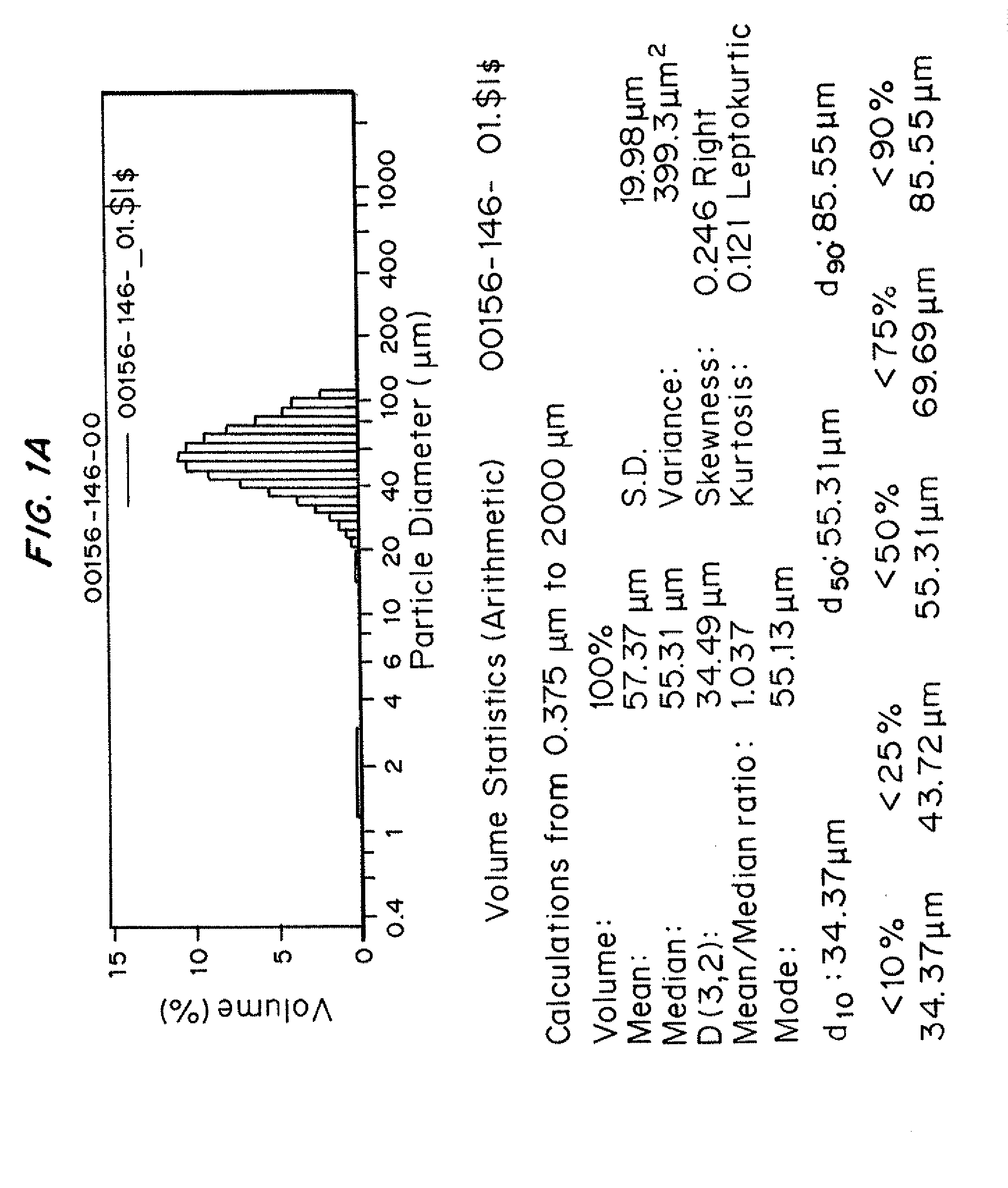
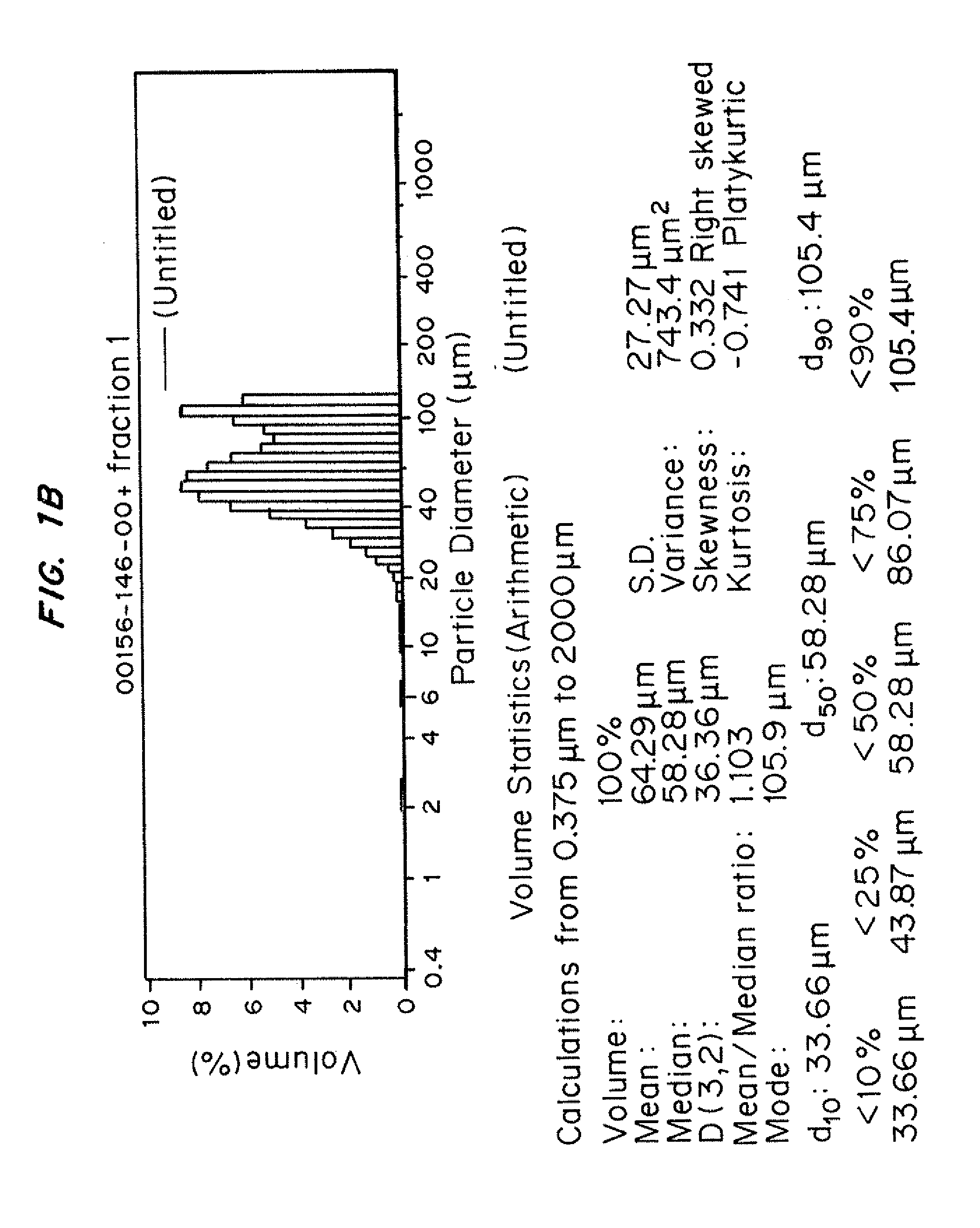
Patents
Literature
393 results about "Poly dl lactide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
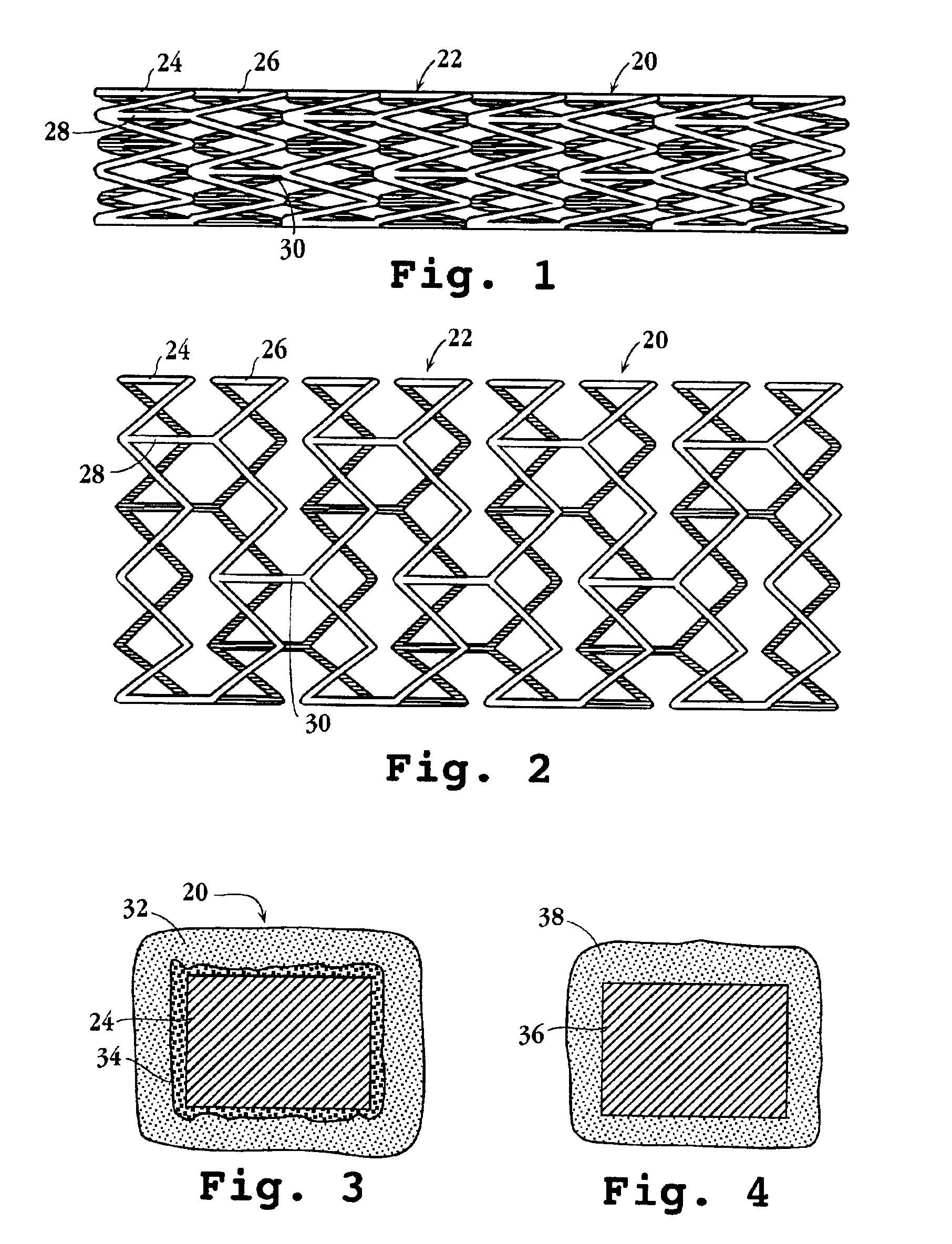
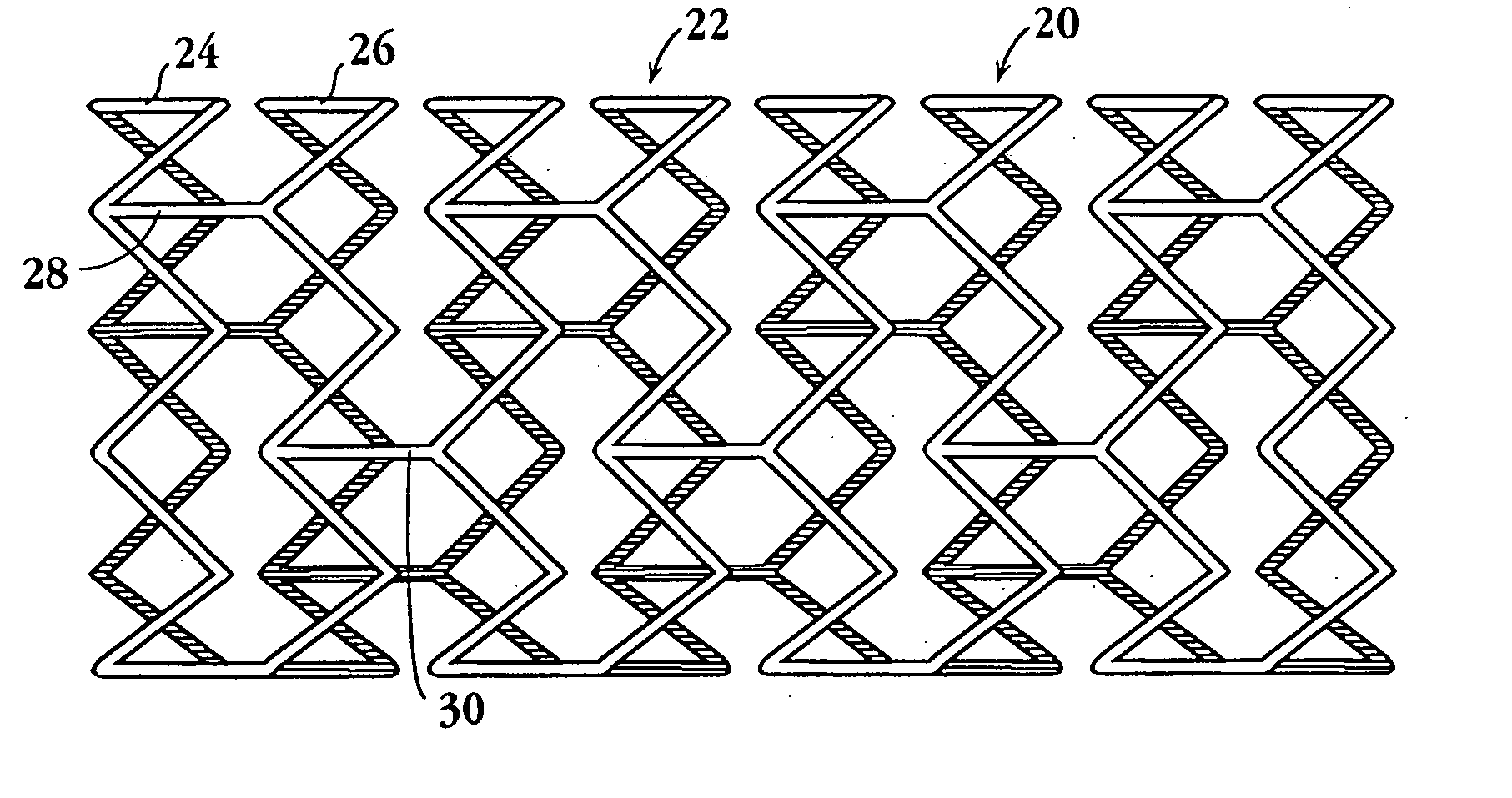
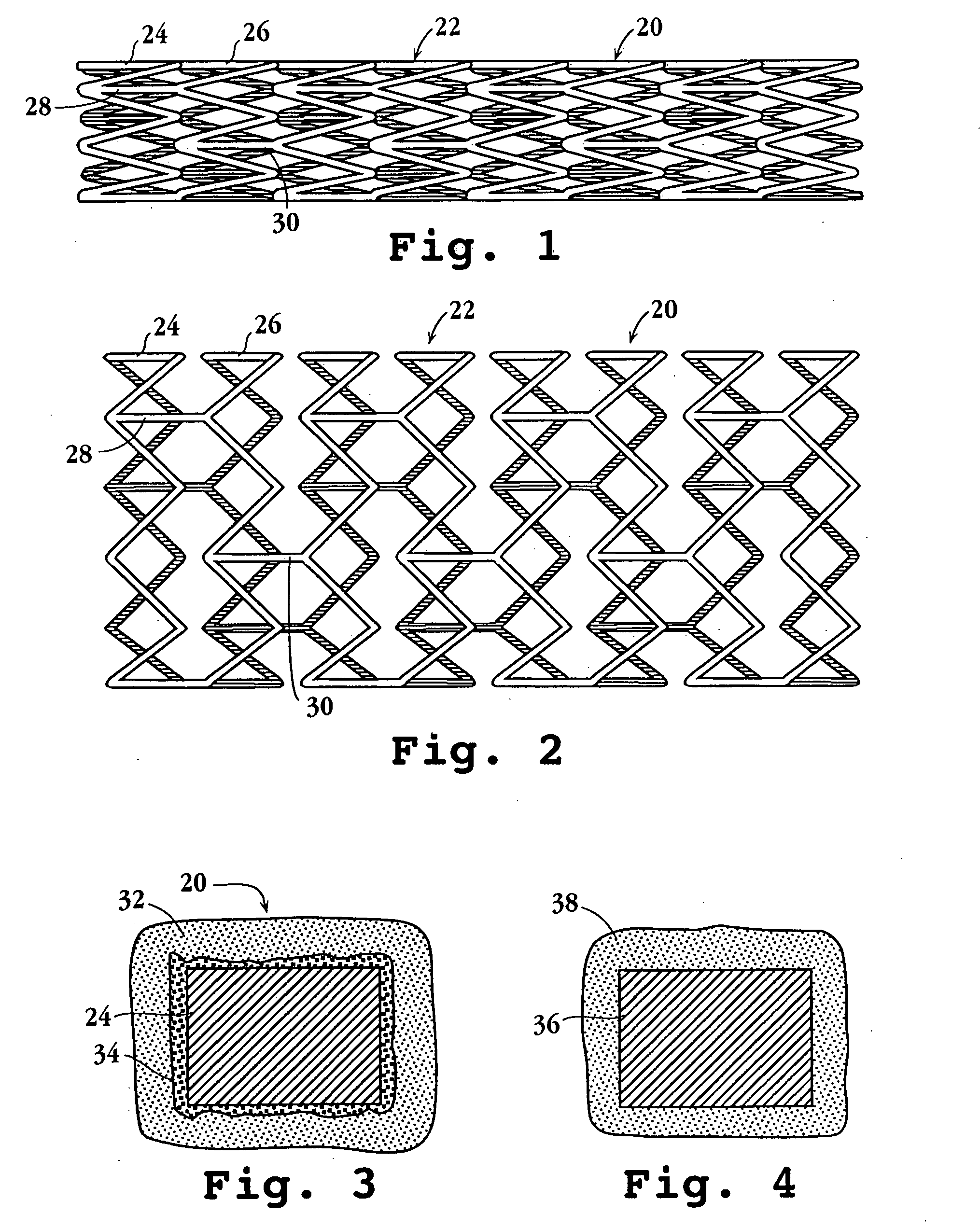
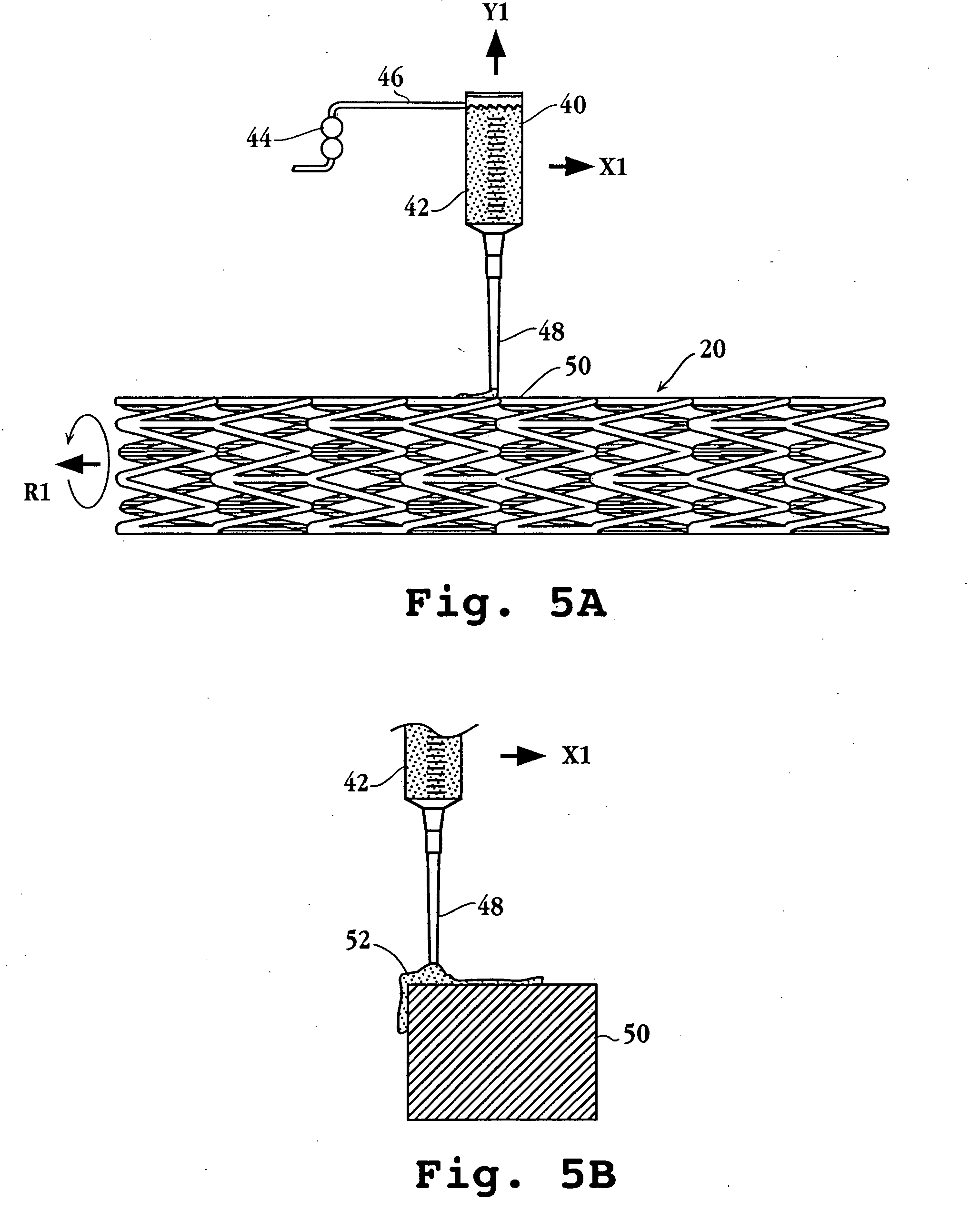
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
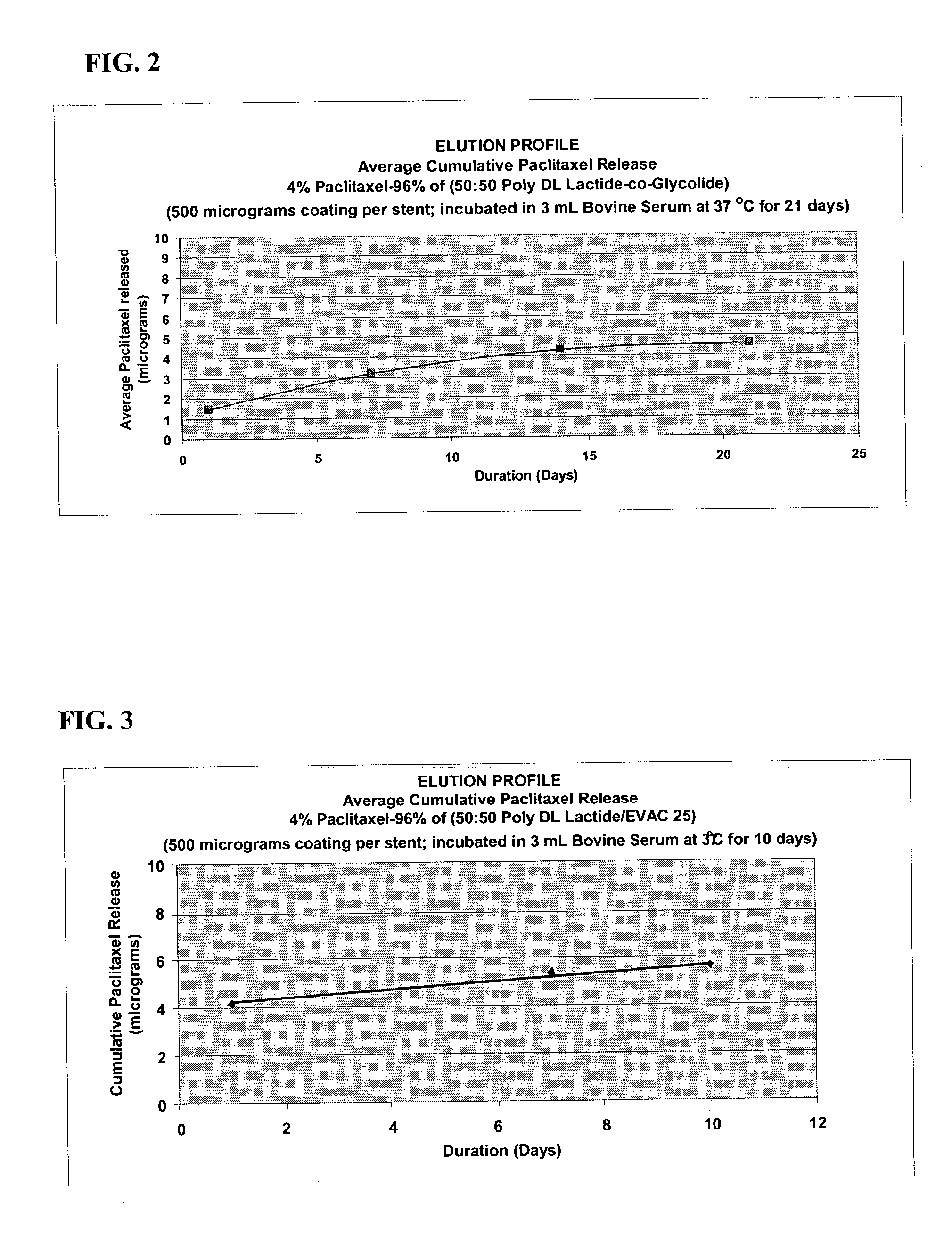
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Drug eluting implantable medical device
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Therapeutic treatment and prevention of infections with a bioactive material(s) encapuslated within a biodegradable-bio-compatable polymeric matrix
InactiveUS6902743B1Induce productionSustained release of active agent over timePowder deliveryPeptide/protein ingredientsTherapeutic treatmentActive agent
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer having a molar composition of lactide / glycolide from 90 / 10 to 40 / 60, which may contain a pharmaceutically-acceptable adjuvant, as a blend of uncapped free carboxyl end group and end-capped forms ranging to ratios from 100 / 0 to 1 / 99.
Owner:ARMY UNITED STATES GOVERNMENT AS REPRESENTED BY THE SEC OF THE
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
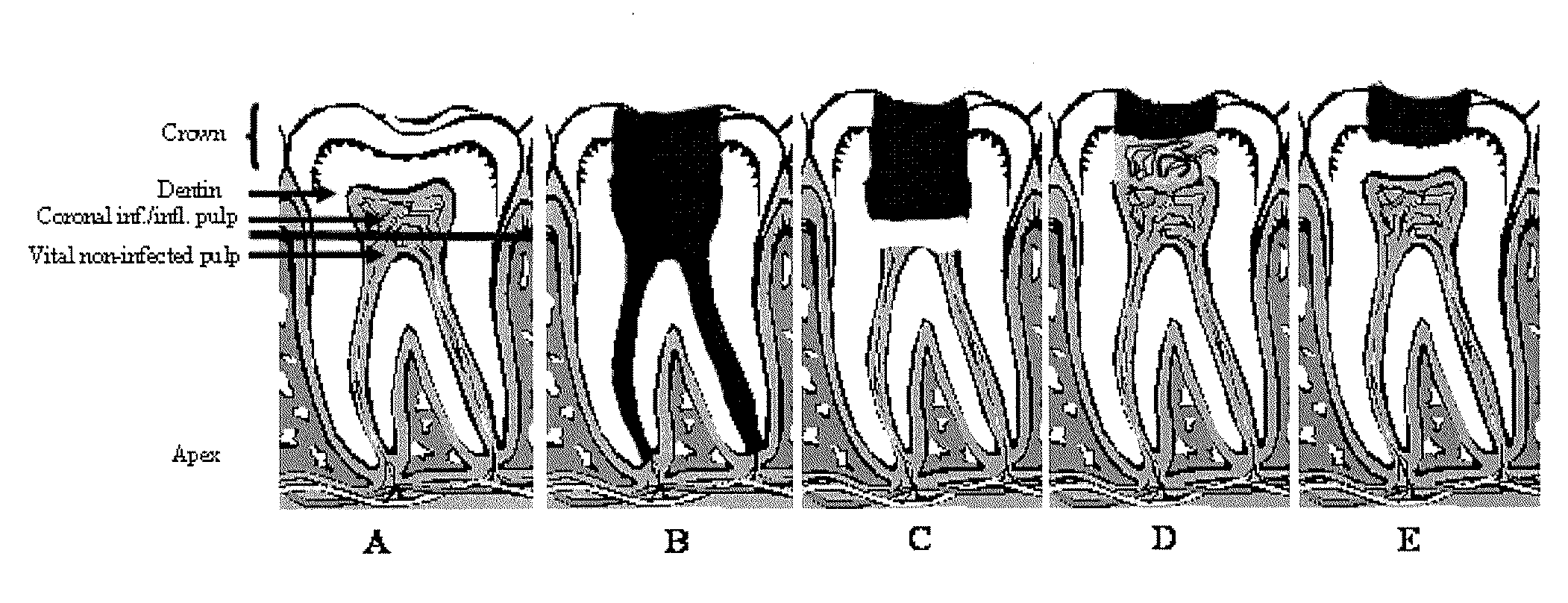
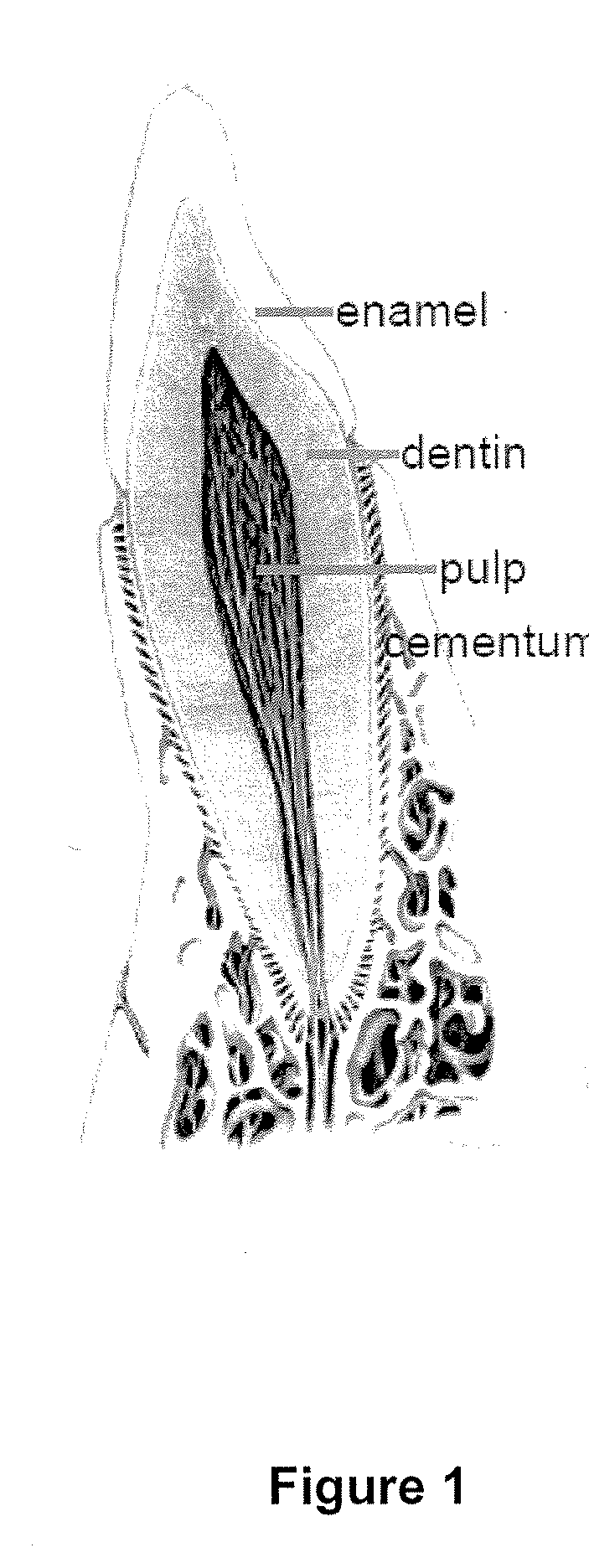
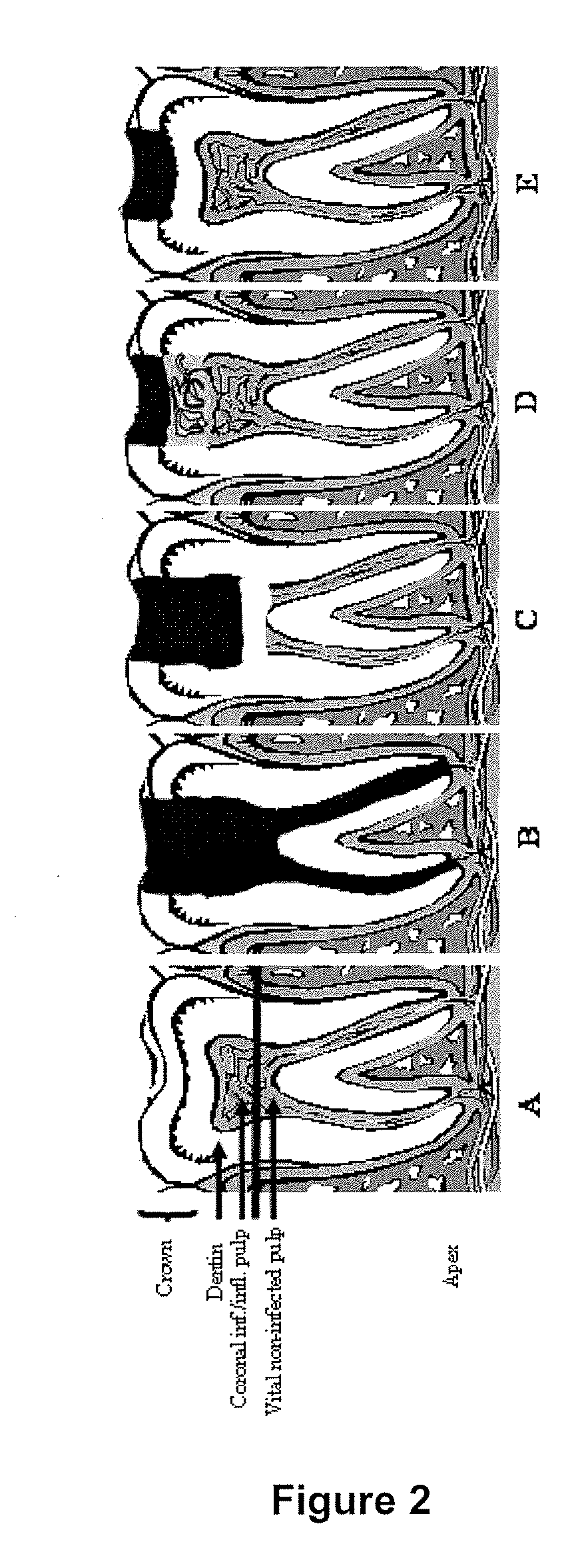
Compositions and methods for treating pulp inflammations caused by infection or trauma
The present disclosed subject matter relates to methods and compositions for restoring a diseased or damaged tooth such that infection is inhibited or eliminated and pulp regeneration is facilitated. The disclosed subject matter also includes a composition comprising a physiologically acceptable matrix seeded with pulp cells. The matrix can be capable of being injected into the pulp chamber of a tooth. In some embodiments, the matrix of a composition includes a hydrogel (e.g., collagen, chitosan, alginate, MATRIGEL™, gelatin, JELL-O®, fibrin), a mesh (e.g., polylactide-coglycolide (PLGA) mesh, polylactide (PLA) mesh, or polyglycolide (PGA) mesh, a cross-linked fiber mesh, a nanofiber mesh, a mesh fabric, biodegradable polymer mesh), a microsphere (biodegradable polymer microsphere, a hydrogel microsphere), or a combination of any of the foregoing. In yet other embodiments, the matrix includes a nanofiber, an artificial three-dimensional scaffold material, or a synthetic three-dimensional scaffold material.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
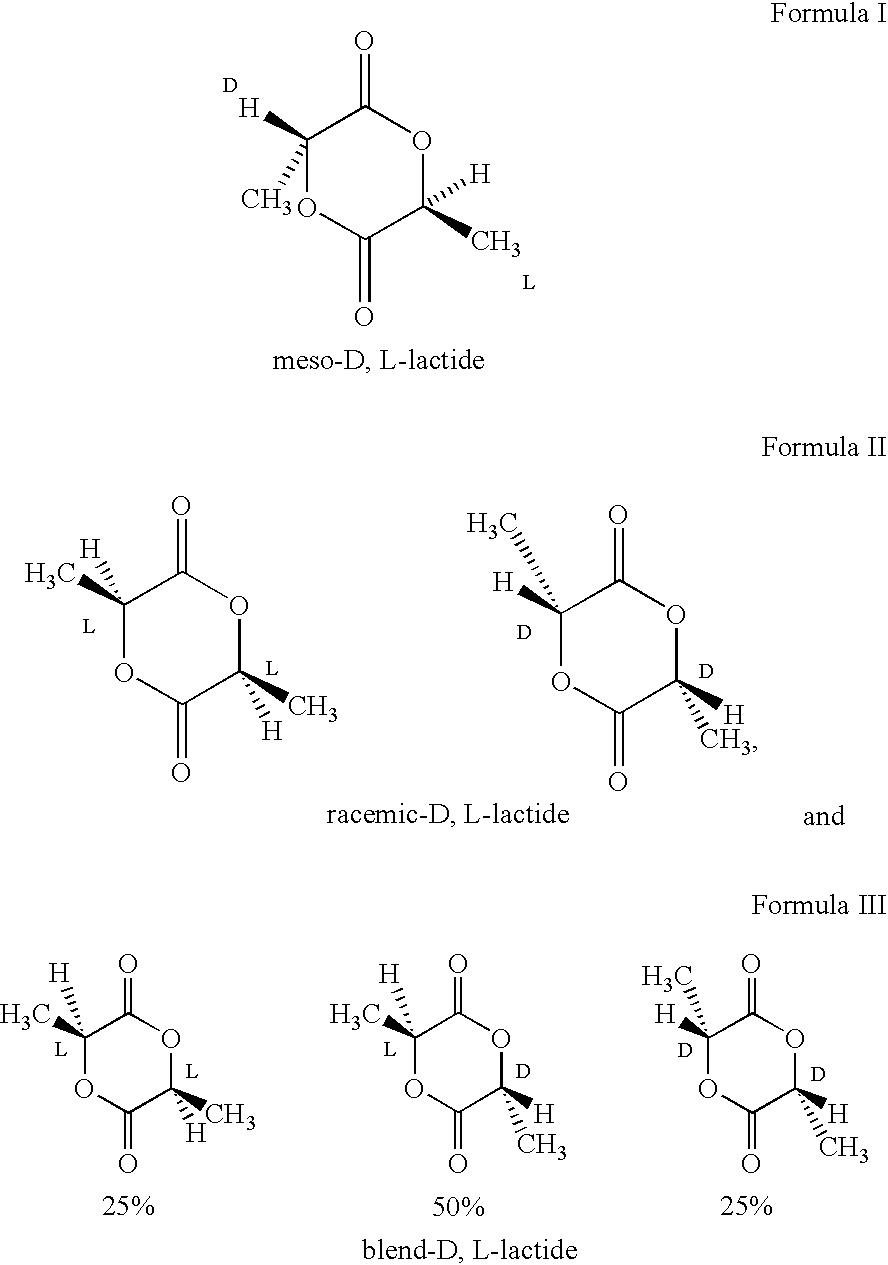
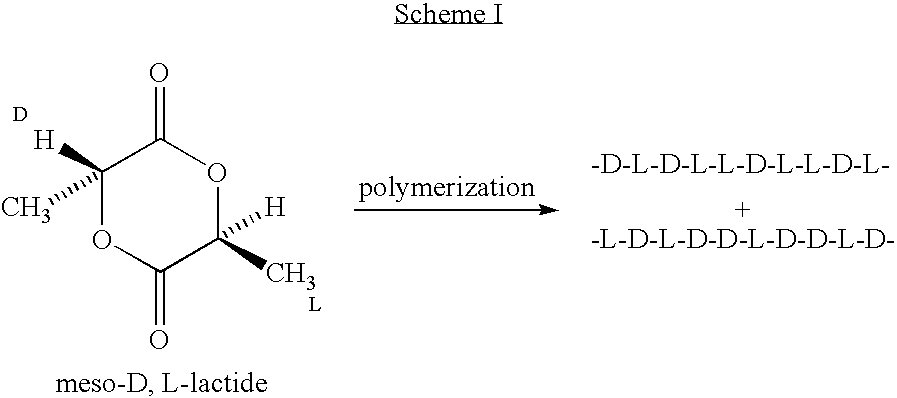
Amorphous poly(D,L-lactide) coating
Implantable devices formed of or coated with a material that includes an amorphous poly(D,L-lactide) formed of a starting material such as meso-D,L-lactide are provided. The implantable device can be used for the treatment, mitigation, prevention, or inhibition of a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Resin composition and molded article made thereof
The present invention provides a resin composition excellent in strength, impact resistance, heat resistance and moldability and further, of low environmental load capable of decreasing CO2 exhaustion in its production.The present invention is a resin composition comprising a styrene-based resin (A), an aliphatic polyester (B) and at least one species selected from a compatibilizer (C) and a dicarboxylic anhydride (D), and the compatibilizer is preferably at least one species described below.(C-1) methyl methacrylate polymer(C-2) a vinyl-based polymer to which epoxy unit or acid anhydride unit is copolymerized(C-3) a graft polymer in which methyl methacrylate unit is grafted to a rubbery polymer(C-4) a block copolymer in which polylactide segment and a vinyl-based polymer segment
Owner:TORAY IND INC
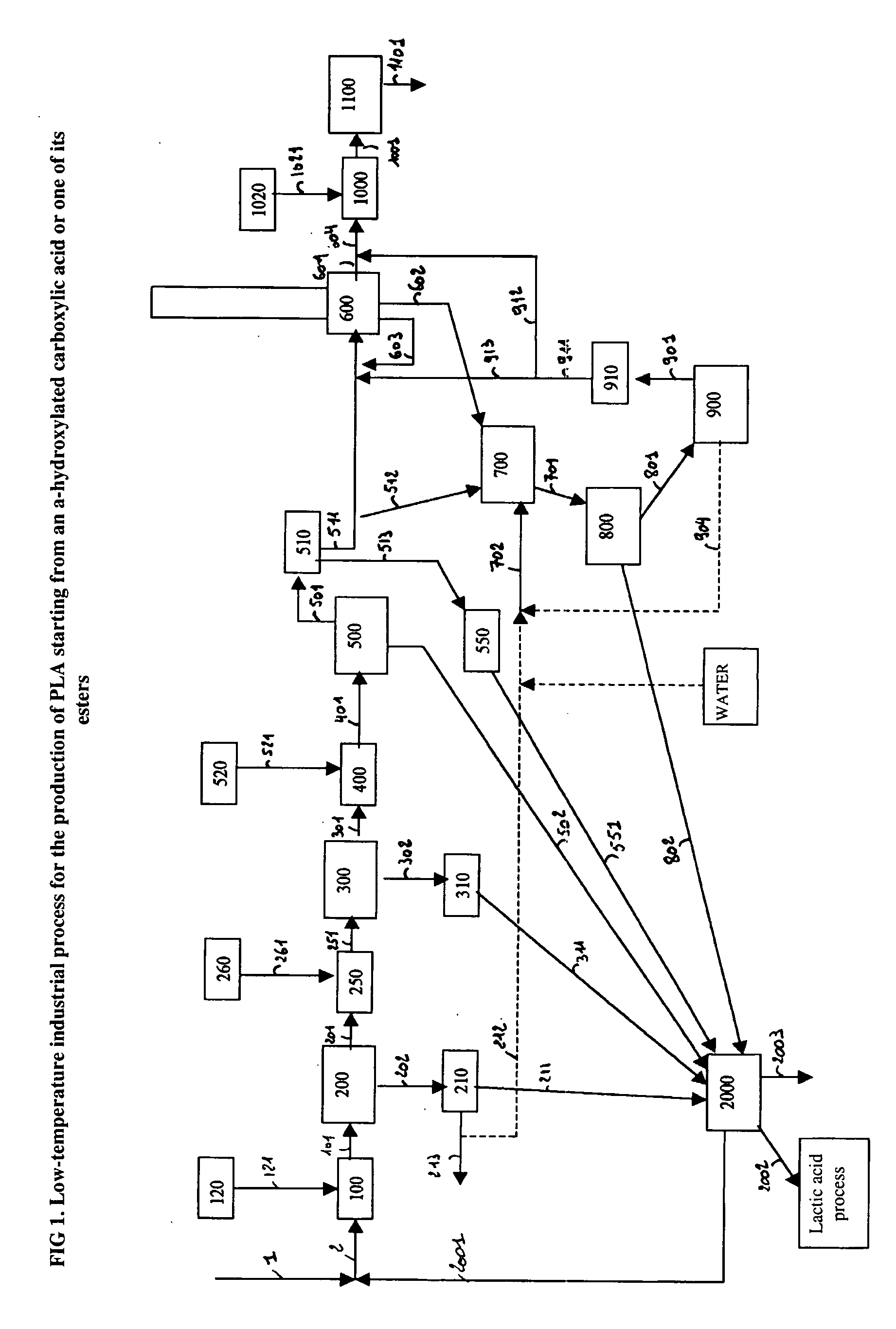
Method for the productiion of polylactide from a solution of lactic acid or one of the derivatives thereof
ActiveUS20060014975A1Easy to handleHigh mixing and dispersing effectivenessOrganic compound preparationCarboxylic acid esters preparationChemistryPolylactic acid
A process for the production of polylactide, the stages of which for the production and purification of lactide, starting from an aqueous solution of lactic acid or of its derivatives, includes evaporation of water with formation of oligomers, depolymerization to give lactide, condensation and then crystallization of the crude lactide product to give purified lactide, aqueous treatment of the residual fractions from the crystallization and polymerization of purified and / or prepurified lactide to give polylactide in an extruder and in the presence of catalysts. An alternative process includes carrying out the aqueous treatment before the crystallization.
Owner:BRUSSELS BIOTECH
Biodegradable polylactide resin composition
This invention provides a novel rapidly biodegradable polylactide resin composition while maintaining the good mechanical strength and physical properties of polylactide. This biodegradable polylactide resin composition comprises at least one protein selected from silk, gelatin, keratin, elastin, gluten, zein, or soybean. This composition further comprises degraded mannan, and furthermore, a filler.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Resorbable thin membranes
Resorbable lactide polymer thin membranes are disclosed. The thin membranes are constructed of polylactide resorbable polymers, which are engineered to be absorbed into the body relatively slowly over time in order to reduce potential negative side effects. The membranes are formed to have very thin thicknesses, for example, thicknesses between about 0.010 mm and about 0.300 mm. The membranes can be extruded from polylactide polymers having a relatively high viscosity property, can be preshaped with relatively thick portions, and can be stored in sterile packages.
Owner:MAST BIOSURGERY
Organic compositions
InactiveUS7141188B2Low dielectric constantNon-metal conductorsLiquid organic insulatorsPolyesterDielectric substrate
The present invention provides a composition comprising: (a) dielectric material; and (b) porogen comprising at least two fused aromatic rings wherein each of the fused aromatic rings has at least one alkyl substituent thereon and a bond exists between at least two of the alkyl substituents on adjacent aromatic rings. Preferably, the dielectric material is a composition comprising (a) thermosetting component comprising (1) optionally monomer of Formula I as set forth below and (2) at least one oligomer or polymer of Formula II as set forth below where Q, G, h, I, I, and w are as set forth below and (b) porogen. Preferably, the porogen is selected from the group consisting of unfunctionalized polyacenaphthylene homopolymer, functionalized polyacenaphthylene homopolymer, polyacenaphthylene copolymer, polynorbornene, polycaprolactone, poly(2-vinylnaphthalene), vinyl anthracene, polystyrene, polystyrene derivatives, polysiloxane, polyester, polyether, polyacrylate, aliphatic polycarbonate, polysulfone, polylactide, and blends thereof. The present compositions are particularly useful as dielectric substrate material in microchips, multichip modules, laminated circuit boards, and printed wiring boards.
Owner:HONEYWELL INT INC
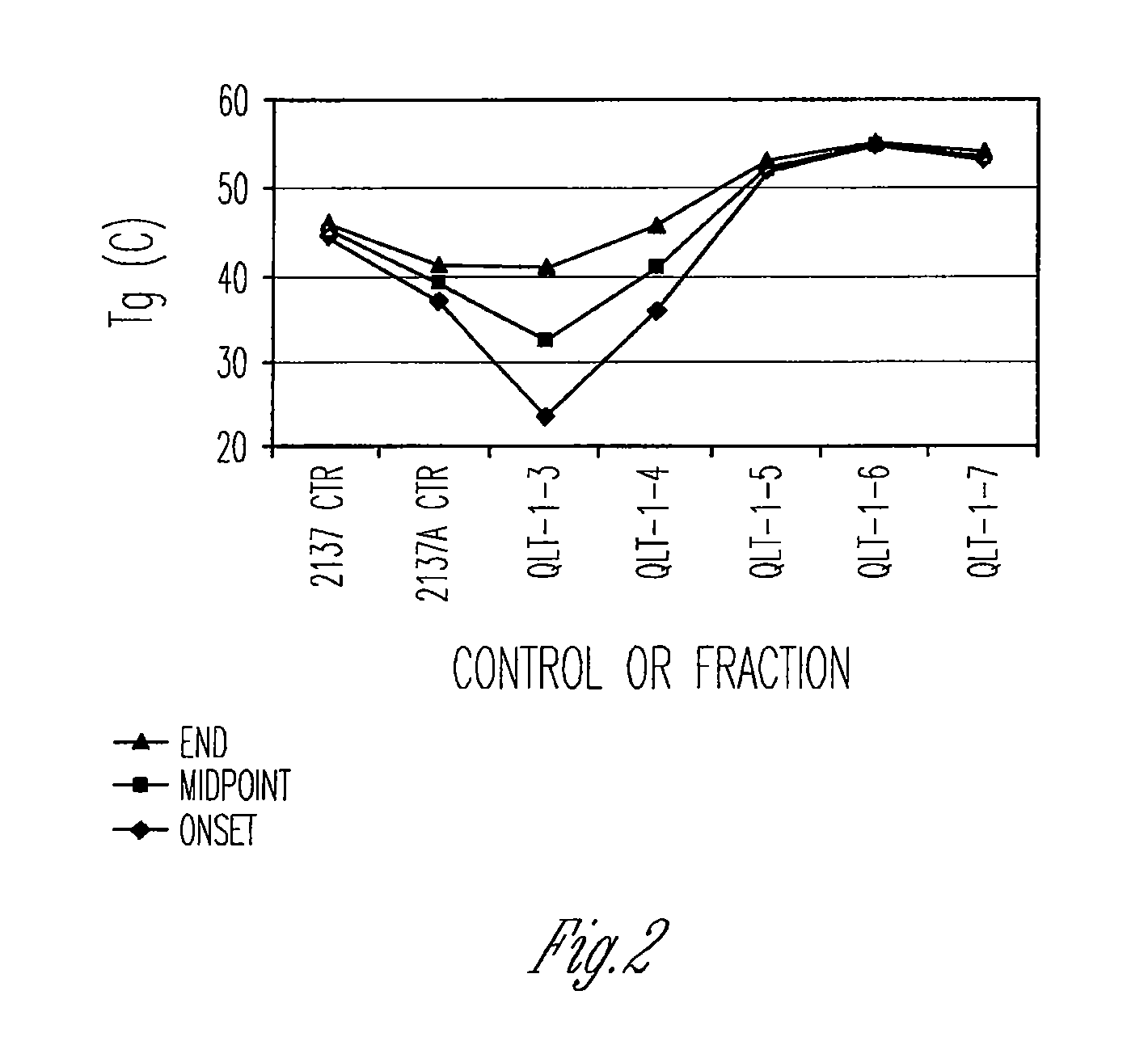
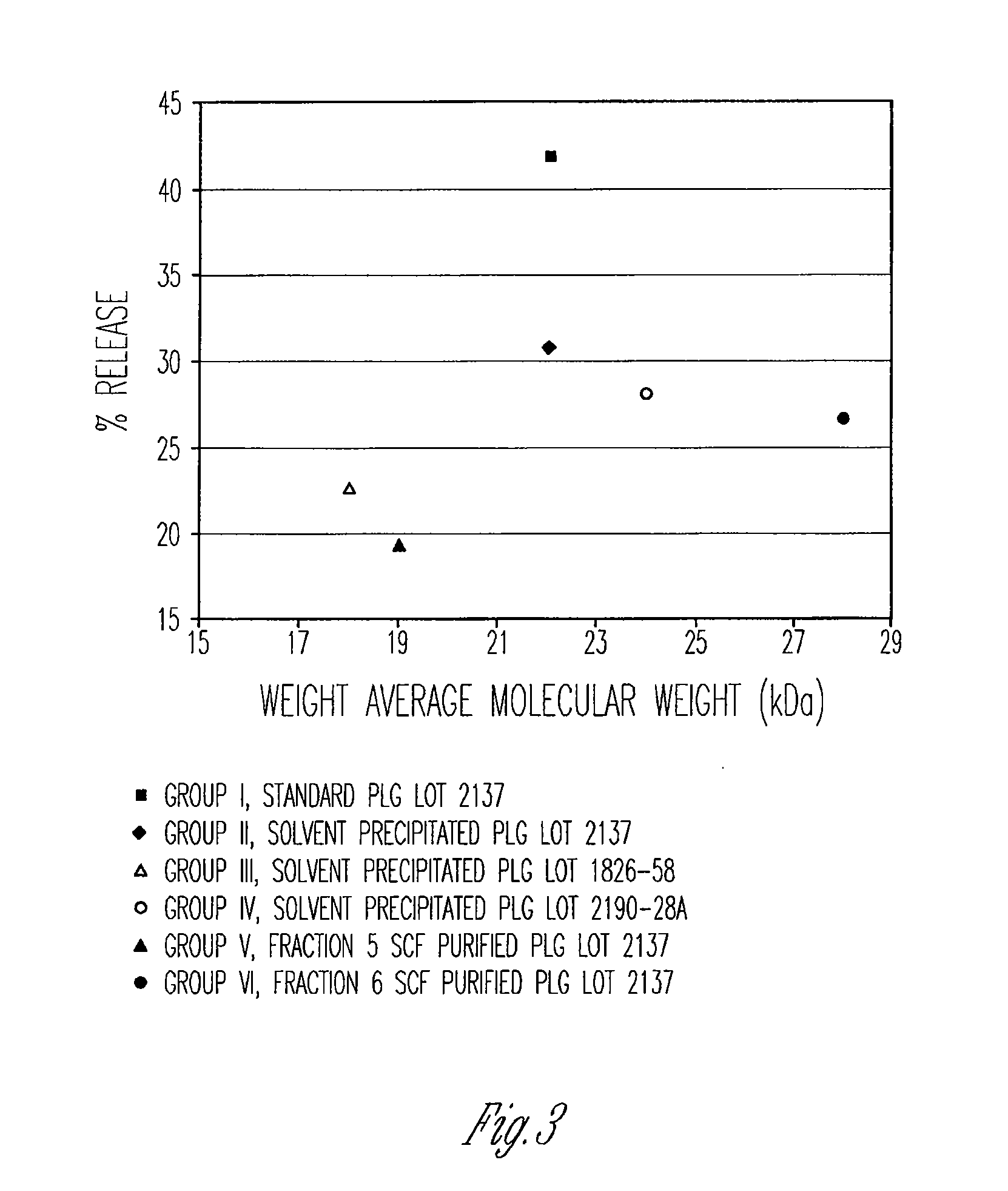
Preparation of biodegradable polyesters with low-burst properties by supercritical fluid extraction
ActiveUS20090305957A1Reduced initial burst effectNarrow molecular weight distributionBiocideOrganic active ingredientsLactideSolvent
The invention provides methods of extracting a biodegradable polyester with a supercritical fluid effective to obtain a purified biodegradable polyester, such as a purified biodegradable poly(lactide-glycolide) (PLG). The supercritical fluid can be carbon dioxide at an elevated pressure, or can be carbon dioxide with one or more cosolvents. Methods for carrying out stepwise purification of the biodegradable polyester at multiple pressures or multiple temperatures, or both, are also provided. When the polyester is PLG, a purified PLG copolymer is obtained having a narrowed molecular weight distribution with respect to the unpurified polyester. The purified PLG copolymer can have a polydispersity index of less than about 1.7, less than about 2% monomers, and less than about 10% oligomers. The purified PLG copolymer can exhibit a reduced initial burst effect when incorporated into a controlled release formulation such as a flowable implant adapted to be injected into body tissues.
Owner:TOLMAR THERAPEUTICS
Thermoplastic resin composition and molded item formed from same
InactiveUS20120329920A1Improve heat resistanceEasy to processDyeing processPolymer scienceMelamine phosphate
Provided is a thermoplastic resin composition comprising 1 to 100 parts by weight of a flame retardant (B) containing any one or more flame retardants (B-1) selected from melamine phosphate, melamine pyrophosphate, and melamine polyphosphate and any one or more flame retardants (B-2) selected from piperazine phosphate, piperazine pyrophosphate, and piperazine polyphosphate, based on 100 parts by weight of a thermoplastic resin (A) comprising polylactide resin, wherein, according to transmission electronic microscopy, the major axis of the largest particle of the flame retardant (B) in the composition is not more than 10 μm, and the ratio of the number of particles of the flame retardant (B) having a major axis of not more than 3 μm relative to the number of particles of the flame retardant (B) per an area of 1,000 μm2 is 70% or more.
Owner:TORAY IND INC
High-stability polyethylene glycol-polyester polymer and application thereof
ActiveCN103601878AImprove hydrophobicityHigh drug loadingPharmaceutical non-active ingredientsPolyesterPolymer science
The invention belongs to the field of medical technology, relates to a high-stability polyethylene glycol-polyester polymer and an application thereof, and particularly relates to an amphiphilic block copolymer having a hydrophilic block and a hydrophobic block with terminal hydroxyl, wherein the terminal hydroxyl of the hydrophobic block is replaced by a cholic acid group. The hydrophilic A block is any one of ethylene glycol monomethyl ether, polyethylene glycol, polyvinyl alcohol and polyvinylpyrrolidone; the hydrophobic B block is any one of polylactide, polylactide-co-glycolide, polyglycollide, polycaprolactone, polylactide-co-caprolactone, polyglycollide-co-caprolactone and polylactide-co-glycolide-cocaprolactone. The polymer provided by the invention can spontaneously form high-stability micelle in a waterborne medium, and can be used as a carrier of various water-insoluble drugs.
Owner:SHENYANG PHARMA UNIVERSITY
Injectable delivery of microparticles and compositions therefore
ActiveUS20100003300A1Flow fastHigh solid contentOrganic active ingredientsPeptide/protein ingredientsPolymer scienceLactide
Compositions and methods of making and using of microparticle compositions that provide faster flow or improved injectability through smaller or small-diameter needles have been developed. Notably, the microparticle compositions can be successfully delivered or administered through smaller-diameter needles than other microparticle compositions prepared from biocompatible or biodegradable polymers including, for example, poly(lactide), poly(lactide-co-glycolide), polycaprolactone, or poly-3-hydroxybutyrate. The microparticle compositions can exhibit a higher solids loading for a given needle size and / or faster flow through needles than other microparticle compositions. Further, blending or mixing the polymer of the microparticle composition with other polymer formulations can enhance the injectability of the resulting formulation.
Owner:EVONIK CORP +1
Methods of promoting enhanced healing of tissues after cardiac surgery
Resorbable polylactide polymer healing membranes and methods of their applications are disclosed. In a broad embodiment, the invention features methods for inducing proper tissue healing after an open heart surgery. In one embodiment, the methods includes a step of forming a patch with a healing membrane over the open pericardium to induce proper tissue healing and placement in other open heart surgery procedures to facilitate re-entry by the surgeon.
Owner:MAST BIOSURGERY
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
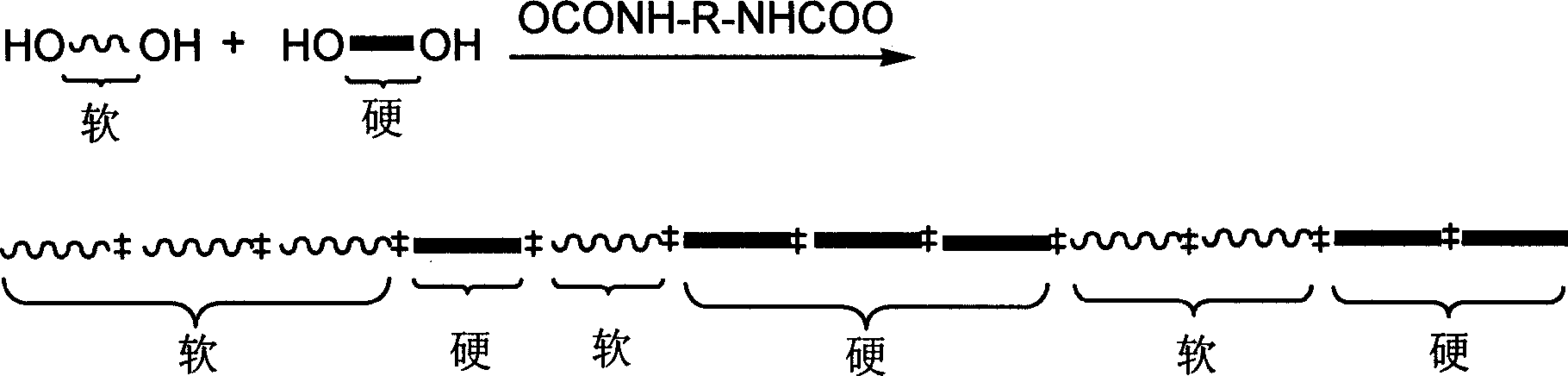
Multi-block polyurethane shape memory high molecule material and preparation method thereof
The invention provides a multi-block polyurethane shape memory polymer material based on lactide and 1, 4-dioxegy cyclohexanone. The polymer material takes hydroxyl terminated poly (lactide-co-1, 4-dioxegy cyclohexanone) as a soft block; a PUU chain segment or uramido chain segment product which is formed by vulcabond and bifunctional micromolecules containing active hydrogen is chosen as a hard block. The temperature of the shape memory is within 20-55 DEG C. The invention further provides the preparation method of the material. The shape memory polymer material based on lactide and 1, 4-dioxegy cyclohexanone provided by the invention integrates good odegradability, biocompatibility, high mechanical strength, flexility and the shape memory performance together. The deformation temperature of the polymer material can be adjusted to be close to human body temperature; therefore, the polymer material is applied to surgical operations or medical appliance implantation materials.
Owner:CHONGQING UNIV +1
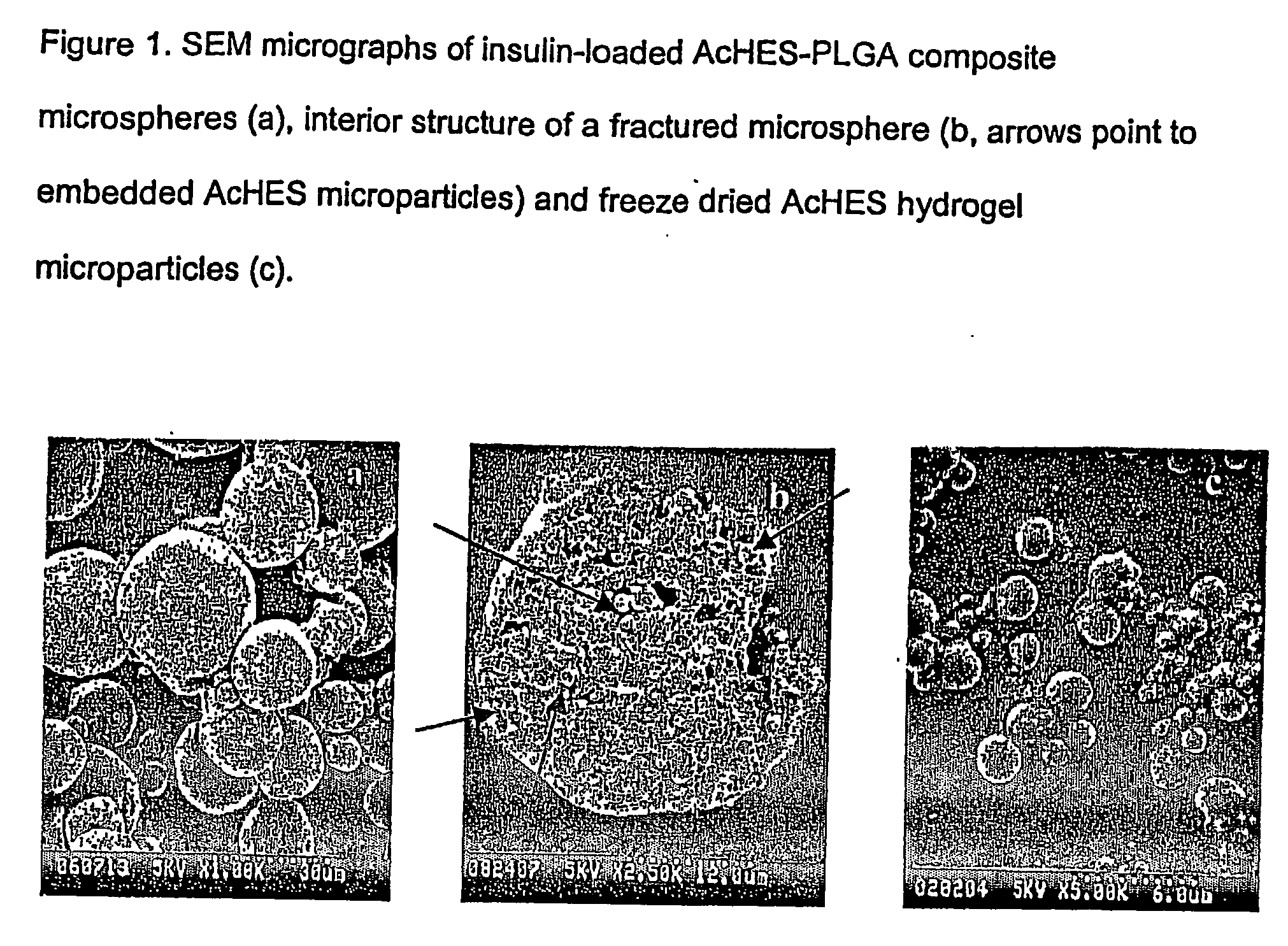
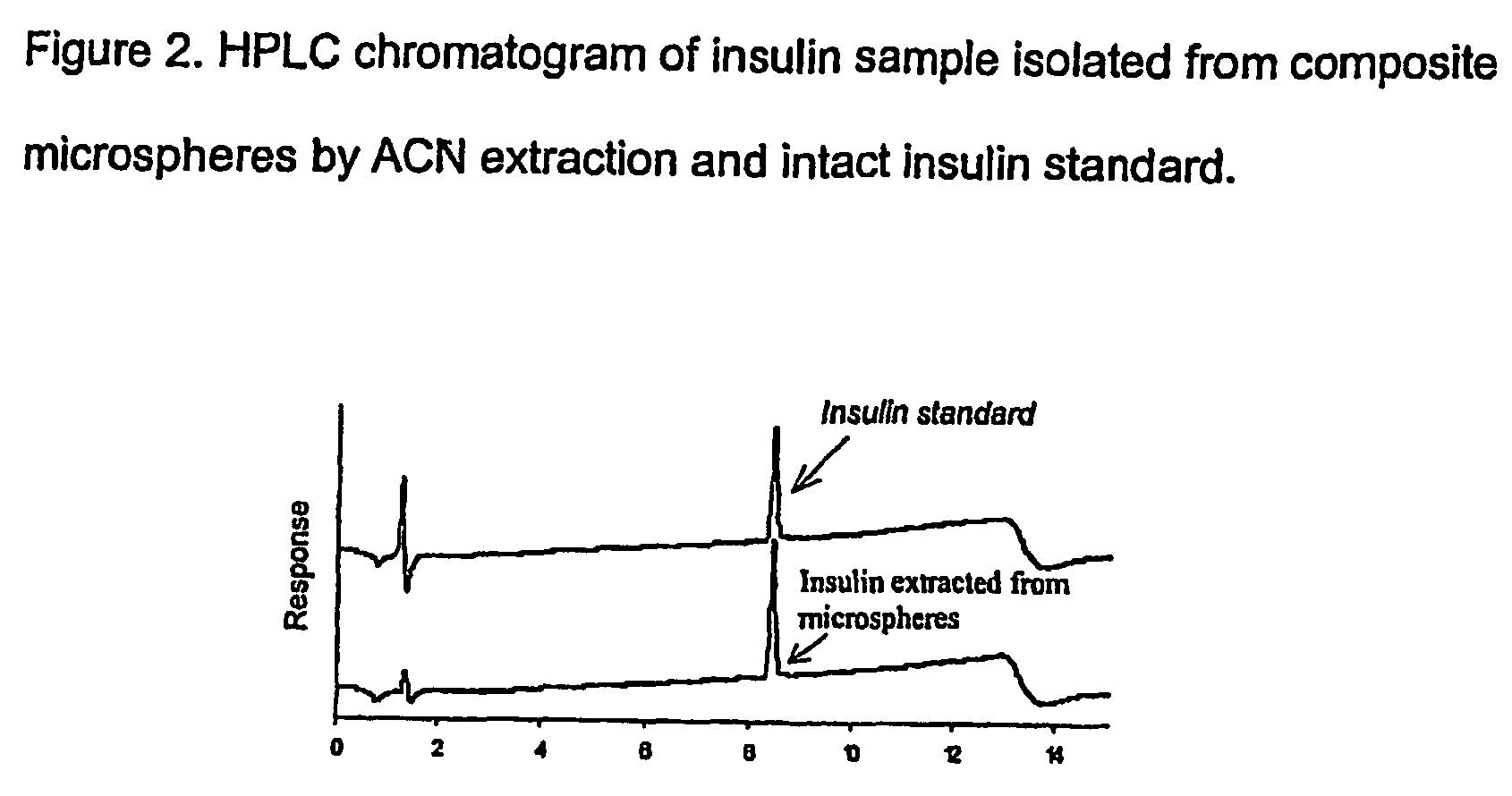
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Aniline oligomer, its aliphatic polyester copolymer and their prepn
InactiveCN1887854AImprove electrical activityGood biocompatibilityAmino compound preparation by condensation/addition reactionsPolyesterPentamer
The present invention provides aniline oligomers, their aliphatic polyester copolymer and their preparation. Two kinds of aniline oligomers are first synthesized with N-phenyl-1, 4-p-phenylene diamine as material, and then copolymerized with aliphatic polyester to obtain electrically active biodegradable polymers. During the preparation, N-phenyl-1, 4-p-phenylene diamine has its end amido group protected with butanedioic anhydride and is then reacted with end amido aniline dimer and phenylene diamine to obtain aniline tetramer with one end carboxyl group and one end amino group and aniline pentamer with two end carboxyl groups; and the aniline oligomers are finally polycondensated with double hydroxyl group terminated aliphatic polyester to obtain the copolymers containing electrically active aniline oligomer block. The copolymers possess the advantages of both aniline oligomer and aliphatic polyester, and is used as biomedicine material mainly.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Method for the removal and recovery of lactide from polylactide
The invention relates to a method for the removal of lactide from polylactide and recovery of lactide from a lactide-containing gas by cooling the hot lactide-containing gas, wherein a polymer melt traveling through a nozzle forms thin threads the surface area of which is so large that in a normal-pressure or vacuum evaporator the lactide evaporates from the polymer rapidly into a hot carrier-gas flow and the polymer settles under gravity onto a collecting device. A hot lactide-containing gas is cooled rapidly to a temperature below 100° C., whereupon the lactide crystallizes from gas, forming lactide crystals, which are separated from the gas.
Owner:HYFLUX IP RESOURCES
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS7501134B2Easy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Anti-cancer sustained-released injection containing epothilone derivate
InactiveCN101396342AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryPoly dl lactidePolyethylene glycol
The invention relates to an anti-cancer sustained release injection containing epothilone derivative, consisting of sustained microspheres and menstruum. The sustained microspheres comprise anti-cancer drugs selected from taxane, alkylating agent and / or plant alkaloid and the like, the epothilone derivative and sustained release auxiliary material. The menstruum is a special menstruum containing suspending agent. The epothilone derivative is selected from epothilone B, epothilone D, iso-epothilone D, BMS-247550, azaepothilone B, furan epothilone D or BMS-310705. The sustained release auxiliary material is selected from poly-dl-lactide, the glycolic acid copolymer of the poly-dl-lactide, polyethyleneglycol, the polylactide copolymer of the polyethyleneglycol, carboxyl terminated polylactide copolymer, fatty acid and decanedioic acid copolymer, etc. The suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The sustained release microsphere can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can release drug at partial position for 40 days approximately, therefore, the sustained release injection improves the local drug concentration selectively and enhances the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy and the like at the same time.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer sustained-released injection containing epothilone derivate
InactiveCN101396340AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryPoly dl lactidePolyethylene glycol
The invention relates to an anti-cancer sustained release injection containing epothilone derivative, consisting of sustained microspheres and menstruum. The sustained microspheres comprise anti-cancer drugs selected from taxane, alkylating agent and / or plant alkaloid and the like, the epothilone derivative and sustained release auxiliary material. The menstruum is a special menstruum containing suspending agent. The epothilone derivative is selected from epothilone B, epothilone D, iso-epothilone D, BMS-247550, azaepothilone B, furan epothilone D or BMS-310705. The sustained release auxiliary material is selected from poly-dl-lactide, the glycolic acid copolymer of the poly-dl-lactide, polyethyleneglycol, the polylactide copolymer of the polyethyleneglycol, carboxyl terminated polylactide copolymer, fatty acid and decanedioic acid copolymer, etc. The suspending agent is selected from carboxymethyl cellulose and the like with the viscosity of 100cp to 3000cp (under the temperature of 25 DEG C to 30 DEG C). The sustained release microsphere can also be made into a sustained release implant. The sustained release injection is injected or arranged in or around the tumour and can release drug at partial position for 40 days approximately, therefore, the sustained release injection improves the local drug concentration selectively and enhances the treatment effect of non-operative treatments, such as radiotherapy, chemotherapy and the like at the same time.
Owner:JINAN SHUAIHUA PHARMA TECH
Multi-block biodegradable shape memory polymeric compound with regular structure and preparation thereof
The invention discloses a regular structure multi-block biodegradable thermic shape memory polymer and a preparation method thereof. The shape memory polymer consists of a plurality of soft blocks and hard blocks which are alternately arranged. The soft blocks and the hard blocks are both copolymers and both have even chain length; the content of the hard blocks is between 50 and 90 weight percent; and the content of the soft blocks is between 10 and 50 weight percent. Each soft block is a crystalline poly(epsilon-caprolactone-glycolide)chain segment which has the fusion point 3 DEG C above a body temperature; and each hard block is a crystalline poly(L-lactide-glycolide)chain segment which has the fusion point 50 DEG C above the body temperature and a controllable deformation recovery rate. The shape memory polymer can relatively independently control the mechanical properties and the degradation rate, thereby having the mechanical properties and the controllable degradation rate matched with the implanted tissue in vivo. With good forming machining performance and excellent biocompatibility, the shape memory polymer can be used as a new functional biological medical material. The preparation method is characterized by simplicity, short reaction time, no residual harmful reagent content, and the like, thereby facilitating the commercialization.
Owner:ZHEJIANG UNIV
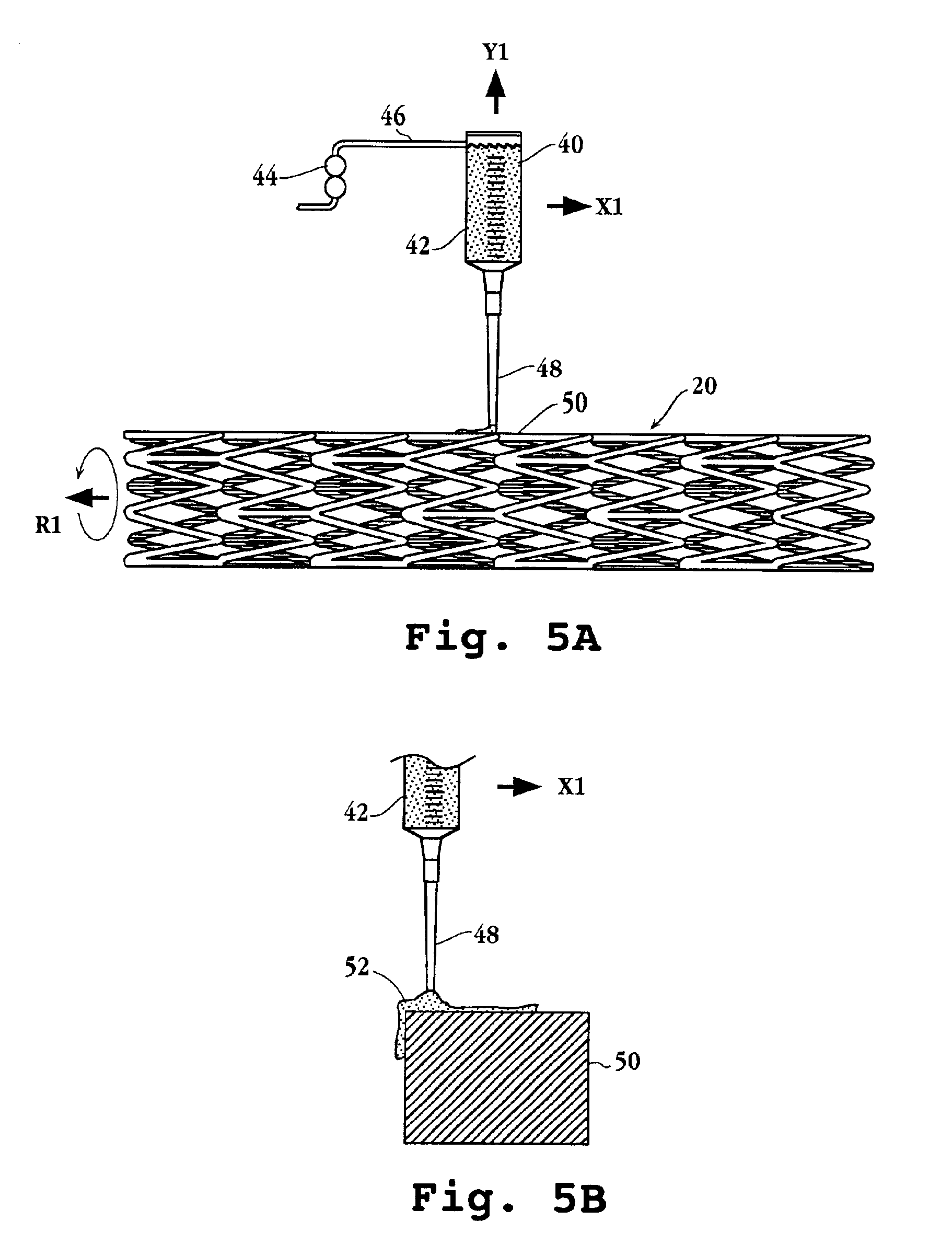
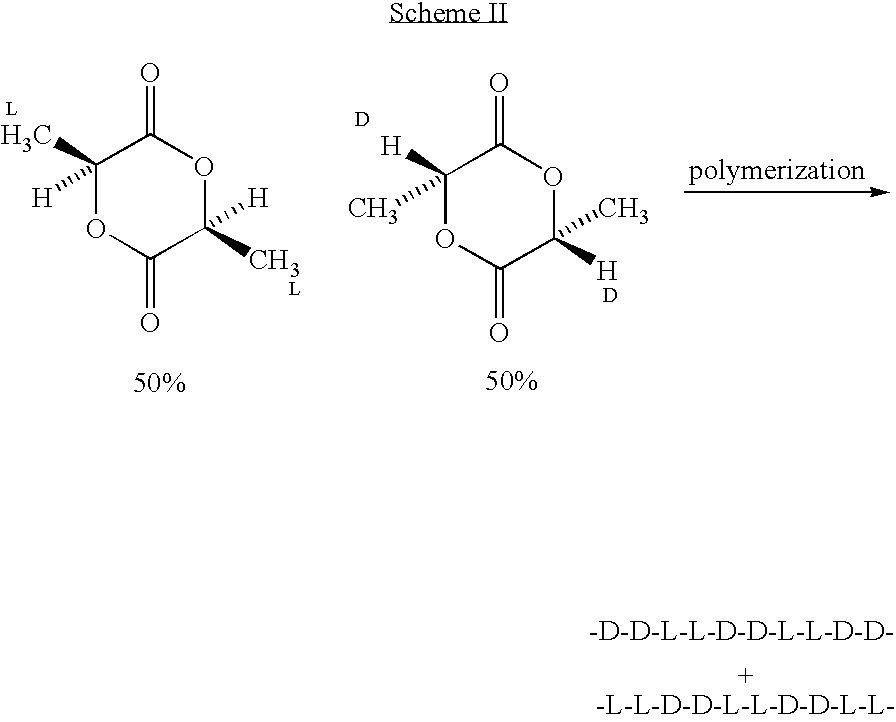
Process for manufacturing a stationary state of crystalline polymer of a biodegradable polymer matrix carrying an active substance and a polymer matrix produced thereby
InactiveUS20080008735A1Good storage stabilityOvercome disadvantagesSurgeryPharmaceutical containersVitrificationCrystallinity
A method for manufacturing a stationary state of polymer crystallinity of an active substance charged polylactide matrix for a stent, comprising the steps of: preparing the active substance charged polylactide matrix containing (a) a polylactide in amorphous or semicrystalline modification or with amorphous domains, and (b) at least one active substance on a surface or in a cavity of the stent communicating with the surface; and beating of the active substance charged polylactide matrix to a temperature ranging from TG−20° C. to TS−10° C., where TG represents a glass transition temperature and TS a melting temperature of the crystallites of the polylactide.
Owner:BIOTRONIK AG
Novel sustained release polymer
A polymer and a method for its preparation are provided. The polymer comprises poly(lactide), poly(lactide / glycolide) or poly(lactic acid / glycolic acid) segments bonded by ester linkages to both ends of an alkanediol core unit. The polymer is for use in a controlled release formulation for a medicament, preferably leuprolide acetate. The controlled release formulation is administered to a patient as a subcutaneous depot of a flowable composition comprising the polymer, a biocompatible solvent, and the medicament. Controlled release formulations comprising the polymer release leuprolide for treatment of prostate cancer patients over periods of 3-6 months.
Owner:TOLMAR INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com