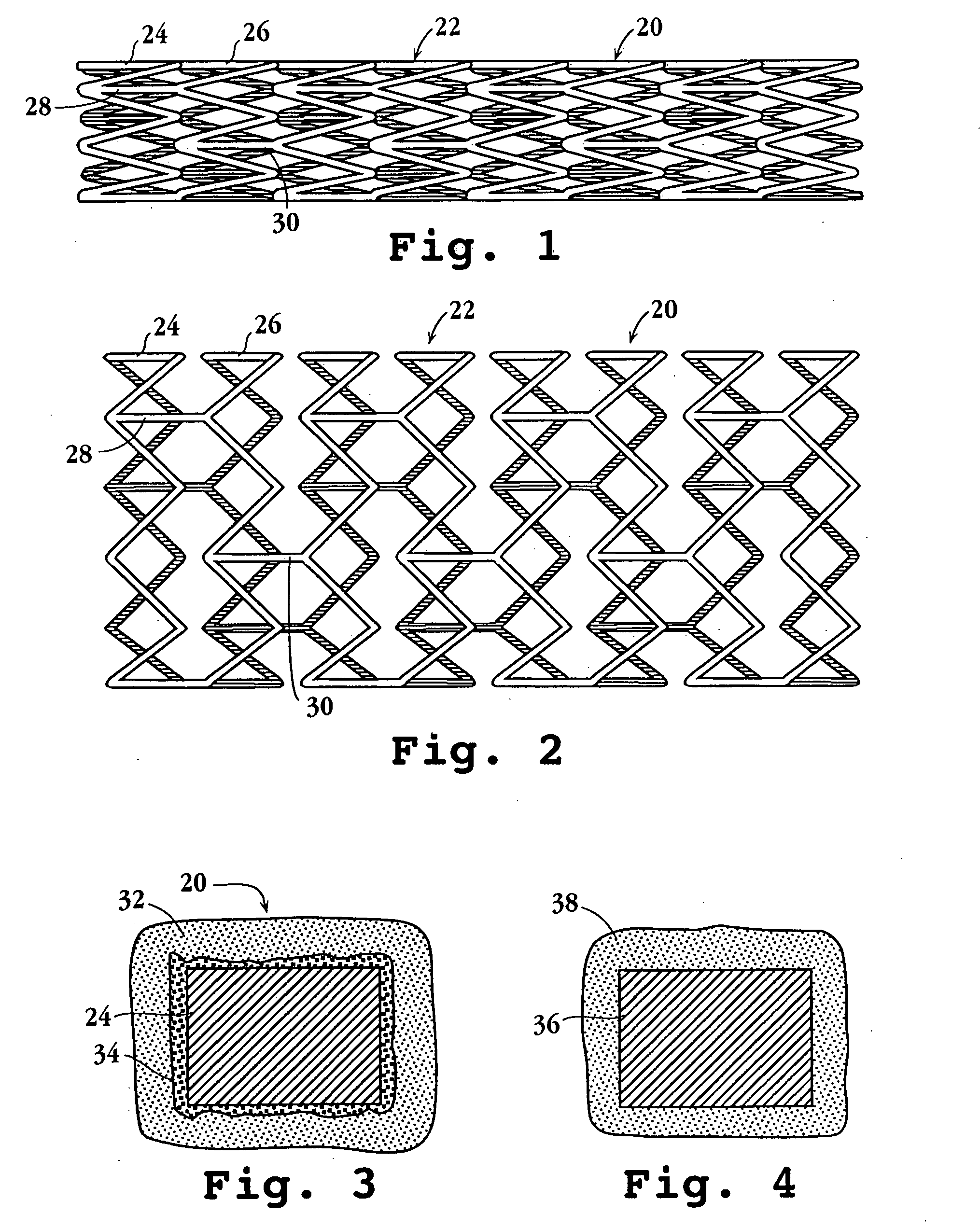
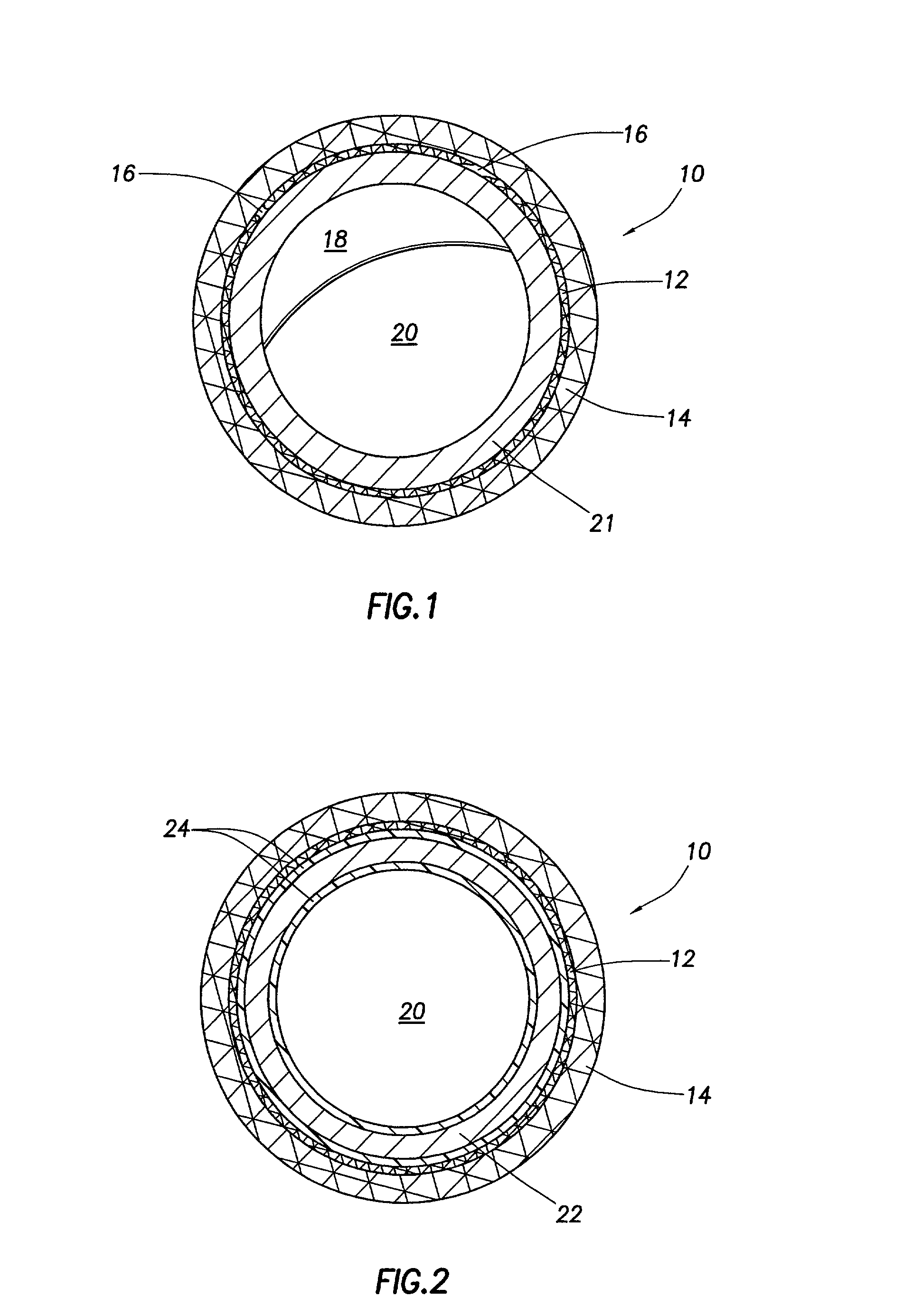
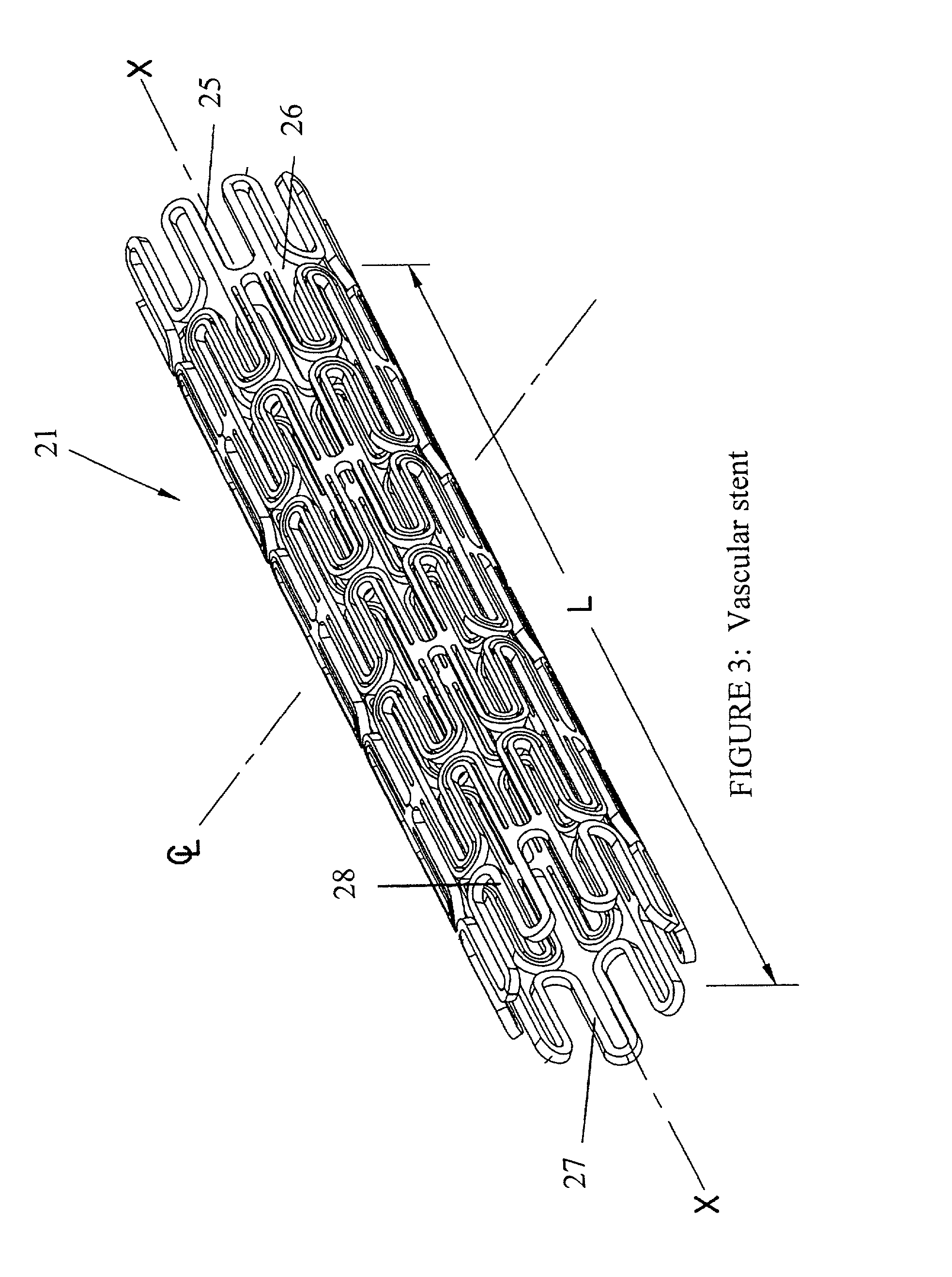
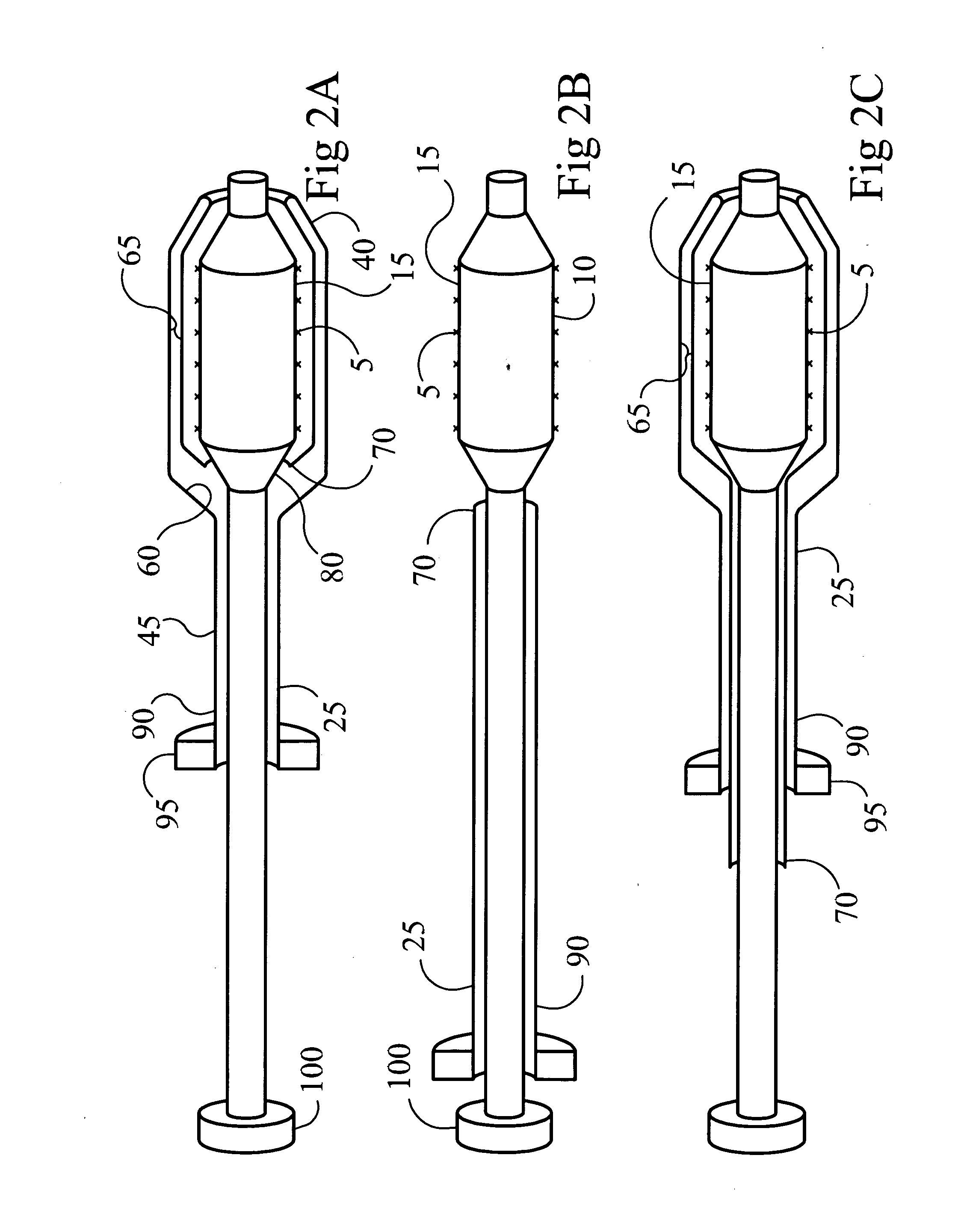
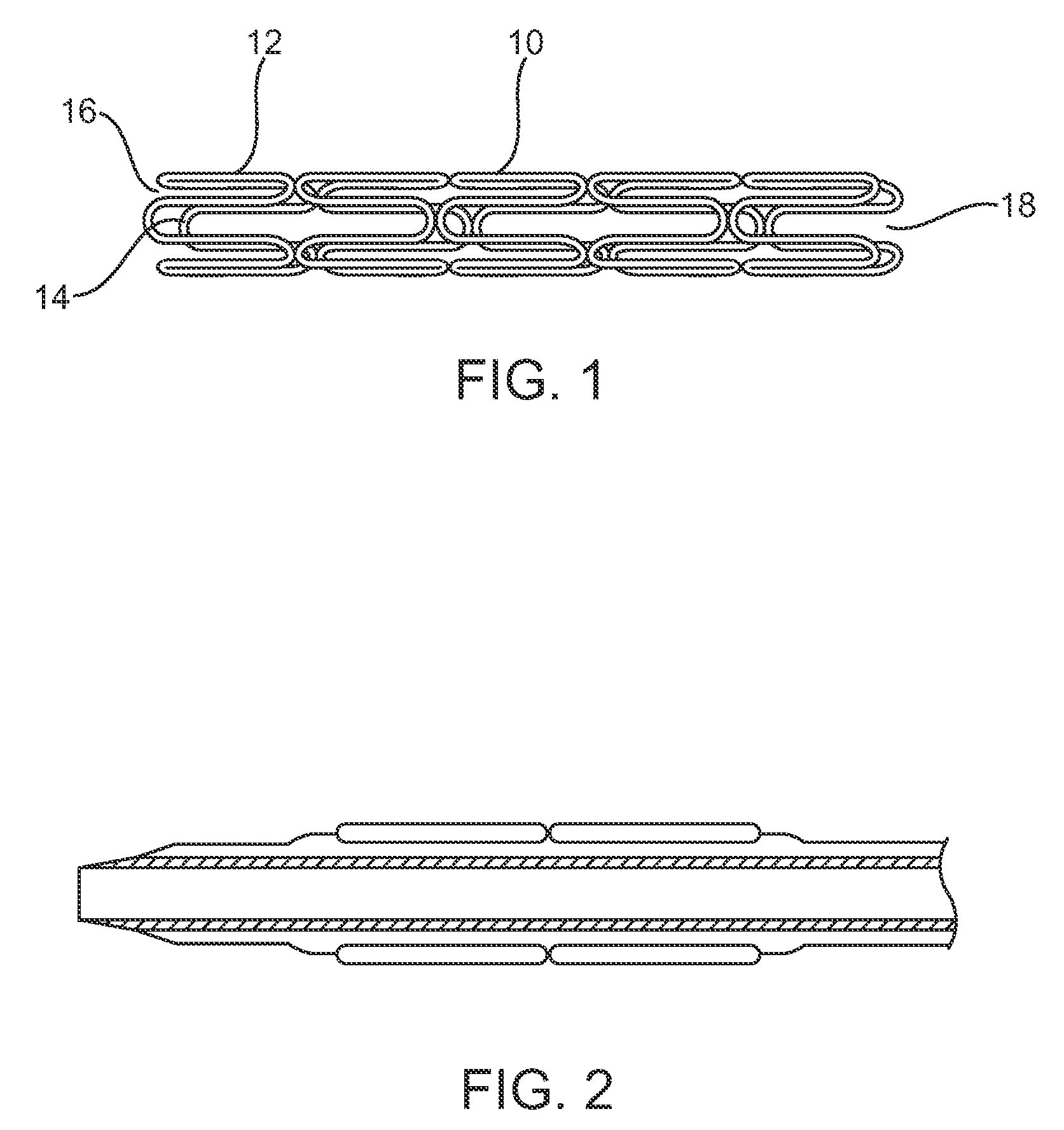
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
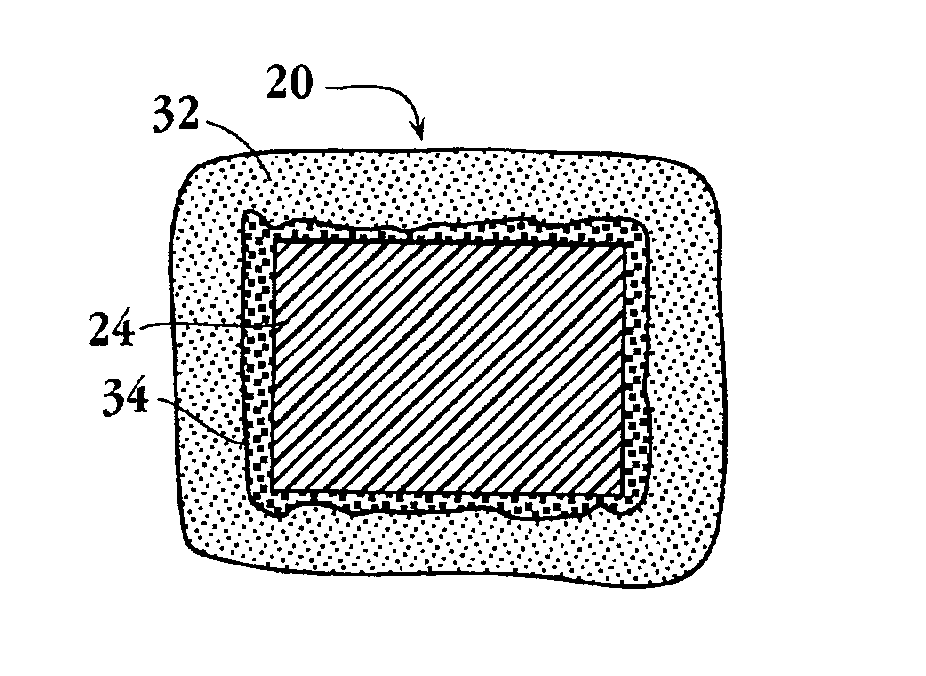
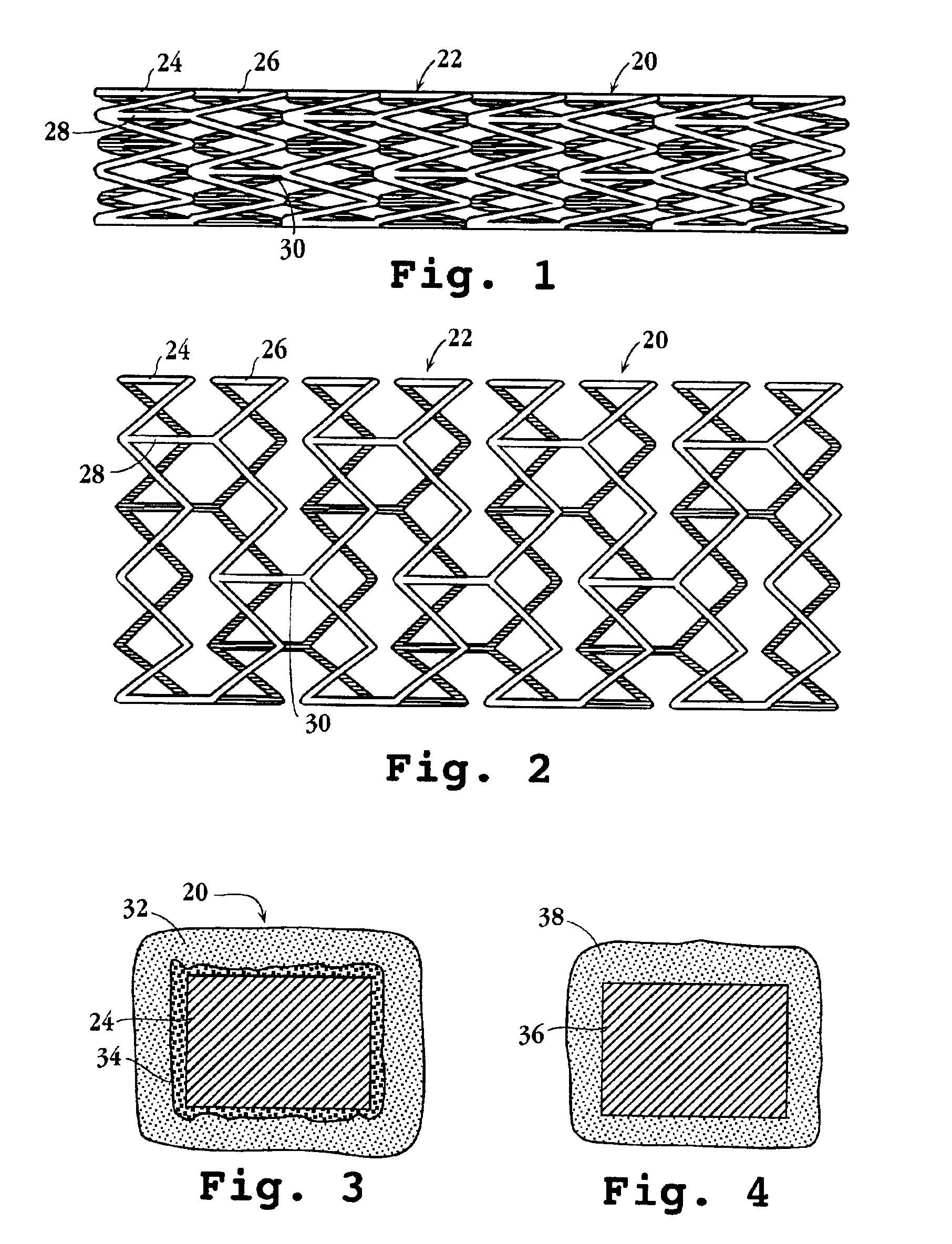
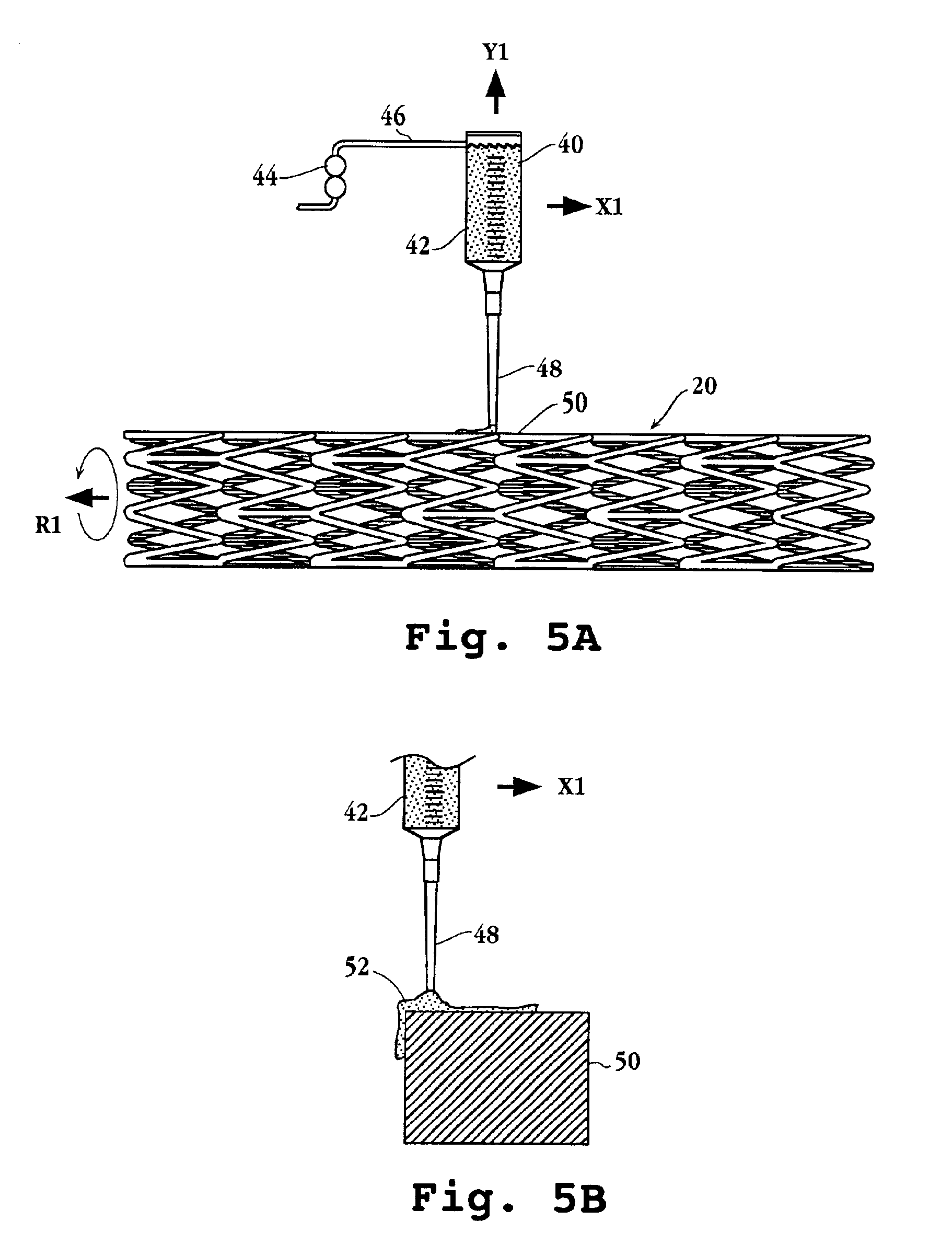
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Methods and compositions for the prevention and treatment of atherosclerosis, restenosis and related disorders
Methods and compositions for the prevention and treatment of all forms of atherosclerosis are described. Administration of compounds such as thalidomide, its analogs, hydrolysis products, metabolites, derivatives and precursors as well as additional compounds capable of inhibiting tumor necrosis factor alpha (TNF-alpha) are used in the invention. Also disclosed is the coating of prosthetic devices, such as stents, with the compounds of the invention for the prevention and / or treatment of restenosis.
Owner:CELGENE CORP
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Stent coatings containing HMG-CoA reductase inhibitors
InactiveUS20030077310A1Hydrolysis can be preventedAvoid accessStentsSurgeryHMG-CoA reductaseDepressant
Stents with coatings comprising a combination of a restenosis inhibitor comprising an HMG-CoA reductase inhibitor and a carrier. Also provided are methods of coating stents with a combination of an HMG-CoA reductase inhibitor and a carrier. A preferred example of a restenosis inhibitor is cerivastatin. The stent coatings have been shown to release restenosis inhibitors in their active forms.
Owner:ZIMMER ORTHOBIOLIGICS
Drug-eluting medical device
The present invention relates to a drug-eluting medical device, in particular a balloon for angioplasty catheters with drug elution to prevent the restenosis of the vessel subjected to angioplasty. More particularly, the present invention relates to a catheter balloon completely or partially coated with paclitaxel in hydrated crystalline form or in hydrated solvated crystalline form, having an immediate release and bioavailability of a therapeutically effective amount of paclitaxel at the site of intervention. The balloon can be made of a polyether-polyamide block copolymer, or a polyester amide, or polyamide-12.
Owner:INVATEC TECH CENT
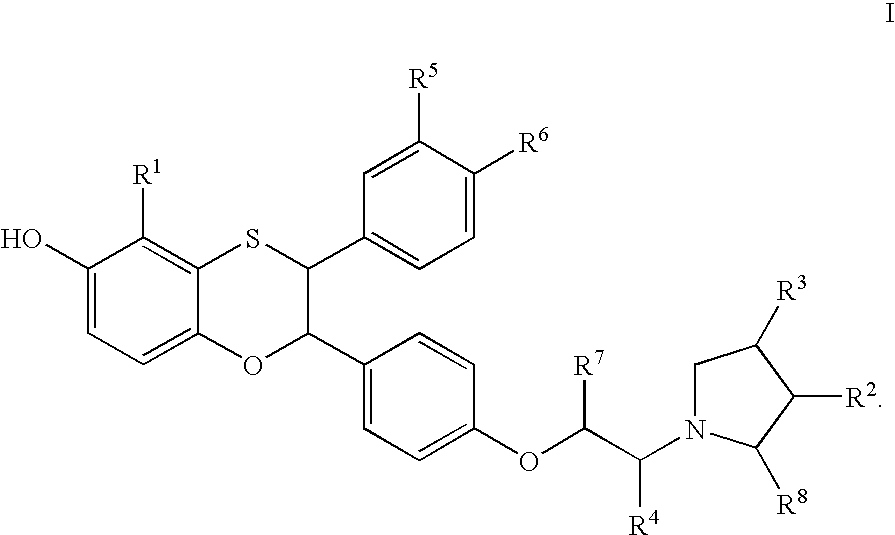
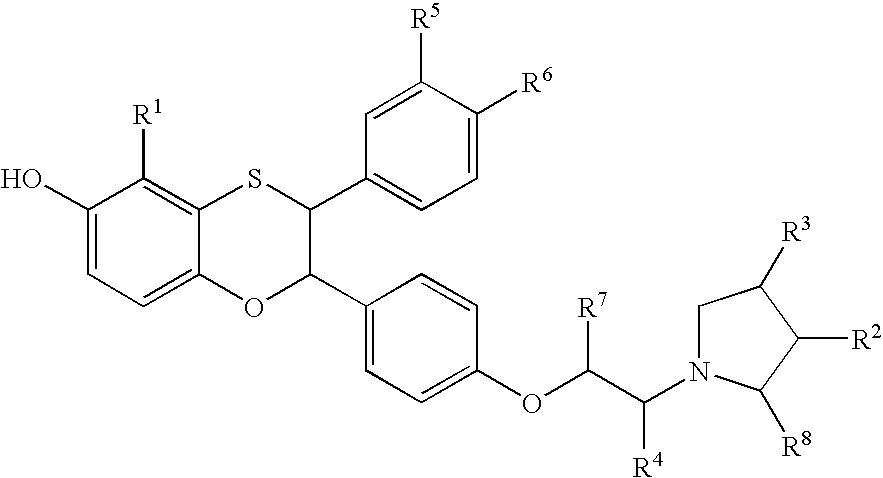
Soluble Guanylate Cyclase Activators
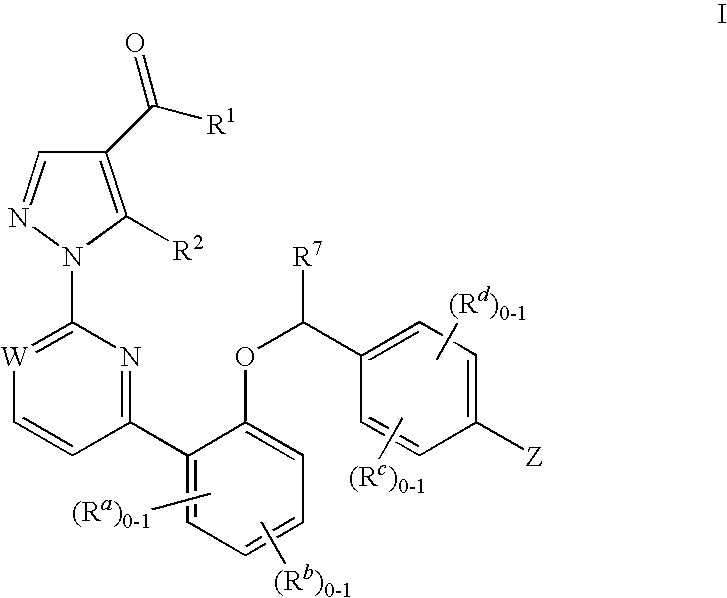
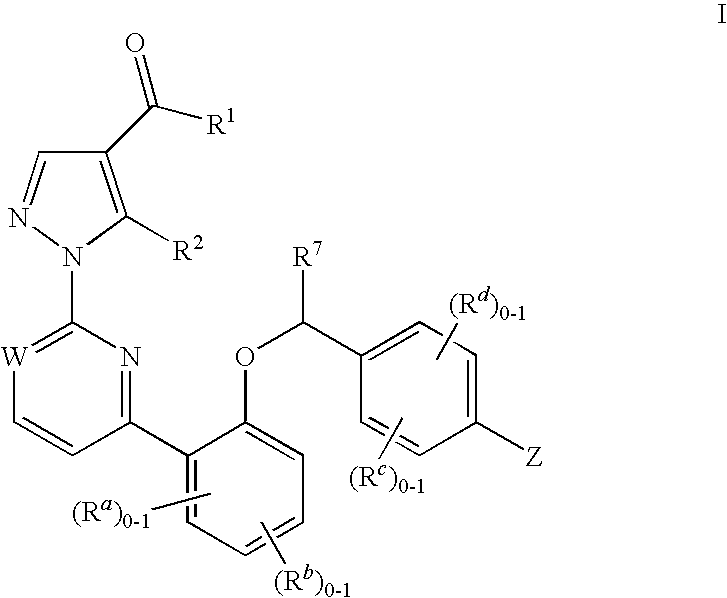
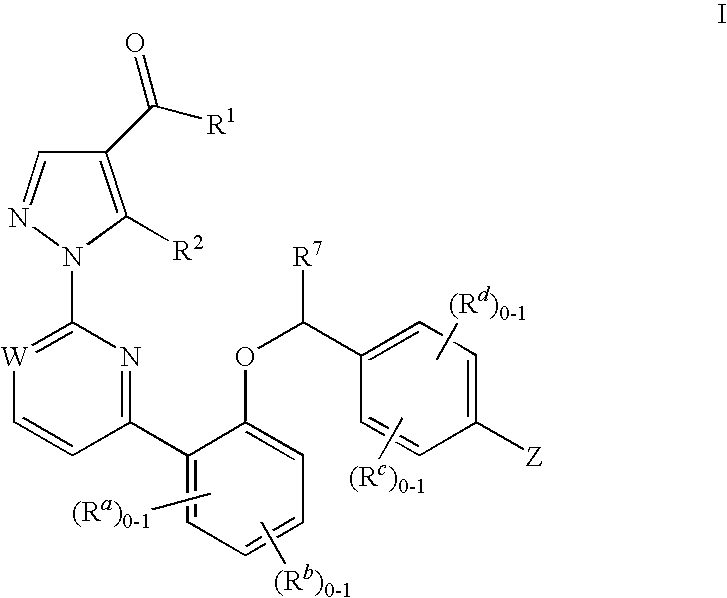
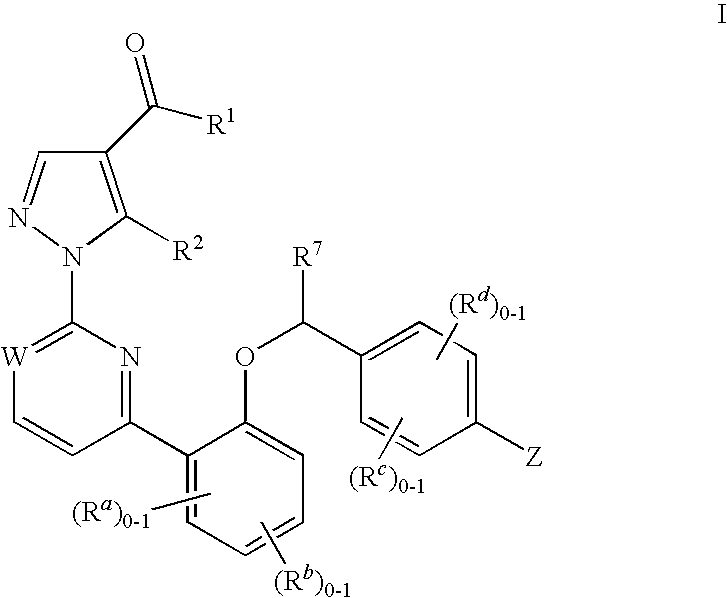
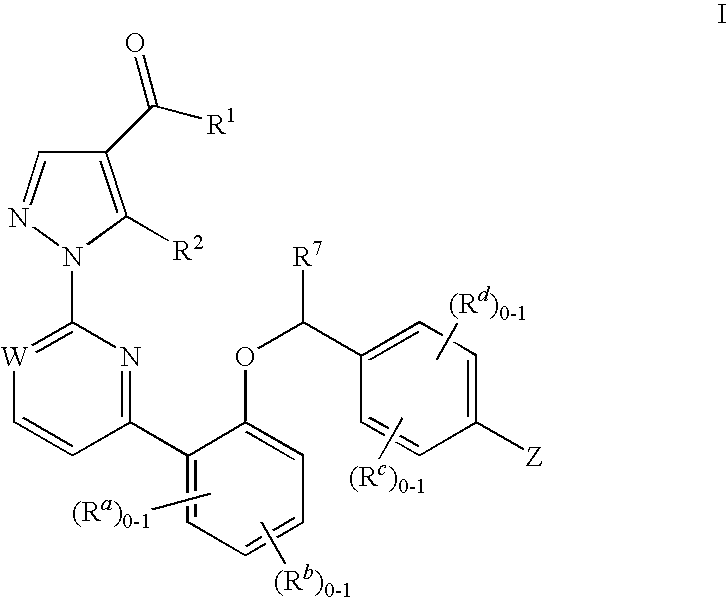
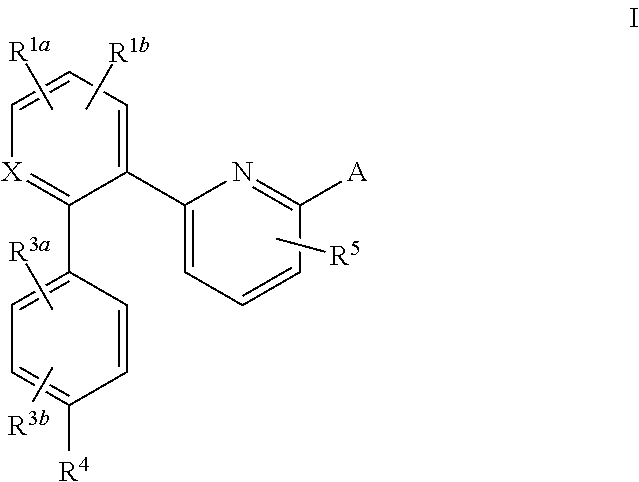
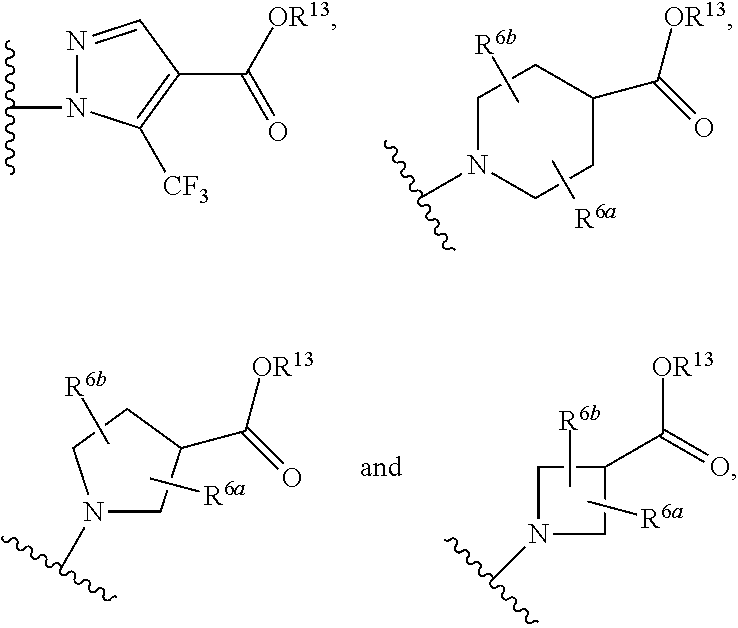
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Drug eluting surface covering
InactiveUS20100228333A1Provide protectionImprove permeabilityStentsBalloon catheterBalloon dilatation catheterLesion site
A thin-walled sheath is placed over a balloon having an antirestenotic drug placed on the balloon of a balloon dilatation catheter. The sheath protects the drug from dissolution into the blood and allows improved delivery to the lesion site. A rolling action of the sheath prevents the drug from loss due to shearing motion. The sheath can also provide a protected surface for carrying the drug and providing exposure to the lesion site for delivery of the drug. The sheath can also serve as a delivery sheath for providing delivery of a stent via a single catheter introduction for drug delivery and stent delivery.
Owner:DRASLER WILLIAM JOSEPH +1
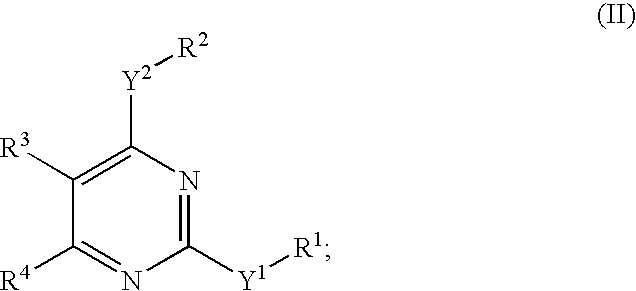
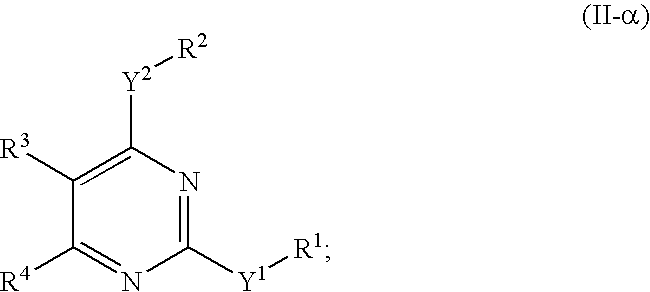
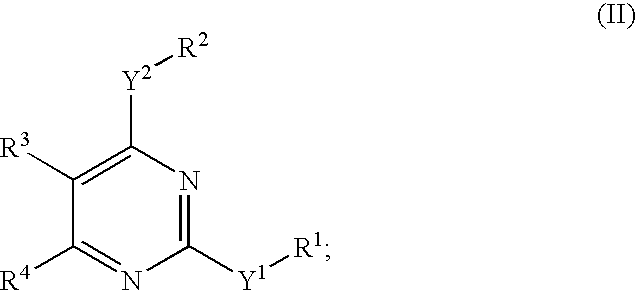
Novel pyrimidine compounds, process for their preparation and compositions containing them
InactiveUS20060084645A1Inhibit inflammationInhibition is effectiveBiocideOrganic chemistryDyslipidemiaNephrosis
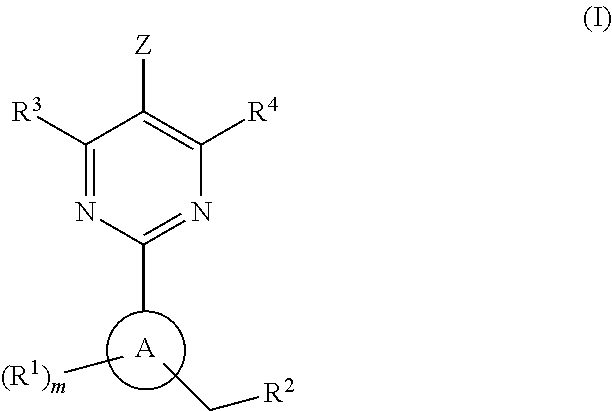
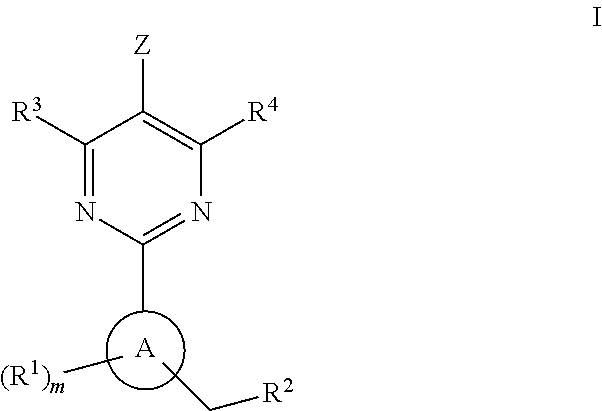
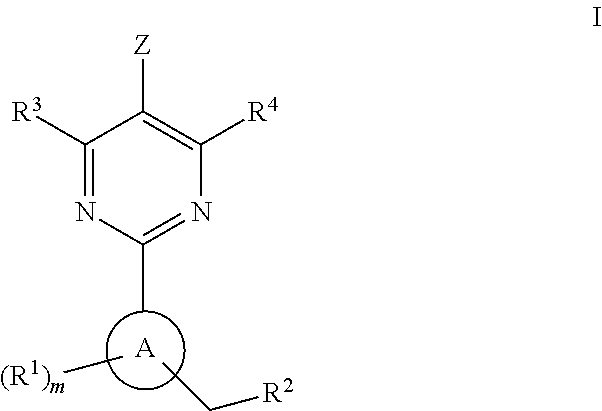
The present invention provides new heterocyclic compounds, particularly substituted pyrimidines, methods and compositions for making and using these heterocyclic compounds, and methods for treating a variety of diseases and disease states, including atherosclerosis, arthritis, restenosis, diabetic nephropathy, or dyslipidemia, or disease states mediated by the low expression of Perlecan.
Owner:DR REDDYS LAB LTD
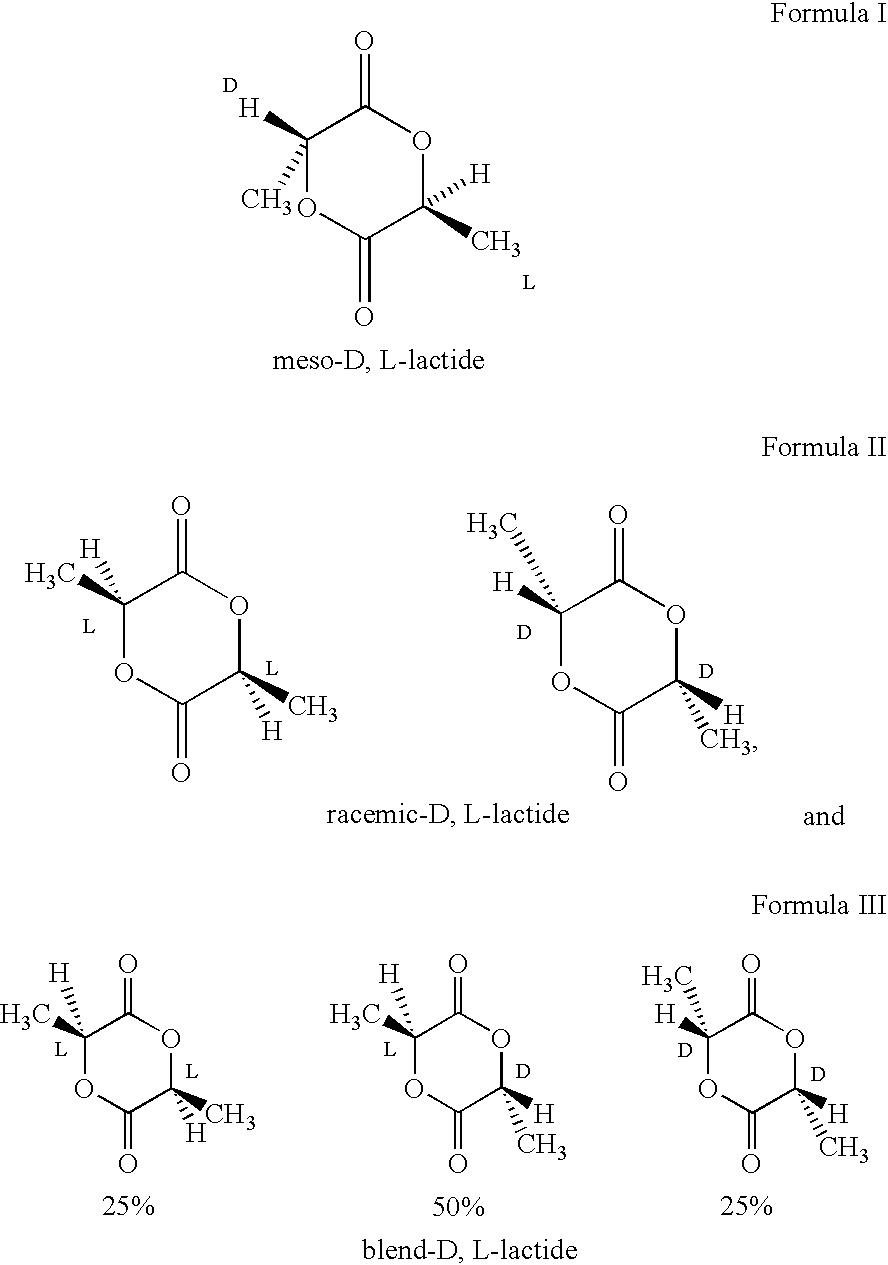
Amorphous poly(D,L-lactide) coating
Implantable devices formed of or coated with a material that includes an amorphous poly(D,L-lactide) formed of a starting material such as meso-D,L-lactide are provided. The implantable device can be used for the treatment, mitigation, prevention, or inhibition of a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Medical stent provided with inhibitors of atp synthesis
A stent provided with a composition having at least one type of inhibitor of ATP synthesis, optionally together with at least one inhibitor of the pentose phosphate pathway is disclosed. The medical stent is useful for treating stenosis and preventing restenosis in vascular ducts and for treating cancerous tumors present in ducts, resectioned cavities and scars and any disorder arising from the proliferation of cells in ducts or cavities.
Owner:INTERSTITIAL THERAPEUTICS
Amidino derivatives and drugs containing the same as the active ingredient
InactiveUS6358960B1BiocideGroup 5/15 element organic compoundsExtracorporeal circulationDisseminated coagulopathy
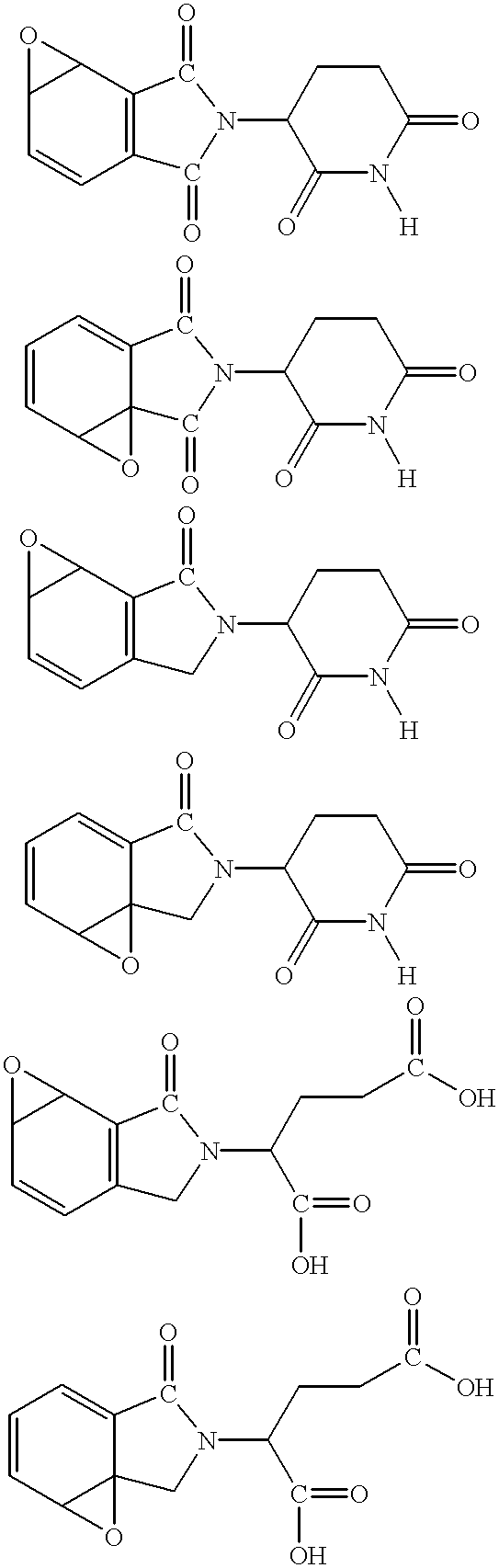
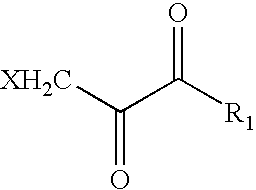
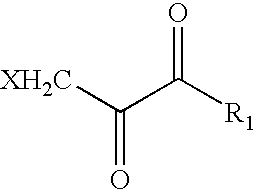
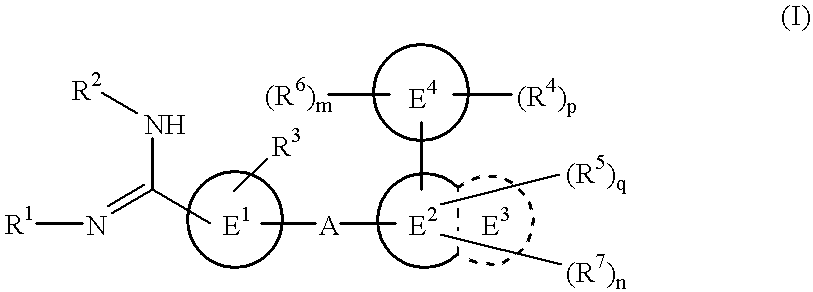
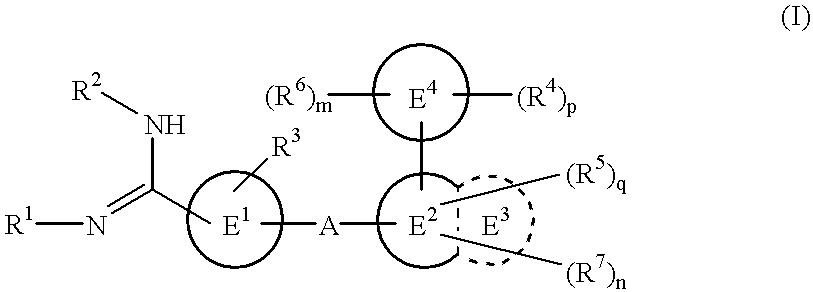
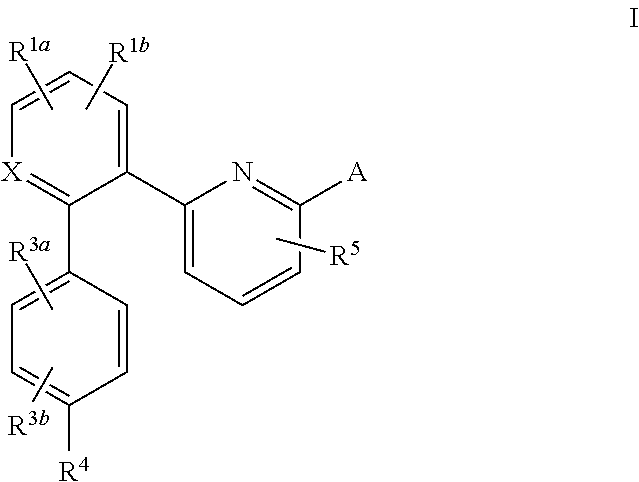
The novel amidino derivatives of the formula (I):wherein all the symbols are as in specification defined;have an inhibitory activity of a blood coagulation factor VIIa and are useful for treatment and / or prevention of several angiopathy caused by enhancing a coagulation activity, such as disseminated intravascular coagulation, coronary thrombosis, cerebral infarction, cerebral embolism, transient ischemic attack, cerebrovascular disorders, pulmonary vascular diseases, deep venous thrombosis, peripheral arterial obstruction, thrombosis after artificial vascular transplantation and artificial valve transplantation, post-operative thrombosis, reobstruction and restenosis after coronary artery bypass operation, reobstruction and restenosis after PTCA or PTCR, thrombosis by extracorporeal circulation and procoagulative diseases such as glomerlonephriitis.
Owner:ONO PHARMA CO LTD
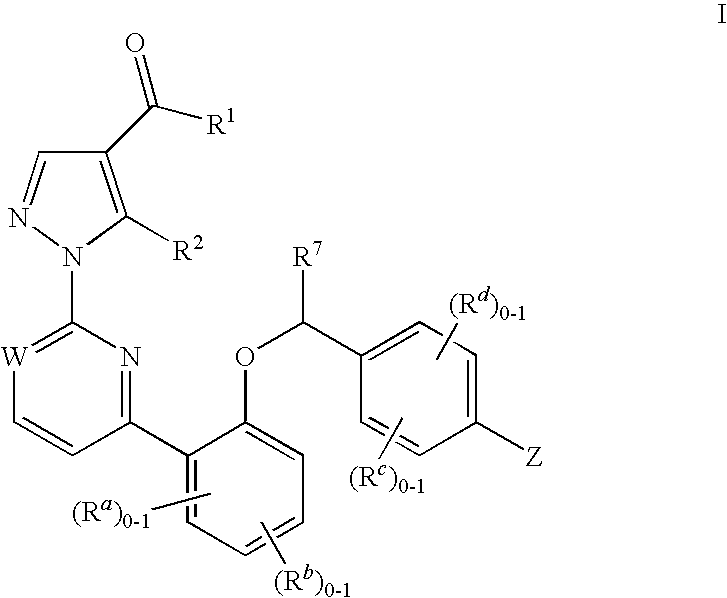
Soluble guanylate cyclase activators
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
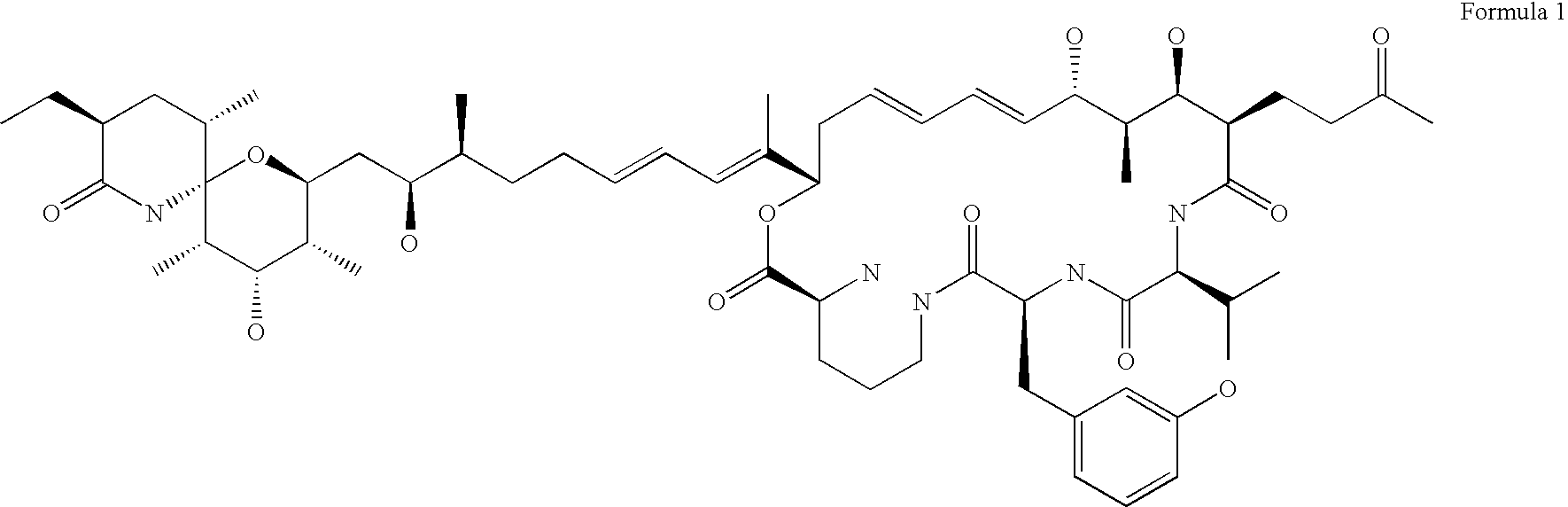
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS8673848B2Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Soluble guanylate cyclase activators
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Compositions and methods for the treatment and diagnosis of cardiovascular disease
InactiveUS6048709AHigh throughputReadily contactFungiBacteriaClinical evaluationPercent Diameter Stenosis
The present invention relates to methods and compositions for the treatment and diagnosis of cardiovascular disease, including, but not limited to, atherosclerosis, ischemia / reperfusion, hypertension, restenosis, and arterial inflammation. Specifically, the present invention identifies and describes genes which are differentially expressed in cardiovascular disease states, relative to their expression in normal, or non-cardiovascular disease states, and / or in response to manipulations relevant to cardiovascular disease. Further, the present invention identifies and describes genes via the ability of their gene products to interact with gene products involved in cardiovascular disease. Still further, the present invention provides methods for the identification and therapeutic use of compounds as treatments of cardiovascular disease moreover, the present invention provides methods for the diagnostic monitoring of patients undergoing clinical evaluation for the treatment of cardiovascular disease, and for monitoring the efficacy of compounds in clinical trials. Additionally, the present invention describes methods for the diagnostic evaluation and prognosis of various cardiovascular diseases, and for the identification of subjects exhibiting a predisposition to such conditions.
Owner:MILLENNIUM PHARMA INC +1
Devices and methods for percutaneous endarterectomy
Devices and methods are provided for percutaneously treating atherosclerotic plaques within blood vessels. Atherosclerotic plaques cause significant morbidity and mortality by narrowing the arteries, which adversely affects blood flow, and by acting as a source for thrombi and emboli thus causing acute organ ischemia. Current treatments include open surgery with its inherent drawbacks, and stenting, which is less invasive but leaves the plaque material in the artery, which promotes restenosis. The present invention combines the advantages of both approaches. In general, the invention provides tools that enable percutaneously dissecting the plaque from the arterial wall and removing it from the body.
Owner:ANGIOWORKS MEDICAL
Compositions and methods for the treatment and diagnosis of cardiovascular disease
InactiveUS6099823ALoss of responseReduce expressionOrganic active ingredientsSenses disorderClinical evaluationPercent Diameter Stenosis
The present invention relates to methods and compositions for the treatment and diagnosis of cardiovascular disease, including, but not limited to, atherosclerosis, ischemia / reperfusion, hypertension, restenosis, and arterial inflammation. Specifically, the present invention identifies and describes genes which are differentially expressed in cardiovascular disease states, relative to their expression in normal, or non-cardiovascular disease states, and / or in response to manipulations relevant to cardiovascular disease. Further, the present invention identifies and describes genes via the ability of their gene products to interact with gene products involved in cardiovascular disease. Still further, the present invention provides methods for the identification and therapeutic use of compounds as treatments of cardiovascular disease. Moreover, the present invention provides methods for the diagnostic monitoring of patients undergoing clinical evaluation for the treatment of cardiovascular disease, and for monitoring the efficacy of compounds in clinical trials. Additionally, the present invention describes methods for the diagnostic evaluation and prognosis of various cardiovascular diseases, and for the identification of subjects exhibiting a predisposition to such conditions.
Owner:MILLENNIUM PHARMA INC
Soluble guanylate cyclase activators
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
MEK inhibiting compounds
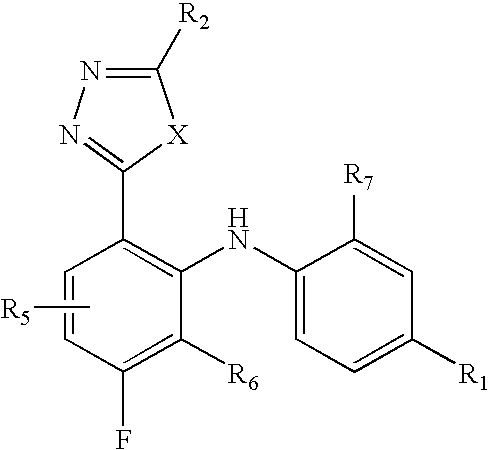
InactiveUS20050004186A1Inhibit phosphorylationAntibacterial agentsBiocidePercent Diameter StenosisImmunomodulations
This invention provides substituted Phenyl-(2-[1,3,4]thiadiazol-2-yl-phenyl)-amine and (2-[1,3,4]Oxadiazol-2-yl-phenyl)phenyl-amine compounds which act as inhibitors of MAPK / ERK Kinase (“MEK”) enzymes and pharmaceutical compositions and methods for their use in immunomodulation and in the treatment and alleviation of inflammation, and proliferative diseases such as cancer and restenosis.
Owner:PFIZER INC
N-acyl sulfamic acid esters useful as hypocholesterolemic agents
PCT No. PCT / US97 / 06725 Sec. 371 Date Aug. 10, 1998 Sec. 102(e) Date Aug. 10, 1998 PCT Filed Apr. 21, 1997 PCT Pub. No. WO97 / 44314 PCT Pub. Date Nov. 27, 1997The instant invention is new compounds of Formula I their use as cerebrovascular agents in diseases such as stroke, peripheral vascular disease, restenosis, and as agents for regulating plasma cholesterol concentrations, for treating hypercholesterolemia and atherosclerosis, and for lowering the serum or plasma level of Lp(a). A pharmaceutical composition is also claimed.
Owner:WARNER-LAMBERT CO
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Variants of C-Type Natriuretic Peptide
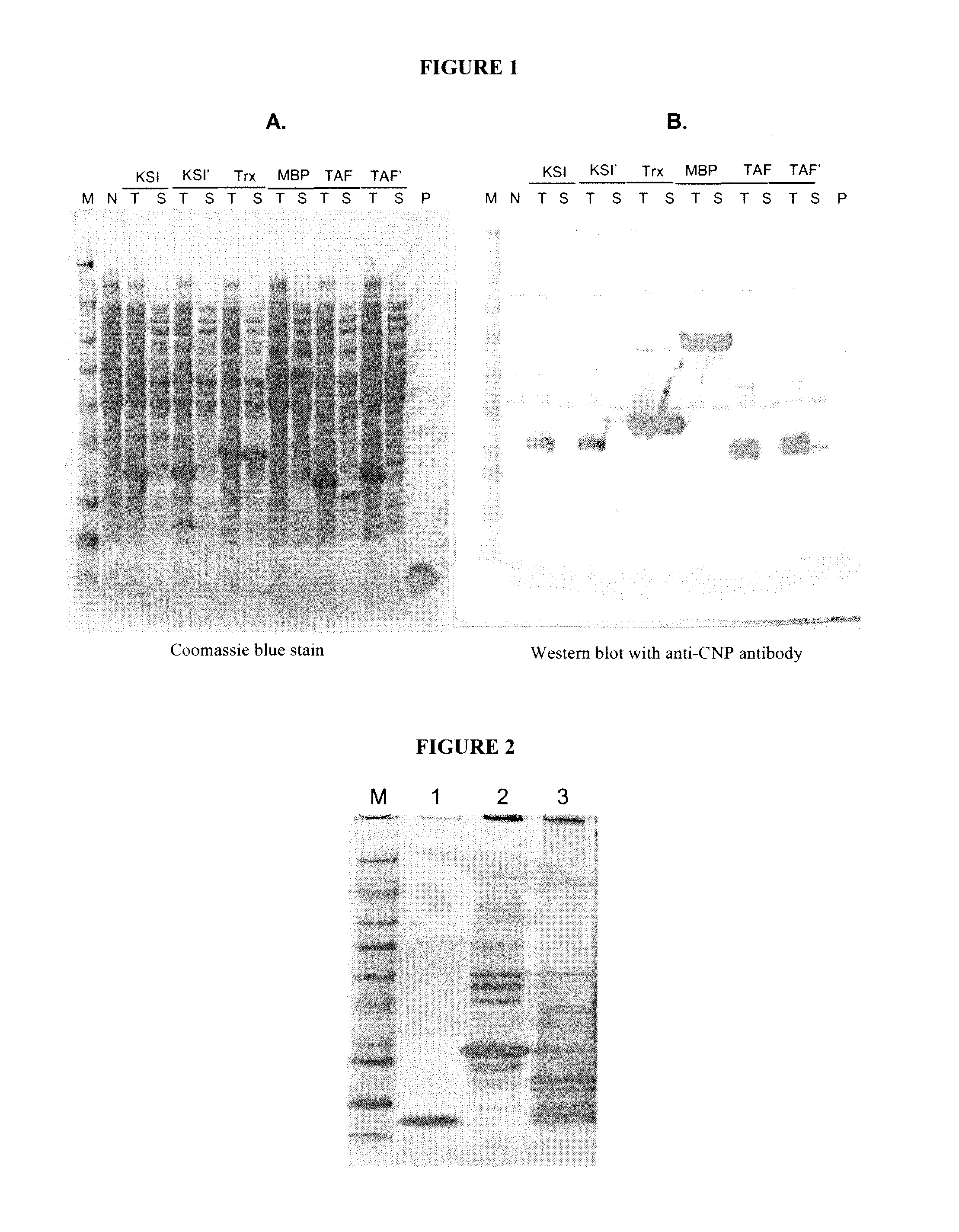
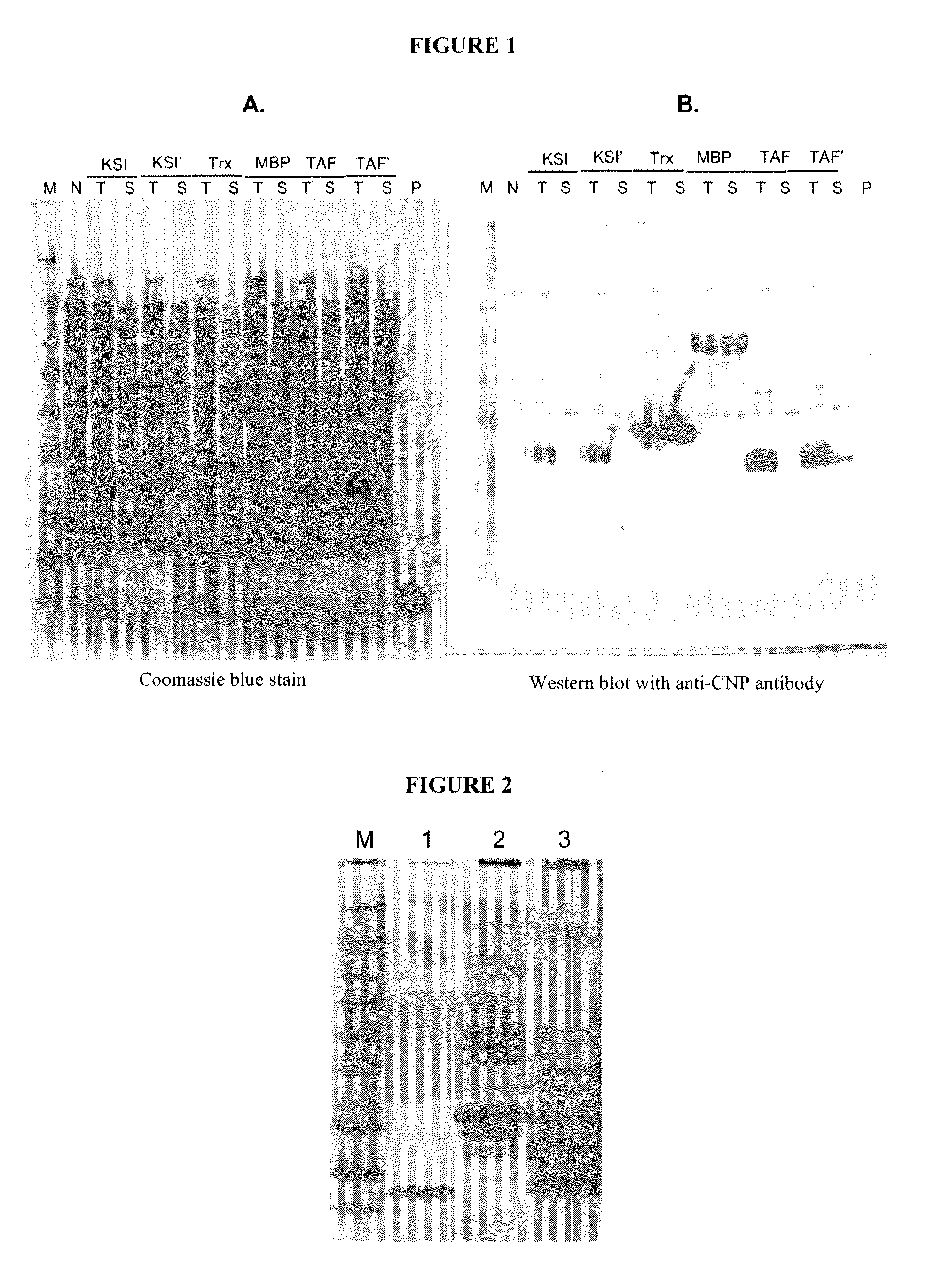
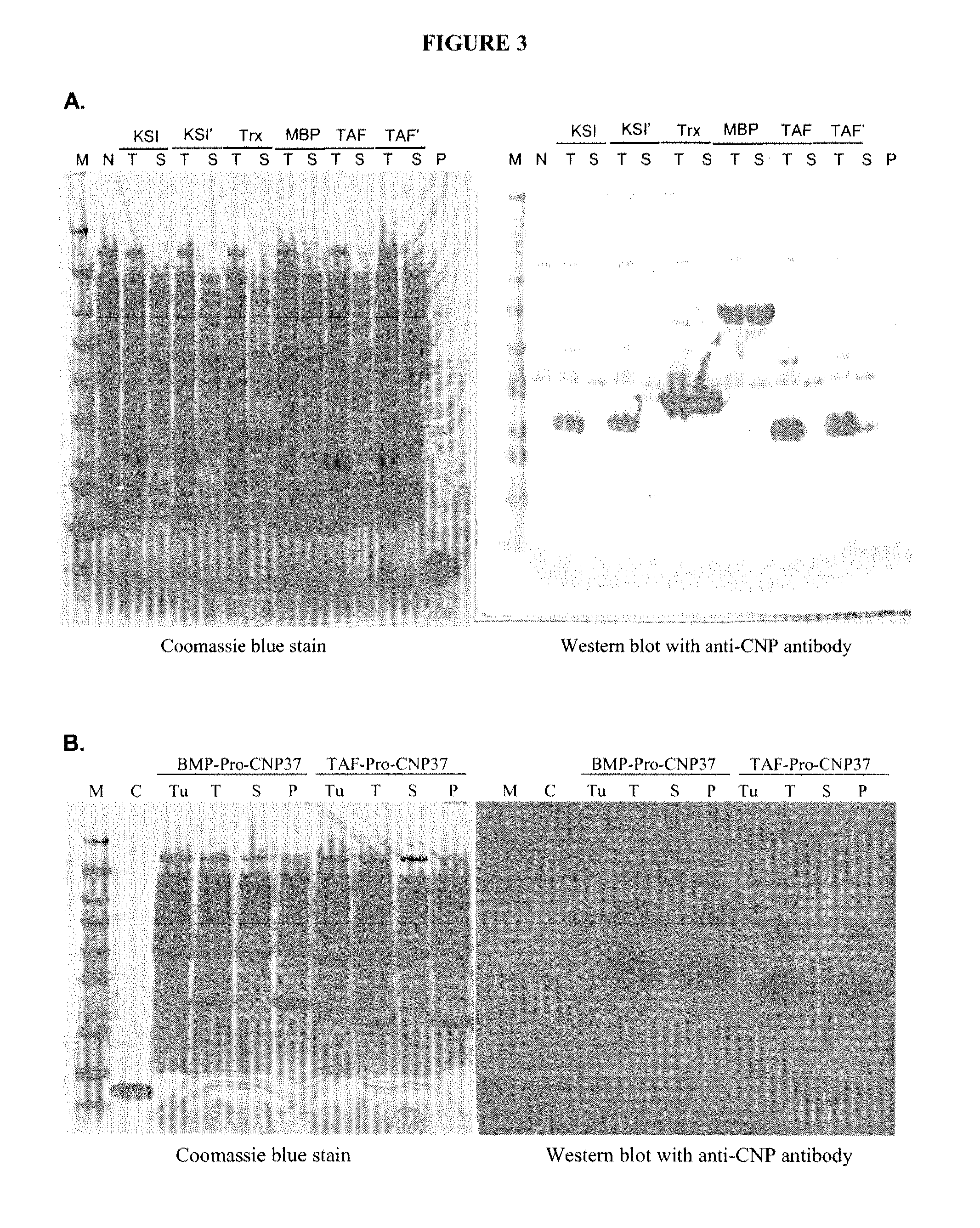
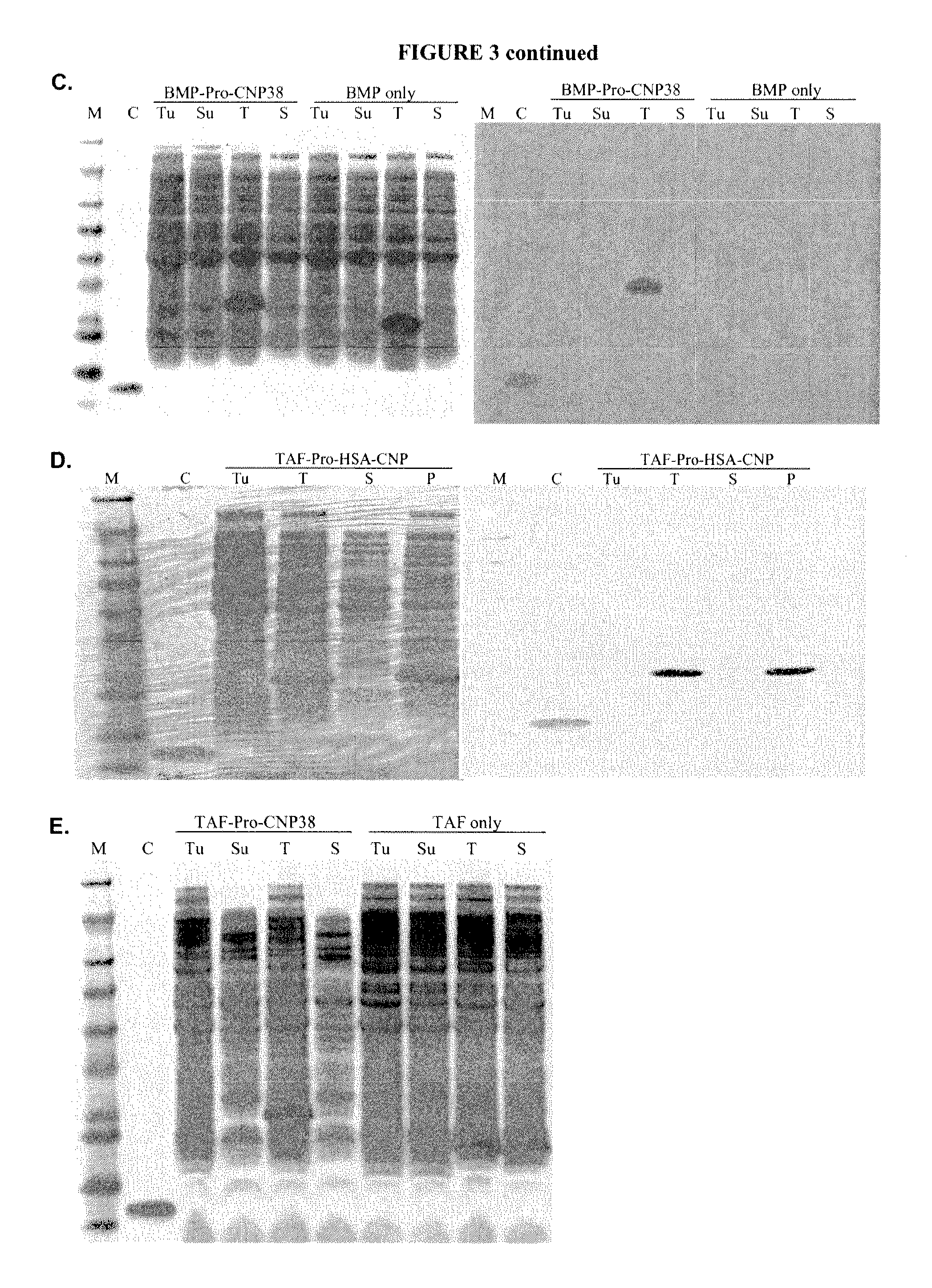
ActiveUS20100297021A1Improve the immunityReduce functionBacteriaPeptide/protein ingredientsDiseaseSmooth muscle
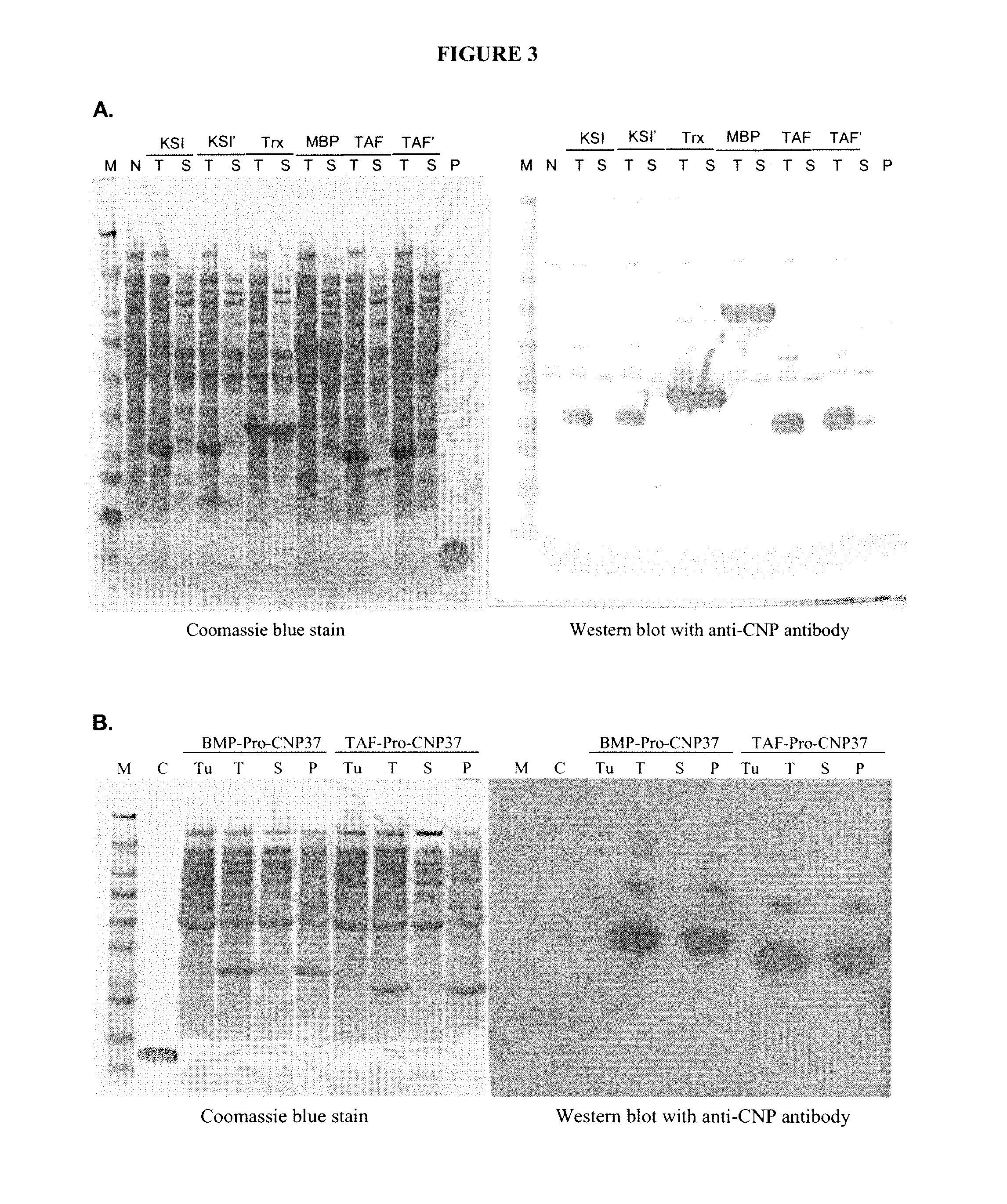
The present disclosure provides variants of C-type natriuretic peptide (CNP), pharmaceutical compositions comprising CNP variants, and methods of making CNP variants. The CNP variants are useful as therapeutic agents for the treatment of diseases responsive to CNP, including but not limited to bone-related disorders, such as skeletal dysplasias (e.g., achondroplasia), and vascular smooth muscle disorders (e.g., restenosis and arteriosclerosis).
Owner:BIOMARIN PHARMA INC
Medical device with coating that promotes endothelial cell adherence and differentiation
InactiveUS20070055367A1Improved prognosisInhibit intimal hyperplasiaMaterial nanotechnologyStentsAntigenProgenitor
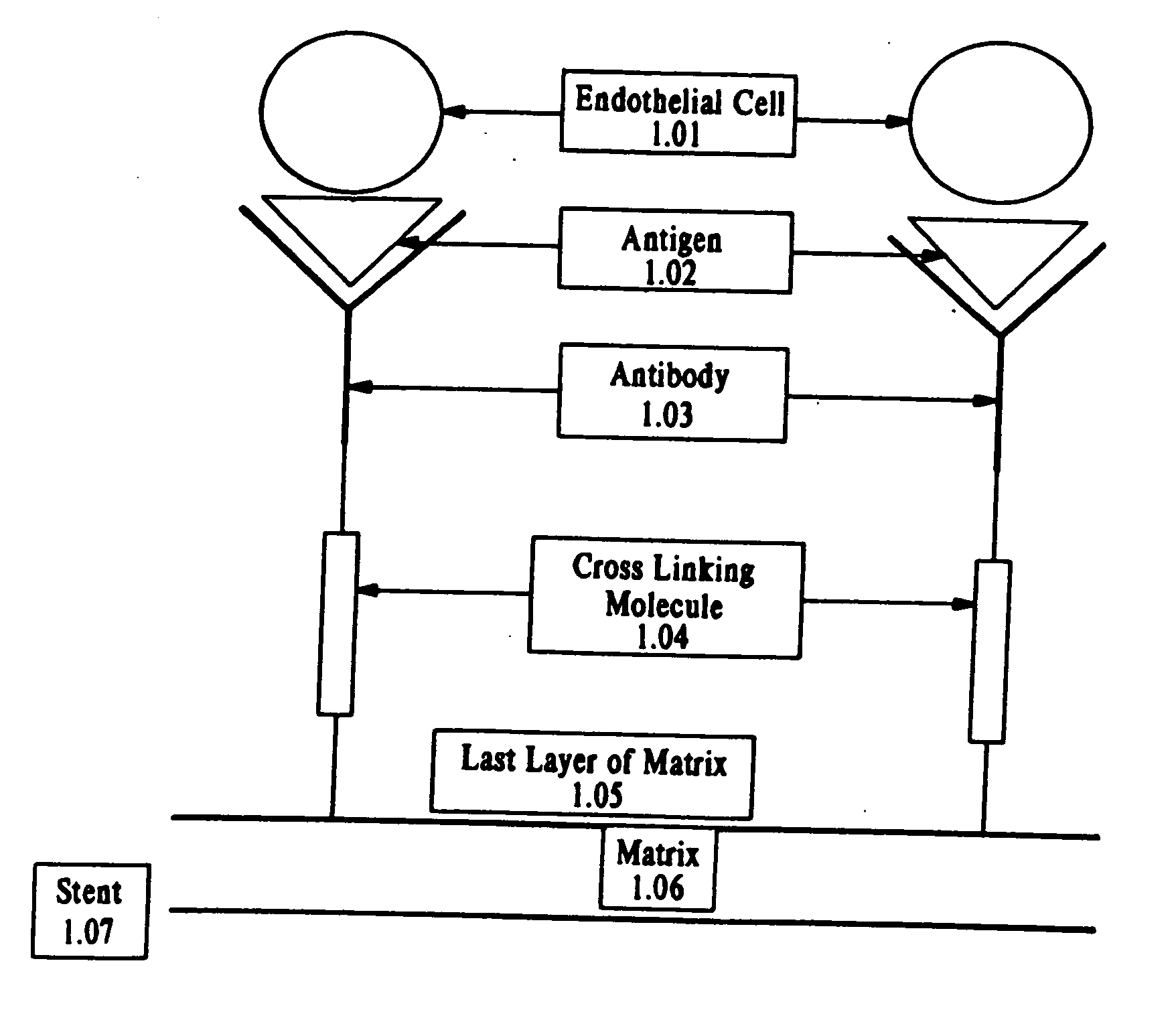
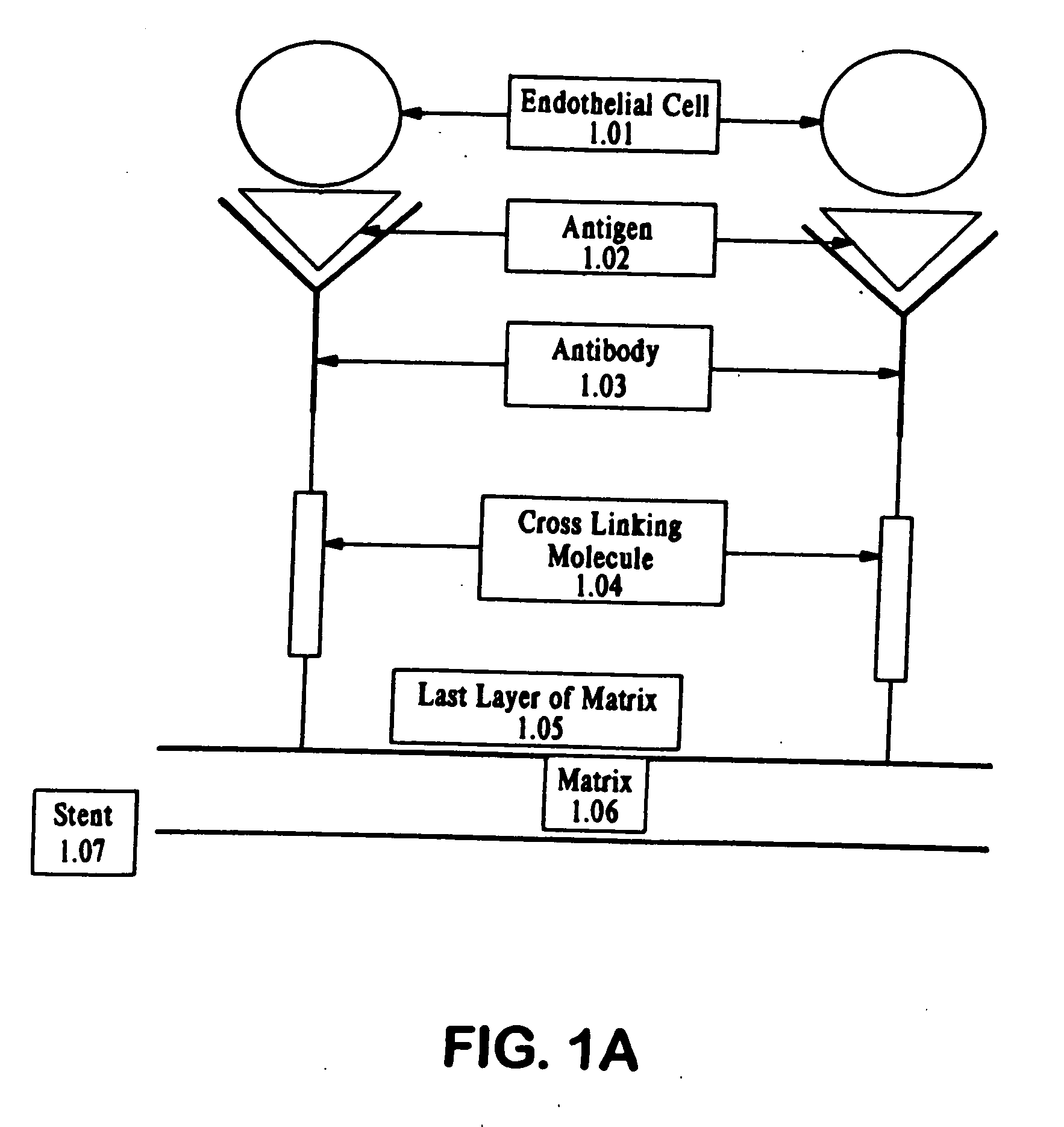
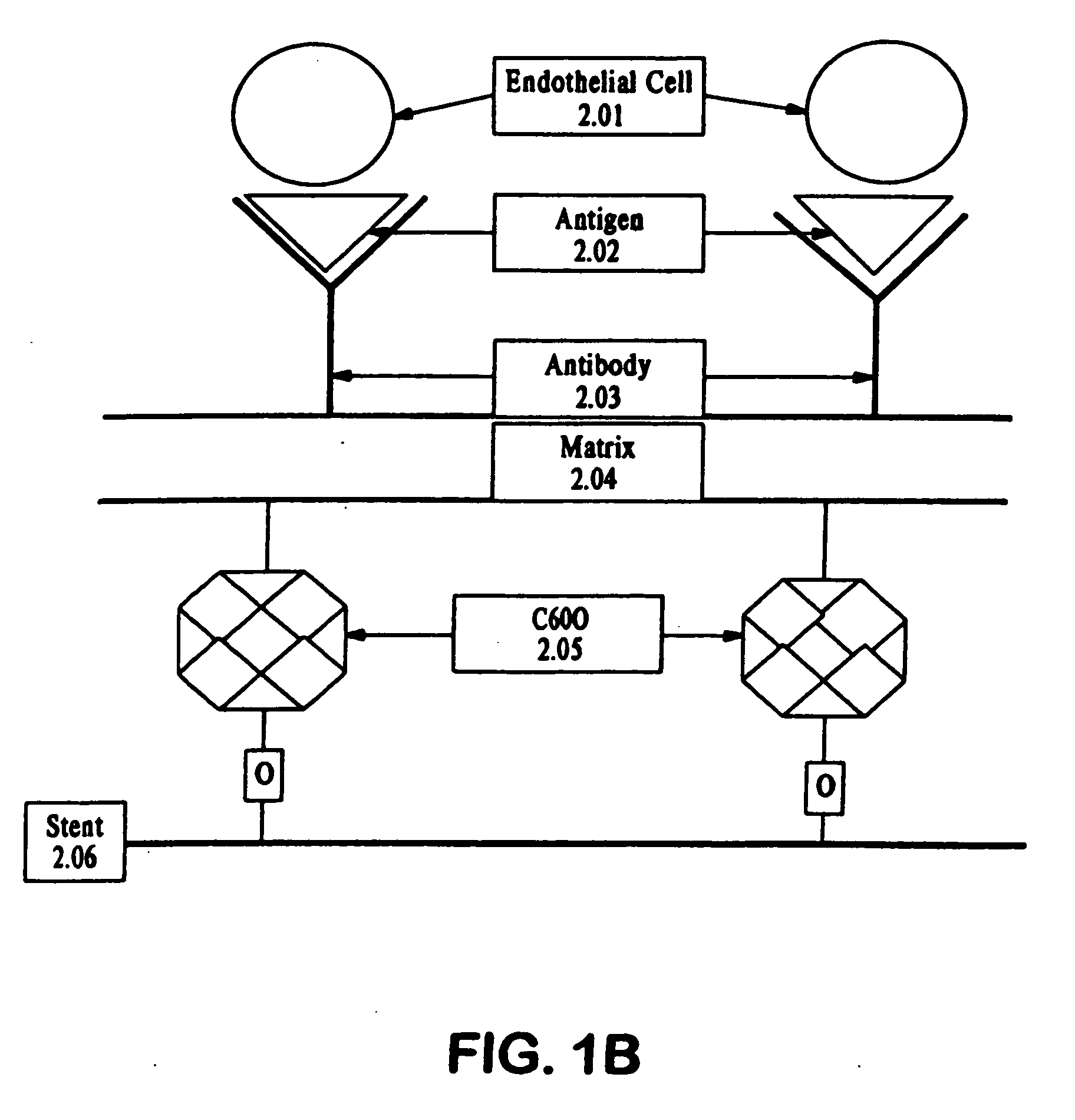
Compositions and methods are provided for producing a medical device such as a stent, a stent graft, a synthetic vascular graft, heart valves, coated with a biocompatible matrix which incorporates antibodies, antibody fragments, or small molecules, which recognize, bind to and / or interact with a progenitor cell surface antigen to immobilize the cells at the surface of the device. The coating on the device can also contain a compound or growth factor for promoting the progenitor endothelial cell to accelerate adherence, growth and differentiation of the bound cells into mature and functional endothelial cells on the surface of the device to prevent intimal hyperplasia. Methods for preparing such medical devices, compositions, and methods for treating a mammal with vascular disease such as restenosis, artherosclerosis or other types of vessel obstructions are disclosed.
Owner:ORBUS MEDICAL TECH +1
Medical devices to treat or inhibit restenosis
Implantable medical devices having anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of certain NF-kappaB inhibitors, particularly certain dialkyl fumarates, are disclosed. Dimethyl fumarate is a particularly preferred dialkyl fumarate. The anti-restenotic medical devices include stents, catheters, micro-particles, probes and vascular grafts. Intravascular stents are preferred medical devices. The medical devices can be coated using any method known in the art including compounding the dialkyl fumarate with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-dialkyl fumarate blends are disclosed. Additionally, medical devices having a coating comprising at least one dialkyl fumarate in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are also disclosed.
Owner:MEDTRONIC VASCULAR INC
Compounds useful in coating stents to prevent and treat stenosis and restenosis
InactiveUS20070037739A1Prevent restenosisBiocideHydroxy compound active ingredientsActive agentPercent Diameter Stenosis
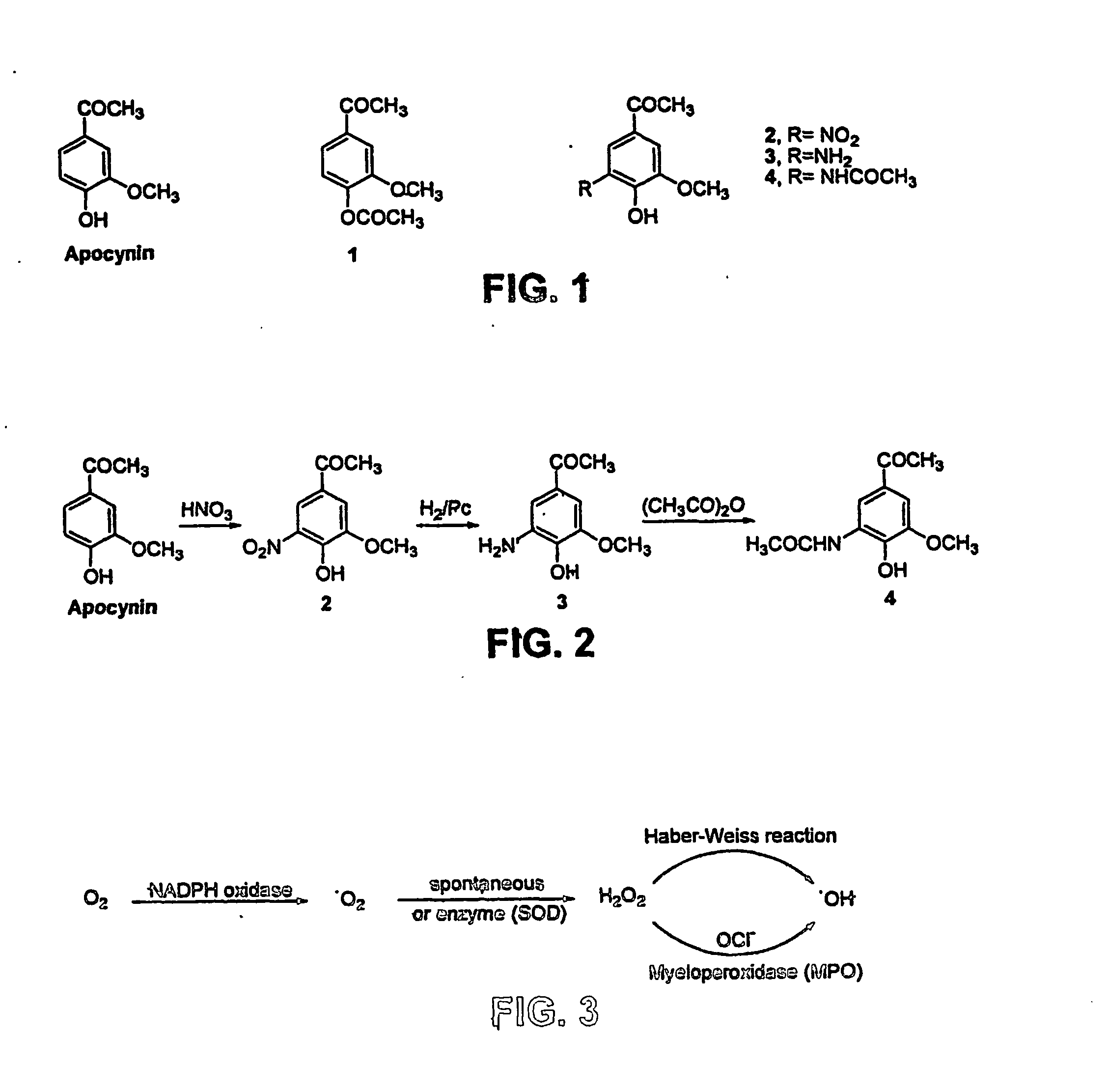
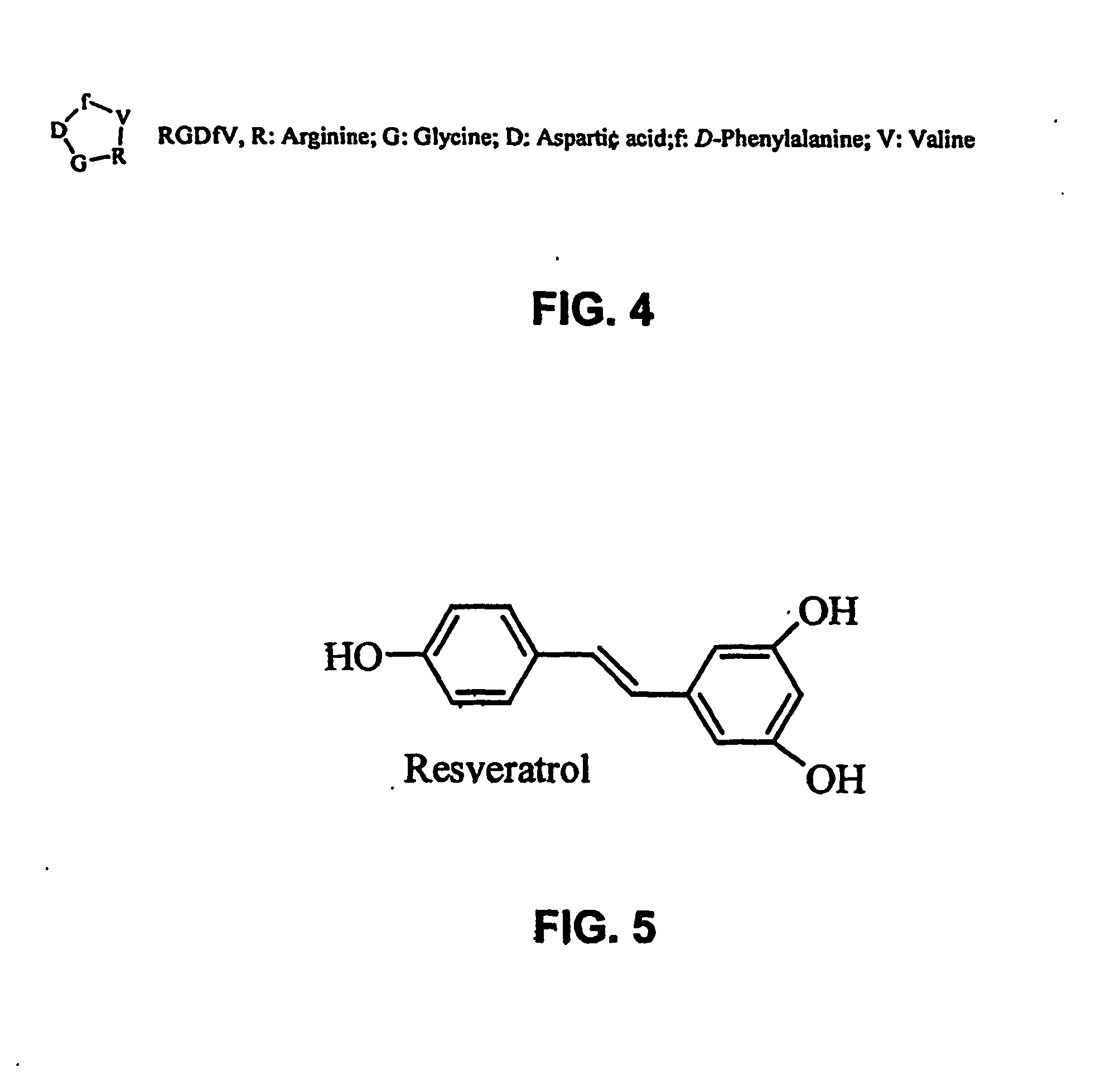
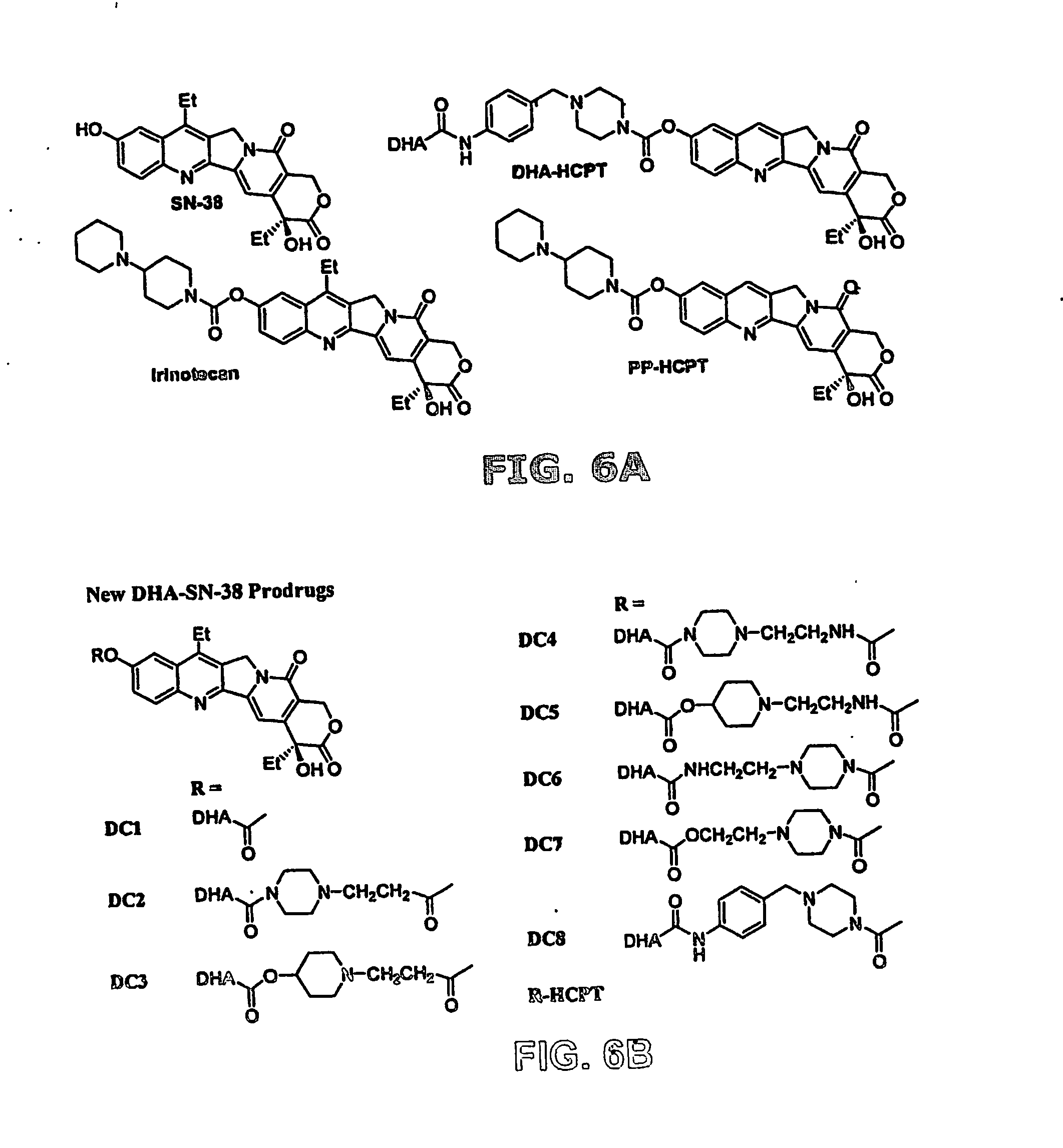
At least one bioactive agent is locally delivered to a location where a stent is implanted within a lumen in a patient's body. The bioactive agent includes a: DNA minor groove binder (such as CC-1065 or Duocarmycin); apocynin; RGD peptide (such as RGDfV); stilbene compound (such as resveratrol); camptothecin; des-aspartate angiotensin I; or ADF; or an analog or derivative thereof; or a combination or blend thereof with at least one other bioactive agent. The bioactive agent is generally locally delivered, such as by elution from the stent. The compounds and methods are of particular benefit for treating or preventing atherosclerosis, stenosis, restenosis, smooth muscle cell proliferation, occlusive disease, or other abnormal lumenal cellular proliferation condition.
Owner:MEDLOGICS DEVICE CORP
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Variants of C-type natriuretic peptide
ActiveUS8198242B2Improve the immunityReduce functionPeptide/protein ingredientsMuscular disorderDiseaseSmooth muscle
Owner:BIOMARIN PHARMA INC
Medical Devices to Prevent or Inhibit Restenosis
InactiveUS20070191934A1Highly effective at preventing or inhibiting restenosisPowder deliveryStentsSanglifehrin DPercent Diameter Stenosis
Implantable medical devices having anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of certain anti-inflammatory agents, are disclosed. The anti-inflammatory agents are selected from the group consisting of Sanglifehrin A, Sanglifehrin B, Sanglifehrin C, Sanglifehrin D, and pharmaceutically acceptable derivatives thereof. The anti-restenotic medical devices include stents, catheters, micro-particles, probes and vascular grafts. Intravascular stents are preferred medical devices. The medical devices can be coated using any method known in the art including compounding the anti-inflammatory agent with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-anti-inflammatory agent blends are disclosed. Additionally, medical devices having a coating comprising at least one anti-inflammatory agent in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the anti-restenotic implantable devices are also disclosed.
Owner:MEDTRONIC VASCULAR INC
Estrogen receptor modulators
The present invention relates to compounds and derivatives thereof, their synthesis, and their use as estrogen receptor modulators. The compounds of the instant invention are ligands for estrogen receptors and as such may be useful for treatment or prevention of a variety of conditions related to estrogen functioning including: bone loss, bone fractures, osteoporosis, cartilage degeneration, endometriosis, uterine fibroid disease, hot flashes, increased levels of LDL cholesterol, cardiovascular disease, impairment of cognitive functioning, cerebral degenerative disorders, restenosis, gynecomastia, vascular smooth muscle cell proliferation, obesity, incontinence, and cancer, in particular of the breast, uterus and prostate.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com